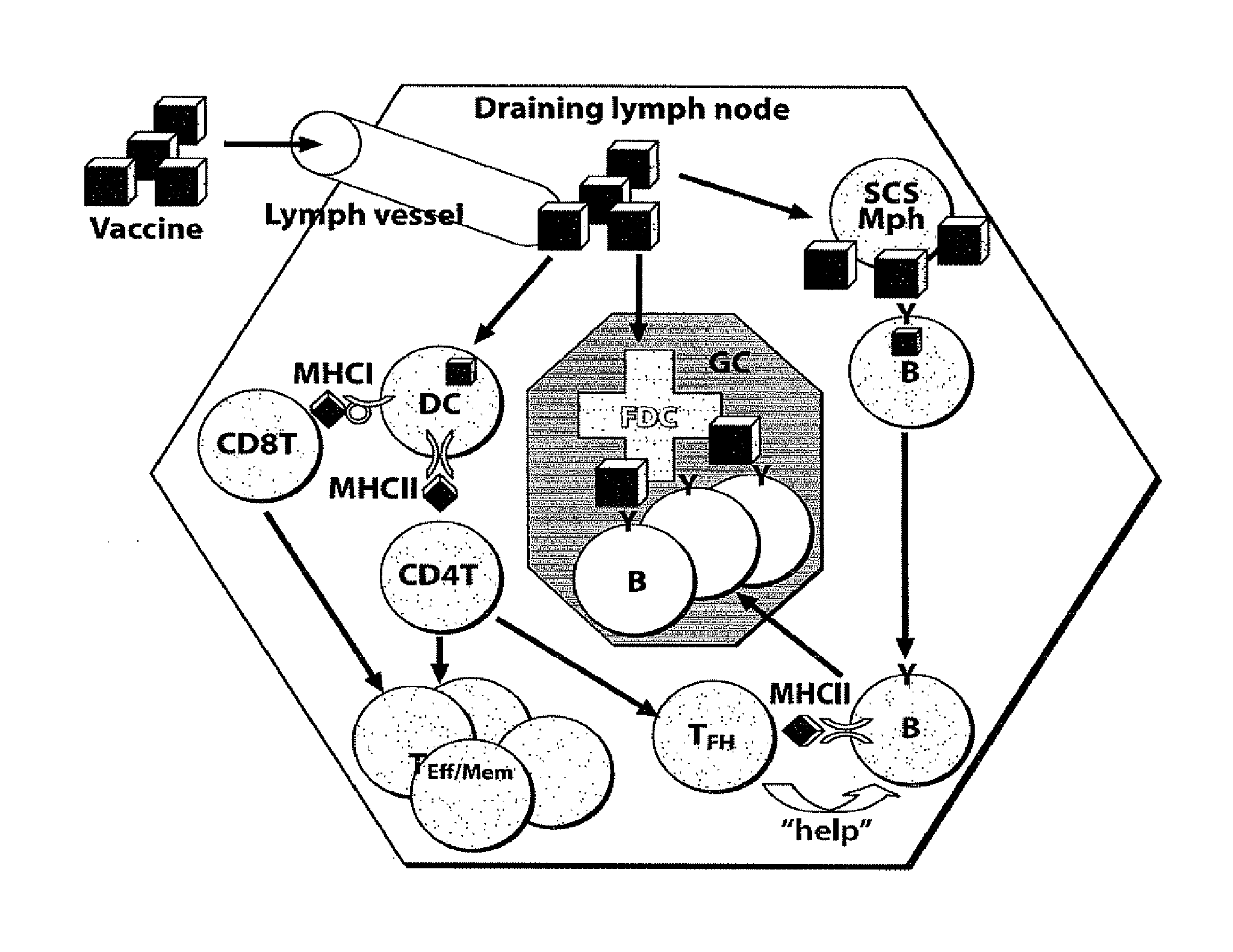
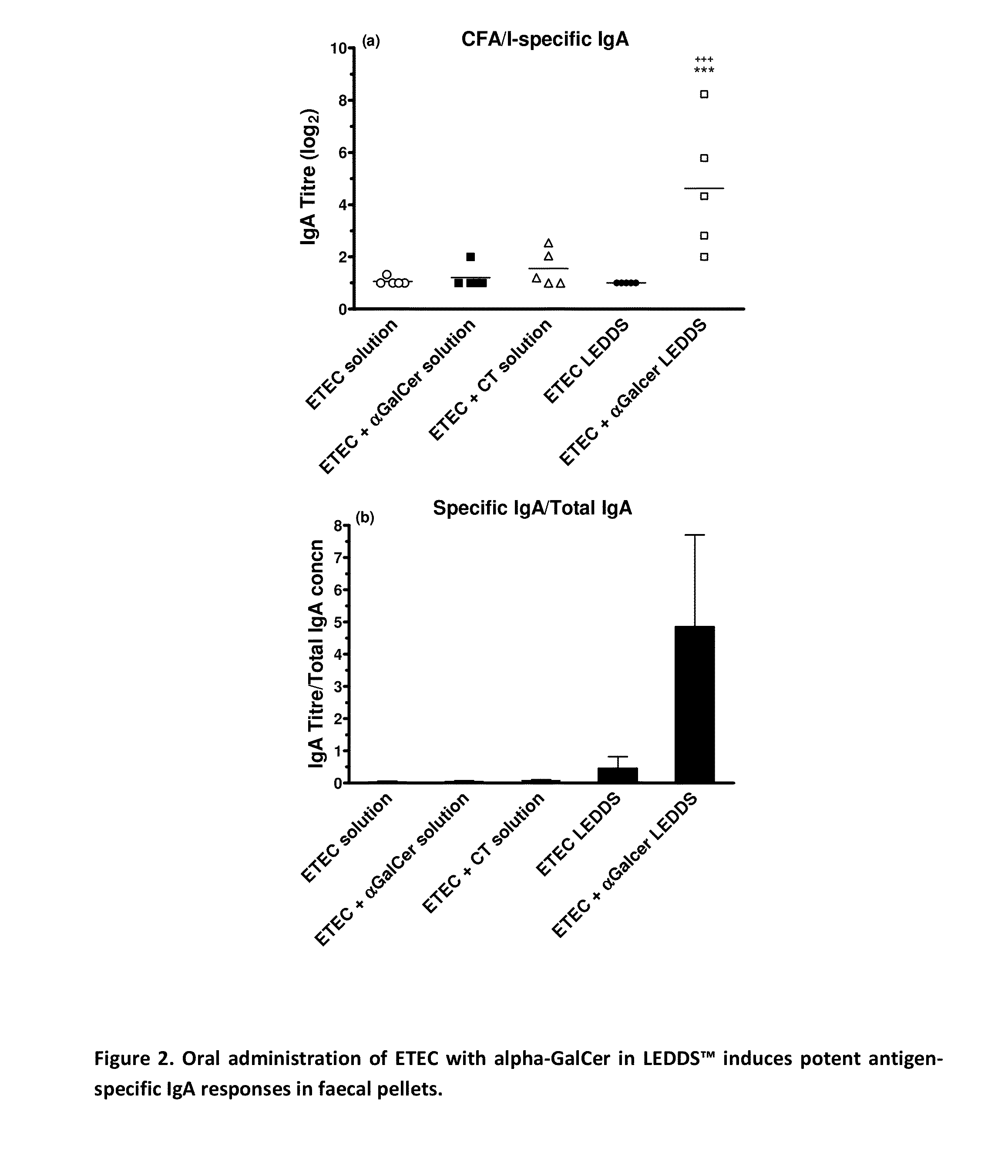
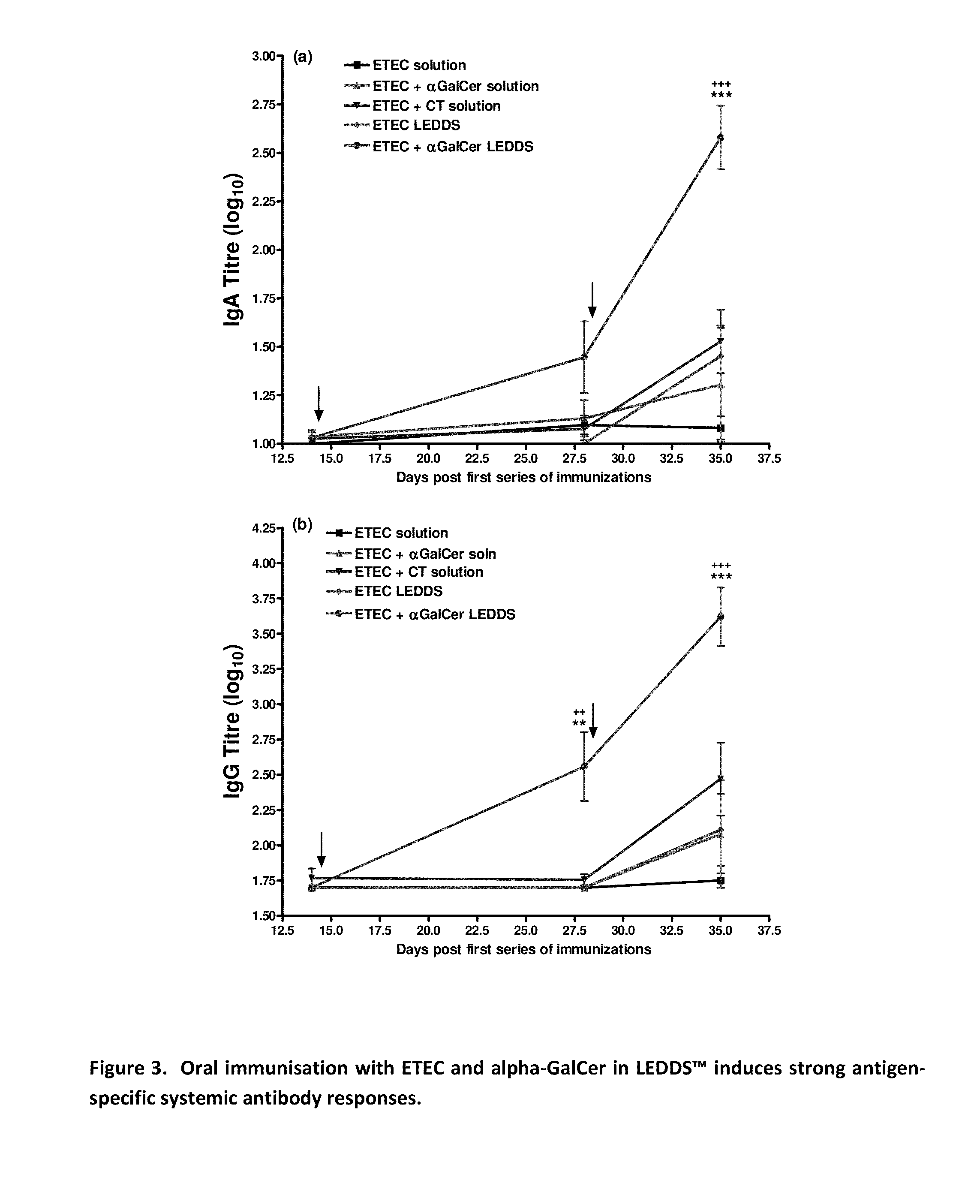
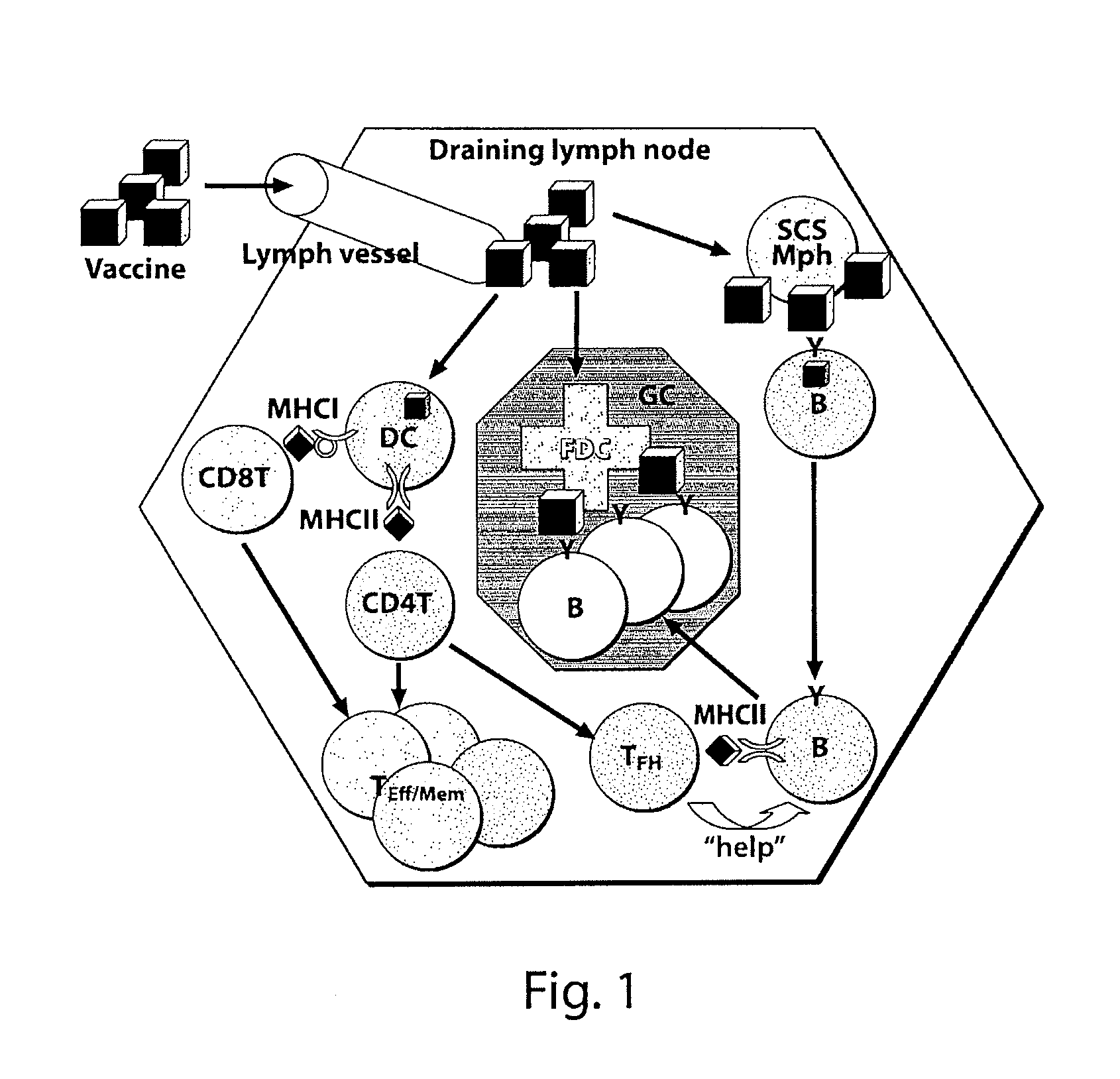
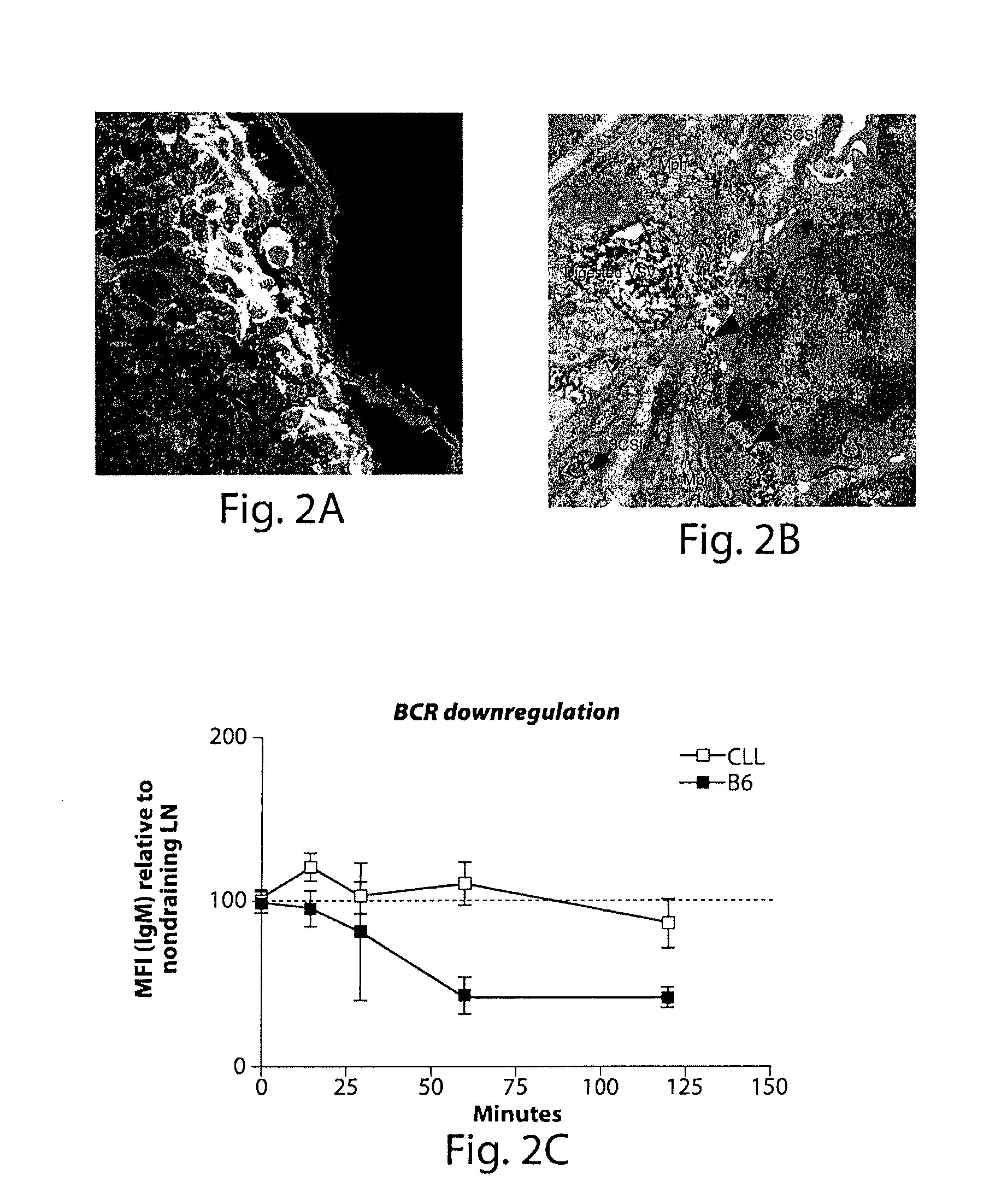
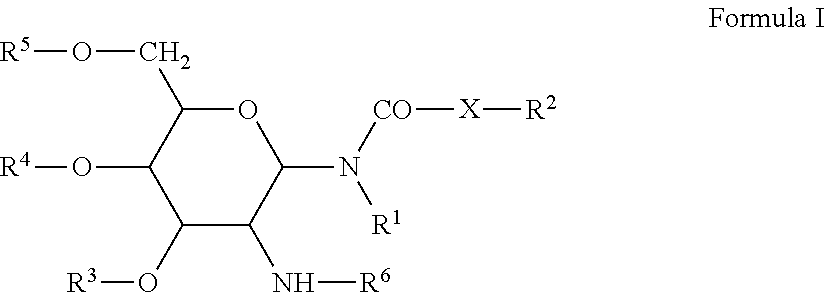
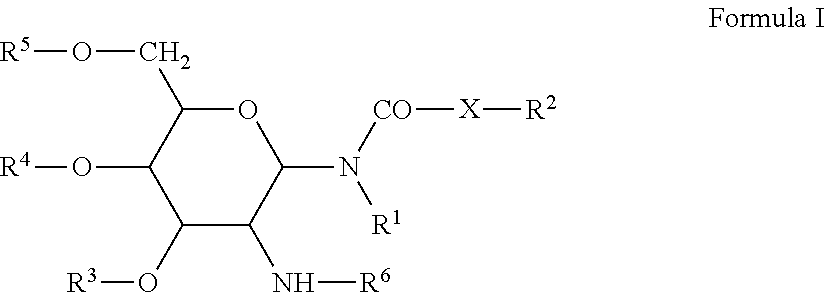
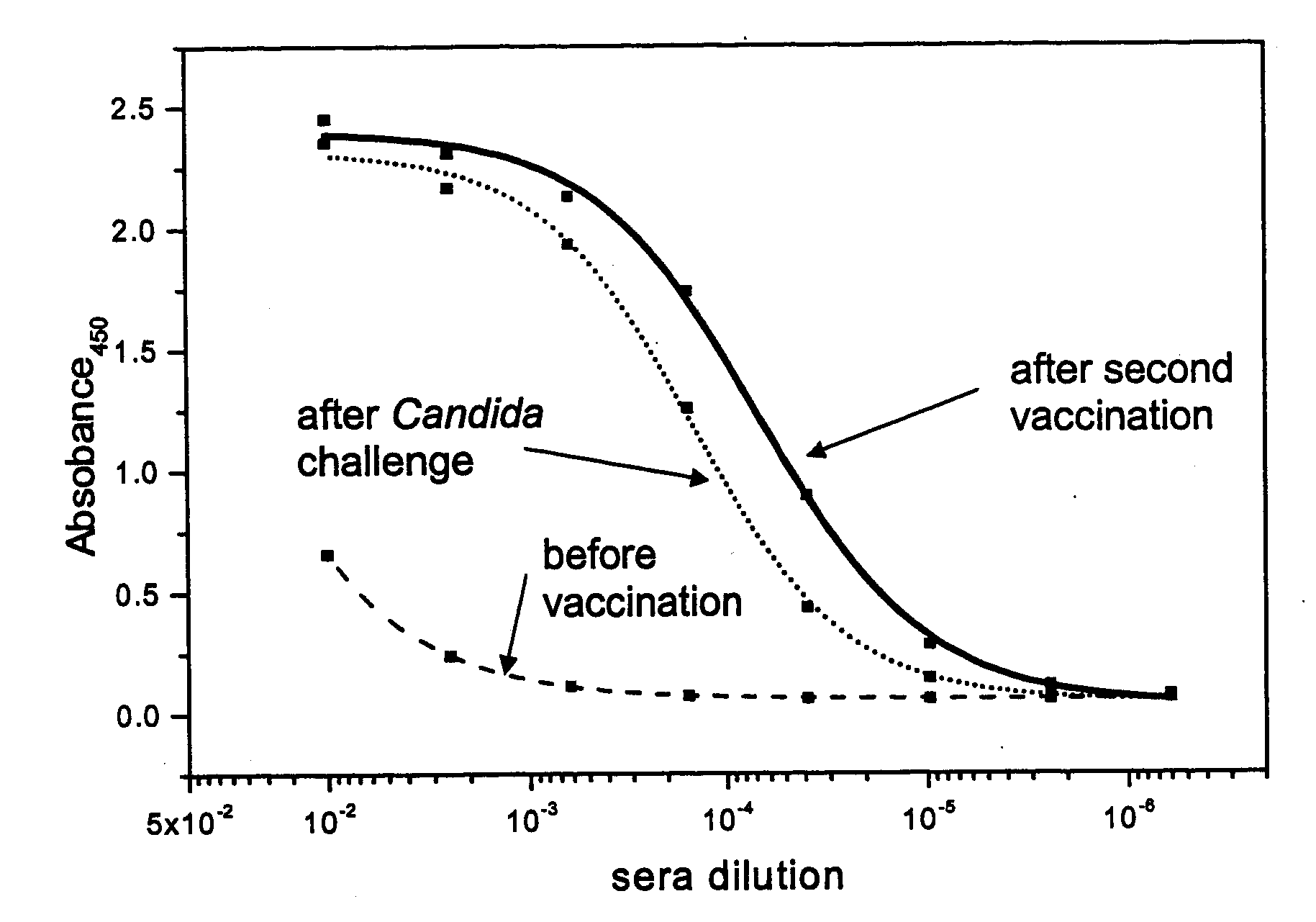
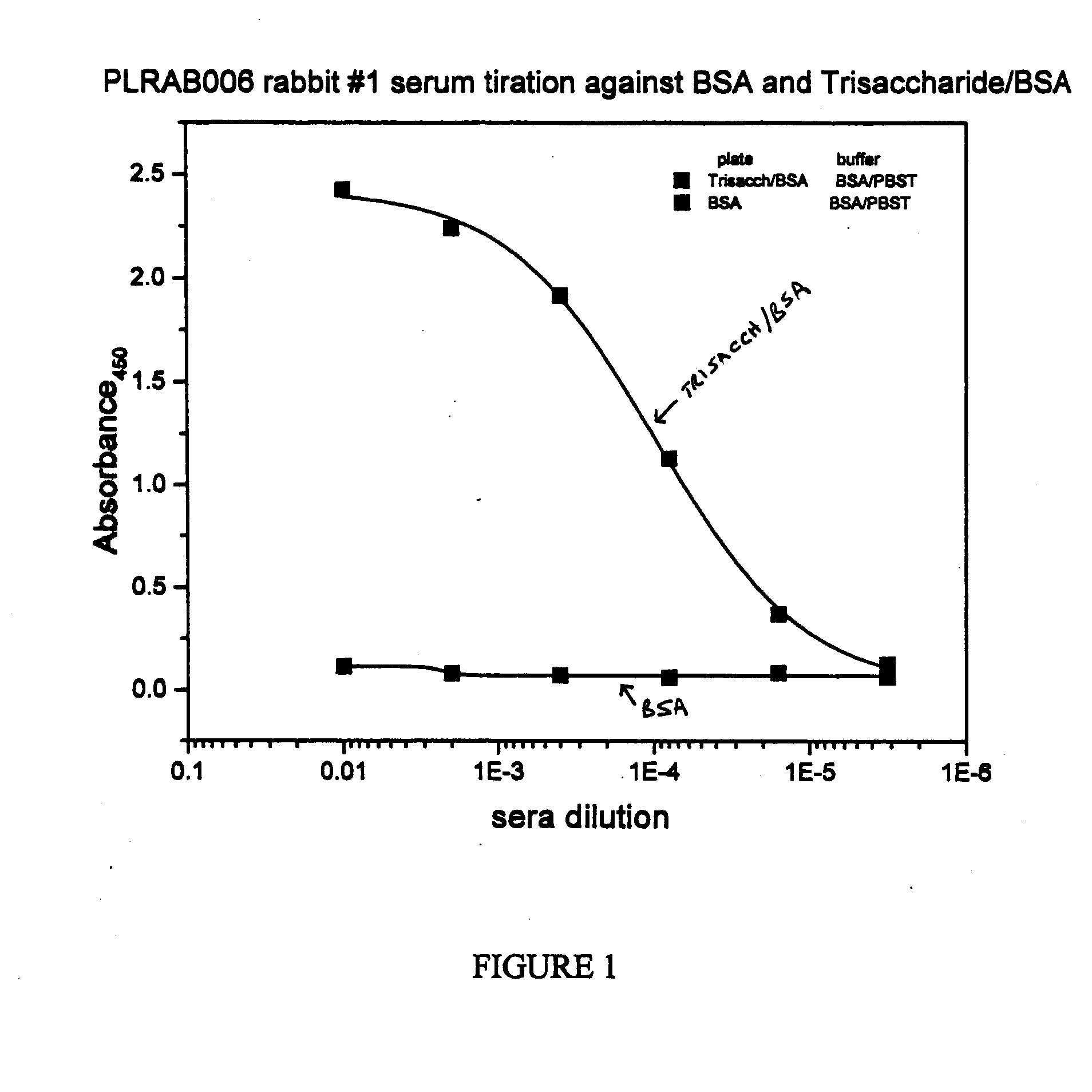
Patents
Literature
206results about "Fungal antigen ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nanoparticle vaccines
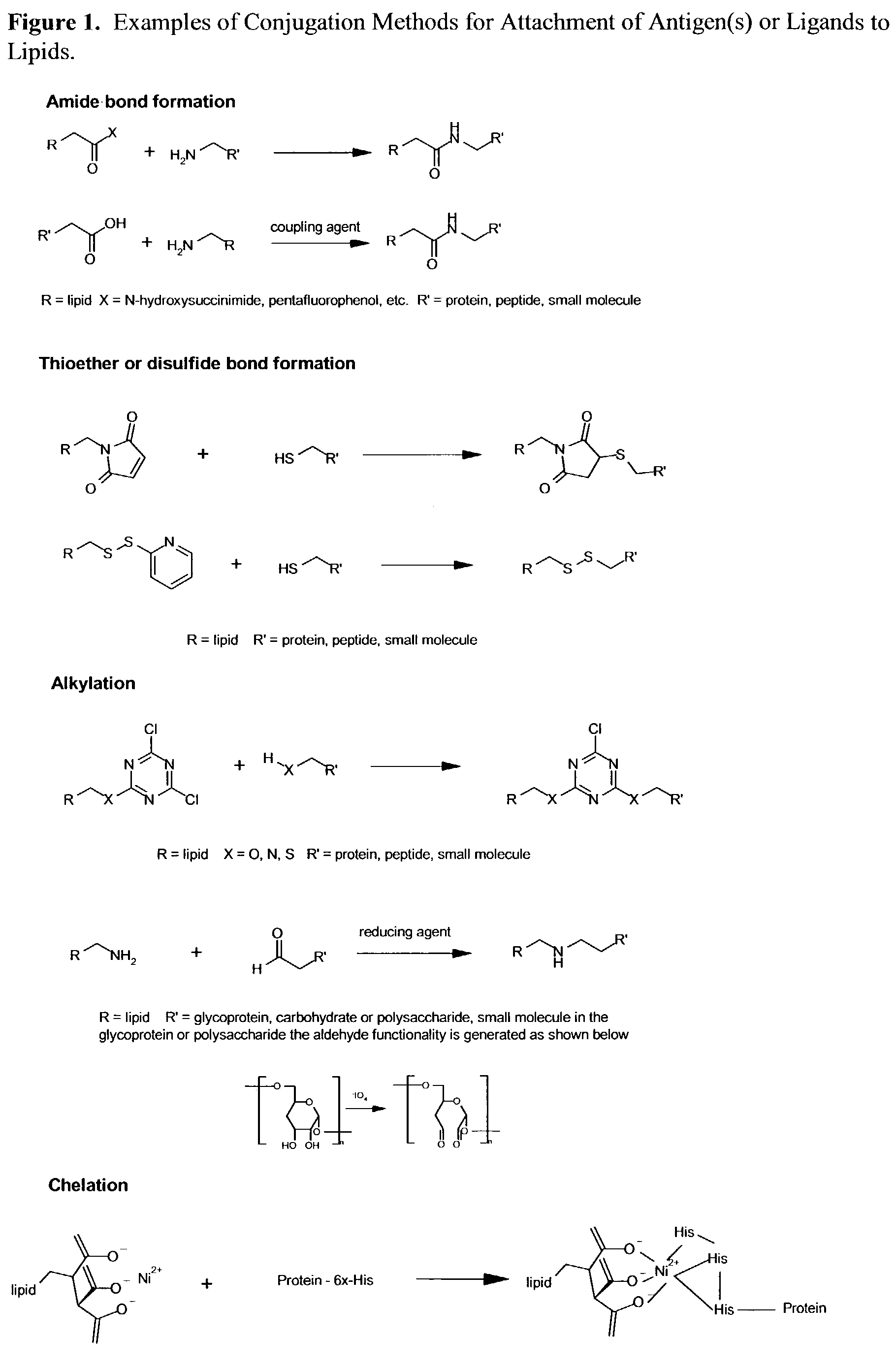
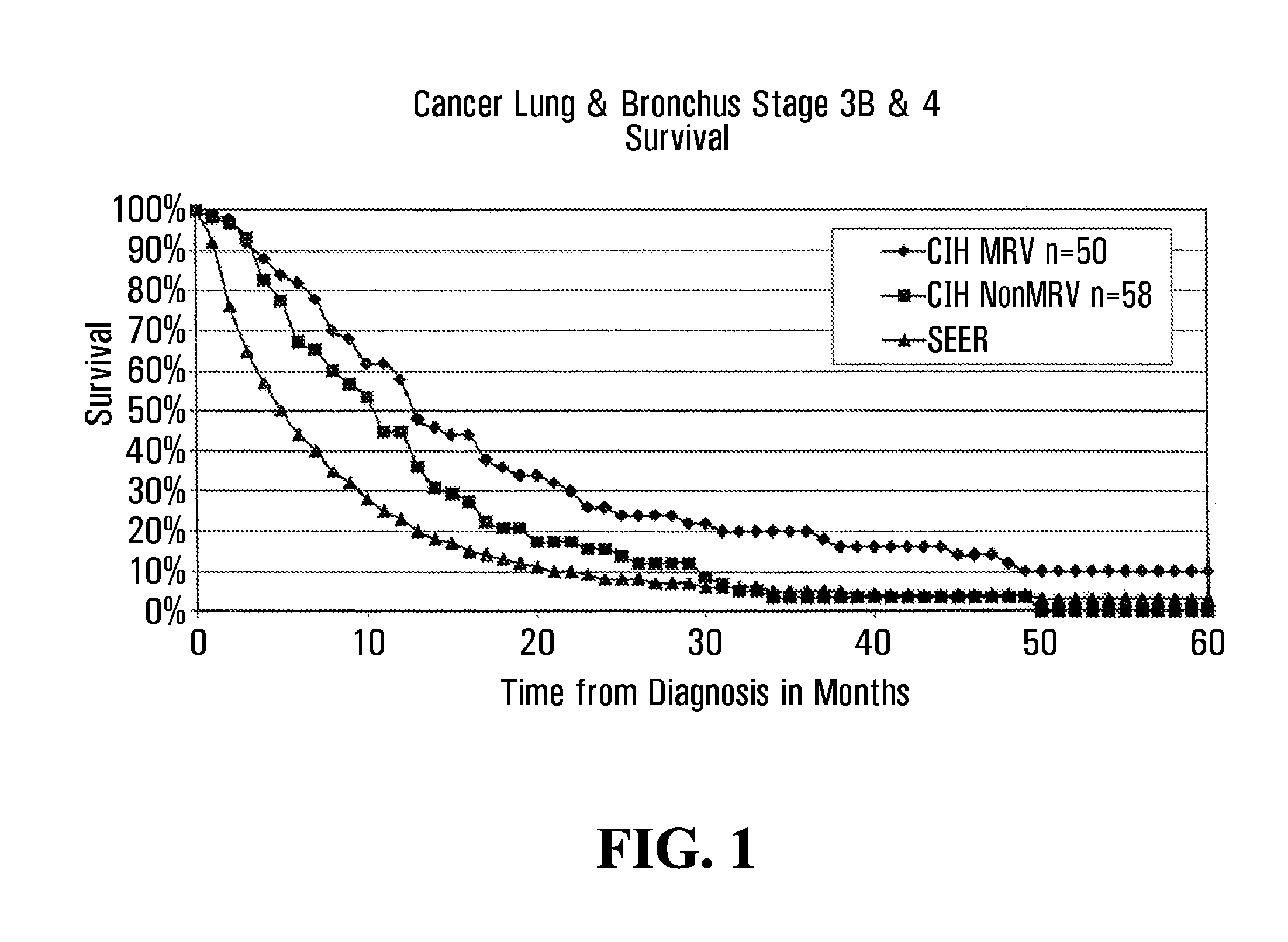
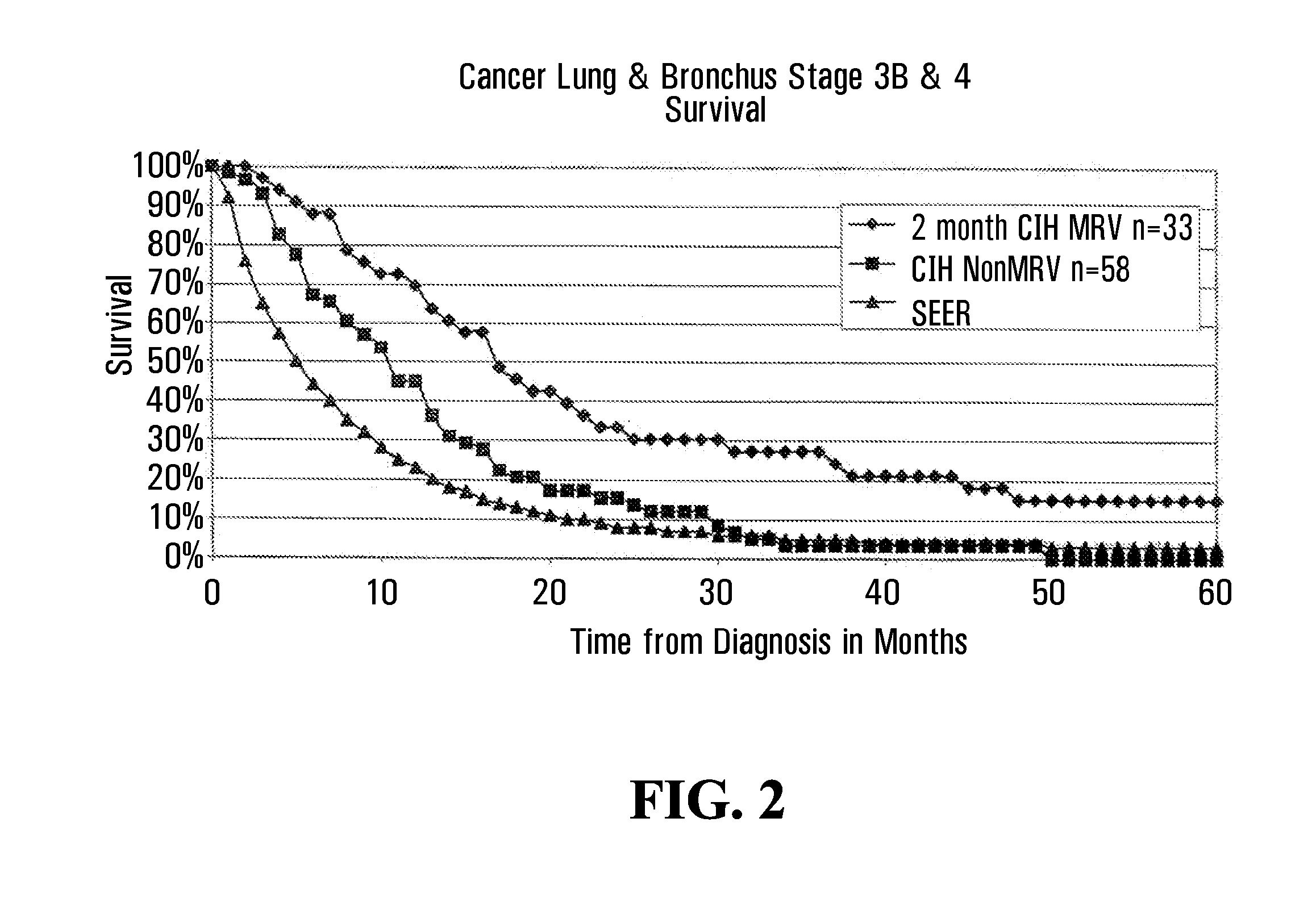
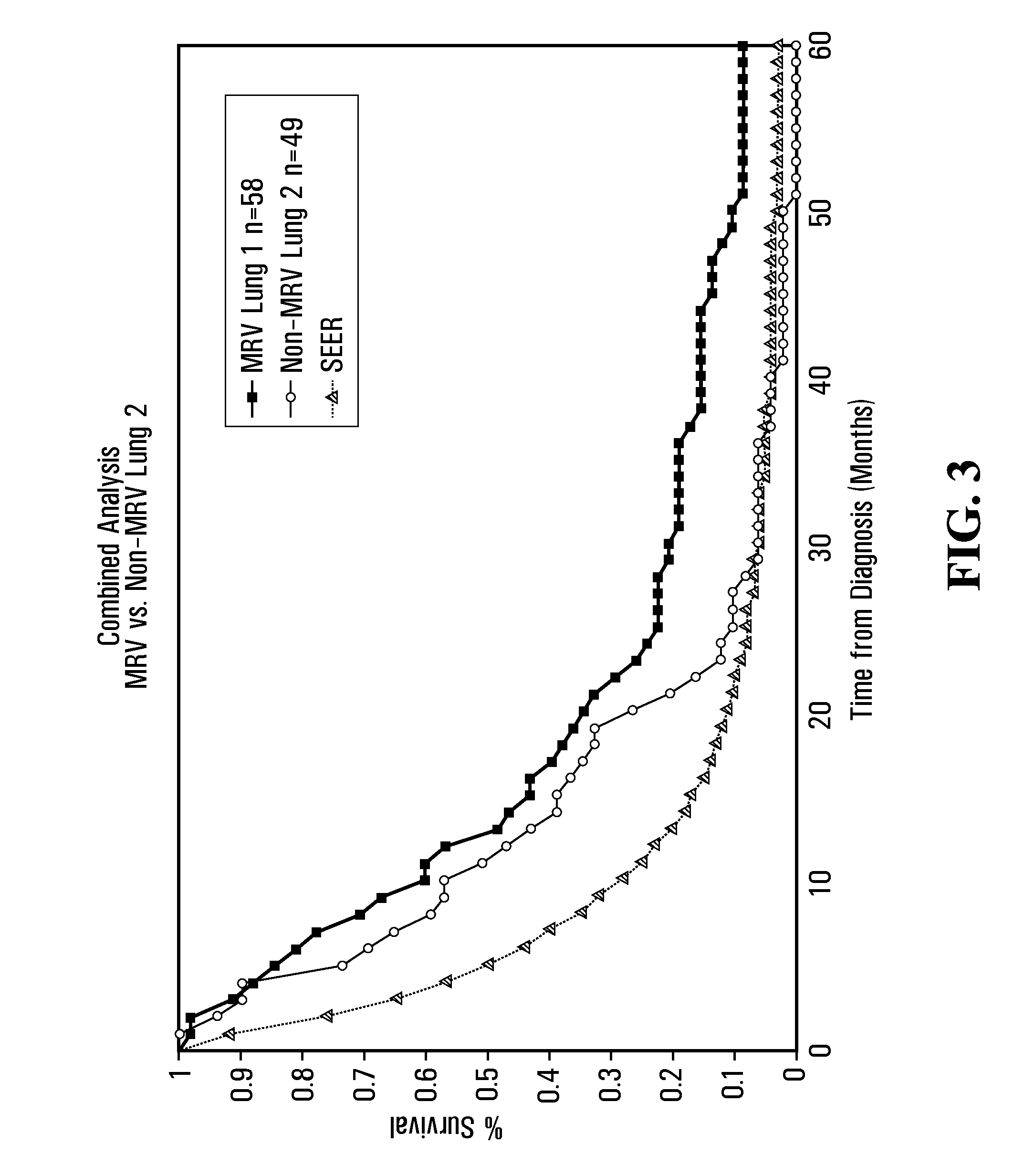
InactiveUS7285289B2Simple and inexpensive to synthesizeEnhance immune responseUltrasonic/sonic/infrasonic diagnosticsPowder deliveryLipid formationAntigen
The present invention relates to nanoparticle vaccines comprised of a carrier, particularly polymerized lipids, having multiple copies of an antigen or combinations of different antigens displayed on the carrier. Such antigen-displaying nanoparticles may also display a targeting molecule on its surface in order to direct it to a specific site or cell type to optimize a desired immune response. The present invention also relates to encapsulating an antigen or combinations of different antigens within such nanoparticles, with or without a targeting molecule displayed on its surface. The antigens used in this invention are effective to produce an immune response against a variety of pathological conditions.
Owner:NANOVALENT PHARMA
Immunonanotherapeutics that Provide IgG Humoral Response Without T-Cell Antigen
ActiveUS20100183727A1Improve responseSsRNA viruses negative-senseAntibacterial agentsNanocarriersImmune therapy
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides synthetic nanocarriers capable of eliciting an immune system response in the form of antibody production, wherein the nanocarriers lack any T cell antigens. In some embodiments, the invention provides nanocarriers that comprise an immunofeature surface, which provides high avidity binding of the nanocarriers to antigen presenting cells. The invention provides pharmaceutical compositions comprising inventive nanocarriers. The present invention provides methods of designing, manufacturing, and using inventive nanocarriers and pharmaceutical compositions thereof.
Owner:MASSACHUSETTS INST OF TECH +2
Immunogenic composition for immune system modulation and use thereof, method for treating and preventing diseases, method for inducing cell regeneration and method for restoring immune response
ActiveUS20140037716A1Effective strikeIncrease opportunitiesSenses disorderNervous disorderBenign tumoursNervous system
The present invention relates to immunogenic compositions for modulating the immune system, comprising a therapeutically effective quantity of two or more immuno-active antigenic agents with pathogen-associated molecular patterns (PAMPs) and / or danger-associated molecular patterns (DAMPs) and one or more physiologically acceptable carriers, excipients, diluents or solvents. The immunogenic compositions according to the present invention are used for producing medicaments for preventing and / or treating, and for preventing and / or treating infectious diseases, auto-immune diseases, allergic diseases, inflammation, arthritis, inflammatory diseases, transplant rejection, affections caused by vascular disorders, diseases caused by haemorrhagic or ischaemic cardiovascular accidents, ischaemia, heart attack and haemorrhagia leading to tissue destruction, heart, kidney, respiratory or liver insufficiency, cancer, malign and benign tumours and neoplasia. The present invention further relates to methods for inducing the regeneration of cells, tissues, organs and organic systems such as the circulatory, nervous and endocrine systems. Finally, the present invention relates to methods for restoring immune response in an animal, in particular a human being.
Owner:NOWILL ALEXANDRE EDUARDO
Pharmaceutical compositions and methods to vaccinate against candidiasis
InactiveUS20030124134A1Treat prevent alleviateToxic reductionPeptide/protein ingredientsSnake antigen ingredientsHospitalized patientsVirulent characteristics
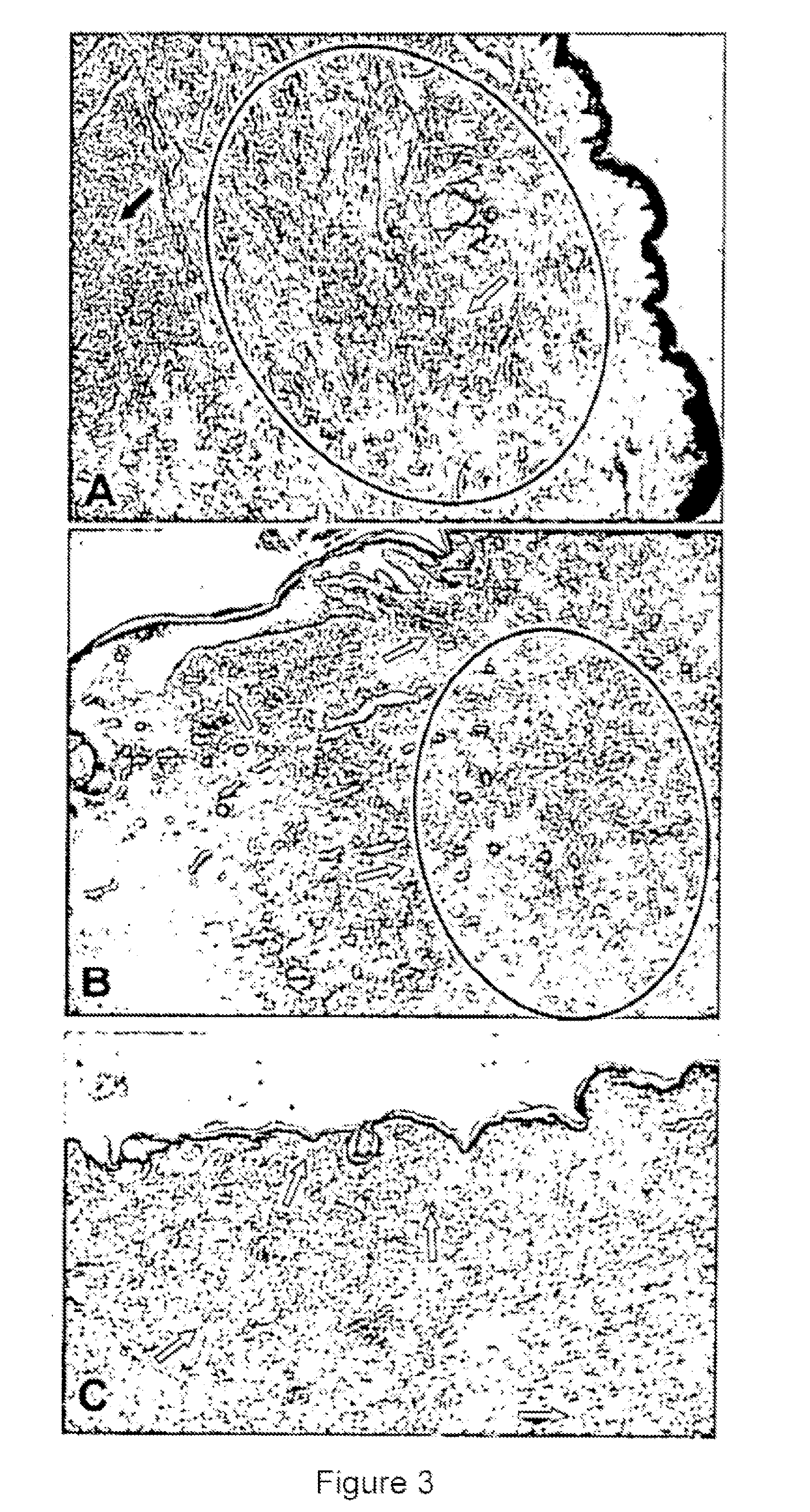
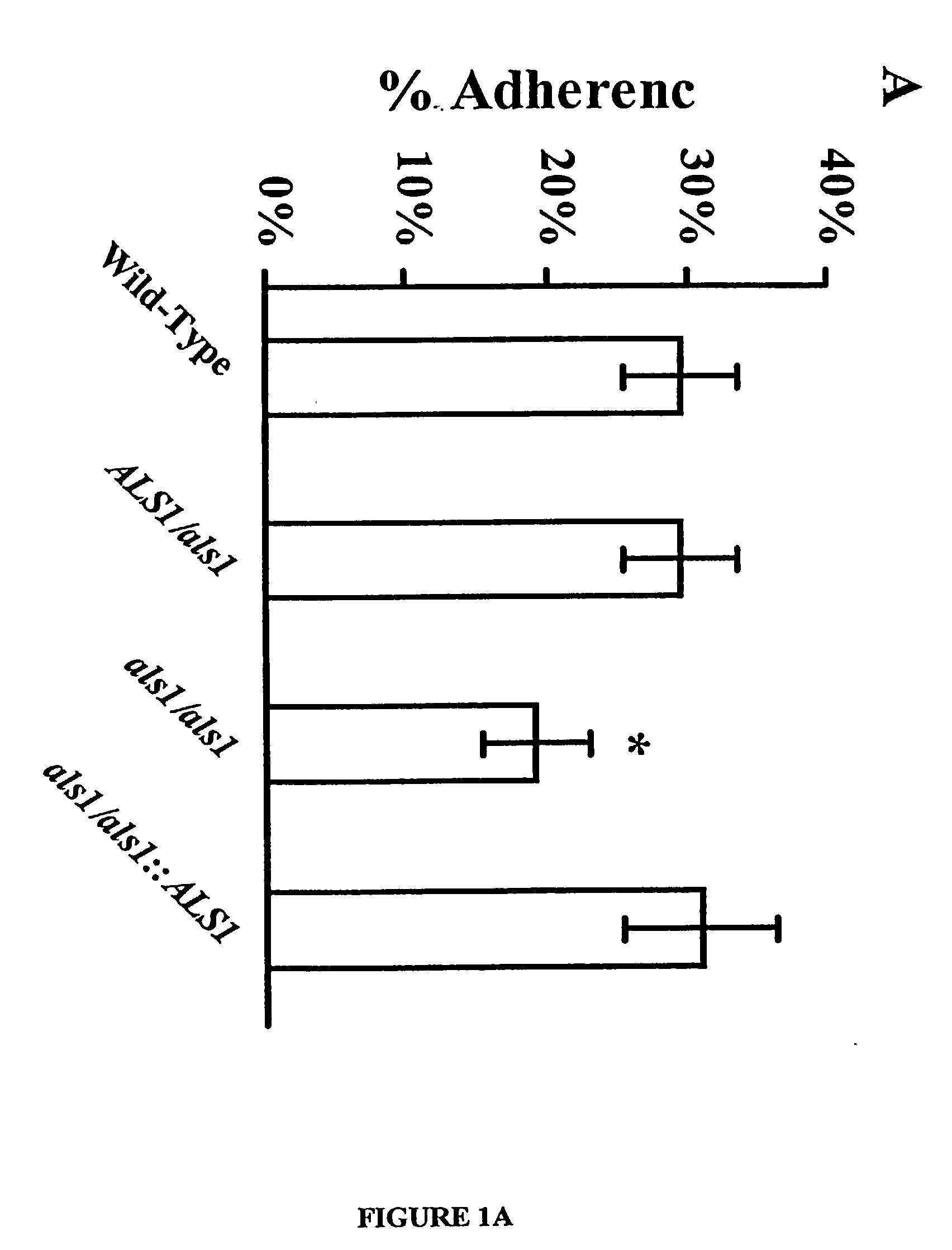
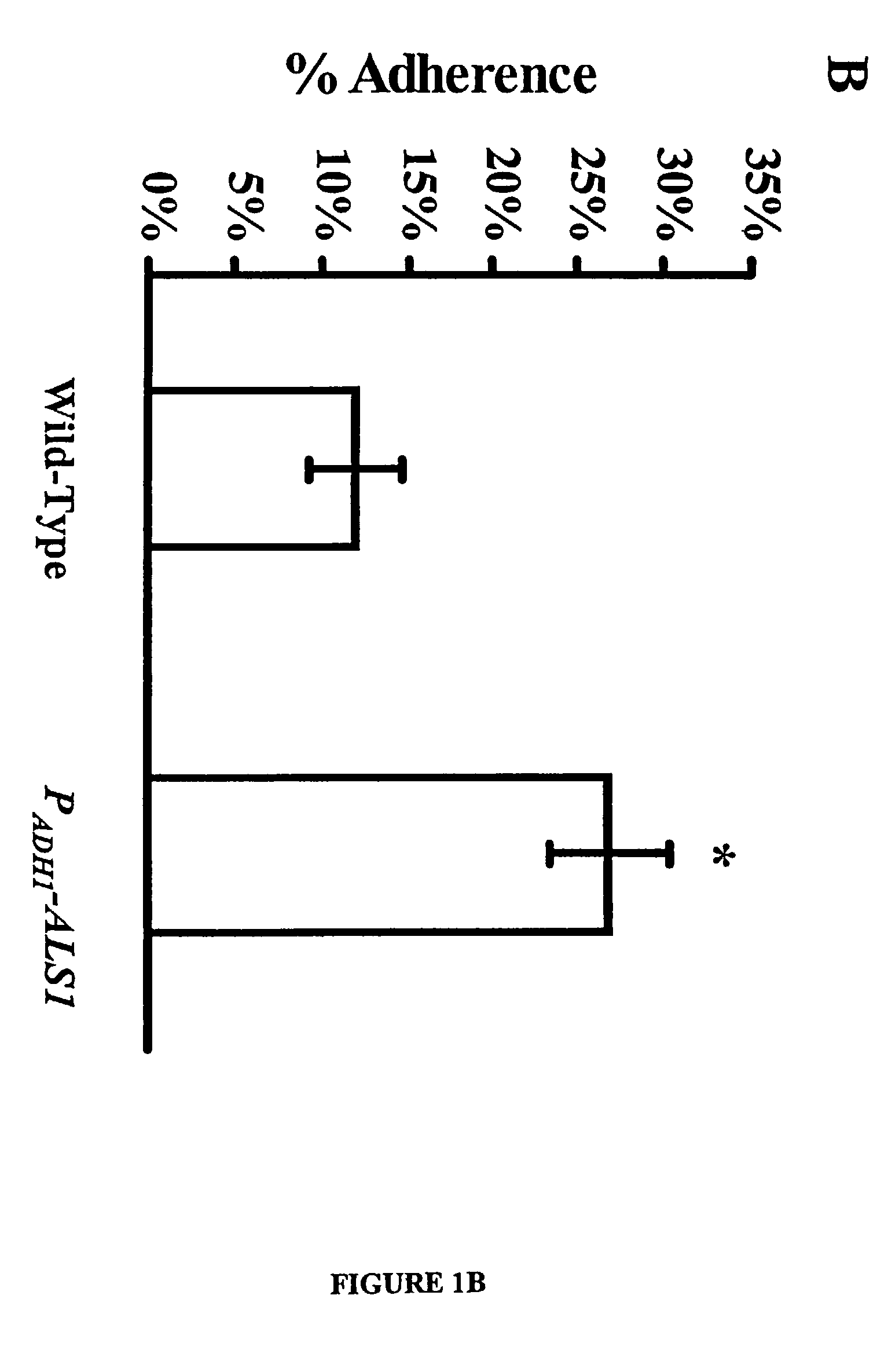
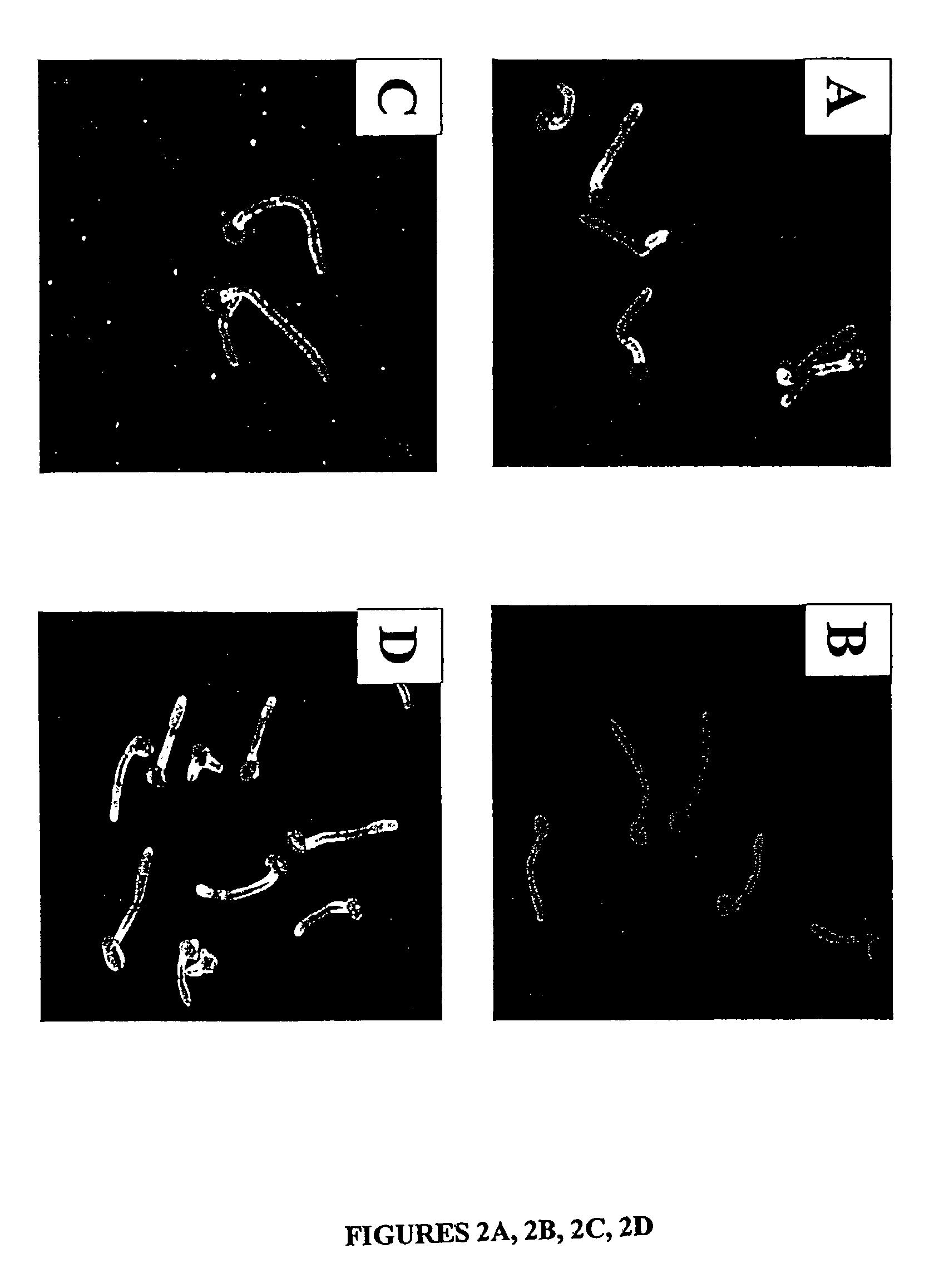
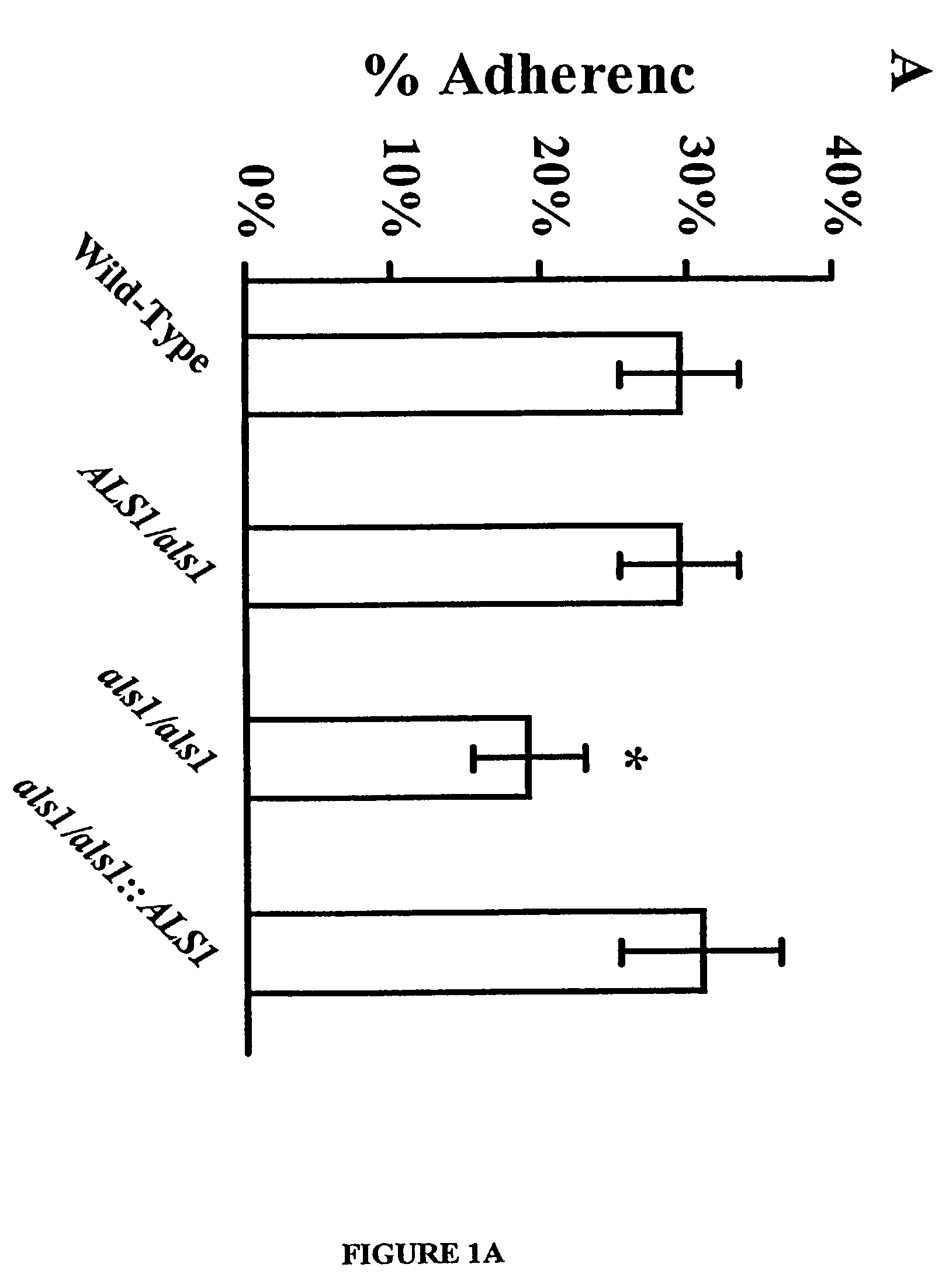
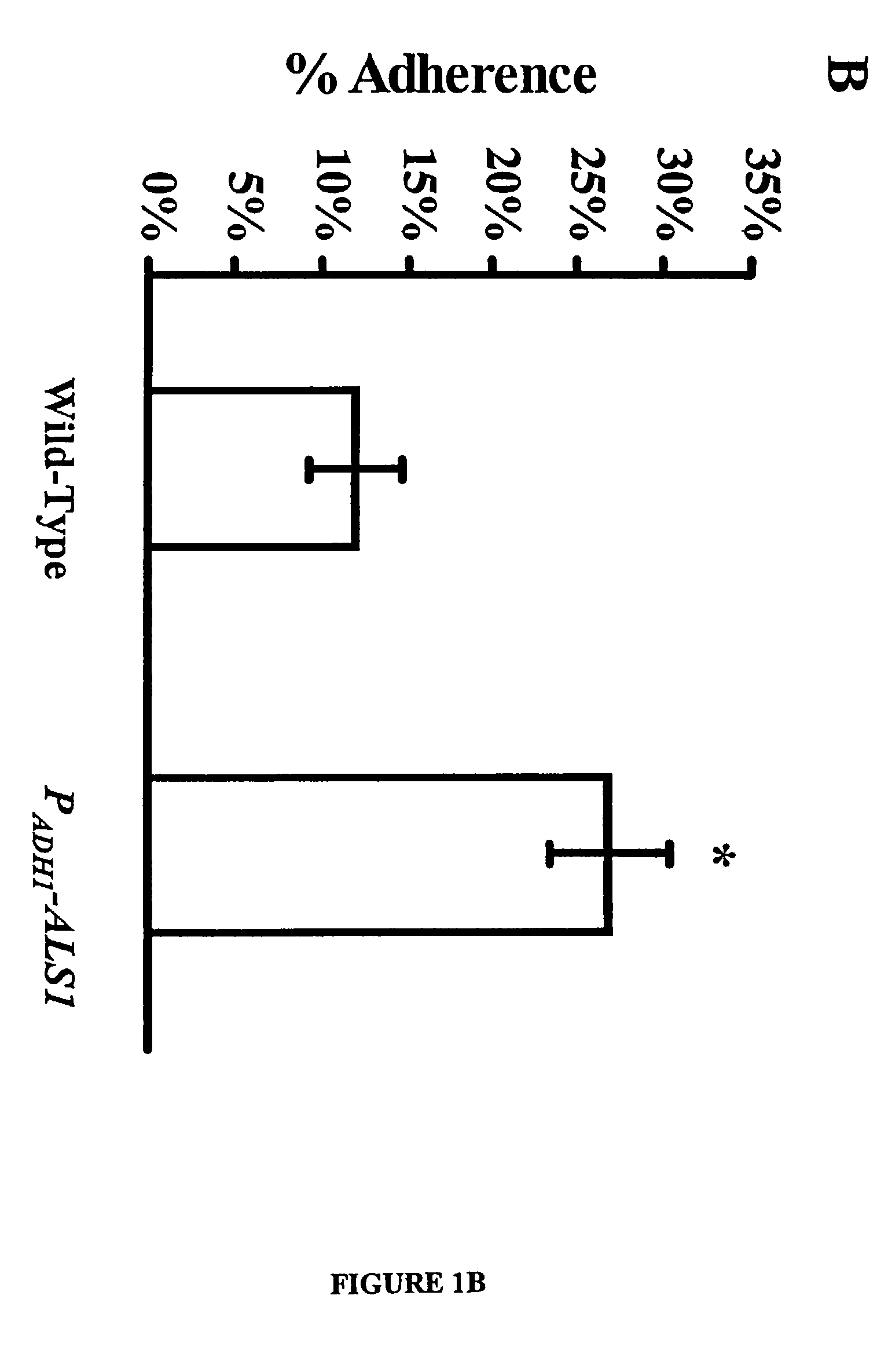
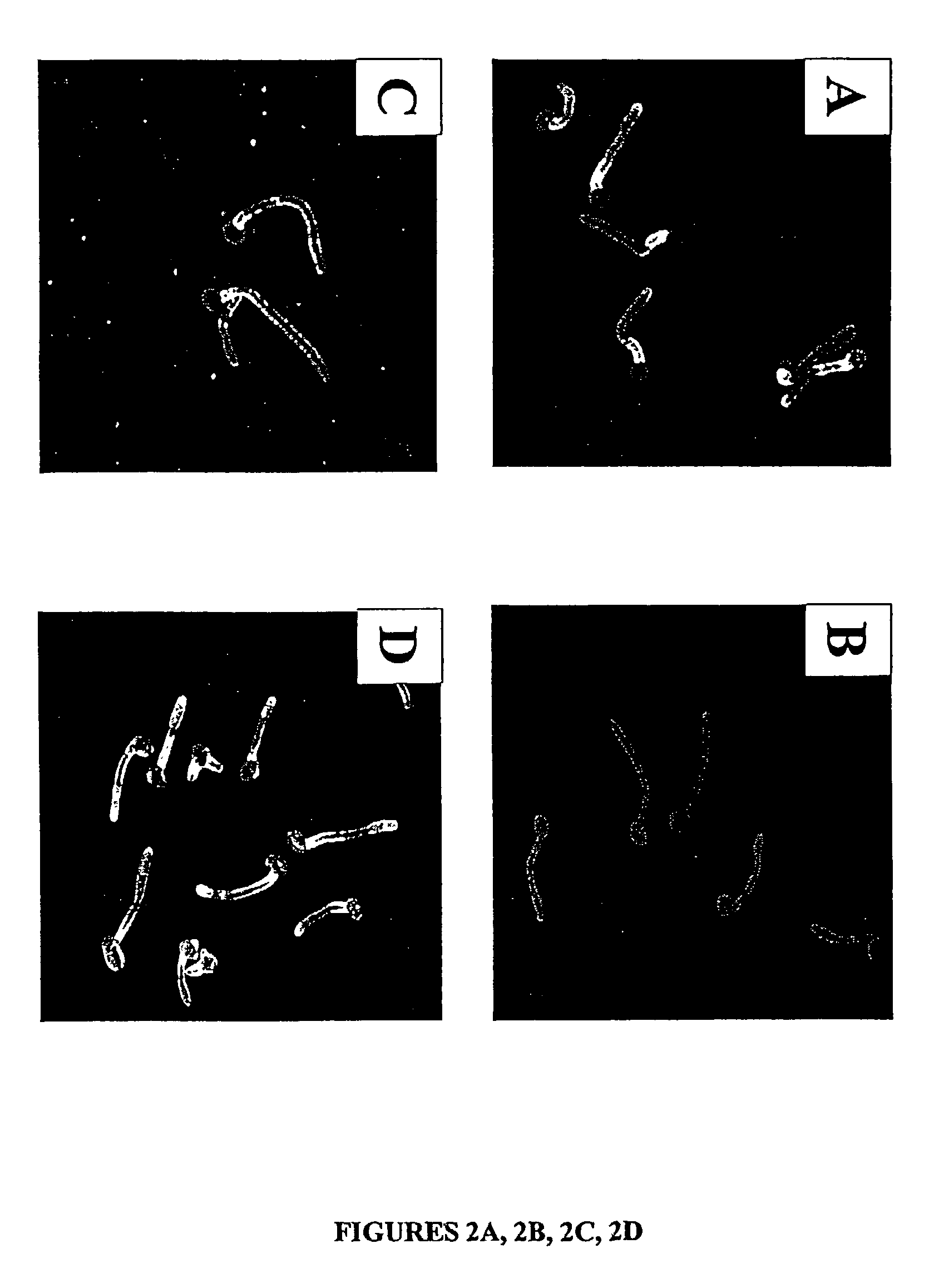
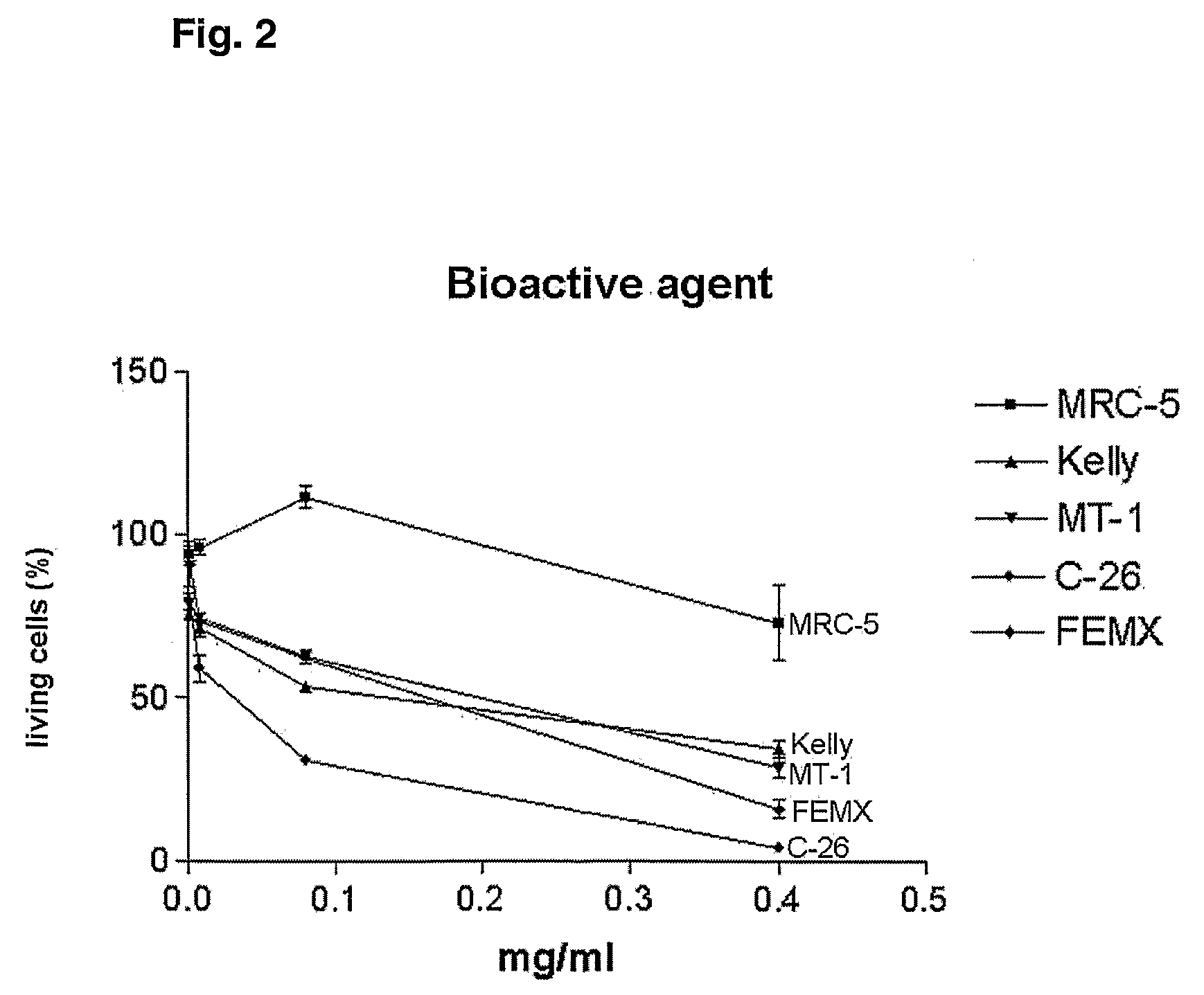
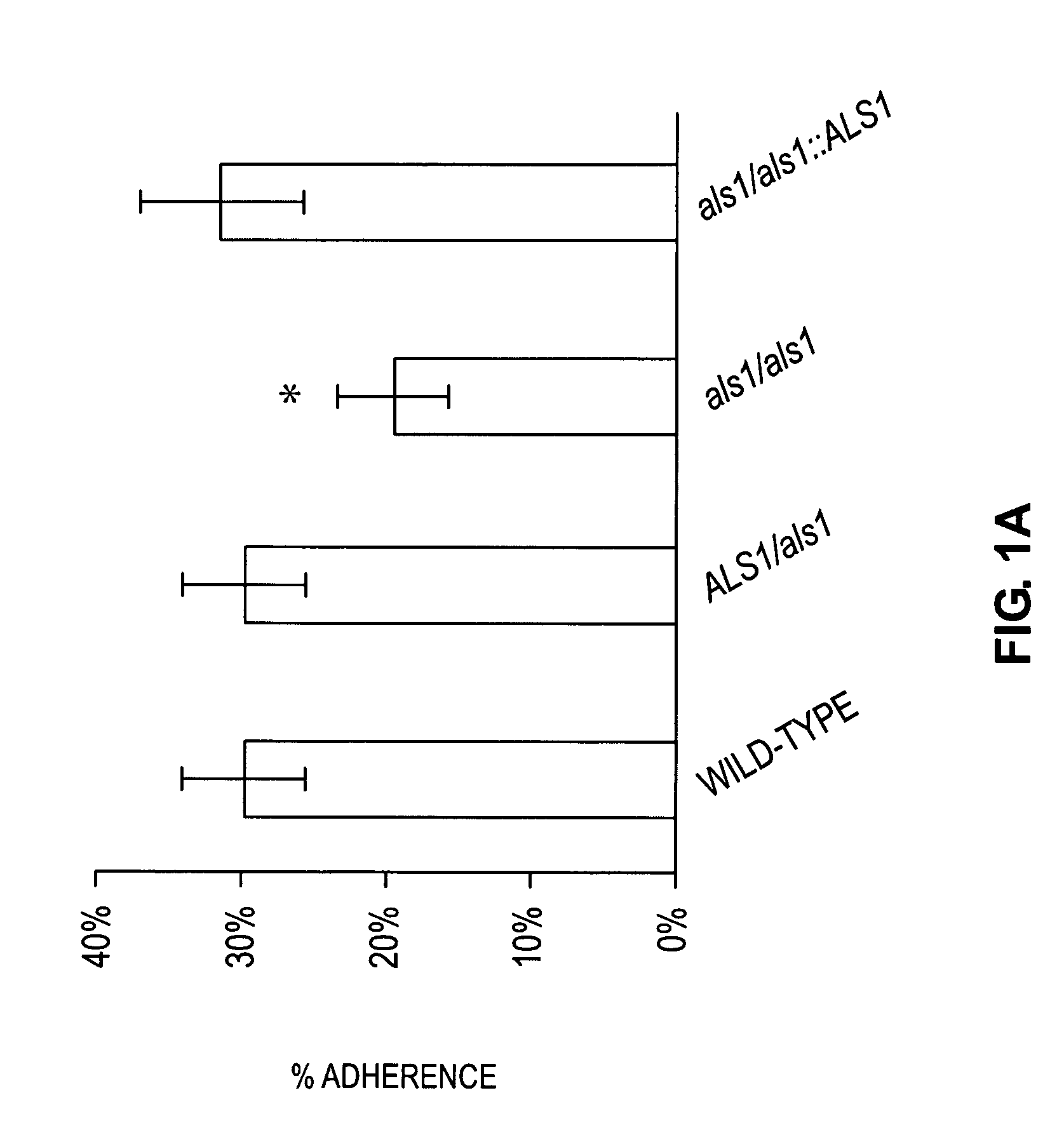
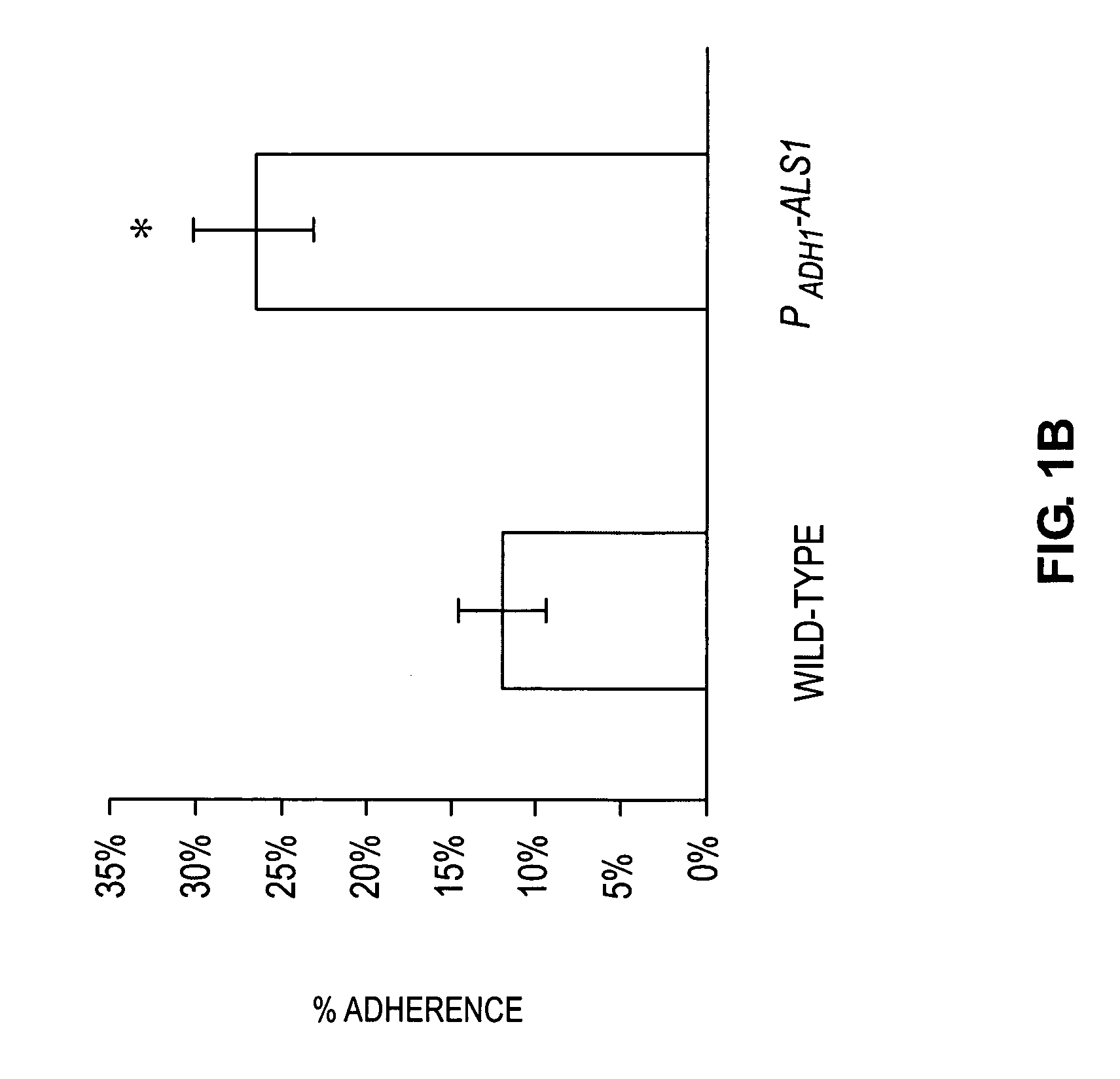
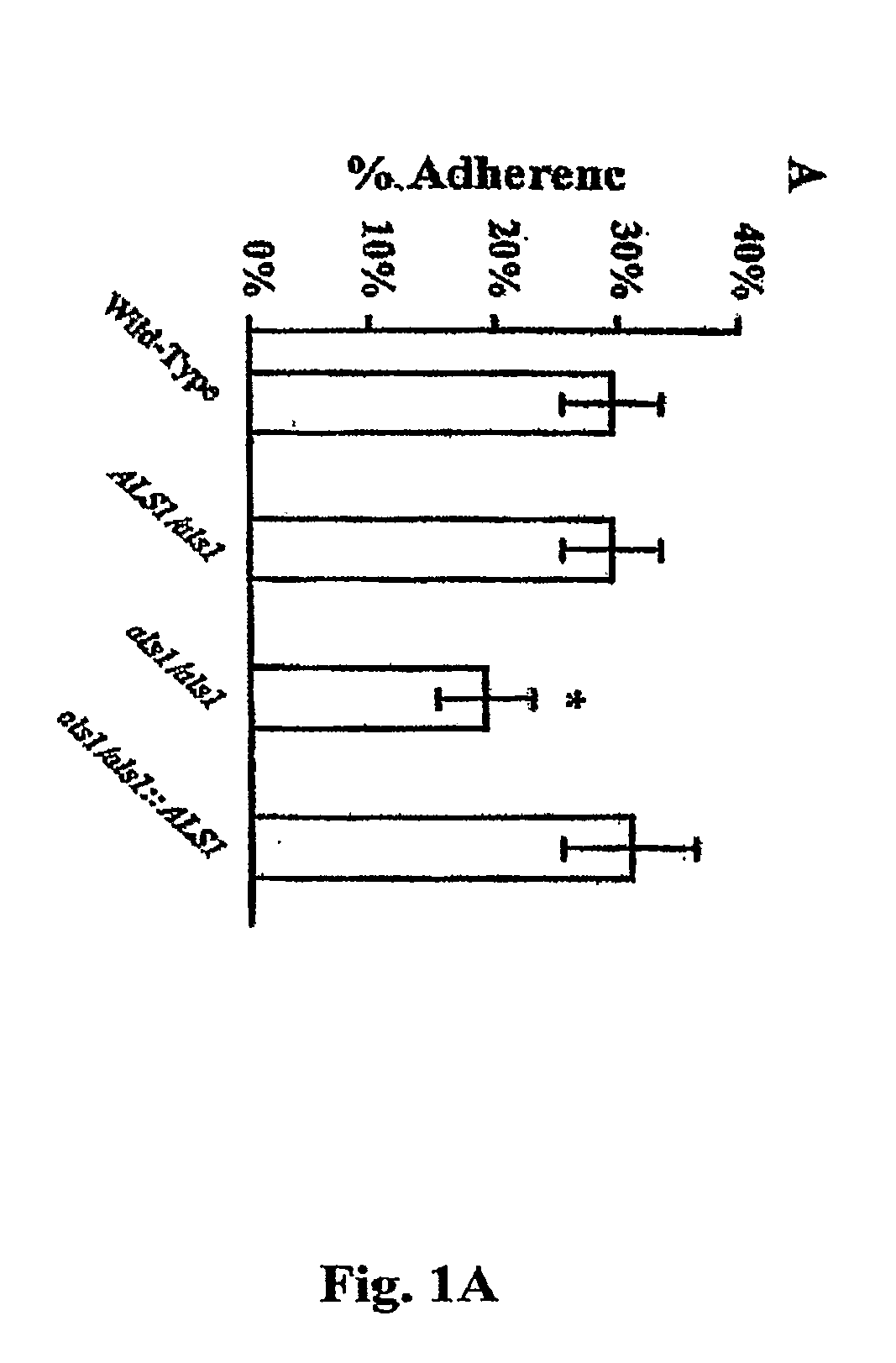
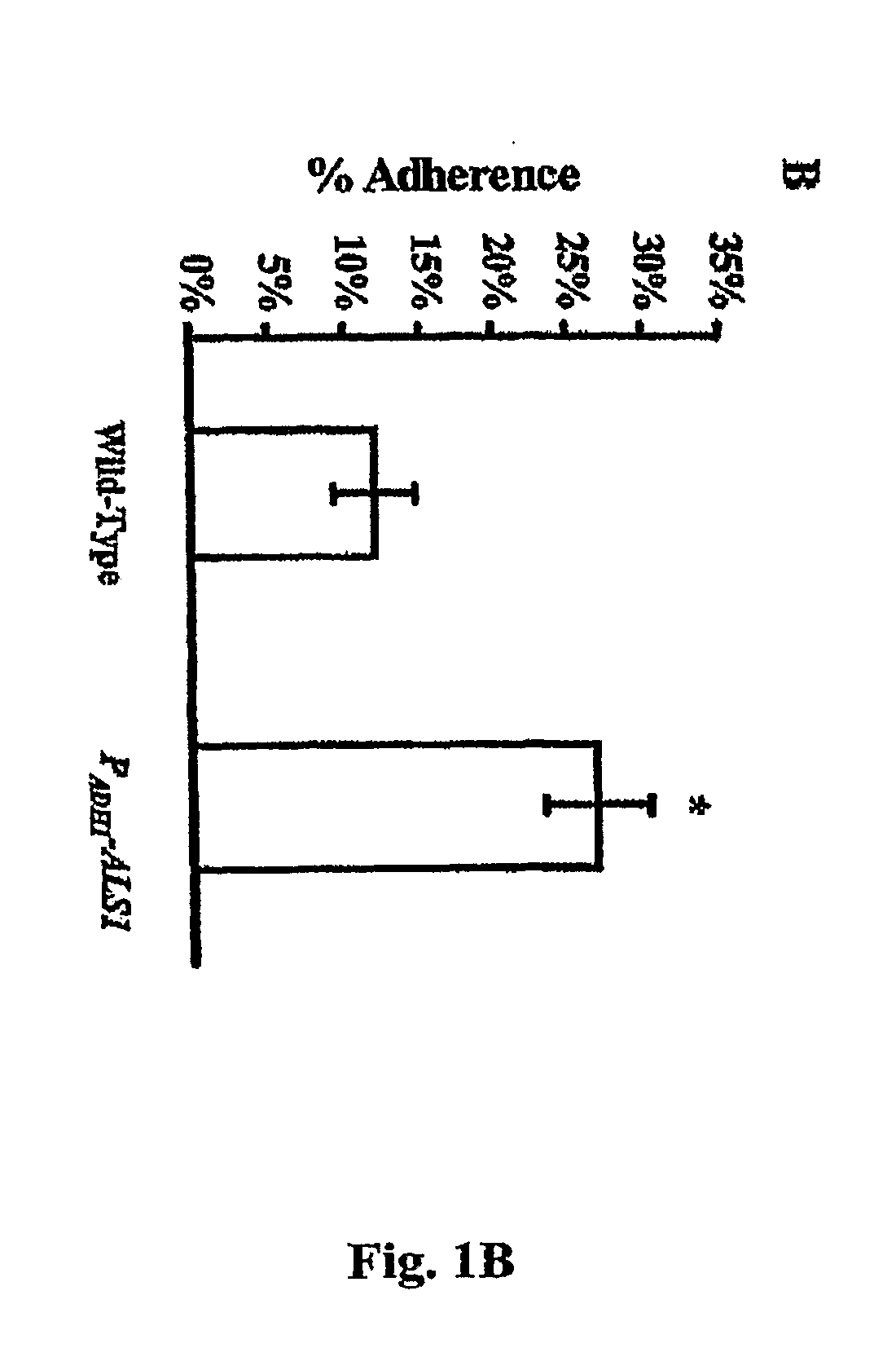
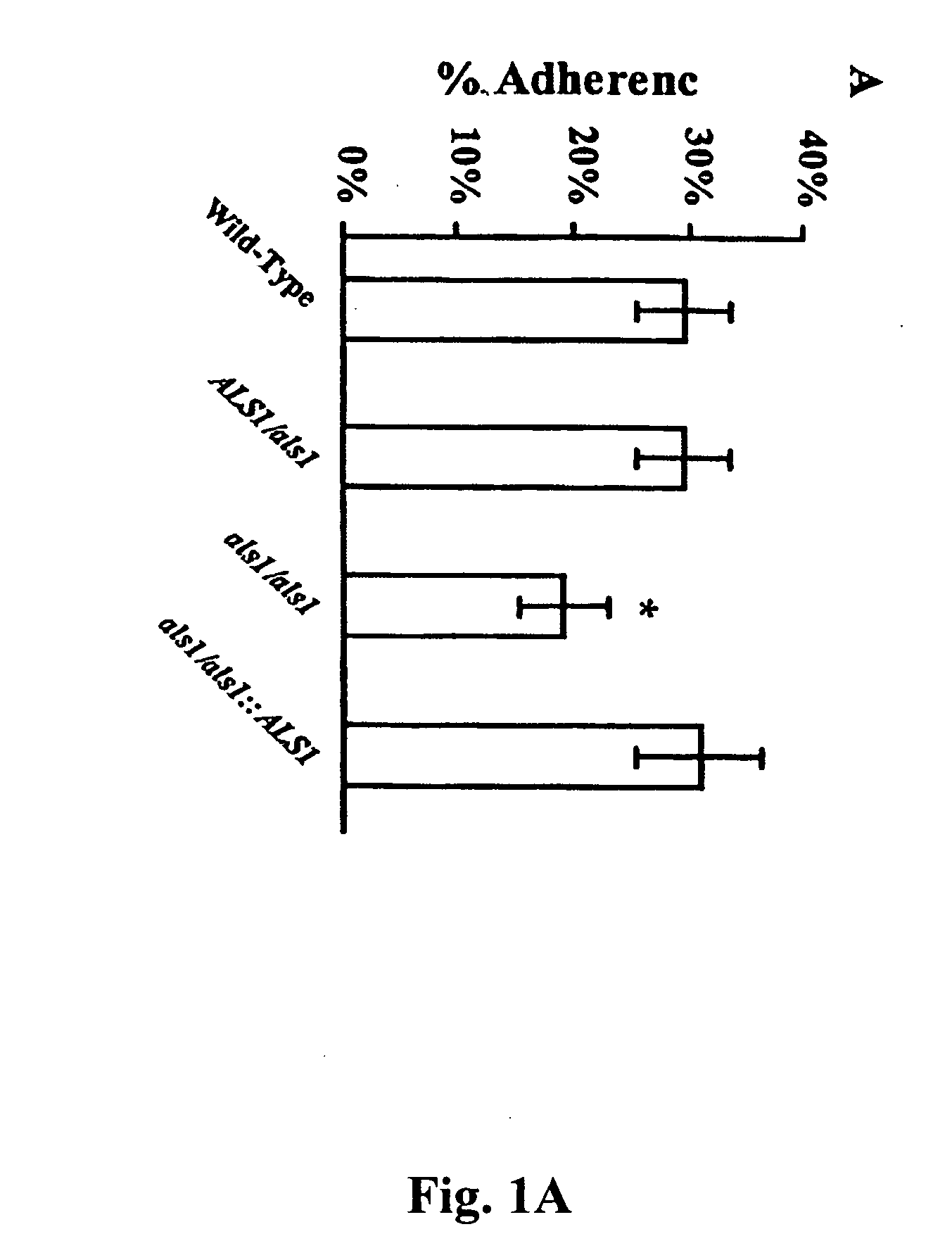
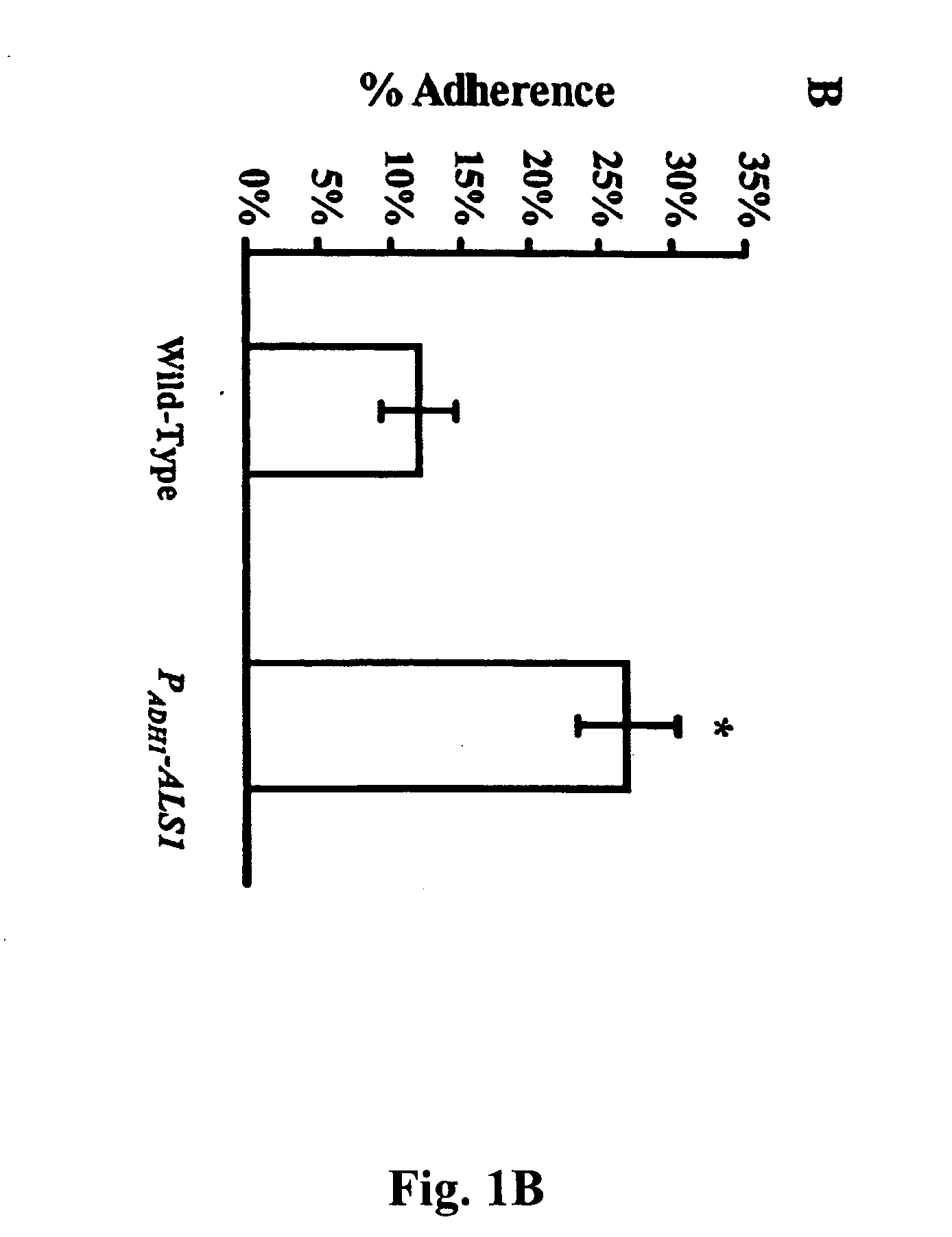
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface proteins in the ALS group are downstream effectors of the filamentation regulatory pathway. Particularly, Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene, gene product, or specific antibody to the ALS gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT UCLA HARBOR MEDICAL CENT +1
Anti-fungal composition
A multivalent fungal vaccine comprising one or more heat-inactivated fungal antigens, wherein at least one fungal antigen is effective in producing an immune response in a host when said vaccine is administered orally at a dose that is sufficient for preventing or treating the fungal disease in said host. Also described are methods for making and using an orally available anti-fungal vaccine.
Owner:IMMUNITOR USA
Adjuvant incorporation in immunonanotherapeutics
ActiveUS20110268805A1Improve responseEffective amountPowder deliveryBacterial antigen ingredientsNanocarriersAdjuvant
Owner:MASSACHUSETTS INST OF TECH +2
Formulations
InactiveUS20140017313A1Large specific surface areaIncrease contactPowder deliveryBacterial antigen ingredientsBiotechnologyMicroorganism
This invention relates to compositions for delivering one or more active ingredients, and more particularly to compositions, e.g. beads, comprising a matrix material which matrix material comprises a microorganism. In particular, the invention relates to compositions comprising a microorganism selected from live, killed, attenuated and inactivated microorganisms. The matrix material may also comprise a surfactant and may further comprise an adjuvant. The invention further relates to the manufacture and use of such compositions, and to other subject matter.
Owner:SUBLIMITY THERAPEUTICS LTD
IMMUNONANOTHERAPEUTICS THAT PROVIDE IgG HUMORAL RESPONSE WITHOUT T-CELL ANTIGEN
ActiveUS20120087890A1Improve responseEffective amountSsRNA viruses negative-senseAntibacterial agentsNanocarriersAntibody production
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides synthetic nanocarriers capable of eliciting an immune system response in the form of antibody production, wherein the nanocarriers lack any T cell antigens. In some embodiments, the invention provides nanocarriers that comprise an immunofeature surface, which provides high avidity binding of the nanocarriers to antigen presenting cells. The invention provides pharmaceutical compositions comprising such nanocarriers. The present invention provides methods of designing, manufacturing maceutical compositions thereof.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Vaccination against Cryptococcus
ActiveUS20150328295A1Reduce and eliminate abilityReduce and eliminate expressionPowder deliverySpray deliveryVaccinationWild type
Vaccines and methods of inoculation for conferring immunity to Cryptococcus infection are disclosed. Strains of Cryptococcus fungi, including Cryptococcus neoformans and Cryptococcus gattii, can be administered to a human or animal subject via inhalation. Cryptococcus fungi that can be used to confer immunity can comprise one or more mutations in genes that contribute to chitosan production, such as genes encoding a chitin deacetylase (cda), a chitin synthase (chs) and / or a regulator of chitin synthase (csr). Inhalation administration of heat-killed Cryptococcus harboring deletions in cda1, cda2 and cda3 genes can confer immunity. In a murine model system, inhalation administration of Cryptococcus neoformans harboring deletions in cda1, cda2 and cda3 genes conferred immunity against subsequent exposure to wild type Cryptococcus neoformans in 100% of test animals. Inhalation administration of heat-killed Cryptococcus grown under conditions leading to reduced chitosan production can also confer immunity.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Method to prevent fertilization in mammals by administering a single dose of zona pellucida derived antigens, liposome and adjuvant
InactiveUSRE37224E1Extended maintenance periodReduce deliveryPeptide/protein ingredientsSnake antigen ingredientsAntigenAdjuvant
A vaccine for the immunocontraception of mammals is described. The vaccine consists of zona pellucida antigens and an adjuvant encapsulated in a liposome delivery system. The liposome delivery system allows for the slow release of antigen resulting in a prolonged immune response. In particular, after a single injection of the vaccine, levels of anti-zona pellucida antibodies were detected for up to 22 months in seals. Thus, the vaccine according to the present invention is effective after a single dose and is therefore very useful in immunocontraceptive protocols.
Owner:IMMUNOVACCINE TECH INC
Allogeneic cellular immunotherapy for invasive pulmonary aspergillosis (IPA)
ActiveUS20060115487A1Snake antigen ingredientsVertebrate antigen ingredientsAllogeneic cellGraft versus host disease induction
A method for stimulating the immune system in immunocompromised patients in order to treat opportunistic infection. The method involves the infusion of intentionally mismatched allogeneic cells. In order to prevent graft vs. host disease complications, the allogeneic cells can be irradiated prior to infusion.
Owner:MIRROR BIOLOGICS INC +1
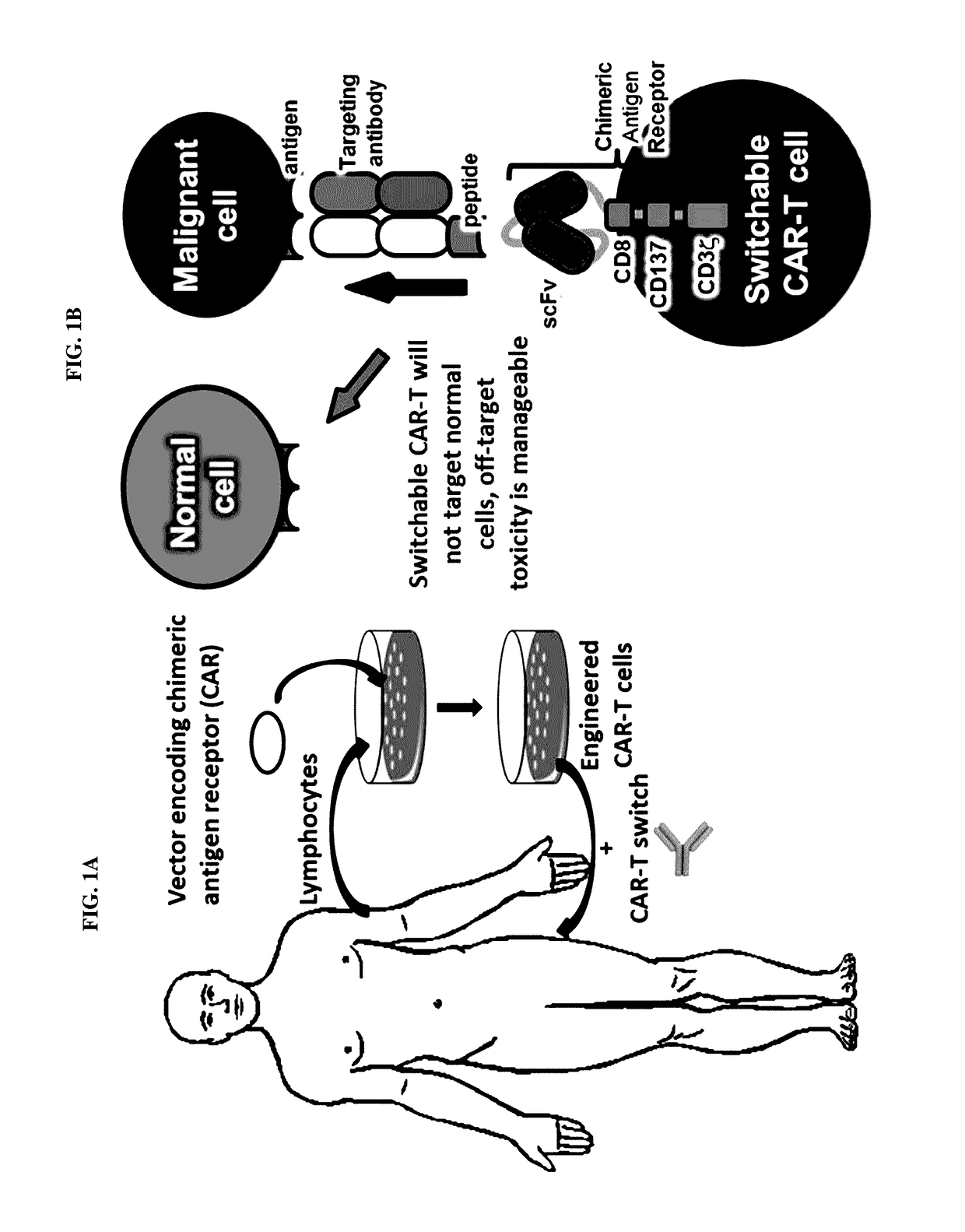
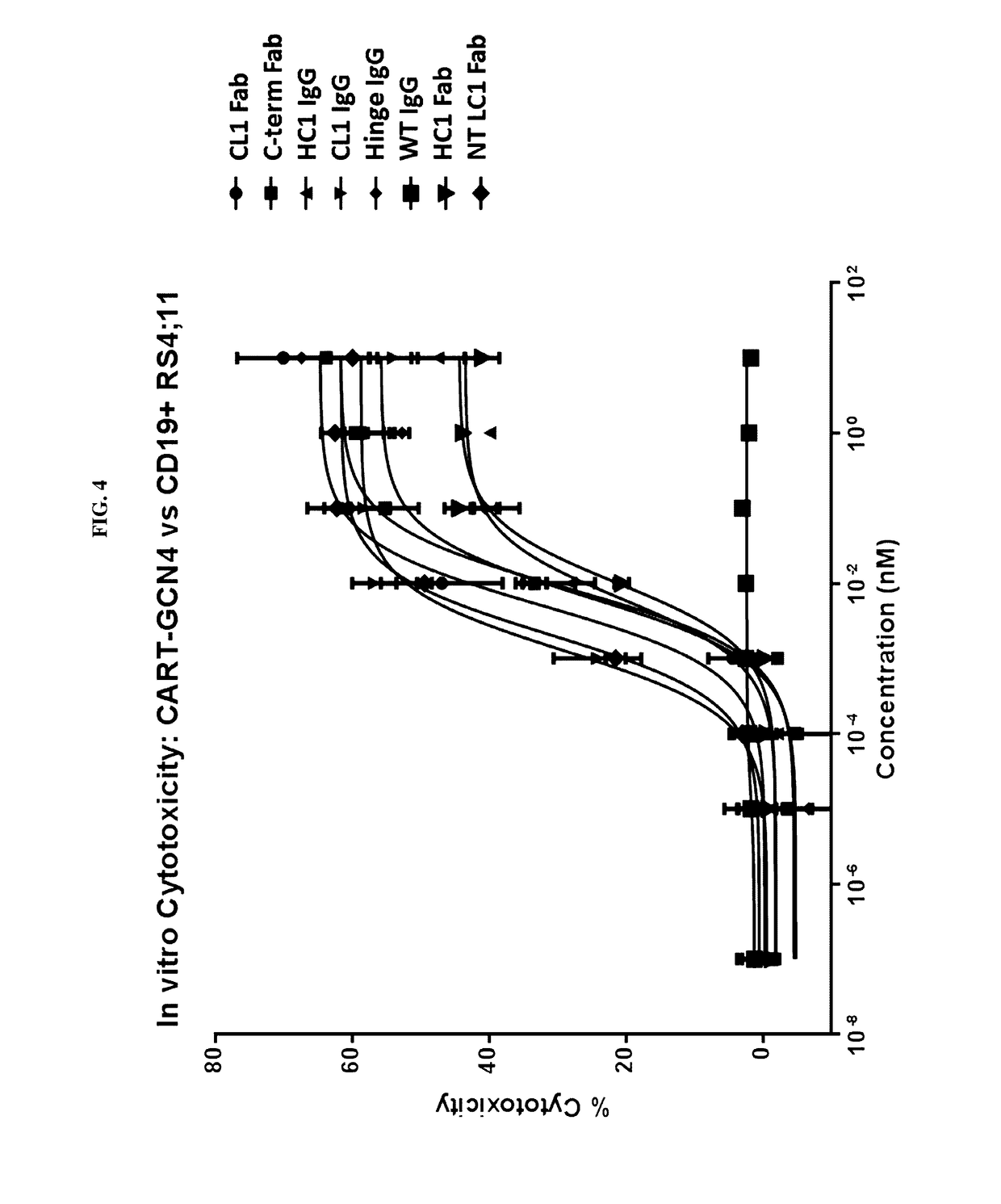
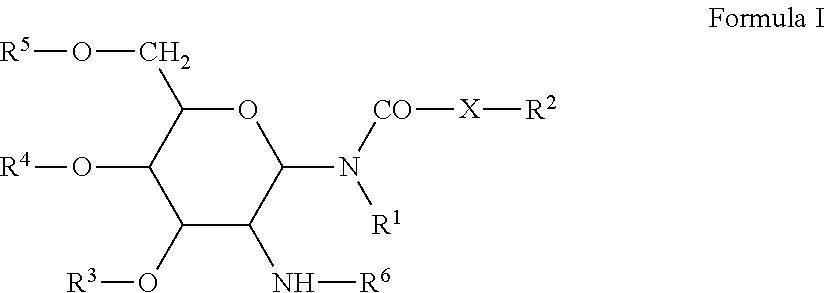
Peptidic chimeric antigen receptor T cell switches and uses thereof
ActiveUS9624276B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsPeptide antigenAntigen receptors
Disclosed herein are chimeric antigen receptor effector cells (CAR-ECs) and CAR-EC switches. The switchable CAR-ECs are generally T cells. The one or more chimeric antigen receptors may recognize a peptidic antigen on the CAR-EC switch. The CAR-ECs and switches may be used for the treatment of a condition in a subject in need thereof.
Owner:THE SCRIPPS RES INST
Oil-based adjuvants
The instant invention provides various formulations comprising combinations of immunostimulating oligonucleotides, polycationic carriers, sterols, saponins, quaternary amines, TLR-3 agonists, glycolipids, and MPL-A or analogs thereof in oil emulsions, use thereof in preparations of immunogenic compositions and vaccines, and use thereof in the treatment of animals.
Owner:ZOETIS SERVICE LLC
Synthetic Anti-Candida Albicans Oligosaccharide Based Vaccines
The present invention provides immunogenic oligosaccharide compositions and methods of making and using them. In particular, the compositions comprise native O-linked and S-linked oligosaccharides coupled to a protein carrier via a linker, wherein the resultant conjugate elicits a protectively immunogenic response, particularly in vaccines against pathogenic Candida species and more particularly against Candida albicans. Preferably the pathogenic Candida species are those that possess cell wall oligosaccharide compositions similar to the β-mannan component of Candida albicans cell walls.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Peptide and DNA immunization against Coccidioides immitis infections
InactiveUS6923973B1Provide protectionCost-effectiveSugar derivativesMicrobiological testing/measurementCoccidioidomycosis infectionDNA construct
Disclosed are peptide and DNA compositions surprisingly found to be effective in generating immune responses against the pathogenic fungi Coccidioides spp., the causative agents of coccidioidomycosis and Valley Fever. The invention thus provides peptides and DNA constructs, combinations and related biological compositions, and prophylactic and therapeutic methods of using such components to generate effective immune responses against Coccidioides spp., including C. immitis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Glycotargeting therapeutics
ActiveUS10046056B2Reduced responseRelieve symptomsPolypeptide with localisation/targeting motifPeptide/protein ingredientsAntigenAutoimmune condition
Several embodiments of the present disclosure relate to glycotargeting therapeutics that are useful in the treatment of transplant rejection, autoimmune disease, food allergy, and immune response against a therapeutic agent. In several embodiments, the compositions are configured to target the liver and deliver antigens to which tolerance is desired. Methods and uses of the compositions for induction of immune tolerance are also disclosed herein.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Yeast-muc1 immunotherapeutic compositions and uses thereof
ActiveUS20130315941A1Improve survivalReduce the burden onTetracycline active ingredientsMucinsYeastCancer research
Owner:GLOBE IMMUNE INC +1
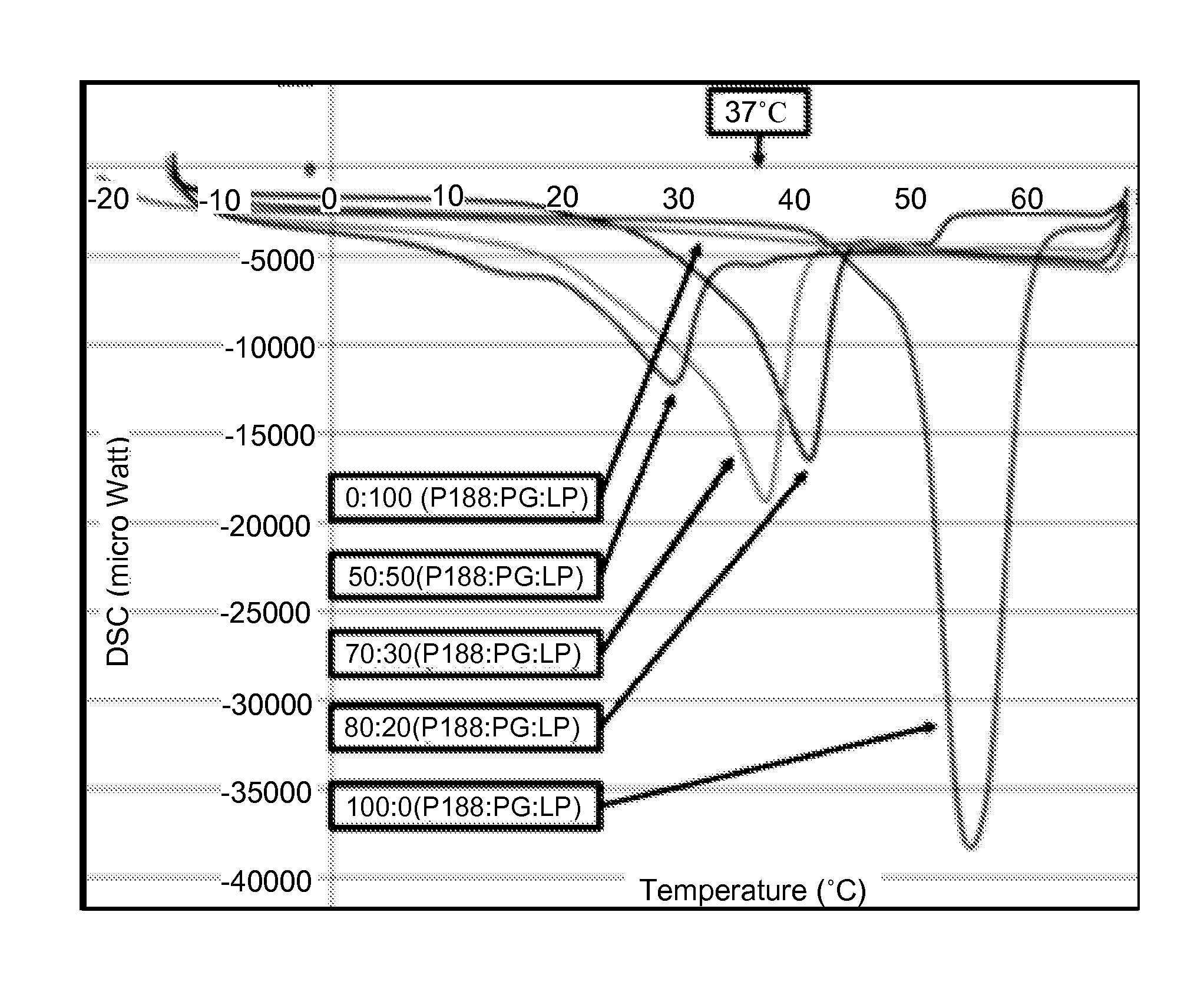
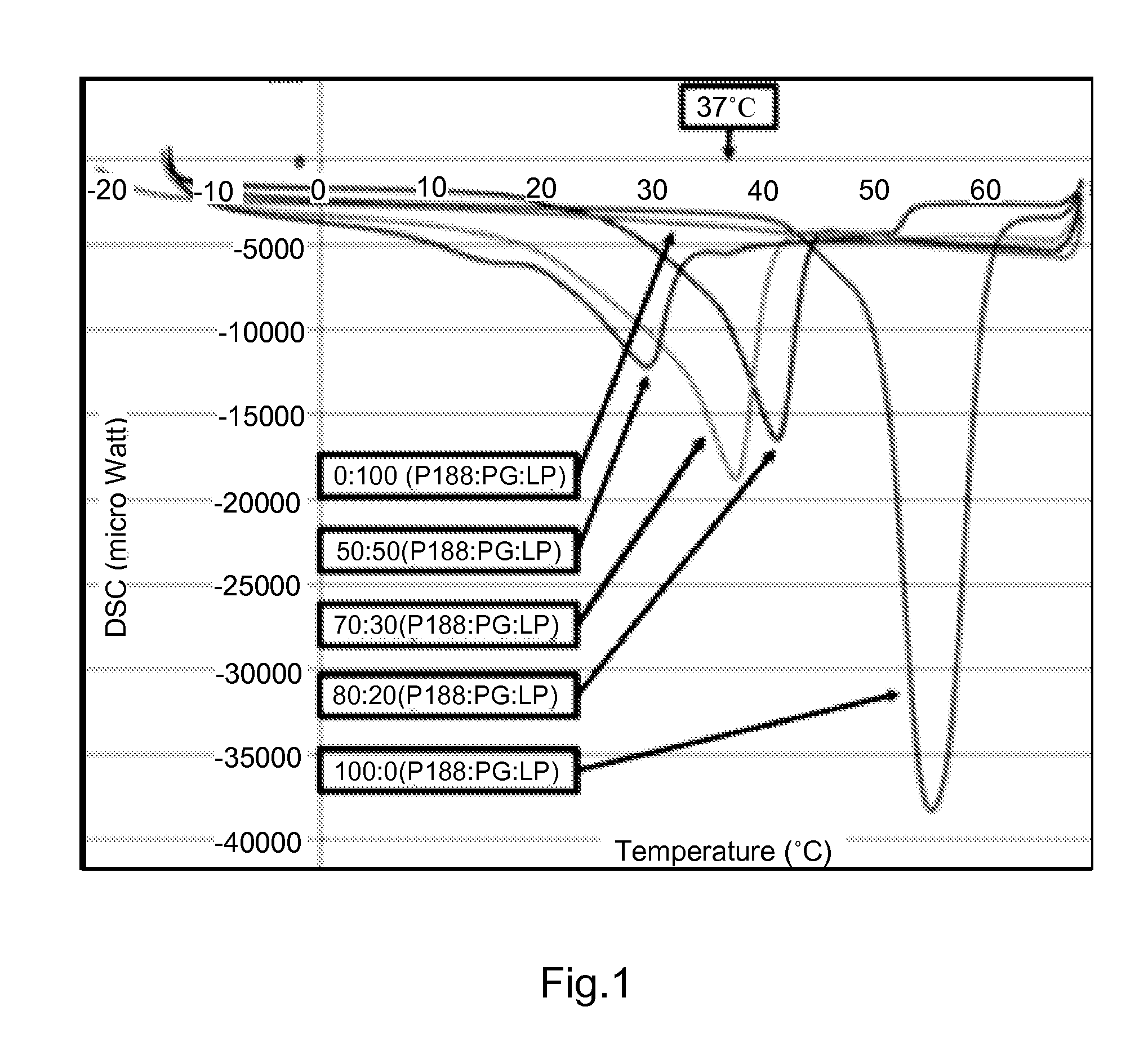
Thermo-sensitive, mucoadhesive or dermoadhesive, and penetration-enhancing formulations for topical delivery of therapeutics
ActiveUS9056137B2Hindering progressReduce deliveryAntibacterial agentsPowder deliveryBiopolymerTopical treatment
The present invention provides thermo-sensitive, mucoadhesive biopolymer formulations that enhance the penetration of therapeutics across the skin or mucosal surfaces. In a preferred embodiment, the biopolymer formulation comprises co-polymer of poloxamer 188 and propylene glycol, laurocapram and, optionally, one or more therapeutic agents. Also provided are uses of the biopolymer formulations for topical therapy of cancer including cervical cancer.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Methods and materials for treating and preventing inflammation of mucosal tissue
The invention involves methods and materials for treating and preventing non-invasive fungus-induced mucositis. Specifically, the invention involves administrating an antifungal agent such that it contact mucus in an amount, at a frequency, and for a duration effective to prevent, reduce, or eliminate non-invasive fungus-induced rhinosinusitis. This invention also provides methods and materials for diagnosing non-invasive fungus-induced rhinosinusitis and culturing non-invasive fungus from a mammalian mucus sample as well as specific antifungal formulations and medical devices for treating and preventing non-invasive fungus-induced rhinosinusitis. In addition, the invention provides methods and materials for treating and preventing other non-invasive fungus-induced mucositis conditions such as chronic otitis media, chronic colitis, and Crohn's disease. Further, the invention involves methods and materials for treating and preventing chronic asthma symptoms.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Production of fungal extracellular immune stimulating compounds
InactiveUS20050163800A1Improve isolationEasy to processBiocideOrganic active ingredientsLiquid mediumCell culture media
A process is described for the production of an immunostimulant by submerged cultivation of Lentinus edodes in which mycelium from agar plates or a fermentation broth is added to a liquid medium in a shake flask or a bioreactor containing nutrients such as malt extract, yeast extract, peptone and glucose having access to air or to which air is added, and which is kept in constant movement at approx. 28° C. At the proper conditions, there will be an increase in the production of extracellular lentinan, which is shown to be a better immunostimulant than intracellular lentinan. The extracellular product is precipitated from the growth medium by means of methods for the precipitation of microbial polysaccharide.
Owner:MEDIMUSH
Pharmaceutical compositions and methods to vaccinate against candidiasis
InactiveUS8541008B2Toxic reductionReduce adhesionPeptide/protein ingredientsSnake antigen ingredientsHospitalized patientsVirulent characteristics
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface proteins in the ALS group are downstream effectors of the filamentation regulatory pathway. Particularly, Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene, gene product, or specific antibody to the ALS gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT UCLA HARBOR MEDICAL CENT +1
Anti-fungal composition
InactiveUS20110171267A1Immunological disordersFungal antigen ingredientsFungal diseaseFungal antigen
A multivalent fungal vaccine comprising one or more heat-inactivated fungal antigens, wherein at least one fungal antigen is effective in producing an immune response in a host when said vaccine is administered orally at a dose that is sufficient for preventing or treating the fungal disease in said host. Also described are methods for making and using an orally available anti-fungal vaccine.
Owner:IMMUNITOR USA
Anti-cancer combination treatment and kit-of-parts
ActiveUS20090143280A1Meaningful patient benefitDesirable effectBiocidePeptide/protein ingredientsDiseaseOncology
Owner:GLYCANOVA
Pharmaceutical compositions and methods to vaccinate against disseminated candidiasis
InactiveUS7067138B1Treat prevent alleviateRetard pathogenesisBiocideAntimycoticsHospitalized patientsVirulent characteristics
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface protein, Als1p, is a downstream effector of the filamentation regulatory pathway and is regulated by Efg1p. Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene product from the ALS1 gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Immunogenic Anti-inflammatory compositions
The invention provides methods of formulating an anti-inflammatory composition for treating inflammatory conditions in a specific organ or tissue. The method involves selecting at least one pathogen that is pathogenic in the specific organ or tissue; producing an antigenic composition comprising antigenic determinants that together are specific for the pathogen; and formulating the antigenic composition for administration as an anti-inflammatory composition capable of eliciting an anti-inflammatory response in the specific organ or tissue. In embodiments of the invention the pathogen may be an endogenous pathogen, such as an endogenous bacterial pathogen. The pathogen may be an exogenous pathogen, such as a bacterial pathogen, viral pathogen, a fungal pathogen, or a helminth pathogen.
Owner:QU BIOLOGICS INC
Pharmaceutical compositions and methods to vaccinate against disseminated candidiasis and other infectious agents
The invention provides a vaccine including an isolated Als protein family member having cell adhesion activity, or an immunogenic fragment thereof, with an adjuvant in a pharmaceutically acceptable medium. The invention also provides a method of treating or preventing hematogenously disseminated or mucocutaneous candidiasis. The method includes administering an immunogenic amount of a vaccine an isolated Als protein family member having cell adhesion activity, or an immunogenic fragment thereof, in a pharmaceutically acceptable medium.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
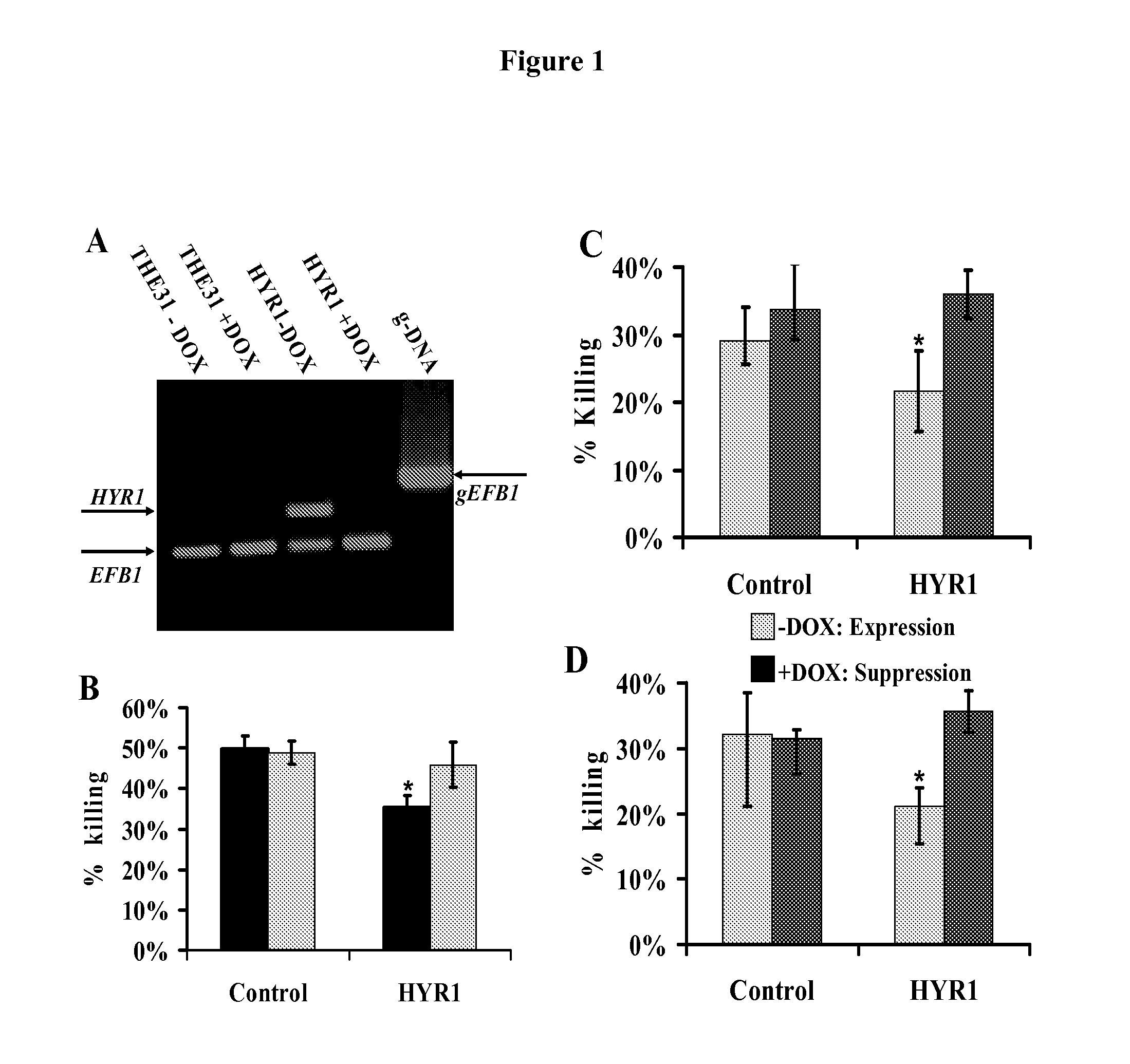
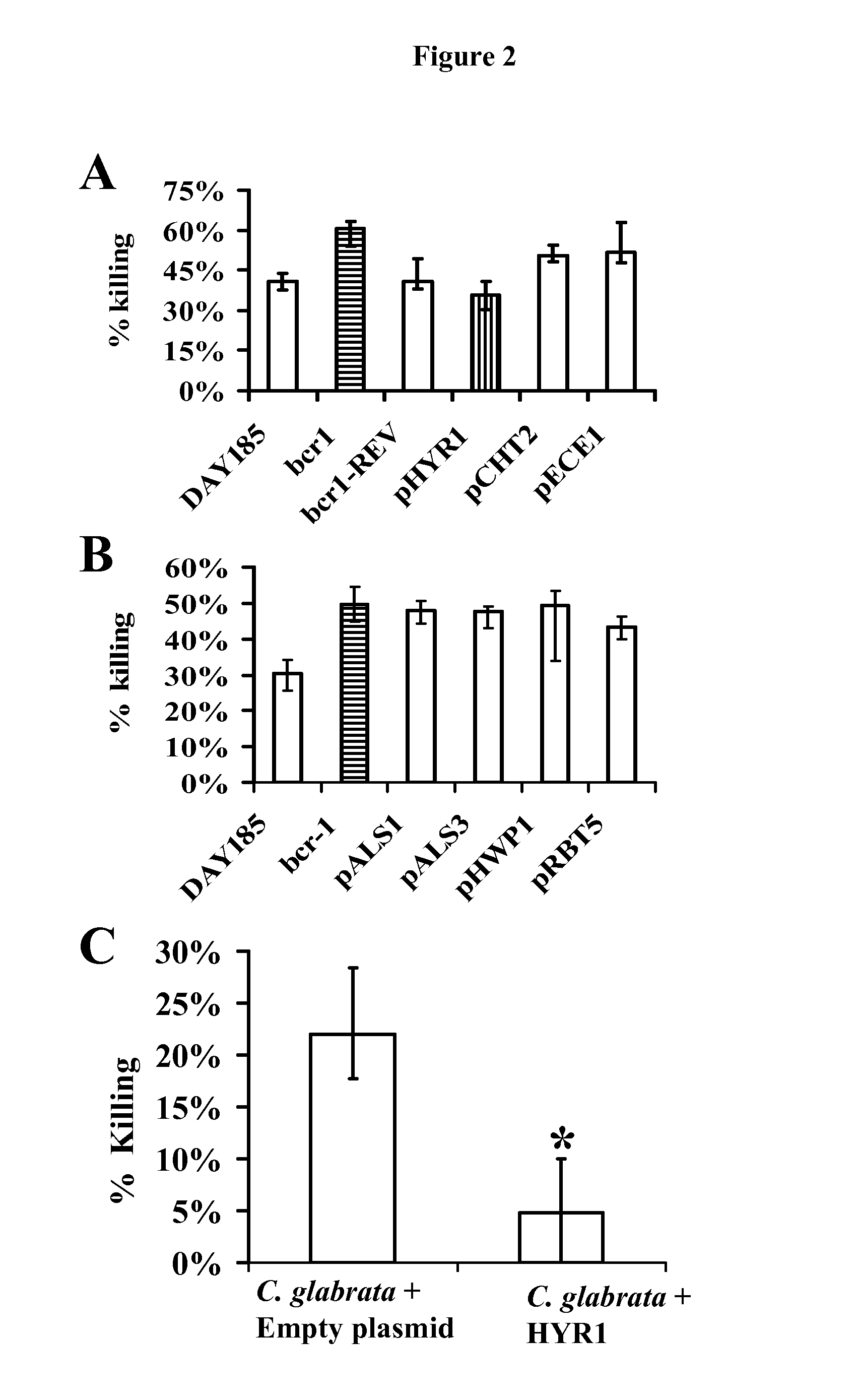
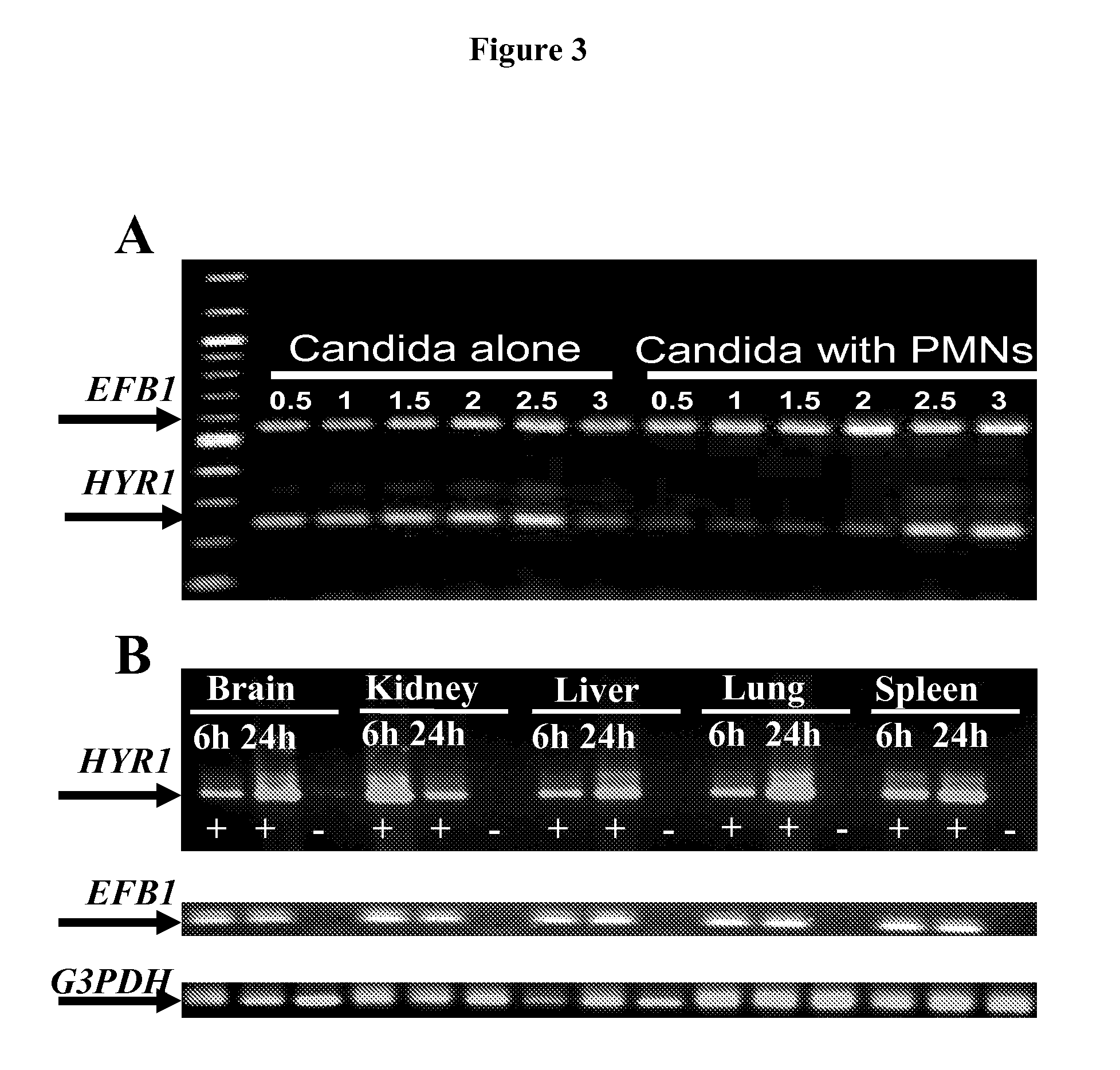
Hyr1 as a target for active and passive immunization against candida
ActiveUS20120237534A1Lower Level RequirementsImprove the immunityAntimycoticsPeptide/protein ingredientsPassive ImmunizationsDisseminated candida
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Vaccine
The disclosure relates to polypeptides and pharmaceutical compositions comprising polypeptides that find use in the prevention or treatment of cancer, in particular breast cancer, ovarian cancer and colorectal cancer. The disclosure also relates to methods of inducing a cytotoxic T cell response in a subject or treating cancer by administering pharmaceutical compositions comprising the peptides, and companion diagnostic methods of identifying subjects for treatment. The peptides comprise T cell epitopes that are immunogenic in a high percentage of patients.
Owner:TREOS BIO LTD
Compositions and methods for prevention and treatment of fungal diseases
InactiveUS20070243209A1Significant clinical importanceEffective adjuvantBiocideOrganic active ingredientsDiseaseFungal disease
The present invention relates to various pharmaceutical compositions that can be used as active or passive vaccines for the treatment or prevention of fungal disease. Methods for prevention and treatment of infectious and allergic fungal diseases in subjects using the pharmaceutical compositions of the present invention are also disclosed.
Owner:HEALTH RES INC
Pharmaceutical compositions and methods to vaccinate against disseminated candidiasis
InactiveUS20060083750A1Treating preventingInhibit bindingAntibacterial agentsFungiCell adhesionAdjuvant
The invention provides a vaccine including an isolated Als protein family member having cell adhesion activity, or an immunogenic fragment thereof, with an adjuvant in a pharmaceutically acceptable medium. The invention also provides a method of treating or preventing disseminated candidiasis. The method includes administering an immunogenic amount of a vaccine an isolated Als protein family member having cell adhesion activity, or an immunogenic fragment thereof, in a pharmaceutically acceptable medium. A method of treating or preventing disseminated candidiasis also is provided that includes administering an effective amount of an isolated Als protein family member having cell adhesion activity, or an functional fragment thereof, to inhibit the binding or invasion of Candida to a host cell or tissue. The Als protein family member can be derived from a Candida strain selected from the group consisting of Candida albicans, Candida krusei, Candida tropicalis, Candida glabrata and Candida parapsilosis and the Als protein family member includes Als1p, Als3p, Als5p, Als6p, Als7p or Als9p.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com