Patents
Literature
45 results about "Clear cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In histology, a clear cell is a cell that shows a clear cytoplasm when stained with hematoxylin and eosin (H&E). Normally, clear cells are secretory cells in the epithelium, and are one of the components of eccrine sweat glands. A clear cell's plasma membrane is highly folded, more so on the apical and lateral surfaces. The cytoplasm of clear cells contains large amounts of glycogen and many mitochondria. Melanocytes appear as clear cells when in the stratum basale of the skin, and Langerhans' cells appear as clear cells in the stratum spinosum.
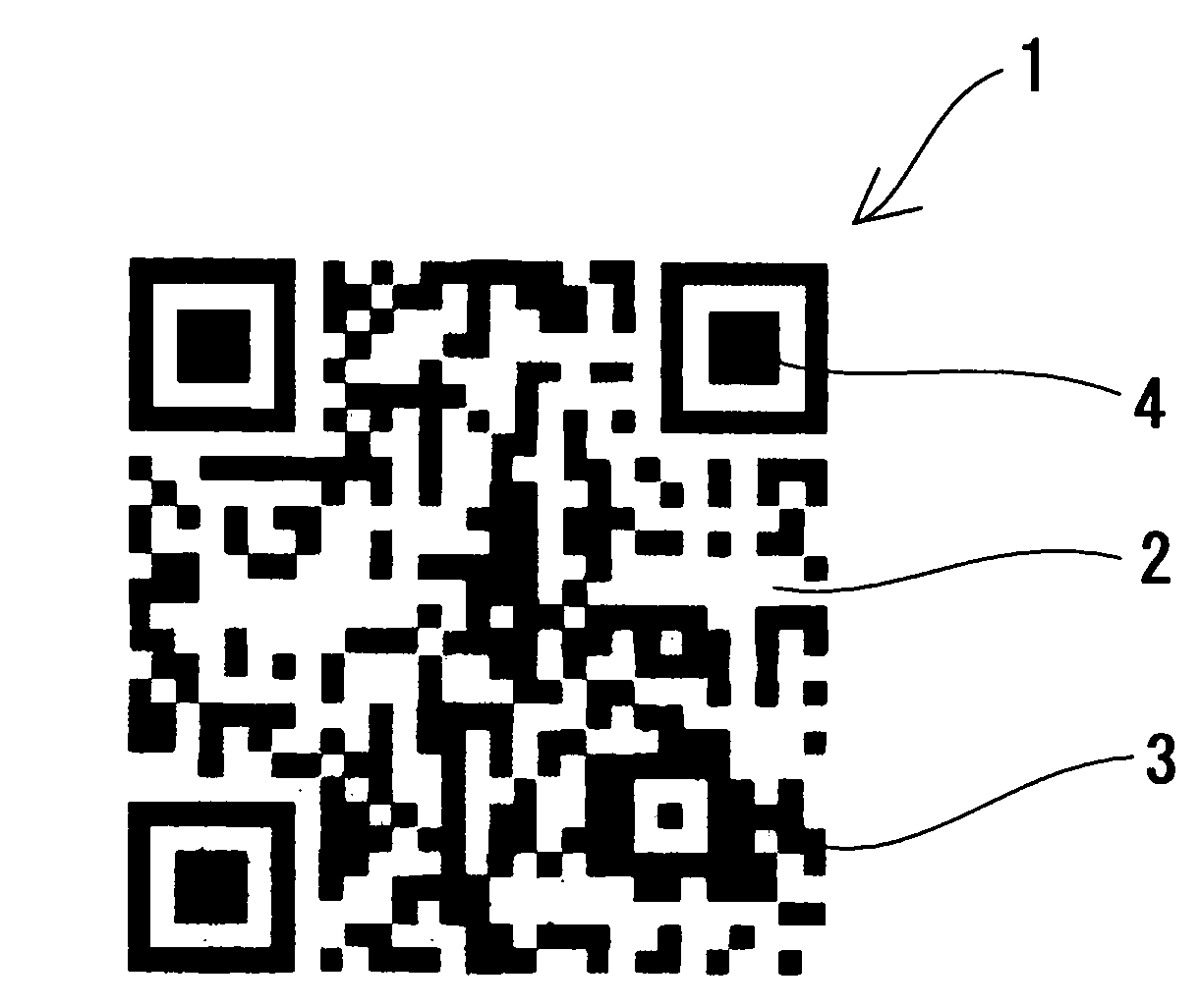
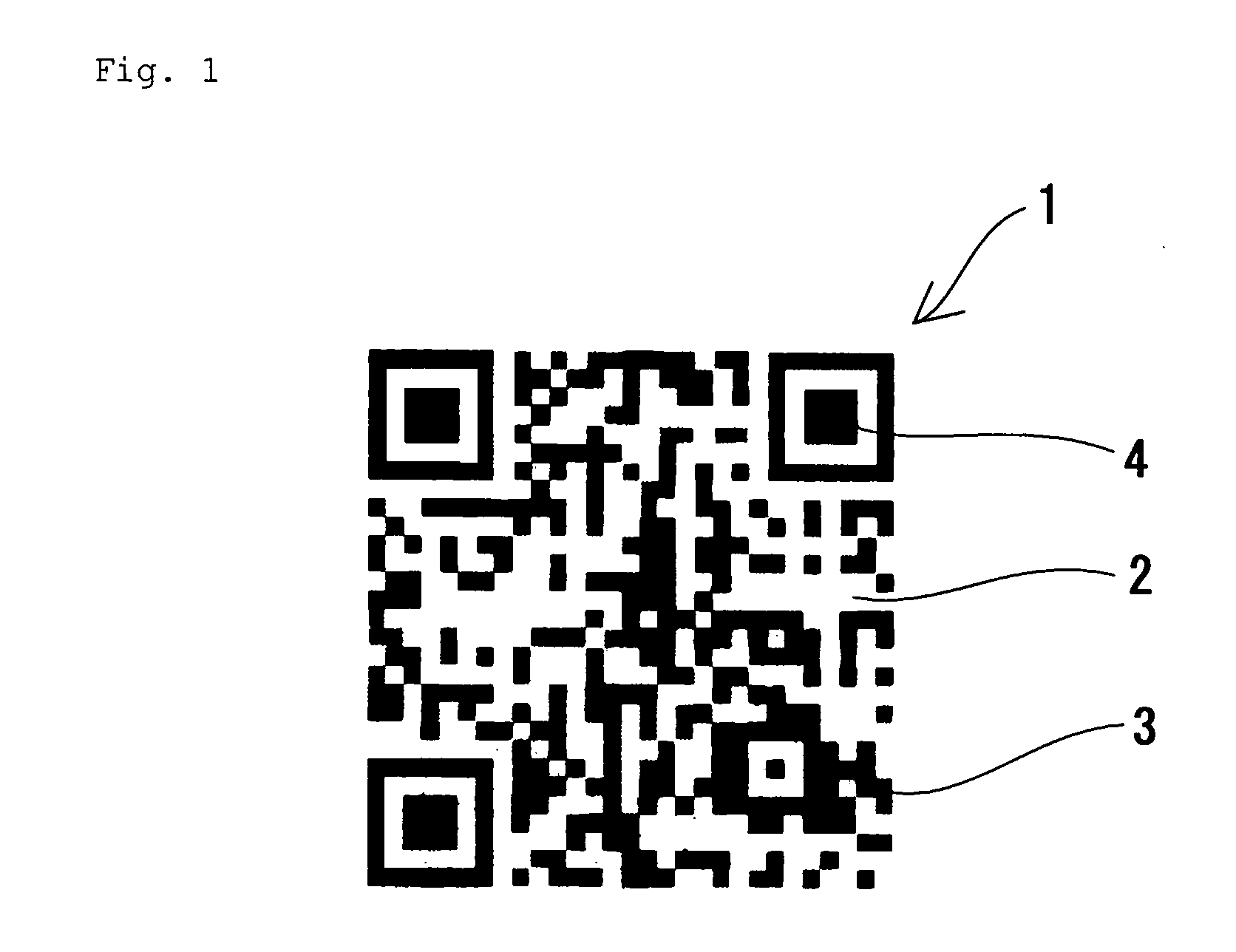
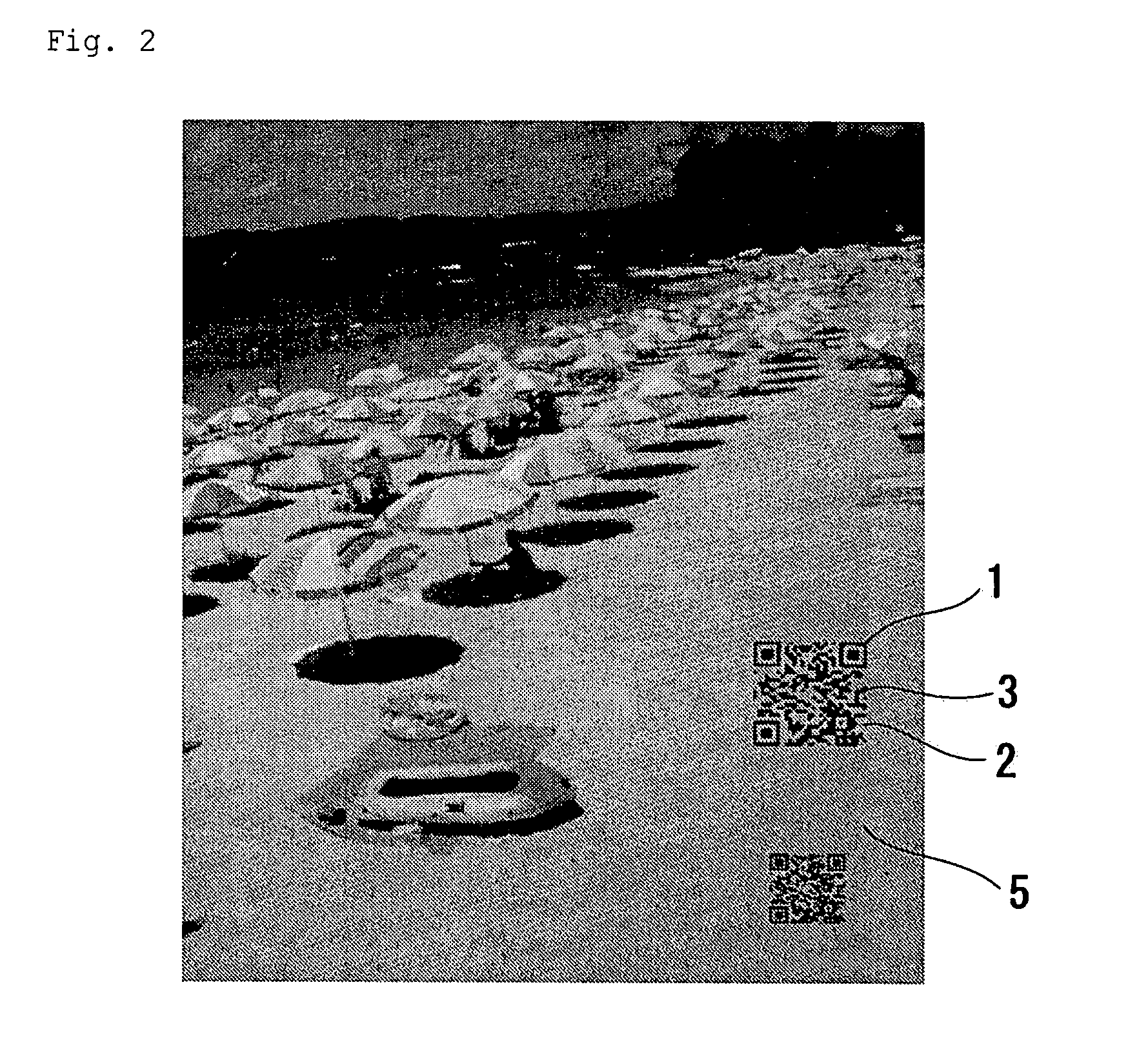
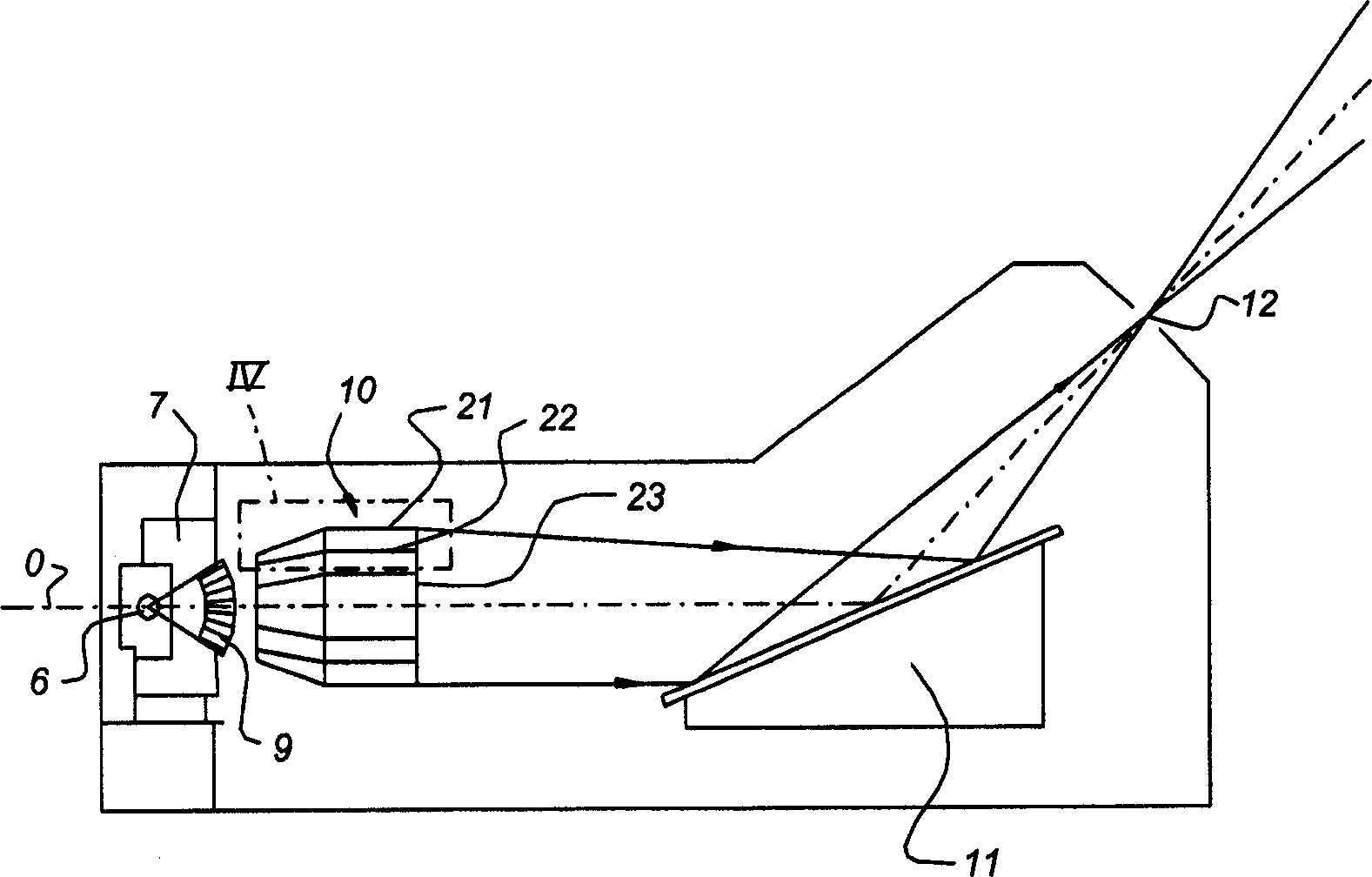
Clear Two-Dimensional Code, Article Having Clear Two-Dimensional Code Attached Thereto, Method for Printing Two-Dimensional Code and Method For Displaying Two-Dimensional Code
InactiveUS20090057420A1Save spaceGood lookingPrintingRecord carriers used with machinesClear cellIdentification device
Owner:CONTENT IDEA OF ASIA
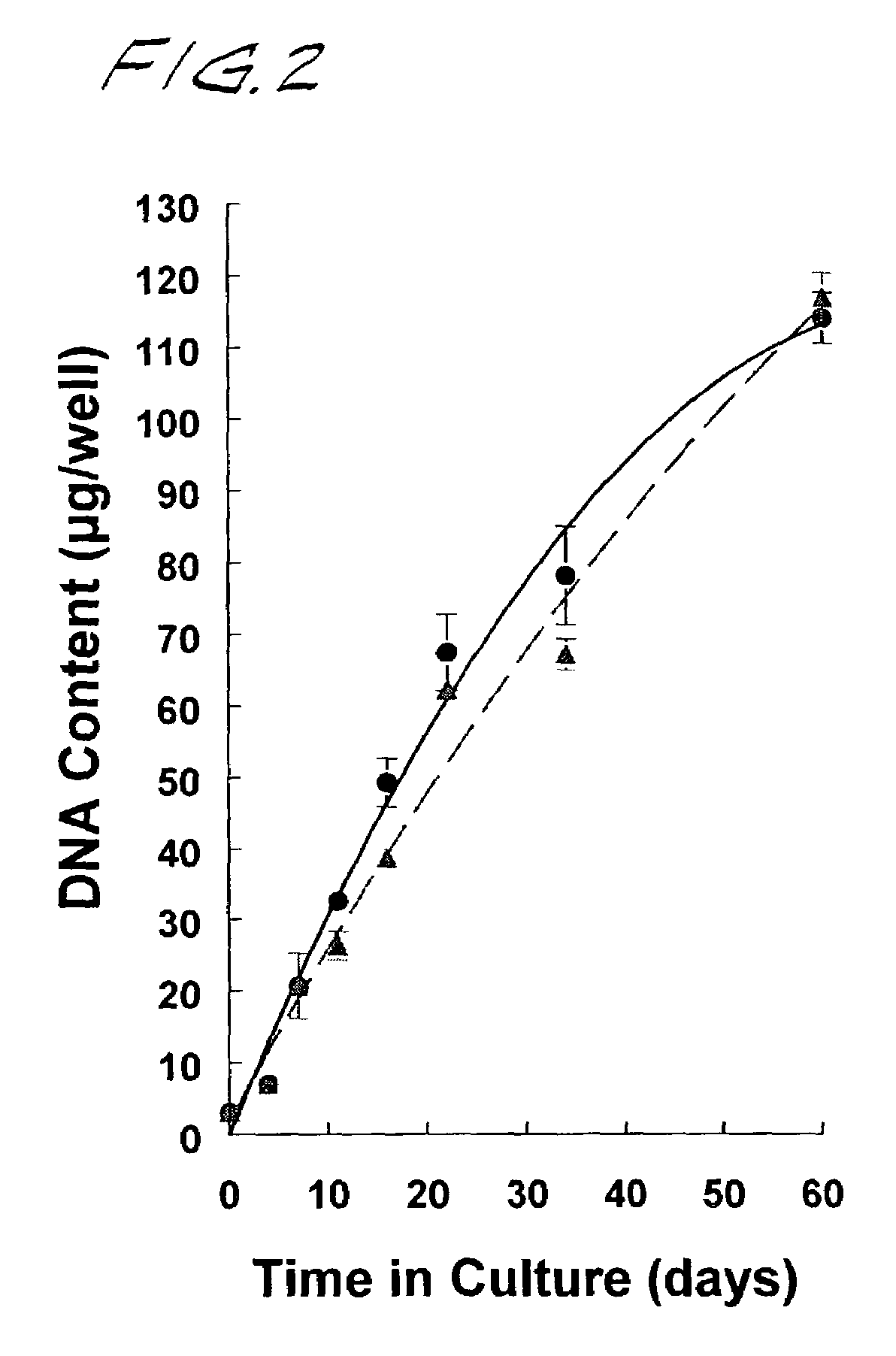
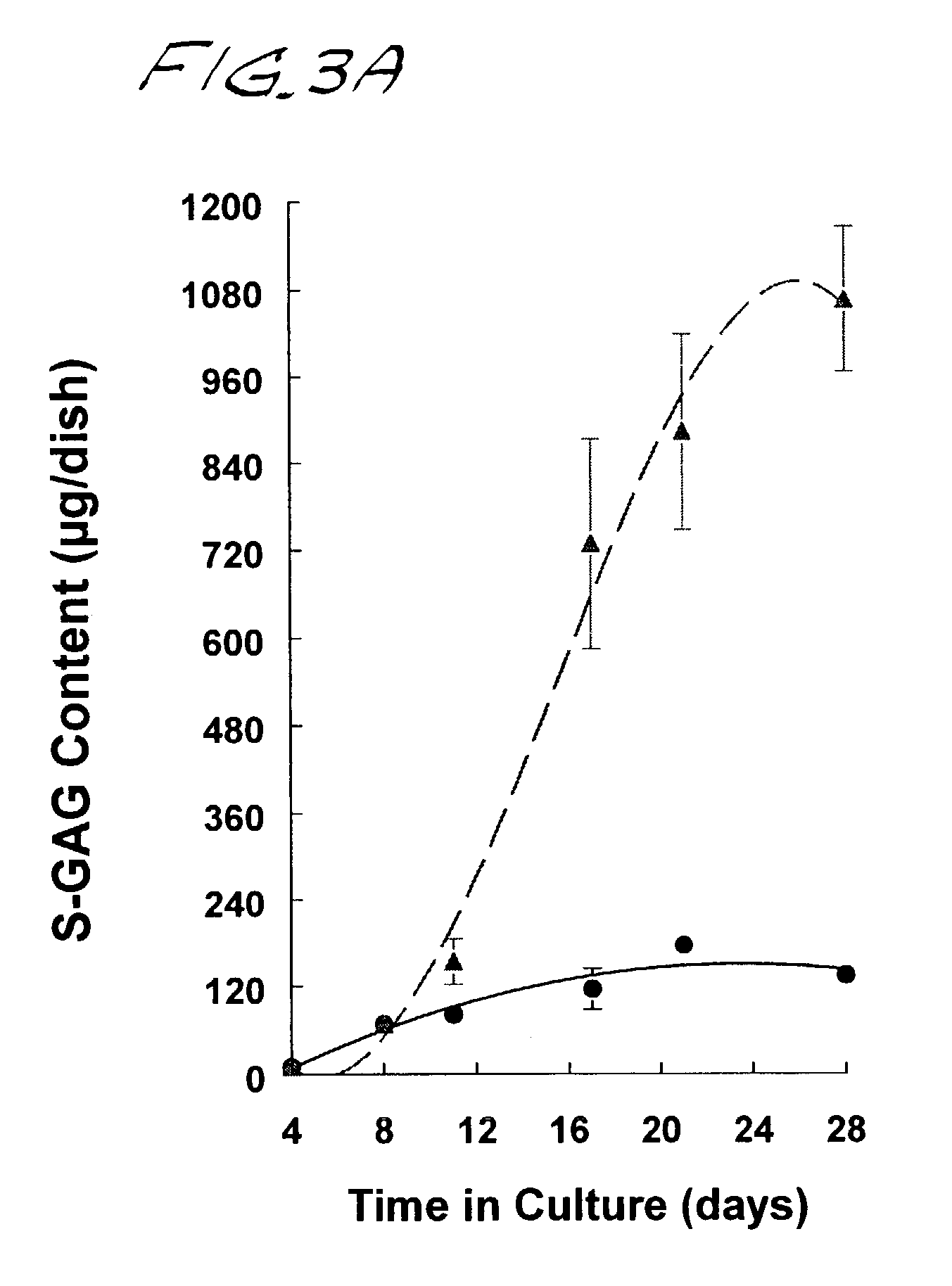
Cartilage composites and methods of use
Disclosed are neocartilage compositions characterized by having multiple layers of cells, said cells being surrounded by a substantially continuous insoluble glycosaminoglycan and collagen-enriched hyaline extra-cellular matrix, and which neocartilage phospholipids are advantageously enriched in anti-inflammatory n-9 fatty acids, particularly 20:3 n-9 eicosatrienoic or Mead acid.Also disclosed are methods of growing neocartilage in substantially serum-free growth media and methods of producing a conditioned growth media containing compounds effective to enhance neocartilage formation.The neocartilage compositions are useful as implants and as replacement tissue for damaged or defective cartilage and as a model system for studying articular cartilage disease and response to natural and synthetic compounds.
Owner:ZIMMER INC
Blood cell component analysis instrument and method
InactiveCN108844906APreparing sample for investigationColor/spectral properties measurementsFailure rateImaging processing
The invention provides a blood cell component analysis instrument and a method. The instrument comprises a sample absorbing and transfer unit, a dyeing liquid adding unit, a stirring and smearing unit, a cleaning unit, a microscanning unit, an image processing unit and a control unit. The sample absorbing and transfer unit is used for absorbing a quantitative blood sample to a glass slide; the dyeing liquid adding unit is used for adding the dyeing liquid to dye cells and dilute the blood sample; a reverse T-shaped stirring and smearing unit is used for mixing the blood sample and the dyeing liquid uniformly and smearing the mixed blood sample; the microscanning unit is used for performing microscopic examination on the smear; the instrument automatically and continuously performs axial scanning on the unclear targets for many times; the image processing unit processes the scanning image and selecting the images with clearest cells or granules in the multi-focus images into a whole image; and the analysis instrument is simple to operate, low in failure rate, accurate in result and few in consumed material reagent, and can perform quantitative analysis on the blood cells conveniently and accurately.
Owner:CLINDIAG SYSTEMS CO LTD
Liquid-based cell preservation liquid and preparation method therefor
InactiveCN105432598AMaintain normal physiological functionReasonable formulaDead animal preservationPreservativeAnticoagulant
The invention discloses a liquid-based cell preservation liquid and a preparation method therefor. Based on the total weight of the cell preservation liquid, the cell preservation liquid comprises the following raw components in parts by weight: 0.05-0.1 parts of a pH buffer, 0.5-1 part of an osmotic pressure retaining agent, 0.3-0.8 parts of a preservative, 25-35 parts of a fixing agent, 0.1-0.6 parts of an anticoagulant, 0.05-0.15 parts of a mucus softener, 0.5-0.9 parts of ammonium chloride and 65-75 parts of de-ionized water. The cell preservation liquid is reasonable in formula, and can effectively keep original morphological structures and physiological functions of cells and effectively remove red blood cell components and mucus components so as to obtain clean field of view; a smear prepared by flaking of an automatic flaking machine is clear and transparent in background and uniform in cell monolayer distribution, thereby facilitating subsequent observation experience of pathologists; and the period of validity of the preservation liquid can reach more than one year.
Owner:湖南品信生物工程有限公司
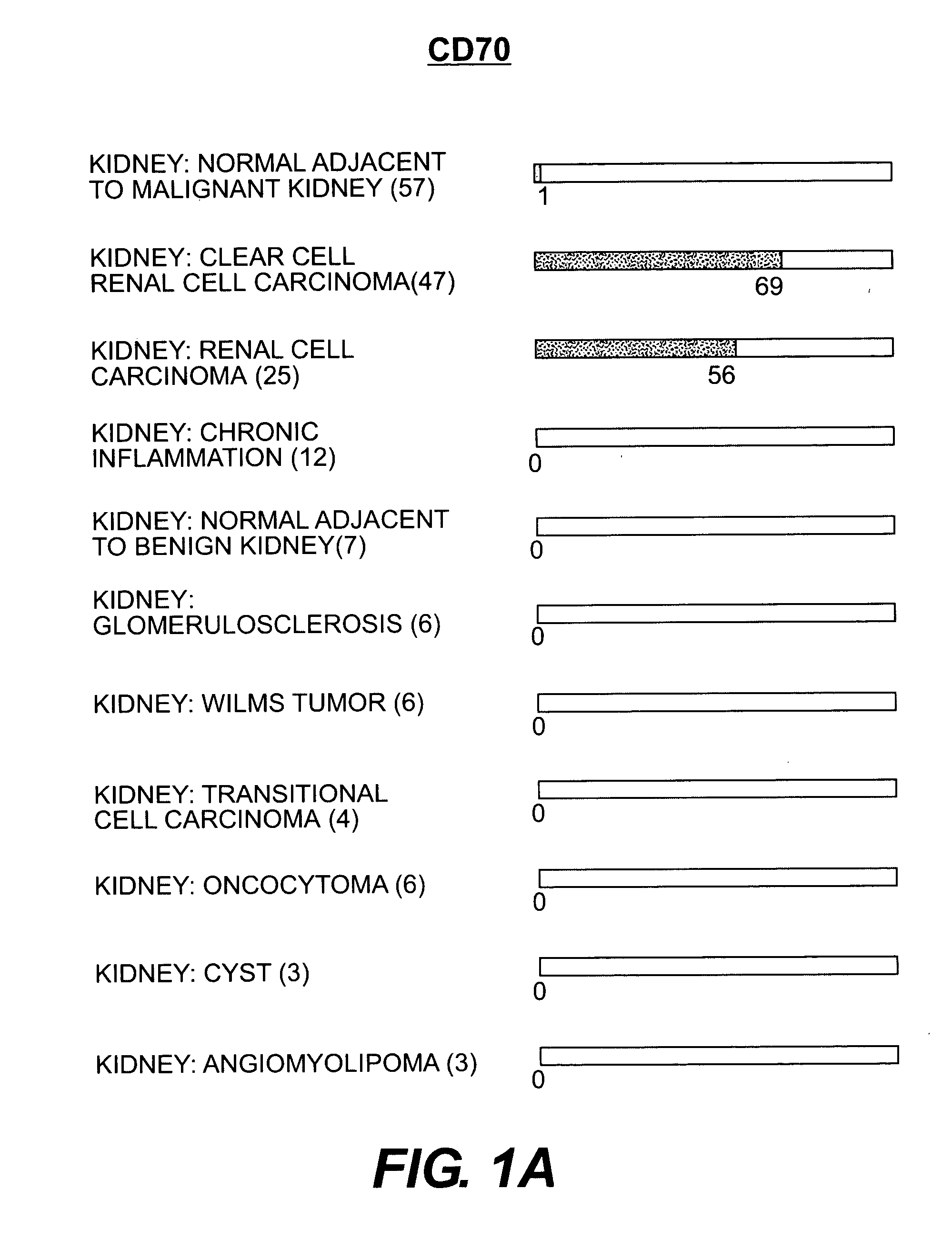
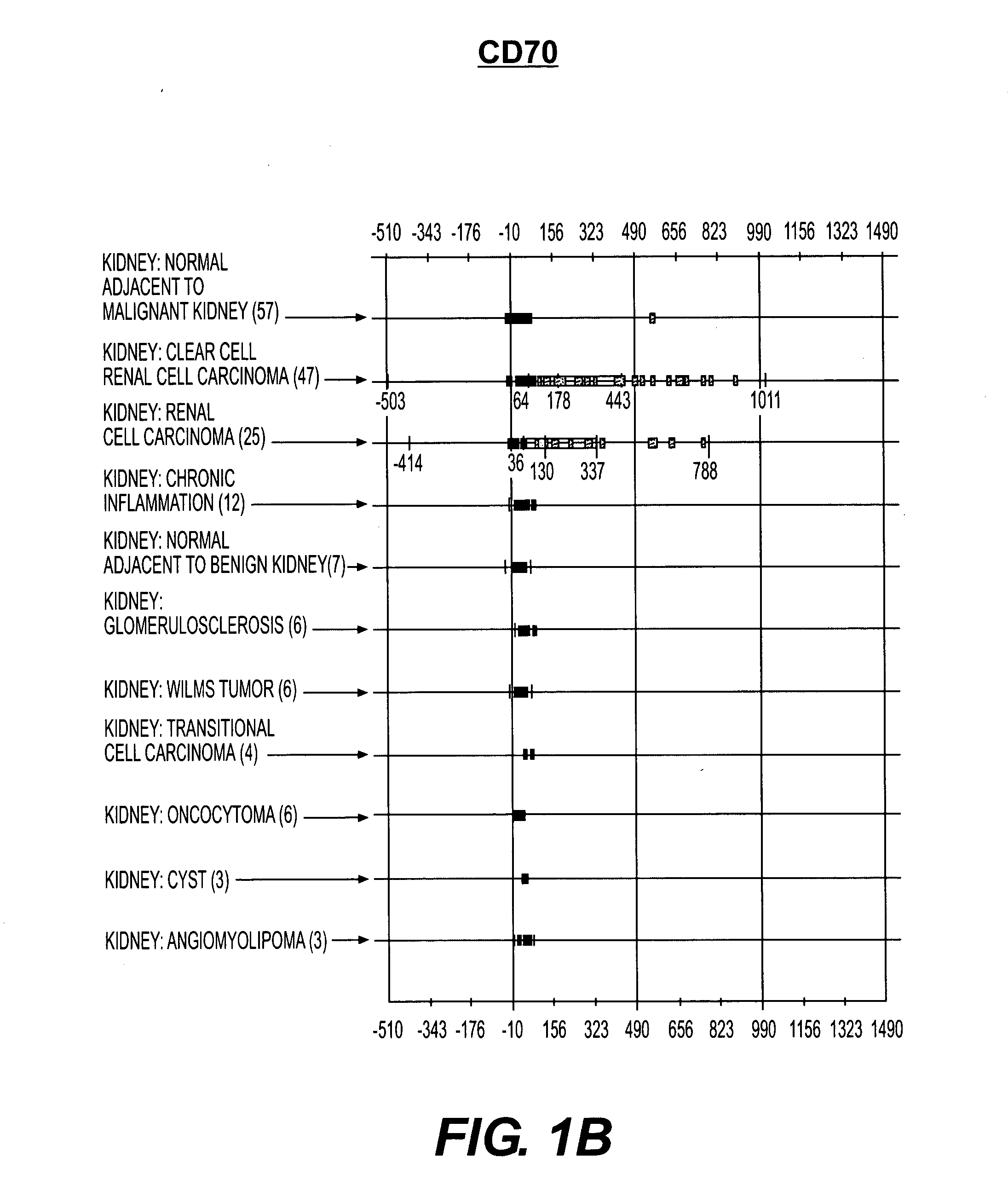
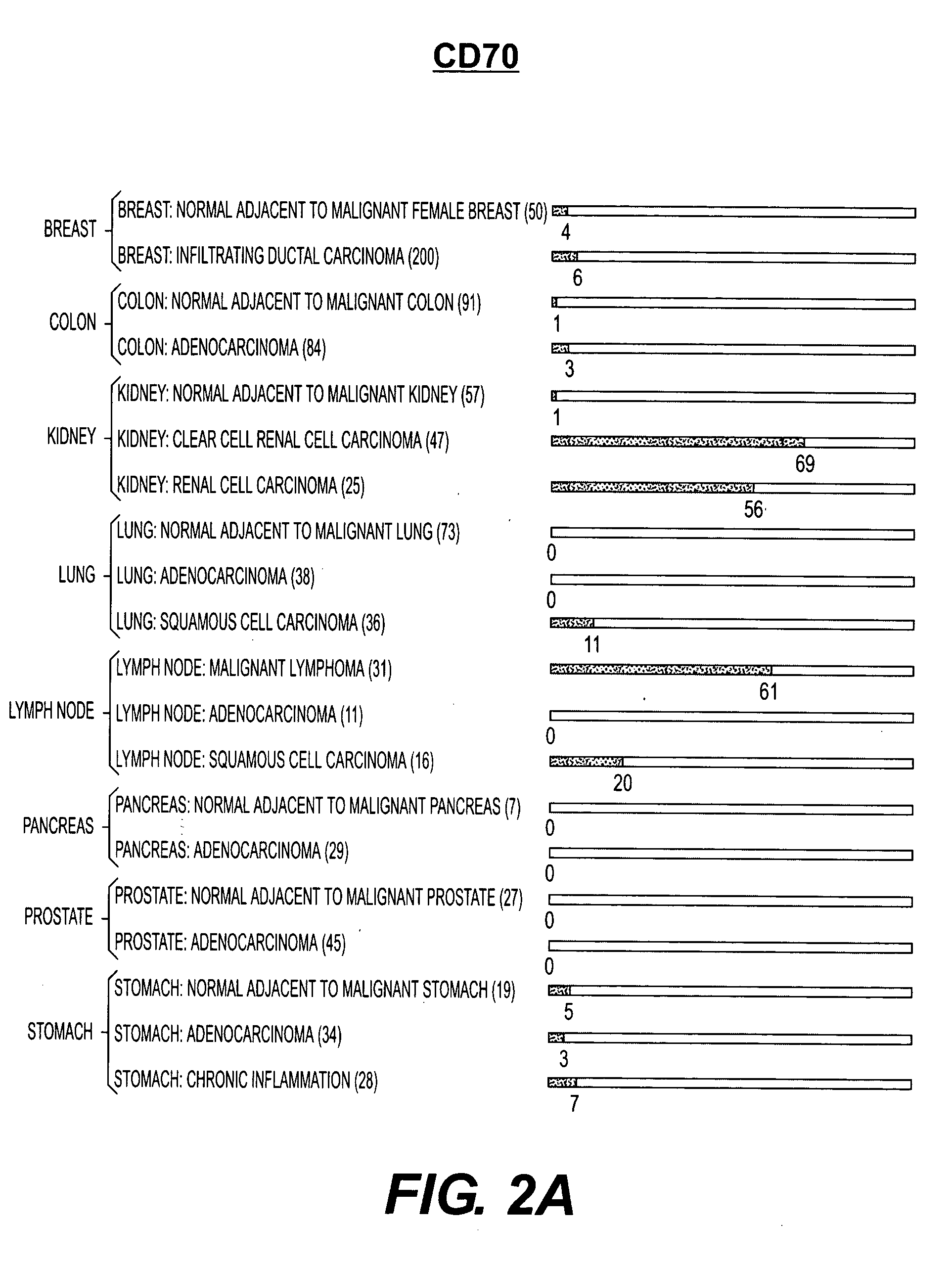
Cell surface molecules as markers and therapeutic agents against kidney cancers
InactiveUS20050282168A1Efficient dosingGrowth inhibitionMicrobiological testing/measurementBiological material analysisClear cell renal cell carcinomaDisease cause
Human cell surface molecules CD70 and CD203c are expressed at higher levels in kidney carcinomas, particularly renal cell carcinomas and clear cell renal cell carcinomas, yet are expressed at low levels in normal kidney and other diseased kidney tissue, and at low levels in other tissues. CD70 and CD203c show specificity towards kidney carcinomas, particularly renal cell carcinomas and clear cell renal cell carcinomas and thus can be used as diagnostic markers and therapeutic targets for these diseases. In addition, antibodies or small molecules against these molecules could be used in treatments towards these diseases.
Owner:WYETH
Blood cell separation method for portunus trituberculatus
ActiveCN108118024AHigh activityEasy to operateCell dissociation methodsInvertebrate cellsGranular cellRed blood cell
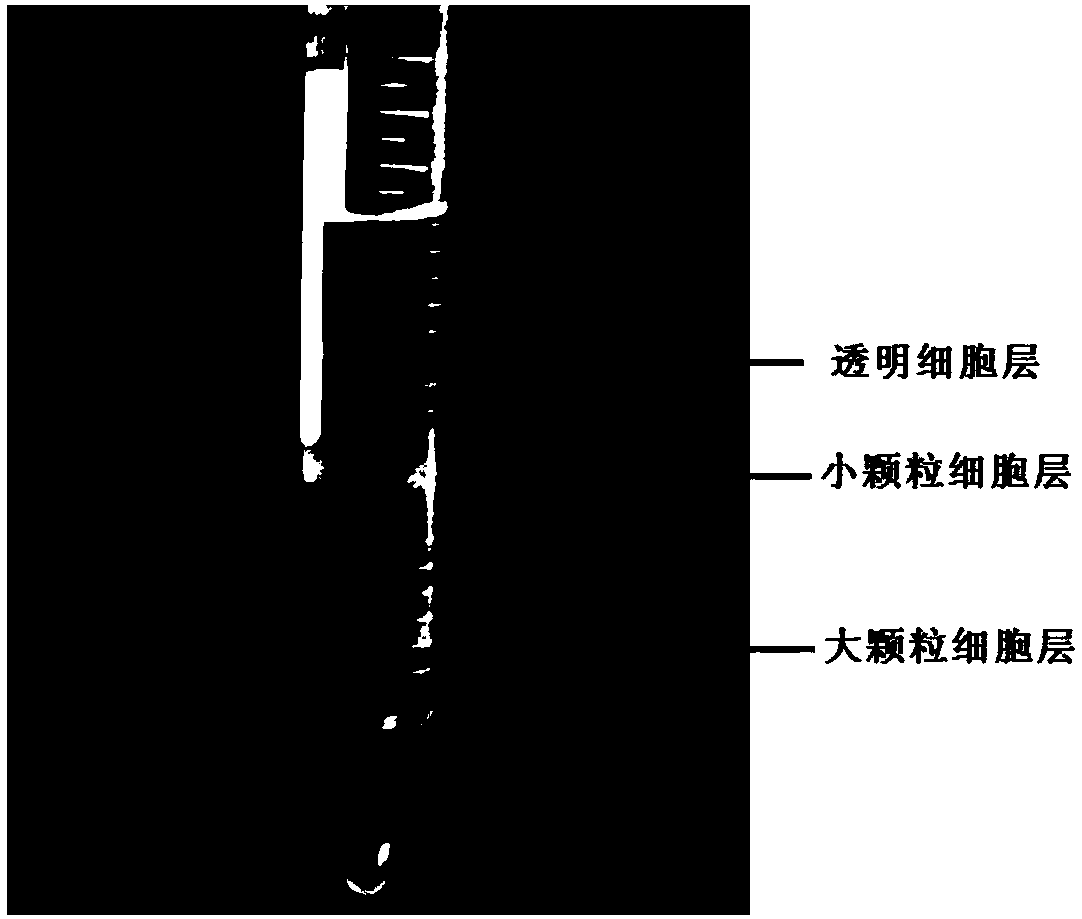
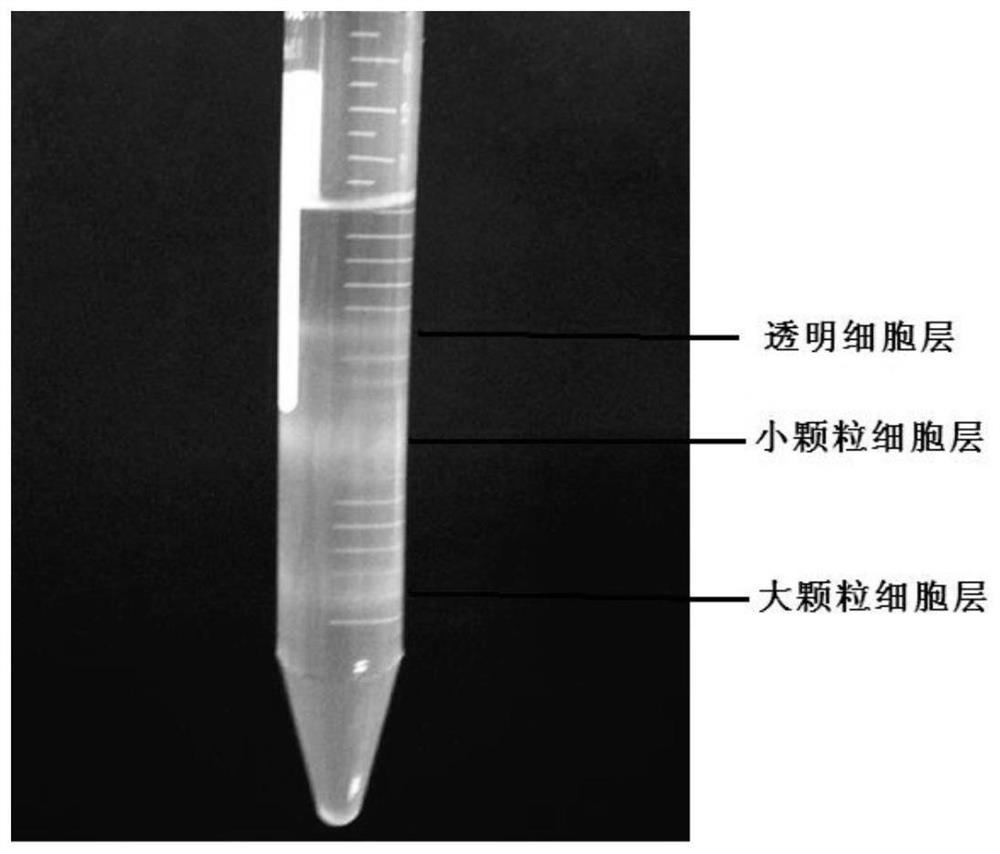
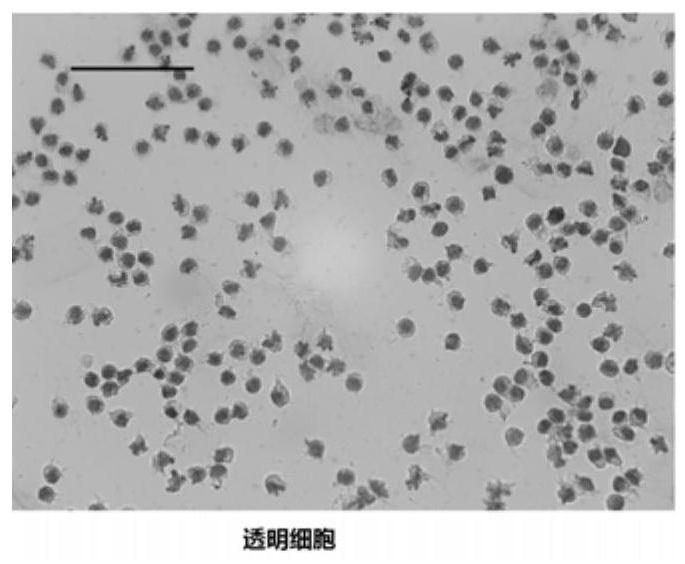
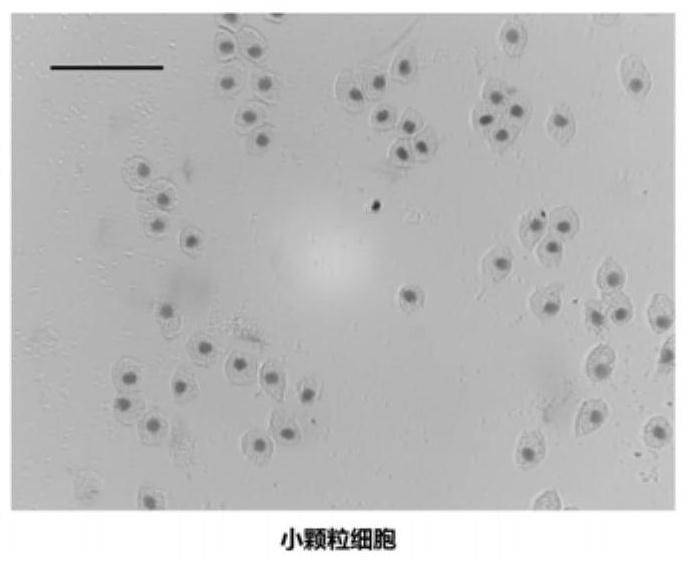
The invention discloses a blood cell separation method for portunus trituberculatus. The method comprises the following steps: diluting iodixanol with an isotonic solution obtained by regulating an osmotic pressure of a PBS buffer solution by utilizing a sodium chloride solution, and performing gradient preparation to obtain three iodixanol solutions of different concentrations; forming gradient solutions of different densities by adopting a cushion technology, and standing to form a continuous density gradient solution to serve as a cell separation solution; collecting blood cells of portunustrituberculatus, and taking mixed cell suspension obtained by diluting the isotonic solution; slowly dropping the mixed cell suspension into the cell separation solution, centrifuging, and dividing the blood cells of portunus trituberculatus into three layers, wherein the uppermost layer refers to clear cells, the intermediate layer refers to small granular cells, and the lowest layer refers to large granular cells. The method has the advantages that only one-step density gradient centrifugation is adopted, the operation is simple, and experiments discover that the separated blood cells haveexcellent viabilities and can be used for cell culture.
Owner:NINGBO UNIV
Application of acetaldehyde dehydrogenase 2 as drug target for treating tumor cells with anthracycline type chemotherapy drugs
InactiveCN103784465AOvercome the shortcomings of only relying on surgical treatmentIncrease lethalityOrganic active ingredientsAntineoplastic agentsCancer cellDrug target
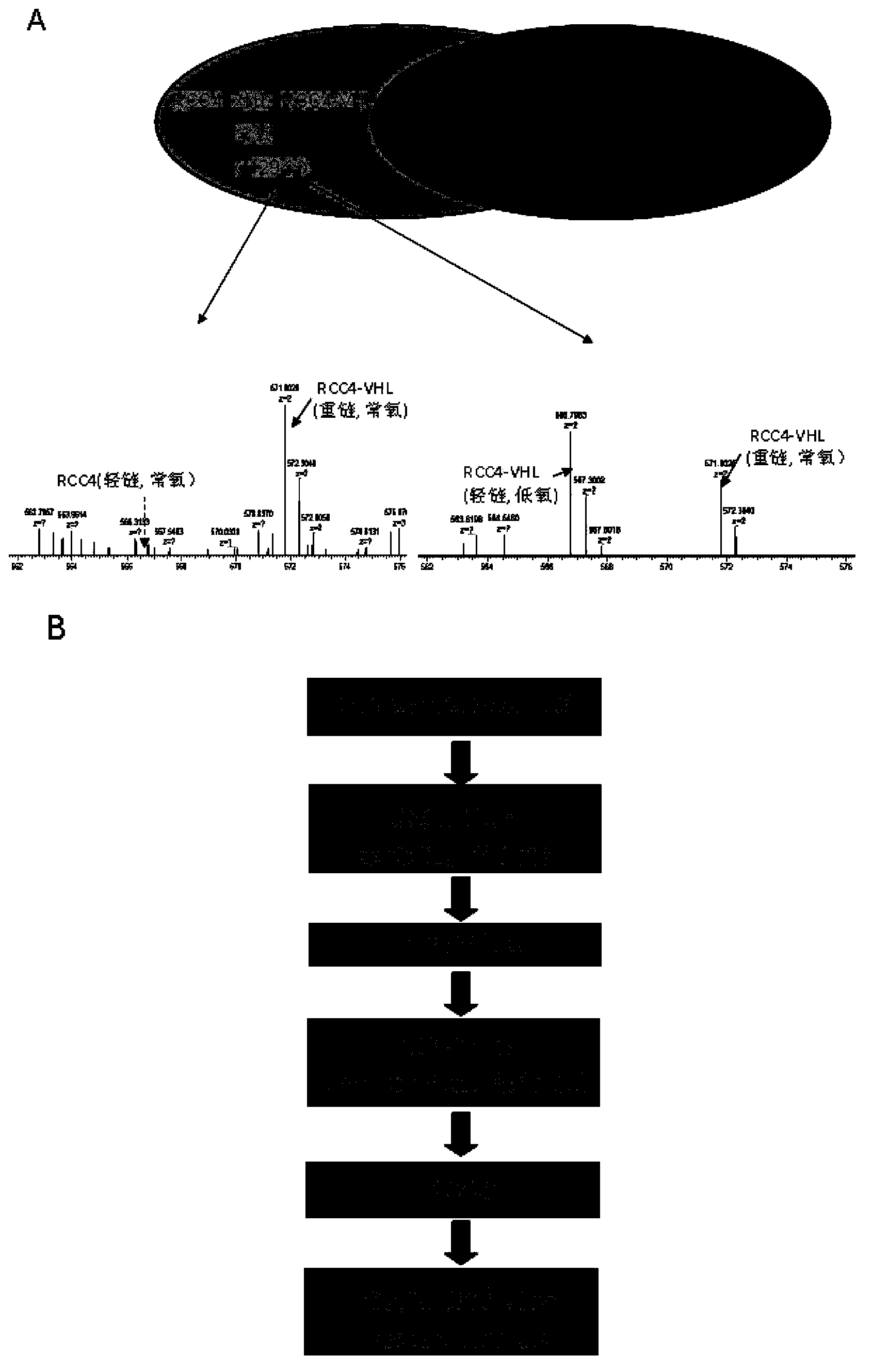
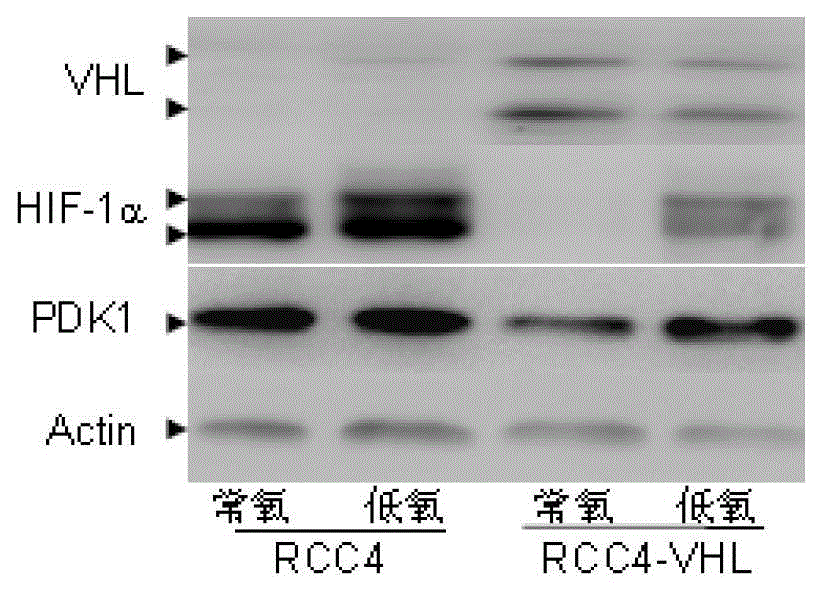
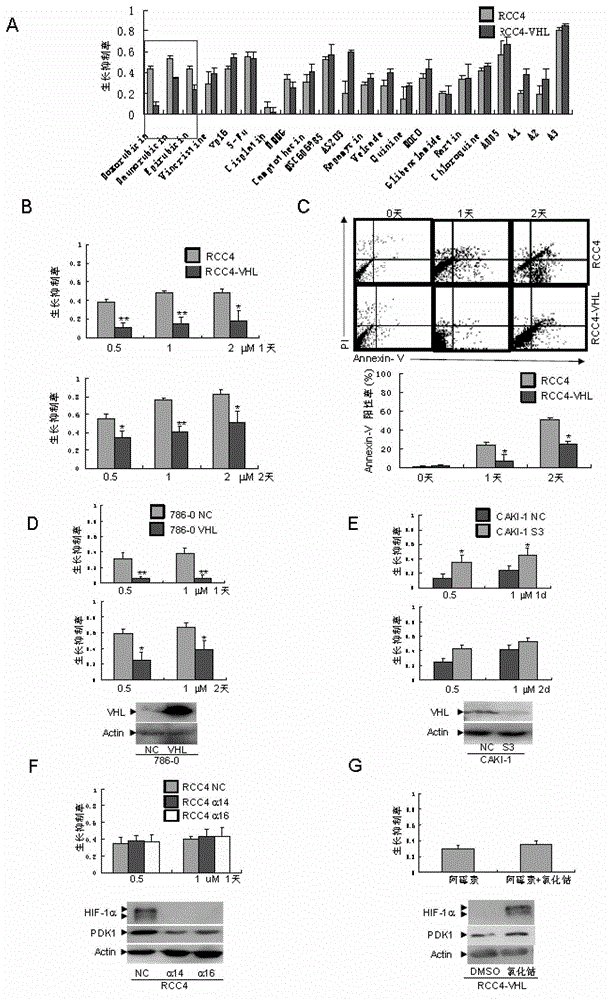
The invention discloses an application of acetaldehyde dehydrogenase 2 as a drug target for treating tumor cells with anthracycline type chemotherapy drugs. Drug screening shows that the anthracycline type chemotherapy drugs can specifically kill renal clear cell cancer cells with VHL deletion or mutation and has small toxicity to normal cells, thus providing an effective choice for chemotherapy of renal cancer and overcoming the disadvantage that the renal cancer is only treated through operations in clinical at present. Further study shows the sensitivity of the acetaldehyde dehydrogenase 2 mediated anthracycline type chemotherapy drugs to tumor cells, thus remaindering the application of the acetaldehyde dehydrogenase 2 as the drug target for treating tumor cells with the anthracycline type chemotherapy drugs. Deletion or mutation of the acetaldehyde dehydrogenase 2 exists in 40% of populations in Asia, so that the application of the acetaldehyde dehydrogenase 2 has a broader application prospect. For patients without the deletion or the mutation of the acetaldehyde dehydrogenase 2, killing effects on tumor cells can be significantly enhanced through combination of the anthracycline type chemotherapy drugs and an inhibitor of the acetaldehyde dehydrogenase 2.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Method for treating biomass for producing cell lysate containing plasmid DNA
InactiveUS7378238B2Easy to handleAvoid disadvantagesSugar derivativesMicrobiological testing/measurementBiomassClear cell
The method of making the clear cell lysate containing plasmid DNA from a biomass obtained in a cell culture process includes filtering the biomass obtained in the cell culture process with a filter medium in the presence of diatomaceous earth acting as a filtering agent to form a filter cake containing the biomass and the filter medium, then thermally digesting the biomass in the filter cake to form a cell lysate containing the plasmid DNA and filtering the cell lysate to form a clear filtrate. The clear filtrate contains the plasmid DNA and has a clarity characterized by an OD600 of at maximum 0.05 U / cm.
Owner:DRM DR MULLER +1
Application of miR-577 for preparing nephrosis diagnosis marker
InactiveCN108624693AMicrobiological testing/measurementAntineoplastic agentsNephrosisTarget analysis
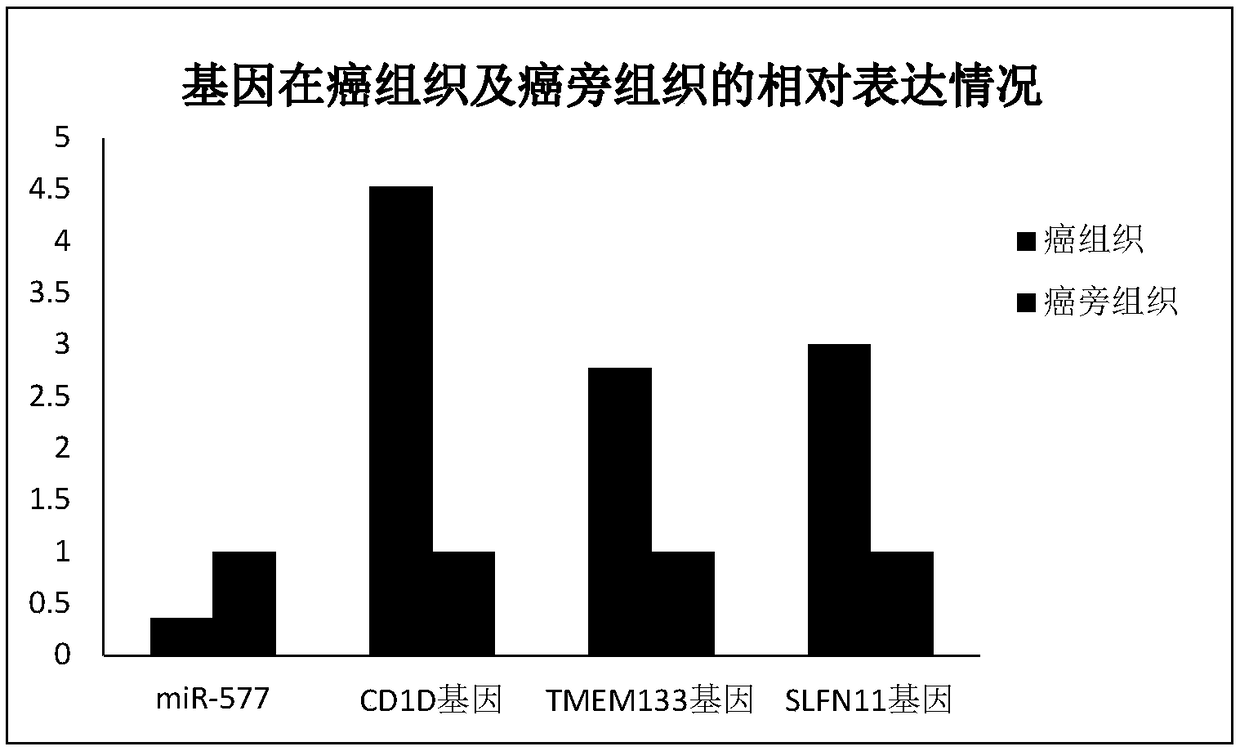
The invention relates to application of miR-577 for preparing a nephrosis diagnosis marker, in particular to application of miR-577 and a target gene thereof in preparing a renal clear cell carcinomadiagnosis marker. Database renal clear cell carcinoma mRNA (Ribonucleic Acid) and miRNA data are retrieved, and miR-577 and a target gene regulated and controlled by the miR-577 are selected to carryout molecular verification through data integration, differential expression analysis and targeted analysis. A result indicates that the miR-577 and the target genes CD1D, TMEM133 and SLFN11 regulatedand controlled by the miR-577 can serve as the diagnosis marker of the renal clear cell carcinoma, and therefore good clinic application value is performed.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
High-transfer kidney clear cell cancer cell system for the Han nationality
InactiveCN101508972AMicrobiological testing/measurementMicroorganism based processesClear cellMetastatic phenotype
The invention discloses a method for constructing the Hans highly metastatic renal clear cell carcinoma cell line HiMet-ccRCC, CCTCC-C200909 with metastatic phenotype in the Hans renal clear cell carcinoma vertebrae metastasis. The invention adopts culture in vitro to construct a human renal clear cell carcinoma cell line having high metastatic potential with vertebrae metastasis of renal clear cell carcinoma patients as tumor source for constructing the line. Tests show that the inventive cell line has the typical characteristics of tumor cell lines, and is capable of leading NOD-SCID mice to generate stable metastasis and being used as the construction of cell model for the screening preparation detection and prophylaxis of Hans renal cell carcinoma pharmaceuticals.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Bone marrow specimen processing method, decalcification solution, and applications
InactiveCN109425523AEasy to sliceSmooth slicePreparing sample for investigationClear cellDistilled water
The invention provides a bone marrow specimen processing method, a decalcification solution, and applications. The bone marrow specimen processing method comprises following processing steps of bone marrow tissues: fixing and primary decalcification, dehydration, paraffin embedding, rough trimming, secondary decalcification, and slicing; the decalcification solution at least comprises formic acid,formaldehyde, and distilled water at a volume ratio of 198-202:48-52:190-210, wherein the mass concentration of formic acid ranges from 97 to 99%. The bone marrow specimen processing method is used for processing bone marrow specimen, so that slices with relatively complete tissue structures and clear cell structures can be obtained, and it is beneficial for observation of slices under microscopes.
Owner:SHANGHAI SIMPLEGENE CLINICAL LAB CO LTD
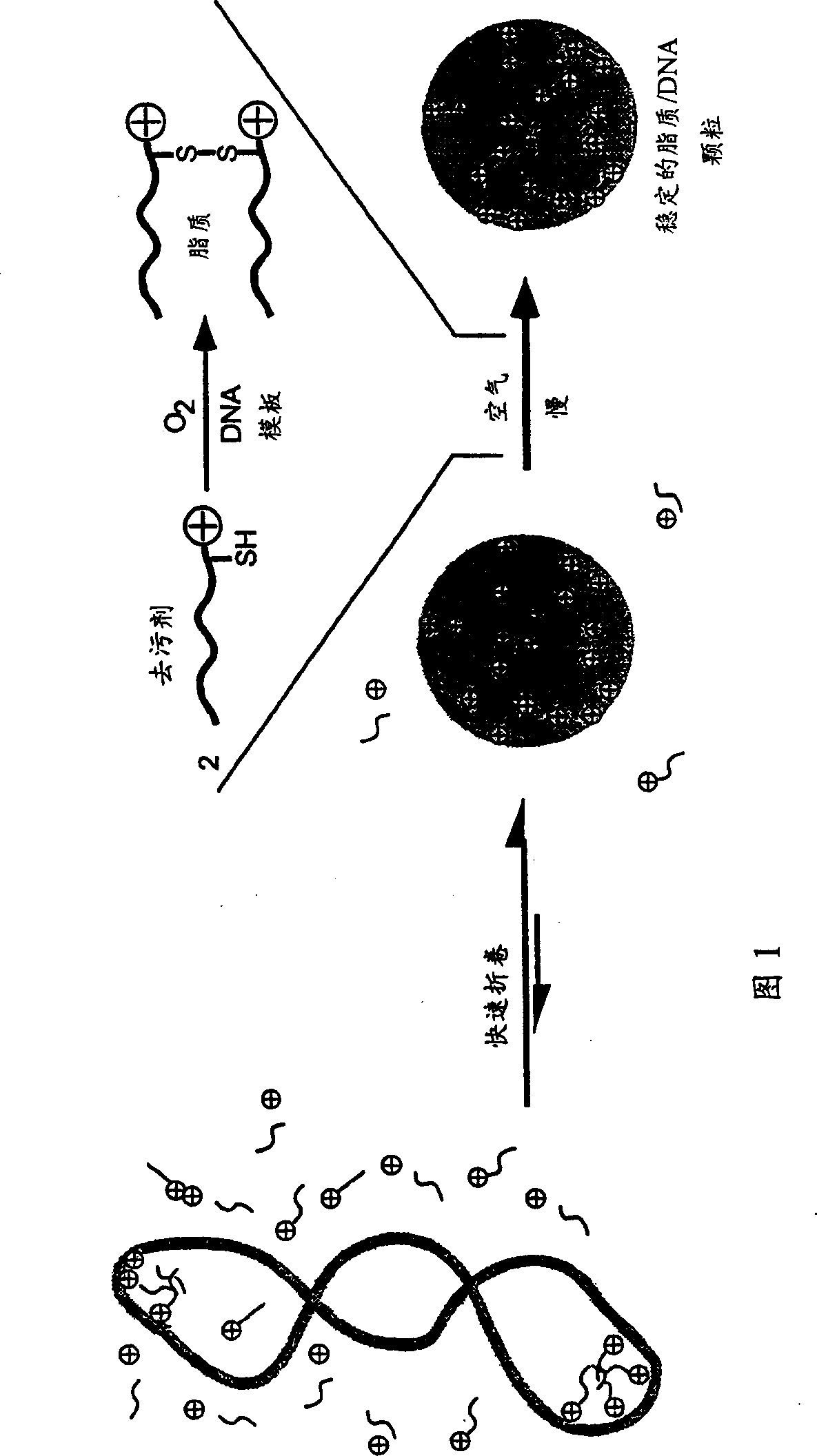
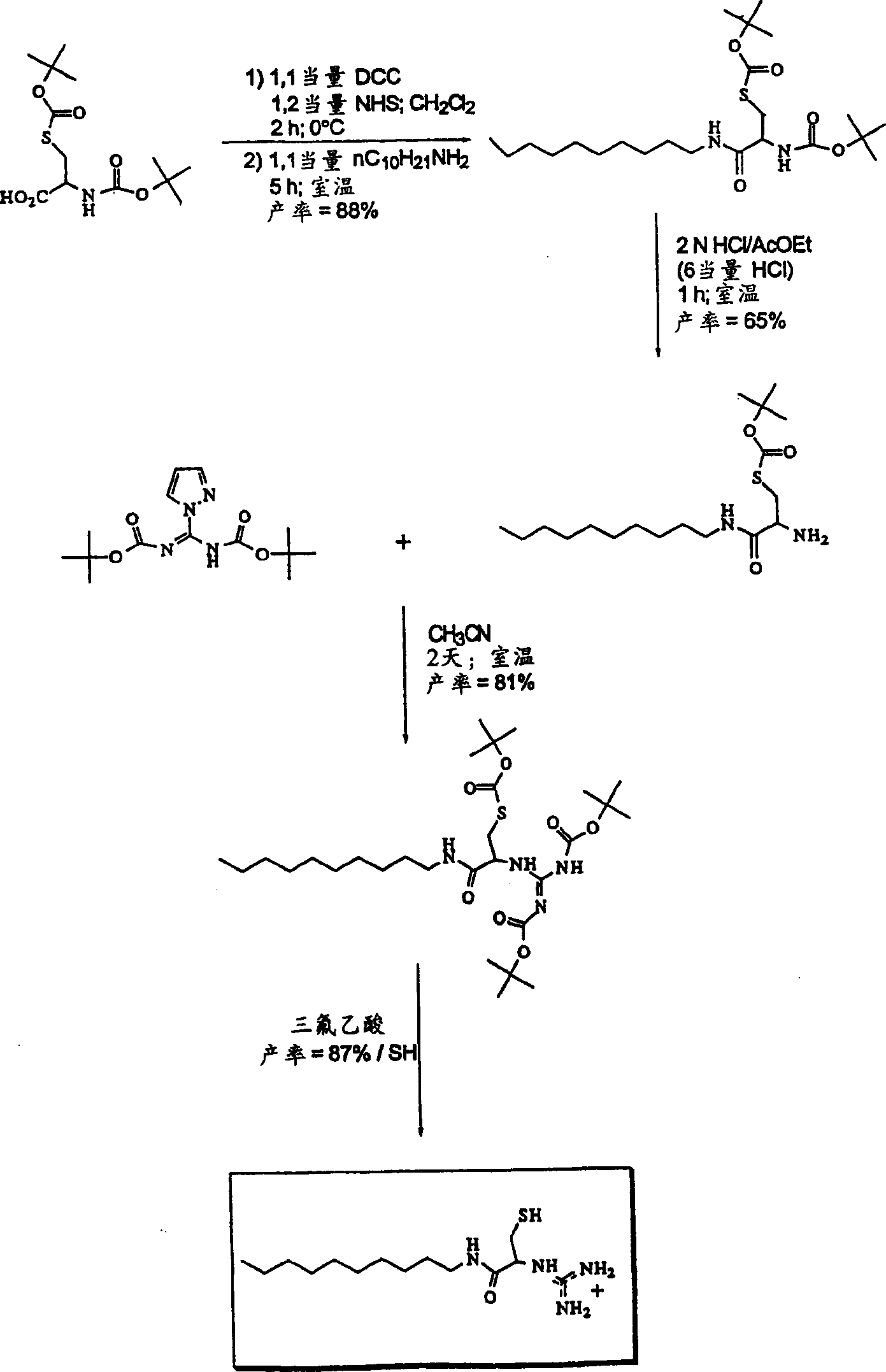
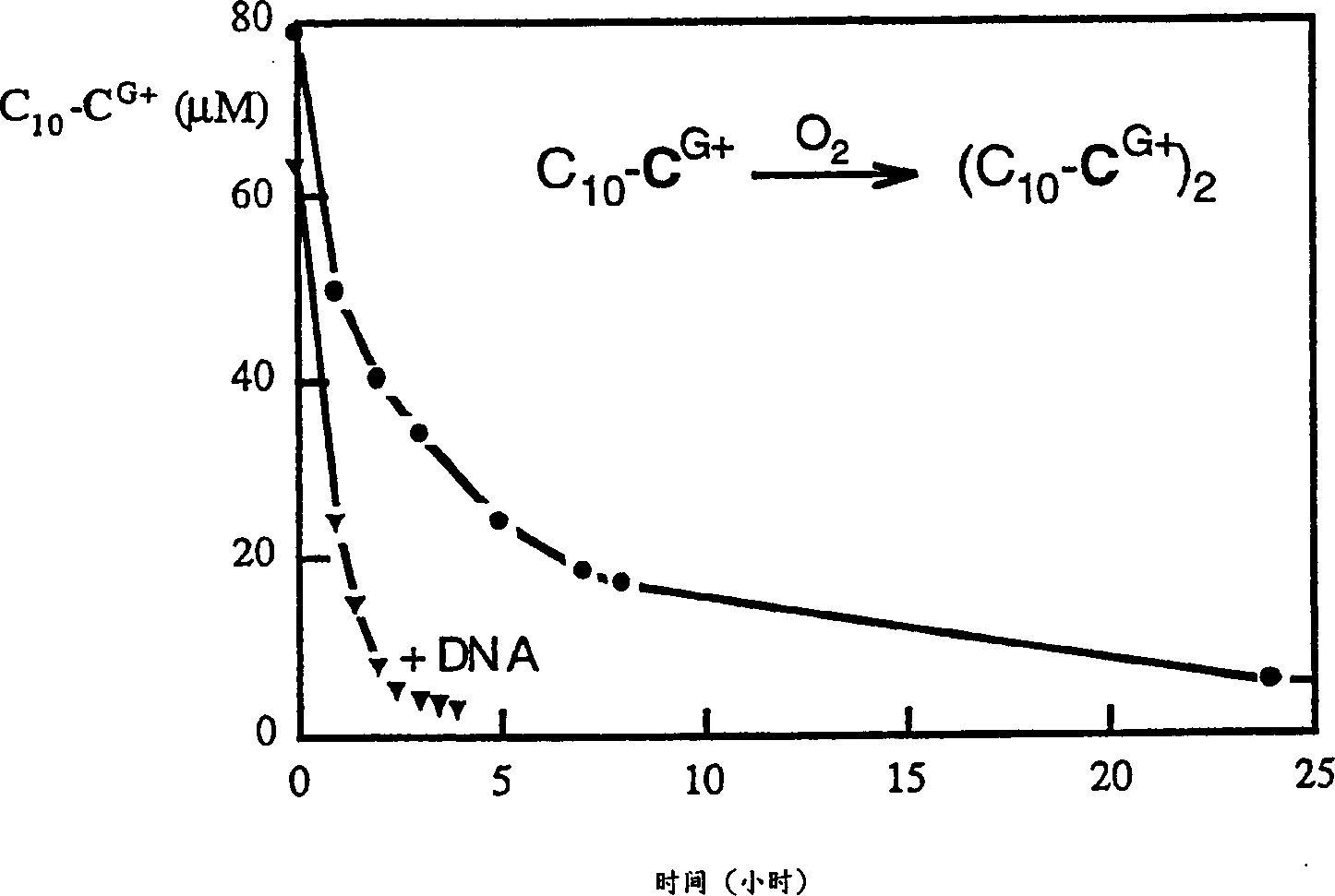
Transfection particles
Transfection particles used to deliver nucleic acids into cells of higher eukaryotes in vivo and in vitro, including one or more nucleic acid molecules condensed by organic cationic molecules. The dispersed stable particles are formed by complexing the nucleic acid molecules with the same or different organic cation precursor molecules, rather than cross-linking between the nucleic acid molecules, and the precursor molecules are covalently bonded to each other on the nucleic acid template and made. For unambiguous cell orientation, the particles are loaded with orientation molecules, such as sugars. Preferred cationic precursor molecules are lipophilic detergents bonded to form lipids. The particle preferably contains only one nucleic acid molecule making it effective for gene therapy and delivery of large DNA molecules.
Owner:BOEHRINGER INGELHEIM INT GMBH +1
Immune agonist and composition, applications of immune agonist and composition, and preparation method of composition
PendingCN107281483AImprove the production effectConvenient inductionOrganic chemistryDigestive systemClear cellGamma interferon
The invention relates to an immune agonist and a composition, applications of the immune agonist and the composition, and a preparation method of the composition. The immune agonist and the composition keep hepatitis B virus specific antibodies of original prophylactic vaccines in animal bodies and human bodies, can inactivate and clear cells infected with hepatitis B viruses, and have double effects of prevention and treatment; and the immune agonist and the composition have stronger immune activation effects than the original HBsAg (Hepatitis B surface antigen), show reinforced immune cell factor induction, higher antibody generating effect and especially high-level mark Th1 type immune generation of gamma-interferon and IgG2a antibodies, and have remarkable innovation and practicability for clearing hepatitis B viruses.
Owner:SHENZHEN UNIV +1
Application of cuprous oxide nanoparticles to preparation of medicine for treating renal cancer diseases
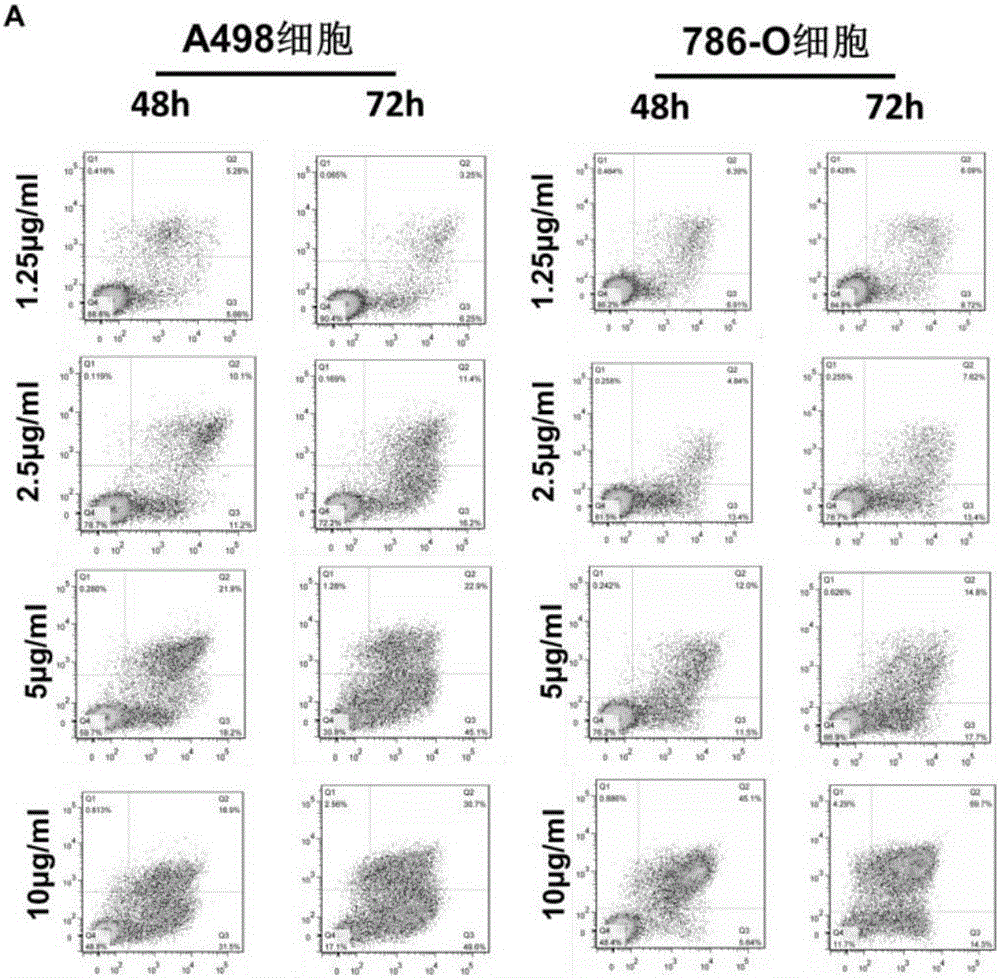
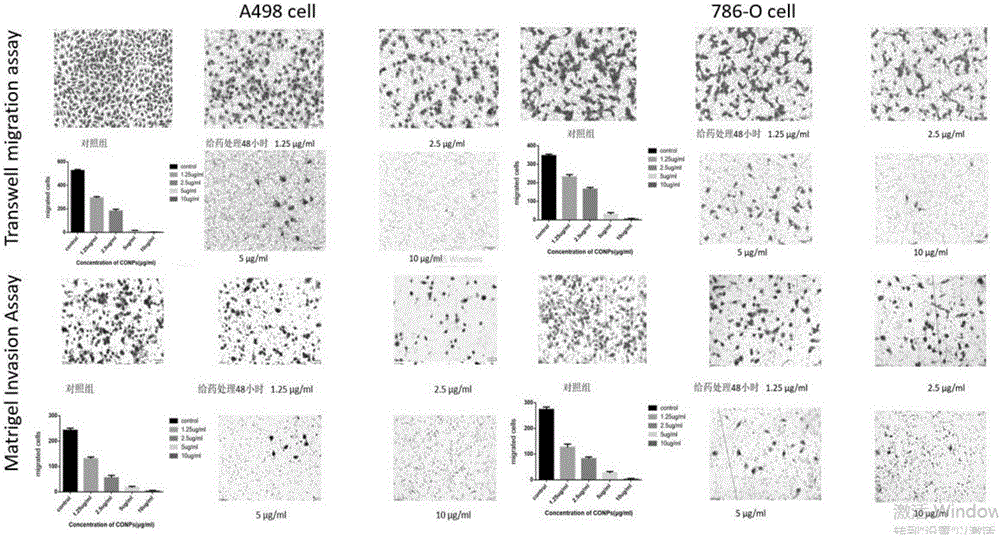
The invention relates to the application of cuprous oxide nanoparticles in the preparation of drugs for treating kidney cancer, and the kidney cancer is clear cell type kidney cancer, granular cell type kidney cancer, mixed cell type kidney cancer and undifferentiated cell type kidney cancer. The invention also provides a medicine for treating kidney cancer. The invention adopts the sequencing of the expression profile after the action of the kidney cancer drug, the in vivo experiment of the drug acting on the cell line, and the in vitro experiment of the subcutaneous tumor bearing in nude mice. Further explain the therapeutic role of CONPs in RCC and related signaling pathways, and lay a theoretical foundation for exploring new treatments for metastatic RCC.
Owner:SHANGHAI CHANGHAI HOSPITAL
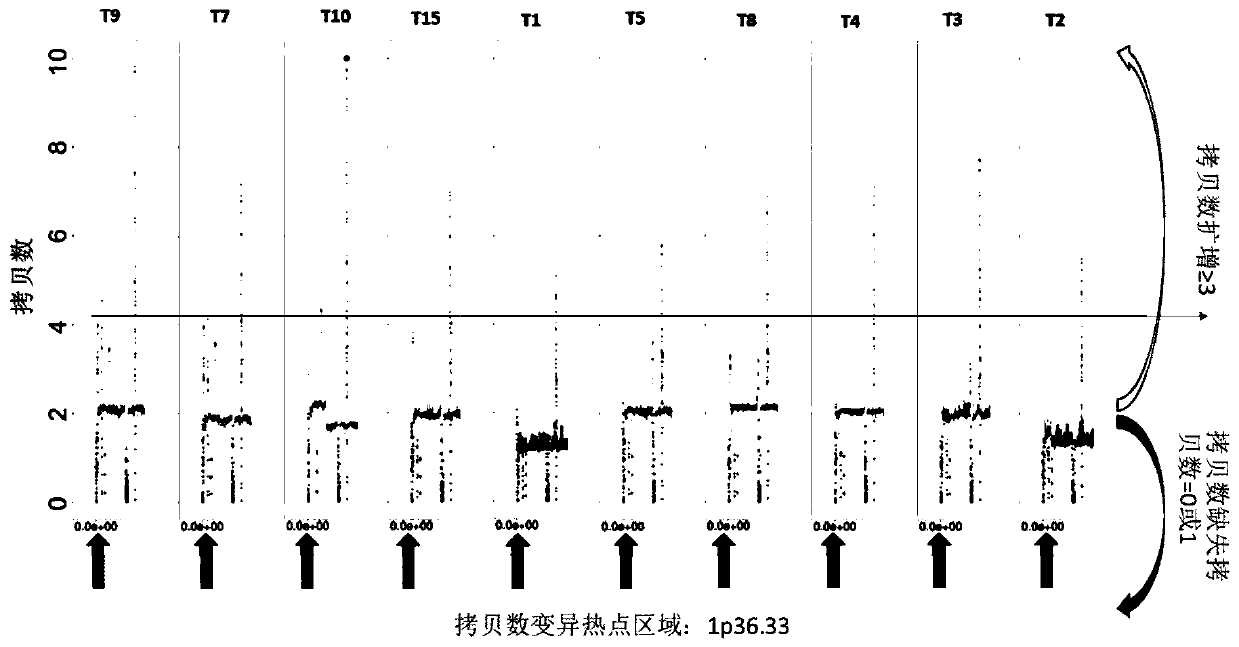
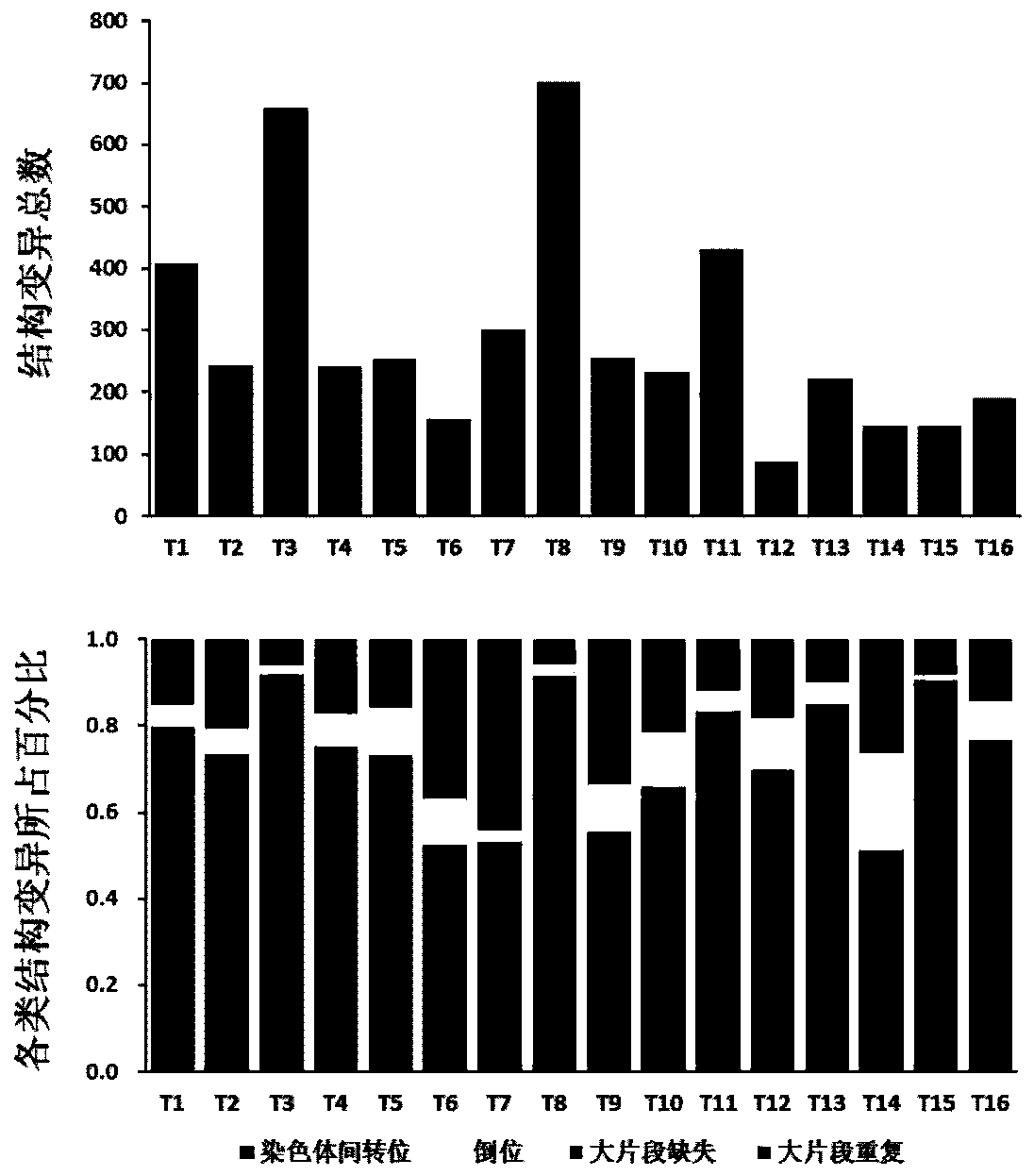
Genome recombination fingerprint for characterizing hHRD homologous recombination deficiency and identification method thereof
The invention relates to a novel method for identifying homologous recombination deficiency and application thereof. More particularly, the present invention relates to a characteristic genome recombination fingerprint which is related to hHRD type homologous recombination repair deficiency and is resolved by using high throughput genome re-sequencing, bioinformatics analysis and statistical correlation analysis. The characteristic hHRD recombination fingerprint is caused by the functional deficiency of a specific homologous recombination repair mechanism (namely CRL4WDR70-H2B mono-ubiquitination pathway), and comprises the total frequency of genome recombination in a single sample, the composition of chromosome structure variation types and site-specific copy number variation. The recombinant fingerprint is used for identifying infectious diseases or tumors with the characteristic mutant fingerprint, and is used for guiding targeted drug treatment of PARP inhibitors. The invention relates to the method and application thereof for diseases including but not limited to breast cancer, ovarian cancer, endometrial (like) cancer, ovarian clear cell cancer, prostate cancer, pancreatic cancer, skin cancer and gastric cancer with such characteristics, as well as hepatitis B virus infection or related liver fibrosis cirrhosis, liver cancer and cholangiocarcinoma.
Owner:成都吉诺迈尔生物科技有限公司 +1
Pap staining solution and application method
InactiveCN108535077AFast coloringRinsing floating color is convenient and simplePreparing sample for investigationClear cellNuclear staining
The invention discloses a Pap staining solution and application thereof, and belongs to the field of biotechnology. Specifically, the Pap staining solution contains a cell nuclear staining solution and a cytosolic staining solution. The Pap staining solution provided by the invention is simple to operate, and ensures uniform staining, clear cell structure after staining and bright cell colors, soas to facilitate diagnostic observation and judgment of clinical pathology.
Owner:南京福怡科技发展股份有限公司
Biomarker based prognostic model for predicting overall survival in patients with metastatic clear cell kidney cancer
The present invention describes a method of using a molecular prognostic signature, to predict overall survival in patients with metastatic clear cell renal cell carcinoma. The present invention also describes a method of selecting therapy and a process for patient risk stratification based on the molecular prognostic signature analysis.
Owner:CEDARS SINAI MEDICAL CENT +1
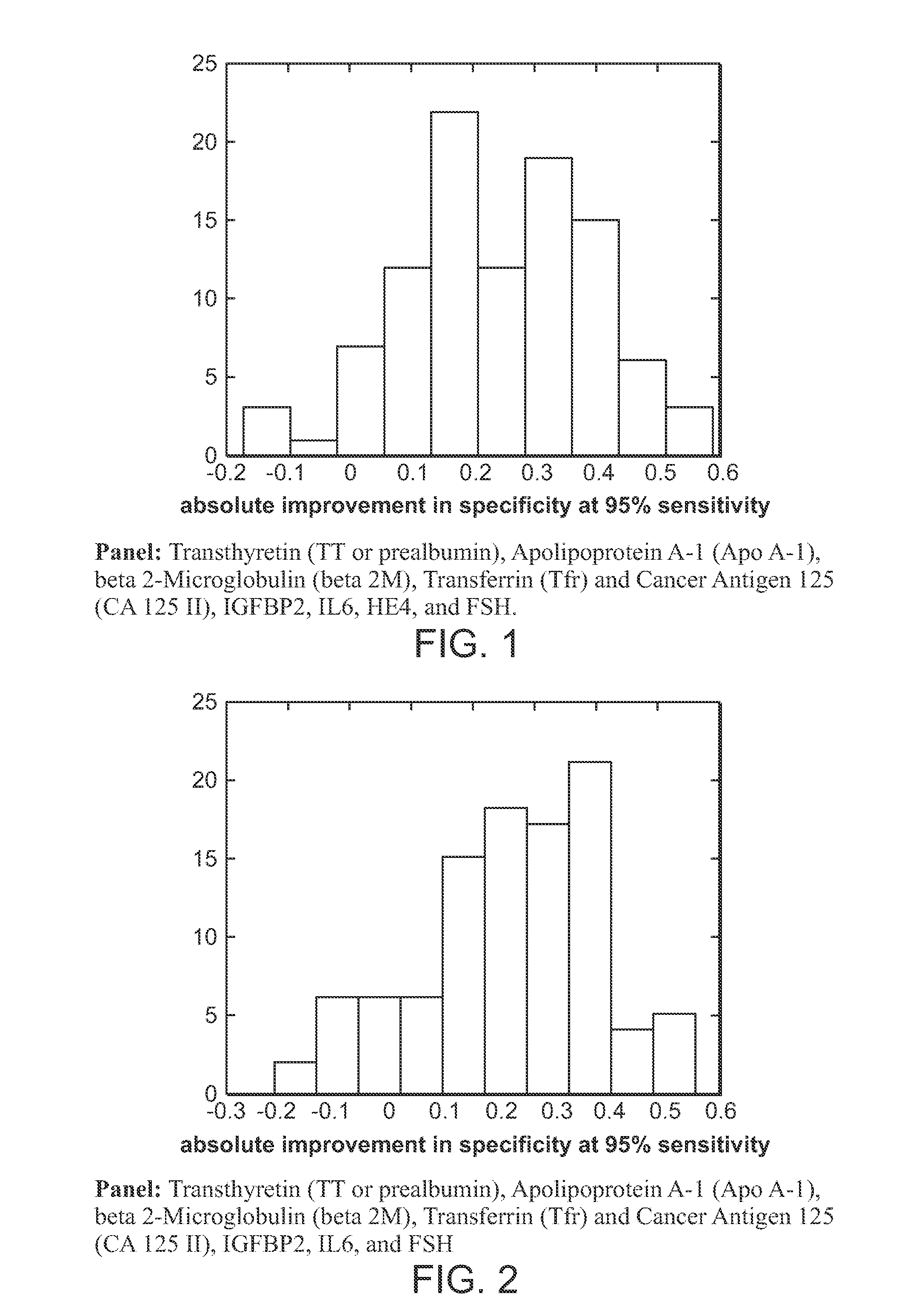
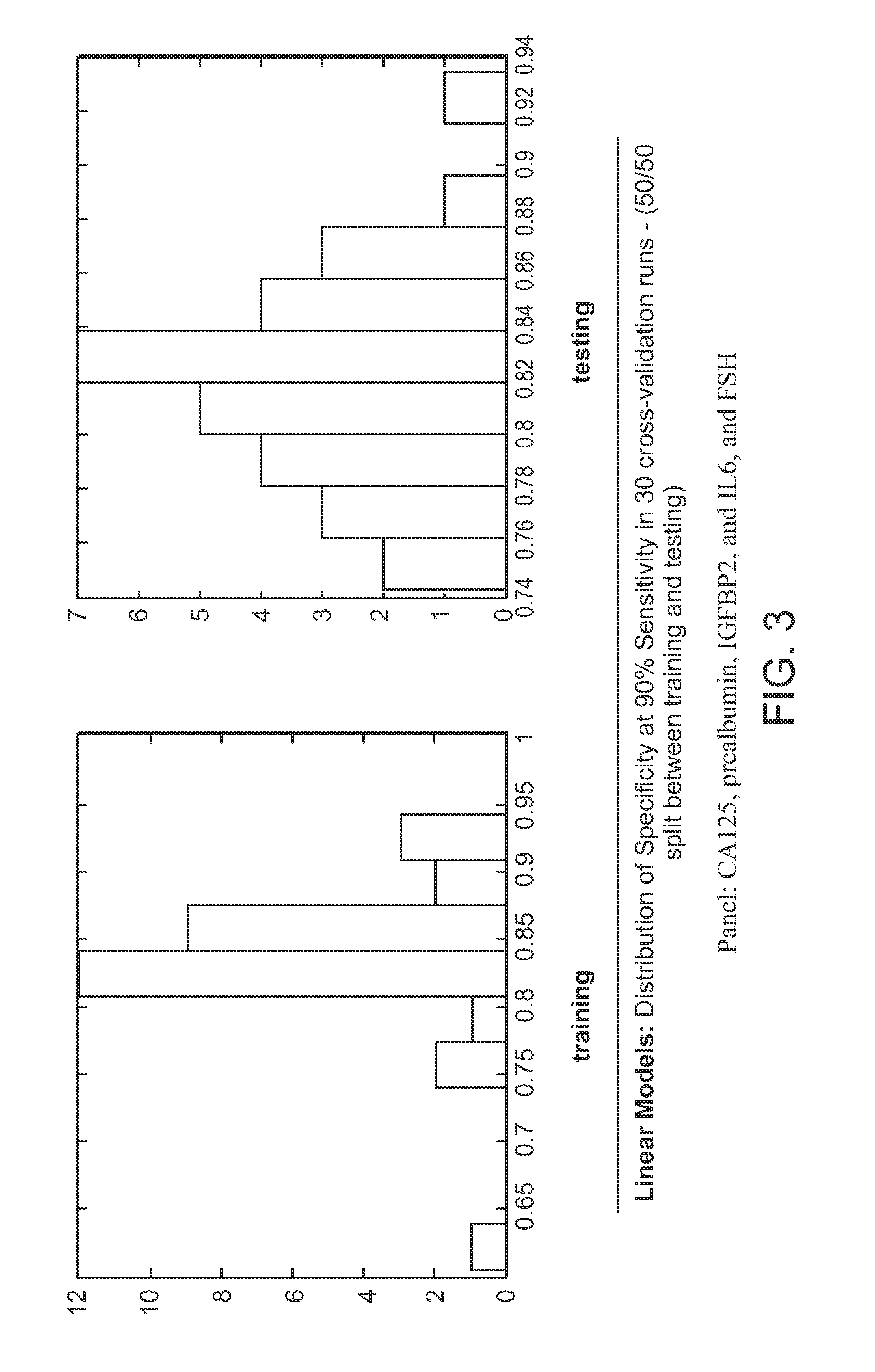
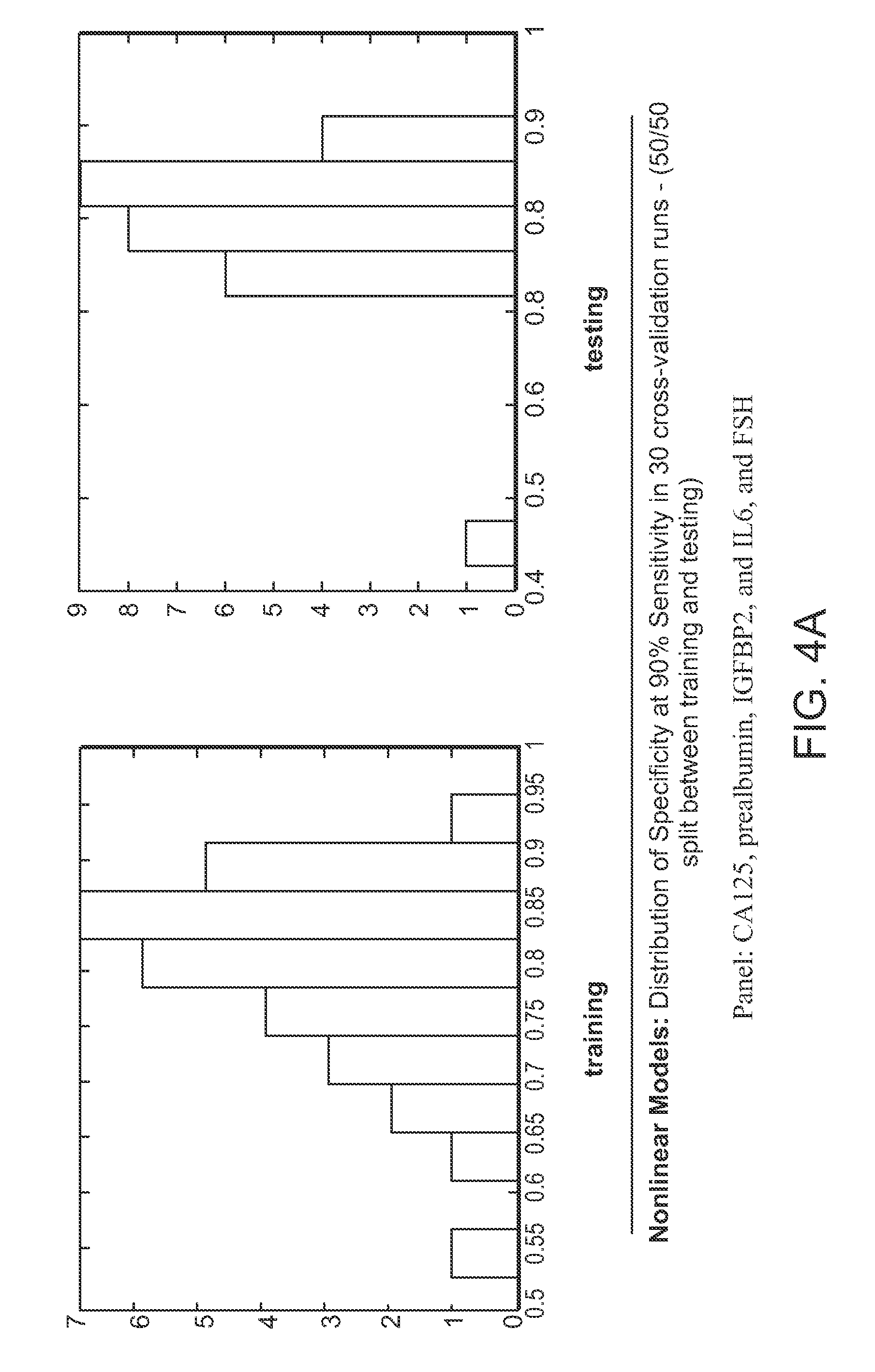
Compositions for ovarian cancer assessment having improved specificty
InactiveUS20160109455A1High degree of sensitivityReduce false alarm ratePeptide librariesLibrary screeningPre-operative evaluationPost menopausal
The present invention provides compositions and methods that provide a high degree of sensitivity and a high degree of specificity for the preoperative assessment of ovarian tumors in a variety of subject's (e.g., pre- and post-menopausal women) having a variety of ovarian cancer types (e.g., clear cell / mucinous, low malignant potential, high malignant potential) and at a variety of disease states (e.g., early and late stage).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Somatic cell culturing method for effectively promoting mongolian oak globular embryogeny
InactiveCN110235785AHigh induction rateHigh somatic embryo induction rateHorticulture methodsPlant tissue cultureMongolian oakClear cell
The invention provides a somatic cell culturing method for effectively promoting mongolian oak globular embryogeny. The method is characterized by comprising the following steps of selection and treatment of an explant, disinfection for the explant, inoculation, callus induction and somatic embryo induction. The somatic cell culturing method for effectively promoting mongolian oak globular embryogeny has the following advantages that the rate of callus induction and the somatic embryo induction rate are high, and embryonic callus with a small cell volume, compact and thick cytoplasm and a large and clear cell nucleus is obtained; the problems are solved that cutting rooting of mongolian oak is difficult, the propagation coefficient is small, the speed is low, and large-scale production cannot be formed for meeting the market requirements, and by utilizing the cell totipotency, the purpose of large-scale cultivation of good plants in a short period is achieved; the possibility is provided for establishment of the asexual propagation system of the mongolian oak and industrialized seedling culturing of the mongolian oak.
Owner:SHENYANG AGRI UNIV
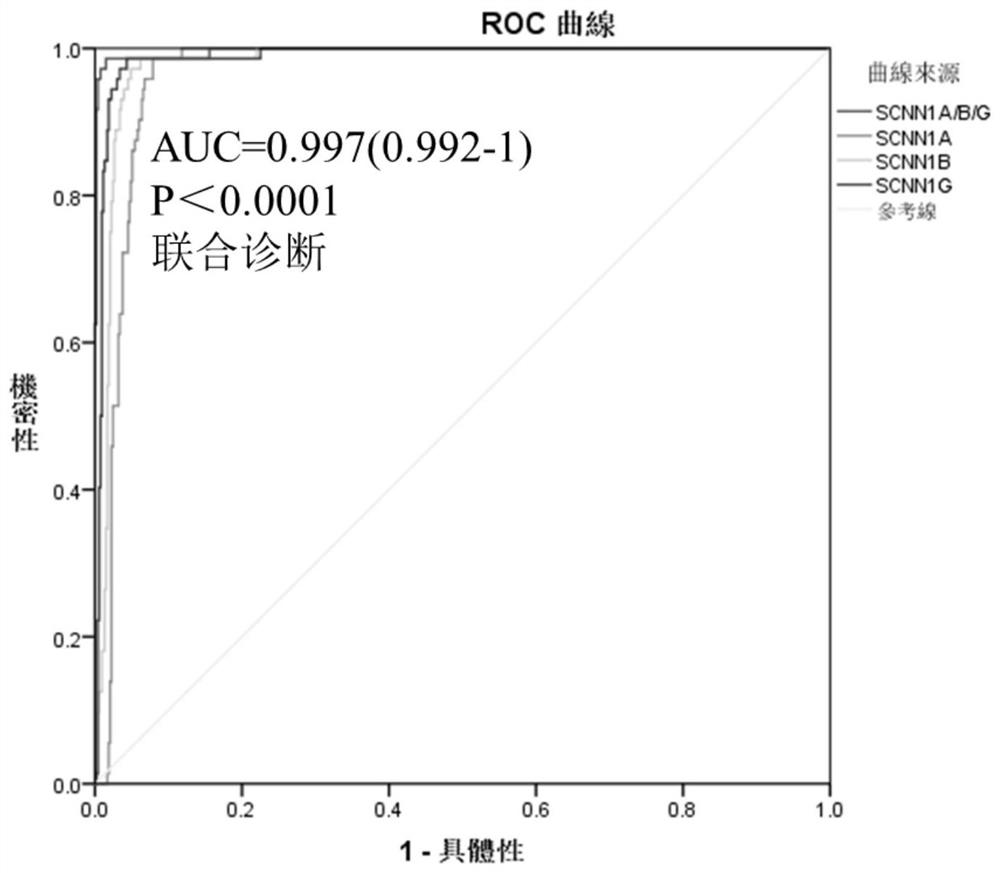
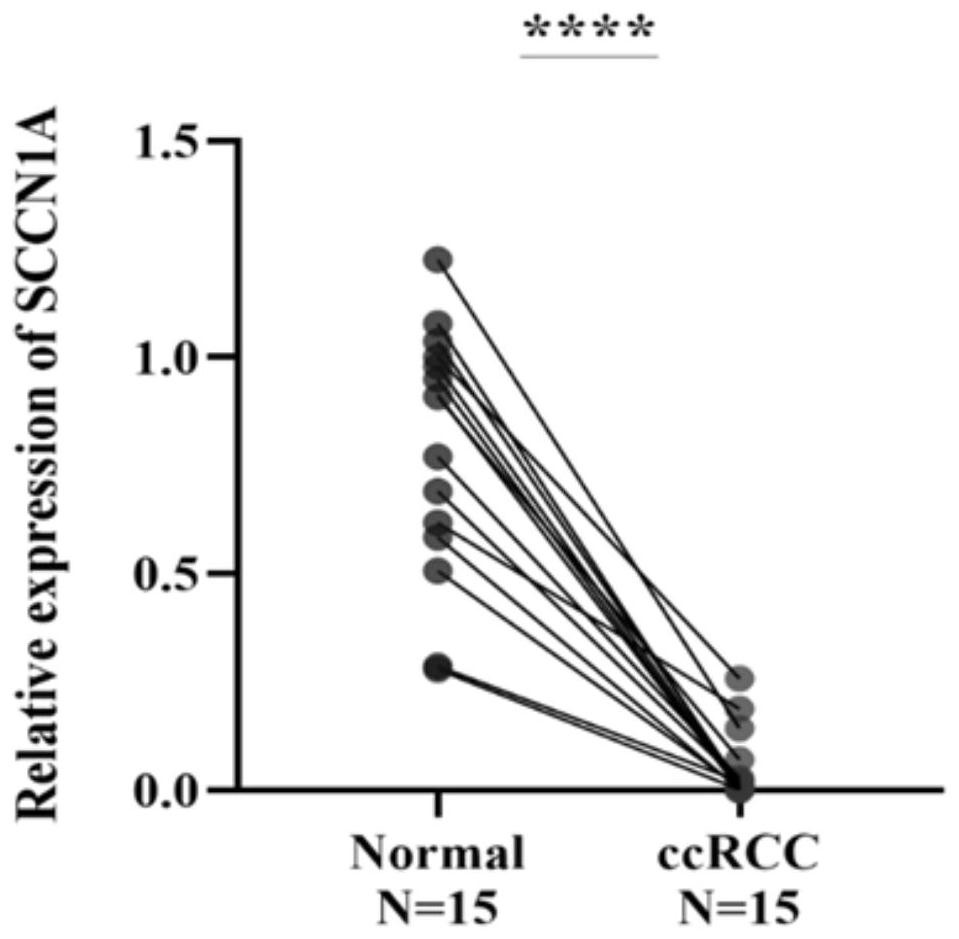
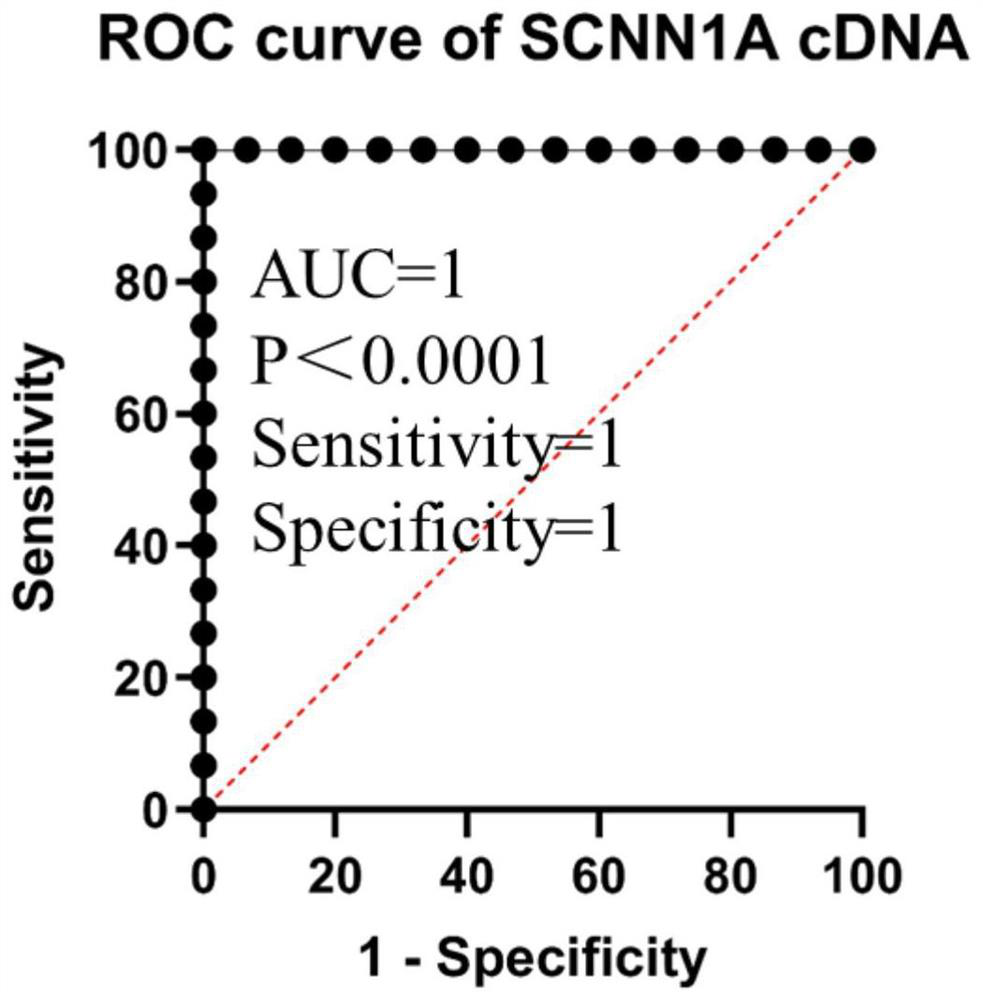
SCNN1 primer and diagnostic kit for clear cell nuclear cell carcinoma and application of SCNN1 primer
ActiveCN112553341AStrong specificityFast and good diagnosticsMicrobiological testing/measurementDNA/RNA fragmentationForward primerNucleotide
The SCNN1 primer for rapidly diagnosing clear cell nuclear cell carcinoma provided by the invention comprises an internal reference primer pair which is GAPDH, mRNA sequence forward primers and reverse primers of SCNN1A genes with nucleotide sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2, mRNA sequence forward primers and reverse primers of SCNN1B genes with nucleotide sequences shown in SEQ IDNO: 3 and SEQ ID NO: 4, and mRNA sequence forward primers and reverse primers of SCNN1G genes with nucleotide sequences shown in SEQ ID NO: 5 and SEQ ID NO: 6, which take human GAPDH as an internal reference control, and forward primers and reverse primers, having nucleotide sequences shown as SEQ ID NO: 7 and SEQ ID NO: 8. A corresponding kit is also prepared. The kit comprises the following reagents: a tissue lysis solution Trizol, an SYBRGreen Master Mix reaction solution, a reverse transcription reaction solution, a negative control and a positive control. The primer and the kit can eliminate false negative and false positive of clinical sample detection, and are safe, reliable, high in accuracy and suitable for clinical popularization.
Owner:GUANGXI MEDICAL UNIVERSITY
Lithographic printing projector containing secondary electronic clear cell
InactiveCN1497358APrevent attractionPrevent secondary electronsSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusPulse beamVoltage pulse
A lithographic projection apparatus is provided wherein an object situated in a pulsed beam of radiation has an electrode in its vicinity and a voltage source connected either to the electrode or to the object. This configuration can provide a negative voltage pulse to the object relative to the electrode. The beam of radiation and the voltage pulse from the voltage source are provided in phase or out of phase. In this way, the object is shielded against secondary electrons generated by radiation beam illumination.
Owner:ASML NETHERLANDS BV
Cells for clearing amyloid polypeptide and application thereof
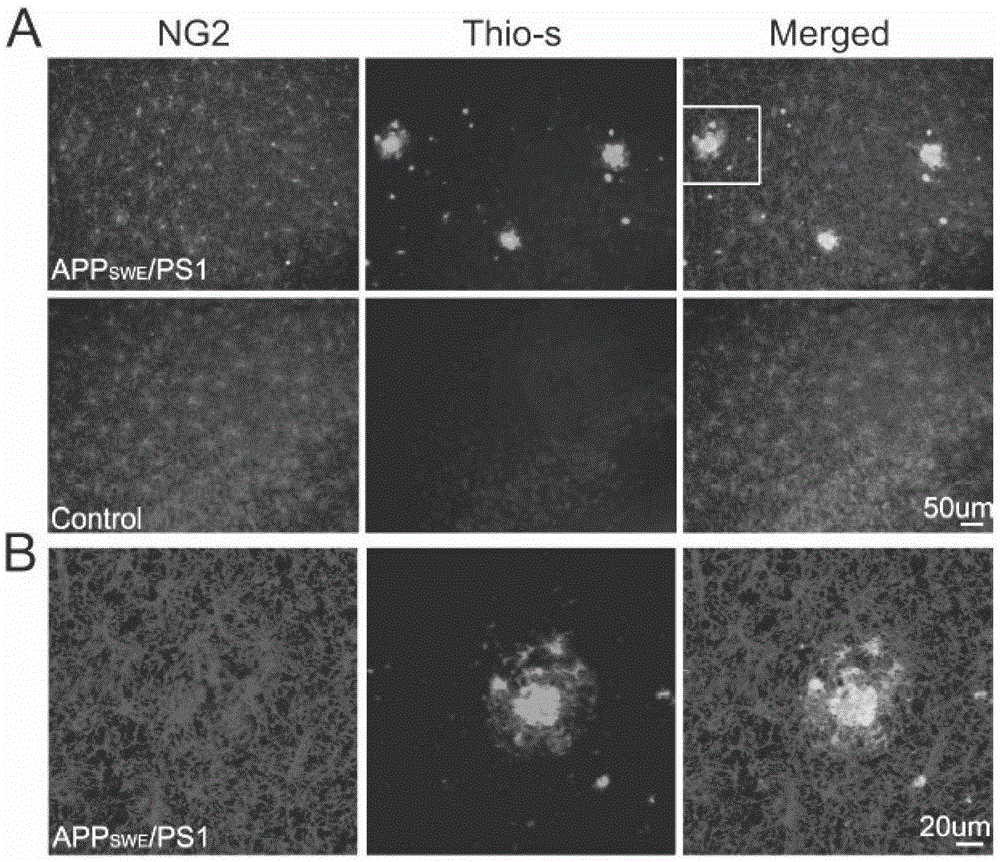
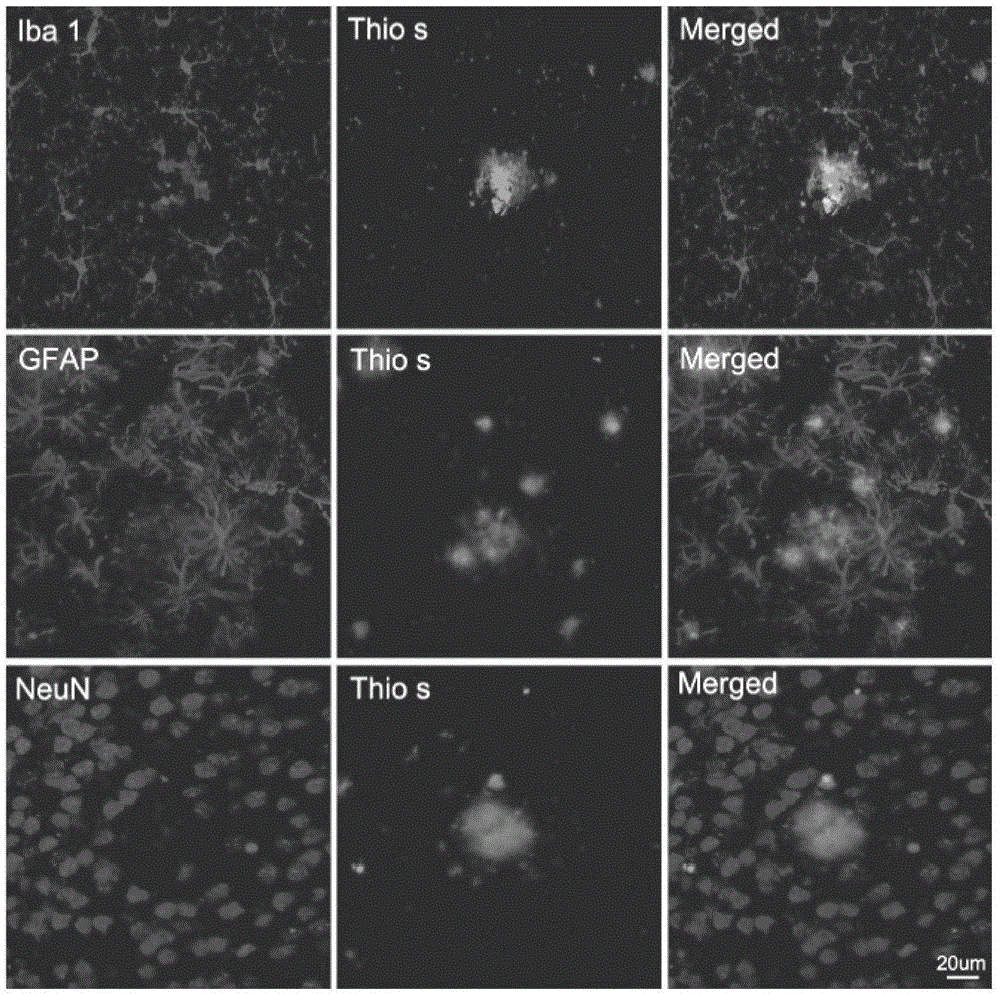
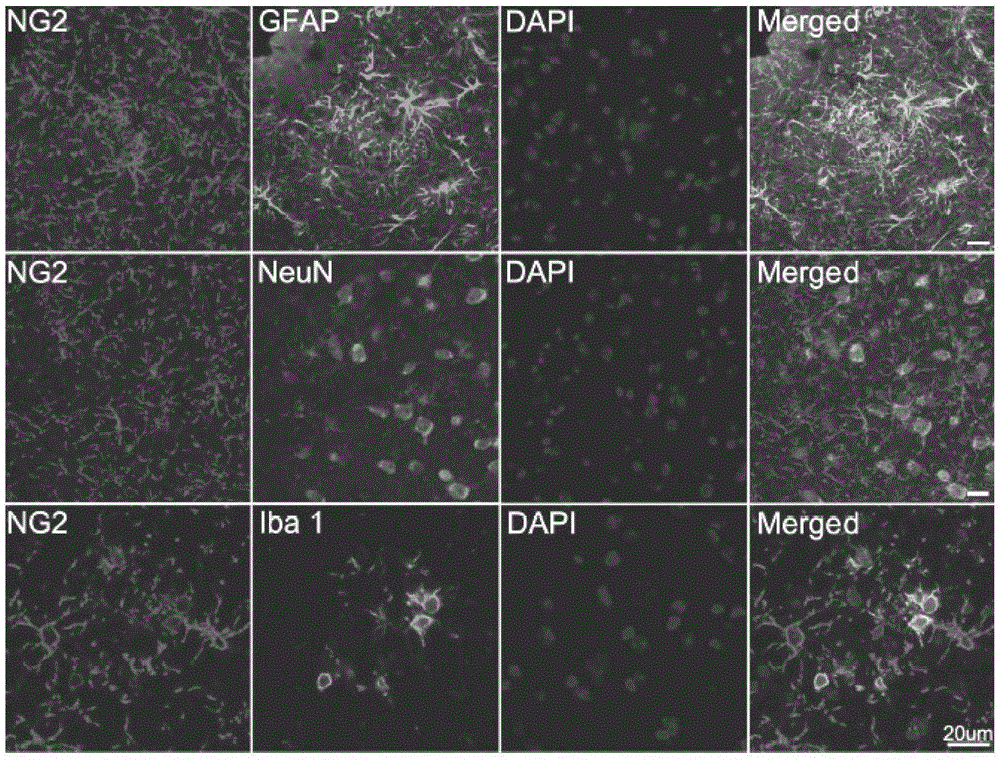
The invention relates to a cell for removing amyloid polypeptide and a use thereof. The invention first discloses that the NG2 cell can be activated by A beta and phagocytose A beta. The NG2 cell can be used as a pharmaceutical target for researching and screening a drug for activation or promotion of NG2 phagocytosis on A beta. The NG2 cell provides an effective approach for treating neurodegenerative diseases caused by excess A beta in the brain.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Screening method of stress-resistance portunus trituberculatus
InactiveCN110029143AGood resistance to screeningStrong stress resistanceMicrobiological testing/measurementClimate change adaptationClear cellScreening method
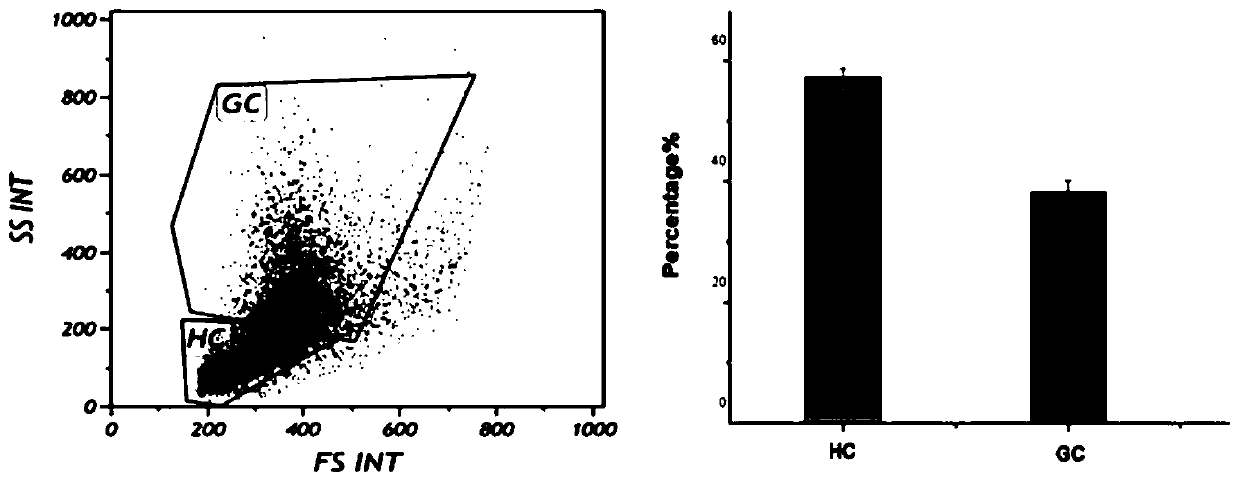
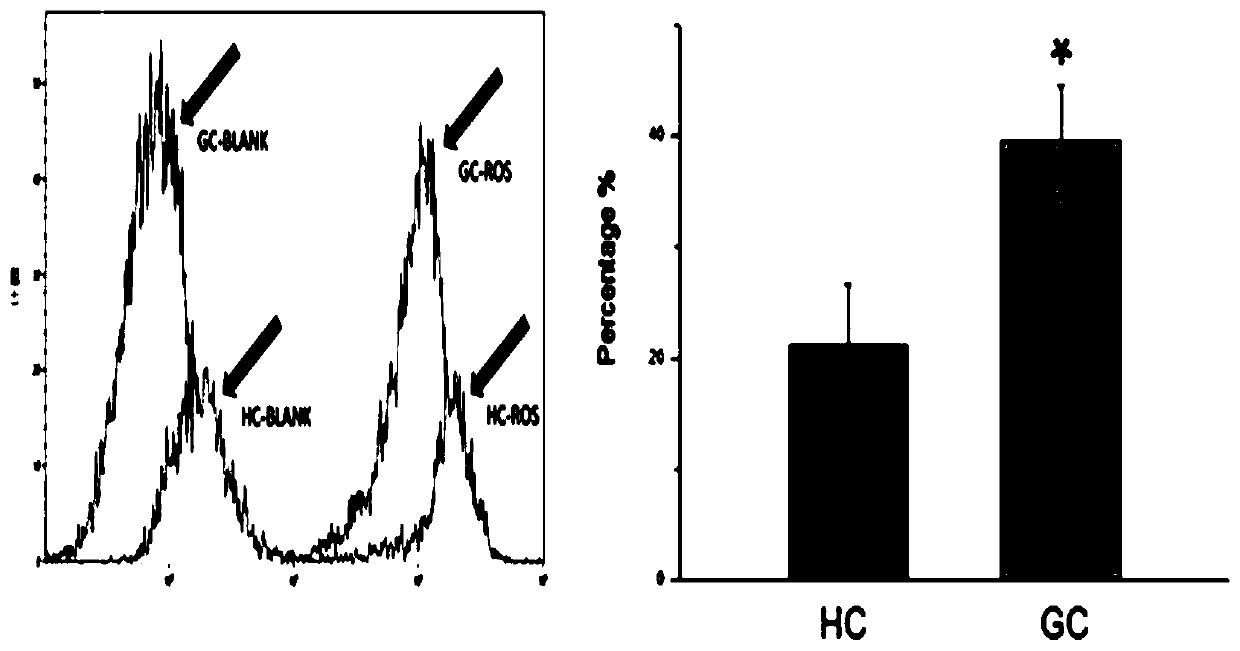
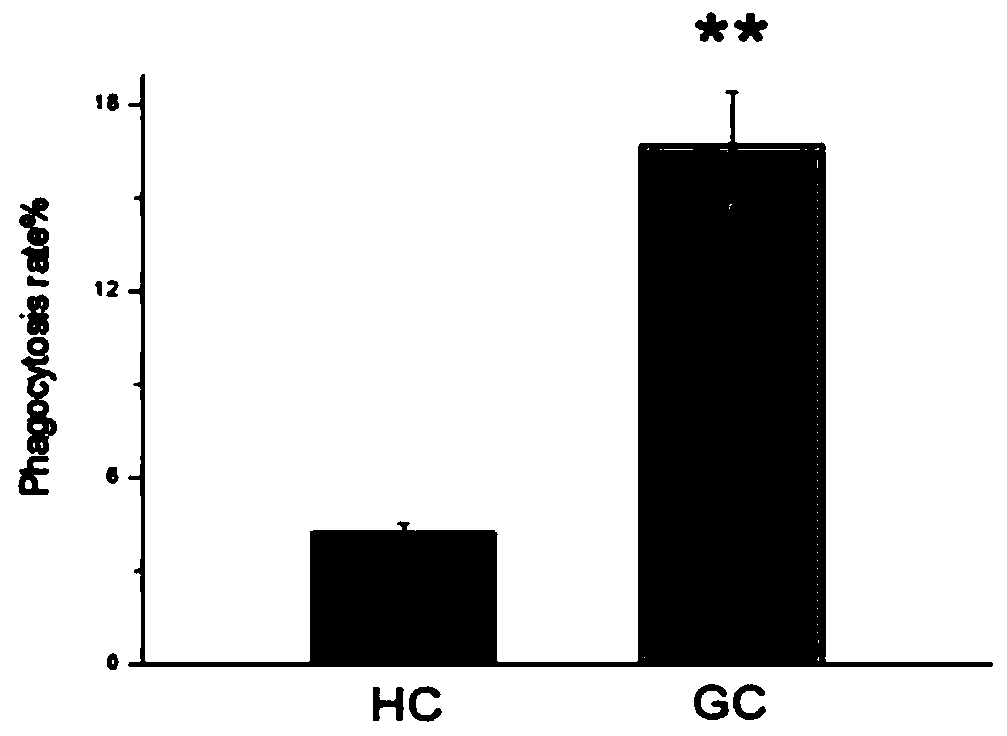
The invention aims to provide a screening method of stress-resistance portunus trituberculatus. Portunus trituberculatus good in stress resistance can be effectively screened for culturing young portunus trituberculatus. The screening method comprises the step of firstly providing a standard for screening stress-resistance portunus trituberculatus, wherein the standard of the stress-resistance portunus trituberculatus includes the following one or several indexes that the content of granulosa cells in blood cells is 38.47+ / -1.78%, the content of clear cells in the blood cells is 57.44+ / -1.28%,the content of active oxygen of the clear cells in the blood cells is not less than 22.7%, the content of active oxygen of the granulosa cells in the blood cells is not less than 40.19%, the phagocytosing rate of the clear cells in the blood cells is not less than 4.67%, the phagocytosing rate of the granulosa cells in the blood cells is not less than 15.29%, and the above standard is used for screening the stress-resistance portunus trituberculatus. According to the screening method disclosed by the invention, the condition for detecting the blood cells of the portunus trituberculatus with aflow cytometer is determined, the standard for screening the stress-resistance portunus trituberculatus is obtained, and the standard can be used for screening portunus trituberculatus varieties having good stress resistance.
Owner:NINGBO UNIV
Method for screening antioxidant by using phascolosoma esculenta
ActiveCN113670875AReal metabolismMorphological results are simpleClimate change adaptationFluorescence/phosphorescenceClear cellCell membrane
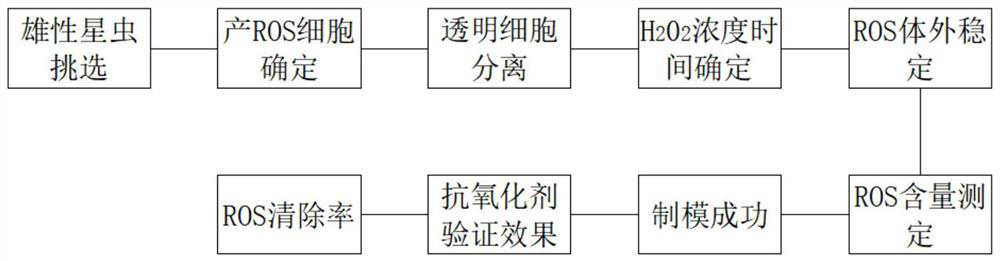
The invention discloses a method for screening an antioxidant by using phascolosoma esculenta, which comprises three steps of phascolosoma esculenta culture, phascolosoma esculenta ROS model establishment and model verification. The establishment of the phascolosoma esculenta ROS model comprises the following six steps: selecting tested phascolosoma esculenta, determining lymphatic blood cells for producing ROS, determining a clear cell separation method, determining the concentration and action time of a positive control compound H2O2, and performing ROS in-vitro stability and reproducibility experiments. The phascolosoma esculenta model is used for screening the antioxidant and has a good application prospect, and the phascolosoma esculenta belongs to closed-tube circulating body cavity animals and can truly reflect organism metabolism; transparent cells of phascolosoma esculenta are thin in membrane, and fluorescent markers and antioxidants are easy to permeate into the transparent cells, so that an anti-oxidation result can be better displayed; the phascolosoma esculenta is easy to breed, the cost is low, and the experimental operation is simple.
Owner:NINGDE NORMAL UNIV
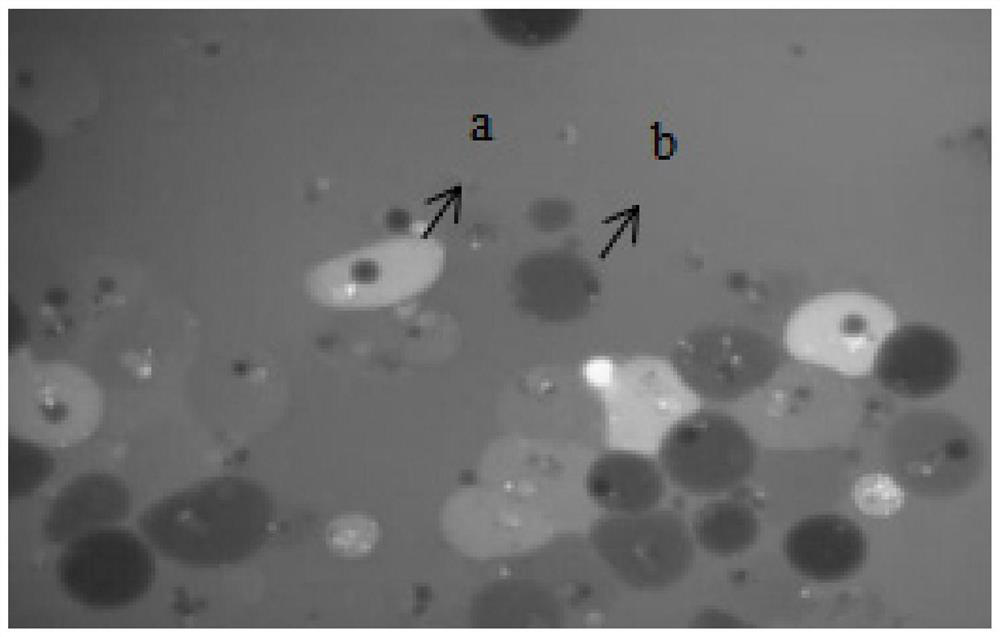
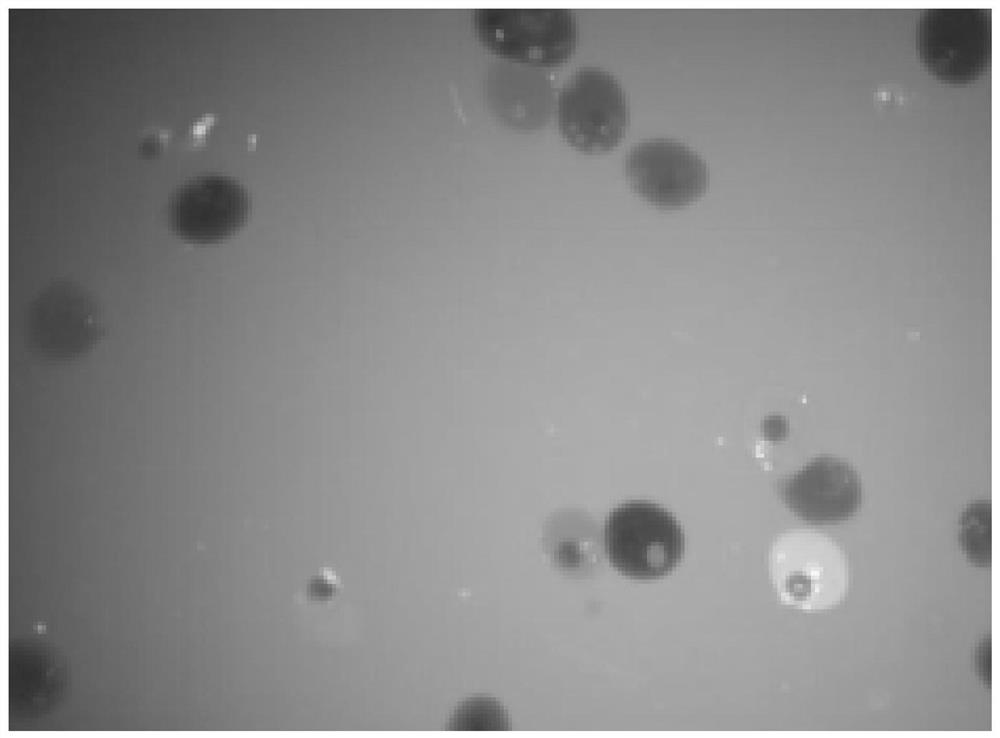
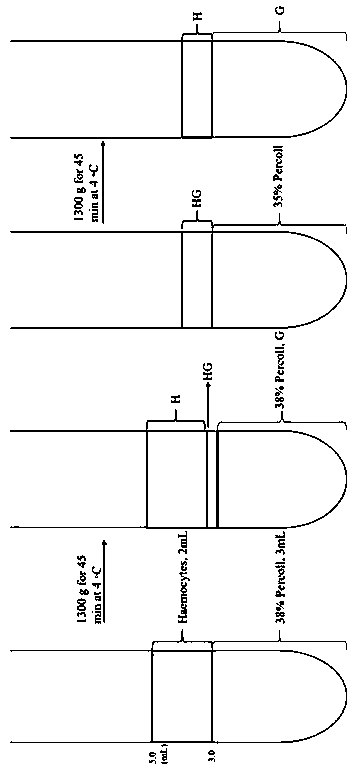
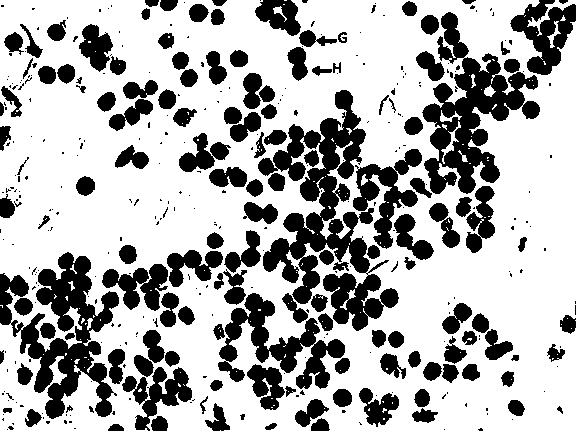
Method for separating babylonia areolata granular cells and clear cells by Percoll serving as medium
PendingCN110129254AReduced Chances of ContaminationSimple methodCell dissociation methodsInvertebrate cellsGranular cellClear cell
The invention belongs to the technical field of babylonia areolata hemocyte separation and discloses a method for separating babylonia areolata granular cells and clear cells by Percoll serving as medium. The technical scheme is characterized in that the method includes steps: taking a 15mL centrifuge tube, adding 3mL Percoll solution in volume fraction of 38% and 2mL of babylonia areolata hemocyte suspension, and centrifuging for 45min under conditions of 1300g and 4 DEG C; after solution is layered into upper and lower layers and an interface layer between the upper and lower layers, takinganother new 15mL centrifuge tube, adding 3mL Percoll solution in volume fraction of 35% and 1mL of interface solution of the upper and lower layers, centrifuging for 45min under conditions of 1300g and 4 DEG C, and collecting upper-layer solution and lower-layer solution respectively to finally realize separation of babylonia areolata granular cells and clear cells. A separation process is simple,convenient, easy in operation, high in specificity and low in cost.
Owner:HENAN NORMAL UNIV
A method for separating blood cells of Portunus trituberculatus
ActiveCN108118024BHigh activityEasy to operateCell dissociation methodsInvertebrate cellsGranular cellClear cell
The invention discloses a blood cell separation method for portunus trituberculatus. The method comprises the following steps: diluting iodixanol with an isotonic solution obtained by regulating an osmotic pressure of a PBS buffer solution by utilizing a sodium chloride solution, and performing gradient preparation to obtain three iodixanol solutions of different concentrations; forming gradient solutions of different densities by adopting a cushion technology, and standing to form a continuous density gradient solution to serve as a cell separation solution; collecting blood cells of portunustrituberculatus, and taking mixed cell suspension obtained by diluting the isotonic solution; slowly dropping the mixed cell suspension into the cell separation solution, centrifuging, and dividing the blood cells of portunus trituberculatus into three layers, wherein the uppermost layer refers to clear cells, the intermediate layer refers to small granular cells, and the lowest layer refers to large granular cells. The method has the advantages that only one-step density gradient centrifugation is adopted, the operation is simple, and experiments discover that the separated blood cells haveexcellent viabilities and can be used for cell culture.
Owner:NINGBO UNIV
Method for removing host DNA in rabies vaccine
PendingCN113881644AEnsure safetyOptimize production and processing methodsSsRNA viruses negative-senseRecovery/purificationClear cellRabies vaccine
The invention discloses a method for removing host DNA in a rabies vaccine. The method comprises the following steps of: firstly selecting Vero cells with clear cell boundaries and few senile cells in a production process, carrying out subculture after cell counting, adjusting to the required cell density, then carrying out virus inoculation, and harvesting a virus solution about 6 days after virus inoculation; on the basis, uniformly mixing the virus solution with EDTA, then carrying out ultrasonic dispersion, wherein EDTA is used as a metal chelating agent and can chelate divalent cations to disperse DNA; then carrying out ultrasonic dispersion and high-pressure homogenization treatment on the basis of the step, wherein ultrasonic dispersion and high-pressure homogenization treatment are combined with each other to shear the host DNA, so that a host DNA chain is broken and broken, and the removal efficiency of the subsequent residual host DNA is improved. According to the scheme, magnetic silicon dioxide is introduced, separation of the magnetic silicon dioxide and the vaccine can be realized through an external magnetic field, the residual DNA is adsorbed in the process for purification, new impurities do not need to be introduced, the quality of the vaccine is greatly improved, and the practicability is higher.
Owner:江苏金迪克生物技术股份有限公司
GN formaldehyde-free rapid stationary liquid and preparation method
InactiveCN111060374ALess irritatingKeep intactPreparing sample for investigationAcetic acidTissue antigens
The invention relates to a GN formaldehyde-free rapid stationary liquid. The GN formaldehyde-free rapid stationary liquid is prepared from the following components in parts by volume: 60 to 80 percentof absolute ethyl alcohol, 10 to 20 percent of glacial acetic acid, 5 to 10 percent of glycerol, 5 to 10 percent of laurocapram and 0.3g / 100ml to 1g / 100ml of potassium sorbate. The beneficial effectsare that compared with the traditional formalin stationary liquid, all the components of the GN formaldehyde-free rapid stationary liquid are non-toxic or low-toxic substances; the GN formaldehyde-free rapid stationary liquid is commonly used in daily life, mild in smell, small in irritation to mucous membranes, high in fixing speed and capable of preserving the tissue form more completely, and has the advantages of more complete tissue antigen preservation, excellent fixing effect, easy coloring of tissue slices, clear cell contour of the tissue slices, easily available raw materials, simplepreparation method, no need of heating and other special conditions and low cost. The invention also provides a preparation method of the GN formaldehyde-free rapid stationary liquid.
Owner:NINGXIA UNIVERSITY
A kind of mouse fibroblast and its use
ActiveCN108504625BStrong value-added vitalityStatus freshEpidermal cells/skin cellsArtificial cell constructsIndividualized treatmentOncology
The invention discloses a mouse fibroblast and its application. in the field of cell biology. The cell is named mouse fibroblast MFC / HL‑041, and the deposit number is CCTCC NO: C201714. Mouse fibroblasts treated with drug mitogenin C or radiation irradiation are used for the separation and subculture of normal primary epithelial cells and primary tumor cells. The primary epithelial cells of human normal lung and lung cancer thus obtained are observed under a microscope, and they are fresh, densely arranged, with clear cell boundaries, strong three-dimensional sense, and polygonal epithelial cells. It can be used for physiological research of human or animal normal cells, virus infection model of normal cells in vitro, drug sensitivity detection of normal cells in vitro for different drugs, drug screening for individualized treatment of cancer patients and research and development of new anticancer drugs.
Owner:深圳涌泰生物科技有限公司
Aldehyde dehydrogenase 2 as a drug target in treatment of tumor cells with anthracycline chemotherapy drugs
InactiveCN103784465BOvercome the shortcomings of only relying on surgical treatmentIncrease lethalityOrganic active ingredientsAntineoplastic agentsCancer cellDrug target
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com