Heat-inactivated vaccinia virus as a vaccine immune adjuvant
A vaccinia virus, heat inactivation technology, applied in the field of virology, immunotherapy, oncology, can solve the problem that the immune system is not activated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
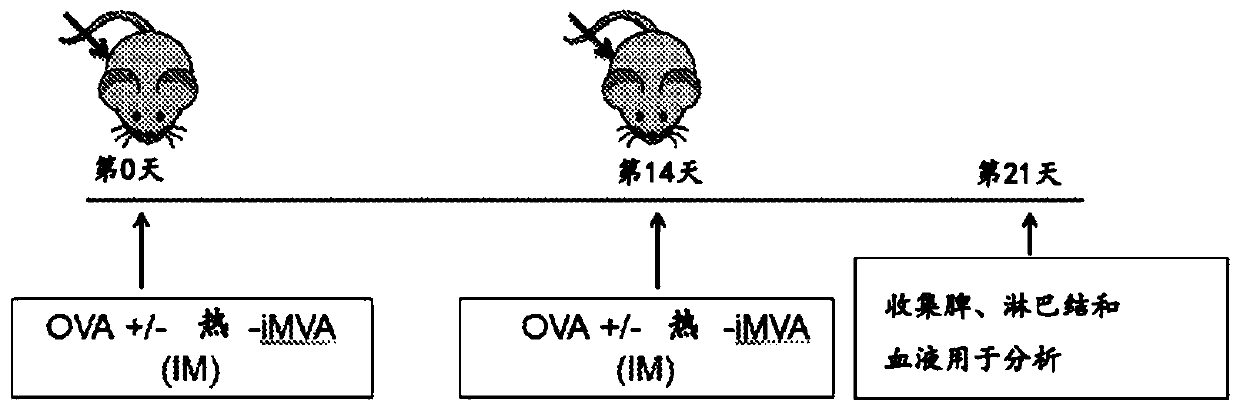
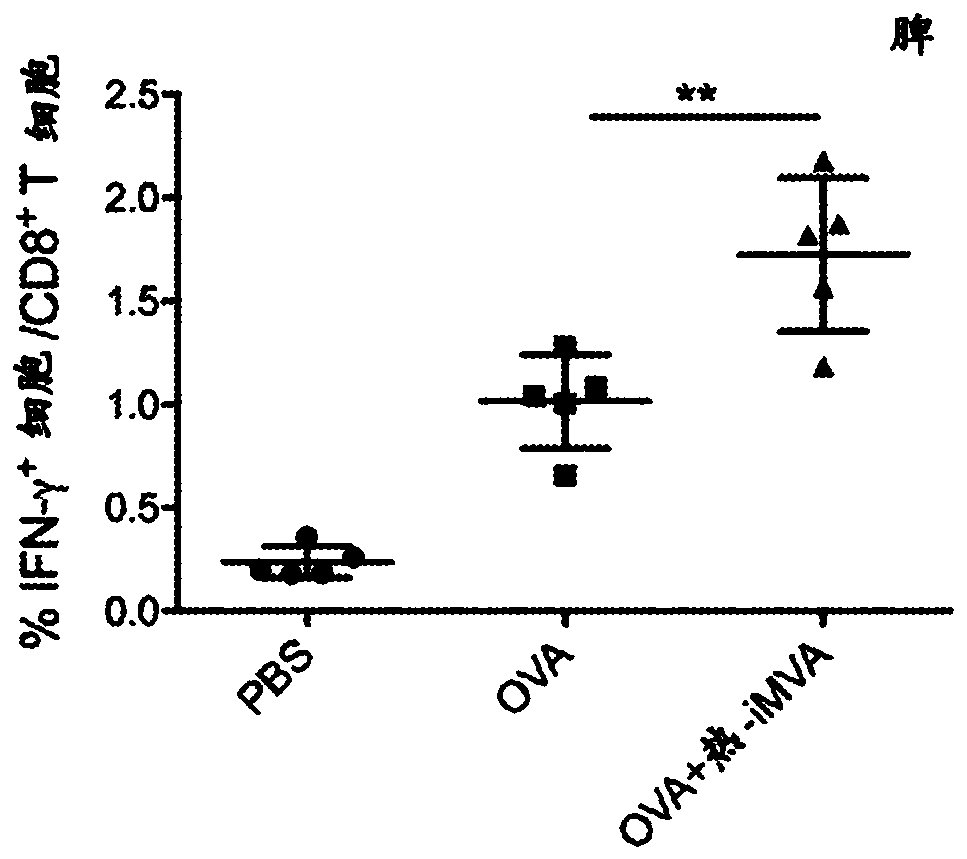
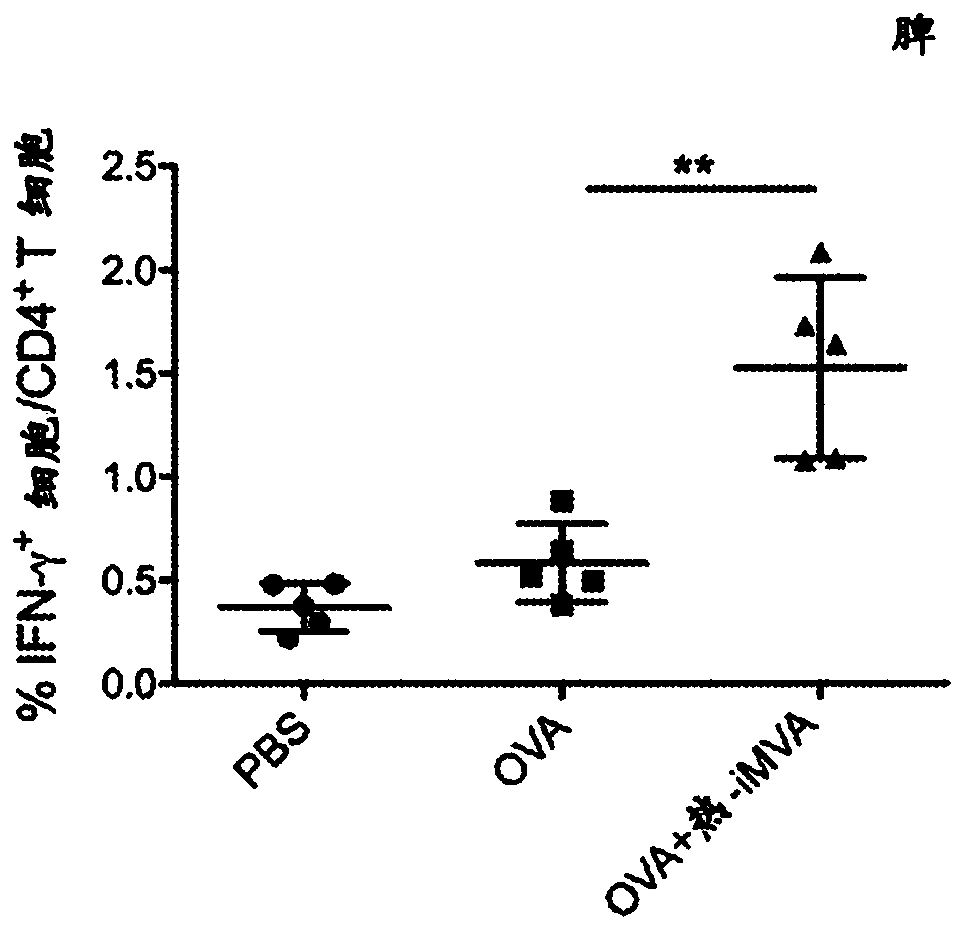
[0147] Example 1: In immunized mice, co-administration of thermal iMVA with the model antigen chicken ovalbumin (OVA) enhanced OVA-specific CD8 in spleen and draining lymph nodes (dLN) + and CD4 + T cells and serum anti-OVA IgG antibody production.
[0148] This example demonstrates that thermal iMVA can act as a vaccine adjuvant to enhance antigen presentation by dendritic cells (DC). Mice were treated with or without heat iMVA (1x 10 7 pfu) together with OVA (10 μg) for two intramuscular (IM) immunizations with an interval of 2 weeks. One week after the second vaccination, mice were euthanized, and spleens, draining lymph nodes (dLNs) and blood were subsequently collected for OVA-specific T cell and antibody assessment ( Figure 1A ). To determine anti-OVA CD8 + T cell response, combining splenocytes (500,000 cells) with MHC class I as OVA (K b ) restricted peptide epitope of OVA 257-264 (SIINFEKL) peptide (SEQ ID NO: 1) was incubated for 12 h, after which it was ...
Embodiment 2
[0150] Example 2: In the production of antigen-specific CD8 + and CD4 + In terms of T cell response, thermal iMVA is superior to complete Freund's adjuvant (CFA).
[0151] Complete Freund's Adjuvant (CFA) contains heat-inactivated Mycobacterium tuberculosis in non-metabolizable oils (paraffin oil and mannidemonooleate). It also contains ligands for TLR2, TLR4 and TLR9. The antigen was co-injected with CFA to induce a Th1 dominant immune response. Currently, the use of CFA in humans is not permitted due to its toxicity profile, and its use in animals is limited to subcutaneous or intraperitoneal routes due to painful responses at the injection site and risk of tissue damage. To test whether thermal iMVA is superior to CFA, mice were vaccinated subcutaneously twice with OVA antigen + thermal iMVA or OVA + CFA 2 weeks apart, and the harvested spleen, dLN and blood were subsequently harvested as described in Example 1 , for anti-OVA CD8 + and CD4 + T cell and antibody r...
Embodiment 3
[0152] Example 3: Batf3-dependent effect of thermal iMVA-mediated vaccine adjuvants on antigen-specific T cell responses sex DC.
[0153] Batf3 is for CD103 + / CD8α + Transcription factor important for the development of lineage DCs that play an important role in the cross-presentation of viral and tumor antigens. Batf3-deficient mice fail to reject highly immunogenic tumors. To test whether STING or Batf3 plays a role in thermal iMVA-mediated vaccine adjuvant effects, WT C57B / 6, STING Gt / Gt or Batf3 - / - Mice were vaccinated twice subcutaneously with OVA+thermal iMVA two weeks apart. Then one week after the last vaccination, spleen, dLN and blood were harvested for analysis of cellular and humoral immune responses ( Figure 3A ). found that CD8 induced by thermal iMVA in the spleen + Anti-OVA IFN-γ in T cells + The percentage of T cells decreased from 1.2% in immunized WT mice to immunized Batf3 - / - 0.38% of mice (P- / - ; Figure 3B ). Additionally, CD8 induced b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com