Patents
Literature
71 results about "Probucol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
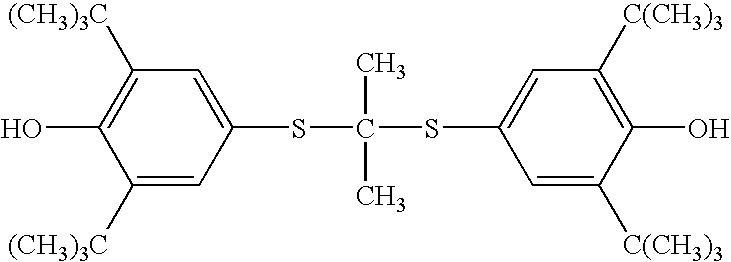
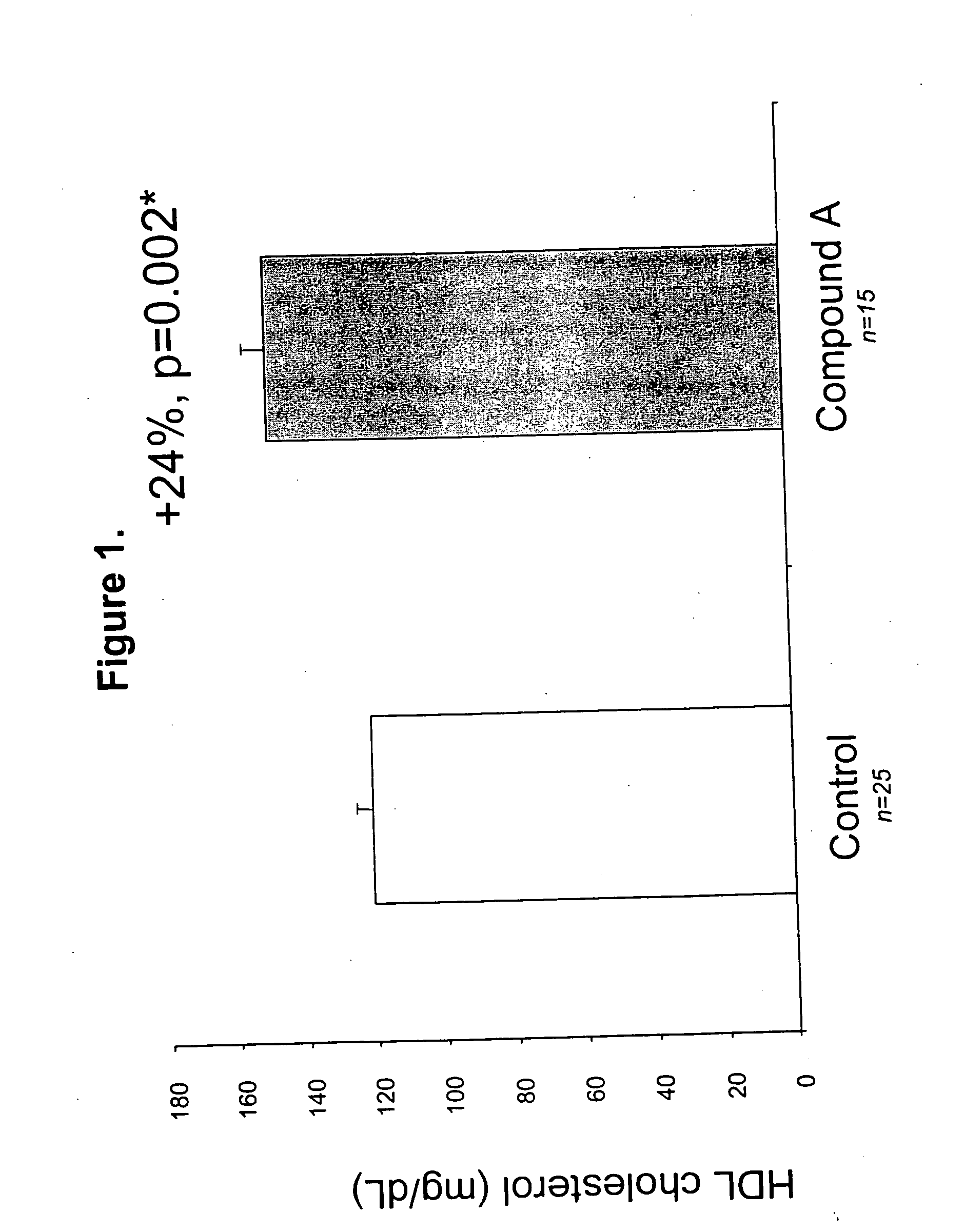
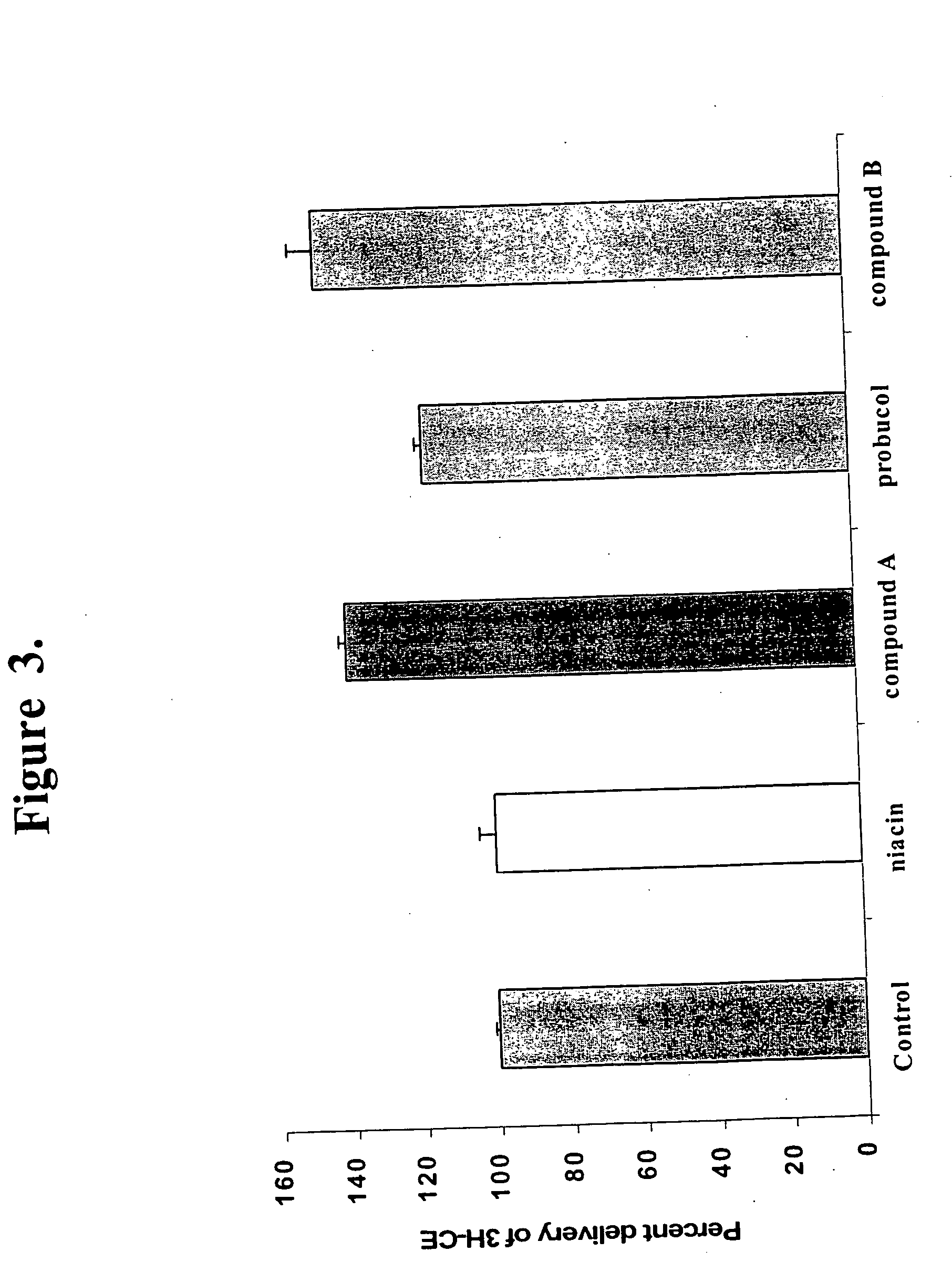
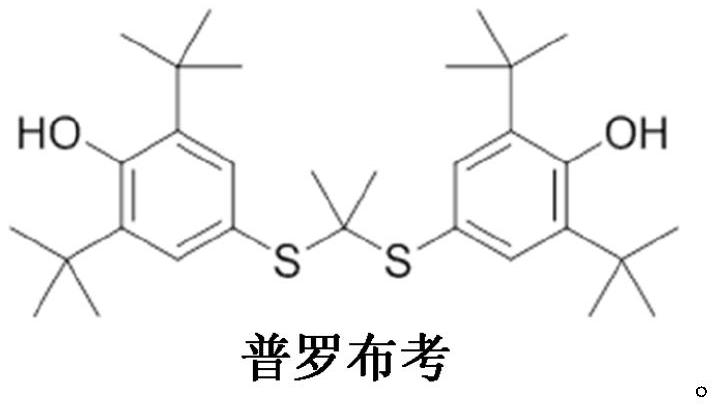
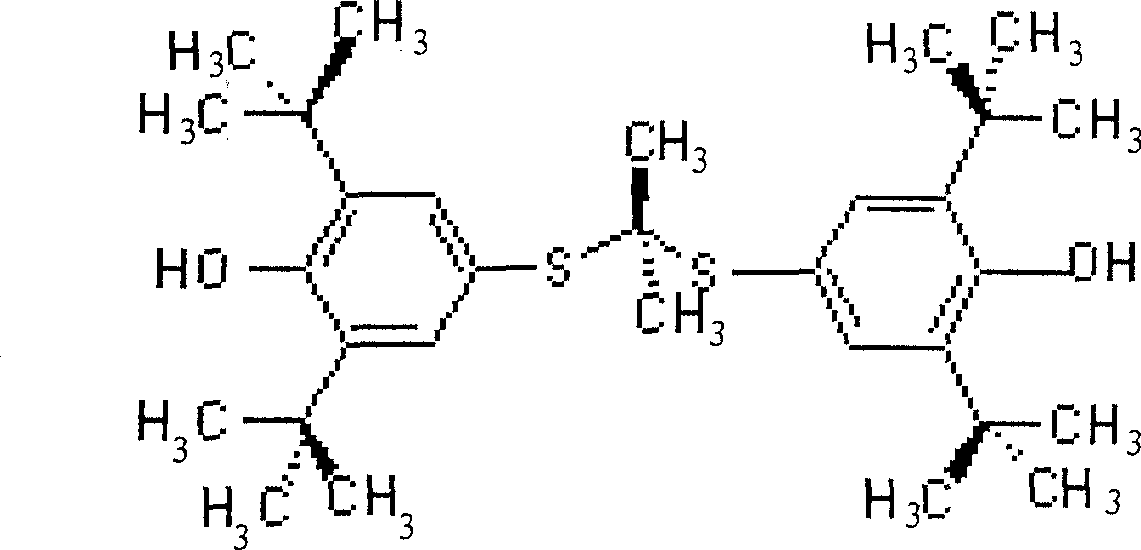
Probucol is an anti-hyperlipidemic drug initially developed in the treatment of coronary artery disease. However, clinical trials were stopped after it was found that it may lower HDL in patients with a previous history of heart disease.
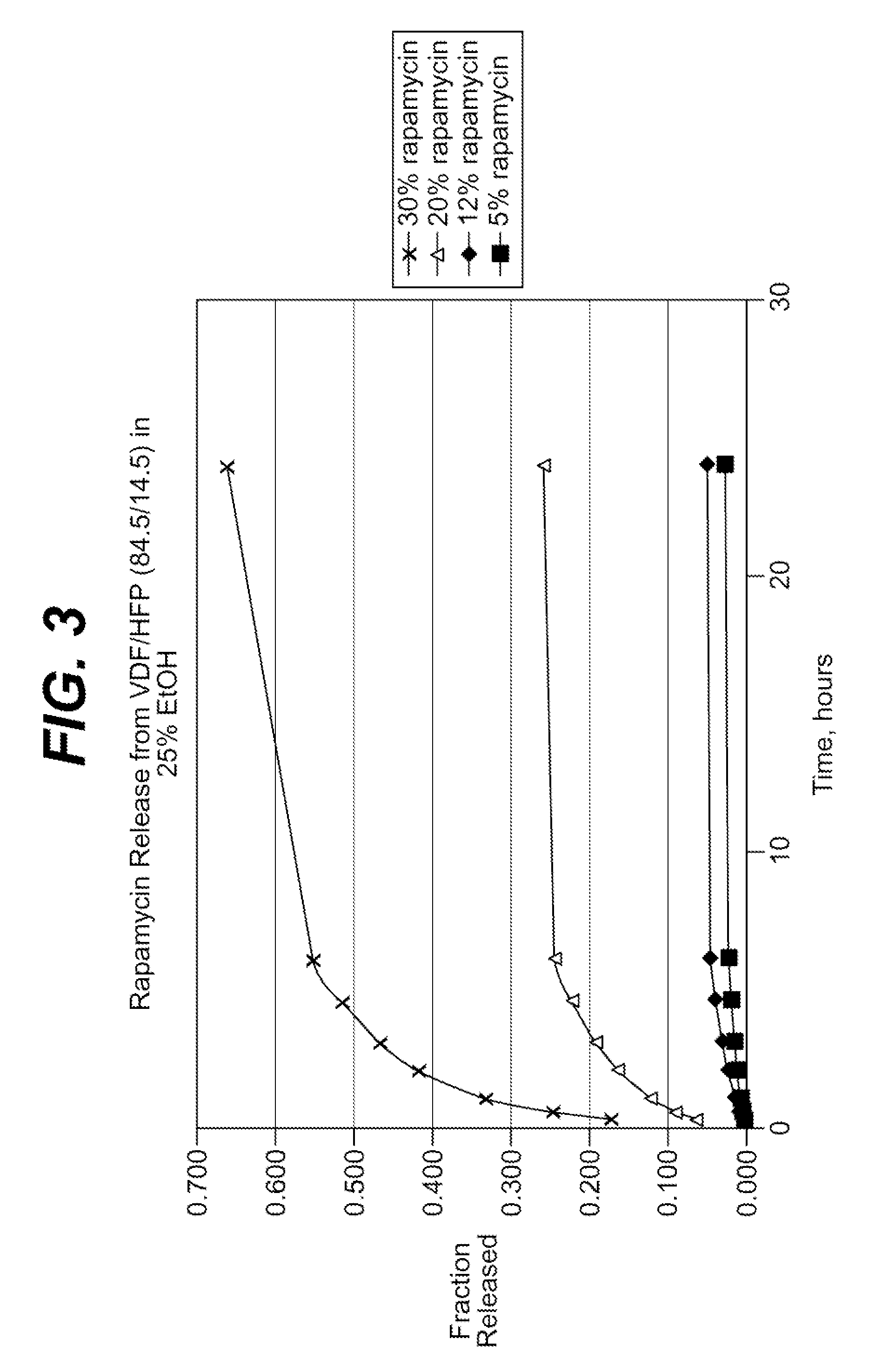
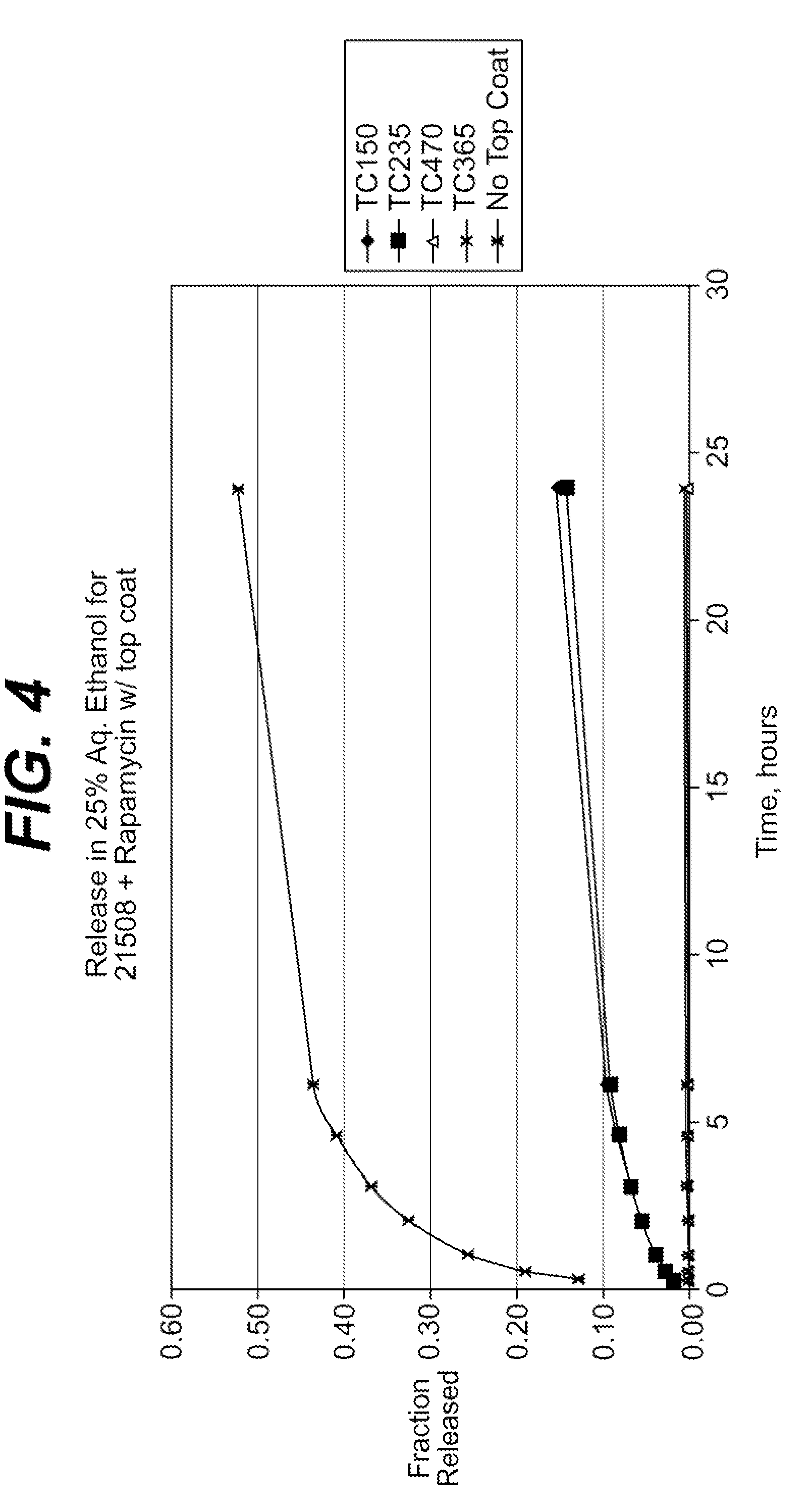
Local vascular delivery of probucol alone or in combination with sirolimus to treat restenosis, vulnerable plaque, aaa and stroke
InactiveUS20080241215A1Minimize potential risk of damageReduce frictionBiocideSurgeryDiseasePercent Diameter Stenosis
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Preparation method of biodegradable medicine composite macromolecular scaffold material
InactiveCN1367023AAvoid combiningPrevent embolismSurgeryPharmaceutical containersPolymer scienceFreeze-drying
A preparation method of biodegradable medicine-compounded macromolecular scaffolding material includes the following steps: dissolving macromolecular polylactic acid, polycaprolactone and restenosis-resisting medicine in the solvent, pouring the prepared solution into a container for film-forming, making the said film into filament, dipping the said filament in the mixed solution prepared with L-lactic acid and diglycolide copolymer, solvent and restenosis-resisting medicine and drying in the air or freeze-drying, then soaking the said filament in anticoagulative solution, drying in the air, making filament wind round the mould, thermosetting and forming so as to obtain the invented product. The described solvent is chloroform, 1,4-dioxane and dimethyl sulfoxide, the restenosis-resisting medicine is taxol, taxadter, arotinoid ethylester, probucol, dectan and cilomosi, and the anticoagulative solution is prepared with carboxylated sulfurnic aid esterified chitin aqueos solution or heparin sodium aqueos solution and acetone through the process of mixing and solvation reaction.
Owner:TSINGHUA UNIV +1
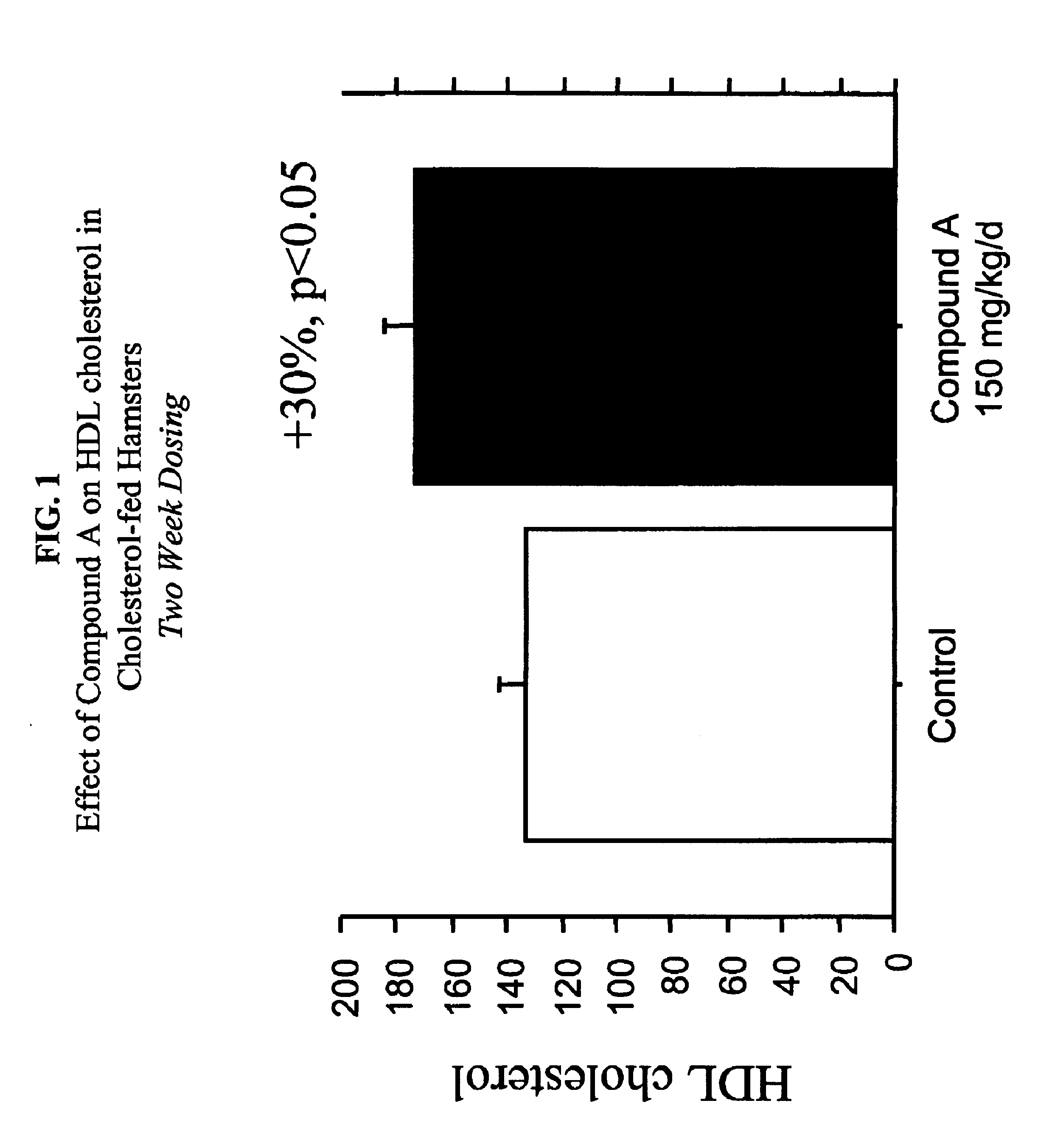
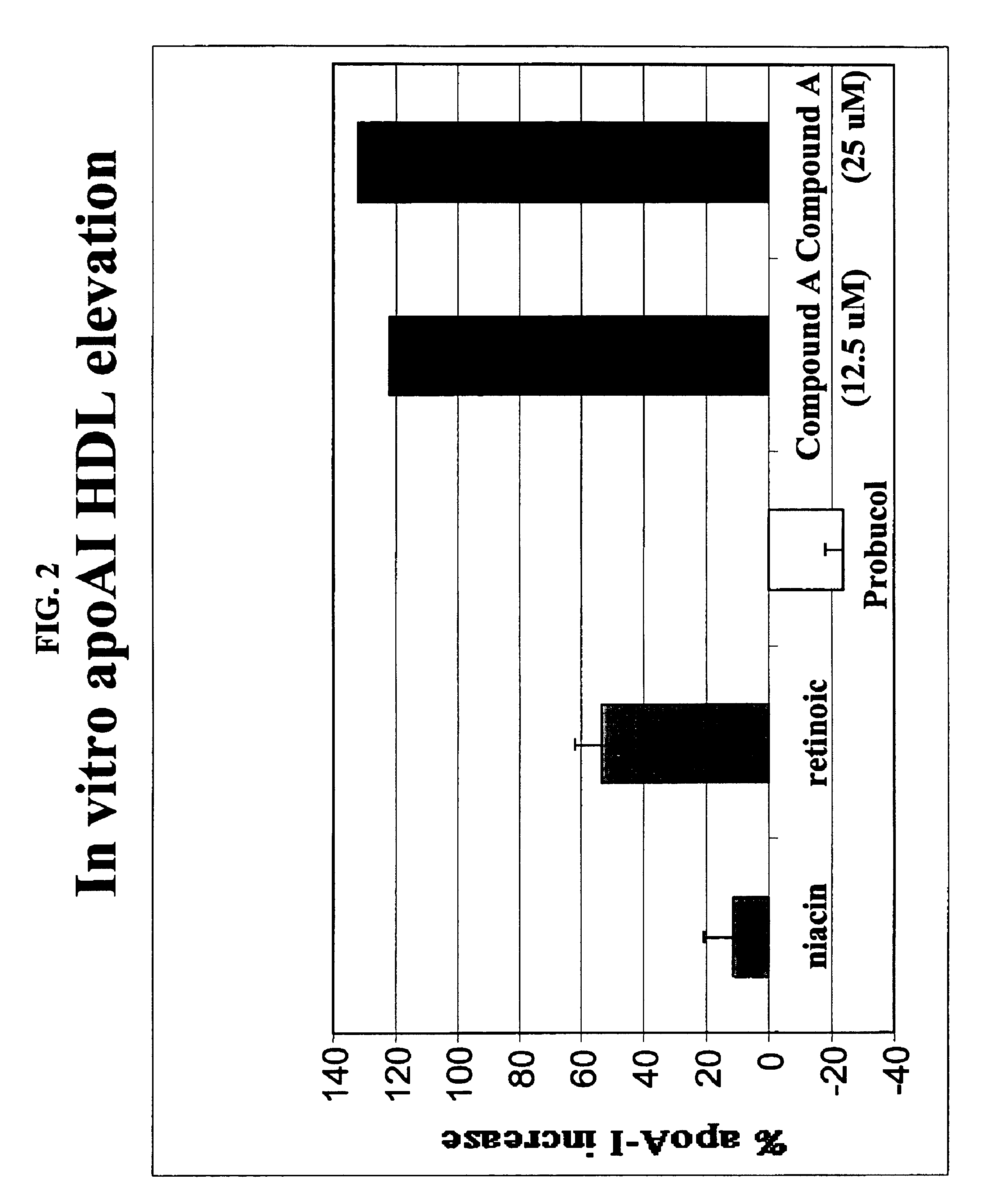
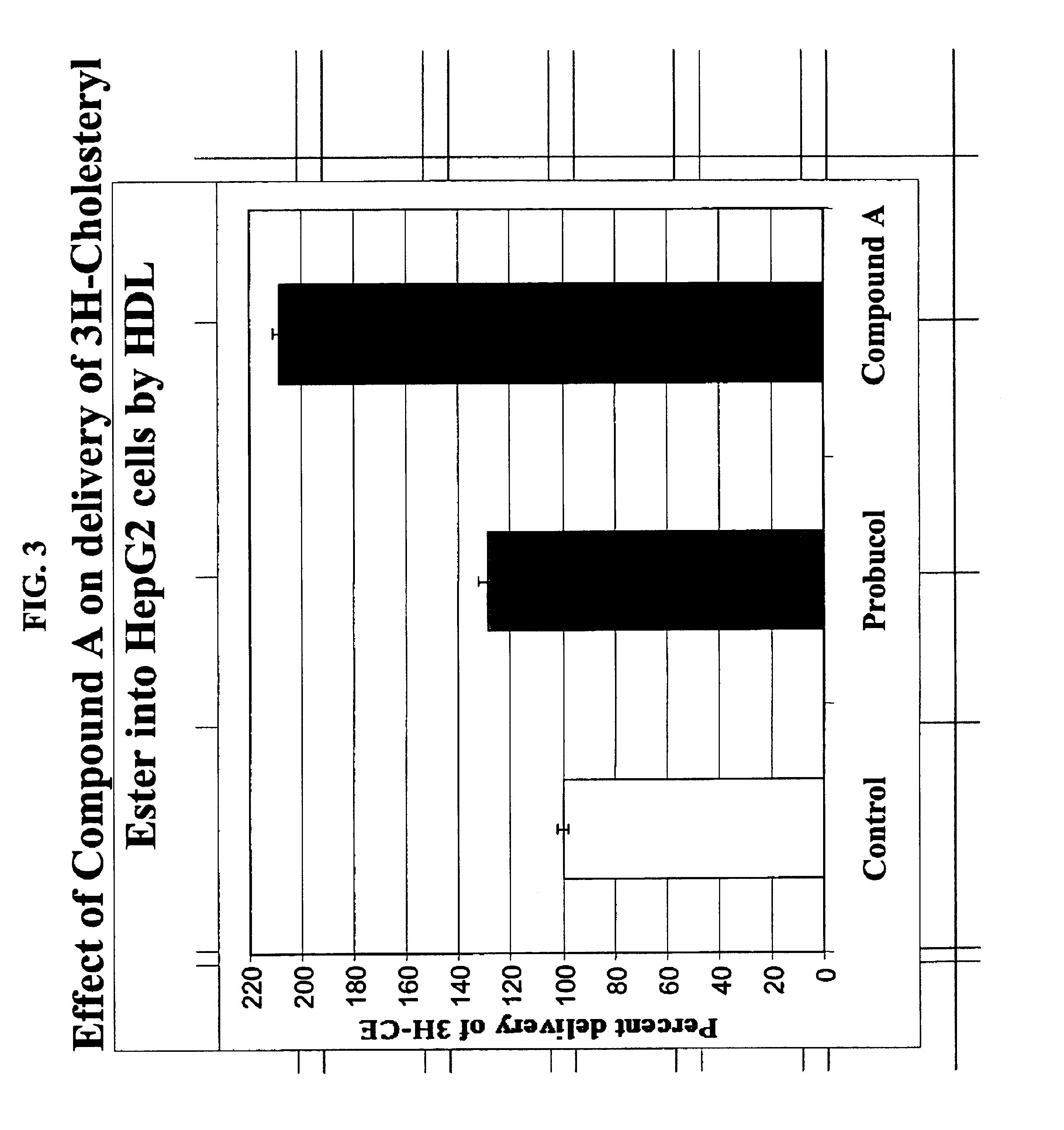
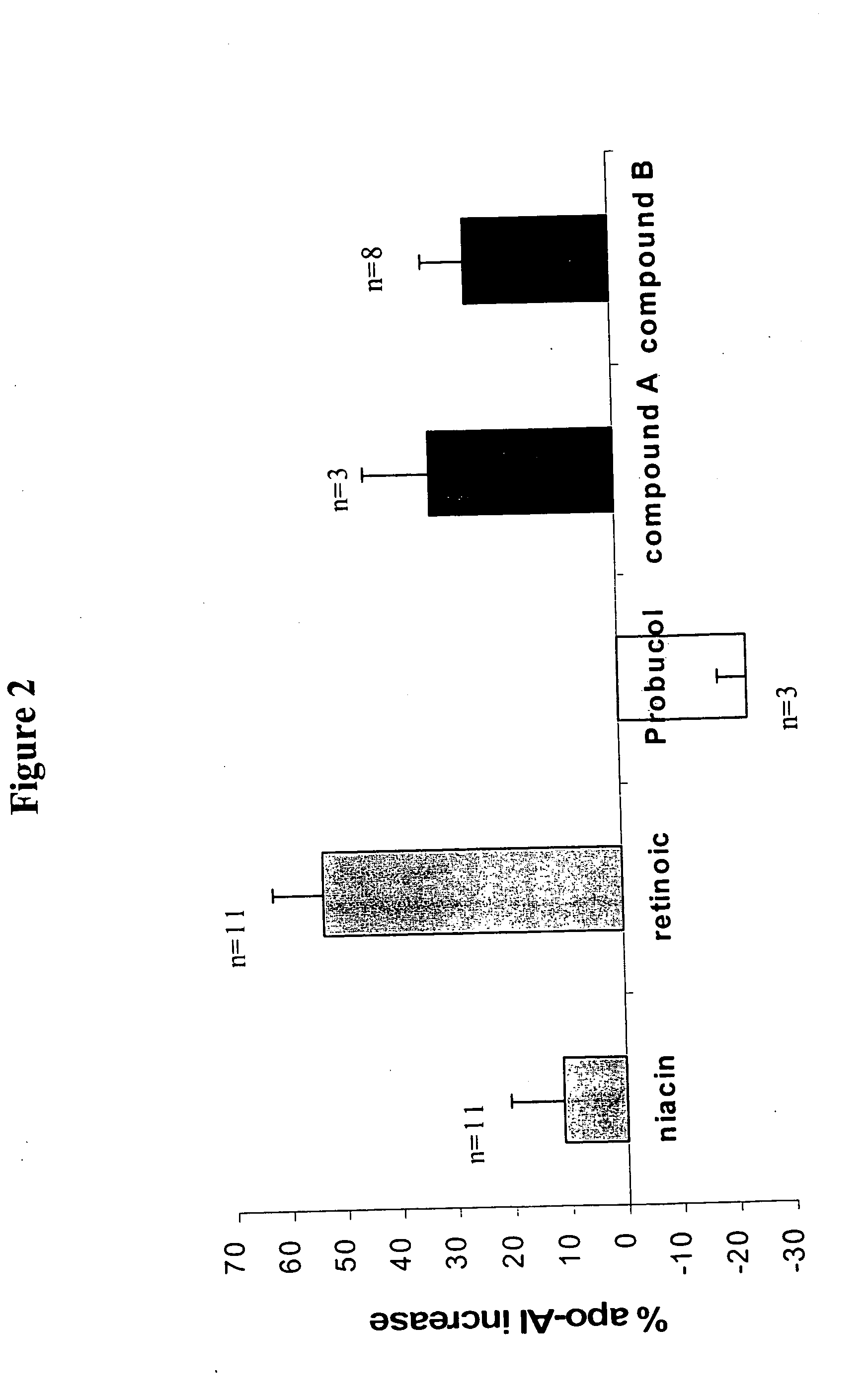
Compounds and methods to increase plasma HDL cholesterol levels and improve HDL functionality
InactiveUS6881860B2Improve the level ofIncreases selective uptakeBiocideCompound screeningHalf-lifeHDL particle
It has been discovered that certain selected ethers of probucol, and their pharmaceutically acceptable salts or prodrugs, are useful for increasing circulating HDL cholesterol. These compounds may also improve HDL functionality by (a) increasing clearance of cholesteryl esters, (b) increasing HDL-particle affinity for hepatic cell surface receptors or (c) increasing the half life of apoAI-HDL.
Owner:SALUTRIA PHARMACEUTICALS LLC
Probucol solid dispersion
InactiveCN101632630ASulfur/selenium/tellurium active ingredientsPharmaceutical delivery mechanismMagnesium stearateDissolution
The invention belongs to the technical field of medicine, and in particular relates to probucol solid dispersion prepared by a solvent method. In the total charging amount, probucol accounts for 5 to 20 percent, PVP K30 accounts for 56 to 88.5 percent, tween-80 accounts for 3 to 15 percent, lauryl sodium sulfate accounts for 0.5 to 1 percent, and polyoxyethylene 40 monostearate accounts for 3 to 8 percent. The solid dispersion, 0.15 to 3 percent of superfine silica powder and 0.3 to 0.9 percent of magnesium stearate are mixed fully and evenly, and are subjected to compressing dry granulation and coating. The dissolution of the solid dispersion in 20 months is over 90 percent, and the bioavailability thereof is 580 percent.
Owner:北京诺美生物科技有限公司
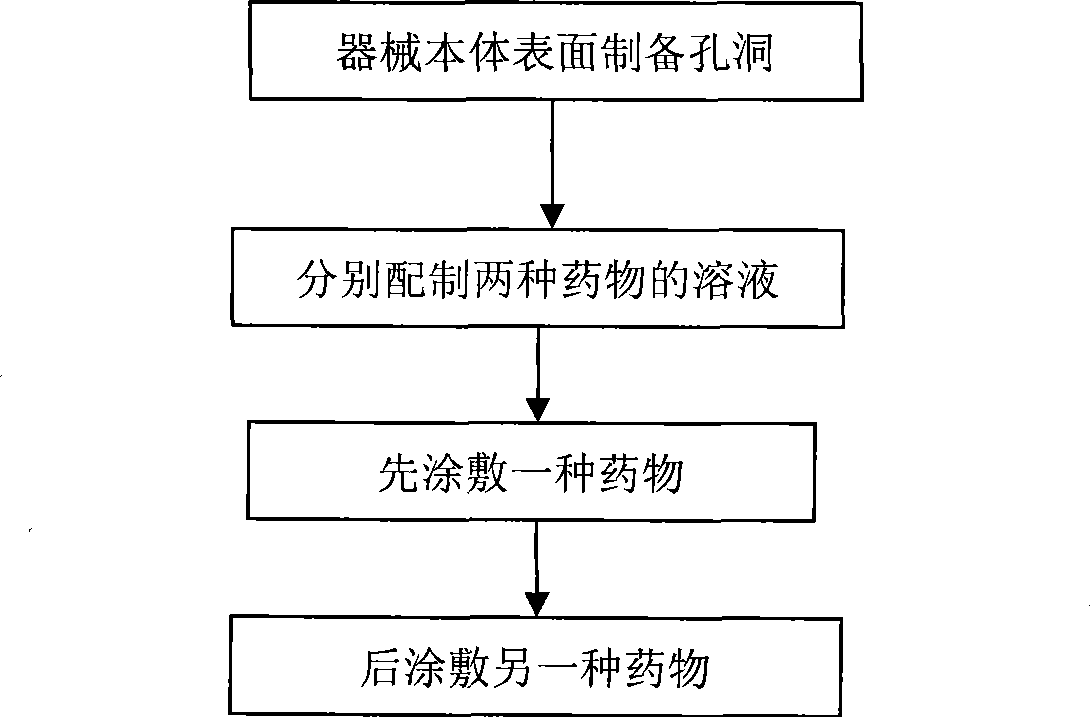
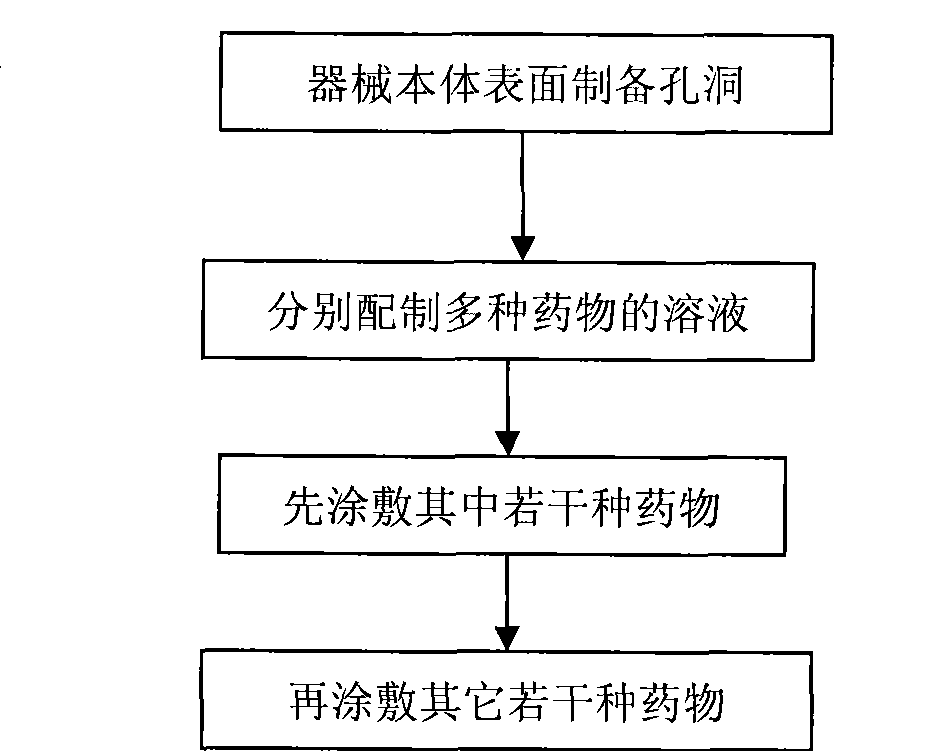
Nano micropore structure capable of storing and releasing various kinds of medicament for medicament eluting instrument and preparation method
ActiveCN101474455AReduce the risk of long-term thrombosisNo effect on mechanical propertiesStentsSurgerySurgical operationAlfaxalone
The invention relates to a nanometer medicinal elution apparatus in microcellular structure for storing and releasing multiple medicines and a preparation method thereof. The multiple medicines in the invention are selected from medicine therapeutic agents, carrier therapeutic genes and bioactive substances, wherein one or two of the medicine therapeutic agents is / are taxol and / or probucol. The preparation method of the medicinal elution apparatus in the invention mainly comprises the followings: 1) nanometer holes are prepared on the surface of the apparatus body; 2) multiple medicines are coated in the nanometer holes and on the surface of the apparatus body. The apparatus body in the invention does not contain polymers, thereby reducing the risk of long-term thrombus possibly caused by polymers; the nanometer holes on the surface of the apparatus body do not have any influence for mechanical property of the body, thereby being capable of efficiently controlling the releasing rate of medicines and obviously reducing the restenosis rate after surgical operations. The invention can be applied to various medicinal elution implanting apparatuses, particularly having good effects for curing vascular lesion and preventing blood vessel restenosis in vascular stents.
Owner:BEIJING TARGET TECH
Officinal composition for lowering blood fat
InactiveCN101766594AMetabolism disorderSulfur/selenium/tellurium active ingredientsImpaired liver functionSide effect
The invention discloses an officinal composition for lowering blood fat. Active ingredients are atorvastatin and probucol. The invention provides a better fat regulating drug for patients clinically suffering from severe lipid metabolism disorders and especially has favorable synergistic effect on patients at high liver function damaging risk level by drug combination; two kinds of drugs complement advantages so as to obviously lower the possibility of side effect occurrence because of increased amount of atorvastatin. The officinal composition for lowering blood fat is prepared from active ingredients and pharmaceutically acceptable supplementary materials, but is not limited in preparations, such as tablets, dispersible tablets, sustained release tablets and the like.
Owner:BEIJING HOPE HUGE PHARM SCI
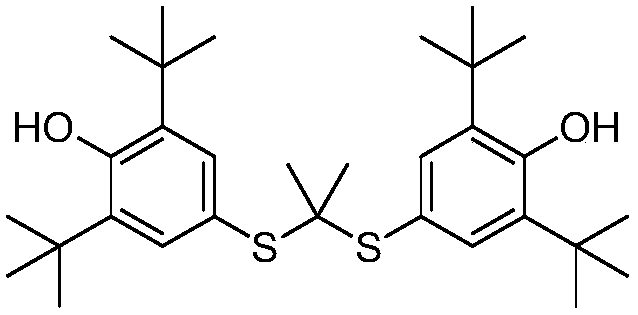
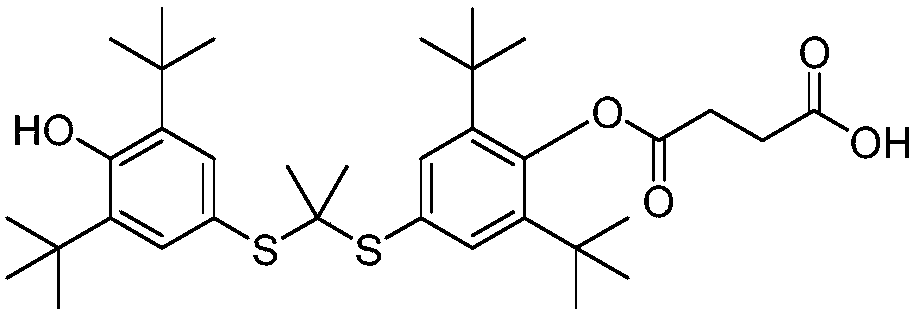
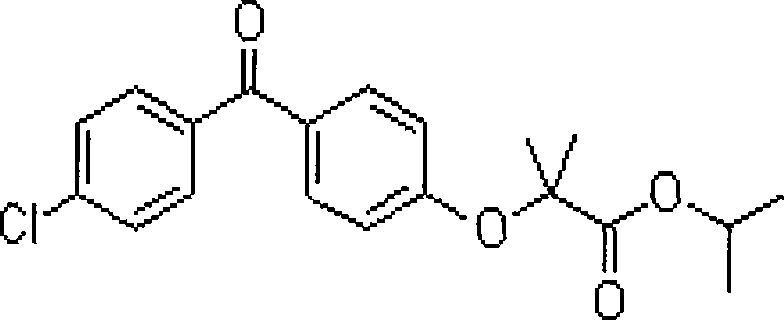
Novel salt forms of poorly soluble probucol esters and ethers
InactiveUS20040082807A1Lower cholesterol levelsDecrease liver cholesterol biosynthesisOrganic active ingredientsSenses disorderEtherMedicinal chemistry
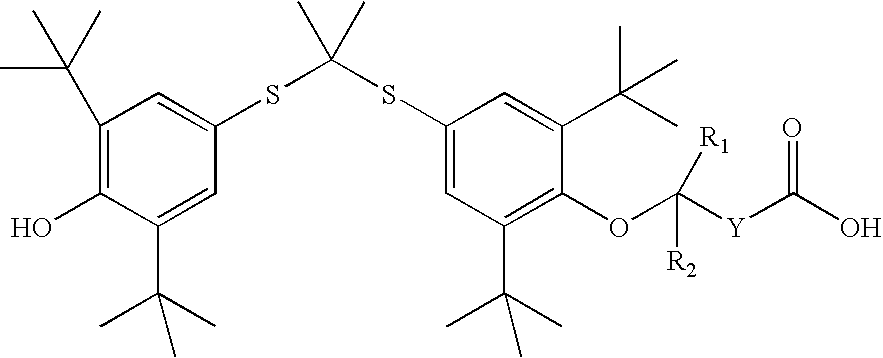
Organic amine salts of compounds of the formula: and their pharmaceutically acceptable salts, and uses in medical therapy are provided.
Owner:SALUTRIA PHARMACEUTICALS LLC
Salt forms of poorly soluble probucol esters and ethers
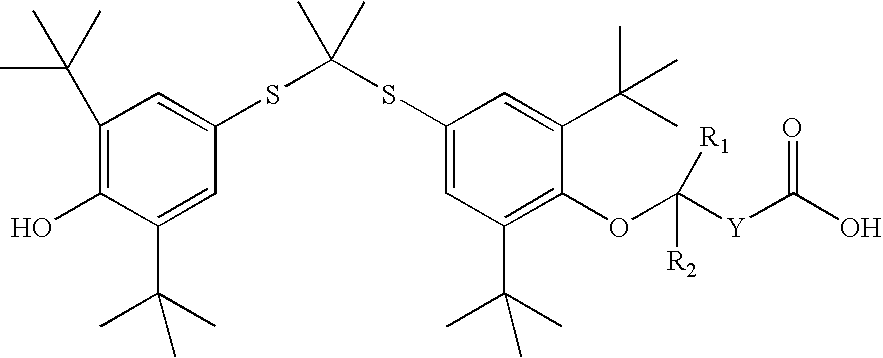
Organic amine salts of compounds of the formula: and their pharmaceutically acceptable salts, and uses in medical therapy are provided.
Owner:SALUTRIA PHARMACEUTICALS LLC
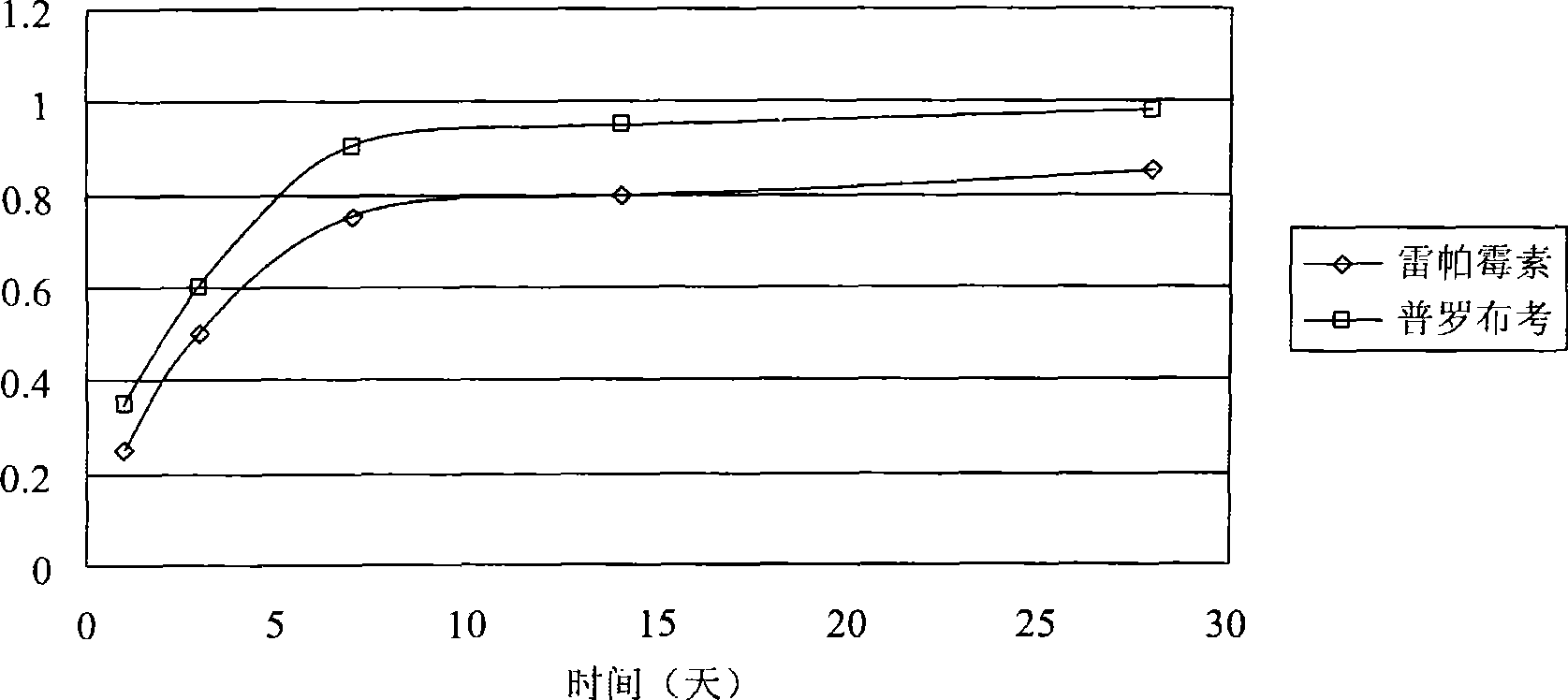
Biological degradable metal stent coated with rapamycin-probucol composite medicament
ActiveCN101485902AStrong controllabilityGood biocompatibilityStentsCoatingsCoronary heart diseaseThrombus
The invention provides a biodegradable rapamycin-probucol composite medicine coated metal stent, which comprises a metal stent, active ingredients which coat on the surface of the metal stent, and a slow release vector, wherein the active ingredients are rapamycin and probucol; and the slow release vector is in vivo degradable fatty cluster polymers. The coated metal stent not only maintains the advantage of preventing restenosis of the medicine coated stent but also solves the problem of late stent thrombus along with complete release of a slow release coating; and the probucol in the coating has the function of inhibiting local inflammatory reaction caused by the vector and a medicine. The coated metal stent is mainly used for interventional therapy of coronary heart disease, prevention of restenosis, and reduction of formation of late thrombus after the stent is implanted.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Process for preparation of probucol derivatives
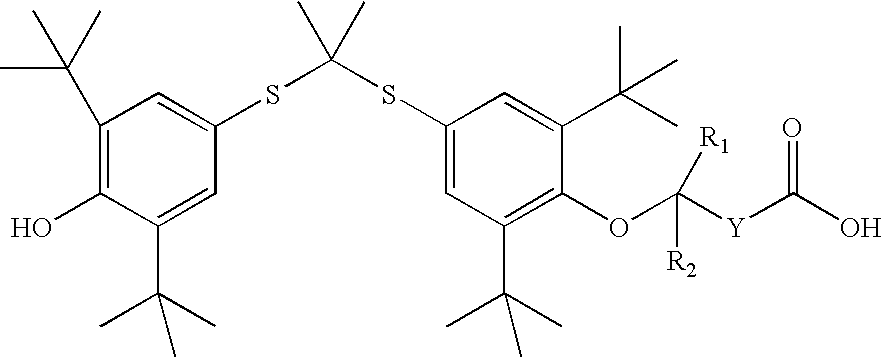
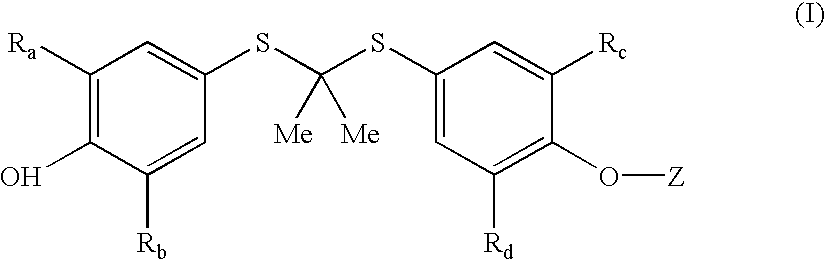
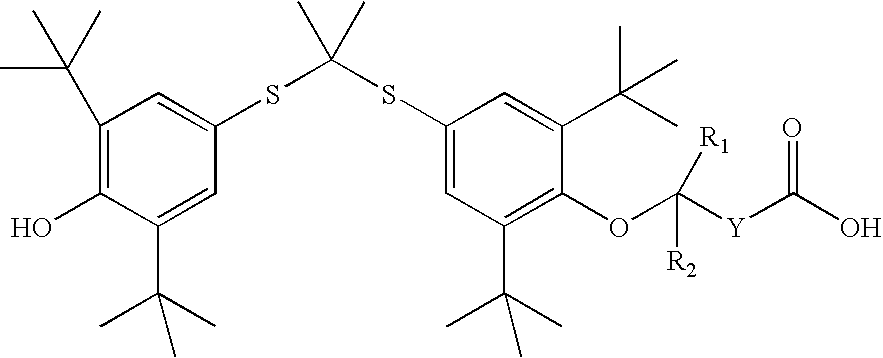
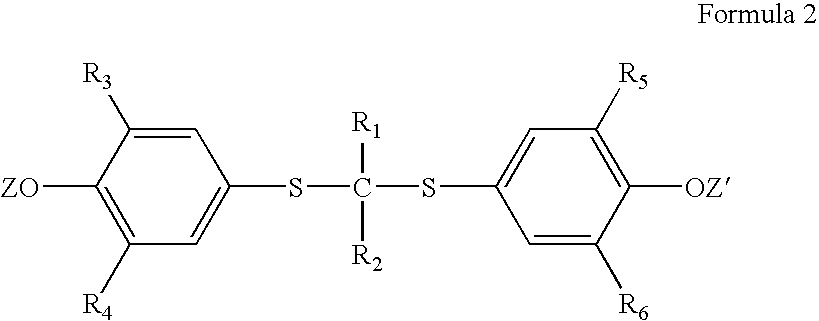
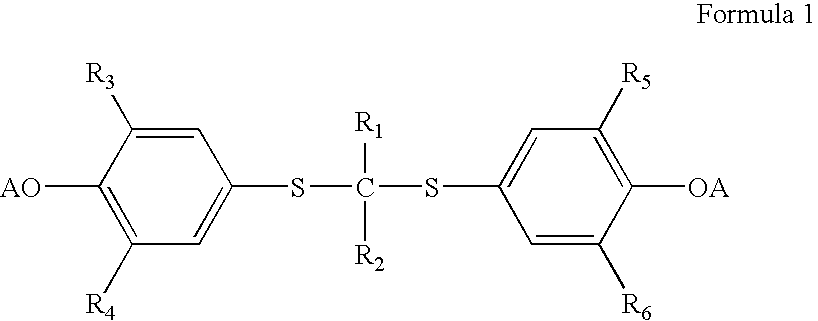
InactiveUS20050228192A1Carbamic acid derivatives preparationOrganic compound preparationWater solubleProbucol
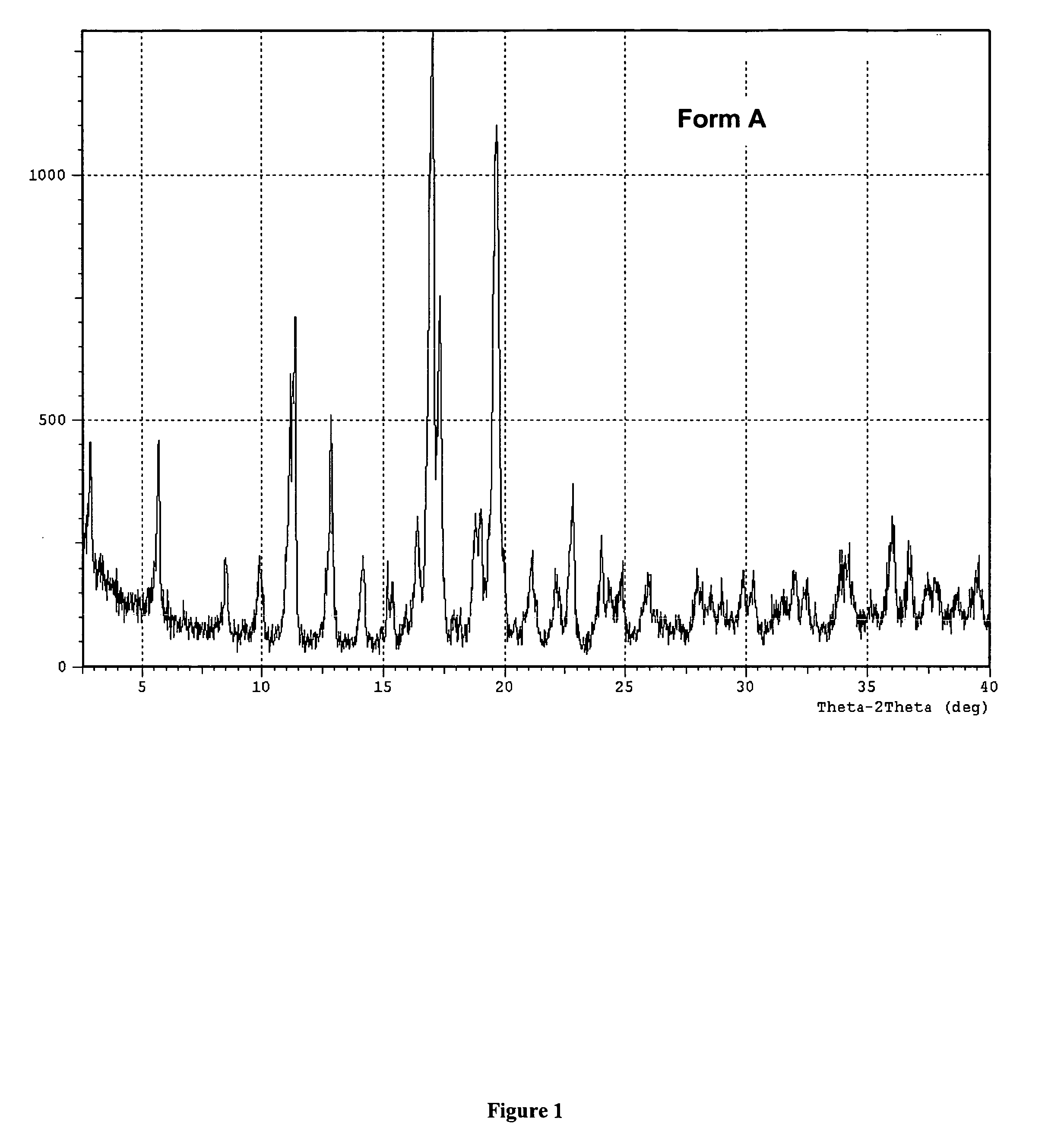
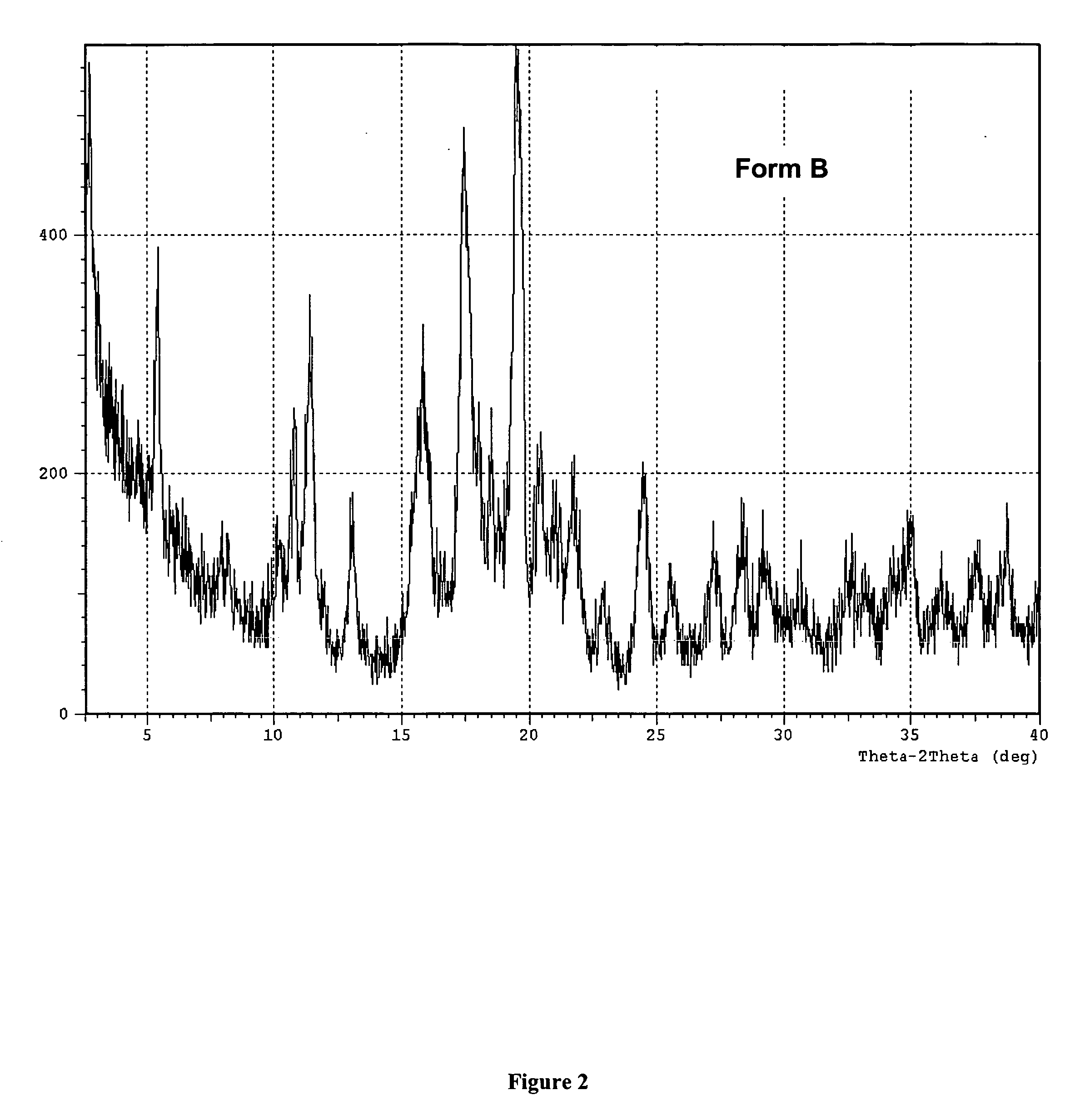
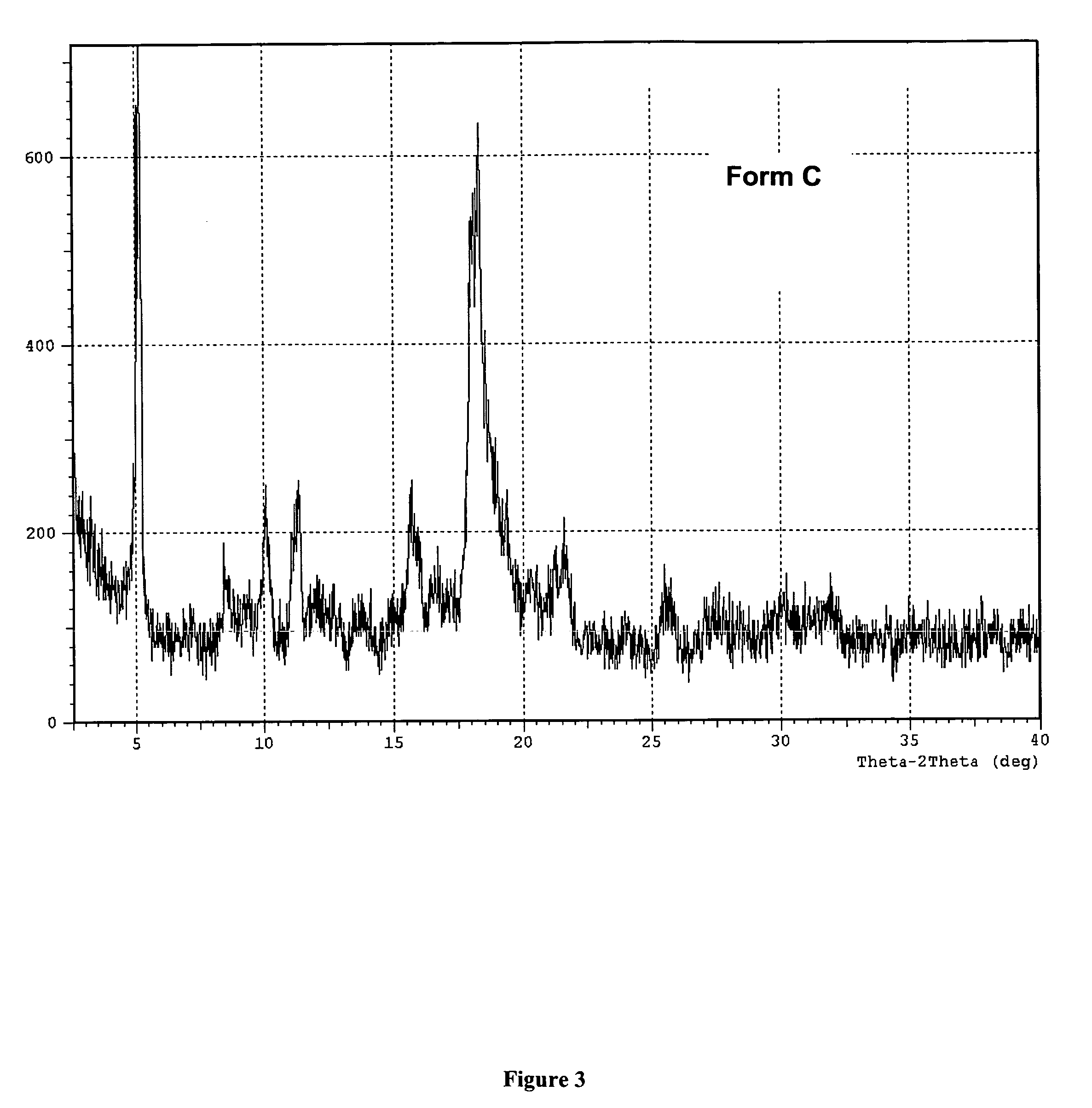
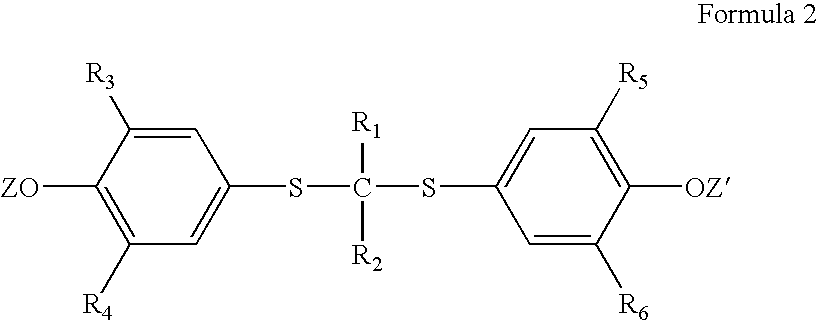
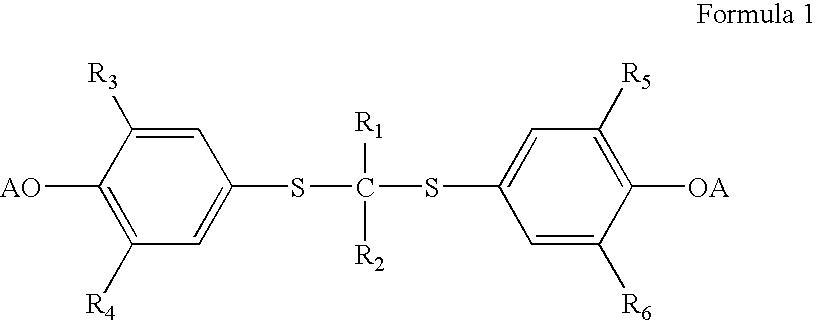
A method is described for the preparation of polymorphic forms of water-soluble derivatives of probucol compounds having the following formula where R1, R2, R3, R4, R5, R6, Z and Z′ are defined herein.
Owner:CAMBREX CHARLES CITY INC
Methods to increase plasma HDL cholesterol levels and improve HDL functionality with probucol monoesters
InactiveUS20050065121A1Improve the level ofFunction increaseBiocideOrganic chemistryHalf-lifeHDL particle
It has been discovered that certain selected probucol monoesters, and their pharmaceutically acceptable salts or prodrugs, are useful for increasing circulating HDL cholesterol. These compounds may also improve HDL functionality by (a) increasing clearance of cholesteryl esters, (b) increasing HDL-particle affinity for hepatic cell surface receptors or (c) increasing the half life of apoAI-HDL.
Owner:SIKORSKI JAMES A +3
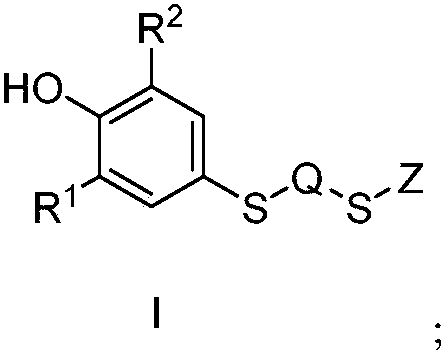
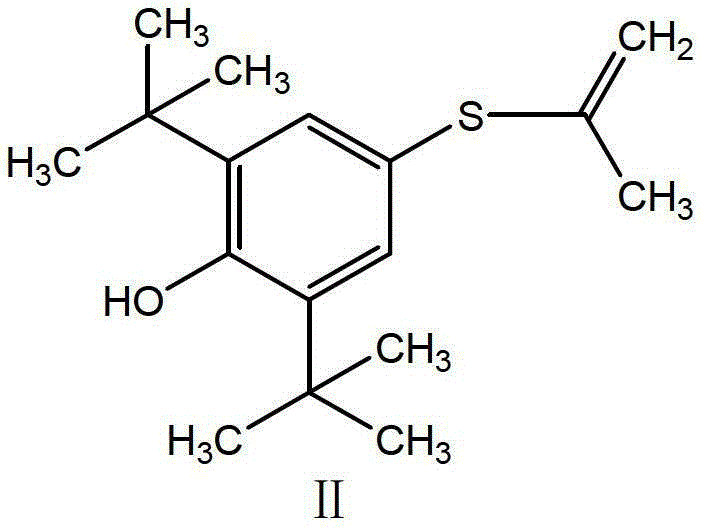
Probucol derivative, preparation method and application thereof
ActiveCN108299263ALower blood sugarLower blood fatSenses disorderMetabolism disorderDiseaseBlood vessel
The invention relates to the fields of compounds, and specifically relates to a probucol derivative, a preparation method and the application thereof. The probucol derivative has a structure shown asa general formula I. The probucol derivative provided by the invention can be used for prevention and curing of vascular diseases such as diabetes and cardiovascular and cerebrovascular diseases or complications thereof, can be used for reducing blood glucose, reducing blood fat, reducing cholesterol, reducing the weight, reducing triglyceride, resisting inflammation and oxidation and the like effectively, and has a wide application prospect.
Owner:DEMOTECH INC
Medicinal composition for treating angiocardiopathy
InactiveCN101411703ASulfur/selenium/tellurium active ingredientsCardiovascular disorderVascular diseaseExcipient
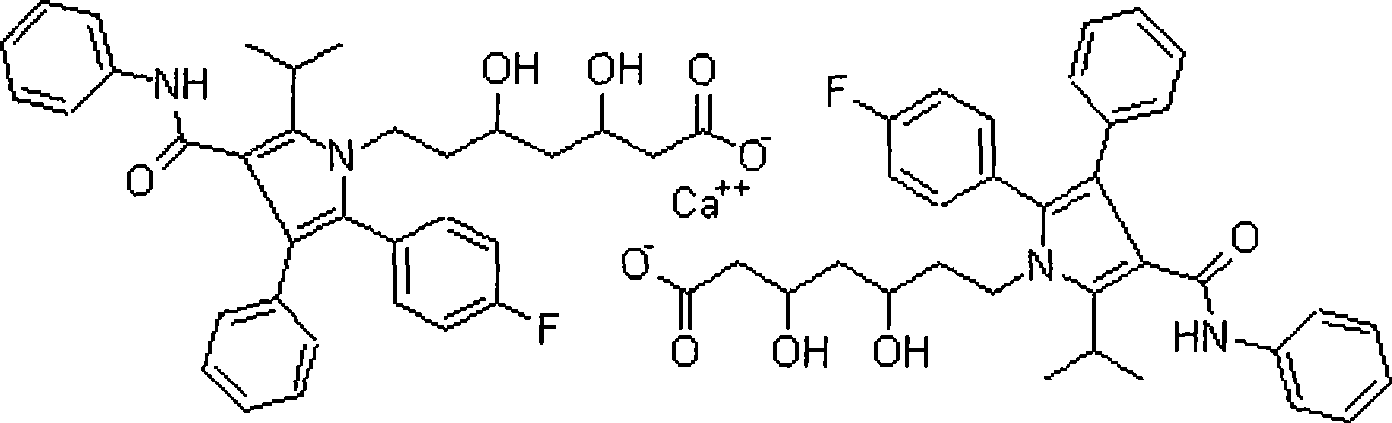
The invention relates to a medicine composition for treating angiocardiopathy. The composition comprises atorvastatin calcium and probucol with therapy dose and pharmaceutically acceptable carriers or excipients.
Owner:北京博时安泰科技发展有限公司
Probucol orally administered nanometer solid preparation and preparation method for same
InactiveCN102475688AImprove in vitro dissolutionImprove bioavailabilityPowder deliveryMetabolism disorderDrugOral medication
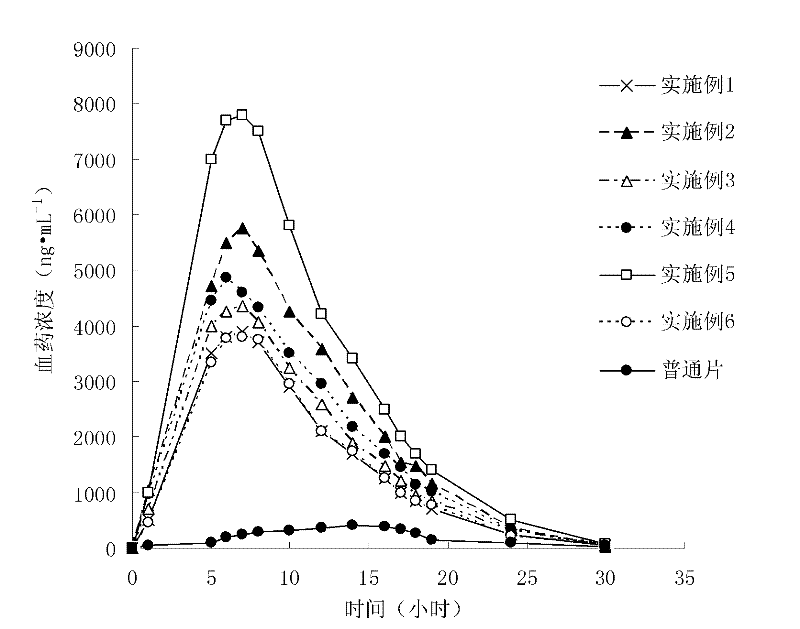
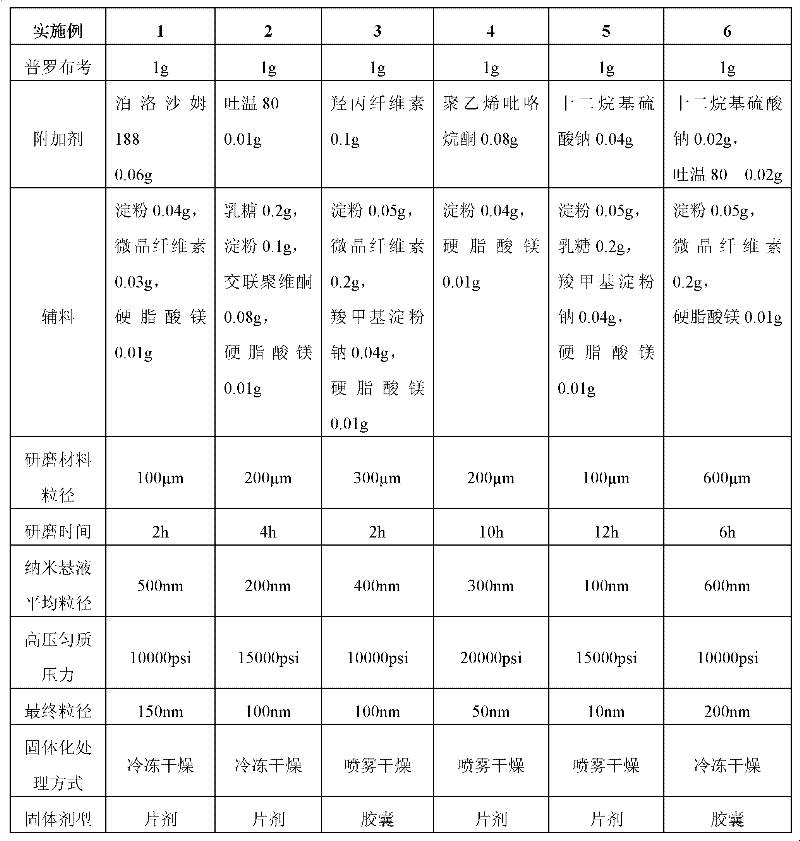
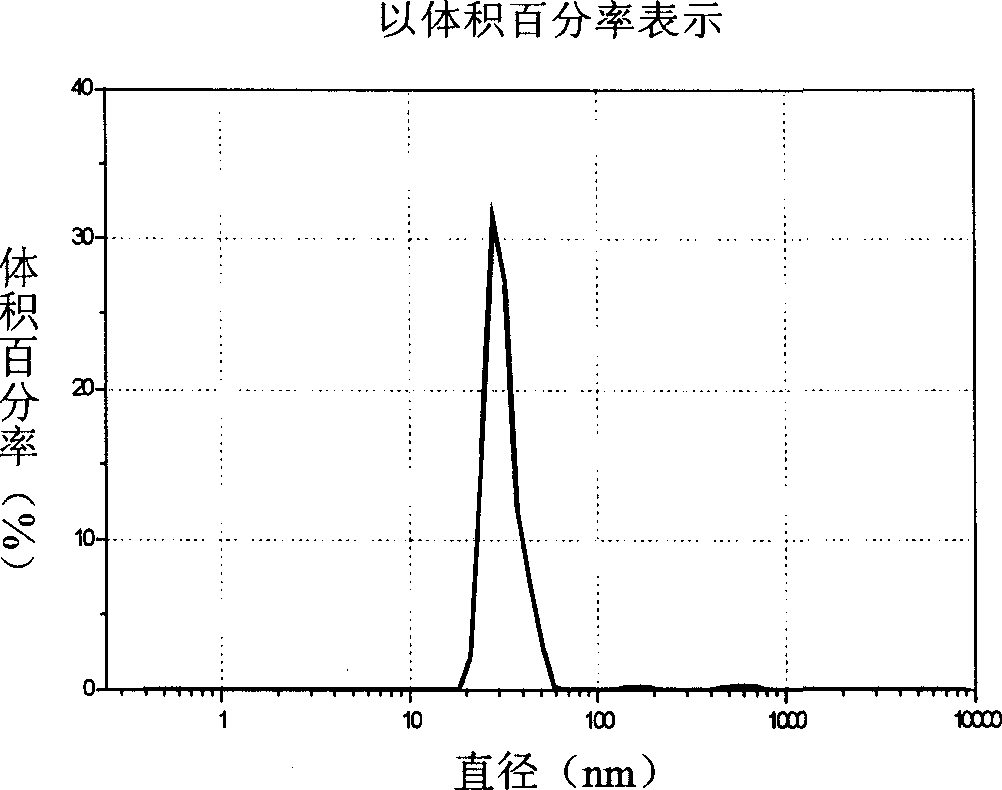
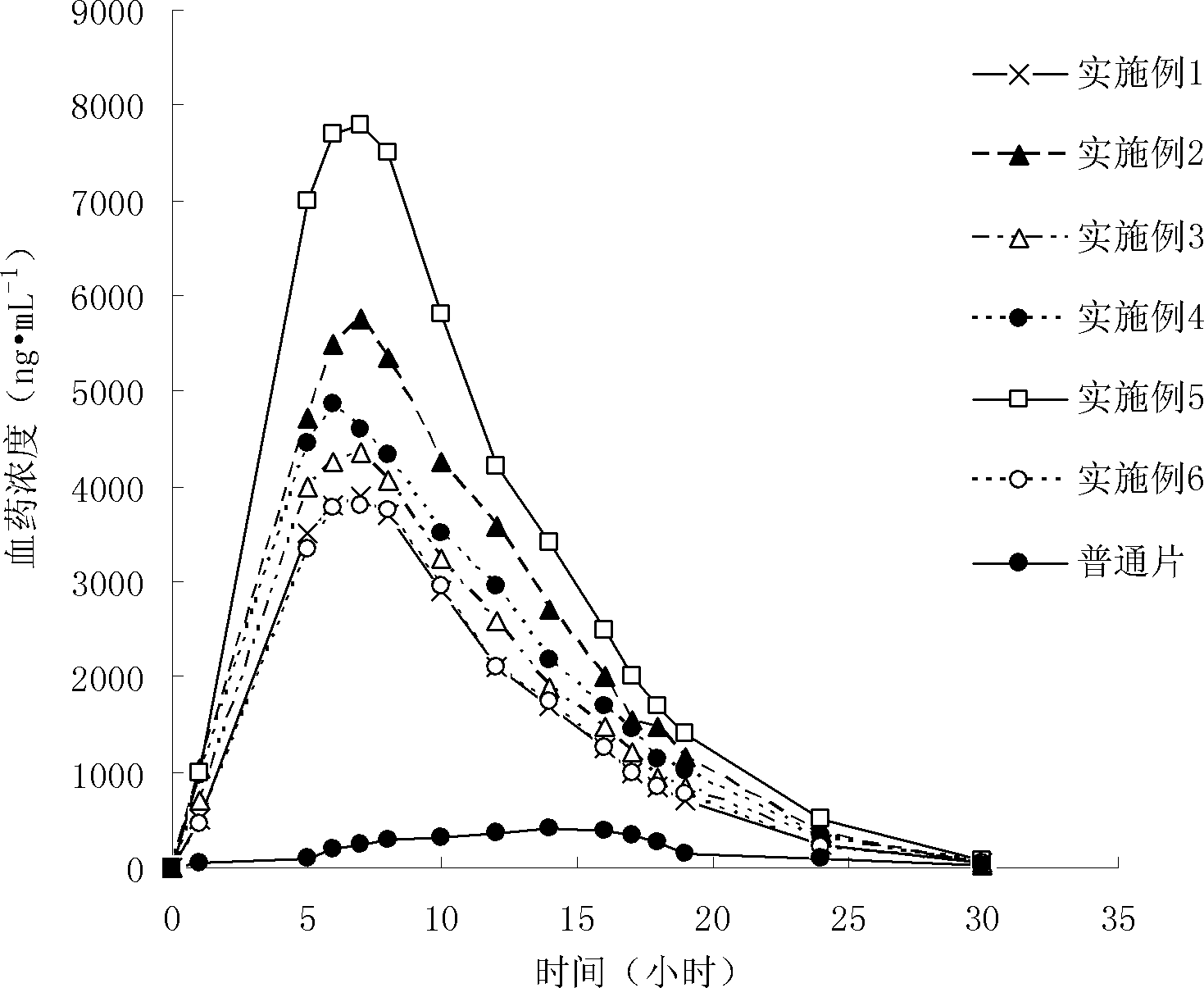
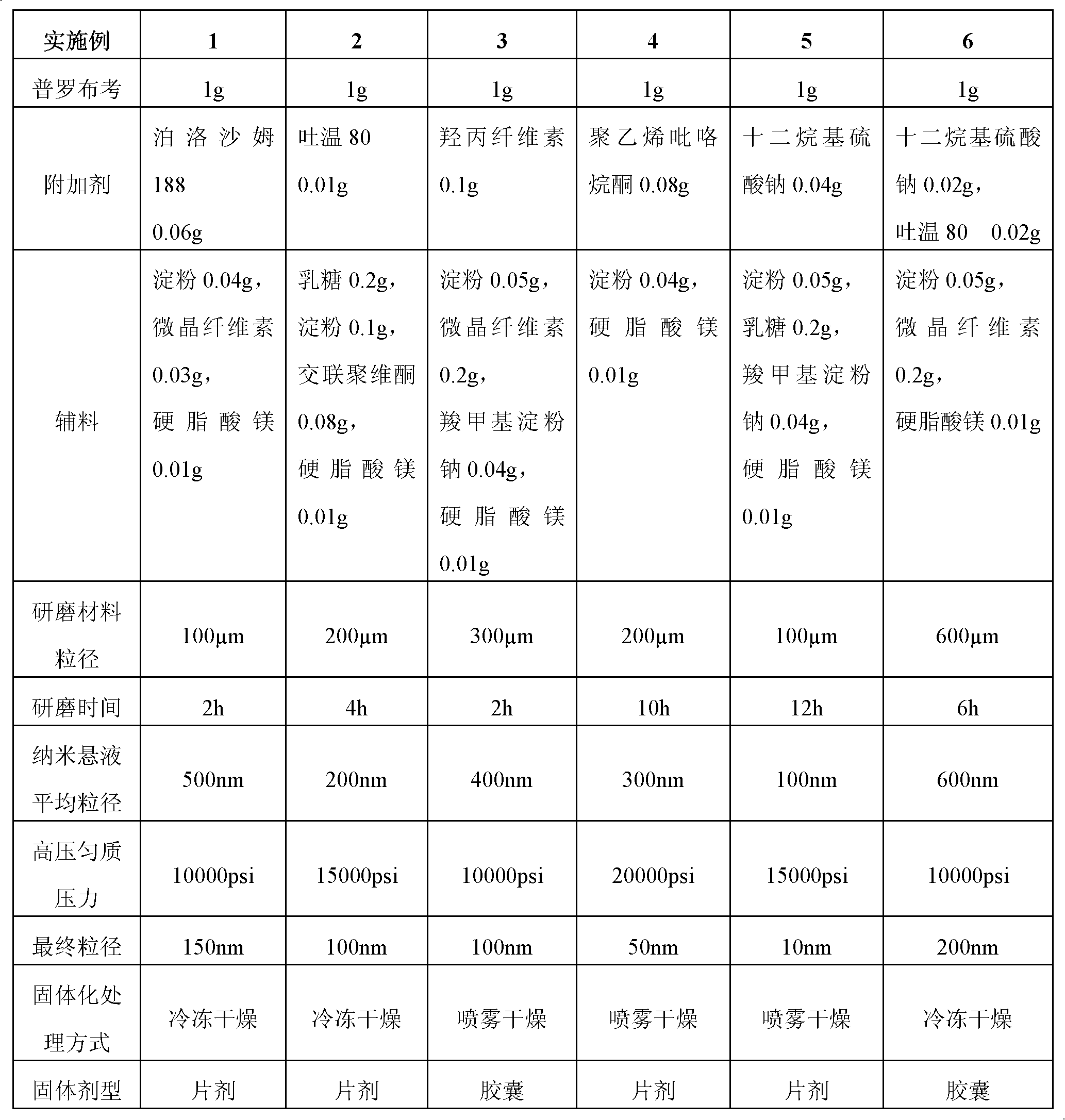
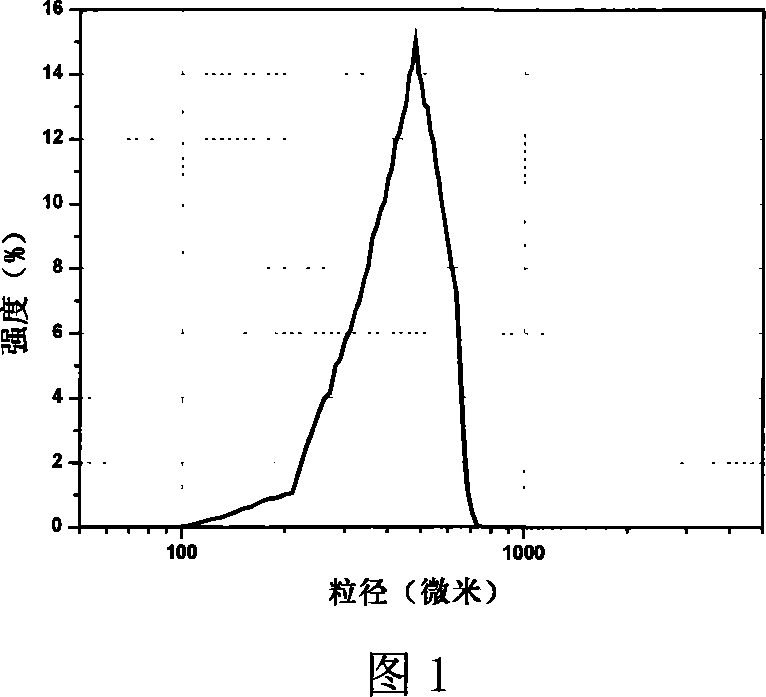
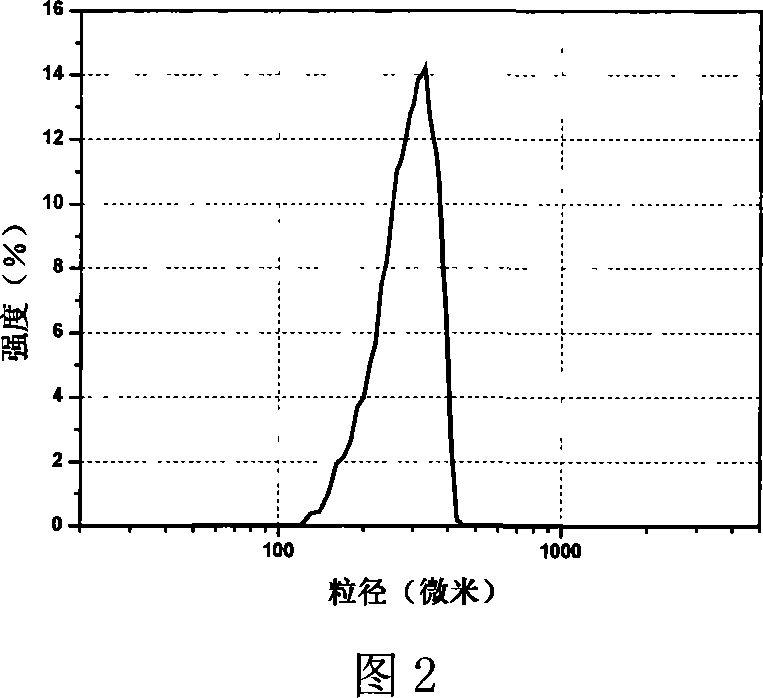
The invention provides a probucol orally administered nanometer solid preparation, which comprises, by weight, 1 part of probucol, 0.01-0.1 part of additive and 0.05-0.4 part of auxiliary materials. The grain size of the probucol ranges from 10nm to 200nm. The invention further provides a preparation method for the probucol orally administered nanometer solid preparation. The preparation method includes that the probucol and water liquor with 5% of additive are prepared into nanometer suspension with the grain size ranging from 100nm to 600nm by a wet grinding method; the nanometer suspension is homogenized into nanometer grains with the grain sizes ranging from 10nm to 200nm by a high-pressure homogenizing machine; and the nanometer suspension which is homogenized is solidified and is added with the auxiliary materials to be prepared into the orally administered nanometer solid preparation. Compared with a common probucol tablet, the preparation has the advantages that dissolution in vitro of the preparation is increased by 20-30 times, and bioavailability after oral administration is improved by 10-20 times. In addition, the drug loading rate of the preparation is high, and the preparation can meet the requirements of tablet weight clinically needed by high-dose probucol administration. Besides, the preparation is stable, and the dissolution of the preparation can be kept unchanged within two years.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Probucol and lovastatin compound composite and preparation method
InactiveCN106176717AIncrease the effective ratioEasy to takeMetabolism disorderSulfur/selenium/tellurium active ingredientsMaterials preparationLovastatin
The invention discloses probucol and lovastatin compound composite and a preparation method. The compound composite comprises, by weight, 15-20 parts of probucol, 1-3 parts of lovastatin, 20-30 parts of diluent, 1-3 parts of surfactant, 20-30 parts of disintegrant and 1-3 parts of lubricant. The preparation method includes material preparation, granulation, granule preparation and tableting. Probucol and lovastatin are combined organically, consumption of pharmaceutic adjuvants is reduced to the uttermost degree, and the compound composite can be used for treating hyperlipidemia. The compound composite is high in effective drug proportion, convenient to take, capable of improving compliance of patients and beneficial to reduction of medicine price and industrial production.
Owner:LIAOCHENG UNIV
Topically applied probucol-containing agent with protective and regenerative effect
InactiveUS20080019957A1Reduce harmRegenerating and vitalizing effect on agingCosmetic preparationsBiocideActive agentTopical preparation
A method of treating skin changes and conditions in human skin including applying a topical preparation that includes an active agent including probucol, a derivative of probucol or a combination of probucol and a derivative of probucol.
Owner:NEUDECKER BIRGIT +2
Medicinal composition for treating cardiovascular and cerebrovascular diseases
InactiveCN101428012AEvenly dispersedShorten or extend mixing timeMetabolism disorderSulfur/selenium/tellurium active ingredientsDiseaseTG - Triglyceride
The invention provides pharmaceutical composition for treating cardiovascular and cerebrovascular diseases. The pharmaceutical composition comprises pantomin and probucol succinic monoester. Pantomin, rather than probucol or probucol succinic monoester, has the effects of reducing triglyceride; therefore, the compounding of the two drugs can realize complementary action.
Owner:张蔚
Process for preparation of probucol derivatives and polymorphic forms thereof
A process is described for the preparation of polymorphic forms of water-soluble derivatives of probucol compounds. The process generally includes reacting a probucol compound with a solution of alkali metal oxide, reacting the salt mixture with a solution of an alkyl or aryl halo-substituted carboxylate, hydrolyzing the resulting compound; and separating a crystalline, polymorphic form of the compound from the hydrolyzing mixture. Polymorphic forms of the probucol compounds are also described.
Owner:STRANGE MATTHEW L
Methods of treating mitochondrial dysfunction
PendingCN112996494AMicrobiological testing/measurementSulfur/selenium/tellurium active ingredientsPharmaceutical medicinePharmacology
This disclosure is directed to methods of treating or preventing mitochondrial dysfunction or mitochondrial disease in a subject comprising administration of probucol, or a pharmaceutically acceptable salt thereof. This disclosure is also directed to methods of diagnosing genetic mitochondrial disease in a subject prior to and in association with said treatment or prevention. This disclosure is also directed to methods of assessing and managing a subject with mitochondrial dysfunction or mitochondrial disease using a composite measurement.
Owner:RIBONOVA INC
Chitin nanometer granule for programmable releasing various kinds of medicine, and its prepn. method
InactiveCN1868541AImprove stabilityIncrease profitOrganic non-active ingredientsPharmaceutical active ingredientsAspirinChitosan nanoparticles
A chitosan nanoparticle able to sequentially release more medicines is proportionally prepared from chitosan, hydrophilic medicine (aspirin), lipophilic medicine (probucol), tween-80 and sodium tripolyphosphate by ion exchange method and microemulsifying method. It features that the aspirin is first released for 24 hr and the probucol is then released for 5 days.
Owner:SHANGHAI JIAO TONG UNIV
Solid dispersion tablet for treating cardiovascular disease and preparation method thereof
The invention discloses a solid dispersible tab used for curing angiocardiopathy, which contains the constituents with the following part by weight: 250 to 300 shares of probucol mono-succinate, 300 to 800 shares of PVPK30, 60 to 120 shares of Tween 80 and 5000 to 16000 shares of magnesium stearate. The invention also discloses a preparation method of the solid dispersible tab: the probucol mono-succinate is prepared into the solid dispersion tab by adopting the solid dispersing technology, thereby leading the quality of the medicine to be guaranteed and having higher bioavailability. Meanwhile, the material quantity is saved, and the economic burden of the patient is relieved.
Owner:广州市元通医药科技有限公司
Medicinal composition for treating angiocardiopathy
InactiveCN101411697ASulfur/selenium/tellurium active ingredientsCardiovascular disorderVascular diseaseMedicine
The invention relates to a medicament composition for treating cardiovascular diseases. The composition contains Fenofibrate, Probucol and pharmaceutically acceptable carrier or excipient with effective dose for treating.
Owner:迈洋致达(北京)科技有限公司
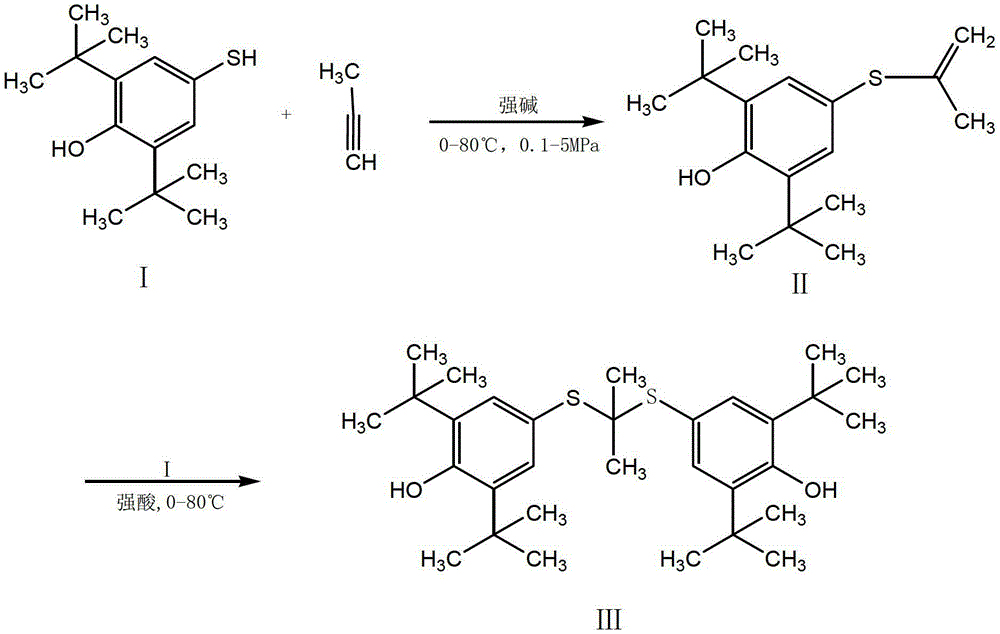
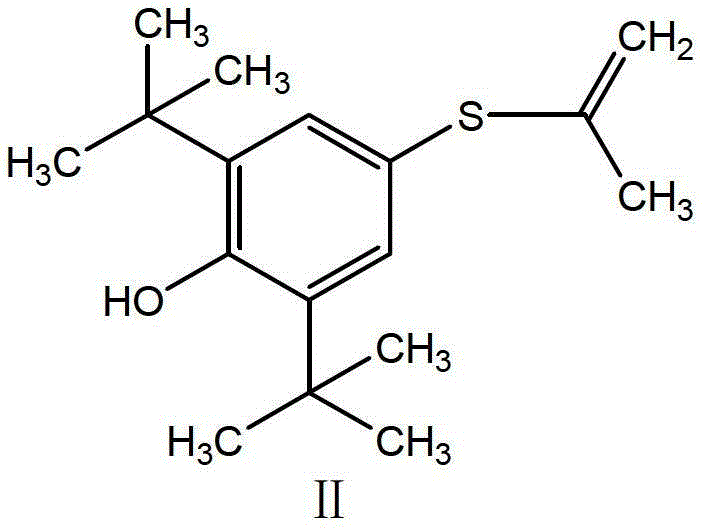
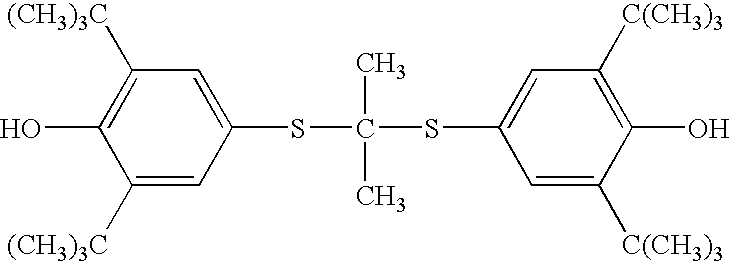
Synthesis method of probucol
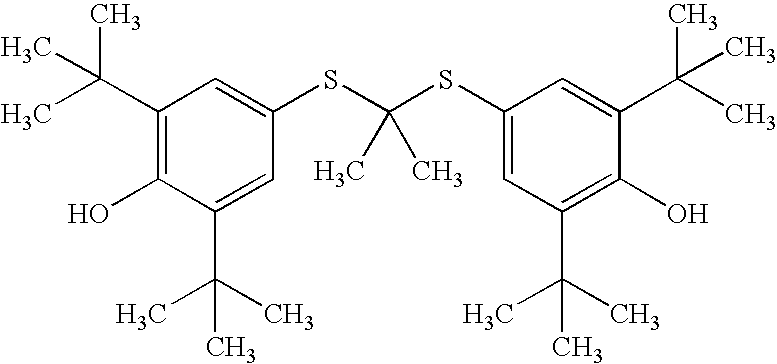
ActiveCN103145595AMild reaction conditionsEasy to operateSulfide preparationSynthesis methodsStrong acids
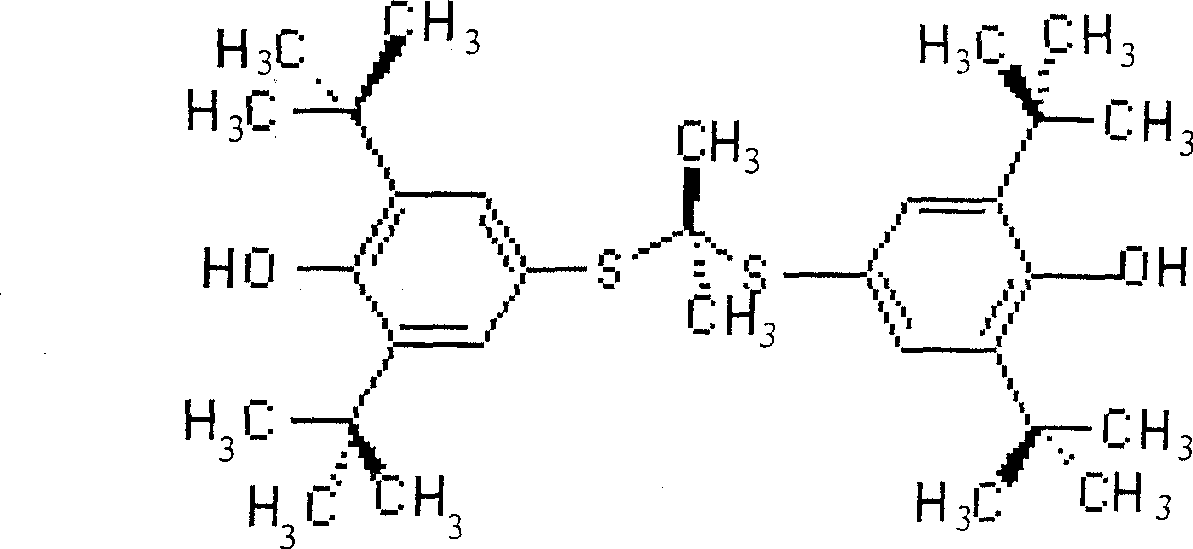
The invention relates to a synthesis method of probucol. The method comprises the following steps of: reacting 2,6-di-tert-butyl-4-mercaptophenol used as a starting material with allylene through pressurization under the catalysis of alkaline conditions, and then reacting the obtained olefin intermediate with 2,6-di-tert-butyl-4-mercaptophenol under the catalysis of a strong acid so as to obtain a product probucol. The method disclosed by the invention is simple in operation, high in product yield and good in purity.
Owner:山东安弘制药有限公司
Quality control method of probucol
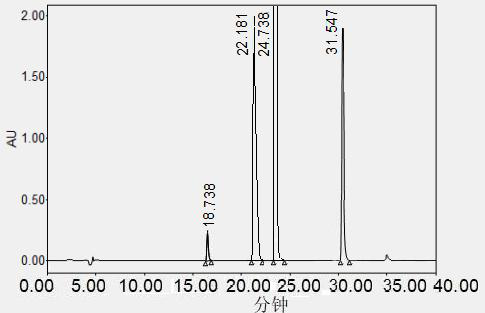
InactiveCN111679004AGuaranteed to be completely separatedEasy to separateComponent separationTert butyl phenolTert butyl
The invention belongs to the technical field of drug analysis. The invention discloses a high performance liquid chromatography separation and determination method for impurities in a probucol bulk drug and a preparation and application. The method is used for simultaneously separating impurities (2,2',6,6'-tetratert-butyldibenzoquinone), impurities B (4,4'-dithiobis(2,6-di-tert-butylphenol) and impurities C ((4-[(3,5-di-tert-butyl-2-hydroxyphenylthio)isopropyl mercaptan]-2,6-di-tert-butylphenol). According to the method, octadecylsilane chemically bonded silica is adopted as a chromatographiccolumn of a filler to prepare a reference substance solution and a test solution, the reference substance solution and the test solution are injected into a liquid chromatograph, an organic phase anda buffer salt solution in a certain proportion are adopted as mobile phases, and bulk drugs in the bulk drugs and related preparations and probucol, impurities A, impurities B and impurities C in thepreparations can be ensured to be completely separated. The method is short in analysis time, provides an effective quality control method for research, development and production of probucol bulk drugs and related preparations, and has popularization value.
Owner:山东同其信息科技有限公司
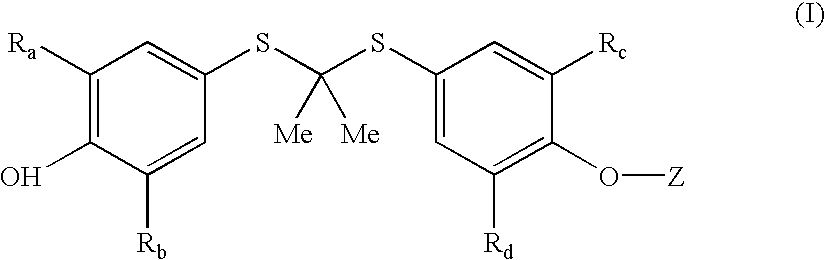
Process for preparation of probucol derivatives
A method is described for the preparation of polymorphic forms of water-soluble derivatives of probucol compounds having the following formula where R1, R2, R3, R4, R5, R6, Z and Z′ are defined herein.
Owner:CAMBREX CHARLES CITY INC
Probucol orally administered nanometer solid preparation and preparation method for same
InactiveCN102475688BImprove in vitro dissolutionImprove bioavailabilityPowder deliveryMetabolism disorderHigh dosesDissolution
The invention provides a probucol orally administered nanometer solid preparation, which comprises, by weight, 1 part of probucol, 0.01-0.1 part of additive and 0.05-0.4 part of auxiliary materials. The grain size of the probucol ranges from 10nm to 200nm. The invention further provides a preparation method for the probucol orally administered nanometer solid preparation. The preparation method includes that the probucol and water liquor with 5% of additive are prepared into nanometer suspension with the grain size ranging from 100nm to 600nm by a wet grinding method; the nanometer suspension is homogenized into nanometer grains with the grain sizes ranging from 10nm to 200nm by a high-pressure homogenizing machine; and the nanometer suspension which is homogenized is solidified and is added with the auxiliary materials to be prepared into the orally administered nanometer solid preparation. Compared with a common probucol tablet, the preparation has the advantages that dissolution in vitro of the preparation is increased by 20-30 times, and bioavailability after oral administration is improved by 10-20 times. In addition, the drug loading rate of the preparation is high, and the preparation can meet the requirements of tablet weight clinically needed by high-dose probucol administration. Besides, the preparation is stable, and the dissolution of the preparation can be kept unchanged within two years.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Preparing method of ethyl cellulose micro-and nano-particles loaded with probucol
InactiveCN101032448ANo adhesionGood ball formingPharmaceutical product form changeDistilled waterOil phase
The preparation process of micron and nanometer probucol supporting ethyl cellulose particle belongs to the field of controlled and slow releasing medicine preparation. The present invention adopts water solution of sodium dodecyl sulfate as the water phase and dichloromethane solution of ethyl cellulose and probucol as the oil phase, adds the oil phase into the water phase, emulsifies and eliminates dichloromethane to obtain micron and nanometer ethyl cellulose particle; and washes the particle with distilled water to neutrality and decompression dries at normal temperature for curing to obtain micron and nanometer probucol supporting ethyl cellulose particle. The present invention has simple operation, and the obtained micron and nanometer probucol supporting ethyl cellulose particle may reach nanometer level size.
Owner:SHANGHAI JIAO TONG UNIV
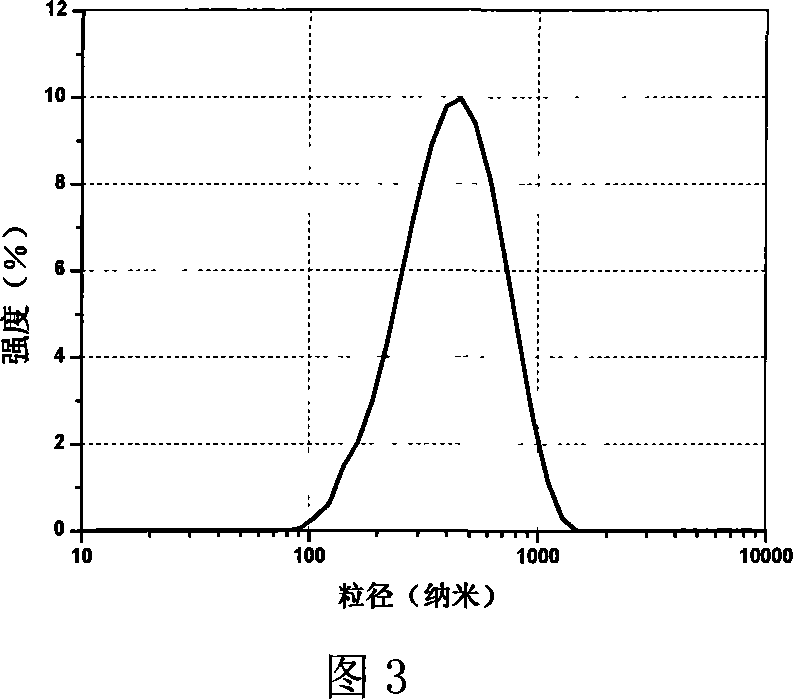
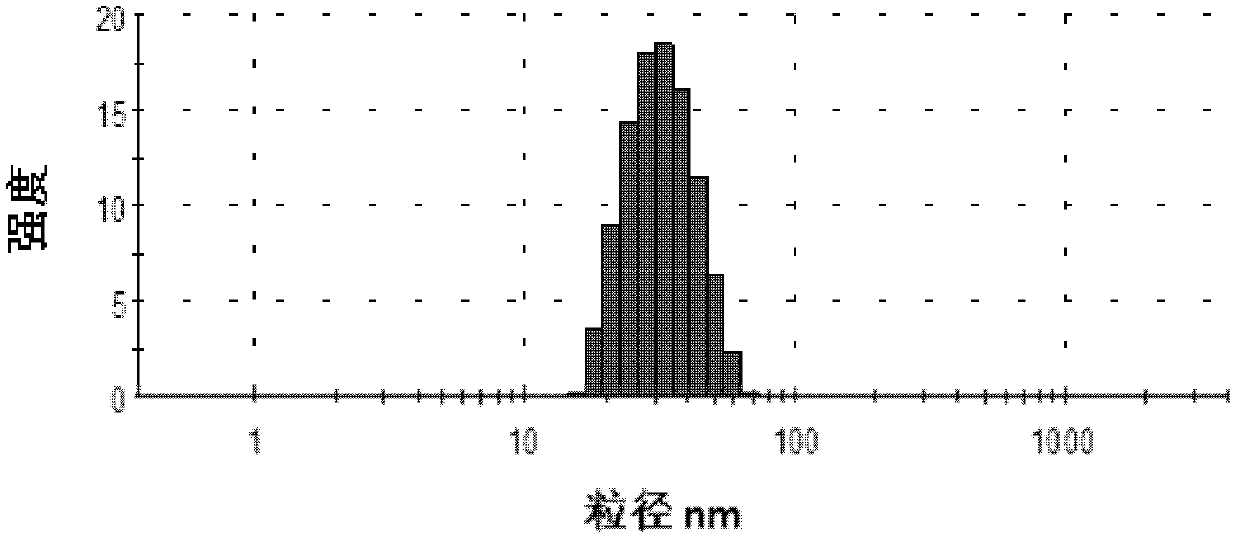
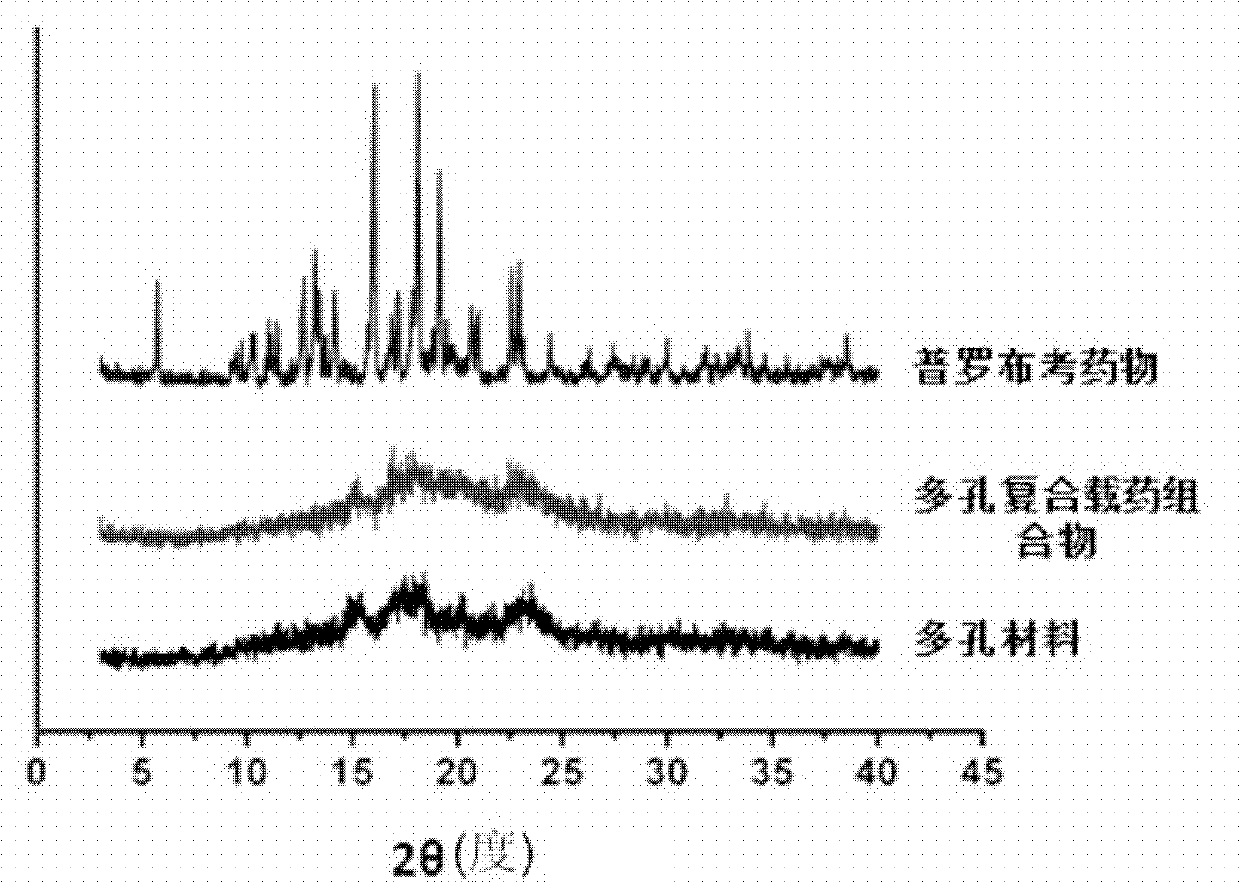
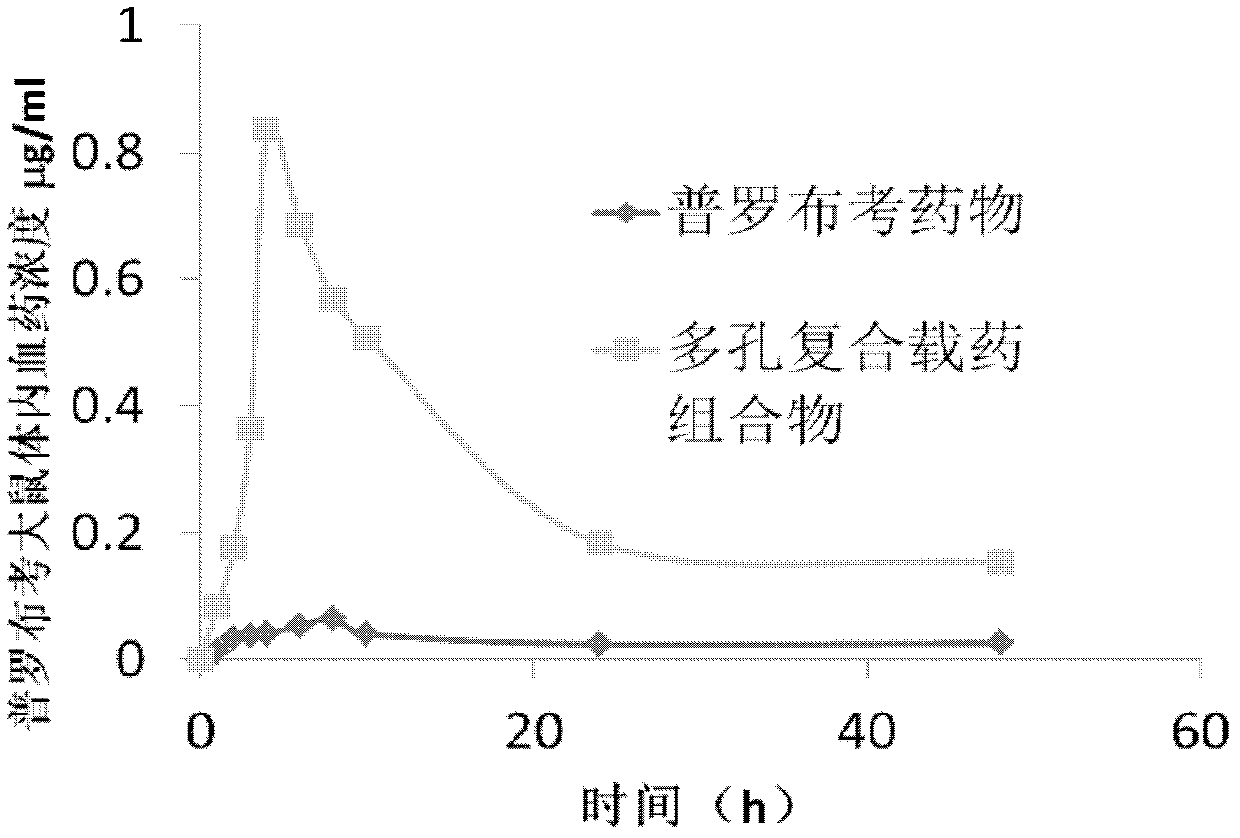
Porous composite medicine-loaded composition for improving oral absorption for probucol, and preparation method of porous composite medicine-loaded composition
ActiveCN103301095ASmall particle sizeImprove solubilityMetabolism disorderInorganic non-active ingredientsSolubilitySide effect
The invention provides a porous composite medicine-loaded composition for improving oral absorption for probucol. The porous composite probucol medicine-loaded composition is stable in thermodynamics, capable of improving the stability of medicaments, stable in gastrointestinal tract environment and free of toxic and side effects, uniform in system dispersion, the solubility of the porous composite medicine-loaded composition in water can be improved by 3-12 times, and the bioavailability of the porous composite medicine-loaded composition can be improved by 2-45 times.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
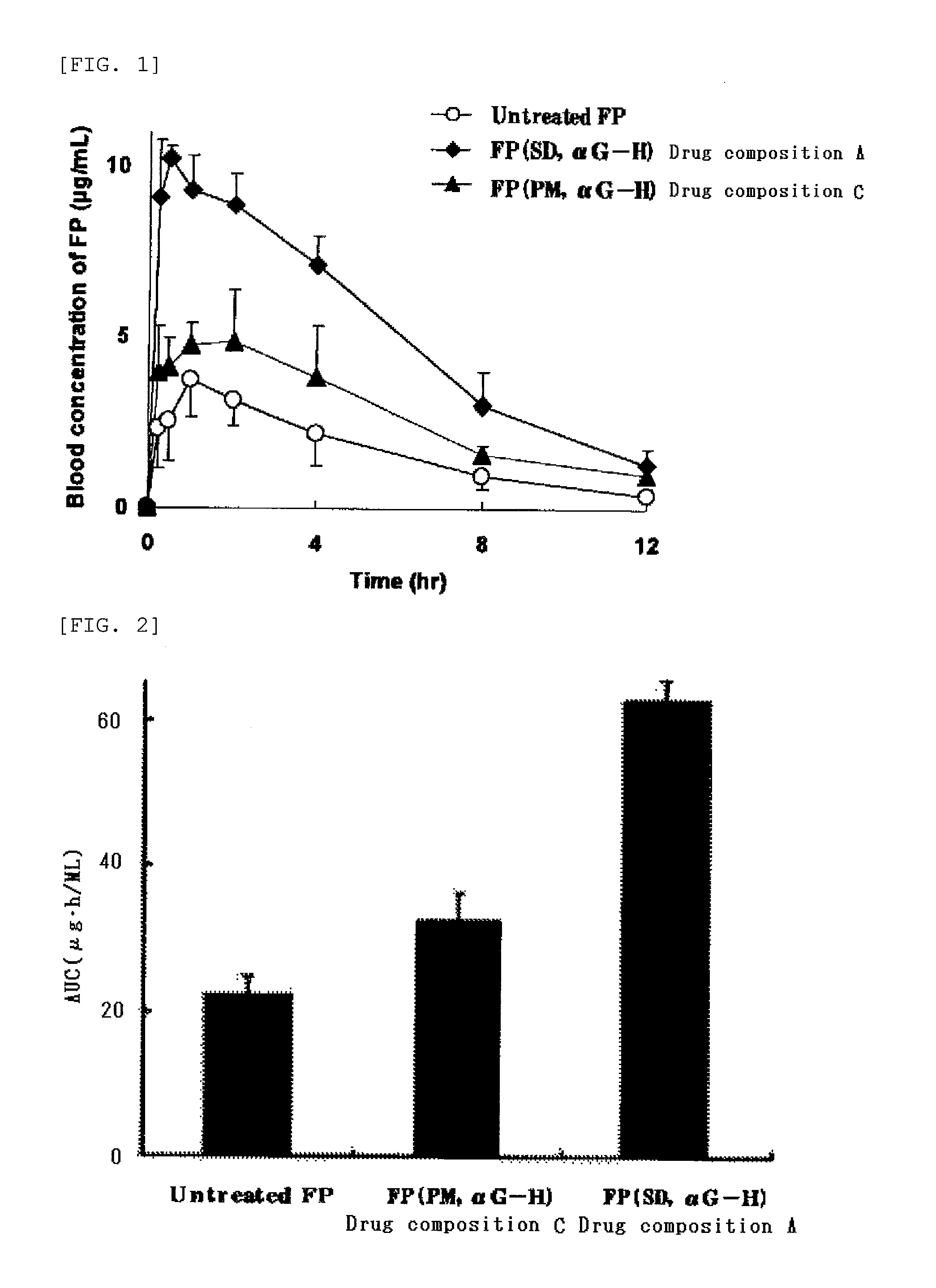
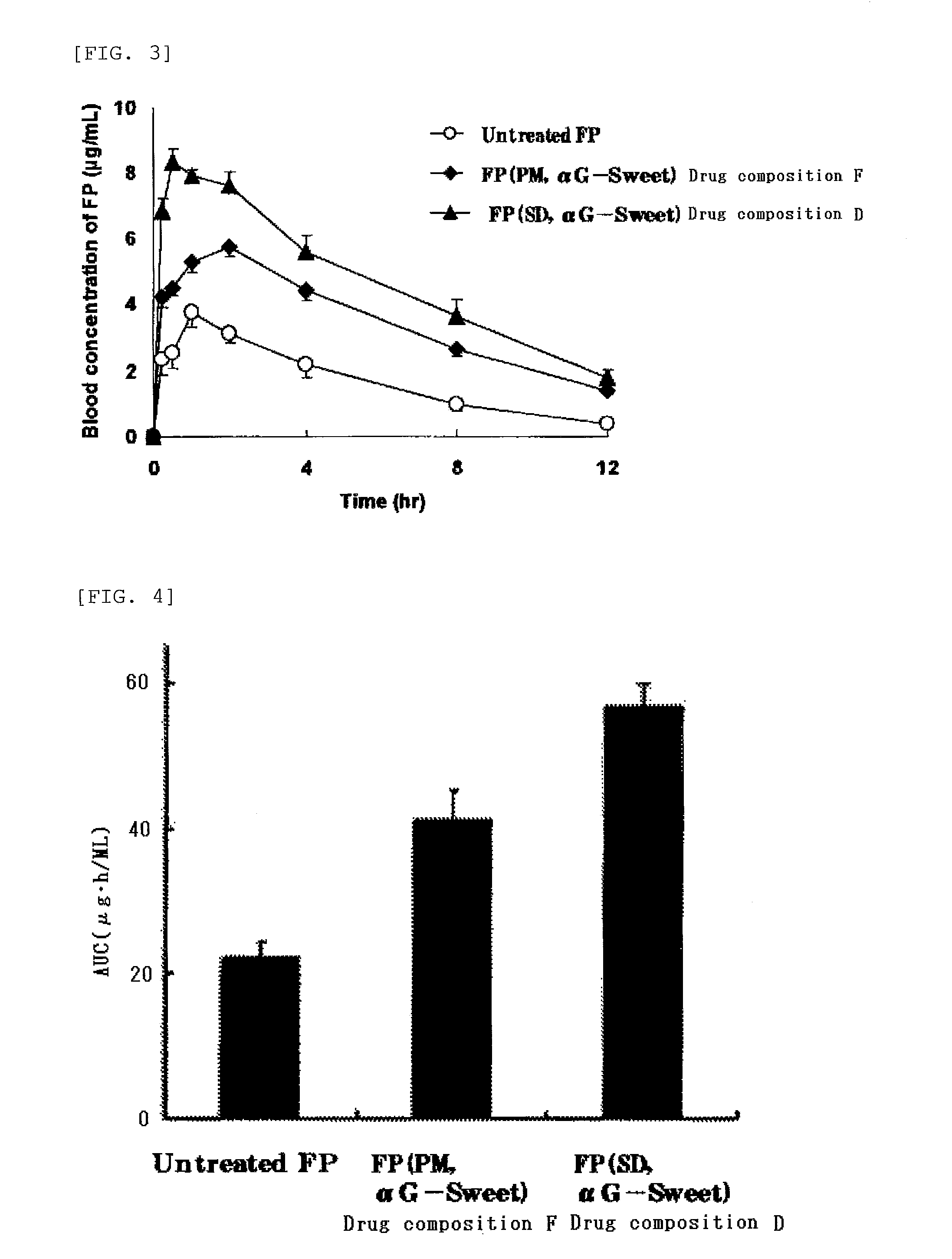
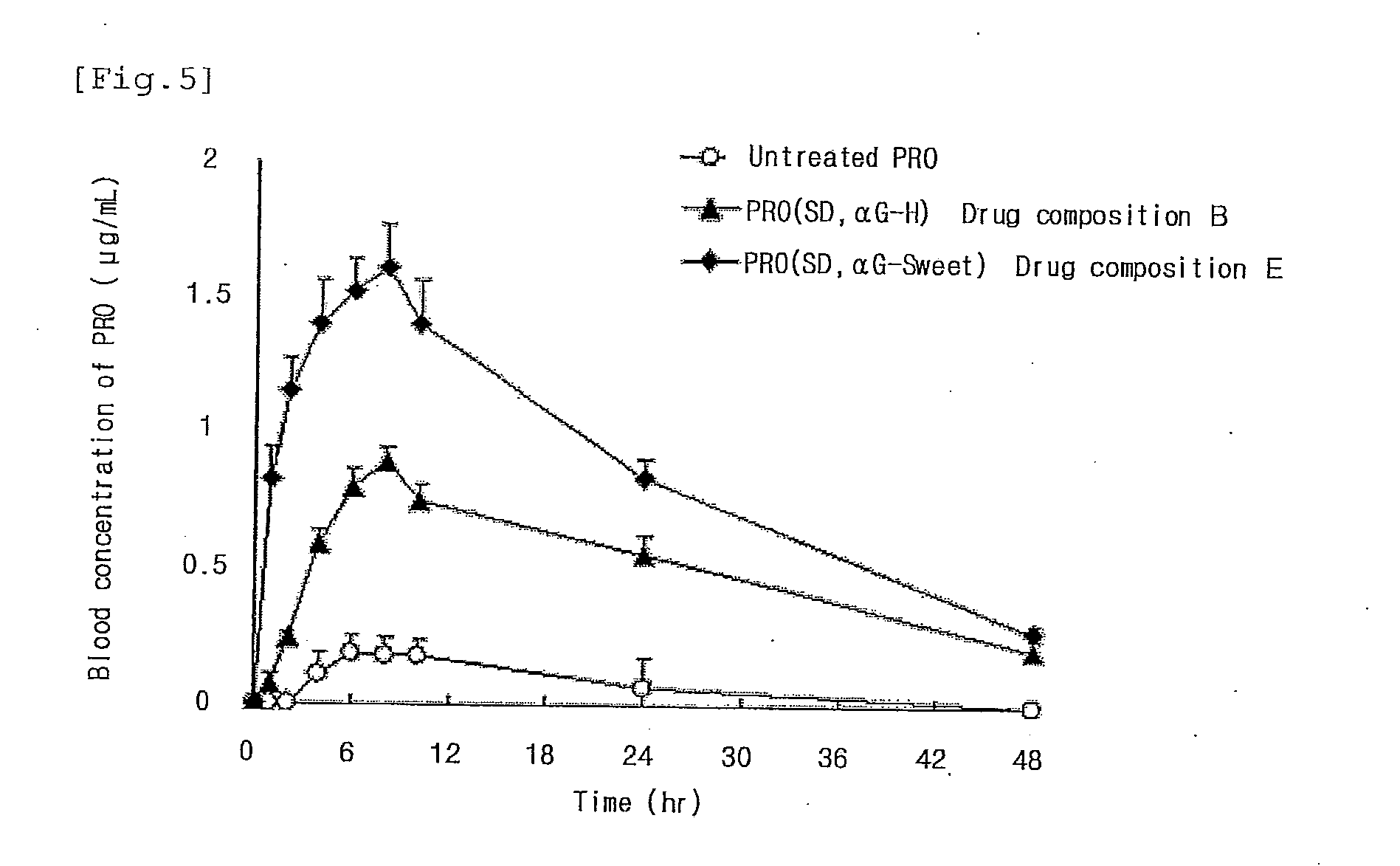
Highly Absorbable Drug Composition and Method of Producing the Same
InactiveUS20110189311A1Sufficient medicinal effectPromote absorptionBiocideAntipyreticWater solubleWater soluble drug
Provided is a highly absorbable drug composition including one or more water-soluble compound (A) selected from an enzyme-treated hesperidin, a stevia extract, or an enzyme-treated stevia and a poorly water-soluble drug (B) selected from flurbiprofen or probucol.
Owner:TOYO SUGAR REFINING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com