Construction method of animal model for biliary atresia of chronic liver disease
A technology of biliary atresia and animal models, applied in animal husbandry and other fields, can solve the problems of low repetition rate, low survival rate, and insufficiency, and achieve the effect of long life cycle, high survival rate and high repetition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
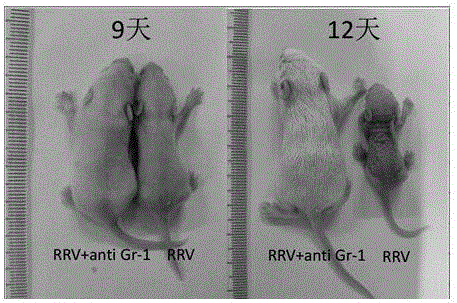
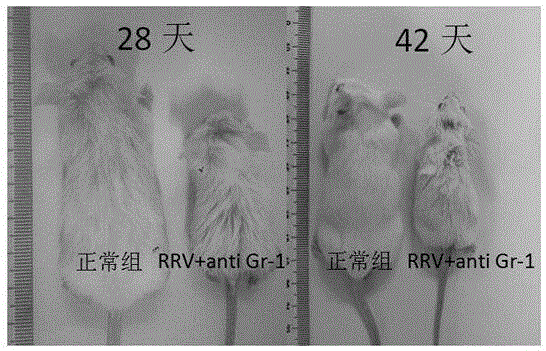
[0029] The experimental group was injection of anti-MDSCs drugs and RRV group (RRV+ anti Gr-1 group), the positive control group was only injection of RRV group (RRV group), and the negative control group was no injection of MDSCs drugs and RRV group (normal group). The construction method of sclerosing animal model, comprises the following steps:
[0030] (1) Balb / c neonatal mice were intraperitoneally injected with an anti-MDSCs drug [Anti-Mouse Ly-6G (Gr-1), referred to as Gr-1 antibody] within 24 hours of birth, and the first dose was 5ng per mouse ( Neonatal mice weigh approximately 1.6 g).
[0031] (2) Four hours after the injection of anti-MDSCs drugs, intraperitoneal injection of rhesus monkey rotavirus (MMU18006 virus strain, the titer of the virus suspension was 1×10 6 PFU), the injection dose was 20 μL per mouse, and the injection time of rhesus monkey rotavirus was also within 24 hours of the birth of Balb / c newborn mice.
[0032] (3) Multiple injections of anti-...
Embodiment 2
[0037] The experimental group was injection of anti-MDSCs drugs and RRV group (RRV+ anti Gr-1 group), the positive control group was only injection of RRV group (RRV group), and the negative control group was no injection of MDSCs drugs and RRV group (normal group). The construction method of sclerosing animal model, comprises the following steps:
[0038] (1) Balb / c neonatal mice were intraperitoneally injected with an anti-MDSCs drug [Anti-Mouse Ly-6G (Gr-1), referred to as Gr-1 antibody] within 24 hours of birth, and the first dose was 20ng per mouse ( Neonatal mice weigh approximately 1.6 g).
[0039] (2) Four hours after the injection of anti-MDSCs drugs, intraperitoneal injection of rhesus monkey rotavirus (MMU18006 virus strain, the titer of the virus suspension was 1×10 6 PFU), the injection dose was 20 μL per mouse, and the injection time of rhesus monkey rotavirus was also within 24 hours of the birth of Balb / c newborn mice.
[0040] (3) Multiple injections of anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com