Recombination VTT and method for detecting vaccinia virus neutralizing antibody by using same
A vaccinia virus and antibody technology, applied in the field of immunology, can solve the problems of strong subjectivity in result analysis, long detection cycle, and high detection cost, and achieve the effects of easy promotion, short detection cycle, and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Construction of recombinant vaccinia virus Tiantan strain comprising firefly luciferase gene
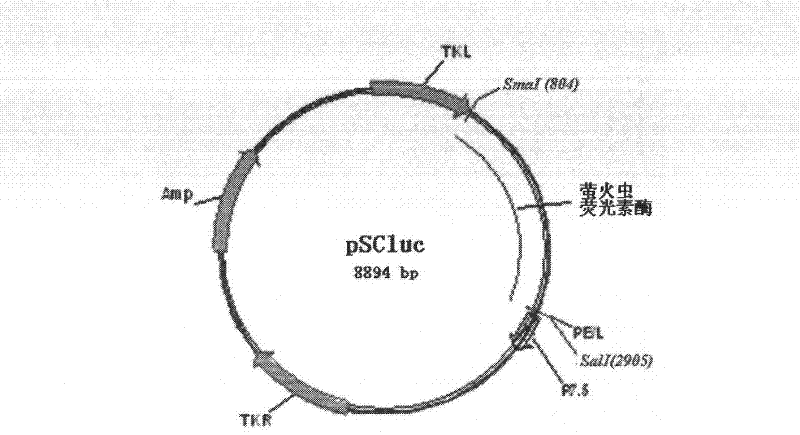
[0051] The construction process of the vaccinia virus Tiantan strain containing the firefly luciferase gene is as follows: First, construct the recombinant plasmid pSCluc containing the firefly luciferase gene using the pSC59 plasmid as a framework; use lipofect2000 liposome technology to transfect the vaccinia virus Tiantan strain (vaccine strain) Infected chicken embryo fibroblasts (CEF), through the X-Gal gene blue-white selection process to obtain recombinant replicative vaccinia virus Tiantan strain rTV-Fluc.
[0052] 1. Construction of recombinant vaccinia virus shuttle plasmid (pSCluc) comprising luciferase gene
[0053] 1.1 Luciferase gene PCR amplification
[0054] Use PrimeSTAR high-fidelity enzyme (TAKARA, Cat: DR010A) to contain the plasmid pLUCF (John T. Schiller, National Cancer Institute) containing the luciferase gene, and the plasmid containing the...
Embodiment 2
[0144] Example 2: Detection of vaccinia virus neutralizing antibody
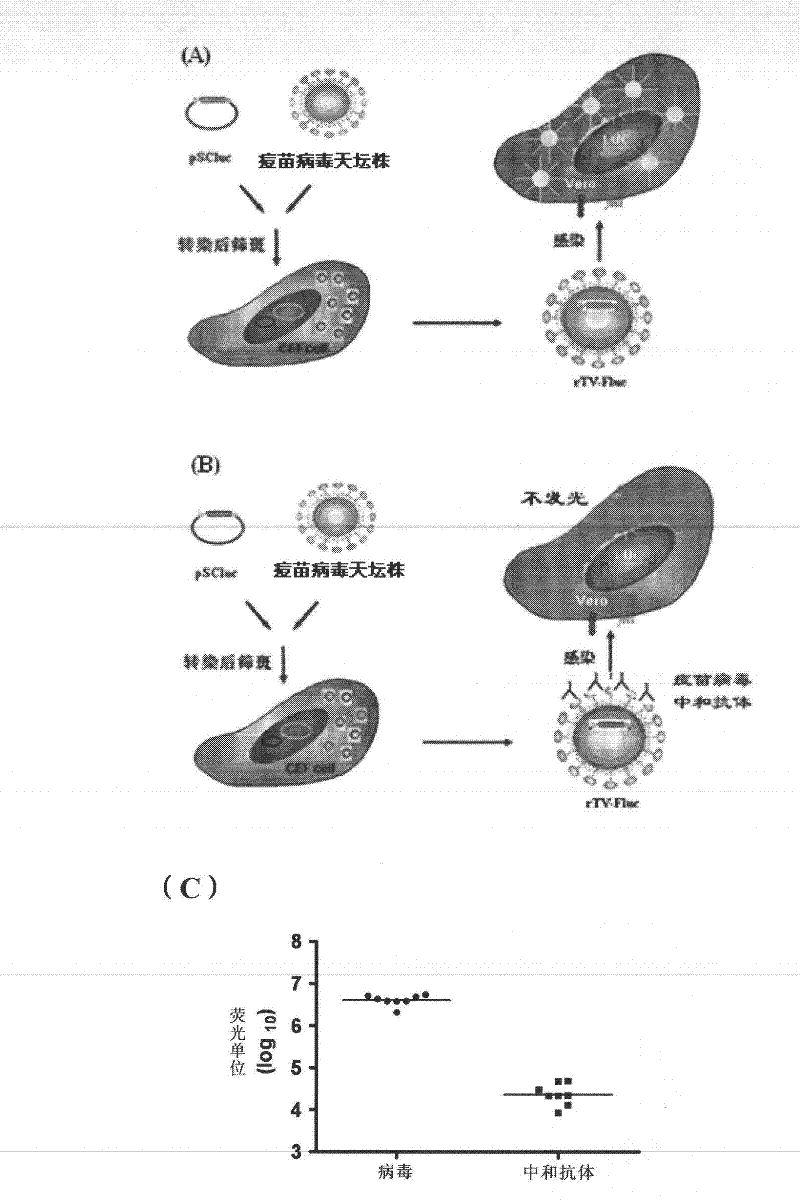
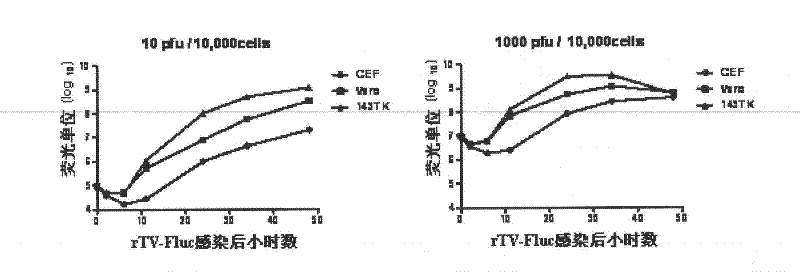
[0145] The vaccinia virus neutralizing antibody detection method of the present invention is established based on the vaccinia virus neutralizing antibody in the sample inhibiting the expression of the recombinant virus firefly luciferase reporter gene.
[0146] This example illustrates the principle of the detection method of the present invention as follows: Vero cells are infected with the rTV-Fluc virus constructed in Example 1 to express firefly luciferase, which catalyzes the luminescent reaction of the substrate luciferin. Since the luminescence value is proportional to the amount of rTV-Fluc virus that infects Vero cells, the luminescence value that can be read by a microplate luminometer reflects the viral infection dose (see figure 1 A). The vaccinia virus-specific neutralizing antibody contained in the serum to be tested can block the cell receptor binding site on the surface of the rTV-Fluc viru...
Embodiment 3
[0168] Embodiment 3 Comparison of the inventive method and the plaque inhibition method
[0169] Plaque inhibition is the most traditional neutralizing antibody detection method and is considered the "gold standard". Therefore, the method of the present invention is compared with the plaque inhibition method to determine the specificity and sensitivity of the method.
[0170] In this example, the positive control sample used was the high-titer rabbit serum immunized with the Tiantan strain of vaccinia virus provided by Beijing Tiantan Biological Products Co., Ltd., labeled as HCS.
[0171] 1. Standardization of Plaque Inhibition Assay
[0172] 1) Serum was inactivated at 56°C for 60 minutes;
[0173] 2) using 3% DMEM medium to dilute the serum in a 5-fold gradient at 1:20, 1:100, 1:500, 1:2500, 1:12500;
[0174] 3) Mix 400 μl of vaccinia virus (rTV-Fluc) at three dilutions of 17, 33 and 66 pfu / ml with 400 μl of serum at each dilution, and incubate at 37°C for 1 hour;
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com