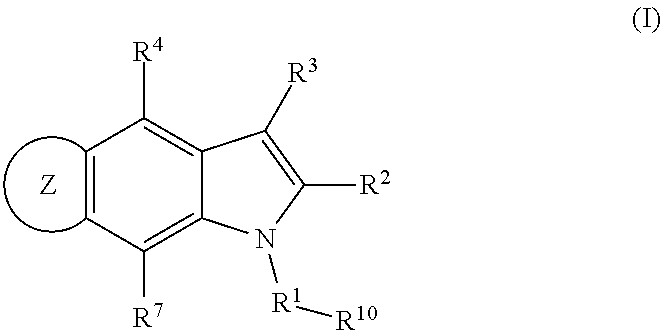
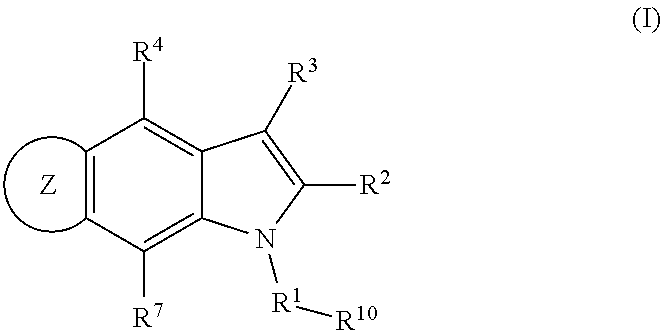
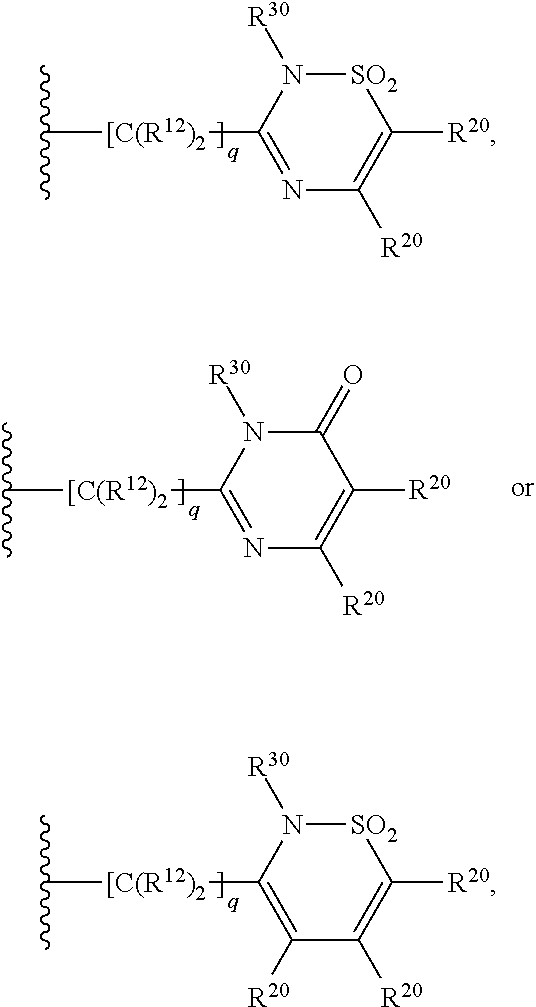
5, 6-ring annulated indole derivatives and use thereof
a technology of indole derivatives and ring annulations, which is applied in the direction of indole-indigos, phosphorous compound active ingredients, drug compositions, etc., can solve the problems of poor treatment effect, poor efficacy of hcv infection approaches, and hcv infection complications, etc., to achieve the effect of improving the safety and efficacy of treatment and improving the safety of treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compound 3
[0467]
Step A
[0468]
[0469]To a solution of 5-aminoindane (3A) (10 g, 75.1 mmol) in EtOAc (50 mL) was added acetic anhydride (8.4 g) and pyridine (6.5 g). The resulting reaction was allowed to stir at room temperature overnight. Et2O (80 mL) was added to the reaction mixture and and the resulting solution was filtered. The collected solid was washed with Et2O (50 mL), then hexane (50 mL) to provide compound 3B (9.92 g) as a gray solid, which was used without further purification. 1H NMR (400 MHz, CDCl3) 7.44 (s, 1H), 7.22 (bs, 1H), 7.14-7.11 (m, 2H), 2.90-2.83 (m, 4H), 2.16 (s, 3H), 2.10-2.02 (m, 2H).
Step B
[0470]
[0471]Bromine (3.4 mL) was added dropwise to a 4° C. solution of compound 3B (9.92 g) in acetic acid (165 mL) at 4° C. When the addition was complete the reaction mixture was stirred for 1 hour at 4° C. and the reaction mixture was then partitioned between EtOAc and 10% aqueous sodium sulfate and the organic phase was separated, washed with saturated s...
example 2
Preparation of Compound 7
[0484]
[0485]To a solution of freshly made sodium methoxide (prepared by dissolving NaOMe (1.72 g, 75.0 mmol) in methanol (30 mL)) was added dropwise to a solution of ethylazidoacetate (3.35 g, 26 mmol), and compound 7A (2 g, 13.6 mmol) in methanol (10 mL) which had been precooled to −20° C. The reaction mixture was stirred at room temperature for 2 hours, then diluted with EtOAc (200 mL). The organic layer was dried (MgSO4), filtered, concentrated in vacuo and purified using flash column chromatography on silica gel (EtOAc / Hexanes) to provide compound 7B (2.7 g) as a colorless liquid.
Step B
[0486]
[0487]To a solution of compound 7B (250 mg) in xylenes (5 mL) was heated at reflux for 30 minutes, then cooled to room temperature and concentrated in vacuo. The resulting residue was purified using flash column chromatography on silica gel using CH2Cl2 / Hexanes (0 to 50% CH2Cl2) to provide compound 7C as a colorless solid which was used in next step without purifica...
example 3
Preparation of Compound 11
[0500]
Step A
[0501]
[0502]A solution of 1,3-benzothiazol-5-amine, (11A, Maybridge, 16 g, 107 mmol) in concentrated HCl (180 mL) was cooled to −10° C. and to the cooled solution was added very slowly a solution of sodium nitrite (7.66 g,111 mmol) in water (35 mL). After the addition was complete, the reaction mixture was vigorously stirred at −5° C. to 0° C. for 30 minutes. To the reaction mixture was then added, dropwise, a solution of tin(II) chloride (81.0 g, 359 mmol) in concentrated HCl (60 mL). The internal reaction temperature was maintained at or below −5° C. during the addition. The resulting suspension was stirred at −10° C. for about 90 minutes, during which time the reaction mixture was allowed to warm to room temperature. The resulting precipitates were filtered off and the flask was rinsed with small amount of water. The collected solids were dissolved into water (100 mL), and Na2S.9H2O (39 g) was added. The aqueous layer was adjusted to pH 11 u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com