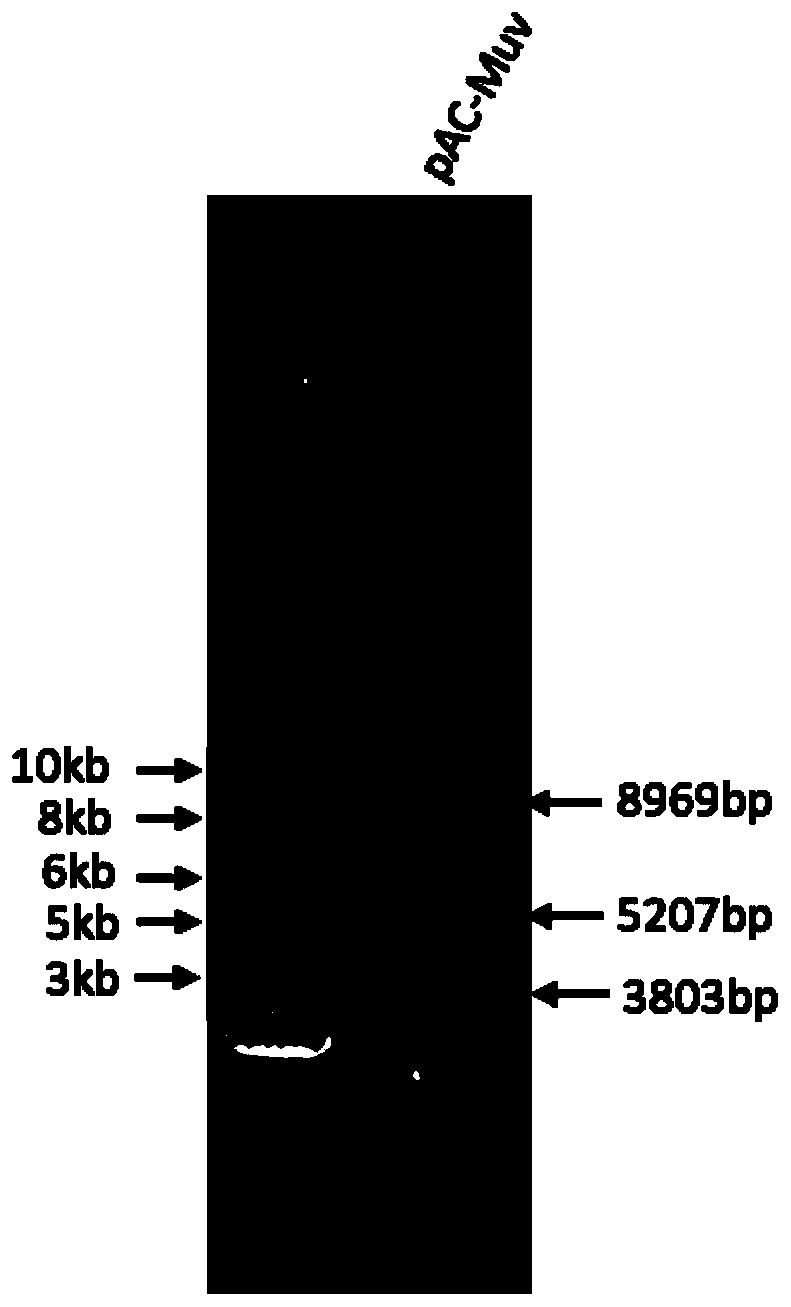
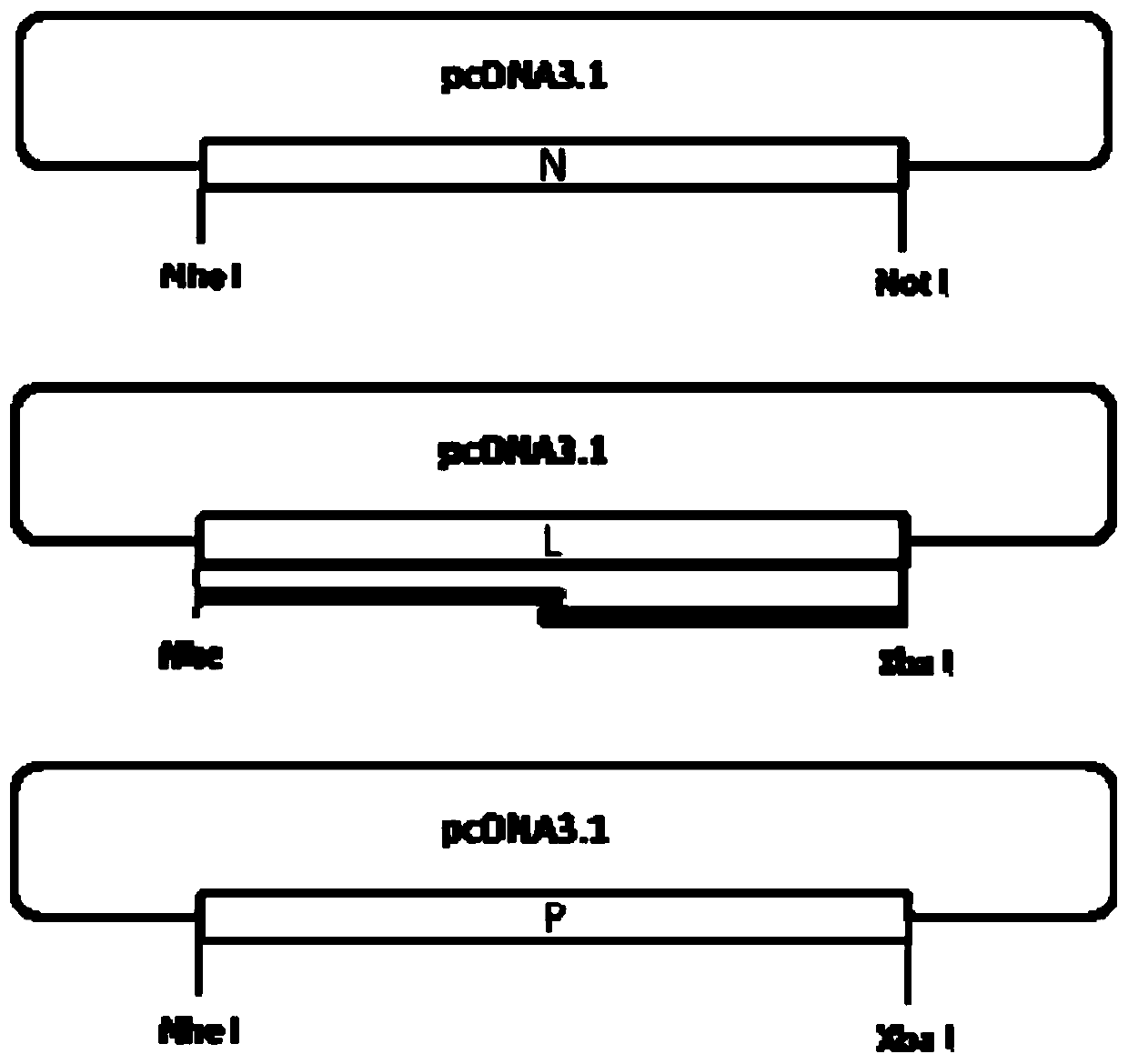
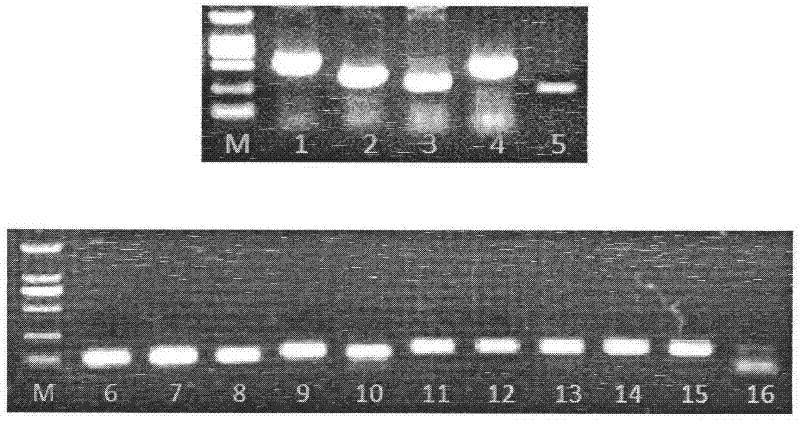
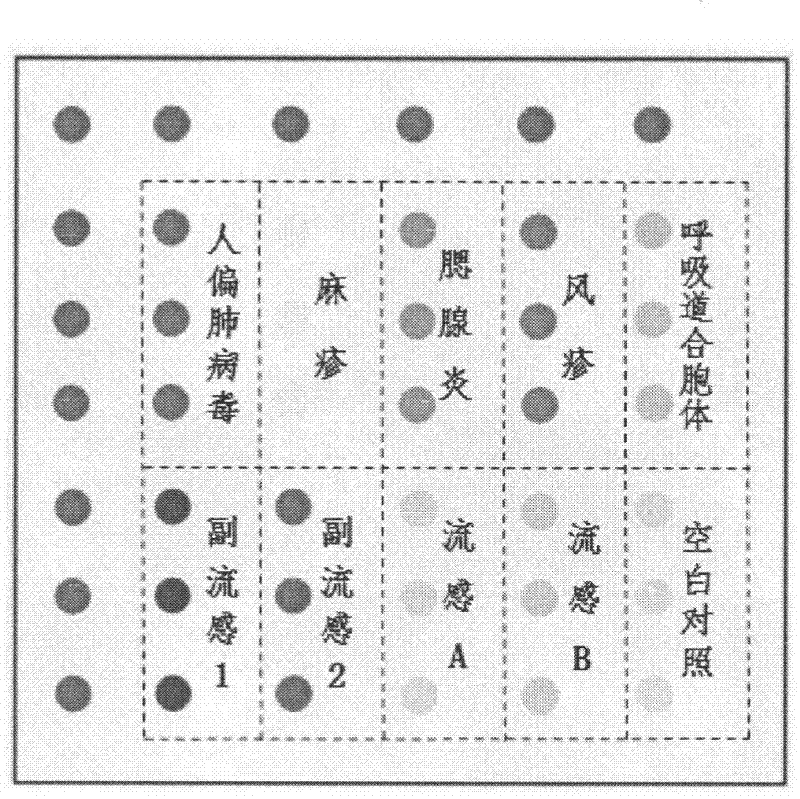
Patents
Literature
75 results about "Mumps virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mumps rubulavirus is the causative agent of mumps. The signs of mumps include swelling of the parotid glands, salivary glands and other epithelial tissues. Symptoms of mumps are fatigue, body aches, headache, loss of appetite, low grade fever, swelling of the salivary glands. Mumps can also result in muscle pain, deafness, meningitis, pancreatitis, swelling of testicles or ovaries, and death. Most people who contract mumps show symptoms of the virus, however there are few who show no or very few symptoms. Natural infection is currently restricted to humans and the virus is transmitted by direct contact, droplet spread, or contaminated objects.
Anti-viral treatment and assay to screenfor Anti-viral agent
InactiveUS20130085133A1Improve palatabilityImprove stabilityBiocideSugar derivativesCytopathic effectAntiviral drug
The present disclosure relates to novel compounds of formulas (1) through (19) and to a method for treating humans infected with a virus including various respiratory viruses such as members of the Paramyxoviridae family (respiratory syncytial virus (RSV), human metapneumovirus (HMPV), human parainfluenza virus (HPIV), measles virus, and mumps virus) with a compound of formulas (1) through (19). The present disclosure also relates to a cytopathic effect (CPE)-based assay that will assess virus-induced CPE for screening of compounds for treating viral diseases or inhibiting a virus.
Owner:SOUTHERN RES INST & IP
F genotype mumps virus attenuated strain as well as construction method and application thereof
The invention provides an F genotype mumps virus attenuated strain as well as a construction method and application thereof. Specifically, the invention provides an F genotype mumps virus attenuated strain and the attenuated strain is a mumps virus QS-F-SH2 with an accession number of CCTCC NO: V201950. The invention also provides a vaccine composition containing the F genotype mumps virus attenuated strain as an active ingredient and a preparation method thereof. The mumps virus attenuated vaccine strain disclosed by the invention can match the F type mumps virus predominantly popular in China, and the level of the mumps virus attenuated vaccine strain is equivalent to that of the current vaccine strain in the aspects of growth characteristics, immunogenicity, neurotoxicity and the like.In addition, the mumps virus genetic engineering attenuated strain screened by the invention can be stably produced in chick embryo cells, and the safety is high.
Owner:SHANGHAI KING CELL BIOTECHNOLOGY CO LTD +1
Immunization Compositions and Methods
ActiveUS20110189226A1SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
Preparation method of antiviral oral liquid
InactiveCN102764405AMatching scienceAvoid wastingAntiviralsAluminium/calcium/magnesium active ingredientsDiseaseInfection disease
The invention relates to the field of traditional Chinese medicines, especially to a preparation method of an antiviral oral liquid. The antiviral oral liquid is prepared from Chinese herbal medicines, has effects of releasing heat, eliminating dampness, cooling the blood and detoxifying, and is used to treat wind-heat cold, fever caused by warm disease, upper respiratory tract infection, influenza and mumps virus infection diseases. The preparation method of the antiviral oral liquid is simple to operate and is suitable for large-scale production. The ratio of Chinese herbal medicines is scientific. And by the adoption of the preparation method, waste of the Chinese herbal medicines is avoided.
Owner:顾建光
Preparation method and application of gene chip for detecting important respiratory pathogenic viruses
InactiveCN102586475AStrong specificityImprove the consistency rateMicrobiological testing/measurementOligonucleotide chipMeasles virus IgG
The invention relates to a gene chip for detecting important respiratory pathogenic viruses. A preparation method of the gene chip comprises the following steps of: preparing a specific primer; preparing a virus specific probe; preparing an oligonucleotide chip; establishing an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) system; establishing a hybrid system; preparing a visual detection reagent; and establishing a developing method. The gene chip prepared by the invention can be used for simultaneously discriminating nine general respiratory pathogenic viruses comprising A and B type influenza viruses, parainfluenza viruses type 1 and 2, human metapneumovirus, respiratory syncytial virus, measles virus, rubella virus and mumps virus, provides a new solution for quickly detecting the general respiratory pathogenic viruses at high throughout and can provide guidance for monitoring, clinically diagnosing and treating the respiratory pathogenic viruses.
Owner:深圳市普瑞康生物技术有限公司 +1
Composition of starwort sulphonic acid or vitriolic acid polyoses ester total phenolic glycoside and method of preparing the same and antiviral application
The invention relates to a kind of natural medicine of broad spectrum antibiotic. At present, the broad spectrum antibiotic medicine with high effect and safety is at shortage all round the world. The invention is intended to extract laminarinsulphate or sulphonic acid sugar ester or sulphosalts from plant chickweed or other chickweed plant with two resin adsorption methods or a water extraction and alcohol precipition method. The spectrum antibiotic in the invention is distributed under 50,000 in the formula weight formed by carbon glycosidic bond and / or oxide glycosidic bond with phenol, especially the total flavones comprising apigenin. However, the invention mainly acts as total phenolic glycoside with the formula weight under 4,000. Besides, the invention can form brownish compound with the total flavones comprising apigenin and the glycosidic ingredients without sulfur element, so as to be applied as broad spectrum antibiotic drug. Therefore, the compound in the invention can be applied to cure ADIS virus, hepatitis virus, influenza virus and parainfluenza virus comprising SARS, adenovirus, verruca acuminate virus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus and varicella. No toxic effect has been found in the application. What is more, the invention can be made into 10 sorts of formulation, disinfector and health-improving products.
Owner:朱耕新
Process for preparing broad spectrum anti-virus medicine and its use
The broad spectrum anti-virus medicine is a composition of general organic phenolic acid and / or general organic phenolic salt and its glucoside extracted from chickweed and other stellaria plant through two extraction processes. It may be used to inhibit several viruses of RNA and DNA. It may be used in preparing medicine for inhibiting HIV, hepatitis virus, flu virus, parainfluenza virus, adenovirus and other viruses. It has no toxicity. It may be used in preparing at least ten kinds of medicine.
Owner:朱耕新
Protein chip for detecting blood and cerebro spinal fluid pathogen antibody, and its preparing method and use
InactiveCN1641355ASmall sample sizeSolve the detection speed is slowMaterial analysisAntigenChickenpox
The invention discloses a protein chip for detecting pathogen antibodies of blood and cerebrospinal fluid and its preparing method, and the protein chip includes substrate and peculiar-culture or shape protein or polypeptide antigen of array distributed pathogen and contrasted dot coating, where the substrate is a glass substrate; and the antigen and contrasted dot coating refers to 13 antigens with ten indexes including HSV-I, HSV-II, VZV, CMV, EBV, RV, JEV, MV, MT and LP, uniformly distributed on the glass substrate in a dot matrix form, and positive contrast, negative contrast and blank contrast. The protein chip of the invention can obtain multiple-index reacting result only by one reaction, judges infection of different pathogens and has the characters of quickness, high efficiency, accuracy and parallel diagnosis.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Use of extract of Fructus Pithecellobii clypeoriae conrmon Apes Ear-ring Fruit
The present invention relates to an application of pithecellobium clypearia extract in medicine and food. It adopts pithecellobium clypearia aqueous extract or ethyl alcohol extract as effective component and adds conventional medicinal auxiliary or food additive, and can make them into tablet, capsul, granule preparation, pills preparation and oral liquor preparation. Said pithecellobium clypearia extract has strong inhibition effect for influenza virus, parainfluenza virus, enteric virus, rhinovirus and mumps virus and has strong antioxidation activity.
Owner:SHANHE PHARMA GUANGZHOU CITY
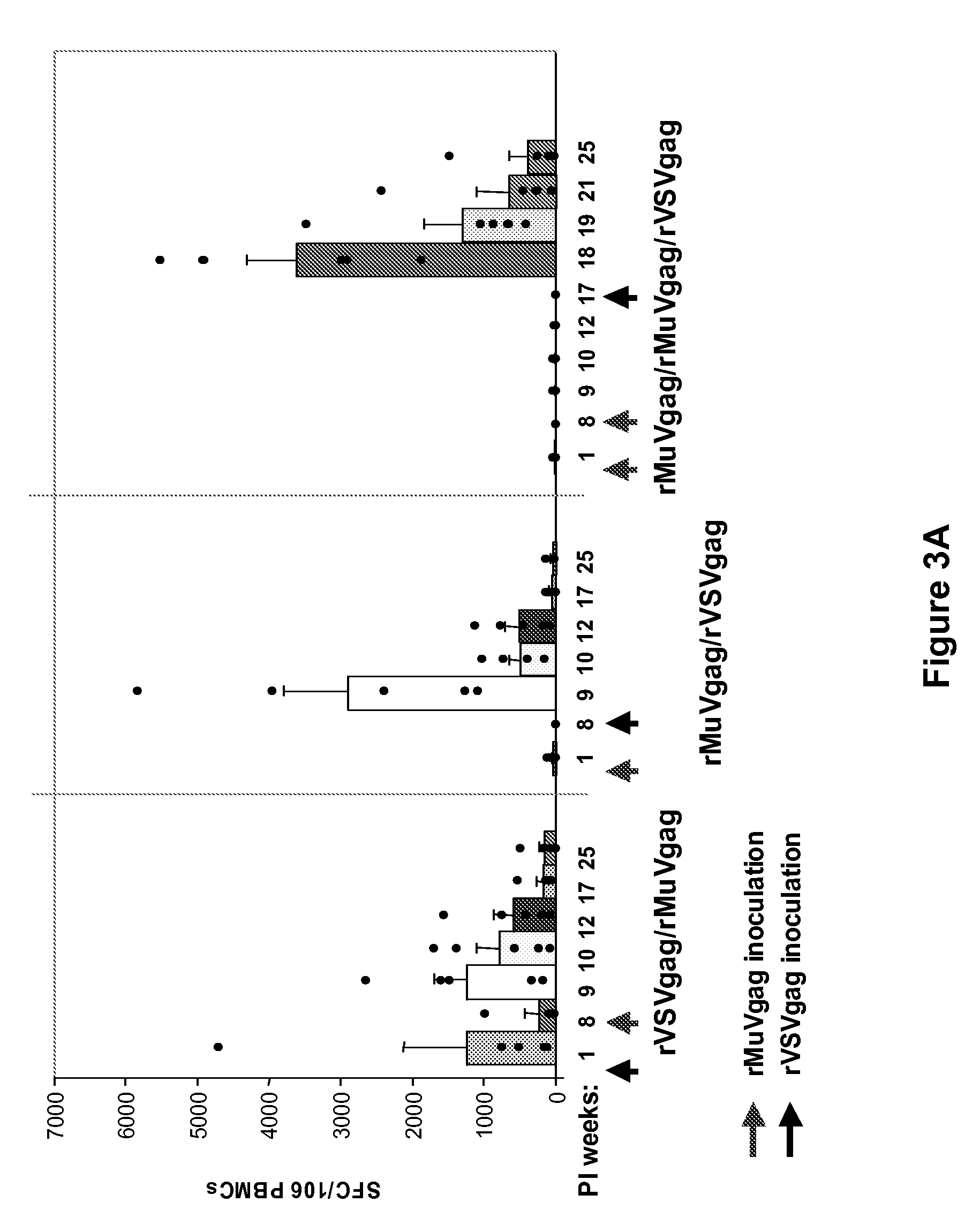
Heterologous prime-boost immunization regimen
InactiveUS20090092635A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsRegimenPrime boost
The present invention is directed to a method for generating an antigen-specific immune response in a subject in general and in particular to administering a priming dose of an immunogenic composition of a recombinant mumps virus (rMuV) that encodes an antigen followed by administering a boosting dose of recombinant vesicular stomatitis virus (rVSV) encoding an antigen.
Owner:WYETH
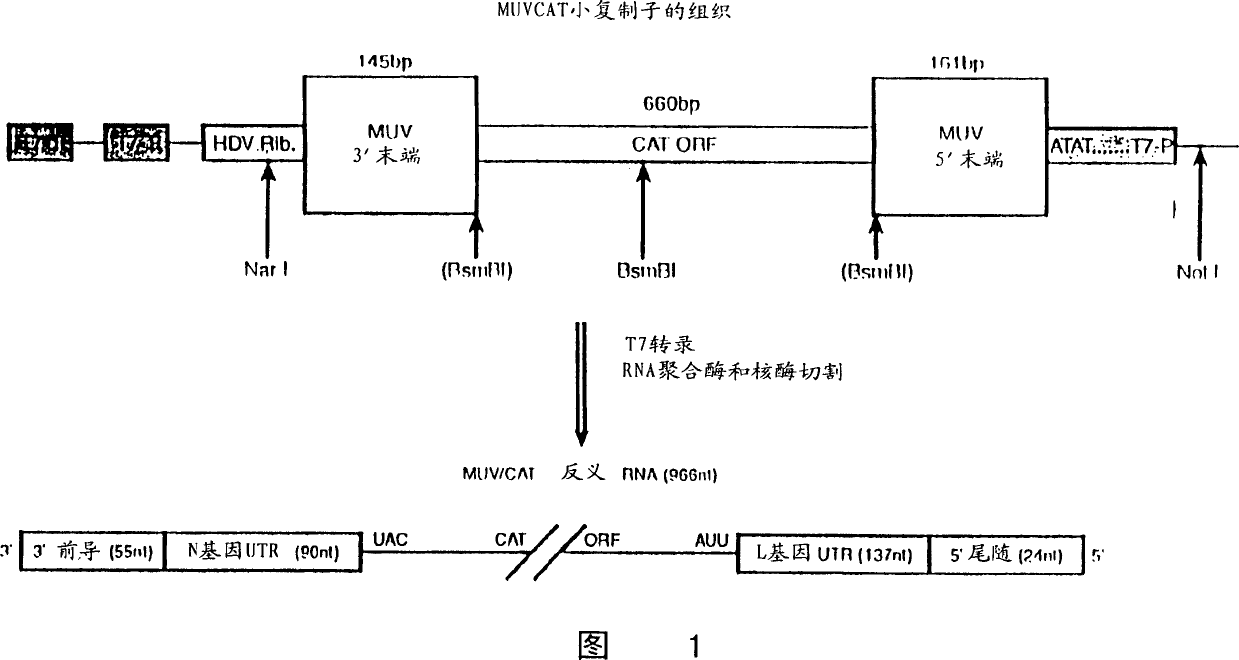
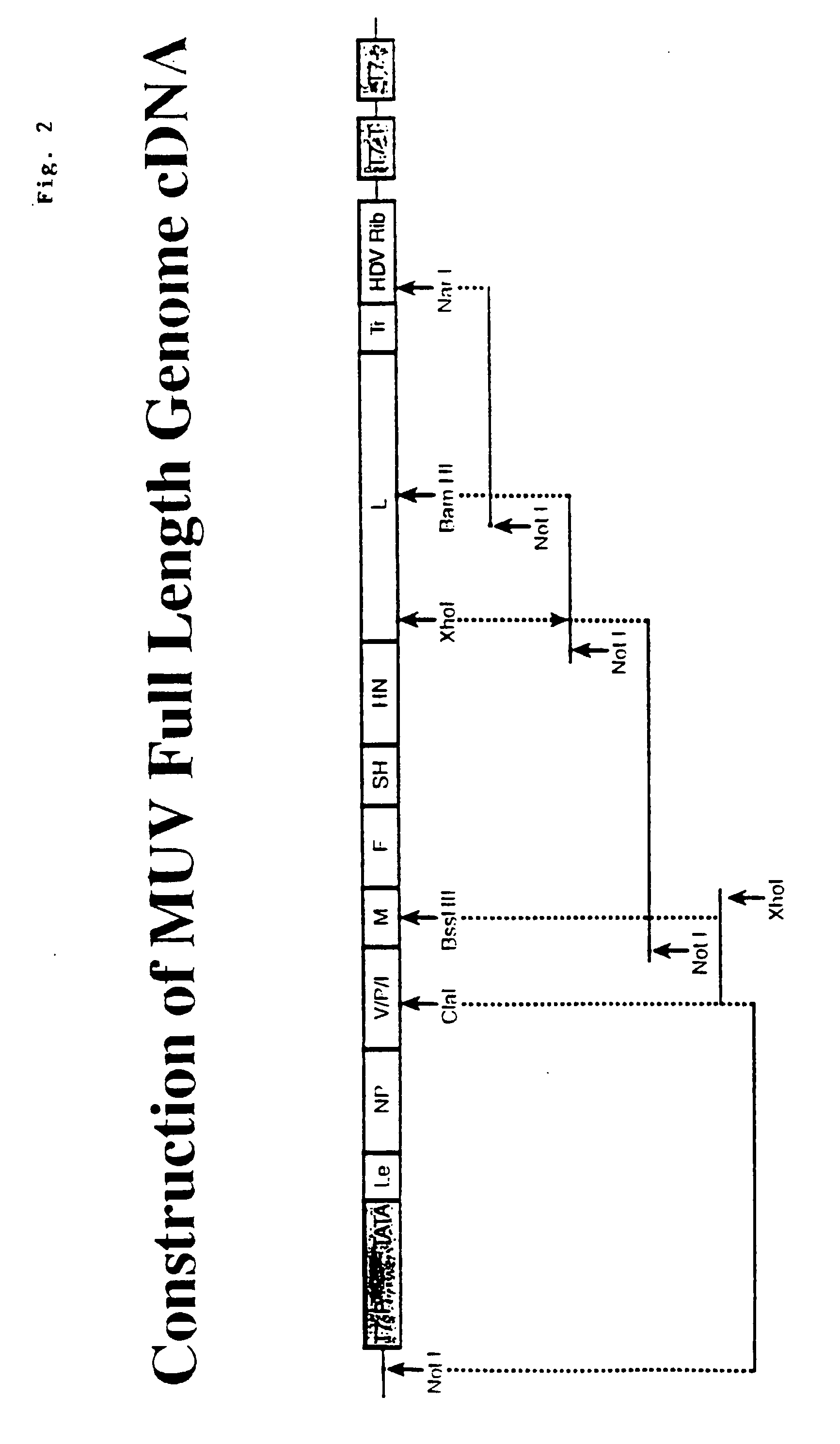
Rescue of mumps virus from cDNA
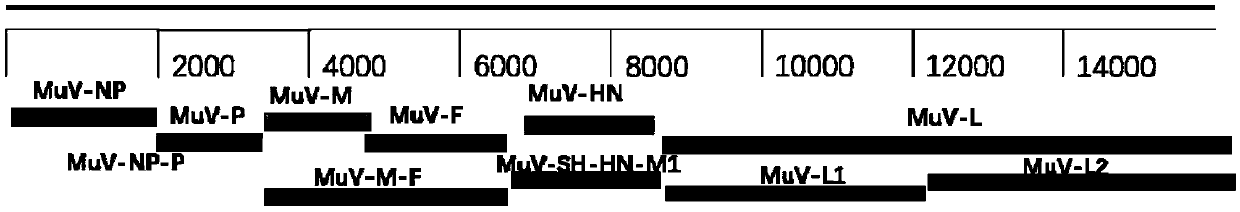
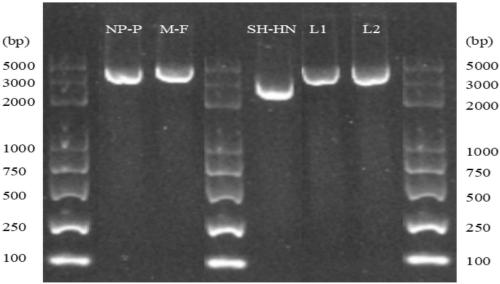
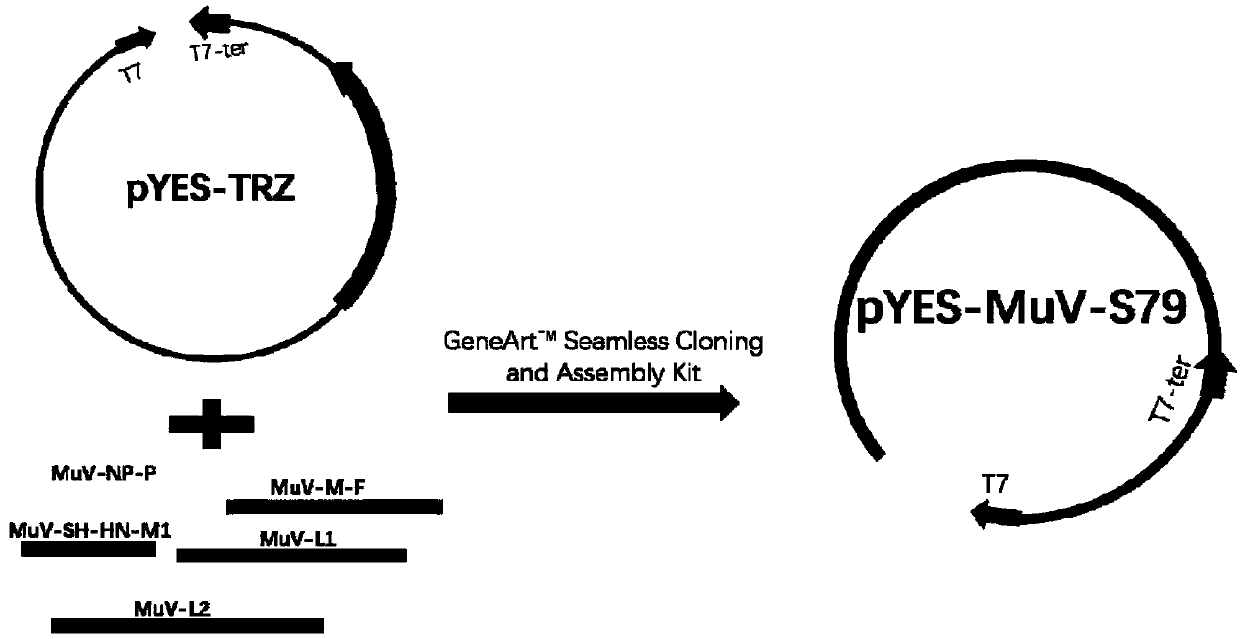
The present invention relates to a method of recombining and producing immunogenic composition by rescuing non-segmented antisense single-stranded RNA virus-mumps virus. Other embodiments relate to methods of making attenuated and / or infectious mumps viruses. Prepare recombinant viruses from cDNA clones to make specific changes, including deletions, insertions, substitutions and rearrangements of nucleotides / polynucleotides within the genome.
Owner:WYETH LLC
MRNA methyltransferase-deficient mumps virus and preparation method and application thereof
ActiveCN109628414AImprove securityImprove stabilitySsRNA viruses negative-senseViral antigen ingredientsMumps virus vaccineVirulent characteristics
The invention discloses an mRNA methyltransferase-deficient mumps virus and a preparation method and application thereof, and belongs to the technical field of reverse genetics. The mRNA methyltransferase-deficient mumps virus is prepared through site-directed mutation of a mumps virus vaccine S79 strain, wherein the amino acid at the site 1792 of the L protein is mutated from K to A, or the aminoacid at the site 1814 of the L protein is mutated from A to G, or the amino acid at the site 1816 is mutated from G to A, or the amino acid at the site 1818 is mutated from G to A, or the amino acidat the site 1892 is mutated from D to A, or the amino acid at the site 1917 is mutated from D to A, or the amino acid at the site 1953 is mutated from K to A, or the amino acid at the site 1990 is mutated from E to A. According to the invention, through site-directed mutation, the rescued recombinant mumps virus is weakened in virulence and good in immunogenicity, so that the immunogenicity of themumps virus attenuated vaccine is retained, and the safety of the mumps virus attenuated vaccine is improved.
Owner:ZHEJIANG UNIV
Compound of sulfoacid flavonecosid component in chickweed and the antivirus application and the preparing method
The invention relates to a wide-spectrum antiviral natural drug, while present world is lack in high-effect safe wide-spectrum antiviral natural drug. The invention, via two the adsorptions of two different resins, extracts a compound from plant FanLu, while the compound contains oligosaccharide or / and polyose and flavonoid as brown flavonoid glycoside with sulfonic acid group formed with apione, whose molecule weight is lower than 4, 000. The inventive compound can be used to treat AIDS, hepatitis viruse, influenza virus, or the like, without toxicity, while it can be made into 10 kinds of agent and health-care products.
Owner:朱耕新
Rescue of mumps virus from cDNA
InactiveUS7361496B1SsRNA viruses negative-senseViral antigen ingredientsSingle-Stranded RNANucleotide
This invention relates to a method for recombinantly producing, via rescue of mumps virus, a nonsegmented, negative-sense, single-stranded RNA virus, and immunogenic compositions formed therefrom. Additional embodiments relate to methods of producing the mumps virus as an attenuated and / or infectious virus. The recombinant viruses are prepared from cDNA clones, and, accordingly, viruses having defined changes, including nucleotide / polynucleotide deletions, insertions, substitutions and re-arrangements, in the place of the genome are obtained.
Owner:WYETH
System used for rescuing mumps virus and rescue method
PendingCN109593784AShorten the timeLow costSsRNA viruses negative-senseFermentationVaccine ProductionComplementary deoxyribonucleic acid
Owner:ZHEJIANG UNIV
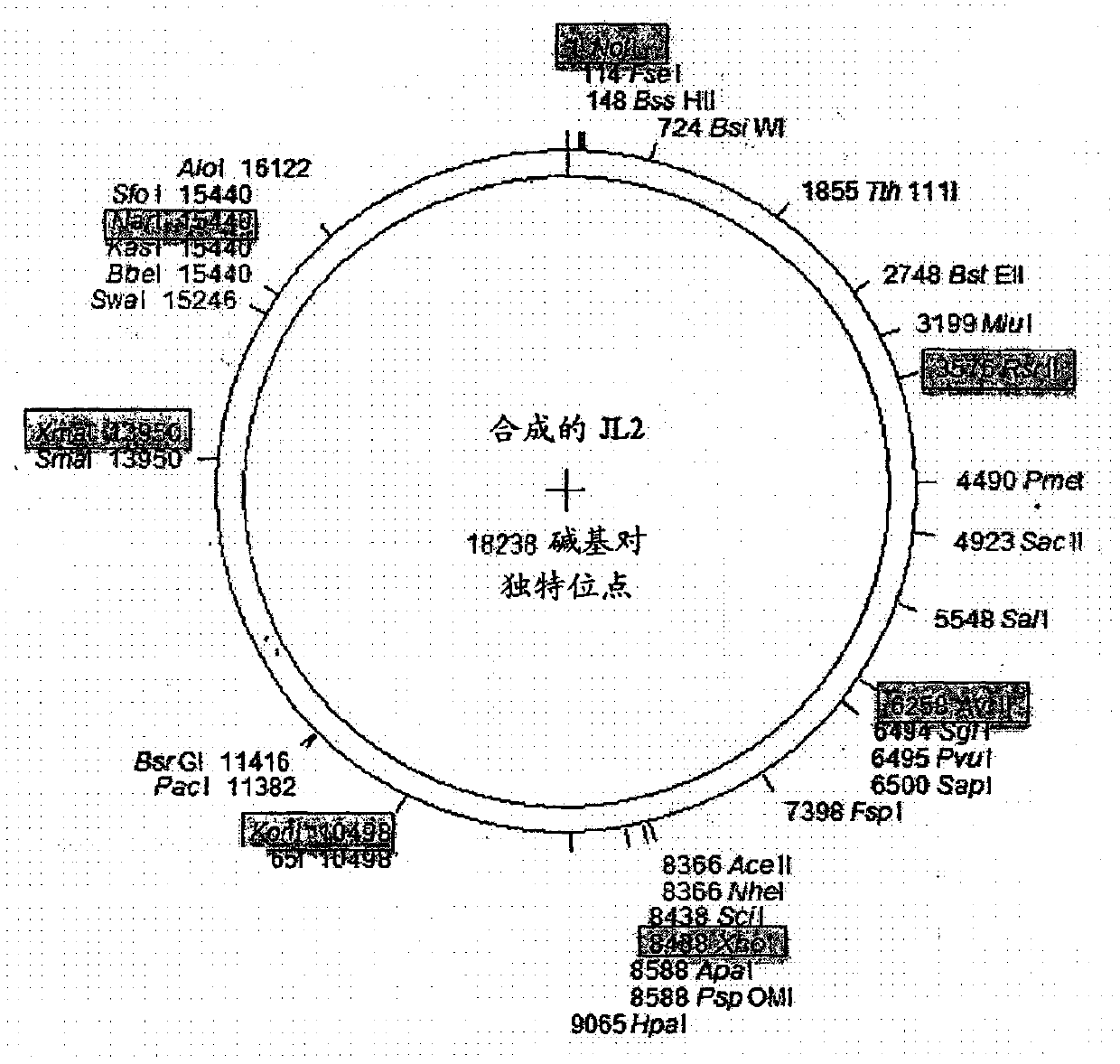
Recombinant mumps virus jeryl lynn 2 based vaccine
InactiveCN107667175AProperly obtainedSsRNA viruses negative-senseSenses disorderJeryl LynnImmunogenicity
The present invention provides a novel vaccine against mumps composed of highly immunogenic rMuVJL2 (recombinant mumps virus Jeryl Lynn2 based). Further, method to develop said immunogenic compositionis described in the present invention. The vaccine according to the present invention is safe, cost effective, highly efficacious and stable and consistent in terms of productivity.
Owner:CADILA HEALTHCARE LTD
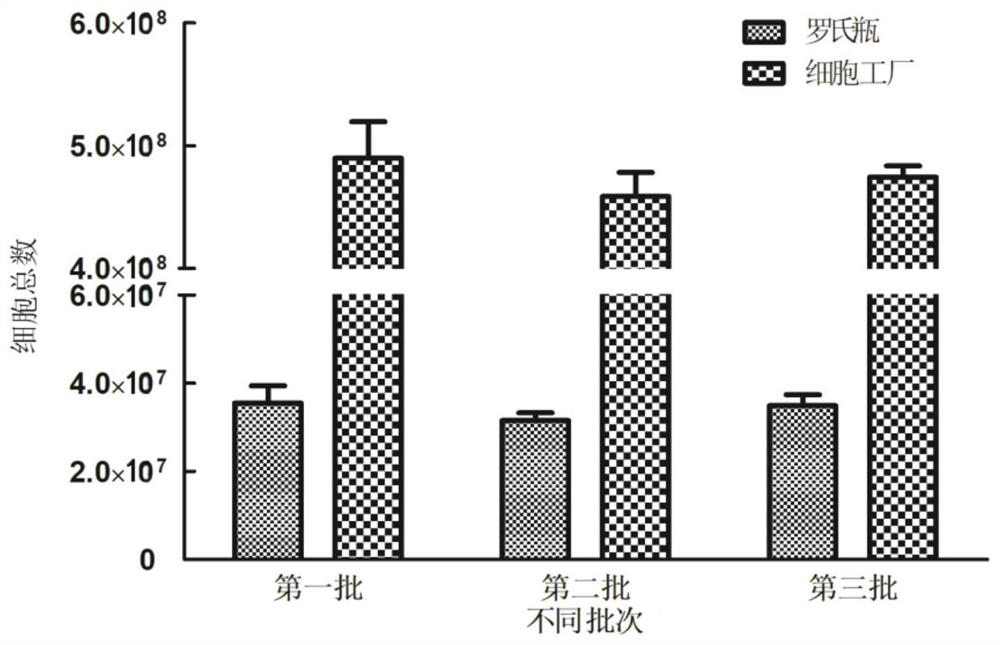
Large-scale preparation method of F genotype mumps attenuated live vaccine
PendingCN111840534AImprove effectivenessEnsure safetySsRNA viruses negative-senseViral antigen ingredientsCell factoryGenotype
The invention discloses a large-scale preparation method of an F genotype mumps attenuated live vaccine. The preparation method comprises the following steps: passage and counting of KMB-17 cells, preparation of a virus harvesting liquid, preparation of a vaccine stock solution, preparation of a vaccine semi-finished product and preparation of a finished product. The F genotype mumps virus attenuated live vaccine is prepared on a large scale by taking human diploid cells as a matrix in a cell factory for the first time, so that the production cost can be reduced and the production time can besaved while the safety and the quality stability of the vaccine are ensured. Compared with traditional Roche flask culture, the method has the advantages that the amount of required virus seeds is small, 60000 vaccine finished products can be obtained only by 60-84 mL of virus seeds in each batch, the yield is high, the cost is low, the quality is stable, and the like, and the method has good application prospects in preventing and controlling infection and transmission of epidemic parotitis in China.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Immunotherapy of epithelial tumors using intralesional injection of antigens that induce a delayed type hypersensitivity reaction
InactiveUS20050175634A1Successful and long lasting resultIncrease potential valueViral antigen ingredientsFungal antigen ingredientsAbnormal tissue growthVirus warts
The pharmaceutical composition is useful for treating epithelial tumors in a subject and contains at least two antigens and a pharmaceutically acceptable carrier, where each of the antigens induces or is capable of inducing a cutaneous delayed type hypersensitivity (DTH) response in the subject. This composition is particularly useful in treating epithelial tumors, such as warts or verrucae, that are induced by or related to papillomavirus. Antigens useful in the present pharmaceutical composition are anergy panel antigens, such as killed mumps virus, candida extract, trichophyton extract or comparable antigenic extracts. An additional pharmaceutical composition, also useful for treating epithelial tumors, contains at least one antigen that induces or is capable of inducing a cutaneous DTH response in a subject, at least one cytokine or colony stimulating factor and a pharmaceutically acceptable carrier. Kits containing these pharmaceutical compositions are useful for this immunotherapy.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Mumps virus ingredient vaccine for human, and its preparation method and uses
ActiveCN1843507AImproving immunogenicityImprove qualityAntiviralsAntibody medical ingredientsAntigenDecomposition
The invention relates to a mumps virus ingredient vaccine for human, and its preparation method and uses, wherein the preparing process comprises isolating a parotiditis virus from human body infected by parotiditis virus through conventional methods, adapting the virus to Vero cell culture to prepare single-layer static or rotary Vero cell culture, carrying out virus deactivation, decomposition and purification, predicating the two antigenic components of HN and NP by means of molecular weights, using the antigen composition as the semi-finished vaccine after protein content measurement according to the semi-finished vaccine protein concentration, diluting to 100mul / ml with physiological saline, formulating Al(OH)3 with a concentration of 10.87mg / ml simultaneously, finally mixing the two formulations.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Composition of starwort total glycopeptides and total flavone and preparation method and uses thereof
InactiveCN101390921ASafe broad-spectrum antiviral drug actionHigh-efficiency broad-spectrum antiviral drugsBiocideOrganic active ingredientsAlkaneDisease
Disclosed is a dark brown combination with the molecular of 1,000-100,000, which is composed of total glycopeptides ingredient and total flavonoid ingredient, and extracted from stellaria through three methods and used as safer and more efficient natural broad-spectrum anti-viral medicine. The total peptide part accounts for 15%-25% and is composed of 17 types of amino acids respectively according to the proportions, such as by proportion, such as aspartic acid, methionine and isoleucine; the total sugar accounts for 15%-30% and is composed of glucose, galactose and arabinose; the total flavonoid which is mainly apigenin glycoside accounts for 10%-40%; Oxygenated alkane which contains alcohols accounts for 25%-40%. The combination can be used as the medicines for the treatment of the diseases, including HIV, hepatitis viruses, influenza virus such as Highly-pathogenic avian influenza virus, condyloma virus, herpes virus and mumps virus, and have no toxicity after application; the combination can be made into more than 10 types of medicinal preparations and health care products, and avoid environmental pollution during the production process.
Owner:朱耕新
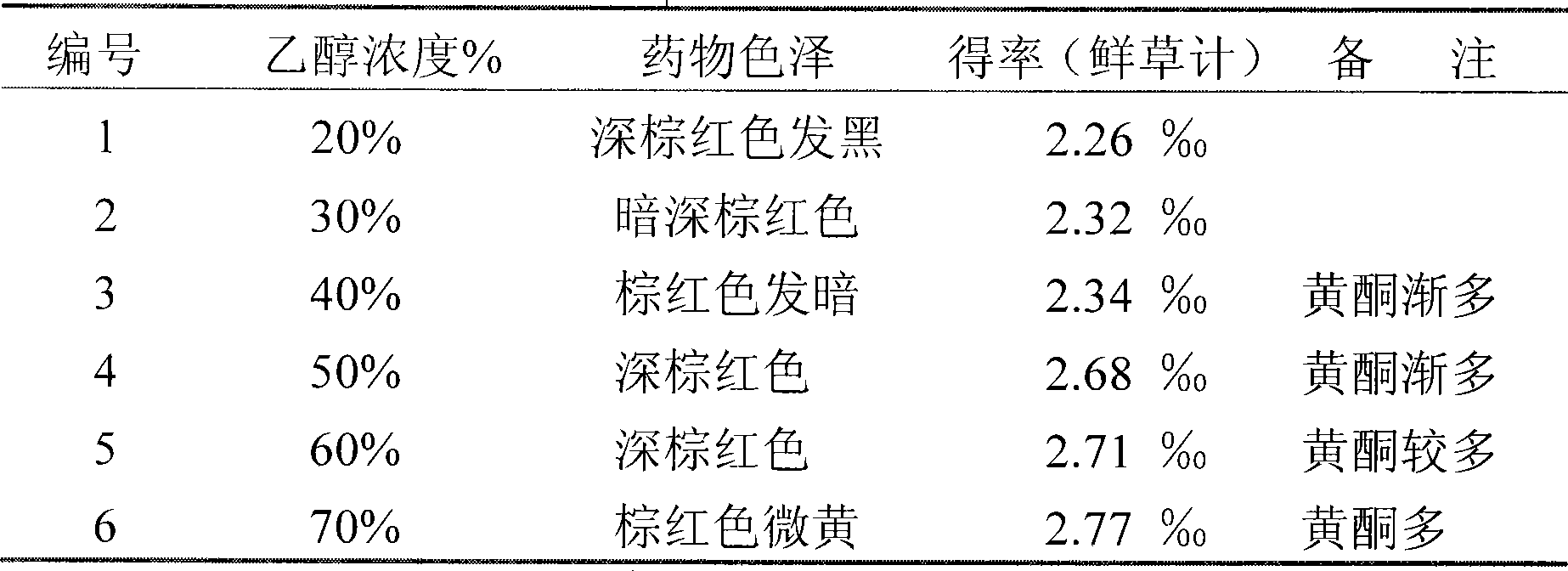
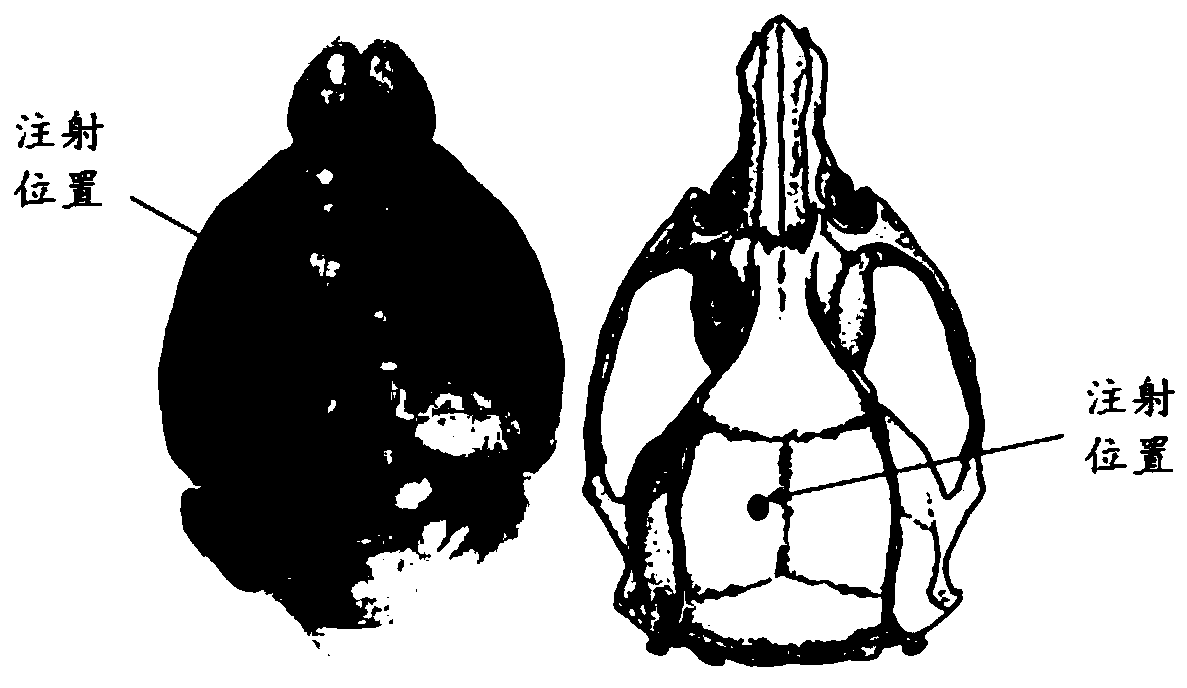
Evaluation model for rat neurotoxicity of parotitis virus
ActiveCN110736654AEasy to operateLow costSsRNA viruses negative-senseImage analysisTGE VACCINEMumps virus
The invention provides an evaluation model for rat neurotoxicity of parotitis virus, concretely a device for evaluating the neurotoxicity of the parotitis virus. The model comprises(I) a virus inoculation module for performing virus inoculation of the parotitis virus to be evaluated to the lateral ventricle of a rat; (II) a processing module for carrying out vibration slicing on the fixed rat brain; (III) an imaging module for scanning and imaging an obtained mouse brain slice; and (IV) an analysis module used for calculating a neurotoxicity index through a formula I: the neurotoxicity index =S1 / S0 *100 (formula I) according to the cross sectional area S1 of a cavity formed by hydrocephalus in the longitudinal section of the rat brain and the total cross sectional area S0 of the rat brainin the obtained image. The results show that the result is stable, the stability is high, wild strains can be distinguished from vaccine strains, and compared with an existing monkey body neurotoxicity model, the animal cost and the operation difficulty are greatly reduced.
Owner:SHANGHAI KING CELL BIOTECHNOLOGY CO LTD +1
Parotiditis virus fluorencent amplification detection reagent box and detection method
InactiveCN1948507AImprove isolationEasy diagnosisMicrobiological testing/measurementFluorescence/phosphorescenceDiseaseHemagglutinin
The invention offers detecting reagent boxes for fluorescent augment of mumps virus, which include hemagglutinin gene standards of mumps virus, detecting reagent of fluorescent augment, DNA polymerase and reverse transcriptase. The detecting reagent of fluorescent augment mainly contains buffer solution of one-step RT-PCR, specificity exciters and probes, mixture of deoxidizing triphosphoric acid and nucleoside. The partial sequence of hemagglutinin gene standards of mumps virus is 5'- CTCAAGGACTGTTTGCCTCTTACACCACAACCACCTGCTTTCAAGATACCGGTGATGCTAGTG-3'. The specificity exciters have two sequences: the sequence of upstream exciters is 5'-CTCAAGGACTRTYTGCYTCSTA-3'and the sequence of downstream exciters is 5'-CTCTRGCAT CACCGGTATCTTGAA-3'. The equence of specificity probes is 5'-FAM-ACCACAACCACCTGC-NFQ-MGB-3', in which FAM is reporting fluorescent metakliny, NFQ is non-fluorescent annihilation metakliny, MGB is modifying metakliny. The detecting reagent boxes can detect pathogen nucleic acid directly. At the early stage of mumps disease, specificity gene of mumps virus can be detected, which facilitates early isolation, diagnosis and therapy.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Immunization compositions and methods
ActiveUS8697353B2SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
Recombinant mumps virus vaccine
ActiveUS20140010840A1SsRNA viruses negative-senseSenses disorderMumps virus vaccineOpen reading frame
The present invention provides the complete genomic sequence of the epidemic mumps virus (MuV) strain MuVIowa / us / 06. Further, a reverse genetics system was constructed and used to rescue recombinant viral constructs that are attenuated compared to MuVIowa / us / 06 and JL vaccine viruses. Such constructs include viral constructs lacking the open reading frame (ORF) of the SH gene (rMuVΔSH) and / or incapable of expressing the V protein (rMuVΔV).
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES FOOD & DRUG ADMINISTRATION +1
Chinese herbal medicinal disinfection incense and production method thereof
InactiveCN105994428AImprove the immunityNo pollutionBiocideDead animal preservationSide effectAdhesive
The invention discloses a Chinese herbal medicinal disinfection incense, and relates to the technical field of live pig farming. The Chinese herbal medicinal disinfection incense is produced from 50-60 parts of wood powder, 15-18 parts of an alcohol extract liquid of Atractylodes lancea, 16-20 parts of an alcohol extract liquid of Artemisia argyi, 10-15 parts of an alcohol extract liquid of dahurian angelica root, 12-14 parts of an alcohol extract liquid of Acorus calamus Linn, 8-15 parts of an alcohol extract liquid of Juncus effusus, 10-16 parts of a Gleditsia sinensis extract liquid, 10-14 parts of a sweet wormwood seed extract liquid, 11-15 parts of a dried orange peel extract liquid, 3-8 parts of a combustion improver and 9-20 parts of an adhesive. The Chinese herbal medicinal disinfection incense is used for disinfecting air in hog houses, has an obvious antibacterial effect on bacteria and fungi, has an inhibition effect on adenoviruses, rhinoviruses, herpes viruses, influenza viruses and mumps viruses, is reasonable and safe, is convenient for enhancing the immunity of pigs, and also has the advantages of disease prevention and resistance, no toxic or side effects, no pollution to environment, fast action and low price.
Owner:ANHUI YU WANG CULTURE
Rescue of mumps virus from CDNA
This invention relates to a method for recombinantly producing, via rescue of mumps virus, a nonsegmented, negative-sense, single-stranded RNA virus, and immunogenic compositions formed therefrom. Additional embodiments relate to methods of producing the mumps virus as an attenuated and / or infectious virus. The recombinant viruses are prepared from cDNA clones, and, accordingly, viruses having defined changes, including nucleotidelpoly / nucleotide deletions, insertions, substitutions and re-arrangements, in the place of the genome are obtained.
Owner:WYETH LLC
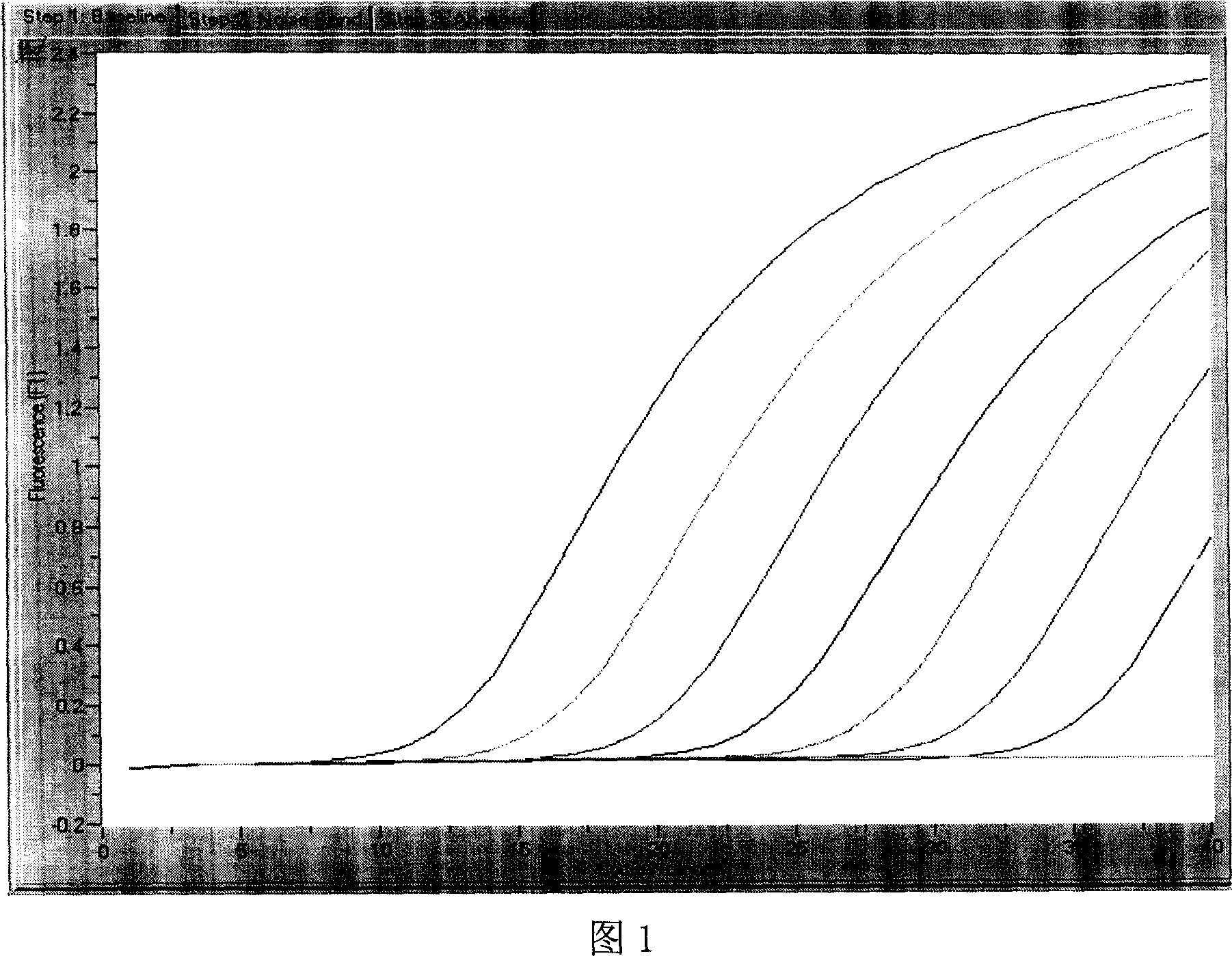
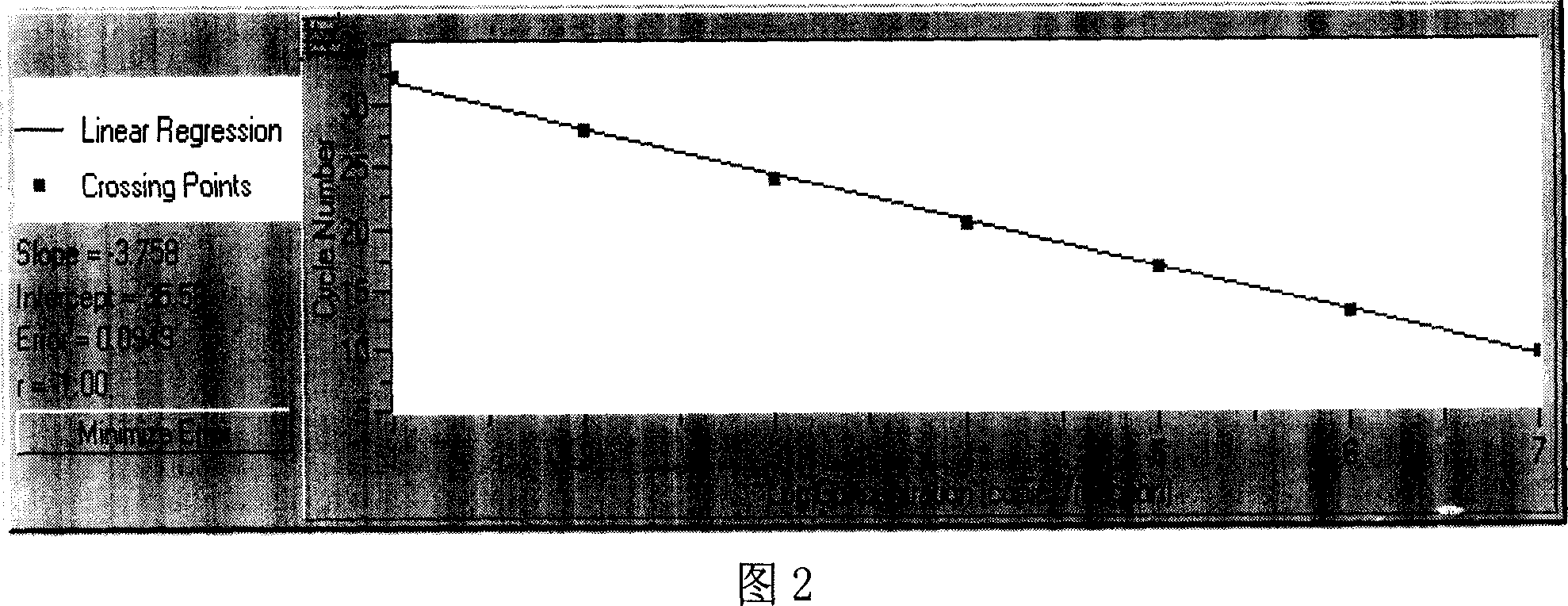
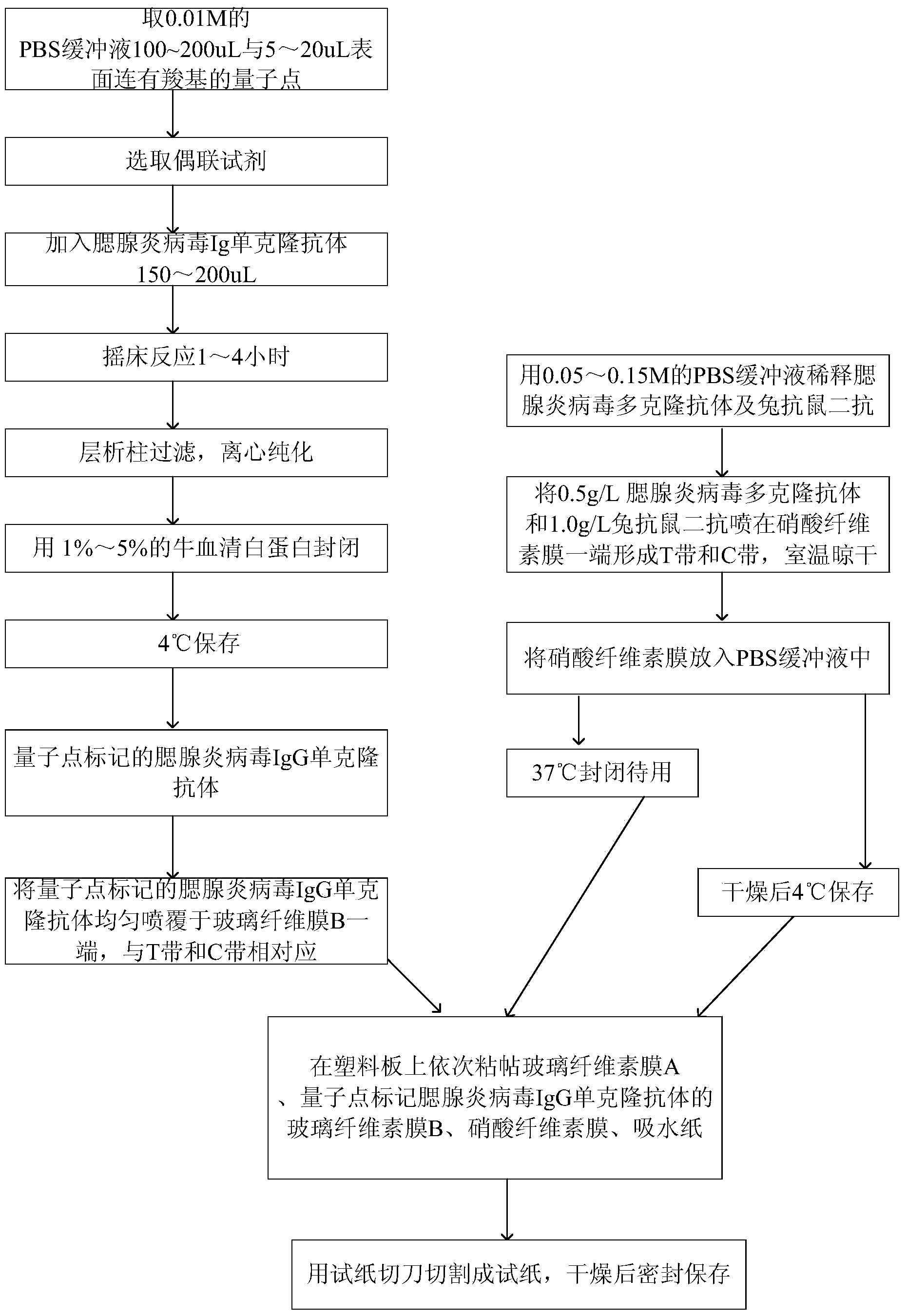
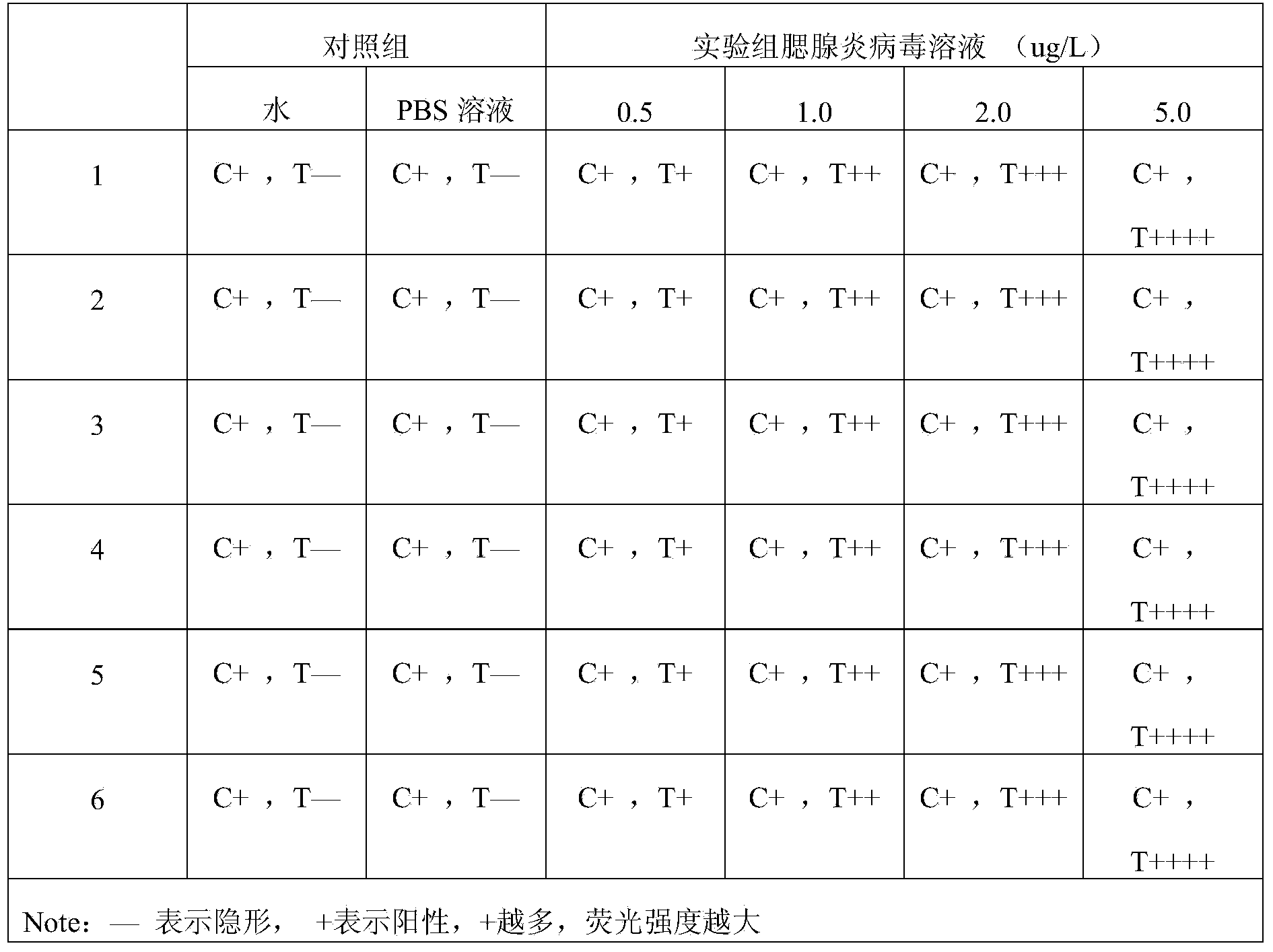
Method for detecting mumps virus, quantum-dot labeled immunochromatography test paper and preparation method thereof
ActiveCN103529216AHigh detection sensitivityHigh detection sensitivity than a rapid detection method commonly used at present - the detection sensitivity of colloidal goldBiological material analysisGlass fiberCellulose
The invention relates to a medical immunodetection method and particularly relates to a method for using quantum-dot labeled immunochromatography test paper to detect mumps virus by an immunological method. The quantum-dot labeled immunochromatography test paper is characterized in that a plastic board is stuck with a glass cellulose membrane A, a glass cellulose membrane B of a quantum-dot labeled mumps virus IgG monoclonal antibody, a nitrocellulose membrane and water absorbing paper from bottom to top in sequence, wherein one end of the nitrocellulose membrane is provided with mumps virus polyclonal antibody and rabbit antimouse second antibody so as to form a detecting band T and a quality control band C; the quantum-dot labeled mumps virus IgG monoclonal antibody is positioned at one end of the glass cellulose membrane and corresponds to the detecting band T and the quality control band C, and the quantum-dot labeled mumps virus IgG monoclonal antibody is positioned at one end of a sampling point. The method has the advantage that the detection sensitivity is about 1000 times higher than that of the commonly-used method.
Owner:北京华卫骥生物医药有限公司
Primer, probe and kit all used for detecting mumps virus
InactiveCN104593523AHigh sensitivityAvoid subjectivityMicrobiological testing/measurementMicroorganism based processesForward primerBiology
The invention discloses a primer, a probe and a kit all used for detecting mumps virus, and belongs to the technical field of kits. The primer and the probe comprise a forward primer for detecting mumps virus, a reverse primer for detecting mumps virus and a probe for detecting mumps virus. The kit comprises the primer and the probe. The primer, the probe, the kit and a detection method possess the characteristic of high sensitivity, the detection speed is fast, and the whole detection process only needs 2-3 h.
Owner:湖北永邦医疗科技有限公司
Recombinant virus and application thereof in mumps virus neutralizing antibody detection
ActiveCN113999822AShielding limitationsThe result is highly consistentSsRNA viruses negative-sensePeptidesNeutralising antibodyTiter
The invention relates to a recombinant virus and application thereof in mumps virus neutralizing antibody detection, and belongs to the technical field of biology. The invention provides a recombinant virus and a method for detecting a neutralizing antibody of a mumps virus by using the recombinant virus as a neutralizing virus. The recombinant virus takes the mumps virus as a live vector to express a marker gene; the traditional mumps virus neutralizing antibody detection method needs 7 days for final judgment of the result, while the neutralizing antibody detection method disclosed by the invention only needs 3 days, and the result conformity is extremely high; moreover, according to the neutralizing antibody detection method, the neutralizing titer of the antibody in the to-be-detected sample is calculated through fluorescence, the neutralizing antibody detection method can be used in a high-flux fluorescence detection instrument, data can be automatically and electronically stored, and meanwhile, the limitation of human factors in result judgment is shielded.
Owner:BEIJING CELL FUSION BIOTECHNOLOGY CO LTD
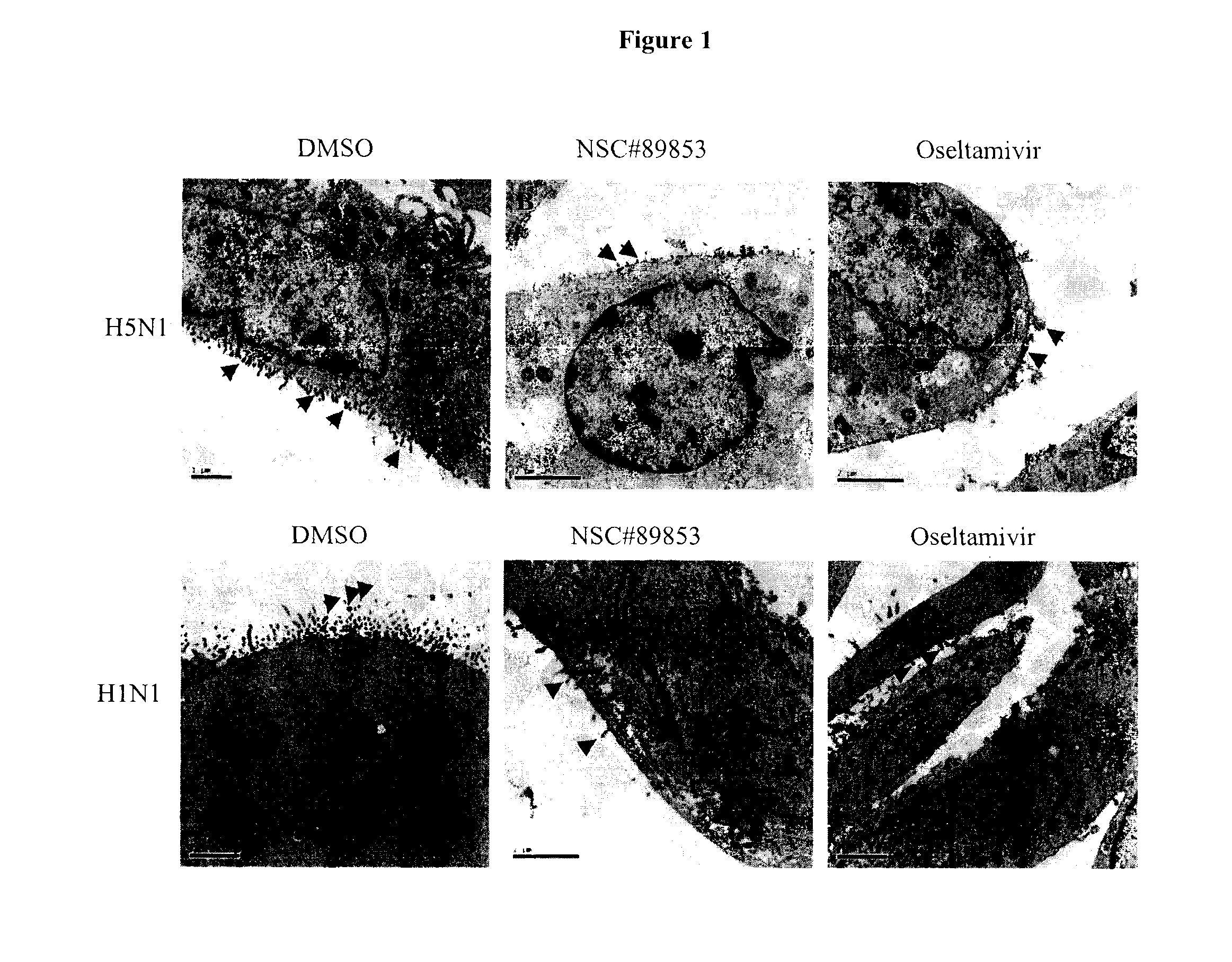
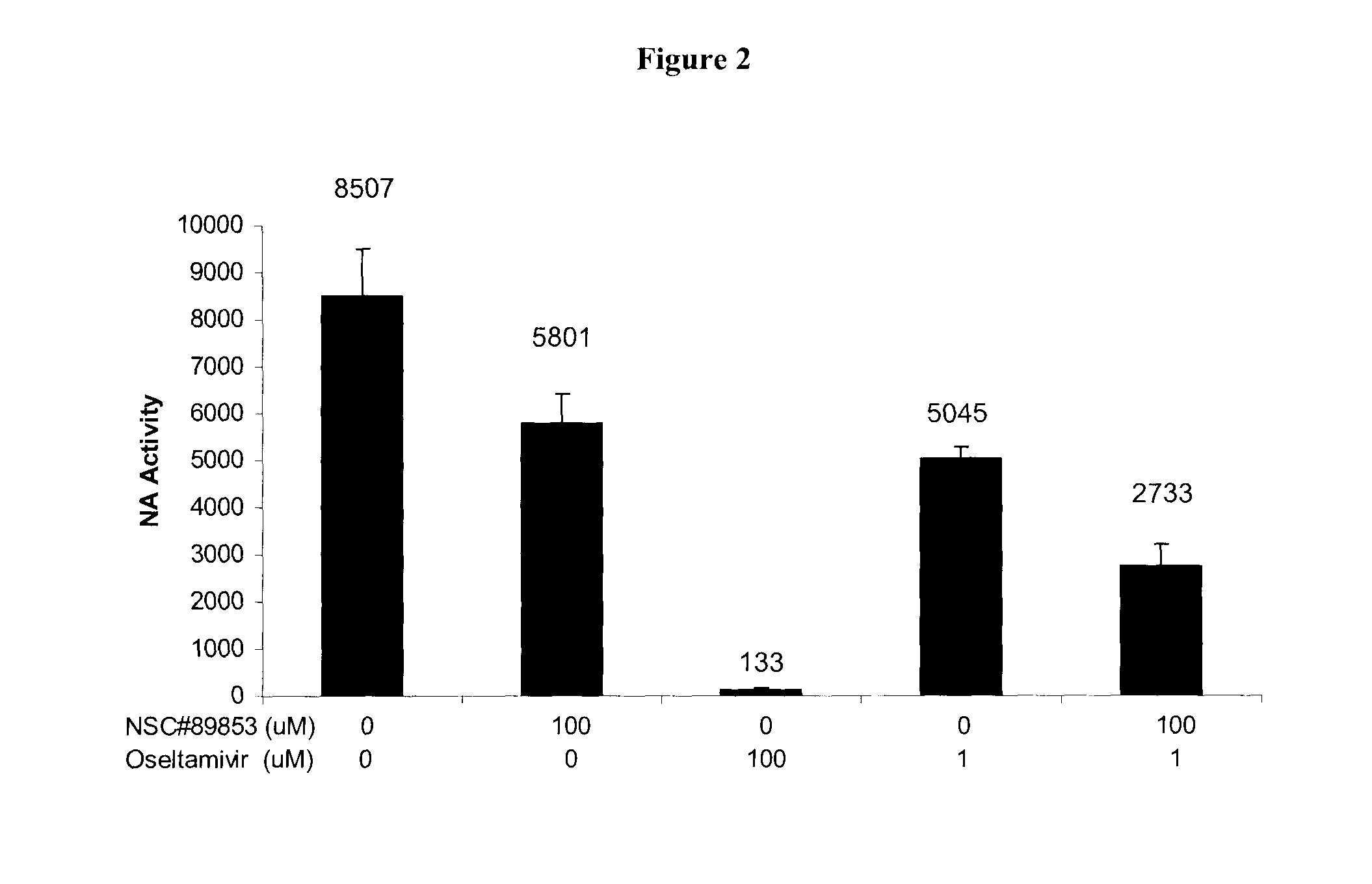
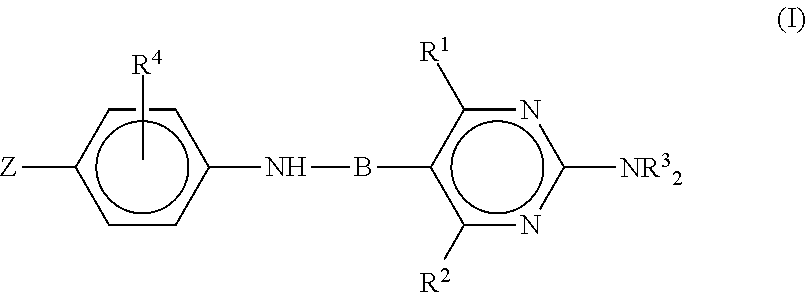
Anti-influenza compounds
The present invention provides pyrimidinyl compounds of formula (I) and pharmaceutically acceptable salts thereof. These compounds may be used for the inhibition of influenza. In particular, the compounds of the invention may be used for the treatment or prophylaxis of influenza A, most particularly H1N1 or H5N1 influenza. The compounds of the invention can also be used for the treatment or prophylaxis of a disease caused by Vibrio cholerae, Clostridium perfringens, Streptococcus pneumoniae, Arthrobacter sialophilus, an orthomyxovirus, a paramyxovirus, a parainfluenza virus, mumps virus, Newcastle disease virus, fowl plague virus or Sendai virus.
Owner:VERSITECH LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com