Patents
Literature
51 results about "Vaginal delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A vaginal delivery is the birth of offspring (babies in humans) in mammals through the vagina. It is the natural method of birth for all mammals except monotremes, which lay eggs into the external environment. The average length of a hospital stay for a normal vaginal delivery is 36–48 hours or with an episiotomy (a surgical cut to widen the vaginal canal) 48–60 hours, whereas a C-section is 72–108 hours.
Coated vaginal devices for vaginal delivery of therapeutically effective and/or health-promoting agents
A vaginal device for delivering therapeutical and / or health-promoting agents. The vaginal device partly or completely coated by, covered by or combined with a coating or covering comprising a film, foam, strip, cap, cup or particles. The coating of the device comprises a mucoadhesive composition comprising a therapeutical and / or health-promoting agent.
Owner:UNIVERSITY OF MINNESOTA DULUTH
Localized vaginal delivery without detrimental blood levels
The invention relates to a pharmaceutical composition for vaginal administration of a treating agent normally associated with undesired side effects at detrimental blood levels. The composition releases the treating agent at a rate to achieve local tissue concentrations without such detrimental blood levels by using a therapeutically effective amount of the treating agent and a bioadhesive, cross-linked water swellable, but water-insoluble polycarboxylic acid polymer. Using this composition and the method of treatment provides sufficient local levels of the drug to provide therapeutic efficacy, but avoids many untoward adverse events. The invention also relates to a pharmaceutical composition for use during menses that includes a treating agent and a bioadhesive, cross-linked water swellable, but water-insoluble polycarboxylic acid polymer.
Owner:COLUMBIA LABORATORIES INC
Vaginal delivery system
InactiveUS20090142313A1Convenient and highly drug deliverySuitable for useAntibacterial agentsBiocideDiseaseControlled release
The present invention is related to an intravaginal delivery system for the controlled release of drospirenone and an estrogen, optionally also comprising one or more therapeutically active or a health-promoting substance capable of giving and / or enhancing protection against bacterial and fungal infections, and / or enhancing protection against sexually transmitted diseases. The delivery system consists of one or more compartments, one of each comprising a core and a membrane encasing the core, said core and membrane essentially consisting of a same or different polymer composition, wherein at least one compartment comprises drospirenone ant at least one compartment which may be the same or different from the one comprising drospirenone, comprises an estrogen or a mixture of drospirenone and an estrogen, and wherein the membrane or the surface of the membrane or at least one of the cores comprises said therapeutically active or a health-promoting substance.
Owner:BAYER OY
Vaginal delivery of chemotherapeutic agents and inhibitors of membrane efflux systems for cancer therapy
Owner:FEMINA PHARMA
Nasal Delivery of Agents with Nested Balloon Catheter
ActiveUS20150290438A1Convenient infusionConvenient treatmentBalloon catheterEndoscopesNasal cavityDiagnostic agent
A method of localized delivery of a therapeutic and / or diagnostic agent to nasal tissue or cavities includes inserting a catheter into a nasal cavity, the catheter having an outer balloon with at least one opening therethrough and an inner surface, and an inner balloon disposed in the outer balloon and at least partially enclosing an inflation chamber and having an outer surface defining a space between the outer surface of the inner balloon and the inner surface of the outer balloon, supplying the agent to the space between the outer surface of the inner balloon and the inner surface of the outer balloon via a first lumen of the catheter, and inflating the inner balloon by supplying fluid to the inflation chamber via a second lumen of the catheter to urge the agent out of the opening in the wall of the outer balloon and into nasal tissue.
Owner:SANOVAS
Vaginal delivery of chemotherapeutic agents and inhibitors of membrane efflux systems for cancer therapy
InactiveUS20030049302A1Significant gastrointestinalReduced activitySuppositories deliveryMedical devicesTumor targetWhole body
Owner:FEMINA PHARMA
Vaginal delivery system
InactiveUS20100285097A1Convenient and highly drug deliverySuitable for useAntibacterial agentsBiocideDiseaseDrospirenone
The present invention is related to an intravaginal delivery system for the controlled release of drospirenone and an estrogen, optionally also comprising one or more therapeutically active or a health-promoting substance capable of giving and / or enhancing protection against bacterial and fungal infections, and / or enhancing protection against sexually transmitted diseases. The delivery system consists of one or more compartments, one of each comprising a core and a membrane encasing the core, said core and membrane essentially consisting of a same or different polymer composition, wherein at least one compartment comprises drospirenone ant at least one compartment which may be the same or different from the one comprising drospirenone, comprises an estrogen or a mixture of drospirenone and an estrogen, and wherein the membrane or the surface of the membrane or at least one of the cores comprises said therapeutically active or a health-promoting substance.
Owner:BAYER OY
Nasal delivery
A nasal delivery device for and method of delivering substance to a nasal cavity of a subject, the delivery device comprising: a nosepiece unit including a nosepiece for fitting to a nostril of a subject and a nozzle through which substance is in use delivered, preferably substantially axially to a longitudinal axis of the nosepiece, to the respective nasal cavity, wherein at least a tip element of the nosepiece has, at least in one configuration, an elongate lateral section which has a longer dimension in a first, sagittal direction than a second direction orthogonal to the sagittal direction, such that, when the nosepiece is inserted in the nasal cavity of the subject, the longer dimension of the nosepiece acts to engage lower and upper surfaces of the nasal cavity, preferably at the nasal valve, and expand the same in the sagittal plane; and a delivery unit for delivering substance through the nozzle of the nosepiece.
Owner:OPTINOSE INC
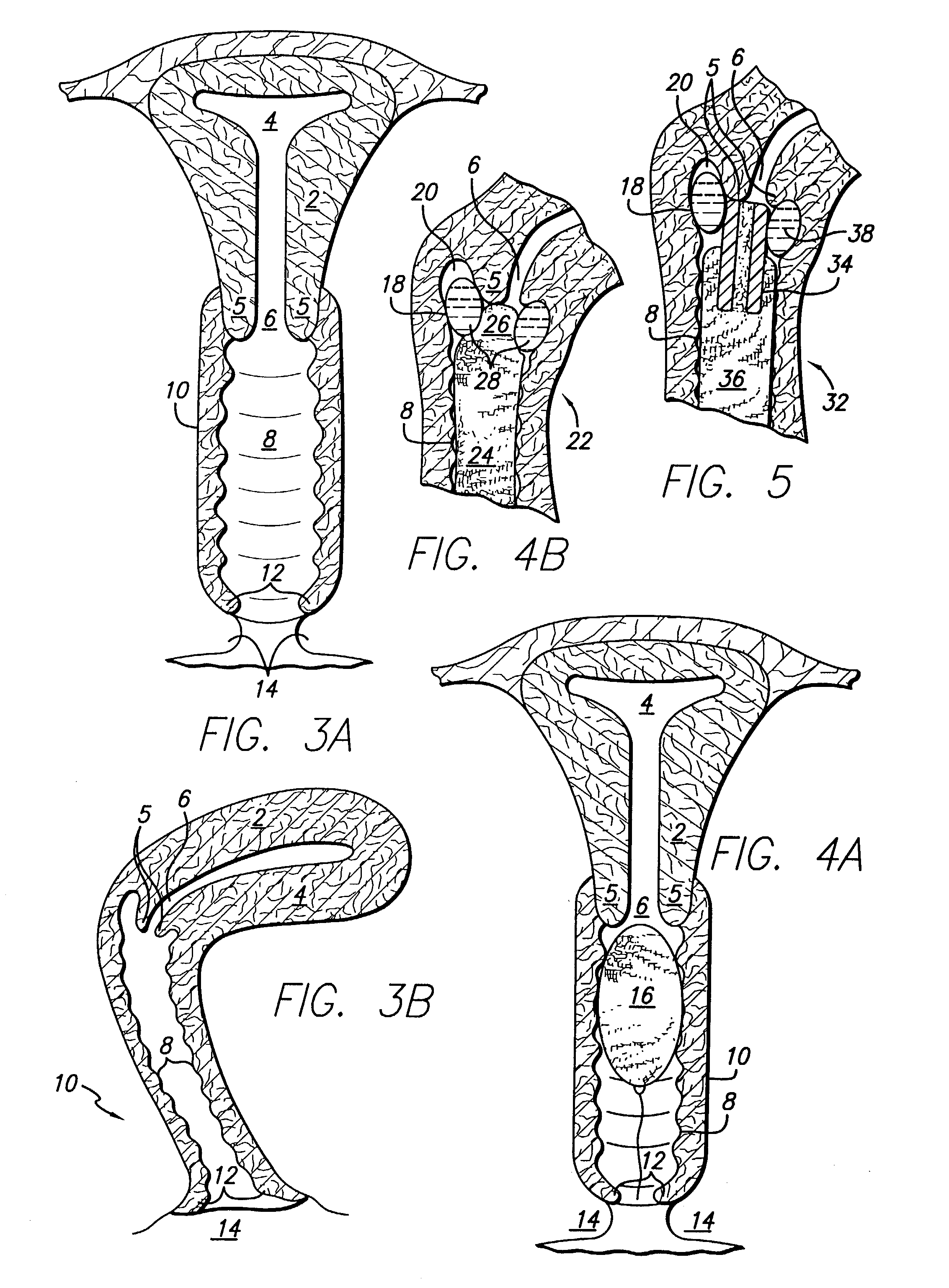
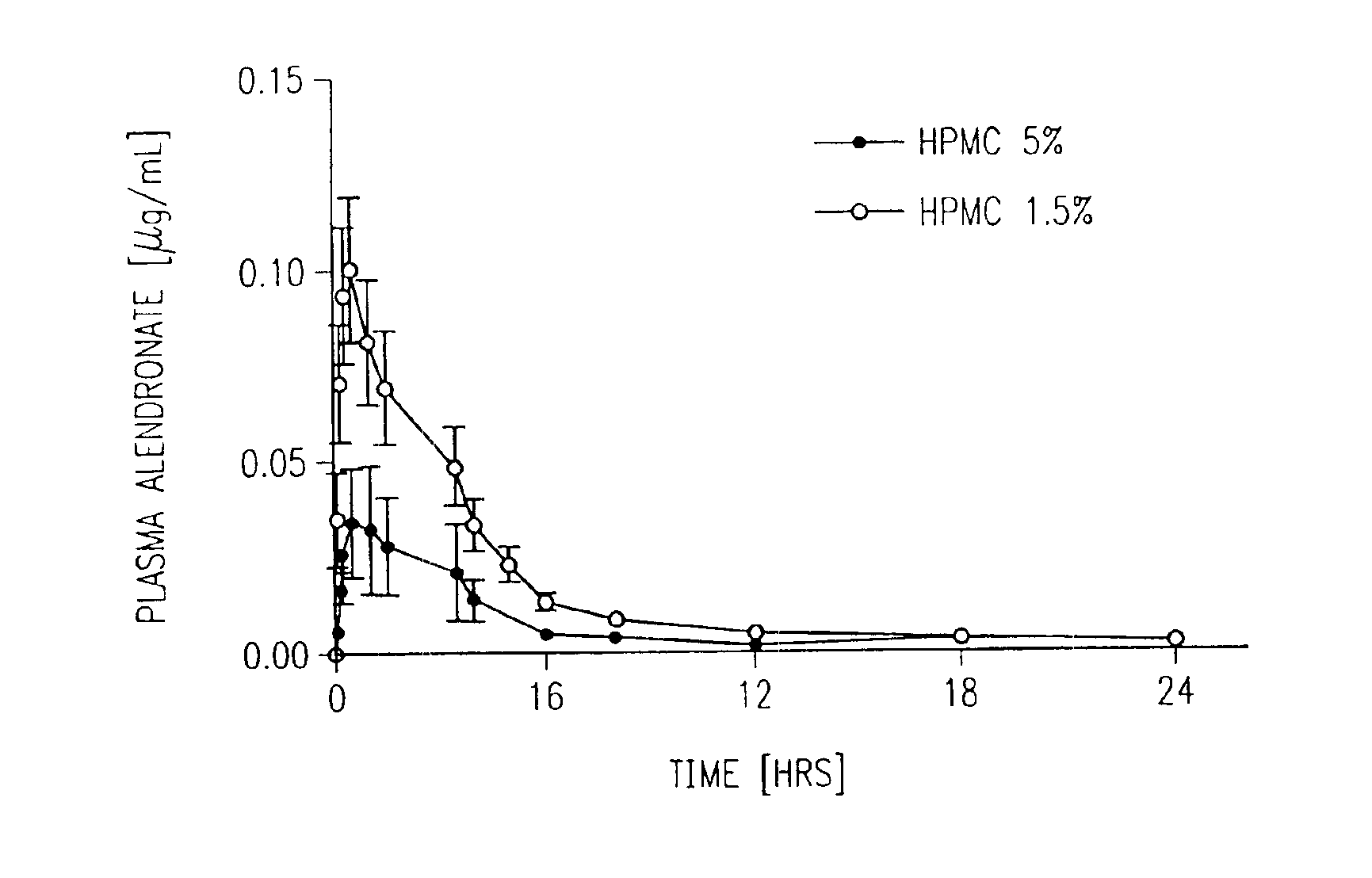
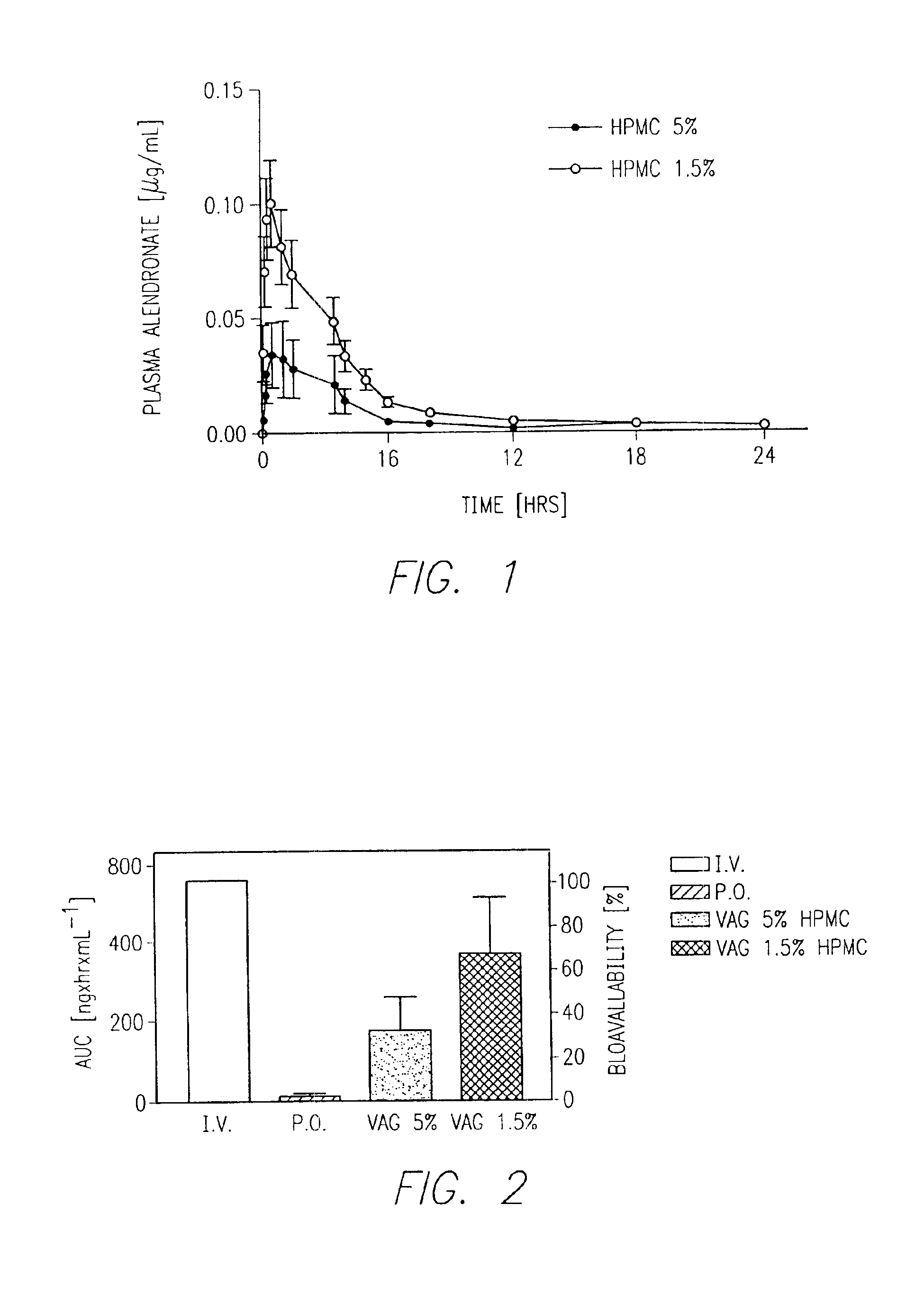
Formulations for transmucosal vaginal delivery of bisphosphonates
InactiveUS6905701B2Effective transmucosal absorptionAvoid developmentBiocideSuppositories deliveryDiseaseIntravaginal administration
Devices, methods, and improved formulation for transmucosal vaginal delivery of bisphosphonates. A targeted site delivery of bisphosphonates to the systemic circulation using a vaginal device comprising an improved bisphosphonate formulation for transmucosal delivery. A method for treatment of osteoporosis and related bone and skeleton diseases, for prevention of bone breakdown and loss of bone mass and strength by intravaginal administration of bisphosphonates to the vagina and transmucosal delivery of bisphosphonates to the general circulation.
Owner:FEMINA PHARMA
Apparatus and method for nasal delivery of compositions to birds
Owner:NOVA TECH ENG INC
Novel delivery assisting gel and preparation method and application of novel delivery assisting gel
ActiveCN102784416AGood biocompatibilityNon-irritatingSurgeryChitosan-g-poly(ethylene glycol)Polyethylene glycol
Disclosed is a composition with a lubricating effect. The composition comprises chitosan, polyethylene glycol, a water-soluble thickener, a humectant and optional water. The composition is used for preparing a medical product used as a lubricant for vaginal delivery, has good safety and antibacterial effects, and can effectively assist delivery, so that delivery is carried out easily, and a delivery birth process and delivery time are shortened.
Owner:山西纳德西生物科技有限公司
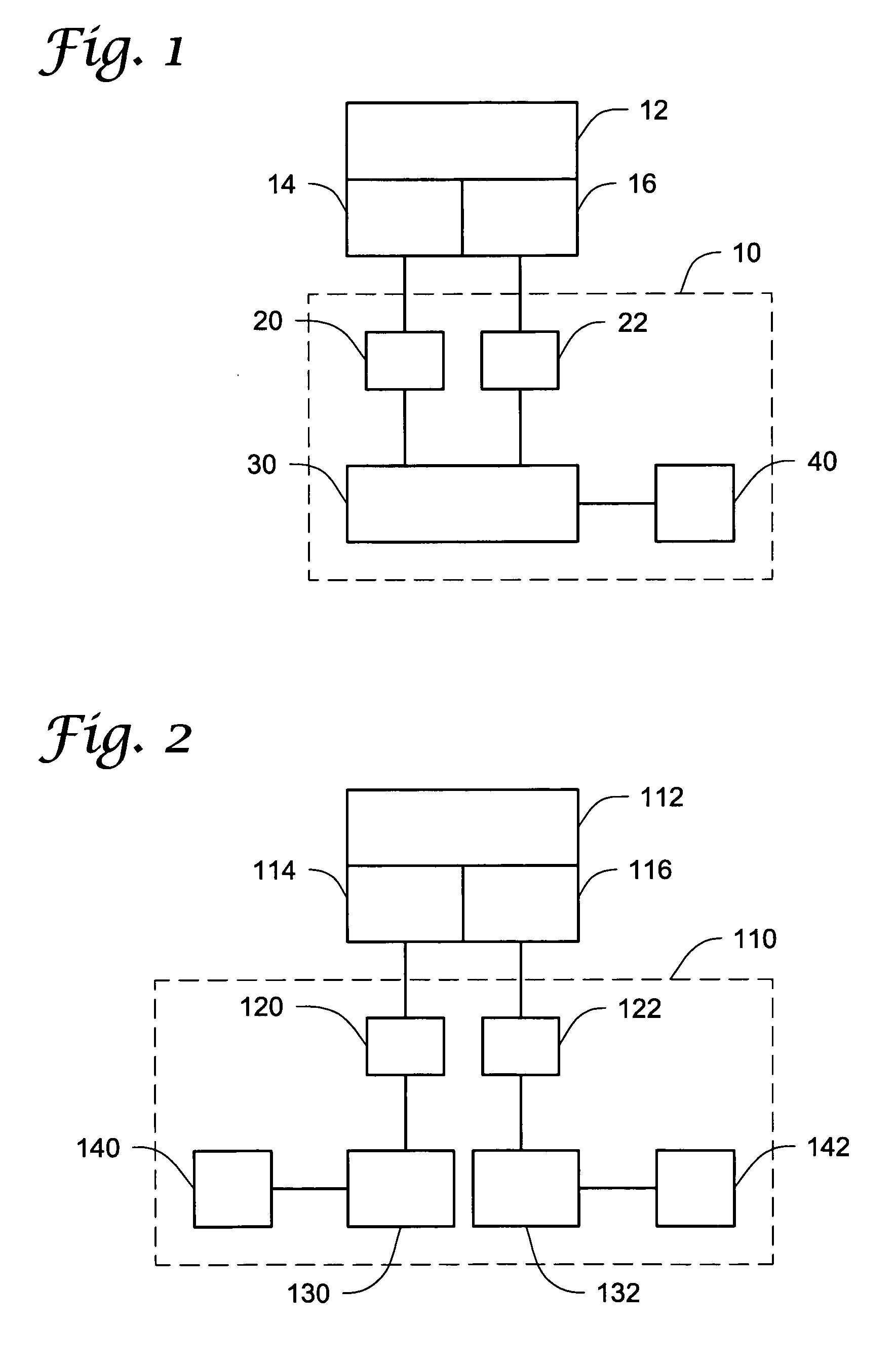
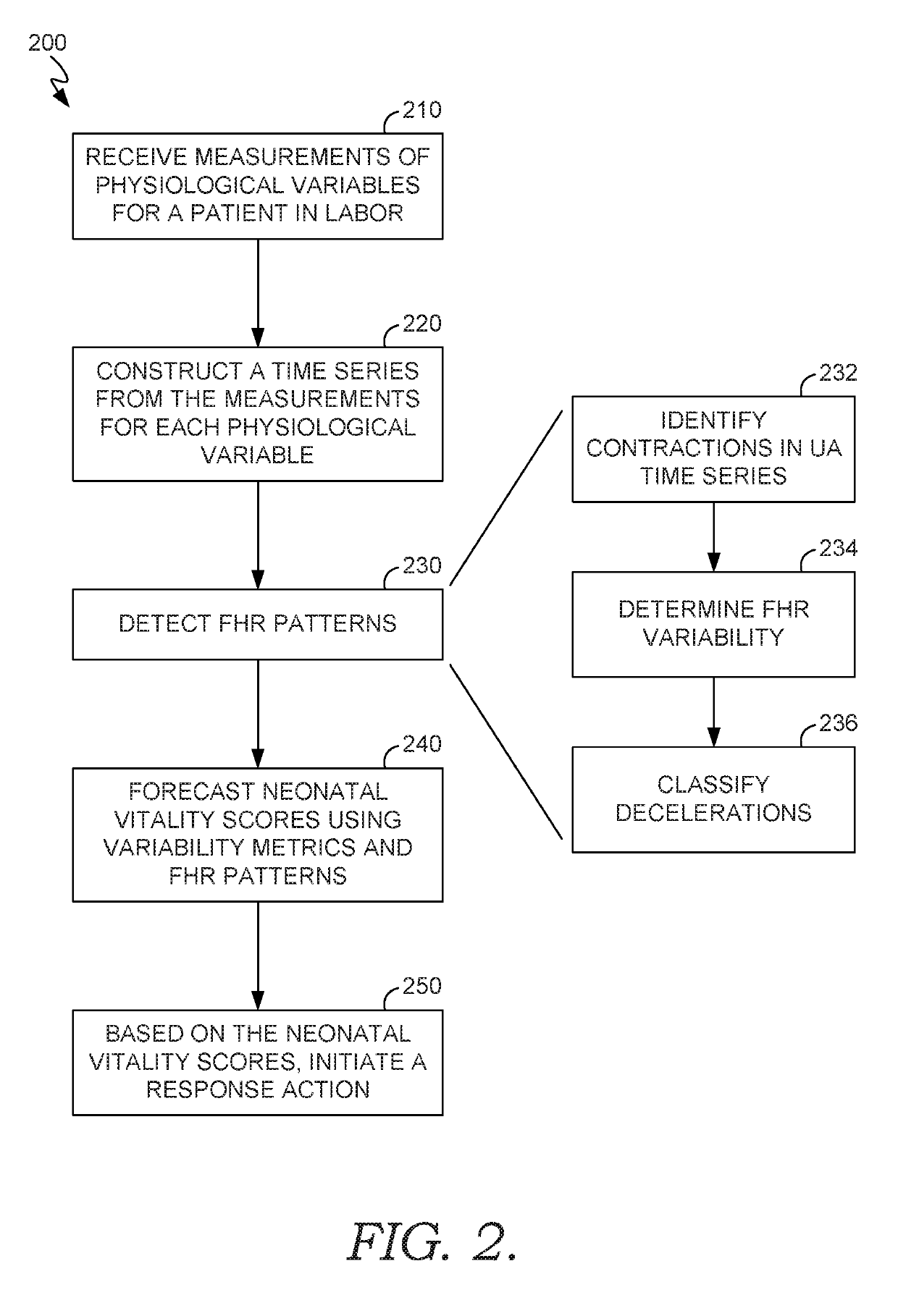
Forecasting neonatal vitality
ActiveUS20190133536A1Effective treatmentEffective careHealth-index calculationEvaluation of blood vesselsObstetricsFetal Heart Rate Variability
A decision support tool is provided for predicting the neonatal vitality scores of a fetus during delivery, the scores being an indicator of future health for the infant anticipated to be born within a future time interval, measured as time to birth. The predicted neonatal vitality score is determined from measurements of physiological variables monitored during labor, such as uterine activity and fetal heart rate. Fetal heart rate variability and patterns may be detected and computed using the monitored physiological variables, and neonatal vitality scores may be predicted based, at least in part, on the variability metrics and fetal heart rate patterns. Scores may be predicted for different delivery methods, such as vaginal delivery or cesarean delivery, for different time-to-birth intervals. In this way, these scores may be used for decision support for care plans during labor, such as increased monitoring and / or modifying the delivery type.
Owner:CERNER INNOVATION
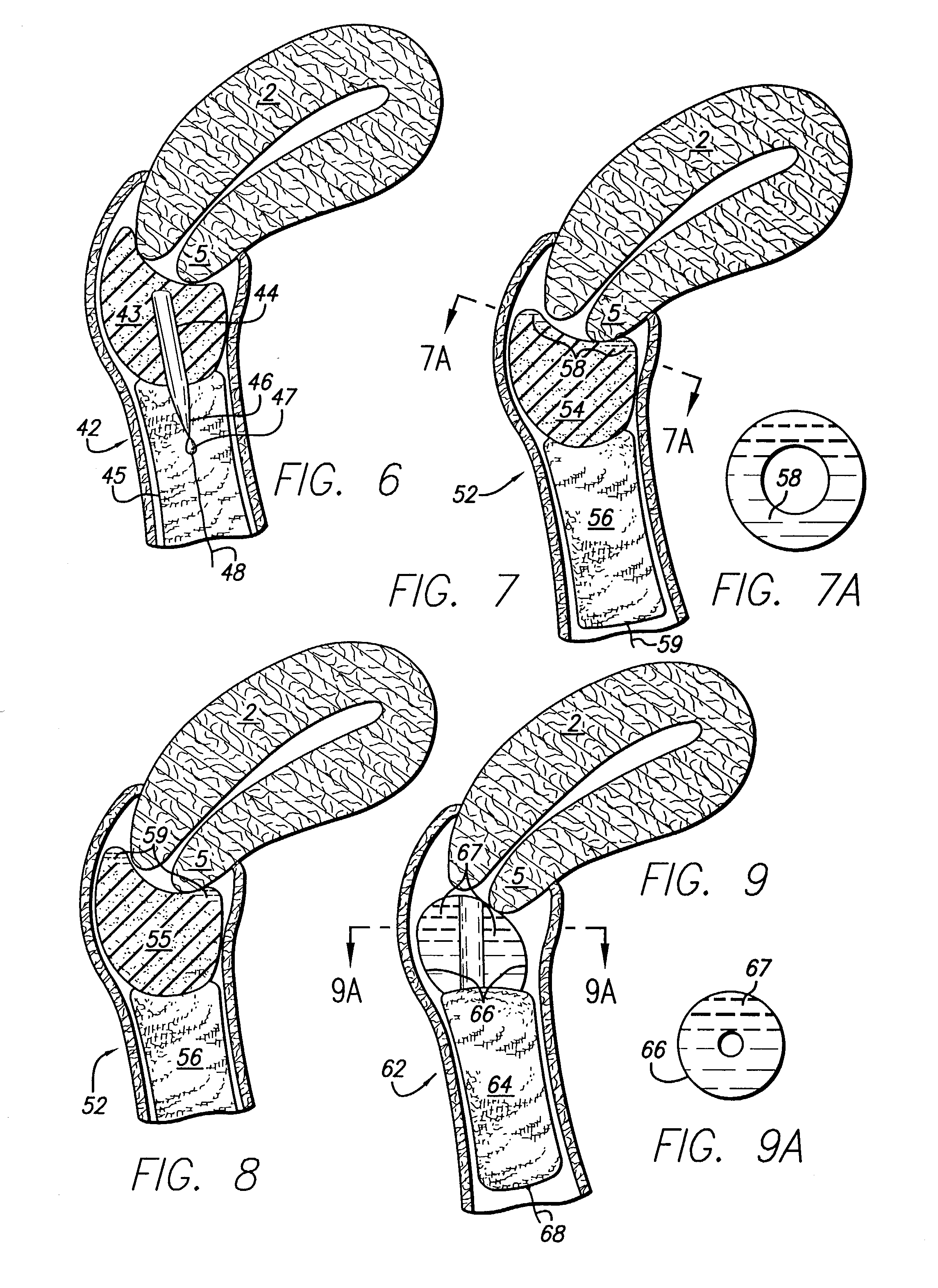
Vaginal drug delivery system and method
InactiveUS20070293837A1Low densityEasy to insertFemale contraceptivesMedical devicesDevice formCervix part
A vaginal drug delivery system includes a device formed of porous material that holds a flowable therapeutic formulation. The device, preferably in a soft, prewetted state, is inserted into the vagina to reside typically at or near the cervix where it continuously releases the flowable therapeutic formulation through its outer surface which is in contact with the vaginal surfaces. In operation, the flowable therapeutic formulation migrates via capillary forces from a reservoir that is centrally located in the device and through a covering that envelopes the reservoir.
Owner:FAMILY HEALTH INT
Formulations and methods for vaginal delivery of antiprogestins
The subject matter of the present invention is pertinent to the field of vaginal delivery of pharmaceutically active agents. Embodiments of the instant invention disclose methods for treating a variety of progesterone related disorders by vaginal administration of a pullulan capsule comprising one or more antiprogestins.
Owner:APTALIS PHARMA
Transvaginal Delivery of Drugs
InactiveUS20110003000A1Reduce systemic levelEliminate side effectsPowder deliveryBiocideOxybutyninDisease
Drug delivery compositions which are suitable for transvaginal administration for the treatment of diseases and disorders of the urogenital tract are described. The drug delivery compositions are administered directly to the vagina using a convenient transvaginal application that deposits a very small volume of drug at the desired site for delivery. This method of administration reduces the systemic levels of the drugs and decreases the side effects which are associated with systemic administration. In the preferred embodiment, the compositions are in the form of a gel. The formulation is administered in volumes of less than or equal to 1 milliliter. In the preferred embodiment, the composition contains an antimuscarinc drug, such as oxybutynin.
Owner:FEMMEPHARMA HLDG CO INC
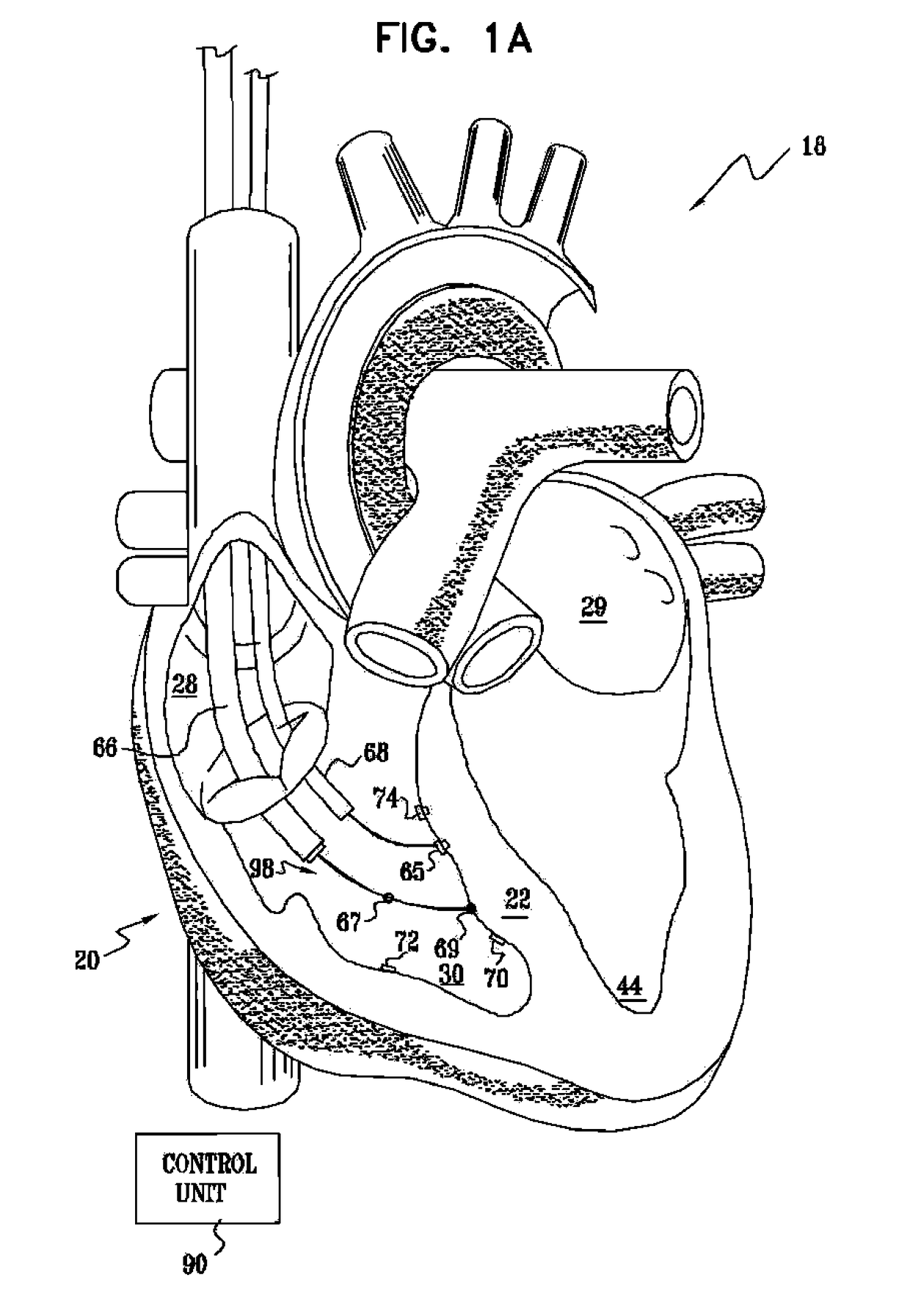
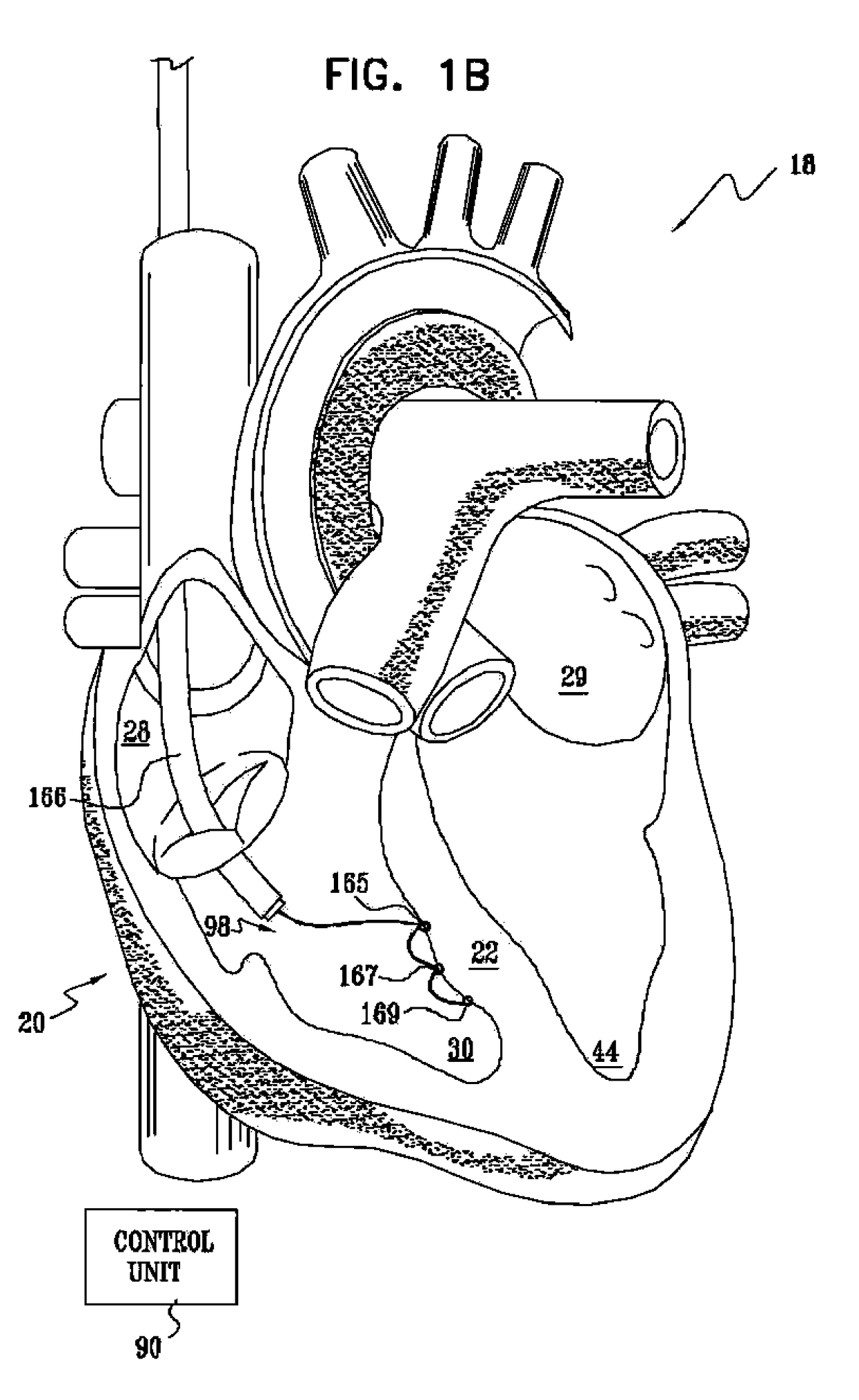
Signal delivery through the right ventricular septum
InactiveUS9713723B2Improve heart functionReduced contractilityHeart stimulatorsVeinLeft ventricle wall
Owner:IMPULSE DYNAMICS NV
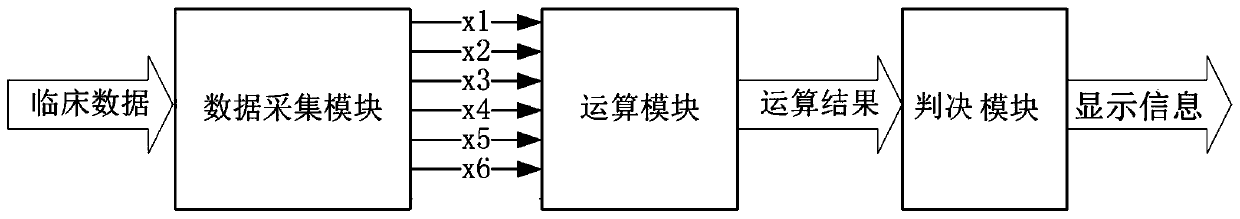
Obstetric operation resource allocation prediction system
ActiveCN110534184AHigh sensitivityImprove featuresHealthcare resources and facilitiesObstetric historyData acquisition
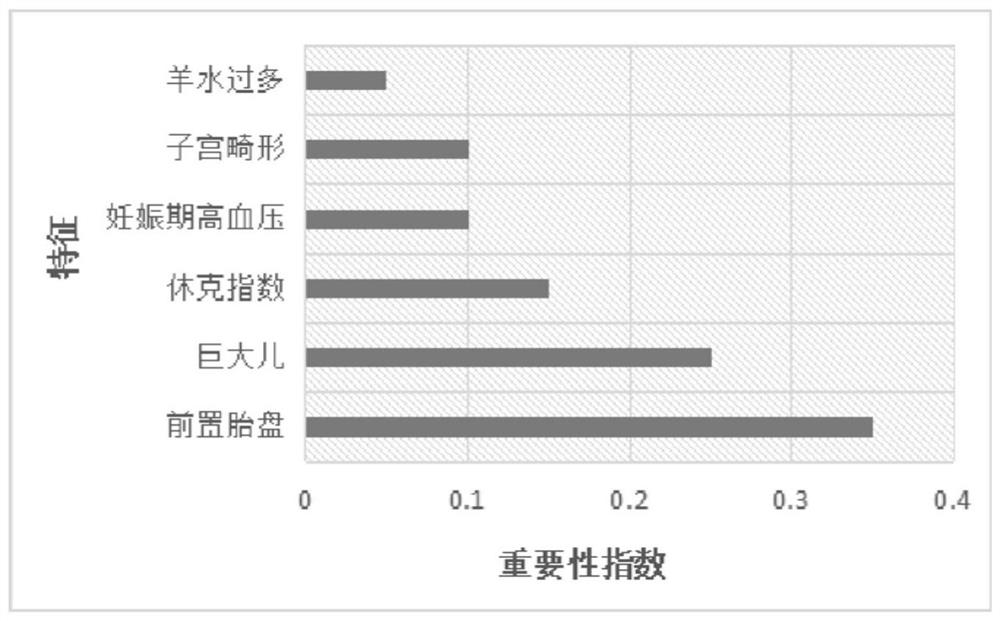
The invention belongs to the field of biological medicine, and relates to an operation resource allocation prediction system, in particular to an obstetric operation resource allocation prediction system. The prediction system comprises a computer. A data acquisition module and an operation module are arranged in the computer; the data acquisition module is provided with an information acquisitionchannel for risk factors, the risk factors include the age of a pregnant woman, the number of previous parturition times, the number of previous abortion times, the previous vaginal delivery history,the week of pregnancy termination and the relationship between the placenta of pregnancy and the uterus, and the operation module calculates a risk value according to the risk factor information. Thesystem can accurately evaluate and predict whether the pregnant and lying-in woman with the preposed placenta during pregnancy has serious postpartum hemorrhage or not, and medical staff take certainmedical resource allocation measures according to risk conditions. The system can be applied to practice of surgical resource allocation of pregnant and preposed placenta pregnant and lying-in womenin the obstetrics and gynecology department of hospitals.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
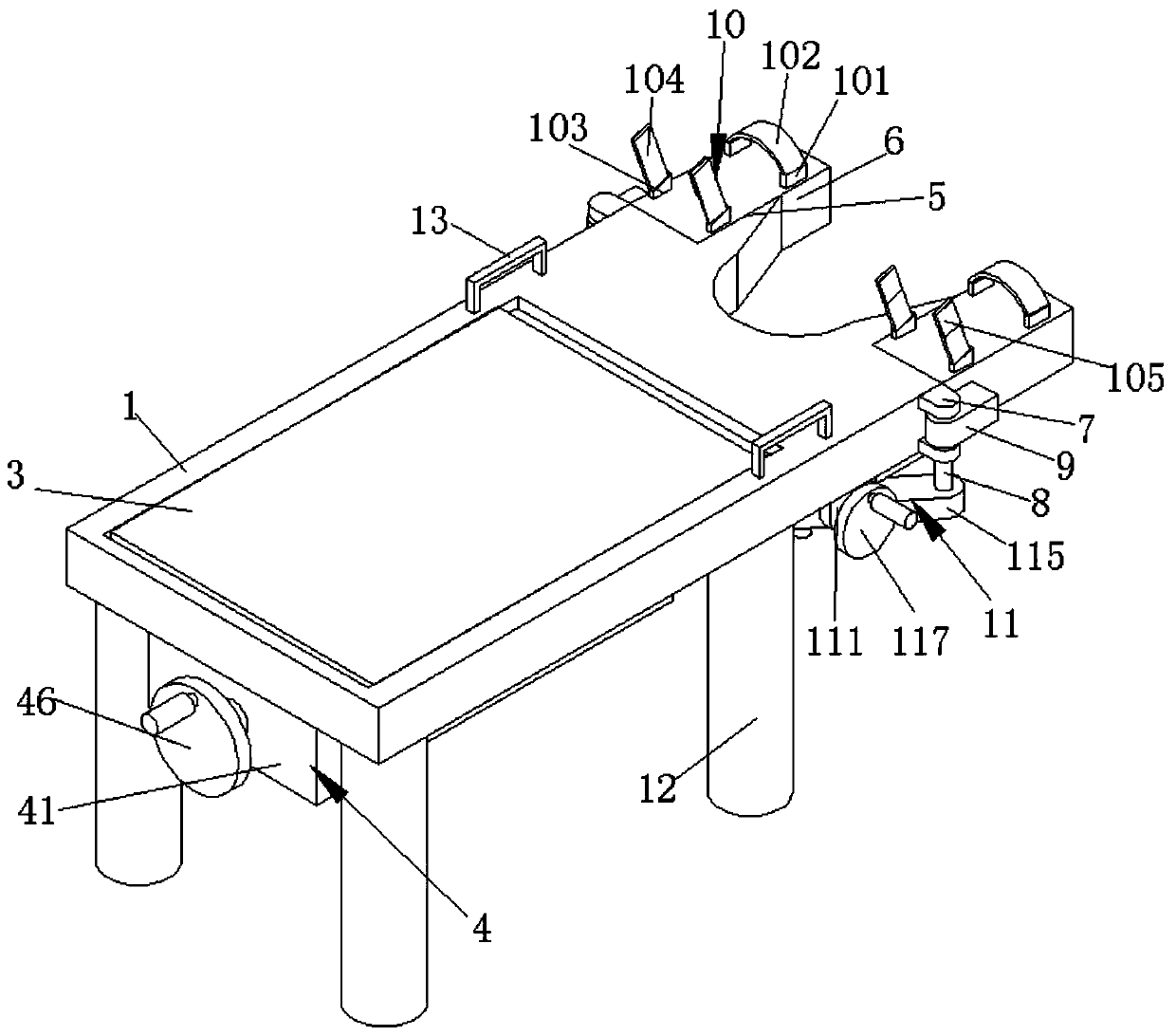
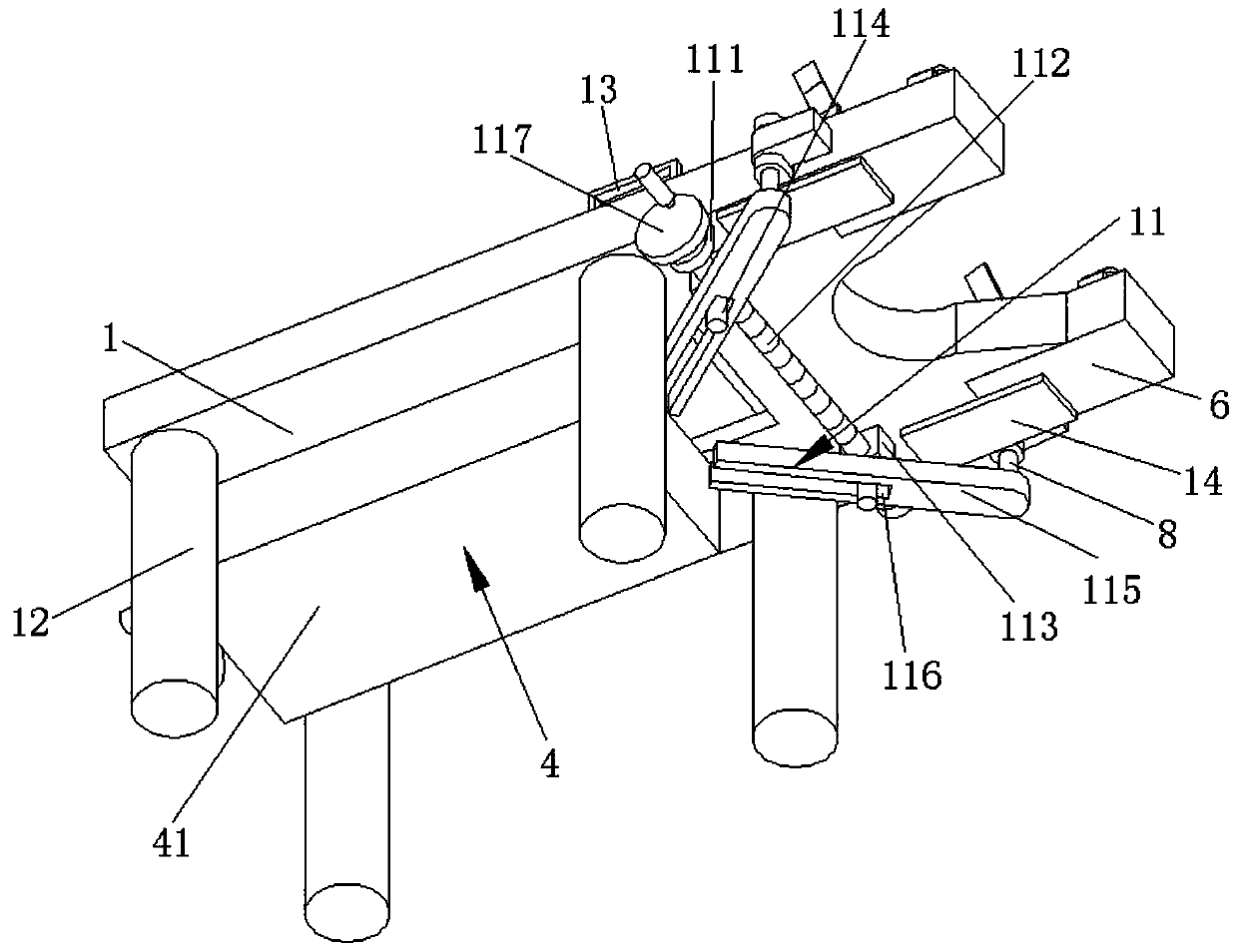
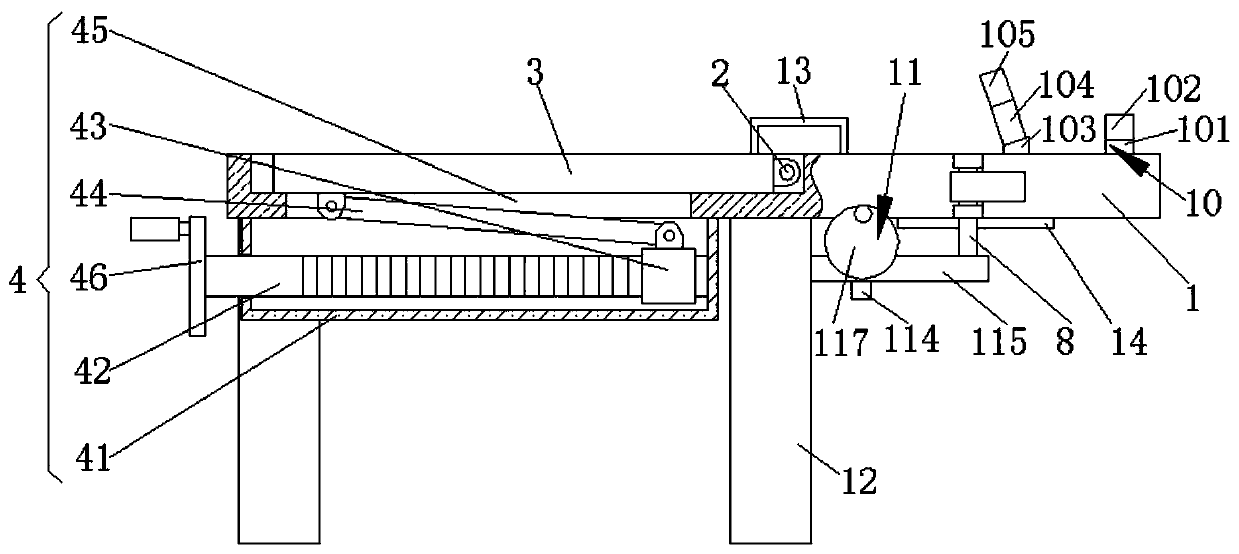
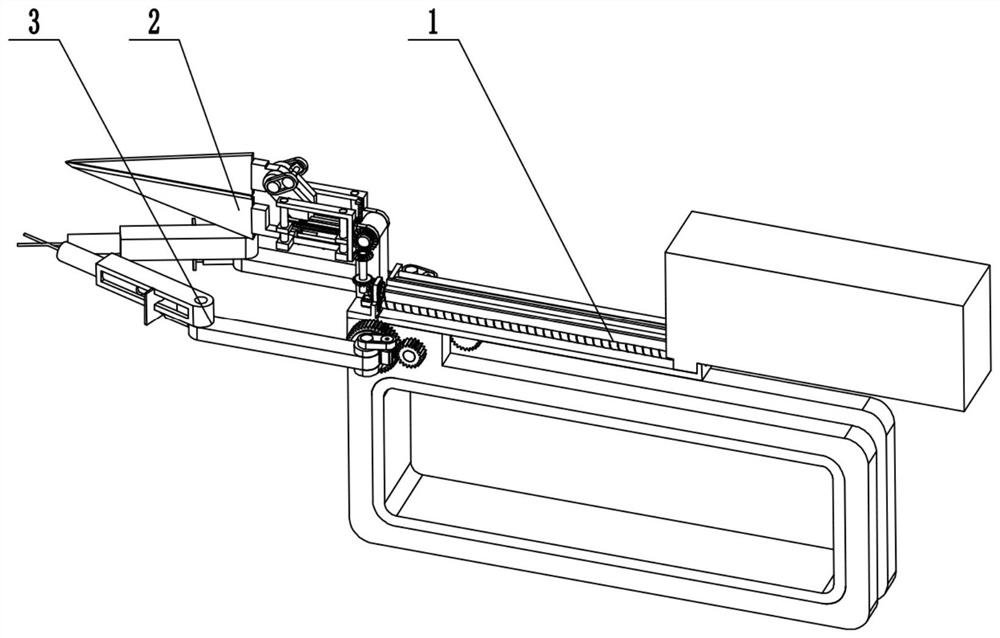
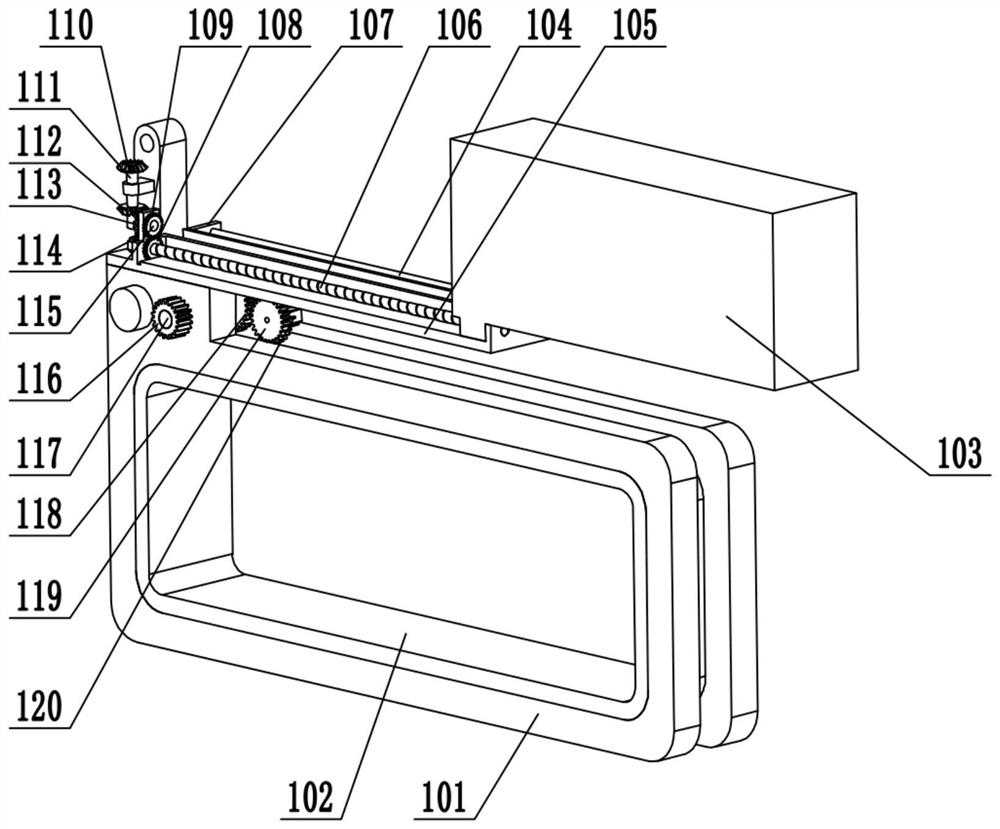
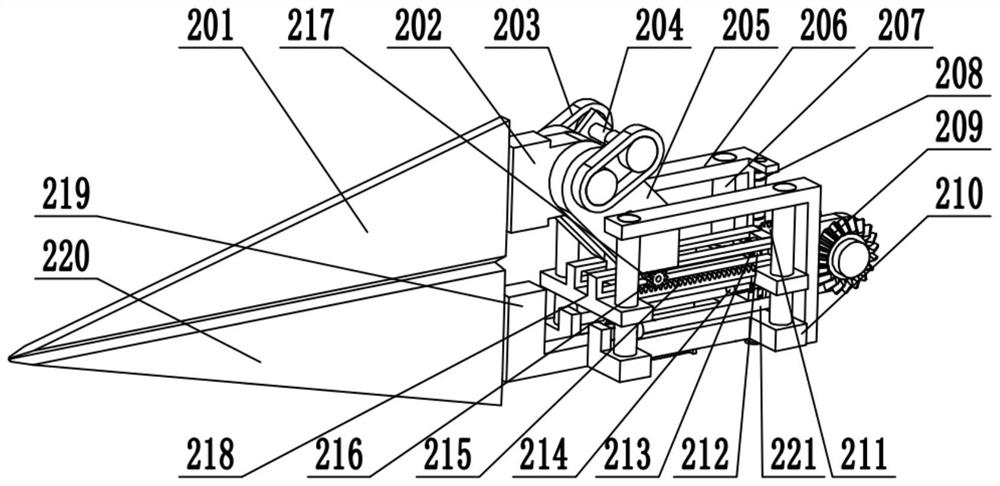
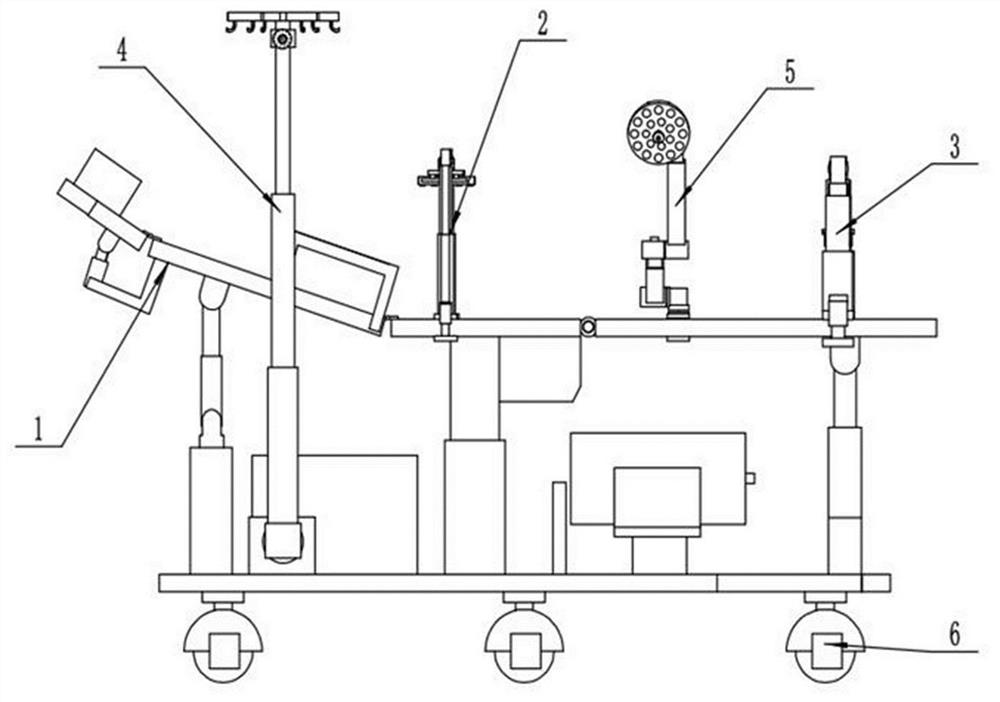
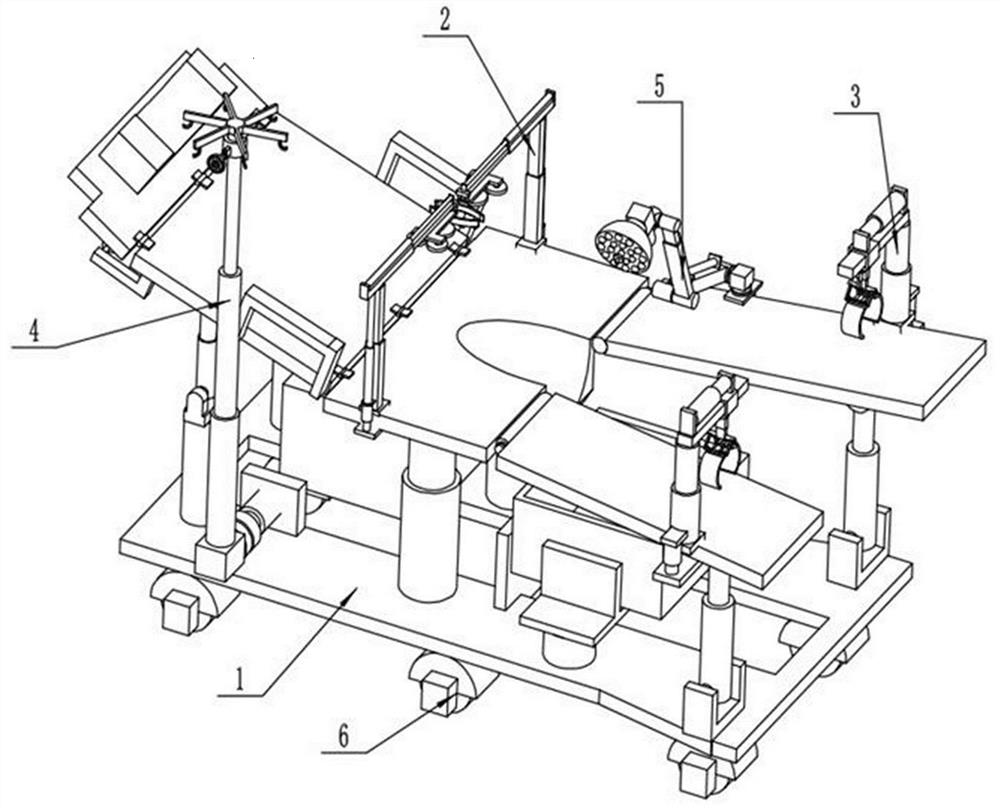
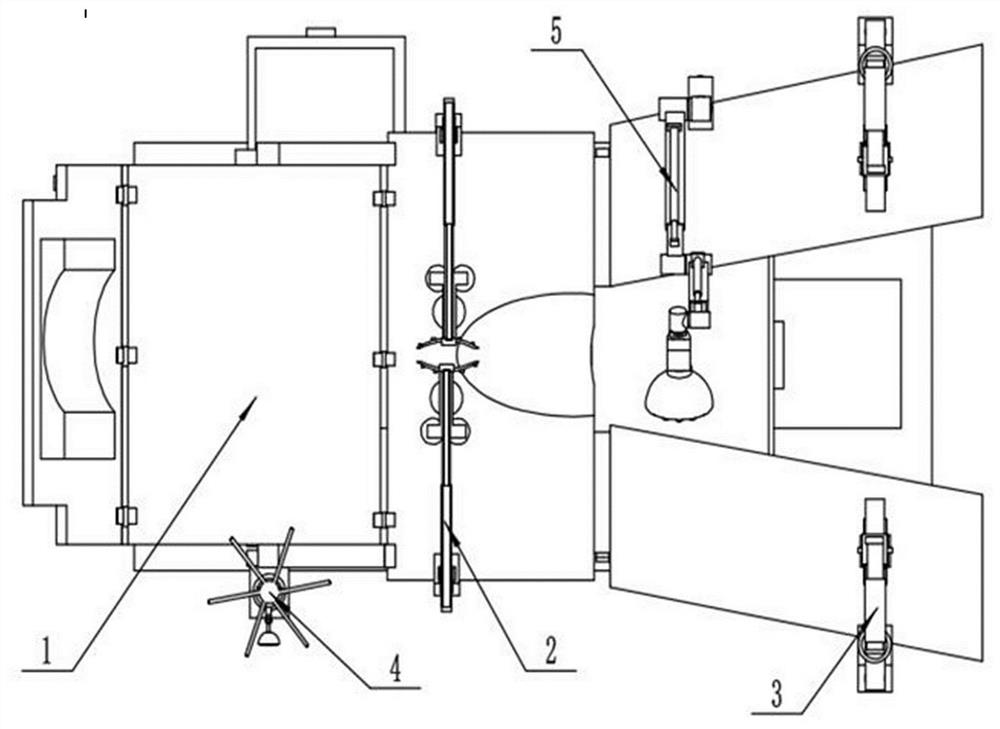
Obstetric clinical auxiliary vaginal delivery device
InactiveCN111419614AAvoid close situationsEasy to adjustOperating tablesMedical transportGeneral surgeryLying
The invention discloses an obstetric clinical auxiliary vaginal delivery device. The device comprises a delivery bed; an inner cavity of the delivery bed is fixedly connected with a rotating shaft rodI; the surface of the rotating shaft rod I is rotatably connected with a movable bed plate; the movable bed plate is positioned in the inner cavity of the delivery bed; and an adjusting mechanism I is arranged on the left side of the bottom of the delivery bed. According to the invention, feet of a lying-in woman are fixed through a strap mechanism, and then regulation is carried out through a regulating mechanism II. A placing plate rotates outwards to a proper position, so that the situation that the feet are close when the lying-in woman is delivered can be avoided, convenience is broughtto delivery, and the purpose of convenient regulation can be achieved. The invention solves the problems that the foot placing position of a lying-in woman on an obstetric clinical auxiliary transvaginal delivery device is fixed and cannot be adjusted according to requirements, feet of the lying-in woman cannot be fixed, and when the lying-in woman is delivered, the feet of the lying-in woman areclose to the middle and great inconvenience is brought to delivery of the lying-in woman.
Owner:张婷
Lyophilized pharmaceutical compositions for vaginal delivery
ActiveUS20190314274A1Easy to usePatient compliance is goodAntibacterial agentsAntimycoticsGynecologyCrystal structure
Disclosed herein is a solid lyophilized vaginal dosage form that can have an effective amount of at least one active ingredient, a crystalline structure forming agent in an amount of about 5 wt. % to about 40 wt. %, based on the total weight of the lyophilized dosage form, and at least one polymeric mucoadhesive matrix forming agent. The dosage form can have a pH of about 4.0 to 5.0, and can disintegrate within 120 seconds after being contacted with a vaginal mucosa. A method of delivering an active ingredient to the vaginal mucosa using the disclosed solid dosage form is also described.
Owner:CATALENT U K SWINDON ZYDIS LTD
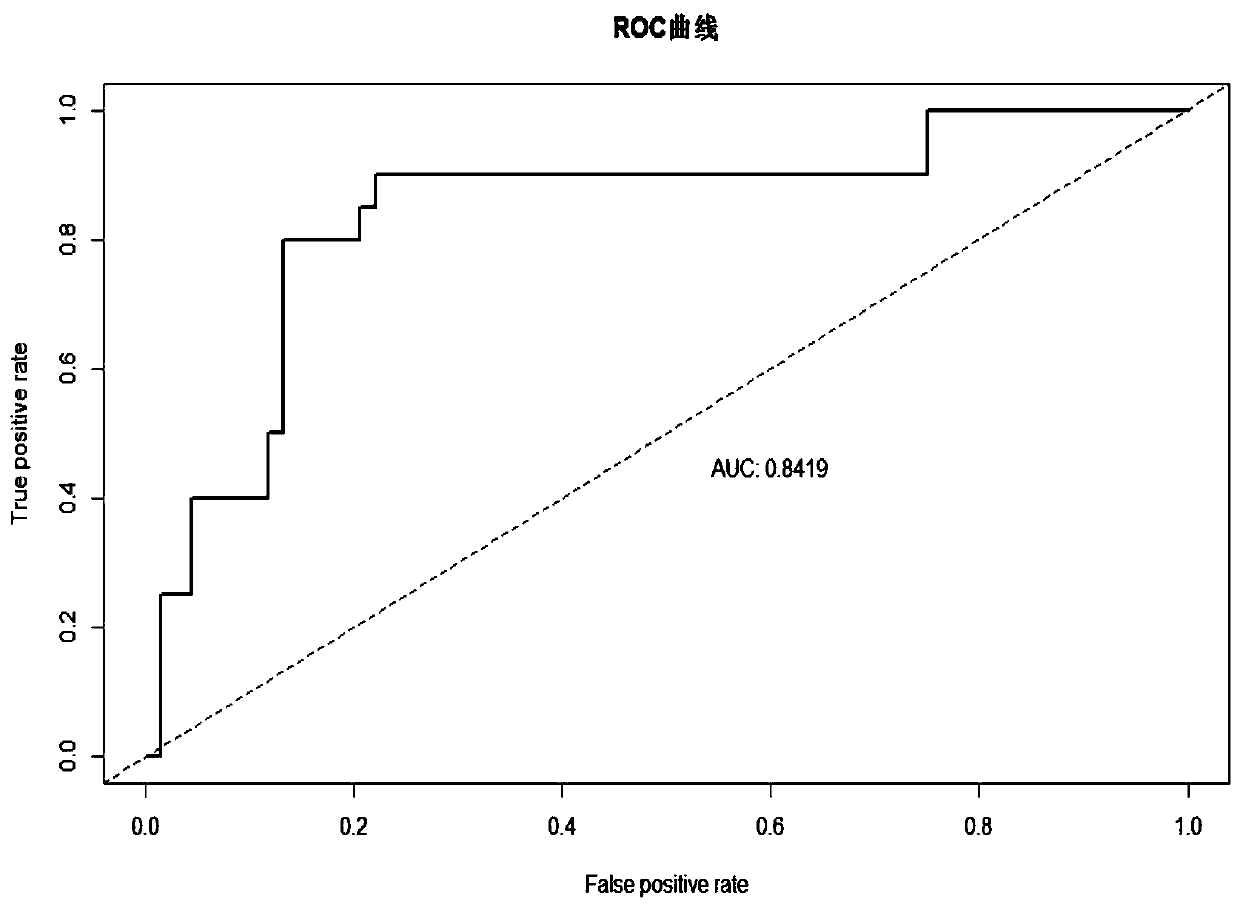
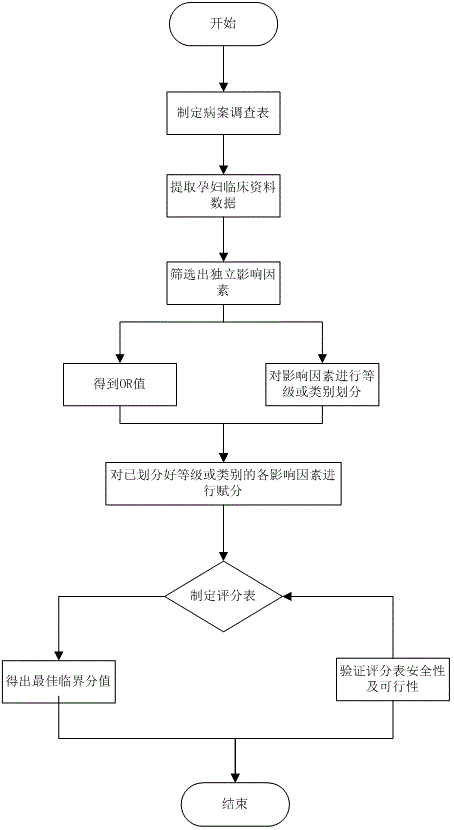
Method for predicting re-pregnant vaginal birth after cesarean delivery
InactiveCN106264462ABest cut-off scoreQuality improvementDiagnostic recording/measuringSensorsObstetricsReceiver operating characteristic
The invention relates to a method for predicting re-pregnant vaginal birth after cesarean delivery. The method comprises the following steps: S1: collecting a large number of clinical data of pregnant and lying-in women experiencing re-pregnant trial vaginal birth after cesarean delivery; S2: comparing the clinical data of two groups of pregnant and lying-in women, screening independent influence factors for influencing successful re-pregnant trial vaginal birth after cesarean delivery, and calculating an odds ratio (OR) of each influence factor; S3: grading and classifying the independent influence factors, scoring the obtained odds ratios (OR) in combination with Logistic regression analysis so as to establish a predictive scoring table used before the re-pregnant vaginal birth after cesarean delivery; S4: according to the predictive scoring table, scoring the collected independent influence factors of each pregnant and lying-in woman, calculating a total score value, and drawing the total score values of all the pregnant and lying-in women into an ROC (receiver operating characteristic) curve to obtain an optimal critical score value, namely a cut-off point, wherein the cut-off point is a critical score value for judging whether a pregnant woman can accomplish the vaginal birth. By the method, a safe and feasible before-birth predictive scoring model for VBAC (vaginal birth after cesarean delivery) is established.
Owner:福建省妇幼保健院
Method and system for predicting success rate of delivery of scar uterine
InactiveCN106845063AIncrease success rateHigh feasibilityHealth-index calculationMedical automated diagnosisObstetricsPredictive methods
The present invention discloses a method and a system for predicting the success rate of delivery of a scar uterine. The method comprises the steps of: carrying out basic data collection and registration on a pregnant woman who is enrolled in the hospital, wherein the basic data comprises age, height, the pre-pregnancy BMI, the BMI in hospital, and the history record of vaginal delivery; collecting cervix maturity parameters of the pregnant woman who is enrolled in the hospital, wherein the cervix maturity parameters comprise the degree of dilation of the cervix and the degree of disappearance of the cervical canals; and taking the collected parameters as input parameters to input into a trained mathematical prediction model for calculation, and finally obtaining the success rate of vaginal delivery of the pregnant woman. The method and system disclosed by the present invention have a high success rate of prediction and good feasibility, is easy to obtain the data that is required to be collected, is very applicable to the actual situation of scar uterine pregnancy in our country, and can be widely used in the field of data analysis of obstetrics and gynecology.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Method of protecting the pelvic floor during vaginal childbirth
ActiveUS20200315659A1Reduce coefficient of frictionPrevent or mitigate ischemic pressure necrosis/ischemic traumaDiagnostic recording/measuringSensorsPelvic diaphragm muscleEngineering
A method of using an apparatus to protect the tissues, muscles and nerves of the female pelvic floor from trauma during vaginal childbirth, the apparatus having a softly expansible intravaginal component and a support component with a handle attached to the intravaginal component, the method including deploying the intravaginal device into a vaginal canal and under a fetal head in the vaginal canal during vaginal childbirth, attaching the handle device to the central body, and delivering fluid through the handle device into the intravaginal device and inflating the intravaginal device with the fluid to deploy the wings on the intravaginal device from the stored configuration into the deployed configuration to provide support and stabilization to at least one from among a perineal, perianal, and anal region and preventing or mitigating the subsequent development of abnormal fistulous communications between the vagina and either the urinary bladder or the rectum or both.
Owner:PARTURA MEDICAL INC
Apparatus to protect the pelvic floor during vaginal childbirth
ActiveUS20190125406A1Prevent or mitigate ischemic pressure necrosis/ischemic traumaReduce coefficient of frictionDiagnostic recording/measuringSensorsObstetricsVaginal canal
An apparatus to protect the tissues, muscles and nerves of the female pelvic floor from trauma during vaginal childbirth, the apparatus having a softly expansible intravaginal component capable of insertion into a vaginal canal and under a fetal head while in either a folded or rolled up configuration and to unfold or unroll into a deployed configuration in the vaginal canal and under the fetal head, and a support component with a handle capable of attachment to the intravaginal component, of deploying and inflating the intravaginal, preferably with pressurized air or liquid, and further sized and shaped to provide support and stabilization to at least one from among a perineal, perianal, and anal region when maintained against a female perineal body and perianal region while attached to the intravaginal device that is deployed beneath the fetal head within the vaginal canal, or, in cases of obstructed vaginal childbirth, being subsequently detached, temporarily leaving in situ within the vagina the deployed, inflated intravaginal component for the purpose of preventing or mitigating the subsequent development of abnormal fistulous communications between the vagina and either the urinary bladder or the rectum or both.
Owner:PARTURA MEDICAL INC
Uterine filling hemostatic balloon and use method thereof
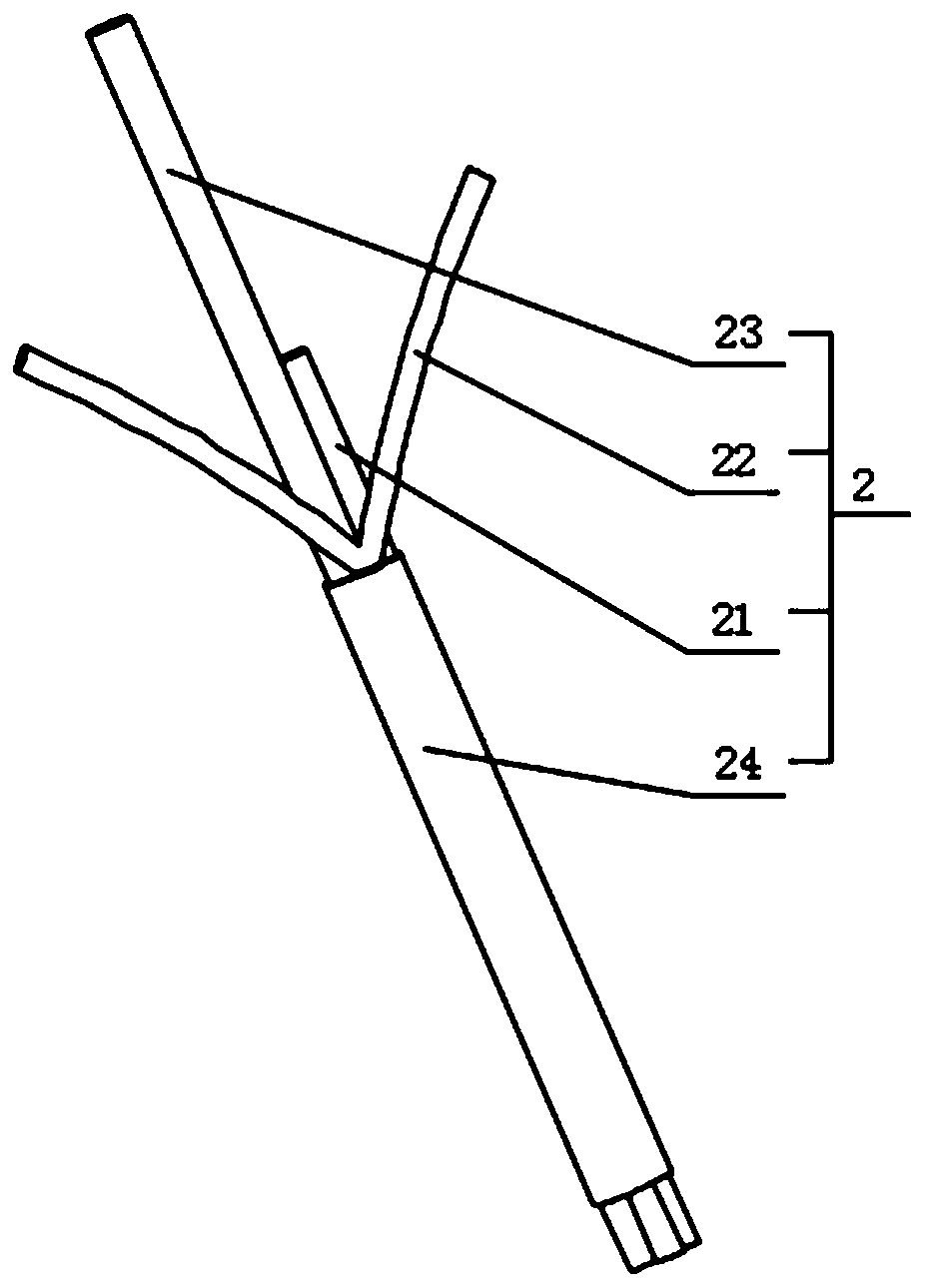
PendingCN111166411AReduce bleedingSolve the problem of not being able to effectively compress the uterine horn and effectively stop bleedingOcculdersObstetrical instrumentsUterine bleedingDrainage tubes
The invention belongs to the technical field of interventional medical instruments, and in particular relates to a uterine filling hemostatic balloon and a use method thereof. The uterine filling hemostatic balloon comprises a balloon and a catheter group; the balloon comprises a body cavity sac and two uterine corneal sacs; and the uterine corneal sacs are fixedly attached to the outer surface ofthe balloon and do not communicate with the inside of the body cavity sac. The use method of the uterine filling hemostatic balloon is characterized by comprising the following steps: A, after a placenta is delivered during vagina delivery, checking the bleeding condition and judging the uterine bleeding position; B, conveying the uterine filling hemostatic balloon into a uterine cavity through acervicial opening; C, connecting a drainage bag to the lower end of a drainage tube, and regularly recording the bleeding amount of the uterine cavity; D, injecting physiological saline into different sacs according to the uterine bleeding parts; and E, taking out the balloon after bleeding is stopped. The invention can solve the problem that no effective hemostasis measures are available for placental adhesion and uterine contraction weakness caused by the fact that a placenta is attached to the cornual uterus during clinical use.
Owner:天津市中心妇产科医院
Cell transplantation pipe for nasal delivery and use method thereof
The present invention discloses a cell transplantation pipe for nasal delivery and a use method thereof. The cell transplantation pipe for nasal delivery comprises a transplantation pipe joint which communicates with a syringe and a transplantation hose for transplanting cells into a nasal mucosa area enriched with olfactory nerve, wherein the transplantation hose is located under a sieve plate atthe deep portion of a nasal cavity. One end of the transplantation hose is a connecting end, and the connecting end communicates with the transplantation pipe joint to form one body. The other end ofthe transplantation hose is an intervention end, and the intervention end is of a circular head structure, a medicine delivery hole is formed in the side wall 2-4 mm away from the intervention end; and the diameter of the cross section of the medicine delivery hole decreases gradually from the outside to the inside. The cell transplantation pipe for the nasal delivery is specially designed for cell medicine delivery, and the technical scheme has the following beneficial effects that for cell transplantation into a central nervous system, compared with a traditional traumatic medicine deliverymethod, the nasal delivery can alleviate the pain of patients, reduce the injury of patients, and reduce the risk of patients; and a cell transplantation pipe can penetrate into the nasal cavity, themedicine wastage is reduced during medicine delivery, the nasal delivery efficiency is improved, the medicine wastage is small, and the cell transplantation pipe for nasal delivery is suitable for cell nasal delivery and other medicines which need low-dosage nasal delivery.
Owner:SHANGHAI ANGECON BIOTECH
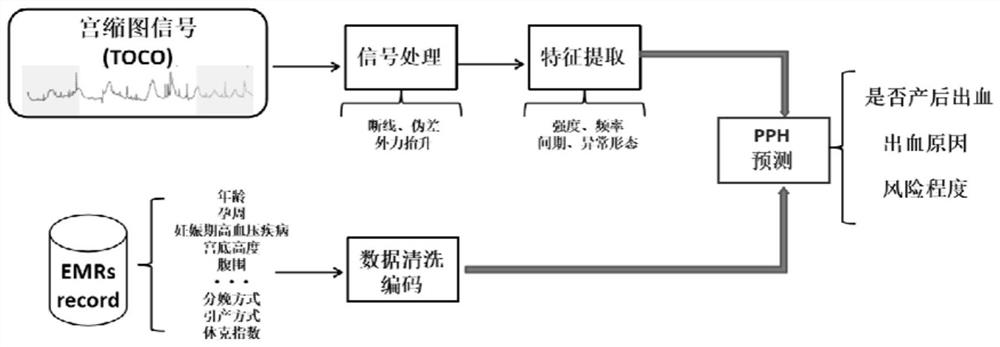
Postpartum hemorrhage risk prediction method and early warning system based on fetal monitoring uterine contraction graph and high-risk factors
PendingCN113611419AEasy to observeReduce the incidence of bleedingMedical simulationMedical data miningMedical recordHigh risk factors
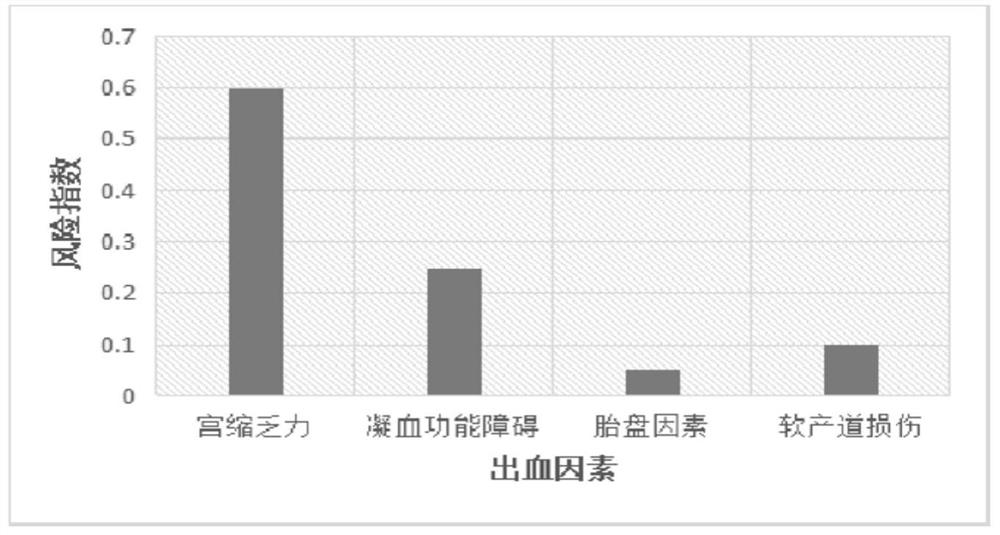
The invention discloses a postpartum hemorrhage risk prediction method and early warning system based on a fetal supervision uterine contraction graph and high risk factors. The method comprises the following steps: S1, collecting data of a hospital fetal supervision uterine contraction graph and an electronic medical record system; S2, screening pregnant and lying-in women during vaginal delivery, and extracting required patient information; S3, establishing a hemorrhage risk prediction model within 24 hours after delivery of the puerpera by applying a machine learning method; S4, evaluating the prediction value of each model, and selecting an optimal prediction model. According to the method, the prediction model is established through a machine learning method, and the method is of great significance to clinical treatment decisions including timely identification of uterine contraction hypodynamia, prevention of soft birth canal injury, correction of blood coagulation dysfunction and the like and reduction of the occurrence rate of bleeding.
Owner:JINAN UNIVERSITY
Progestin therapy with controlled bleeding
InactiveCN1282250AAvoid side effectsSide effects are uncommonOrganic active ingredientsPharmaceutical non-active ingredientsRegimenSide effect
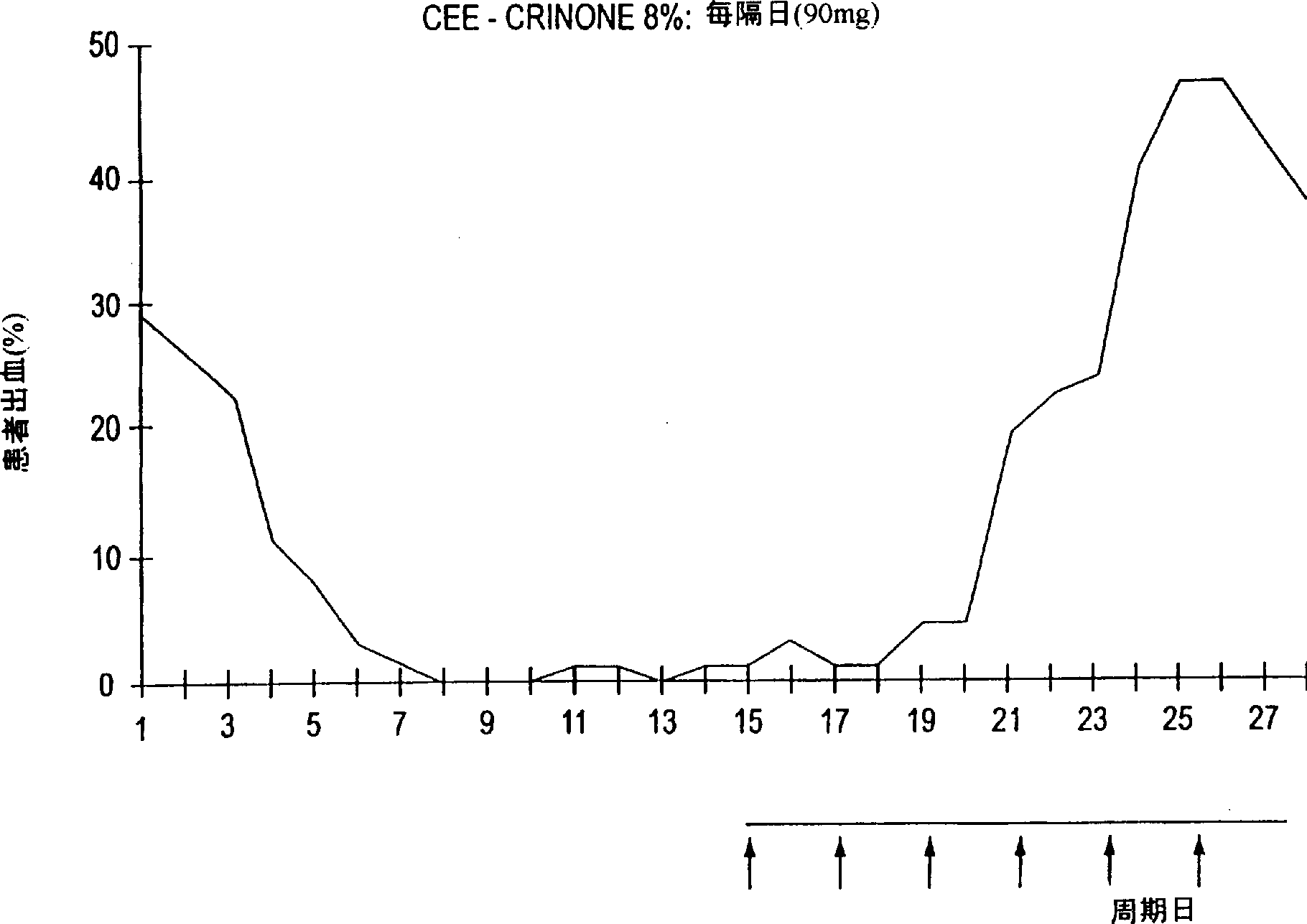
The present invention teaches that daily, cyclical vaginal delivery of progestin may be used to provide regular, predictable withdrawal bleeding during hormone replacement therapy. The present invention also teaches that constant administration of progestin in a water-insoluble, water-swellable cross-linked polycarboxylic acid polymer may be used to maintain amenorrhea. Either regimen is accompanied by a significant decrease in adverse side effects.
Owner:COLUMBIA LAB BERMUDA
A kind of automatic vaginal scissors
InactiveCN112515750BEasy to cutRelieve painAutomatic syringesSurgical scissorsEngineeringFetal injury
The invention discloses a full-automatic vaginal scissors, including a hand-held system, a scissors system, and an anesthesia system. The hand-held system is provided with a hand-held sheath, which can prevent doctors from slipping when using the invention, and hold the hand-held system so that the scissors can reach the puerpera's vagina Nearby, the anesthesia system is used to perform local anesthesia around the puerpera's vagina, and then the vagina is cut open by the scissor system to make the delivery smoother and reduce the risk of fetal injury and death. Wait for multiple actions to cut the vagina easily, and the hand-held system is equipped with a scissors cover, which can automatically cover the scissors when the scissors are not in use, reducing the risk of accidental injury.
Owner:孙礼鹃
Special hospital bed for maternity emergencies
InactiveCN109875822BReduce deliveryReduce the risk of vaginal deliveryOperating tablesInfusion devicesSpecial bedEngineering
Owner:尹红梅 +1
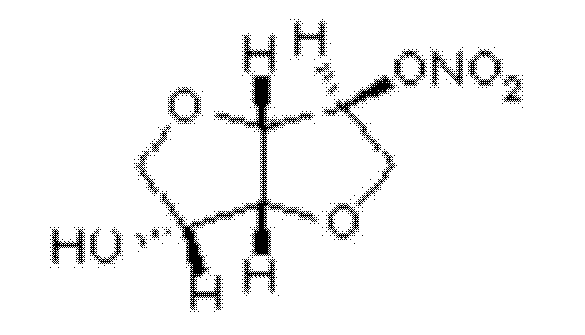
New application of isosorbide mononitrate
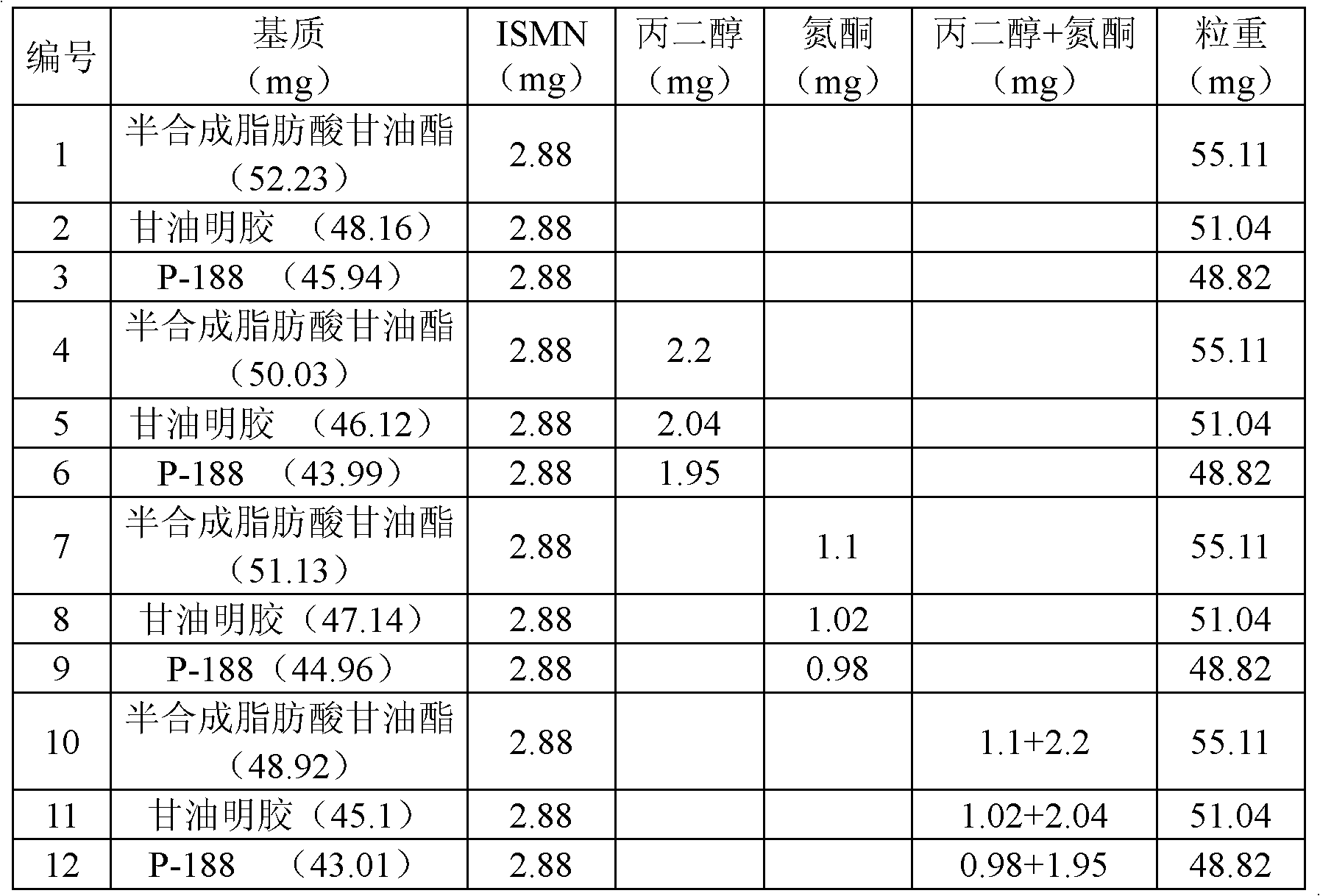
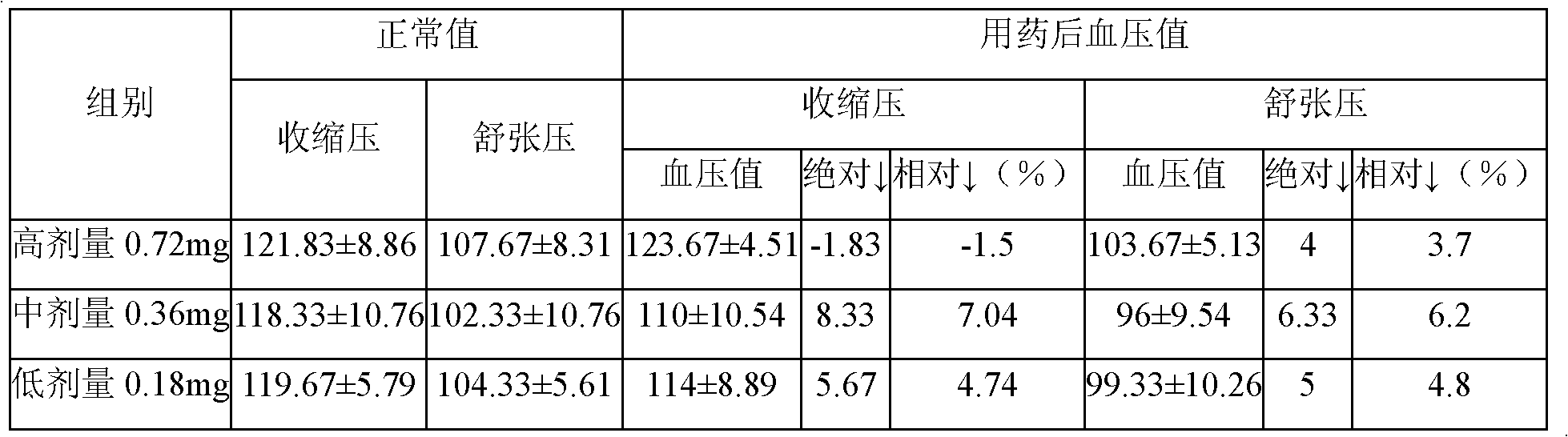
InactiveCN102283827AImprove blood supplyPromote repairSexual disorderHeterocyclic compound active ingredientsSide effectEndometrium thickness
The invention belongs to the field of medicine, and in particular relates to a new application of isosorbide mononitrate and a new dosage form corresponding to the new application; specifically, it is the preparation of isosorbide mononitrate in the preparation of drugs for promoting endometrium development use. The promotion of endometrial development is specifically achieved by repairing and improving the endometrium to increase the thickness of the endometrium, or by promoting the development of uterine glands, or by promoting the development of uterine stroma. The drug prepared to adapt to the above four new uses is a topical vaginal administration preparation, that is, a preparation prepared by adding an effective amount of isosorbide mononitrate as an active ingredient and adding pharmaceutically acceptable auxiliary materials. After vaginal administration of isosorbide mononitrate, it can not only promote the development of endometrium tissue and improve the endometrial receptivity, but also effectively avoid the side effect of isosorbide mononitrate blood pressure drop. A new option is provided.
Owner:SICHUAN WEIXIN MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com