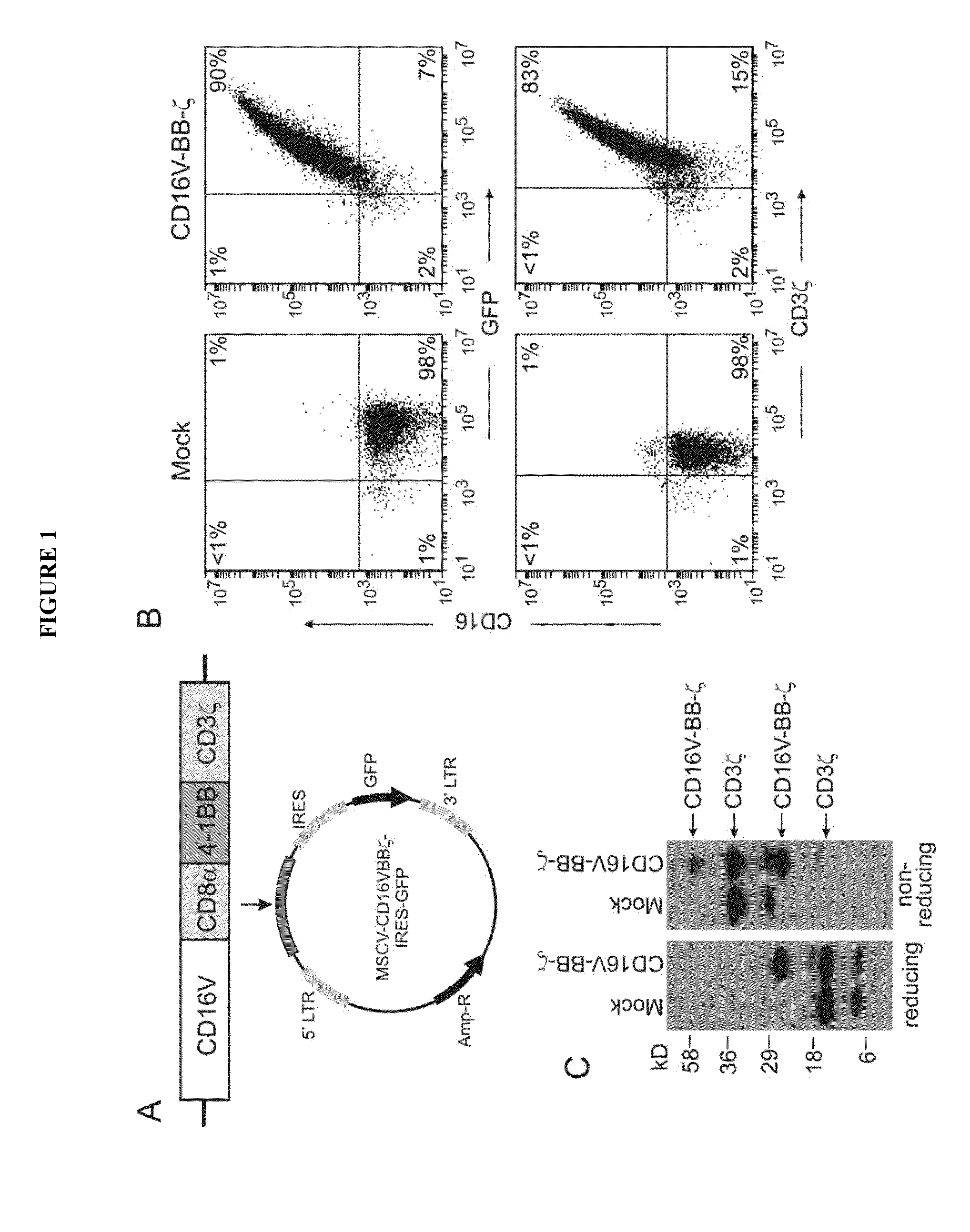
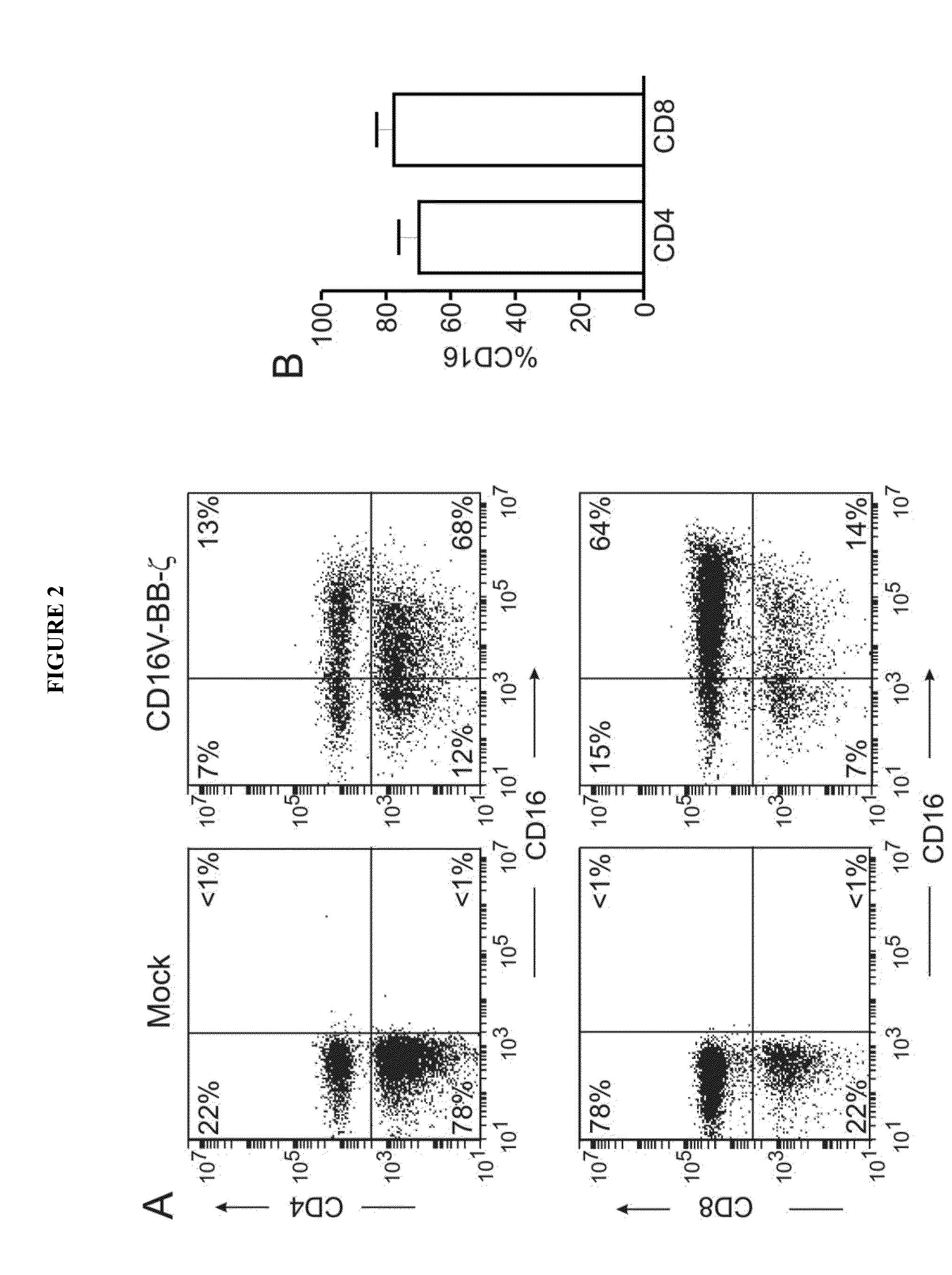
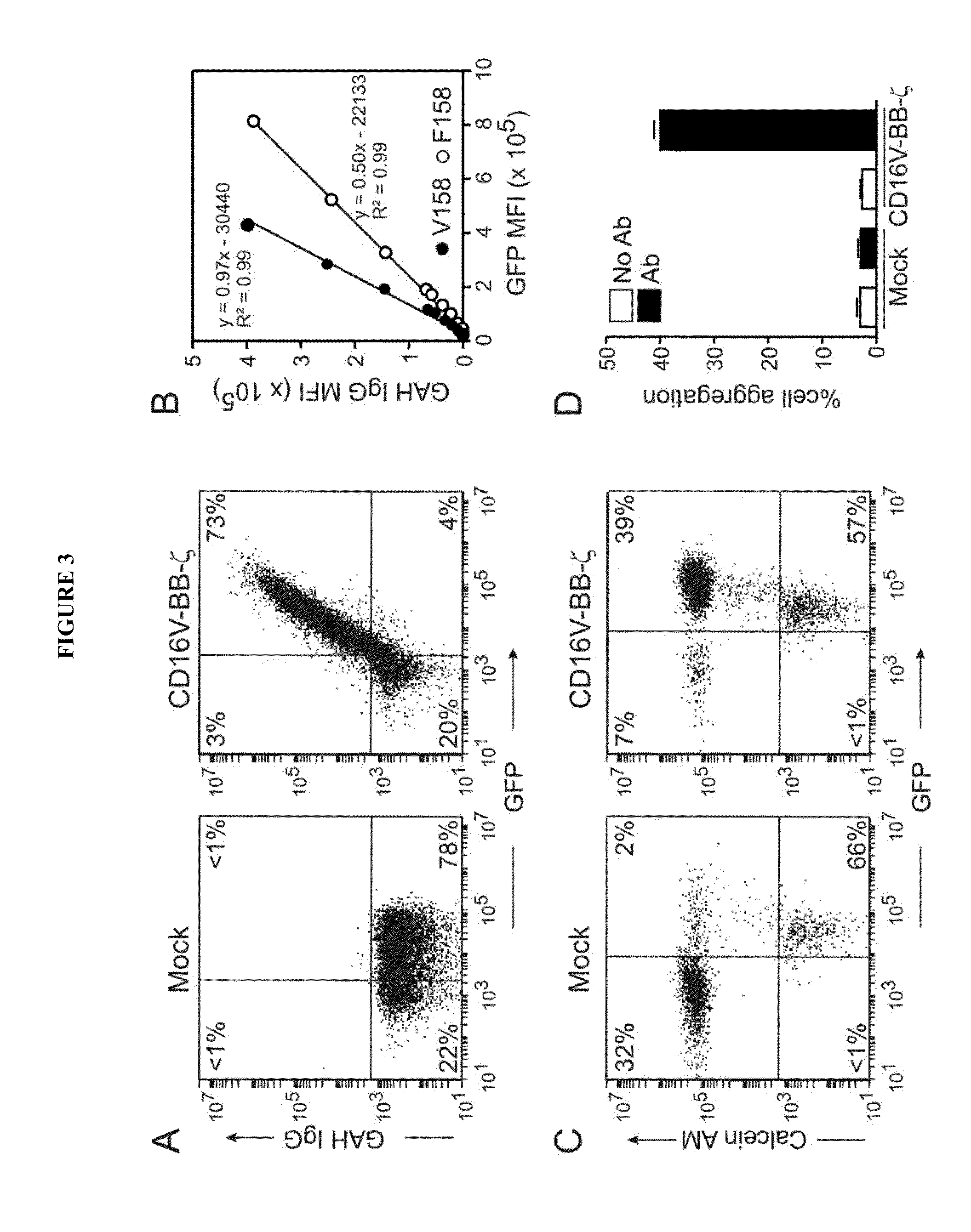
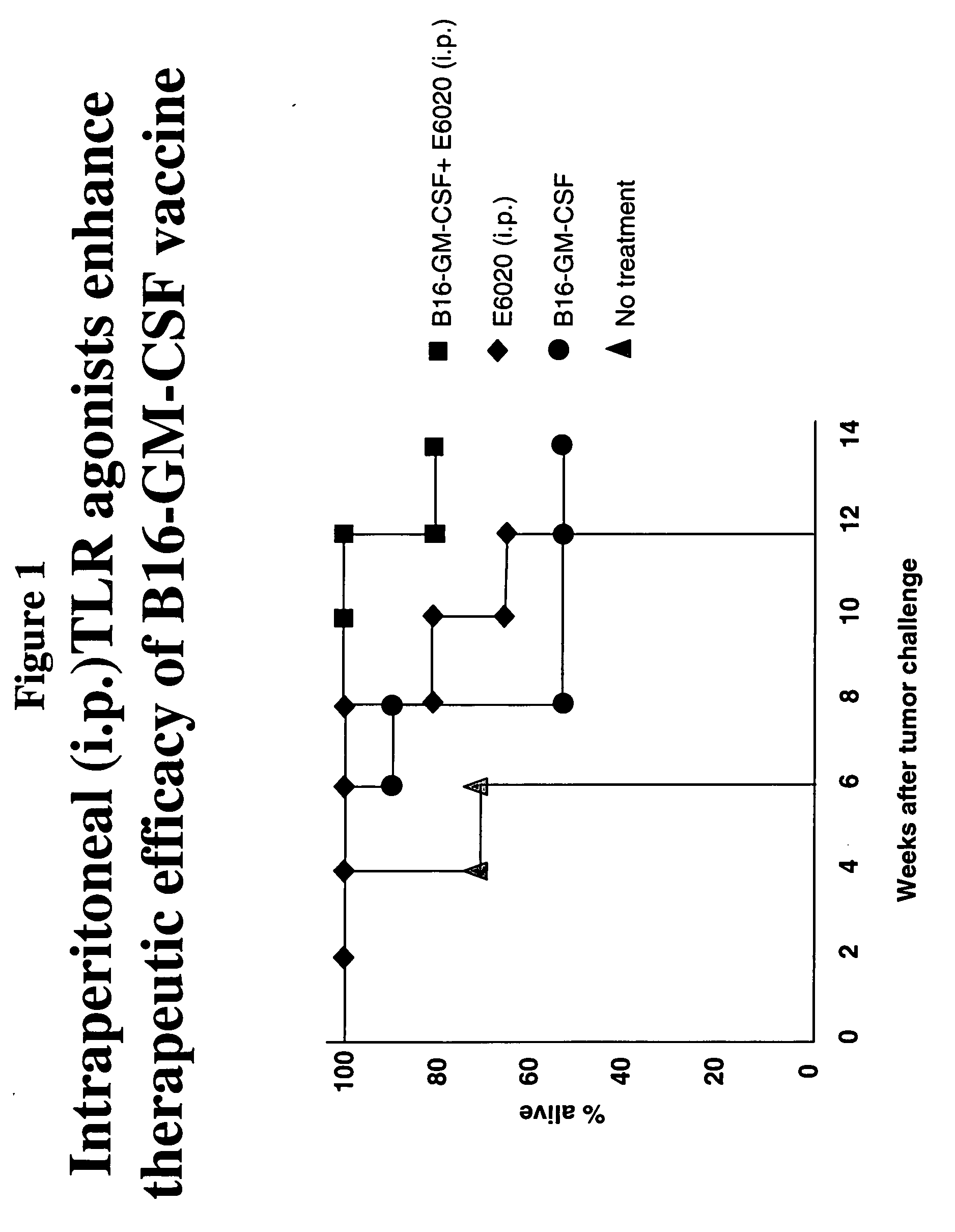
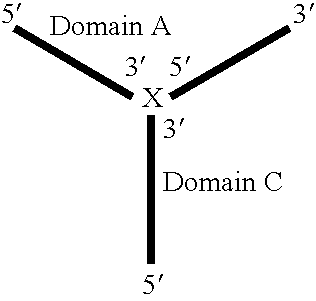
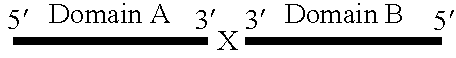Patents
Literature
201 results about "Immune therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunotherapy, also called biological therapy, is a type of treatment that uses the body's immune system to fight cancer. The therapy mainly consists of stimulating the immune system to help it do its job more effectively. Immunotherapy is a comparatively new type of therapy in the fight against many cancers.
Chimeric receptors and uses thereof in immune therapy
ActiveUS20150139943A1Good curative effectEnhanced ADCC activityVirusesPeptide/protein ingredientsCytotoxicityCD8
Owner:COGENT BIOSCIENCES INC +2
Compositions and methods for cancer immunotherapy
The invention relates to immunotherapeutic compounds and to methods for stimulating an immune response in a subject individual at risk for developing cancer, diagnosed with a cancer, in treatment for cancer, or in post-therapy recovery from cancer or the compounds of the invention can be administered as a prophylactic to a subject individual to prevent or delay the development of cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
Combining Radioimmunotherapy and Antibody-Drug Conjugates for Improved Cancer Therapy
InactiveUS20110070156A1Organic active ingredientsHybrid immunoglobulinsParanasal Sinus CarcinomaAntiendomysial antibodies
Described herein are compositions and methods of use of radionuclide-antibody conjugates (for RAIT) and drug-antibody conjugates (ADC). The combination of RAIT and ADC was more efficacious than either RAIT alone, ADC alone, or the sum of effects of RAIT and ADC. The unexpected synergy resulted in decreased tumor growth rate and increased survival, with a high incidence of tumor-free survival in Capan-1 human pancreatic cancer xenografts in nude mice.
Owner:IMMUNOMEDICS INC
Stress proteins and uses therefor
InactiveUS6338952B1Preventing and reducing adverse effectEnhance immune responseVirusesAntibody mimetics/scaffoldsAntigenImmunotherapeutic agent
The present invention relates to stress proteins and methods of modulating an individual's immune response. In particular, it relates to the use of such stress proteins in immune therapy and prophylaxis, which results in an induction or enhancement of an individual's immune response and as an immunotherapeutic agent which results in a decrease of an individual's immune response to his or her own cells. The present invention also relates to compositions comprising a stress protein joined to another component, such as a fusion protein in which a stress protein is fused to an antigen. Further, the present invention relates to a method of generating antibodies to a substance using a conjugate comprised of a stress protein joined to the substance.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES INC
Immunonanotherapeutics that Provide IgG Humoral Response Without T-Cell Antigen
ActiveUS20100183727A1Improve responseSsRNA viruses negative-senseAntibacterial agentsNanocarriersImmune therapy
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides synthetic nanocarriers capable of eliciting an immune system response in the form of antibody production, wherein the nanocarriers lack any T cell antigens. In some embodiments, the invention provides nanocarriers that comprise an immunofeature surface, which provides high avidity binding of the nanocarriers to antigen presenting cells. The invention provides pharmaceutical compositions comprising inventive nanocarriers. The present invention provides methods of designing, manufacturing, and using inventive nanocarriers and pharmaceutical compositions thereof.
Owner:MASSACHUSETTS INST OF TECH +2
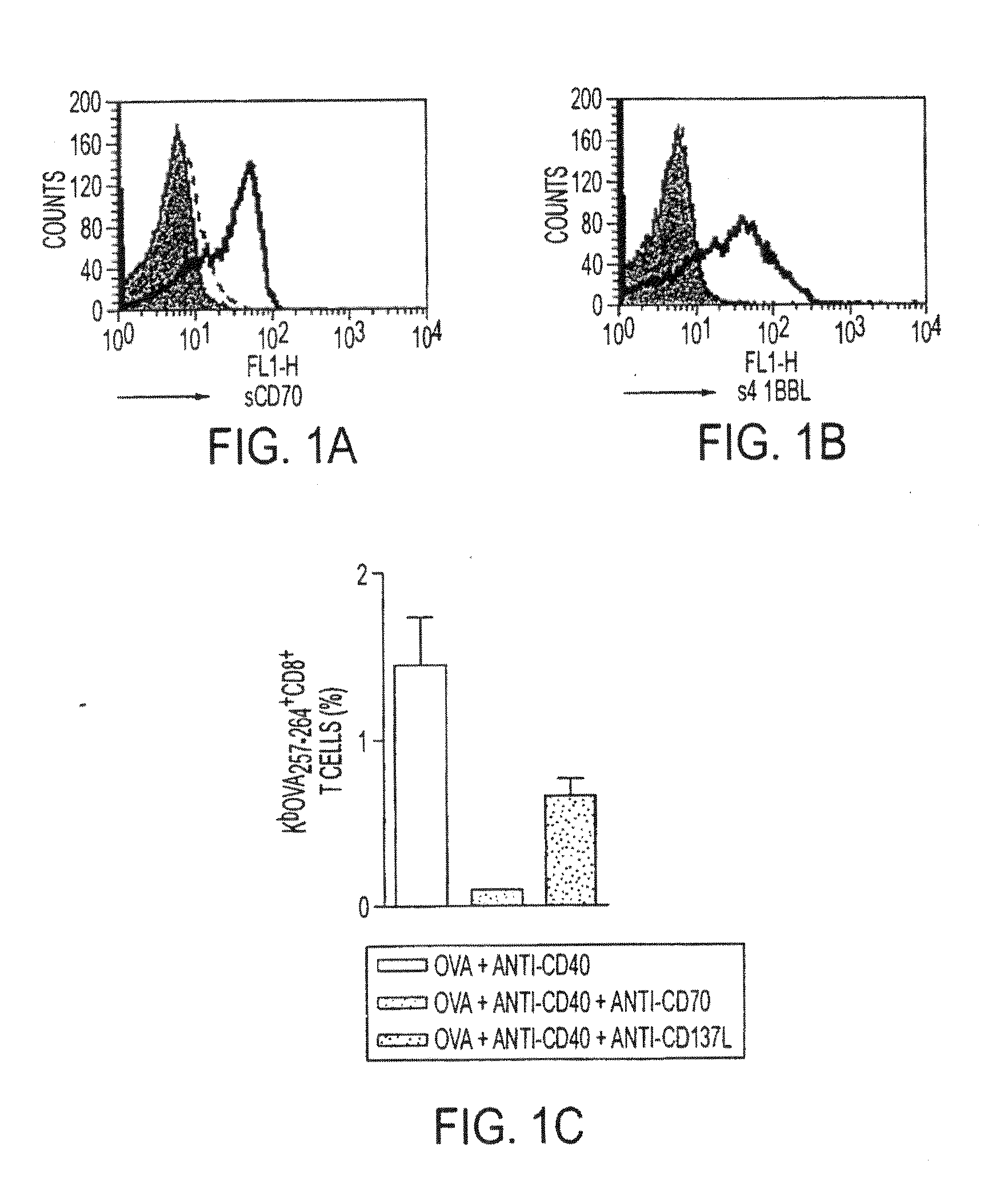
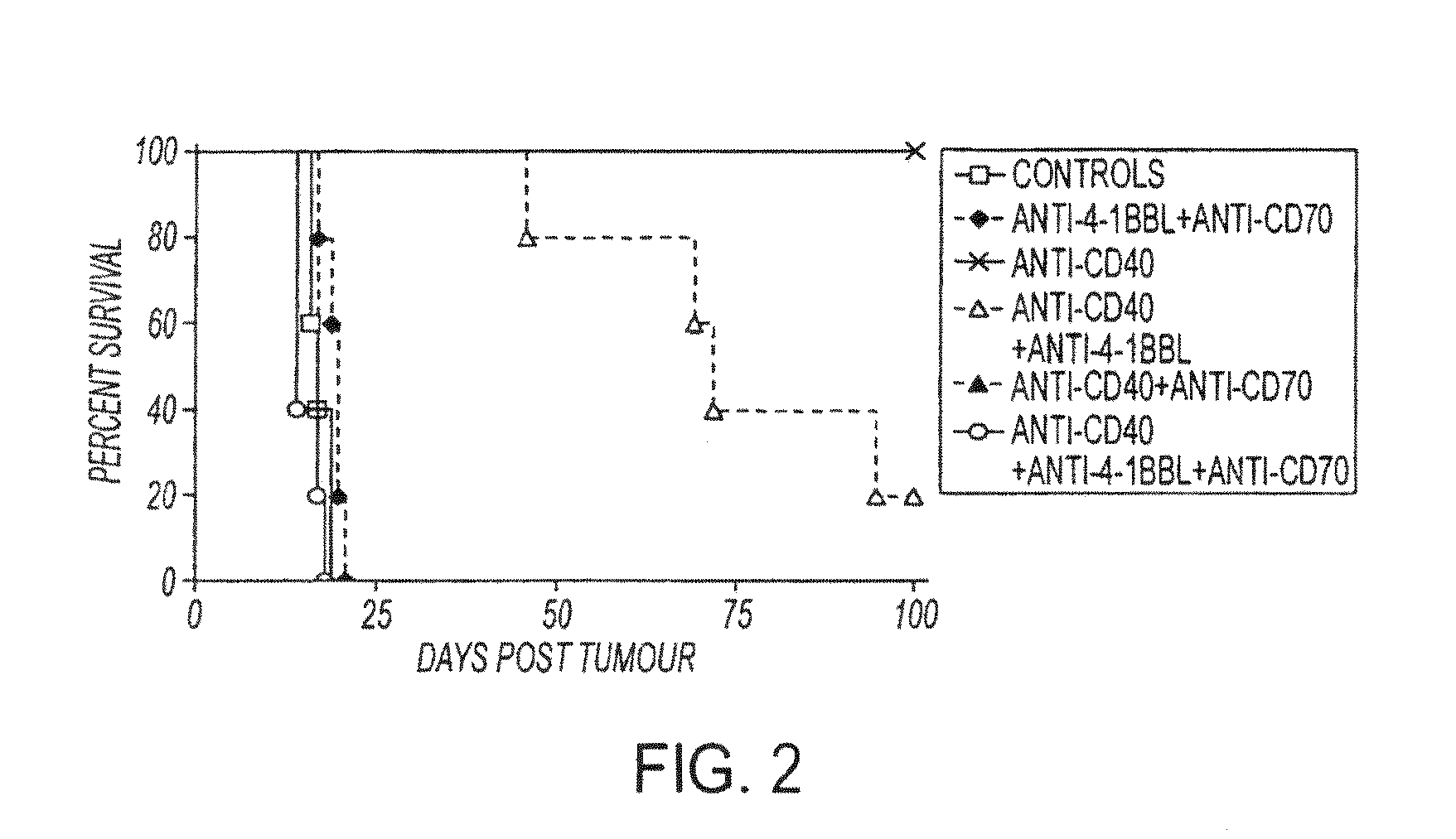
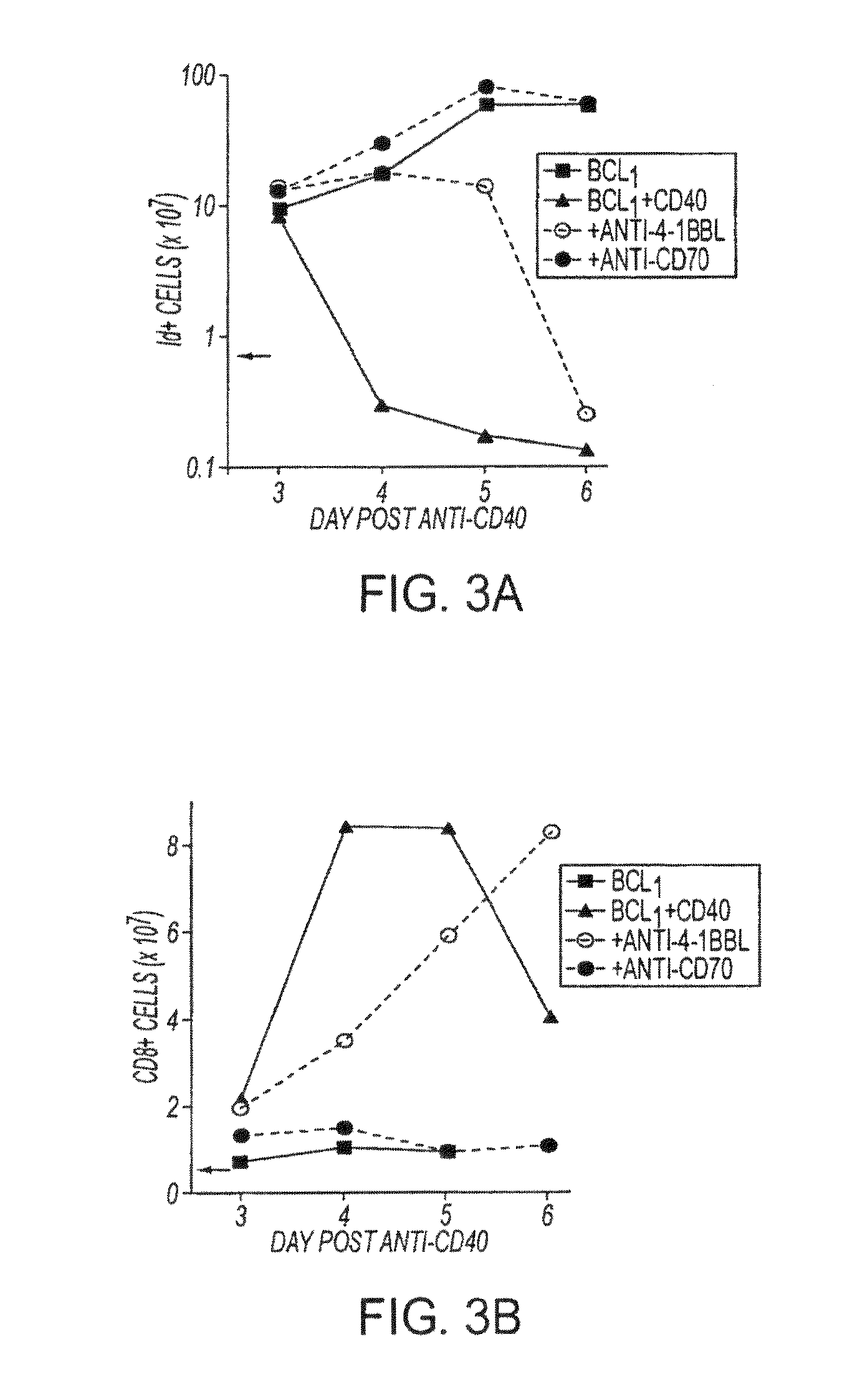
Human immune therapies using a CD27 agonist alone or in combination with other immune modulators
ActiveUS8481029B2Promotes strong expression of 4-1BBImprove responseAntibacterial agentsNervous disorderAutoimmune responsesImmune modulator
Methods of inducing T cell proliferation and expansion in vivo for treating conditions wherein antigen-specific T cell immune response are therapeutically desirable such as cancer, infection, inflammation, allergy and autoimmunity and for enhancing the efficacy of vaccines are provided. These methods comprise the administration of at least one CD27 agonist, preferably an agonistic CD27 antibody, alone or in association with another moiety such as immune stimulant or immune modulator such as an anti-CD40, OX-40, 4-IBB, or CTLA-4 antibody or an agent that depletes regulatory cells, or a cytokine. These mono and combination therapies may also optionally include the administration of a desired antigen such as a tumor antigen, an allergen, an autoantigen, or an antigen specific to an infectious agent or pathogen against which a T cell response (often CD8+) is desirably elicited.
Owner:UNIV OF SOUTHAMPTON
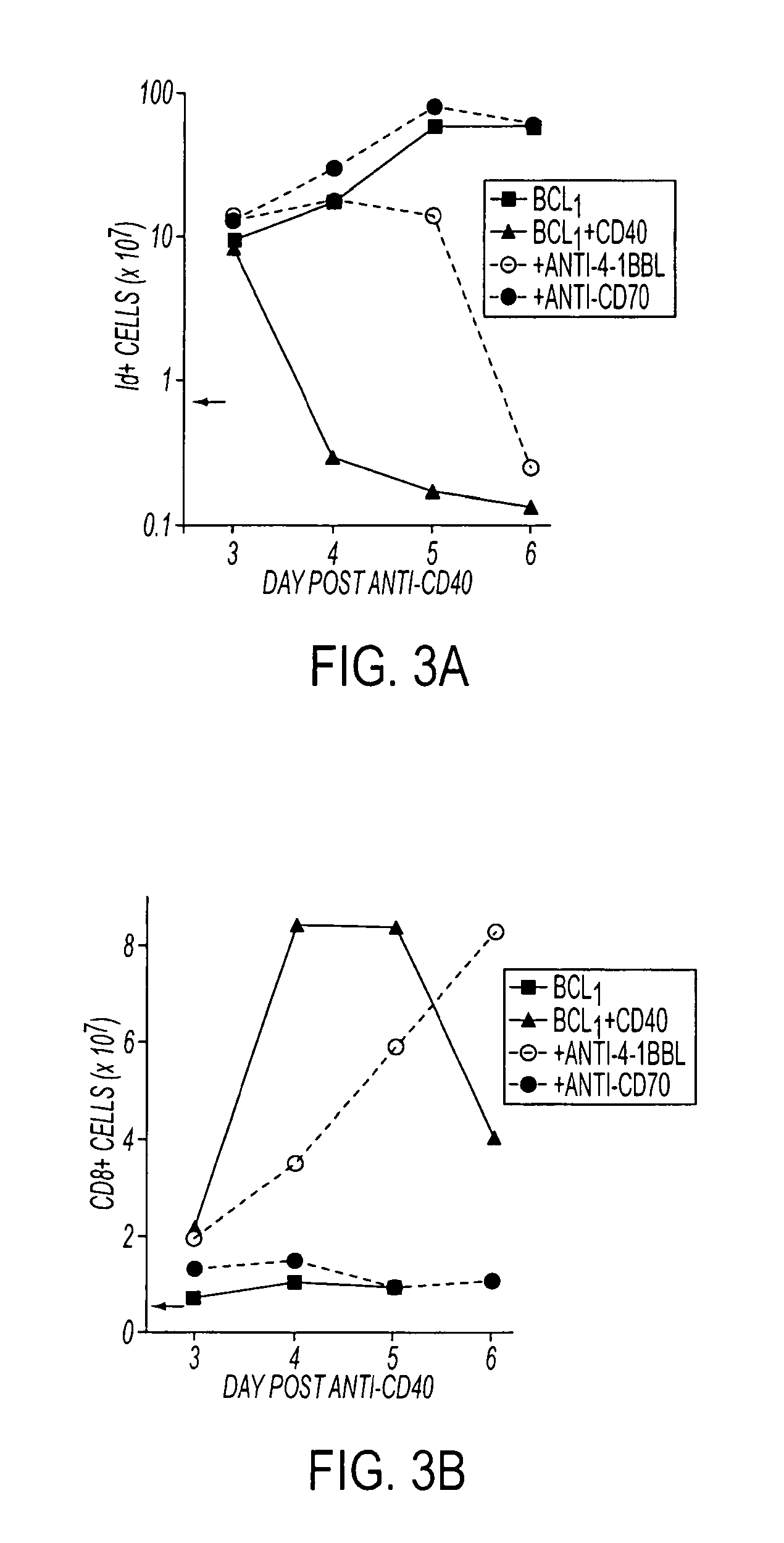
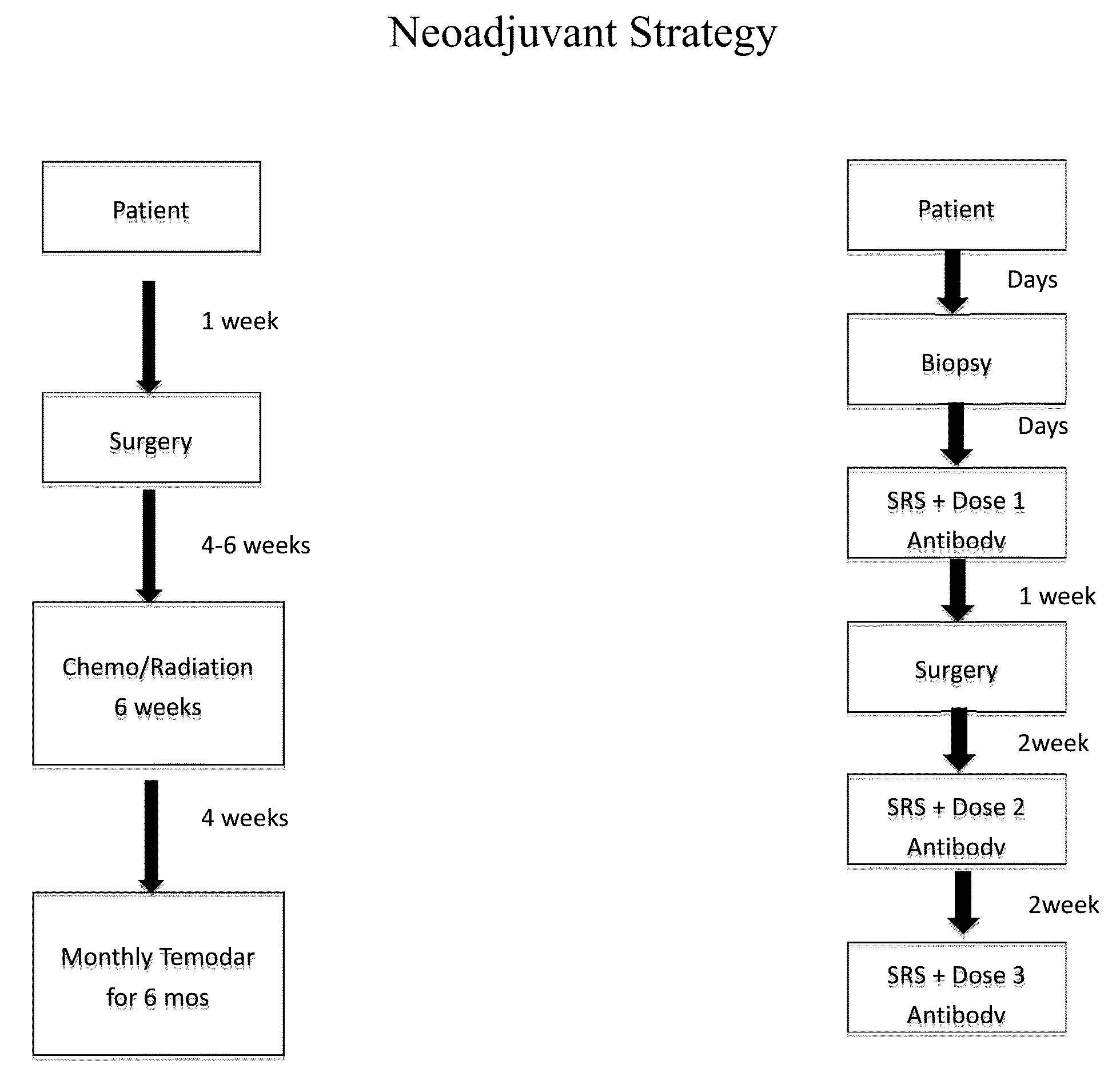
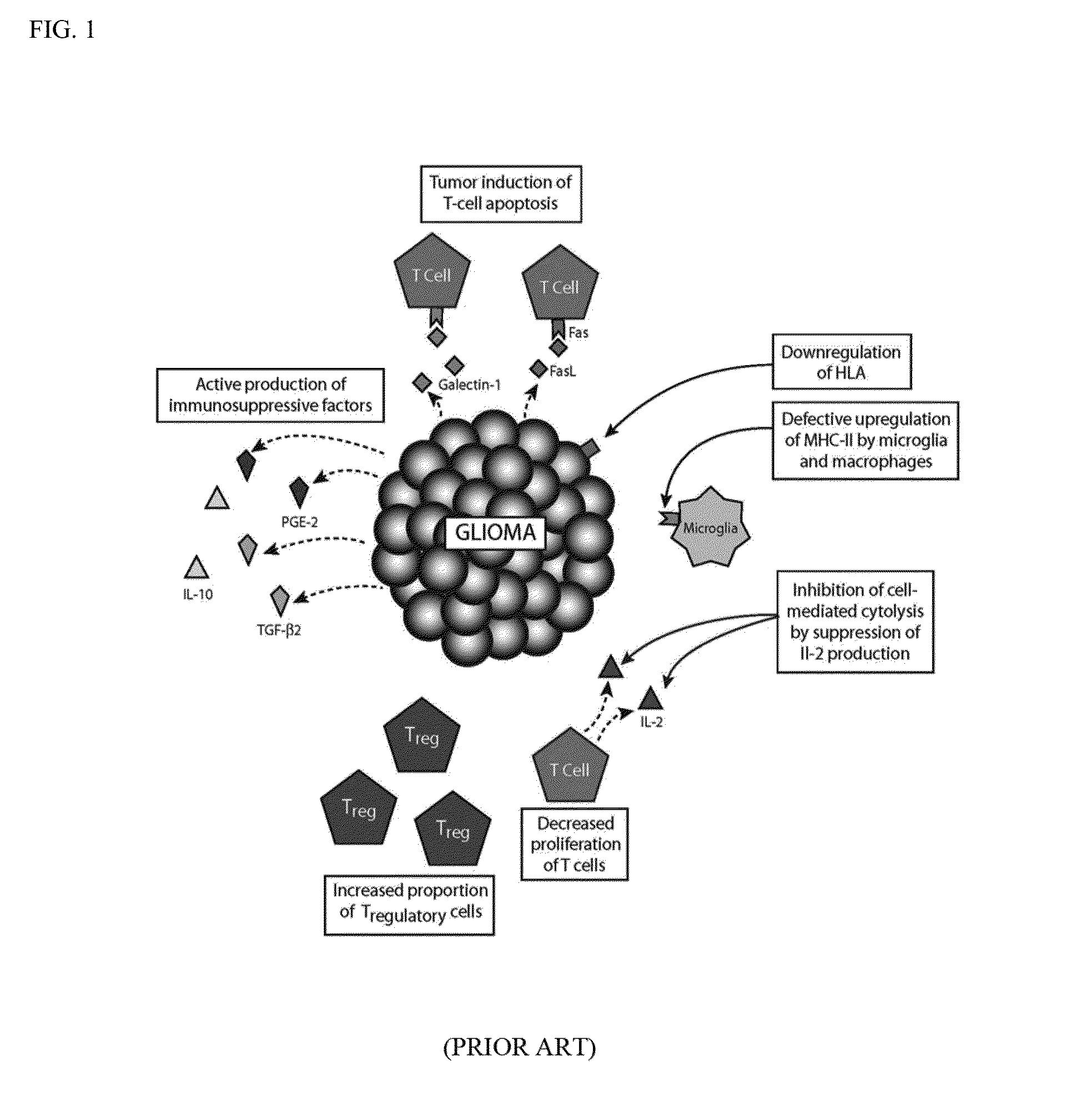
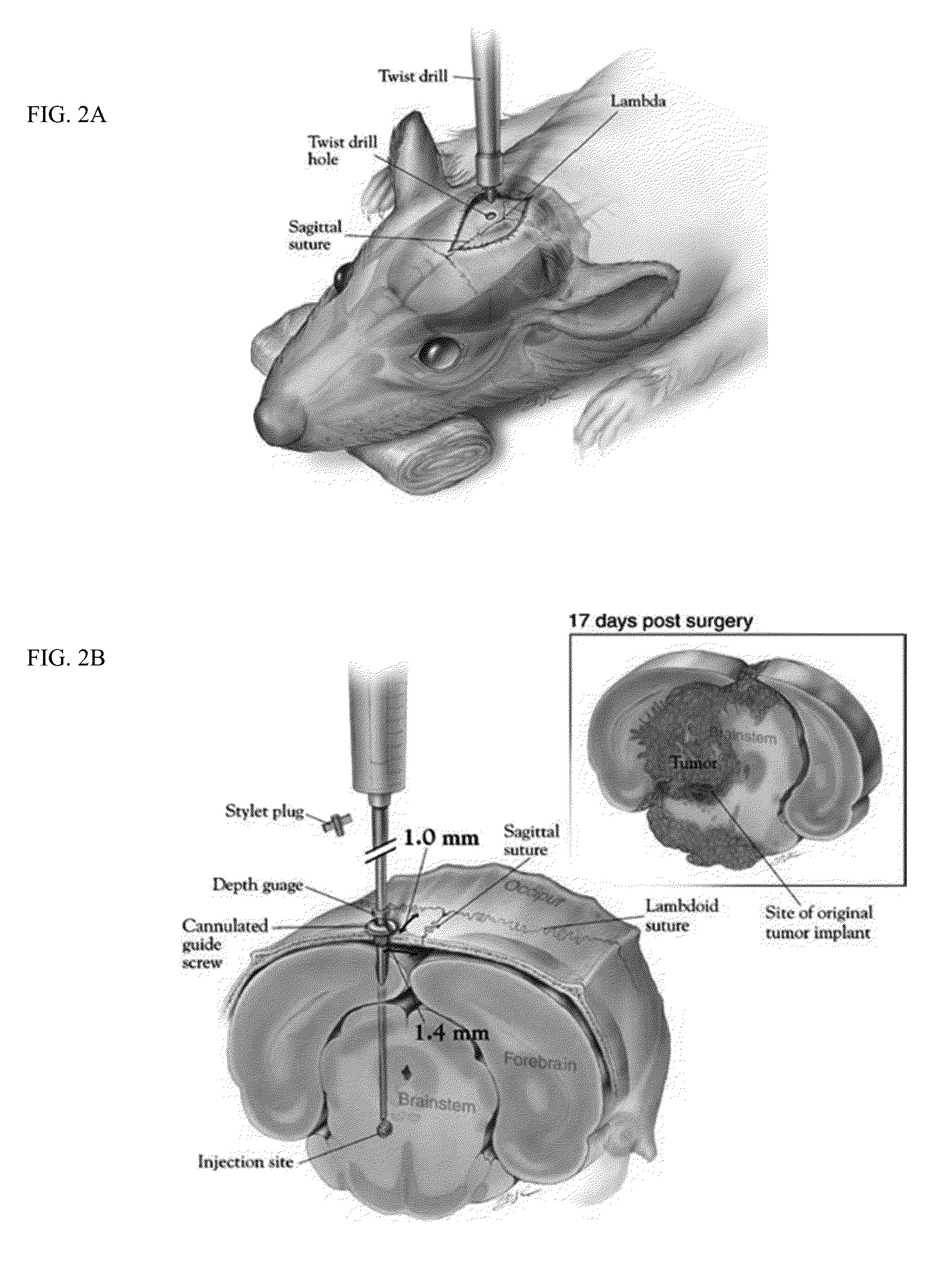
Focused radiation for augmenting immune-based therapies against neoplasms
ActiveUS9132281B2Inhibiting, reducing, or counteracting an immunosuppressive effect of a tumor in a subjectEliminate the effects ofEnergy modified materialsImmunoglobulins against cytokines/lymphokines/interferonsAdjuvantImmunotherapeutic agent
An approach combining immune-based therapies with focused radiation, including stereotactic radiation, to treat cancers is disclosed. The use of focused radiation primes the immune system in a similar manner to vaccines to augment immune-based therapies and can counteract the suppressive effects of a tumor. The combination of focused radiation and immune-based therapies, including administration of at least one immunotherapeutic agent, improves survival compared to each therapy alone and can, in some cases, lead to a durable cure. Accordingly, focused radiation can be an adjuvant for immune-based therapies for treating cancers.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Heterodimeric fusion proteins useful for targeted immune therapy and general immune stimulation
InactiveUS20050137384A1Extended half-lifeAltering pharmacology and biodistributionPeptide/protein ingredientsAntibody mimetics/scaffoldsWhite blood cellStepwise approach
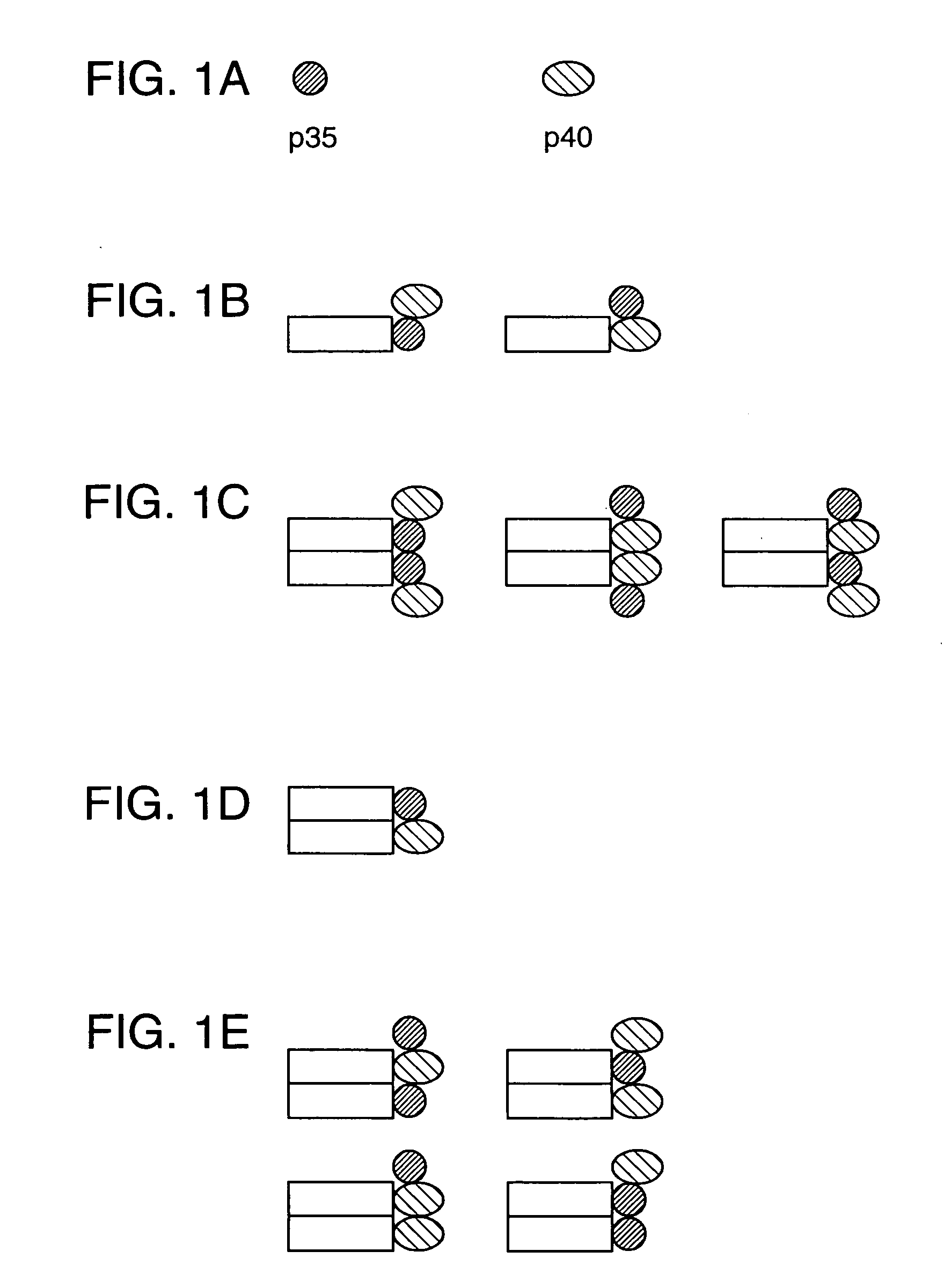
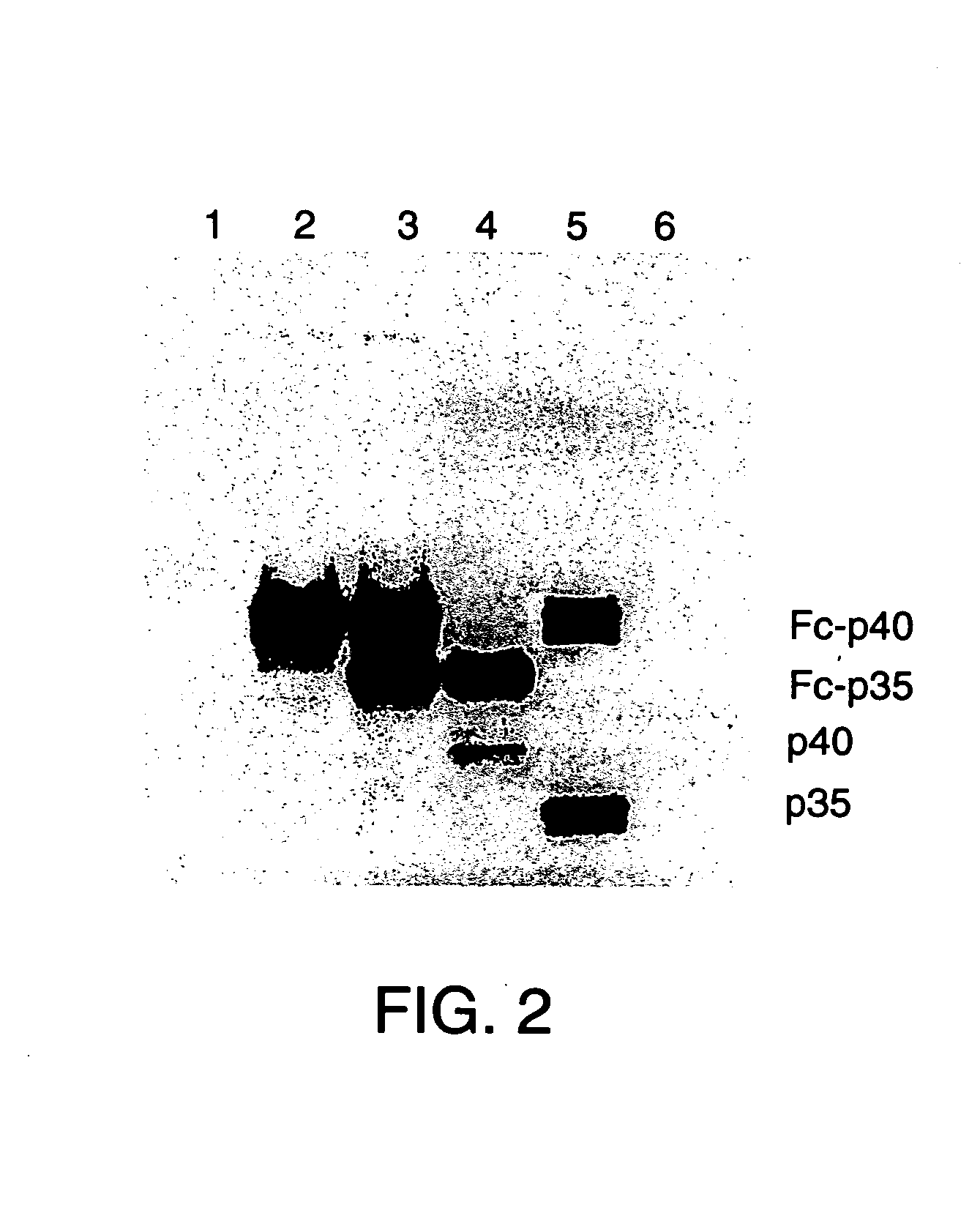
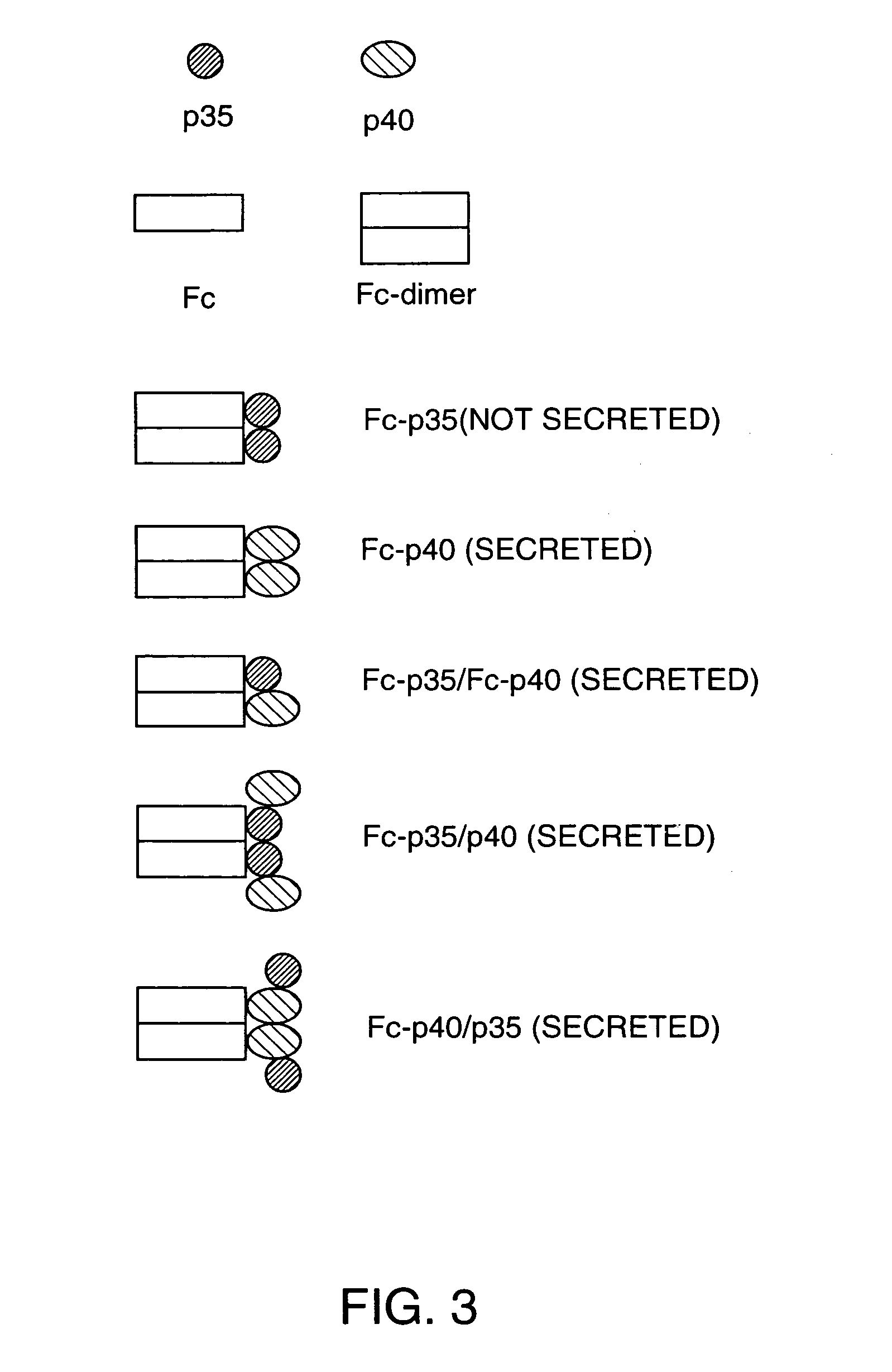
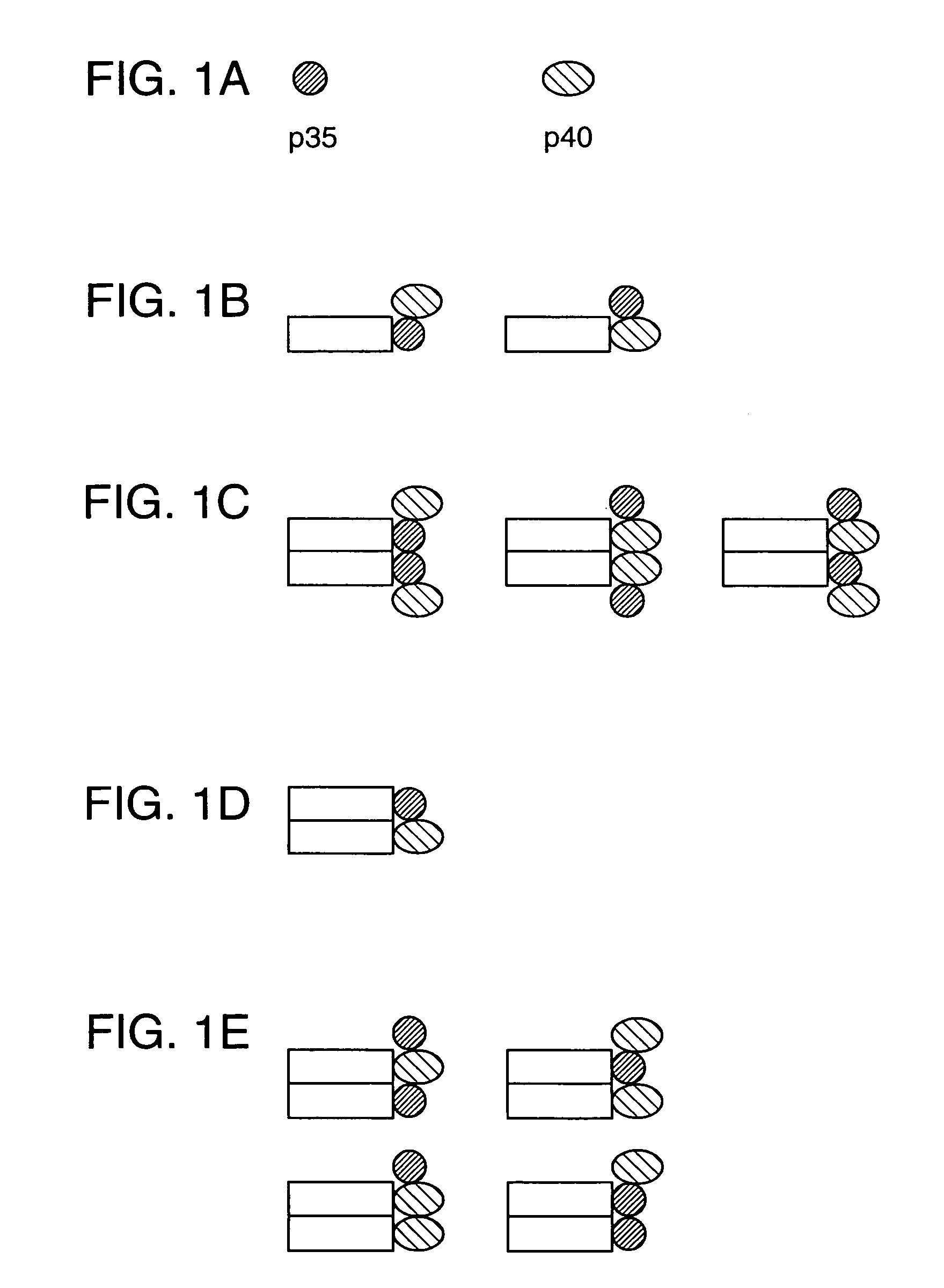
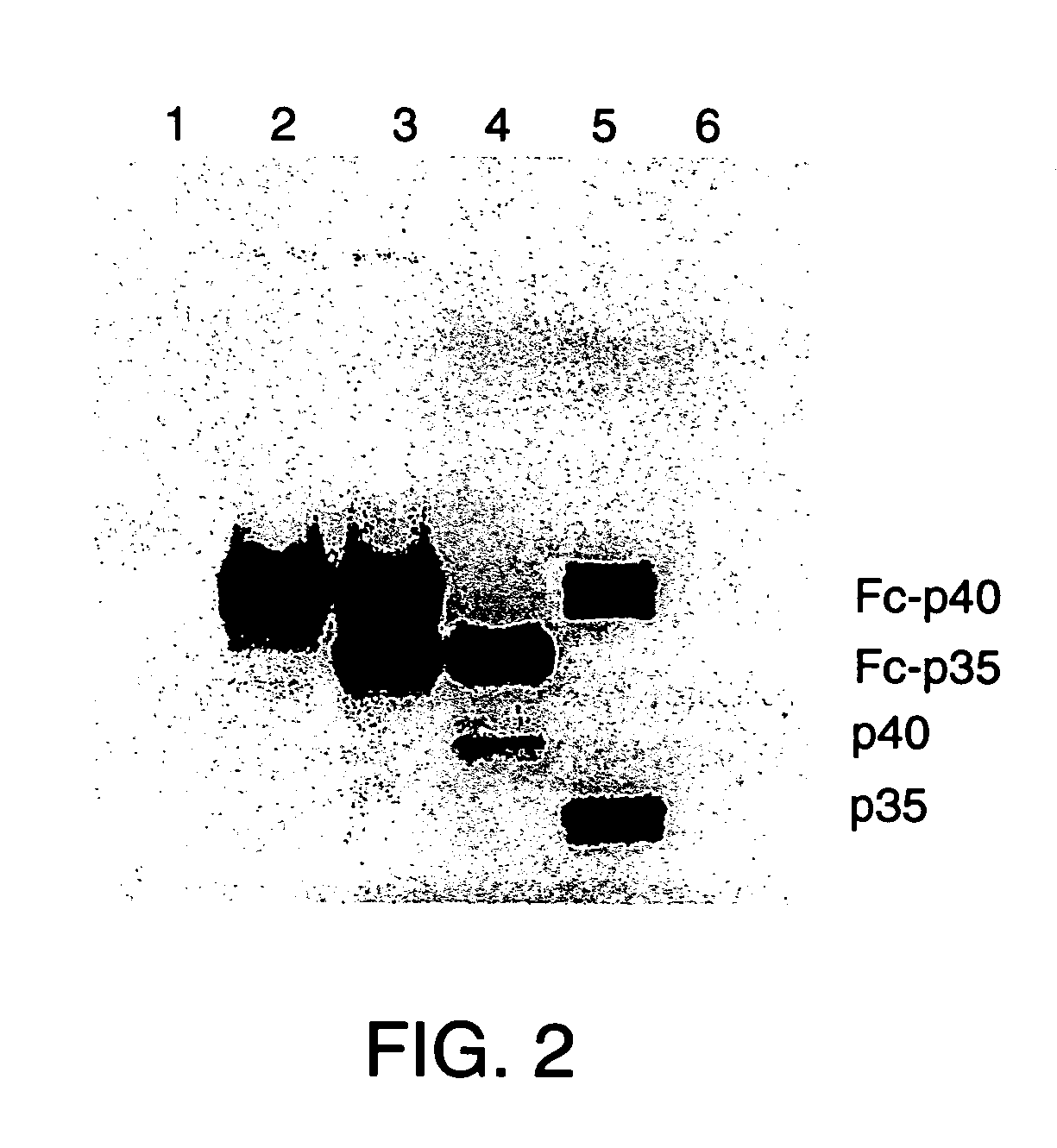
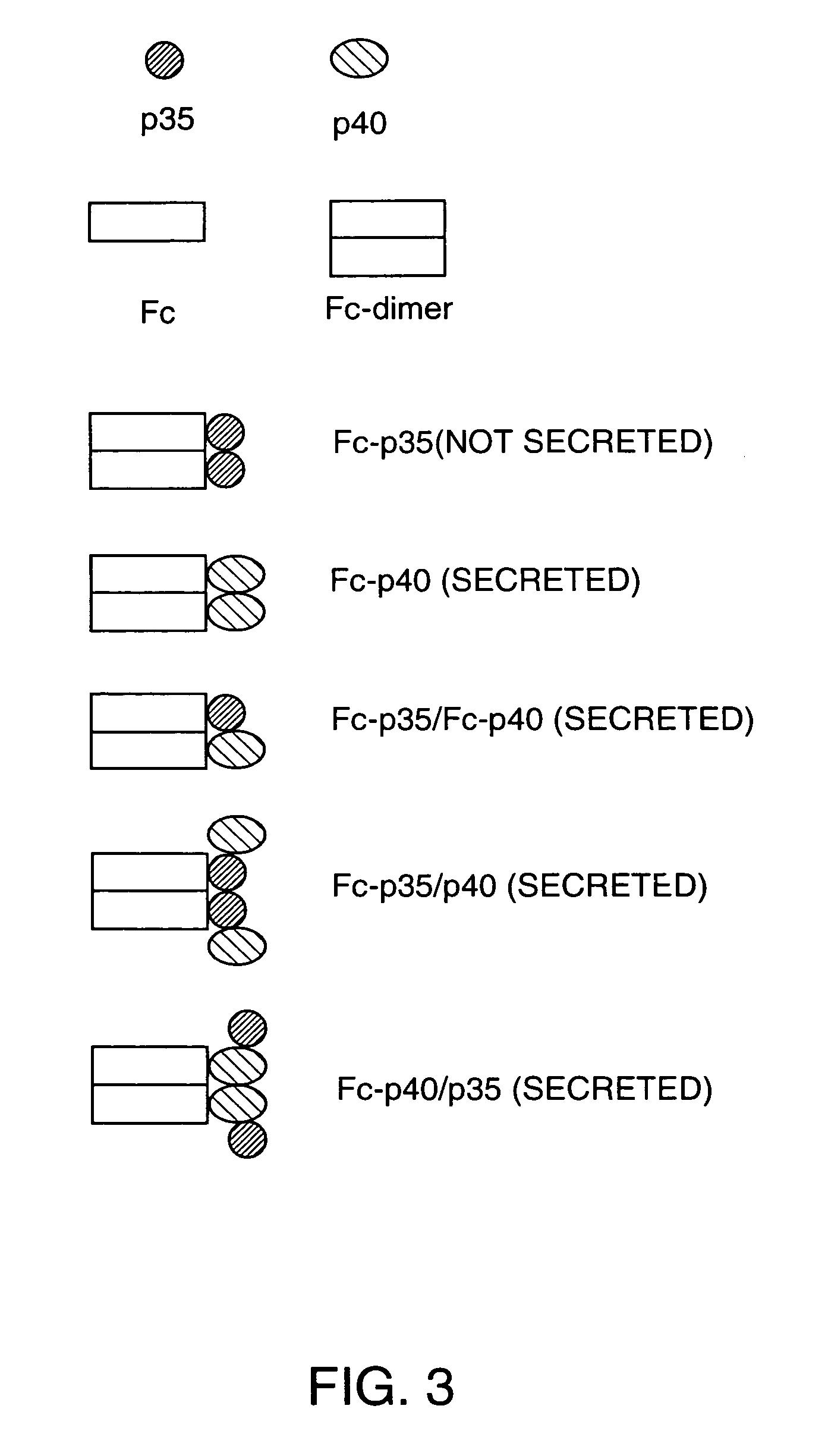
Disclosed are methods for producing fusion proteins with the heterodimeric cytokine, interleukin-12. In order to insure that the proper ratio of fused and non-fused subunits are obtained in the fusion protein, a specific stepwise approach to genetic engineering is used. This consists of first expressing the non-fused p40 IL-12 subunit in a production cell line, followed by or simultaneously expressing in the same cell, a second recombinant fusion protein consisting of the fused polypeptide linked by a peptide bond to the p35 subunit of IL-12. Molecules containing the p35 fusion protein cannot be secreted from the transfected mammalian cell without first complexing in a one to one ratio with the p40 subunit, thus ensuring the production of active heterodimeric fusion proteins.
Owner:MERCK PATENT GMBH
Synthetic rna-based agonists of tlr7
The invention relates to the therapeutic use of novel stabilized oligoribonucleotides as immune modulatory agents for immune therapy applications. Specifically, the invention provides novel RNA-based oligoribonucleotides with improved nuclease and RNase stability and that selectively induce immune modulatory activity through TLR7.
Owner:IDERA PHARMA INC
Compositions and methods for cancer immunotherapy
ActiveUS20150010613A1Improve propertiesHigh activityOrganic active ingredientsPeptide/protein ingredientsAntigenHuman tumor
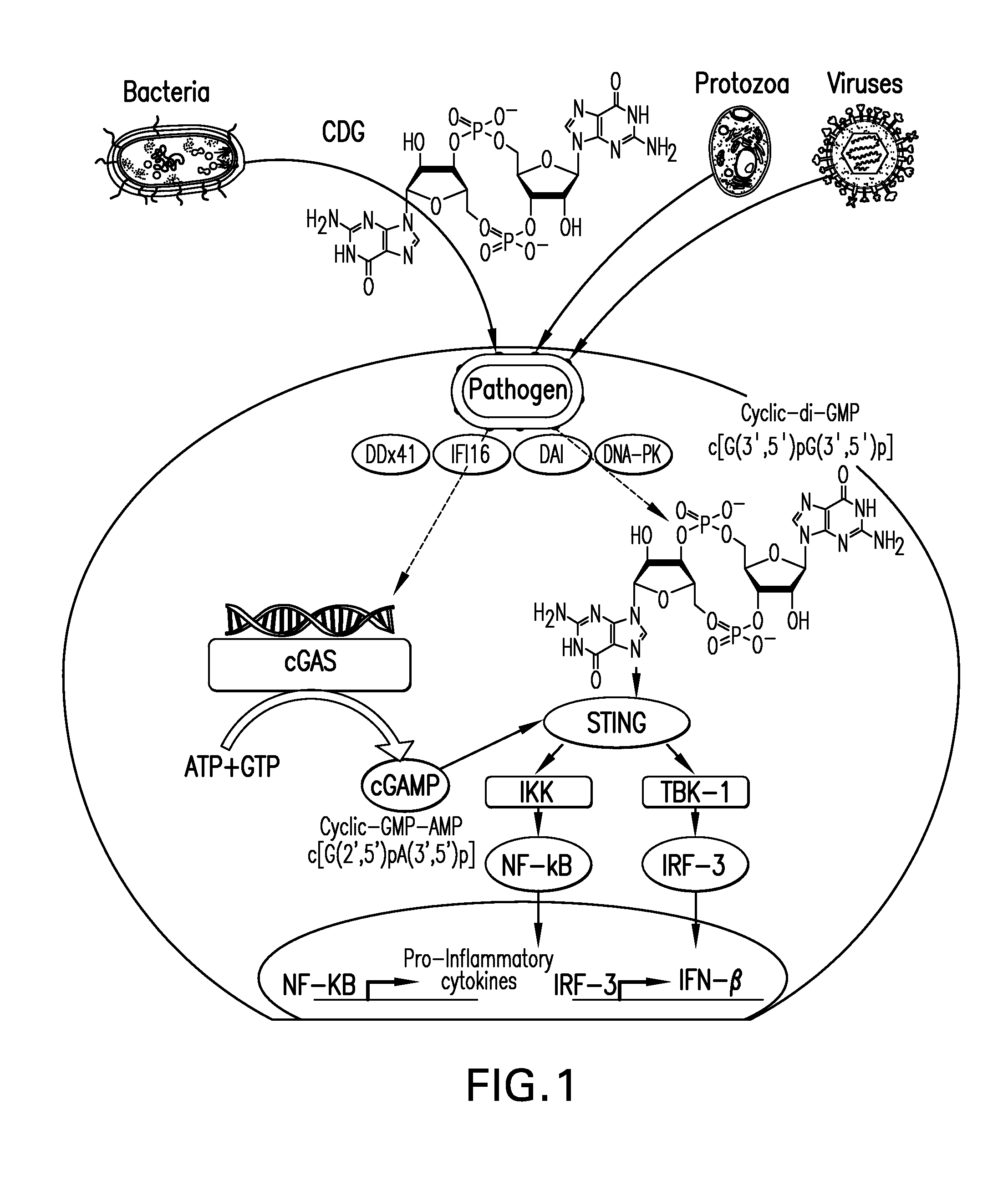
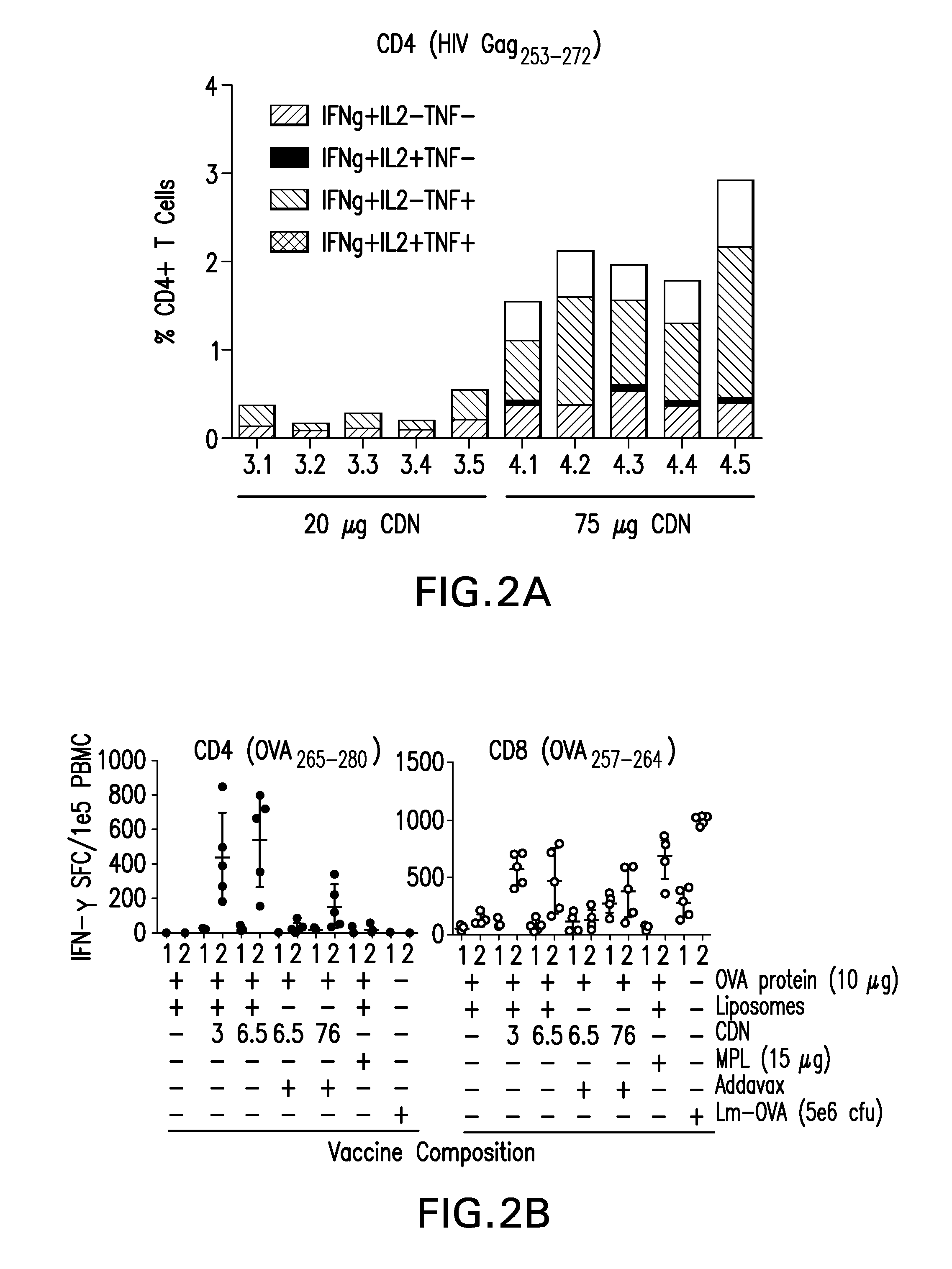
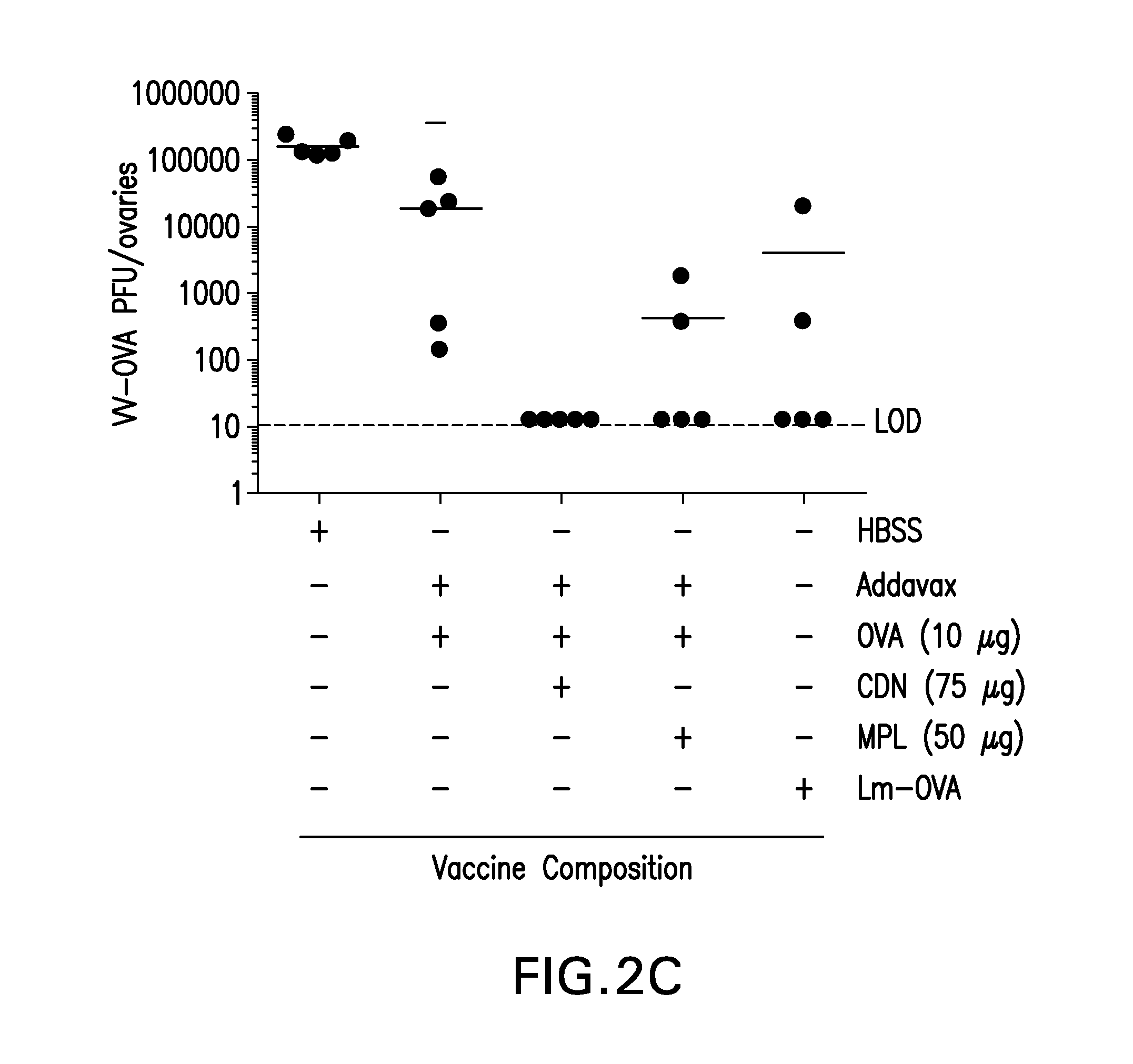
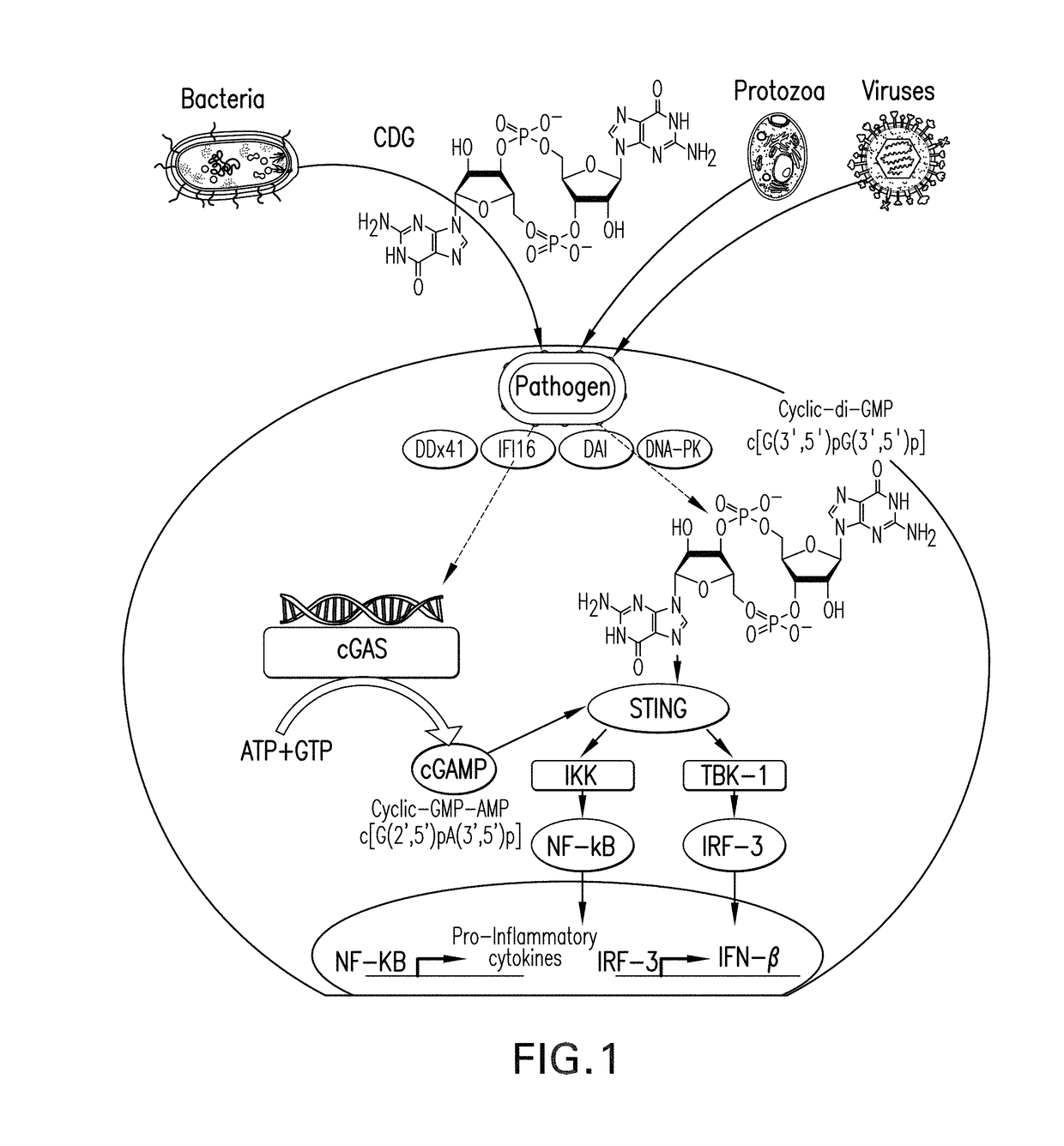
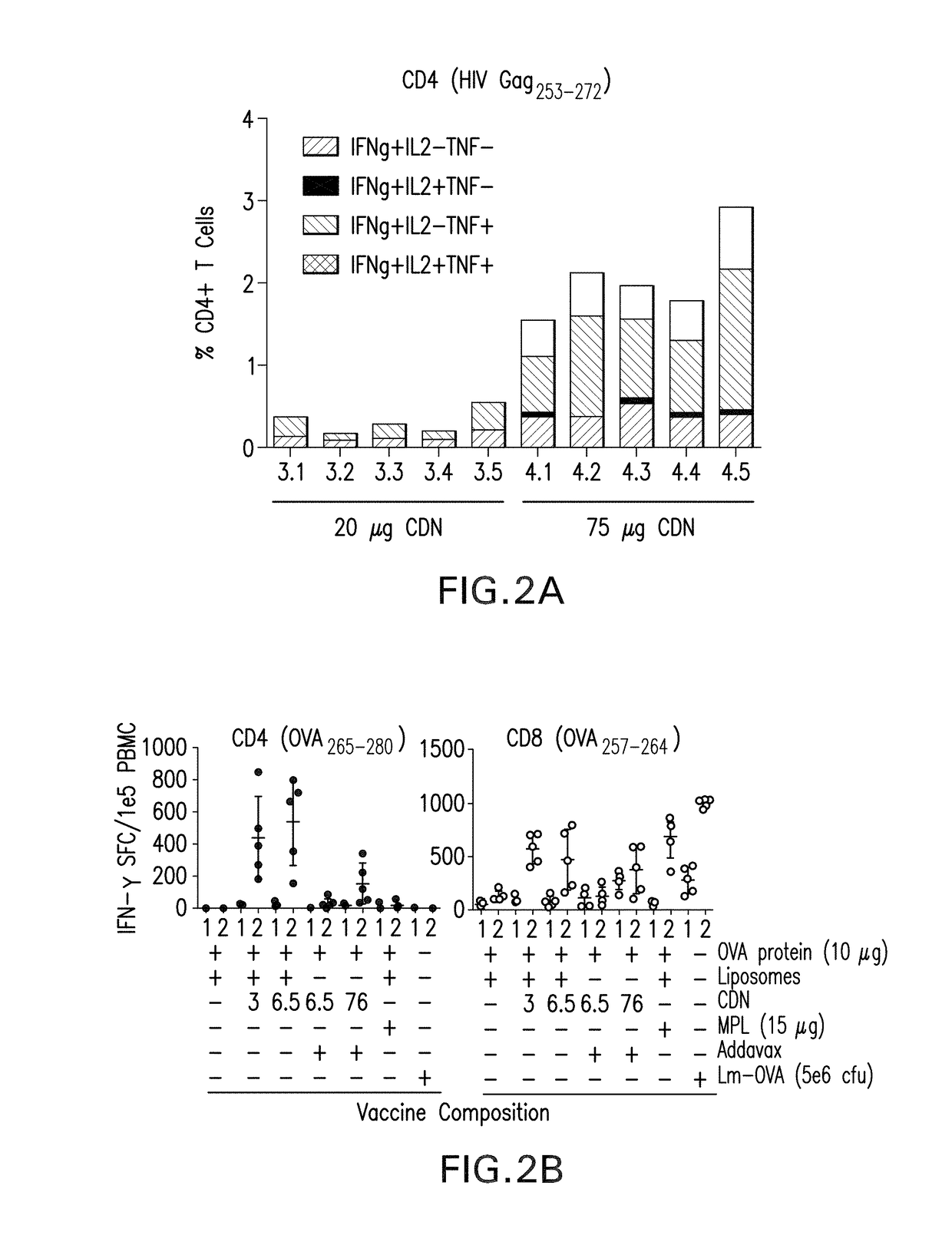
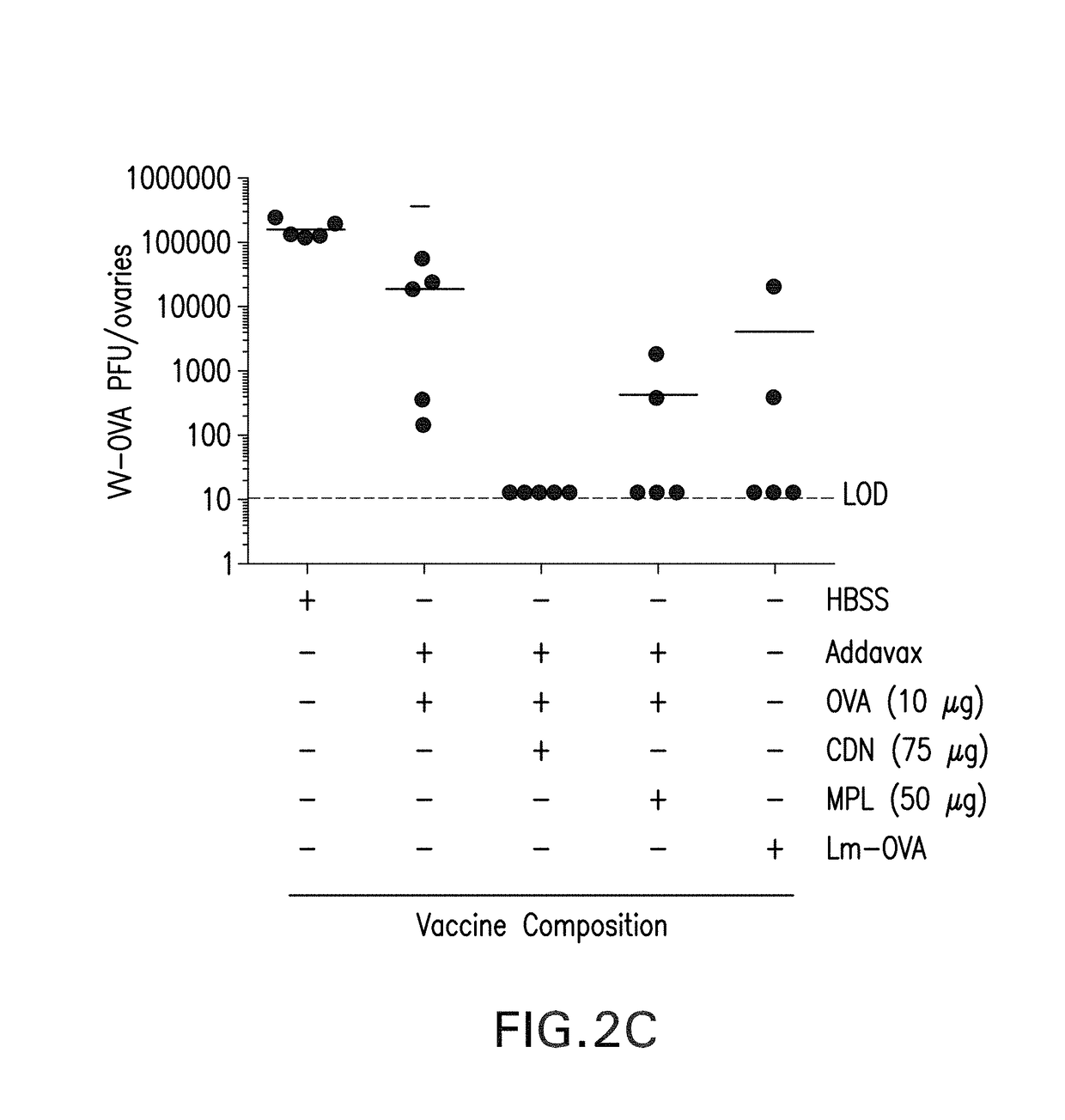
The present invention provides a combination therapy which relies on a small molecule immune stimulator—cyclic-di-nucleotide (CDN)—that activates DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes) formulated with allogeneic human tumor cell lines engineered to secrete high amounts of GM-CSF. This combination therapy can provide an ideal synergy of multiple tumor associated antigens, DC recruitment and proliferation, coupled with a potent DC activation stimulus.
Owner:ADURO BIOTECH +2
Neutralizing monoclonal antibodies against severe acute respiratory syndrome-associated coronavirus
InactiveUS20060240551A1Reduce the binding forceAvoid infectionAnimal cellsMicrobiological testing/measurementAntigenicitySpike Protein
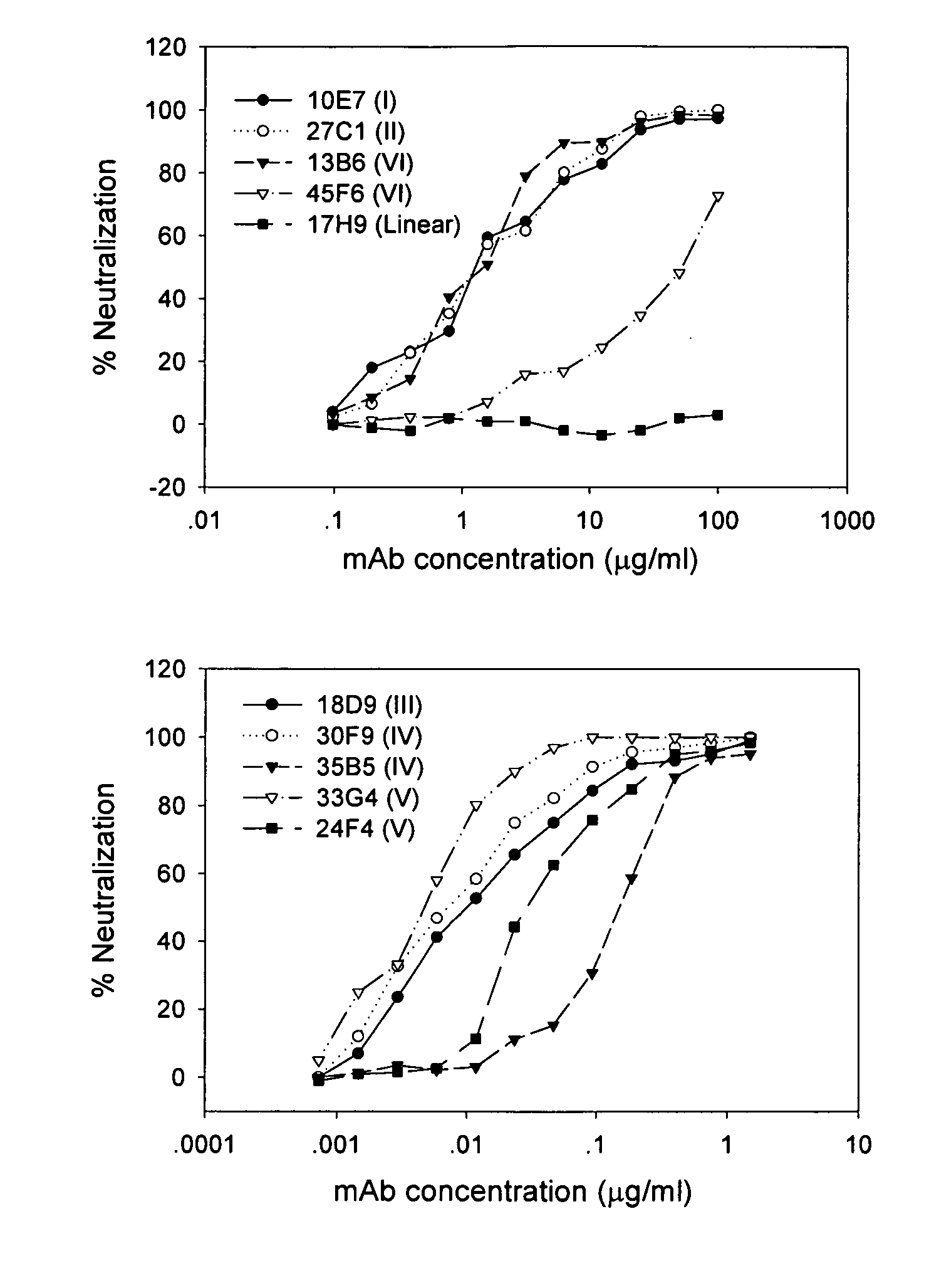
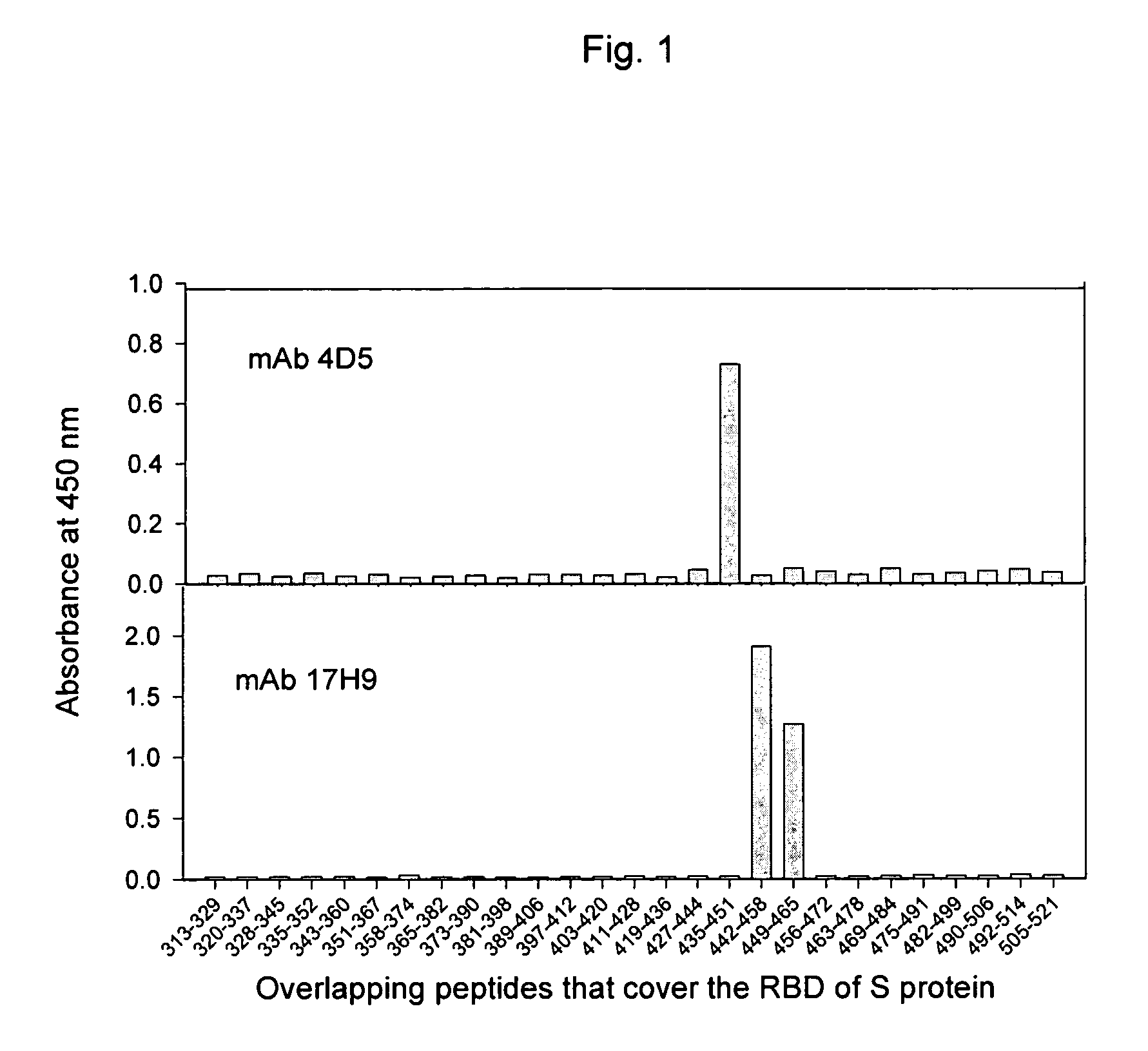
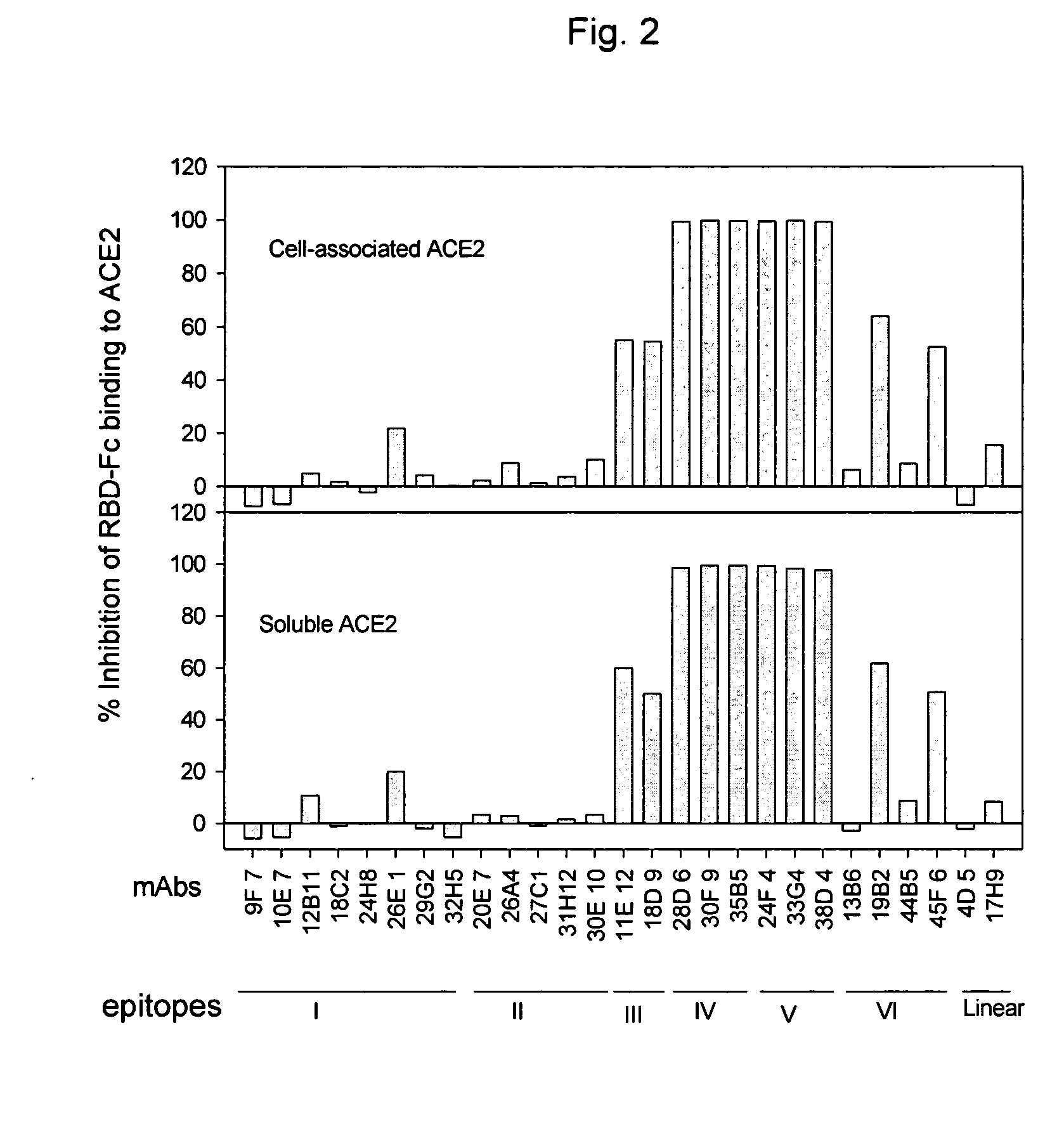
The present invention provides an isolated antibody capable of binding to the receptor-binding domain of the spike protein of the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) so as to competitively inhibit the binding of the SARS-CoV to host cells. These mAbs or substances can be used: 1) as passive-immunizing agents for prevention of SARS-CoV infection; 2) as biological reagents for diagnosis of SARS-CoV infection; 3) as immunotherapeutics for early treatment of SARS-CoV infection; and 4) as probes for studying the immunogenicity, antigenicity, structure, and function of the SARS-CoV S protein.
Owner:NEW YORK BLOOD CENT
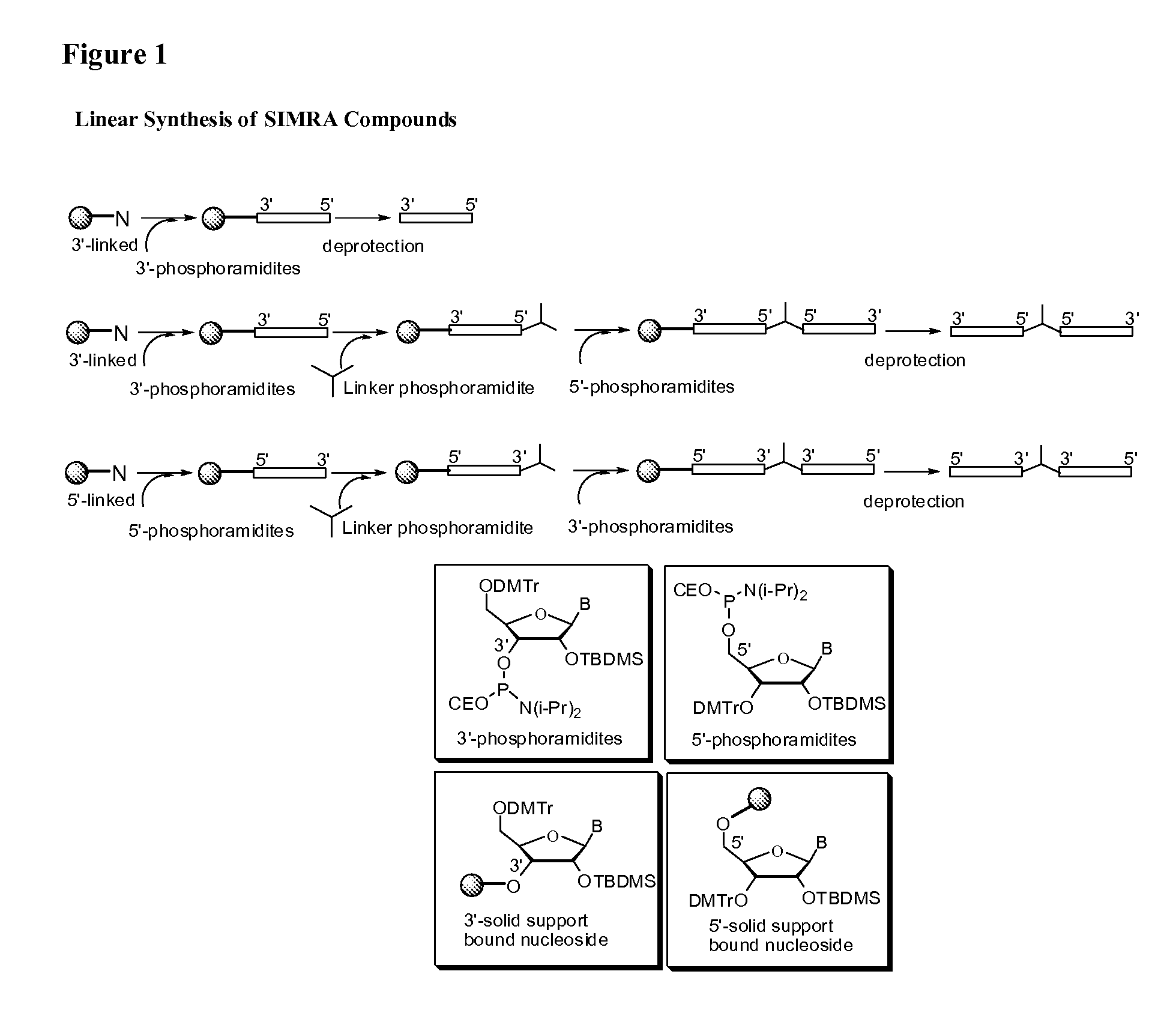
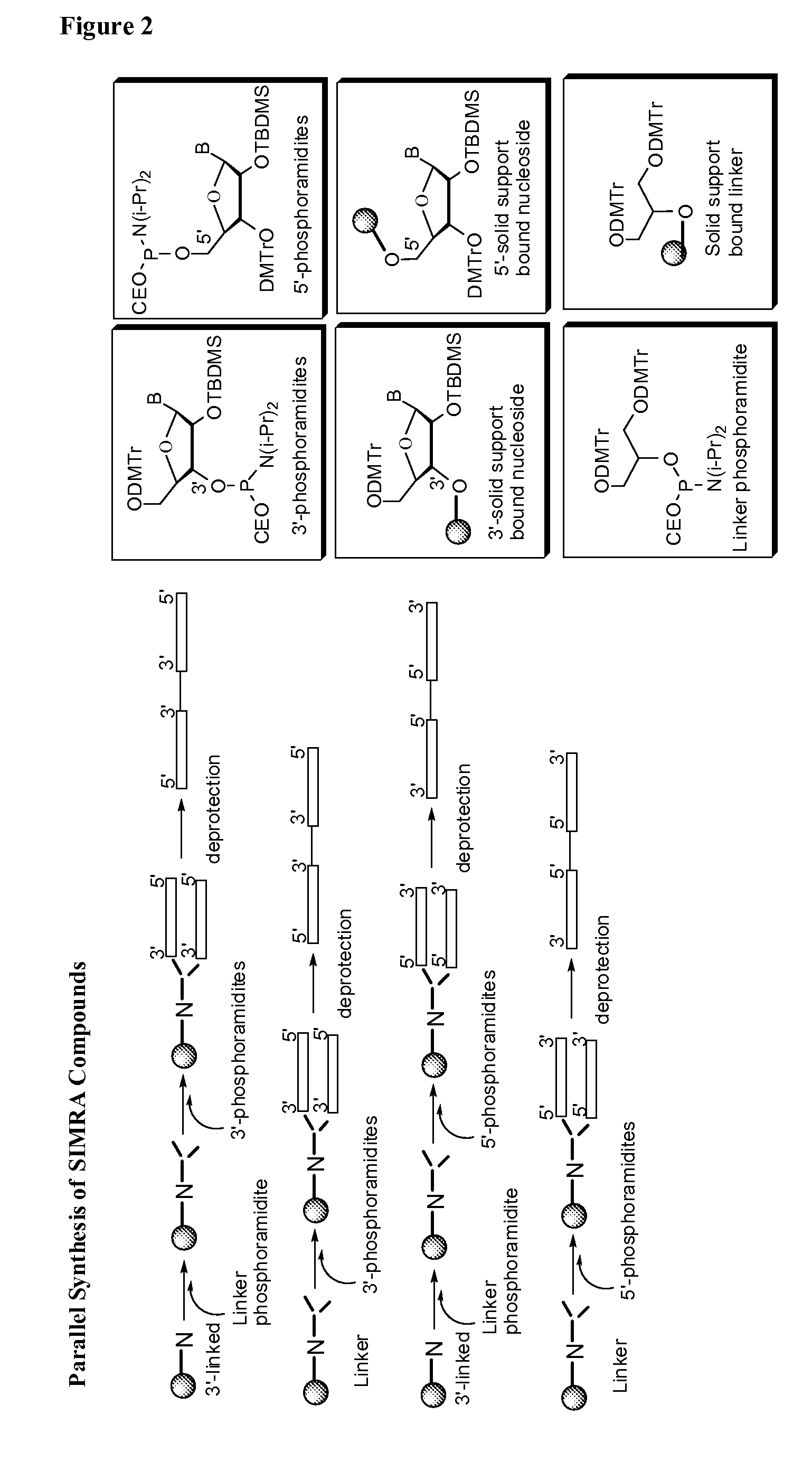
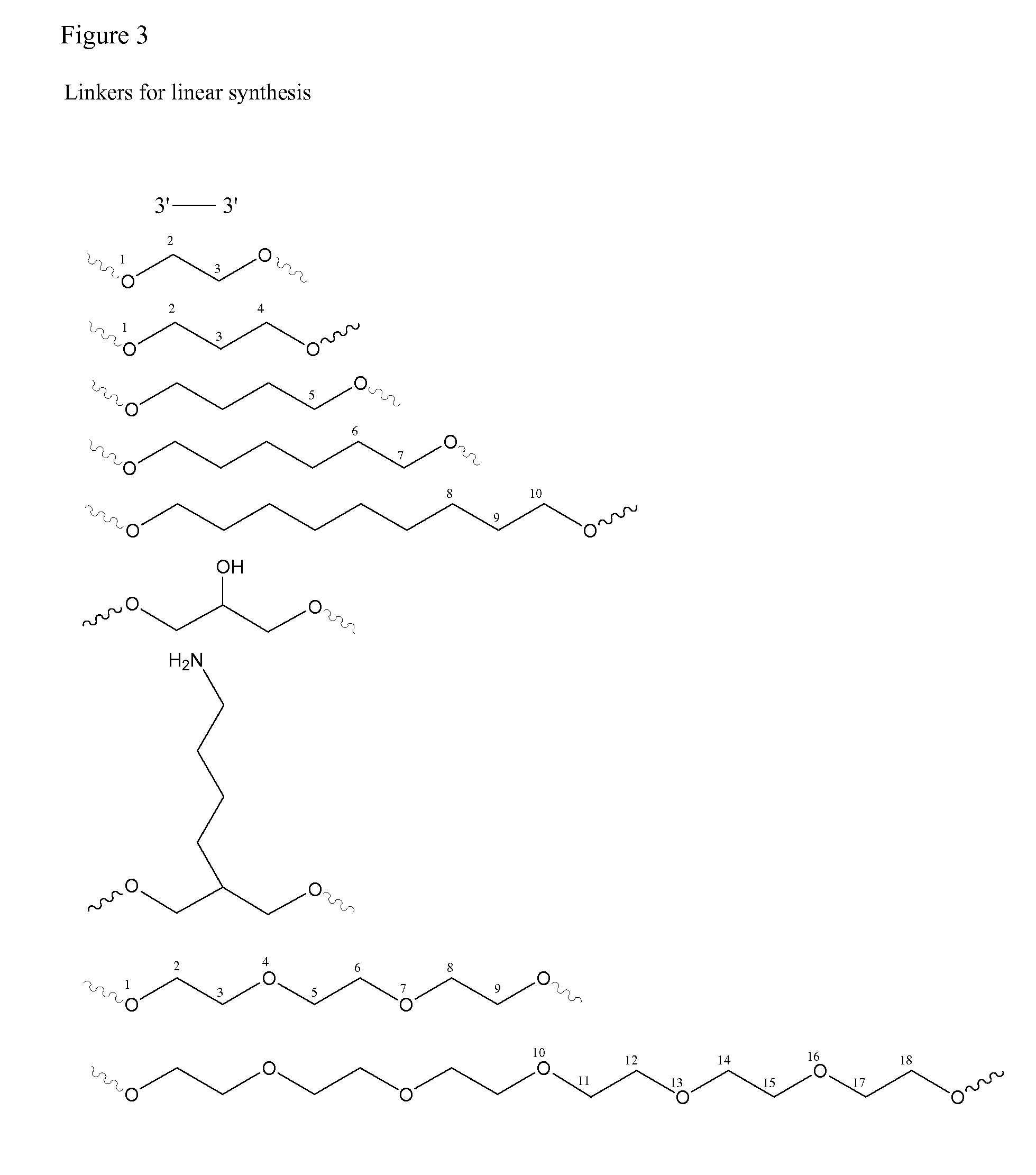
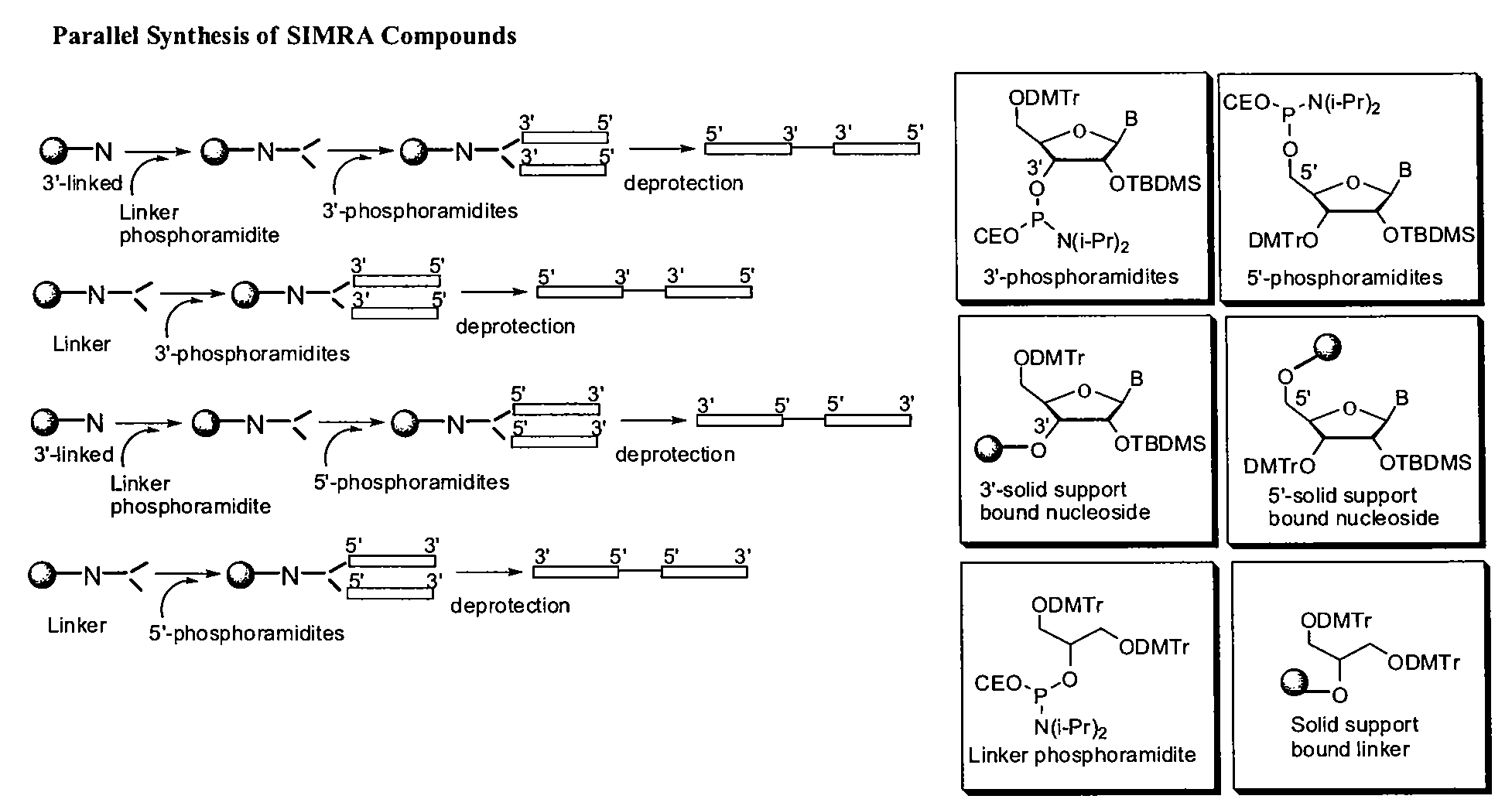
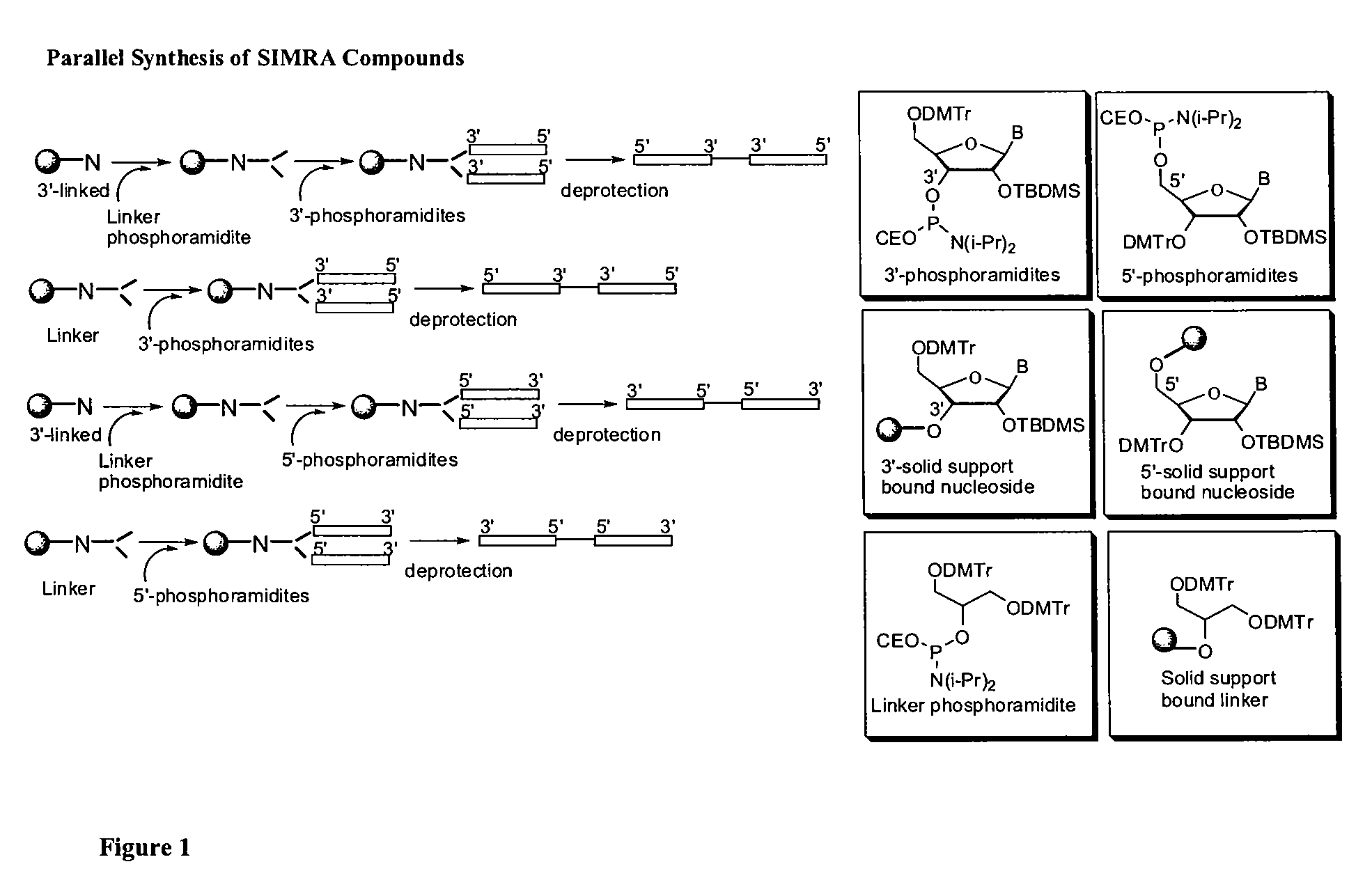
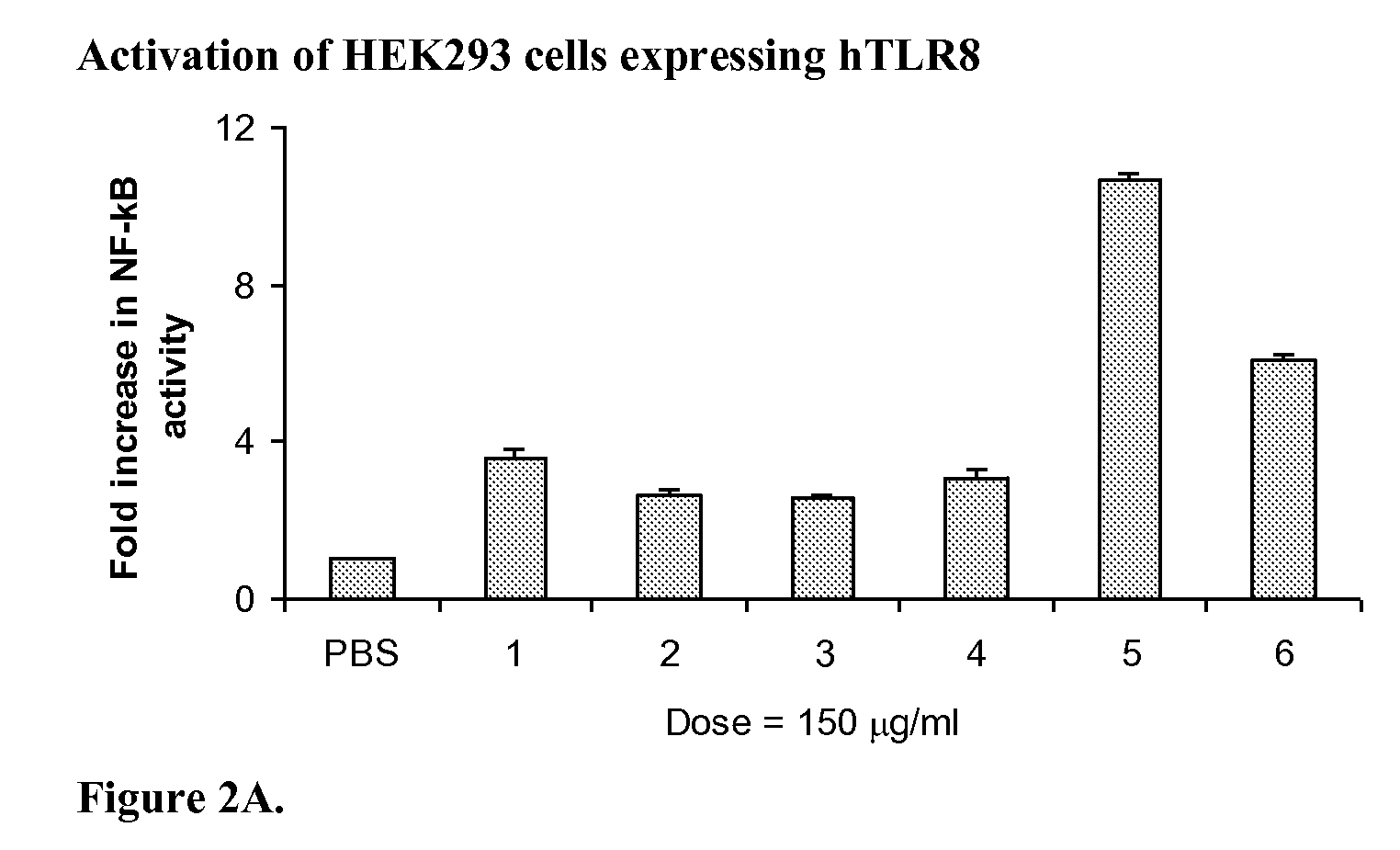
Stabilized immune modulatory RNA (SIMRA) compounds for tlr7 and tlr8
ActiveUS20080171712A1Improve stabilityImprove vaccination effectAntibacterial agentsOrganic active ingredientsOligoribonucleotidesTLR8
The invention relates to the therapeutic use of stabilized oligoribonucleotides as immune modulatory agents for immune therapy applications. Specifically, the invention provides RNA based oligoribonucleotides with improved nuclease and RNase stability and that have immune modulatory activity through TLR7 and / or TLR8.
Owner:IDERA PHARMA INC
Human immune therapies using a cd27 agonist alone or in combination with other immune modulators
InactiveUS20130336976A1Promotes strong expression of 4-1BBImprove responseAntibacterial agentsAntimycoticsIMMUNE STIMULANTSCD8
Methods of inducing T cell proliferation and expansion in vivo for treating conditions wherein antigen-specific T cell immune response are therapeutically desirable such as cancer, infection, inflammation, allergy and autoimmunity and for enhancing the efficacy of vaccines are provided. These methods comprise the administration of at least one CD27 agonist, preferably an agonistic CD27 antibody, alone or in association with another moiety such as immune stimulant or immune modulator such as an anti-CD40, OX-40, 4-1BB, or CTLA-4 antibody or an agent that depletes regulatory cells, or a cytokine. These mono and combination therapies may also optionally include the administration of a desired antigen such as a tumor antigen, an allergen, an autoantigen, or an antigen specific to an infectious agent or pathogen against which a T cell response (often CD8+) is desirably elicited.
Owner:UNIV OF SOUTHAMPTON
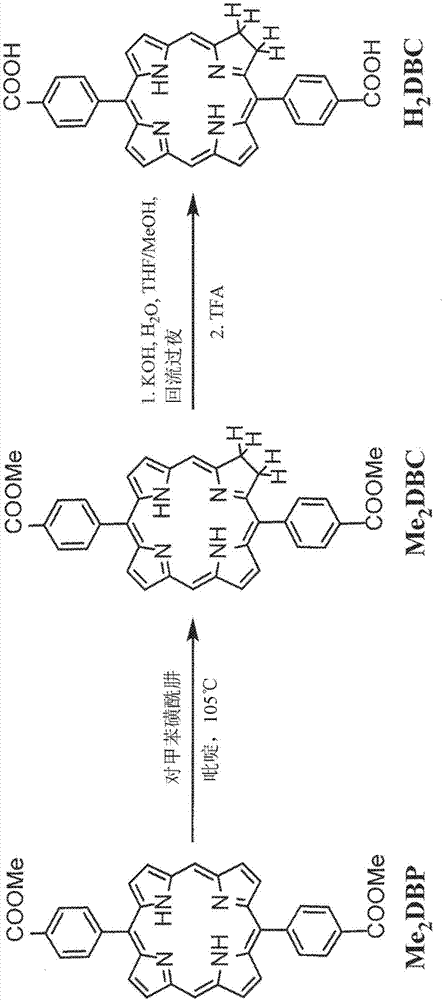
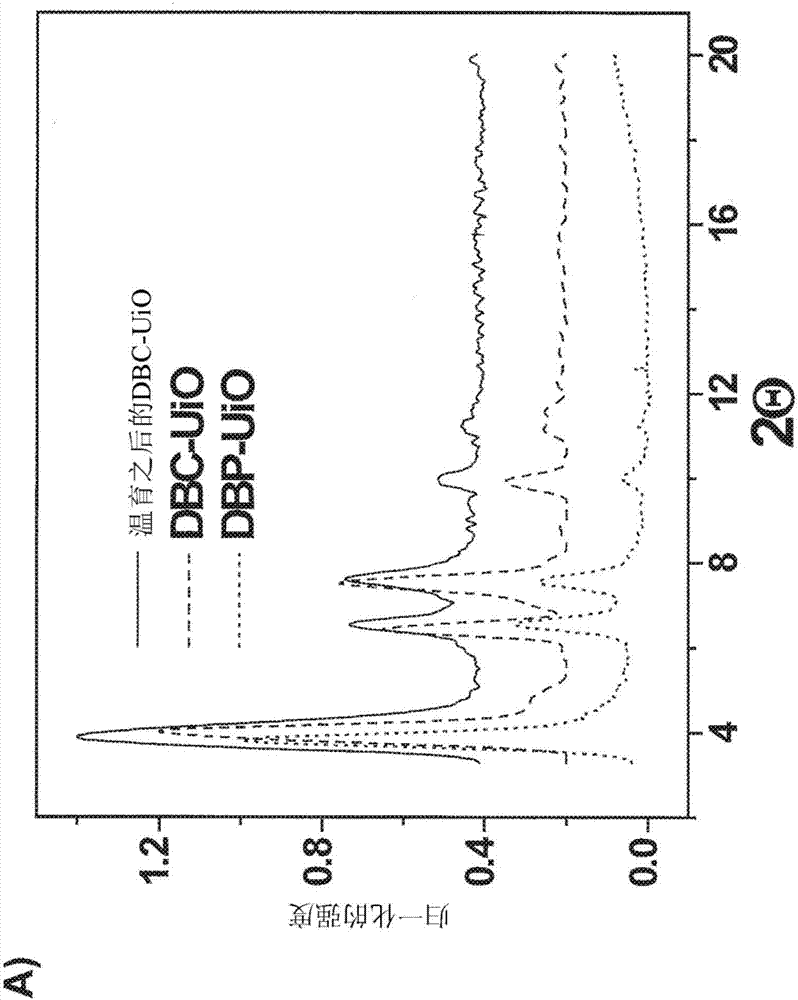
Nanoparticles for photodynamic therapy, x-ray induced photodynamic therapy, radiotherapy, chemotherapy, immunotherapy, and any combination thereof
ActiveCN107001031AExhibit nanorod morphologyPowder deliveryPhotodynamic therapyCo administrationImmunotherapeutic agent
Metal-organic frameworks (MOFs) comprising photosensitizers are described. The MOFs can also include moieties capable of absorbing X- rays and / or scintillation. Optionally, the photosensitizer or a derivative thereof can form a bridging ligand of the MOF. Further optionally, the MOF can comprise inorganic nanoparticles in the cavities or channels of the MOF or can be used in combination with an inorganic nanoparticle. Also described are methods of using MOFs and / or inorganic nanoparticles in photodynamic therapy or in X-ray induced photodynamic therapy, either with or without the co-administration of one or more immunotherapeutic agent and / or one or more chemotherapeutic agent.
Owner:UNIVERSITY OF CHICAGO
Heterodimeric fusion proteins useful for targeted immune therapy and general immune stimulation
InactiveUS7226998B2Altering pharmacology and biodistributionIncreasing its circulating half-life and its affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsStepwise approachImmune therapy
Disclosed are methods for producing fusion proteins with the heterodimeric cytokine, interleukin-12. In order to insure that the proper ratio of fused and non-fused subunits are obtained in the fusion protein, a specific stepwise approach to genetic engineering is used. This consists of first expressing the non-fused p40 IL-12 subunit in a production cell line, followed by or simultaneously expressing in the same cell, a second recombinant fusion protein consisting of the fused polypeptide linked by a peptide bond to the p35 subunit of IL-12. Molecules containing the p35 fusion protein cannot be secreted from the transfected mammalian cell without first complexing in a one to one ratio with the p40 subunit, thus ensuring the production of active heterodimeric fusion proteins.
Owner:MERCK PATENT GMBH
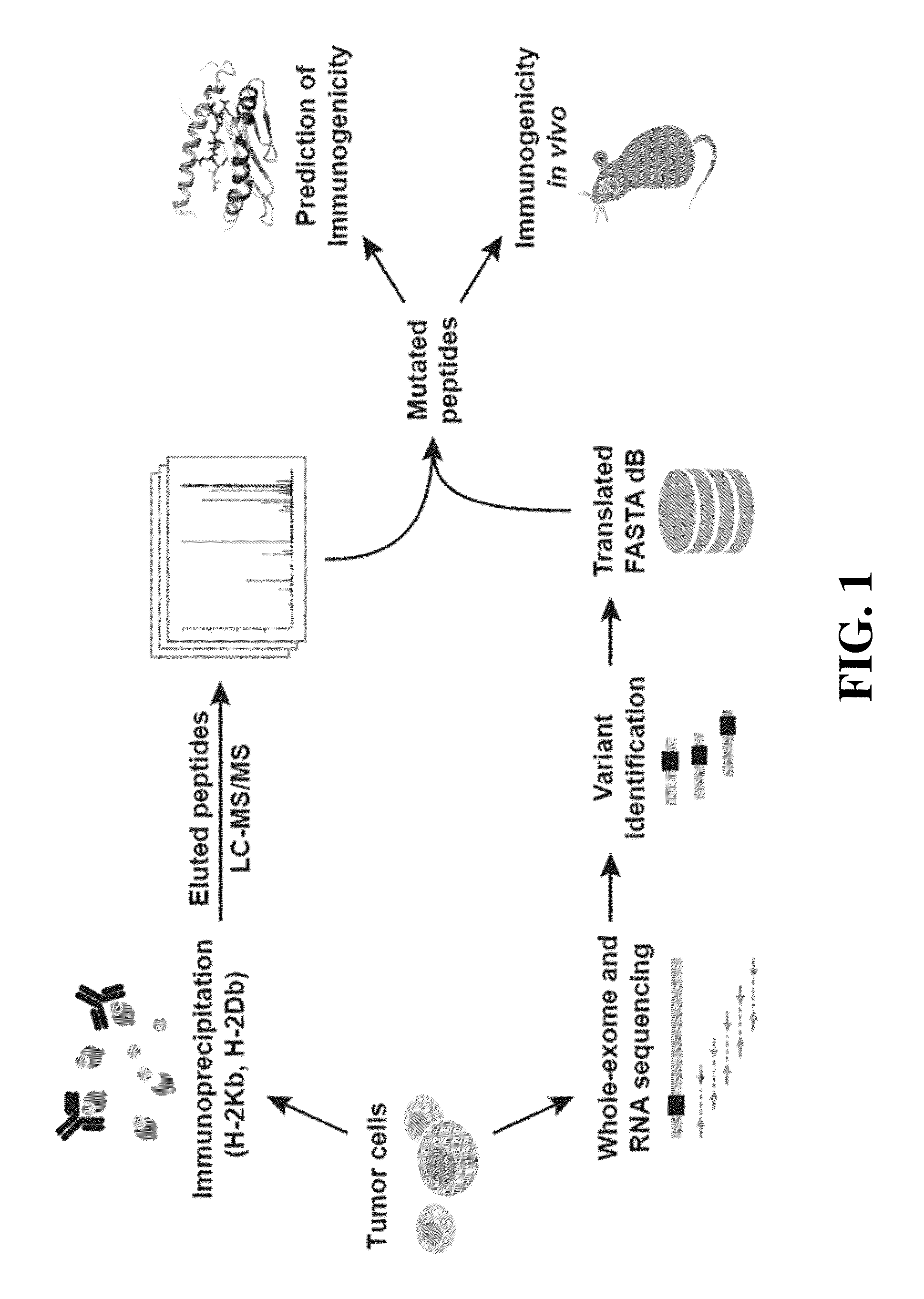
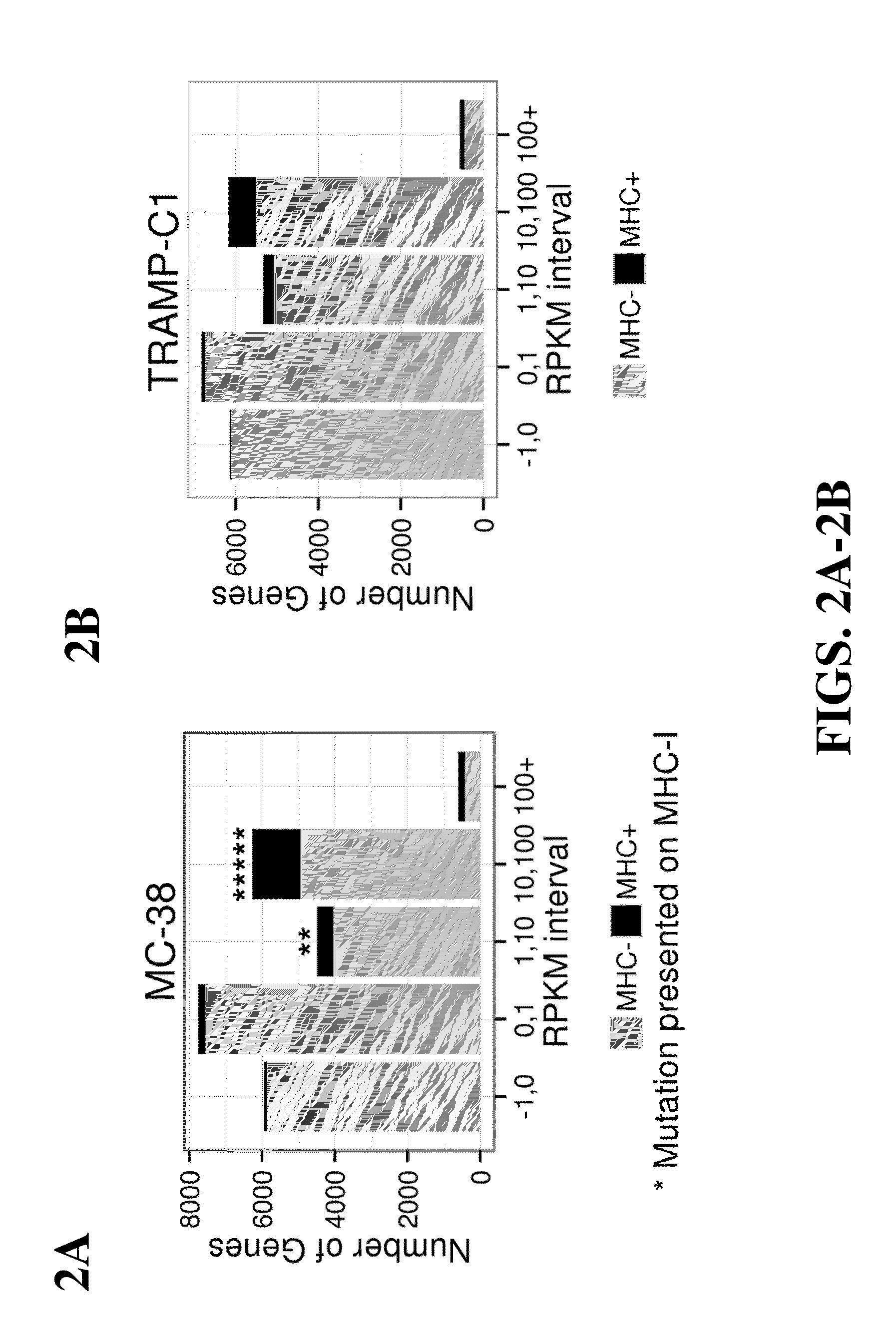
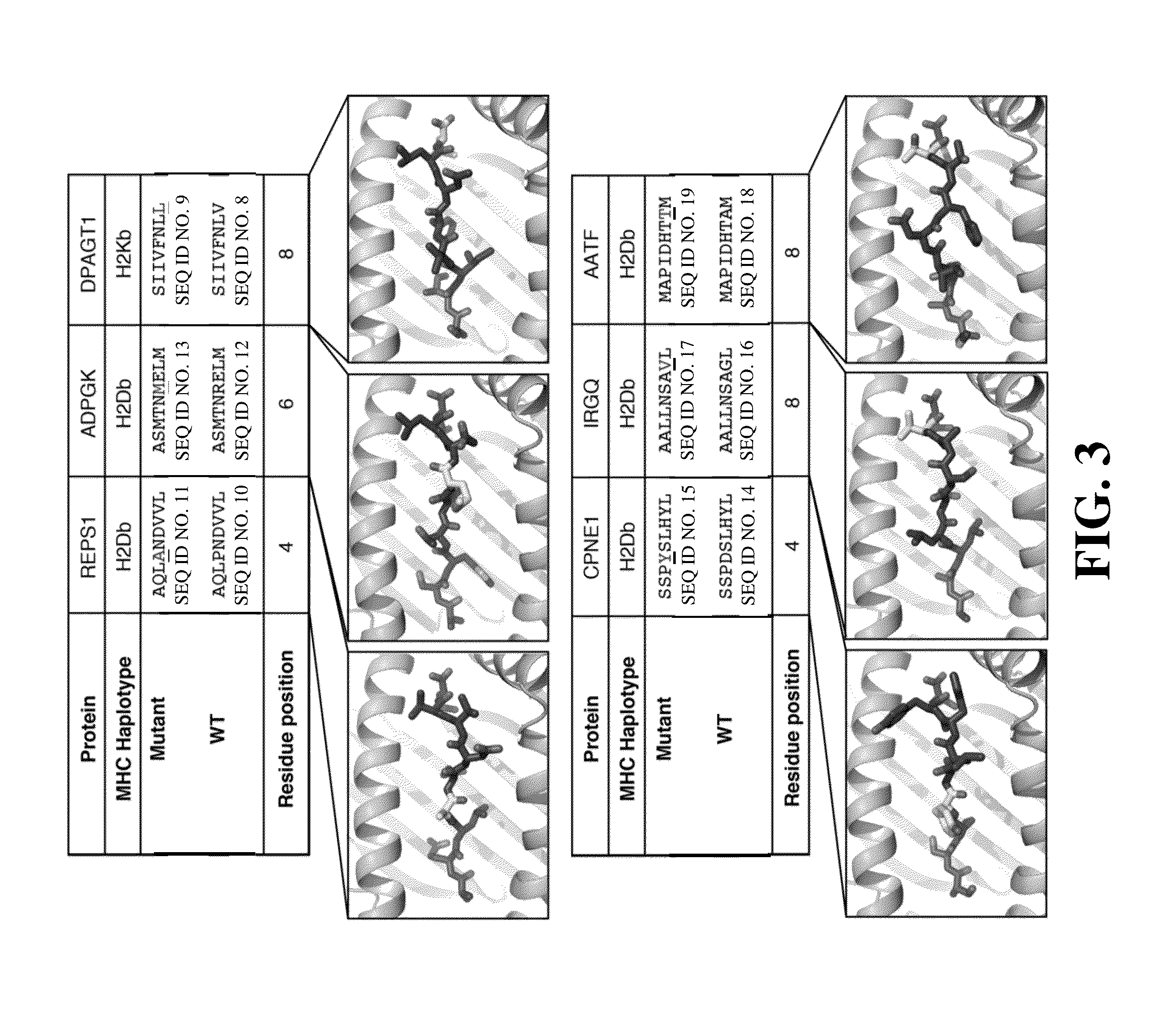
Immunogenic mutant peptide screening platform
The present disclosure provides methods of identifying a disease-specific immunogenic peptide through a series of selection steps. Immunogenic epitopes identified by methods of the present disclosure are applicable for use in peptide-based immunotherapy, preferably cancer therapy. Furthermore, the methods of the present disclosure may be performed in a high-throughput manner and serve as a means of personalized vaccine development and therapy. Also provided are compositions of immunogenic peptides as well as methods of treatment comprising said compositions.
Owner:GENENTECH INC
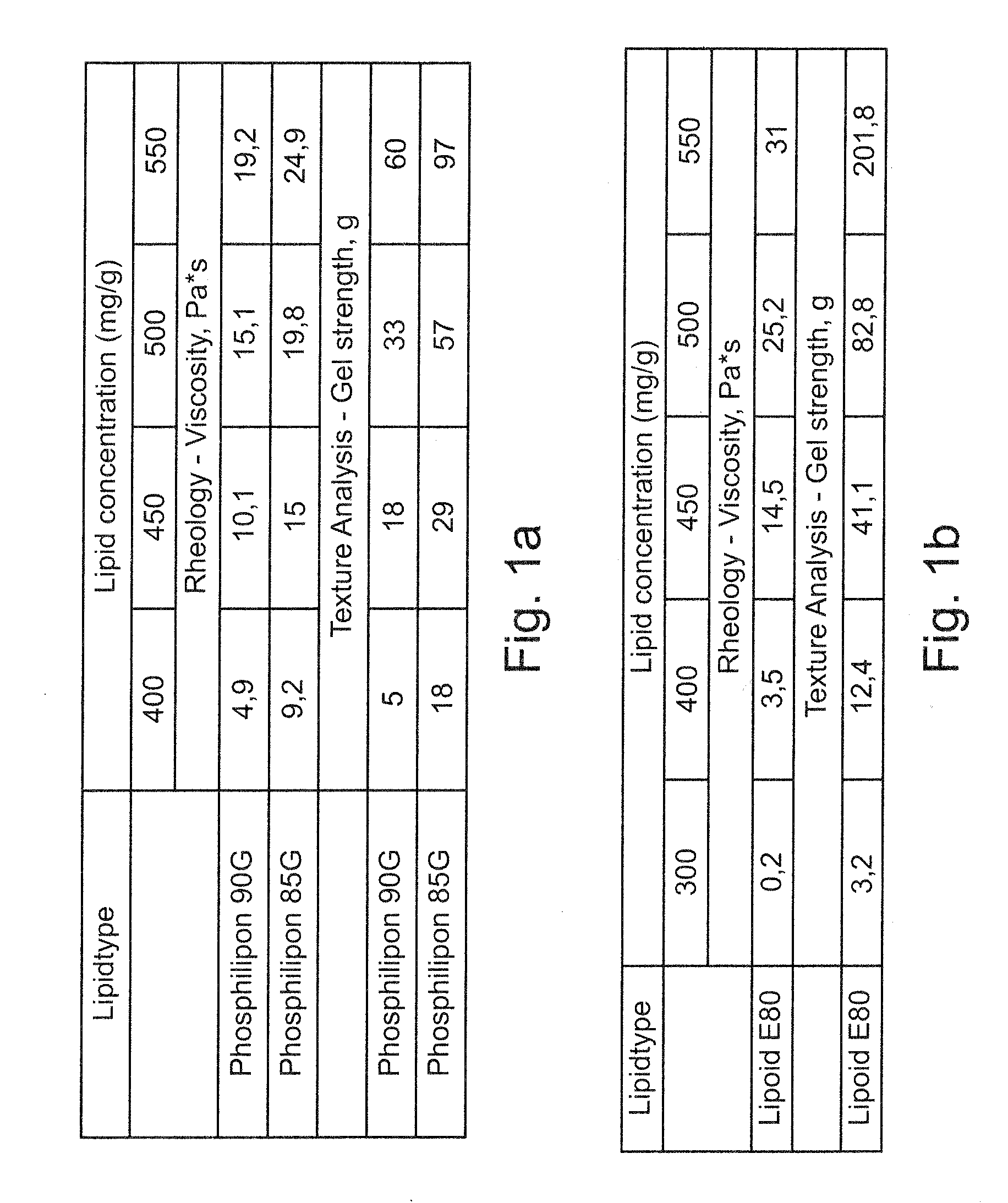
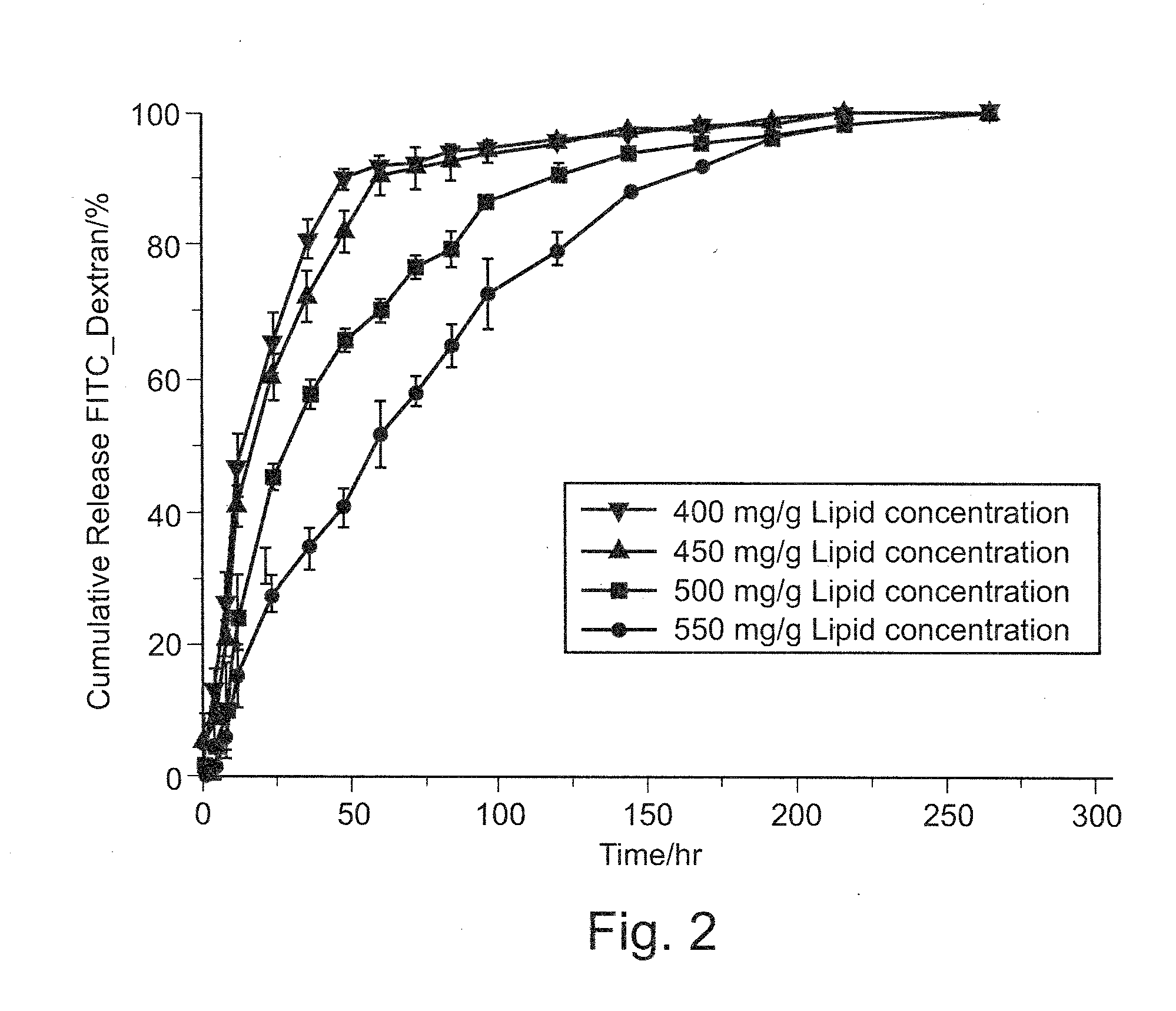
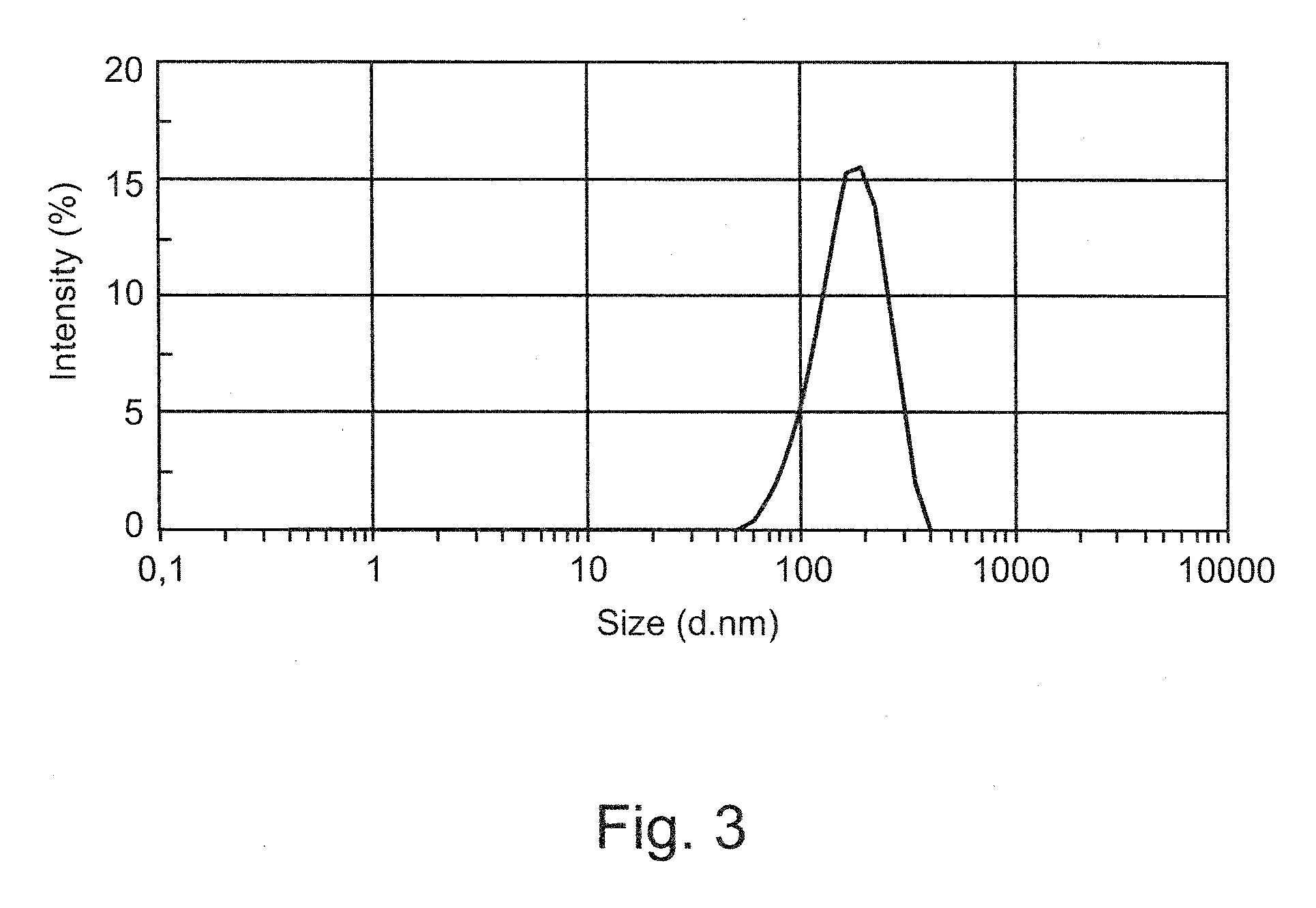
Vesicular phospholipid gels comprising proteinaceous substances
The present invention relates to a pharmaceutical composition for sustained release of a pharmaceutically active compound, the composition comprising a vesicular phospholipid gel. More particularly, the invention relates to a pharmaceutical composition comprising at least one proteinaceous substance as the pharmaceutically active compound in encapsulated form, the at least one proteinaceous substance being a biologically active protein, peptide or polypeptide. Furthermore, the present invention relates to a method for the production of said pharmaceutical composition comprising dual asymmetric centrifugation and to the use of said pharmaceutical composition for immunotherapy and / or for stimulating selective tissue regeneration in the treatment of surgical defects in the course of surgical interventions.
Owner:WINTER GERHARD DR
Compositions and methods for cancer immunotherapy
ActiveUS9770467B2Improve propertiesHigh activityPeptide/protein ingredientsViral antigen ingredientsAntigenHuman tumor
The present invention provides a combination therapy which relies on a small molecule immune stimulator—cyclic-di-nucleotide (CDN)—that activates DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes) formulated with allogeneic human tumor cell lines engineered to secrete high amounts of GM-CSF. This combination therapy can provide an ideal synergy of multiple tumor associated antigens, DC recruitment and proliferation, coupled with a potent DC activation stimulus.
Owner:ADURO BIOTECH +2
Polysaccharide Microparticles Containing Biological Agents: Their Preparation and Applications
A method of preparing polysaccharide glassy microparticles which are less than 10 μum in diameter and contain structurally delicate agents, such as proteins, peptides, gene materials, vaccines, antibodies, viruses and liposomes using low-temperature aqueous-aqueous emulsification (free of polyelectrolytes) and freezing-induced phase separation. When delicate agents are added to a polysaccharide-PEG two phase system followed by homogenization (or other shear adding process), the agents partition into the polysaccharide dispersed phase preferentially. These processes help to avoid aggregation of proteins caused by interaction with charged polyelectrolytes used for stabilizing the polysaccharide dispersed phase in our previously reported aqueous-aqueous emulsion. When this system is frozen and lyophilized, glassy particles less than 10 μm in diameter containing delicate agents can be formed. These fine polysaccharide particles protect proteins within their hydrophilic glassy matrix, and can therefore be easily suspended in hydrophobic polymer solutions and formulated to various forms of sustained release devices such microsphere, sheets, fibers, coating layers, and scaffolds. The particles can also be dispersed in hydrophilic gels to improve releasing kinetics and to deliver vaccines and antibodies for immune therapy.
Owner:BIODVERY PHARMATECH LTD
Adjuvant immune therapy in the treatment of solid tumors through modulation of signaling pathways following engagement of humoral and cell mediated responses
InactiveUS20020187130A1Evaluating efficacyAccurate assessmentBiocideSaccharide peptide ingredientsAdjuvantWhite blood cell
Owner:KINDNESS GEORGE +2
Stabilized immune modulatory RNA (SIMRA) compounds
ActiveUS20090053205A1Exonuclease digestionOrganic active ingredientsActivity regulationOligoribonucleotidesTLR8
The invention relates to the therapeutic use of novel stabilized oligoribonucleotides as immune modulatory agents for immune therapy applications. Specifically, the invention provides novel RNA-based oligoribonucleotides with improved nuclease and RNase stability and that have immune modulatory activity through TLR7 and / or TLR8.
Owner:IDERA PHARMA INC
Medicament for preventing and controlling alzheimer's disease
InactiveCN101152576AAvoid repeated dosingLow cost of treatmentNervous disorderGenetic material ingredientsSingle-Chain AntibodiesSide effect
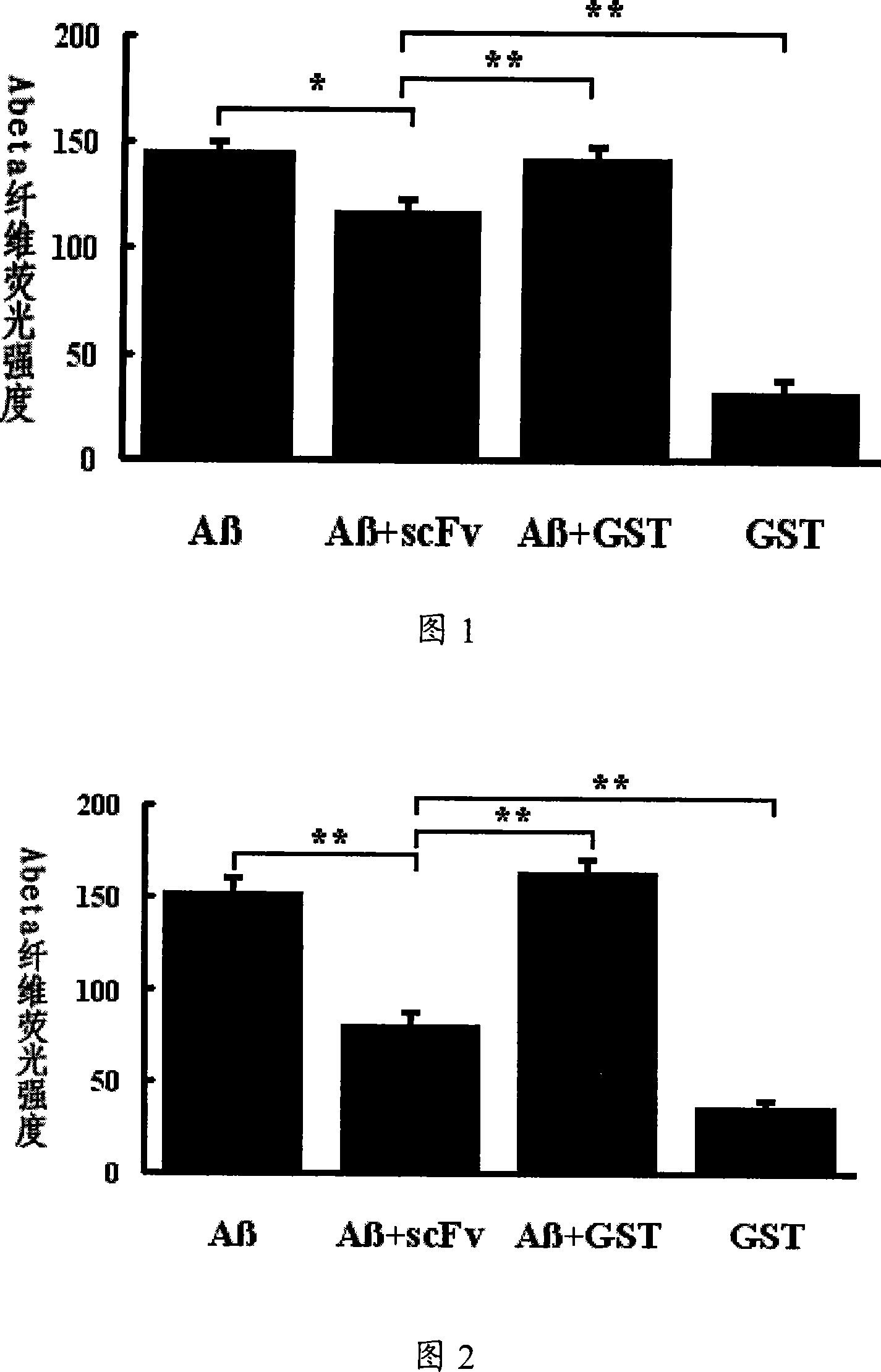
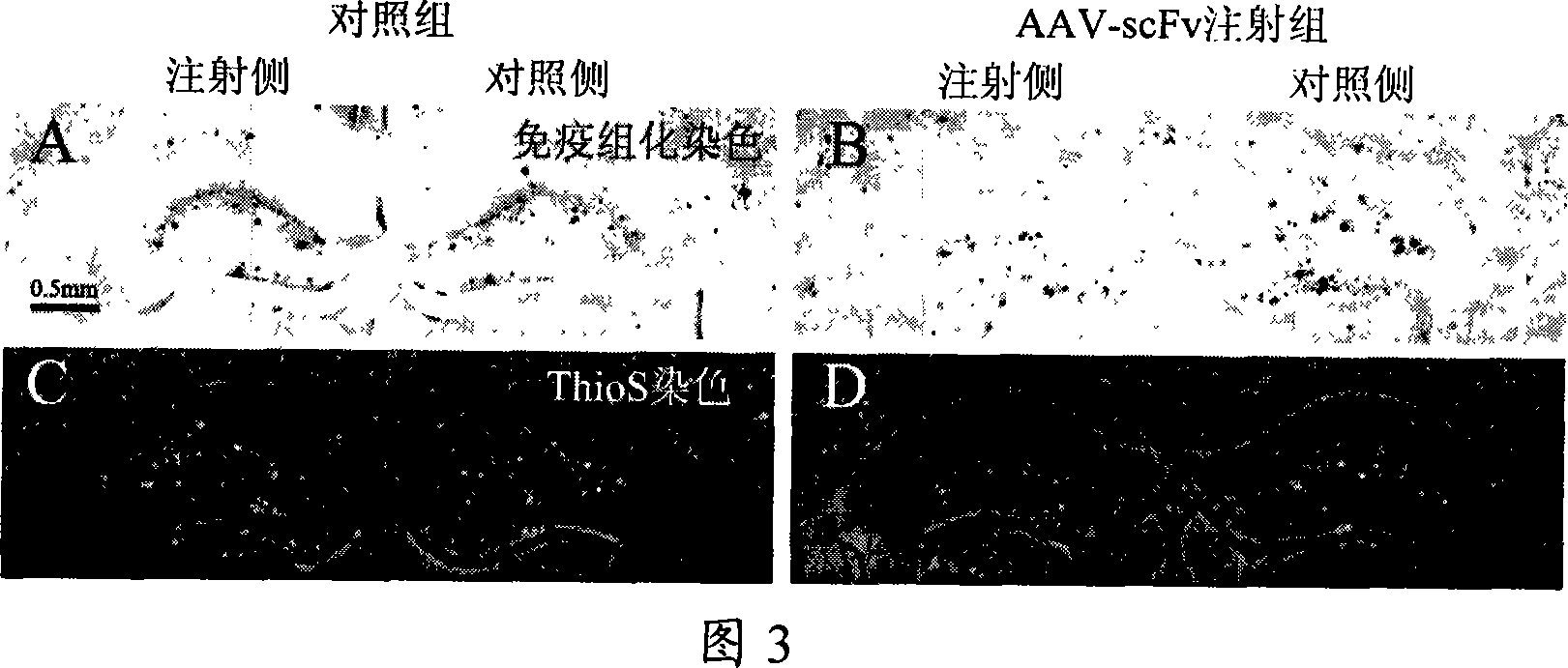
A medicine for curing alzheimer disease is characterized in that a recombinant virus of gene transfection is regarded as a carrier; the front end of one, two or a plurality of anti A beta single-chain antibody genes is respectively connected with a secretion signal peptide dna sequence; and then the anti A beta single-chain antibody genes are inserted into the recombinant virus gene; finally a packaged product is formed through the recombinant virus. The invention can produce anti A beta single-chain antibody in the body by transfecting the anti A beta single-chain antibody genes capable of prohibiting A beta monomer isomerization and promoting A beta polymer depolymerization on the periphery and in the brains, so as to express scFv in the body for a long time, to effectively remove the A beta in the brains with alzheimer disease, prevent inflammatory reaction of the central nervous system and side effect of capillary bleeding produced in the anti A beta immune therapy, avoid repeated feeding of the anti A beta medicine, and reduce the curing cost. The invention provides a safe and effective novel technology of curing alzheimer disease which works for long and has broad clinical application prospect.
Owner:王延江 +2
Inhibition of cxcr4 signaling in cancer immunotherapy
InactiveUS20150216843A1Reduce immunosuppressionReduce rejectionBiocidePeptide/protein ingredientsAbnormal tissue growthCXCR4
Owner:CAMBRIDGE ENTERPRISE LTD
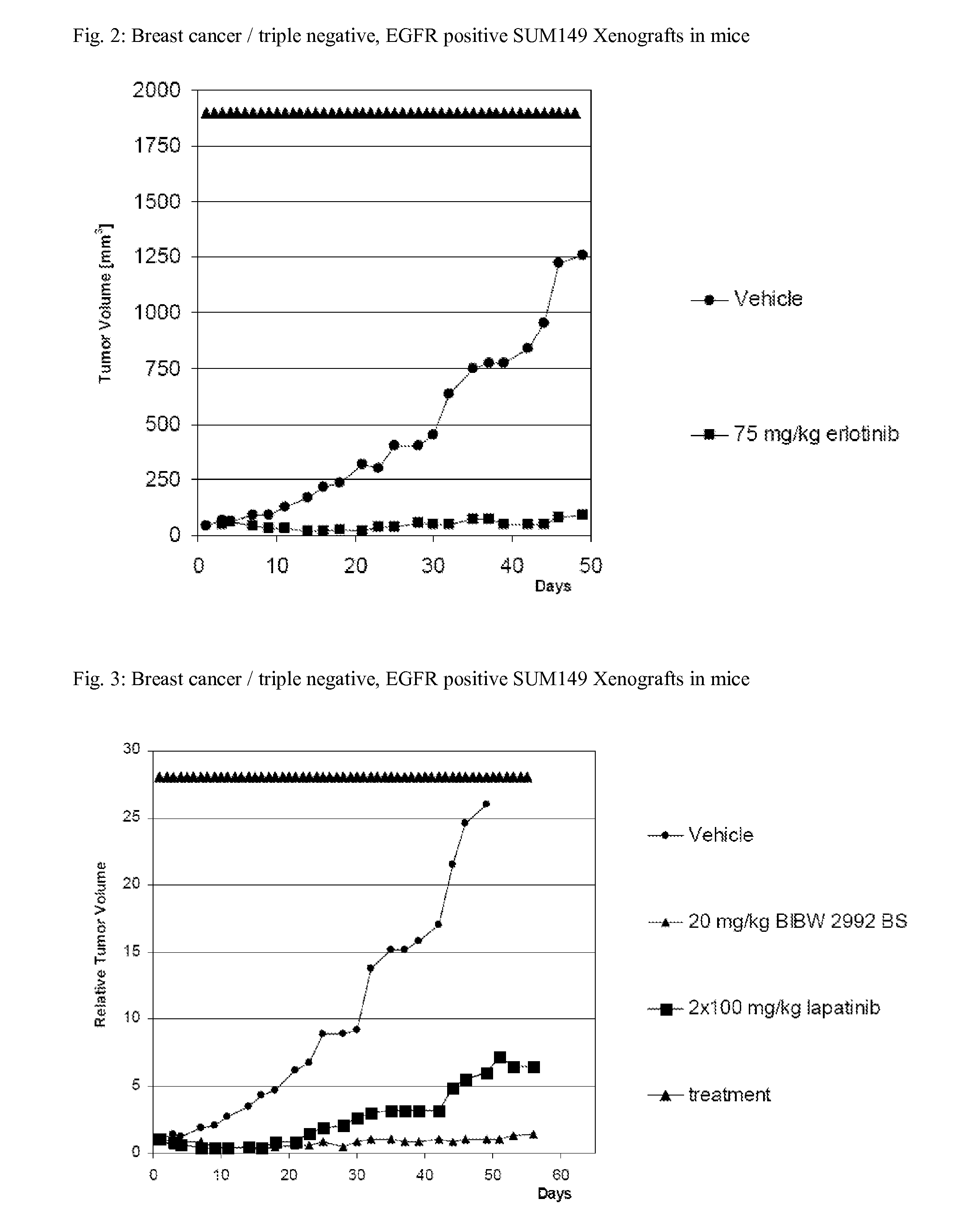
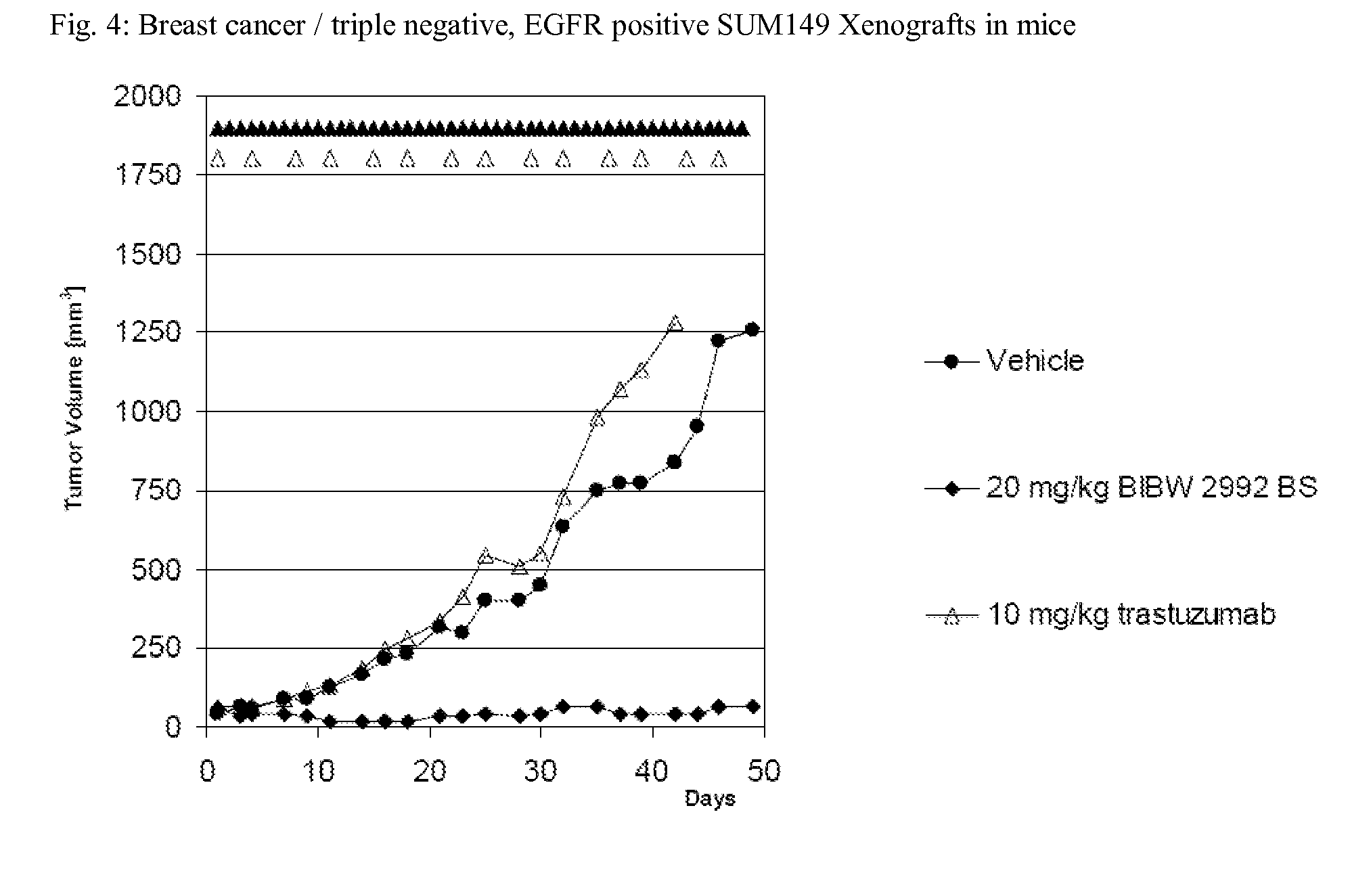
Bibw 2992 for use in the treatment of triple negative breast cancer
InactiveUS20130012465A1Increase growthBiocideHeavy metal active ingredientsWilms' tumorTriple-negative breast cancer
The present invention relates to a method of treating patients suffering from triple negative breast cancer comprising administration of an effective amount of the irreversible EGFR / HER1 and HER2 inhibitor BIBW 2992 (1) to a person in need of such treatment, optionally in combination with the administration of a further chemotherapeutic agent (2), in combination with radiotherapy, radio-immunotherapy and / or tumour resection by surgery.
Owner:BOEHRINGER INGELHEIM INT GMBH
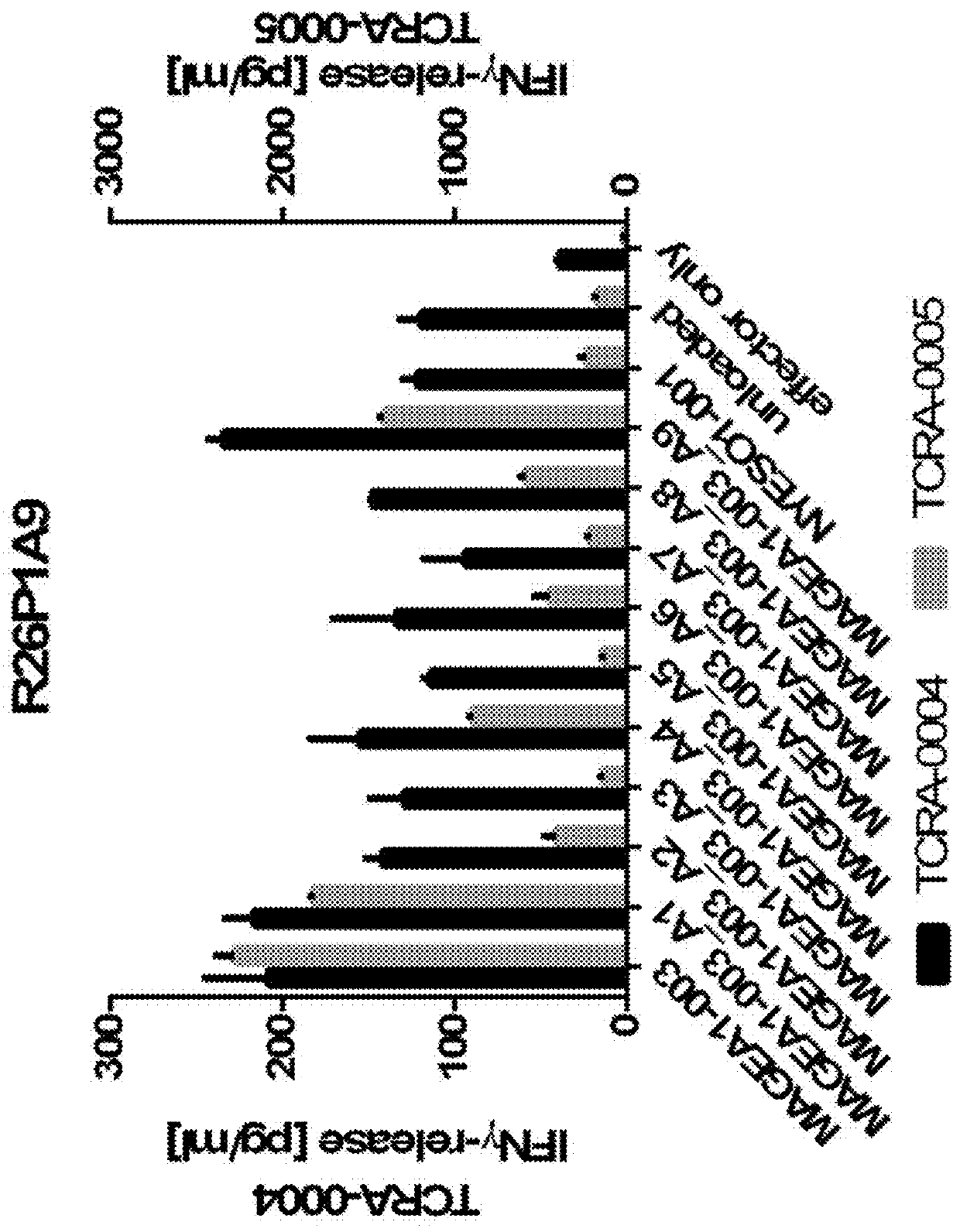
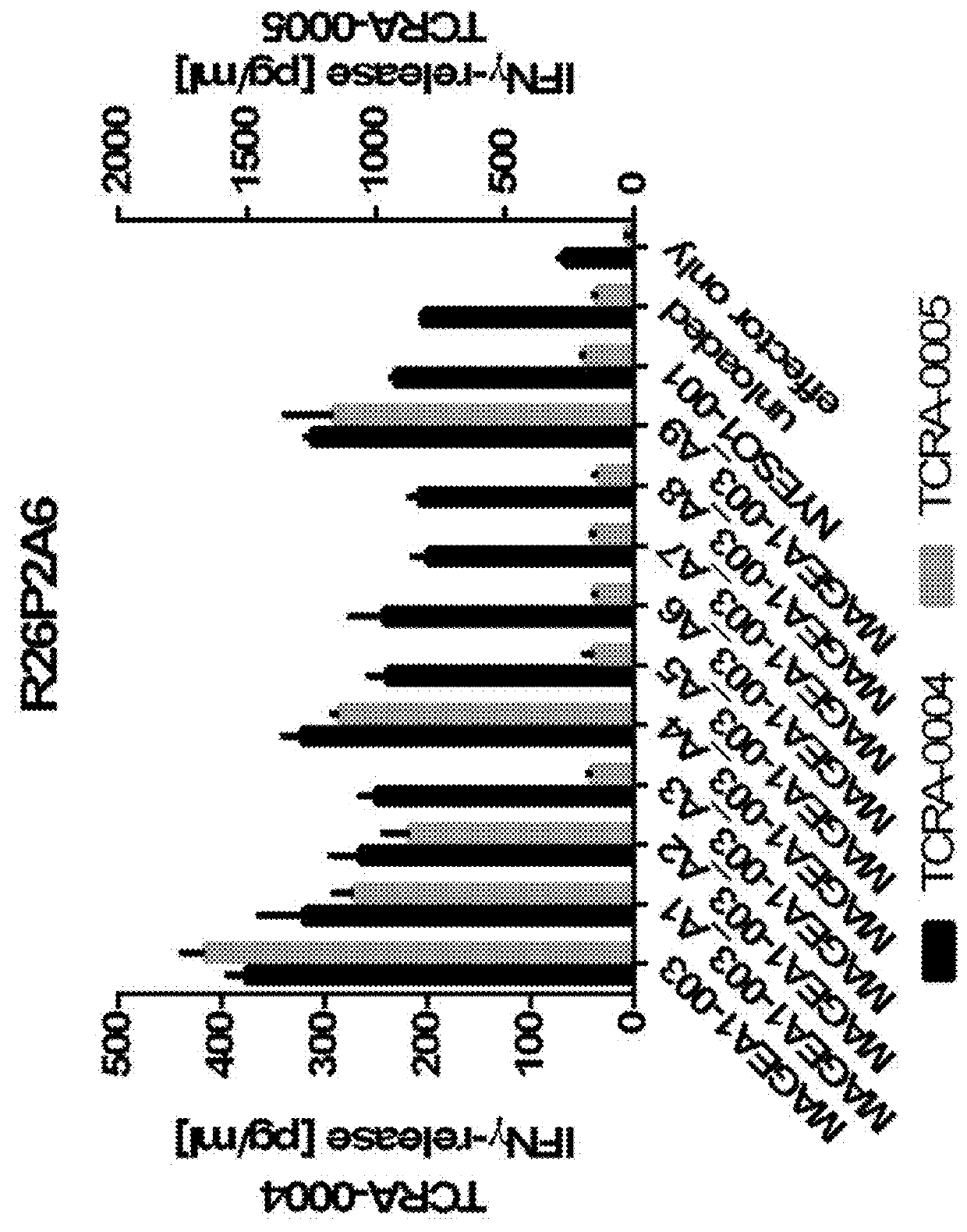
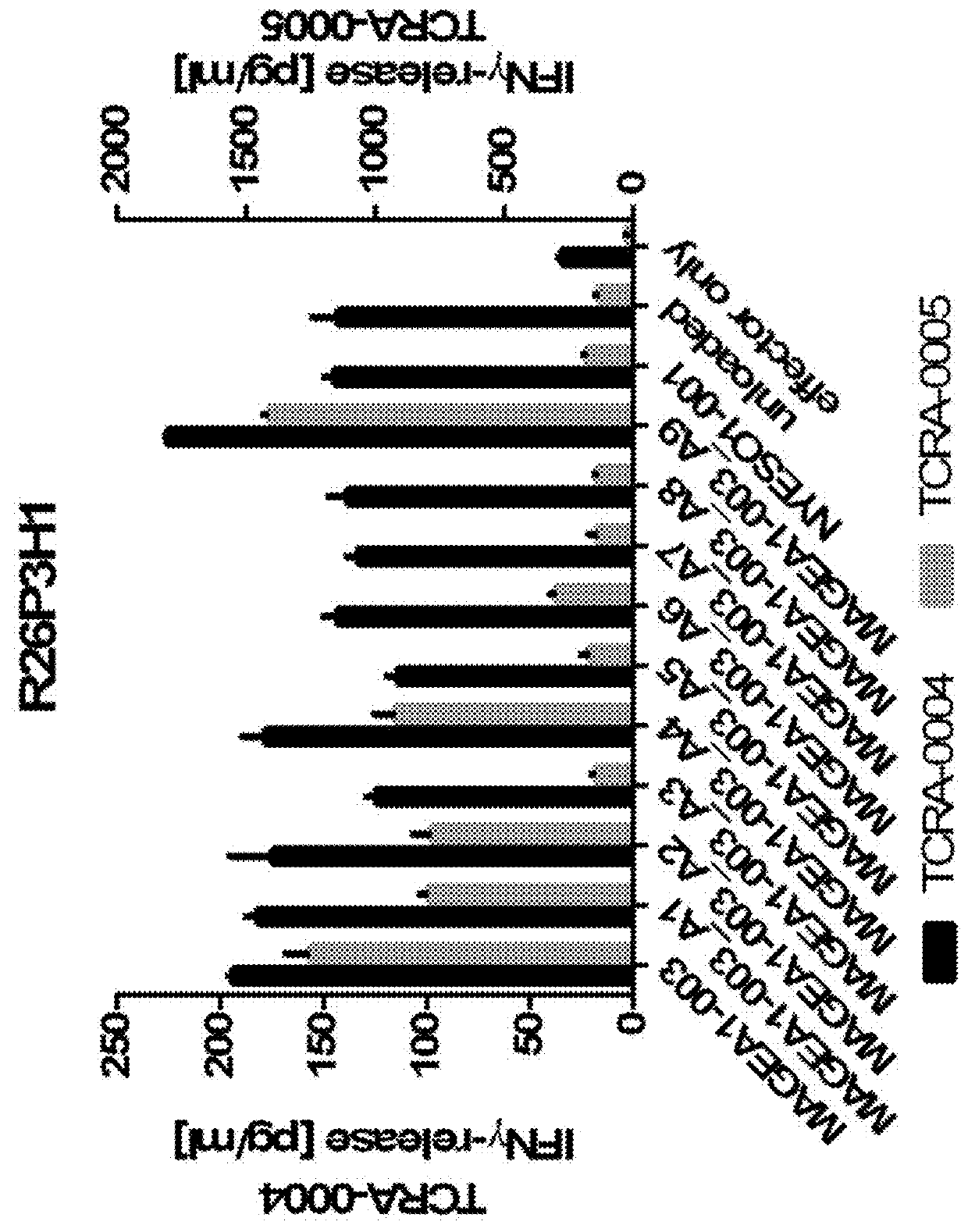
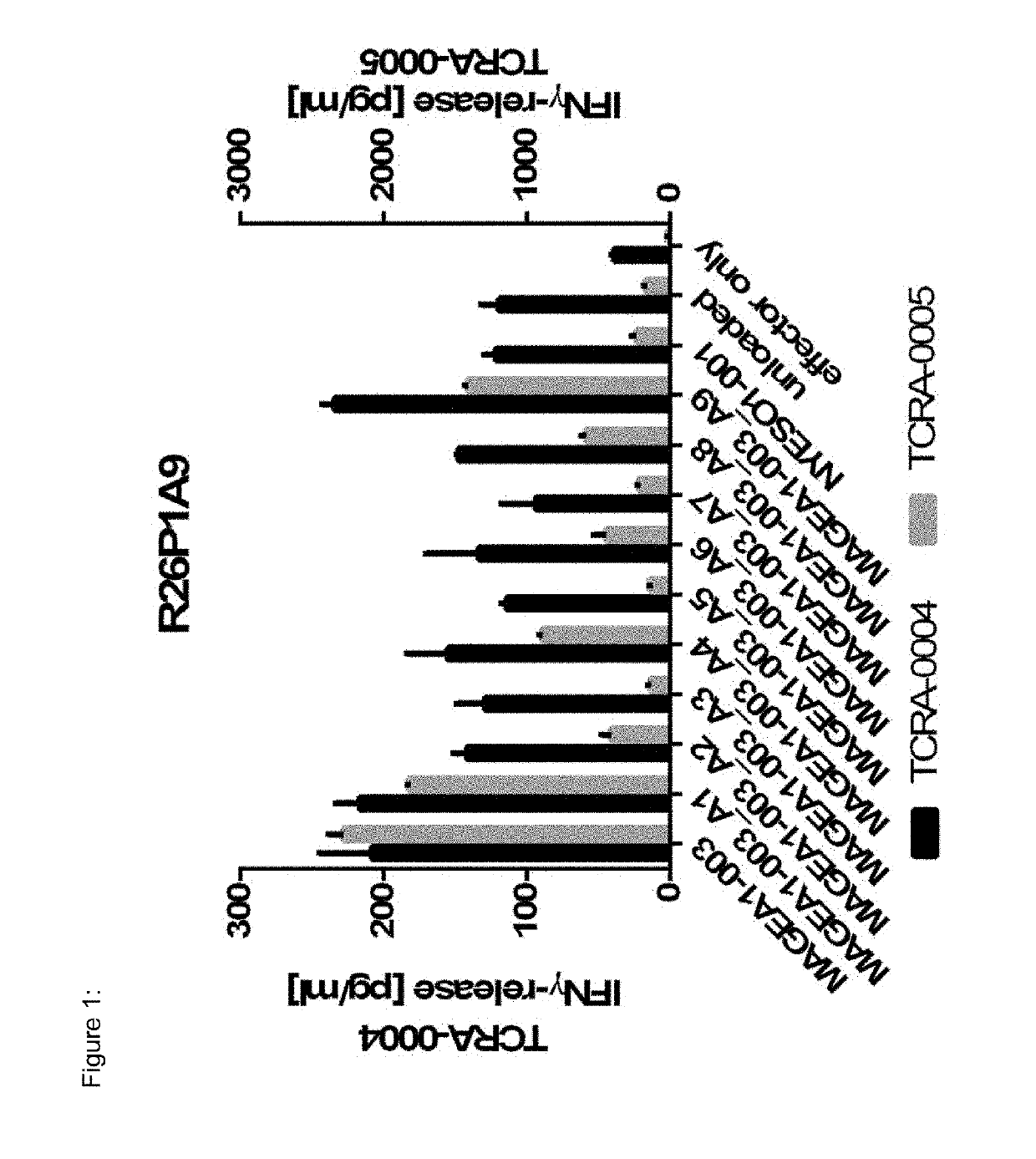
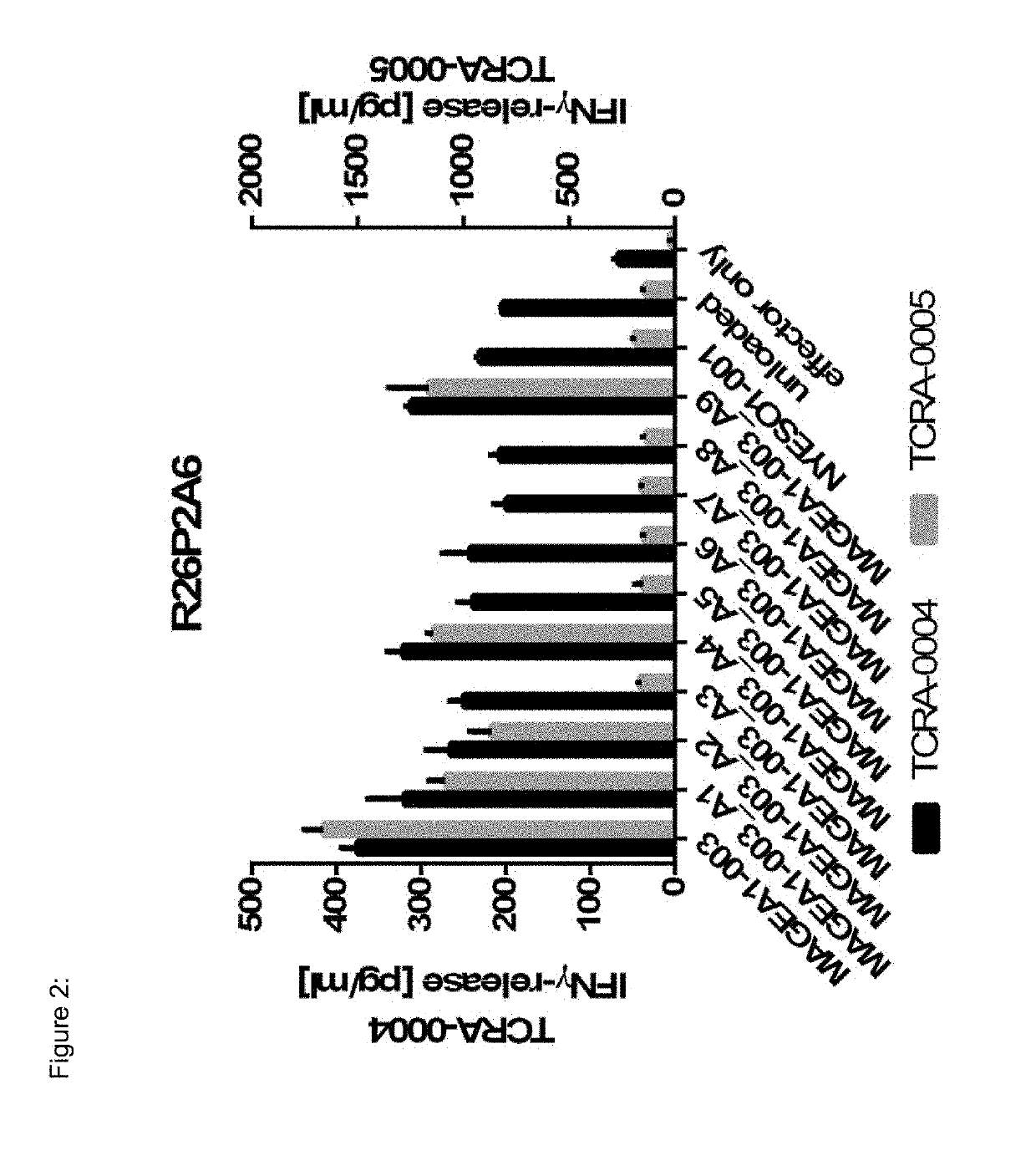
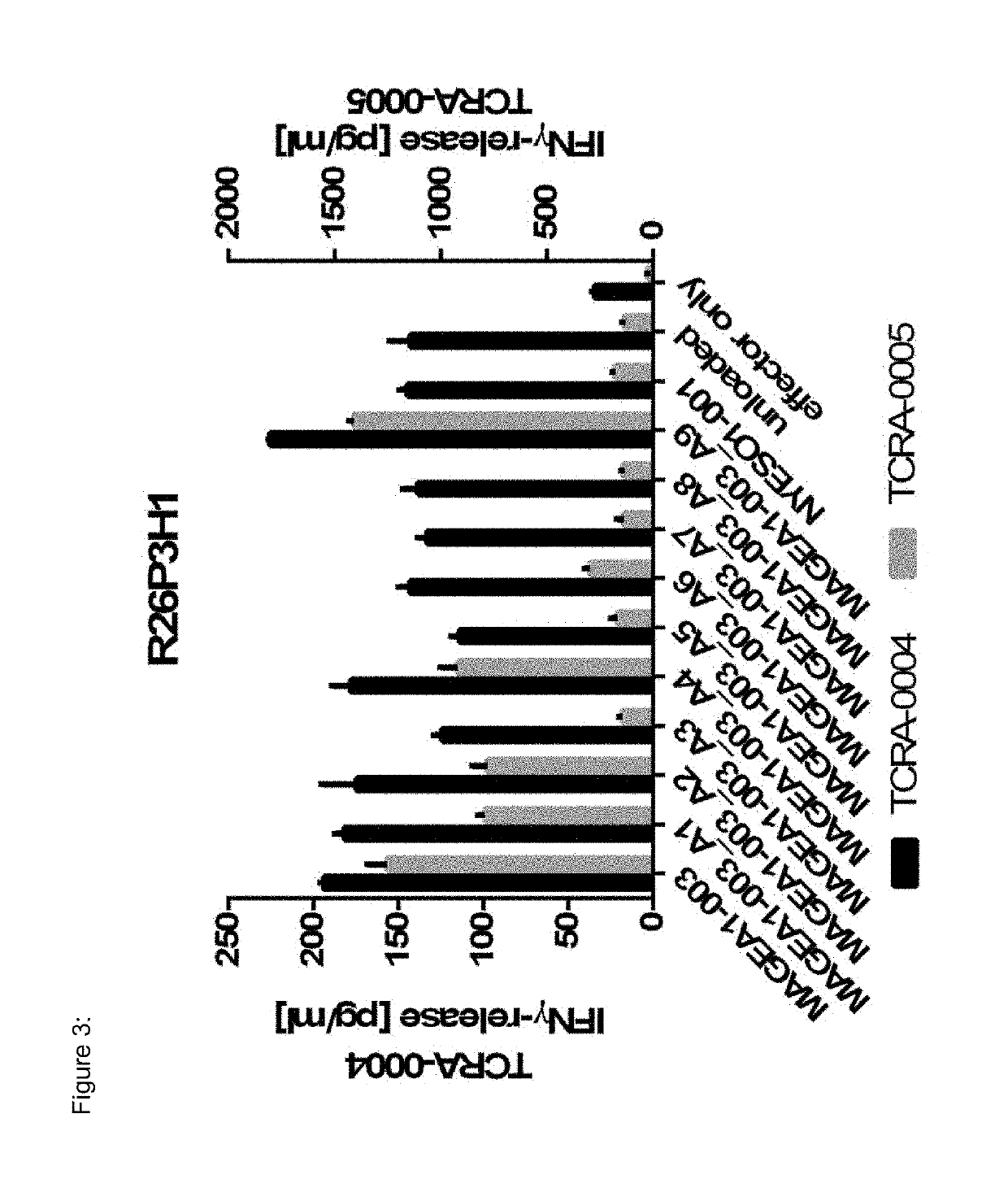
Novel t cell receptors and immune therapy using the same
ActiveUS20180161396A1Immunoglobulin superfamilyTumor rejection antigen precursorsDiseaseImmune therapy
The present invention pertains to antigen recognizing constructs against tumor associated antigens (MAGEA1). The invention in particular provides novel T cell receptor (TCR) based molecules which are selective and specific for the tumor expressed antigen of the invention. The TCR of the invention, and TAA binding fragments derived therefrom, are of use for the diagnosis, treatment and prevention of TAA expressing cancerous diseases. Further provided are nucleic acids encoding the antigen recognizing constructs of the invention, vectors comprising these nucleic acids, recombinant cells expressing the antigen recognizing constructs and pharmaceutical compositions comprising the compounds of the invention.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Novel t cell receptors and immune therapy using the same
ActiveUS20190321478A1Tumor rejection antigen precursorsPeptide/protein ingredientsDiseaseImmune therapy
The present invention pertains to antigen recognizing constructs against tumor associated antigens (MAGEA1). The invention in particular provides novel T cell receptor (TCR) based molecules which are selective and specific for the tumor expressed antigen of the invention. The TCR of the invention, and TAA binding fragments derived therefrom, are of use for the diagnosis, treatment and prevention of TAA expressing cancerous diseases. Further provided are nucleic acids encoding the antigen recognizing constructs of the invention, vectors comprising these nucleic acids, recombinant cells expressing the antigen recognizing constructs and pharmaceutical compositions comprising the compounds of the invention.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Methods and means to suppress symptoms of an allergic disease by inhibiting the glucocorticoid-induced tumor necrosis factor receptor (GITR or TNFRSF18)
InactiveUS20060051350A1Inhibitory responseIncrease productionOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsTolerabilityGlucocorticoid
The invention relates to the field of immunology, more in particular to the field of immune therapy, even more particularly to a method for regulating tolerance to an allergen in a subject and even more specifically, to methods which involve regulation of a glucocorticoid-induced tumor necrosis factor receptor (GITR). Provided is methods of treating allergic disorder and compositions for use therein.
Owner:UTRECHT UNIVERSITY
Bursa of Fabricius heptapeptide with immune regulation effect
InactiveCN101830968ASynthetic technology is matureImprove efficiencyPeptide preparation methodsAntibody medical ingredientsSide effectImmunologic Competence
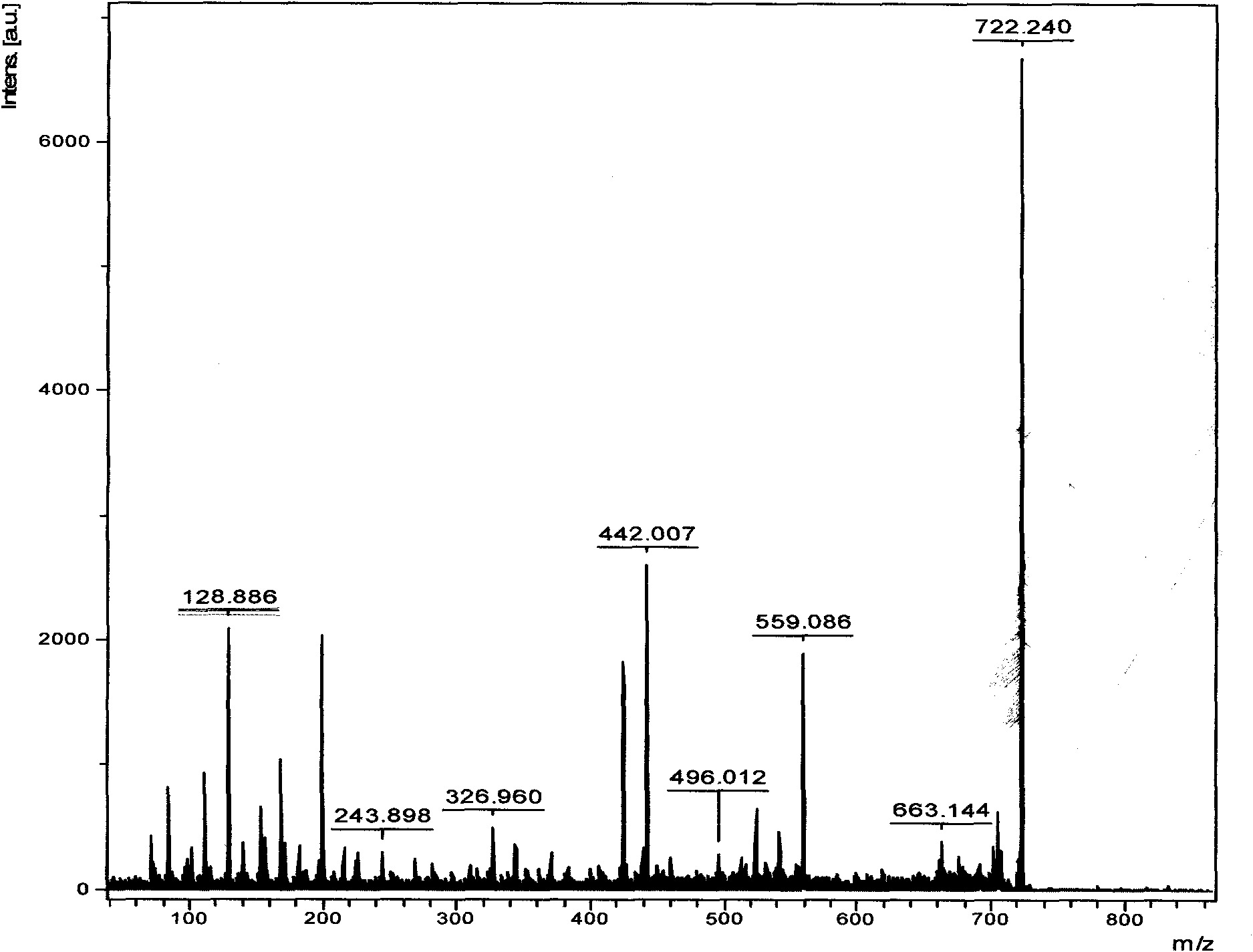
The invention relates to bursa of Fabricius heptapeptide with immune regulation effect and application thereof in immunity (in improving the immune capability of animals, improving the immune effect of vaccines and affecting the activity of tumor cells), and belongs to the field of immunology. The molecular weight of the separated heptapeptide is 722.240, the amino acid sequence is EPASGMM, and the heptapeptide has a simple structure, no toxic or side effect and extremely weak immunogenic property. The heptapeptide can be separated and extracted from bursa of Fabricius, also can be chemically synthesized, has low cost, and can be massively produced. The bursa of Fabricius heptapeptide has induction effect on the production of antibody and subtype thereof, and meanwhile can regulate the production of cell factors, partition of T lymphocytes and proliferation of spleen cells, and promote the immune reaction of the cells. The bursa of Fabricius heptapeptide is an immune regulation factor on functions, has wide application prospect on the aspects of immune regulation, immune therapy and the like, and can be applied in the fields of basic immune research, clinical application research and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for in vitro culture of lymphocytes and composition for use in immune therapy
InactiveUS20040161433A1Reduce expressionEasily damagedBiocideGenetically modified cellsAntigenCancer cell
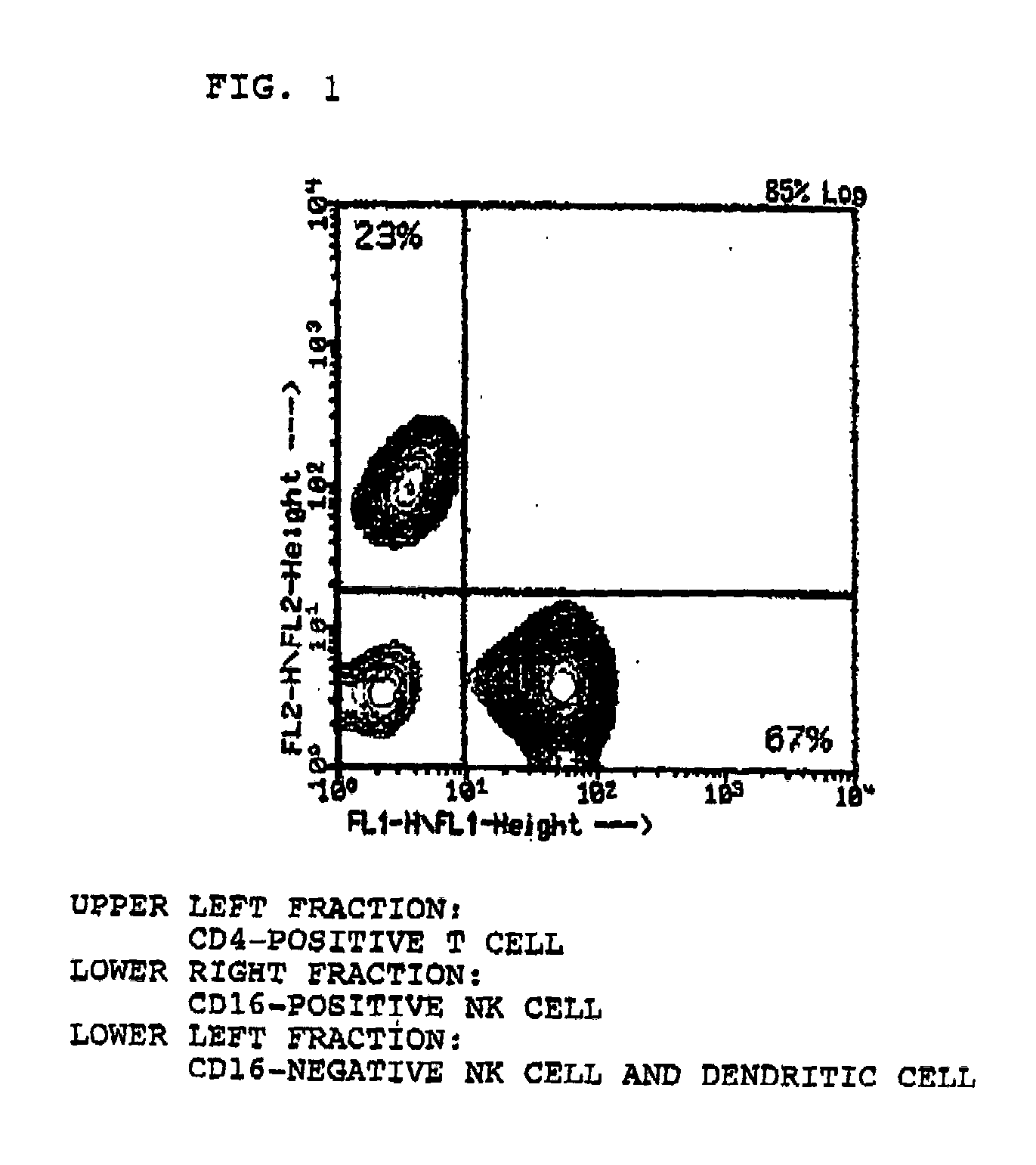
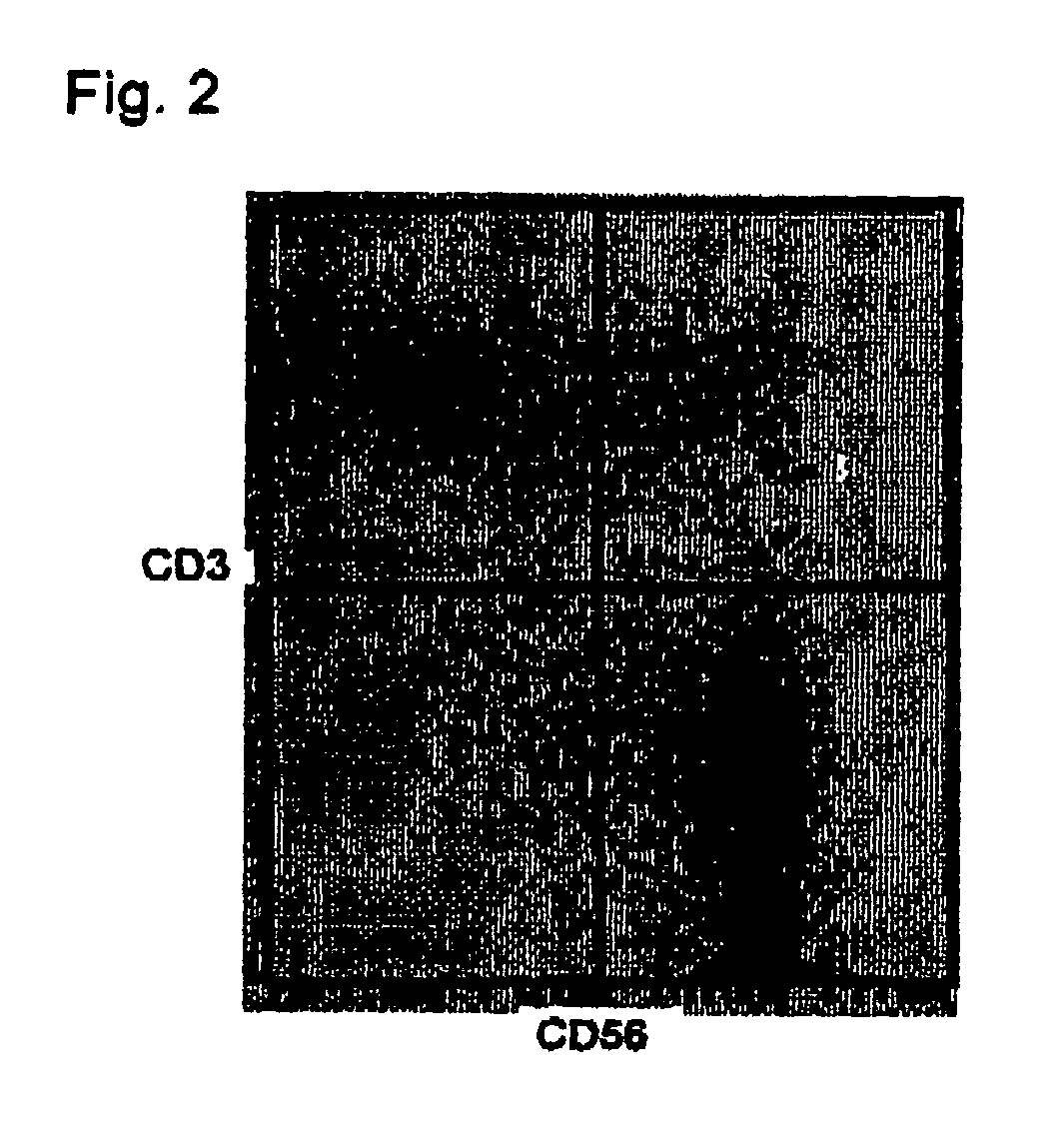
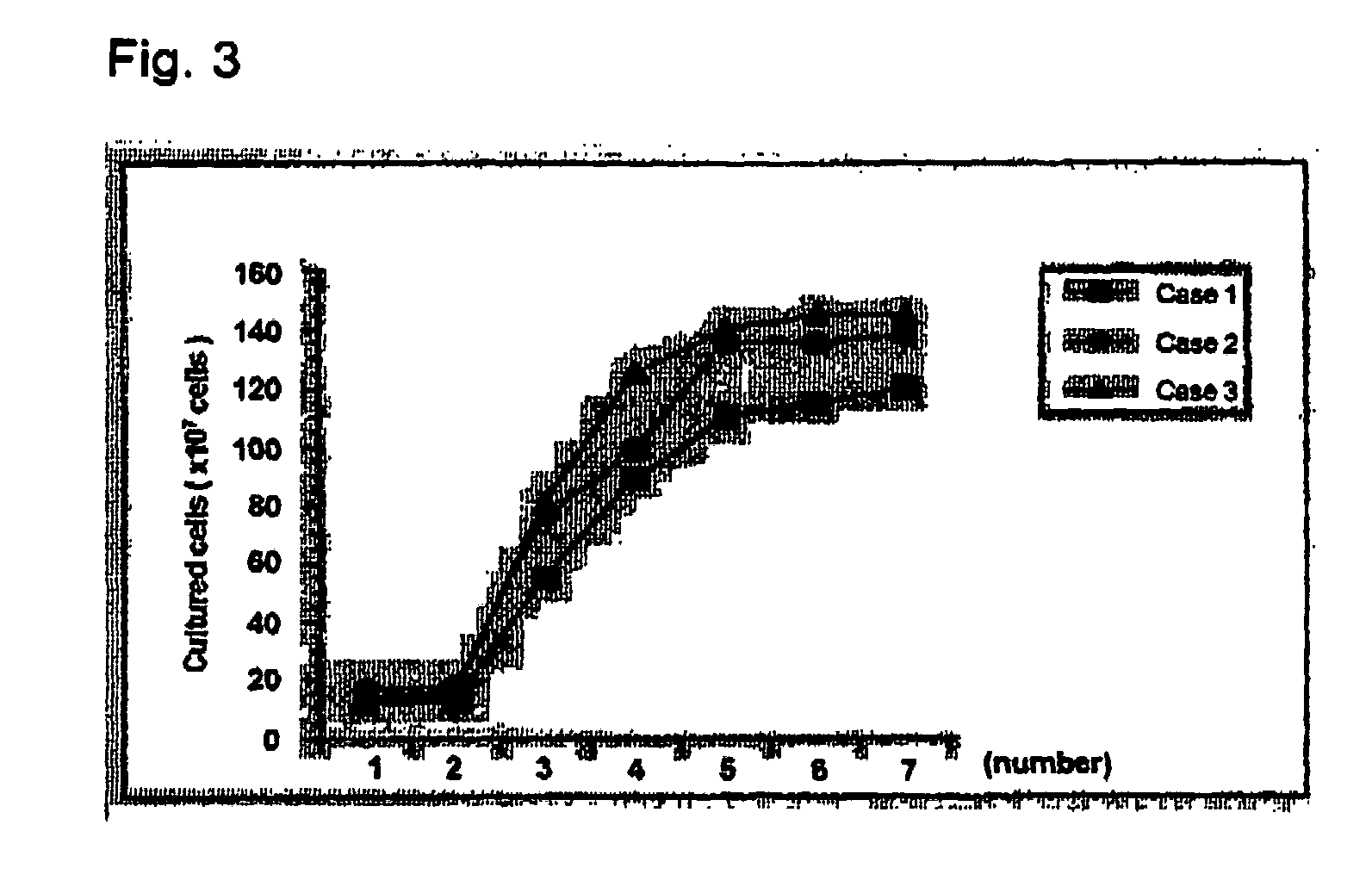
The method for in vitro culture of lymphocytes involves incubating lymphocytes and cells with a particular gene expressed in a particular cancer cell line or cells, which are deficient or lowered in expression of a class 1 antigen and the particular gene has already been expressed, to amplify mainly NK cells or non-MHC-bound or MHC-bound killer T cells and then to amplify killer T cells specific to an antigen to a cancer. The in vitro culture of the lymphocytes can produce a group of lymphocytes composed mainly of killer cells. The group of the lymphocytes so produced can be used for immune therapy effective even for patients with cancer in the terminal stage to whom conventional cancer treatment and cancer curing agents.
Owner:TESHIGAWARA KEISUKE +1
Preparation and application of anti-human programmed death factor 1 (PD-1) monoclonal antibody
ActiveCN106046162AHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenAutoimmune disease
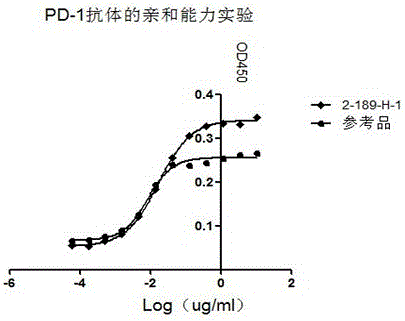
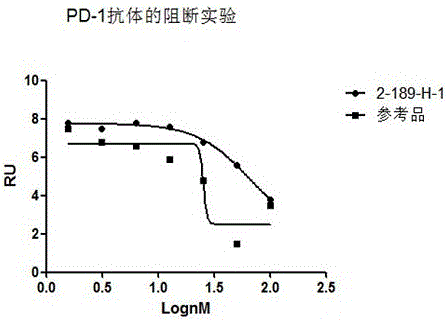
The invention discloses preparation, a variable-region sequence and application of an anti-human programmed death factor 1 (PD-1) monoclonal antibody. The invention provides the anti-human PD-1 monoclonal antibody which comprises gene sequences of heavy-chain variable regions of the monoclonal antibody and gene sequences of light-chain variable regions of the monoclonal antibody. The amino acid sequences of the heavy-chain variable regions are as shown in site 20th to site 134th of SEQ ID NO.3; the amino acid sequences of the light-chain variable regions are shown in site 20th to site 131th of SEQ ID NO.4. The invention discloses the monoclonal antibody 189-H-1 capable of blocking a human PD-1 function and a coding gene thereof. The monoclonal antibody 189-H-1 can be specifically combined with human PD-1 antigen, has median effective concentration EC50 being 1.0667 nM, can specifically block a PD-1 / PD-L inhibition signal, and therefore, the monoclonal antibody 189-H-1 can be used as a blocker of a PD-1 access, so that the anti-human PD-1 monoclonal antibody becomes a novel drug for tumor immune therapy, chronic virus infective disease treatment and autoimmune disease treatment.
Owner:大庆东竺明生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com