Pharmaceutical composition for the treatment of acute disorders
a technology for acute disorders and pharmaceutical compositions, applied in the direction of drug compositions, immunological disorders, metabolism disorders, etc., can solve the problems of many pharmaceutically active agents, long onset times of rectal or sublingual formulations, and inability to control acute disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of a Rapidly Disintegrating Tablet with Bio / Mucoadhesion Promoting Properties
[0059] A batch of 1000 tablets was produced from the following composition: 91.0 g of mannitol (granular quality of a particle size from 250 to 450 .mu.m) and 1.0 g of sodium lauryl sulfate and 500 mg of micronized fentanyl were mixed in a V-mixer over a period of 24 hours. Thereafter, 5.0 g of Avicel.RTM. PH101 and 2.0 g of Ac-Di-Sol.RTM. (here used both as a disintegrant and as a bio / mucoadhesion promoting agent) was admixed for an additional 2 hours. Finally, 0.5 g of magnesium stearate was admixed for 2 minutes. The resulting tablet mass was compacted into tablets at a compaction pressure of 130 Mpa, each tablet containing 0.5 mg of fentanyl.
[0060] The disintegration time was tested with the use of the apparatus described in Ph.Eur. (latest edition)
[0061] It was found that the disintegration time was less than 15 seconds.
[0062] For comparison, conventional rapidly dissolving tablets were als...
example 3
Evaluation of Uptake in Sublingual Administration
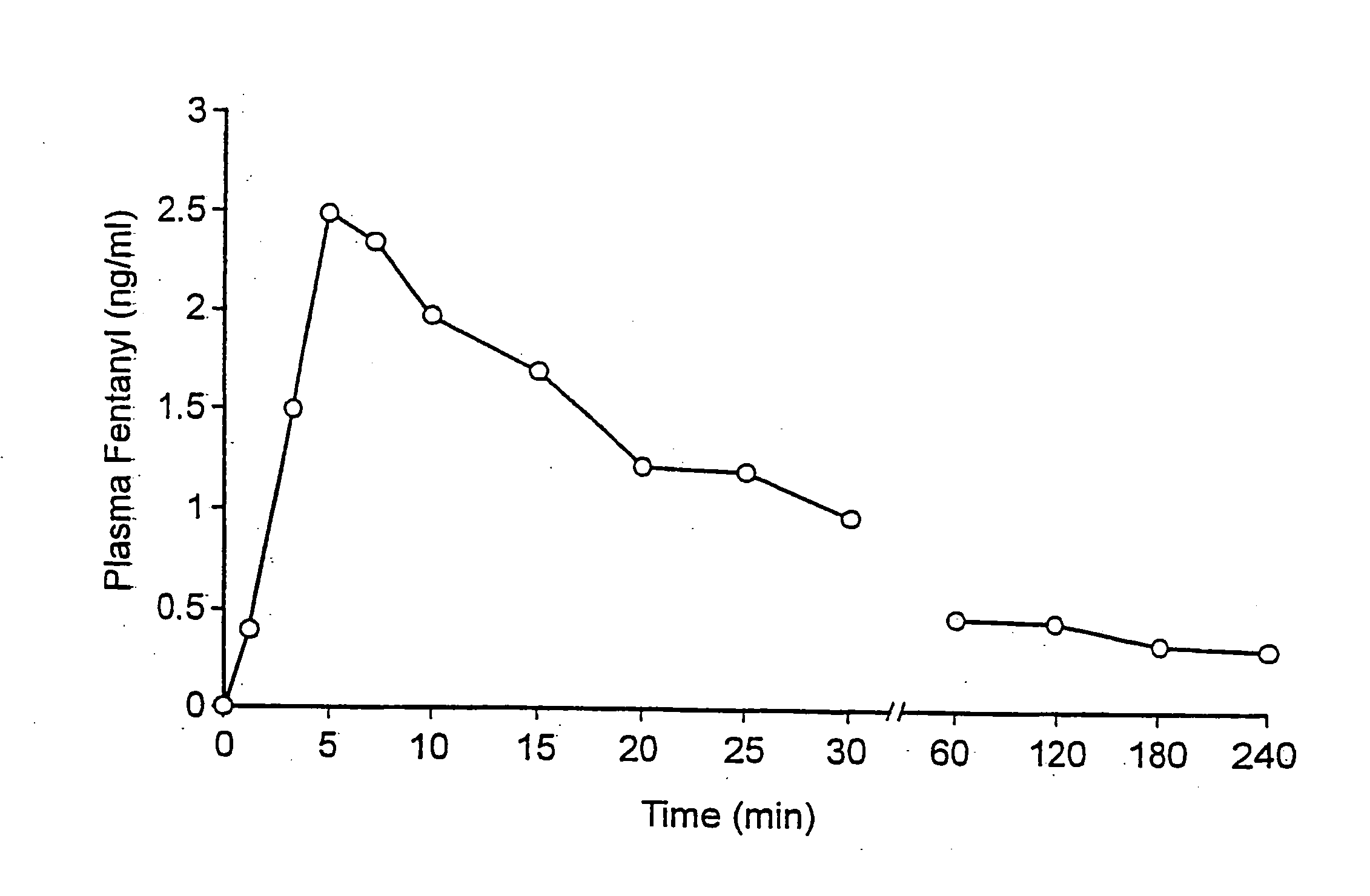
[0064] To one patient suffering from breakthrough pain due to cancer was administered 400 .mu.g of fentanyl as a sublingual tablet formulated as described in Examole 1. The plasma concentration of fentanyl was monitored for a time of 240 minutes after the administration, and the results are shown in the accompanying figure. It will be seen that the uptake of fentanyl was rapid, with the maximum value attained already after 5 minutes. This shows that a sublingual preparation according to the invention gives a rapid uptake of the active agent, even though a very small volume of liquid is available for dissolution in this route of administration.
example 4
Evaluation of Bio / Mucoadhesive Properties
[0065] For in vitro evaluation of the bio / mucoadhesive properties of the formulation according to the present invention, a method permitting evaluation of bio / mucoadhesion promoting properties directly on finished dosage forms (Sala, G. E. et al., Proc. Int. Symp. Contr. Release Bioact. Mat. 16:420, 1989) was used. The evaluation was based on measurements of the flow of water required to remove the active substance from a rabbit intestinal membrane. A strip of rabbit mucosa was placed horizontally in a suitable temperature controlled chamber set at 37.degree. C. The tissue was first washed with predetermined volumes of water by means of a peristaltic pump. Pre-compressed compositions according to Example 1 (5-15 mg) were then placed on the tissue and allowed to remain there for 2 minutes to ensure proper dissolution. Upon this followed an elution with water fed by a peristaltic pump during 10 minutes. Rinsed-off fentanyl was collected, and it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean diameter | aaaaa | aaaaa |
| mean sieve diameter | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com