Method for removing pyrogen from injections
An injection and filtration technology, applied in the field of medicine, can solve the problems of backward production technology, affecting the penetration of excipient ingredients and drug ingredients, and affecting industrial development, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
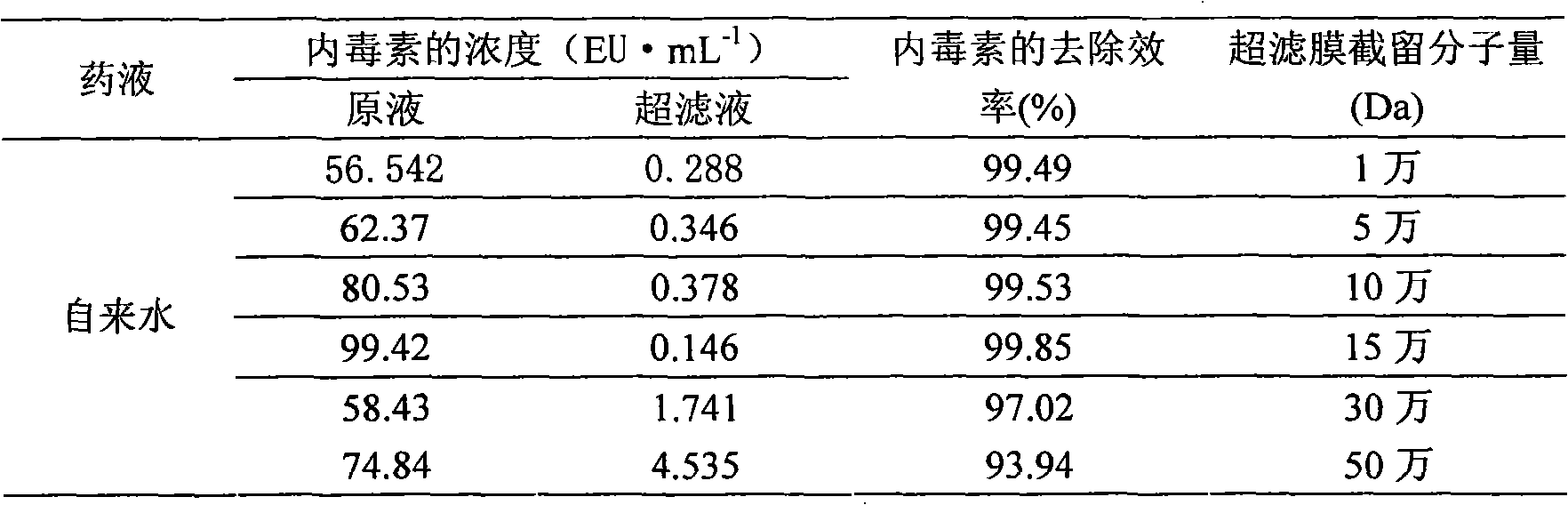
[0011] Example 1: Different molecular weight cut-off ultrafiltration membranes remove pyrogen test in tap water
[0012] Take 10L of tap water respectively, add a certain amount of Escherichia coli (containing endotoxin) culture solution to make it contain a certain amount of bacterial endotoxin, use 1-500,000 Dalton ultrafiltration membrane to ultrafilter, sample the original solution and ultrafiltration For the filtrate, the content of pyrogens before and after ultrafiltration of the liquid medicine was detected by dynamic turbidity method. The results are shown in Table 1.
[0013] Table 1 The effect of ultrafiltration membranes with a membrane pore size of 150,000-200,000 Daltons in removing pyrogens from tap water
[0014]
[0015] The results show that the ultrafiltration membrane with a membrane pore size of 10,000 to 500,000 Daltons has a high removal efficiency for endotoxin as an indicator of pyrogens. It is speculated that although the molecular weight of pyroge...
Embodiment 2
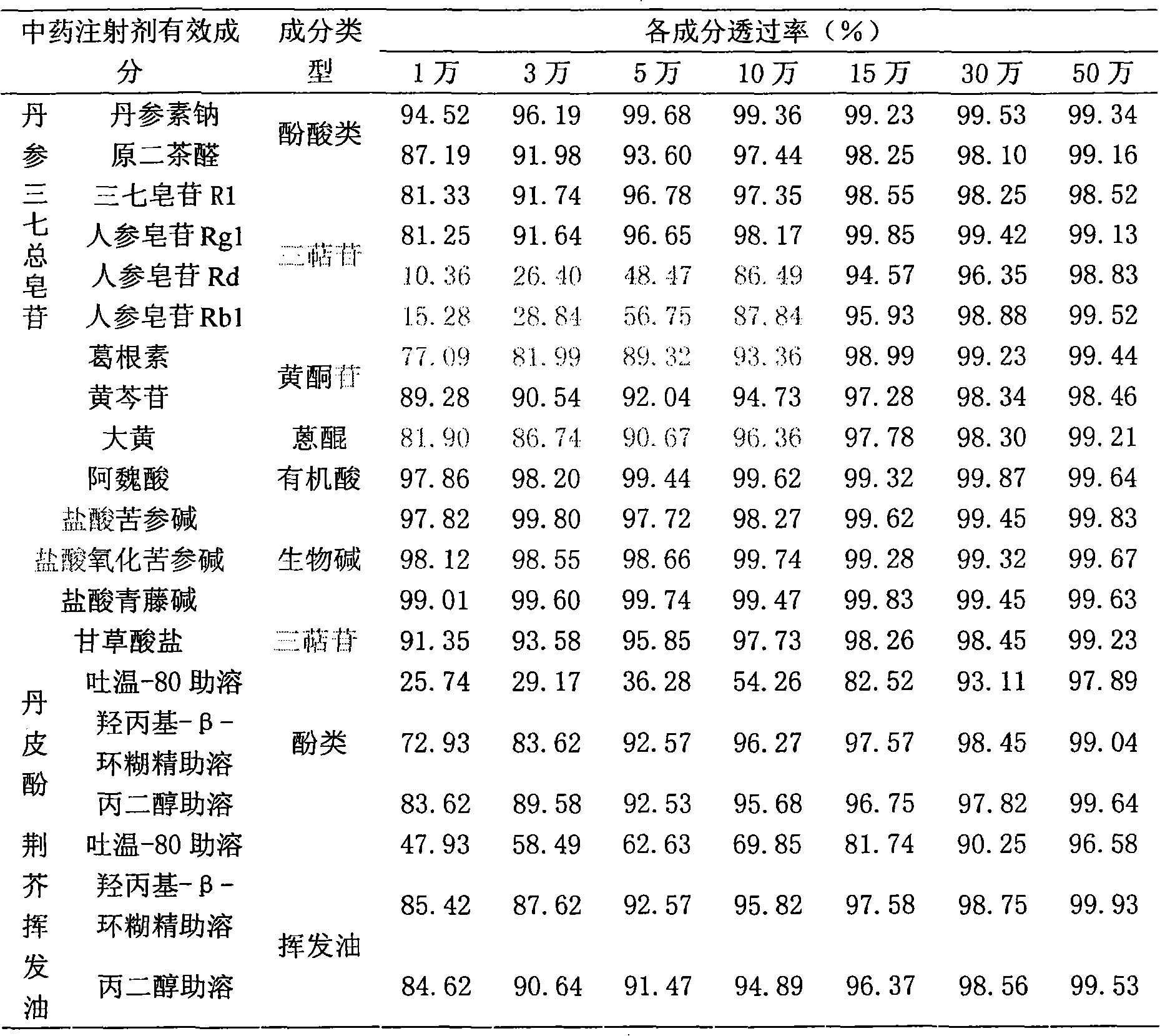
[0016] Example 2: Adaptability of active ingredients of traditional Chinese medicine injection to ultrafiltration membranes with different membrane pore sizes
[0017] Ultrafiltration experiment and sampling: select representative medicinal solutions (V) of various types of ingredients, put them in ultrafiltration membrane systems of 10,000 to 500,000 Daltons, and balance the ultrafiltration cycle for 30 minutes, and sample the equilibrium solution (V 平 ). After the ultrafiltration, when the ultrafiltration begins, the volume of the ultrafiltrate is 1 / 3 of the total volume of the stock solution and the volume of the ultrafiltrate is 2 / 3 of the total volume of the stock solution, the retentate and the ultrafiltrate are sampled respectively (V 0截 -V 0 , V 1 / 3截 -V 1 / 3 , V 2 / 3截 -V 2 / 3 ). Detect the concentration of C 0截 -C 0 、C 1 / 3截 -C 1 / 3 、C 2 / 3截 -C 2 / 3 . C 0 / C 0截 ×100%, C 1 / 3 / C 1 / 3截 ×100%, C 2 / 3 / C 2 / 3截 ×100% is the dynamic transmittance at the beginning of ...
Embodiment 3
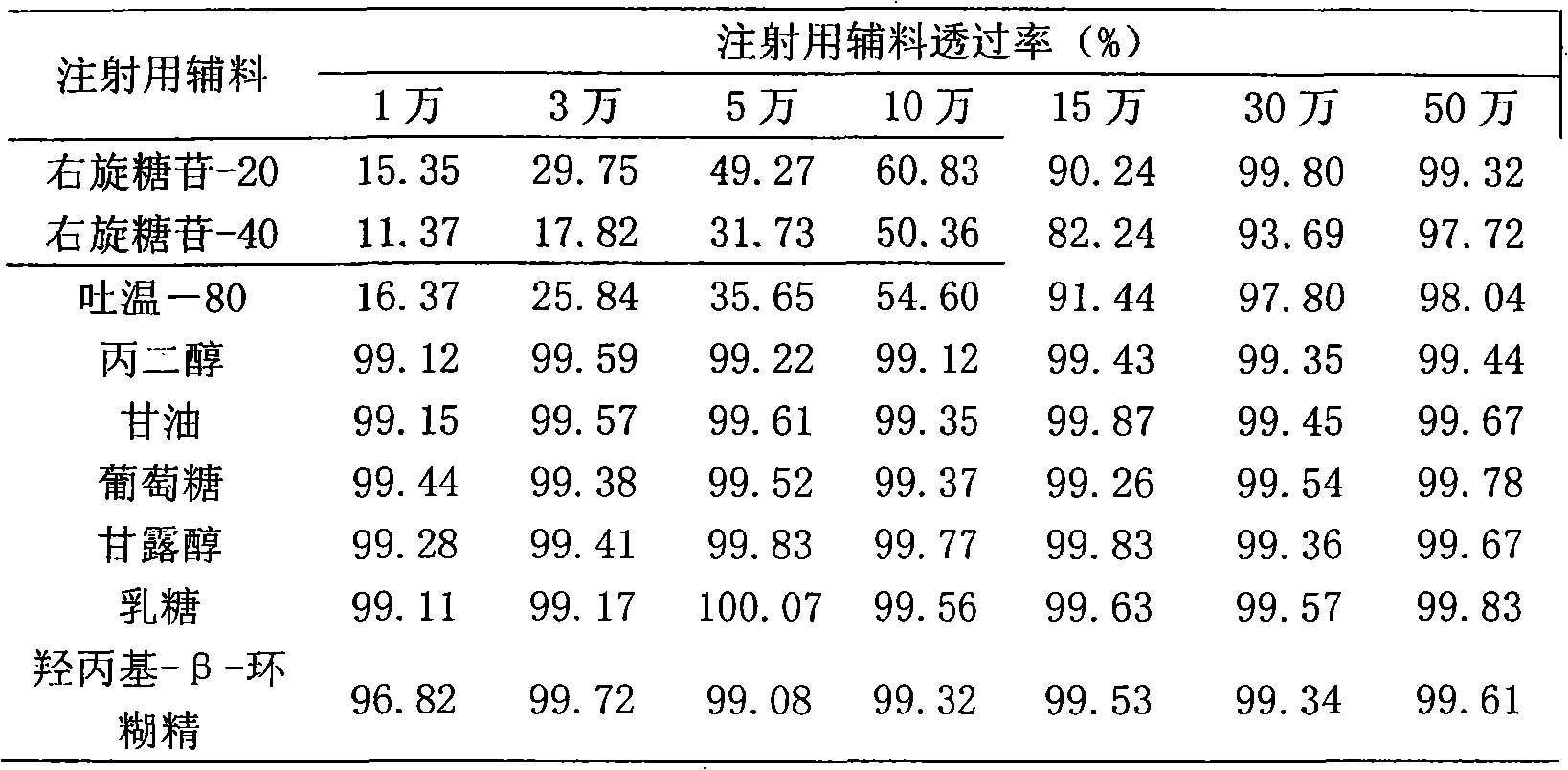
[0021] Example 3: Adaptability of macromolecular drugs and injection excipients to ultrafiltration membranes with different membrane pore sizes
[0022] Ultrafiltration experiment and sampling: Take the experimental solution (V) of each injection excipient, put them in an ultrafiltration membrane system of 10,000 to 500,000 Daltons, and balance the ultrafiltration cycle for 30 minutes, and sample the equilibrium solution (V 平 ). After the ultrafiltration, when the ultrafiltration begins, the volume of the ultrafiltrate is 1 / 3 of the total volume of the stock solution and the volume of the ultrafiltrate is 2 / 3 of the total volume of the stock solution, the retentate and the ultrafiltrate are sampled respectively (V 0截 -V 0 , V 1 / 3截 -V 1 / 3 , V 2 / 3截 -V 2 / 3 ). Detect the concentration of C 0截 -C 0 、C 1 / 3截 -C 1 / 3 、C 2 / 3截 -C 2 / 3 . C 0 / C 0截 ×100%, C 1 / 3 / C 1 / 3截 ×100%, C 2 / 3 / C 2 / 3截 ×100% is the dynamic transmittance at the beginning of ultrafiltration, when the vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com