Application of miRNA in preparation of chemotherapy drug resistance evaluation kit for high-grade serous epithelial ovarian cancer
A technology for drug resistance and ovarian cancer, applied in the field of biomedicine, can solve the problems of different histological features and stages, insufficient identification ability to guide clinical decision-making, and influence the identification ability of prediction models
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
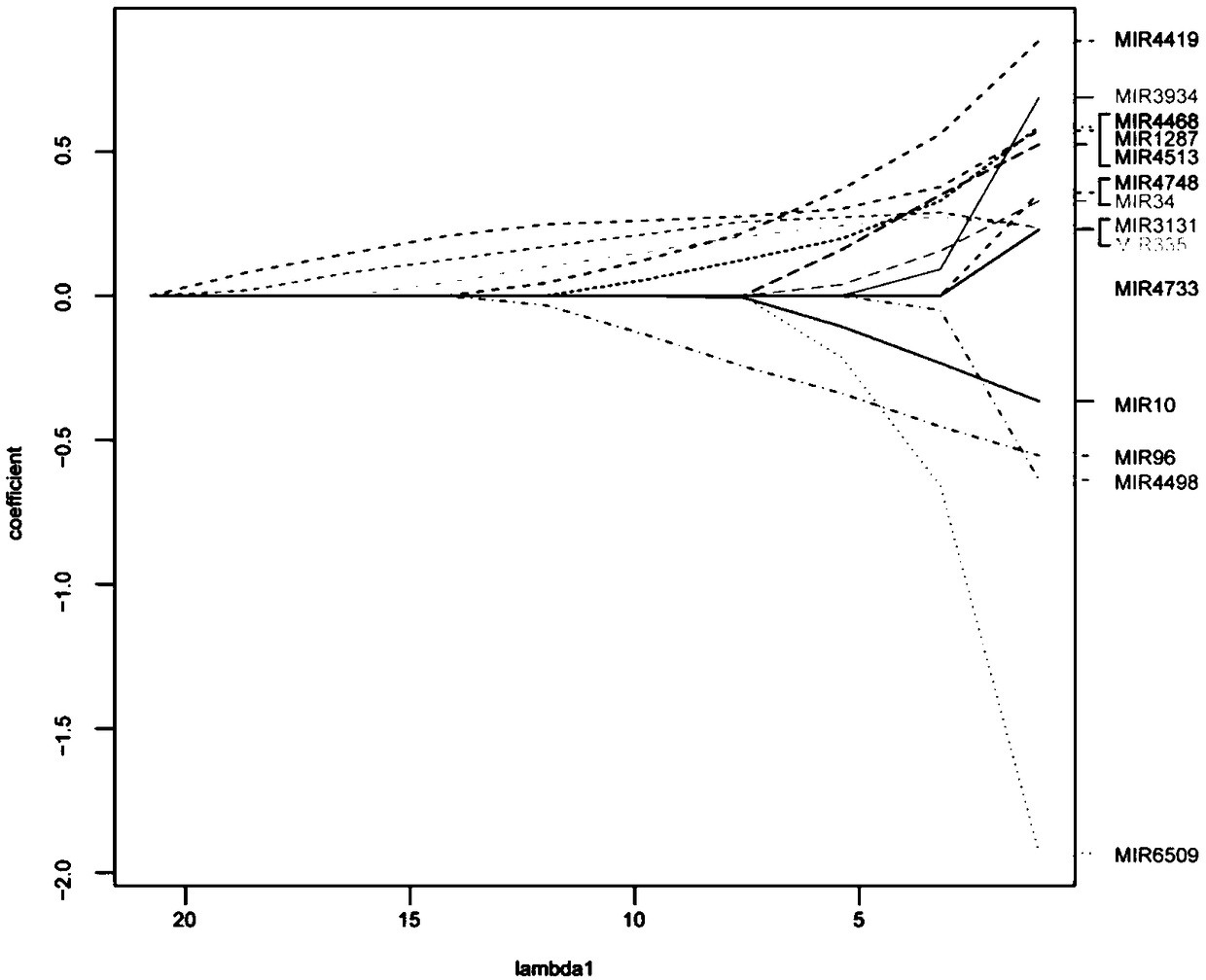
[0045] Example 1: Screening of miRNA markers related to platinum chemotherapy resistance in high-grade serous ovarian cancer
[0046] Application of miRNA in preparing reagents for evaluating chemotherapy resistance or recurrence of advanced serous epithelial ovarian cancer:
[0047] 1. Collection of patient tissue and clinical pathological data
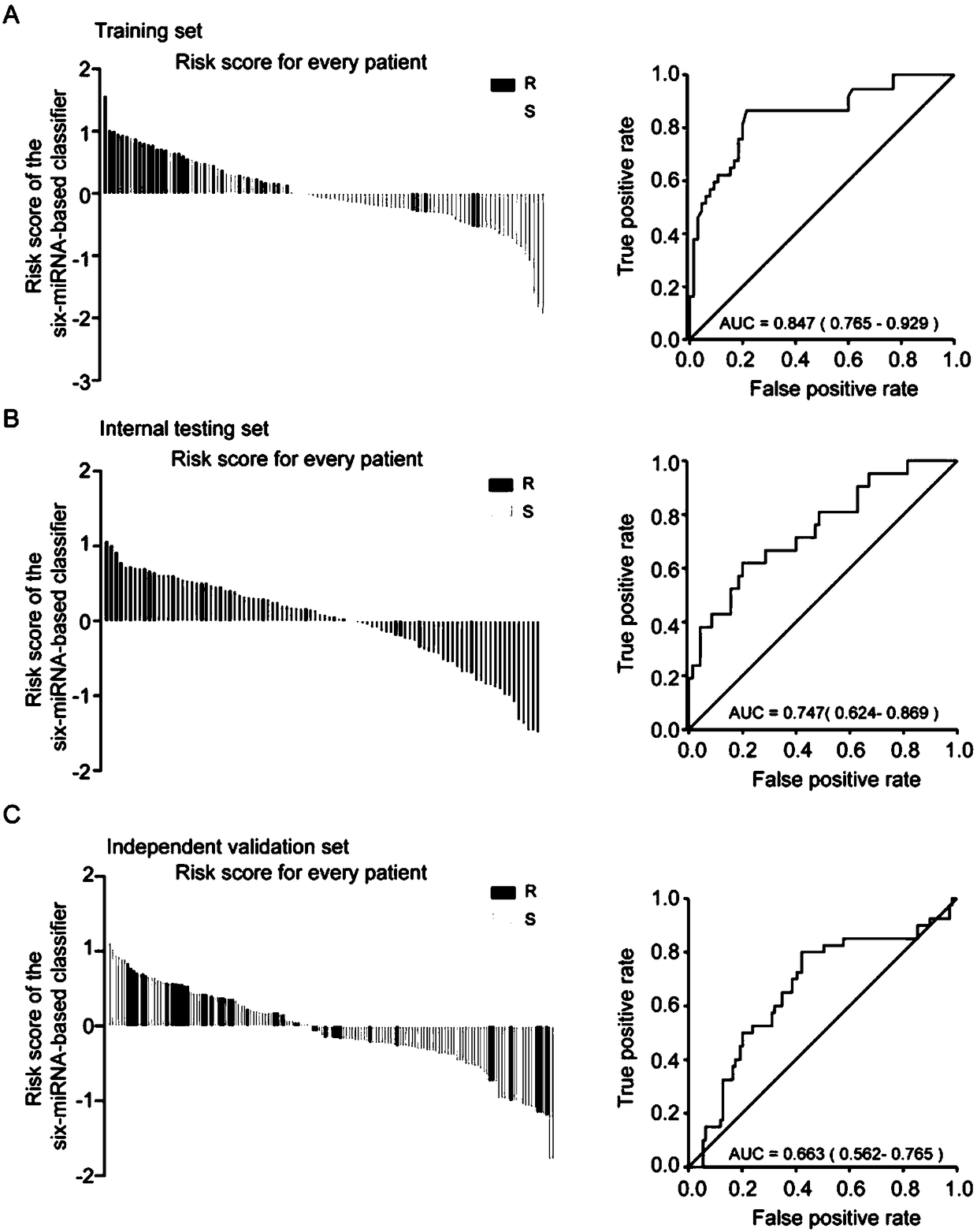
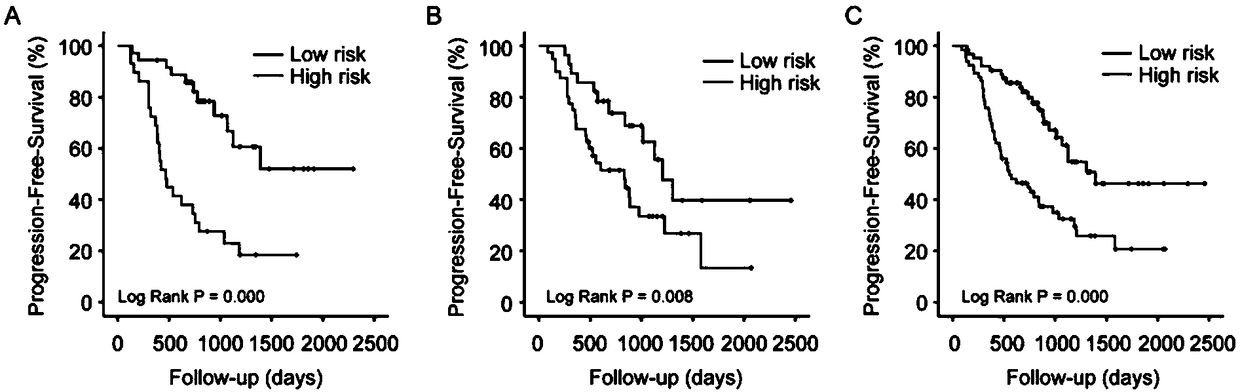
[0048] Among 349 patients with high-grade serous epithelial ovarian cancer, 101 were used as the training set, 98 were used as the internal validation set, and 150 were used as the independent validation set.
[0049] All included specimens meet the following entry criteria:
[0050] (1) All patients were primary high-grade serous ovarian cancer and did not receive any anti-cancer treatment before surgery.
[0051] (2) After the staging of ovarian cancer, the pathological type of the specimen was reviewed by the pathologist as high-grade serous ovarian cancer.
[0052] (3) The specimens used for RNA extraction are four 10 μm paraffin specimens, ...
Embodiment 2
[0084] Example 2. Preparation of miRNA detection kit
[0085] Kit based on RT-qPCR technology
[0086] In this embodiment, the miRNA detection kit based on nucleic acid amplification includes the following components:
[0087] (1) Total RNA extraction reagent: environmentally friendly dewaxing agent, tissue lysis buffer, proteinase K, Trizol solution, chloroform, isopropanol, ethanol, DEPC water;
[0088] (2) miRNA reverse transcription reagents: reverse transcriptase, RNase inhibitor, reverse transcription buffer, dNTP mixture.
[0089] (3) miRNA reverse transcription primers: miR-1287, miR-3131, miR-335, miR-4419, miR-4468, miR-96 reverse transcription primers, and U6 reverse transcription primers used as internal controls.
[0090] (4) PCR detection reagents for miRNA: Taq enzyme, Mg2+, PCR buffer, dNTP mixture, nucleic acid dye.
[0091] (5) miRNA nucleic acid amplification primers: miR-1287, miR-3131, miR-335, miR-4419, miR-4468, miR-96 upstream primer, miRNA qPCR universal downstrea...
Embodiment 3
[0095] Example 3. Clinical application of miRNA detection based on RT-qPCR technology
[0096] The paraffin sections of 50 subjects were obtained, and the RT-qPCR method as in Example 2 was used to detect miR-1287, miR-3131, miR-335, miR-4419, miR-4468, and miR-96. Judge the subject’s platinum sensitivity based on the measured results, and the results are as follows:
[0097] Among the 50 subjects, 29 were platinum-sensitive patients and 21 were platinum-resistant patients. According to the cut-off value in Example 1, 22 of the 29 platinum-sensitive patients were detected as platinum-sensitive patients, and 16 of the 21 platinum-resistant patients were detected as platinum-resistant patients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com