Patents
Literature
100results about How to "Avoid virus infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
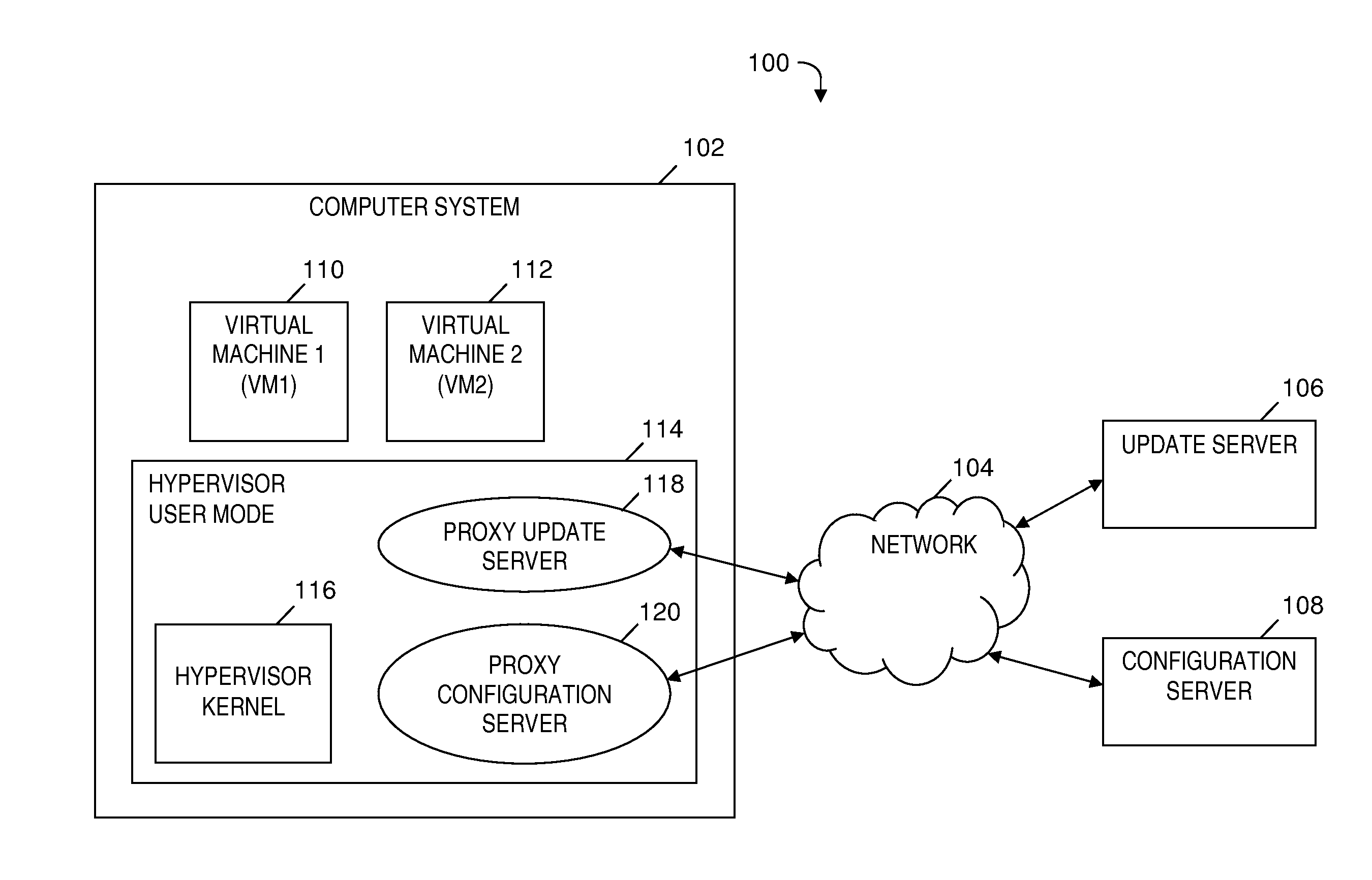
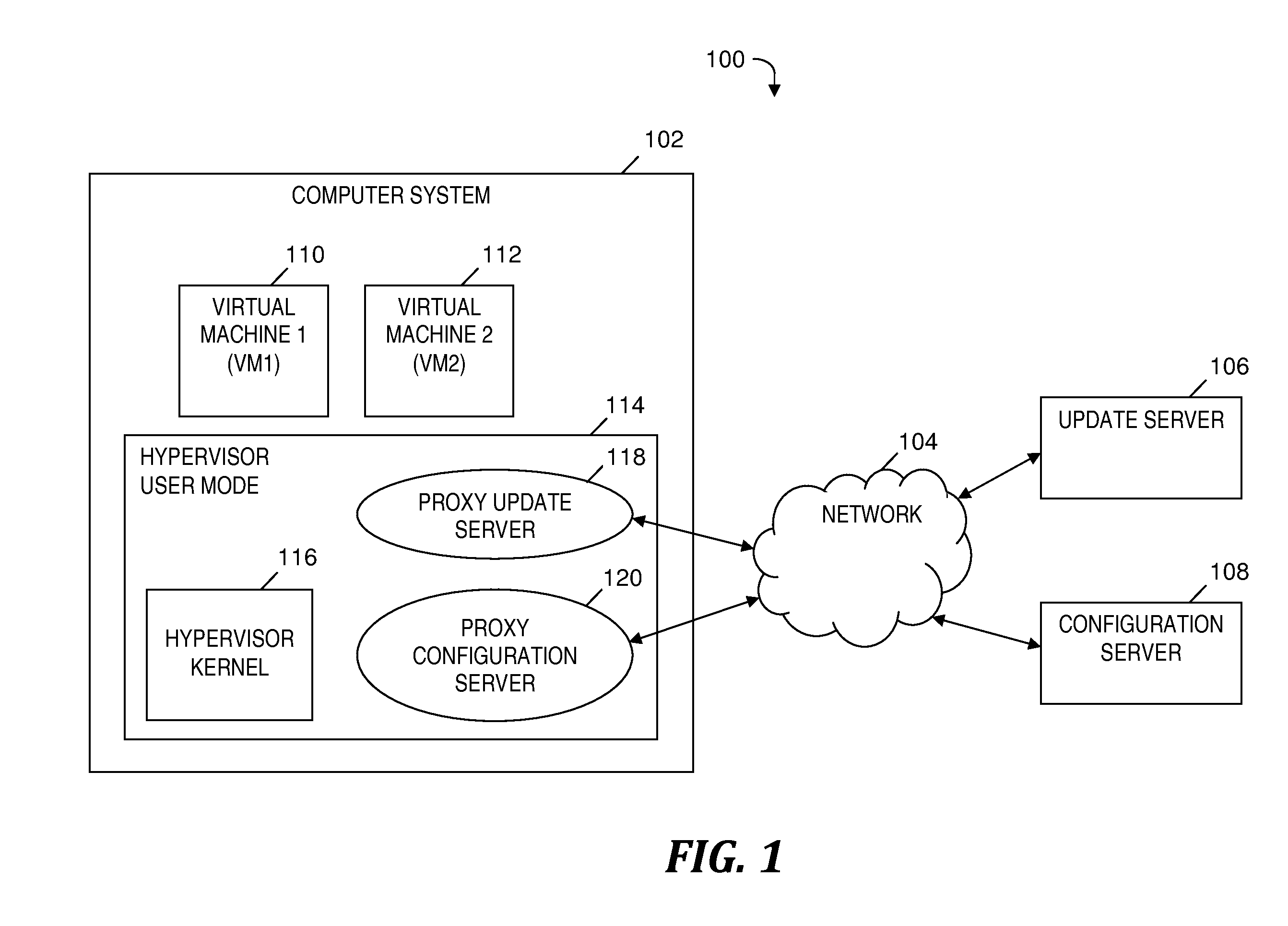
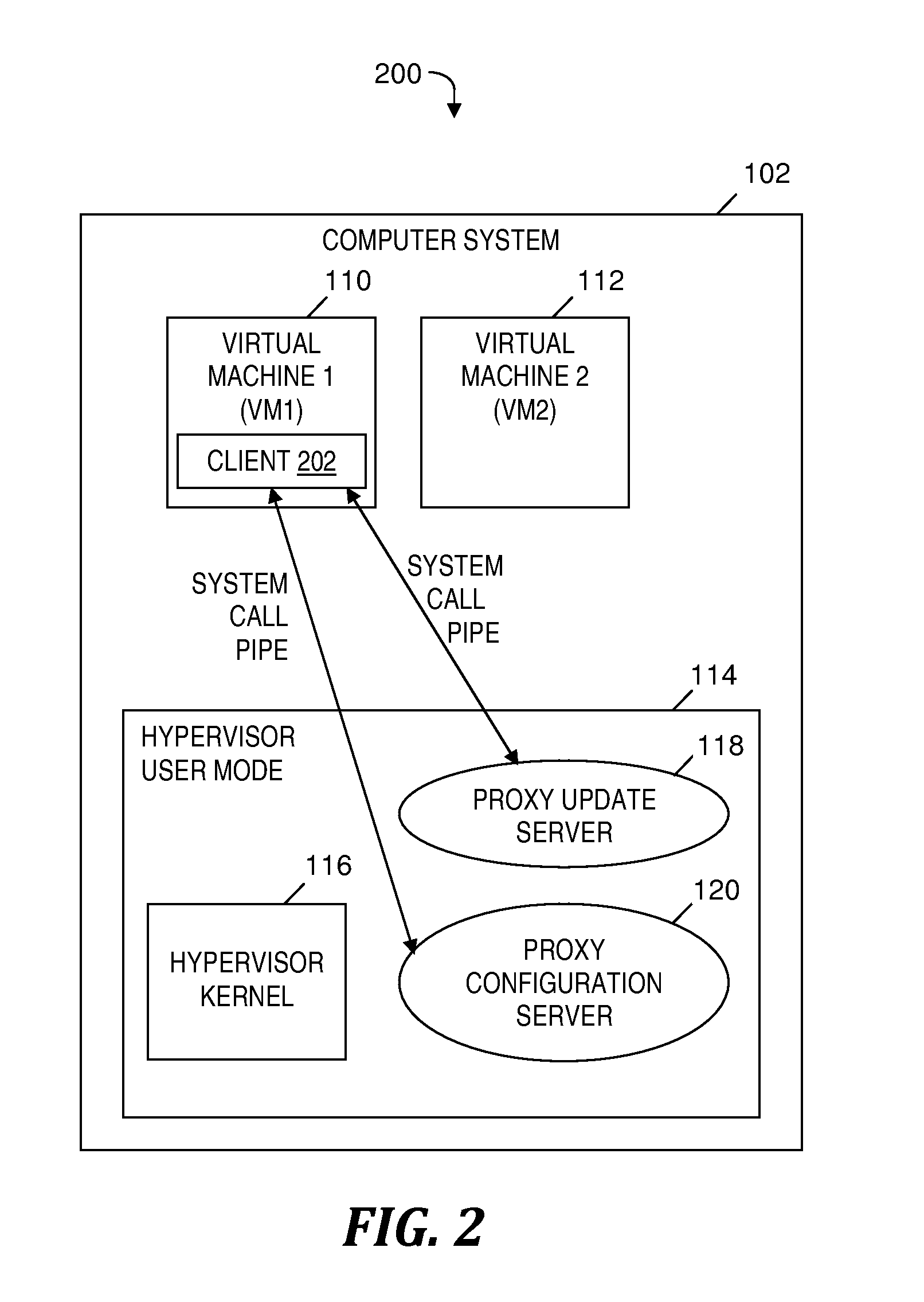
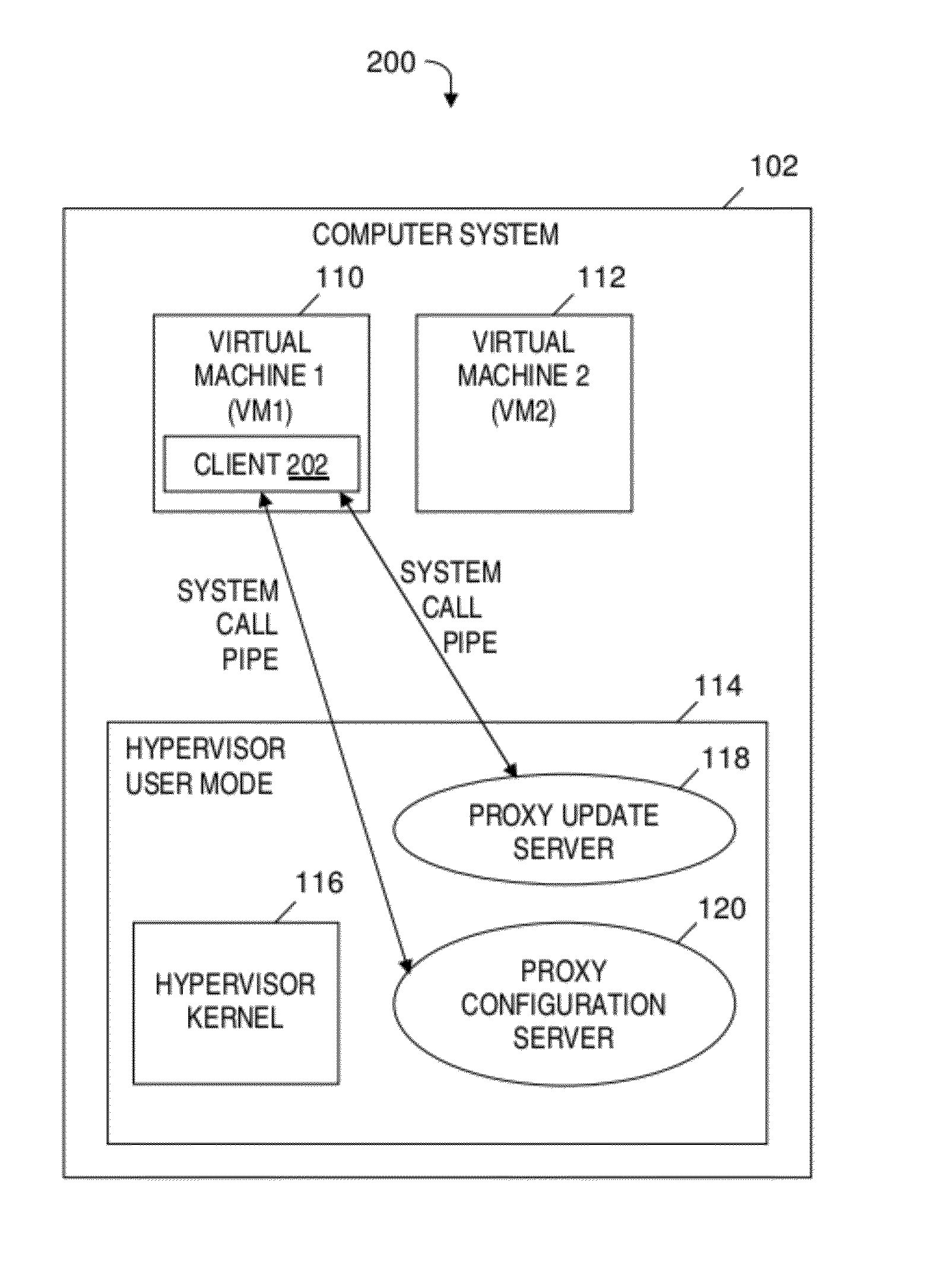
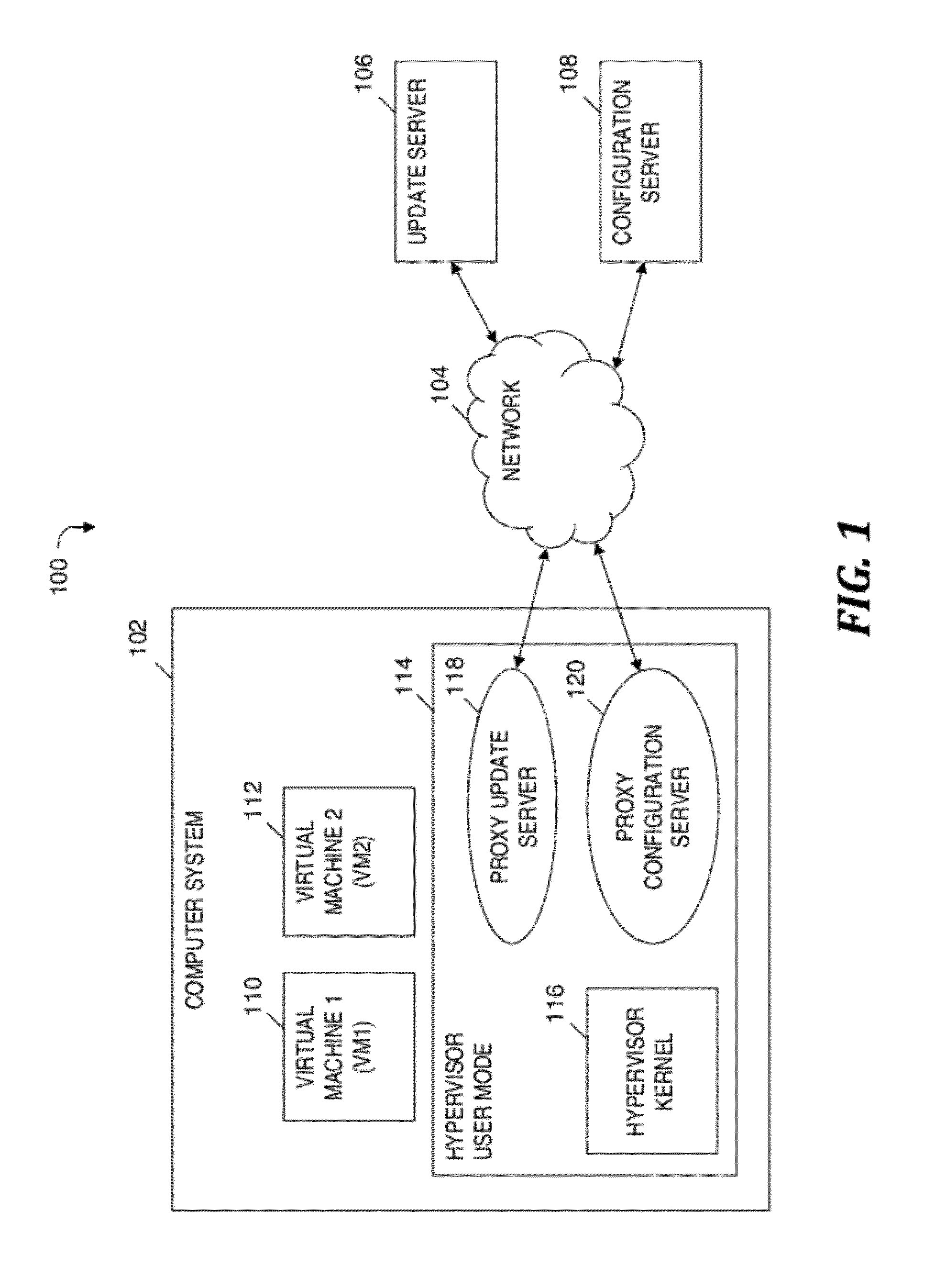
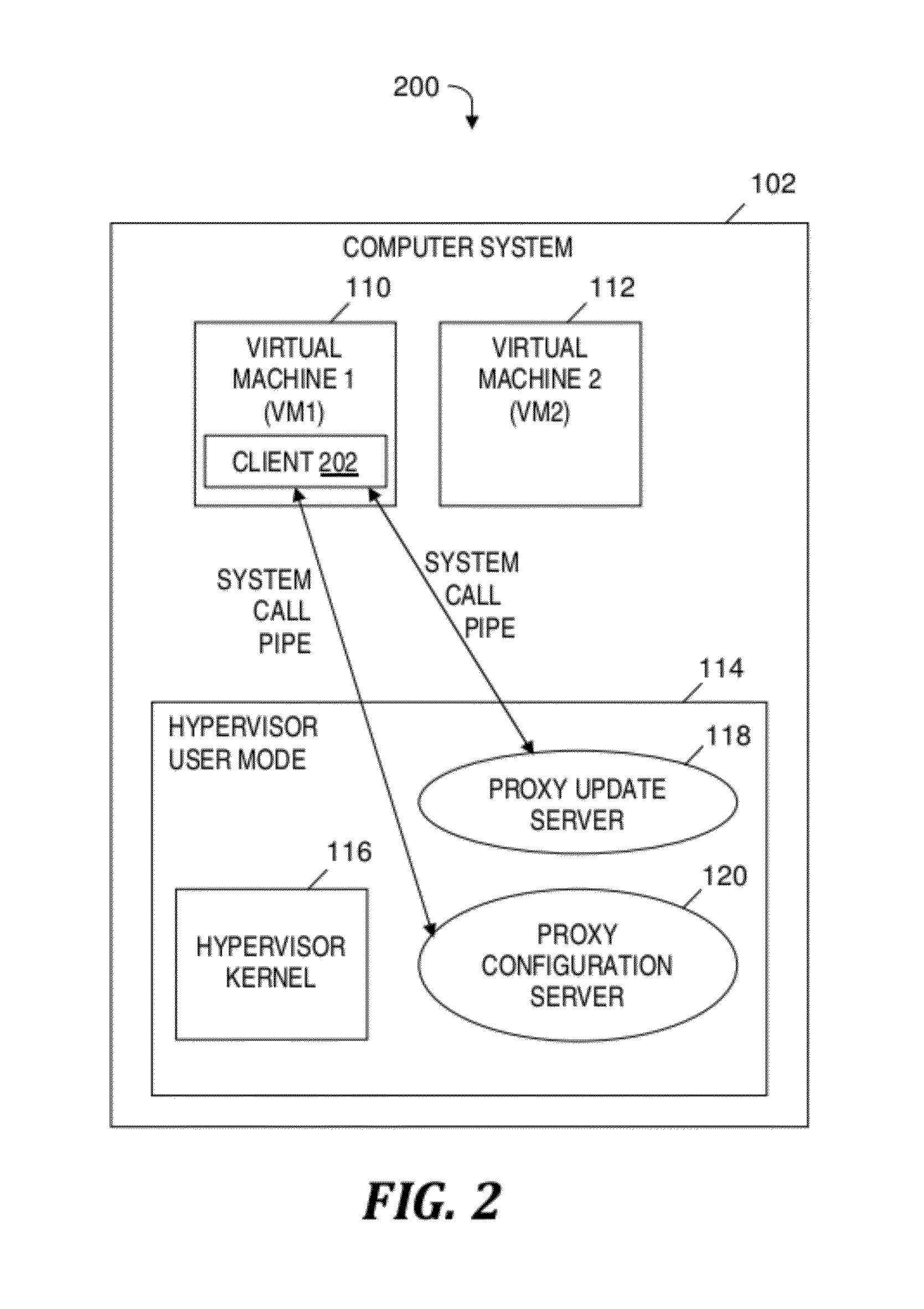
Automatically and securely configuring and updating virtual machines
InactiveUS20120174095A1Avoid virus infectionAvoiding software piracy issuesSoftware simulation/interpretation/emulationMemory systemsVirtual machineSoftware
A method and program product for automatically and securely updating software on a virtual machine (VM). A VM coming online in a virtualized server is detected. A current version of the software that is installed on the VM is determined. The current version is determined to not match an updated version of the software available from a remote update server via a network. The updated version of the software is received from the remote update server and via the network without the VM being connected to the network. A confirmation is received indicating that the updated version of the software is installed on the VM. In response to receiving the confirmation, the VM is connected to the network.
Owner:IBM CORP
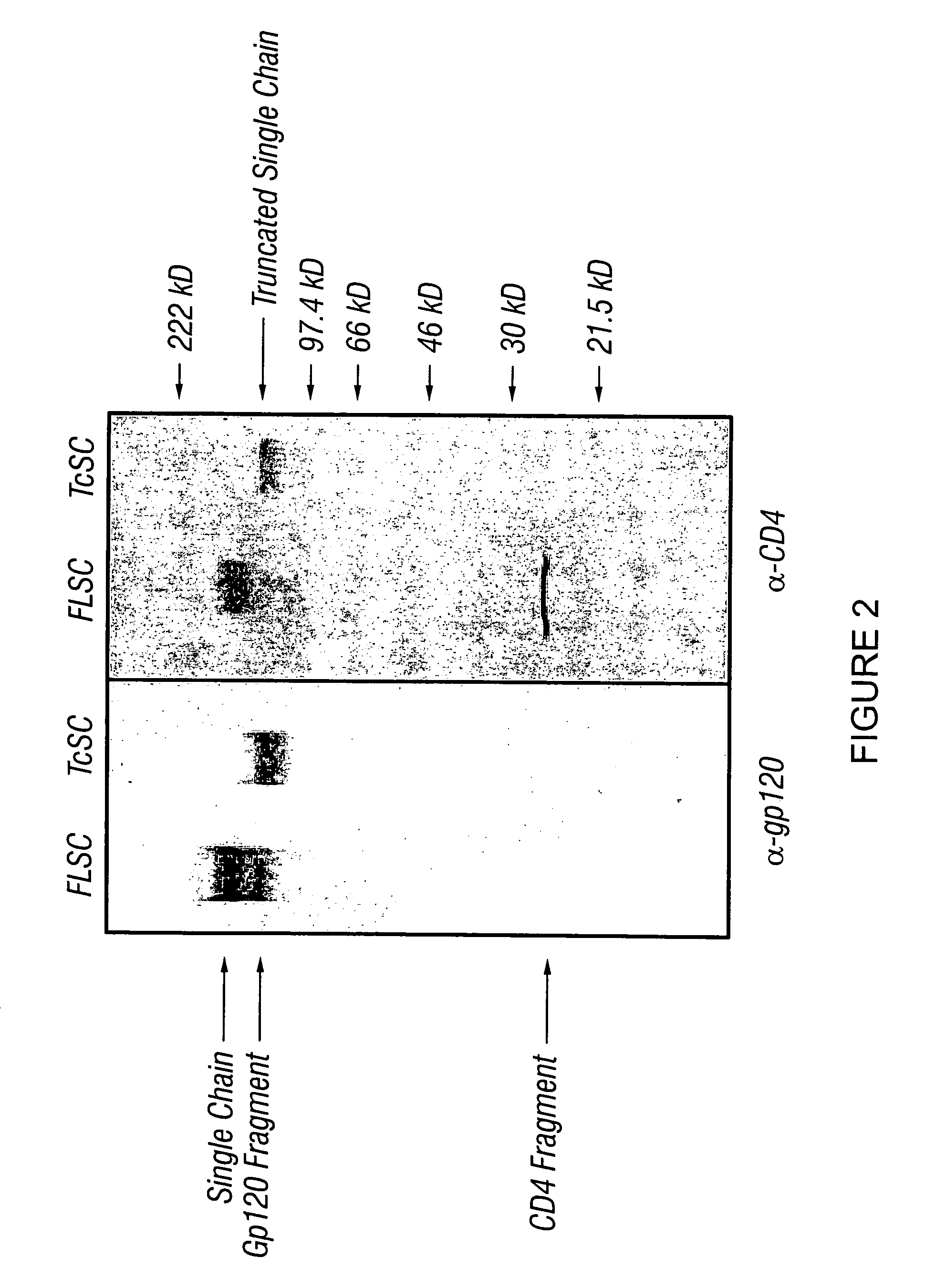
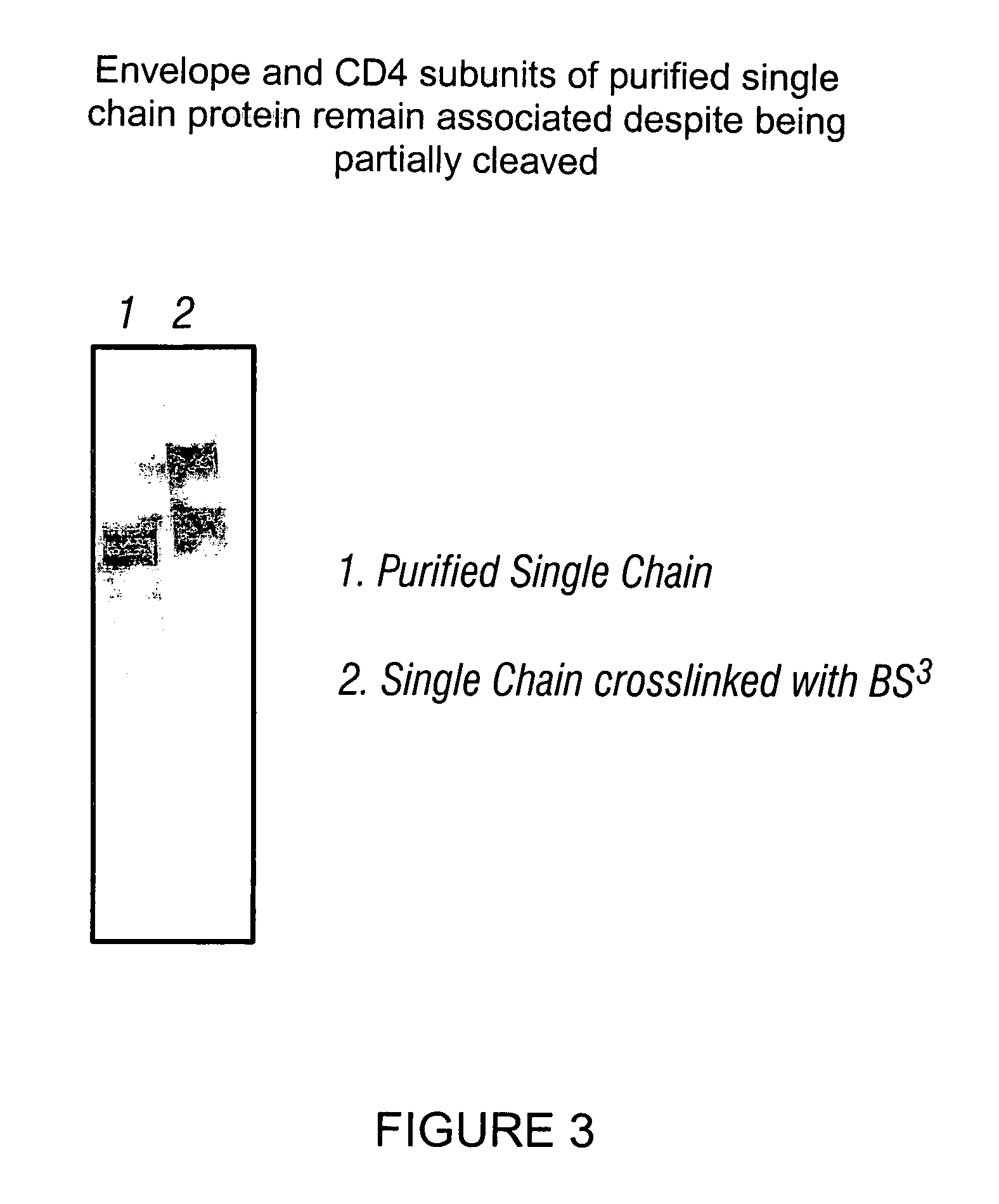
Virus coat protein/receptor chimeras and methods of use
InactiveUS7311920B1Sufficient amountMethod can be usedCompound screeningApoptosis detectionCXCR4Immunodeficiency virus
The invention relates to chimeric molecules comprising a virus coat sequence and a receptor sequence that can interact with each other to form a complex that is capable of binding a co-receptor. Such chimeric molecules therefore exhibit functional properties characteristic of a receptor-coat protein complex and are useful as agents that inhibit virus infection of cells due to occupancy of co-receptor present on the cell, for example. In particular aspects, the chimeric polypeptide includes an immunodeficiency virus envelope polypeptide, such as that of HIV, SIV, FIV, FeLV, FPV and herpes virus. Receptor sequences suitable for use in a chimeric polypeptide include, for example, CCR5 and CXCR4 sequences.
Owner:MARYLAND BIOTECH INST UNIV OF
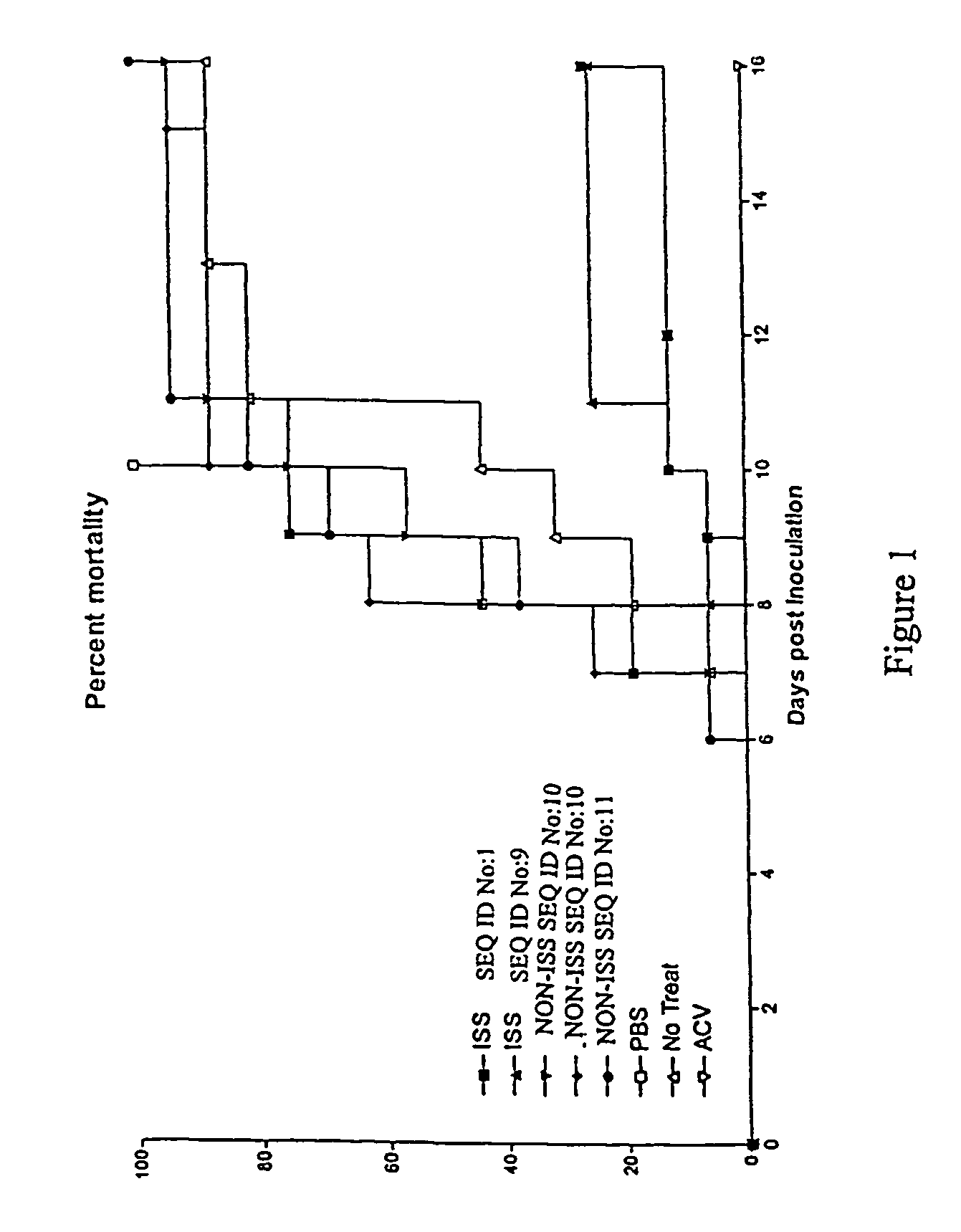
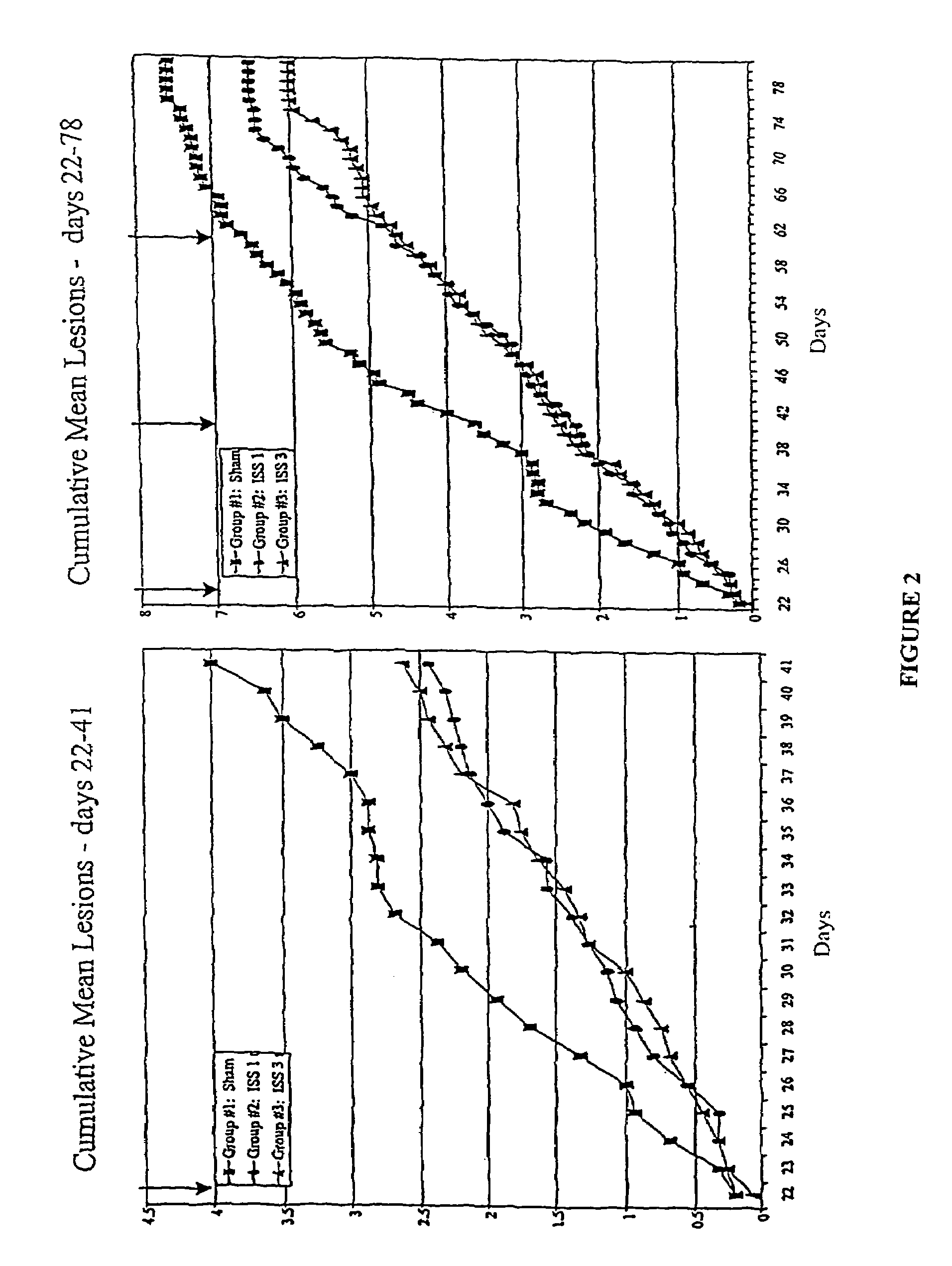
Methods of ameliorating symptoms of herpes infection using immunomodulatory polynucleotide sequences
InactiveUS7157437B2Many symptomReduce incidenceOrganic active ingredientsSugar derivativesAntigenHerpes simplex virus DNA
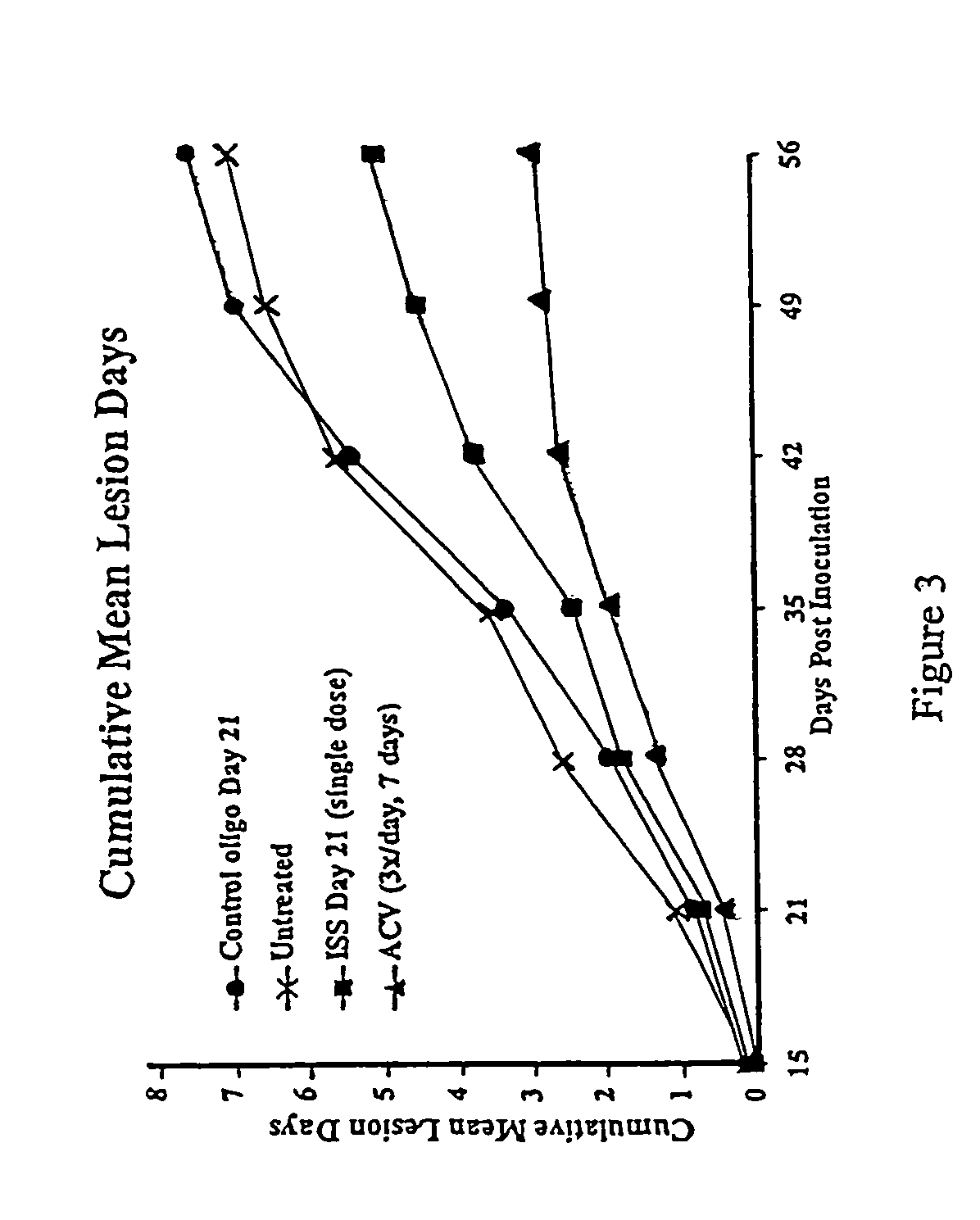
The invention provides new methods of preventing and / or treating herpes virus infections, particularly reducing infection, one or more symptoms and recurrence of one or more symptoms of herpes simplex virus infection. A polynucleotide comprising an immunostimulatory sequence (an “ISS”) is administered to an individual which is at risk of being posed to alphaherpesvirinae, has been exposed to alphaherpesvirinae or is infected with alphaherpesvirinae. The ISS is administered without any alphaherpesvirinae antigens. Administration of the ISS results in reduced incidence, recurrence, and severity of one or more symptoms of alphaherpesvirinae infection.
Owner:DYNAVAX TECH CORP
Treating viral infection at smallpox vaccination site
InactiveUS7288265B1Useful in treatmentAvoid infectionAdhesive dressingsAbsorbent padsVaccinationAdhesive
An adhesive patch is provided wherein the patch includes a porous backing having a front side and a back side. The patch also includes a therapeutic formulation located on the front side of the backing. The backing includes a flexible sheet of water insoluble porous material. The therapeutic formulation includes a combination of a antiviral agent useful for treating a viral infection in a mammal (e.g., human), a medicament that relieves topical discomfort, an adhesive, and a solvent. The solvent can preferably include a fragrance.
Owner:LECTEC CORP
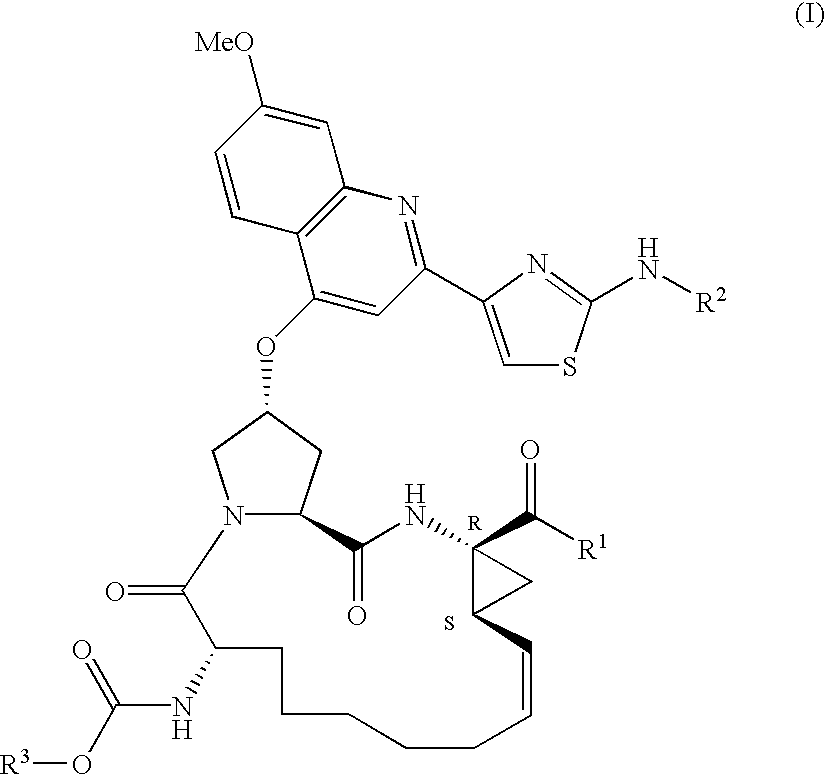
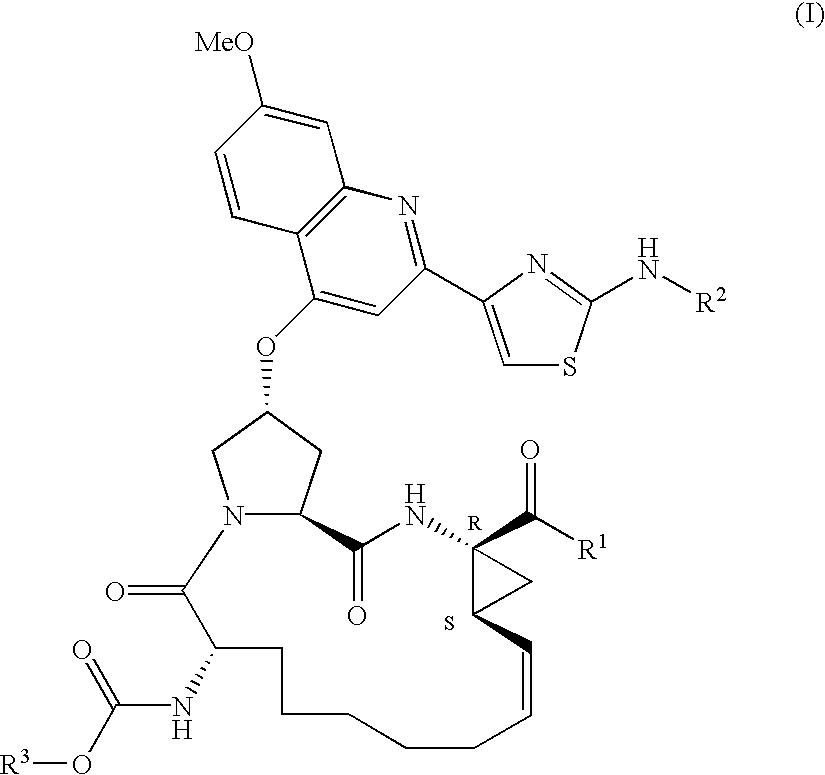
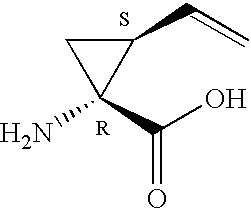
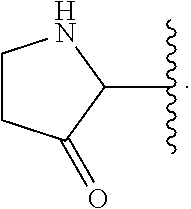
Macrocyclic peptides active against the hepatitis C virus
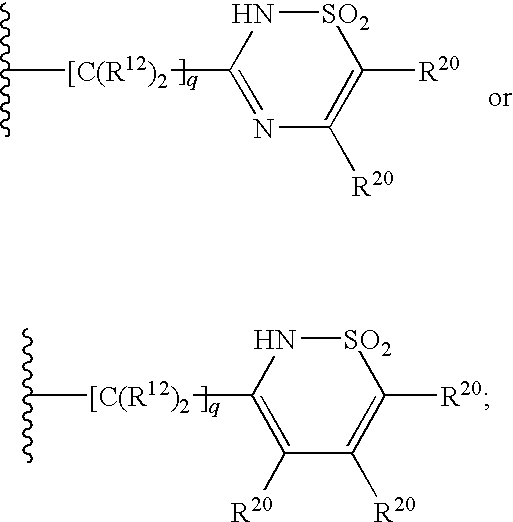
Compounds of formula I:wherein R1 is hydroxy or NHSO2R1A wherein R1A is (C1-8)alkyl, (C3-7)cycloalkyl or {(C1-6)alkyl-(C3-7)cycloalkyl}, which are all optionally substituted from 1 to 3 times with halo, cyano, nitro, O(C1-6)alkyl, amido, amino or phenyl, or R1A is C6 or C10 aryl which is optionally substituted from 1 to 3 times with halo, cyano, nitro, (C1-6)alkyl, O(C1-6)alkyl, amido, amino or phenyl; R2 is (C5-6)cycloalkyl and R3 is cyclopentyl; or a pharmaceutically acceptable salt thereof, useful as inhibitors of the HCV NS3 protease.
Owner:BOEHRINGER INGELHEIM CANADA LTD
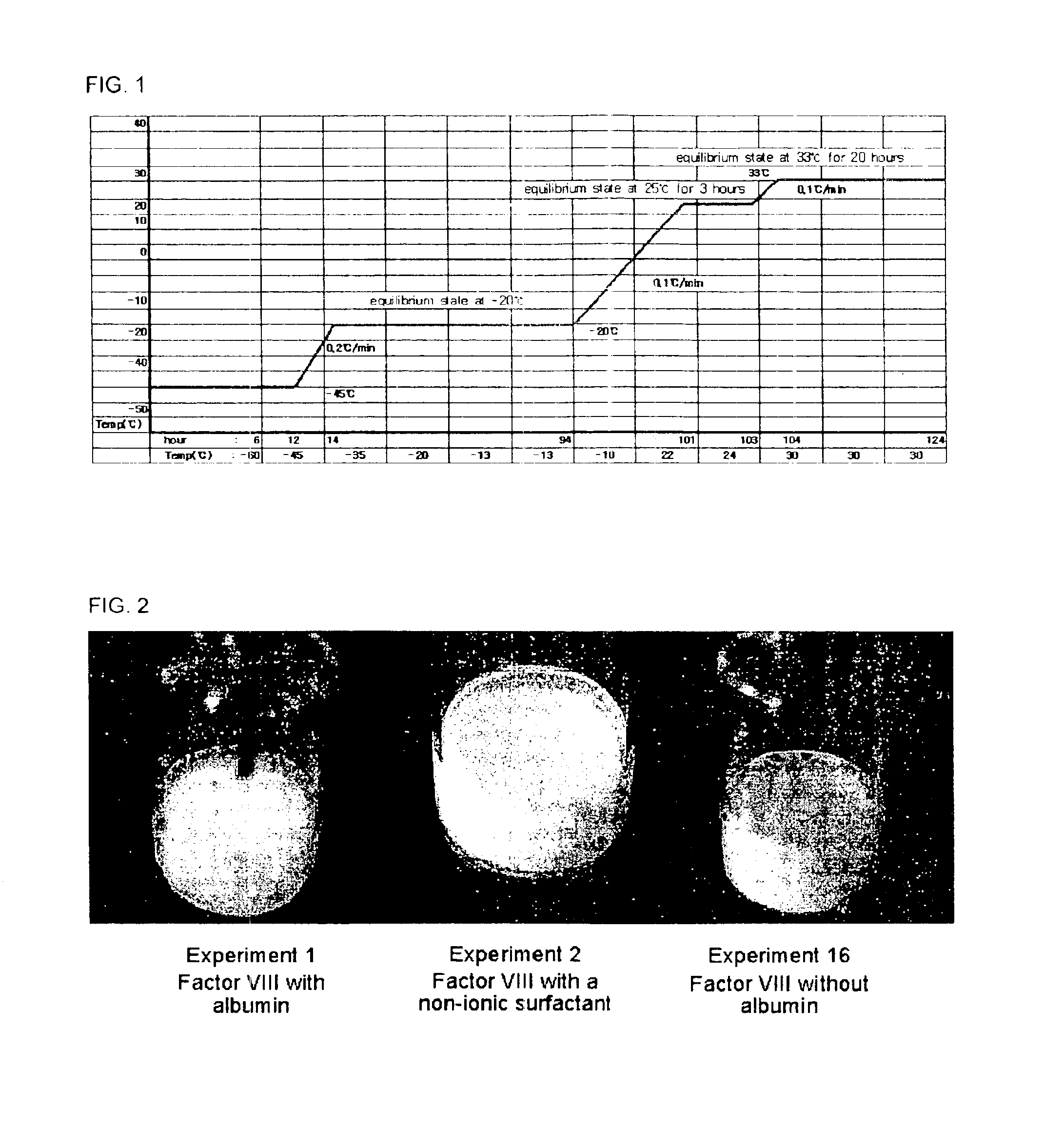
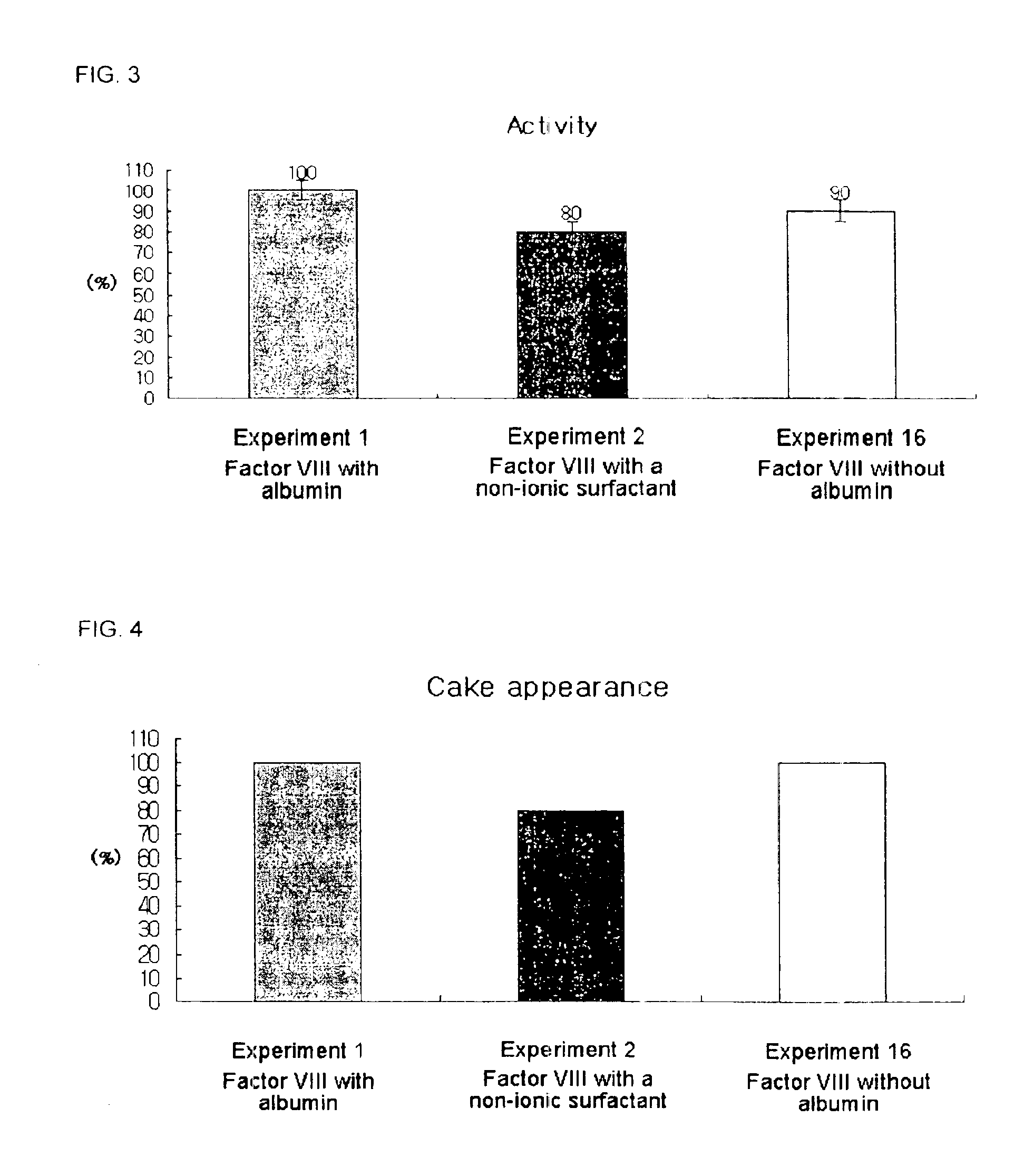
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
ActiveUS6887852B1Same pharmaceutical efficacyAvoid virus infectionFactor VIIPowder deliveryMedicineArginine
Disclosed is a lyophilized preparation of recombinant factor VIII used as a therapeutic preparation of hemophilia A. The lyophilized preparation of recombinant factor VIII is prepared by performing lyophilization using a mixture comprising 6 to 100 mM of L-arginine, 3.5 to 50 mM of L-isoleucine, and 10 to 100 mM of L-glutamic acid as a stabilizer for stabilizing the recombinant factor VIII which exhibits an unstable activity during lyophilization, rather than using human blood derived albumin.
Owner:KOREA GREEN CROSS CORP
Antiviral patch
InactiveUS20070026056A1Useful in treatmentAvoid infectionHalogenated hydrocarbon active ingredientsBiocideSolventDrug
An adhesive patch is provided wherein the patch includes a porous backing having a front side and a back side. The patch also includes a therapeutic formulation located on the front side of the backing. The backing includes a flexible sheet of water insoluble porous material. The therapeutic formulation includes a combination of a antiviral agent useful for treating a viral infection in a mammal (e.g., human), a medicament that relieves topical discomfort, an adhesive, and a solvent. The solvent can preferably include a fragrance.
Owner:LECTEC CORP
Delivery of proteins using adeno-associated virus (AAV) vectors
ActiveUS8865881B2Reducing and inhibiting infection riskReduce the risk of infectionSugar derivativesGenetic material ingredientsADAMTS ProteinsAdeno-associated virus
Disclosed herein are compositions, systems and methods for delivery of proteins of interest using adeno-associated virus (AAV) vectors.
Owner:CALIFORNIA INST OF TECH
Virus coat protein/receptor chimeras and methods of use
InactiveUS6908612B2Raise the potentialAvoid virus infectionOrganic active ingredientsFungiImmunodeficiency virusHerpes simplex virus DNA
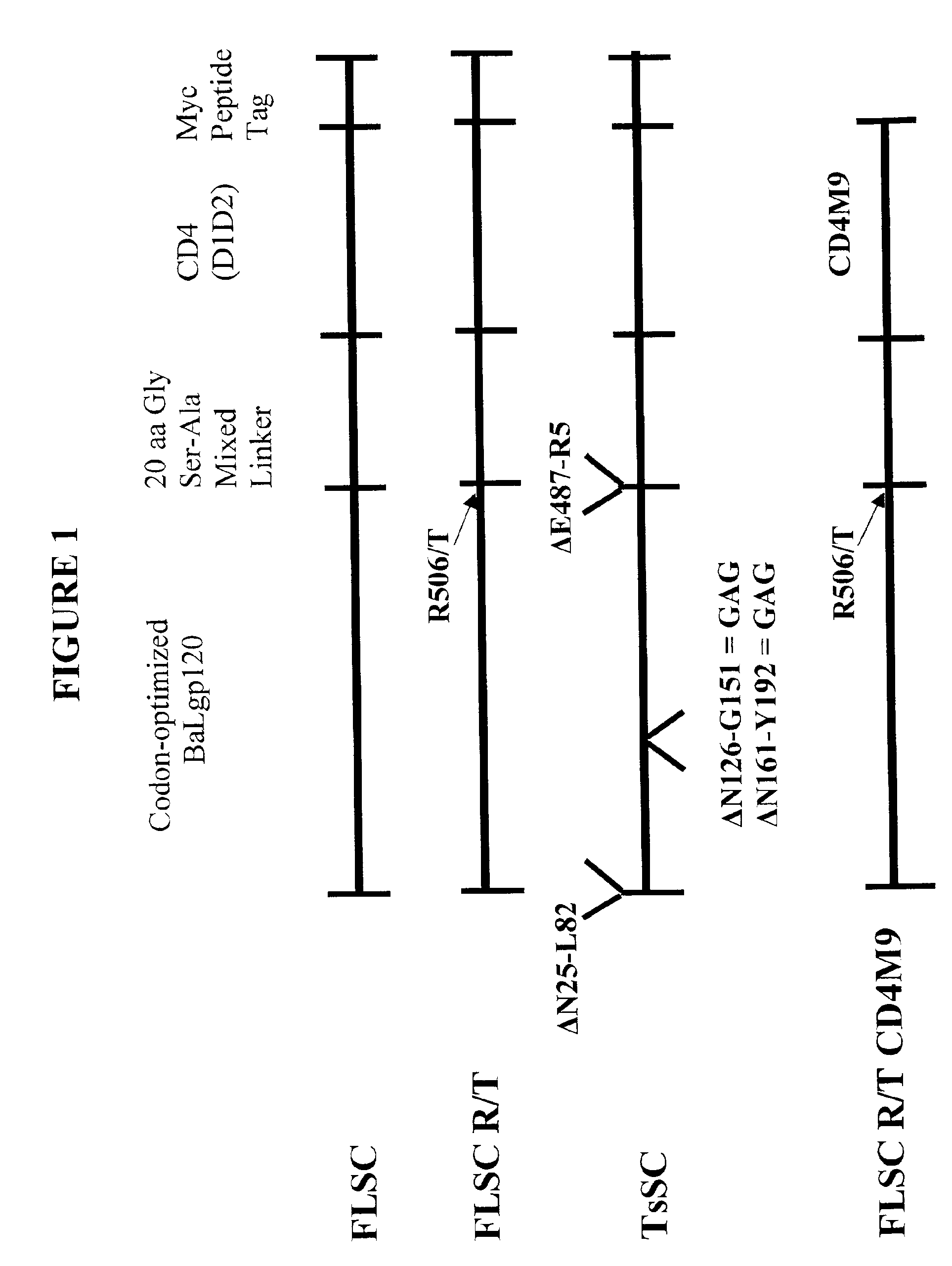
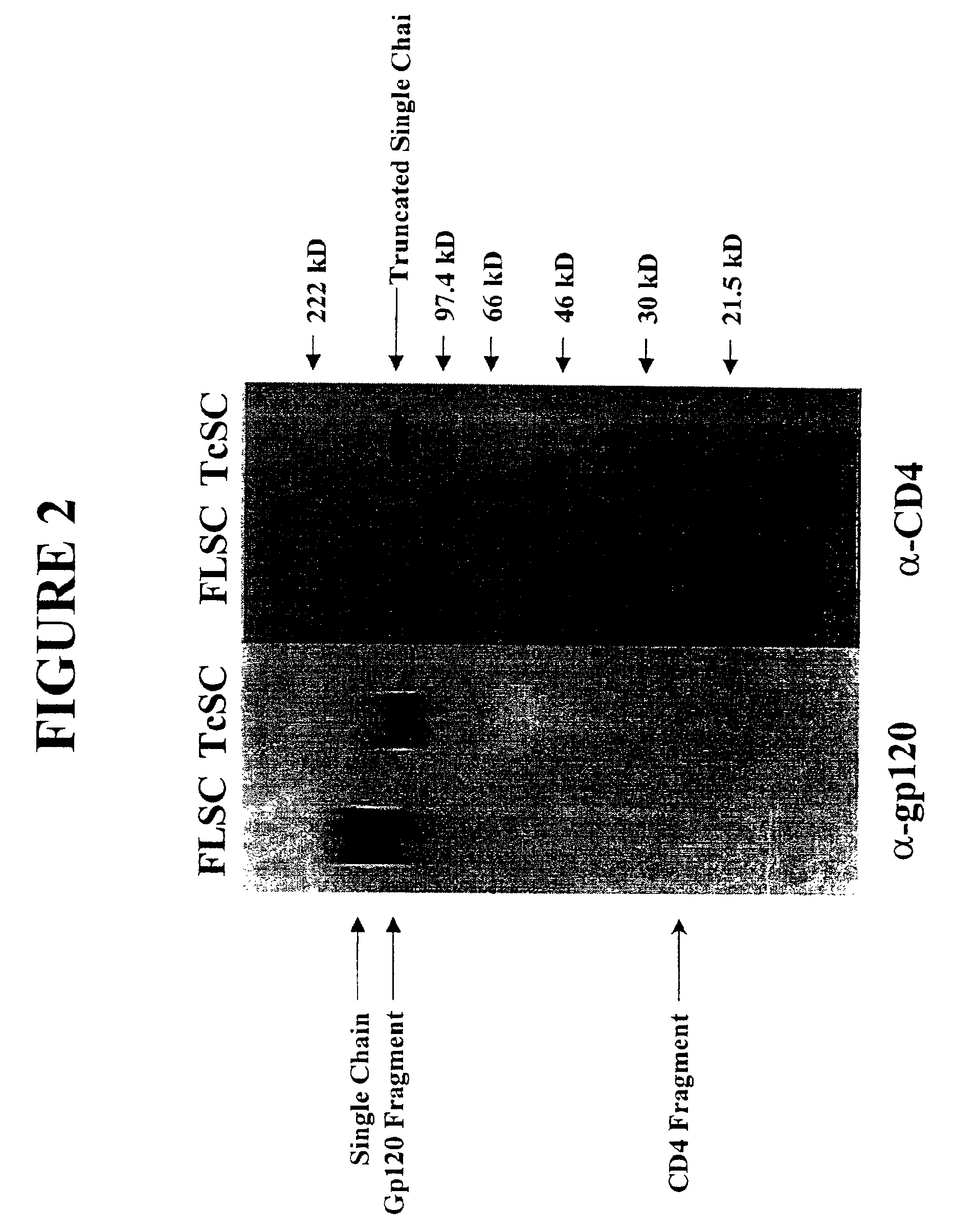
The invention relates to chimeric molecules comprising a virus coat sequence and a receptor sequence that can inter-act with each other to form a complex that is capable of binding a co-receptor. Such chimeric molecules therefore exhibit functional properties characteristic of a receptor-coat protein complex and are useful as agents that inhibit virus infection of cells due to occupancy of a co-receptor present on the cell. In particular aspects, the chimeric polypeptide includes an immunodeficiency virus envelope polypeptide, such as that of HIV, SIV, FIV, FeLV, FPV and herpes virus. Receptor sequences suitable for use in a chimeric polypeptide include, for example, CD4 D1D2 and CD4M9 sequences.
Owner:MARYLAND UNIV OF BIOTECH INST
Compositions and methods for the treatment of viral infections
ActiveUS20110318352A1Restore native alpha-helical structureImprove stabilitySsRNA viruses negative-senseBiocideViral infectionProteolysis
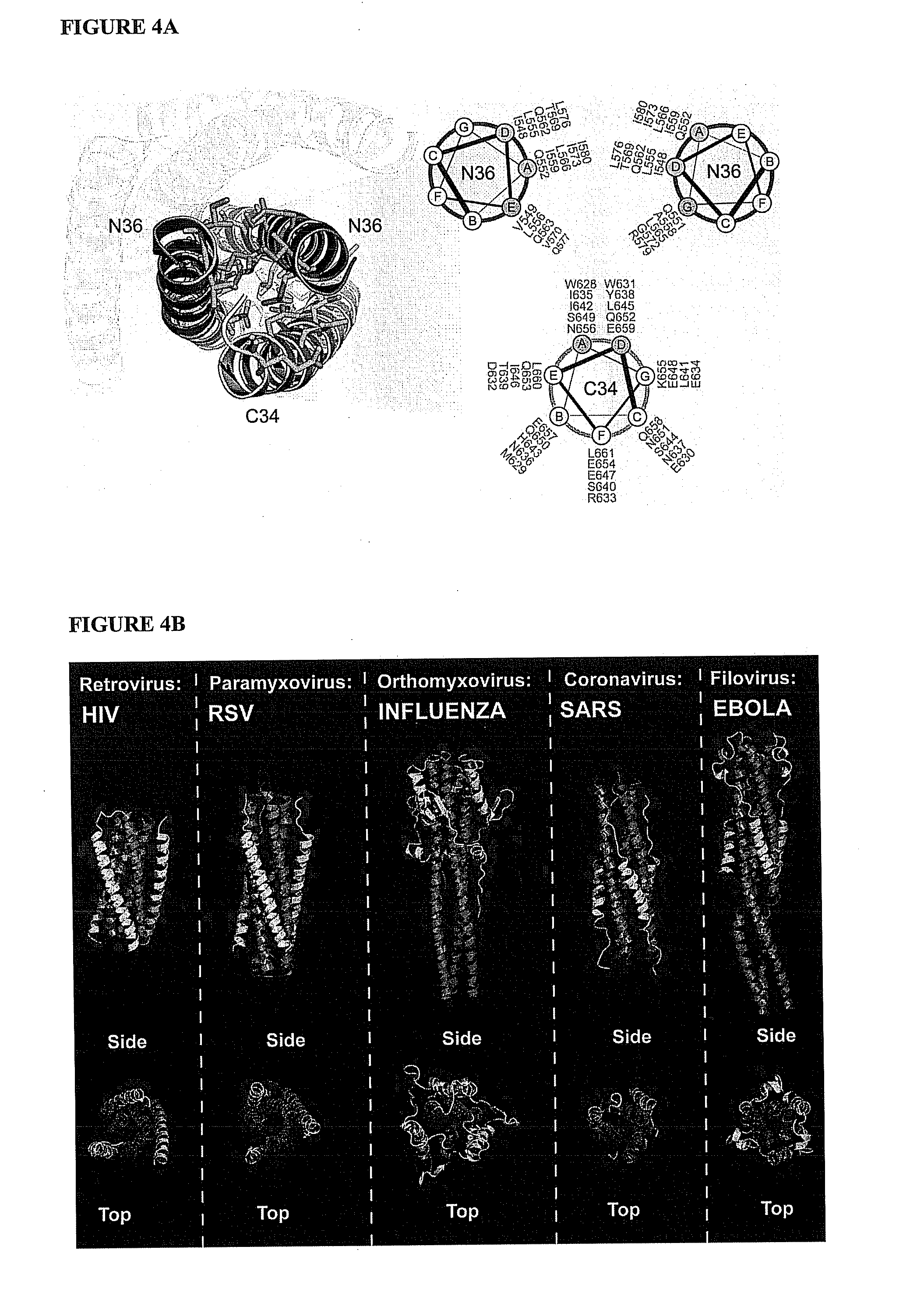
The invention provides compositions, kits and methods utilizing polypeptides having a viral alpha-helix heptad repeat domain in a stabilized a-helical structure (herein also referred to as SAH). The compositions are useful for treating and / or preventing viral infections. The invention is based, at least in part, on the result provided herein demonstrating that viral hydrocarbon stapled alpha helical peptides display excellent proteolytic, acid, and thermal stability, restore the native alpha-helical structure of the peptide, are highly effective in interfering with the viral fusogenic process, and possess superior pharmacokinetic properties compared to the corresponding unmodified peptides.
Owner:DANA FARBER CANCER INST INC
Automatically and securely configuring and updating virtual machines
InactiveUS8578376B2Avoid virus infectionAvoiding software piracy issuesSoftware simulation/interpretation/emulationMemory systemsSoftwareVirtual machine
Owner:INT BUSINESS MASCH CORP
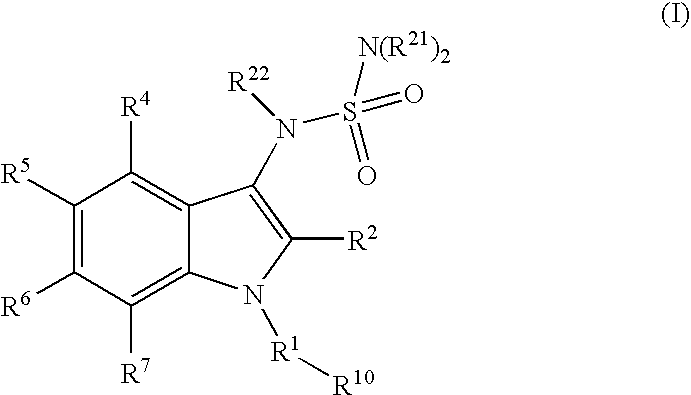
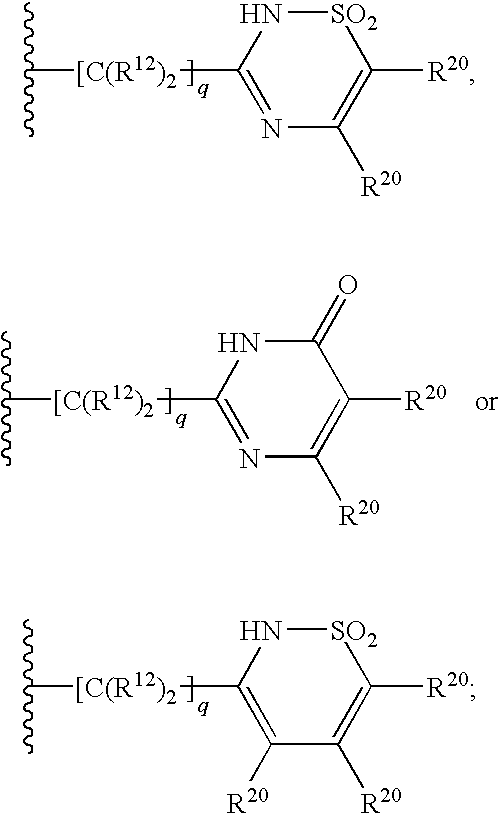
Tetracyclic indole derivatives and their use for treating or preventing viral infections
InactiveUS20110104109A1Avoid virus infectionBiocideOrganic chemistryMolecular biologyPerylene derivatives
The present invention relates to Tetracyclic Indole Derivatives, compositions comprising at least one Tetracyclic Indole Derivative, and methods of using the Tetracyclic Indole Derivatives for treating or preventing a viral infection or a virus-related disorder in a patient.
Owner:MERCK SHARP & DOHME CORP
Hepatitis B Virus Compositions and Methods of Use
ActiveUS20110257080A1Avoid virus infectionBiocideOrganic active ingredientsHepatocyteHepatitis B virus
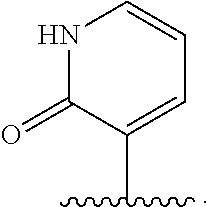
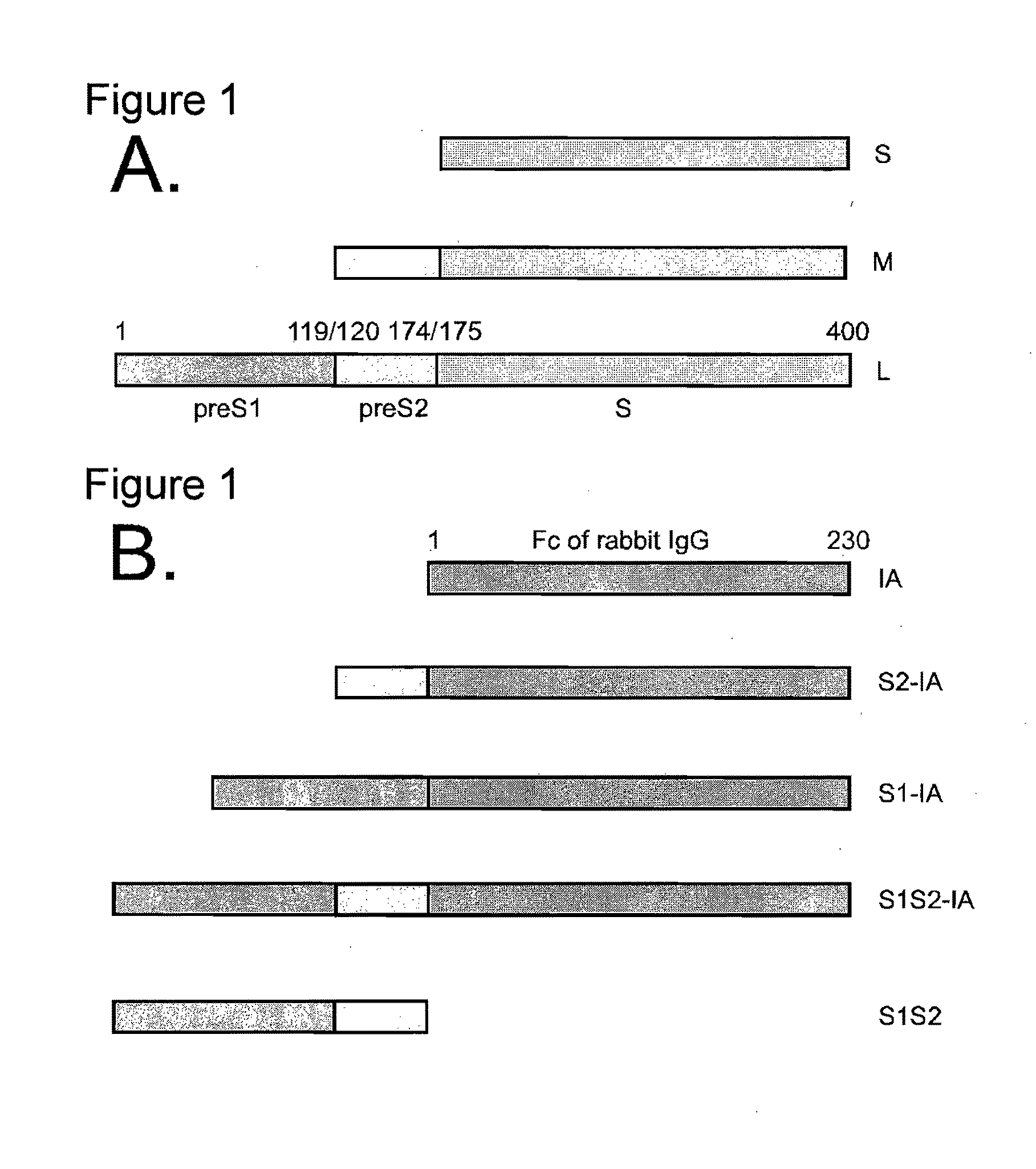
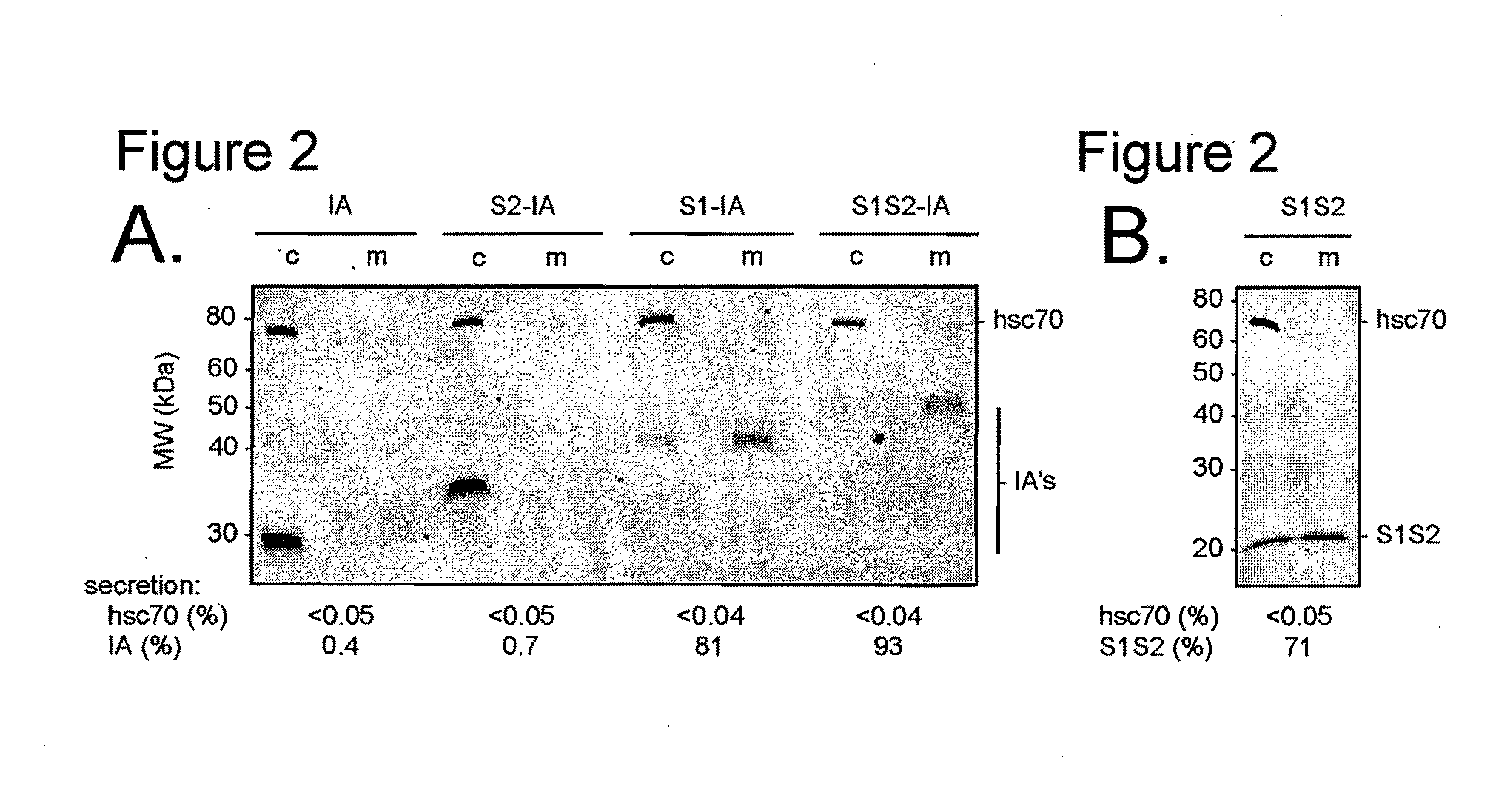
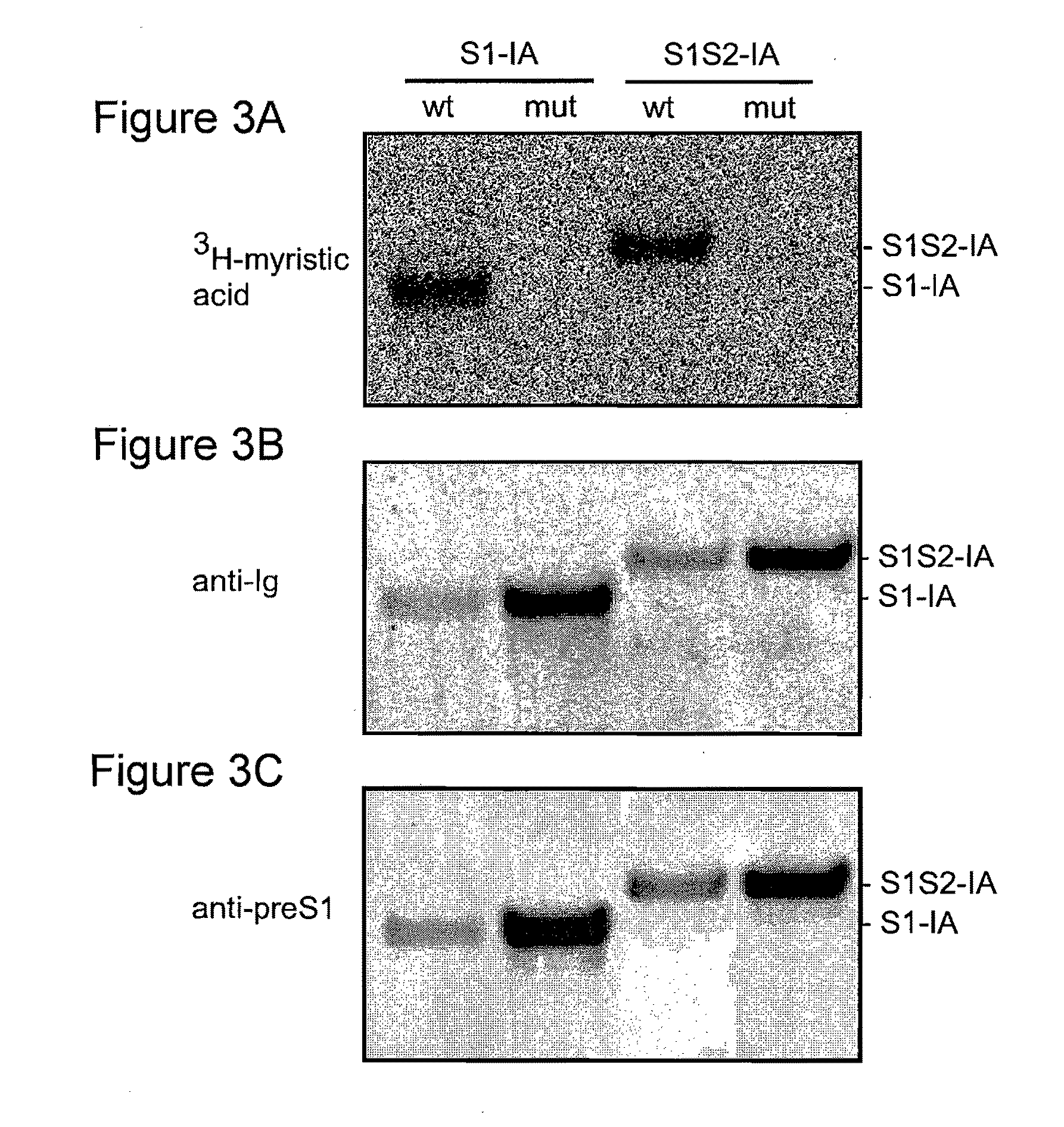
A polypeptide comprising a preS1 region of hepatitis B virus (HBV), or a fragment thereof, and / or the preS2 region of HBV or a fragment thereof, and methods of use to inhibit virus infection are disclosed. A lentivirus comprising hepatitis B virus (HBV) envelope proteins, or a fragment thereof, and / or the L envelope protein of HBV and / or the M envelope protein of HBV or a fragment thereof, and / or the S envelope protein of HBV or a fragment thereof, and methods of use of this lentivirus HBV pseudovirus as a gene therapy to target hepatocytes for the administration of therapeutic agents are also disclosed.
Owner:INST FOR CANCER RES
Methods for treating herpes virus infections
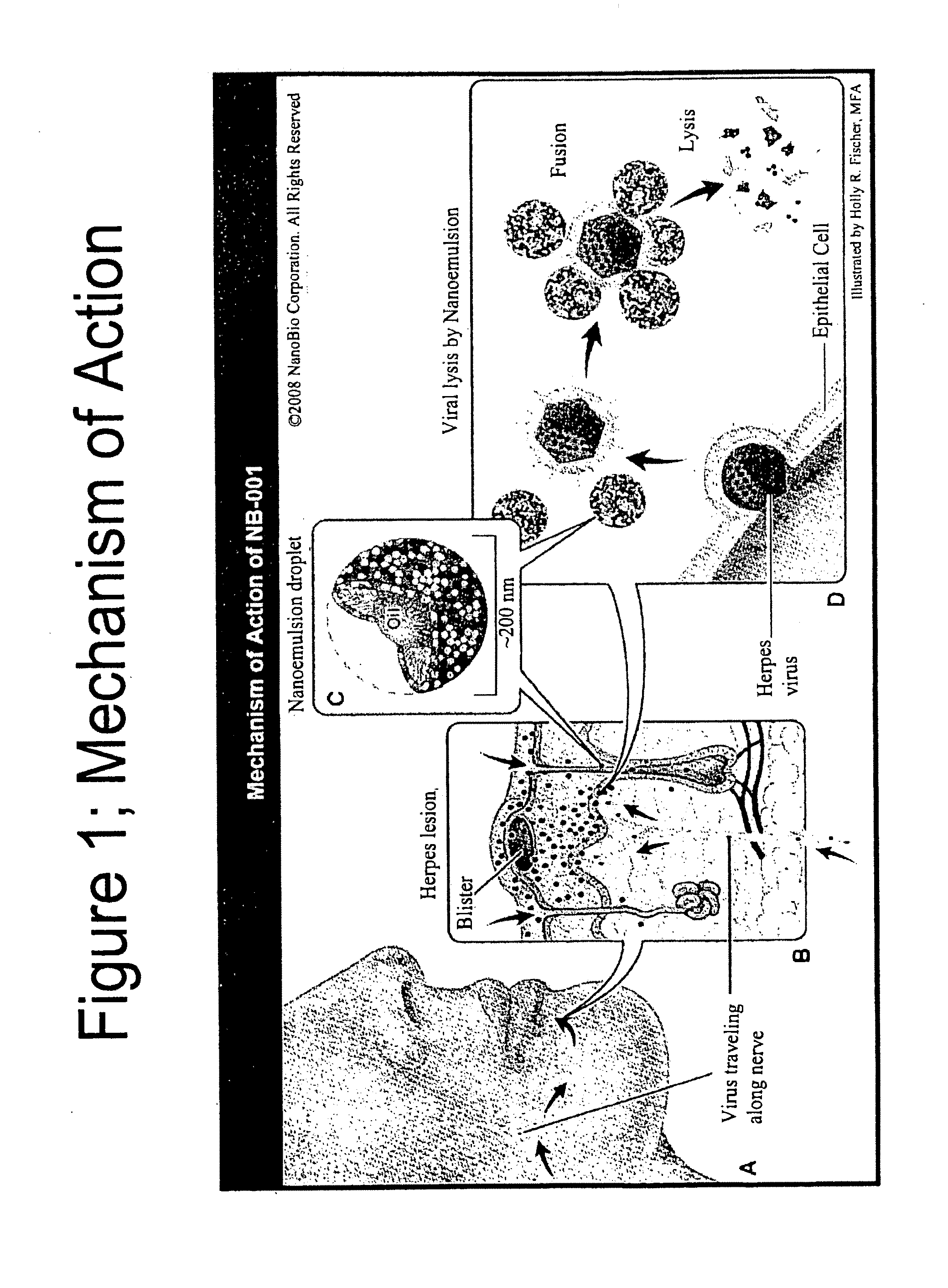
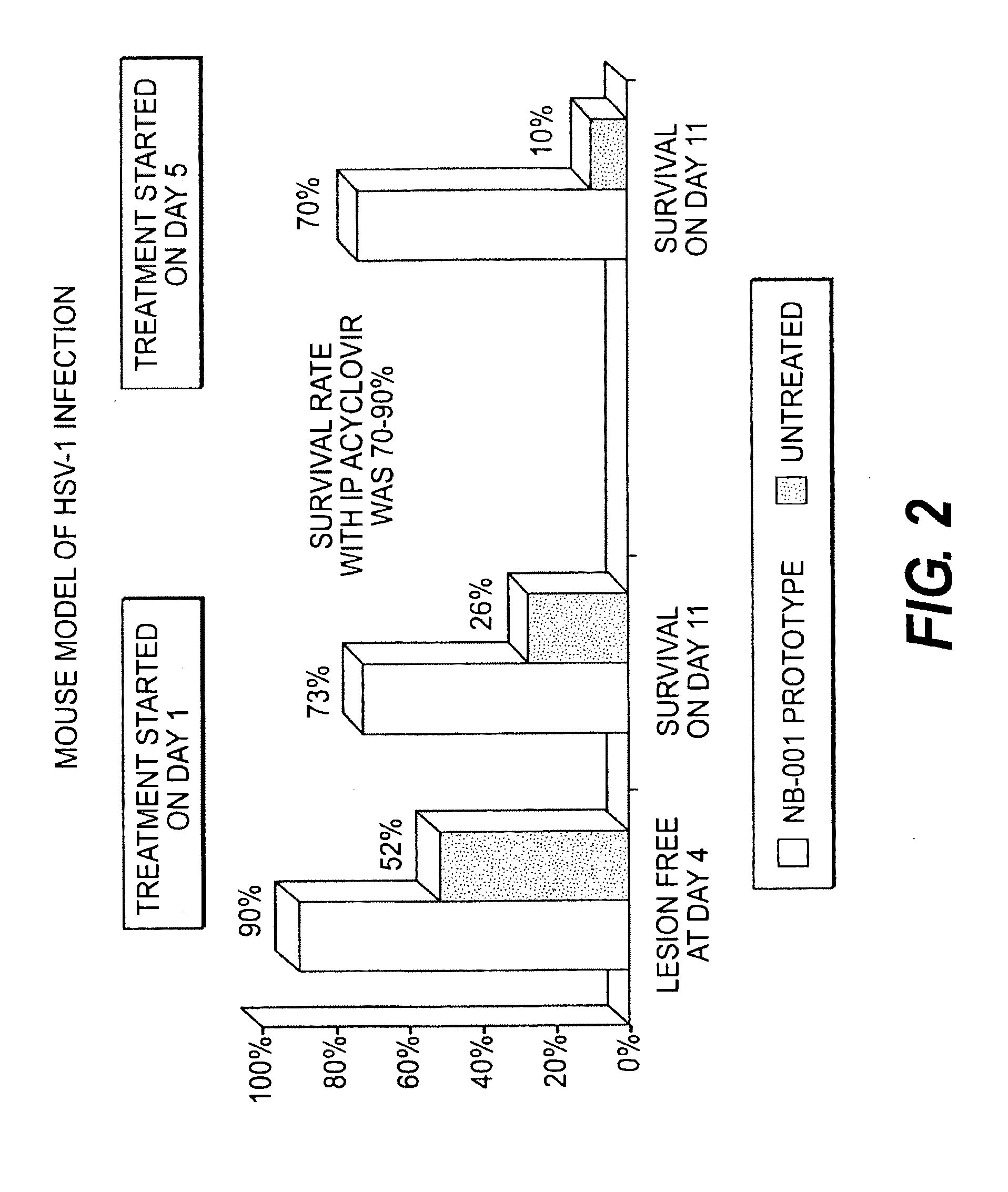
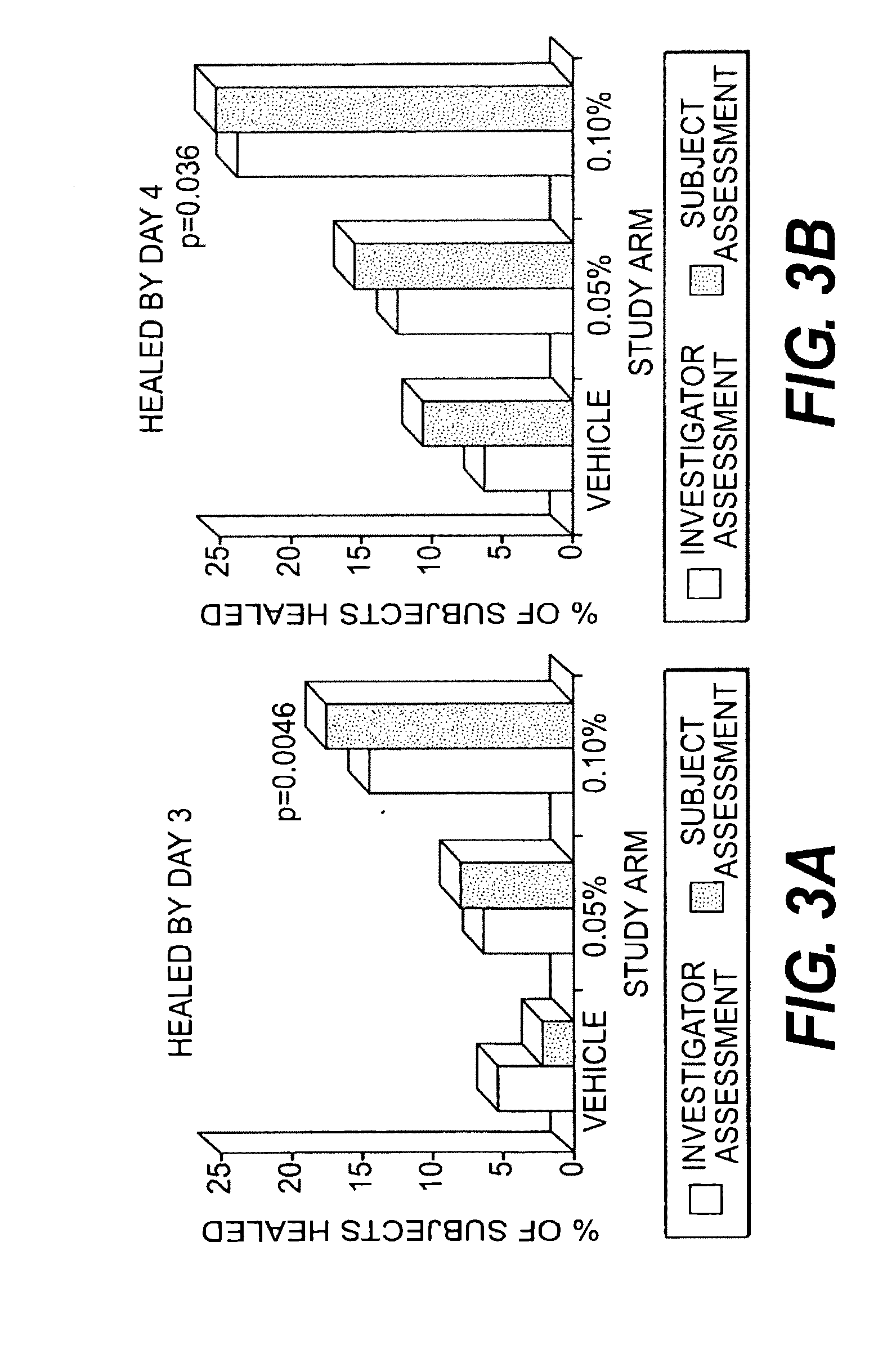
InactiveUS20120219602A1Minimizing reactivationAvoid spreadingBiocideNanomedicineHerpes simplex virus DNAViral infection
Owner:NANOBIO CORP
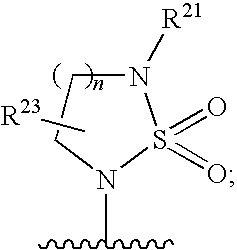
3-aminosulfonyl substituted indole derivatives and methods of use thereof
ActiveUS20100260711A1Avoid virus infectionBiocideOrganic chemistryViral infectionMedicinal chemistry
The present invention relates to 3-Aminosulfonyl Substituted Indole Derivatives, compositions comprising at least one 3-Aminosulfonyl Substituted Indole Derivative, and methods of using the 3-Aminosulfonyl Substituted Indole Derivatives for treating or preventing a viral infection or a virus-related disorder in a patient.
Owner:MERCK SHARP & DOHME CORP
L- beta -dioxolane uridine analogs and methods for treating and preventing Epstein-Barr virus infections
InactiveUS6022876ALow toxicityMinimal toxicityBiocideGroup 5/15 element organic compoundsHigh activityUridine Nucleotides
The present invention relates to the discovery that certain beta -L-dioxolane nucleoside analogs which contain a uracil base, and preferably, a 5-halosubstituted uracil base, exhibit unexpectedly high activity against Epstein-Barr virus (EBV), Varciella-Zoster virus (VZV) and Herpes Virus 8 (HV-8). In particular, the compounds according to the present invention show potent inhibition of the replication of the virus (viral growth) in combination with very low toxicity to the host cells (i.e., animal or human tissue). Compounds are useful for treating EBV, VZV and HV-8 infections in humans.
Owner:GEORGIA UNVERSITY OF RES FOUND INC THE +1
Lipopeptide inhibitors of hiv-1
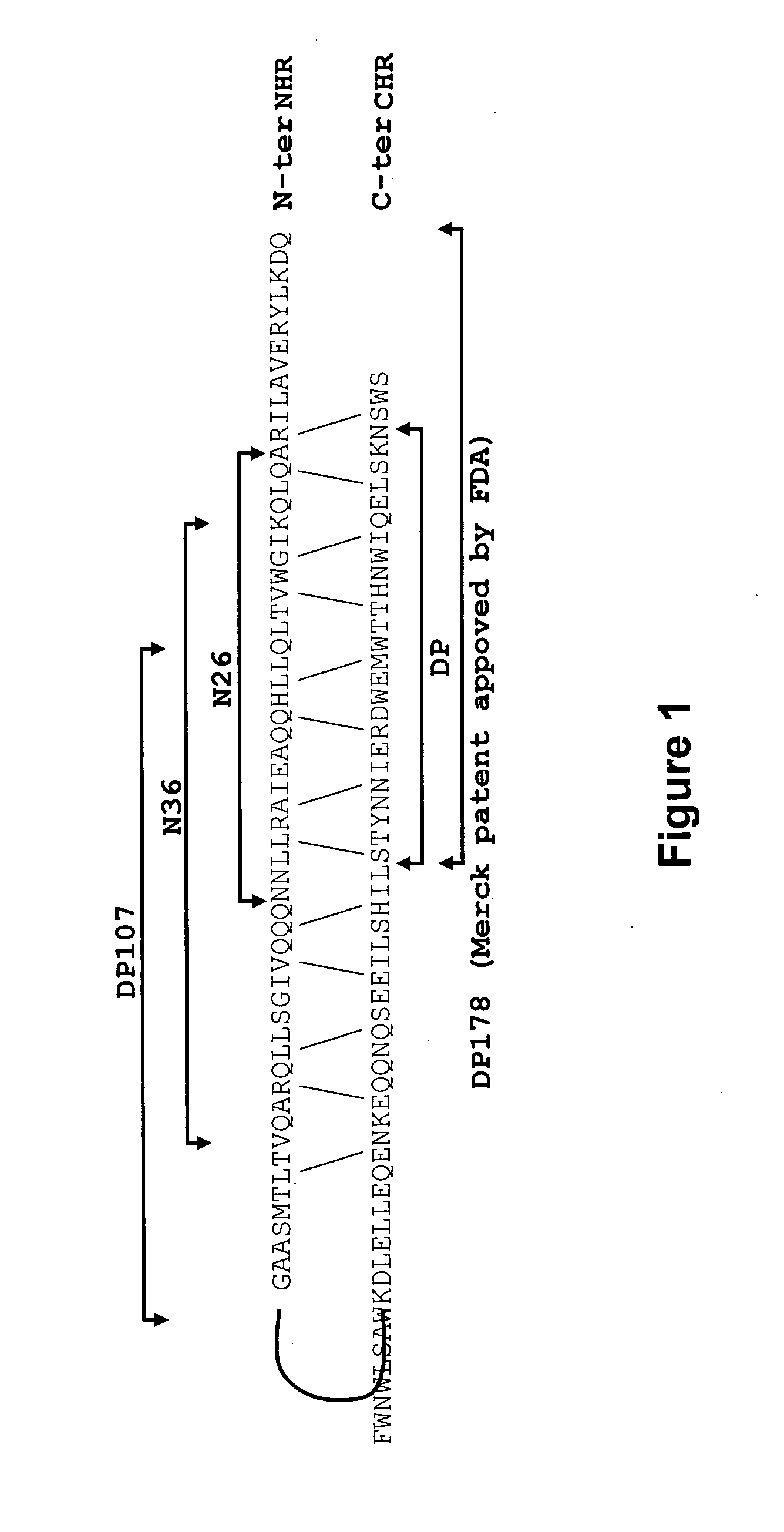
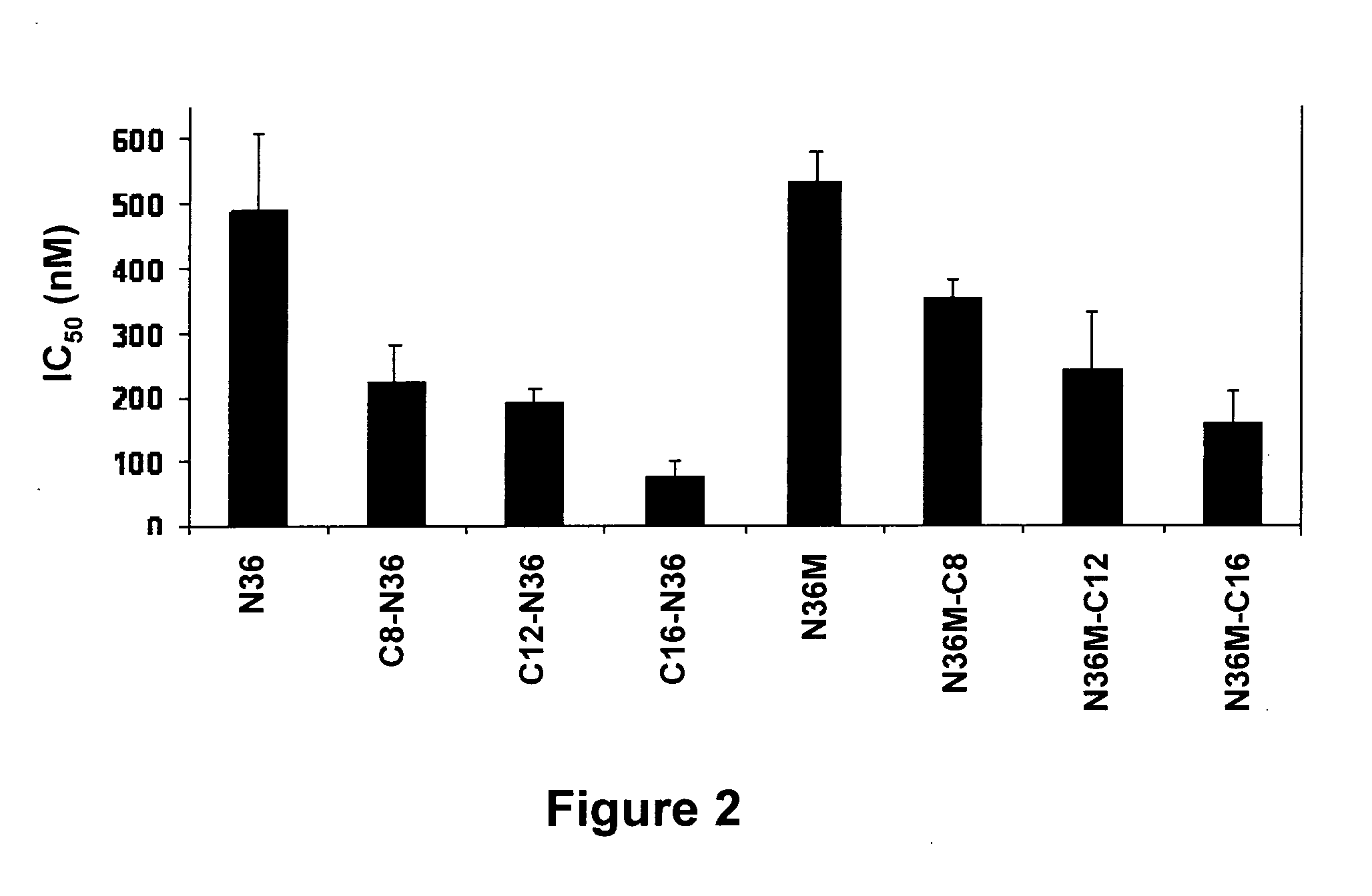
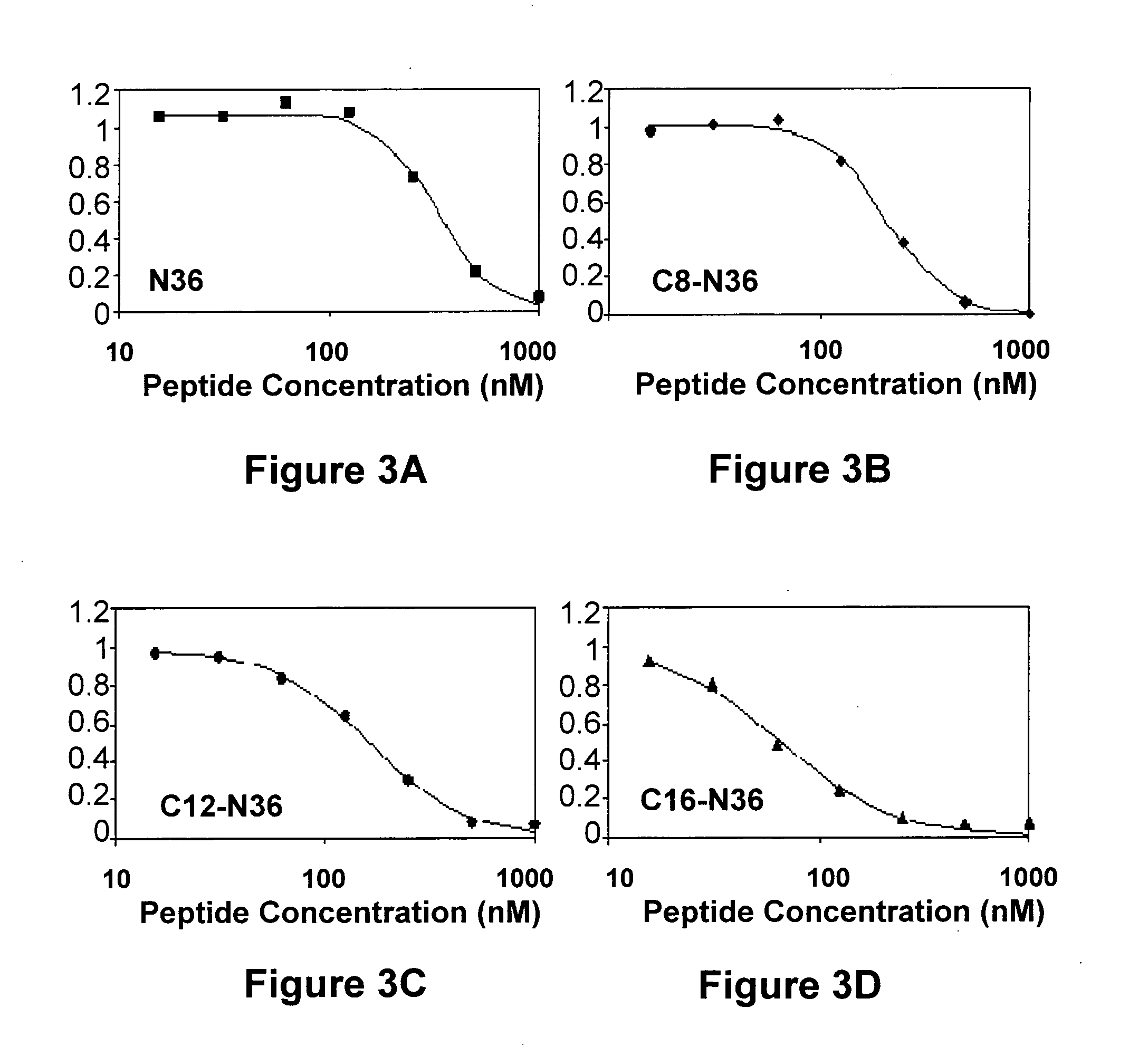
InactiveUS20120028887A1Avoid fusesGood pharmacologicalBiocidePeptide/protein ingredientsCrystallographyHeptad repeat
The invention provides lipophilic conjugates comprising a short isolated peptide coupled to a hydrophobic moiety, the peptide comprising a sequence derived from the HIV-1 gp41 N-terminal heptad repeat domain, said peptide after conjugation to the hydrophobic moiety possesses anti-fusogenic activity higher than prior to conjugation. The lipophilic conjugates are suitable for treatment of infections caused by human and non-human retroviruses, especially HIV.
Owner:YEDA RES & DEV CO LTD
Delivery vehicles, bioactive substances and viral vaccines
InactiveUS20060177468A1Reduce riskEnhanced securitySsRNA viruses negative-senseViral antigen ingredientsActive agentBioactive substance
The invention relates to compositions and methods for the safe delivery of a bioactive agent to an animal. Preferably, the bioactive agent is a vaccine, and more preferably, the bioactive agent is a virus.
Owner:PHILADELPHIA HEALTH & EDUCATION CORP +1
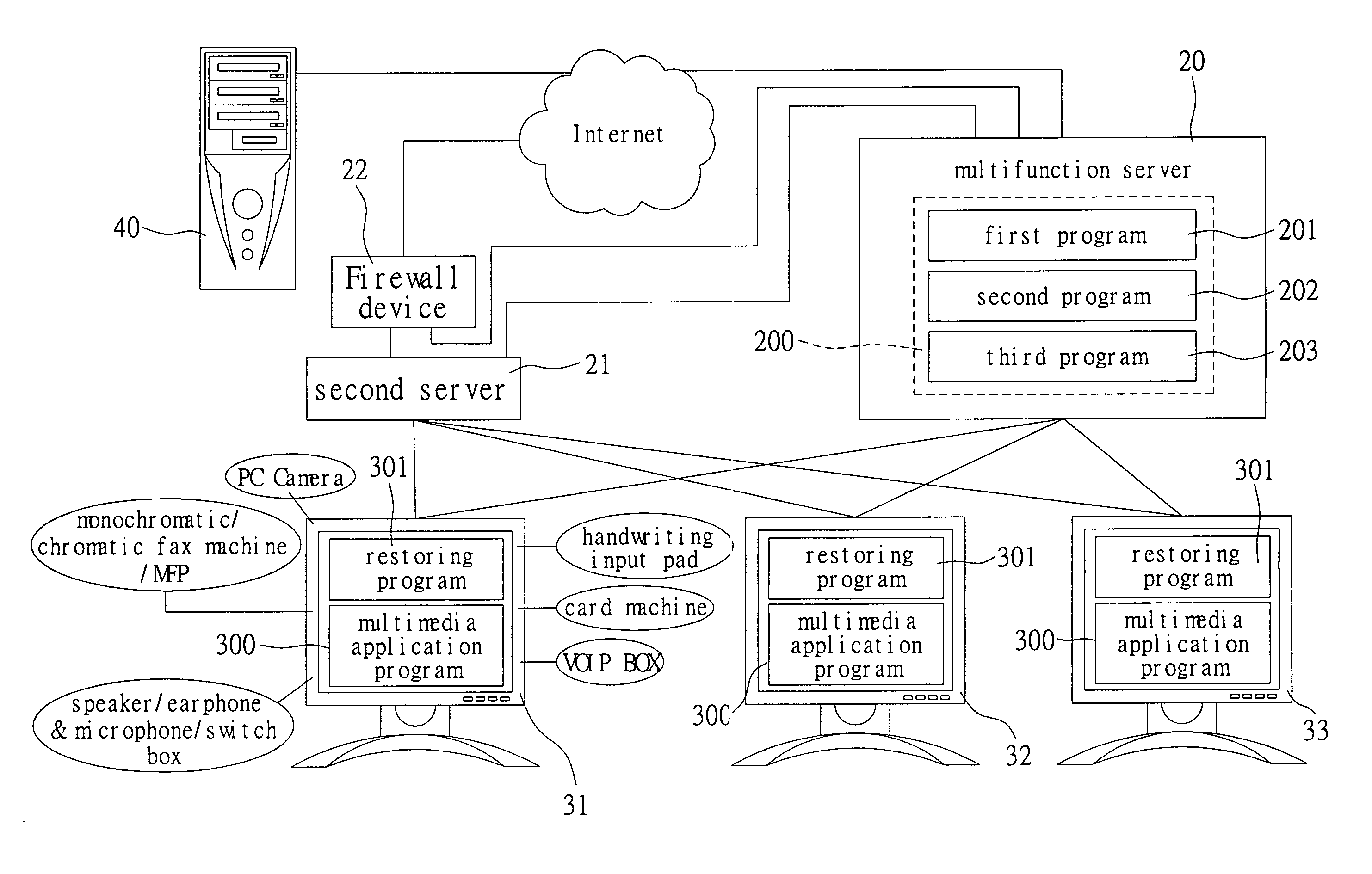
Multifunction server system
InactiveUS20060272012A1Good performance in processingImprove performanceDigital data processing detailsUser identity/authority verificationData securityMultiple function
A multifunction server system is proposed in the present invention to eliminate the drawbacks of conventional server systems. More particularly, the present invention is related to a multifunction server that connects with at least a host computer to form an enterprise intranet and provides services, such as bandwidth management, data management and data security, via software programs installed therein. The present invention performs better when processing multimedia data.
Owner:WU CHAO HUNG
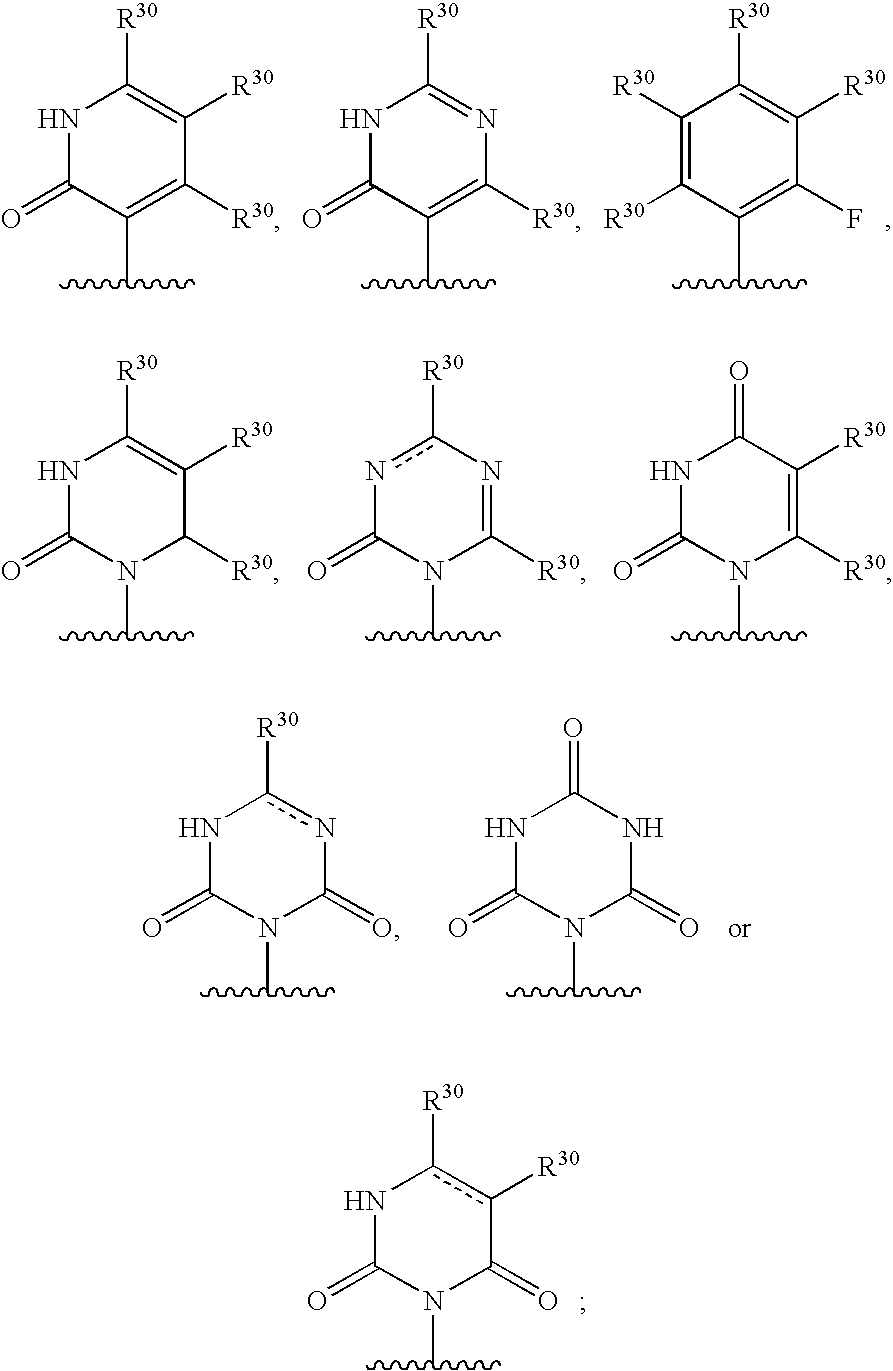
5, 6-ring annulated indole derivatives and use thereof
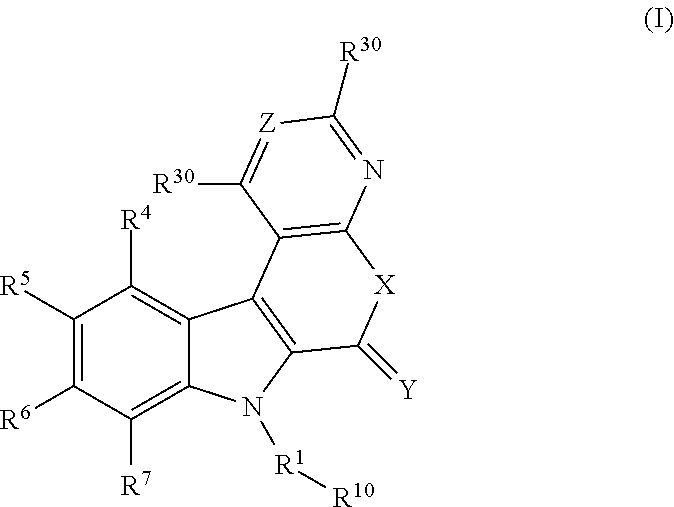
ActiveUS20100322901A1Avoid virus infectionBiocideOrganic chemistryViral infectionMedicinal chemistry
The present invention relates to 5,6-ring annulated indole derivatives of the formula (I), compositions comprising at least one 5,6-ring annulated indole derivatives, and methods of using the 5,6-ring annulated indole derivatives for treating or preventing a viral infection or a virus-related disorder in a patient.
Owner:MERCK SHARP & DOHME CORP
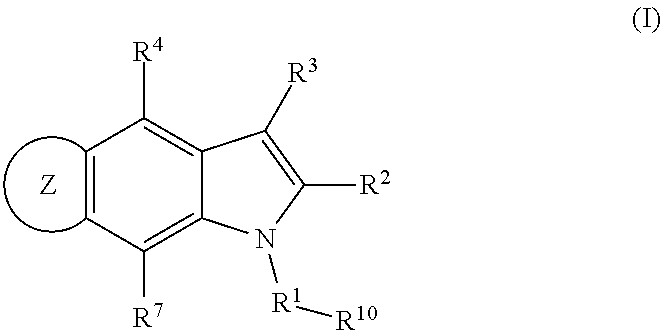
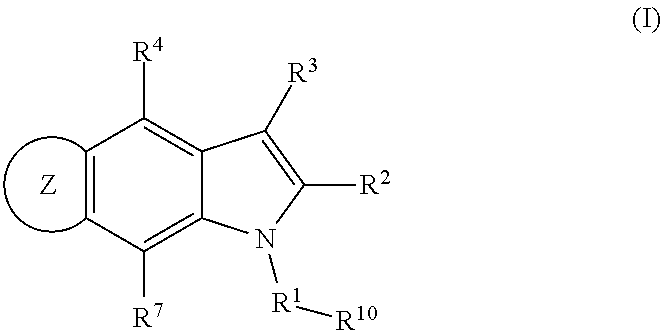
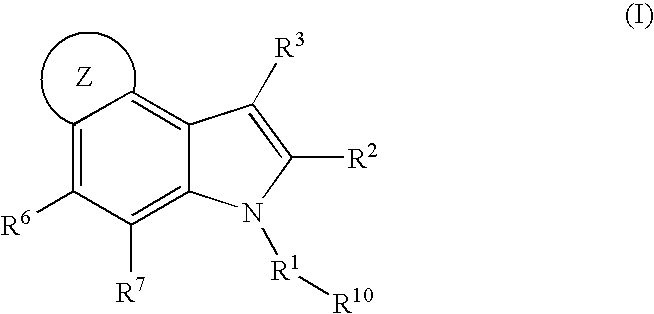
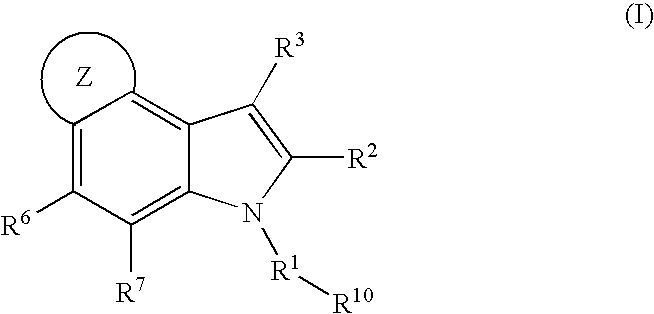
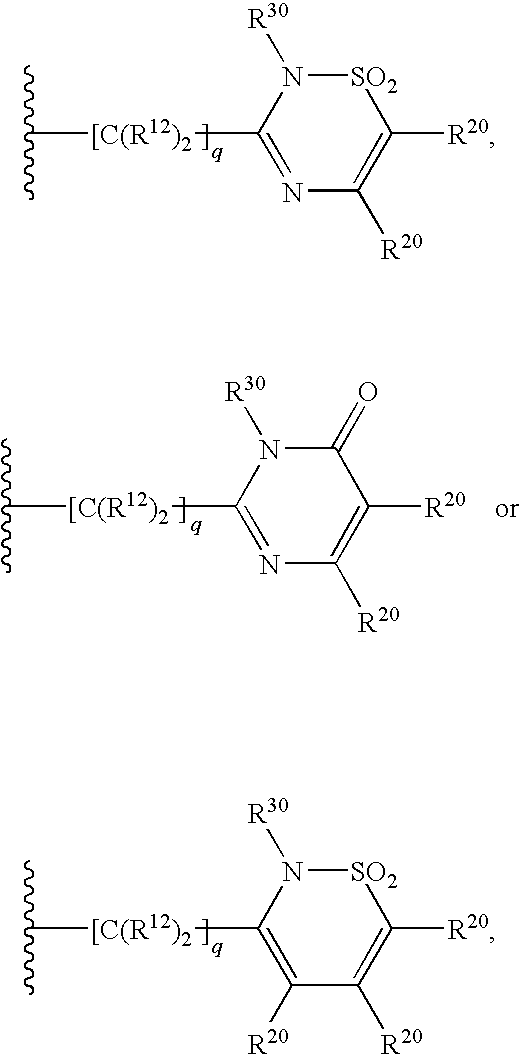
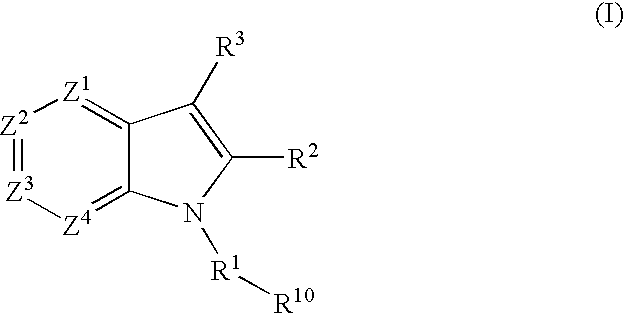
4,5-ring annulated indole derivatives for treating or preventing of hcv and related viral infections
The present invention relates to 4,5-ring annulated indole derivatives, compositions comprising at least one 4,5-ring annulated indole derivatives, and methods of using the 4,5-ring annulated indole derivatives for treating or preventing a viral infection or a virus-related disorder in a patient. Wherein ring Z, of formula (I), is a cyclopentyl, cyclopentenyl, 5-membered heterocycloalkyl, 5-membered heterocycloalkenyl or 5-membered heteroaryl ring.
Owner:MERCK SHARP & DOHME CORP
Antivirals that target transporters, carriers, and ion channels
InactiveUS20110038852A1Avoid virus infectionOrganic active ingredientsCompound screeningSelect agentAntiviral drug
This invention provides methods for preventing or treating infection by viruses, in particular an influenza virus by modulating transporters, carriers, and ion channels. Methods to identify, validate, and classify the cellular proteins required by viruses during infection of host cells in order to select agents which can inhibit viral infection are described herein. The method employs a siRNA screening platform and uses gene silencing to map the ‘viral infectome’—a compilation of cellular proteins that the virus needs to establish infection and drive the infectious cycle. Charting the infectome provides information on the viral biology by the identification of host cell proteins involved in viral infection and allows the development of novel anti-viral drugs that prevent the viruses from establishing productive infection in cells.
Owner:3 V BIOSCI INC
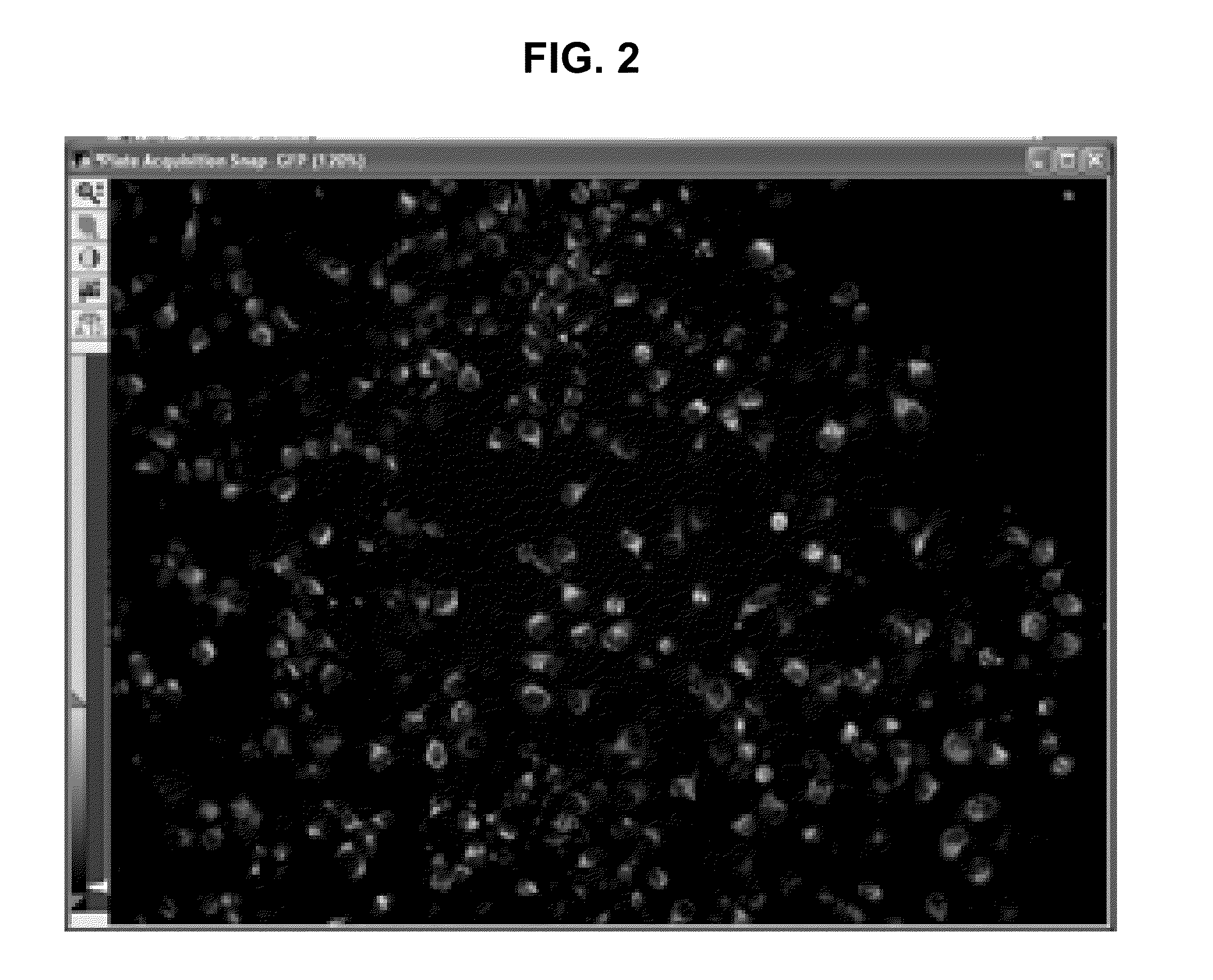
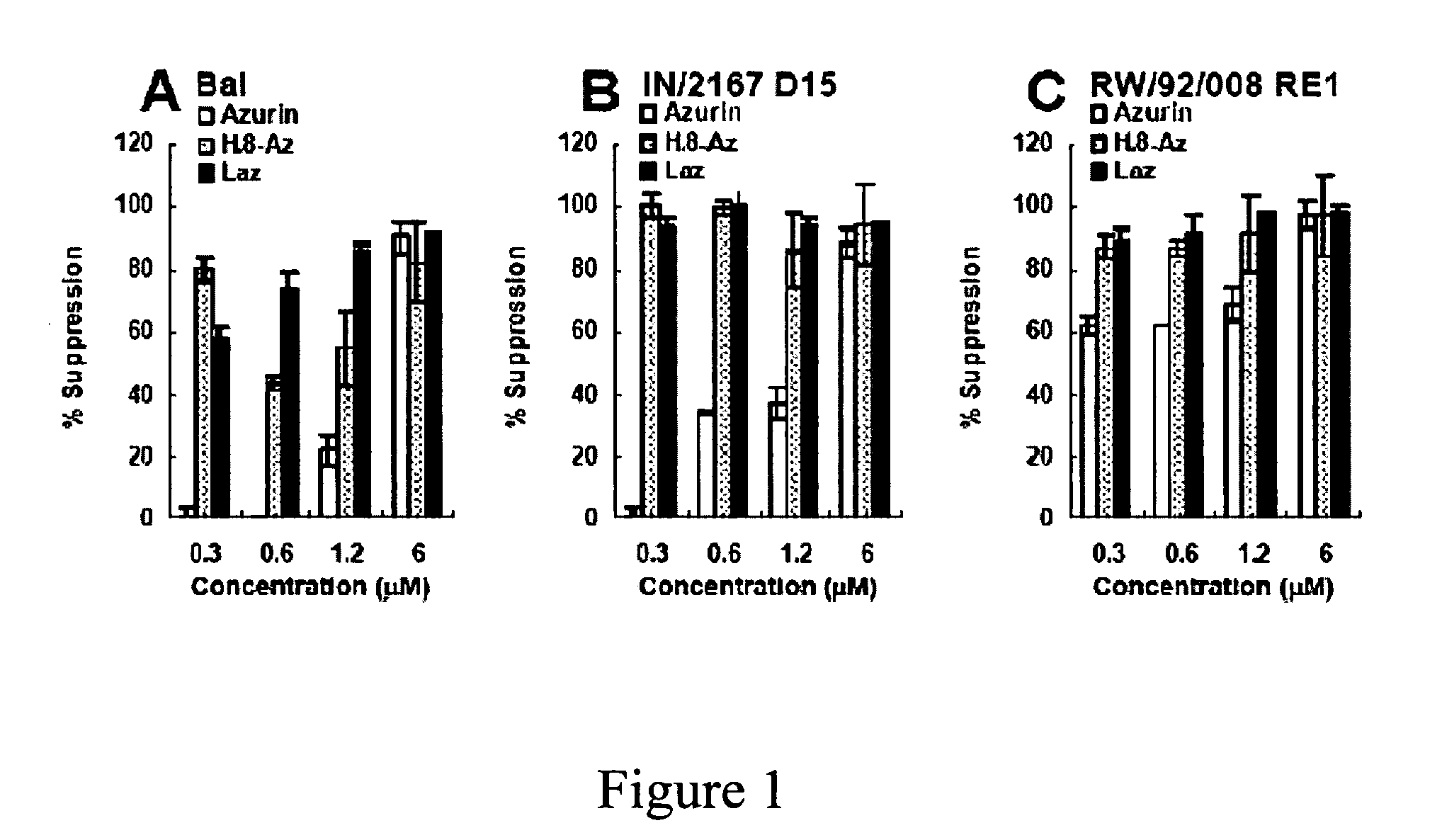
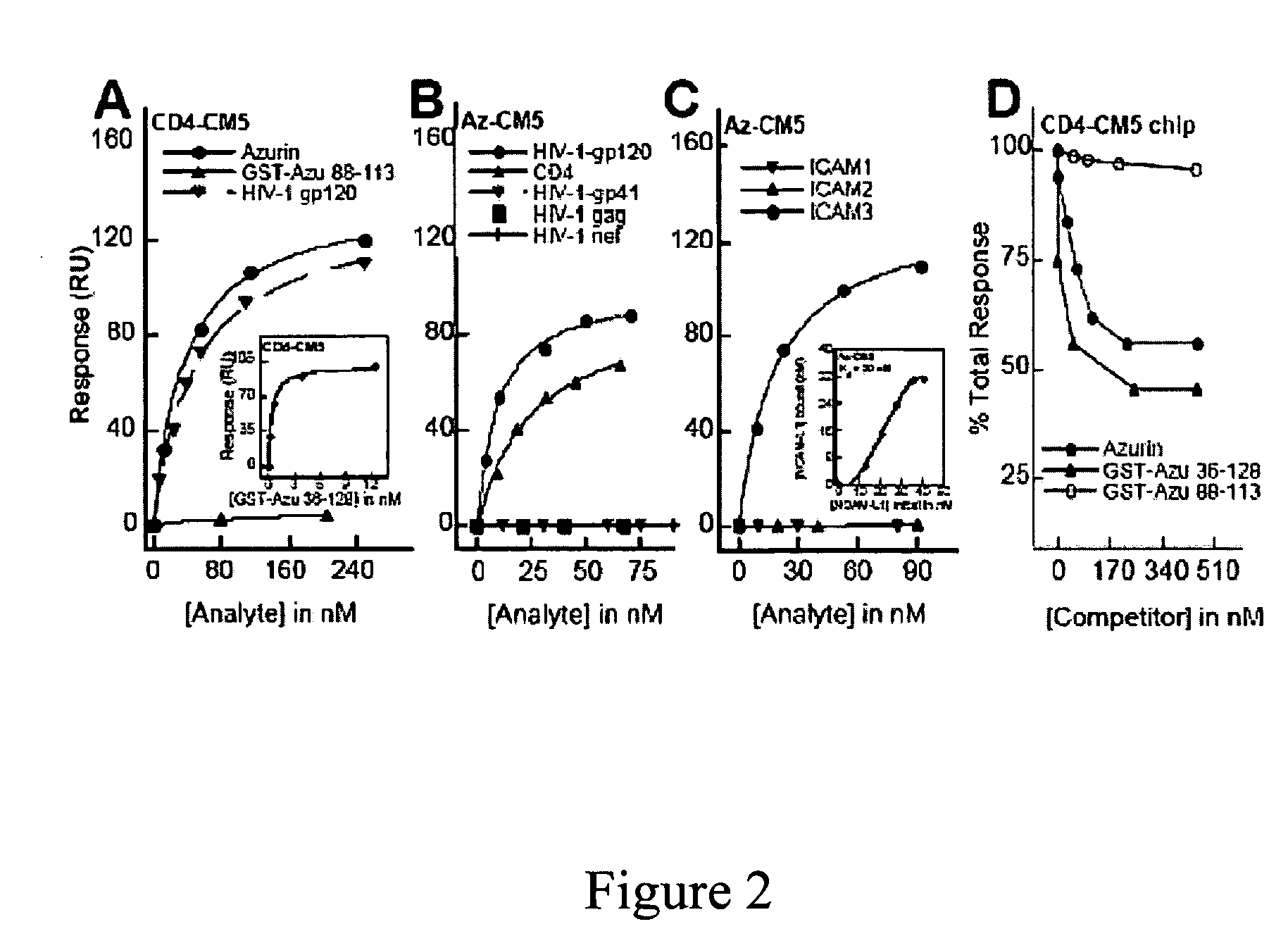
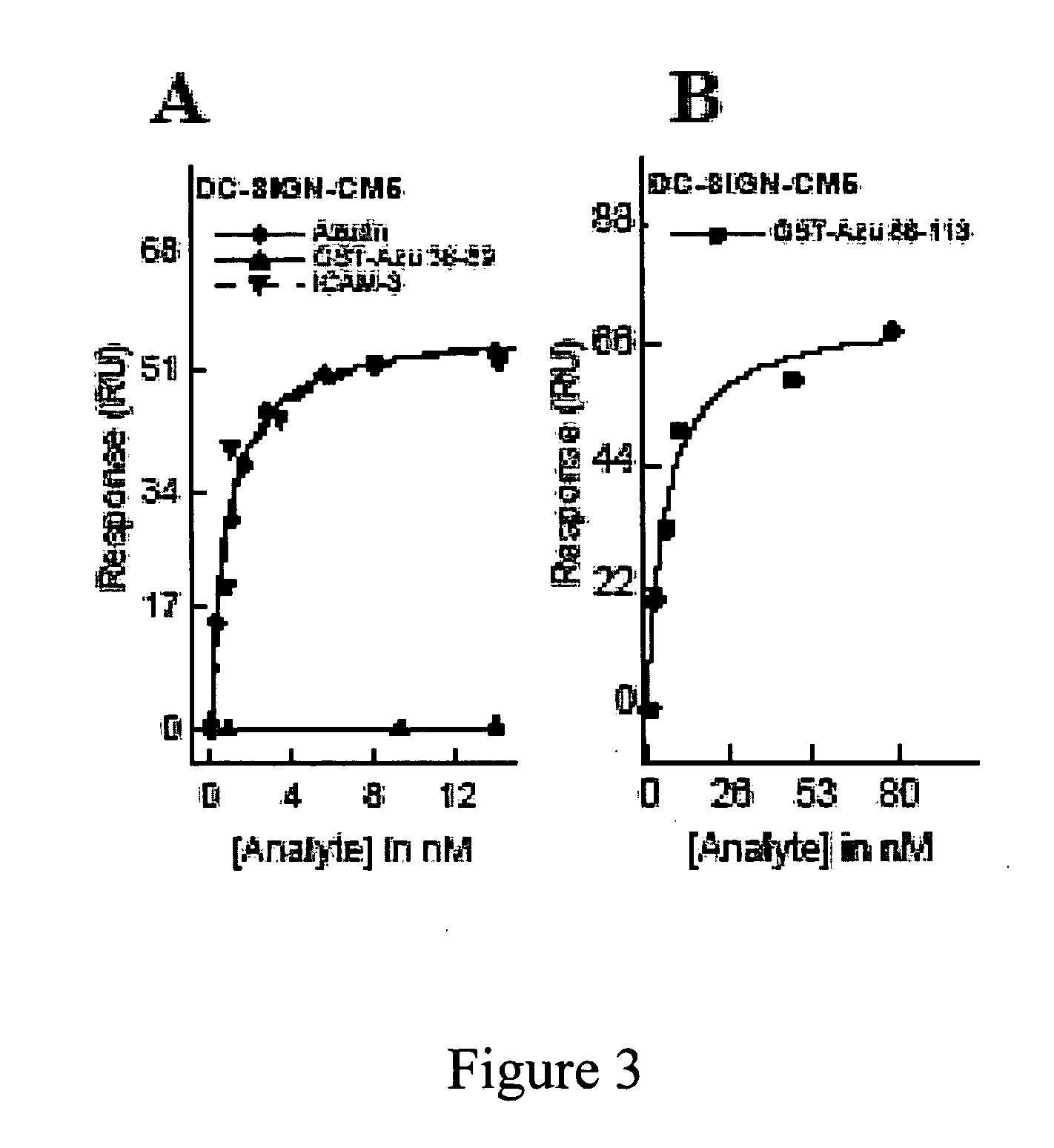
Compositions and methods for treating HIV infection with cupredoxin and cytochrame C
InactiveUS20060251639A1Avoid virus infectionGrowth inhibitionCompound screeningApoptosis detectionBiologyPseudomonas aeruginosa
The present invention relates to cupredoxin, specifically Pseudomonas aeruginosa azurin, and / or Pseudomonas aeruginosa cytochrome C551 and their use in inhibiting of viral infection, and in particular infection of mammalian cells by the Human Immunodeficiency Virus (HIV). The invention also relates to variants and derivatives of cupredoxin and cytochrome c that retain the ability to inhibit viral infection, and in particular infection by the Human Immunodeficiency Virus (HIV). The invention also relates to research methods for studying viral and bacterial infection in mammalian cells.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
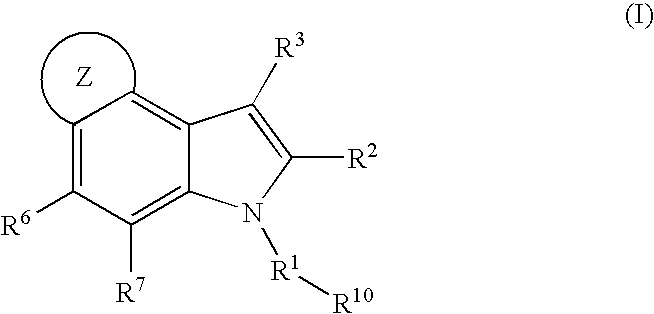
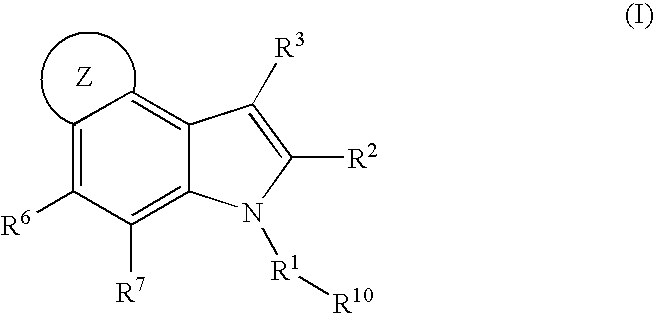
4,5-ring annulated indole derivatives for treating or preventing of HCV and related viral infections
The present invention relates to 4,5-ring annulated indole derivatives, compositions comprising at least one 4,5-ring annulated indole derivatives, and methods of using the 4,5-ring annulated indole derivatives for treating or preventing a viral infection or a virus-related disorder in a patient. Wherein ring Z, of formula (I), is a cyclopentyl, cyclopentenyl, 5-membered heterocycloalkyl, 5-membered heterocycloalkenyl or 5-membered heteroaryl ring.
Owner:MERCK SHARP & DOHME CORP
Heavy metal contaminated soil remediation agent and preparation method and application method thereof
InactiveCN110564425AEnhance the activity of organic matterBoost Activation RegulatorAgriculture tools and machinesContaminated soil reclamationPesticide residueMicrobial agent
The invention discloses a heavy metal contaminated soil remediation agent. The remediation agent is prepared from the following raw materials in parts by weight: 100-200 parts of humic acid, 50-100 parts of biochar, 20-30 parts of composite minerals, 1-5 parts of a compound enzyme preparation and 1-5 parts of a compound microbial agent. The preparation method comprises the following steps:(1)weighing the raw materials;(2)mixing the humic acid, the biochar and the composite minerals, crushing, sieving, adding water, heating, stirring, and cooling to obtain a mixture A for later use;(3)mixing the compound enzyme preparation and the compound microbial agent, adding water and a carbon source, and performing habituated culture to obtain a mixture B; and(4)uniformly mixing the mixture A and themixture B, and drying to obtain the product. According to the remediation agent, mild enzymes and microbial strains are adopted for treating soil, heavy metal and pesticide residues are degraded and adsorbed, and the soil quality condition of the soil is obviously improved.
Owner:赵龙
2,3-substituted azaindole derivatives for treating viral infections
Owner:MERCK SHARP & DOHME LLC
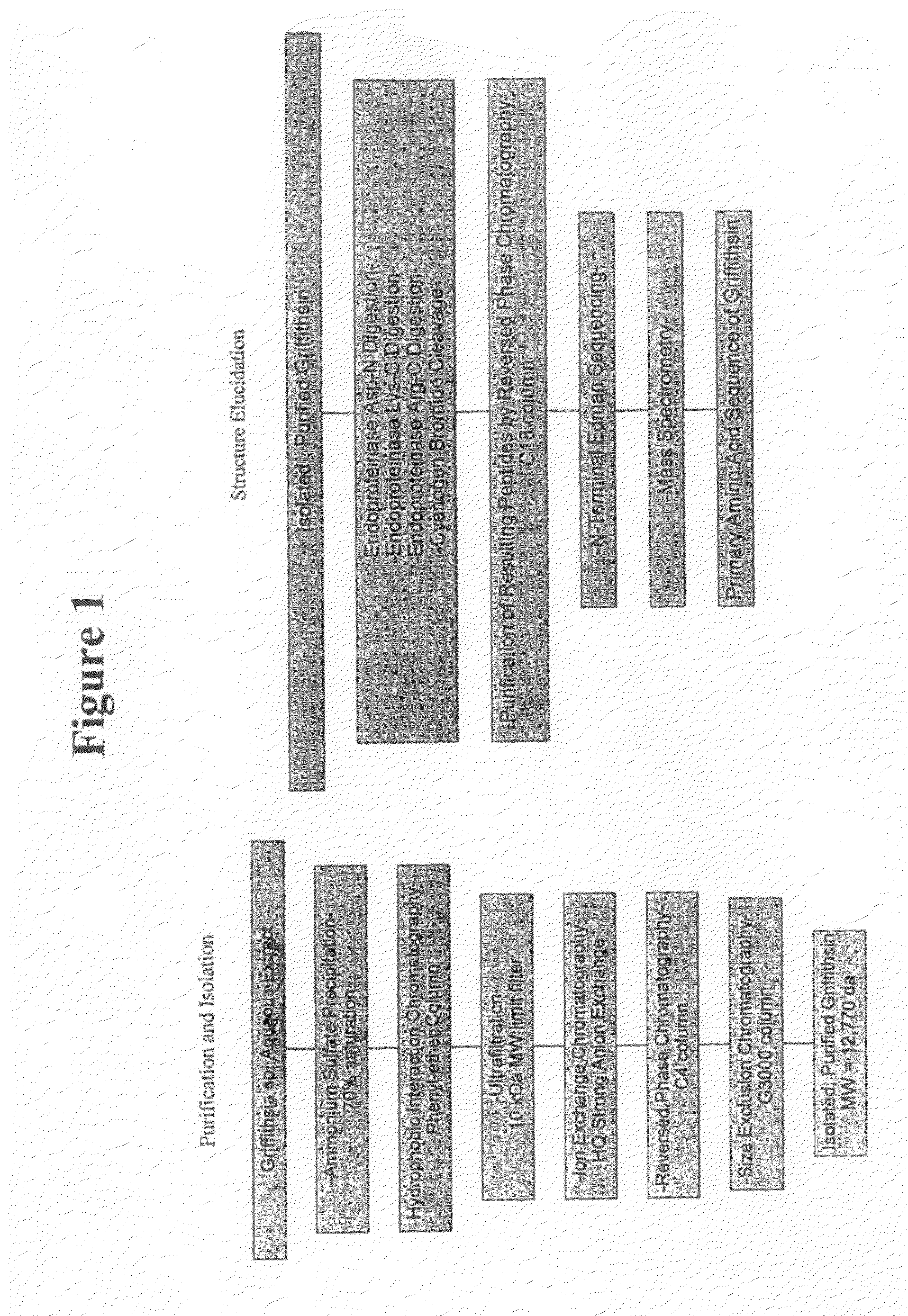
Griffithsin, glycosylation-resistant griffithsin, and related conjugates, compositions, nucleic acids, vectors, host cells, methods of production and methods of use
ActiveUS20090092557A1Avoid virus infectionAvoid infectionOrganic active ingredientsPeptide/protein ingredientsViral infectionMethods of production
An isolated and purified nucleic acid molecule that encodes a polypeptide comprising at least eight contiguous amino acids of SEQ ID NO: 3, wherein the at least eight contiguous amino acids have anti-viral activity, as well as an isolated and purified nucleic acid molecule that encodes a polypeptide comprising at least eight contiguous amino acids of SEQ ID NO: 3, wherein the at least eight contiguous amino acids have anti-viral activity, and, when the at least eight contiguous amino acids comprise amino acids 1-121 of SEQ ID NO: 3, the at least eight contiguous amino acids have been rendered glycosylation-resistant, a vector comprising such an isolated and purified nucleic acid molecule, a host cell comprising the nucleic acid molecule, optionally in the form of a vector, a method of producing an anti-viral polypeptide or conjugate thereof, the anti-viral polypeptide itself, a conjugate or fusion protein comprising the anti-viral polypeptide, and compositions comprising an effective amount of the anti-viral polypeptide or conjugate or fusion protein thereof. Further provided are methods of inhibiting prophylactically or therapeutically a viral infection of a host.
Owner:UNITED STATES OF AMERICA
Multivalent avian influenza vaccines
ActiveUS20060204976A1Rapid onsetPreventing or ameliorating avian influenzaSsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus
A vaccine composition and method which is effective in preventing or ameliorating Avian Influenza Virus infection is set forth herein. The vaccine contains at least two inactivated strains of avian influenza virus, wherein the combined haemoagglutinin (HA) total is at least about 200 HA / dose of the vaccine composition, and wherein each of the strains presents at least about 128 HA / dose, and further wherein one of the strains has the same HA subtype as that of a challenge virus, and wherein at least one of the strains has a different NA subtype than the challenge virus.
Owner:ZOETIS SERVICE LLC
Methods of reducing a viral infection and kits therefore
InactiveUS20090104209A1Lower Level RequirementsHigh activityBiocideCompound screeningSubtilisinKexin
A method for treating and / or preventing a proprotein convertase subtilisin / kexin type 9 preproprotein (PCSK9)-susceptible viral infection comprising increasing a PCSK9 activity and / or expression in a biological system infected by the virus, whereby the increased PCSK9 activity and / or expression treats and / or prevents the viral infection in the biological system. Methods of classifying subjects, methods of screening and kits therefore.
Owner:INSTITUT NATIONAL DE LA RECHERCHE SCIENTIFIQUE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com