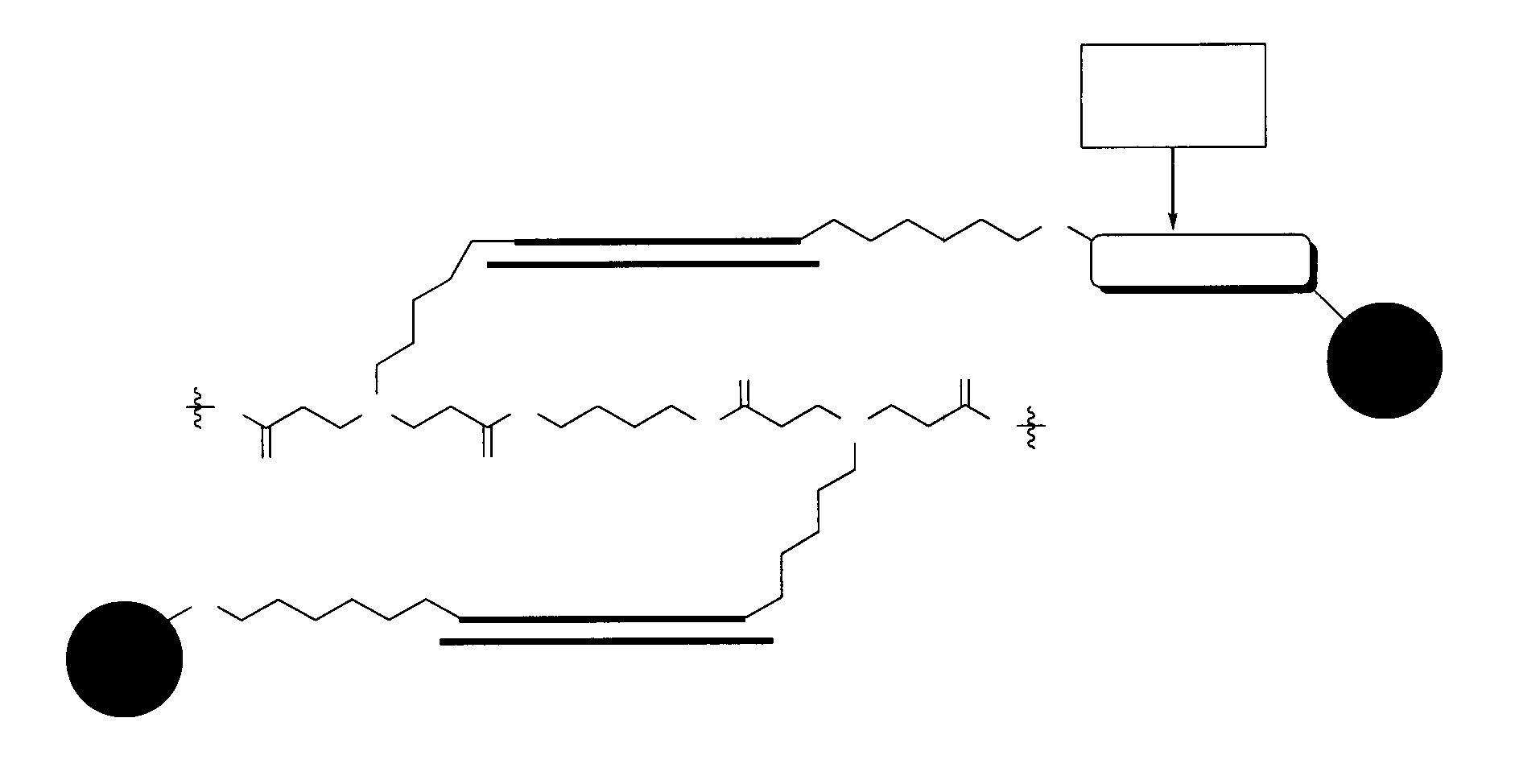
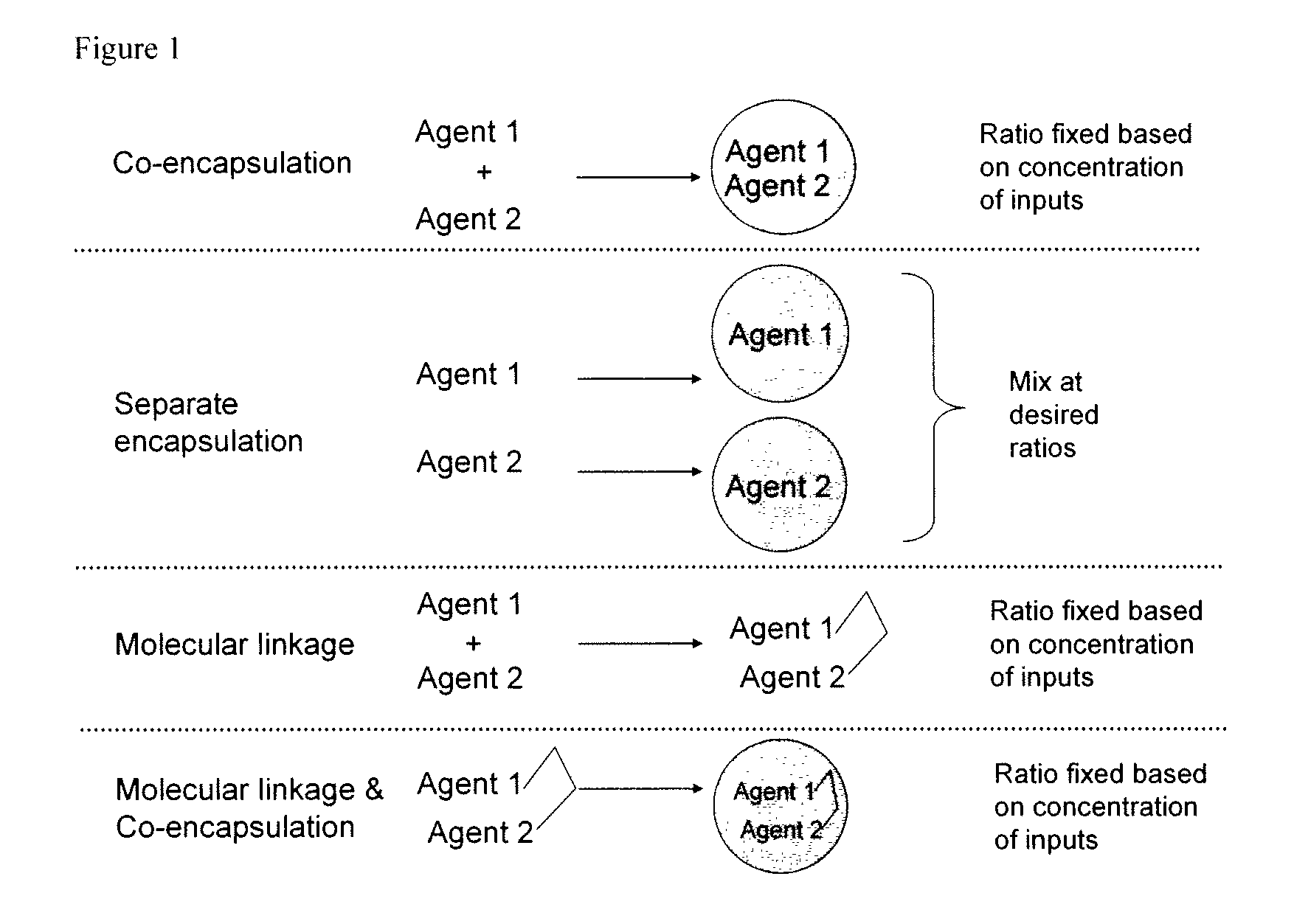
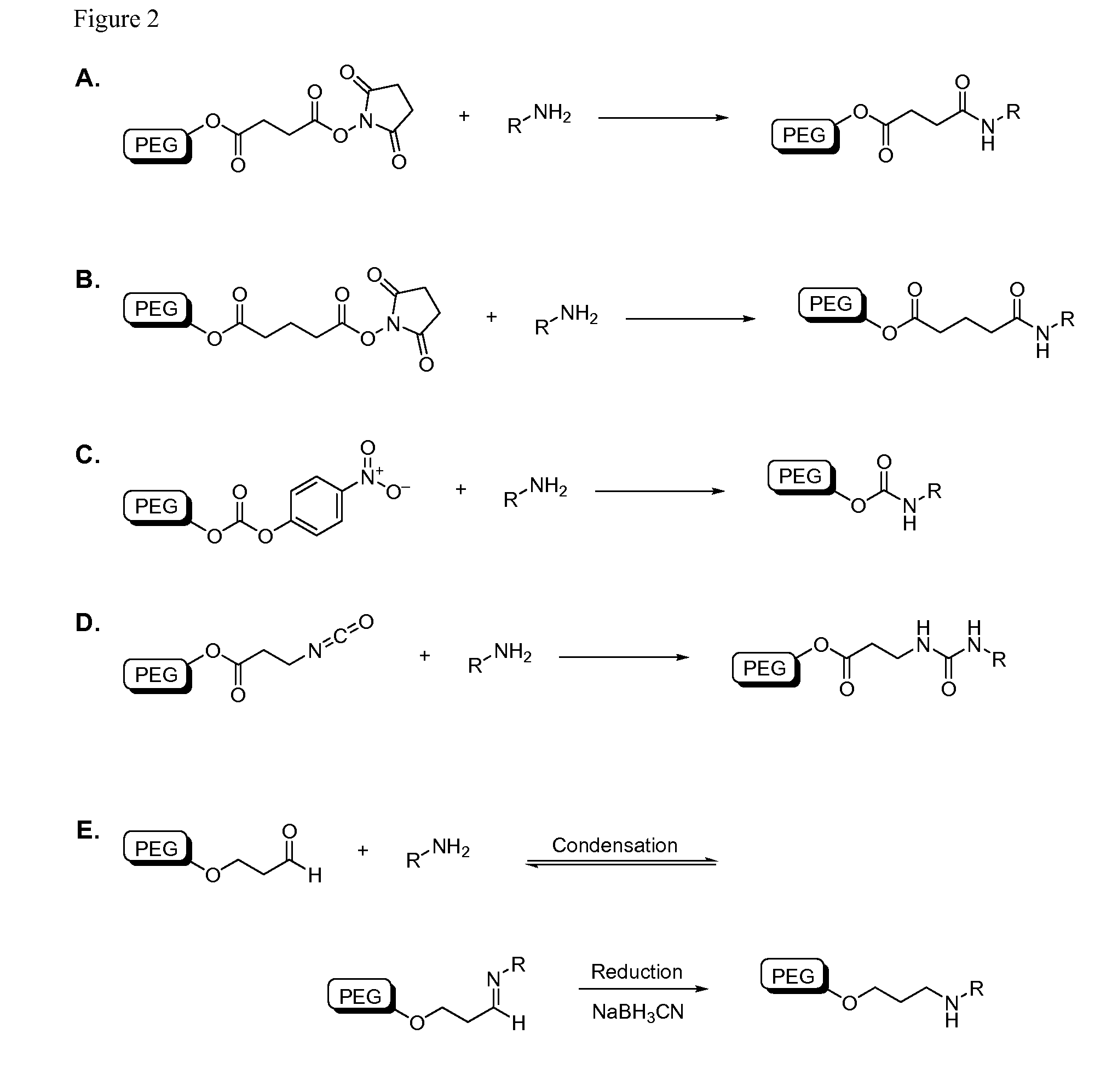
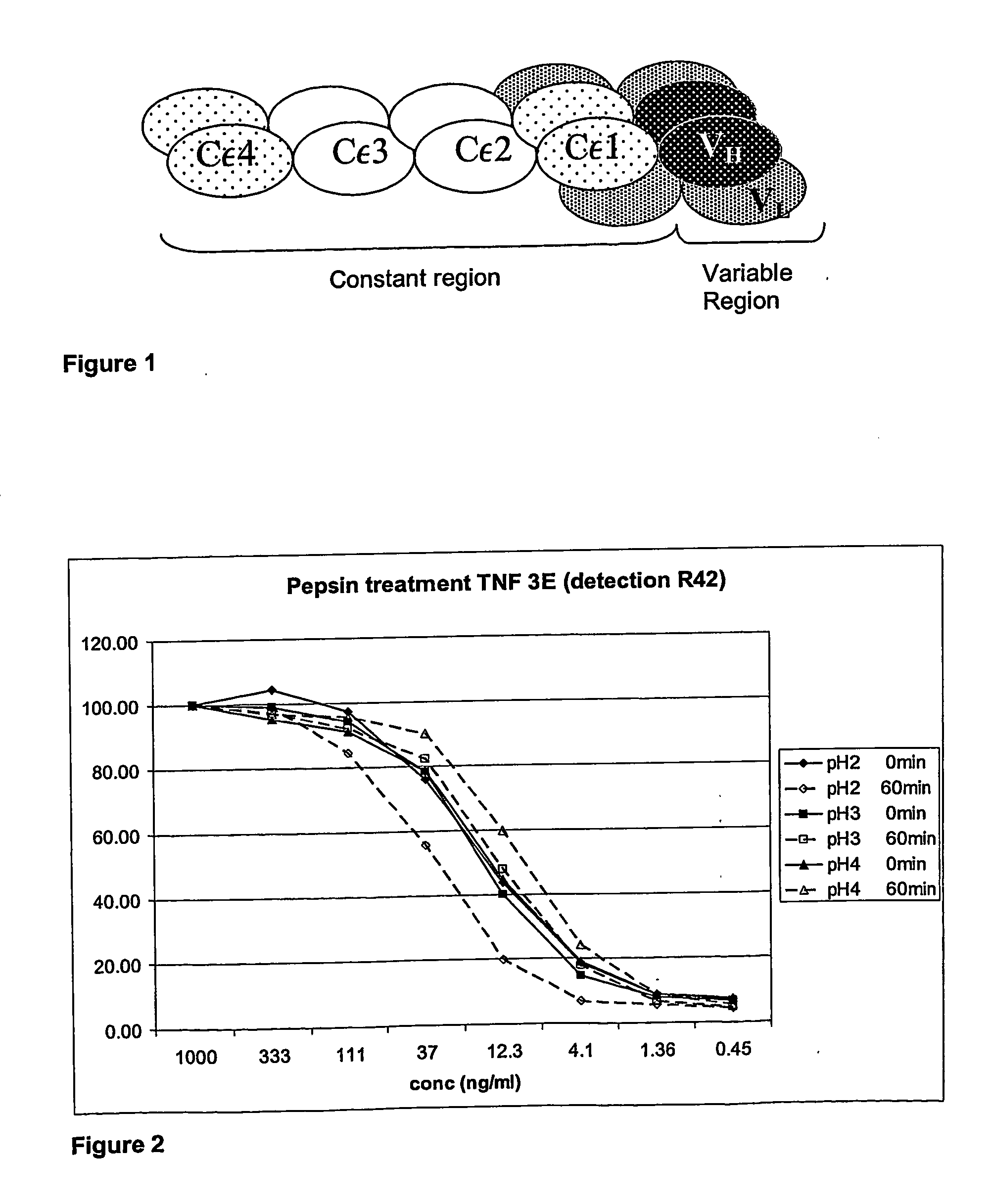
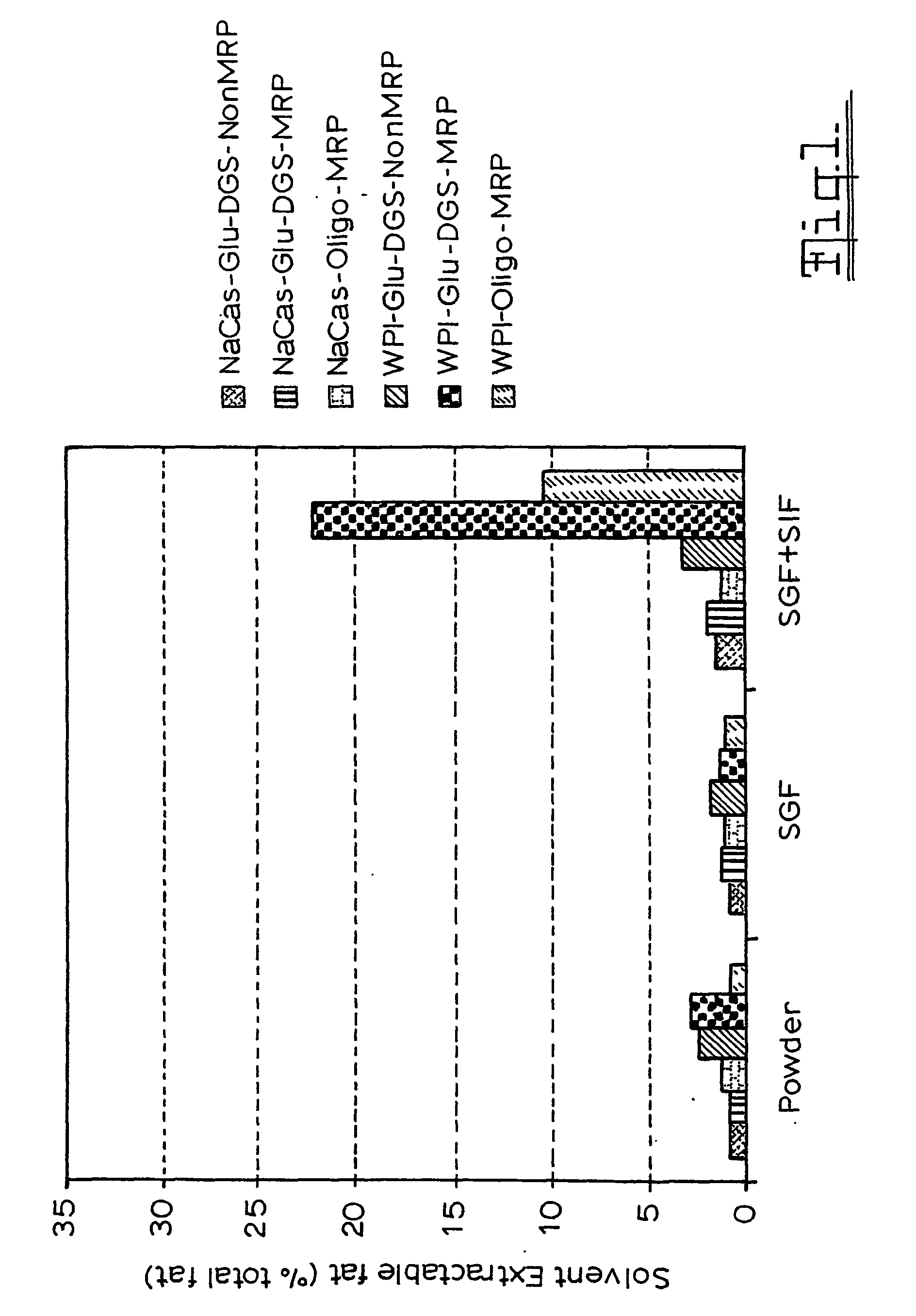
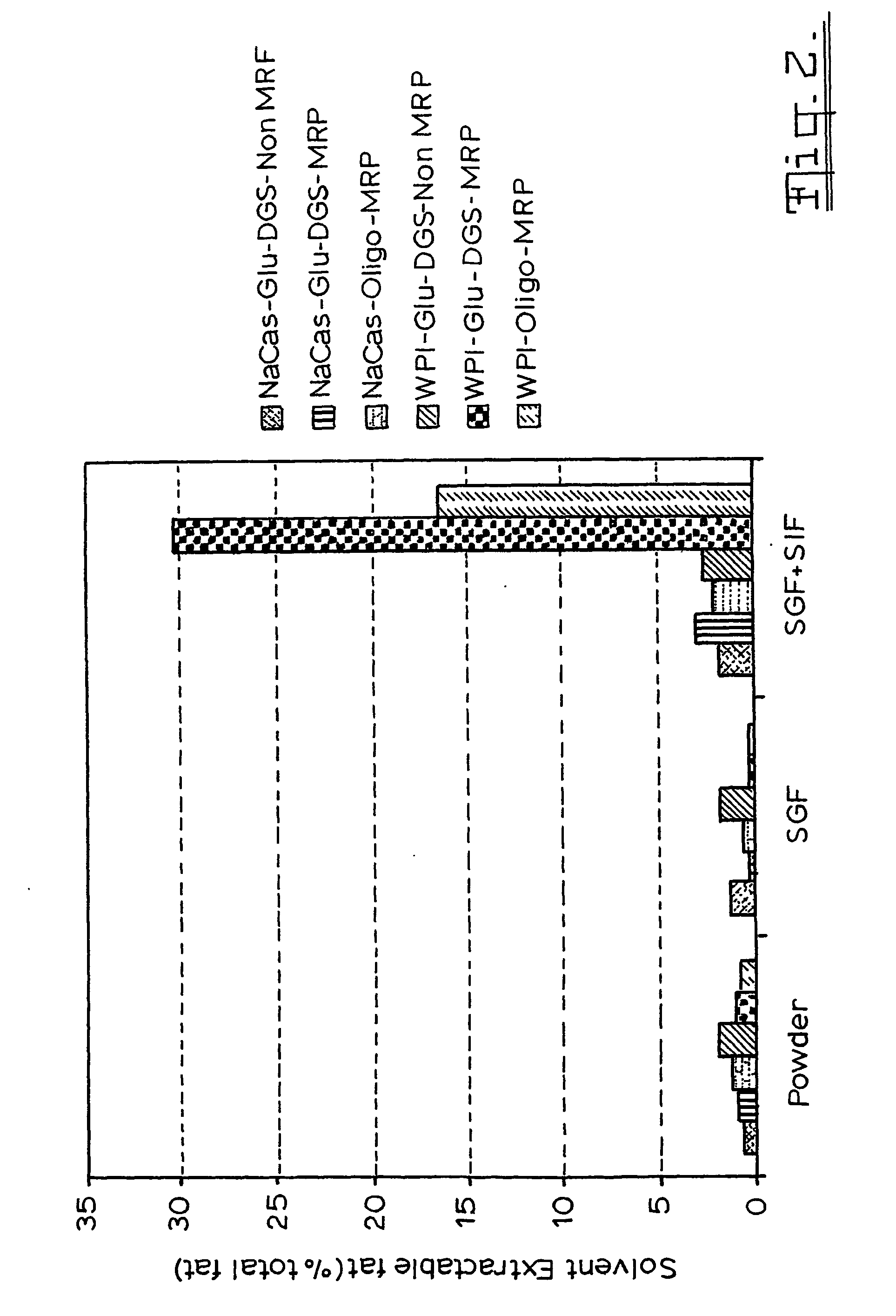
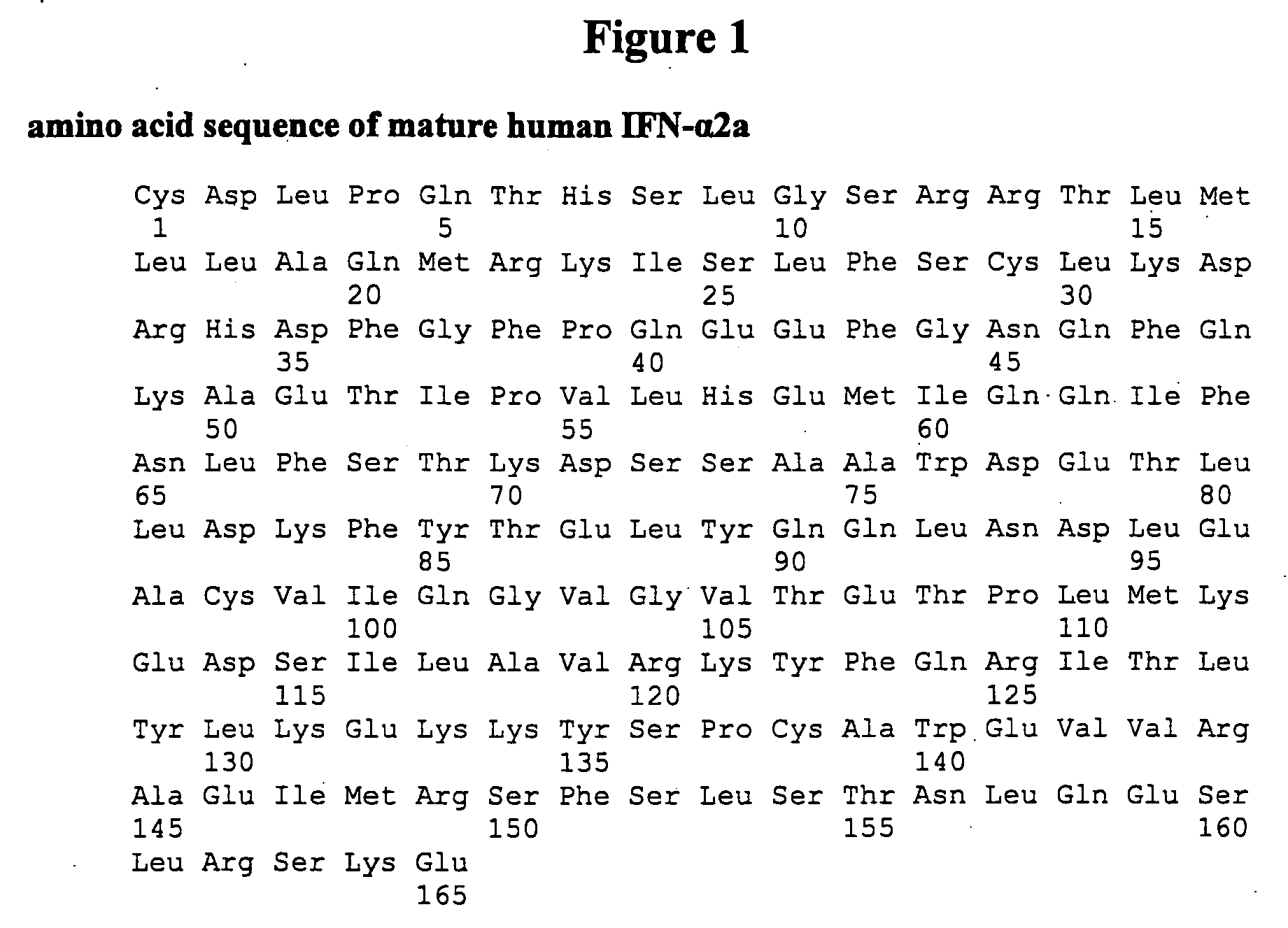
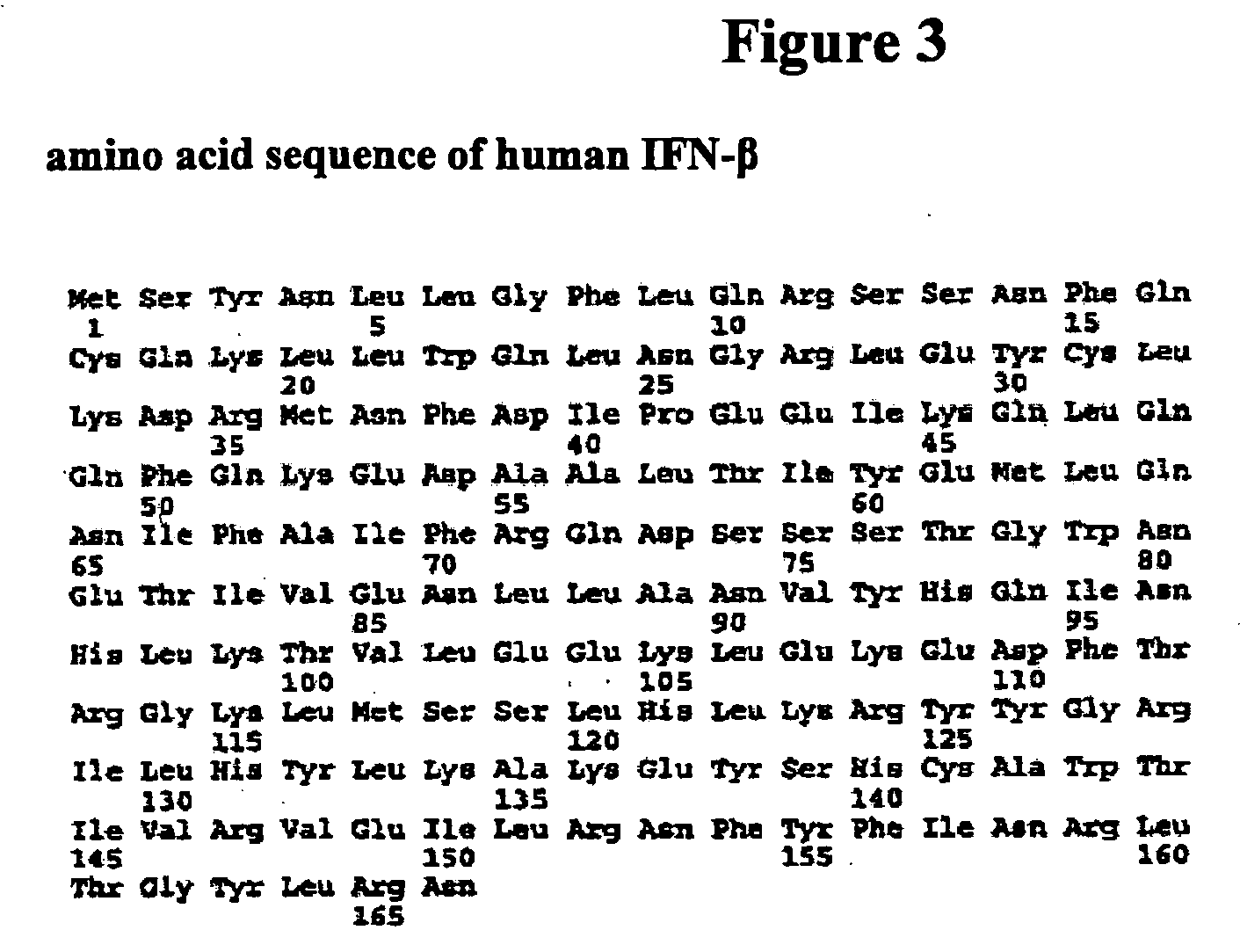
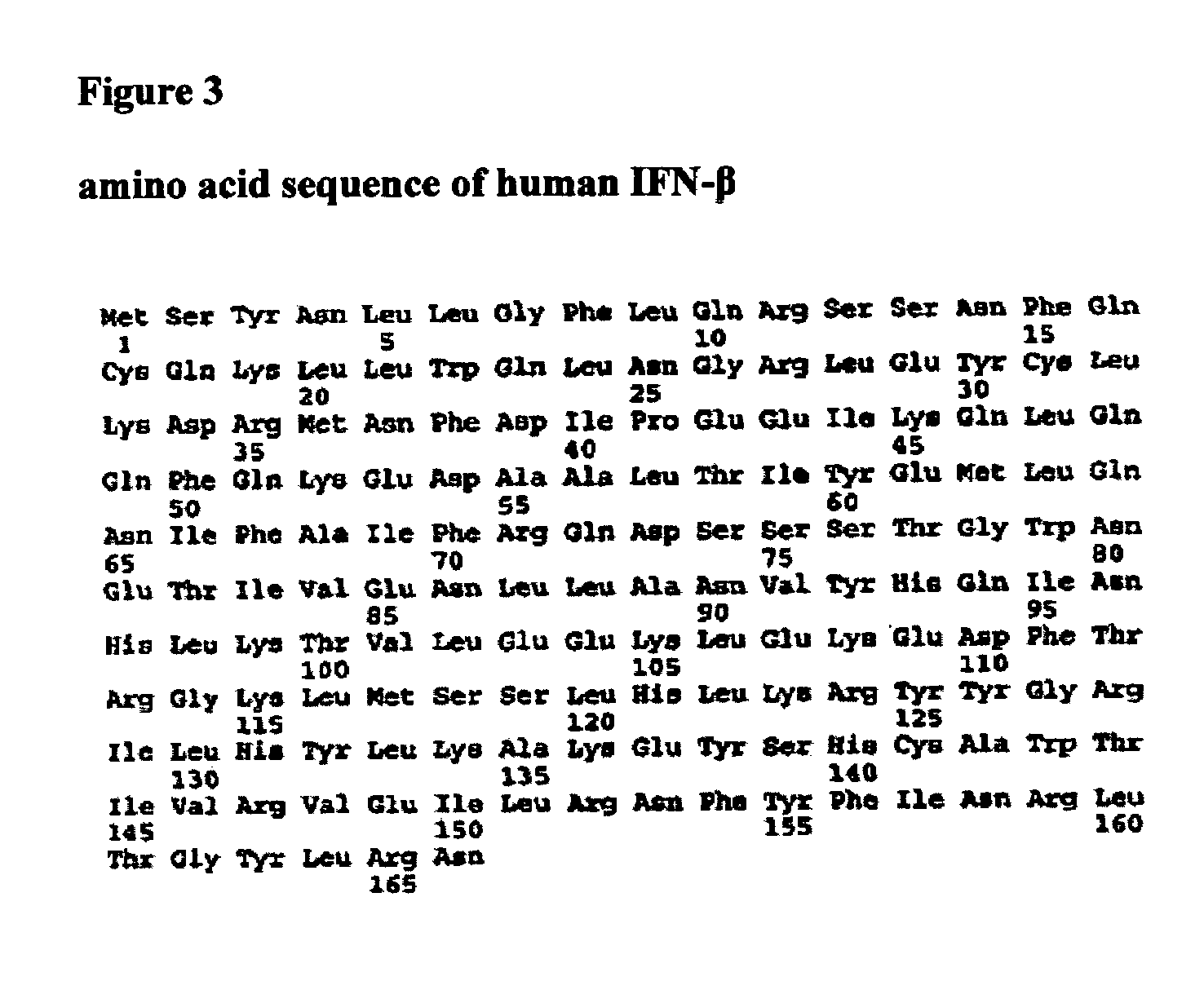
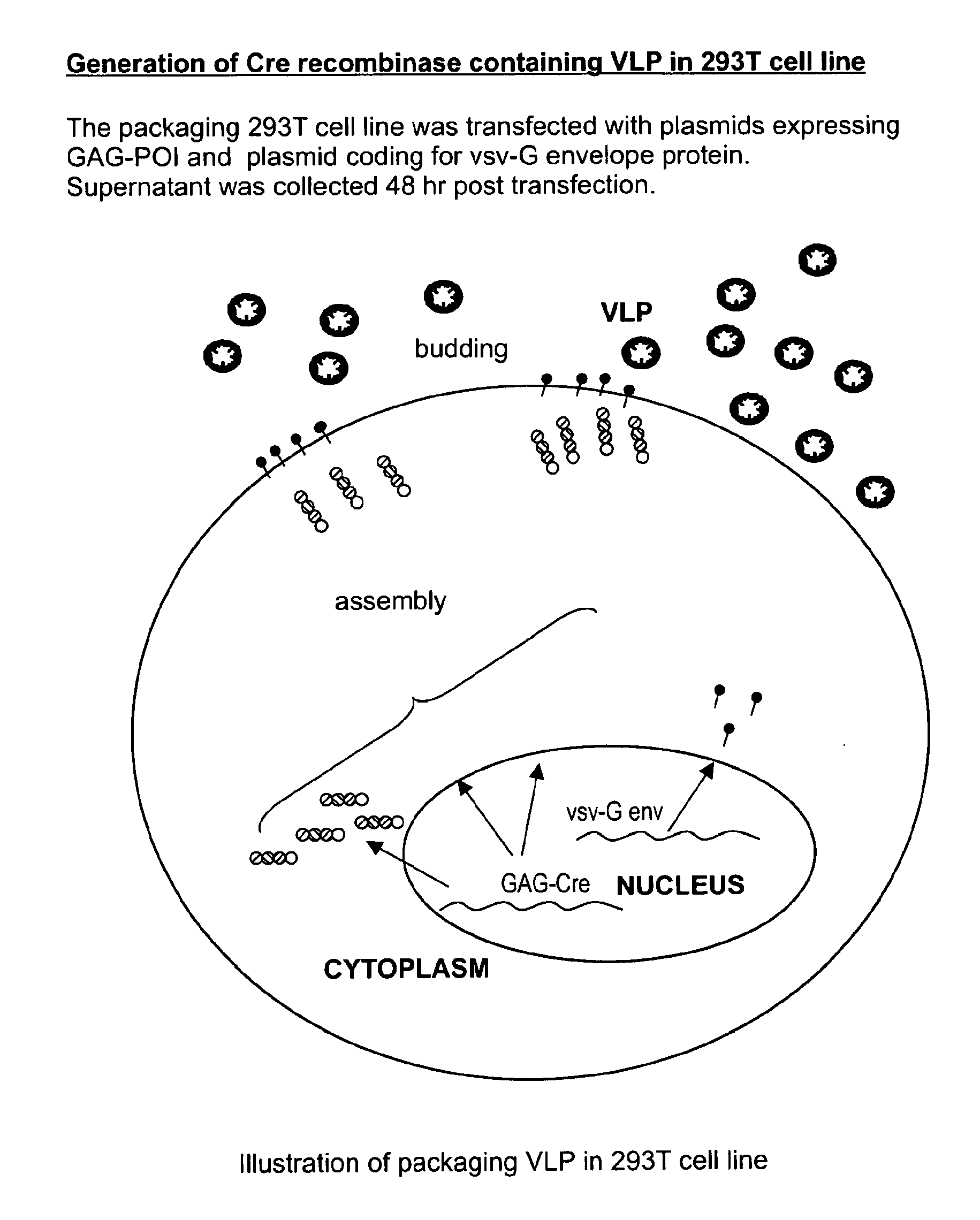
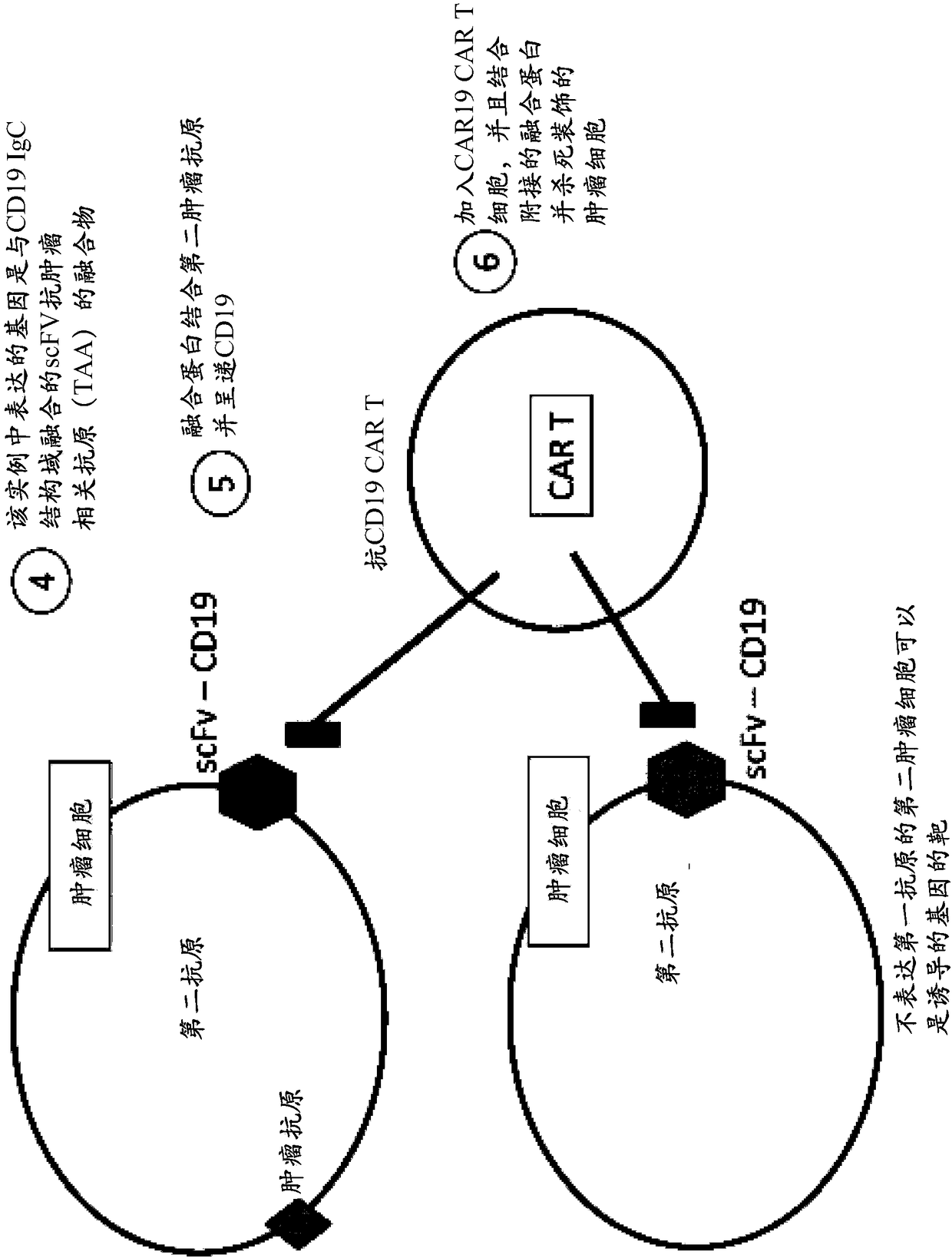
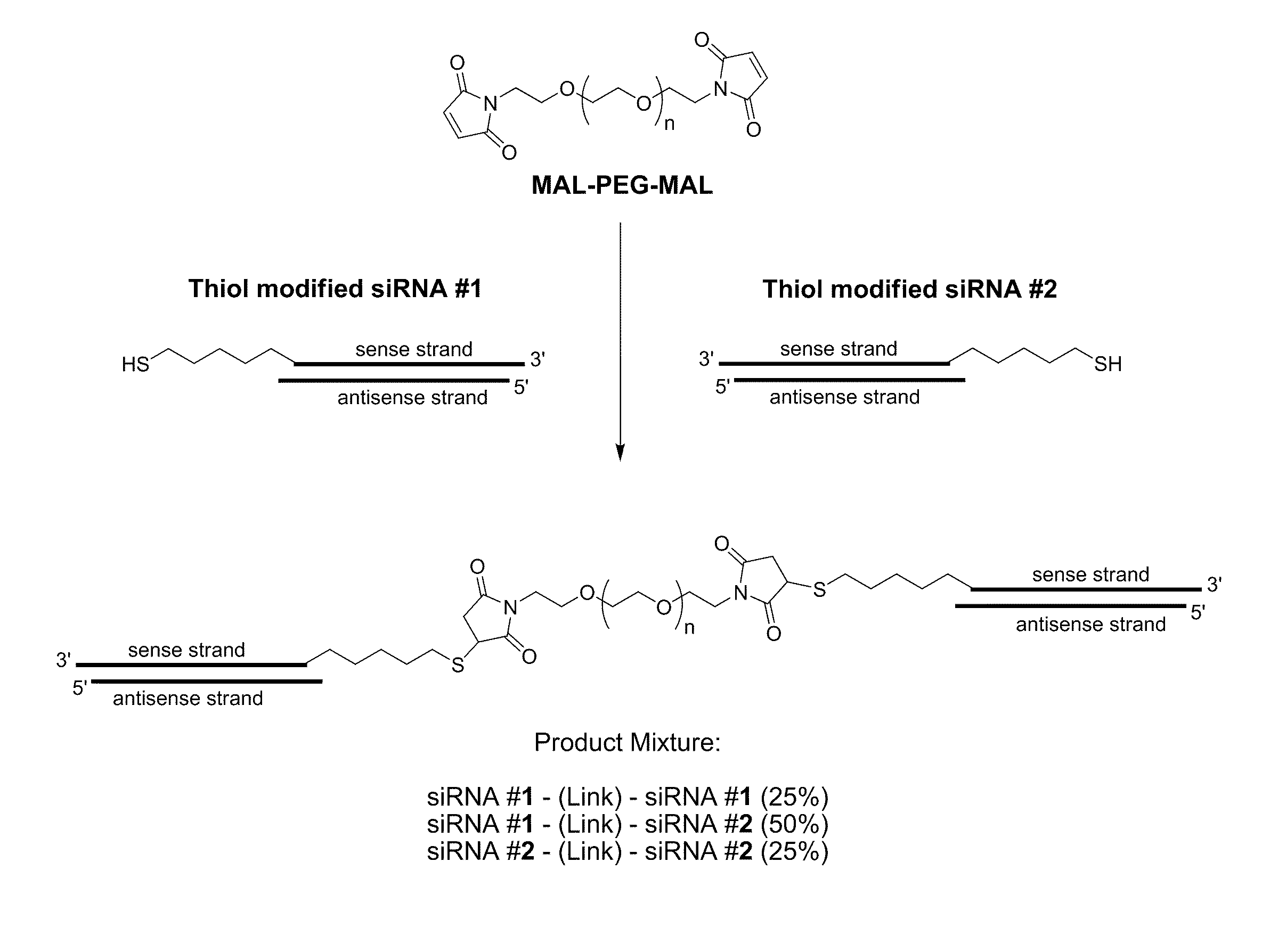
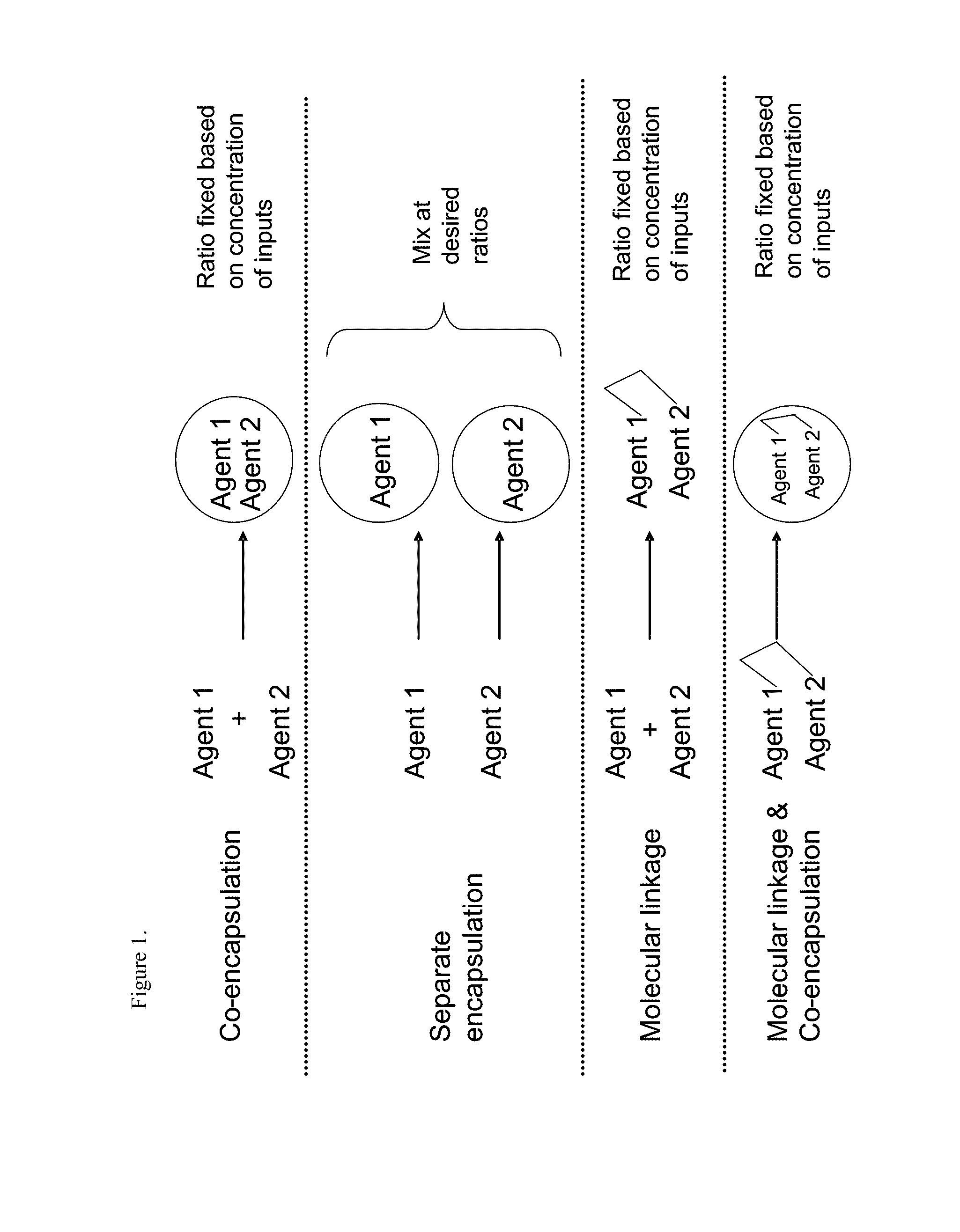
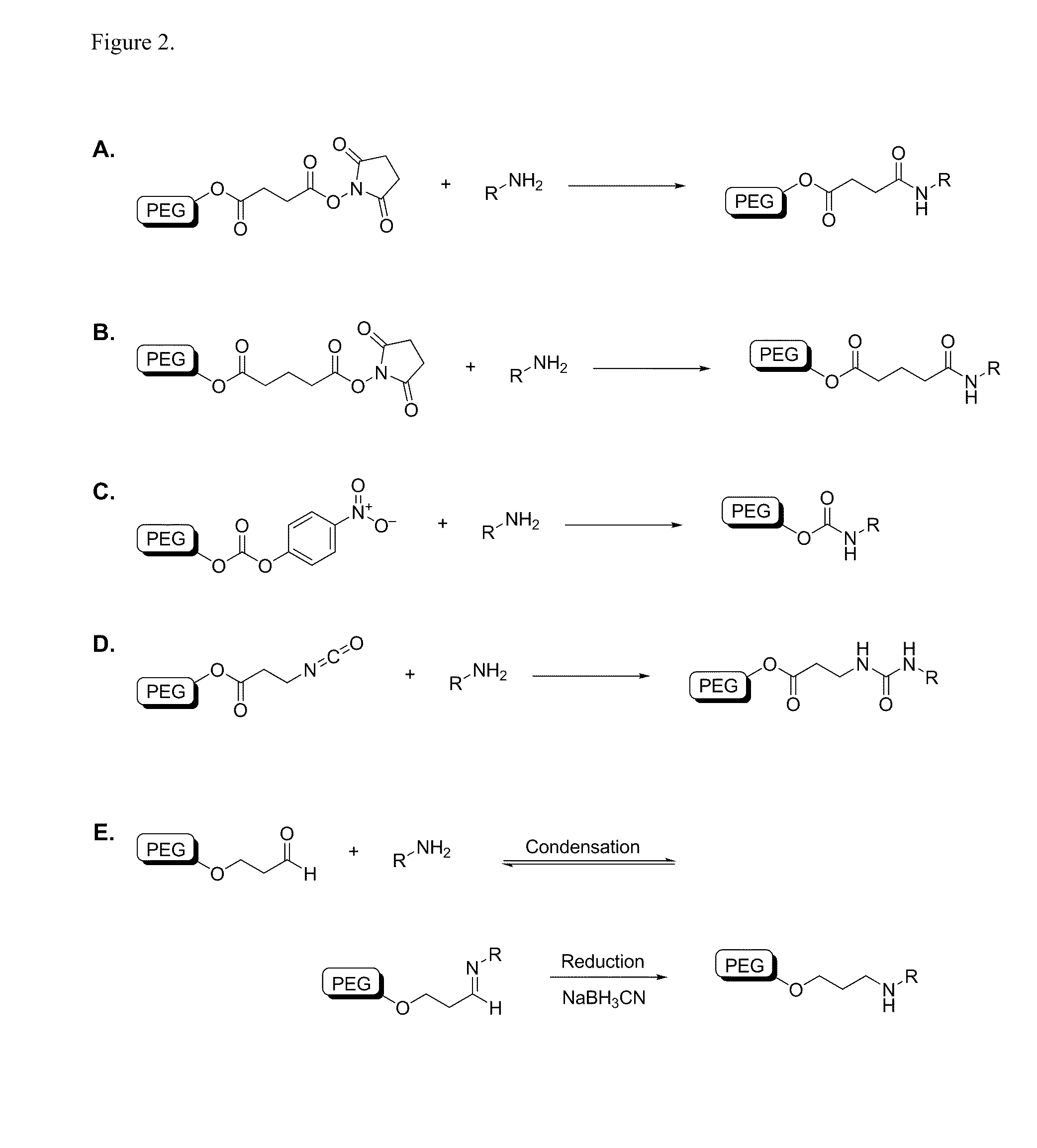
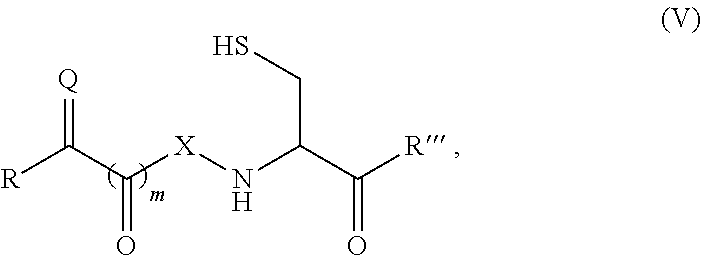
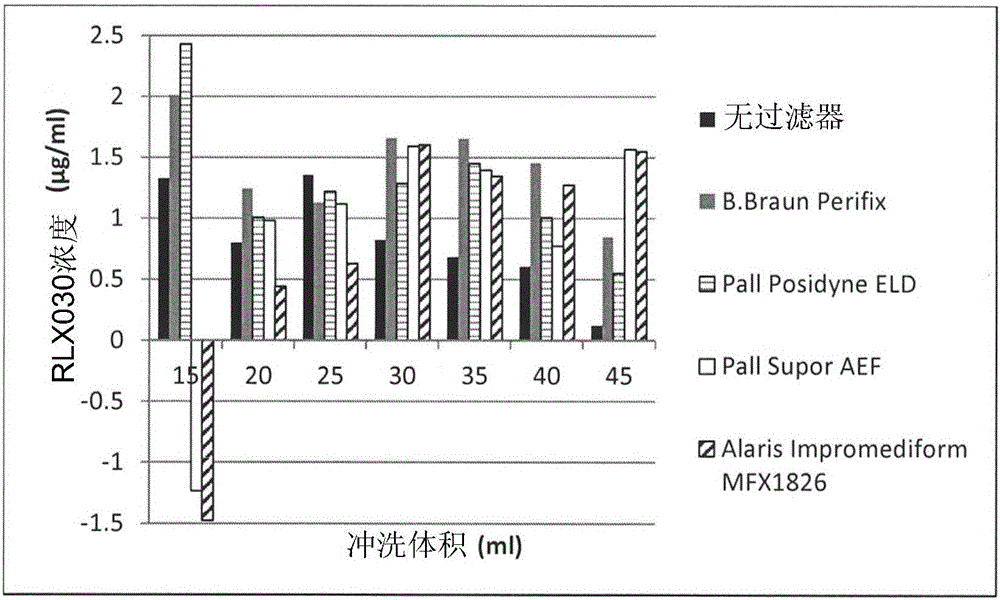
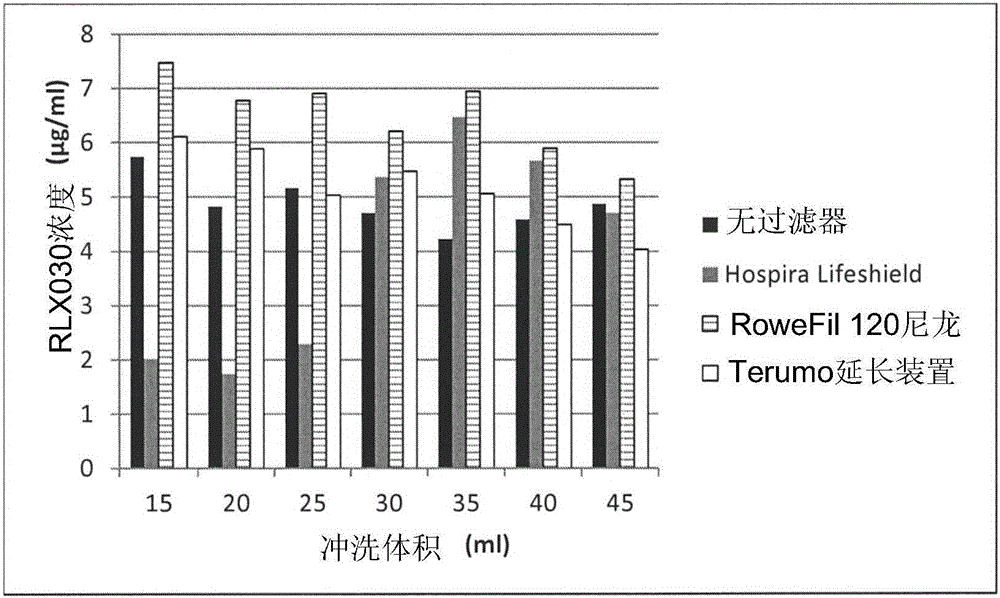
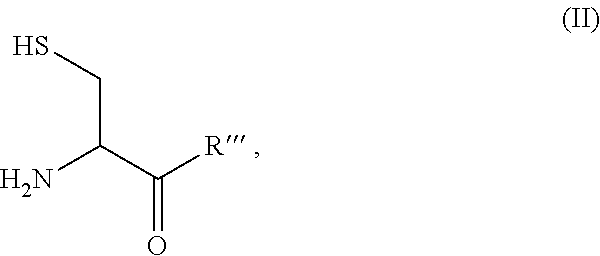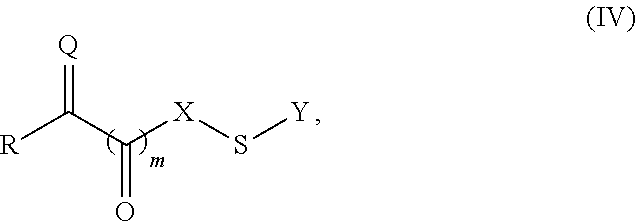Patents
Literature
87 results about "Protein therapeutics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein therapeutics: a summary and pharmacological classification. Leader B(1), Baca QJ, Golan DE. ... Protein therapeutics already have a significant role in almost every field of medicine, but this role is still only in its infancy. This article overviews some of the key characteristics of protein therapeutics, summarizes the more than 130 ...
METHODS AND COMPOSITIONS FOR IMPROVED THERAPEUTIC EFFECTS WITH siRNA
InactiveUS20080311040A1Improve in vivo stabilityImprove efficacyBiocidePeptide/protein ingredientsTherapeutic effectProtein
The present invention relates to chemically modified, linked double-stranded (ds)RNA compositions comprising two or more double-stranded (ds) oligoribonucleotides linked by at least one linking moiety and methods of formulating and delivering such compositions to modulate gene expression through target-specific RNA co-interference (RNAco-i). The compositions of the invention may optionally comprise a conjugation or a complex with one or more small molecule drugs, protein therapeutics, or other dsRNA molecules. The present invention is directed at the methods of production for, methods of use of, and therapeutic utilities for RNAi co-interference therapy utilizing the compositions of the invention.
Owner:FLAGSHIP VENTURES
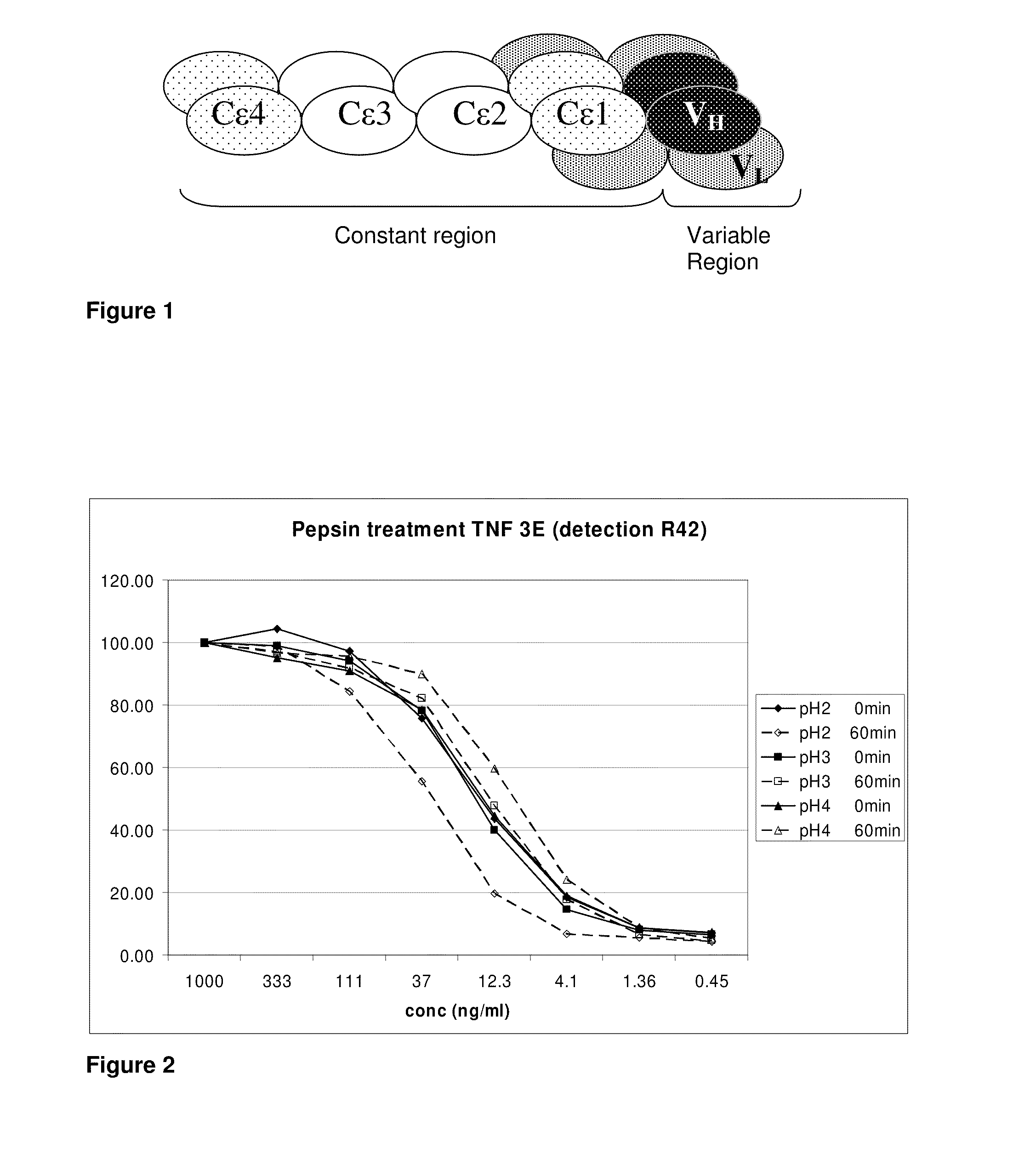
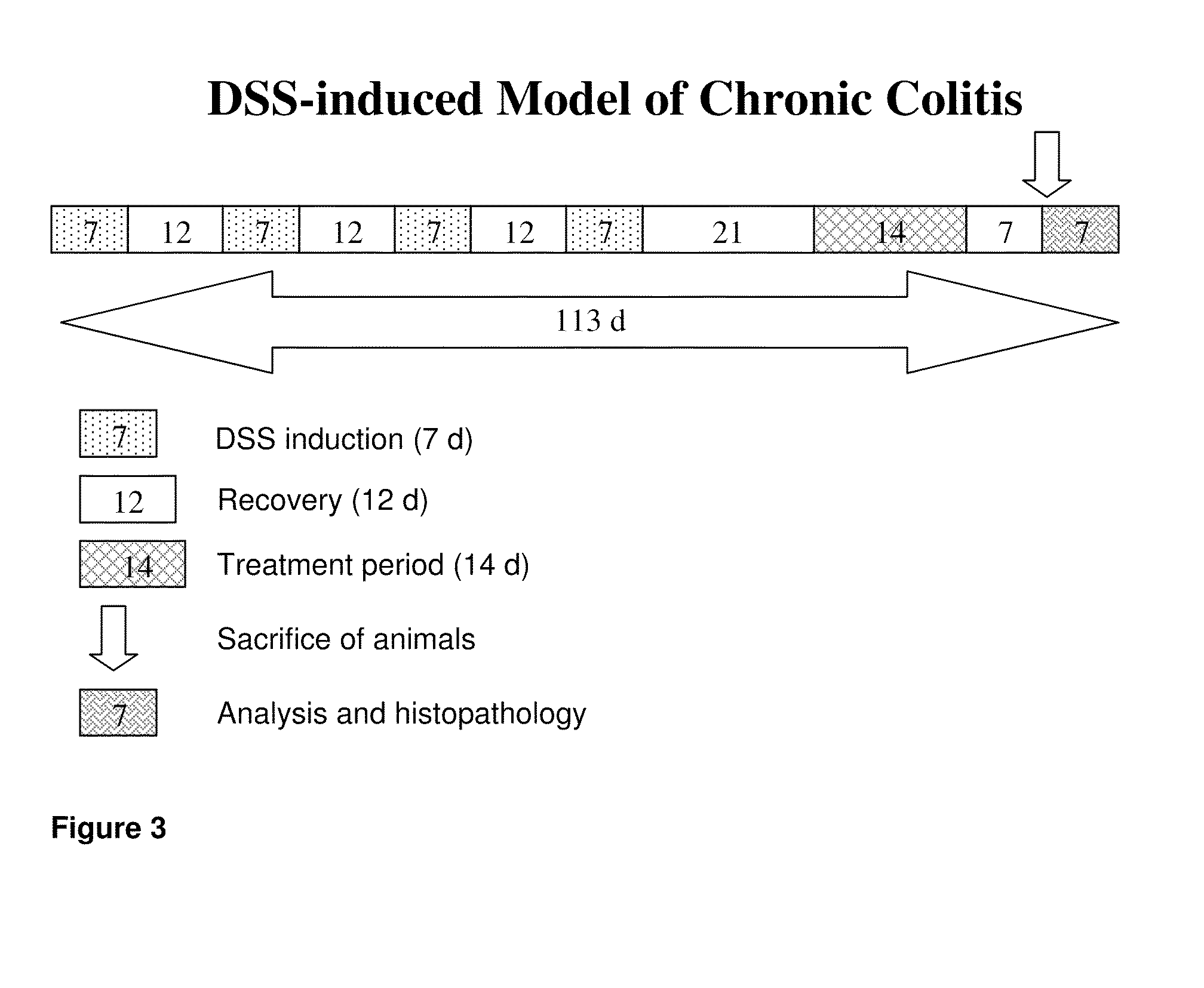
Camelidae antibodies against imminoglobulin e and use thereof for the treatment of allergic disorders
InactiveUS20060115470A1Preventing and alleviating symptomReduce deliveryAntibacterial agentsSenses disorderInhalationProtein therapeutics
The invention relates to a method suitable for administering protein therapeutic molecules orally, sublingually, topically, intravenously, subcutaneously, nasally, vaginally, rectally or by inhalation so as to avoid inactivation, by using VHH polypeptides derived from Camelidae antibodies. The invention further relates to the said therapeutic molecules. The invention further a method for delivering therapeutic molecules to the interior of cells. The invention furthe relates to anti-IgE therapeutic molecules.
Owner:ABLYNX NV
Macromolecular drug complexes having improved stability and therapeutic use of the same
InactiveUS20050147581A1Safe and easy and effective deliveryAlleviate and eliminate and reverse complicationPowder deliveryOrganic active ingredientsDiseaseSomatotropic hormone
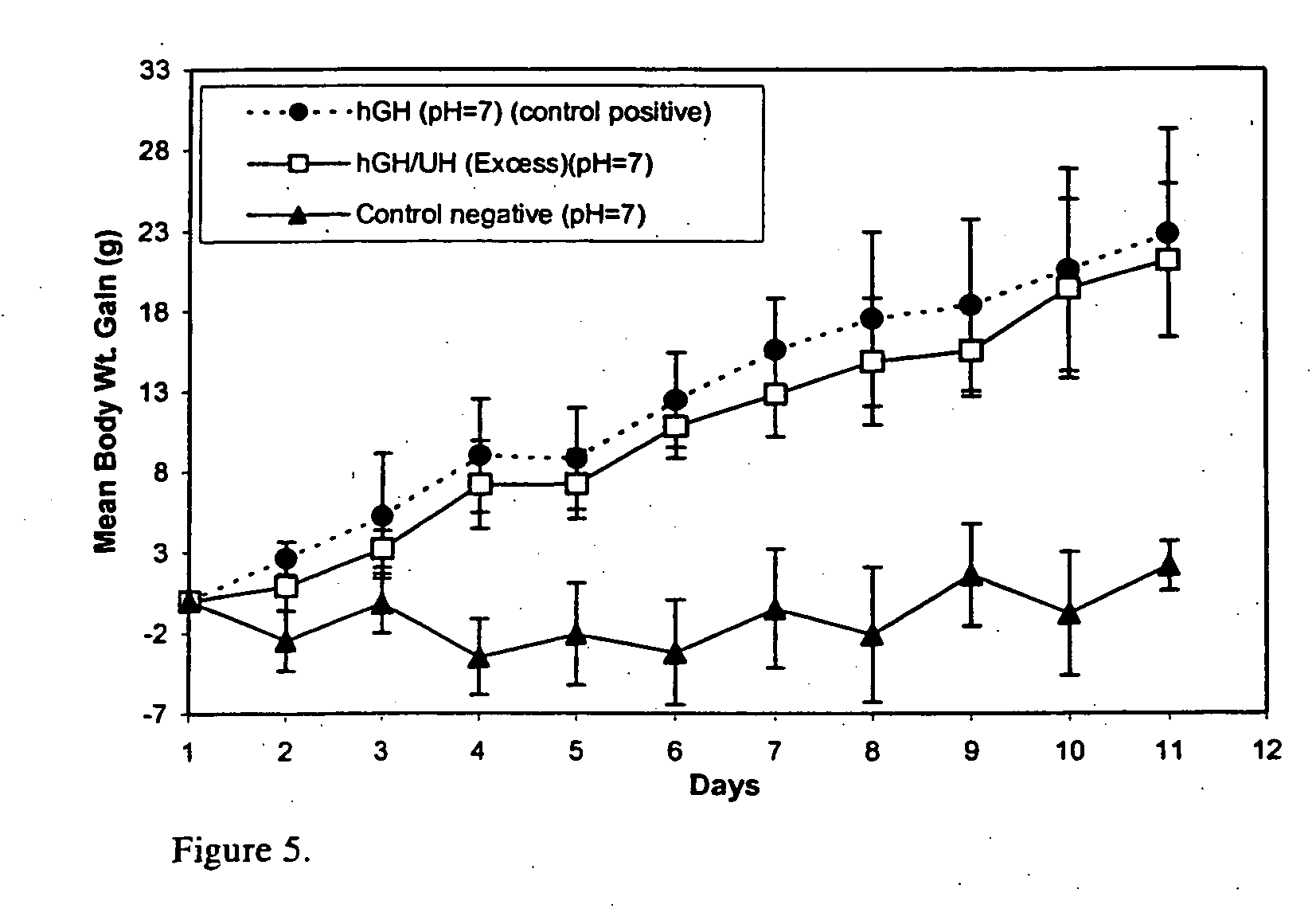
Macromolecular drug complexes containing a protein therapeutic, like human growth hormone, and an excess stoichiometric molar amount of a polymer, like heparin, and compositions containing the same, are disclosed. Compositions containing the macromolecular drug complexes are administered, including via pulmonary delivery, to individuals suffering from a disease or condition, and the complexes release the protein therapeutic, in vivo, to treat the disease or condition.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Generating protein pro-drugs using reversible PPG linkages
InactiveUS20050079155A1Reduce and block activityEliminate side effectsPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectProtecting group
The invention relates to methods for modulating the side effects, including immunogenicity, of a protein therapeutic via derivatization with a protein protecting group using reversible or labile linkages.
Owner:XENCOR
Integrated approach for generating multidomain protein therapeutics
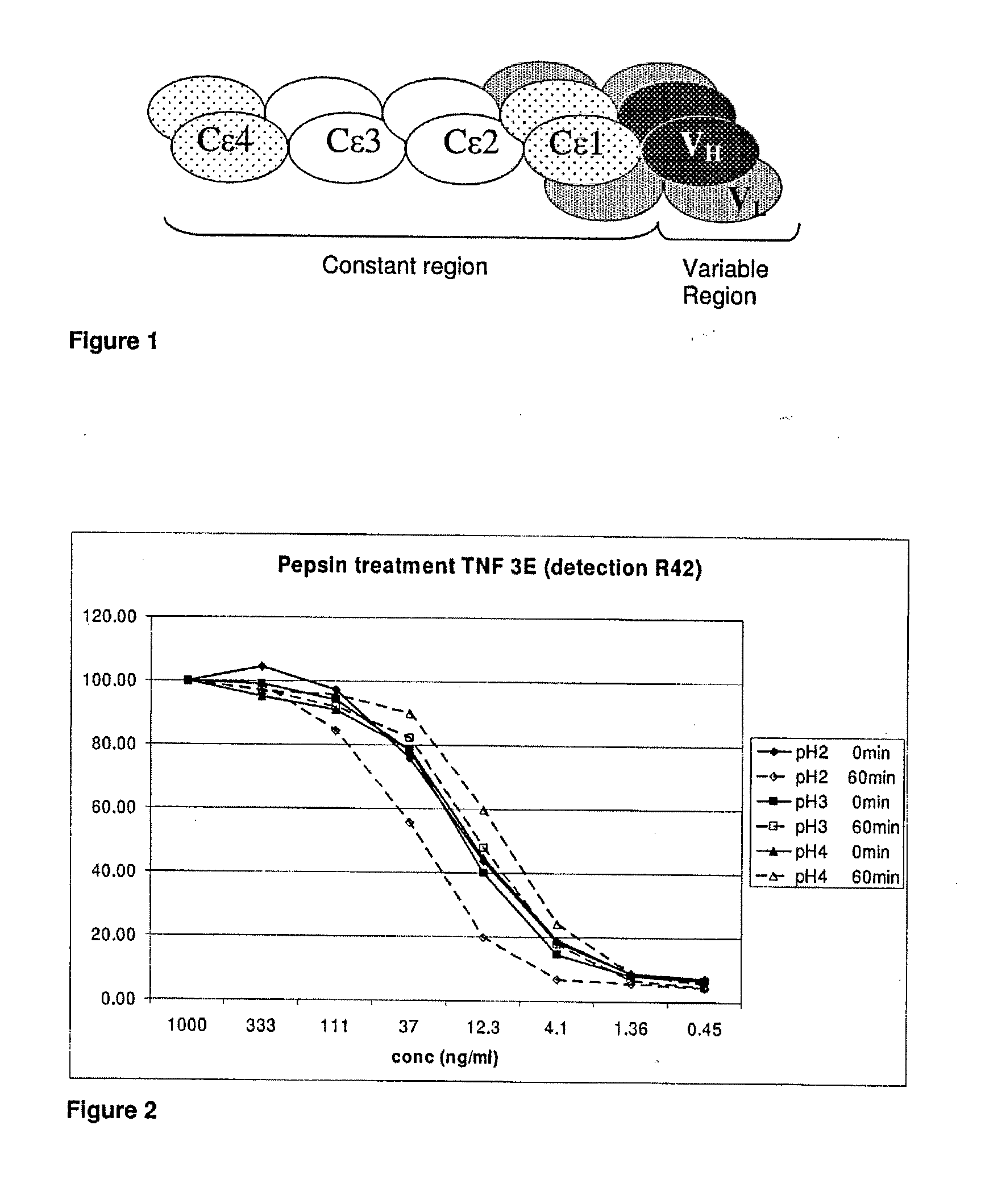
InactiveUS8309690B2Good curative effectImprove development efficiencyAntibody ingredientsImmunoglobulinsThermal denaturationTherapeutic protein
Owner:MEDIMMUNE LLC
Compositions For the Treatment of Diabetes
A supramolecular insulin assembly and supramolecular exendin-4 assembly, which is useful as a protein therapeutic agent for the treatment of metabolic disorders particularly diabetes. The supramolecular assemblies disclosed in the present invention consists of insoluble and aggregated oligomers the protein. The invention also provides pharmaceutical compositions comprising the supramolecular assembly.
Owner:EXTENDED DELIVERY PHARMA
Chimeric activators: quantitatively designed protein therapeutics and uses thereof
ActiveUS20110274658A1Reduced cell-activating propertyAvoid and reduce unwanted side effectObesity gene productsPeptide/protein ingredientsChimerin ProteinsApoptosis
Aspects of the invention provide methods for harnessing the potential of proteins that occur naturally (e.g., in humans) and that have serious but finite toxicity. Aspects of the invention relate to a quantitative systems-biological and structural approach to design a class Mof chimeric proteins that avoid the toxicity of protein drugs while retaining their desired activities. In particular, chimeric proteins containing a variant form of a natural protein fused to a targeting moiety may be administered to a subject to target a signal (e.g., induction of apoptosis) to particular cells without having a generalized toxic effect
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
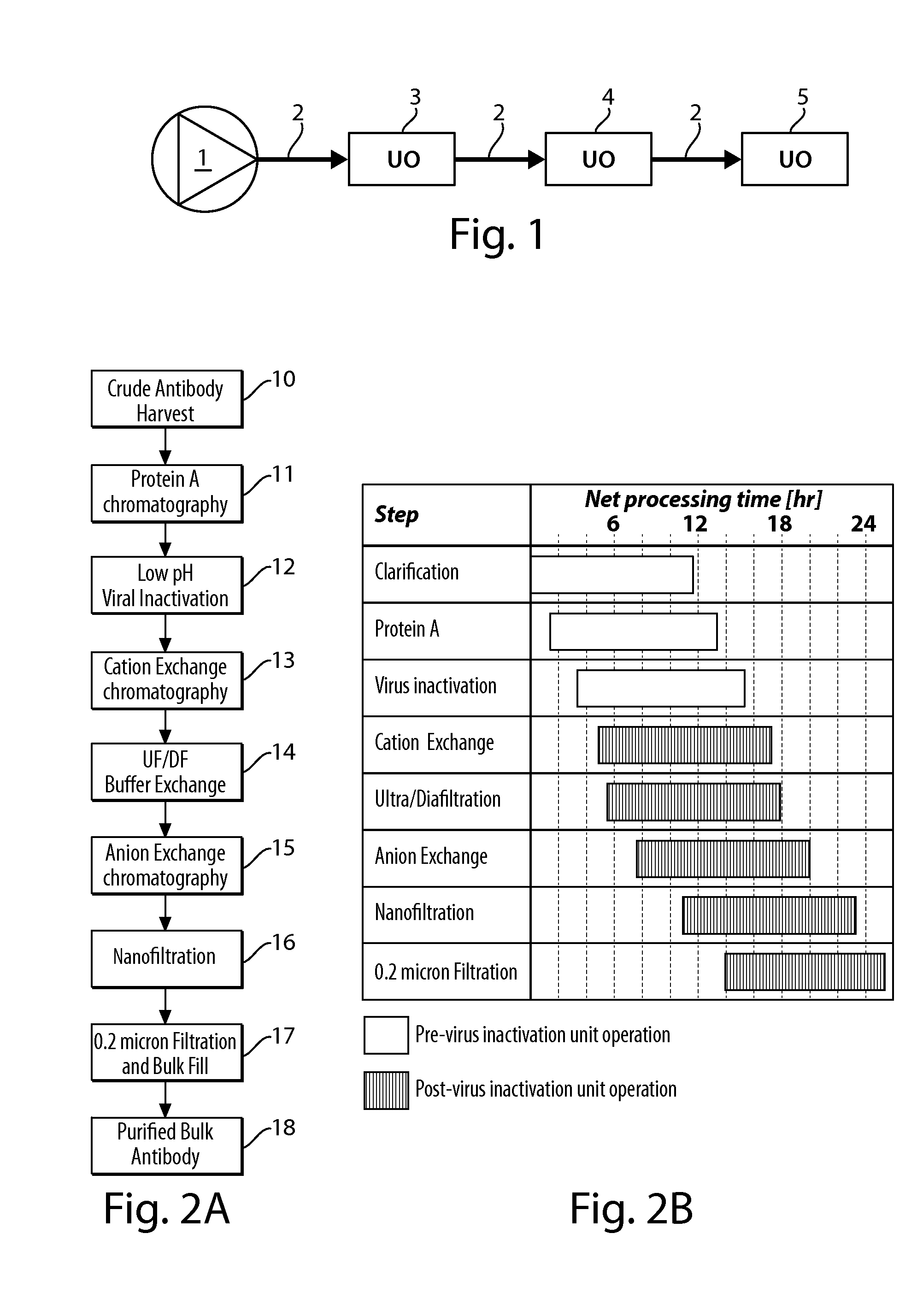
Continuous processing methods for biological products
InactiveUS20130260419A1Improve manufacturing productivityImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyMonoclonal antibody
The present invention is directed to the development of continuous processing technology for the purification of biopharmaceuticals and biological products, such as monoclonal antibodies, protein therapeutics, and vaccines. Methods for continuous processing of a biological product in a feed stream toward formulation of a purified bulk product are described.
Owner:SARTORIUS STEDIM CHROMATOGRAPHY SYST LTD
Therapeutic peptide formulations with improved stability
InactiveUS20060188555A1Improved and optimal physical stabilityExtended shelf lifePeptide/protein ingredientsAntipyreticBiocompatible coatingStratum corneum
Compositions of and methods for formulating and delivering peptide, polypeptide and protein therapeutic agent formulations having enhanced physical stability, and wherein fibril formation is minimized and / or controlled, to yield a consistent and predictable composition viscosity. The compositions of and methods for formulating and delivering peptide, polypeptide and protein therapeutic agents of the present invention further facilitate their incorporation into a biocompatible coating which can be employed to coat a stratum-corneum piercing microprojection, or a plurality of stratum-corneum piercing microprojections of a delivery device, for delivery of the biocompatible coating through the skin of a subject, thus providing an effective means of delivering the peptide therapeutic agents.
Owner:ALZA CORP
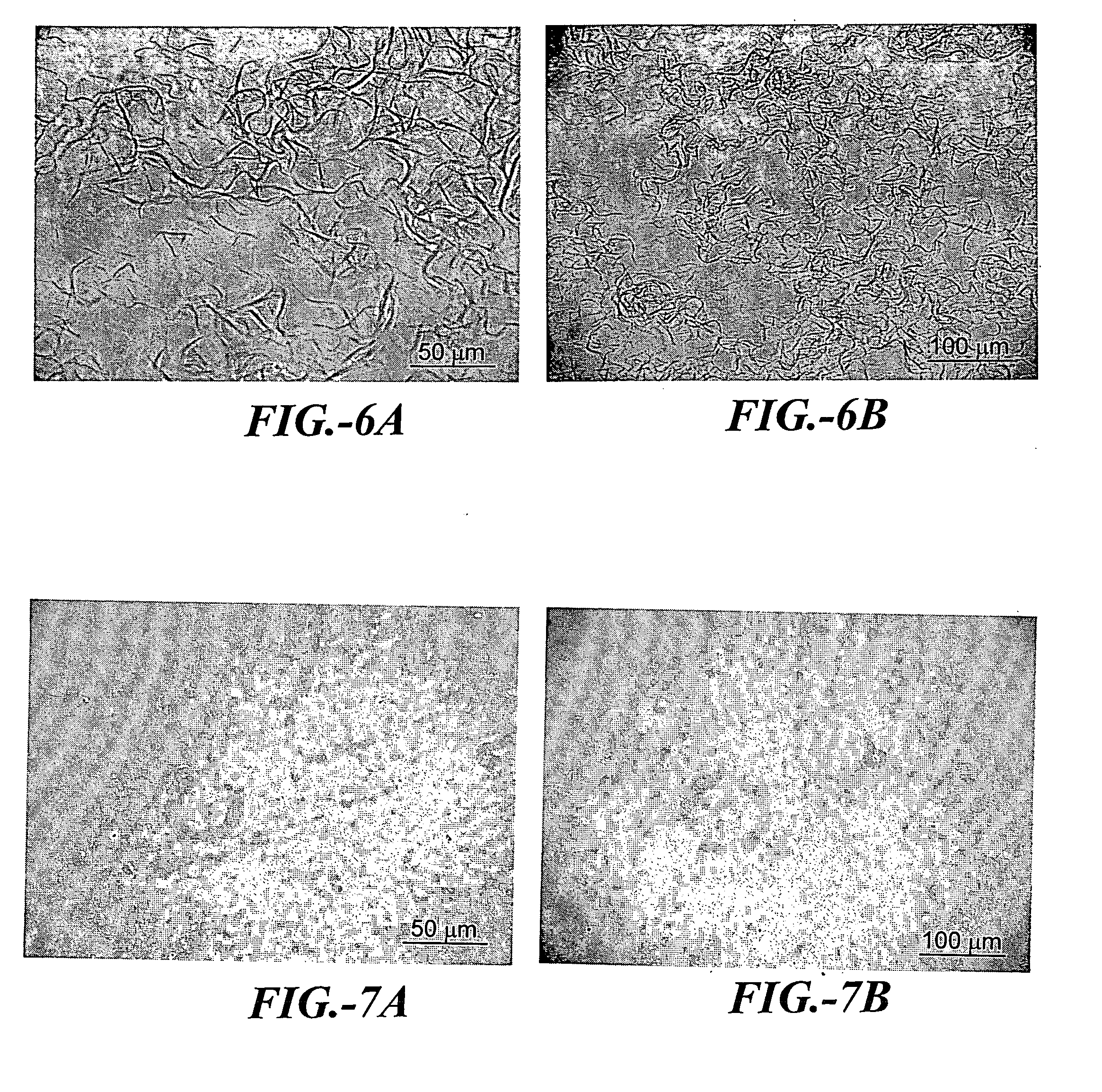
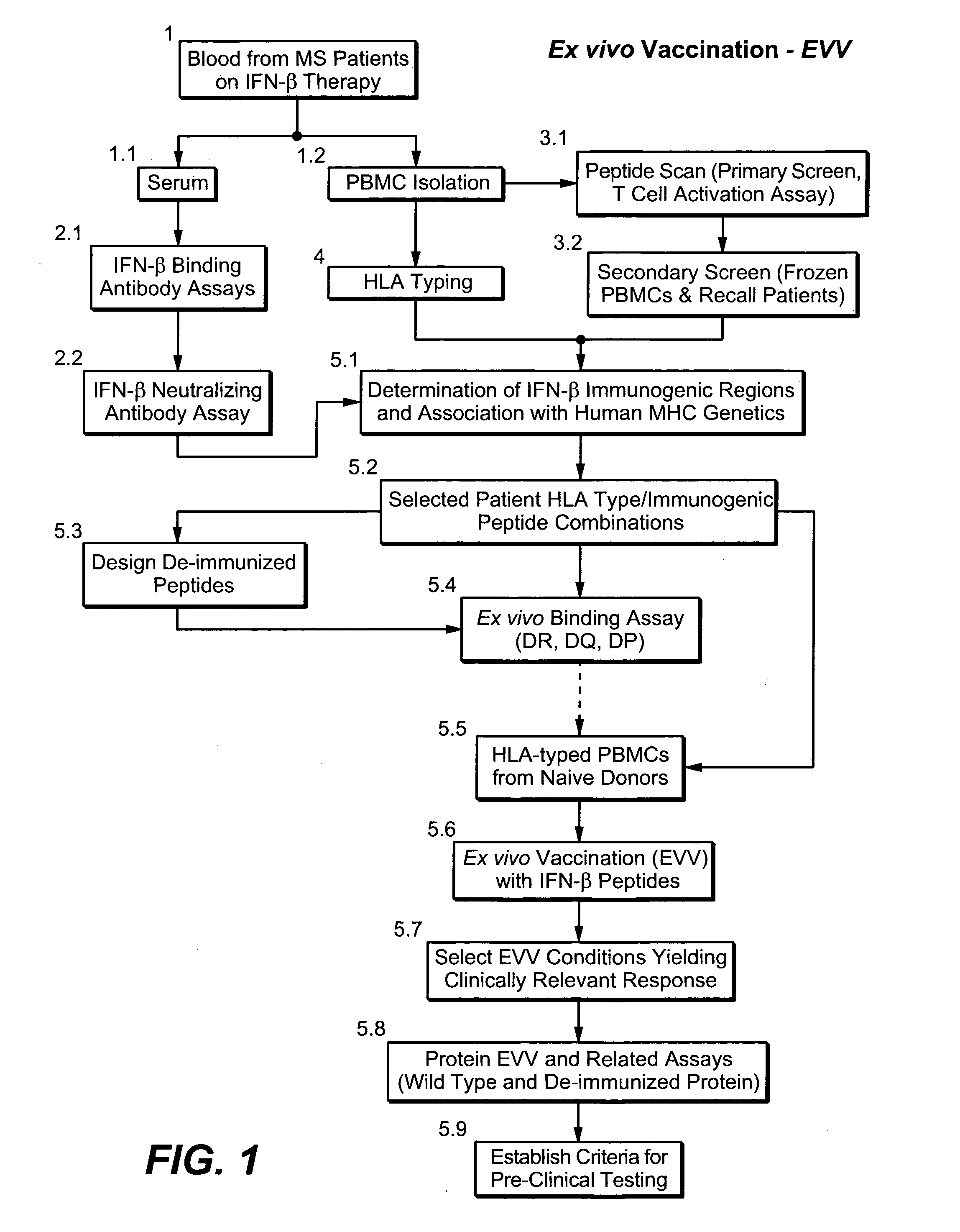
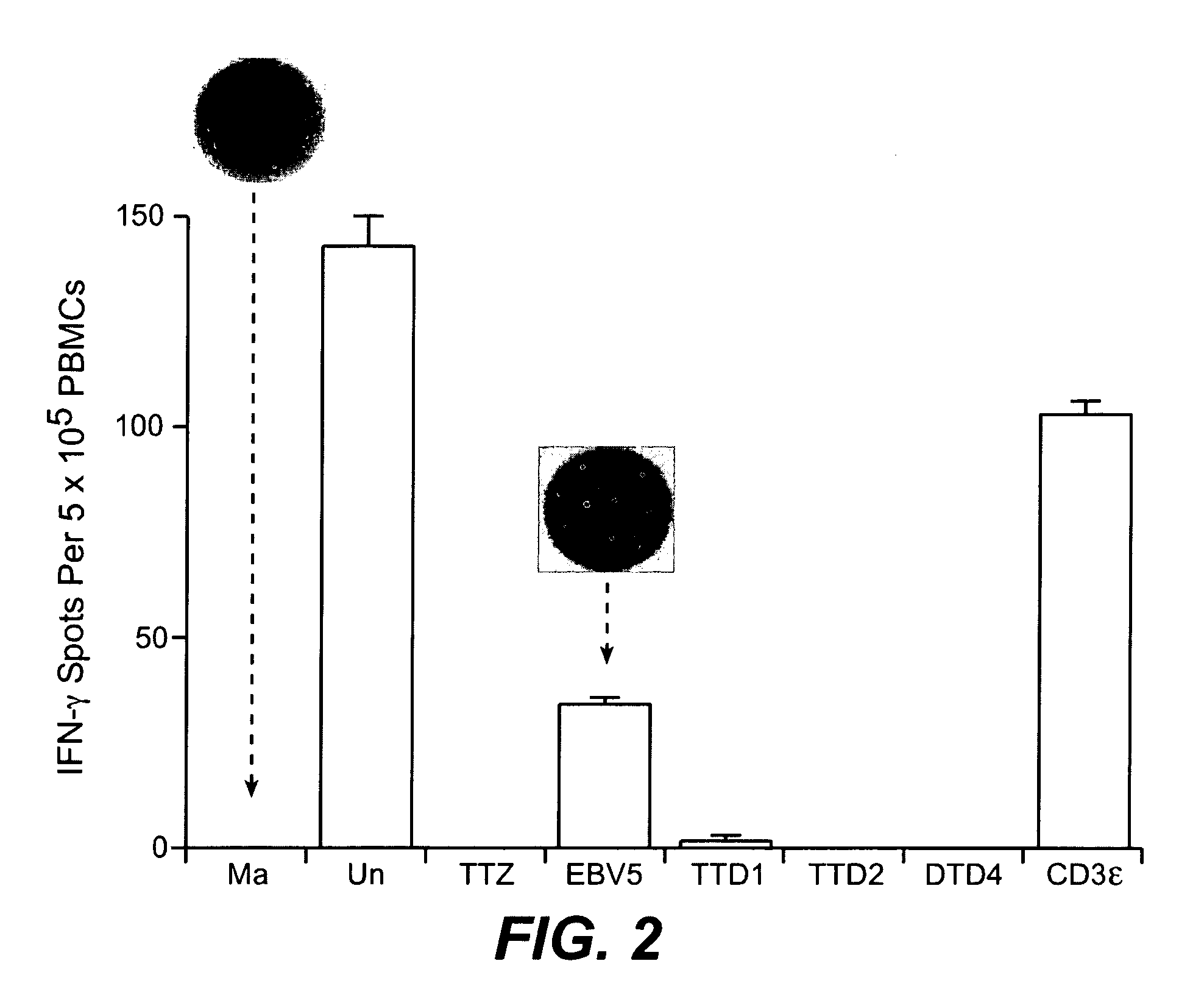
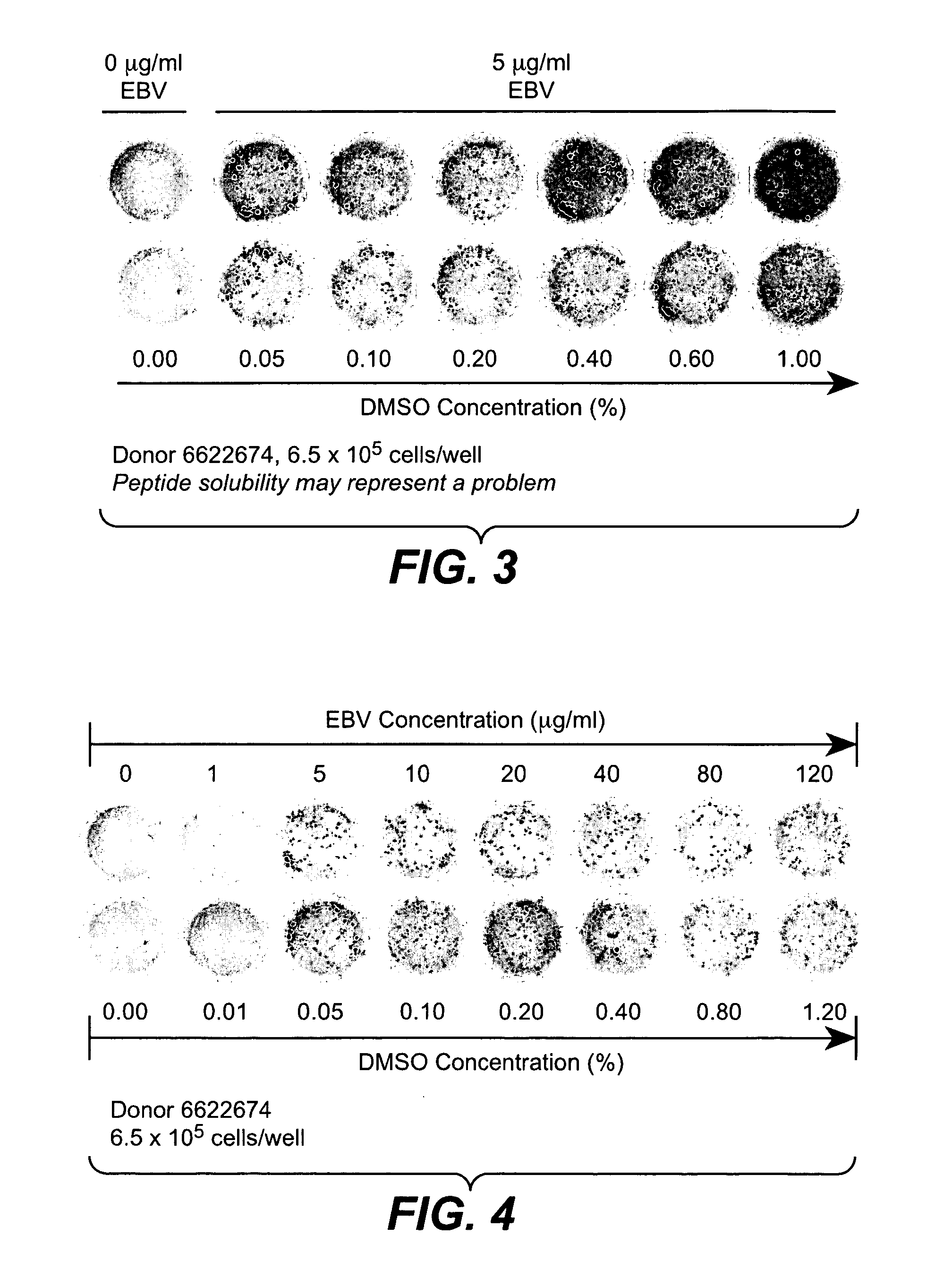
Prediction and assessment of immunogenicity
InactiveUS20060148009A1Low immunogenicityBiological material analysisVaccine efficacyImmunocompatibility
A system and method to predict and assess immunogenicity, especially prior to on-set of immunogenic conditions. Also disclosed are methods to identify relevant peptides associated with the formation of antibodies in patients treated with a given protein therapeutic. In various aspects, the present application is directed to methods of determining the immunological compatibility of a subject with a therapeutic agent such as a proteinaceous therapeutic agent, methods of determining vaccine efficacy by determining the immunological compatibility of a subject with a therapeutic agent, and selecting a therapeutic agent for a subject in need of treatment. Methods of designing a therapeutic agent with reduced immunogenicity for a subject and methods for designing vaccines with enhanced immunogenicity for a subject are also contemplated.
Owner:XENCOR
Biodegradable polymer vesicles and preparation and application thereof
InactiveCN101792516AEfficient packagingEliminate drug resistancePharmaceutical non-active ingredientsPolymer sciencePolyethylene glycol
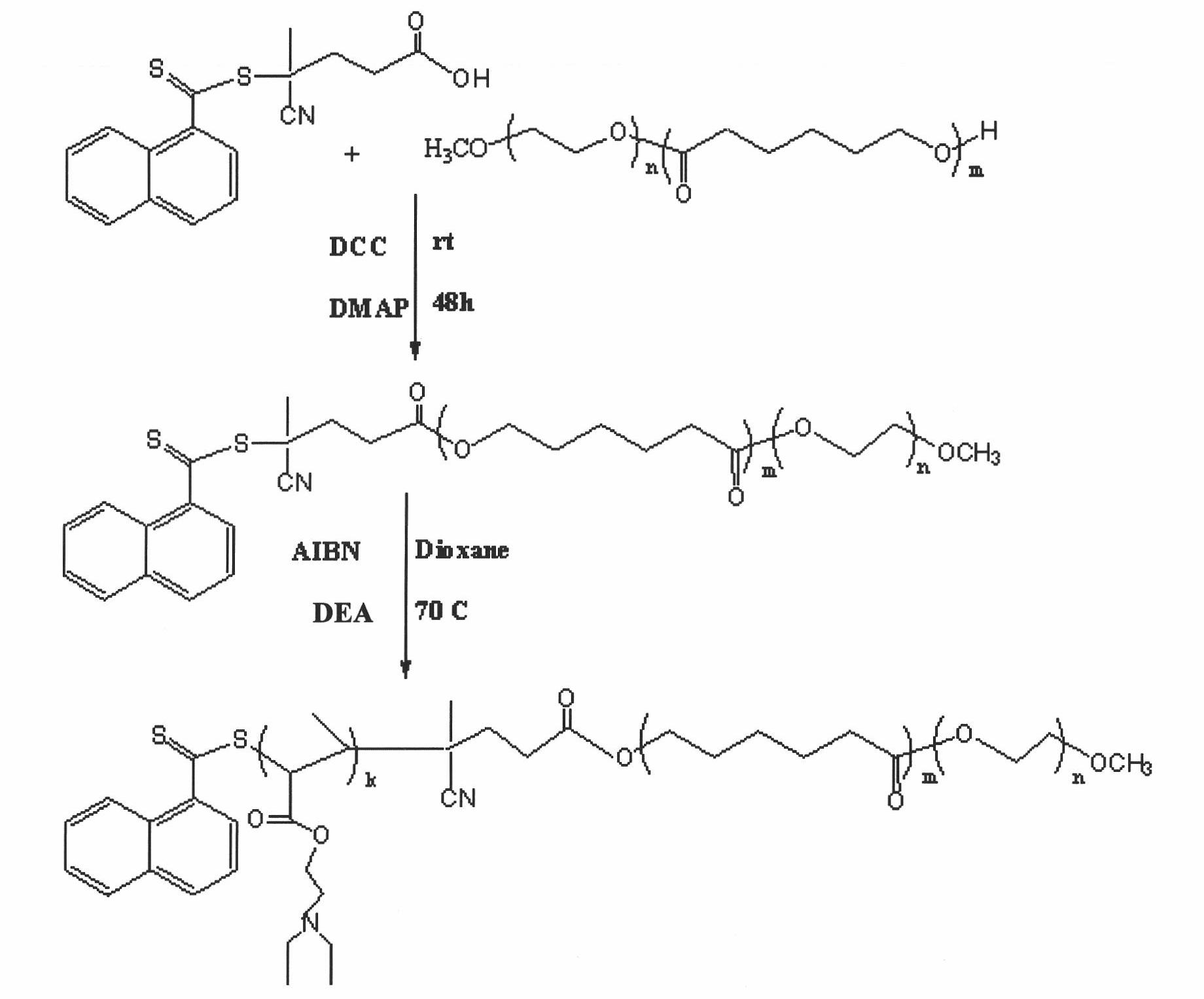
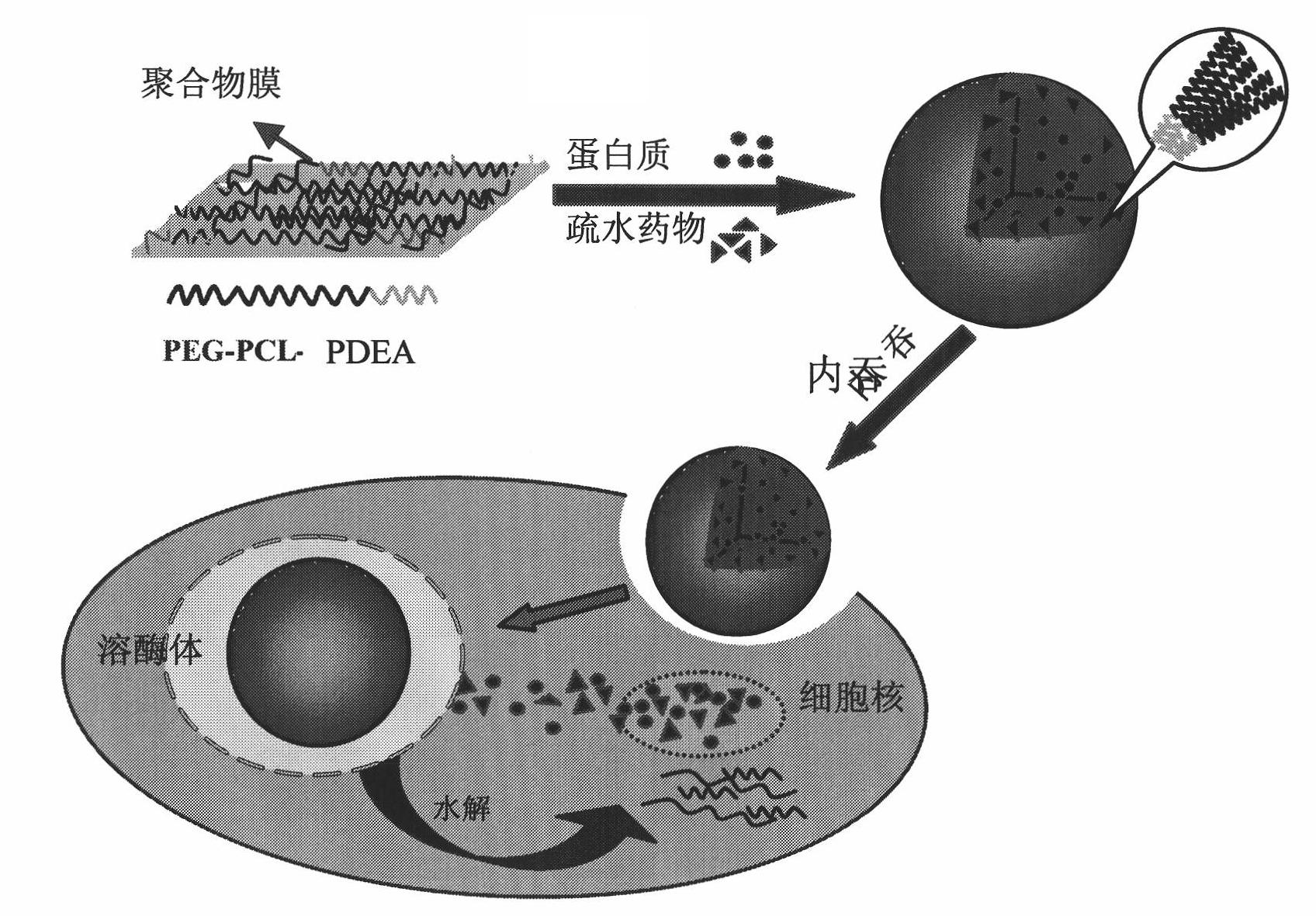
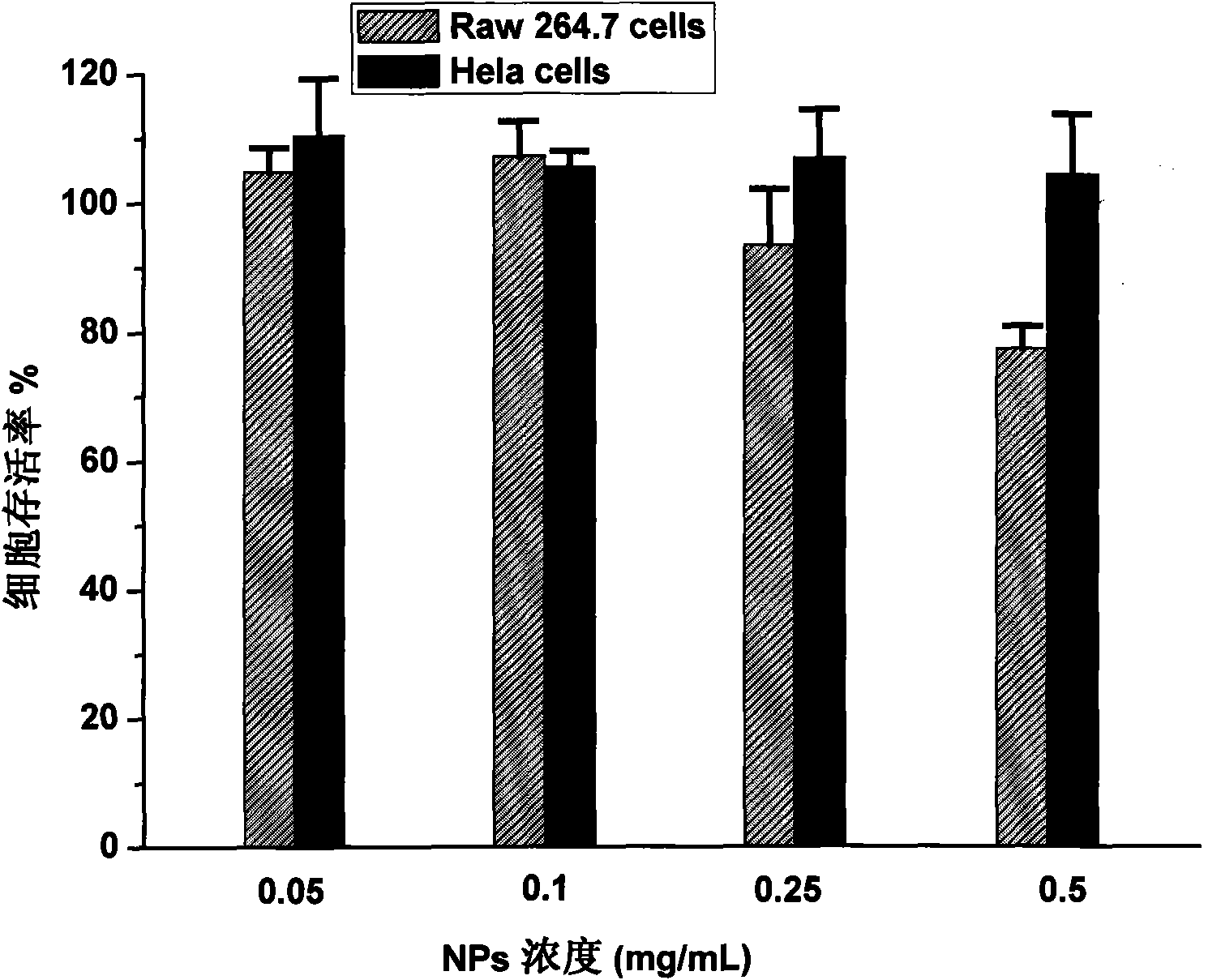
The invention relates to a medicament carrier and a preparation method thereof, in particular to a medicament delivery system comprising biodegradable polymer vesicles with asymmetric membranes. The invention discloses the biodegradable polymer vesicles and preparation and application thereof. The biodegradable polymer vesicles are prepared from A-B-C type block polymer, wherein a block A is polyethylene glycol (PEG) distributed on outer surfaces of the vesicles; a block B is hydrophobic biodegradable polymer to form the nucleuses of the vesicles; and a block C is polyelectrolyte distributed on inner walls of the vesicular membranes and used for efficiently loading medicaments with opposite electric charge. The biodegradable polymer vesicles are formed in aqueous solution directly, can efficiently load protein and polypeptide medicaments, nucleic acid medicaments and micro-molecular medicaments, and are expected to be applied to the protein therapy and the combination therapy of cancers.
Owner:SUZHOU UNIV
Method and apparatus to measure particle mobility in solution
ActiveUS20110210002A1Reduce harmMinimizing electrochemical degradationSludge treatmentVolume/mass flow measurementPhotodetectorFree solution
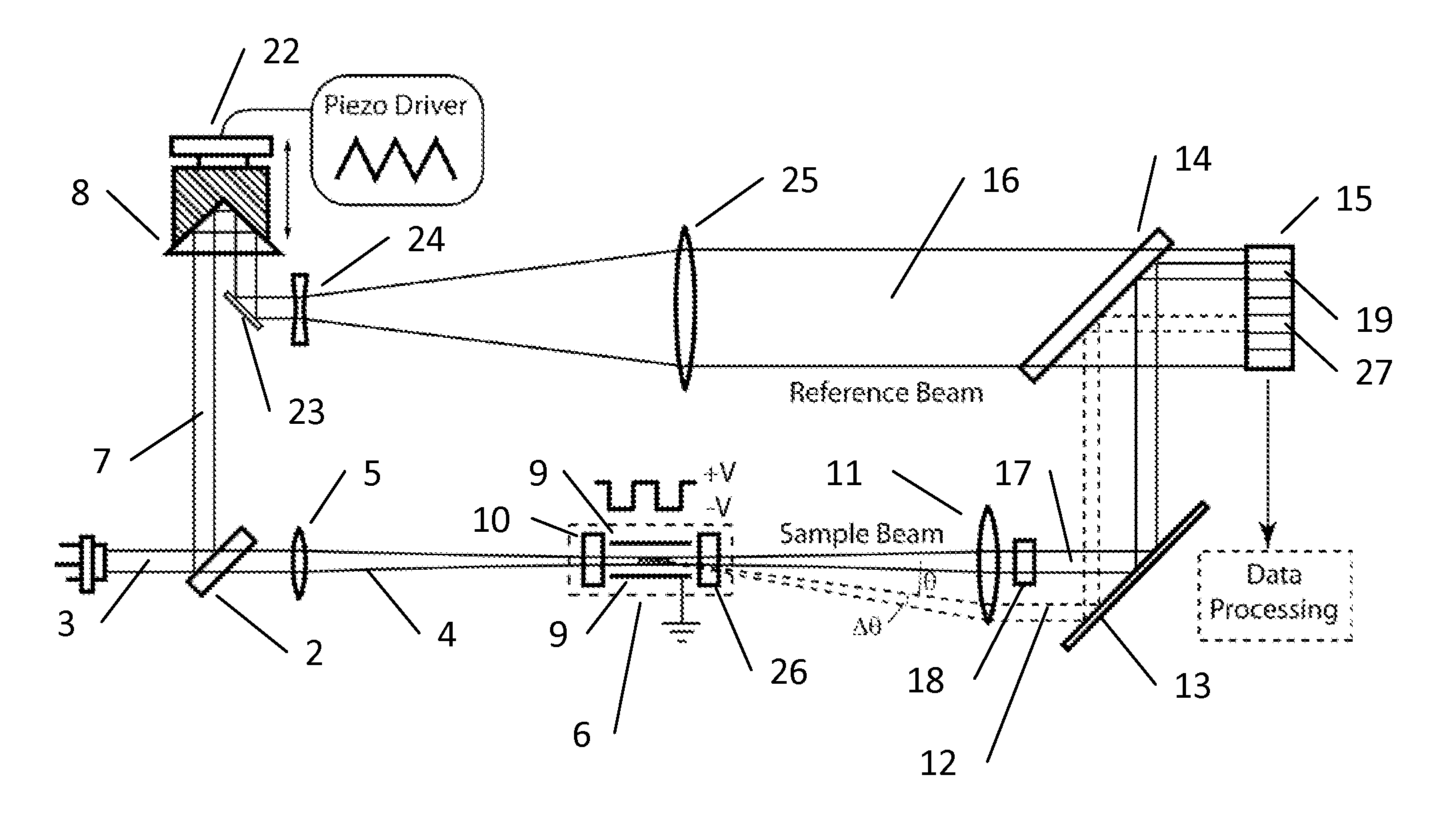
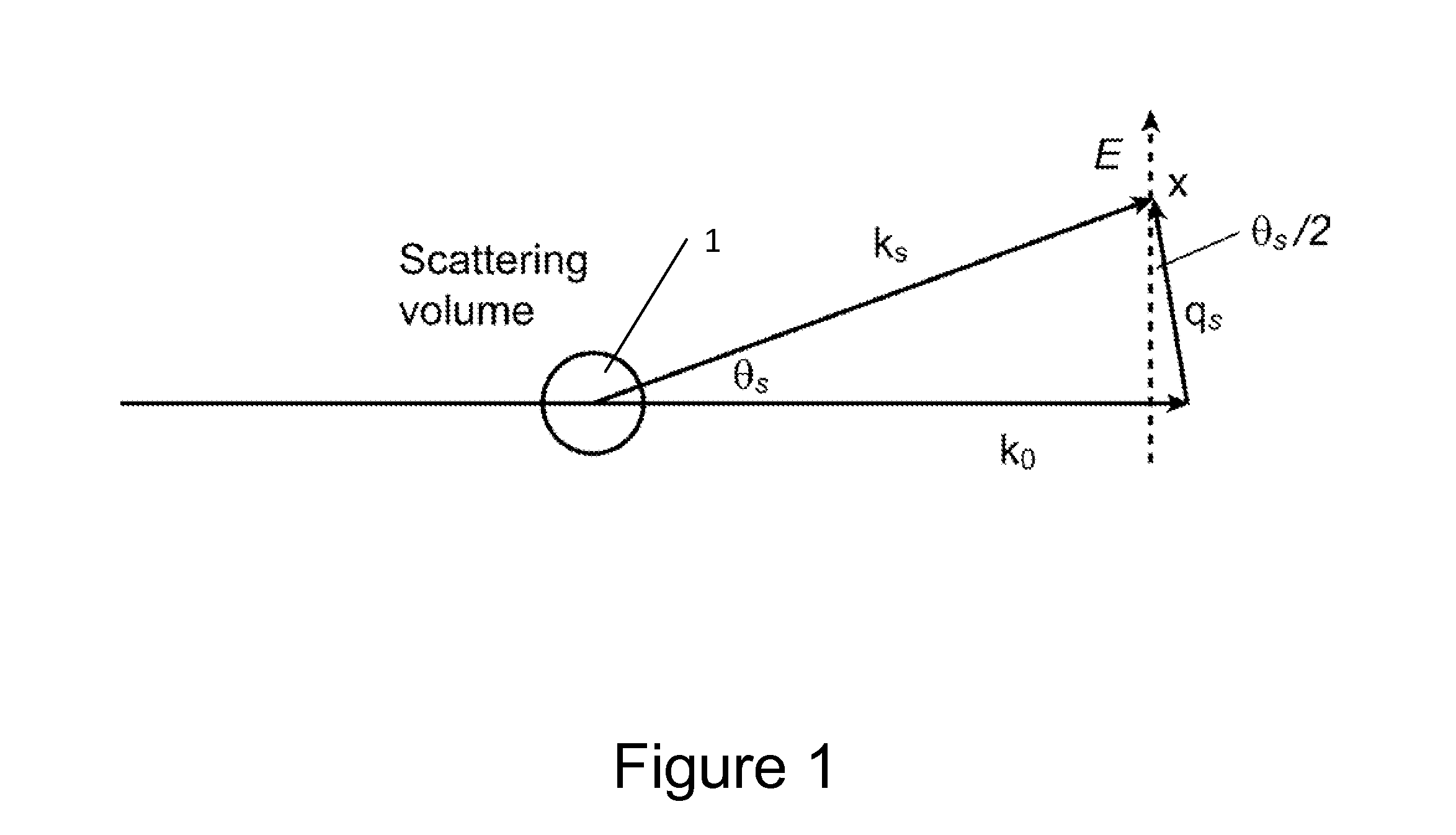
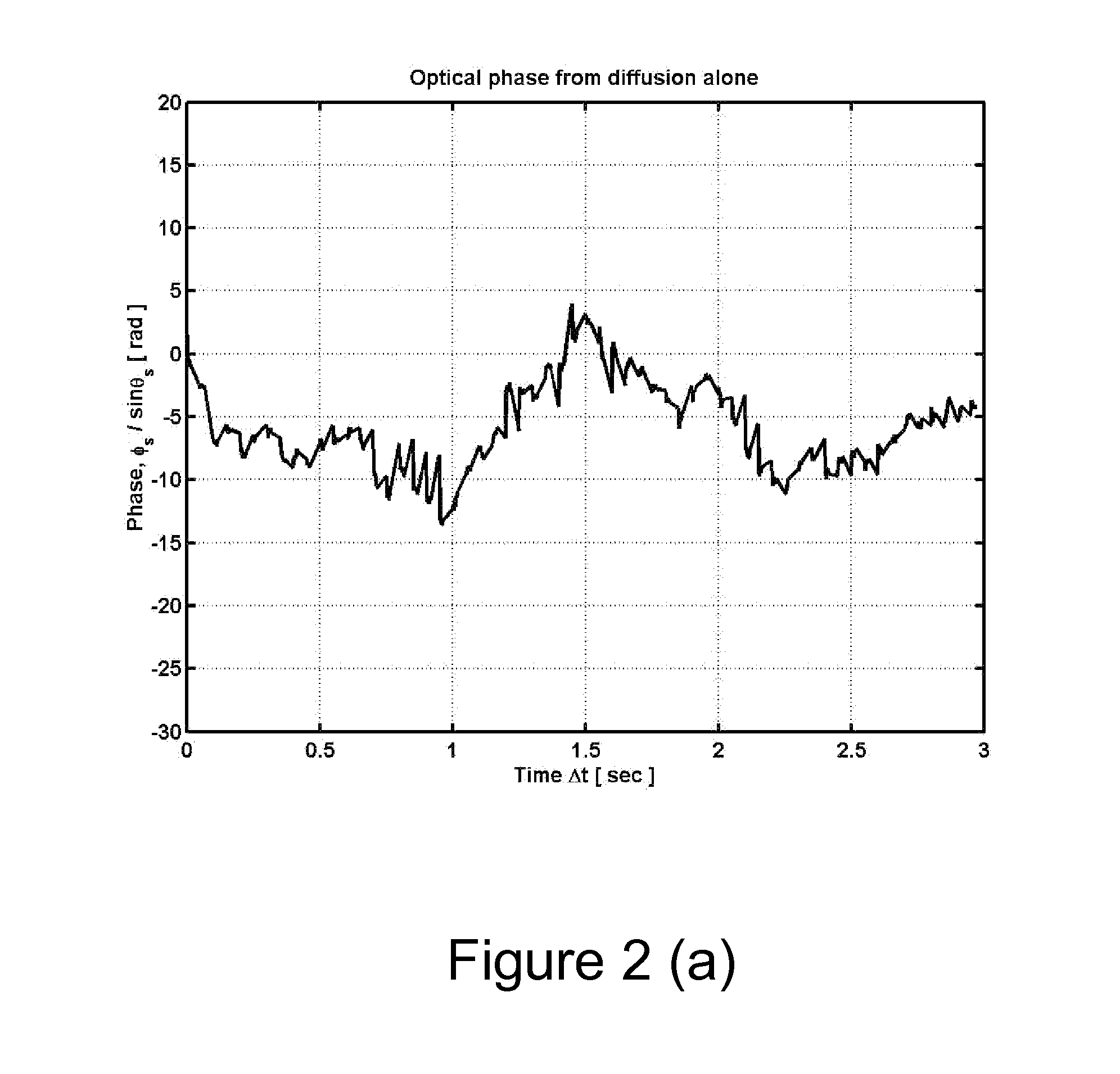
A method and apparatus is disclosed for measurement of the electrophoretic mobility of particles and molecules in solution. A sample of particles is placed in a cell containing two electrodes that apply an alternating electric field. A monochromatic light beam passes through the sample. Light scattered by the particles, along with the unscattered beam, is collected and collimated as it exits the cell. This beam is combined in free space with a phase modulated reference beam. The interference forms a frequency modulated speckle pattern, which is detected by a photodetector array. Each array element collects a narrow range of well-defined scattering angles. The signal from each is demodulated to extract the optical phase information providing a first-principle measurement of the electrophoretic mobility of the scattering particles. Each detector element provides a simultaneous independent measurement. This inherent parallelism drastically increases the amount of information available in a given time. The resulting increased sensitivity extends the mobility measurement to particles below one nanometer, reduces the required concentration and electric field compared to previous methods. This minimizes damage to fragile samples, increases the electrode useful life, and reduces joule heating. Electrophoretic mobility is a critically important parameter for predicting the stability of nanoparticle suspensions and pharmaceutical formulations such as protein therapeutics. This invention enables reliable free-solution phase measurement of these samples.
Owner:WYATT TECH
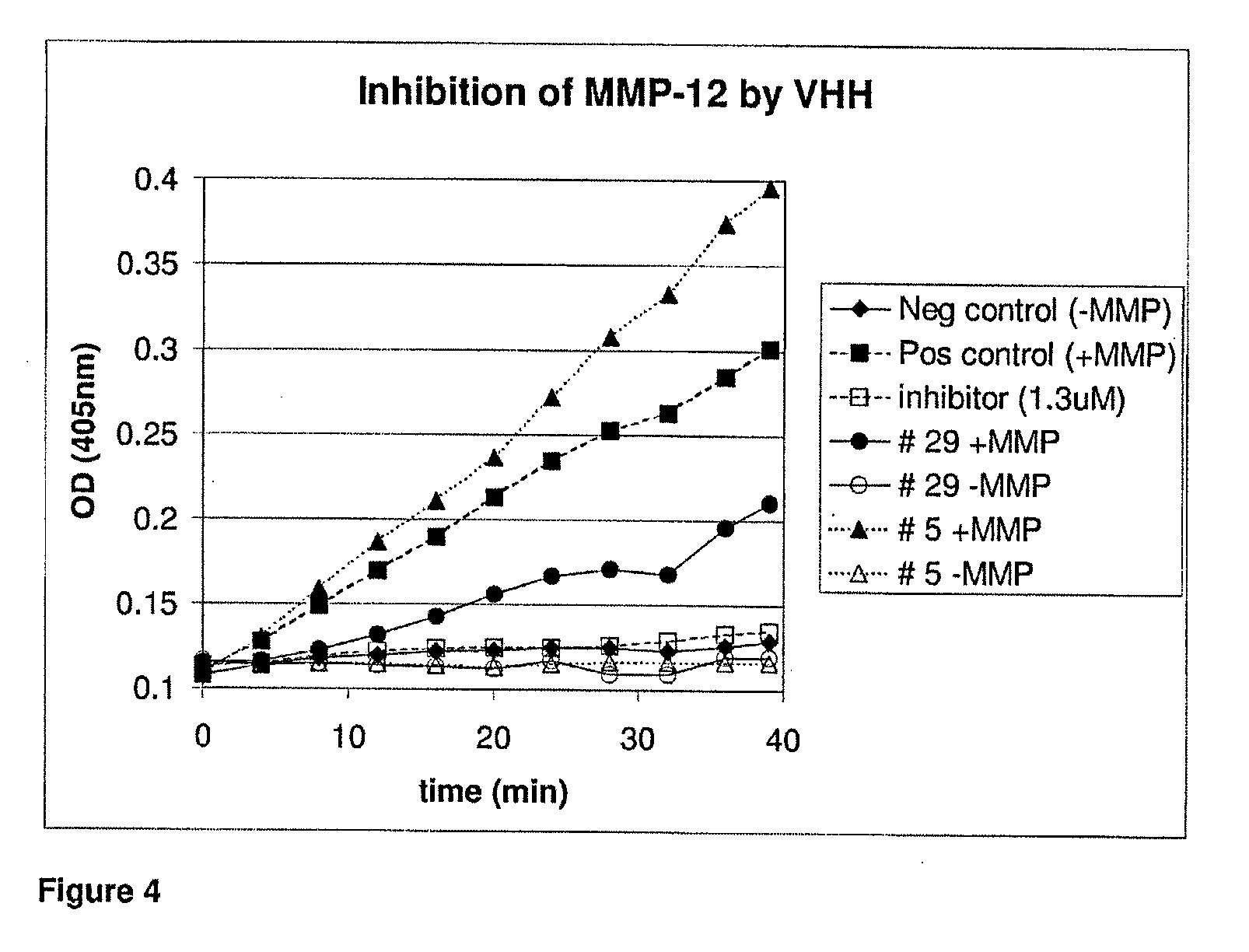
Pulmonary administration of immunoglobulin single variable domains and constructs thereof
ActiveUS20130136744A1Overcomes shortcomingIncrease exposure timeImmunoglobulins against blood coagulation factorsAntibody mimetics/scaffoldsInhalationWhole body
In one aspect, the invention relates to a method suitable for administering protein therapeutic molecules orally, sublingually, topically, intravenously, subcutaneously, nasally, vaginally, rectally or by inhalation so as to avoid inactivation, by using VHH polypeptides derived from Camelidae antibodies. The invention further relates to the said therapeutic molecules. The invention further a method for delivering therapeutic molecules to the interior of cells. The invention further relates to anti-IgE therapeutic molecules.In one aspect, the present invention relates to a method wherein an immunoglobulin single variable domain (such as a Nanobody) and / or construct thereof are absorbed in pulmonary tissue. More particularly, the invention provides systemic delivery of an immunoglobulin single variable domain and / or construct thereof via the pulmonary route.
Owner:ABLYNX NV
Integrated approach for generating multidomain protein therapeutics
InactiveUS20100105873A1Improves overall drug development efficiencyGood curative effectLibrary screeningAntibody ingredientsThermal denaturationTherapeutic protein
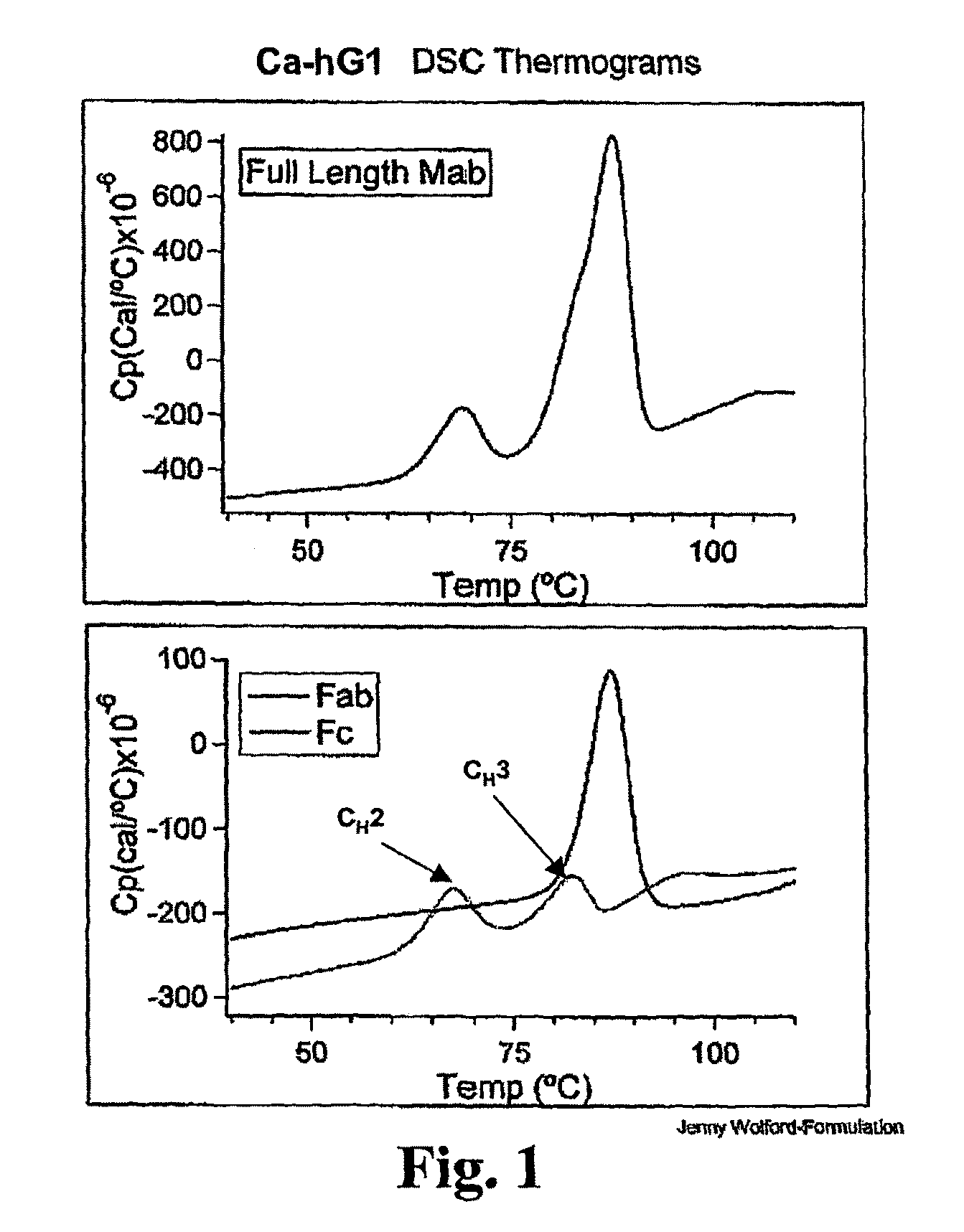
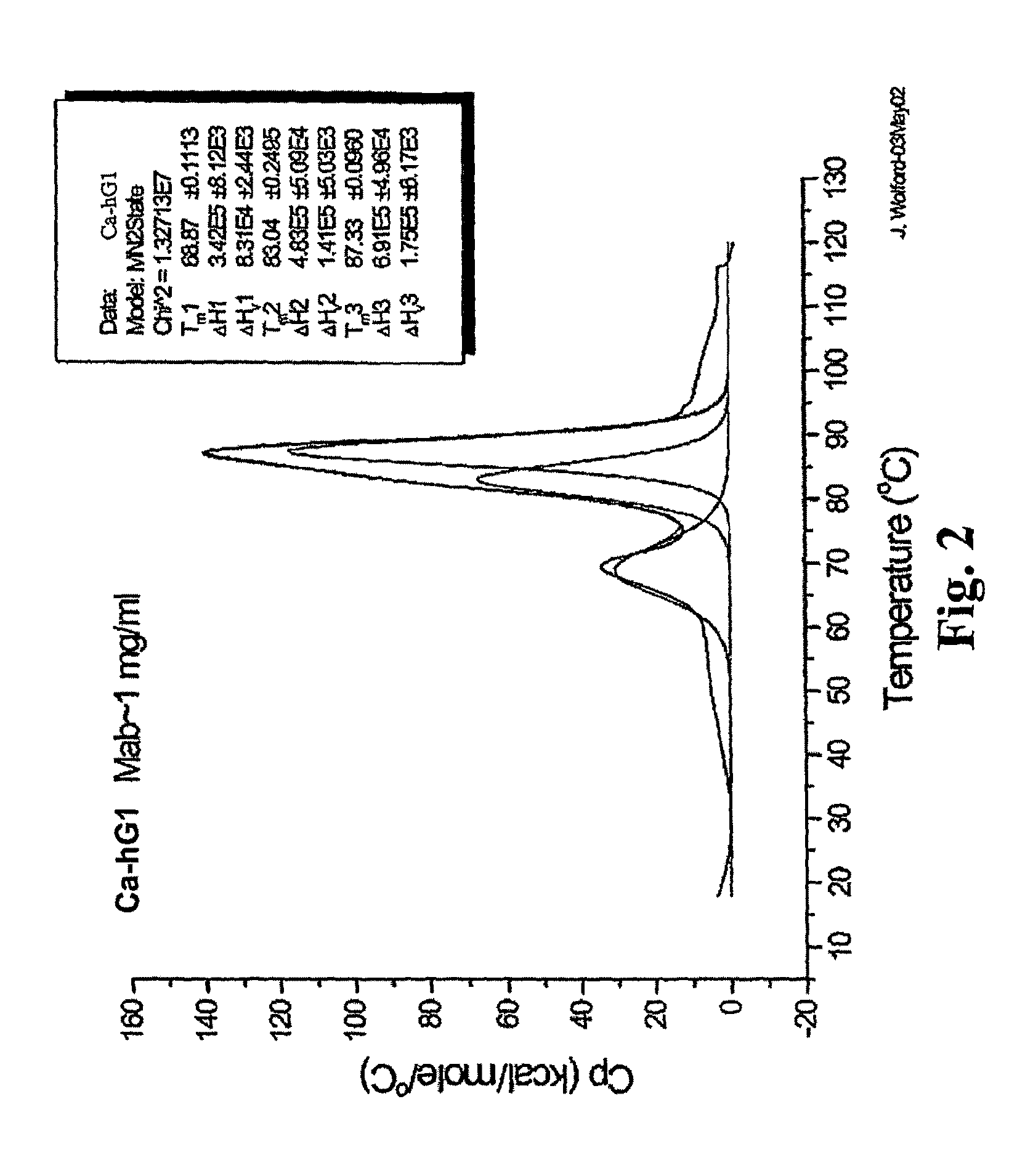
The invention provides method for therapeutic protein drug development that incorporates therapeutic and / or formulation and / or manufacturing considerations in the early screening process. The approach involves screening a plurality of different variants of a domain that have been determined to have the desired therapeutic property to identify one or more variants that have desired therapeutic and / or formulation characteristics, and constructing the full multidomain proteins using the identified domain variants. The present invention also provides a method for determining the shelf life of multidomain proteins in formulations. The method comprises determining a thermal denaturation and / or renaturation curve of a domain of the protein whose unfolding leads to aggregation of the protein in a solution. The method evaluates the shelf life of the multidomain protein based on the denaturation / renaturation curve. The invention also provides methods for engineering multidomain proteins to improve their therapeutic and / or formulation characteristics.
Owner:MEDIMMUNE LLC
Synthetic hyperglycosylated, and hyperglycosylated protease-resistant polypeptide variants, oral formulations and methods of using the same
ActiveUS20060204473A1Increased protease resistanceNervous disorderPeptide/protein ingredientsHybrid typeInterferon receptor
The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites. The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites, as well as erythropoietin and darbepoetin alfa, each of which are linked to a penetrating peptide that facilitates translocation of a substance across a biological barrier as well as pharmaceutical compositions, including oral formulations, of the same. The present invention further provides oral formulations of hyperglycosylated or protease-resistant, hyperglycosylated polypeptide variants, which polypeptide variants lack at least one protease cleavage site found in a parent polypeptide, and thus exhibit increased protease resistance compared to the parent polypeptide, which polypeptide variants further include (1) a carbohydrate moiety covalently linked to at least one non-native glycosylation site not found in the parent protein therapeutic or (2) a carbohydrate moiety covalently linked to at least one native glycosylation site found but not glycosylated in the parent protein therapeutic. The present invention further provides compositions, including oral pharmaceutical compositions, comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides containers, devices, and kits comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides therapeutic methods involving administering an effective amount of an oral pharmaceutical composition comprising a synthetic Type I interferon receptor polypeptide agonist, a hyperglycosylated polypeptide variant, or a hyperglycosylated, protease-resistant polypeptide variant to an individual in need thereof.
Owner:ALIOS BIOPHARMA INC +1
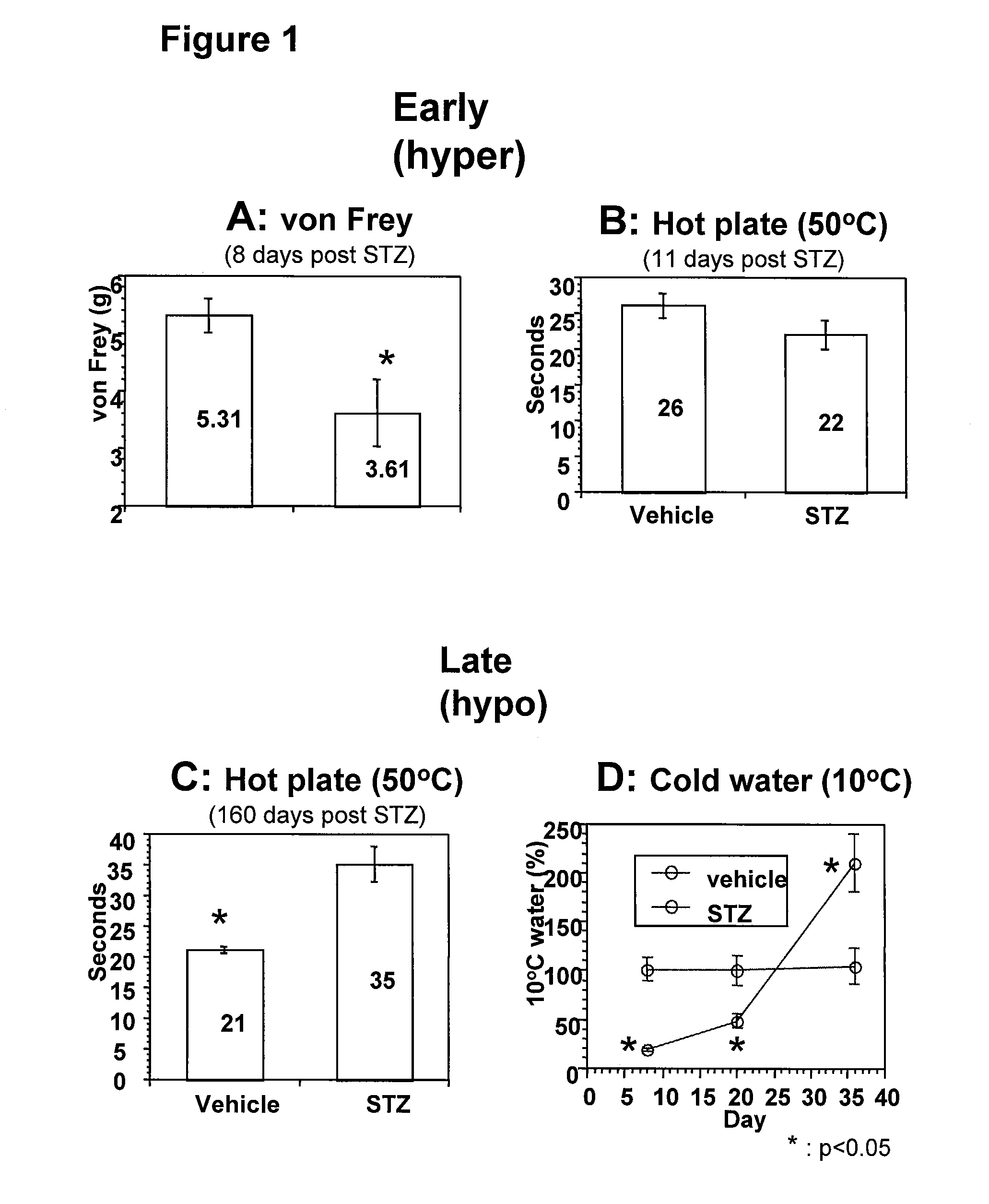
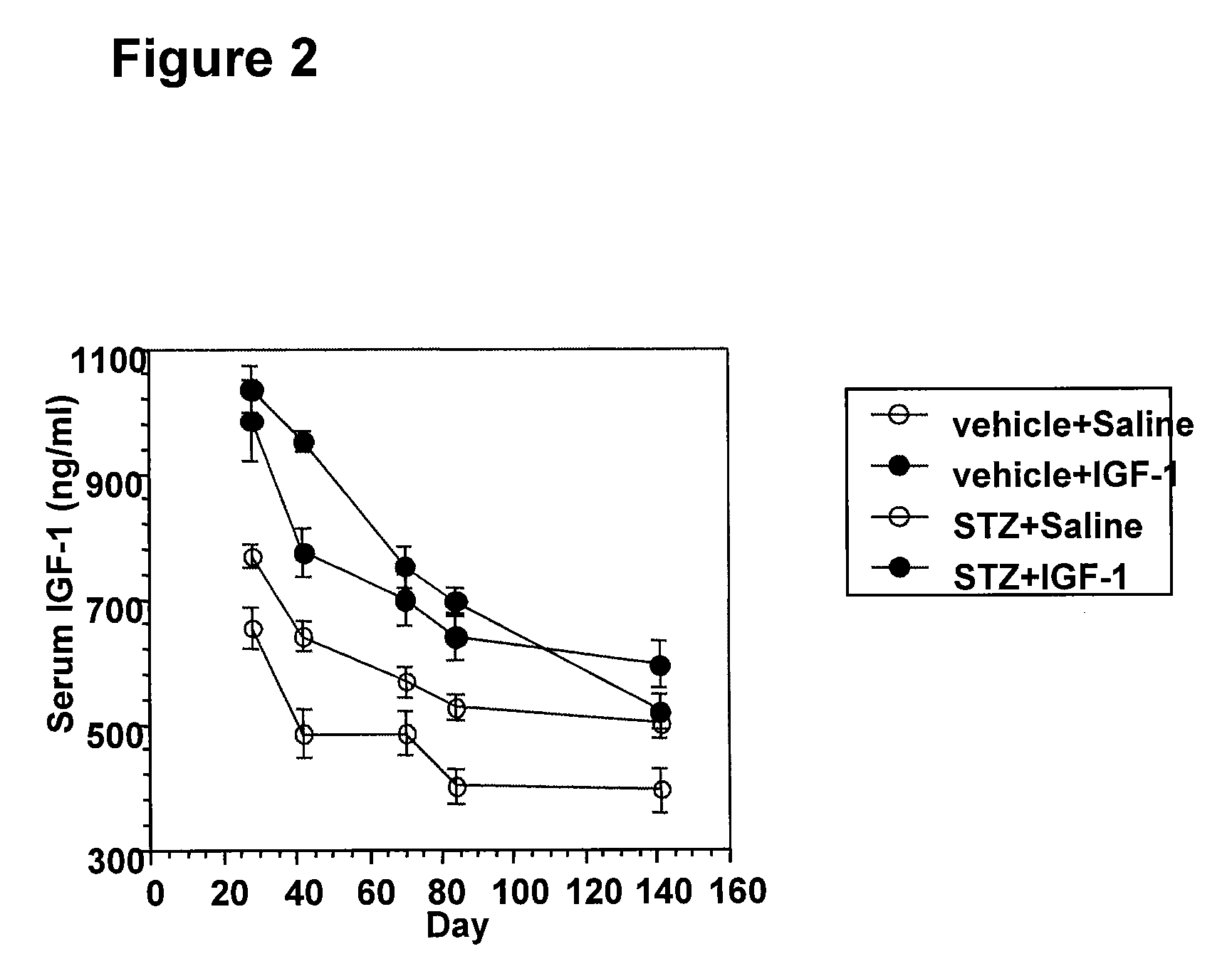
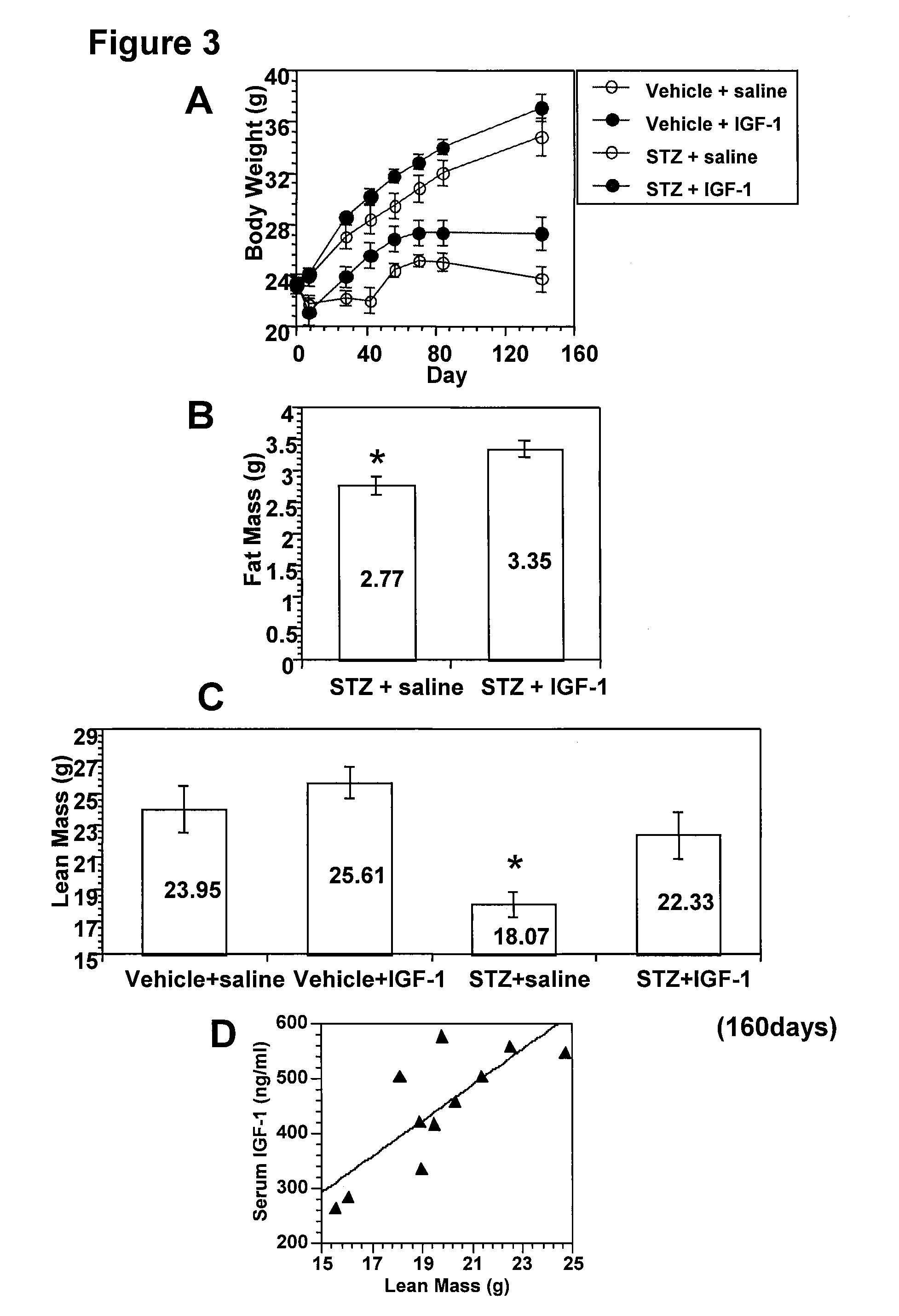
Systemic insulin-like growth factor-1 therapy reduces diabetic peripheral neuropathy and improves renal function in diabetic nephropathy
InactiveUS20100216709A1Prevents subsequent hyposensitivityEasy maintenanceOrganic active ingredientsNervous disorderInsulin-like growth factorHyperglycemic disorder
The present invention provides methods of treatment of patients suffering from the complications of blood sugar disorders: diabetic peripheral neuropathy and diabetic nephropathy by administration of IGF-1 via protein therapy or gene therapy. It relates to methods of treating an individual having a diabetic disorder or a hyperglycemic disorder, comprising administering to the individual an effective amount of a DNA vector expressing IGF-1Eb or IGF-1Ec in vivo or an effective amount of at the IGF-1Eb or IGF-1Ec protein in the early hyperalgesia stage or in patients that have advanced to the hyposensitivity stage. Treatment at the early hyperalgesia stage prevents subsequent hyposensitivity with increases or maintenance of sensory nerve function. IGF-1Eb or IGF-1Ec treatment also increases muscle mass and improves overall mobility, which indicates a treatment-related improvement in motor function. Treatment with IGF-1Eb or IGF-1Ec at the hyposensitivity stage reverses hyposensitivity and improves muscle mass and overall health. Systemic IGF-1 provides a therapeutic modality for treating hyposensitivity associated with DPN. In addition, IGF-1Eb or IGF-1Ec provides a therapeutic modality for treating diabetic nephropathy. IGF-1Eb or IGF-1Ec improves renal function as evidenced by a modulation in serum albumin concentration and a reduction in urine volume and protein levels. IGF-1Eb or IGF-1Ec also reduces diabetic glomerulosclerosis.
Owner:GENZYME CORP
Polypeptide constructs for nasal administration
InactiveUS20090324512A1Reduce deliveryReadily assess potencyAntibacterial agentsPowder deliveryNasal cavityNose
The invention relates to a method suitable for administering protein therapeutic molecules orally, sublingually, topically, intravenously, subcutaneously, nasally, vaginally, rectally or by inhalation so as to avoid inactivation, by using VHH polypeptides derived from Camelidae antibodies. The invention further relates to the said therapeutic molecules. The invention further a method for delivering therapeutic molecules to the interior of cells. The invention further relates to anti-IgE therapeutic molecules.
Owner:ABLYNX NV
Gi Track Delivery Systems
ActiveUS20070218125A1Effective matrixEasy to adaptSugar food ingredientsMetabolism disorderLipid formationAdditive ingredient
A micro encapsulation material for use with storage unstable, therapeutic and nutritional agents which release the therapeutic and nutritional agents in predetermined locations in the gastro intestinal tract in which the microencapsulation material is formed by combining a food grade treated carbohydrate with a water soluble food grade protein. The therapeutic and nutritional agents form an oil phase which is emulsified with the water dispersed or dissolved encapsulant to encapsulate the therapeutic and nutritional agents. These agents may be oils or oil soluble or oil dispersible. The agents that may be encapsulated Include lipids (oils Including oxygen sensitive oils, fatty acids, triglycerides) and oil soluble and oil dispersible ingredients (including pharmaceuticals, probiotics, protein therapeutics and bioactives). The protein used may include any film forming water soluble protein or hydrolysed protein and includes milk proteins such as casein and its derivatives or whey proteins. The carbohydrate component may be those containing reducing sugar groups, oligosaccharides and starches (raw, modified, resistant, acetylated, proprionated and butyrated starches).
Owner:COMMONWEALTH SCI & IND RES ORG
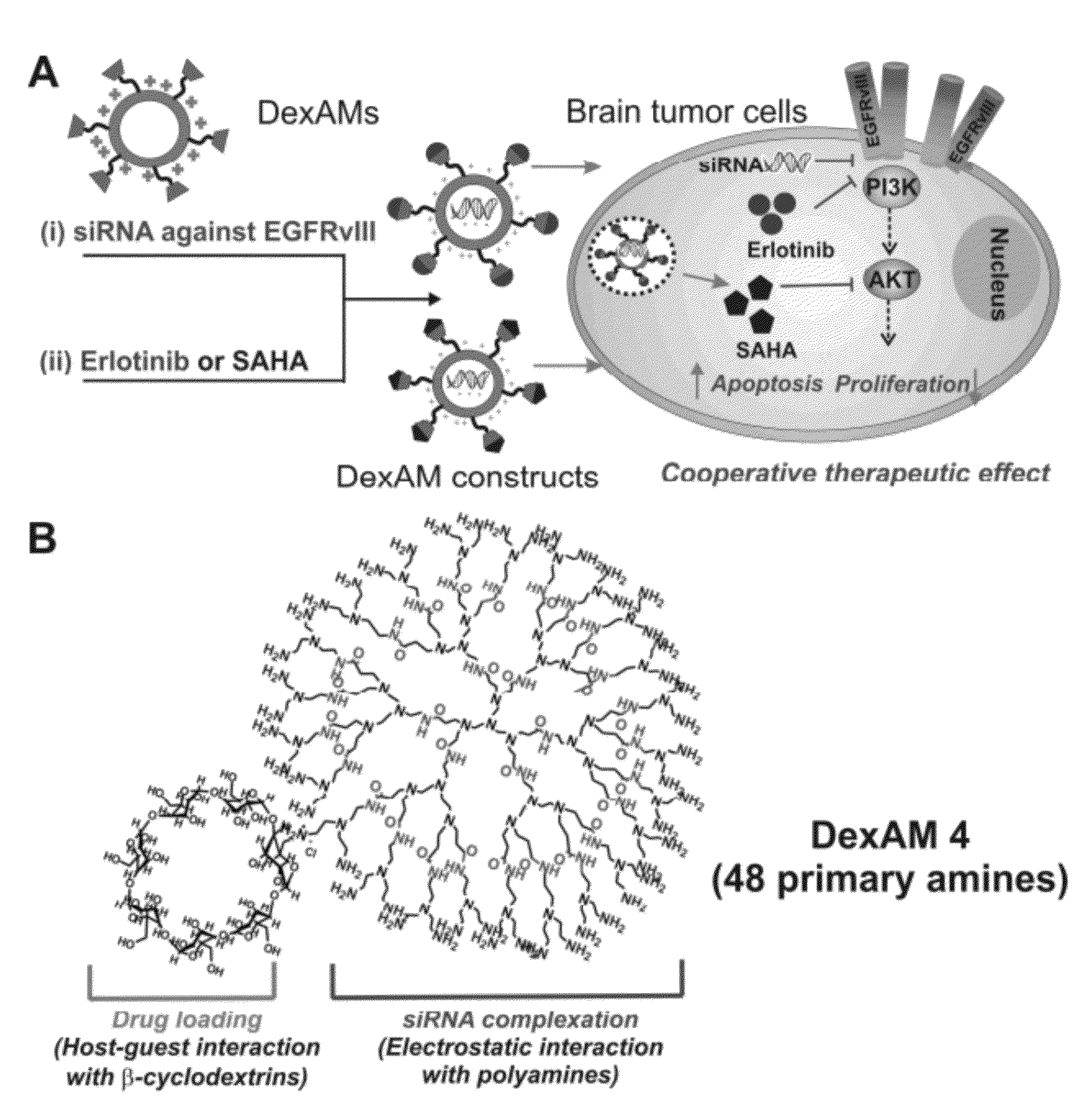
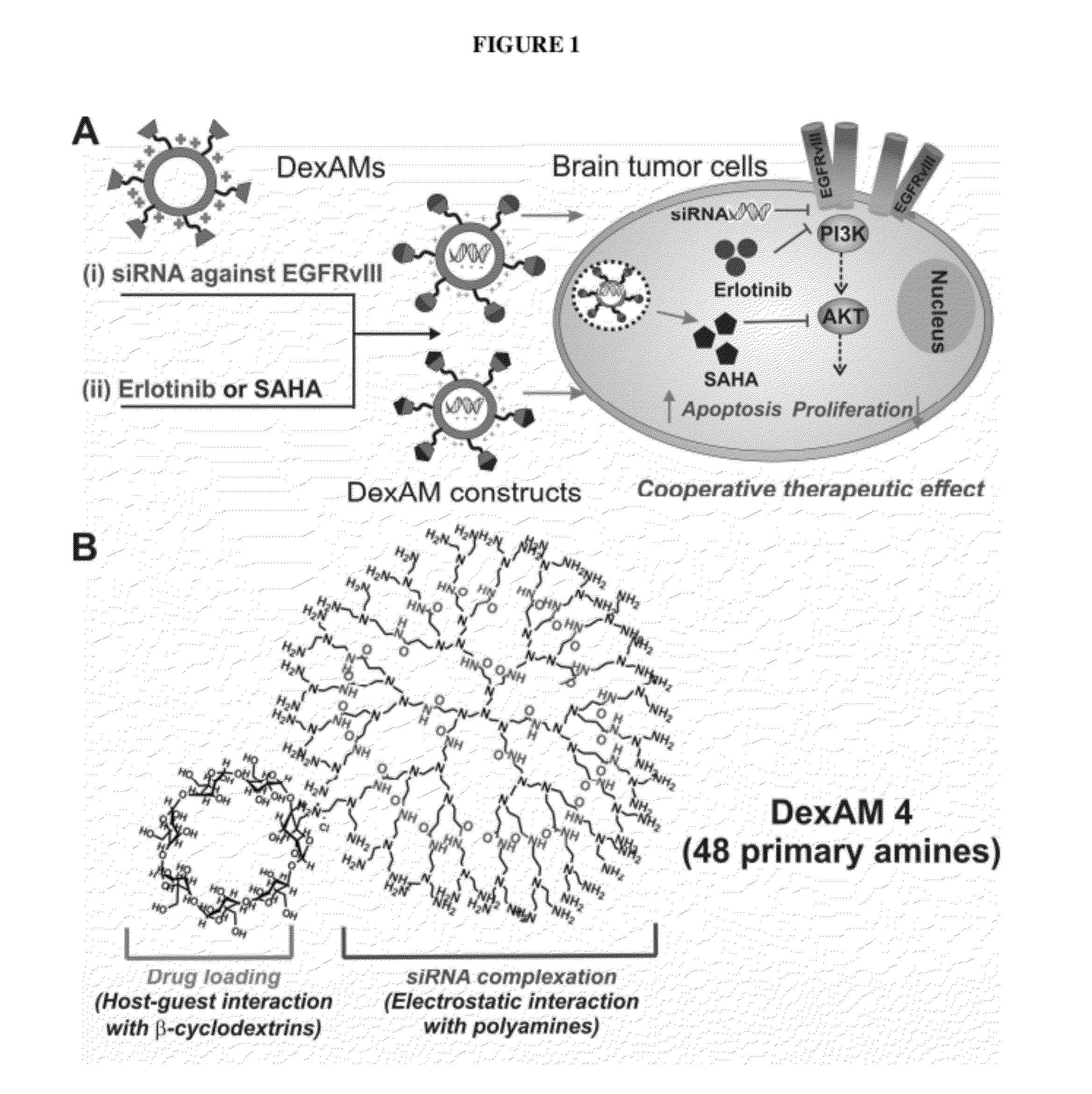
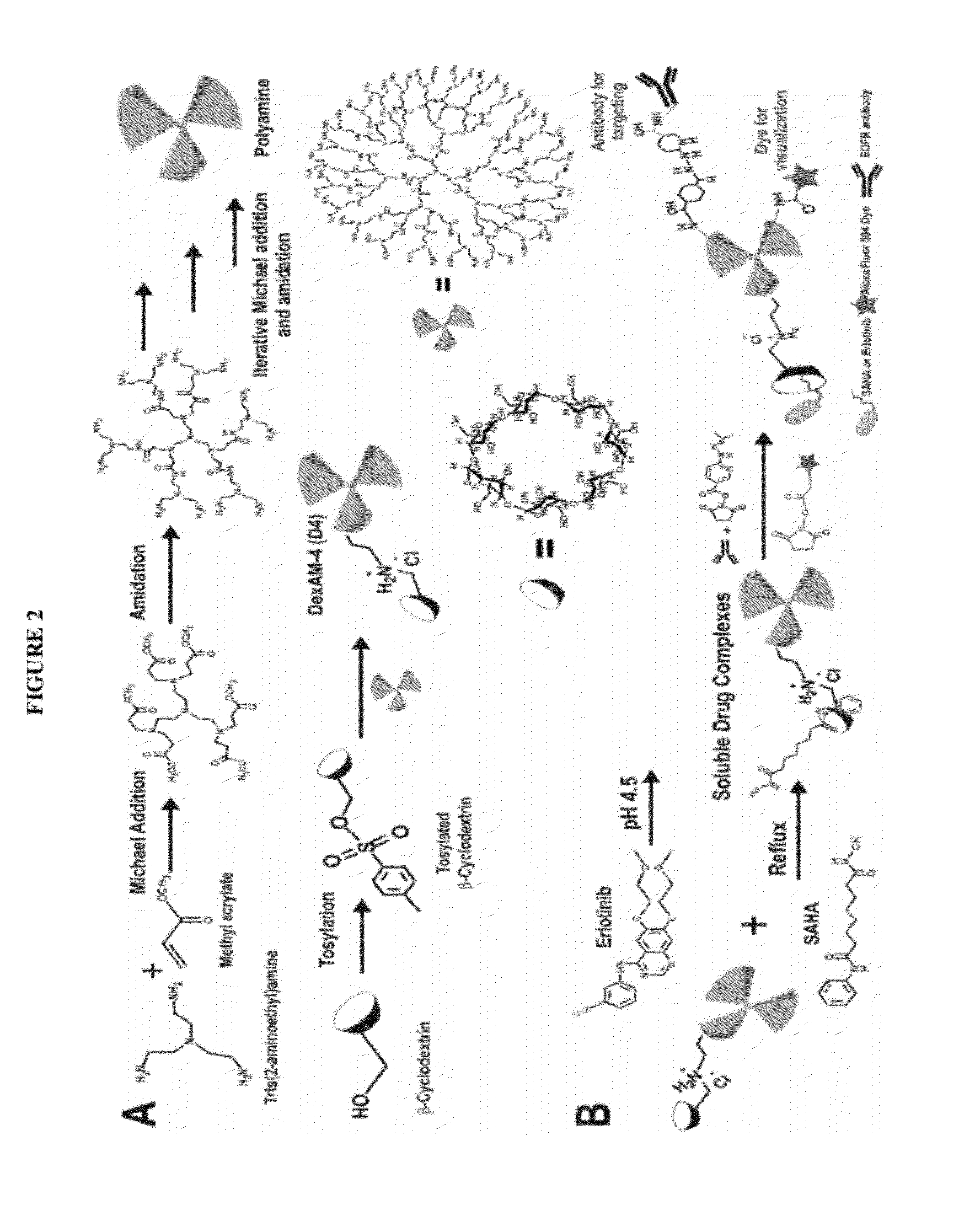
Cyclodextrin-modified polyamines for delivery of therapeutic molecules
ActiveUS20120115962A1Efficient translocationPharmaceutical non-active ingredientsTumor/cancer cellsDelivery vehicleCyclodextrin
The present invention is directed to drug delivery vehicles comprising one or more cyclodextrin moieties conjugated to a dendritic polyamine for the delivery of small molecule and protein therapeutic molecules and nucleic acid therapeutic molecules, and methods of making and using the delivery vehicles.
Owner:RUTGERS THE STATE UNIV
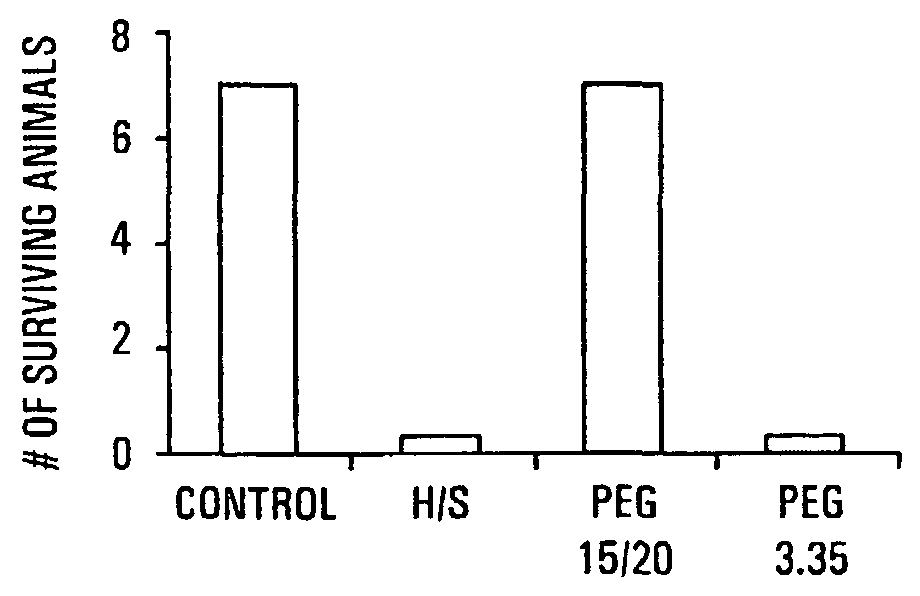
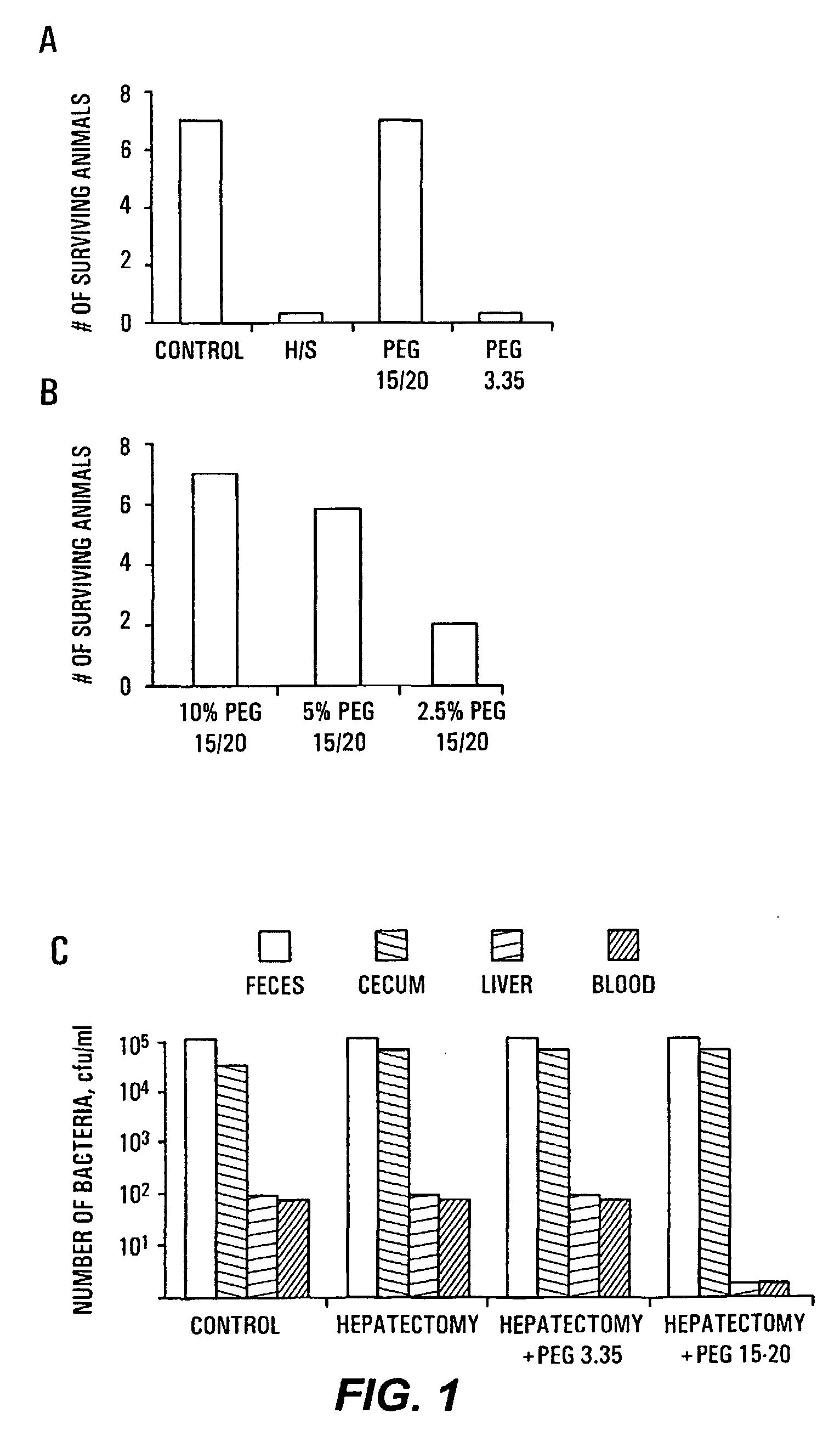
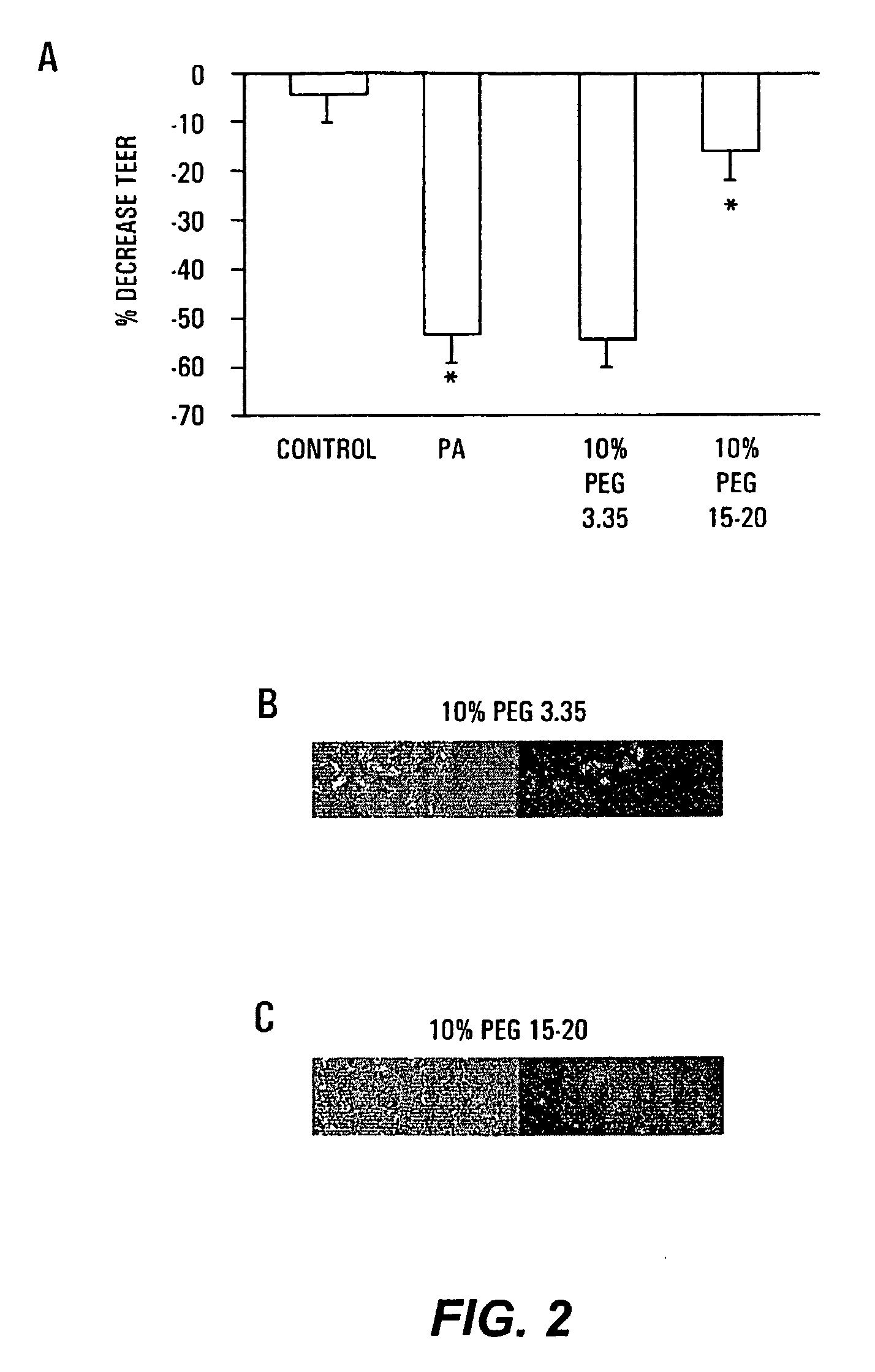
Therapeutic Delivery System Comprising a High Molecular Weight Peg-Like Compound
InactiveUS20080206188A1Suppresses virulence expressionEasy to operateAntibacterial agentsNervous disorderEpitheliumPolyethylene glycol
The present invention provides a system for delivering a wide range of chemical and biological therapeutics, including protein therapeutics, via transepithelial routes. The system comprises a high molecular weight polyethylene glycol-like (HMW PEG-like) compound for use with a therapeutic compound. Optionally, the system comprises a composition containing one or more HMW PEGlike compounds and one or more therapeutics, supplemented with a protective polymer such as dextran and / or essential pathogen nutrients such as L-glutamine. Administered alone, the HMW PEG-like compounds also provide therapeutic benefits. Also provided are methods for preventing or treating epithelial diseases, disorders, or conditions, such as an epithelium at risk of developing gut-derived sepsis attributable to an intestinal pathogen, as well as methods for monitoring the administration of HMW PEG-like compounds.
Owner:UNIVERSITY OF CHICAGO
Synthetic hyperglycosylated, protease-resistant polypeptide variants, oral formulations and methods of using the same
InactiveUS20060182716A1Skeletal disorderSaccharide peptide ingredientsInterferon receptorCarbohydrate moiety
The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites. The present invention further provides oral formulations of protease-resistant or protease-resistant, hyperglycosylated polypeptide variants, which polypeptide variants lack at least one protease cleavage site found in a parent polypeptide, and thus exhibit increased protease resistance compared to the parent polypeptide, which polypeptide variants further include (1) a carbohydrate moiety covalently linked to at least one non-native glycosylation site not found in the parent protein therapeutic or (2) a carbohydrate moiety covalently linked to at least one native glycosylation site found but not glycosylated in the parent protein therapeutic. The present invention further provides compositions, including oral pharmaceutical compositions, comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, the protease-resistant polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides containers, devices, and kits comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, the protease-resistant polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides therapeutic methods involving administering an effective amount of an oral pharmaceutical composition comprising a synthetic Type I interferon receptor polypeptide agonist, a hyperglycosylated polypeptide variant, a protease-resistant polypeptide variant, or a hyperglycosylated, protease-resistant polypeptide variant to an individual in need thereof.
Owner:LAWRENCE M BLATT +1
Hyperglycosylated polypeptide variants and methods of use
The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites. The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites, as well as erythropoietin and darbepoetin alfa, each of which are linked to a penetrating peptide that facilitates translocation of a substance across a biological barrier as well as pharmaceutical compositions, including oral formulations, of the same. The present invention further provides oral formulations of hyperglycosylated or protease-resistant, hyperglycosylated polypeptide variants, which polypeptide variants lack at least one protease cleavage site found in a parent polypeptide, and thus exhibit increased protease resistance compared to the parent polypeptide, which polypeptide variants further include (1) a carbohydrate moiety covalently linked to at least one non-native glycosylation site not found in the parent protein therapeutic or (2) a carbohydrate moiety covalently linked to at least one native glycosylation site found but not glycosylated in the parent protein therapeutic. The present invention further provides compositions, including oral pharmaceutical compositions, comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides containers, devices, and kits comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides therapeutic methods involving administering an effective amount of an oral pharmaceutical composition comprising a synthetic Type I interferon receptor polypeptide agonist, a hyperglycosylated polypeptide variant, or a hyperglycosylated, protease-resistant polypeptide variant to an individual in need thereof.
Owner:ALIOS BIOPHARMA INC +1
Methods and compositions for protein delivery
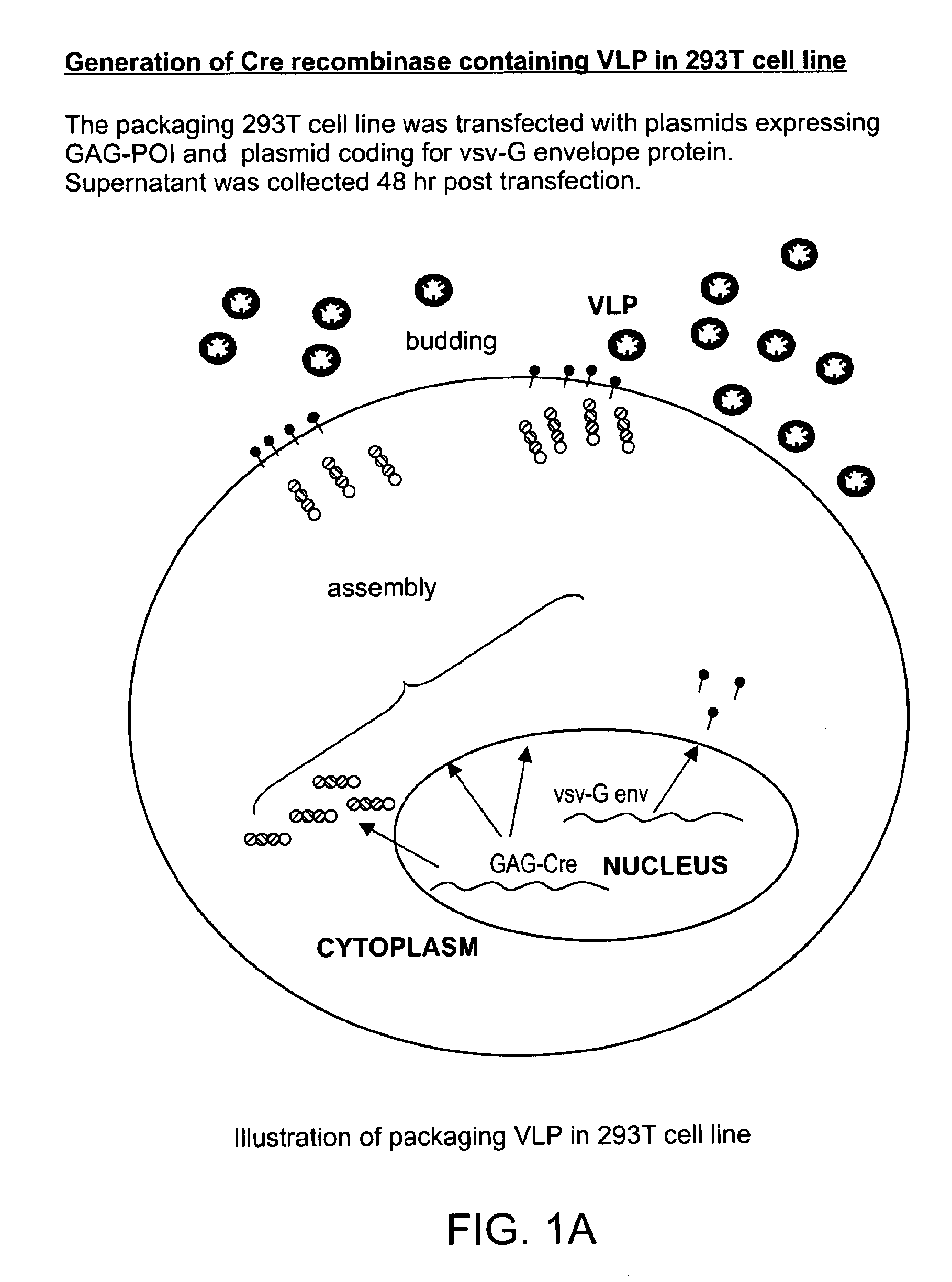
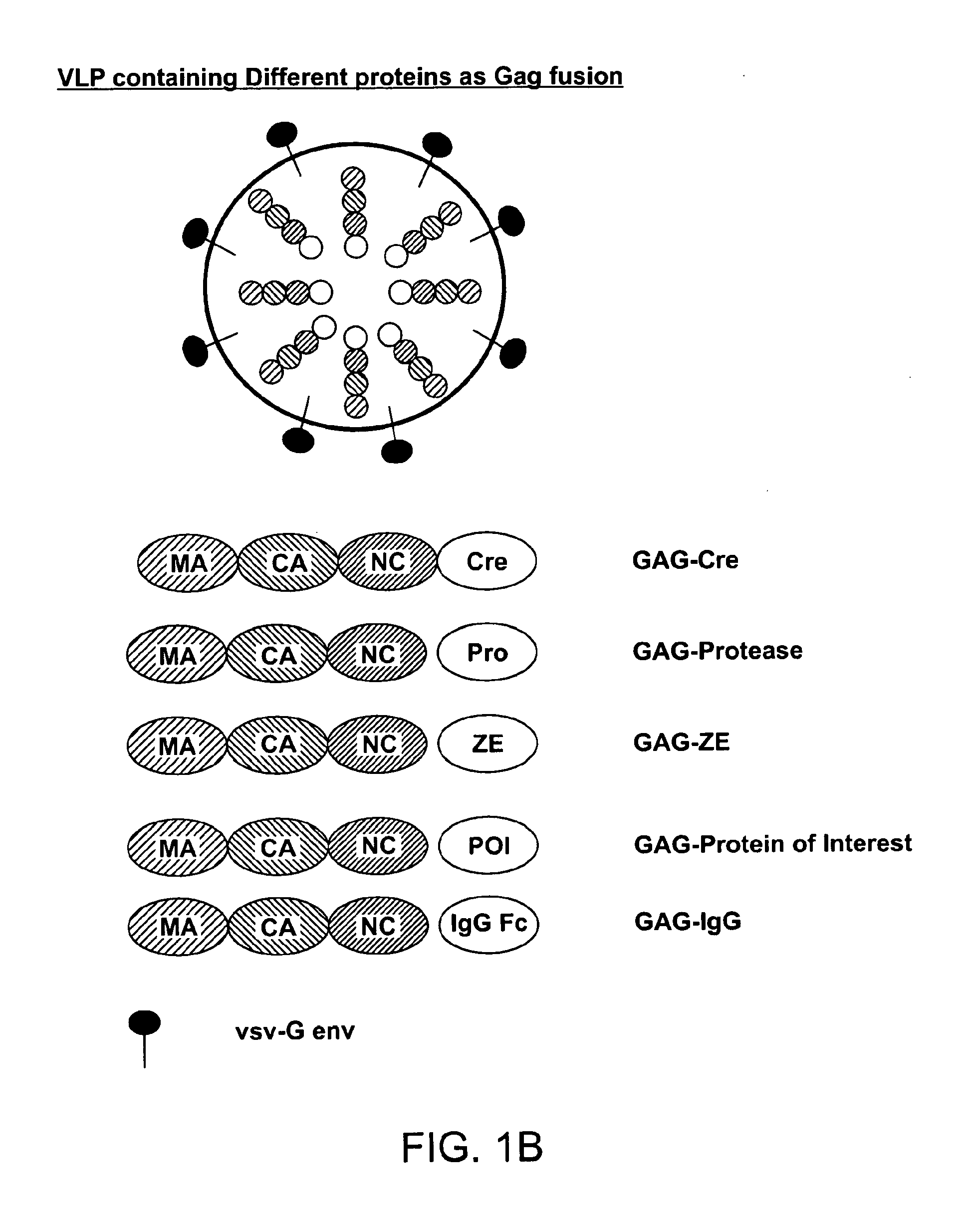
The present invention provides methods and compositions for protein delivery. The invention features virus like particles, methods of making virus like particles and methods of using virus like particles to deliver proteins to a cell, to provide protein therapy and to treat diseases or disorders. The invention also features methods of targeting a protein to a cell, methods of protein therapy and methods of treating diseases or disorders using a TUS protein, a NLS or NES identified from full length TUS.
Owner:UNITED STATES OF AMERICA
Compositions and methods for treatment of cancer
PendingCN108602872ATumor rejection antigen precursorsAntibody mimetics/scaffoldsOncologyProtein therapeutics
Owner:ALETA BIOTHERAPEUTICS INC
METHODS AND COMPOSITIONS FOR IMPROVED THERAPEUTIC EFFECTS WITH siRNA
InactiveUS20100330155A1Improve in vivo stabilityImprove efficacyOrganic active ingredientsSugar derivativesOligoribonucleotidesTherapeutic effect
The present invention relates to chemically modified, linked double-stranded (ds)RNA compositions comprising two or more double-stranded (ds) oligoribonucleotides linked by at least one linking moiety and methods of formulating and delivering such compositions to modulate gene expression through target-specific RNA co-interference (RNAco-i). The compositions of the invention may optionally comprise a conjugation or a complex with one or more small molecule drugs, protein therapeutics, or other dsRNA molecules. The present invention is directed at the methods of production for, methods of use of, and therapeutic utilities for RNAi co-interference therapy utilizing the compositions of the invention.
Owner:FLAGSHIP VENTURES
Site-specific modification of proteins through chemical modification enabling protein conjugates, protein dimer formation, and stapled peptides
ActiveUS20110263832A1Diminishment of extentAvoid spreadingHormone peptidesHybrid immunoglobulinsLupus erythematosusAdduct
The present invention generally provides methods for the site-specific modification of peptides, polypeptides, and proteins, e.g., granulocyte macrophage colony-stimulating factor, human superoxide dismutase, annexin, leptin, antibodies and the like, cytokines and chemokines, at their N-termini and at sites at which unnatural aminoacids have been introduced along the protein framework. The modifications described herein can be used for the synthesis and application of the adducts in radio-labeling, molecular imaging and protein therapeutic applications, and the treatment of disorders such as rheumatoid arthritis, lupus erythematosus, psoriasis, multiple sclerosis, type-1 diabetes, Crohn's disease, and systemic sclerosis, Alzheimer disease, cancer, liver disease (e.g., alcoholic liver disease), and cachexia.
Owner:ADVANCED PROTEOME THERAPEUTICS
Filters for infusion sets
In an acute care setting, rapid onset of the therapeutic effects of medication is highly desirable. The invention provides in-line filters suitable for rapid delivery of a positively charged protein therapeutic via intravenous administration.
Owner:NOVARTIS AG
Protein therapy for treatment of retinal diseases
InactiveUS20180236035A1High expressionEasy transferHormone peptidesPeptide/protein ingredientsMammalApoptosis
The present invention encompasses methods, compositions, and devices for treating an ocular disease, disorder or condition in a mammal. The invention includes polypeptides that possess anti-inflammatory, anti-apoptotic, immune modulatory and anti-tumorigenic properties, and their application in the treatment of eye disease, particularly diseases of the retina. In particular aspects, the invention includes administration of a therapeutic polypeptide such as a stanniocalcin family member protein for the treatment of an eye disease. Also included are fusion proteins and cells stimulated or modified to express the therapeutic polypeptides as set forth herein.
Owner:SCOTT & WHITE HEALTHCARE
Processes and devices for delivery of fluid by chemical reaction
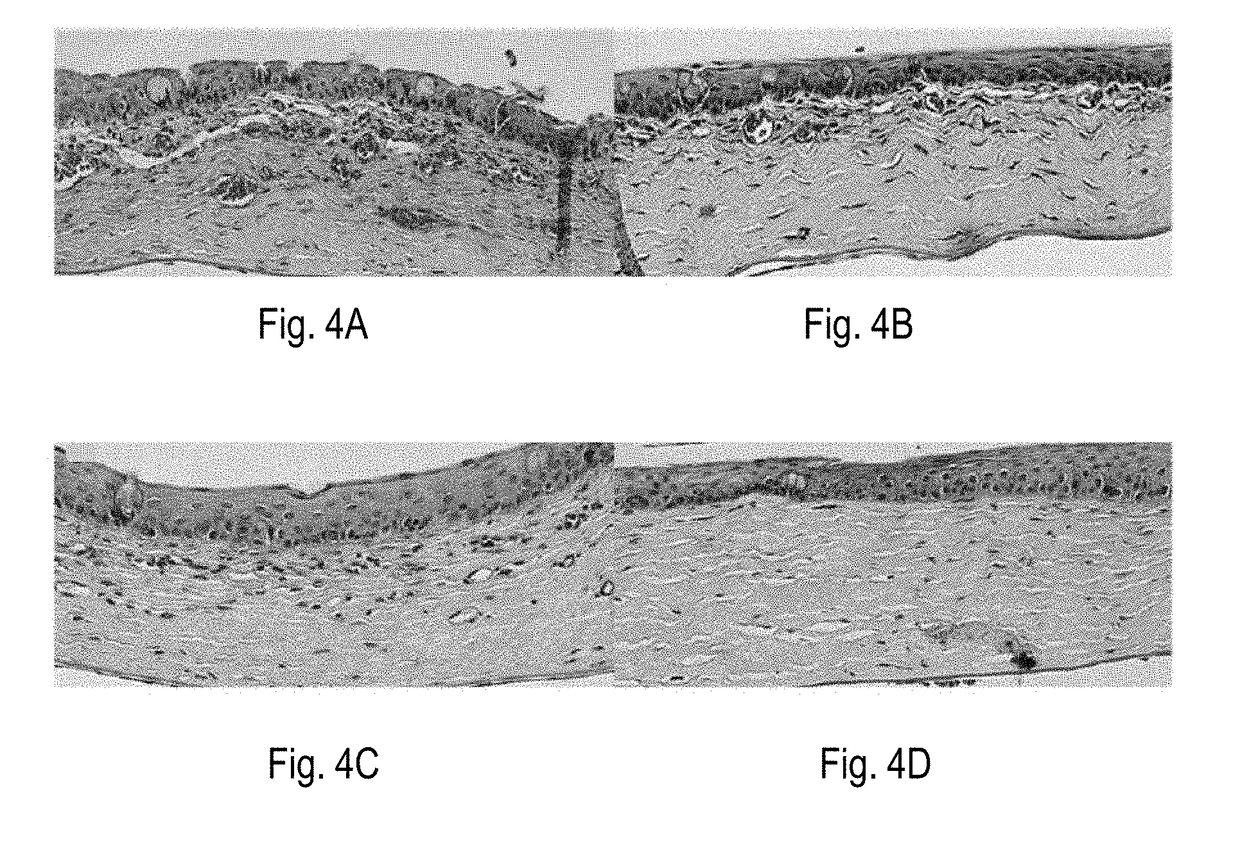
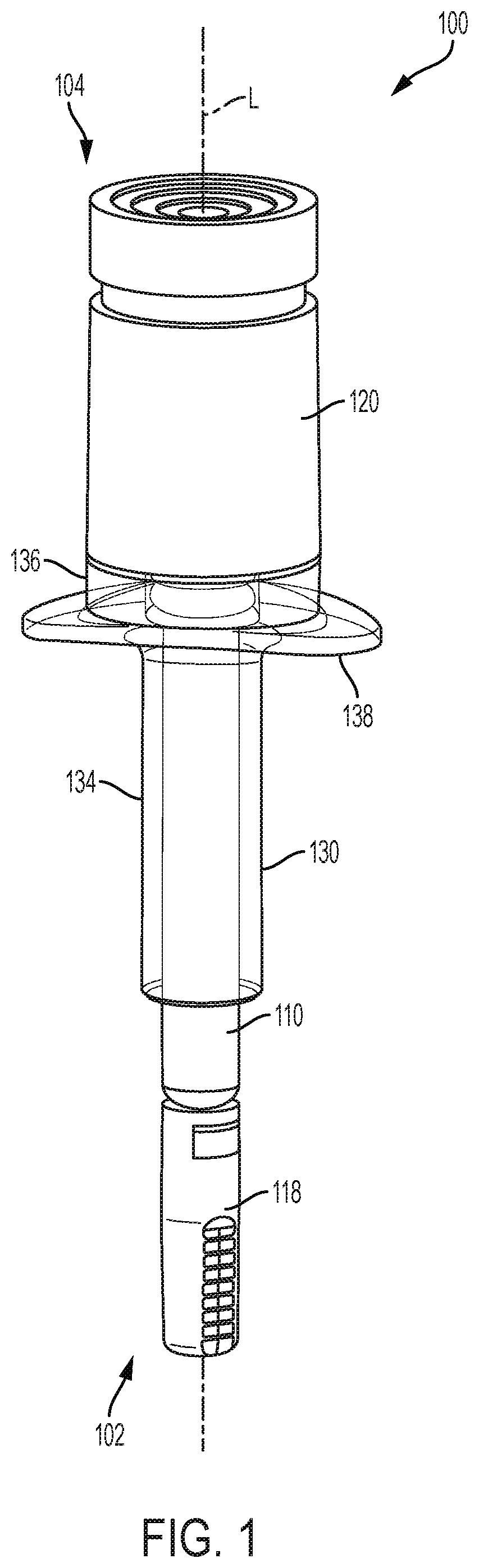
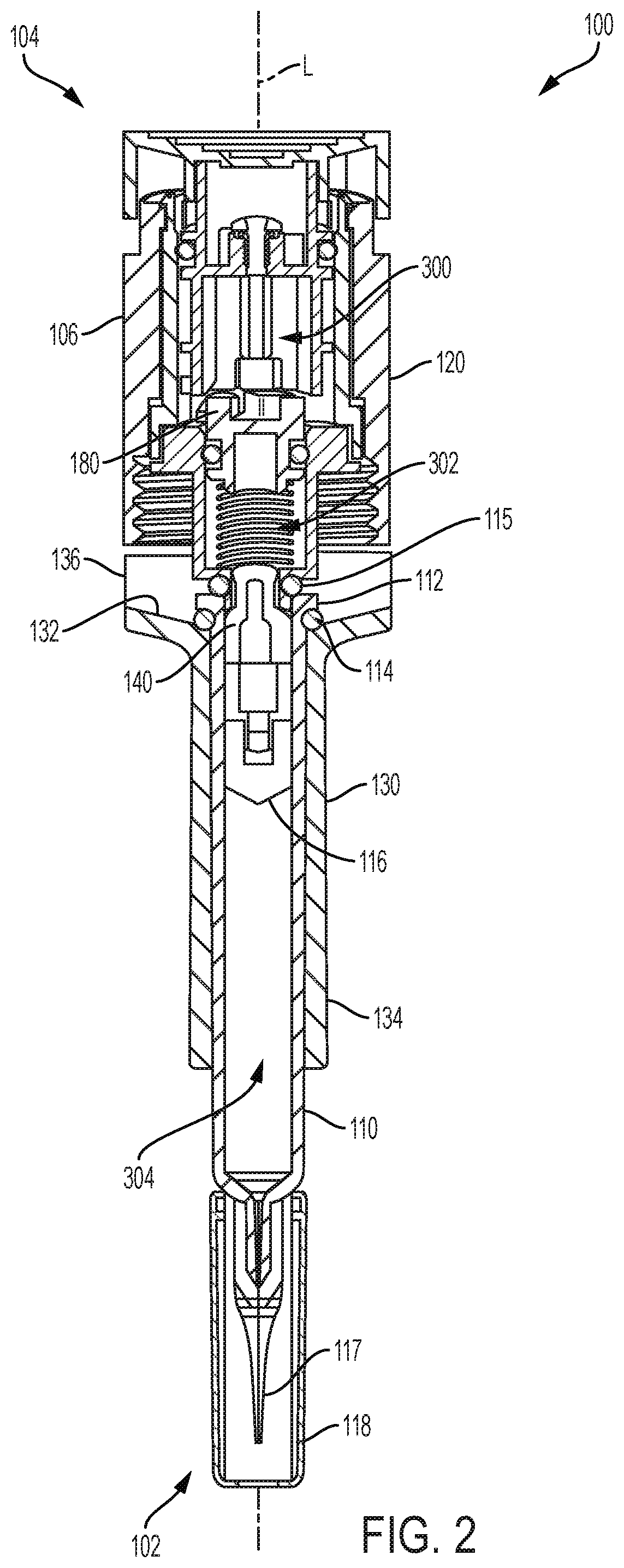
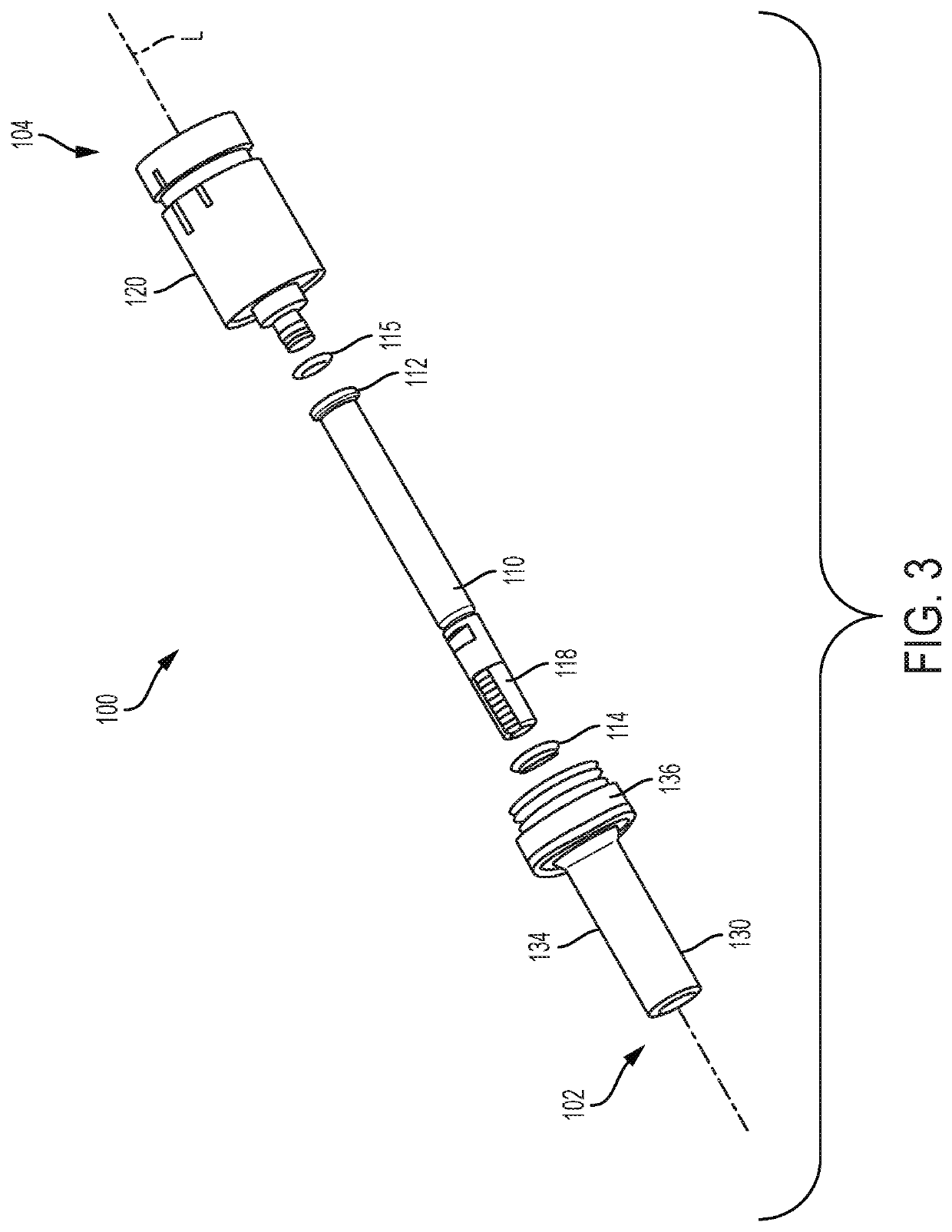
PendingUS20200030537A1Prevent movementAutomatic syringesMedical devicesHyperviscosityChemical reaction
Processes and devices are disclosed for parenteral delivery of therapeutic fluids, in particular high-viscosity therapeutic fluids (e.g., protein therapeutics), by a chemical reaction that generates a gas. The device may include a first actuation chamber containing a first reagent, a second reaction chamber containing a second reagent, and a third therapeutic fluid chamber containing the therapeutic fluid. In a loaded configuration, a plunger separates the first chamber from the second chamber. In a delivery configuration, the plunger allows the first reagent from the first chamber to communicate and react with the second reagent from the second chamber. The generated gas acts upon a plunger to deliver the therapeutic fluid from the third chamber.
Owner:ELI LILLY & CO
N-glycosylated human growth hormone with prolonged circulatory half-life
InactiveCN102131825AProlonged Circulatory Half-LifePeptide/protein ingredientsFusion with post-translational modification motifDiseaseSomatotropic hormone
The present invention relates to novel human growth hormone (h GH) variant(s) with one or more N-glycans. The hGH variants of the invention comprises an amino acid sequence which includes at least one N-glycosylation motif (N-X-S / T) arising from one or more mutations not present in the wild type hGH. The h GH variants of the invention have a prolonged circulatory half-life and thus can be effectively used as a protein therapeutic for disease states that will benefit from increased levels of h GH. The process of obtaining the hGH variants is also encompassed by the invention.
Owner:NOVO NORDISK HEALTH CARE AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com