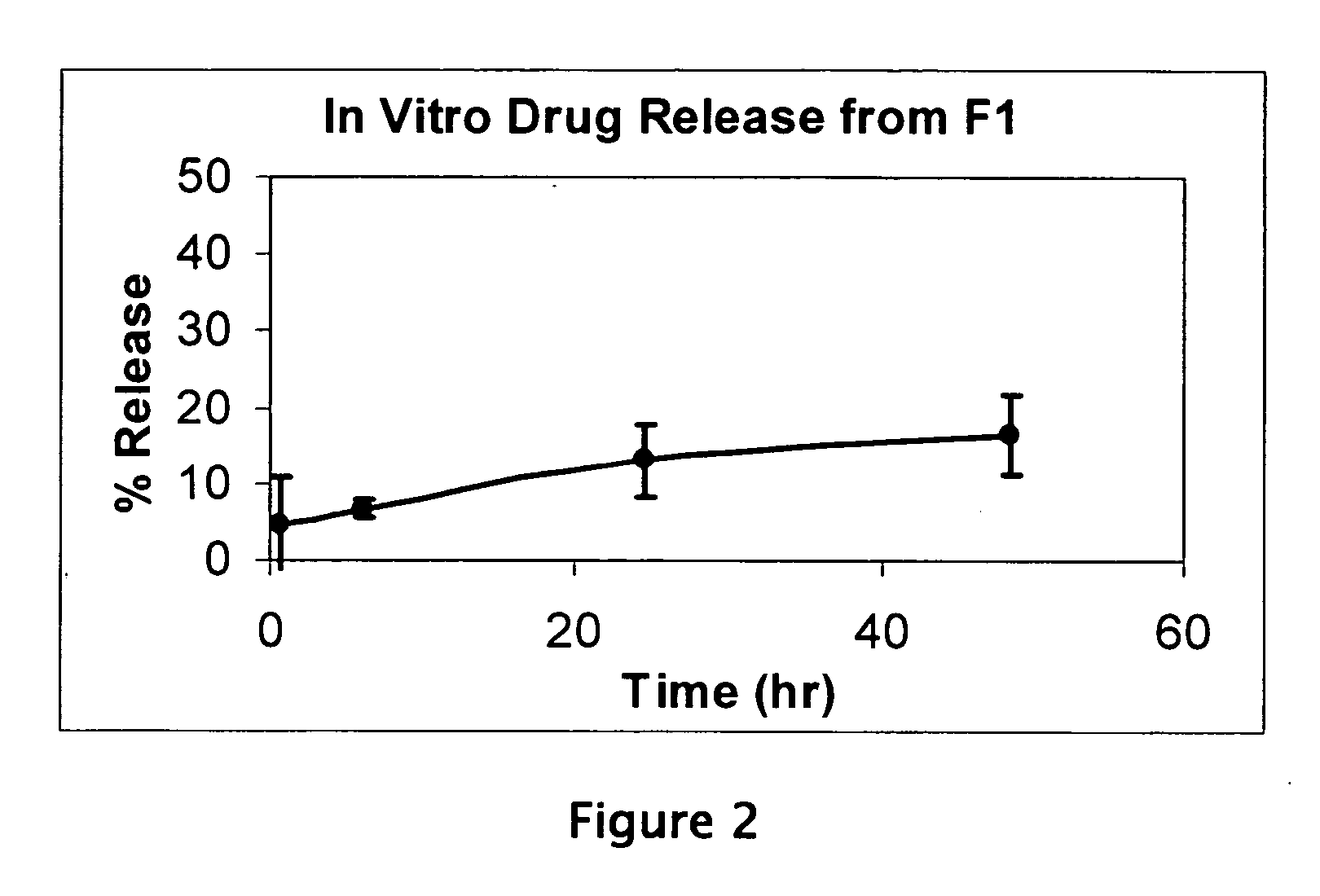
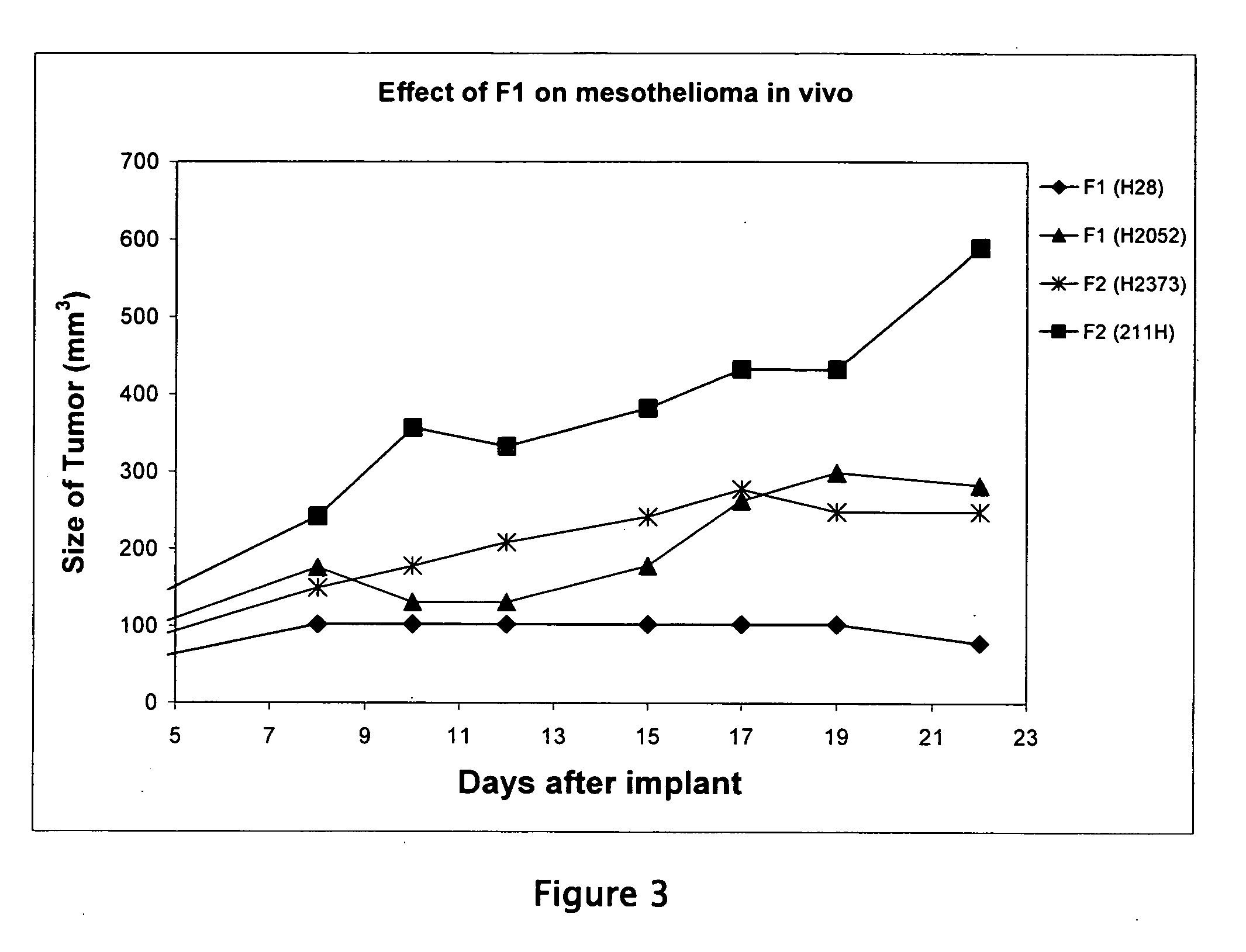
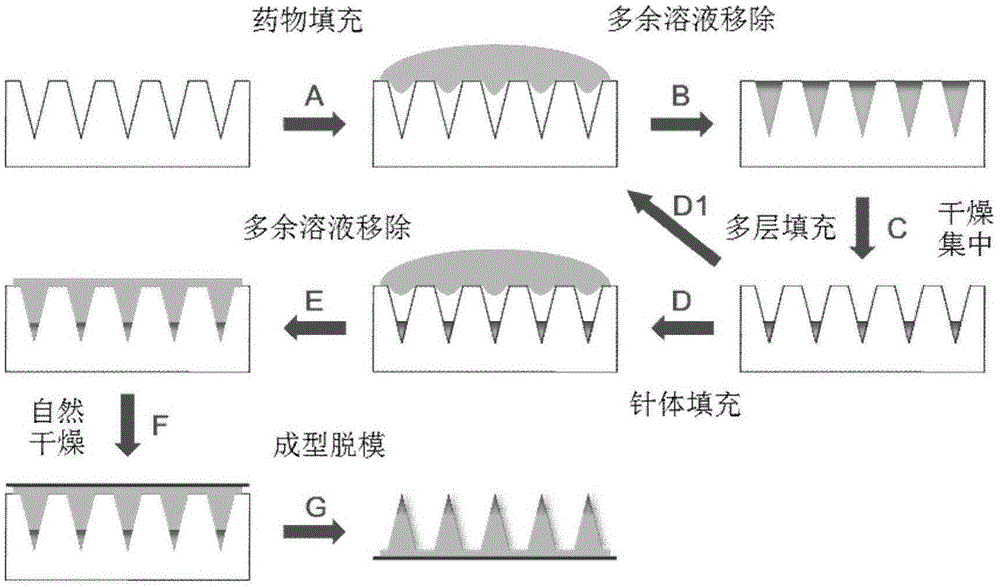
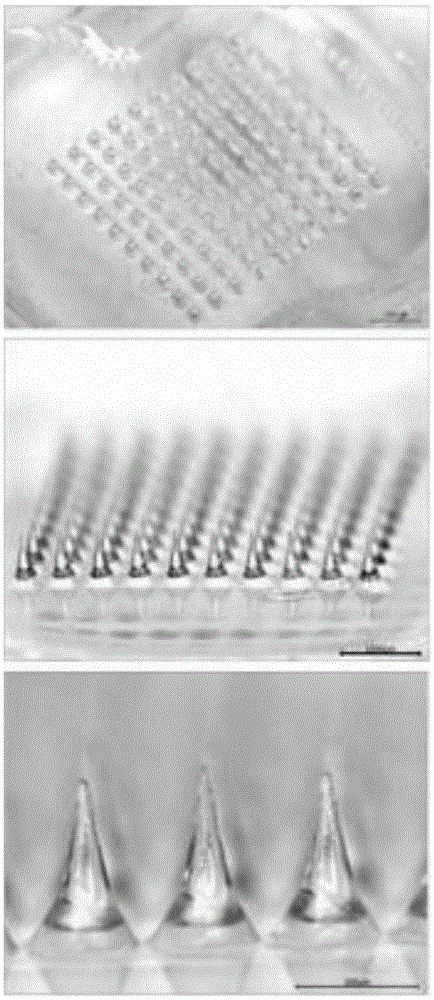
Patents
Literature
198 results about "Macromolecular drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Macromolecular drugs, such as peptides, proteins, and antibodies, offer a newer class of drugs that can treat diseases of the GI tract, such as inflammatory bowel disease (IBD). These drugs have demonstrated improved efficacy and represent a new paradigm of treatments for IBD.
Macromolecular drug complexes having improved stability and therapeutic use of the same
InactiveUS20050147581A1Safe and easy and effective deliveryAlleviate and eliminate and reverse complicationPowder deliveryOrganic active ingredientsDiseaseSomatotropic hormone
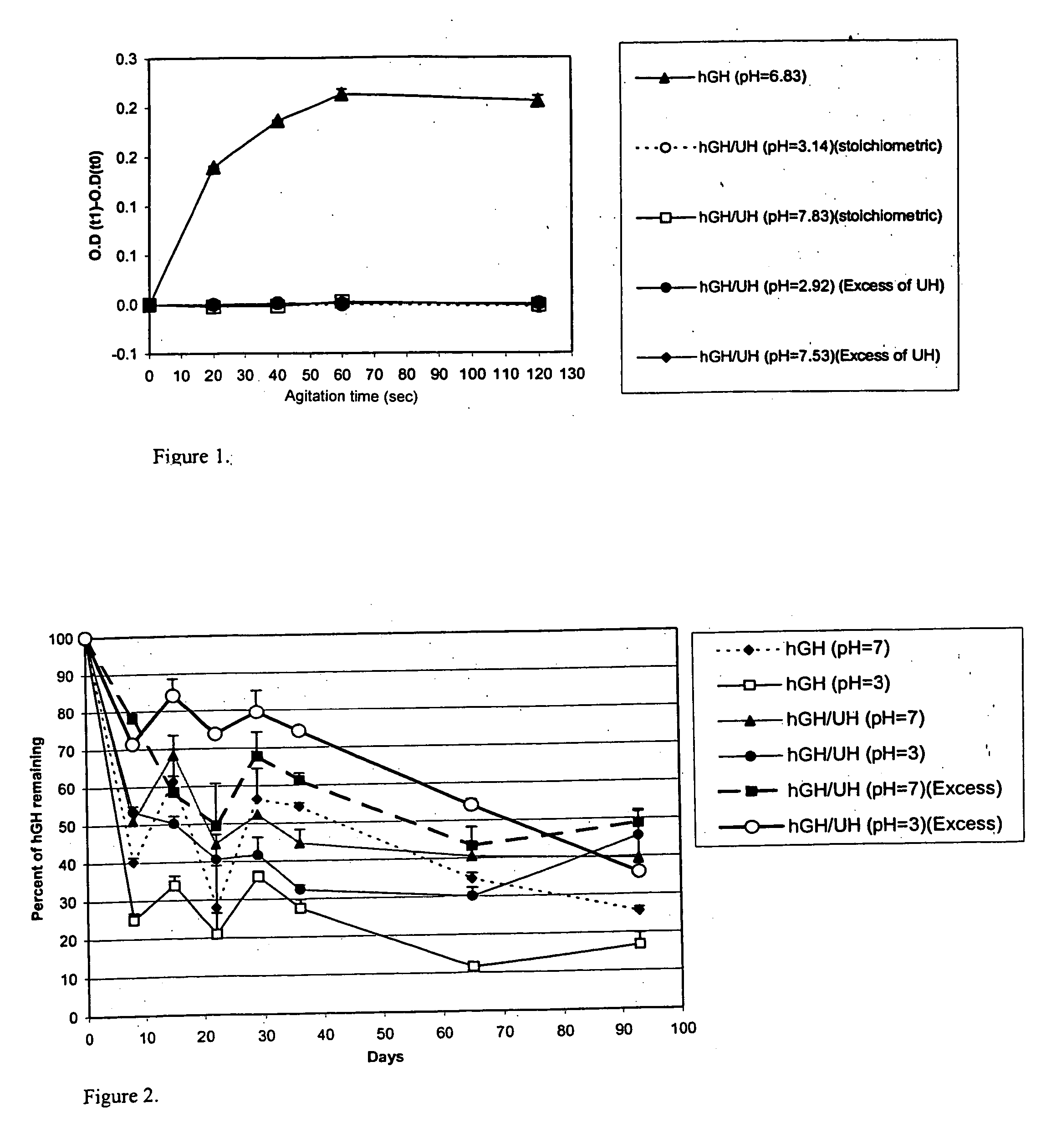
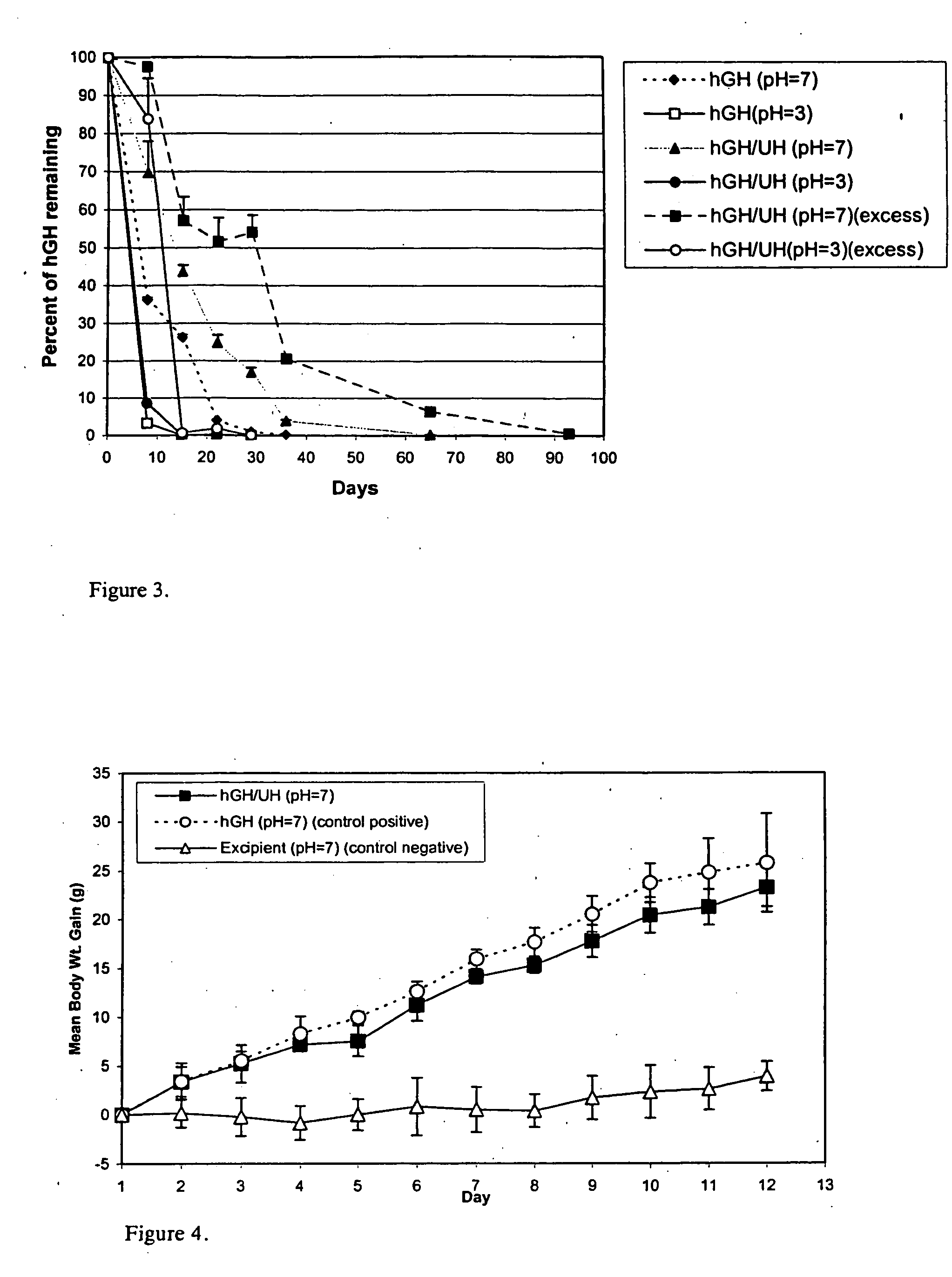
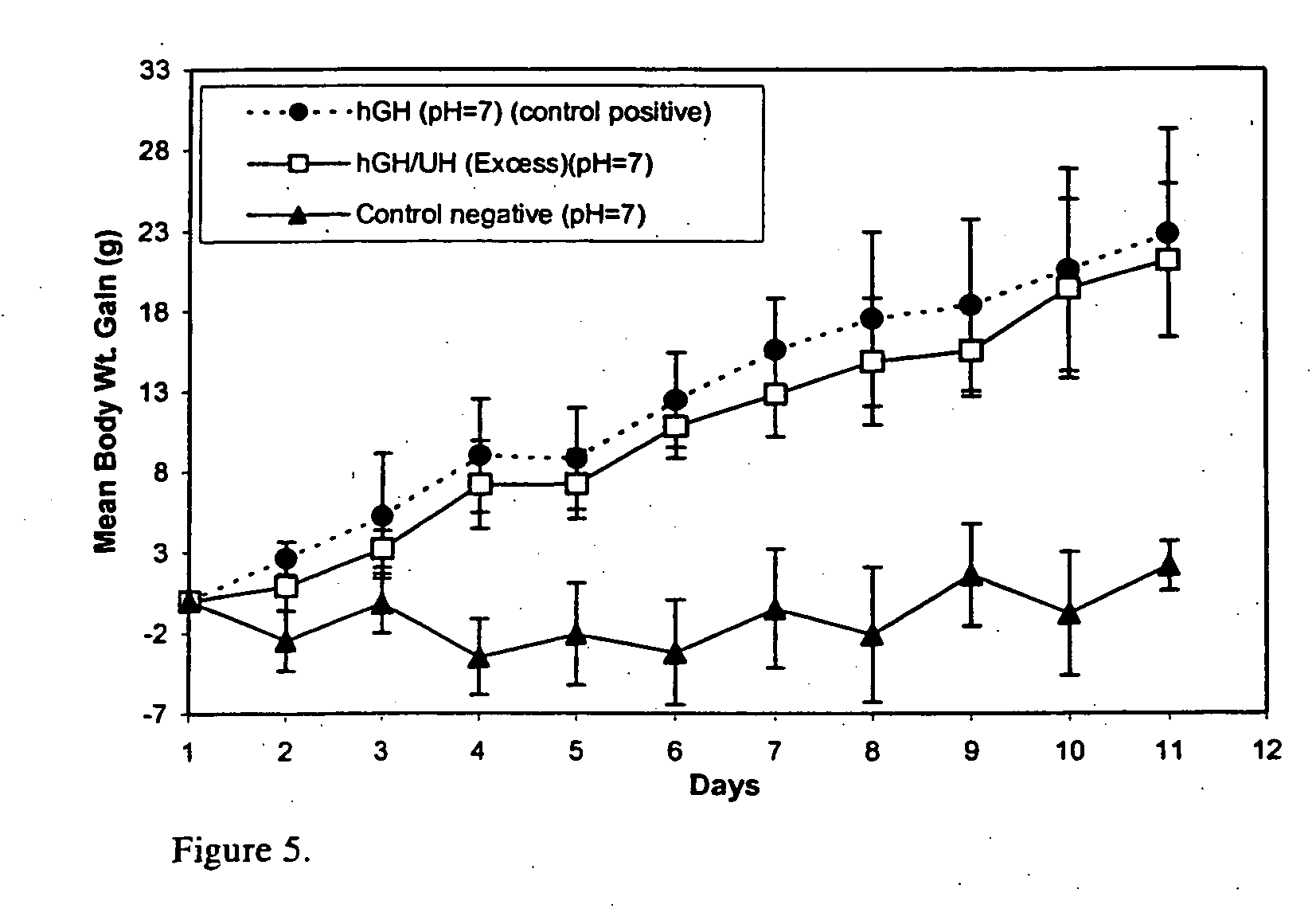
Macromolecular drug complexes containing a protein therapeutic, like human growth hormone, and an excess stoichiometric molar amount of a polymer, like heparin, and compositions containing the same, are disclosed. Compositions containing the macromolecular drug complexes are administered, including via pulmonary delivery, to individuals suffering from a disease or condition, and the complexes release the protein therapeutic, in vivo, to treat the disease or condition.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Liposome compositions for the delivery of macromolecules
InactiveUS20050260260A1Good curative effectReduce penetrationLiposomal deliveryLipid formationSterol
This invention provides for a liposome composition which demonstrates greatly increased therapeutic efficacy when used to deliver encapsulated macromolecular drugs. The liposome composition excludes the use of sterols, sterol derivatives, and cationic lipids, contrary to conventional formulations. The invention liposome is also unique in that it utilizes low gel to fluid phase transition temperature lipids in its membrane.
Owner:KISAK EDWARD +1
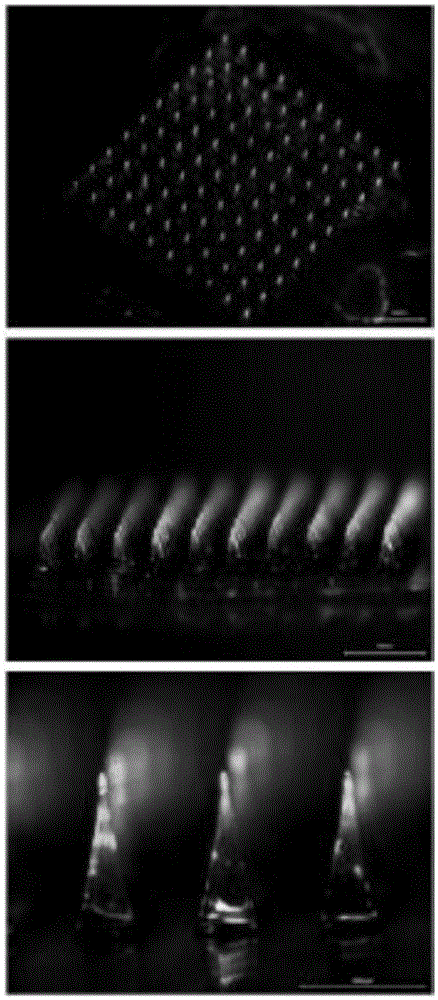
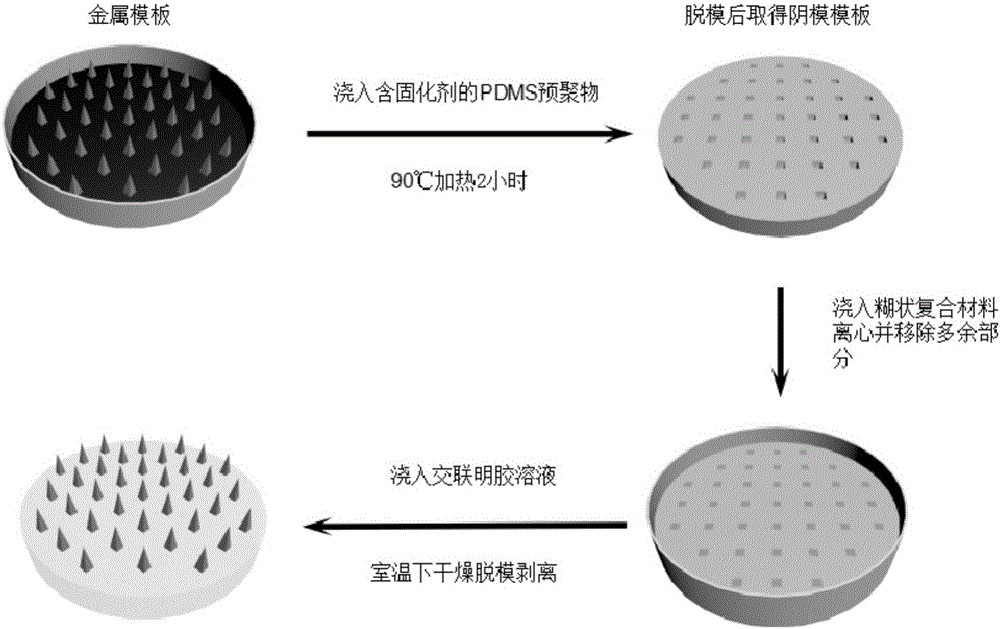
Macromolecular soluble microneedle used for cutaneous penetration of polypeptide and protein medicines and preparation method of macromolecular soluble microneedle
InactiveCN105078880AHigh concentrationThe preparation process conditions are simplePeptide/protein ingredientsPharmaceutical delivery mechanismSide effectHigh volume manufacturing
The invention discloses a macromolecular soluble microneedle used for polypeptide and protein medicines. The macromolecular soluble microneedle comprises a base as well as a needle body on the base and a needle point on the needle body, wherein the base and the needle body are made of a mixture of a high polymer material and a stabilizer; and solid active peptide or protein medicines are embedded into the needle point. The concentration degree of the medicines embedded in the needle point of the macromolecular soluble microneedle is relatively high, and nearly no residual medicines exist on the bottom of the needle body, so that the administration efficiency and the administration accuracy are greatly improved; in addition, the part entering the subcutaneous tissue of the needle body can rapidly dissolve, and further degrade to be completely absorbed by the tissues without toxic or side effects, so that the possible needle breaking risk existing in the microneedle made of other materials such as metal, glass and silicon is avoided. The preparation method of the macromolecular soluble microneedle is carried out under simple technological conditions, is low in price, and suitable for volume production, and the macromolecular soluble microneedle can be widely applied to the field of cutaneous penetration of macromolecular medicines.
Owner:BEIJING UNIV OF CHEM TECH
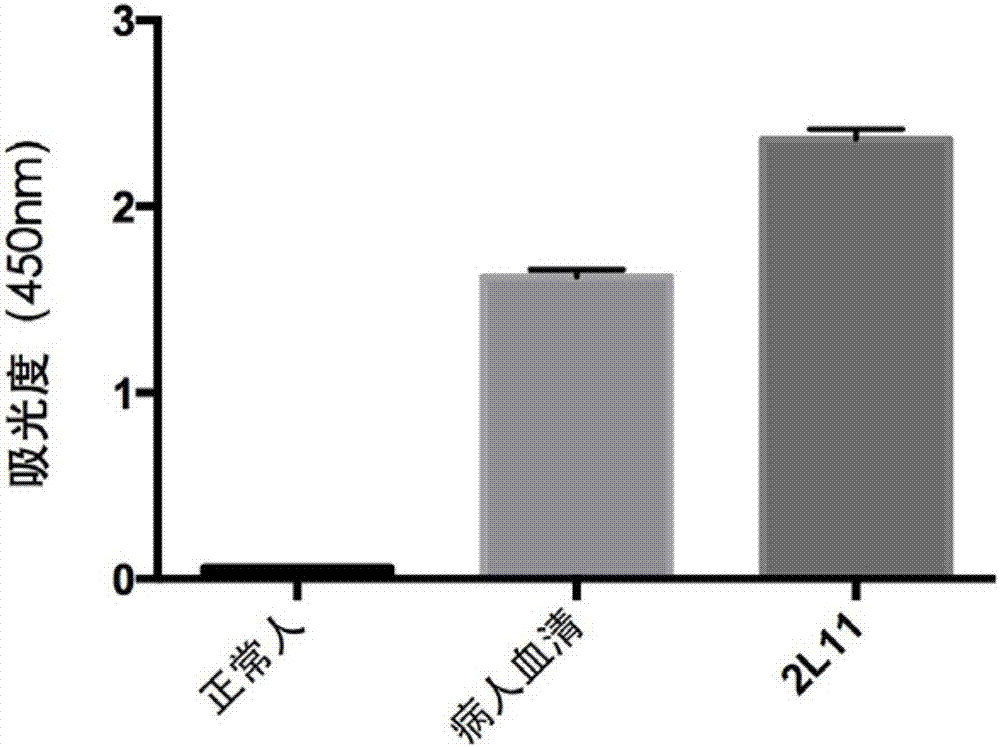
An anti-H7N9 completely humanized monoclonal antibody 2L11, and a preparing method and applications thereof
ActiveCN107353340AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininHeavy chain
The invention relates to an anti-H7N9 completely humanized monoclonal antibody 2L11, and a preparing method and applications thereof. The amino acid sequence of the heavy chain variable region of the antibody is shown as SEQ ID NO:2, or an amino acid sequence having similar functions and obtained by subjecting the sequence shown as the SEQ ID NO:2 to replacement, deletion or addition of one or more amino acids; and / or the amino acid sequence of the light chain variable region of the antibody is shown as SEQ ID NO:4, or an amino acid sequence having similar functions and obtained by subjecting the sequence shown as the SEQ ID NO:4 to replacement, deletion or addition of one or more amino acids. The antibody 2L11 can be bonded in a targeted manner with hemagglutinin HA of H7N9 viruses. Compared with mouse-sourced antibodies, genes of the completely humanized antibody are completely derived from human genes and do not comprise components of other species, and therefore no side or toxic effect such as anti-mouse antibodies is generated in a human body, and the antibody 2L11 has better biocompatibility, and is more suitable and has higher potential as a macro-molecule medicine treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Anti-H7N9 all-human-derived monoclonal antibody 2J17, and preparation method and application thereof
ActiveCN107337732AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininSide effect
The invention relates to an anti-H7N9 all-human-derived monoclonal antibody 2J17, and a preparation method and application thereof. The heavy chain variable region amino acid sequence of the antibody is disclosed as SEQ ID NO:2, or an amino acid sequence with the same function, which is formed by performing substitution, deletion or addition of one or more amino acids on the sequence; and / or the light chain variable region amino acid sequence of the antibody is disclosed as SEQ ID NO:4, or an amino acid sequence with the same function, which is formed by performing substitution, deletion or addition of one or more amino acids on the sequence. The antibody 2J17 can be bound with hemagglutinin HA of the H7N9 virus in a targeted way. Compared with the rat-derived antibody, genes of the all-human-derived antibody are completely derived from human genes without components of other species, so that the all-human-derived antibody does not have rat antiantibody resistance and other toxic or side effects in the human body, has better biocompatibility, and has higher adaptability and potential to become the macromolecular drug for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI +1
Micro-needle array chip, percutaneous administration device, percutaneous administration patch and preparation method thereof
The invention relates to a micro-needle array chip, a transdermal delivery device, a transdermal delivery patch and a preparation method thereof, wherein a micro-needle is obliquely fixed on a substrate at a set angle, the upper surface of the needle is an elliptic plane parallel to the substrate or obliquely provided with an acute angle, the needle is further treated to have more edge angles, a metal wire bar or tube penetrates a non-metallic material substrate such as polymer and the like along a certain angle, and then a nick of the metal wire bar or tube on the substrate is grinded and polished; and photosensitive resist patterns are formed nearby the nick at one end by adopting photoetching and etching technology, then the metal wire bar or tube is subjected to chemical or electrochemical corrosion to form a needle point and the photosensitive resist is removed, the surface of the manufactured solid micro-needle array chip can be covered with drugs, and the manufactured solid micro-needle array chip can deliver liquid medicine or extract body fluid. The method avoids the skin plugging a transfusion hole, is easy to adjust and control the maximum penetrating depth of the micro-needle, and is suitable for percutaneous transportation of biological macromolecular drugs and cosmetics and skincare and new percutaneous preparation of the prior drugs.
Owner:TSINGHUA UNIV
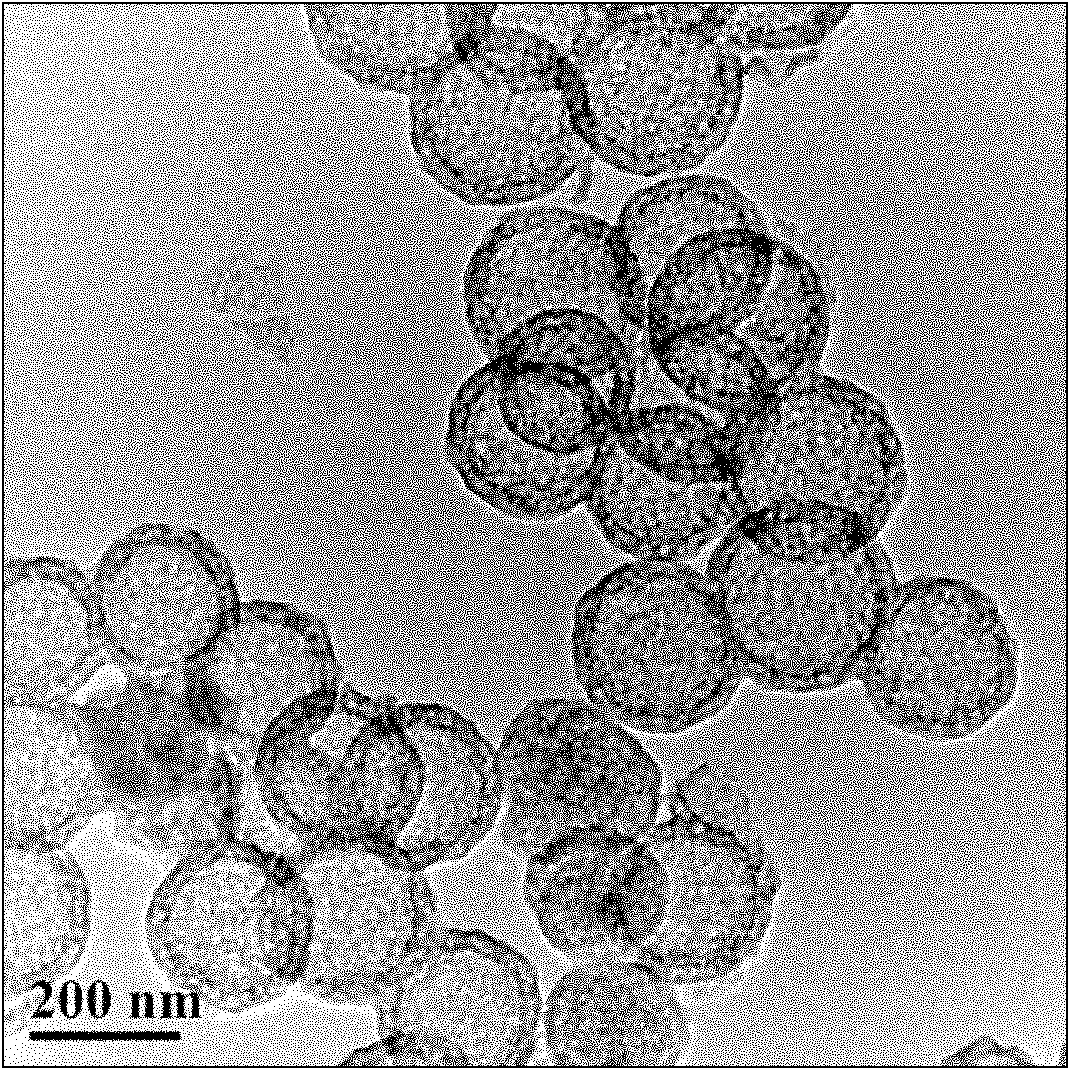
Large-pore-wall cage-shaped silica hollow sphere and preparation method thereof
InactiveCN102381715AOvercoming technical difficultiesLarge specific surface areaSilicaDispersityMicroreactor
The invention relates to a large-pore-wall cage-shaped silica hollow sphere and a preparation method thereof. A spherical wall of the silica hollow sphere is of a mesoporous structure, and the aperture of mesopores is 2.5-11nm. The preparation method comprises the steps of: firstly, synthesizing solid core / mesoporous shell SiO2 spheres with high dispersity and uniform particle sizes by using a sol-gel method and a surfactant orienting method; and then, skillfully removing solid cores in the SiO2 spheres while maintaining the completeness of the mesoporous layers of shells by performing a simple postprocessing method in an alkaline / acid solution to obtain the large-pore-wall cage-shaped SiO2 hollow spheres. The preparation method disclosed by the invention is simple and feasible, has no pollution, and is high in yield, low in cost, high in efficiency and extremely easy for industrialized production; and the prepared large-pore-wall cage-shaped SiO2 structure has wide application prospects in the fields of macromolecular medicament transportation, deoxyribonucleic acid (DNA) and small interfering ribonucleic acid (siRNA) loading and transporation, catalysis, microreactors, adsorption, separation, color spectrum and the like.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Anti-H7N9 full-human-derived monoclonal antibody 5J13 and preparation method and application thereof
ActiveCN106519027AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininSide effect
The invention relates to an anti-H7N9 full-human-derived monoclonal antibody 5J13 and a preparation method and application thereof. Amino acid sequences of heavy and light chain CDR1, CDR2 and CDR3 of the antibody are GFSFSNYG in the heavy chain CDR1 area, ISYDGTNK in the heavy chain CDR2 area, AKGRGPYCSSSICYHGMDV in the heavy chain CDR3 area, QSVLSGSINMNY in the light chain CDR1 area, WAS in the light chain CDR2 area and QQYYSTPLT in the light chain CDR3 area correspondingly. The antibody can be combined with hemagglutinin A of the H7N9virus in a targeted mode and has remarkable neutralization activity in resisting H7N9 virus infection. Compared with a murine antibody, genes of the full-human-derived antibody are completely from human genes and have no components of other species, toxic and side effects such as the anti-mouse anti-antibody are avoided, the biocompatibility is better, and the anti-H7N9 full-human-derived monoclonal antibody 5J13 is more suitable and has more potential in becoming a macromolecular drug for treating influenza virus.
Owner:SHENZHEN INST OF ADVANCED TECH
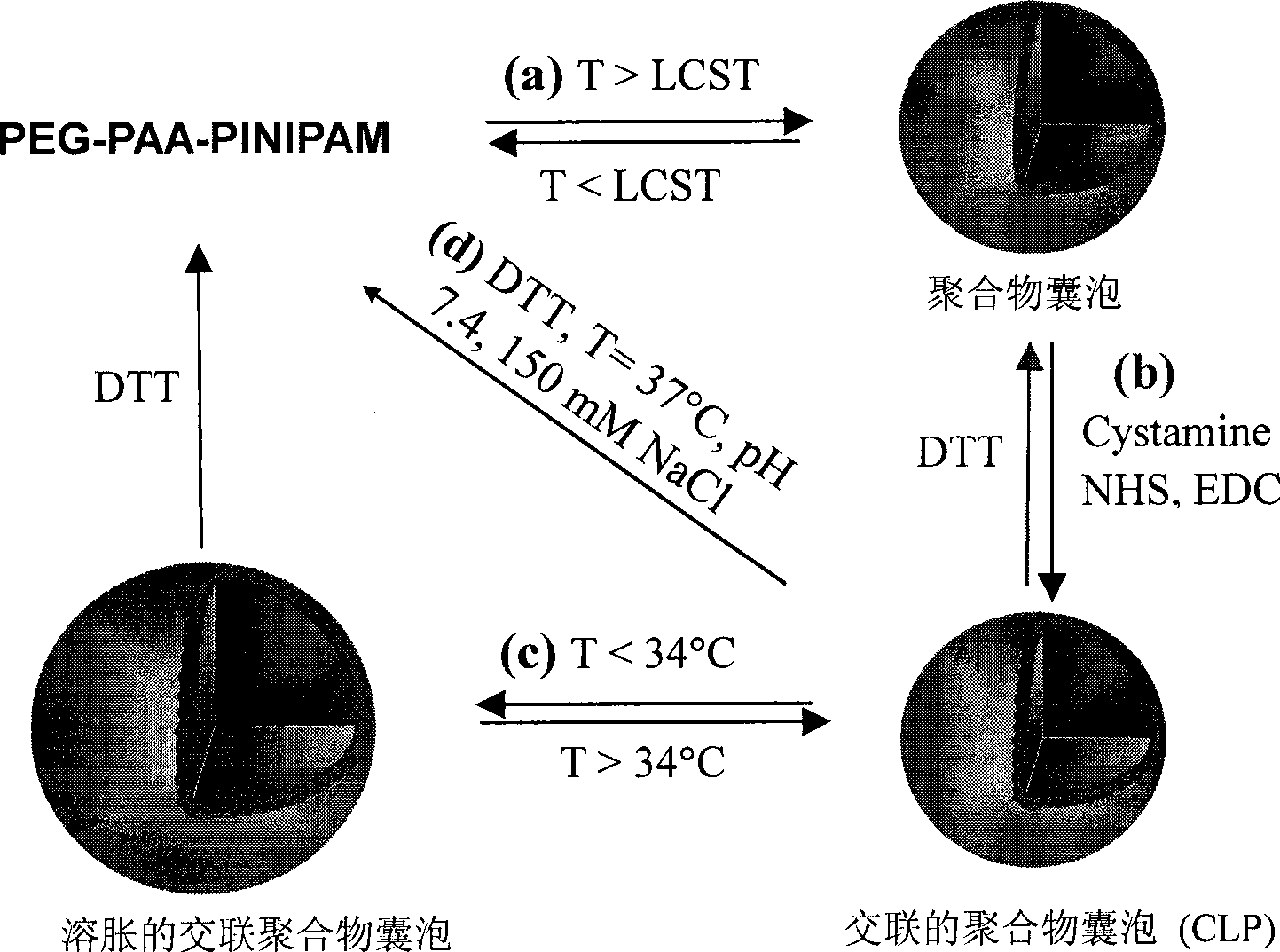
Interface-cross-linked temperature-sensitive polymer vesicle and use thereof
InactiveCN101519495AConvenient and Efficient PackingOvercome efficiencyPharmaceutical non-active ingredientsCross-linkIn vivo
The invention discloses an interface-cross-linked temperature-sensitive polymer vesicle and a method for preparing the same. The interface-cross-linked temperature-sensitive polymer vesicle is formed by a block copolymer which at least comprises a hydrophilic block, a cross-lined interstrand block and a temperature sensitive block, wherein the hydrophilic block forms the membrane shell of the polymer vesicle, the temperature sensitive block forms the membrane nuclear of the polymer vesicle, and the cross-lined interstrand block forms the interface of the polymer vesicle to cross-link the interface of the polymer vesicle to stabilize the structure of the vesicle, and thus the interface-cross-linked temperature-sensitive polymer vesicle is formed; as the vesicle is formed in an aqueous solution system and the interface of vesicle is cross-linked, the small molecule drug, macromolecular drug and probe molecule entrapment efficiency of the polymer vesicle, the circulation stability in vivo blood and the efficiency of endocytosis by tumor cells are improved; and as a result, the bioavailability of drugs is improved and the polymer vesicle can be expelled out of the body conveniently.
Owner:SUZHOU UNIV
Preparation of keratin-sodium alginate composite microporous gel and application of gel as drug carrier
InactiveCN104892864APromote swellingGood deswellabilityOrganic active ingredientsMacromolecular non-active ingredientsPolymer scienceCross linker
The invention provides preparation of a keratin-sodium alginate composite microporous gel, belonging to the fields of composite materials and biotechnology. The preparation method comprises the following step: carrying out self-crosslinking polymerization on the raw materials biocompatible natural high-polymer protein and polysaccharide (keratin and sodium alginate) under the actions of an organic crosslinking agent, an inorganic crosslinking agent an initiator. The composite microporous gel has favorable swelling and deswelling properties, is sensitive to pH value, and has slow-release effects on both low-molecular and high-molecular drugs. The in-vitro drug release performance experiment indicates that the composite microporous gel can implement controlled release of drug molecules by utilizing the acid sensitiveness, and thus, can be used as a drug carrier in drug controlled release.
Owner:NORTHWEST NORMAL UNIVERSITY
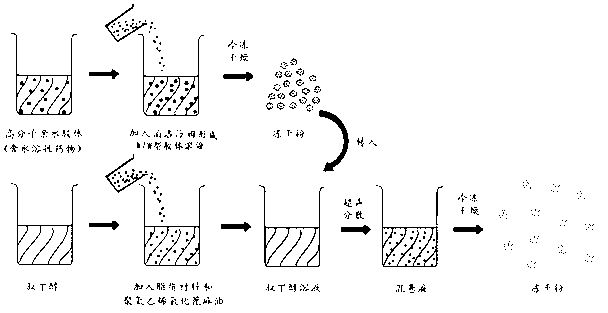
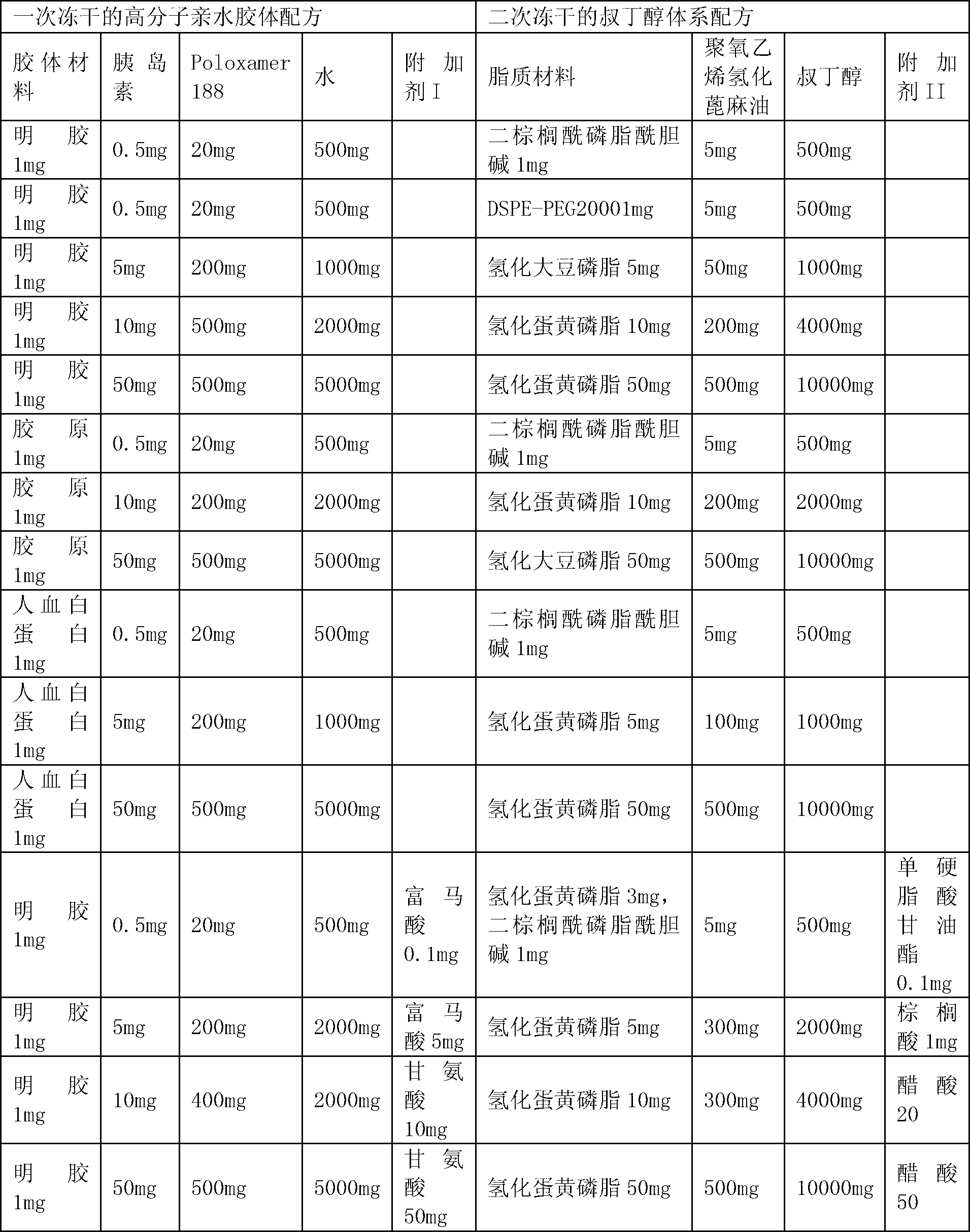
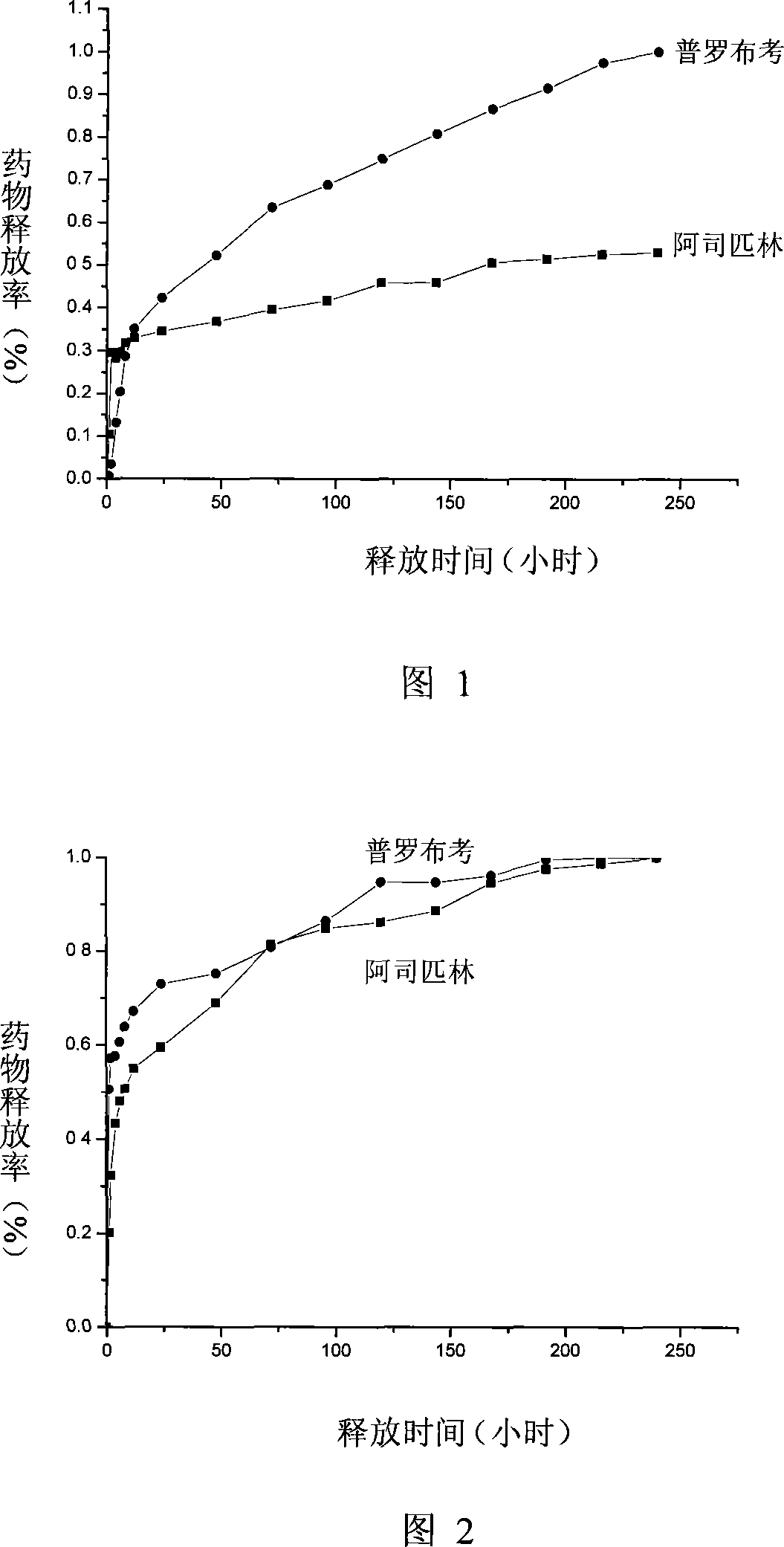
Preparation method of liposome entrapping water-soluble medicines
InactiveCN102935068AGood dispersionGuaranteed stabilityPharmaceutical product form changeMacromolecular non-active ingredientsWater soluble drugEntrapment
The invention relates to a preparation method of a liposome entrapping water-soluble medicines. According to the invention, a water-soluble medicine is dissolved in amino-acid-structure-containing high-molecular hydrophilic colloids such as gelatin, collagen or albumin; poloxamer is added and the mixture is well mixed, such that a W / W type colloidal solution is formed; the solution is lyophilized; the formed lyophilized powder is transferred into a tert-butanol solution containing a liposome material, and the mixture is well dispersed; and the mixture is subjected to lyophilization, such that high-entrapment-efficiency liposome entrapping the water-soluble medicine is formed. According to the liposome preparation method, W / W type colloid is combined with a two-step lyophilization preparation technology, such that problems such as low water-soluble medicine load, low entrapment efficiency and poor stability of existing preparation methods of liposome entrapping water-soluble medicines are solved. The method provided by the invention is used for preparing liposome used for entrapping water-soluble medicines, and is especially suitable for preparing liposome used for entrapping poor-thermal-stability or macromolecular medicines. The method can also be used for covering medicine bitterness or odor, and for separating medicines from other components.
Owner:ZHEJIANG HISUN PHARMA CO LTD +1
Method for preparing quaternary ammonium salt modified chitosan medicine-carried nano particles
InactiveCN101085358AImprove stabilityIncrease profitPharmaceutical non-active ingredientsMacromolecular drugNanoparticle
A preparation method of quarternary ammonium salt modified chitosan drug-loaded nanoparticle belongs to medical engineering field. The method comprises lead-in quaternary amine group to 6-hydroxy of chitosan under the protection of 2-amino to complete quaternary amine modification of C6-OH on chitosan and obtain quarternary ammonium modified chitosan, and wrapping under the existence of sodium tripolyphosphate at room temperature through controlling quarternary ammonium modified chitosan / TPP of 6 / 1-3 / 1 to complete preparation of drug-loaded nanoparticle. The invention can be used as carrier material of macromolecular medicament to promote the molecule passing through tissue epithelia and increase absorption of macromolecular medicament in tissue; used for transporting micromolecular medicament; and has significant clinical application value and potential application.
Owner:SHANGHAI JIAO TONG UNIV
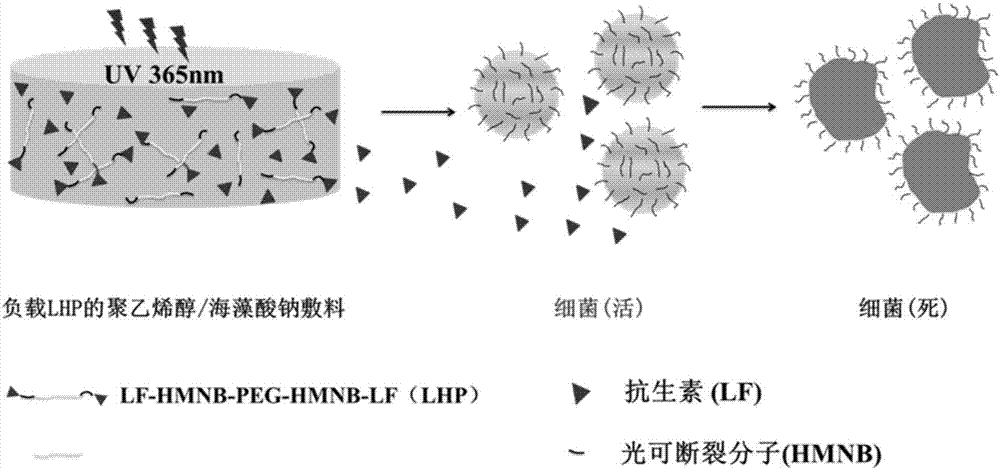
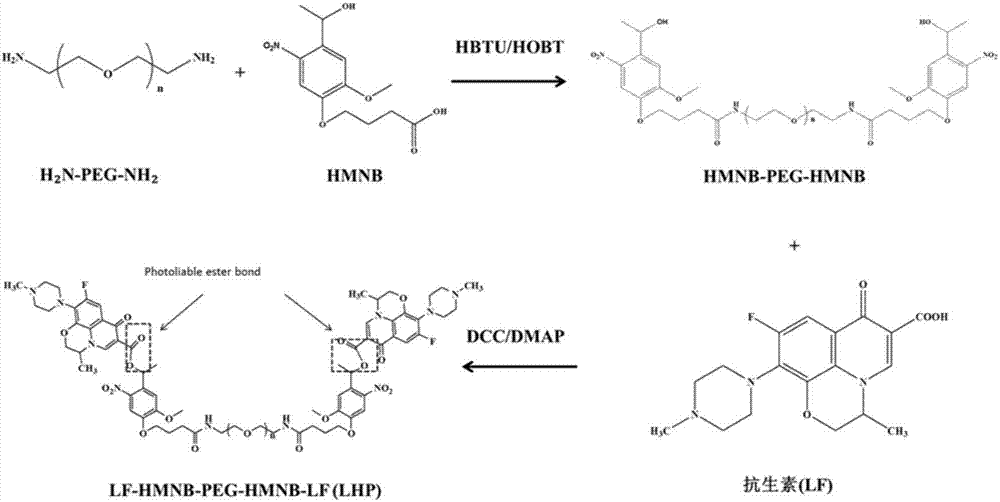
Polyvinyl alcohol/sodium alginate medicine-loading hydrogel dressing having light-responsive bacteria resistance and preparation method of polyvinyl alcohol/sodium alginate medicine-loading hydrogel dressing
ActiveCN107496975AWith mechanical propertiesGood biodescriptiveAntibacterial agentsOrganic active ingredientsBiocompatibility TestingUltraviolet lights
The invention relates to polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing having light-responsive bacteria resistance and a preparation method of the polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing, wherein a macromolecular medicine realizing fracturing under ultraviolet light is distributed inside the hydrogel dressing. The preparation method of the polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing comprises the following steps: grafting micromolecule antibiotic, namely, levofloxacin to polyethylene glycol macromolecular chains with amino groups at two ends through molecules with ultraviolet light response property, thus obtaining the macromolecular medicine realizing fracturing under ultraviolet light; and carrying out repeated freezing and thawing on a mixed solution of polyvinyl alcohol solution, sodium alginate solution and the macromolecular medicine realizing fracturing under ultraviolet light, thus obtaining the polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing having light-responsive bacteria resistance. The adopted raw materials have good biocompatibility, the obtained hydrogel dressing has certain mechanical property and has the ultraviolet-light-responsive antibacterial property, namely, the hydrogel dressing can realize controlled release of antibiotics under irradiation of 365nm ultraviolet light, and thus the polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing is suitable for protecting and treating the wound surface.
Owner:ZHEJIANG UNIV
A preparing method for slow release hydrogel carrier material and an application thereof
ActiveCN108384031AStable structureGood slow releaseOrganic active ingredientsPeptide/protein ingredientsPolyelectrolyteMacromolecular drug
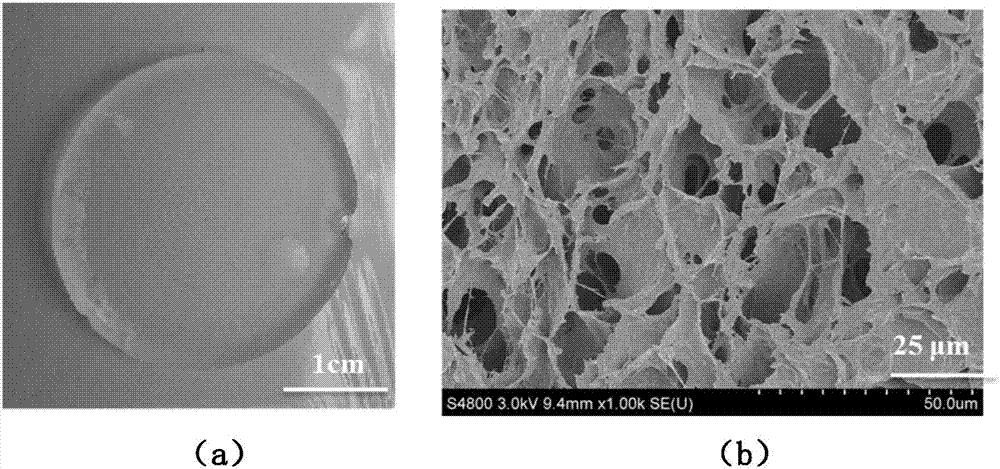
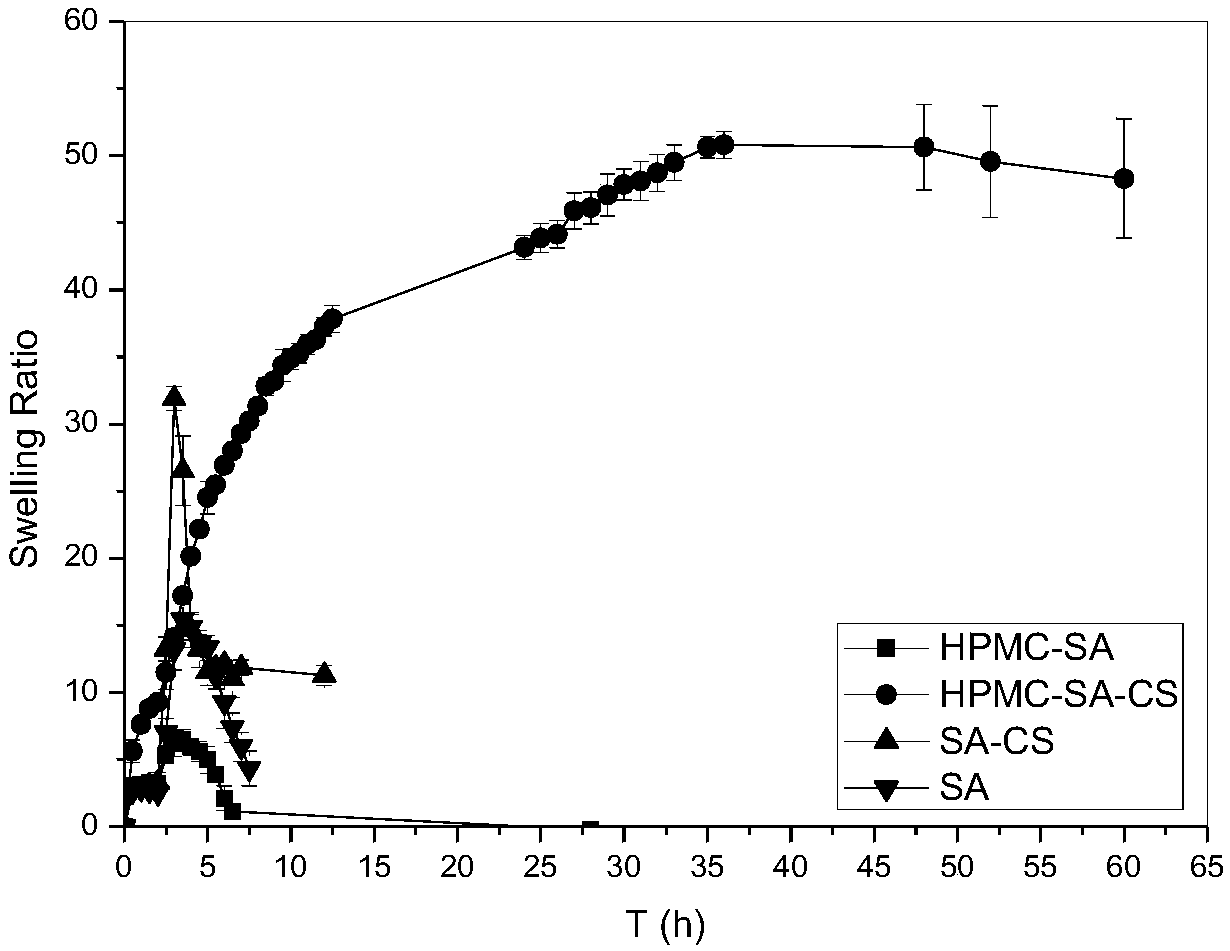
The invention belongs to the field of a medicine slow release carrier material and specifically discloses a preparing method for slow release hydrogel carrier material with a semi-interpenetrating structure modified by a polyelectrolyte membrane and an application thereof. The slow release hydrogel material is comprised of HPMC and SA as raw materials, firstly an HPMC-SA semi-interpenetrating composite gel is prepared by a Ca2+ physical crosslinking method and polyelectrolyte membrane modification is performed on the HPMC-SA hydrogel with low molecular weight CS so as to increase the stabilityof the HPMA-SA hydrogel, so that the HPMA-SA-CS hydrogel is obtained. The hydrogel has advantages of mild preparing condition, simple method, less reagent and no violent reactions. BSA and micromolecule indissolvable IDM are regarded as model medicine and the slow release effect of the HMPC-SA-CS composite hydrogel material on macro-molecule medicine and micromolecule medicine is investigated. The result shows that the gel system continuously swell for 50 hours, the swellability reaches above 52 times and the slow release effect of the protein medicine and IDM is increased.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Group of polypeptides having percutaneous and transmembrane penetration promoting effect, and applications thereof
ActiveCN103145799AEnhanced penetration enhancer activityImprove percutaneousAerosol deliveryOintment deliveryMacromolecular drugBiological macromolecule
Owner:DALIAN UNIV OF TECH
Application of N-arginine chitosan as percutaneous sorbefacient
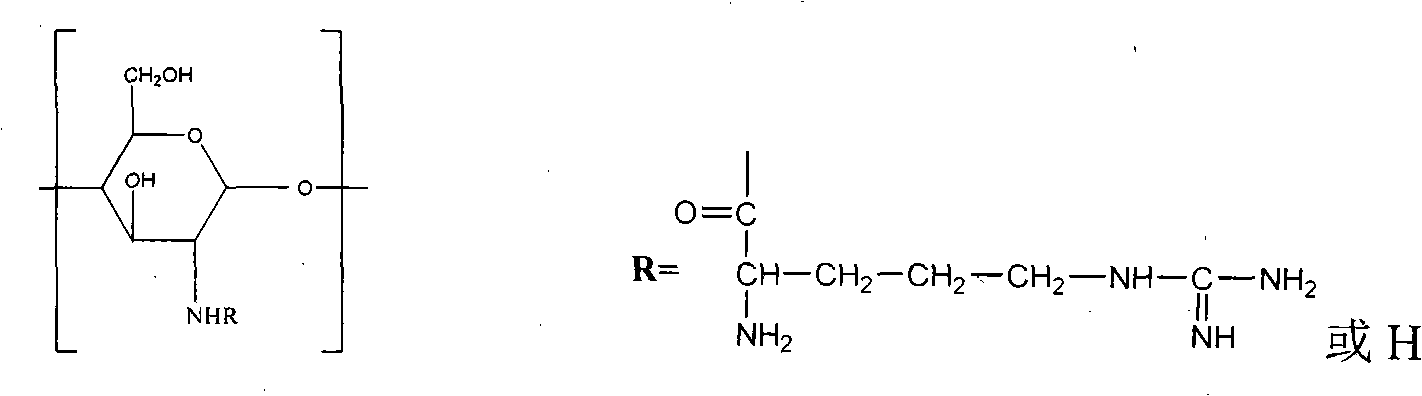
The invention belongs to biomedical field, particularly relates to an application of a chitosan derivative N-arginine chitosan as percutaneous sorbefacient. The invention has advantages of providing a chitosan derivative which has simple structure and biodegradable property and can well promote percutaneous absorption of macromoleculare medicines and medicines with poor permeability.
Owner:CHINA PHARM UNIV
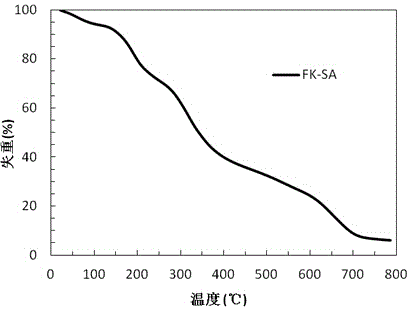
Microcapsule lyophilized powder and preparation method thereof
InactiveCN102552215AHigh encapsulation efficiencyAvoid heatPharmaceutical non-active ingredientsMicrocapsulesQuick FreezeFreeze-drying
The invention relates to microcapsules and a preparation method thereof. Each microcapsule has a structure of taking a polymeric hydrophilic colloid as a capsule core and a biodegradable polymer material as a capsule membrane; a water-soluble medicine is dispersed into the hydrophilic colloid capsule core; the microcapsules are prepared by the combination of multiple technologies such as a microemulsion technology, an ultra-low temperature quick-freezing technology and the like and a freeze-drying process; the particle sizes of the microcapsules can be controlled; the microcapsules are high in medicine loading capacity and medicine entrapment efficiency; and the microcapsules are uniform in particle size, microcapsule particles cannot be gathered, and the medicine release behavior is controllable. The microcapsules are applicable to the entrapment of water-soluble medicines, are particularly applicable to medicines with low thermal stability and macromolecular medicines such as protein, polypeptides, polysaccharides and the like, and can be applied to the injection and oral administration of multiple formulations for local or systemic treatment of mucous membranes, skins, wounds and orifices.
Owner:ZHEJIANG HISUN PHARMA CO LTD
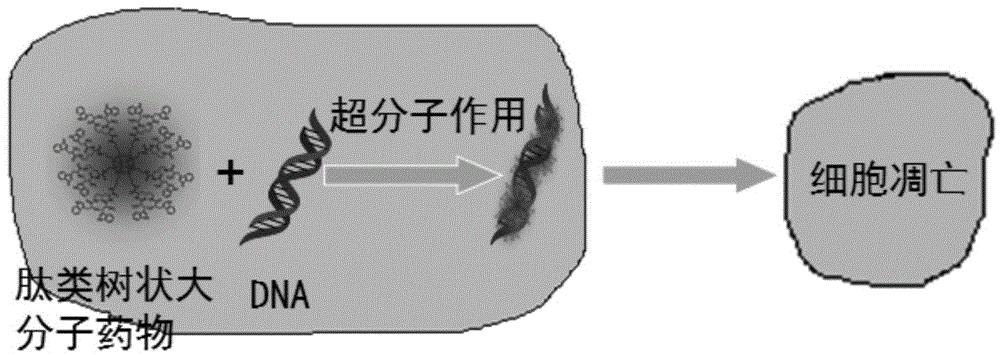
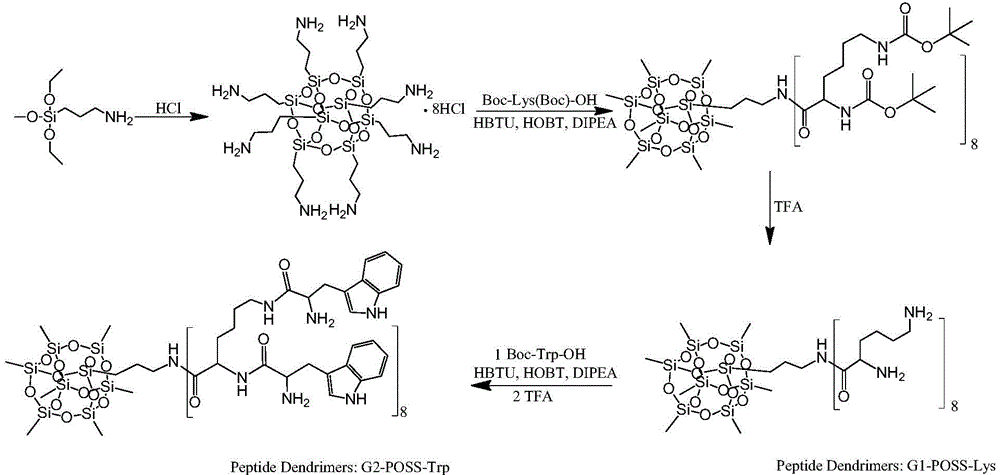
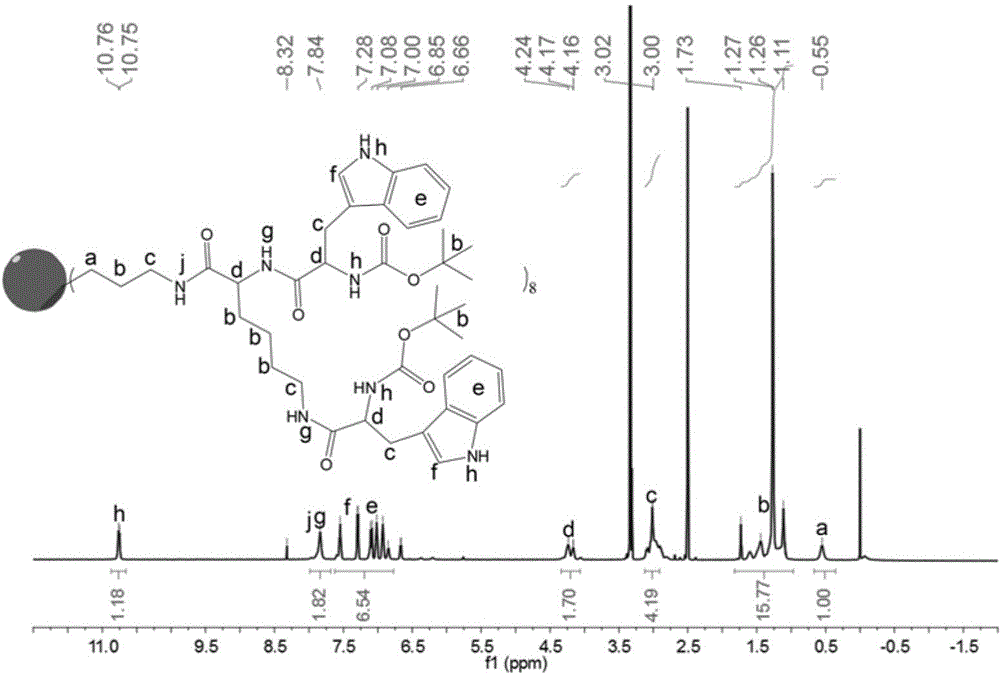
Peptide dentritic macromolecular drug and preparation method and application thereof
InactiveCN104650194AGood water solubilitySolve permeabilityPeptide/protein ingredientsDepsipeptidesSolubilityEnd-group
The invention discloses a peptide dentritic macromolecular drug and a preparation method and application thereof, and belongs to the field of biomedicine. The drug comprises a peptide dentritic macromolecular skeleton, and terminal-group functionalized groups. The biological activity of the drug is ensured through the terminal-group functionalized groups; and the periphery of the peptide dentritic macromolecular drug can be grafted with a lot of terminal-group functionalized groups, so that the biological effect of the terminal-group functionalized groups is effectively amplified by the amplification effect of dendritic macromolecules. The drug has an accurate molecular structure and abundant surface functional groups, is good in water solubility, and also has the biological characteristics of being similar to globulin, easy to be taken in by cells and the like; the biological effect of the peptide dentritic macromolecules is enriched; and the development of peptide drugs is promoted. The preparation method is simple in technology; and the structure of the peptide dentritic macromolecular drug is accurately controlled by controlling preparation of various raw materials.
Owner:SICHUAN UNIV
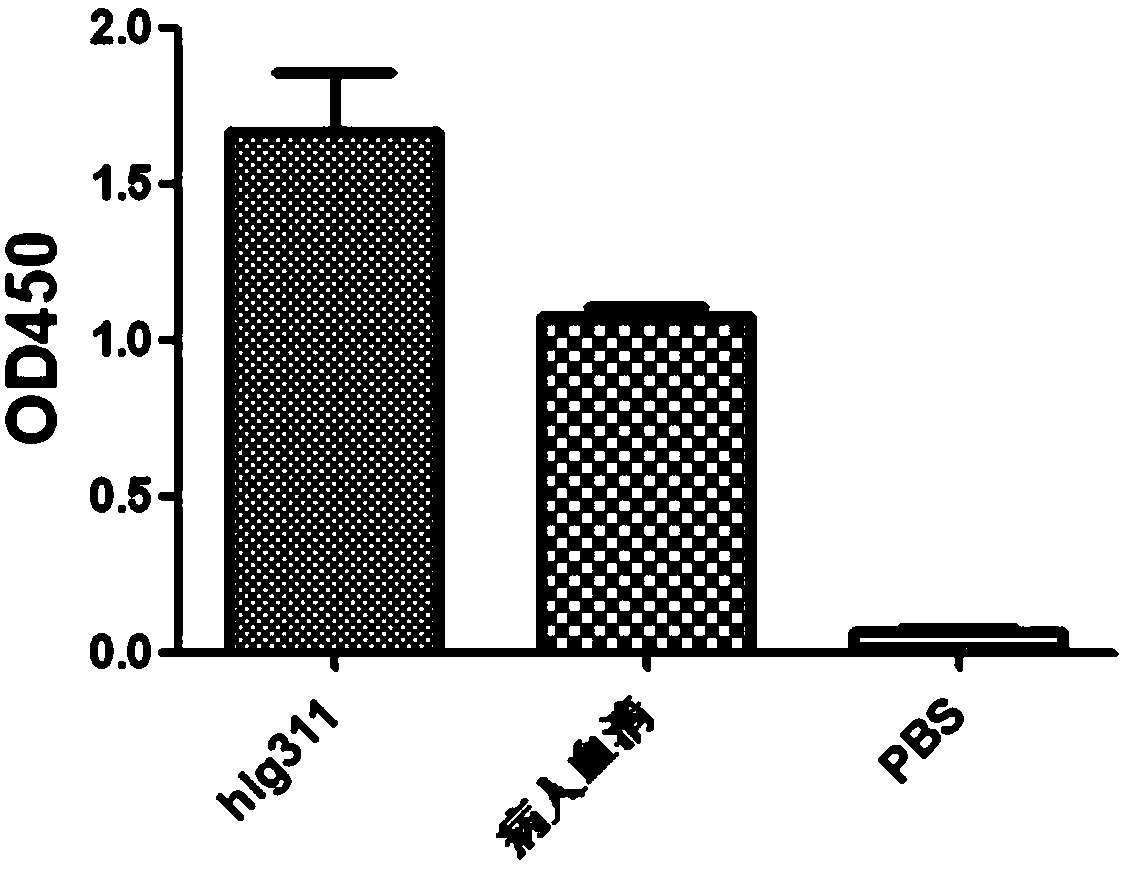
Anti-H7N9 full human derived monoclonal antibody hIg311 and preparation method and application thereof
ActiveCN110746503AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsHemagglutininAntiendomysial antibodies
The application relates to an anti-H7N9 full human derived monoclonal antibody hIg311 and a preparation method and application thereof. A memory B cell PCR method is used for quickly screening a fullhuman derived monoclonal antibody hIg311, and the anti-H7N9 human derived monoclonal antibody hIg311 is free from any mouse derived components. The antibody disclosed by the application can realize target combination of hemagglutinin HA of H7N9 viruses, and has notable anti H7N9 viral infection neutralization activity. The antibody disclosed by the application does not generate toxic and side effects for resisting mouse resisting antibodies, has good biocompatibility, and is suitable for becoming and has potential to become macromolecular drugs for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Multiple-response polymer microsphere drug carrier material and a preparation method thereof
InactiveCN104587478APotential application value is goodEasy to prepareEnergy modified materialsMacromolecular non-active ingredientsResponse effectMicrosphere
The invention discloses a preparation method of a polymer microsphere drug slow-release carrier with three responses to an oxidant, glucose and an electric field. The microsphere material consists of a polymer with beta-cyclodextrin, ferrocene-modified polyether and alpha-cyclodextrin. The preparation method of the polymer microsphere drug carrier comprises the following steps: firstly, synchronously dissolving the polymer with beta-cyclodextrin and the ferrocene-modified polyether in an aqueous solution, and stirring uniformly to ensure that the beta-cyclodextrin and ferrocene are fully compounded; and then adding alpha-cyclodextrin, and further stirring for a certain period of time to ensure that alpha-cyclodextrin and polyether are fully compounded. Along with the progress of compounding, a system gradually becomes cloudy, and polymer microspheres are formed. The polymer microspheres have good performance of wrapping macromolecular drugs and performance of effectively disintegrating the oxidant, glucose and electric field stimulation, so that gradual disintegration and slow release of a drug are realized. The polymer microsphere drug slow-release carrier disclosed by the invention is simple in preparation method and good in response effect, is environment-friendly and economical, and has a relatively great application value in the field of drug controllable slow-release.
Owner:QUZHOU UNIV
Iontophoretic transdermal drug delivery system
ActiveCN102430195AImprove compatibilityGood penetration enhancing effectElectrotherapyMedical devicesMacromolecular drugMiddle frequency
The invention discloses an iontophoretic transdermal drug delivery system which comprises a low and middle frequency pulse electronic generator, a conductor wire and a hydrosol pulse electrode; the low and middle frequency pulse electronic generator is connected with the hydrosol pulse electrode through the conductor wire; a drug carrier of the hydrosol pulse electrode of the system is prepared from a medical hydrosol and has the characteristics of good compatibility and permeability promoting property to drugs and good compatibility with human bodies; and the drug carrier can be used as a transdermal drug delivery carrier for macromolecular drugs, micromolecular drugs, hydrophilic drugs, lipophilic drugs and the like and can also be used as a transdermal drug delivery carrier of some biological drugs of polypeptide, protein and the like. Low and middle frequency pulse electrons generated by the system have a good permeability promoting action and a directional introduction function to the drugs.
Owner:太原市怀诚医疗器械有限公司
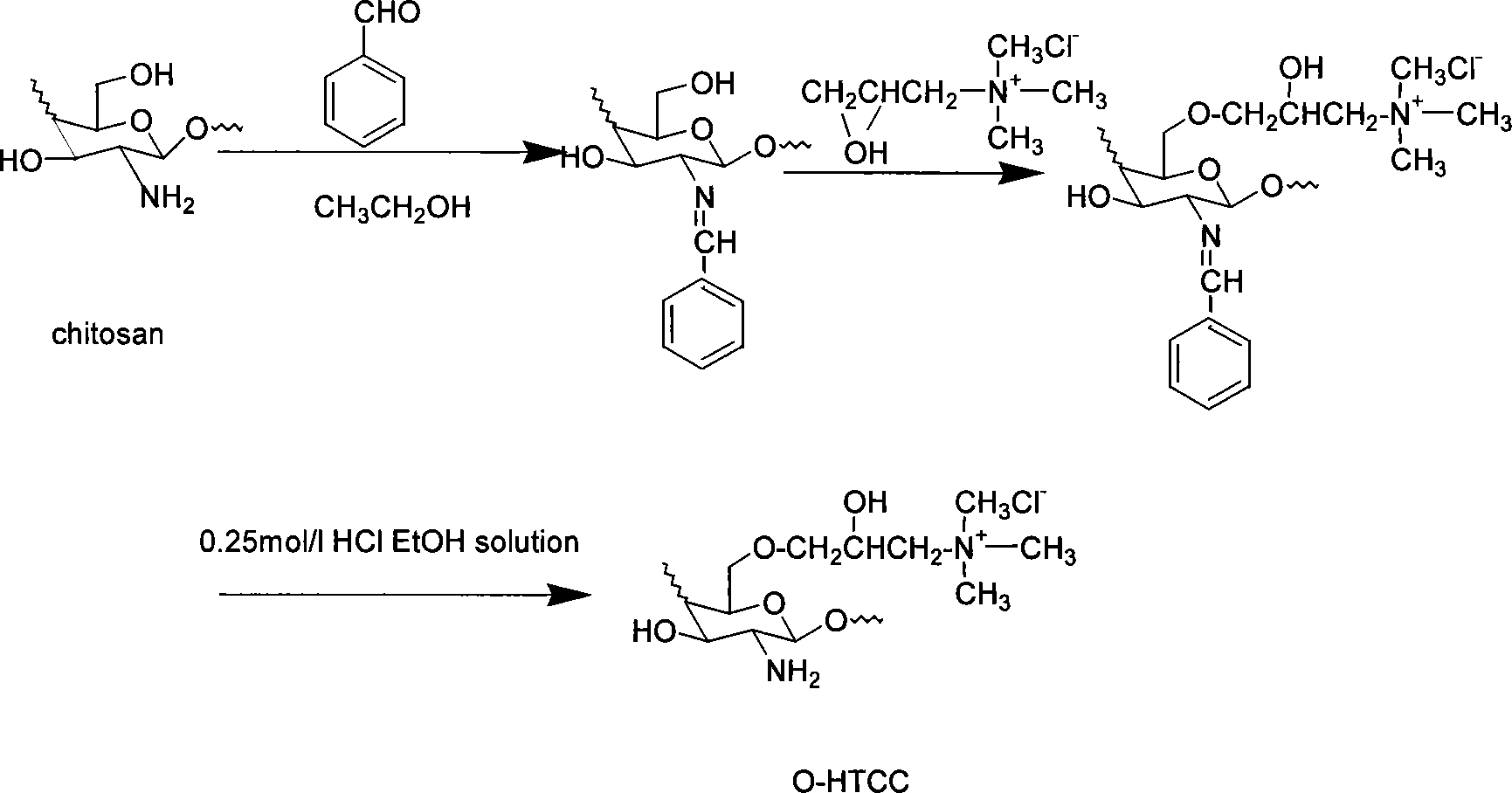
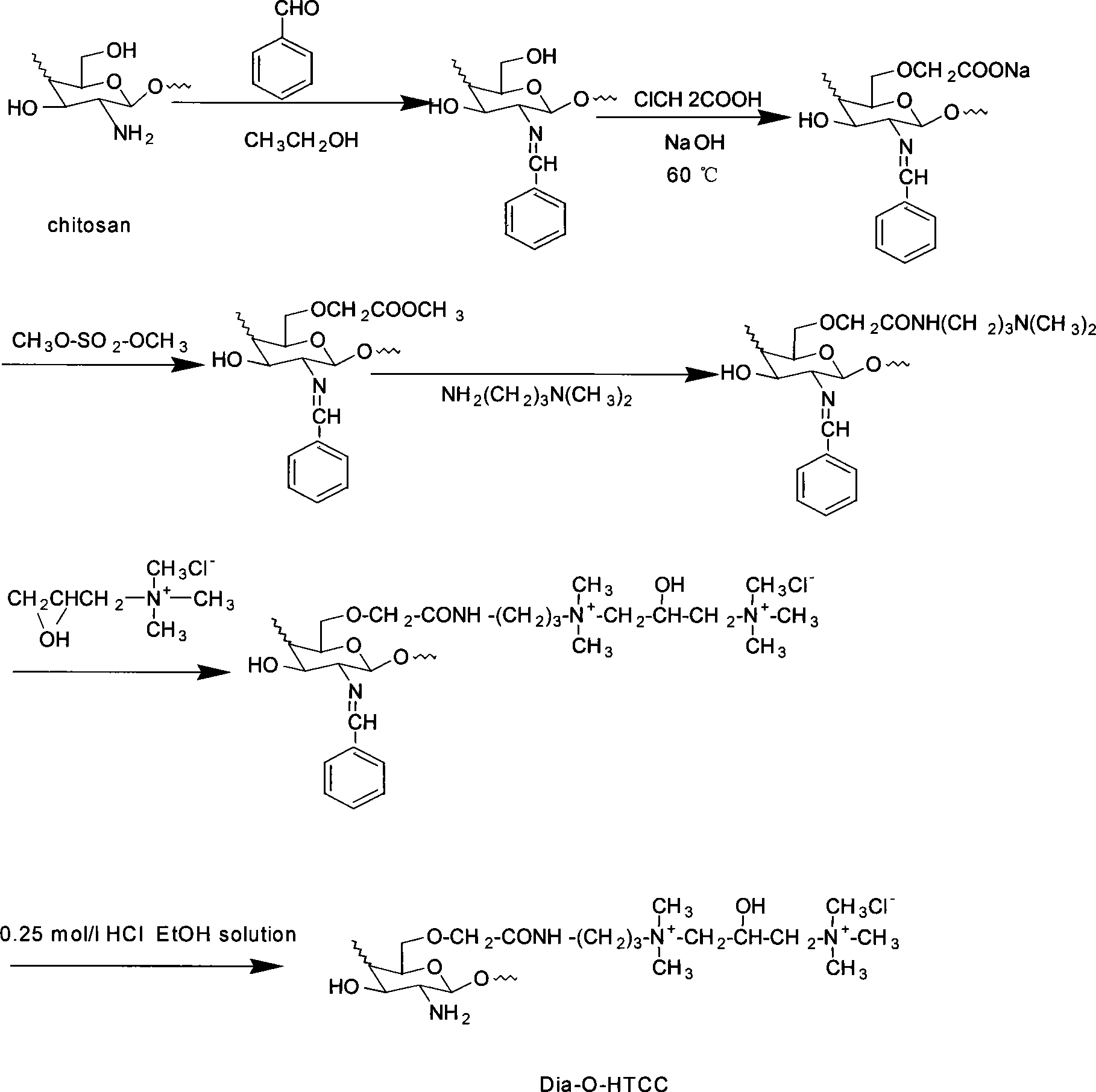
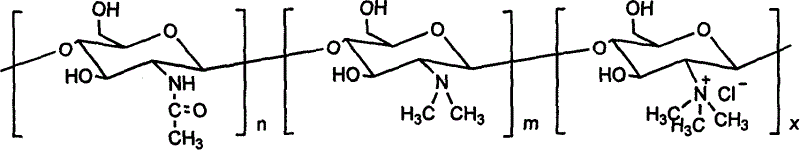
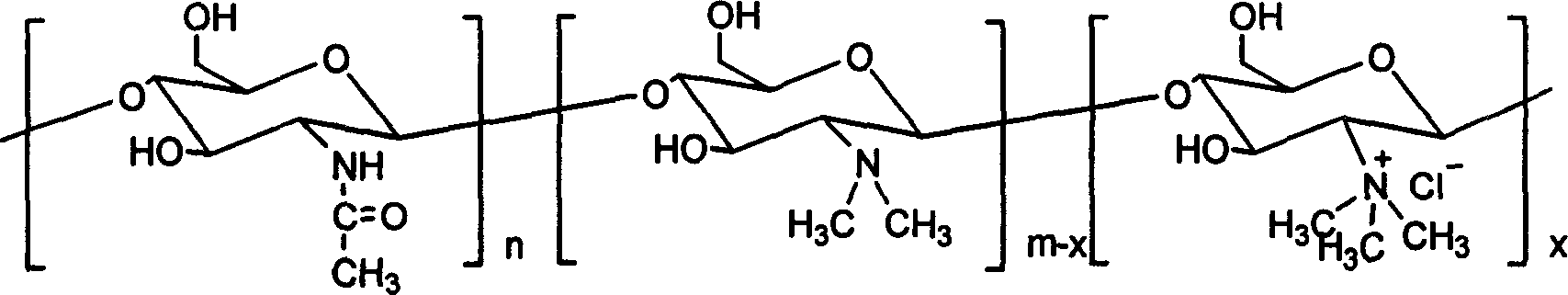
Chloro-N-trimethyl chitin hyamine
A chloride-n-trimethide chitose quaternary ammonium salt is characterized by the molecular formula is (C8H13NO5)n (C8H17NO4)m-x (C9H20NO4Cl)x, n=0.07-0.19, x=0.39-0.75, n+m+x=1. The production is carried out by expanding dissolving chitose into 1-methyl-2- pyrrolidone, adding sodium iodide, adding iodomethane, keeping temperature 50-65deg.C, agitating reacting, reacting liquid cooling, precipitating settling, washing deposit matter with acetone methyl - water solution, dissolving the deposit matter into water, dripping chlorhydric acid - alcohol solution and ion exchanging, adding acetone methyl into reacting liquid, washing the deposit matter, dissolving into water, adding into acetone methyl and settling, washing with acetone methyl - water solution, washing with acetone methyl, and drying. It achieves low cost, simple operation, and good water soluble performance.
Owner:OCEAN UNIV OF CHINA
Formula of oral mucosa absorbing dosage form of medicines of polypeptides and proteins, and preparation method and application thereof
InactiveCN101843595AAvoid lostIncrease lossPeptide/protein ingredientsPharmaceutical non-active ingredientsMacromolecular drugFreeze-drying
The invention relates to a medicament formula of macromolecular medicines of polypeptides and proteins and the like delivered by oral mucosa, and a preparation method and application thereof. The medicament comprises the following components: medicines of polypeptides and proteins, a penetration and absorbing enhancer, a bioadhesive agent, a protease inhibitor and an additive. The medicament is an oral adhesive two-layer piece with an adhesive layer and a waterproof layer, and the preparation technique is a freeze drying method. The medicament is suitable for various medicines of proteins and polypeptides. The invention aims at improving bioavailability of the medicines of polypeptides and proteins absorbed by the oral mucosa, enhancing the stability of medicine preparation of polypeptides and proteins and lays the foundation for developing a non-injection administration preparation of the medicines of polypeptides and proteins.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
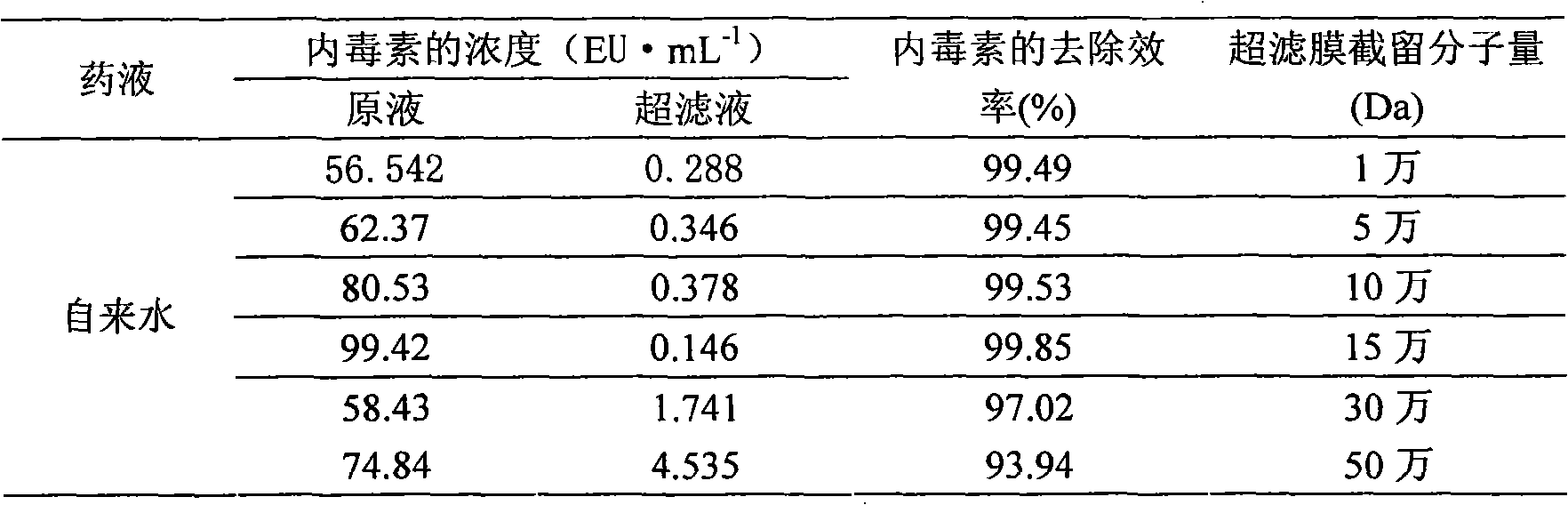
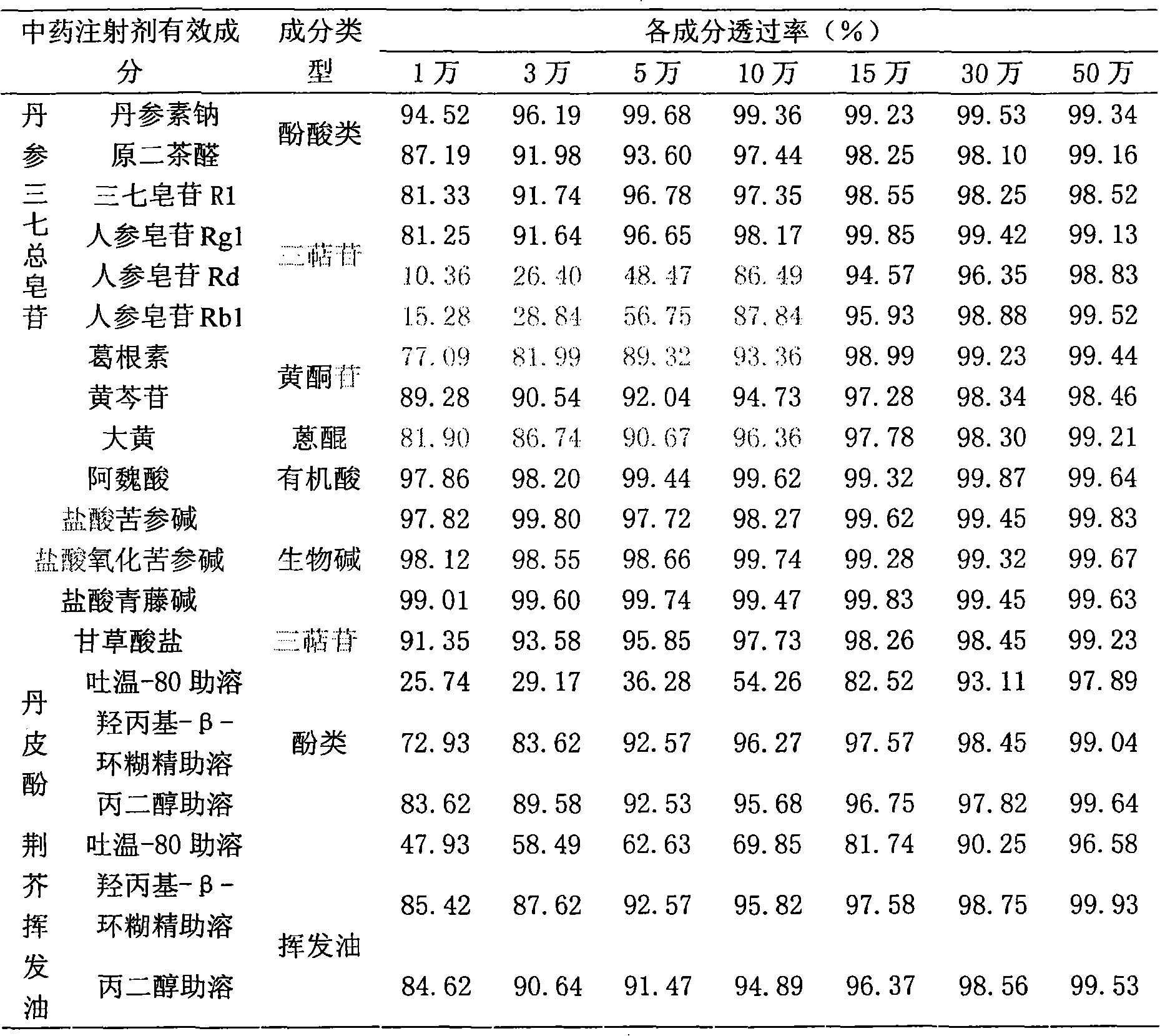
Method for removing pyrogen from injections
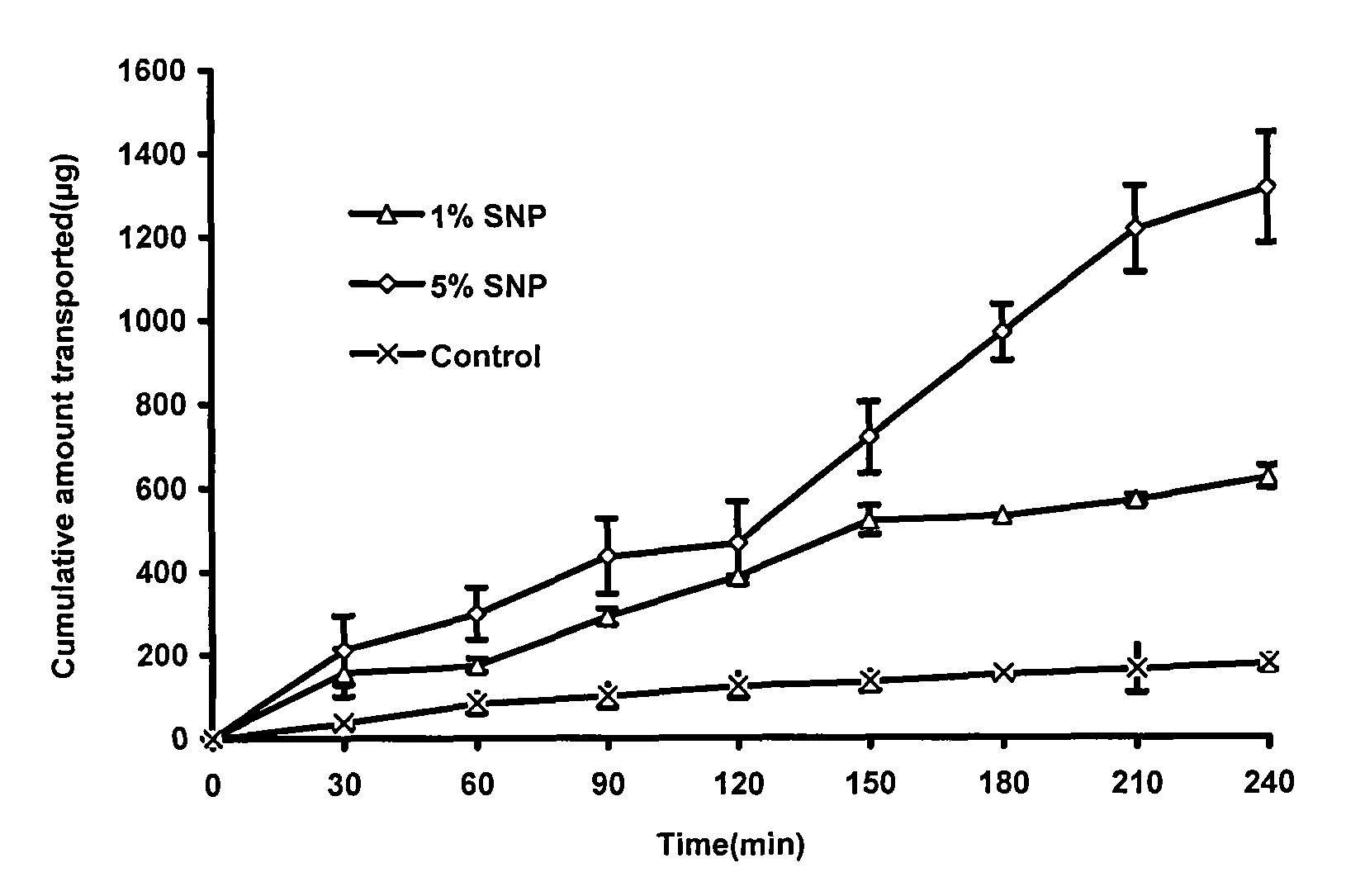
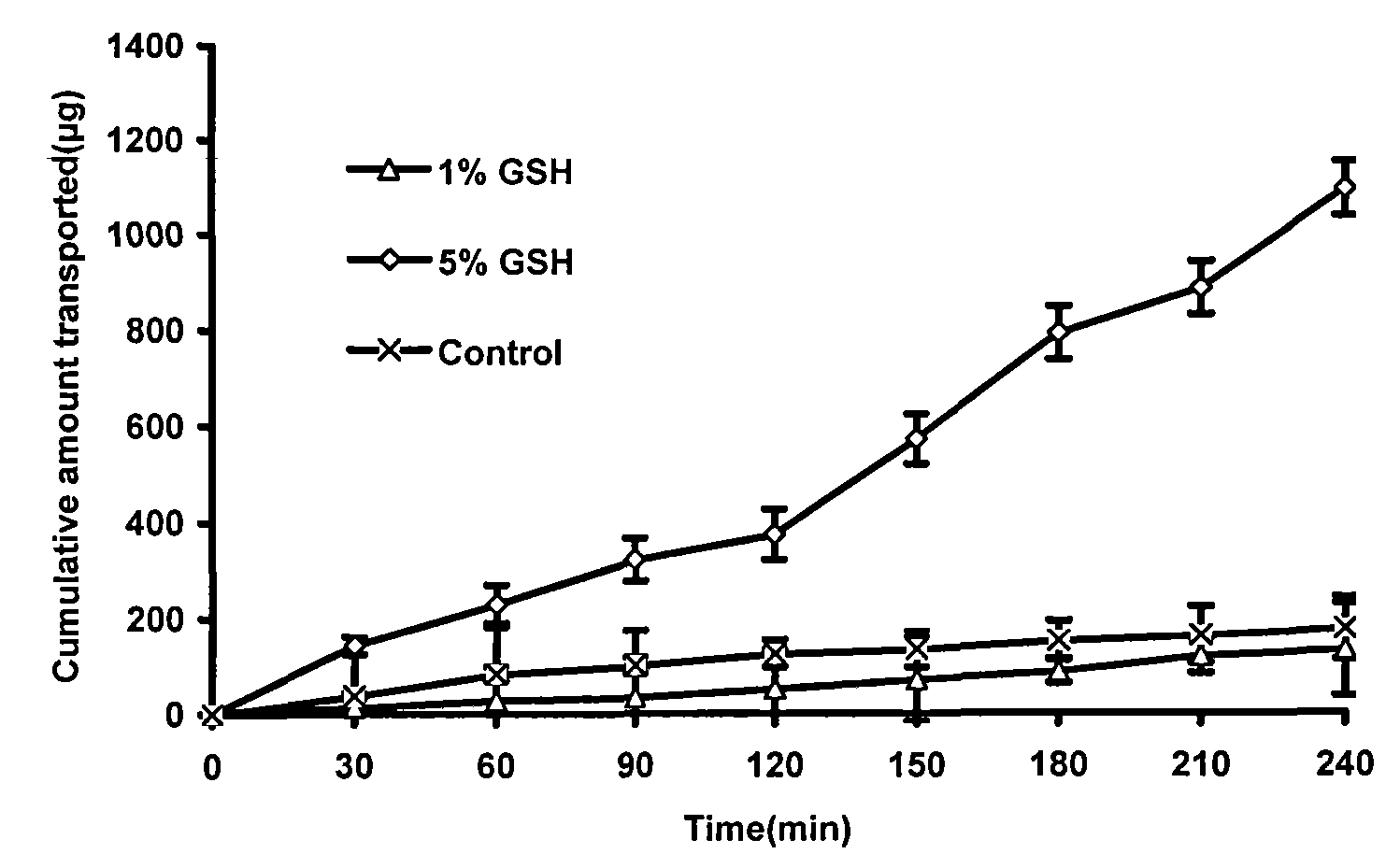
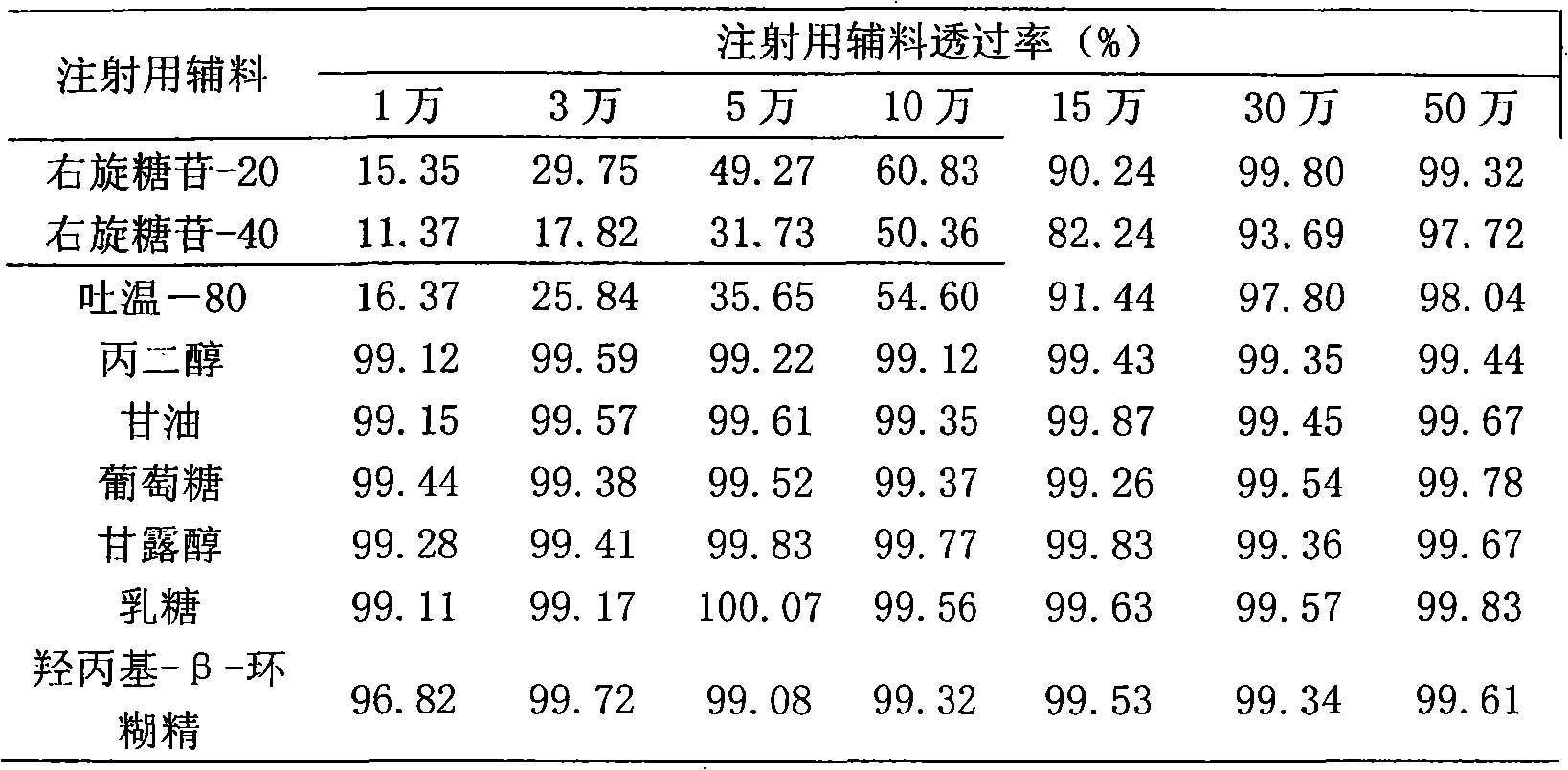
InactiveCN101683318AEfficient removalReservation of validityUltrafiltrationPharmaceutical delivery mechanismMacromolecular drugUltrafiltration
The invention relates to a method for removing pyrogen from injections, which adopts an ultrafiltration membrane filtering method. According to the ultrafiltration membrane filtering method, iquid medicine passes through an ultrafiltration membrane piece, the liquid passing through the ultrafiltration membrane piece is collected and prepared into the injections. The invention aims to overcome theprejudices of the prior theory and technology and design the membrane aperture of the ultrafiltration membrane piece to be 100000-500000 Daltons. The prior theory considers that the molecular weight of the pyrogen is below 100000 Daltons, and all technicians at home and abroad use the membrane with the aperture in the molecular weight range below 100000 Daltons for ultrafiltration, although the pyrogen can be effectively removed, for some specific injections, such as injections containing macromolecular medicines or auxiliary materials, or most of Chinese medicine injections containing complexcomponents, the effective medicine components can be lost in the ultrafiltration process. The ultrafiltration membrane with the membrane aperture of 100000-500000 Daltons not only can remove the pyrogen from the injections, but also can keep the effective components and auxiliary materials in the injections more effectively.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
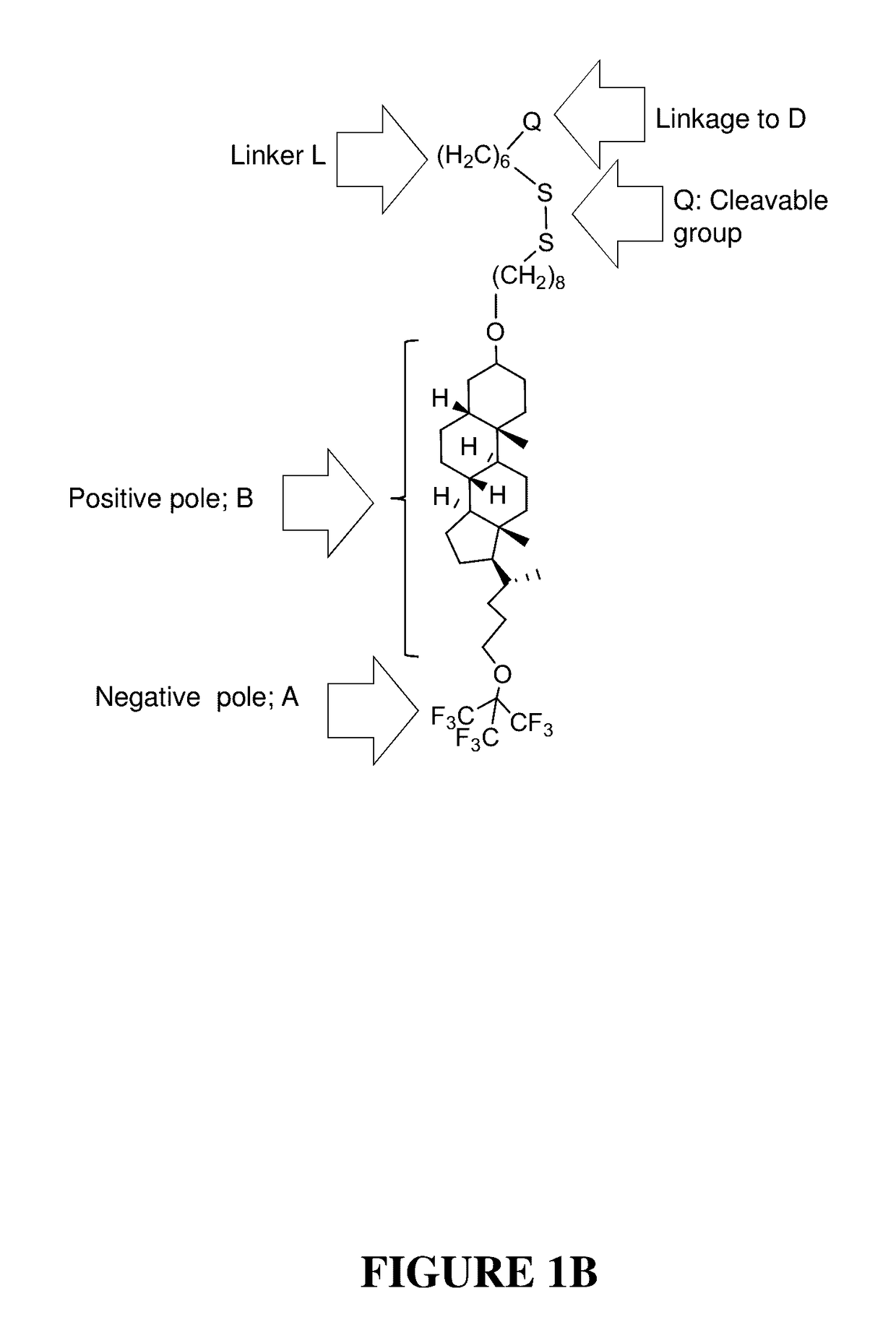
Compounds and methods for trans-membrane delivery of molecules
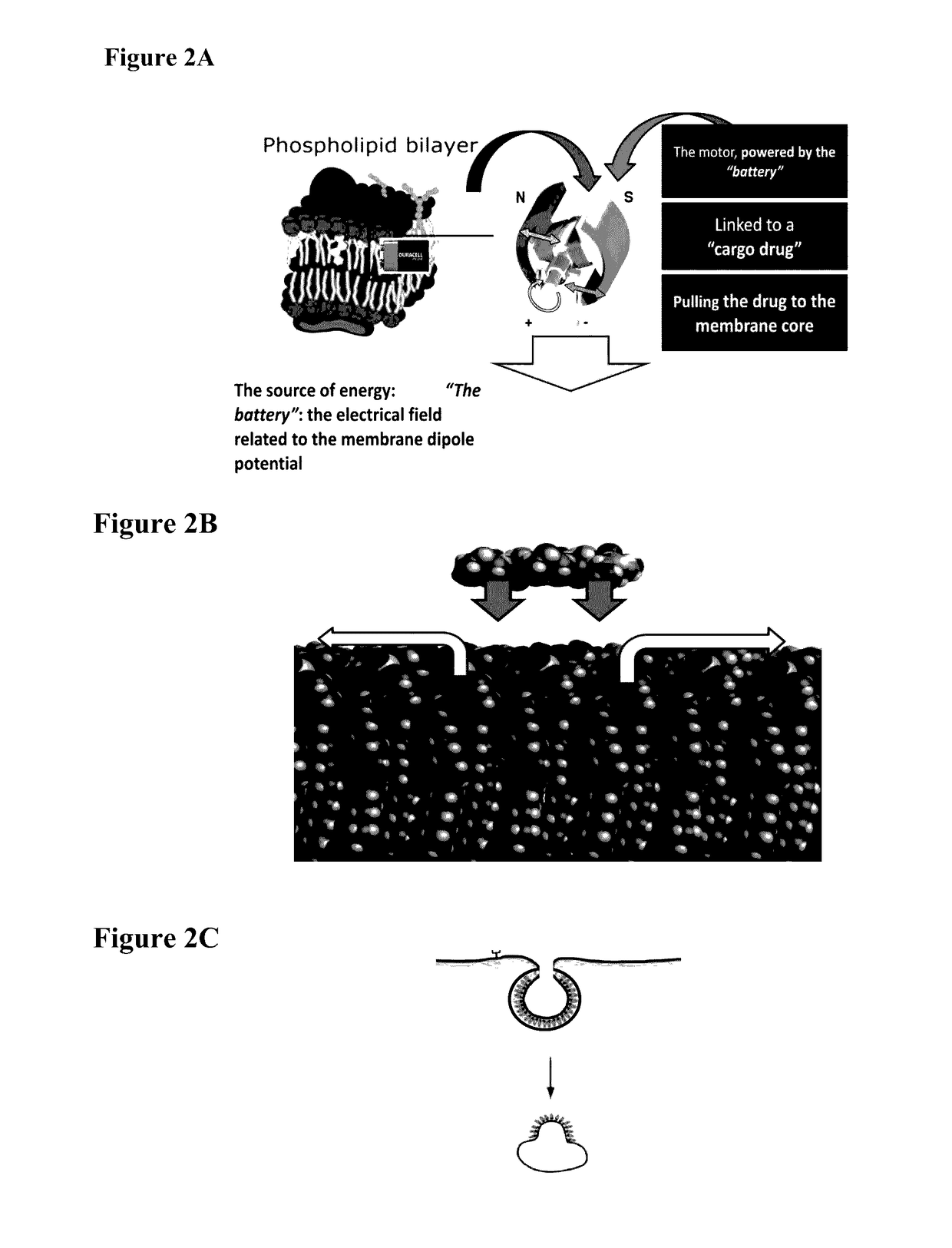
ActiveUS20170100486A1Organic active ingredientsPeptide/protein ingredientsGene deliveryMacromolecular drug
A novel delivery system for drugs, and especially macromolecules such as proteins or oligonucleotides through biological membranes is provided, and specifically delivery of siRNA. The delivery system comprises conjugation of the macromolecule drug to a moiety that enables effective passage through the membranes. Respectively, novel compounds and pharmaceutical compositions are provided, utilizing said delivery system. In one aspect of the invention, the compounds may be utilized in medical practice, for example, in delivery of siRNA or antisense oligonucleotides across biological membranes for the treatment of medical disorders.
Owner:APOSENSE
Drug-reservoir type scar-removing silicone gel paster and preparation method thereof
InactiveCN101756941AEasy to prepareEasy to operatePharmaceutical non-active ingredientsDermatological disorderSilicone membraneSilicone Gels
The invention relates to a drug-reservoir type scar-removing silicone gel paster and a preparation method thereof. The drug-reservoir type scar-removing silicone gel paster is characterized by comprising a silicone membrane and a silicone gel layer, wherein a drug reservoir is arranged in the silicone gel layer, and the inner part of the drug reservoir contains drugs and organic silicon polymer mixed liquid. The drug reservoir is directly arranged in the silicone gel layer, so the drug-reservoir type scar-removing silicone gel paster solves the problem that active ingredients in the drugs are isolated by the silicone membrane to cause macromolecular drugs to difficultly penetrate. The drugs in the drug-reservoir can better penetrate the silicone gel layer and play a role of treatment for scars, so that the paster shortens the treatment cycle and increases the treatment effect for the scars. In addition, the drug-reservoir type scar-removing silicone gel paster has simple preparation method, does not need complex molding equipment and is easy to produce and operate.
Owner:云南白药集团无锡药业有限公司
Biodegradable calcium sulfate/gelatin composite micro needle array patch and preparation method thereof
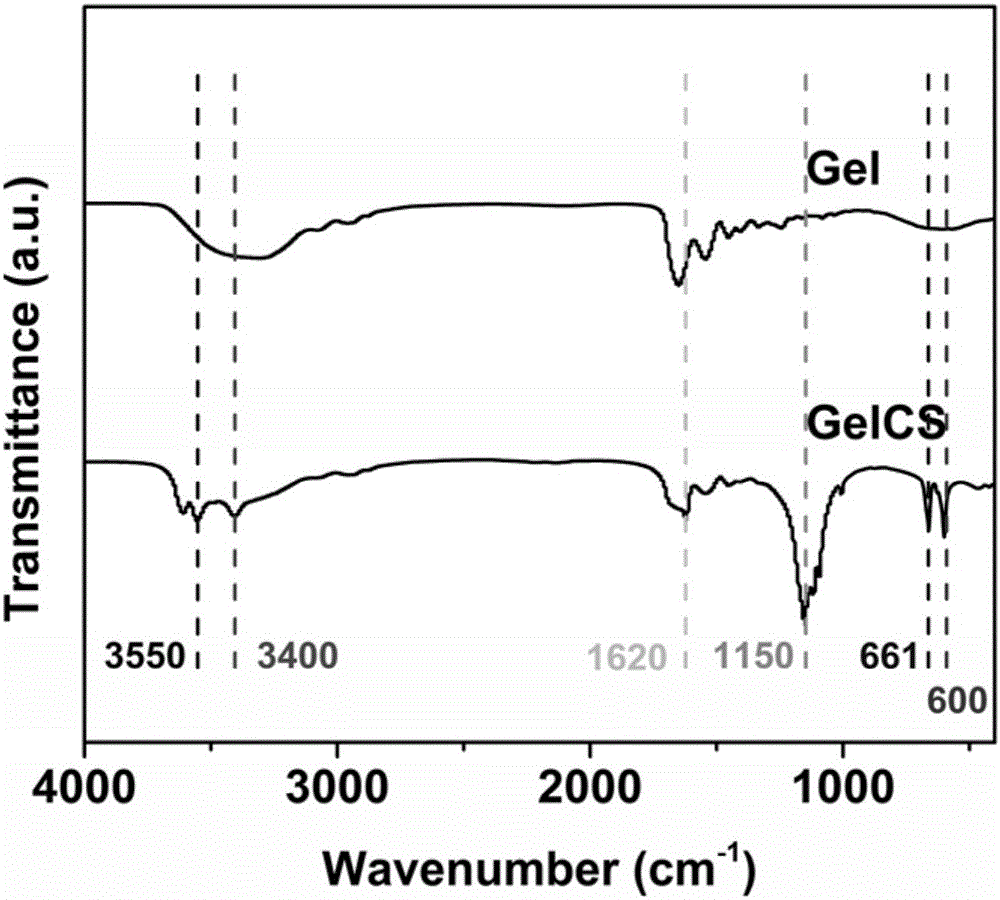
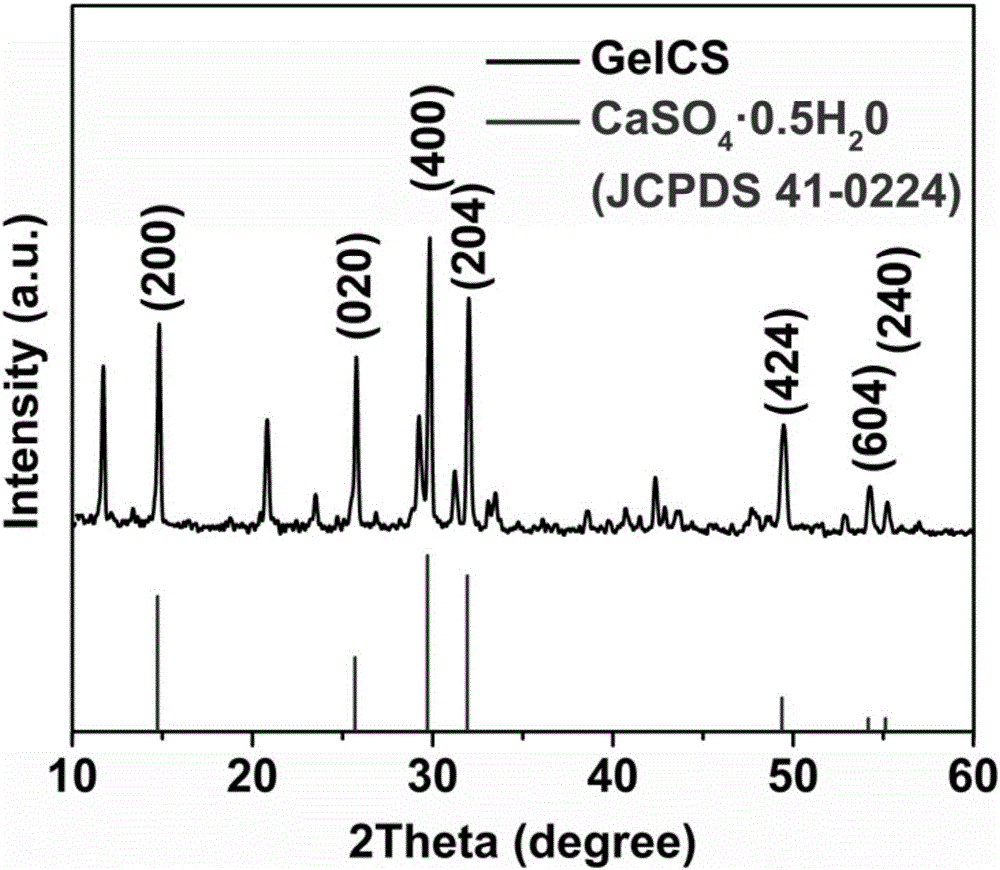
ActiveCN105879213AStrong enoughSolve the lack of hardnessMicroneedlesMedical devicesCross-linkHigh volume manufacturing
The invention belongs to the technical field of micro needle transdermal delivery equipment, and particularly relates to a biodegradable calcium sulfate / gelatin composite micro needle array patch and a preparation method thereof. The composite micro needle array patch comprises a cross-linked gelatin flexible patch and biodegradable calcium sulfate / gelatin composite micro needles; the cross-linked gelatin flexible patch is prepared from gelatin and a cross-linking stabilizer; the biodegradable calcium sulfate / gelatin composite micro needles are prepared from calcium sulfate, the gelatin and the cross-linking stabilizer; the biodegradable calcium sulfate / gelatin composite micro needles are arranged on the cross-linked gelatin flexible patch in an array mode; solid protein macromolecular drugs are embedded in the biodegradable calcium sulfate / gelatin composite micro needles. The biodegradable calcium sulfate / gelatin composite micro needle array patch disclosed by the invention has good biocompatibility, is safe and pollution-free, is simple in preparation process, is rapid to form, is suitable for mass production, and can be widely applied to the field of transdermal delivery of the protein macromolecular drugs.
Owner:ZHEJIANG SCI-TECH UNIV
Synthesis and preparation method of disulfiram entrapped polylactic acid microspheres having pH responsiveness and application thereof
InactiveCN108743559ARealize the effect of property applicationImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsMacromolecular drugMicrosphere
Provided are a synthesis and preparation method of disulfiram entrapped polylactic acid microspheres having pH responsiveness and application thereof. A pH sensitive polylactic acid material containing acetal bonds and disulfiram are prepared as model drugs by preparing lactide, polylactic acid macromolecules containing acetal bonds serve as a carrier material, the polylactic acid microspheres having pH responsiveness can be obtained by preparing polylactic acid drug carrying microspheres through a W1 / O / W2 double-emulsification solvent evaporation method, the synthesized and prepared disulfiram entrapped polylactic acid microspheres having pH responsiveness are applied to inhibition of tumor activity, biodegradable macromolecular drug carrying microspheres can be prepared by injecting thepolylactic acid material containing acetal bonds and coat disulfiram and Cu<2+>, the specificity is improved, and the success rate of tumor treatment is greatly improved.
Owner:常荷玥
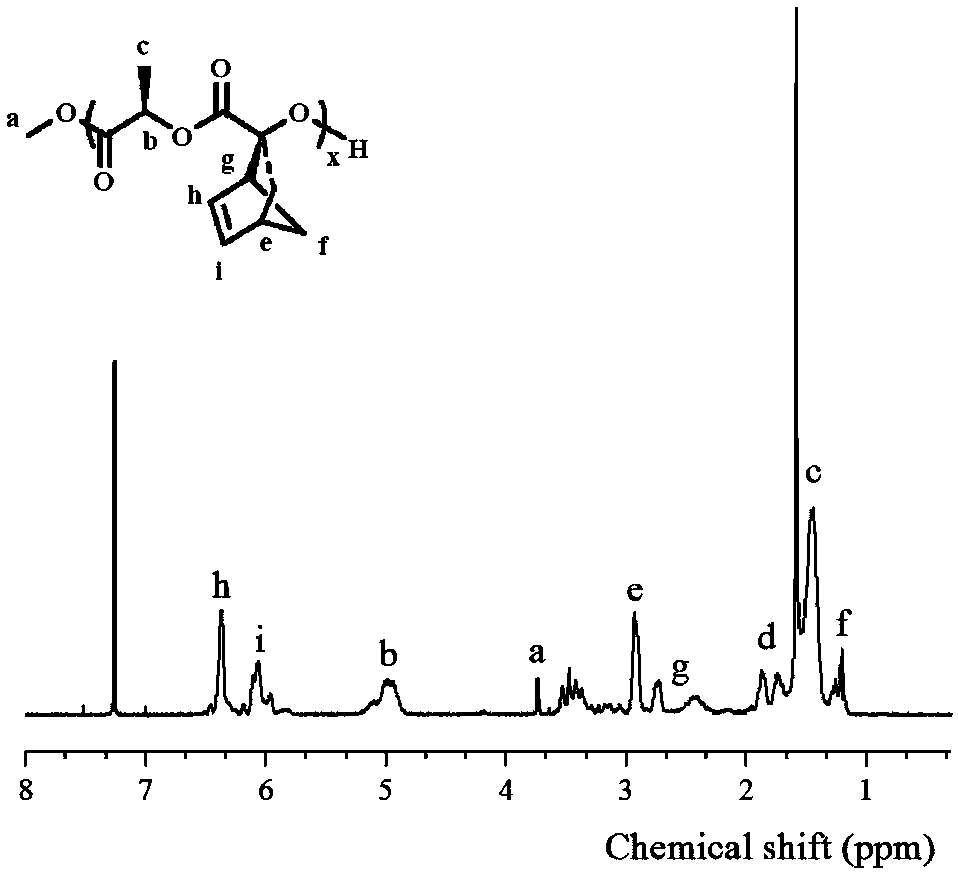
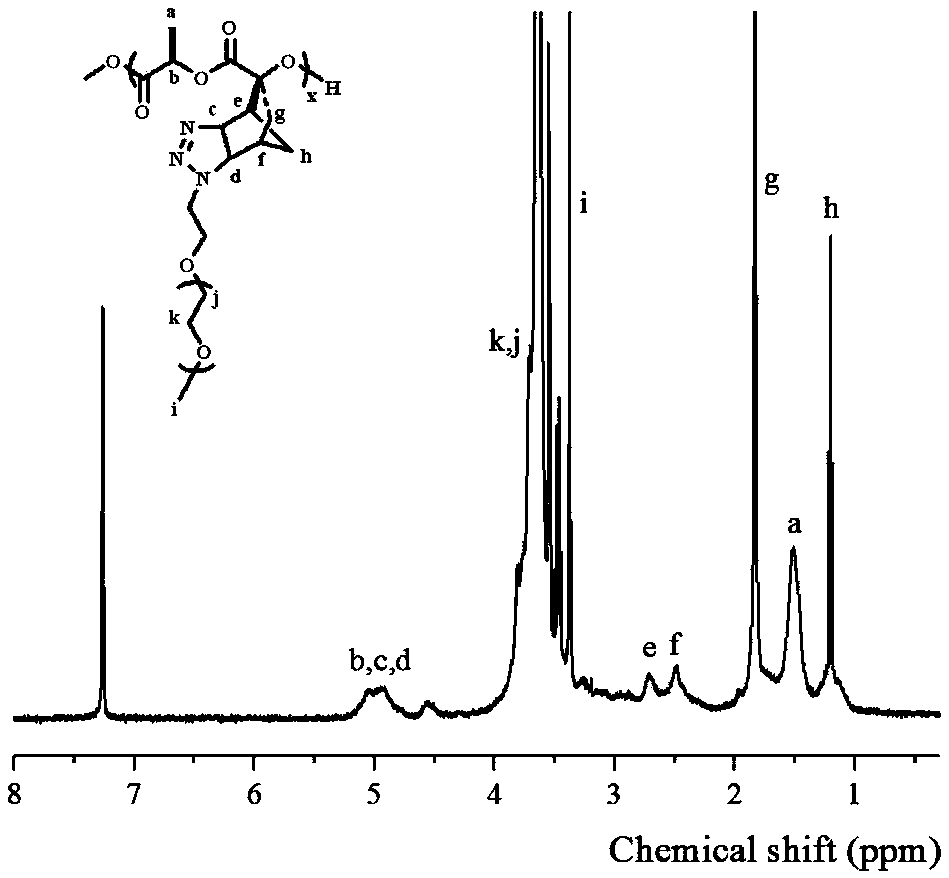
Fully-degradable and anti-tumor macromolecular drug with multi-drug synergistic effect and preparation method of fully-degradable and anti-tumor macromolecular drug
InactiveCN107670049AReduce usageUsage did not decreaseOrganic active ingredientsPharmaceutical non-active ingredientsSide effectThio-
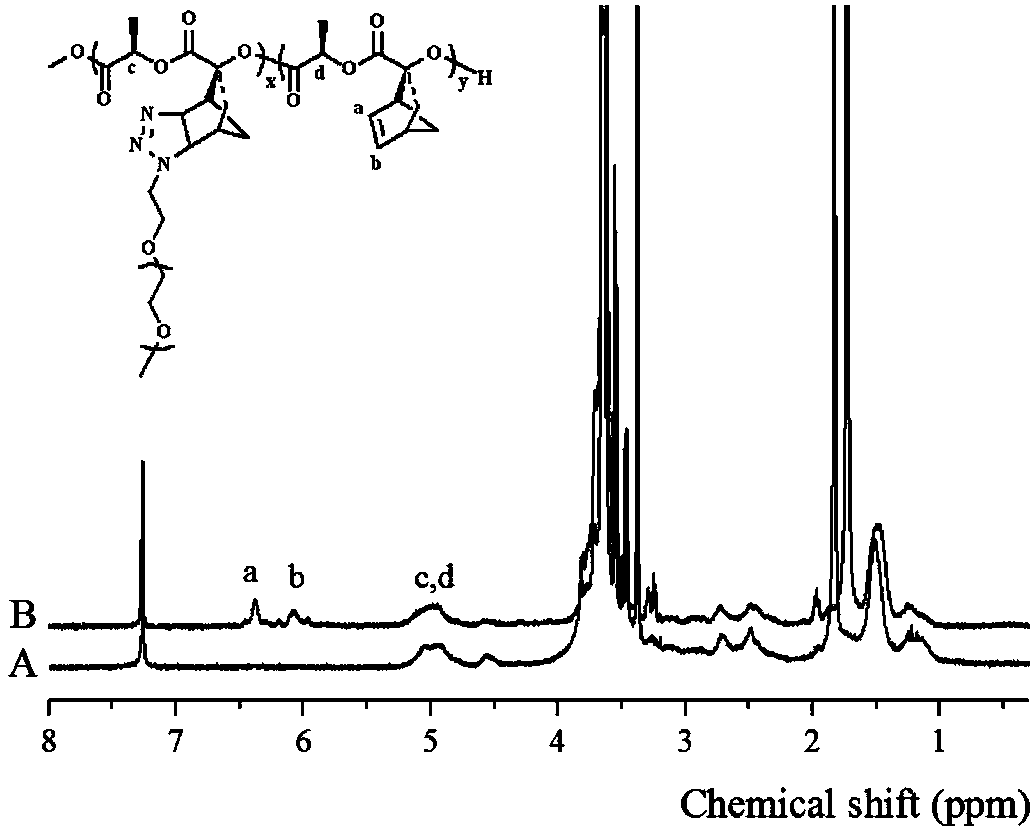
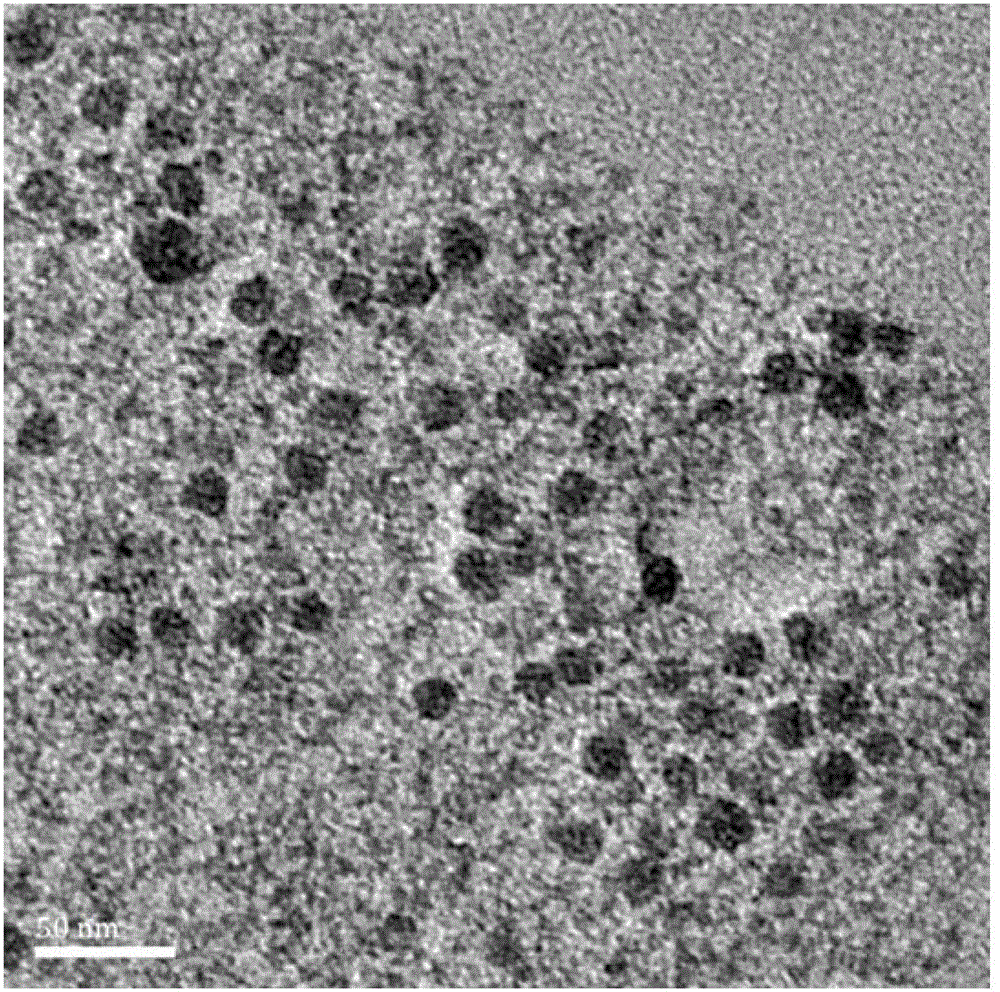
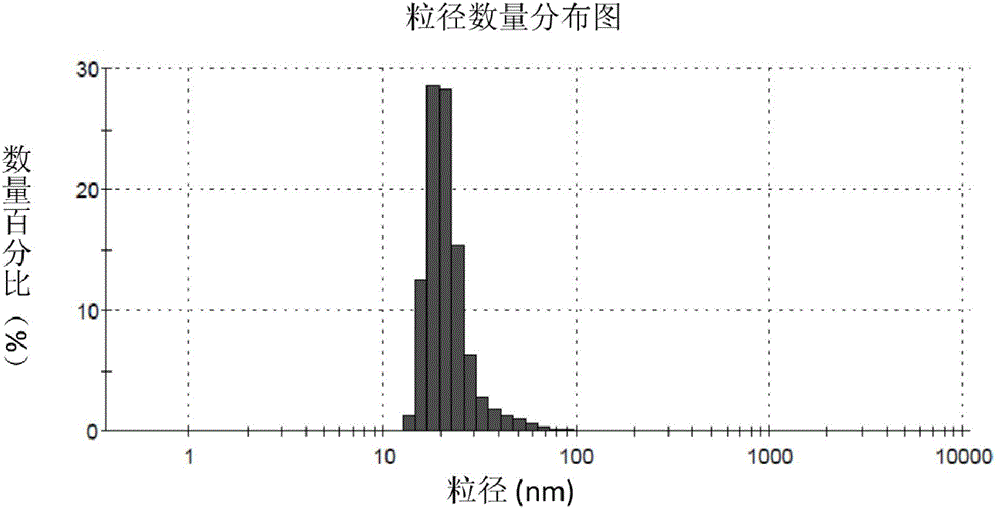
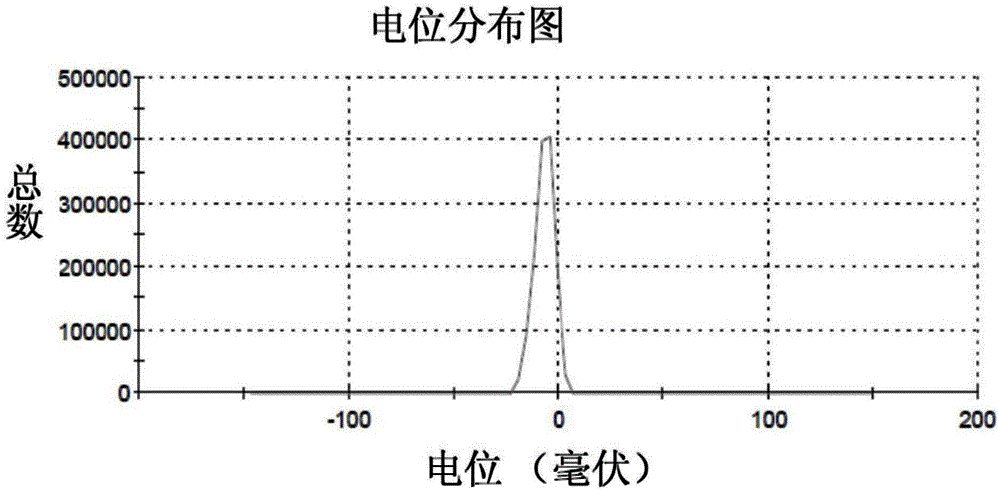
The invention discloses a fully-degradable and anti-tumor macromolecular drug with a multi-drug synergistic effect and a preparation method of the fully-degradable and anti-tumor macromolecular drug.The anti-tumor macromolecular bonded drug takes polylactide as a main chain, a hydrophilic branch chain of polyethylene glycol is introduced, and various anti-tumor drugs are grafted. The preparationmethod of the anti-tumor drug comprises the steps as follows: firstly, polylactide containing norbornene side groups is prepared, methoxypolyethylene glycol branch chains are introduced through an azide-ene click reaction sequentially, azide side groups are modified by a photo-click reaction and an esterification reaction of thio-ene, and finally, anti-tumor drug molecules are introduced by a force actuated azide-alkyne cycloaddition reaction. According to the method, the anti-tumor macromolecule bonded drug is synthesized with various click reactions, and the method has the characteristics ofsimplicity, high efficiency, mild reaction conditions, large drug loading amount, high purity and the like; the anti-tumor macromolecular bonded drug has the advantages that the anti-cancer activityis improved through the synergistic action of multiple drugs, and the dosage of each drug can be reduced due to the combined action of the multiple drugs, and accordingly, toxic and side effects of chemotherapy are reduced.
Owner:XIANGTAN UNIV
Nano particles modified by alendronic acid functionalized polyethylene glycol and preparation method of nano particles
ActiveCN104147614AExtension of timeLow costGenetic material ingredientsPharmaceutical non-active ingredientsCalcium biphosphateMacromolecular drug
The invention provides nano-particles modified by alendronic acid functionalized polyethylene glycol. The nano particles are calcium phosphate particles or calcium carbonate particles wrapped with target delivery matters; the target delivery matters are selected from DNA, siRNA and protein acromolecular drugs or micro-molecular chemical drugs; alendronic acid functionalized polyethylene glycol covers the surfaces of the nano particles; and the particle size of the nano particles modified by alendronic acid functionalized polyethylene glycol is 20-50 nm. The nano particles modified by alendronic acid functionalized polyethylene glycol are small in particle size; polyalendronic acid-polyethylene glycol can stably cover the surfaces of phosphate-genetic particles, and therefore, the nano particles can be effectively stabilized for a long time; the body circulation time is prolonged; the nano particles disclosed by the invention are low in cost and toxicity, safe and effective. The invention further provides a preparation method of the nano particles modified by the alendronic acid functionalized polyethylene glycol.
Owner:SHENZHEN INST OF ADVANCED TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com