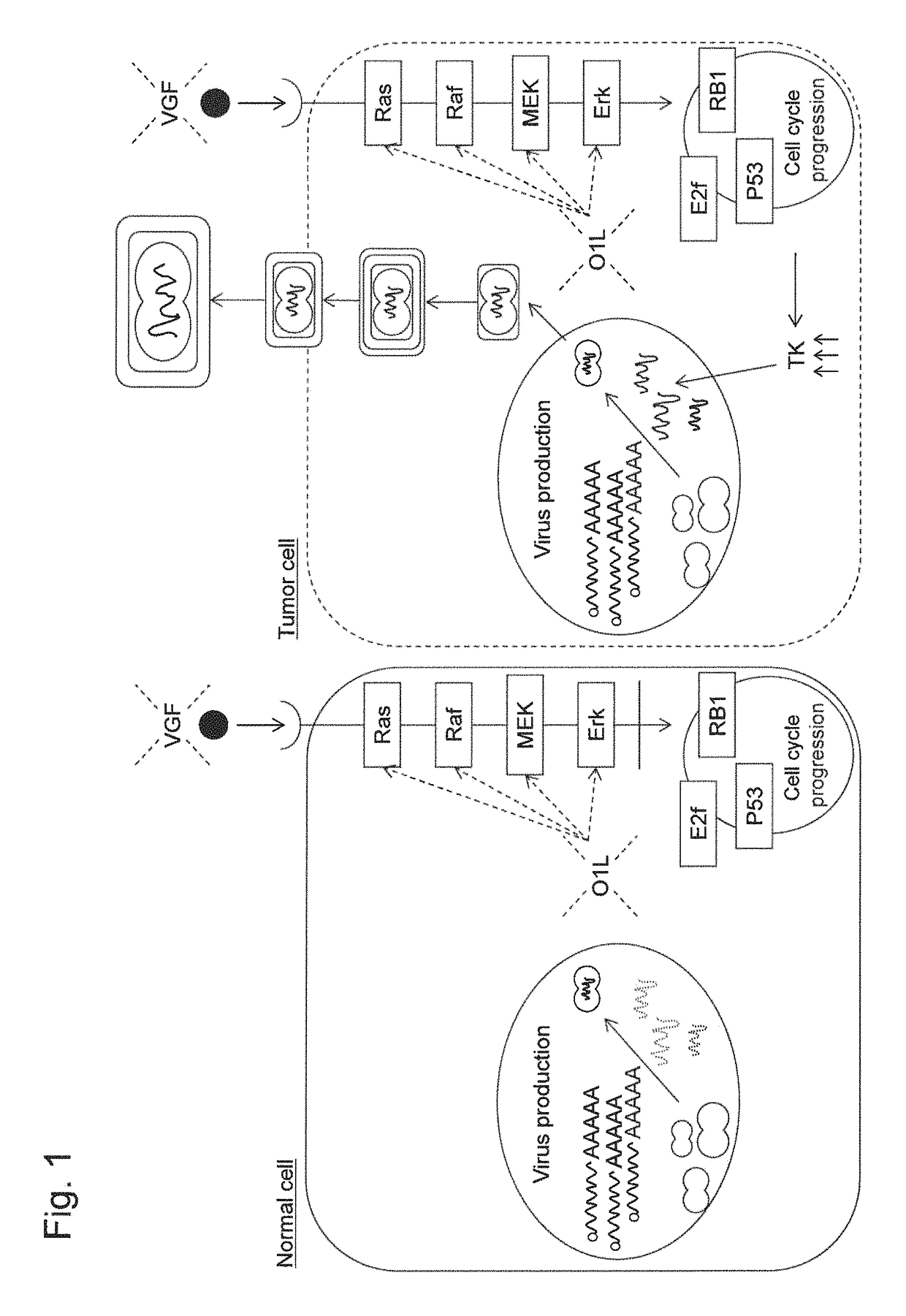
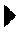Patents
Literature
82 results about "Recombinant vaccinia virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccinia Virus as a Tool for Vaccine Research. Recombinant vaccinia viruses provide a powerful means of dissecting the immune responses of humans and experimental animals to individual gene products of infectious agents.
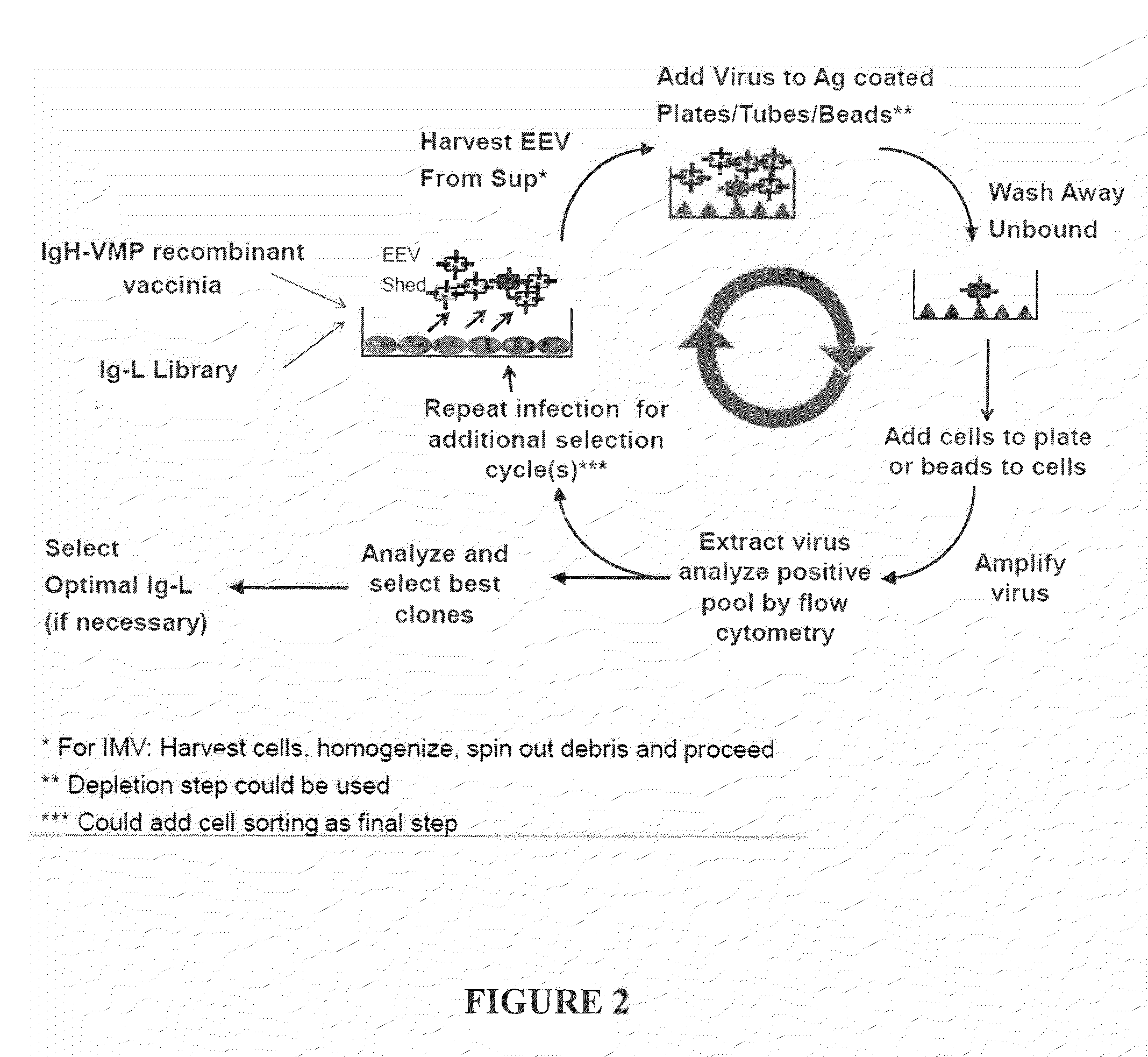
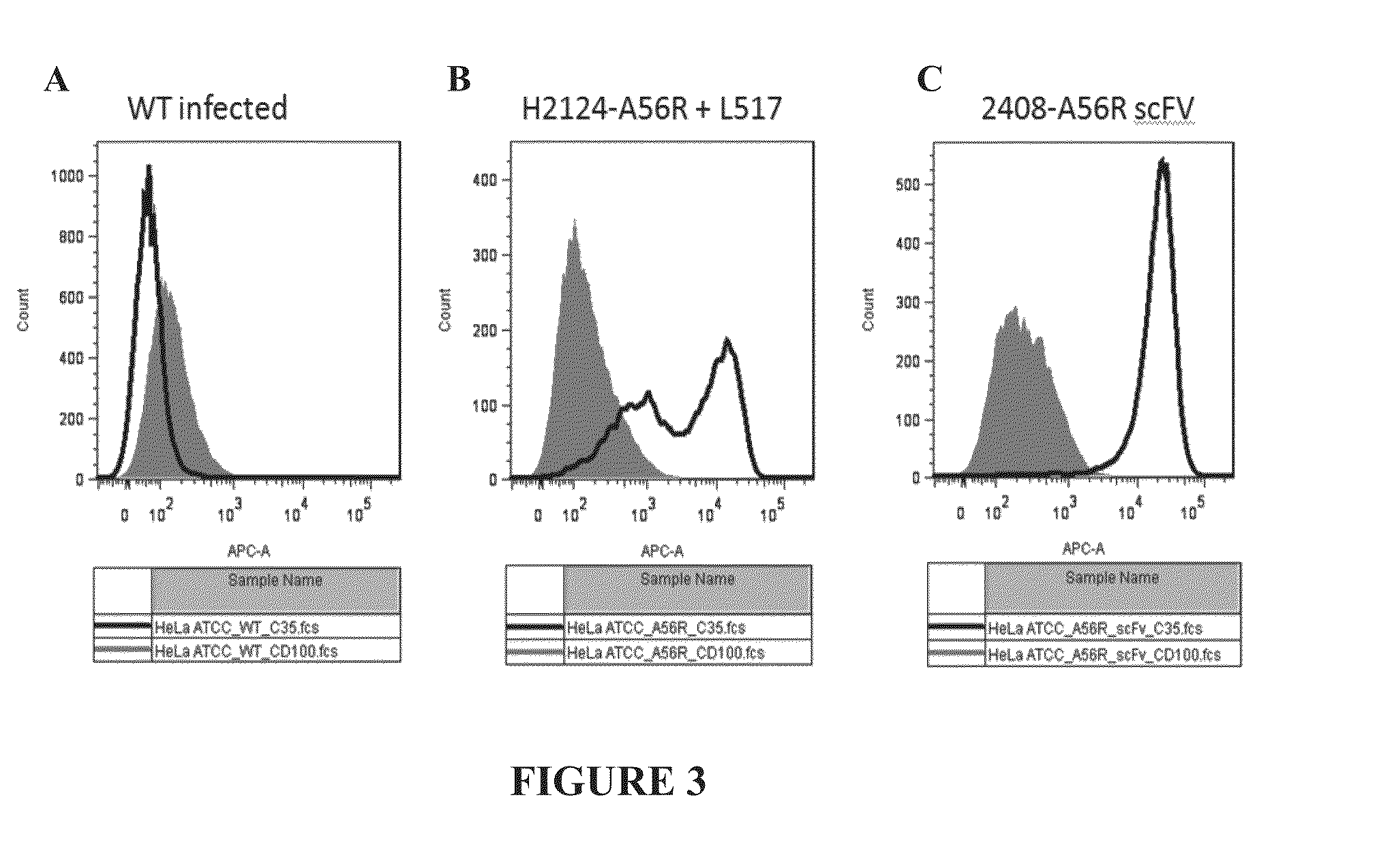
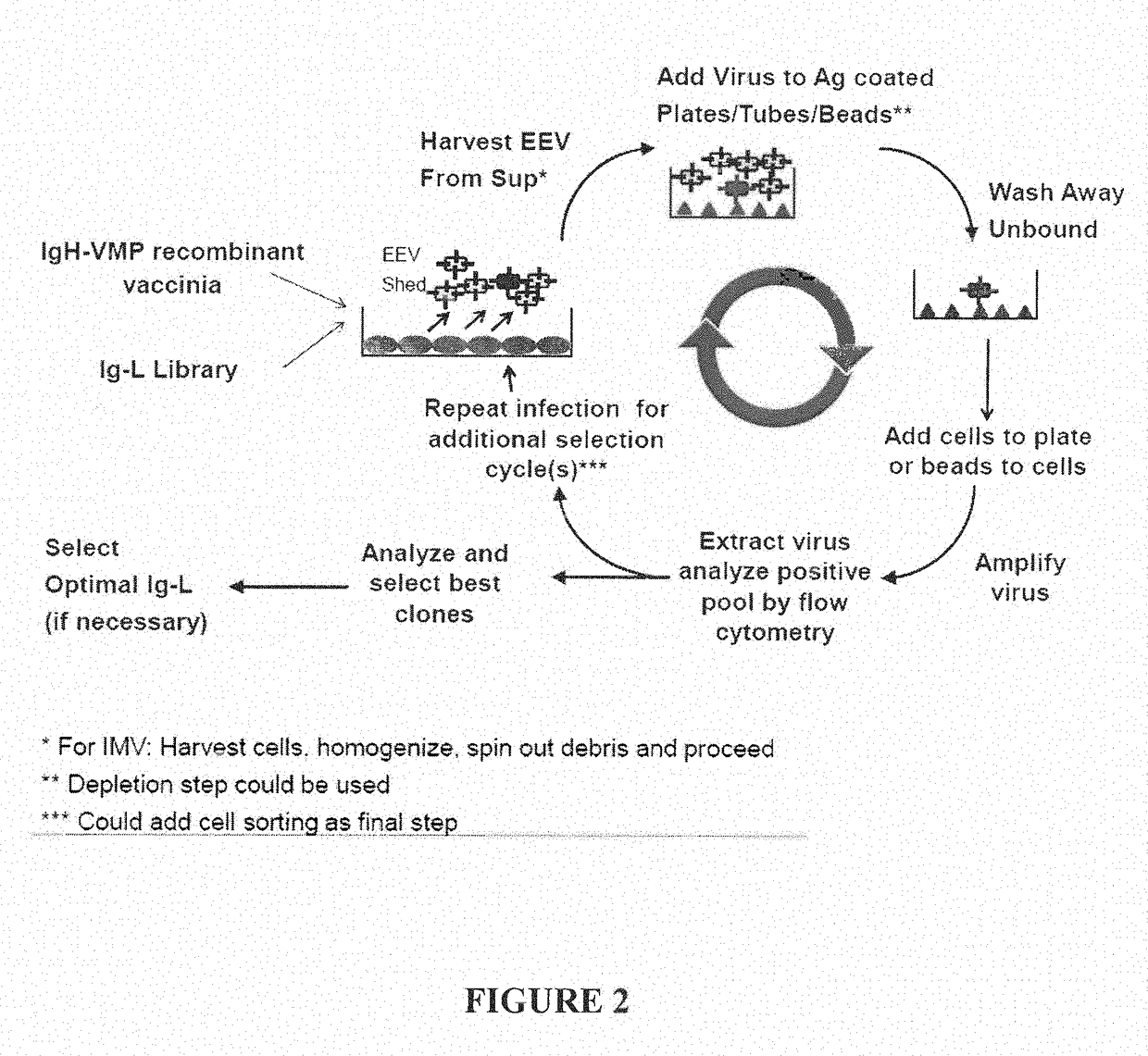
Fusion Proteins to Facilitate Selection of Cells Infected with Specific Immunoglobulin Gene Recombinant Vaccinia Virus
ActiveUS20130288927A1Library screeningVirus peptidesImmunoglobulin heavy chainRecombinant vaccinia virus
The present invention relates to a high efficiency method of expressing immunoglobulin molecules in eukaryotic cells. The invention is further drawn to a method of producing immunoglobulin heavy and light chain libraries, particularly using the trimolecular recombination method, for expression in eukaryotic cells. The invention further provides methods of selecting and screening for antigen-specific immunoglobulin molecules, and antigen-specific fragments thereof. The invention also provides kits for producing, screening and selecting antigen-specific immunoglobulin molecules. Finally, the invention provides immunoglobulin molecules, and antigen-specific fragments thereof, produced by the methods provided herein.
Owner:VACCINEX
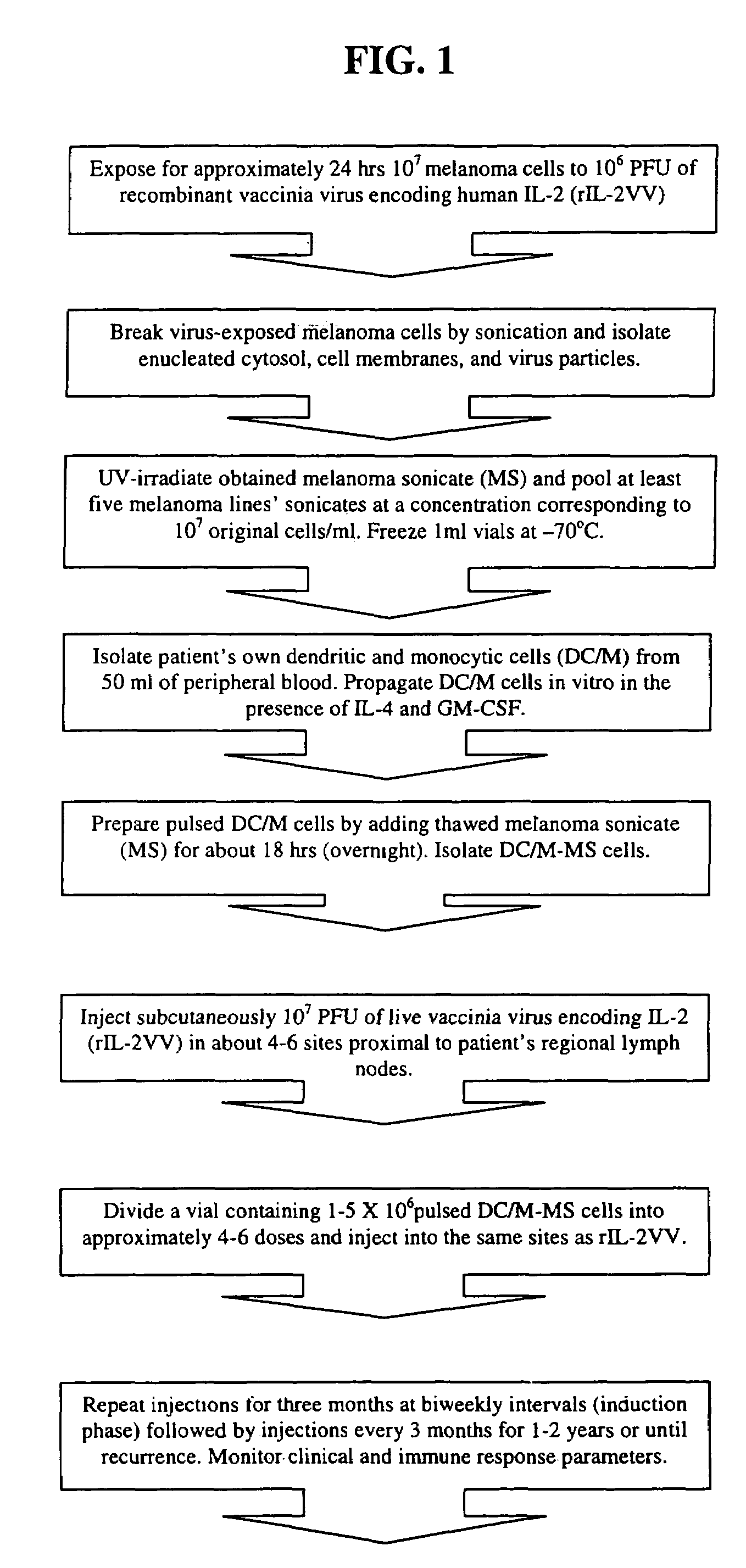
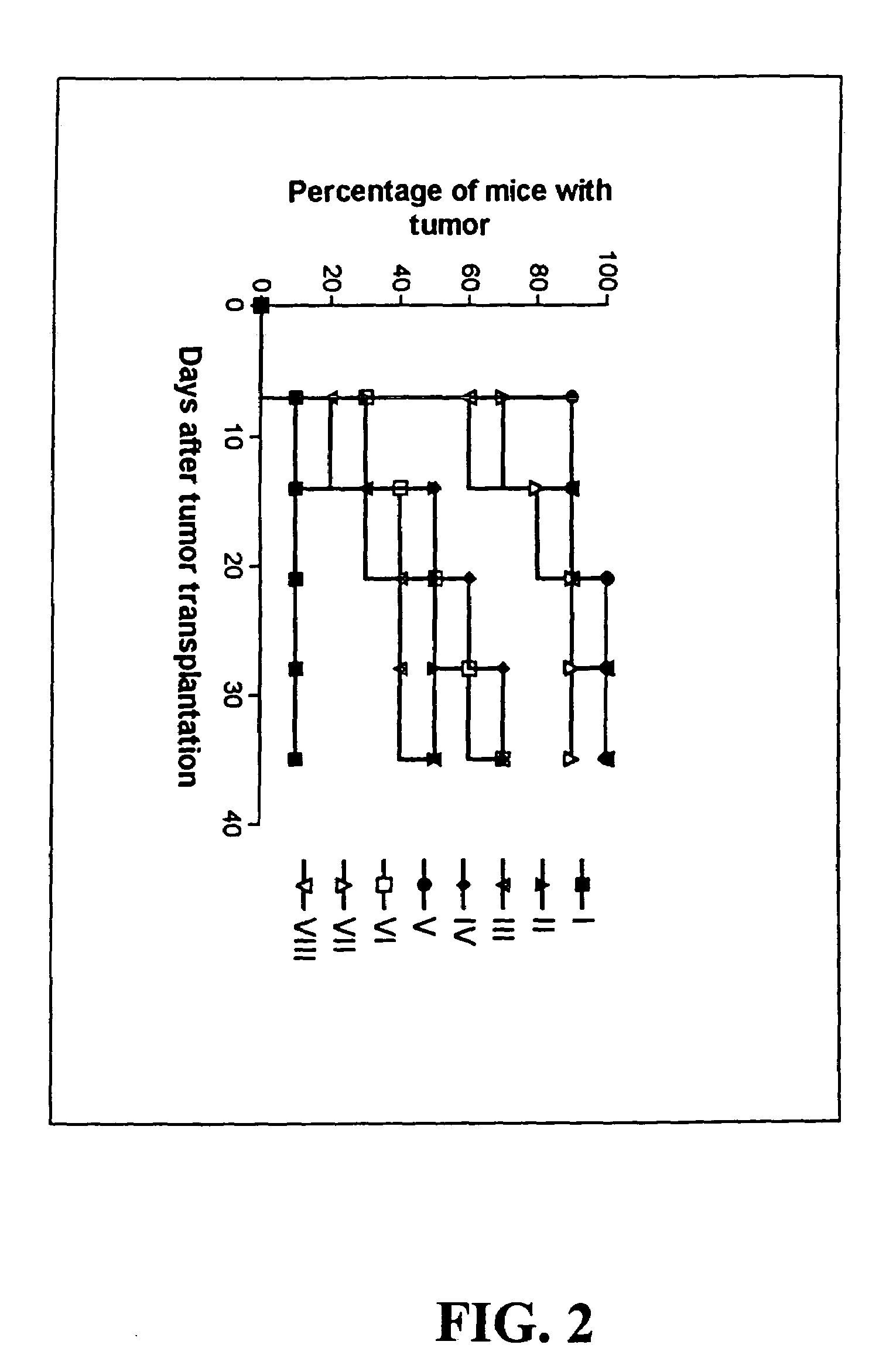
Melanoma vaccine and methods of making and using same
Owner:WALLACK MARC K
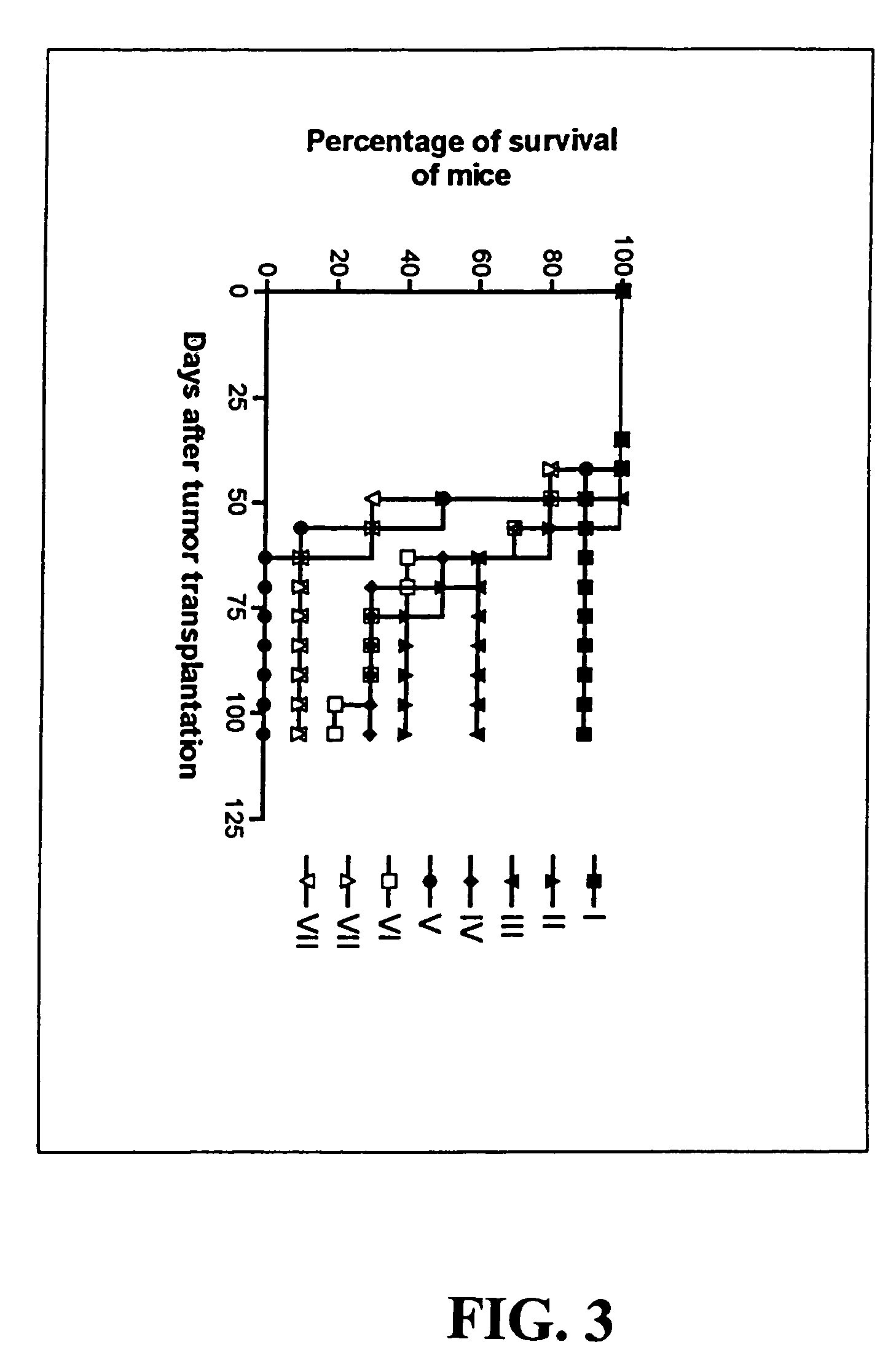
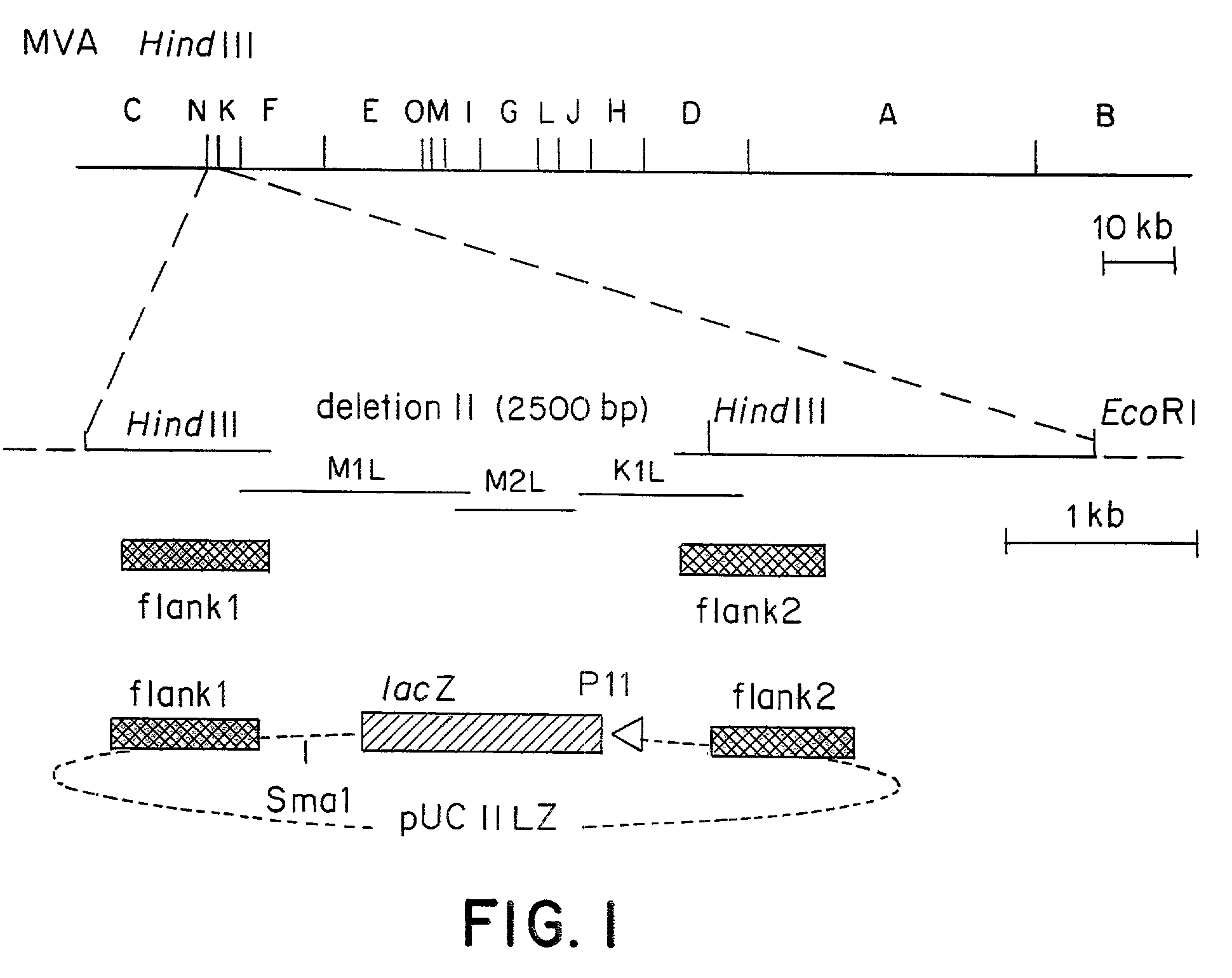
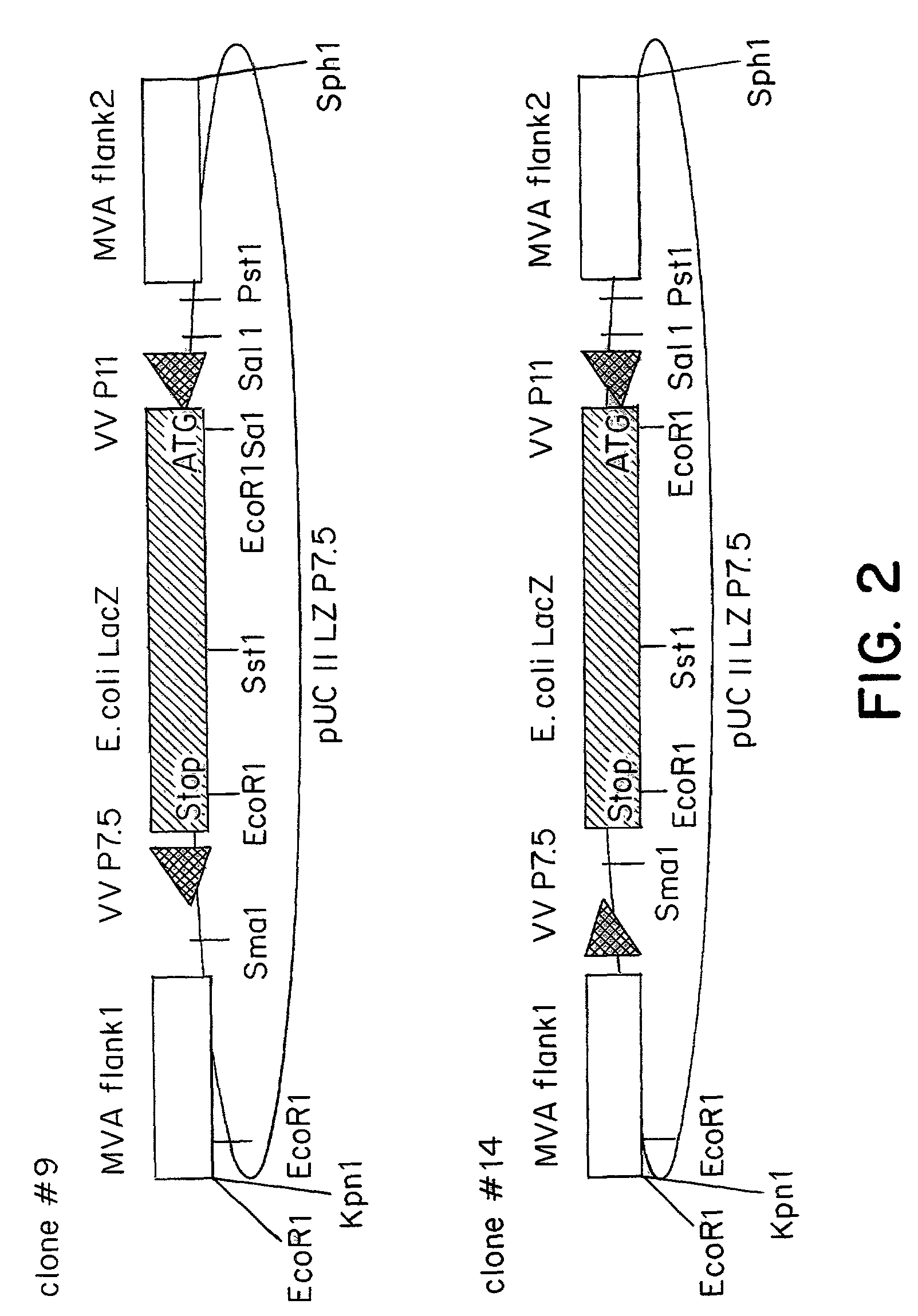
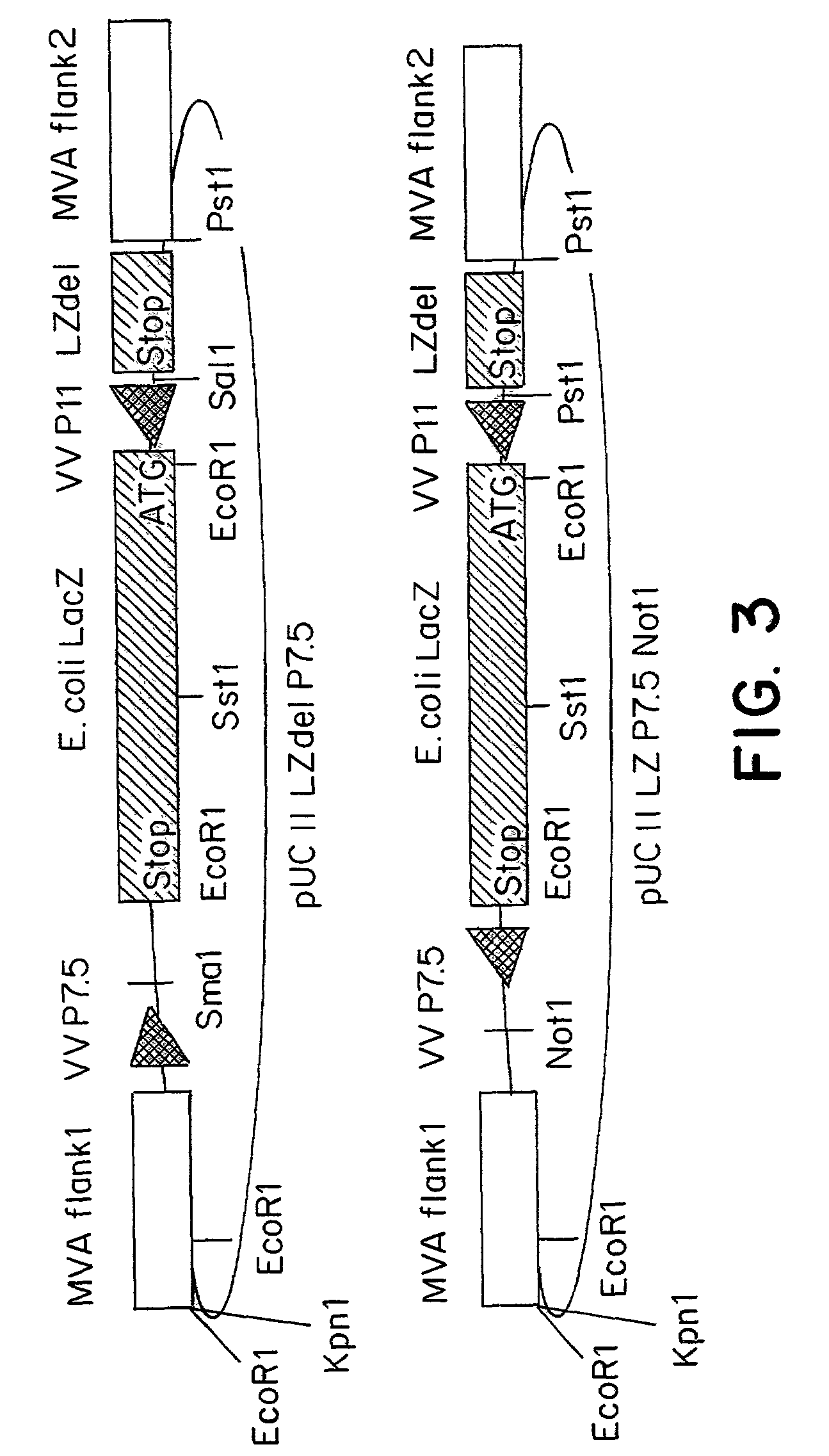
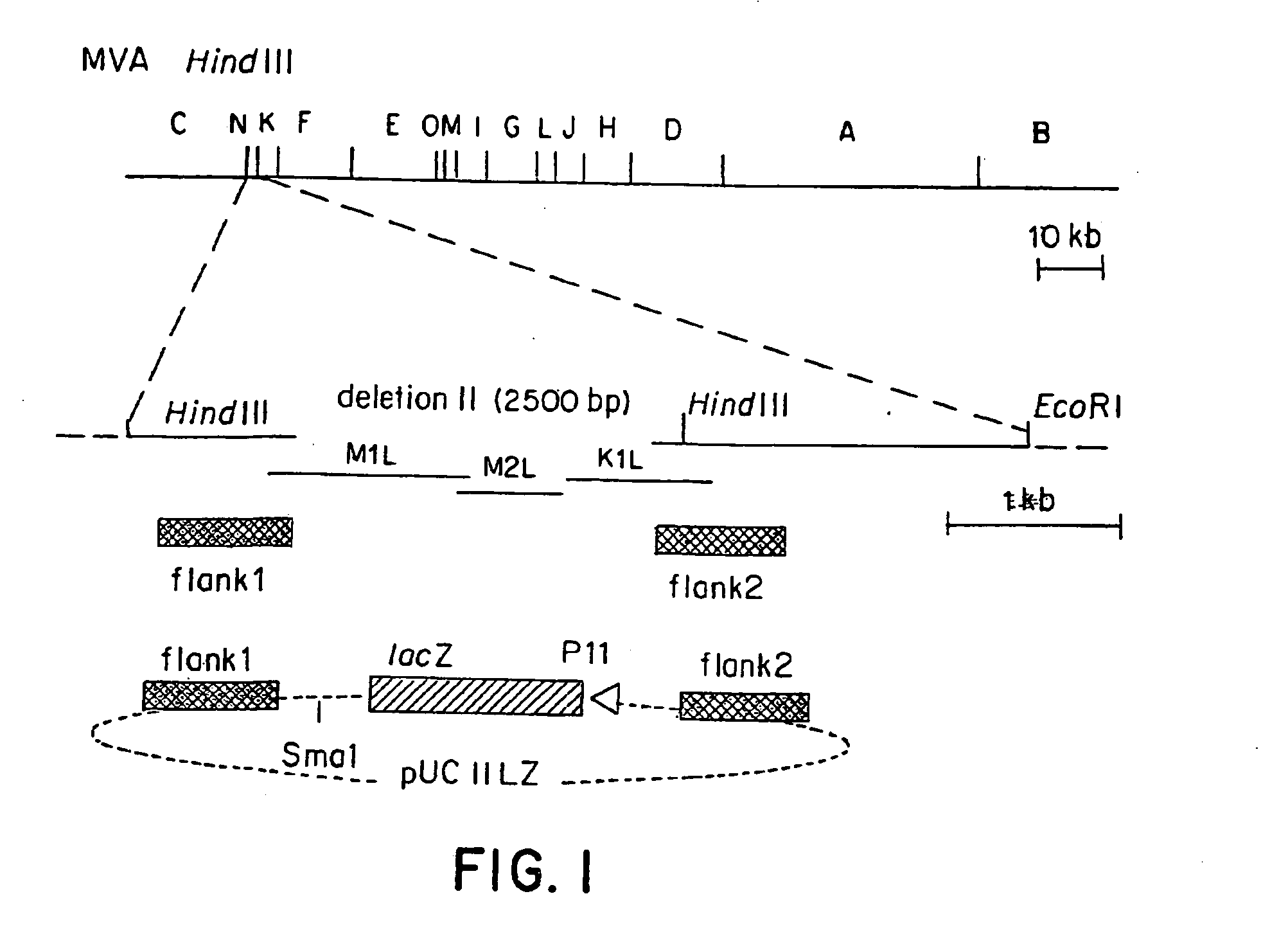
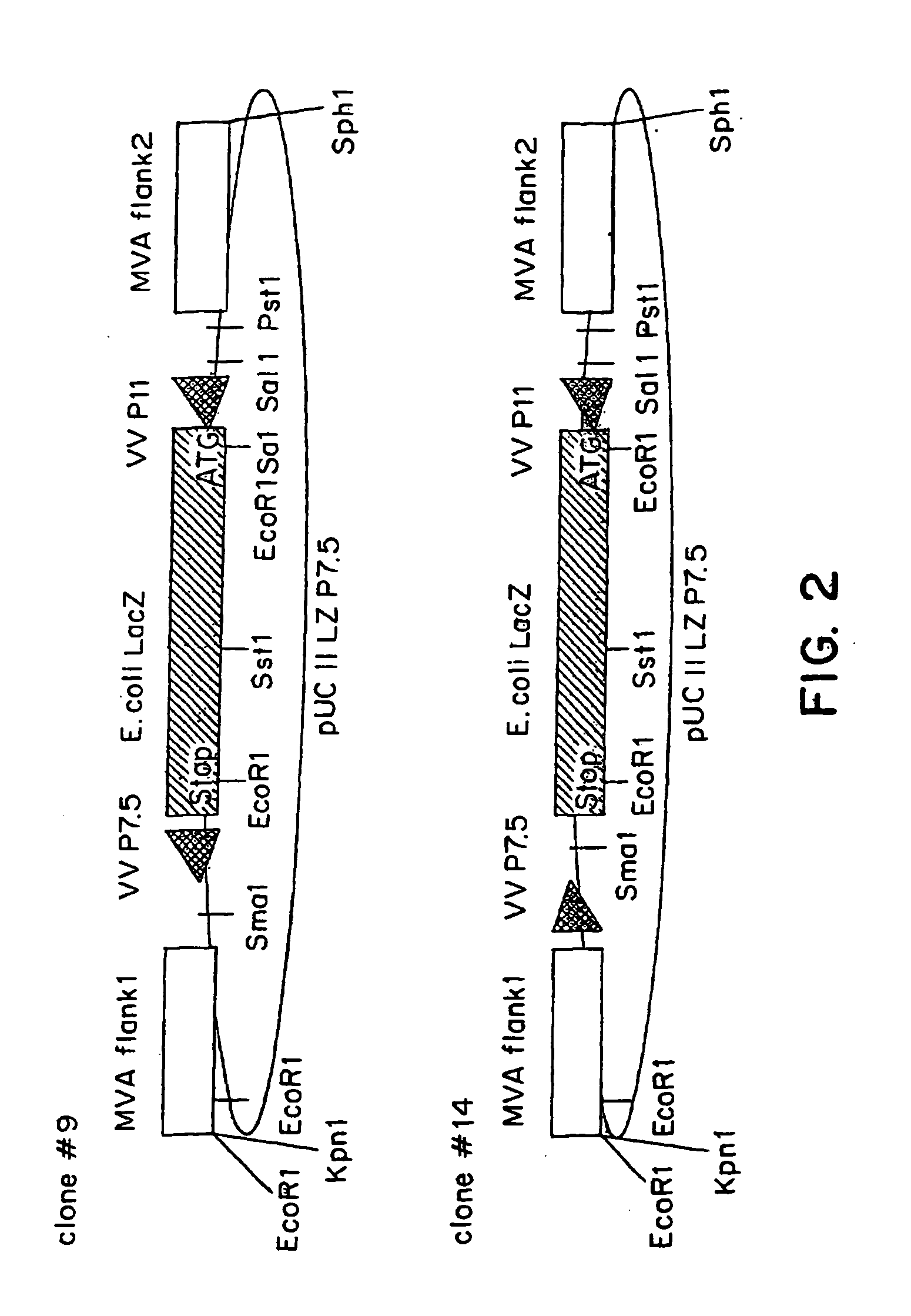
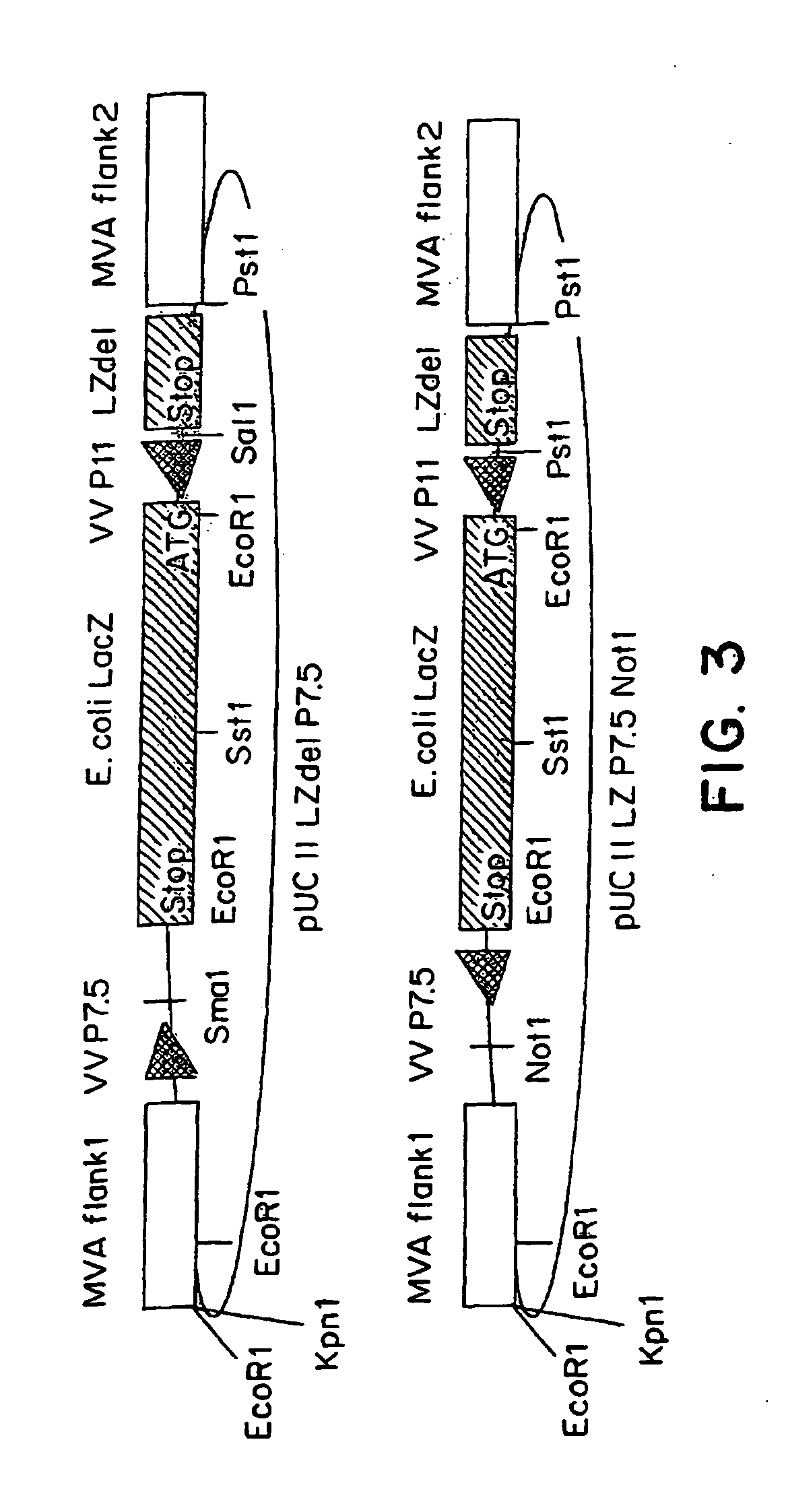
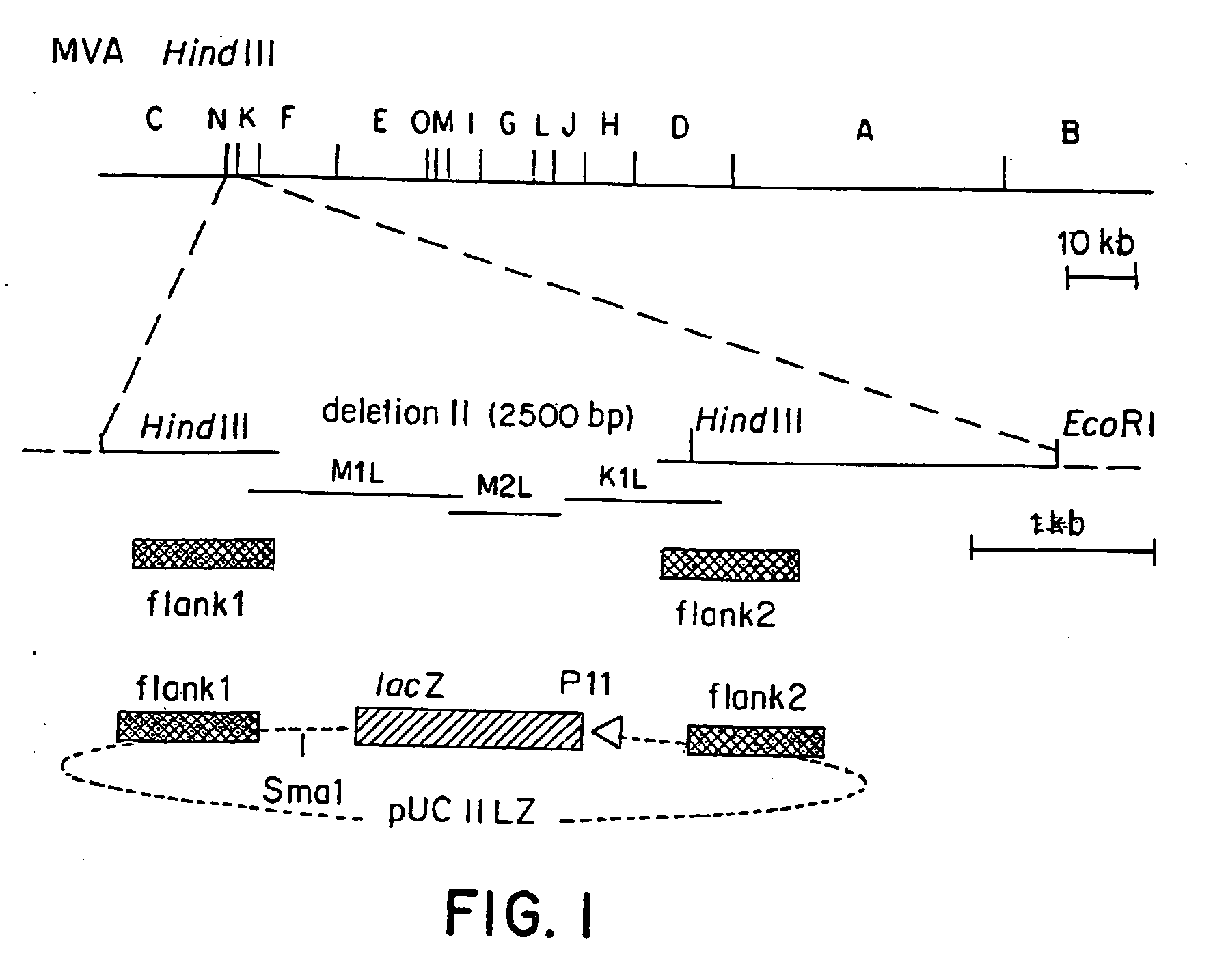
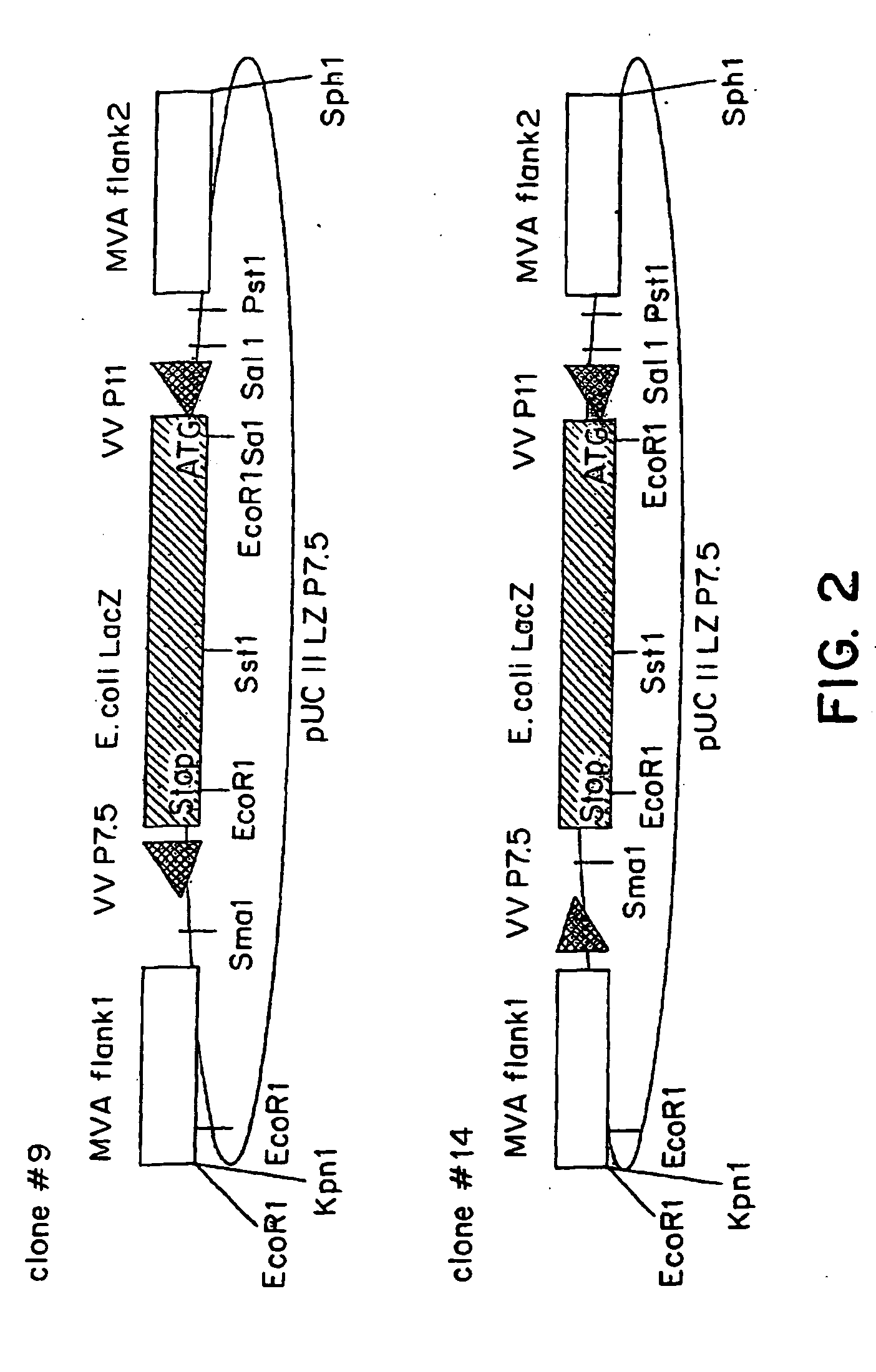
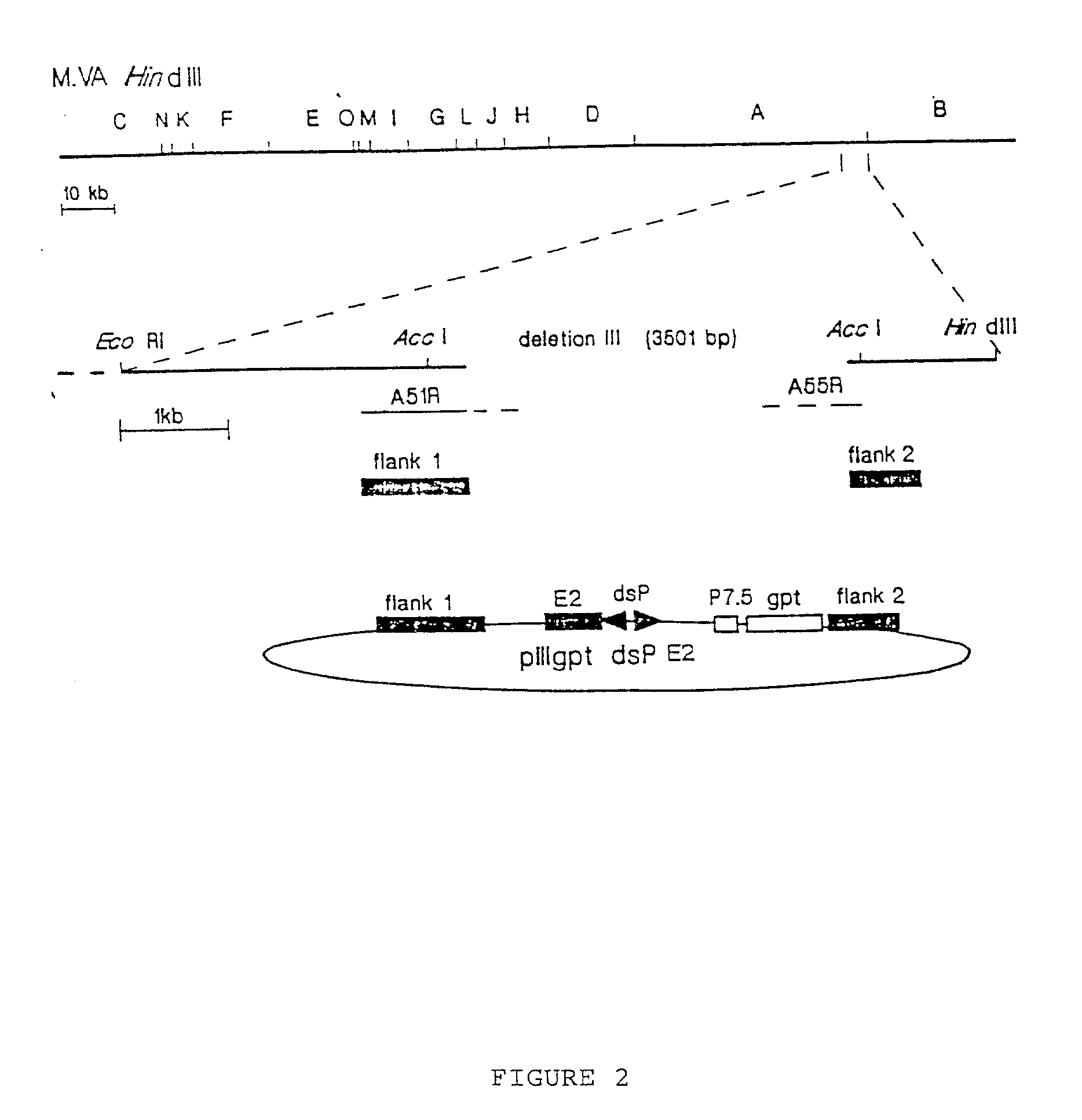
Recombinant MVA virus, and the use thereof
InactiveUS7198934B2Improve efficiencyReduce the amount of solutionOrganic active ingredientsVirusesAntigenViral vector
The present invention relates to recombinant vaccinia viruses derived from the modified vaccinia virus Ankara (MVA) and containing and capable of expressing foreign genes which are inserted at the site of a naturally occurring deletion in the MVA genome, and the use of such recombinant MVA viruses for the production of polypeptides, e.g. antigens or therapeutic agents, or viral vectors for gene therapy, and the use of such recombinant MVA viruses encoding antigens as vaccines.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
Recombinant MVA virus, and the use thereof
InactiveUS20070071769A1Improve efficiencyReduce the amount of solutionOrganic active ingredientsVirusesAntigenViral vector
The present invention relates to recombinant vaccinia viruses derived from the modified vaccinia virus Ankara (MVA) and containing and capable of expressing foreign genes which are inserted at the site of a naturally occurring deletion in the MVA genome, and the use of such recombinant MVA viruses for the production of polypeptides, e.g. antigens or therapeutic agents, or viral vectors for gene therapy, and the use of such recombinant MVA viruses encoding antigens as vaccines.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
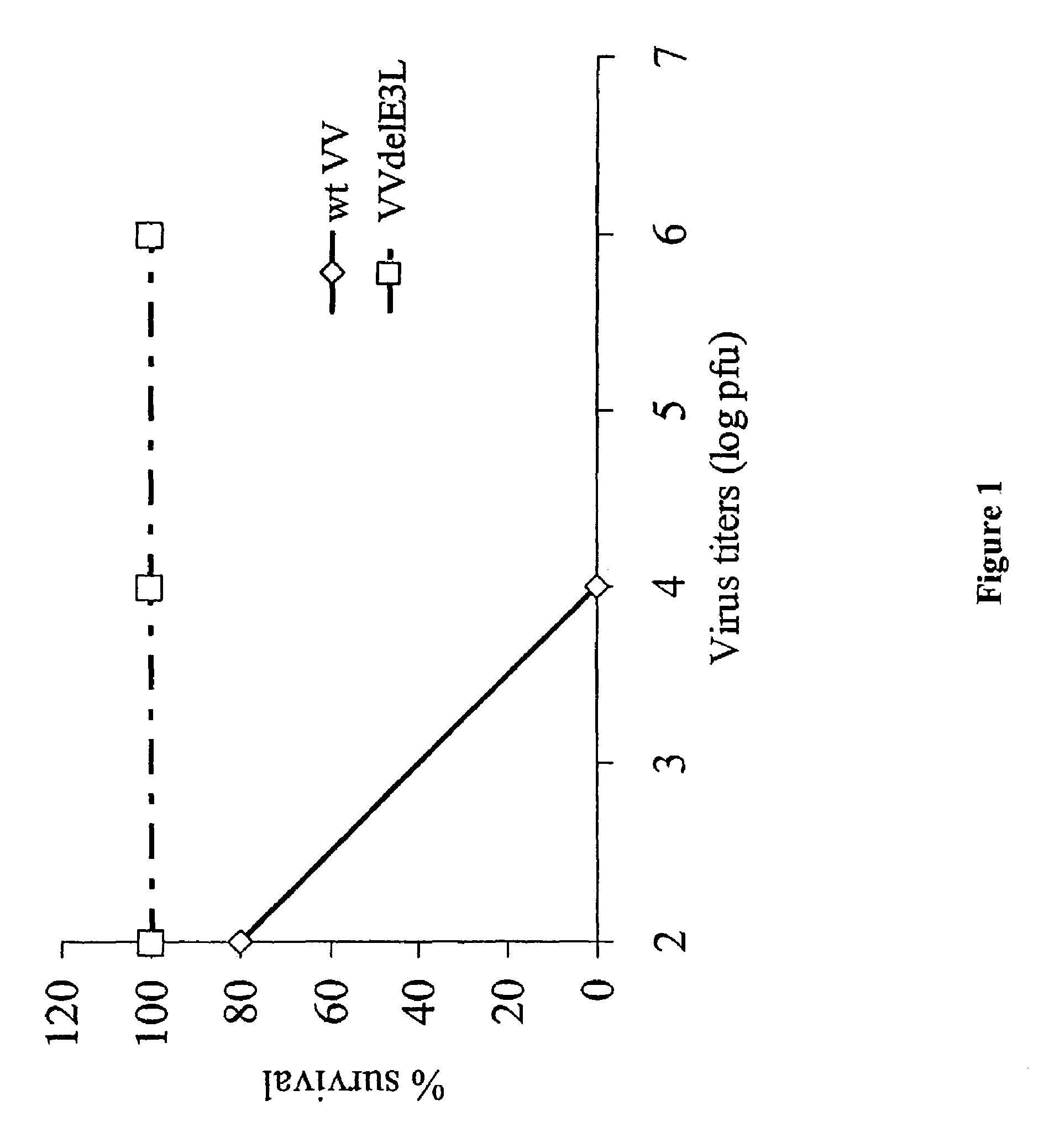
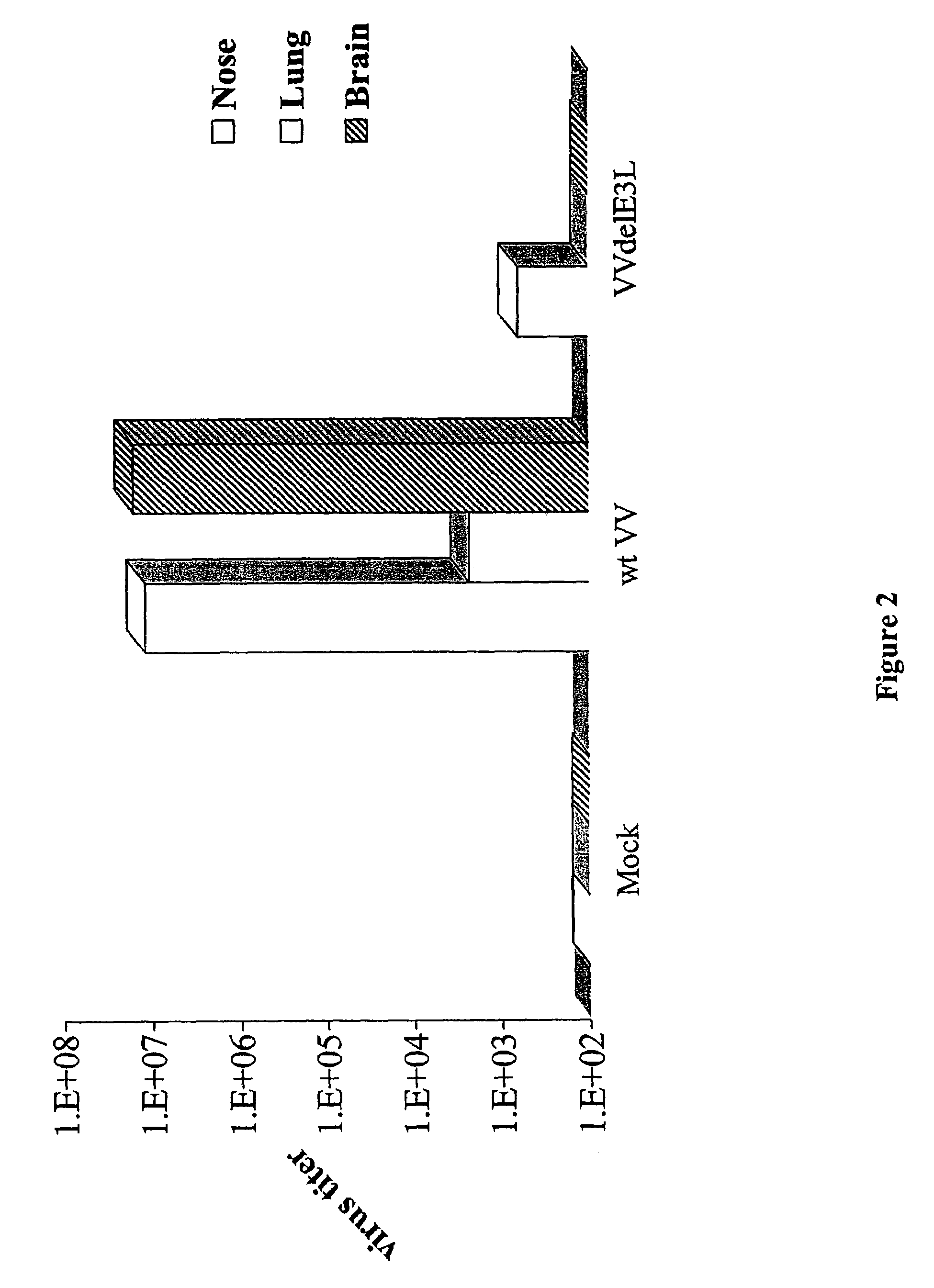
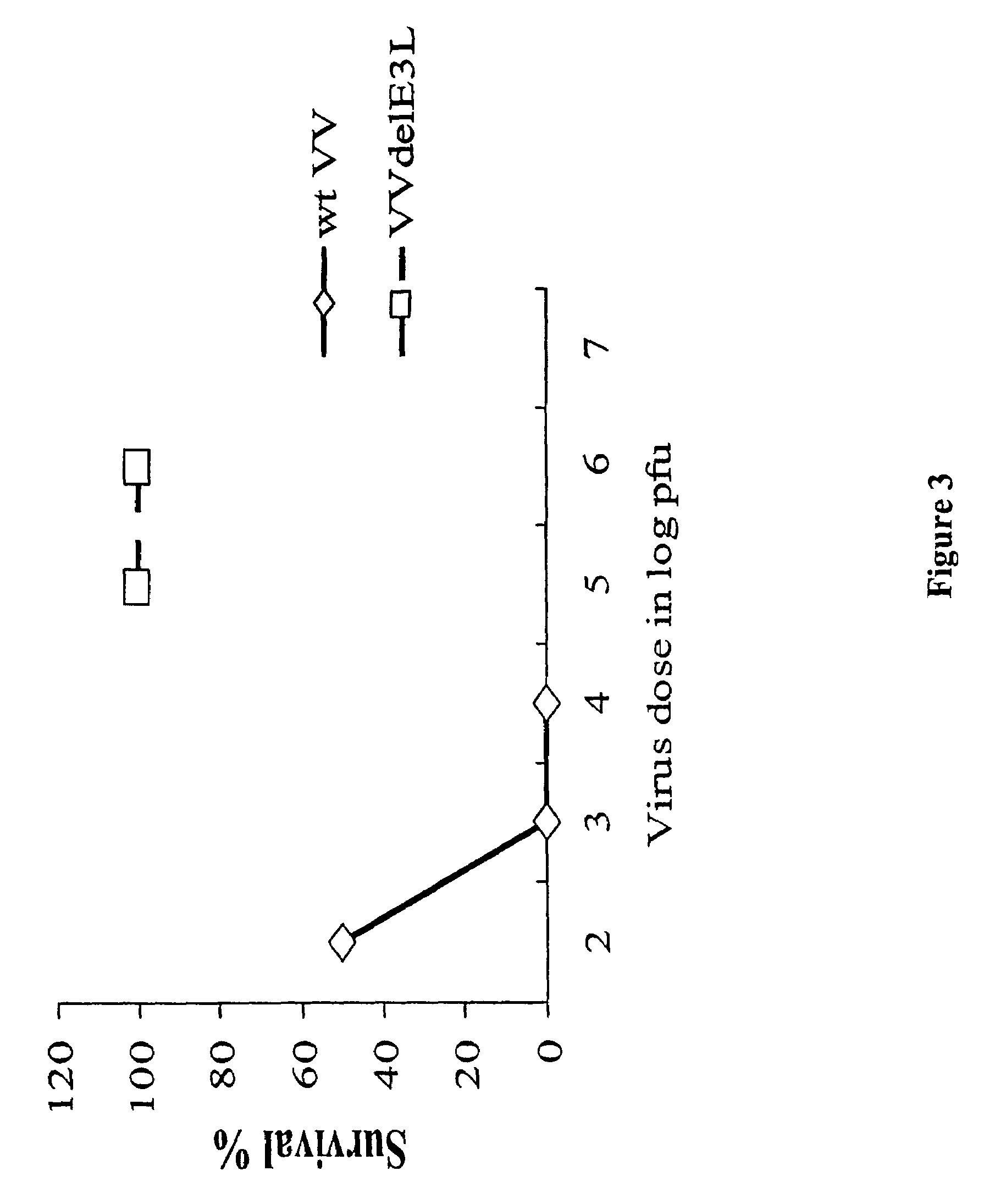
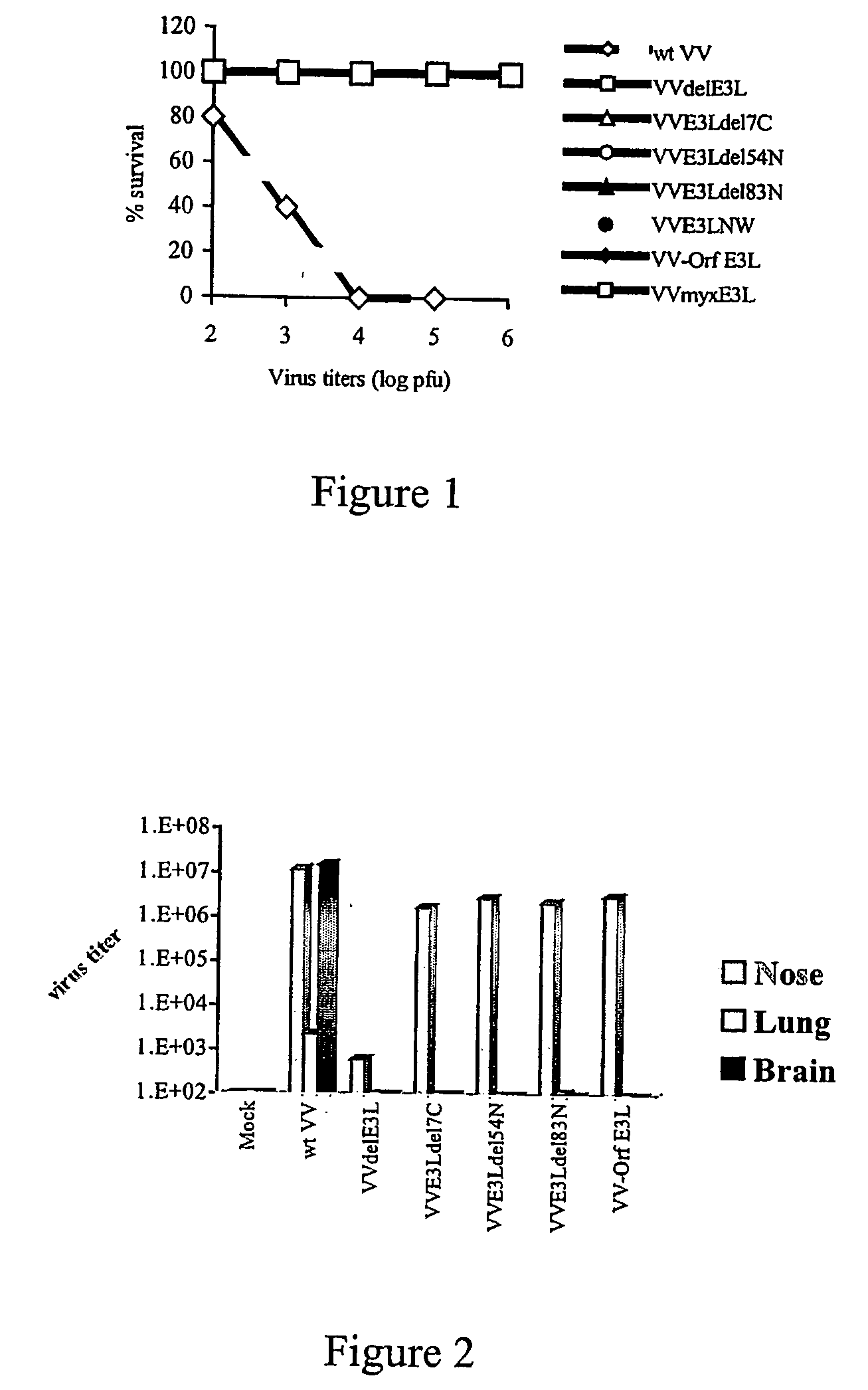
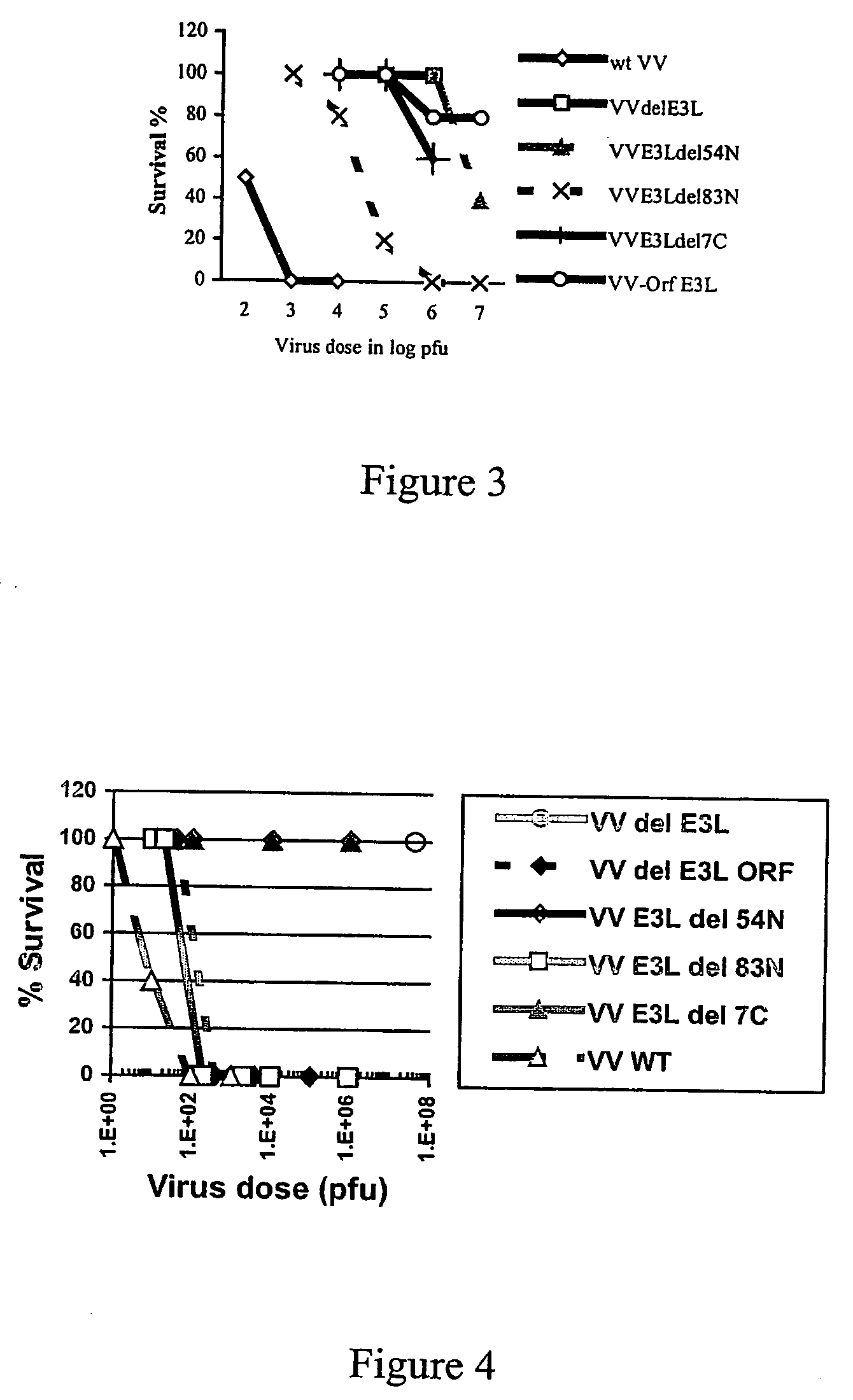
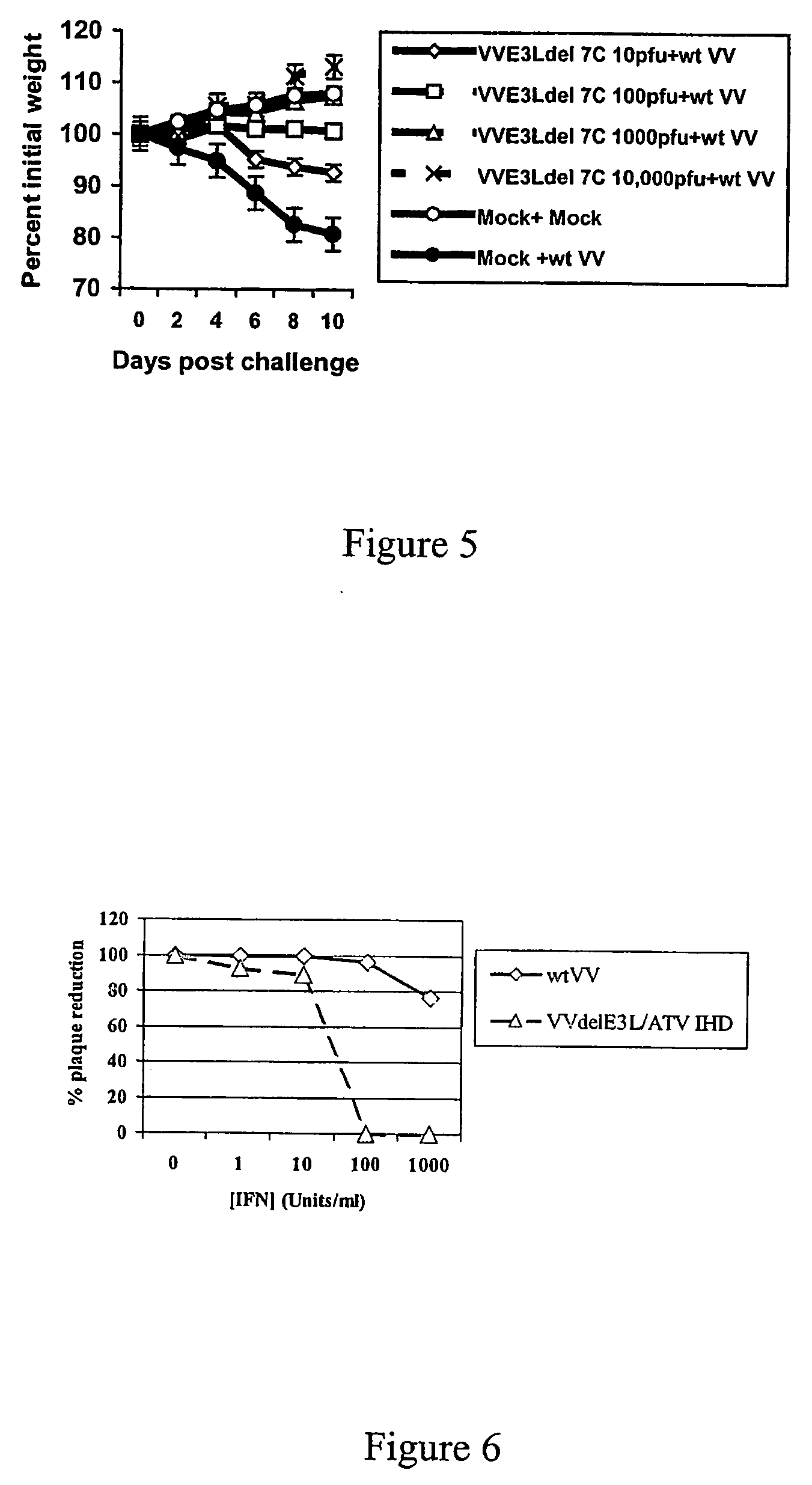
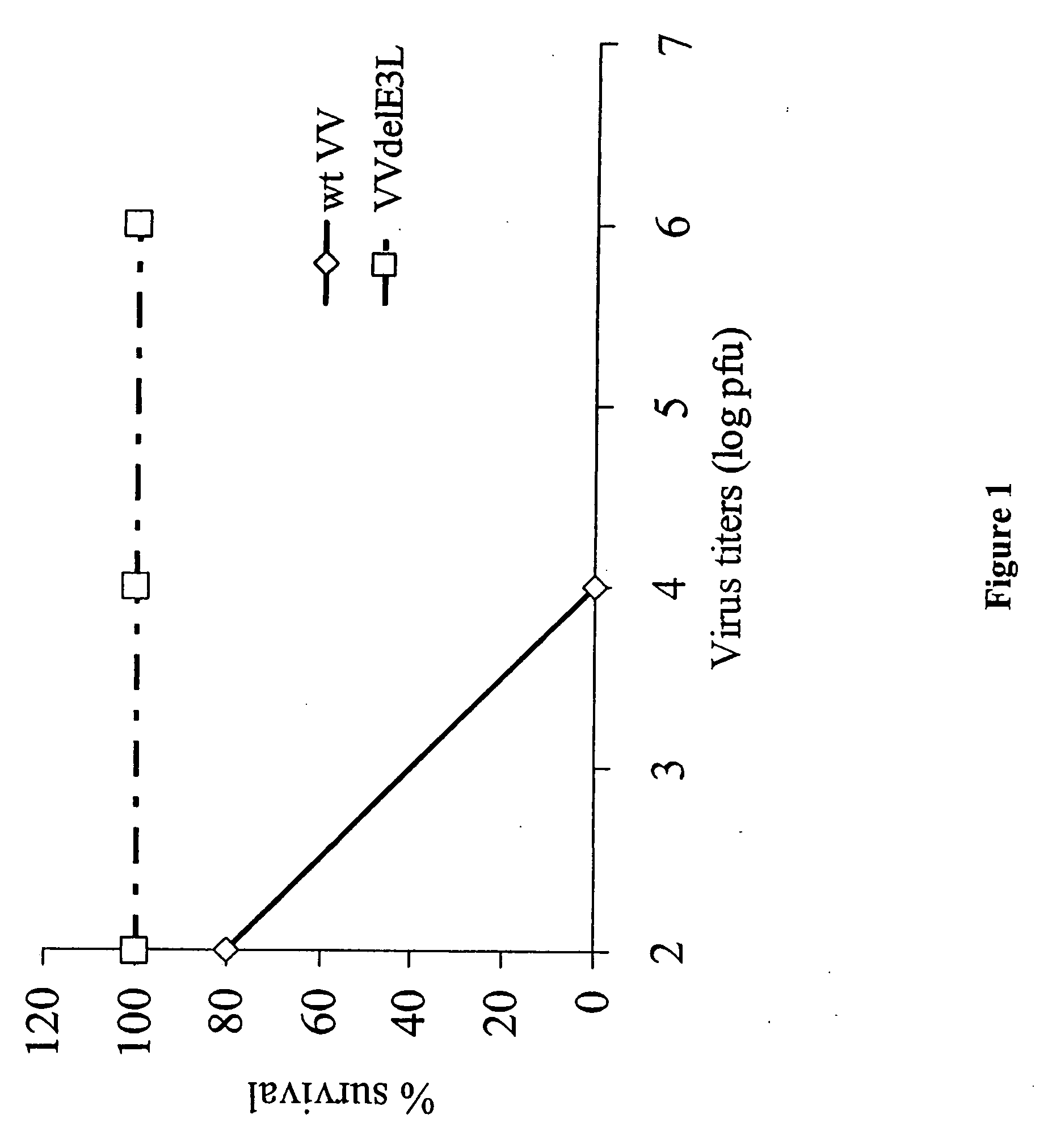
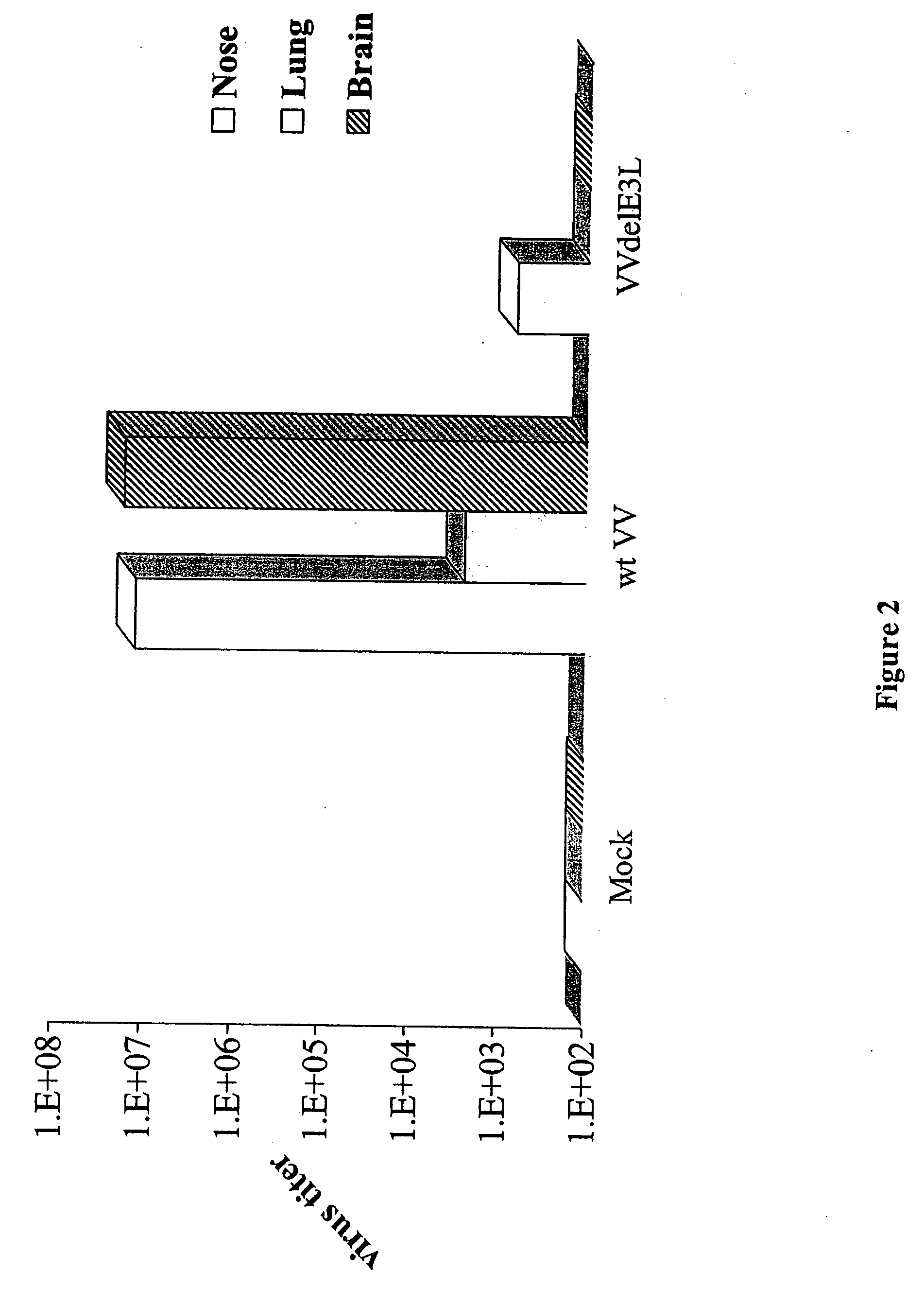
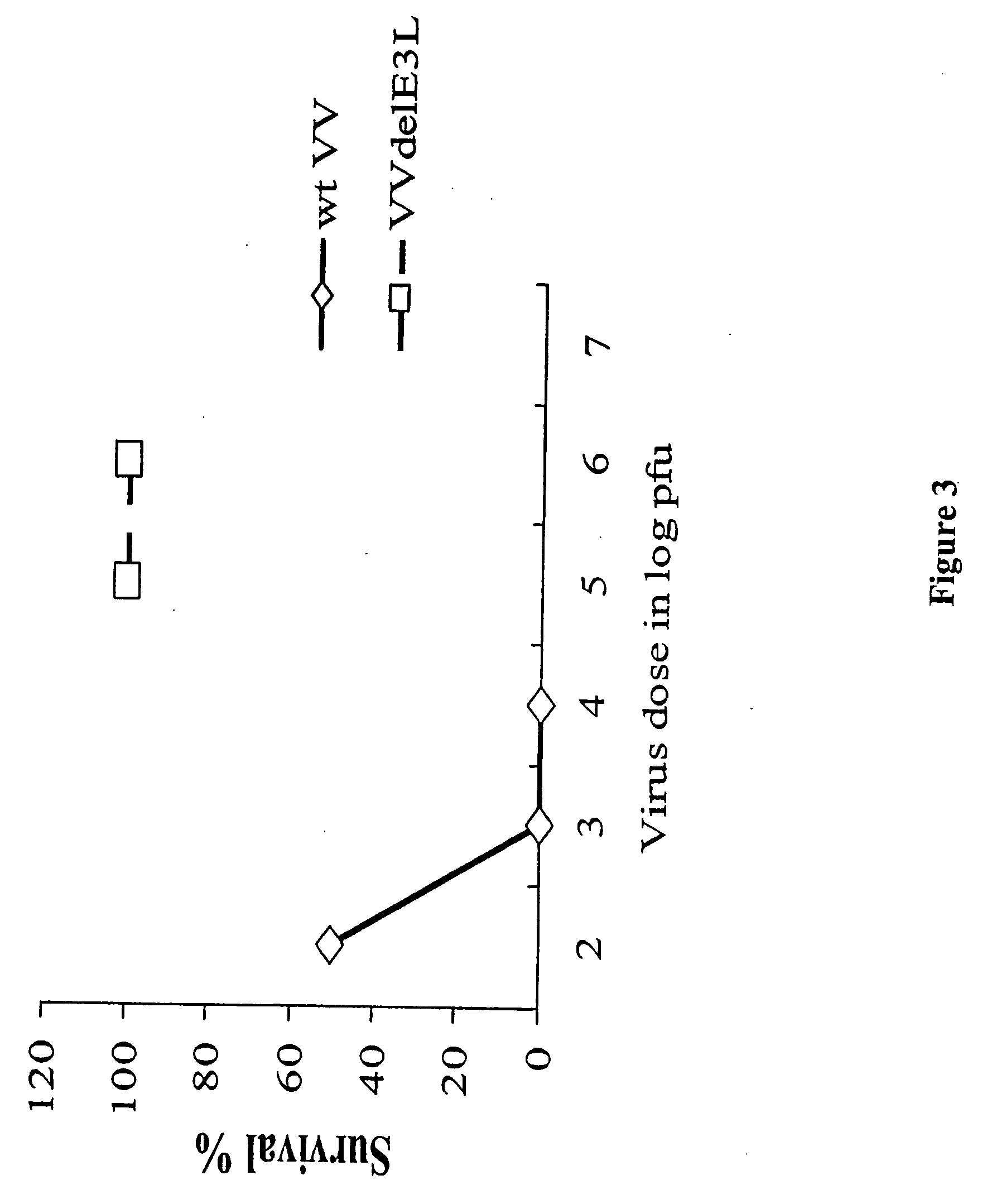
Use of vaccinia virus deleted for the E3L gene as a vaccine vector
The present invention relates to vaccines having an increased level of safety comprising recombinant vaccinia viruses containing an inactivated E3L region. The invention also relates to methods for stimulating a protective immune response in an immunized host using the vaccines of the invention. The invention is based on the discovery that vaccinia virus mutants having deletions in the E3L region exhibit dramatically reduced pathogenesis while remaining highly immunogenic. In addition, the invention relates to modified recombinant vaccinia viruses engineered to express heterologous polypeptides and the use of such viruses in vaccines designed to stimulate a protective immune response against such polypeptides in a host. The invention further relates to an interferon-sensitive recombinant vaccinia virus with broad host range wherein a salamander eIF2α is inserted into the viral genome in place of at least a portion of the E3L gene.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Mutants of replication competent vaccinia virus
The present invention relates to vaccines having an increased level of safety comprising recombinant vaccinia viruses. The invention also relates to methods for stimulating a protective immune response in an immunized host using the vaccines of the invention. The vaccines and recombinant vaccinia viruses of the invention comprise a first nucleic acid comprising an expression control sequence and a second nucleic acid comprising an exogenous nucleic acid encoding a conditional replication gene product, wherein the expression control sequence is operably linked to the exogenous nucleic acid. The exogenous nucleic acid may, by its expression or non-expression, confer upon the recombinant vaccinia virus either sensitivity or dependence upon an exogenous molecule (e.g. a drug) or a condition. Importantly, to allow the recombinant vaccinia viruses of the invention to replicate normally under permissive conditions, the exogenous nucleic acid is inserted into a non-essential locus, e.g., the E2L / E3L inter-genic locus, K1L / K2L inter-genic locus, the superoxide dismutate locus, and the 7.5 K locus.
Owner:ARIZONA STATE UNIVERSITY
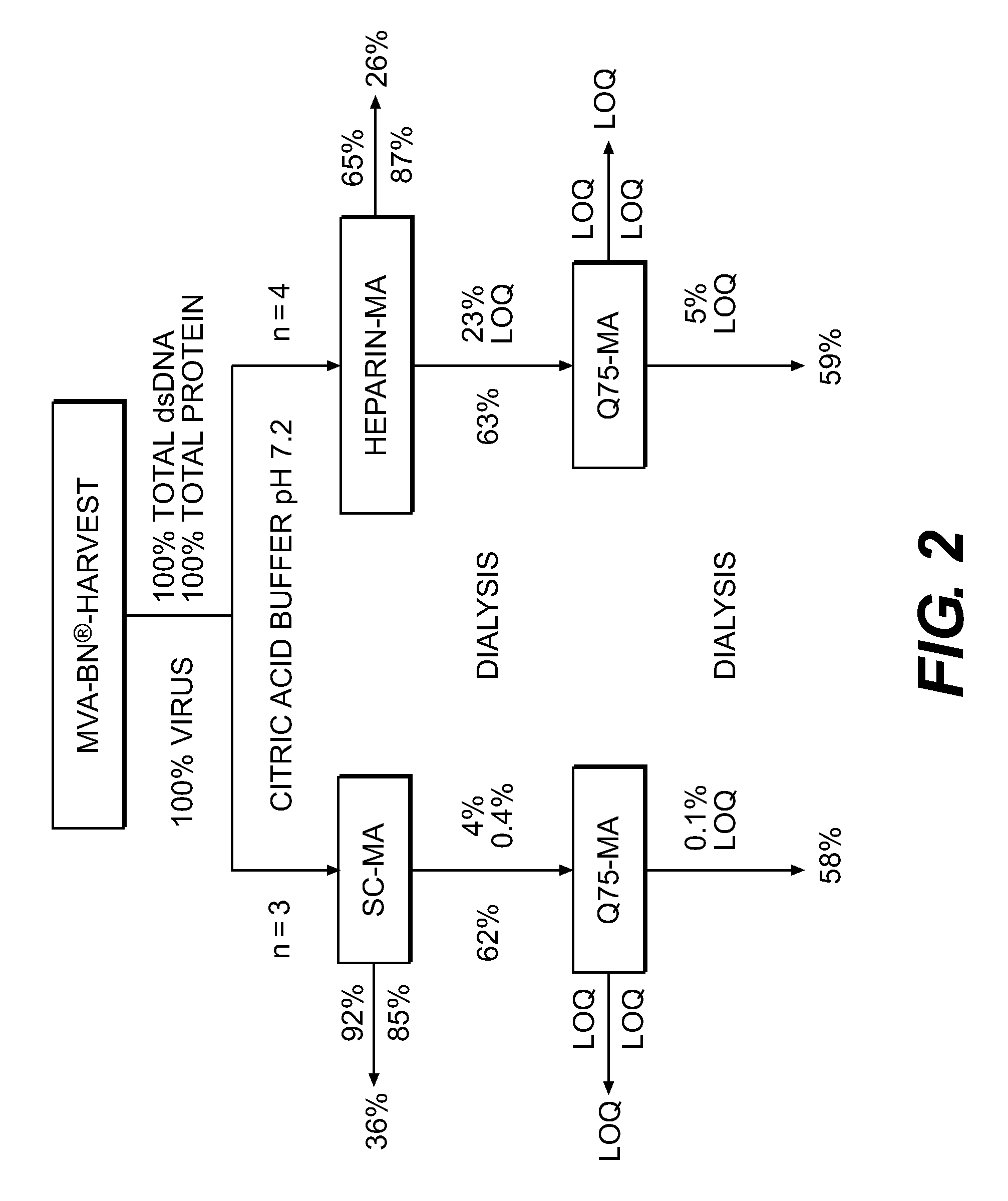
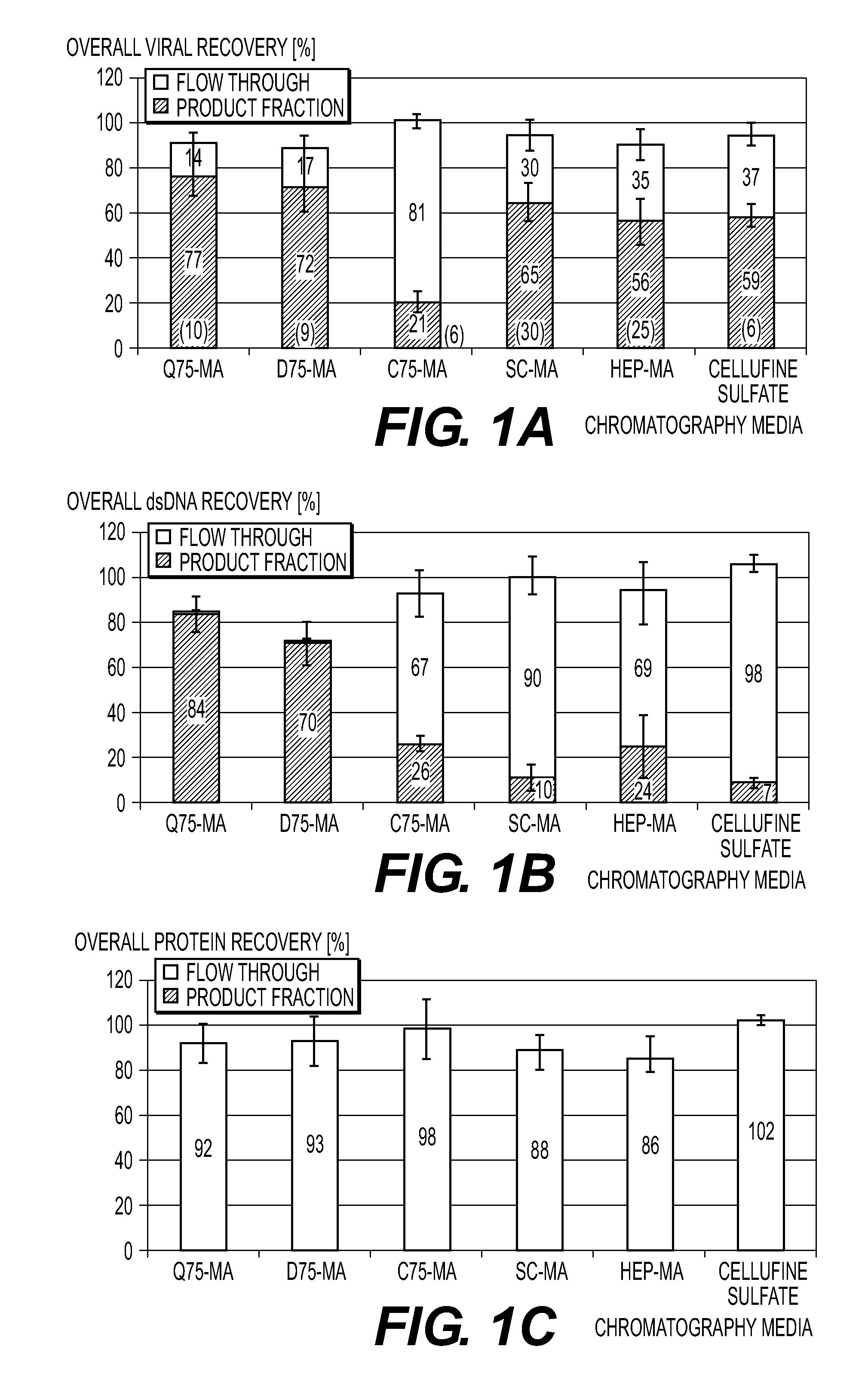
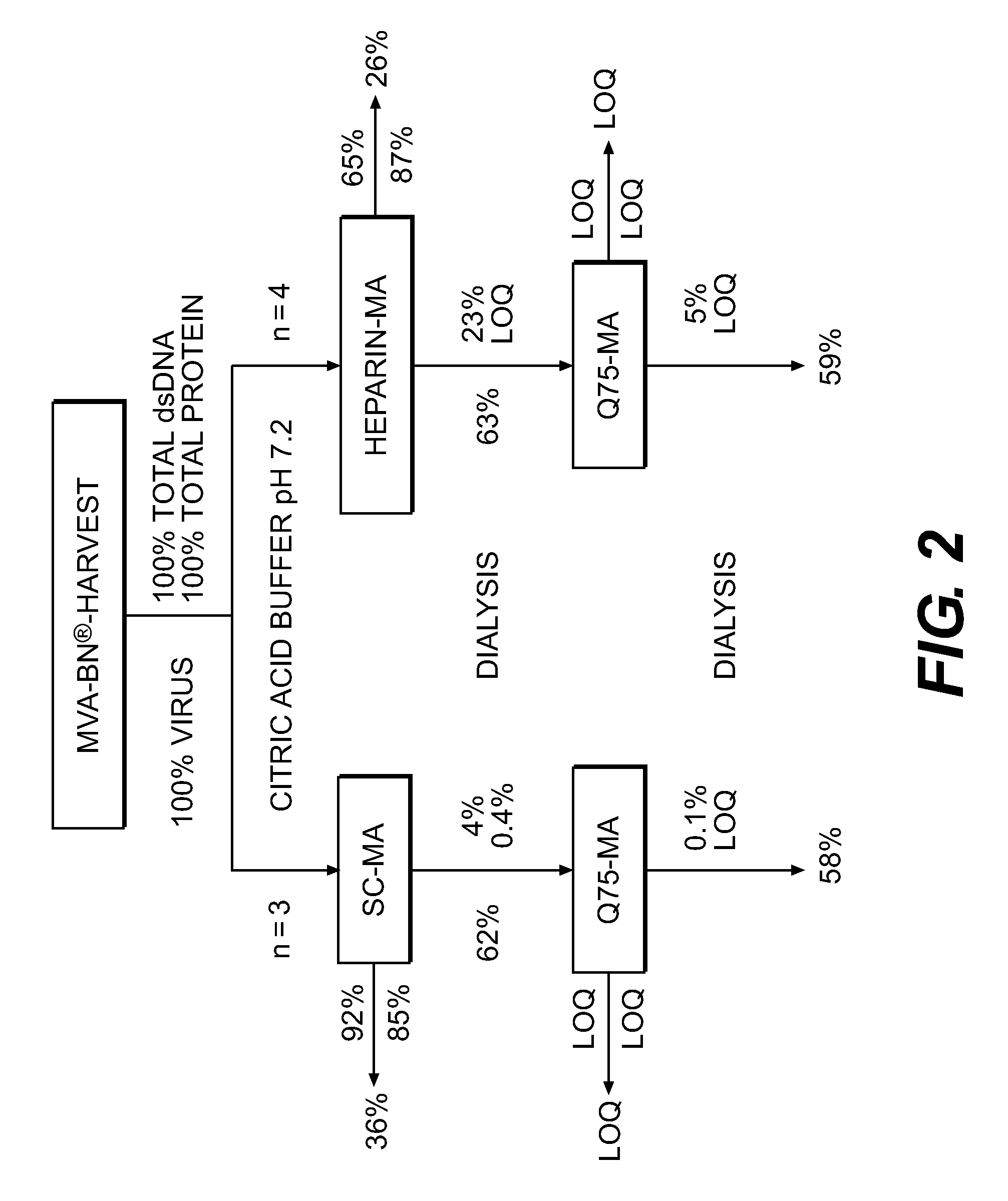
Purification of vaccinia virus- and recombinant vaccinia virus-based vaccines
ActiveUS8003363B2Reduce the amount requiredViral antigen ingredientsRecovery/purificationOrganismVaccinia viruses
The present invention relates to methods for purification of Vaccinia viruses (VV) and / or Vaccinia virus (VV) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS
Vaccination of skunks and/or mongooses against rabies
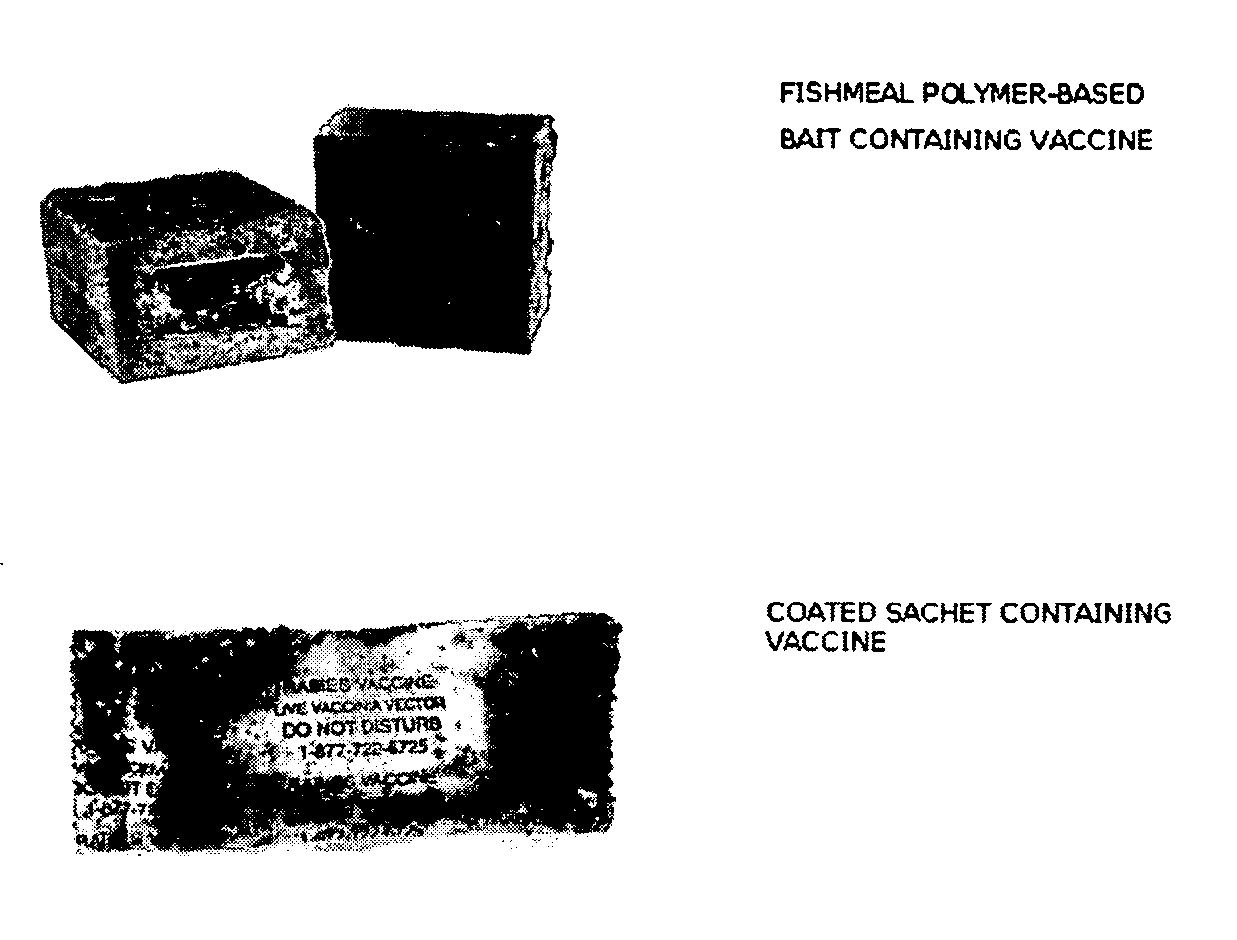
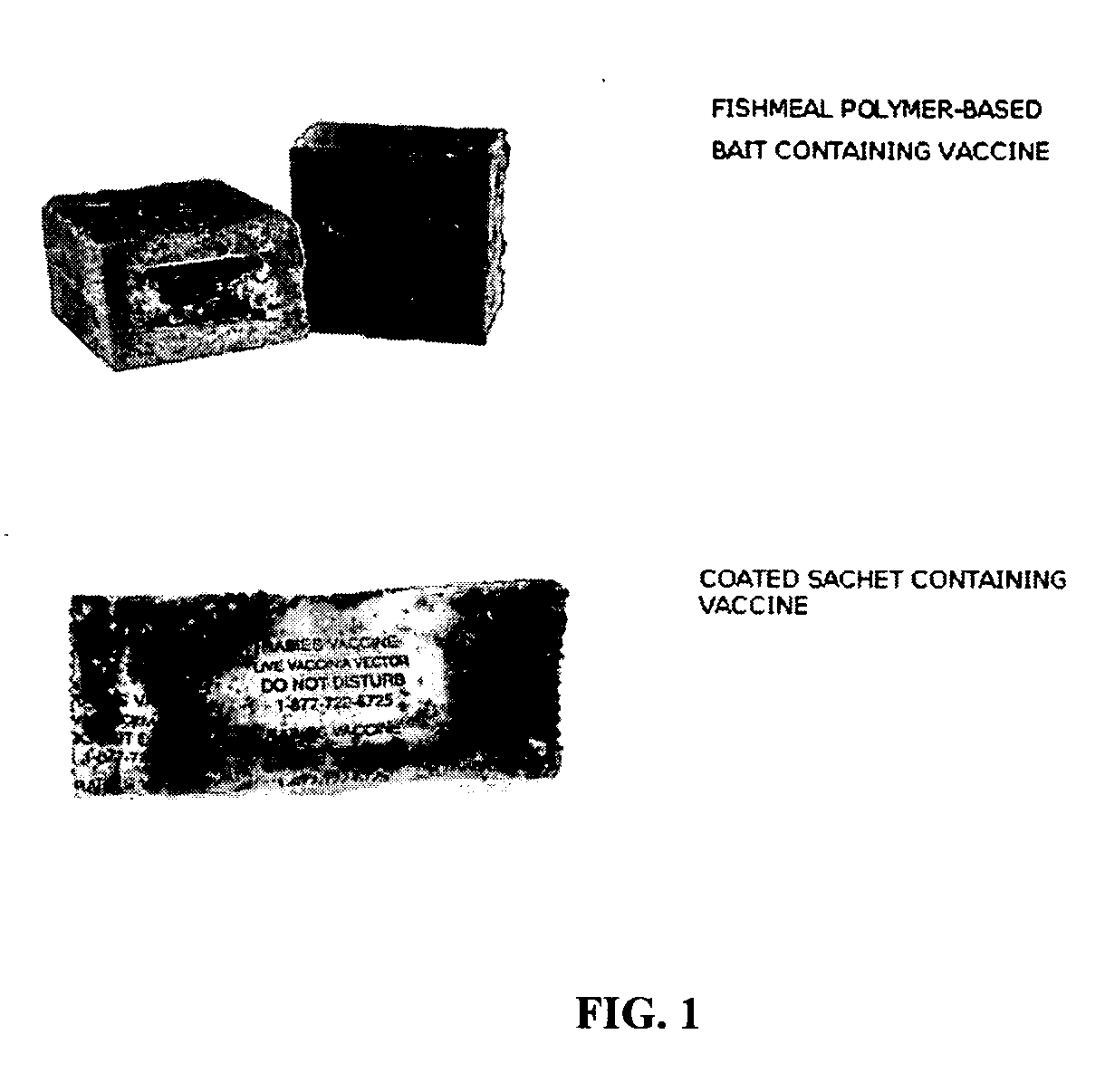
The present invention relates to recombinant anti-rabies vaccines and the oral administration of such vaccines to skunks and / or mongooses. Advantageously, the anti-rabies vaccine may comprise a recombinant vaccinia virus containing a rabies glycoprotein gene. The invention encompasses methods of vaccinating skunks and / or mongooses by administration of an anti-rabies vaccines which may comprise a recombinant vaccinia virus containing a rabies glycoprotein gene.
Owner:MERIAL LTD
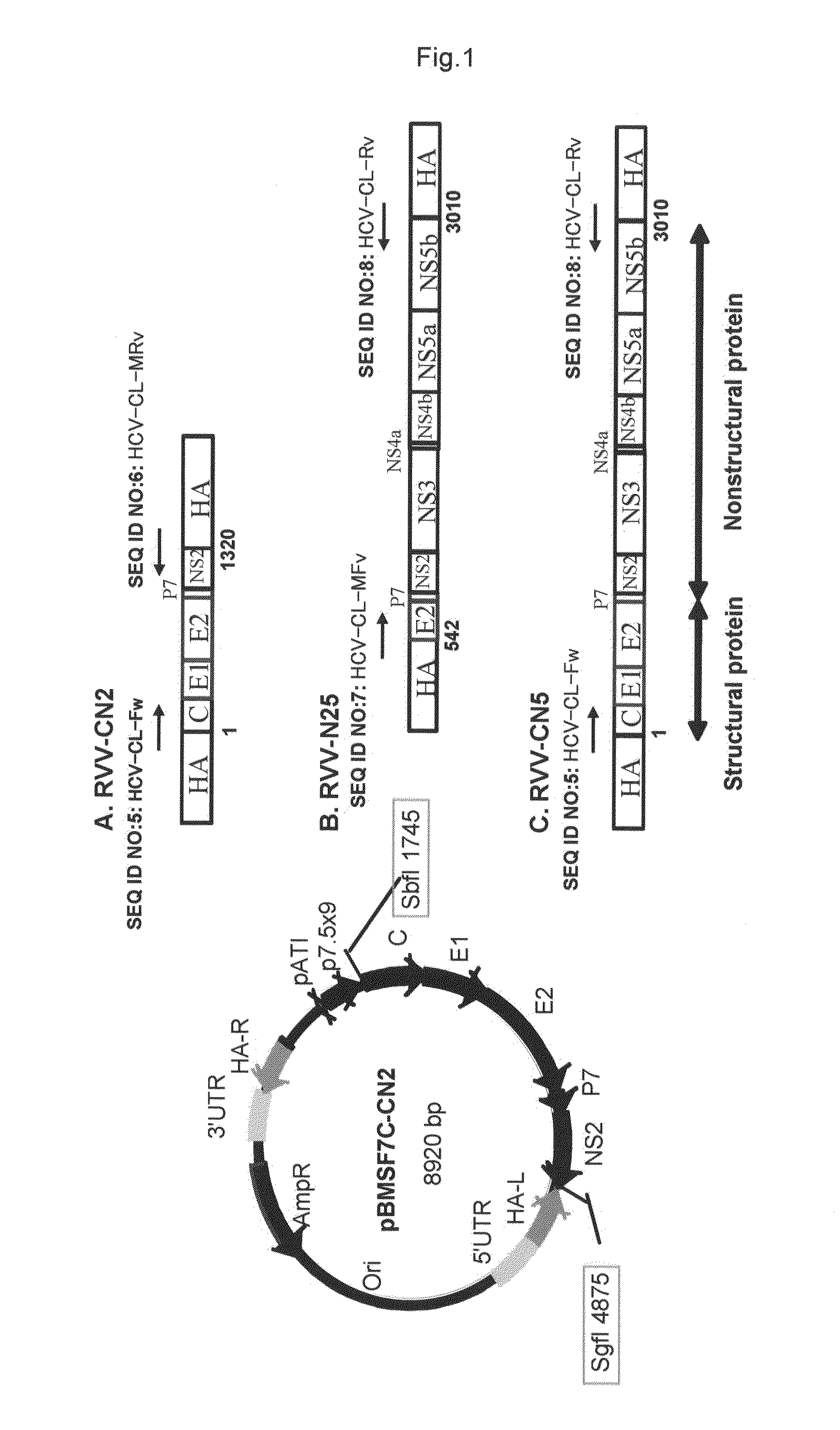
Recombinant vaccinia virus having hepatitis c virus gene
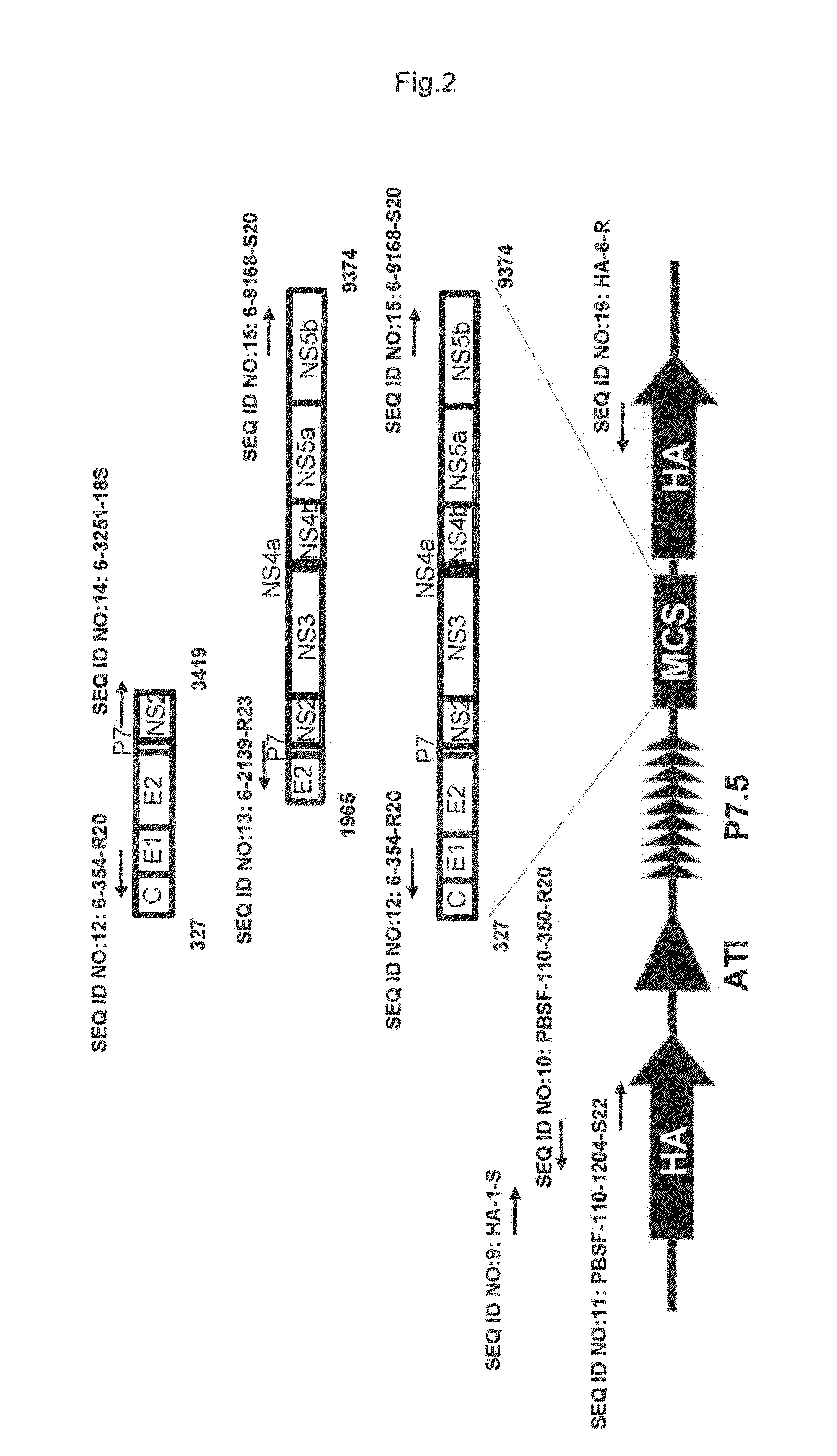
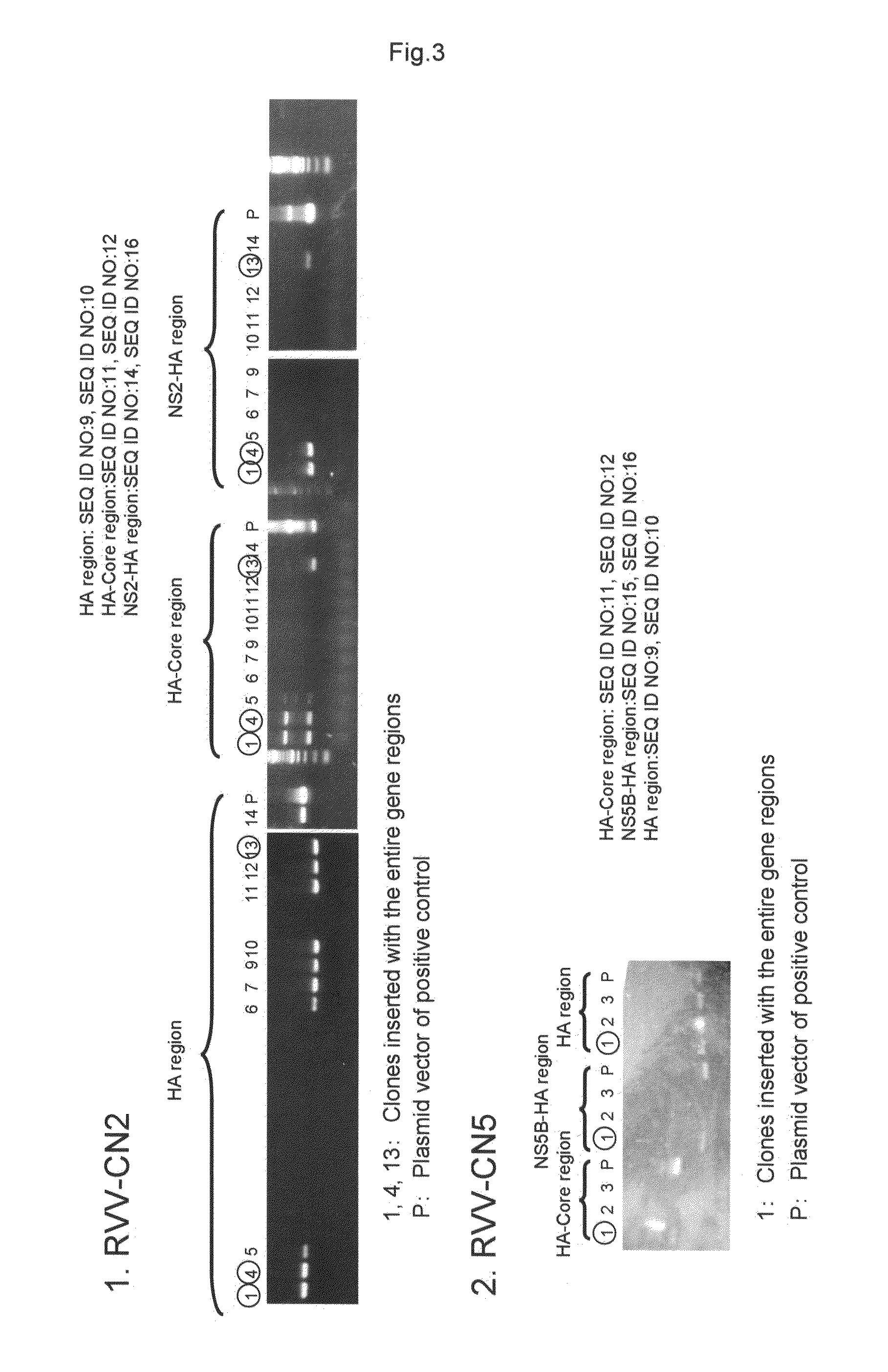
InactiveUS20110275139A1Potent hepatitis C infection prevention methodAvoid infectionSsRNA viruses positive-senseViral antigen ingredientsHepacivirusGene
Provided is a recombinant virus which is efficacious in preventing the onset of hepatitis C infection and has a high safety. Also provided is a vaccine for hepatitis C virus which contains the recombinant virus. A recombinant vaccinia virus which can express hepatitis C virus gene. The hepatitis C virus vaccine as described above contains the recombinant virus as described above.
Owner:KM BIOLOGICS CO LTD +1
Recombinant MVA virus, and the use thereof
InactiveUS20070071770A1Improve efficiencyReduce the amount of solutionOrganic active ingredientsVirusesAntigenRecombinant vaccinia virus
The present invention relates to recombinant vaccinia viruses derived from the modified vaccinia virus Ankara (MVA) and containing and capable of expressing foreign genes which are inserted at the site of a naturally occurring deletion in the MVA genome, and the use of such recombinant MVA viruses for the production of polypeptides, e.g. antigens or therapeutic agents, or viral vectors for gene therapy, and the use of such recombinant MVA viruses encoding antigens as vaccines.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
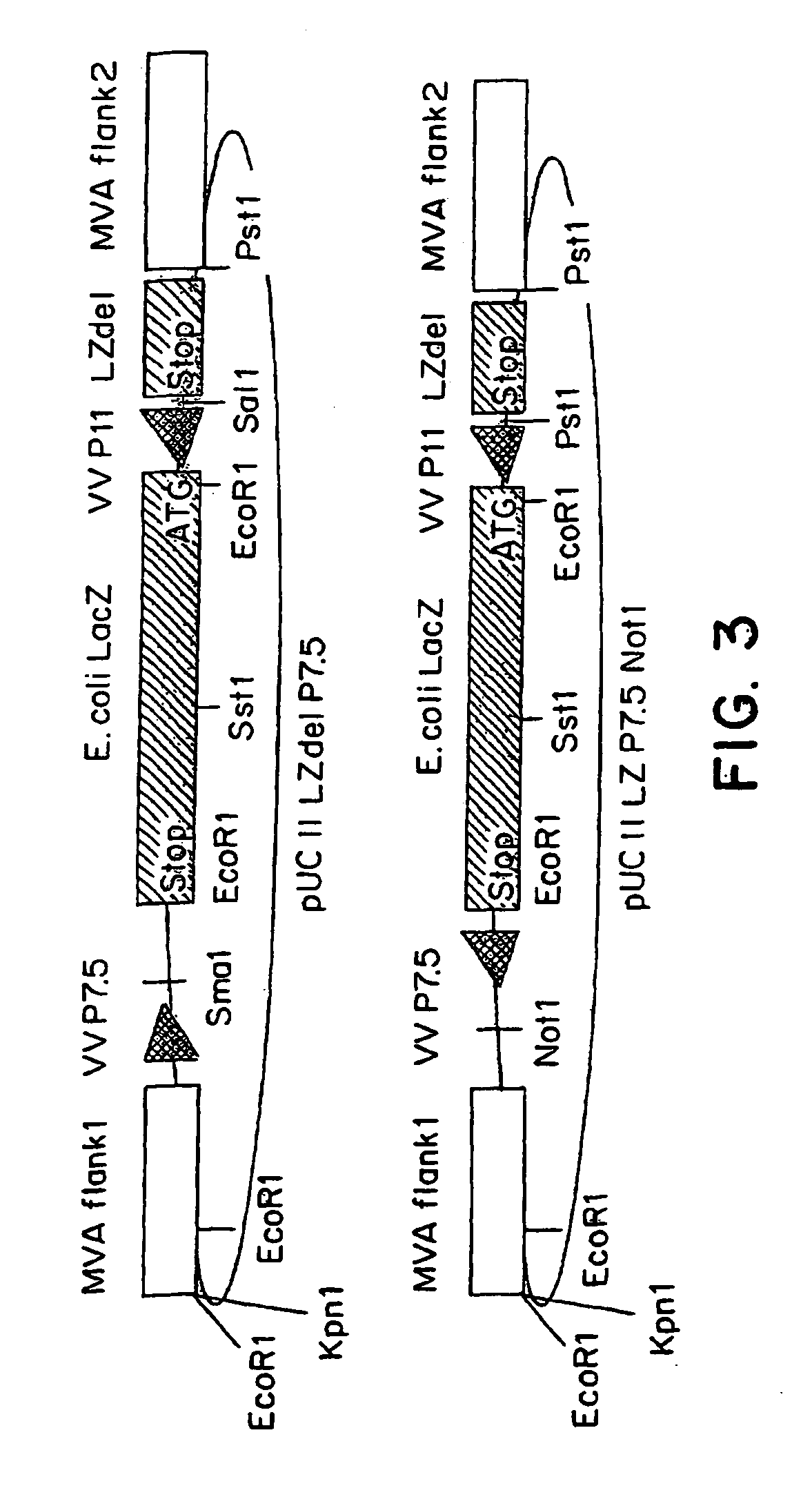
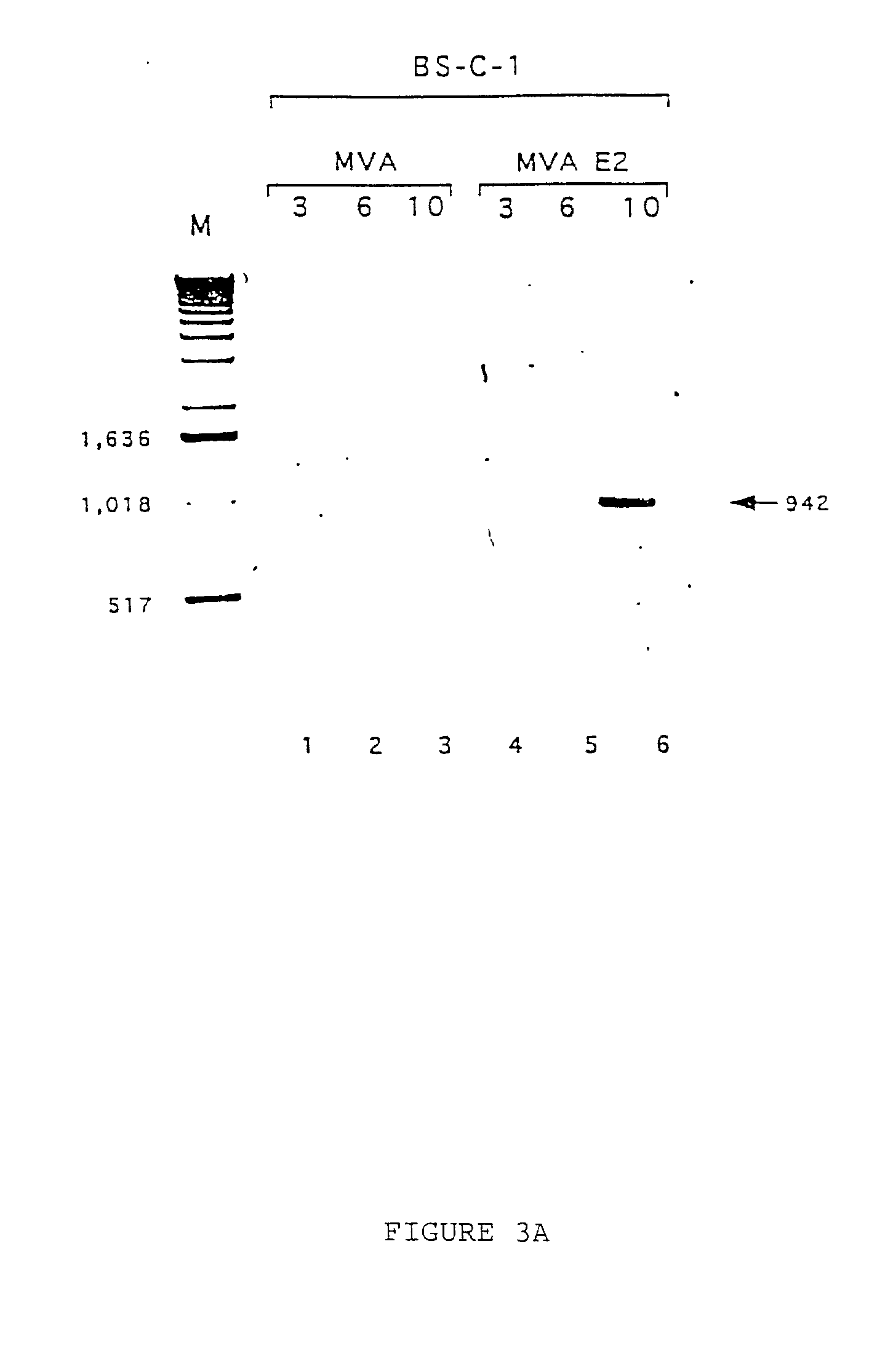
Recombinant MVA virus expressing the E2 gene of Bovine papillomavirus and its use in the therapy of tumors generated by papillomaviruses
InactiveUS20020028498A1Improve efficiencyBiocideGenetic material ingredientsAlphapapillomavirusWilms' tumor
A recombinant vaccinia virus derived from the vaccinia virus Ankara (MVA) encoding and capable of expressing the E2 gene of Bovine papillomavirus. Also, the use of the virus in the treatment of lesions caused by papillomavirus.
Owner:LEMERY DE C V
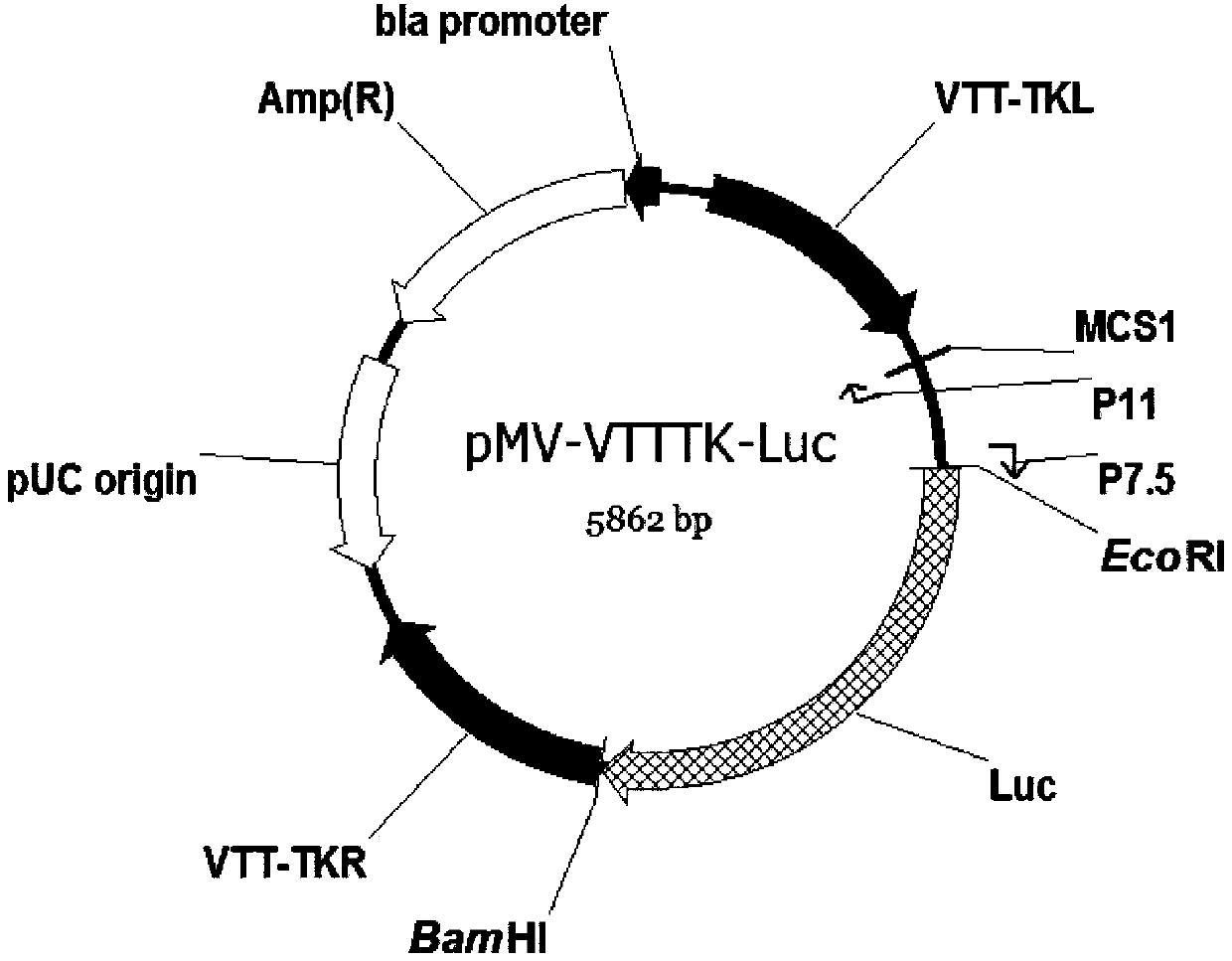
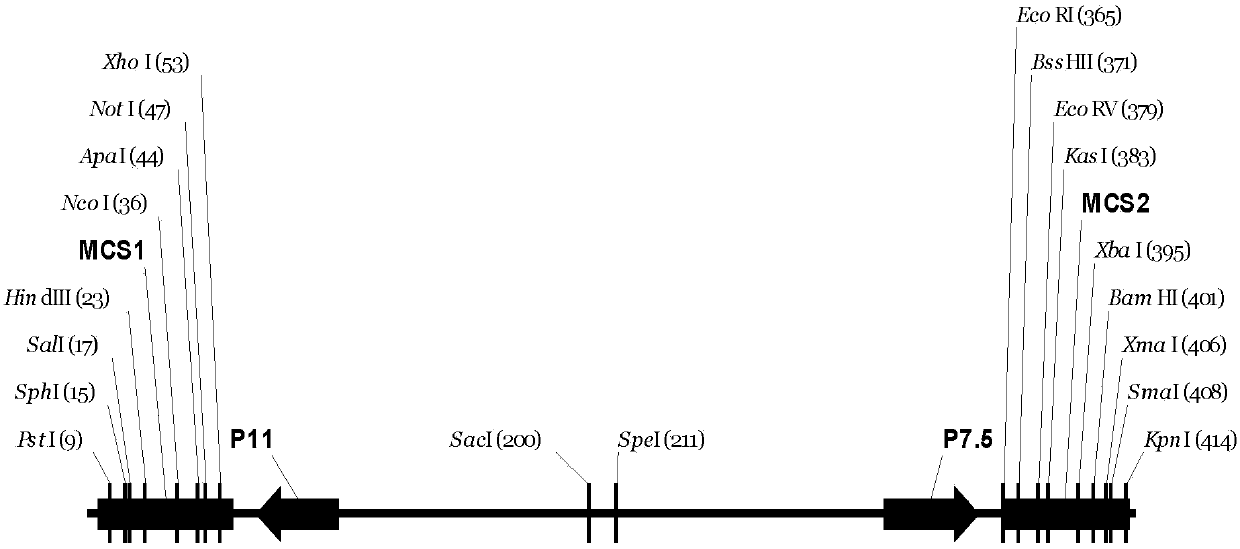
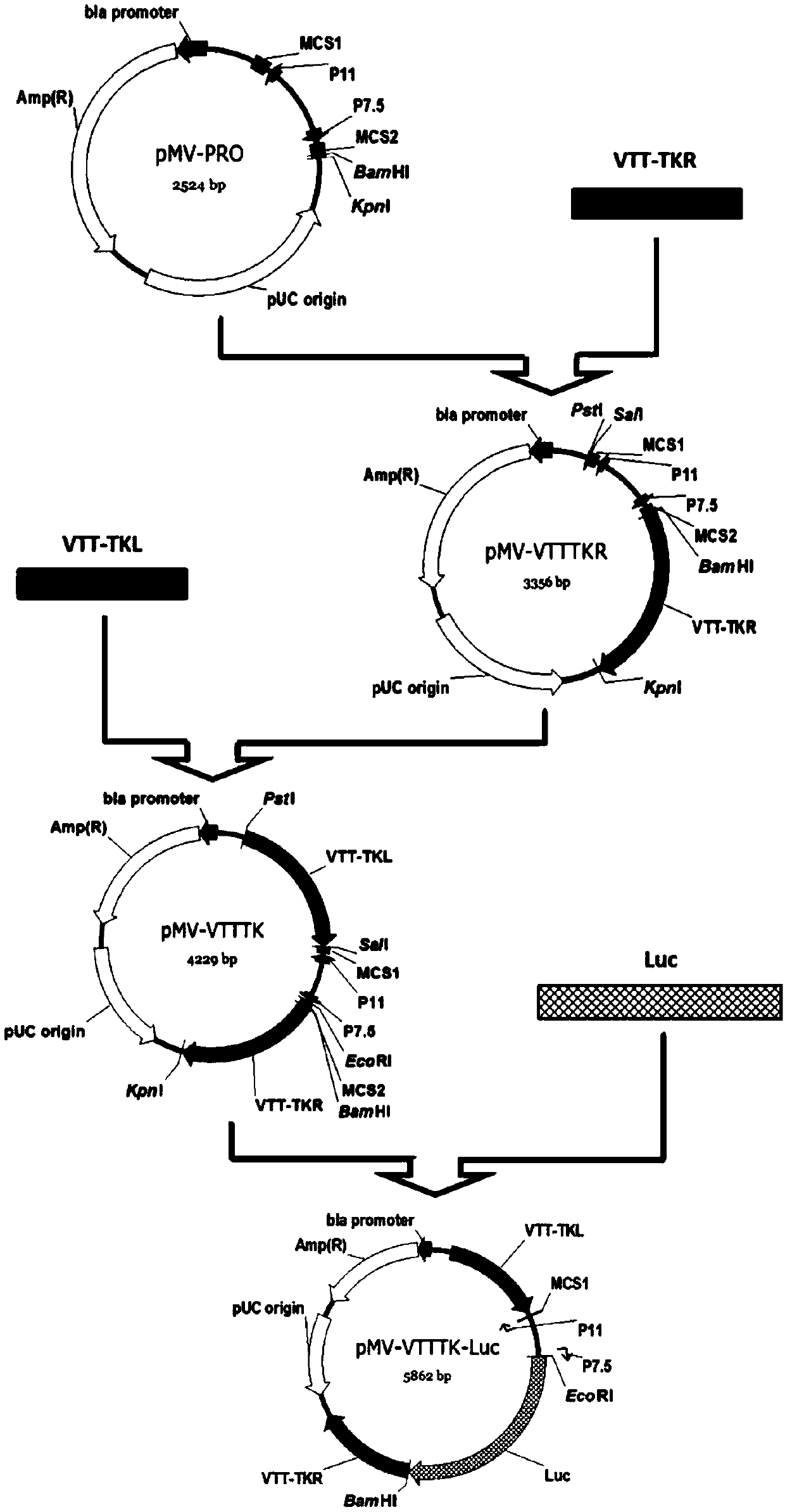
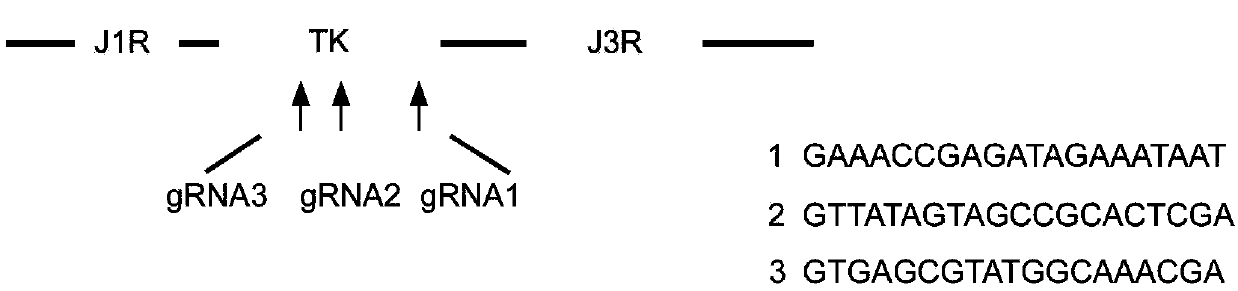
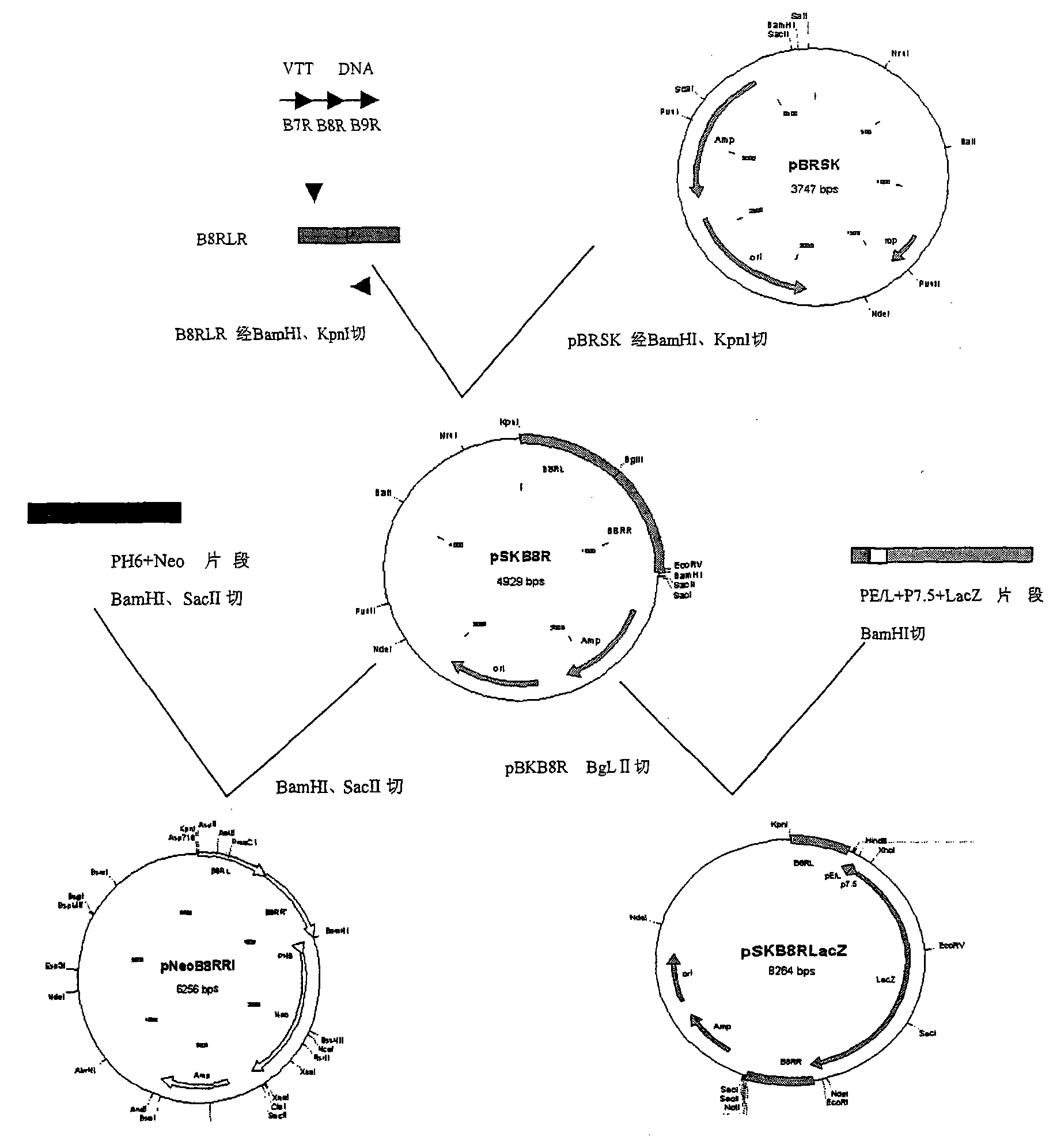
Tracer target plasmid for vaccinia virus Tian Tan TK gene and preparation method thereof
InactiveCN107604004AMeet the needs of different needsFermentationGenetic engineeringRestriction Enzyme Cut SiteNucleotide
The invention discloses a tracer target plasmid for a vaccinia virus Tian Tan TK gene. The tracer target plasmid for the vaccinia virus Tian Tan TK gene comprises a starting vector, an upstream homologous recombination arm VTT-TKL of the vaccinia virus Tian Tan TK gene and a downstream homologous recombination arm VTT-TKR of the vaccinia virus Tian Tan TK gene; a vaccinia virus early / late promoterP11 and a late promoter P7.5 exist between the VTT-TKL and the VTT-TKR; a connecting sequence between the promoter P11 and the promoter P7.5 is provided with two restriction enzyme cutting sites; each of the downstream of the promoter P11 and the downstream of the promoter P7.5 comprises a poly-cloning site; a tracer gene Luciferase is inserted at the poly-cloning site behind the promoter P7.5; the nucleotide sequence of the tracer target plasmid is as shown in the sequence 1. According to the tracer target plasmid disclosed by the invention, the problem that the distribution situation of viruses in vivo cannot be analyzed by the existing recombinant vaccinia virus is solved. The invention also provides a preparation method for the tracer target plasmid.
Owner:XIAN MEDICAL UNIV
Fusion proteins to facilitate selection of cells infected with specific immunoglobulin gene recombinant vaccinia virus
ActiveUS9708601B2Library screeningVirus peptidesImmunoglobulin heavy chainRecombinant vaccinia virus
The present invention relates to a high efficiency method of expressing immunoglobulin molecules in eukaryotic cells. The invention is further drawn to a method of producing immunoglobulin heavy and light chain libraries, particularly using the trimolecular recombination method, for expression in eukaryotic cells. The invention further provides methods of selecting and screening for antigen-specific immunoglobulin molecules, and antigen-specific fragments thereof. The invention also provides kits for producing, screening and selecting antigen-specific immunoglobulin molecules. Finally, the invention provides immunoglobulin molecules, and antigen-specific fragments thereof, produced by the methods provided herein.
Owner:VACCINEX
GFP (Green Fluorescent Protein) tracing system of vaccinia virus and application of GFP tracing system
The invention discloses a GFP (Green Fluorescent Protein) tracing system of a vaccinia virus and an application of the system. The system disclosed by the invention is a recombinant vaccinia virus which is a recombinant virus obtained by subjecting wild-type vaccinia virus genome DNA (Deoxyribonucleic Acid) to replacement or insertion, wherein the replacement is to replace any segment of a segment a in the wild-type vaccinia virus genome DNA by a segment b; the insertion is to insert the segment b at any locus of the segment a into the wild-type vaccinia virus genome DNA, the segment a is the DNA segment shown in the sequence 1 in a sequence table; and the segment b is the DNA segment containing GFP encoding gene. The V.V. (Vaccinia Virus)-GFP disclosed by the invention can be applied to a research in parallel with a wild-type virus; and in addition, by virtue of the signal amplification effect of the GFP, the V.V. -GFP can greatly improve the virus monitoring sensitivity, provide a new concept for establishing a novel low-dosage virus subclinical infection animal model, and provide a new technical platform for the application researches on screening of anti-V.V medicines and the like.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
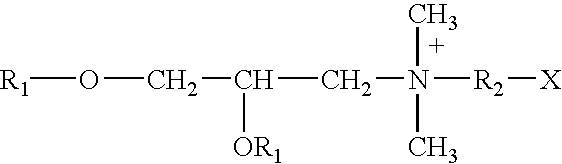
Adjuvanted rabies vaccine with improved viscosity profile
ActiveUS9216213B2Good curative effectHigh viscositySsRNA viruses negative-senseViral antigen ingredientsAdjuvantRabies vaccination
Owner:MERIAL INC +1
Efficient recombinant vaccinia virus vector and establishing method thereof
PendingCN109536529AImprove recombination efficiencySave time and costTransferasesStable introduction of DNAFluorescenceWestern blot
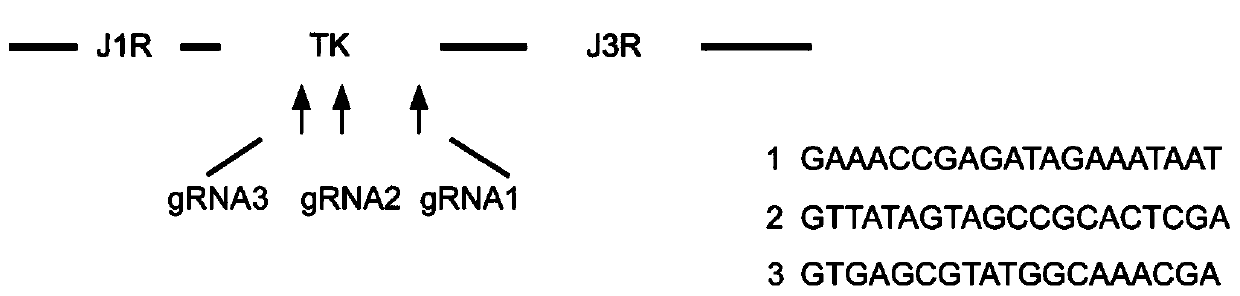
The present invention establishes a system for an efficient recombinant vaccinia virus vector and comprises an efficient recombinant vaccinia virus vector and an establishing method thereof. A CRISPR-Cas9 technology is used to knock out a TK region of a vaccinia virus Tiantan strain and then the vaccinia virus Tiantan strain is transfected with a recombinant plasmid pJ2R-EGFP carrying EGFP. A recombinant virus with deletion of TK and insertion of EGFP is obtained by fluorescence screening to establish the system for the efficient recombinant vaccinia virus vector. A molecular cloning technology, a Western Blot experiment, an immunofluorescence and a viral plaque formation test find that a recombination rate in a first round of a recombinant virus screening process is improved compared witha traditional homologous recombination method. Further, the recombinant virus obtained by a PCR technology, an agarose gel electrophoresis and a sequencing verification is accurately modified at specific sites. The vaccinia virus vector is improved in the recombination rate and provides application value in vaccine vector construction, tumor immunotherapy, etc.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Recombinant vaccinia virus of Tiantan strain with IFN-gamma receptor gene (B8R) deletion and applications thereof
InactiveCN101671695ALow toxicityImprove securityMicroorganism based processesAntiviralsAntigenViral Vaccine
The invention relates to an attenuated carrier of recombinant vaccinia virus of Tiantan strain with IFN-gamma receptor gene (B8R) deletion, and AIDS vaccines of recombinant vaccinia virus of Tiantan strain with IFN-gamma receptor gene (B8R) deletion used for expressing various antigens (univalent or polyvalent) of HIV-1 and constructed based on same, a recombinant vaccinia virius VTKgpe recombinant vacciniavirus VTKgpe CGMCC.NO.1099 and another hepatitis B virus HBSAg antigen, and hepatitis B virus vaccines of recombinant vaccinia virus of Tiantan strain of IL-2. The invention has an importantmeaning to the use for preparation of recombinant vaccinia virus vaccines for treating virus diseases.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Purification of vaccinia virus- and recombinant vaccinia virus-based vaccines
ActiveUS20100112001A1Reduce the amount requiredViral antigen ingredientsRecovery/purificationViral VaccineOrganism
The present invention relates to methods for purification of Vaccinia viruses (VV) and / or Vaccinia virus (VV) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS
Recombinant vaccinia virus, vaccinia virus vector vaccine and application and preparation method of recombinant vaccinia virus
InactiveCN113151196AImprove stabilityBlock bindingSsRNA viruses positive-senseViral antigen ingredientsShuttle vectorAdjuvant
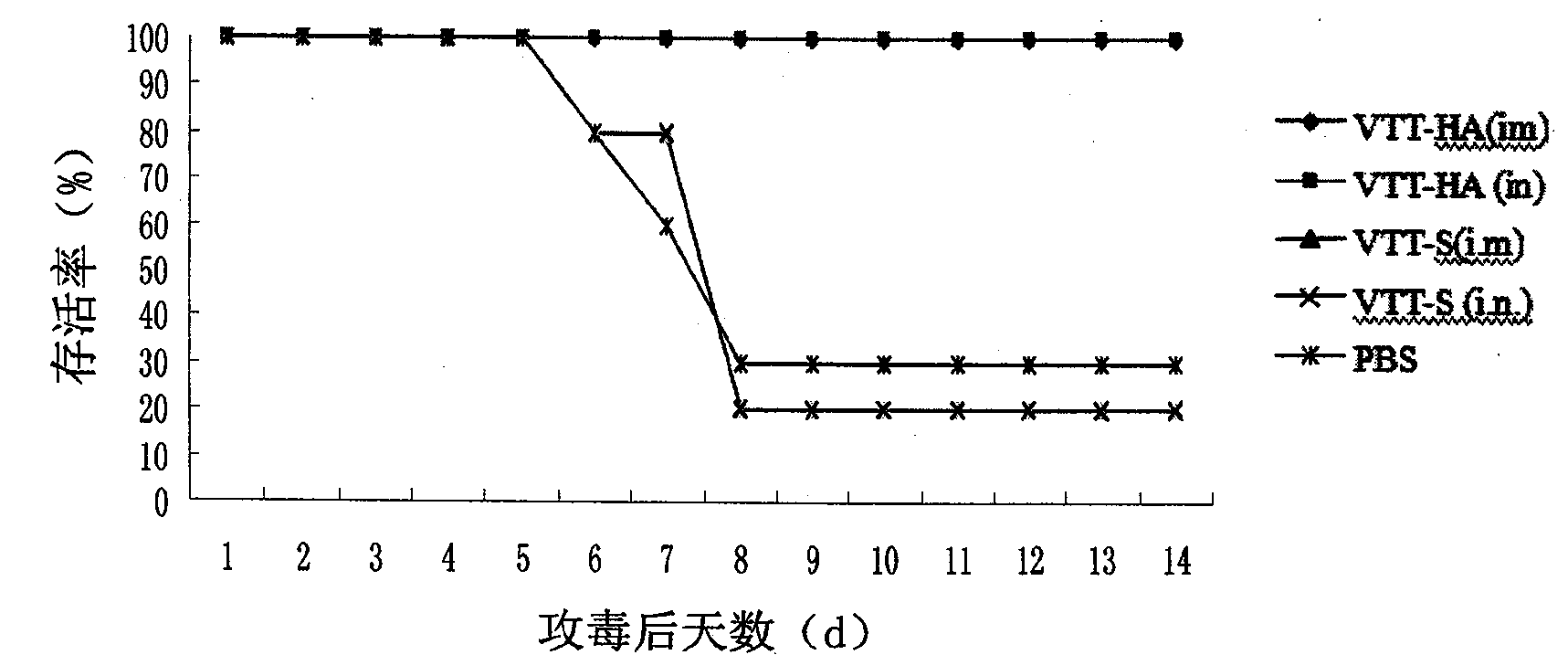
The invention discloses a recombinant vaccinia virus, a vaccinia virus vector vaccine and an application and preparation method of the recombinant vaccinia virus. The recombinant vaccinia virus comprises: a combined defect of a thymidine kinase (TK) gene and an L025 gene, and a recombinant nucleic acid sequence encoding a first nucleic acid sequence in the S gene of a novel coronavirus, the first nucleic acid sequence is inserted into the genome of the recombinant vaccinia virus. The recombinant vaccinia virus vector vaccine comprises the recombinant vaccinia virus and a pharmaceutically acceptable vector or adjuvant. The method for preparing the recombinant vaccinia virus comprises the following steps: introducing the recombinant nucleic acid sequence between a left arm and a right arm of a target gene of a genome of the vaccinia virus to obtain a recombinant shuttle vector, and transfecting the recombinant shuttle vector into a host cell to form the recombinant vaccinia virus. The invention further discloses a recombinant nucleic acid sequence for coding the novel coronavirus S protein and a recombinant virus vector.
Owner:SHENZHEN HUA YAO KANG MING BIOPHARMACEUTICAL CO LTD
Purification of vaccinia virus- and recombinant vaccinia virus-based vaccines
ActiveUS8012738B2High yieldMore cost-effectiveBiocideViral antigen ingredientsViral VaccineVaccinia viruses
The present invention relates to methods for purification of Vaccinia viruses (W) and / or Vaccinia virus (W) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS
Purification of vaccinia virus- and recombinant vaccinia virus-based vaccines
ActiveUS20100129326A1Improve purification effectImprove efficiencyBiocideViral antigen ingredientsViral VaccineDrug biological activity
The present invention relates to methods for purification of Vaccinia viruses (W) and / or Vaccinia virus (W) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS
In-vivo imaging tracing system of vaccinia virus and application of system
The invention discloses an in-vivo imaging tracing system of a vaccinia virus and an application of the system. The system disclosed by the invention is a recombinant vaccinia virus which is a recombinant virus obtained by subjecting wild-type vaccinia virus genome DNA (Deoxyribonucleic Acid) to replacement or insertion, wherein the replacement is to replace any segment of a segment a in the wild-type vaccinia virus genome DNA by a segment b; the insertion is to insert the segment b at any locus of the segment a in the wild-type vaccinia virus genome DNA; the segment a is the DNA segment shown in the sequence 1 in a sequence table; and the segment b is the DNA segment containing Gaussia luciferase encoding gene. The V.V. (Vaccinia Virus)-LUC (Luciferase) disclosed by the invention can be applied to a research in parallel with a wild-type virus; and in addition, by virtue of the signal amplification effect of the Gaussia luciferase, the V.V.-LUC can greatly improve the virus monitoring sensitivity, provide a new concept for establishing a novel low-dosage virus subclinical infection animal model, and provide a new technical platform for the application researches on screening of anti-V.V medicines and the like.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
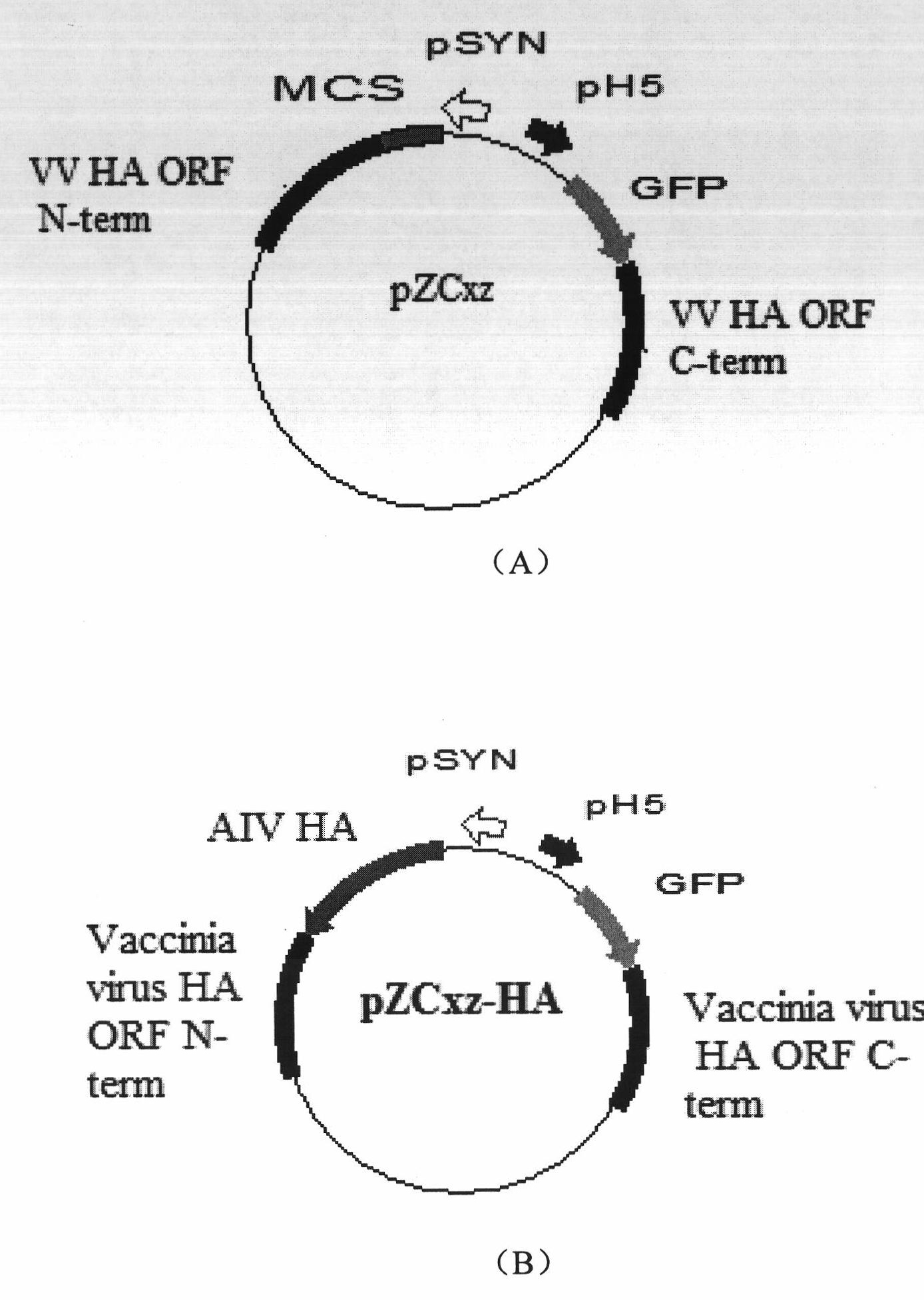
Recombinant vaccinia virus having hemagglutinin protein genes derived from novel influenza viruses
InactiveUS20130288338A1Avoid symptomsInhibition is effectiveSsRNA viruses negative-senseOrganic active ingredientsHemagglutinin proteinInfluenza vaccine
Provided are a highly-safe recombinant vaccinia virus that is effective in preventing the onset of symptoms due to infection by novel influenza viruses, and a vaccine for the novel influenza viruses containing the recombinant vaccinia virus. This recombinant vaccinia virus is capable of expressing the hemagglutinin protein genes of the novel influenza virus. This novel influenza vaccine contains the recombinant vaccinia virus.
Owner:TOKYO METROPOLITAN INST OF MEDICAL SCI +2
Efficiently recombinant vaccinia virus vector without screening markers and establishment method of vaccinia virus vector
InactiveCN110029128AImprove recombination efficiencyRaise the possibilityTransferasesStable introduction of DNAWestern blotViral vector
The invention discloses an efficiently recombinant vaccinia virus vector without screening markers and an establishment method of the vaccinia virus vector. The TK region of vaccinia virus Tiantan strain is knocked out by CRISPR-Cas9 technology and then the processed vaccinia virus Tiantan strain is transfected with a recombinant plasmid pJ2R-EGFP-LoxP with EGFP. By fluorescent screening, a recombinant virus lacking TK and with inserted EGFP is obtained. Through calculation, the efficiency of the recombination is dozens of times higher than that of conventional homologous recombination, and anefficiently recombinant vaccinia virus vector system is established. Based on the recombinant viral vector, a screening marker, EGFP, in the recombinant viral vector is eliminated by a Cre-LoxP system. Through molecular cloning technology, a Western Blot experiment, immunofluorescence, PCR technology, etc., EGFP is accurately eliminated at a specific site of the resulting recombinant virus. The invention establishes the efficiently recombinant vaccinia virus vector system, the screening marker of the vaccinia virus vector is eliminated, and therefore the application value to vaccine vector construction, tumor immunotherapy and other aspects is improved.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Recombinant vaccinia virus vaccine
InactiveUS20050053620A1Low cytotoxicityImprove securityViral antigen ingredientsVirus peptidesViral VaccineVaccinia virus vaccine
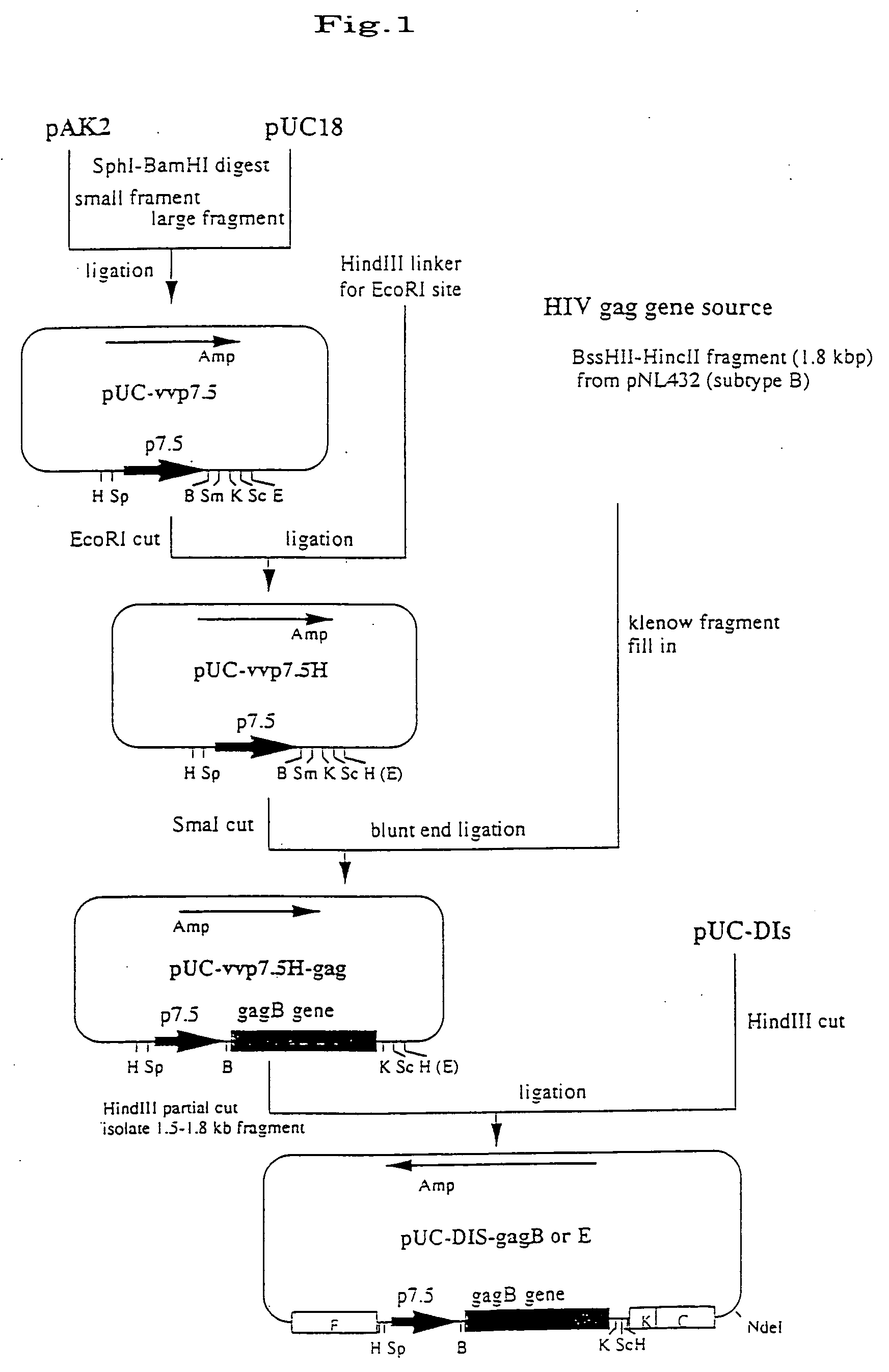
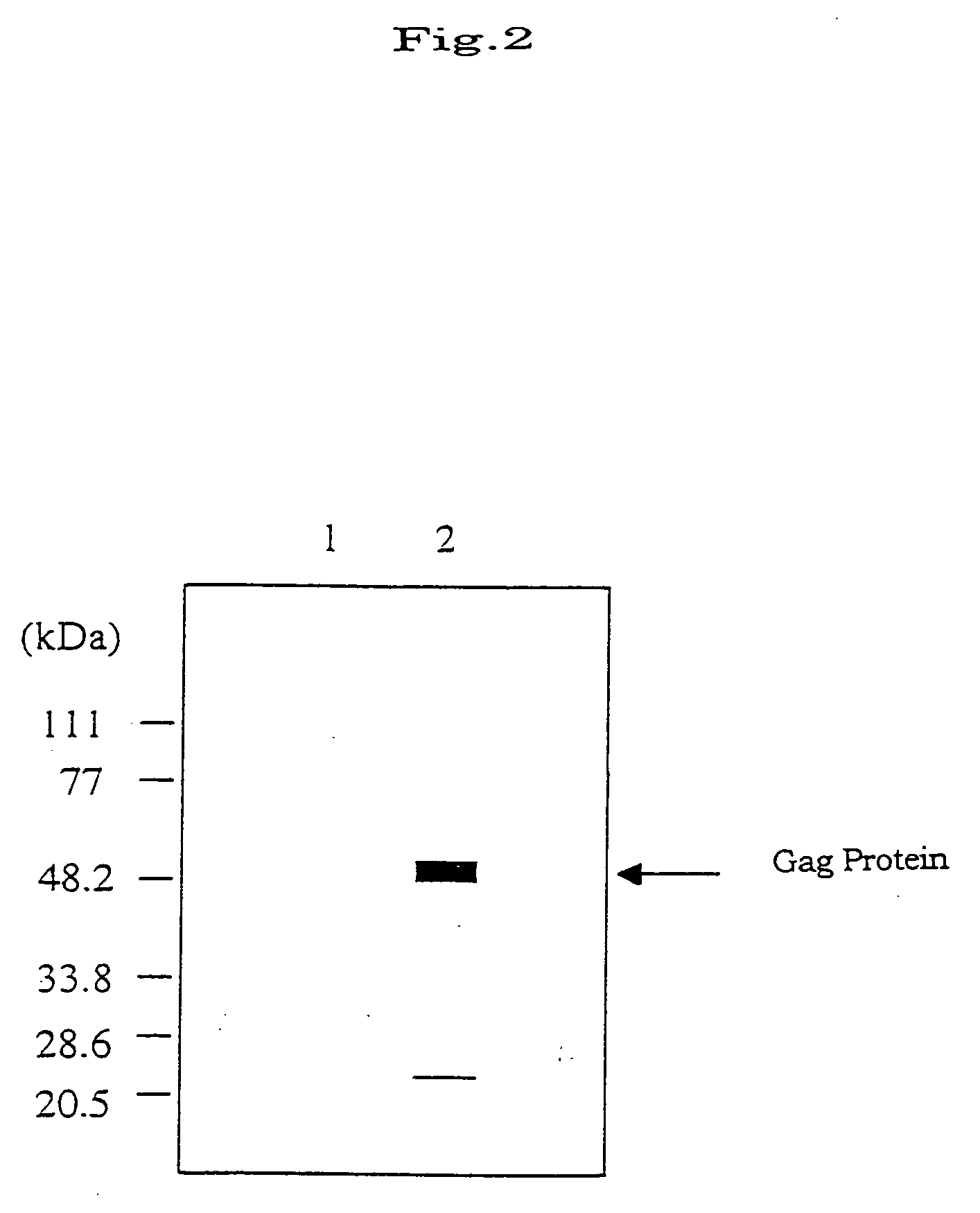
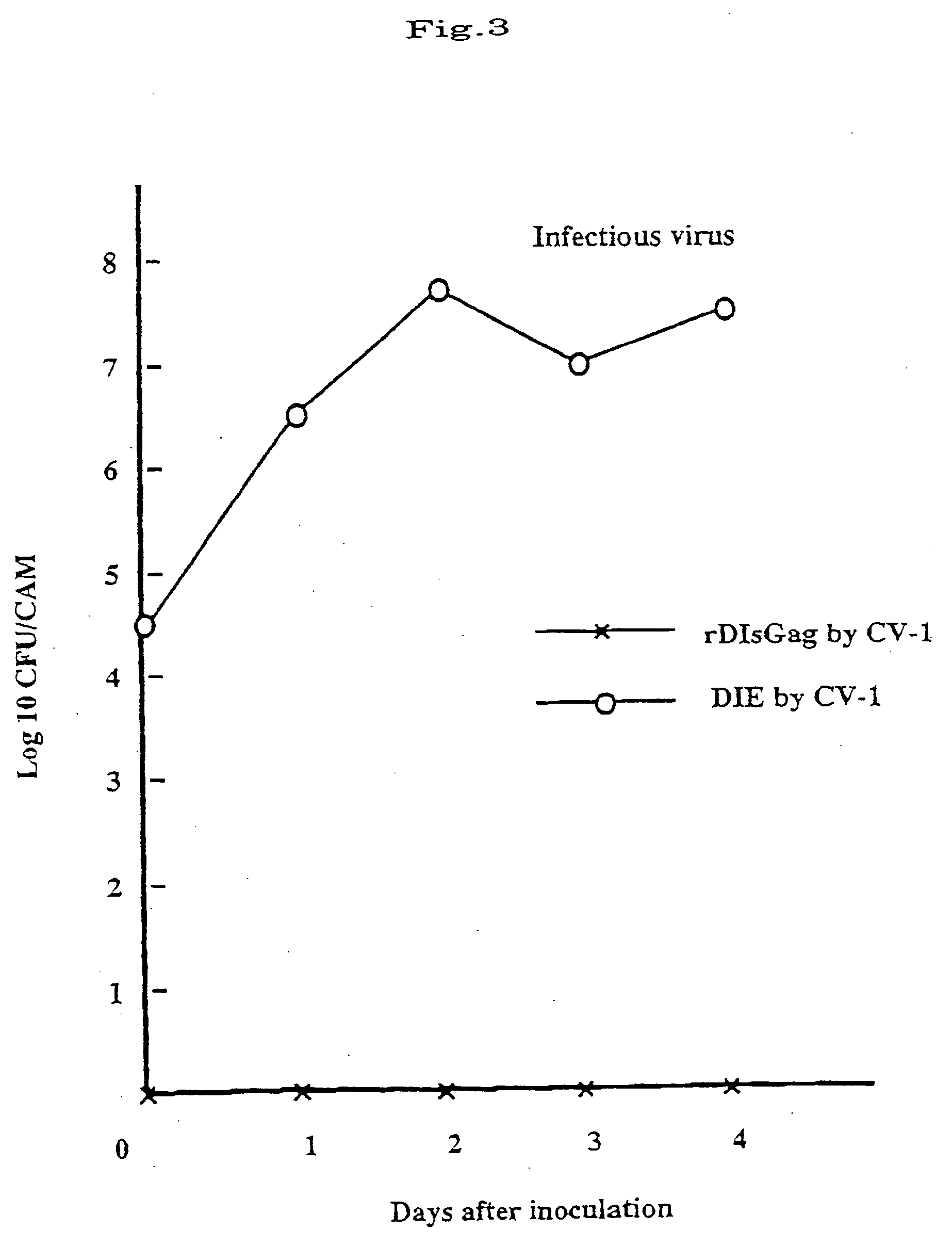
The invention provides a recombinant vaccinia virus strain DIs that possesses a polynucleotide encoding a foreign antigenic protein in the non-essential gene region of the chromosome DNA and expresses the antigenic protein; and provides a highly-safe, vaccinia virus vaccine containing the recombinant virus strain DIs as the active ingredient. The invention also provides a method of using the vaccinia virus strain DIs as a vector for protein expression.
Owner:JAPAN SCI & TECH CORP +1
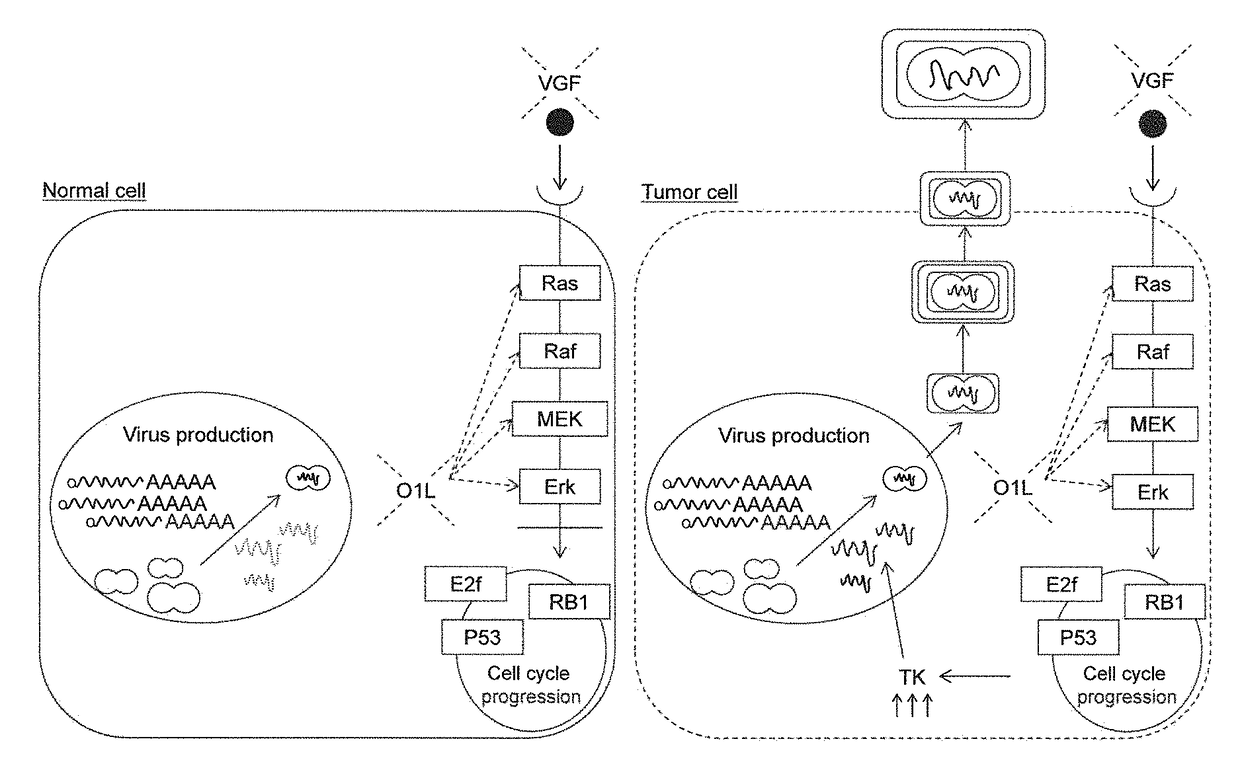
Mitogen-activated protein kinase-dependent recombinant vaccinia virus (MD-RVV) and use thereof
Owner:KM BIOLOGICS CO LTD +1
Bird flu vaccine by using attenuated vaccinia Tian Tan as vector
InactiveCN102140441AImprove securityImproving immunogenicityGenetic material ingredientsMicroorganism based processesHemagglutininBird flu
The invention relates to a recombinant vaccinia virus expressing the hemagglutinin (HA) protein of H5N1 bird flu virus and the recombinant vaccinia virus can be used as the bird flu vaccine. Specifically, the bird flu vaccine is constructed on the basis of attenuated vaccinia Tian Tan (CCTCC: V200416). The invention provides a vector for constructing the bird flu vaccine. The invention also relates to an inoculation scheme by using the bird flu vaccine.
Owner:高福 +1
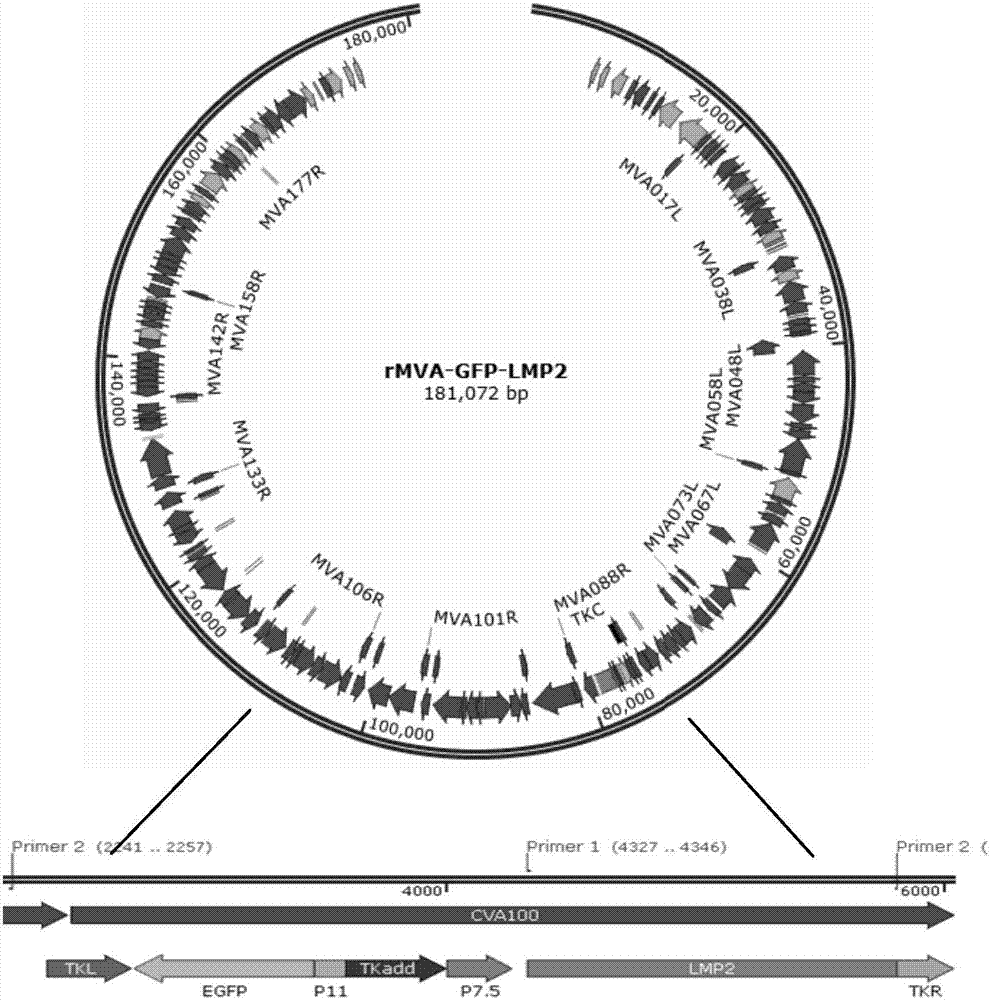
Recombinant vaccinia virus carrying EB virus latent membrane antigen 2 gene and application of recombinant vaccinia virus
InactiveCN107488677AEasy to take offEasy to markAntiviralsBlood/immune system cellsLymphocyteMembrane antigen
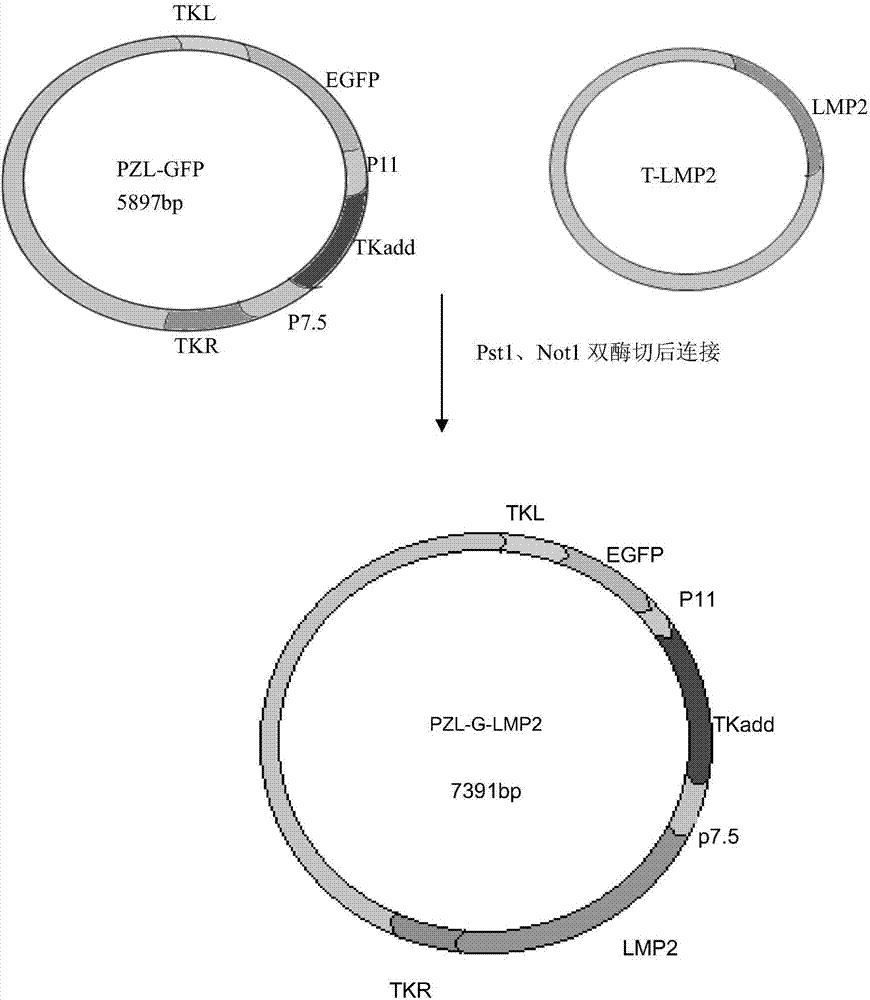
The invention provides a recombinant vaccinia virus carrying an EB (epstein-barr) virus latent membrane antigen 2 gene and an application of the recombinant vaccinia virus. A preparation method of the recombinant virus comprises the steps of constructing a joint recombinant plasmid and MVA (modified vaccinia virus ankara) infection BHK-21 cell carrying an EBV (epstein-barr virus) LMP2 exogenous gene and a green fluorescin exogenous gene, and obtaining an MVA recombinant virus (MVA-LMP2A) only carrying the EBV LMP2 exogenous gene. Mouse spleen lymphocytes immunized by MVA-LMP2A are detected by an IFN (interferon)-gamma immunodotting method, and a result shows that MVA-LMP2A can well induce EBV LMP2 specific immune response generated in a body of a mouse.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Mutant vaccinia viruses and use thereof
The present invention discloses recombinant vaccinia virus (VV) virions that are resistant to antiviral defenses and have enhanced anti-tumor activities. In one embodiment, the recombinant VV comprise one or more variant VV proteins that have mutations at one or more neutralizing antibody epitopes, thereby conferring viral escape from the neutralizing antibodies. In another embodiment, the recombinant VV is resistant to complement-mediated neutralization due to the expression of a regulator of complement activation (e.g. CD55). In another embodiment, the recombinant VV has enhanced anti-tumor activities due to the expression of bi-specific antibodies co-targeting cancer cells and immune effector cells, or the expression of a polypeptide blocking the PD-1 pathway. The recombinant vaccinia virus virions can be used to treat cancer in a subject.
Owner:ICELLKEALEX THERAPEUTICS LLC
Use of vaccinia virus deleted for the E3L gene as a vaccine vector
InactiveUS20060008470A1Reduce pathogenicityMaintain immunogenicityViral antigen ingredientsVirus peptidesHeterologousProtection sex
The present invention relates to vaccines having an increased level of safety comprising recombinant vaccinia viruses containing an inactivated E3L region. The invention also relates to methods for stimulating a protective immune response in an immunized host using the vaccines of the invention. The invention is based on the discovery that vaccinia virus mutants having deletions in the E3L region exhibit dramatically reduced pathogenesis while remaining highly immunogenic. In addition, the invention relates to modified recombinant vaccinia viruses engineered to express heterologous polypeptides and the use of such viruses in vaccines designed to stimulate a protective immune response against such polypeptides in a host. The invention further relates to an interferon-sensitive recombinant vaccinia virus with broad host range wherein a salamander eIF2α is inserted into the viral genome in place of at least a portion of the E3L gene.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com