Progestin therapy with controlled bleeding
A progestogen and polymer technology, applied in the field of progestogen therapy with the characteristics of controlling bleeding, can solve the problems of reducing systemic action, prolonging retention, etc., and achieving the effects of avoiding side effects, no side effects, and avoiding bleeding subsidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] Preparation of the formulation involves hydration of the polymer, mixing the water soluble ("water phase") and oil soluble ("oil phase") components separately, heating and mixing the two phases, and homogenizing the mixture. All of the ingredients listed above are known and can be readily purchased from suppliers well known in the art.
[0039] The polymer phase can usually be prepared by mixing added water, sorbic acid, acrylic resin and carbomer. The polymer is hydrogenated by mixing for several hours, usually about 2-3 hours, until a uniform, smooth, homogeneous mixture free of lumps is obtained. When the polymer is fully hydrated, the progesterone is added and mixed until a homogeneous suspension is obtained.
[0040] The oily phase can usually be prepared by heating glycerin and mineral oil together to melt at 75-78°C. The mixture was cooled to 60°C while the polymer phase was warmed to approximately the same temperature. The polymer is then added to the heated ...
Embodiment 1
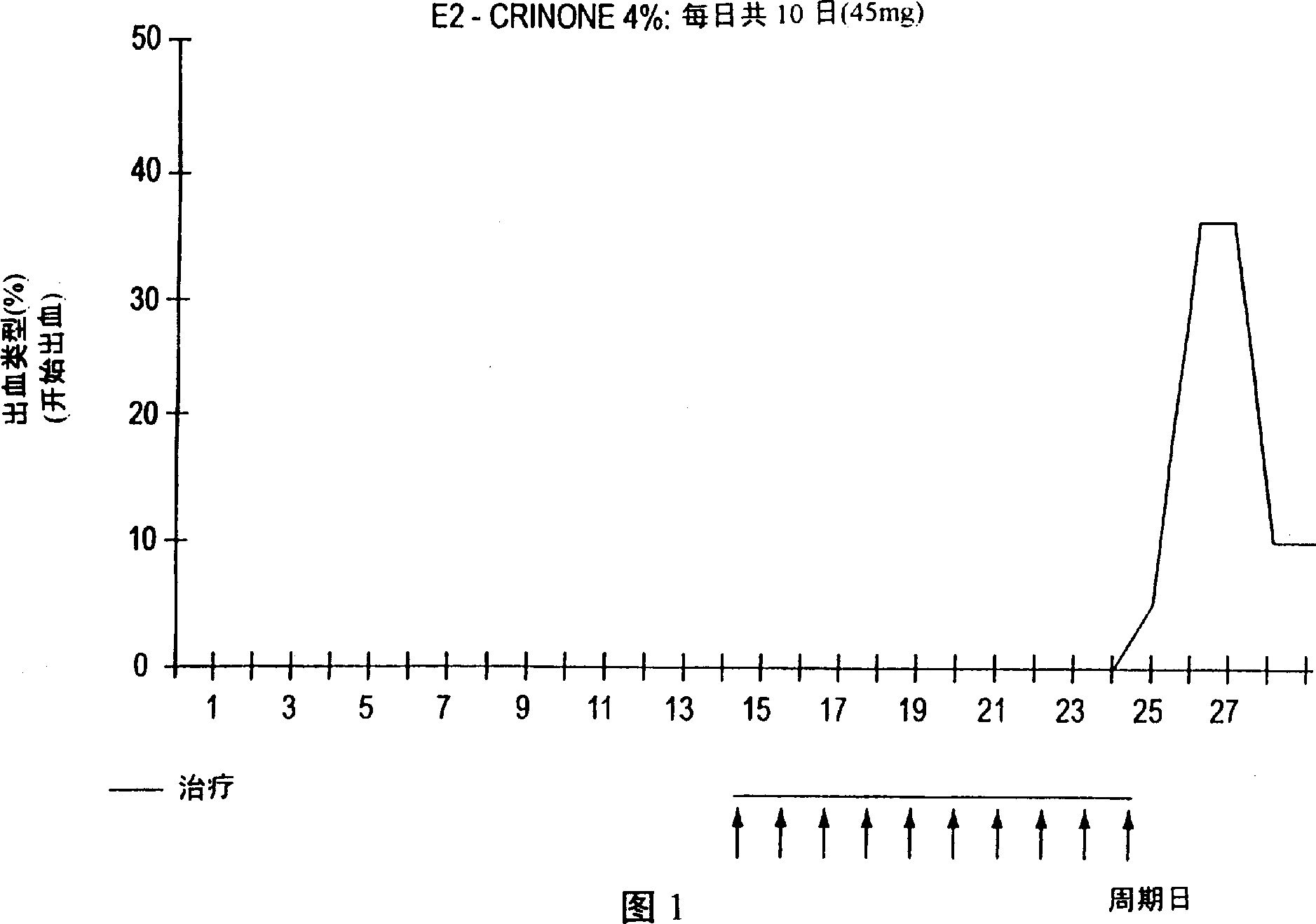
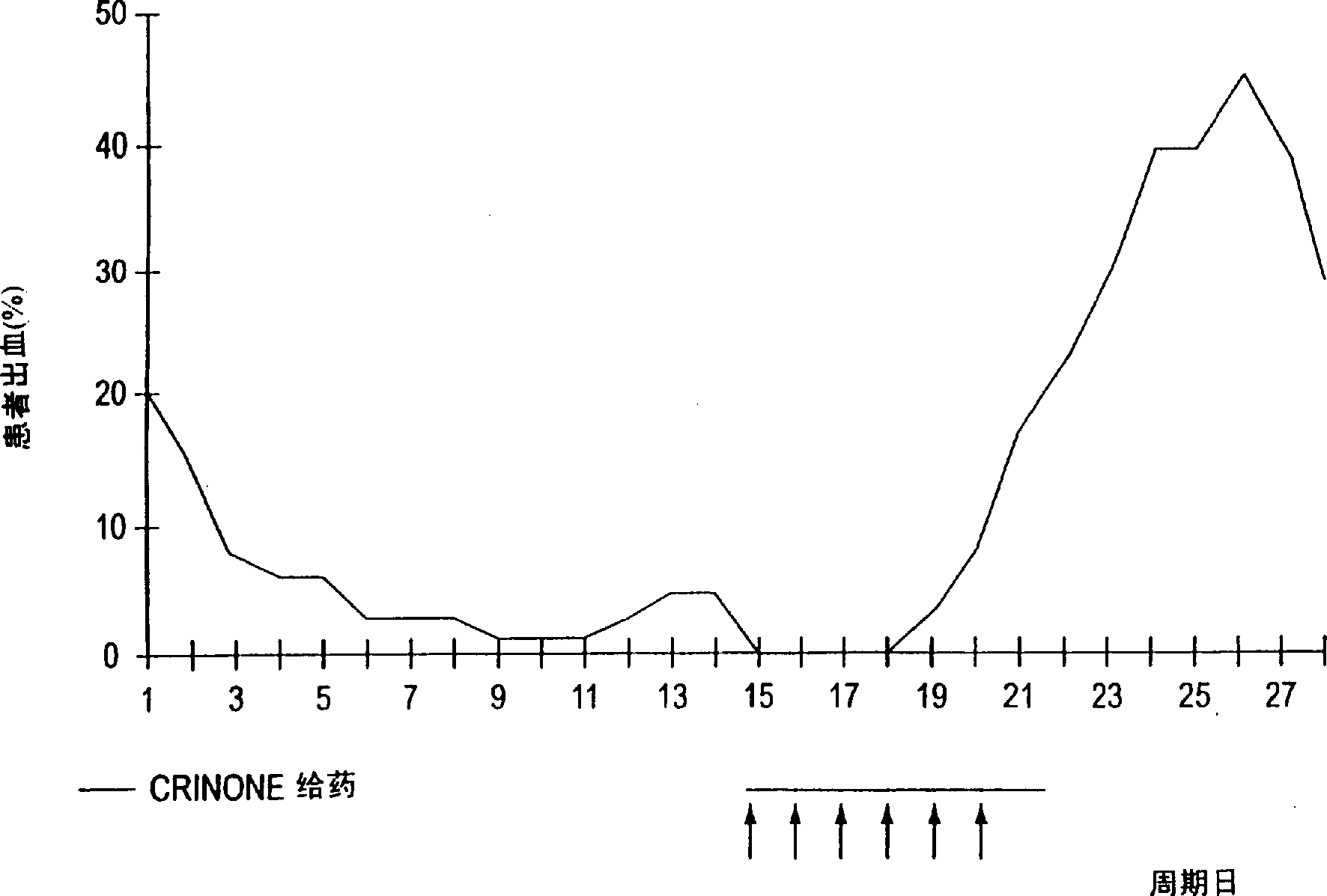
[0043] Example 1 Comparison of Continuous Progesterone Dosing in Daily and Twice-Weekly Cycles
[0044] The study was designed to examine the use of CRINONE Progesterone Gel as part of Hormone Replacement Therapy ("HRT") during menopause during estrogen-related cycle therapy and continuous dosing in combination with estrogen to achieve a bleeding-free regimen . The results of the first cohort of cases in this study are reported below (the study continued with other cases and the full results of all cases, including those discussed in Example 1, are reported in Example 3 below).
[0045] The women were divided into two groups of 20 each. Group I ranged in age from 38-55 years, and each woman presented with menopausal symptoms or was undergoing HRT. Group II age range 50-64 years, each woman had been menopausal (absence of menstruation) for 3 years, or was undergoing HRT with periodic bleeding. None of the women in either group exhibited abnormal bleeding or any other uterine...
Embodiment 2
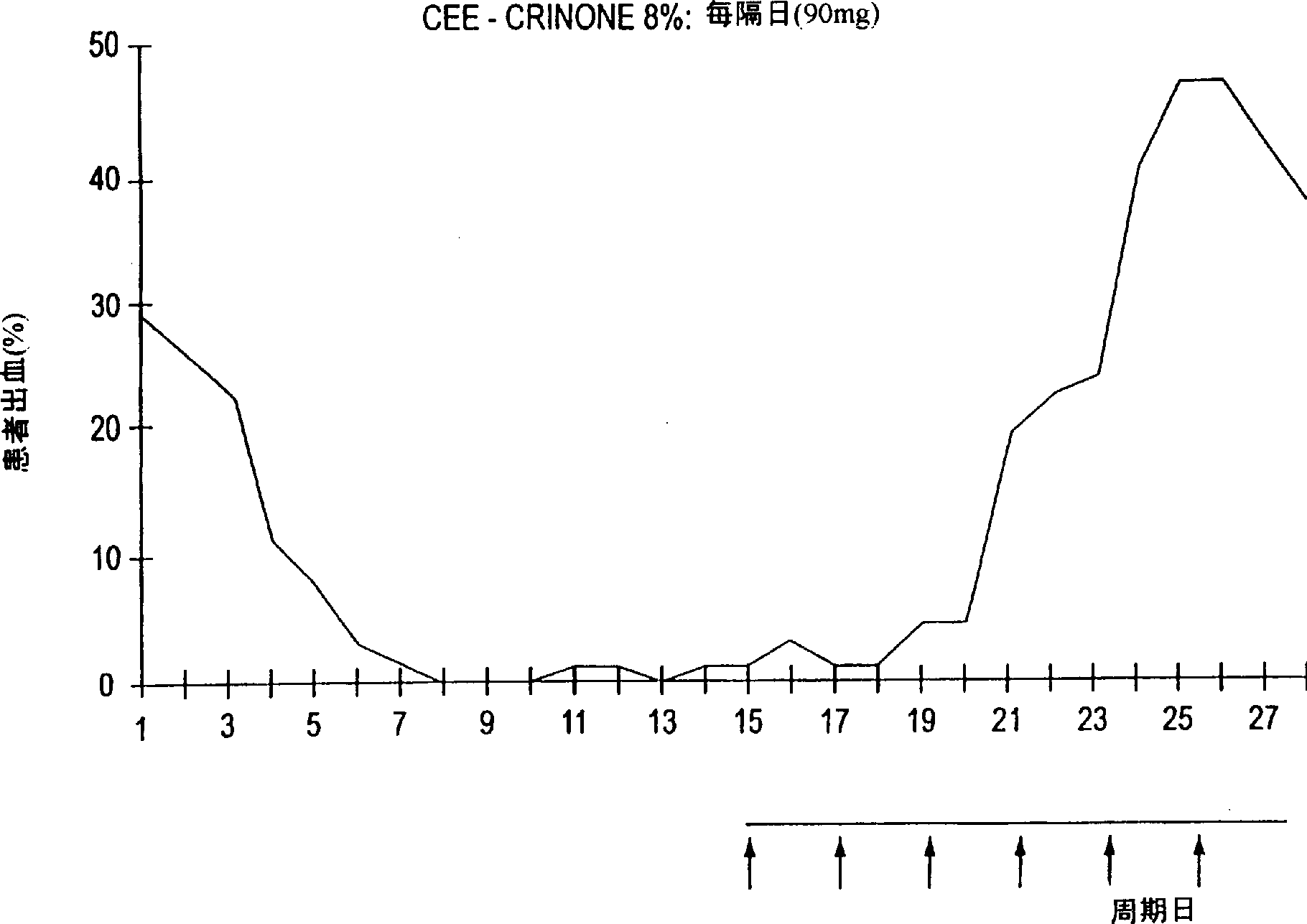
[0054] Example 2 Continuous progesterone administration
[0055] 18 women who have been amenorrhea for at least 3 years or are at least 53 years of age continue to receive supraconventional estrogen therapy (ESTRADERM TTS (50mg) or ESTRADERM MX (50mg) estrogen small tablets) with 1.125g of CRINONE 8% twice a week Progesterone Gel (90mg Progesterone). Ultrasound was performed at 6 months.
[0056] After 6 months of treatment, 13 of 18 cases remained amenorrhea. Of the 5 bleeding patients, 4 had only intermittent bleeding, and only 1 had severe bleeding. At 6 months all women had an endometrium less than 5 mm thick. Of the 13 amenorrhea women, 10 were satisfied to continue treatment, 2 changed to a different HRT regimen, and 1 stopped HRT.
[0057] With continued administration of CRINONE progesterone gel twice weekly, most patients remained completely amenorrhea.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com