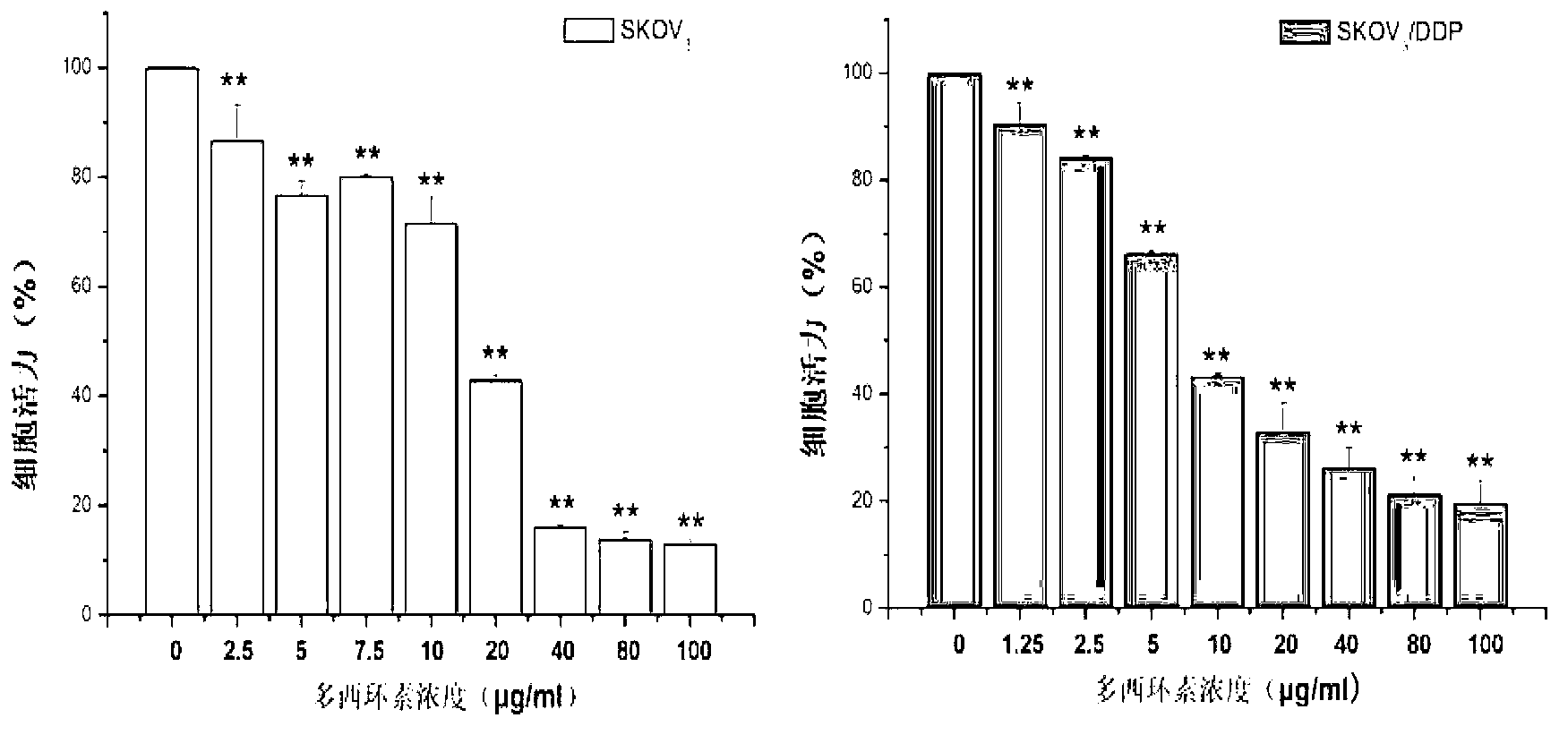Patents
Literature
65 results about "Epithelial ovarian cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Most epithelial ovarian tumors are benign (noncancerous). There are several types of benign epithelial tumors, including serous adenomas, mucinous adenomas, and Brenner tumors. Cancerous epithelial tumors are carcinomas - meaning they begin in the tissue that lines the ovaries.
Prevention of ovarian cancer by administration of progestin products
InactiveUS6977250B2High activityEliminate side effectsOrganic active ingredientsBiocideProgestin therapyEpithelial ovarian cancer
The present invention relates to methods for preventing the development of epithelial ovarian cancer by administering progestin products, either alone or in combination with other agents such as estrogen products.
Owner:NEW LIFE PHARMA
Novel peptides and combination of peptides for use in immunotherapy against epithelial ovarian cancer and other cancers
ActiveUS20170035807A1High error rateOrganic active ingredientsPeptide/protein ingredientsReceptorOncology
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Methods for generating predictive models for epithelial ovarian cancer and methods for identifying eoc
InactiveUS20140156573A1Improve forecast accuracyMagnetic measurementsDigital computer detailsNMR - Nuclear magnetic resonancePredictive methods
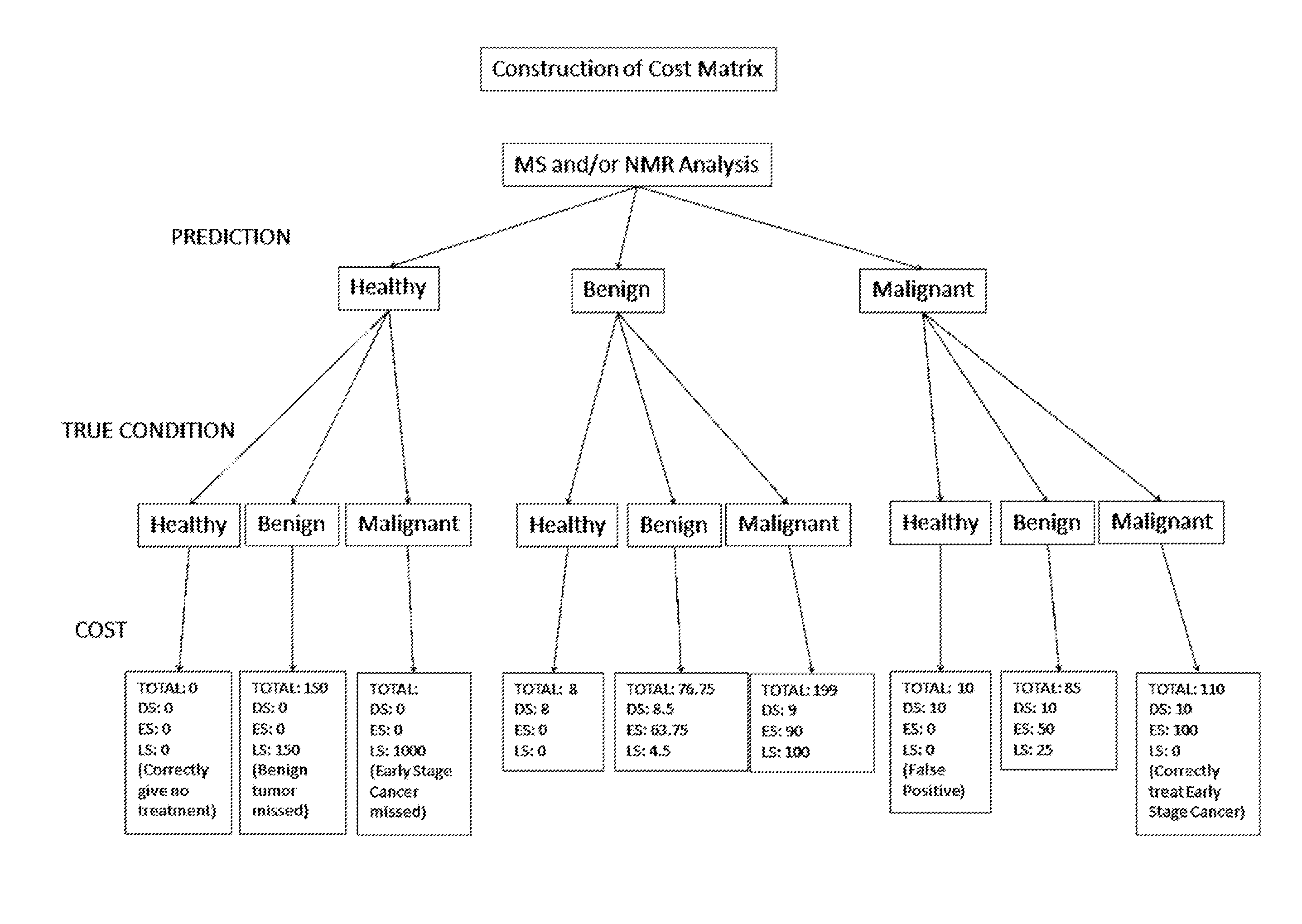
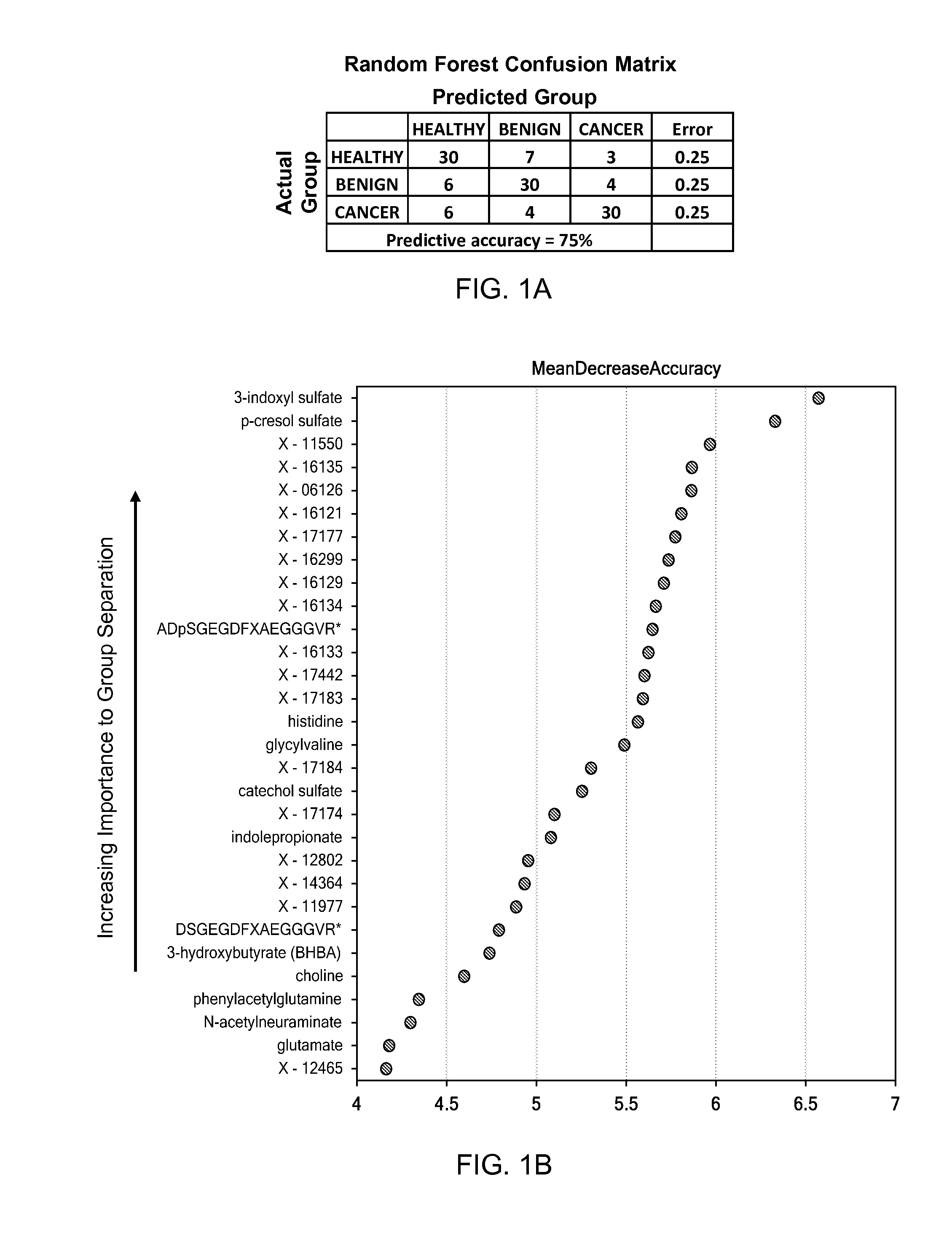
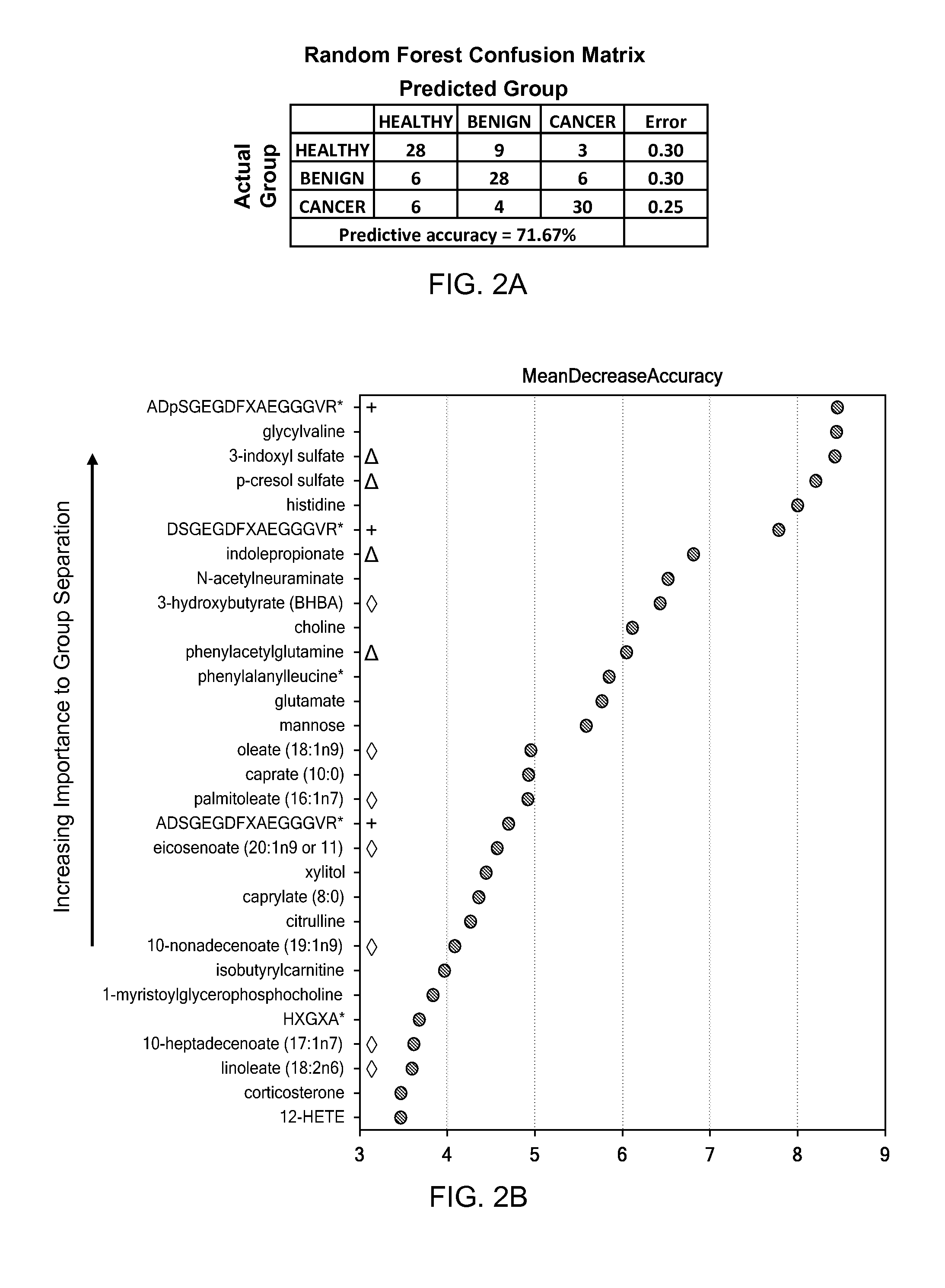
A method for generating a model for epithelial ovarian cancer is presented, comprising the steps of obtaining a mass spectrum for each of a plurality of samples, segmenting each of the mass spectra into “bins,” and determining a plurality of relationships between two or more bins. One are more statistically significant factors are identified according to the determined plurality of relationships, and a predictive model is generated as a function of the one or more identified factors. A method of the present invention may further comprise the step of obtaining one or more nuclear magnetic resonance spectra of each of the samples, which are segmented into a plurality of bins. Combinations of mass spectra and NMR spectra may be used to determine the plurality of relationships. In other embodiments, methods for identifying the presence of EOC indicated by a biological sample of an individual are presented.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Method of prognosis and stratification of ovarian cancer
InactiveCN104854247AEasy to studyGenetic material ingredientsMicrobiological testing/measurementSurvival prognosisMicroRNA
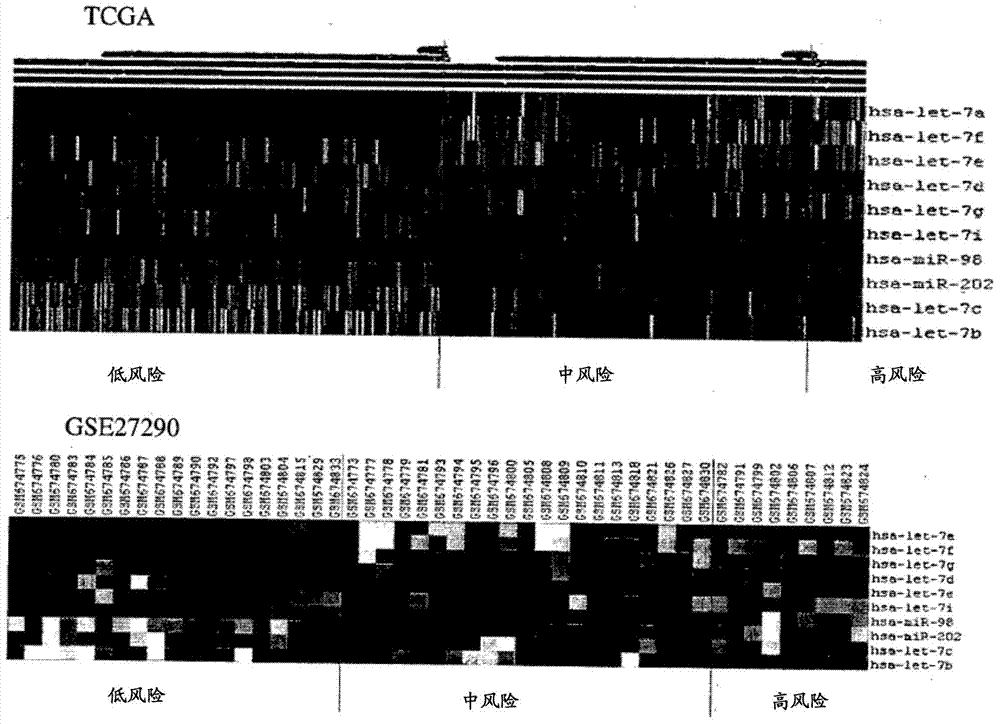
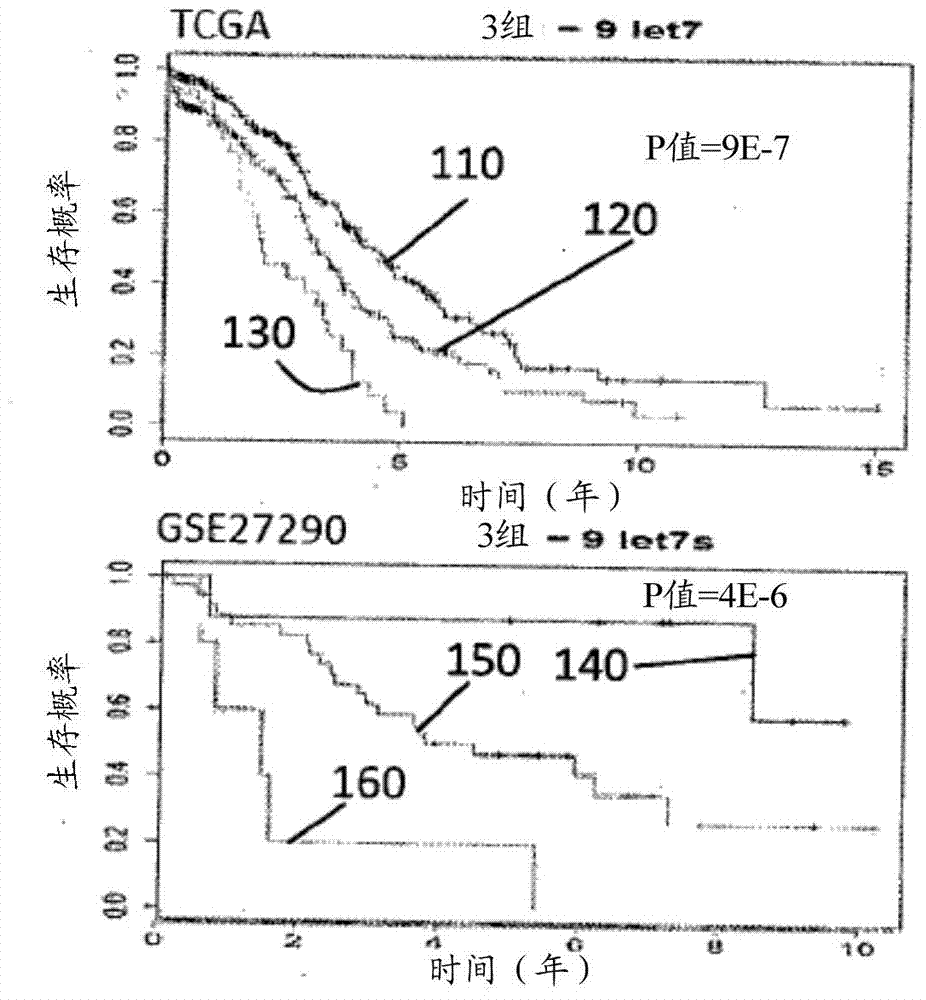
A method for the prognosis of overall survival or prediction of therapeutic outcome for a patient suffering from epithelial ovarian cancer (EOC), comprising: a. providing a sample from the patient, b. determining the expression level of microRNA family lethal-7b (let-7b) in the sample; c. using the expression level of the let-7b to obtain the prognosis of overall survival or prediction of therapeutic outcome for the patient.
Owner:AGENCY FOR SCI TECH & RES
Quantitative test to detect disease progression markers of epithelial ovarian cancer patients
InactiveUS20090123932A1Avoided technical biasMicrobiological testing/measurementOncologyDisease progression
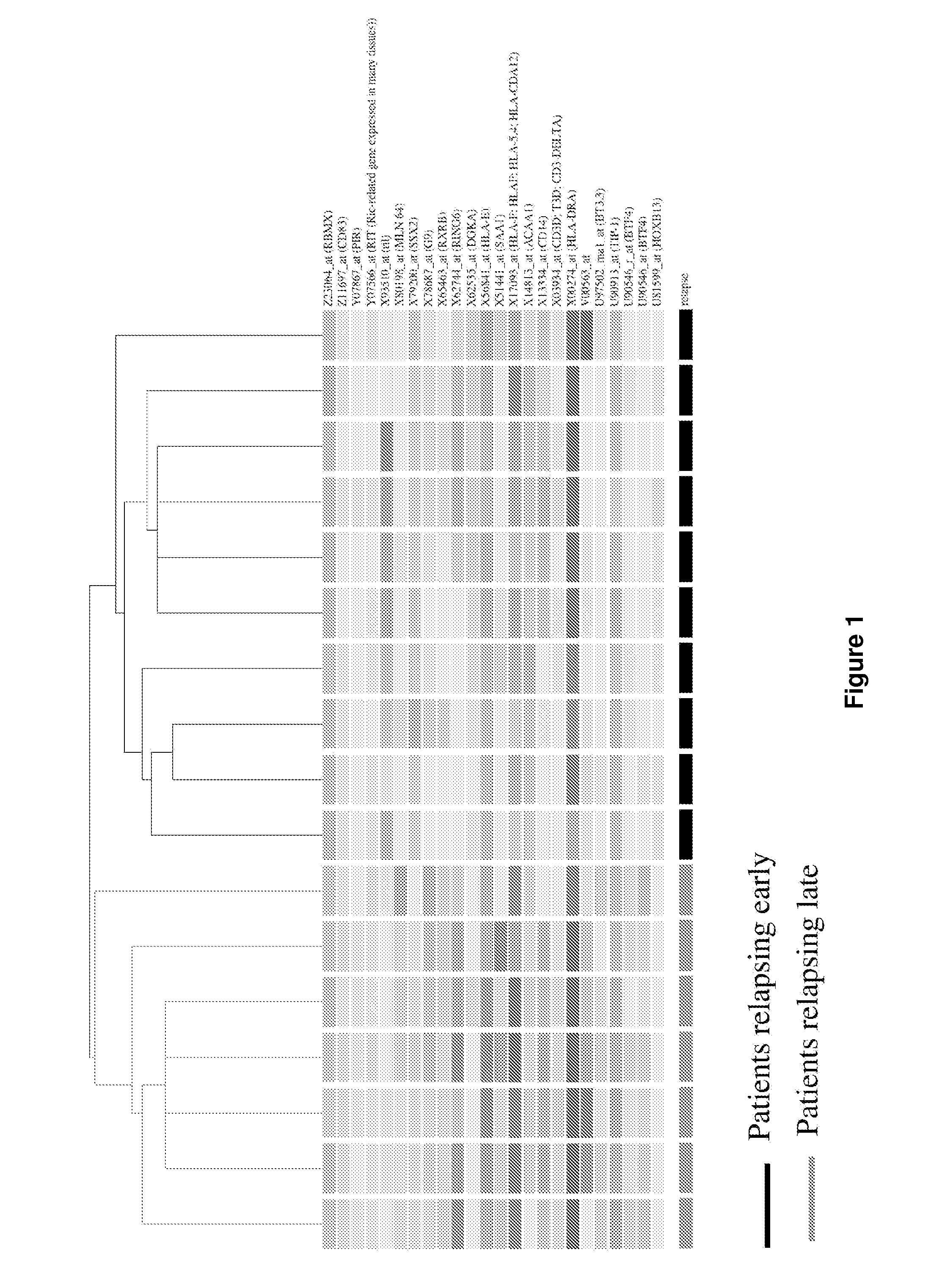
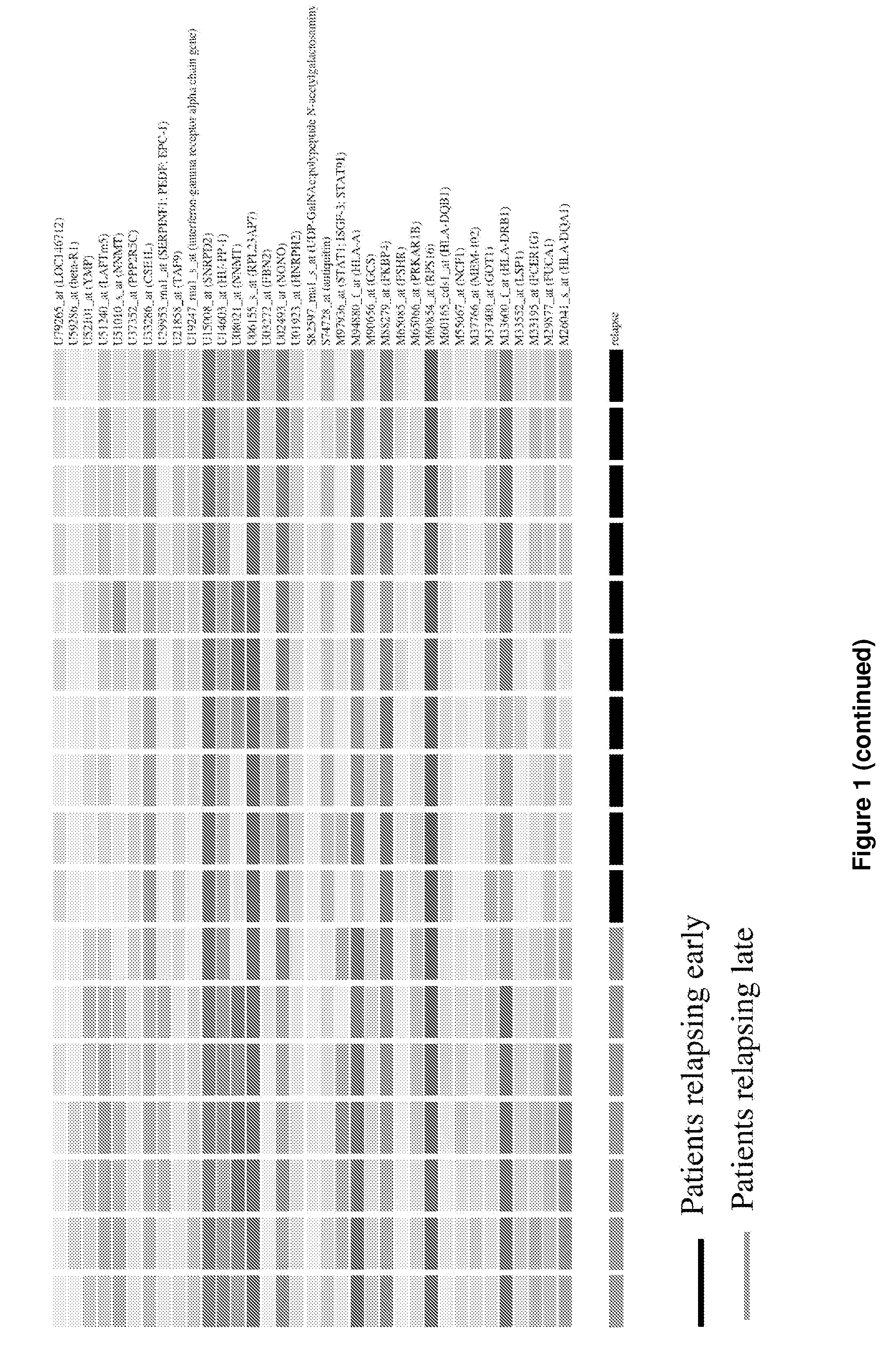
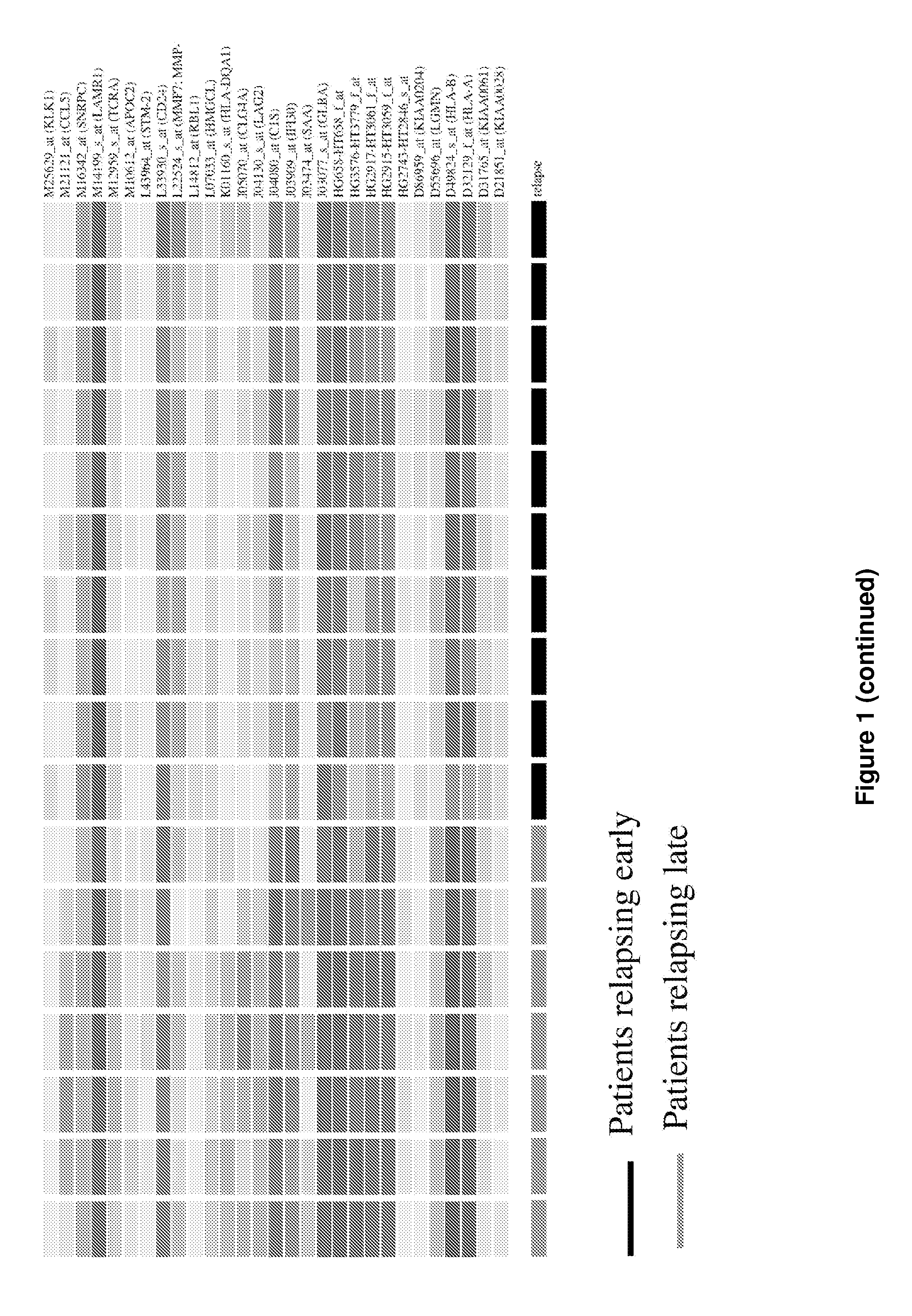
The present invention concerns a method of prognosing the risk of early ovarian cancer relapse in a subject having ovarian cancer comprising: a) detecting the level of at least one marker selected from the group consisting of BTF4, GCS and HLA-DRbeta1; and b) comparing the level of the above at least one marker with that of a corresponding control sample, wherein the detection of a lower level of the at least one marker compared to that in the control sample is indicative that the subject is at risk of early cancer relapse. Also provided is a method of stratifying a subject suffering from ovarian cancer based on the expression levels of the disclosed markers and kits for practicing the methods of the present invention.
Owner:LE CENT HOSPITALER DE LUNIV DE MONTREAL
Prevention of ovarian cancer by administration of agents that induce biologic responses
InactiveUS20070213543A1Avoid developmentReduce riskOrganic active ingredientsOrganic chemistryEpitheliumOncology
The present invention relates to compositions and methods for preventing the development of epithelial ovarian cancer by administering compounds in an amount capable of regulating TGF-β expression in the ovarian epithelium and / or capable of optimally altering expression of other surrogate biomarkers identified by microarray technology. HRT and OCP regimens comprising such compositions and methods are disclosed.
Owner:RODRIGUEZ GUSTAVO C
Peptides and combination of peptides for use in immunotherapy against epithelial ovarian cancer and other cancers
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Antibody for detecting epithelial ovarian cancer marker and method for diagnosing epithelial ovarian cancer
ActiveUS20140242607A1High accuracy rateEasy to detectAnimal cellsFused cellsEpitopeGlycosyltransferase
It is intended to find a highly specific epithelial ovarian cancer marker and to provide an antibody capable of specifically recognizing and detecting the marker or a fragment of the antibody. The present invention provides an anti-β1,6-N-acetylglucosaminyltransferase 5B antibody for diagnosis of epithelial ovarian cancer, i.e., an antibody for detection of a glycosyltransferase β1,6-N-acetylglucosaminyltransferase 5B as an epithelial ovarian cancer marker. The antibody recognizes, as an epitope, a part of a polypeptide of the enzyme consisting of the amino acid sequence represented by SEQ ID NO: 1.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Method of Diagnosing or Prognosing Epithelial Ovarian Cancer
InactiveUS20110217238A1Improved recurrence-free survivalImprove the level ofCompound screeningApoptosis detectionAntigenAntigen Binding Fragment
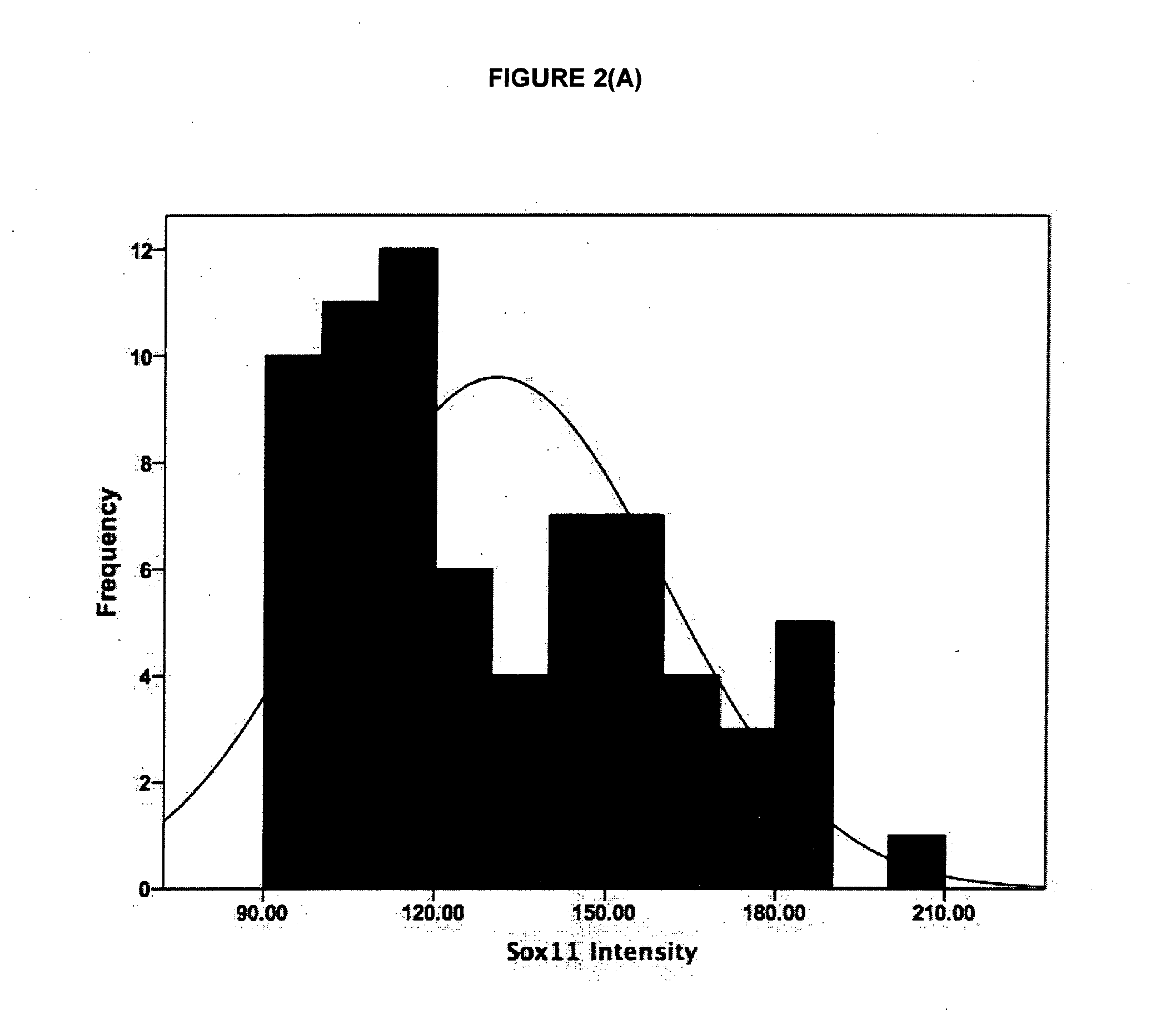
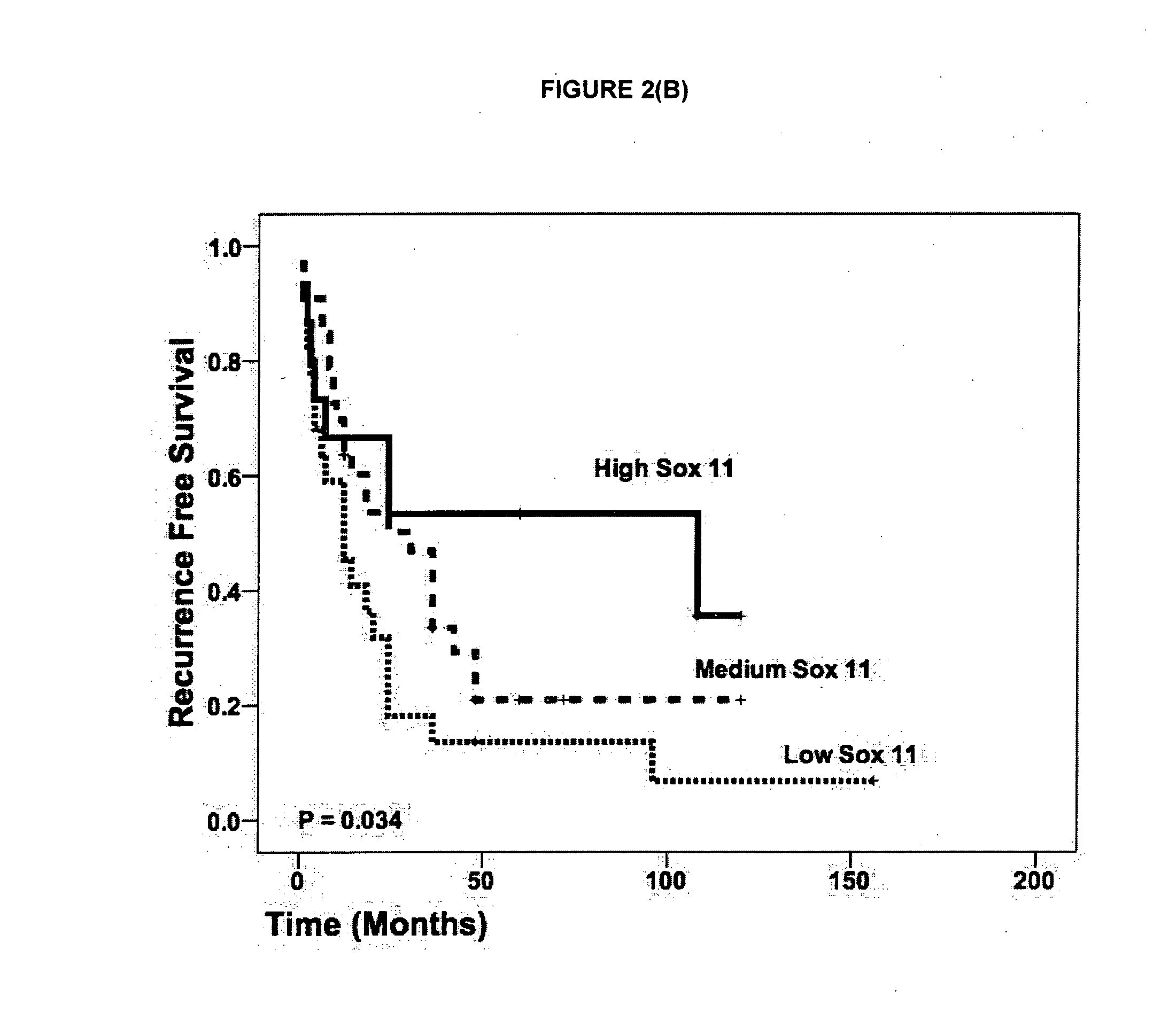
The present invention provides a binding moiety which selectively binds to Sox11 protein and / or mRNA for imaging, diagnosis or prognosis of epithelial ovarian cancer (EOC). Optionally, the moiety is an antibody or antigen-binding fragment thereof. Advantageously, moiety comprises a further, readily detectable moiety. The invention also provides methods of imaging EOC cells as well as methods of diagnosing or prognosing EOC in an individual. A further aspect of the present invention provides a method of identifying cells associated with EOC, the method comprising analysing the pattern of gene expression in a sample of cells to be tested and comparing it to the pattern of gene expression in a sample of known lymphomas cells. Preferably, the cells to be tested are identified as EOC cells if the expression of Sox11 is up-regulated compared to normal B-cells. Preferably EOC cells are identified as improved recurrence-free survival-associated if expression of Sox11 is up-regulated compared with non-cancerous epithelial ovarian cells. Preferably, EOC cells are identified as diminished recurrence-free survival-associated if expression of Sox11 is similar to, or down-regulated, compared with non-cancerous epithelial ovarian cells.
Owner:BORREBAECK CARL ARNE KRISTER +2
Application of miRNA in preparation of chemotherapy drug resistance evaluation kit for high-grade serous epithelial ovarian cancer
PendingCN109337978AMicrobiological testing/measurementDrug referencesDrug resistanceChemotherapy resistance
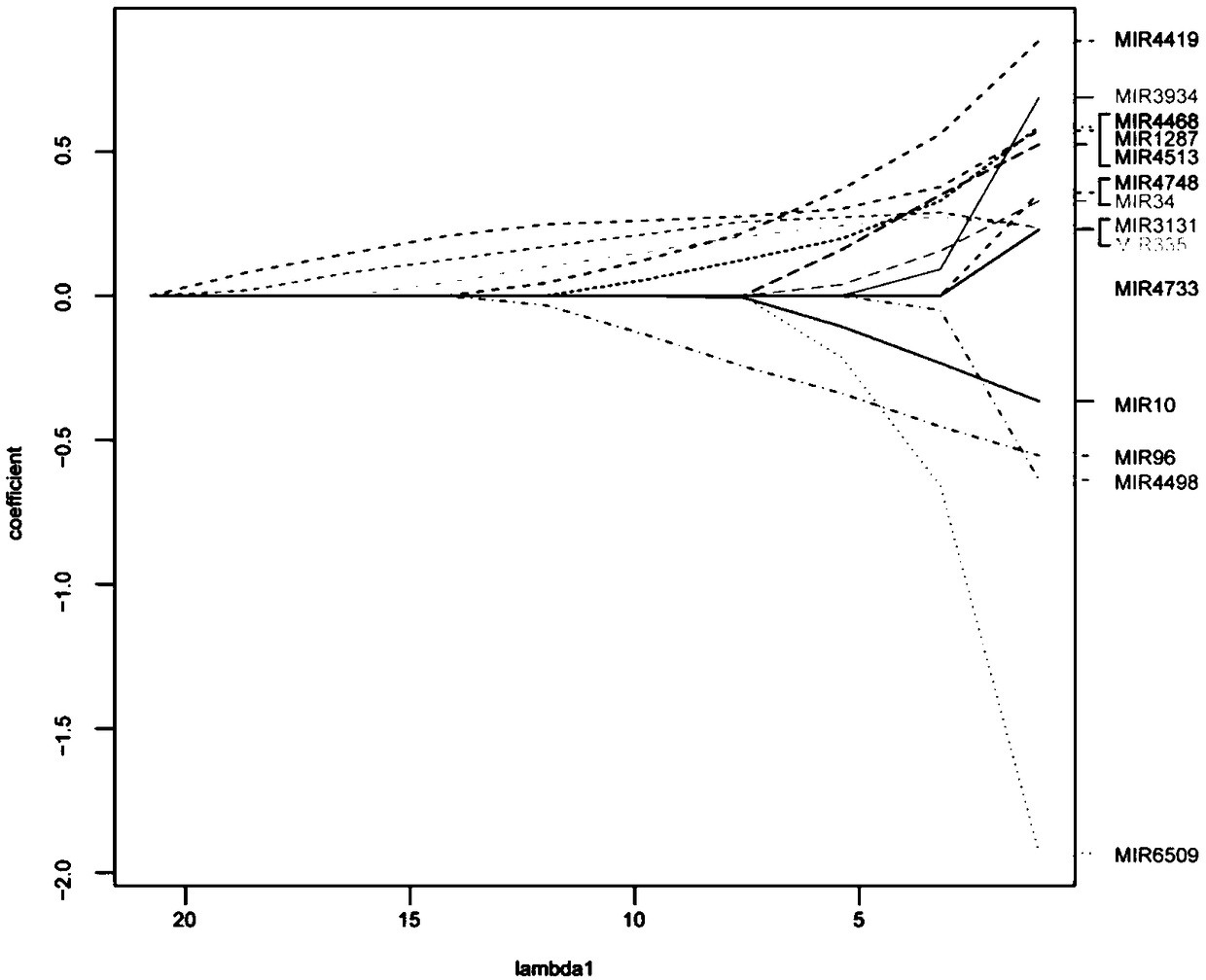
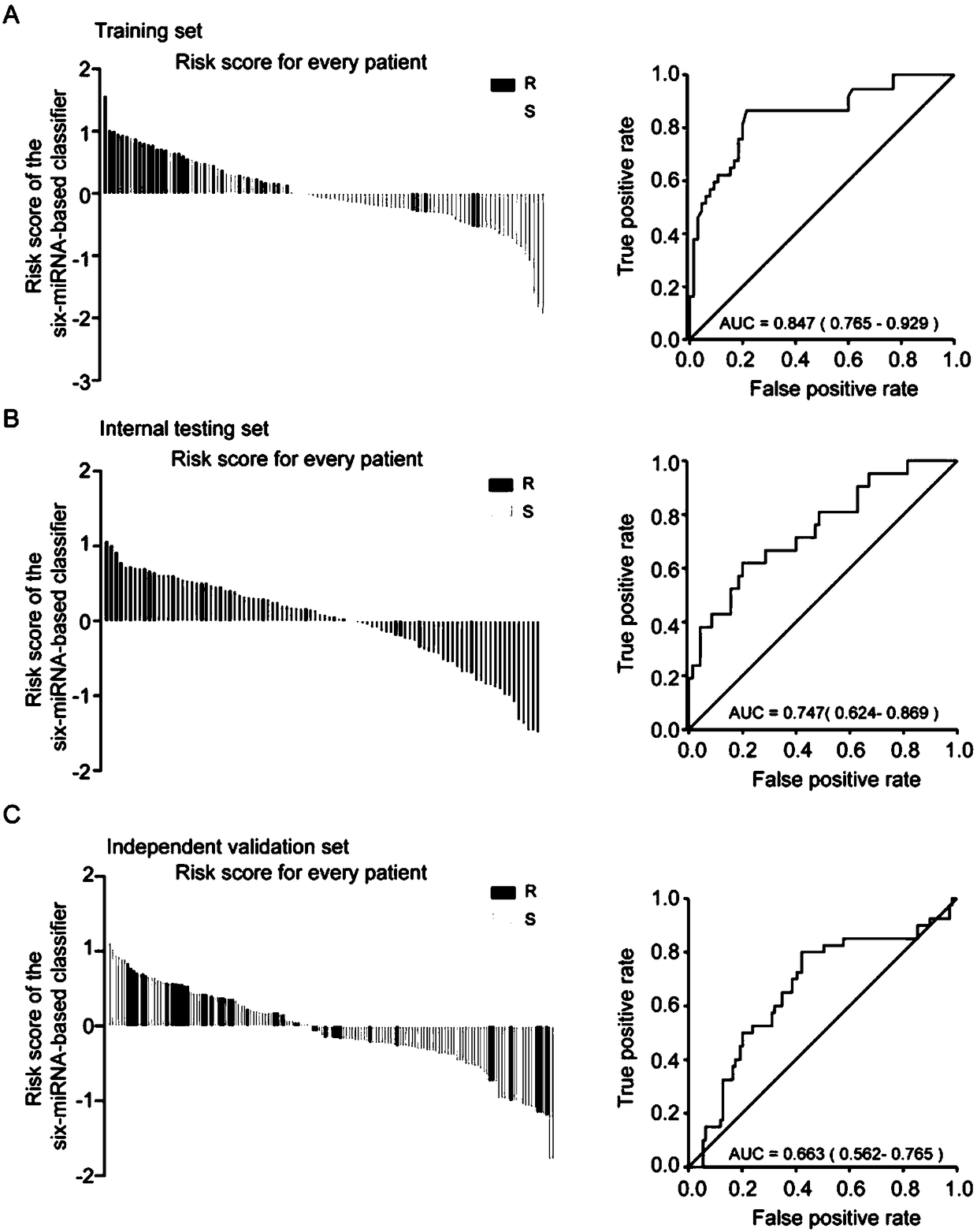
The invention relates to application of miRNA in preparation of a diagnostic reagent for predicting platinum chemotherapy drug resistance of high-grade serous ovarian cancer. The miRNA comprises miR-1287, miR-3131, miR-335, miR-4419, miR-4468 and miR-96. The invention also provides application for analyzing and detecting platinum chemotherapy drug resistance of high-grade serous ovarian cancer onthe basis of the determination of the expression quantity of miR-1287, miR-3131, miR-335, miR-4419, miR-4468 and miR-96, and the application has better accuracy than the existing method for predictingplatinum chemotherapy drug resistance of high-grade serous ovarian cancer.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Methods for diagnosis and/or prognosis of gynecological cancer
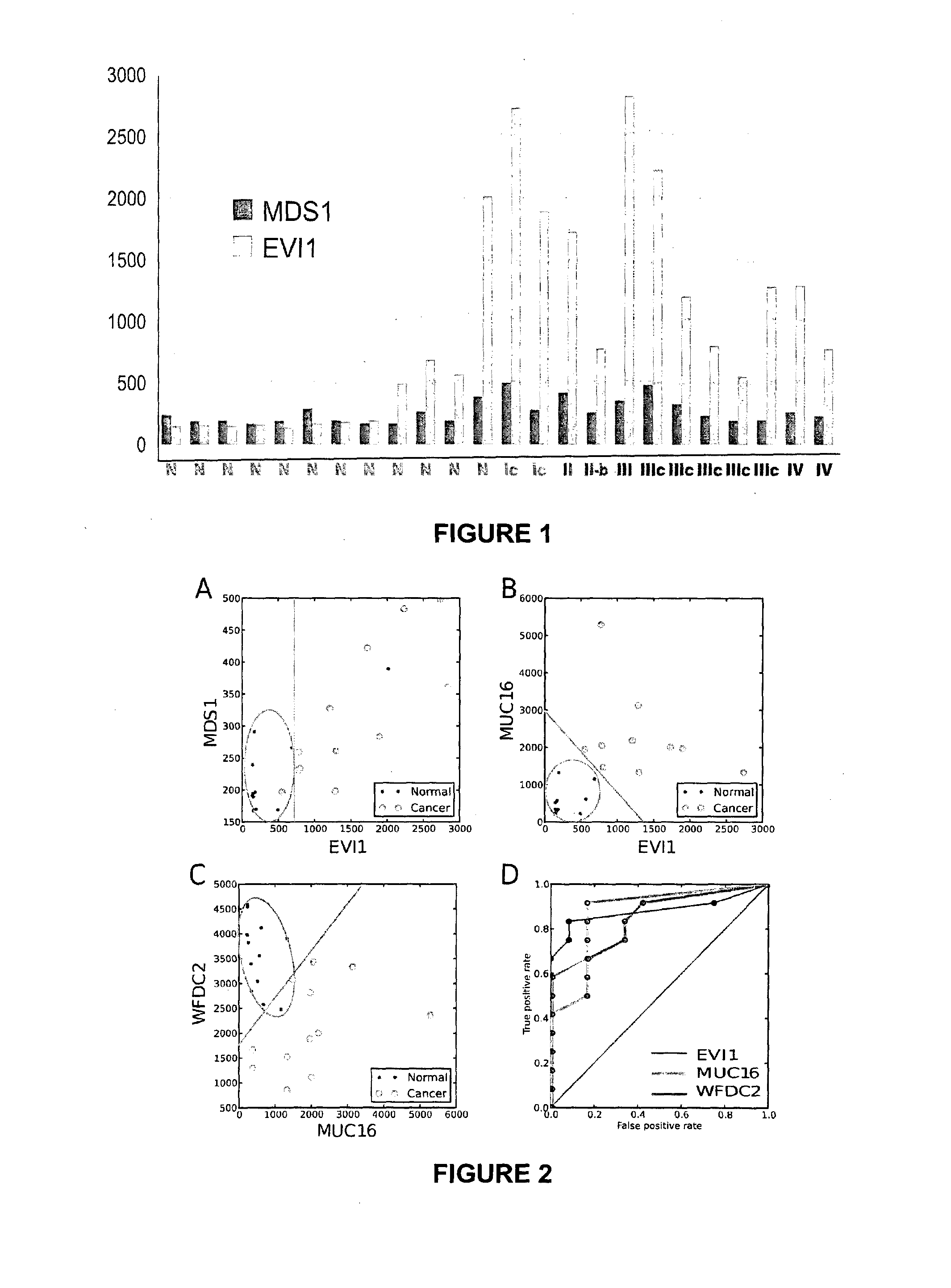
InactiveCN104080928AMicrobiological testing/measurementGene expression levelClear Cell Renal Carcinoma
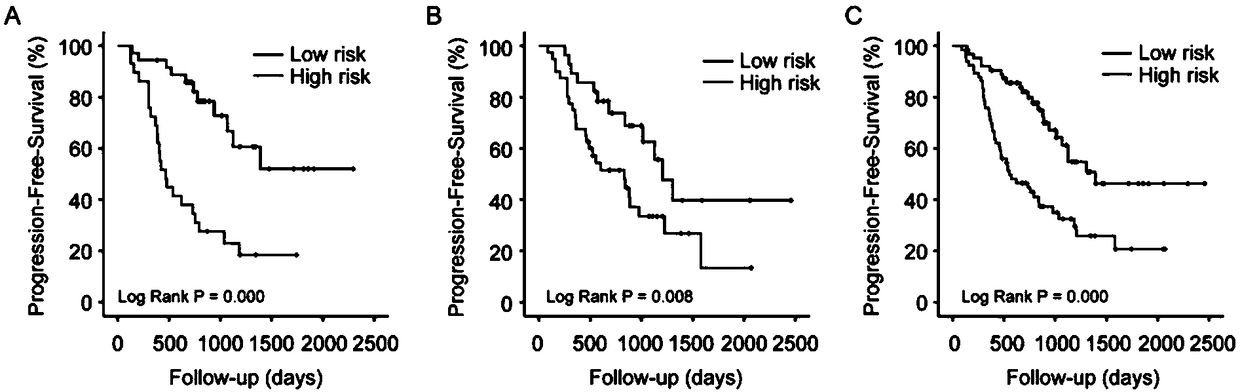
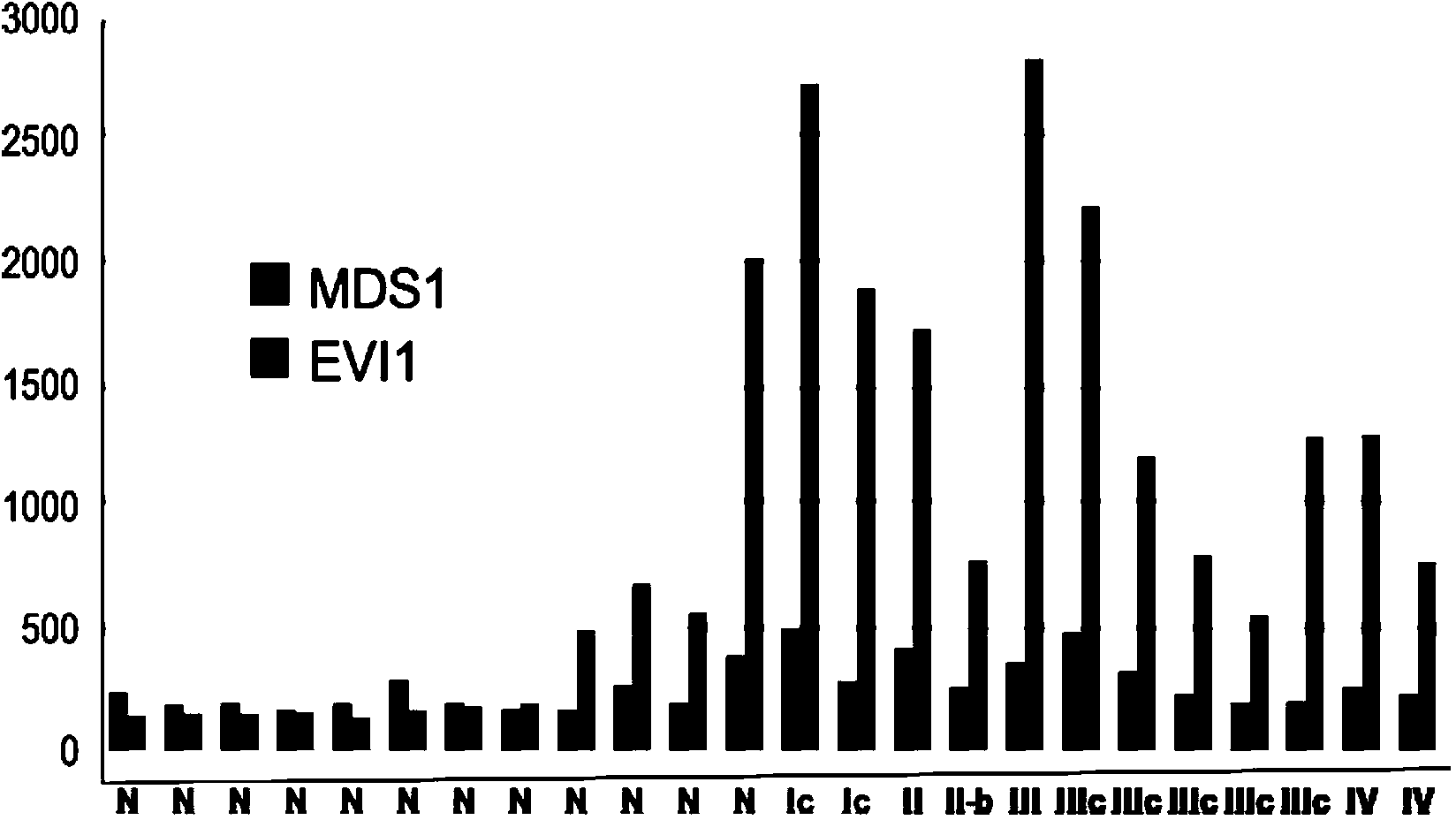
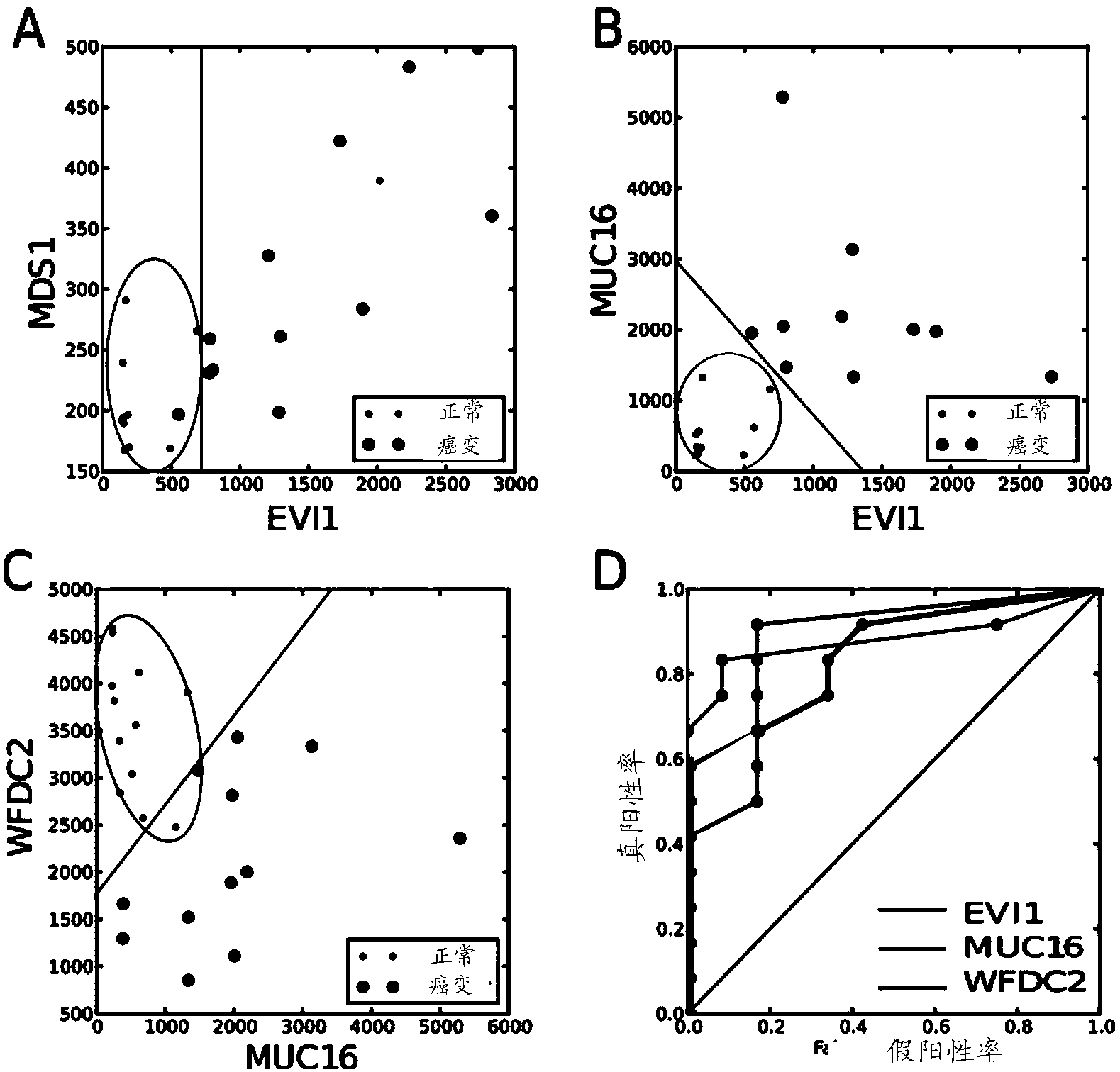
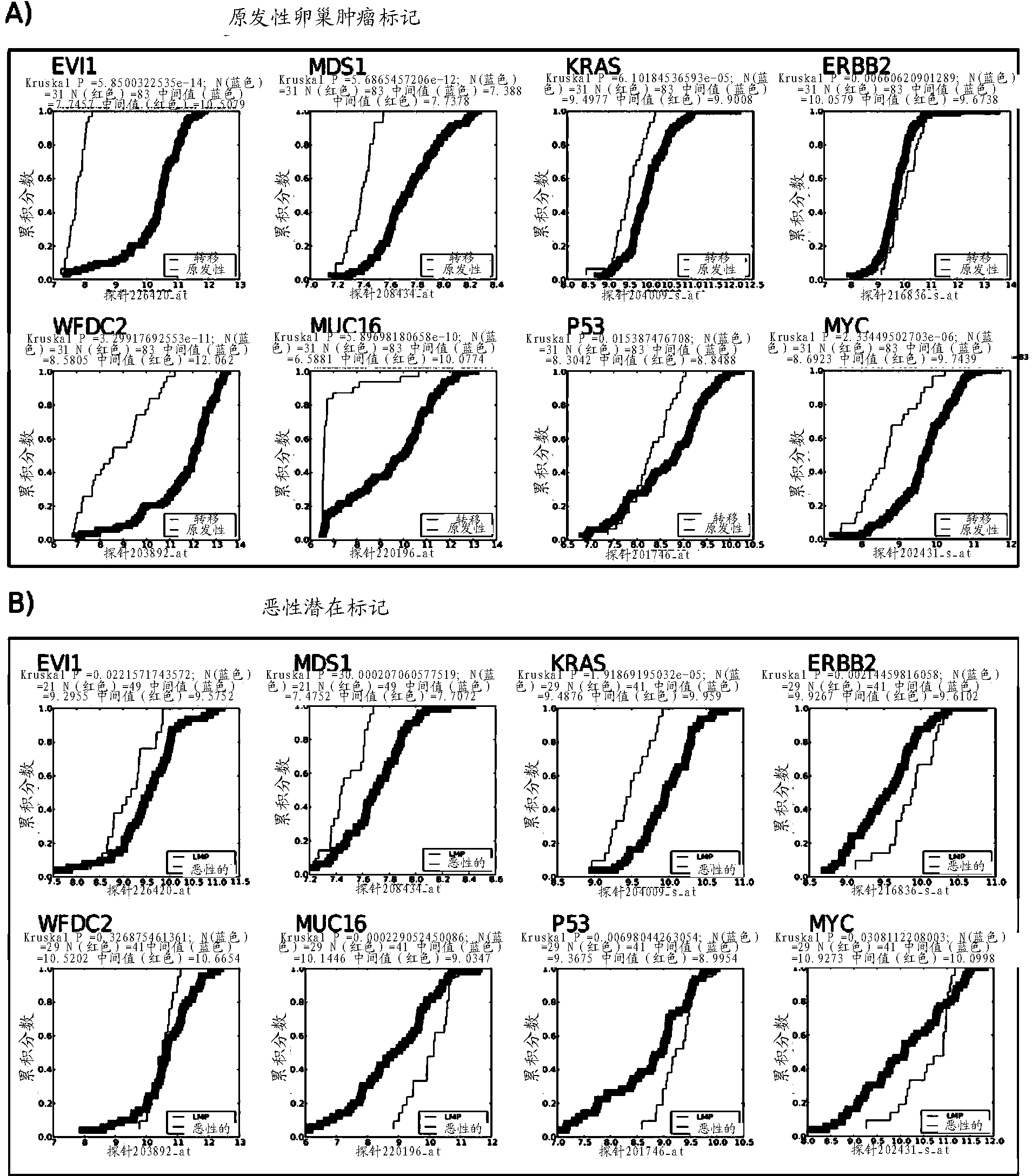
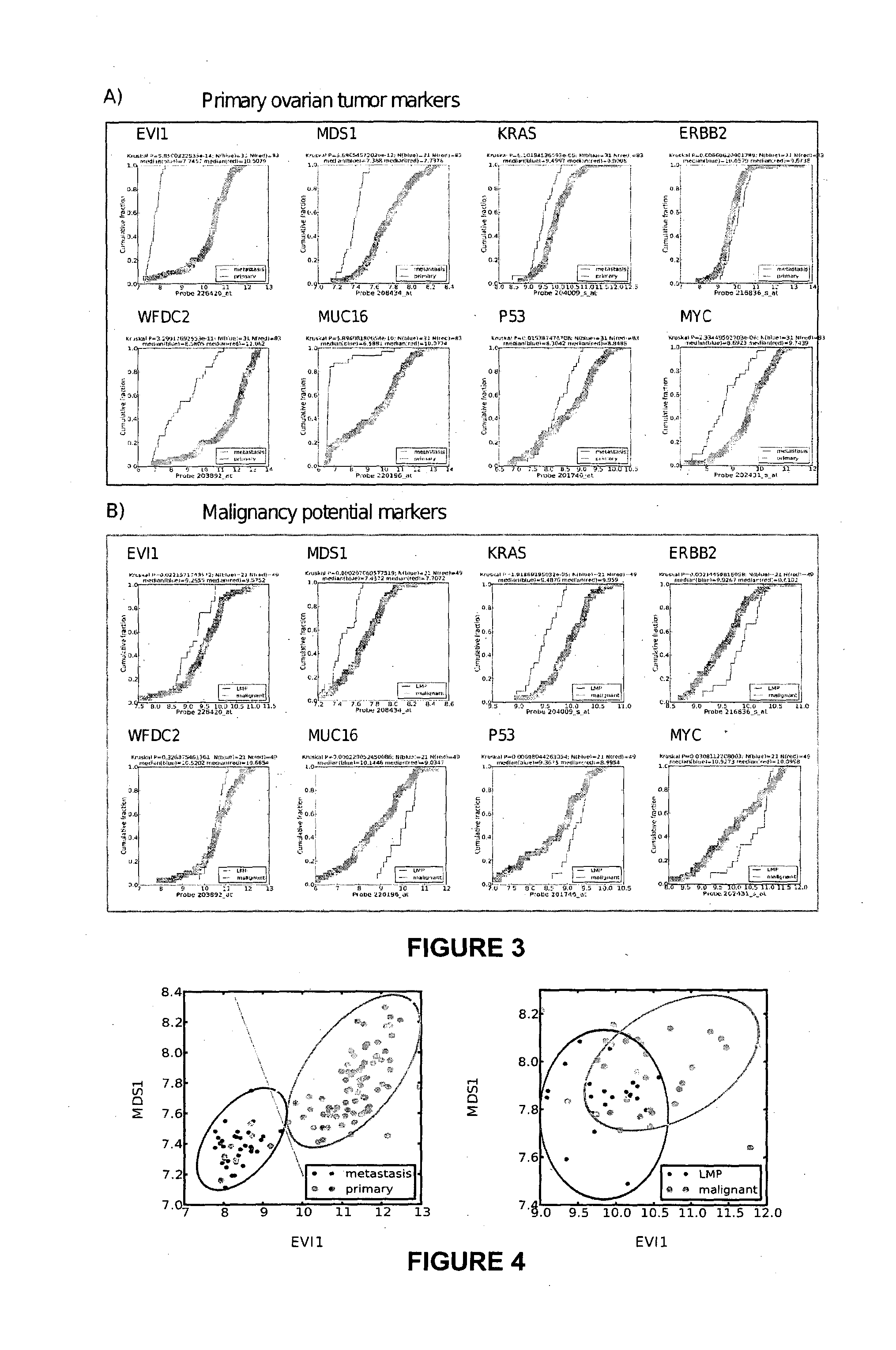
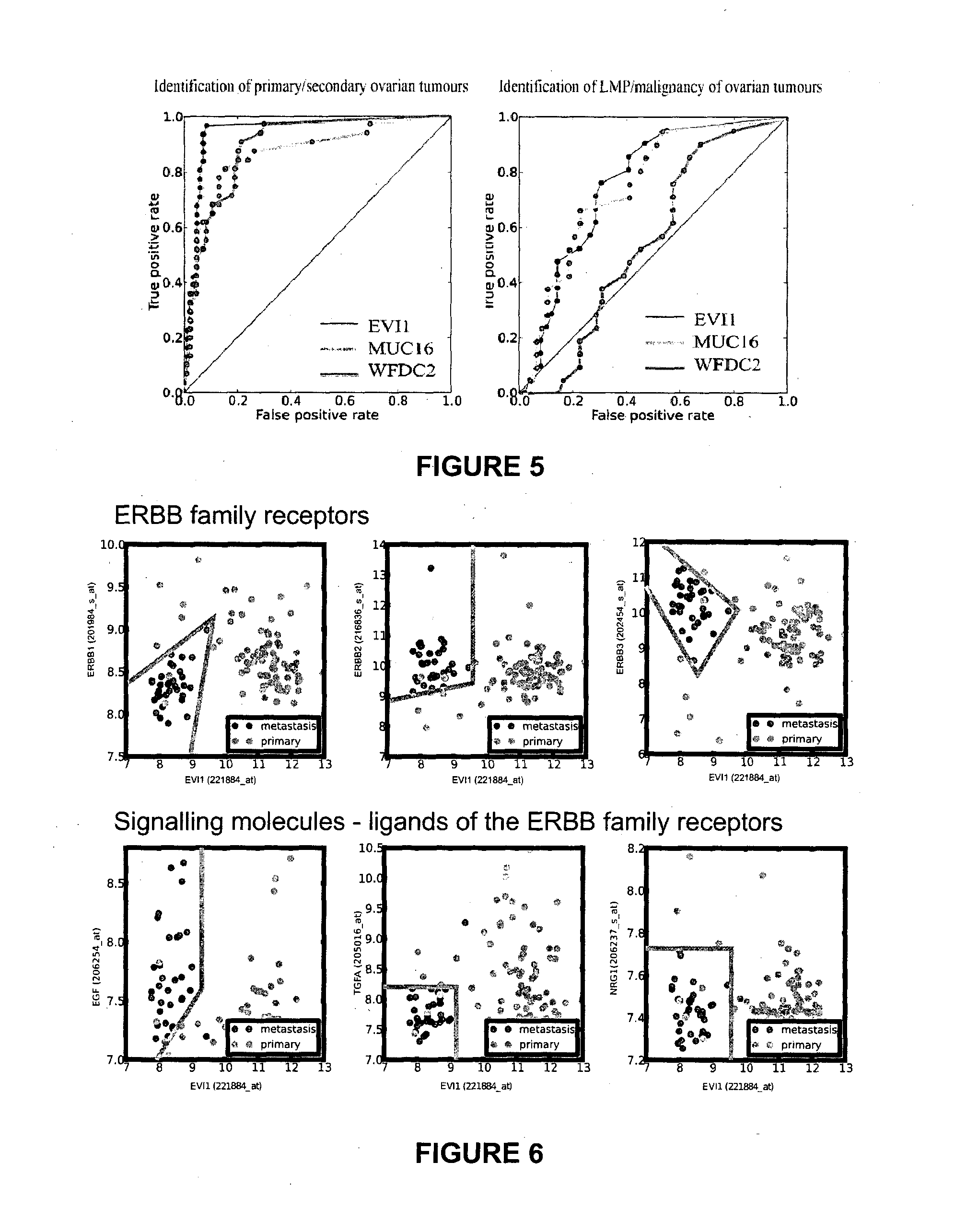
The present invention relates to an in vitro method for diagnosing epithelial ovarian cancer, cervical cancer, endometriosis, clear cell renal carcinoma and / or predisposition to epithelial ovarian cancer in a subject, the method comprising determining in a sample of the subject gene expression level of at least one gene in the MDS 1 and EVI1 complex (MECOM) locus, and / or copy number of at least one gene in the MECOM locus, wherein the level against at least one expression cutoff value and / or copy number against at least one copy number cutoff value are indicative of the subject having epithelial ovarian cancer, cervical cancer, endometriosis, clear cell renal carcinoma and / or a predisposition to epithelial ovarian cancer cervical cancer, endometriosis, clear cell renal carcinoma and / or determining whether the ovarian cancer in the subject is primary or secondary ovarian cancer and / or a risk of the disease progression after surgery treatment, and / or an effectiveness of post-surgery chemotherapy.
Owner:AGENCY FOR SCI TECH & RES
Gene probe composition and kit for detecting epithelial ovarian cancer
ActiveCN103276060AOvercoming a gap in the marketEasy to detectMicrobiological testing/measurementDNA preparationFluorescenceBrca1 gene
The invention relates to a gene probe composition and a kit for detecting epithelial ovarian cancer. The gene probe composition comprises a c-myc gene probe, an Rb1 gene probe, a Chk2 gene probe, a p53 gene probe and a BRCA1 gene probe. Another objective of the invention is to provide a kit for fluorescence in situ hybridization (FISH) detection of the epithelial ovarian cancer, and the kit comprises the gene probe composition as described above. The kit can detect 5 kinds of genes in a same sample even a single tumor cell of the epithelial ovarian cancer at the same time, and thereby substantially improving detection capability and efficiency. The invention further provides an optimal sample selection strategy and a slide preparation method for an ovarian cancer FISH research.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE +1
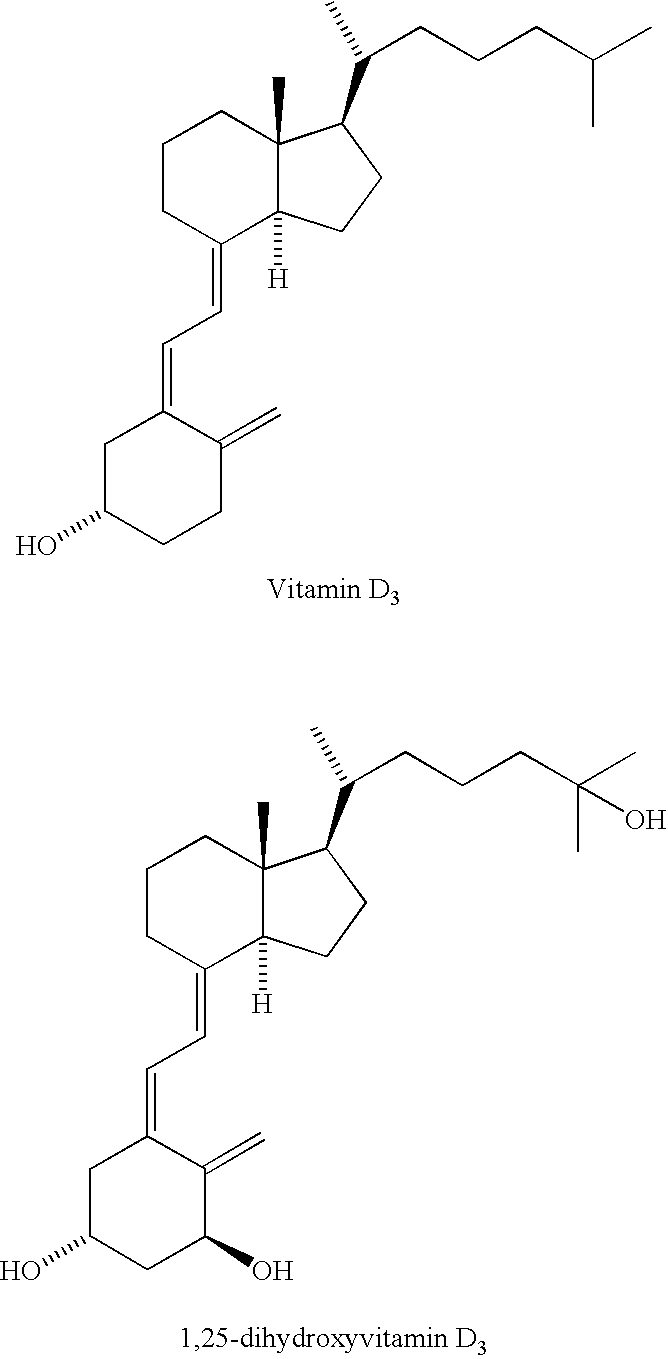
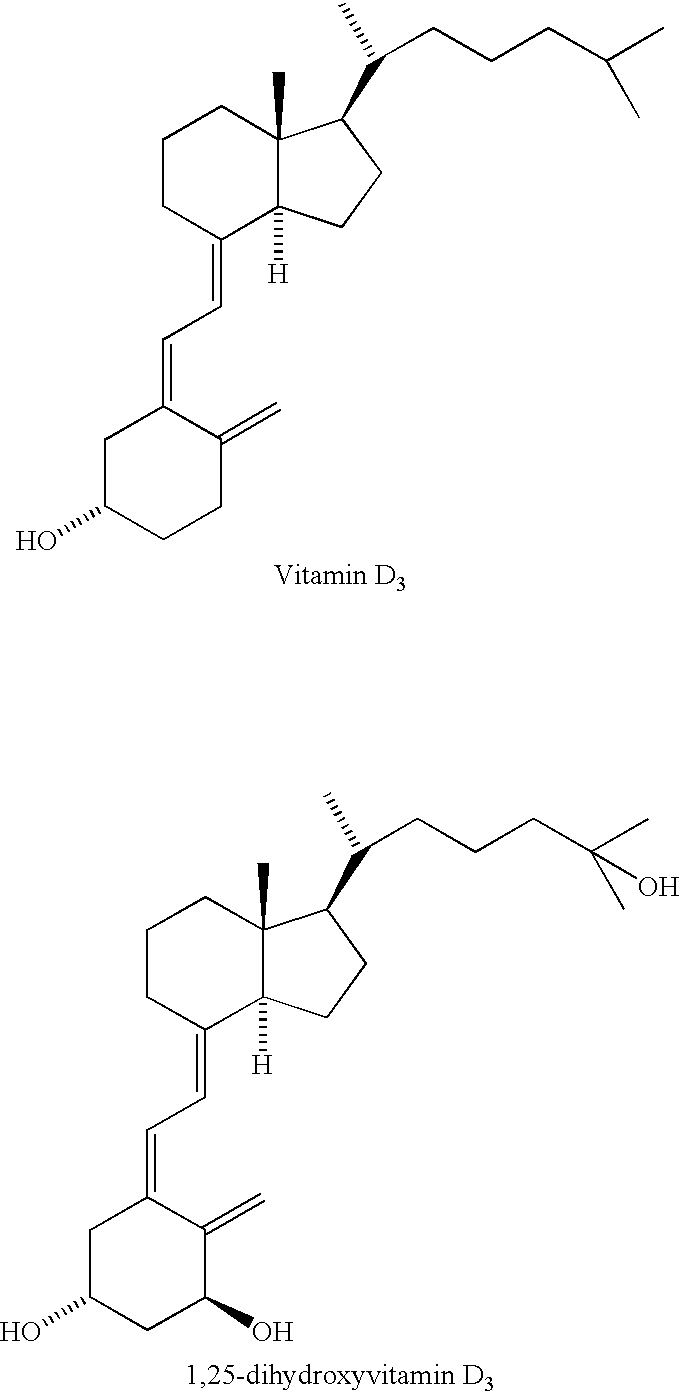
Prevention of ovarian cancer by administration of a Vitamin D compound
InactiveUS7053074B2Avoid developmentIncrease ratingsBiocideAnimal repellantsApoptosisRectal epithelium
The present invention relates to methods for preventing the development of epithelial ovarian cancer by administering a Vitamin D compound in an amount capable of increasing apoptosis in non-neoplastic ovarian epithelial cells of the female subject.
Owner:NEW LIFE PHARMA
siRNA for inhibiting growth of epithelial ovarian cancer as well as recombinant vector and application thereof
InactiveCN103146703AGrowth inhibitionGenetic material ingredientsAntineoplastic agentsGynautoceraMortality rate
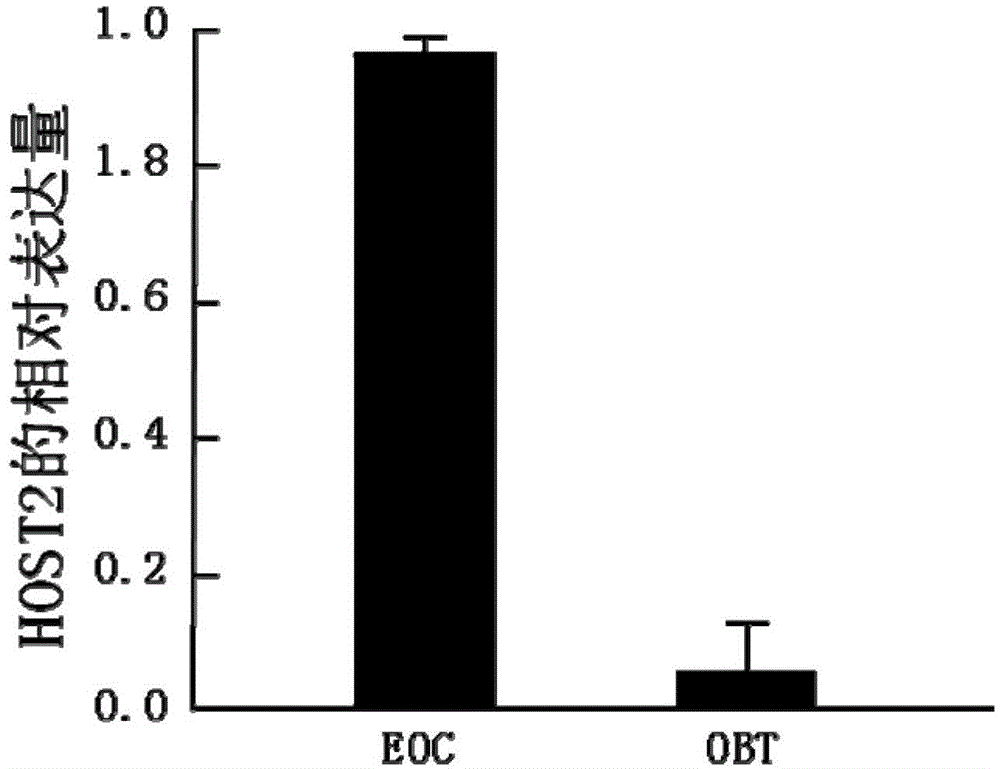
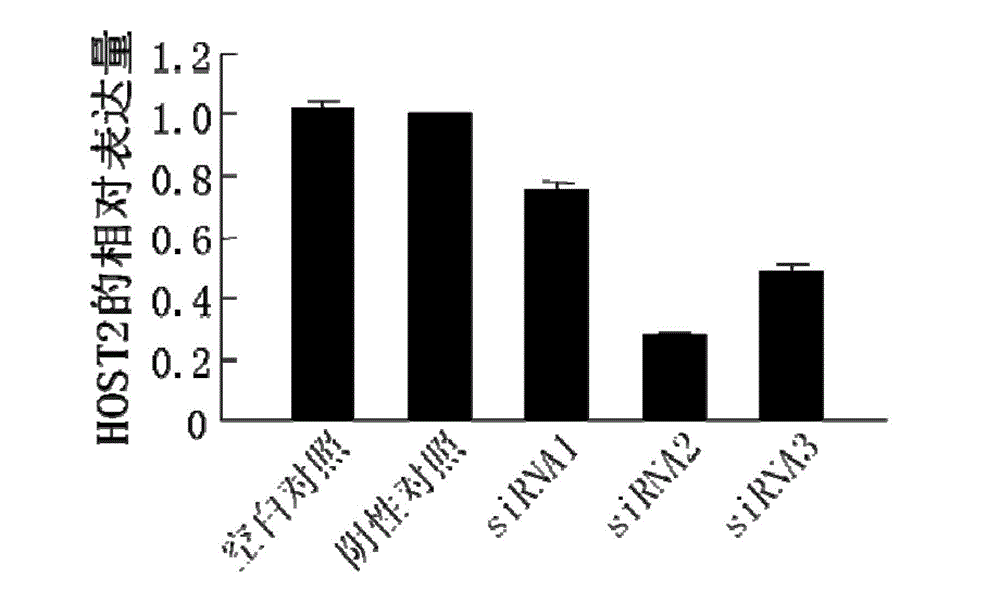
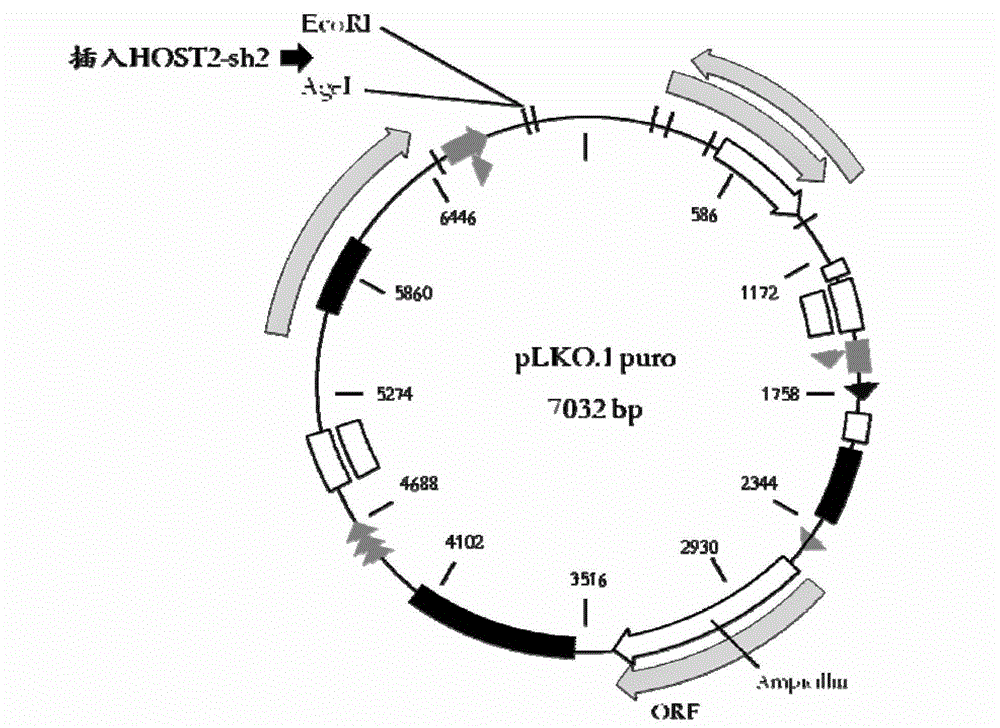
The invention belongs to the technical field of genetic engineering. The epithelial ovarian cancer (EOC) is the most common ovarian cancer; the morbidity ranks the third in malignant tumors of gynecological reproductive organs and ranks only second to cervical cancer and endometrial cancer, but the death rate can be up to 70%, and ranks the first. An interference sequence for inhibiting the expression of the epithelial ovarian cancer HOST2 gene is screened; and the RNA (ribonucleic acid) sequence of the HOST2 gene is shown in SEQ ID NO:4 or 5. According to the invention, a recombinant vector including the interference sequence is also built; in vivo and in vitro experiments show that the recombinant vector can inhibit the specific high-expression recombinant HOST2 in an EOC cell, so as to inhibit the growth of the tumor. The siRNA and the recombinant vector provided by the invention provide a new gene treatment medicament for clinic treatment of EOC.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Application of doxycycline in preparing medicament for treating epithelial ovarian cancer
InactiveCN103006675AEasy to administerLittle side effectsTetracycline active ingredientsAntineoplastic agentsSide effectApoptosis
The invention relates to the technical field of medicines, and in particular relates to a novel application of doxycycline in preparing a medicament for treating epithelial ovarian cancer. Animal experiments and in-vitro cell experiments prove that doxycycline can inhibit multiplication and invasion of ovarian cancer cells, can prevent epithelial ovarian cancer cells from transferring, can promote apoptosis of epithelial ovarian cancer cells, has a remarkable inhibition effect to ovarian cancer cells, in particular platinum-based drug-resistance ovarian cancer cells, and can inhibit growth of ovarian cancer tissues and formation of ascites, so that doxycycline can be used for preparing a medicament for treating epithelial ovarian cancer. The invention provides a novel application to doxycycline, and compared with other chemotherapeutic drugs, doxycycline has the advantages of convenient administration and small side effect in the field of treating epithelial ovarian cancer.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
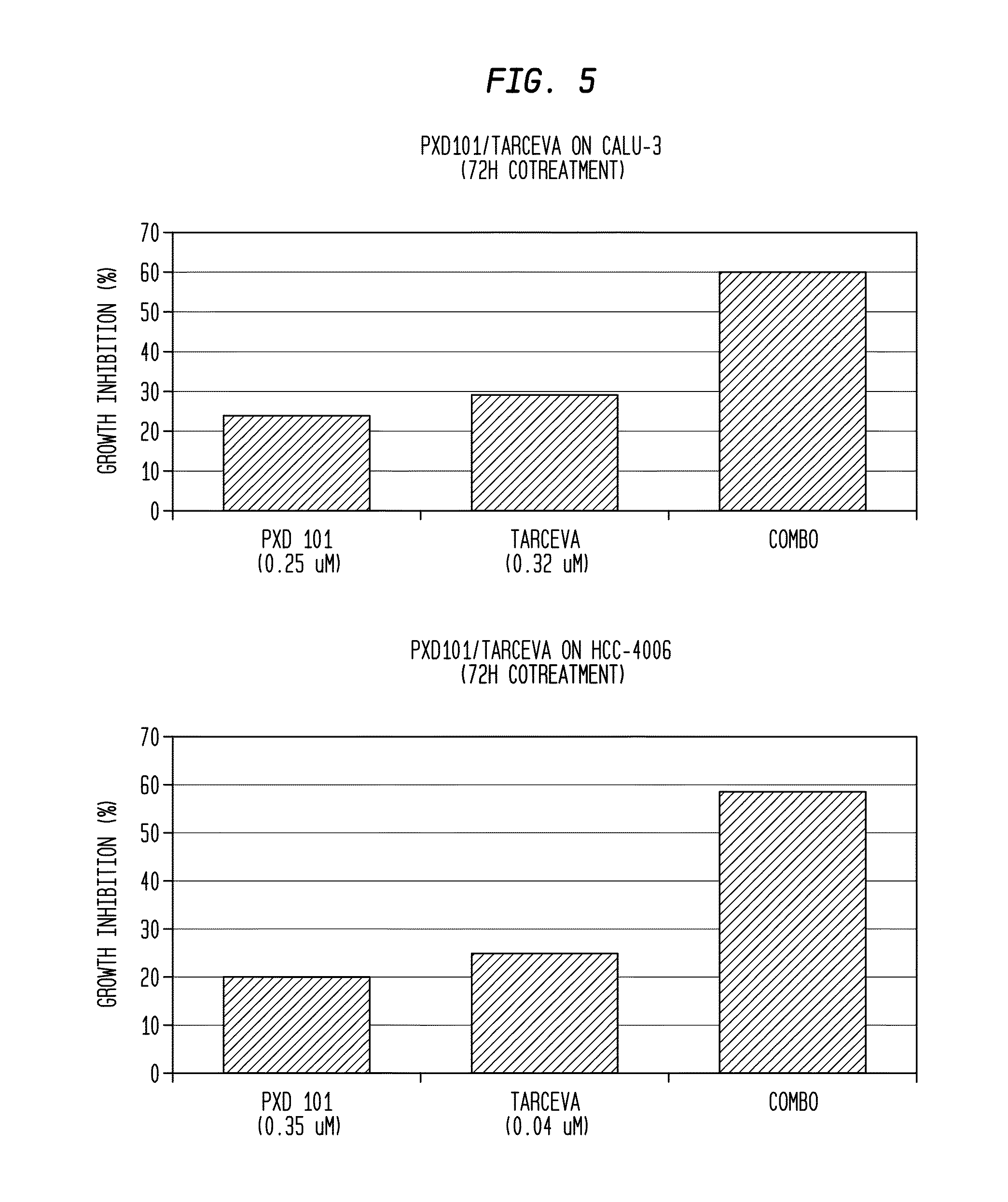
Combination therapies using HDAC inhibitors
ActiveUS20150231096A1Without increasing patient morbidityAchieve effectHeavy metal active ingredientsBiocideLung cancerCombination therapy
Owner:TOPOTARGET UK LTD
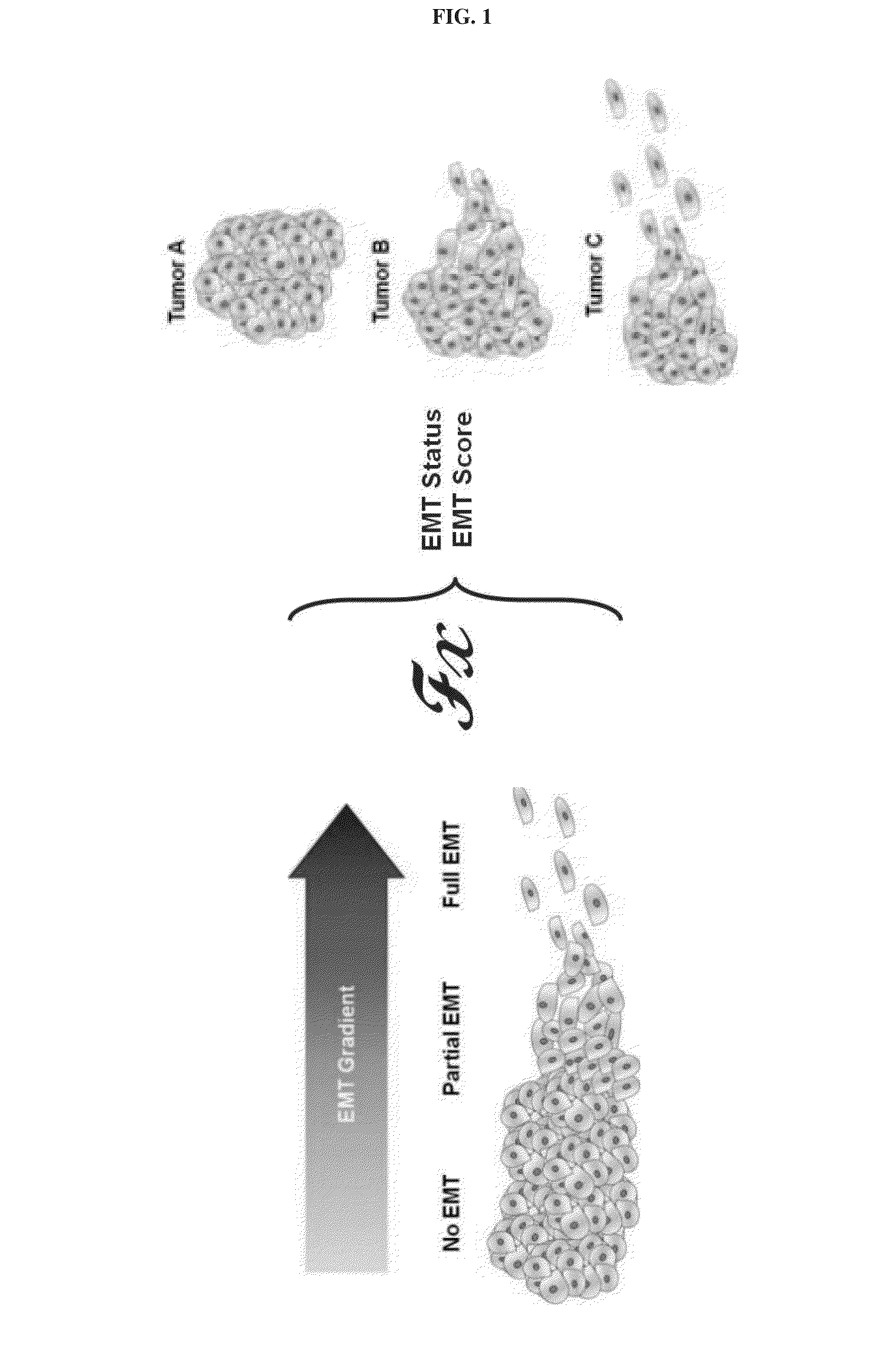
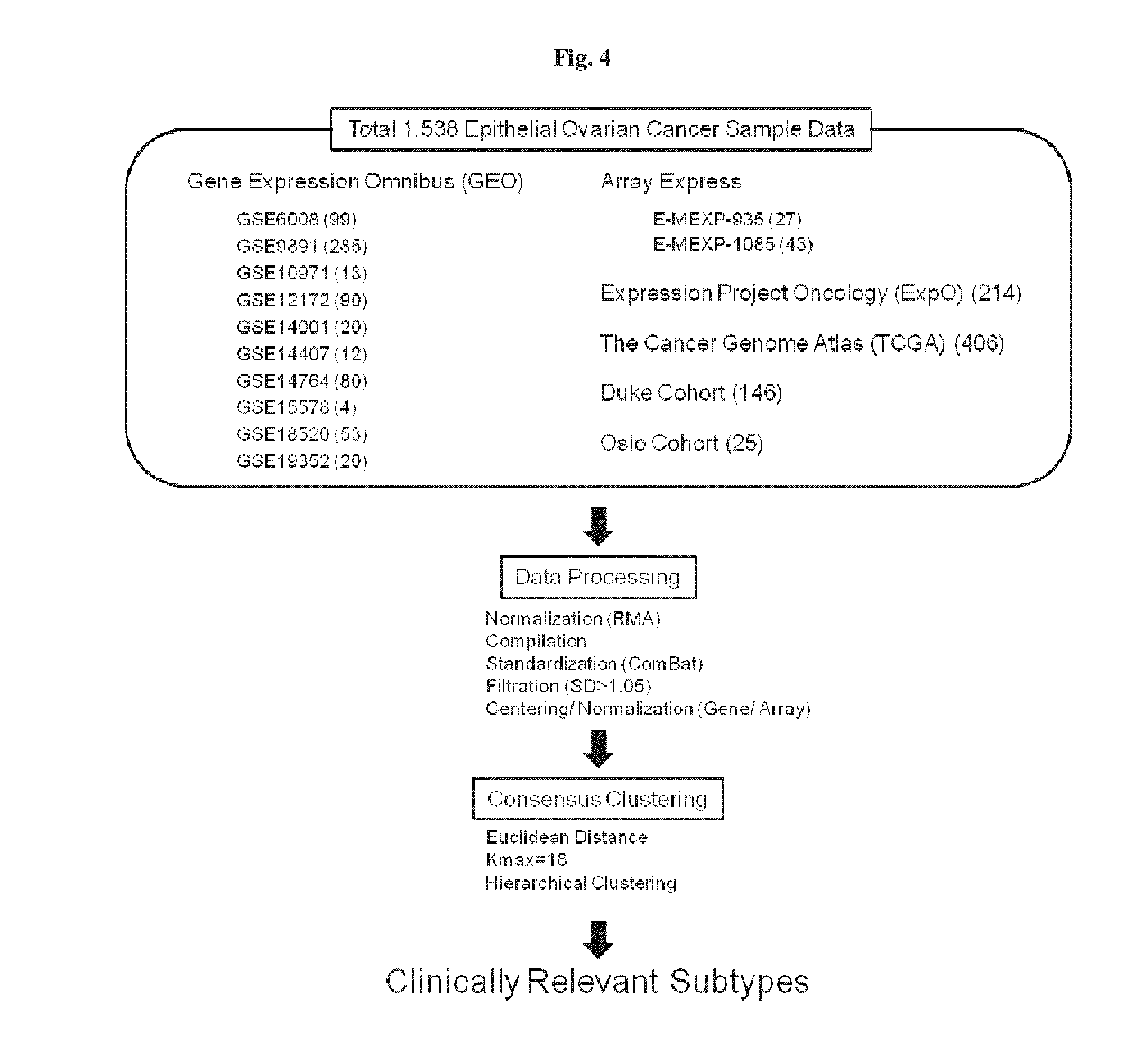
Patient stratification and determining clinical outcome for cancer patients
ActiveUS20140236495A1Increased susceptibilityMicrobiological testing/measurementProteomicsPatient stratificationOncology
In a first aspect the present invention is directed to a method of generating a scheme allowing classification of a cancer of an individual patient for estimating a clinical outcome for said patient. It also refers to a method of estimating a clinical outcome of a patient suffering from epithelial ovarian cancer (EOC). The present invention also refers to a method of determining whether the epithelial mesenchymal score of a patient suffering from a cancer can be changed by administering an EMT reversal agent to increase patients susceptibility for an anti-cancer treatment.
Owner:AGENCY FOR SCI TECH & RES +1
Epithelial ovarian cancer differentiation marker
InactiveUS20150293104A1Detected inexpensively and convenientlyReduce intrusionBiological material analysisBiological testingGlycanOncology
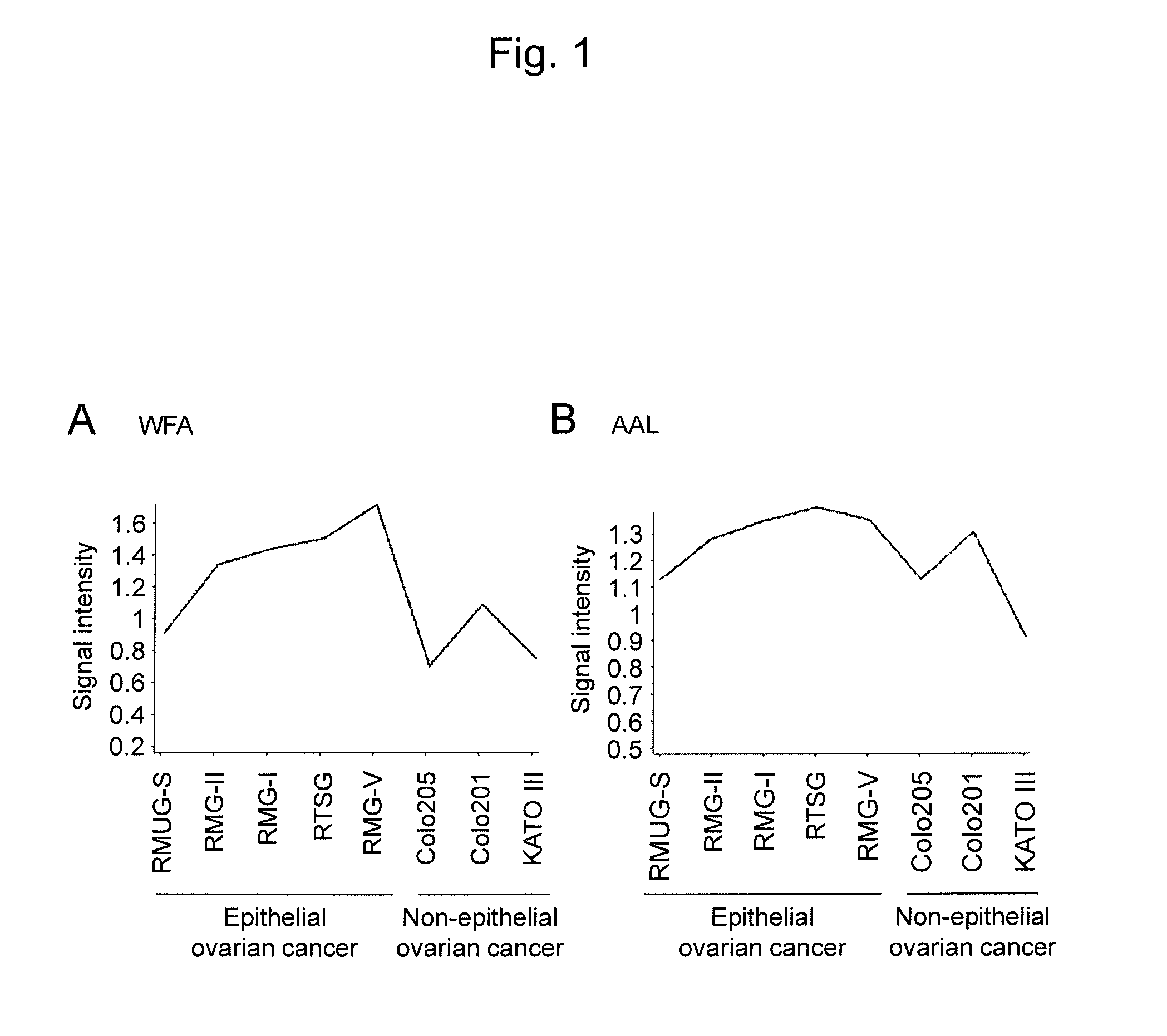
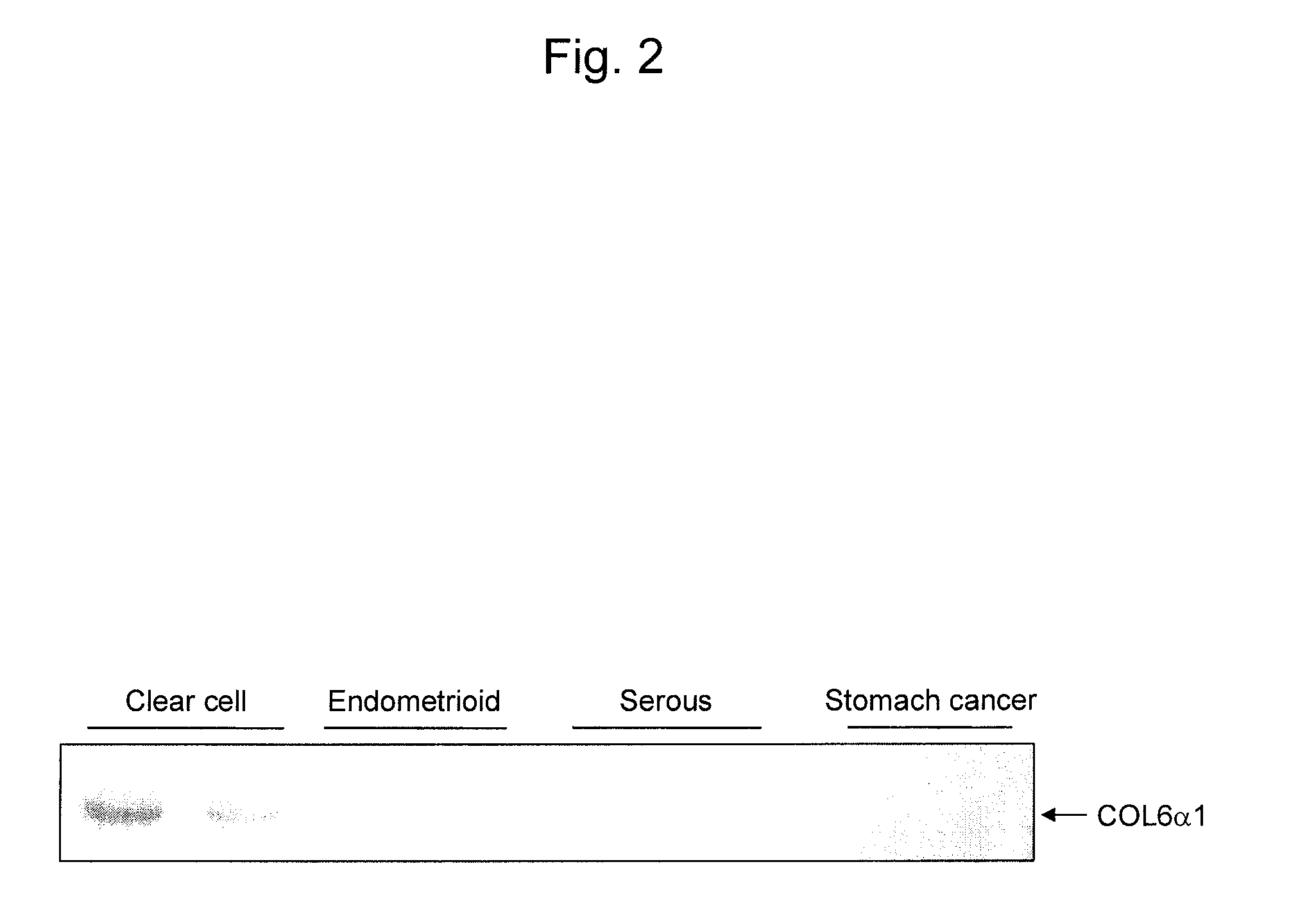
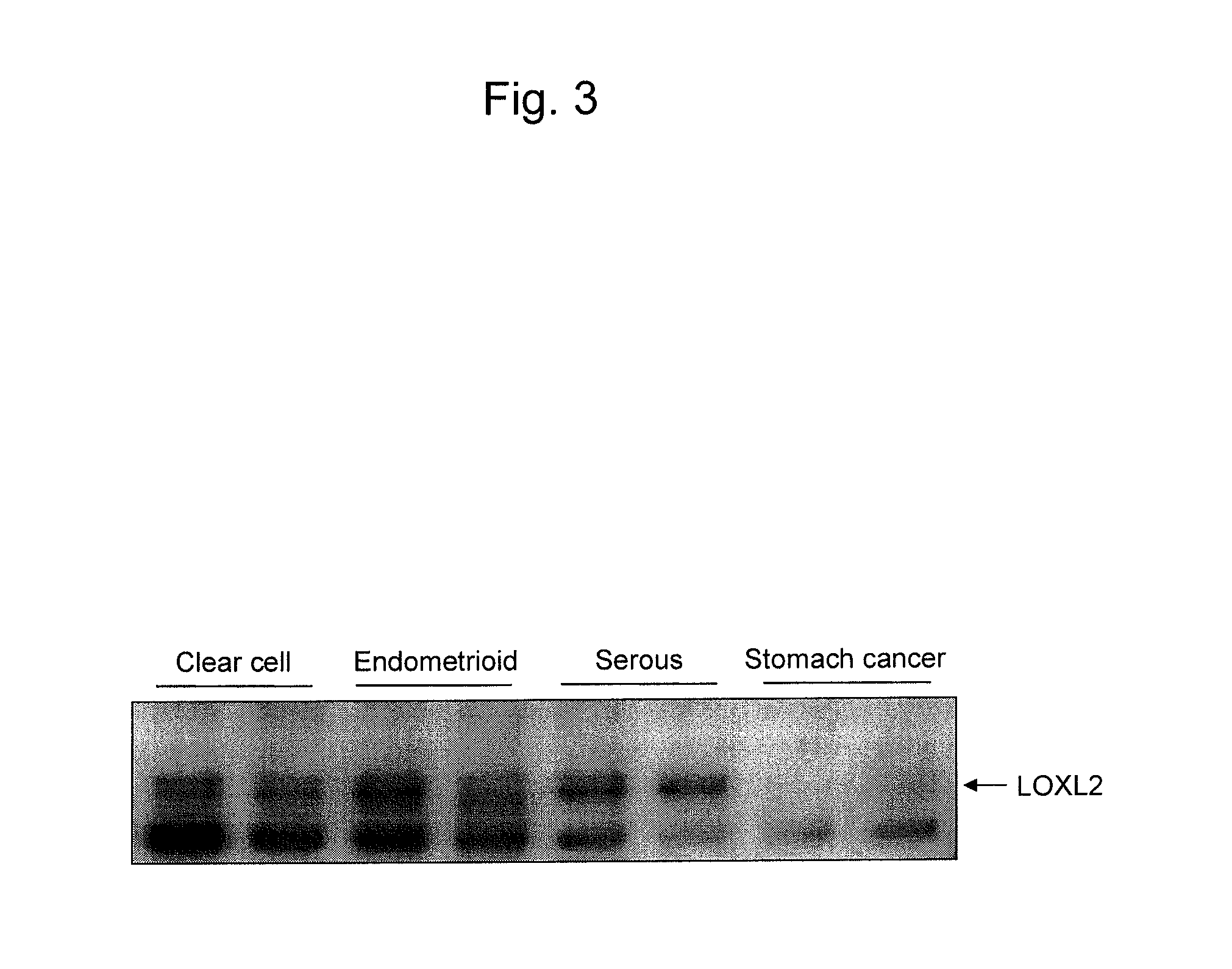
An object of the present invention is to develop and provide an epithelial ovarian cancer diagnosis marker with which epithelial ovarian cancer can be detected inexpensively, conveniently, and low invasively with high accuracy, and a method for determining the presence or absence of epithelial ovarian cancer using the marker. The present invention provides a glycoprotein having a glycan-linked asparagine residue at a particular site of the glycoprotein secreted from an epithelial ovarian cancer cell, or a fragment thereof having the glycan as an epithelial ovarian cancer diagnosis marker. The present invention also provides a method for determining the presence or absence of epithelial ovarian cancer using the glycoprotein.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Apoptosis-Based Evaluation Of Chemosensitivity In Cancer Patients
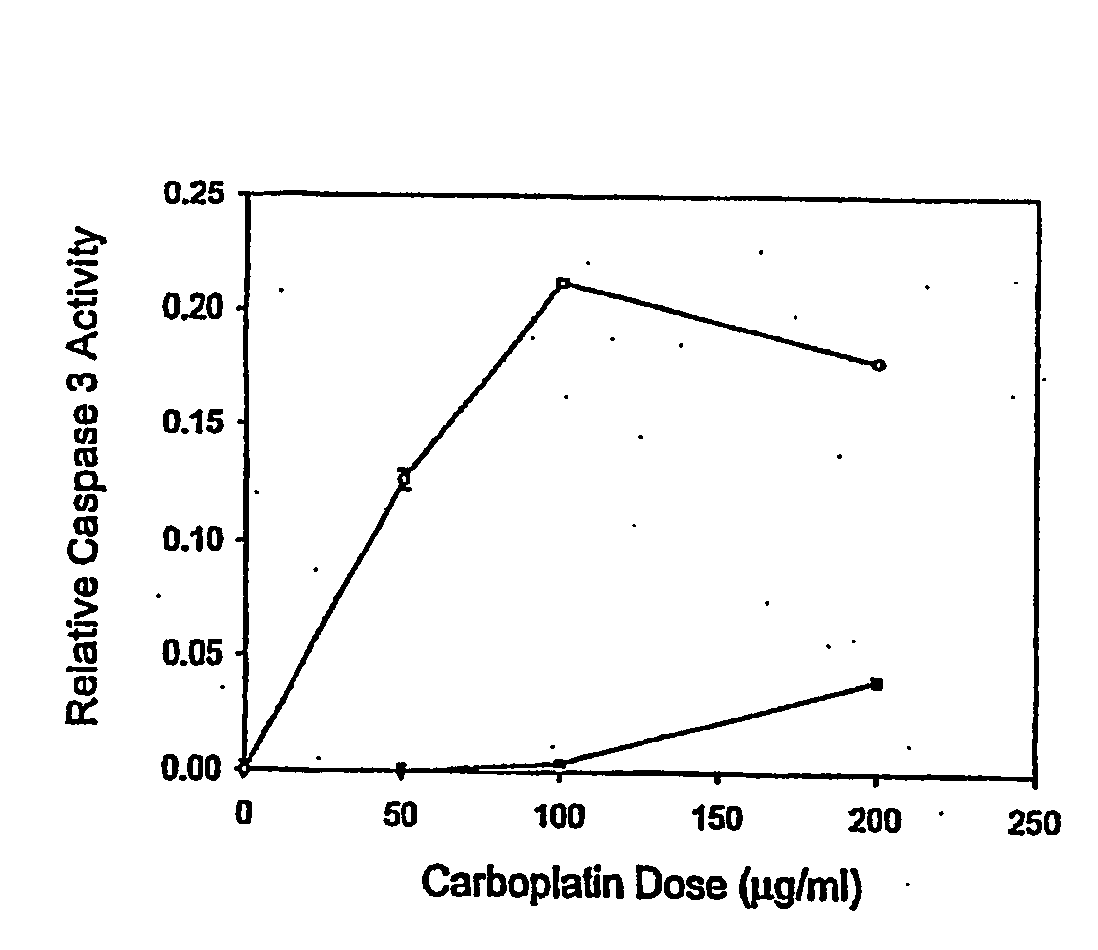
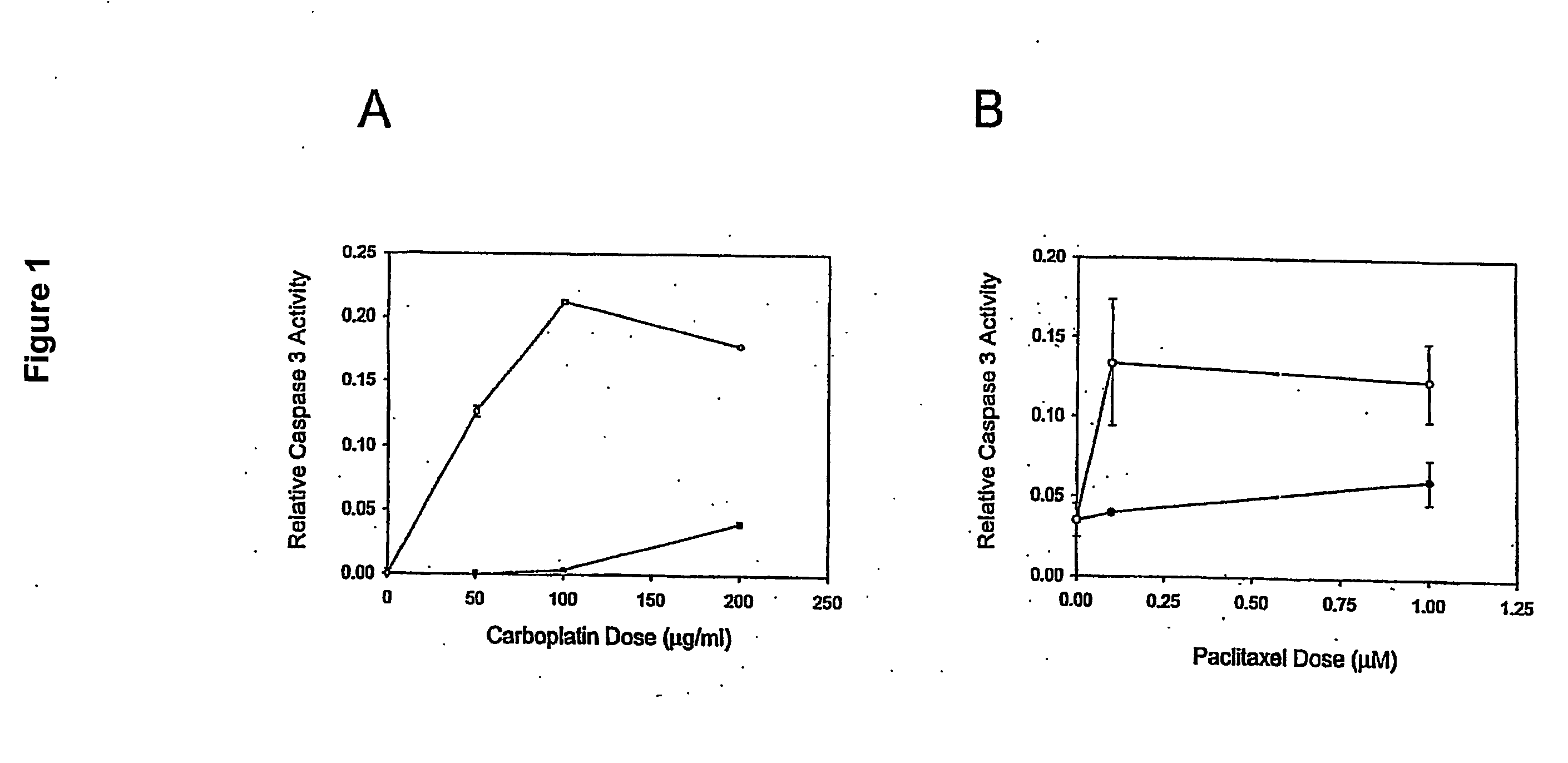
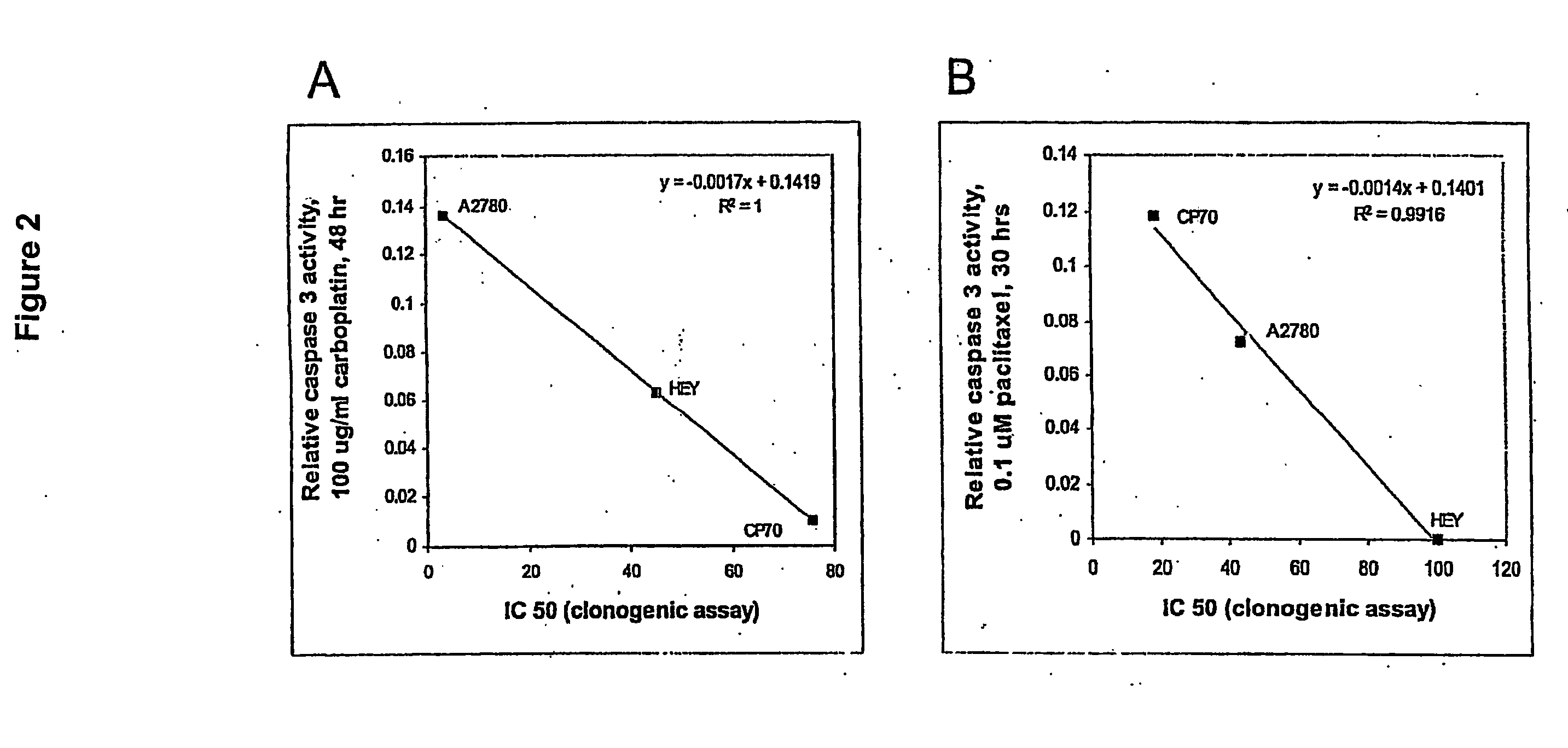
ActiveUS20070275434A1Microbiological testing/measurementPreparing sample for investigationCarboplatinPositive predicative value
Induction of apoptosis in target cells is a key mechanism by which chemotherapy induces cell killing. An in vitro system has been established for determining carboplatin and paclitaxel (Taxol) chemosensitivity of epithelial ovarian cancer cells, where measurements of caspase-3 activation are surrogate markers for activation of chemotherapy-induced programmed cell death. To validate the assay as a predictor of clinical chemotherapy-induced programmed cell death. To validate the assay as a predictor of clinical chemosensitivity in vitro apoptotic response were compared to the clinical response of the patients from whom the tumor cells were isolated. Caspase-3 activation in response to in vitro chemotherapy to both drugs was shown to have an 83% positive predictive value and a 71% negative predictive value. Markers of apoptosis such as caspase-3 activation can be quantitated and utilized to predict the clinical response to chemotherapy.
Owner:YALE UNIV
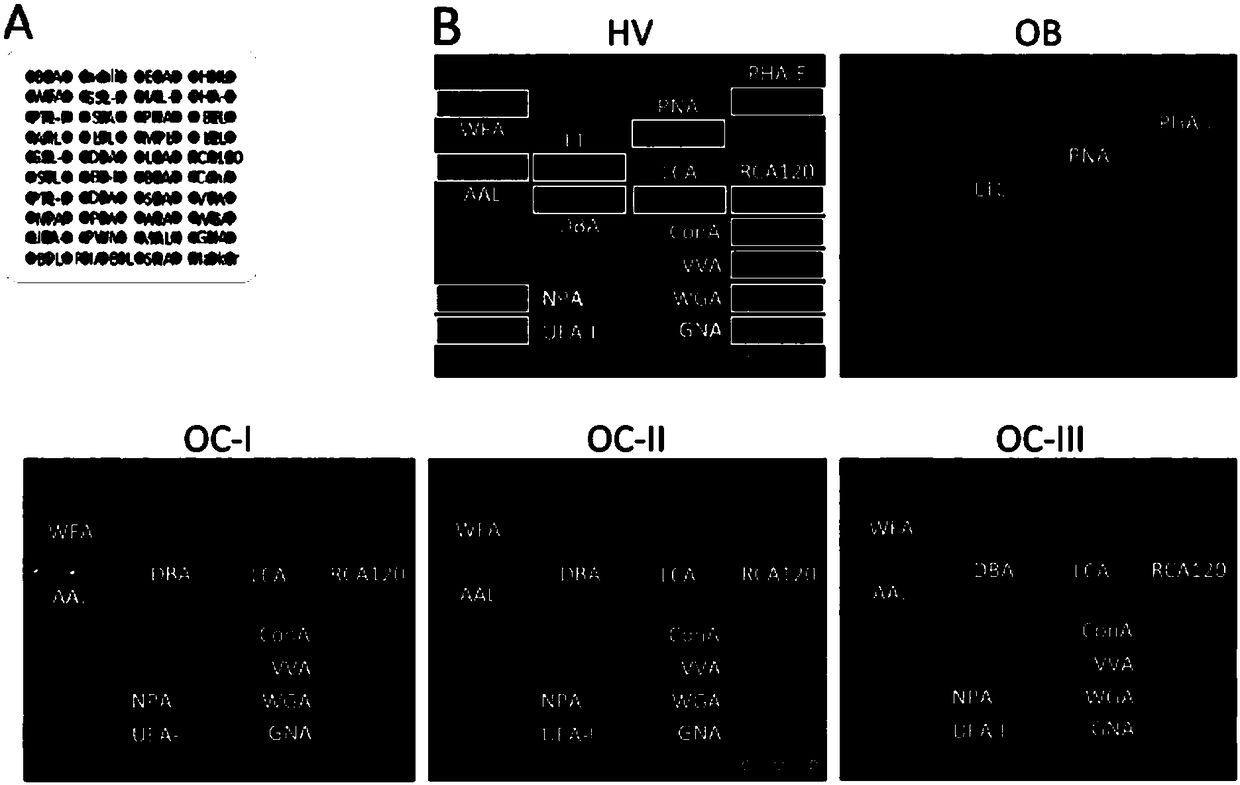
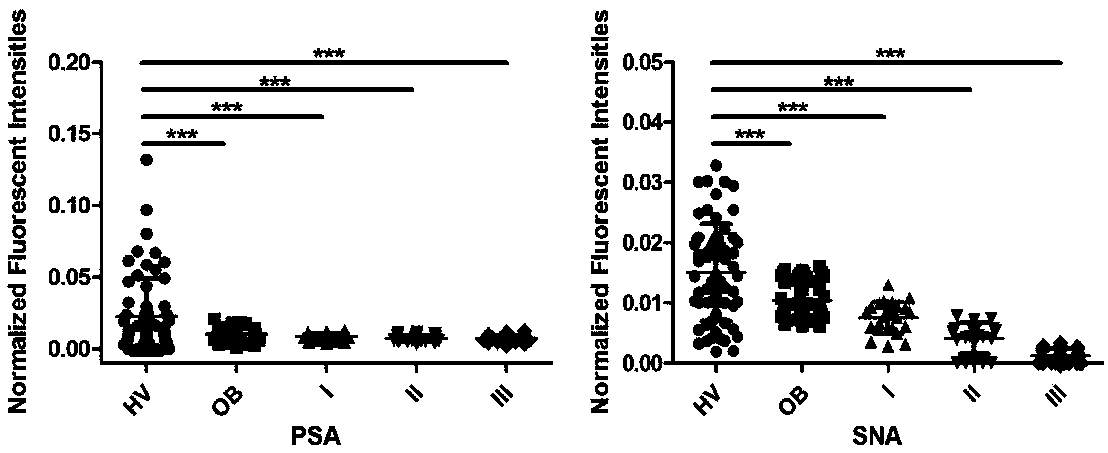
Application of specific lectin composition in preparing test carrier for identifying epithelial ovarian cancer, and kit
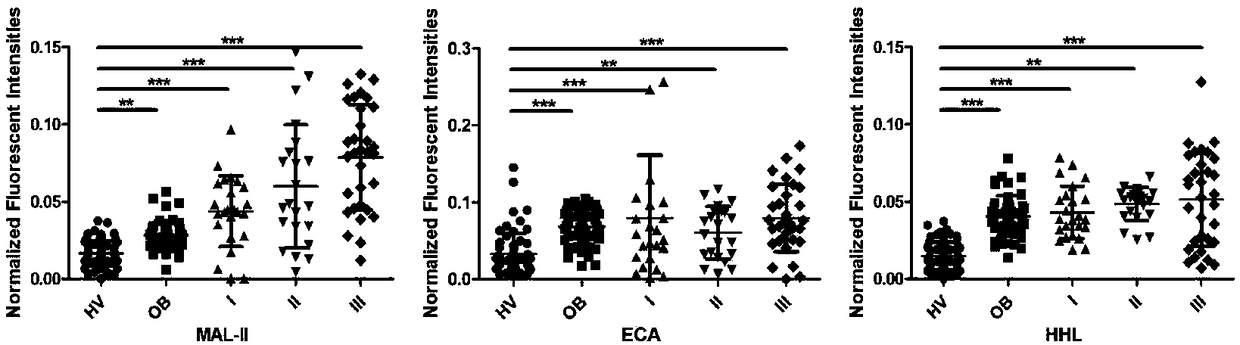
The invention provides application of a specific lectin composition in preparing a test carrier for identifying epithelial ovarian cancer, and a kit. Aiming at a saliva sample, the specific lectin composition comprises (1) a composition of PSA, SNA, MAL-II, ECA and HHL for a healthy crowd; (2) a composition of PHA-E, LTL and PNA for patients with benign ovarian diseases; (3) a composition of RCA120, UEA-I, ConA, LCA, AAL, WFA, WGA, DBA, VVA, NPA and GNA for patients with epithelial ovarian diseases. Through specific combining of lectin and glycoprotein carbohydrate chain in saliva, glycoprotein and a carbohydrate chain structure in differential expression in the saliva sample of a patient can be quickly detected, and multiple glycoprotein and carbohydrate chain structures in the sample canbe quickly detected in high flux.
Owner:深圳格道糖生物技术有限公司
Method for diagnosis and prognosis of epithelial cancers
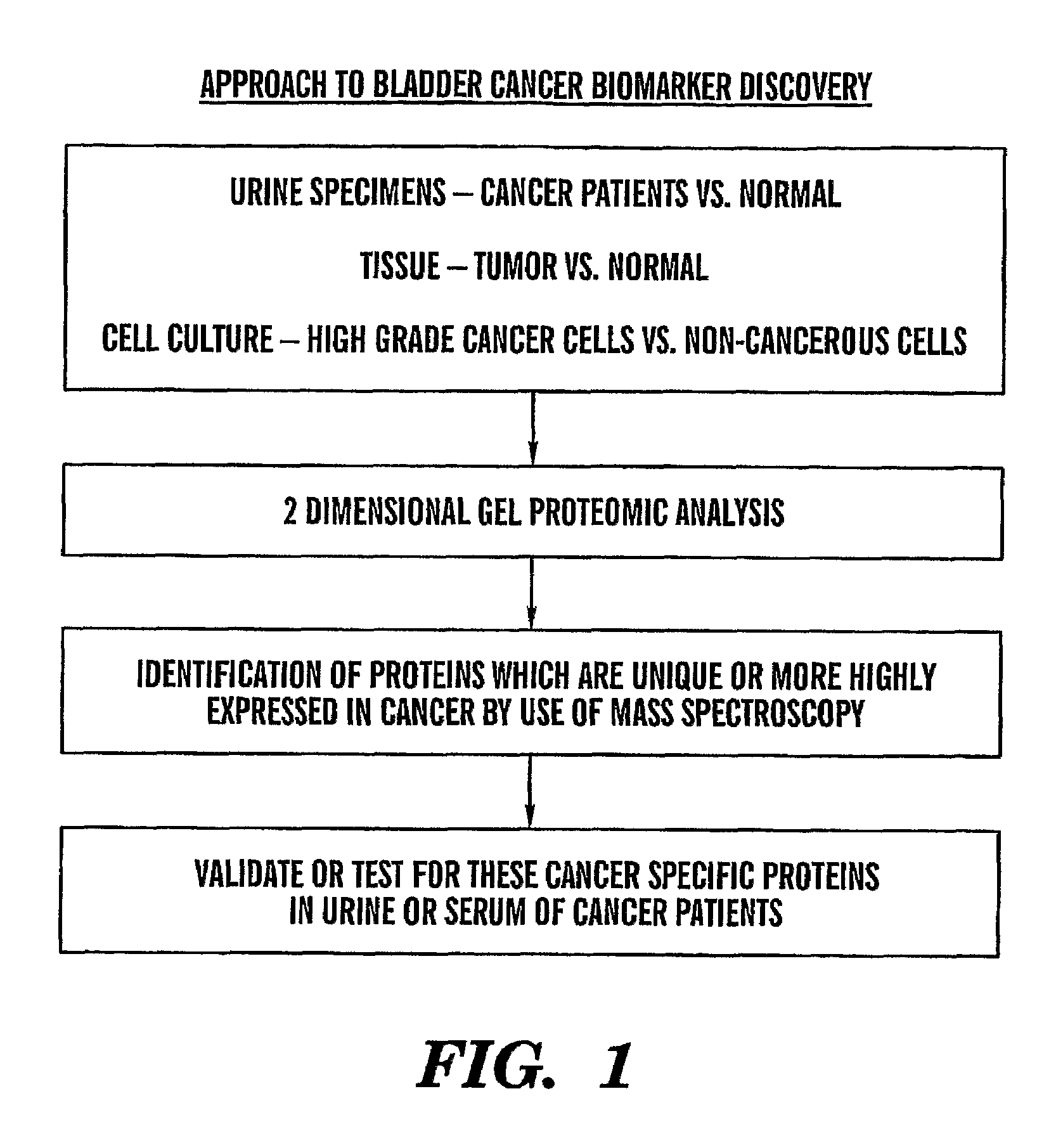
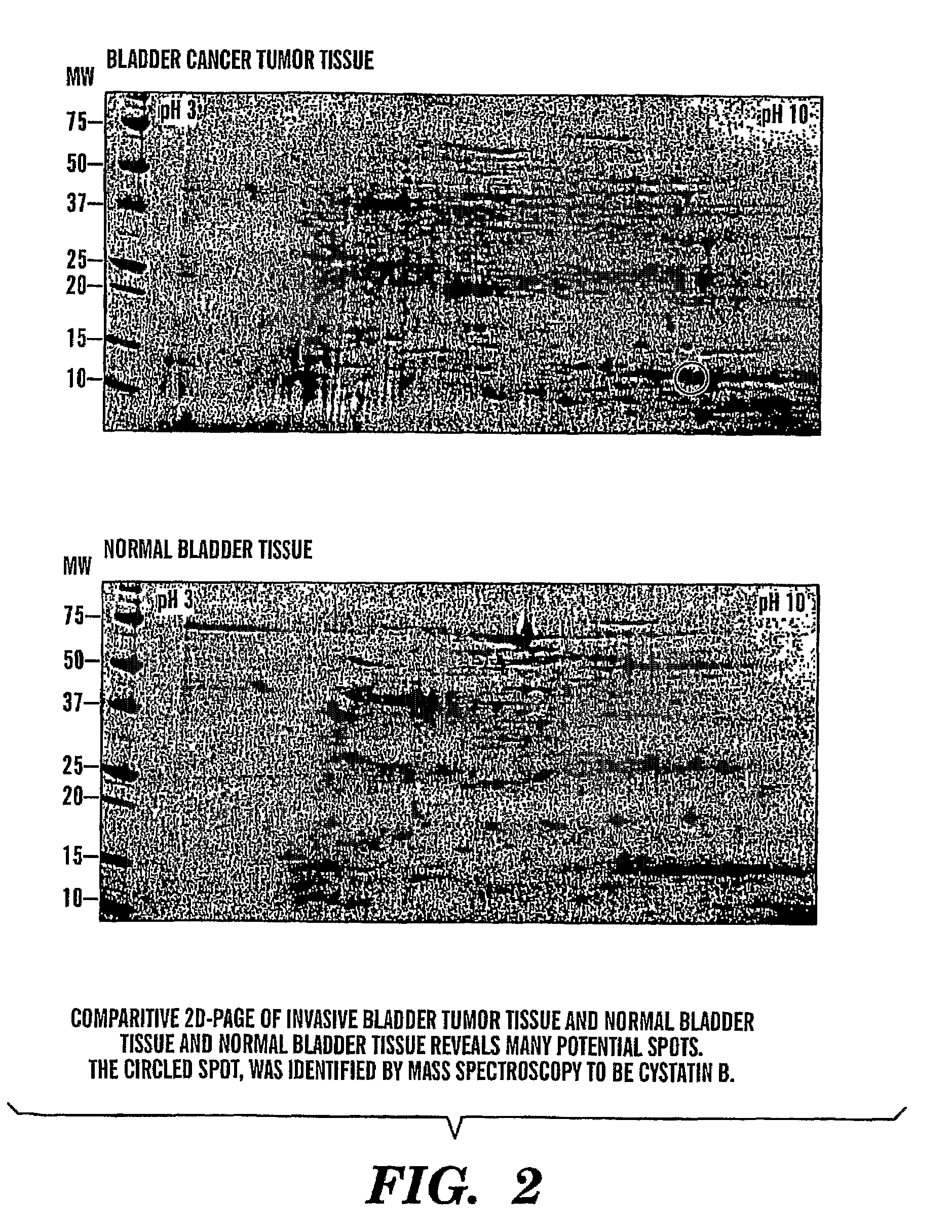
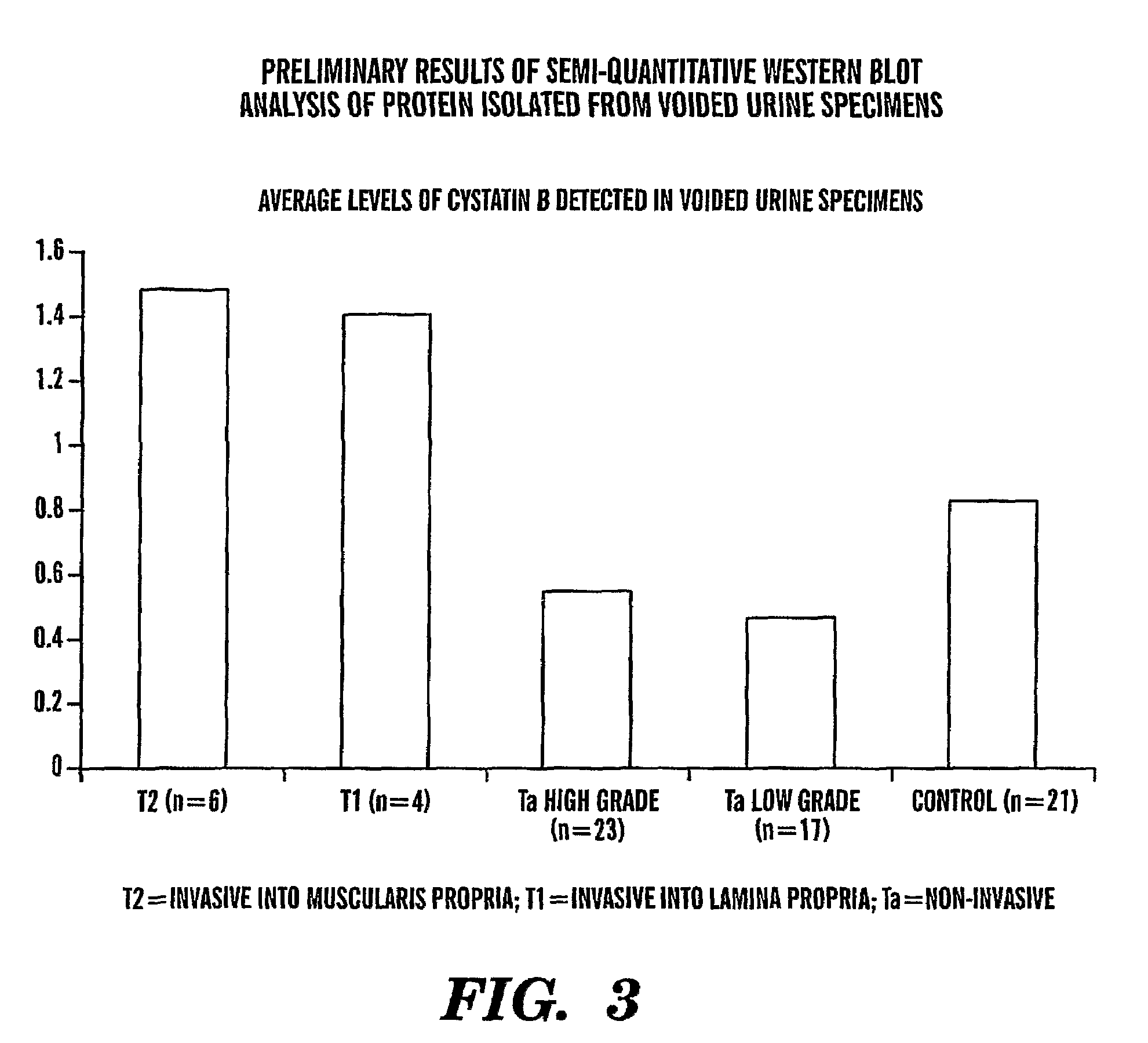
InactiveUS8685659B2Easy diagnosisQuick and easy and safeBiological material analysisBacteriuriaBladder cancer patient
The present invention is based on the discovery that three proteins, Cystatin B, Chaperonin 10, and Profilin are present in the urine of patients with bladder cancer, a cancer of epithelial origin. Accordingly, the present invention is directed to methods for prognostic evaluation of cancers of epithelial origin and to methods for facilitating diagnosis of cancers of epithelial origin by monitoring the presence of these markers in biological samples. The invention is also directed to markers for therapeutic efficacy.
Owner:THE GENERAL HOSPITAL CORP +1
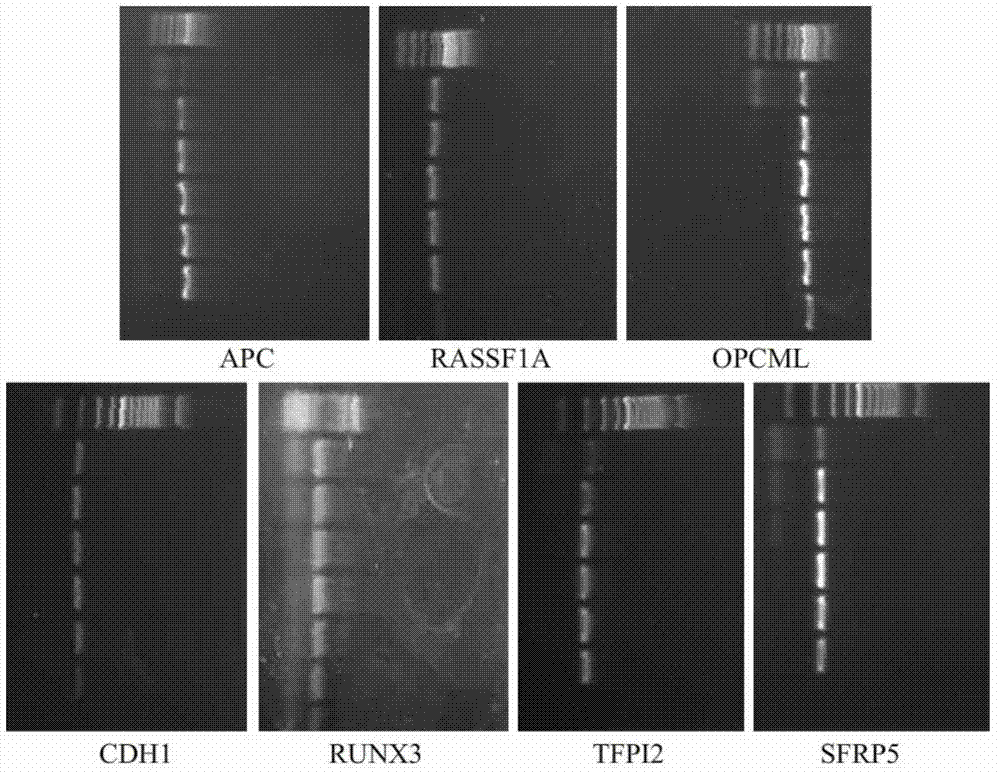
Multiplex nested methylation specific PCR (Polymerase Chain Reaction) detection kit, using method and application thereof
InactiveCN102732637AAvoid formingGuaranteed specificityMicrobiological testing/measurementSerum freeNucleotide
The invention discloses a multiplex nested methylation specific PCR (Polymerase Chain Reaction) detection kit which comprises the following substances: a primer, of which the nucleotide sequence is shown as SEQ ID NO.1-42, and a PCR working solution. A using method for the multiplex nested methylation specific PCR detection kit comprises the steps of obtaining serum of a patient to extract DNA; (2) obtaining a DNA solution to carry out methylation beautification; (3) carrying out PCR detection by using the kit; and (4) judging the result, that is, taking an obtained product, carrying out agarose gel electrophoresis detection, putting gel in a gel imaging system for shooting and analysis, and judging the reaction result by naked eyes. The defects of low sensitivity and low specificity of serum free DNA trace and single methylation markers are overcome to the greatest extent by the multiplex nested methylation specific PCR (MN-MSP) detection technology disclosed by the invention, the methylation states of seven target genes are efficiently detected, the multiplex nested methylation specific PCR detection kit has the advantages of strong specificity, high accuracy and the like and can be used as an effective diagnosis and detection means for early epithelial ovarian cancers.
Owner:SHANDONG UNIV QILU HOSPITAL
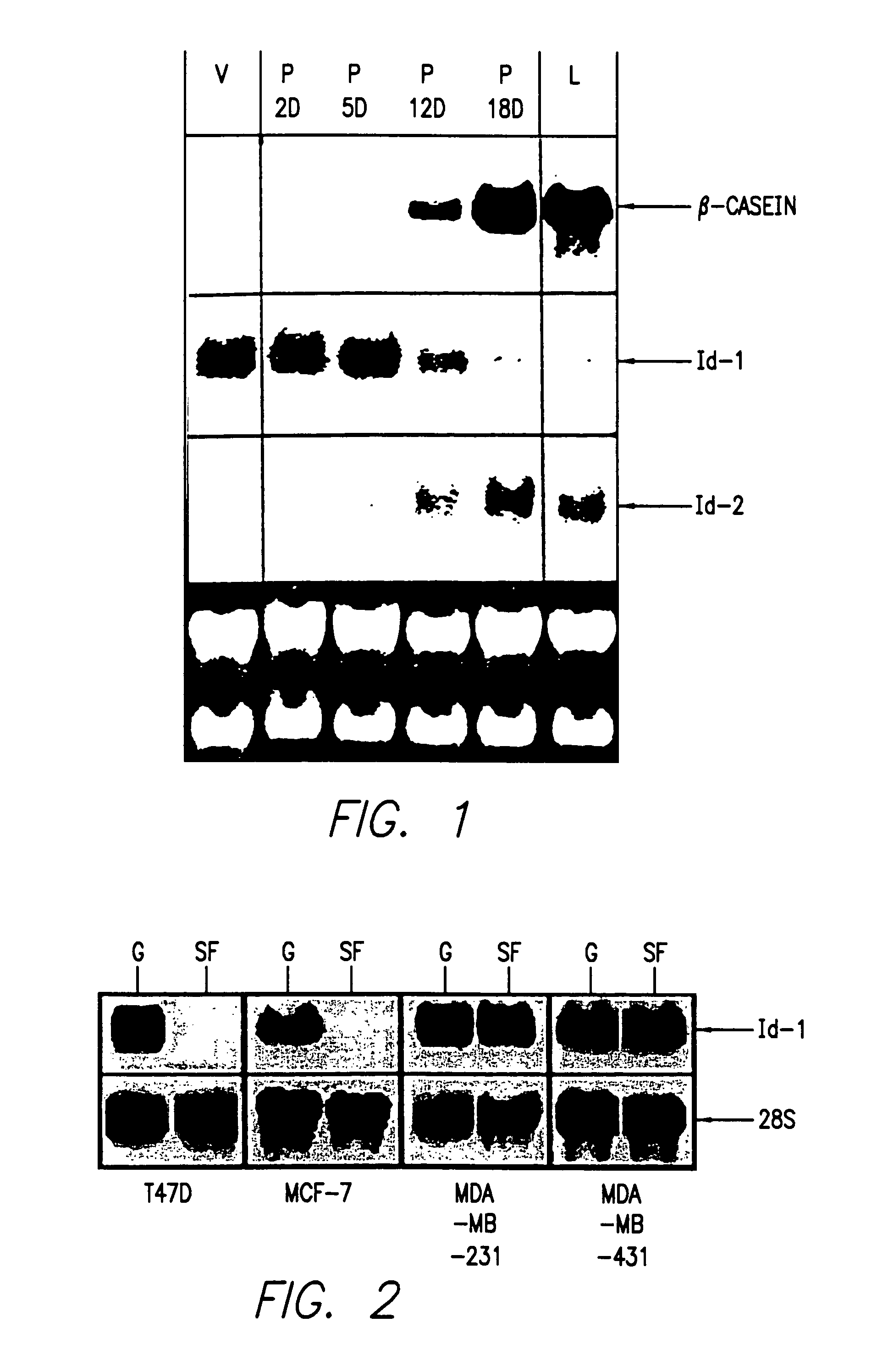
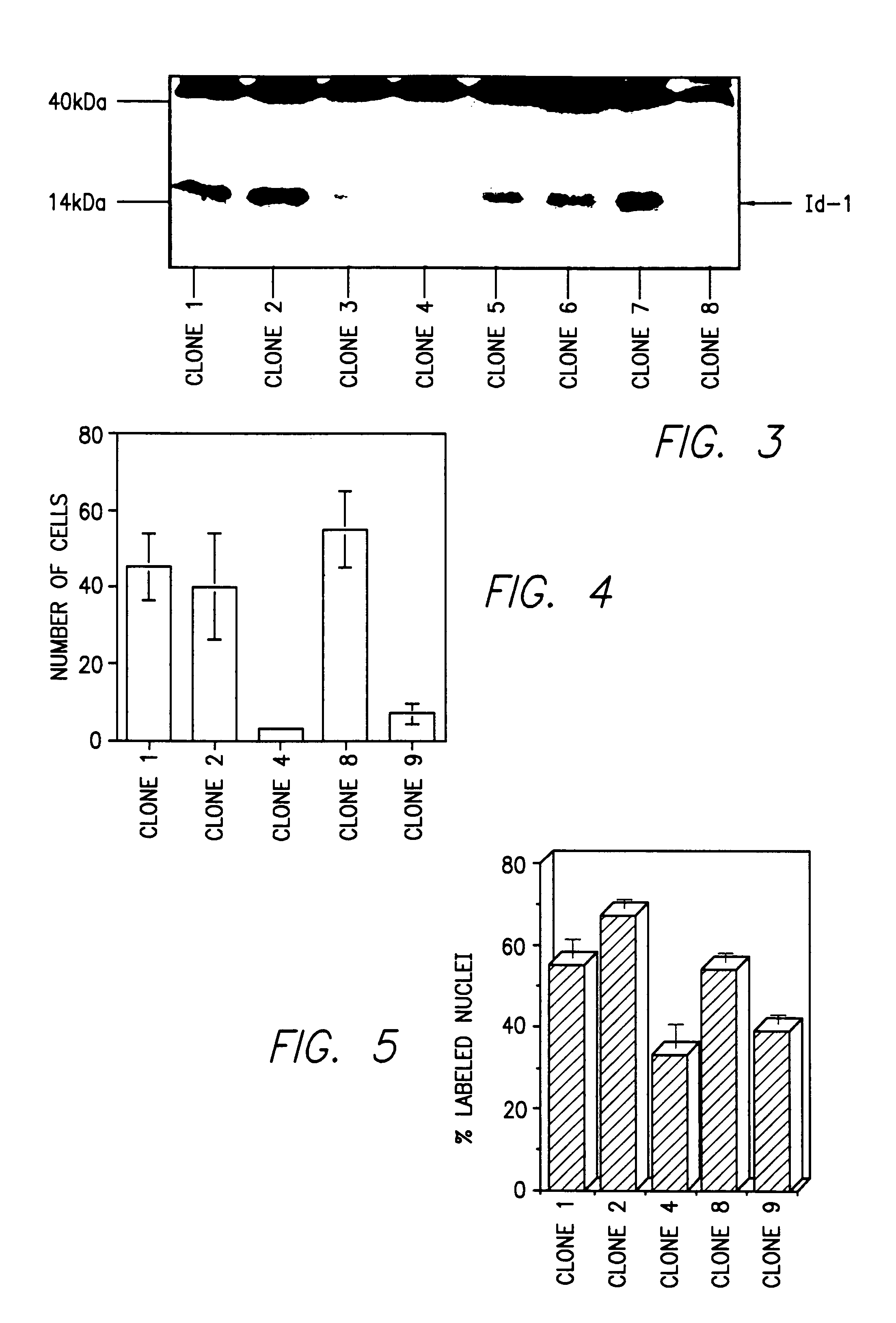
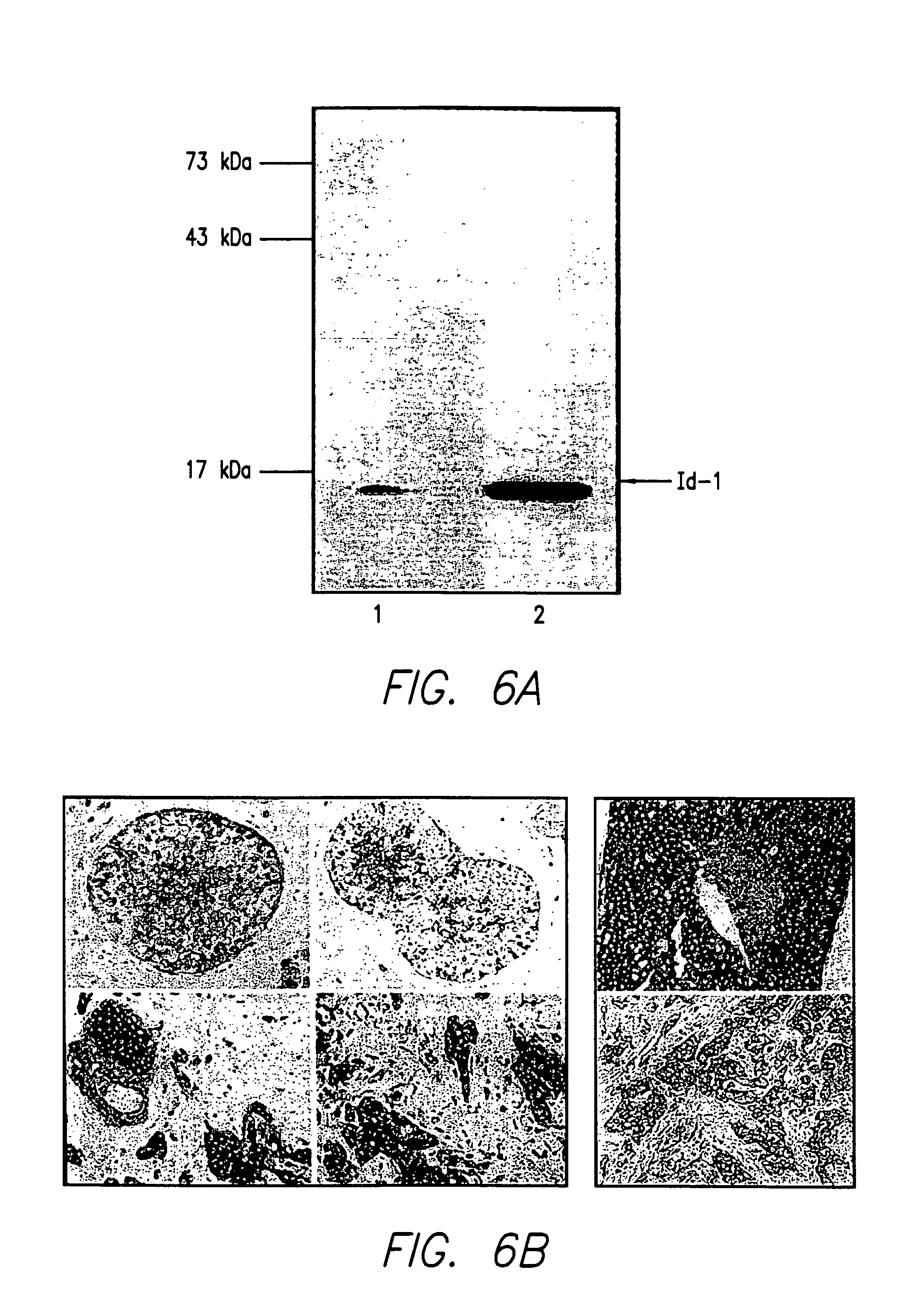
Id-1 and Id-2 genes and products as markers of epithelial cancer
A method for detection and prognosis of breast cancer and other types of cancer. The method comprises detecting expression, if any, for both an Id-1 and an Id-2 genes, or the ratio thereof, of gene products in samples of breast tissue obtained from a patient. When expressed, Id-1 gene is a prognostic indicator that breast cancer cells are invasive and metastatic, whereas Id-2 gene is a prognostic indicator that breast cancer cells are localized and noninvasive in the breast tissue.
Owner:RGT UNIV OF CALIFORNIA +1
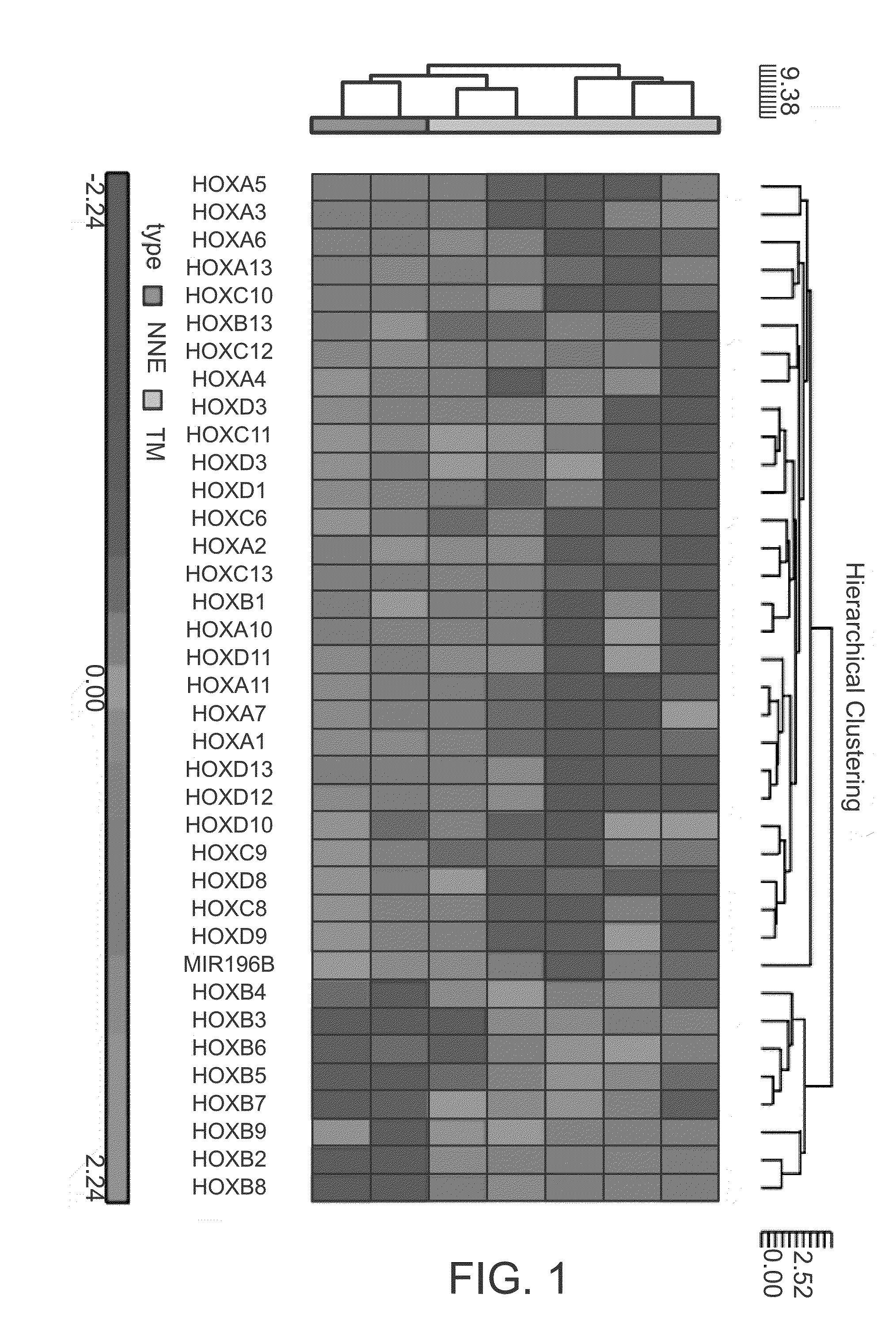
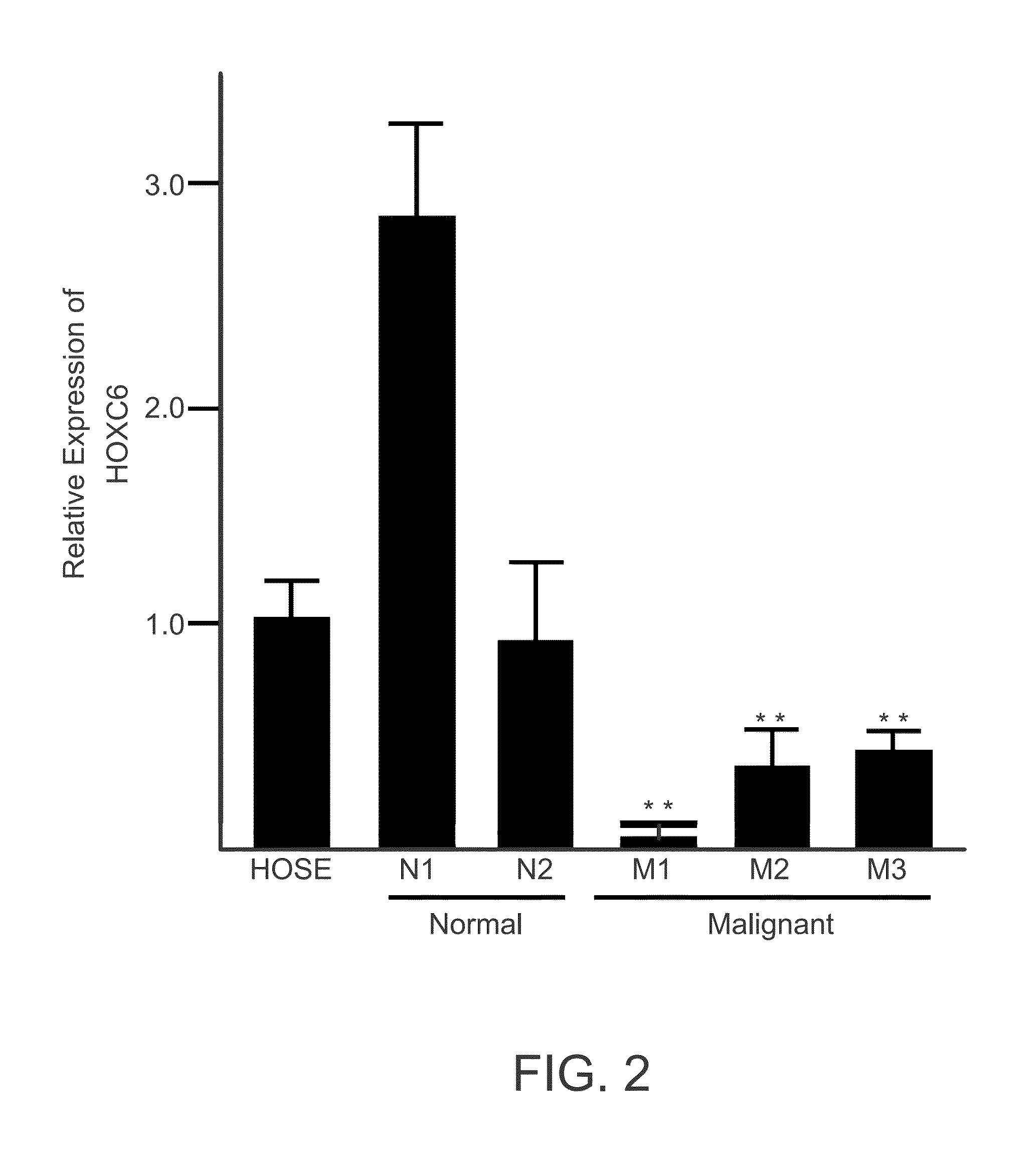
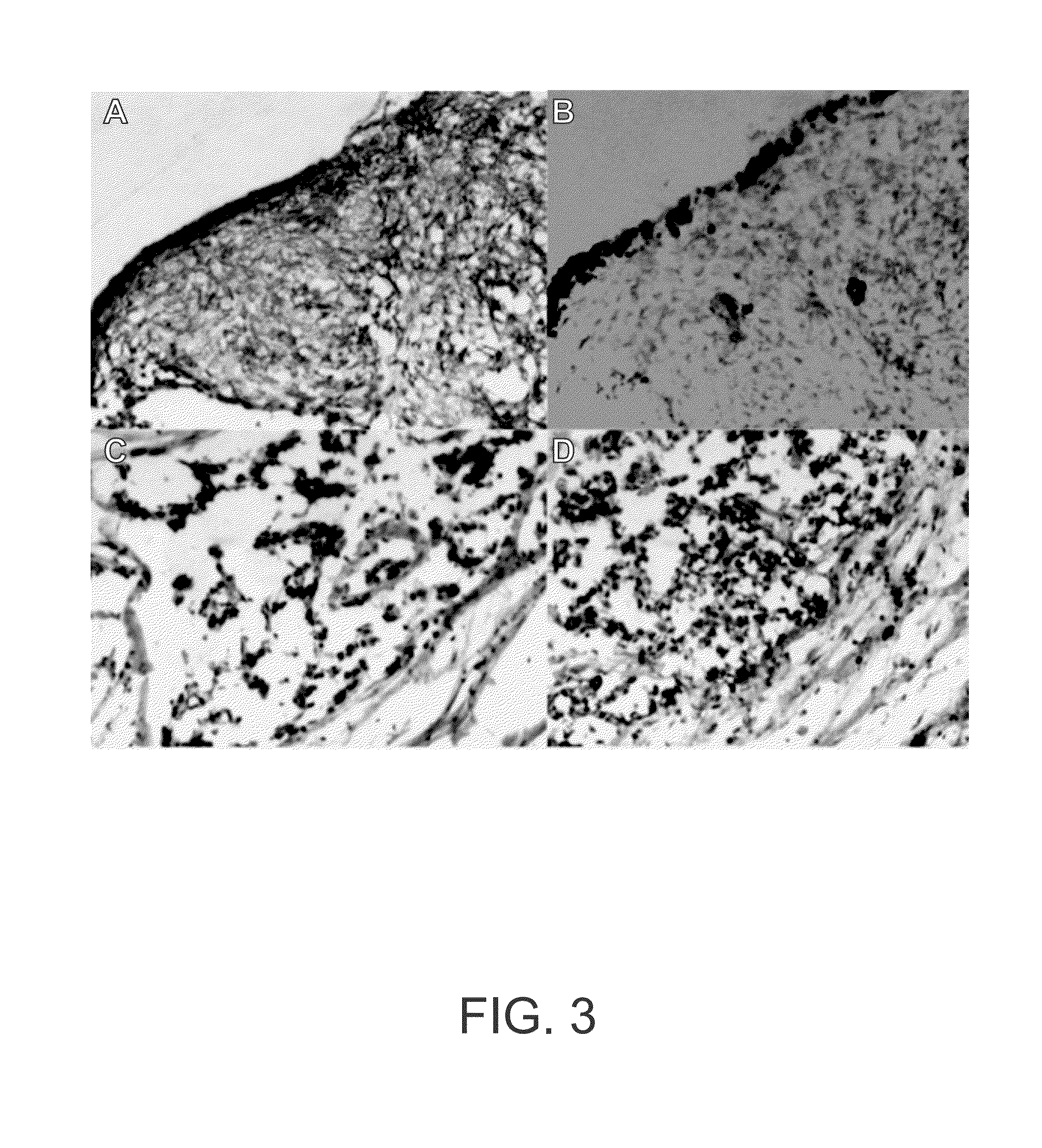
Hoxc6 and ovarian cancer methods and uses thereof
InactiveUS20150191798A1Microbiological testing/measurementLibrary screeningElisa kitTranscriptional expression
The present invention comprises novel methods, systems, devices, and kits to detect cancer in a patient using HOXC6. Methods and systems are presented herein to (1) determine differential gene expression in cancer tissue (e.g. human epithelial ovarian cancer) using Exon microarray analysis and confirm select gens using qPCR; (2) to correlate transcriptional expression from part 1 with potential protein using IHC; and (3) to confirm specific proteins in sera by ELISA process. In some embodiments, a ELISA kit is provided. More specifically, the inventors developed a cancer screen test based on significant changes in HOXC6 protein in blood serum of ovarian cancer human subjects. Industry available standard protocols and reagents for the detection of HOXC6 in patient blood serum provided highly variable results that would be unsatisfactory for clinical diagnostic testing and screening. Thus the inventors developed and optimized a protocol for indirect sandwich ELISA to detect the HOXC6 protein in blood serum suitable for clinical use.
Owner:CHARLOTTE MECKLENBURG HOSPITAL AUTHORITY +1
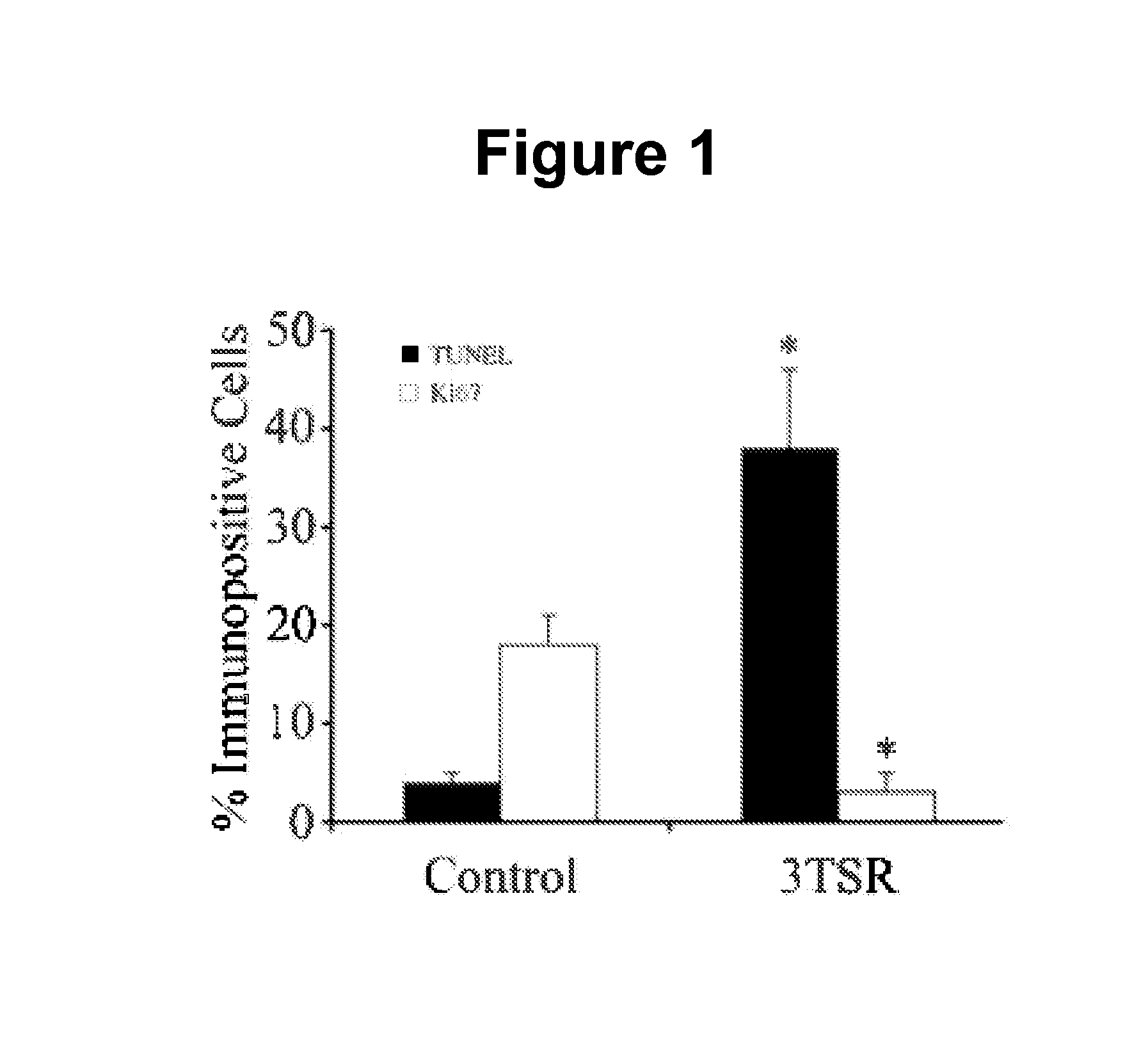
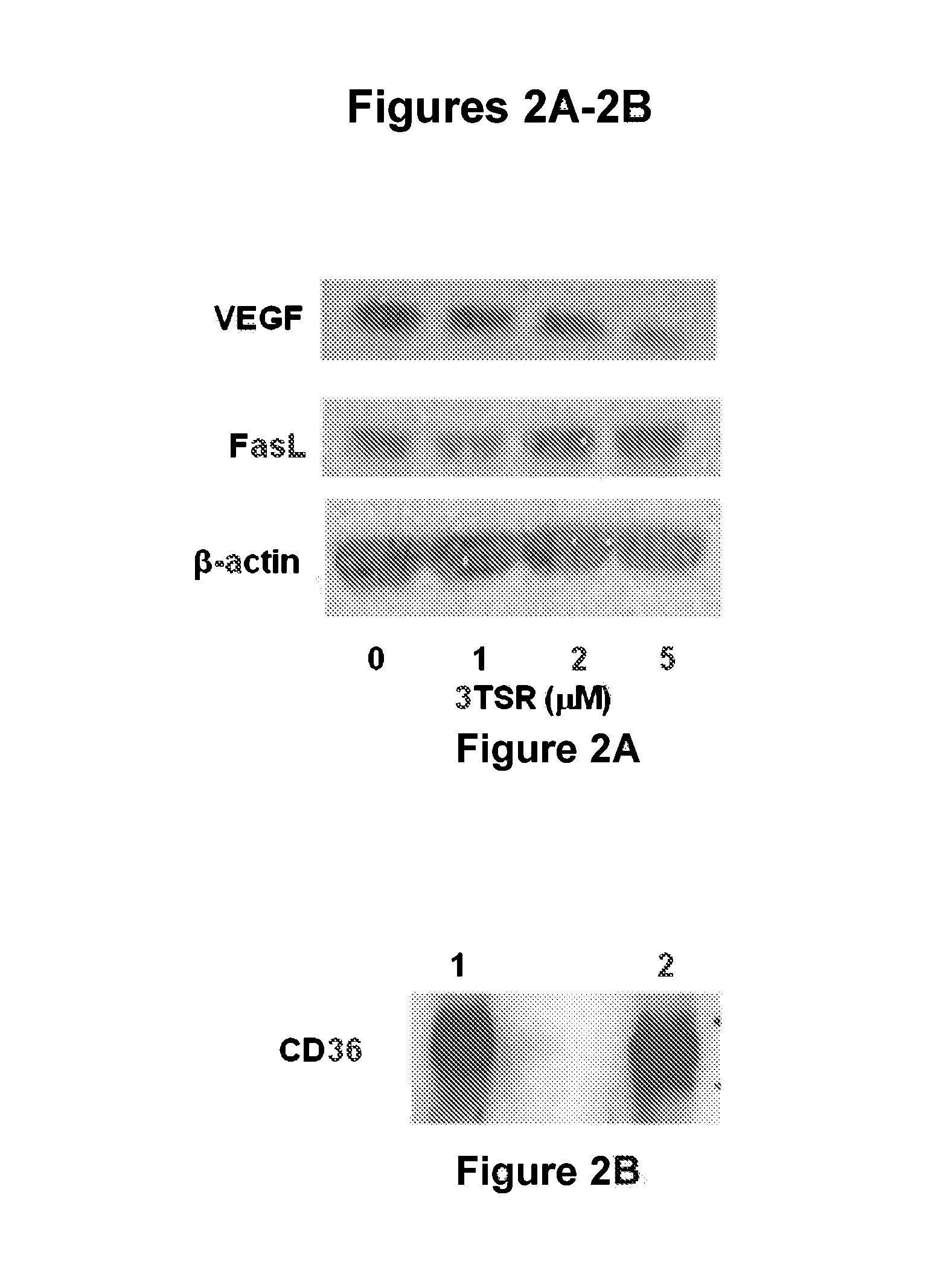
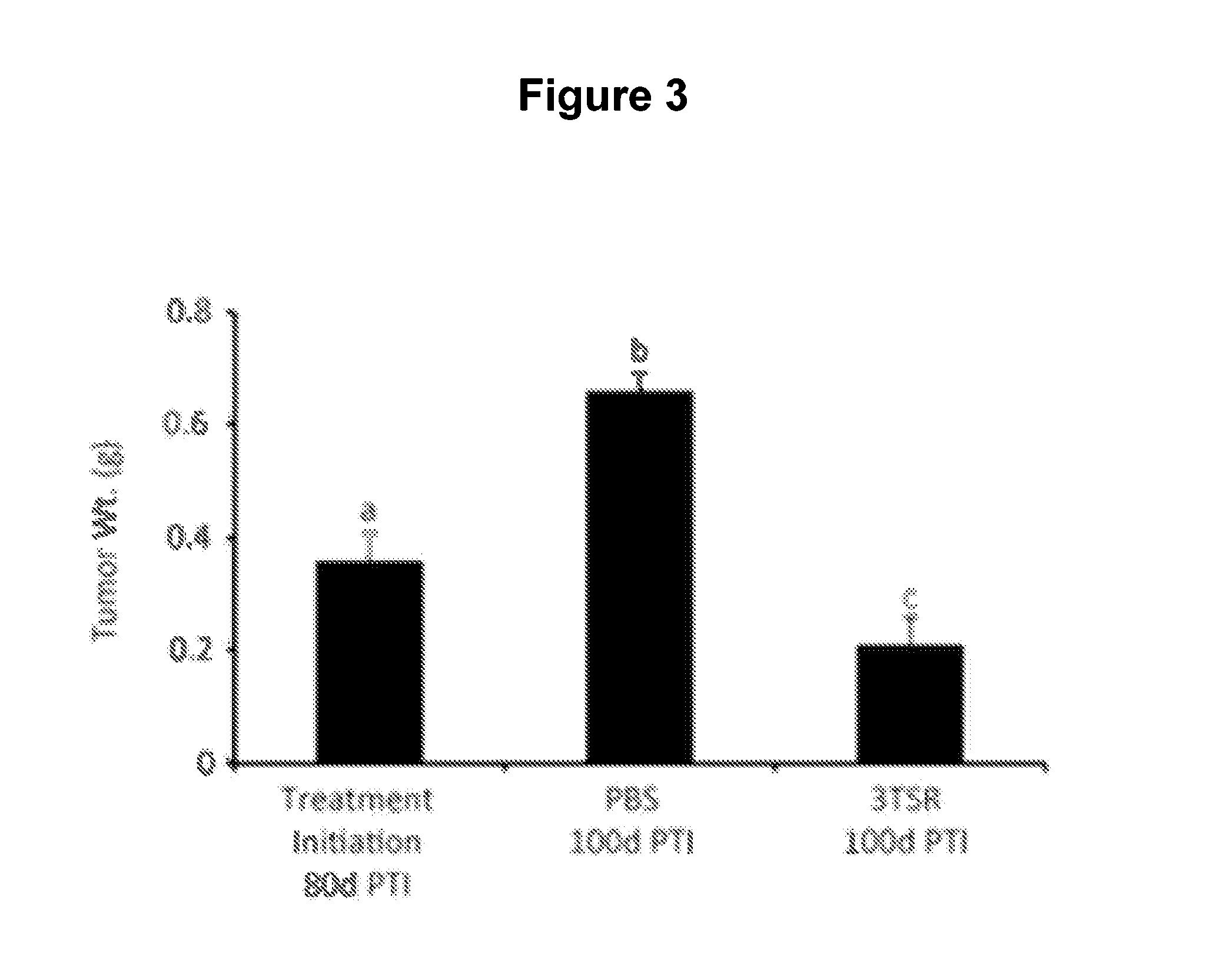
Thrombospondin-1 polypeptides and methods of using same
The invention features thrombospondin-1 (TSP-1) polypeptides (e.g., 3TSR-Fc fusion proteins), nucleic acid molecules encoding the TSP-1 polypeptides, and compositions thereof. The invention also features methods of making and using the TSP-1 polypeptides of the invention (e.g., using 3TSR-Fc fusion proteins to treat a subject having a disorder associated with pathological angiogenesis, e.g., cancer, e.g., epithelial ovarian cancer (EOC)).
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Methods of treating ovarian cancer
Disclosed are methods of treating ovarian cancer (e.g., epithelial ovarian cancer) using lonafarnib, a taxane (e.g., paclitaxel or docetaxel) and a platinum coordinator complex (e.g., carboplatin, cisplatin or oxaliplatin). Also disclosed are methods of treating ovarian cancer (e.g. epithelial ovarian cancer) using lonafarnib, paclitaxel and carboplatin. Also disclosed are methods of treating ovarian cancer using lonafarnib in combination with a liposomal doxorubicin.
Owner:SCHERING CORP
Biological marker of epithelial ovarian cancer and application of biological marker
InactiveCN108085392AMicrobiological testing/measurementBiomarker (petroleum)Epithelial ovarian cancer
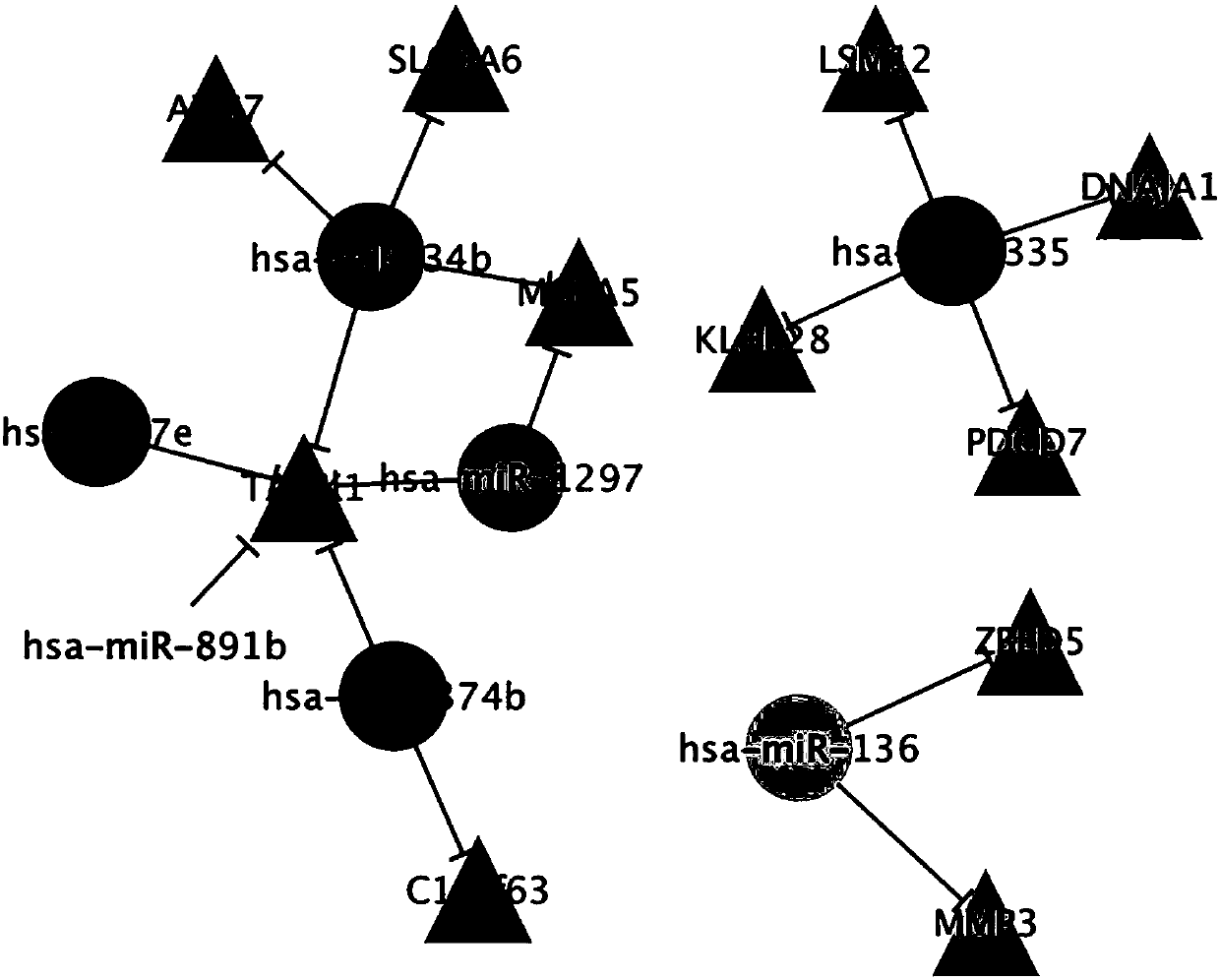
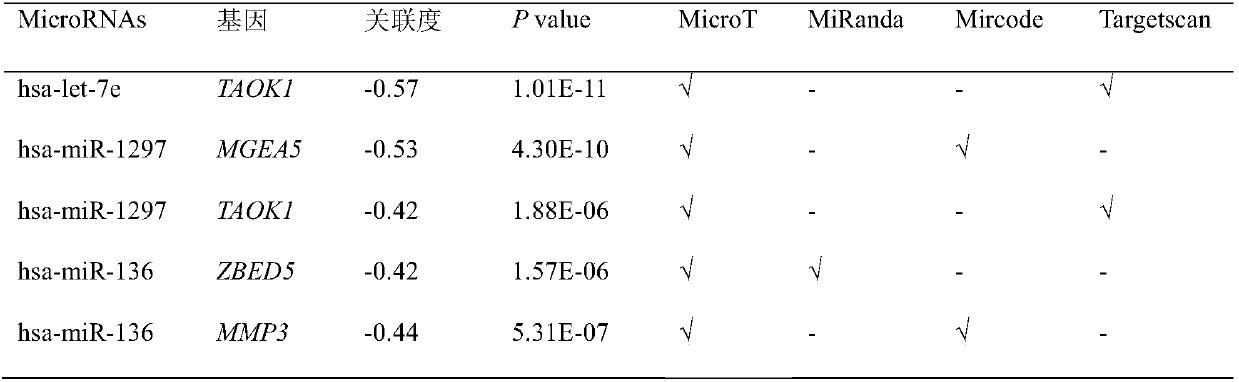
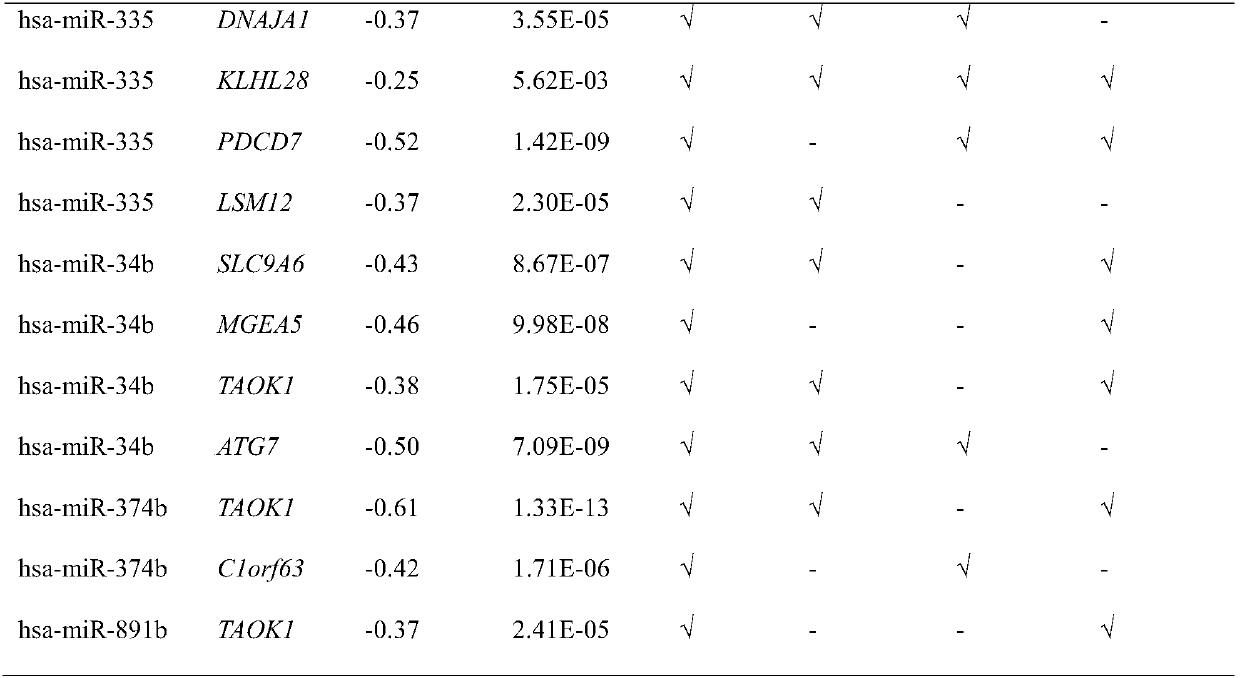
The invention provides a biological marker of epithelial ovarian cancer and application of the biological marker. The invention provides a method for researching and detecting the epithelial ovarian cancer by the combination of the following miRNA: hsa-miR-34b, hsa-miR-335, hsa-miR-136, hsa-let-7e, hsa-miR-1297, hsa-miR-374b and hsa-miR-891b. The invention also provides a kit and a device for themethod for researching and detecting the epithelial ovarian cancer.
Owner:SDIVF R&D CENT LTD
Novel chemoimmunotherapy for epithelial cancer
ActiveUS20180264004A1Inhibit bindingReduce in quantityOrganic active ingredientsDipeptide ingredientsChemoimmunotherapyOncology
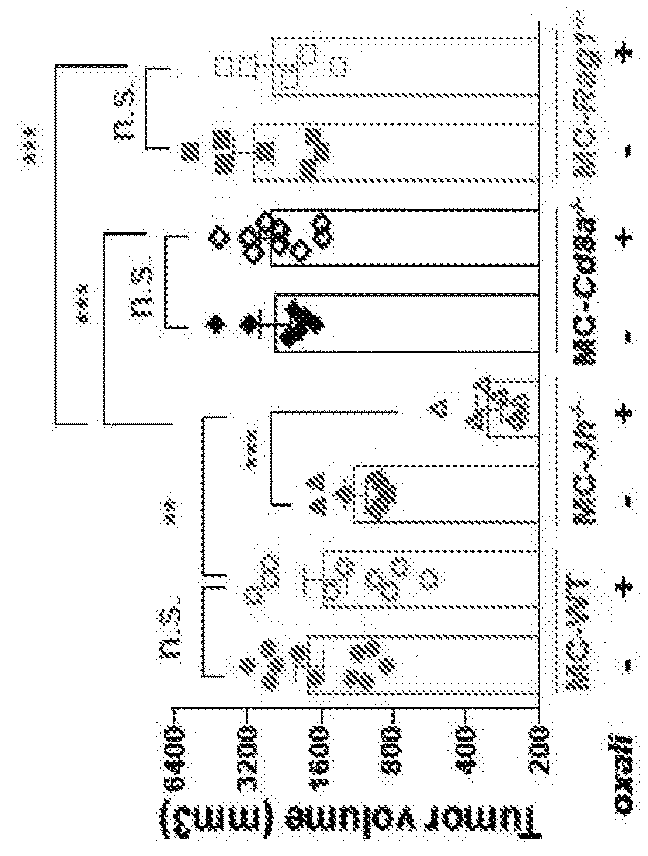
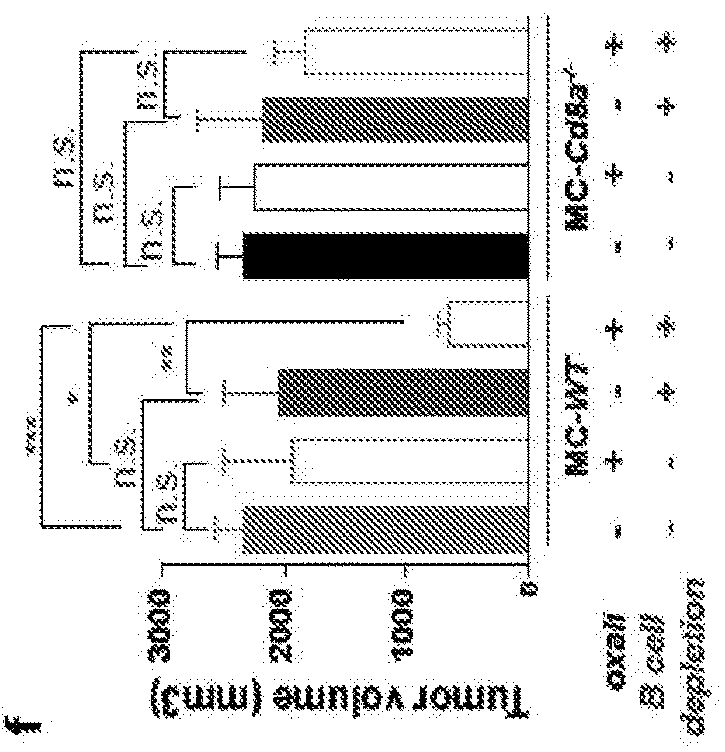
The invention provides a method for treating cancer in a subject in need thereof, wherein said subject comprises cancer tissue that ‘contains epithelial cancer cells and immunosuppressive 8 cells, and wherein said method comprises administering to said subject a therapeutically effective amount of a) one or more first composition that pauses—immunogenic eel’ death and / or of said epithelial cancer cells, and b) one or more second composition that reduces one or both of the number and function of said immunosuppressive B cells in said cancer.
Owner:RGT UNIV OF CALIFORNIA
Prevention of ovarian cancer by administration of a vitamin D compound
InactiveUS20070010500A1Increase ratingsEfficient removalOrganic active ingredientsBiocideApoptosisRectal epithelium
The present invention relates to methods for preventing the development of epithelial ovarian cancer by administering a Vitamin D compound in an amount capable of increasing apoptosis in non-neoplastic ovarian epithelial cells of the female subject.
Owner:RODRIGUEZ GUSTAVO C +1
Methods for diagnosis and/or prognosis of gynecological cancer
InactiveUS20150024956A1Sugar derivativesMicrobiological testing/measurementDisease progressionMolecular biomarkers
Ovarian, cervical cancer, endometriosis, clear cell renal carcinoma cancers are very heterogeneous diseases which lack robust diagnostic, prognostic and predictive clinical biomarkers. Conventional clinical biomarkers (stages, grades, tumor mass etc) and molecular biomarkers (CA125, KRAS, p53 etc) are not appropriate for early diagnostics, differential diagnostics, prediction and prognosis of the disease outcome for individual patients. The most common type of the human ovarian cancers is human epithelial ovarian cancer (EOC). This cancer is characterized with one of the lowest survival rates compared to other cancers. The present invention relates to an in vitro method for diagnosing epithelial ovarian cancer, cervical cancer, endometriosis, dear cell renal carcinoma and / or predisposition to epithelial ovarian cancer in a subject, the method comprising determining in a sample of the subject gene expression level of at least one gene in the MDS1 and EVI1 complex (MECOM) locus; and / or copy number of at least one gene in the MECOM locus; wherein the level against at least one expression cutoff value and / or copy number against at least one copy number cutoff value are indicative of the subject having epithelial ovarian cancer, cervical cancer, endometriosis, clear cell renal carcinoma and / or a predisposition to epithelial ovarian cancer, cervical cancer, endometriosis, dear cell renal carcinoma and / or determining whether the ovarian cancer in the subject is primary or secondary ovarian cancer and / or a risk of the disease progression after surgery treatment, and / or an effectiveness of post-surgery chemotherapy.
Owner:AGENCY FOR SCI TECH & RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com