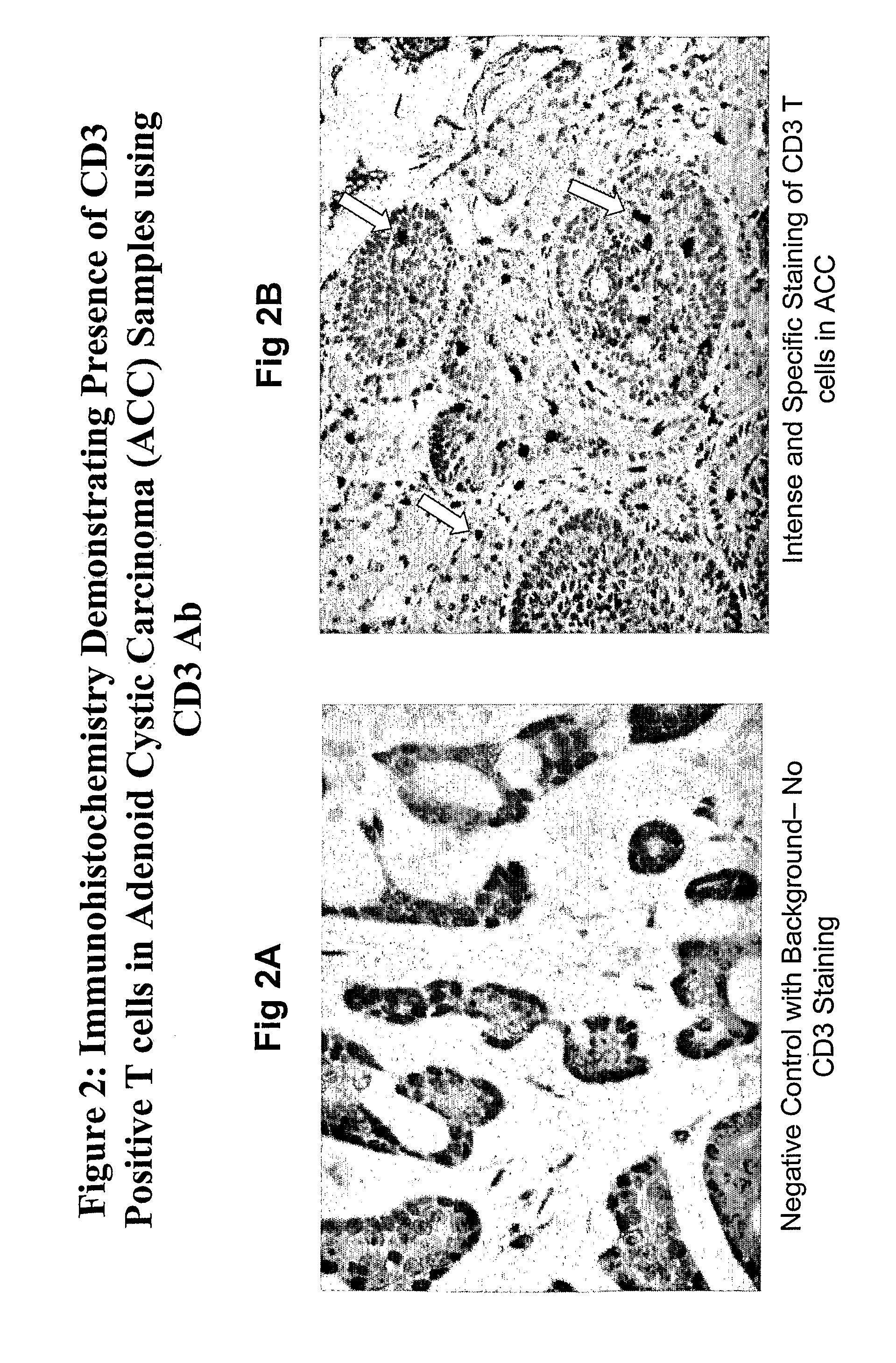
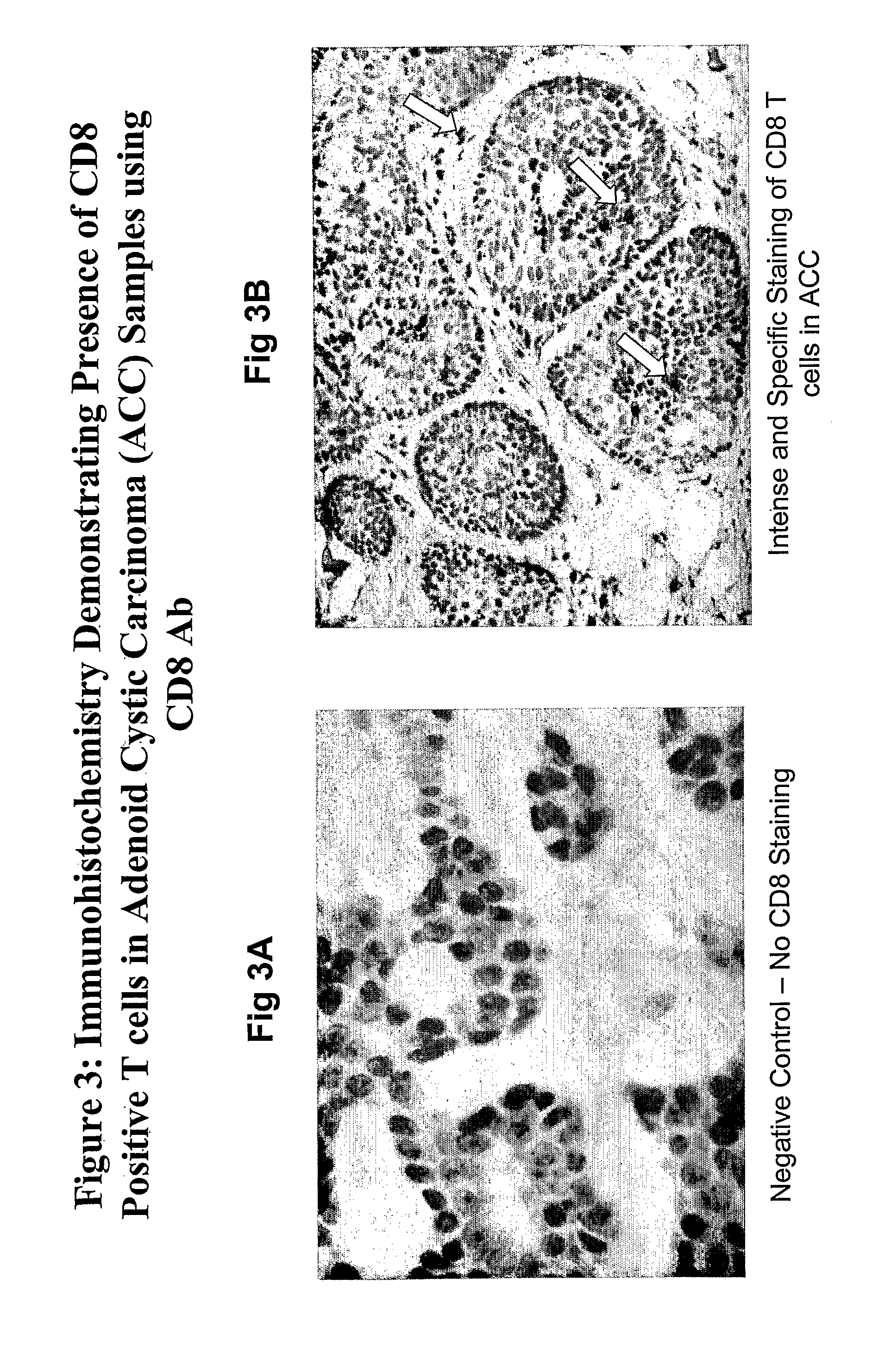
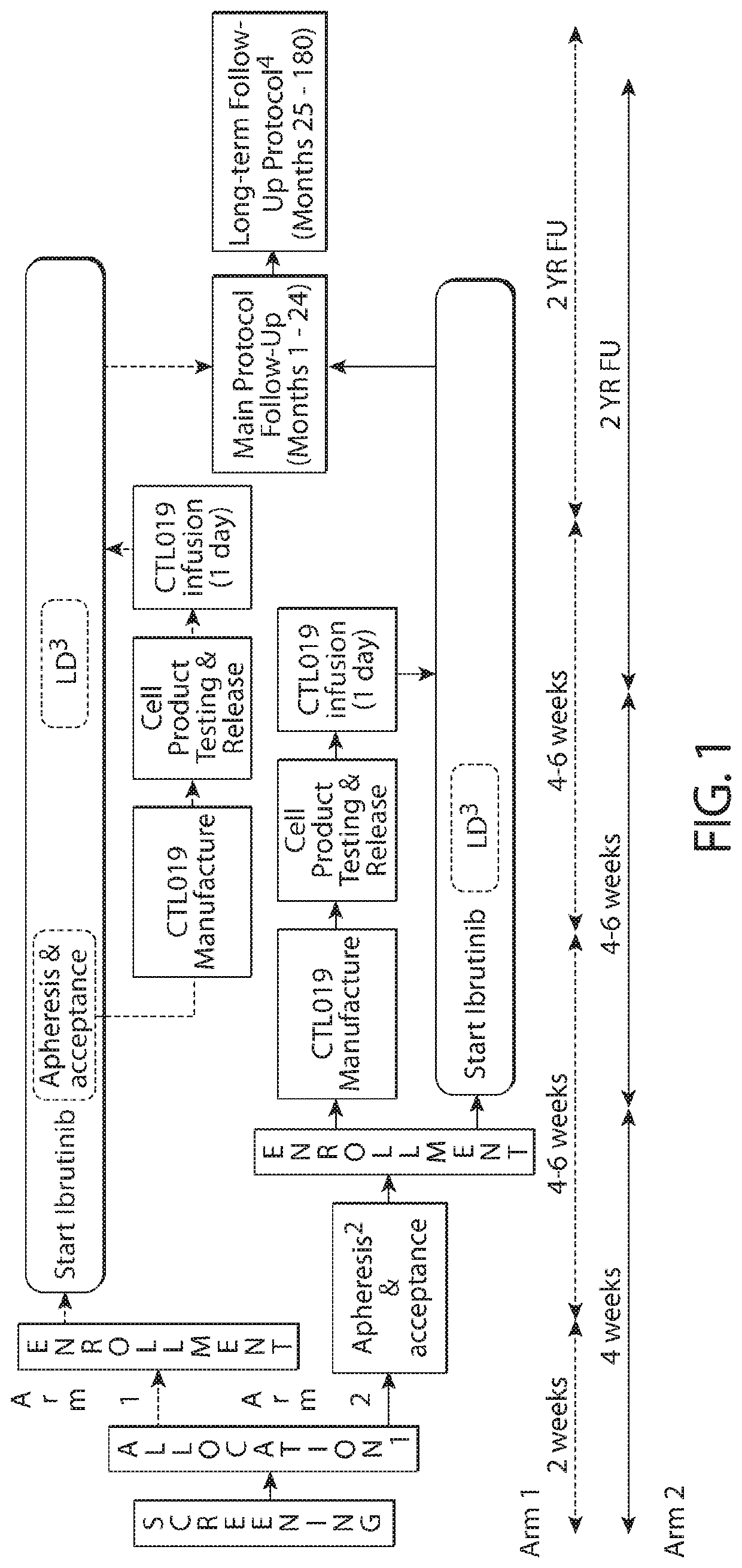
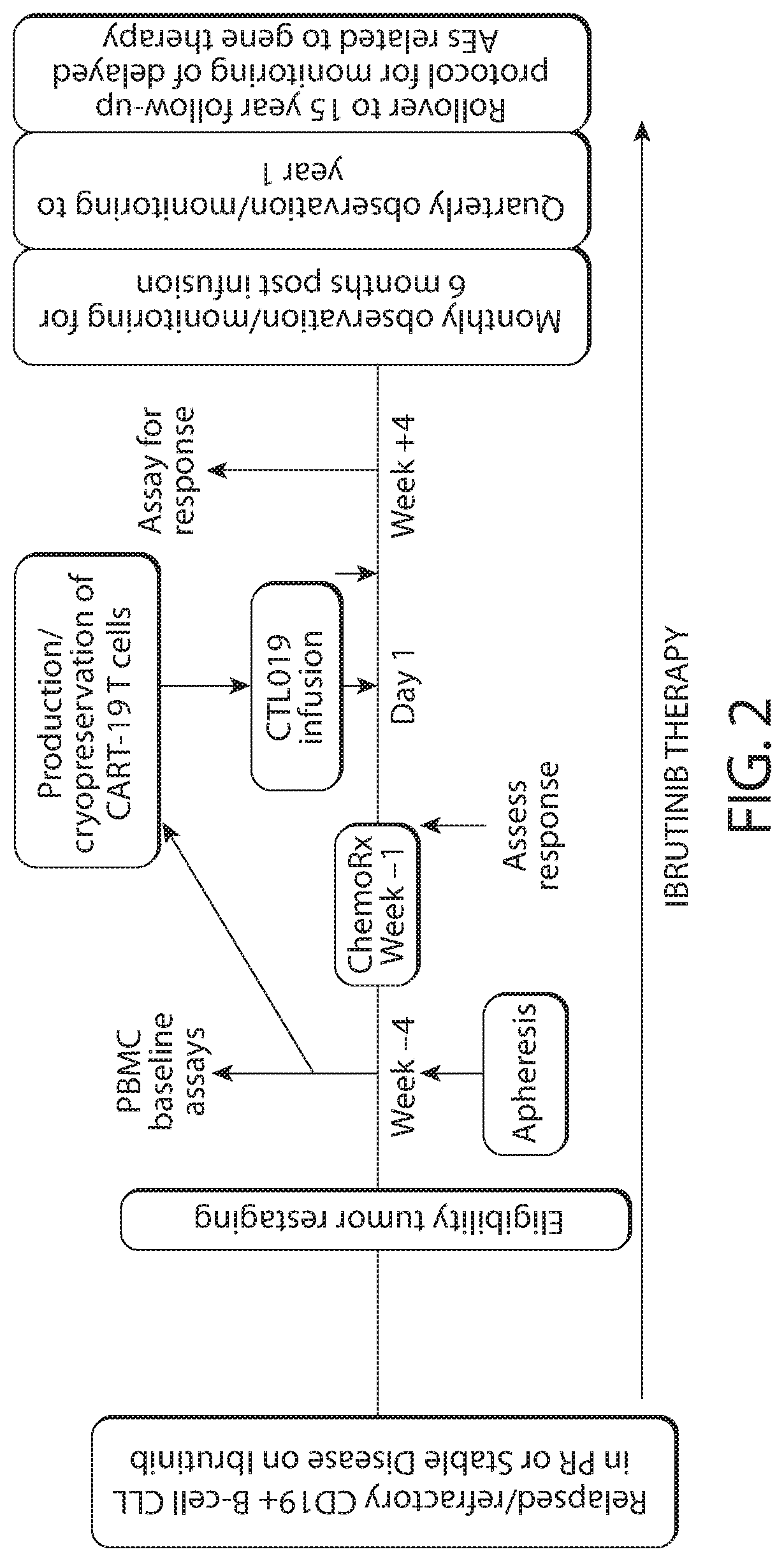
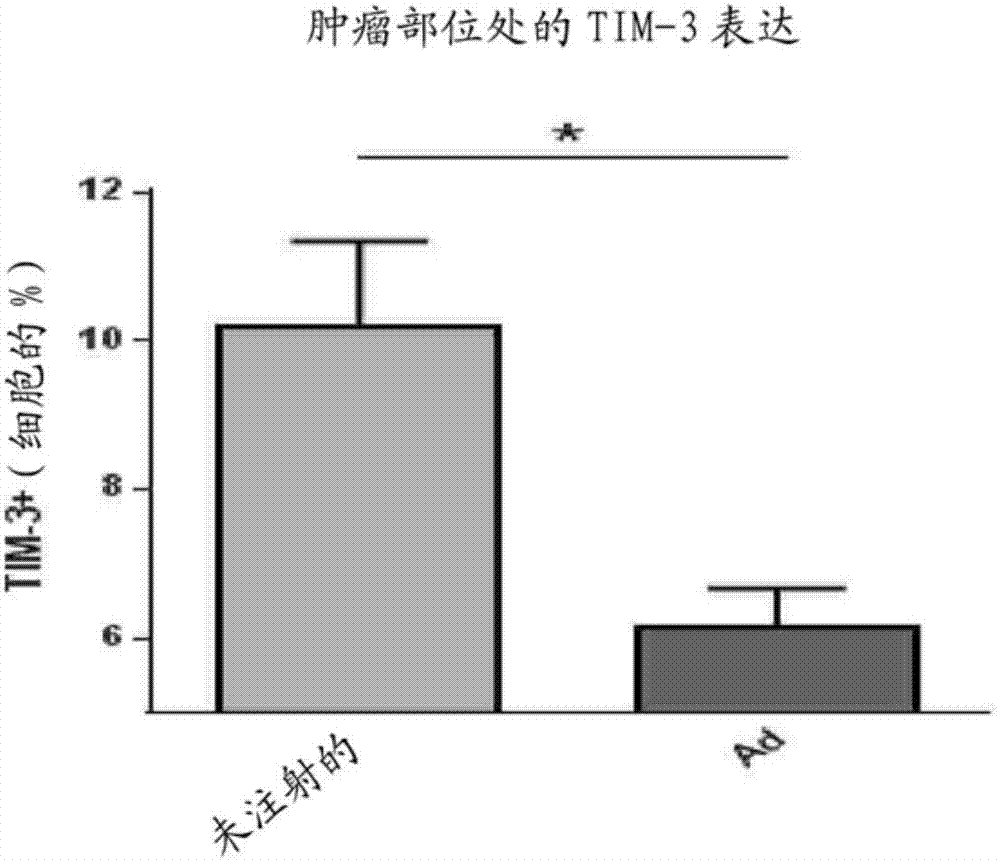
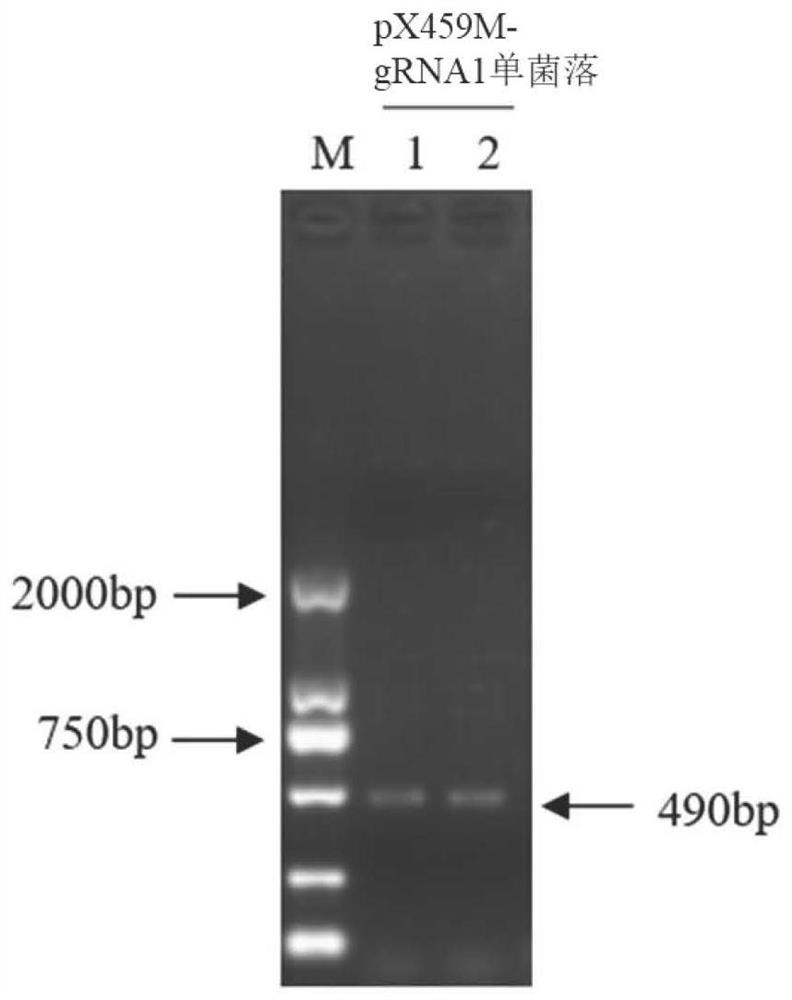
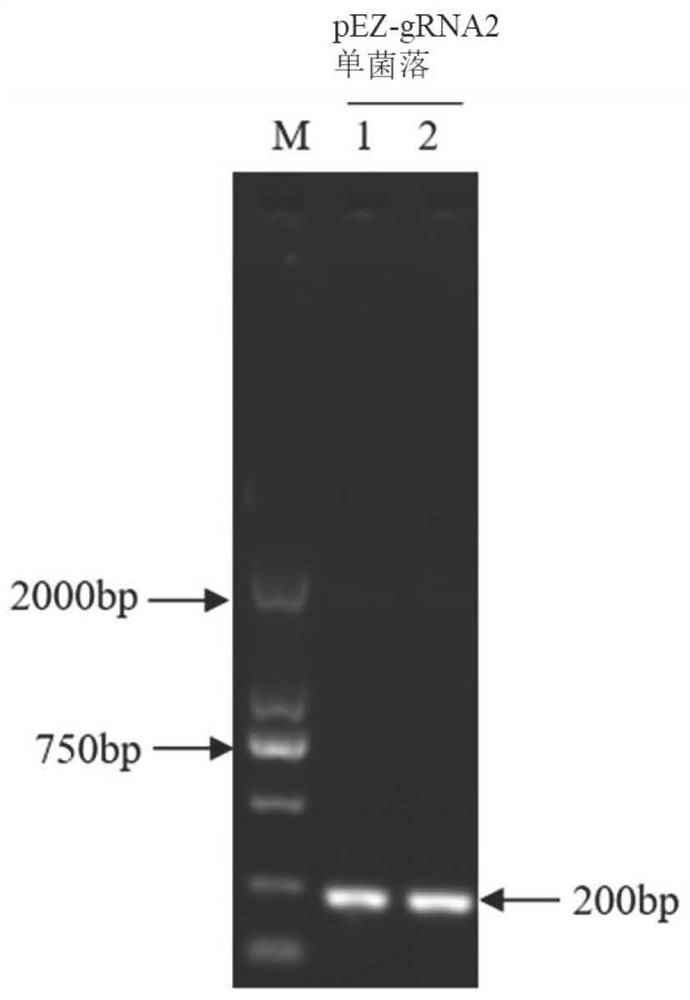
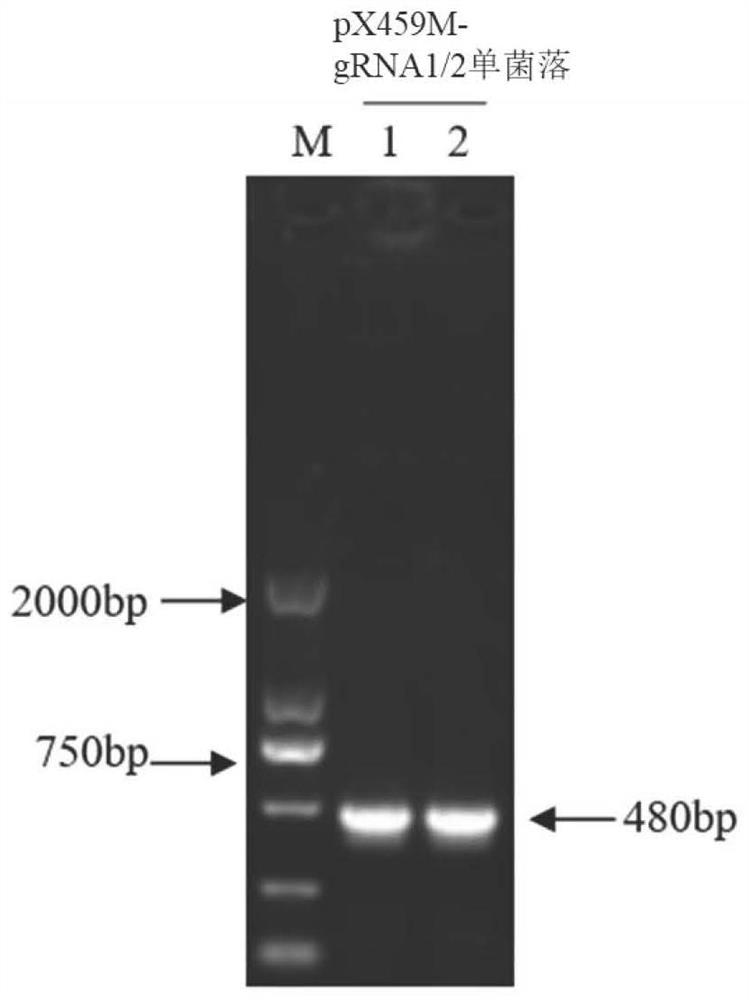
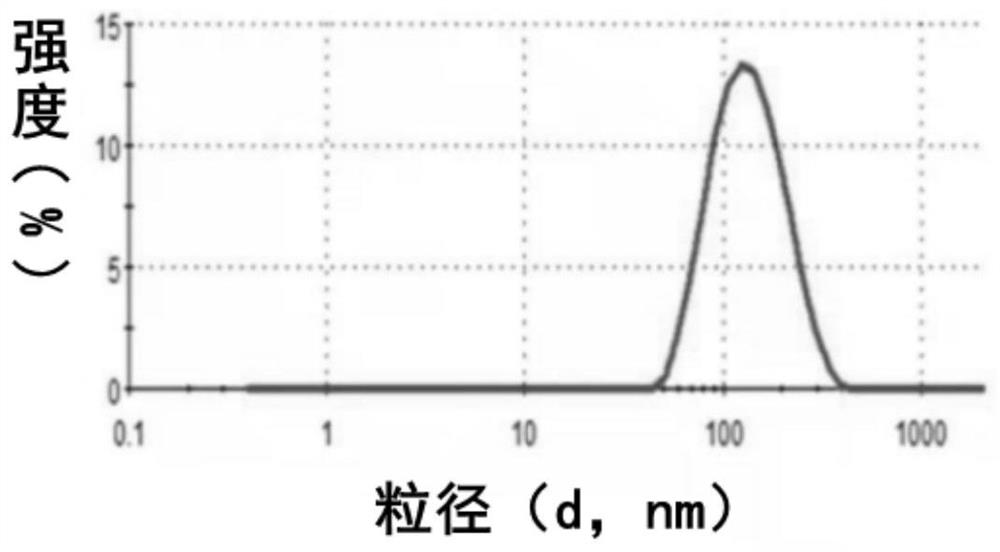
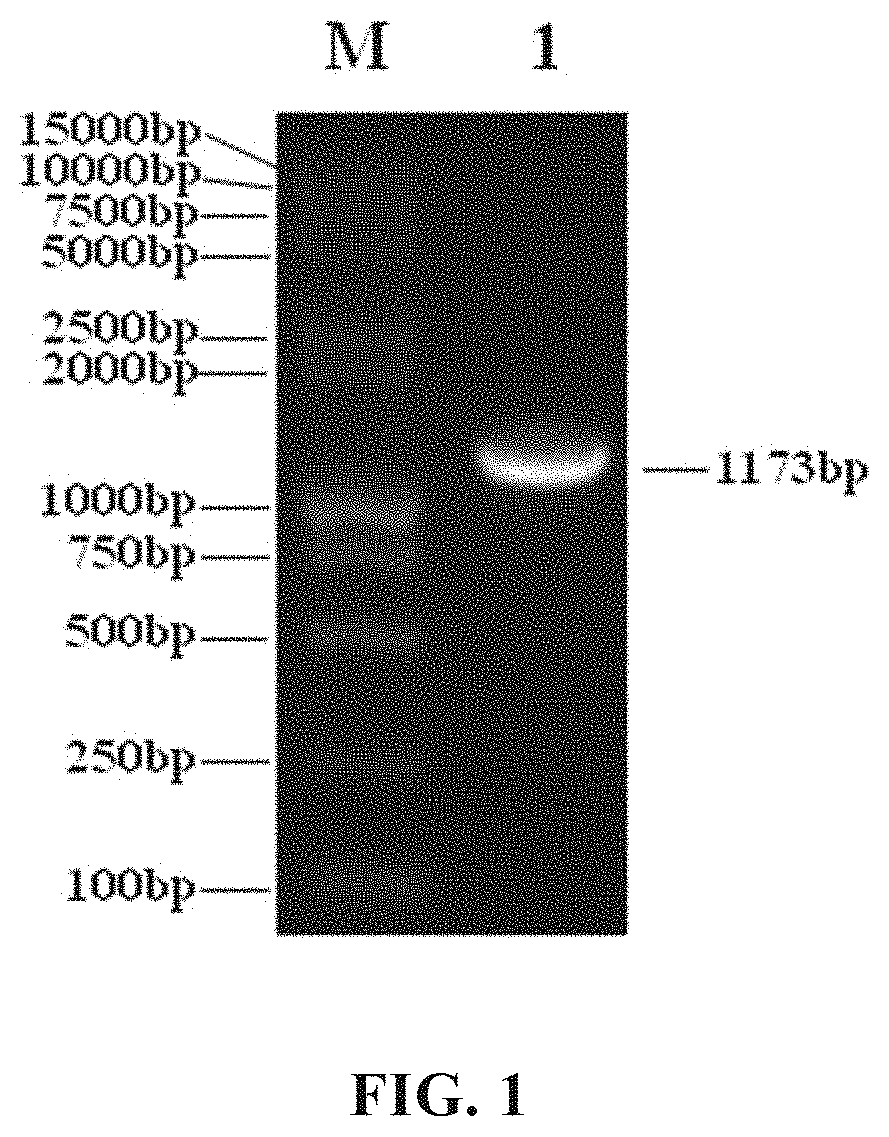
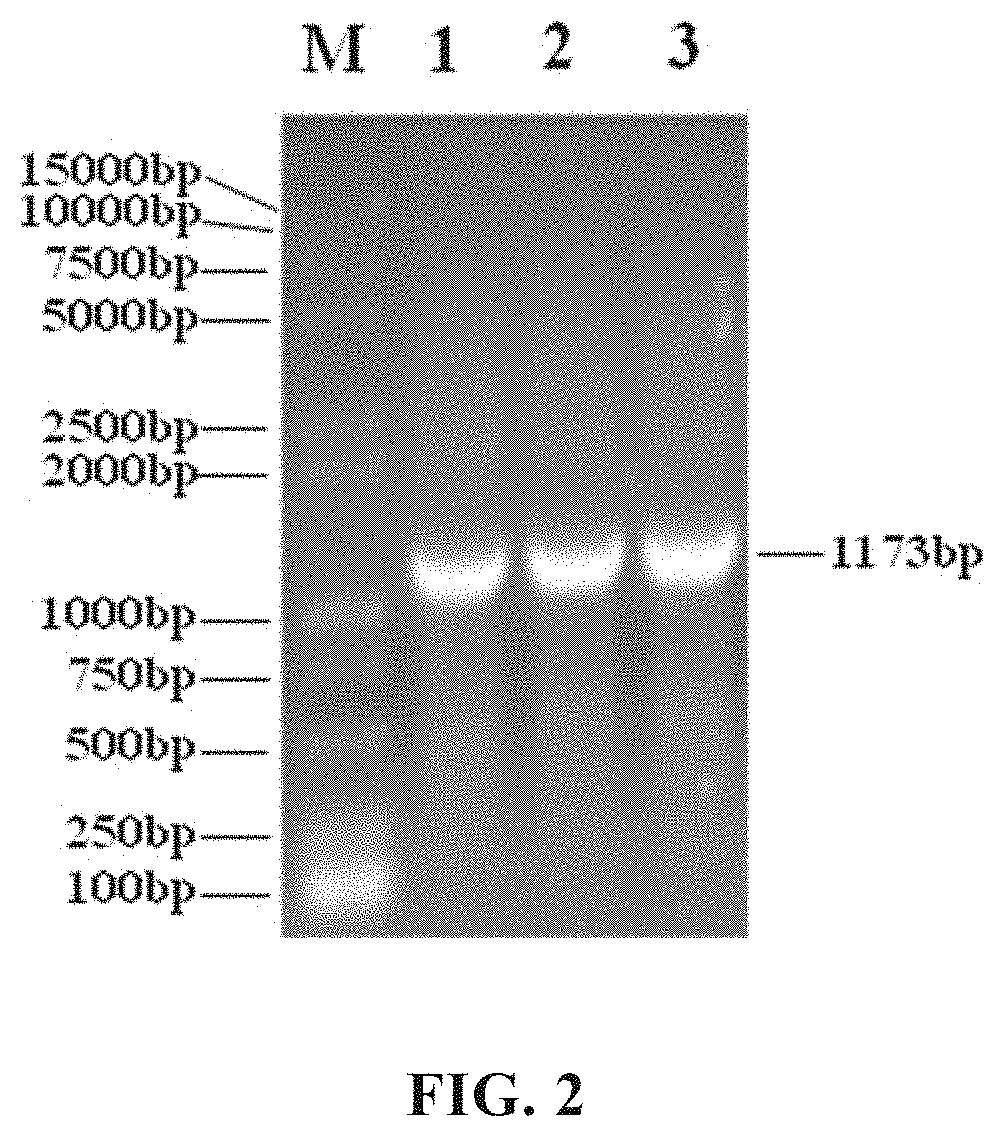
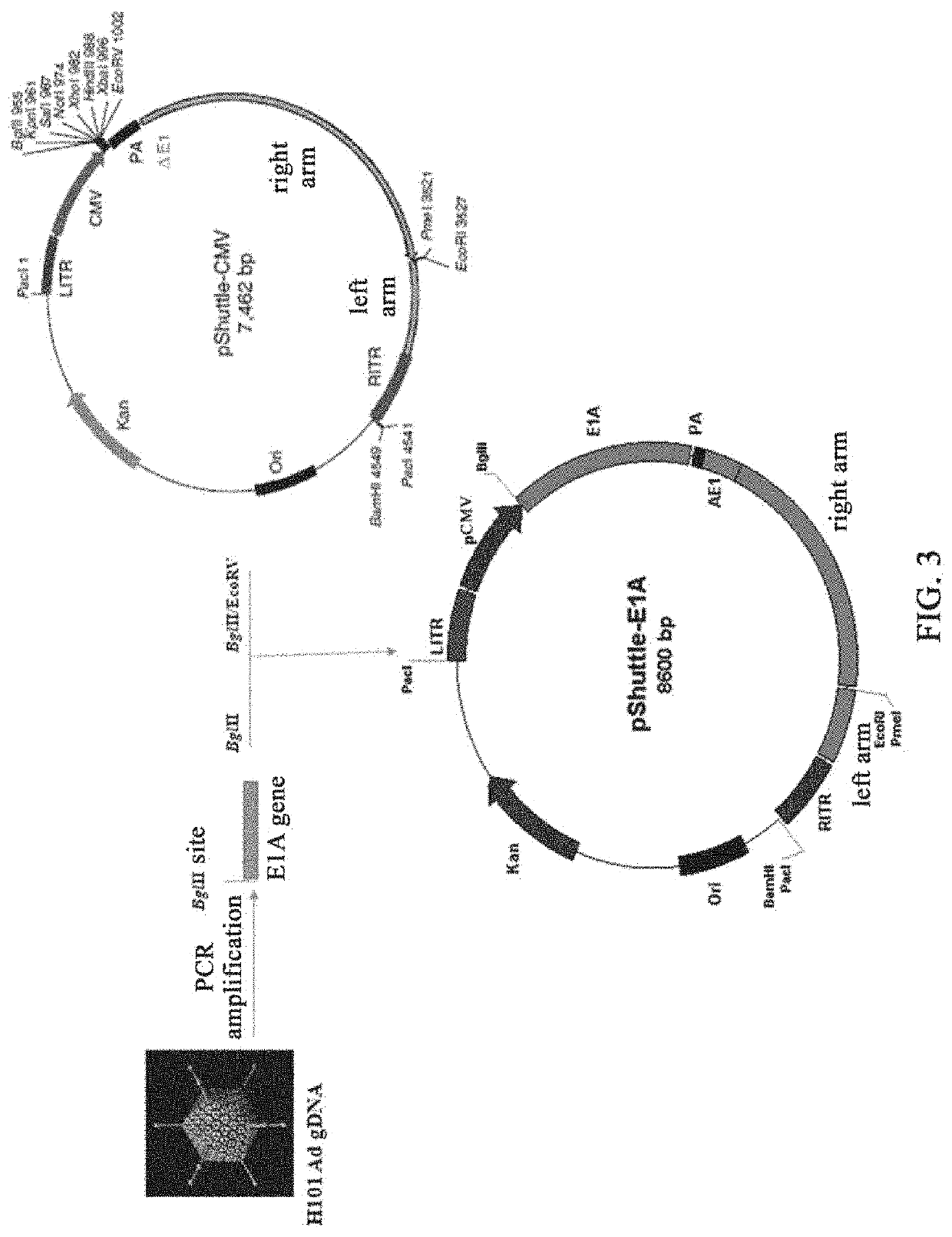
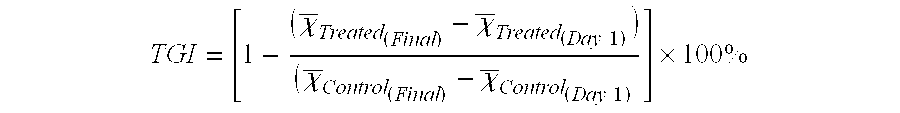Patents
Literature
79results about How to "Reduce immunosuppression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
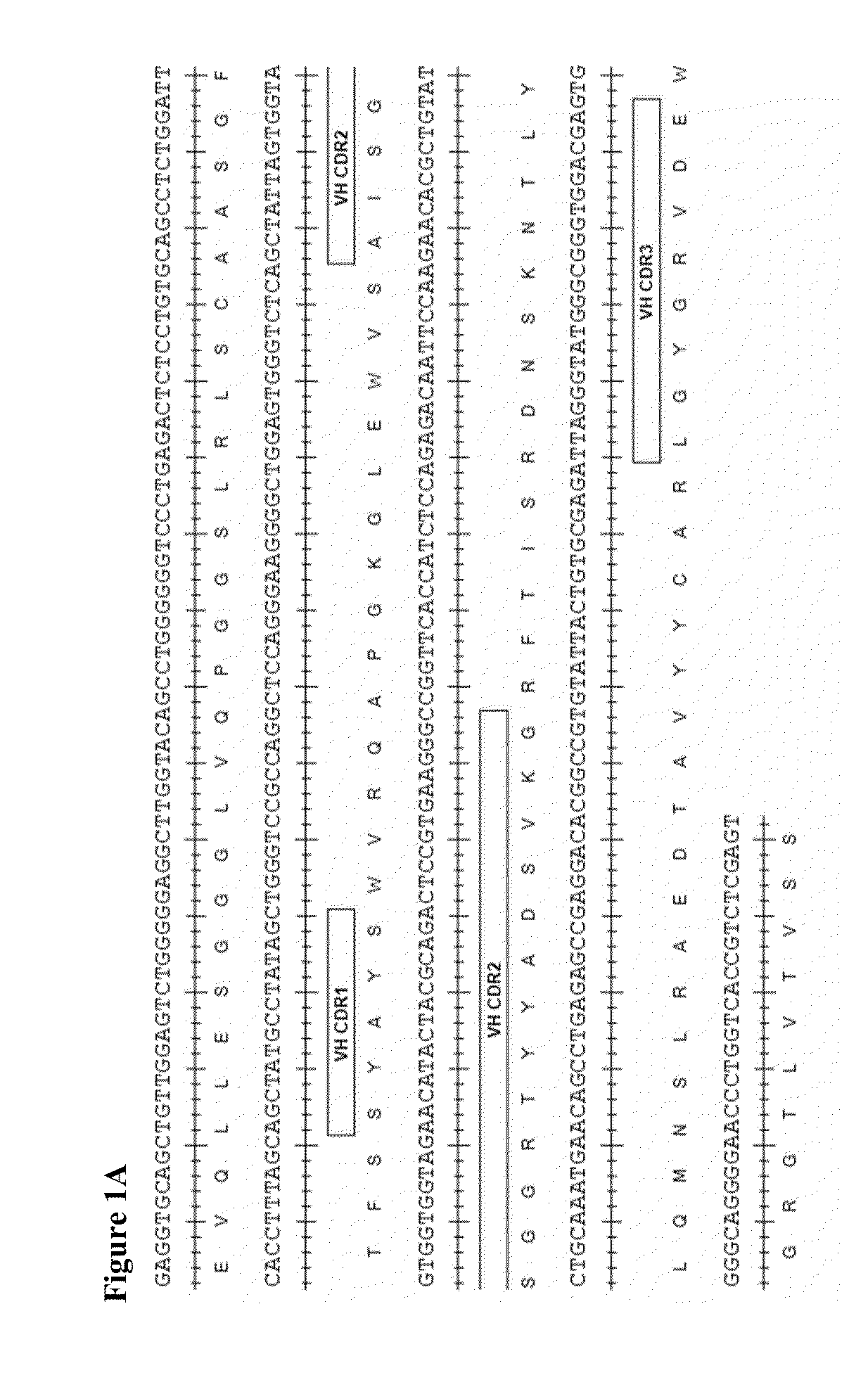
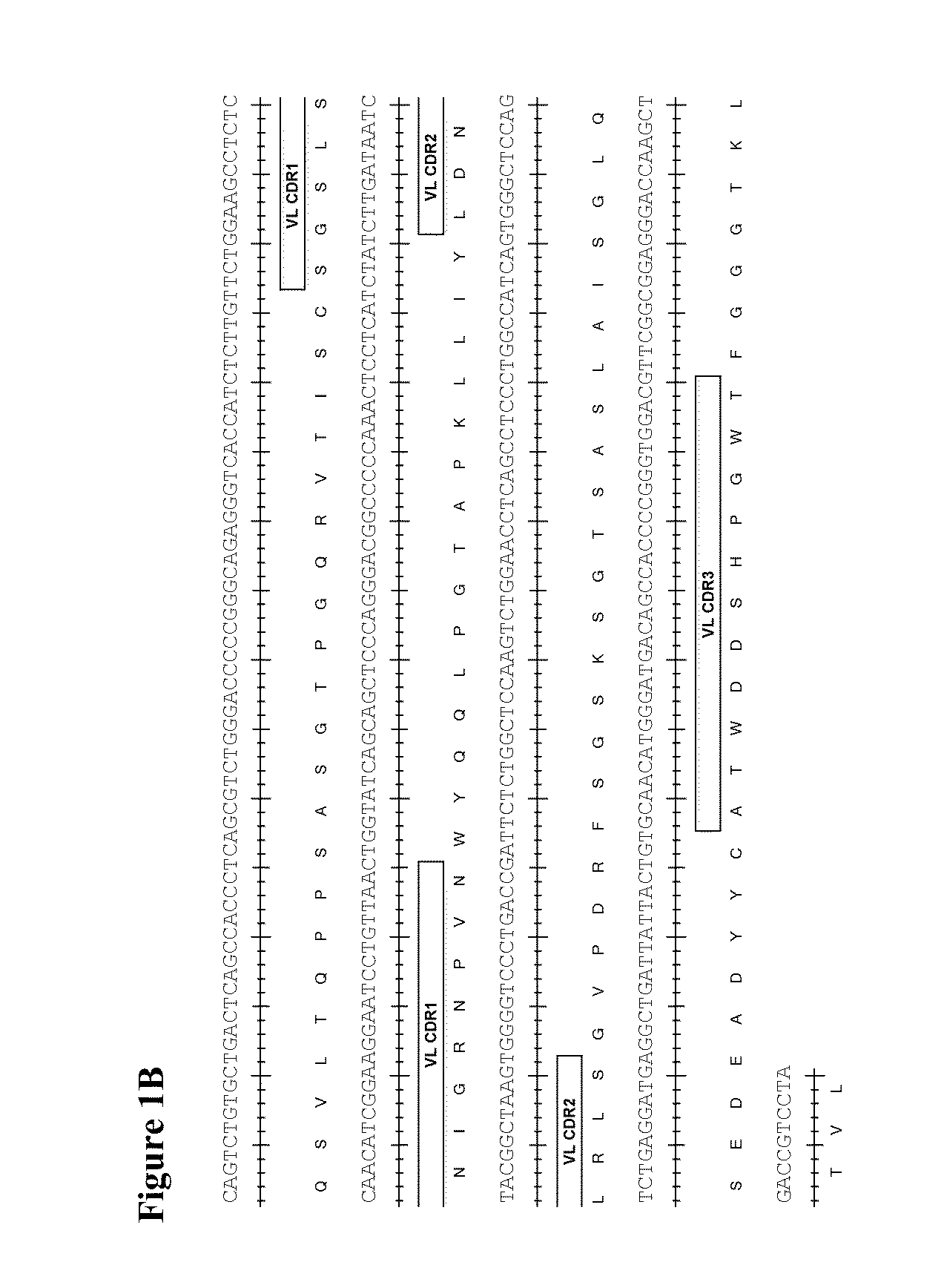
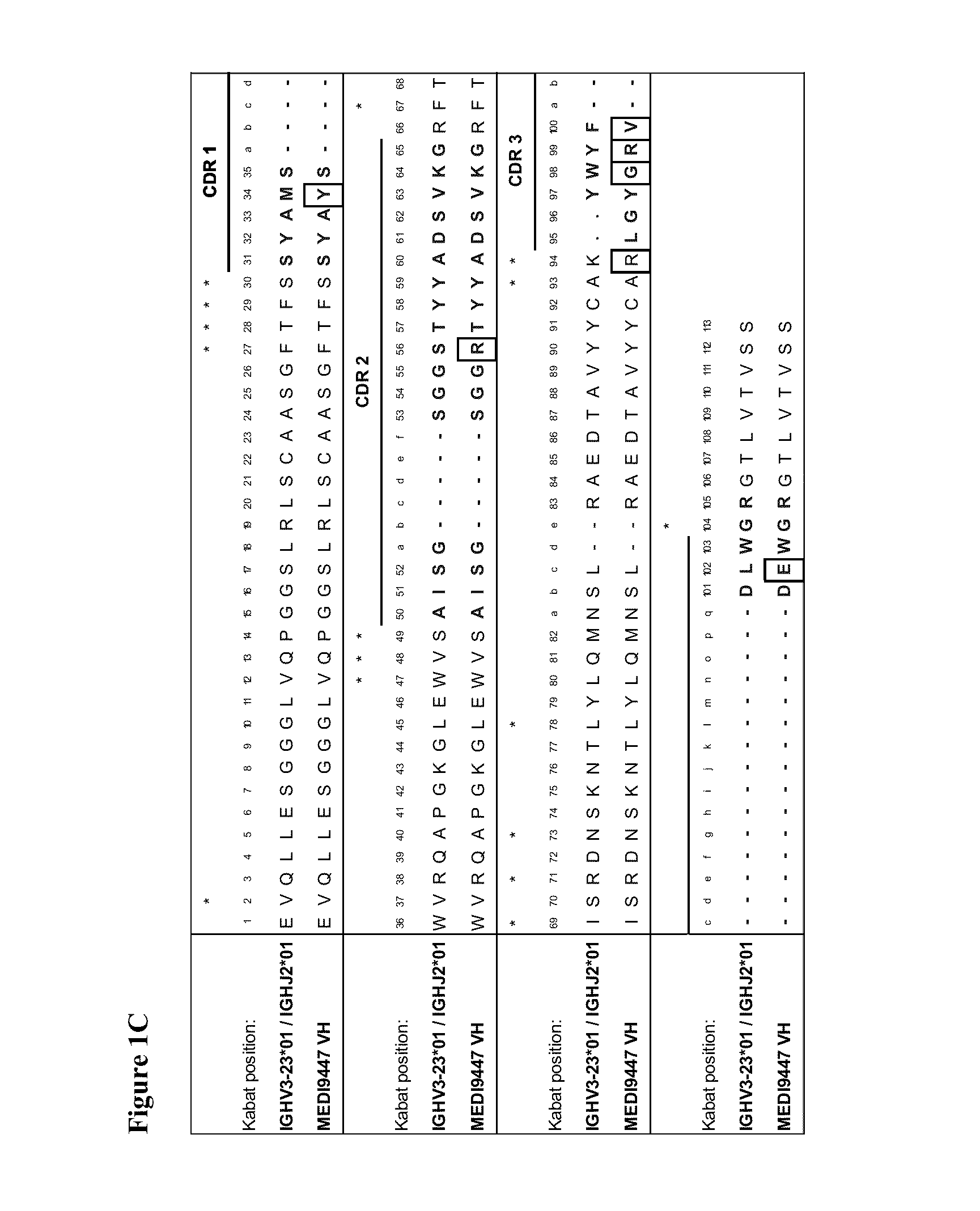
Therapeutic combinations comprising Anti-cd73 antibodies and uses thereof
InactiveUS20160129108A1Increase survivalIncrease immune responseOrganic active ingredientsAntibody ingredientsWilms' tumorAntibody
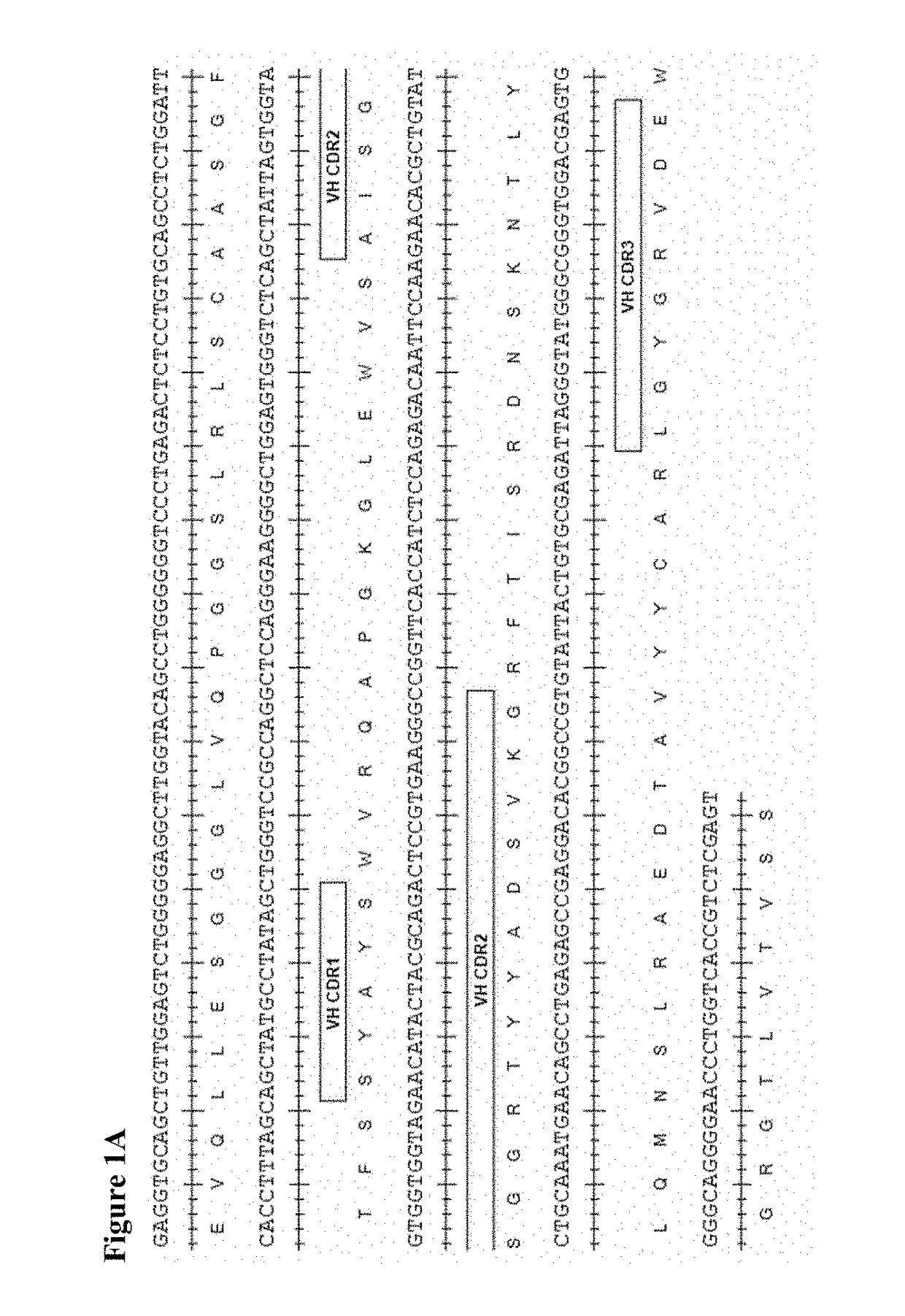
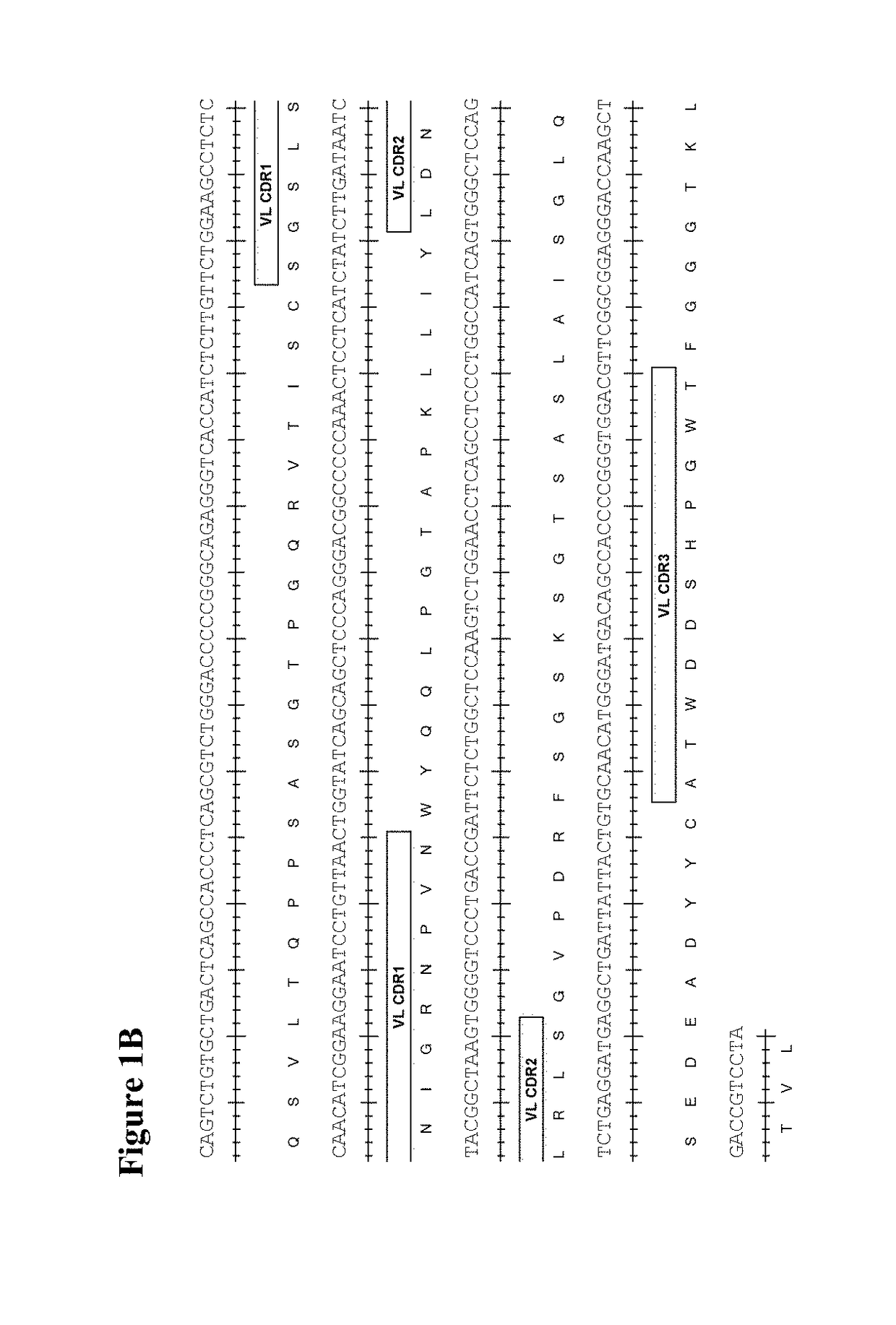
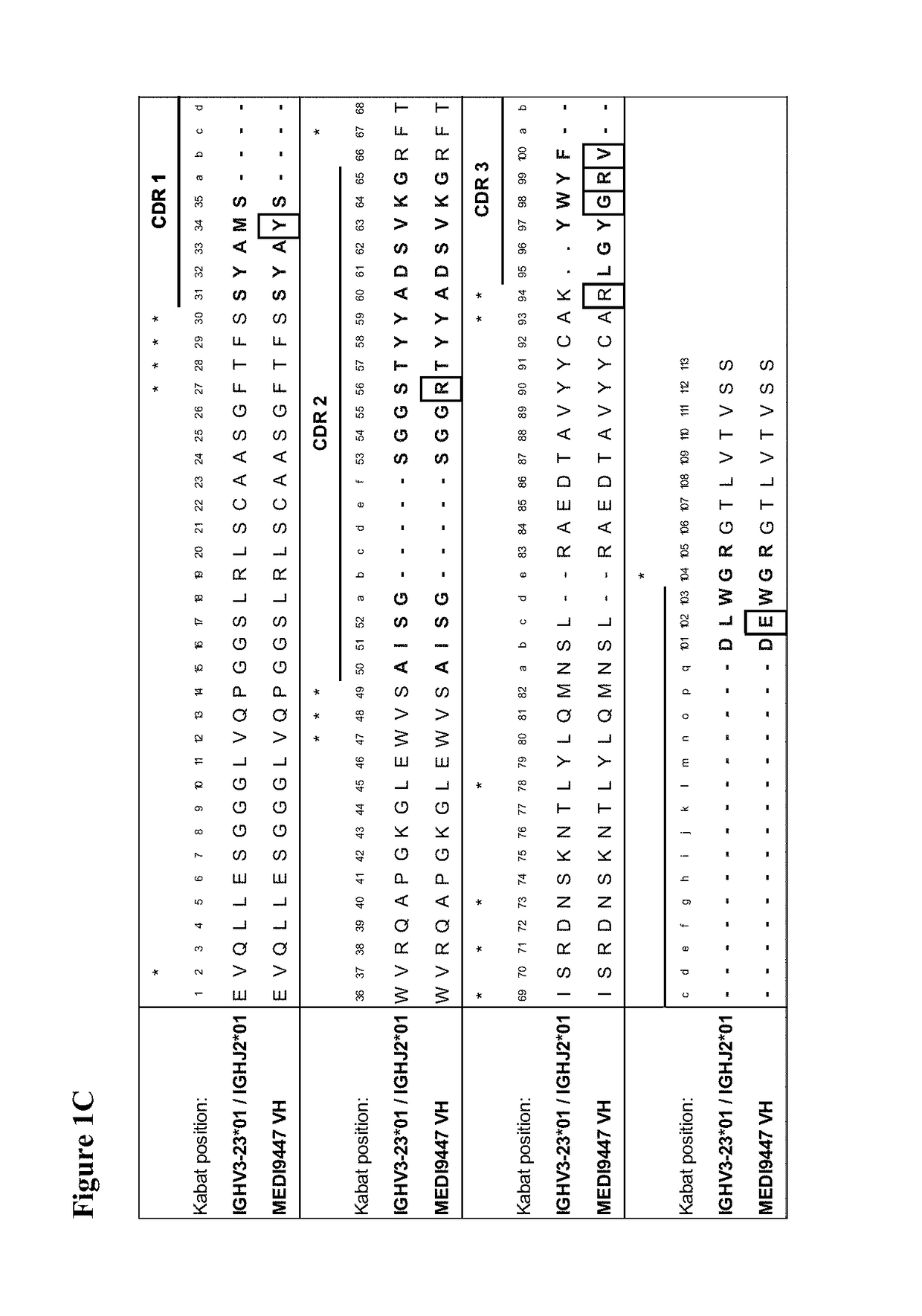
The present invention provides therapeutic combinations featuring anti-CD73 antibodies (e.g., MEDI9447) and A2A receptor inhibitors and methods of using such combinations for reducing tumor-mediated immunosuppression.
Owner:MEDIMMUNE LTD
OVR110 Antibody Compositions and Methods of Use
ActiveUS20100136009A1Suppressed immune functionReduce immunosuppressionNervous disorderTissue cultureCancer cellMammal
Isolated antibodies and antigen binding fragments thereof directed against Ovrl 10 which is expressed by head and neck, ovarian, endometrial, kidney, pancreatic, lung or breast cancer are provided. Also provided are cells and methods for their production as well as methods for their use in killing an Ovrl 10-expressing cancer cells and alleviating or treating an Ovrl 10-expressing cancer in a mammal. The anti-Ovrl 10 antibodies modulate Ovrl 10 function or internalize upon binding to Ovrl 10 expressed by mammalian cells in vitro and in vivo. Compositions comprising an anti-Ovrl 10 antibody and a carrier as well as articles of manufacture or kits thereof are also provided. In addition, isolated nucleic acids encoding an anti-Ovrl 10 antibody, expression vectors containing the isolated nucleic acids, and host cells containing the vectors are provided.
Owner:DIAZYME LAB INC
Inhibition of cxcr4 signaling in cancer immunotherapy
InactiveUS20150352208A1Increases proximity and frequency of T-cellsReduce immunosuppressionOrganic active ingredientsPeptide/protein ingredientsCancer cellCXCR4
Owner:CAMBRIDGE ENTERPRISE LTD
Genetic engineering of macrophages for immunotherapy
ActiveUS20170087185A1Promote growthWide applicabilityAntibacterial agentsHydrolasesWhite blood cellTumor therapy
Disclosed are methods of making a genetically modified immune cell for modifying a tumor microenvironment (TME) and methods of modifying a tumor microenvironment (TME). In some embodiments, the method can include delivering a first vector to an immune cell, wherein the first vector comprises a nucleic acid encoding a protein that induces T-cell proliferation, promotes persistence and activation of endogenous or adoptively transferred NK or T cells and / or induces production of an interleukin, an interferon, a PD-1 checkpoint binding protein, HMGB1, MyD88, a cytokine or a chemokine. Methods of modulating the suppression of the immune response in a tumor microenvironment, minimizing the proliferation of tumor and suppressive cells, and increasing the efficiency of an anti-cancer therapy, anti-infection therapy, antibacterial therapy, anti-viral therapy, or anti-tumoral therapy are also provided.
Owner:SEATTLE CHILDRENS HOSPITAL (DBA SEATTLE CHILDRENS RES INST)
Method of cancer treatment using sirna silencing
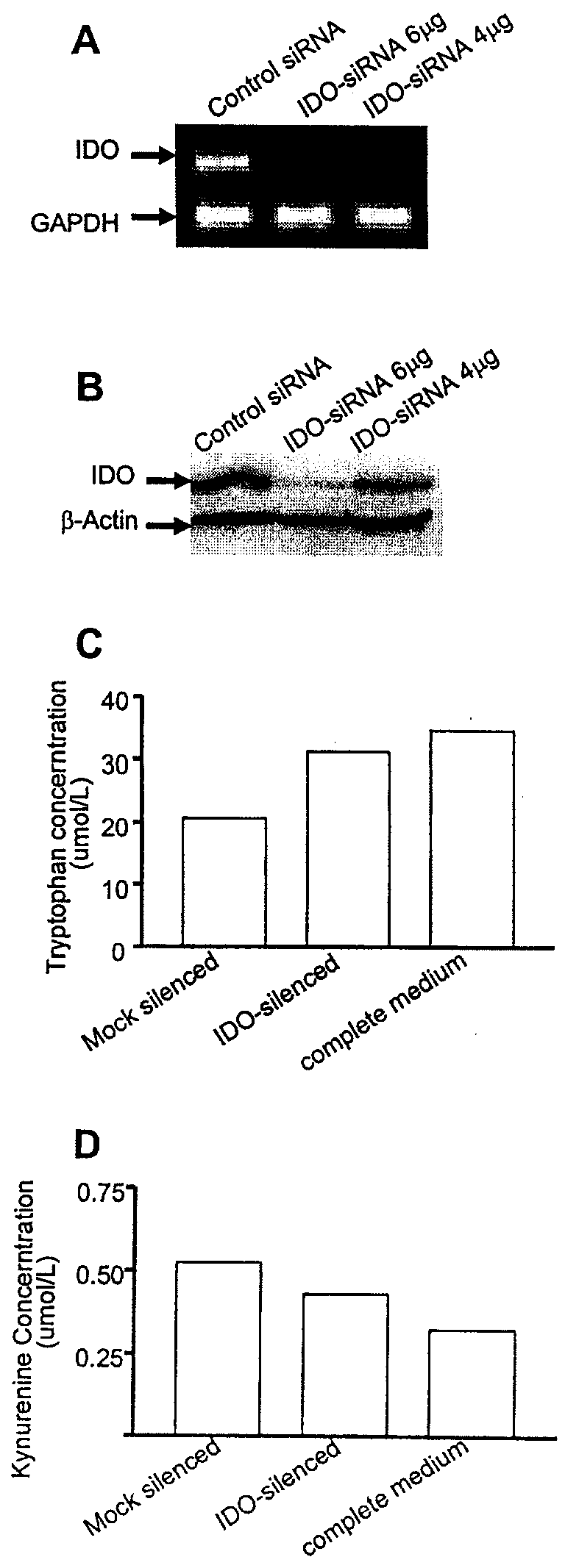
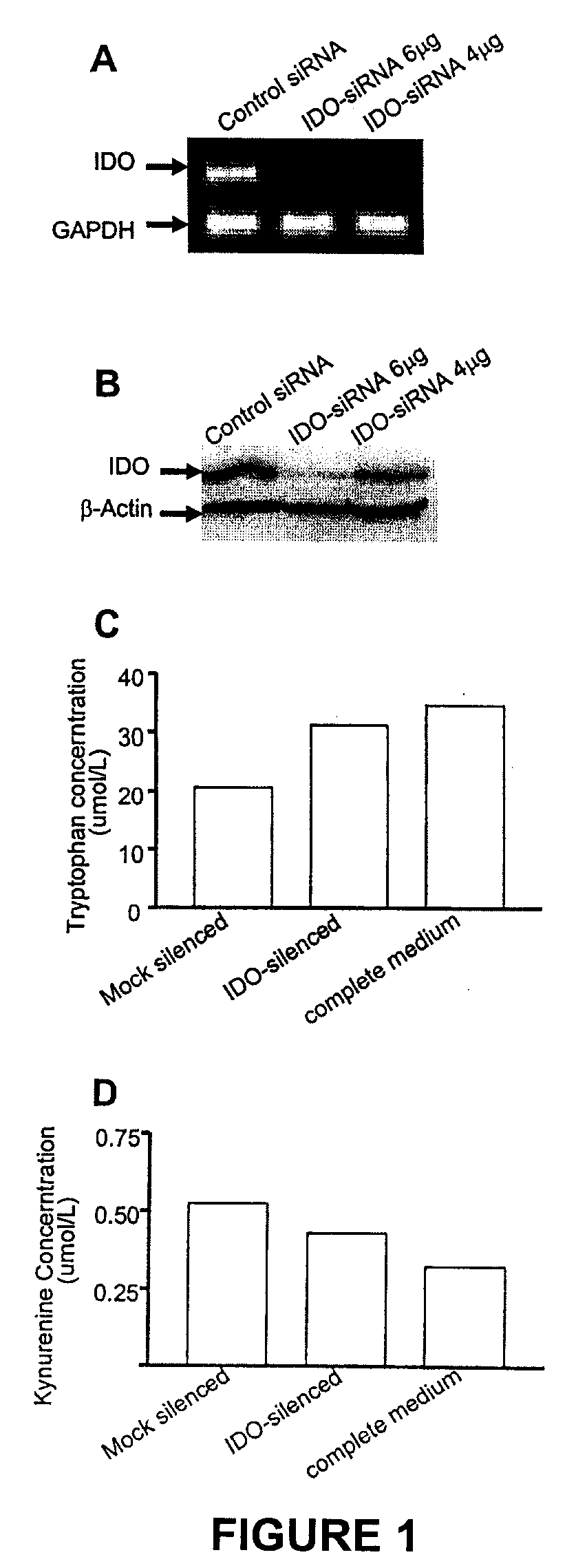
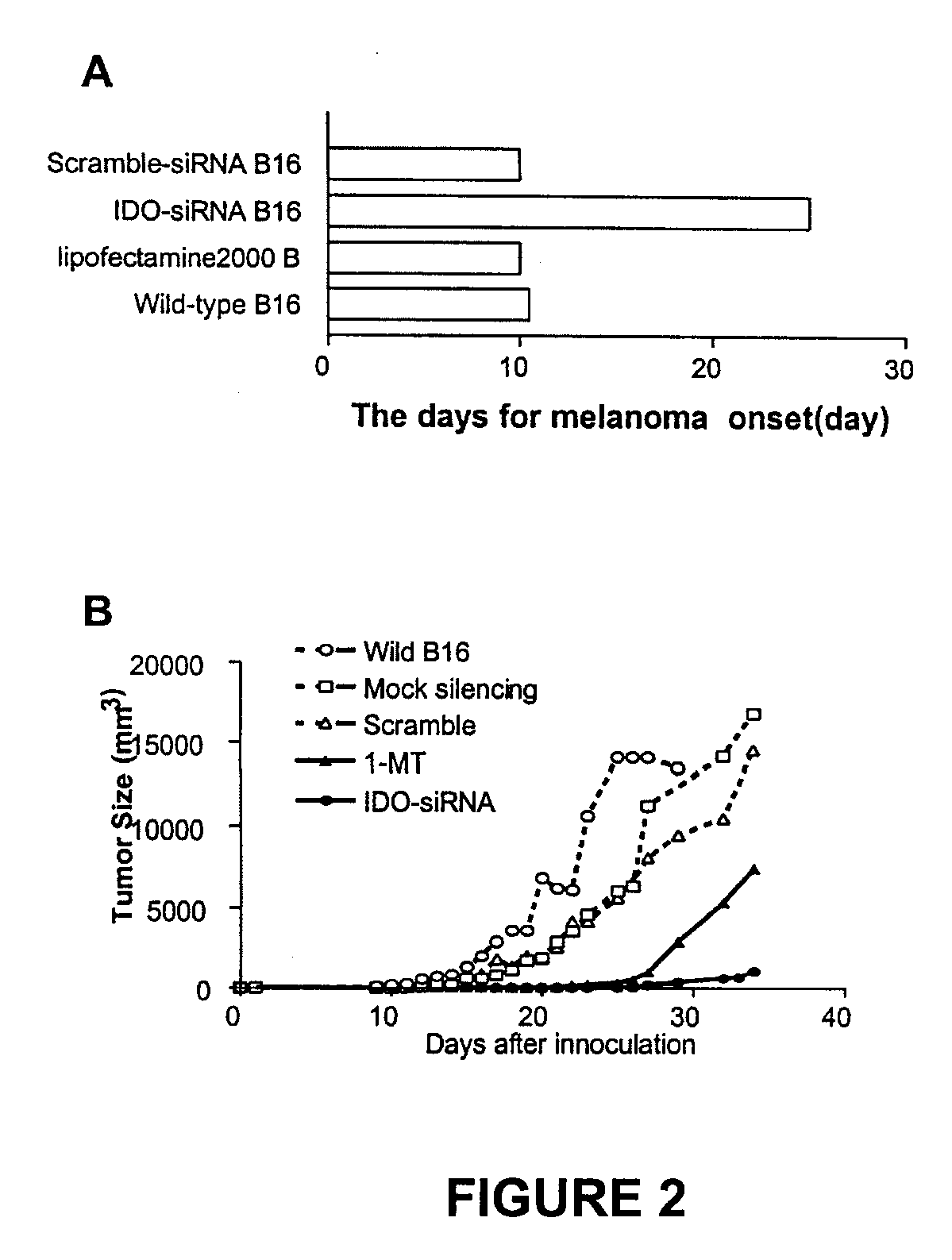
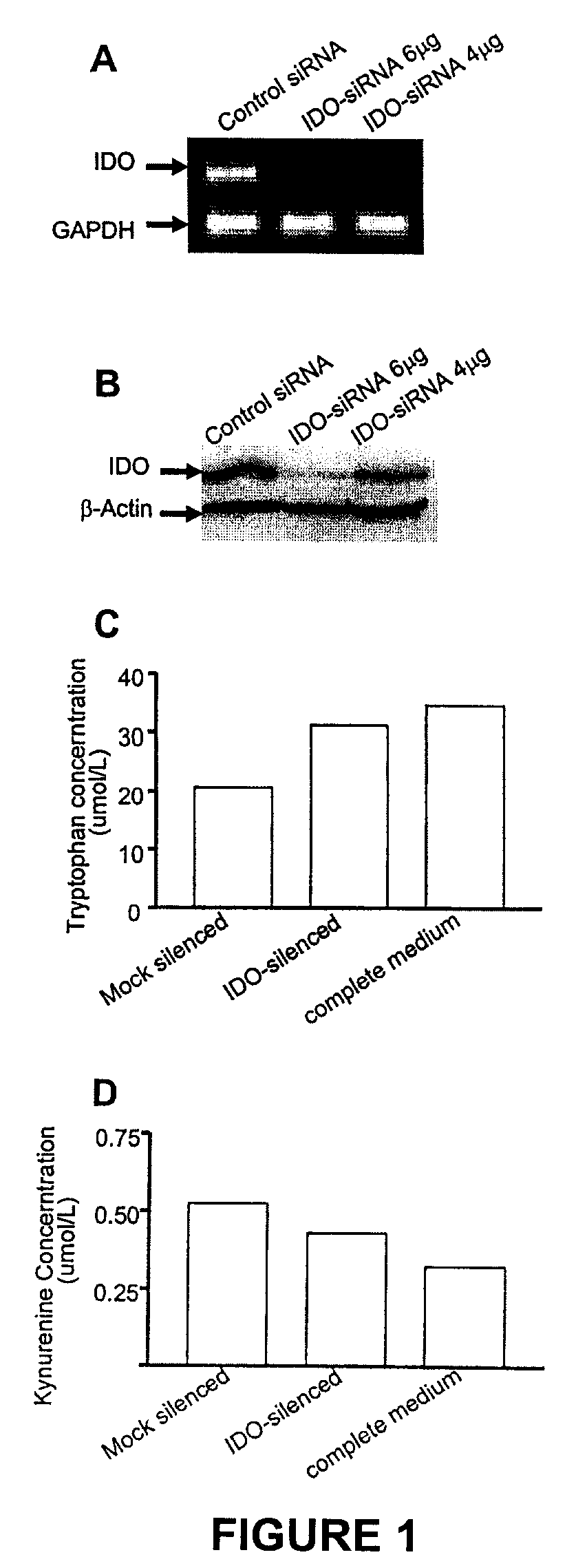
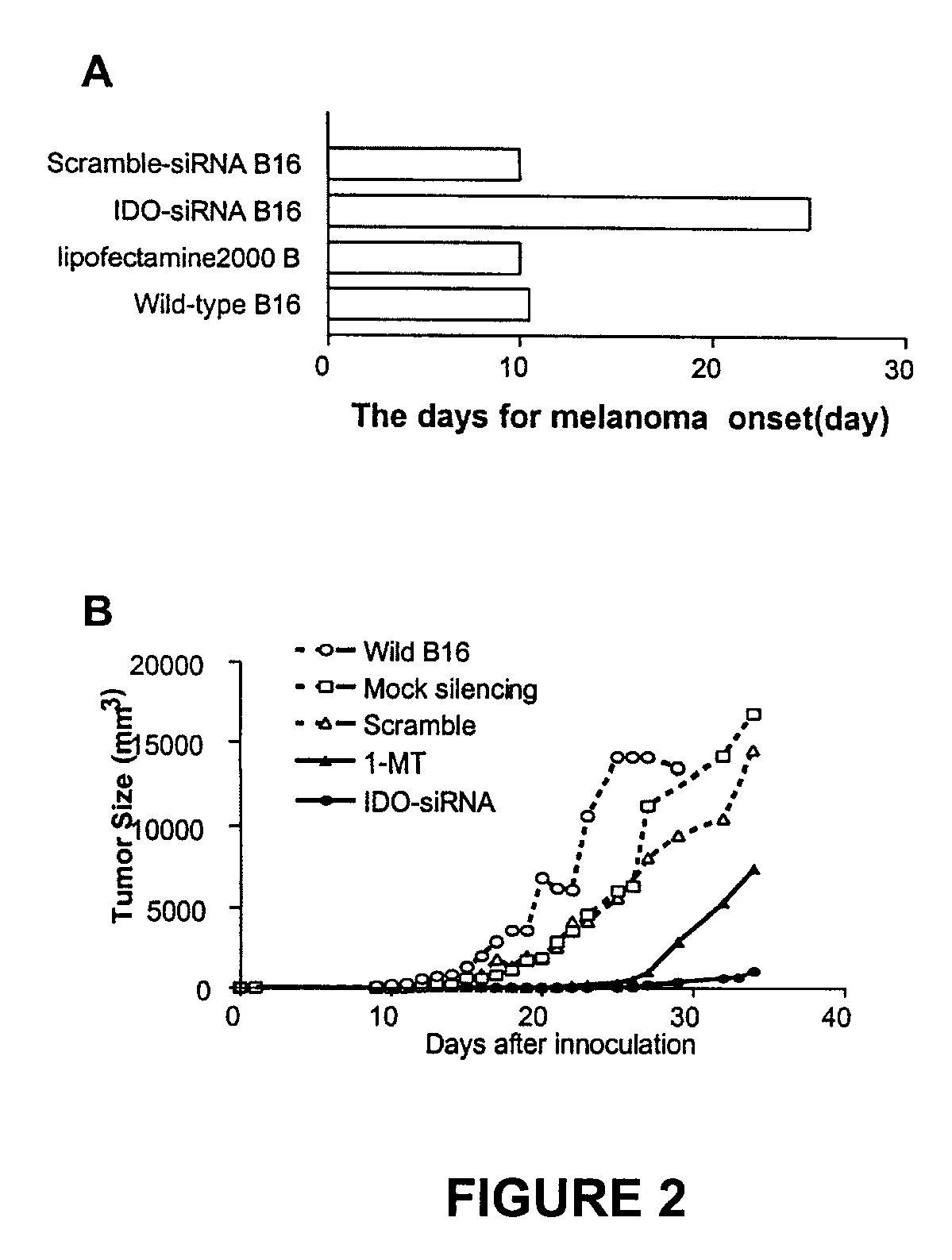
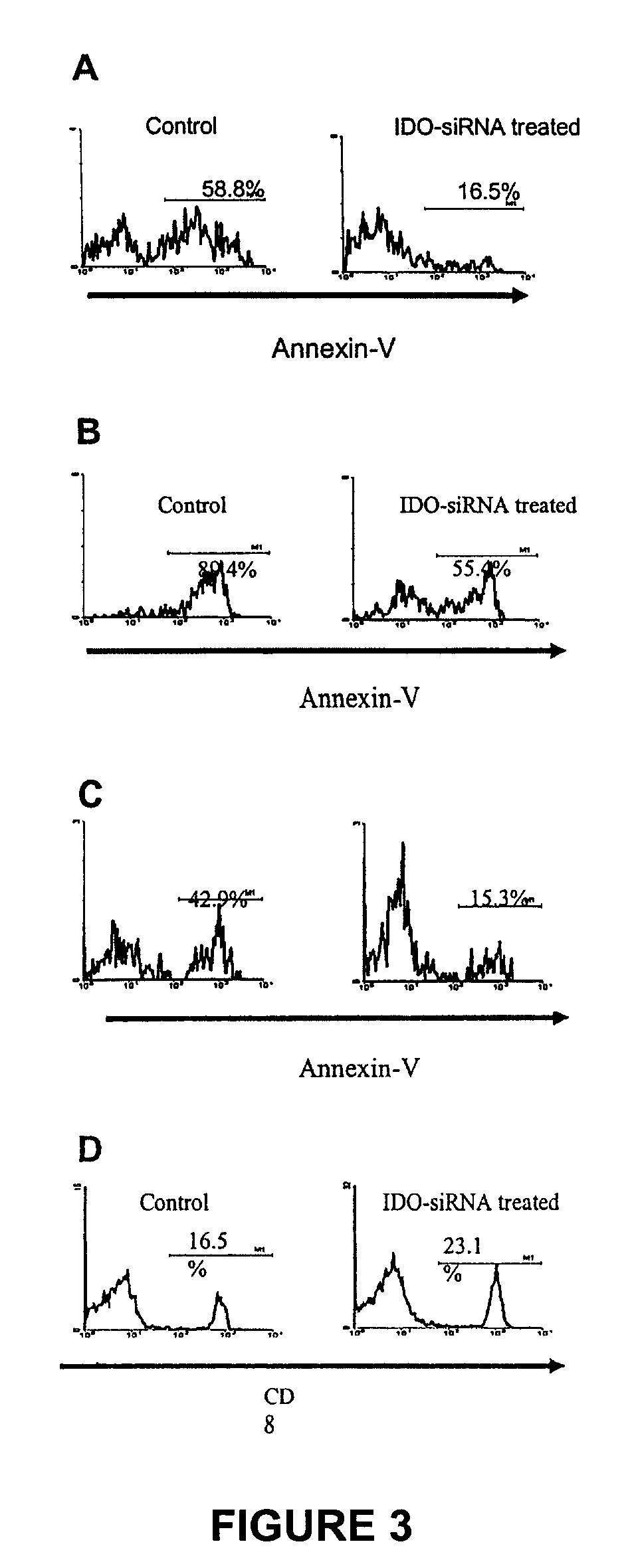
ActiveUS20090220582A1Reduction in IDO expressionDecrease in IDO-directed tumoral immunosuppressionOrganic active ingredientsSugar derivativesSirna silencingWilms' tumor
The present invention is a method for the treatment of cancer involving tumor derived immunosuppression in a subject. The method comprises administering to a subject one or more siRNA constructs capable of inhibiting the expression of an immunosuppressive molecule. The invention also provides siRNA constructs and compositions.
Owner:REGEN BIOPHARMA
Method of cancer treatment using siRNA silencing
ActiveUS8389708B2Inhibit and reduce expressionIncreasing T-cell proliferationOrganic active ingredientsSugar derivativesSirna silencingWilms' tumor
Owner:REGEN BIOPHARMA
Reversal of the suppressive function of specific t cells via toll-like receptor 8 signaling
InactiveUS20080026986A1Improve therapeutic efficacyImprove responseOrganic active ingredientsPeptide/protein ingredientsOligonucleotideTreg cell
CD8+ regulatory T (Treg) cells and γδ Treg cells profoundly suppress host immune responses and thus protect against autoimmune disease while restricting desired immune responses such as antitumor immunity. Synthetic phosphorothioate-protected, guanosine-containing oligonucleotides can directly reverse the suppressive activity of Treg cells without involving dendritic cells. This effect appears to be transduced by signaling through Toll-like receptor (TLR) 8 and engagement of the MyD88 and IRAK4 molecules in Treg cells, in specific embodiments. Stimulation of Treg cells with natural ligands for human TLR8 recapitulated the effect of the synthetic guanosine-containing oligonucleotides.
Owner:BAYLOR COLLEGE OF MEDICINE
Separated recombinant oncolytic adenovirus, drug composition and application of separated recombinant oncolytic adenovirus to drug for treating tumor and/or cancer
ActiveCN109576231AImprove tumor killing abilityLow replication capacitySpecial deliveryMammal material medical ingredientsOncolytic adenovirusPrimary cell
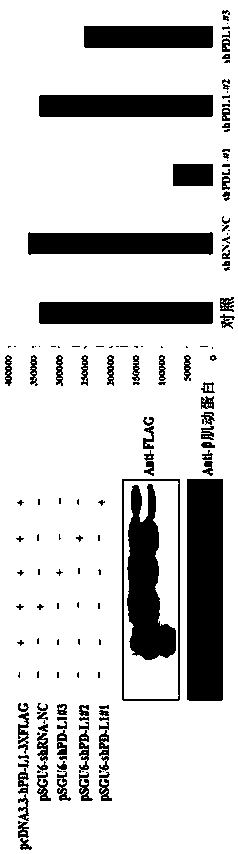
The invention provides a separated recombinant oncolytic adenovirus, a drug composition and application of the separated recombinant oncolytic adenovirus to a drug for treating tumor and / or cancer. The recombinant oncolytic adenovirus is a selective replication type oncolytic adenovirus, and a genome of the recombinant oncolytic adenovirus is integrated with a coded sequence of exogenous shRNA capable of inhibiting PDL1 expression in tumor cells. The replication ability of the separated recombinant oncolytic adenovirus in normal primary cells is far lower than that of the separated recombinantoncolytic adenovirus in the tumor cells, the high-expression PDL1 protein level in the tumor cells can be significantly lowered by expressed shPDL1, and thus the oncolytic killing effect of an oncolytic virus and the antitumor immunostimulation effect of T lymphocyte achieve a synergetic effect.
Owner:BEIJING CONVERD CO LTD
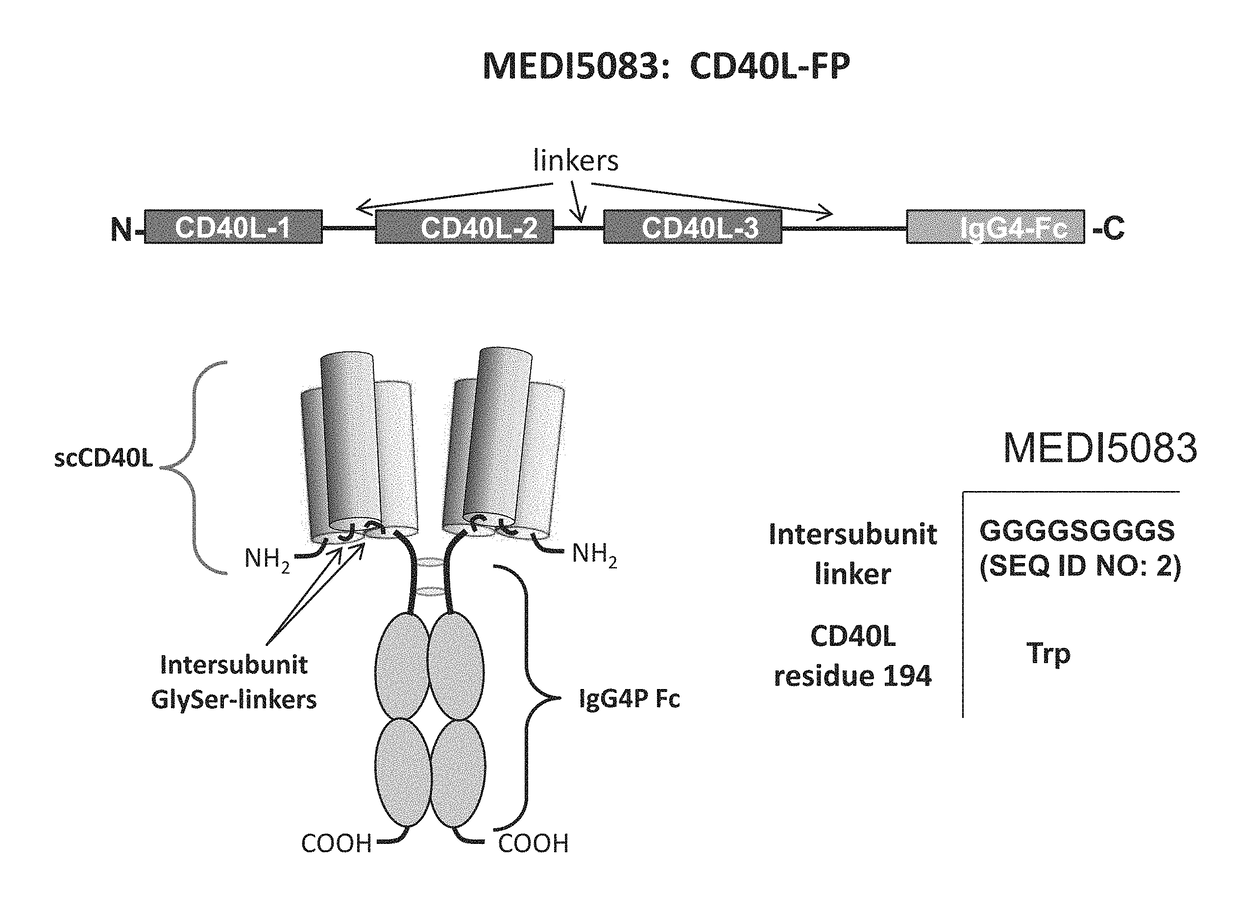
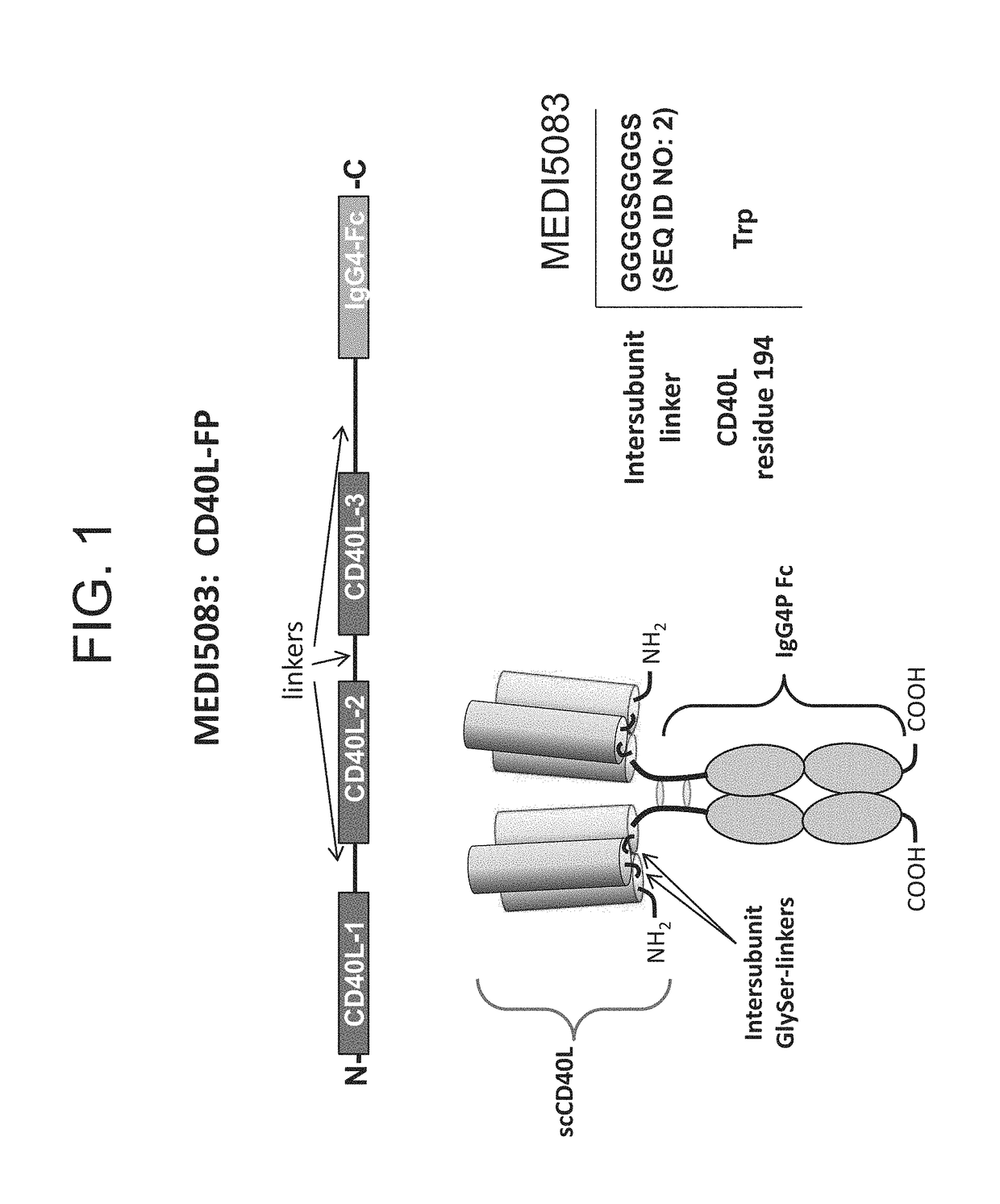
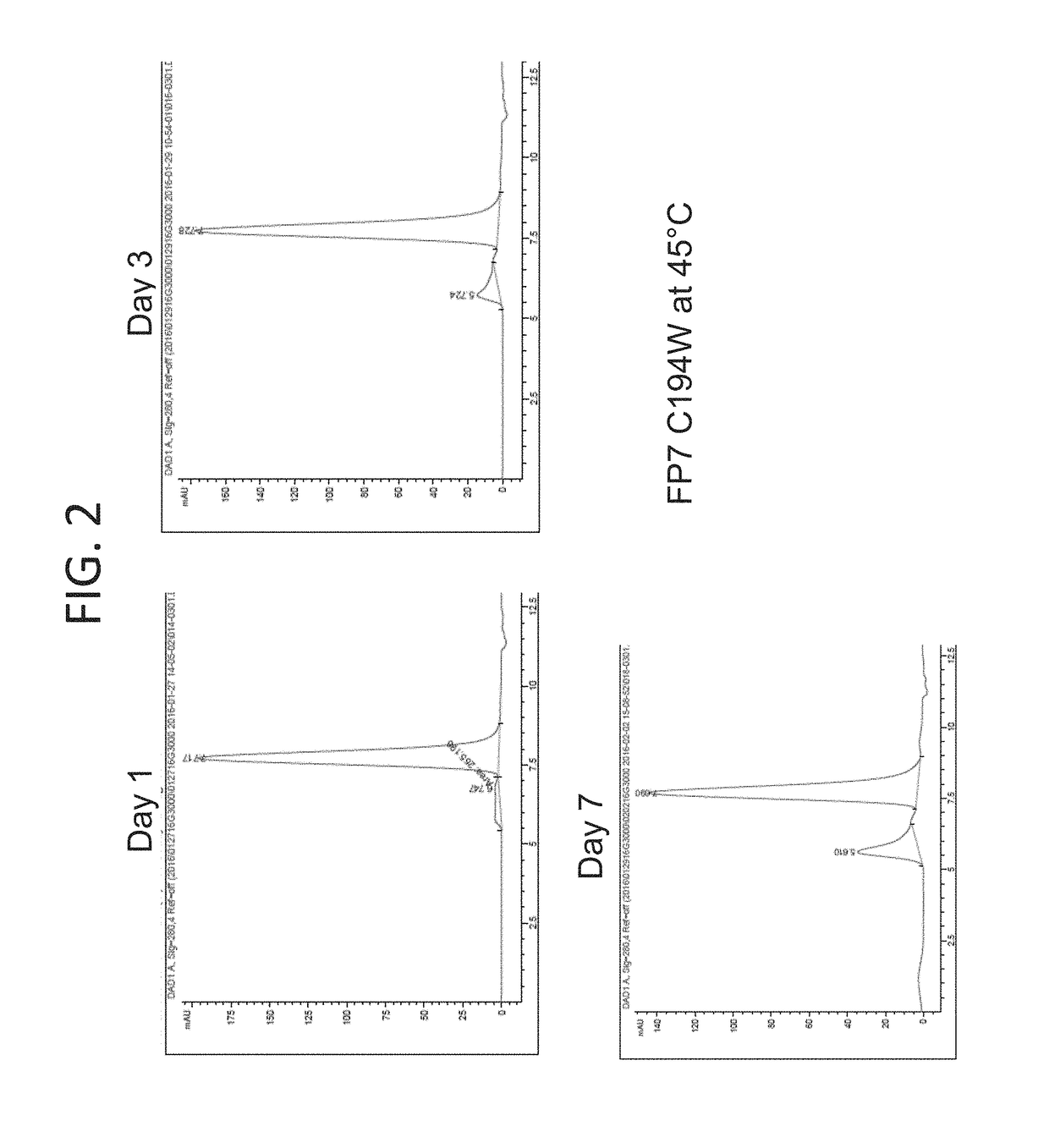
CD40L-Fc Fusion Polypeptides And Methods Of Use Thereof
ActiveUS20170327588A1Enhance immune responseReduce immunosuppressionPeptide/protein ingredientsAntibody mimetics/scaffoldsMedicinePD-L1
Provided herein is a CD40L-Fc fusion protein and methods of using the fusion protein in the treatment of cancer comprising administering the CD40L-Fc fusion protein or the CD40L-Fc fusion protein in combination with one or more immune checkpoint inhibitors (e.g., an anti-CTLA4 antibody, anti-PD-L1 antibody).
Owner:MEDIMMUNE LLC
Ligand polypeptide specifically combined with MDSCs (Myeloid-Derived Suppressor Cells) and drug delivery system
ActiveCN103626846AImprove bindingPromote differentiationEnergy modified materialsGenetic material ingredientsMedicineDrug carrier
The invention discloses a ligand polypeptide specifically combined with MDSCs (Myeloid-Derived Suppressor Cells) and a drug delivery system. The ligand polypeptide specifically combined with MDSCs comprises an amino acid sequence as shown in SEQ ID NO.1. The drug delivery system disclosed by the invention comprises the ligand polypeptide, a drug carrying system and at least one active or developing substance, wherein the drug carrying system is specifically lipidosome; the at least one active substance is any substance which is needed to be delivered to a special in-vivo position. The ligand polypeptide disclosed by the invention has good MDSCs-targeting effect, and can be combined with lipidosome to be used as a targeting drug carrier, so that a targeting drug delivery system for tumors is developed.
Owner:SHANGHAI JIAO TONG UNIV
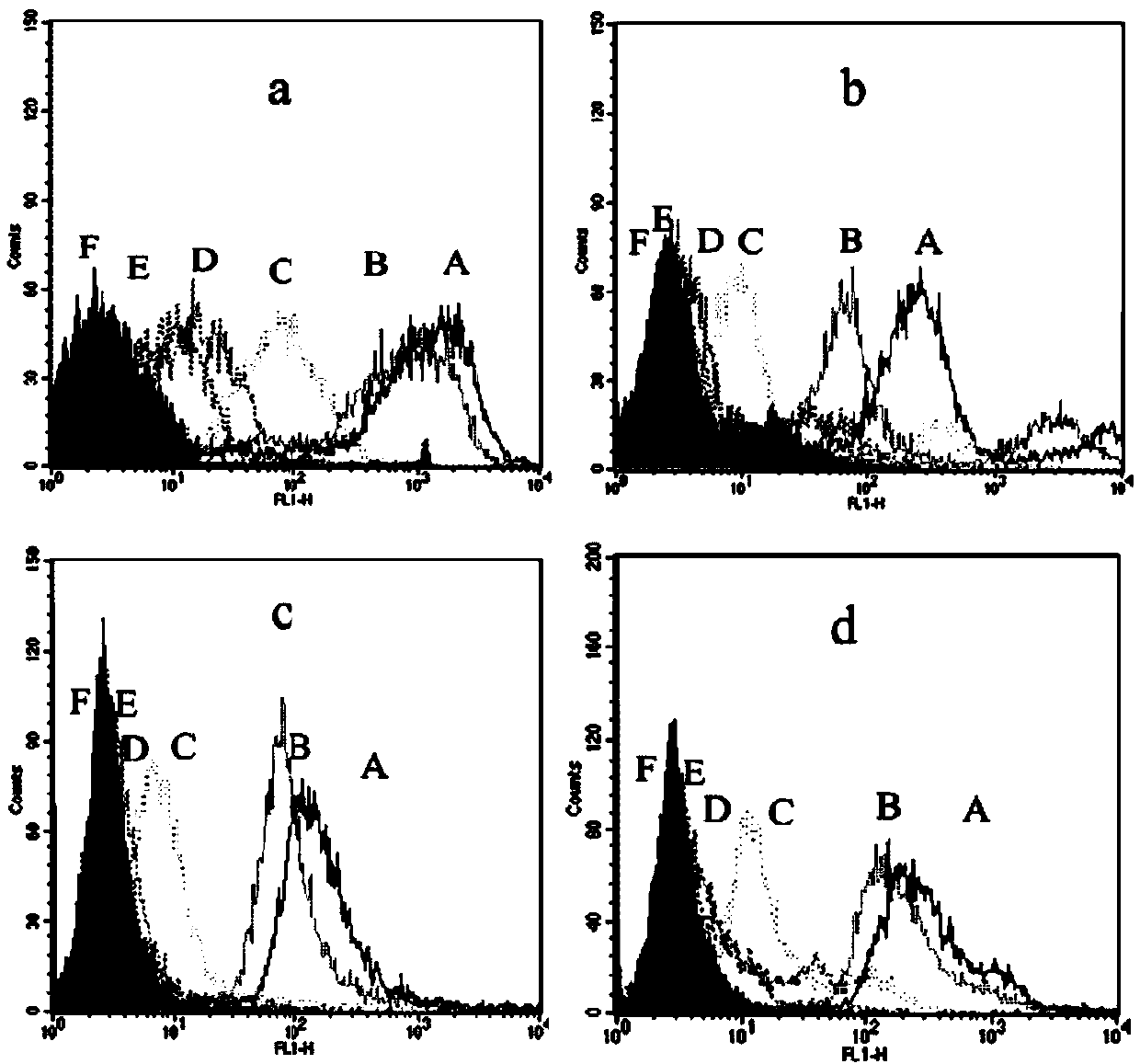
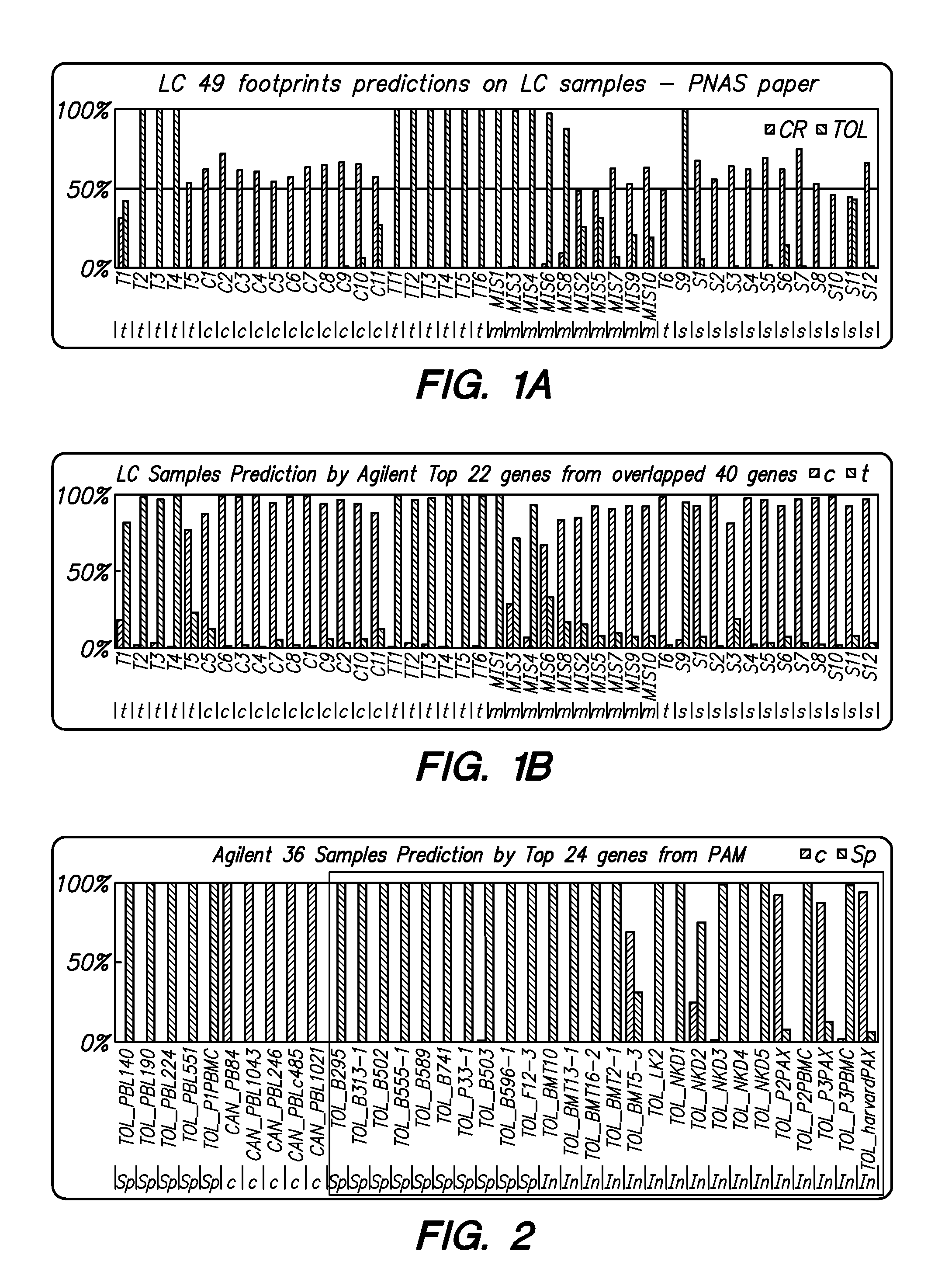
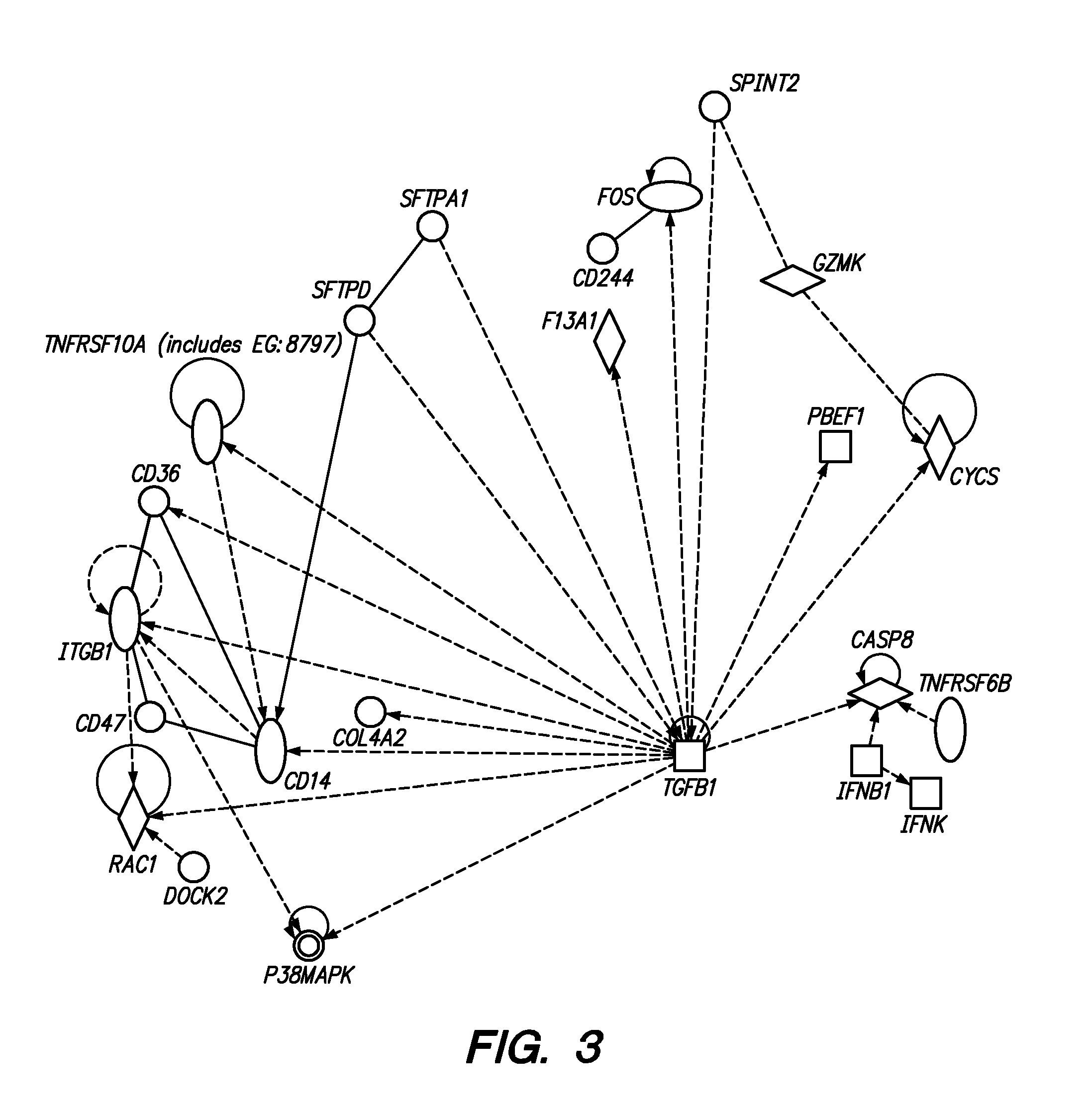
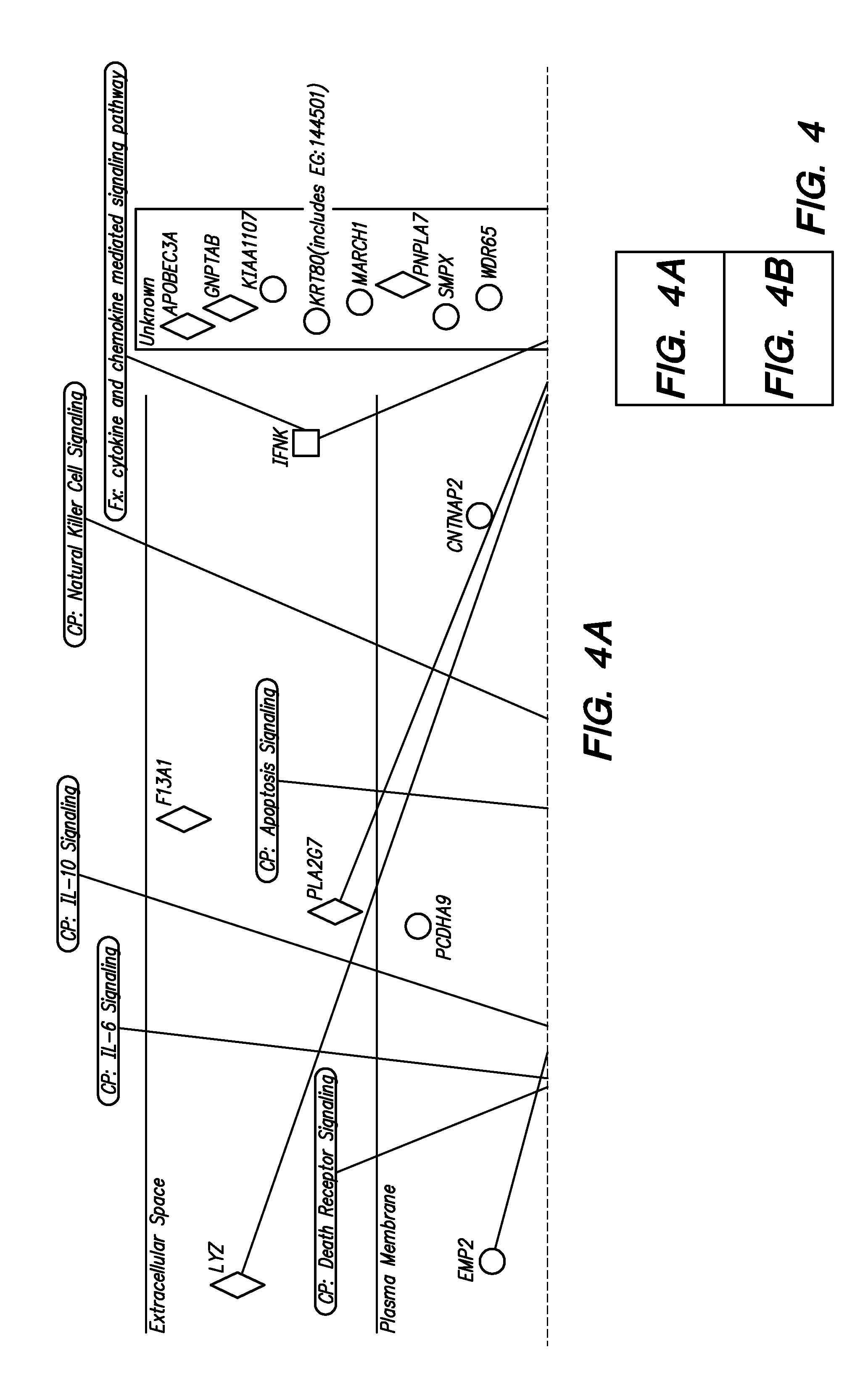
Methods and Compositions for Determining a Graft Tolerant Phenotype in a Subject
InactiveUS20110201519A1Reduce immunosuppressionMicrobiological testing/measurementLibrary screeningGraft ToleranceRegimen
Methods are provided for determining whether a subject has a graft tolerant phenotype. In practicing the subject methods, the expression of at least 5 genes in a sample from the subject, e.g., a blood sample, is assayed to obtain a gene expression result for the at least 5 genes. The obtained gene expression result for the at least 5 genes is then employed to determine whether the subject has a graft tolerant phenotype. Also provided are compositions, systems and kits that find use in practicing the subject methods. The methods and compositions find use in a variety of applications, including the determination of an immunosuppressive therapy regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Ovr 110 Antibody Compositions and Methods of Use
ActiveUS20080199461A1Suppressed immune functionReduce immunosuppressionIn-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenCancer cell
The invention provides isolated anti-head and neck, ovarian, pancreatic, lung, endometrial or breast cancer antigen (Ovr110) antibodies that internalize upon binding to Ovr110 on a mammalian in vivo. The invention also encompasses compositions comprising an anti-Ovr110 antibody and a carrier. These compositions can be provided in an article of manufacture or a kit. Another aspect of the invention is an isolated nucleic acid encoding an anti-Ovr110 antibody, as well as an expression vector comprising the isolated nucleic acid. Also provided are cells that produce the anti-Ovr110 antibodies. The invention encompasses a method of producing the anti-Ovr110 antibodies. Other aspects of the invention are a method of killing an Ovr110-expressing cancer cell by contacting the cancer cell with an anti-Ovr110 antibody and a method of alleviating or treating an Ovr110-expressing cancer in a mammal by administering a therapeutically effective amount of the anti-Ovr110 antibody to the mammal.
Owner:DIAZYME LAB INC
Ovr110 antibody compositions and methods of use
ActiveUS7964195B2Suppressed immune functionReduce immunosuppressionIn-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenCancer cell
The invention provides isolated anti-head and neck, ovarian, pancreatic, lung, endometrial or breast cancer antigen (Ovr110) antibodies that internalize upon binding to Ovr110 on a mammalian in vivo. The invention also encompasses compositions comprising an anti-Ovr110 antibody and a carrier. These compositions can be provided in an article of manufacture or a kit. Another aspect of the invention is an isolated nucleic acid encoding an anti-Ovr110 antibody, as well as an expression vector comprising the isolated nucleic acid. Also provided are cells that produce the anti-Ovr110 antibodies. The invention encompasses a method of producing the anti-Ovr110 antibodies. Other aspects of the invention are a method of killing an Ovr110-expressing cancer cell by contacting the cancer cell with an anti-Ovr110 antibody and a method of alleviating or treating an Ovr110-expressing cancer in a mammal by administering a therapeutically effective amount of the anti-Ovr110 antibody to the mammal.
Owner:DIAZYME LAB INC
Combination therapy with chimeric antigen receptor (CAR) therapies
PendingUS20210213063A1Well clinical effectivenessImprove efficacyOrganic active ingredientsMicrobiological testing/measurementCAR - Chimeric antigen receptorMolecular biology
The invention provides a method of treating an adult subject having a hematological cancer, comprising administering to the subject selected dosage regimens comprising a plurality of immune effector cells expressing a CAR molecule in combination with a BTK inhibitor.
Owner:NOVARTIS AG +1
Agents for treating tumors, use and method thereof
InactiveUS20150344577A1Reduce tumor burdenEnhance anti-tumor immunityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsRadiation therapyTumor cells
The present disclosure provides agents for treating and / or preventing resistance of tumor cells to radiation therapy (RT), the use and relevant method thereof.
Owner:SUZHOU DINGFU BIOTARGET CO LTD
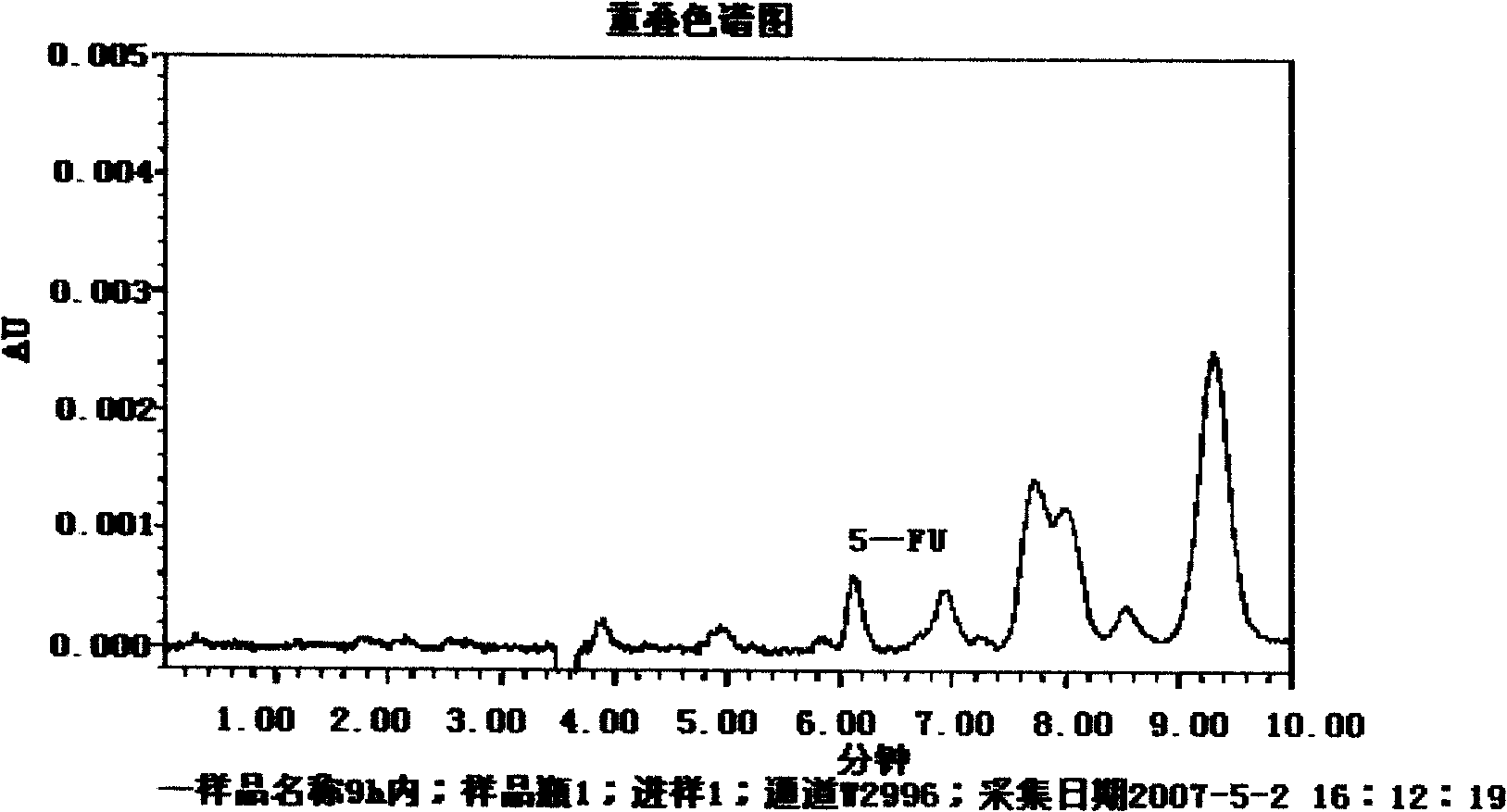
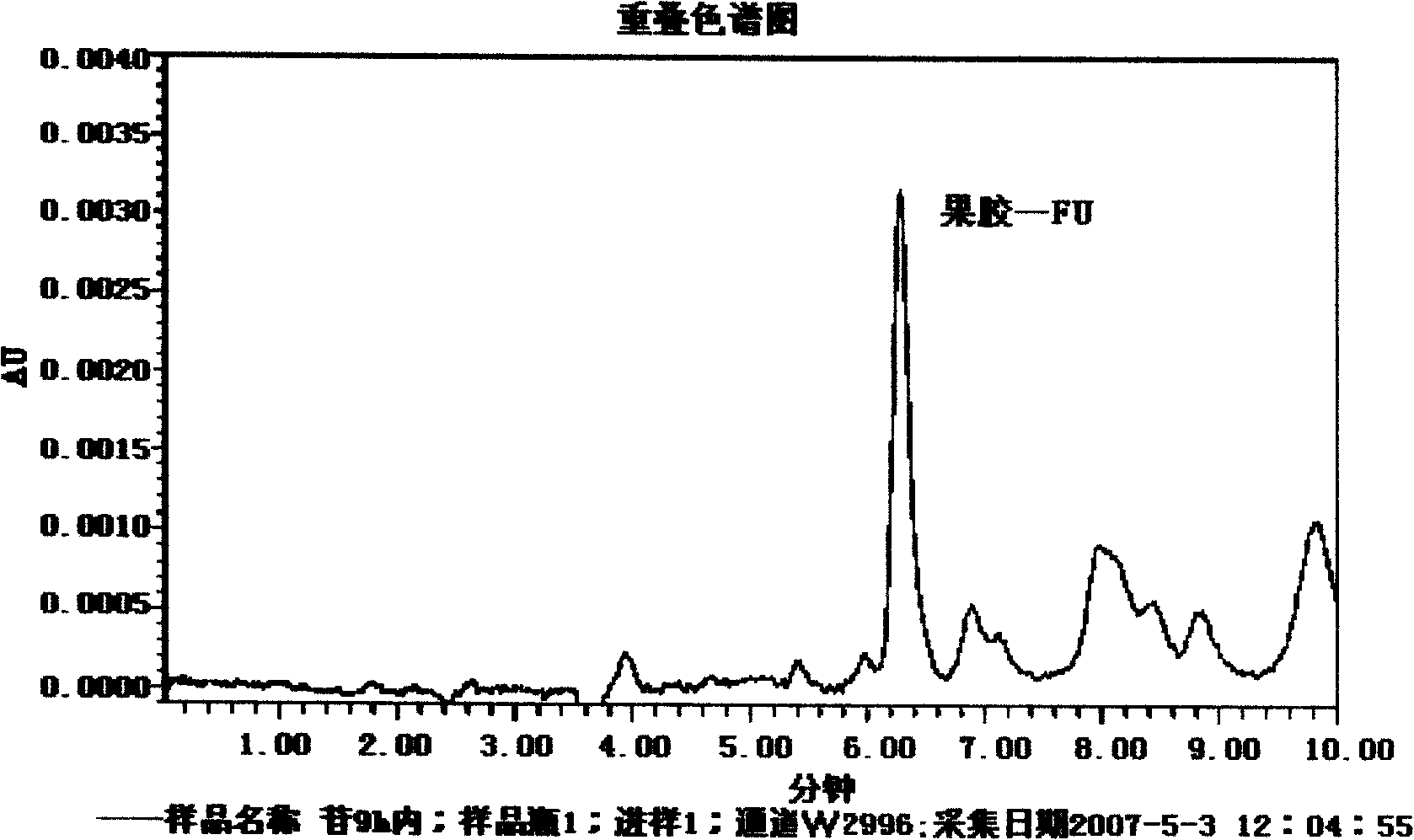
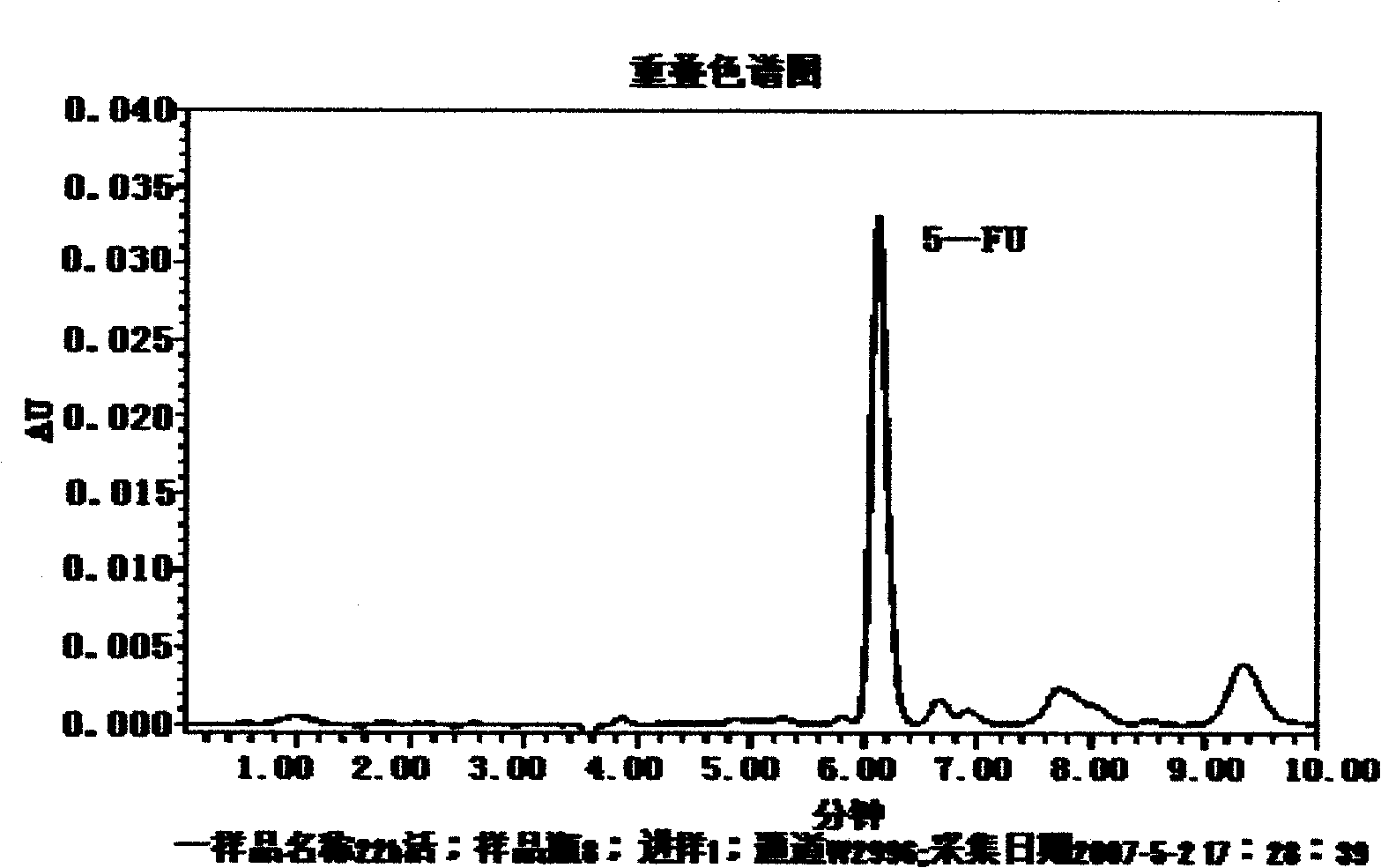
Pectin-5-efudix colon cancer double-target ahead body medicament and preparation method
InactiveCN101269087AGood treatment effectIncrease healing effect, improve selectivityOrganic active ingredientsPharmaceutical non-active ingredientsLymphatic SpreadColon cancer cell
The invention relates to a pectin-5-fluorouracil colon cancer two-targeting prodrug and the preparation method thereof, and mainly synthesizes a two-targeting prodrug for colon cancer by utilizing an anti-cancer drug, 5-fluorouracil (5-FU), and pectin. The two-targeting prodrug for colon cancer is used for treating colon cancer and is characterized in that the 6-digit carboxyl is utilized to be combined with 5-FU directly or through different bridging groups to synthesize a series of two-targeting prodrugs for colon cancer. The prodrug first targets 5-FU to the colon by utilizing pectin to realize colon positioned release, and then 5-FU-galactose is identified through high expression of colon cancer galactin-3, and then 5-Fu is targeted to the colon cancer cells to realize two-targeting of colon cancer to treat colon cancer. The prodrug improves the selectivity of 5-FU greatly, enhances the curative effect and reduces adverse reactions. In addition, the hydrolysis fragments of pectin play a role in resisting tumor metastasis and can have a synergic action with 5-FU. The pectin-5-fluorouracil two-targeting prodrug for colon cancer focuses on the drug design ideas of drug delivery, targeting and coordination; therefore, the prodrug achieves the goal of high selectivity, high efficiency and low toxicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Therapeutic agents comprising isolated recombinant oncolytic adenoviruses and immune cells and uses thereof
PendingCN111743923AImprove tumor killing abilityLow replication capacityMammal material medical ingredientsGenetic engineeringOncolytic adenovirusAntigen receptors
The present invention provides a therapeutic agent comprising isolated recombinant oncolytic adenoviruses and immune cells and uses thereof. The therapeutic agent comprises: a tfirst composition, wherein the first composition comprises a first active ingredient located in a first pharmaceutically acceptable carrier, wherein the first active component comprises an isolated recombinant oncolytic adenovirus, the recombinant oncolytic adenovirus is a selective replication type oncolytic adenovirus, and a coding sequence of exogenous shRNA capable of inhibiting PDL1 expression in tumor cells is integrated in a genome of the recombinant oncolytic adenovirus; and a second composition wherein the second composition comprises a second active ingredient located in a second pharmaceutically acceptable carrier, the second active ingredient comprising T cell receptor modified immune cells or chimeric antigen receptor modified immune cells. According to the invention, the oncolytic adenovirus and the immune cells are combined for use to realize a synergistic treatment effect, and the administration mode enriches the treatment mode and treatment means of tumor treatment.
Owner:BEIJING CONVERD CO LTD
Oncolytic adenoviruses coding for bi-specific antibodies and methods and uses related thereto
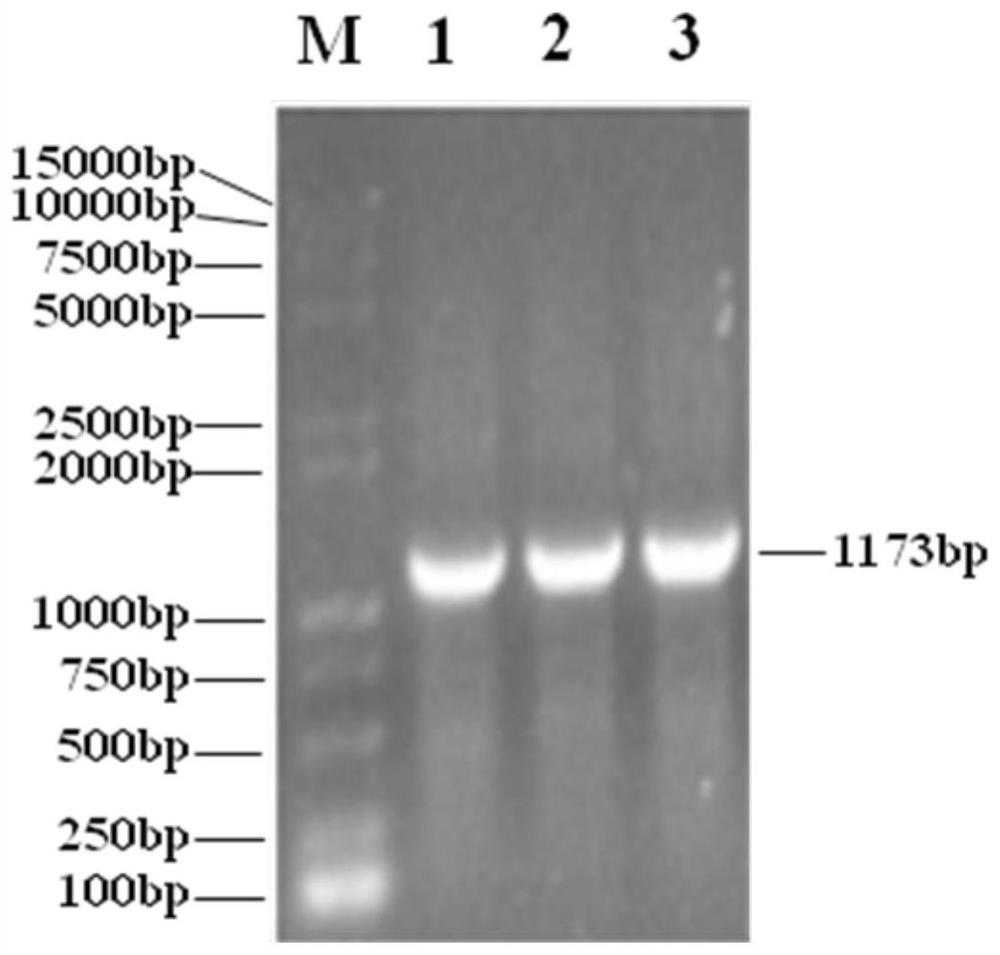
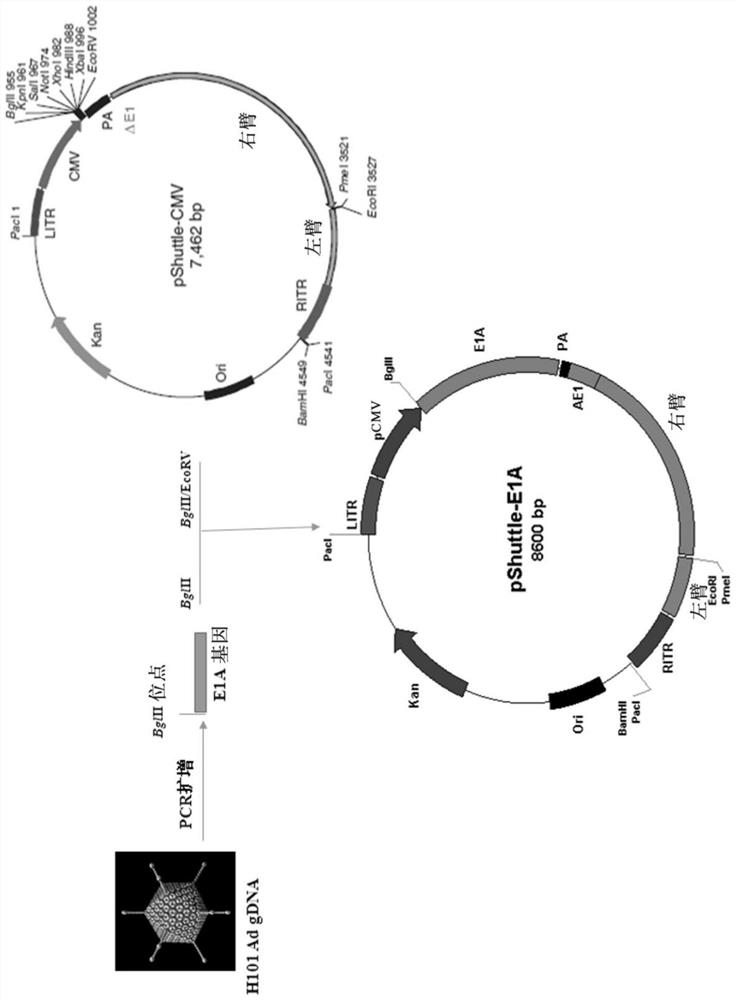
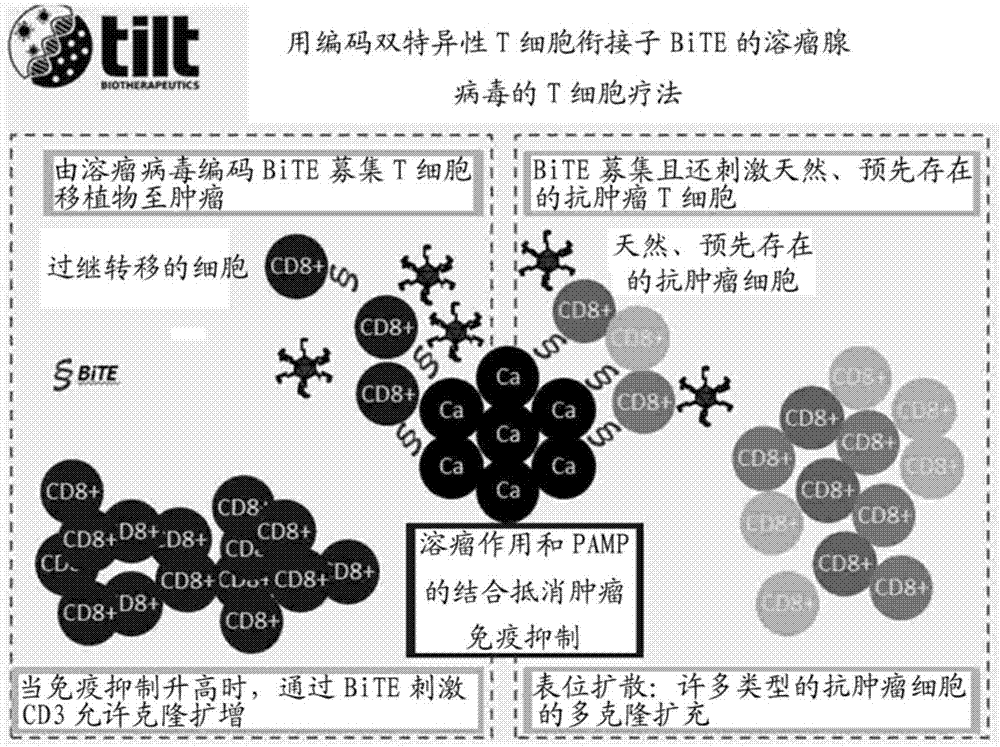
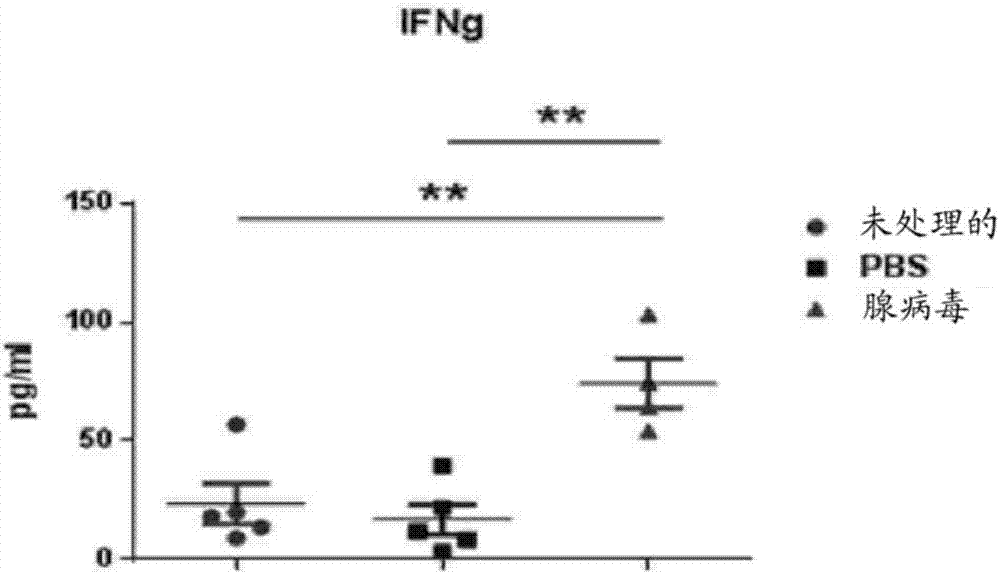
PendingCN107406859AIncrease useReduce immunosuppressionImmunoglobulins against cell receptors/antigens/surface-determinantsUnknown materialsAdoptive cellular therapyAntiendomysial antibodies
The present invention relates to the fields of life sciences and medicine. Specifically, the invention relates to cancer therapies of humans. More specifically, the present invention relates to an oncolytic adenoviral vector encoding a bispecific monoclonal antibody. Furthermore, the present invention relates to methods and uses utilizing the oncolytic adenoviral vectors, also together with adoptive cell therapies.
Owner:TILT BIOTHERAPEUTICS OY
Method for constructing pseudorabies virus gene-deleted attenuated strain and application of attenuated strain
ActiveCN111635891AReduce immunosuppressionViral antigen ingredientsTransferasesDeletion mutationPseudorabies
The invention provides a method for constructing a pseudorabies virus gene-deleted attenuated strain, a recombinant virus constructed by the method and application. According to the method, a pseudorabies virus gene-deleted attenuated strain is constructed by utilizing a CRISPR / Cas9 gene editing technology, and the pseudorabies virus gene-deleted attenuated strain constructed by the method is subjected to specific deletion mutation at a UL13 gene part. The expression quantity of the I-type IFN gene induced by the obtained pseudorabies virus gene-deleted attenuated strain is remarkably increased, a stronger natural immune reaction can be generated, and the replication of the virus is not remarkably different. Therefore, the UL13 gene deletion mutation can significantly reduce immunosuppression caused by the pseudorabies virus, and has important potential application value.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Therapeutic combinations comprising Anti-cd73 antibodies and uses thereof
ActiveUS20180125973A1Reduce immunosuppressionReduce transferOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsWilms' tumorAntibody
The present invention provides therapeutic combinations featuring anti-CD73 antibodies (e.g., MED19447) and A2A receptor inhibitors and methods of using such combinations for reducing tumor-mediated immunosuppression.
Owner:MEDIMMUNE LTD
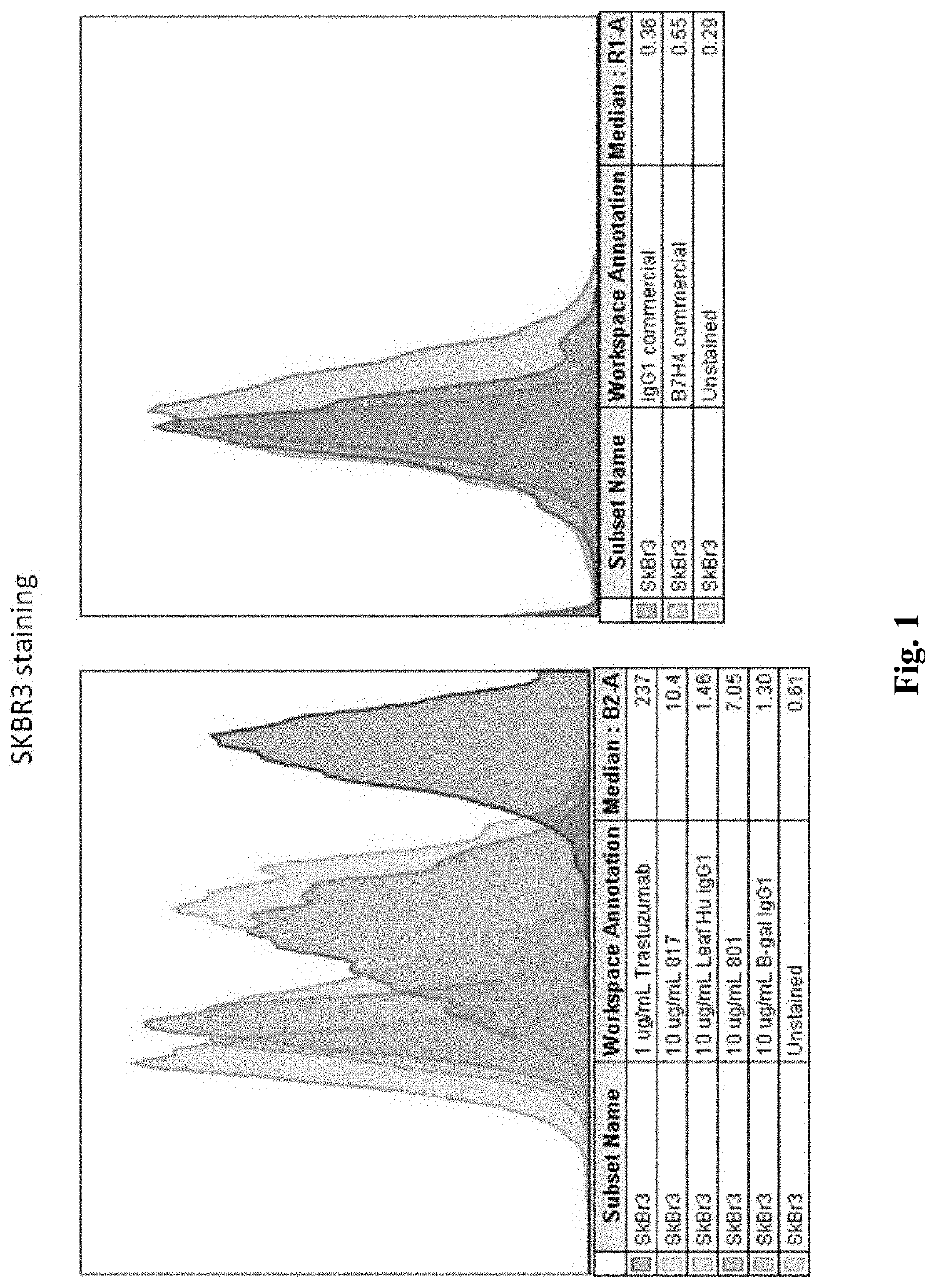
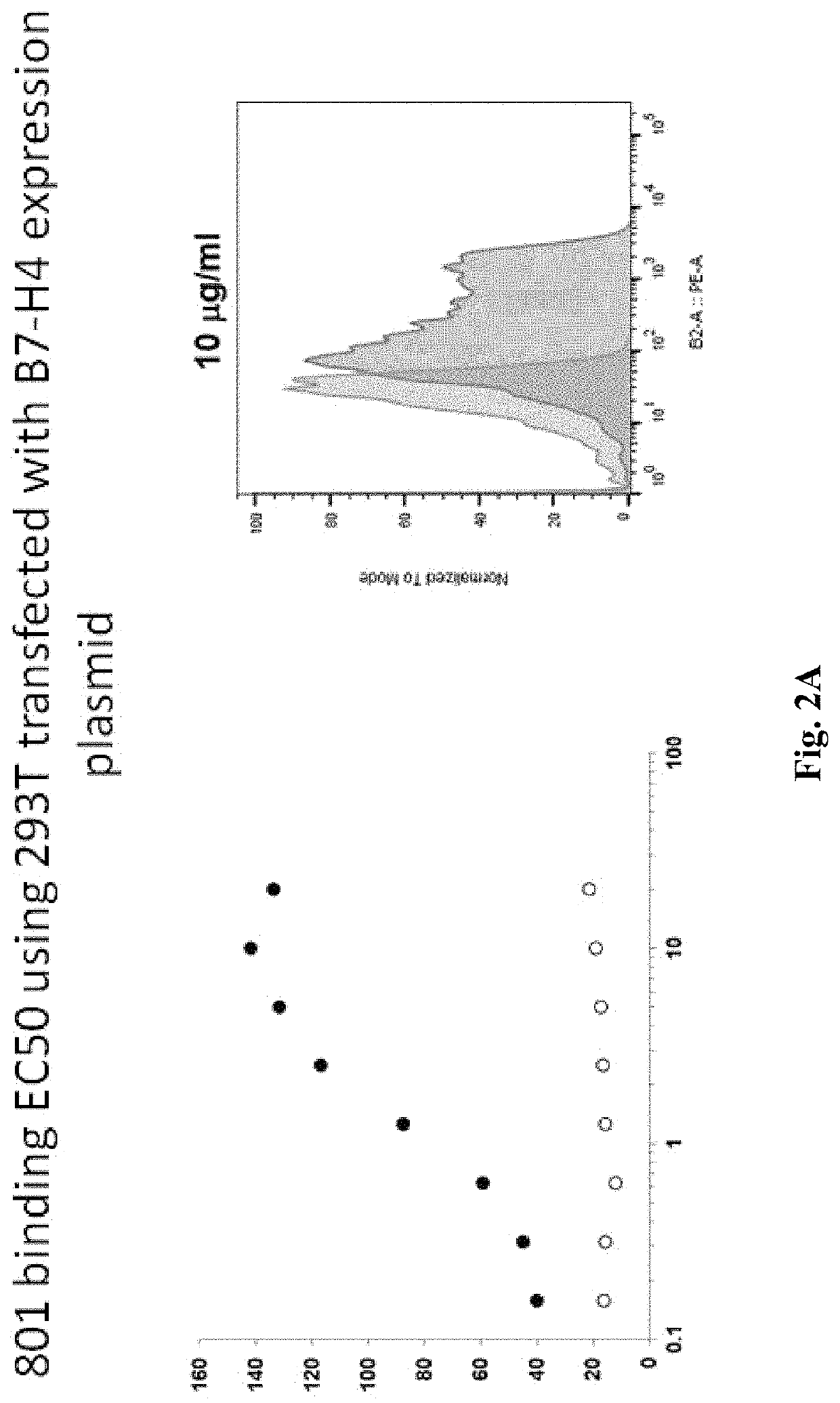
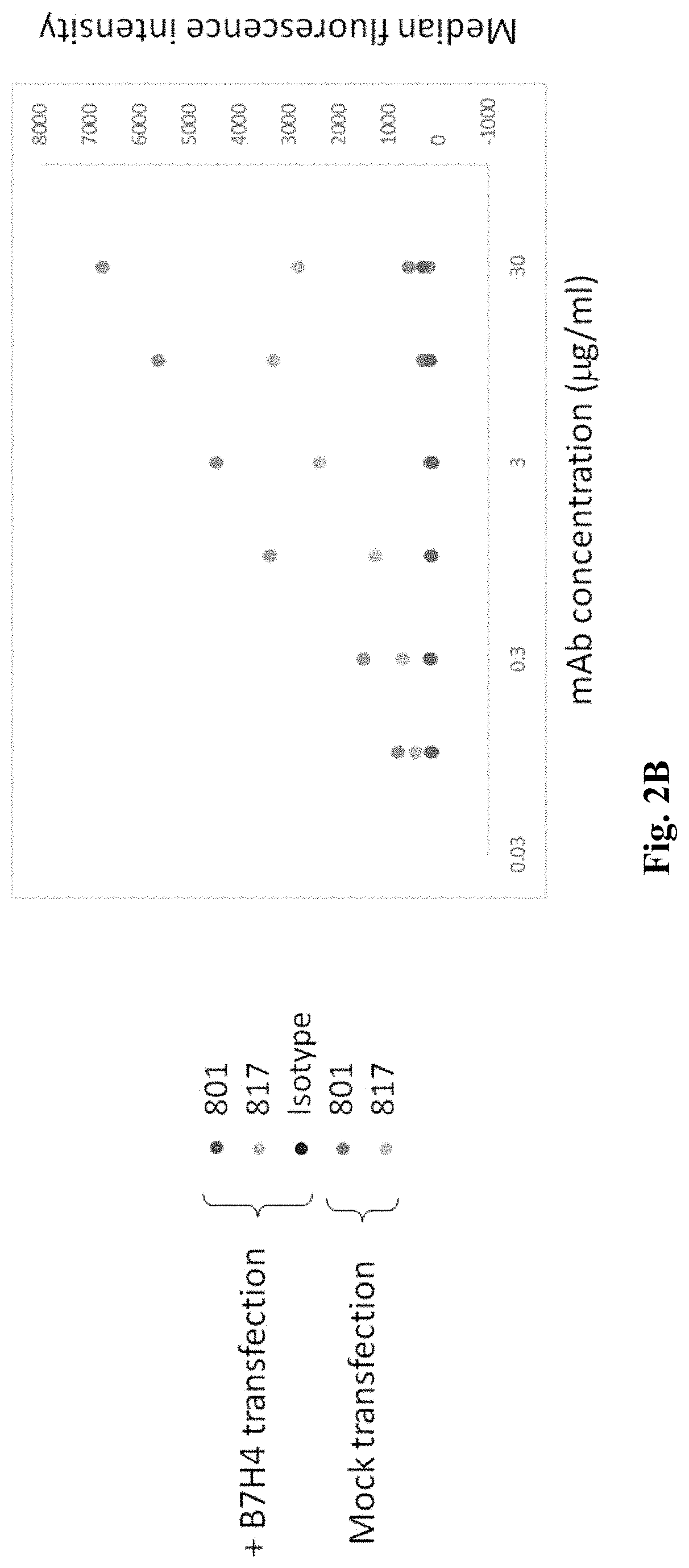
Anti-B7-H4 antibodies and methods
ActiveUS10814011B1Reduce immunosuppressionImprove efficiencyPolypeptide with localisation/targeting motifImmunoglobulins against cytokines/lymphokines/interferonsAntiendomysial antibodiesActivation cells
Compositions, methods, and uses of recombinant B7-H4 protein or binding motifs to B7-H4 protein that can reduce the immune suppression effect of B7-H4 in relation to T cell activation, proliferation, and conversion. Preferably, the recombinant B7-H4 protein has a plurality of mutations in N-glycosylation residue to reduce its inhibitory effect to T cell activation and proliferation. The recombinant protein having binding motifs to B7-H4 protein preferably includes a binding motif to IgC domain and another binding motif to IgV domain such that the immune-suppressive effects by two Ig-like domains are concurrently reduced.
Owner:NANTBIO INC
OVR110 antibody compositions and methods of use
ActiveUS8444971B2Suppressed immune functionReduce immunosuppressionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellAntigen Binding Fragment
Isolated antibodies and antigen binding fragments thereof directed against Ovr110 which is expressed by head and neck, ovarian, endometrial, kidney, pancreatic, lung or breast cancer are provided. Also provided are cells and methods for their production as well as methods for their use in killing an Ovr110-expressing cancer cells and alleviating or treating an Ovr110-expressing cancer in a mammal. The anti-Ovr110 antibodies modulate Ovr110 function or internalize upon binding to Ovr110 expressed by mammalian cells in vitro and in vivo. Compositions comprising an anti-Ovr110 antibody and a carrier as well as articles of manufacture or kits thereof are also provided. In addition, isolated nucleic acids encoding an anti-Ovr110 antibody, expression vectors containing the isolated nucleic acids, and host cells containing the vectors are provided.
Owner:DIAZYME LAB INC
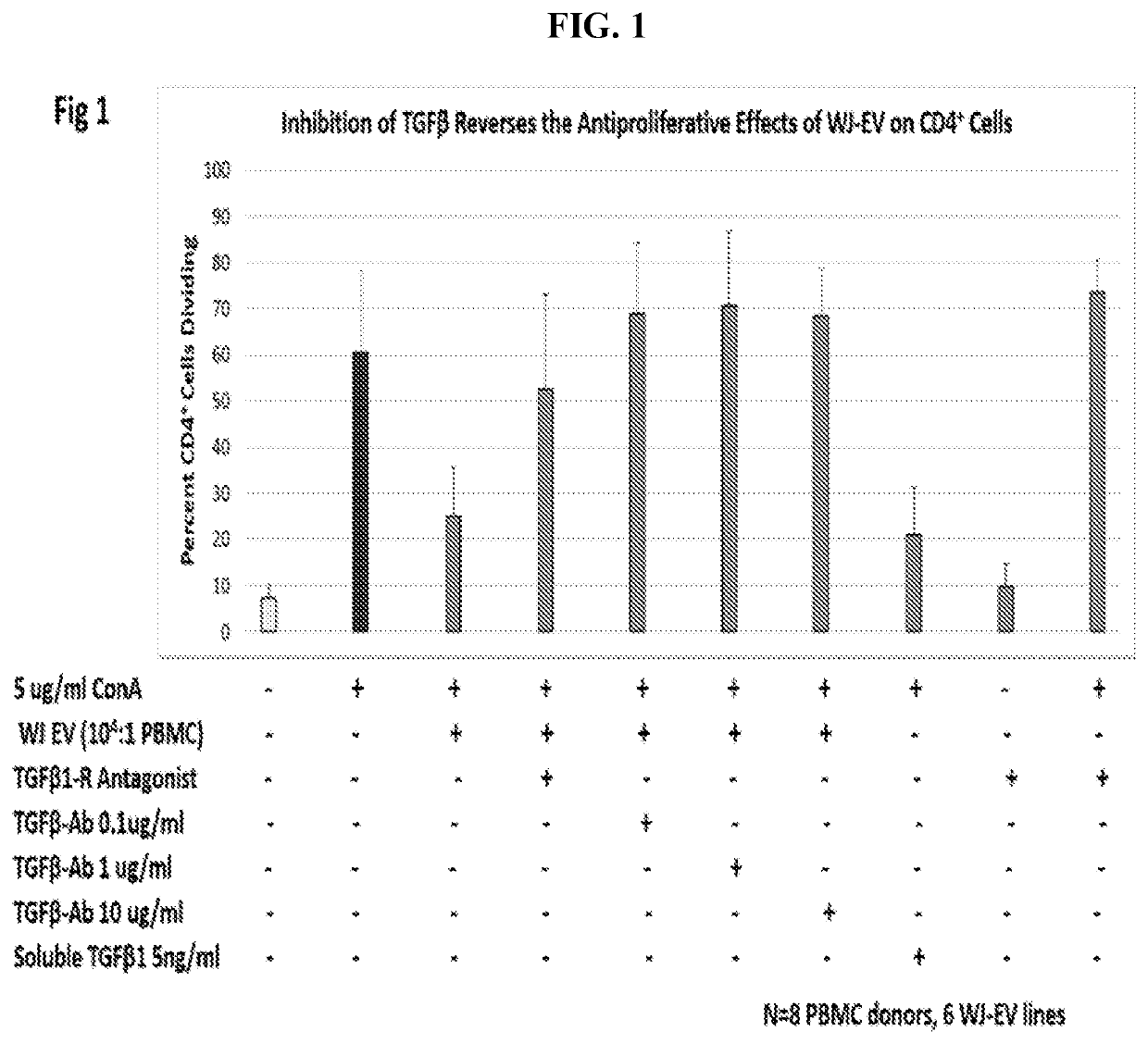
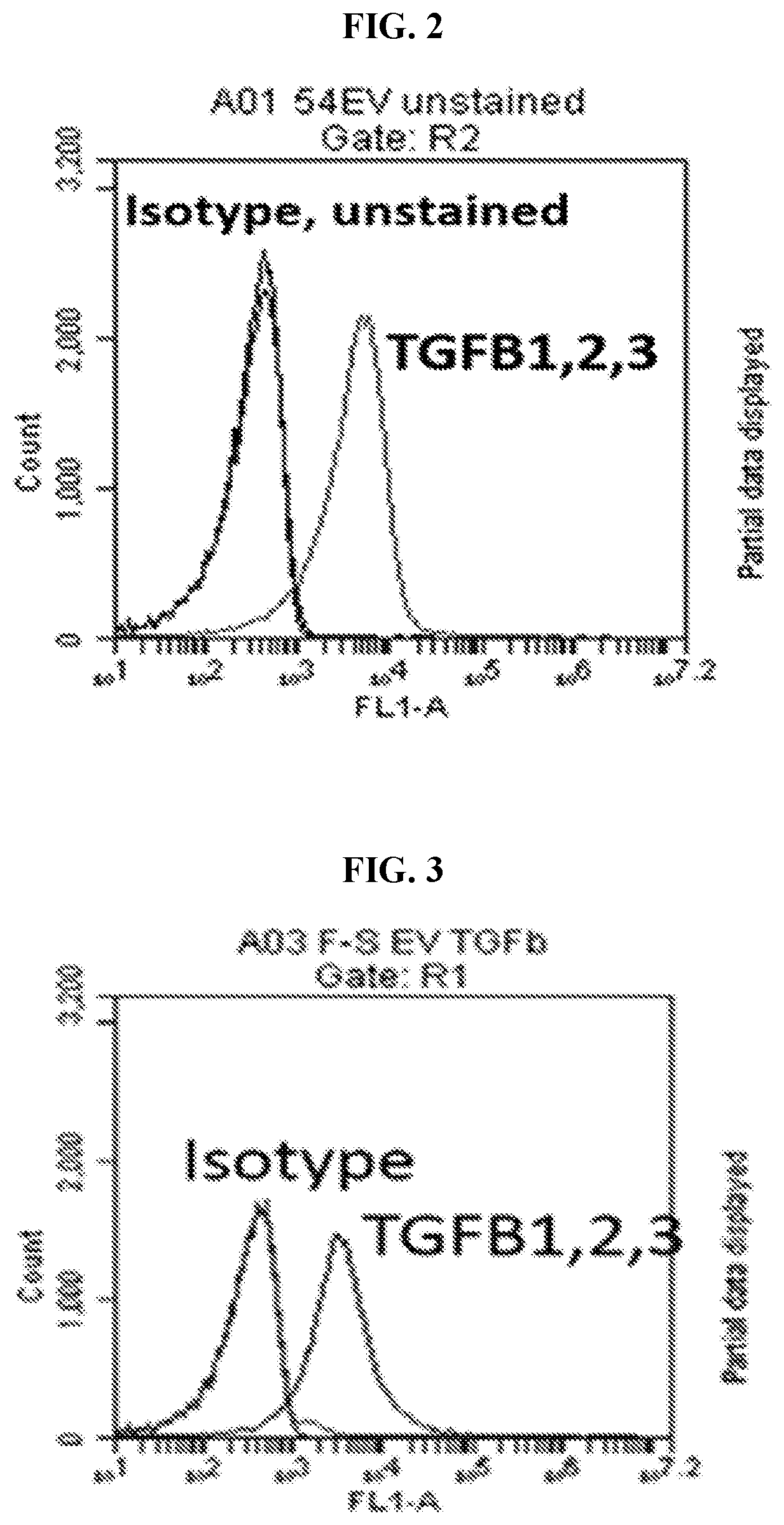
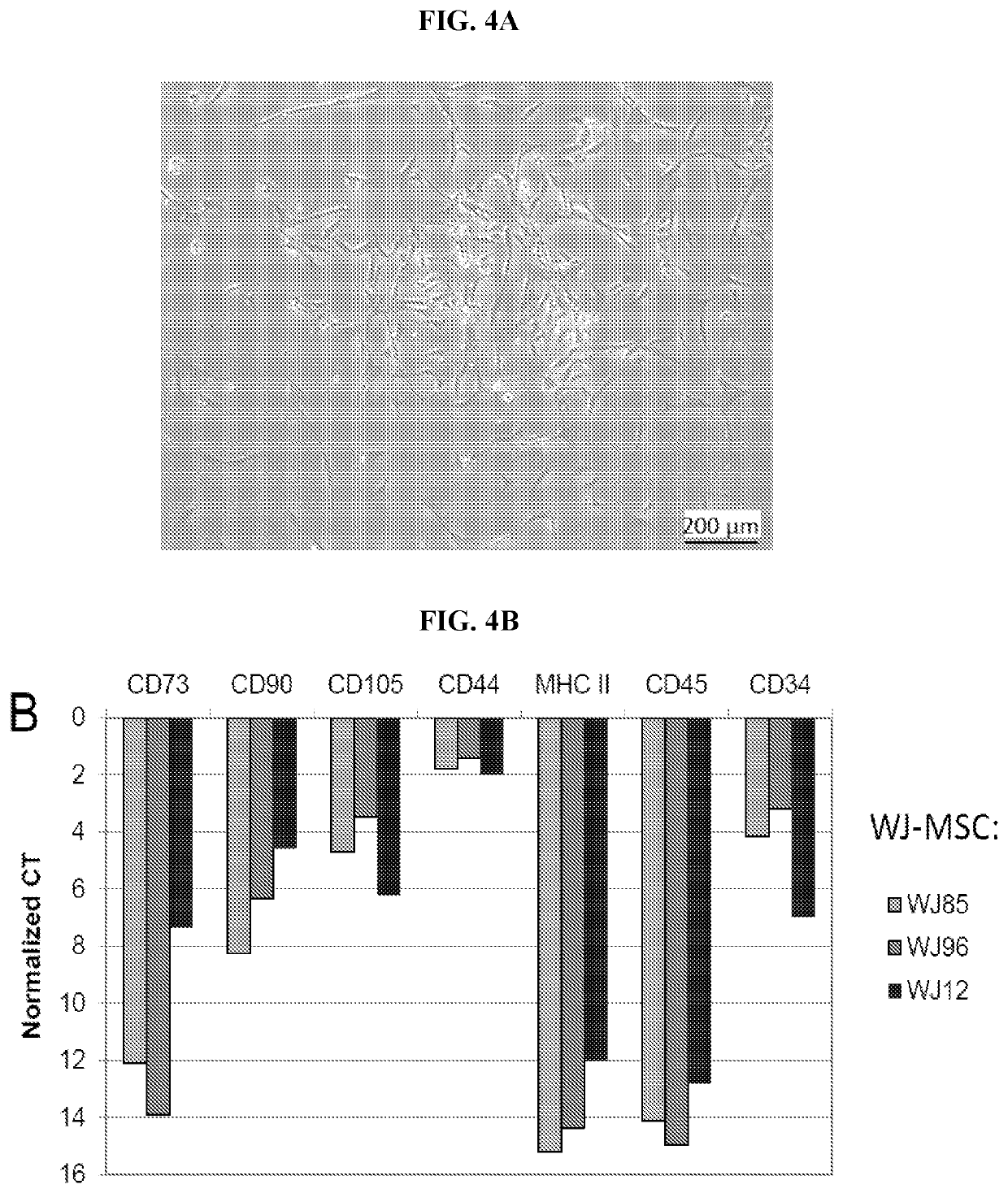
Extracellular vesicles comprising membrane-tethered tgf-beta, compositions and methods of use thereof
PendingUS20200392219A1Effective therapyEffective diagnostic biomarkerPeptide/protein ingredientsGenetically modified cellsActive agentImmunotherapeutic agent
Provided are mesenchymal stromal cell (MSC)-derived extracellular vesicles (EV) having tethered (membrane-bound) TGF-β (MSC-derived membrane-tethered TGF-β EV), and compositions containing such EV for use as therapeutics and immunomodulatory agents. Provided also are diagnostic methods and methods of assessing or monitoring disease status and / or progression in patients using membrane-tethered TGF-β derived from a variety of cell sources that serve as detectable, quantifiable biomarkers in biological samples. The MSC-derived membrane-tethered TGF-β EV can also be used to deliver various bioactive agents to a target cell or tissue for treating various diseases. The level of TGF-β tethered to the membrane of the EV can also be modified or manipulated in vitro or ex vivo. Such modified MSC-derived membrane-tethered TGF-β EV are useful as immunotherapeutic agents in the treatment or management of certain diseases, particularly those involving inflammation, autoimmunity, transplant rejection and cancer.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
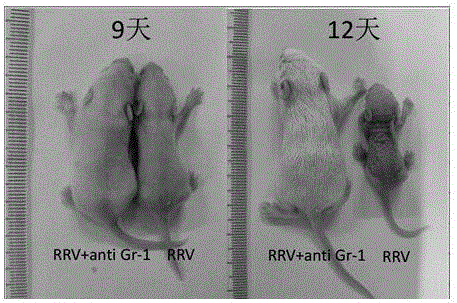
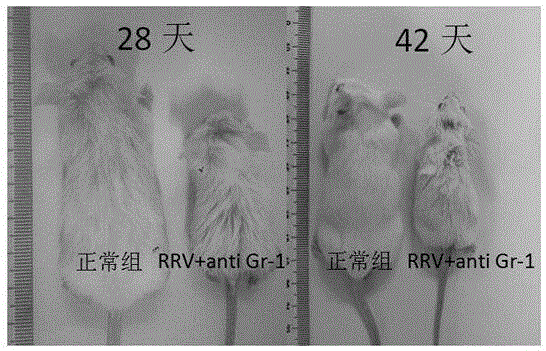
Construction method of animal model for biliary atresia of chronic liver disease
The invention relates to a construction method of an animal model for biliary atresia of chronic liver disease firstly utilizing a Gr-1 antibody to construct the construction method of the animal model for biliary atresia of chronic liver disease. The method comprises following steps: injecting rhesus monkey rotavirus within the 24 hours of neonatal mouse to induce a classic animal model for biliary atresia, firstly injecting the Gr-1 antibodies before rhesus monkey rotavirus is injected; injecting the Gr-1 antibodies multiple times after mouse are born on the 14th day to maintain the effective concentration range of the Gr-1 antibodies in the mouse; and injecting 5-20ng / 1.6g of Gr-1 antibodies to the mouse each time. The construction method of an animal model for biliary atresia of chronic liver disease has following beneficial effects: the model is capable of simulating the process of biliary atresia, development and prognosis, especially in the end-stage hepatocirrhosis phase.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Allogenic tumor cell vaccine
ActiveUS20200330596A1Reduce tumor burdenImprove clinical outcomesPolypeptide with localisation/targeting motifOrganic active ingredientsVaccinationAutologous tumor cell
The described invention provides allogeneic tumor cell vaccines comprising tumor cell lines or tumor cell line variants that are genetically engineered to express a core group of three immunomodulatory molecules, and optionally additional R immunomodulatory polypeptides for induction of one or more subpopulations of PBMCs to proliferate in response to the expressed immunomodulatory molecules and to then enter an effector phase for killing of tumor cells. According to some embodiments, the tumor cell vaccine candidate can induce an immune response in the recipient cancer patient that cross reacts with the patient's own (autologous) tumor cells, the effects of which are sufficient to result in enhanced anti-tumor immunity contributing to the increased survival of a vaccinated patient cohort compared to a matched unvaccinated patient cohort.
Owner:ALLOPLEX BIOTHERAPEUTICS INC
Direct Reversal Of The Suppressive Function Of CD4+Regulatory T Cells Via Toll-Like Receptor 8 Signaling
InactiveUS20090209620A1Inhibiting immunosuppressive capacityDecrease Treg cell mediated immunosuppressionOrganic active ingredientsSugar derivativesAntitumor immunityTLR8
CD4+ regulatory T (Treg) cells profoundly suppress host immune responses and thus protect against autoimmune disease while restricting desired immune responses such as antitumor immunity. Synthetic phosphorothioate-protected, guanosine-containing oligonucleotides can directly reverse the suppressive activity of Treg cells without involving dendritic cells. This effect appears to be transduced by signaling through Toll-like receptor (TLR) 8 and engagement of the MyD88 and IRAK4 molecules in Treg cells. Stimulation of Treg cells with natural ligands for human TLR8 recapitulated the effect of the synthetic guanosine-containing oligonucleotides .
Owner:WANG RONG FU +2
PKS type-I polyketones compound with immunosuppression activity and preparation method and application of PKS type-I polyketones compound
ActiveCN109467579ALow priceSignificant calmodulin phosphatase inhibitory activitySugar derivativesMicroorganism based processesDiseaseImmunosuppressive drug
The invention relates to a PKS type-I polyketones compound Efophylin B with immunosuppression activity and a preparation method and application of the PKS type-I polyketones compound. Streptomyces sp.DSM 4137 is fermented by an SFM solid culture medium, and a fermented product is separated and purified to obtain the compound. Experiments prove that the compound has remarkable calmodulin phosphatase suppression activity, the immunosuppression effect of the compound is higher than that of cyclosporin A as an immunosuppressor used clinically and widely, toxicity to spleen cells is quite weak, andthe PKS type-I polyketones compound is an efficient low-toxicity immunosuppressor, and can be used for preparing a second-generation immunosuppressive drug for organ transplantation and autoimmune diseases.
Owner:HAINAN UNIVERSITY
Patient selection for enhancement of Anti-tumor immunity in cancer patients
PendingUS20220175787A1Enhance immune activationPromote antitumor immunityMicrobiological testing/measurementAntibody ingredientsChemotherapy combinationsPharmaceutical medicine
A method for increasing the progression free survival or overall survival of a patient with cancer comprising: determining if the cancer has a surrounding microenvironment that is favorable to immune modulation; determining if the chemotherapy regimen induces immunogenic cell death, and if both are yes, administering an effective amount of a CDK 4 / 6 inhibitor selected from Compounds I, II, III, IV, or V, or a pharmaceutically acceptable salt thereof, wherein the CDK4 / 6 inhibitor is administered prior to the administration of the chemotherapy or optionally prior to and concurrently with chemotherapy; and, wherein the increase in progression free survival or overall survival is in comparison to the progression free survival or overall survival based on administration of the chemotherapy alone, either based on literature or otherwise publicly available evidence, a comparative during preclinical or clinical trials, or other means accepted by persons skilled in the field.
Owner:G1 THERAPEUTICS INC
Pharmaceutical composition containing IL-15 cationic liposome compound and celecoxib liposome as well as preparation method and application of pharmaceutical composition
PendingCN113694217AReduce tumor immunosuppressionImprove stabilityOrganic active ingredientsPeptide/protein ingredientsFolic acidImmunosuppression
The invention belongs to the field of medicines, and particularly relates to a pharmaceutical composition containing an IL-15 cationic liposome compound and a celecoxib liposome as well as a preparation method and application of the pharmaceutical composition. In the pharmaceutical composition, the IL-15 cationic liposome compound is a delivery system of a folic acid PEGylation DOTAP cationic liposome nuclear transport gene drug, which is designed by taking plasmid DNA (pcDNA3.1 / IL-15) capable of editing and releasing a cell factor IL-15 as a gene drug, after the IL-15 cationic liposome compound and Celecoxib liposome which is released in a targeted manner in TIME are used in a combined manner, Celecoxib promotes NK-cells to be recruited into a tumor microenvironment, infiltration of the NK-cells is greatly improved, IL-15 factors promote proliferation and activation of the NK-cells in the tumor microenvironment, the number of Treg cells is remarkably reduced, the tumor microenvironment is changed, the immunosuppression is relieved to a certain degree, the two components are combined for use, so that the inherent immune anti-tumor effect can be better played, and the safe and low-toxicity synergistic tumor immunotherapy effect is played.
Owner:SHENYANG PHARMA UNIVERSITY
Isolated Recombinant Oncolytic Adenoviruses, Pharmaceutical Compositions, and Uses Thereof for Drugs for Treatment of Tumors and/or Cancers
PendingUS20200276252A1Inhibiting PDL expressionEnhance tumor killing effectSpecial deliveryMammal material medical ingredientsOncolytic adenovirusPharmaceutical drug
The present disclosure provides an isolated recombinant oncolytic adenovirus, a pharmaceutical composition, and uses thereof for drugs for treatment of tumors and / or cancers. The recombinant oncolytic adenovirus is a selectively replicating oncolytic adenovirus, and the genome of the recombinant oncolytic adenovirus is integrated with a coding sequence of exogenous shRNA capable of inhibiting PDL1 expression in tumor cells. The replication capability of the virus in normal primary cells is much lower than the replication capability of the virus in tumor cells. Moreover, the expressed shPDL1 can significantly reduce the level of PDL1 protein highly expressed in tumor cells. Thus, the oncolytic killing effect of the oncolytic virus and the anti-tumor immunostimulatory effect of immune cells produce a synergistic effect.
Owner:HANGZHOU CONVERD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com