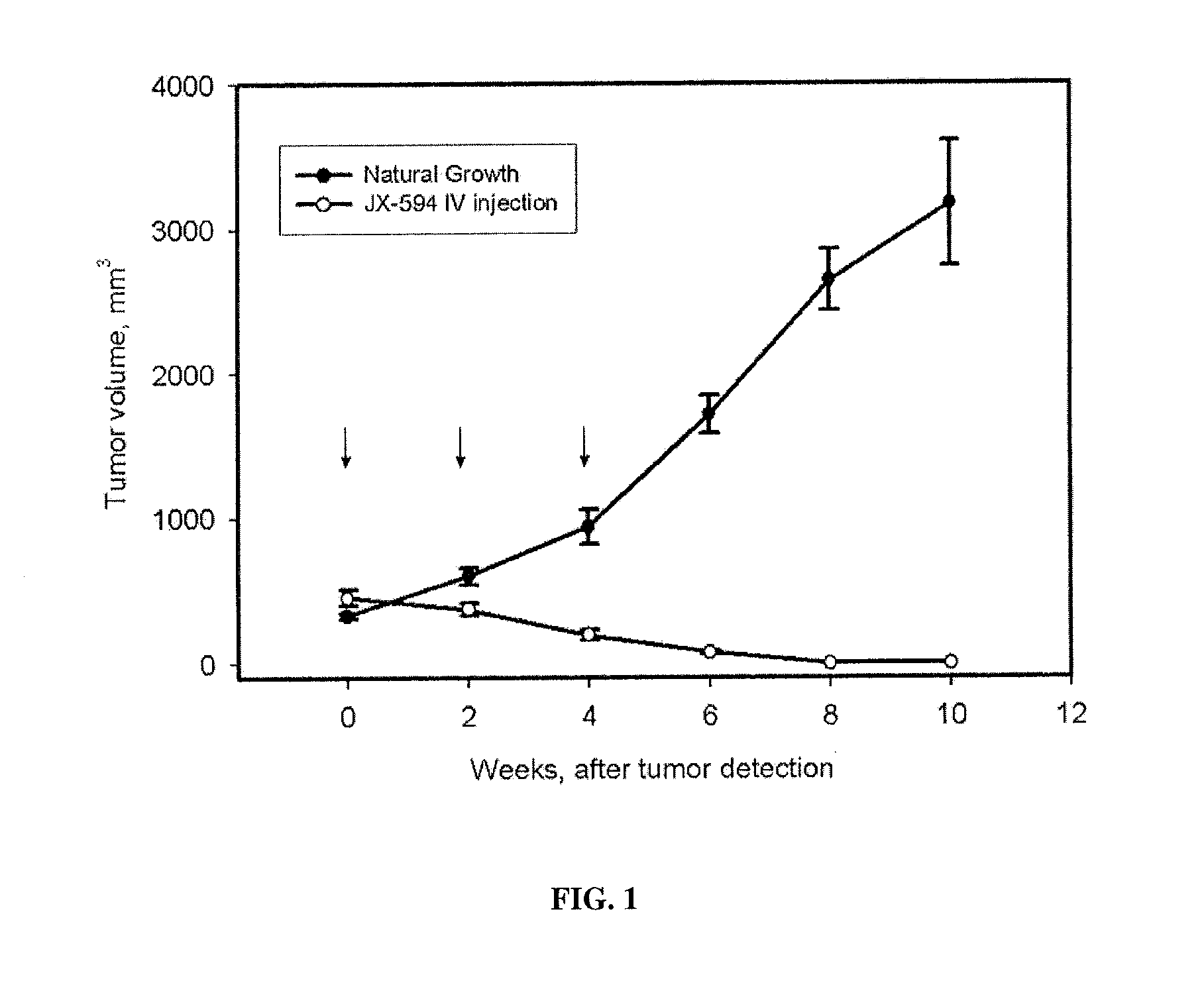
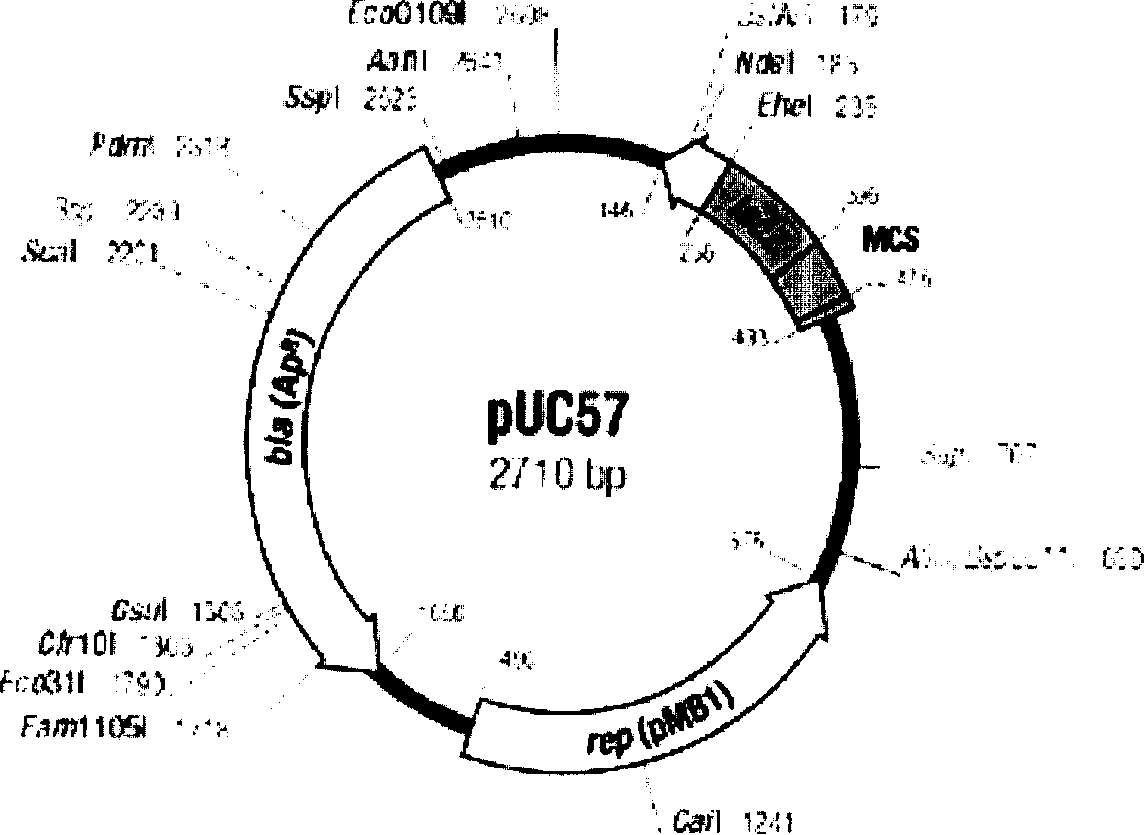
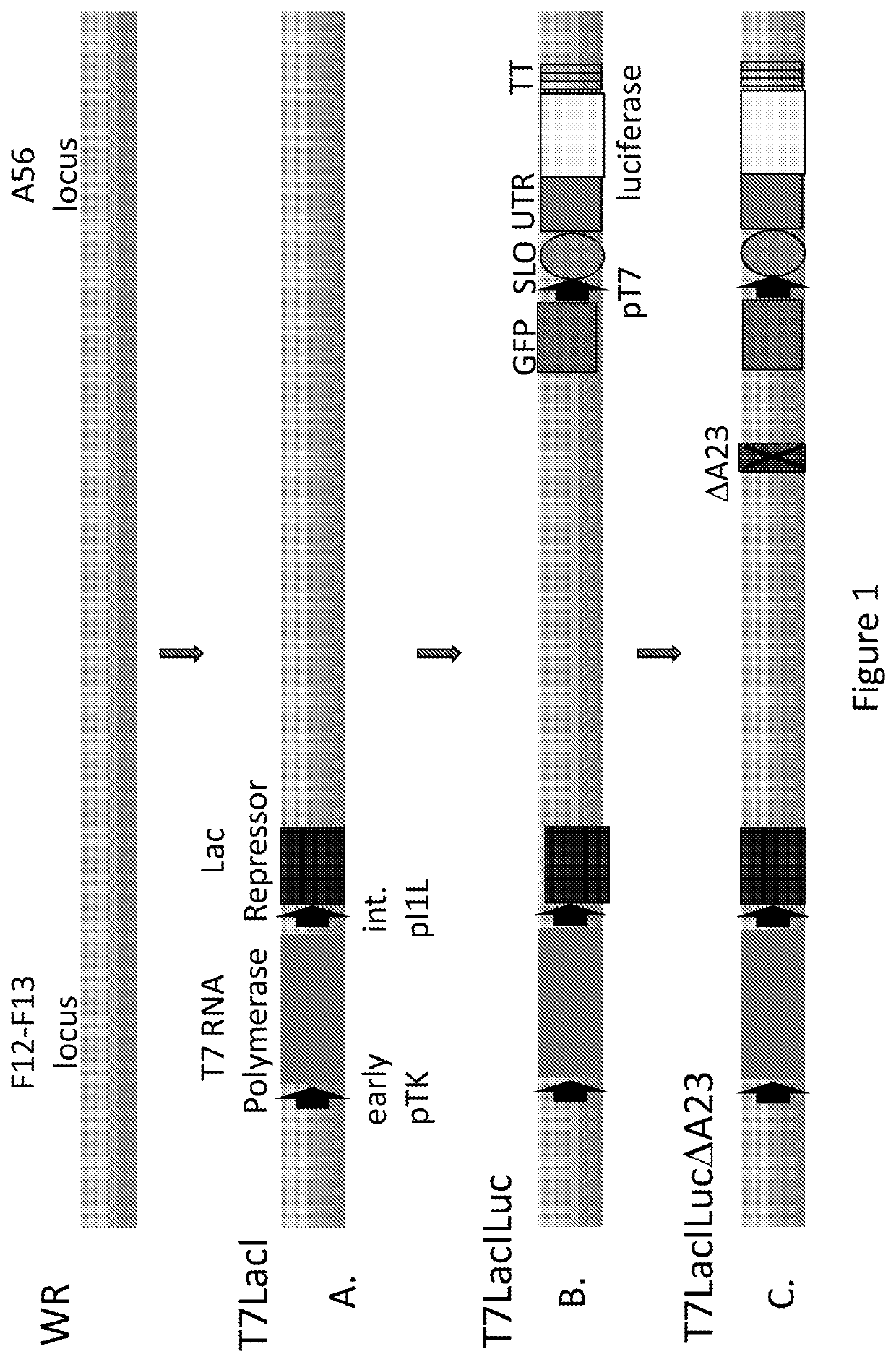
Patents
Literature
74 results about "Cowpox virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A species of ORTHOPOXVIRUS that is the etiologic agent of COWPOX. It is closely related to but antigenically different from VACCINIA VIRUS.
Cony fur containing biological active substances and use thereof
InactiveCN1613305AInhibit inflammationImprove the activation effectSlaughtering/stunningAnimal husbandryCowpox virusDrug product
A rabbit hide containing novel bioactive material for preparing medicine and health-care product is produced through inoculating the cowpox virus in the body of rabbit, raising it until the cowpox is better developed in skin tissue, and killing the rabbit.
Owner:VANWORLD PHARMA (RUGAO) CO LTD
Oral smallpox vaccine production and methods to evaluate safety, efficacy, and potency of orally delivered vaccine
InactiveUS20040175398A1Efficient responseSafety and efficacyViral antigen ingredientsMicrobiological testing/measurementHuman useDiagnostic test
This invention relates to methods and systems for generating a safe and effective oral smallpox vaccine for humans using a genetically defective strain of vaccinia virus to confer immunity following oral delivery of the vaccine. This invention is one that expands on current use of vaccinia virus propagation developed for gene therapy applications, and pharmaceuticals and nutraceuticals packaging and formualtion technologies. The vaccine invention can be delivered as a live virus with the ability to express viral proteins but unable to achieve complete, lytic virus replication, or it may be derived from such a virus, contain additional immunogens, or be delivered as viral antigens. Furthermore, the invention establishes innovative methods for formulation and packaging and for preclinical testing of the vaccine invention for safety, efficacy and potency with the use of human intestinal and other test cells and diagnostic test systems and kits.
Owner:INCELLS
Vaccinia virus for diagnosis and therapy of tumors
InactiveUS8642257B2Easy to identifyImprove securityUltrasonic/sonic/infrasonic diagnosticsVirusesAbnormal tissue growthCytotoxicity
Described are diagnostic and pharmaceutical compositions comprising a microorganism or cell containing a DNA sequence encoding a detectable protein or a protein capable of inducing a detectable signal, e.g. a luminescent or fluorescent protein, and, in a particular embodiment, furthermore (a) DNA sequence(s) encoding (a) protein(s) suitable for tumor therapy and / or elimination of metastatic tumors, e.g. a cytotoxic or cytostatic protein.
Owner:GENELUX
Identification of gene sequences and proteins involved in vaccinia virus dominant T cell epitopes
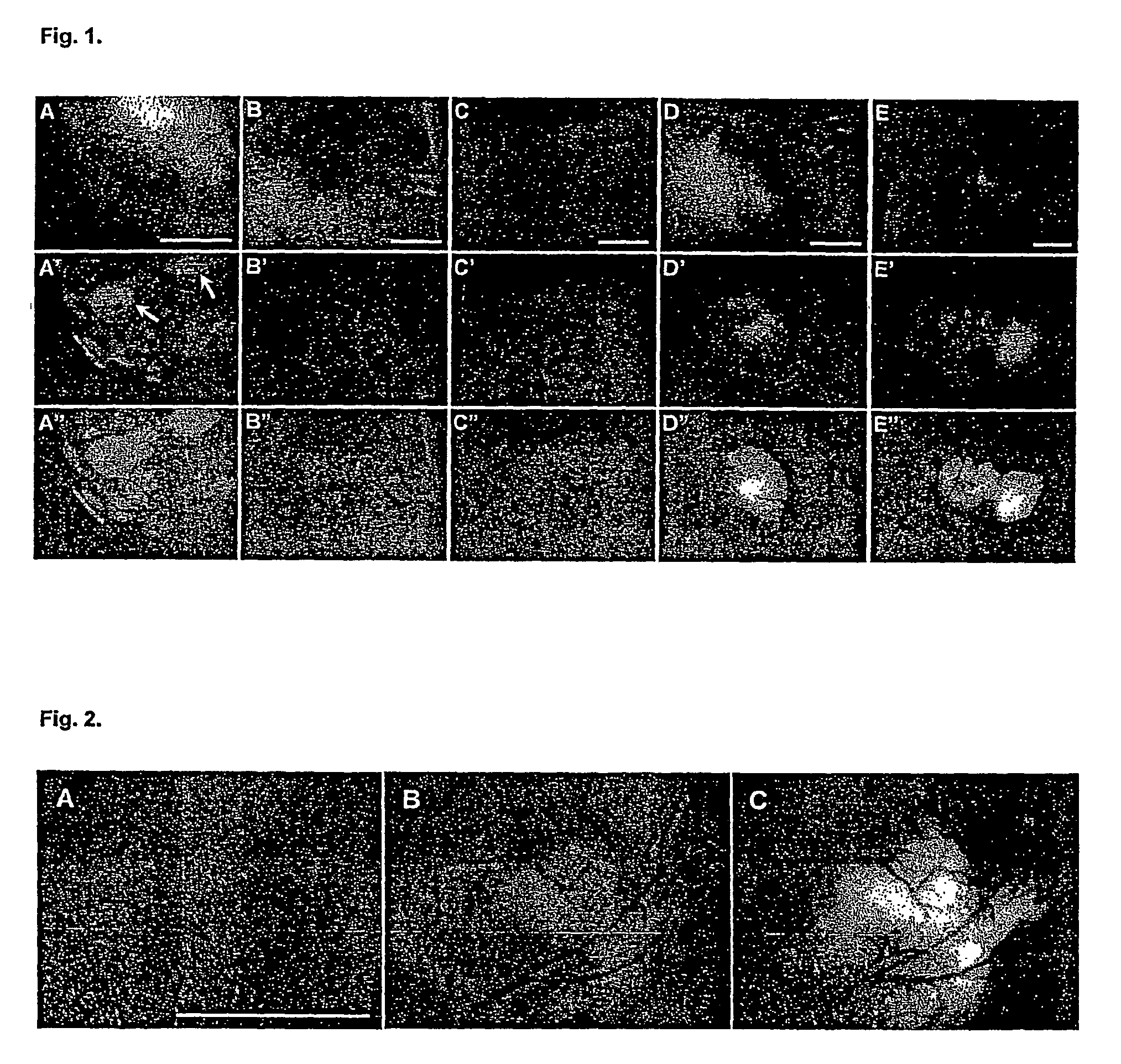
The present invention relates to the identification of gene sequences and proteins involved in vaccinia virus dominant T cell epitopes. Two vaccinia virus CD8+ T cell epitopes restricted by the most common human MHC class I allele, HLA-A0201 have been identified. Both epitopes are highly conserved in vaccinia and variola viruses. The induction of the T cell responses following primary vaccination is demonstrated by the kinetics of epitope specific CD8+ T cells in 3 HLA-A0201 individuals. This information will be useful for the design and analyses of the immunogenicity of experimental vaccinia vaccines, and for basic studies of human T cell memory.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
Oncolytic vaccinia virus cancer therapy
Owner:SILLAJEN BIOTHERAPEUTICS
Recombinant modified vaccinia ankara (MVA) vaccinia virus containing restructured insertion sites
ActiveUS20120263750A1Viral antigen ingredientsVirus peptidesModified vaccinia AnkaraOpen reading frame
The present invention relates to recombinant modified vaccinia Ankara (MVA) virus containing restructured sites useful for the integration of heterologous nucleic acid sequences into an intergenic region (IGR) of the virus genome, where the IGR is located between two adjacent, essential open reading frames (ORFs) of the vaccinia virus genome, wherein the adjacent essential ORFs are non-adjacent in a parental MVA virus used to construct the recombinant MVA virus, and to related nucleic acid constructs useful for inserting heterologous DNA into the genome of a vaccinia virus, and further to the use of the disclosed viruses as a medicine or vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Porcine reproductive and respiratory syndrome virus M protein CTL cell epitopes and application thereof
The invention discloses porcine reproductive and respiratory syndrome virus M protein CTL cell epitopes and application thereof. The identification of the epitopes comprises the following steps of: fusing and cloning a M protein gene of a porcine reproductive and respiratory syndrome virus CH-1a strain and a mouse ubiquitin gene to form a DNA vaccine and also form recombination viruses, expressing an M protein, of a WR strain vaccinia virus; immunizing a BALB / c mouse according to a Priming-Boosting policy, separating the mouse splenic lymphocyte, culturing and stimulating a CTL short peptide in vitro predicted and synthesized by a bioinformatic method, and using the flow cytometry and enzyme-linked immunospot technology to identify two CTL epitopes that are K93FITSRCRL and F57GYMTFVHF. The identification of the PRRSVM protein CTL cell epitopes lays a certain theoretical foundation for the PRRS cell immune mechanism and the novel epitope pepetide vaccine and has an instructing significance for the theoretical study on PRRS preventing and control technology.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Virus vector for prime/boost vaccines, which comprises vaccinia virus vector and sendai virus vector
InactiveUS20130302367A1Avoid infectionEffective preventionSsRNA viruses negative-senseViral antigen ingredientsCowpox virusPrime boost
[Problem]To provide a set of virus vectors which can be used for producing a prime / boost vaccine that can activate both cellular immunity and humoral immunity and is effective on infections by pathogenic microorganisms and malignant tumors which are generally believed to be difficult to be treated by vaccine therapy.[Solution]Provided is a set of virus vectors for prime / boost vaccines, comprising the following virus vector (a) and virus vector (b): (a) a vaccinia virus vector which carries a gene encoding an immunogenic polypeptide in such a manner that the gene can be expressed; and (b) a Sendal virus vector which carries the gene encoding the immunogenic polypeptide in such a manner that the gene can be expressed.
Owner:HOKKAIDO UNIVERSITY +1
Vaccinia virus strains
InactiveUS20090169512A1Suitable for usePrevention and treatmentBiocideViral antigen ingredientsVaccinia virus vaccineVirus strain
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Ankara vaccinia virus genetic engineering vaccine for pig replication and respiration complex
InactiveCN101209351AImprove immunityHigh protein expressionGenetic material ingredientsAntiviralsBALB/cCowpox virus
The invention discloses a porcine reproductive and respiratory syndrome-Ankara vaccinia virus genetic engineering vaccine, the preparation method is that: 1. the PCR amplification of GP5 gene is carried out from the natural porcine reproductive and respiratory syndrome virus; 2. the porcine preferred codon is used for replacing the codon in the natural GP5 gene, so as to carry out GP5 gene optimization and the synthesis of the optimized sequence GP5A; 3. the epitope A in the optimized GP5A gene is replaced by the epitope B of the GP5 gene, so as to obtain the GP5-DB gene; 4. the GP5 and GP5-DB genes are respectively cloned to the expression carrier JN-2; 5. the constructed JN-2-GP5 and the JN-2-GP5-DB plasmid are recombined with Ankara vaccinia virus, so as to obtain the porcine reproductive and respiratory syndrome GP5 and GP5-DB gene Ankara vaccinia virus recombinant vaccine. Experiments confirm that the invention has excellent immune protection in BALB / c mice and pigs.
Owner:WUCHANG SHIPBUILDING IND
Recombinant Modified Vaccinia Ankara (MVA) Vaccinia Virus Containing Restructured Insertion Sites
The present invention relates to recombinant modified vaccinia Ankara (MVA) virus containing restructured sites useful for the integration of heterologous nucleic acid sequences into an intergenic region (IGR) of the virus genome, where the IGR is located between two adjacent, essential open reading frames (ORFs) of the vaccinia virus genome, wherein the adjacent essential ORFs are non-adjacent in a parental MVA virus used to construct the recombinant MVA virus, and to related nucleic acid constructs useful for inserting heterologous DNA into the genome of a vaccinia virus, and further to the use of the disclosed viruses as a medicine or vaccine.
Owner:UNITED STATES OF AMERICA
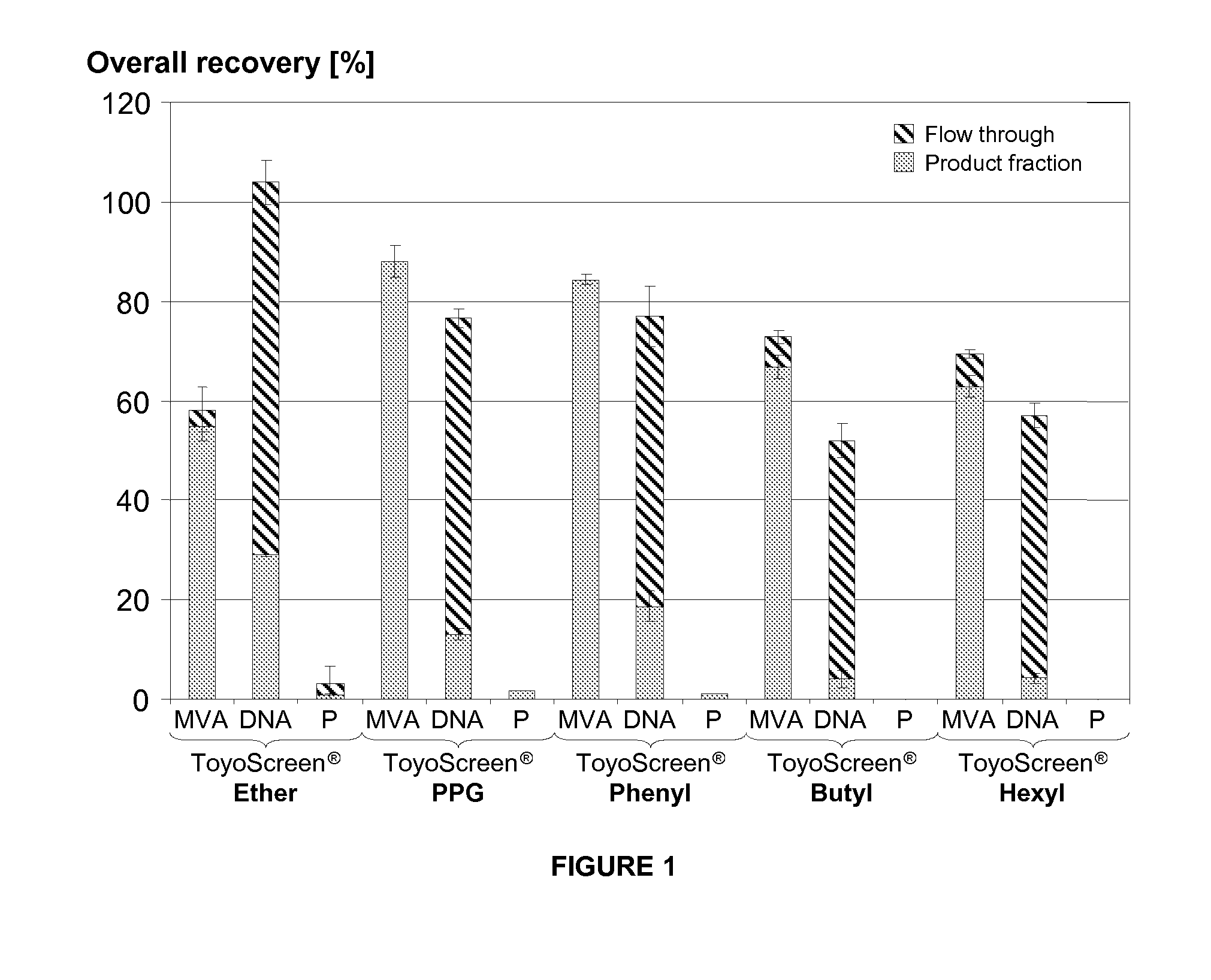
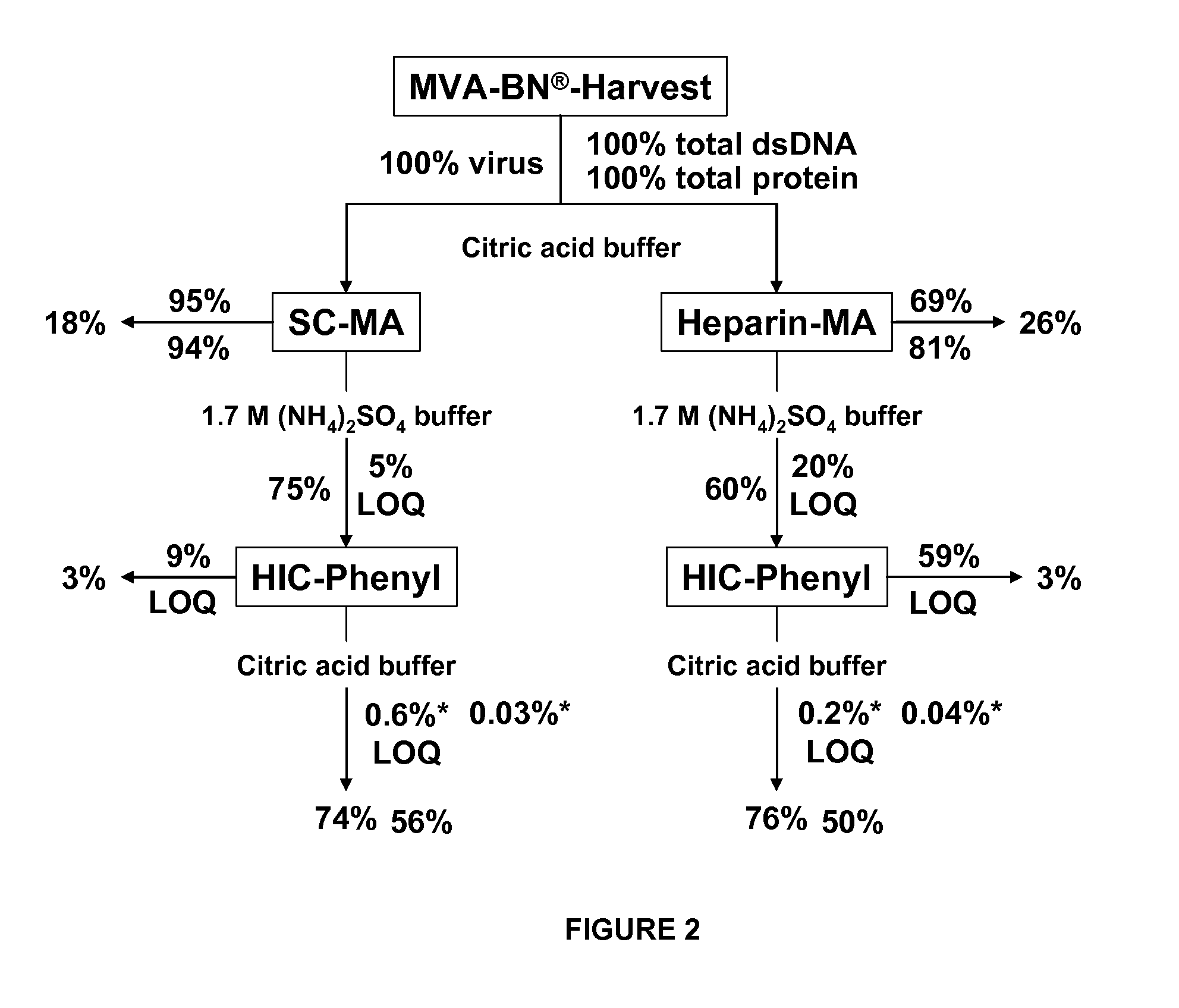
Purification of vaccinia viruses using hydrophobic interaction chromatography
ActiveUS20110306114A1Reduce the amount requiredViral antigen ingredientsAntiviralsCowpox virusVaccinia viruses
The present invention relates to methods for purification of Vaccinia viruses (VV) and / or Vaccinia virus (VV) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS +2
Vaccinia viral vectors encoding chimeric virus like particles
ActiveUS20200289633A1SsRNA viruses negative-senseSsRNA viruses positive-senseModified vaccinia AnkaraDisease
Owner:GEOVAX INC
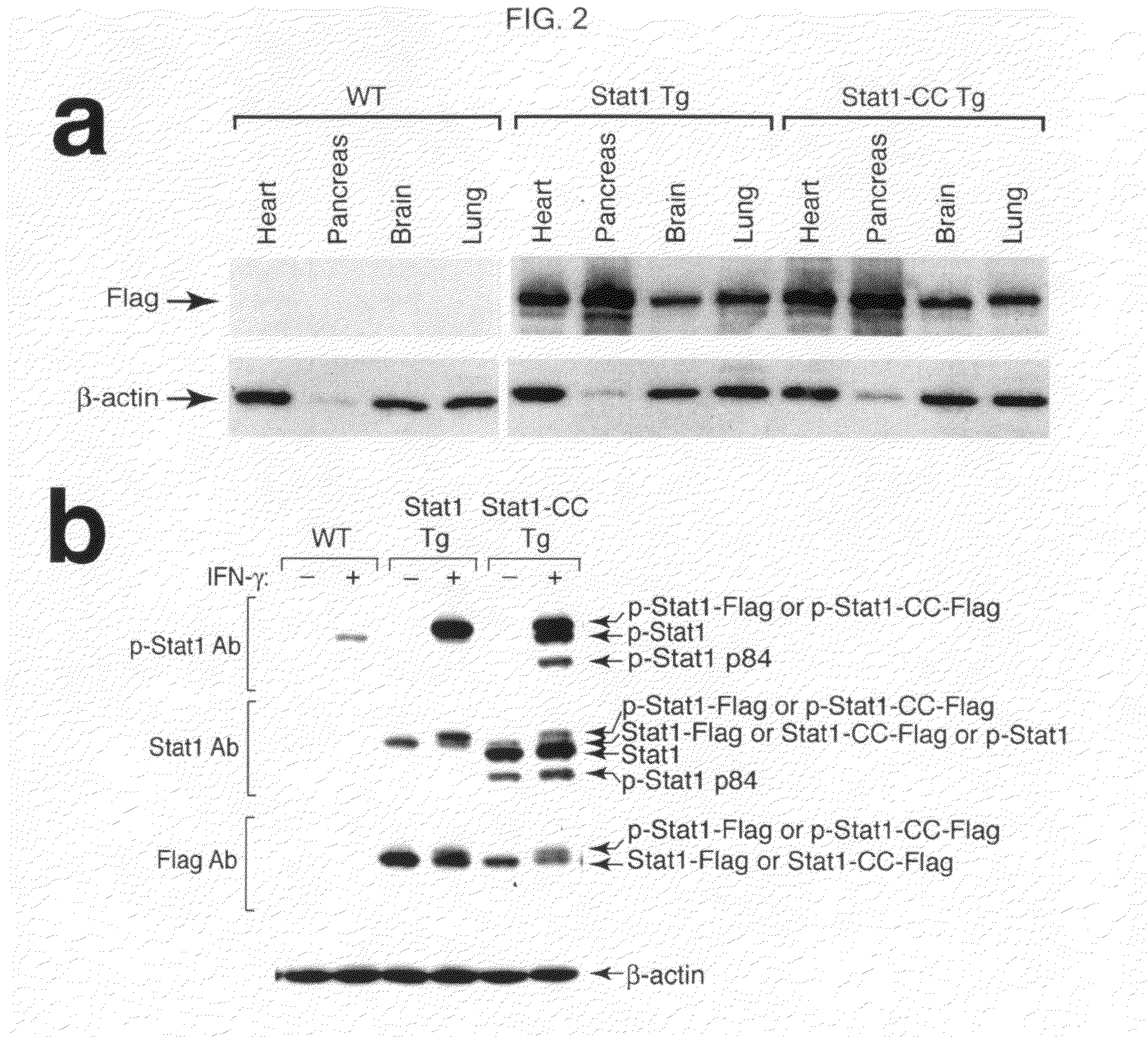
Modified stat1 transgene that confers interferon hyperresponsiveness, methods and uses therefor
InactiveUS20100015091A1Increase probabilityIncreased activationOrganic active ingredientsPeptide/protein ingredientsCowpox virusSystemic lupus erythematosus
Methods of enhancing cellular responses to interferons are disclosed. These methods comprise administering to a subject a vector comprising a Stat1-CC transgene, such as an AAV5 vector comprising a reporter operably linked to a nucleic acid sequence encoding a Stat1-CC polypeptide. The methods can be used in the treatment of diseases that involve interferon responses, such as multiple sclerosis, amyotrophic lateral sclerosis, and lupus; viral infections such as infection by hepatitis C virus, influenza A virus, cowpox virus, Sendai virus or Encephalomyocarditis virus; respiratory disorders; and cancers.
Owner:WASHINGTON UNIV IN SAINT LOUIS
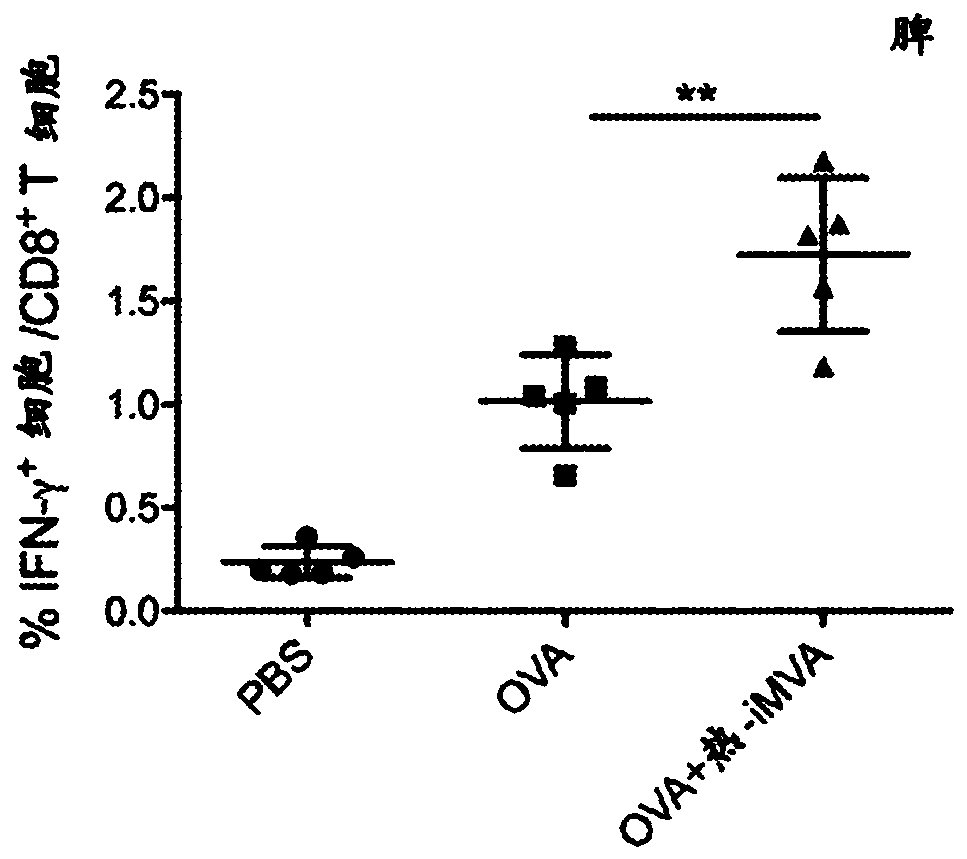
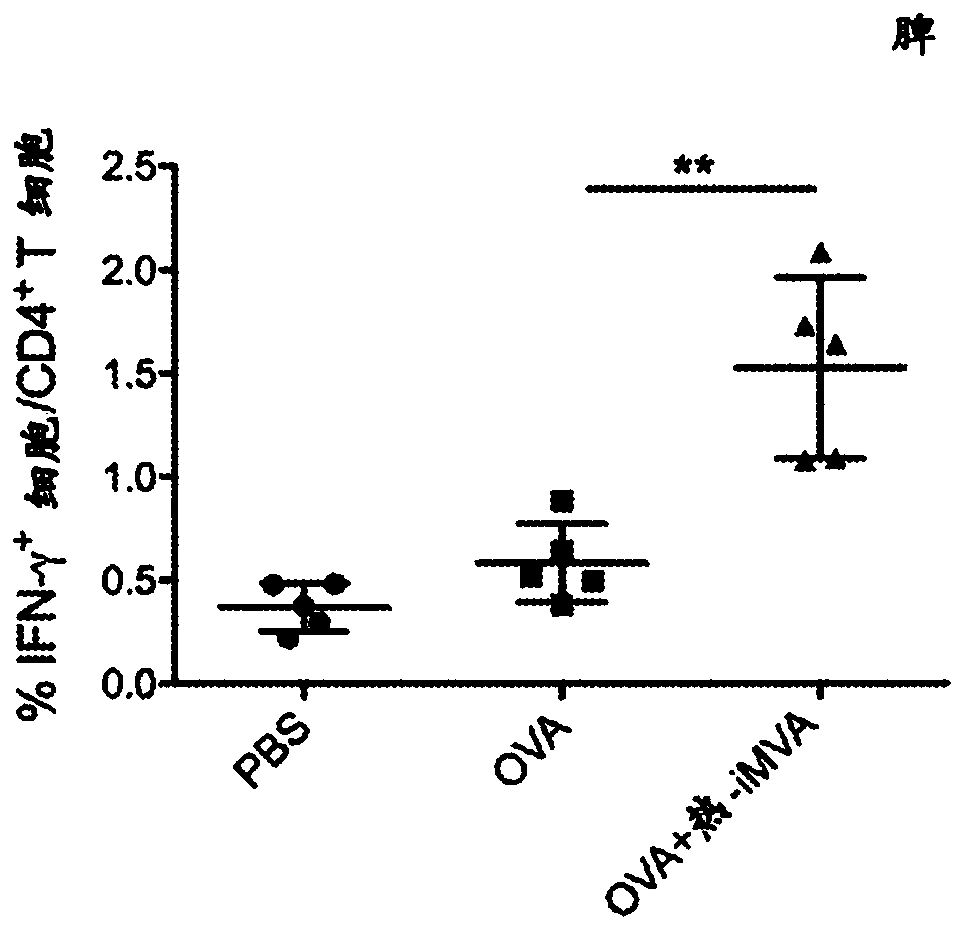
Heat-inactivated vaccinia virus as a vaccine immune adjuvant
The technology of the present disclosure relates to the use of Heat-inactivated modified vaccinia Ankara (MVA) virus (Heat-iMVA) or Heat-inactivated vaccinia virus as a vaccine immune adjuvant. In particular, the present technology relates to the use of Heat-iMVA as a vaccine adjuvant for tumor antigens in cancer vaccines alone or in combination with immune checkpoint blockade (ICB) antibodies foruse as a cancer immunotherapeutic.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Nucleic acid combination product, detection kit and micro-fluidic chip
PendingCN113061665ABioreactor/fermenter combinationsBiological substance pretreatmentsFeline parvovirusFeline immunodeficiency virus
The invention relates to a nucleic acid combination product, a detection kit and a micro-fluidic chip. The nucleic acid combination product comprises at least two of the following detection primer pairs: a feline parvovirus primer pair, a feline coronavirus primer pair, a feline herpesvirus type I primer pair, a feline calicivirus primer pair, a feline leukemia virus primer pair, a feline immunodeficiency virus primer pair, a feline astrovirus primer pair, a feline rotavirus primer pair, a cat vaccinia virus primer pair and a cat reovirus primer pair. The nucleic acid combination product can be used for detecting at least two feline pathogenic viruses in the sample to be detected at one time, and is good in specificity and relatively high in detection sensitivity.
Owner:深圳市刚竹医疗科技有限公司
Mutant vaccinia viruses and use thereof
The present invention discloses recombinant vaccinia virus (VV) virions that are resistant to antiviral defenses and have enhanced anti-tumor activities. In one embodiment, the recombinant VV comprise one or more variant VV proteins that have mutations at one or more neutralizing antibody epitopes, thereby conferring viral escape from the neutralizing antibodies. In another embodiment, the recombinant VV is resistant to complement-mediated neutralization due to the expression of a regulator of complement activation (e.g. CD55). In another embodiment, the recombinant VV has enhanced anti-tumor activities due to the expression of bi-specific antibodies co-targeting cancer cells and immune effector cells, or the expression of a polypeptide blocking the PD-1 pathway. The recombinant vaccinia virus virions can be used to treat cancer in a subject.
Owner:ICELLKEALEX THERAPEUTICS LLC
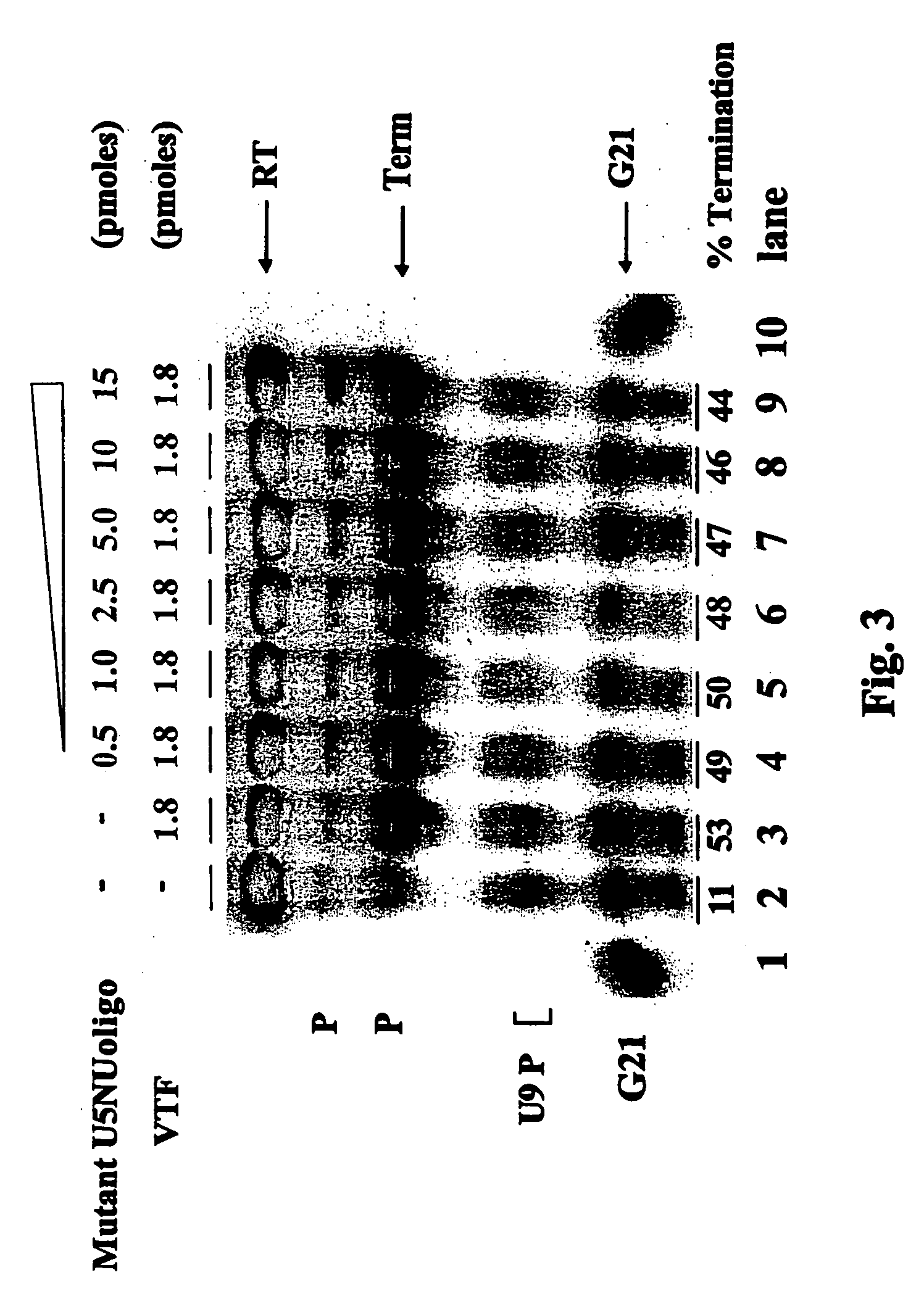
Novel inhibitors of poxvirus replication
InactiveUS20050085435A1Sugar derivativesGenetic material ingredientsVariola virusRibonucleotide synthesis
The present invention provides compositions and methods for generation of premature transcription termination products. Premature termination of transcription interferes with the normal transcription and termination of early phase genes of the poxviruses and results in inhibition of replication of the poxviruses. The compositions of the present invention are oligonucleotides comprising a U5NU sequence flanked by ribonucleotides, deoxyribonucleotides or modifications thereof. This invention also provides methods for inhibition of replication of poxvirus including the small poxvirus, Monkeypox virus and vaccinia virus.
Owner:NILES EDWARD +1
Recombinant MVA virus expressing the E2 gene of Bovine papillomavirus and its use in the therapy of tumors generated by papillomaviruses
InactiveUS6582693B2Efficient expressionImprove efficiencyBiocideGenetic material ingredientsAlphapapillomavirusFhit gene
A recombinant vaccinia virus derived from the vaccinia virus Ankara (MVA) encoding and capable of expressing the E2 gene of Bovine papillomavirus. Also, the use of the virus in the treatment of lesions caused by papillomavirus.
Owner:LEMERY DE C V
Oncolytic virus improved in safety and anticancer effect
PendingCN111556899AReduce in quantityImprove securityOrganic active ingredientsVirus peptidesAnticarcinogenic EffectCancer cell
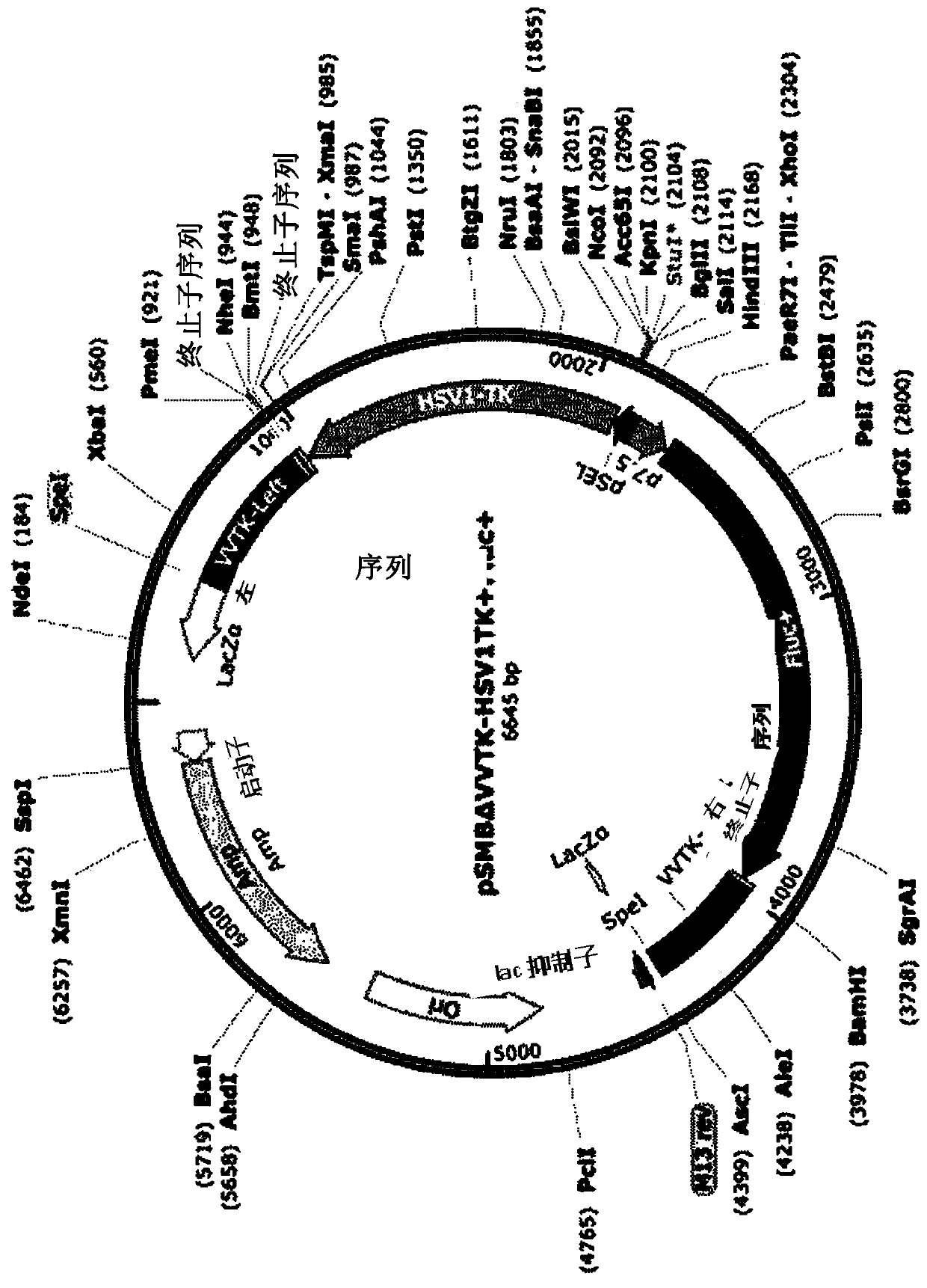
The present invention relates to an oncolytic virus improved in safety and anticancer effect and a use thereof. The oncolytic virus improved in safety and anticancer effect of the present invention isobtained by inserting an HSV-TK fragment-encoding gene into a TK gene region to delete TK of Vaccinia virus. In addition, the oncolytic virus of the present invention expresses an HSV-TK fragment tophosphorylate GCV so that cancer cells infected with the oncolytic virus and even their neighboring cancer cells can be killed. In addition, GCV is also involved in the suppression of viral proliferation and thus can control side effects caused by a virus even upon the administration of a high dose of the virus. Furthermore, an anticancer effect is increased even though the number of viral particles is reduced due to the suppression of GCV against virus proliferation. Therefore, the oncolytic virus improved in safety and anticancer effect of the present invention can be effectively used for the treatment of cancer.
Owner:拜耳诺克斯有限公司
Mutant vaccinia viruses and use thereof
PendingUS20210388388A1High killing efficiencyHeightened tumor specificityVirus peptidesUnknown materialsCancer cellCowpox virus
The present invention discloses recombinant vaccinia virus (VV) virions that are resistant to antiviral defenses and have enhanced anti-tumor activities. In one embodiment, the recombinant VV comprise one or more variant VV proteins that have mutations at one or more neutralizing antibody epitopes, thereby conferring viral escape from the neutralizing antibodies. In another embodiment, the recombinant VV is resistant to complement-mediated neutralization due to the expression of a regulator of complement activation (e.g. CD55). In another embodiment, the recombinant VV has enhanced anti-tumor activities due to the expression of bi-specific antibodies co-targeting cancer cells and immune effector cells, or the expression of a polypeptide blocking the PD-1 pathway. The recombinant vaccinia virus virions can be used to treat cancer in a subject.
Owner:ICELLKEALEX THERAPEUTICS LLC
Therapeutic Peptides For Cerebrovascular Diseases
The present invention provides a method for treating or ameliorating cerebrovascular diseases in a subject by using peptides isolated from extracts from rabbit skin inflamed by vaccinia virus. The present invention also provides a peptide comprising an amino acid sequence having at least 70% identity to SEQ ID NO: 1, or variants, mutants, derivatives, or fragments thereof.
Owner:PRIME BIO DRUG DEV LTD
Surface glucoprotein gp160 of recombination expression human acquired immunity defact virus 1
InactiveCN1718733AFacilitate efficient separationRaw yieldVirus peptidesPeptide preparation methodsCowpox virusWild type
A process for preparing the modified surface glucoprotein gp160 of human acquired immune deficiency virus 1 in the cowpox virus system, the cowpox virus used to prepare the protein gp160, and the prepared protein gp160 are disclosed. Its advantages are high output and high purity.
Owner:上海安久生物科技有限公司
Virus-based expression vectors and uses thereof
Expression vectors ideal for use in vaccinating individuals against disease based on vaccinia virus and other chordopoxviruses having high expression of recombinant genes and low expression of vector genes in target animals, and low expression of recombinant genes and high expression of vector genes in cells used for propagation.
Owner:UNITED STATES OF AMERICA
New genetically-modified vaccinia virus
The invention provides a genetically-modified vaccinia virus that is effective for the prevention or treatment of cancer. Specifically, the invention provides: a vaccinia virus containing two polynucleotides which are a polynucleotide that codes for IL-7 and a polynucleotide that codes for IL-12; a combination kit including two vaccinia viruses which are a vaccinia virus containing a polynucleotide that codes for IL-7 and a vaccinia virus containing a polynucleotide that codes for IL-12; and a combination use of the two vaccinia viruses.
Owner:ASTELLAS PHARMA INC +1
A kind of protein vaccinia vaccine and its efficacy detection method
ActiveCN109172818BImproving immunogenicityEnhance immune responseViral antigen ingredientsAntiviralsEndotoxin removalCowpox virus
The invention relates to the field of vaccines, and discloses a protein vaccinia vaccine and a method for detecting its effectiveness. The protein vaccine contains viral recombinant protein D8L expressed by yeast and with a purity >90% of endotoxin removed. The preparation method is as follows: expressing yeast with a purity >90% 90% of the recombinant protein D8L of vaccinia virus has been treated to remove endotoxin to obtain a recombinant protein D8L solution with an endotoxin content <5 EU / ml. Efficacy testing method is as follows: the recombinant protein D8L solution that removes endotoxin is injected subcutaneously twice to immunize mice. After 21 days, specific antibodies and neutralizing antibodies can be detected in the mouse serum, indicating that the mice have acquired anti-vaccinia virus immunity. immune protection. The protein vaccine of the present invention does not require additional vaccine adjuvants, and can theoretically replace traditional live vaccinia virus vaccines, and play a role in resisting pox virus infections such as smallpox virus.
Owner:CARBIOGENE THERAPEUTICS CO LTD
Virus-based expression vectors and uses thereof
Expression vectors ideal for use in vaccinating individuals against disease based on vaccinia virus and other chordopoxviruses having high expression of recombinant genes and low expression of vector genes in target animals, and low expression of recombinant genes and high expression of vector genes in cells used for propagation.
Owner:UNITED STATES OF AMERICA
Synthetic chimeric vaccinia virus
The invention relates in various aspects to a synthetic chimeric vaccinia virus or compositions comprising such viruses, and the development and use of systems and methods for producing such synthetic chimeric vaccinia viruses. The synthetic chimeric vaccinia viruses are well suited, among others, as virus vaccines or to generate an oncolytic response and pharmaceutical formulations.
Owner:TONIX PHARMA LTD
Use of extract from rabbit skin inflamed by vaccinia virus in treatment of cancer
PendingUS20220354900A1Promote secretion of cytokinesEliminate the effects ofOrganic active ingredientsUnknown materialsAnticarcinogenCowpox virus
The present invention relates to the therapeutic use of extract from rabbit skin inflamed by vaccinia virus. More specifically, the present invention relates to the use of the extract for preventing or treating cancer, or the use of the extract for stimulating secretion of cytokine by somatic cells in a patient. In addition, the present invention also relates to a drug combination comprising the extract as the first anticancer agent and a second anticancer agent as well as its use for preventing or treating cancer.
Owner:MEGA WINNING LTD
Vacpox virus capping enzyme mutant, recombinant vector, recombinant engineering bacterium and application of vaccpox virus capping enzyme mutant, recombinant vector and recombinant engineering bacterium
The invention relates to a vaccinia virus capping enzyme mutant, a recombinant vector, a recombinant engineering bacterium and application of the vaccinia virus capping enzyme mutant. The vaccinia virus capping enzyme mutant comprises: (a) a polypeptide formed by deleting, replacing or adding one or more amino acids to an amino acid sequence as shown in SEQ ID No.2; or (b) a polypeptide having at least 90% of homology with the polypeptide composed of the amino acid sequence as shown in SEQ ID No.2. The half-life period of the vaccinia virus capping enzyme mutant at 45 DEG C is 35 min or above and is higher than that of a wild type vaccinia virus capping enzyme mutant, and when mRNA is capped at a high temperature, the vaccinia virus capping enzyme mutant still shows excellent catalytic activity and can play an important role in the fields of clinical medicine, influenza vaccines, tumor vaccines, biological pharmacy and the like.
Owner:苏州瀚源新酶生物科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com