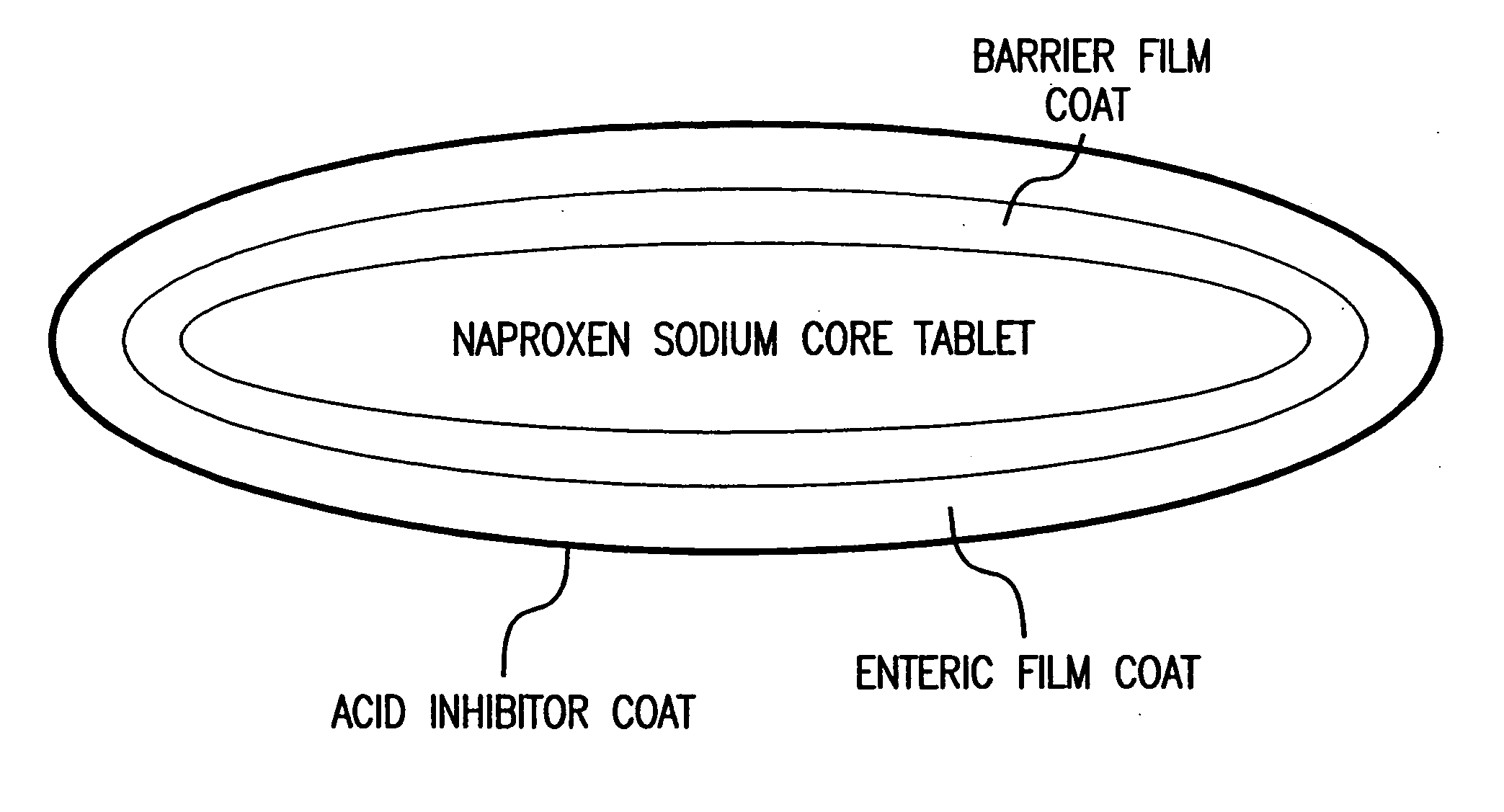
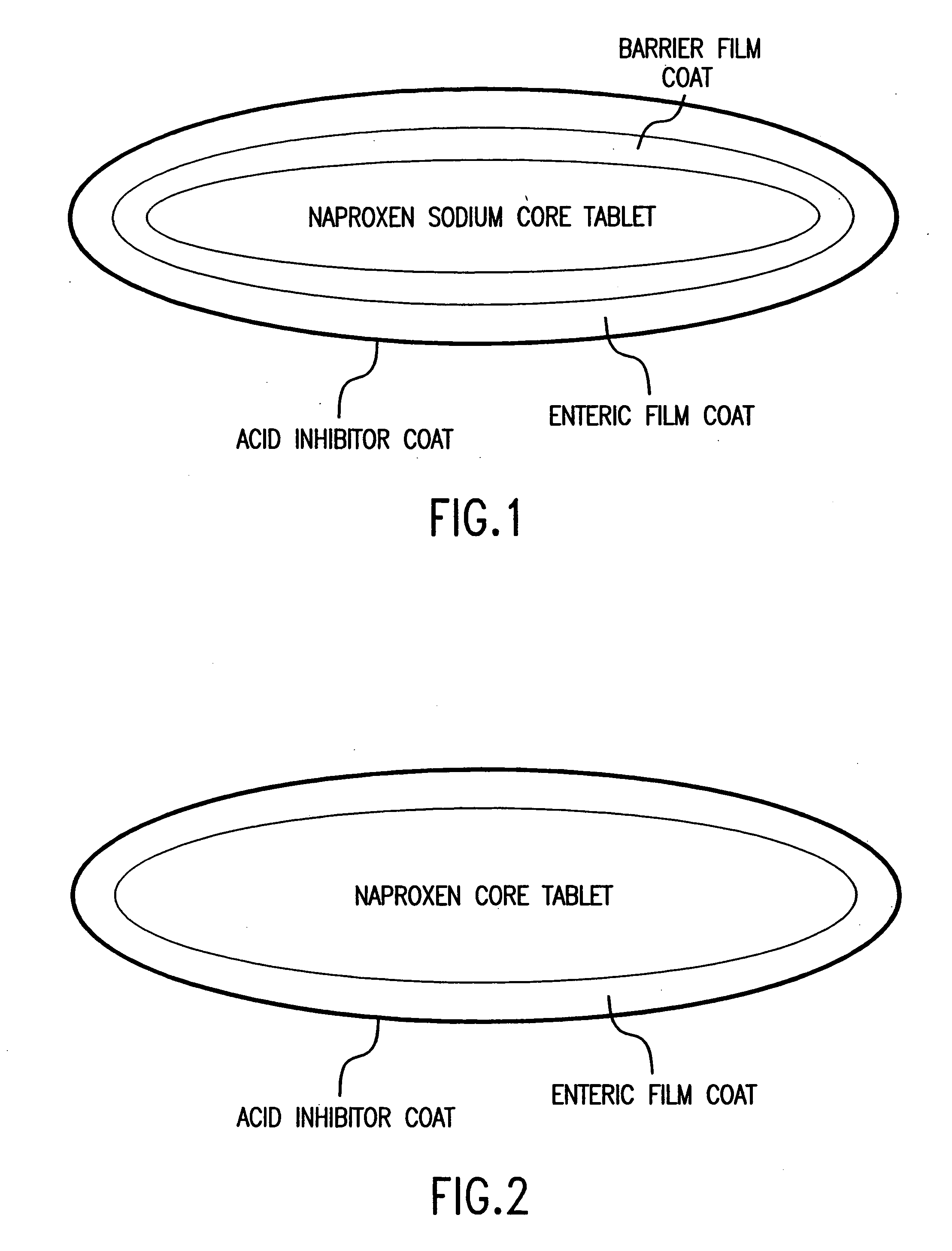
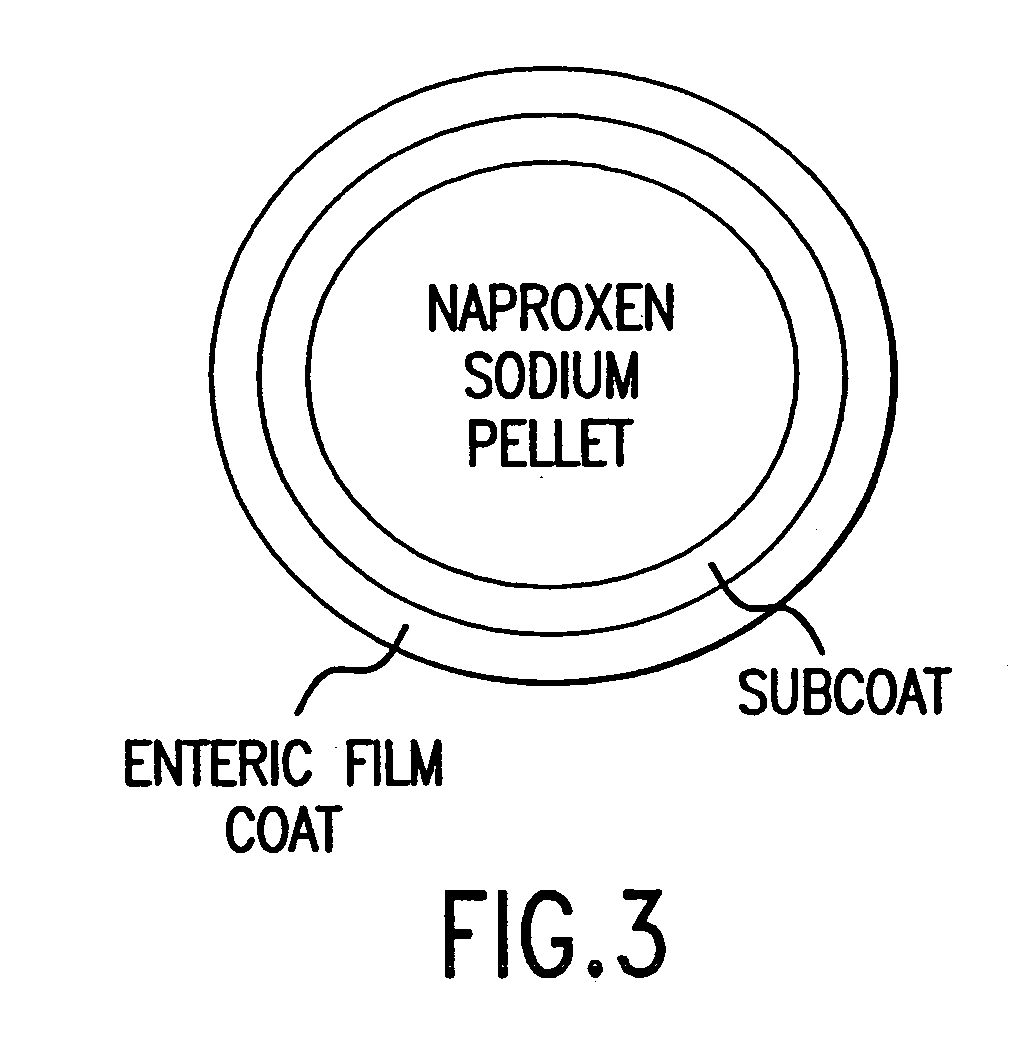
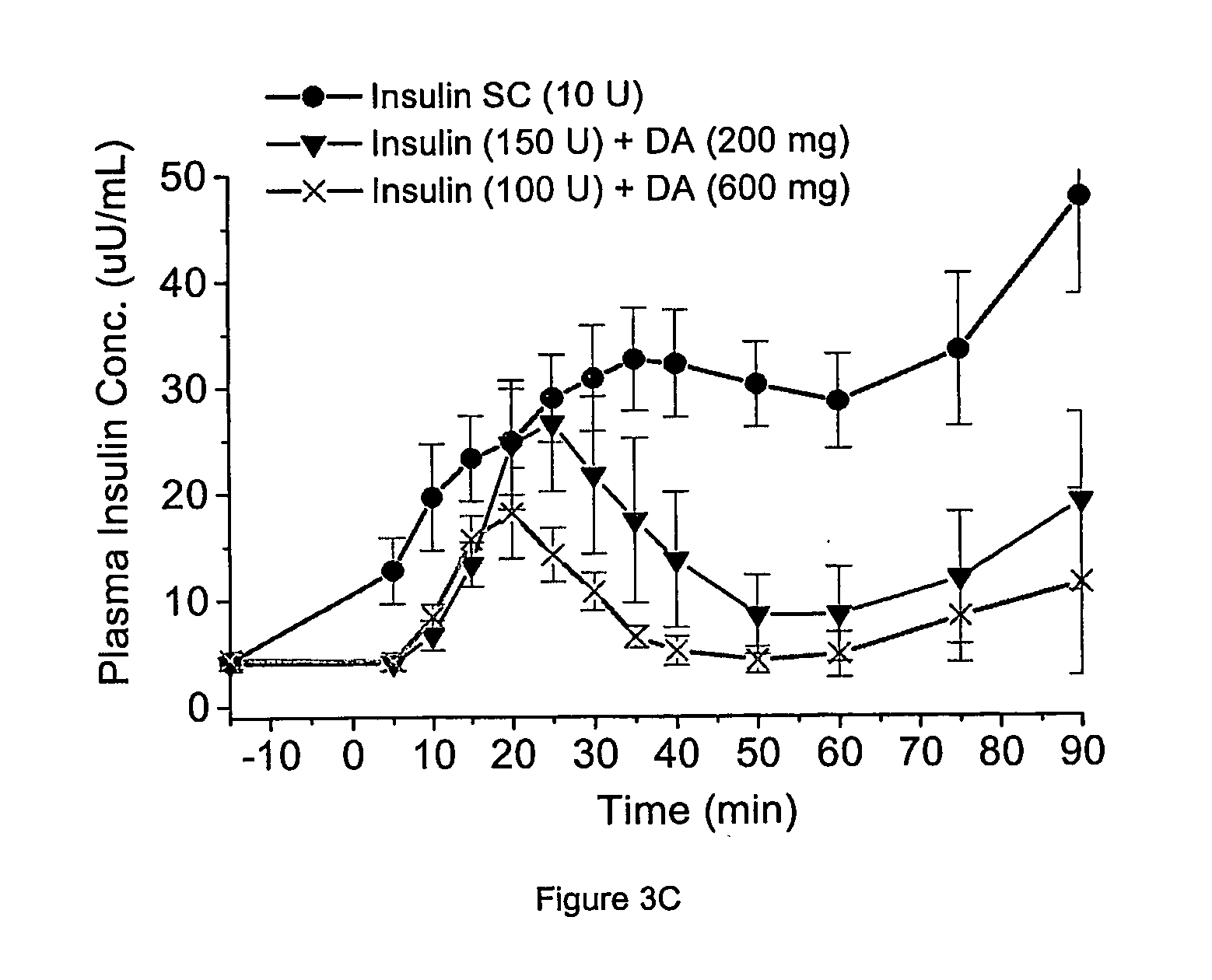
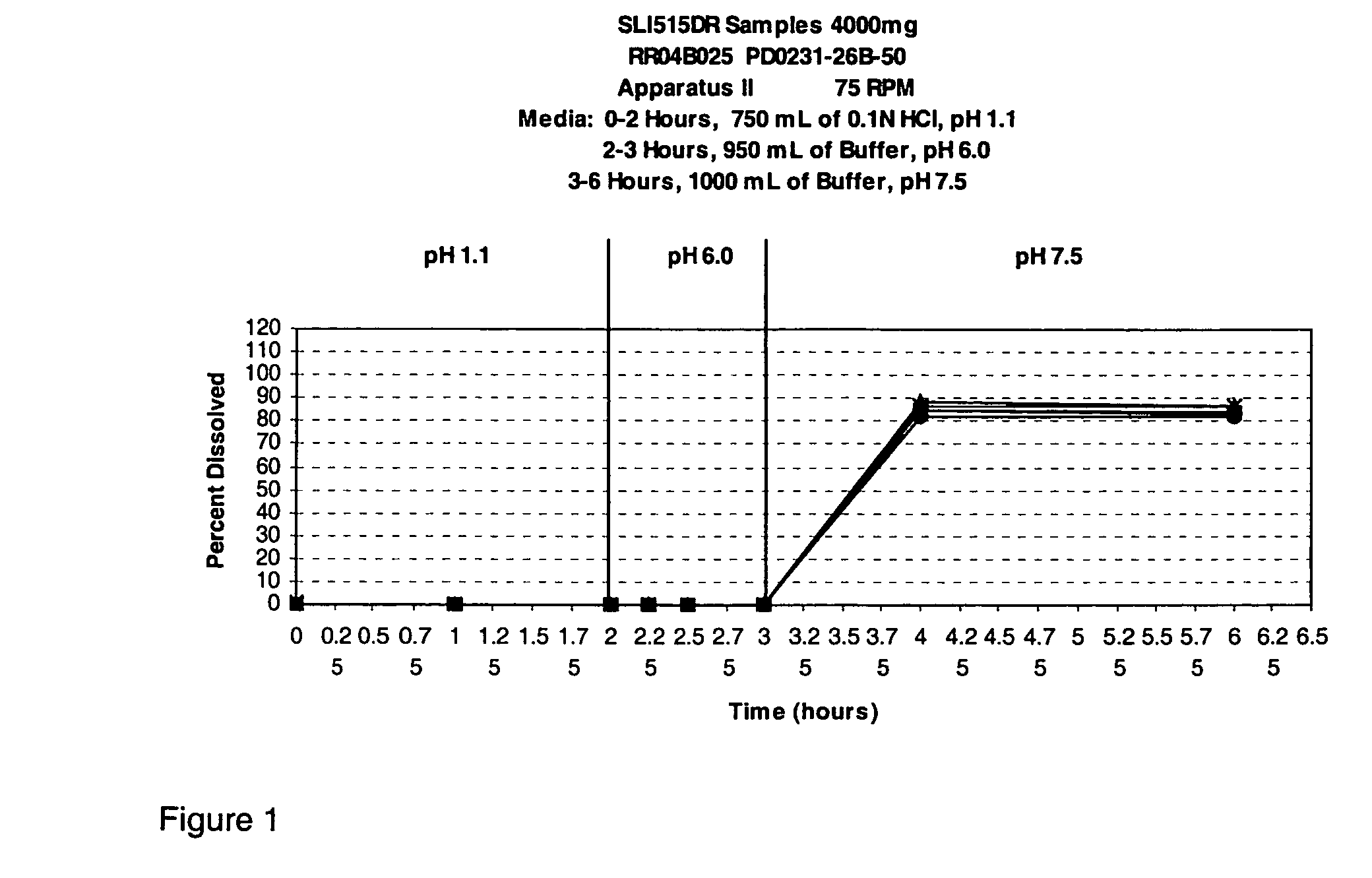
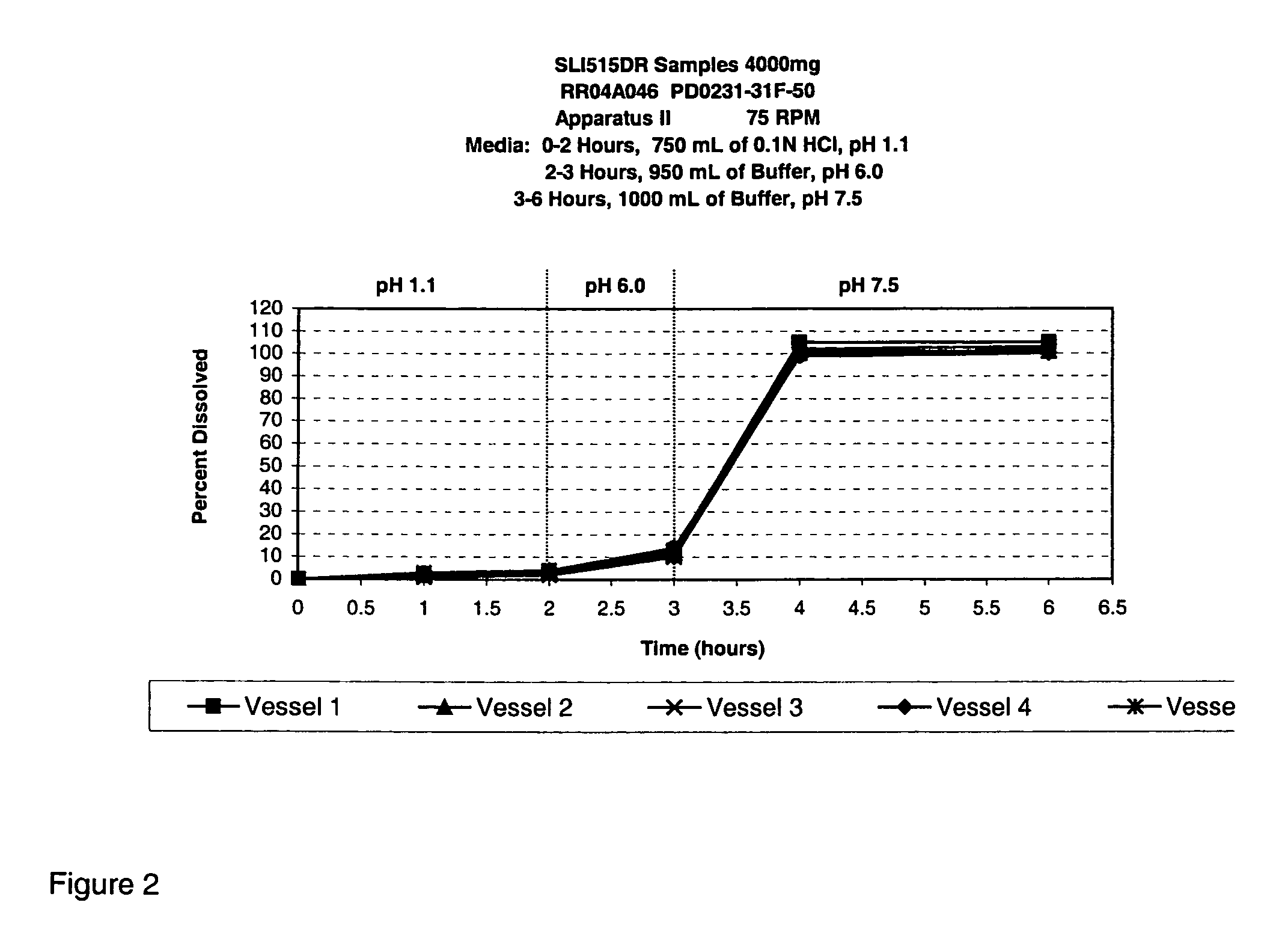
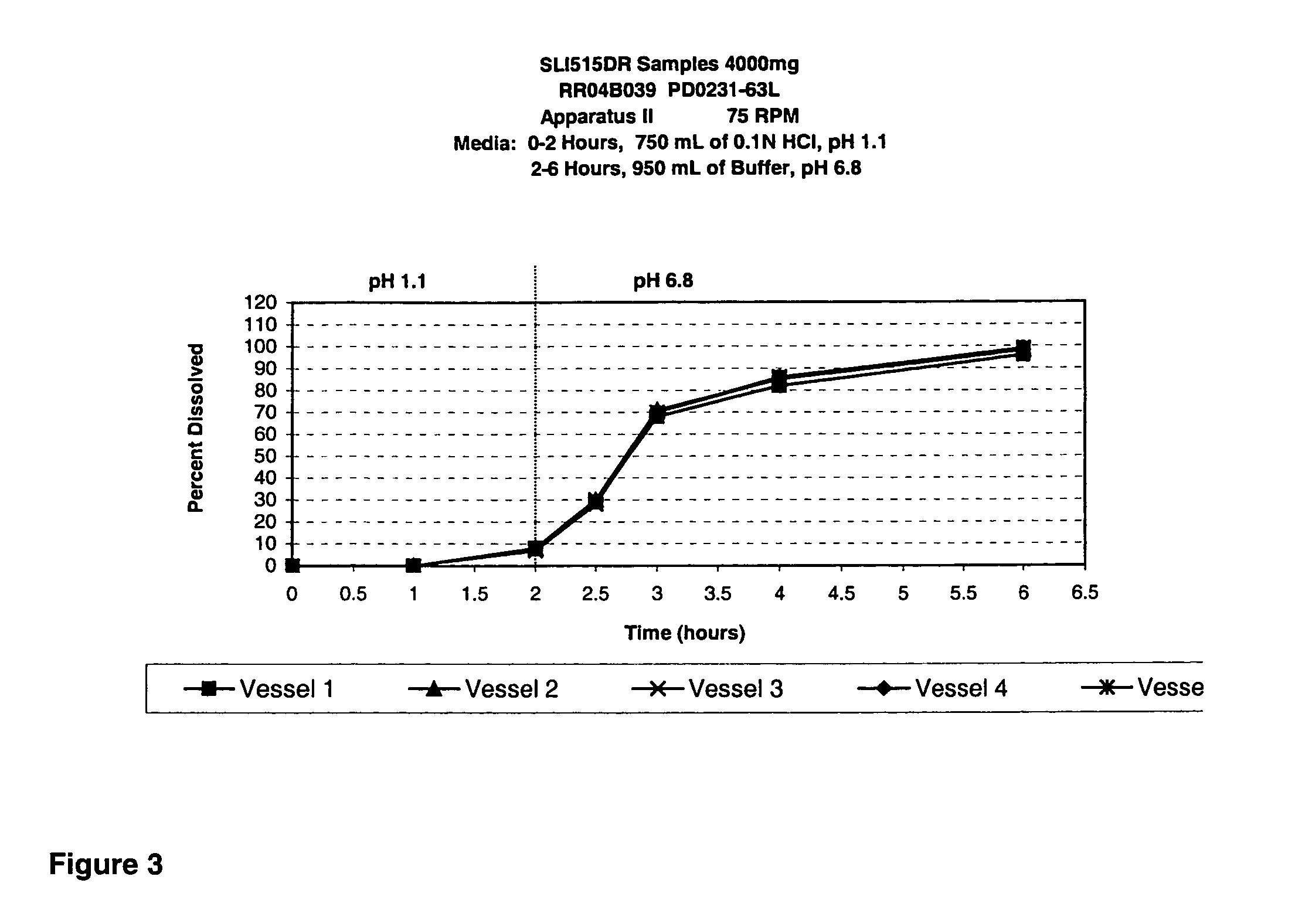
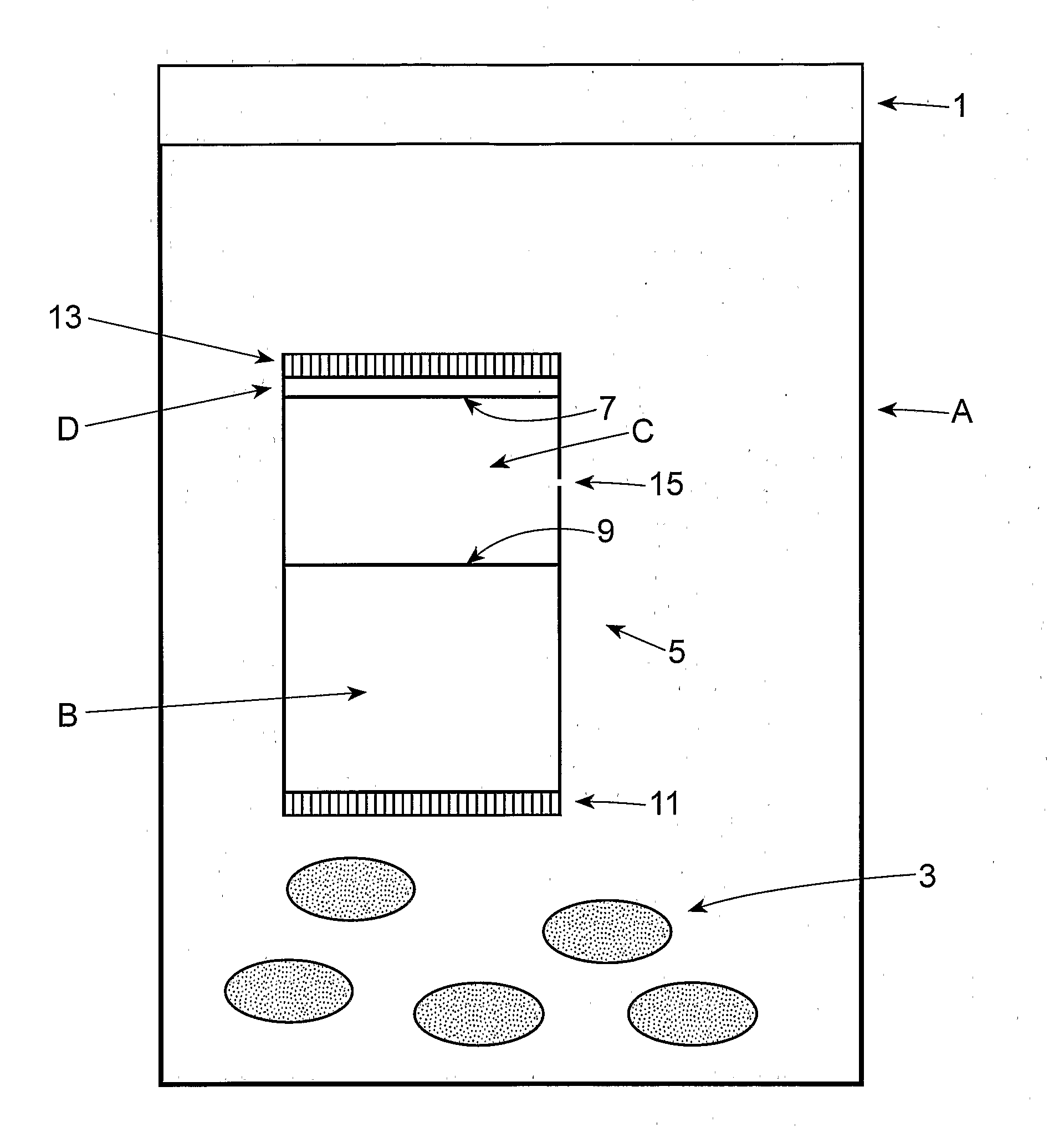
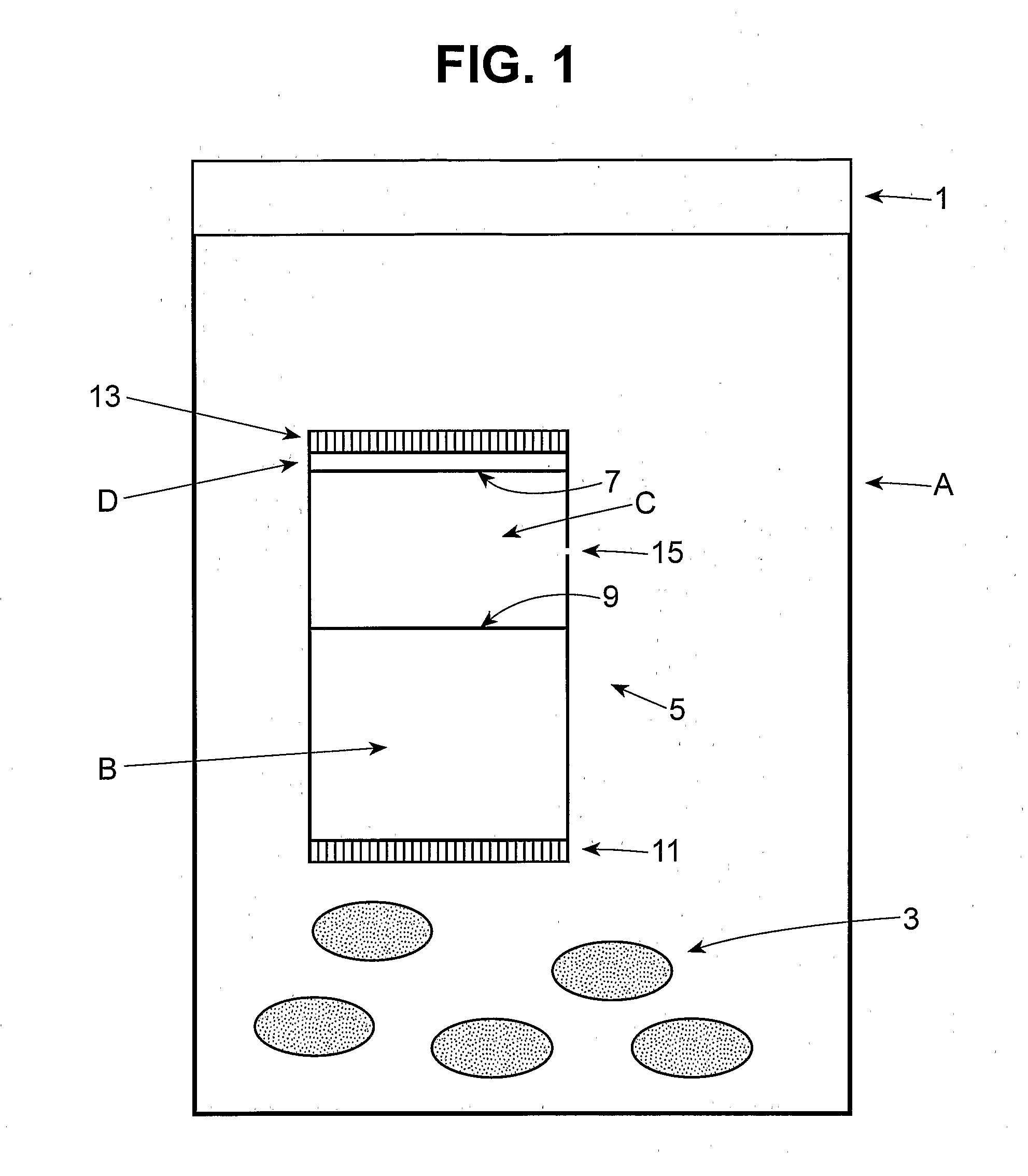
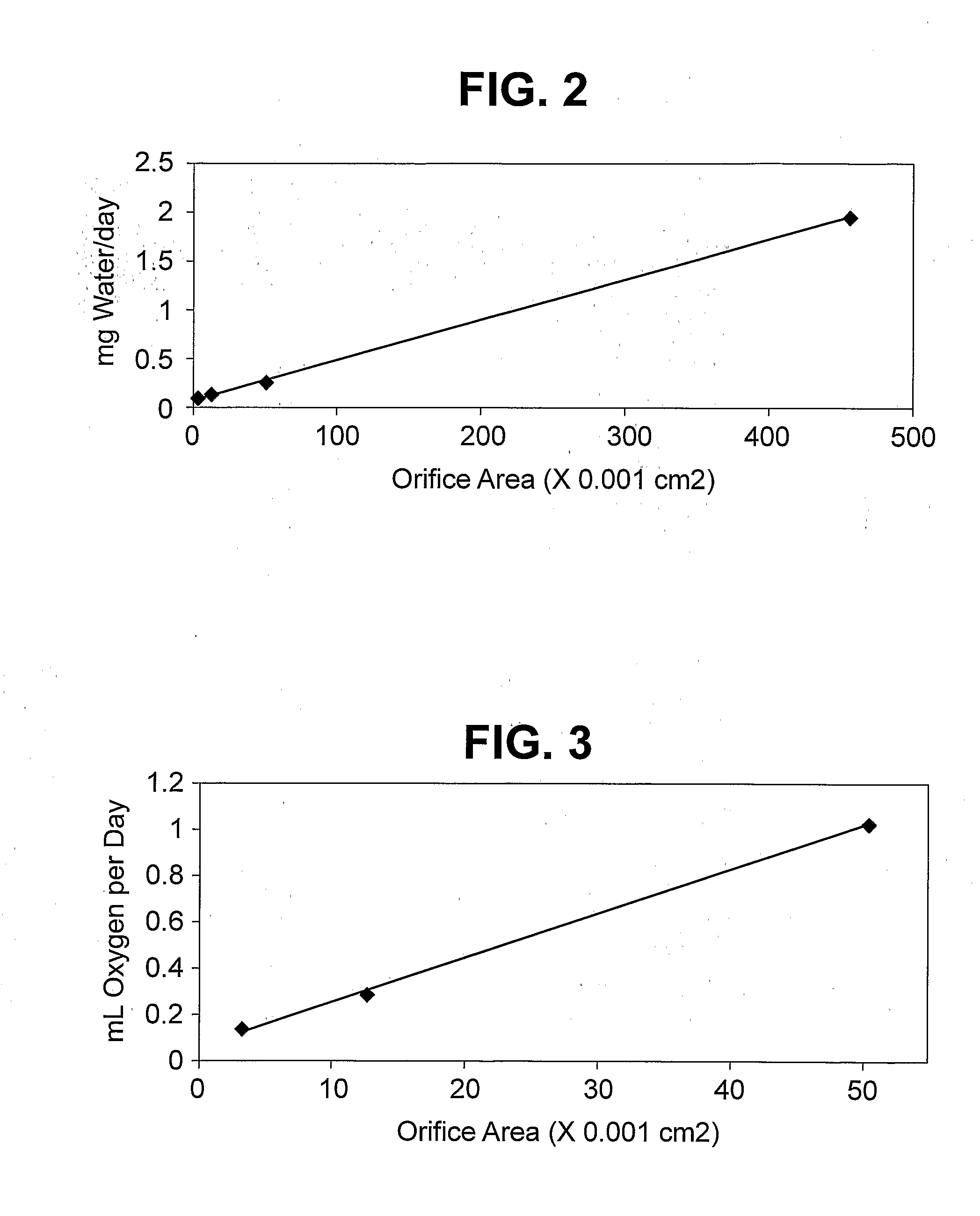
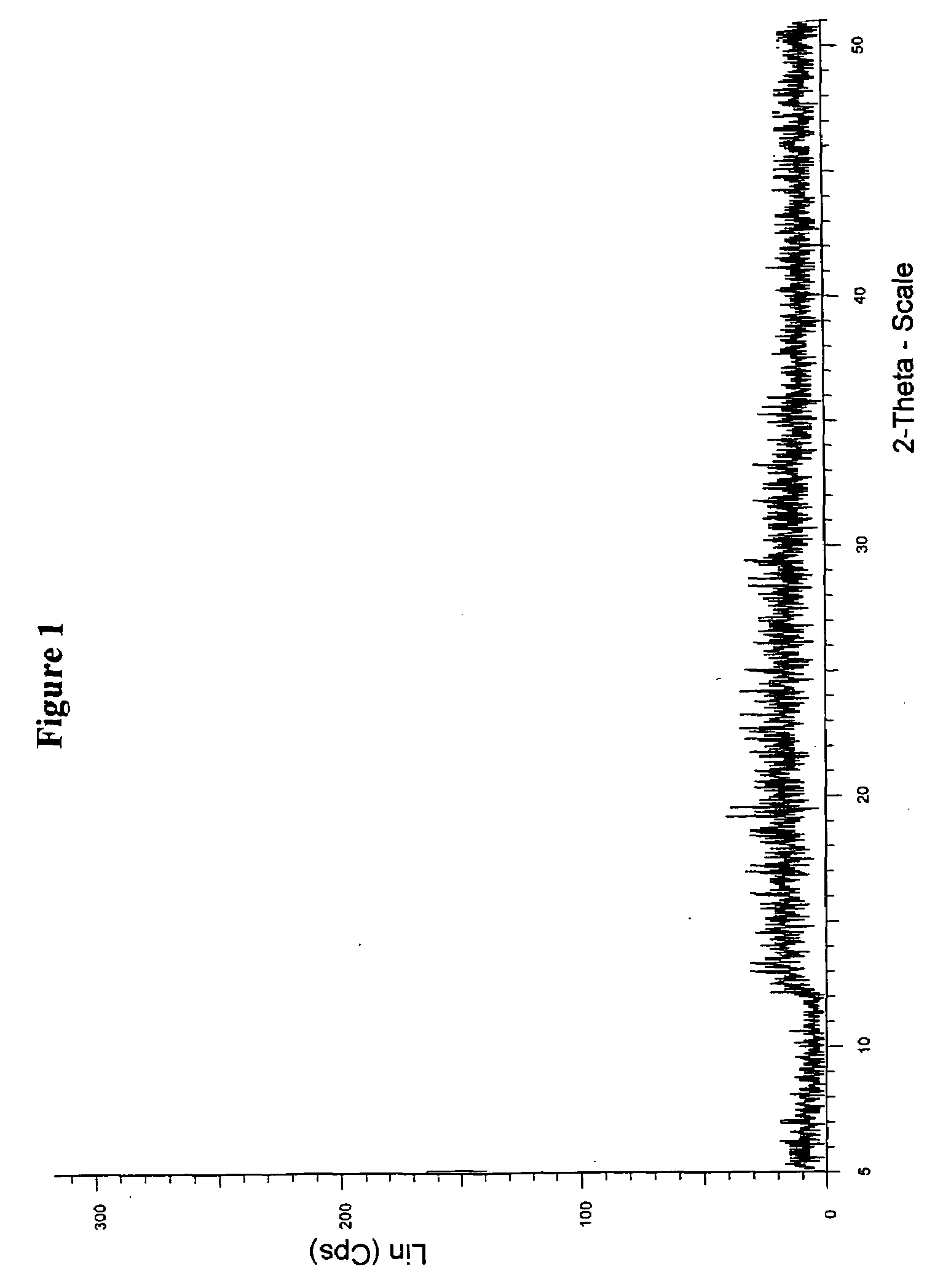
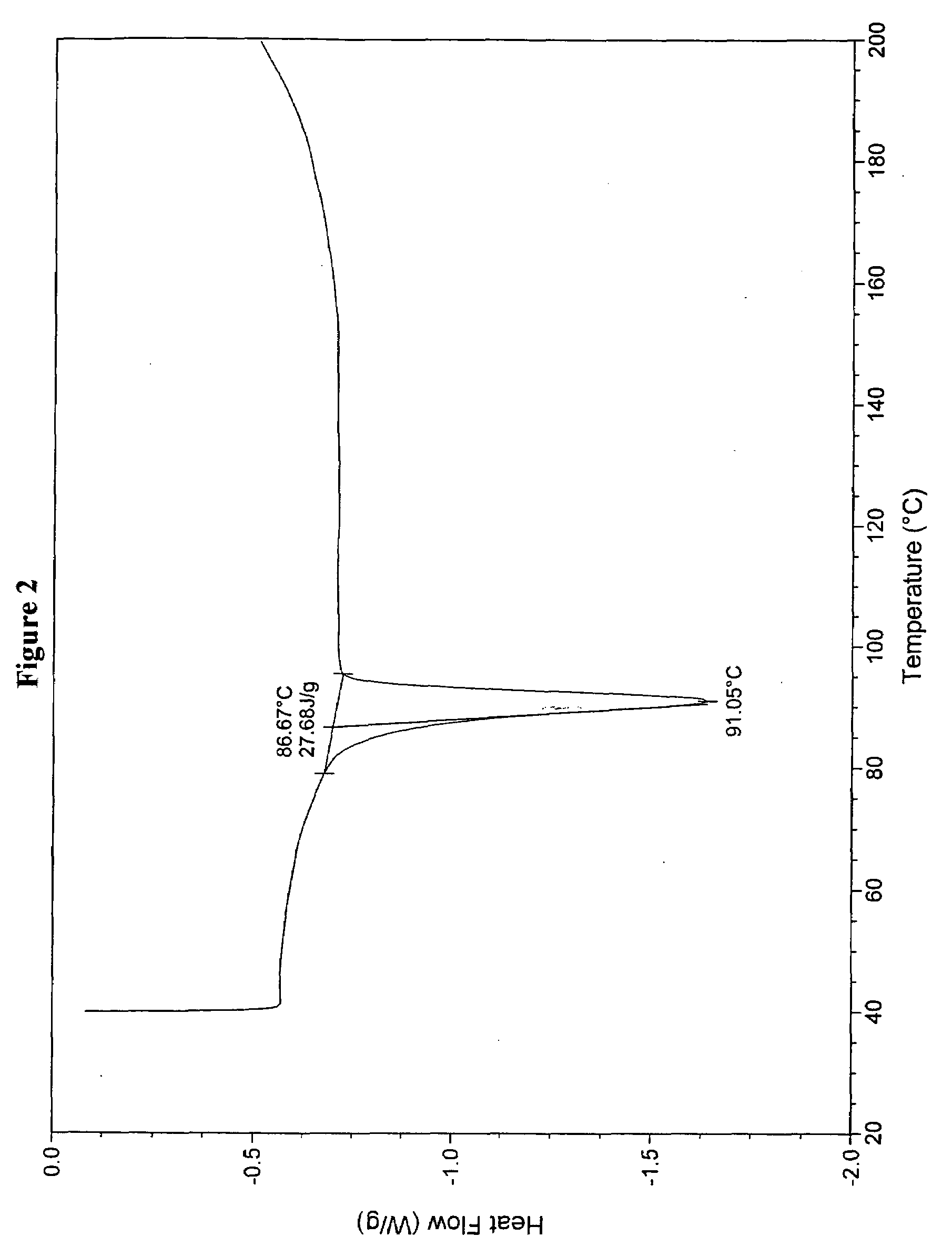
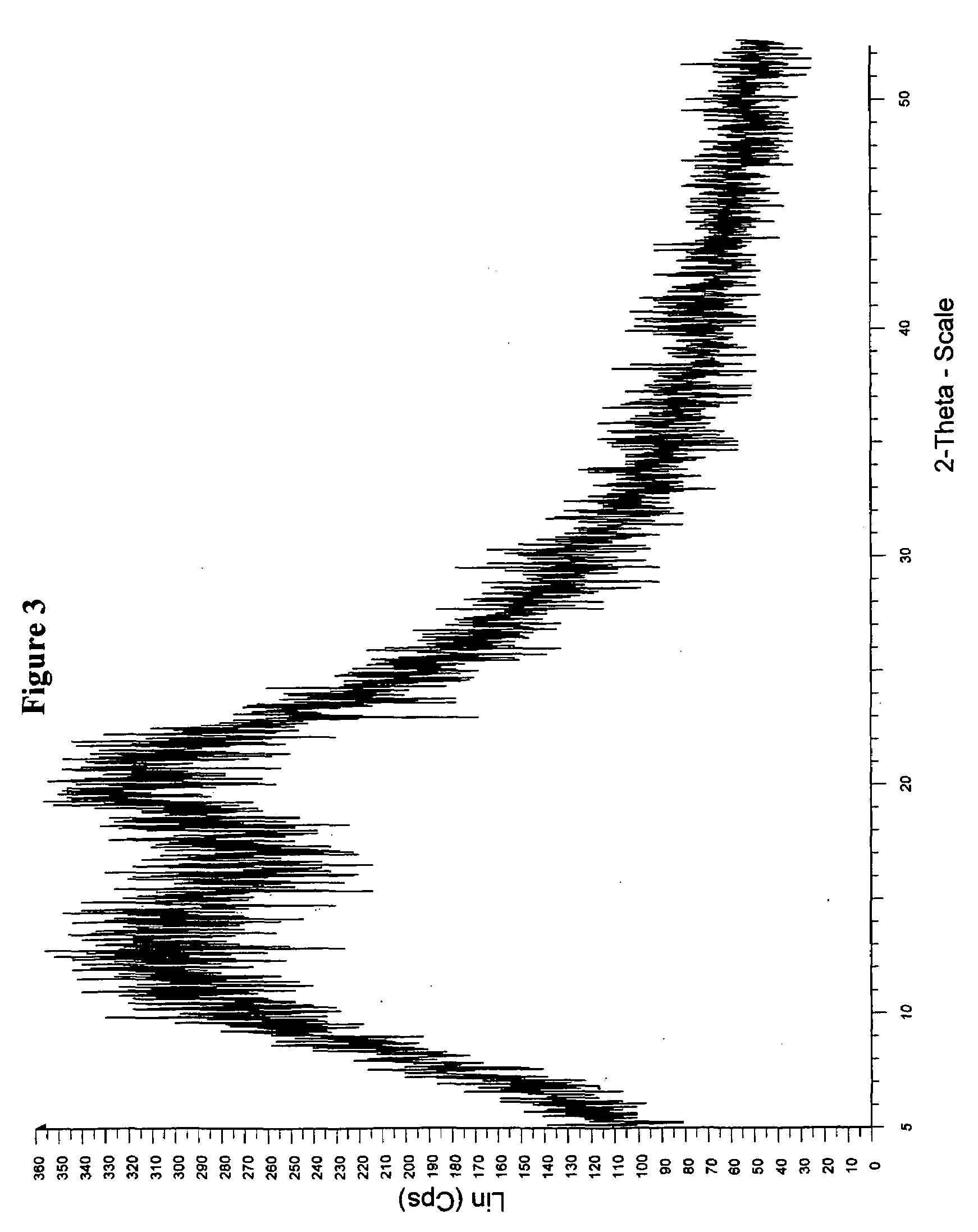
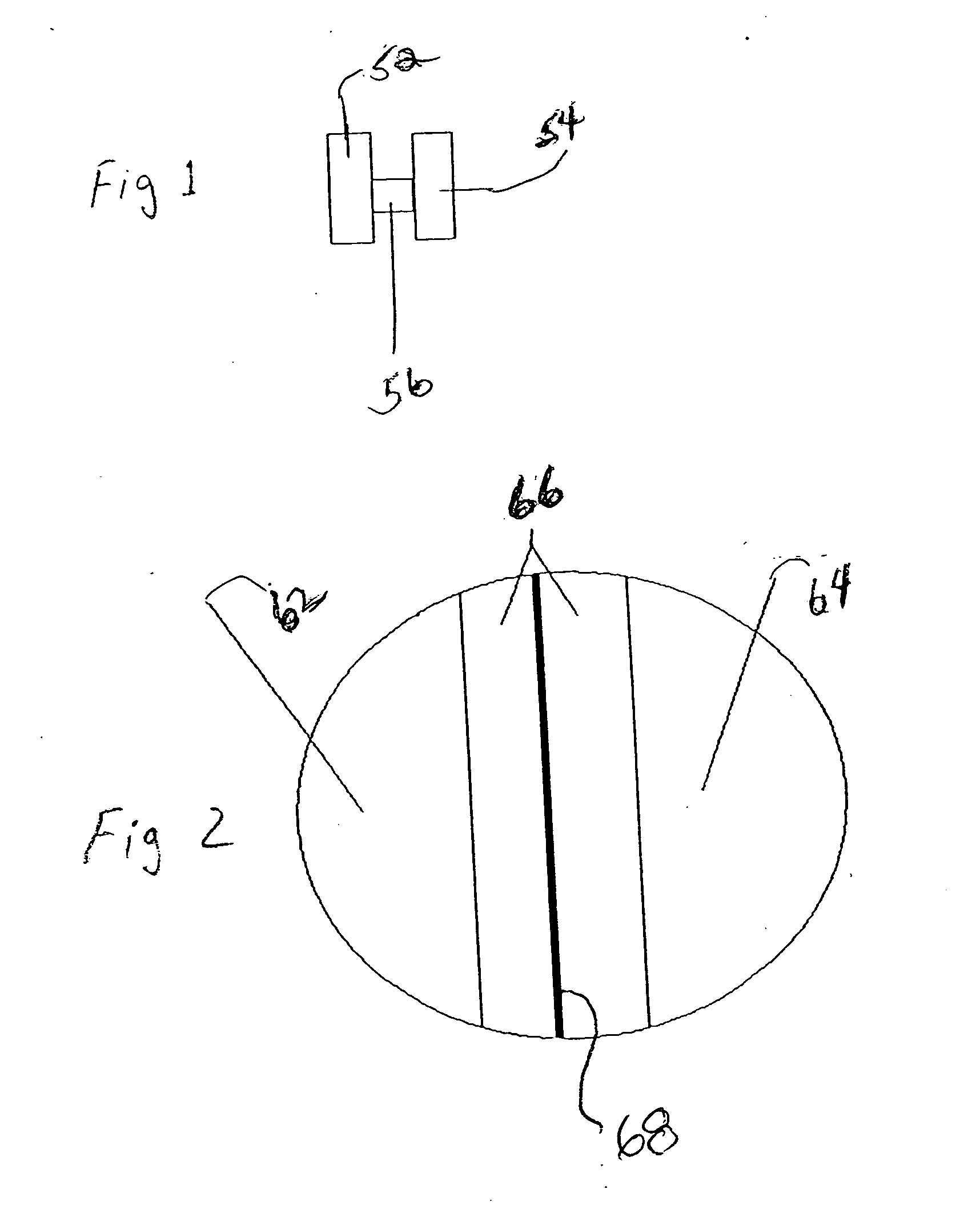
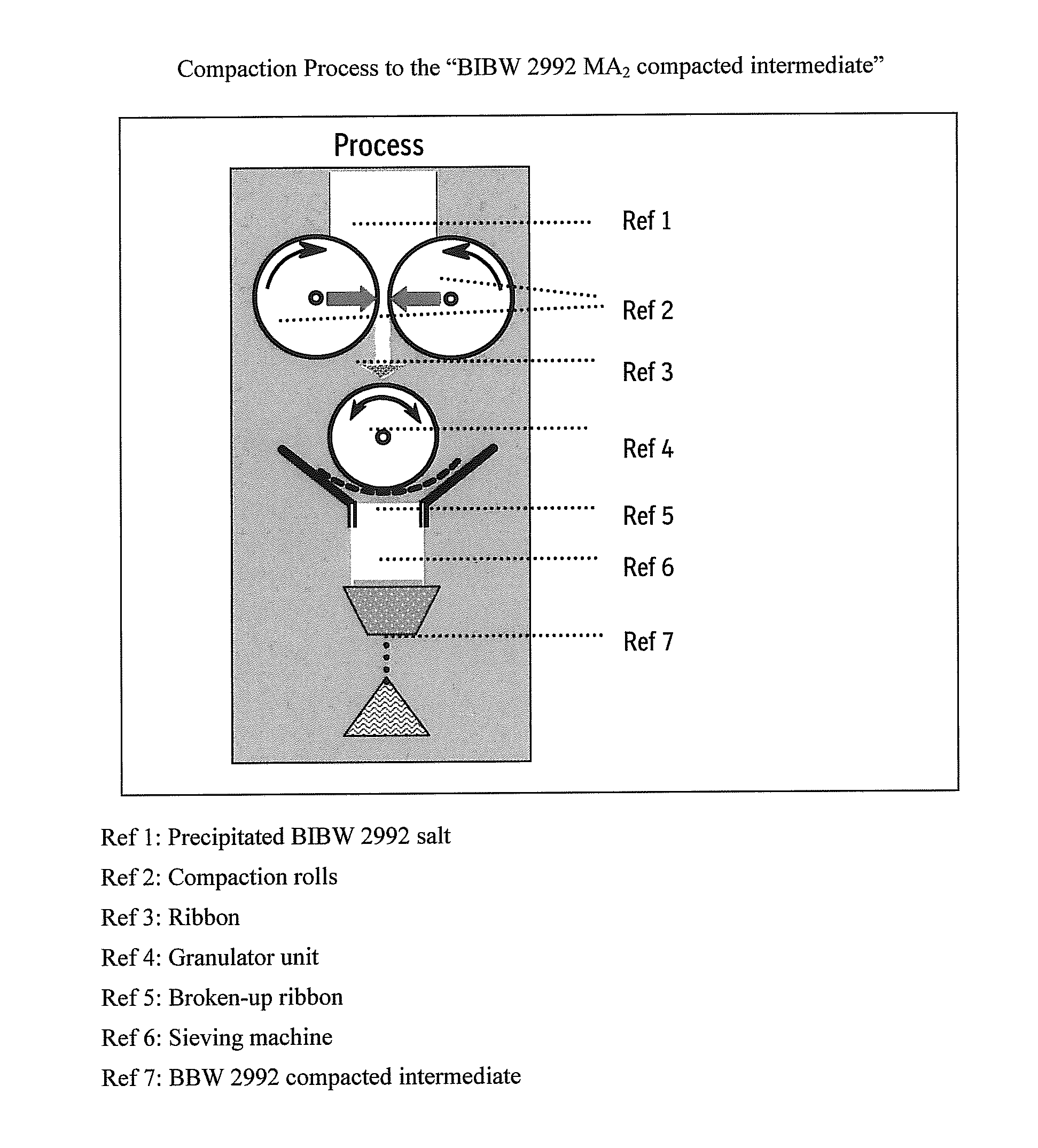
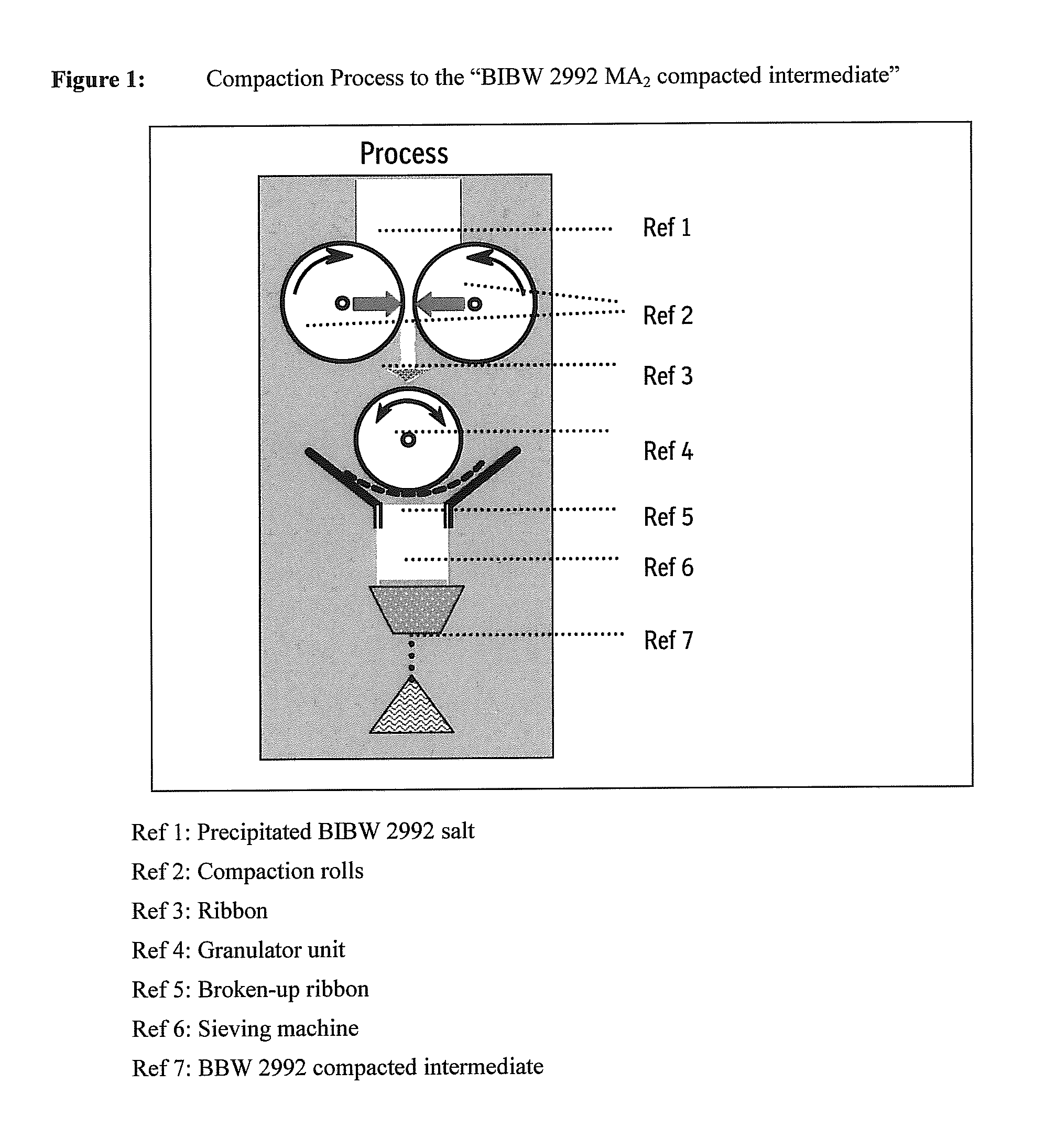
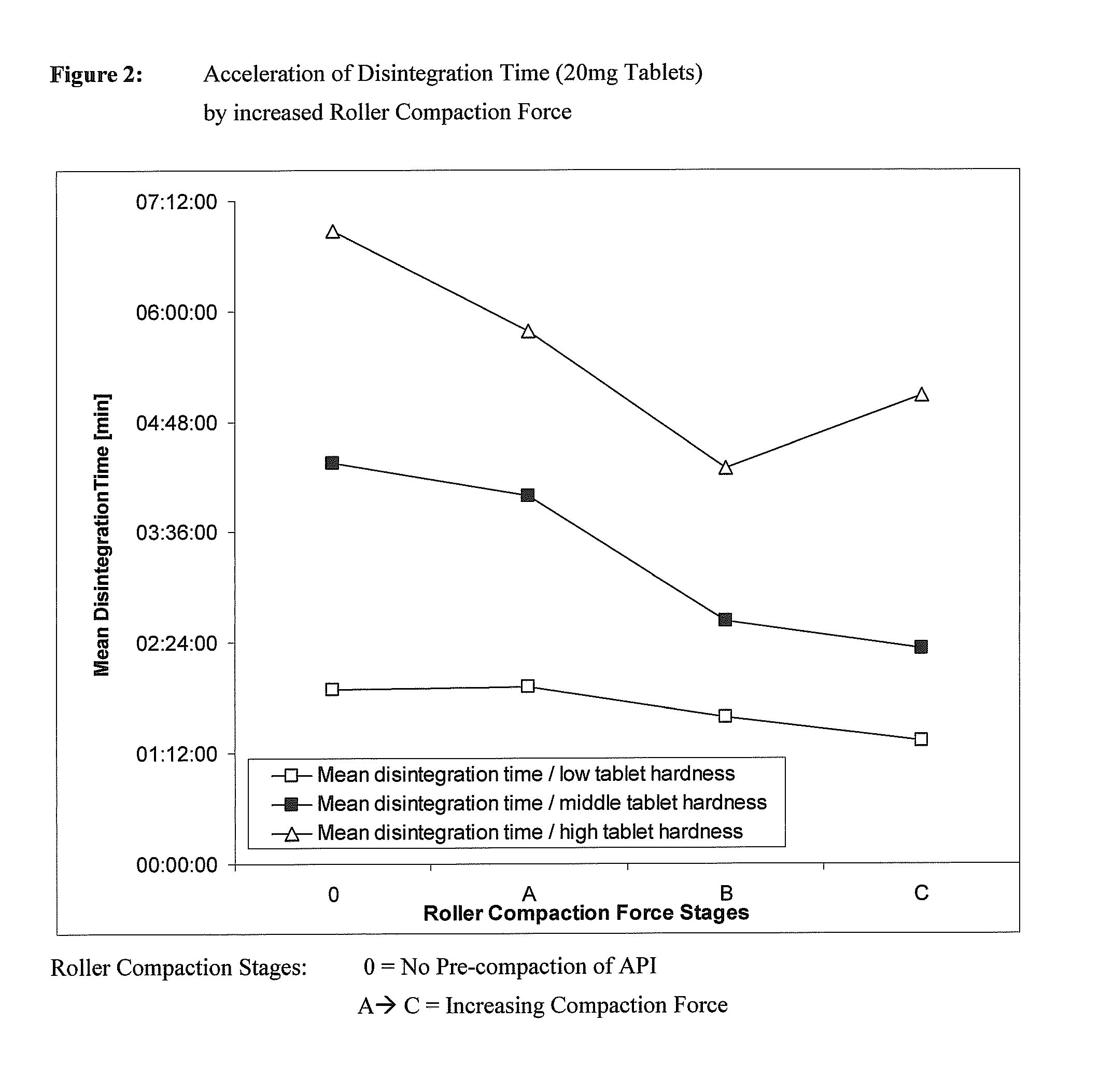
Patents
Literature
191 results about "Pharmaceutical Dose Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical compositions for the coordinated delivery of NSAIDs
InactiveUS20050249811A1Improve complianceReduce in quantityBiocideAntipyreticGastrointestinal InjuryArthritis
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO +1
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Systems and methods for administration of small volume sufentanil drug dosage forms to the sublingual mucosa of a subject using a device are disclosed. The dispensing device includes a lock-out feature and a means to retard or prevent saliva and / or moisture ingress such that the drug dosage forms in the device remain dry prior to administration.
Owner:ACEIRX PHARM INC
Oral insulin therapy
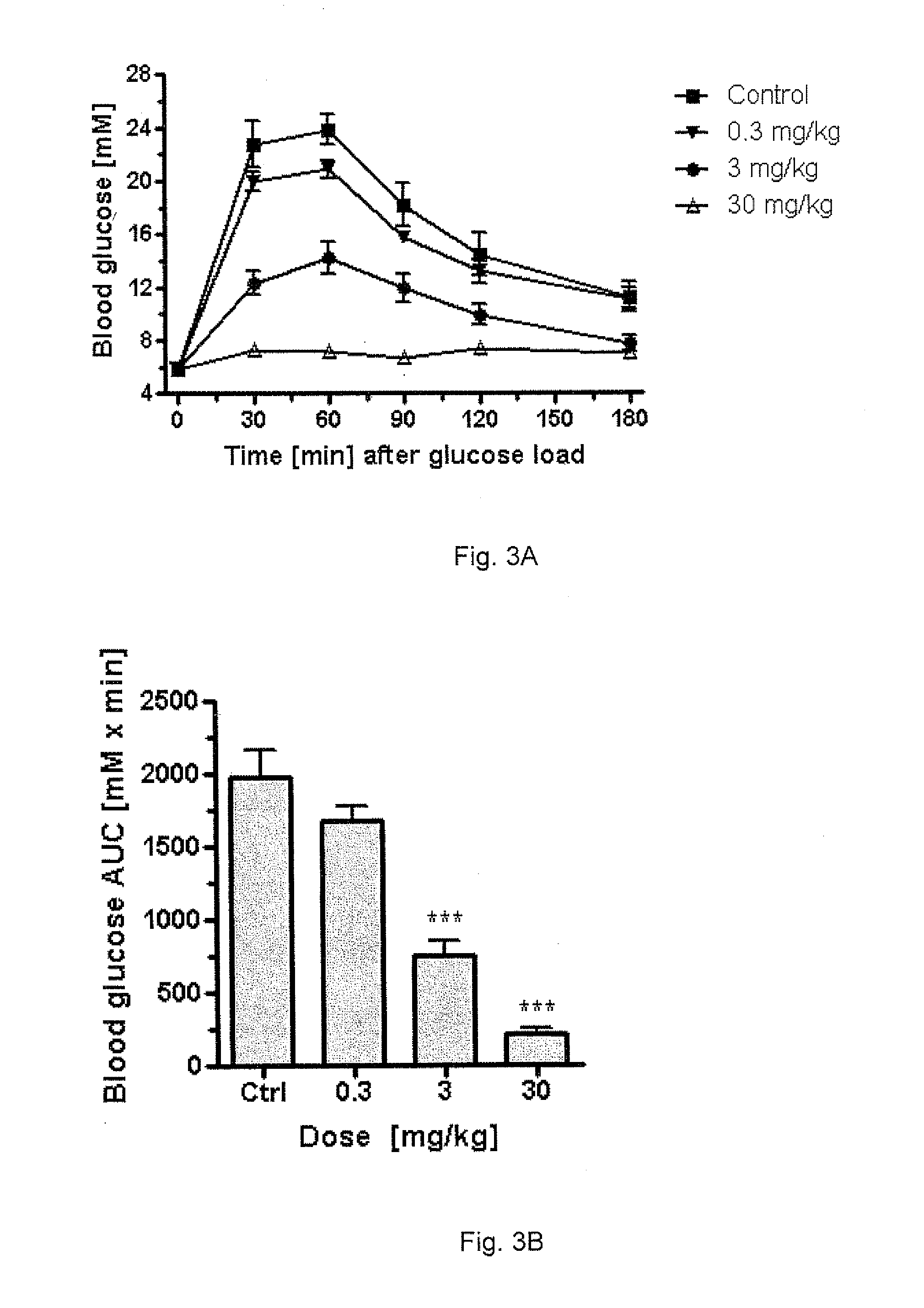
ActiveUS20060234913A1Reduce morbidityDosing is convenientBiocideOrganic active ingredientsDrugOral medication
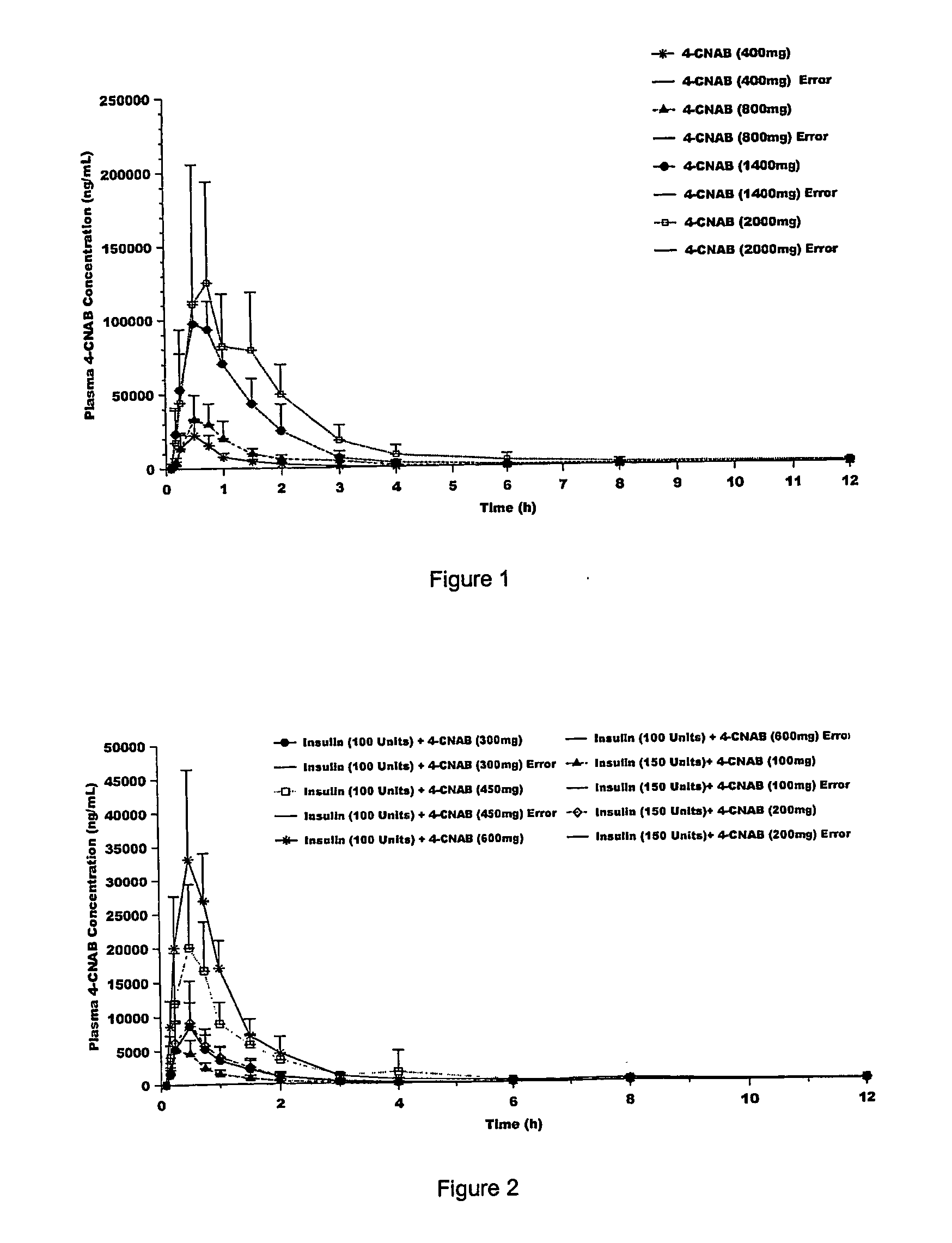
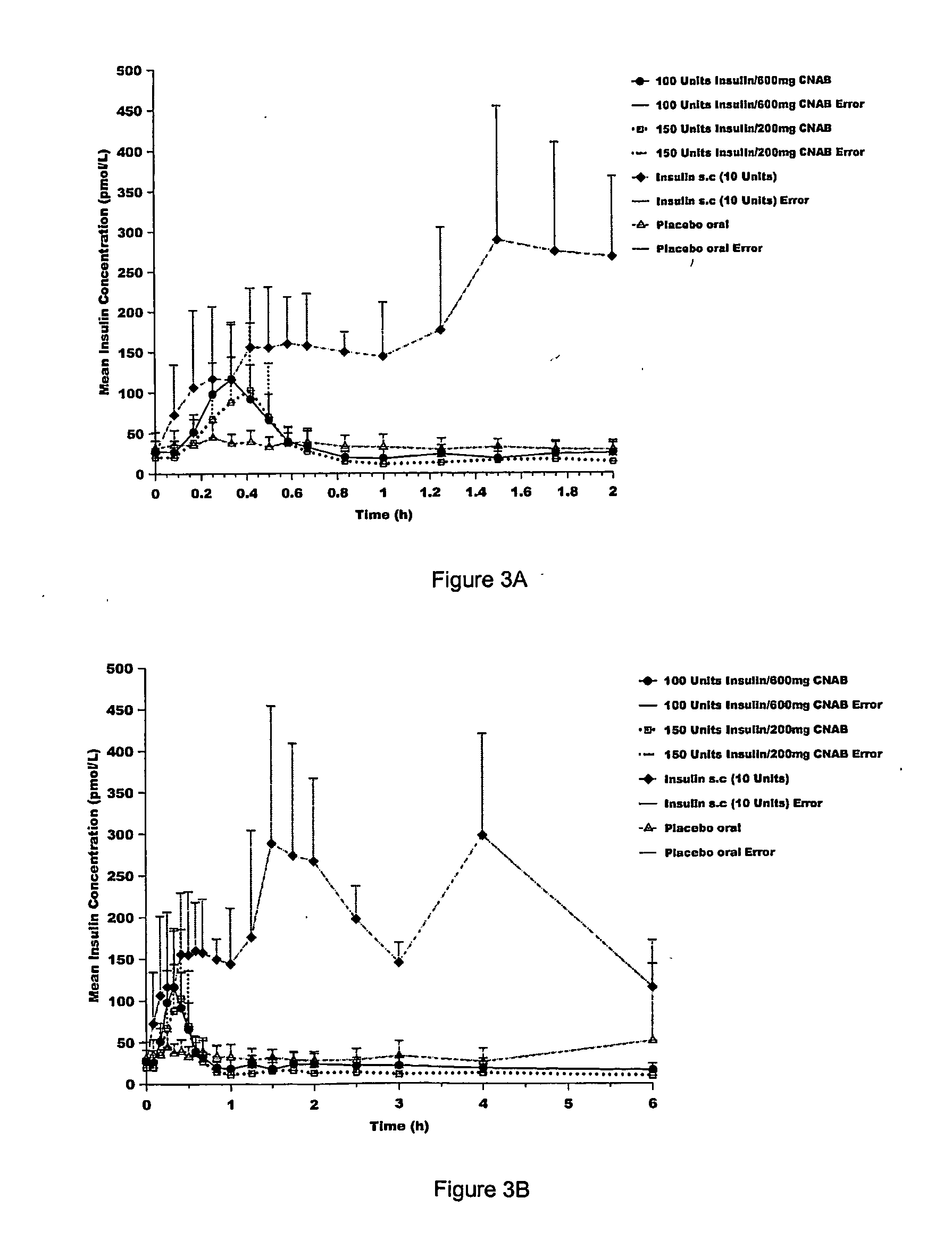
Pharmaceutical dosage forms for oral administration to a patient for the treatment of diabetes, comprising insulin and a delivery agent that facilitates insulin transport in a therapeutically effective amount to the bloodstream and that result in a lower incidence of vascular diseases associated with the repeated administration of insulin are disclosed. Also disclosed is a method of attenuating the undesirable incidence of diseases associated with chronic dosing of insulin is provided whereby the oral administration to a patient of insulin along with a suitable delivery agent that facilitates the absorption of insulin from the gastrointestinal tract of the patient in a therapeutically effective amount, for treatment of diabetes.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Controlled release compositions of gamma-hydroxybutyrate
ActiveUS8193211B2Reduce in quantityReduce the possibilityBiocideNervous disorderControl releaseImmediate release
The present invention is directed to oral pulse-release pharmaceutical dosage form containing an immediate release component of gamma-hydroxybutyric acid, and one or more delayed / controlled release components of gamma-hydroxybutyric acid.
Owner:SUPERNUS PHARM INC
Rapidly expanding composition for gastric retention and controlled release of therapeutic agents, and dosage forms including the composition
InactiveUS20040234608A1Improved gastric retentionHigh retention ratePowder deliveryOrganic active ingredientsGastric fluidAttention deficits
The present invention provides a pharmaceutical composition for use in a dosage form for oral administration to a patient. The composition expands upon contact with gastric fluid and promotes retention of the dosage form in the patient's stomach for a prolonged period of time. The present invention further provides pharmaceutical dosage forms containing an active ingredient, and the pharmaceutical composition. The forms are adapted for immediate or controlled release of the active ingredient. The dosage forms may be used advantageously in the treatment of Parkinson's disease with levodopa and hyperactivity and attention deficit disorder with methylphenidate.
Owner:TEVA PHARM USA INC
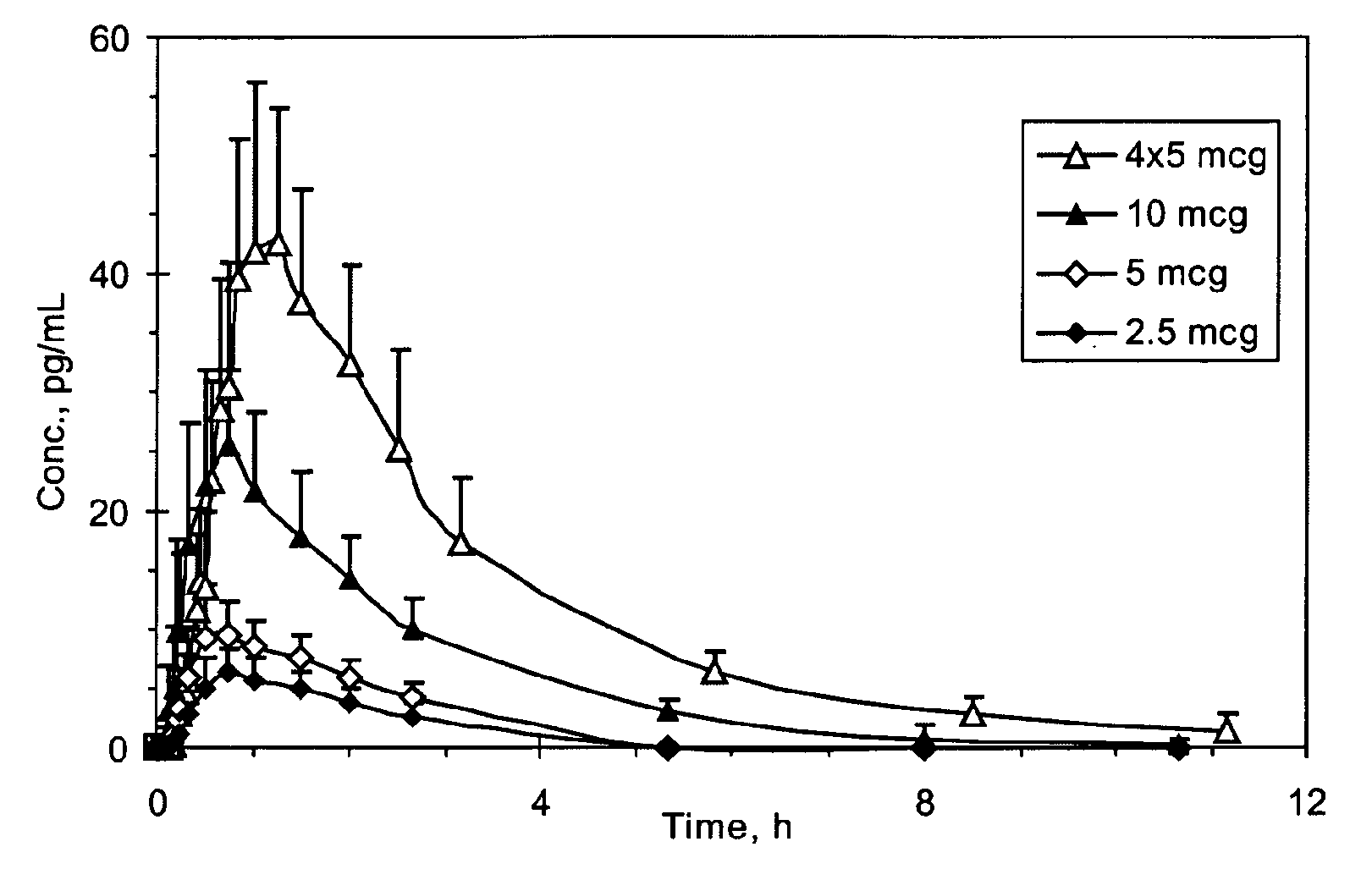
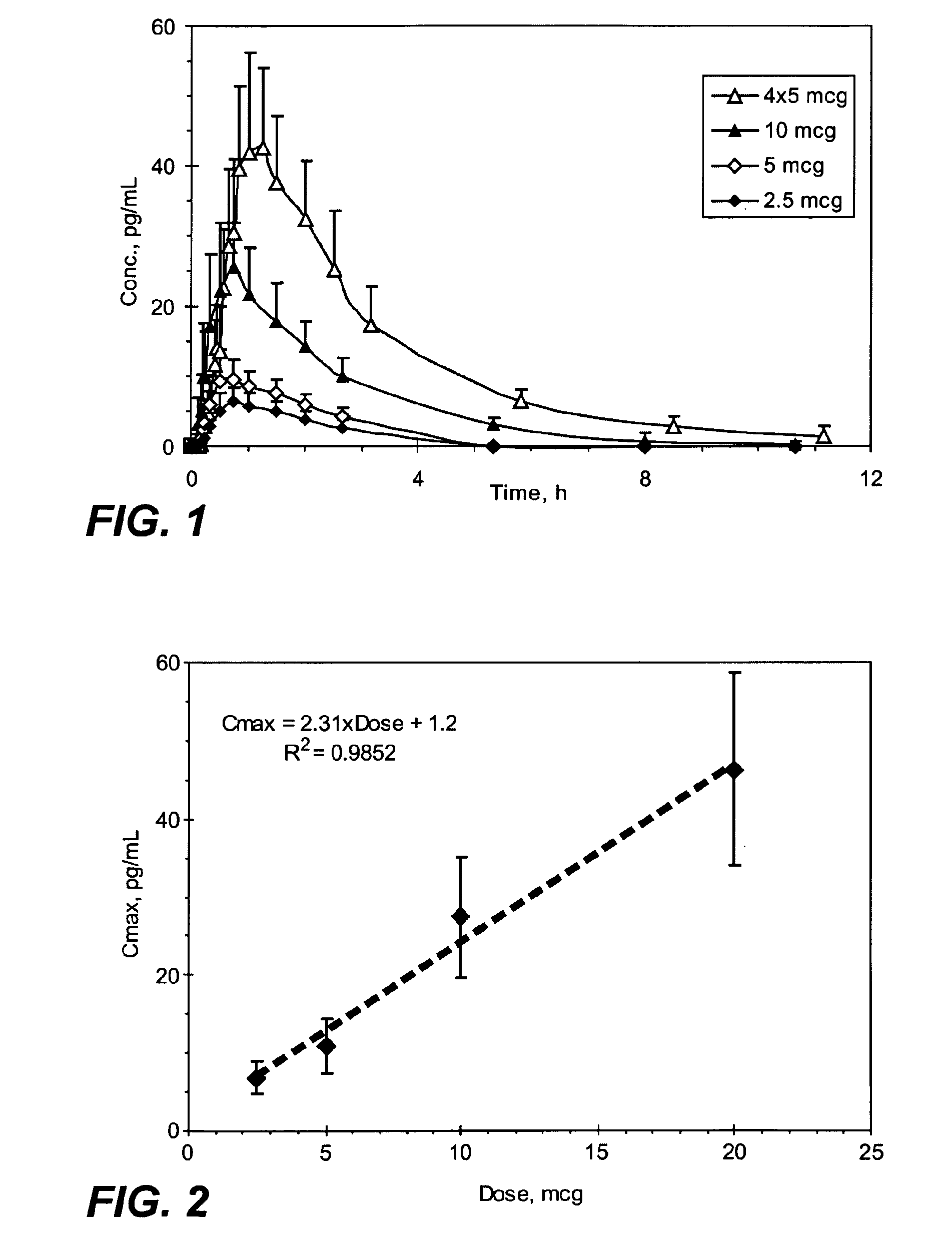
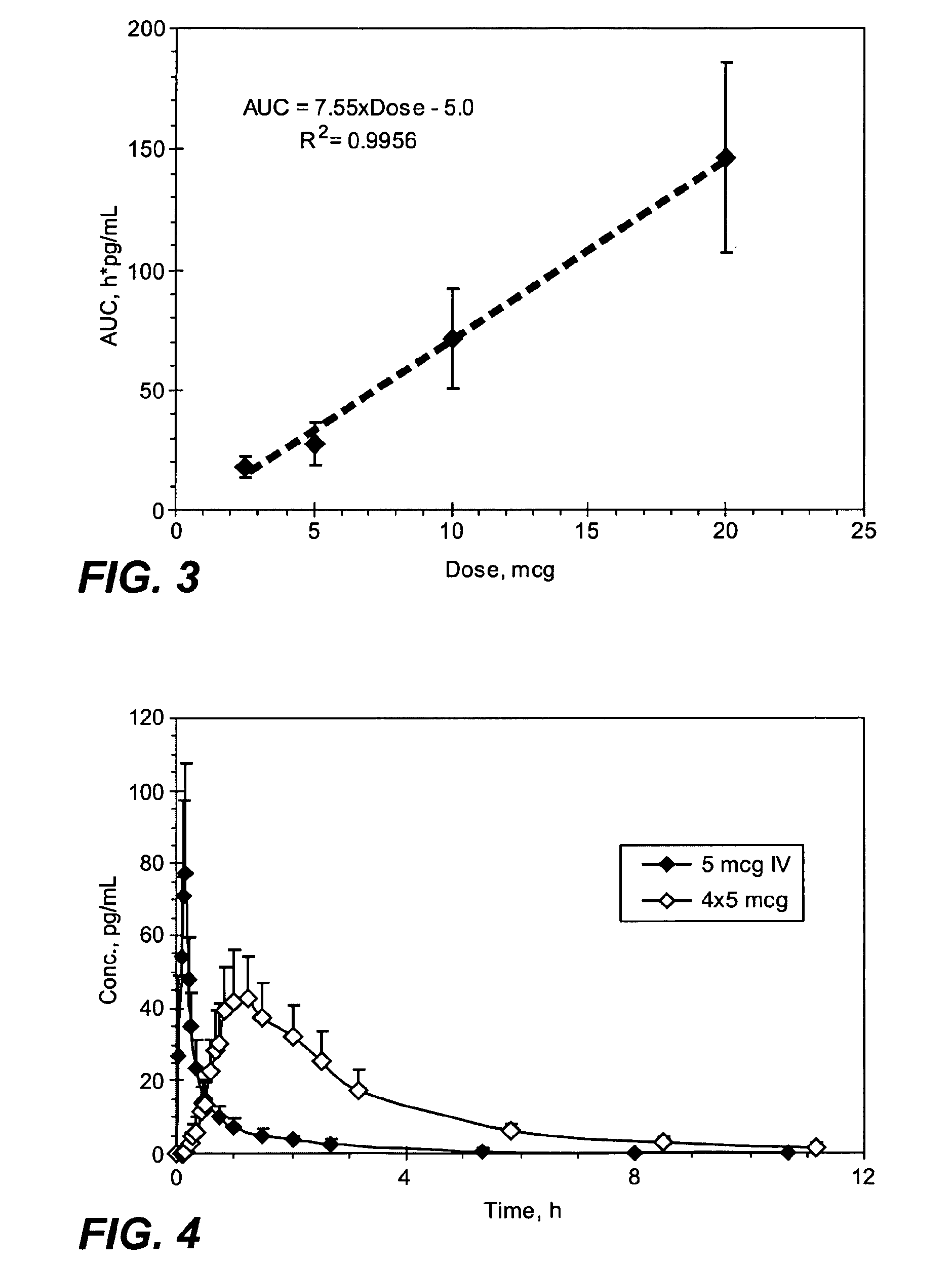
Small Volume Oral Transmucosal Dosage Forms Containing Sufentanil for Treatment of Pain
Compositions, methods and systems for administration of small volume sufentanil-containing drug dosage forms to the oral mucosa of a subject are disclosed.
Owner:VERTICAL PHARMA LLC
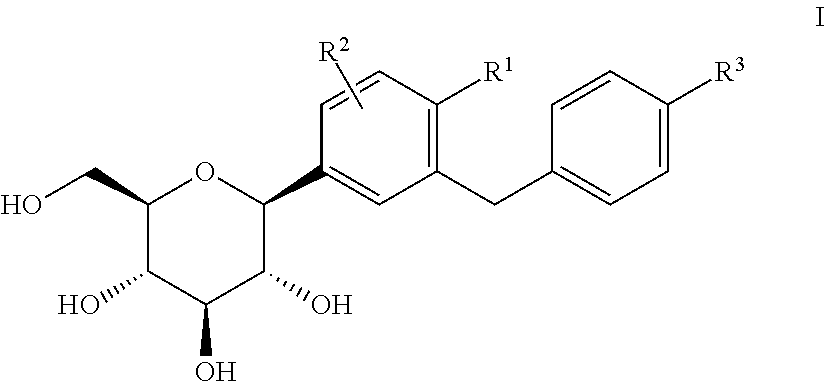
Multiparticle Pharmaceutical Dosage Form for a Low-Soluble Active Substances and Method for Producing Said Pharmaceutical Dosage Form
ActiveUS20080166416A1Easy to processAffect permeabilityBiocidePowder deliveryDrug additiveActive agent
The invention relates to an oral multiparticle pharmaceutical dosage form in the form of a receptacle reducing the pH values of stomach, containing a plurality of pellets, particles, granules or agglomerates whose mean diameter ranges from 50 to 2500 μn substentially consisting of a) an internal matrix layer containing an active agent which is neither peptide or protein, nor the derivatives or conjugates thereof, a lipophilic matrix whose melting point is greater than 37° C. and a polymer with mucoadhesive effect, b) an external film coating substentially consisting of a polymer or an anionic copolymer which is optionally formulated with conventional pharmaceutical additives, wherein the active agent has a water solubility according to DAB 10, of at least 30 volume parts of water for one part by weight of the active agent and is coated with the lipophilic matrix and said active agent-containing lipophilic matrix is coated with a matrix made of a polymer with mucoadhesive effect. A method for producing the inventive multiparticle pharmaceutical dosage is also disclosed.
Owner:EVONIK OPERATIONS GMBH
Tamper resistant dosage forms
InactiveUS20130251797A1Reduces and prevents stickingPowder deliveryBiocidePharmaceutical drugPharmaceutical Dose Form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Pharmaceutical composition, pharmaceutical dosage form, process for their preparation, methods for treating and uses thereof
InactiveUS20110014284A1Good effectFew compliancePowder deliveryBiocidePharmaceutical drugMedicinal chemistry
Owner:BOEHRINGER INGELHEIM INT GMBH
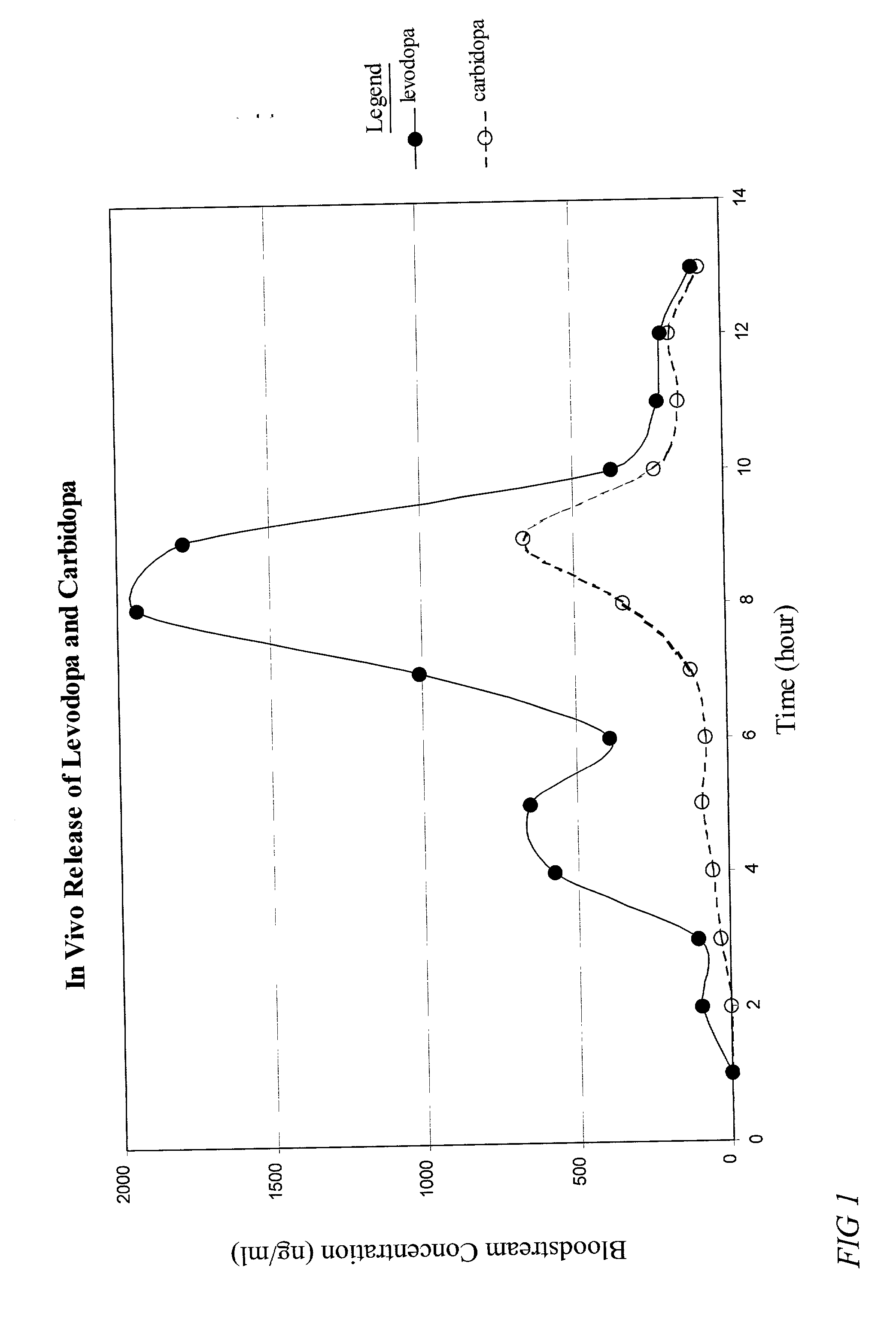
Combination immediate release controlled release levodopa/carbidopa dosage forms
The present invention relates to pharmaceutical dosage forms of a combination of carbidopa and levodopa comprising both immediate release and controlled release components for the treatment of ailments associated with depleted amounts of dopamine in a patient's brain tissue, which dosage forms display no food effect or at least substantially avoid the food effect, and a related method of treatment for a patient in which the bioavailability of levodopa under non-fasting conditions is equivalent to the bioavailability under fasting conditions.
Owner:IMPAX LAB INC
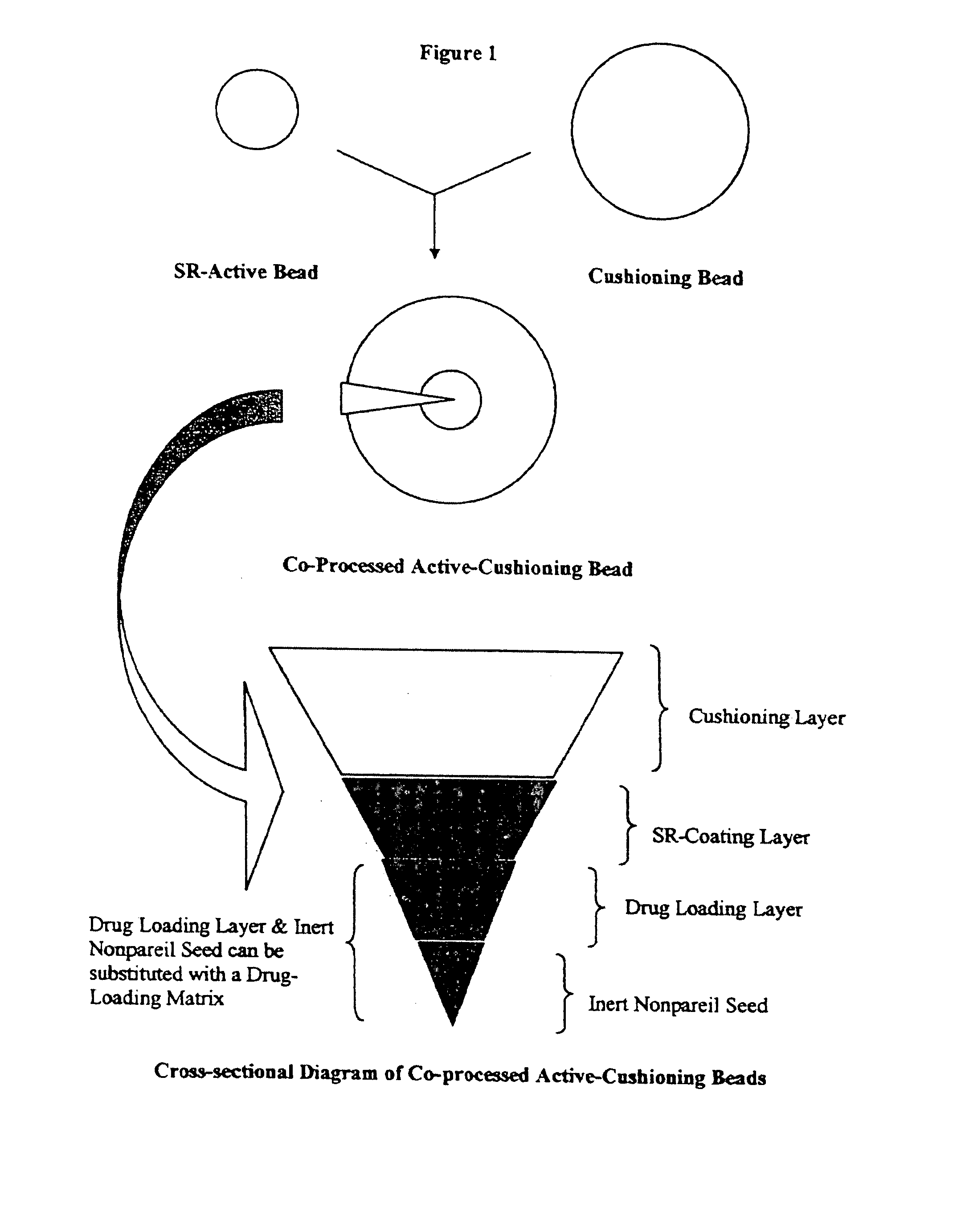
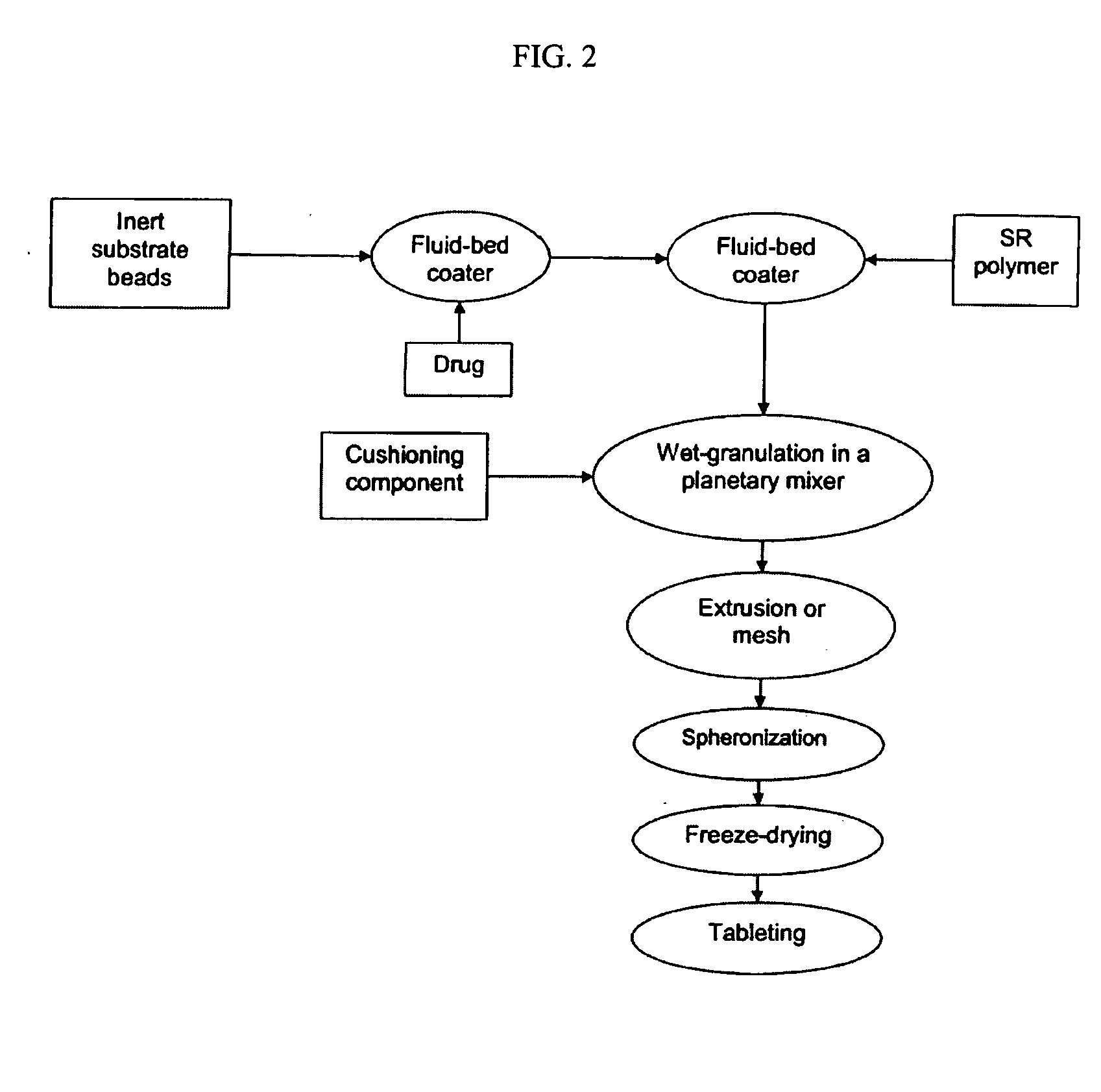
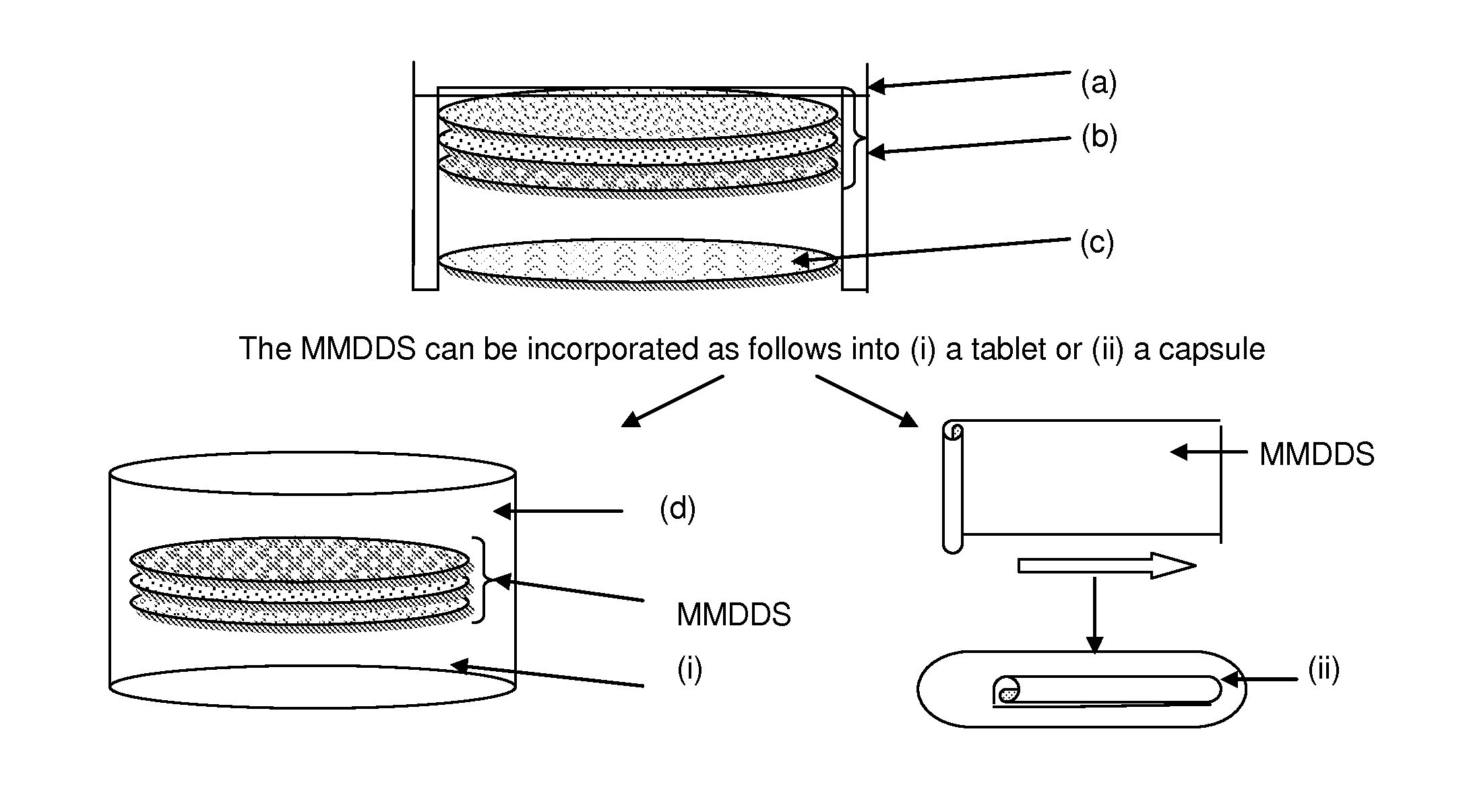
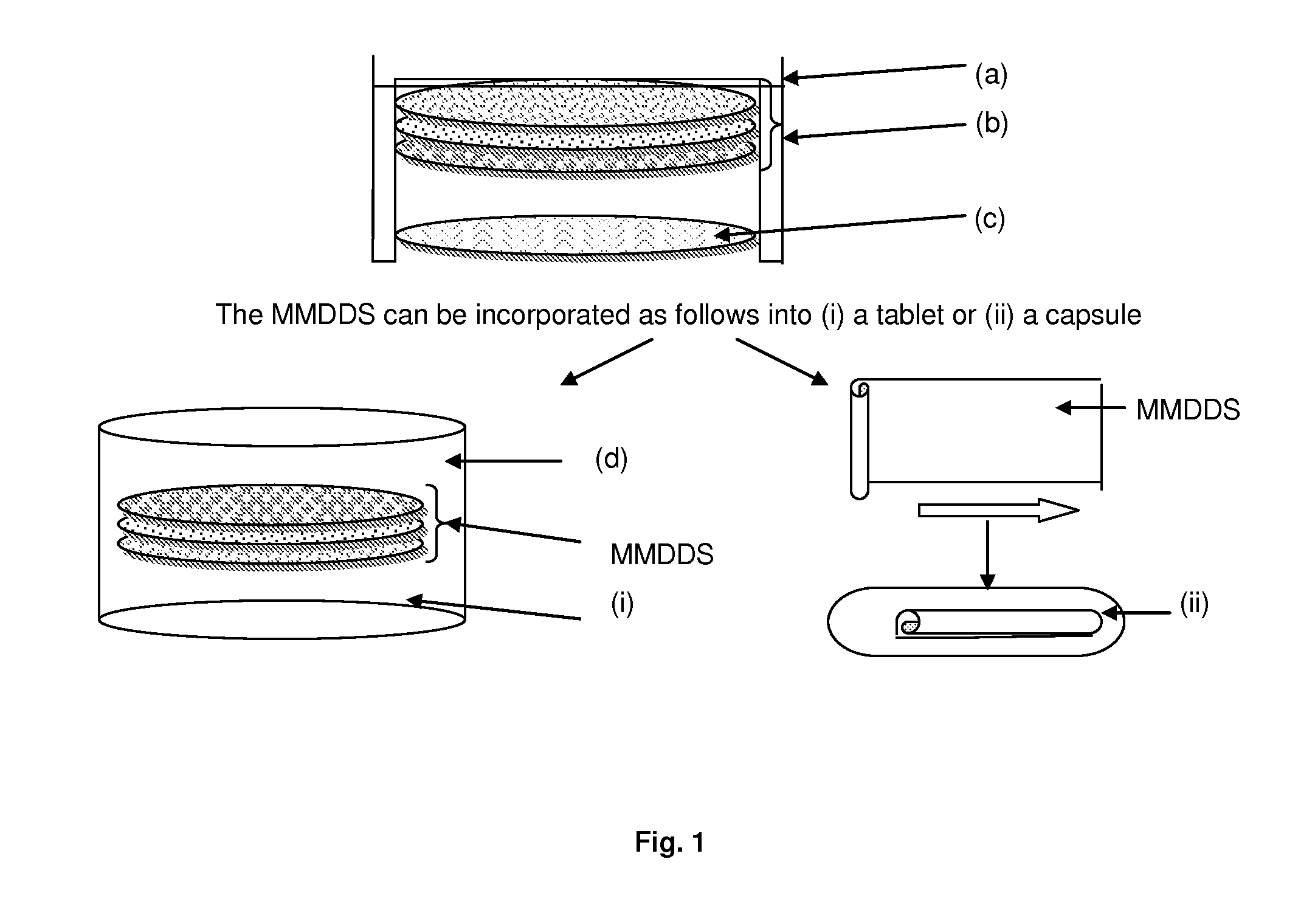
Methods for making pharmaceutical dosage forms containing active cushioning components
InactiveUS20050019393A1Uniform compositionPowder deliveryOrganic active ingredientsCushioningDosage form
Novel methods for making dosages form comprising a cushioning component. The methods of the present invention provides dosage forms which can be compressed to form compressed dosage forms that are substantially uniform in composition and robust and exhibit reduced friability. The invention also relates to methods for making fast-disintegrating dosage forms.
Owner:FOTONATION VISION +1
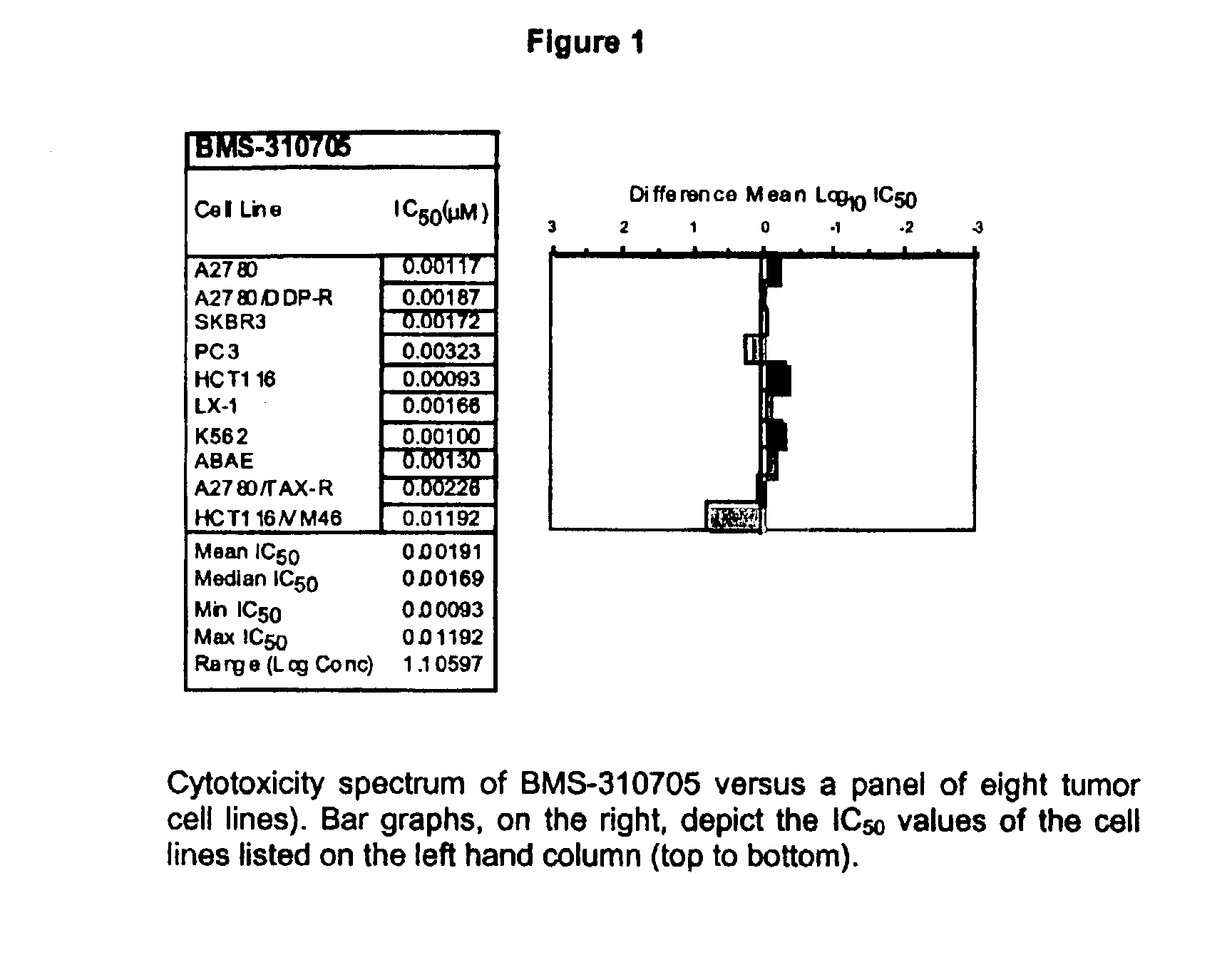
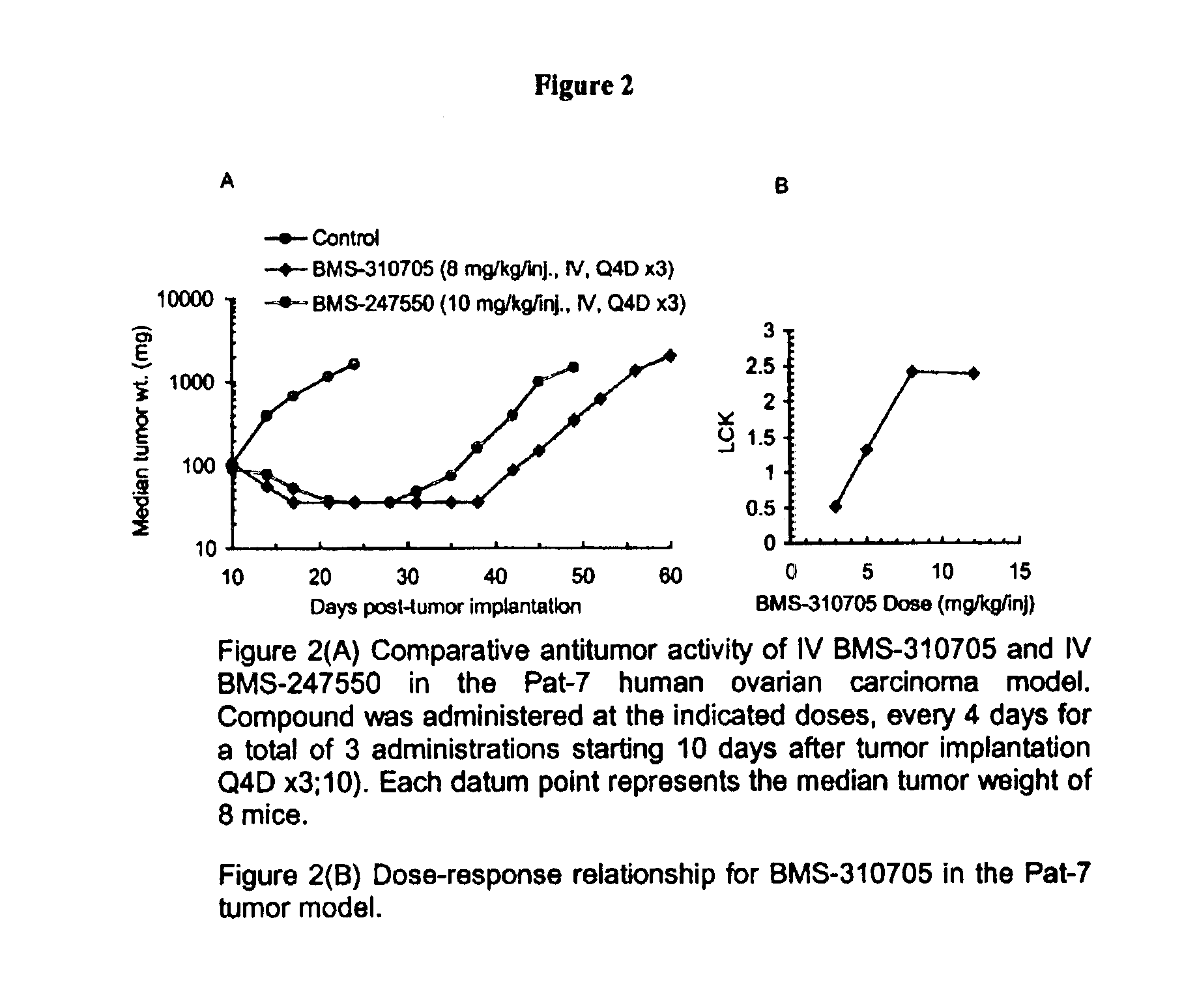
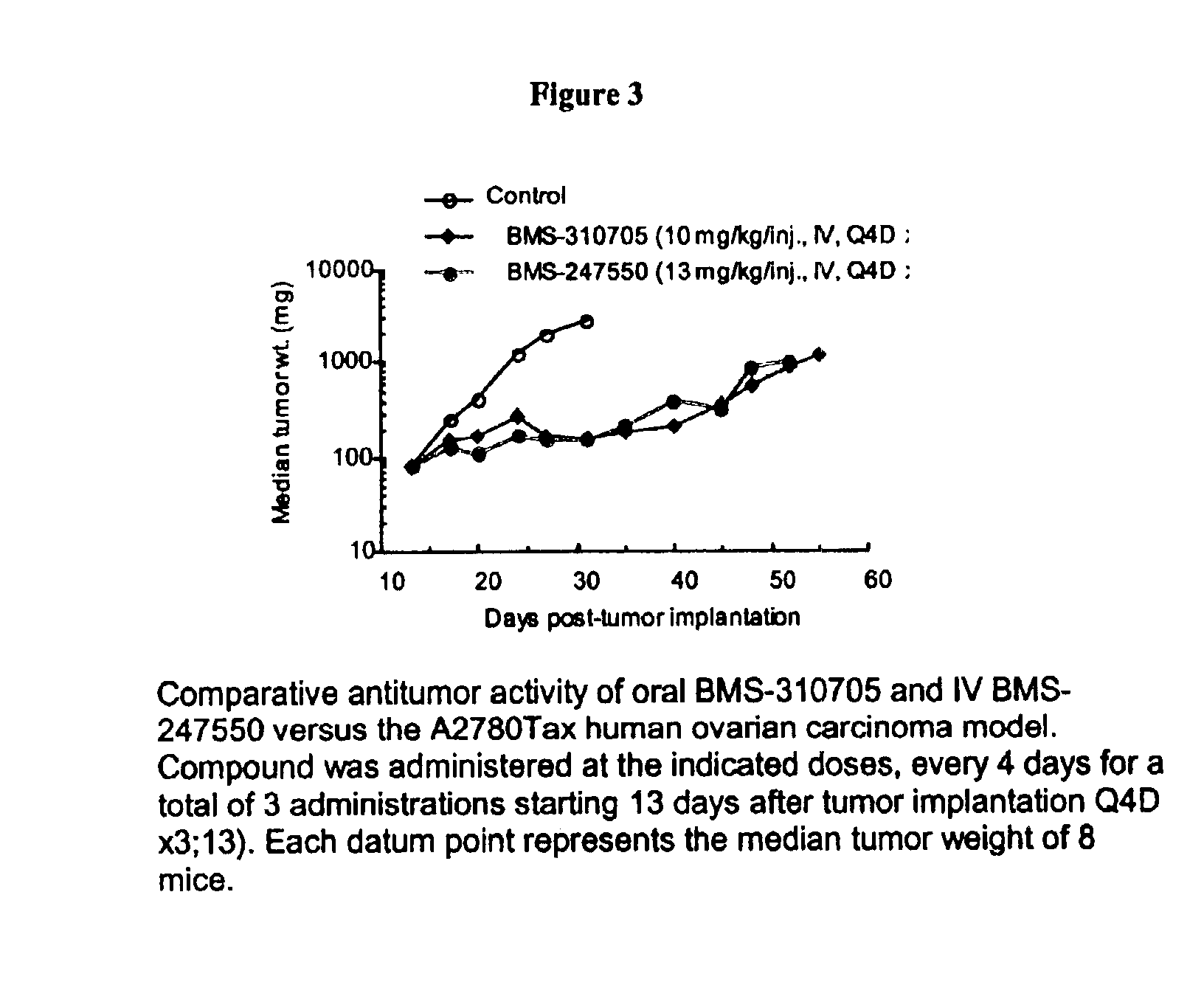
Oral administration of epothilones
The invention relates to methods of increasing the bioavailability of orally administered epothilones. Epothilones administered by the methods of the invention are sufficiently bioavailable to have a pharmacological effect. The invention further relates to pharmaceutical compositions, pharmaceutical dosage forms, and kits for use in the methods of the invention.
Owner:BRISTOL MYERS SQUIBB CO
Tamper resistant dosage forms
InactiveUS20130251800A1Reduces and prevents stickingBiocideNervous disorderPharmaceutical drugPharmaceutical Dose Form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Package and device for simultaneously maintaining low moisture and low oxygen levels
InactiveUS20070163917A1Low moisture levelLower levelSmall article dispensingPharmaceutical containersDesiccantBottle
The present invention relates to a device for reducing the oxygen content of the air surrounding pharmaceutical dosage forms contained within an oxygen-permeable bottle, while also maintaining a relatively low moisture level in said air during the shelf-life of the product. Accordingly, a pharmaceutical package contains a dessicant in a first sub-container and a self-activated oxygen-absorber in a second sub-container.
Owner:BEND RES
Compressed core for pharmaceutical composition
A compressed core for a pharmaceutical dosage form comprising a mixture of (a) at least one pharmaceutically acceptable organic acid, and (b) at least one pharmaceutically acceptable excipient is described. Such compressed core is useful for the preparation of pharmaceutical compositions containing a drug in which dissolution of the drug is favored in acidic environments. Also described are pharmaceutical compositions comprising such compressed core.
Owner:TEVA PHARM USA INC
Pharmaceutical preparation comprising an active dispersed on a matrix
InactiveUS7175854B2Improve stabilityUniform deliveryBiocidePowder deliveryEngineeringBULK ACTIVE INGREDIENT
The present invention relates to the field of pharmaceutical technology and describes a novel advantageous preparation for an active ingredient. The novel preparation is suitable for producing a large number of pharmaceutical dosage forms. In the new preparation an active ingredient is present essentially uniformly dispersed in an excipient matrix composed of one or more excipients selected from the group of fatty alcohol, triglyceride, partial glyceride and fatty acid ester.
Owner:ASTRAZENECA AB
Sublingual buccal effervescent
InactiveUS20050064030A1Organic active ingredientsWood working apparatusOral medicationAbsorption drugs
A pharmaceutical dosage form adapted to supply a medicament to the oral cavity for buccal, sublingual or gingival absorption of the medicament which contains an orally administrable medicament in combination with an effervescent for use in promoting absorption of the medicament in the oral cavity. The use of an additional pH adjusting substance in combination with the effervescent for promoting the absorption drugs is also disclosed.
Owner:CIMA LABS
Preparation of solid coprecipitates of amorphous valsartan
A novel coprecipitate of amorphous valsartan with a pharmaceutically acceptable carrier, e.g. polyvinylpyrolidone (PVP), crosslinked-polyvinylpyrolidone, polyvinylpyrolidone vinyl acetate copolymer (PVP-VA64), a process for the preparation of said novel co-precipitate and the use of said novel coprecipitate in the treatment and / or prophylaxis of hypertension, cardiovascular diseases and conditions associated with thereof and certain complications thereof, are disclosed. A novel solid solution of amorphous valsartan with a pharmaceutically acceptable carrier, preferably with polyethyelene glycol PEG from 4000 to 20,000 of average mol. wt., a process for the preparation thereof and use are disclosed. The said novel coprecipitate of amorphous valsartan and the said novel solid solution of valsartan are stable and may be particularly suitable for pharmaceutical dosage forms.
Owner:MAI DE
Adhesively bonded dosage form
InactiveUS20060003000A1Easily breakablePill deliveryCapsule deliveryPharmaceutical drugPharmacometrics
A solid pharmaceutical dosage form having a plurality of adhesively-joined subunits and also having one or more of the following: A. (i) a first inert tablet subunit and (ii) a second active subunit; B. (i) a first tablet subunit with a pharmacologically inactive layer in which said layer has a mass of at least 20 mg and (ii) a second subunit; C. a tablet subunit that is provided with a separation mark; or D. (i) a first tablet subunit and (ii) a second capsule subunit that is adhesively joined to said first tablet subunit.
Owner:ACCU BREAK TECH
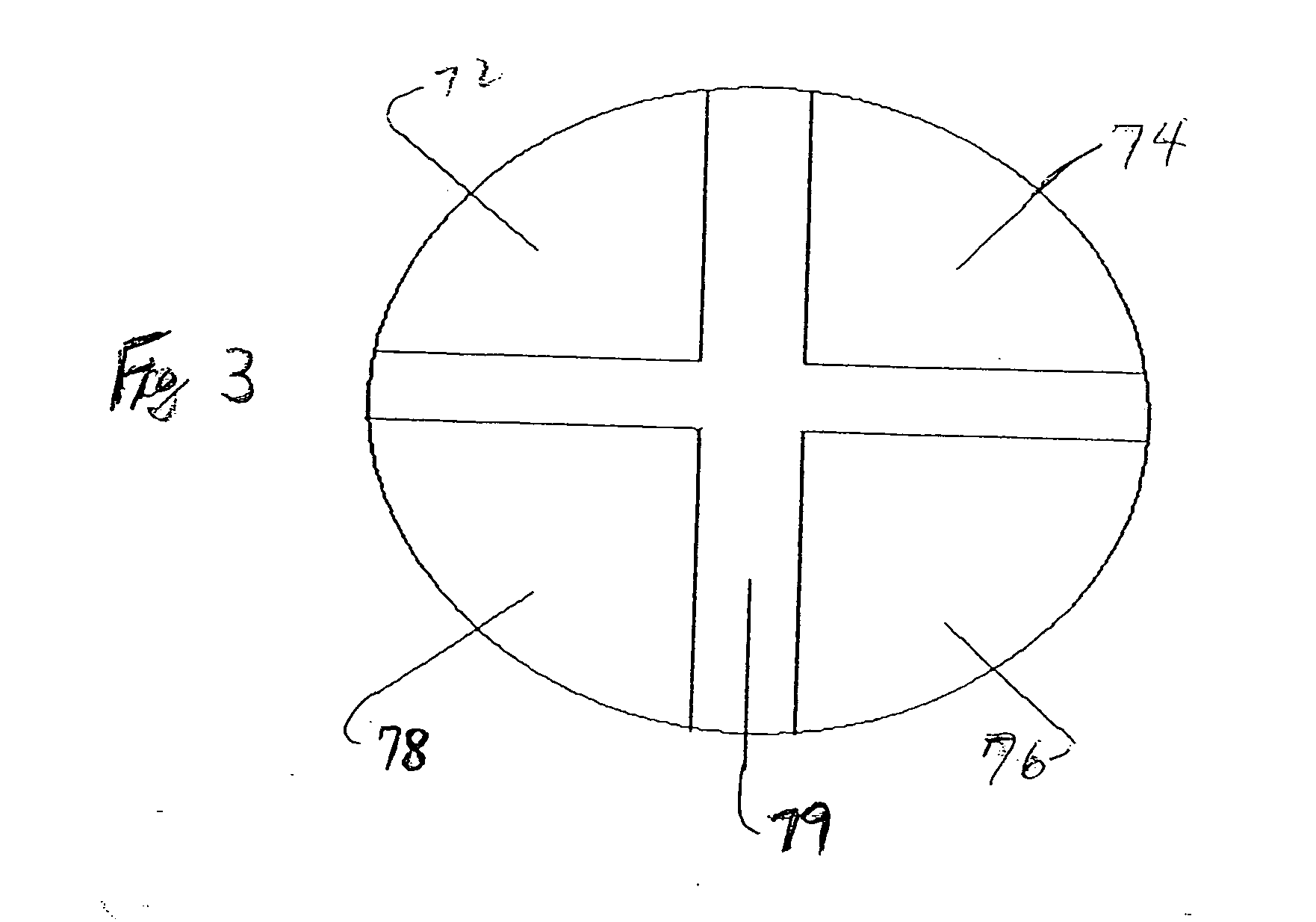
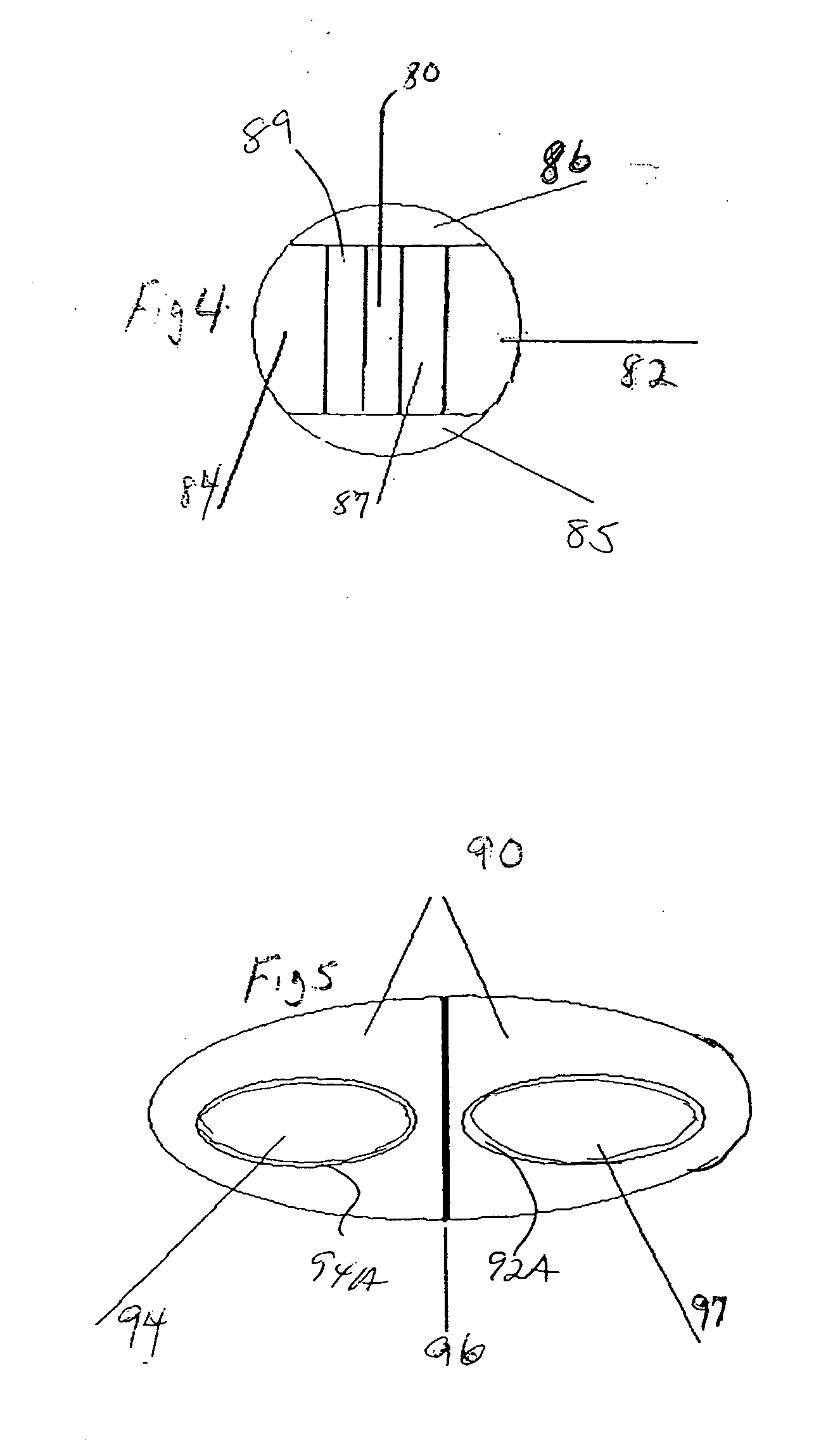
Pharmaceutical dosage form
InactiveUS20130337022A1Reduce releaseReduce deliveryBiocidePeptide/protein ingredientsActive agentMucoadhesion
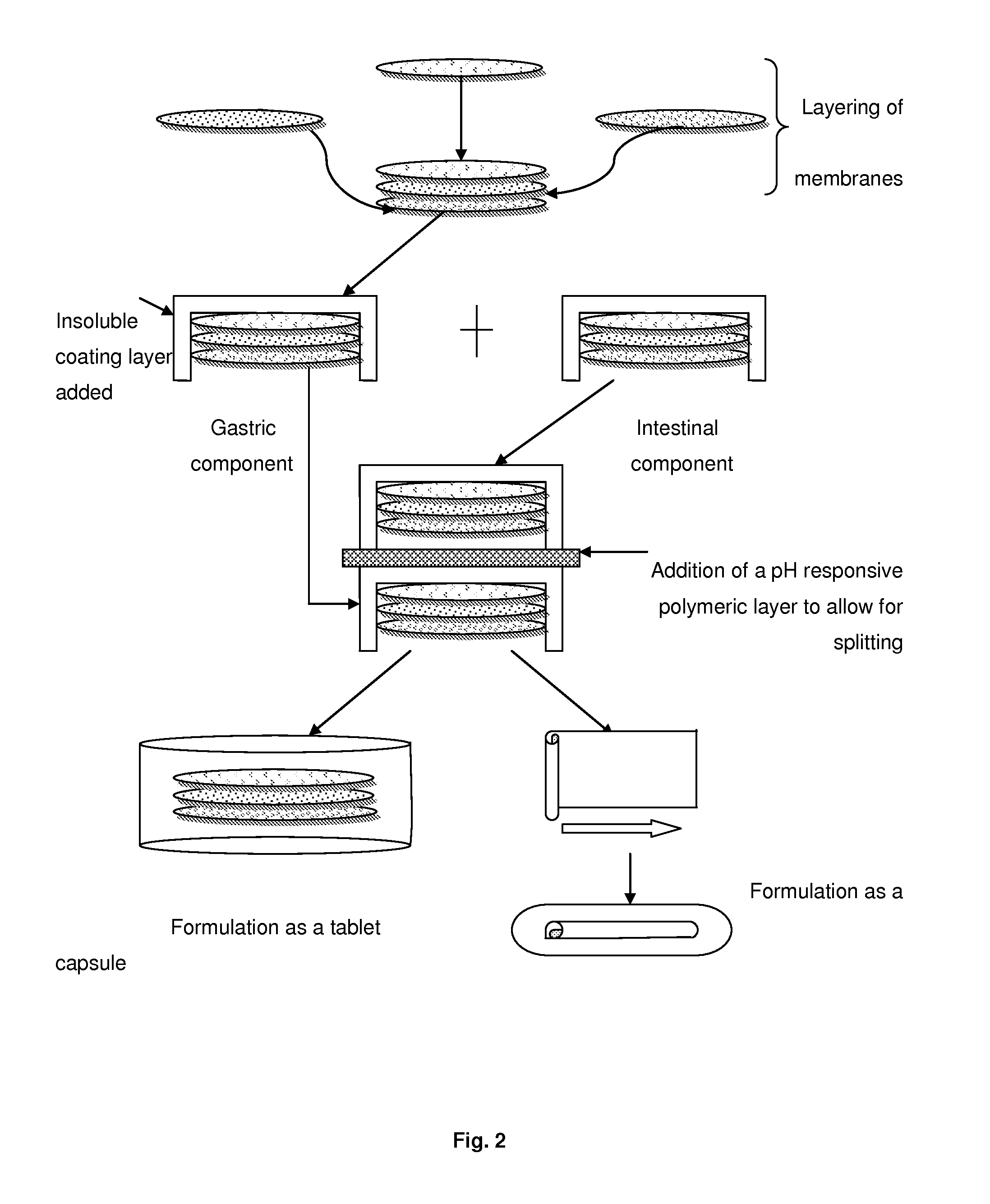
A pH responsive and mucoadhesive pharmaceutical dosage form for the release of a pharmaceutically active agent is described. The dosage form includes a mucoadhesive layer for site-specific mucoadhesion, a water-insoluble outer layer, and an intermediate layer including one or more pharmaceutically active agents for site-specific delivery. The different membranous layers perform different functions in order to create a drug delivery system which is able to deliver a drug to a specific site, for a particular period of time and with a specific drug release pattern. The dosage form can have two or more intermediate layers, each layer comprising an active agent. The mucoadhesive layer can also include an active agents. The dosage form is preferably an oral or buccal delivery form for release of the active agent into the gastro intestinal tract. The intermediate layer can be an electrospun fibrous membrane layer containing the active agent.
Owner:UNIVERSITY OF THE WITWATERSRAND
Storage and dispensing devices for administration of oral transmucosal dosage forms
Owner:VERTICAL PHARMA
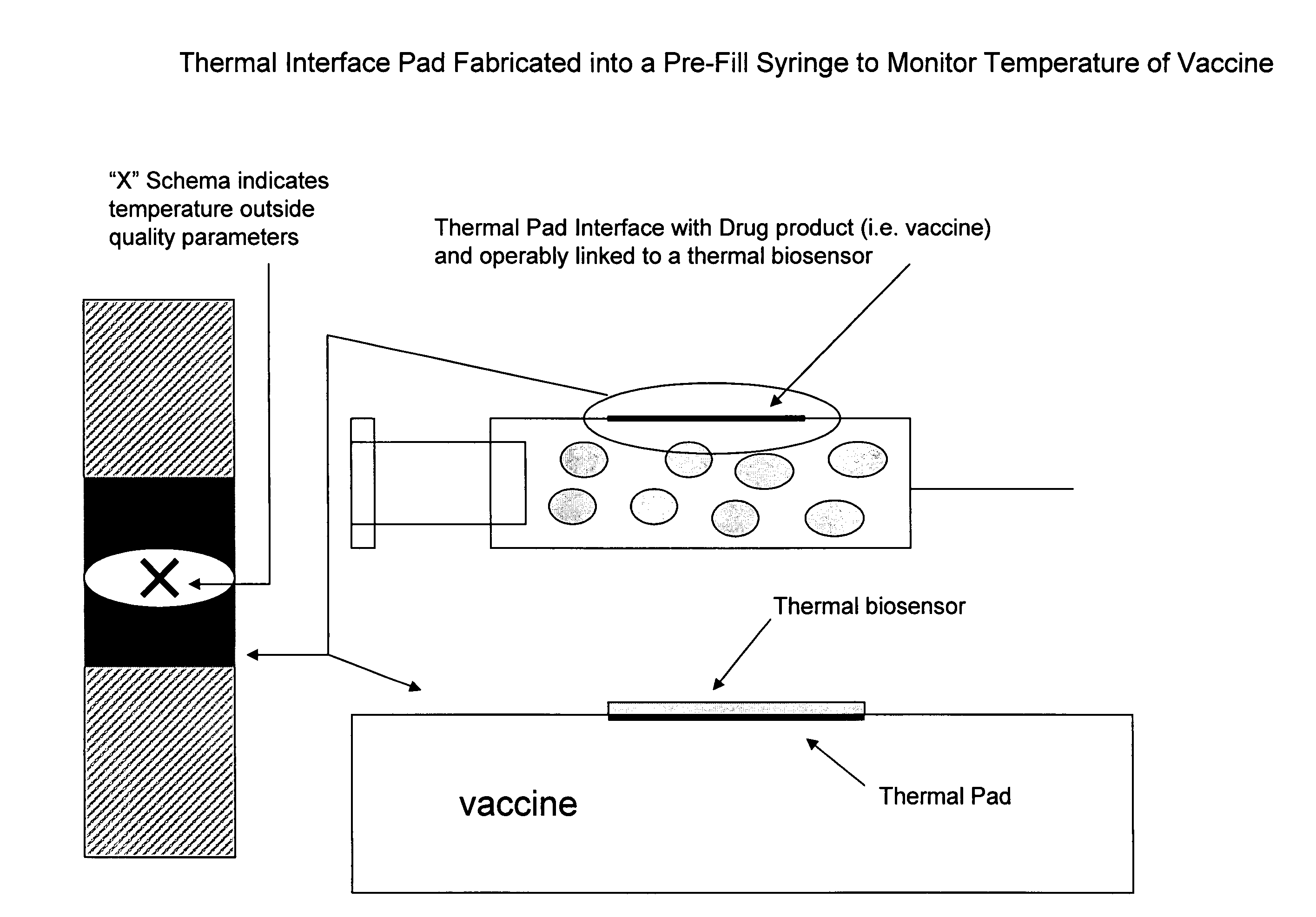
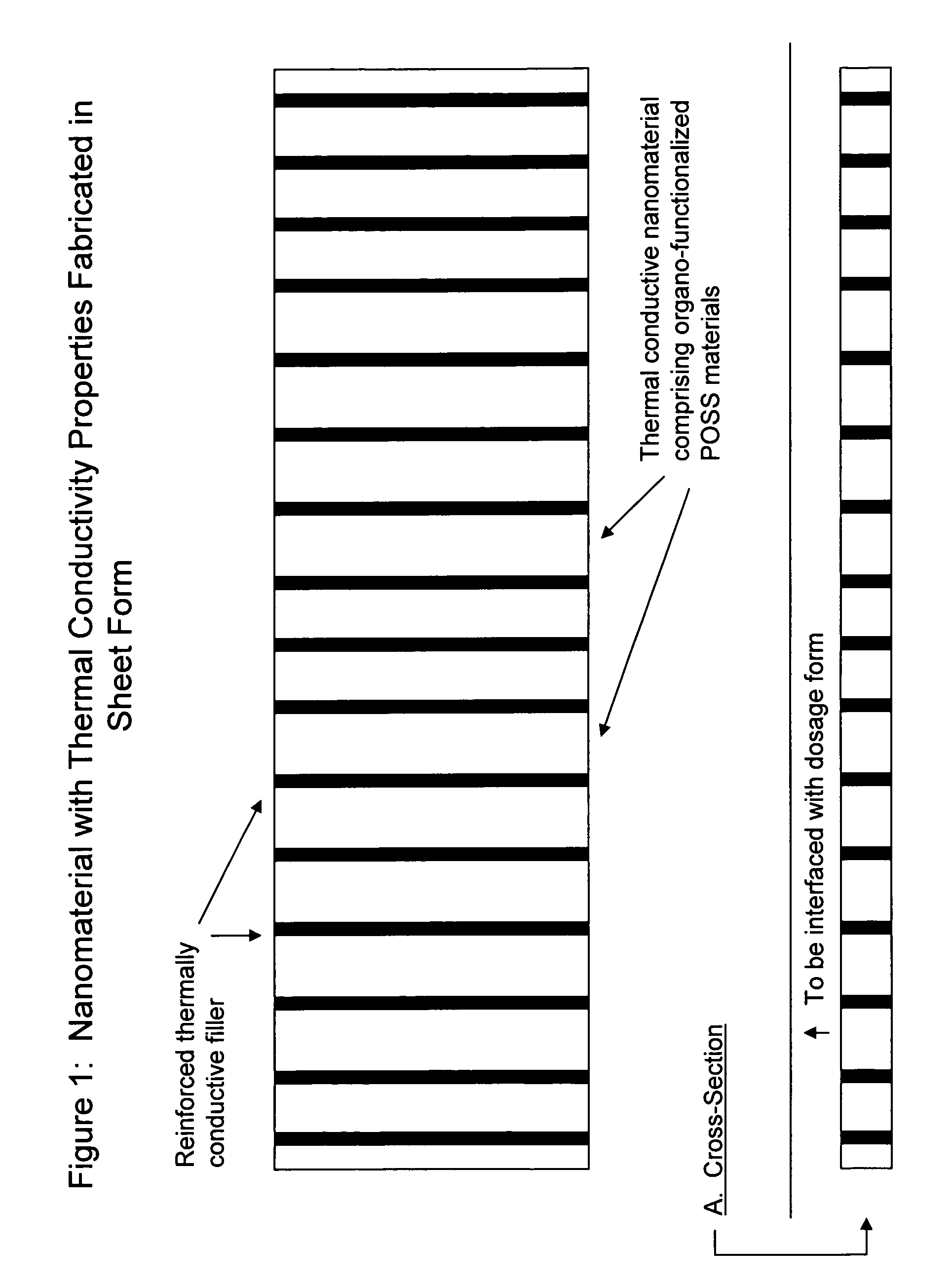
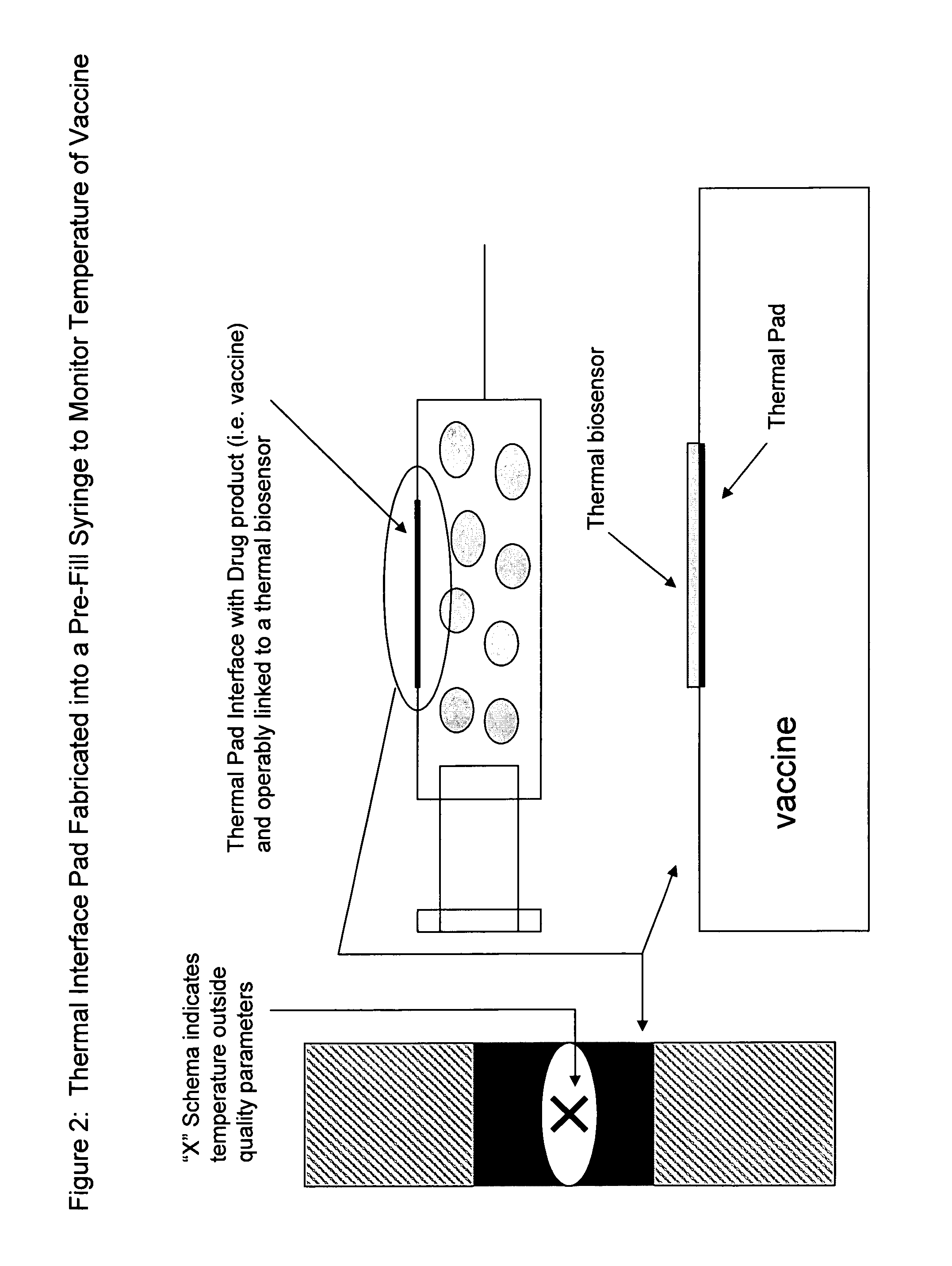
Pharmaceutical dosage forms fabricated with nanomaterials for quality monitoring
InactiveUS20090004231A1Analysis using chemical indicatorsChemical analysis using titrationCold chainMedicine
Nanomaterials fabricated to pharmaceutical dosage forms used to monitor quality of the drug product enclosed therein are disclosed. The nanomaterials are useful to provide a plurality of quality analysis to the dosage form. Consequently, the nanomaterials provide a means to perform quality testing on a continuous basis throughout the supply chain, including the cold chain whereby manufacturers and distributors can achieve greater product integrity and longer shelf life and ultimately minimize cost. The end user benefits in obtaining the highest quality drugs at the time of need.
Owner:SMP LOGIC SYST
Modified release dosage form
The present invention relates to a medicinal dosage form having a first core, a second core, and a shell that surrounds a first portion of each core and a fill material that covers a second portion of at least one core, wherein the fill material that is provided over at least one core is not in contact with any portion of the other core. e. The inventive dosage forms provide modified release of one or more active ingredients contained therein. The present invention also relates to methods for manufacturing such medicinal dosage forms.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Powder inhaler formulations
The present invention relates to new methods for the surface modification of powders. Furthermore the present invention relates to new, improved pharmaceutical dosage forms obtainable by the new methods for surface modification of drugs according to the invention and to the use of these pharmaceutical dosage forms within dry powder inhalation devices (DPI).
Owner:BECHTOLD PETERS KAROLINE +2
Pharmaceutical preparation comprising an active dispersed on a matrix
InactiveUS20070122474A1Improve stabilityUniform deliveryPowder deliveryOrganic non-active ingredientsTriglycerideEngineering
The present invention relates to the field of pharmaceutical technology and describes a novel advantageous preparation for an active ingredient. The novel preparation is suitable for producing a large number of pharmaceutical dosage forms. In the new preparation, an active ingredient is present essentially uniformly dispersed in an excipient matrix composed of one or more excipients selected from the group of fatty alcohols, triglycerides, partial triglycerides and fatty acid esters.
Owner:ASTRAZENECA AB
Tamper resistant dosage forms
ActiveUS20130251799A1Reduces and prevents stickingBiocideNervous disorderPharmaceutical drugPharmaceutical Dose Form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Tamper resistant dosage forms
ActiveUS20130251802A1Reduces and prevents stickingBiocideNervous disorderPharmaceutical drugPharmaceutical Dose Form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Drug delivery systems comprising weakly basic drugs and organic acids
Owner:ADARE PHARM INC
Roflumilast and integrin inhibitor combination and treatement method
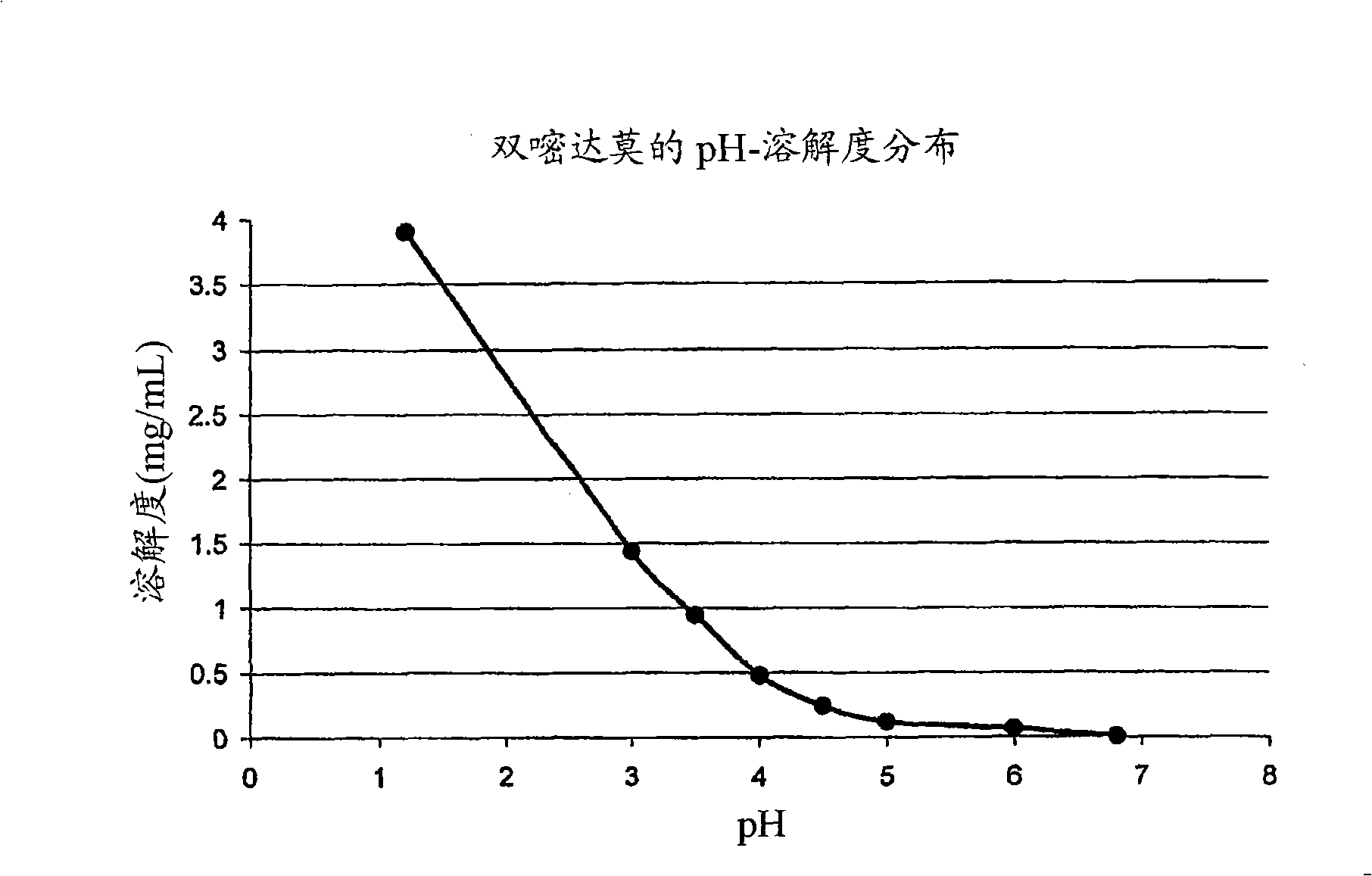
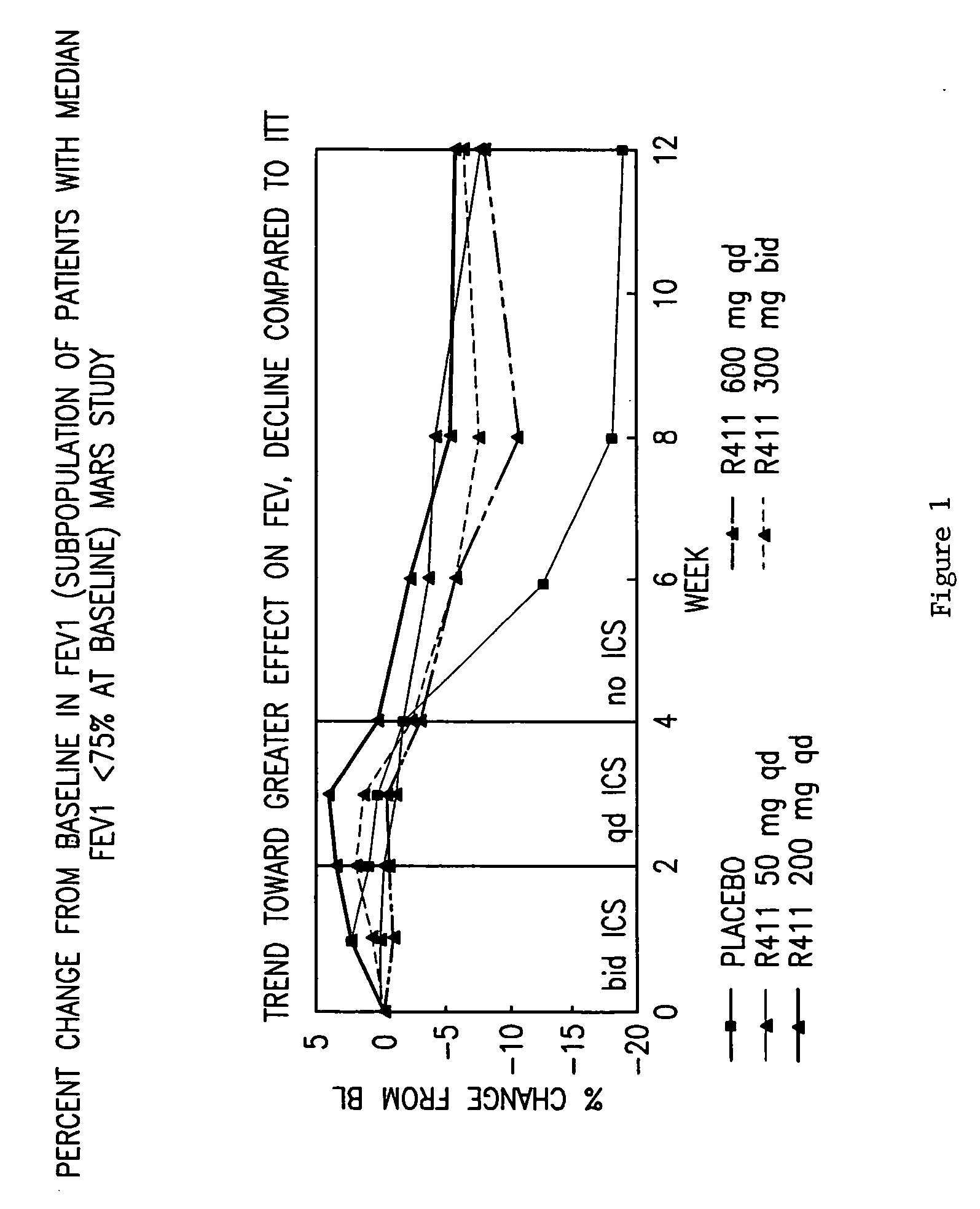
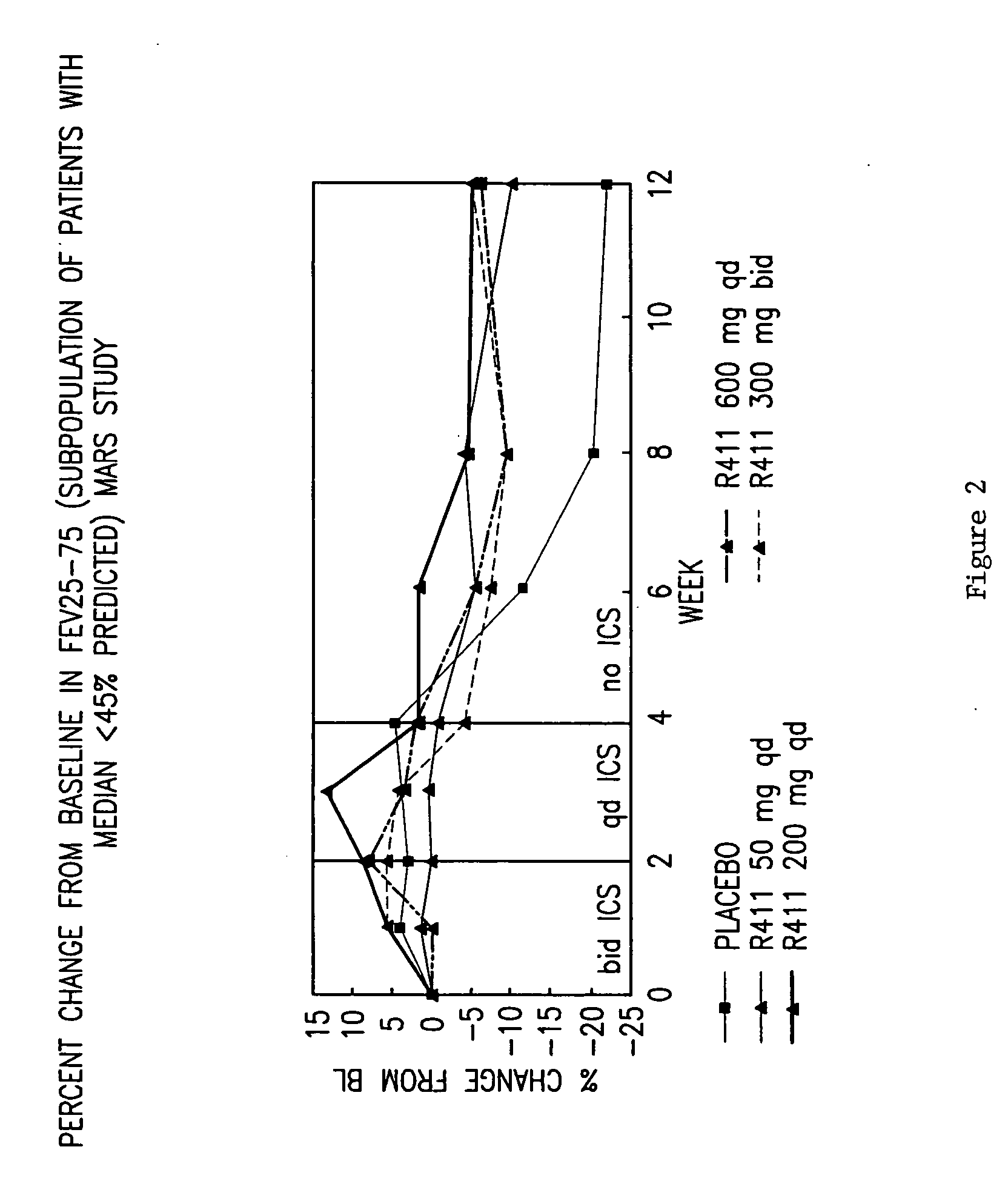
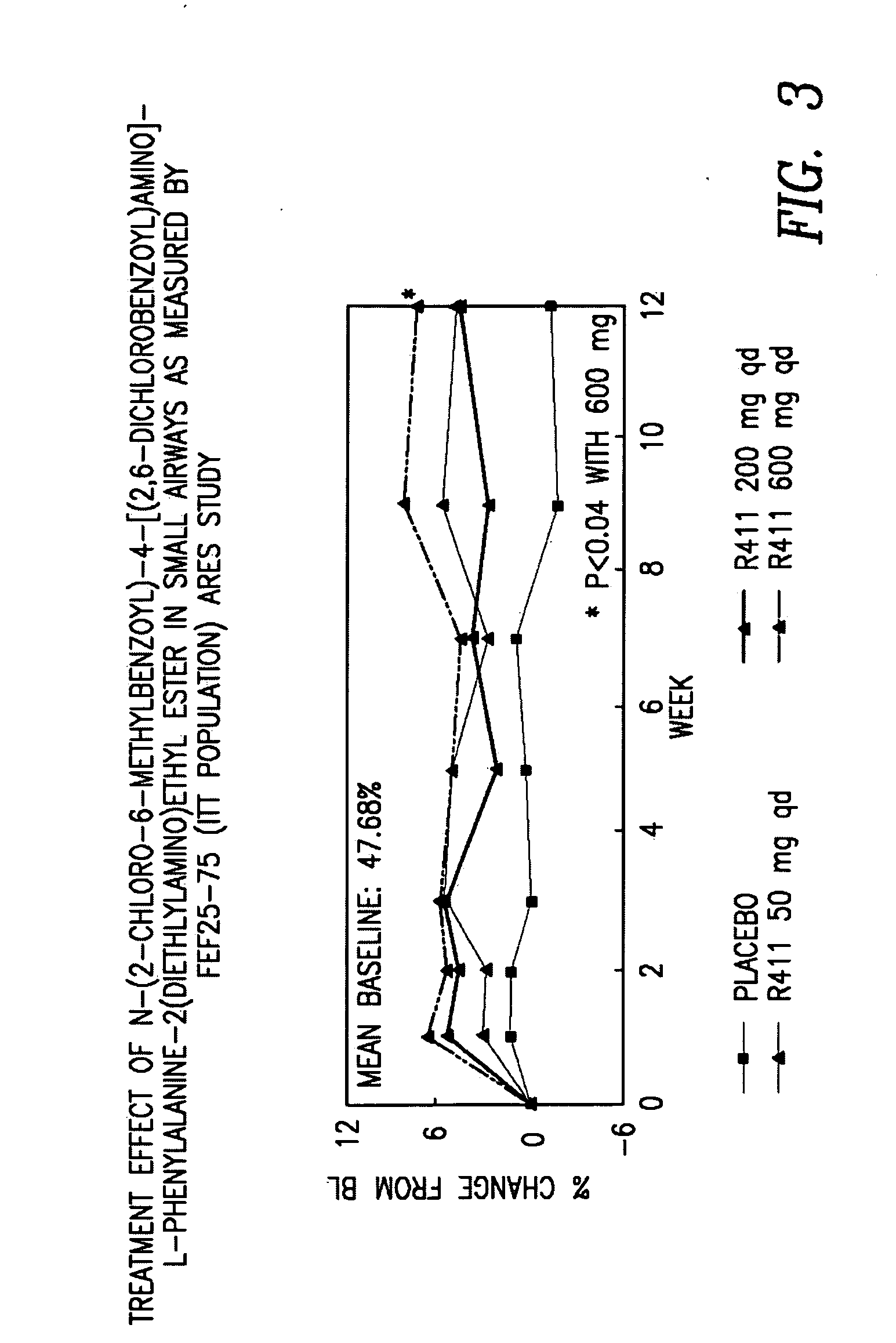
InactiveUS20060198889A1Cell receptors/surface-antigens/surface-determinantsDipeptide ingredientsOral medicationEthyl ester
The present invention provides novel solid pharmaceutical dosage forms for oral administration comprising a therapeutically active amount of roflumilast, or a pharmaceutically acceptable salt thereof, a therapeutically effective amount of N-(2-chloro-6-methylbenzoyl)-4-[(2,6-dichlorobenzoyl)amino]-L-phenylalanine-2-(diethylamino)ethyl ester, or a pharmaceutically acceptable thereof, and one or more pharmaceutically acceptable excipients. These novel solid pharmaceutical dosage forms are useful in the treatment or control of asthma. The present invention also provides a method for treating asthma employing the solid pharmaceutical dosage forms and a method for preparing the pharmaceutical dosage forms.
Owner:SANDHU HARPREET K +1
Solid pharmaceutical formulations comprising BIBW 2992
ActiveUS8545884B2Improve liquidityReduce frictionPowder deliveryOrganic active ingredientsCoated tabletsImmediate release
Owner:BOEHRINGER INGELHEIM INT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com