Microcapsule lyophilized powder and preparation method thereof
A technology of freeze-dried powder and microcapsules, applied in the field of preparation of microcapsules, can solve the problems of poor encapsulation capacity, serious burst release effect, poor thermal stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1: Bevacizumab capsule-core microcapsules
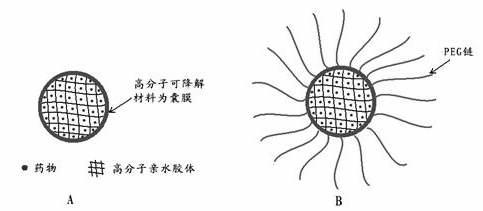
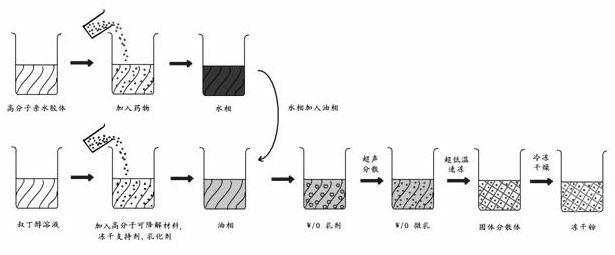
[0099] Antibody drugs have the advantages of small amount and strong effect, but they are easily degraded in the body and last for a short time. In this example, bevacizumab was used as an object to prepare bevacizumab cored microcapsules, and the composition of bevacizumab cored microcapsules is shown in Table 2.
[0100] Table 2 Bevacizumab capsule-core microcapsule composition ratio
[0101] prescription Bevacizumab polymer hydrocolloid material Emulsifier polymer degradable material Freeze-dried support agent tert-butanol Prescription 1 0.05mg Gelatin 1mg Polyoxyethylene hydrogenated castor oil 10mg PLGA with a molecular weight of 20,000 (50:50) 1mg Poloxamer 188 100mg 2g Prescription 2 0.05mg Human albumin 1mg Polyoxyethylene hydrogenated castor oil 10mg PLGA with a molecular weight of 10,000 (50:50) 2mg Poloxamer 188 100mg 2g Prescription 3 0.05mg ...
Embodiment 2
[0106] Embodiment 2: the preparation of doxorubicin capsule core microcapsule
[0107] There are many anti-tumor chemical drugs, including doxorubicin, vincristine, topotecan, etc. Although they have definite anti-tumor effects, they lack targeting in vivo, so they have strong toxic and side effects, and a targeted delivery carrier is needed. In this example, doxorubicin was used as an object to prepare doxorubicin capsule-core microcapsules, and the composition of doxorubicin capsule-core microcapsules is shown in Table 3.
[0108] Table 3 Doxorubicin gel-core microcapsule composition distribution ratio
[0109] prescription Adriamycin polymer hydrocolloid material Emulsifier polymer degradable material Freeze-dried support agent pH regulator co-emulsifier Prescription 1 5mg Gelatin 1mg Polyoxyethylene hydrogenated castor oil 20mg 5 mg of PLGA (50:50) with a molecular weight of 10,000 Poloxamer 188 200mg Hydrochloric acid 0.01 Oleic ...
Embodiment 3
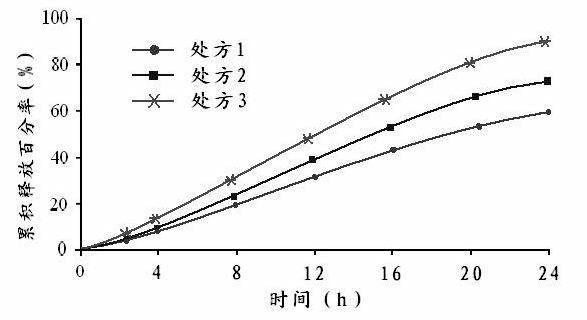
[0113] Example 3: Quality Evaluation of Adriamycin Microcapsules
[0114] In this example, the doxorubicin microcapsule freeze-dried powder prepared in Example 2 and the control group were used for quality comparison.
[0115] Microscopic morphology and particle size analysis: with embodiment 1.
[0116] Determination of encapsulation efficiency: Take 100 mg of the above lyophilized powder and add 1 g of water to form a microcapsule or nanoparticle solution, load the sample on a Sephadex G-50 gel column, use distilled water as the eluent, take different volumes of the eluted fraction, and separate Receive the eluted part of the microcapsules or nanoparticles loaded with doxorubicin, add chloroform to destroy the doxorubicin microcapsules or nanoparticles, extract doxorubicin to make doxorubicin solution, and use HPLC method to detect the content of doxorubicin hydrochloride (chromatographic Conditions: Venusil MP C18 column (416mm×250mm, 5μm), the mobile phase is acetonitrile...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com