Vaccinia virus mutants useful for cancer immunotherapy
A vaccinia, virus strain technology, applied in the field of virology, immunotherapy, oncology, can solve the problem of the immune system is not activated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
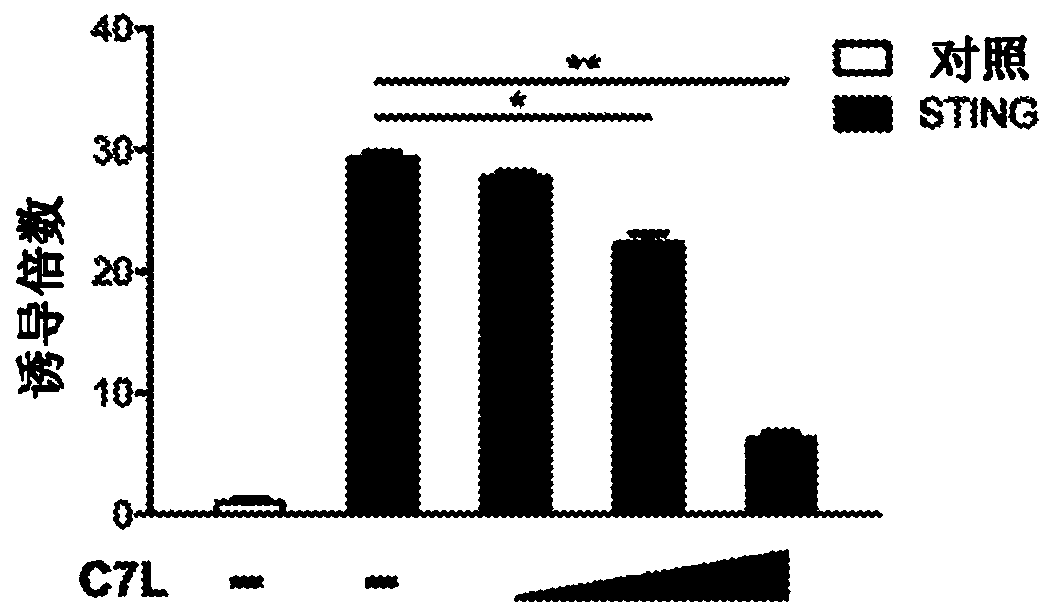
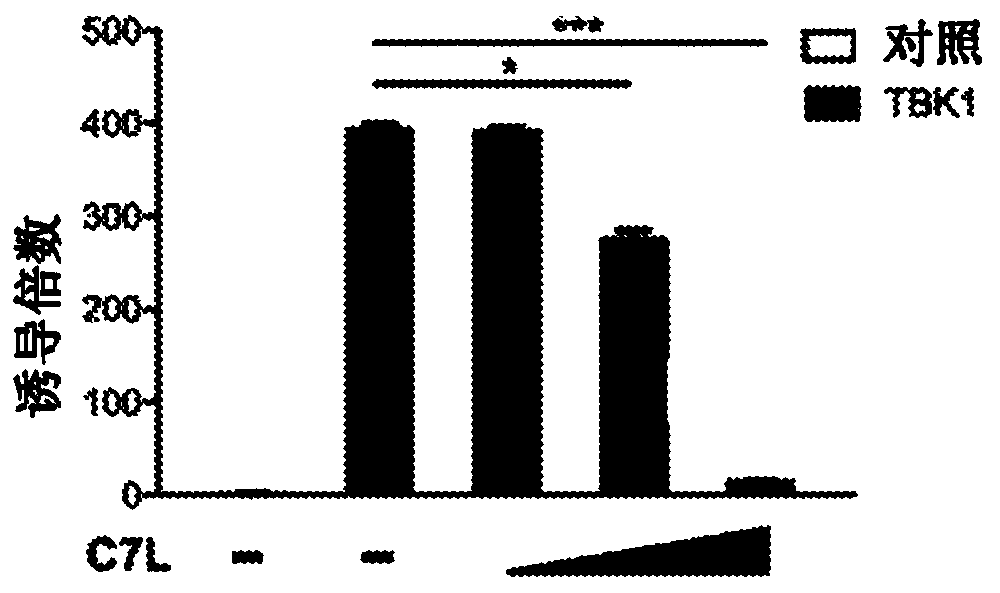
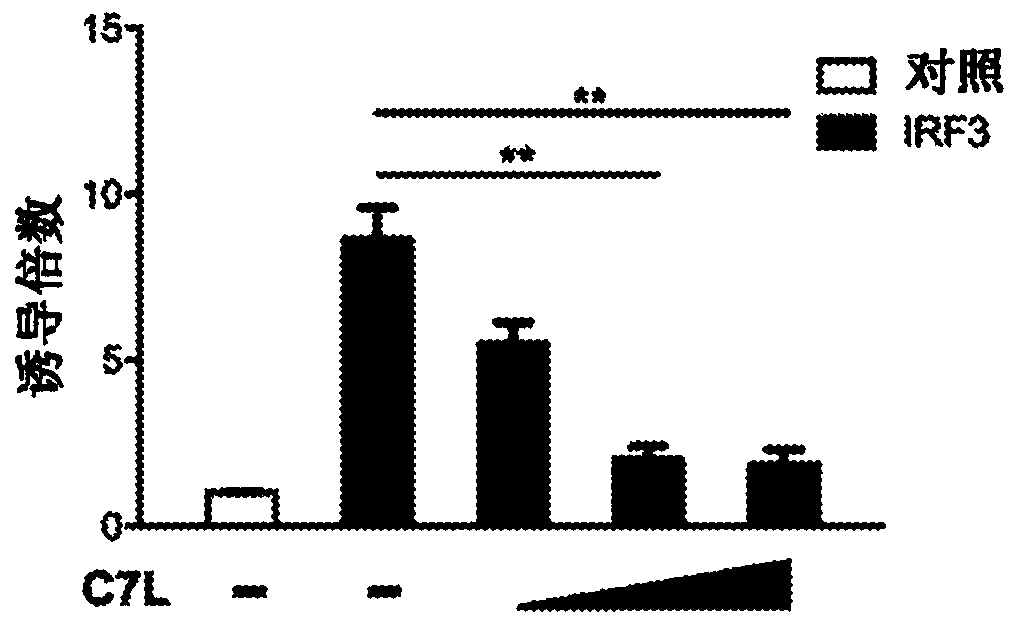
[0182] Example 1: Vaccinia C7 inhibits IFN gene induction mediated by STING, TBK1 and IRF3.
[0183] The dual luciferase assay system was used to evaluate the role of vaccinia C7 in the regulation of STING, TBK1 or IRF3-induced IFNB promoter activation in HEK293T cells, which are human embryonic kidneys transformed with SV40 large T antigen Cell line. HEK293T cells were transfected with a plasmid expressing the IFNB firefly luciferase reporter, a control plasmid pRL-TK expressing Renilla luciferase, STING, and vaccinia C7L as indicated. A dual luciferase assay was performed 24h after transfection. By normalizing the firefly luciferase activity with respect to the Renilla luciferase activity, the relative luciferase activity is expressed in arbitrary units. Compared with the IFNB promoter activity in the control sample without STING, the overexpression of STING resulted in 30-fold induction of IFNB promoter activity. Co-transfection of increasing amounts of C7L expression plas...
Embodiment 2
[0184] Example 2: Vaccinia C7 inhibits poly I:C (TLR3) or TRIF-mediated IFN gene induction.
[0185] The TBK1-IRF3 axis is important for signal transduction from multiple sensing pathways, including cGAS-cGAMP-STING, RIG-I or MDA5-MAVS, TLR3-TRIF, and TLR4-TRIF. In order to test whether vaccinia C7 has the inhibitory effect of TRIF signaling, the inventors transfected HEK293T cells with TLR3 expression plasmid, IFN-β-luc reporter and increasing amounts of C7 expression plasmid (10ng, 50ng or 250ng). After 24h, the cells were treated with poly I:C (5 μg / ml). The luciferase activity was measured 24h after poly I:C treatment. Compared with the empty vector control, TLR3 transfection and poly I:C treatment resulted in a 9-fold induction of IFNB promoter activity ( Figure 2A ). The overexpression of C7 resulted in poly(I:C) / TLR3-induced reduction of IFNB promoter activity by up to 90% ( Figure 2A ). To test whether C7 also inhibits TRIF-induced IFNB promoter activity, HEK293T ce...
Embodiment 3
[0186] Example 3: Overexpression of vaccinia C7 in immune cells inhibits IFNB gene induction.
[0187] To evaluate the role of vaccinia C7 in the induction of IFNB gene in immune cells, we generated two cell lines stably expressing vaccinia C7, including murine macrophages RAW264.7 and human THP-1. THP-1 is a human monocytic leukemia cell line, which has been widely used to study the function and immune regulation of human monocytes and macrophages. Briefly, RAW264.7 and THP-1 were transduced with a retrovirus containing a vaccinia C7 expression construct under the CMV promoter and puromycin selection marker. An empty vector with a drug selection marker was also used to generate control cell lines. The drug-resistant cells were obtained and used in the following experiments. Before the THP-1 stable cell lines expressing C7 or with an empty vector were used in experiments, they were differentiated by phorbol-12-myristate-13-acetate (PMA; 20ng / ml) for 3 days. Cells were infecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com