Progestin co-micronized with a surfactant, pharmaceutical composition comprising same, methods for making same and uses thereof
a technology of surfactant and progestin, which is applied in the field of comicronized progestin, pharmaceutical composition comprising same, methods for making same, etc., can solve the problems of reducing the intrinsic androgenic activity of the molecule, affecting the function, and increasing the endometrial power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Progesterone Co-Micronized with Sodium Lauryl Sulfate
[0067] 1) 97 g of progesterone and 3 g of sodium lauryl sulfate are mixed for 5 minutes in a mixer of the LÖDIGE type.
[0068] 2) The mixture obtained in step (1) is introduced into an Alpine 200 AS airjet mill preset on the following parameters: [0069] injection: 5.5 B [0070] ring: 3.0B [0071] rate: 35 kg / h
[0072] 3) This mixture is ground in order to obtain a co-micronized material of progesterone / sodium lauryl sulfate.
example 2
Tablets Based on Progesterone Co-Micronized with A Surfactant
[0073] The formulations of progesterone tablets according to the invention, containing 100 and 200 mg of progesterone, are given in Table I below.
TABLE 1NAME OF THEUNIT AMOUNTBATCHCOMPONENTFUNCTIONmg / tabletSIZE (kg)MicronizedActive55.000progesterone*principle(sodium lauryl(progestin)200100sulfate (SLS))*(surfactant)6.183.09Povidone K30Binder9.604.802.561Solution at35% (m / m)MannitolDiluent29.8414.927.981CrosslinkedDisintegrating13.006.503.468sodiumagentcarboxymethylcelluloseMagnesiumLubricant1.300.650.347stearate
*Progesterone / SLS are co-micronized according to example 1.
[0074] To prepare the tablets in question, the following procedure was carried out:
Step 1: Preparation of the Wetting Solution
[0075] 4.756 kg of purified water are introduced into a container of suitable volume. 2.561 kg of Povidone K30 are then introduced into the purified water, gradually, and with stirring in a deflocculating-type stirrer (RAYNERI)...
example 3
Dissolving of the Progesterone Tablets Prepared According to the Invention, In Vitro
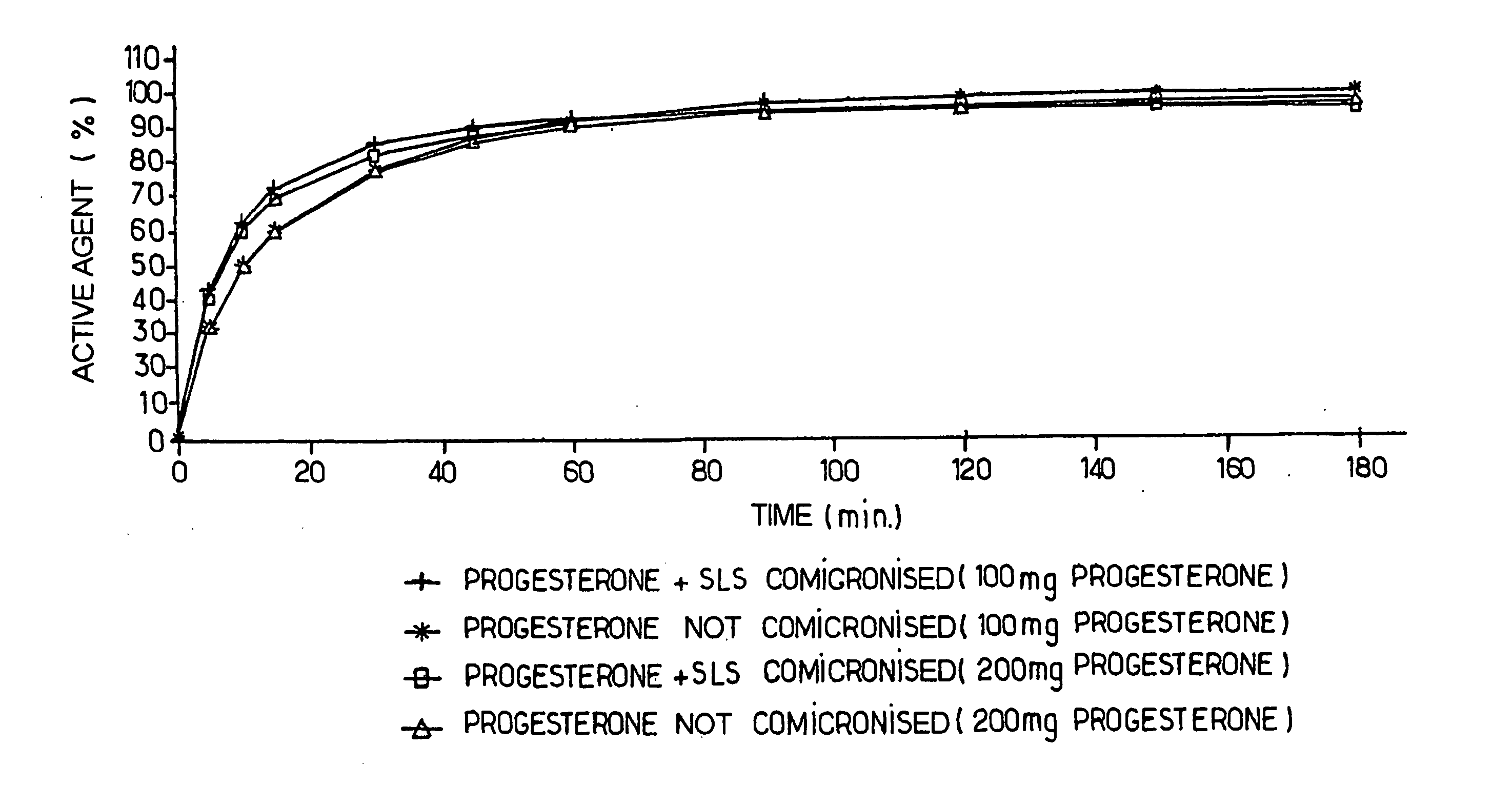
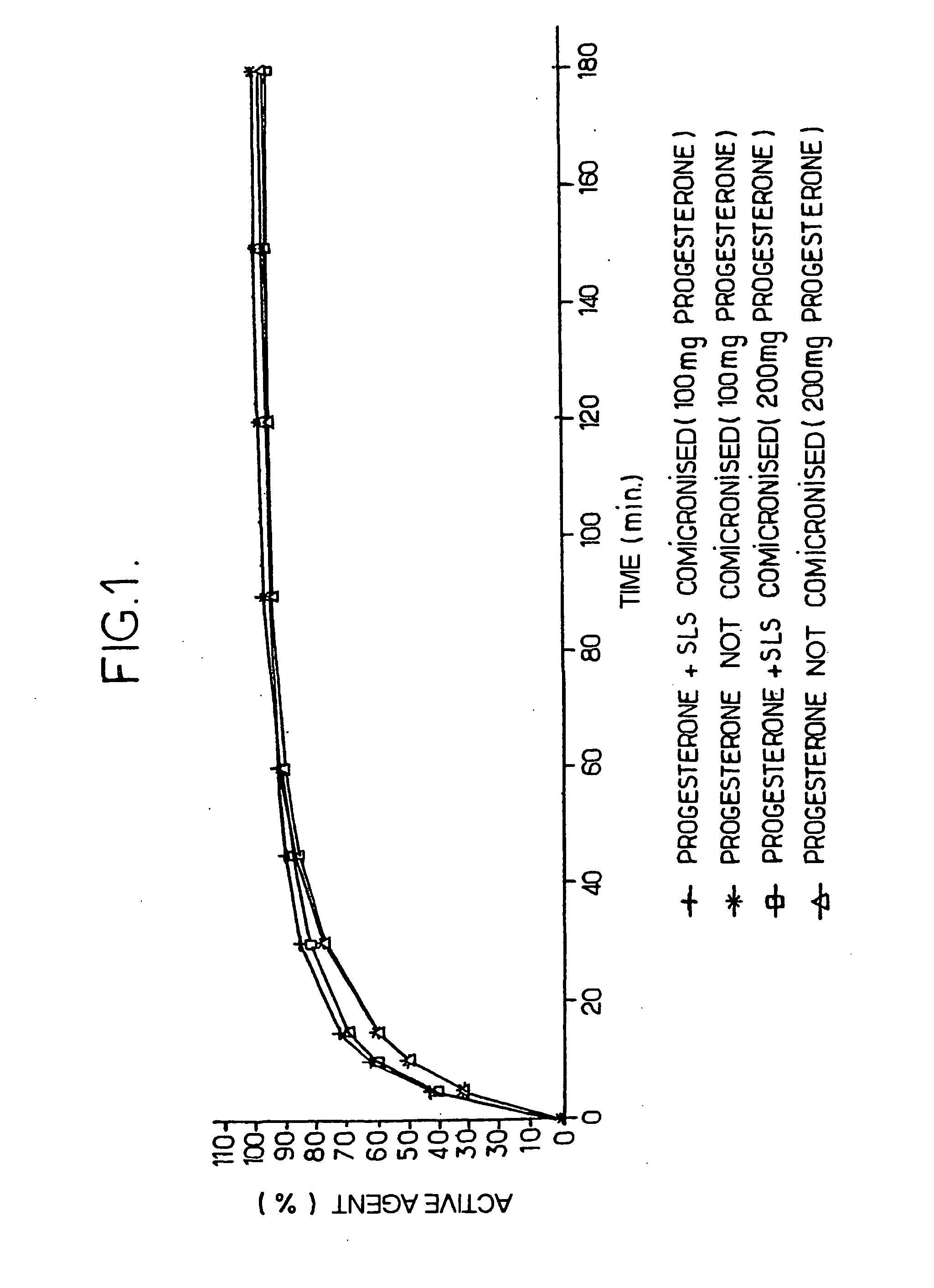
[0103] 100 mg and 200 mg progesterone tablets are prepared according to the method given in example 2, and the following operations are carried out in order to determine the in vitro dissolution curves (see FIG. 1).
[0104] The following material is used: [0105] SOTAX AT7 7-position revolving-paddle dissolution device [0106] PERKIN ELMER lambda 20 spectrophotometer [0107] ISMATEC IPC 12 cassette pump [0108] WINSOTAX data acquisition software.
[0109] The dissolving conditions are as follows: [0110] dissolving medium: 1000 ml of aqueous solution of β-hydroxypropylcyclodextrin having the trade mark KLEPTOSE® at 1% per cell [0111] rotation rate: 150 rpm [0112] temperature: 37° C.±0.5° C. [0113] number of cells: 7 [0114] circulation cell made of quartz with an optical path: 0.1 cm.
[0115] A control is prepared, consisting of a tablet of micronized progesterone:
Progesterone20mgHPLC ethanol20ml1% solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| disintegration time | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com