PKS type-I polyketones compound with immunosuppression activity and preparation method and application of PKS type-I polyketones compound
A polyketide compound, immunosuppressive technology, applied in the field of medicine and biology, can solve the problems of high price, hyperlipidemia, inducing tumors, etc., and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Cultivation and identification of Streptomyces sp.DSM 4137
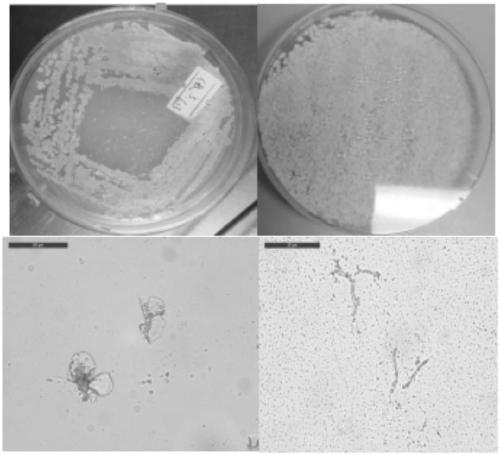
[0043] Zeeck first isolated microbial samples Streptomyces sp.DSM 3816 from soil samples in Greece. The strain Streptomyces sp.DSM 4137 is Streptomyces sp. DSM 3816 or its mutants, provided by the University of Cambridge, UK. The strains were grown in SFM medium for 30 Cultivated at a constant temperature at ℃, extensively branched substrate hyphae (0.3-0.5 μm in diameter) and aerial hyphae were formed, which differentiated into compact chains of helical spores. The appearance of the spores ranged from cylindrical to barrel-shaped (1.3-1.0 x 1.5 pm) with a wrinkled surface. The color of the substrate mycelium tends to be brown / gray or yellow / grey. At maturity, the aerial hyphae differentiate into compact, spirally rugose, cylindrical spore chains. For the results of morphological identification, see figure 1 .
Embodiment 2
[0044] Example 2: Optimization of fermentation conditions for Streptomyces sp.DSM 4137
[0045]Take an appropriate amount of strains, inoculate them on SFM solid plane medium (soybean powder 2%, D-mannitol 2%, agar 2%), and cultivate them in a 30°C incubator for 6 days. Pick appropriate spores and thalline with an inoculation needle, inoculate into a 25mL Erlenmeyer flask containing 5mL of TSBY seed culture solution (trypsin soybean broth 3%, sucrose 10.3%, yeast extract 0.5%), and place in a shaker , cultivated at 30° C. and 150 rpm for 48 hours to obtain a seed culture solution.
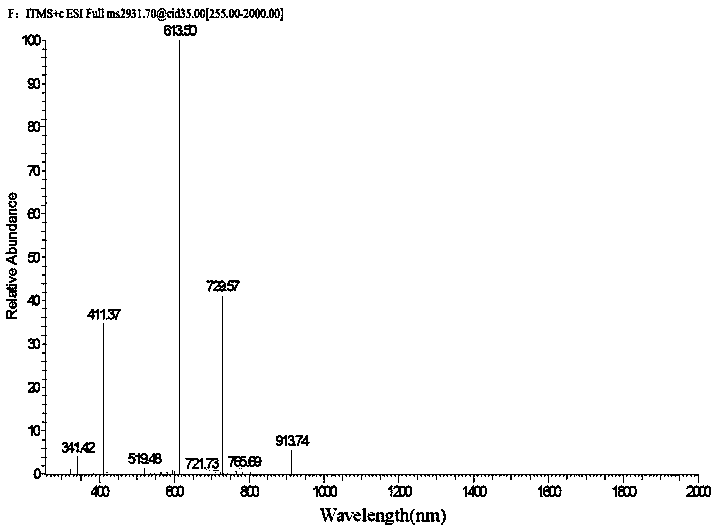
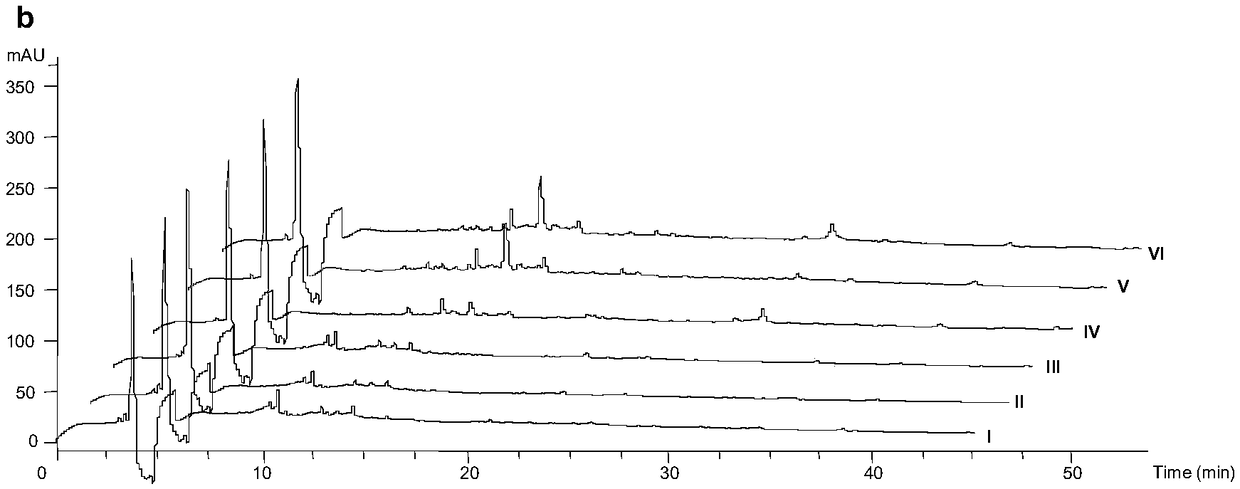
[0046] Get the seed culture solution (0.2mL), inoculate it on TSBY liquid fermentation medium, SFM liquid fermentation medium, TSBY solid fermentation medium, SFM solid fermentation medium, cultivate 2d, 4d, 6d in 30 ℃ incubator, obtain For the fermentation culture of the strain, HPLC-ESI-MS adopts a 5μC18 column (4.6×250mm, Phenomenex), and the solvent system in Table 1 is used for metabolite scr...
Embodiment 3
[0062] Embodiment three, the separation preparation and structural identification of compound Efophylin B (I)
[0063] 3.1 Extraction of strain fermentation products
[0064] After the 6L SFM solid medium was fermented, it was fully soaked in 2 times the volume of methanol for 2 hours, the methanol extract was obtained by filtration, concentrated under reduced pressure, and the methanol was recovered. This was repeated three times to obtain 36 g of the fermentation product of strain Streptomyces sp.DSM 4137.
[0065] 3.2 Thin layer detection (TLC) rapid identification of Efophylin B
[0066] ① point board
[0067] Take a small amount of fermented extract and dissolve it with a small amount of solvent, and use a capillary to spot on the thin layer of silica gel GF 254 board. The diameter of the spot plate is about 2 mm, and the distance from the thin-layer plate is about 1.5 cm. After pointing the sample, wait for it to evaporate to dryness and put it into the chromatograph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com