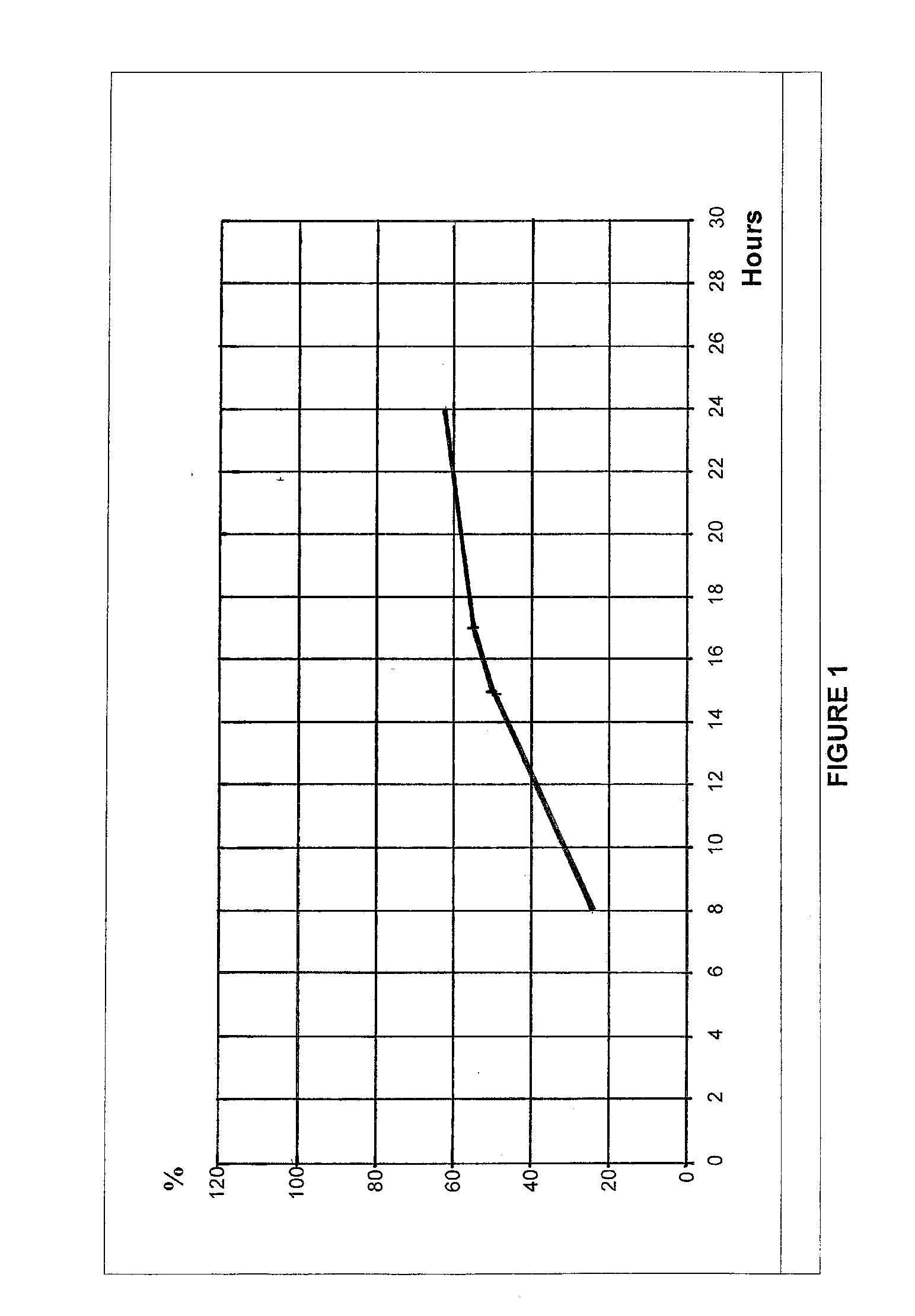
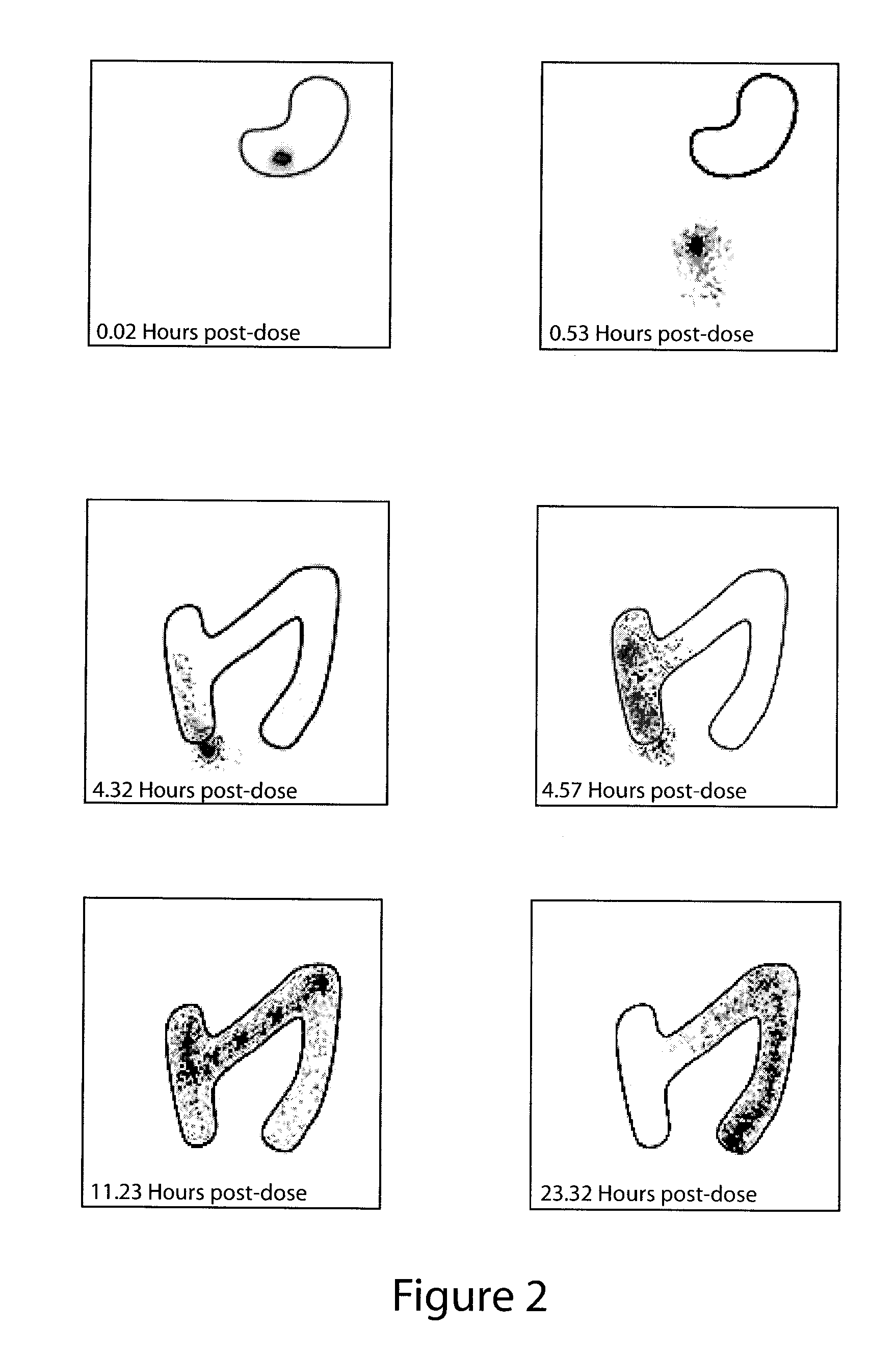
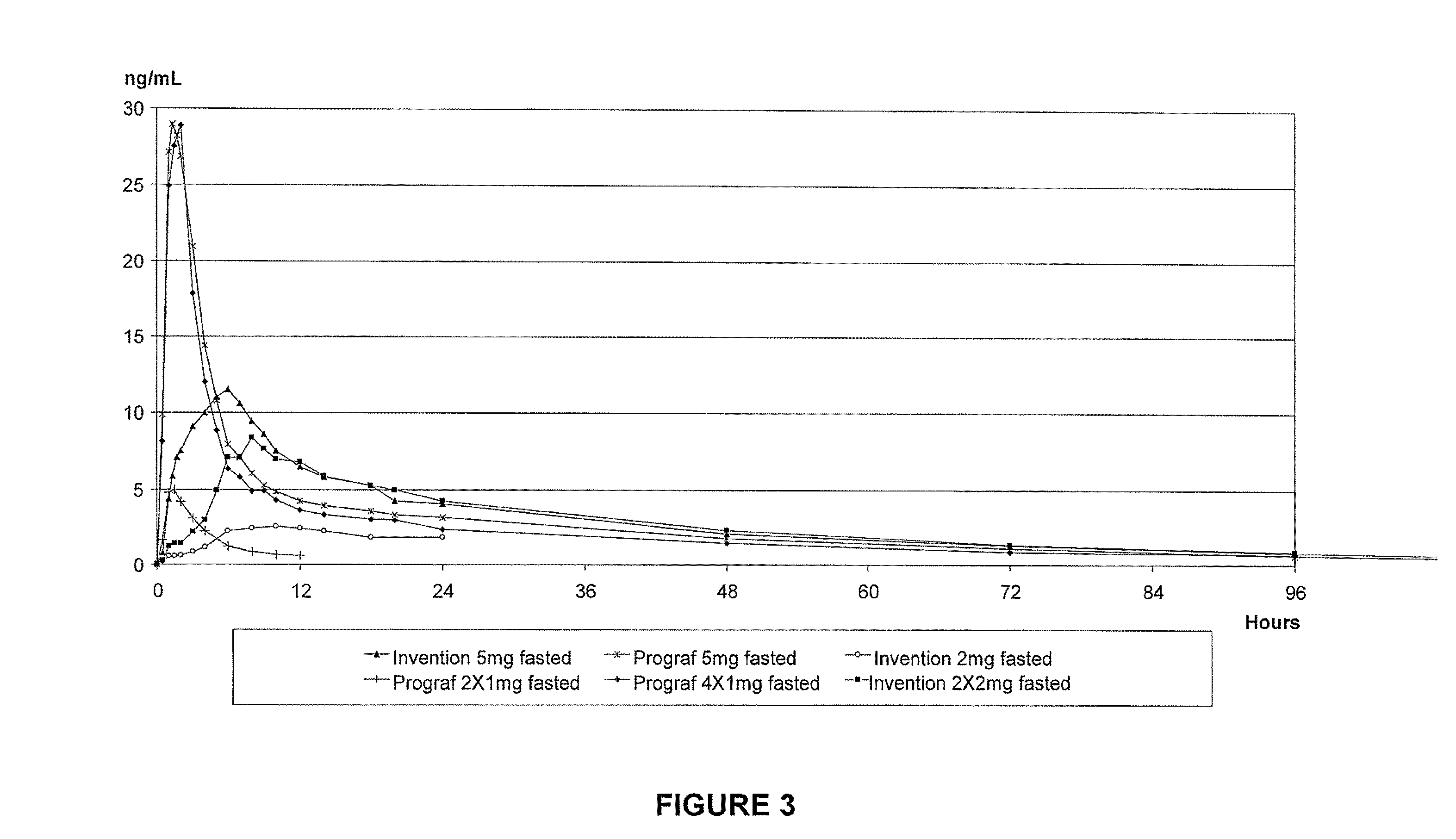
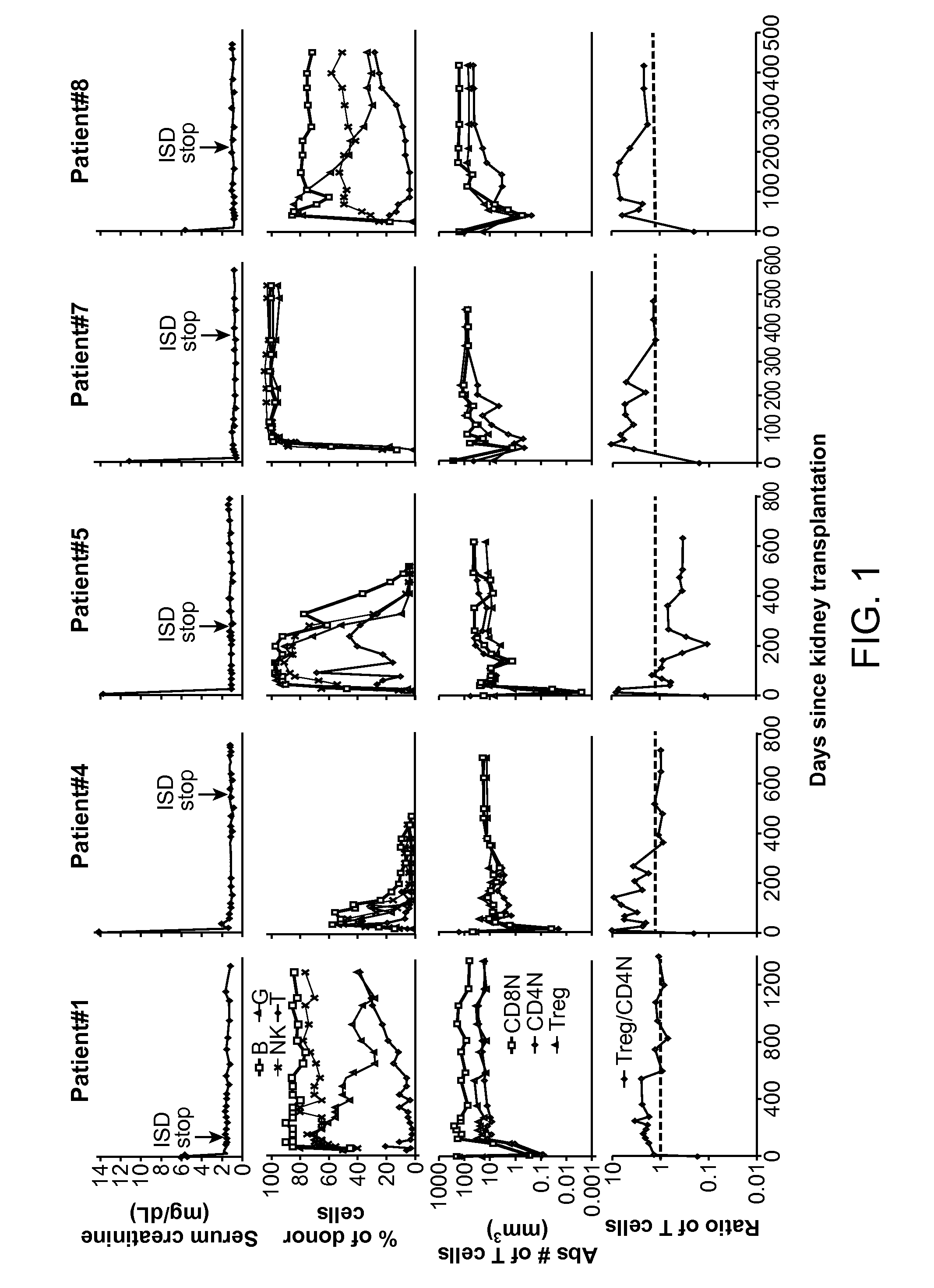
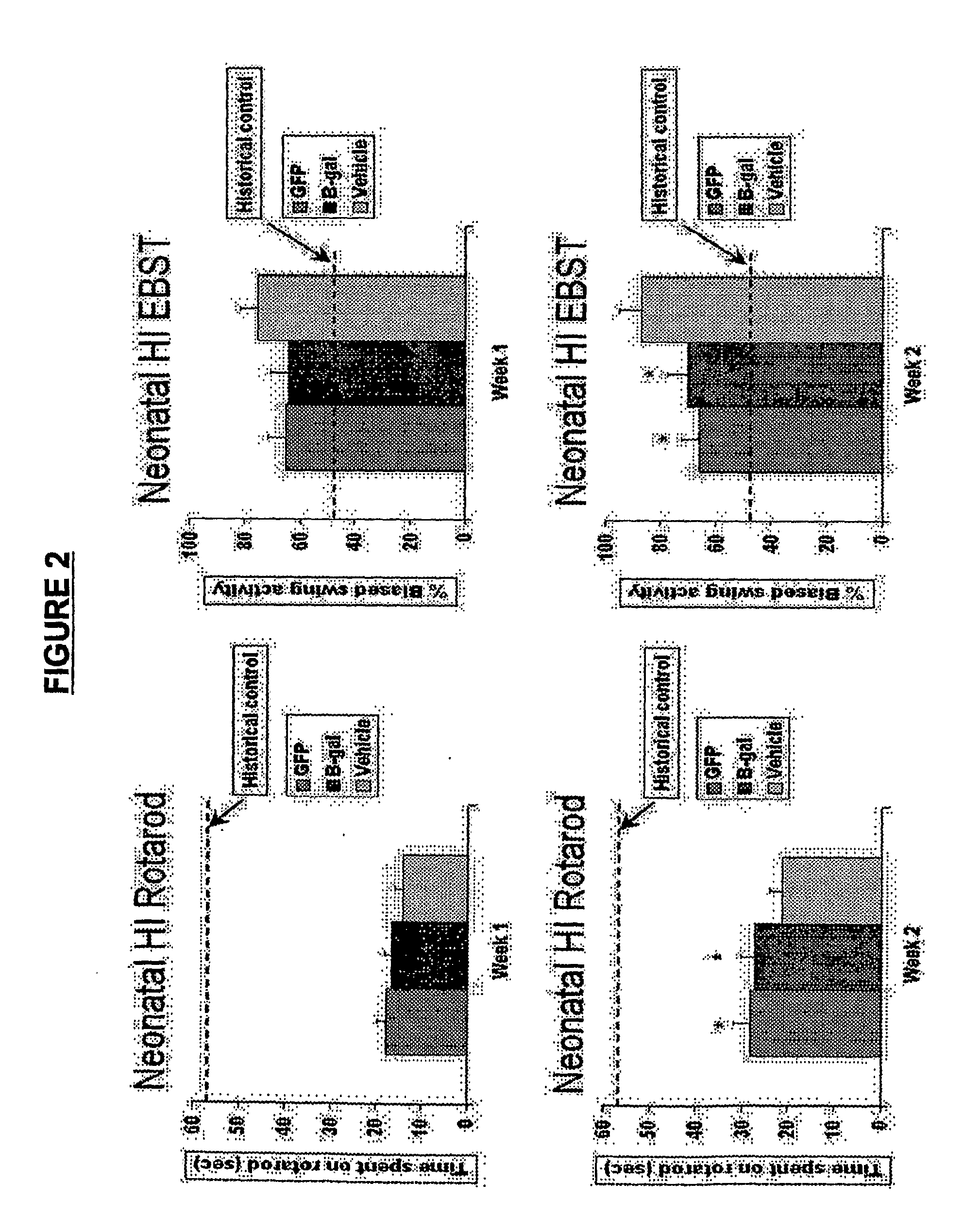
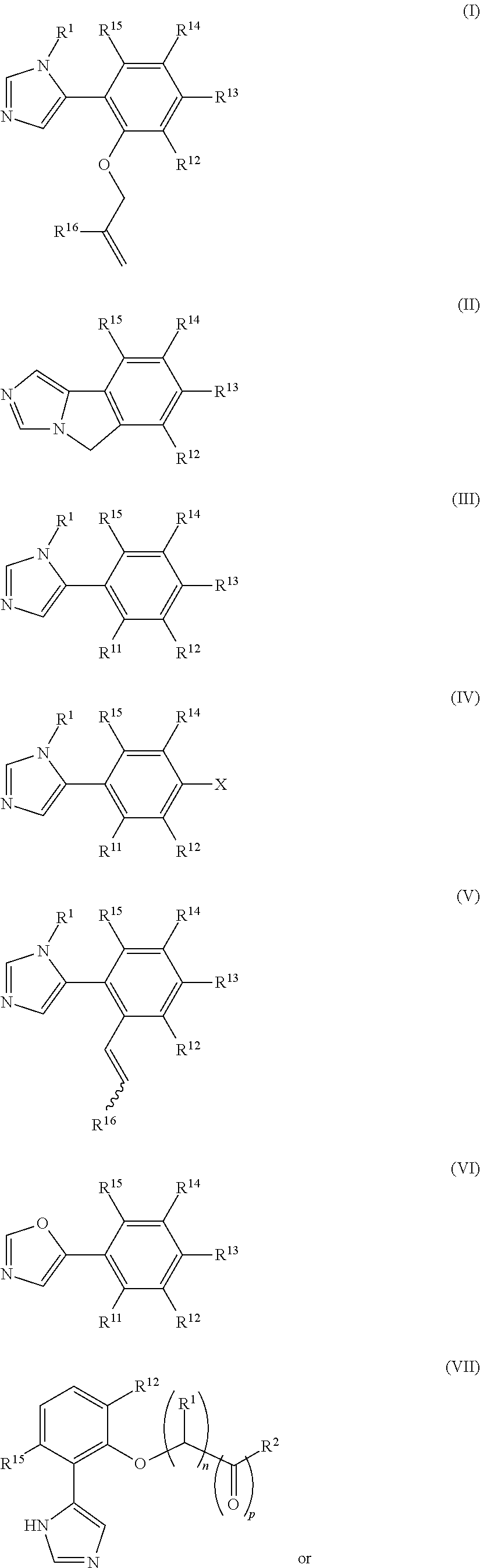
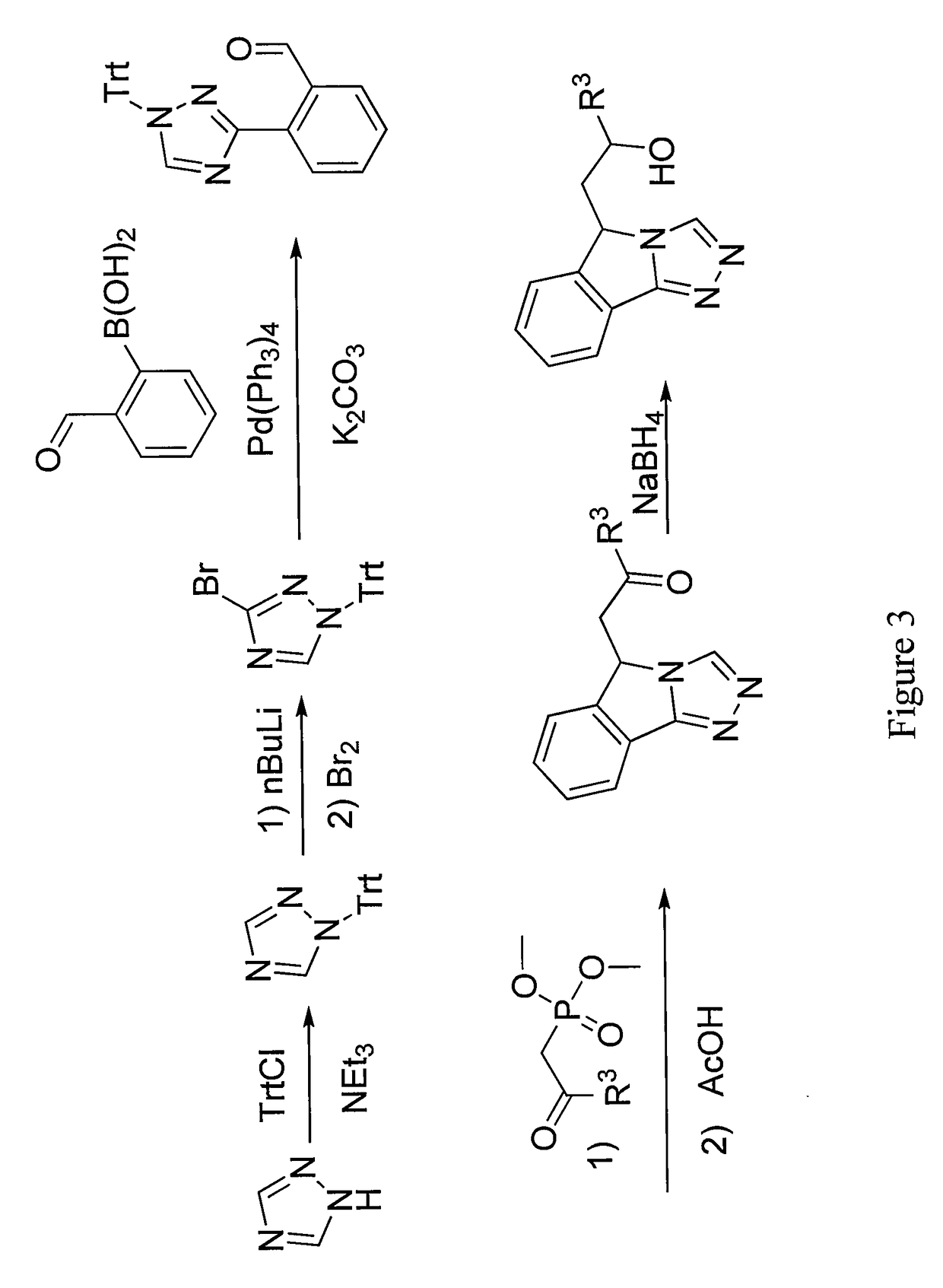
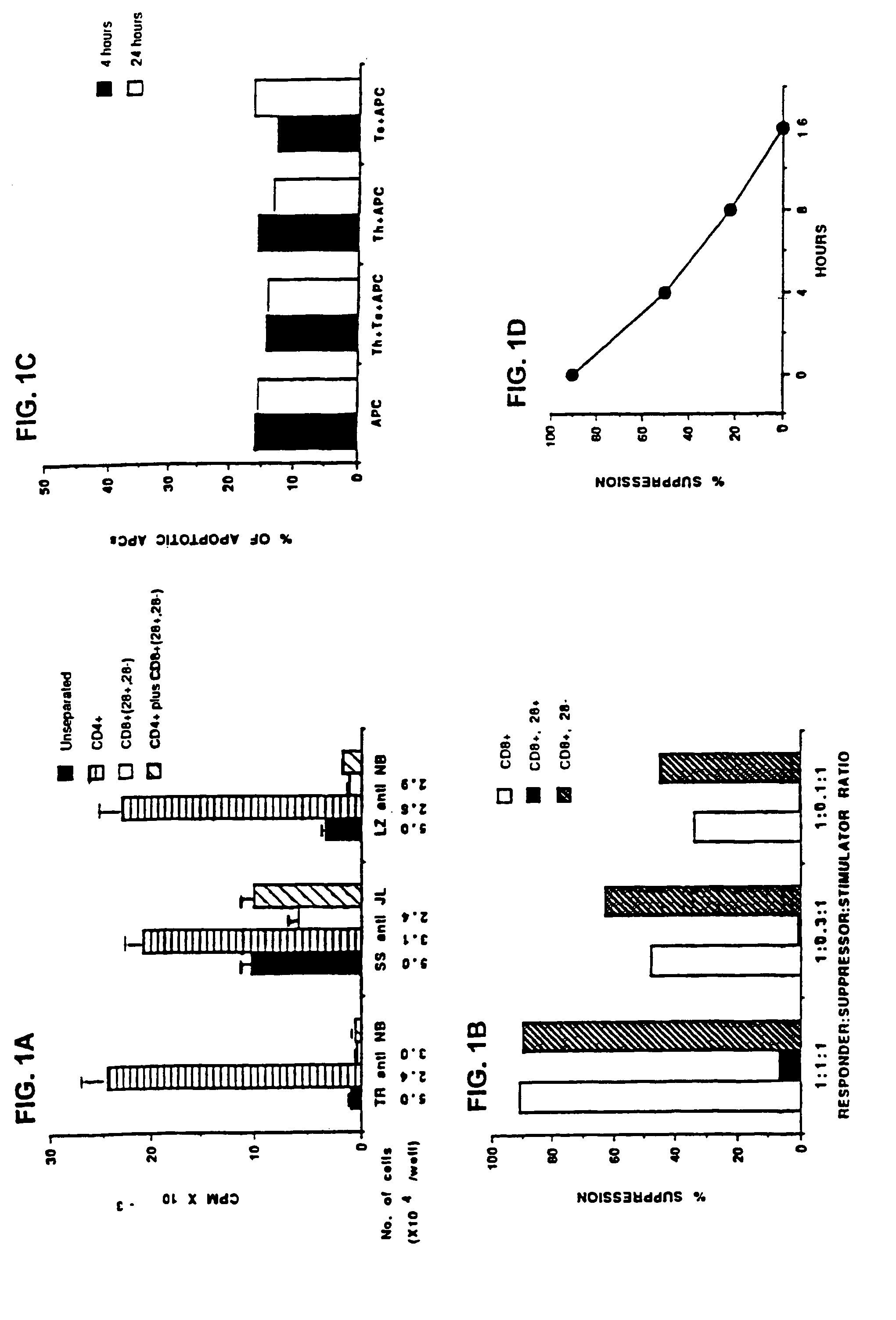
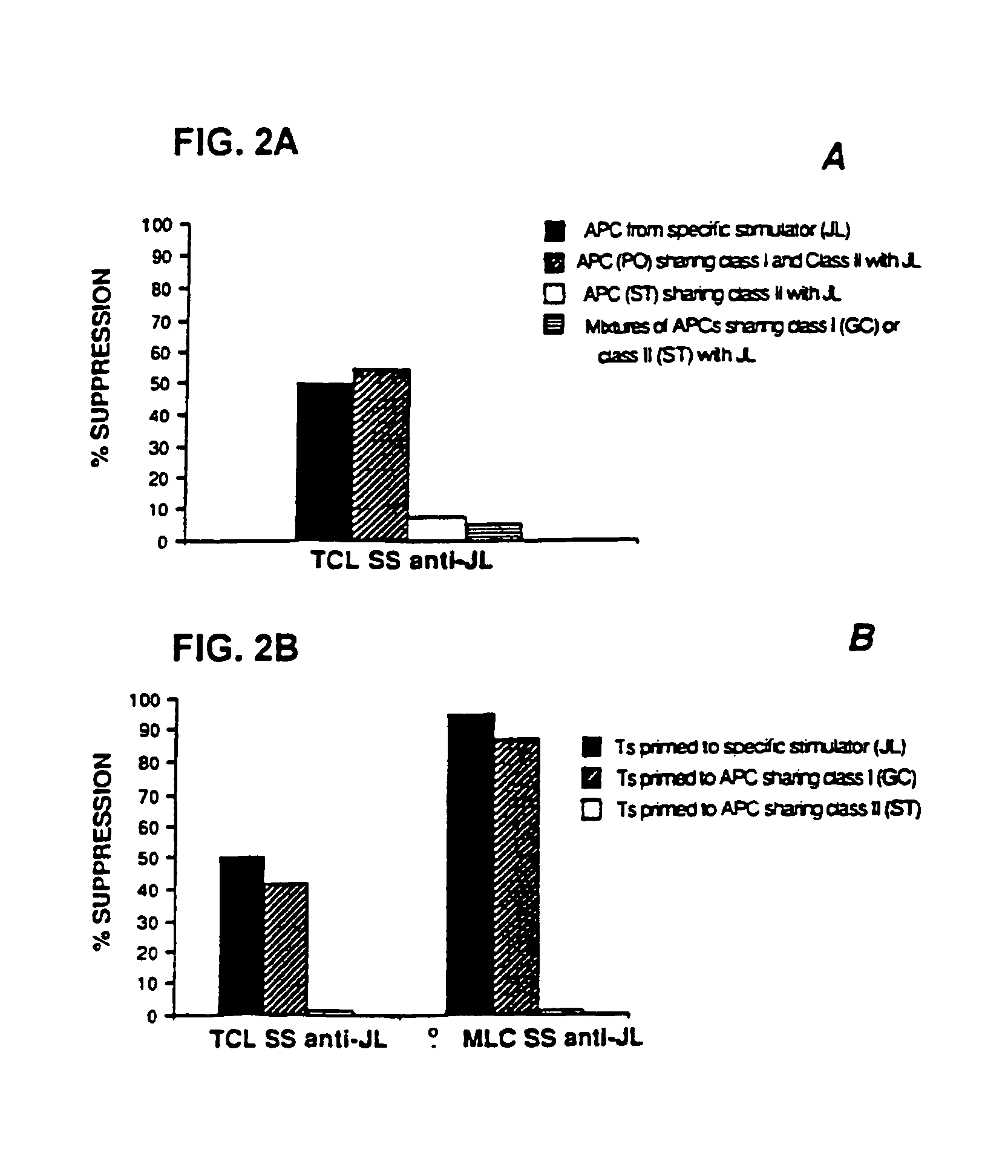
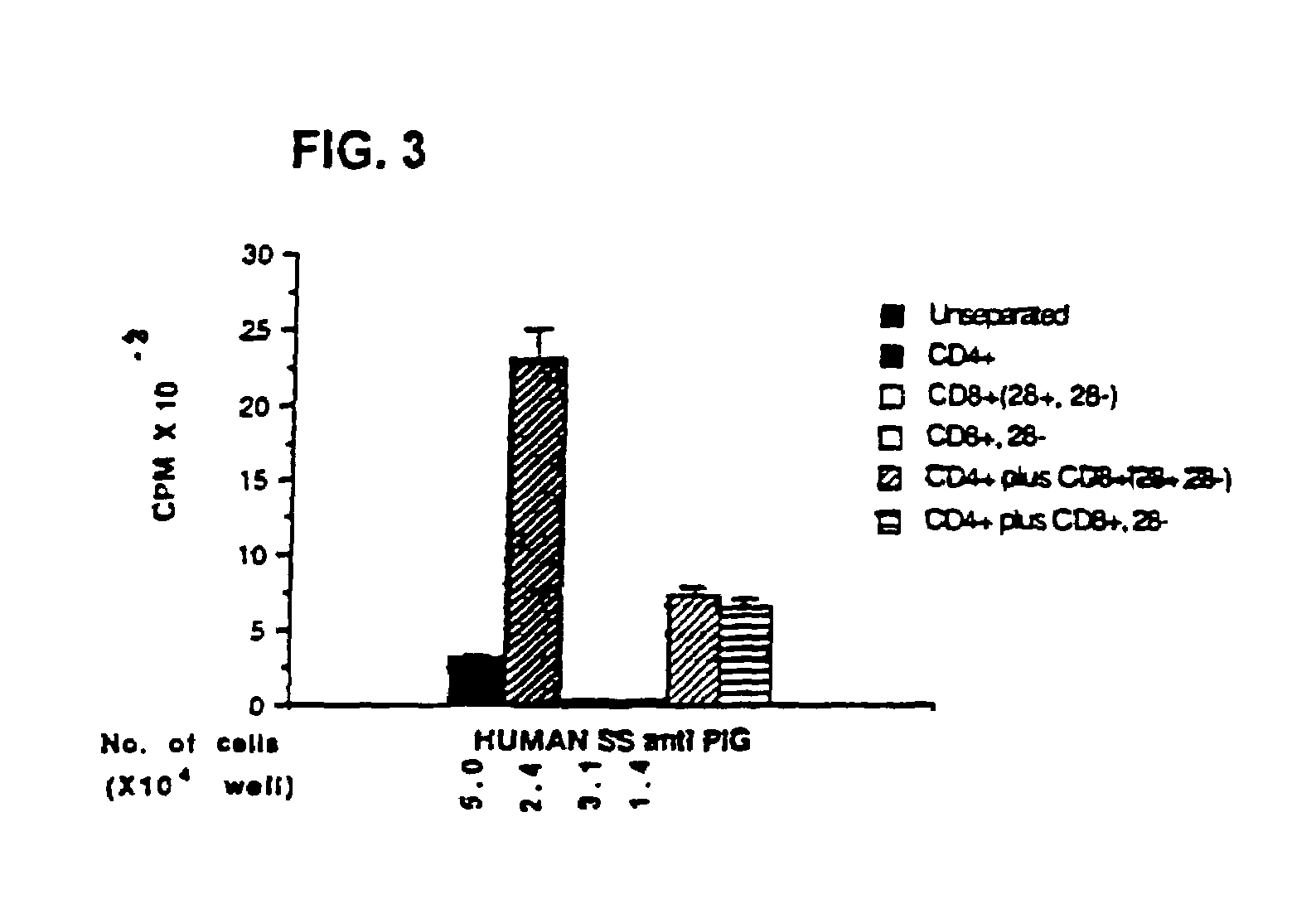
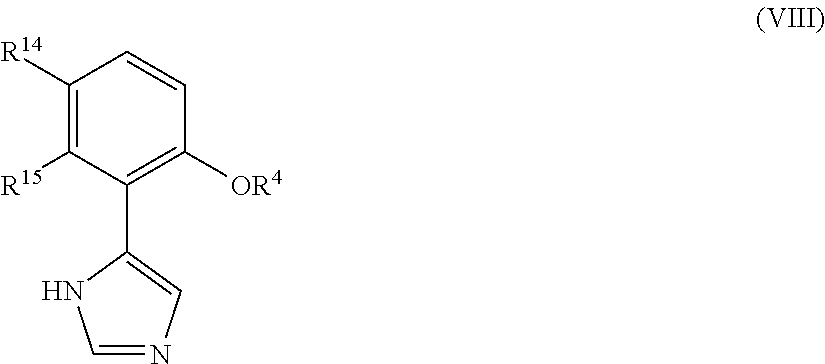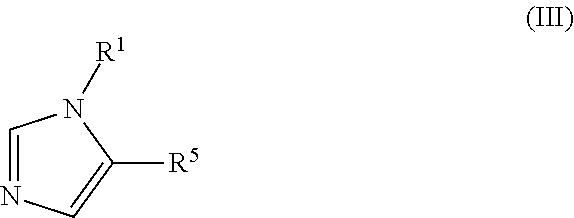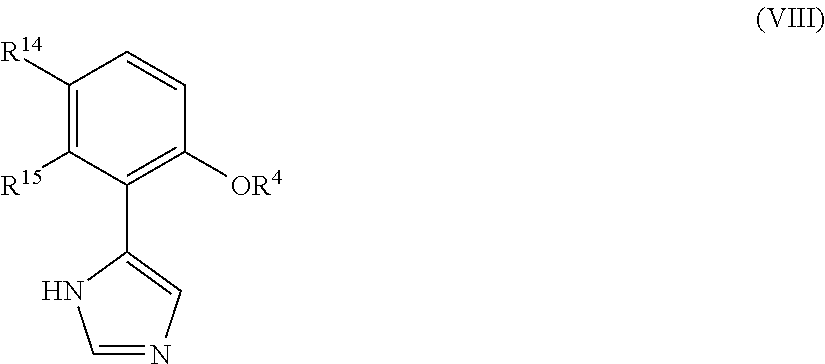Patents
Literature
57 results about "Immunosuppressive treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunosuppressive therapy (treatment): Immunosuppressants are a general class of medications that suppress the immune system. They are often used against diseases of. Immunosuppressive therapy (treatment): A drug given to suppress the natural responses of the body's immune system ....
Tacrolimus for improved treatment of transplant patients
ActiveUS20100105717A1Improve bioavailabilityReduce riskBiocideOrganic chemistryTherapeutic effectIn vivo
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARM INC
Biomarkers for Determining an Allograft Tolerant Phenotype
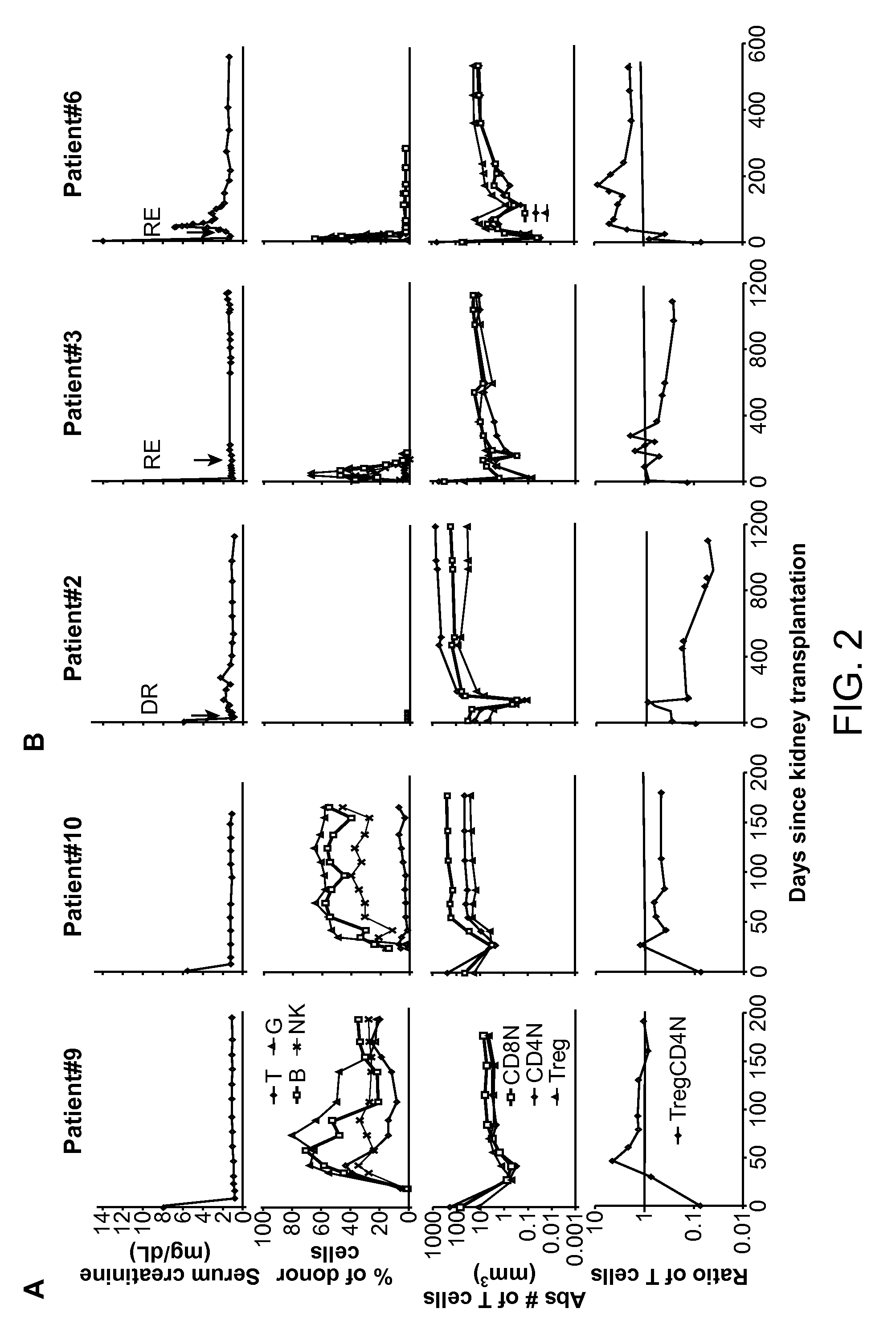
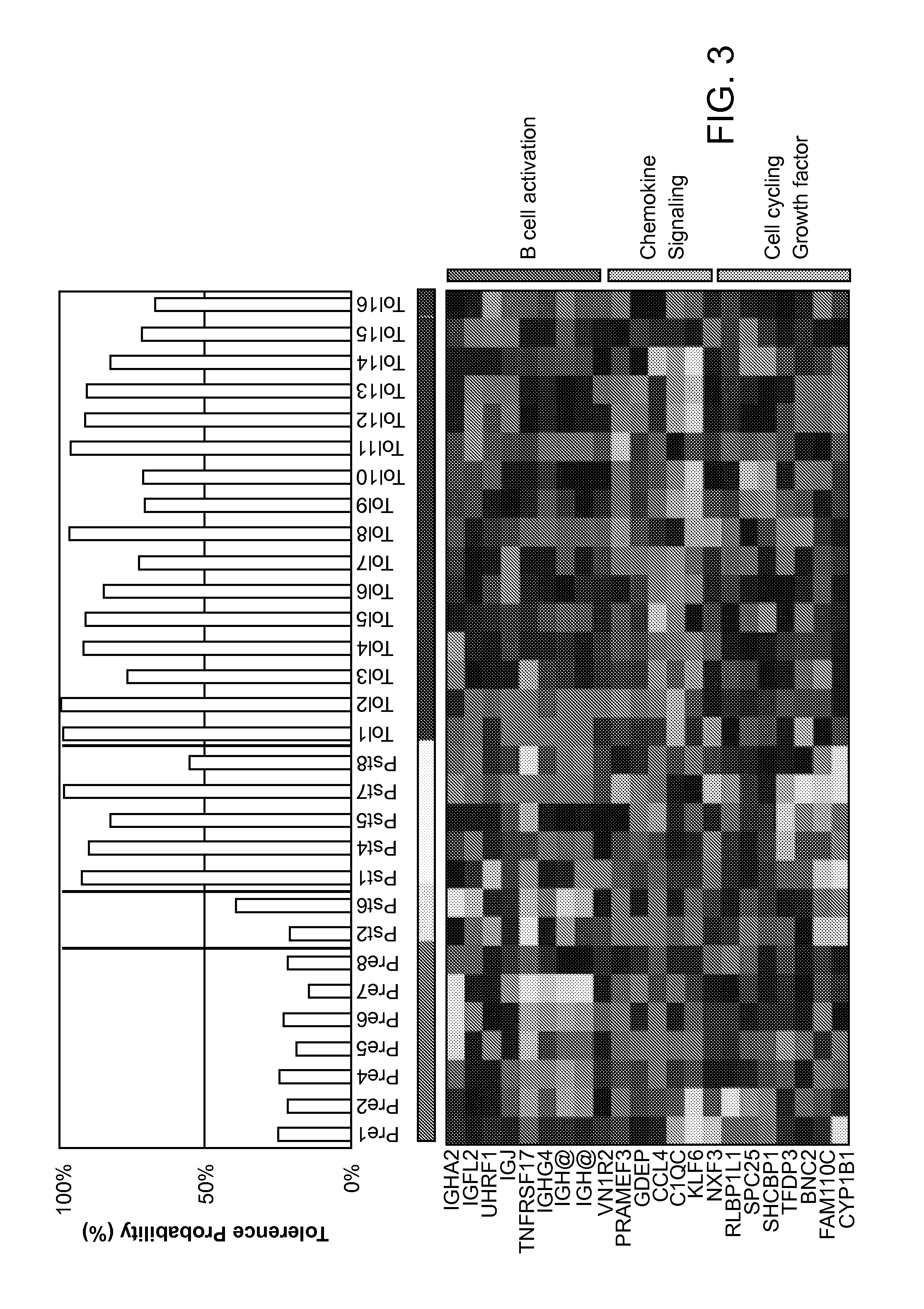
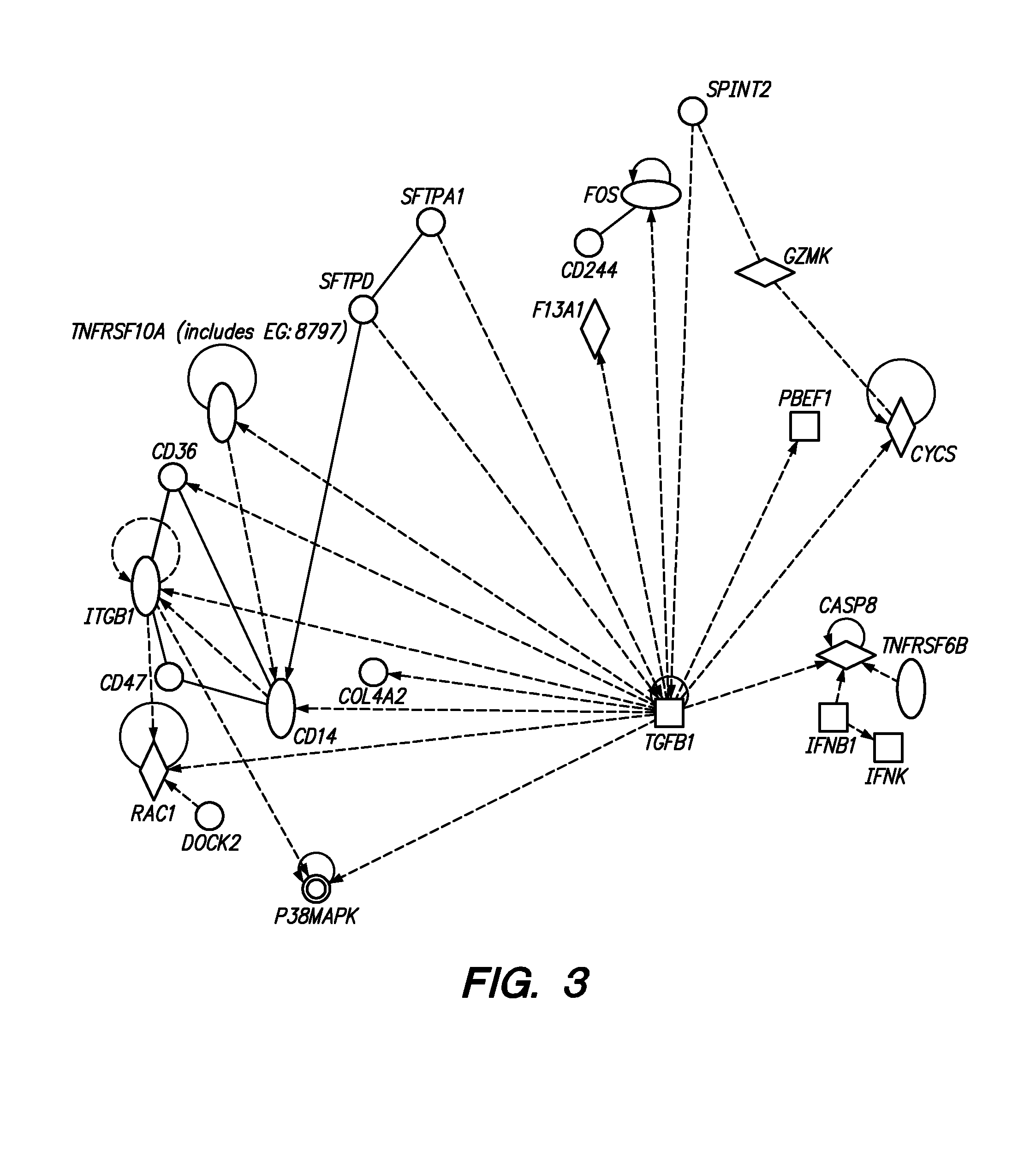
Methods are provided for determining whether a subject has a graft tolerant phenotype. In practicing the subject methods, the expression level of one or more gene in a sample from the subject, e.g., a blood sample, is assayed to obtain a gene expression result, where the gene expression result includes a result for a biomarker of graft tolerance. The obtained gene expression result is then employed to determine whether the subject has a graft tolerant phenotype. Also provided are compositions, systems and kits that find use in practicing the subject methods. The methods and compositions find use in a variety of applications, including the determination of an immunosuppressive therapy regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
MAPC Therapeutics Without Adjunctive Immunosuppressive Treatment
The invention relates to the treatment of various injuries, disorders, dysfunctions, diseases, and the like with MAPCs, without the need for adjunctive immunosuppressive treatment.
Owner:ABT HOLDING COMPANY
Imidazole Derivatives as IDO Inhibitors
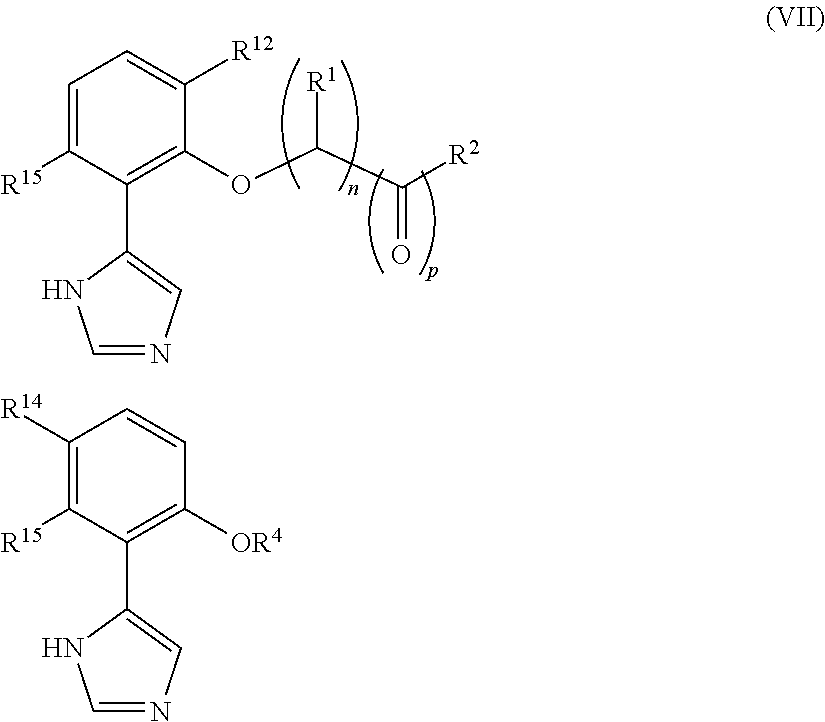
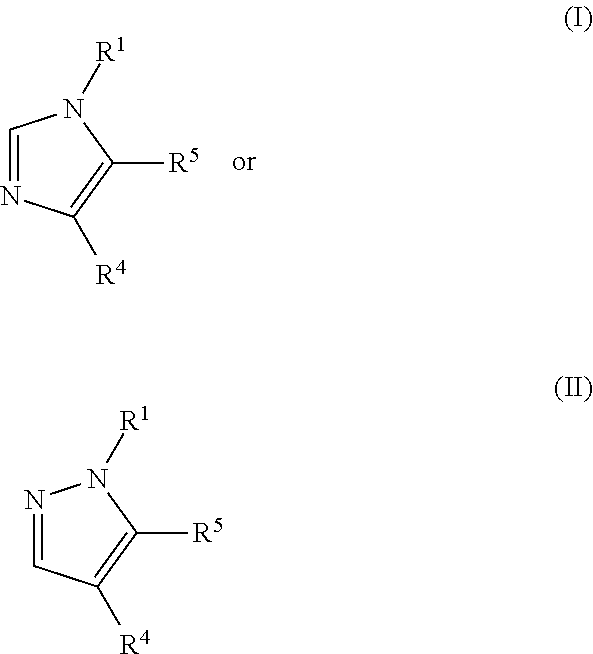
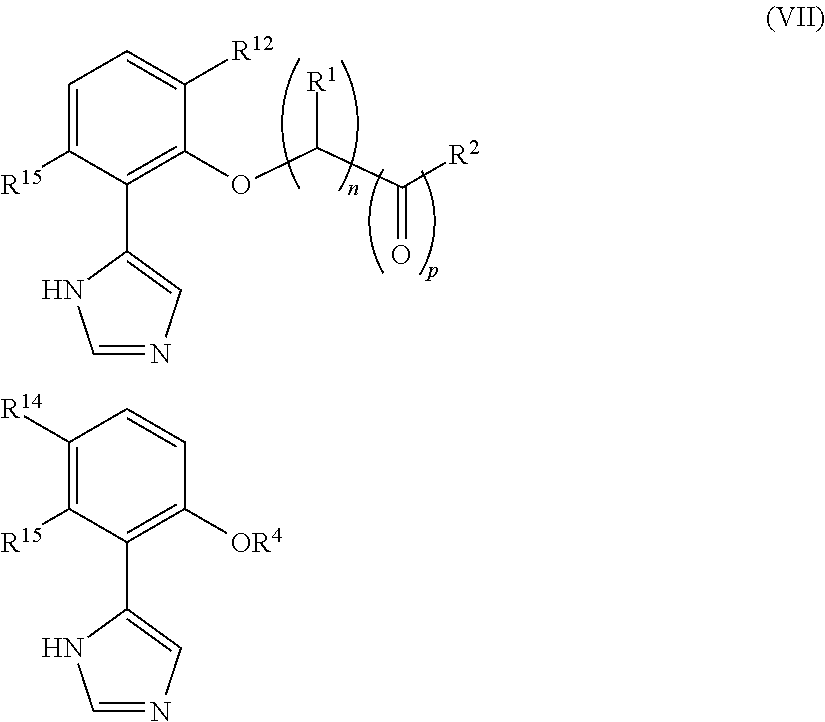
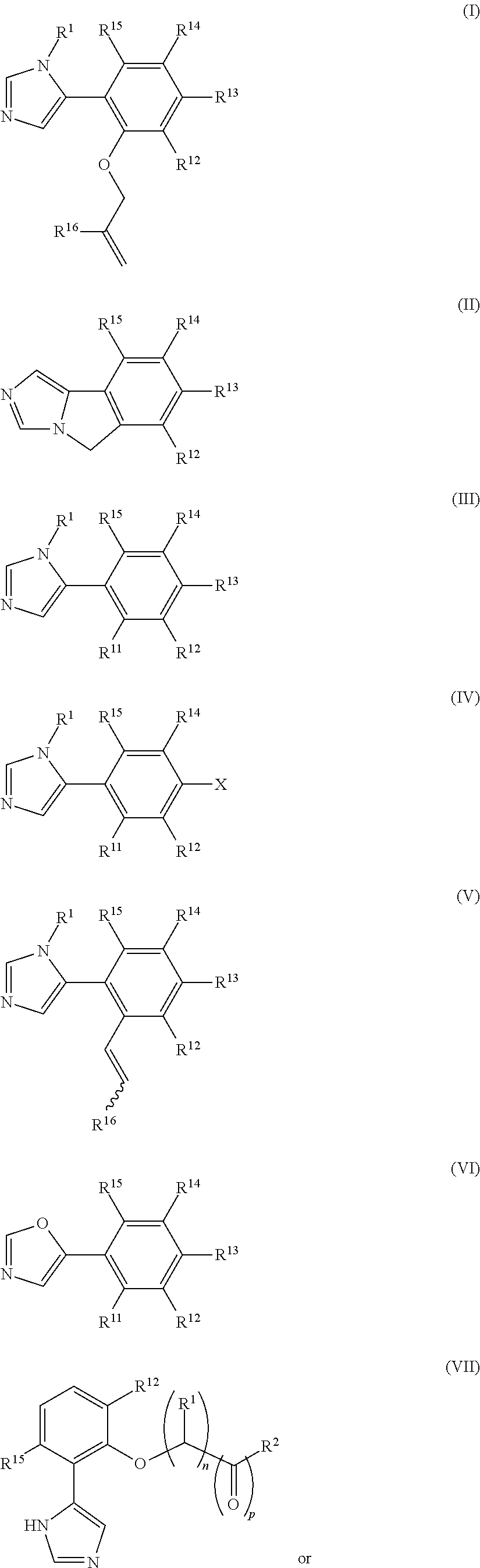
Presently provided are IDO inhibitors of general formulae (VII), (VIII) as shown below and pharmaceutical compositions thereof, useful for modulating an activity of indoleamine 2,3-dioxygenase; treating indoleamine 2,3-dioxygenase (IDO) mediated immunosuppression; treating a medical conditions that benefit from the inhibition of enzymatic activity of indoleamine-2,3-dioxygenase; enhancing the effectiveness of an anti-cancer treatment comprising administering an anti-cancer agent; treating tumor-specific immunosuppression associated with cancer; and treating immunosupression associated with an infectious disease.
Owner:NEWLINK GENETICS
Method of preventing or treating viral infection
InactiveUS20130123344A1Avoid virus infectionBiocideOrganic chemistryChemical compoundPharmaceutical drug
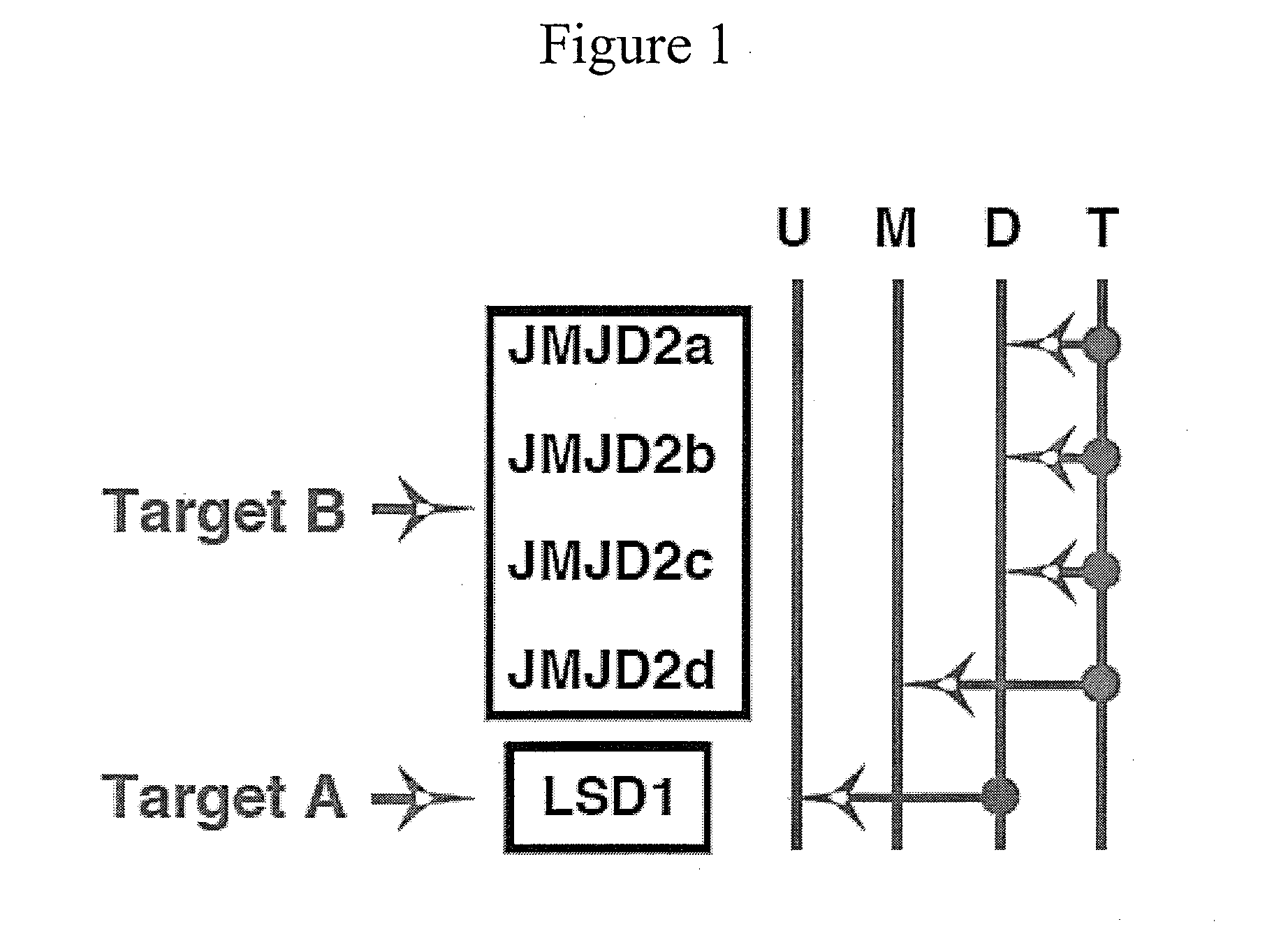
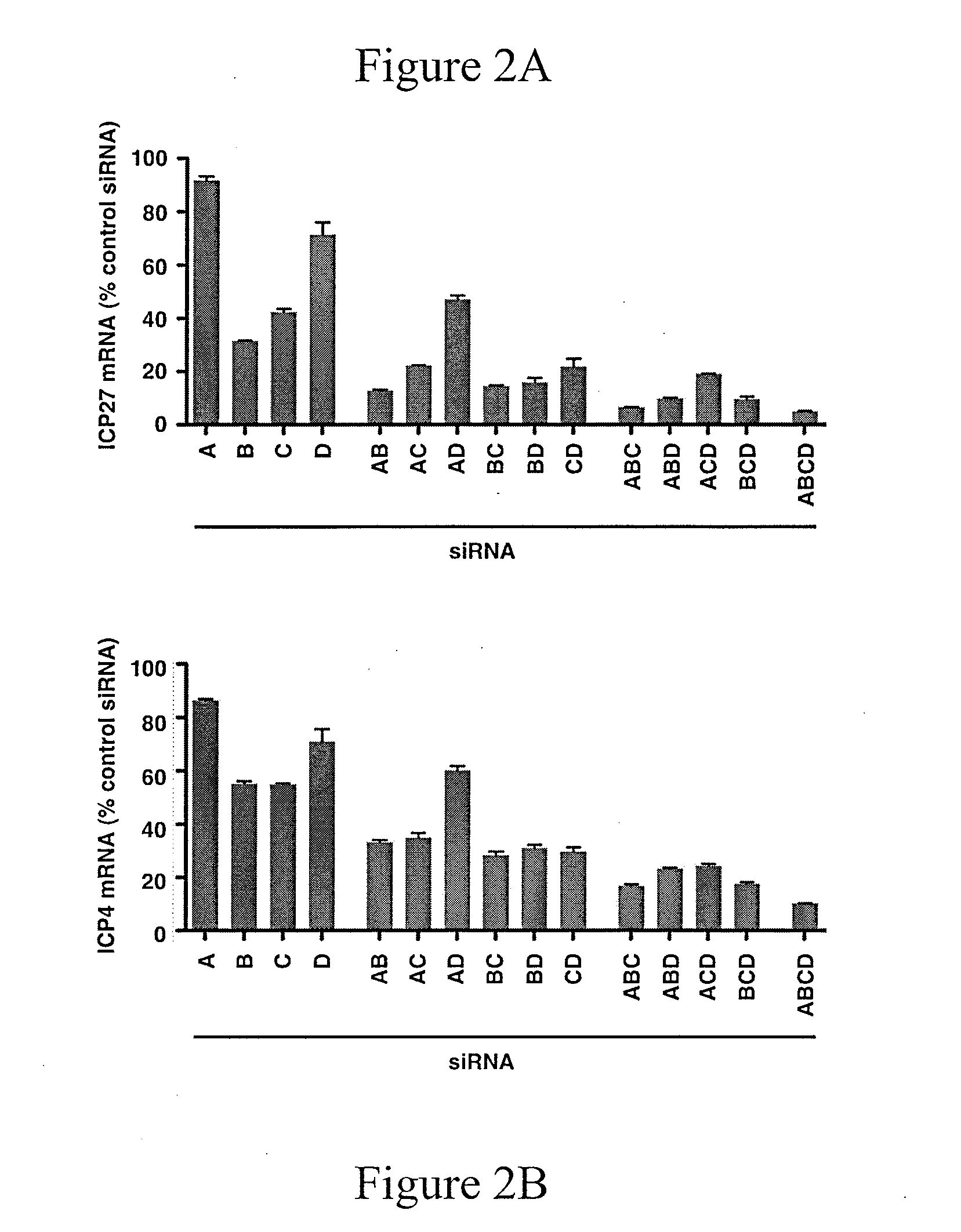
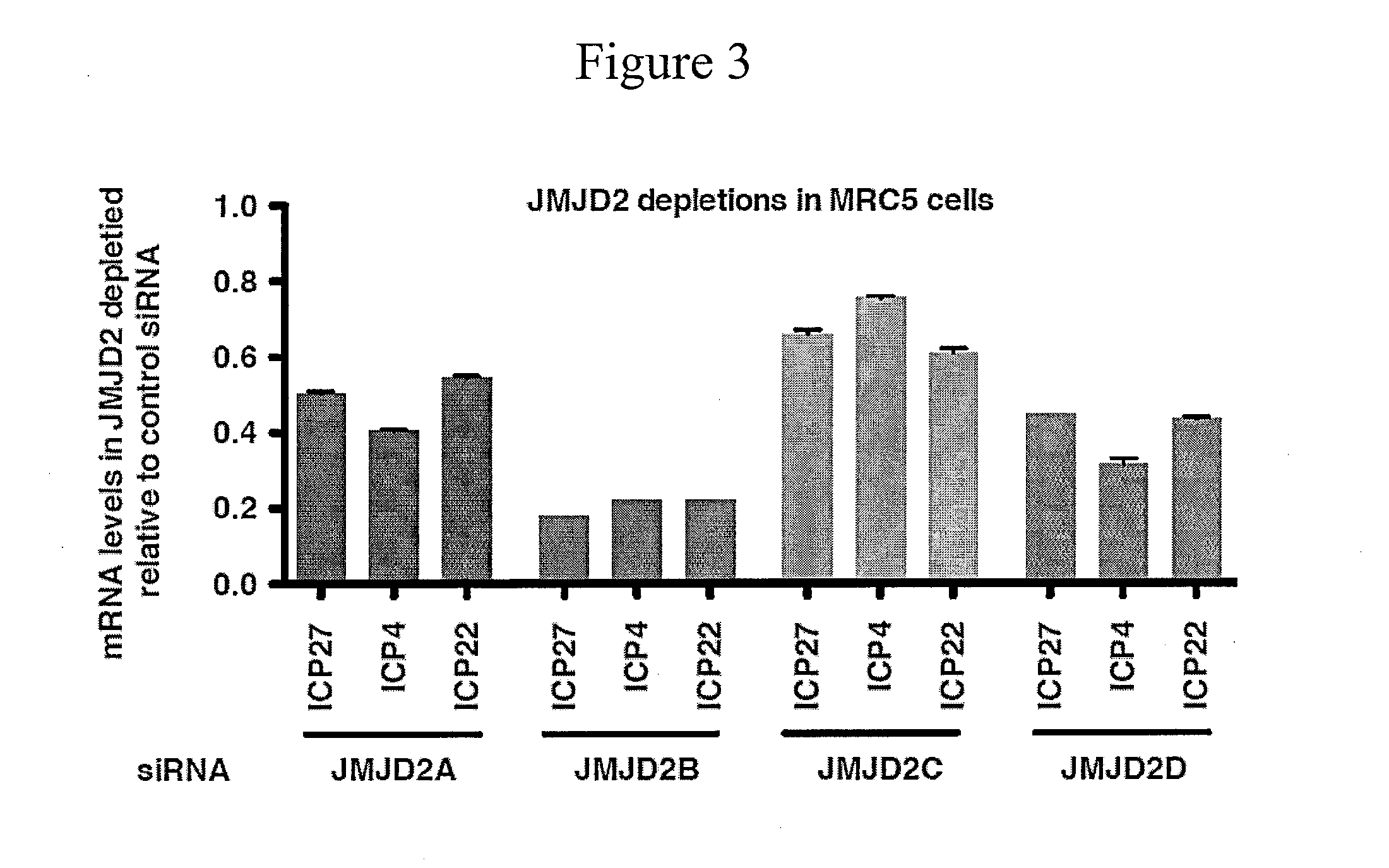
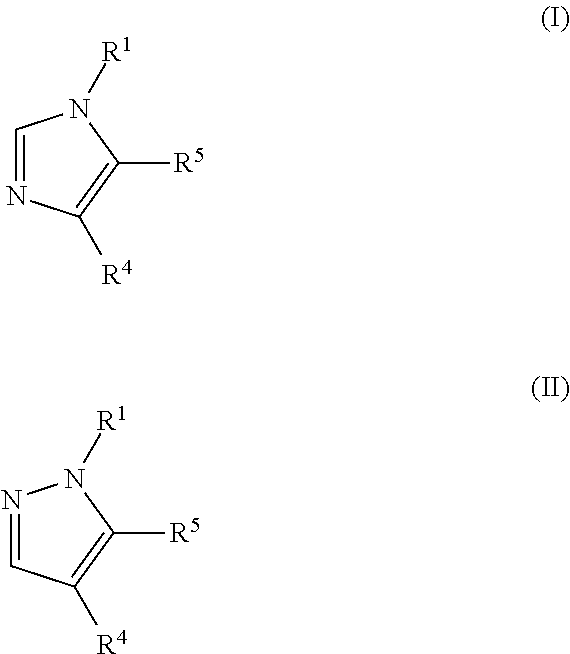
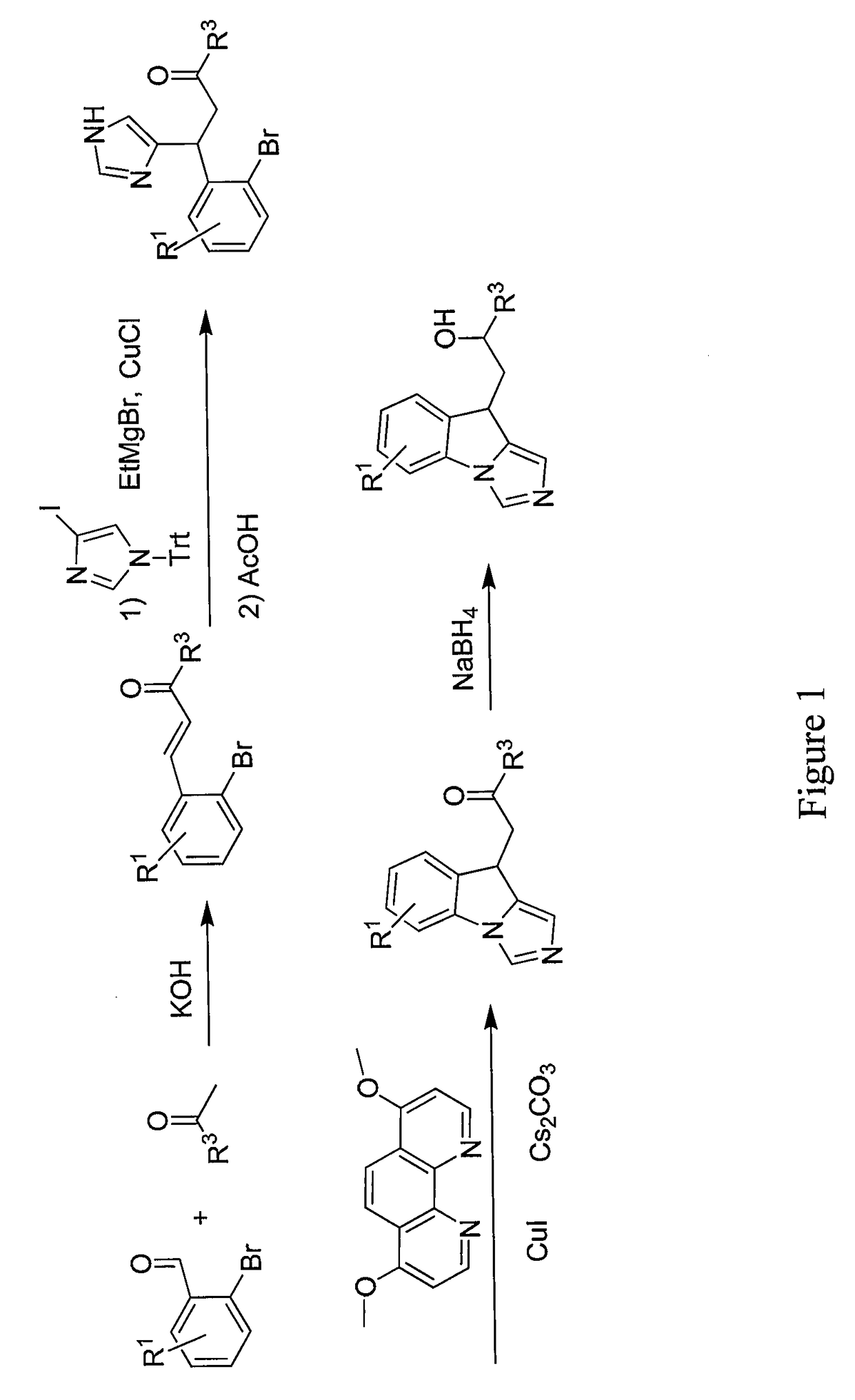
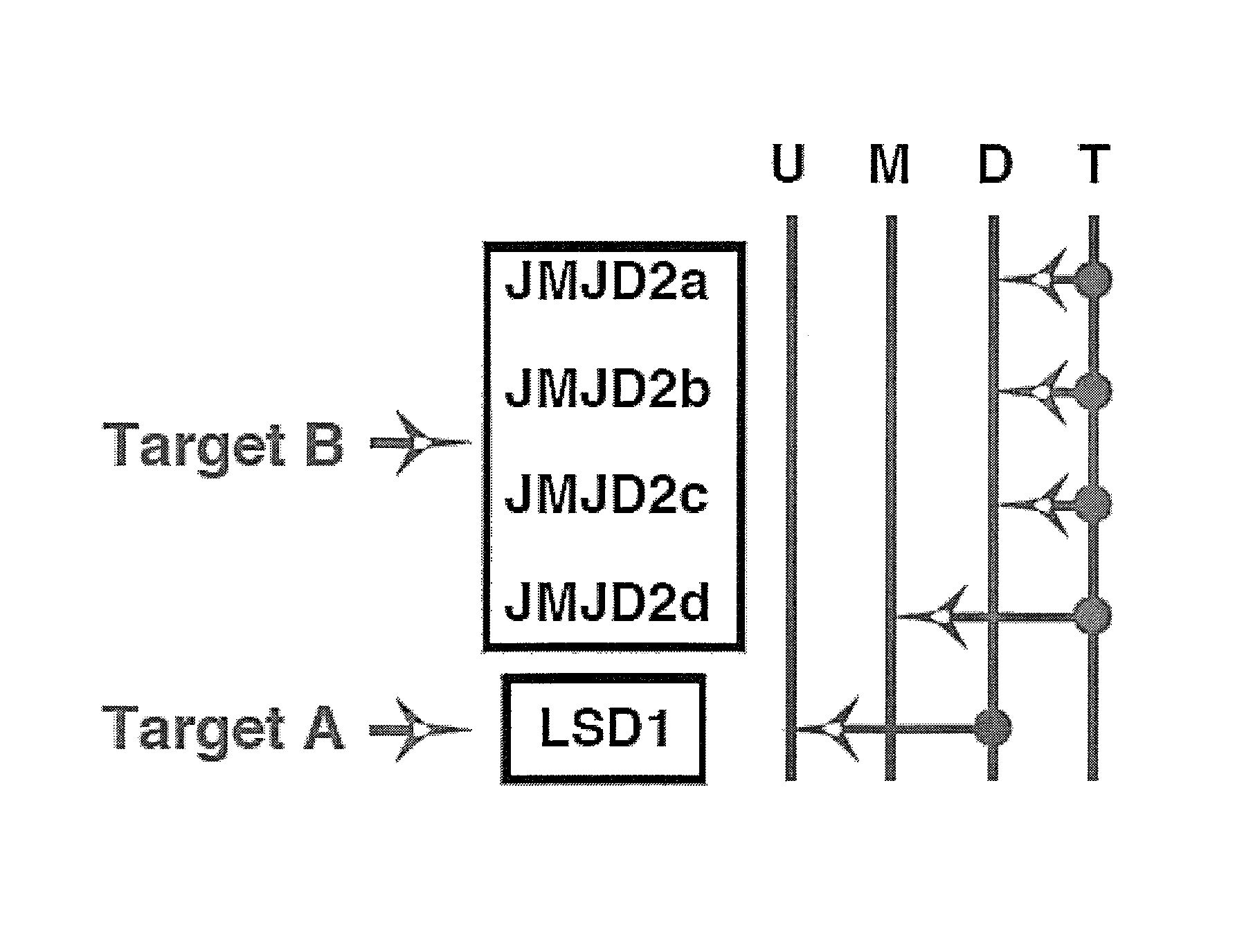
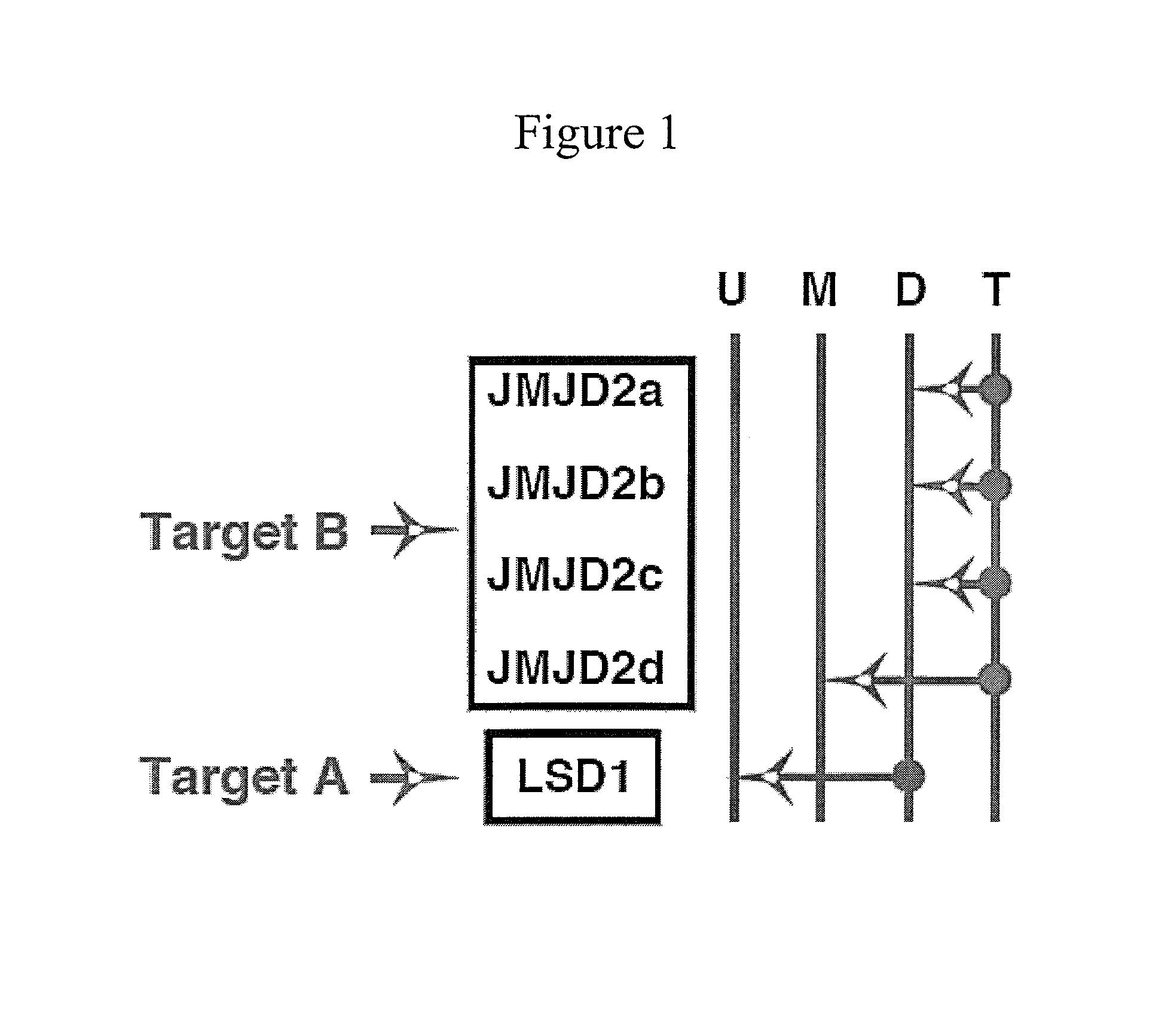
Disclosed are compounds and pharmaceutical compositions containing compounds that inhibit JMJD2 proteins, including those of the formula (I):wherein R1, R2, R3, R4, and R5 are as defined herein or pharmaceutically acceptable salts thereof. Also disclosed is a method of preventing or treating a viral infection of a host, comprising administering to the host an effective amount of an inhibitor of the JMJD2 family of histone demethylases, for example, a compound of the formula (I). The viral infection may be a primary infection, reactivation of a virus after latency in a host, or may be in a mammal that has undergone, is undergoing, or will undergo immunosuppressive therapy.
Owner:UNITED STATES OF AMERICA
IDO inhibitors
Presently provided are compounds according to the formula (I) or (II), and pharmaceutical compositions comprising the compounds, wherein R1, R4, and R5 are defined herein. Such compounds and compositions are useful for modulating an activity of indoleamine 2,3-dioxygenase; treating indoleamine 2,3-dioxygenase (IDO) mediated immunosuppression; treating a medical conditions that benefit from the inhibition of enzymatic activity of indoleamine -2,3-dioxygenase; enhancing the effectiveness of an anti-cancer treatment comprising administering an anti-cancer agent; treating tumor-specific immunosuppression associated with cancer; and treating immunosupression associated with an infectious disease.
Owner:LANKENAU INST FOR MEDICAL RES +1
Tricyclic compounds as inhibitors of immunosuppression mediated by tryptophan metabolization
InactiveUS9617272B2Improve efficiencyEffective inhibiting amountOrganic active ingredientsOrganic chemistryAnticarcinogenOxygenase
Presently provided are inhibitors of IDO and TDO and pharmaceutical compositions thereof, useful for modulating an activity of indoleamine 2,3-dioxygenase and tryptophan 2,3 dioxygenase; treating immunosuppression; treating a medical conditions that benefit from the inhibition of tryptophan degradation; enhancing the effectiveness of an anti-cancer treatment comprising administering an anti-cancer agent; treating tumor-specific immunosuppression associated with cancer; and treating immunosuppression associated with an infectious disease.
Owner:NEWLINK GENETICS
Method of inducing anergic T helper cells
InactiveUS7144728B1Reduce riskLower Level RequirementsMicrobiological testing/measurementGenetically modified cellsDiseaseAutoimmune disease
This invention also provides a method of generating antigen specific human suppressor CD8+CD28− T cells. This invention further provides a method of generating allopeptide antigen specific human suppressor CD8+CD28− T cells. Methods of tent for reduction of risk of rejection of allografts and xenografts and autoimmune diseases using the human suppressor CD8+CD28− T cells so produced are also provided, as are methods of preventing rejection and autoimmune diseases, and vaccines comprising the produced suppressor T cells. Methods of diagnosis to determine whether a level of immuno-suppressant therapy requires a reduction are provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
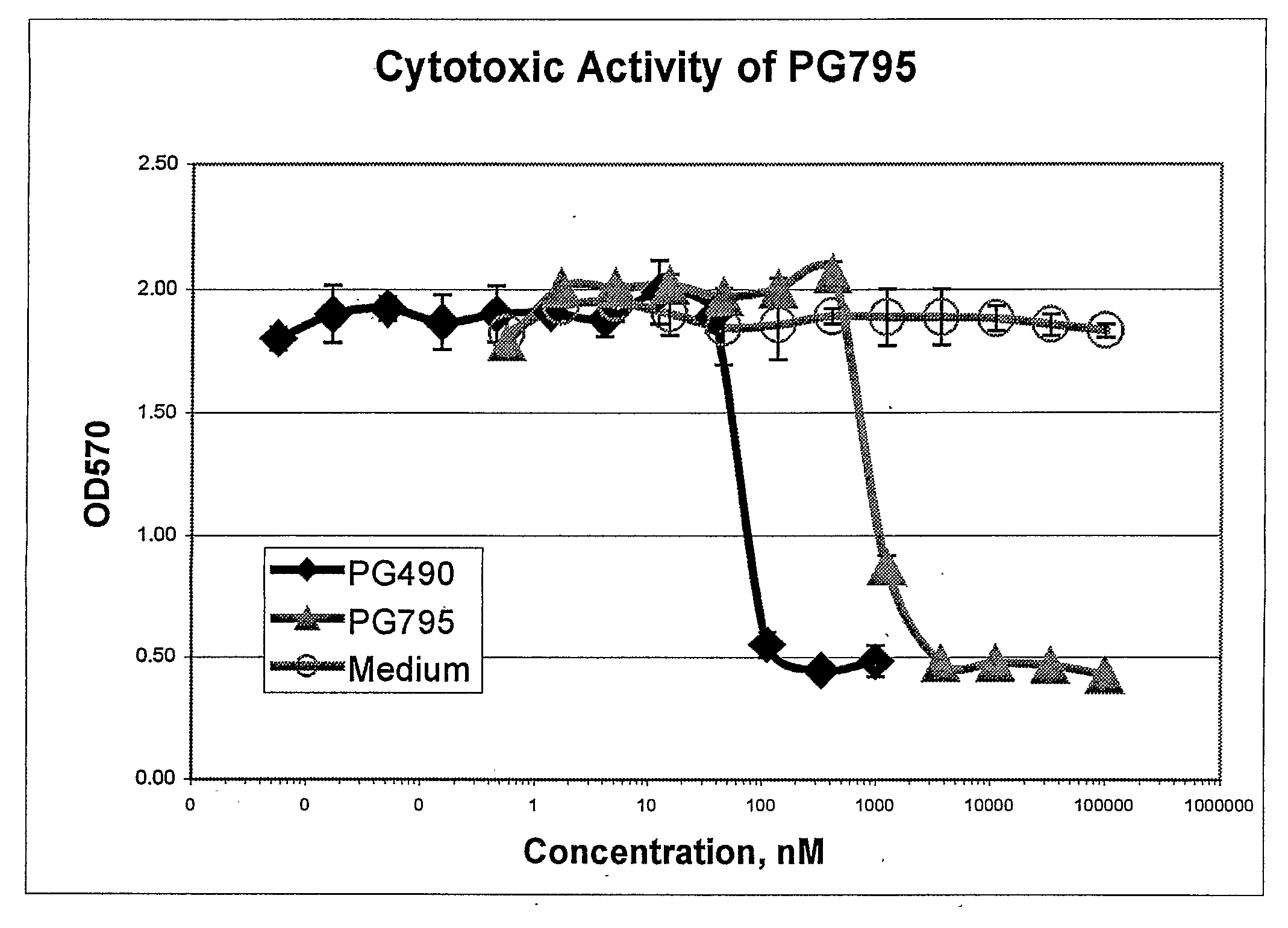
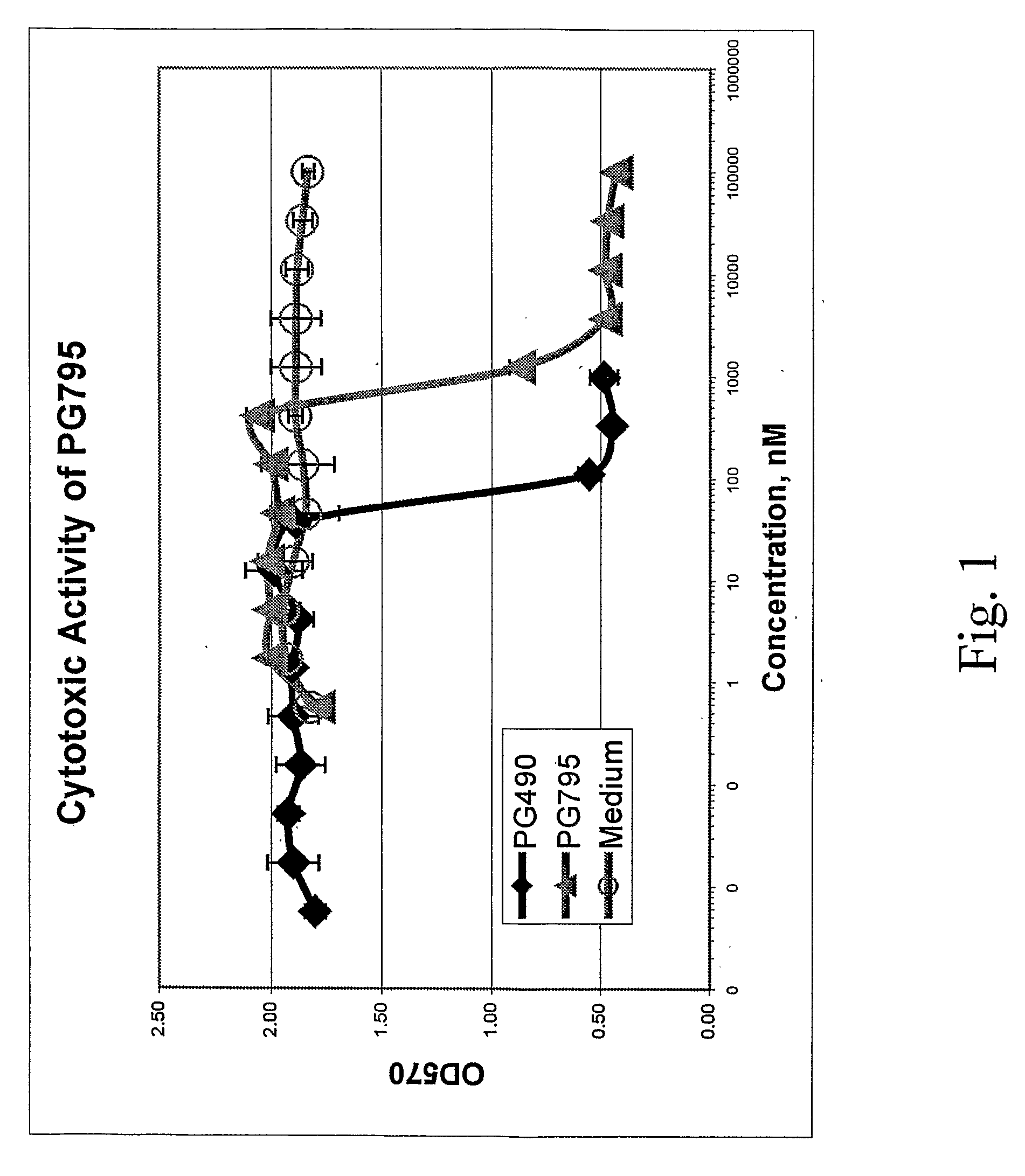
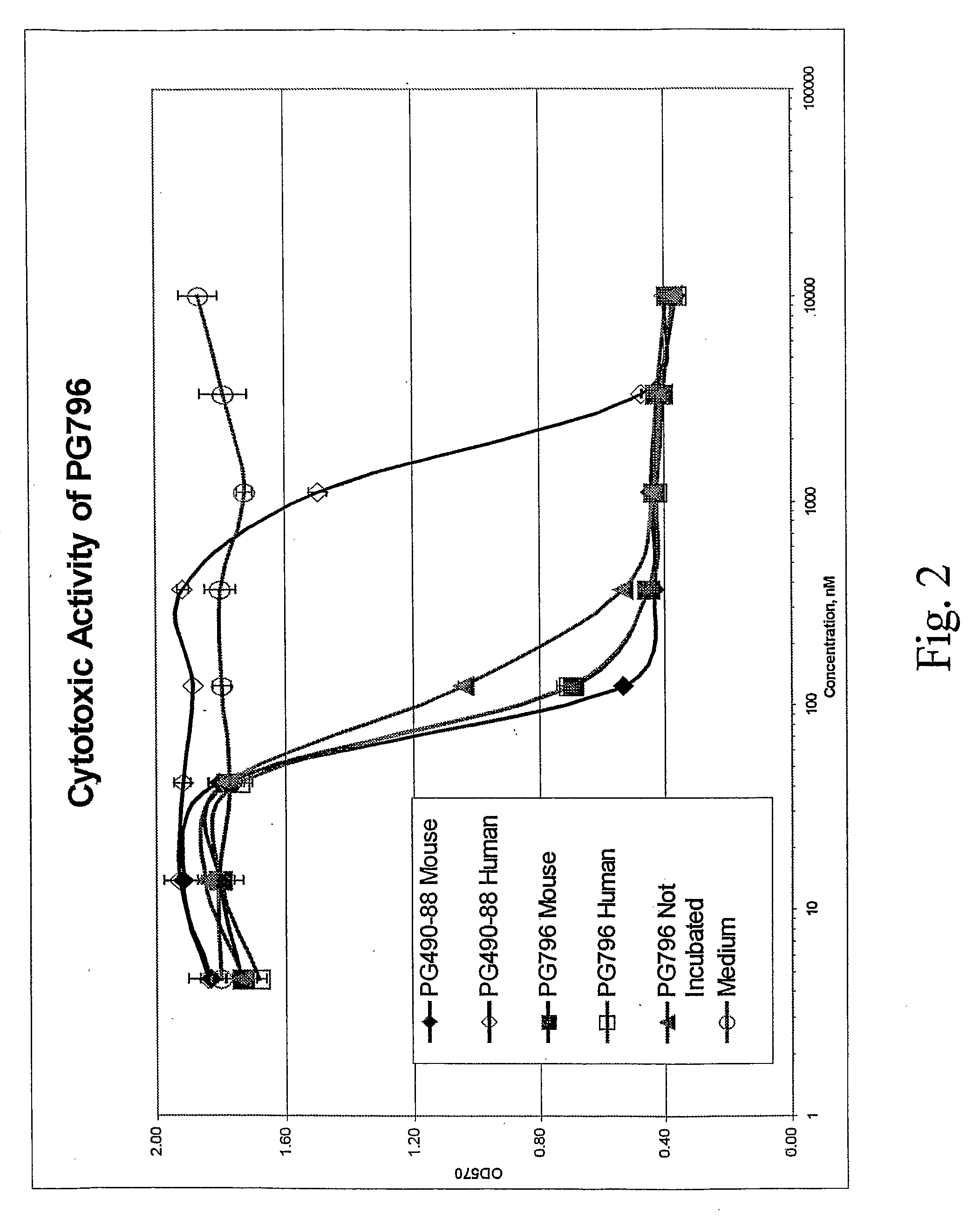
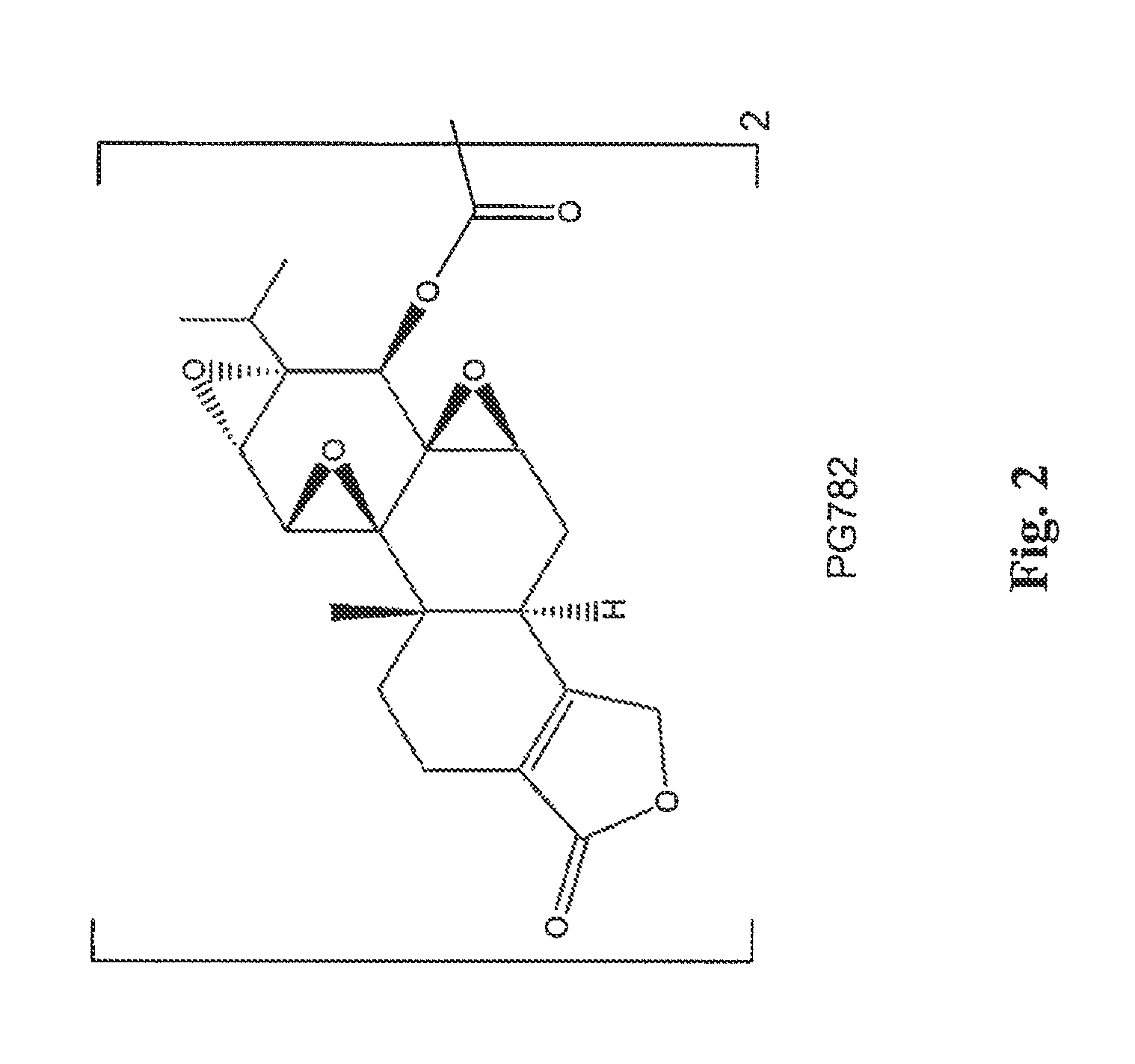
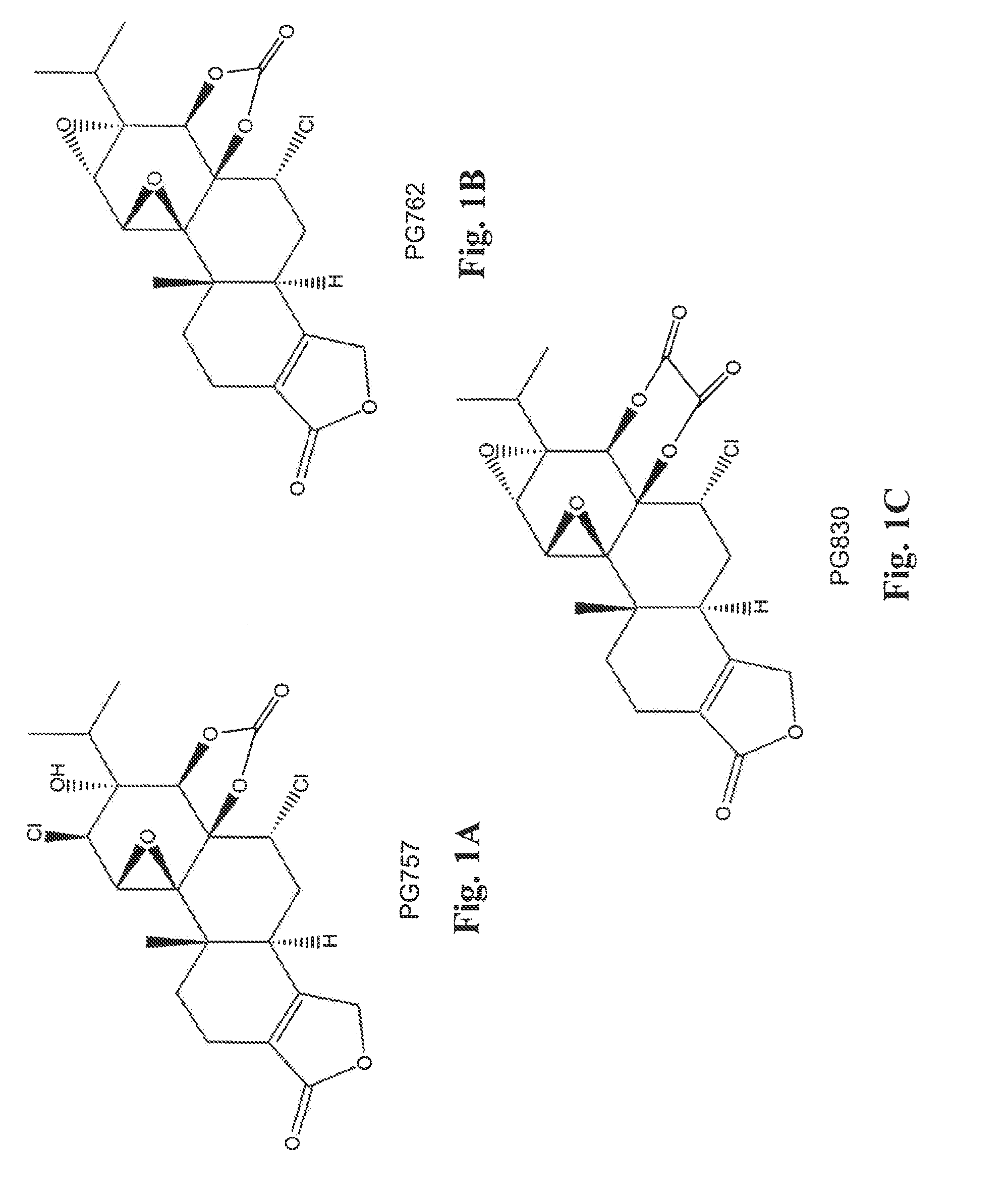
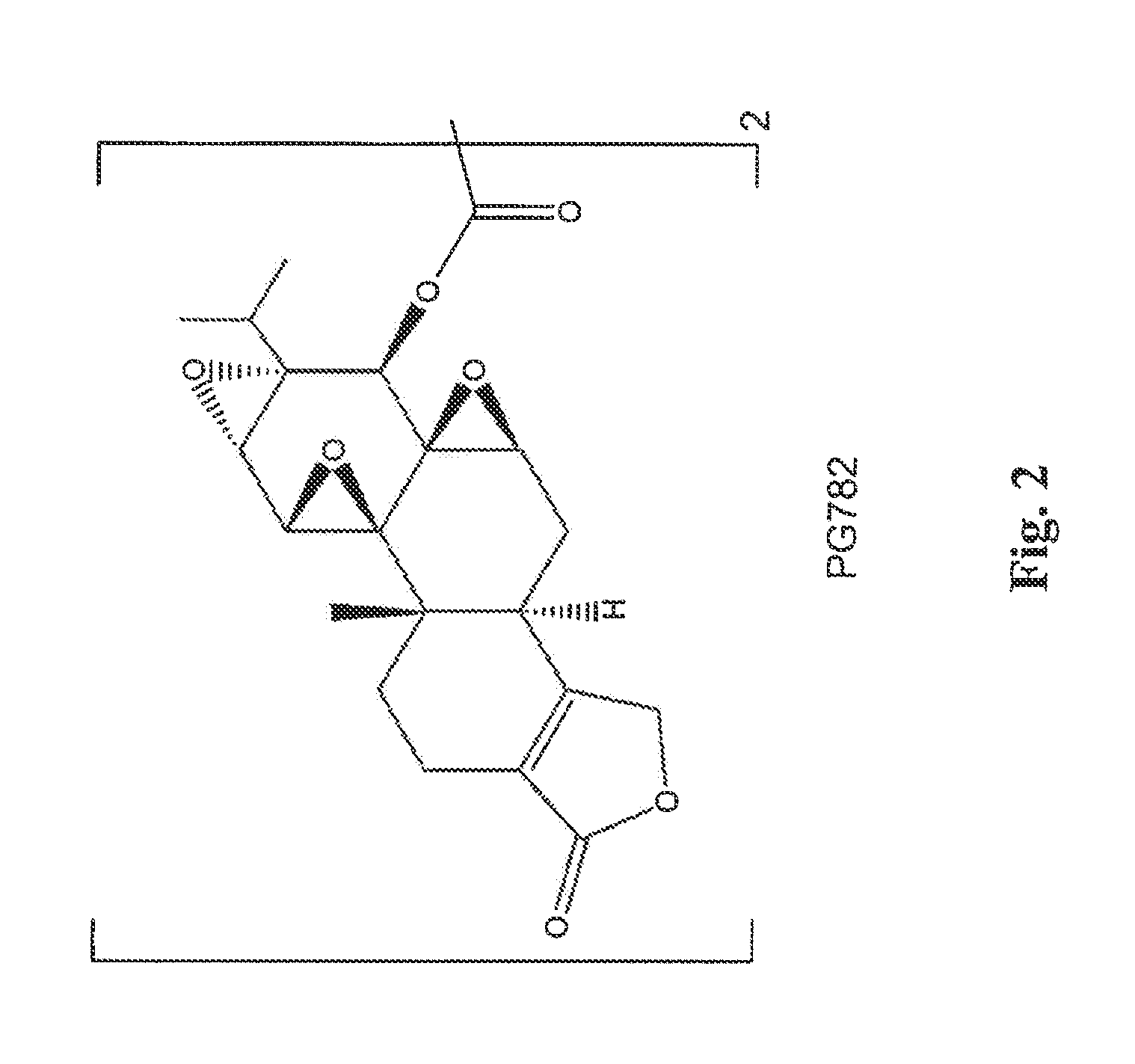
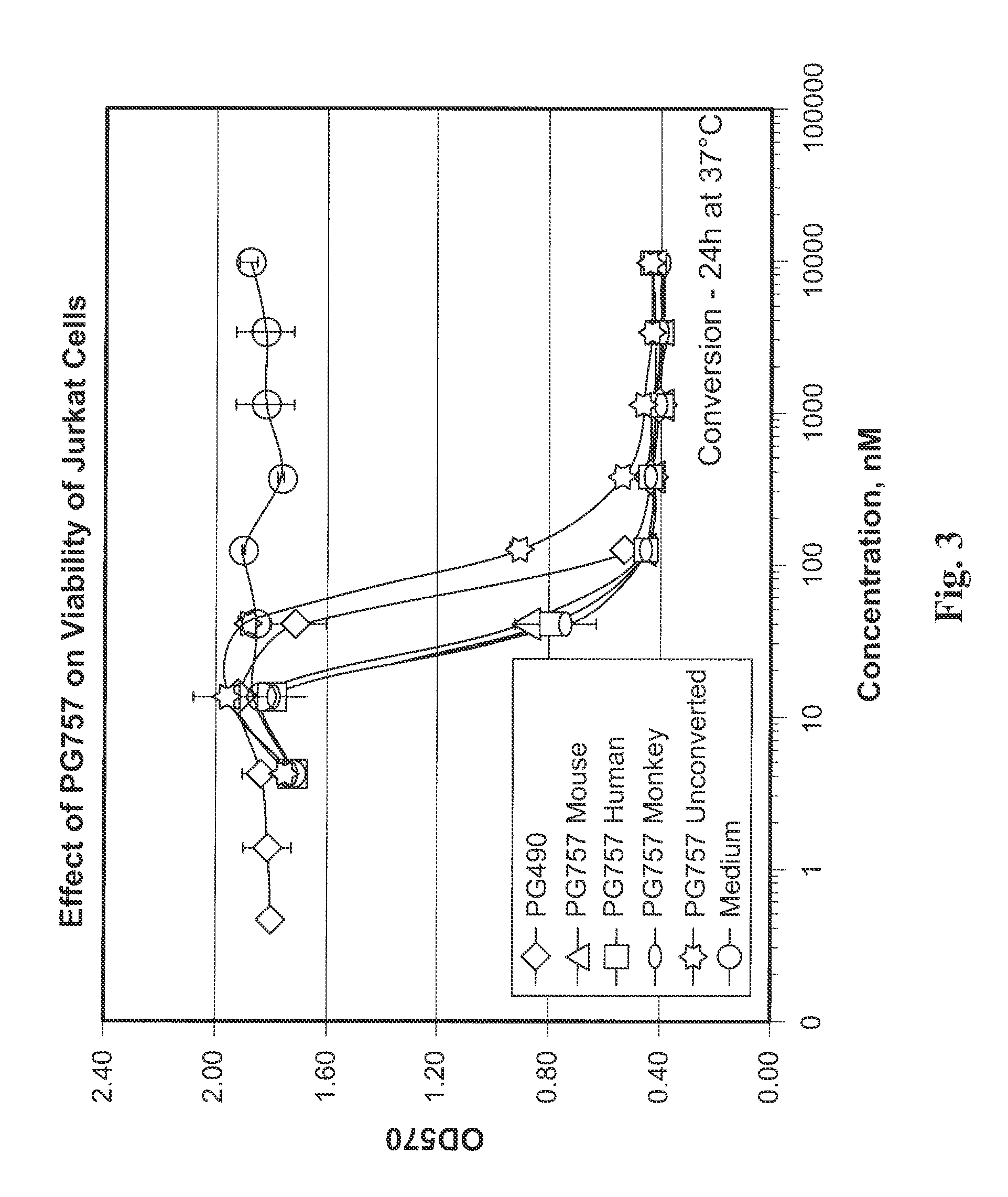
Tripolide Lactone Ring Derivatives as Immunomodulators and Anticancer Agents
Owner:PHARMAGENESIS
Methods and Compositions for Determining a Graft Tolerant Phenotype in a Subject
InactiveUS20110201519A1Reduce immunosuppressionMicrobiological testing/measurementLibrary screeningGraft ToleranceRegimen
Methods are provided for determining whether a subject has a graft tolerant phenotype. In practicing the subject methods, the expression of at least 5 genes in a sample from the subject, e.g., a blood sample, is assayed to obtain a gene expression result for the at least 5 genes. The obtained gene expression result for the at least 5 genes is then employed to determine whether the subject has a graft tolerant phenotype. Also provided are compositions, systems and kits that find use in practicing the subject methods. The methods and compositions find use in a variety of applications, including the determination of an immunosuppressive therapy regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
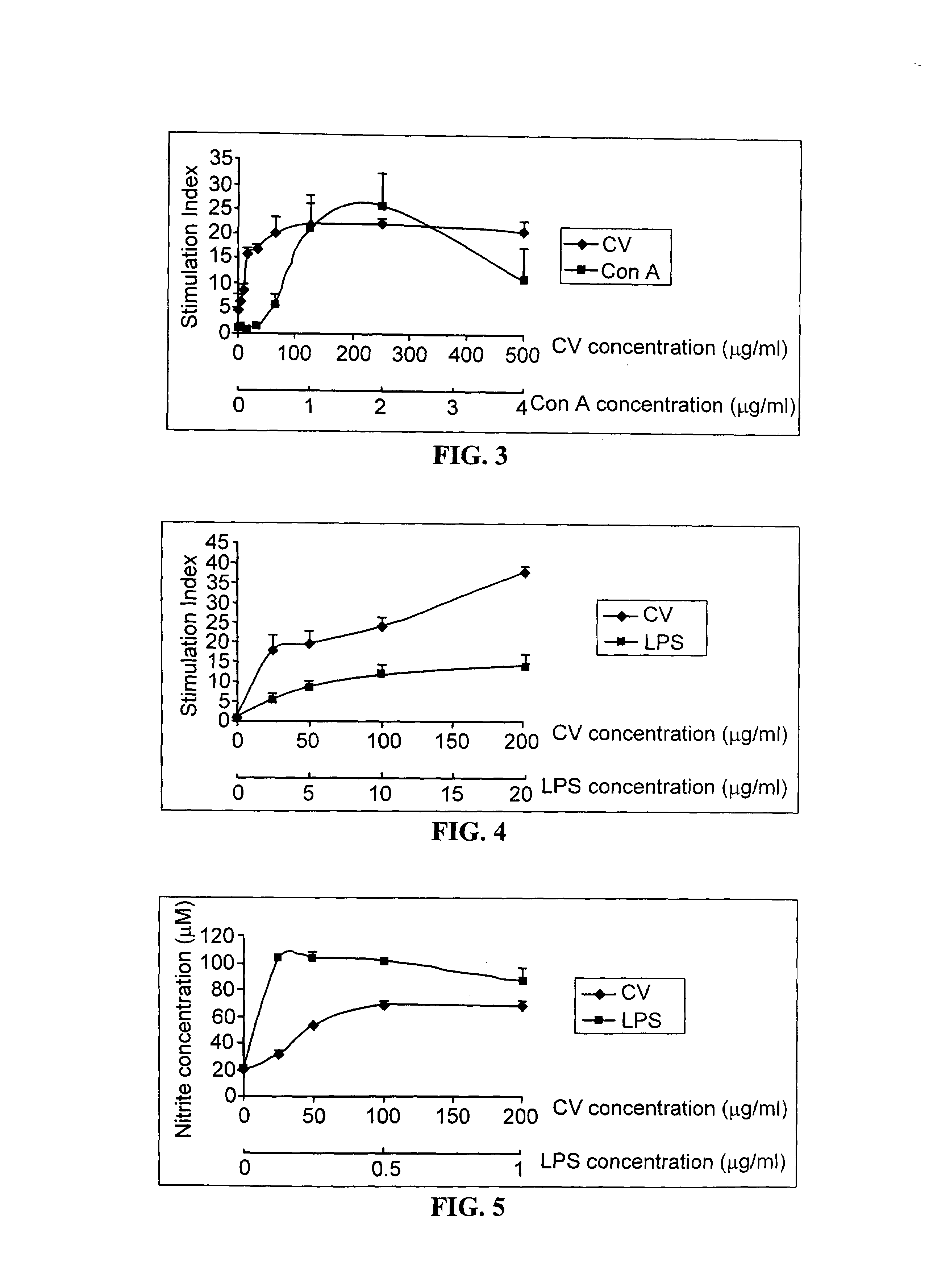
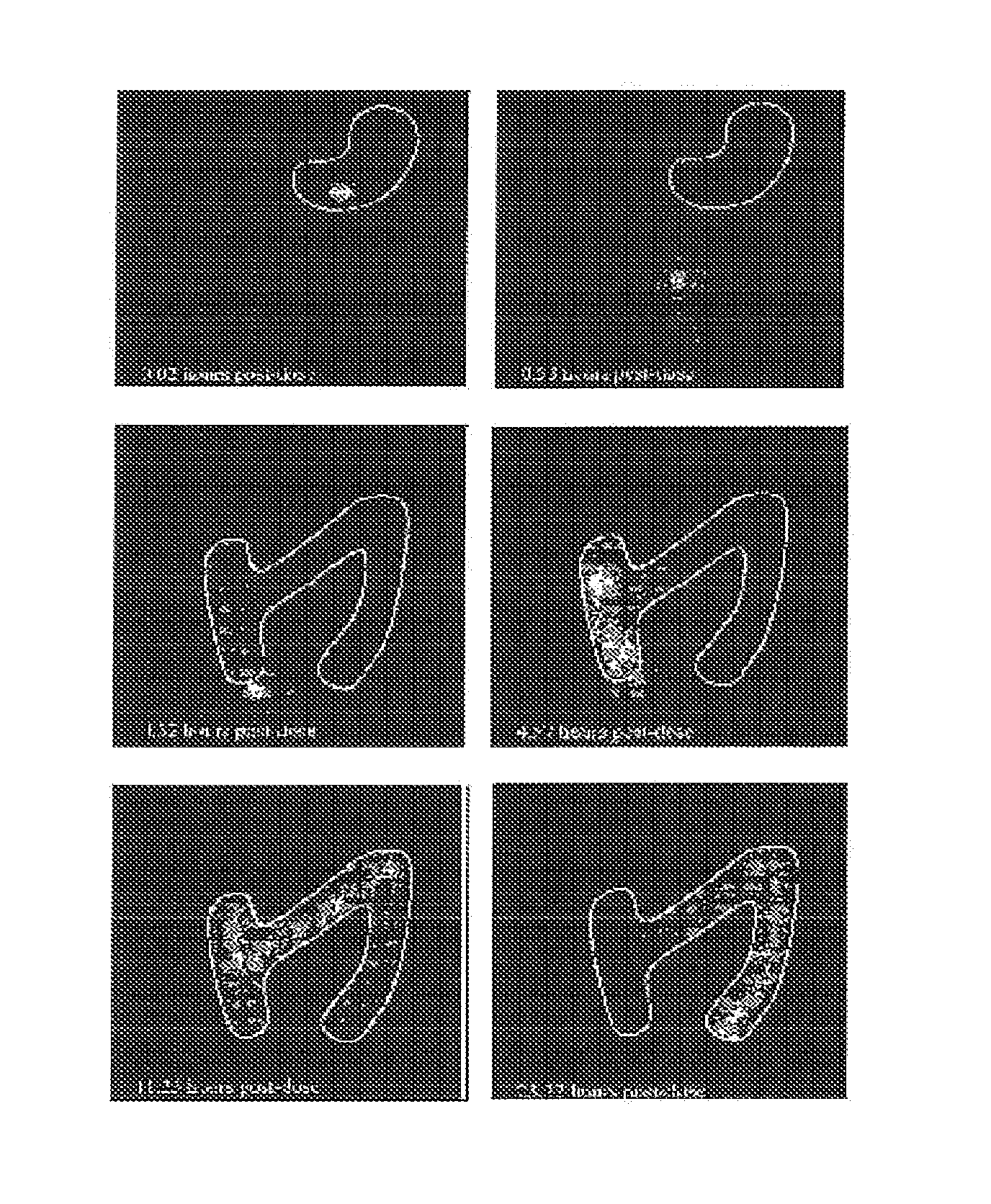
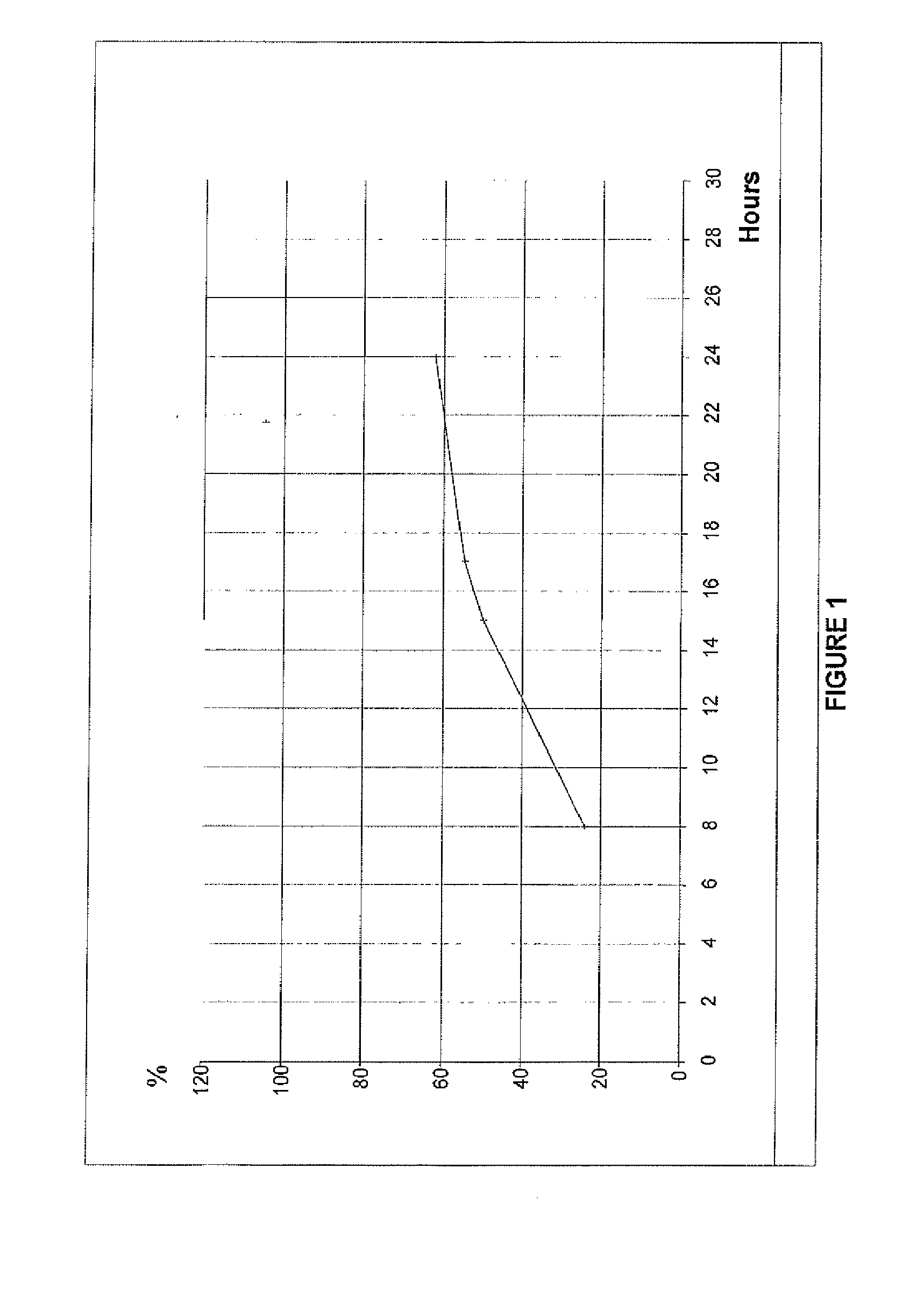
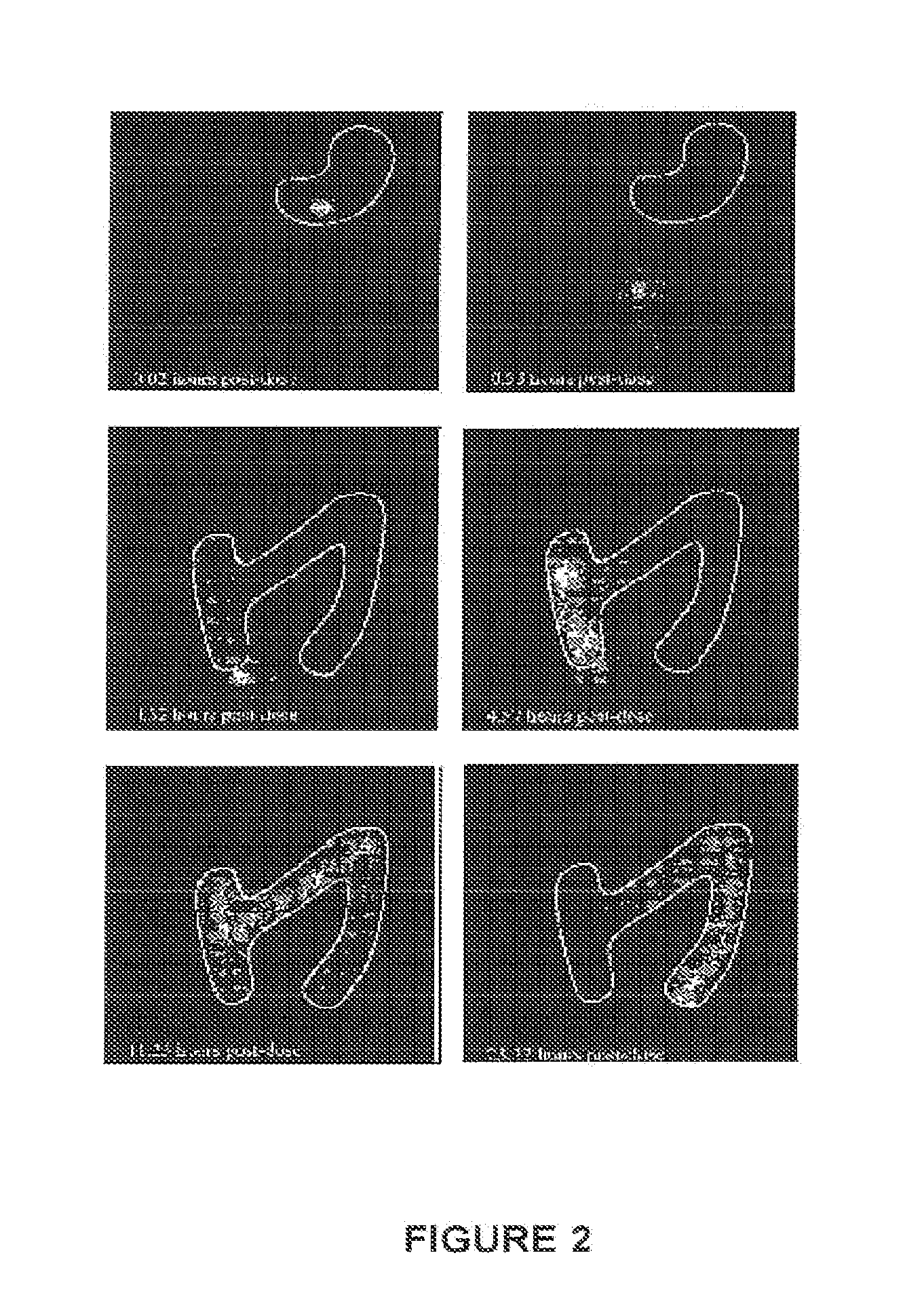
Preparation and standardization of immunomodulatory peptide-linked glucans with verifiable oral absorbability from coriolus versicolor
This invention provides compositions and methods for stimulating the immune system. Such methods include administering an extract, purified peptide-linked glucan or active component thereof from Coriolus versicolor. The methods are particularly useful for prophylactic and therapeutic treatment of secondary immunodeficiency, wherein the immunodeficiency is the result of an infection, a malignant neoplastic disease, an autoimmune disease, a protein losing state, an immunosuppressive treatment, surgery or anesthesia.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Tacrolimus For Improved Treatment Of Transplant Patients
InactiveUS20110275659A1Reduce riskAffect bioavailabilityBiocidePharmaceutical non-active ingredientsIn vivoBioavailability
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARMA
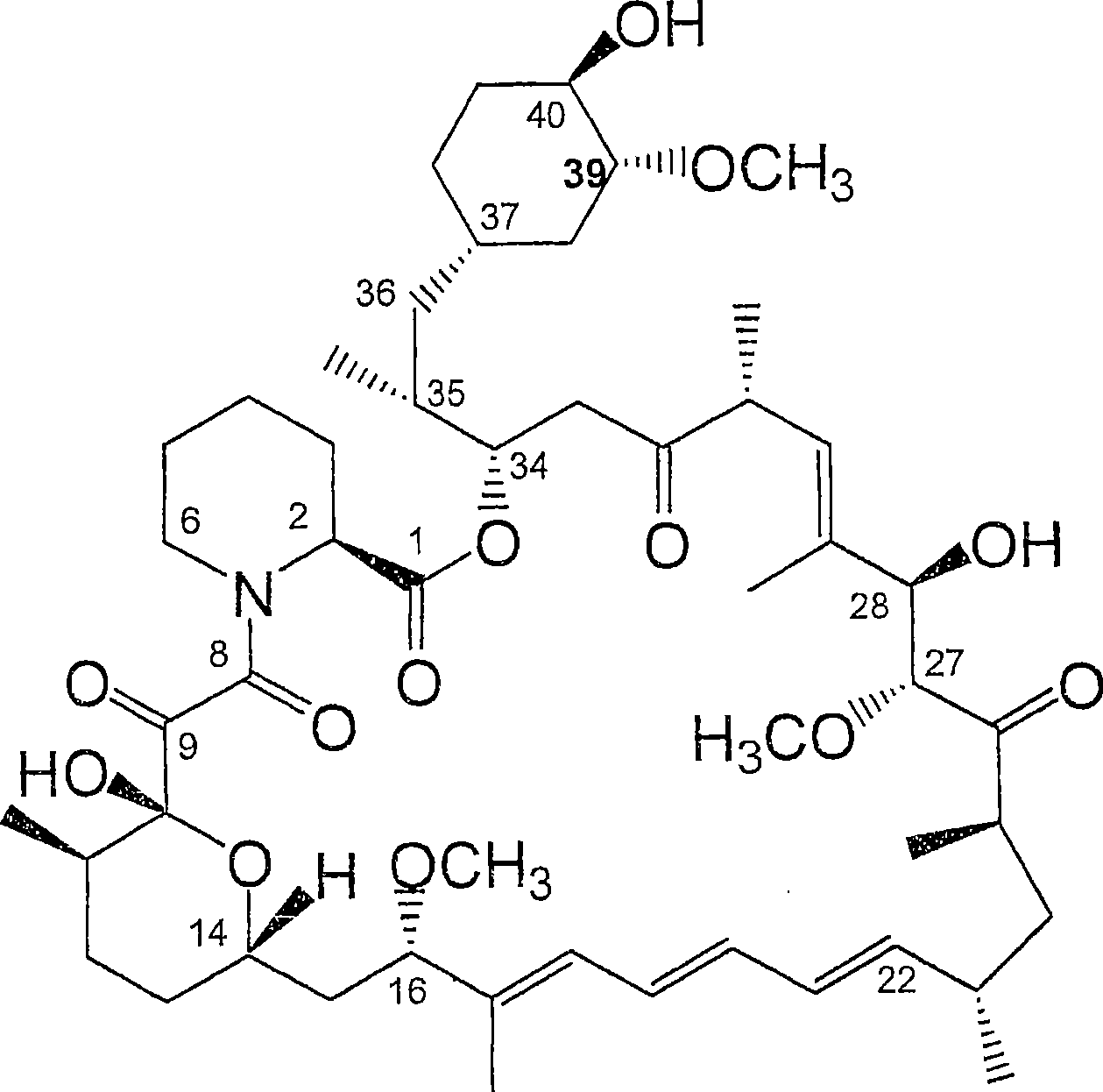
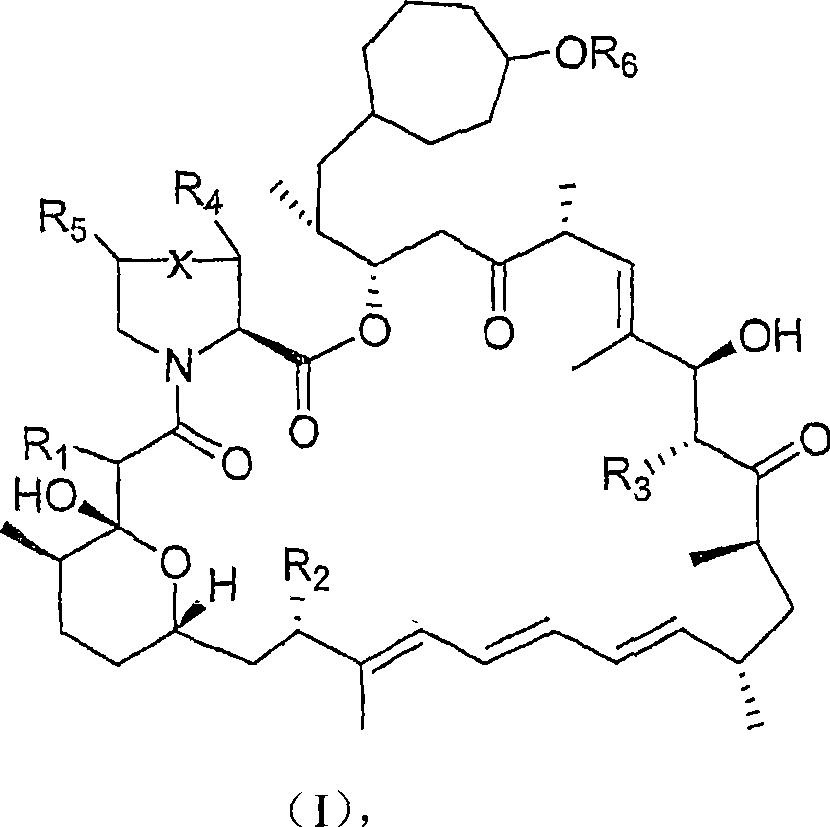
36 -des (3 -methoxy-4 -hydroxycyclohexyl) 36 - (3 -hydroxycycloheptyl) derivatives of rapamycin for the treatment of cancer and other disorders
The present invention relates to novel 36-des(3-methoxy-4-hydroxycyclohexyl)-36-(3-hydroxycycloheptyl)rapamycin derivatives, methods for their production, and uses thereof. In a further aspect the present invention provides for the use of these 36-des(3-methoxy-4-hydroxycyclohexyl)-36-(3-hydroxycycloheptyl)rapamycin derivatives in the treatment of cancer and / or B-cell malignancies, the induction or maintenance of immunosuppression, the treatment of transplantation rejection, graft: vs. host disease, autoimmune disorders, diseases of inflammation, vascular disease and fibrotic diseases, the stimulation of neuronal regeneration or the treatment of fungal infections.
Owner:DEBIOTECH SA
Triptolide C-ring derivatives as anticancer agents and immune modulators
Disclosed are compounds based on C- and D-ring modifications of triptolide and hydroxylated triptolide, for use in therapy, such as antiproliferative, anticancer, and immunosuppressive therapy.
Owner:PHARMAGENESIS
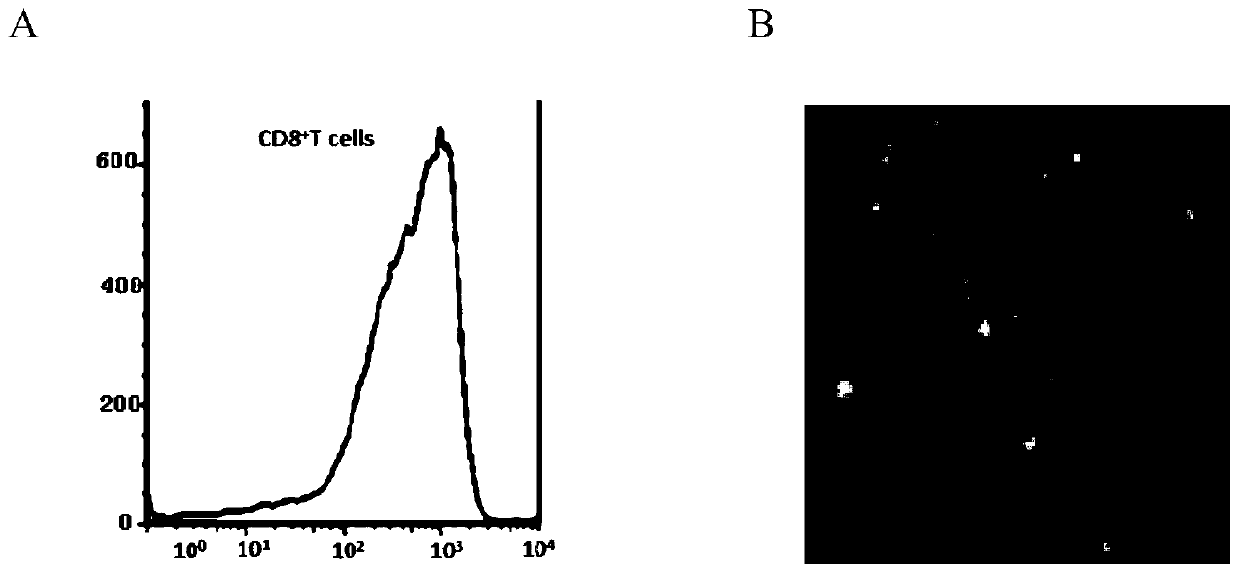
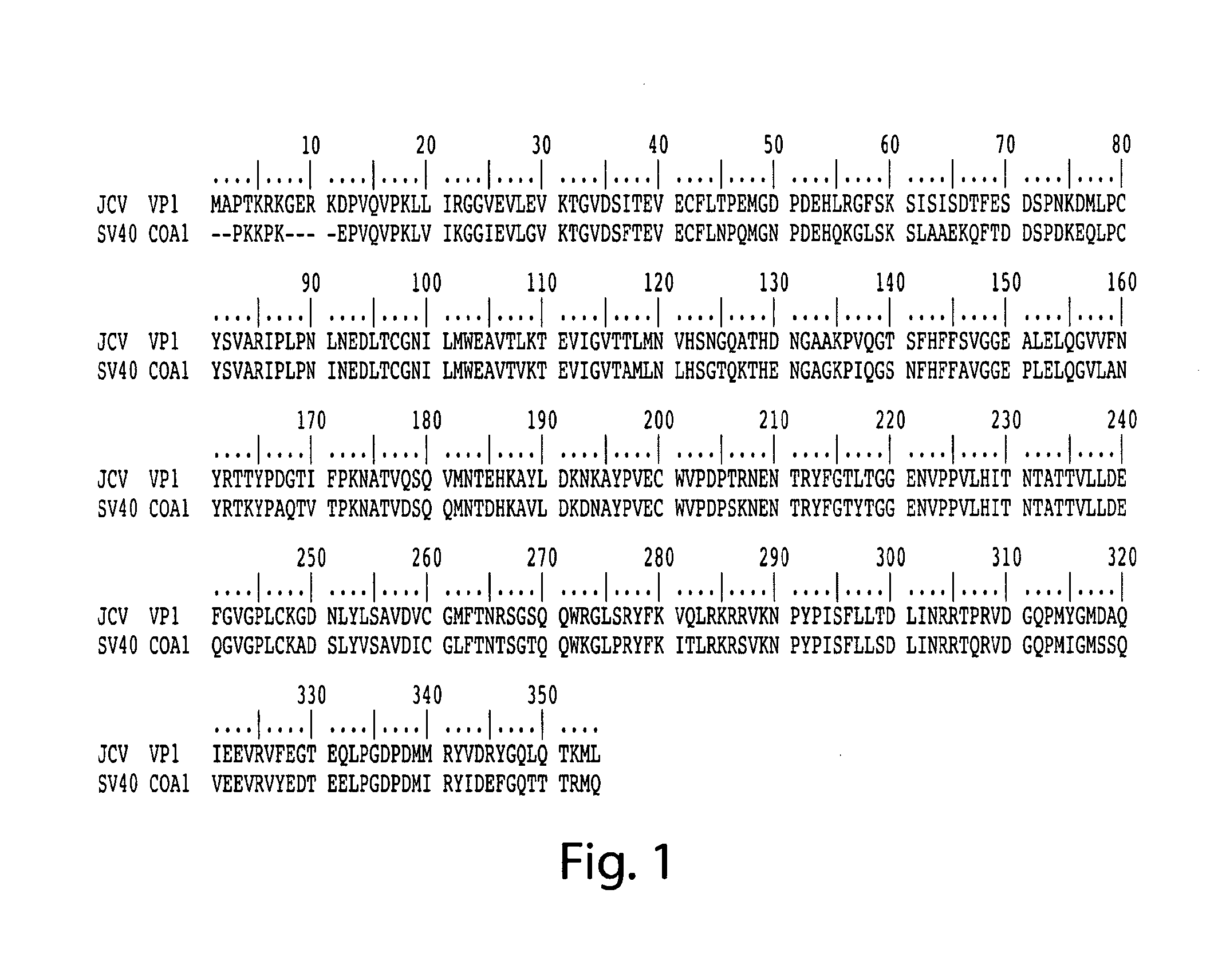
Methods for the detection of jc polyoma virus
InactiveUS20120258443A1Improved profileHigh profileMicrobiological testing/measurementDisease diagnosisSequence variationSialic acid binding
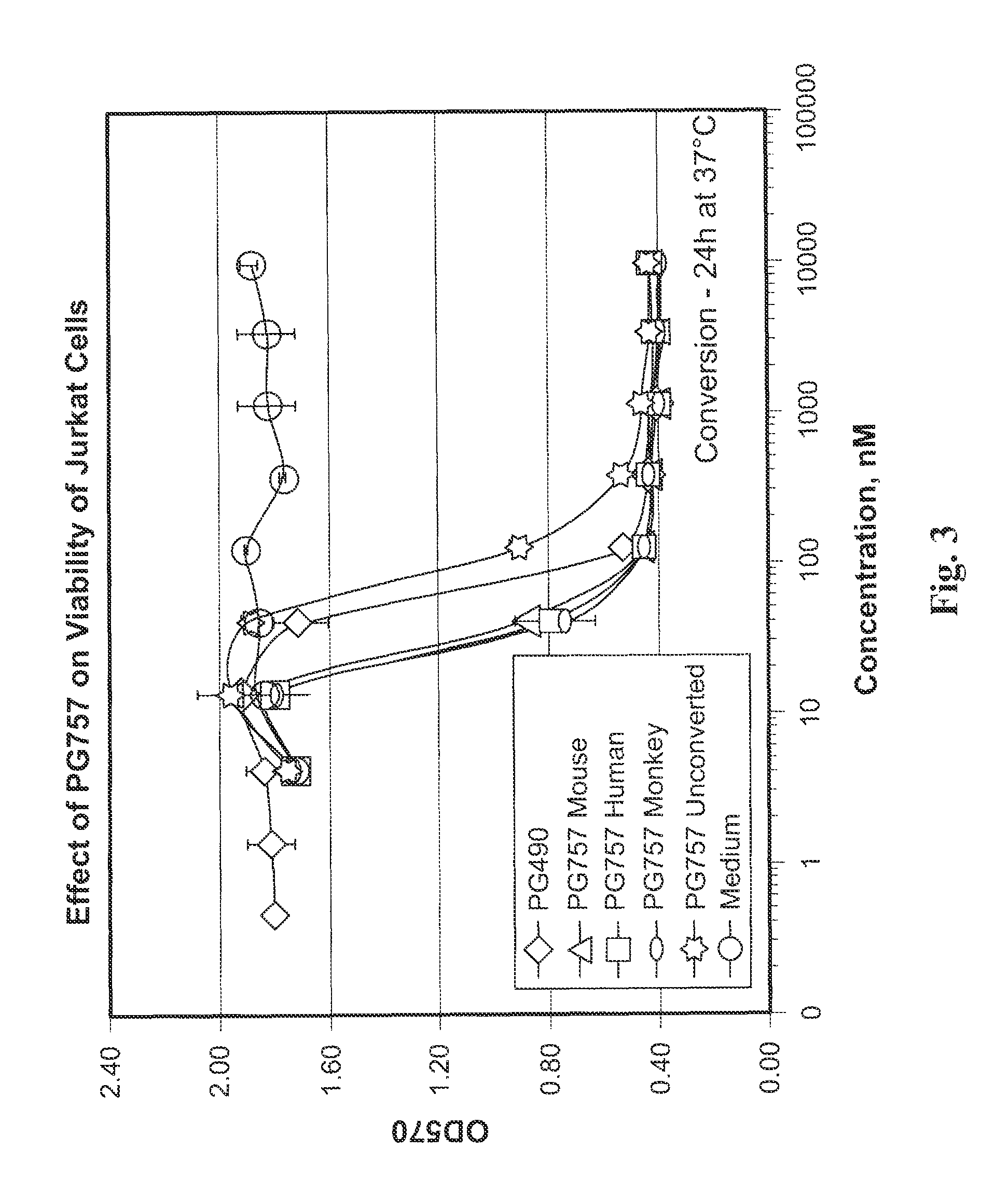
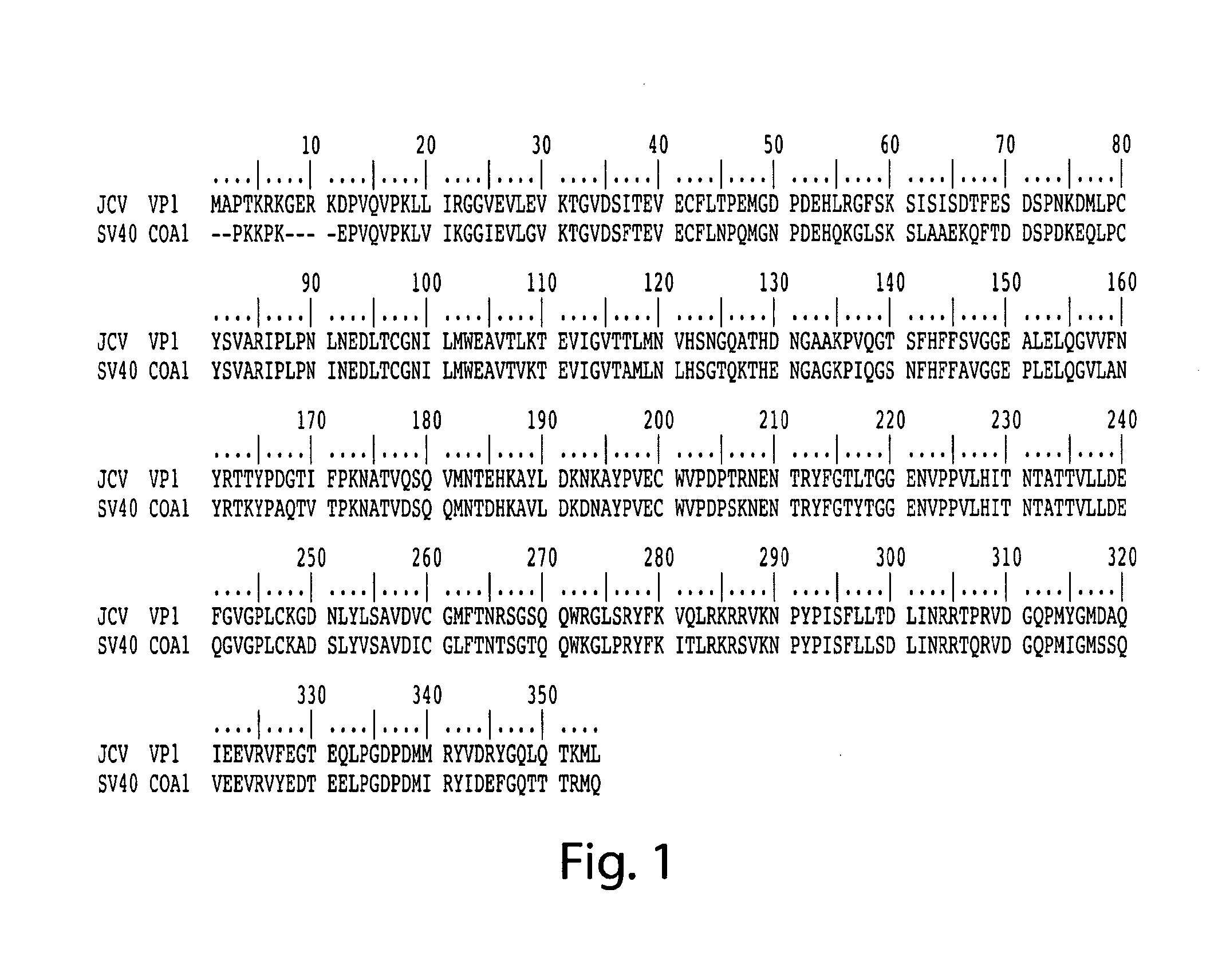
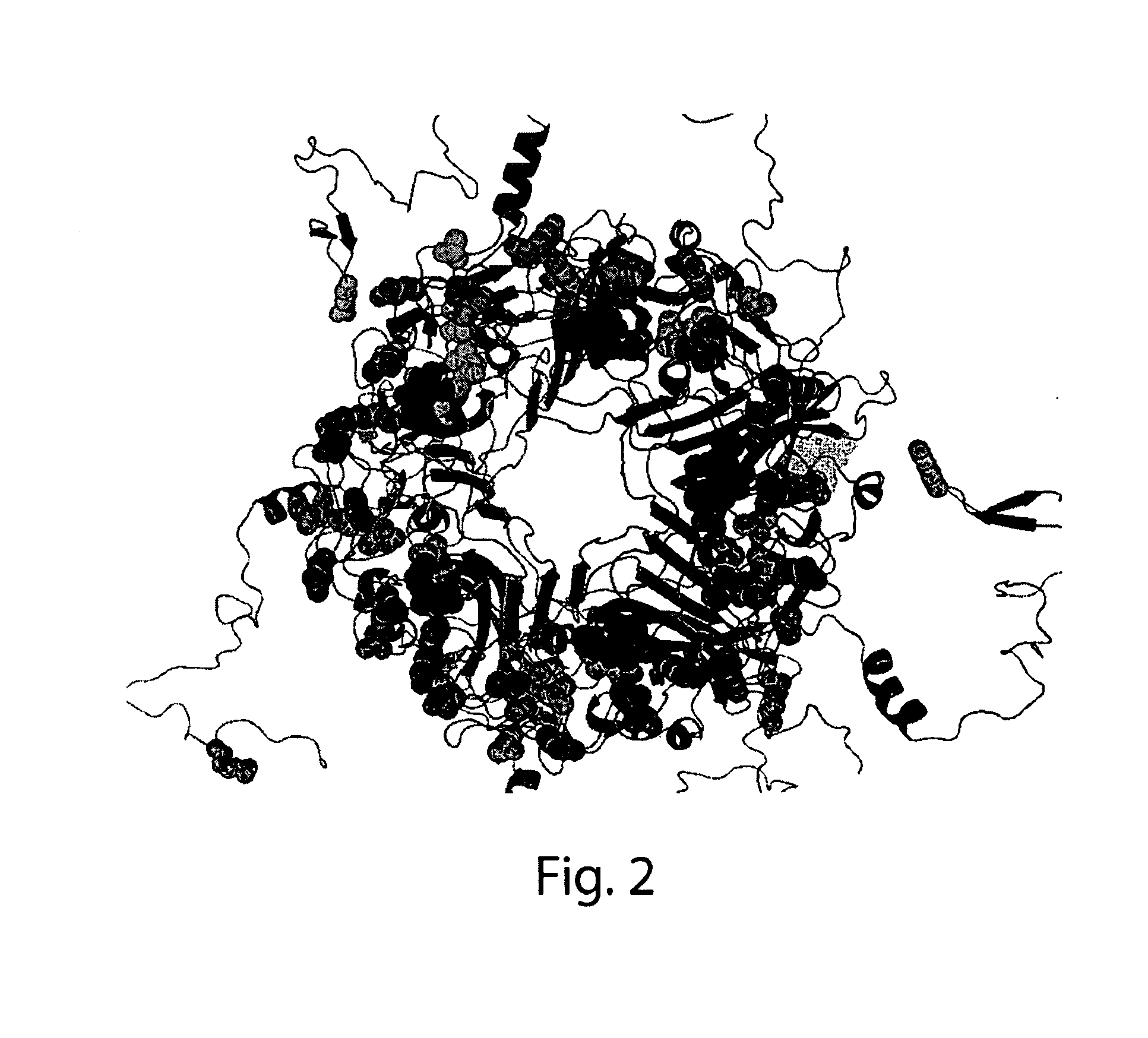
Methods and compositions for determining whether a subject is at risk for PML, including subjects being treated with immunosuppressants, by determining whether the subject harbors a JCV variant with reduced binding for sialic acid relative to a normal JCV, are presented. Furthermore, combinations of JCV-VP1 sequence variations that are associated with PML and that can be used as a basis of an assay for identifying subjects susceptible to PML, subjects with PML (e.g., early stage PML), or subjects at risk of developing PML in response to an immunosuppressive treatment are provided.
Owner:BIOGEN MA INC
Methods for determining the risk of acute graft versus host disease
ActiveUS20150301022A1Increased riskBiocidePhosphorous compound active ingredientsCandidate donorSub populations
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Method of preventing or treating viral infection
Disclosed are compounds and pharmaceutical compositions containing compounds that inhibit JMJD2 proteins, including those of the formula (I):wherein R1, R2, R3, R4, and R5 are as defined herein or pharmaceutically acceptable salts thereof. Also disclosed is a method of preventing or treating a viral infection of a host, comprising administering to the host an effective amount of an inhibitor of the JMJD2 family of histone demethylases, for example, a compound of the formula (I). The viral infection may be a primary infection, reactivation of a virus after latency in a host, or may be in a mammal that has undergone, is undergoing, or will undergo immunosuppressive therapy.
Owner:UNITED STATES OF AMERICA
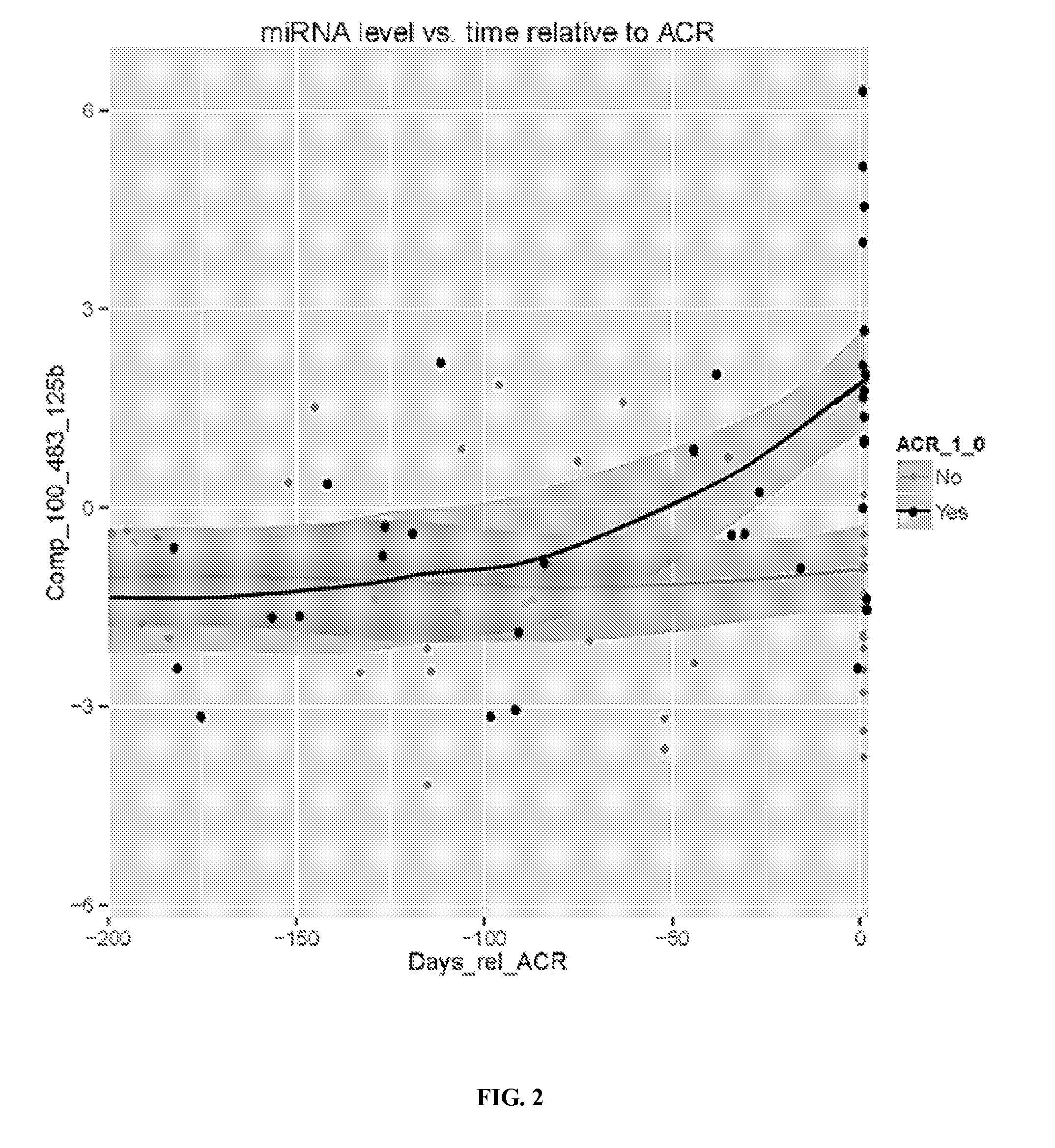
Use of micro-ribonucleic acid (MIRNA) to diagnose transplant rejection and tolerance of immunosuppression therapy
InactiveUS20170032100A1Medical simulationMicrobiological testing/measurementTolerabilityTransplant rejection
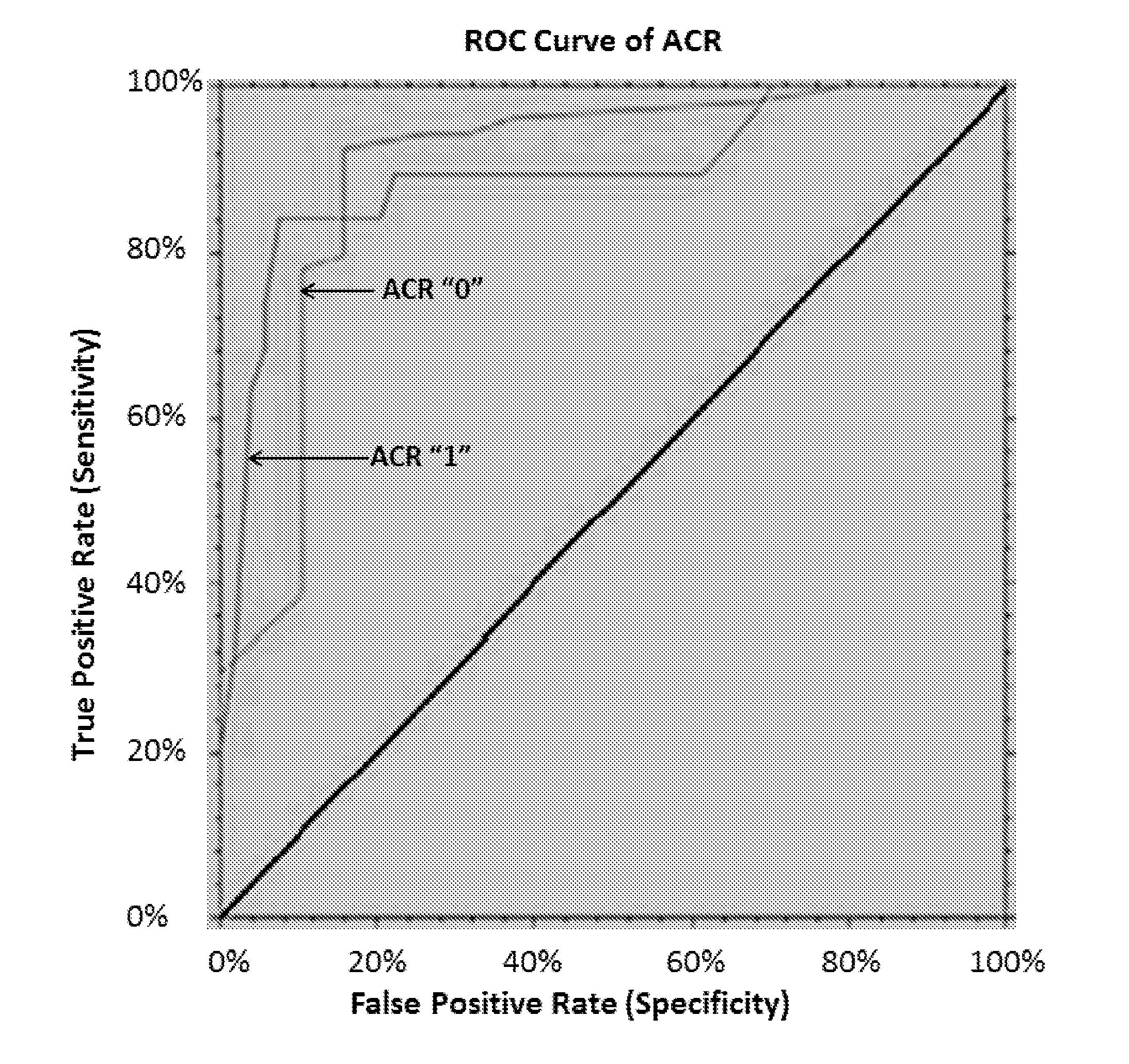
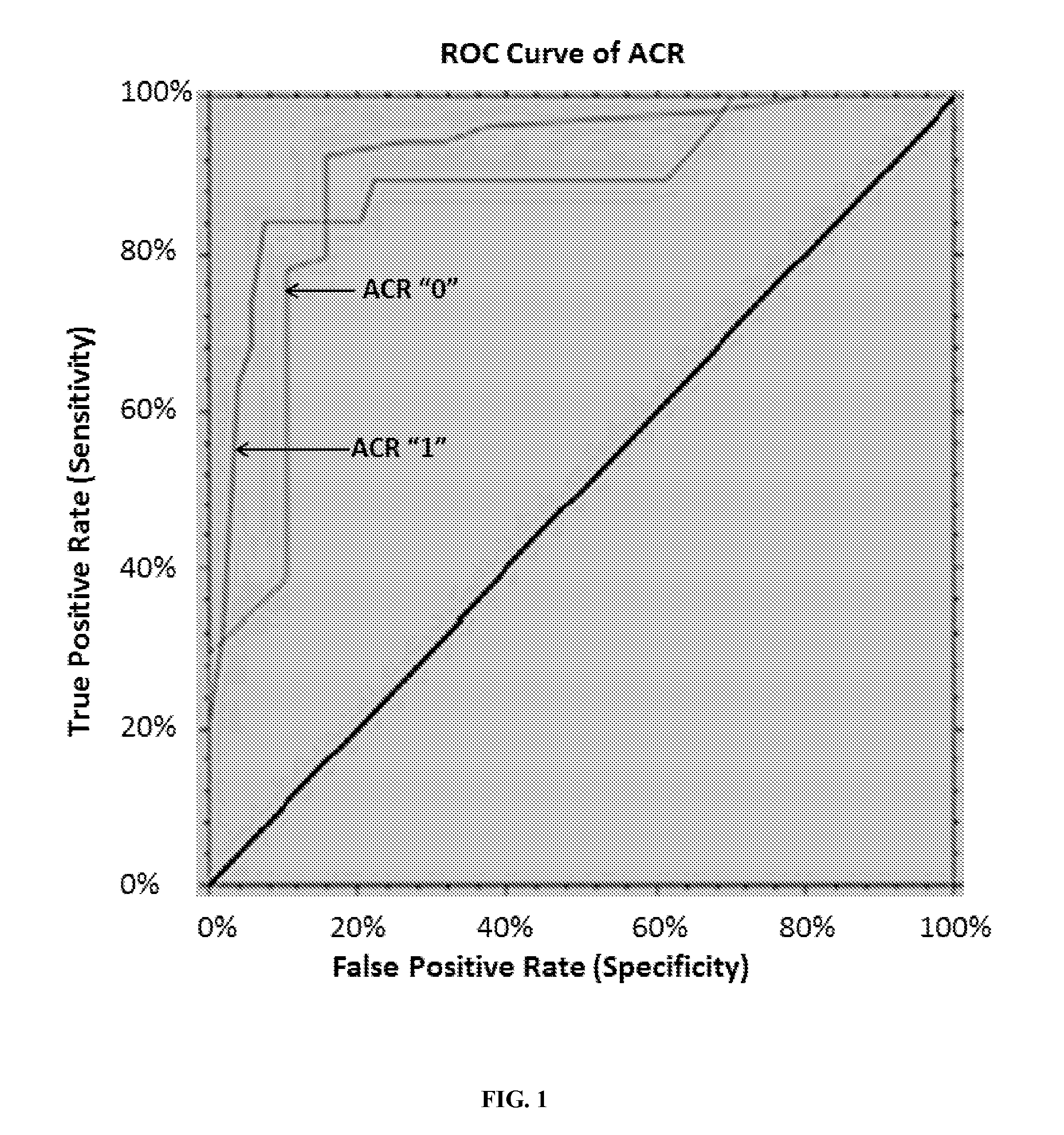
The present invention relates to the discovery that the expression levels of some microRNAs (miRNAs) can use a diagnostic signature to predict transplant outcomes in a transplant recipient. Thus, in various embodiments described herein, the methods of the invention relate to methods of diagnosing a transplant subject for acute rejection such as acute cellular rejection (ACR), methods of predicting a subject's risk of having or developing ACR and methods of assessing in a subject the likelihood of a successful or failure minimization of immunosuppression therapy (IST) dosage from standard ranges.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
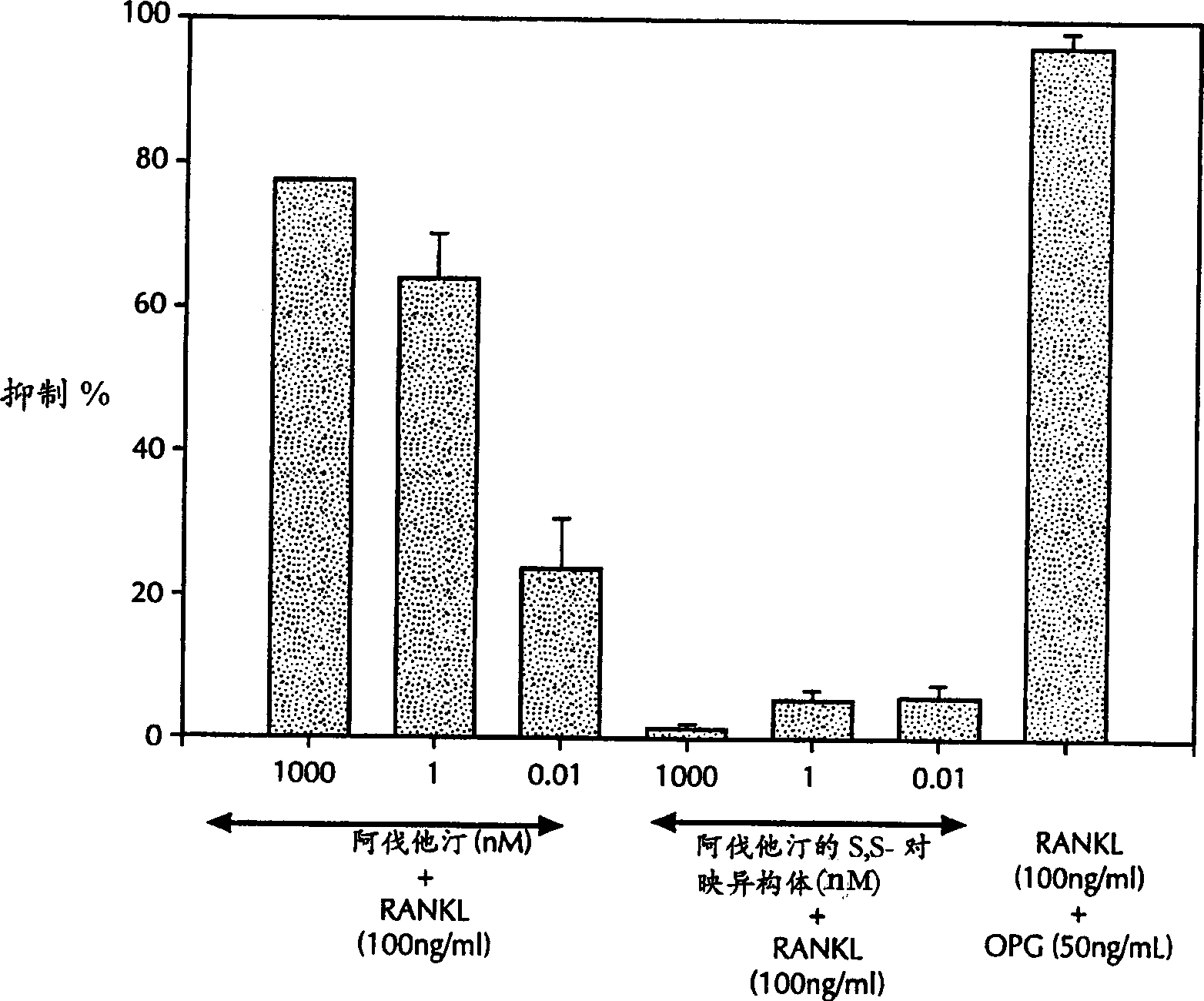
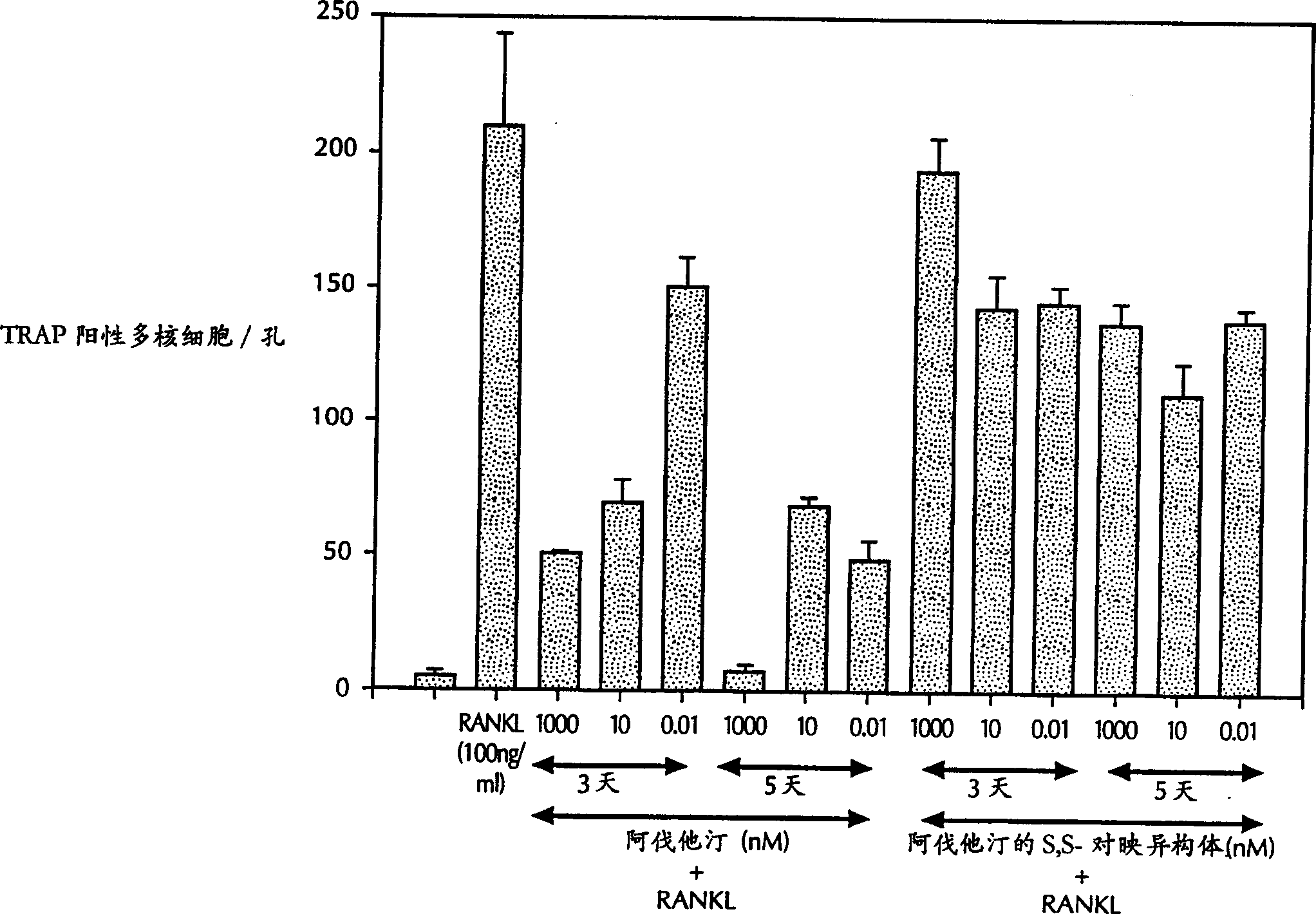
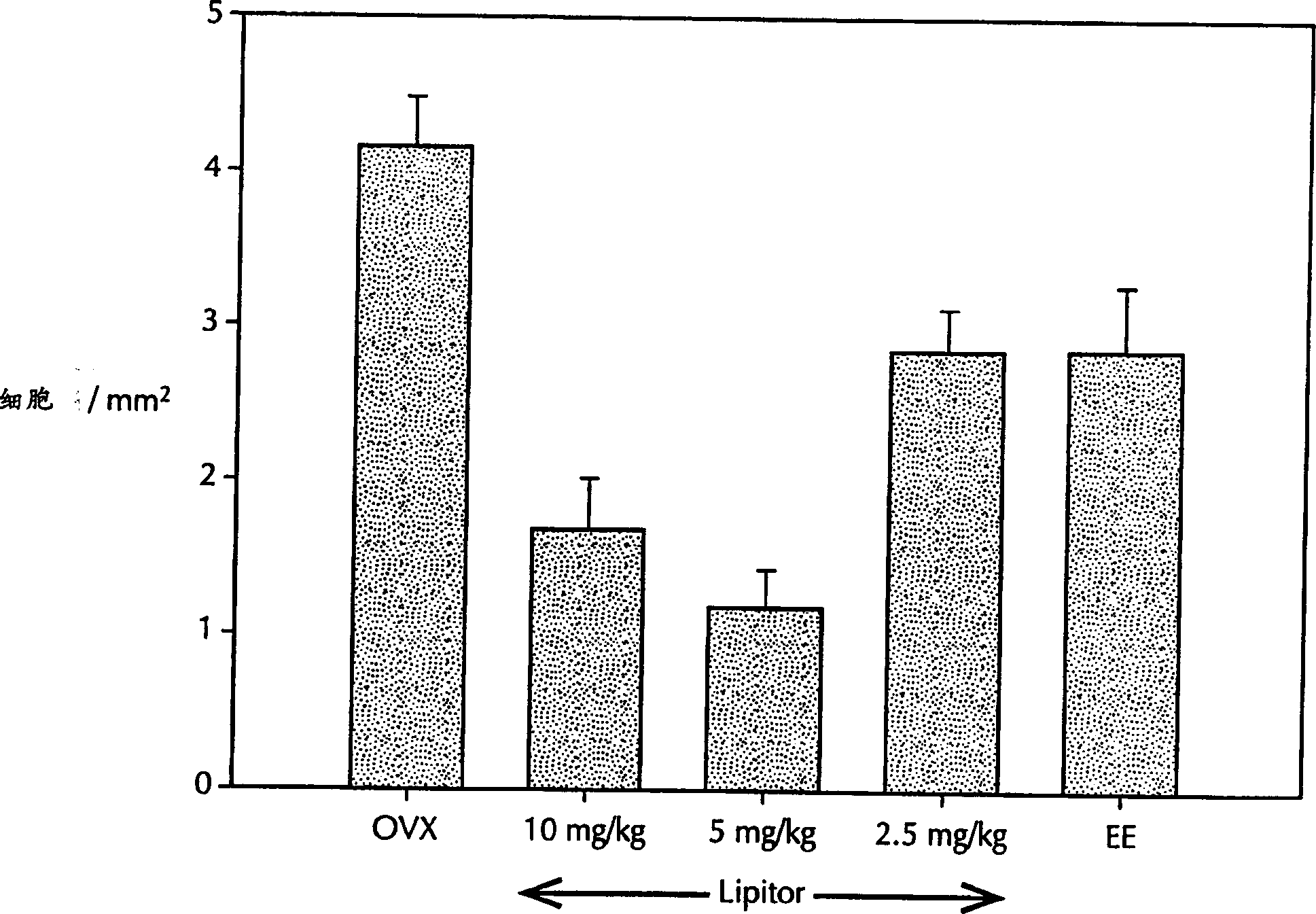
Application of tadins inhibiting formation of osteoclast
A method for inhibiting the formation of osteoclasts comprising administering a therapeutically effective amount of a statin to a mammal in need thereof as well as pharmaceutical compositions, kits for containing such compositions comprising a statin or a method of treating or preventing a disease state selected from the group consisting of osteoporosis, Paget's disease, osteolysis, hypercalcemia of malignancy, osteogenesis imperfecta, osteoarthritis, alveolar bone loss, side effects of immunosuppressive therapy, and side effects of chronic glucocorticoid use by inhibiting the formation of osteoclasts comprising administering a therapeutically effective amount of a statin to a mammal in need thereof.
Owner:WARNER-LAMBERT CO
Targeting phase-change nano-drug system as well as preparation method and application thereof
ActiveCN111557926AReducing ROS levelsRepair effectOrganic active ingredientsOrganic non-active ingredientsNephrosisSide effect
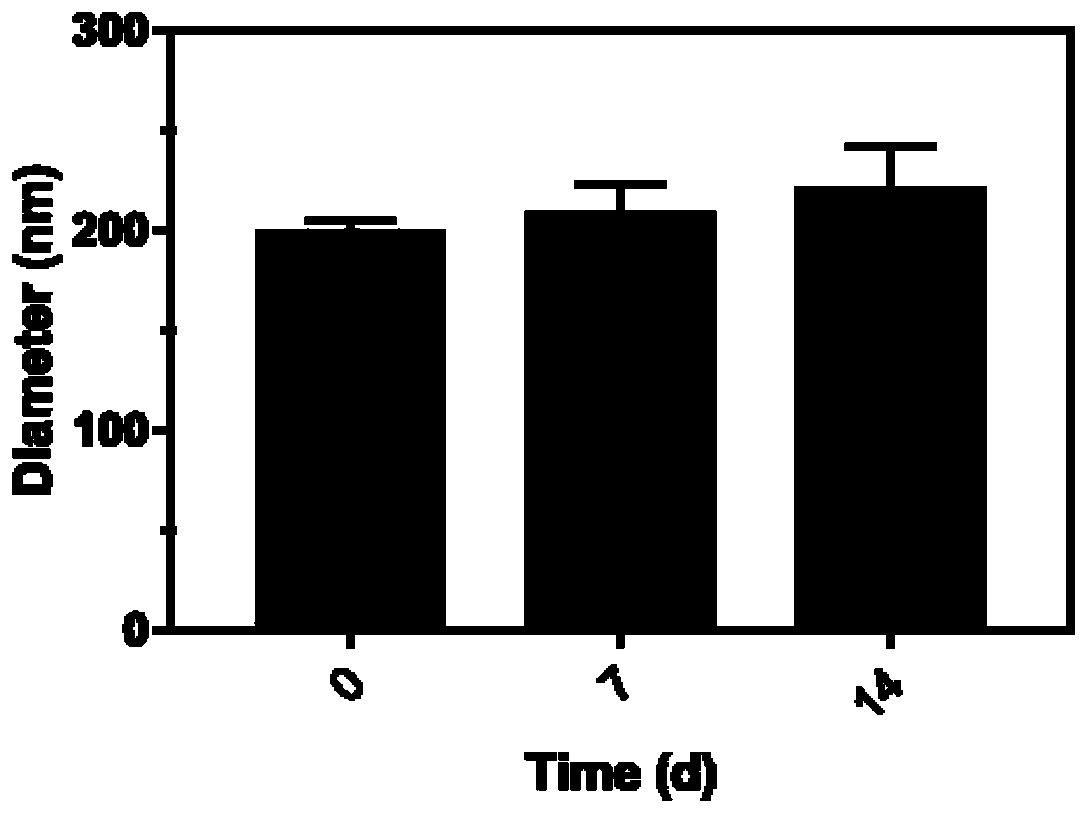
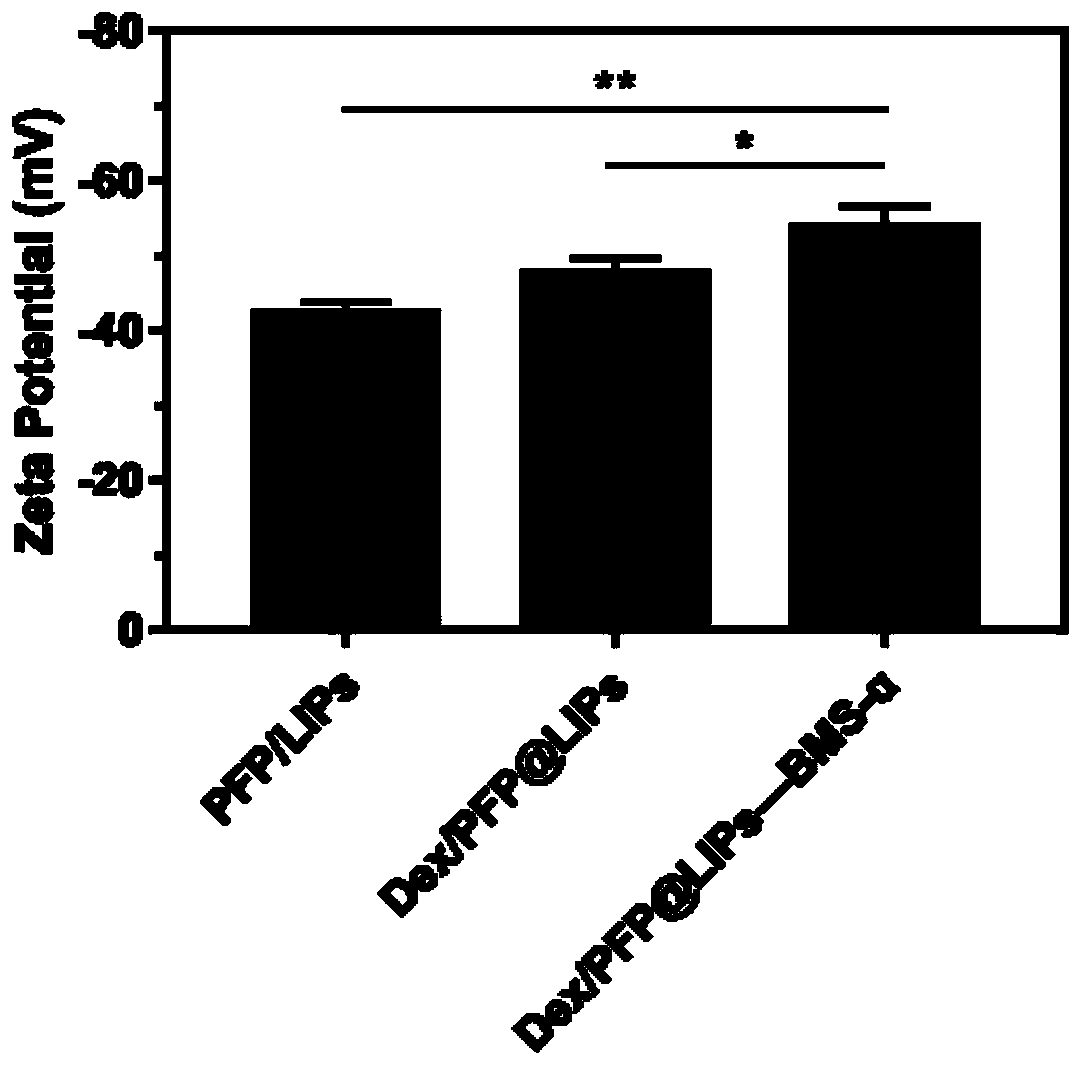
The invention relates to the technical field of biological medicines, in particular to a targeting phase-change nano-drug system as well as a preparation method and application thereof. The targetingphase-change nano-drug system comprises a main body, liquid fluorocarbon is wrapped in the main body, the main body is loaded with a drug, and the main body is covalently connected with BMS-470539. The medicine system can solve the problems of toxic and side effects and weak targeting when immunosuppressive treatment is adopted for immune-related kidney diseases in the prior art. The targeting phase-change nano-drug system can be applied to preparation of related drugs for treating immune nephropathy.
Owner:CHONGQING MEDICAL UNIVERSITY
Methods and compositions for determining a graft tolerant phenotype in a subject
ActiveUS8932808B1Bioreactor/fermenter combinationsBiological substance pretreatmentsGraft ToleranceRegimen
Methods are provided for determining whether a subject has a graft tolerant phenotype. In practicing the subject methods, the expression of at least one gene in a sample from the subject, e.g., a blood sample, is assayed to obtain an expression evaluation for the at least one gene. The obtained expression evaluation is then employed to determine whether the subject has a graft tolerant phenotype. Also provides are compositions, systems and kits that find use in practicing the subject methods. The methods and compositions find use in a variety of applications, including the determination of an immunosuppressive therapy regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Autoimmune hepatitis model and medicine screened by using autoimmune hepatitis model
InactiveCN107661511ACompounds screening/testingPeptide/protein ingredientsMouse hepatitisSuppression treatment
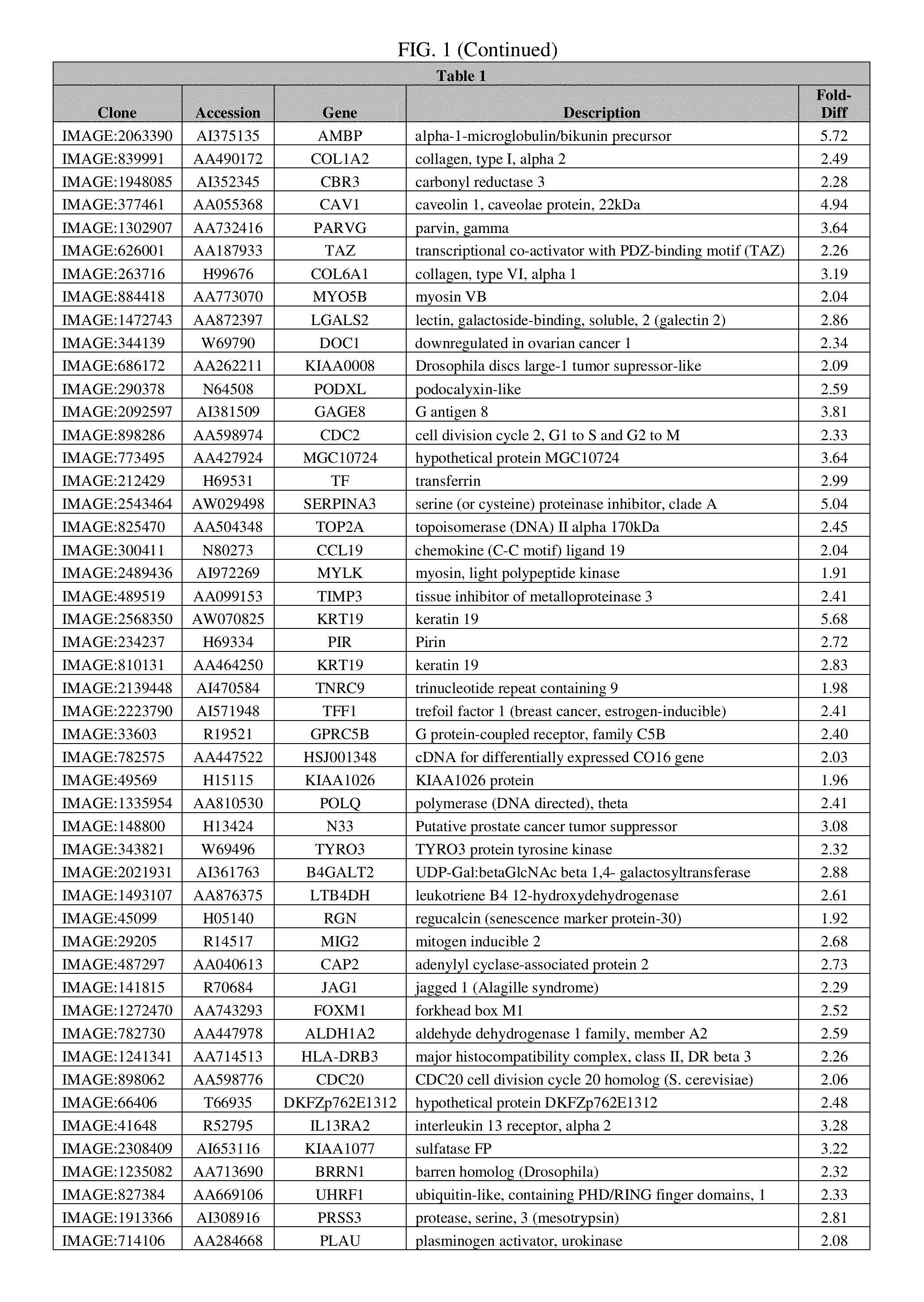
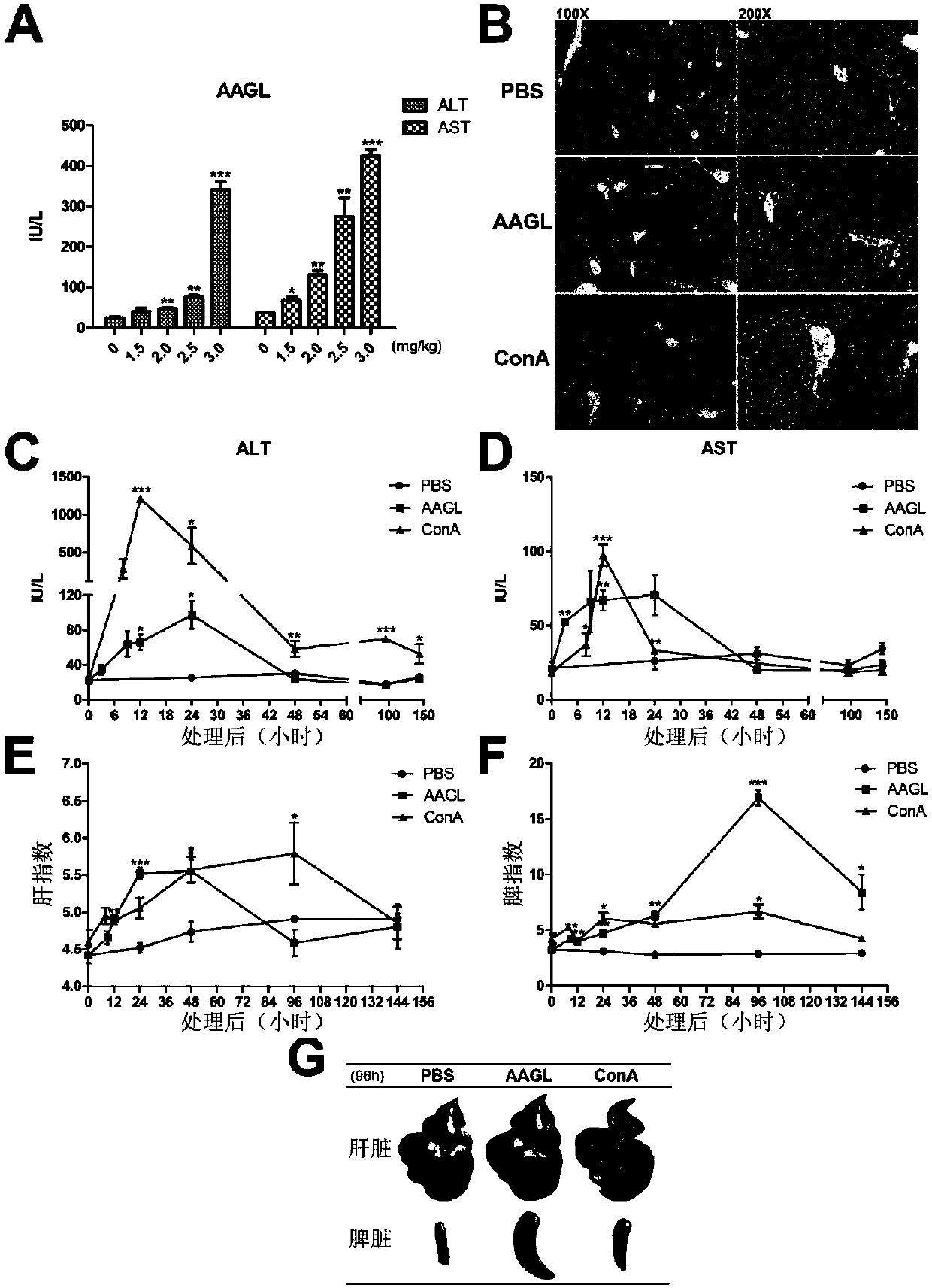
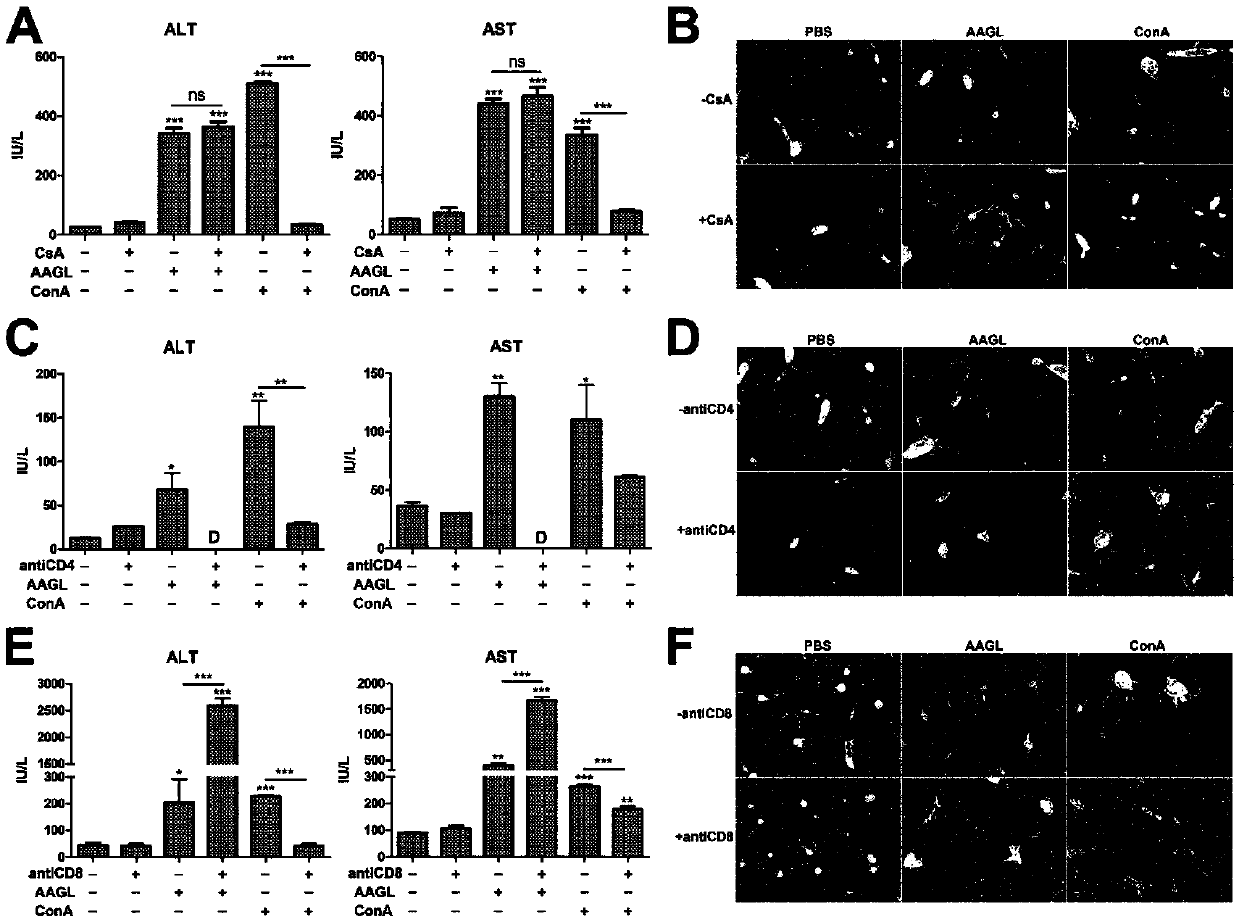
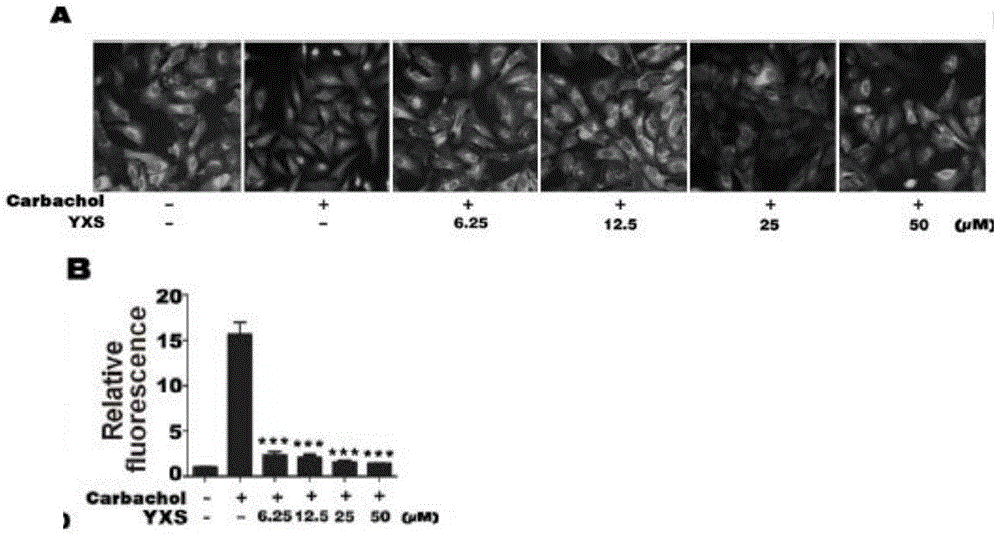
The invention discloses an autoimmune hepatitis model and medicine screened by using the autoimmune hepatitis model. Firstly, an AAGL-induced AIH-like mouse hepatitis model is built; the model is notsensitive on the treatment of a conventional immunosuppressor, and can be clinically used as an animal model for conventional immunological suppression treatment on non-sensitive patients. The invention further provides the medicine screened by using the model for treating the autoimmune hepatitis. The result shows that glatiramer acetate can obviously relieve the autoimmune hepatitis symptom of the animal model. The condition indicates that the glatiramer acetate can be used for treating the autoimmune hepatitis. Therefore the invention provides a novel purpose of the glatiramer acetate. Novel medicine is provided for the treatment of the autoimmune hepatitis.
Owner:WUHAN UNIV
Application of ginkgolic acid in preparation of medicine for preventing and/or treating diseases caused by overactivity of osteoclast
InactiveCN104666313APharmacological target is clearInhibitory activitySalicyclic acid active ingredientsAntipyreticDiseaseGlucocorticoid
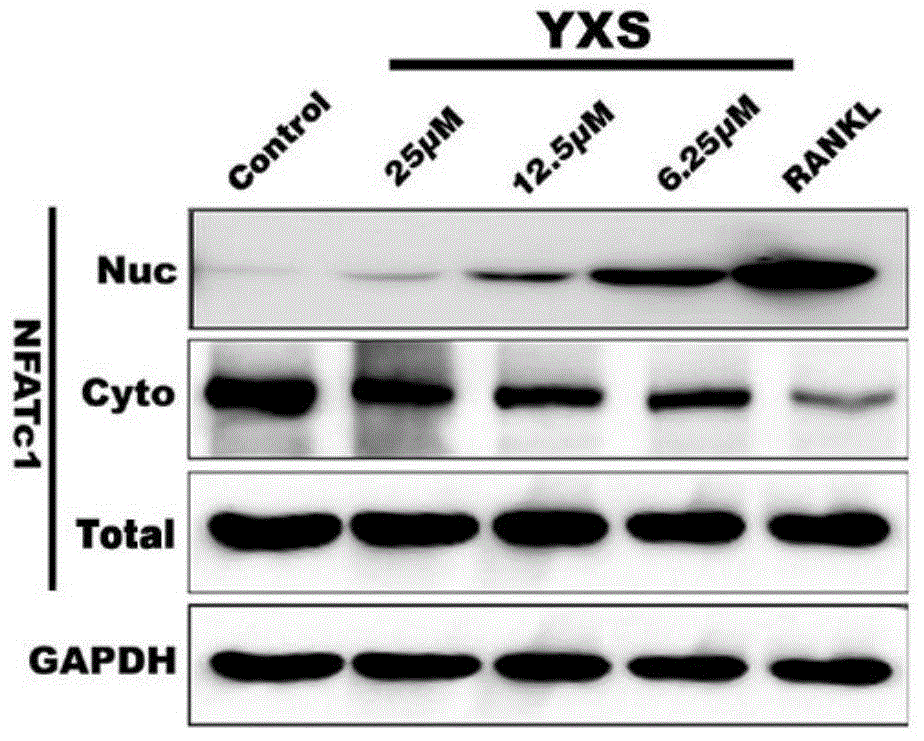
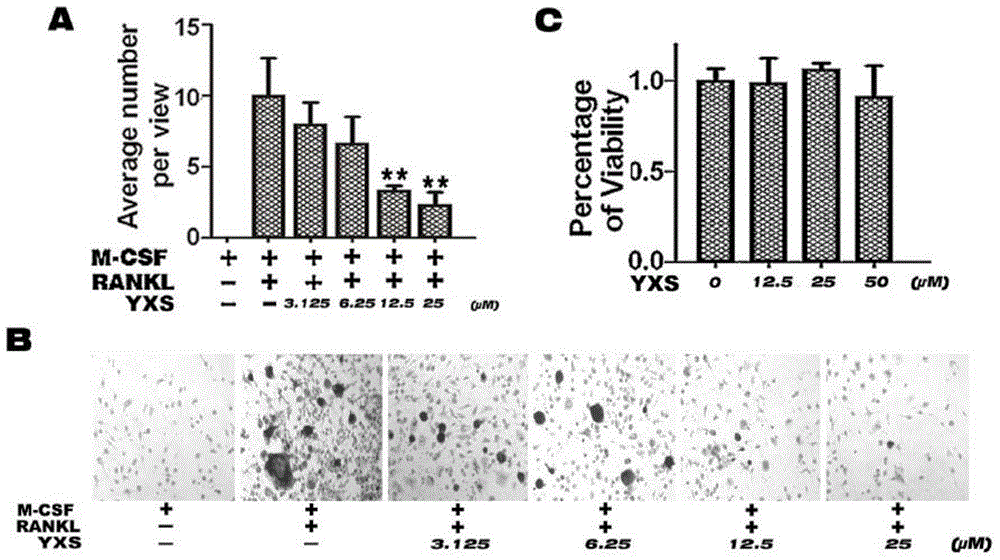
The invention belongs to the field of medicines, relates to novel pharmaceutical application of ginkgolic acid and particularly relates to application of ginkgolic acid (C17:1), which is a syzygiumtetragonum extract having a structural formula show in the specification, in preparation of a medicine for preventing and / or treating diseases caused by overactivity of osteoclast. The ginkgolic acid (C17:1) is capable of preventing nuclear translocation of NFATc1 and inhibiting differentiation and bone erosion of osteoclast and thus is used for preparing the medicine for preventing and treating the diseases including osteoporosis, osteolysis, rheumatoid arthritis, multiple myeloma, Paget's disease, hypercalcemia of tumors, osteogenesis imperfect, alveolar bone deficiency or bone loss caused during immunosuppressive therapy or long-term use of glucocorticoid.
Owner:FUDAN UNIV
Imidazole derivatives as IDO inhibitors
Presently provided are IDO inhibitors of general formulae (VII), (VIII) as shown below and pharmaceutical compositions thereof, useful for modulating an activity of indoleamine 2,3-dioxygenase; treating indoleamine 2,3-dioxygenase (IDO) mediated immunosuppression; treating a medical conditions that benefit from the inhibition of enzymatic activity of indoleamine-2,3-dioxygenase; enhancing the effectiveness of an anti-cancer treatment comprising administering an anti-cancer agent; treating tumor-specific immunosuppression associated with cancer; and treating immunosupression associated with an infectious disease.
Owner:NEWLINK GENETICS
Biomarkers for determining an allograft tolerant phenotype
Methods are provided for determining whether a subject has a graft tolerant phenotype. In practicing the subject methods, the expression level of one or more gene in a sample from the subject, e.g., a blood sample, is assayed to obtain a gene expression result, where the gene expression result includes a result for a biomarker of graft tolerance. The obtained gene expression result is then employed to determine whether the subject has a graft tolerant phenotype. Also provided are compositions, systems and kits that find use in practicing the subject methods. The methods and compositions find use in a variety of applications, including the determination of an immunosuppressive therapy regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Triptolide c-ring derivatives as anticancer agents and immune modulators
Disclosed are compounds based on C- and D-ring modifications of triptolide and hydroxylated triptolide, for use in therapy, such as antiproliferative, anticancer, and immunosuppressive therapy.
Owner:PHARMAGENESIS
Methods and Compositions for Determining a Graft Tolerant Phenotype in a Subject
Methods are provided for determining whether a subject has a graft tolerant phenotype. In practicing the subject methods, the expression of at least 5 genes in a sample from the subject, e.g., a blood sample, is assayed to obtain a gene expression result for the at least 5 genes. The obtained gene expression result for the at least 5 genes is then employed to determine whether the subject has a graft tolerant phenotype. Also provided are compositions, systems and kits that find use in practicing the subject methods. The methods and compositions find use in a variety of applications, including the determination of an immunosuppressive therapy regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Immune tolerance agent
PendingCN109771638AConducive to survivalSustained induction of immune tolerancePeptide/protein ingredientsAntipyreticApoptosisCD8
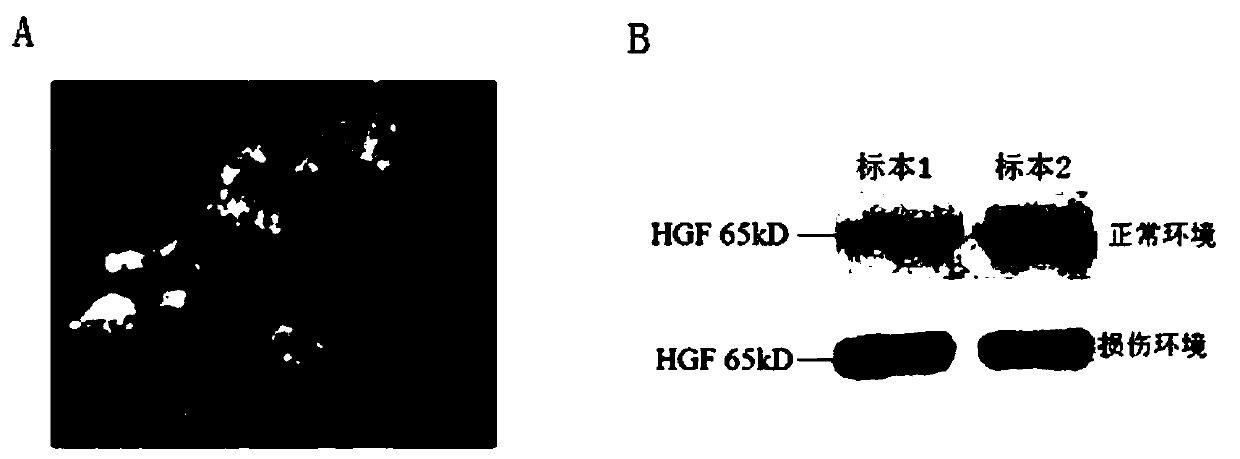
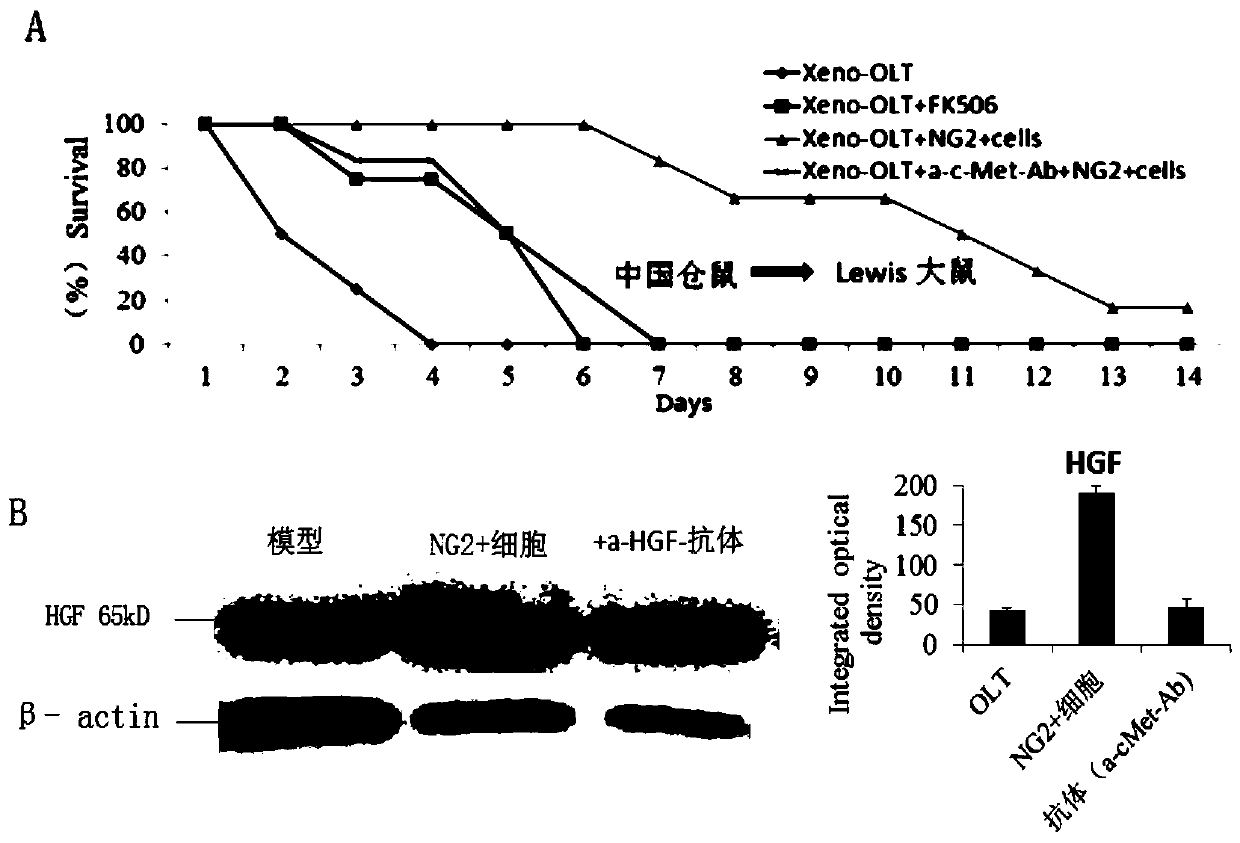
The invention discloses application of a hepatic growth factor (HGF) in the preparation of an organ transplantation immune tolerance agent. The main function is that HGF is expressed in a CD8-cytotoxic-T-lymphocyte (CTL) surface cMet / FAS molecular coupling mode (CD8<+>CTL<cMet / FAS>) through regulating to induce apoptosis of CTL. The CD8<+>T CTL is a major lymphocyte population which attacks heterologous target organs or target antigens. Therefore, stem cells (or drugs) which give HGF or secrete HGF in the allogeneic or xenogeneic organ transplantation process can significantly prolong the survival of transplanted organs and recipients. The application not only broadens the application range of HGF preparations or HGF-secreting cells (or drugs), but also provides a new immunosuppressive treatment drug for clinical organ transplantation application.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Methods for the detection of jc polyoma virus
InactiveUS20130101985A9Improved profileHigh profileMicrobiological testing/measurementDisease diagnosisSequence variationSialic acid binding
Methods and compositions for determining whether a subject is at risk for PML, including subjects being treated with immunosuppressants, by determining whether the subject harbors a JCV variant with reduced binding for sialic acid relative to a normal JCV, are presented. Furthermore, combinations of JCV-VP1 sequence variations that are associated with PML and that can be used as a basis of an assay for identifying subjects susceptible to PML, subjects with PML (e.g., early stage PML), or subjects at risk of developing PML in response to an immunosuppressive treatment are provided.
Owner:BIOGEN MA INC
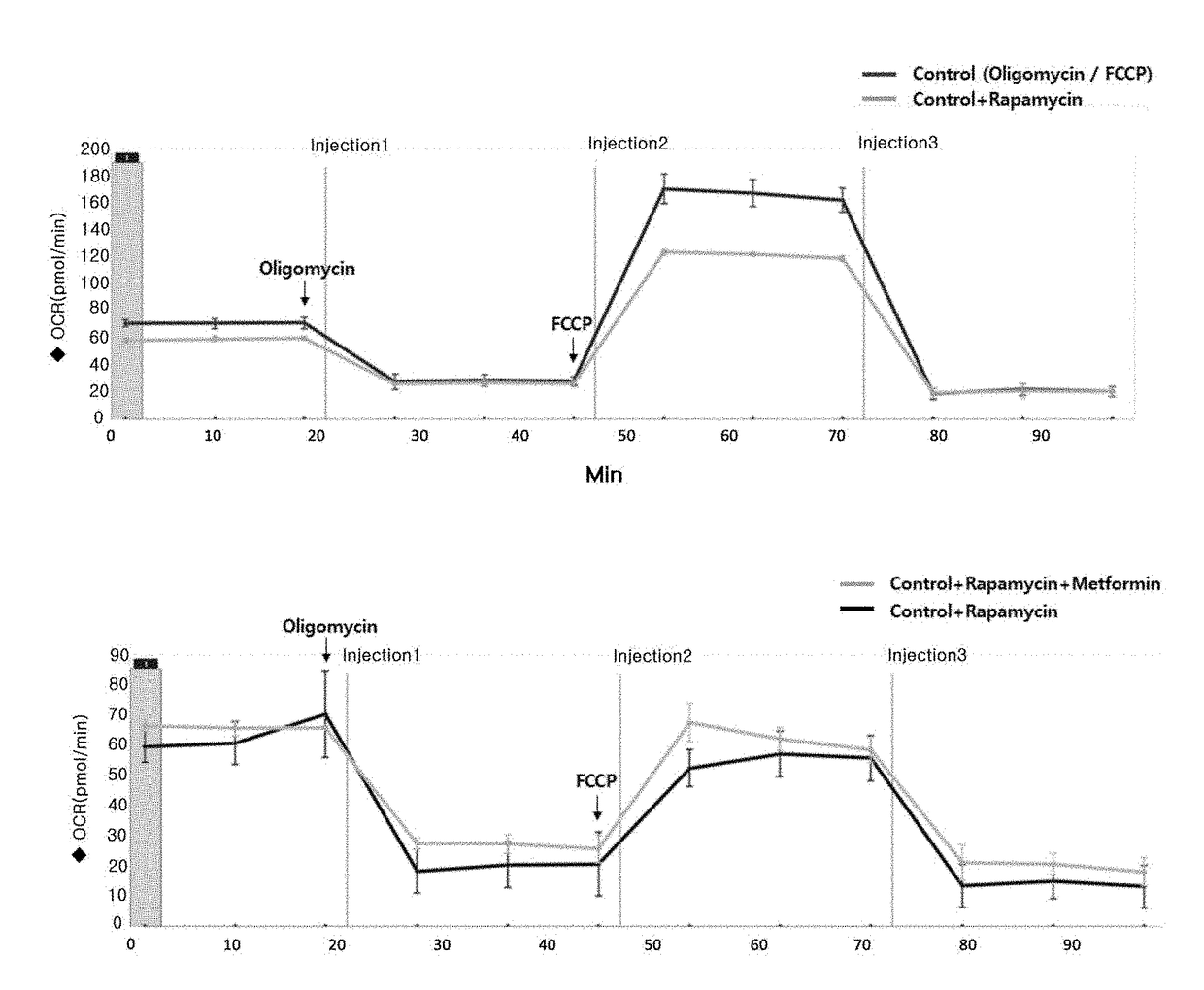
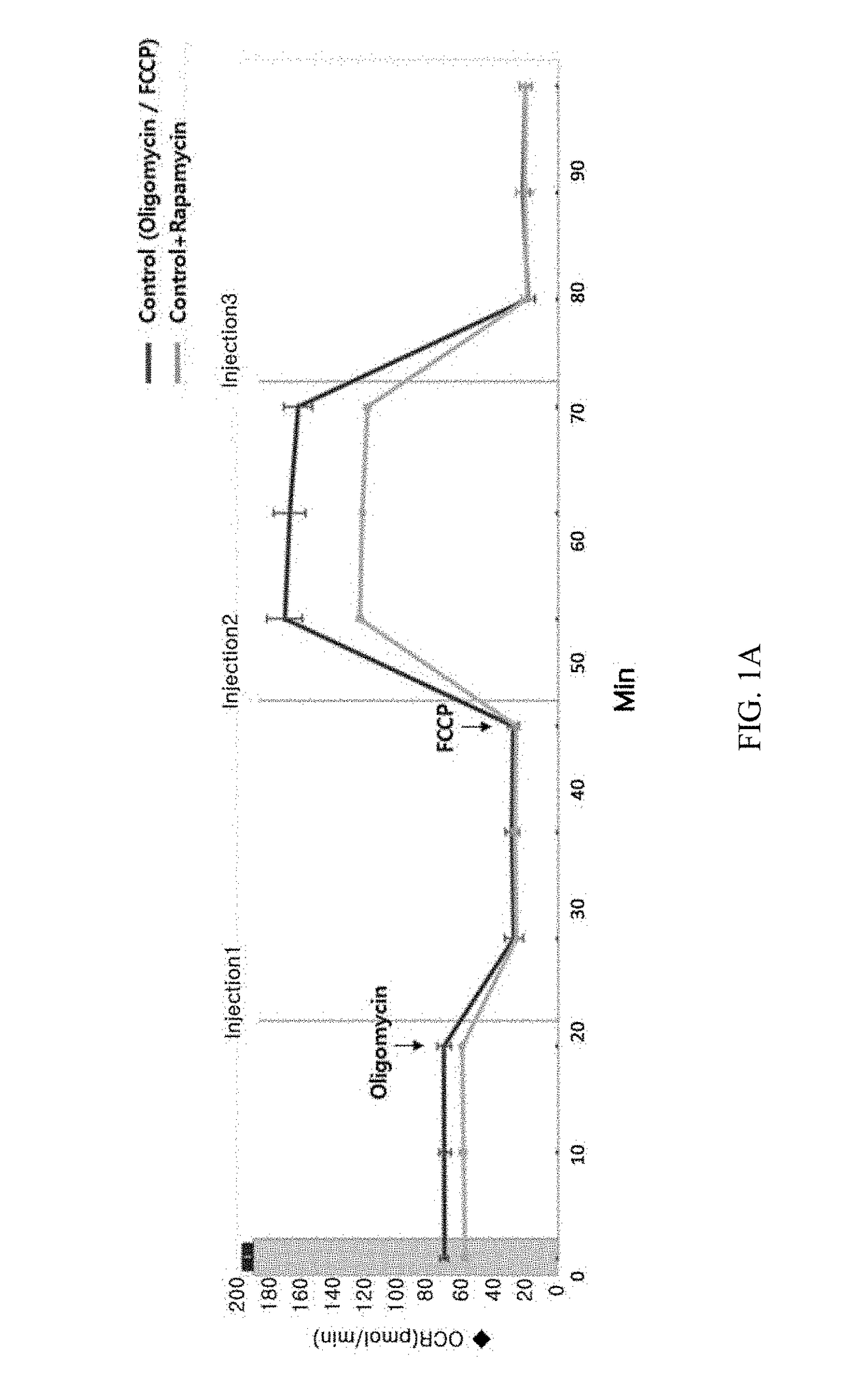
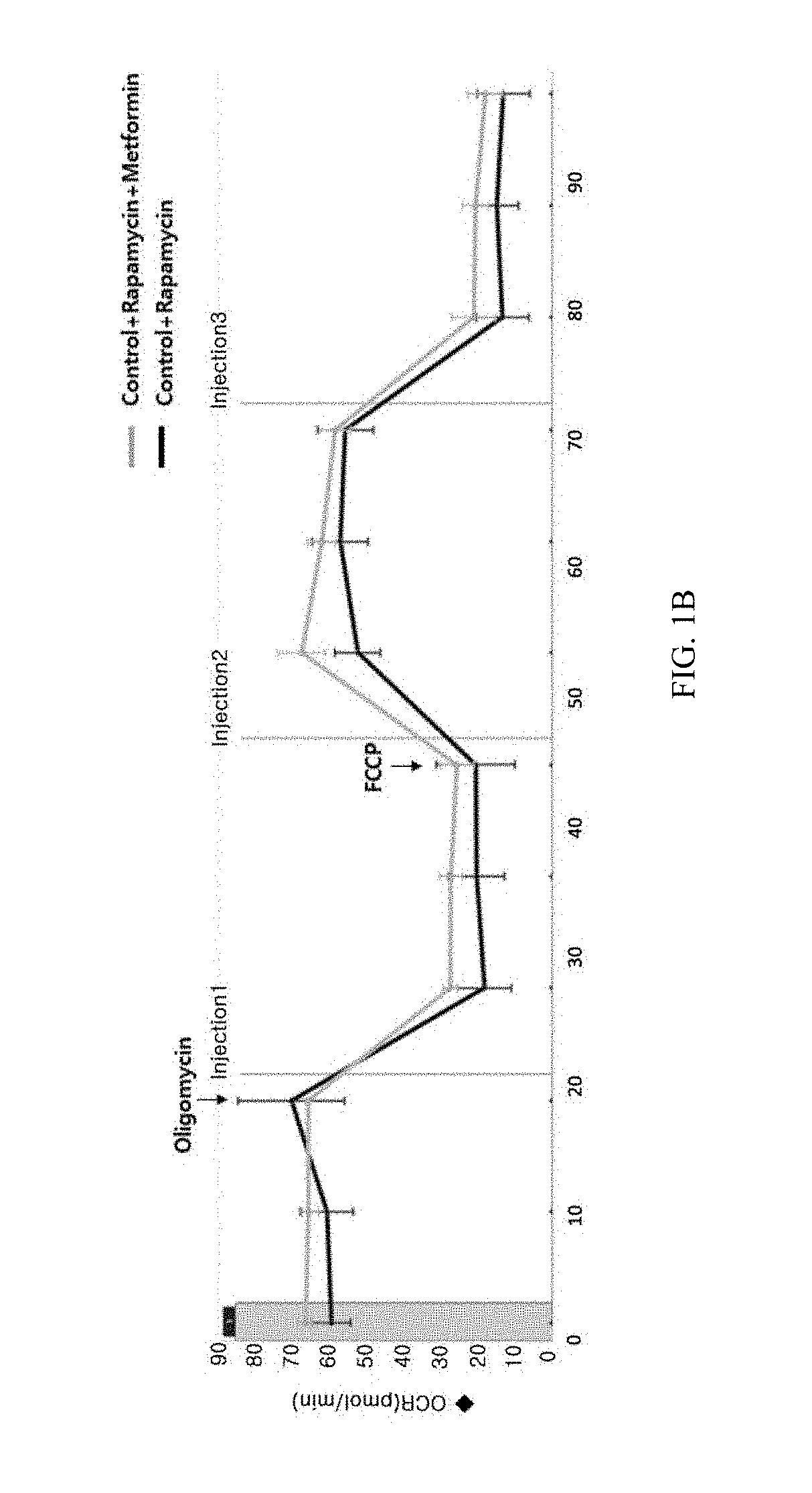
Composition for preventing or treating mitochondrial diseases caused by immunosuppressants, and immune diseases, containing metformin
InactiveUS20180256519A1Easy to useImprove mitochondrial functionOrganic active ingredientsImmunological disordersSide effectAdditive ingredient
The present invention relates to a composition for preventing or treating mitochondrial diseases caused by immunosuppressants, and immune diseases, containing metformin and, more specifically, to a composition for treating mitochondrial diseases caused by immunosuppressants, containing metformin; a pharmaceutical composition for preventing or treating immune diseases, containing, as active ingredients, metformin and an immunosuppressant, which is a target of rapamycin inhibitor (mTOR inhibitor); and a pharmaceutical composite formulation for preventing or treating immune diseases, containing, as ingredients, metformin and a mammalian target of rapamycin inhibitor, wherein the metformin and mammalian target of rapamycin inhibitor are administered simultaneously or separately, or administered in a predetermined sequence. The composition effectively alleviates mitochondrial dysfunction, occurring as a side effect of conventional immunosuppressants, while having a more improved immunosuppressive therapeutic effect, thereby being usable in prevention and treatment of transplant rejection, autoimmune diseases, inflammatory diseases, and the like, all of which require immunosuppression.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com