Patents
Literature
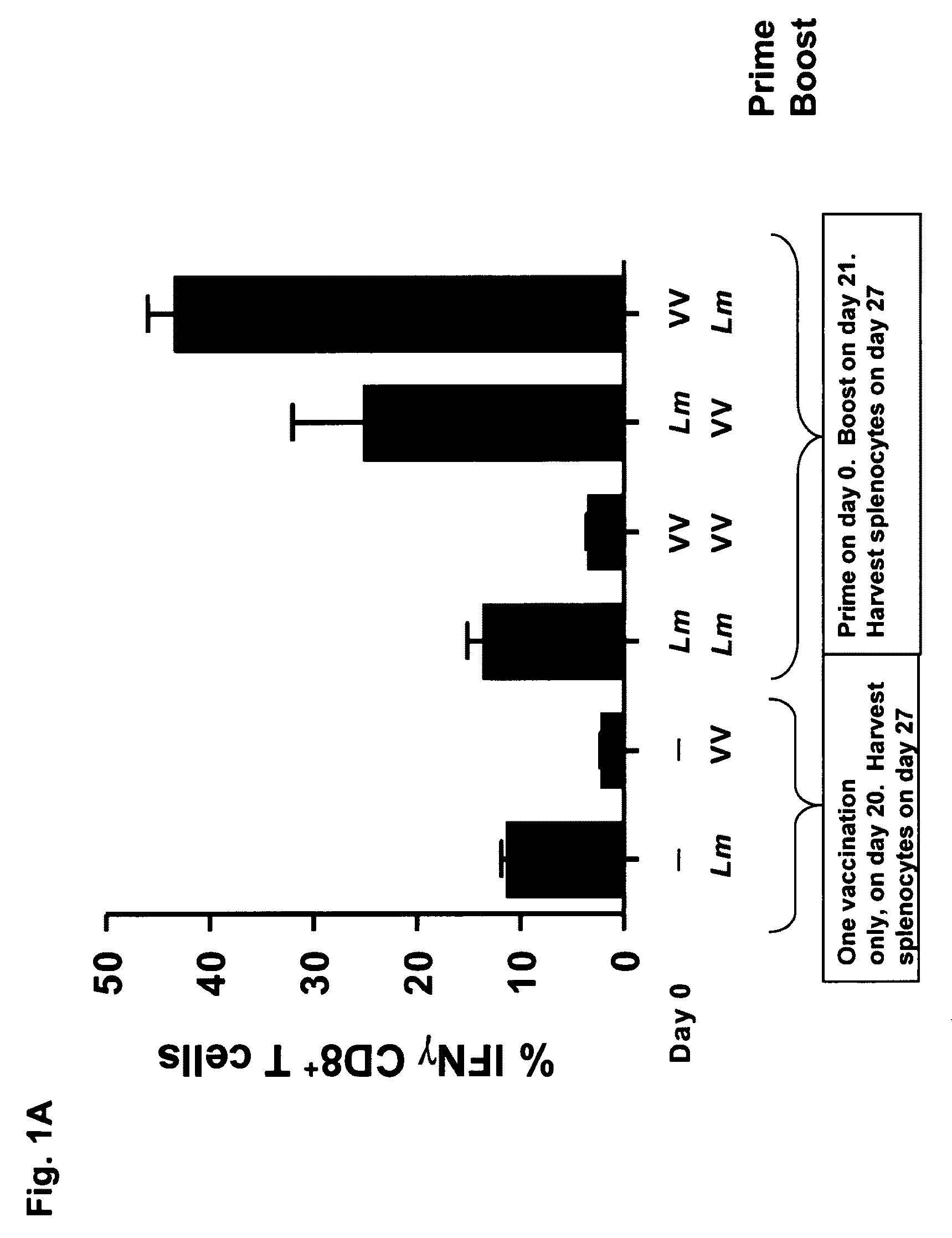
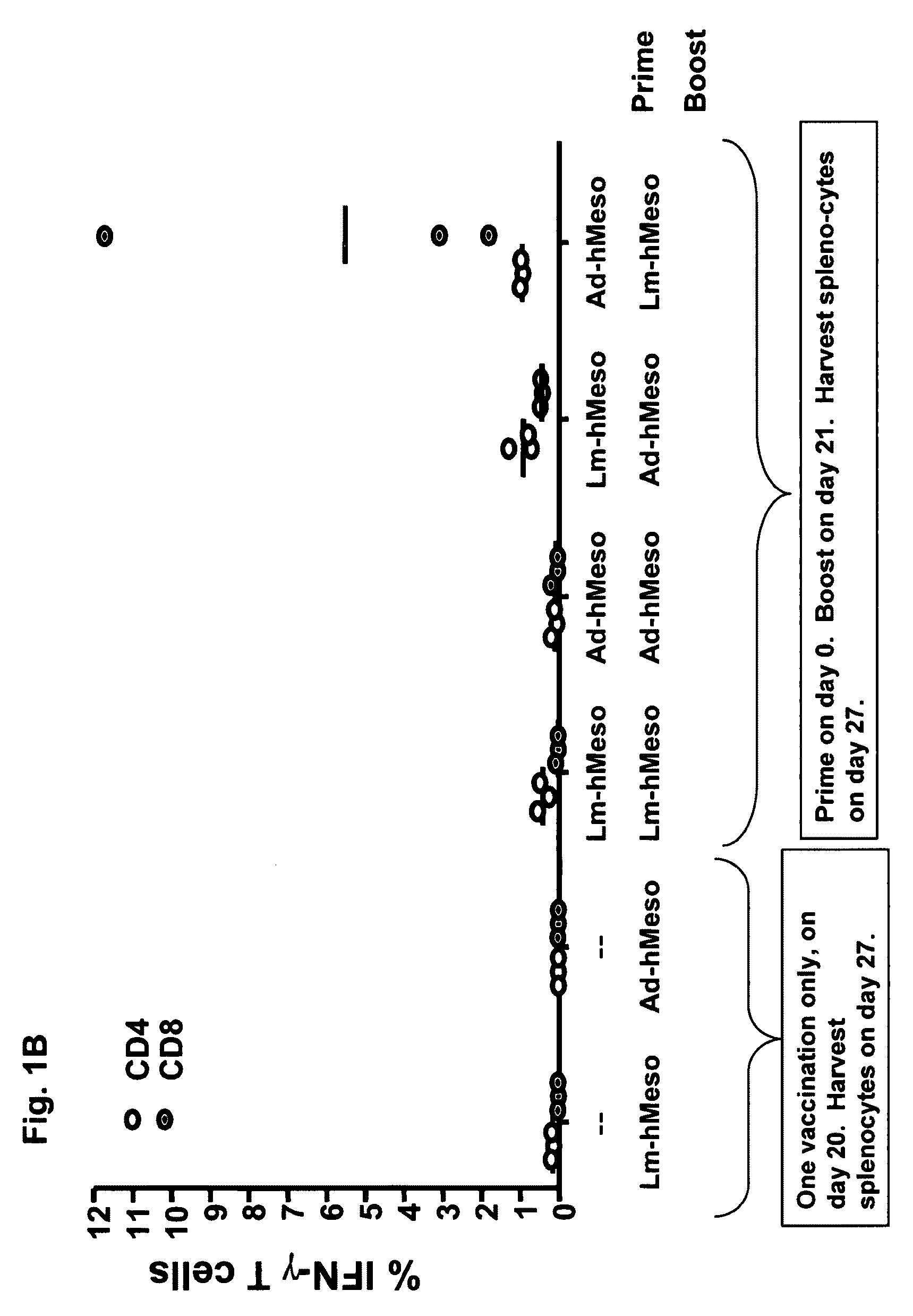
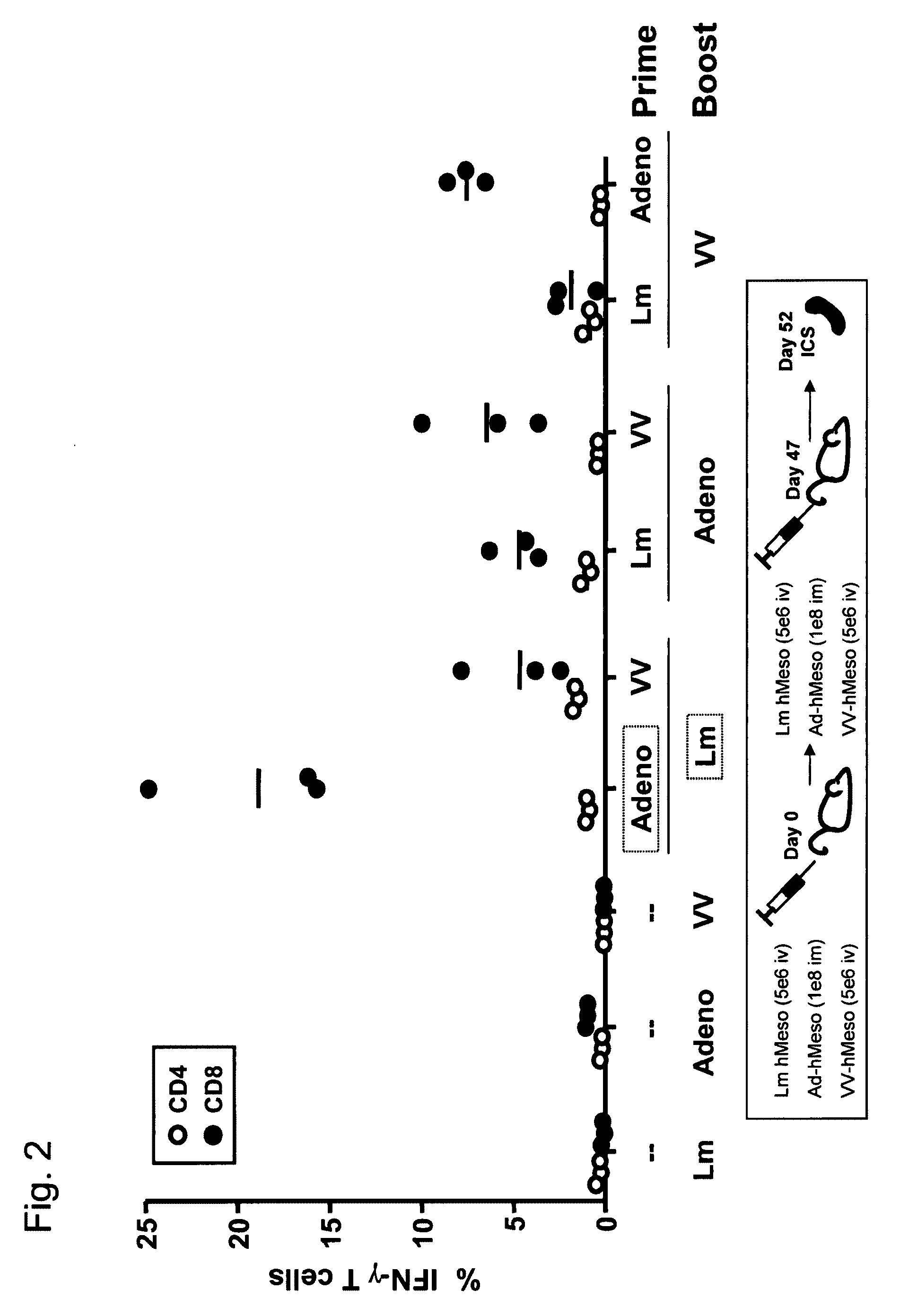
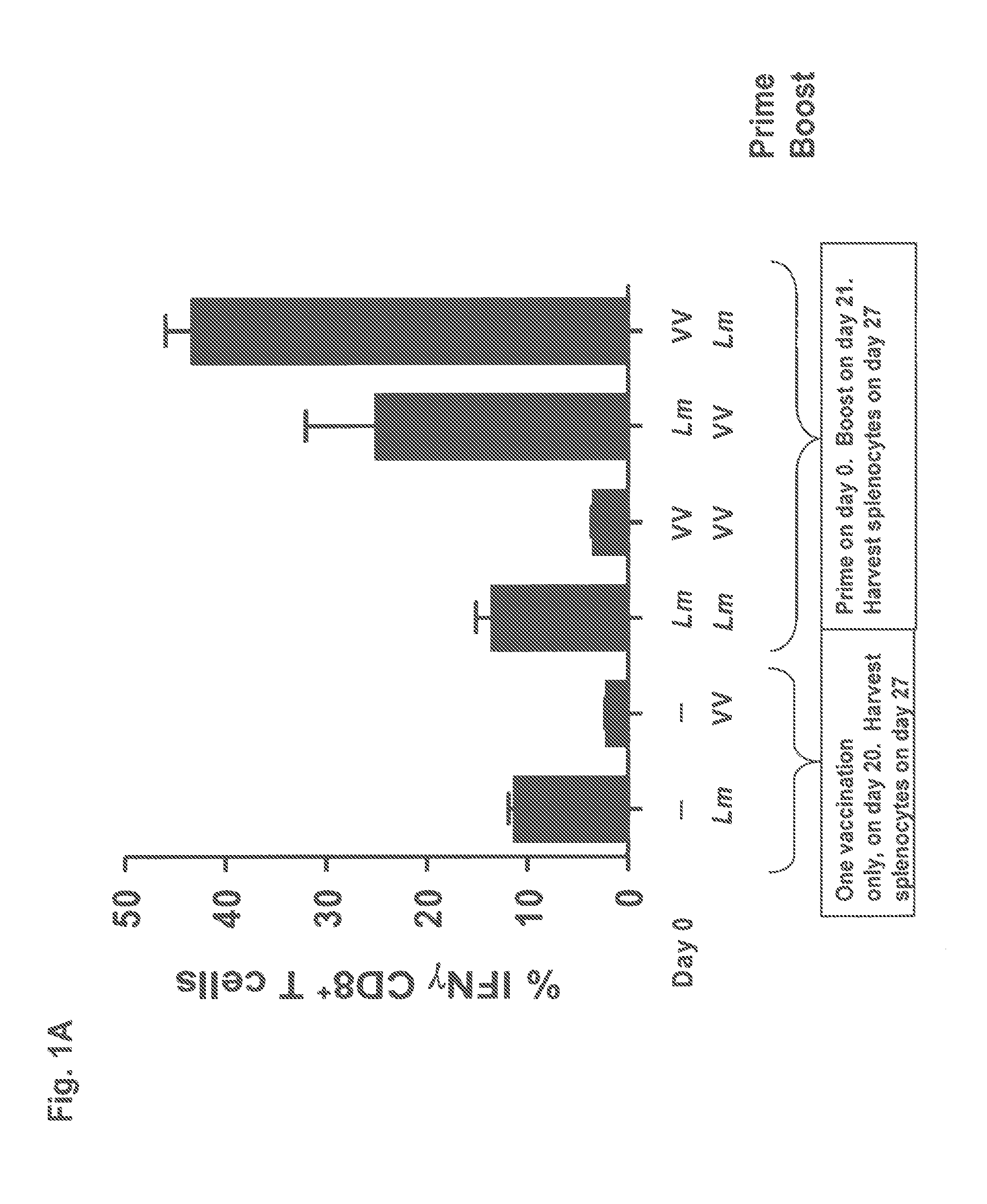
57 results about "Prime boost" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The prime-boost vaccination method appears to be more effective than the conventional single stage vaccination strategy, particularly in vaccinations against the influenza virus. The prime-boost is a vaccination strategy involving several heterogeneous phases.
Polypeptide vaccine and vaccination strategy against mycobacterium
A vaccine is provided wherein a polypeptide or combination of peptides from M. tuberculosis is administered to a subject to elicit an immune response. The polypeptide vaccine is administered as part of a prime-boost strategy with BCG vaccine to increase the immunoprotection in a subject such that prevention or elimination of disease is achieved. Finally, a pharmaceutical package is provided that encompasses a polypeptide vaccine for M. tuberculosis that when administered to a subject elicits immunoprotection.
Owner:EMORY UNIVERSITY +1
Recombinant vaccine against West Nile Virus
ActiveUS20050255127A1Provide securityPractical and convenientSsRNA viruses positive-senseViral antigen ingredientsAdjuvantRecombinant vaccines
An immunogenic or vaccine composition to induce an immune response or protective immune response against West Nile virus (WNV) in an animal susceptible to WNV. The composition includes a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector contains heterologous nucleic acid molecule(s), expresses in vivo in the animal WNV antigen, immunogen or epitope thereof, e.g., WNV E; WNV prM and E; WNV M and E; WNV prM, WNV M and E, WNV polyprotein prM-E, WNV polyprotein M-E, or WNV polyprotein prM-M-E. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL LTD
Recombinant vaccine against bluetongue virus
ActiveUS20070280960A1Provide securityPractical and convenientAntibacterial agentsViral antigen ingredientsAntigenAdjuvant
The present invention relates to an immunogenic or vaccine composition to induce an immune response or protective immune response against Orbiviruses, more specifically bluetongue virus (BTV) in an animal susceptible to BTV infection. The composition may include a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector may contain heterologous nucleic acid molecule(s), expresses in vivo in the animal BTV antigen, immunogen or epitope thereof, e.g., BTV VP2; BTV VP2 and VP5; BTV VP2 and VP5 and VP3 and / or VP7. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.AGACAGTGGTCAATTCCAATGGTACTGTTTGACGATAC
Owner:MERIAL LTD +3
Recombinant viral-based malaria vaccines
Owner:JANSSEN VACCINES & PREVENTION BV
Methods and compositions using Listeria for enhancing immunogenicity by prime boost
Provided herein are prime-boost regimens and materials used therein. The prime-boost regimens enhance the immune response to a target antigen. The vaccines used for boost are comprised of recombinant attenuated metabolically active Listeria that encodes an expressible antigen that is cross-reactive with the target antigen. In some examples, the immune response is a cellular immune response.
Owner:ANZA THERAPEUTICS INC +1
Antiviral agents and vaccines against influenza
InactiveUS20090208531A1SsRNA viruses negative-senseOrganic active ingredientsHemagglutininPrime boost
These vaccines target H5N1, H1, H3 and other subtypes of influenza and are designed to elicit neutralizing antibodies, as well as cellular immunity. The DNA vaccines express hemagglutinin (HA) or nucleoprotein (NP) proteins from influenza which are codon optimized and / or contain modifications to protease cleavage sites of HA which affect the normal function of the protein. Adenoviral constructs expressing the same inserts have been engineered for prime boost strategies. Protein-based vaccines based on protein production from insect or mammalian cells using foldon trimerization stabilization domains with or without cleavage sites to assist in purification of such proteins have been developed. Another embodiment of this invention is the work with HA pseudotyped lentiviral vectors which would be used to screen for neutralizing antibodies in patients and to screen for diagnostic and therapeutic antivirals such as monoclonal antibodies.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Methods and compositions using listeria for adjuvant treatment of cancer
InactiveUS9161974B2Easy to adaptBacterial antigen ingredientsBacteria material medical ingredientsRegimenPrime boost
Provided herein are prime-boost regimens and materials used therein. The prime-boost regimens enhance the immune response to a target antigen. The vaccines used for boost are comprised of recombinant attenuated metabolically active Listeria that encodes an expressible antigen that is cross-reactive with the target antigen. In some examples, the immune response is a cellular immune response.
Owner:ADURO BIOTECH
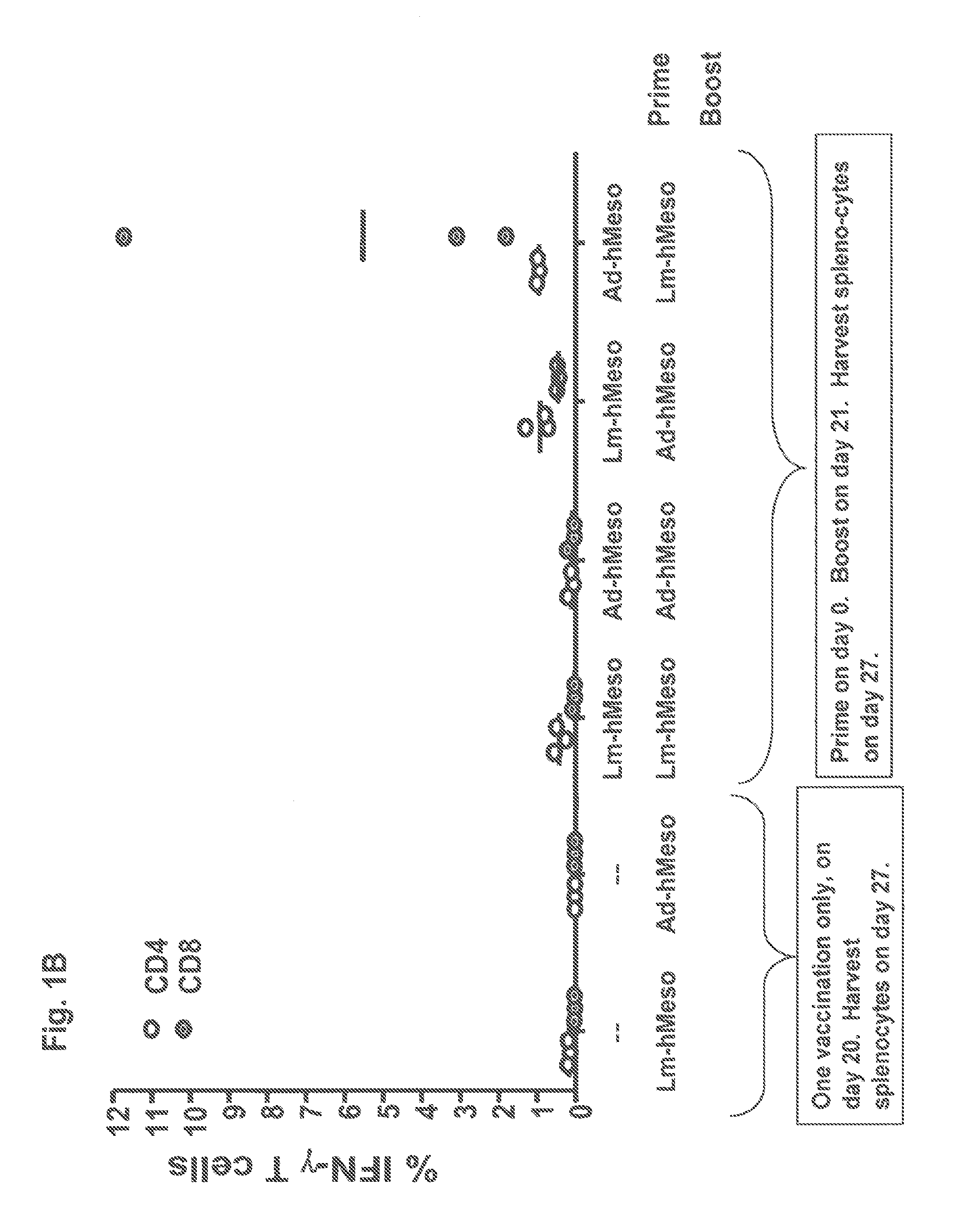
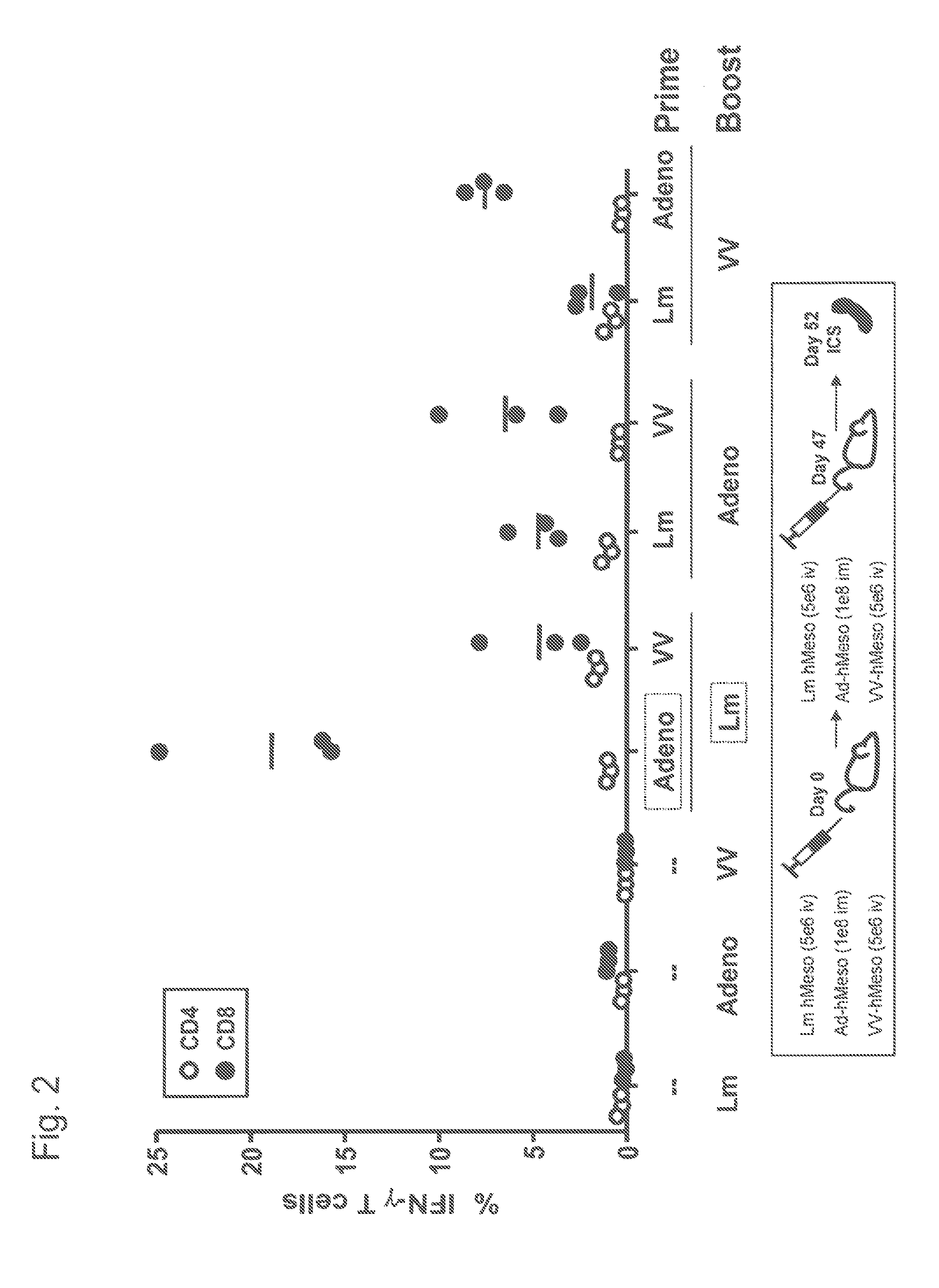
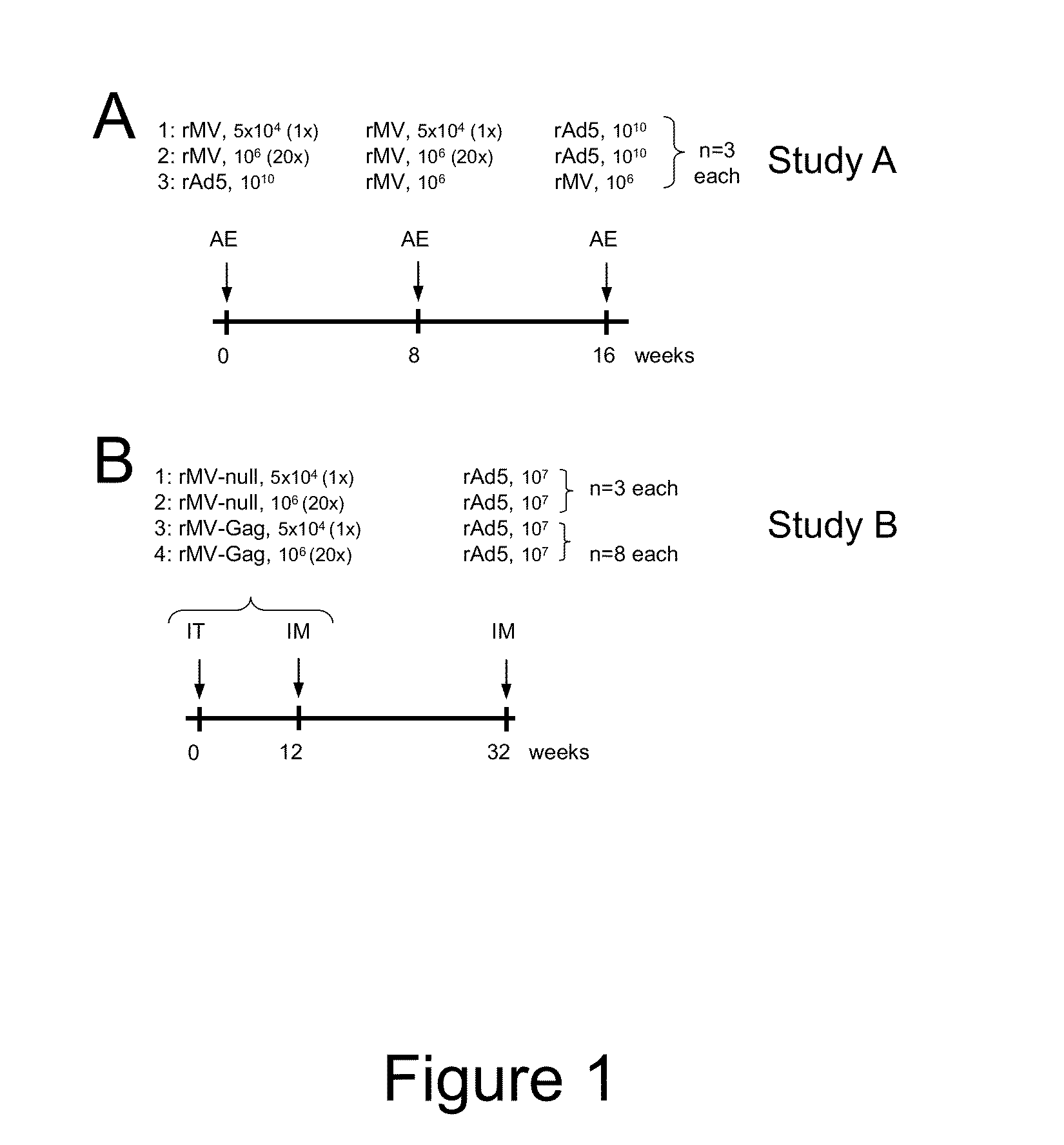
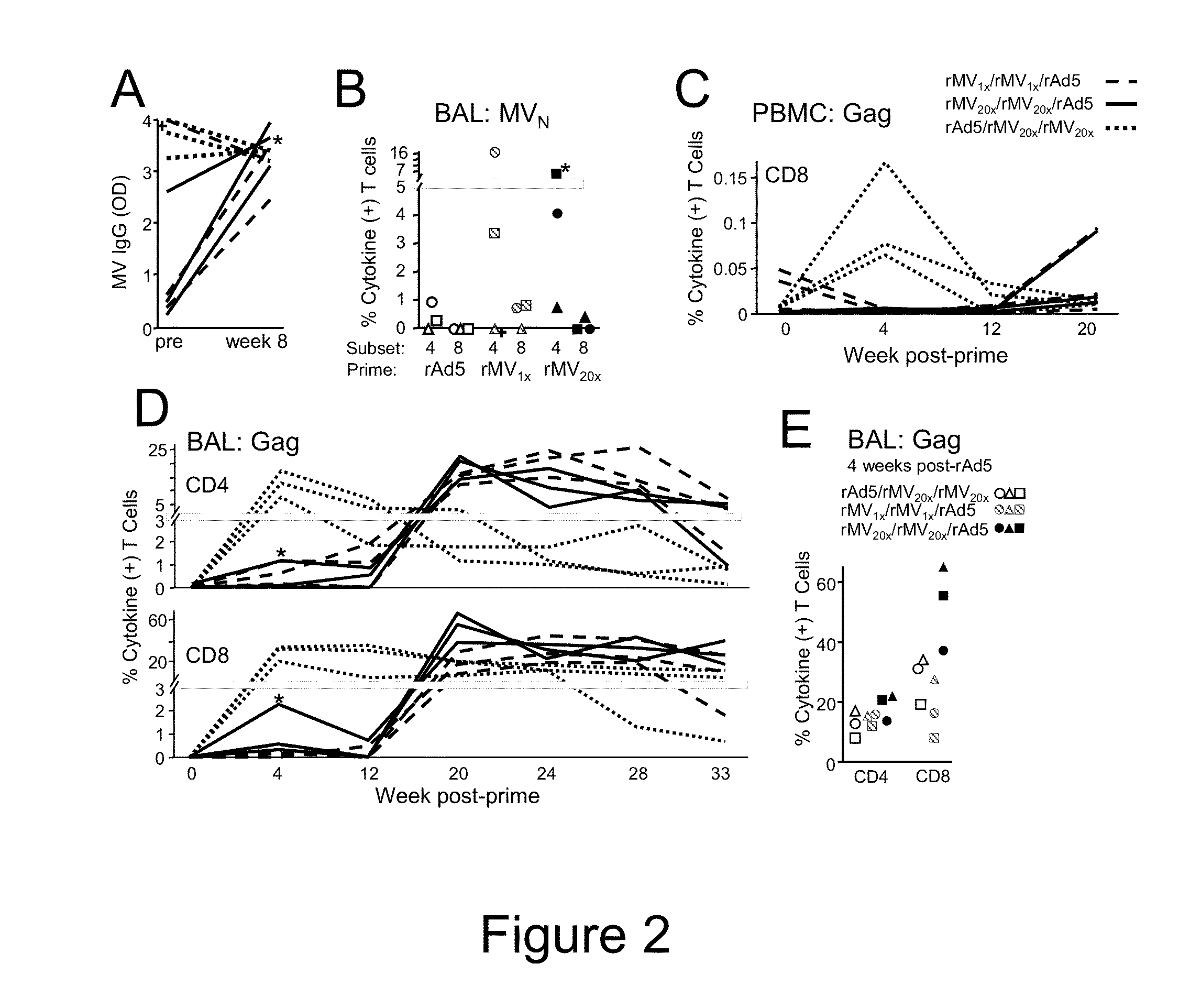
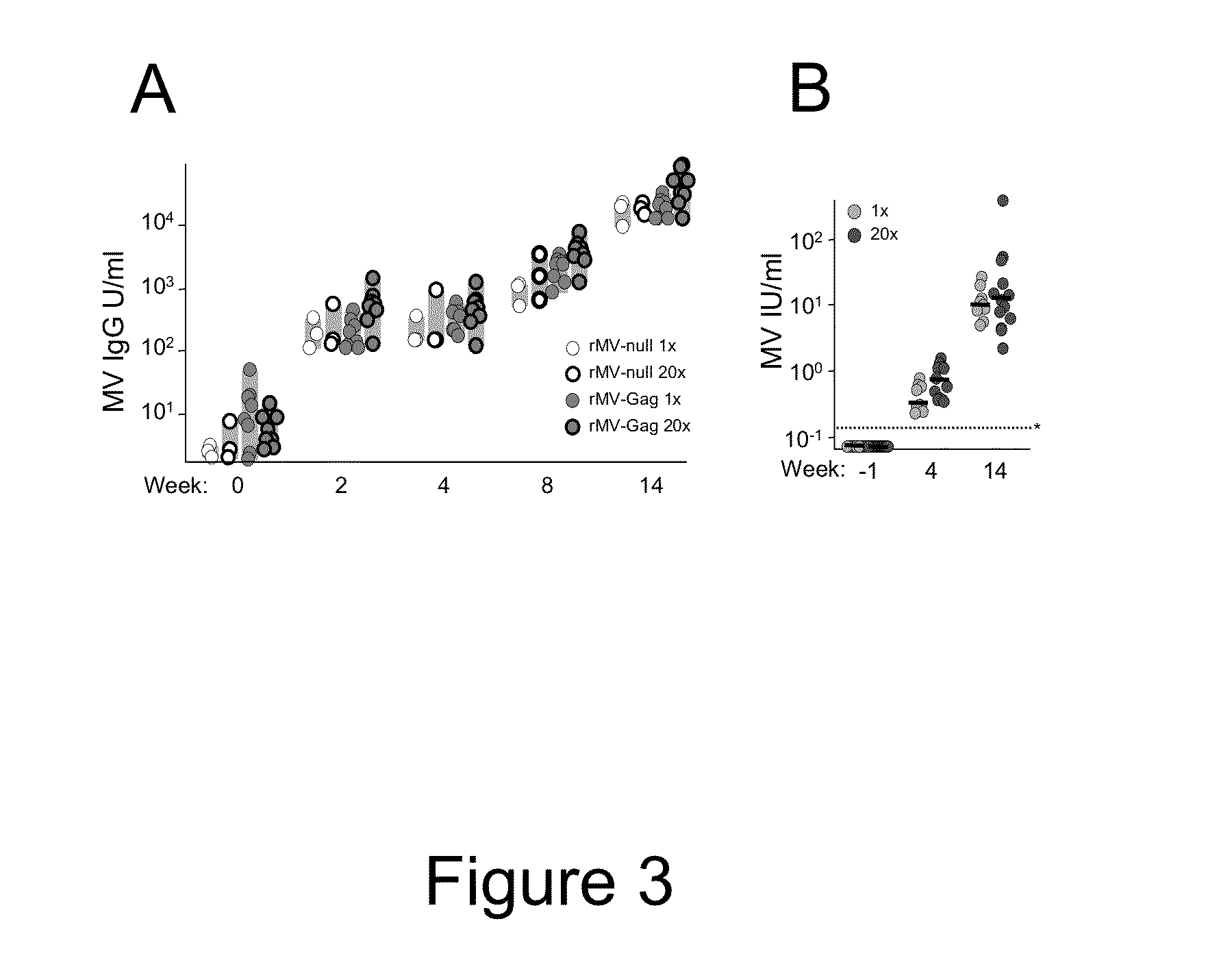
Heterologous prime-boost immunization using measles virus-based vaccines
InactiveUS20130122038A1Enhanced Gag-specificImprove responseSsRNA viruses negative-senseViral antigen ingredientsAntigenPrime boost
The invention provides reagents and methods for heterologous prime-boost immunization regimens. In particular, the invention provides reagents and methods for use in a paramyxovirus-based prime and adenovirus-based boost immunization system, wherein the immunization induces an immune response to a foreign antigen.
Owner:JANSSEN VACCINES & PREVENTION BV +1
Methods and compositions using listeria for enhancing immunogenicity by prime boost
Provided herein are prime-boost regimens and materials used therein. The prime-boost regimens enhance the immune response to a target antigen. The vaccines used for boost are comprised of recombinant attenuated metabolically active Listeria that encodes an expressible antigen that is cross-reactive with the target antigen. In some examples, the immune response is a cellular immune response.
Owner:ANZA THERAPEUTICS INC +1
Recombinant vaccine against West Nile Virus
InactiveUS20050031641A1Provide securityPractical and convenientSsRNA viruses positive-senseViral antigen ingredientsAdjuvantRecombinant vaccines
An immunogenic or vaccine composition to induce an immune response or protective immune response against West Nile virus (WNV) in an animal susceptible to WNV. The composition includes a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector contains heterologous nucleic acid molecule(s), expresses in vivo in the animal WNV antigen, immunogen or epitope thereof, e.g., WNV E; WNV prM and E; WNV M and E; WNV prM, WNV M and E, WNV polyprotein prM-E, WNV polyprotein M-E, or WNV polyprotein prM-M-E. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL LTD A CO LTD BY SHARES +1
Recombinant avian influenza vaccine and uses thereof
ActiveUS20100189731A1Highly immunogenicImprove protectionSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininEpitope
The present invention encompasses influenza vaccines, in particular avian influenza vaccines. The vaccine may be a subunit vaccine based on the hemagglutinin of influenza. The hemagglutinin may be expressed in plants including duckweed. The invention also encompasses recombinant vectors encoding and expressing influenza antigens, epitopes or immunogens which can be used to protect animals against influenza. It encompasses also a vaccination regimen compatible with the DIVA strategy, including a prime-boost scheme using vector and subunit vaccines.
Owner:MERIAL INC
Recombinant ndv antigen and uses thereof
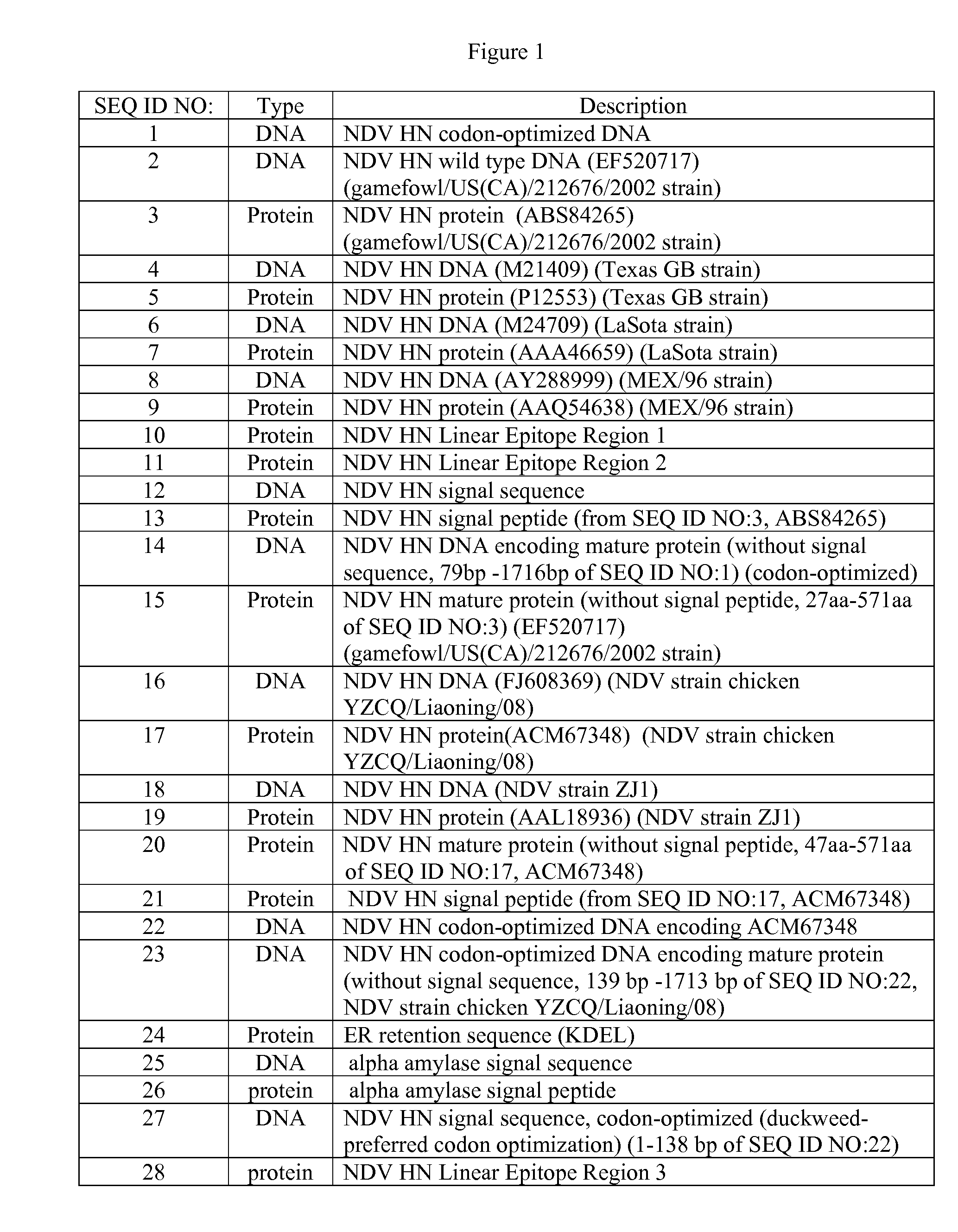
The present invention encompasses NDV vaccines. The vaccine may be a subunit vaccine based on HN of NDV. The NDV HN may be expressed in plants or algae including microalgae. The invention also encompasses recombinant vectors encoding and expressing NDV antigens, epitopes or immunogens which can be used to protect animals against NDV. It encompasses also a vaccination regime compatible with the DIVA strategy, including a prime-boost scheme using viral vector or inactivated vaccines and subunit vaccines.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
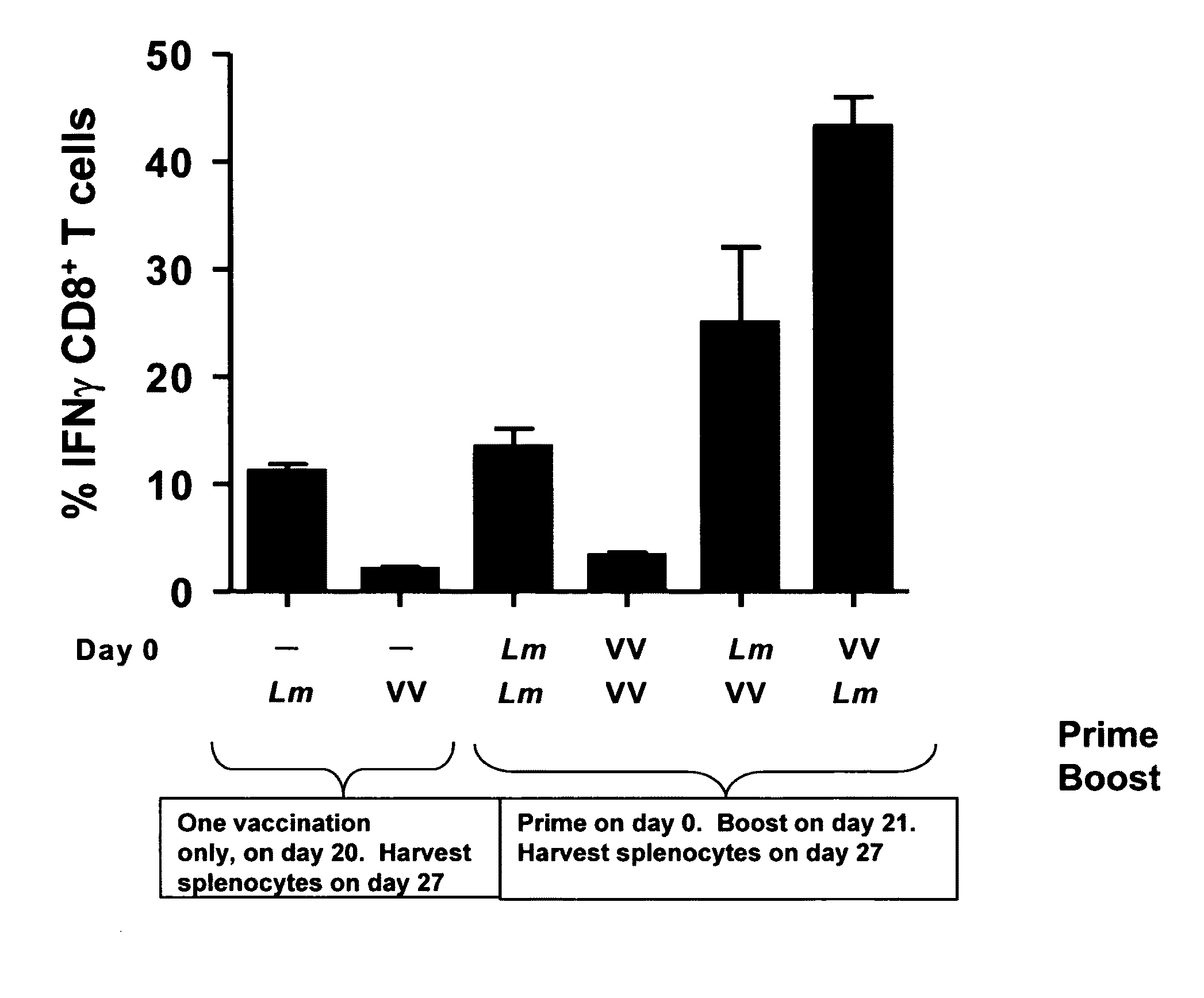
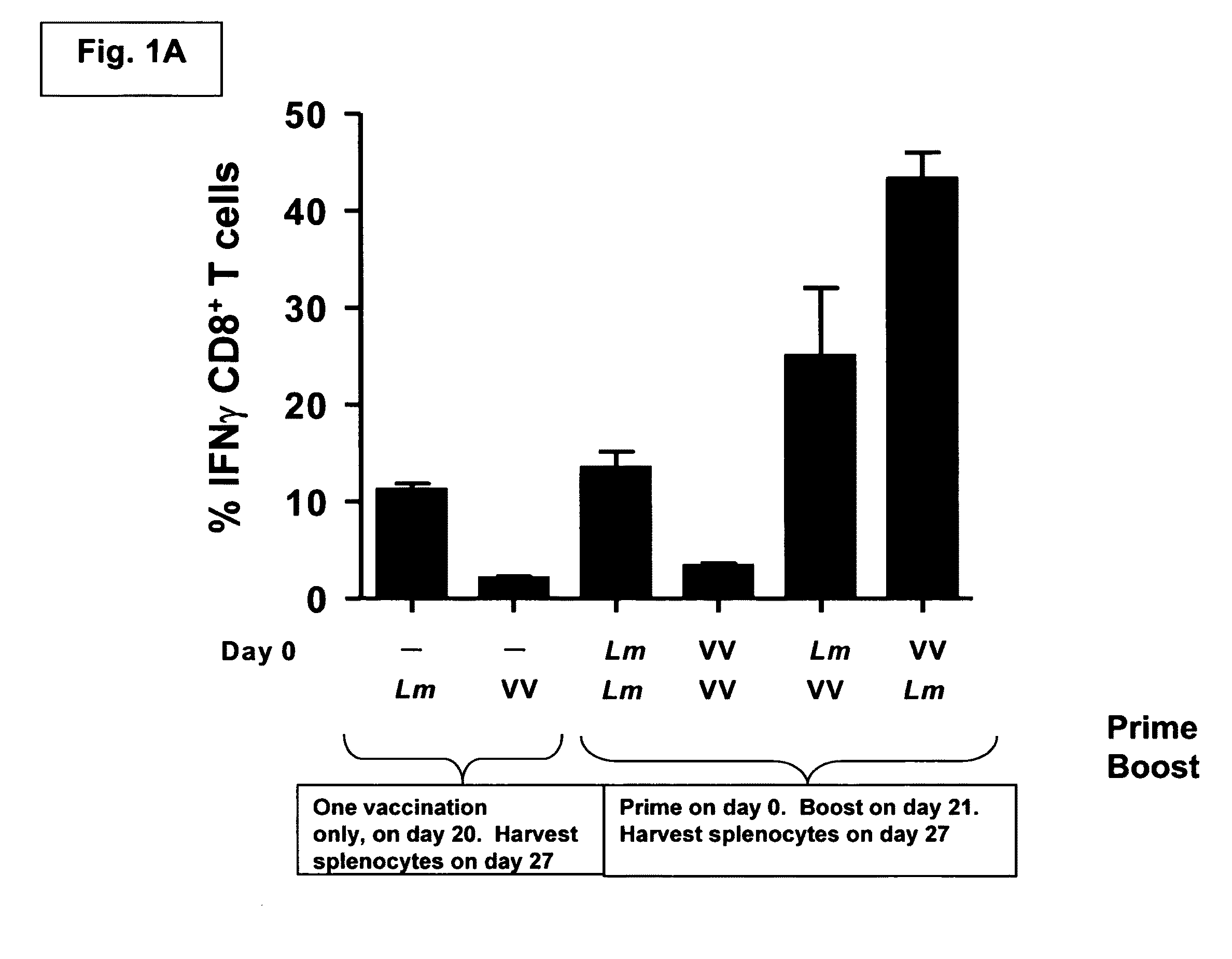
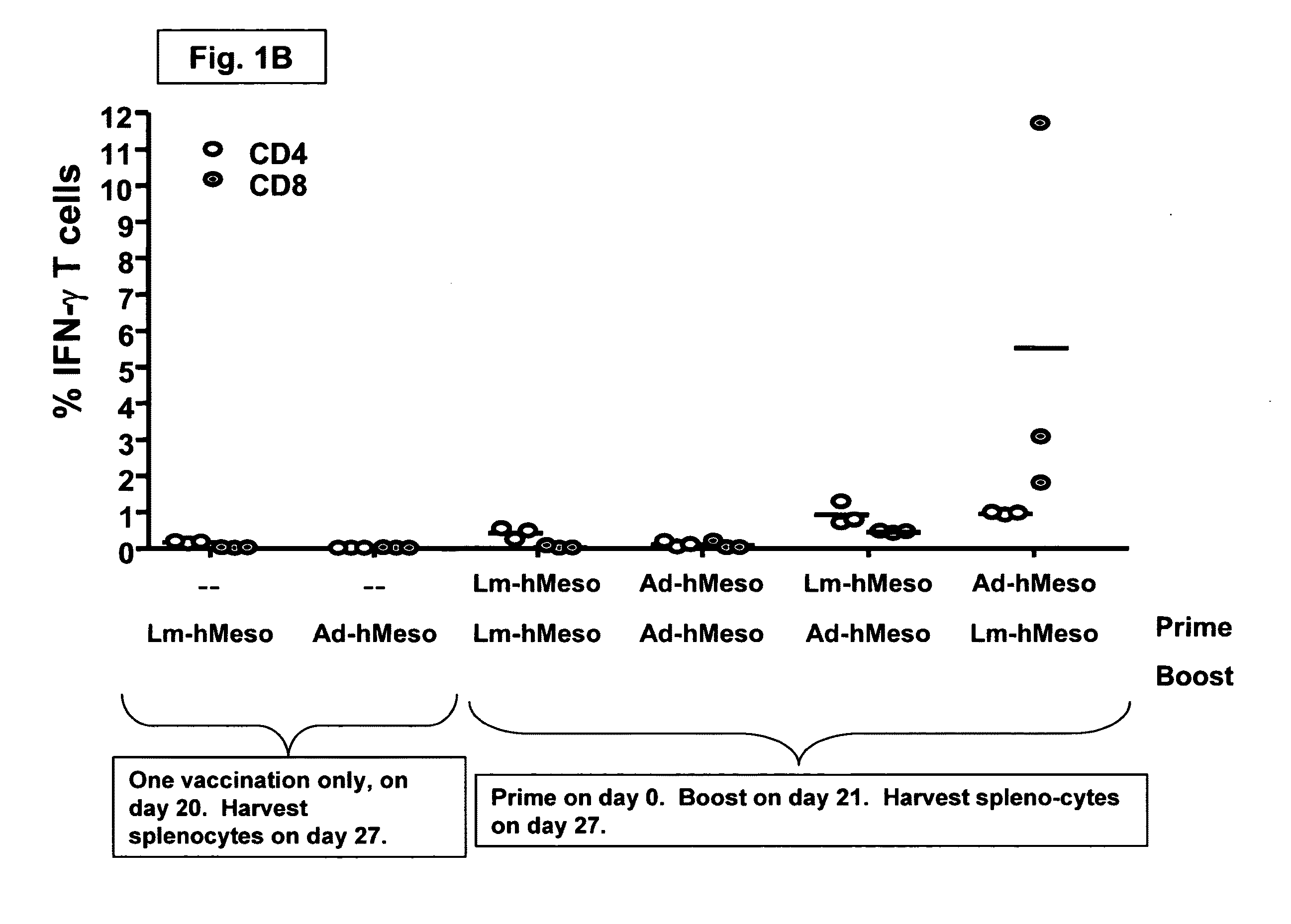
Methods and kits for inducing a ctl response using a prime boost regimen
The present invention relates to the generation of a T cell response against a target antigen using a polypeptide comprising a polyepitope construct as a priming composition in a prime boostregimen.
Owner:INNOGENETICS INC
Polypeptide vaccine and vaccination strategy against mycobacterium
A vaccine is provided wherein a polypeptide or combination of peptides from M. tuberculosis is administered to a subject to elicit an immune response. The polypeptide vaccine is administered as part of a prime-boost strategy with BCG vaccine to increase the immunoprotection in a subject such that prevention or elimination of disease is achieved. Finally, a pharmaceutical package is provided that encompasses a polypeptide vaccine for M. tuberculosis that when administered to a subject elicits immunoprotection.
Owner:EMORY UNIVERSITY +1
Heterologous prime-boost immunization regimen
InactiveUS20090092635A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsRegimenPrime boost
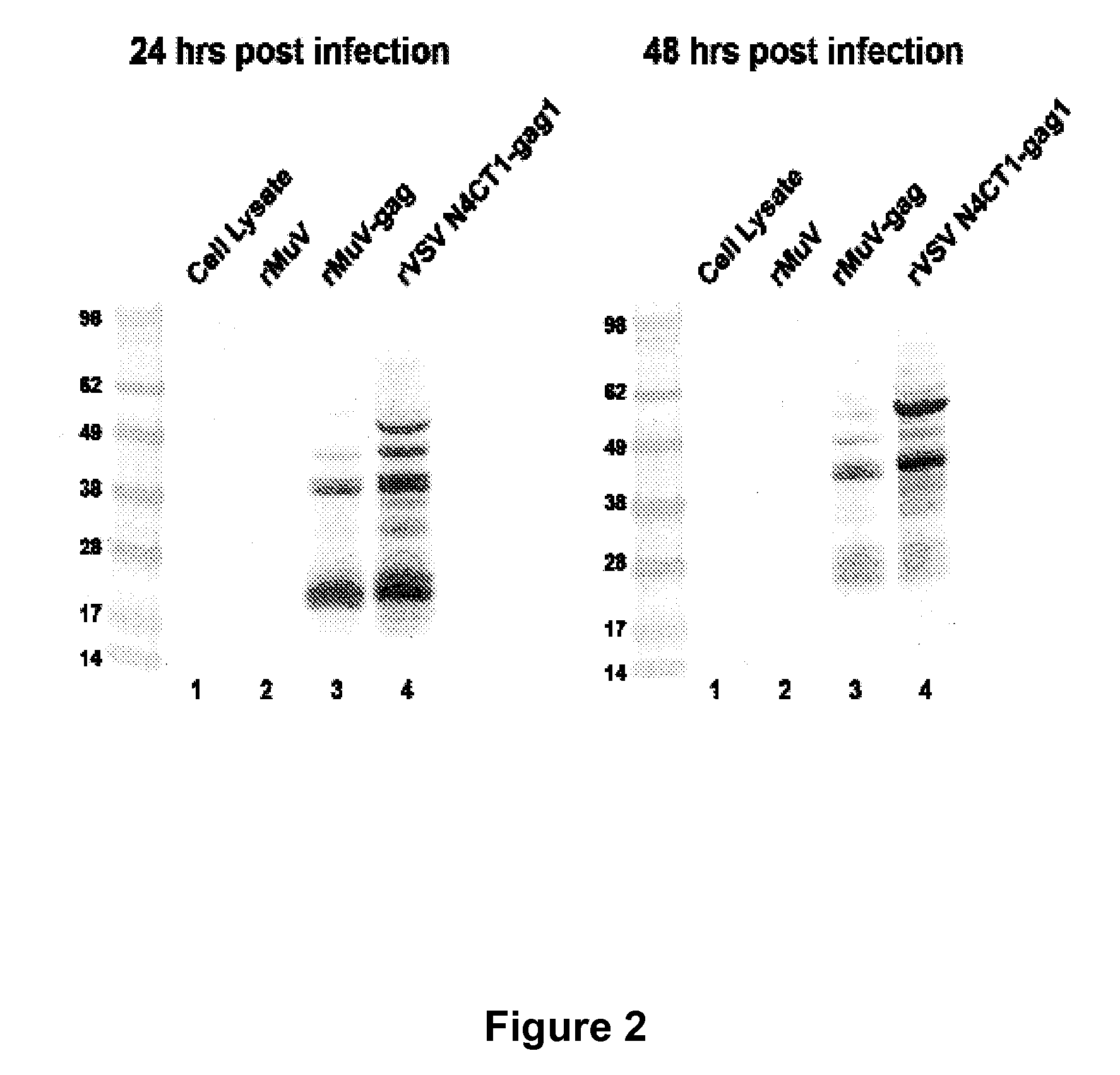
The present invention is directed to a method for generating an antigen-specific immune response in a subject in general and in particular to administering a priming dose of an immunogenic composition of a recombinant mumps virus (rMuV) that encodes an antigen followed by administering a boosting dose of recombinant vesicular stomatitis virus (rVSV) encoding an antigen.
Owner:WYETH
Recombinant vaccine against bluetongue virus
ActiveUS7862821B2Provide securityPractical and convenientAntibacterial agentsViral antigen ingredientsAdjuvantRecombinant vaccines
Owner:MERIAL LTD +3
Recombinant vaccine against West Nile Virus
InactiveUS7740863B2Provide securityPractical and convenientSsRNA viruses positive-senseViral antigen ingredientsAdjuvantRecombinant vaccines
An immunogenic or vaccine composition to induce an immune response or protective immune response against West Nile virus (WNV) in an animal susceptible to WNV. The composition includes a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector contains heterologous nucleic acid molecule(s), expresses in vivo in the animal WNV antigen, immunogen or epitope thereof, e.g., WNV E; WNV prM and E; WNV M and E; WNV prM, WNV M and E, WNV polyprotein prM-E, WNV polyprotein M-E, or WNV polyprotein prM-M-E. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL LTD
Novel prime-boost combinations of attenuated mycobacterium
InactiveUS20090304750A1Effective protectionOrganic active ingredientsBacterial antigen ingredientsPrime boostMucosal vaccine
The present invention provides vaccine compositions for effective induction of both mucosal and systemic immunity to pathogenic Mycobacterium species. Vaccination protocols are provided in which both parenteral and mucosal vaccine formulations are administered to a host. The parenteral and mucosal formulations comprise live, attenuated Mycobacteria.
Owner:HONE DAVID +1
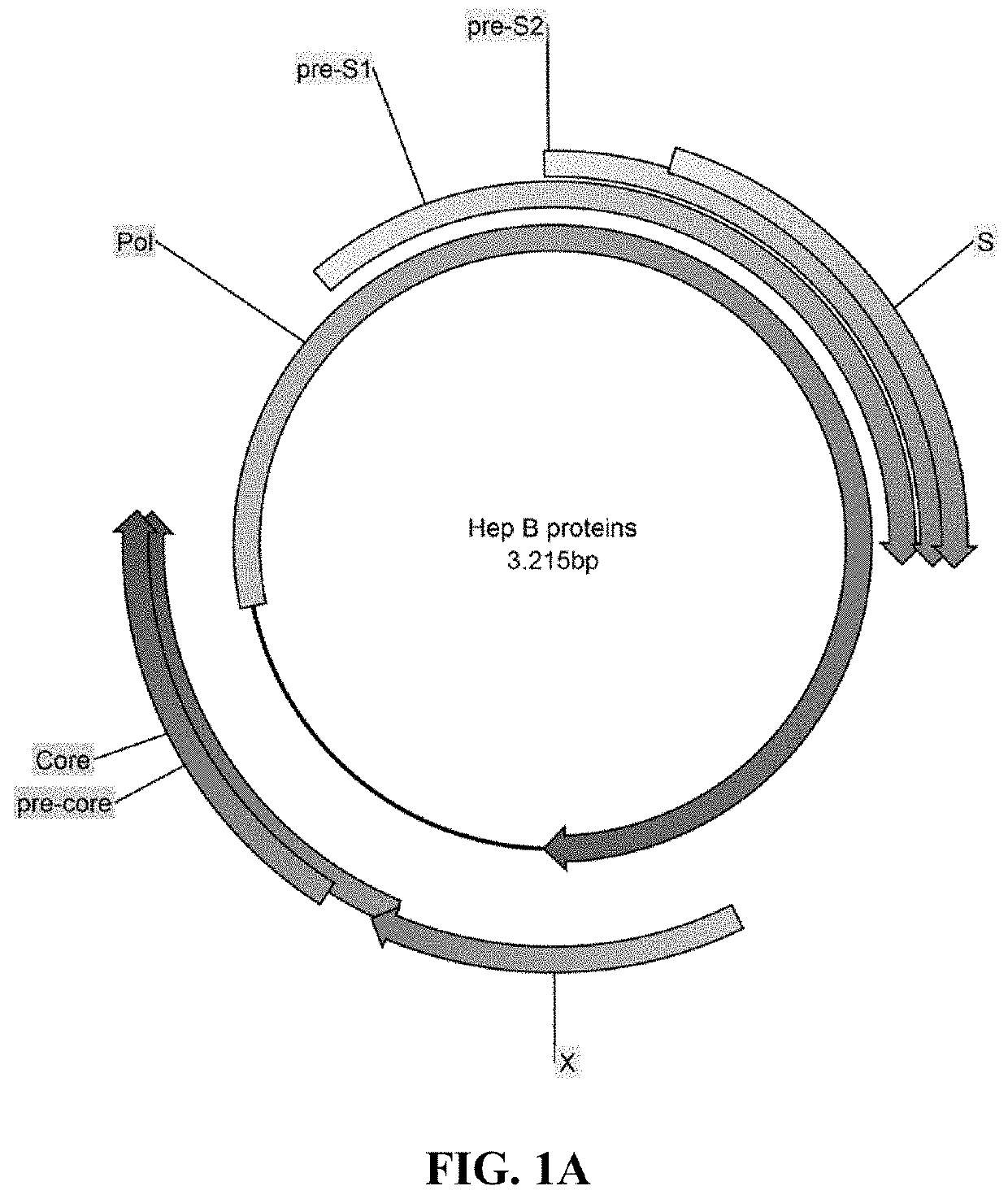
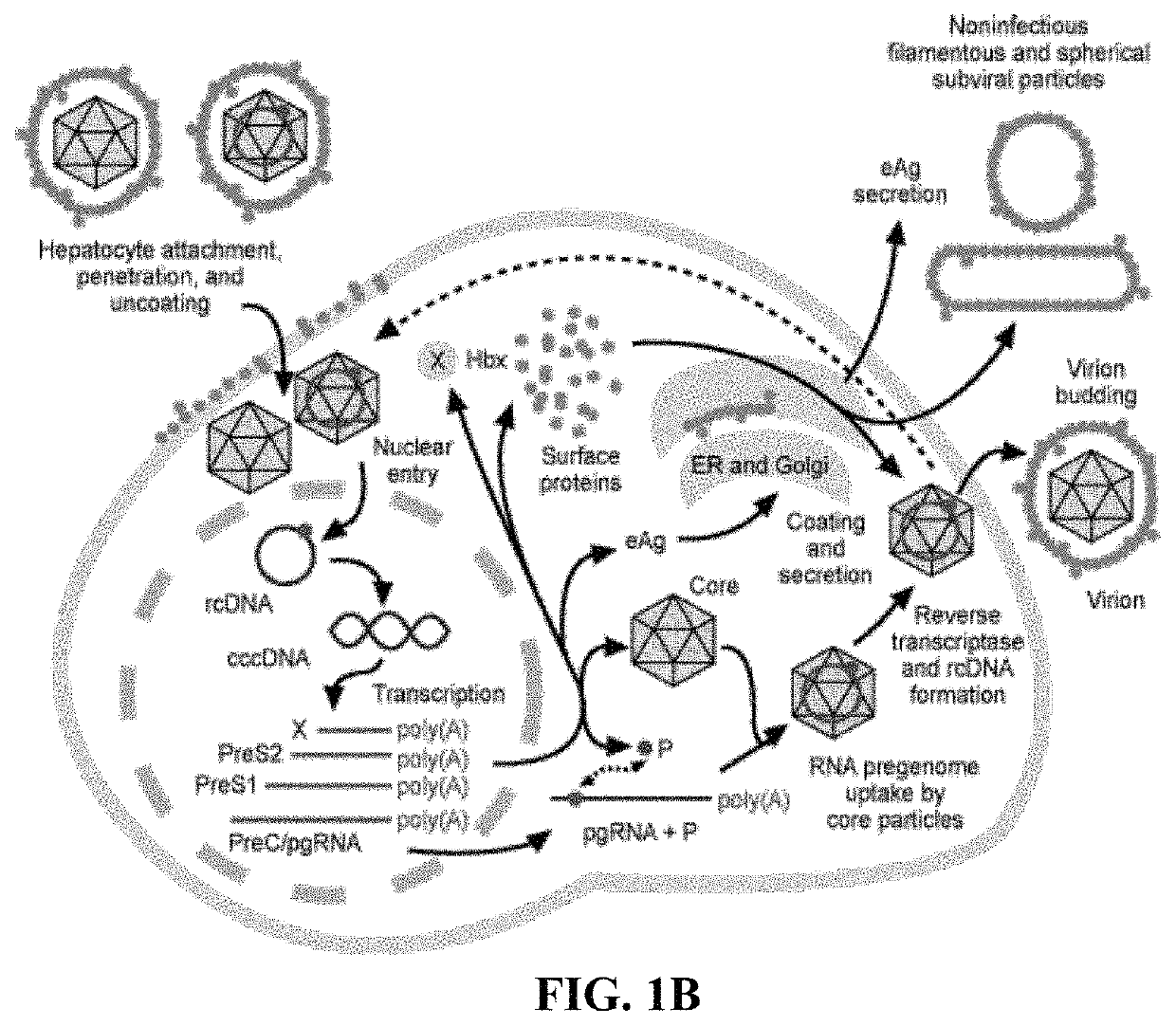
Methods and compositions for inducing an immune response against Hepatitis B Virus (HBV)
Provided herein are Modified Vaccinia Ankara (MVA) vectors and adenovirus vectors encoding HBV antigens. Also provided are methods of enhancing an immune response in a human subject by utilizing the MVA and adenovirus vectors encoding HBV antigens in a prime / boost regimen to the enhance the immune response in the human subject.
Owner:JANSSEN SCI IRELAND UC +1
Methods and compositions using listeria for adjuvant treatment of cancer
InactiveUS20130315950A1Bacterial antigen ingredientsBacteria material medical ingredientsRegimenPrime boost
Owner:ADURO BIOTECH
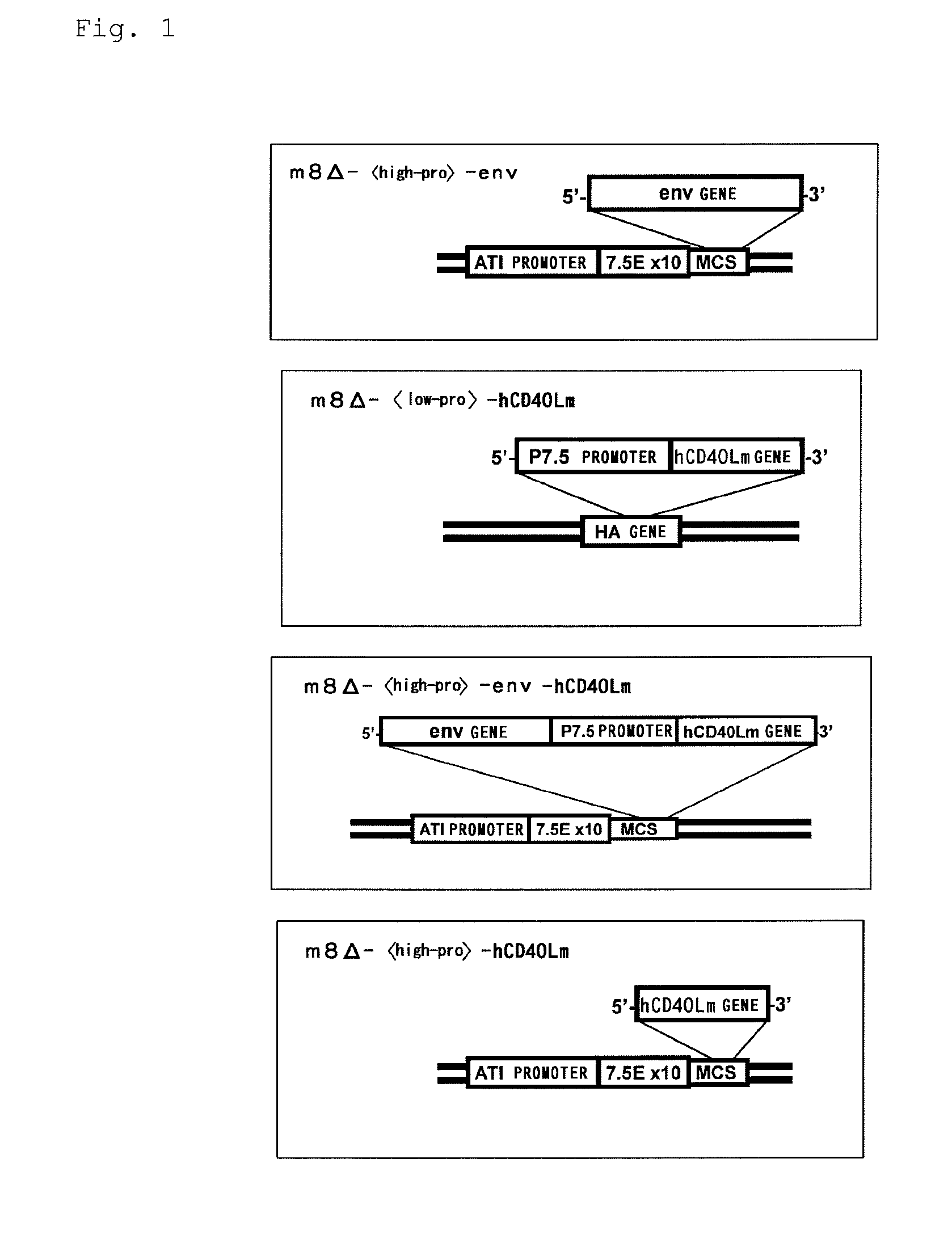
Virus vector for prime/boost vaccines, which comprises vaccinia virus vector and sendai virus vector
InactiveUS20130302367A1Avoid infectionEffective preventionSsRNA viruses negative-senseViral antigen ingredientsCowpox virusPrime boost
[Problem]To provide a set of virus vectors which can be used for producing a prime / boost vaccine that can activate both cellular immunity and humoral immunity and is effective on infections by pathogenic microorganisms and malignant tumors which are generally believed to be difficult to be treated by vaccine therapy.[Solution]Provided is a set of virus vectors for prime / boost vaccines, comprising the following virus vector (a) and virus vector (b): (a) a vaccinia virus vector which carries a gene encoding an immunogenic polypeptide in such a manner that the gene can be expressed; and (b) a Sendal virus vector which carries the gene encoding the immunogenic polypeptide in such a manner that the gene can be expressed.
Owner:HOKKAIDO UNIVERSITY +1
Vaccination or immunization using a prime-boost regimen
InactiveUS20040002472A1Genetic material ingredientsPharmaceutical delivery mechanismVaccinationRegimen
Disclosed and claimed are methods and compositions and kits for the vaccination or immunization of an animal, such as a mammal, advantageously a bovine, involving a prime-boost regimen.
Owner:MERIAL LTD +1
Binary composition for prime-boost release of active ingredients like vaccines
InactiveUS20050002969A1Presented quicklyPresent some limitBiocideOrganic active ingredientsActive agentPrime boost
Therapeutic compositions in solid dose form that comprise a mixture of first amorphous or non-crystalline microparticles comprising a bioactive agent and second amorphous or non-crystalline microparticles that comprise the same or different bioactive agent. The compositions provide a primary pharmacological response (first microparticles) and a second “boosting” effect (second microparticles) produced by releasing the agent over a linger period.
Owner:QUADRANT DRUG DELIVERY
Vaccines against vesicular stomatitis
Owner:MERIAL INC
Prime-boost regimens with a tlr4 agonist adjuvant and a lentiviral vector
InactiveUS20170196954A1High transduction efficiencySignificantly more efficientlySsRNA viruses positive-sensePharmaceutical delivery mechanismAdjuvantPrime boost
Owner:IMMUNE DESIGN CORP
Polypeptide vaccine and vaccination strategy against mycobacterium
A vaccine is provided wherein a polypeptide or combination of peptides from M. tuberculosis is administered to a subject to elicit an immune response. The polypeptide vaccine is administered as part of a prime-boost strategy with BCG vaccine to increase the immunoprotection in a subject such that prevention or elimination of disease is achieved. Finally, a pharmaceutical package is provided that encompasses a polypeptide vaccine for M. tuberculosis that when administered to a subject elicits immunoprotection.
Owner:U S GOVERNMENT DEPT OF HEALTH & HUMAN SERVICES SECRETARIAT
Recombinant avian influenza vaccine and uses thereof
ActiveUS8394384B2Highly immunogenicImprove protectionSsRNA viruses negative-sensePeptide/protein ingredientsEpitopeHemagglutinin
Owner:MERIAL INC
Novel prime-boost combinations of attenuated mycobacterium
The present invention provides vaccine compositions for effective induction of both mucosal and systemic immunity to pathogenic Mycobacterium species. Vaccination protocols are provided in which both parenteral and mucosal vaccine formulations are administered to a host. The parenteral and mucosal formulations comprise live, attenuated Mycobacteria.
Owner:AERAS GLOBAL TB VACCINE FOUND
Method of inducing an enhanced immune response against hiv
InactiveUS20050106123A1Enhance immune responseIncrease probabilityBiocideGenetic material ingredientsTreatment effectMammal
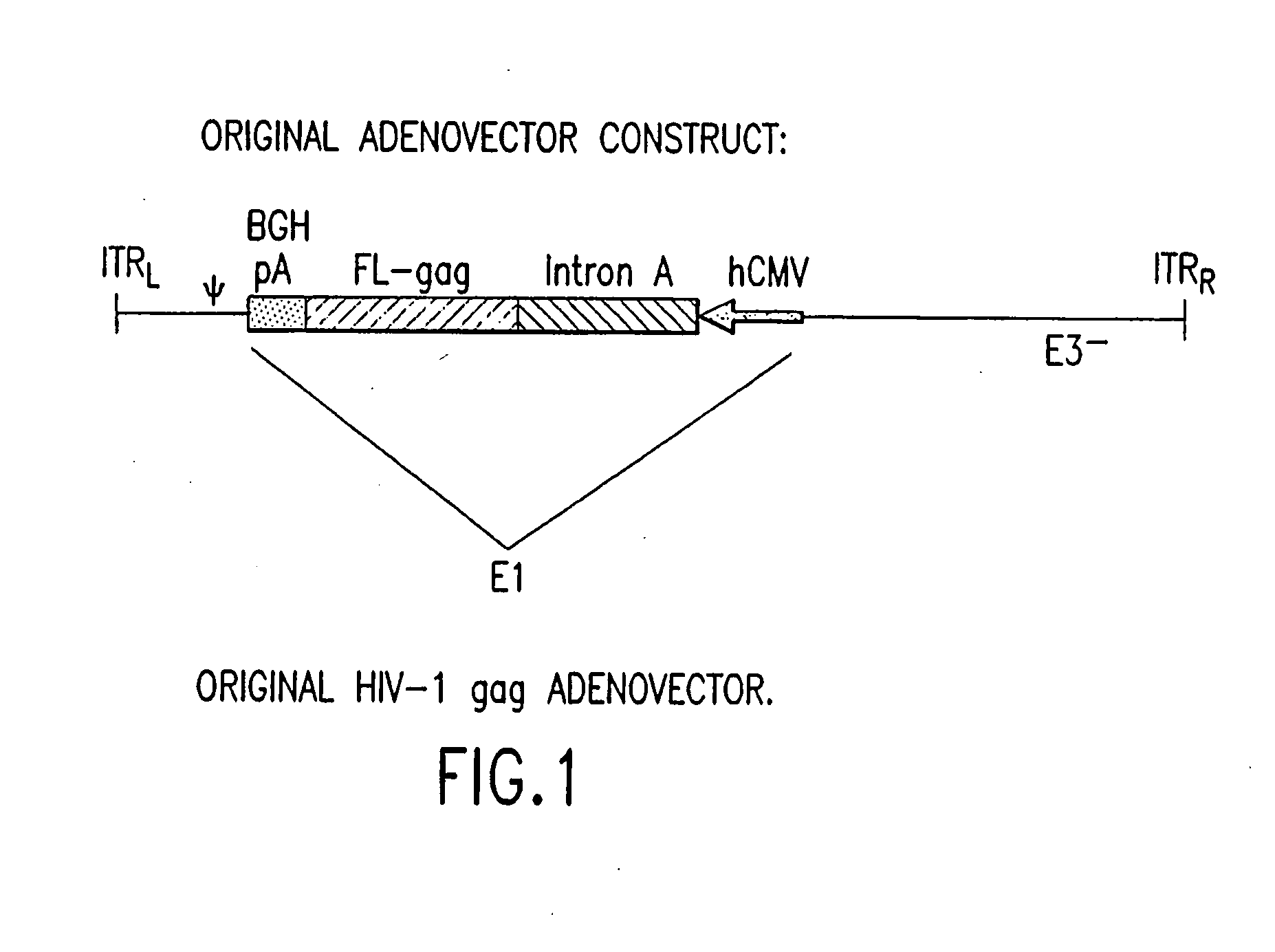
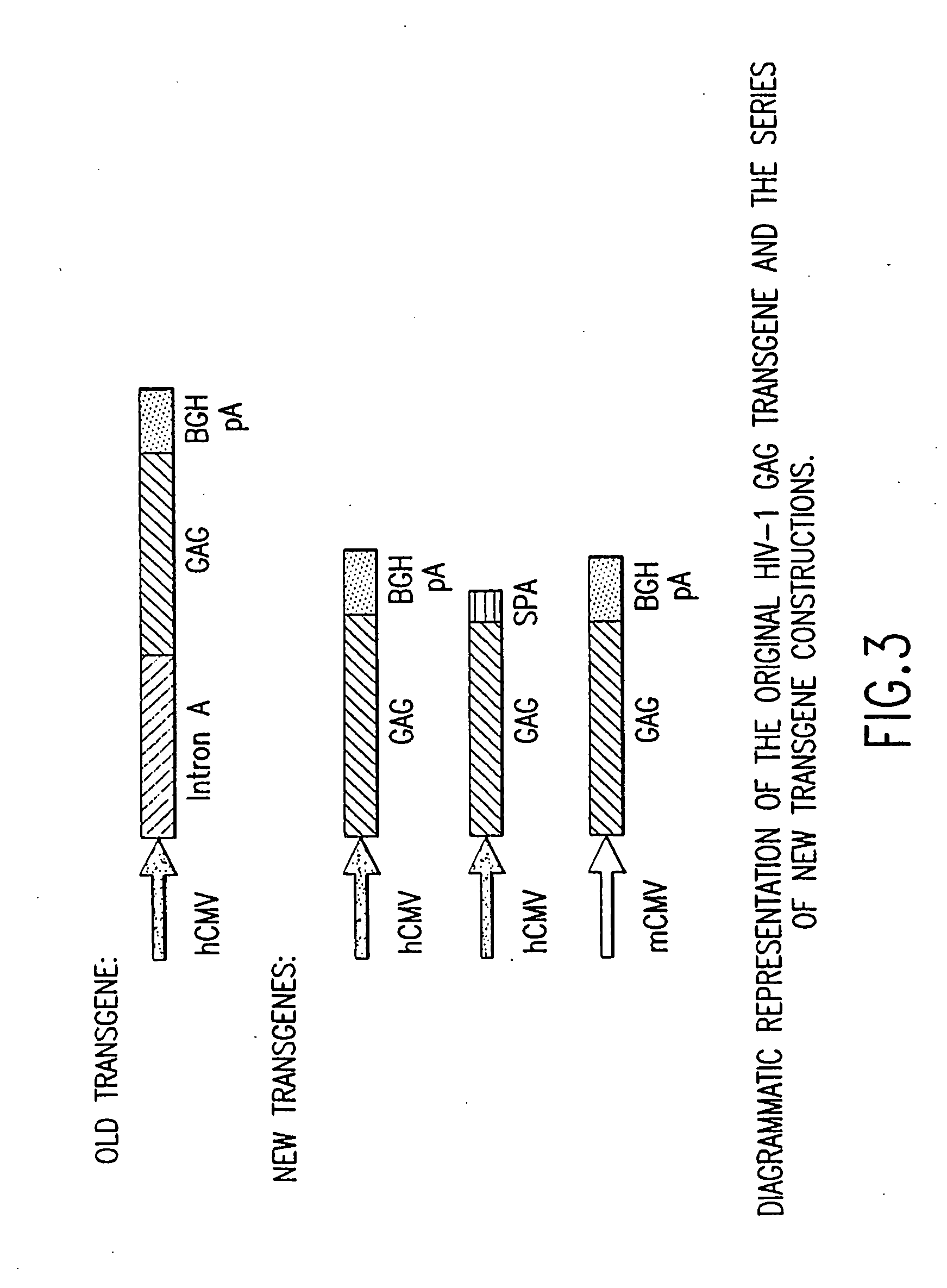
An efficient means of inducing an immune response against human immunodeficiency virus (“HIV”) utilizing specific prime-boost regimes is disclosed. The specific prime-boost regimes employ a heterologous prime-boost protocol wherein recombinant adenoviral and poxvirus vectors comprising exogenous genetic material encoding a common HIV antigen are administered in that order. Vaccines administered into living vertebrate tissue in accordance with the disclosed regimes, preferably a mammalian host such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV-1 antigen (e.g., Gag), inducing a cellular immune response which specifically recognizes HIV-1. It is believed that the disclosed prime / boost regime will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO +5
Vaccines against vesicular stomatitis
The present invention relates to an immunogenic or vaccine composition to induce an immune response or protective immune response against vesicular stomatitis virus (VSV) in an animal susceptible to VSV. The composition may include a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector may contain at least one heterologous nucleic acid molecule(s), expresses in vivo in the animal VSV antigen(s), immunogen(s) or epitope(s) thereof, e.g., VSV G protein and / or VSV N protein and / or VSV M protein. The heterologous nucleic acid molecule(s) may be adjusted to the vector / mammalian cell system by codon optimization. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com