Application of N-arginine chitosan as percutaneous sorbefacient
A technology of absorption enhancer, arginine, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
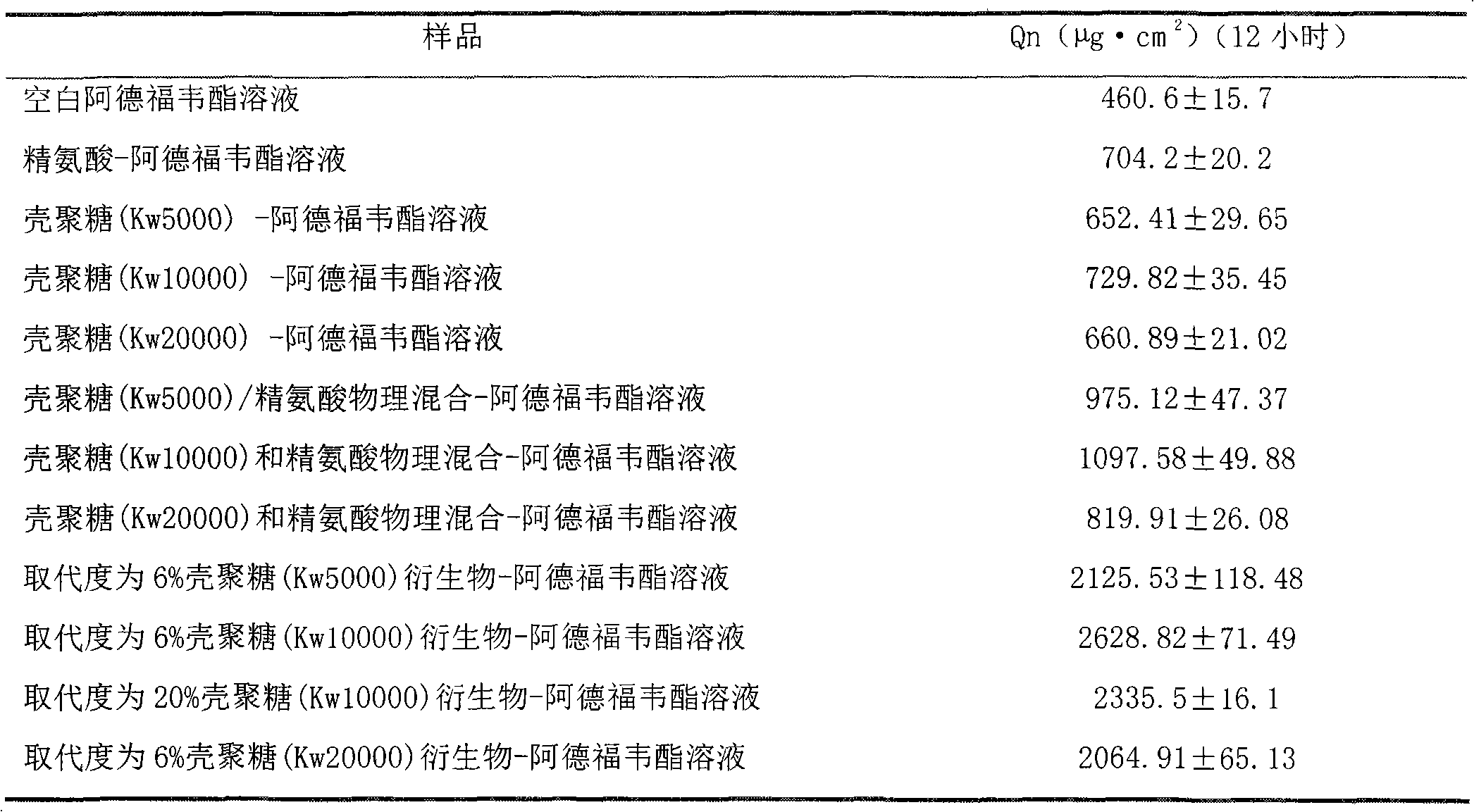
[0018] Implementation example 1: Experimental research on the promotion of adefovir transdermal absorption by N-arginine chitosan
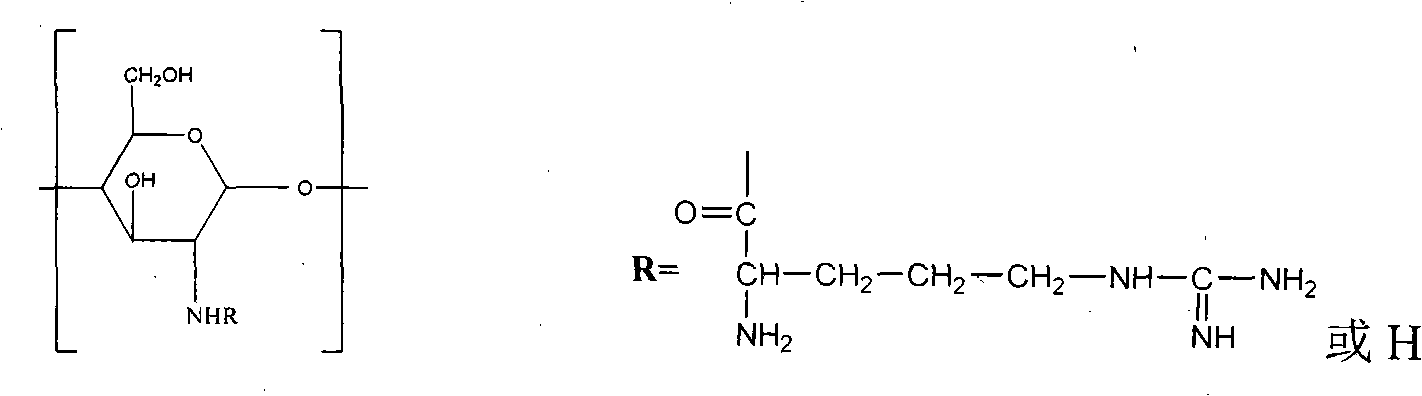
[0019] 1. Synthesis of N-arginine chitosan
[0020] (1) BOC protection of arginine side chain amino group: Weigh 5 grams of arginine and dissolve it in 200ml deionized water, and then weigh 4 grams of Boc anhydride and dissolve it in 200ml tetrahydrofuran, and add arginine to the tetrahydrofuran solution dissolved in Boc anhydride In the aqueous acid solution, stir evenly, add dropwise 1mol / L NaOH solution to adjust the pH value to 9-10, stir and react at room temperature for 24 hours, put the reaction solution on a rotary evaporator to remove the tetrahydrofuran-water mixture, and obtain N-tert-butyl Oxycarbonyl arginine, take 200ml deionized water and dissolve it.
[0021] (2) Synthesis of N-arginine chitosan: take by weighing 10 grams of chitosan with a deacetylation degree of 90% and a molecular weight of 10,000, add it to 1% glacial acetic a...
Embodiment 2
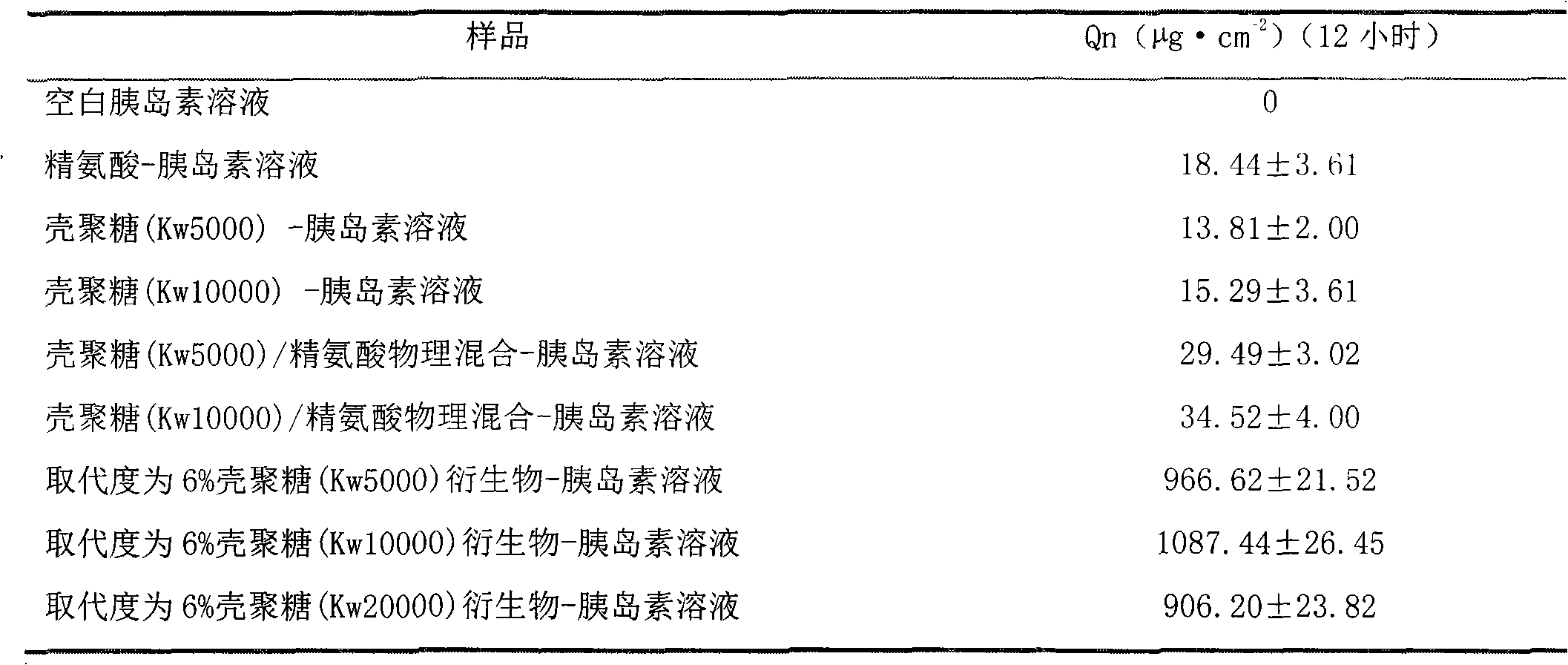
[0032] Implementation example 2 insulin
[0033] 1, the synthesis of N-arginine chitosan: with embodiment 1.
[0034] 2. Transdermal experiment
[0035] Preparation of sample solution: Weigh 30mg of insulin and dissolve it in 18ml (0.1mol / L) HCl solution, divide the drug solution into 6 parts on average, adjust the pH value of the solution to 7 with 1mol / L NaOH, add distilled water to the full amount Take arginine, polysaccharide, derivatives of arginine and chitosan, and 60 mg of N-arginine chitosan after refining and purification, and dissolve them in the above solution to obtain arginine, poly Derivatives of sugar, arginine and chitosan and insulin solution at 2% concentration of N-arginine chitosan.
[0036] Transdermal test: take the above-prepared solution for transdermal test, and the test method is the same as in Example 1.
[0037] 3. Experimental results and data
[0038] Using different molecular weight chitosan, mixture of chitosan and arginine, and N-arginine ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of deacetylation | aaaaa | aaaaa |
| degree of deacetylation | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com