Methods and Compositions for Determining a Graft Tolerant Phenotype in a Subject
a graft tolerance and subject technology, applied in the field of methods and compositions for determining a graft tolerance phenotype in a subject, can solve the problems of increased risk of developing neoplastic disease conditions, unsatisfactory side effects of such agents, etc., and achieve the effect of reducing immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
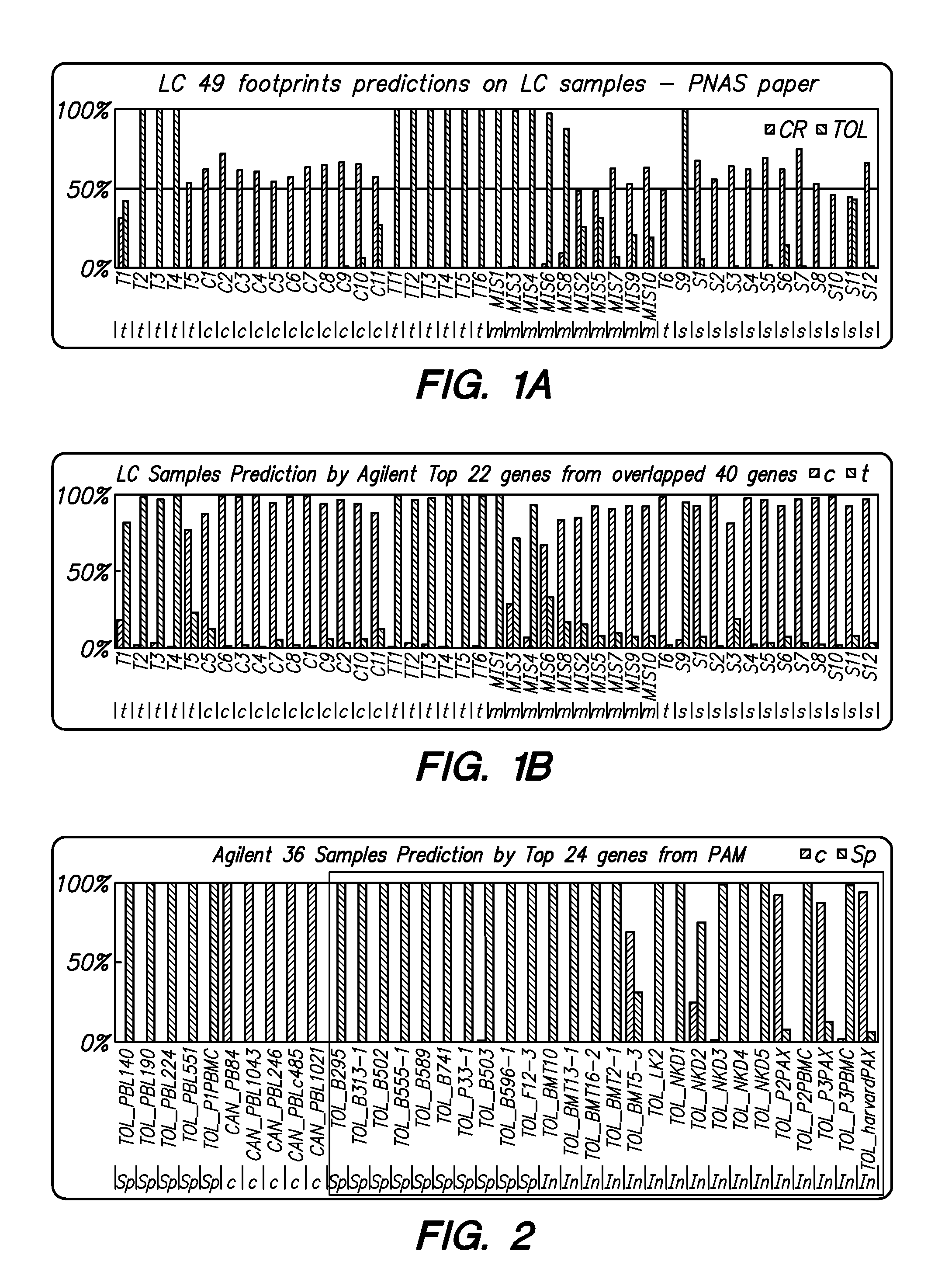
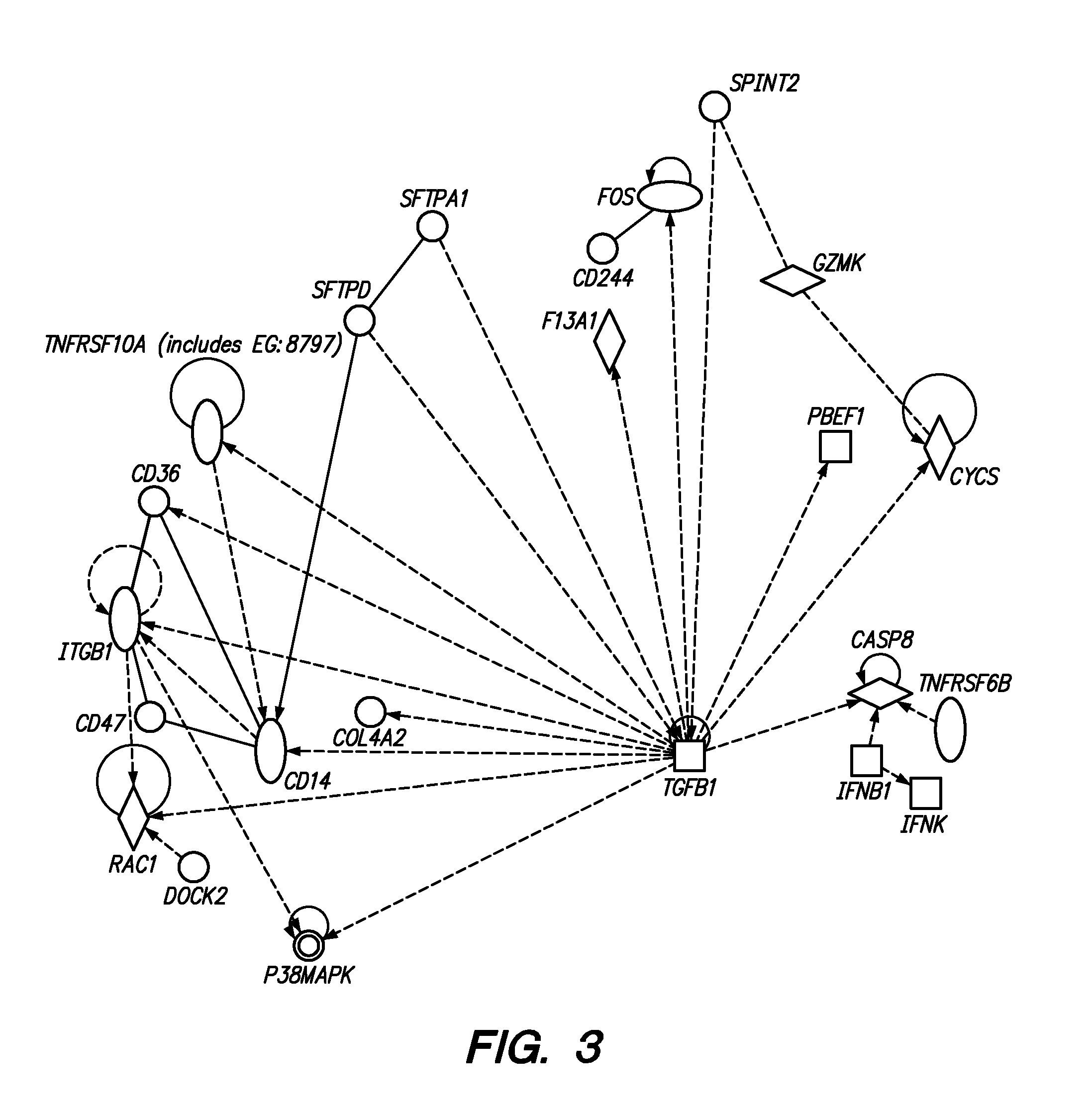
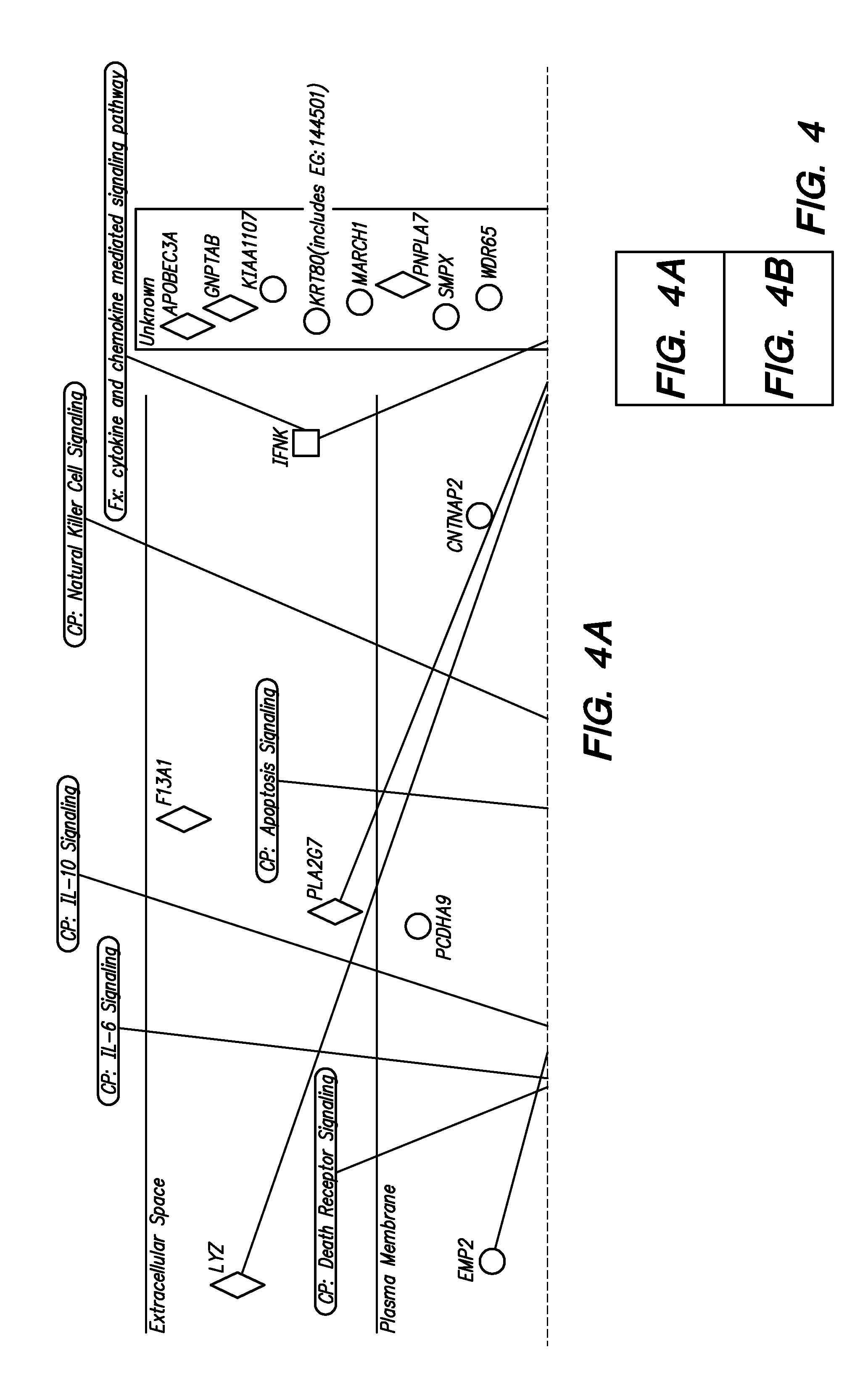
[0022]Methods are provided for determining whether a subject has a graft tolerant phenotype. In practicing the subject methods, the expression of at least one gene in a sample from the subject, e.g., a blood sample, is assayed to obtain an expression evaluation for the at least one gene. The obtained expression evaluation is then employed to determine whether the subject has a graft tolerant phenotype. Also provided are compositions, systems and kits that find use in practicing the subject methods. The methods and compositions find use in a variety of applications, including the determination of an immunosuppressive therapy regimen.
[0023]Before the present invention is described in greater detail, it is to be understood that this invention is not limited to particular embodiments described, as such may, of course, vary. It is also to be understood that the terminology used herein is for the purpose of describing particular embodiments only, and is not intended to be limiting, since ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com