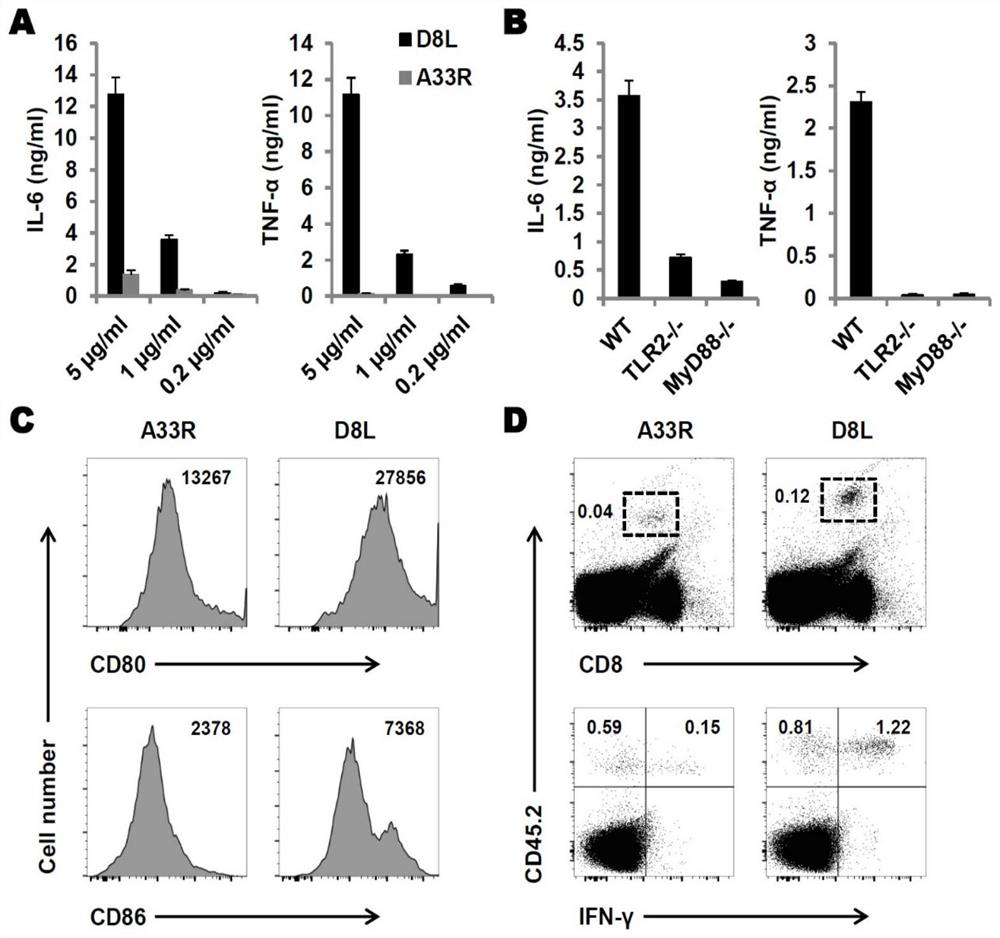
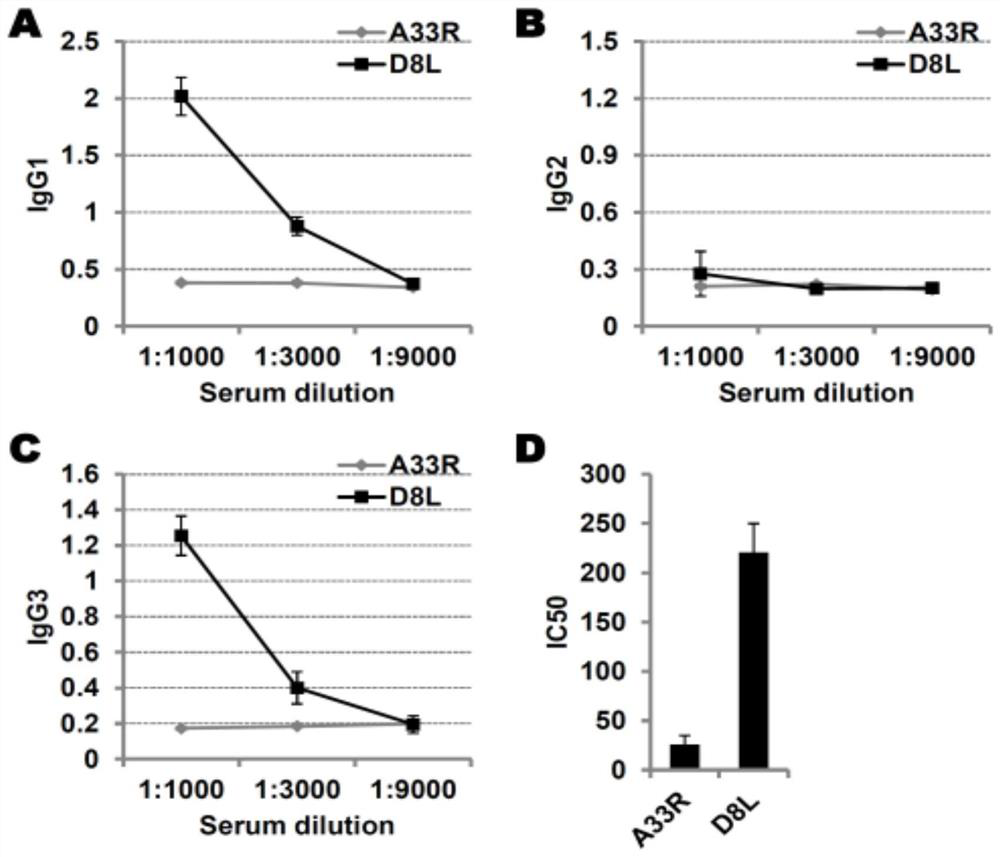
A kind of protein vaccinia vaccine and its efficacy detection method
A vaccinia vaccine and protein technology, applied in the field of vaccines, can solve problems such as unclear mechanism of action, and achieve the effect of preventing smallpox virus infection and excellent immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The preparation method of above-mentioned protein vaccinia vaccine comprises the following steps:
[0056] 1) Spin column treatment: regenerate and balance the resin in the spin column after the first use and after each use, the specific method is:
[0057] 1.1) Return the spin column to room temperature;
[0058] 1.2) Unscrew the switch at the bottom of the spin column, unscrew the top cap, place the spin column in the collection tube, centrifuge, remove the storage liquid in the spin column, and discard the liquid in the collection tube;
[0059] 1.3) Remove the top cap, plug the bottom plug, add 0.15-0.25 N NaOH to the spin column for regeneration, put on a new top cap, invert the spin column and mix several times until the resin in the spin column is completely suspended in the liquid , standing overnight at room temperature;
[0060] 1.4) Loosen the top cap, remove the bottom plug, place the spin column in the collection tube, centrifuge, and discard the liquid i...
Embodiment 1
[0085] 1. The endotoxin removal steps of virus recombinant protein:
[0086] Use the "Pierce™ High Capacity Endotoxin RemovalSpin Columns, 0.25 mL" kit from Thermo Scientific for endotoxin removal. The following is the specific operation procedure:
[0087] 1. The resin in the spin column needs to be regenerated and balanced after the first use and after each use. The specific operation is as follows:
[0088] 1.1. Return the spin column to room temperature.
[0089] 1.2. Unscrew the switch at the bottom of the spin column, unscrew the top cap, place the spin column in the collection tube, and centrifuge at 500xg for 1 min to remove the storage solution in the spin column. Discard the liquid in the collection tube.
[0090] 1.3. Take off the top cap, plug the bottom plug, add 0.2 N NaOH to the spin column for regeneration, put on a new top cap, turn the spin column upside down and mix several times until the resin in the spin column is completely suspended in the liquid . ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com