N-glycosylated human growth hormone with prolonged circulatory half-life
A technology of human growth hormone and glycosylation, which is applied in the direction of growth hormone, hormone peptides, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0125] CLAIMS 1. A human growth hormone variant, wherein said variant comprises an amino acid sequence comprising one or more N-glycosylation motifs (N-X-S / T) that are absent in wild-type human growth hormone.
[0126] 2. The human growth hormone variant according to embodiment 1, wherein at least one of said N-glycosylation motifs (N-X-S / T) absent in wild-type human growth hormone has been selected from the group consisting of Produced by mutations: S55N, Q69N, E74S, E74T, R77N, I83N, L93N, A98N, L101S, L101T, G104N, S106N, Y111S, Y111T, I121N, D130N, K140N, T142N, G161S, G161T, and E186N.
[0127] 3. The human growth hormone variant according to embodiment 1, wherein at least one of said N-glycosylation motifs (N-X-S / T) absent in wild-type human growth hormone has been selected from the group consisting of Generated by one or more mutations / mutation pairs: K41N, Q49N, S55N, E65T, E65T, E65N, Q69N, E74S, E74T, R77N, I83N, L93N, A98N, L101S, L101T, G104N, S106N, Y111S, Y111T, ...
Embodiment 1
[0260] Construction of vectors for expression of wild-type human growth hormone in mammalian cells
[0261] By means of flanking the sequence Hind III and Eco RI site, will Figure 1A The indicated nucleotide sequence was inserted into plasmid pEE14.4, resulting in plasmid pGB039. In pGB039, the nucleotide sequence encoding growth hormone was placed under the transcriptional control of a cytomegalovirus (CMV) promoter.
[0262] By inserting the pTT5 Hind III and not Between the I sites, the nucleotide sequence encoding growth hormone in pGB039 was subcloned, resulting in plasmid pTVL01.
Embodiment 2
[0264] Transient expression of wild-type human growth hormone in mammalian HEK293 cells
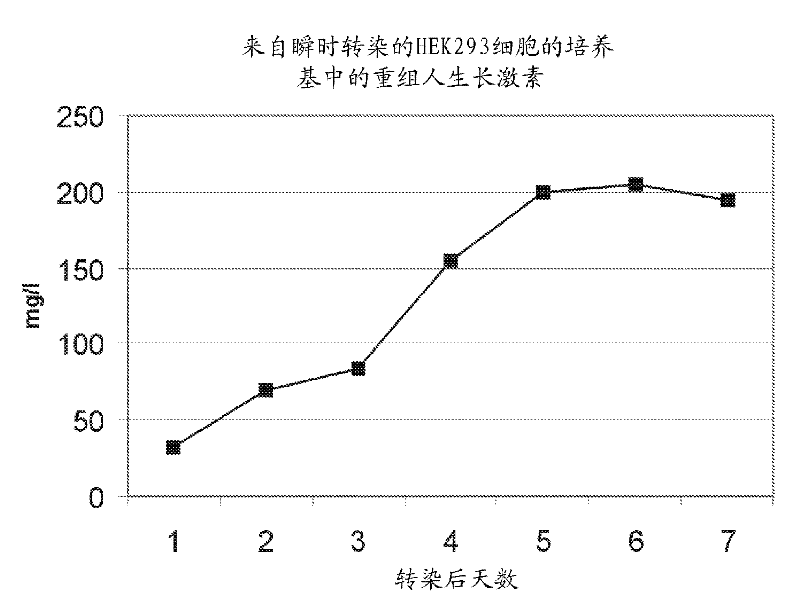
[0265] Suspension-adapted human embryonic kidney (HEK293F) cells (Freestyle, Invitrogen) were transfected with the pGB039 expression plasmid encoding wild-type human growth hormone according to the manufacturer's instructions. Briefly, 30 μg of plasmid was incubated with 40 μl of 293fectin (Invitrogen) for 20 min and added to 3 X 10 7 cell. in a shaking incubator (37°C, 8% CO 2 and 125 rpm) for 7 days. Media samples were collected daily and analyzed for human growth hormone using an ELISA kit (Roche).
[0266] The result of ELISA is as follows figure 2 shown, and demonstrated that transiently transfected mammalian cells are efficient producers of human growth hormone. The media harvested 7 days after transfection and dilutions of purified recombinant human growth hormone produced in bacteria were loaded onto SDS-PAGE gels and electrophoresed. Gels were stained with SimpleBlue Saf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com