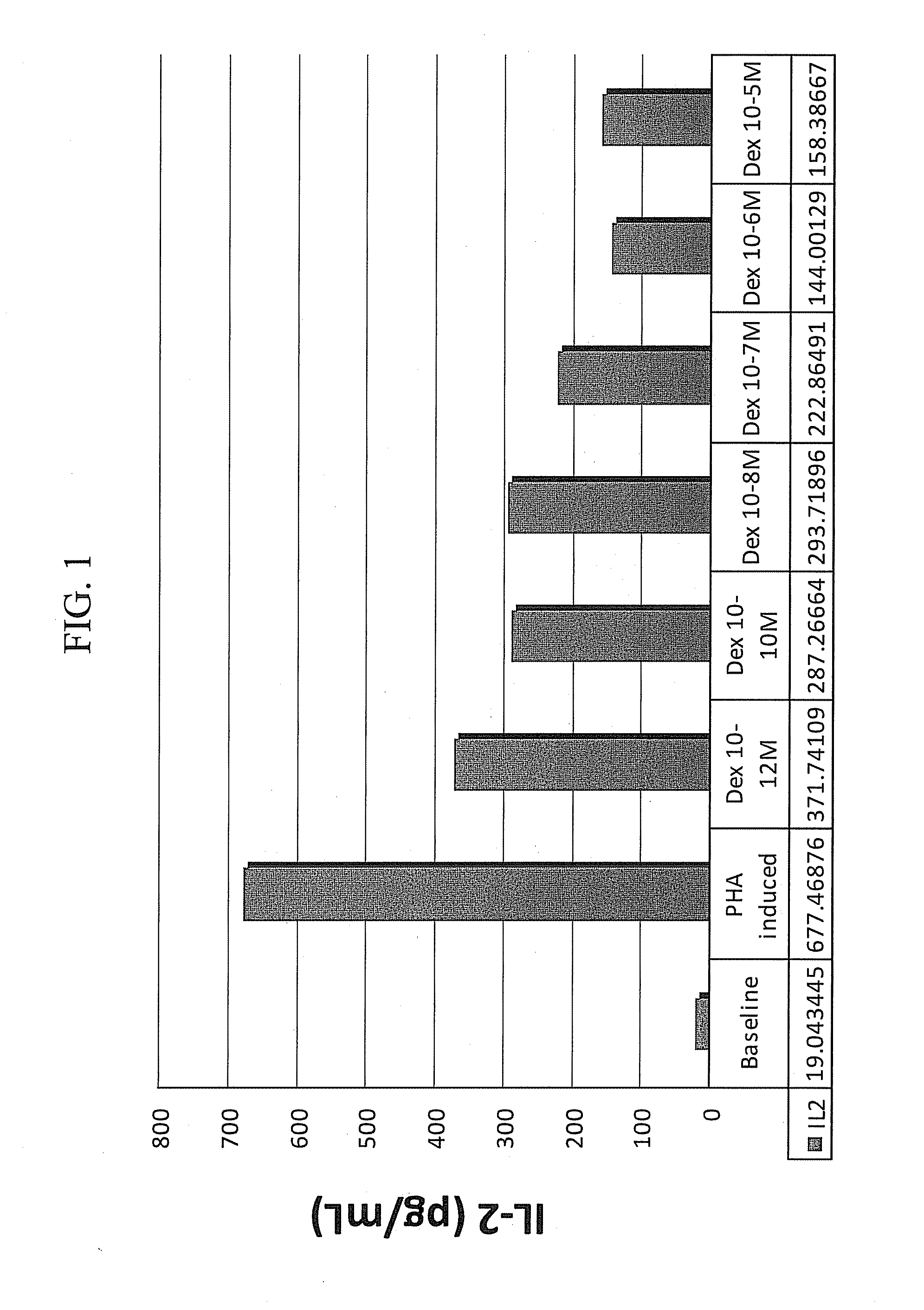
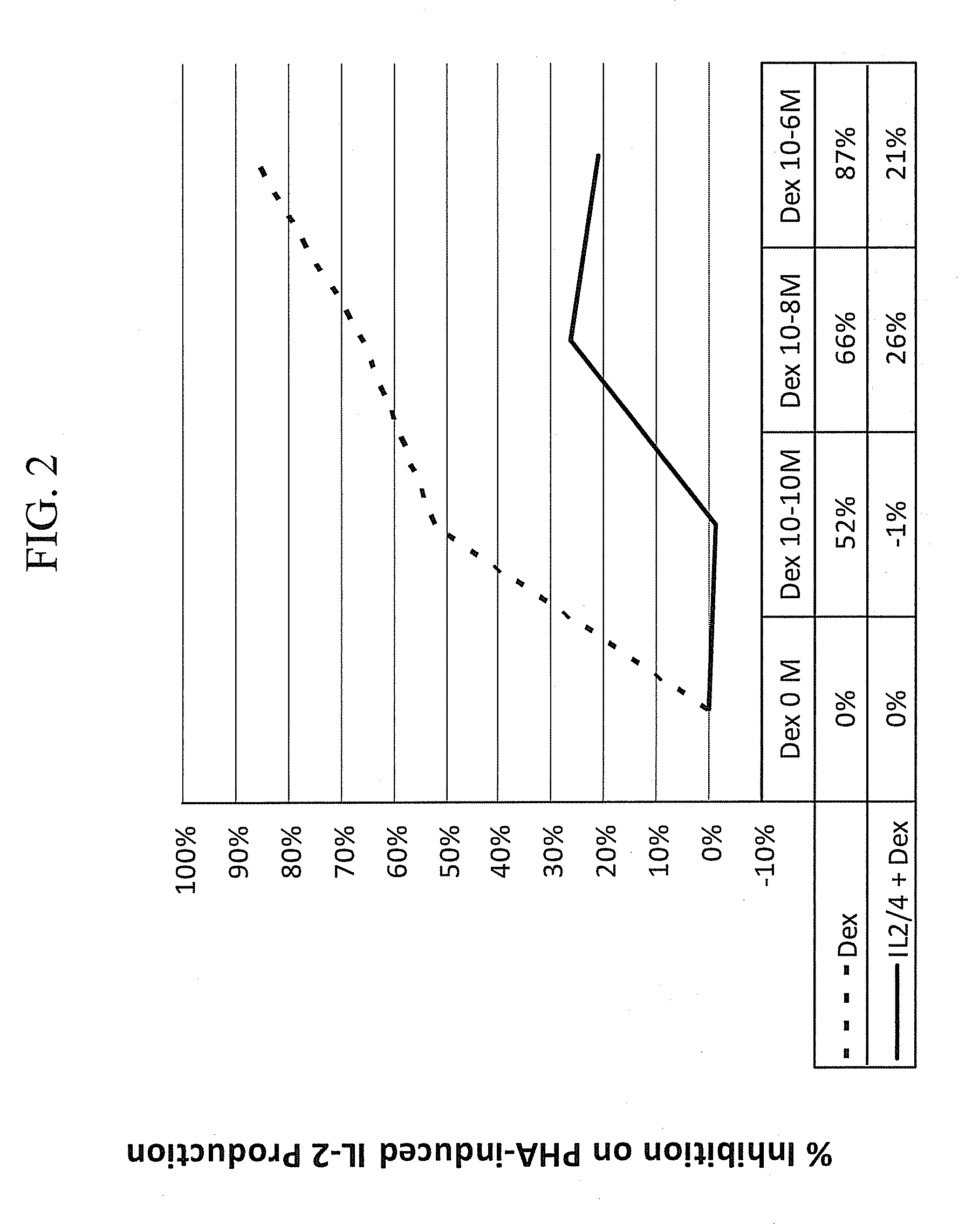
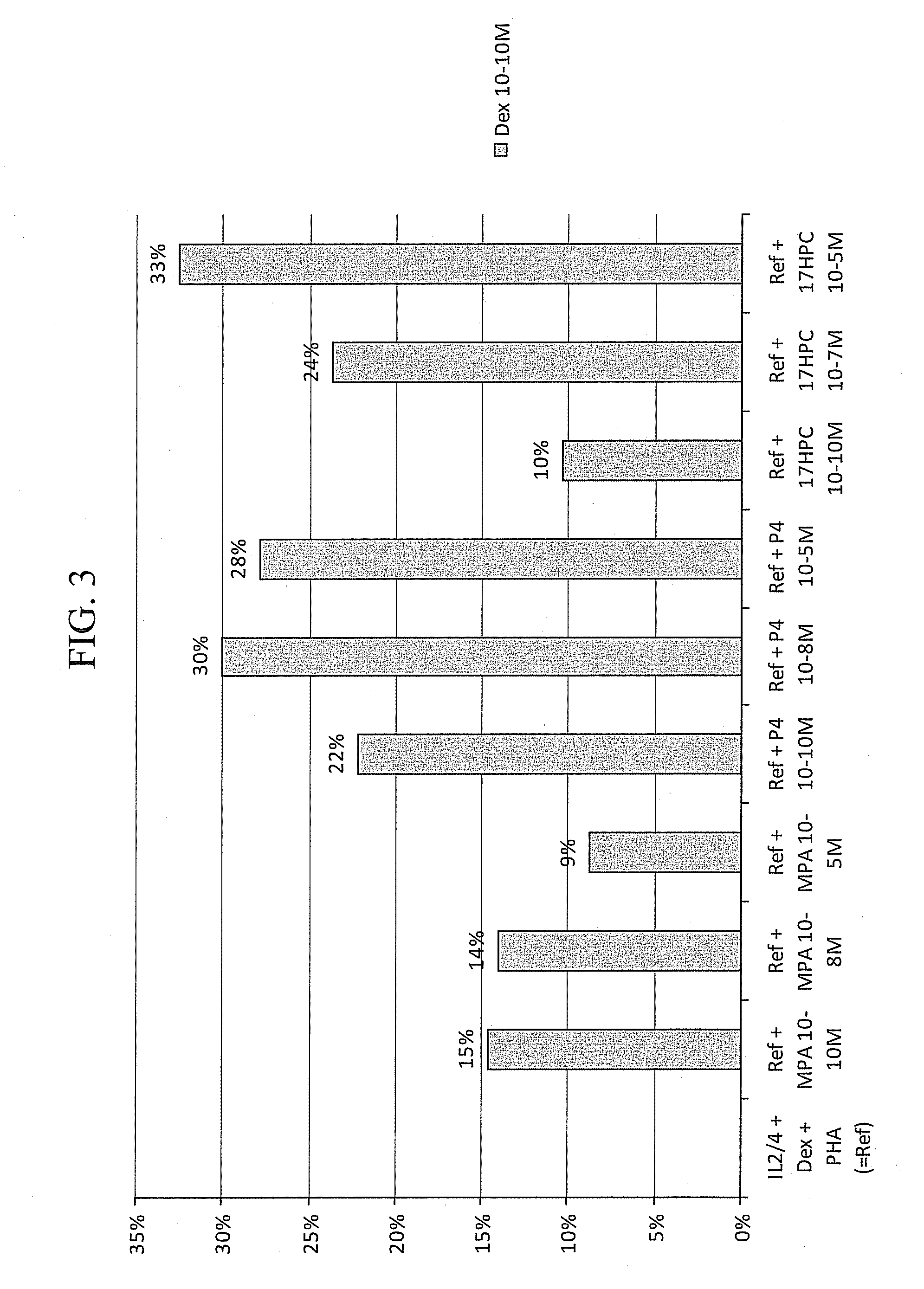
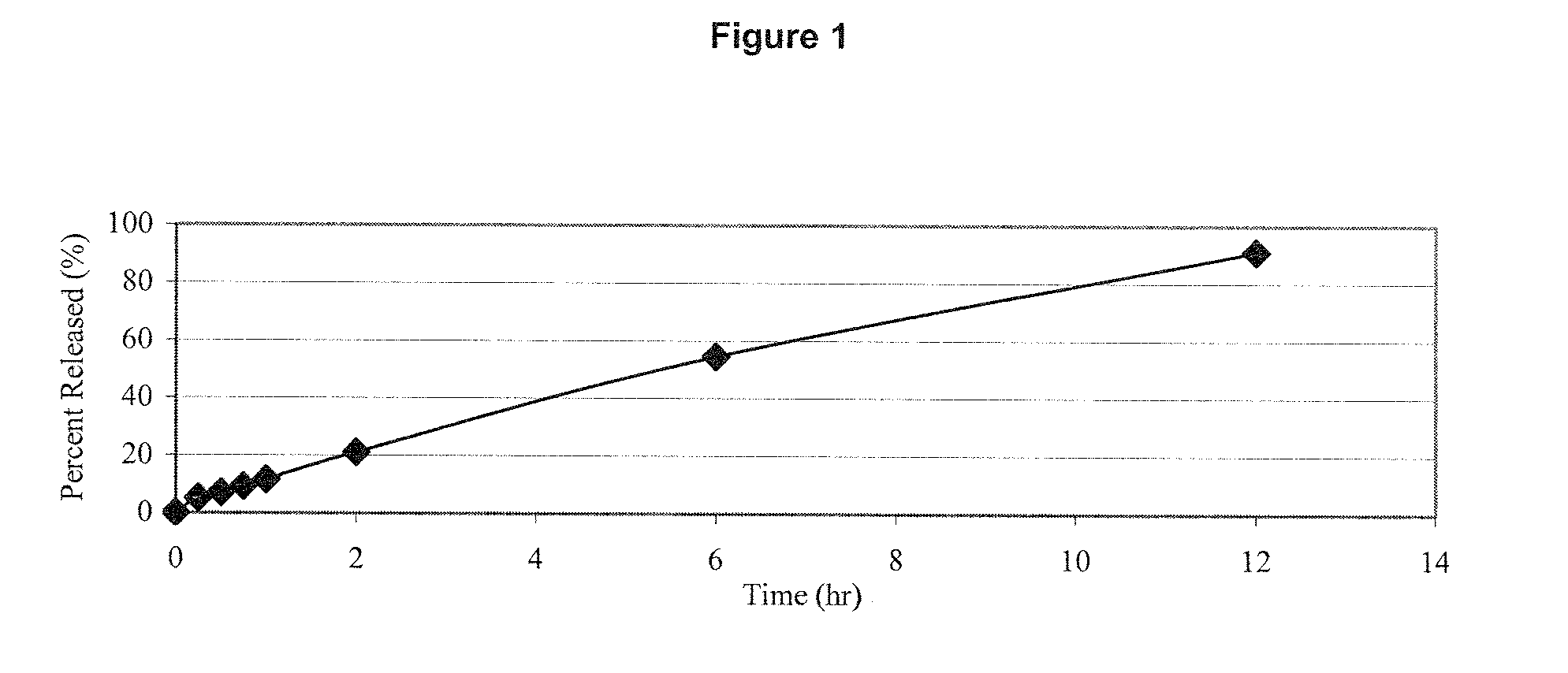
Patents
Literature
140 results about "Progestin therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In Wikidata. A progestin is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications.
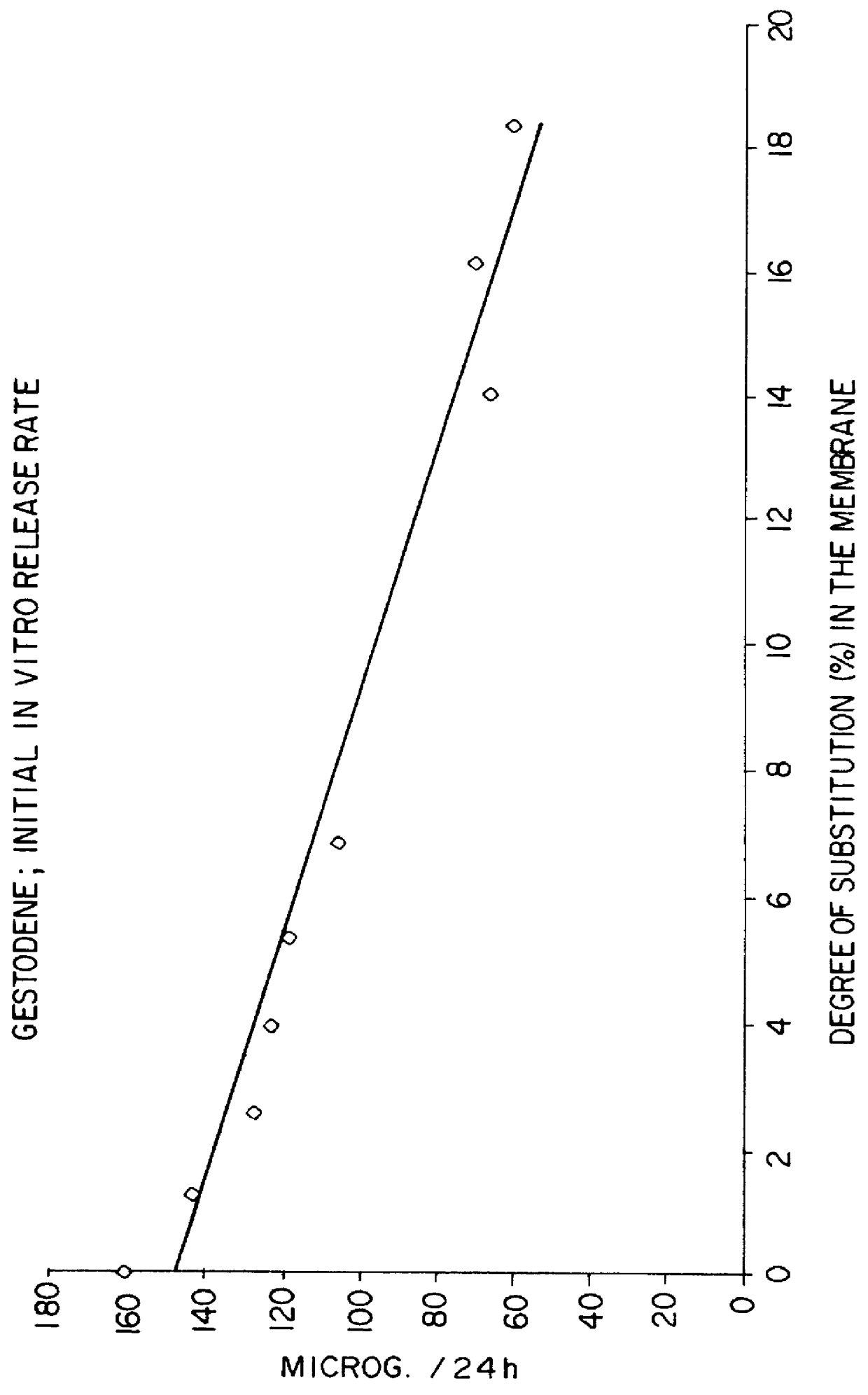
Molecular dispersions of drospirenone
InactiveUS20050220825A1Improve bioavailabilityGood chemical stabilityPowder deliveryPill deliveryParticulatesDrospirenone
Described are pharmaceutical compositions comprising at least one steroidal drug such as a progestin (e.g. drospirenone, progesterone, eplerenone, etonogestrel) and / or an estrogen (estradiol and esters thereof) in molecularly dispersed form. The composition comprises a steroidal drug, preferably drospirenone, which is present in the composition in a non-particulate form. Preferably, the drug is present in a dissolved state in the excipient. The molecularly dispersed drug will be released very fast as dissolution takes place instantly when the dosage unit has disintegrated. Also described are methods for preparing the pharmaceutical compositions and methods of using the compositions.
Owner:BAYER SCHERING PHARMA AG
Extended use combination comprising estrogens and progestins
InactiveUS20050113350A1Good effectIncrease of the estrogen and/or progestin dosageOrganic active ingredientsBiocidePhysiologyPharmaceutical formulation
A pharmaceutical preparation to obtain a continuous hormonal treatment over a desired period of time longer than 21-28 days comprising a first composition containing at least one estrogen and / or at least one progestin in a predetermined amount to be administered in the first 21-28 days and a second composition which contains at least one estrogen and / or at least one progestin in a predetermined amount higher than the amount of the first composition and comprises a mono or multiphase sequence of pharmaceutical dosages.
Owner:DUESTERBERG BERND +2
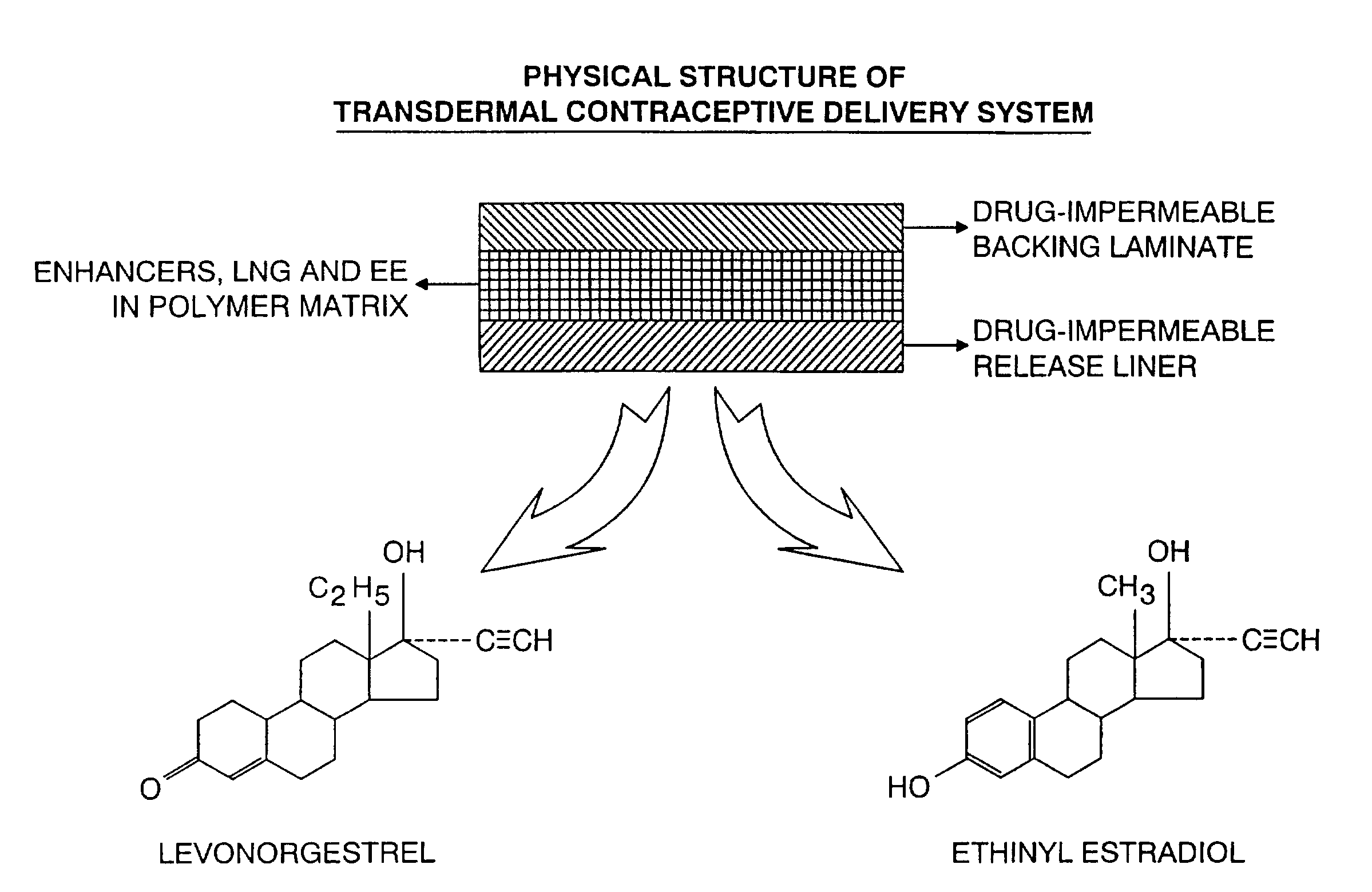
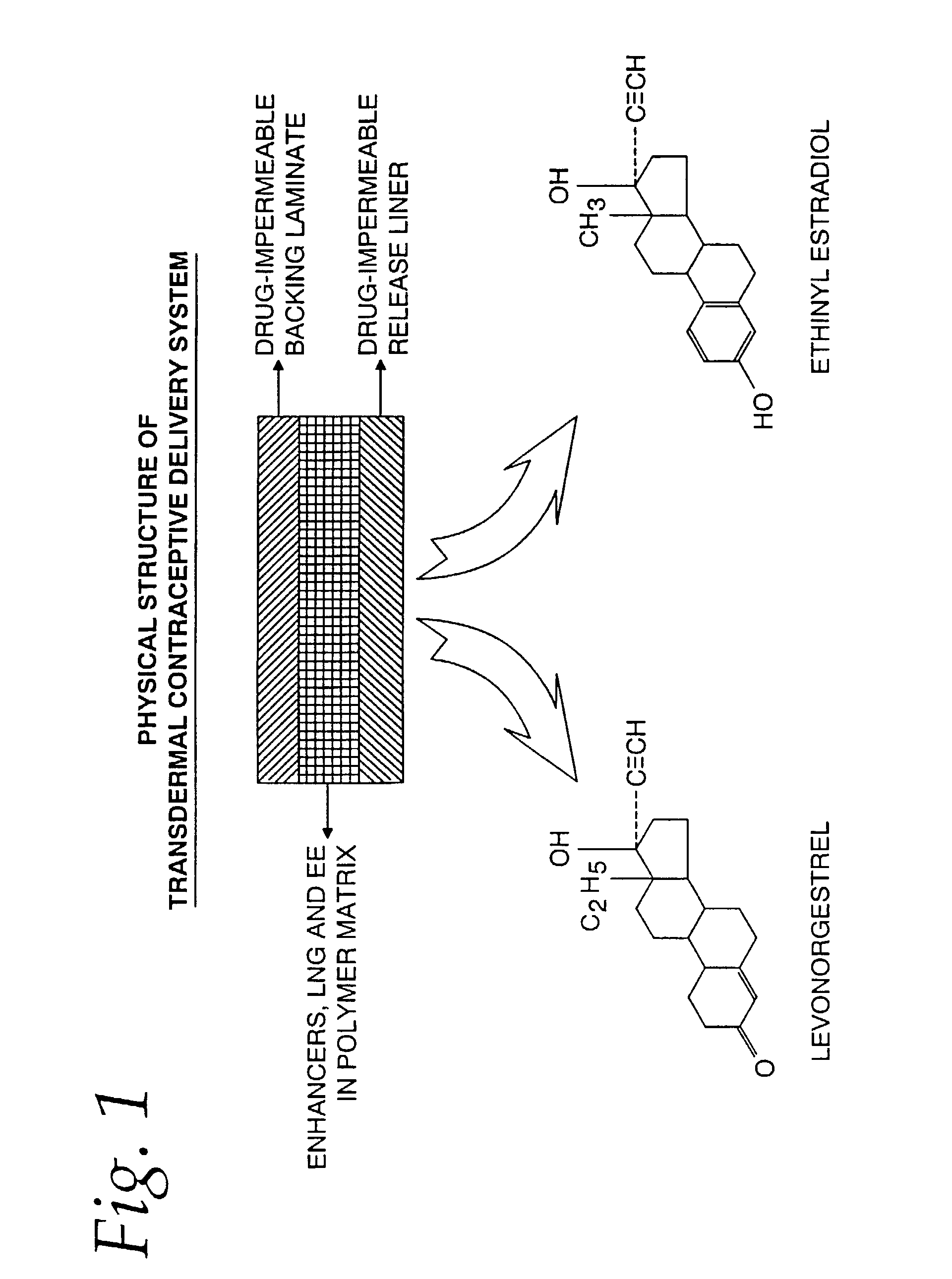
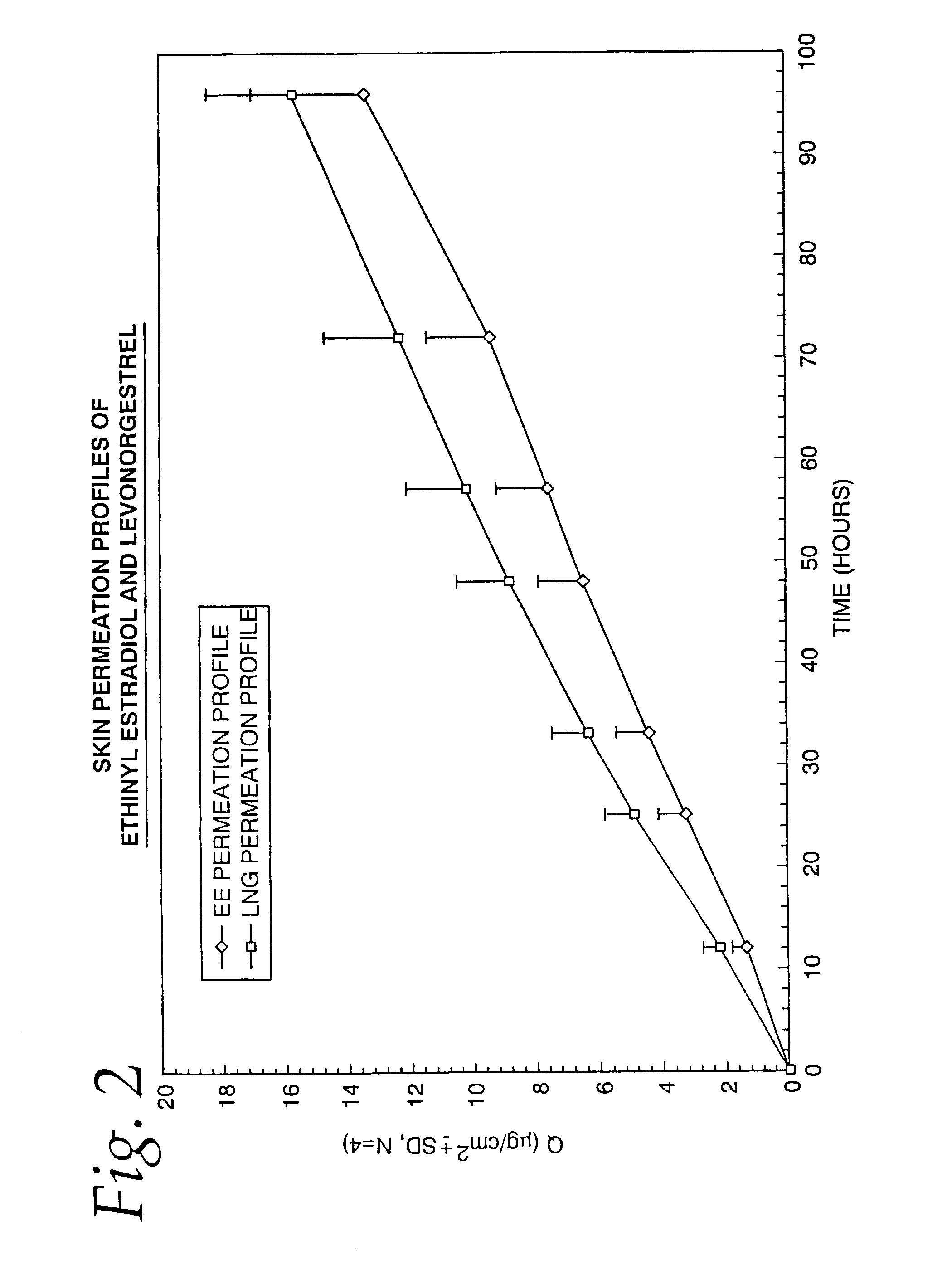
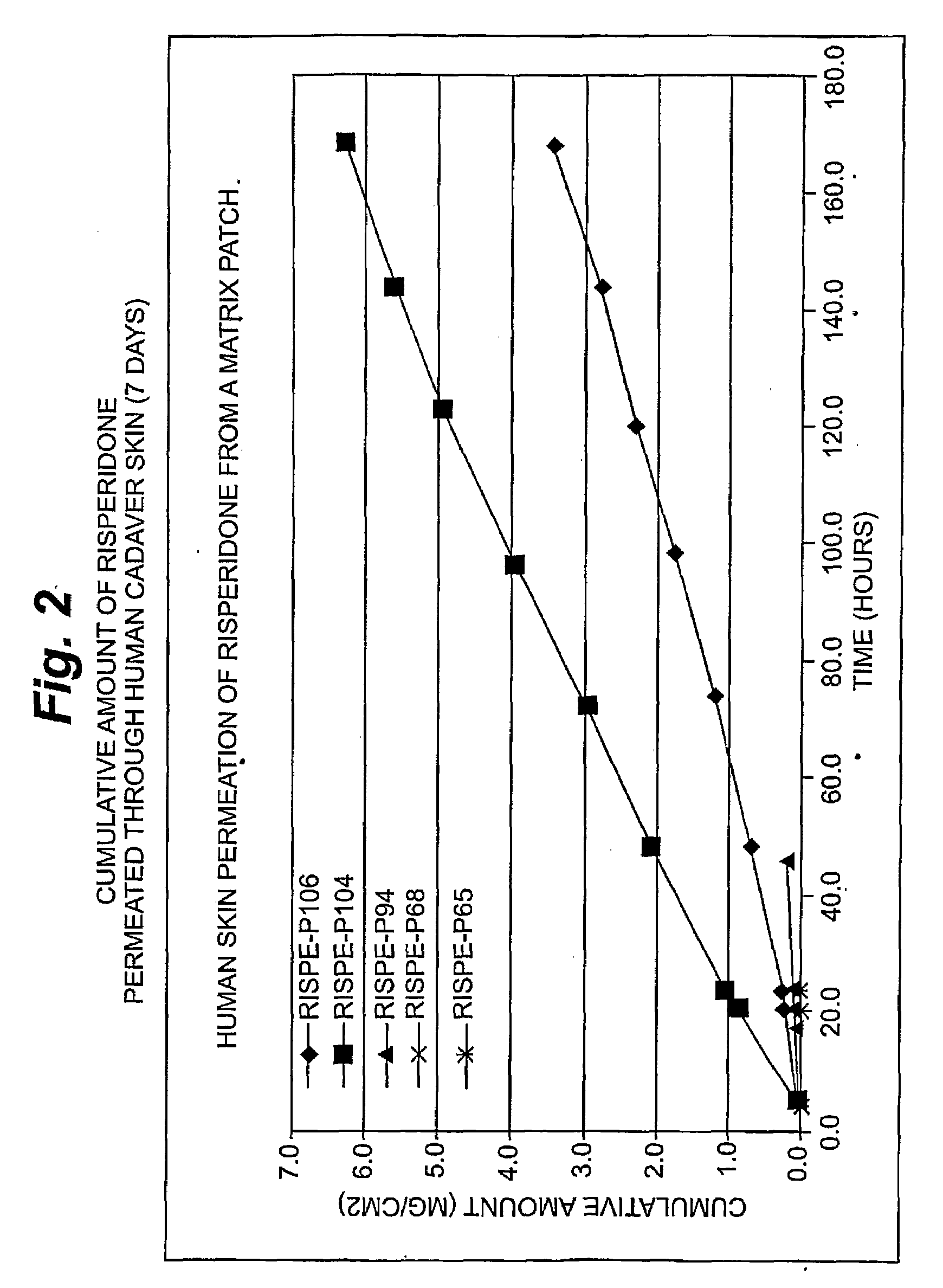
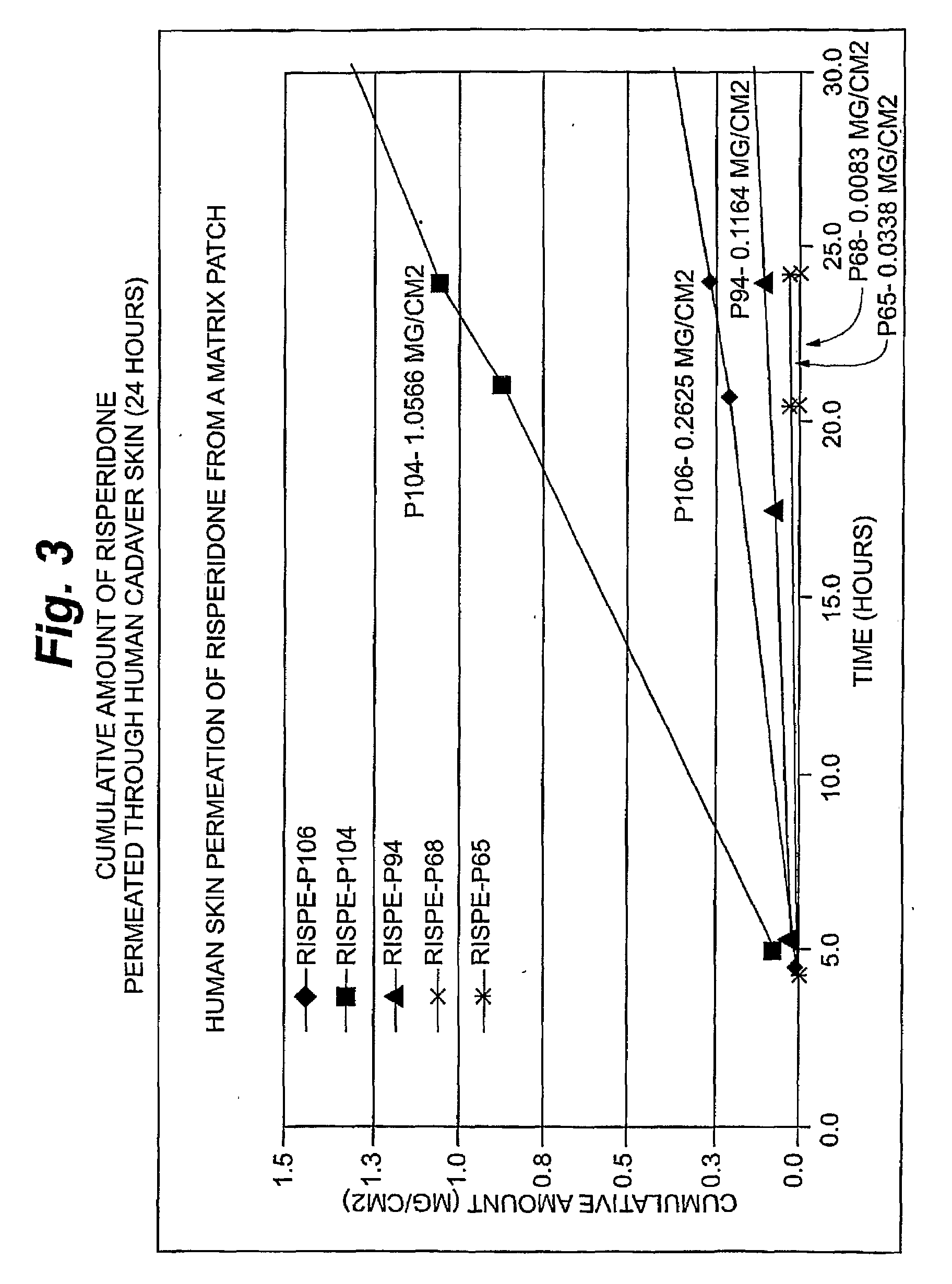
Transdermal contraceptive delivery system and process
InactiveUS7045145B1Cut skinReduce concentrationOrganic active ingredientsAdhesive dressingsObstetricsAdhesive
A transdermal contraceptive delivery system (TCDS) for fertility control in women is described. It comprises a backing layer, an adjoining layer of a solid absorption adhesive polymer matrix in which effective daily doses of an estrogen and a progestin are dispersed and released for transdermal absorption. Presently preferred is the use of the synthetic estrogen, ethinyl estradiol, and the synthetic progestin, levonorgestrel. Along with these two steroidal contraceptive agents, a combination of several chemical skin permeation enhancing agents, including capric acid, blended at specific weight ratios, ranging from 2:1:1:0.8 to 6:1:1:0.8, are homogeneously dispersed in the adhesive polymer matrix. The invention also provides a method of fertility control utilizing the transdermal contraceptive delivery system.
Owner:AGILE THERAPEUTICS
Drug delivery device especially for the delivery of progestins and estrogens
InactiveUS6063395AEasy to adjustSmall sectionOrganic active ingredientsPowder deliveryElastomerControl release
The invention relates to a delivery device for the controlled release of a therapeutically active agent, especially a progestin or an estrogen, over a prolonged period of time, said device including a core which contains the therapeutically active agent, and a membrane encasing the core wherein said membrane is made of an elastomer. According to the invention, the elastomer is a siloxane-based elastomer which includes 3,3,3-trifluoropropyl groups attached to the Si-atoms of the siloxane units.
Owner:OY SCHERING +1
Hormone replacement therapy method
InactiveUS6551611B2Lower energy requirementsOrganic active ingredientsAerosol deliveryHormone replacementPhysician attending
Varying the daily dose of either or both of the estrogen and the progestogen administered for hormone replacement therapy (HRT) is readily and inexpensively accomplished, without the necessity of the physician prescribing a new product each time the daily dose of the estrogen or progestogen is changed, by administering preferably transdermally the estrogen and the progestogen contained in separate extrudable pharmaceutical compositions from a dispenser which contains means, preferably adjustable only by the attending physician or dispensing pharmacist, for varying the volume of either or both of the respective compositions which is dispensed as a single dose from the dispenser in response to a defined digital dispensing manipulation of the dispenser thereby facilitating optimal compliance to a combination of HRT with individually adjusted dosages of the estrogen and progestogen.
Owner:BAYER SCHERING PHARMA AG
Methods for the use of progestogen as a glucocorticoid sensitizer
ActiveUS20110195031A1Improve responsivenessImprove toleranceBiocideSenses disorderSterolGlucocorticoid Sensitivity
Provided are methods and kits for administering progestogen as a glucocorticoid sensitizer to restore corticosteroid sensitivity or reverse the glucocorticoid insensitivity or enhance glucocorticoid sensitivity, in order to treat one or more glucocorticoid insensitivity related diseases or conditions. For example, these include methods for reversing the glucocorticoid insensitivity in a subject having no history of menstrual cycle-related exacerbation or allergy to self-hormones, particularly progesterone, such as premenstrual or perimenstrual deterioration in the symptoms, e.g., premenstrual worsening of atopic dermatitis or premenstrual exacerbations of asthma, and exhibiting relatively or totally refractory responses to glucocorticoid therapy, e.g., glucocorticoid resistance. The methods and kits provide for the administration of a sex hormone to the subject who is corticosteroid dependent or corticoid resistant or unresponsive or intolerant to corticosteroids.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
Estrogen/serm and estrogen/progestin bi-layer tablets
The present invention is directed to bi-layer tablets comprising at least one estrogen in a first layer and a therapeutic agent in a second layer, and processes for their preparation.
Owner:WYETH LLC
Pharmaceutical composition for use in hormone replacement therapy
ActiveUS8048869B2Interaction can be difficultReliable efficacyBiocideOrganic active ingredientsAnti-ProgestinPresent method
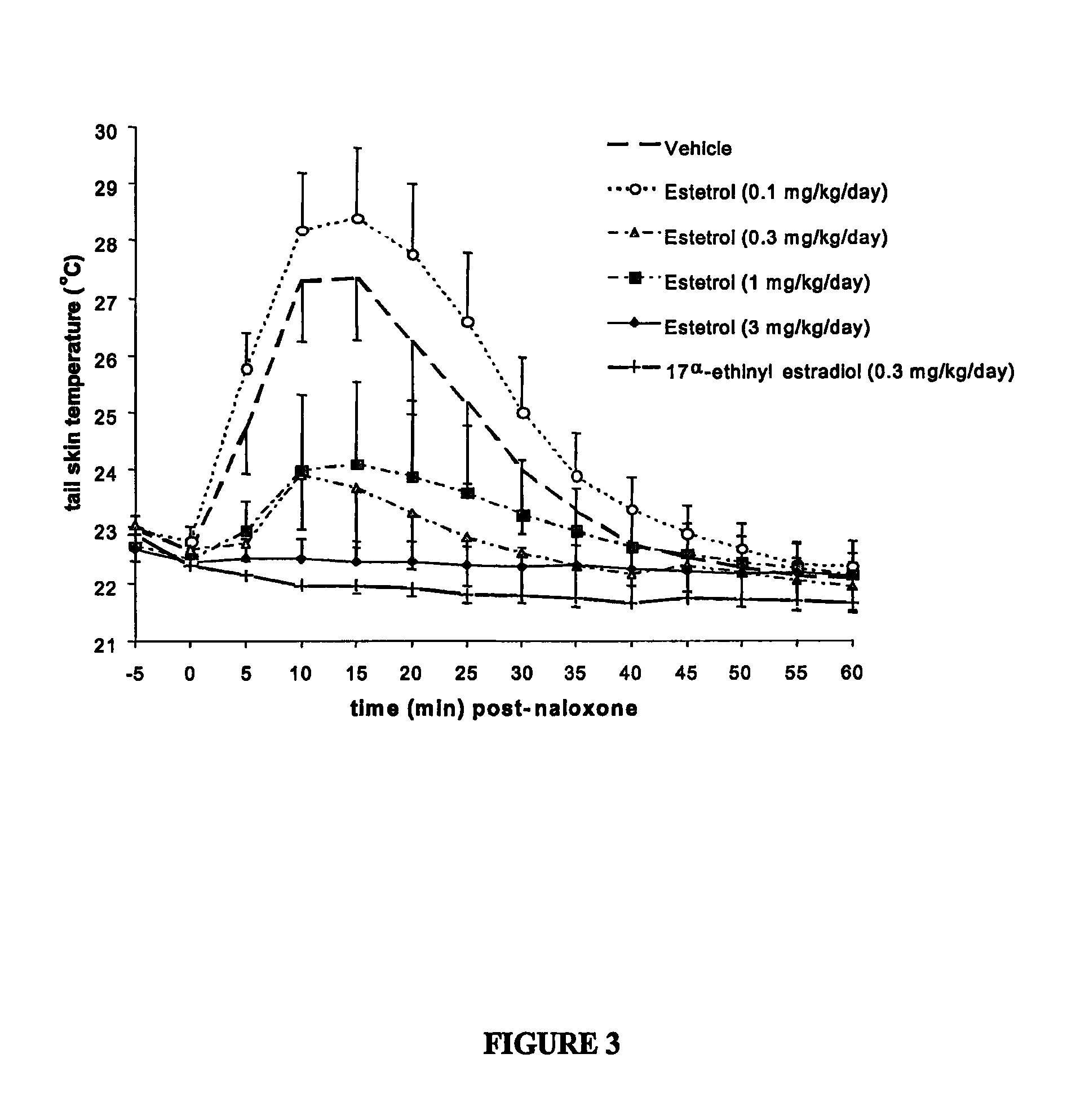
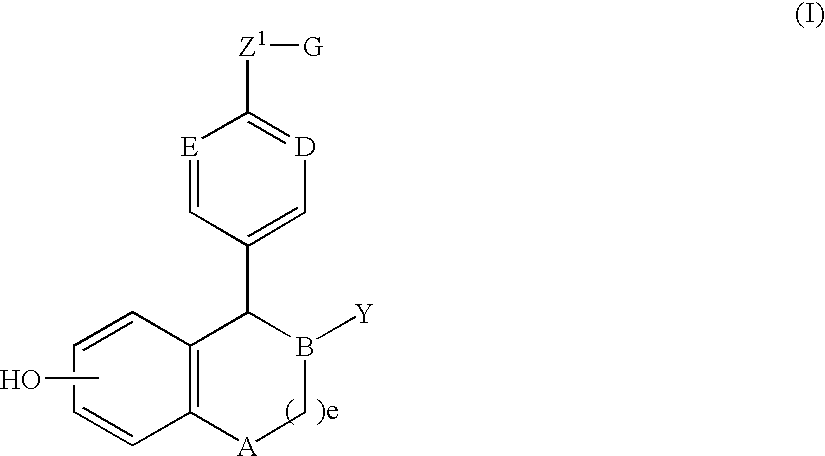
One aspect of the invention is concerned with a method of hormone replacement therapy, which method comprises administering to a person in need of such a therapy an effective amount of an estrogenic component selected from the group consisting of: substances represented by the formulain which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alokxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method, and mixtures thereof; said composition containing virtually no progestogen or anti-progestin.Another aspect of the invention relates to a drug delivery system for enteral or parenteral administration that contains at least 1 μg of the aforementioned estrogenic component and virtually no progestogen or anti-progestin.
Owner:ESTETRA SRL
Prevention of ovarian cancer by administration of progestin products
InactiveUS6977250B2High activityEliminate side effectsOrganic active ingredientsBiocideProgestin therapyEpithelial ovarian cancer
The present invention relates to methods for preventing the development of epithelial ovarian cancer by administering progestin products, either alone or in combination with other agents such as estrogen products.
Owner:NEW LIFE PHARMA
Pharmaceutical compositions, kits and methods comprising combinations of estrogen agonists/antagonists, estrogens and progestins
The present invention relates to pharmaceutical compositions, kits and methods comprising combinations of (−)-cis-6-phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5,6,7,8-tetrahydro-naphthalene-2-ol or nontoxic pharmacologically acceptable acid addition salts thereof and estrogens. The present invention also relates to pharmaceutical compositions, kits and methods comprising combinations of (−)-cis-6-phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5,6,7,8-tetrahydro-naphthalene-2-ol or nontoxic pharmacologically acceptable acid addition salts thereof, estrogens and progestins.
Owner:PFIZER INC
Transdermal Delivery of Hydrophobic Bioactive Agents
InactiveUS20080262445A1Improve solubilityImprove permeabilityBiocideAdhesive dressingsSerotoninFlumazenil
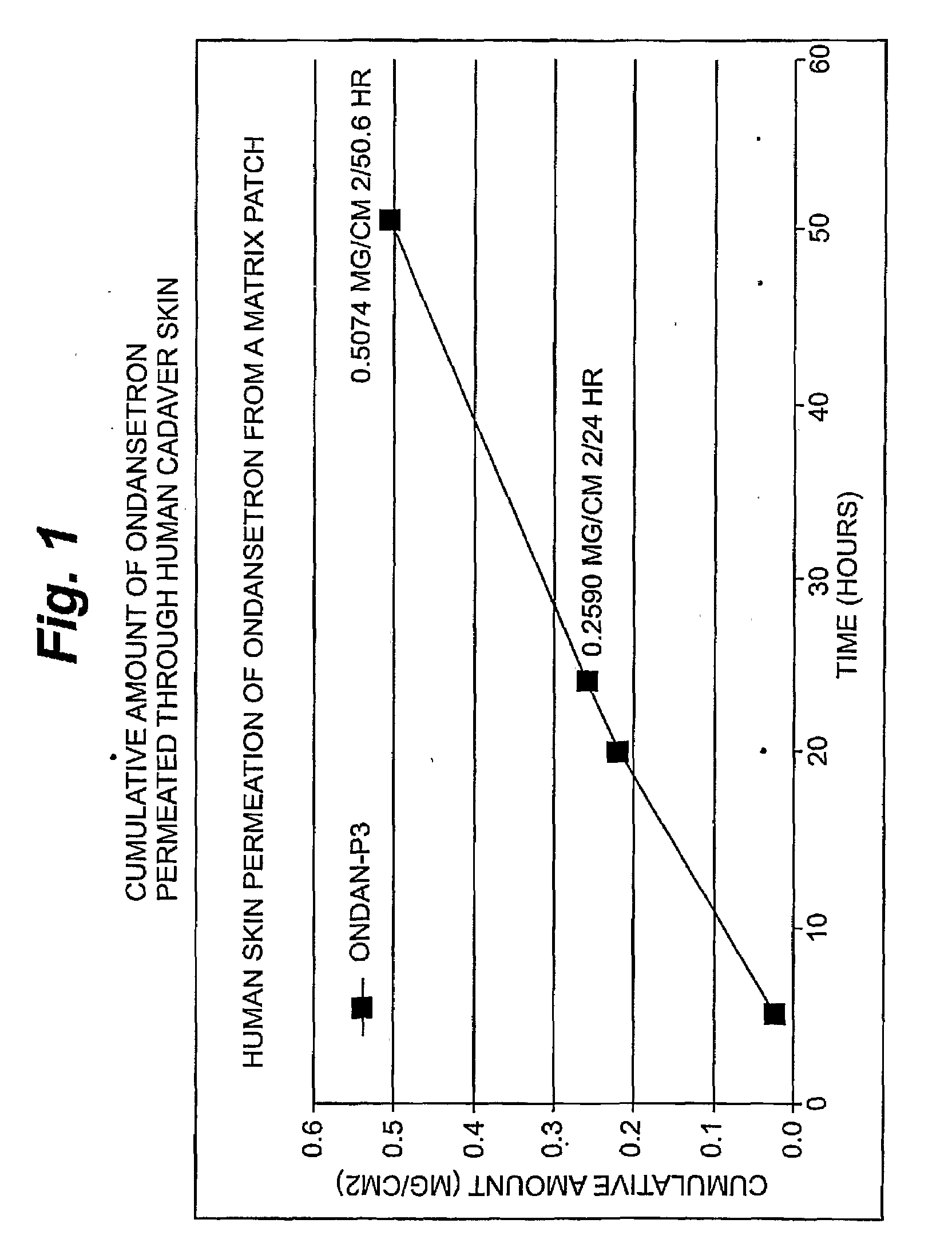
A method and related compositions, including the use of N-acyl derivatives of sarcosine, provide for the delivery of bioactive agents through tissue surfaces such as the skin. The method and composition are particularly well suited for hydrophobic active agents such as serotonin (5HT3) receptor antagonists (e.g., ondansetron), antipsychotic agents (e.g., risperidone), benzodiazepines (e.g., flumazenil), and progestins (e.g., levonorgestrel).
Owner:DERMATRENDS INC
Process for producing a pharmaceutical preparation for therapeutic treatment of endometriosis containing a combination of a gestagen and (6S)-5-methyltetrahydrofolate
InactiveUS20090060997A1Reduce endometriosisEffective therapeutic treatmentBiocidePill deliveryBone densityObstetrics
A combination of an anti-androgenic gestagen at a daily dose of from an ovulation-inhibiting dose up to at most twice the ovulation-inhibiting dose and from 0.1 to 10 mg of (6S)-5-methyltetrahydrofolate are used to produce a pharmaceutical preparation for therapeutically treating endometriosis while simultaneously reducing therapy side effects including the negative effect on bone density and / or bone metabolism, reducing the risk of osteoporosis and, in the event of pregnancy, reducing the risk of congenital malformations, such as medullary tube defects, cleft lip, cleft jaw, or cleft palate, and the risk of pregnancy complications, such as detachment of the placenta and premature birth. The preparation is suitable for long-term administration, which continues daily for at least 169 days to at least two years.
Owner:BAYER SCHERING PHARMA AG
Extended Use Combination Comprising Estrogens And Progestins
InactiveUS20080234240A1Good effectIncrease of the estrogen and/or progestin dosageBiocideOrganic active ingredientsPhysiologyProgestin therapy
A pharmaceutical preparation to obtain a continuous hormonal treatment over a desired period of time longer than 21-28 days comprising a first composition containing at least one estrogen and / or at least one progestin in a predetermined amount to be administered in the first 21-28 days and a second composition which contains at least one estrogen and / or at least one progestin in a predetermined amount higher than the amount of the first composition and comprises a mono or multiphase sequence of pharmaceutical dosages.
Owner:BAYER SCHERING PHARMA AG
Progestin-containing drug delivery system
The present invention relates to drug delivery compositions in the form of thin water-soluble films (wafers), which contain small particles that comprise at least one progestin and at least one protective agent. The protective agent provides effective taste-masking of the progestin due to limited release of the progestin in the mouth. The progestin is hence not absorbed via the buccal route, but rather via the enteral (per-oral) route.
Owner:BAYER SCHERING PHARMA AG
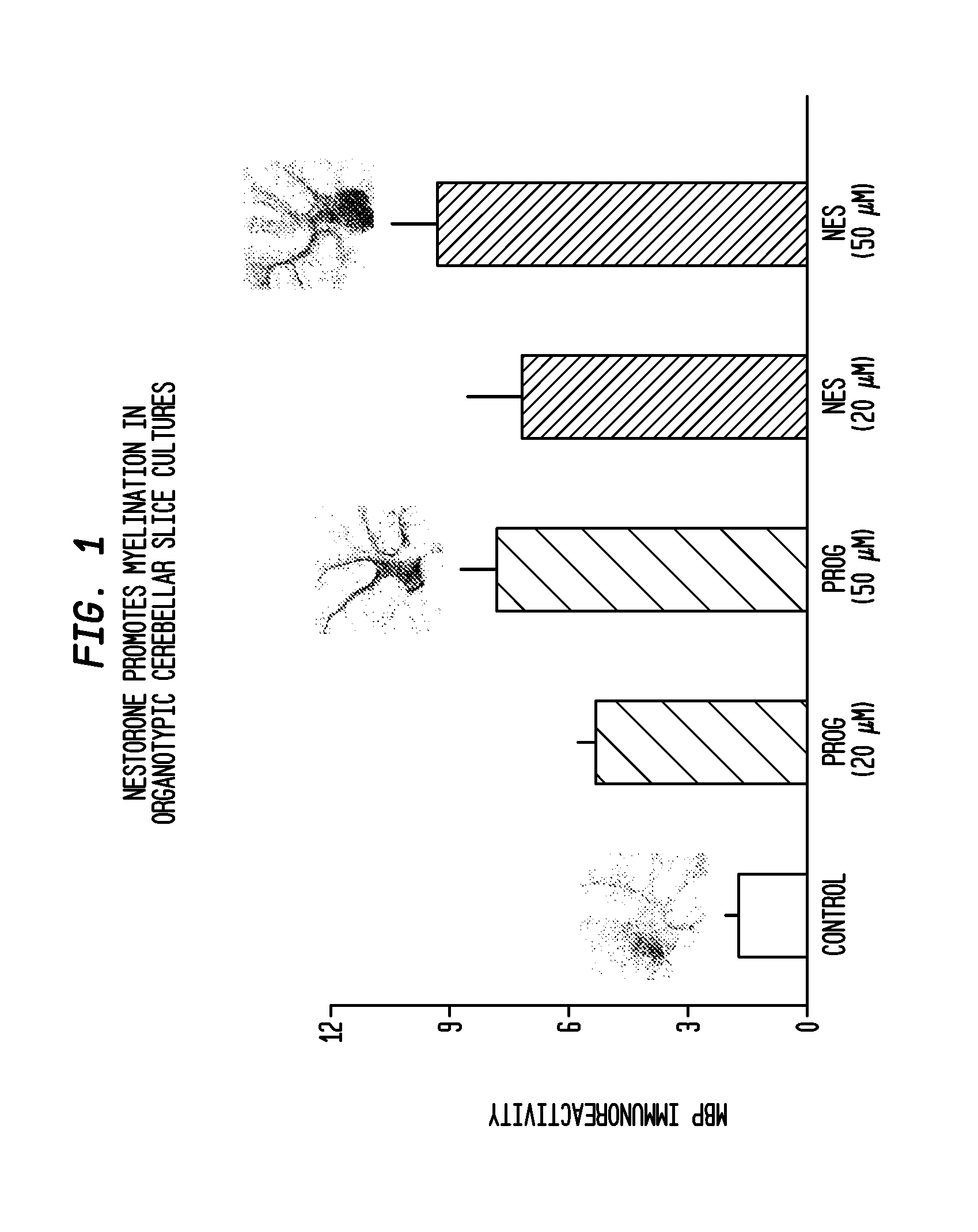
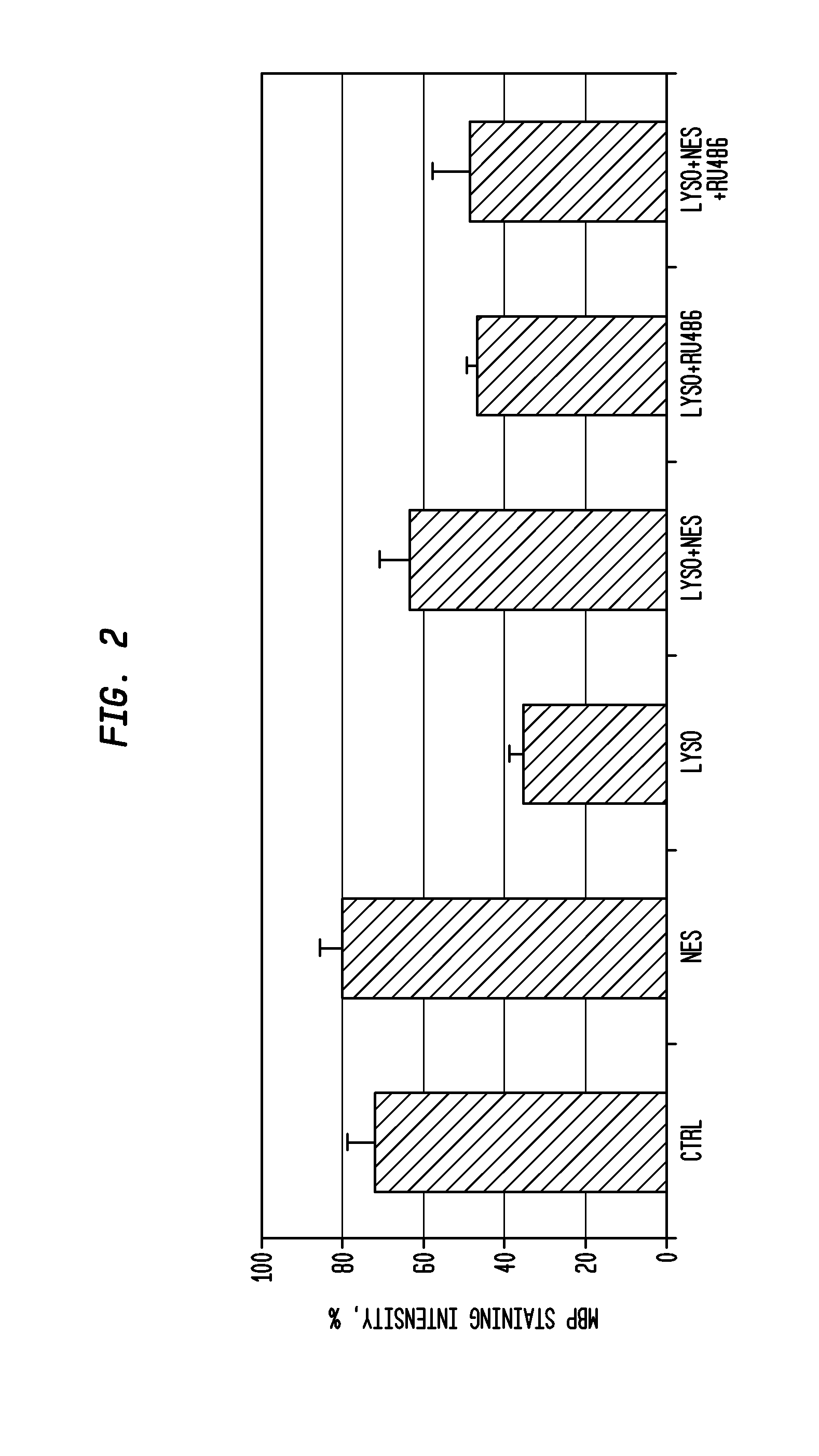
Neuroprotection and myelin repair using nestorone®
ActiveUS20120231052A1Neurodegeneration is prevented and reducedGood effectBiocideOrganic active ingredientsPR - Progesterone receptorAndrogen
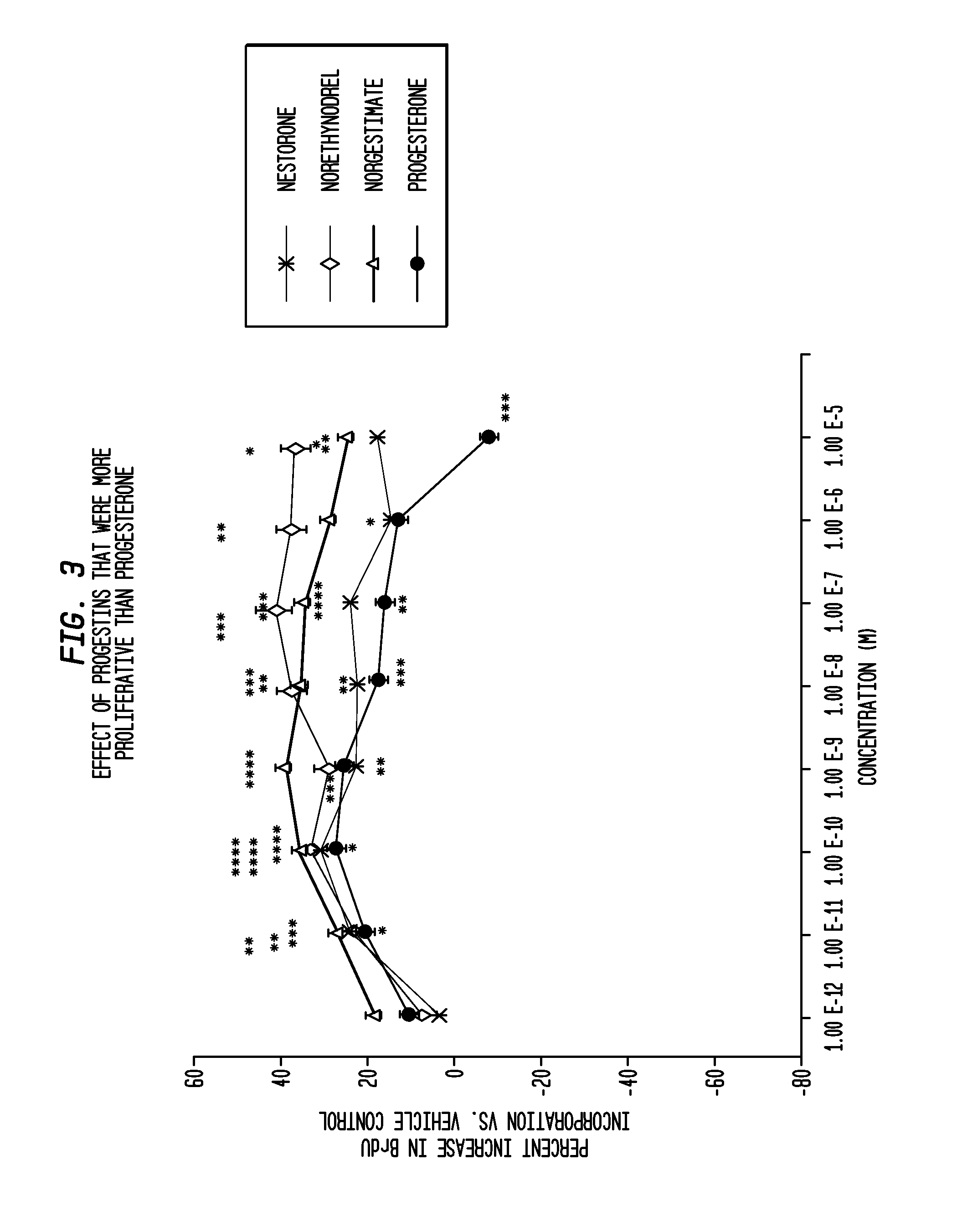
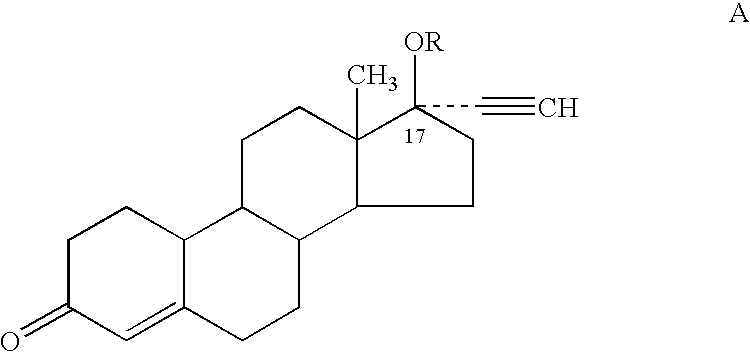
Methods for treating neurodegeneration and / or myelination in patients are disclosed comprising treating the patient with a progestin compound which exerts binding to progesterone receptors and elicits progesterone-receptor-induced biological responses without interacting with the androgen receptor and without inducing androgen or glucocorticoid biological responses at a dosage sufficient to prevent or reduce neurodegeneration. The progestin compound preferably comprises 16-methylene-17α-acetoxy-19-norpregn-4-ene-3,20-dione, and the methods include combining the progestin compound with an estrogen compound to provide both contraception and treatment for myelin repair and neurodegeneration.
Owner:THE POPULATION COUNCIL INC
Method of preventing or treating benign gynaecological disorders
ActiveUS8071576B2Few side-effectsLow recurrence rateBiocideOrganic active ingredientsDiseaseGynecological disorders
The present invention relates to a method of preventing or treating benign estrogen sensitive gynecological disorders in a female mammal, wherein the method comprises the administration to said female mammal of a combination of progestogen and androgen in an amount that is therapeutically effective to prevent or reduce the symptoms of these disorders. The present method is particularly suitable for preventing or treating disorders selected from the group consisting of endrometriosis, adenomyosis, uterine fibroids, dysmenorrhoea, menorrhagia and metrorrhagia. Another aspect of the invention relates to a pharmaceutical kit comprising a plurality of oral dosage units which comprise a progestogen in an amount equivalent to 3-500 μg levonorgestrel and either 5 to 250 mg dehydroepiandrosterone or 1 to 50 mg testosterone undecanoate.
Owner:PANTARHEI BIOSCI
Male contraceptive formulation comprising norethisterone
A formulation for male contraception comprising a progestin possessing both estrogenic and androgenic properties is remarkably effective for spermatogenesis suppression in males. The progestin Norethisterone (NET), particularly its derivatives Norethisterone acetate and Norethisterone enanthate in sufficient doses induce oligozoospermia or azoospermia in males. Formulations further comprising an androgen, such as a testosterone derivative such as a testosterone ester, particularly testosterone undecanoate, are especially effective male contraceptive formulations.
Owner:NIESCHLAG EBERHARD +5
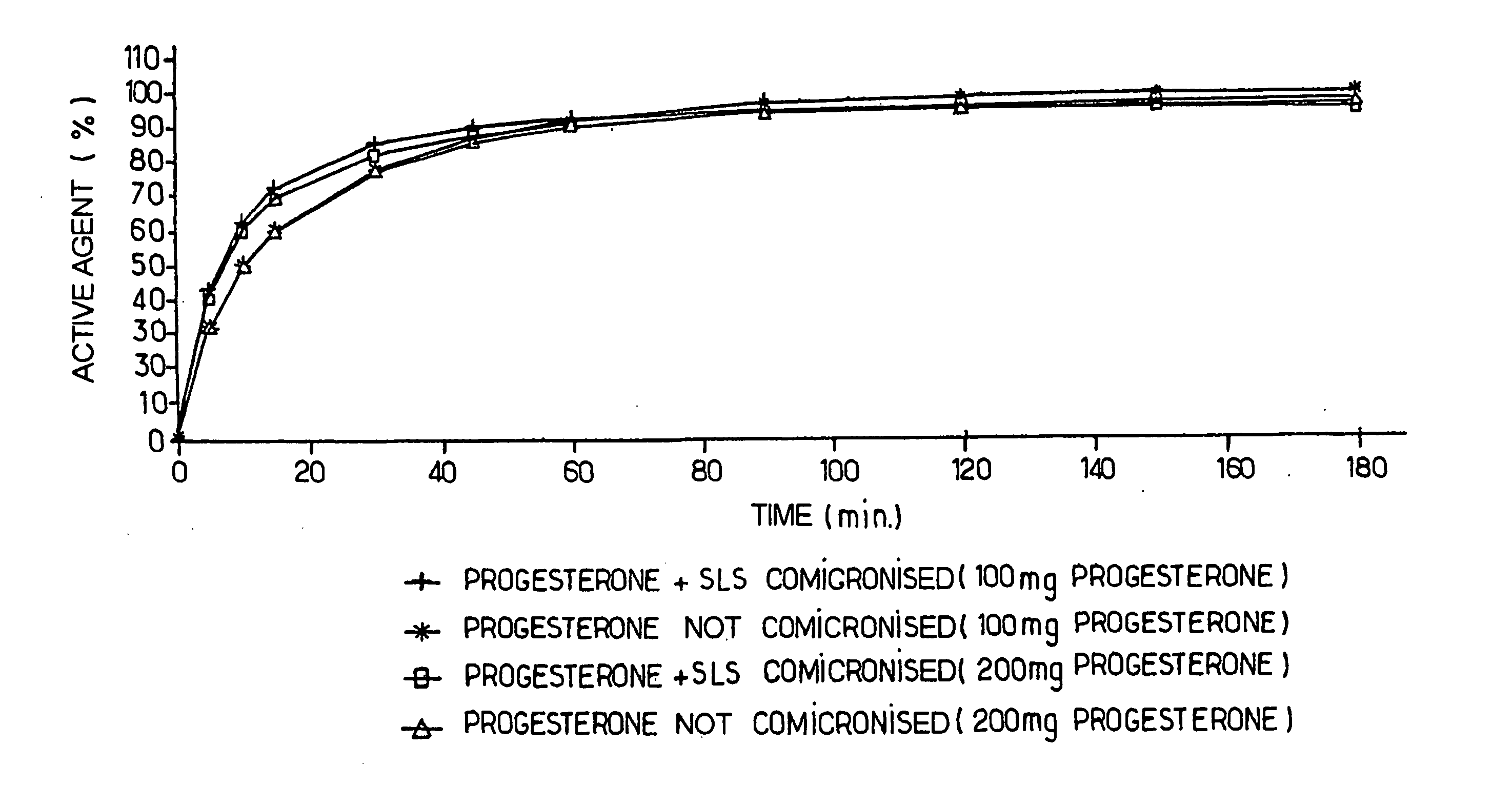
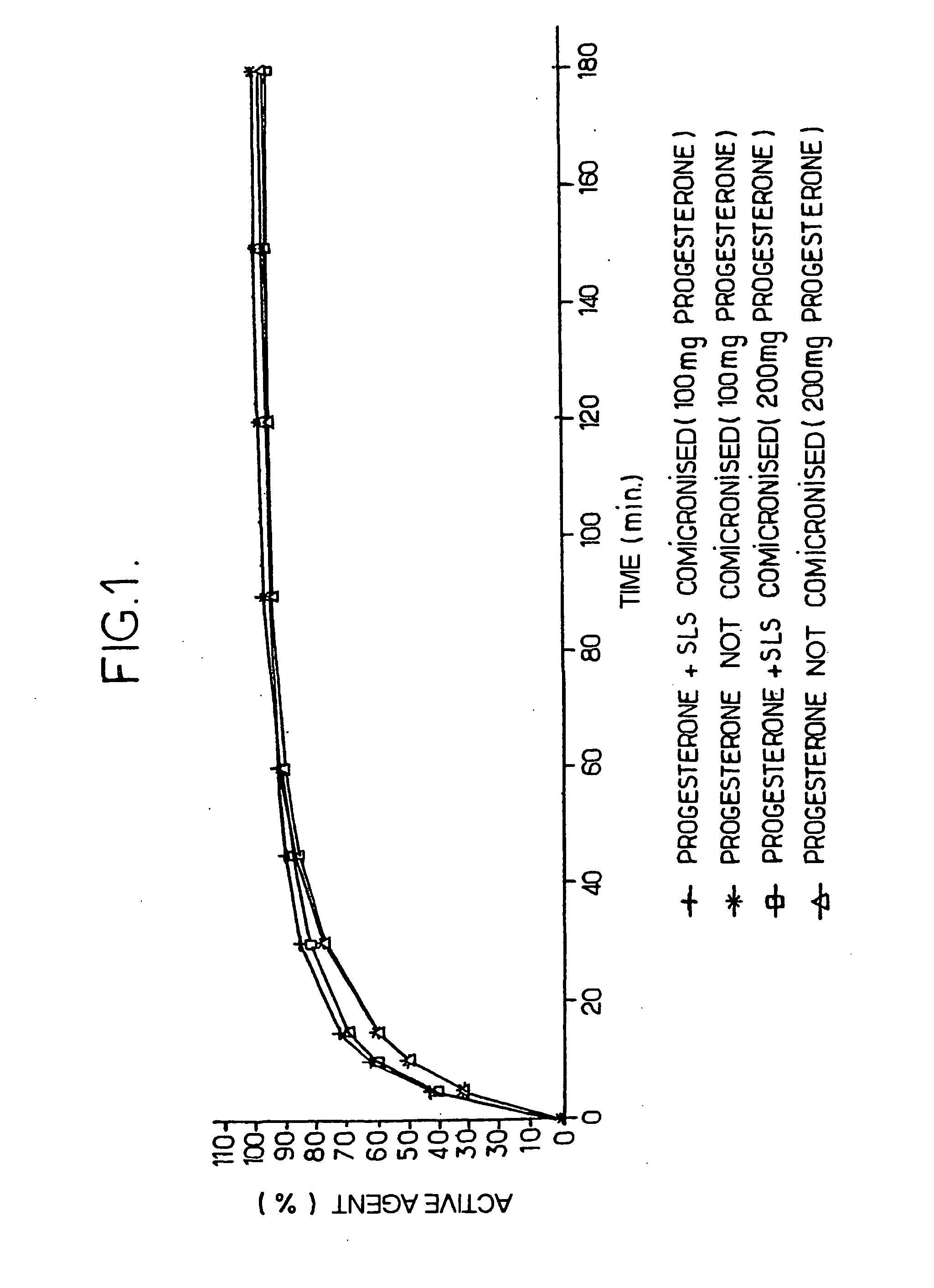
Progestin co-micronized with a surfactant, pharmaceutical composition comprising same, methods for making same and uses thereof
The invention concerns a progestin co-micronized with a surfactant and a pharmaceutical composition comprising said gestagenic. The invention also concerns methods for preparing same.
Owner:BESINS INT BELGIQUE
Pharmaceutical preparation containing a gestagen, and kit and method for treating endometriosis using the preparation
InactiveUS20080214512A1Significant positive effectEndometriosis can be reducedOrganic active ingredientsBiocideSide effectBone density
The pharmaceutical preparation for treating endometriosis contains at least 28, preferably 30, daily dose units, each of which contain dienogest, cyproterone acetate, or chlormadinone acetate at a daily dose that is at most twice that required to inhibit ovulation together with one or more pharmaceutical aids and / or carriers. The daily dose units are administered in a method of prophylaxis and / or therapy of endometriosis continuously during a time interval of at least 169 days or 25 weeks, preferably more than two years. The method effectively reduces endometriosis and associated pain, while undesirable side effects including bone density decrease are reduced or eliminated.
Owner:BAYER SCHERING PHARMA AG
Methods and compositions for treating benign gynecological disorders
InactiveUS6960337B2Increased riskPrevent endometrial hyperplasiaOrganic active ingredientsBiocideDiseaseGynecology
An improvement in a method of treating benign gynecological disorders is described. In the method, treatment of a benign gynecological disorder with a composition comprised of a gonadotropin releasing hormone (GnRH) compound and an estrogenic compound, and optionally, an androgenic compound, is extended to premenopausal women who are not receiving an exogenously supplied progestin on a regular or periodic basis. Treatment in accord with the invention does not increase significantly the risk of endometrial hyperplasia. The method is also suitable for contraception.
Owner:BALANCE PHARMA
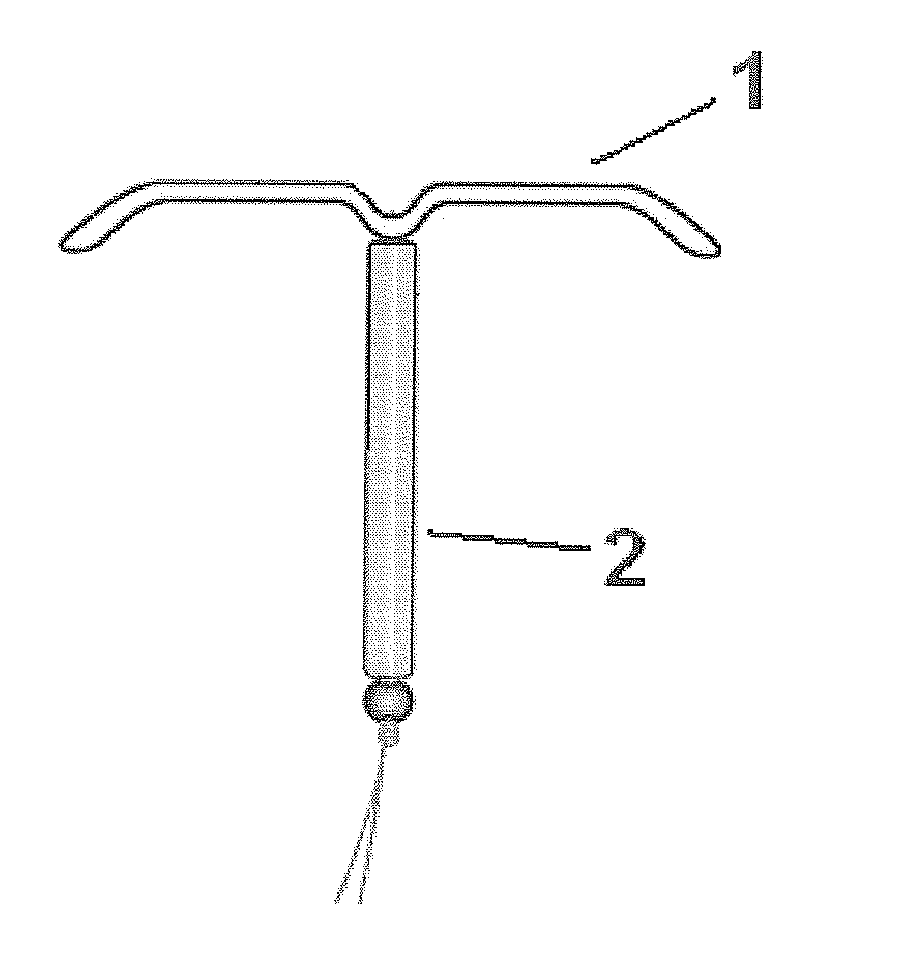
Intrauterine delivery system for contraception
ActiveUS20140127280A1High success rateMinimal to no side-effects or related complicationsBiocideOrganic active ingredientsControl releaseEndometrium
The invention relates to a method for contraception and for reducing menstrual problems and inducing amenorrhea, wherein an intrauterine delivery device is used for the controlled release of a combination of progestogen or a drug having a progestogenic activity and at least one therapeutically active substance capable of preventing or suppressing abnormal and / or irregular endometrial bleeding over a prolonged period of time.
Owner:BAYER OY
Antiprogestin method for reducing side effects associated with low dosage HRT and oral contraception
InactiveUS7704983B1Avoid bleedingAvoid problemsOrganic active ingredientsBiocideSide effectBreak-through bleeding
Menses regulation and, when desired, contraception is achieved at low doses of estrogen and progestin, which otherwise would create episodes of breakthrough bleeding and / or withdrawal amenorrhea, by periodically inducing menses with an antiprogestin.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
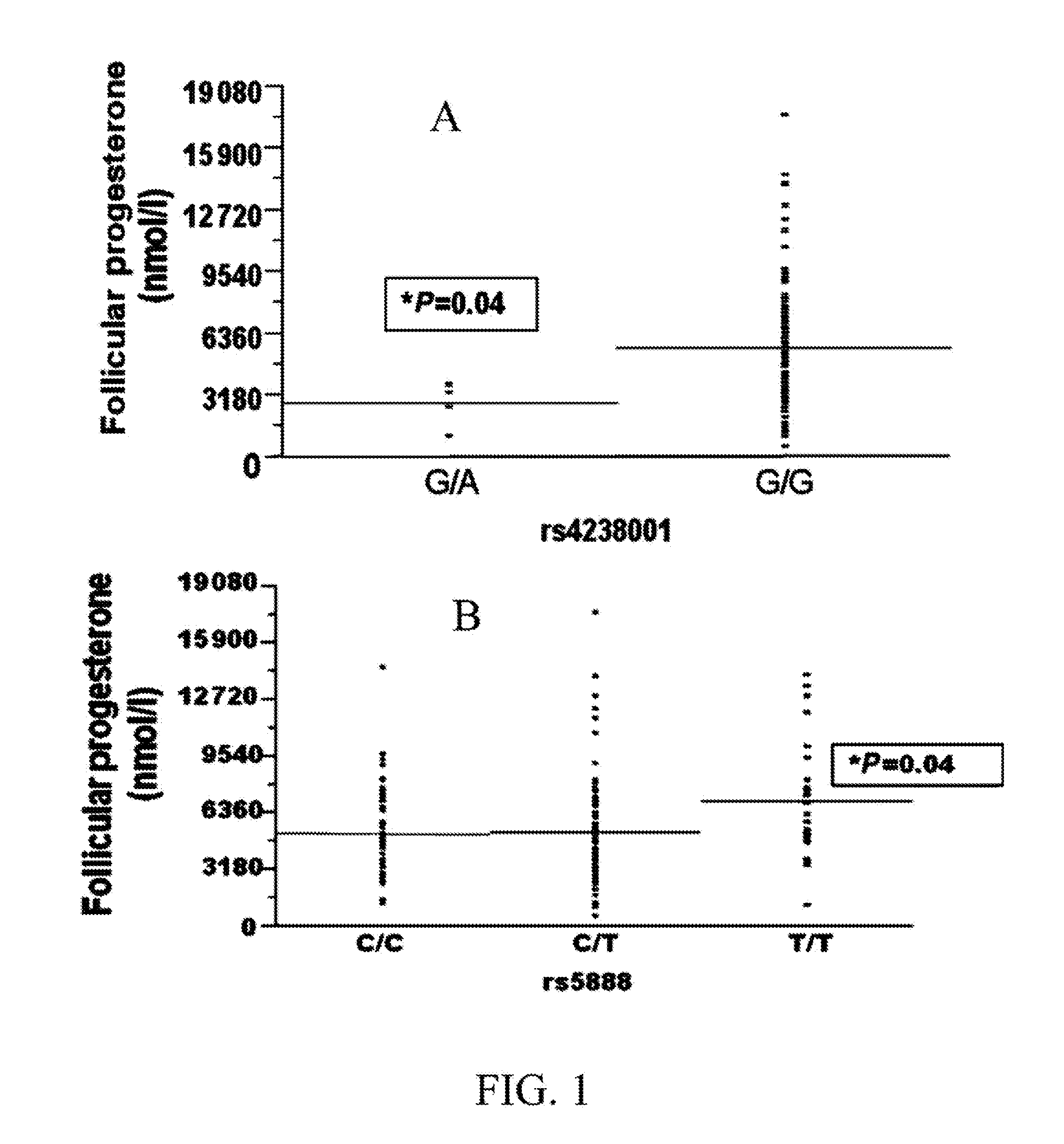
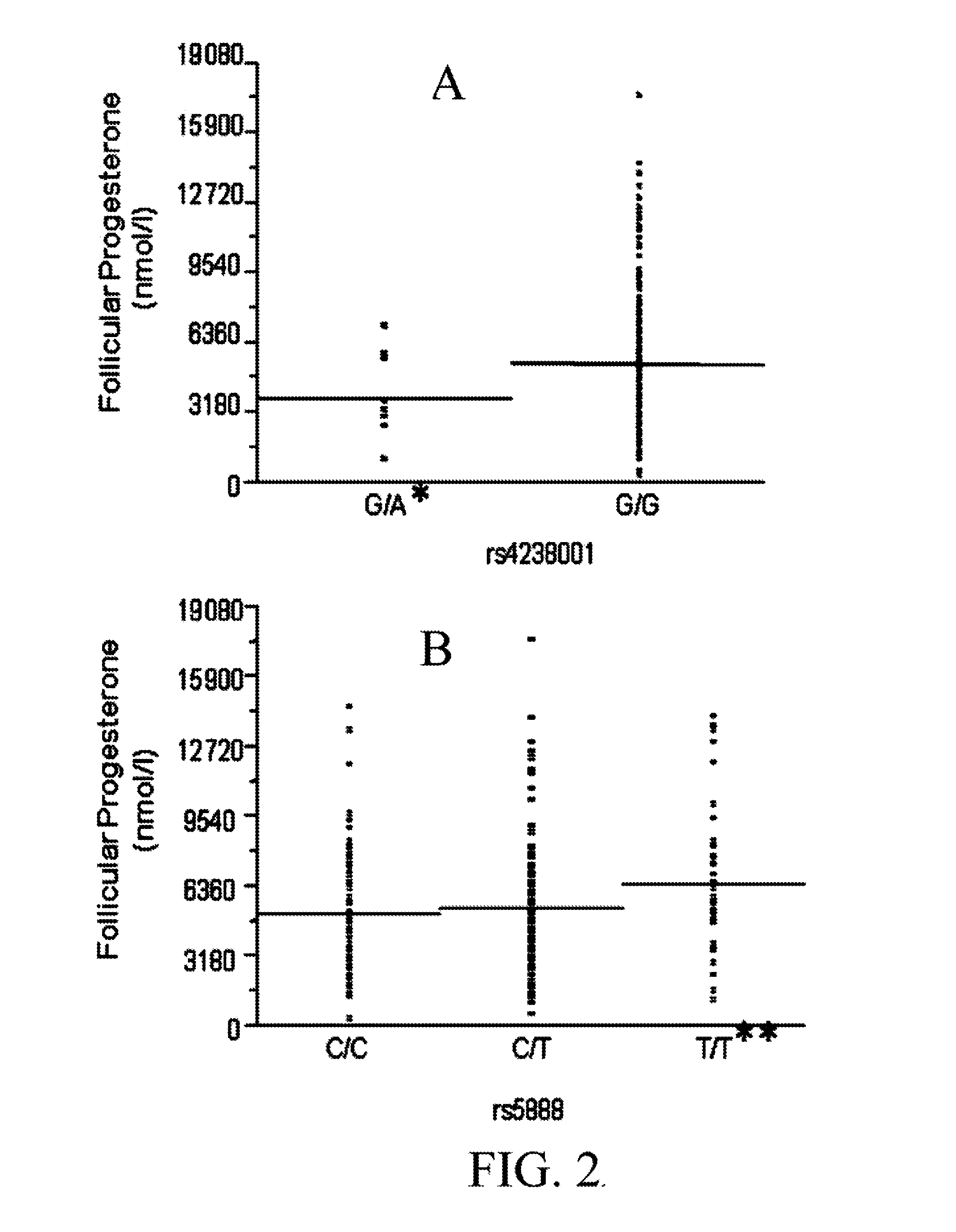
Method for pre-screening and correlation of underlying scarb1 gene variation to infertility in women and therapeutic use of progestational and other medications in treatment
ActiveUS20130345187A1Restore fertilityMicrobiological testing/measurementLibrary screeningCholesterolProgesterones
A method of genotyping women experiencing infertility for non-physical reasons in order to identify the presence of the rs4238001 and / or rs10846744 mutation of the SCARB1 gene and, upon identifying the presence of one or both genetic mutations, administering a tailored therapeutic regimen to restore fertility by either one or a combination of 1) mediating the flux of cholesterol resulting from the mutation by therapeutic use of the cholesterol medication probucol and / or other cholesterol altering medications, and / or 2) amplifying the presence of hormone progesterone by therapeutic use of progestational and progestin medications.
Owner:RODRIGUEZ OQUENDO ANNABELLE
Compositions and methods for treatment of premenstrual dysphoric disorder
InactiveUS20050272712A1Effective amountEffective contraceptionOrganic active ingredientsBiocidePhysiologyPremenstrual dysphoric disorder
The present invention relates to a method for treating premenstrual dysphoric disorder through administration of at least one progestin and at least one estrogen to a female subject.
Owner:WYETH
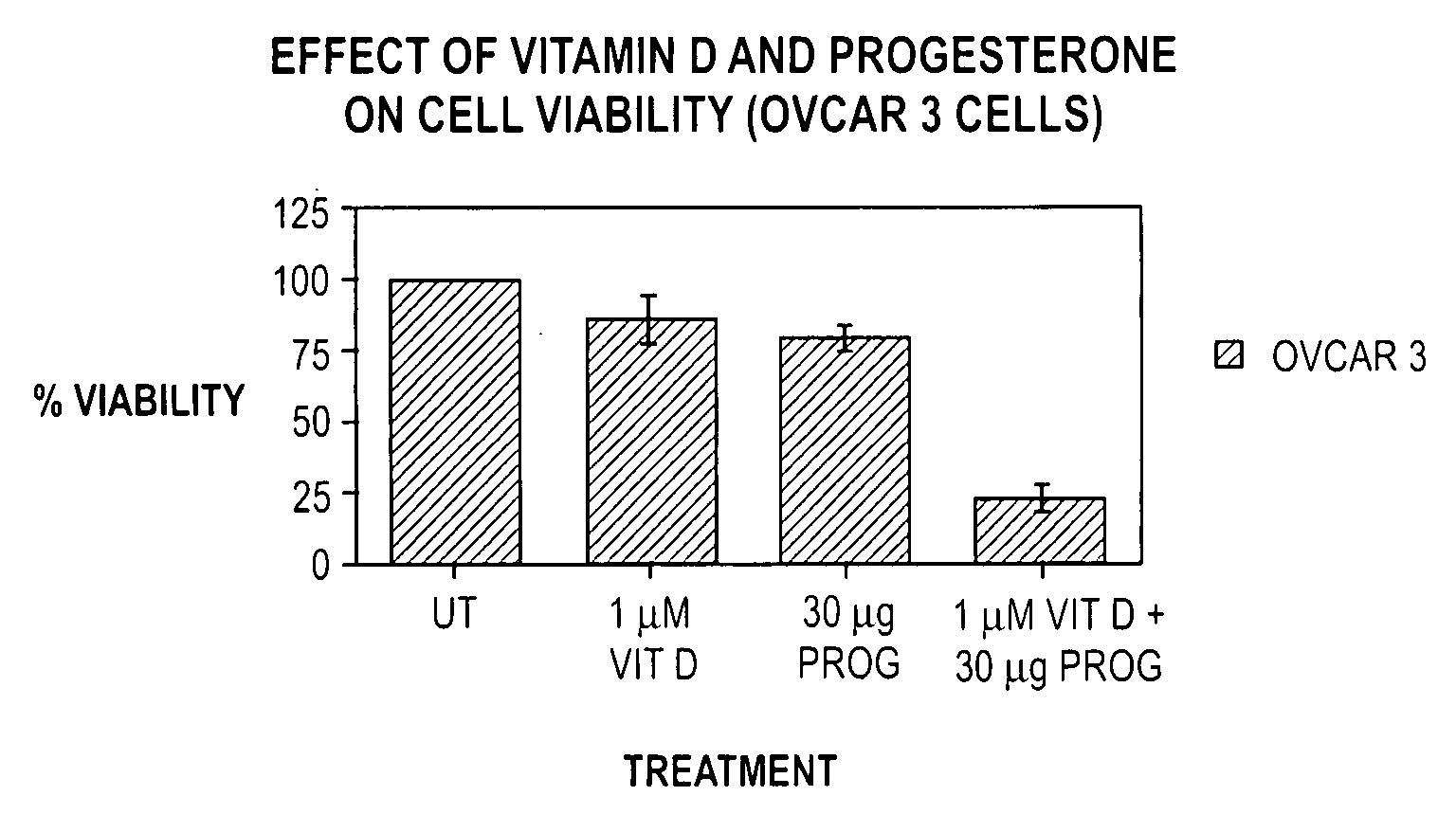
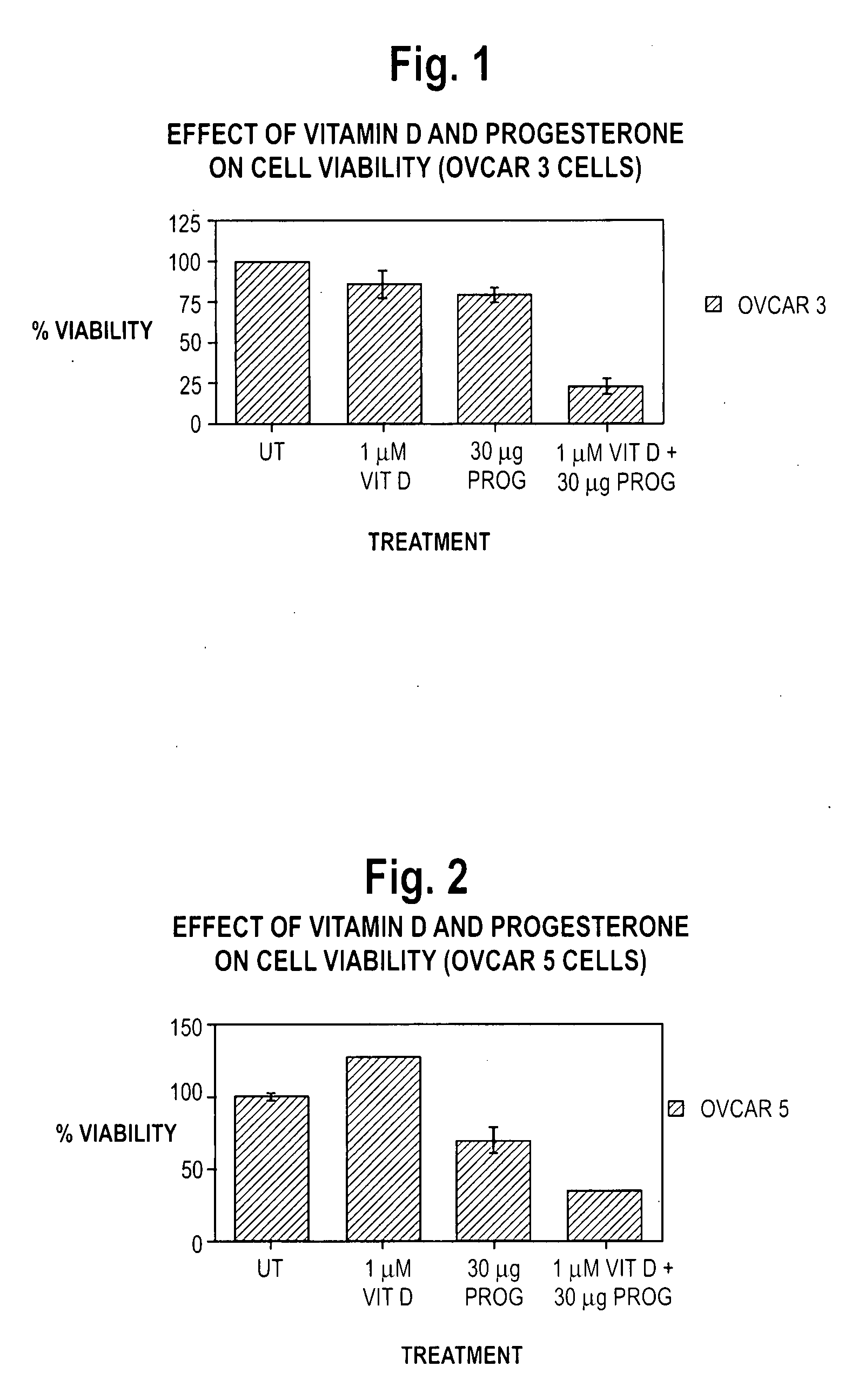
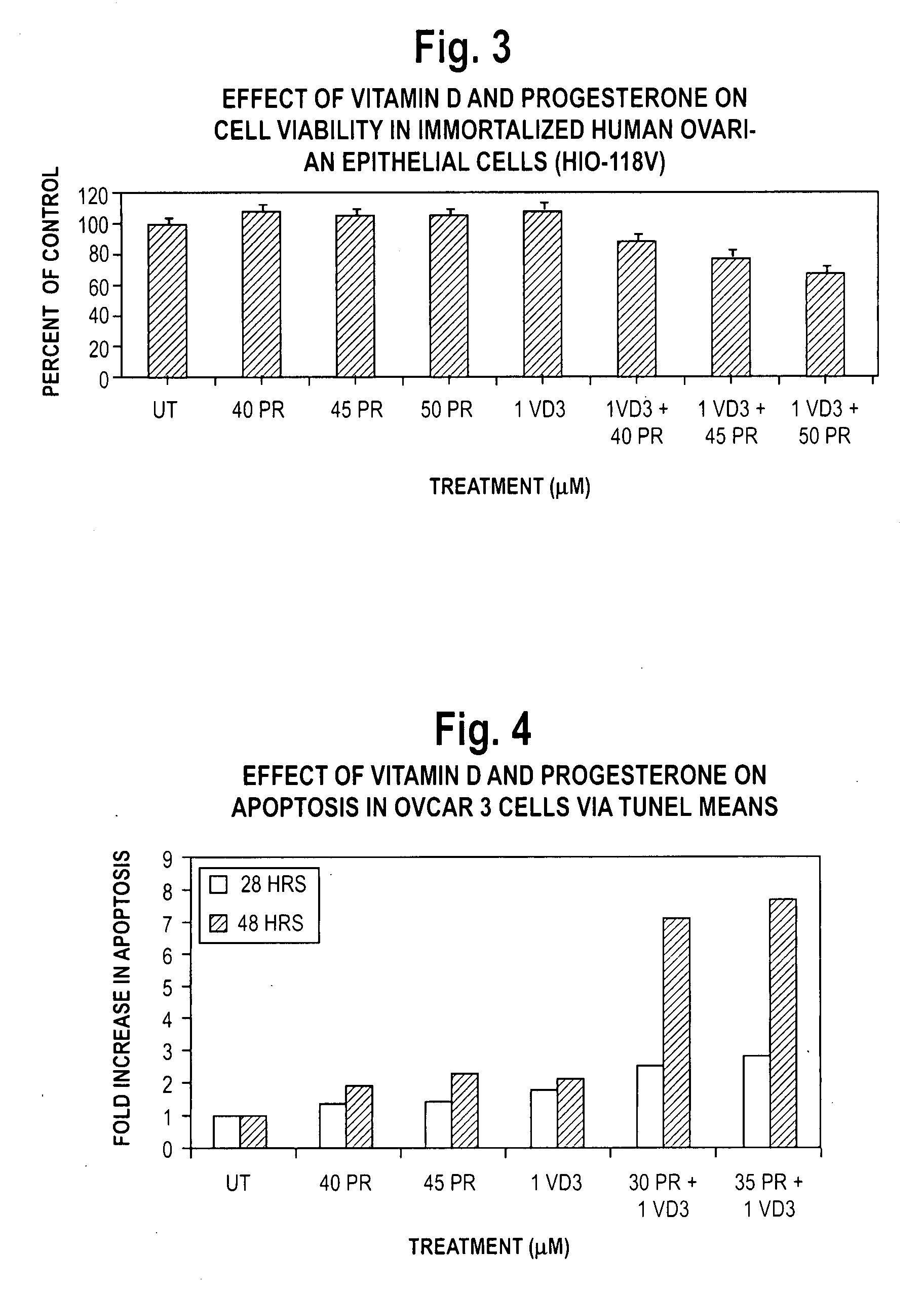
Pharmaceutical products containing hormones and a 25-hydroxy vitamin d compound
InactiveUS20080312198A1Maximum safetyGood effectOrganic active ingredientsBiocideMedicineProgesterone agent
The present invention relates to pharmaceutical products containing progestins in combination with the 25 hydroxy Vitamin D compounds. The 25 hydroxy Vitamin D compounds are preferably administered with the progestins. In OC and HRT regimens, the 25 hydroxy Vitamin D compounds can be administered daily, or on a non-daily basis, and if so, preferably when the progestin dosages are the highest in the cycle.
Owner:RODRIGUEZ GUSTAVO C
Oral modified release formulations
InactiveUS20100086599A1High dose of drugReduce doseBiocidePowder deliveryDrospirenoneImmediate release
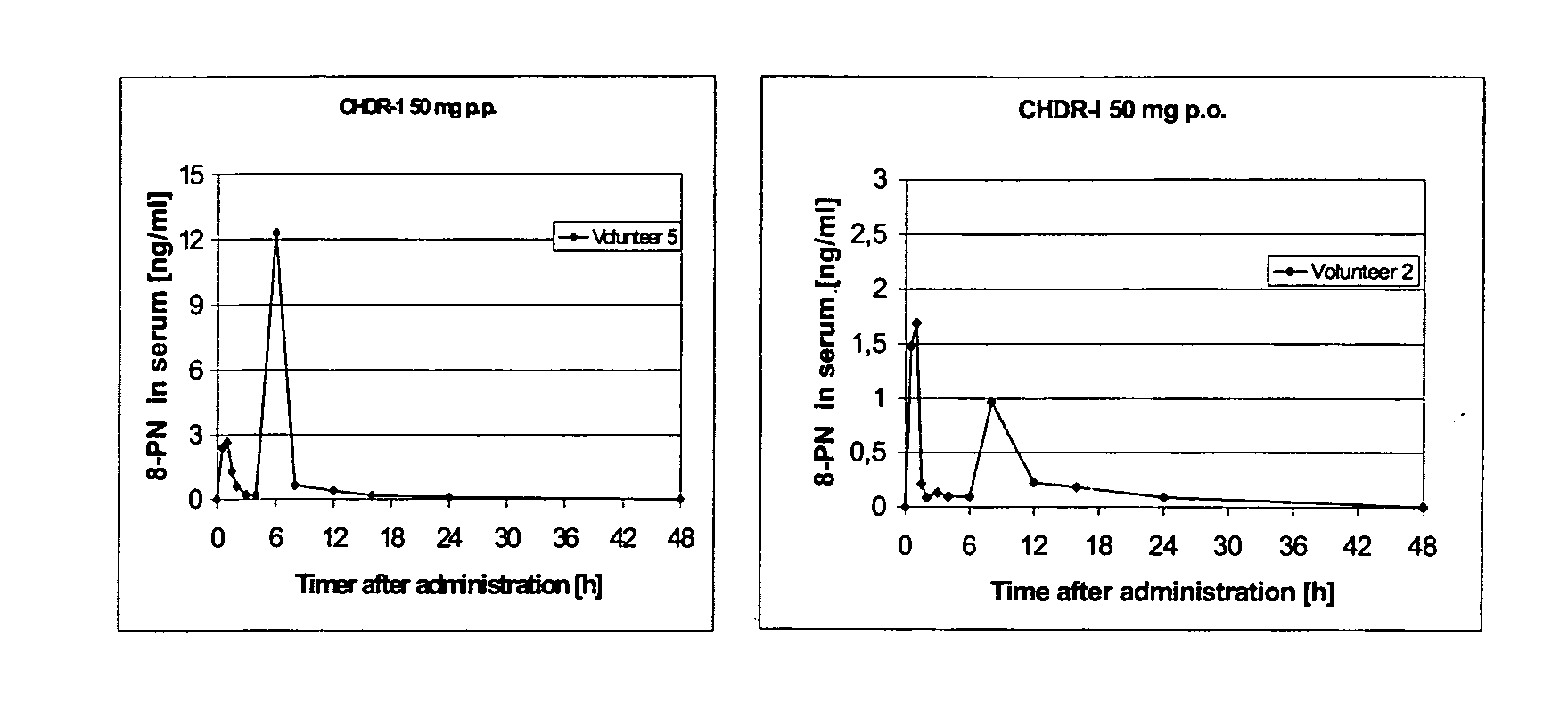
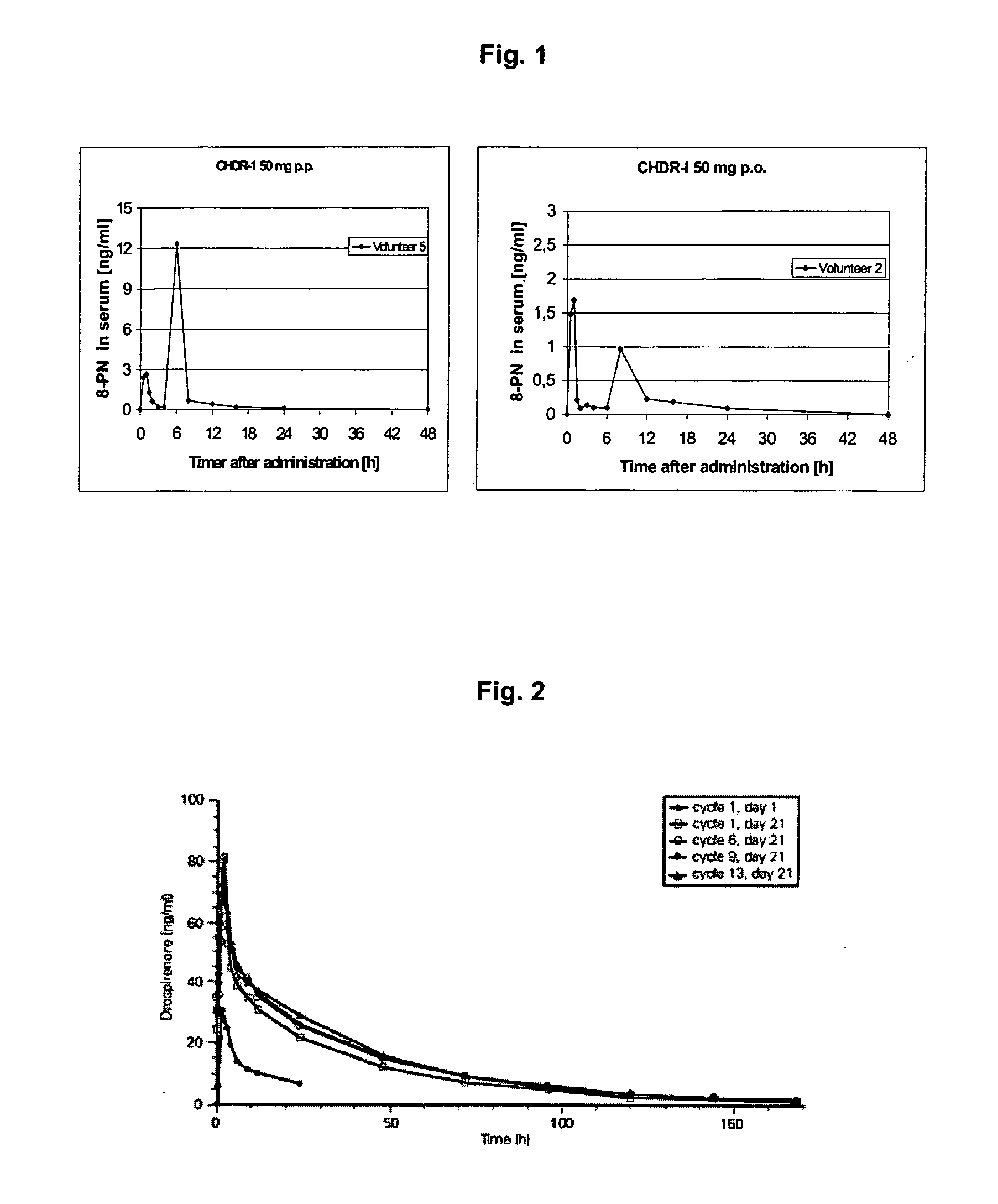
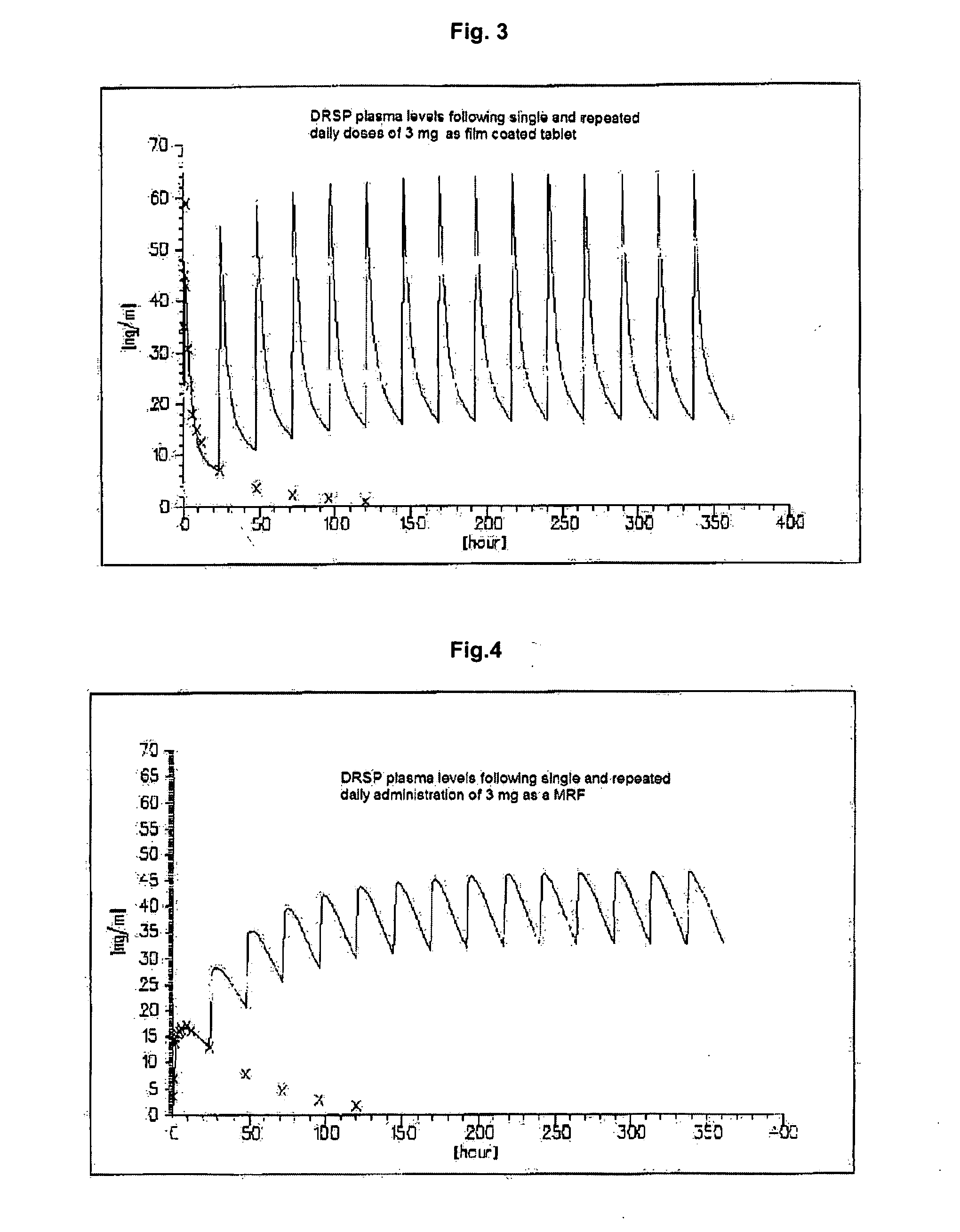
This invention is directed to an oral modified release formulation of the phytoestrogen 8-Prenylnaringenin in combination with a progestin, preferably with Drospirenone, and several uses thereof. In another aspect of the invention an oral modified formulation of 8-Prenylnaringenin with an immediately releasing progestin, like Drospirenone, is provided as well as several uses thereof.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Treatment of Prevention of Unscheduled Bleeding in Women on Progestogen Containing Medication
InactiveUS20090197843A1Increased chronotropismIncreased inotropismBiocidePeptide/protein ingredientsPhysiologyProgestogen
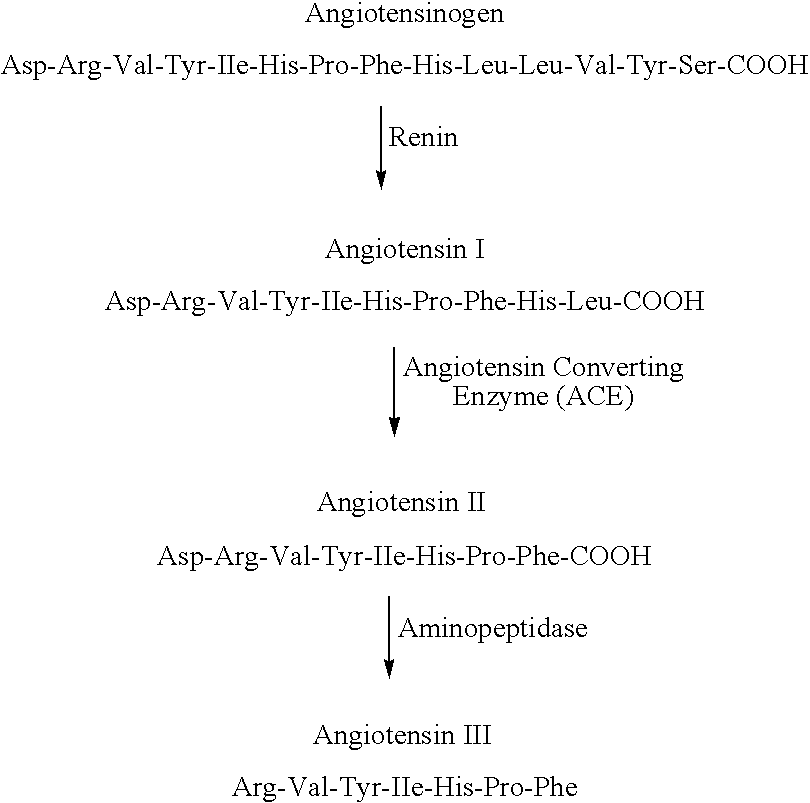
The present invention relates to a method of treating or preventing unscheduled bleeding in women, the unscheduled bleeding being the result of repeated administration of a hormonal composition that contains a progestogen, wherein the method includes the administration of an effective amount of Renin Angiotensin System (RAS) suppressor selected from angiotensin converting enzyme inhibitors; angiotensin II receptor antagonists; renin inhibitors and combinations thereof. Other aspects of the invention relate to a pharmaceutical composition containing a RAS suppressor and a progestogen and to a pharmaceutical kit having a plurality of dosage units, wherein at least one dosage unit contains a progestogen; at least one dosage unit contains an estrogen; and at least one dosage unit contains a RAS suppressor.
Owner:PANTARHEI BIOSCI
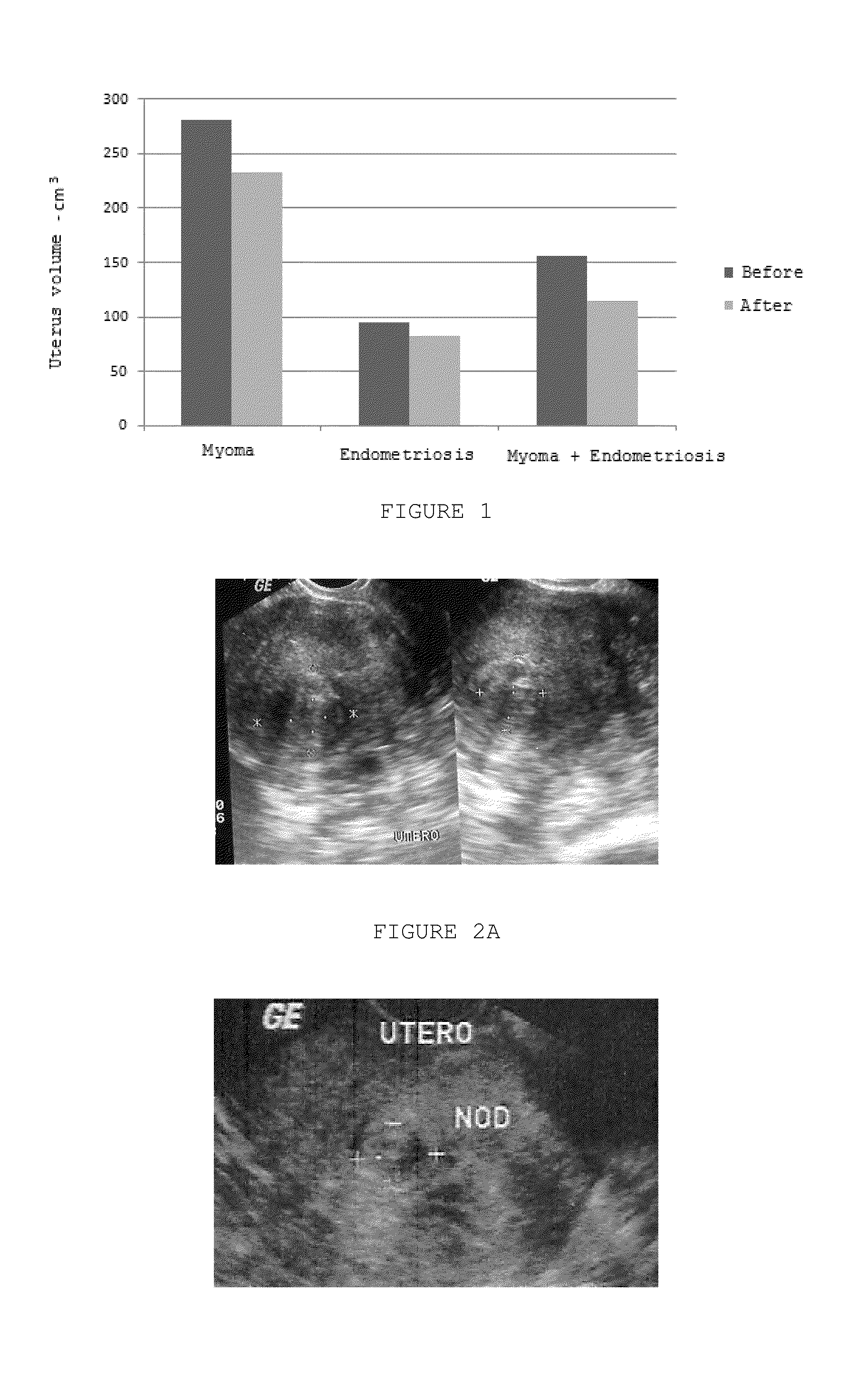
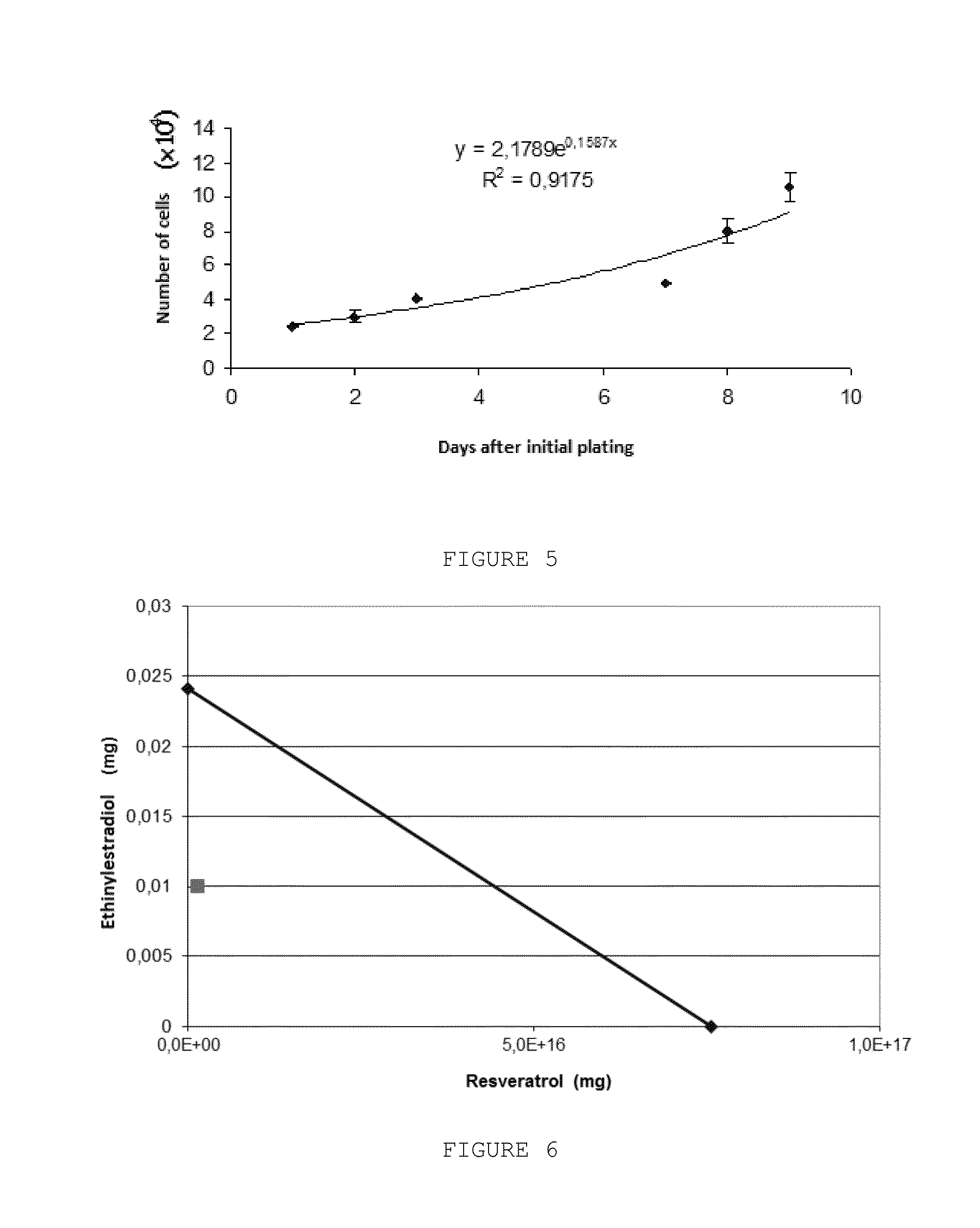
Pharmaceutical Combination of Resveratrol and Progestin to Treat and/or Prevent Myoma and/or Endometriosis
The present invention relates to a combination of resveratrol with progestogen, and medicaments and pharmaceutical compositions containing the same, which are useful in the treatment and / or prevention of myoma and / or endometriosis, providing their size reduction and the control of related symptoms. Said association may additionally comprise an estrogen component. A treatment method and a kit are also objects of this invention.
Owner:LIBBS FARM
Pharmaceutical products containing hormones and a 25-hydroxy vitamin d compound
InactiveUS20080312197A1Maximum safetyGood effectBiocideOrganic active ingredientsMedicineVitamin D+Metabolites
The present invention relates to pharmaceutical products containing progestins in combination with the 25 hydroxy Vitamin D compounds. The 25 hydroxy Vitamin D compounds are preferably administered with the progestins. In OC and HRT regimens, the 25 hydroxy Vitamin D compounds can be administered daily, or on a non-daily basis, and if so, preferably when the progestin dosages are the highest in the cycle.
Owner:RODRIGUEZ GUSTAVO C
Hormone replacement therapy method and hormone dispenser
InactiveUS6083528AAvoid possibilityPrevent projectionOrganic active ingredientsClosuresHormone replacementPhysiology
Varying the daily dose of either or both of the estrogen and the progestogen administered for hormone replacement therapy (HRT) is readily and inexpensively accomplished, without the necessity of the physician prescribing a new product each time the daily dose of the estrogen or progestogen is changed, by administering preferably transdermally the estrogen and the progestogen contained in separate extrudable pharmaceutical compositions from a dispenser which contains means, preferably adjustable only by the attending physician or dispensing pharmacist, for varying the volume of either or both of the respective compositions which is dispensed as a single dose from the dispenser in response to a defined digital dispensing manipulation of the dispenser thereby facilitating optimal compliance to a combination of HRT with individually adjusted dosages of the estrogen and progestogen.
Owner:BAYER SCHERING PHARMA AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com