Patents
Literature
70 results about "Benzocaine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
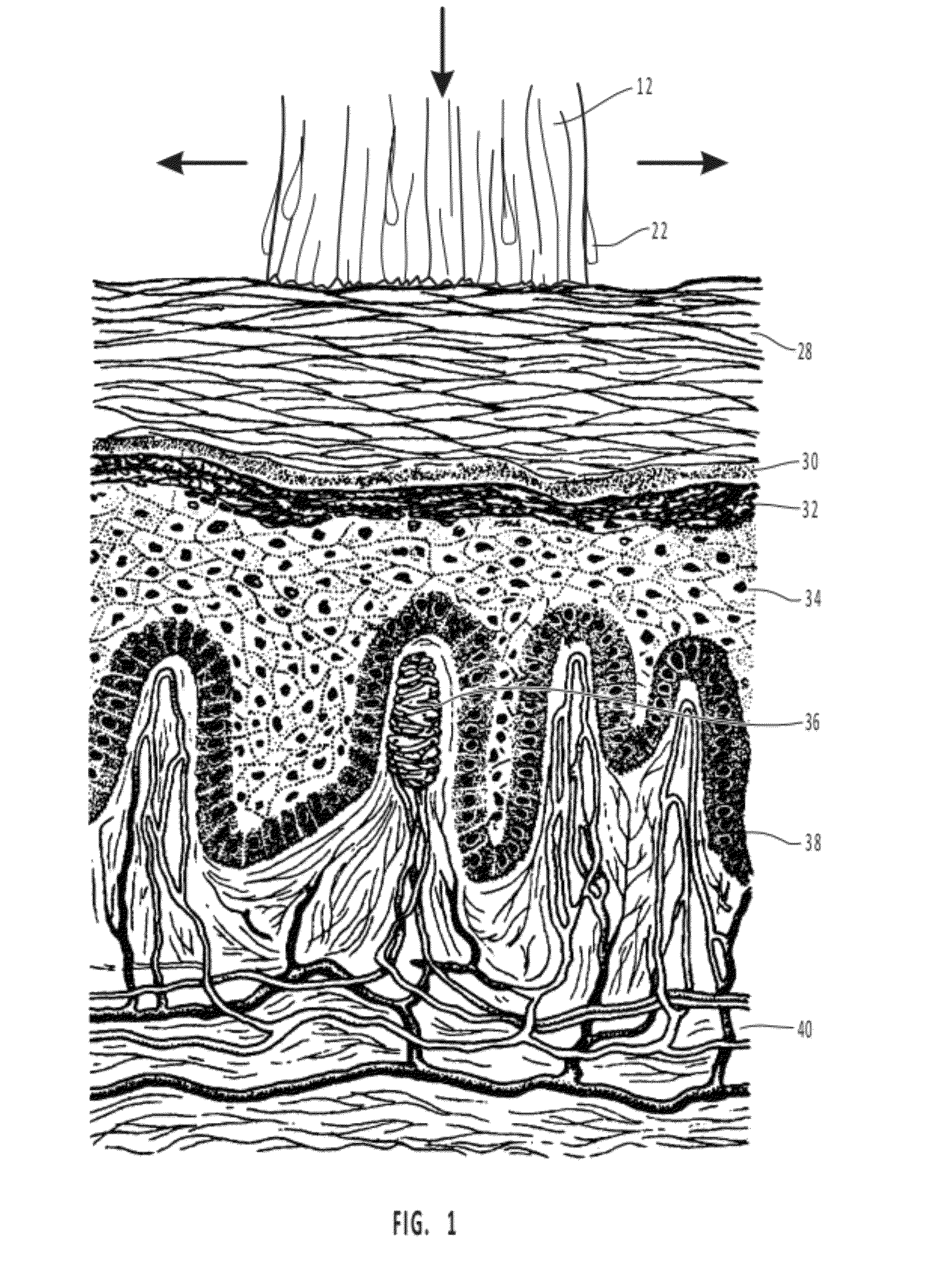
This medication is used on the skin to stop itching and pain from certain skin conditions (such as scrapes, minor burns, insect bites) and to treat minor discomfort and itching caused by hemorrhoids and certain other problems of the genital/anal area (such as anal fissures, itching around the vagina/rectum).
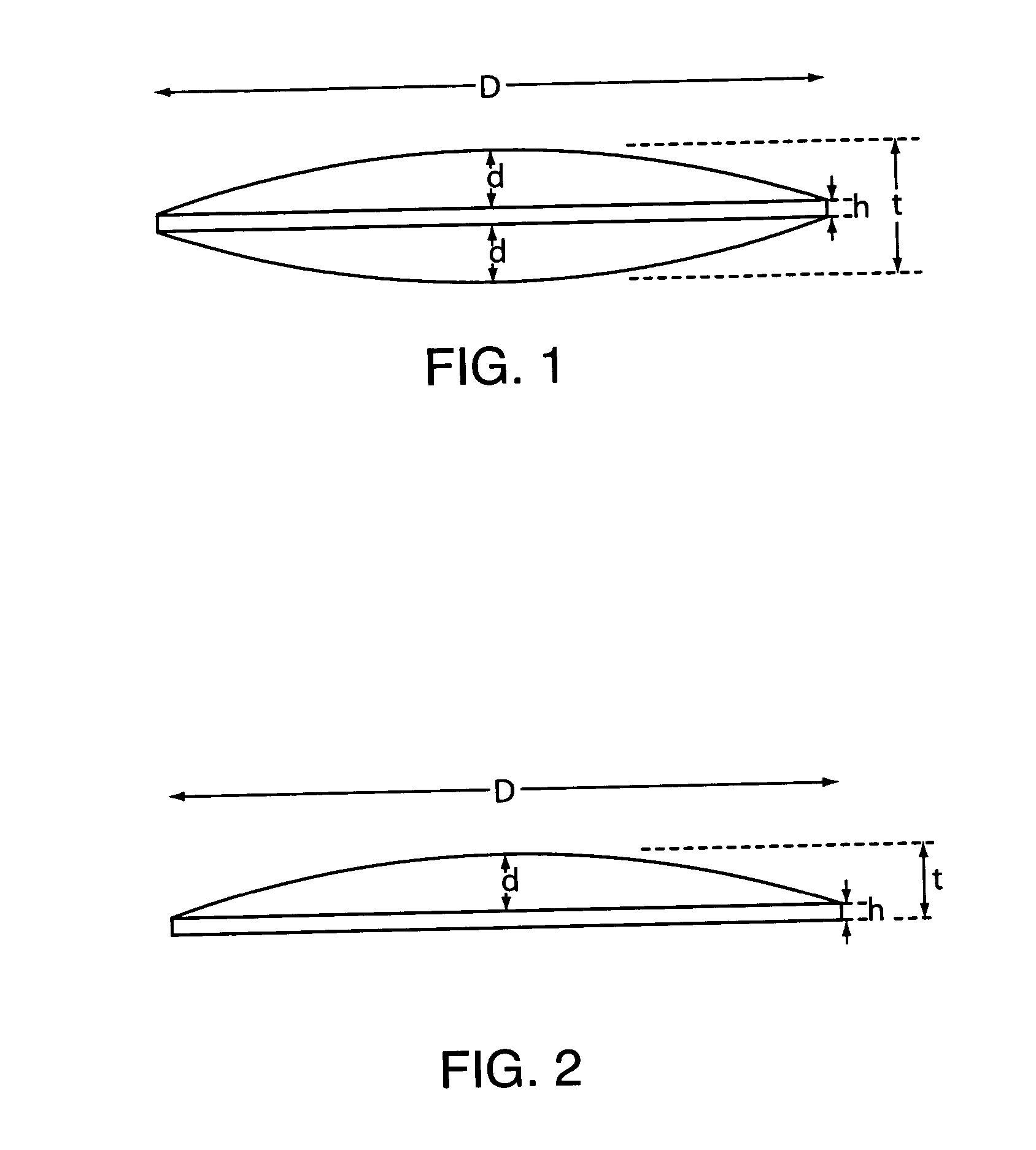
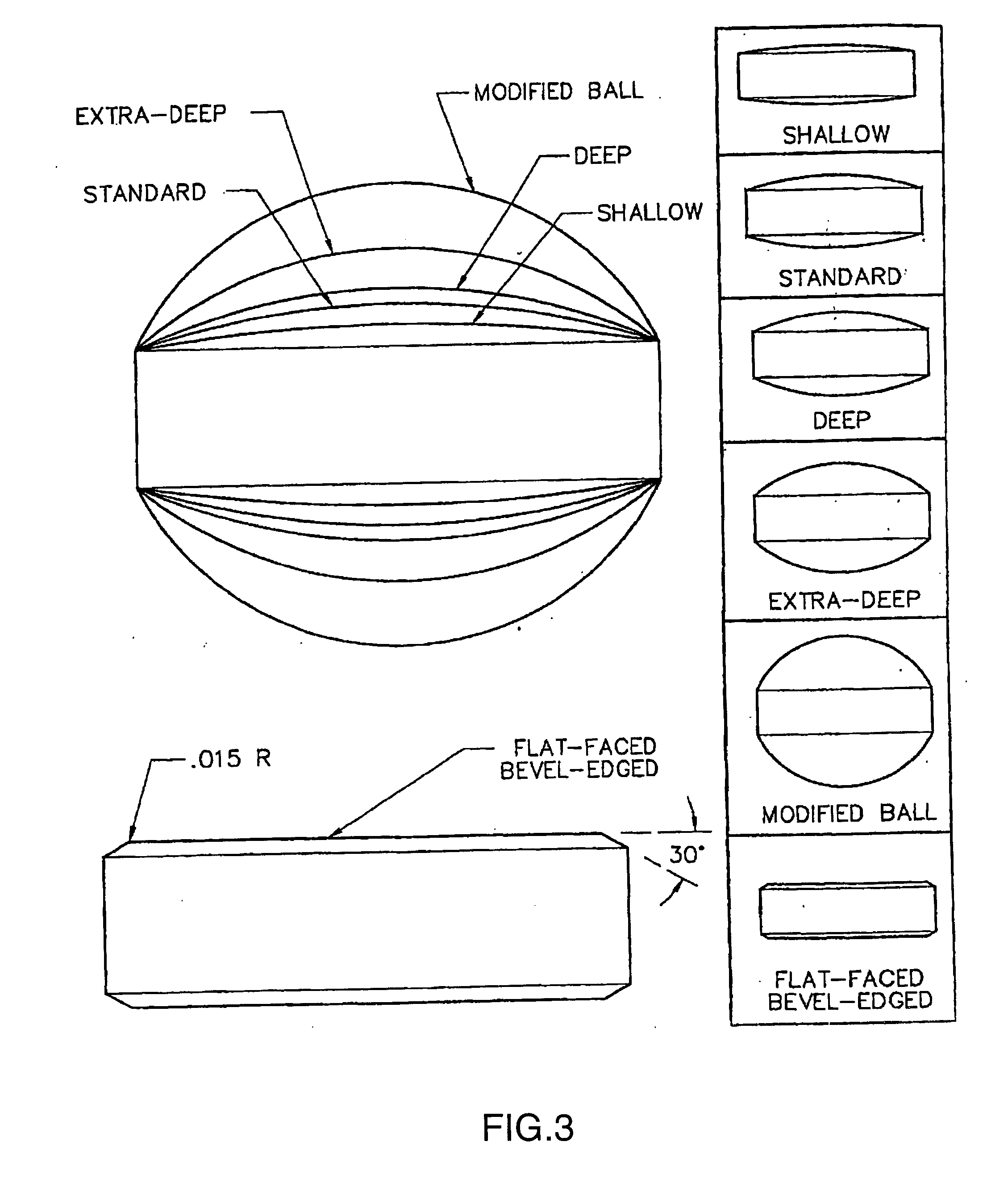
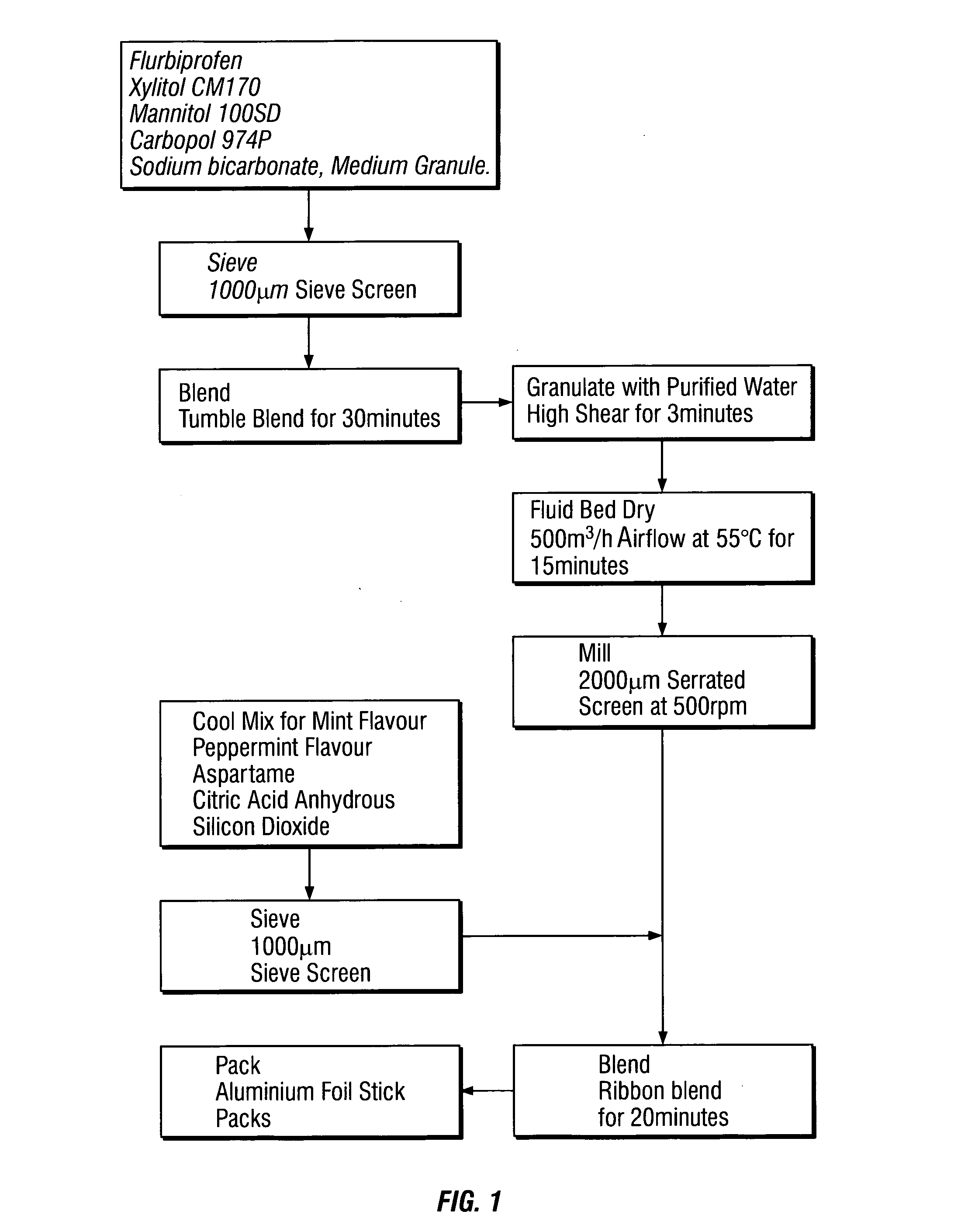
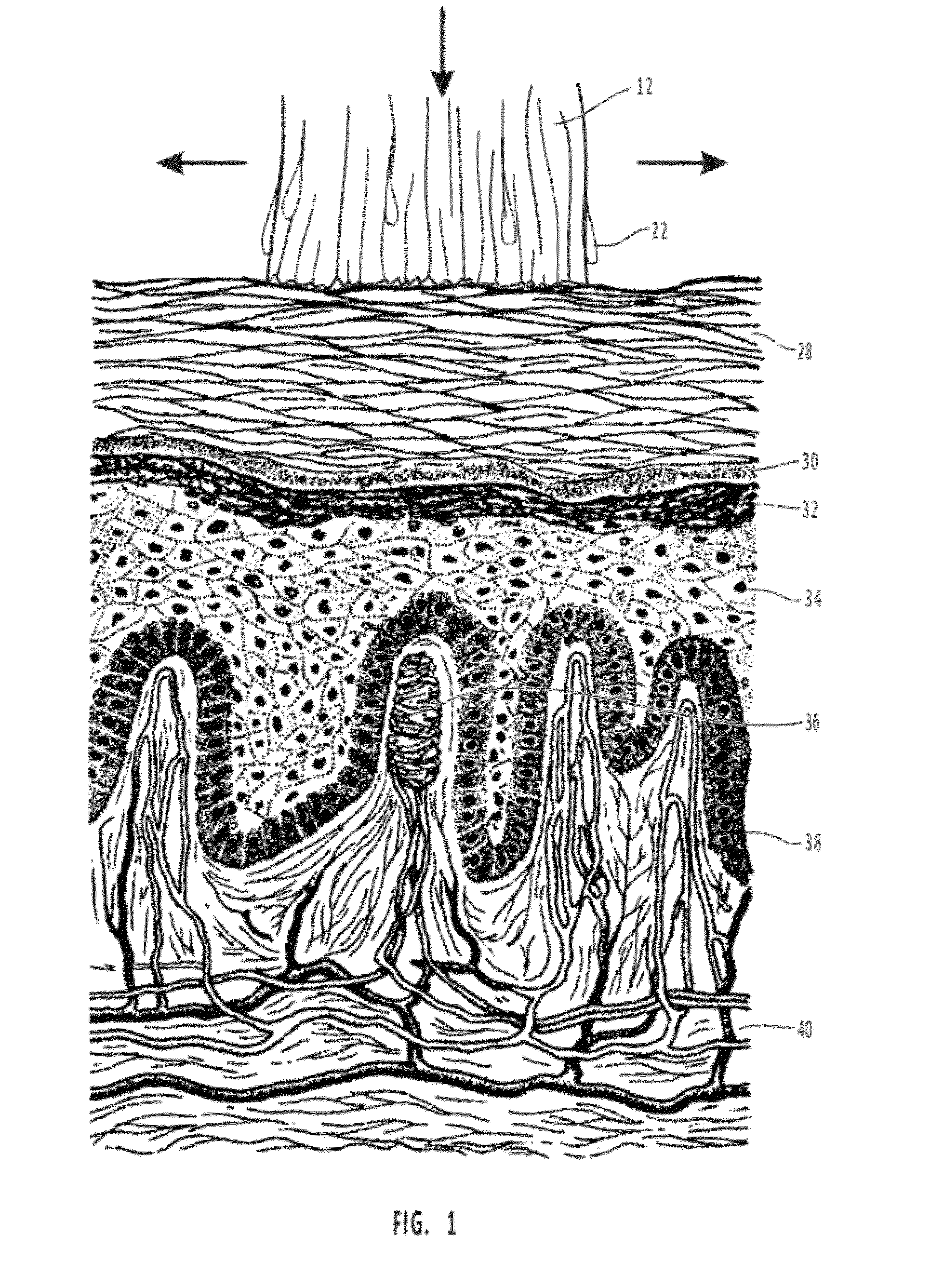
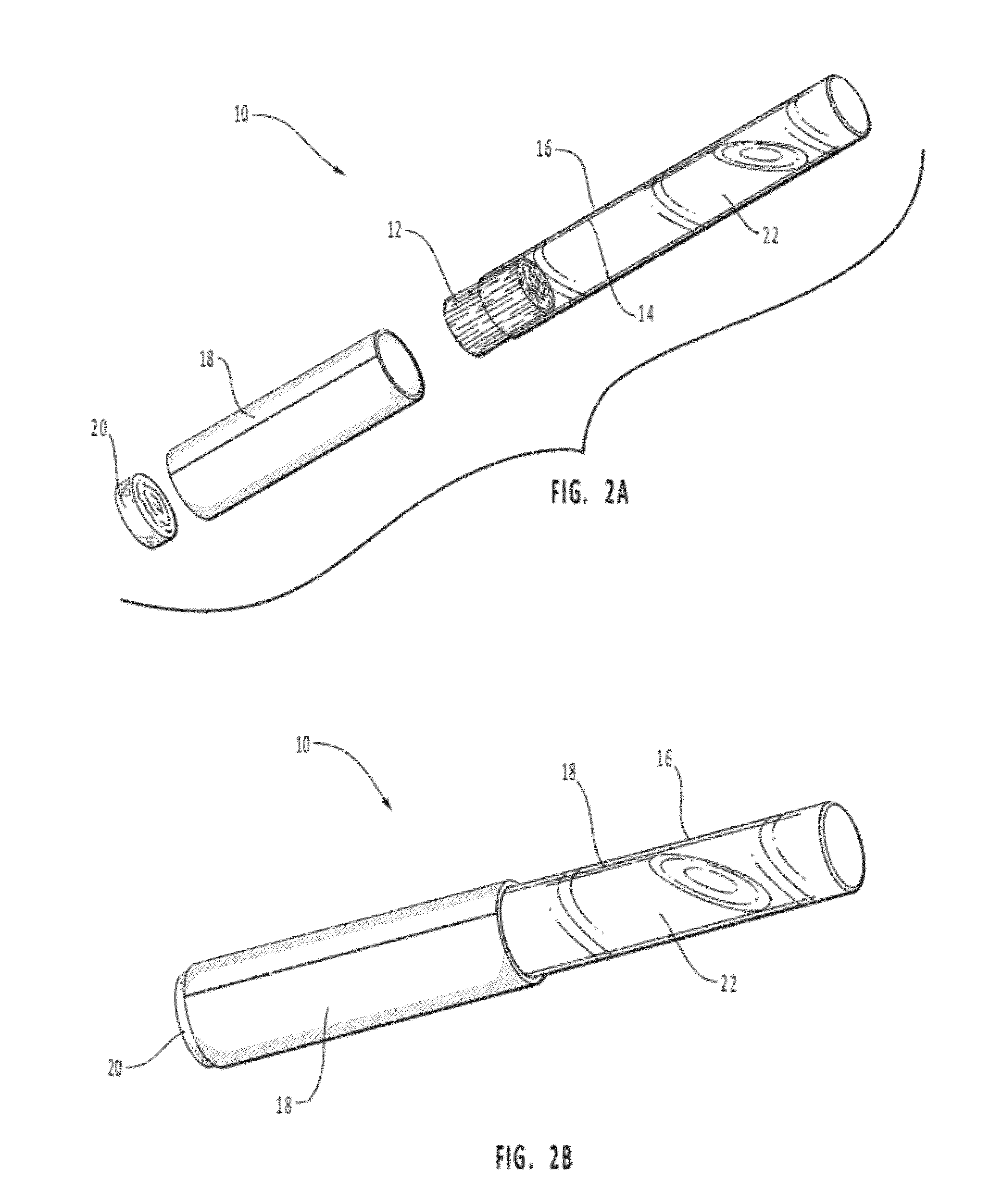
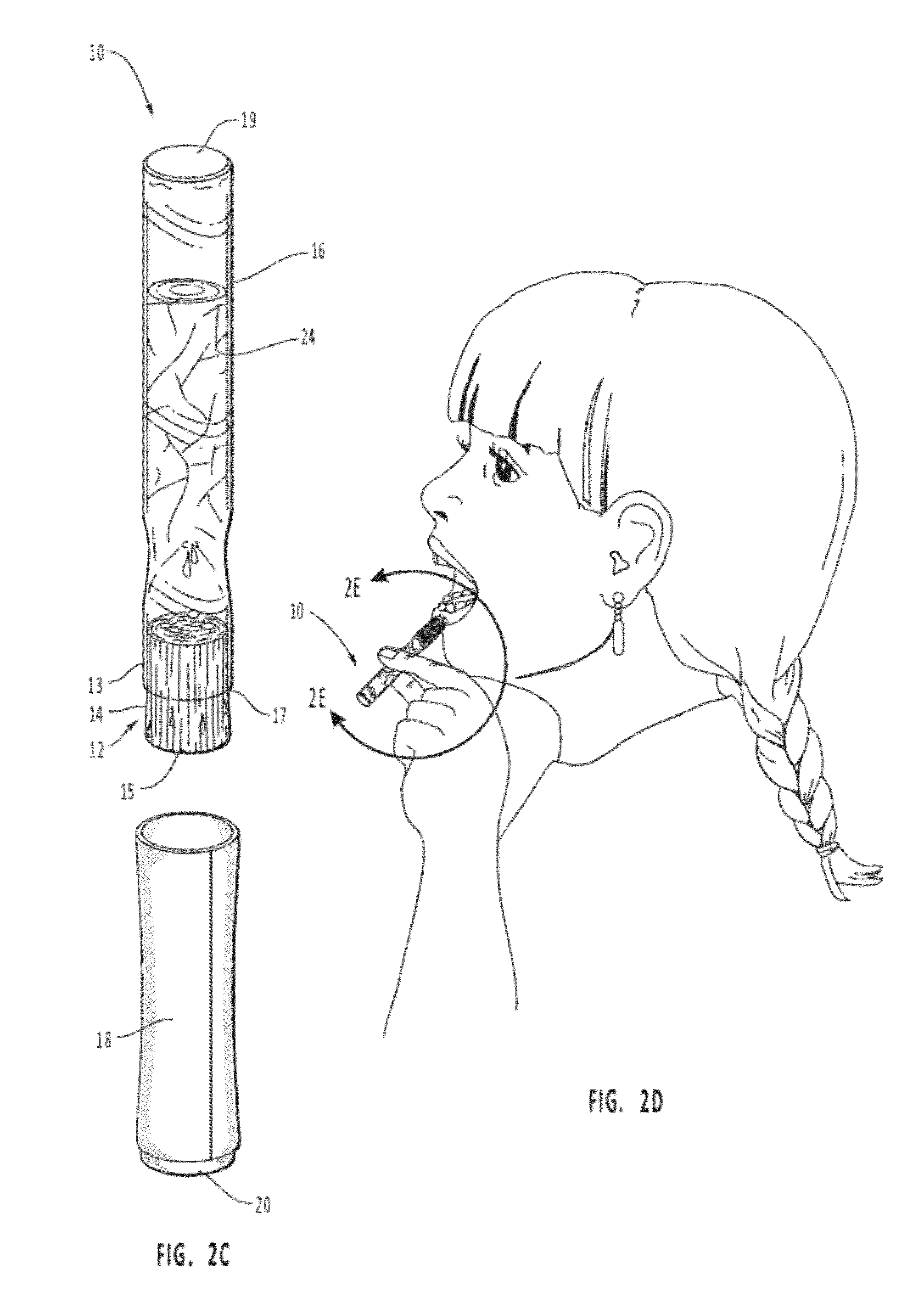
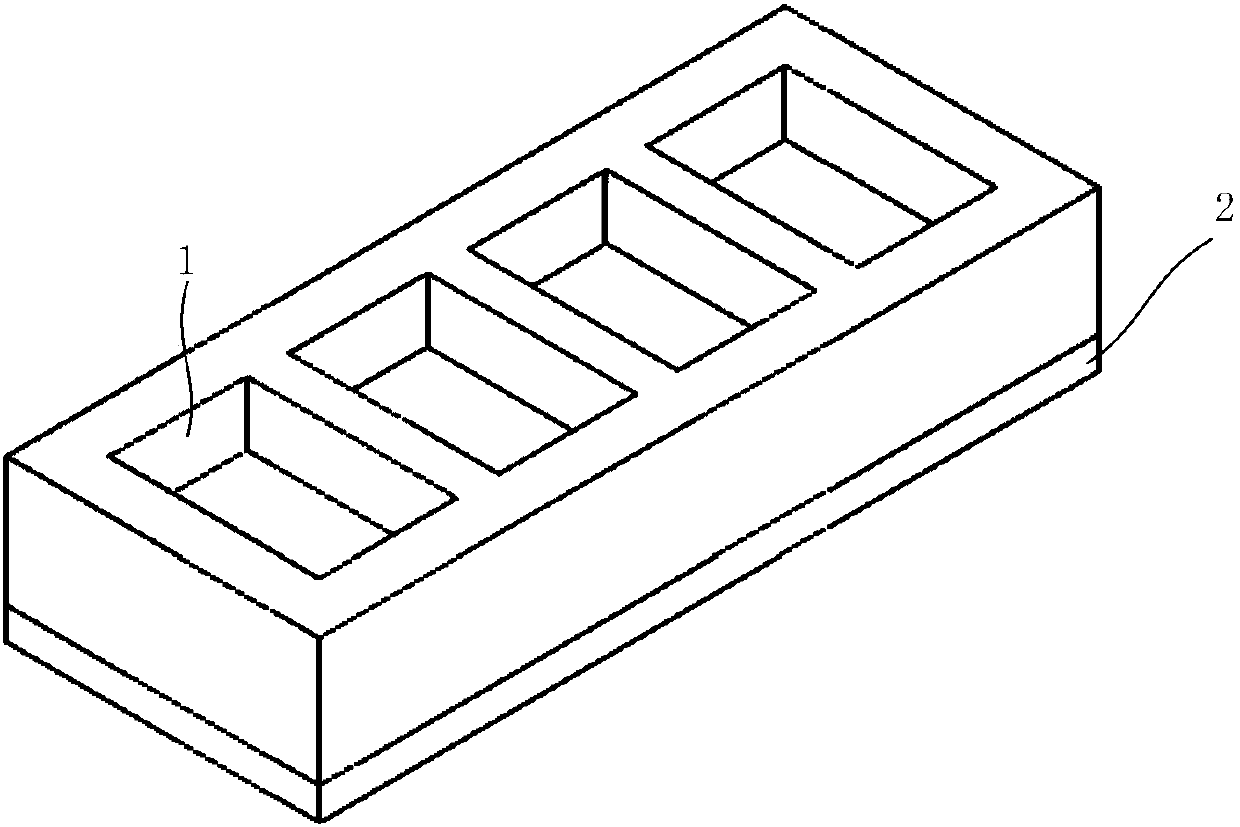
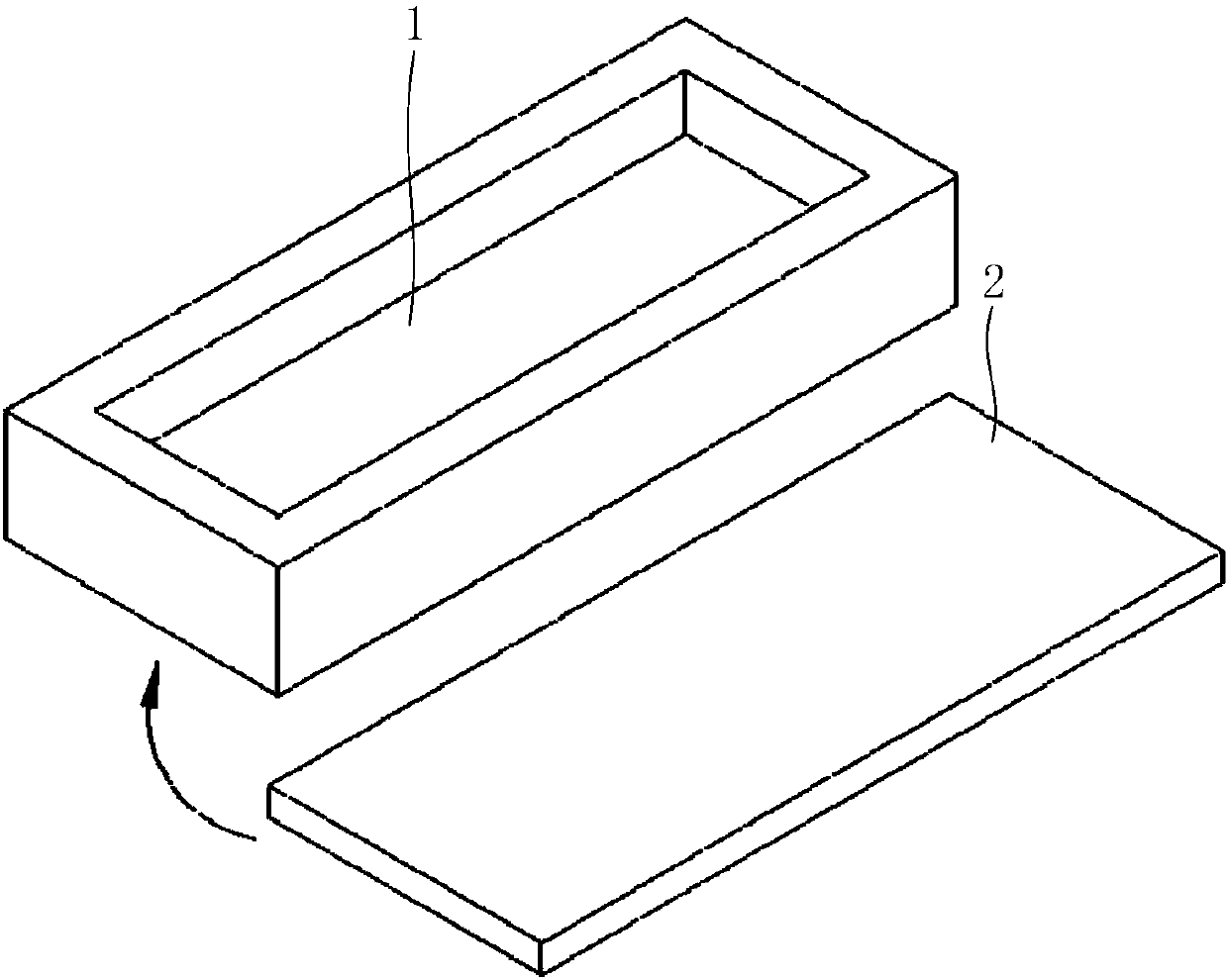
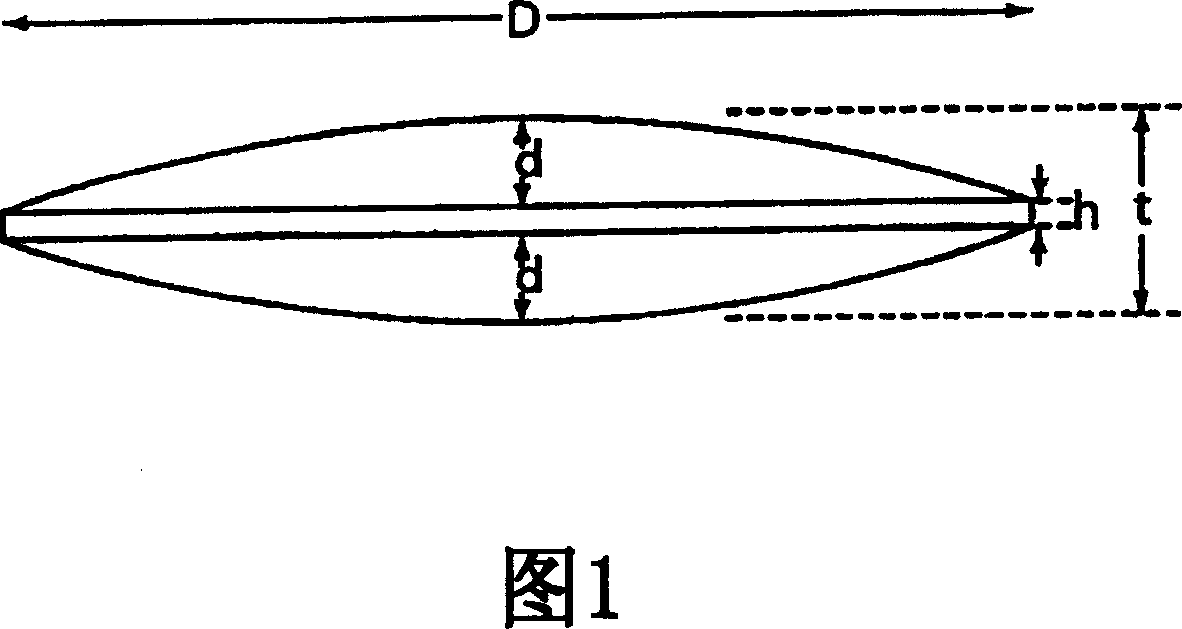
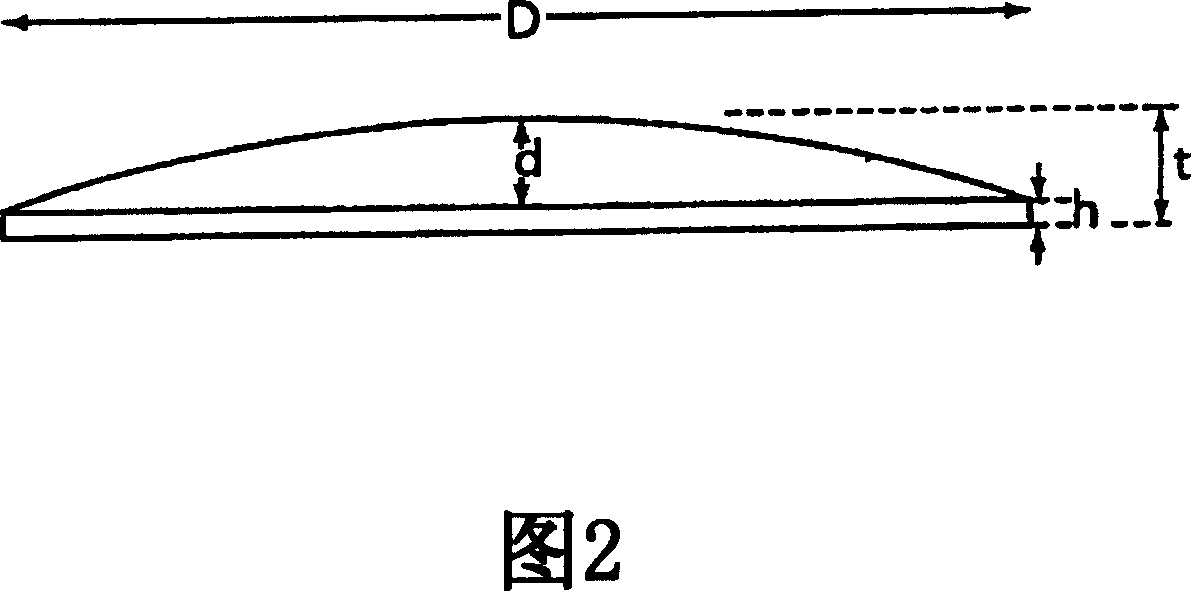
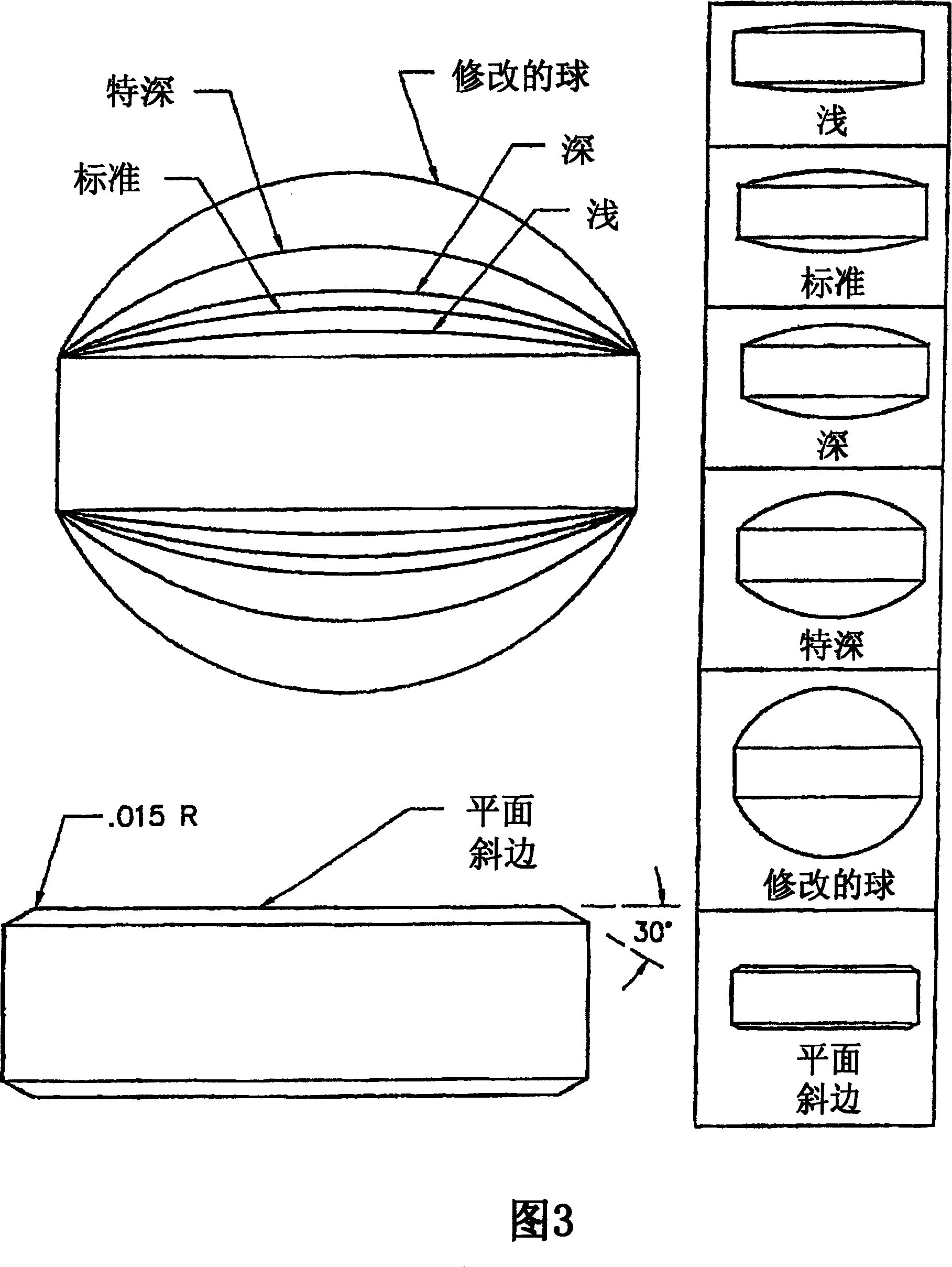
Mucosal delivery tablet
InactiveUS20070048369A1Reduce exposureResists brittle fractureBiocideAntipyreticMucoadhesionBiomedical engineering
Mucoadhesive tablets have a convex surface, a diameter to cup depth ratio of 4-20 and a cup depth to edge thickness ratio of greater than 0.75 swiftly adheres and conforms to the contacting tissue. The tablets are used to administer actives such as, for example, benzocaine.
Owner:HENKEL KGAA
Pain relief and soothing cream and method for making same
emollient and methyl salicylate, where said methyl salicylate comprises between 10 and 30 percent by weight of said cream; a skin stimulant comprising menthol crystals and two or more reactants selected from a group of camphor, cinnamomum cassia, diclofenac, eucalyptus, and ginger to cause a hot and cool stimulus on an epidermal surface; a pain reliever comprising two or more agents selected from a group of benzocaine, emu oil, eucalyptus oil, methylsulfonylmethane and glucosamine; and an inflammatory comprising two or more agents selected from a group of artemisia vulgaris, amica Montana flower extract, licorice root extract, oat extract, and thyme oil.
Owner:TRAN HUYNH D
Liniment for treating mouth and tooth diseases and applicator
InactiveCN102198126AObvious hyperplasiaPrevent proliferationAntipyreticInorganic active ingredientsNasopharyngeal catheterDentistry
The invention discloses compound benzocaine externally-applied medicinal liquid, gel or ointment. The medicine liquid, gel or ointment, which contains a compound medicine, is filled into an applicator to be applied on oral wound surfaces, ulcer surfaces and mucosa surfaces, treat tooth diseases, narcotize and relieve itching caused by haemorrhoids, treat itch and lubricate a nasopharyngeal catheter, inner root sight glass and the like for relieving pain.
Owner:广西星银迪智药业有限公司
Non-flammable topical anesthetic liquid aerosols
A topical liquid aerosol formulation for accurate metered dose delivery has been developed which includes a concentrate comprising a local anesthetic in a non-alcohol solvent and a hydrofluorocarbon (HFC) propellant. In the preferred embodiment, the concentration of the non-alcohol solvent in the concentrate is between about 75% and 85% by weight of the formulation. In the most preferred embodiment, the non-alcohol solvent is a water-soluble polyol such as ethylene glycol, propylene glycol, glycerol, diethylene glycol, dipropylene glycol, oligoalkylene glycols, liquid polyalkylene glycols, or combinations thereof. In one embodiment, the concentration of the local anesthetic in the concentrate is between about 15% and 25% by weight. In the preferred embodiment, the hydrofluorocarbon propellant is 1,1,1,2-tetrafluoroethane 1,1,1,2,3,3,3-heptafluoropropane or combinations thereof, in a concentration between about 35% and 65% by weight of the final formulation, more preferably between about 45% and 55% by weight of the final formulation. A particularly preferred formulation includes benzocaine, tetracaine, and butylaminobenzoate, wherein the concentration of benzocaine in the concentrate is 14% by weight, the concentration of tetracaine in the concentrate is 2% by weight, and the concentration of butylaminobenzoate in the concentrate is 2% by weight. It has been found that the formulation is more stable in the substantial absence of oxygen. The formulation is preferably administered using a metered dose device for release of a controlled amount of the local anesthetic.
Owner:PRECISION DERMATOLOGY
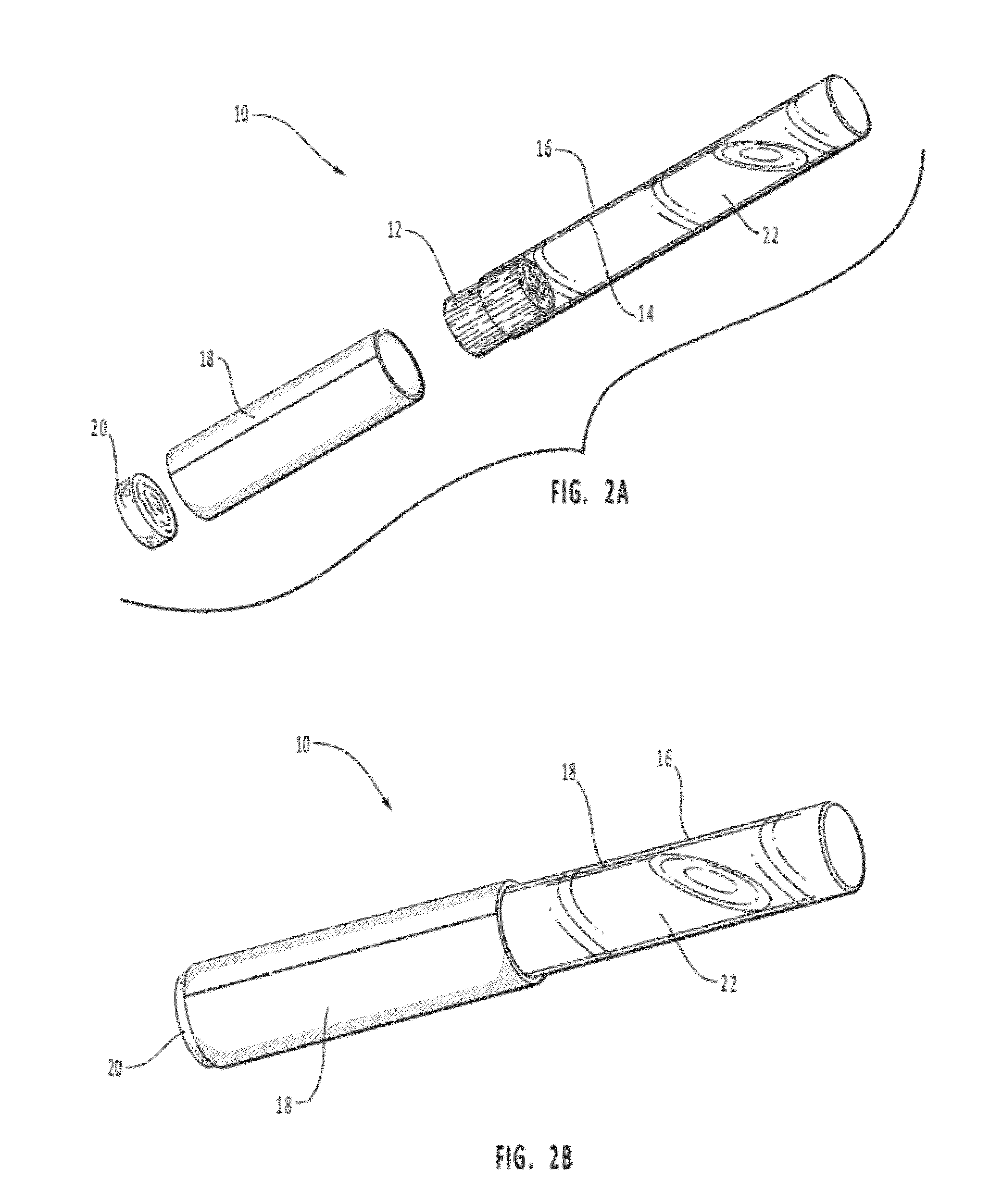
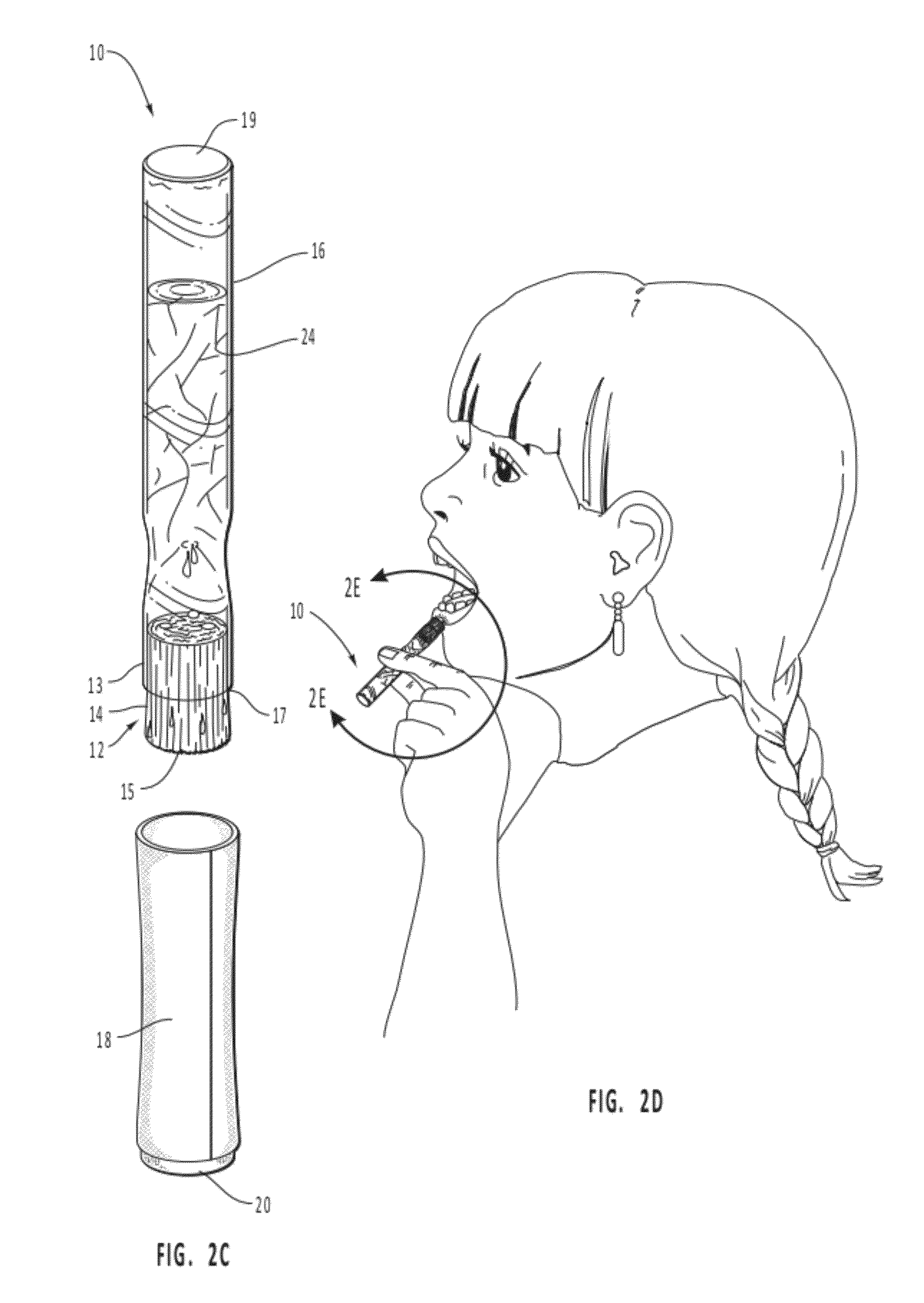
Stimulant/desensitizer swabs
InactiveUS20060029648A1Quickly stimulate the penisQuick installationBiocideAnimal repellantsPenisStimulant
A method and pharmaceutical composition for treating the sexual dysfunction of premature ejaculation is disclosed. The pharmaceutical composition consists of a solution of one or more stimulants and a desensitizer. The stimulant is selected from the group comprising spearmint solution, peppermint solution, cool mint solution, pepper solution, alcohol, rubbing alcohol, ether, or any solution that will stimulate the penis to cause an erection. The desensitizer is selected from the group comprising benzocaine and lidocaine. The pharmaceutical composition is enclosed within a cotton swab applicator and is applied directly to the penis with the cotton swab applicator.
Owner:TSAUR GARRY
Inflammation-eliminating, pain-stopping, bacteria-resisting, pain-relieving soluble hemostatic gauze and its processing method
ActiveCN1799639AAnti-stickWith sterilizationAbsorbent padsSynthetic polymeric active ingredientsCelluloseAntimicrobial drug
A soluble hemostatic gauze for anti-inflammation and pain relieving and the preparing method, relating to field of medical sanitary goods. The hemostatic gauze comprises the following substance according to the percentage by weight: dibasic sodium phosphate 0.1-0.3%, sodium alginate 1-1.8%, carbomu 0.5-25, antibacterial drugs 1-10%, polyethylene glycol 1-2%, tuwen 1-4%, dimethicone 0.1-1%, sodium carboxymethylcellulose 0.2-0.8%, americaine 0.05% and the left is ethanol solution. The product possesses functions of special pain relieving, preventing adhesion after surgery besides functions of hemostatic, broad spectrum antibiotic and promoting wound healing, which is of great importance for relieving patient pain and improving their survival quality.
Owner:青岛中惠圣熙生物工程有限公司
Highly penetrating compositions and methods for treating pathogen-induced disordered tissues
InactiveUS20120190715A1Simple compositionRelieve painAntibacterial agentsBiocideActive agentMedicine
Compositions and methods for treating disordered tissues, such as caused by pathogens and / or by toxins. The treatment compositions include an anti-infective active agent, a liquid carrier, and benzocaine in an amount so that the treatment composition penetrates more quickly into disordered tissue compared to the treatment composition in the absence of the benzocaine. In addition, the benzocaine can increase residence time of the anti-infective active in the treatment area. The preferred anti-infective active agent can be an organohalide, such as a quaternary ammonium halide compound, an example of which is benzalkonium chloride. The treatment compositions and methods may employ the use of an applicator adapted for use in promoting penetration of the treatment composition and / or agitation of the disordered tissue to further enhance penetration.
Owner:QUADEX PHARMA
Condom with localized active agent
InactiveCN102083395AMale contraceptivesPharmaceutical delivery mechanismAdditive ingredientVascular dilatation
Provided are condoms and methods of making and using the same, the condoms having an elastomeric layer and a low melt wax bead which comprises an active ingredient mixed therein, wherein the bead is affixed on the interior surface of the elastomeric layer in the tip of the condom. Active ingredients can include spermicides such as nonoxynol-9 and vasodilators such as niacin, sildenafil citrate, and nitroglycerin and male desensitizing agents such as benzocaine. The bead can comprise a natural wax in combination with polyethylene glycol or it can be a polyethylene glycol-based wax. The exterior surface of the elastomeric layer is substantially free of the active ingredient.
Owner:ANSELL HEALTHCARE PRODS
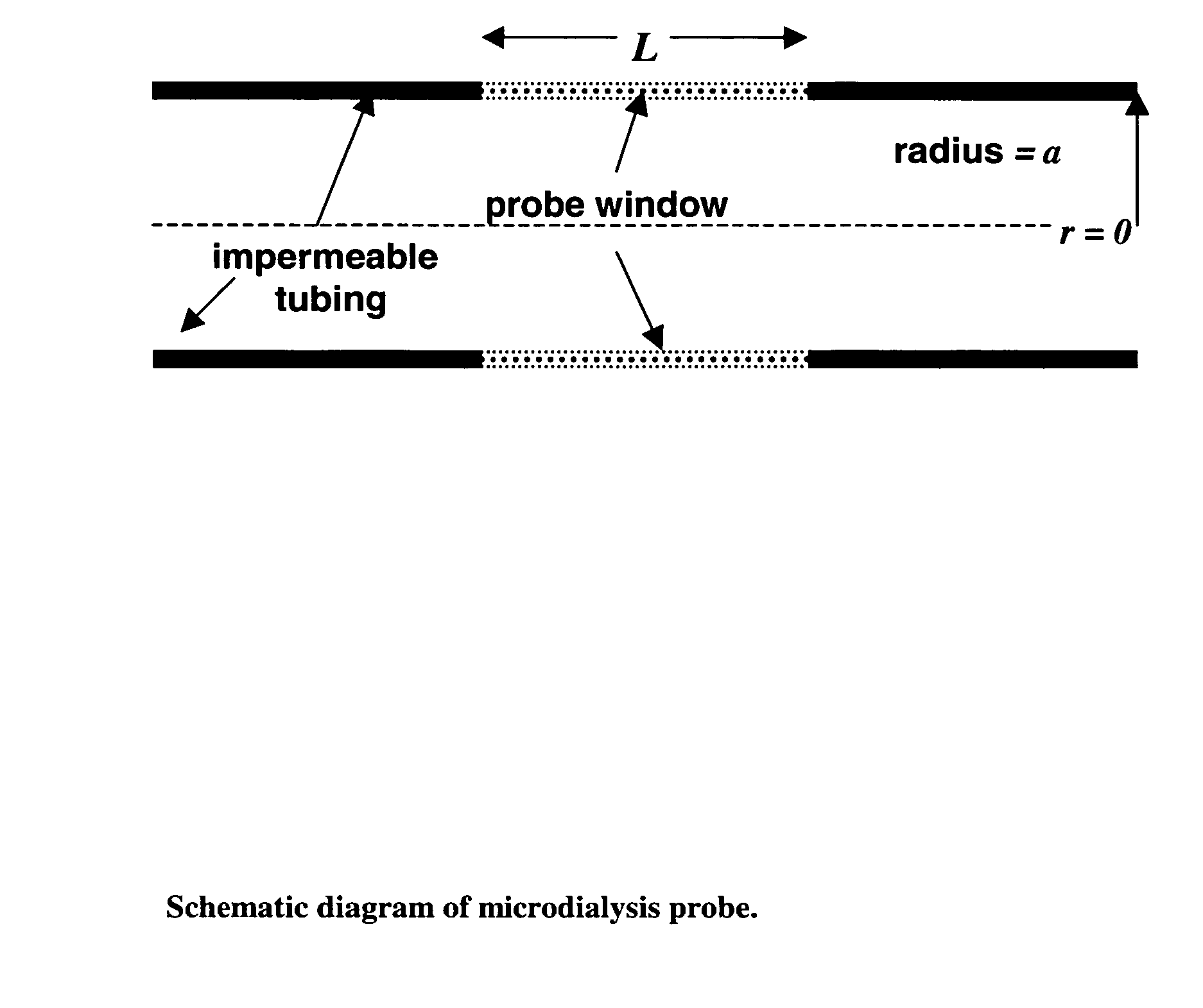
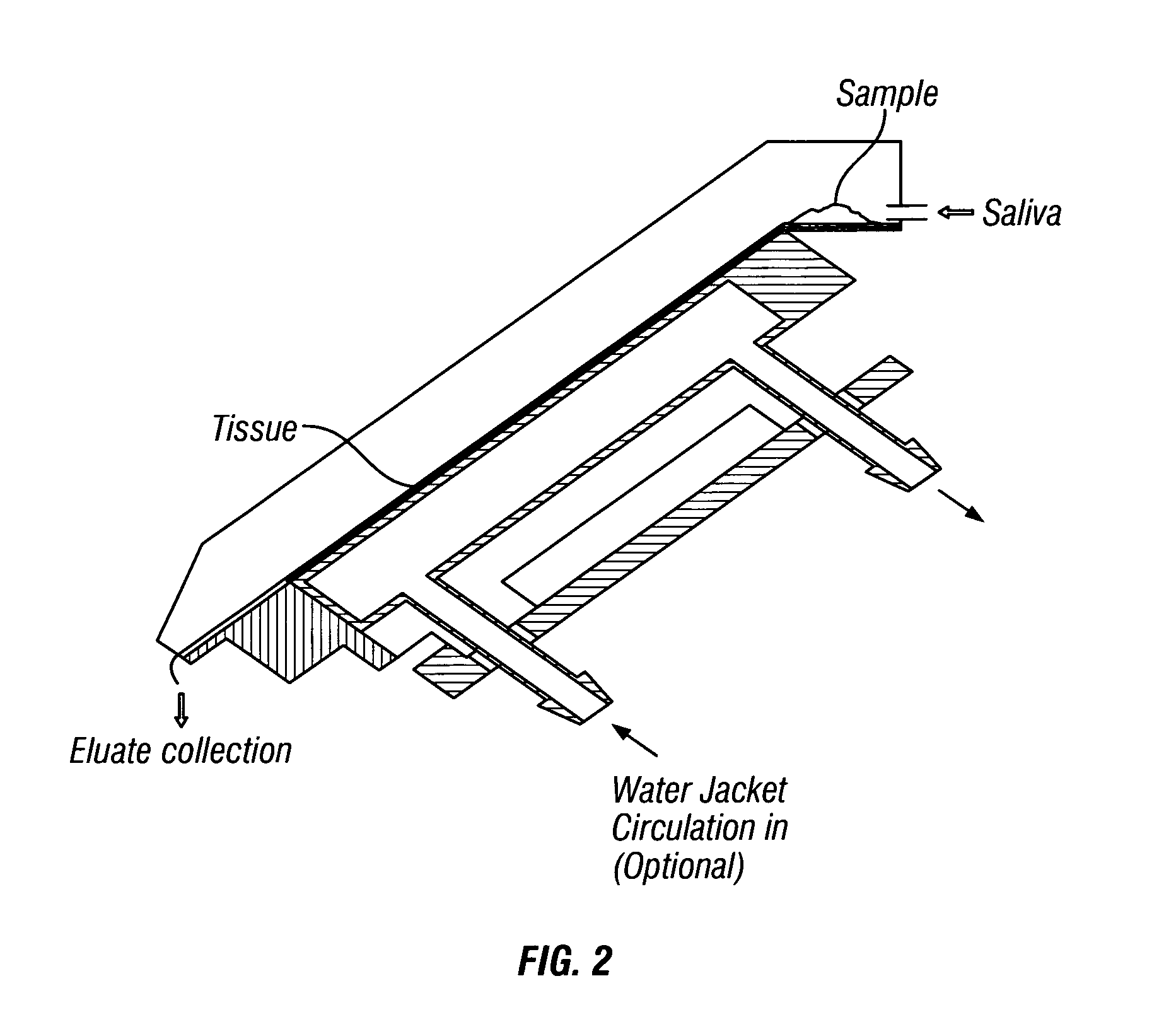
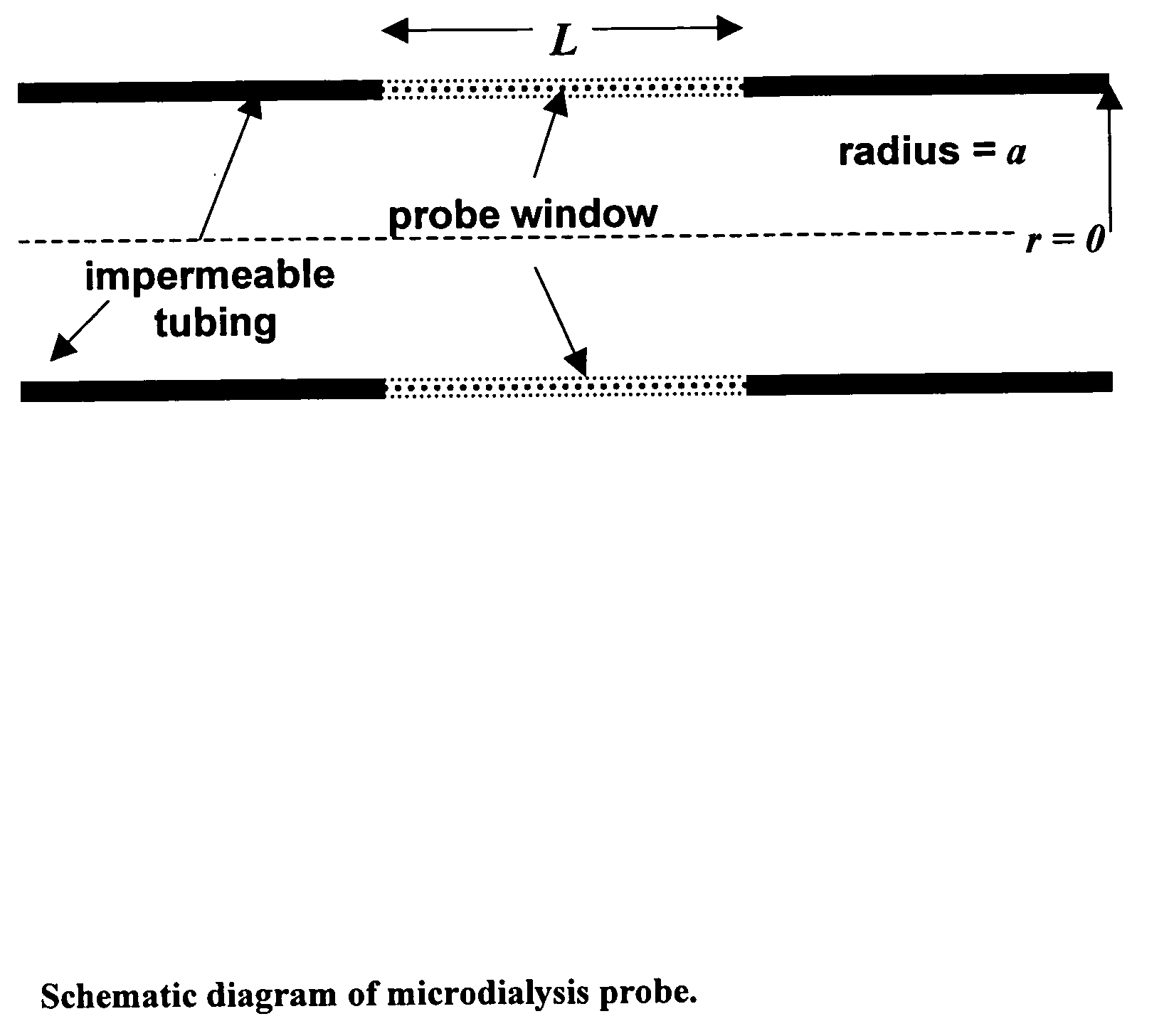
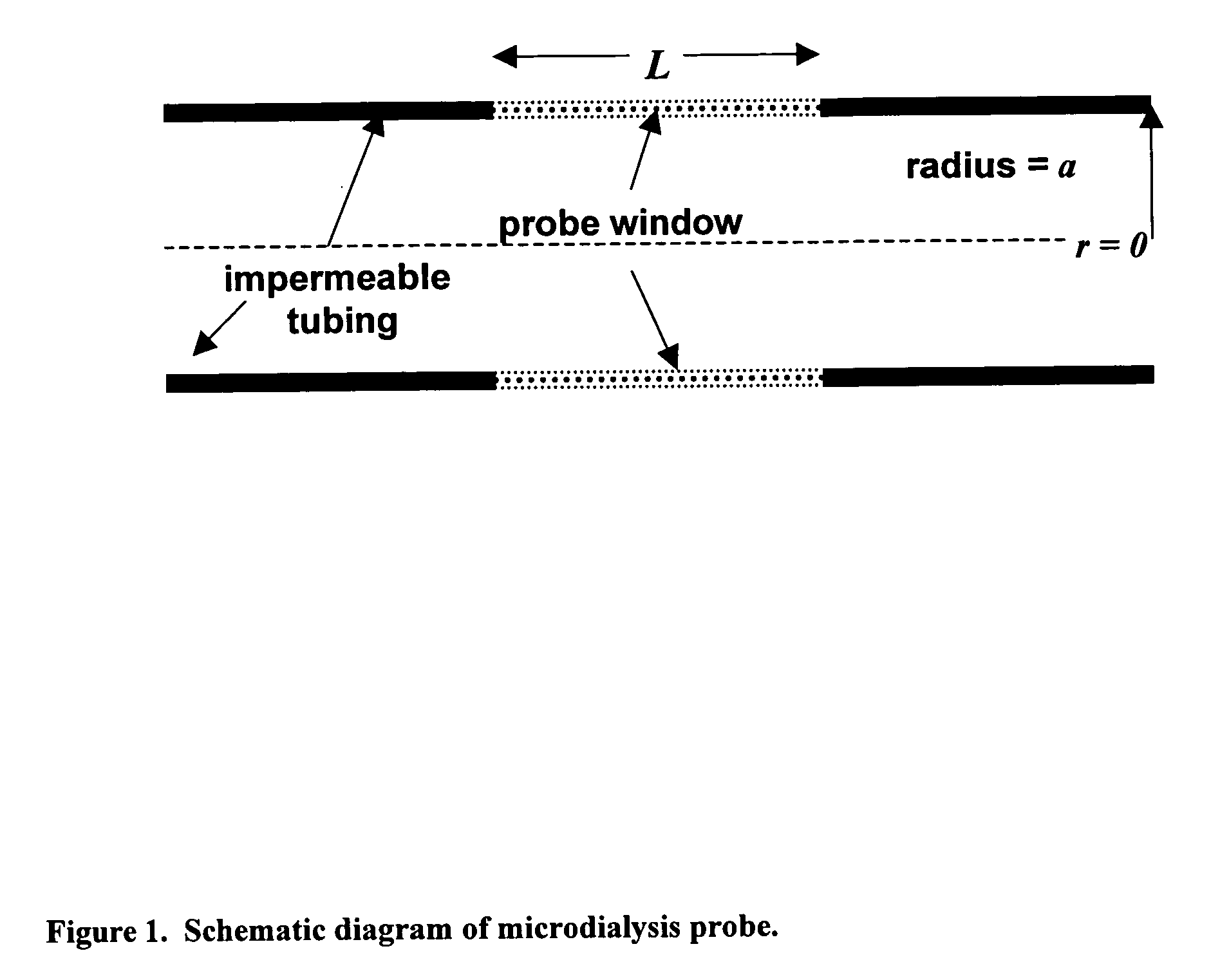
Method for use of microdialysis
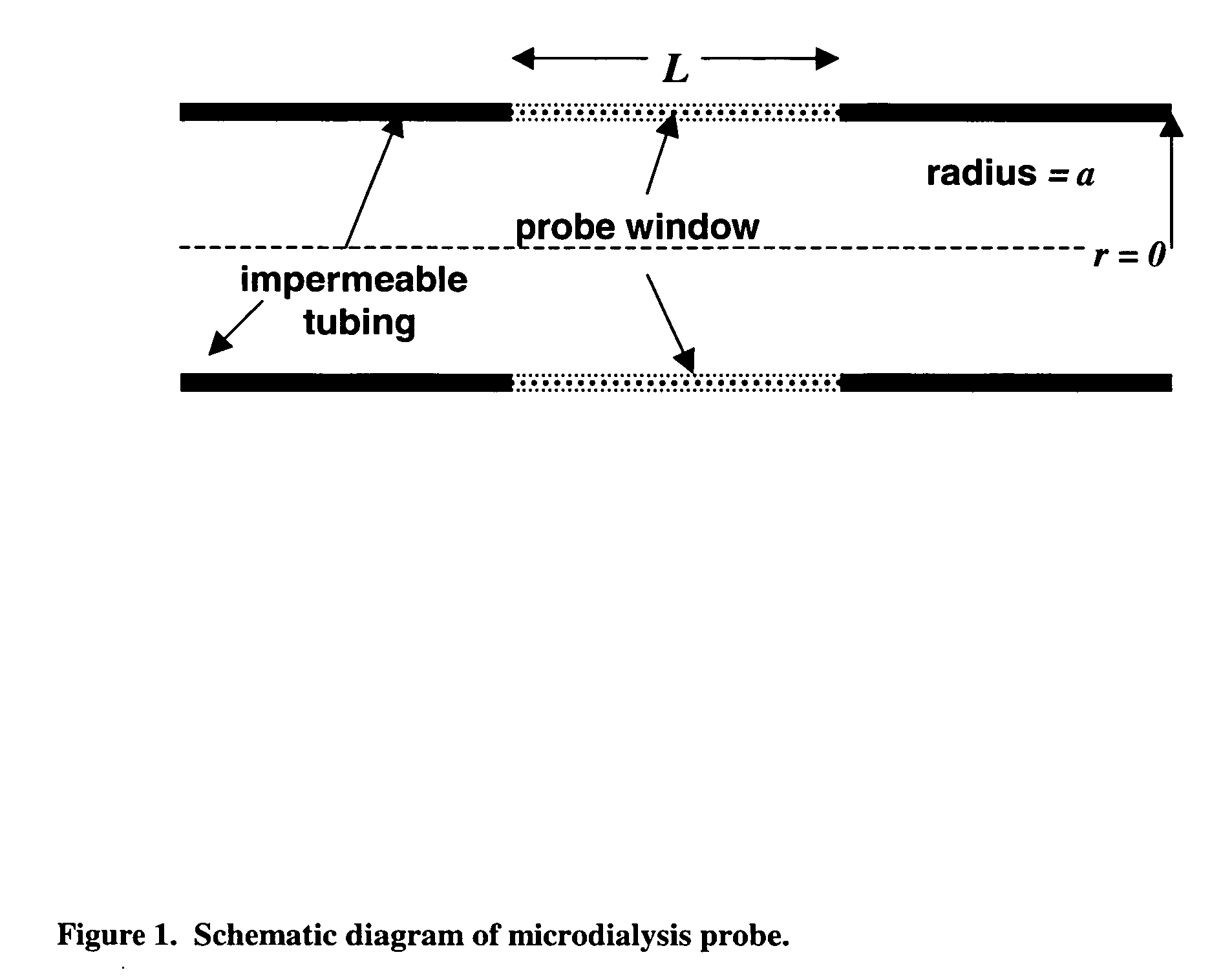
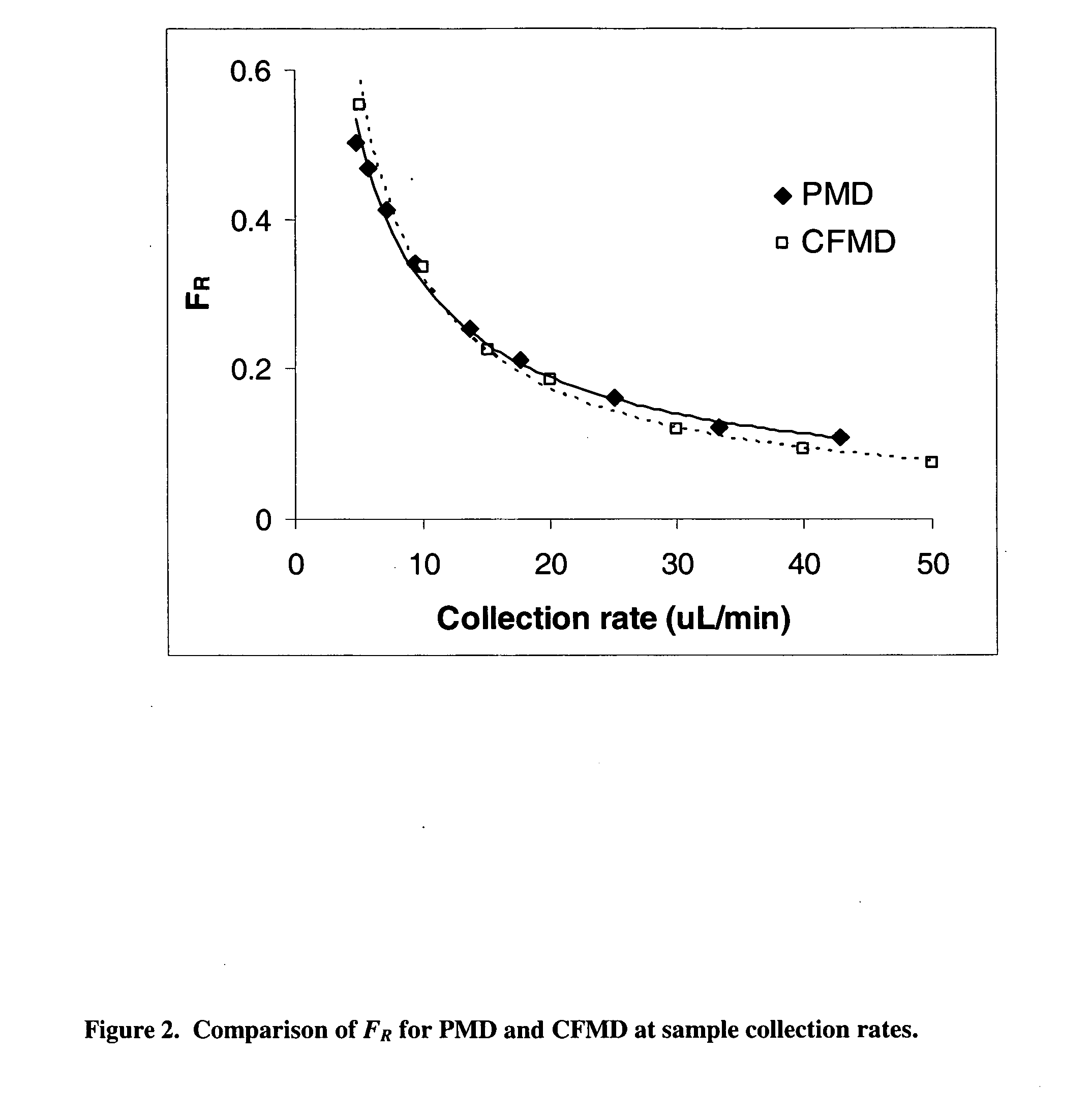
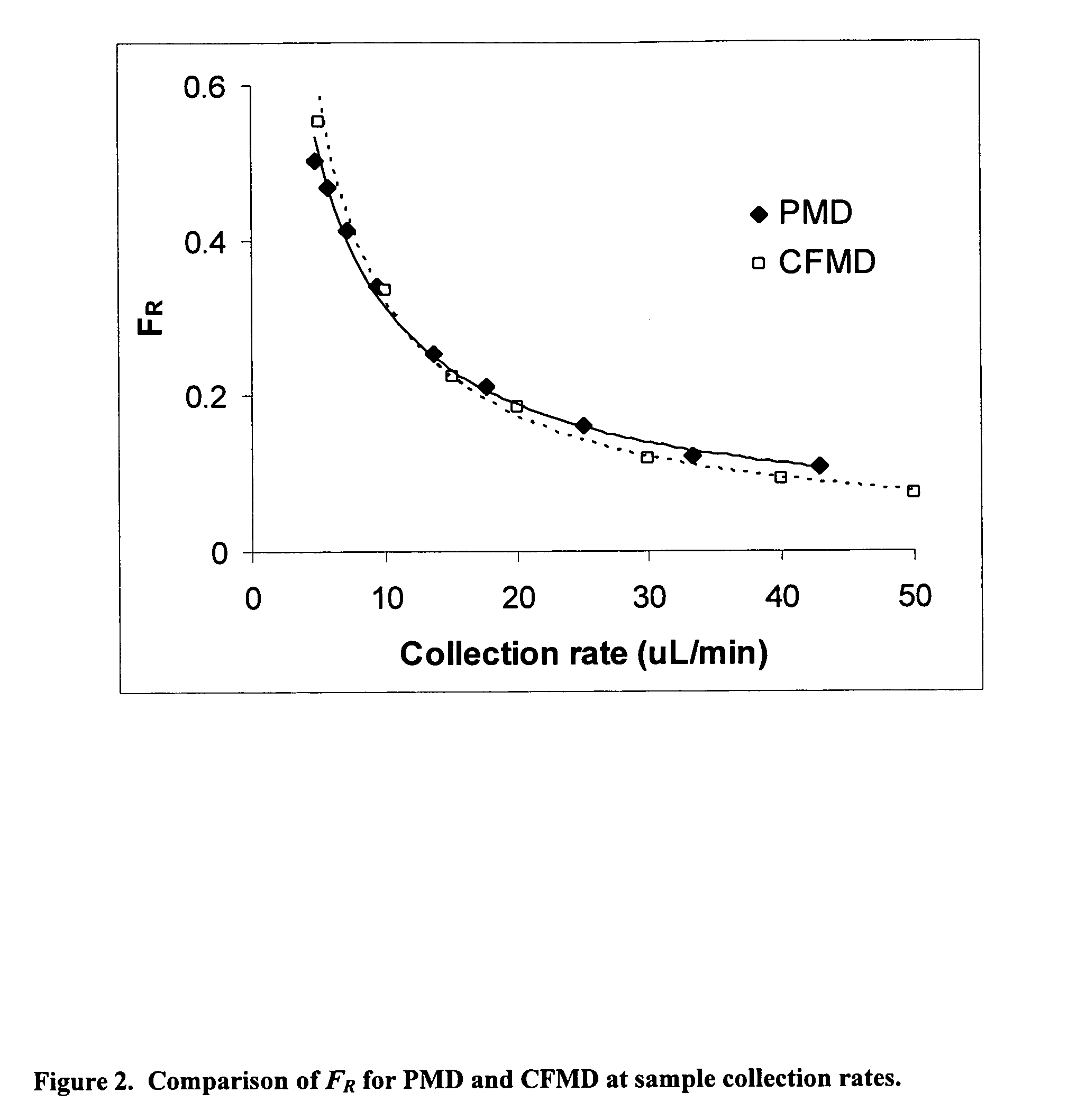
InactiveUS20070106140A1Fast resultsAccurately permeabilitySensorsBlood characterising devicesResting timeHigh rate
It has been surprisingly found that very accurate measurements of mass transfer can be made rapidly by permitting diffusion of an agent desired to be measured into a small, known volume of receiver or out of a known volume of donor, then rapidly pumping or flushing (“pulsing”) the receiver with a known volume of fluid. More specifically, a novel method of transferring small quantities of a contained material (either dissolved or suspended) between two media, based on such pulsing, hereinafter called pulsatile microdialysis (PMD), is disclosed. In a preferred embodiment, one medium (the dialysate) is inside a small, permeable tube (microdialysis probe window) and the other (external medium) is outside. The transfer of material between the two media can be utilized, for example, to sample drug concentrations in the external medium, or the release of drugs from systems within the dialysate, or for other measurements as disclosed herein. In PMD, a dialysate fluid is pumped into a microdialysis probe window, allowed to occupy the probe window while at rest for some resting time, and then flushed at a high rate out as a single pulse. A model that is based on a Fick's Laws was solved, and equations were derived to calculate the effects of various experimental parameters. The models were verified against experimental data using methazolamide, warfarin and benzocaine as test drugs. The data followed the mathematical models. For cases in which the concentration of free drug in the medium outside the probe was constant or changed very slowly, the concentration calibration plots were linear. In simulated first order uptake studies, the PMD and direct donor sampling data were in nearly exact agreement with the theoretical values of k=0.09 min−1. In another experiment, the free concentration of warfarin sodium in the medium outside the probe was made to decline rapidly in a known first order manner, with rate constants as high as 0.077 sec−1. The concentration in the external medium calculated from the PMD data was in nearly exact agreement with the known concentration at various times, and the experimental rate constants were in nearly exact agreement with the theoretical rate constants. For binding of methazolamide to activated charcoal, and for the binding of sodium warfarin to bovine serum albumin, PMD was able to generate sufficient data points to accurately characterize the rapid initial binding. This invention demonstrates that PMD is an accurate method of sampling drug concentrations and measuring rates and extents of a number of processes, including protein binding, adsorption to binding agents such as activated charcoal, release from microemulsion drug delivery systems, and the determination of drug diffusion coefficients, and for various other purposes which will occur to those skilled in the art. Compared to known methods such as traditional (continuous) microdialysis, the present invention offers the ability to sample more frequently, and over much shorter time intervals, thereby accurately obtaining data not heretofore available.
Owner:BELLANTONE ROBERT ARTHUR
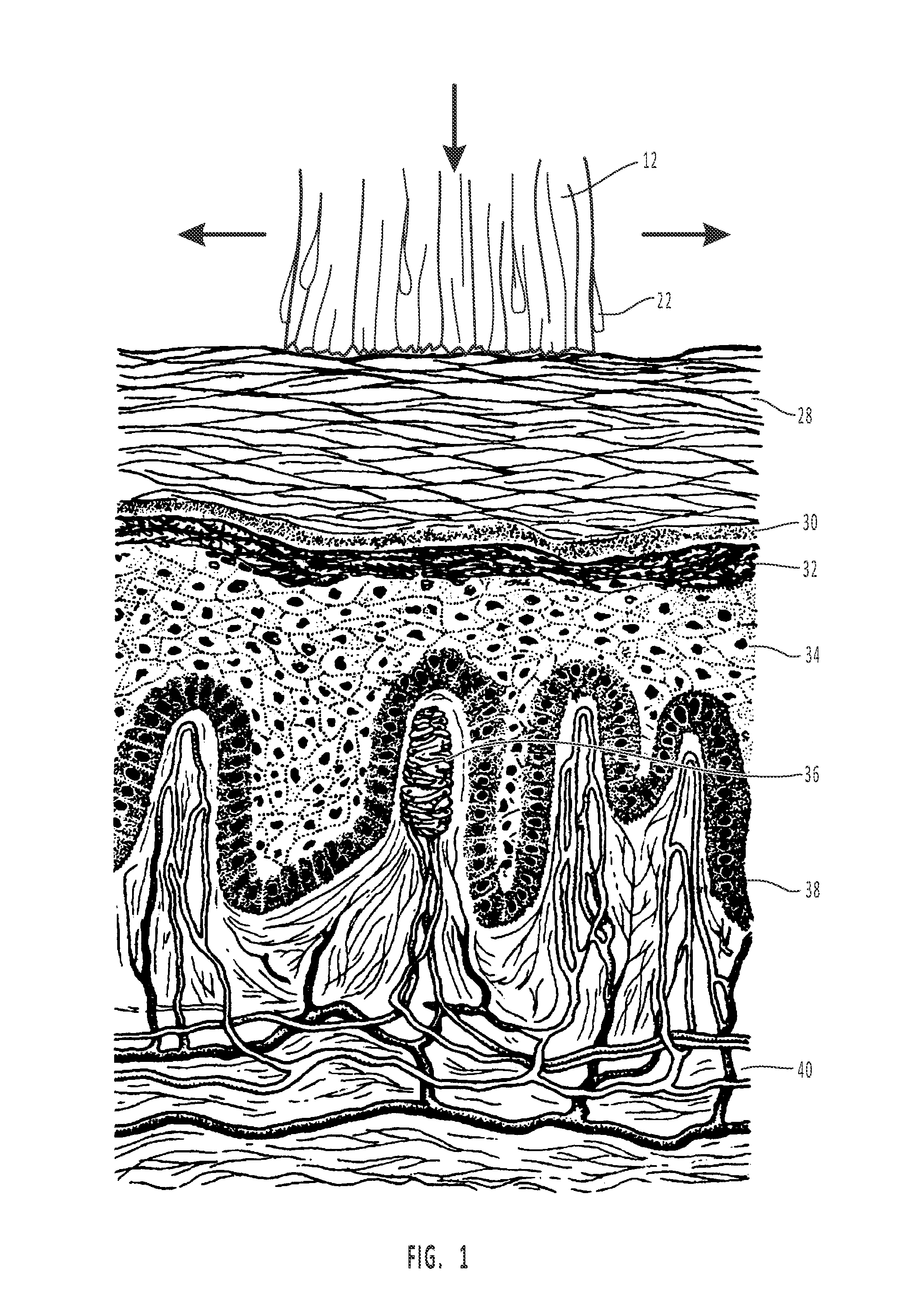
Bioadhesive compositions for epithelial drug delivery
ActiveUS20130131166A1Superior of analgesiaImprove adhesionBiocidePharmaceutical delivery mechanismDiseaseBioadhesive
Disclosed are compositions and methods for treating a disease, such as infection, pain, or inflammation, by using the compositions. Particularly, disclosed is a method of treating oral pain, wherein, the above-described compositions are applied to the oral mucosa; the compositions undergo in-situ gelation, optimal adhesion to the oral mucosa, controlled erosion and controlled release of the active ingredient, i.e., benzocaine, which provides a superior degree of pain relief or analgesia for an extended period of time.
Owner:TRILOGIC PHARMA
Benzocaine hapten, artificial antigen and antibody, and preparation and application thereof
ActiveCN110938011AReserve space structureStrong specificityTransferrinsOvalbuminAntiendomysial antibodiesBiochemistry
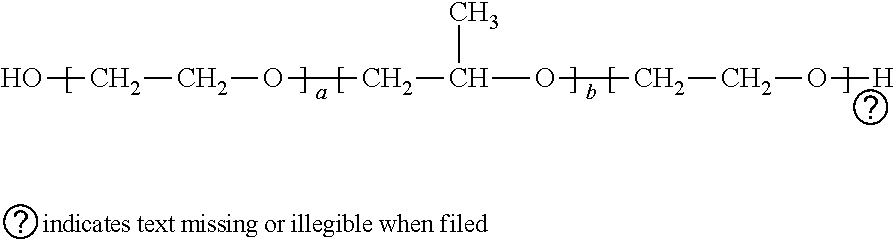
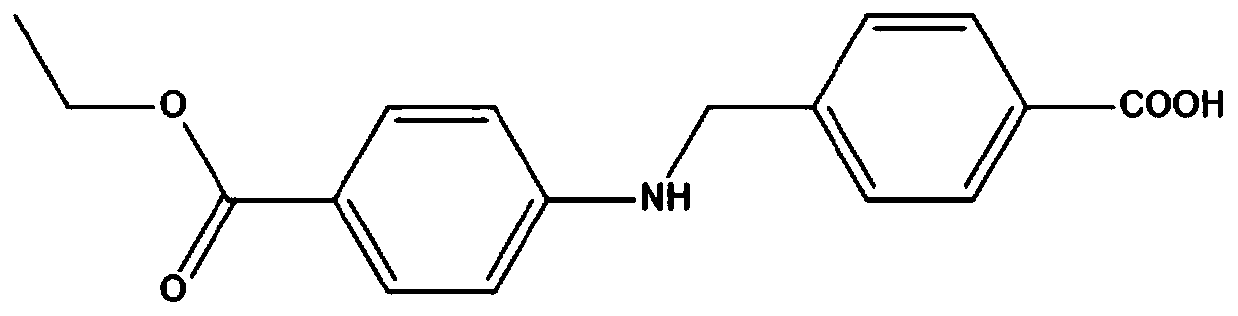
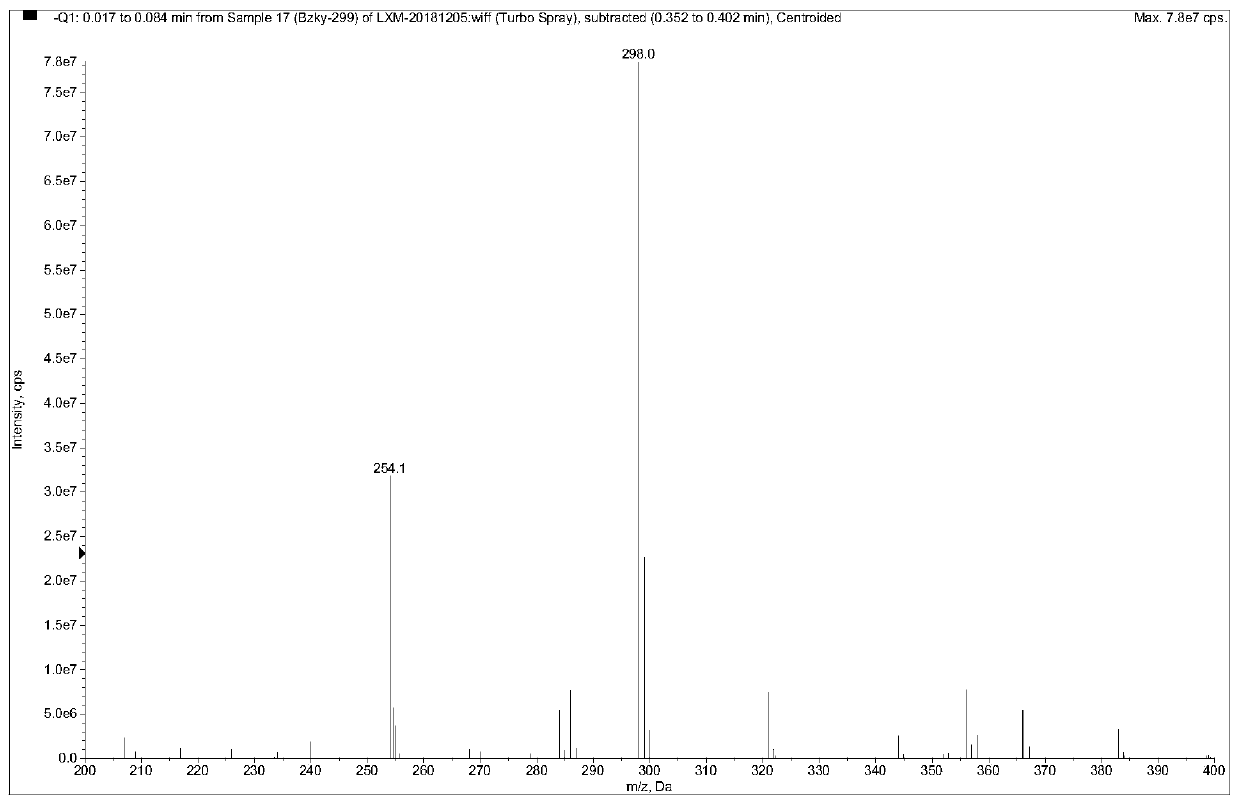
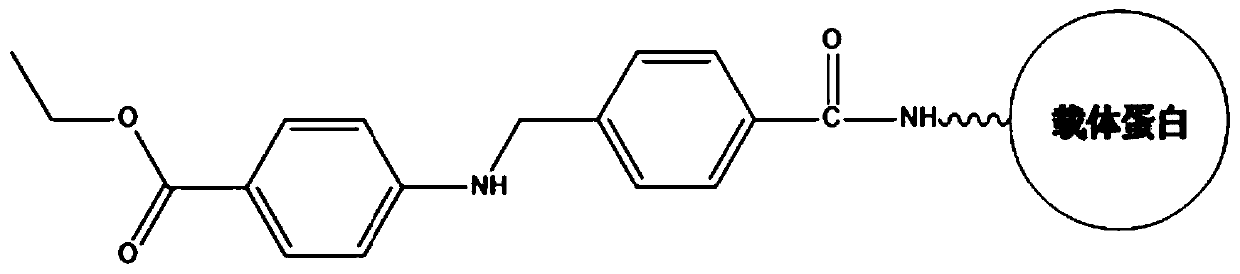
The invention provides benzocaine hapten, artificial antigen and antibody, and preparation and application thereof. The benzocaine hapten has the structure represented as the formula (I), and the benzocaine artificial antigen has the structure represented as the formula (II) or (III). Because there is less limit standard of the benzocaine, reporting of the synthesis of hapten and artificial antigen of the benzocaine is rare, and there is less related immunoassay method of the benzocaine, and the existing detection technology of benzocaine mainly includes instrument-based methods which are complex in operation and high in pretreatment demand and cost, the antiserum prepared from animals immunized by the artificial antigen has high titer, so that the antibody is high in specificity and sensitivity and is good in accuracy.
Owner:SOUTH CHINA AGRI UNIV
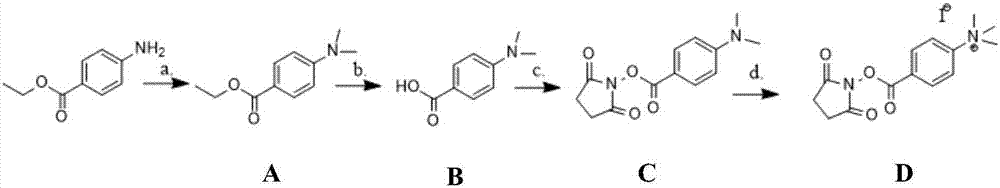
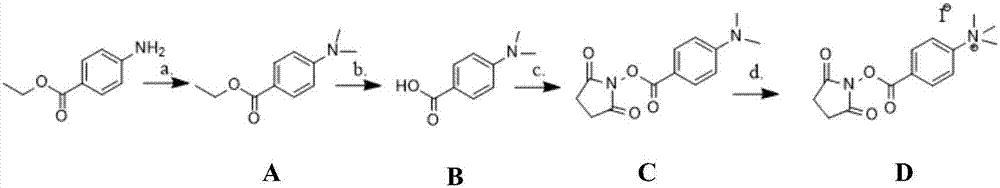
Green preparation process of benzocaine
PendingCN108314627AReduce usageHigh purityOrganic compound preparationAmino-carboxyl compound preparationOrganic solventP-nitrobenzoic acid
The invention discloses a green preparation process of benzocaine. The process comprises the steps that p-nitrobenzoic acid and ethanol are subjected to esterification under the catalysis of concentrated sulfuric acid to obtain ethyl p-nitrobenzoate; ethyl p-nitrobenzoate is subjected to catalytic hydrogenation to obtain the benzocaine, wherein catalytic hydrogenation reaction is performed in water; the catalytic hydrogenation reaction temperature is 90 to 110 DEG C; the catalytic hydrogenation reaction pressure is 0.2 to 1.0MPa; the use quantity of water is 3 to 7 times of the weight of ethylp-nitrobenzoate; the weight ratio of ethyl p-nitrobenzoate to a catalyst is (1:0.001)-(1:0.1); the catalyst is a Pd / C catalyst with the mass fraction being 3 to 10 weight percent. Through the catalytic hydrogenation reaction, the use of organic solvents is avoided, so that the whole benzocaine preparation becomes a green process; the proper use quantity of water is selected, so that high reactionyield and product purity can be obtained.
Owner:CHANGZHOU YONGHE FINE CHEM
Soluble hemostatic gauze for diminishing inflammation, killing bacteria and calming pain, and its making method
ActiveCN1803203AAnti-stickWith sterilizationAbsorbent padsAmine active ingredientsPolyethylene glycolSurface-active agents
Disclosed is a soluble hemostatic gauze for diminishing inflammation, killing bacteria and calming pain, and its making method, which comprises the following raw materials (by weight portion), 0.1-0.3% of disodium hydrogen phosphate, 1-1.8% of sodium alginate, 0.5-2% of Carbomer, 1-10% of cationic surface active agent, 1-2% of polyethylene glycol, 1-4% of Tween-80, 0.1-1% of dimethyl silicon oil, 0.2-0.8% of sodium carboxymethylcellulose, 0.05% of benzocain and balancing ethanol solution.
Owner:青岛中惠圣熙生物工程有限公司
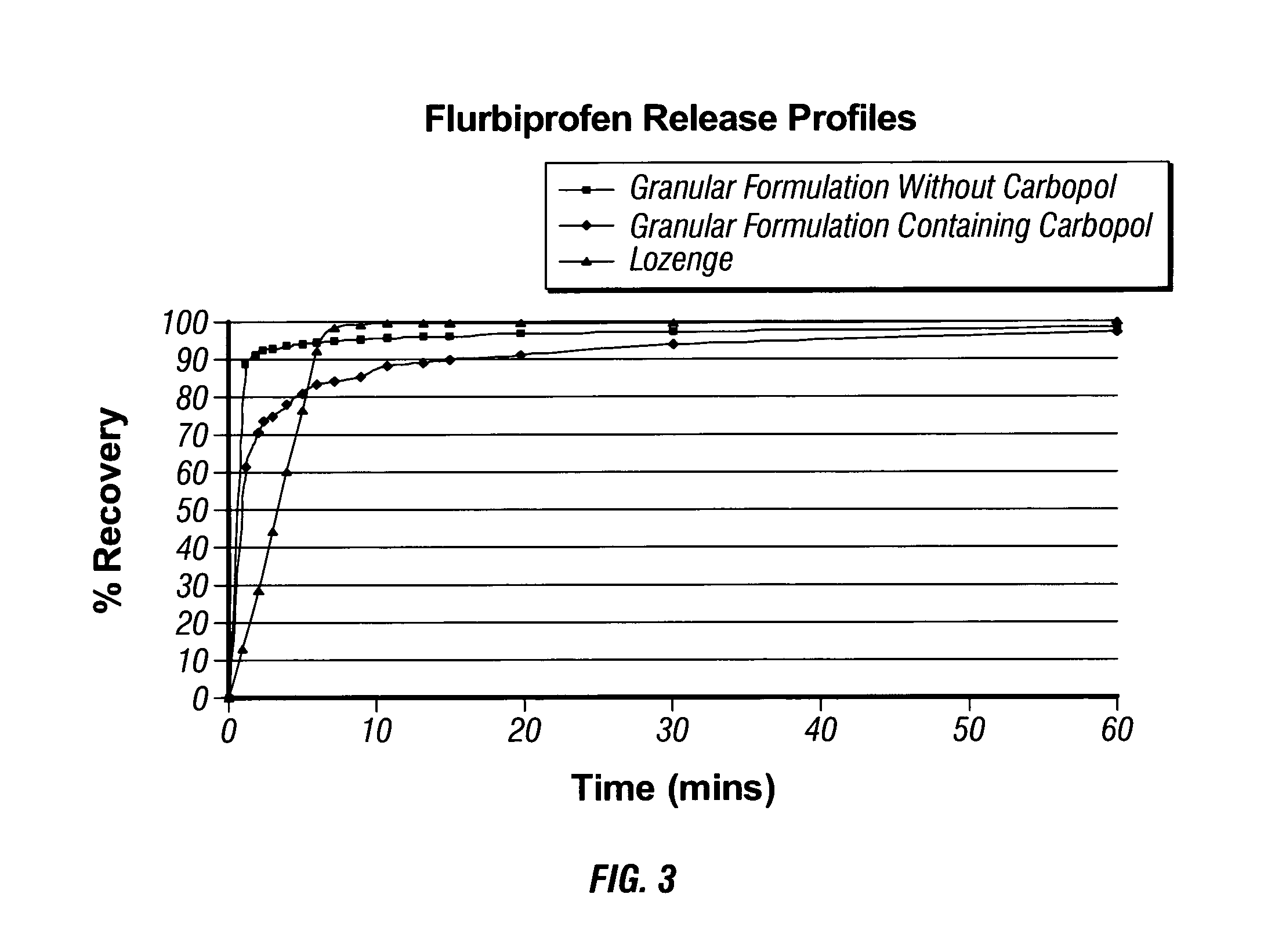
Compositions
ActiveUS8569375B2Reduce painLess discomfortBiocideSmall article dispensingParticulatesParticle composition
An ingestible particulate composition comprises: a) at least one compound selected from the group consisting of 2,4-dichlorobenzyl alcohol, amylmetacresol, cetylpyridinium chloride, hexitidine, hexylresorcinol, flurbiprofen, lidocaine, benzocaine, ibuprofen, paracetamol, pectin, menthol, and benzydamine; and b) one or more bioadhesive materials. Resulting particulate compositions have excellent flow characteristics, dust suppression, organoleptic properties and stability. They are highly suitable for administration direction into a patient's mouth, and ingested to alleviate the symptoms of a sore throat.
Owner:RECKITT BENCKISER HEALTHCARE (UK) LTD
Highly penetrating compositions and methods for treating disordered tissues
InactiveUS20140364496A1Improve efficiencyQuickly contact and killAntibacterial agentsBiocideMedicineActive agent
Compositions and methods for treating disordered tissues, such as caused by pathogens and / or by toxins. The treatment compositions include an anti-infective active agent, a liquid carrier, and benzocaine in an amount so that the treatment composition penetrates more quickly into disordered tissue compared to the treatment composition in the absence of the benzocaine. In addition, the benzocaine can increase residence time of the anti-infective active in the treatment area. The preferred anti-infective active agent can be an organohalide, such as a quaternary ammonium halide compound, an example of which is benzalkonium chloride. The treatment compositions and methods may employ the use of an applicator adapted for use in promoting penetration of the treatment composition and / or agitation of the disordered tissue to further enhance penetration.
Owner:QUADEX PHARMA
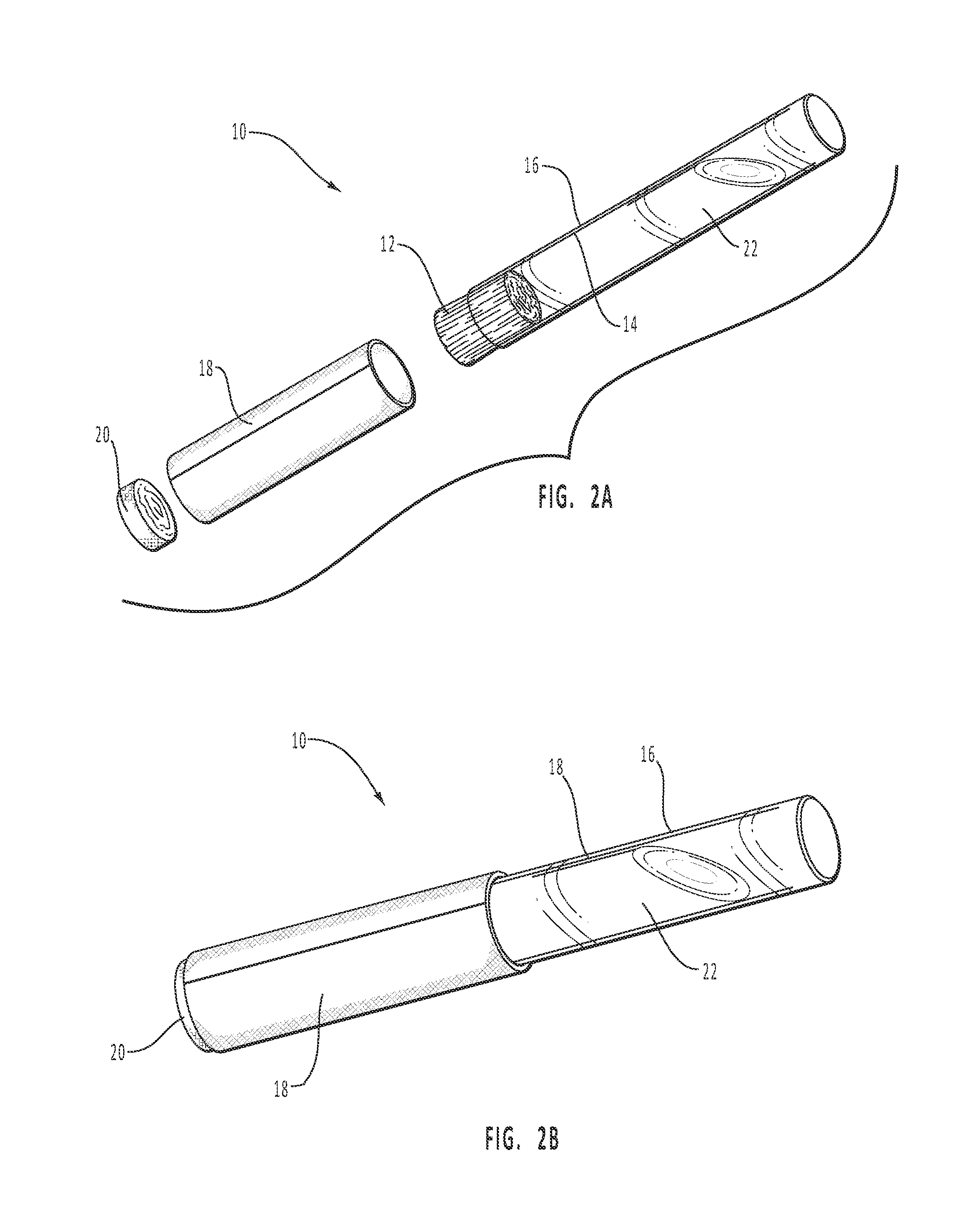
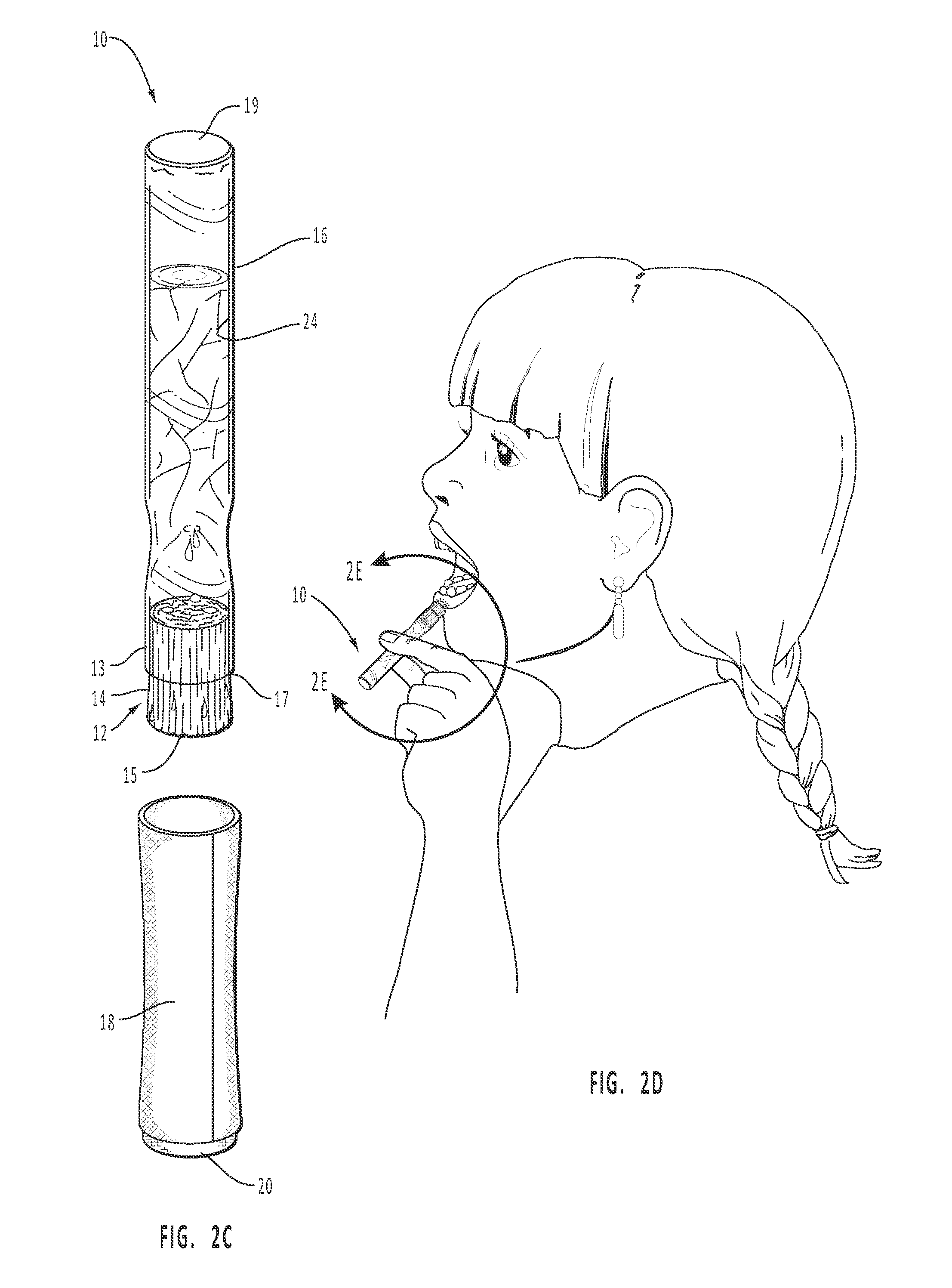
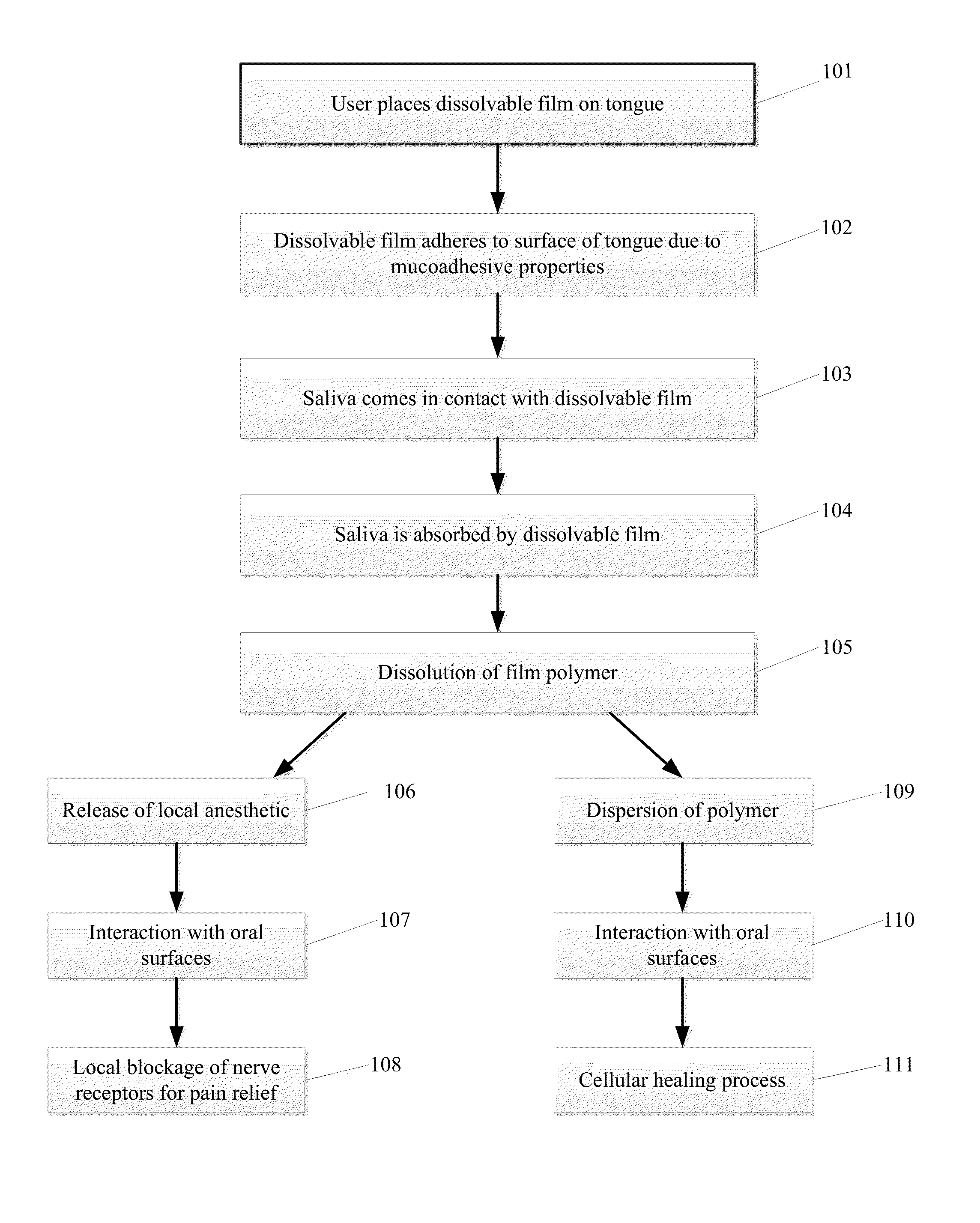
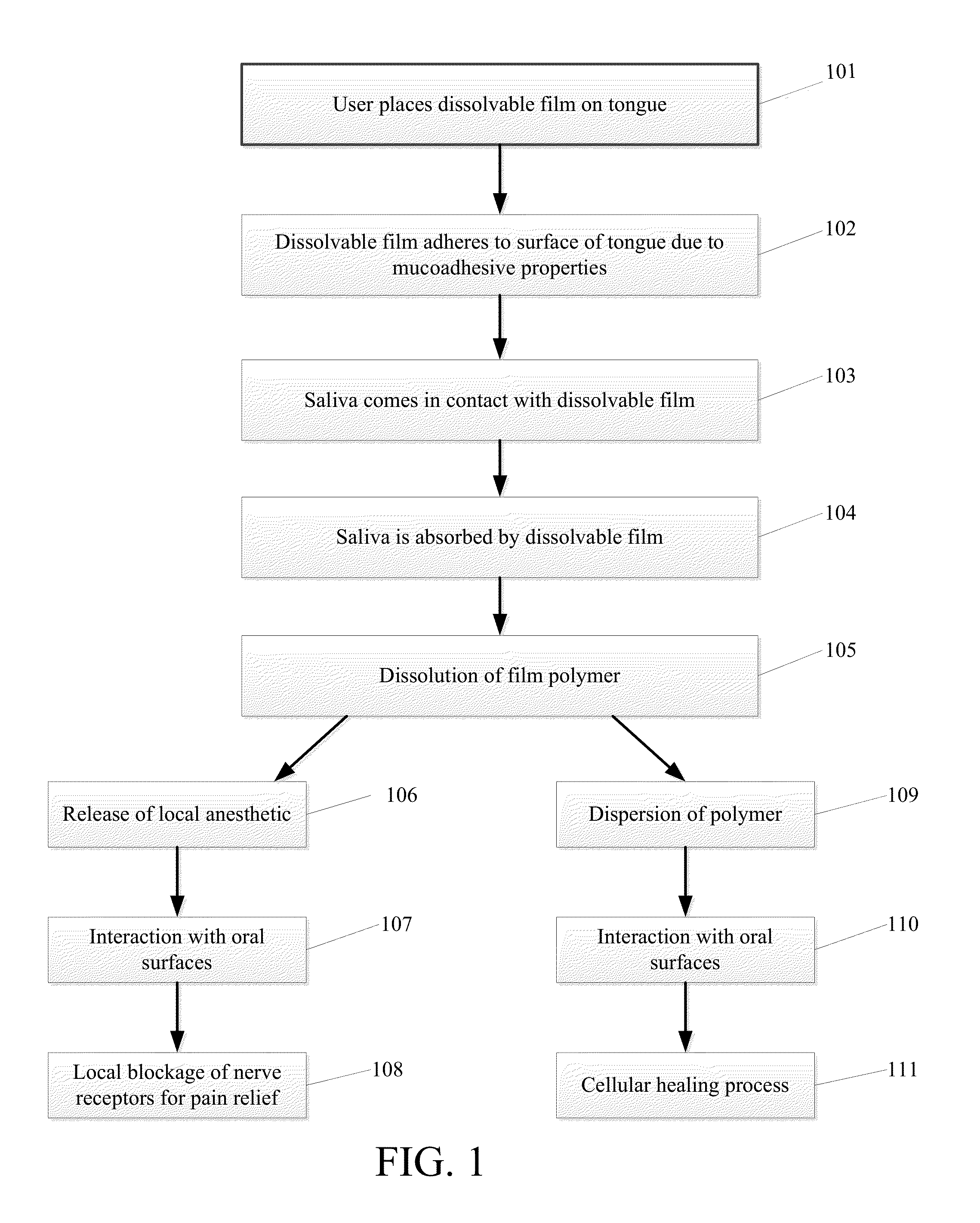
Dissolvable Strip for Treatment of Oral Thermal Burns
InactiveUS20150104493A1High tensile strengthFavorable consumer useHydroxy compound active ingredientsPharmaceutical delivery mechanismImmediate releaseDentistry
A consumable film adapted to adhere and to dissolve in the oral cavity that provides a local anesthetic and therapeutic agents for the treatment of oral burns or injuries. The film is designed to instantly release benzocaine, or other types of local anesthetic or therapeutic agent, upon adhesion to the affected areas of the mouth, and will continue to release sufficient quantities for pain relief and for healing over an extended period of time.
Owner:MCDONALD III ROBERT W +4
Synthesis method of benzocaine
InactiveCN106699579AHigh yieldImprove teaching effectOrganic compound preparationAmino-carboxyl compound preparationP-nitrobenzoic acidSynthesis methods
Relating to the field of chemical engineering, the invention in particular relates to a synthesis method of benzocaine. The method includes the steps of: synthesis of p-nitrobenzoic acid; synthesis of ethyl 4-nitrobenzoate; and synthesis of benzocaine. Through improvement of benzocaine synthetic experiment method, the invention specifically solves the problems of low yield, tedious reaction aftertreatment and the like in conventional preparation experiments, and the improved experimental operation method not only can achieve high yield preparation of benzocaine, enhance experimental efficiency, but also effectively improves the teaching effects of the experimental course, and is suitable for chemical experimental course teaching.
Owner:SHAANXI ALLIANCE LOGISTICS
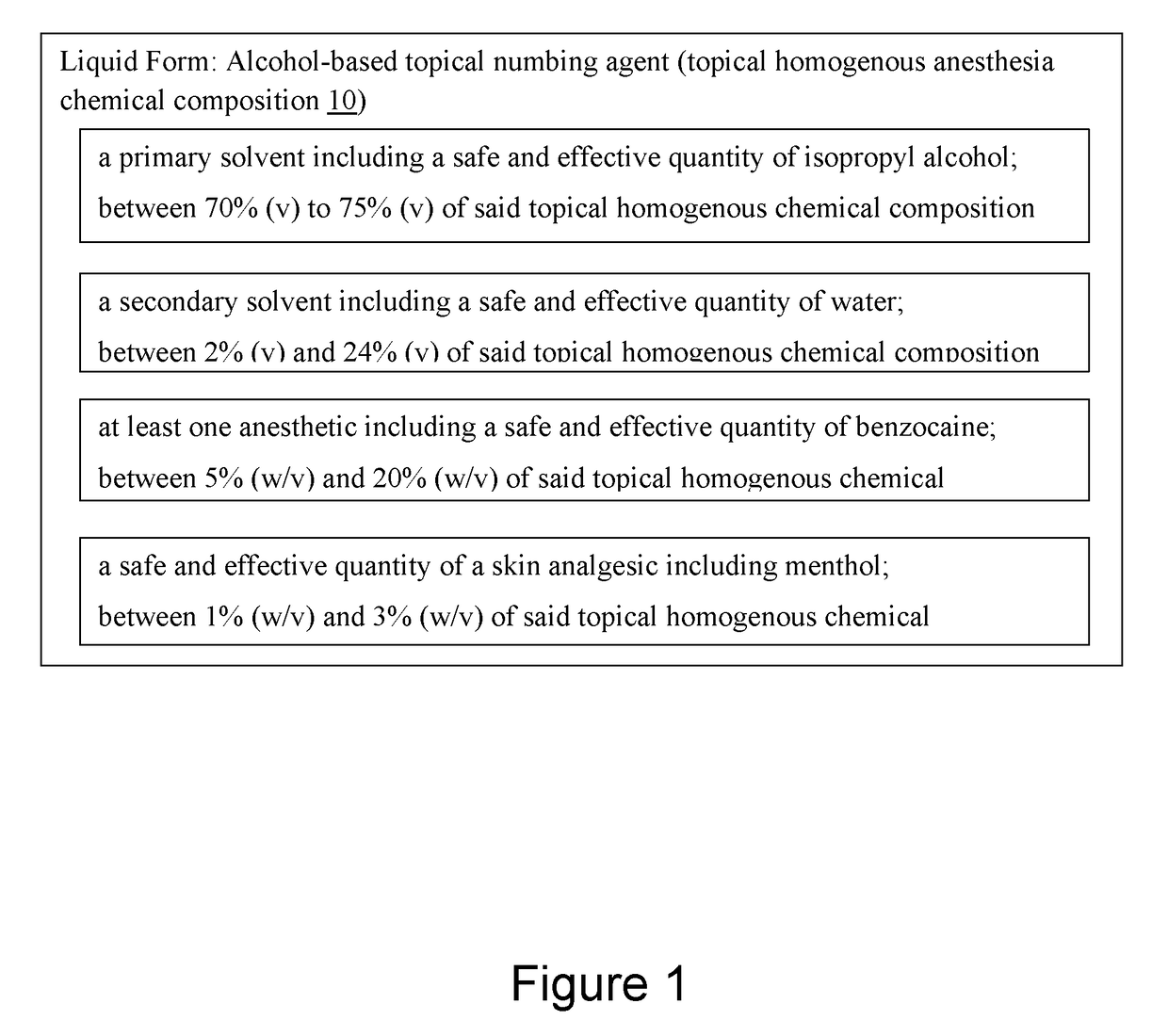
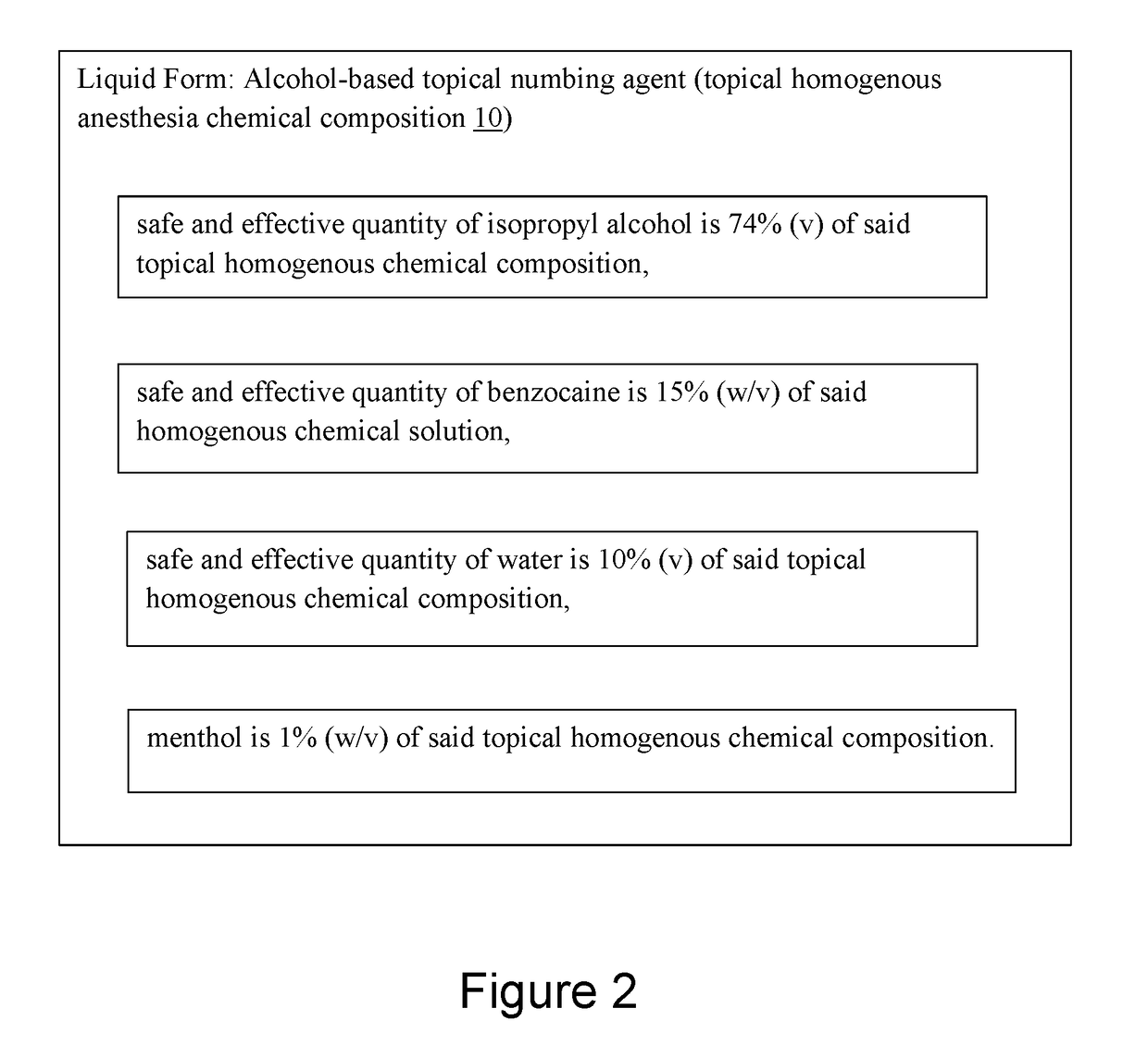
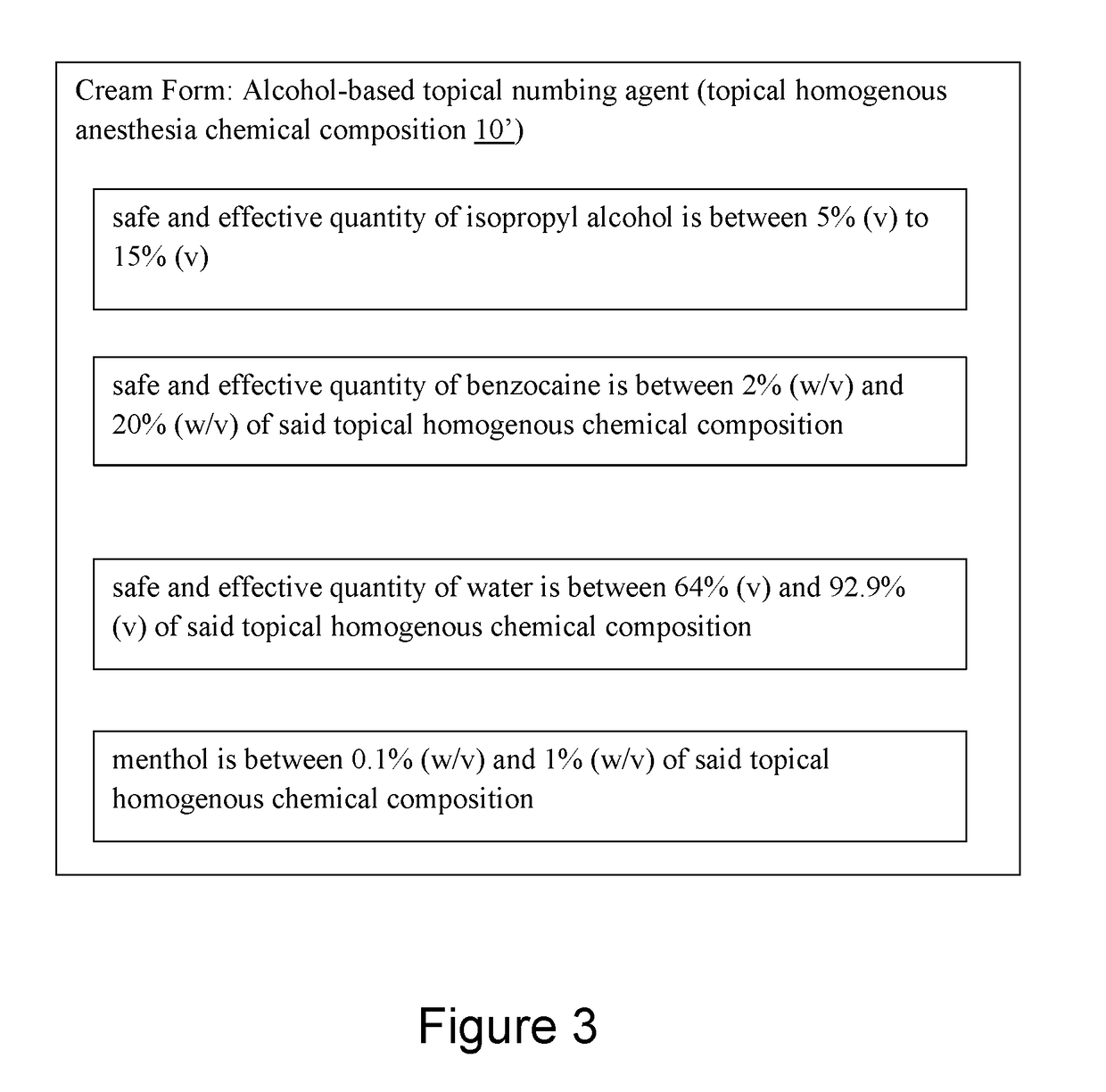
Alcohol-based local anesthesia and associated use thereof
An alcohol-based topical numbing agent can be applied to an epithelial surface via a cotton swab or other suitable applicator for providing an anesthetic and analgesic effect on skin tissue. The alcohol-based topical numbing agent includes a topical homogenous chemical composition including a primary solvent including a safe and effective quantity of isopropyl alcohol; a secondary solvent including a safe and effective quantity of water; at least one anesthetic including a safe and effective quantity of benzocaine; and a safe and effective quantity of a skin analgesic including menthol.
Owner:ENDOLOGIC LLC
Artificial Saliva
ActiveUS20190070135A1Cosmetic preparationsOrganic active ingredientsDibasic sodium phosphateMonobasic sodium phosphate
A composition for mixing with water for use as an oral rinse, comprising monobasic sodium phosphate, dibasic sodium phosphate, sodium chloride, calcium chloride, and an analgesic / anaesthetic such as benzocaine / lidocaine.
Owner:FORWARD SCI TECH
Highly penetrating compositions and methods for treating pathogen-induced disordered tissues
InactiveUS8846725B2Improve efficiencyQuickly contact and killAntibacterial agentsBiocideActive agentMedicine
Compositions and methods for treating disordered tissues, such as caused by pathogens and / or by toxins. The treatment compositions include an anti-infective active agent, a liquid carrier, and benzocaine in an amount so that the treatment composition penetrates more quickly into disordered tissue compared to the treatment composition in the absence of the benzocaine. In addition, the benzocaine can increase residence time of the anti-infective active in the treatment area. The preferred anti-infective active agent can be an organohalide, such as a quaternary ammonium halide compound, an example of which is benzalkonium chloride. The treatment compositions and methods may employ the use of an applicator adapted for use in promoting penetration of the treatment composition and / or agitation of the disordered tissue to further enhance penetration.
Owner:QUADEX PHARMA
Pharmaceutical composition, patch, and preparation methods and application of pharmaceutical composition and patch
PendingCN110038130AGuaranteed thicknessGet Continuous Pain ReliefNervous disorderAntipyreticProcaineBULK ACTIVE INGREDIENT
Owner:张洁
Method for use of microdialysis
ActiveUS20100021932A1Accurately permeabilityAccurate measurementSensorsBiological testingWarfarinContinuous flow
Very accurate measurements of mass transfer can be made rapidly by permitting diffusion of an agent desired to be measured into or out of a small, very precisely known volume of a microdialysis probe, then rapidly pumping or flushing (“pulsing”) the probe with a known volume of fluid as a single pulse. The diffusion and pulsing may be repeated. The method, hereinafter called pulsatile microdialysis (PMD) to distinguish it from prior art continuous flow microdialysis, is useful for measurements in a number of processes, including protein binding, adsorption to binding agents such as activated charcoal, release from microemulsion drug delivery systems, determination of drug diffusion coefficients and concentrations, and for various other purposes.The method is based on mathematical manipulation of Fick's Laws. Resulting equations were verified against experimental data using methazolamide, warfarin and benzocaine as test drugs.
Owner:PHYSICAL PHARMA
Compound medicament with double effects of alleviating pain and inhibiting bacteria
InactiveCN103006699AReduce adverse reactionsLow cost of treatmentAntibacterial agentsOrganic active ingredientsUse medicationPharmaceutical drug
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method of chemical intermediate benzocaine
InactiveCN106366009AHigh catalytic efficiencyReduced catalytic efficiencyOrganic compound preparationAmino-carboxyl compound preparationRefluxFiltration
The invention discloses a preparation method of chemical intermediate benzocaine. The preparation method of the chemical intermediate benzocaine comprises the steps: S1, adding 4-nitrobenzoic acid, absolute ethanol, a solid catalyst and a water-carrying agent into a reaction container with a water segregator and performing hot reflux for 1 to 6 hours, wherein the solid catalyst takes magnesium aluminum hydrotalcite as a precursor and modified nanometer bentonite as a carrier, the weight of the precursor is 25 to 45 percent that of the carrier, and the solid catalyst is prepared by grinding and mixing the precursor and the carrier uniformly and calcining at 500 to 700 DEG C for 2 to 4 hours; S2, discharging water out of the water segregator, replenishing the absolute ethanol and continuously performing reflux and water segregation until no water drops occur; S3, after the reaction is finished, filtering when the reaction liquid is hot and putting the filtrate into a hydrogenation kettle to perform hydrogenation reaction; S4, after the hydrogenation is finished, performing heat filtration to remove the catalyst from the system, cooling and filtering under the protection of nitrogen, and drying to obtain the chemical intermediate benzocaine. The preparation method of the benzocaine, provided by the invention, is high in conversion rate; the solid catalyst has high catalytic efficiency and can be repeatedly used, and the catalytic efficiency is not obviously reduced after the catalyst is repeatedly used for 50 times.
Owner:安徽金邦医药化工有限公司
Mucosal delivery tablet
Mucoadhesive tablets have a convex surface, a diameter to cup depth ratio of 4-20 and a cup depth to edge thickness ratio of greater than 0.75 swiftly adheres and conforms to the contacting tissue. The tablets are used to administer actives such as, for example, benzocaine.
Owner:HENKEL KGAA
Western medicine composition for repairing scars and preparation method thereof
InactiveCN106728555ASpeed up circulationPromote absorptionSalicyclic acid active ingredientsInanimate material medical ingredientsSalicylic acidKetoconazole
The invention discloses a western medicine composition for repairing scars. The western medicine composition is prepared from the following raw materials in parts by weight: 2 to 10 parts of glycopyrronium bromide, 2 to 8 parts of aspirin, 5 to 10 parts of lomustine, 10 to 15 parts of aloe vera gel, 2 to 8 parts of vaseline, 2 to 5 parts of salicylic acid, 5 to 8 parts of sesame oil, 5 to 10 parts of metronidazole, 2 to 5 parts of ketoconazole, 2 to 5 parts of gentamicin, 1 to 3 parts of rhizoma bolbostemmae total saponin, 2 to 5 parts of monocrotalinum hydrochloride, 2 to 5 parts of anisodamine hydrobromide, 1 to 3 parts of benzocaine and 2 to 5 parts of oil-in-water emulsifiable paste. The western medicine composition disclosed by the invention is capable of effectively repairing the scars and enabling skin to be smooth again, meanwhile, the western medicine composition is capable of treating all kinds of pimples, acne marks cannot be generated, and the purposes of fundamentally curing the scars and the acne marks can be achieved; the skin can be tender and smooth by using the western medicine composition for a long time.
Owner:ZHENGZHOU RENHONG PHARMA CO LTD
Preparation method of amboryl ester type sugar marker with permanent charges and application
InactiveCN107400078AMild reaction conditionsHigh detection sensitivityOrganic chemistryComponent separationOrganic synthesisHigh-performance liquid chromatography
Owner:EZHOU INST OF IND TECH HUAZHONG UNIV OF SCI & TECH +1
Dissolvable Strip for Treatment of Oral Thermal Burns
InactiveUS20140120150A1High tensile strengthUniform compositionHydroxy compound active ingredientsPharmaceutical delivery mechanismImmediate releaseDentistry
A consumable film adapted to adhere and to dissolve in the oral cavity that provides a local anesthetic and therapeutic agents for the treatment of oral burns or injuries. The film is designed to instantly release benzocaine, or other types of local anesthetic or therapeutic agent, upon adhesion to the affected areas of the mouth, and will continue to release sufficient quantities for pain relief and for healing over an extended period of time.
Owner:2010 MFI
Anti-inflammatory analgesic adhesive patch
InactiveCN1805739AGood anti-inflammatory and analgesic effectLess irritatingSalicyclic acid active ingredientsNervous disorderAdditive ingredientIrritation
An anti-inflammatory analgesic adhesive patch which has an excellent anti-inflammatory analgesic effect and is reduced in unpleasant irritant feeling during wear. It is characterized by containing, as active ingredients, benzocaine and an ingredient having a counter-irritation effect, wherein the ingredient having a counter-irritation effect comprises one or more members selected from the group consisting of 1-menthol, dl-menthol, dl-camphor, d-camphor, methyl salicylate, glycol salicylate, peppermint oil, eucalyptus oil, capsaicin, capsicum extract, and nonylic acid vanillamide and the benzocaine is contained in an amount of 0.5 to 20 wt.%.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Compositions
InactiveUS20140039057A1Relieve painQuick effectSmall article dispensingPowder deliveryParticle compositionDICHLOROBENZYL ALCOHOL
An ingestible particulate composition including at least one compound selected from the group consisting of 2,4-dichlorobenzyl alcohol, amylmetacresol, cetylpyridinium chloride, hexitidine, hexylresorcinol, flurbiprofen, lidocaine, benzocaine, ibuprofen, paracetamol, pectin, menthol, and benzydamine and one or more bioadhesive materials. Resulting particulate compositions have excellent flow characteristics, dust suppression, organoleptic properties and stability. They are highly suitable for administration direction into a patient's mouth, and ingested to alleviate the symptoms of a sore throat.
Owner:RECKITT BENCKISER HEALTHCARE (UK) LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com