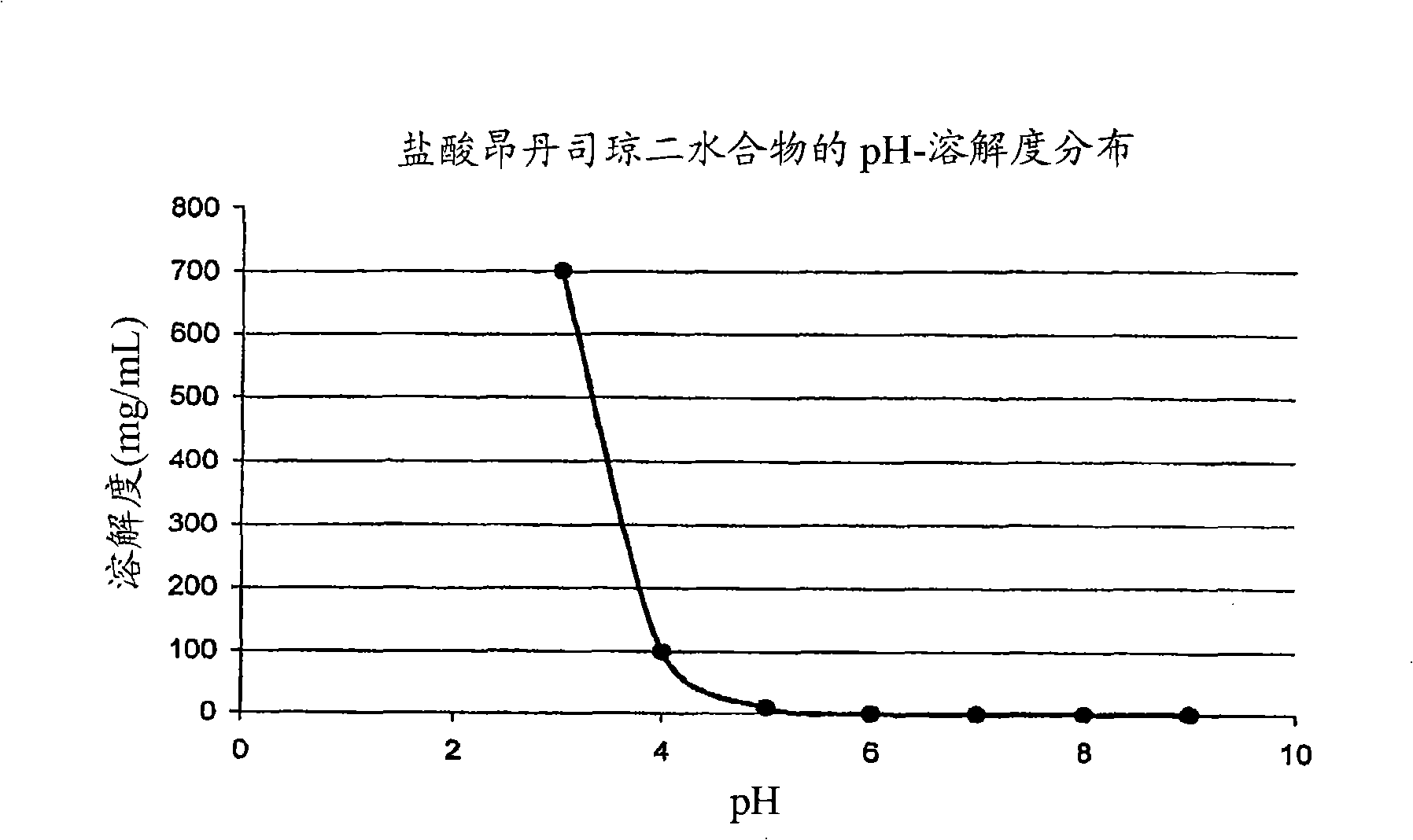
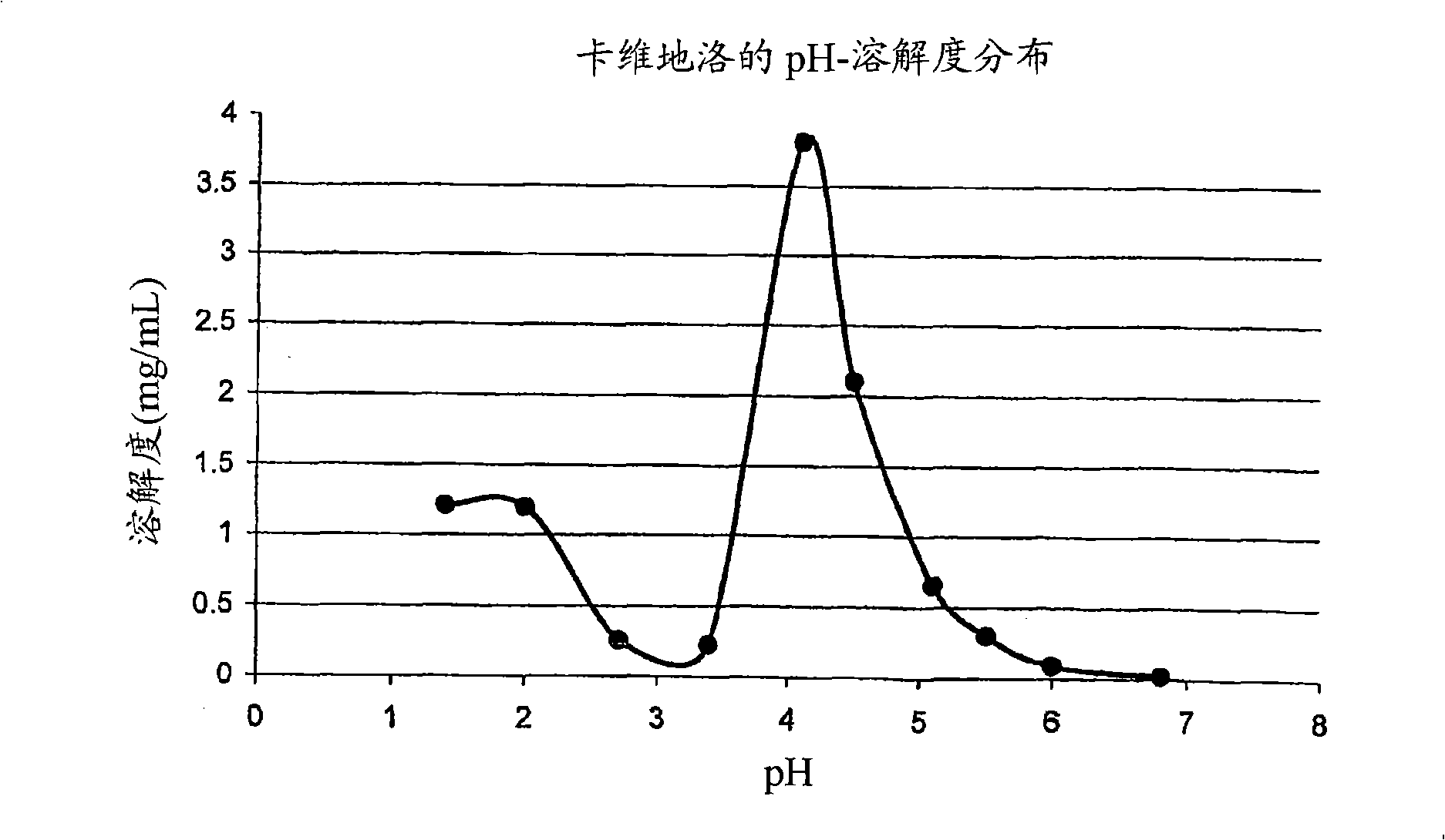
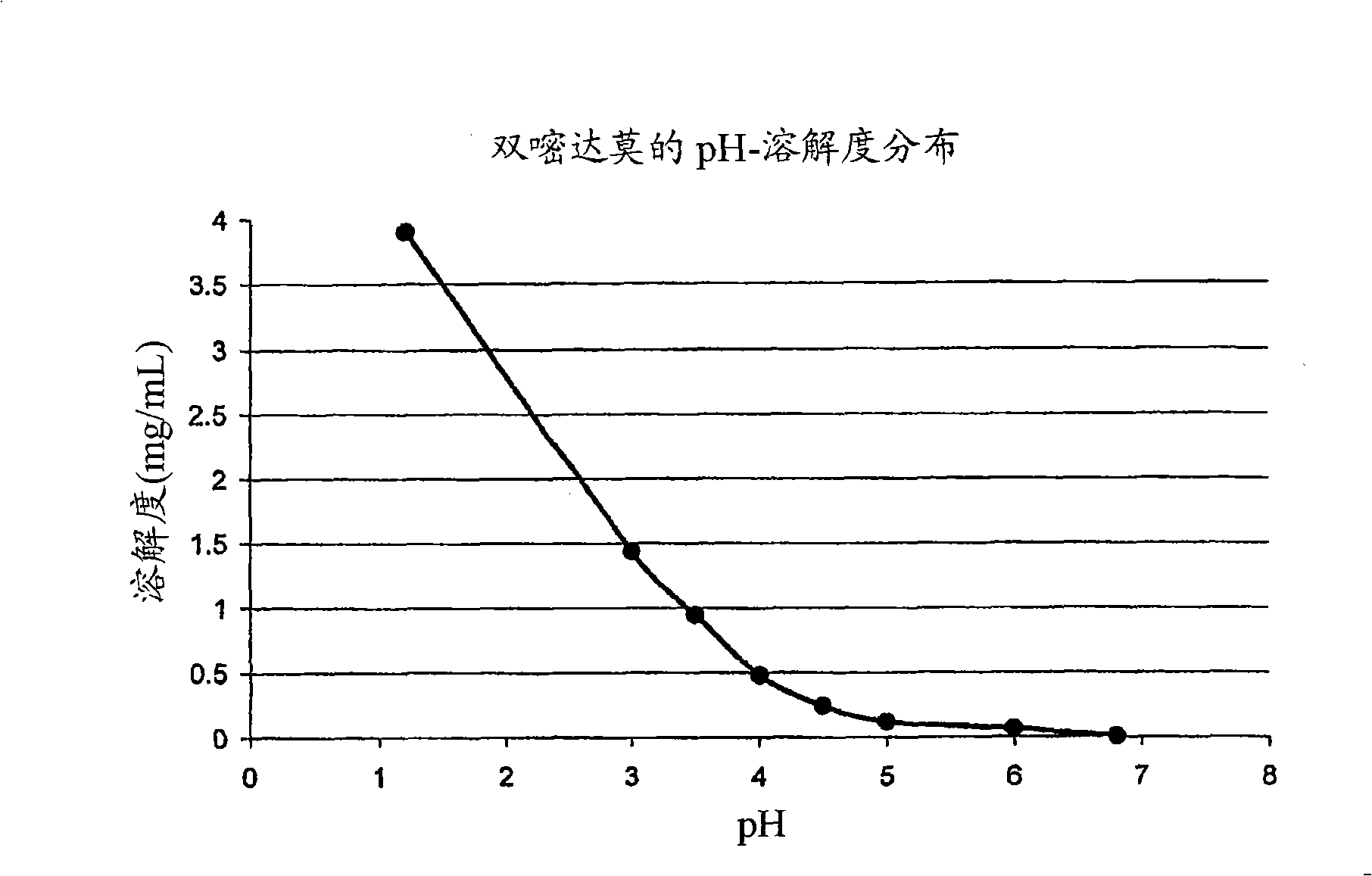
Drug delivery systems comprising weakly basic drugs and organic acids
A weakly alkaline, organic acid technology, applied in drug delivery, drug combination, digestive system, etc., can solve problems such as the inability to maintain plasma distribution and dosing regimens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0079] According to one embodiment of the present invention, the method may include the following steps:
[0080] a. Provide organic acid containing core particles (e.g. organic acid crystals with desired size distribution or particles comprising inert particles (e.g. sugar spheres, cellulose spheres, silica spheres) layered with polymer organic acids in the binder solution);
[0081] b. Core particles containing organic acids are coated with an SR coating film consisting of a single water-insoluble polymer (e.g. EC-10 (ethylcellulose with an average viscosity of 10 cps)) or a combination of a water insoluble polymer with a water soluble polymer such as povidone or PEG 400 or an enteric polymer such as hydroxypropylmethylcellulose phthalate (eg HP-55);
[0082] c. Coating a weakly basic drug layer on the SR-coated organic acid-containing core particles to form IR beads;
[0083] d. coating the barrier coating film on the IR beads with a solution of the water insoluble polyme...
Embodiment 1
[0110] A. Fumaric Acid SR Beads
[0111] 40-80 mesh fumaric acid crystals (3750 g) were loaded into a fluid bed coater Glatt GPCG 5 equipped with a 9" bottom spray Wurster insert, column length 10" and tubing 16 mm . 250g of ethyl cellulose (Ethocel Premium, 10cps, hereinafter referred to as EC-10) and 166.7g of polyethylene glycol (PEG 400) (the ratio of the two is 60 / 40) are dissolved in 98 / 2 acetone / water ( 6528.3 g), the resulting solution (6% solids concentration) was used to coat the above-mentioned acid crystals with a weight gain of up to 10% by weight. The treatment conditions are as follows: atomization air pressure is 2.0 bar; nozzle diameter is 1.00mm; bottom distribution plate is B; spray / shake interval is 30s / 3s; product temperature is maintained at 35±1°C; (inlet air volume) was 145-175 cubic feet per minute (cfm); and the spray rate was increased from about 8 g / min to 30 g / min.
[0112] In addition, fumaric acid crystals were coated with different ratios of ...
Embodiment 2
[0116] A. Cores containing fumaric acid
[0117] Hydroxypropyl cellulose (Klucel LF, 33.3 g) was slowly added to 90 / 10 denatured alcohol SD 3C 190 Proof (Denatured SD 3C 190 Proof Alcohol) (Denatured SD 3C 190 Proof Alcohol) (Water) at a solids concentration of 4% while stirring vigorously to dissolve, then slowly added Fumaric acid (300g) was dissolved. A Glatt GPCG 3 equipped with a 6" bottom spray Wurster insert and an 8" distribution column was packed with 866.7 g of 25-30 mesh sugar spheres. The sugar spheres were layered with the fumaric acid solution while maintaining a product temperature of about 33-34°C and maintaining an air inlet velocity of about 3.5-4.5 m / s. Dry the acid cores in the unit for 10 minutes to remove residual solvent / moisture, then pass through a 20-30 mesh screen.
[0118] B. SR coated fumaric acid core
[0119] Acid cores (1080 g) from above were prepared with 95 / 5 acetone / water solution (solid Concentration is 7.5%) carry out coating, weight g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com