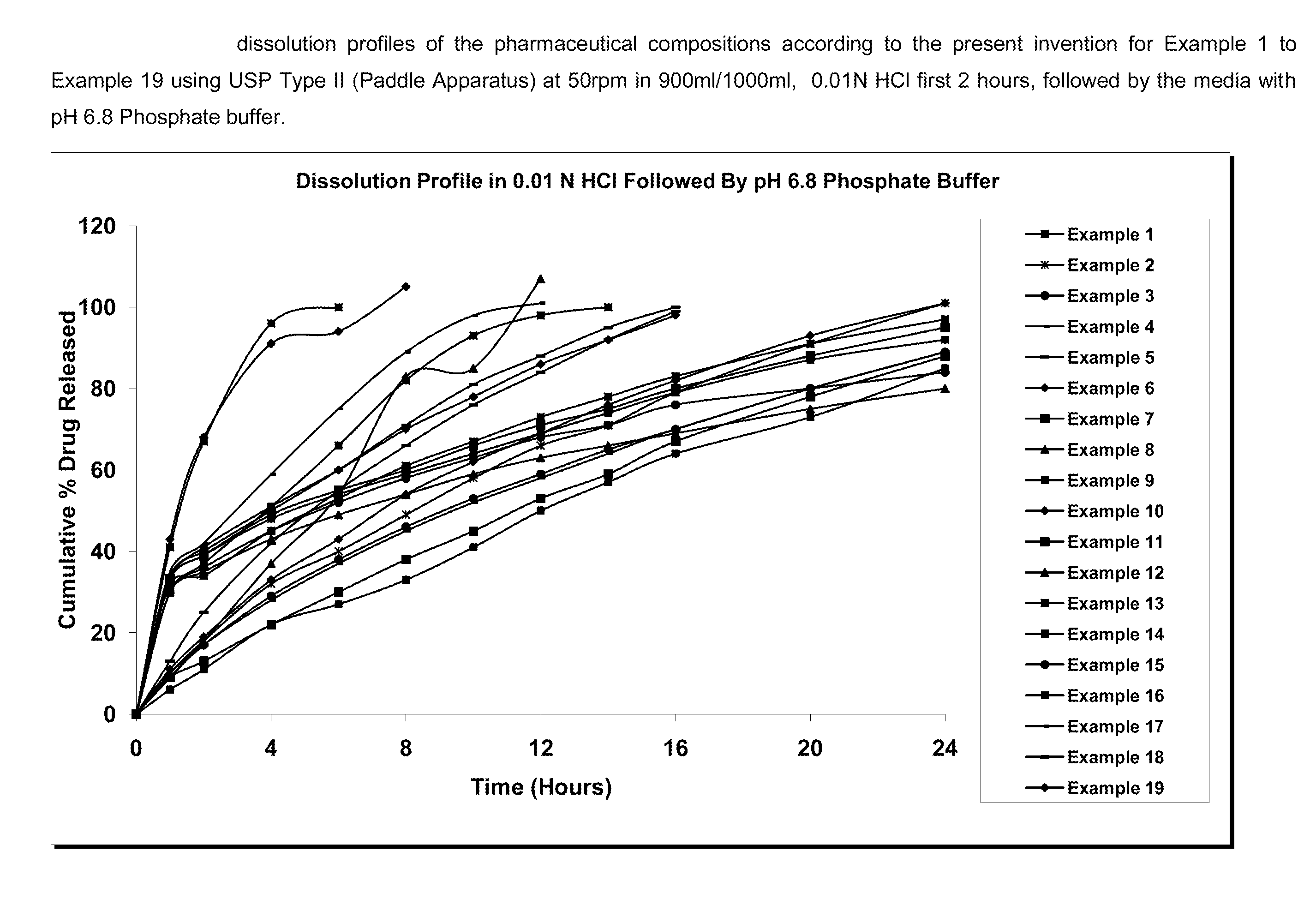
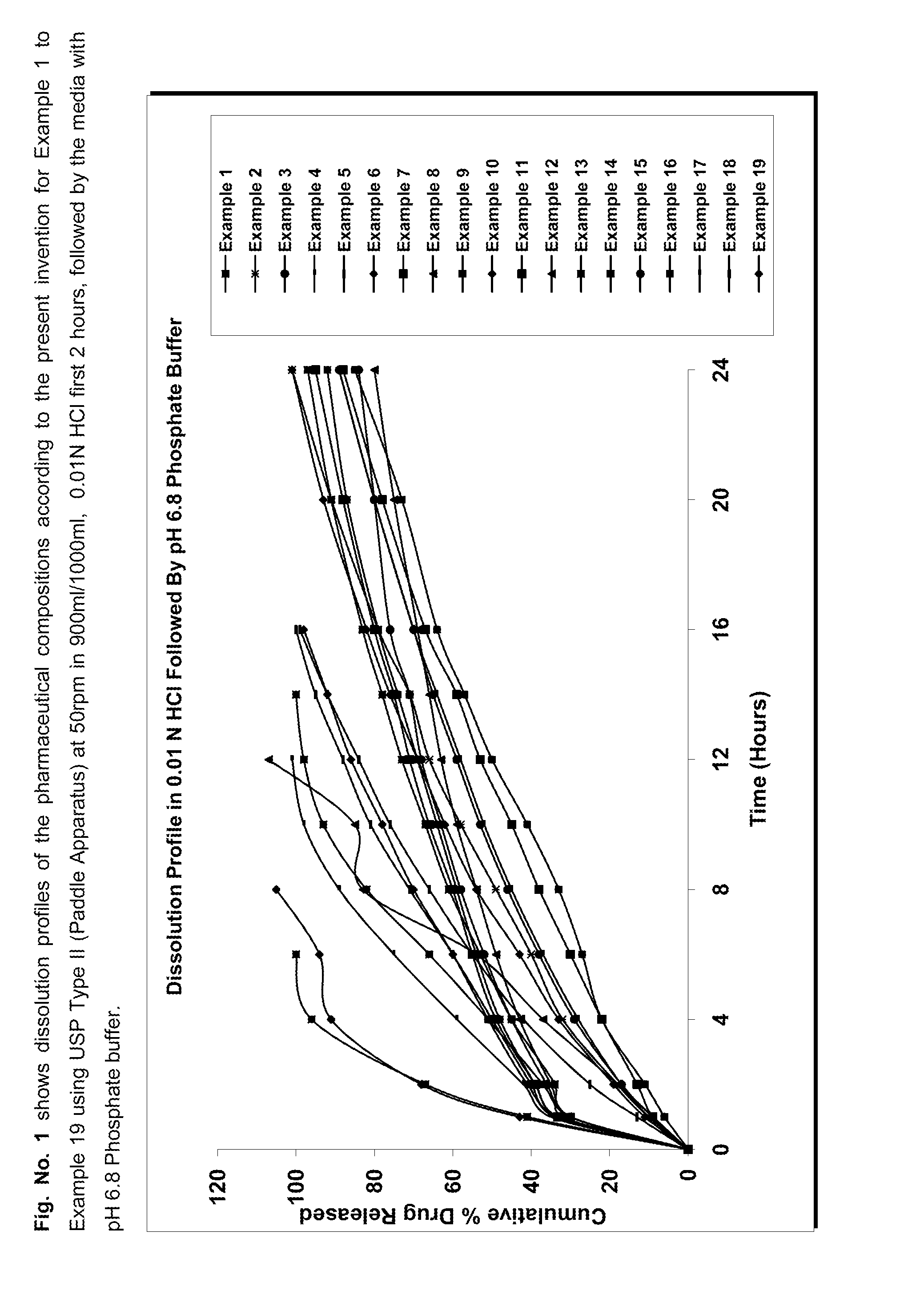
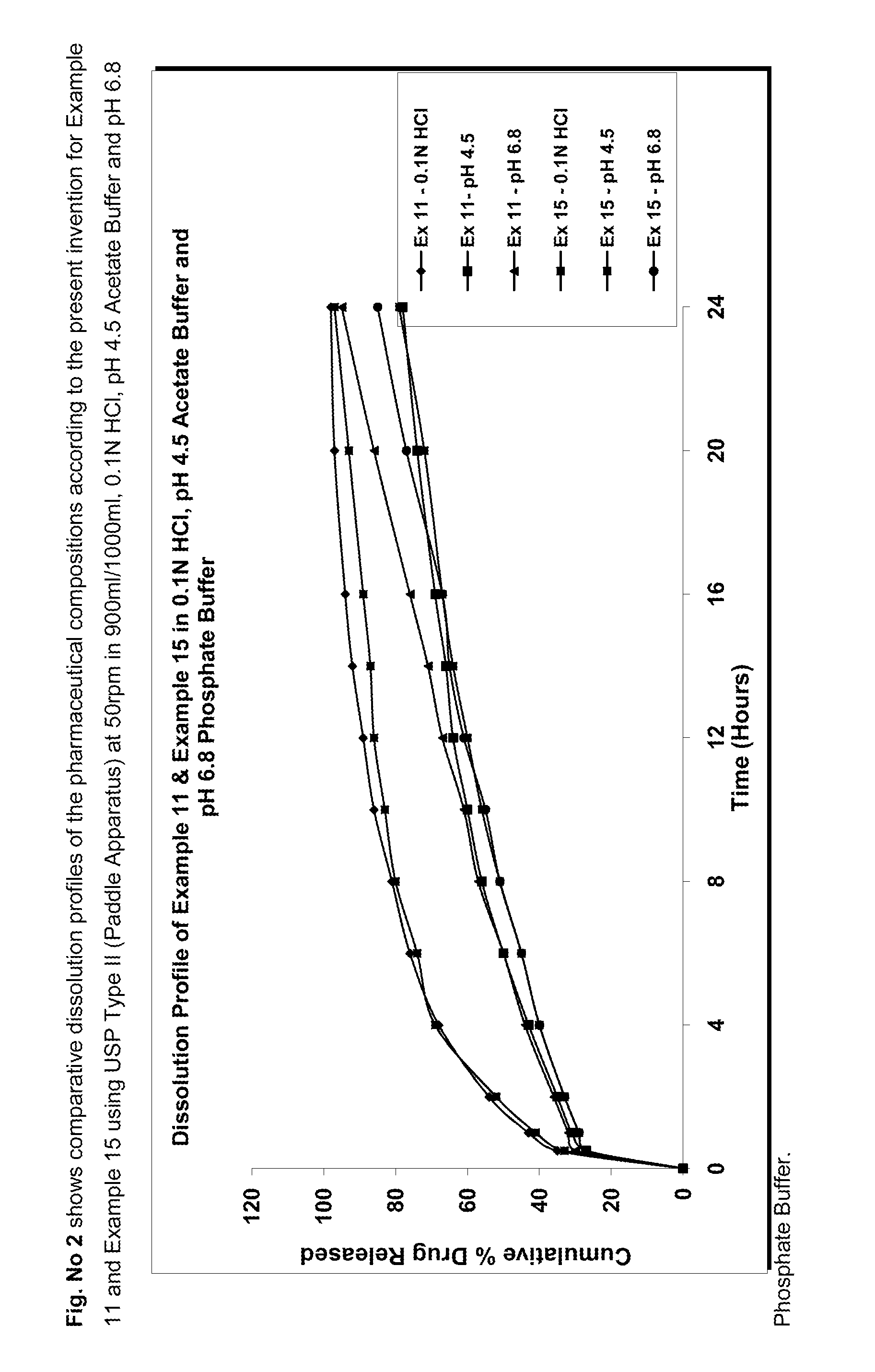
Patents
Literature
46 results about "Once daily dosing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
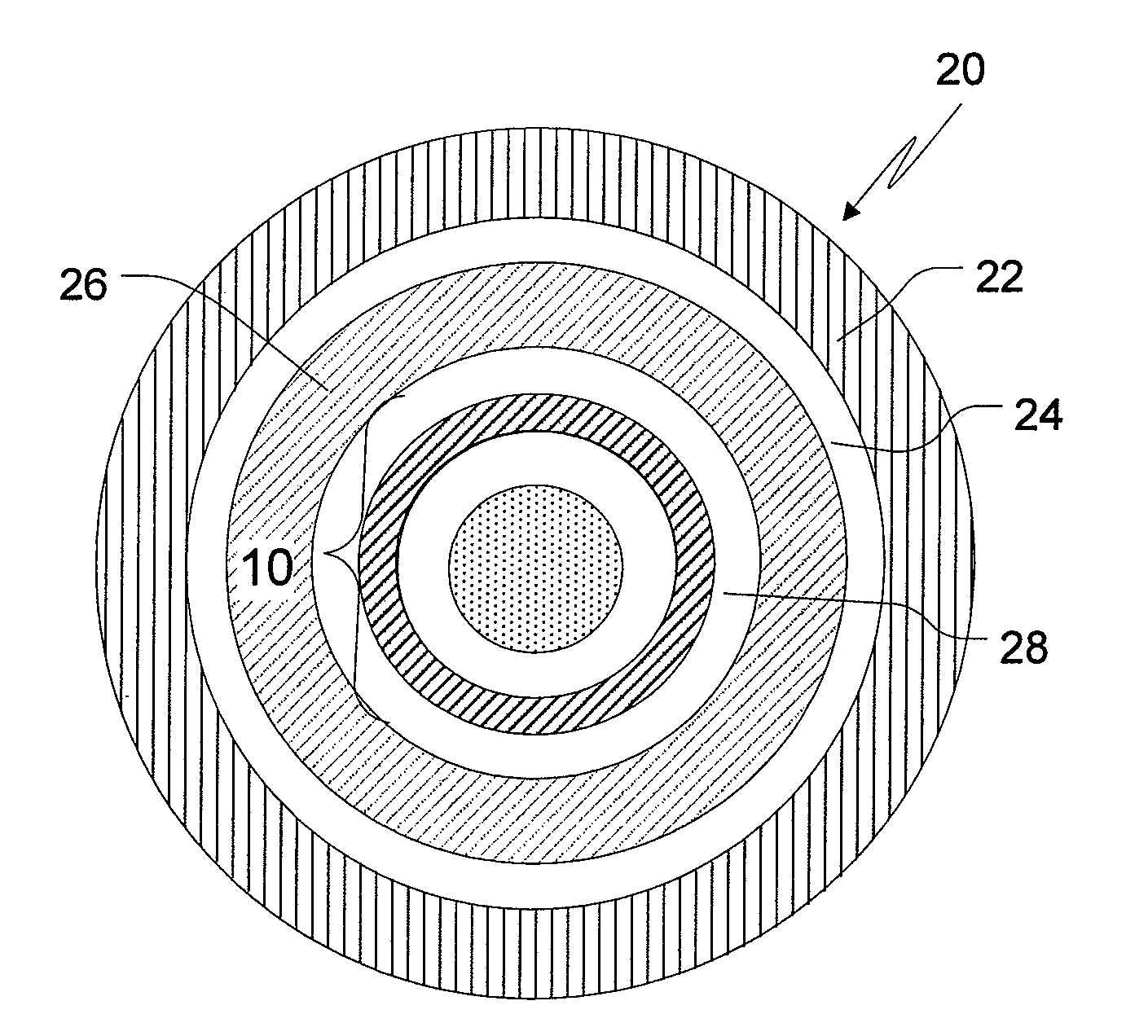
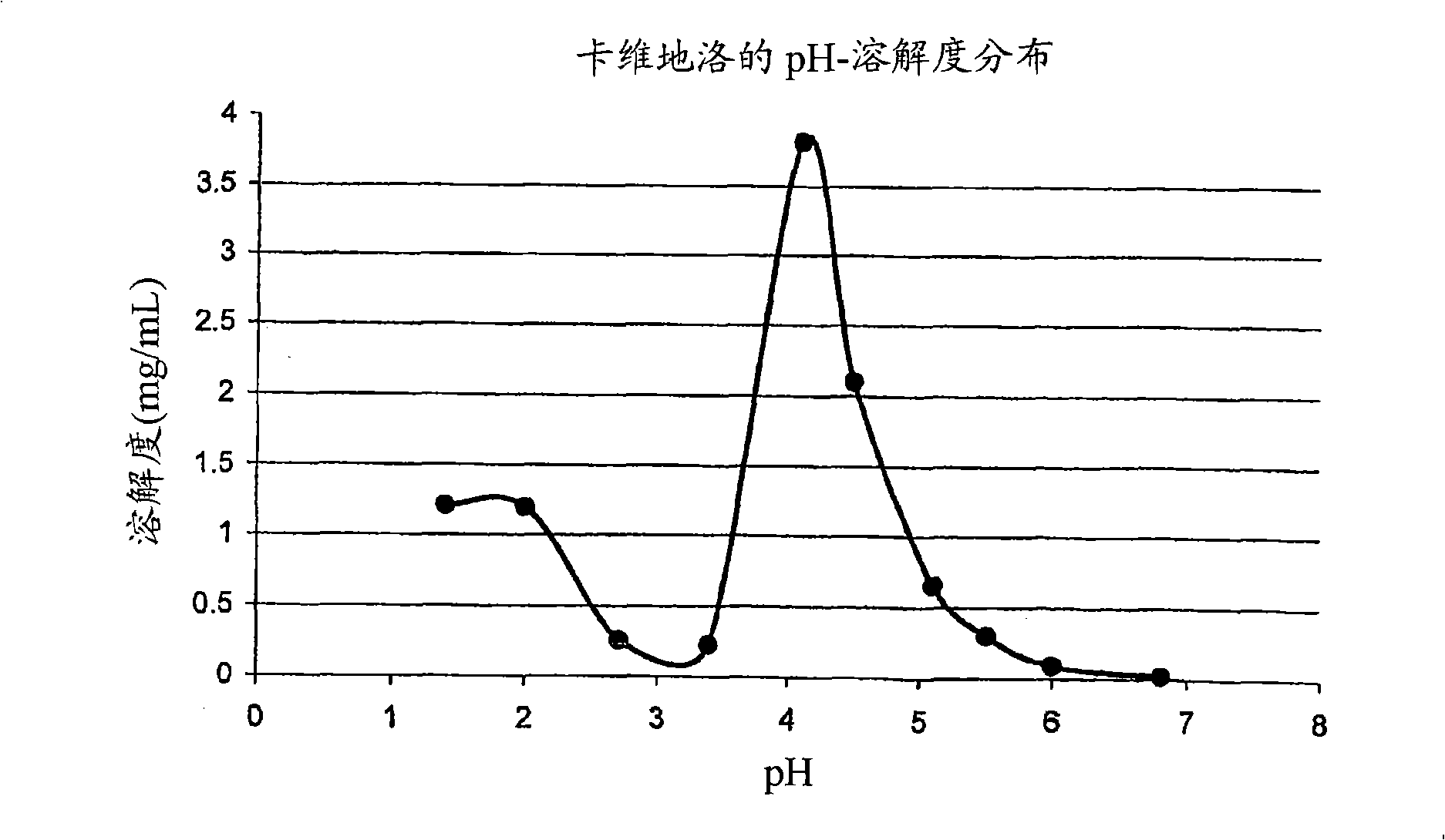
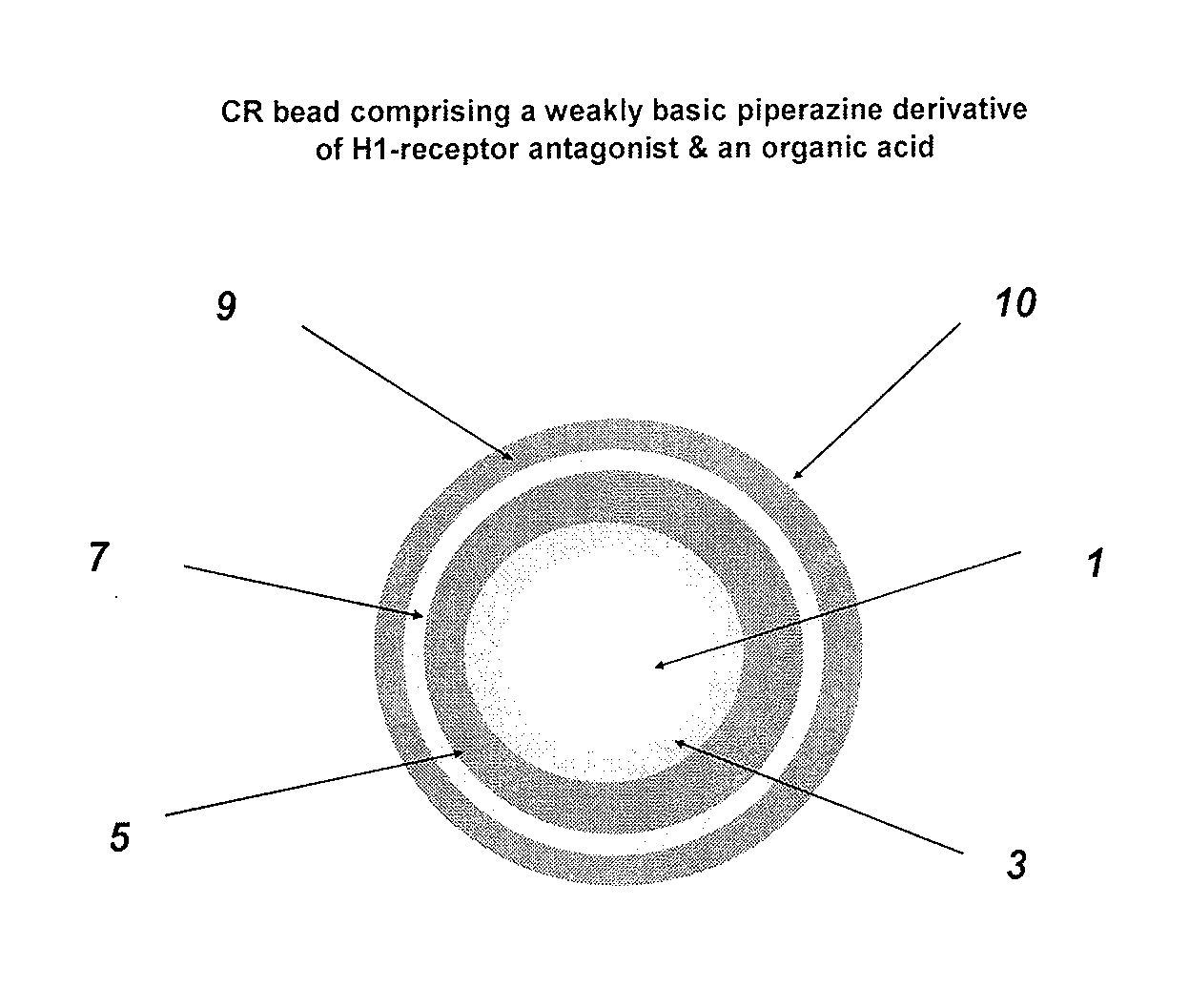
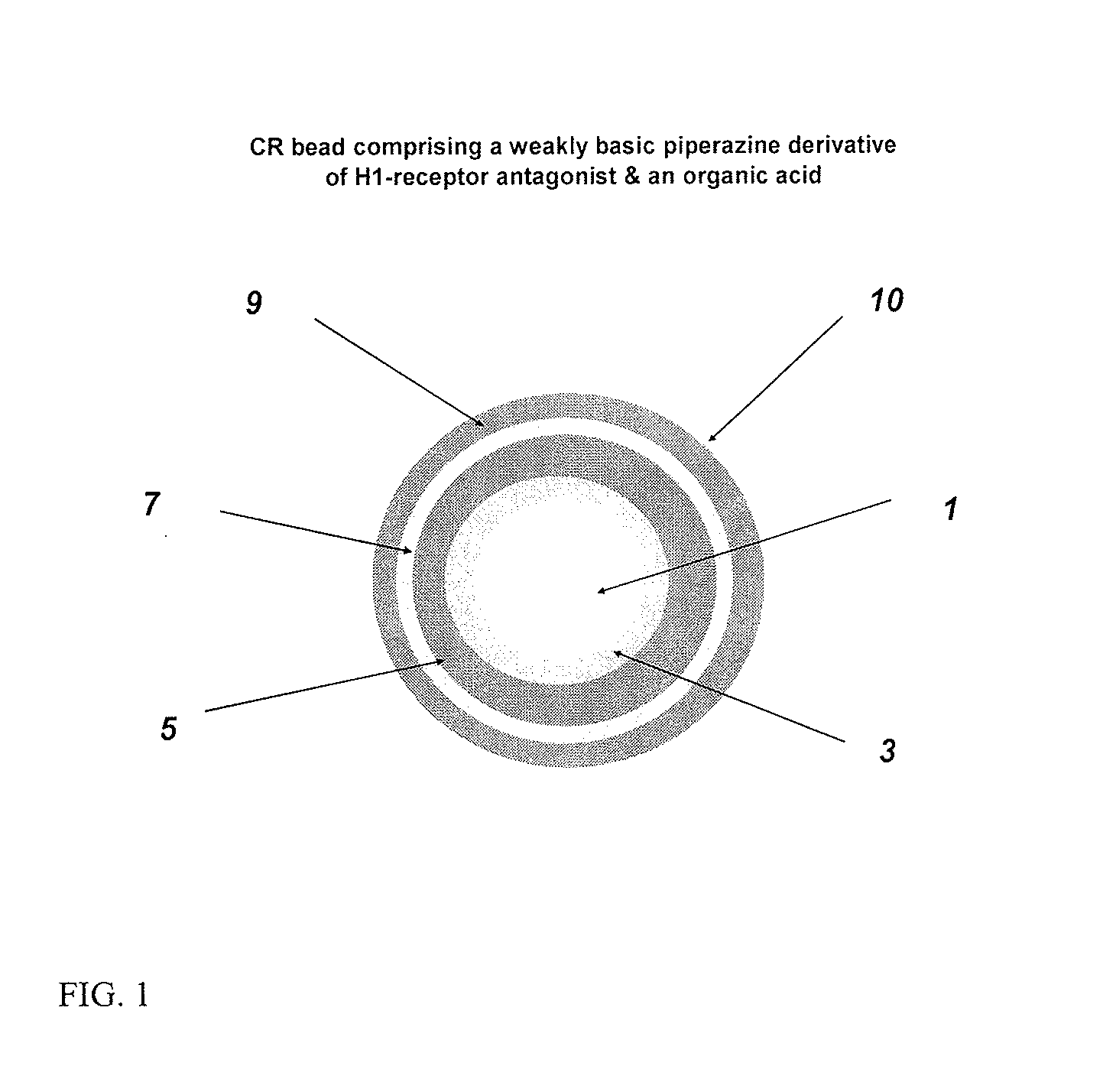
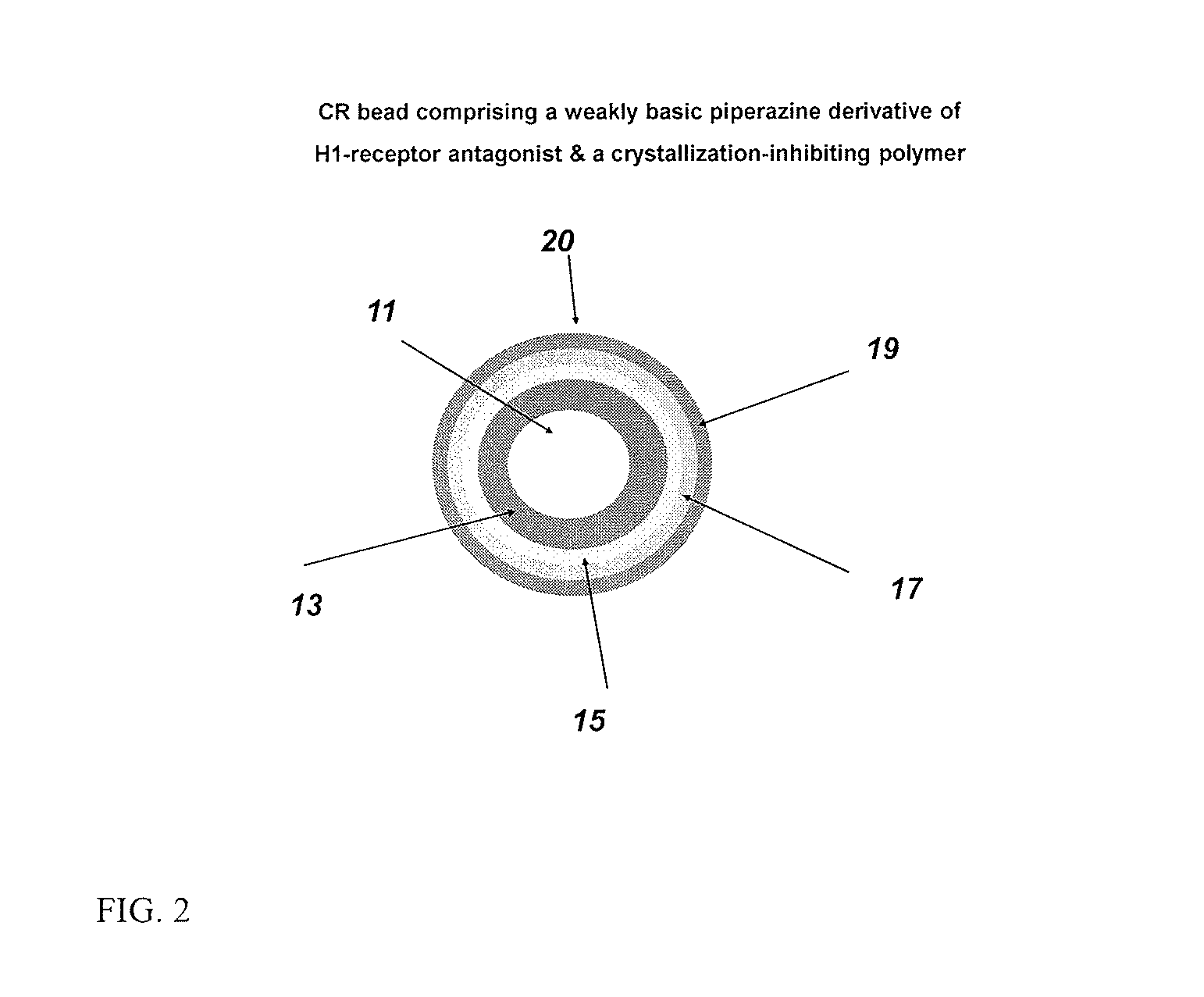
Drug delivery systems comprising weakly basic drugs and organic acids
InactiveUS20070196491A1Increase probabilityAvoid eliminationPowder deliveryNervous disorderParticulatesRegimen
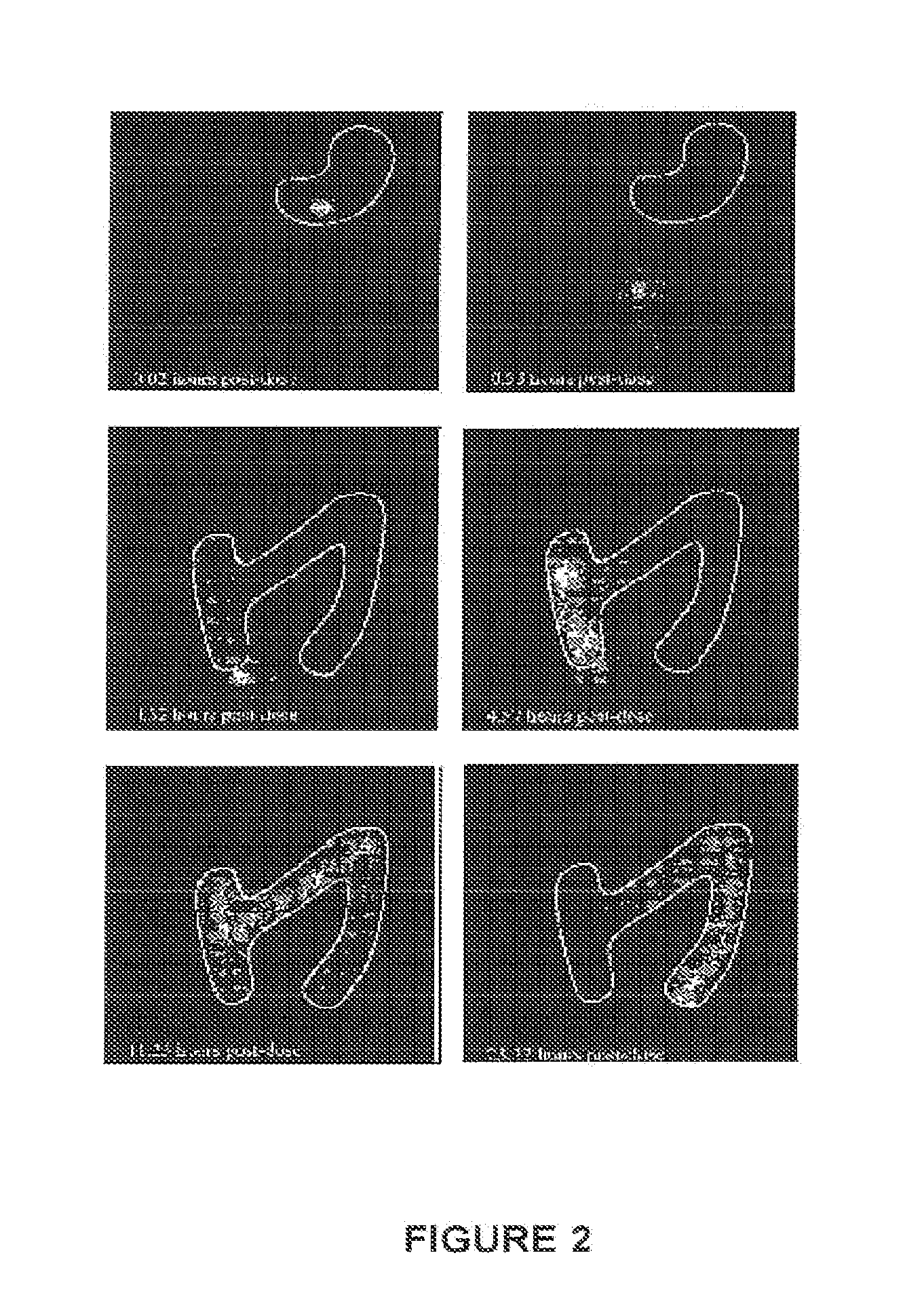
A pharmaceutical dosage form such as a capsule, a conventional or orally disintegrating tablet capable of delivering a nitrogen (N)-containing therapeutic agent having a pKa in the range of from about 5 to 14 into the body in a sustained-released fashion, in order to be suitable for a twice- or once-daily dosing regimen, comprises at least one organic acid, which solubilizes the therapeutic agent the drug prior to releasing it into the hostile intestinal environment wherein said weakly basic drug is practically insoluble. The unit dosage form is composed of a multitude of multicoated particulates (i.e., immediate-release beads, sustained-release beads and / or one or more timed, pulsatile-release bead populations) is designed in such a way that said weakly basic drug and said organic acid do not come into close contact during processing and / or storage for in-situ formation of acid addition compounds while ensuring that the acid is not depleted prior to completion of the drug release.
Owner:ADARE PHARM INC
Oral dosage form safeguarded against abuse
The present invention relates to an abuse-proofed, oral dosage form with controlled opioid-release for once daily administration, characterised in that it comprises at least one opioid with potential for abuse (A), at least one synthetic or natural polymer (C), optionally delayed-release matrix auxiliary substances, physiologically acceptable auxiliary substances (B), optionally a wax (D) and optionally at least one delayed-release coating, component (C) or (D) in each case exhibiting a breaking strength of at least 500 N, preferably of at least 1000 N.
Owner:GRUNENTHAL GMBH
Tacrolimus for improved treatment of transplant patients
ActiveUS20100105717A1Improve bioavailabilityReduce riskBiocideOrganic chemistryTherapeutic effectIn vivo
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARM INC
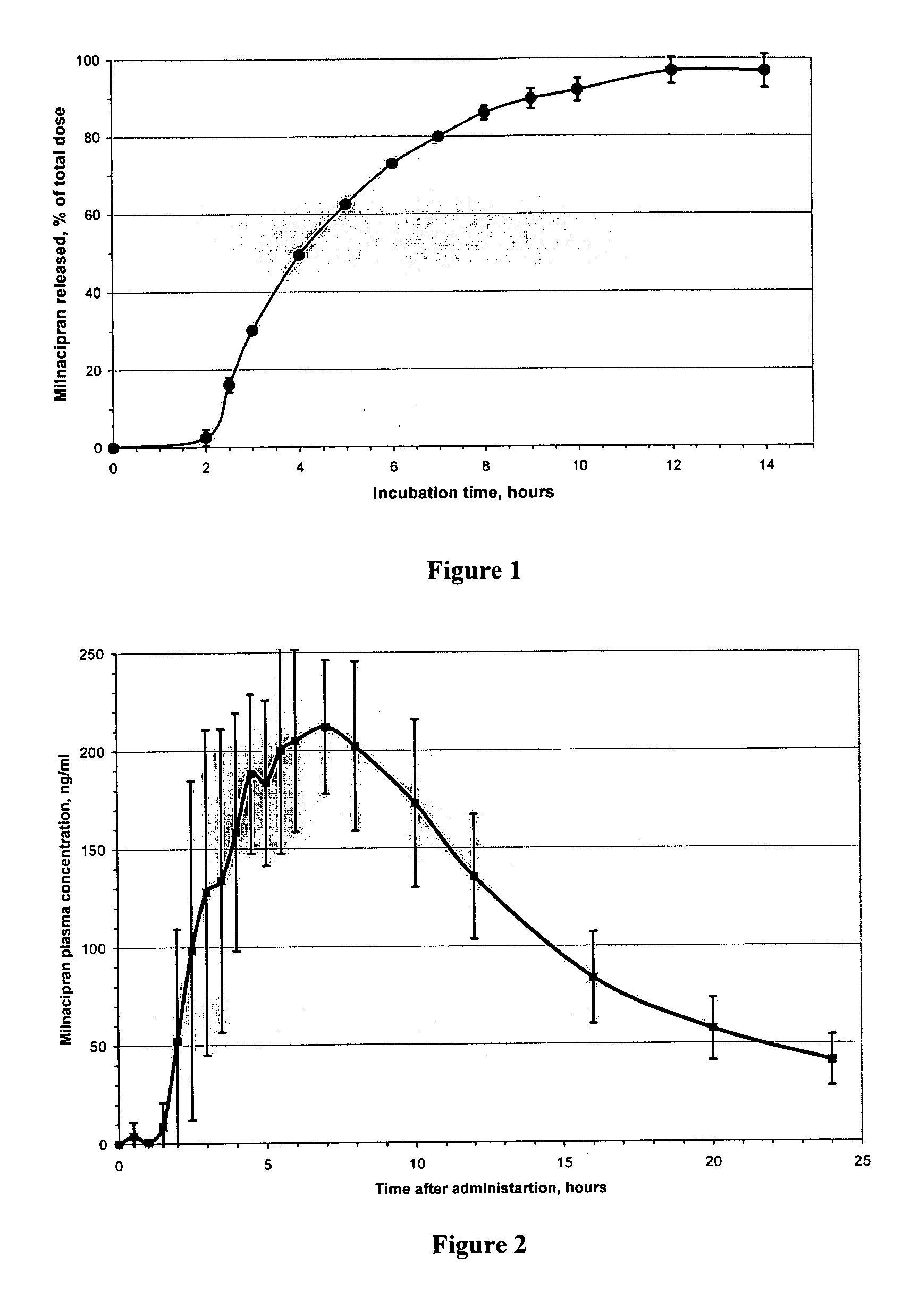
Modified release compositions of milnacipran
InactiveUS20060024366A1Reduce incidenceDiminished decreased intensityCoatingsDrageesPalpitationsRash
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
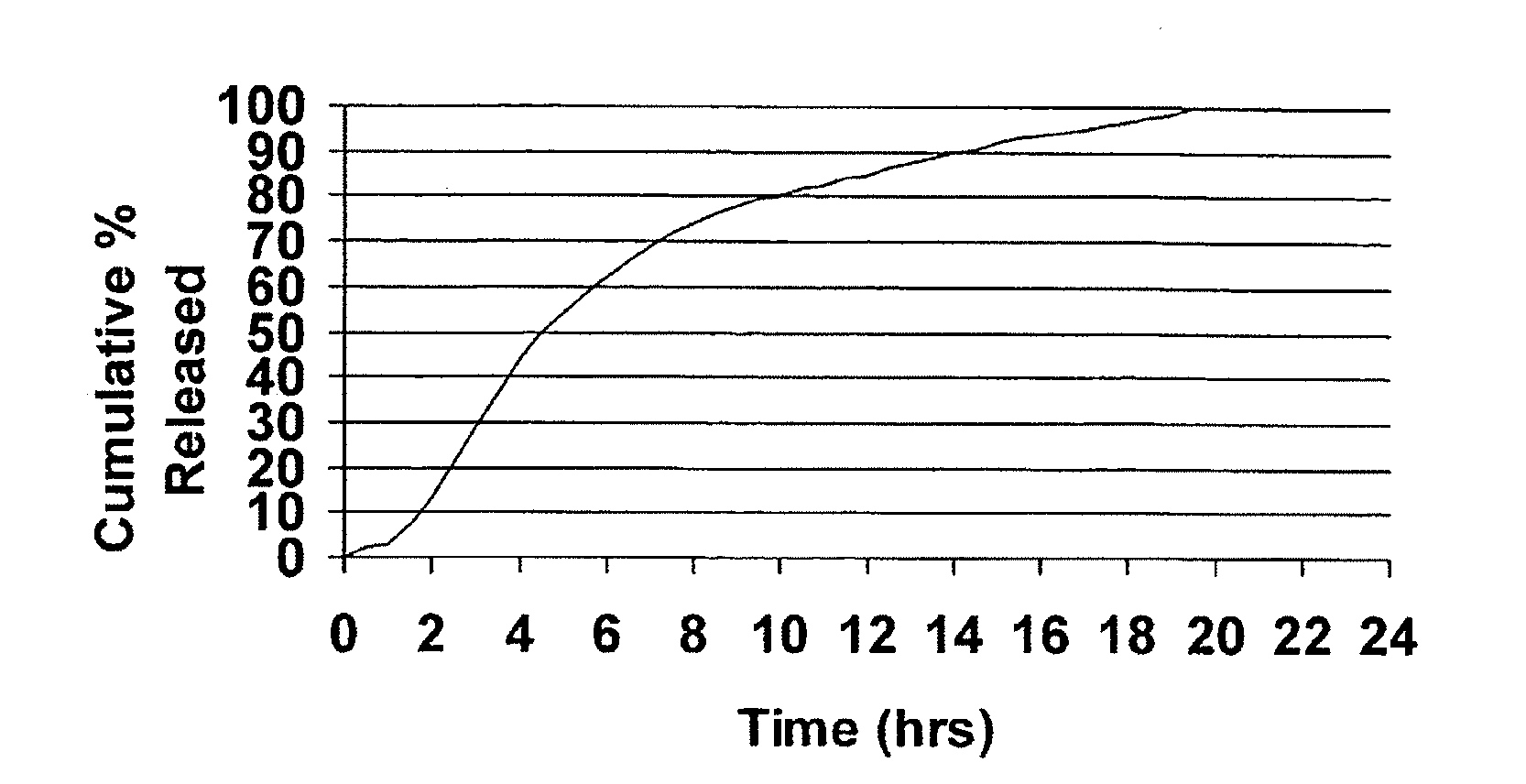
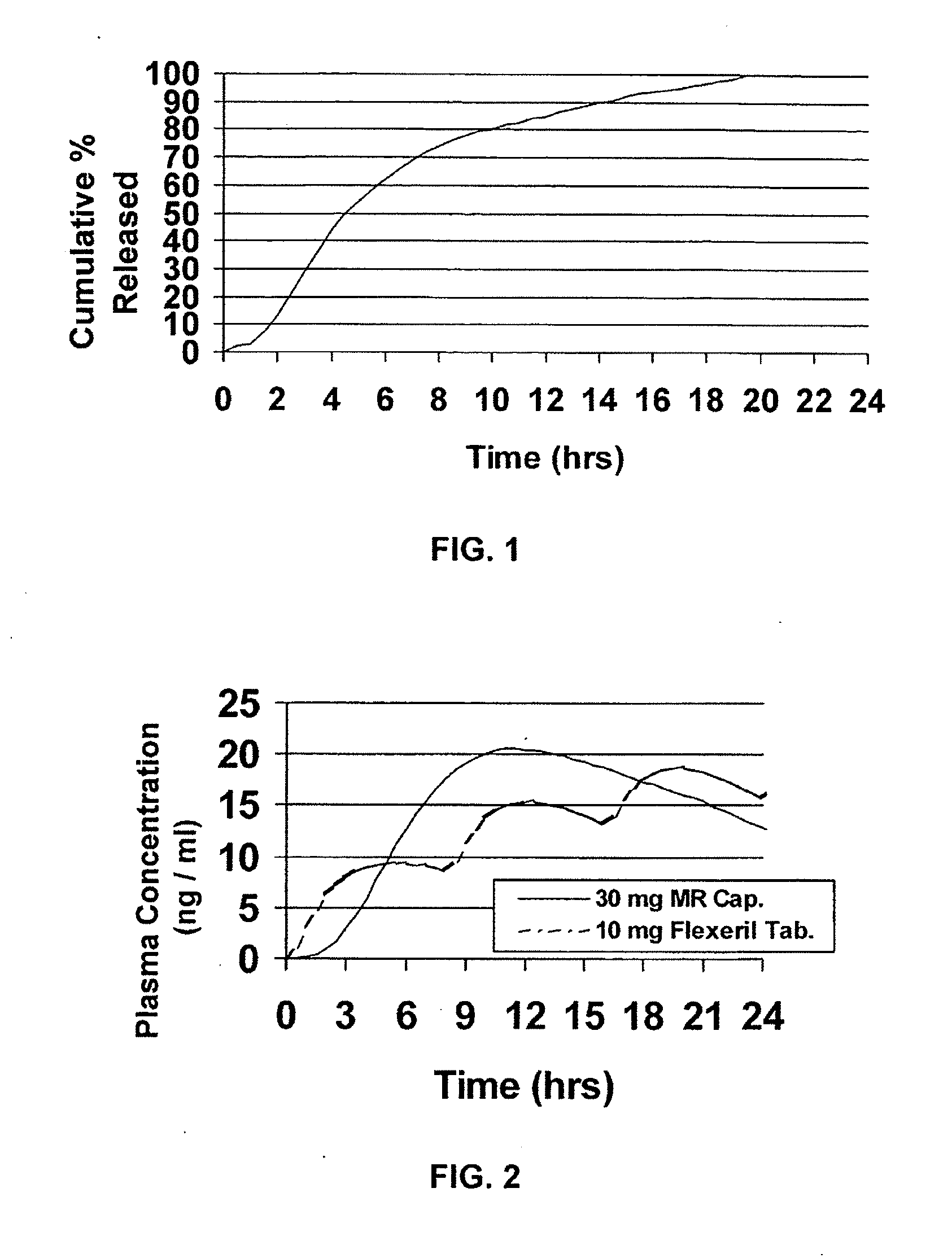
Modified release dosage forms of skeletal muscle relaxants
InactiveUS20080124398A1Patient compliance is goodEfficient ConcentrationPowder deliveryOrganic active ingredientsDiseaseModified Release Dosage Form
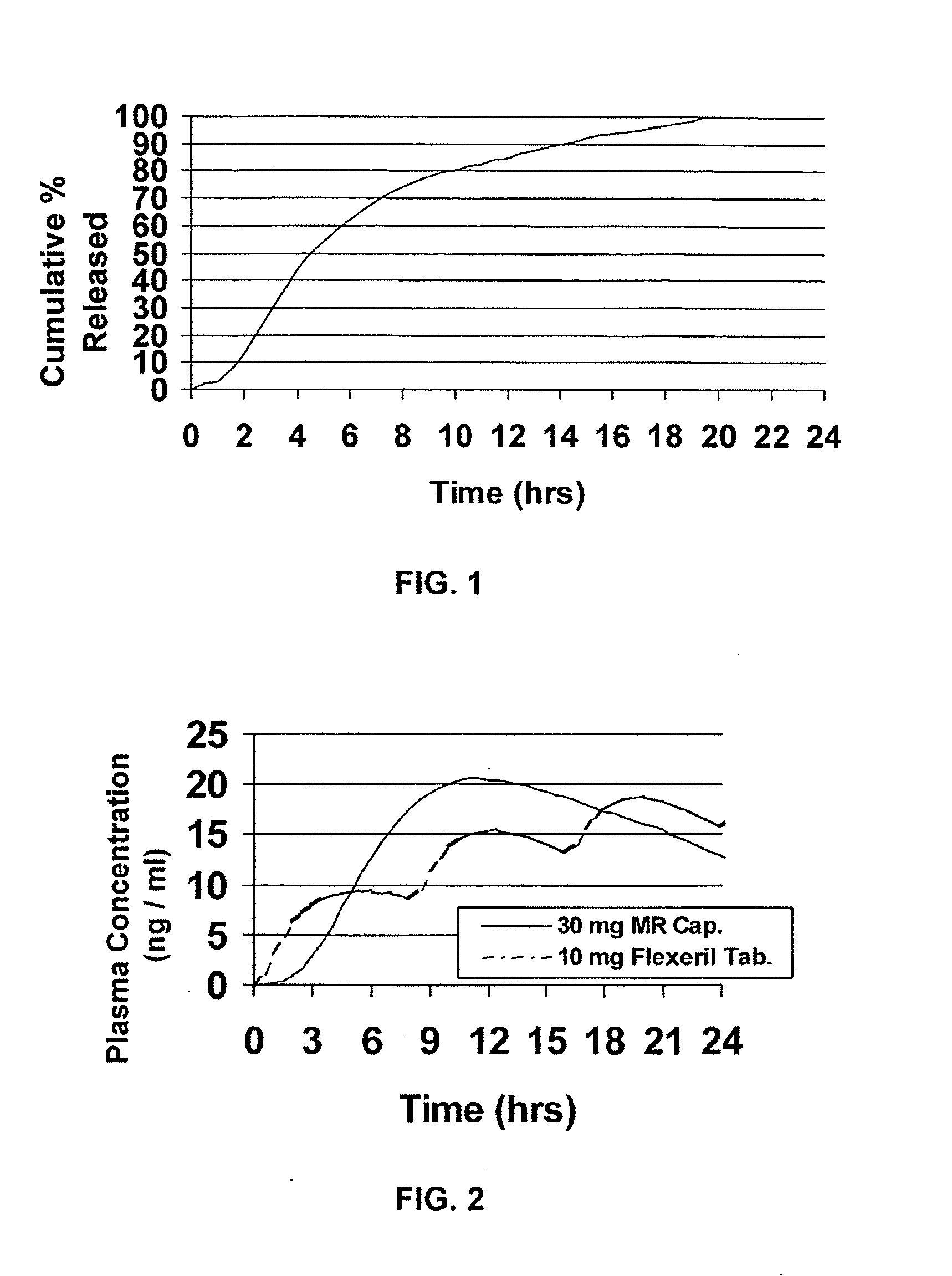
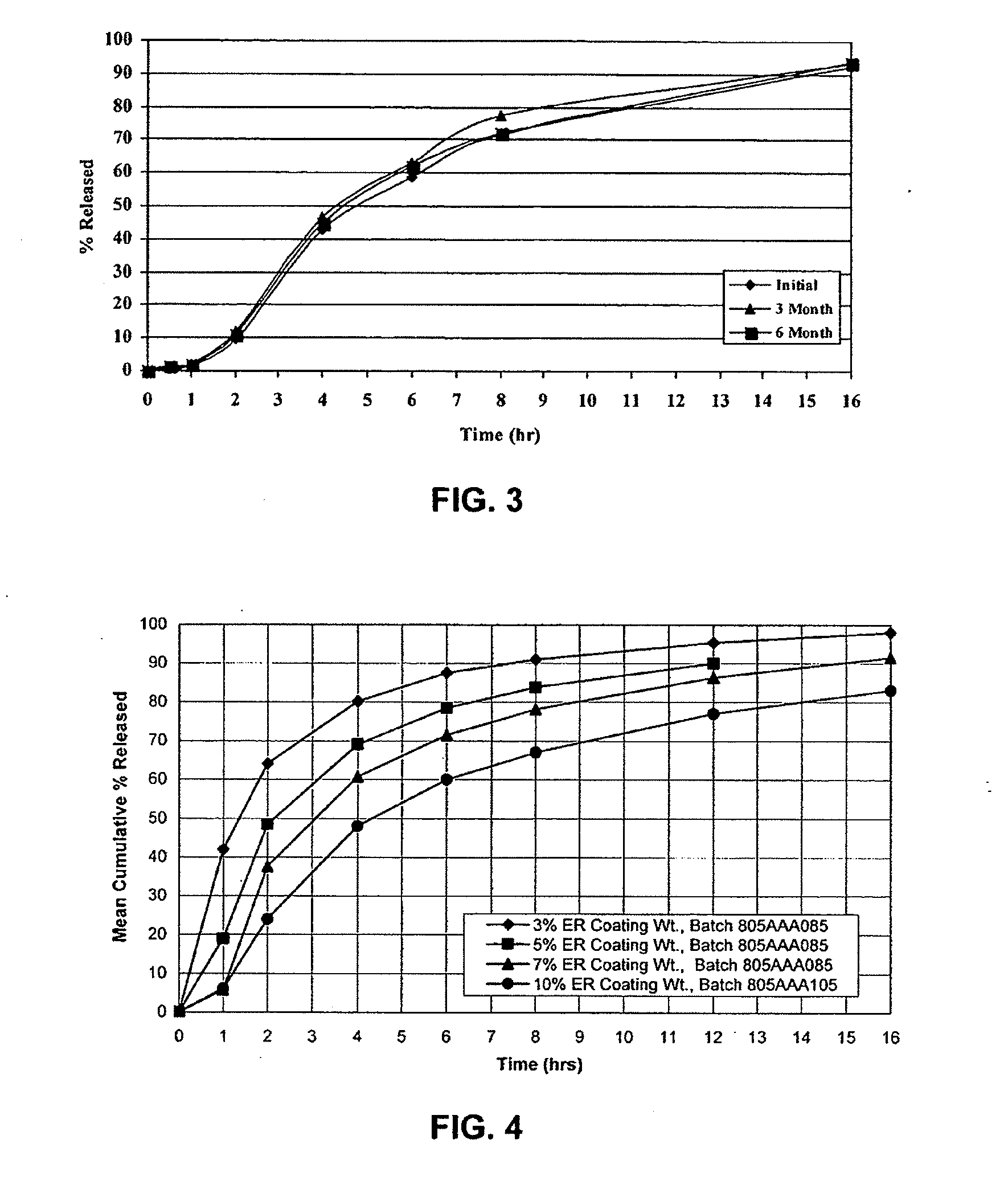
A unit dosage form, such as a capsule or the like, for delivering a skeletal muscle relaxant, such as cyclobenzaprine hydrochloride, into the body in an extended or sustained release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. At least one bead population exhibits a pre-designed sustained release profile. Such a drug delivery system is designed for once-daily oral administration to maintain an adequate plasma concentration—time profile, thereby providing relief of muscle spasm associated with painful musculoskeletal conditions over a 24 hour period.
Owner:ADARE PHARM INC
Controlled release formulation of divalproex sodium
A controlled release tablet formulation which permits once daily dosing in the treatment of epilepsy comprises from about 50 weight percent to about 55 weight percent of an active ingredient selected from the group consisting of valproic acid, a pharmaceutically acceptable salt or ester of valproic acid, divalproex sodium, and valpromide; from about 20 weight percent to about 40 weight percent hydroxypropyl methylcellulose; from about 5 weight percent to about 15 weight percent lactose, from about 4 weight percent to about 6 weight percent microcrystalline cellulose, and from about 1 weight percent to about 5 weight percent silicon dioxide having an average particle size ranging between about 1 micron and about 10 microns; all weight percentages based upon the total weight of the tablet dosage form. Also disclosed are pre-tableting granular formulations, methods of making the granular formulations and tablets, and a method of treating epilepsy employing the controlled release tablet formulations of the invention.
Owner:ABBOTT LAB INC
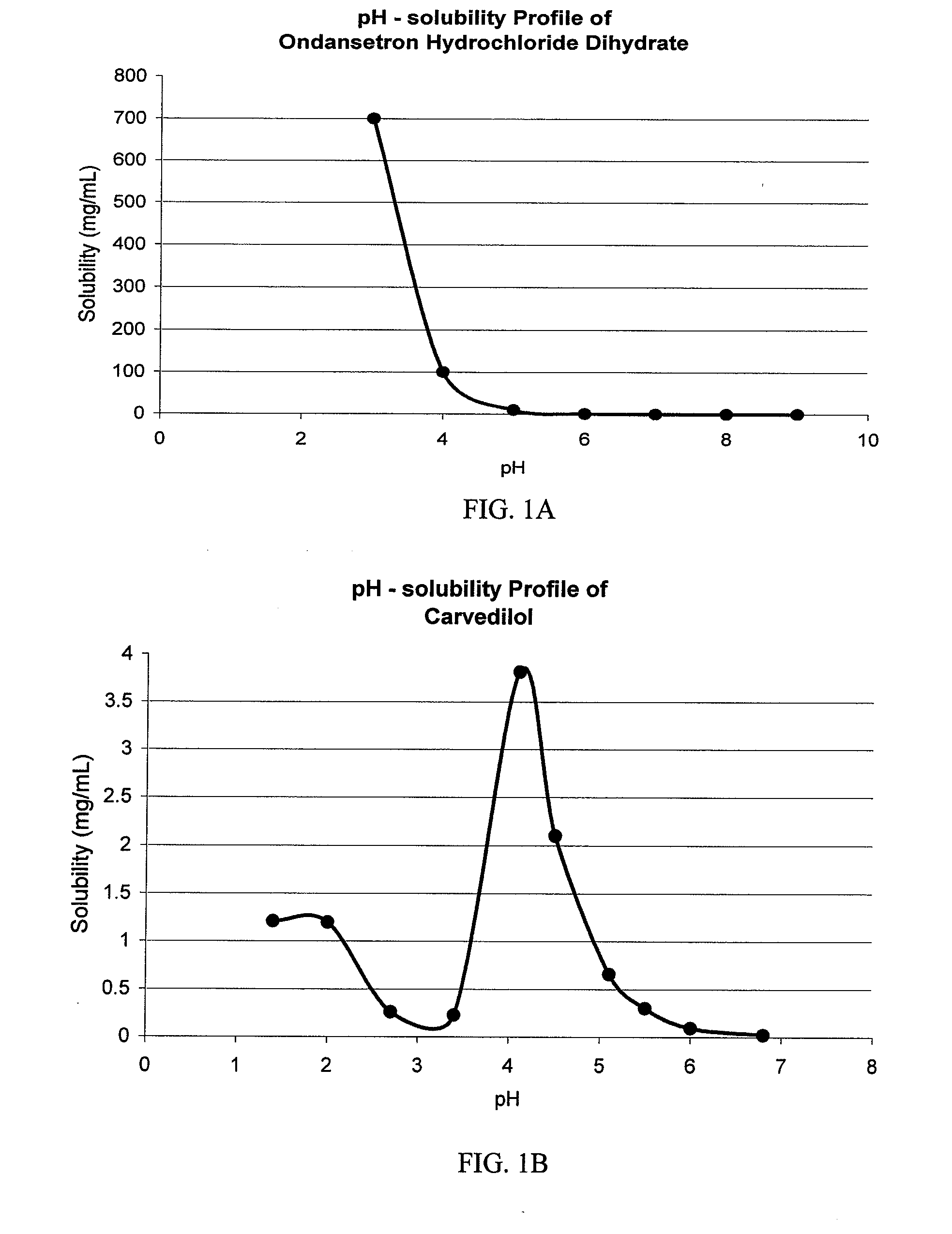
Ondansetron Orally Disintegrating Tablet Compositions for Prevention of Nausea and Vomiting
InactiveUS20110135724A1Disperse fastEasy to swallowOrganic active ingredientsBiocideSerotoninRegimen
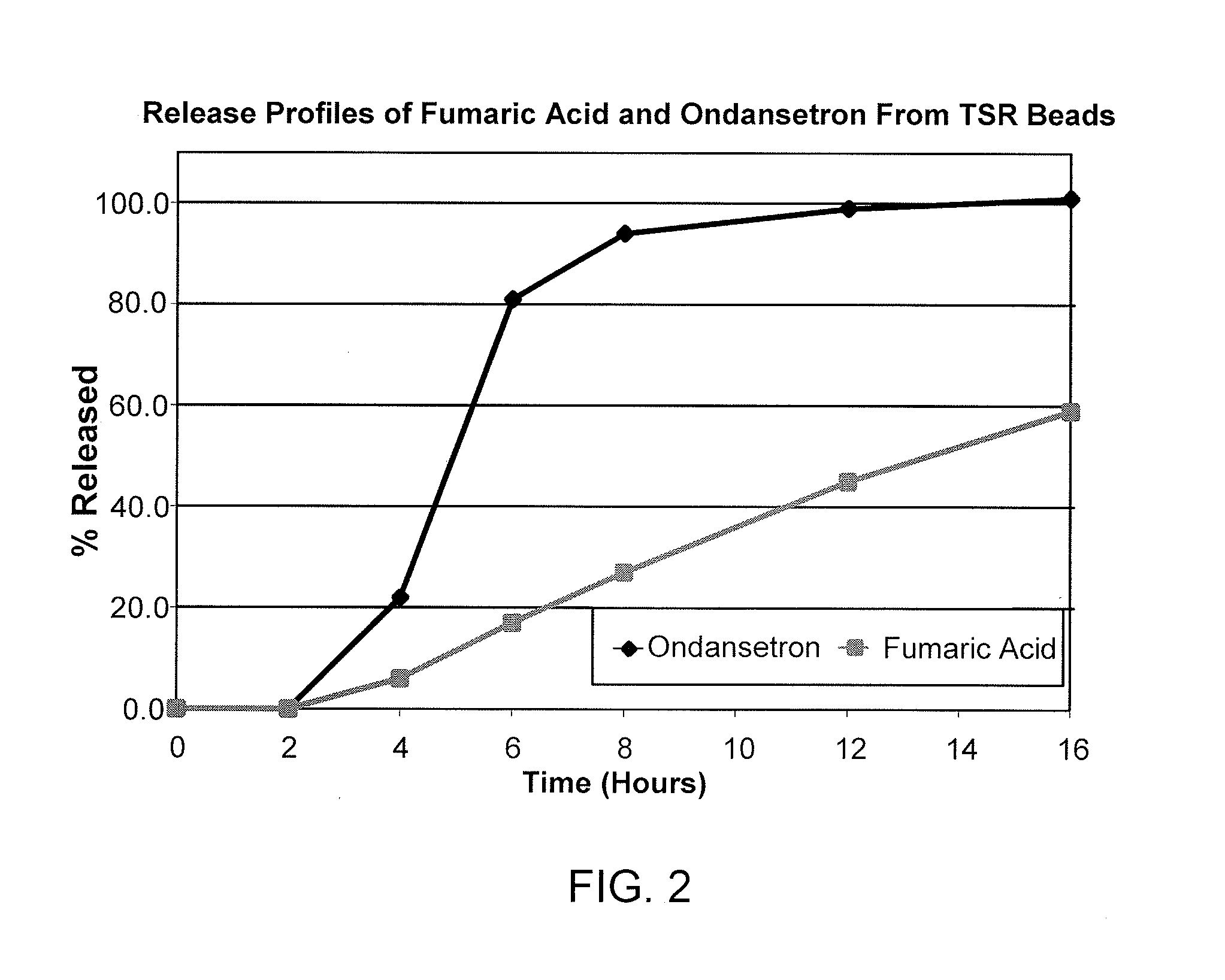
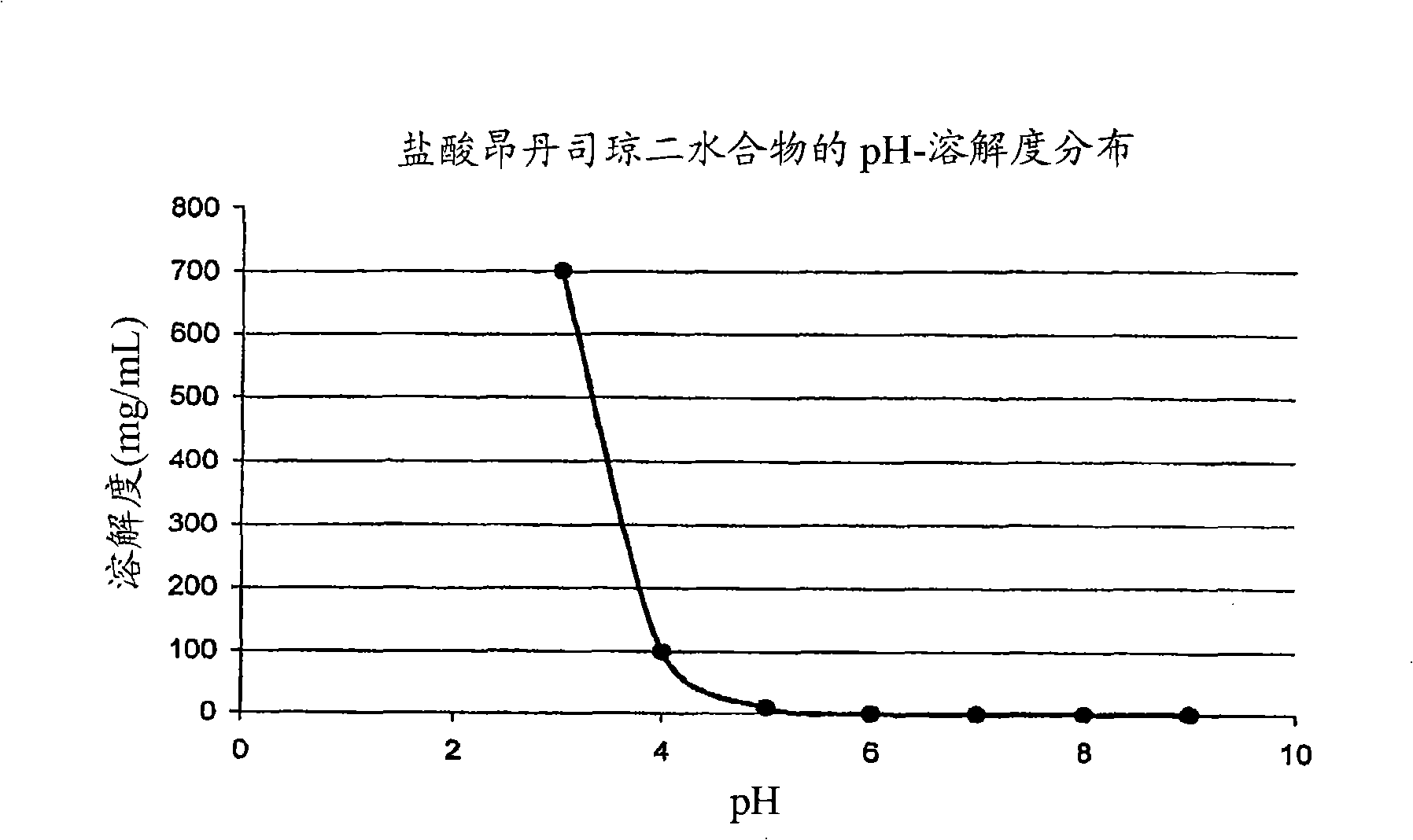
This invention is related to a pharmaceutical composition in the patient-friendly orally disintegrating tablet form comprising a weakly basic, selective serotonin 5-HT3 blocking agent for the prevention of nausea and / or vomiting for up to 24 hrs postdosing in cancer patients prior to undergoing moderately emetogenic chemotherapy or partial or whole body radiotherapy or in subjects at moderate to high risk of postoperative or postdischarge nausea and / or vomiting prior to inpatient or outpatient ambulatory surgery. The unit dosage form comprising a multitude of immediate-release drug particles providing dissolution profiles similar to that of reference drug product, and one or more timed, pulsatile-release bead populations, comprising at least one organic acid, which solubilizes said weakly basic selective serotonin 5-HT3 blocking agent prior to releasing it into the hostile intestinal environment, wherein the blocking agent is practically insoluble, is capable of delivering said antiemetic agent in patients in need thereof in a sustained-released fashion to be suitable for a once-daily dosing regimen.
Owner:APTALIS PHARMATECH
Modified-release compositions of at least one form of venlafaxine
InactiveUS20050244498A1Reduce morbidityReduce releaseBiocideAnimal repellantsControlled releaseOral medication
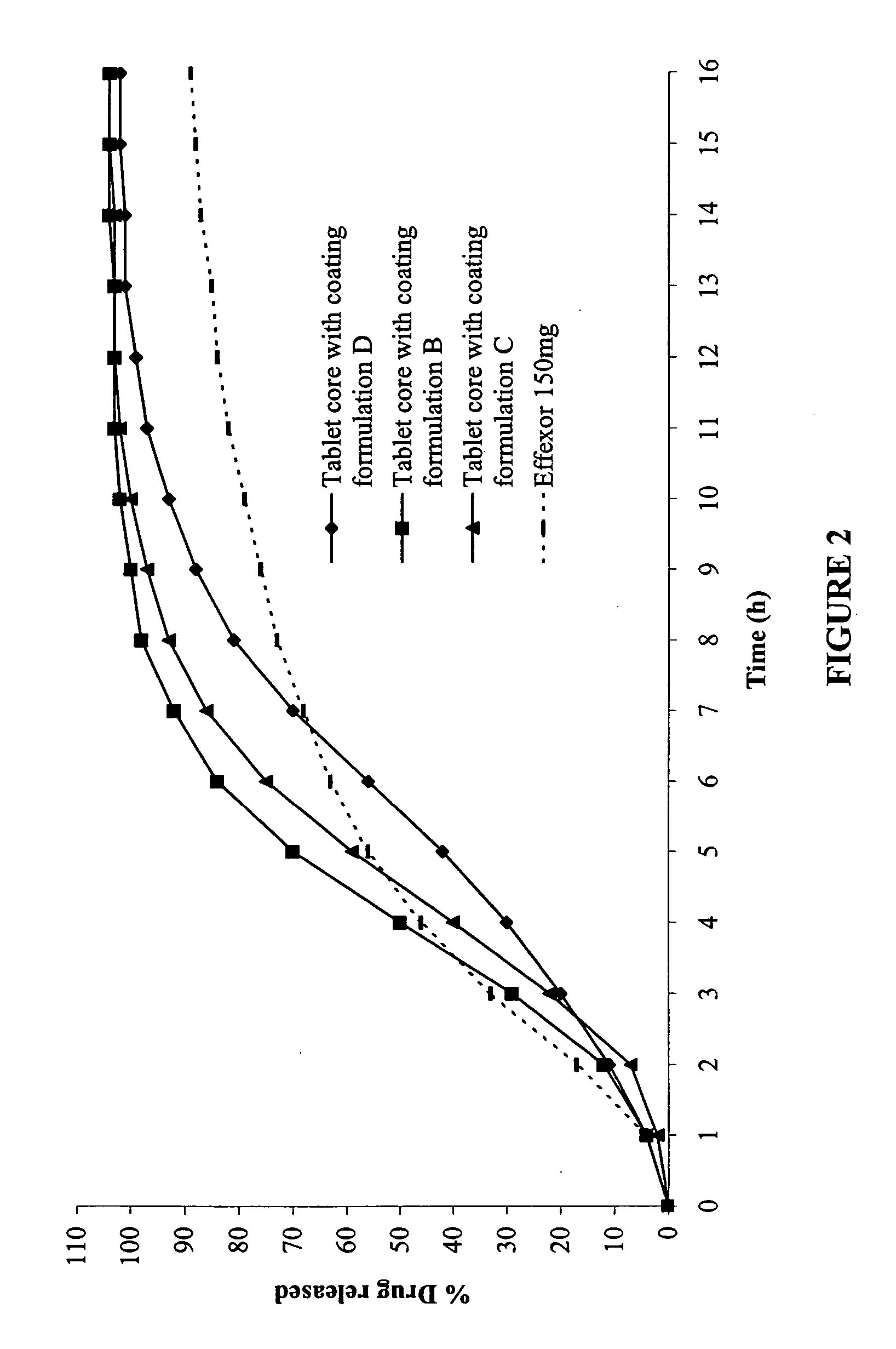
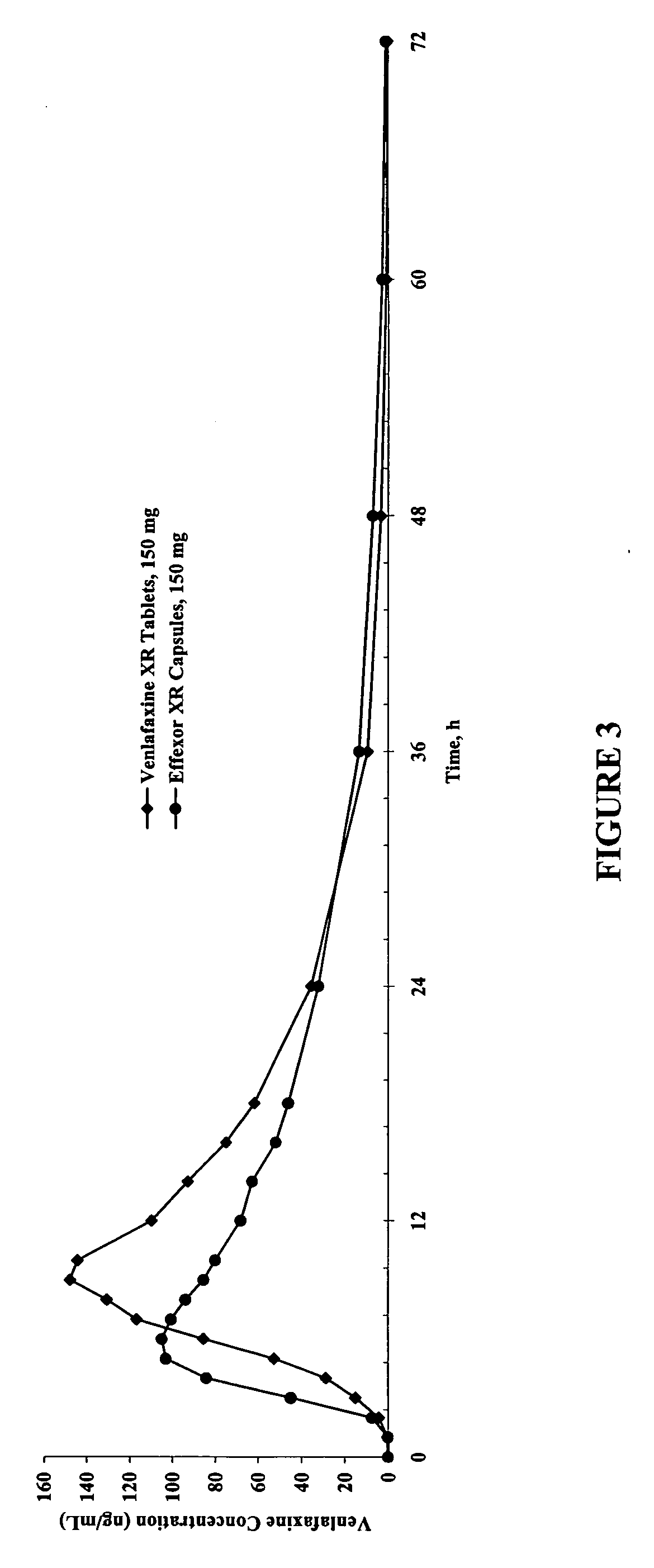
The present invention relates to a modified release composition of at least one form of venlafaxine, which is an enhanced absorption delayed controlled release composition for oral administration suitable for once daily dosing. The composition comprises a core comprising at least one form of venlafaxine selected from the group consisting of venlafaxine, an active metabolite of venlafaxine, a pharmaceutically acceptable salt of venlafaxine, a pharmaceutically acceptable salt of an active metabolite of venlafaxine, and combinations thereof, and a pharmaceutically acceptable excipient. The composition further comprises a modified release coating which substantially surrounds the core. The compositions of the invention provide enhanced absorption delayed controlled release of the at least one form of venlafaxine such that the combined geometric mean ratio of the composition of the invention to the reference product for the AUC0-t or the Cmax for venlafaxine and its active metabolite O-desmethylvenlafaxine is greater than 2 after first administration of the composition under fed or fasting conditions.
Owner:BIOVAIL LAB INT SRL
Drug delivery systems comprising weakly basic drugs and organic acids
Owner:ADARE PHARM INC
Sustained release dosage forms of oxcarbazepine
This invention relates to a once a day sustained release dosage form suitable for oral administration of oxcarbazepine. The once a day sustained release dosage form of oxcarbazepine includes oxcarbazepine and hydroxypropyl methylcellulose (HPMC) having a viscosity of 11,000 to 25,000 cps.
Owner:RAMAKRISHNAN SANKAR +3
Sutained release formulation for venlafaxine hydrochloride
InactiveUS20060182797A1Quick releaseOrganic active ingredientsNervous disorderSustained Release CapsuleMini tablets
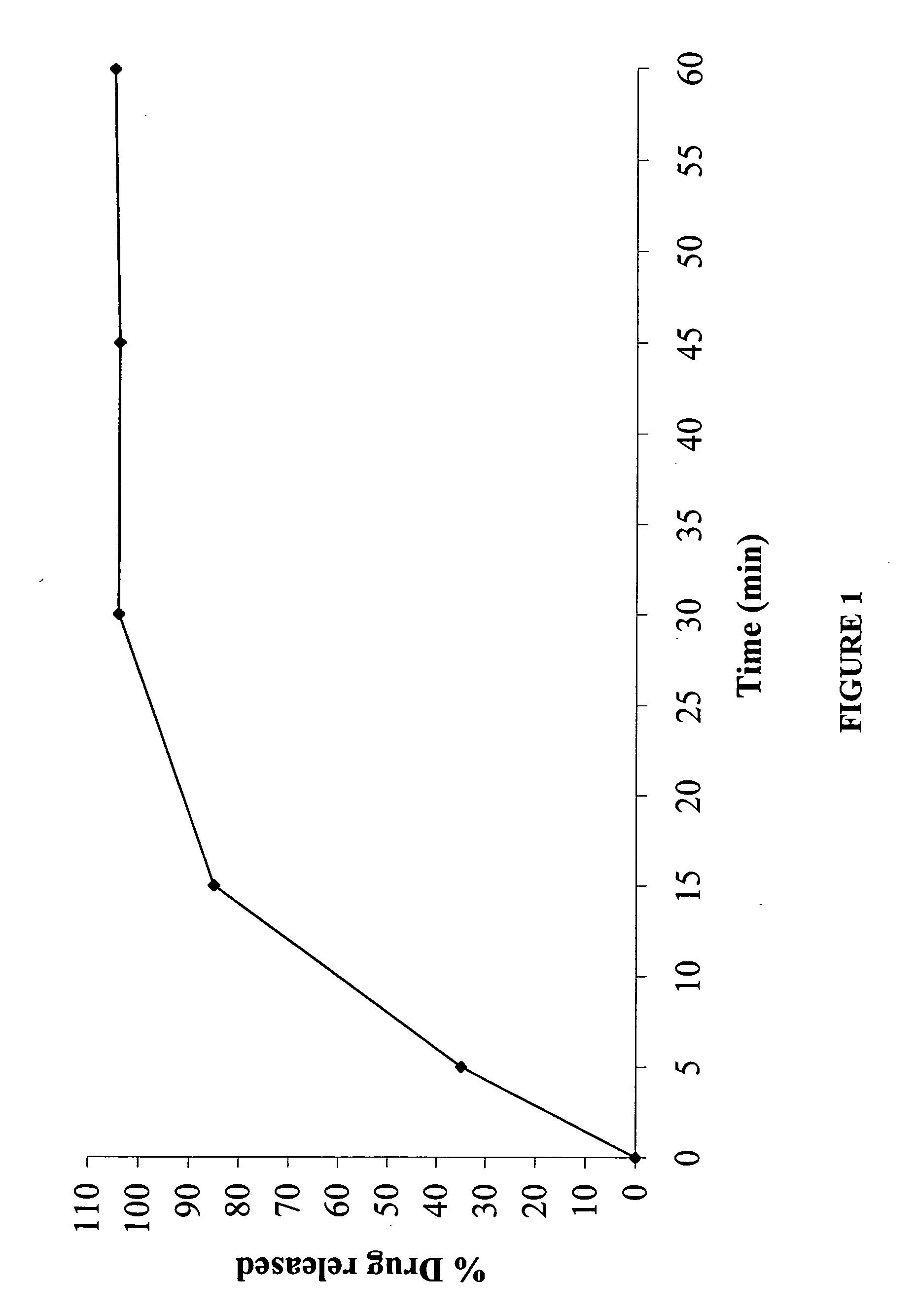
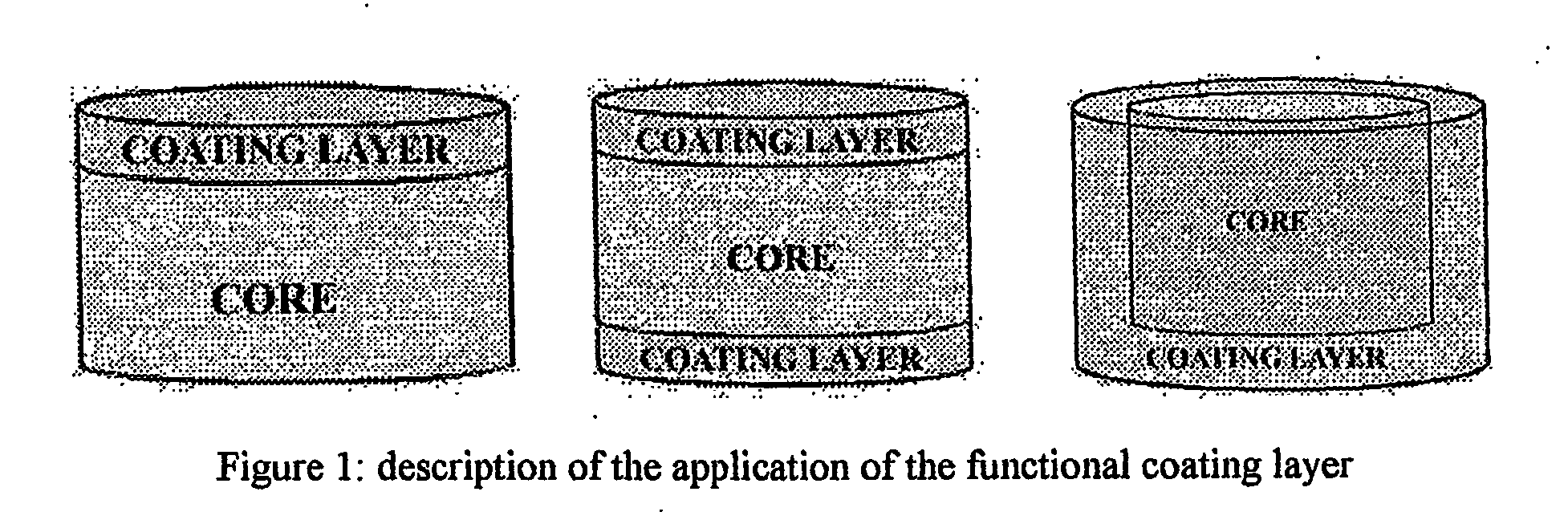
The invention provides a sustained release composition that; 1. Is free of initially increased drug delivery that occurs (in sustained release systems containing the water soluble drug venlafaxine HCl, known as burst phenomenon, by using a functional core partially or totally coated by a functional coating layer or film. 2. Delivers the drug substance within 24 hours and is therefore suitable for once daily administration of the said drug substance. 3. Exhibits linearity between the strength dosage form and the (total mass of the dosage form, by proportional increase of the amounts of the drug substance and the excipients in the formulation. 4. Is possible to be divided in smaller doses, without affecting the release of the drug substance. The invention provides a sustained release capsule formulation containing an appropriate number of functional complex mini tablets comprising of: I. A functional core comprising the active ingredient, especially the water-soluble drug Venlafaxine HCl and appropriate excipients. 2. A functional coating layer or film that reduces the initial surface of the core that is available for the release of the water-soluble drug Venlafaxine HClt phenomenon.
Owner:PHARMATHEN
Drug delivery systems comprising weakly basic selective serotonin 5-HT3 blocking agent and organic acids
Owner:ADARE PHARM INC
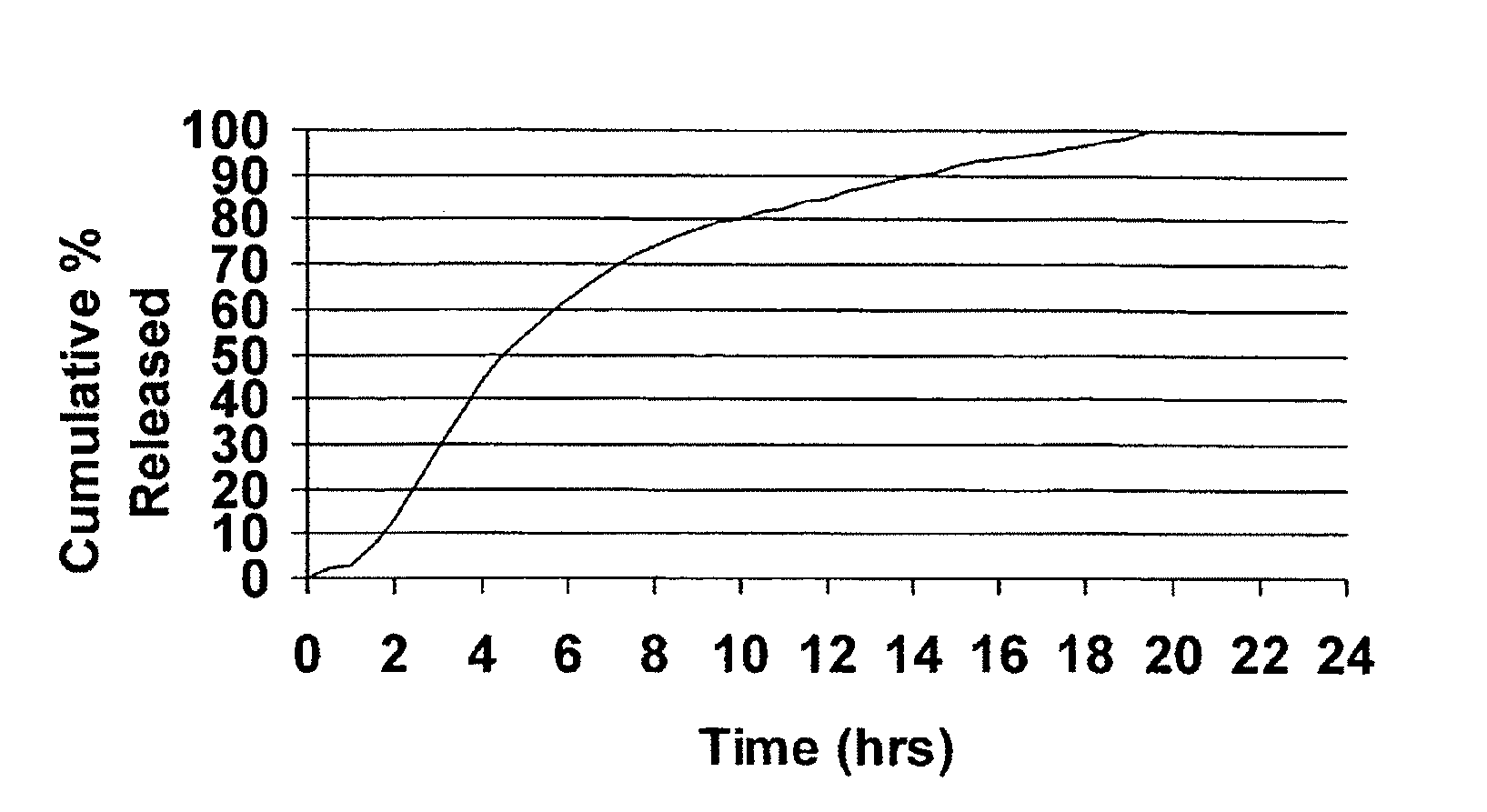
Controlled release compositions comprising meclizine or related piperazine derivatives
The present invention provides pharmaceutically acceptable compositions for once-daily dosing comprising a piperazine derivative of H1-receptor antagonists, or its salt, and / or solvate and methods of making and using the compositions in the treatment of treating vertigo and other diseases. The present invention also provides once-a-day dosage forms as orally disintegrating tablets comprising compositions of the present invention.
Owner:EURAND INC +1
Modified release dosage forms of skeletal muscle relaxants
InactiveUS20080124399A1Patient compliance is goodEfficient ConcentrationPowder deliveryCosmetic preparationsDiseaseModified Release Dosage Form
A unit dosage form, such as a capsule or the like, for delivering a skeletal muscle relaxant, such as cyclobenzaprine hydrochloride, into the body in an extended or sustained release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. At least one bead population exhibits a pre-designed sustained release profile. Such a drug delivery system is designed for once-daily oral administration to maintain an adequate plasma concentration-time profile, thereby providing relief of muscle spasm associated with painful musculoskeletal conditions over a 24 hour period.
Owner:ADARE PHARM INC
Tacrolimus For Improved Treatment Of Transplant Patients
InactiveUS20110275659A1Reduce riskAffect bioavailabilityBiocidePharmaceutical non-active ingredientsIn vivoBioavailability
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARMA
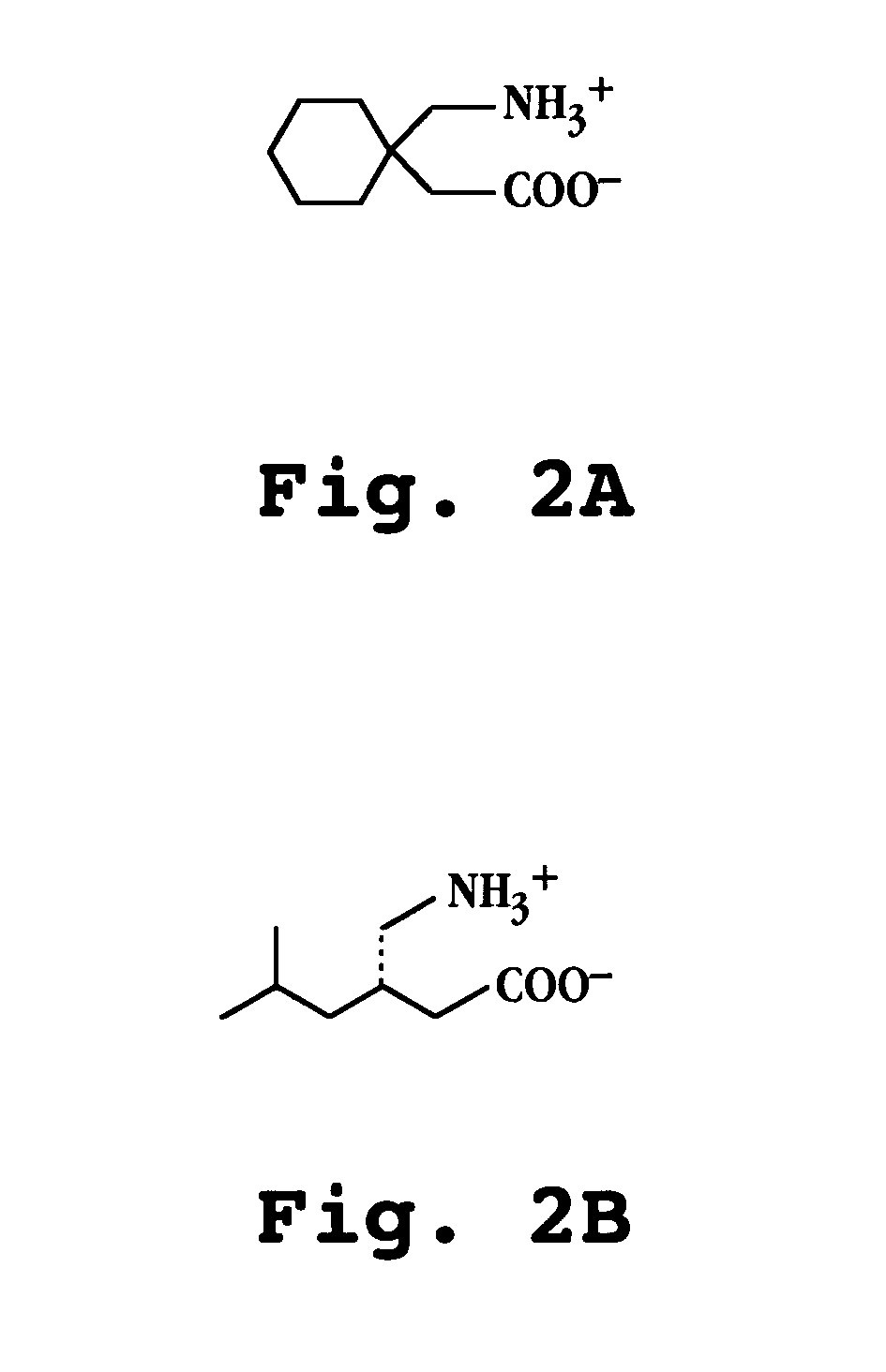
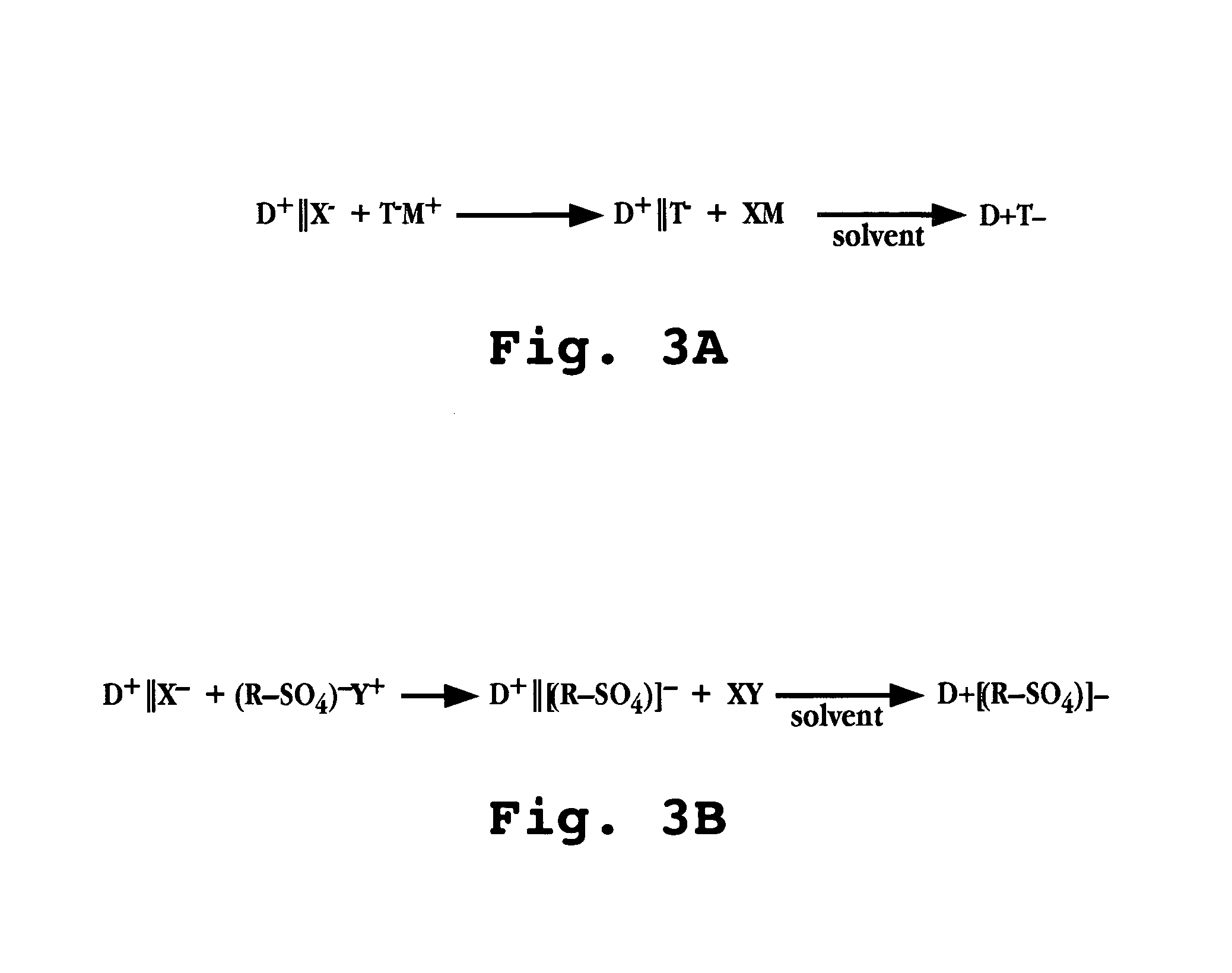
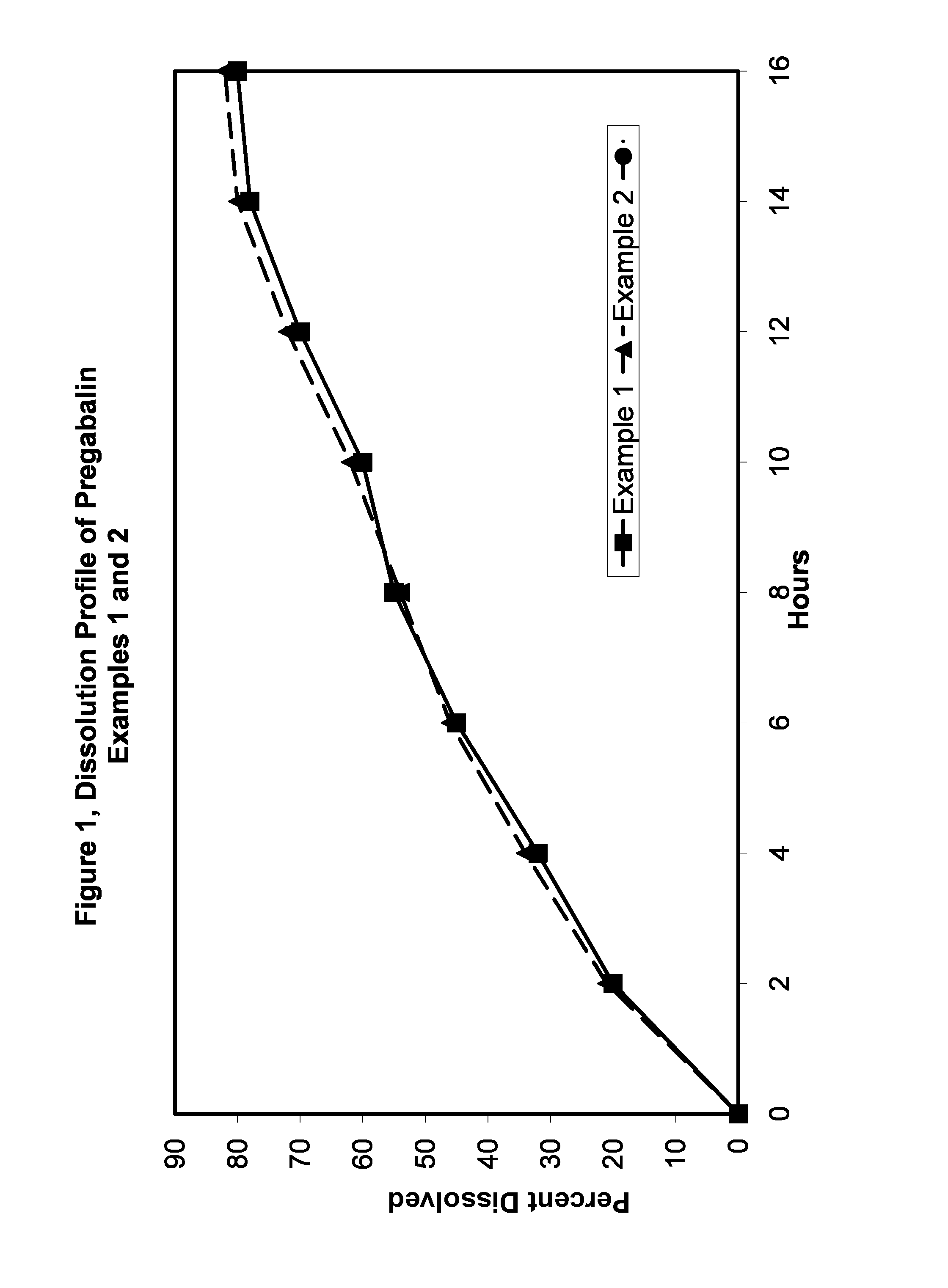
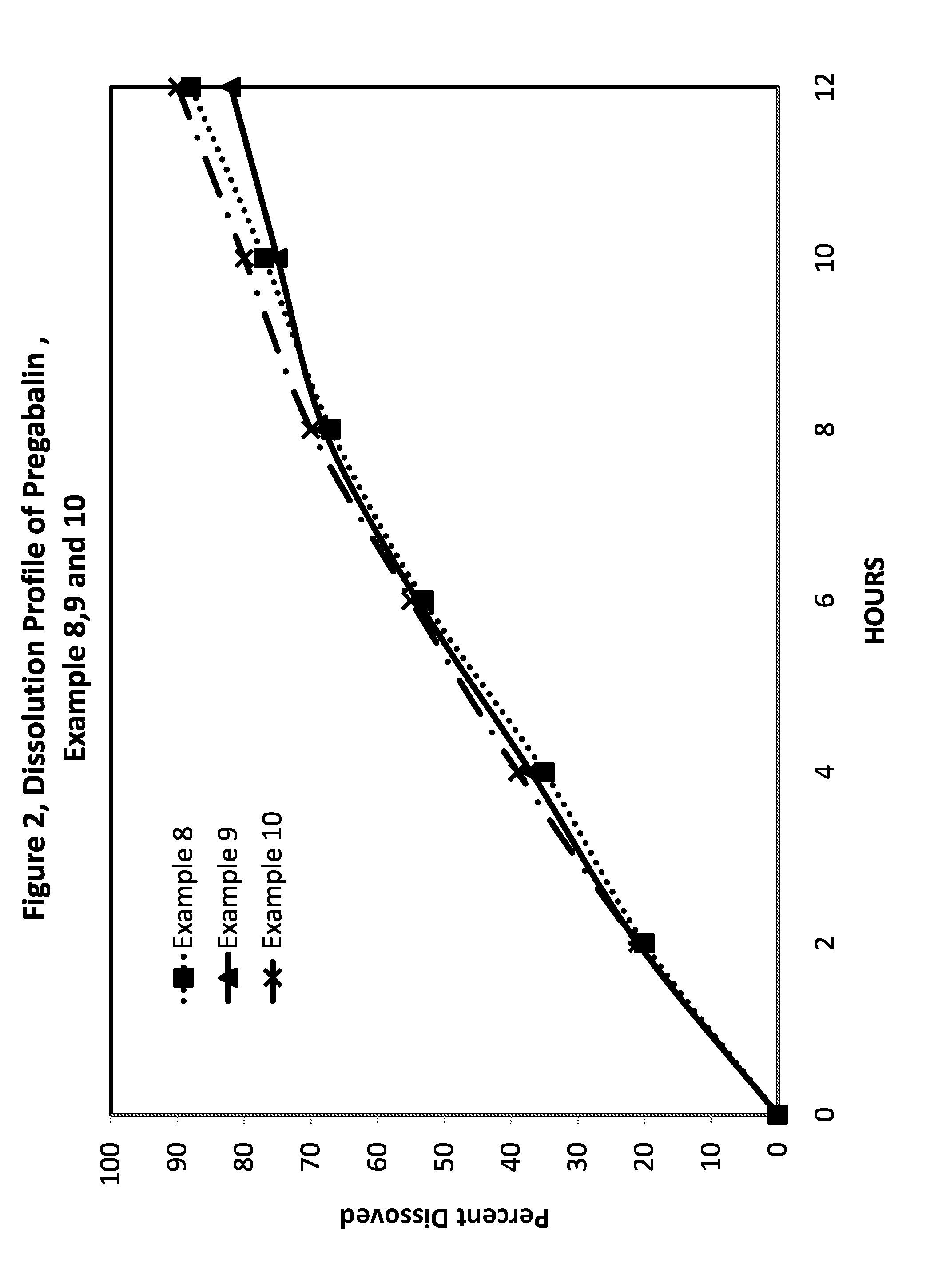
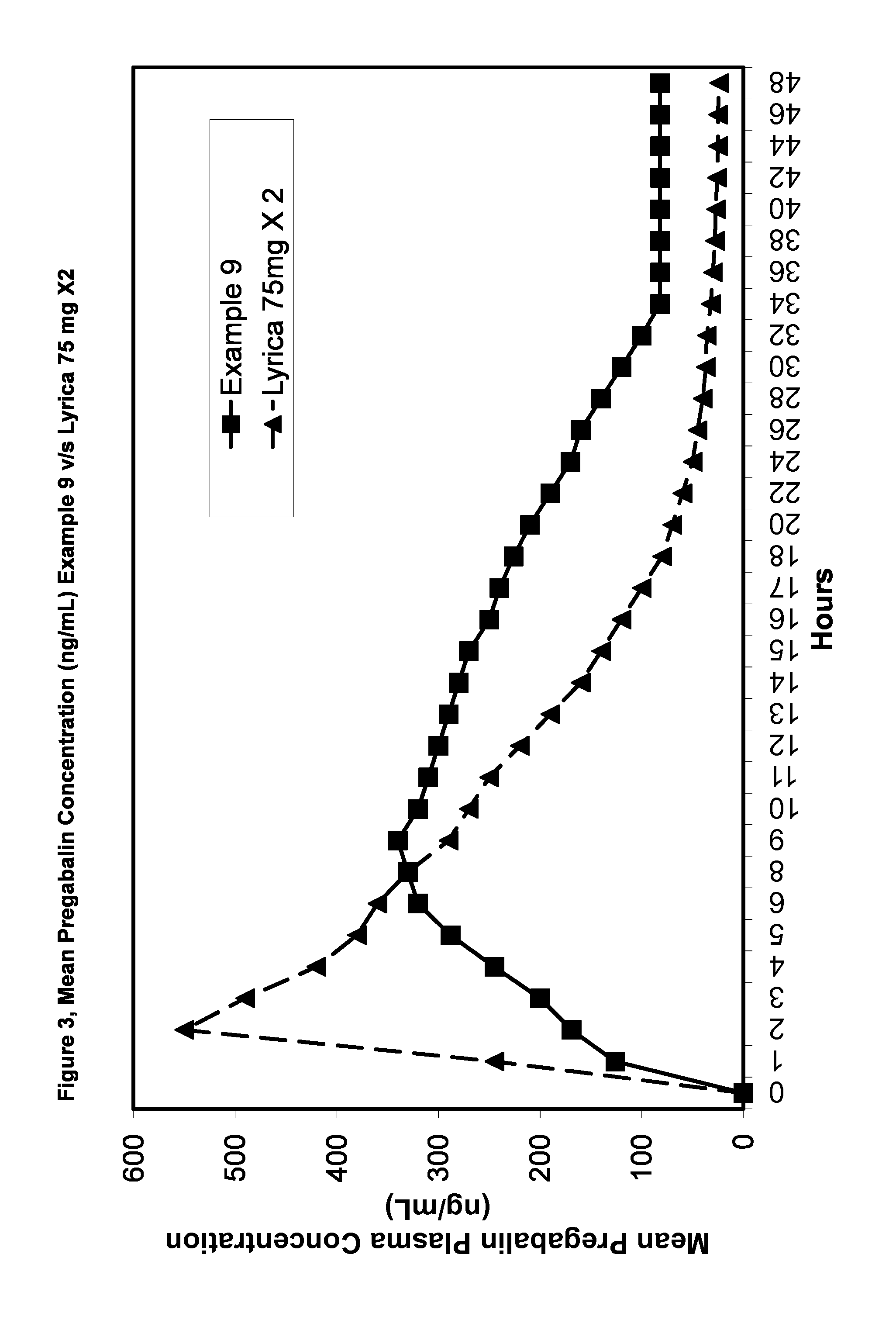
Compositions and dosage forms for enhanced absorption of gabapentin and pregabalin
A complex comprised of gabapentin or pregabalin and a transport moiety, such as an alkyl sulfate, is described. The complex has an enhanced absorption in the gastrointestinal tract, particularly the lower gastrointestinal tract. The complex, and compositions and dosage forms prepared using the complex, provide for absorption by the body of the drug through a period of ten to twenty-four hours, thus enabling a once-daily dosage form for gabapentin or pregabalin.
Owner:ALZA CORP
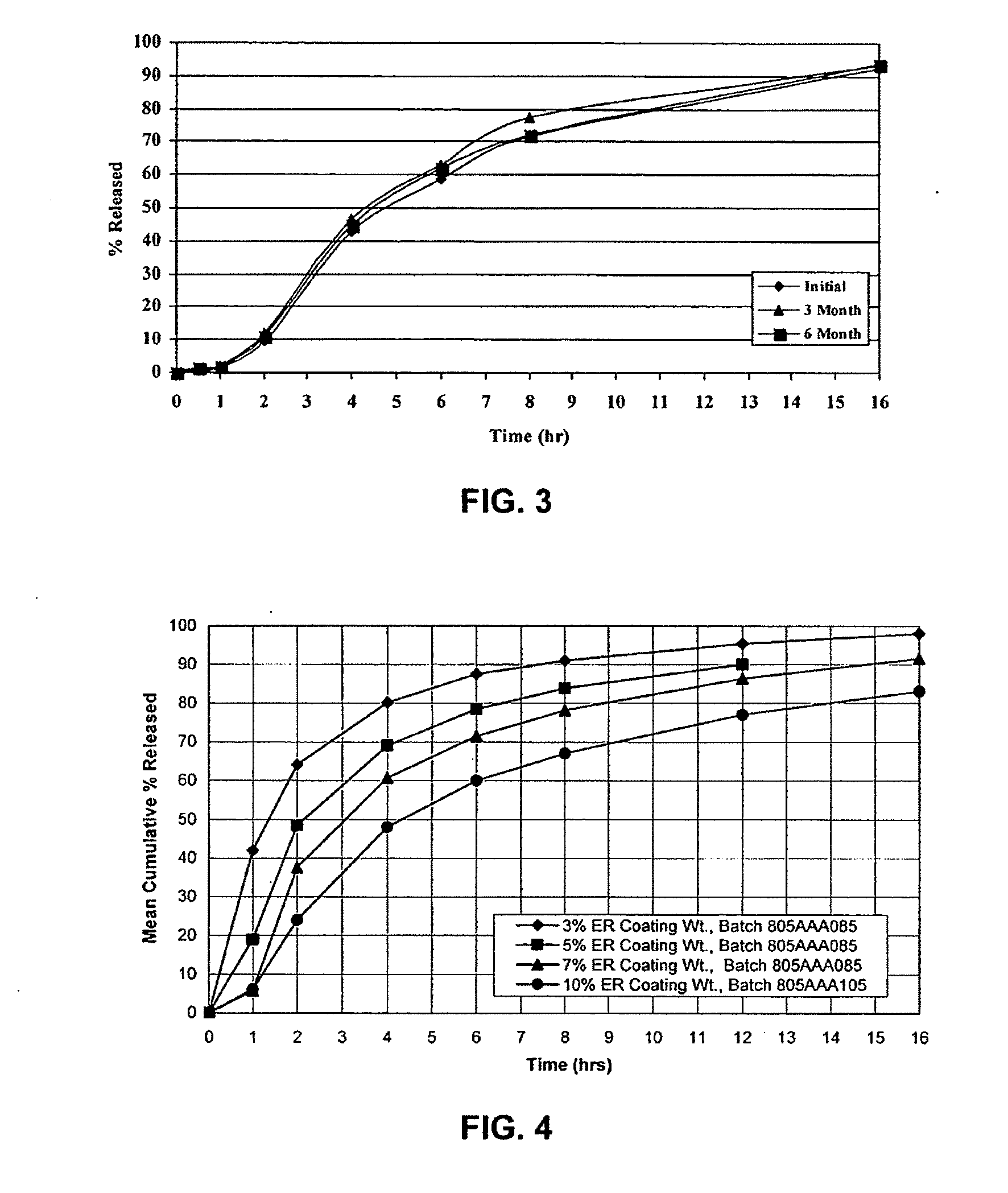
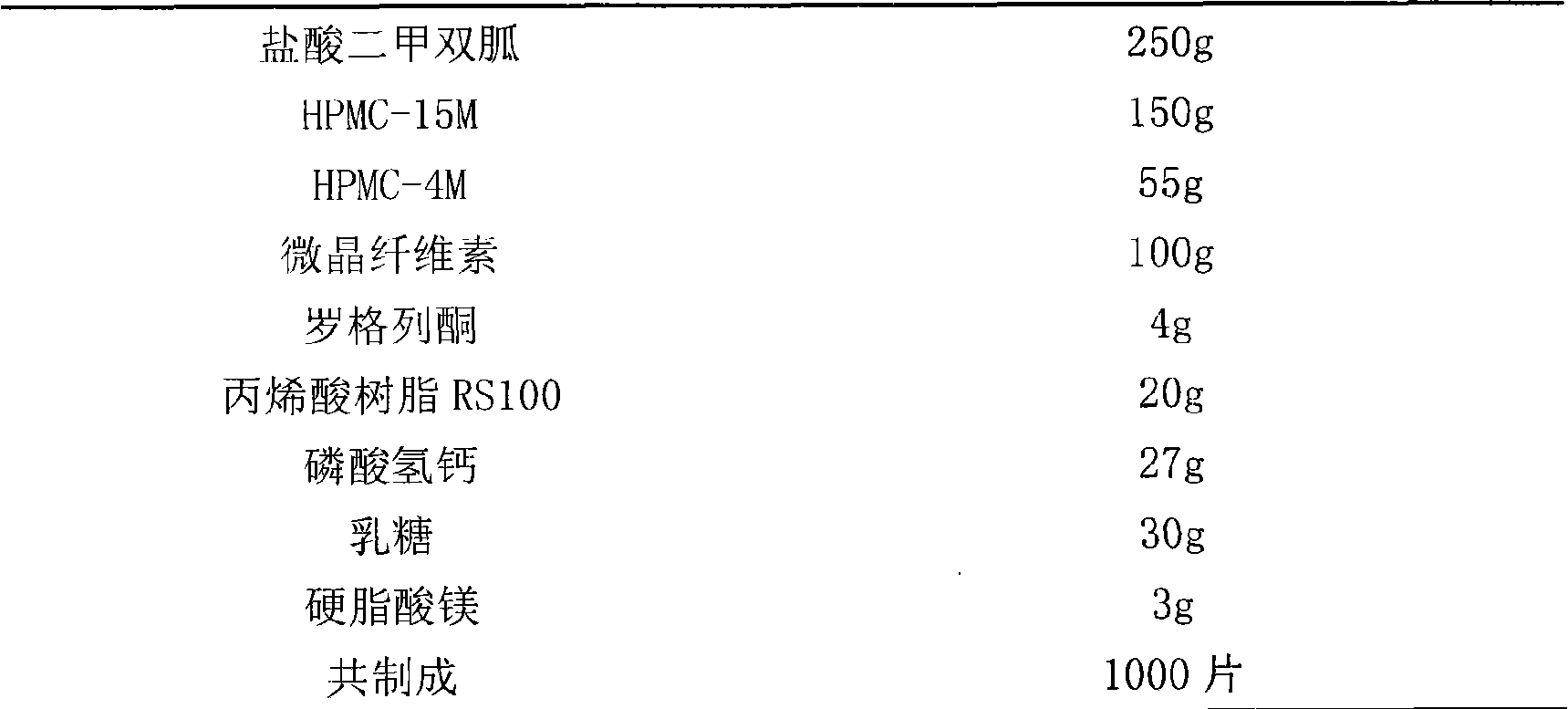
Sustained-release preparation of compound metformin hydrochloride rosiglitazone and preparation method thereof
InactiveCN101683340AImprove complianceReduce the frequency of takingOrganic active ingredientsMetabolism disorderMalaiseMedicine
The invention relates to a sustained-release preparation of compound metformin hydrochloride rosiglitazone and a preparation method thereof; wherein, rosiglitazone and metformin hydrochloride have slow releasing characteristic in an in-vitro dissolution test, the rosiglitazone is released by 10-30 percent for a first hour, and is released by 60-80 percent for a fourth hour, and is released by morethan 80 percent for an eighth hour; the metformin hydrochloride is released by 15-40 percent for a first hour, is released by 50-70 percent for a fourth hour, and is released by more than 75 percentfor an eighth hour. The formula is mild and has prolonged action of glucose reduction, so as to avoid adverse reaction such as hypoglycemia and gastrointestine malaise, reduce taking times (one or twotimes each day), and improve compliance of a patient. The invention discloses the in-vitro drug-releasing characteristic of the sustained-release preparation and the preparation method thereof.
Owner:北京利乐生制药科技有限公司
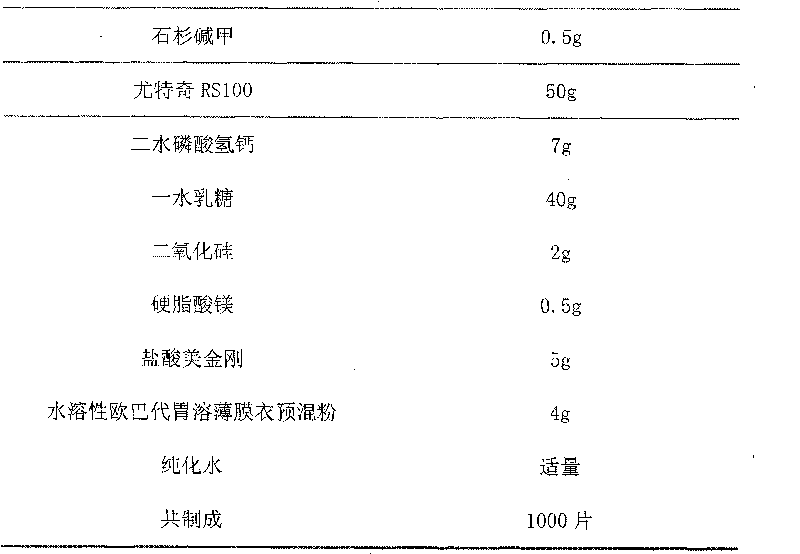
Sustained release preparation containing memantine hydrochloride and huperzine A and preparation method thereof
InactiveCN101756970ANervous disorderAmine active ingredientsMemantine HydrochlorideImmediate release
The invention relates to sustained release preparation containing memantine hydrochloride and huperzine A and preparation method thereof. The sustained release preparation includes quick release component and slow release component, wherein the memantine hydrochloride is the quick release component which represents the characteristic of quick release in vitro dissolution test and can be released by more 80% 30min later, while huperzine A represents the characteristic of slow release in vitro dissolution test and is released by 10-30% in the first hour, by 40-60% in 6 hours, by 60-80% in 12 hours and by more than 80% in 18 hours. The sustained release preparation is mild, has long-term effect and can reduce the application time (only once a day) and improve the compliance of the patient. The invention further discloses the vitro release characteristic and the preparation method of the sustained release preparation.
Owner:北京利乐生制药科技有限公司
Novel gastro-retentive dosage forms
InactiveUS20120009261A1Reduce doseSubstantial patient complianceBiocidePowder deliveryDrugGastro retentive
A gastro-retentive pharmaceutical dosage form of a therapeutically effective amount of at least one GABA Analog, at least one opioid, and at least one pharmaceutically acceptable excipient wherein the said dosage form is retained in the stomach for at least four hours and is suitable for formulating for once daily or twice daily administration. Further provided is a method of treating a disorder by administering to a patient in need thereof, a gastro-retentive pharmaceutical dosage form of a therapeutically effective amount of at least one GABA Analog, at least one opioid, and at least one pharmaceutically acceptable excipient wherein the said dosage form is retained in the stomach for at least four hours and is suitable for once daily or twice daily administration. The opioid is either in slow release form or in immediate release form.
Owner:GRUNENTHAL GMBH
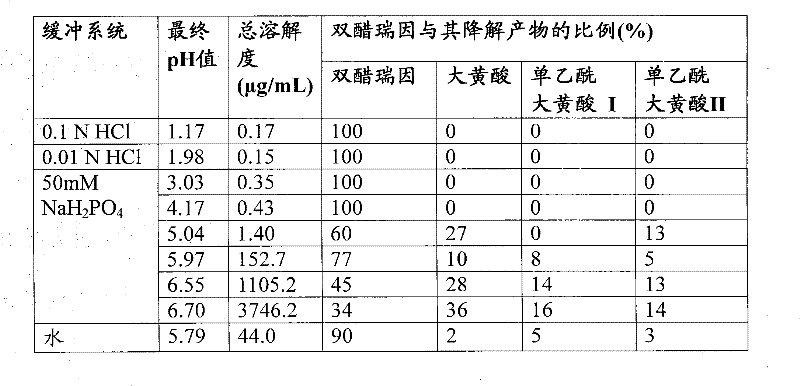
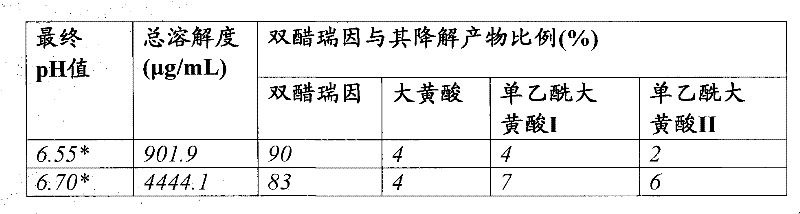
Pharmaceutical compositions containing diacerein
A once-daily controlted-release formulation of diacerein for treating inflammatory or autoimmune diseases or their complications, with reduced adverse side effects and methods of treating such diseases are disclosed.
Owner:TWI BIOTECH
Modified release formulations of at least one form of tramadol
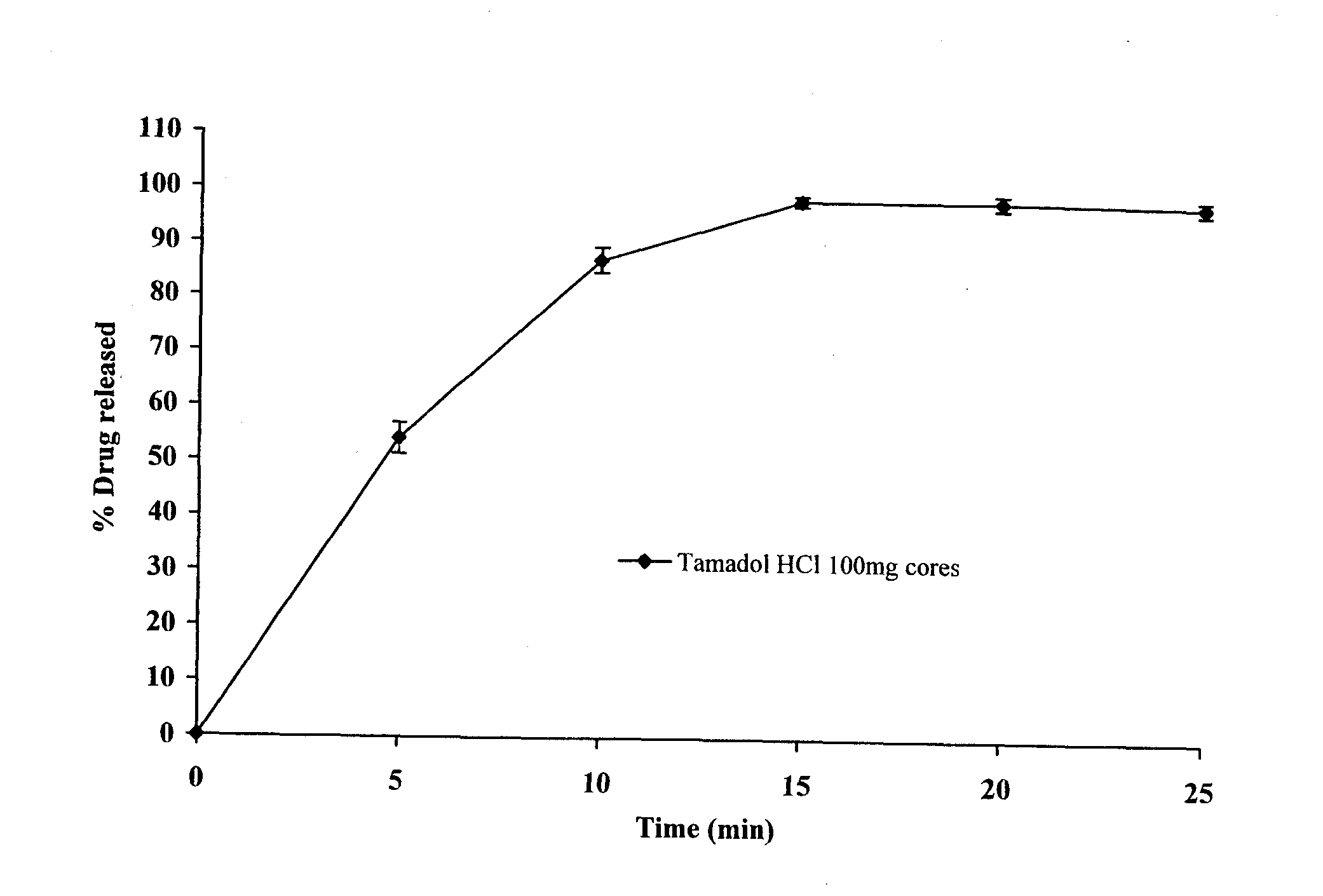
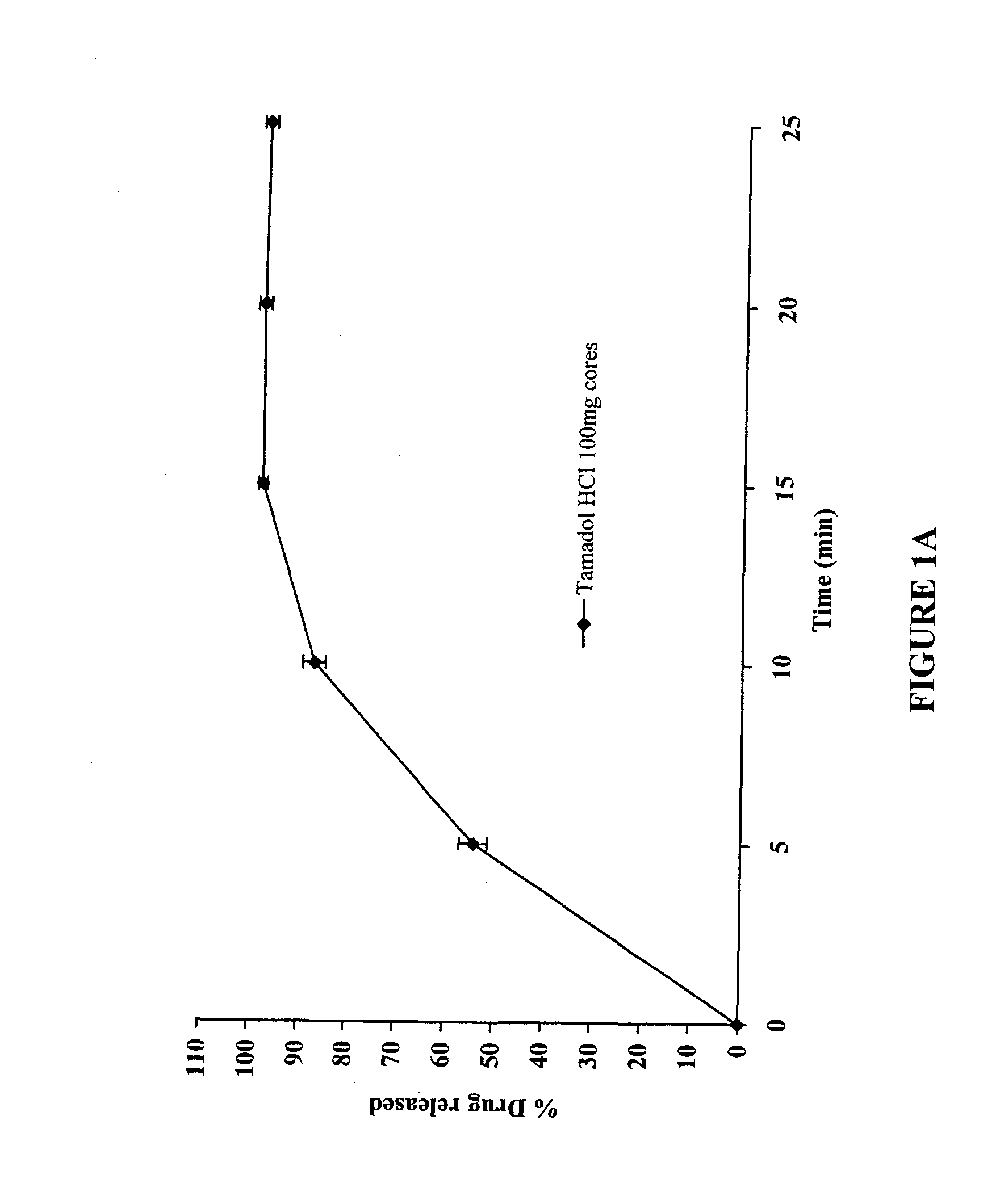
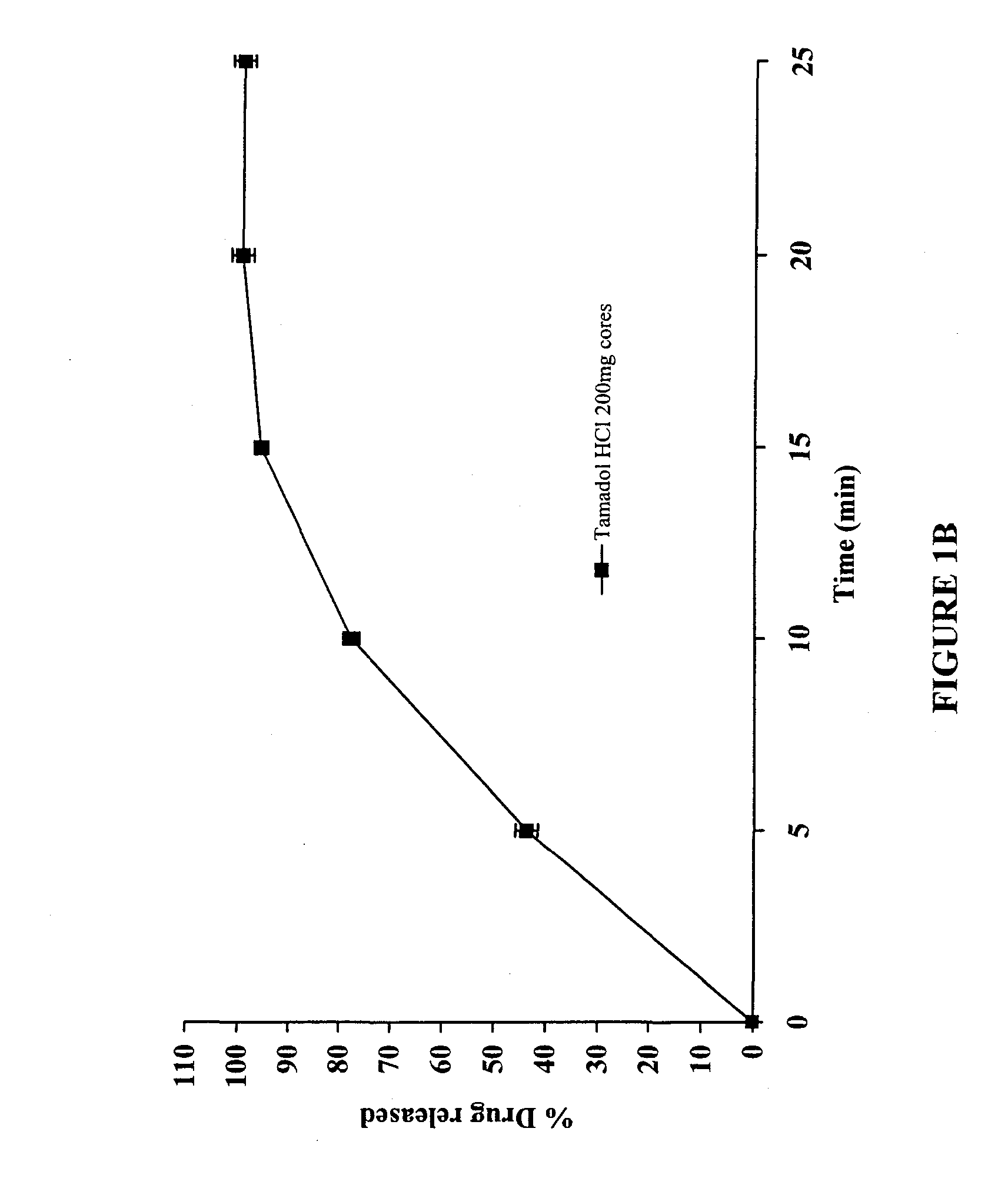
InactiveUS20080069888A1Reduction in adverse eventReduction in adverse eventsOrganic active ingredientsBiocideOral medicationBlood plasma
The present invention relates to a modified release composition of at least one form of tramadol which is a delayed and extended release composition for oral administration suitable for once daily dosing. That composition comprises a core comprising at least one form of tramadol selected from the group consisting of tramadol, racemic mixtures thereof, enantiomers thereof, pharmaceutically acceptable salts thereof, and combinations thereof in combination with a pharmaceutically acceptable excipient. That composition further comprises a modified release coating which substantially surrounds said core. The compositions of the invention provide delayed and extended release of said at least one form of tramadol such that the mean plasma concentration of the at least one form of tramadol reaches a therapeutically effective level at a time which is after at least about 3 hours after first administration.
Owner:VALEANT INT BERMUDA
Controlled Release Pharmaceutical Composition and a Process for Preparing the Same
InactiveUS20070231385A1Reduce frequencyReduce pill loadBiocideAntiviralsNucleoside Reverse Transcriptase InhibitorControlled release
A three-drug antiretrovial pharmaceutical composition having a selective combination of a controlled release active formulation and an immediate release active formulation for once daily administration. The composition provides desired dosages of the actives lamivudine, zidovudine or pharmaceutically acceptable derivatives thereof, and the immediate release formulation including at least one selective Non-nucleoside Reverse Transcriptase Inhibitor (NNRTI), preferably nevirapine or a pharmaceutically acceptable derivative thereof along with pharmaceutically acceptable excipients. The once daily composition would favour patient compliance and effective treatment. A method of reducing the pill burden in a patient suffering from HIV infection and / or Acquired Immunodeffieciency Syndrome by administering a once daily dose of the three-drug antiretroviral pharmaceutical composition.
Owner:LUPIN LTD
Extended release pharmaceutical composition comprising linezolid and process for preparing the same
The present invention provides an extended release pharmaceutical composition suitable for once daily dosing comprising Linezolid or pharmaceutically acceptable salt, derivative, prodrug, metabolite and polymorph thereof and one or more pharmaceutically acceptable excipients and a process of preparing the same. The present invention further provides a method of treating bacterial infections in a mammal comprising administering an extended release, pharmaceutical composition suitable for once daily dosing comprising Linezolid capable of maintaining T>MIC for at least 24 hours. The present invention further provides an extended release pharmaceutical composition suitable for once daily dosing comprising Linezolid so that upon oral administration the maximum concentrations (Cmax) of Linezolid in plasma are statistically significantly lower than the immediate release formulation given twice daily, and area under the plasma concentration-time curve (AUC) and the minimum plasma concentrations are maintained over 24 hours.
Owner:MICRO LABS
Dosage forms and methods using ethacrynic acid
The invention is directed to a method of treating systemic arterial hypertension which preferably uses enter administration of a once-daily dose of ethacrynic acid of less than 50 mg / day to a patient afflicted with arterial hypertension. Doses of less than 25 mg / day ethacrynic acid are also indicated for treating hypertension. The invention also includes novel dosage forms comprising from 2.0 to 20 mg of ethacrynic acid.
Owner:ACCU BREAK TECH
Therapeutic applications for C-peptide
InactiveUS7964558B2Effective treatmentSenses disorderPeptide/protein ingredientsDiabetic complicationC-peptide
Owner:CREATIVE PEPTIDES SWEDEN
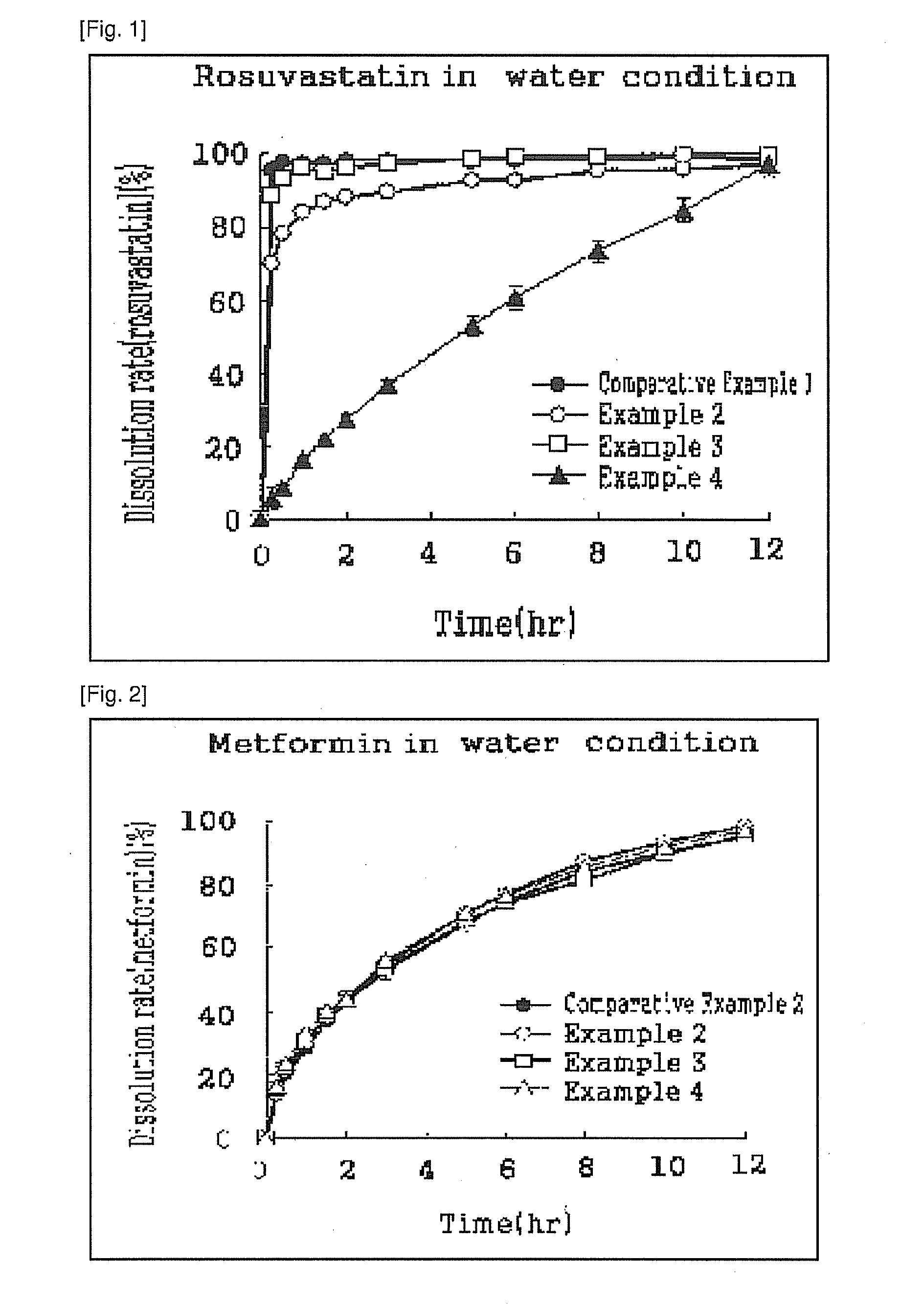
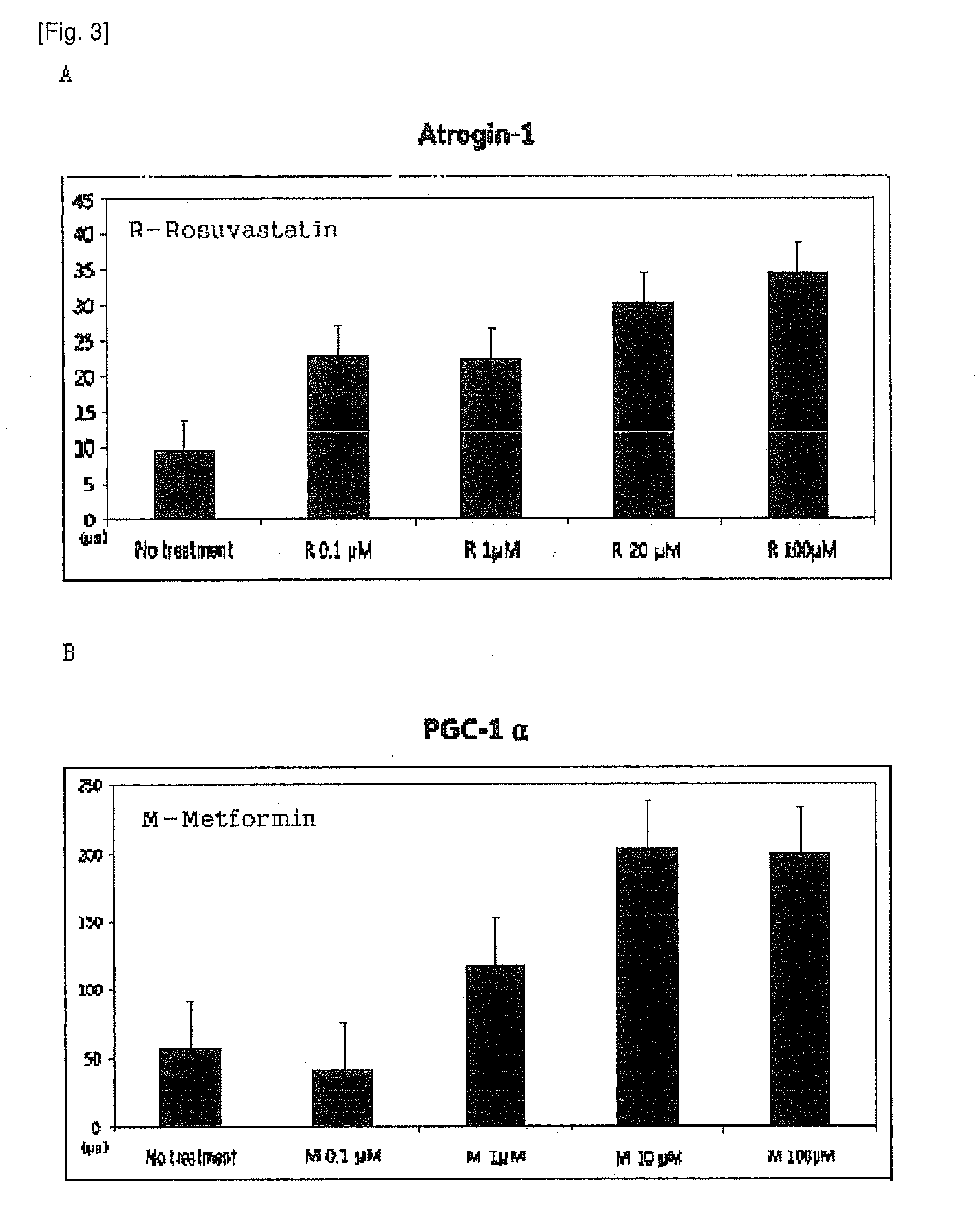
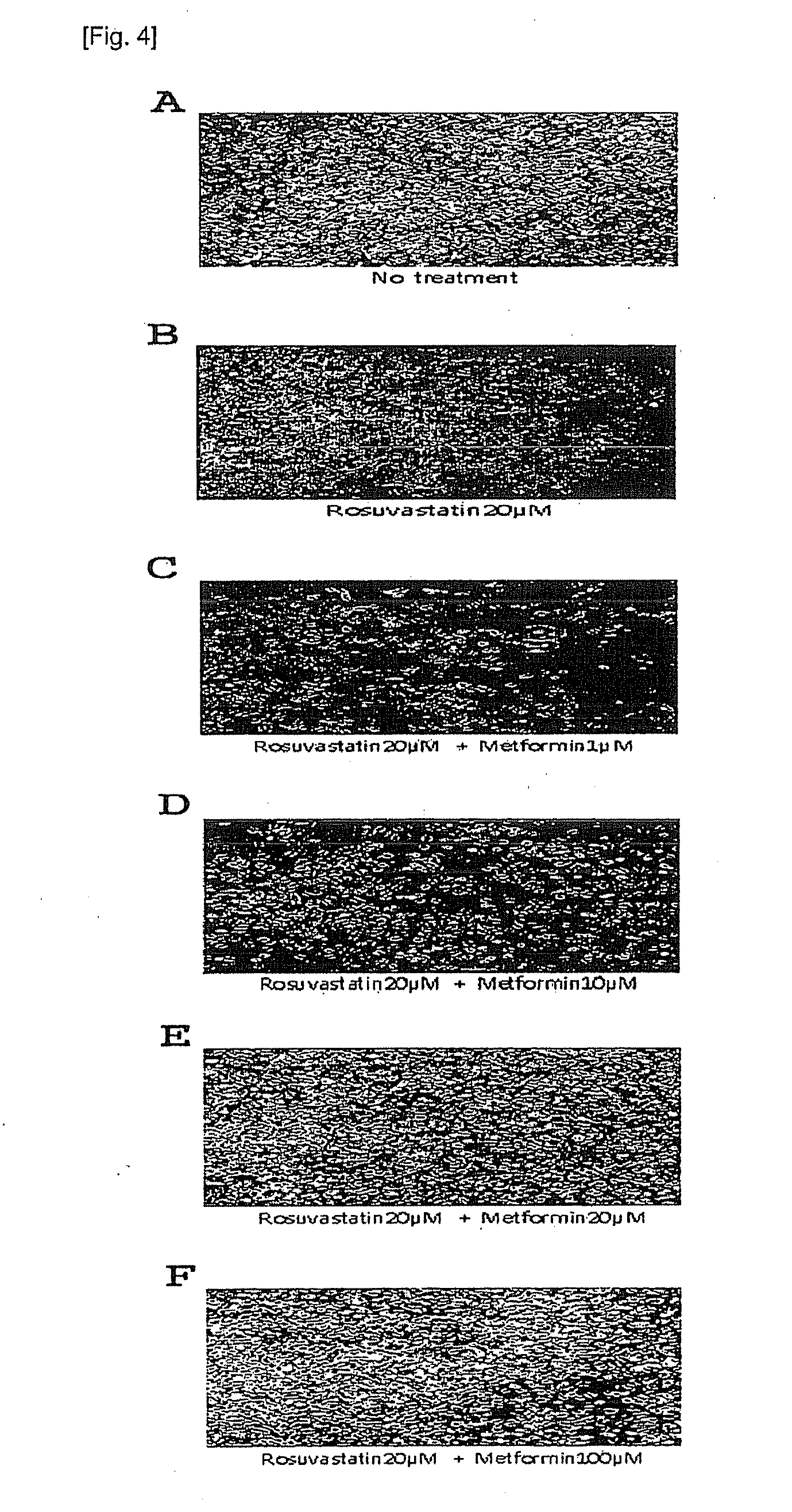
Pharmaceutical composition comprising metformin and rosuvastatin
ActiveUS20130035316A1Eliminate side effectsImprove securityBiocideSalicyclic acid active ingredientsControlled releaseSide effect
This invention relates to the oral pharmaceutical composition of metformin and rosuvastatin. In detail, this invention comprising metformin, rosuvastatin, sustained release carriers and / or excipients reduces the side effects caused by statins and enhances safety, patients' convenience and compliance with its one-per-day dosage. In addition, regulation of an early effective blood concentration of the drug and maintenance of the drug's concentration at a steady level in vivo by a controlled-release can be advantageously used as a pharmaceutical composition for preventing and treating hyperlipidemia.
Owner:BCWORLD PHARMA
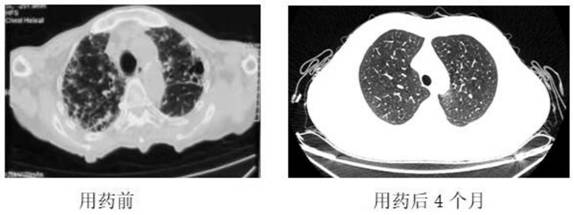
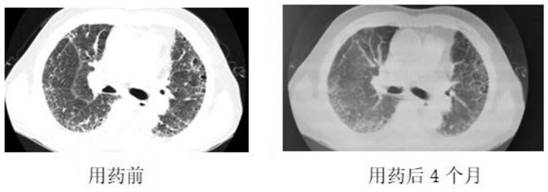
Pharmaceutical composition for idiopathic pulmonary fibrosis and application thereof
ActiveCN112823795AReduced interstitial fibrosisImprove the quality of lifeOrganic active ingredientsRespiratory disorderOral medicineFibrosis
The invention discloses a pharmaceutical composition for idiopathic pulmonary fibrosis and application thereof. The pharmaceutical composition for the idiopathic pulmonary fibrosis comprises pirfenidone and dextromethorphan. The content of the pirfenidone is 200 to 600 mg, and the content of the dextromethorphan is 7.5 mg. The pharmaceutical composition for resisting the idiopathic pulmonary fibrosis is an oral medicine. An administration mode of the pharmaceutical composition for resisting the idiopathic pulmonary fibrosis is that the single dose of the pirfenidone is 200-600 mg, and the pirfenidone is taken for three times a day; and the single dose of the dextromethorphan is 7.5 mg, and the dextromethorphan is taken once a day. According to the administration dosage of the pirfenidone, the single dose of the pirfenidone is increased by 200-400 mg per week. The dosage of the pirfenidone is increased to 400-600 mg each time within two weeks. According to the invention, acute exacerbation of pulmonary fibrosis can be slowed down, pulmonary interstitial fibrosis is relieved compared with the previous condition, and the life quality is improved.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
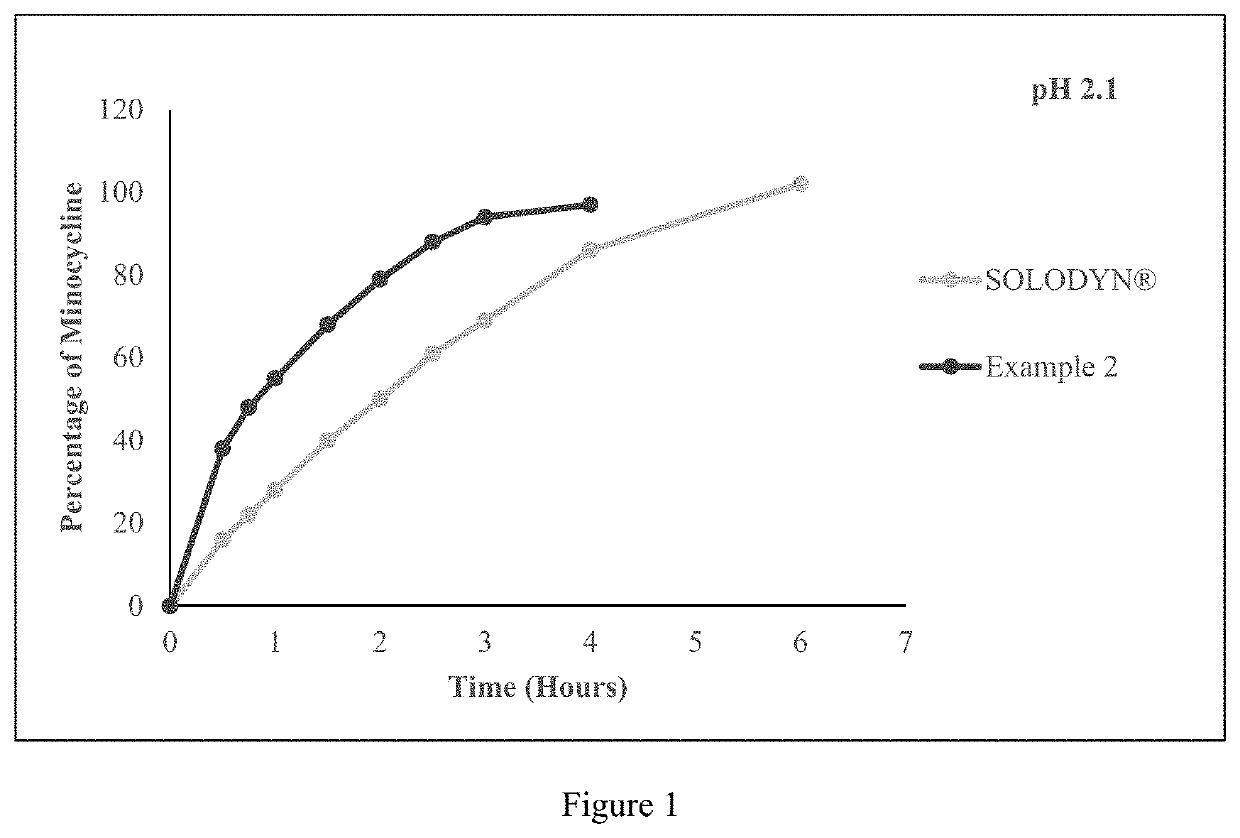
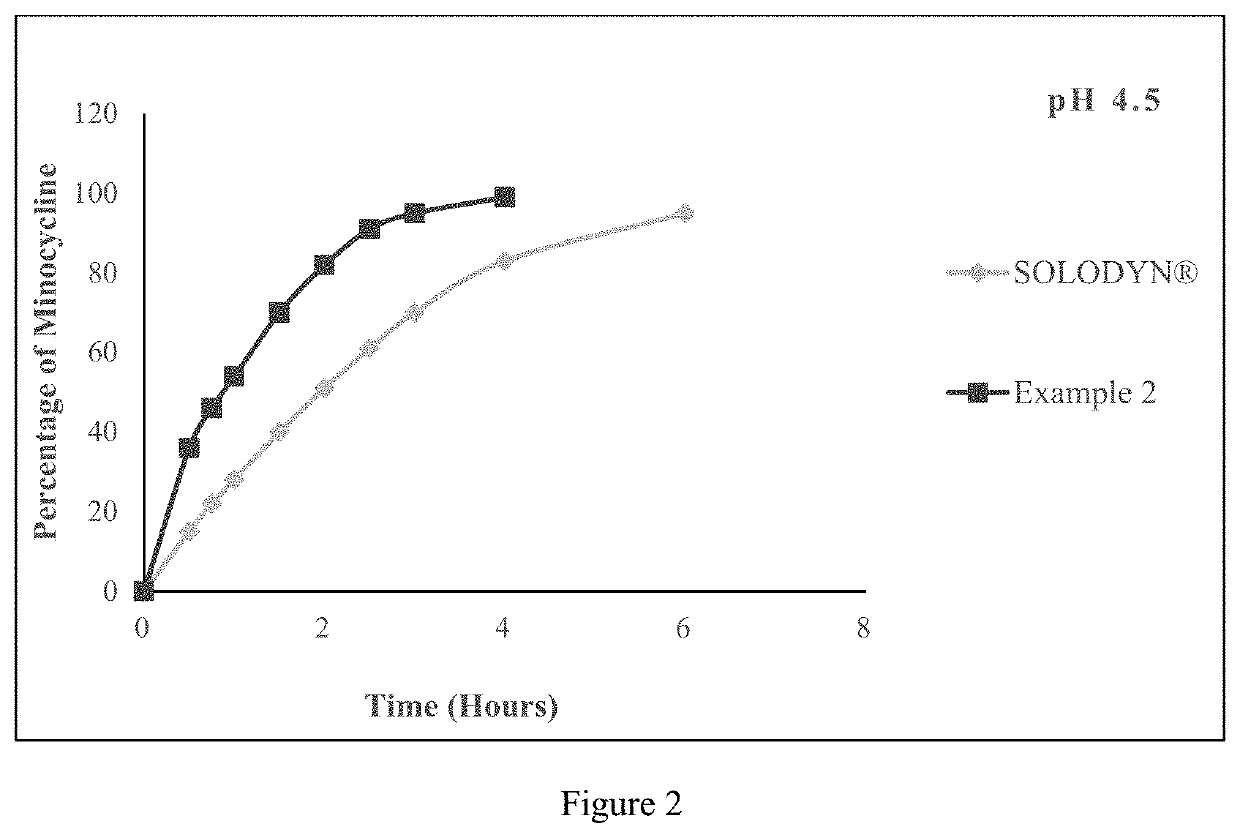
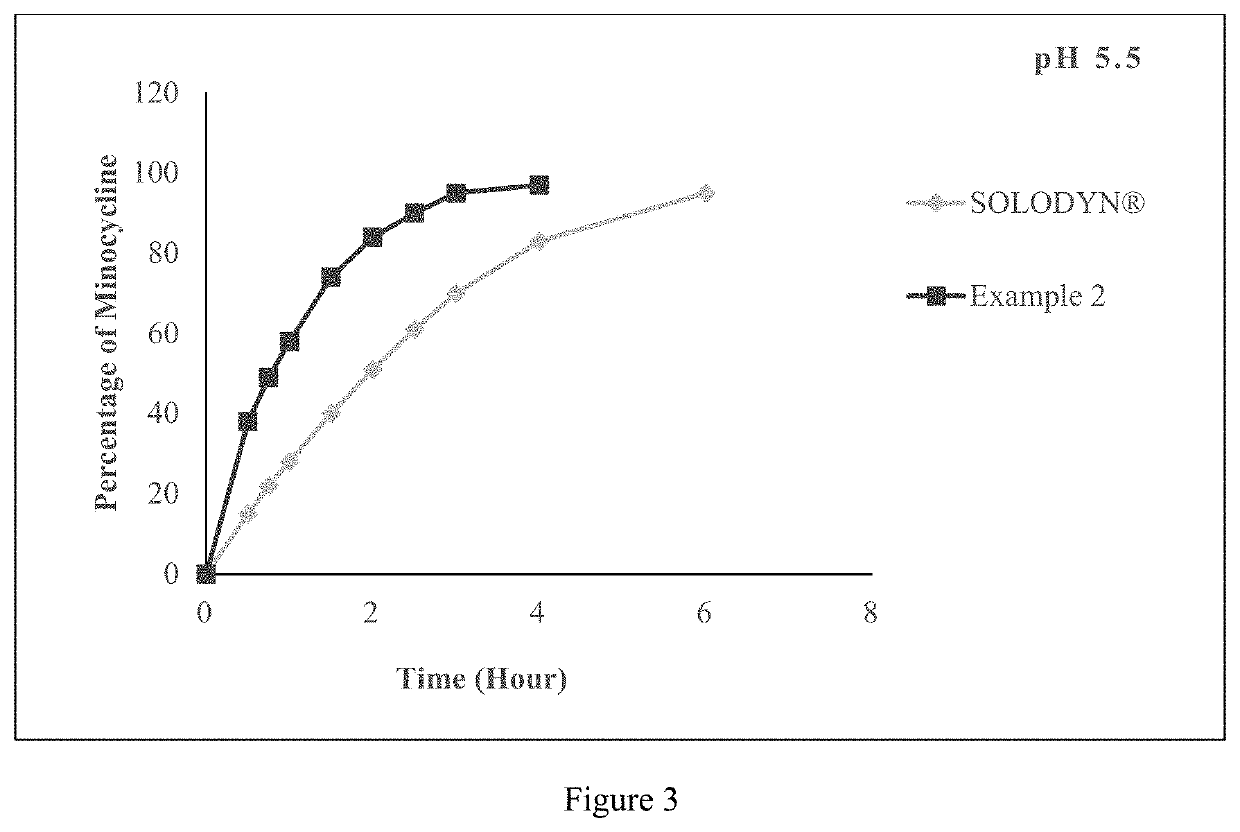
Pharmaceutical compositions for minocycline
PendingUS20210353649A1Reduces SKUsEasy to stockAntibacterial agentsTetracycline active ingredientsLactosePharmaceutical Substances
The present application relates to a method of orally administering once daily tablet of minocycline to a subject in need thereof, wherein said tablet is substantially free of lactose. The present application also relates to processes for preparing said once daily tablet of minocycline that provides reduced stock keeping units with improved inventory by supplying multiple doses of minocycline in single tablet.
Owner:DR REDDYS LAB LTD
Pregabalin-containing, high swellable, sustained-release triple layer tablet
ActiveUS11007153B2Good expansion performanceFast deliveryOrganic active ingredientsNervous disorderPregabalinMedicinal chemistry
Disclosed a pregabalin-containing, high swellable, oral sustained-release triple layer tablet suitable for administration once daily.
Owner:GL PHARMTECH CO LTD
Extended release pharmaceutical compositions of riociguat
Provided herein are the extended release pharmaceutical composition suitable for once or twice daily dosing comprising riociguat and at least one or more pharmaceutically acceptable excipients. The present invention also relates to method for preparing extended release composition and method of using these dosage forms for the treatment of pulmonary hypertension and related diseases. The present invention provides extended release composition of riociguat which are expected to exhibit desired technical attributes such as assay, stability and release profile suitable for once or twice daily administration.
Owner:JUBILANT PHARM HLDG INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com