Chitin nanometer granule for programmable releasing various kinds of medicine, and its prepn. method
A chitosan nanometer and nanoparticle technology, which is applied in the field of medical engineering, can solve the problems of no time lag effect, the microspheres cannot reach the nanometer level, and the programmed release of various drugs cannot be realized, so as to reduce the pain of patients and improve medical treatment. cost, improved utilization, improved drug stability and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the chitosan nanoparticle of the programmed release multiple medicines to be prepared, specific weight percentage is: chitosan 58.52%, hydrophilic medicine 10.53%, lipophilic medicine 5.26%, Tween-800.01%, Sodium tripolyphosphate 10.53%, the preparation process is as follows:
[0036] (1) Preparation of aspirin-chitosan nanoparticles
[0037]Take by weighing 10mg aspirin and concentration be that 2mg / ml chitosan acetic acid solution 25ml phase mix, add 1ml concentration after stirring evenly and be the surfactant Tween-80 of 1wt%, under room temperature magnetic stirring (500r / s), drop 10 ml of 1 mg / ml sodium tripolyphosphate solution was continuously stirred for 1 hour to obtain a solution of chitosan nanoparticles loaded with aspirin. The aspirin-chitosan drug-loaded nanoparticles are obtained by centrifugal separation and freeze-drying.
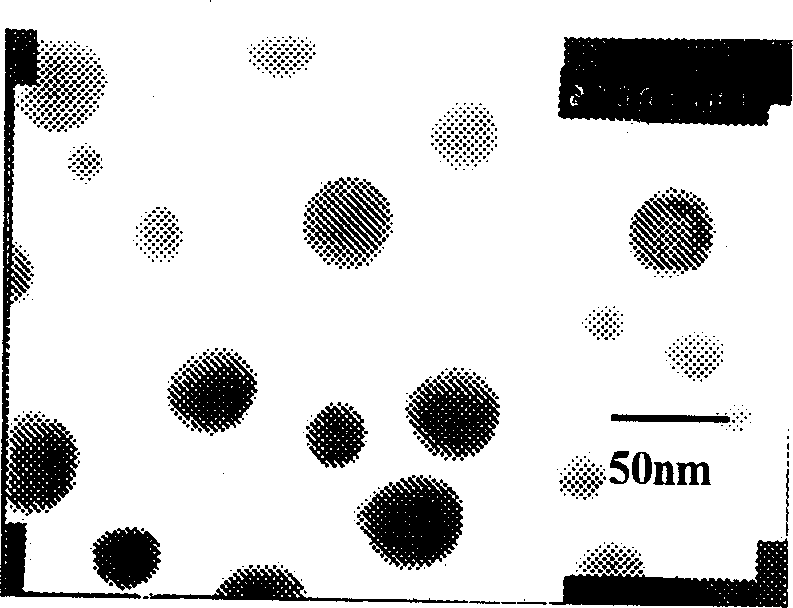
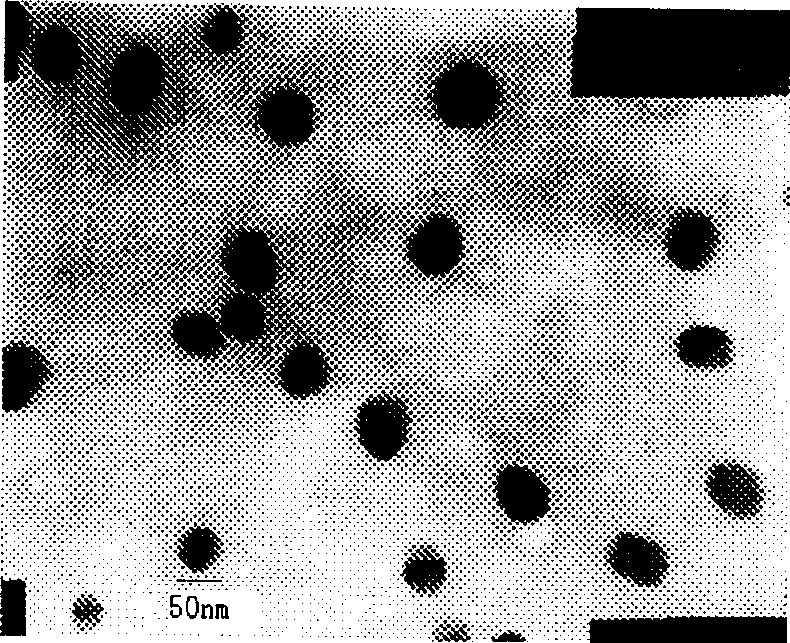
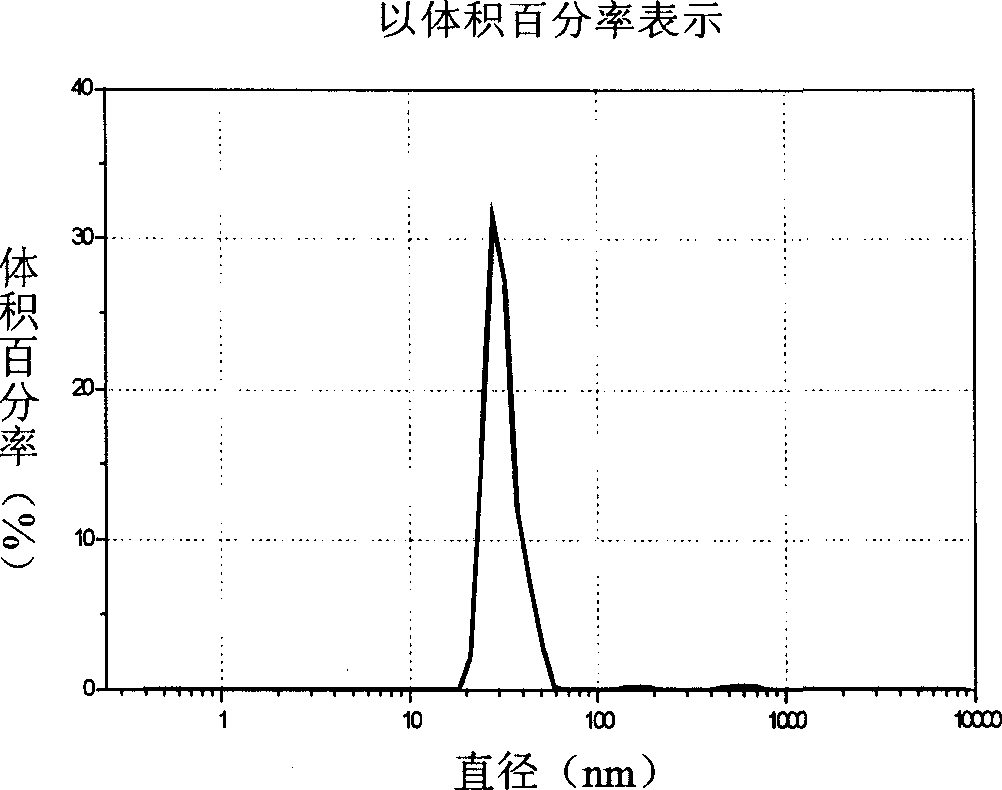
[0038] figure 1 It is an electron micrograph of the nanoparticle of the present invention (prepared as described ...
Embodiment 2
[0049] Embodiment 2: the chitosan nanoparticle of the programmed release multiple medicines to be prepared, specific weight percent is: chitosan 66.10%, hydrophilic medicine 15.02%, lipophilic medicine 6.29%, Tween-800.03%, Sodium Tripolyphosphate 12.58%. The preparation process is as follows:
[0050] (1) Preparation of aspirin-chitosan nanoparticles
[0051] Get 25mg of aspirin medicine and concentration is that 25ml of chitosan acetic acid solution of 3mg / ml are mixed, after stirring, add 1ml concentration and be the tensio-active agent Tween-80 of 3wt%, under room temperature magnetic stirring (500r / s), drop 10ml of 2mg / ml sodium tripolyphosphate solution was continuously stirred for 1 hour to obtain the aspirin-loaded chitosan nanoparticle solution. The aspirin-chitosan drug-loaded nanoparticles are obtained by centrifugal separation and freeze-drying.
[0052] (2) Preparation of probucol chitosan nanoparticles
[0053] Get the chitosan solution 25ml of 3mg / ml, add 1m...
Embodiment 3
[0058] Embodiment 3: the chitosan nanoparticle of the programmed release multiple medicines to be prepared, specific weight percentage is: chitosan 73.67%, hydrophilic medicine 19.51%, lipophilic medicine 7.31%, Tween-800.06%, Sodium Tripolyphosphate 14.63%. The preparation process is as follows:
[0059] (1) Preparation of aspirin-chitosan nanoparticles
[0060] Get 40mg of aspirin medicine and concentration is that 25ml of chitosan acetic acid solution of 4mg / ml are mixed, add 1ml concentration after stirring evenly and be the tensio-active agent Tween-80 of 6wt%, under room temperature magnetic stirring (500r / s), add dropwise 10ml of 2mg / ml sodium tripolyphosphate solution was continuously stirred for 1 hour to obtain the aspirin-loaded chitosan nanoparticle solution. The aspirin-chitosan drug-loaded nanoparticles are obtained by centrifugal separation and freeze-drying.
[0061] (2) Preparation of probucol chitosan nanoparticles
[0062] Get the chitosan solution 25ml ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com