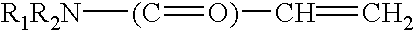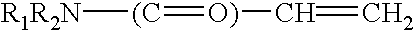Patents
Literature
248 results about "Mucoadhesion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
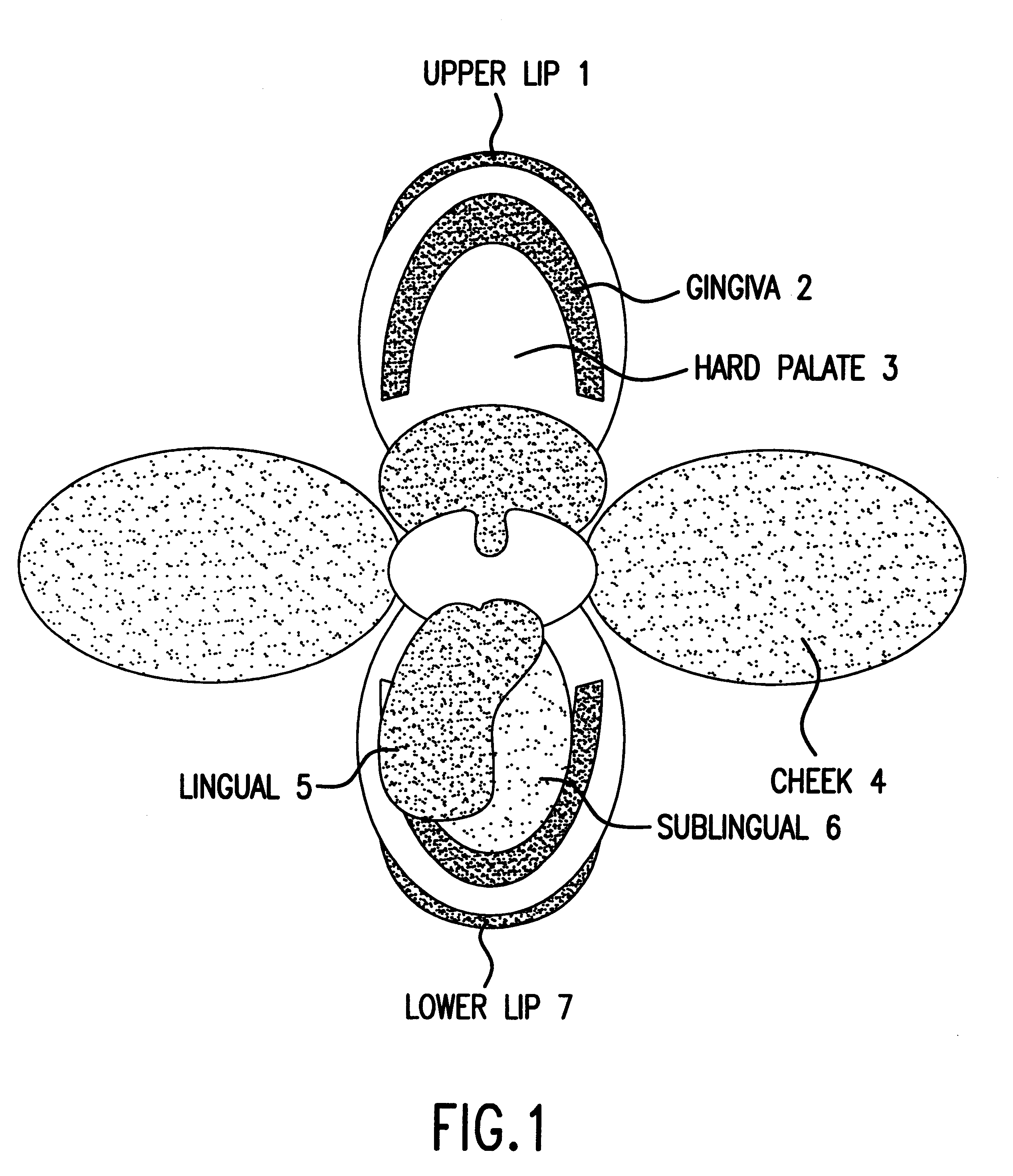
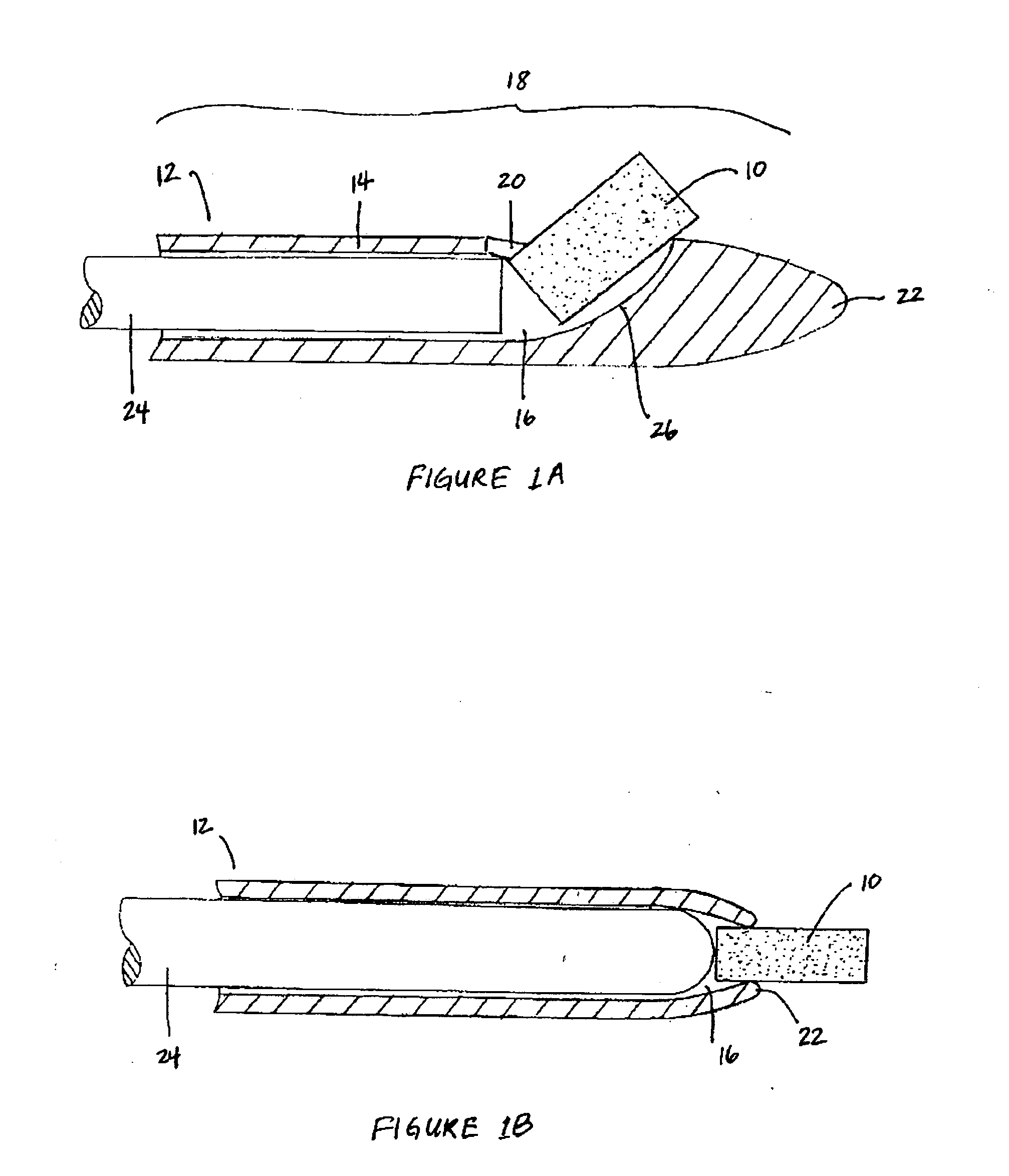
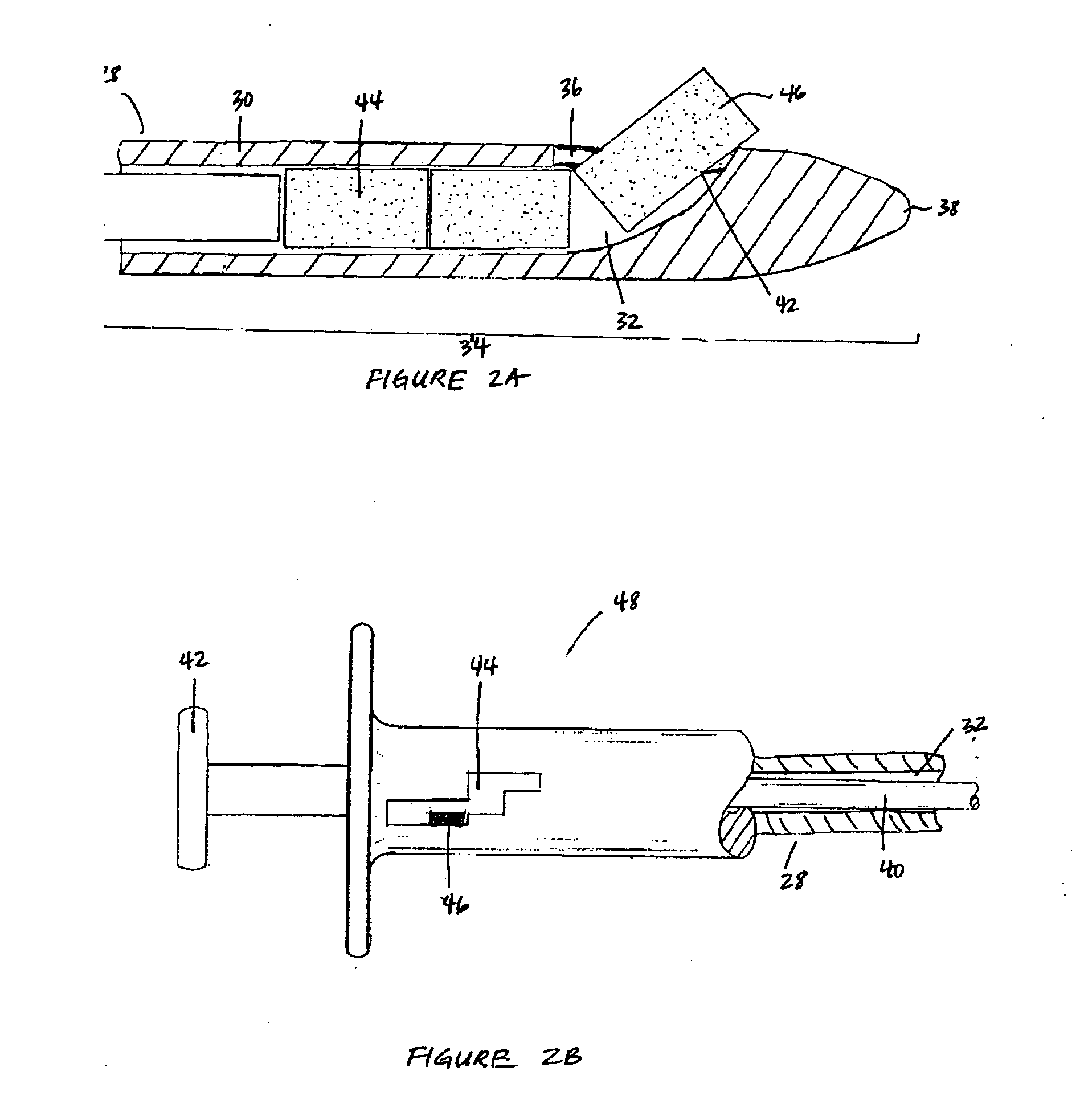
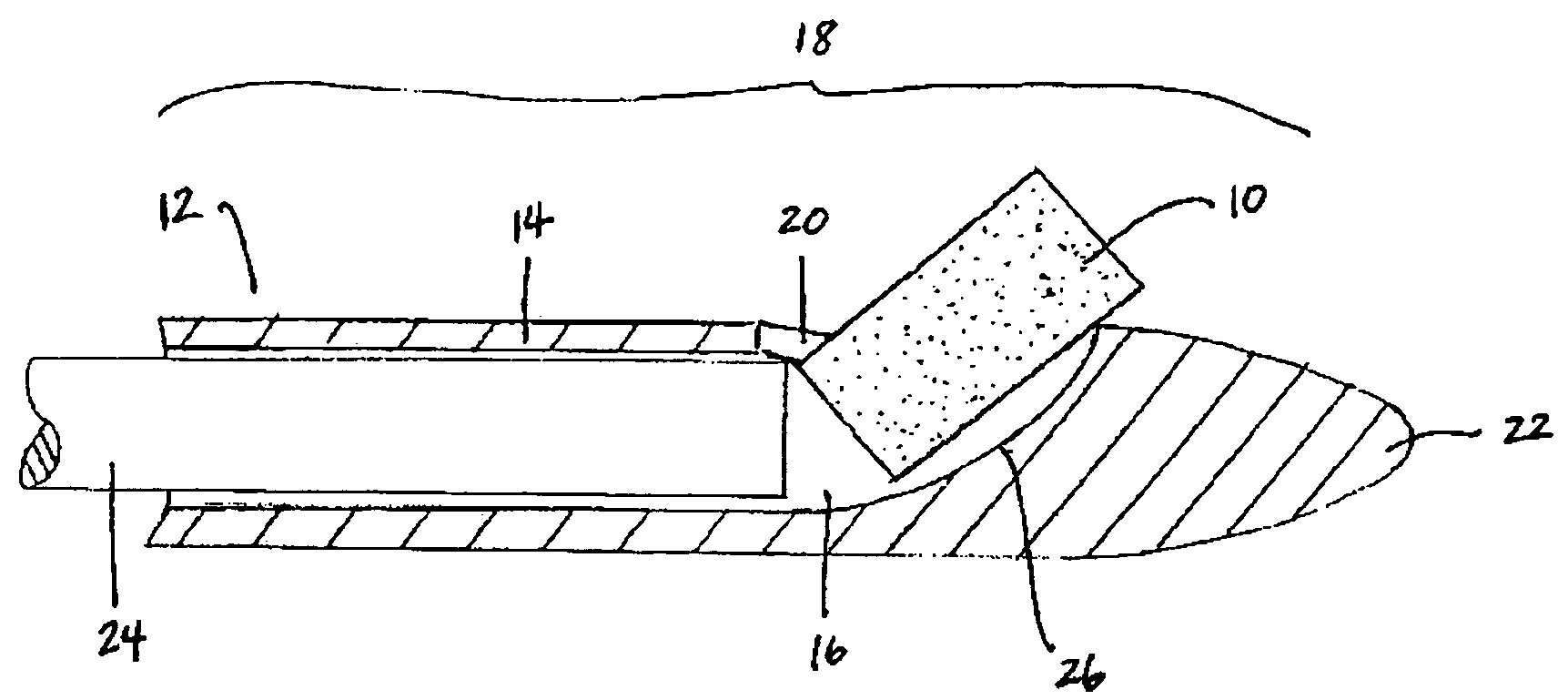
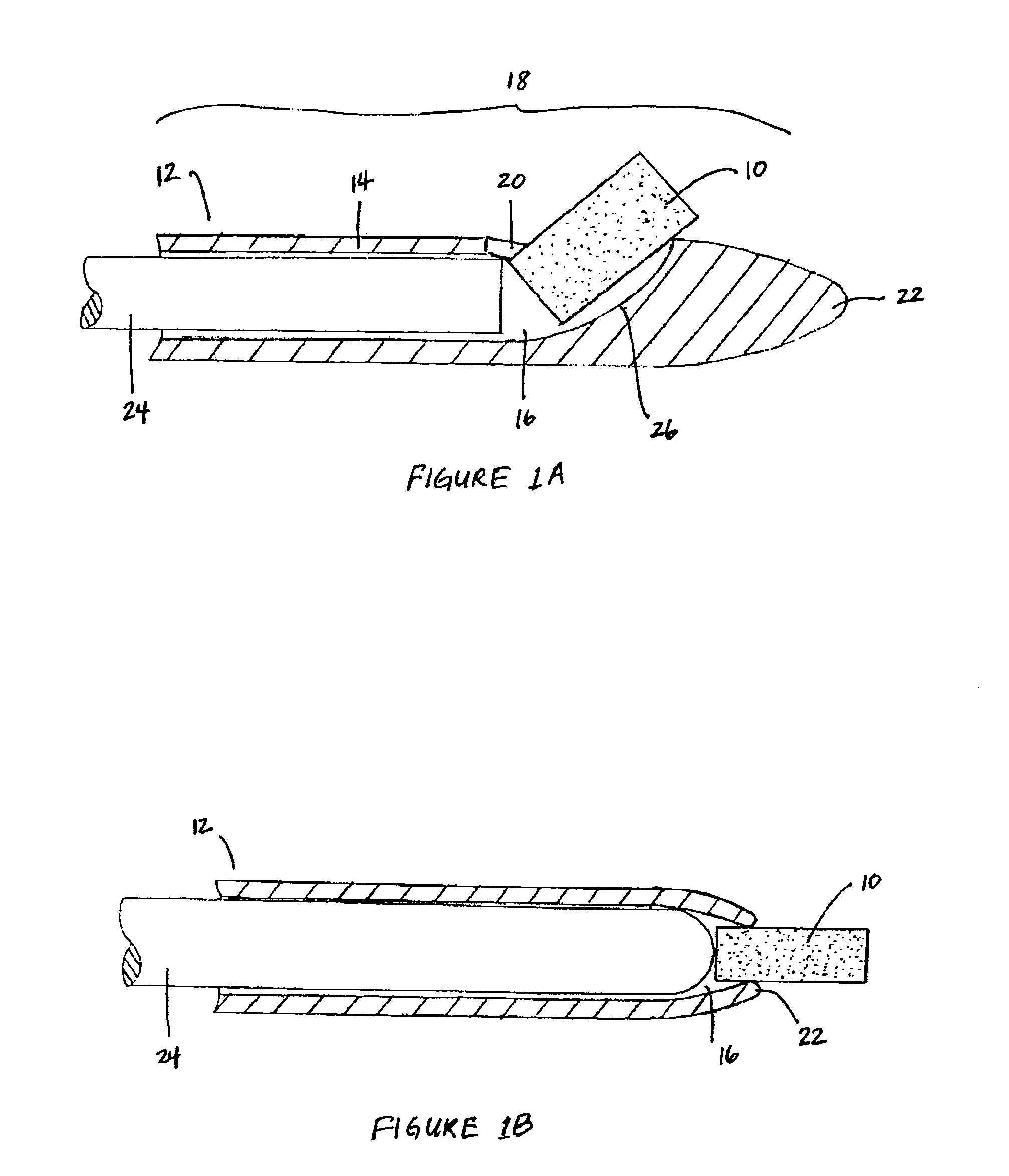
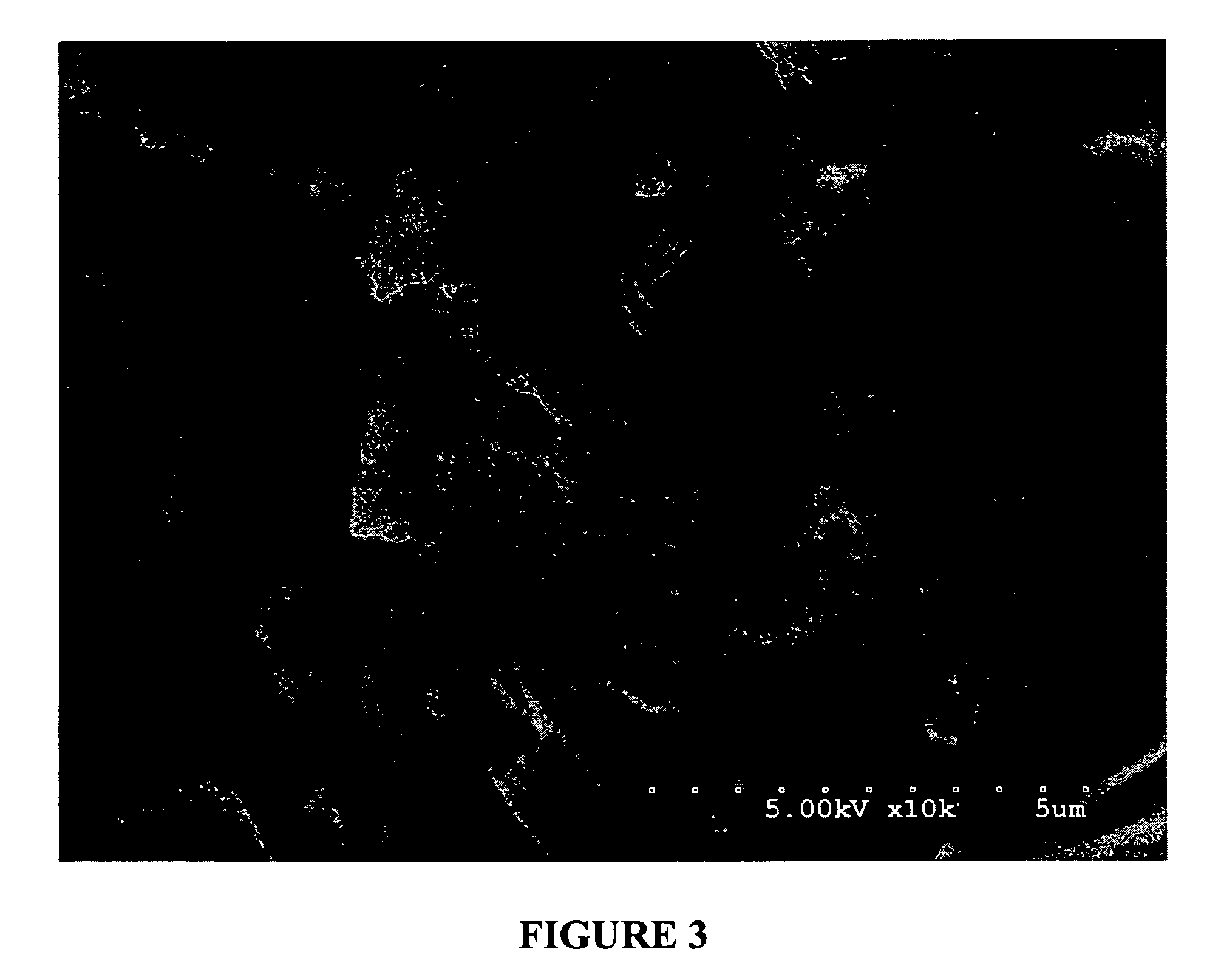
Bioadhesion is the mechanism by which two biological materials are held together by interfacial forces. When relating this mechanism to the pharmaceutical sciences, mucoadhesion describes the attractive forces between a biological material and mucus or mucous membrane. Mucous membranes adhere to epithelial surfaces such as the gastrointestinal tract (GI-tract), the vagina, the lung, the eye, etc. They are generally hydrophilic as they contain many hydrogen macromolecules due to the large amount of water (approximately 95%) within its composition. However, mucin also contains glycoproteins that enable the formation of a gel-like substance. Understanding the hydrophilic bonding and adhesion mechanisms of mucus to biological material is of utmost importance in order to produce the most efficient applications. For example, in drug delivery systems, the mucus layer must be penetrated in order to effectively transport micro- or nanosized drug particles into the body.
Compositions and methods for mucosal delivery
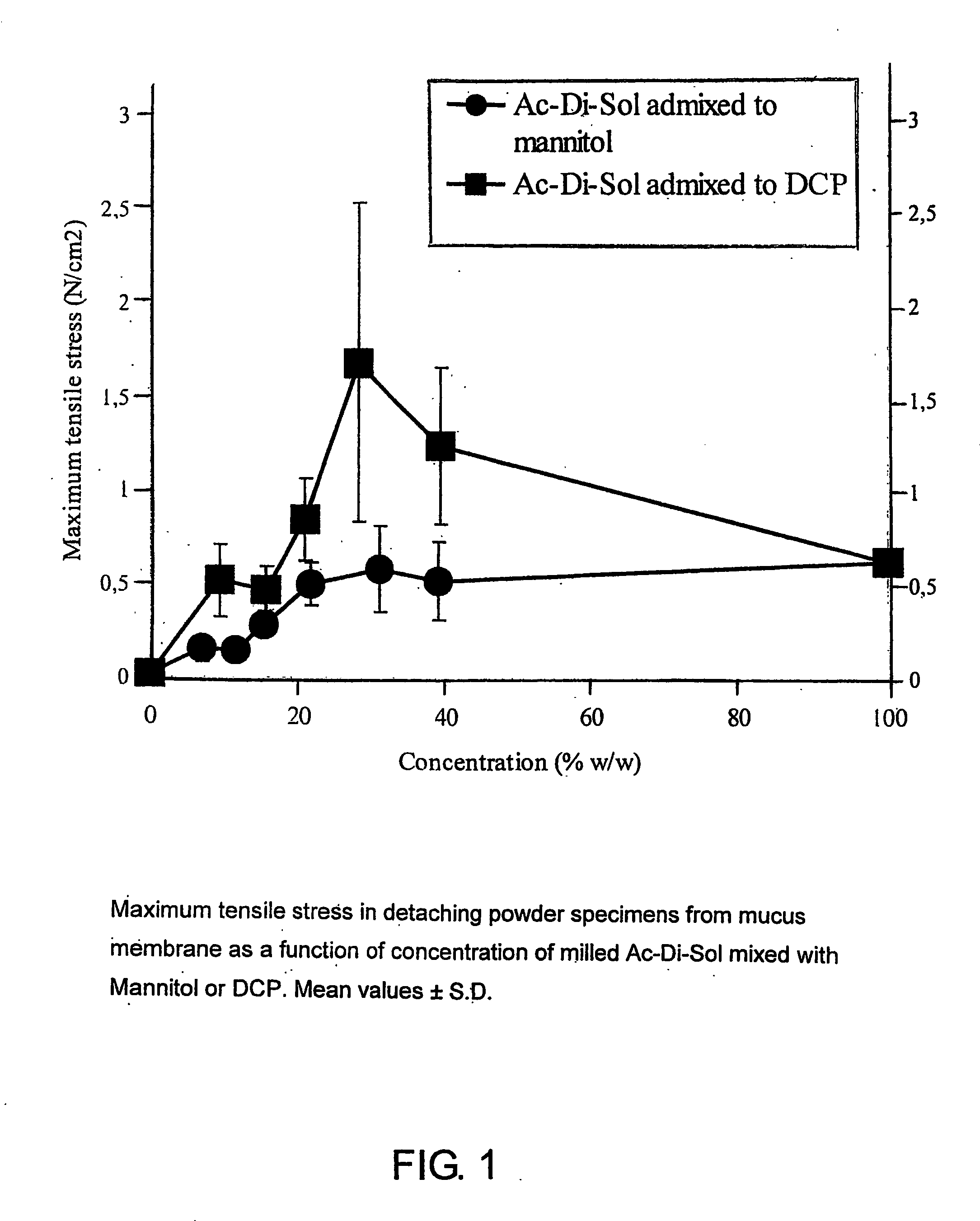
Mucosal surface-coat-forming film dosage units containing a water-soluble hydrocolloid, an effective dose of an active agent and a mucosal adhesion enhancer; wherein the active agent is encapsulated within a polymer which is chemically or physically distinct from the hydocolloid; wherein the mucosal adhesion enhancer is a starch graft copolymer; wherein the film exhibits a dry tack value of less than 3.5 g, a wet tack of greater than 35 g, a gelation temperature that is greater than 70° C. for a 2% polymer solution, a dry film thickness of less than 20 mil, a water content of 0.5 to 10%, a tensile strength greater than 1500 psi, a modulus in the range of 35,000 to 300,000 psi, a % elongation of less than 20%, a tear probagation resistance of 0.001 to 1 N, and a dissolution time in the range of 1 to 600 seconds upon application to an oral mucosal surface.
Owner:THALLIUM HLDG CO LLC
Coated vaginal devices for vaginal delivery of therapeutically effective and/or health-promoting agents
A vaginal device for delivering therapeutical and / or health-promoting agents. The vaginal device partly or completely coated by, covered by or combined with a coating or covering comprising a film, foam, strip, cap, cup or particles. The coating of the device comprises a mucoadhesive composition comprising a therapeutical and / or health-promoting agent.
Owner:UNIVERSITY OF MINNESOTA DULUTH
Compositions and methods relating to reduced mucoadhesion
InactiveUS20120121718A1Reduced mucoadhesionDiffusion fastOrganic active ingredientsPowder deliveryMedicineParticle composition
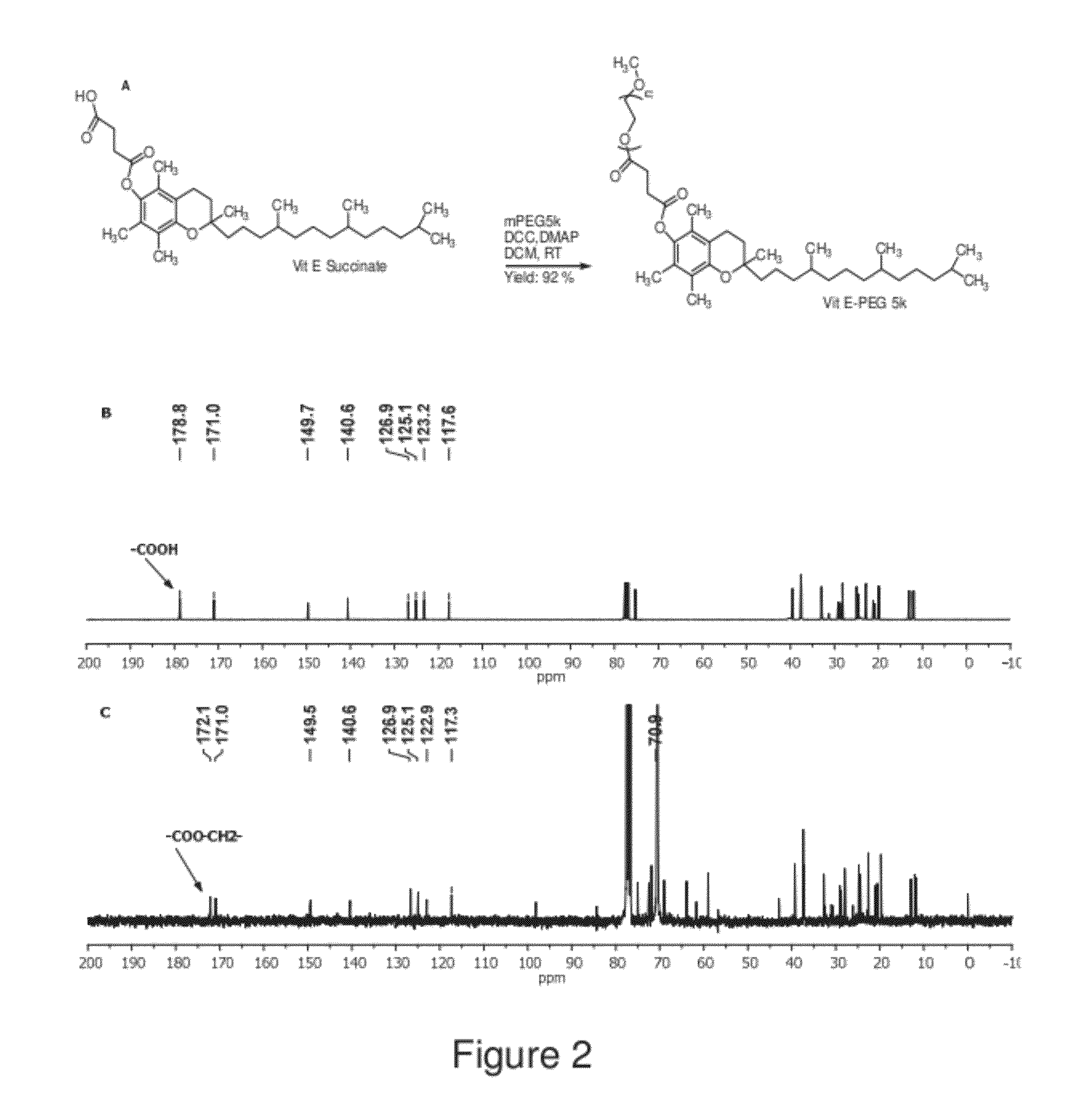
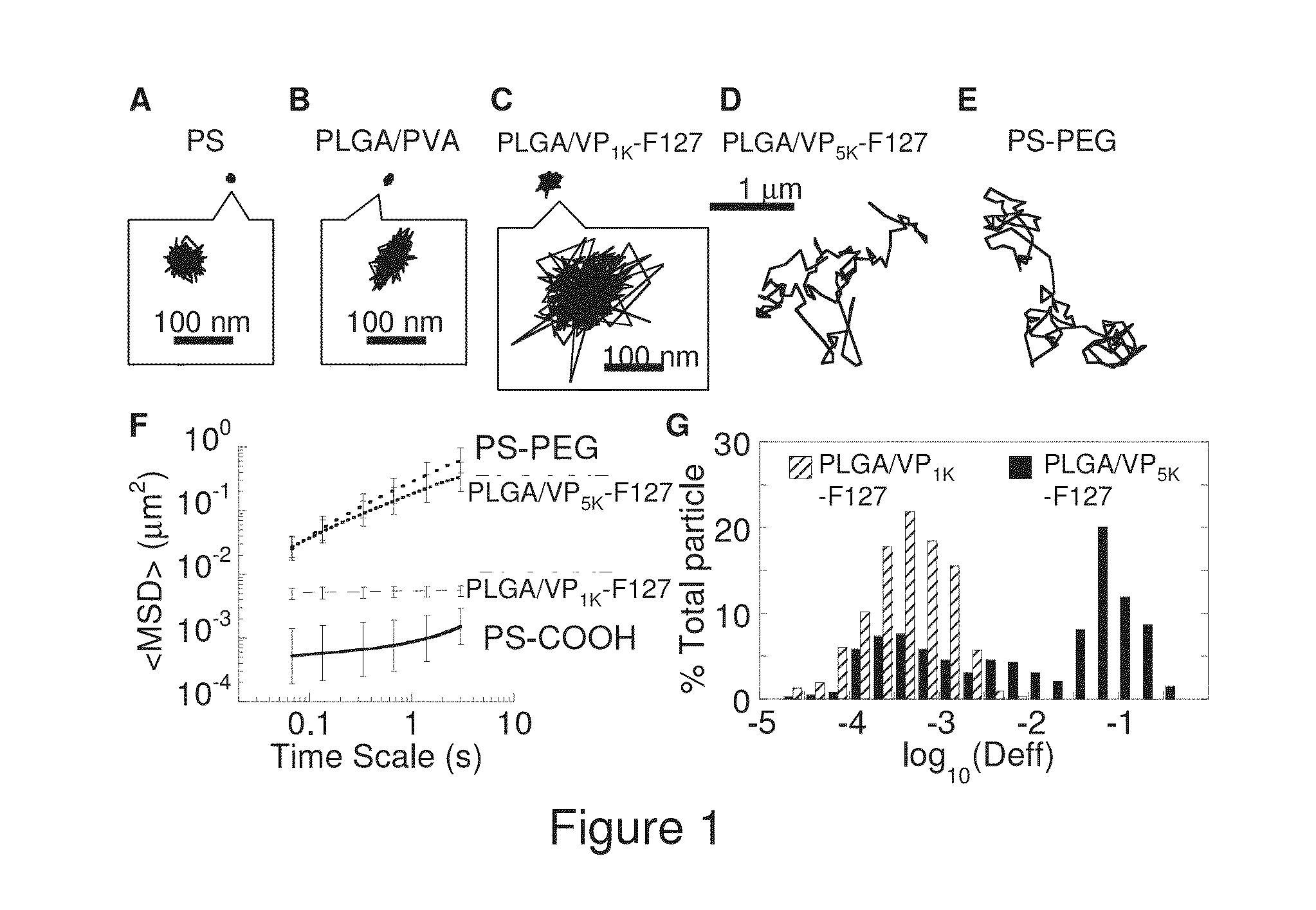
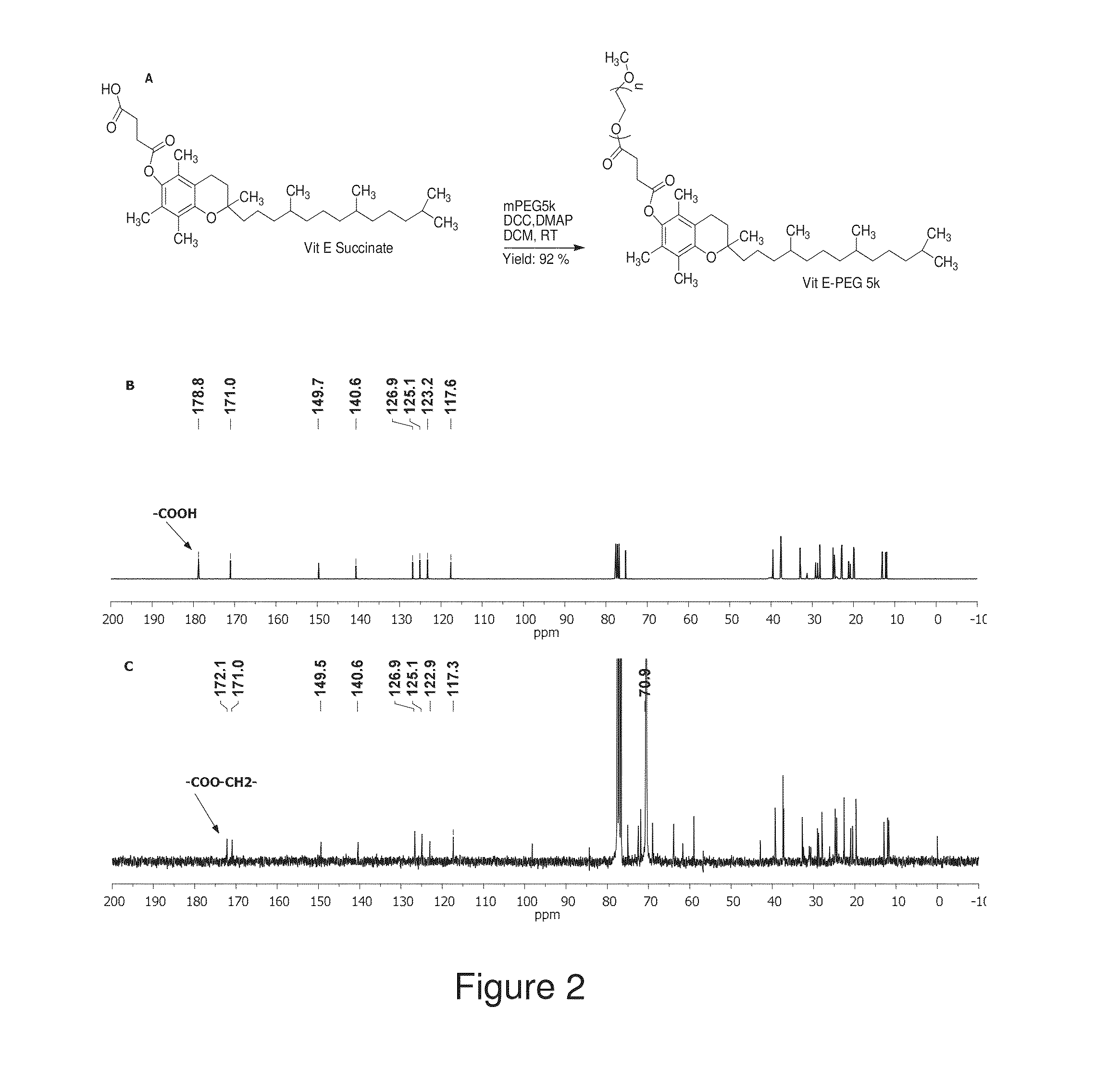
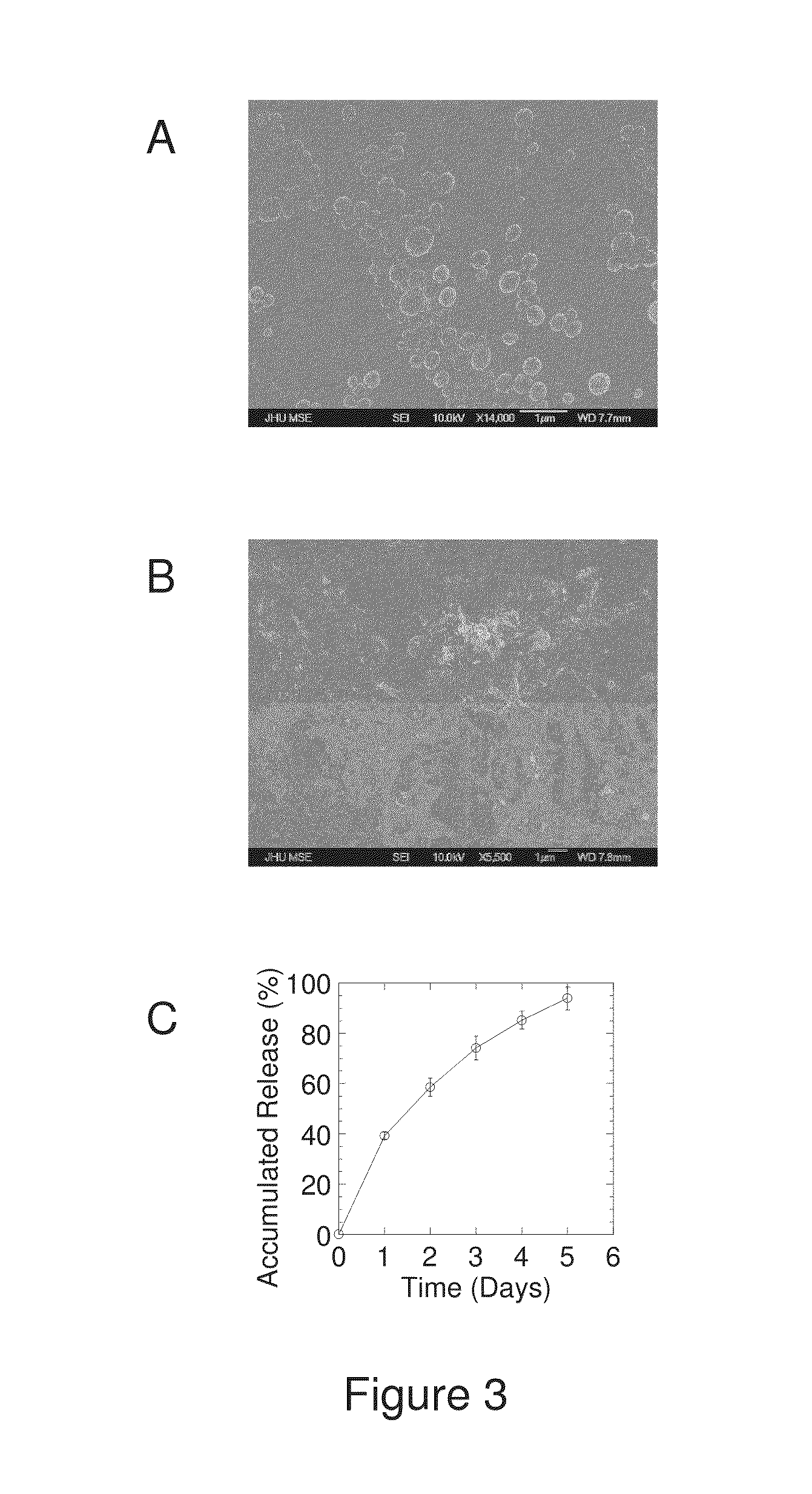
The present invention generally relates to reducing the mucoadhesive properties of a particle. In some embodiments, the particle is coated with and / or associated with a (poly(ethylene glycol))-(poly(propylene oxide))-(poly(ethylene glycol)) triblock copolymer. Methods for preparing inventive particles using a poly(ethylene glycol)-vitamin E conjugate as a surfactant are also provided. In some embodiments, methods are provided comprising administering to a subject a composition of particles of the present invention. Such particles with reduced mucoadhesive properties are useful in delivering agents to mucosal tissues such as oral, ophthalmic, gastrointestinal, nasal, respiratory, and genital mucosal tissues.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
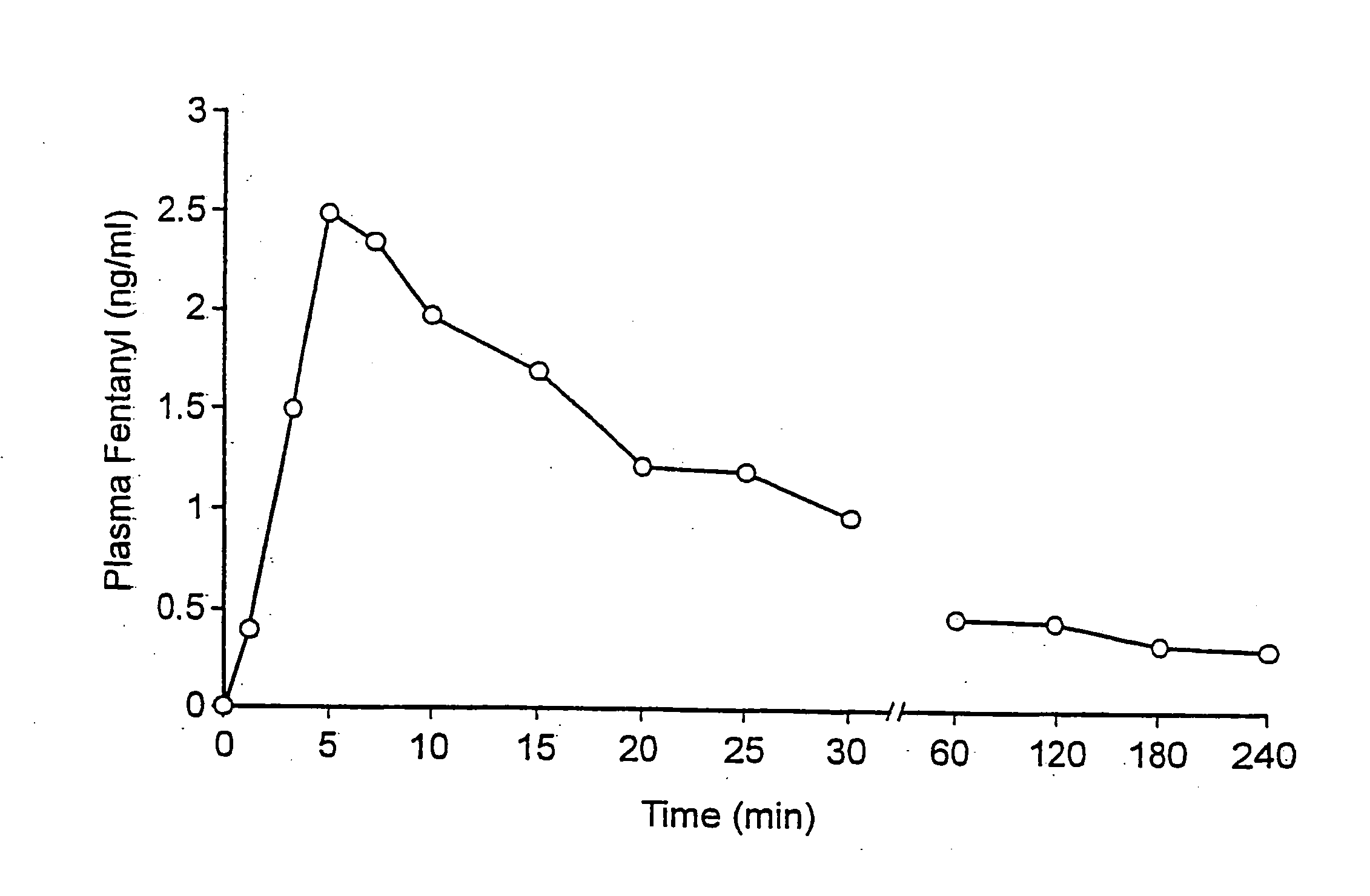
Fentanyl composition for the treatment of acute pain
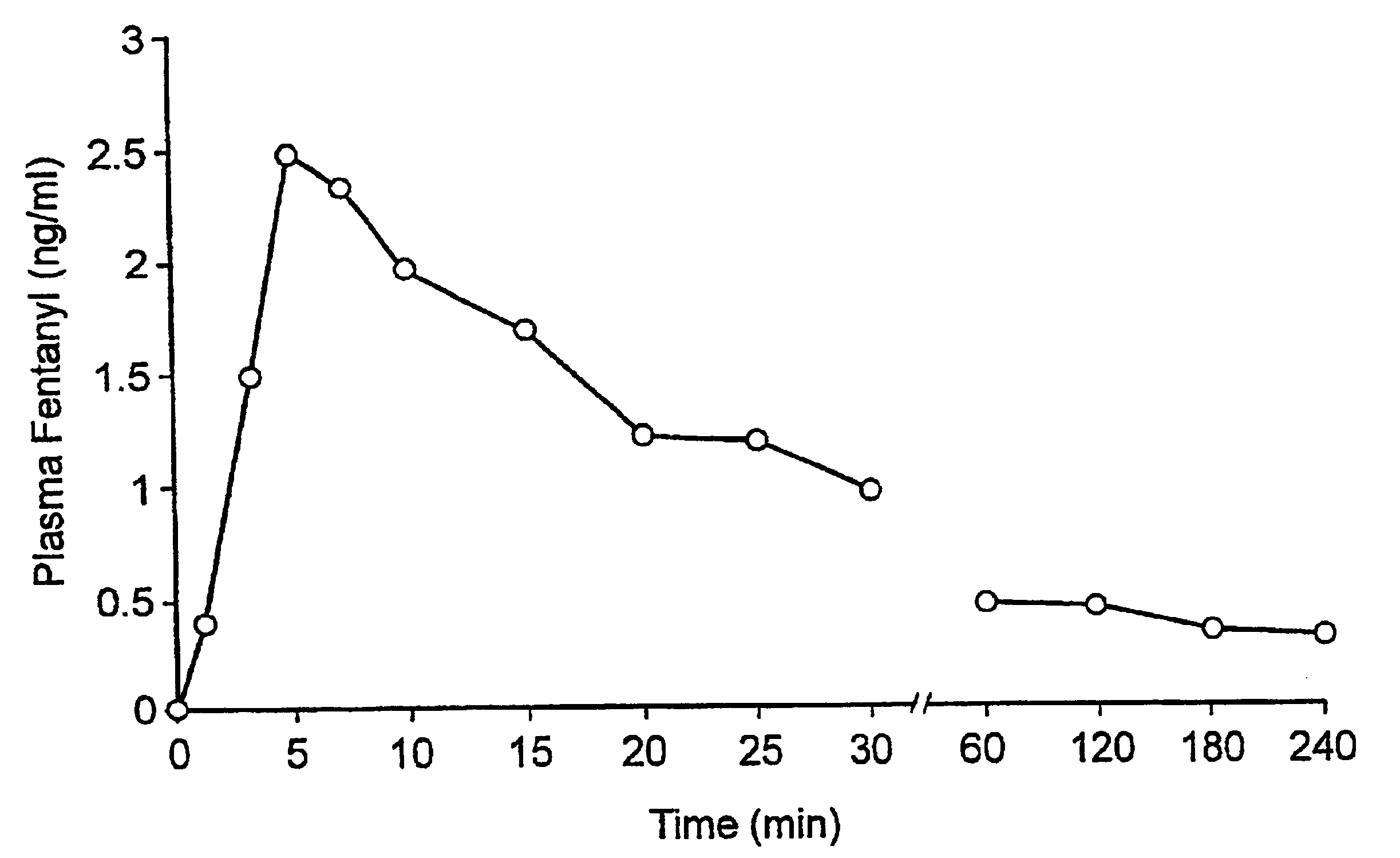
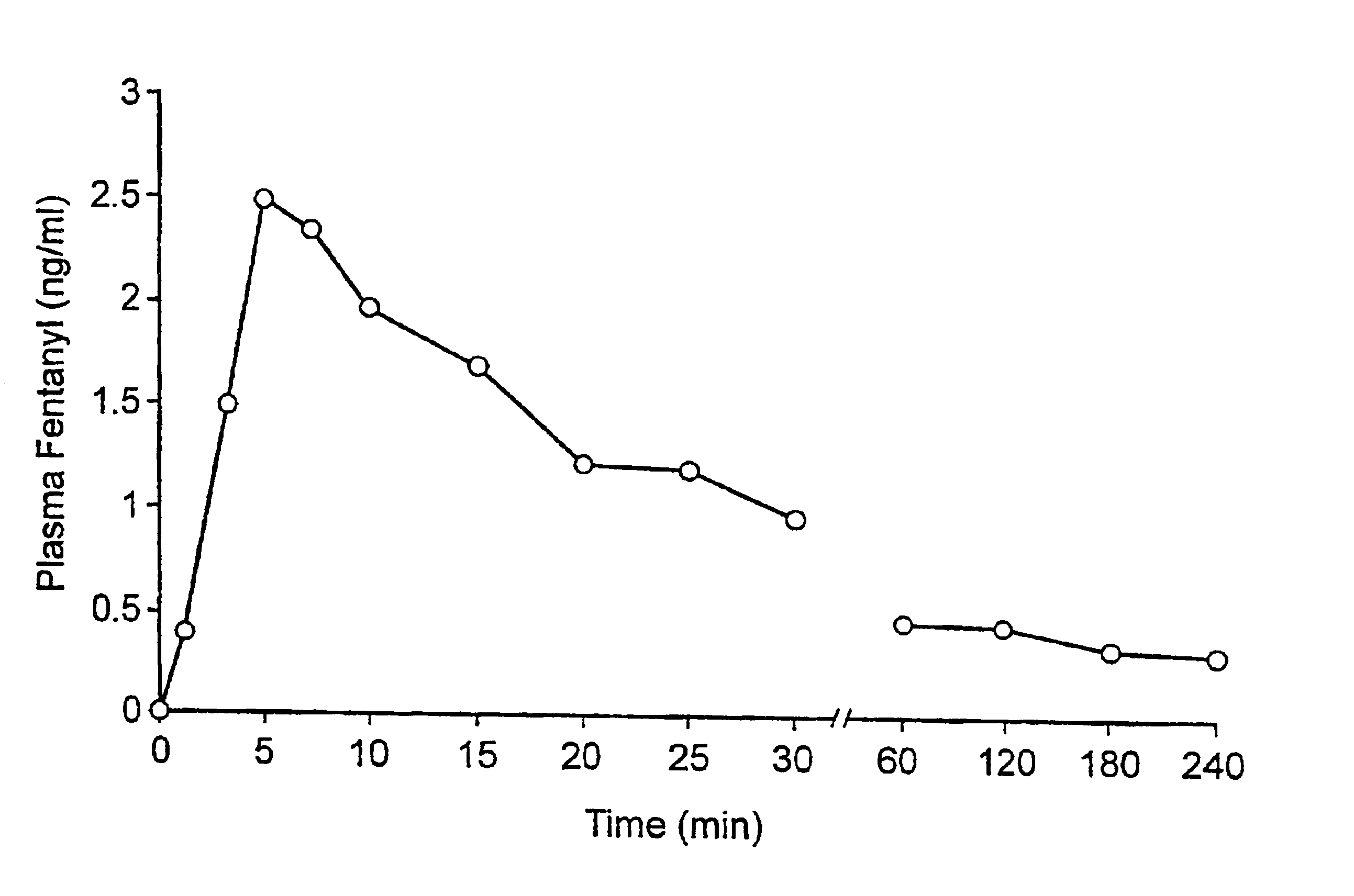
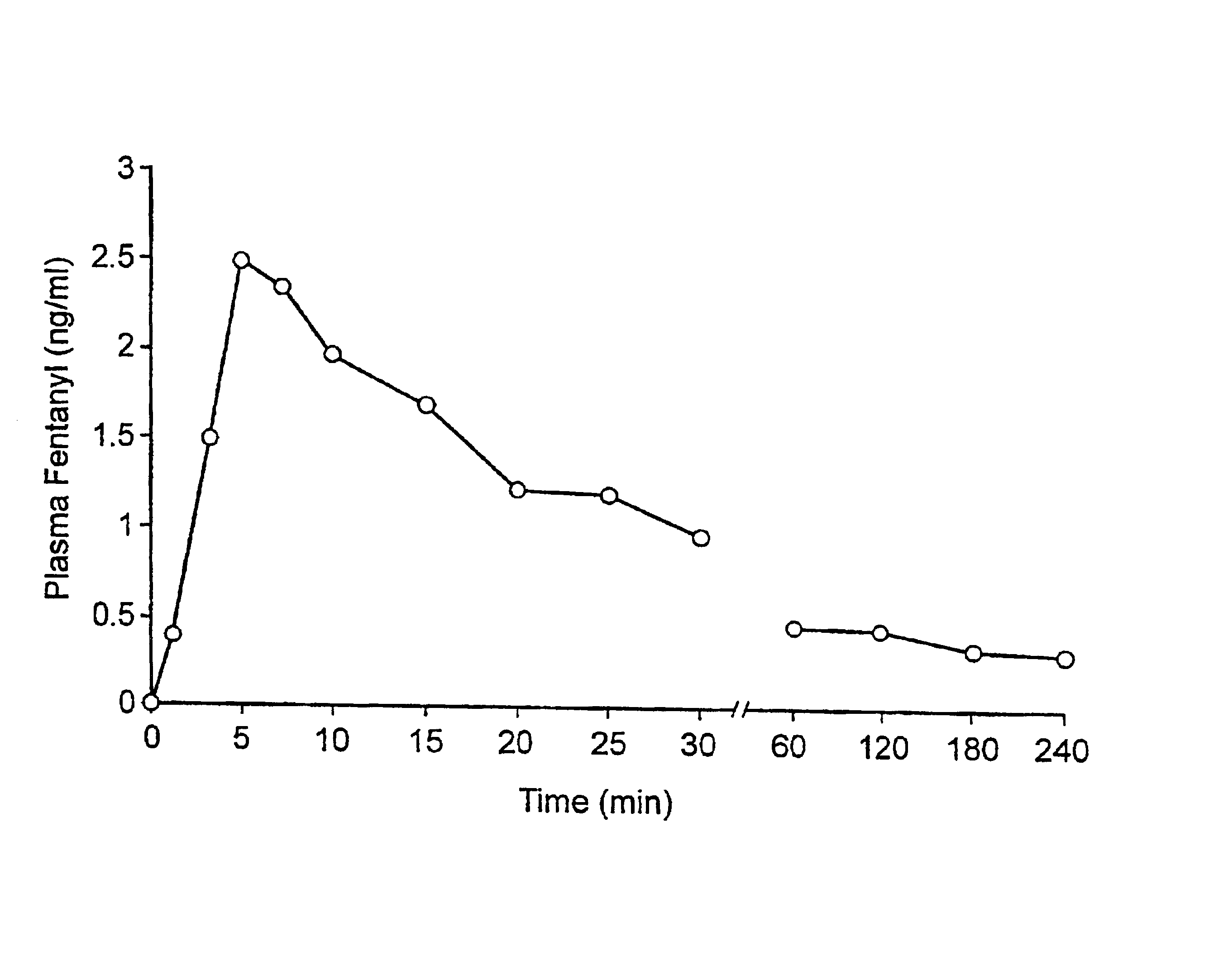
A pharmaceutical composition for the treatment of acute pain by sublingual administration is described. The composition comprises an essentially water-free, ordered mixture of fentanyl or a pharmaceutically acceptable salt thereof in the form of microparticles which are adhered to the surface of carrier particles which are substantially larger than the particles of fentanyl, and are essentially water-soluble. In a preferred embodiment, the composition also contains a bioadhesion and / or mucoadhesion promoting agent. The invention also relates to the preparation of the composition, and to the use of the composition for the treatment of acute pain.
Owner:DIABAKT AB
Oral transmucosal delivery of drugs or any other ingredients via the inner buccal cavity
InactiveUS6210699B1Avoid irritationAvoid the tasteAdhesive dressingsPill deliverySide effectAdditive ingredient
A device and method for the oral transmucosal delivery of active substances to the oral cavity utilizing an unplasticized polyvinyl pyrrolidone polymer (PVP) as the primary mucoadhesive. The device is applied and adheres to the mucosa of the oral cavity without causing side effects or leaving an unpleasant taste. Preferably the device is a bilayer tablet having a mucoadhesive layer and an overlying active substance containing layer. The mucoadhesive layer may contain PVP as the only adhesive or may be combined with other hydrophilic polymeric substances. The active layer also contains a hydrophilic polymer carrier. The layers in the device dissolve and release the active substance to the oral cavity and is particularly adapted for the delivery of substances active in the oral cavity such as breath fresheners and substances to combat dry mouth. It is also useful for the delivery of ionic drugs such as peptides.
Owner:WATSON PHARMA INC +1
Sinus delivery of sustained release therapeutics
ActiveUS20050043706A1Easy accessReduce inflammationMedical devicesPharmaceutical delivery mechanismSinusitisMicroparticle
The invention provides biodegradable implants for treating sinusitis. The biodegradable implants have a size, shape, density, viscosity, and / or mucoadhesiveness that prevents them from being substantially cleared by the mucociliary lining of the sinuses during the intended treatment period. The biodegradable implants include a sustained release therapeutic, e.g., an antibiotic, a steroidal anti-inflammatory agent, or both. The biodegradable implants may take various forms, such as rods, pellets, beads, strips, or microparticles, and may be delivered into a sinus in various pharmaceutically acceptable carriers.
Owner:INTERSECT ENT INC
Sinus delivery of sustained release therapeutics
The invention provides biodegradable implants for treating sinusitis. The biodegradable implants have a size, shape, density, viscosity, and / or mucoadhesiveness that prevents them from being substantially cleared by the mucociliary lining of the sinuses during the intended treatment period. The biodegradable implants include a sustained release therapeutic, e.g., an antibiotic, a steroidal anti-inflammatory agent, or both. The biodegradable implants may take various forms, such as rods, pellets, beads, strips, or microparticles, and may be delivered into a sinus in various pharmaceutically acceptable carriers.
Owner:INTERSECT ENT INC
Film-Shaped Mucoadhesive Administration Forms For Administering Cannabis Agents
InactiveUS20060039959A1Improving acceptance and complianceQuick effectOrganic active ingredientsBiocideCannabisActive agent
A film-shaped, mucoadhesive administration form having a content of at least one active agent. The active agent is a cannabis agent.
Owner:LTS LOHMANN THERAPIE-SYST AG
Compositions and methods relating to reduced mucoadhesion
InactiveUS20130236556A1Reduced mucoadhesionDiffusion fastBiocidePowder deliveryMedicineParticle composition
The present invention generally relates to reducing the mucoadhesive properties of a particle. In some embodiments, the particle is coated with and / or associated with a (poly(ethylene glycol))-(poly(propylene oxide))-(poly(ethylene glycol)) triblock copolymer. Methods for preparing inventive particles using a poly(ethylene glycol)-vitamin E conjugate as a surfactant are also provided. In some embodiments, methods are provided comprising administering to a subject a composition of particles of the present invention. Such particles with reduced mucoadhesive properties are useful in delivering agents to mucosal tissues such as oral, ophthalmic, gastrointestinal, nasal, respiratory, and genital mucosal tissues.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Pharmaceutical composition for the treatment of acute disorders
A pharmaceutical composition for the treatment of acute disorders is described. The composition comprises an essentially water-free, ordered mixture of at least one pharmaceutically active agent in the form of microparticles which are adhered to the surfaces of carrier particles which are substantially larger than the particles of the active agent or agents, and are essentially water-soluble, in combination with the bioadhesion and / or mucoadhesion promoting agent. The invention also relates to a method for preparing the composition and to the use of the composition for the treatment of acute disorders.
Owner:DIABAKT AB
Mucoadhesive drug delivery devices and methods of making and using thereof
ActiveUS20050196440A1Improve drug absorptionPromote resultsPowder deliveryOrganic active ingredientsActive agentSolvent
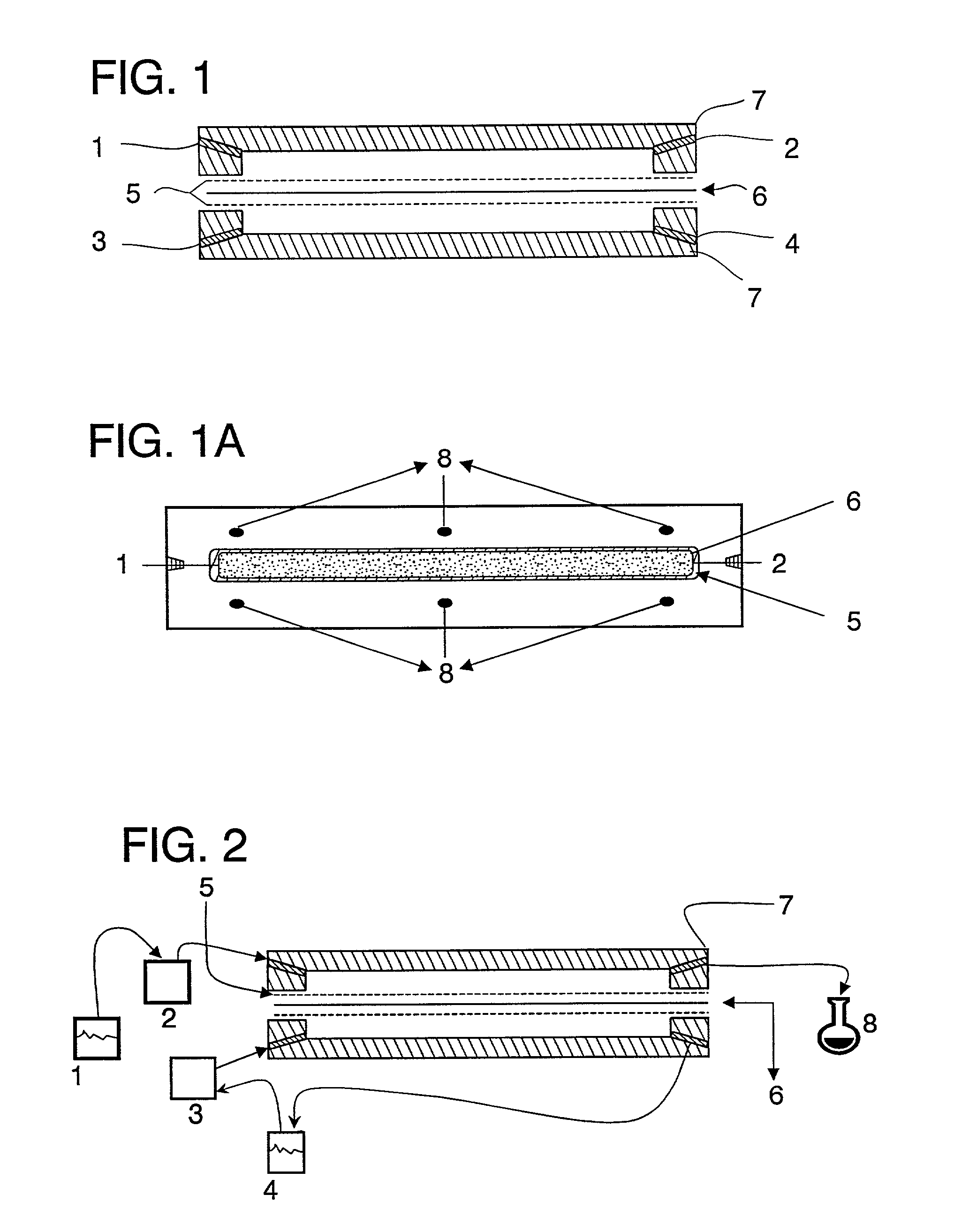
The present invention relates to mucoadhesive drug delivery devices and their methods of preparation and use. More specifically the present invention relates to mucoadhesive drug delivery devices comprising one or more biocompatible purified proteins combined with one or more biocompatible solvents and one or more mucoadhesive agents. The mucoadhesive drug delivery devices may also include one or more pharmacologically active agents. The drug delivery devices of the present invention adhere to mucosal tissue, thereby providing a vehicle for delivery of the pharmacologically active agent(s) through such tissue.
Owner:PETVIVO HLDG INC
Sinus delivery of sustained release therapeutics
The invention provides biodegradable implants for treating sinusitis. The biodegradable implants have a size, shape, density, viscosity, and / or mucoadhesiveness that prevents them from being substantially cleared by the mucociliary lining of the sinuses during the intended treatment period. The biodegradable implants include a sustained release therapeutic, e.g., an antibiotic, a steroidal anti-inflammatory agent, or both. The biodegradable implants may take various forms, such as rods, pellets, beads, strips, or microparticles, and may be delivered into a sinus in various pharmaceutically acceptable carriers.
Owner:INTERSECT ENT INC
Pharmaceutical composition for the treatment of acute disorders
A pharmaceutical composition for the treatment of acute disorders is described. The composition comprises an essentially water-free, ordered mixture of at least one pharmaceutically active agent in the form of microparticles which are adhered to the surfaces of carrier particles which are substantially larger than the particles of the active agent or agents, and are essentially water-soluble, in combination with the bioadhesion and / or mucoadhesion promoting agent. The invention also relates to a method for preparing the composition and to the use of the composition for the treatment of acute disorders.
Owner:OREXO AB
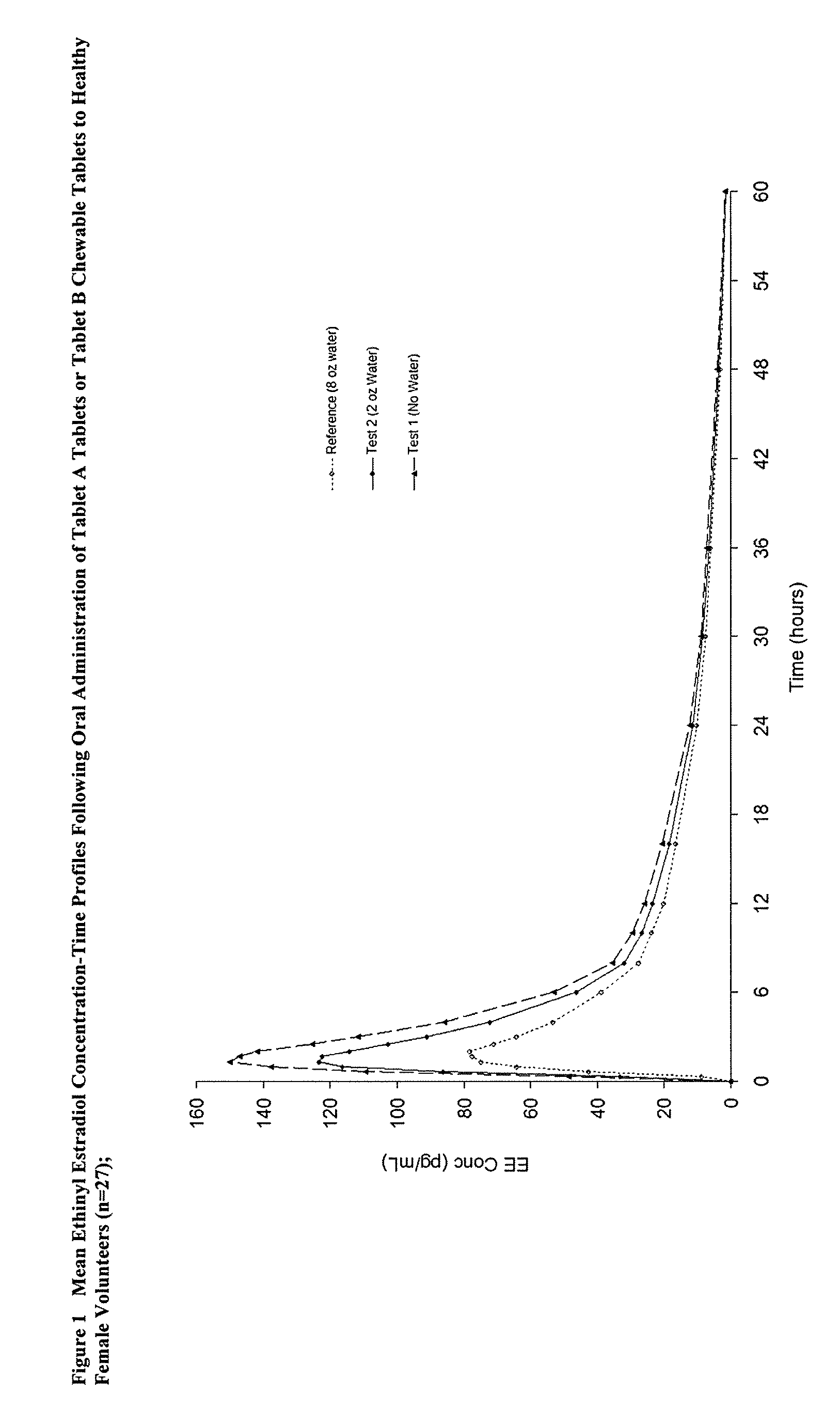
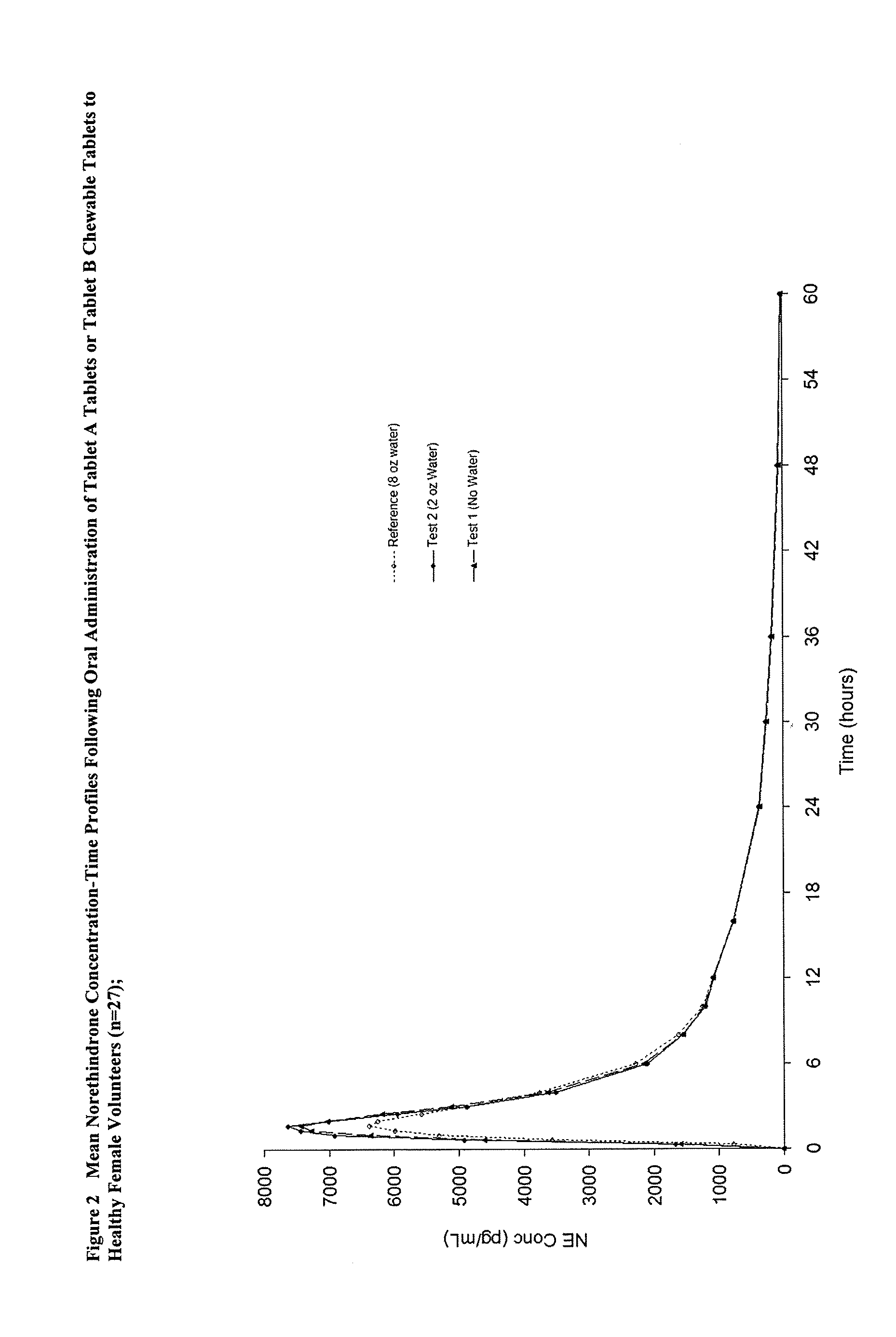
Methods to administer ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20070286819A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPill deliveryHormone replacementBioavailability
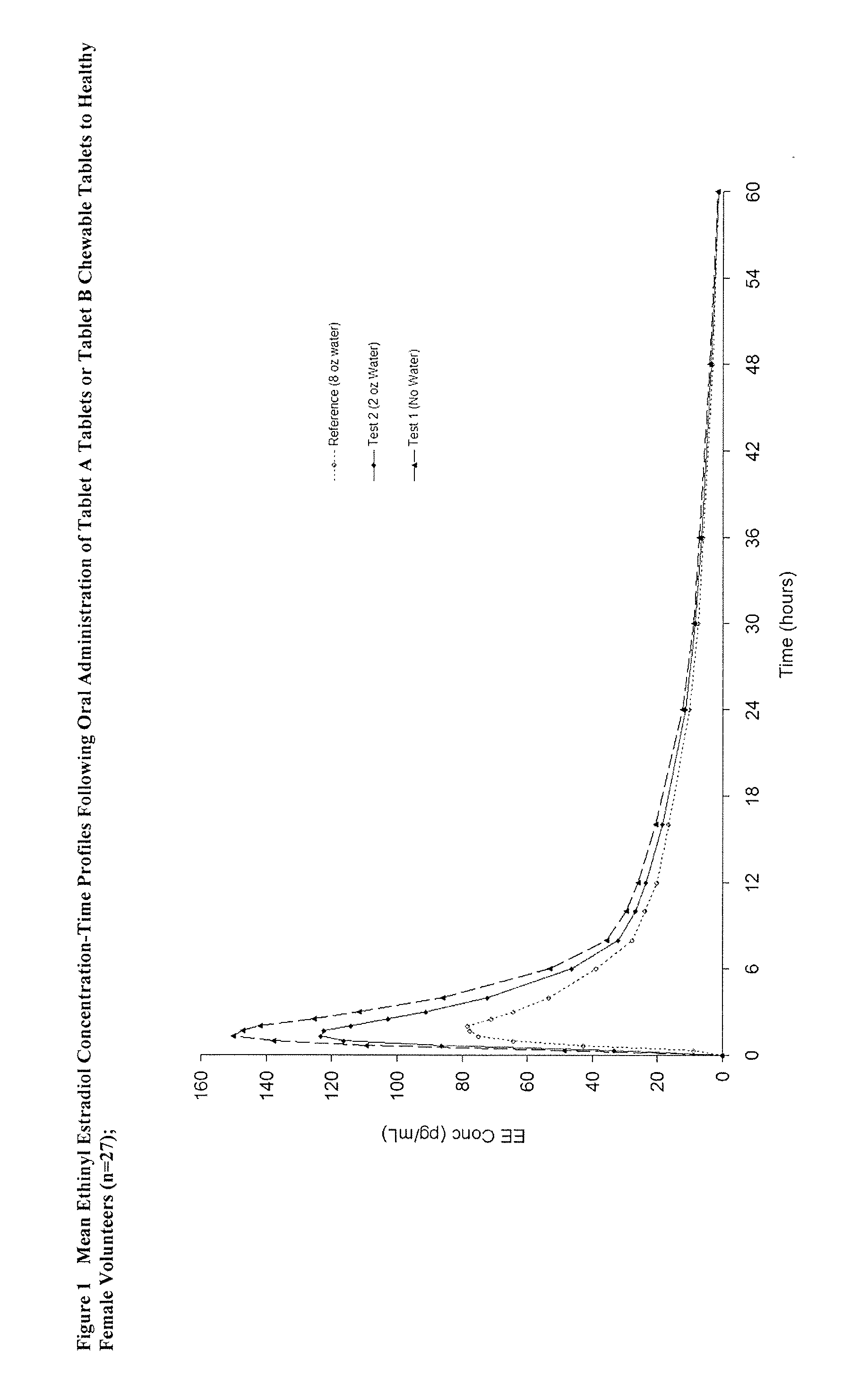
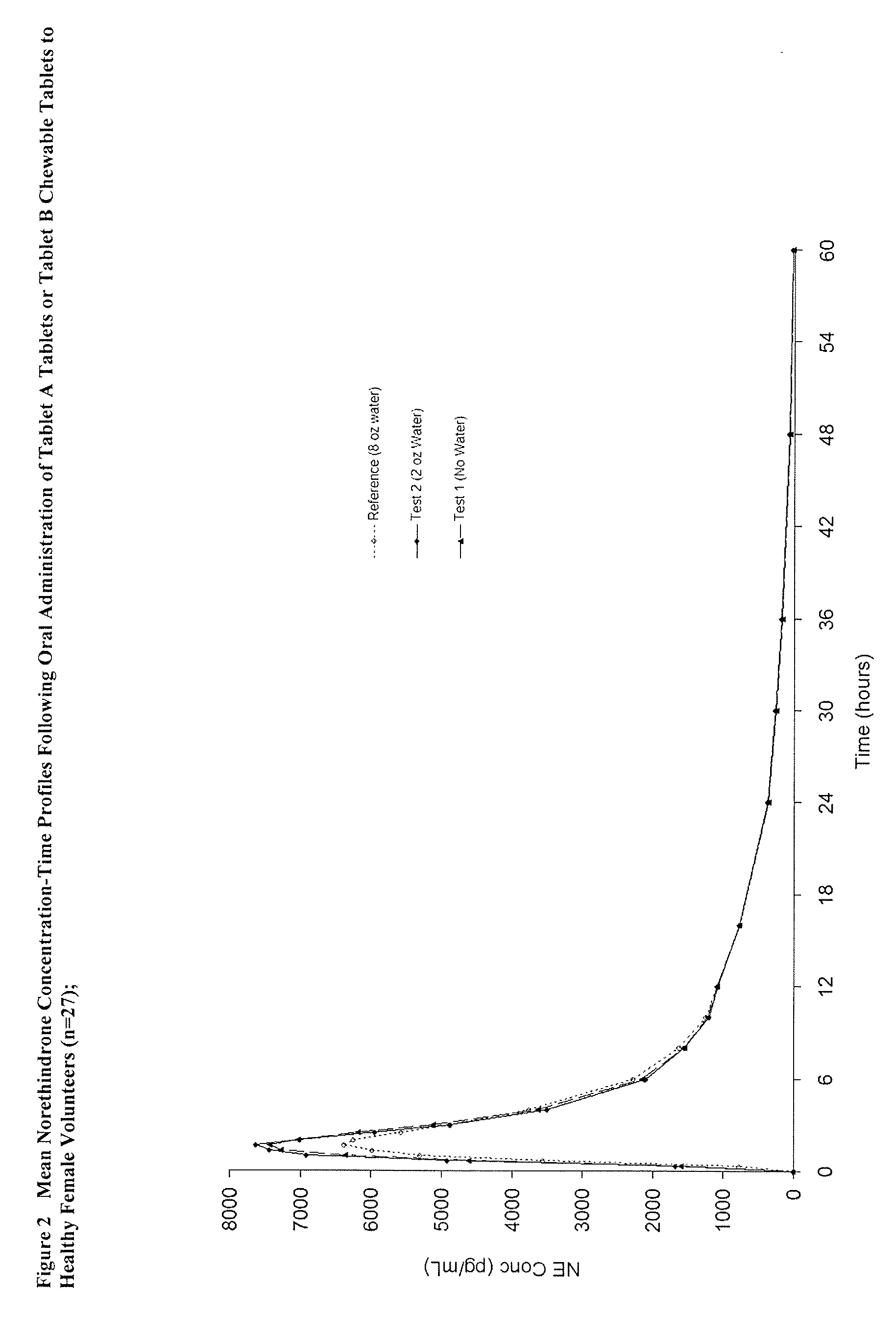
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:WARNER CHILCOTT CO LLC
Sustained release and long residing ophthalmic formulation and the process of preparing the same
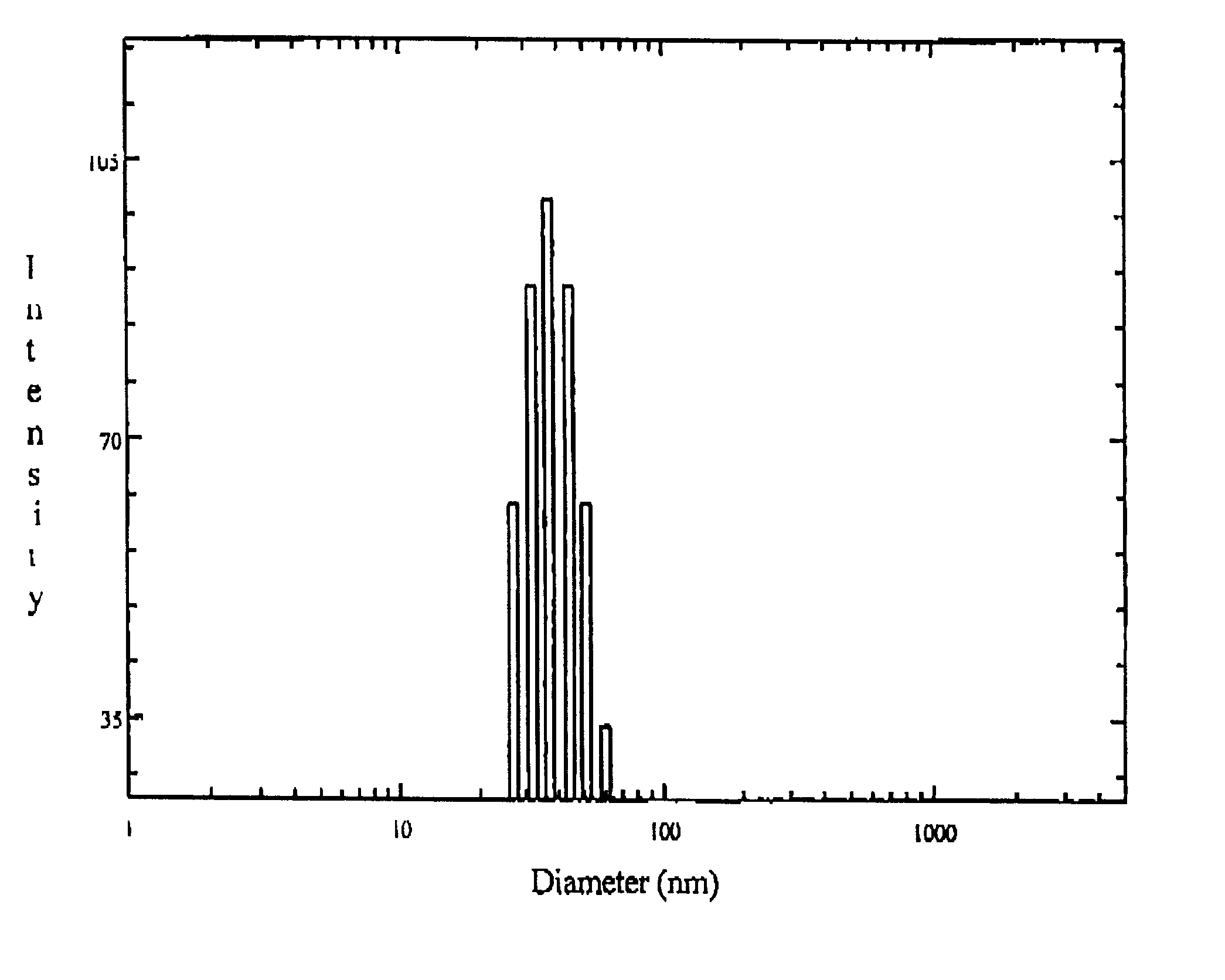
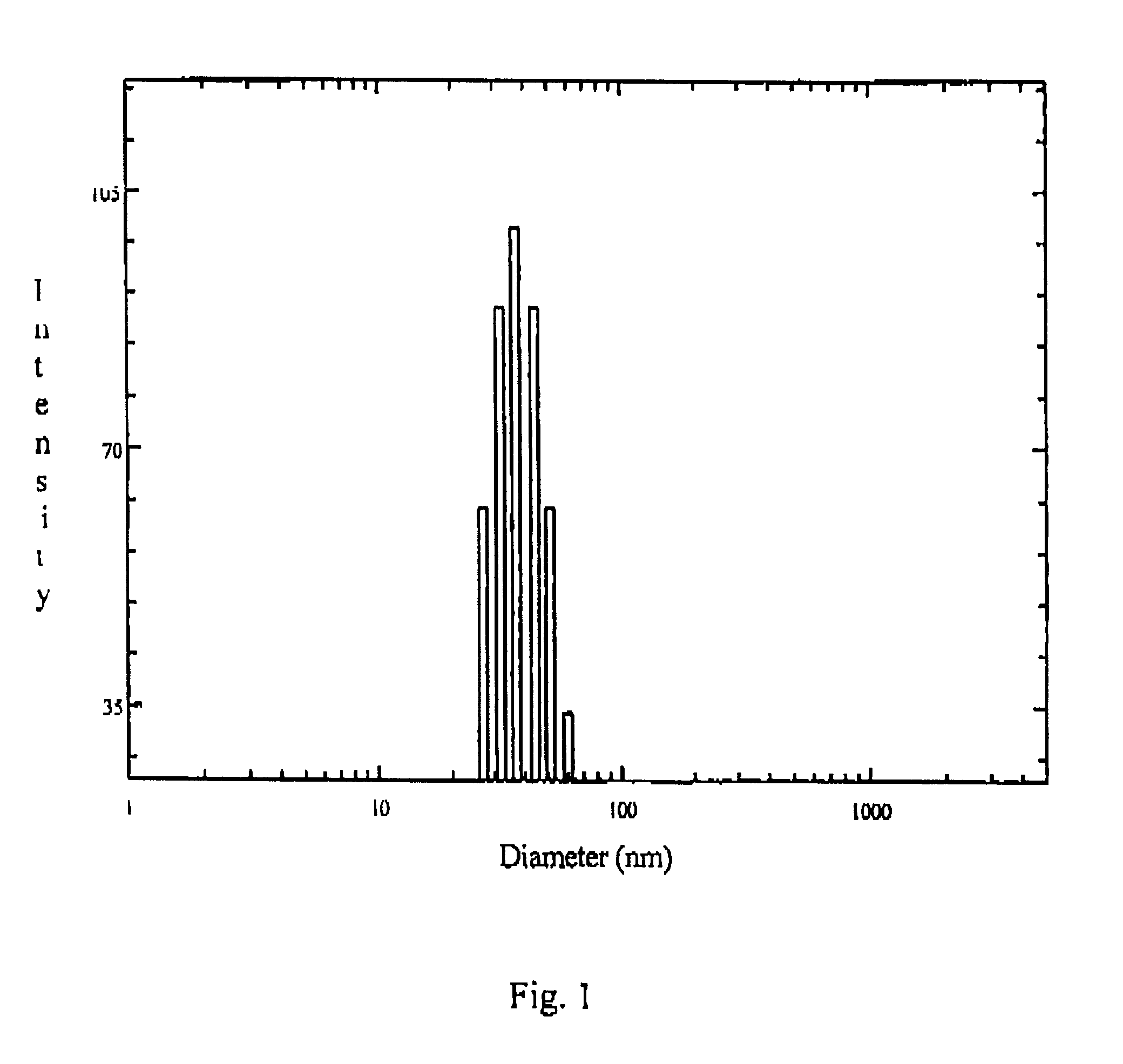
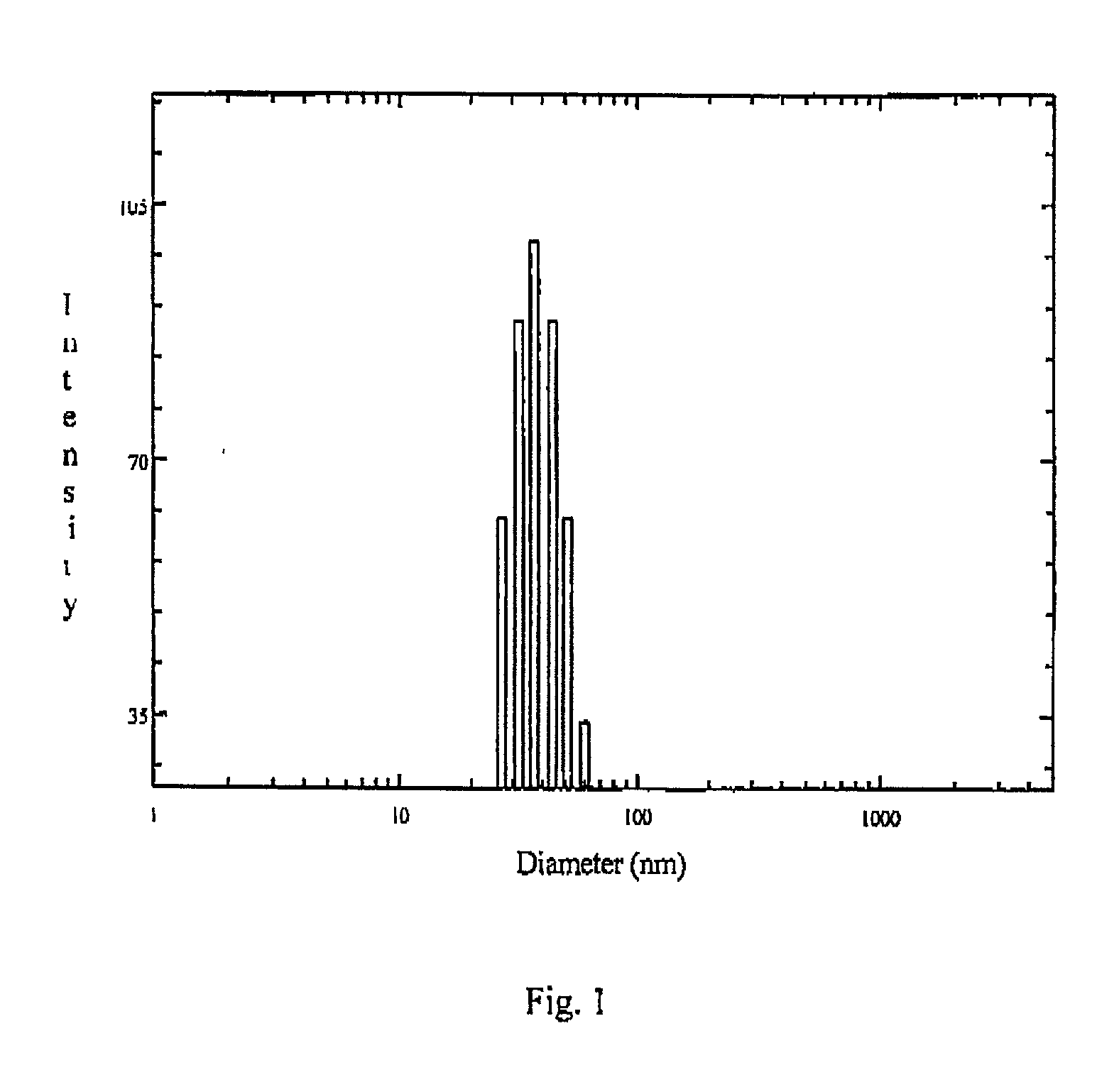
The present invention relates to sustained release and long residing opthalmic formulation having thermosensitivity, mucoadhesiveness, hydro gel properties and small particle size. The said formulation comprises micelle solution of random block co-polymer having a hydrophobic component and a hydrophillic component of general formula -(X+Y+Z-)m, and at least one hydrophobic drug with the block co-polymer solution. The invention also provides a process of preparing said formulation.
Owner:UNIVERSITY OF DELHI
Adhesive bioerodible transmucosal drug delivery system
InactiveUS20070207192A1Suitable bioadhesive capabilityRapid onsetAntibacterial agentsBiocideWhole bodyIrritation
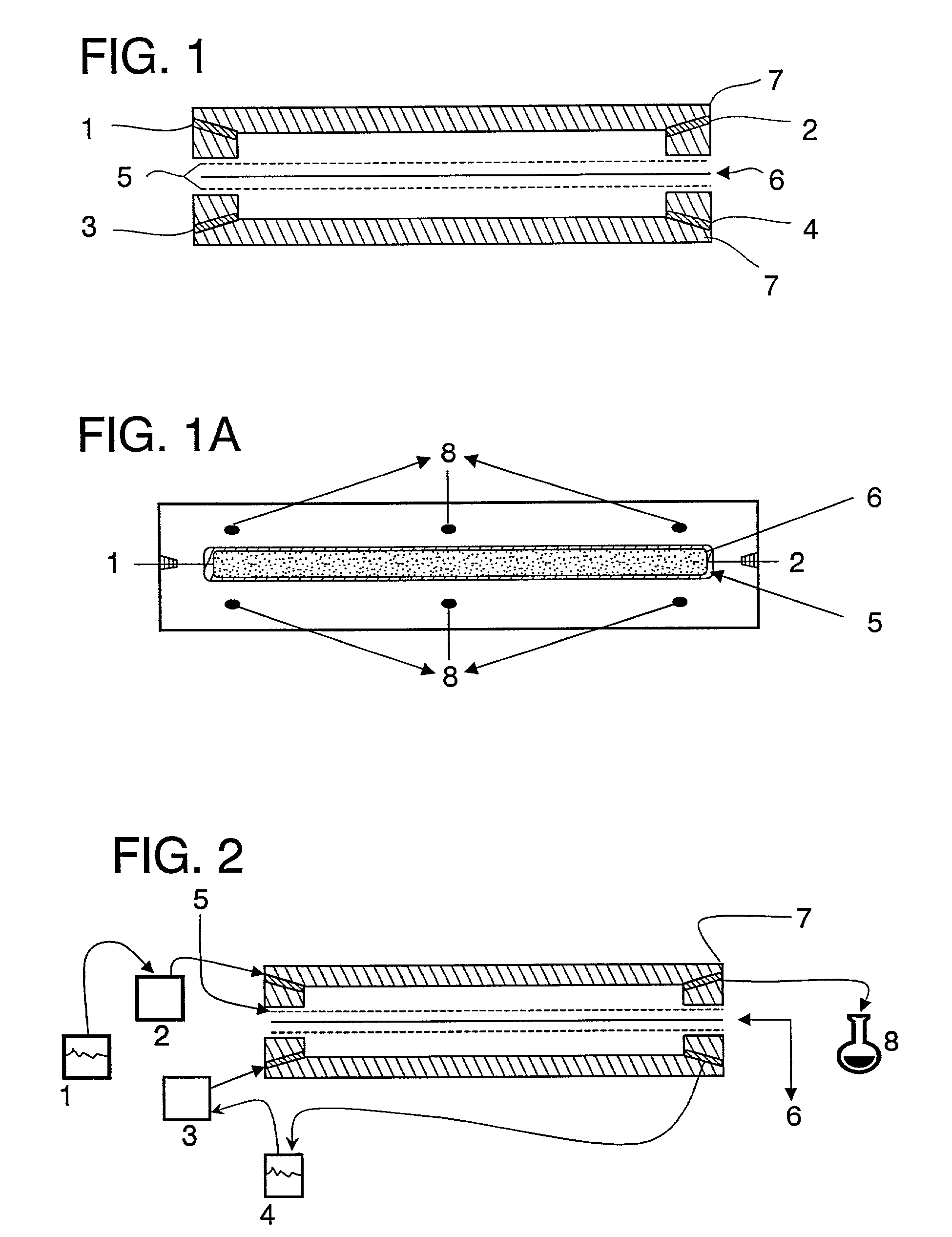
The present invention is directed to a mucoadhesive delivery system for the local or systemic administration of a pharmaceutical agent. The delivery system of the invention effectively and facilely enables transport of the pharmaceutical agent through mucosal membranes and into the vasculattire of the mucosa. The delivery system includes an at least partially water soluble bioadhesive layer and an at least partially water soluble backing layer. Incorporated within either or both of these layers are the pharmaceutical agent and a mucosal penetration enhancing agent. The mucosal penetration enhancing agent displays localized tissue irritation properties. The mucoadhesive delivery system may be in the form of a gel, film, disc or patch. It may be applied to any mucosal membrane of a patient including but not limited to those of the buccal and nasal cavities, throat, eye, vagina, alimentary tract and peritoneum.
Owner:ARIUS TWO
Fast dissolving composition with prolonged sweet taste
A novel fast dissolving pharmaceutical composition in solid dosage form with prolonged sweet taste which comprises (a) At least one pharmaceutically active agent, (b) At least one water soluble sugar, (c) At least one non-sugar sweetner in normal fast release form and (d) At least one non-sugar sweetner in a mucoadhesive slow release form.
Owner:PANACEA BIOTEC
Drug Delivery Formulations For Targeted Delivery
The size and location of microsphere uptake / delivery are important determinants of the final biodistribution of oral microsphere systems. Formulations, kits, methods of administering the formulations, and using the kits are described herein. The formulations are oral dosage formulations. In one embodiment, the formulations contain microparticles and / or nanoparticles having a homogenous size range selected to optimize uptake in a specific region of the GI tract and target drug delivery to specific organs. In some embodiments, the dosage formulation contains an enteric coating and / or a magnetic material. In a preferred embodiment, the formulation contains a magnetic material and an active agent to be delivered, optionally the active agent is in the form of micro- or nano-particles. In some embodiments metallomucoadhesive materials and / or magnetic materials are employed as magnetic and / or mucoadhesive sources. Formulations containing magnetic materials can be localized using the kits and methods disclosed herein. In one embodiment, the method includes orally administering the formulation and applying an extracorporeal magnet to a site on the outside surface of the patient's body in an area that closely apposes the location in the gastrointestinal tract to which delivery of the formulation is desired. The extracorporeal magnet is applied for a suitable time period to allow for the drug to be released from the formulation and / or to allow for the formulation to adhere to the site. Both magnetic and mucoadhesive forces may be utilized to site-direct and retain the dosage form in the region of the gastrointestinal (GI) tract most suitable for the desired delivery.
Owner:PEROSPHERE INC
Sustained release and long residing ophthalmic formulation and the process of preparing the same
The present invent relates to sustained release and long residing ophthalmic formulation having thermosensitivity, mucoadhesiveness, hydro gel properties and small particle size. The said formulation comprises of micelle solution of random block co-polymer having a hydrophobic component and a hydrophilic component of general formula -(X+Y+Z-)m, wherein m is an integer greater than 2 X is a monomer which will provide hydrogel formation properties of the co-polymer to reduce the irritability of the eye and is selected from vinyl group of compounds Y is a monomer which will provide thermosensitivity properties of the co-polymer having a general formula R1-R2N-(C=O)-CH=CH2, R1=a proton or CnH2n+1 in which n may have the value from 3 to 6 and R2=alkyl group having chain length of C3 to C6 Z is a monomer which will provide mucoadhesiveness and pH-sensitivity properties to the co-polymer and is selected from acrylate based monomers at least one hydrophobic drug with the said block co-polymer solution; The invention also provides a process of preparing said formulation.
Owner:UNIVERSITY OF DELHI
Mucoadhesive drug delivery devices and methods of making and using thereof
ActiveUS8529939B2Promote resultsReduce deliveryPowder deliveryOrganic active ingredientsActive agentSolvent
The present invention relates to mucoadhesive drug delivery devices and their methods of preparation and use. More specifically the present invention relates to mucoadhesive drug delivery devices comprising one or more biocompatible purified proteins combined with one or more biocompatible solvents and one or more mucoadhesive agents. The mucoadhesive drug delivery devices may also include one or more pharmacologically active agents. The drug delivery devices of the present invention adhere to mucosal tissue, thereby providing a vehicle for delivery of the pharmacologically active agent(s) through such tissue.
Owner:PETVIVO HLDG INC
Rapid-acting pharmaceutical composition
A pharmaceutical composition for the treatment of acute disorders is described. The composition includes an essentially water-free, ordered mixture of at least one pharmaceutically active agent in the form of microparticles which are adhered to the surfaces of carrier particles which are substantially larger than the particles of the active agent or agents, and are essentially insoluble or sparingly soluble in water, in combination with a bioadhesion and / or mucoadhesion promoting agent adhered to the surfaces of the carrier particles. The composition is primarily intended for sublingual or intranasal administration. The invention also relates to a method for preparing the composition and to the use of the composition for the treatment of acute disorders.
Owner:OREXO AB
Methods for improving lid margin and tear film function and treatment of lid margin disease using tetracycline family antibiotics
InactiveUS20110059925A1Improving lid margin functionRelieve ocular discomfortBiocideSenses disorderDiseaseOphthalmology
The present invention provides a mucoadhesive broad spectrum antibiotic with anti-inflammatory characteristics with strong tissue penetration for improving lid margin function and the treatment of diseases associated therewith. The present invention further provides compositions and methods for treating and / or preventing the signs and / or symptoms of blepharitis and dry eye disease.
Owner:DONNENFELD ERIC
Methods to administer solid dosage forms of ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20080113953A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPharmaceutical delivery mechanismEthinyl oestradiolHormone replacement
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:APTALIS PHARMA
Liquid formulations for the prevention and treatment of mucosal diseases and disorders
Stable, viscous, mucoadhesive aqueous compositions which are useful for the prevention and treatment of ulcerative, inflammatory, and / or erosive disorders of mucous membranes and / or the delivery of pharmaceutically active compounds to mucosal surfaces for topical treatment or transfer to the systemic circulation.
Owner:AMAG PHARMA
Multidirectional mucosal delivery devices and methods of use
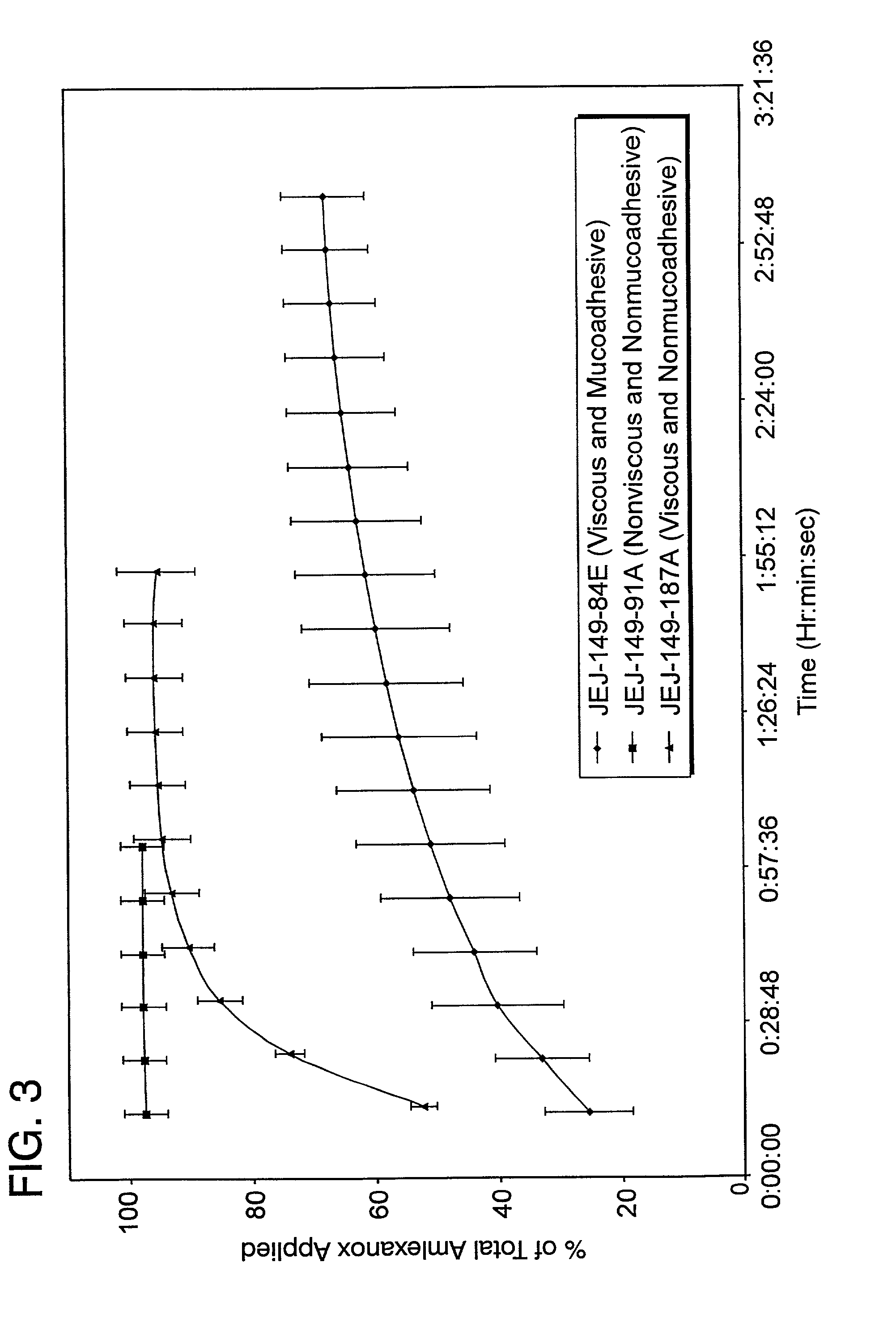
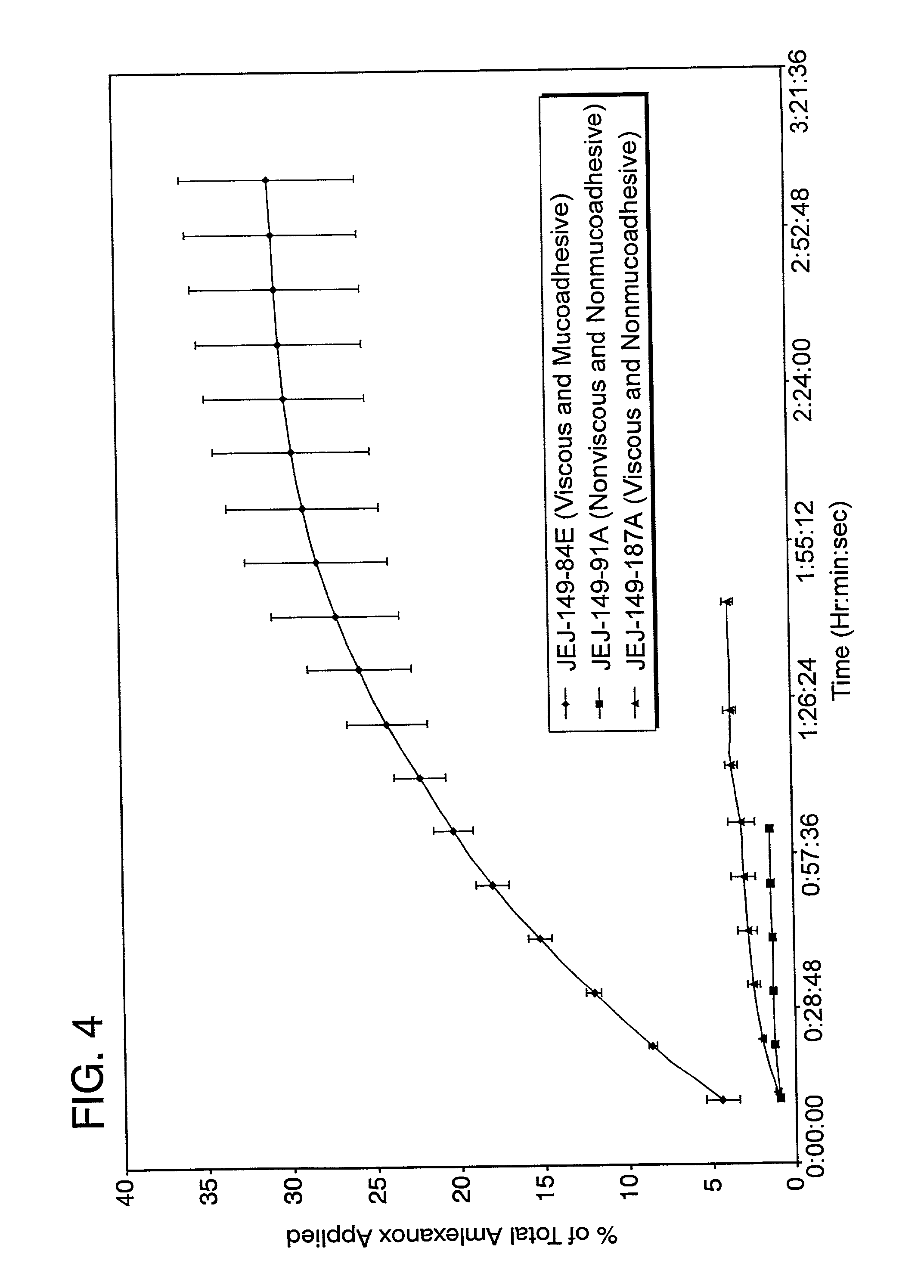
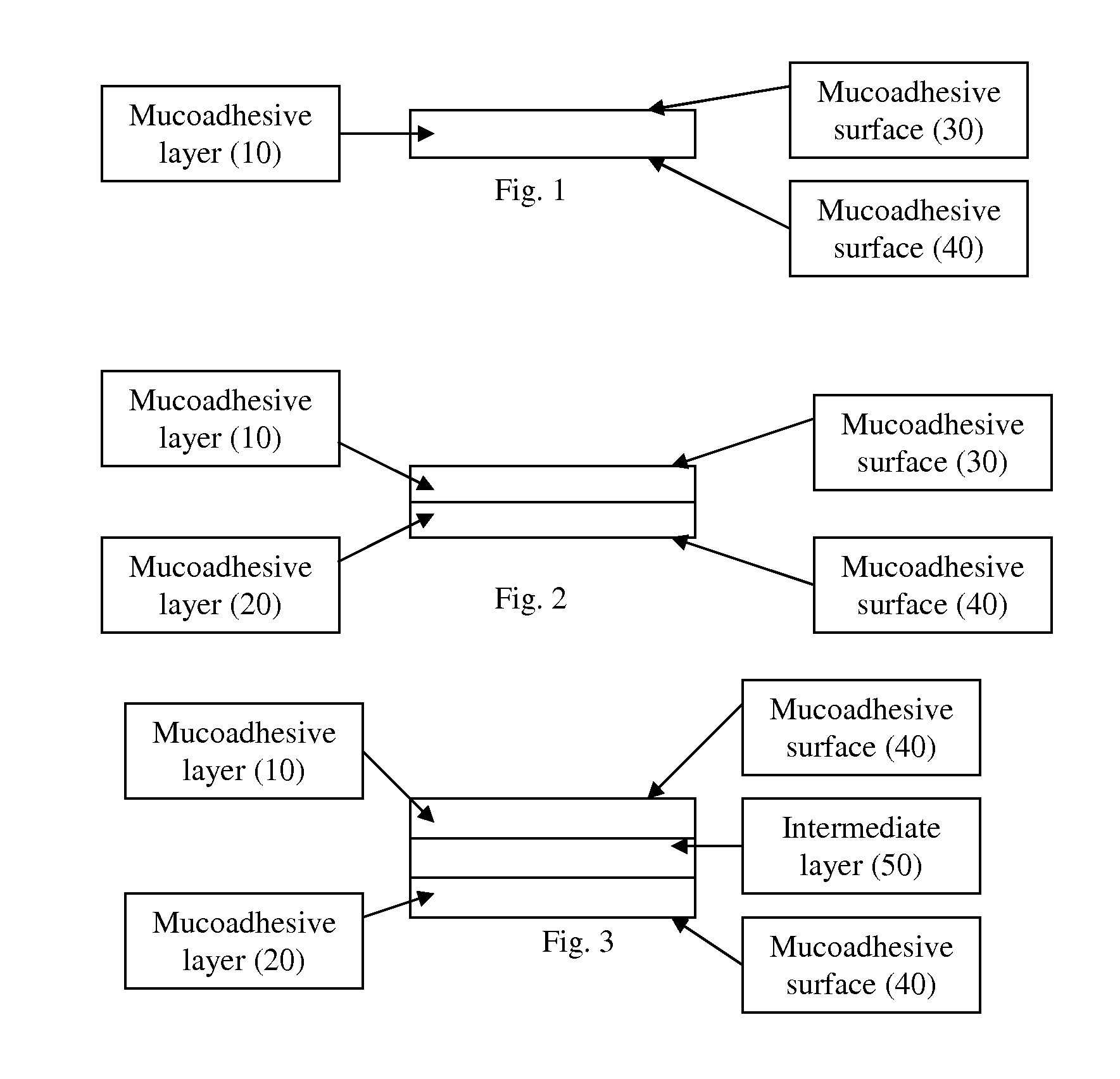
The present invention relates to a pharmaceutical dosage form for transmucosal delivery of an active agent to two or more mucosal surfaces. The dosage form is presented as a transmucosal delivery device. The devices of the invention may include at least two mucoadhesive surfaces. The devices may further include an intermediate layer disposed between the mucoadhesive layers. The pharmaceutical can be incorporated in any one or all of the mucoadhesive layers or the intermediate layer. Upon application, the device adheres to at least two surfaces, providing transmucosal delivery of the drug to at least two surfaces.
Owner:BIODELIVERY SCI
Mucoadhesive vesicles for drug delivery
InactiveUS20080305149A1Prolong residenceSolution deliveryPharmaceutical non-active ingredientsActive agentMedicine
Vesicles for delivery of active macromolecules which are formed from amphiphilic segmented copolymers having one or more mucoadhesive groups or regions and which can be used for delivery of an active agent to an area of the body having a mucous membrane, such as but not limited to the gastrointestinal tract.
Owner:BIOCURE
Liquid formulations for the prevention and treatment of mucosal diseases and disorders
Stable, viscous, mucoadhesive aqueous compositions which are useful for the prevention and treatment of ulcerative, inflammatory, and / or erosive disorders of mucous membranes and / or the delivery of pharmaceutically active compounds to mucosal surfaces for topical treatment or transfer to the systemic circulation.
Owner:AMAG PHARMA
Multiparticle Pharmaceutical Dosage Form for a Low-Soluble Active Substances and Method for Producing Said Pharmaceutical Dosage Form
ActiveUS20080166416A1Easy to processAffect permeabilityBiocidePowder deliveryDrug additiveActive agent
The invention relates to an oral multiparticle pharmaceutical dosage form in the form of a receptacle reducing the pH values of stomach, containing a plurality of pellets, particles, granules or agglomerates whose mean diameter ranges from 50 to 2500 μn substentially consisting of a) an internal matrix layer containing an active agent which is neither peptide or protein, nor the derivatives or conjugates thereof, a lipophilic matrix whose melting point is greater than 37° C. and a polymer with mucoadhesive effect, b) an external film coating substentially consisting of a polymer or an anionic copolymer which is optionally formulated with conventional pharmaceutical additives, wherein the active agent has a water solubility according to DAB 10, of at least 30 volume parts of water for one part by weight of the active agent and is coated with the lipophilic matrix and said active agent-containing lipophilic matrix is coated with a matrix made of a polymer with mucoadhesive effect. A method for producing the inventive multiparticle pharmaceutical dosage is also disclosed.
Owner:EVONIK OPERATIONS GMBH
Methods of treating epithelial lesions
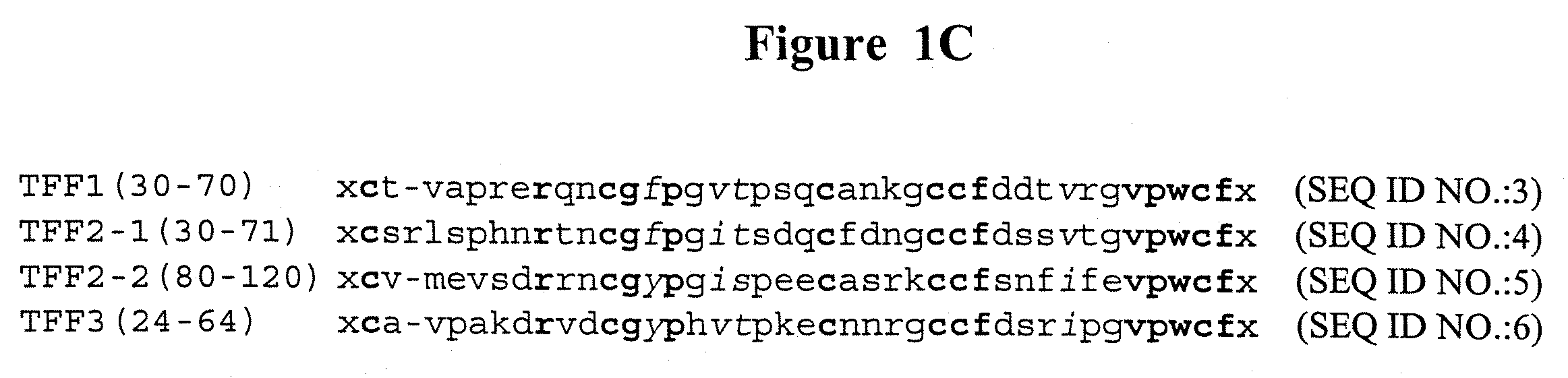
InactiveUS20060188471A1Altered pharmacokinetic and pharmacodynamic profileIncreased serum half-lifeBiocideSenses disorderDiseaseTrefoil domain
The invention features methods of preventing or treating epithelial cell lesions in a mammal by administering a composition containing a therapeutically effective amount of a trefoil domain-containing polypeptide, or a trefoil peptide fragment, and a mucoadhesive excipient. The invention further features methods of preventing or treating an eye disorder, e.g., dry eye, by topically administering to the eye a composition containing a therapeutically effective amount of a trefoil domain-containing polypeptide, or a trefoil peptide fragment, and a mucoadhesive excipient. Compositions containing a trefoil domain-containing polypeptide, or a trefoil peptide fragment, and a mucoadhesive excipient may be formulated in combination with one or more additional therapeutic agents and used in the methods of the invention.
Owner:THE GENERAL HOSPITAL CORP +1
Mucosal delivery tablet
InactiveUS20070048369A1Reduce exposureResists brittle fractureBiocideAntipyreticMucoadhesionBiomedical engineering
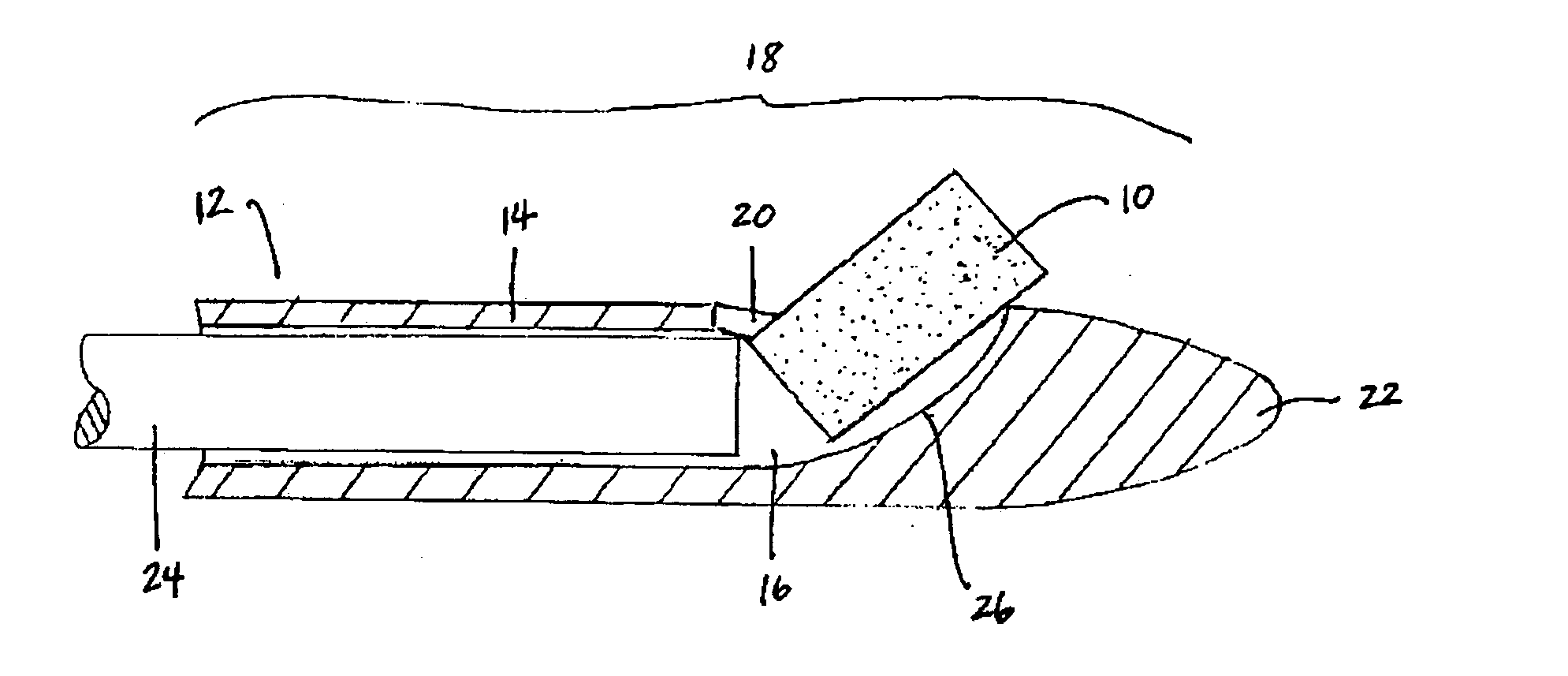
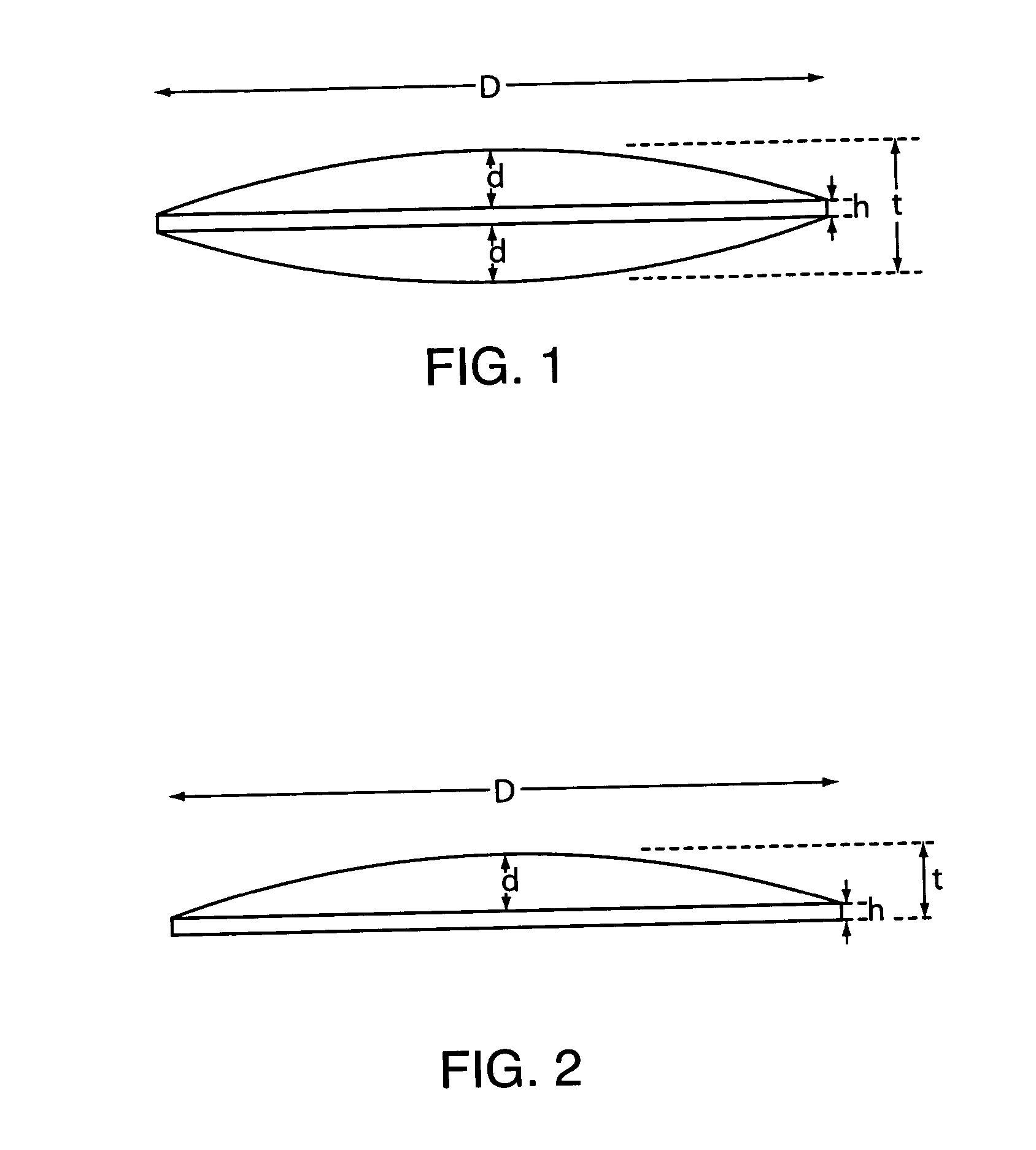
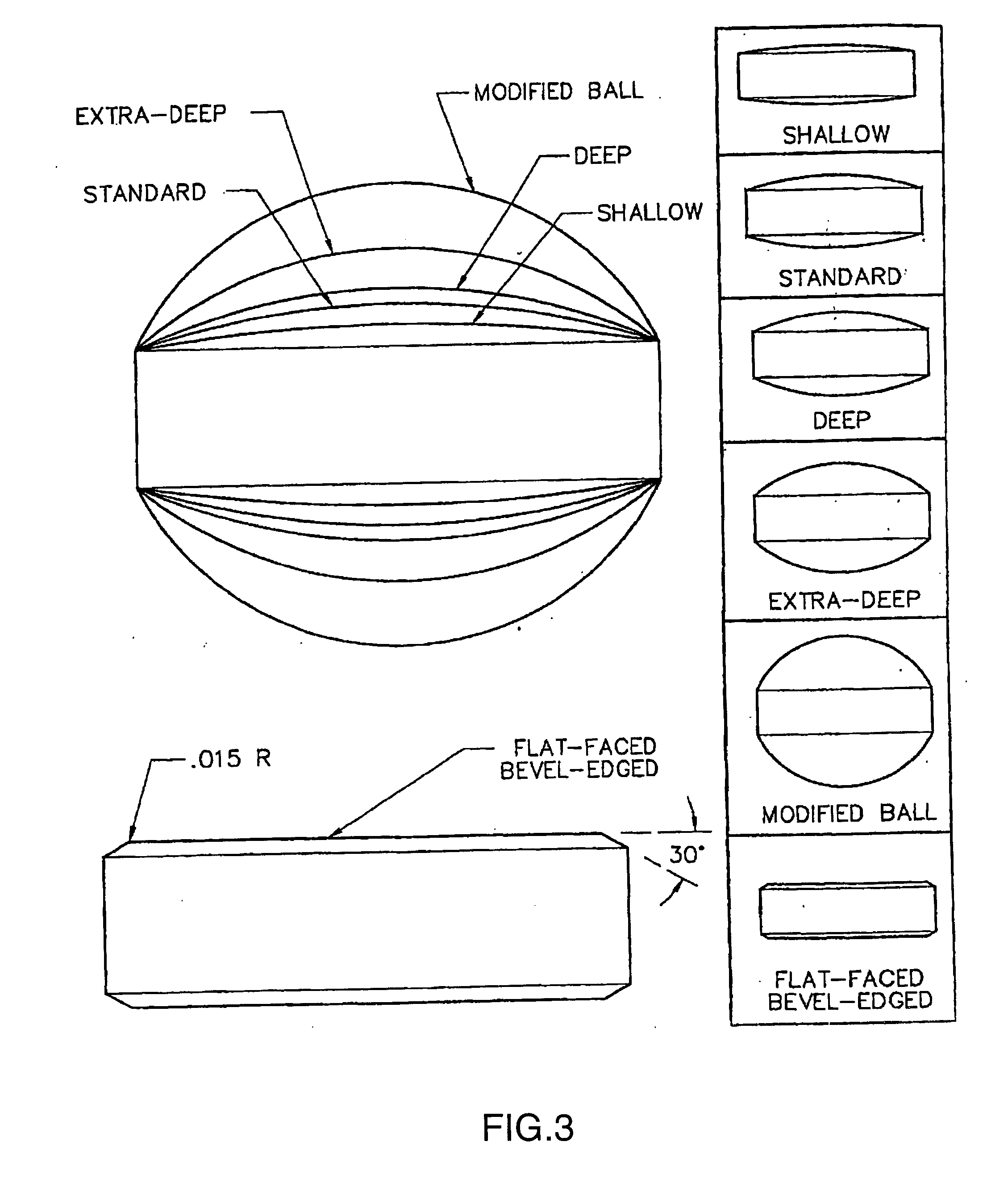
Mucoadhesive tablets have a convex surface, a diameter to cup depth ratio of 4-20 and a cup depth to edge thickness ratio of greater than 0.75 swiftly adheres and conforms to the contacting tissue. The tablets are used to administer actives such as, for example, benzocaine.
Owner:HENKEL KGAA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com