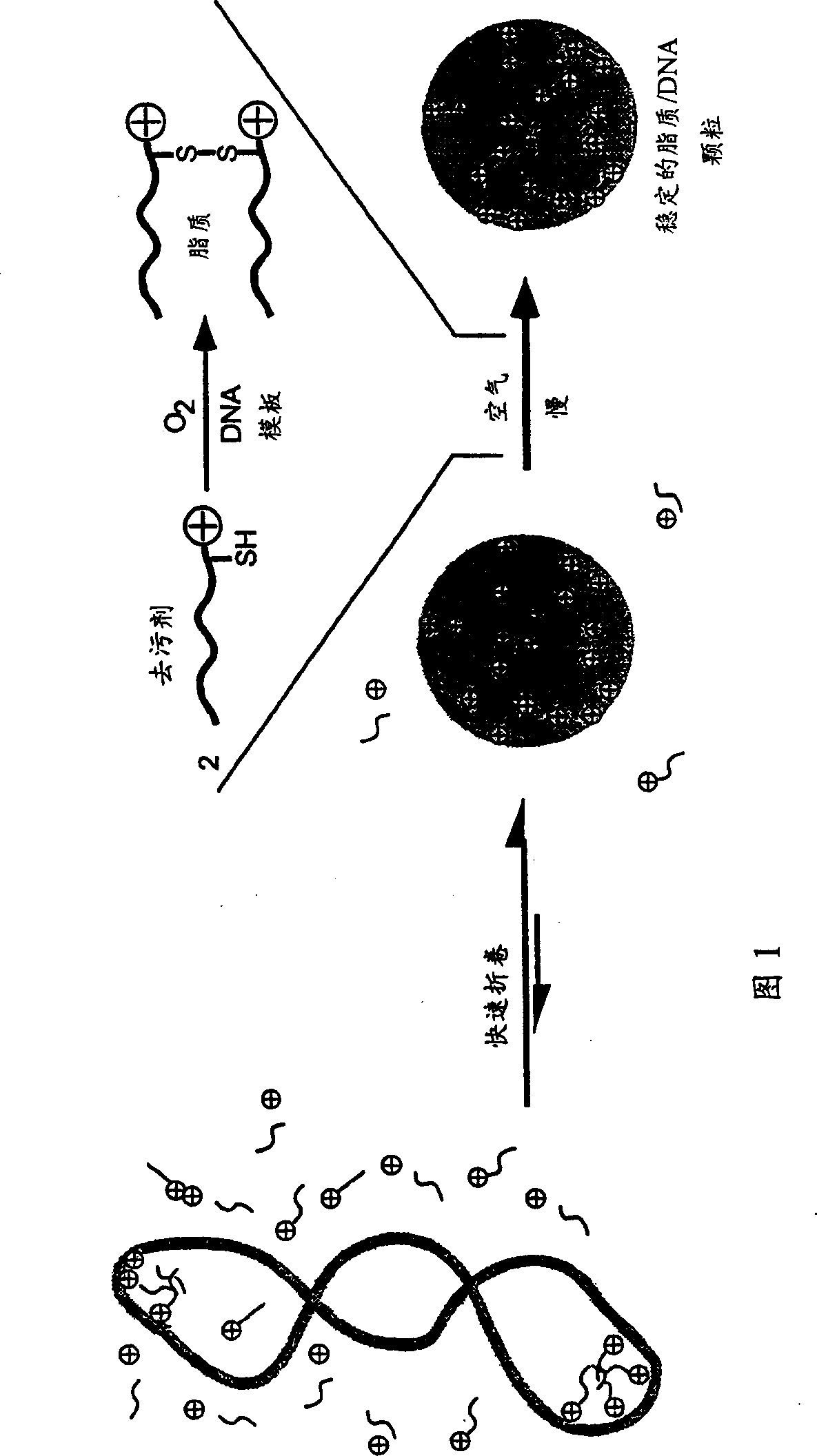
Transfection particles
A particle and molecular technology, applied in the field of transfection particles, which can solve the problems of DNA decondensation and cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
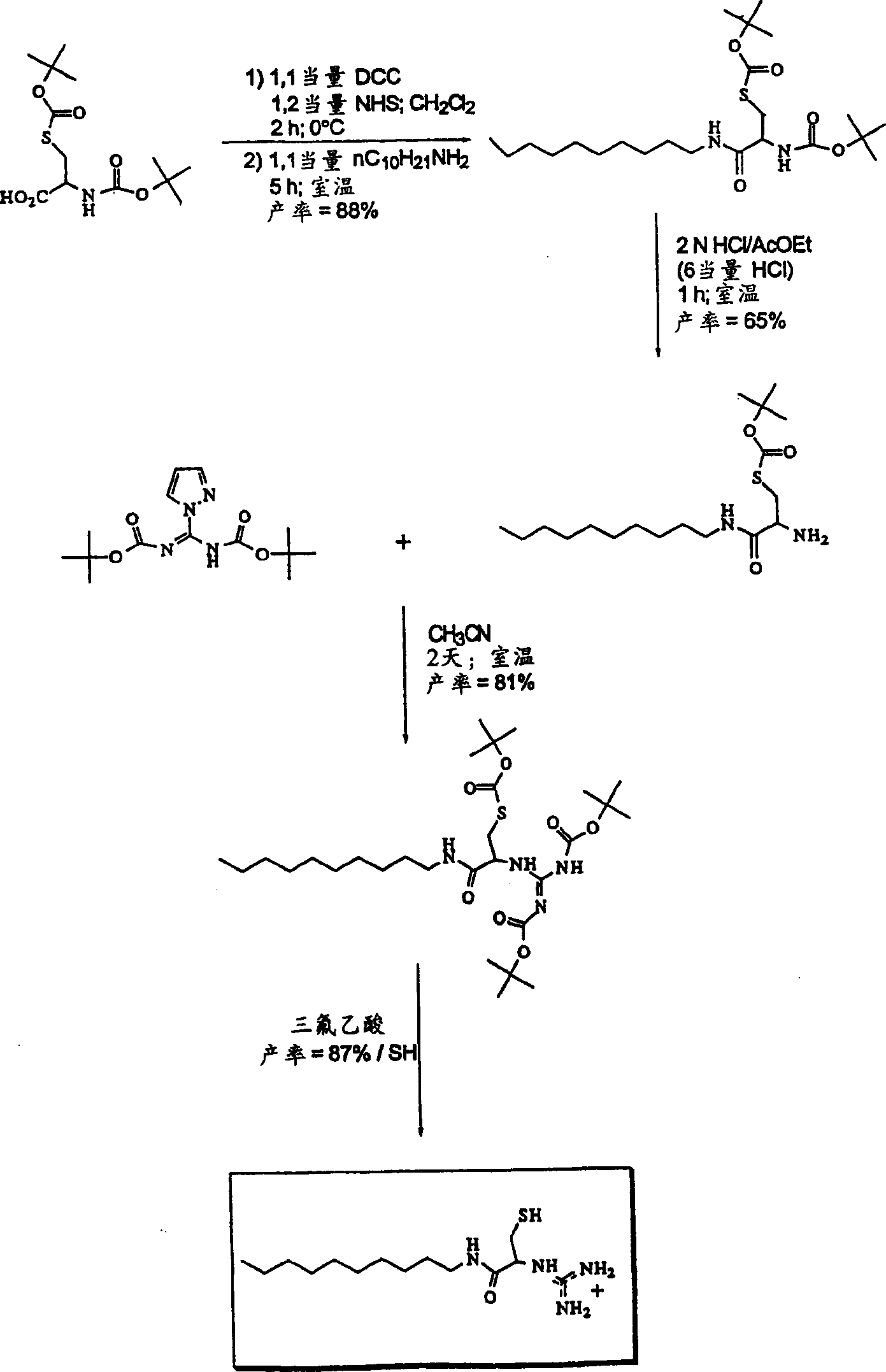
[0232] b) Synthesis of lipophilic guanidine-cysteine amide detergent
[0233] according to figure 2 The shown route was used to synthesize the lipophilic compound.
[0234] step 1
[0235]Preparation of N-tert-butoxycarbonyl-pyrazole-1-carboxamidine
[0236] Add 11.3ml of N,N-diisopropylethylamine (66mmol) and 4.40g of 1H-pyrazole to a solution of di-tert-butyl carbonate (7.43g, 33mmol) in 100ml of dichloromethane - 1-Formamidine hydrochloride (30 mmol). The solution was stirred at room temperature for 3 hours and evaporated to dryness. The resulting white solid was dissolved in 200 ml of ethyl acetate, the organic phase was washed with 5% (w / w) sodium bicarbonate, dried over magnesium sulfate, and finally evaporated to dryness. The residue was dissolved with boiling hexane. The solution was stored overnight at room temperature and then stored at 4°C for 24 hours. The resulting crystals were recovered by filtration and washed in cold hexane (0°C). After drying, 5.2...
Embodiment 2
[0290] a) Synthesis of lipophilic alcohol cysteine ester detergent
[0291] according to Figure 9 The synthesis was carried out according to the indicated route.
[0292] step 1
[0293] Preparation of N,S-di-tert-butoxycarbonyl-L-cysteine
[0294] 945 mg of L-cysteine chlorohydrate (6 mmol) was dissolved in 10 ml of deionized water, where the gas had been removed by vacuum. To this solution was added 2.625 g of BocOBoc (12 mmol) in 10 ml of THF and 5.4 ml of triethylamine (39 mmol). After stirring for 2 days at room temperature under an argon atmosphere, the reaction medium is evaporated to a volume of slightly less than 10 ml. Then 10 ml of deionized water was added, and the liquid phase was washed with ether to remove residual unreacted BocOBoc. The aqueous phase was then acidified to pH 3 with saturated citric acid and extracted with diethyl ether (3 x 60ml). The ether phase was washed with 0.5M citric acid (2 x 100ml), dried over magnesium sulfate, and evapor...
Embodiment 3
[0317] Synthesis of lipophilic alcohol guanidino-cysteine ester detergent
[0318] according to Figure 13 The synthesis was carried out according to the indicated route.
[0319] step 1
[0320] Preparation of S-tert-butoxycarbonylcysteine decyl ester
[0321] 231 mg of N,S-di-tert-butoxycarbonyl-cysteine decyl ester (0.5 mmol) were dissolved in 1.1 ml of 2.2N HCl / AcOEt. The solution was stirred at room temperature for 1 hour and then evaporated to dryness. The obtained residue was dissolved in 50 ml of ethyl acetate. The organic phase was washed twice with 25 ml of 5% (w / w) sodium bicarbonate, dried over magnesium sulfate and finally evaporated to dryness. The resulting oil was purified by silica gel chromatography (eluting with 0-3% methanol in dichloromethane) to give 141 mg of a yellow oil (0.39 mmol, 78% yield).
[0322] C 18 h 35 NO 4 S: 361.55g / mol
[0323] NMR 1 H(CDCl 3 ); δppm: 0.90 (t, J=7Hz, 3H, CH 3 ); 1.20-1.42 (m, 14H, CH 3 -O(CH 2 ) 7 -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com