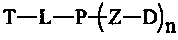Patents
Literature
87results about How to "Prolonged Circulatory Half-Life" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reversible pegylated drugs
ActiveUS7585837B2Prolonged Circulatory Half-LifeLoss of biological and pharmacological potenciesAntibacterial agentsOrganic active ingredientsPhosphate9-fluorenylmethoxycarbonyl
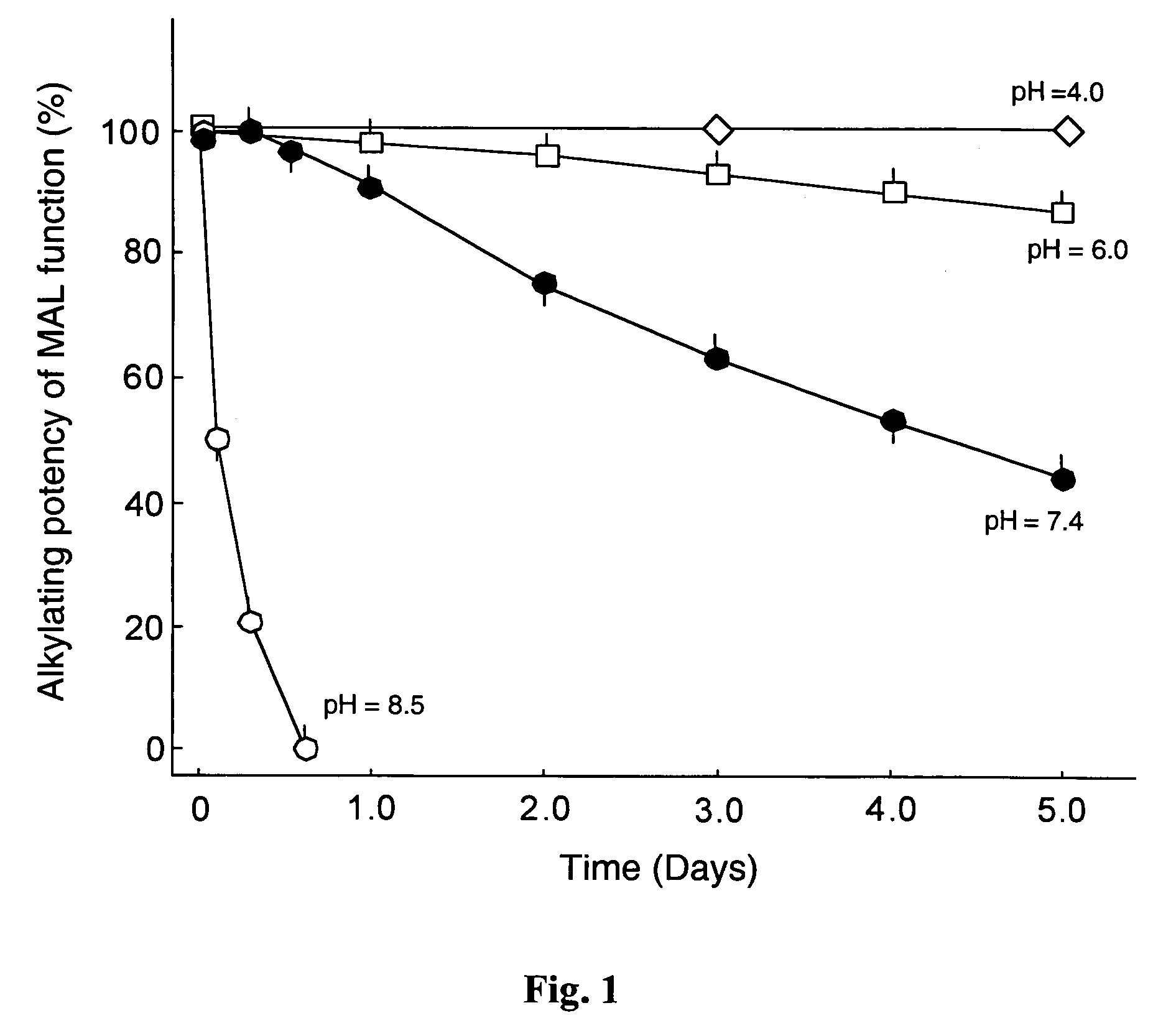
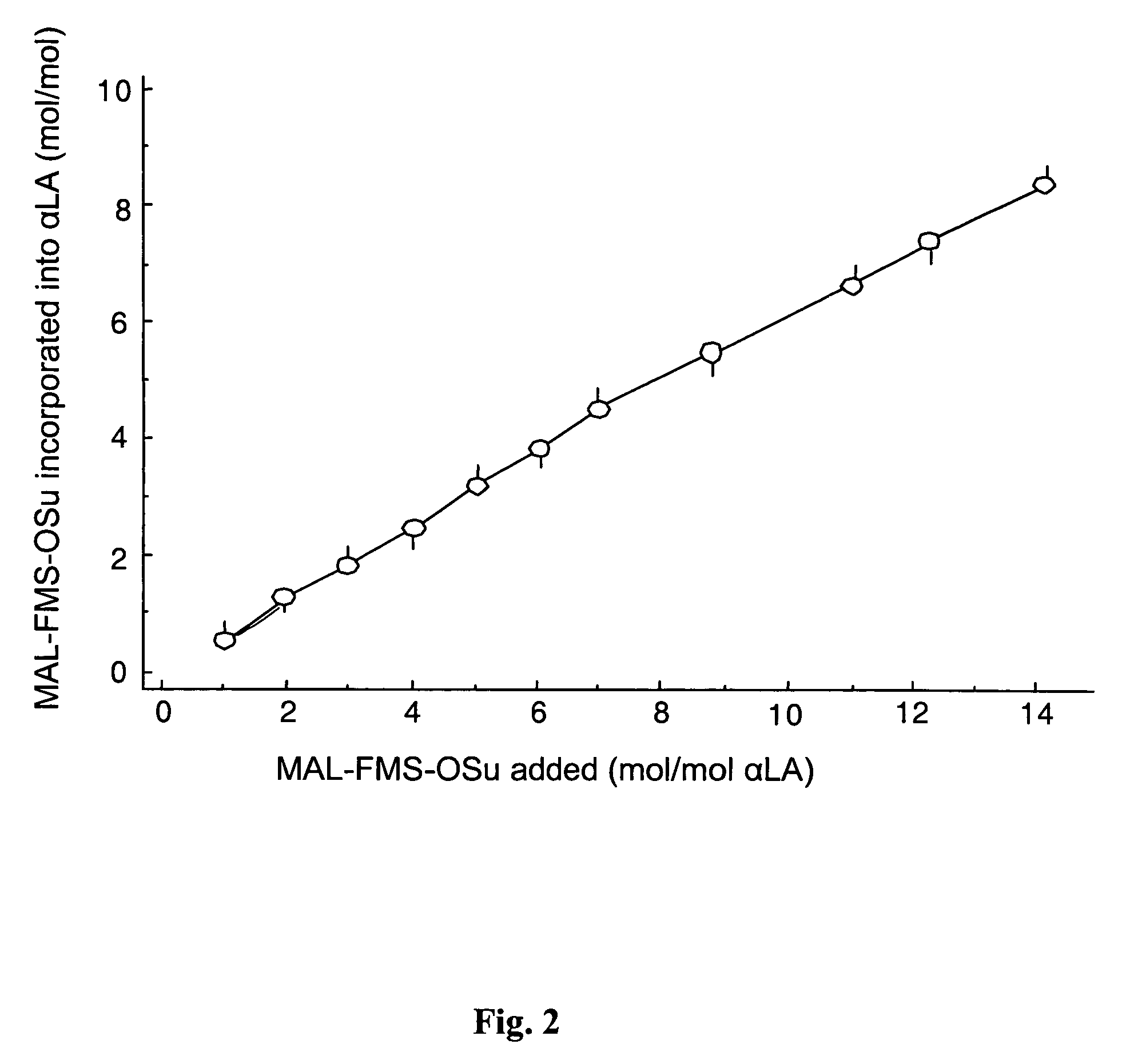
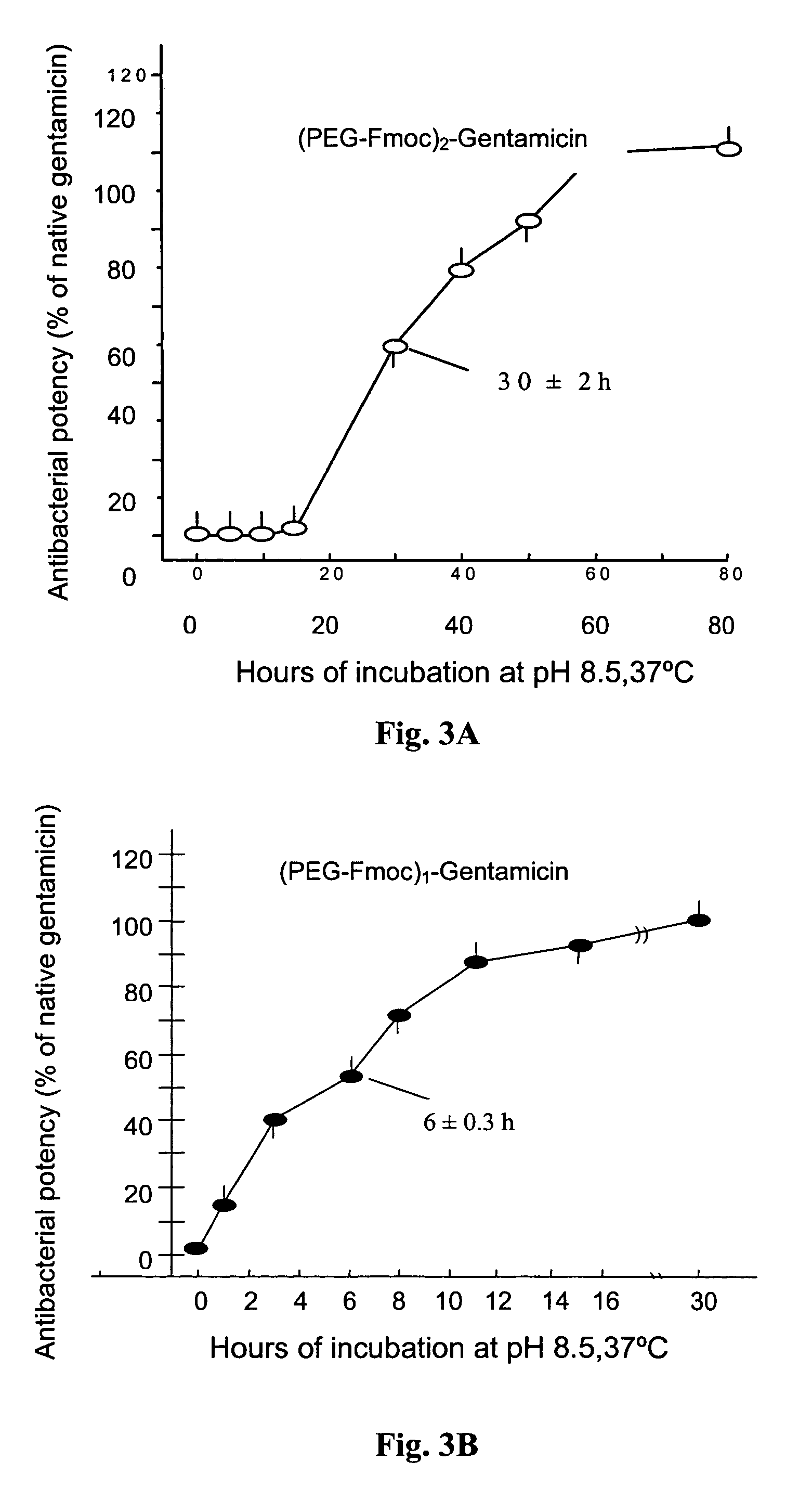
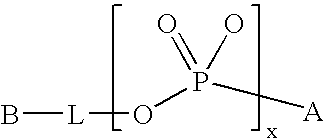
Reversible pegylated drugs are provided by derivatization of free functional groups of the drug selected from amino, hydroxyl, mercapto, phosphate and / or carboxyl with groups sensitive to mild basic conditions such as 9-fluorenylmethoxycarbonyl (Fmoc) or 2-sulfo-9-fluorenylmethoxycarbonyl (FMS), to which group a PEG moiety is attached. In these pegylated drugs, the PEG moiety and the drug residue are not linked directly to each other, but rather both residues are linked to different positions of the scaffold Fmoc or FMS structure that is highly sensitive to bases and is removable under physiological conditions. The drugs are preferably drugs containing an amino group, most preferably peptides and proteins of low or medium molecular weight. Similar molecules are provided wherein a protein carrier or another polymer carrier replaces the PEG moiety.
Owner:YEDA RES & DEV CO LTD
PEG-modified uricase
InactiveUS6913915B2Prolonged Circulatory Half-LifeEnhancing anti-uric acid activityPeptide/protein ingredientsHydrolasesPolyethylene glycolDisease
The present invention is directed to uricase modified with polyethylene glycol and to methods of treating different illnesses characterized by increased circulating uric acid levels, including but not limited to, hyperuricemia and tumor lysis syndrome.
Owner:SHENYANG SUNSHINE PHARMA
Modified Proteins
InactiveUS20080108557A1Prolonged Circulatory Half-LifeReduce in quantityPeptide/protein ingredientsAlbumin peptidesGlycosyltransferasePeptide
Owner:NOVO NORDISK AS
Modified proteins
InactiveUS20100056428A1Prolonged Circulatory Half-LifeReduce in quantityFactor VIIPeptide/protein ingredientsGlycoprotein iOrganic chemistry
Method of conjugating glycoproteins by means of chemical modification is provided as well as new modified glycoproteins.
Owner:NOVO NORDISK AS
Methods for treating viral infection using il-28 and il-29 cysteine mutants
InactiveUS20080075693A1Prolonged Circulatory Half-LifeLow immunogenicityPeptide/protein ingredientsAntipyreticInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Assembly and folding of Fc-interferon-beta fusion proteins
InactiveUS20060228332A1Good biological propertiesImprove solubilityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseSolubility
Disclosed are Fc-interferon-beta (Fc-IFN-β) fusion proteins and nucleic acid molecules encoding them. The Fc-IFN-β fusion proteins include variants of the interferon-beta (IFN-β) protein that are altered to achieve enhanced biological activity, prolonged circulating half-life and greater solubility. Also disclosed are methods of producing the fusion proteins and methods of using the fusion proteins and / or nucleic acid molecules for treating diseases and conditions alleviated by the administration of interferon-beta.
Owner:MERCK PATENT GMBH
Use of hmgb1 for the activation of dendritic cells
InactiveUS20040242481A1Reduced expression levelHigh expressionUltrasonic/sonic/infrasonic diagnosticsBiocideDendritic cellNucleotide
Use of the protein HMGB1 or a variant or fragment thereof or a polynucleotide encoding therefor for inducing the activation of an antigen presenting cell (APC).
Owner:FOND CENT SAN RAFFAELLE DEL MONTE TABOR
Compositions and methods for less immunogenic protein-lipid complexes
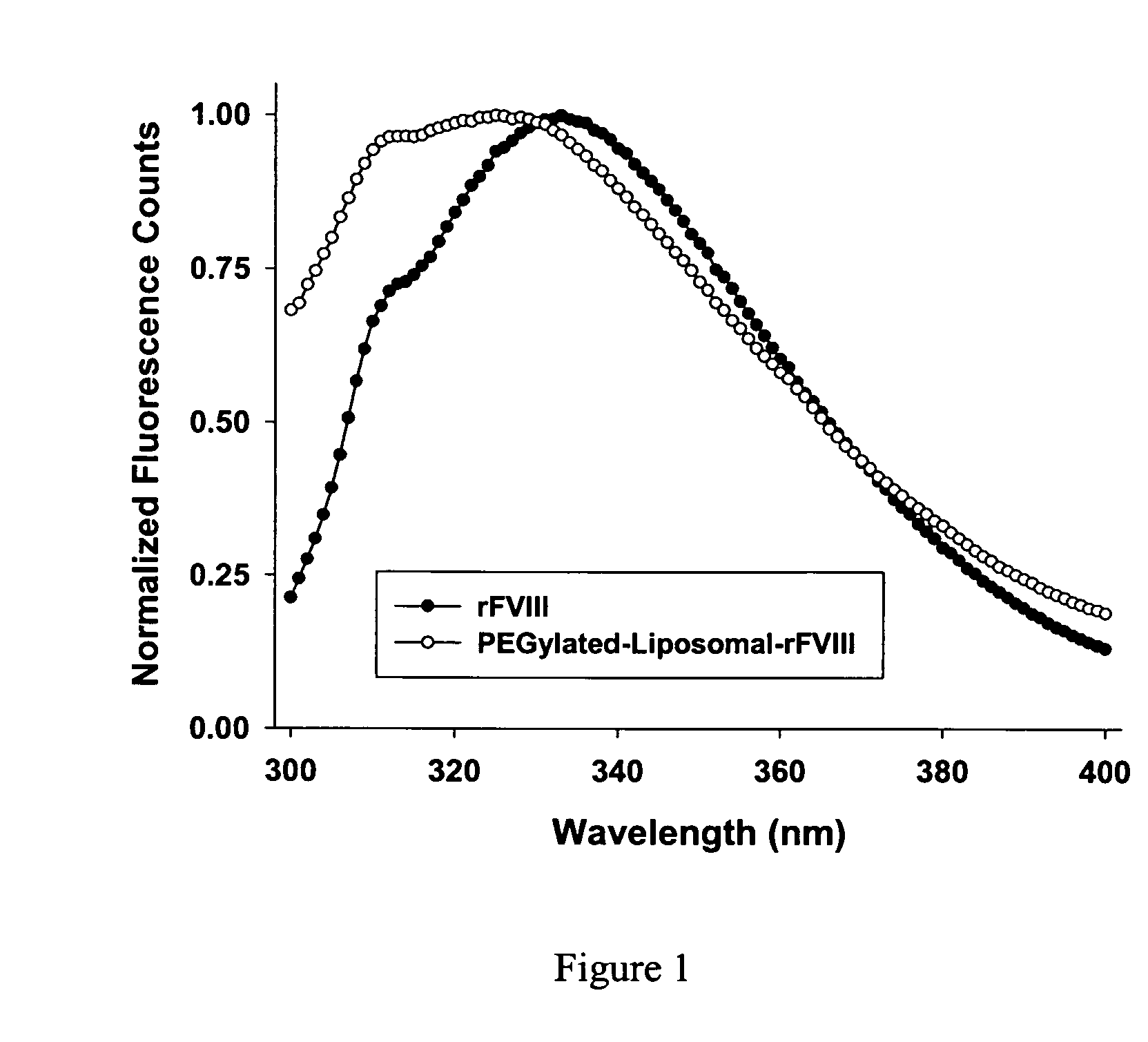
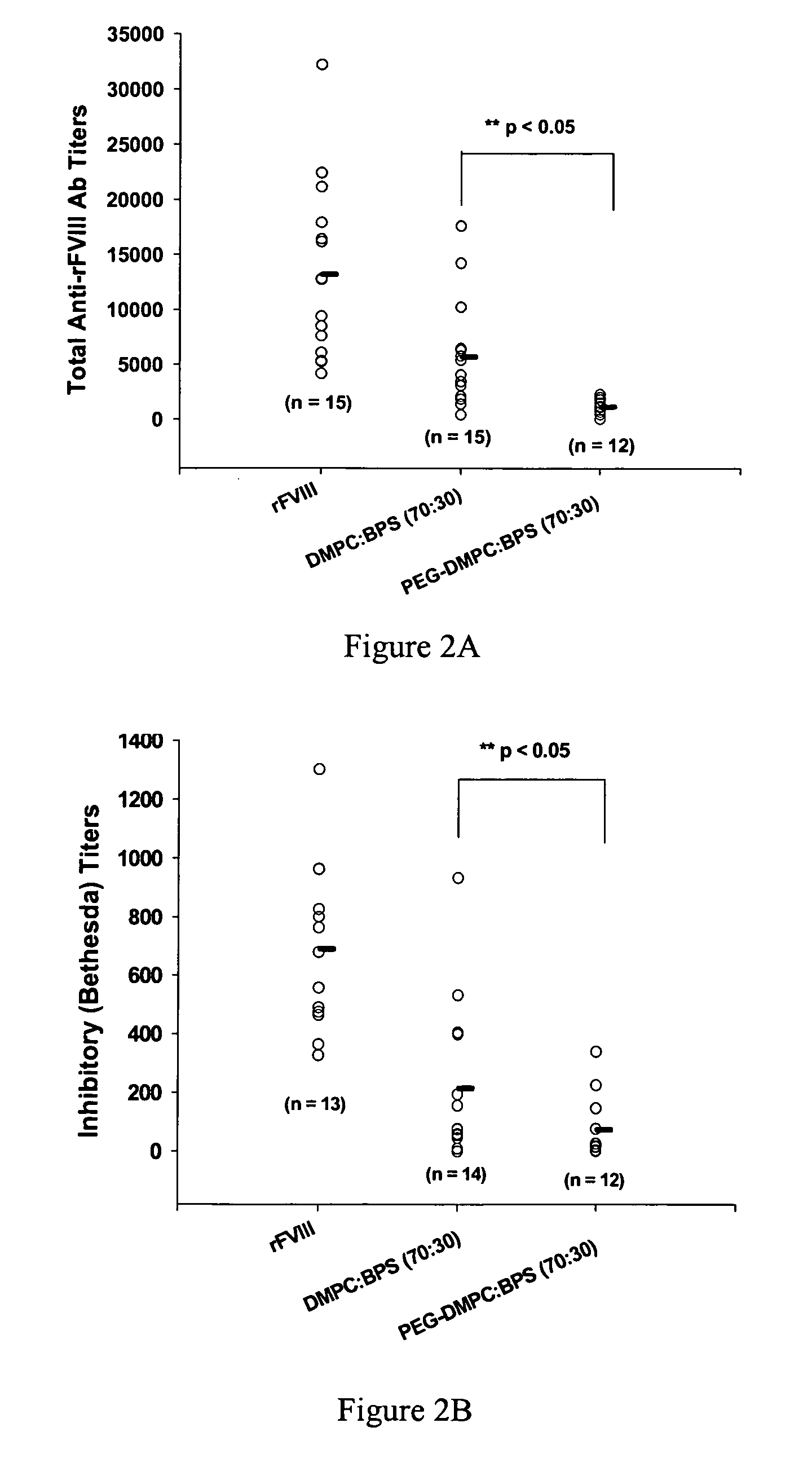
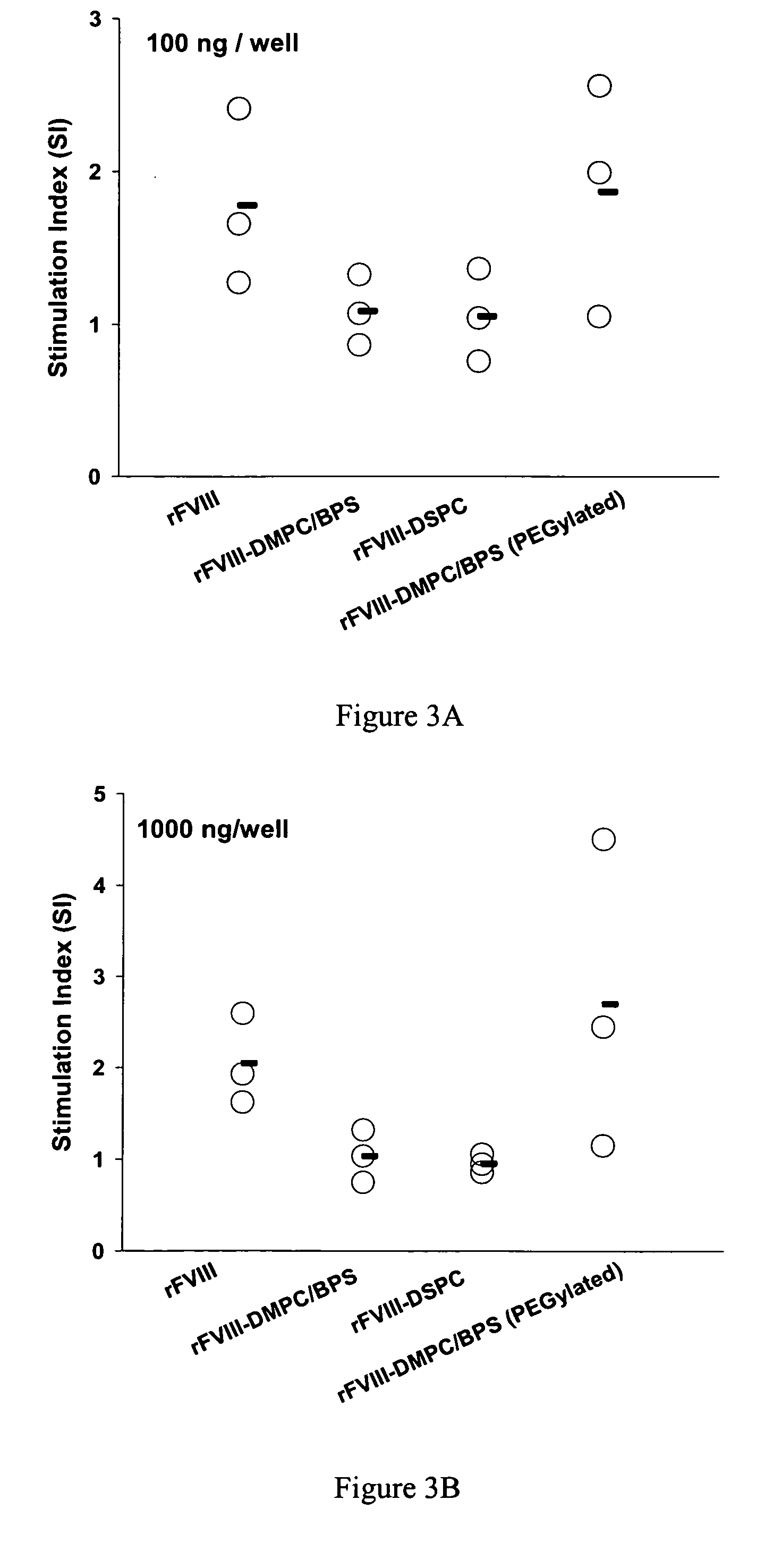
InactiveUS20090053297A1Lower titerLow immunogenicityPeptide/protein ingredientsMicroencapsulation basedLipid formationHalf-life
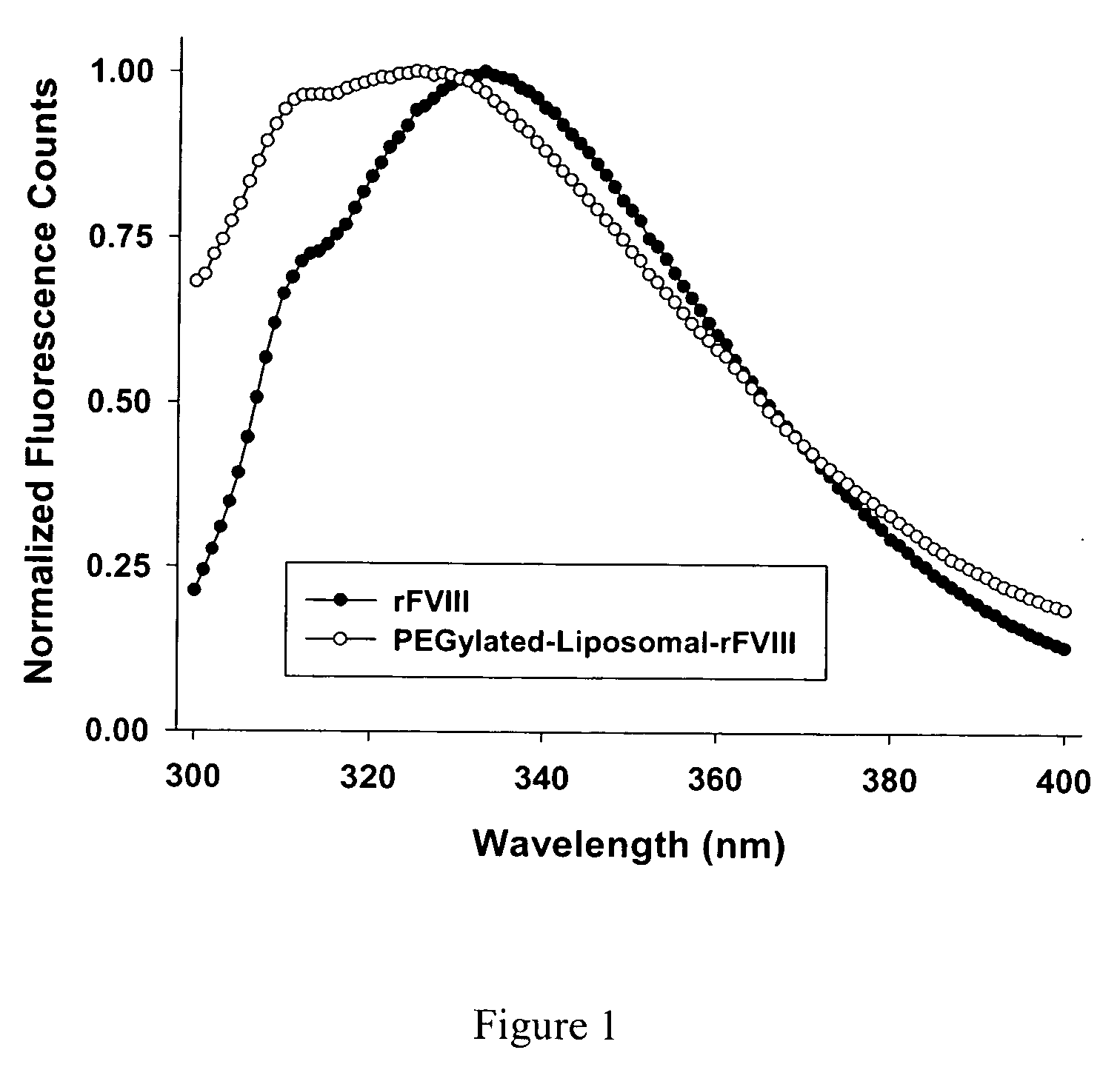
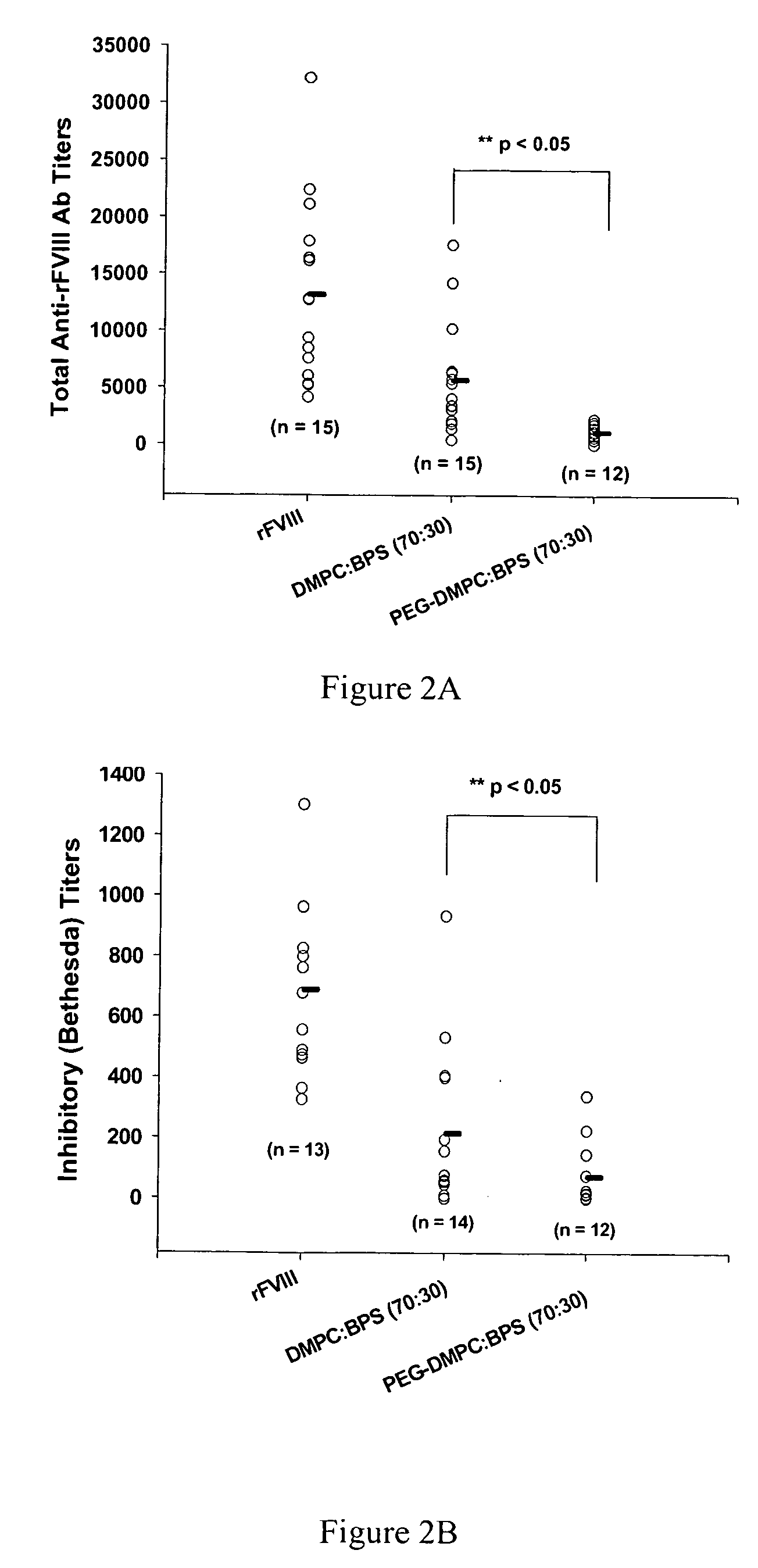
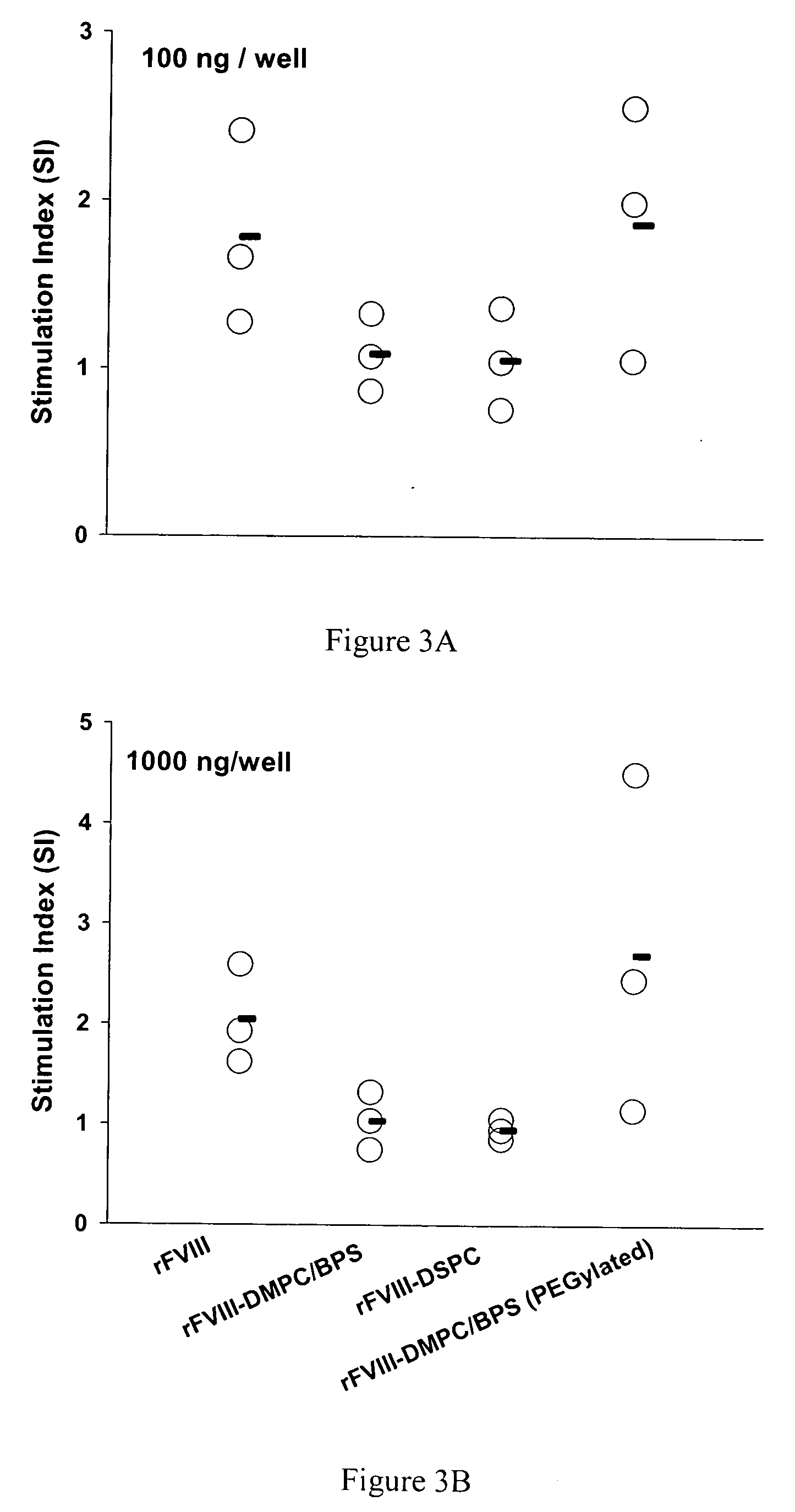
The present invention provides compositions and methods for reducing the immunogenicity and increasing the circulating half-life of therapeutic proteins such as Factor VIII. The compositions comprise lipidic structures such as liposomes, micelles and cochleates comprising a negatively charged lipid and polyethylene glycol derivatized phosphatidyl ethanolamine.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
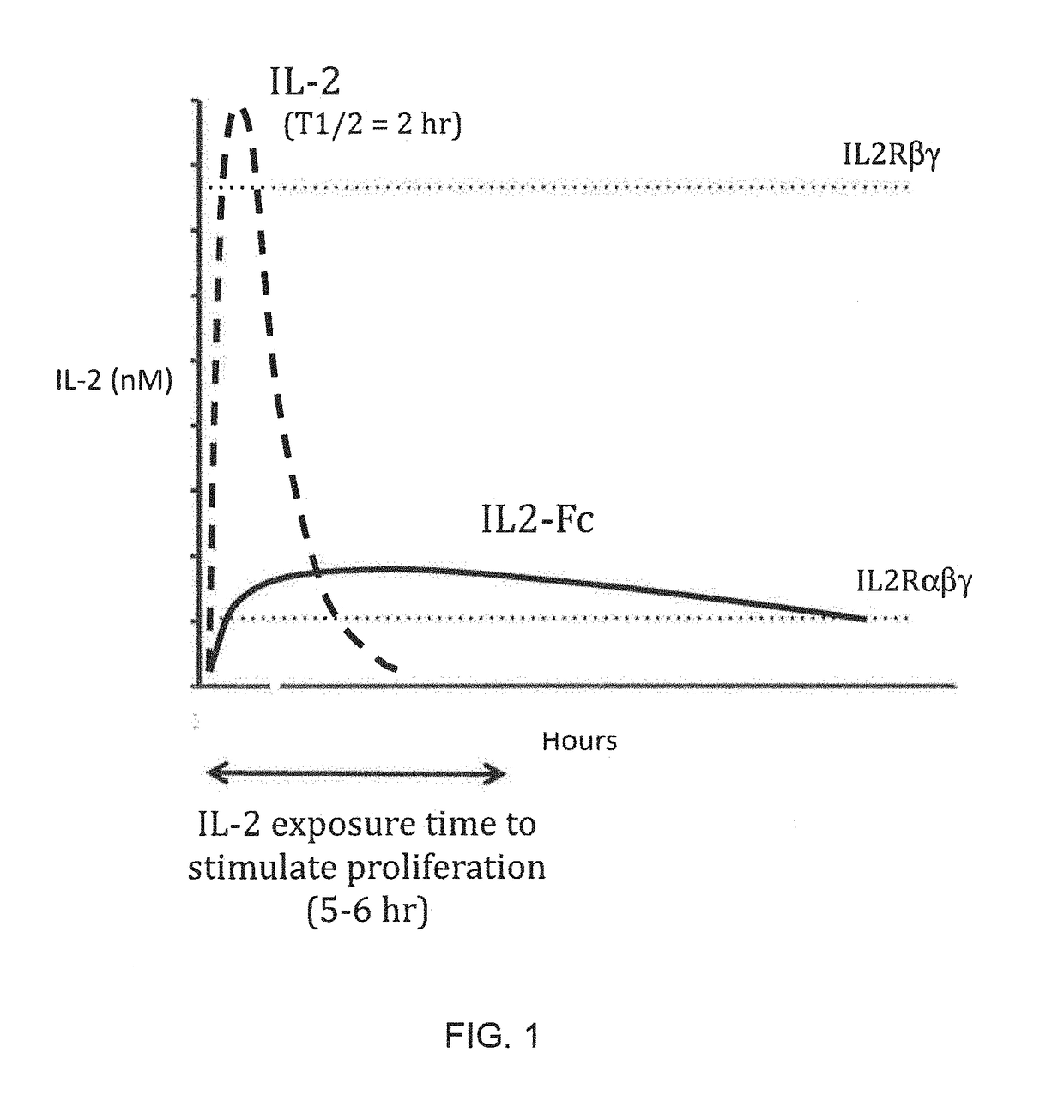
Molecules that selectively activate regulatory t cells for the treatment of autoimmune diseases
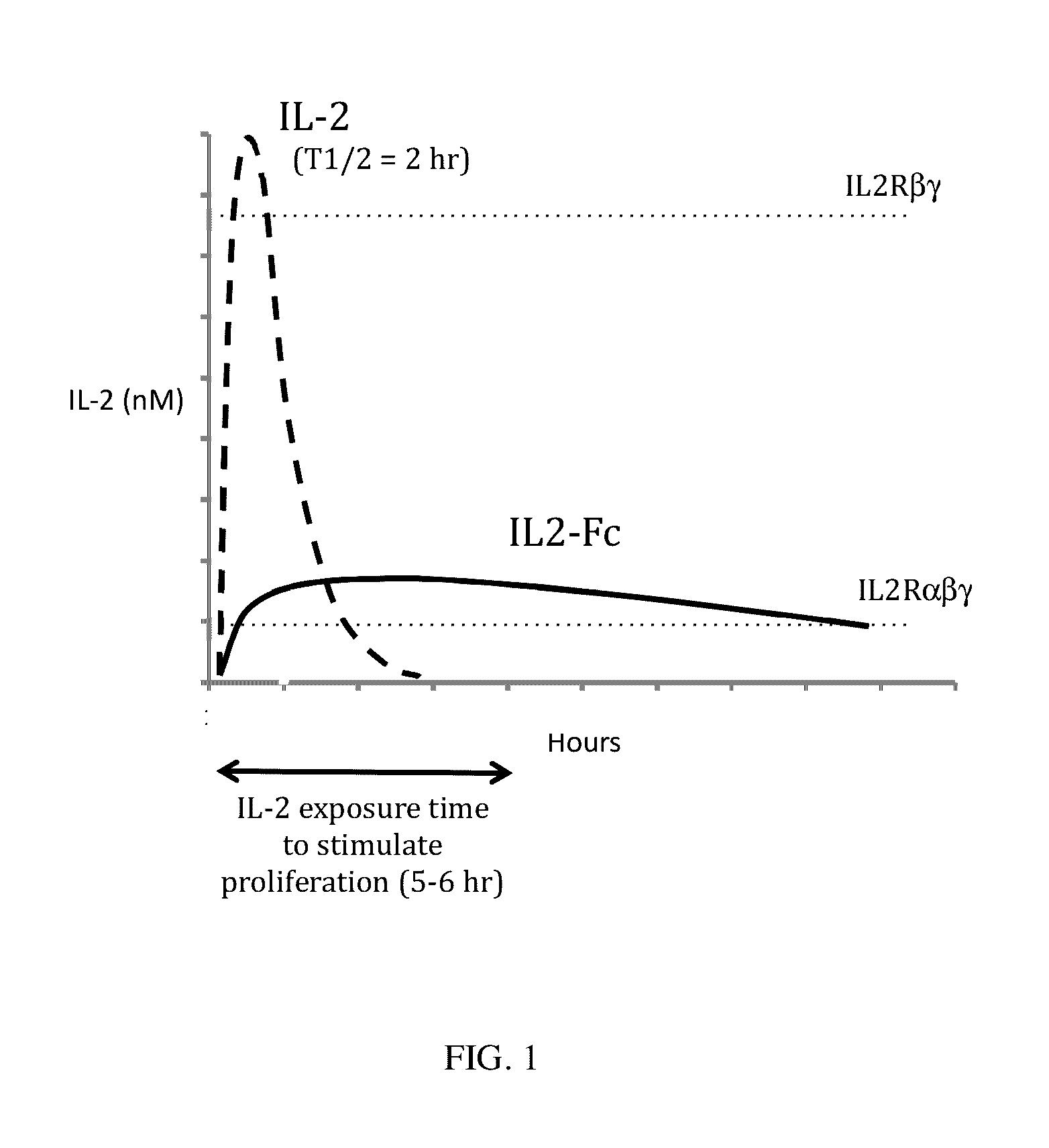
InactiveUS20170051029A1Prolonged Circulatory Half-LifeExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsImmunologic disordersRegulatory T cell
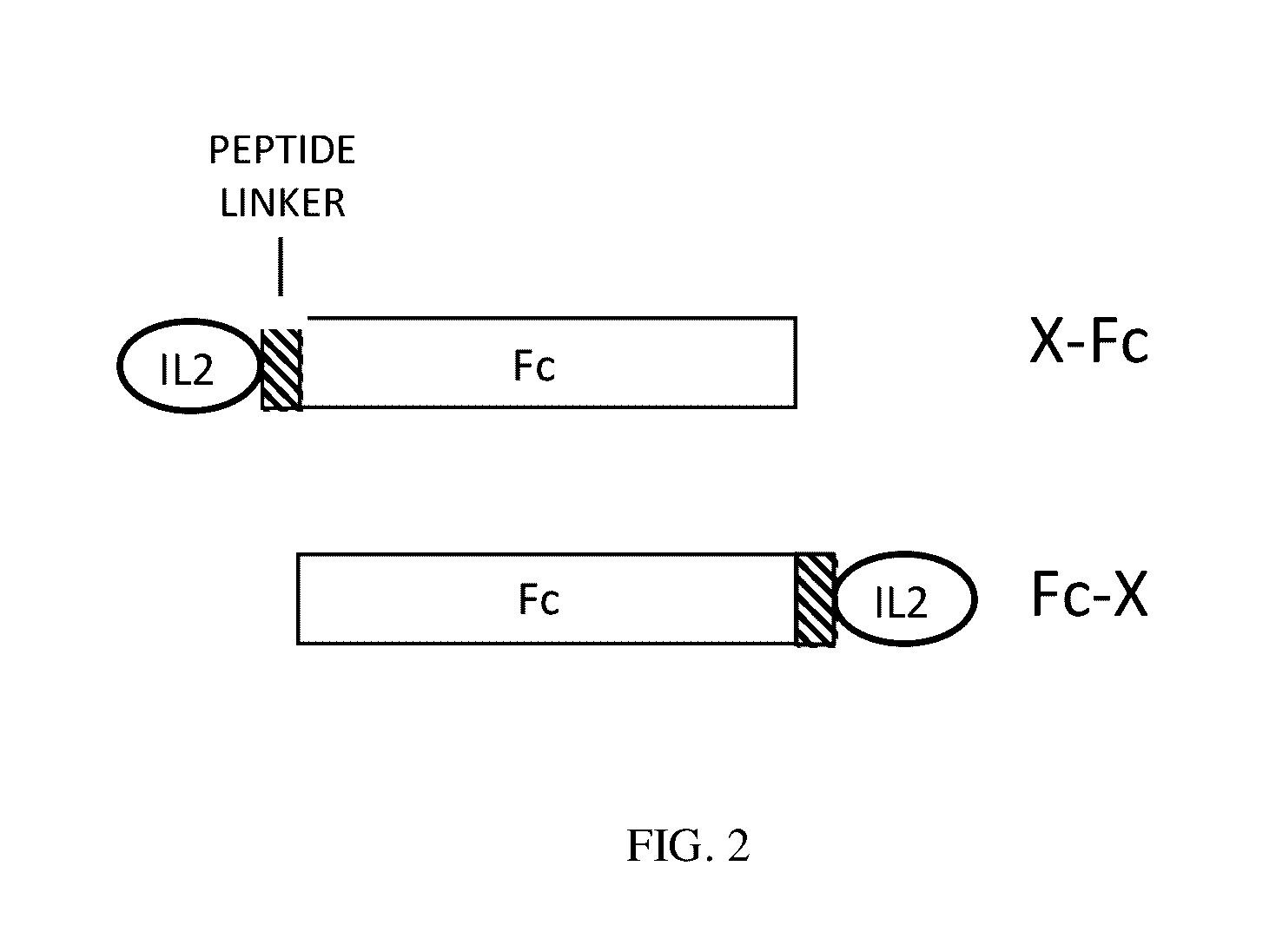
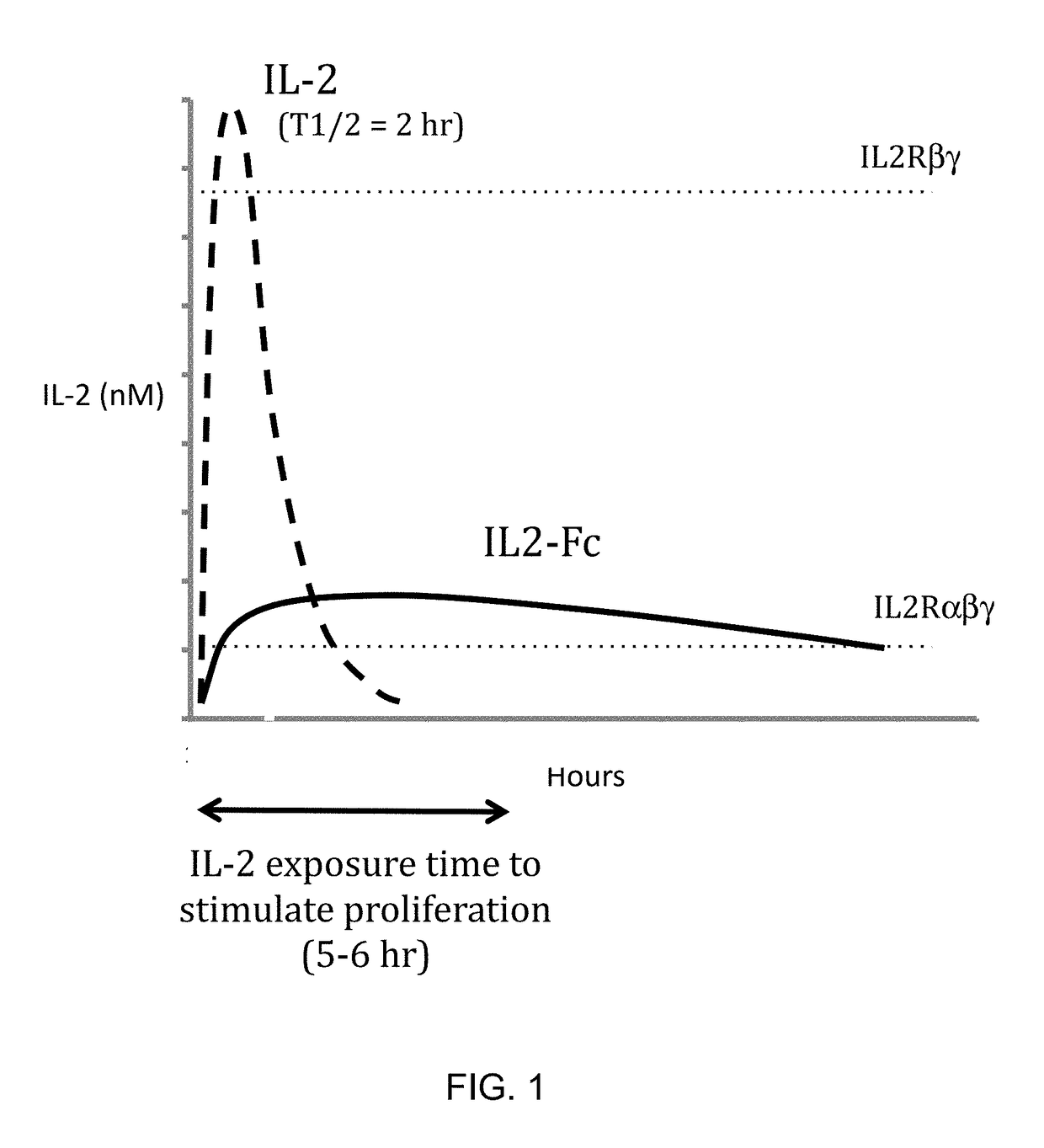
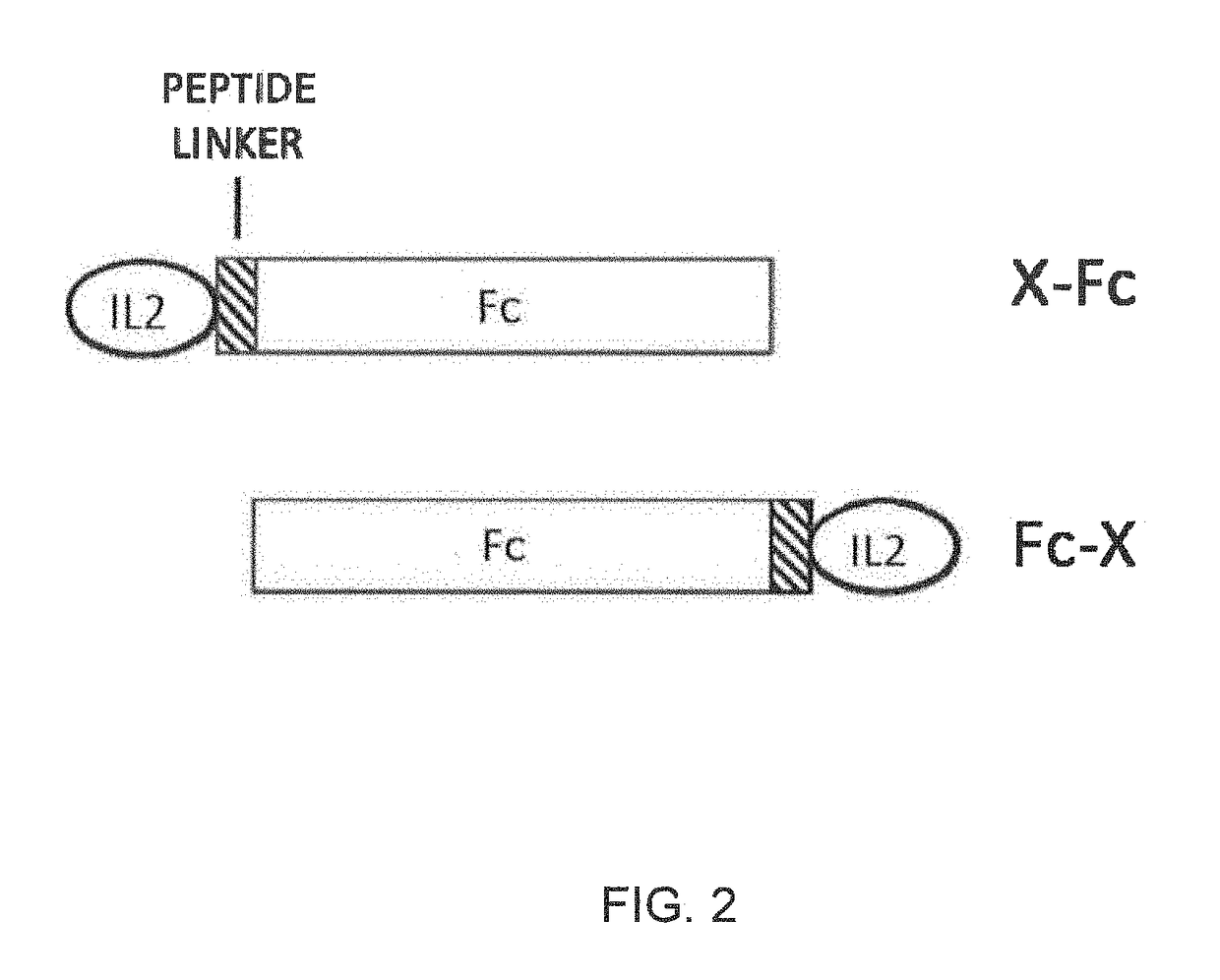
This invention provides for a fusion protein between an IL2αβγ Selective Agonist protein (IL2 Selective Agonist) and a IgG Fc protein. The IL2 Selective Agonist moiety provides a therapeutic activity by selectively activating the IL2αβγ form of the receptor, thus selectively stimulating Tregs. The Fc moiety provides a prolonged circulating half-life compared to the circulating half-life of IL-2 or an IL2SA protein.
Owner:DELINIA
Antibodies against H5N1 strains of influenza A virus
InactiveUS8124092B2Easy transferAvoid difficultySugar derivativesImmunoglobulins against virusesVirologyAntibody
Provided are human antibodies that can neutralize a H5N1 strain of influenza A virus. Also provided are antibodies that can neutralize a strain of influenza A virus in clade 2 of the H5 subtype, that can neutralize a H5N1 strain of influenza A virus and have a lambda light chain, and that are IgG antibodies (but not with a IgG1 heavy chain) that can neutralize a H5N1 strain of influenza A virus.
Owner:HUMABS LLC
Molecules that selectively activate regulatory t cells for the treatment of autoimmune diseases
InactiveUS20170204154A1Prolonged Circulatory Half-LifeExtended half-lifePeptide/protein ingredientsMetabolism disorderDiseaseRegulatory T cell
This invention provides for a fusion protein between an IL2αβγ Selective Agonist protein (IL2 Selective Agonist) and a IgG Fc protein using a linker. The IL2 Selective Agonist moiety provides a therapeutic activity by selectively activating the IL2αβγ form of the receptor, thus selectively stimulating Tregs. The Fc moiety provides a prolonged circulating half-life compared to the circulating half-life of IL-2 or an IL2SA protein.
Owner:DELINIA
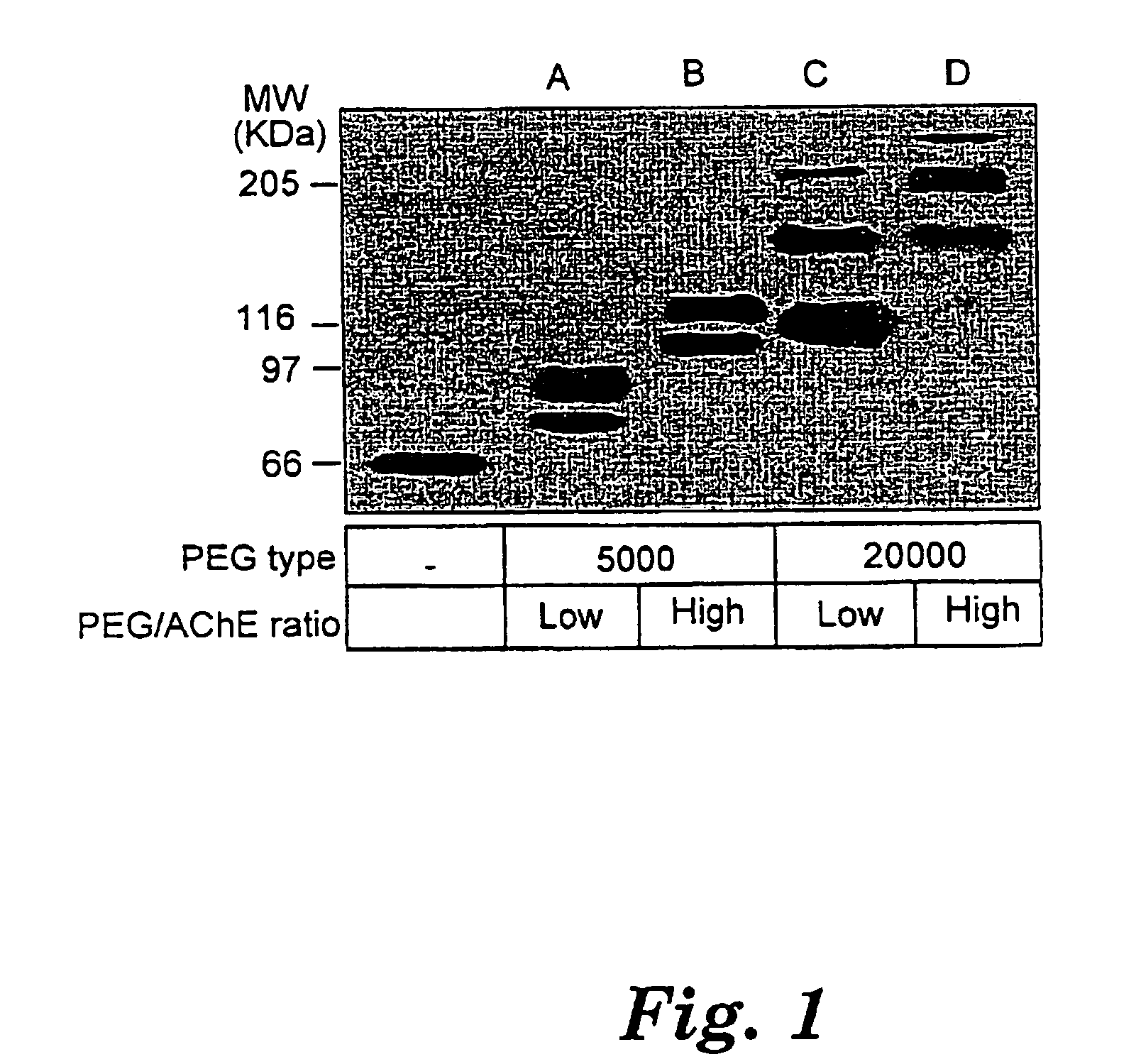
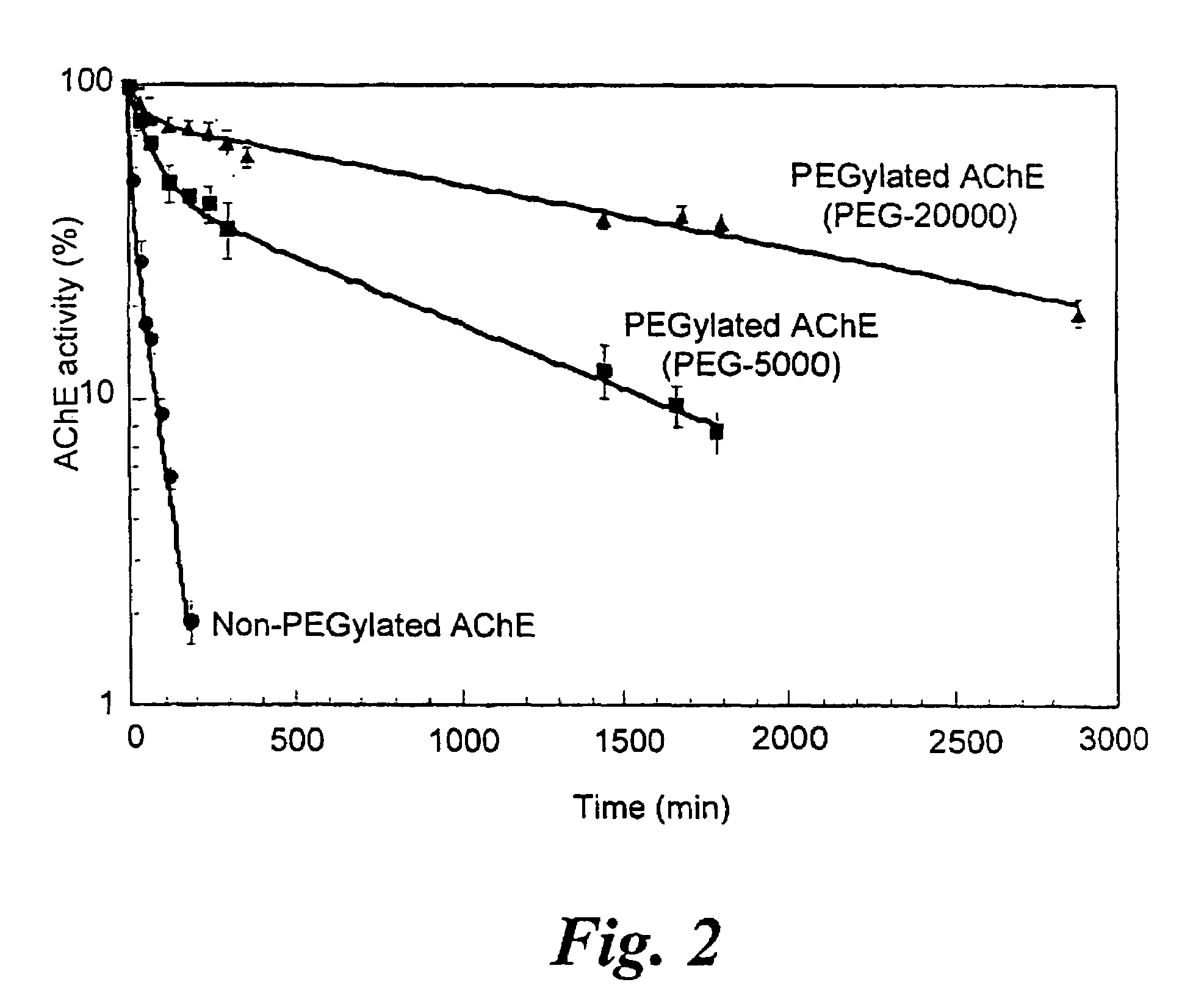
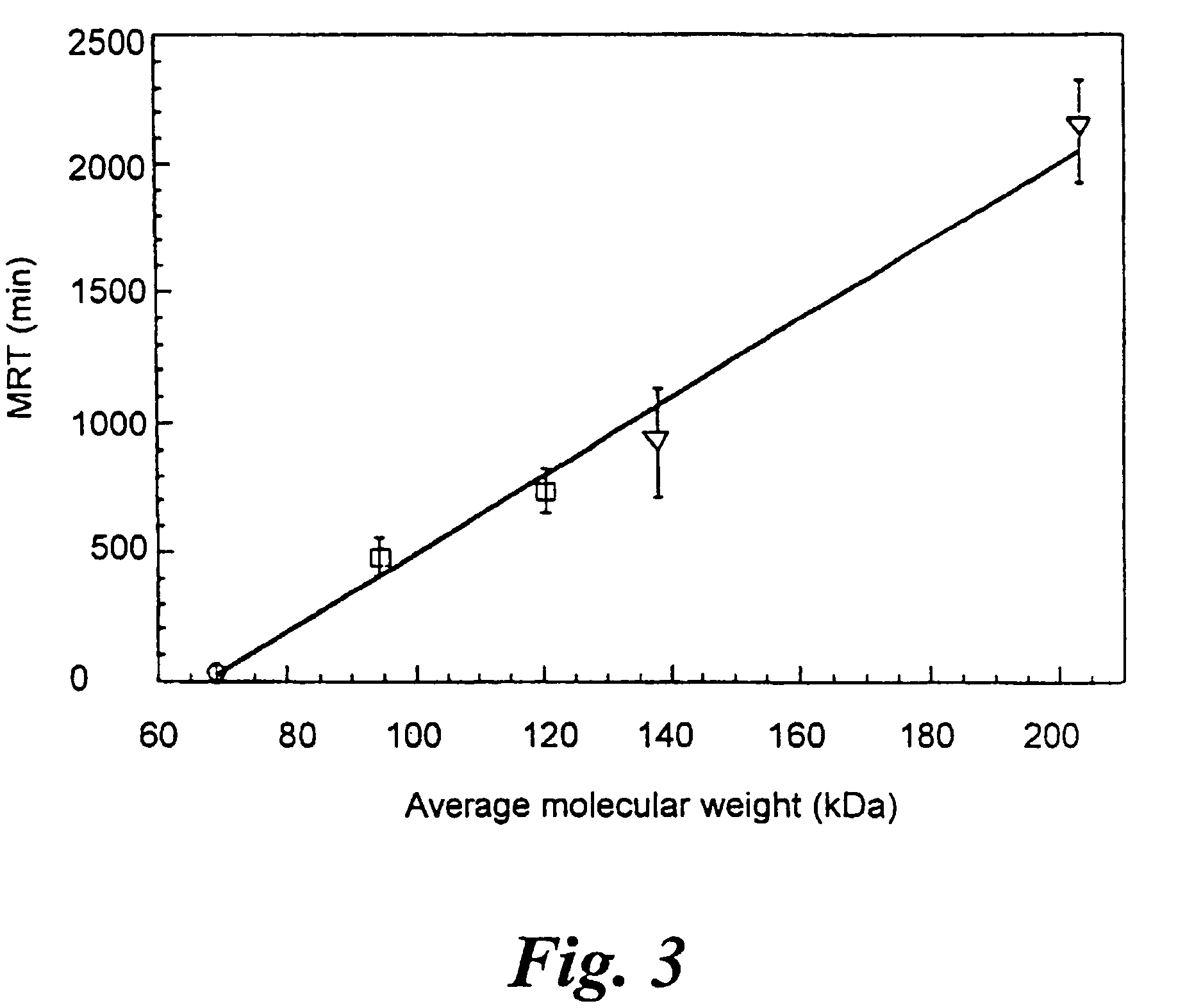
Uses of chemically-modified cholinesterases for detoxification of organophosphorous compounds
InactiveUS7572764B2Prolonged Circulatory Half-LifeBiocidePeptide/protein ingredientsCholinesteraseMammal
A circulatory long-lived cholinesterase (ChE) protein, such as acetylcholinesterase (AChE) or butyrylcholinesterase (BChE), which is a ChE protein modified with a non-antigenic polymer. The ChE may be AChE, such as native AChE of mammalian origin or of non-mammalian origin, or recombinant AChE. The recombinant AChE may be mutated at one or more amino-acid residues. The BChE may be native BChE of mammalian origin or of non-mammalian origin.
Owner:STATE OF ISRAEL MINIST OF AGRI & RURAL DEV AGRI RES ORG (A R O) (VOLCANI CENT)
Molecules that selectively activate regulatory t cells for the treatment of autoimmune diseases
ActiveUS20180037624A1Prolonged Circulatory Half-LifeExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseRegulatory T cell
Owner:DELINIA
Compositions and methods for the prevention or treatment of cancer and bone loss associated with cancer
InactiveUS20060019887A1Inhibiting bone resorptionPrevent and treat lossOrganic active ingredientsPeptide/protein ingredientsVeterinary medicineMultiple myeloma
The present invention relates to compositions and methods for the prevention and / or treatment of bone loss associated with cancer. More particularly, the invention relates to OPG compositions and methods for the prevention and / or treatment of bone loss comprising said compositions. The invention also relates to the use of OPG compositions for the treatment of multiple myeloma.
Owner:AMGEN INC
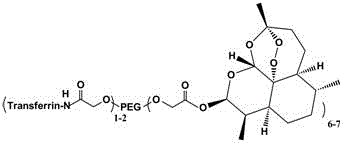
Target hydrophilic polymer-triptolide conjugate
InactiveCN103816548AAchieve active targetingProlonged Circulatory Half-LifeOrganic active ingredientsPharmaceutical non-active ingredientsPolymer scienceAqueous solubility
The invention provides a target hydrophilic polymer-triptolide conjugate (I), wherein P represents a water-soluble polymer; D represents triptolide; T represents a target molecule; and L and Z represent linking groups. The conjugate improves water solubility of triptolide, reduces toxicity of triptolide and prolongs circulating half-life period of triptolide in a living body.
Owner:BEIJING FORESTRY UNIVERSITY
Combination treatment with t-PA variant and low molecular weight heparin
InactiveUS7084118B2Prolonged Circulatory Half-LifeRetained fibrin bindingData processing applicationsFibrinogenPLG - PlasminogenRegimen
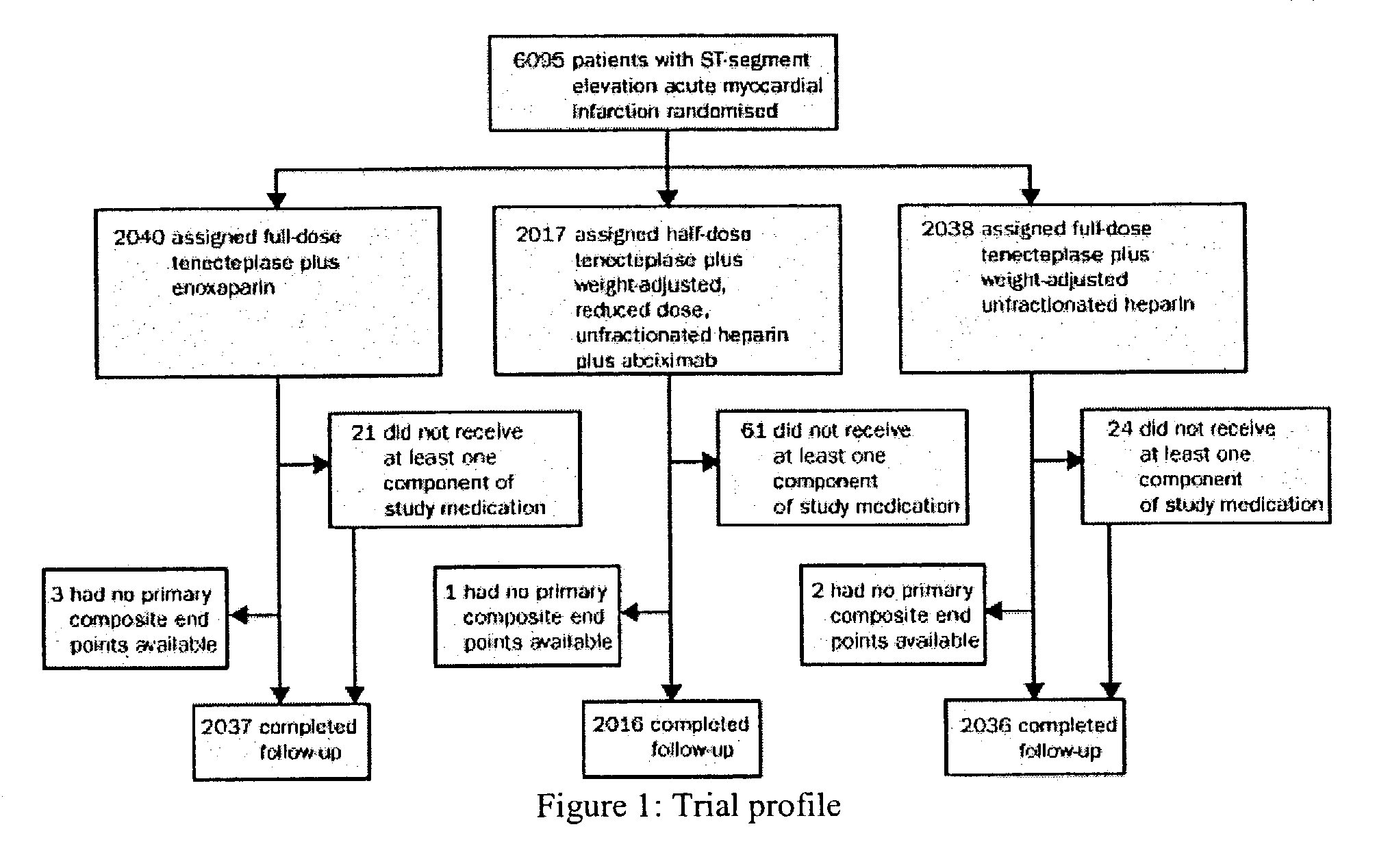
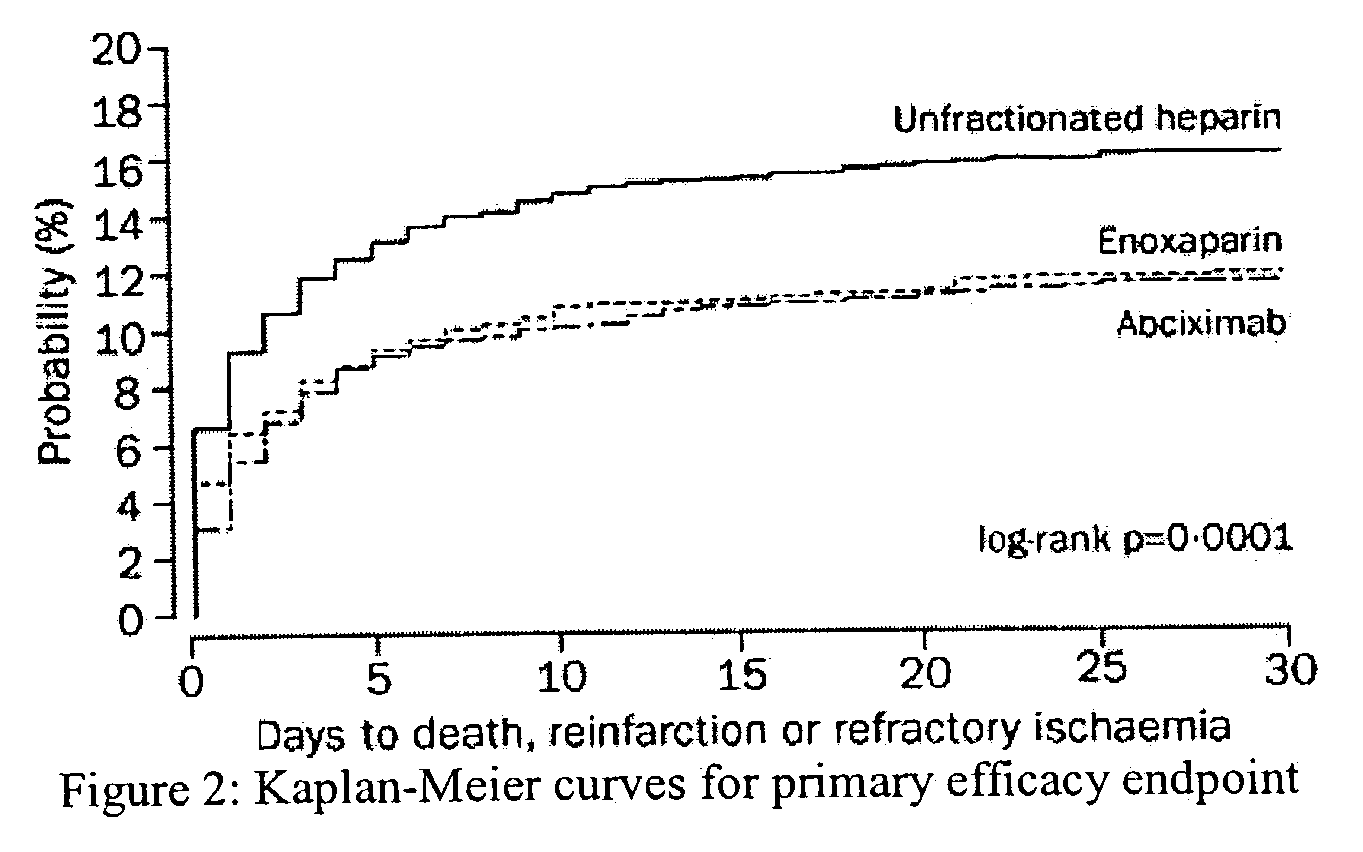
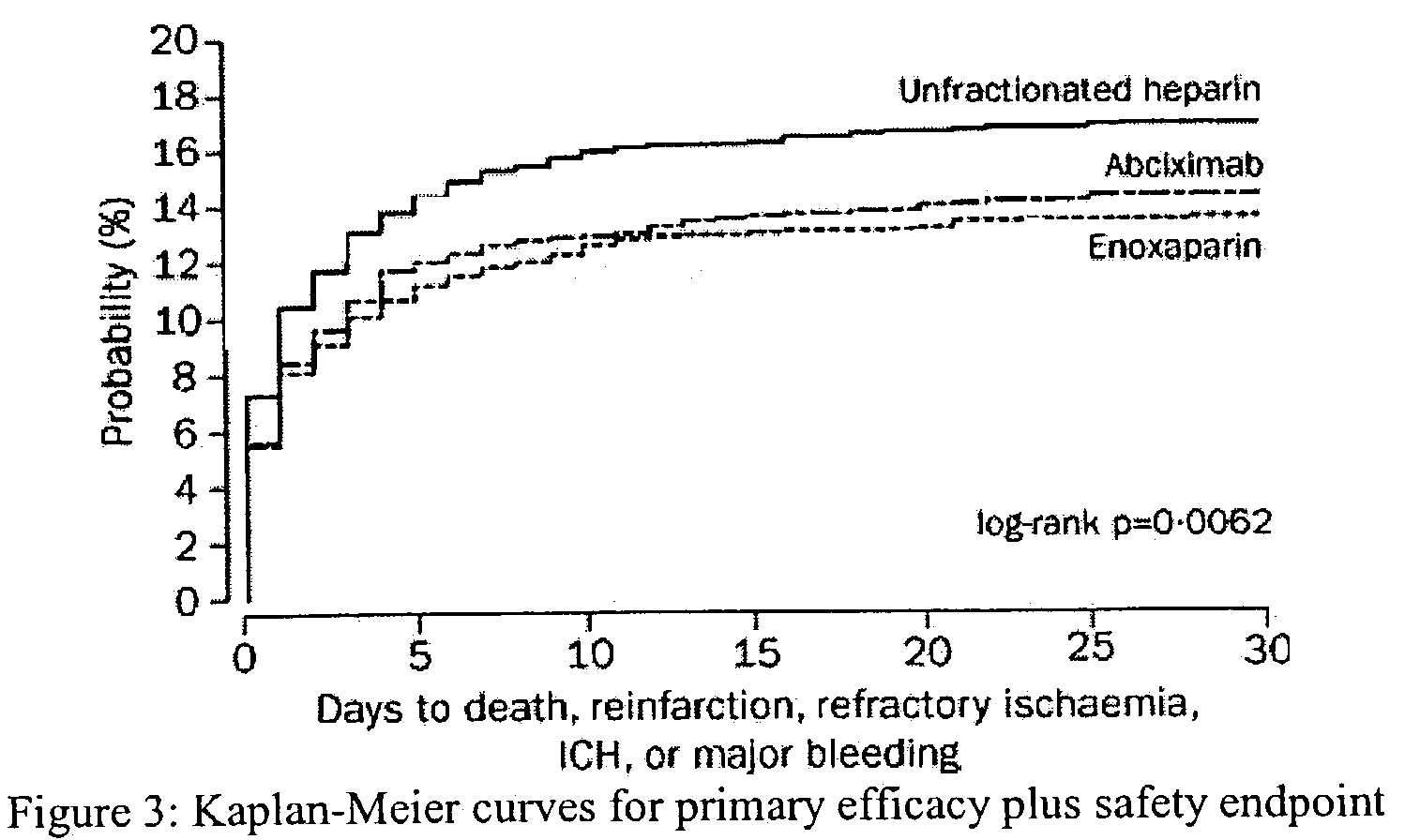
The invention concerns an improved therapeutic regimen for the treatment of thrombolytic disorders, such as acute myocardial infarction (AMI). In particular, the present invention concerns the treatment of thrombolytic disorders, e.g. AMI, with a combination of a tissue plasminogen activator (t-PA) variant having improved fibrin specificity and extended plasma half-life when compared with wild-type human t-PA and a low molecular weight heparin.
Owner:AVENTIS PHARMA SA (US) +2
Compositions and methods for less immunogenic protein-lipid complexes
InactiveUS20070141135A1Low titerReduce immunogenicityFactor VIIPeptide/protein ingredientsHalf-lifeTherapeutic protein
The present invention provides compositions and methods for reducing the immunogenicity and increasing the circulating half-life of therapeutic proteins such as Factor VIII. The compositions comprise lipidic structures such as liposomes, micelles and cochleates comprising a negatively charged lipid and polyethylene glycol derivatized phospatidyl ethanolamine.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Soluble epidermal growth factor receptor-like proteins and their uses in cancer detection methods
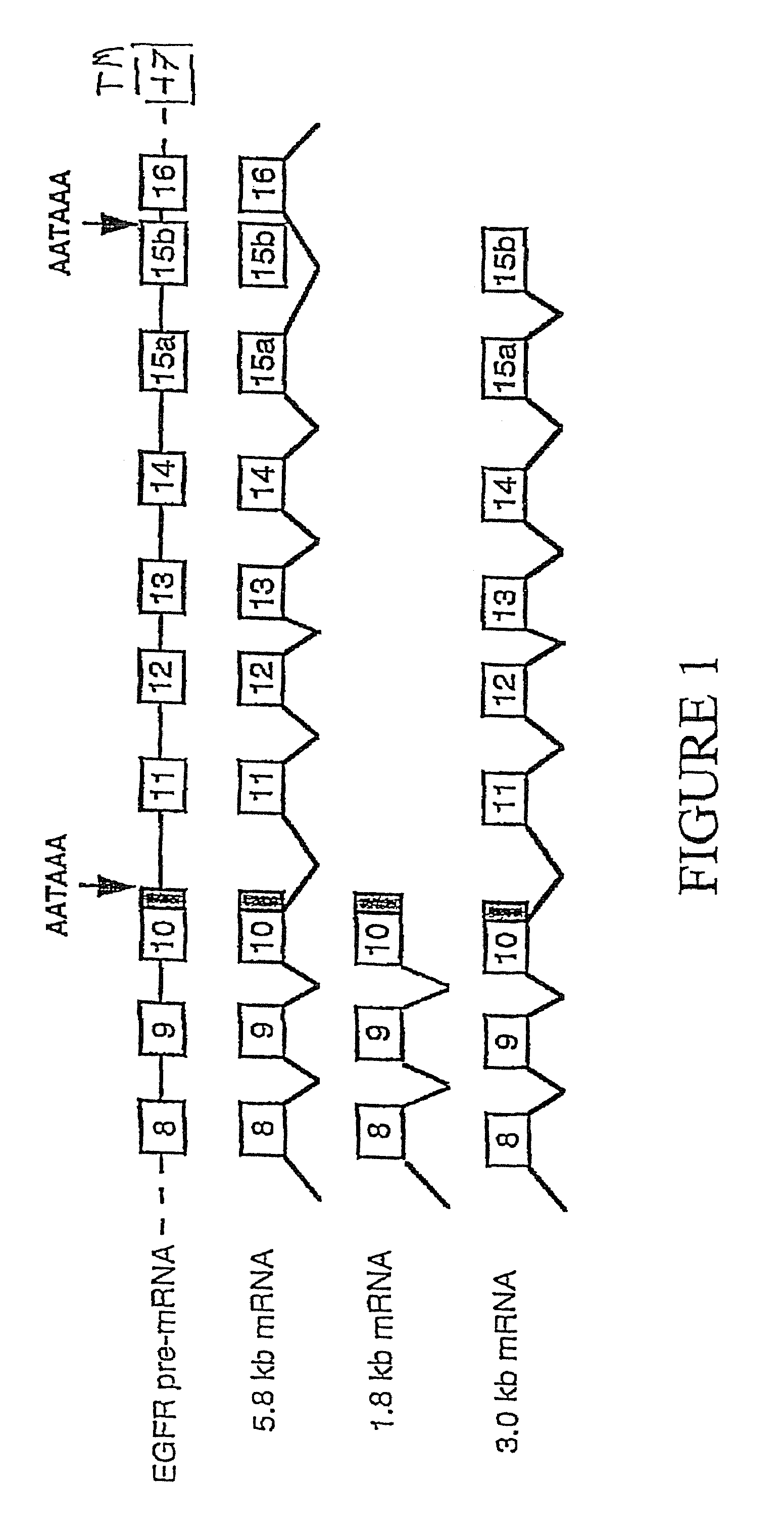
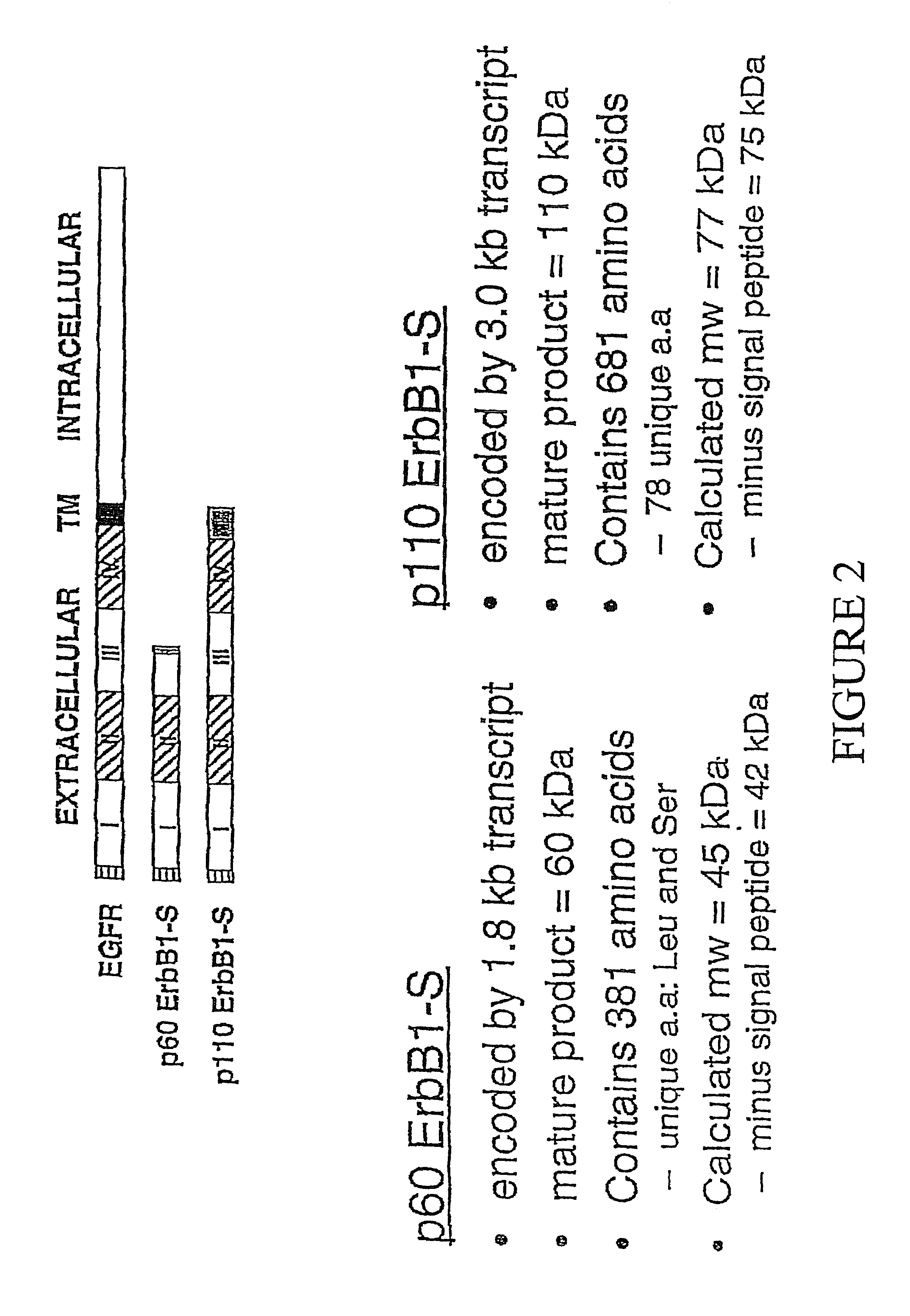
InactiveUS7732157B1Increase decrease half-lifeInhibiting ligand degradationBiological material analysisBiological testingGene productHuman epidermal growth factor receptor
The present invention relates to the discovery of soluble isoforms of an Epidermal Growth Factor Receptor, or sErbB 1 / HER1 variants, the provision of the sequences of nucleic acids encoding these isoforms, purified recombinant proteins, novel antibodies specific for these isoforms, and the use of immunoassay and gene expression assay techniques to measure the concentration of these gene products in a patient biological sample. The present invention also provides methods for determining the presence of an ovarian carcinoma in the patient by assaying the concentration of soluble EGFR / ErbB1 variants in a biological sample from a patient.
Owner:TBIG
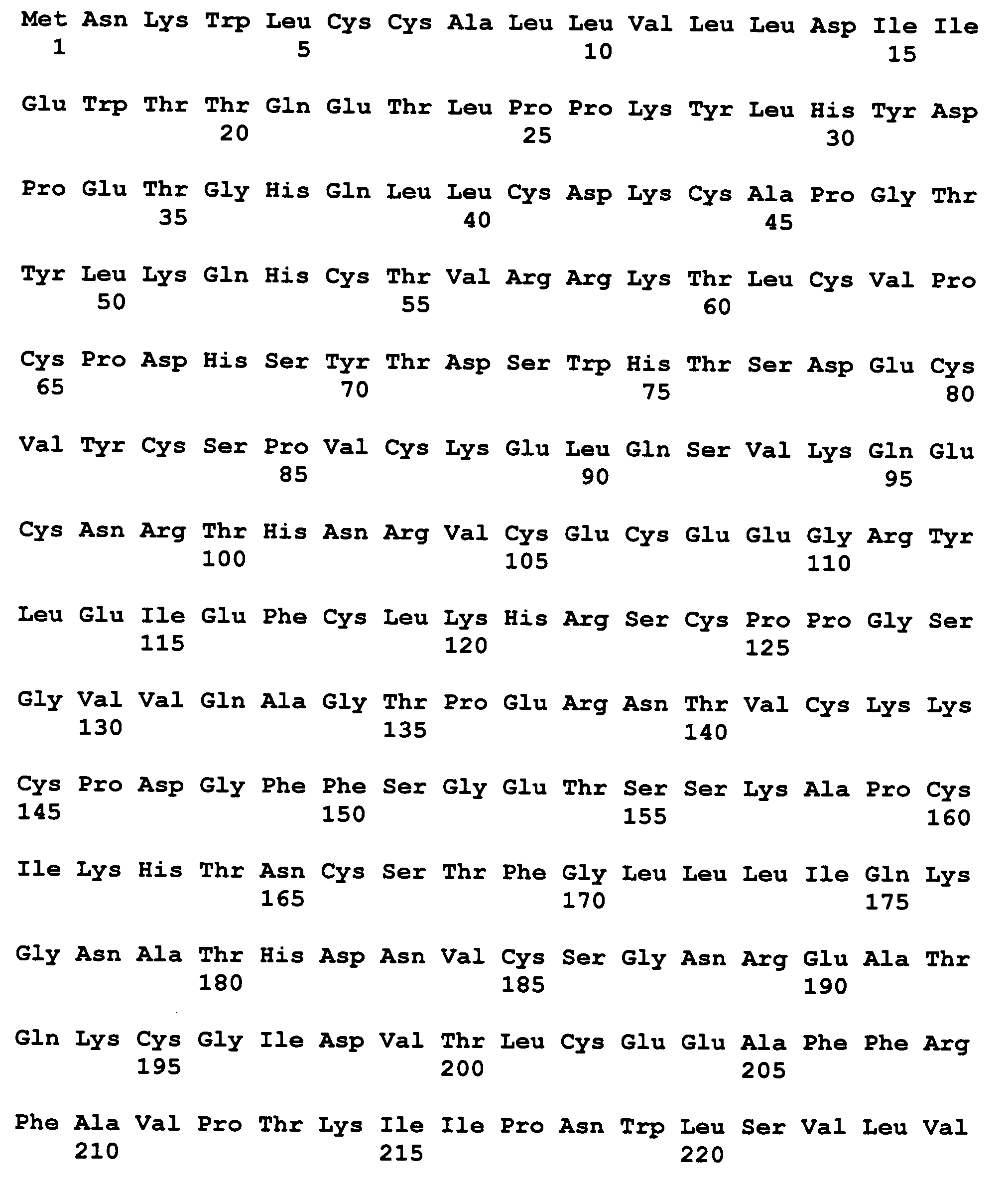
N-glycosylated human growth hormone with prolonged circulatory half-life
InactiveCN102131825AProlonged Circulatory Half-LifePeptide/protein ingredientsFusion with post-translational modification motifDiseaseSomatotropic hormone
The present invention relates to novel human growth hormone (h GH) variant(s) with one or more N-glycans. The hGH variants of the invention comprises an amino acid sequence which includes at least one N-glycosylation motif (N-X-S / T) arising from one or more mutations not present in the wild type hGH. The h GH variants of the invention have a prolonged circulatory half-life and thus can be effectively used as a protein therapeutic for disease states that will benefit from increased levels of h GH. The process of obtaining the hGH variants is also encompassed by the invention.
Owner:NOVO NORDISK HEALTH CARE AG
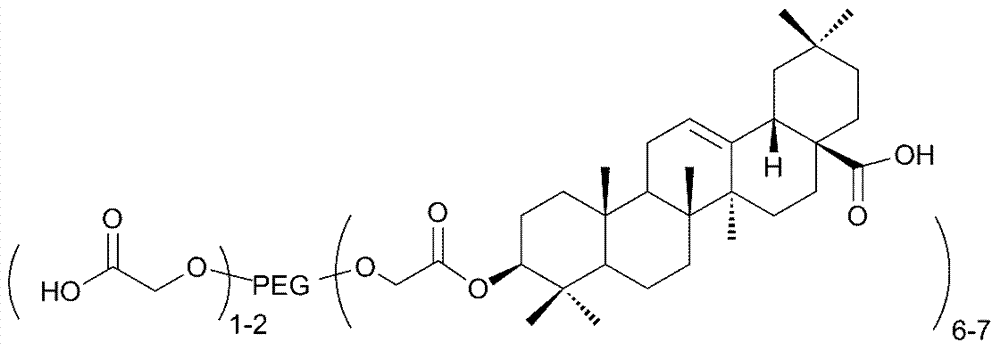
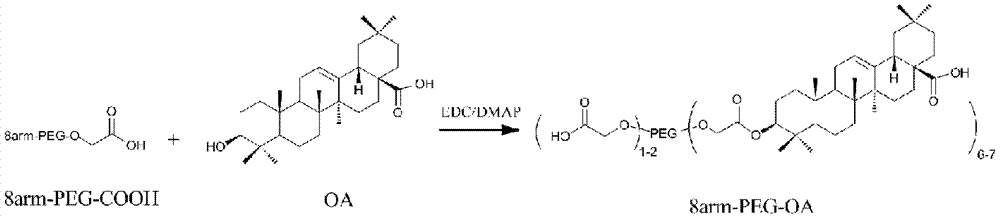
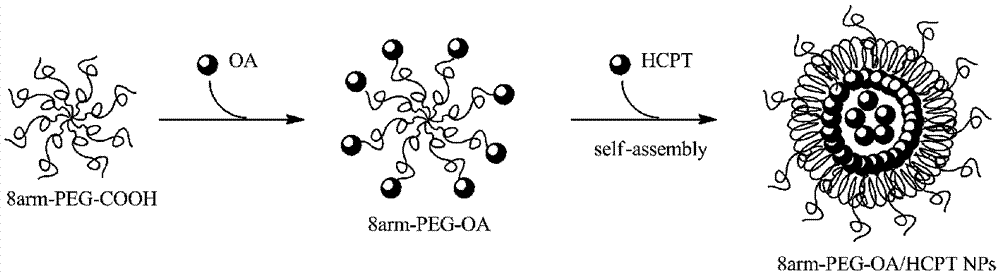
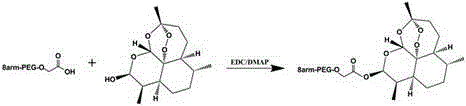
Nanoparticles of eight-arm PEG (polyethylene glycol)-oleanolic acid drug carrier and preparation
InactiveCN107115323AGood water solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention discloses a preparation method of nanoparticles of an eight-arm PEG (polyethylene glycol)-oleanolic acid drug carrier. The preparation method comprises steps as follows: eight-arm PEG and oleanolic acid are subjected to an esterification reaction, and a conjugate of eight-arm PEG-oleanolic acid is obtained; 10-hydroxycamptothecine is wrapped with eight-arm PEG-oleanolic acid through self-assembly, and the nanoparticles are obtained. The nanoparticles have a double-layer structure, the outer layer is hydrophilic PEG, and the inner layer is hydrophobic oleanolic acid drug and 10-hydroxycamptothecine. The preparation method has the advantages that the drug loading capacity is greatly increased with the adoption of PEG; the water solubility and stability of oleanolic acid are increased, and the half-life period of oleanolic acid is prolonged; pH sensitive release of the drug in tumor cells can be realized; the toxic and side effects on normal tissue are reduced; the preparation process is simple and easy to operate.
Owner:BEIJING FORESTRY UNIVERSITY
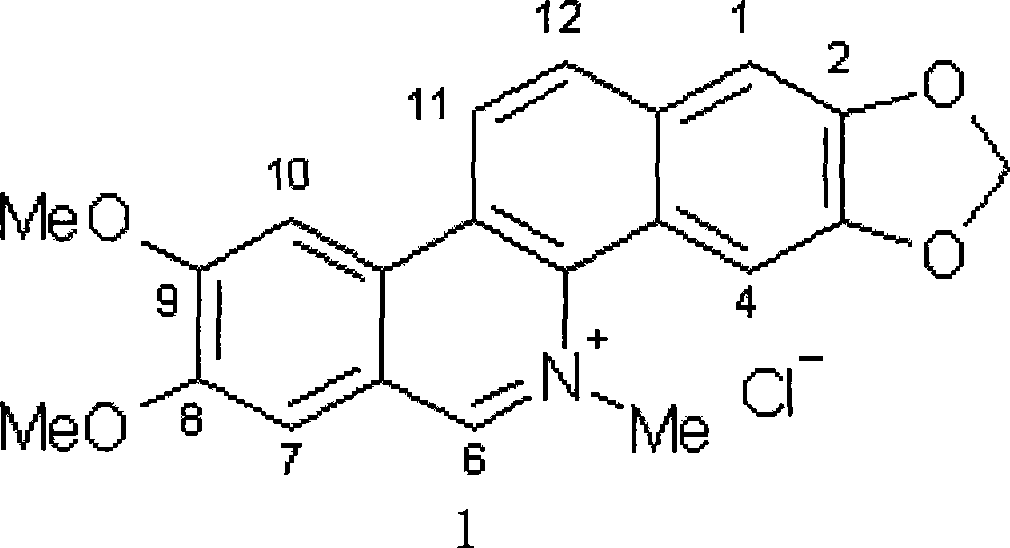
Preparation method of targeting antineoplastic medicine nitidine chloride complexes, product thereof and injection containing the product
InactiveCN101474183AImprove solubilityImprove hydrophilicityPowder deliveryOrganic active ingredientsSolubilityCarbon nanotube
The invention discloses a method for preparing target antineoplastic drug chlorinated nitidine compound, products thereof and an injection containing the products. The method for preparing the target antineoplastic drug chlorinated nitidine compound comprises the steps of pre-treating the chlorinated nitidine to acquire carboxylic acid-modified chlorinated nitidine, then using amphiphilic polymers to implement non-covalent functionalized modification on the outer wall of a single-wall carbon nano tube that is cut to be 20-500 nm long, and coupling the carboxylic acid-modified chlorinated nitidine with the single-wall carbon nano tube after the non-covalent functionalized modification by means of esterification and amidation reactions to acquire the compound. The preparation method of the compound is simple, the solubility of the resulting products can reach 5.0mg / mL, and the antineoplastic target is strong, thus favorably settling the problems of low solubility and strong toxicity of the chlorinated nitidine; in addition the invention can be applied clinically as a substitute for the chlorinated nitidine and provides a new water-soluble compound for the clinical trial of the chlorinated nitidine.
Owner:刘华钢 +1
Formation of novel erythropoietin conjugates using transglutaminase
InactiveUS6995245B2Increase circulation half-lifeImproved erythropoietic potencyPeptide/protein ingredientsPeptide sourcesHalf-lifeOrganic molecules
The invention provides biologically active erythropoietin (EPO) conjugate compositions wherein a transglutaminase reaction is employed to covalently and site specifically conjugate the EPO molecule to a non-antigenic hydrophilic polymer that can also be covalently linked to an organic molecule either of which modification increases the circulating serum half-life of the composition.
Owner:CENTOCOR
Chlorotoxin variants, conjugates, and methods for their use
ActiveUS9944683B2Prolonged Circulatory Half-LifeExtended half-lifeSaccharide peptide ingredientsImmunoglobulinsChemistry
Chlorotoxin variants, chlorotoxin variant conjugates, compositions that include the chlorotoxin variants or conjugates, and methods for using the chlorotoxin variants, conjugates, and compositions.
Owner:FRED HUTCHINSON CANCER CENT
Chimeric IL-10
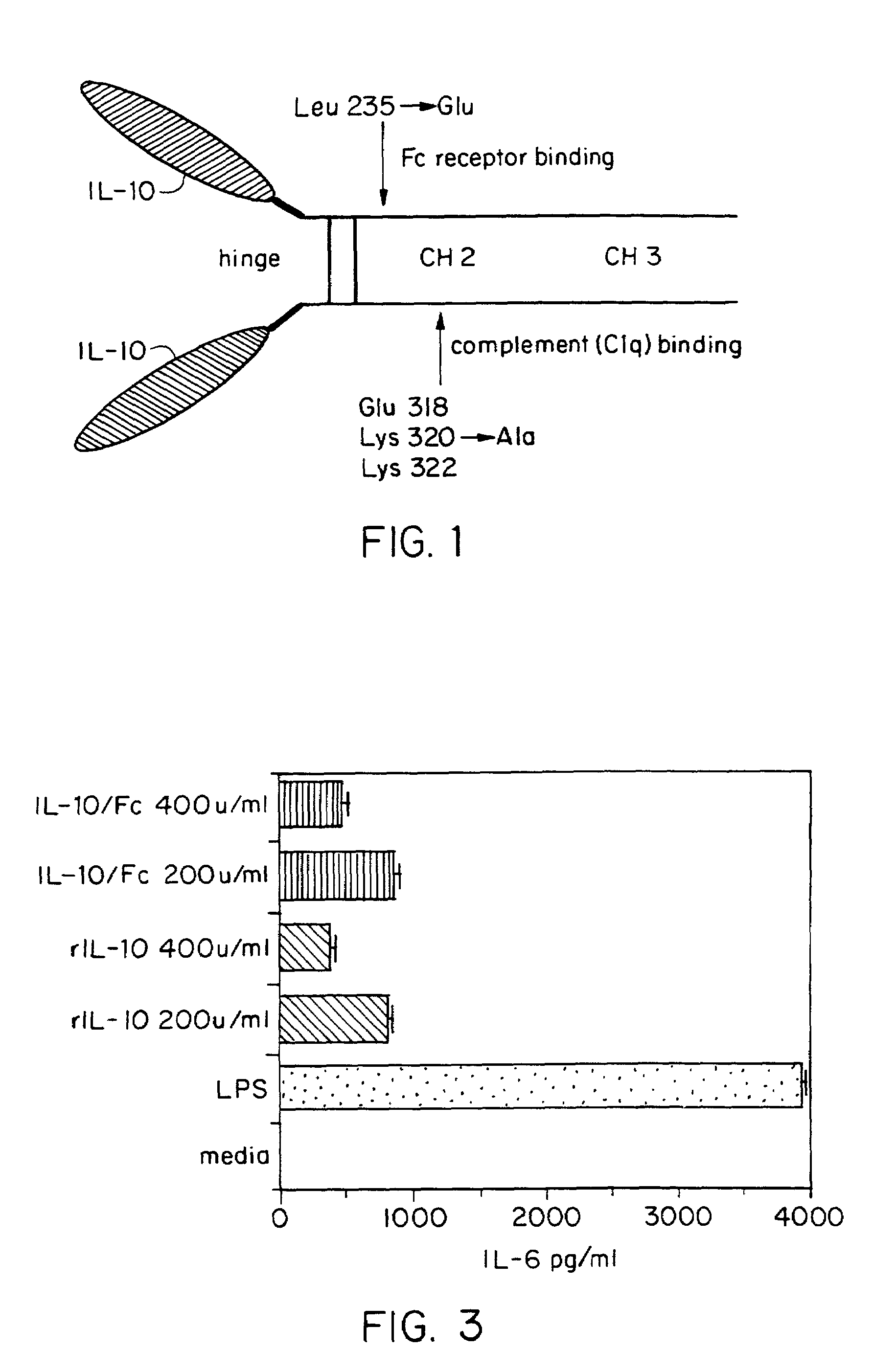
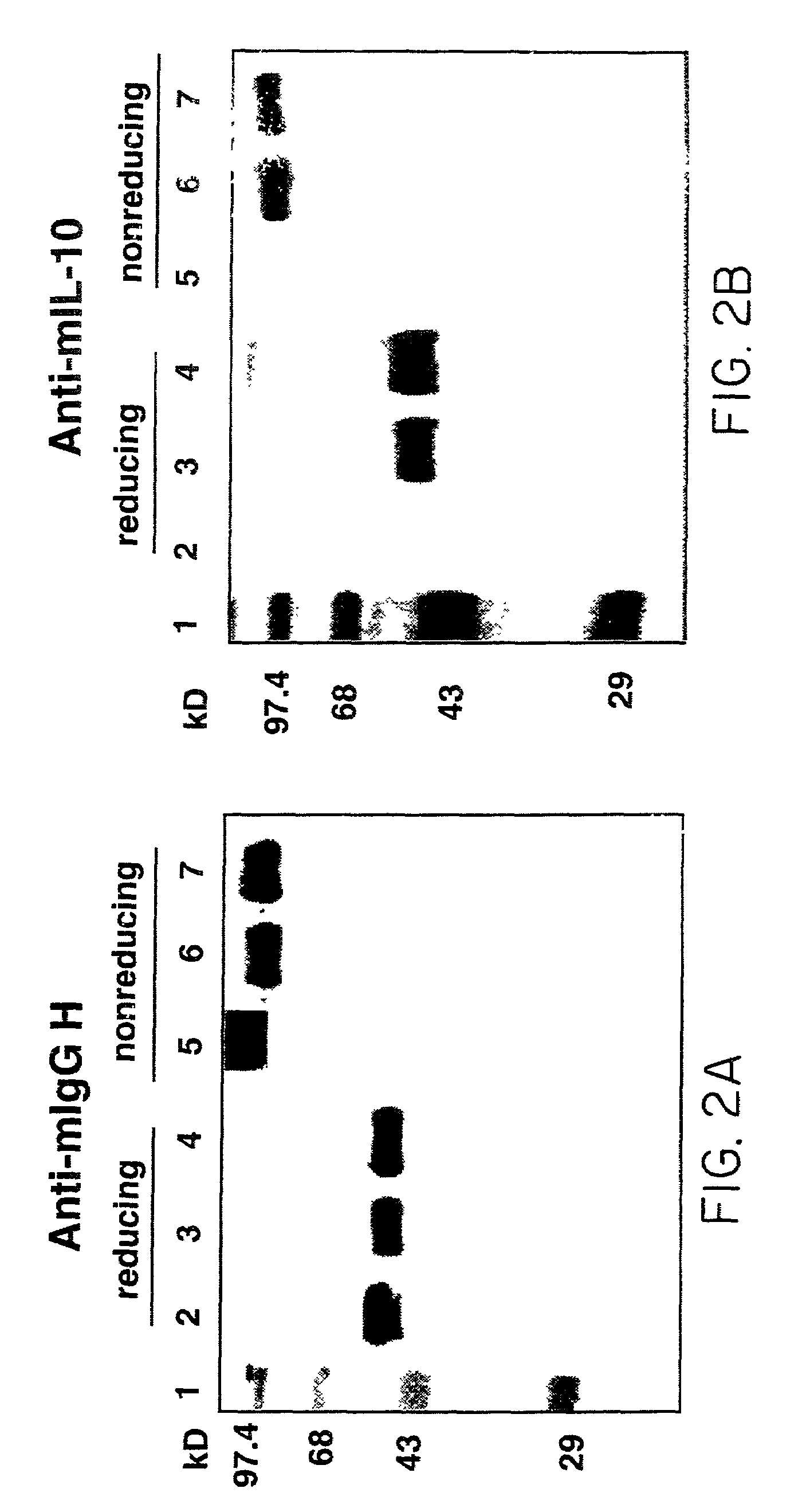
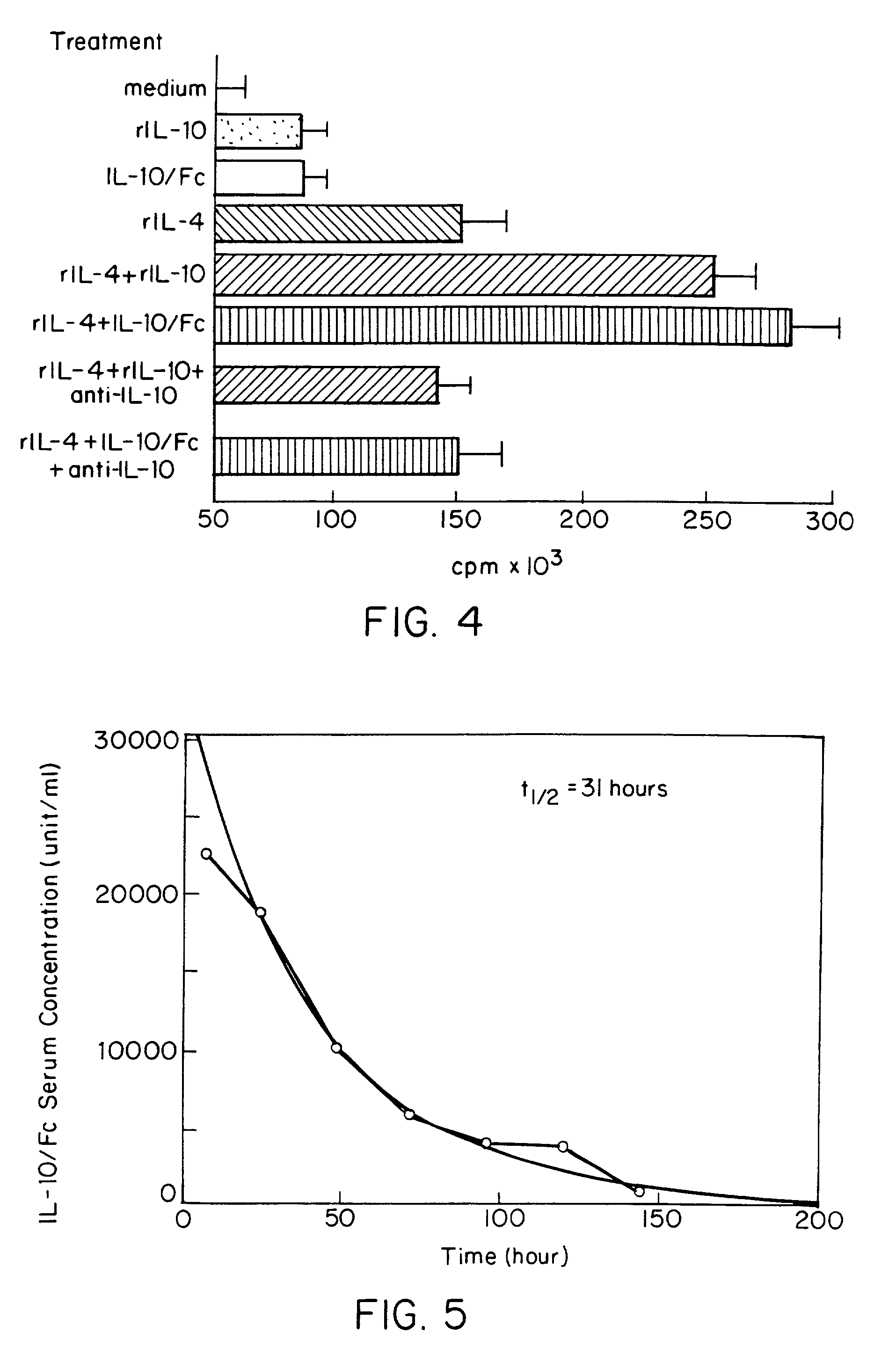
InactiveUS7018626B2Prolonged Circulatory Half-LifeLong-term protectionPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeMedicine
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
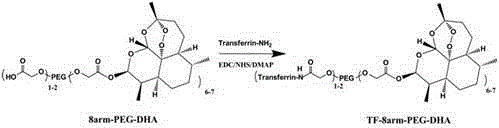
Self-assembled targeting drug carrier nanoparticle and preparation method thereof
InactiveCN105879050AProlonged Circulatory Half-LifeHigh drug loadingPowder deliveryOrganic active ingredientsTarget–actionFreeze-drying
The invention discloses a method for preparing self-assembled targeting nano drug carrier particles, comprising: esterifying eight-armed carboxypolyethylene glycol with dihydroartemisinin to obtain eight-armed polyethylene glycol-dihydroartemisinin The eight-arm polyethylene glycol-dihydroartemisinin conjugate is activated by NHS; the activated conjugate is further chemically linked to the targeting molecule transferrin by using an amide bond; the eight-arm modified transferrin The polyethylene glycol-dihydroartemisinin conjugate removes impurities by dialysis, is filtered and freeze-dried; self-assembles to obtain transferrin-modified nano drug carrier particles. The nanoparticle has a double-layer structure, the outer layer is hydrophilic polyethylene glycol, and the inner layer is hydrophobic small molecule drug dihydroartemisinin. The advantages of the present invention: the use of eight-arm carboxypolyethylene glycol greatly increases the drug loading; the targeting effect of drugs on tumor cells and the pH-sensitive release in tumor cells can be realized; the targeted therapy reduces the toxic and side effects on normal tissues ; The preparation process is simple and easy to operate.
Owner:BEIJING FORESTRY UNIVERSITY
Antibodies against h5n1 strains of influenza a virus
InactiveUS20100278834A1Easy to useEasy transferSugar derivativesImmunoglobulins against virusesAntibodyHeavy chain
Provided are human antibodies that can neutralize a H5N1 strain of influenza A virus. Also provided are antibodies that can neutralize a strain of influenza A virus in clade 2 of the H5 subtype, that can neutralize a H5N1 strain of influenza A virus and have a lambda light chain, and that are IgG antibodies (but not with a IgG1 heavy chain) that can neutralize a H5N1 strain of influenza A virus.
Owner:HUMABS LLC
Modified IL-2 variants that selectively activate regulatory T cells for the treatment of autoimmune diseases
ActiveCN107106654AProlonged Circulatory Half-LifePeptide/protein ingredientsDepsipeptidesRegulatory T cellHalf-life
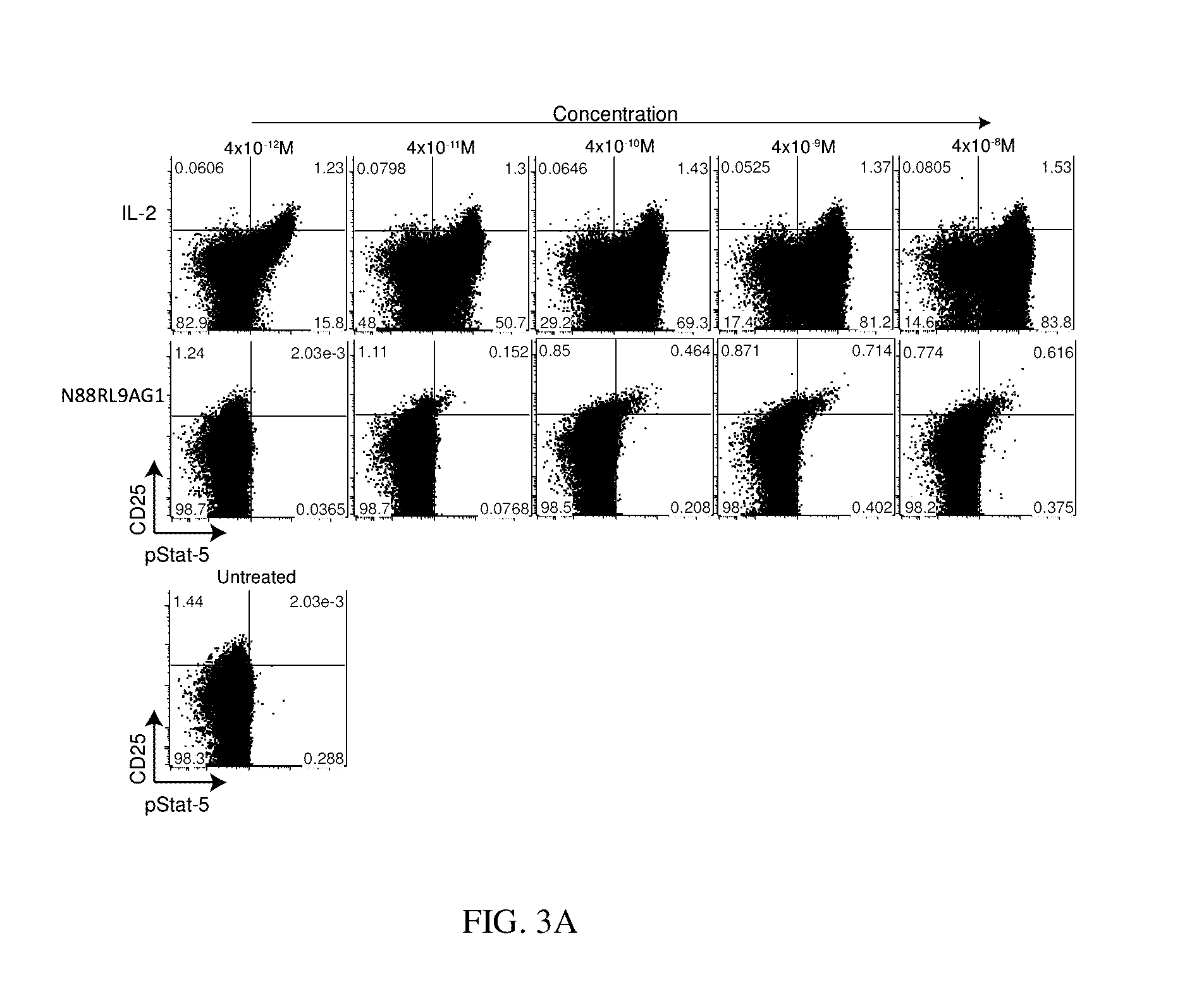
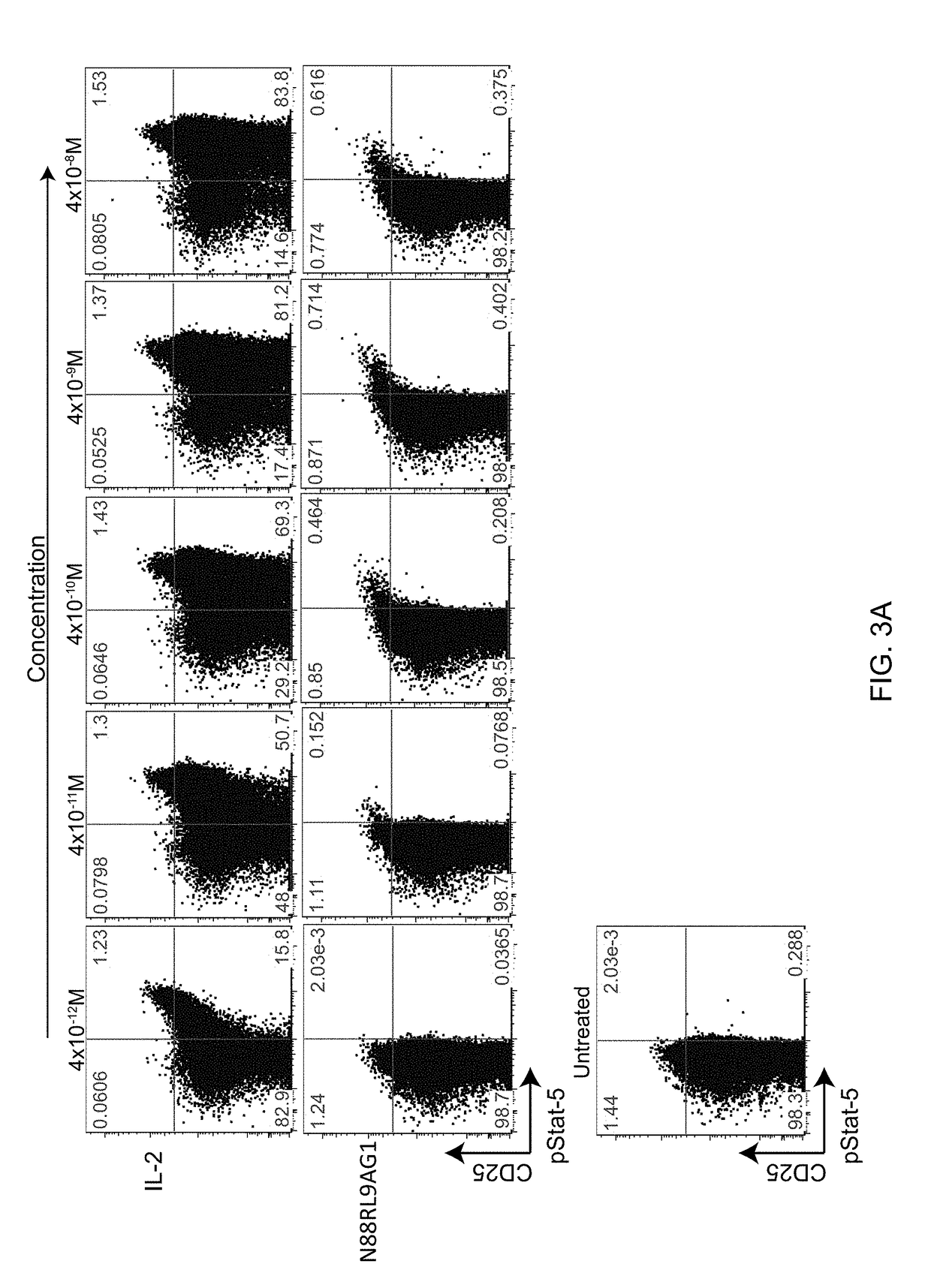
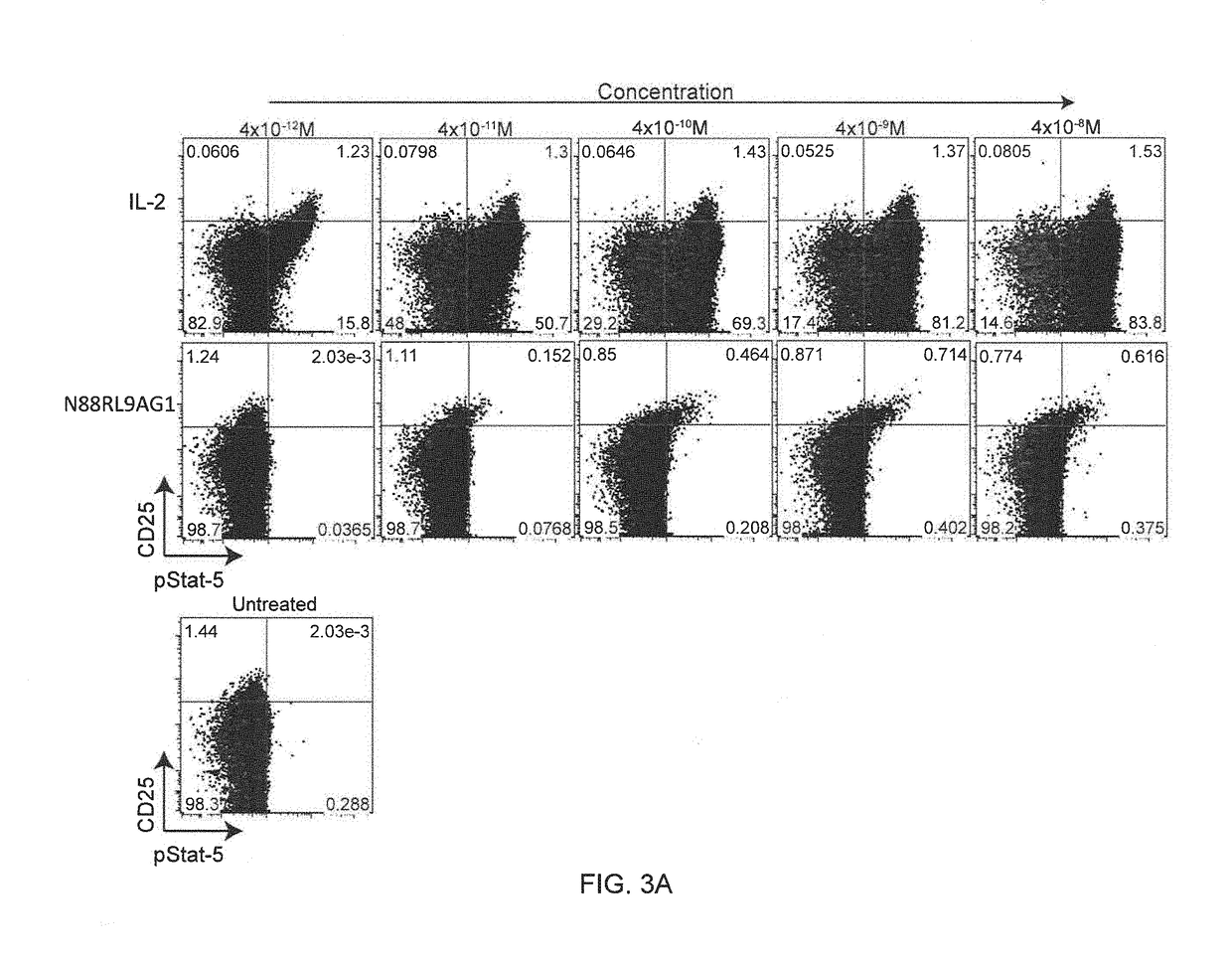
The invention described herein is a novel IL-2 protein with selective agonist activity for Regulatory T cells and with an additional amino acid substitution that enable chemical conjugation with Polyethyene Glycol (PEG) that increase circulating half-life compared to the IL-2 selective agonist alone. A preferred IL-2 selective agonist variant is IL2 / N88R / C125S / D109C.
Owner:DELINIA
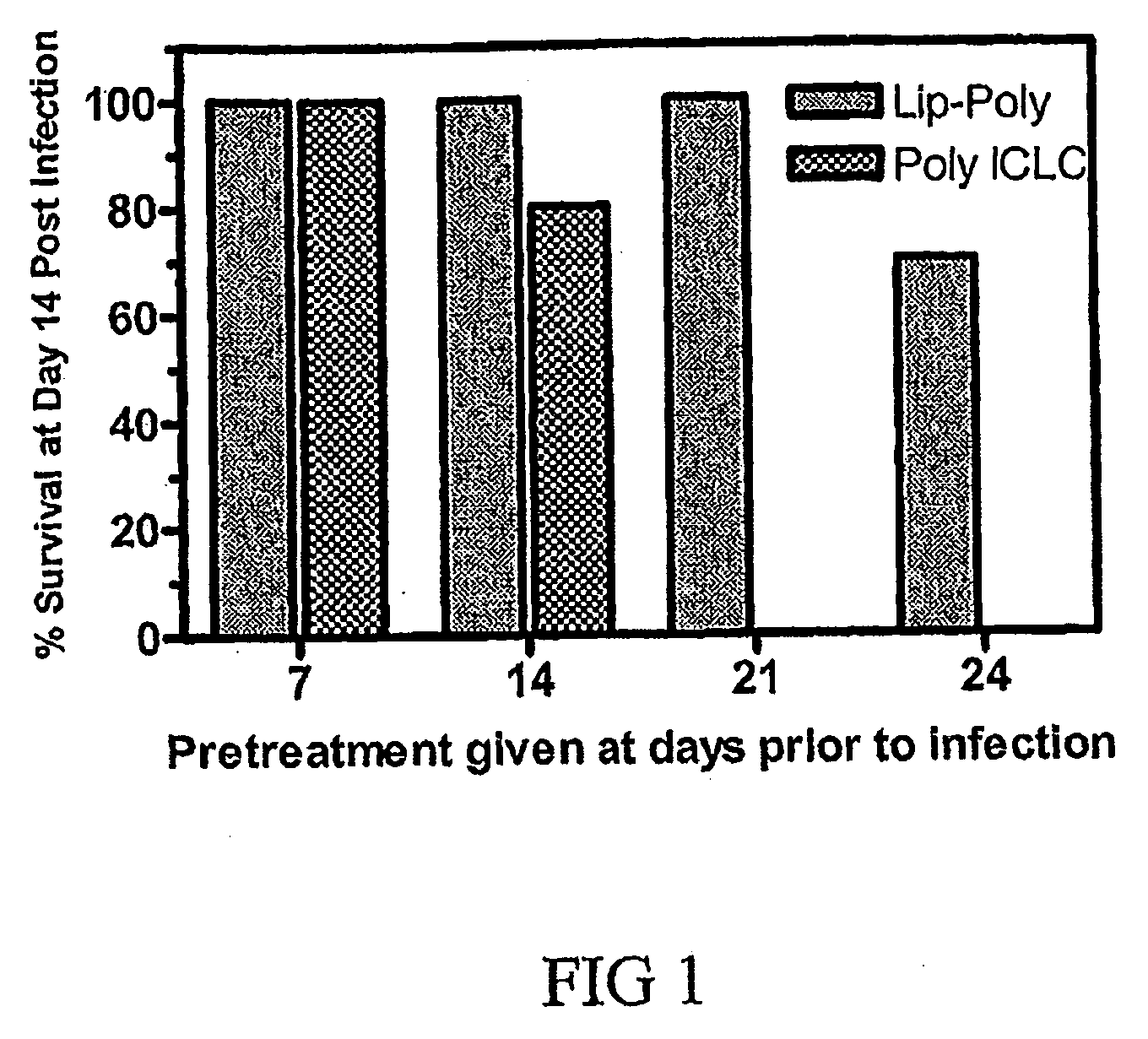
Liposome encapsulated poly-iclc method to prophylactically treat an avian influenza viral infection
InactiveUS20090214638A1Enhancing immunologicalImprove biological activityOrganic active ingredientsAntiviralsInfections siteProphylactic treatment
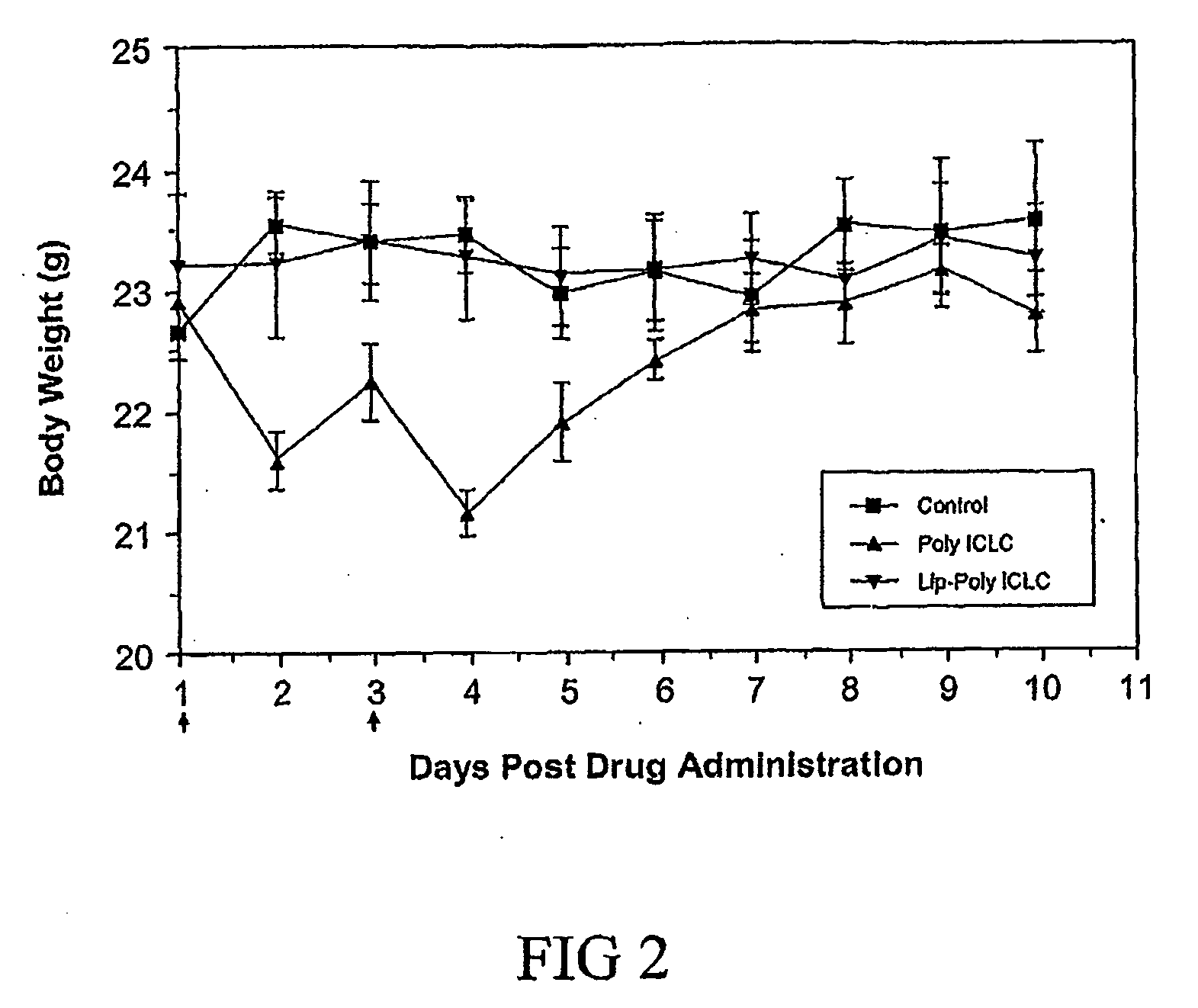
A method of treating an avian influenza viral infection using a poly ICLC formulation with improved therapeutic efficacy is disclosed. The poly ICLC is encapsulated within liposomes which provides a drug delivery system with slow sustained release characteristic and which has the ability to target the drug to sites of viral infection without causing systemic burden to normal tissues, thereby enhancing the immunological and biological activities of poly ICLC. This poly ICLC formulation has been found to be particularly effective in treating an avian influenza viral infection and in particular, an avian H5N1 influenza viral infection.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
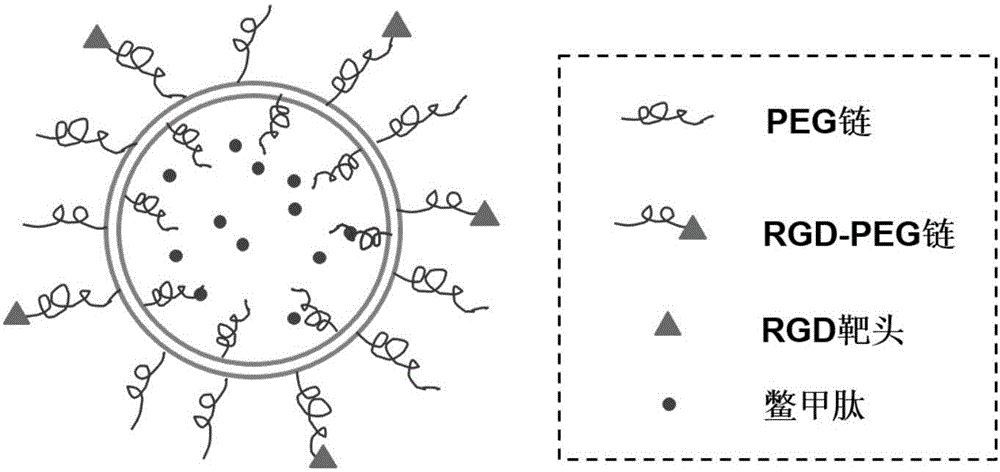
RGD-SSL lipidosome for packaging turtle shell peptide and preparation method of lipidosome
InactiveCN106310220AOvercome degradabilityOvercome the problems of low bioavailability and short half-lifePeptide/protein ingredientsDigestive systemDiseaseHalf-life
The invention discloses an RGD-SSL lipidosome for packaging turtle shell peptide and a preparation method of the lipidosome. The turtle shell peptide long-cycle lipidosome modified by RGD comprises a lipidosome body and the turtle shell peptide (HGRFG) in the lipidosome body. The lipidosome body is prepared from egg yolk lecithin (EPC), cholesterol (Chol), mPEG2000-DSPE and RGD-PEG2000-DSPE according to the molar ratio of 50:25:1:0.6, and the weight ratio of the turtle shell peptide (HGRFG) to the EPC is 1:5 to 1:30. The defects that turtle shell peptide and other oligopeptides can easily degrade when orally taken and are low in bioavailability and short in half-life period are overcome; the RGD modified turtle shell peptide long-cycle lipidosome (RGD-SSL-turtle shell peptide) is obtained by packaging the turtle shell peptide with the granularity and RGD modified dual-control hepatic targeting lipidosome, the local biological effect and cycle half-life period are improved, the dosage frequency is reduced, and the lipidosome is suitable for treatment of hepatic fibrosis and other hepatic diseases.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
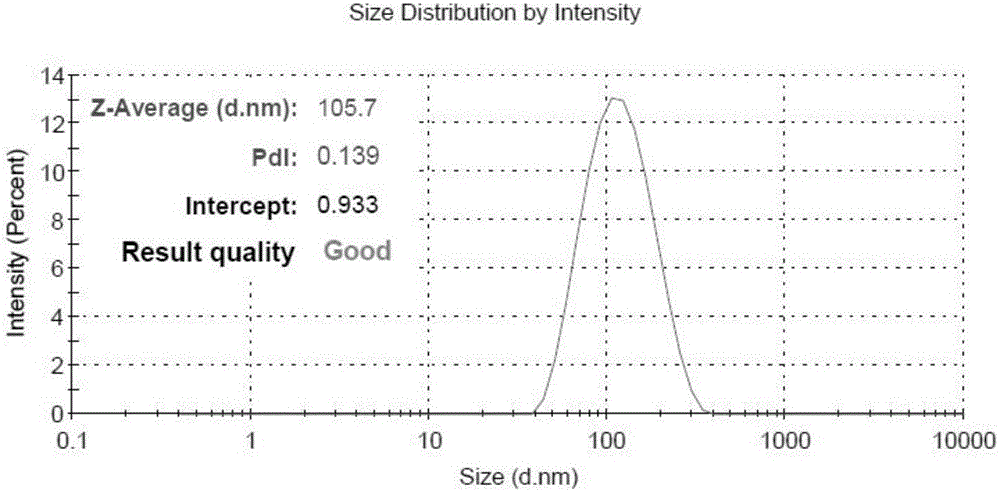
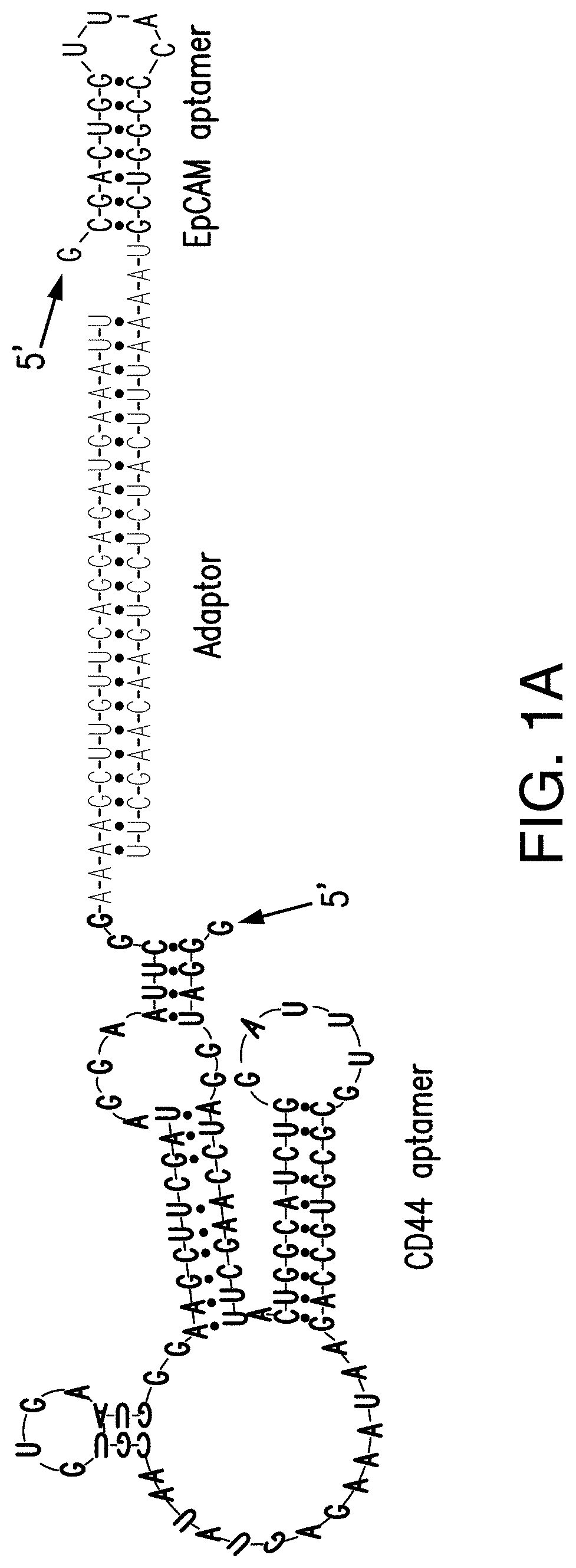
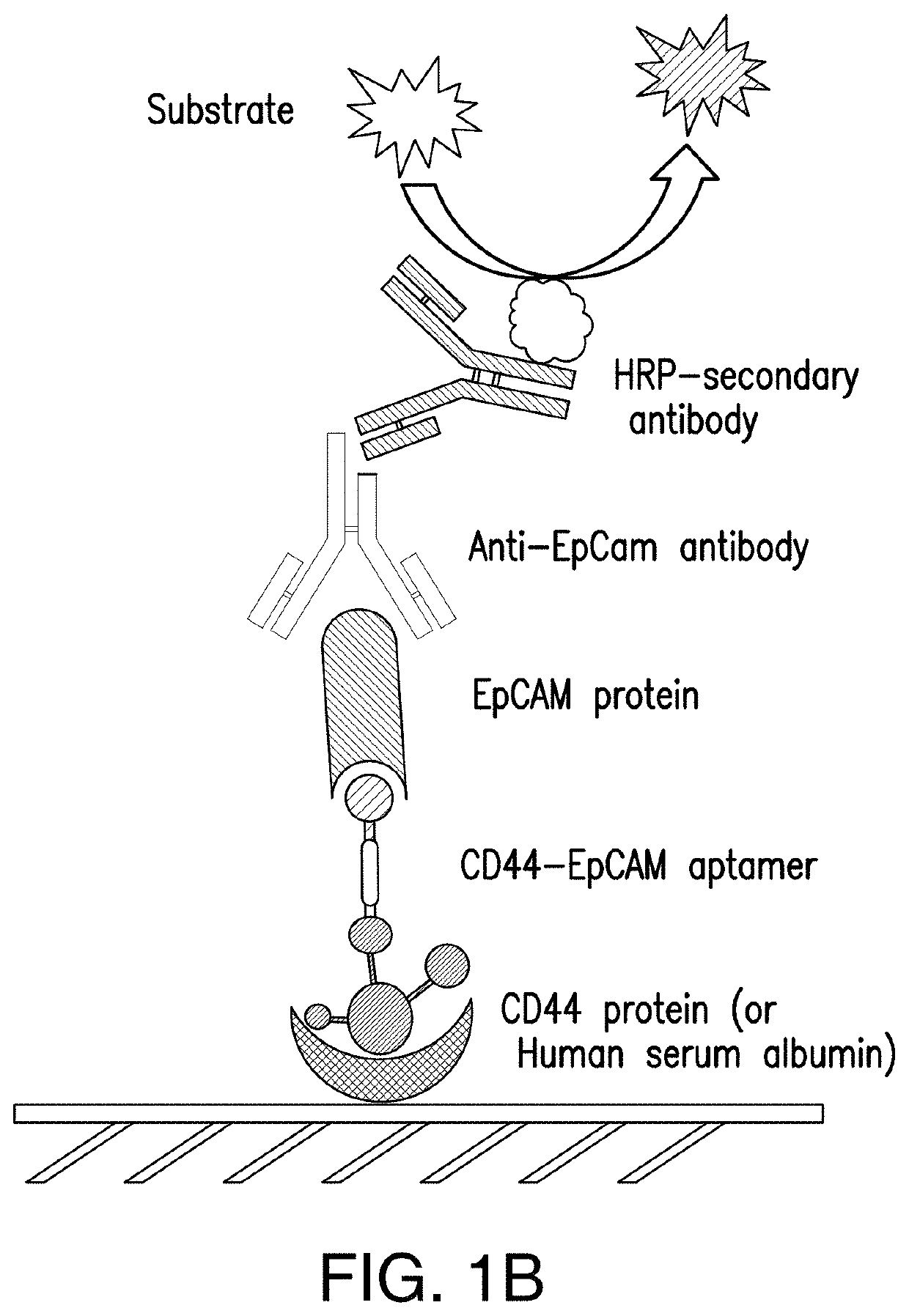
Bispecific Aptamer for Treating Cancer
ActiveUS20200157542A1Prolonged Circulatory Half-LifeReduced renal filtrationGenetic material ingredientsAntineoplastic agentsAptamerVirus Protein
Bispecific aptamers having a first end that specifically binds to a first tumor specific marker, tumor antigen, or viral protein and a second end that specifically binds to a second tumor specific marker, tumor antigen, or viral protein are provide. The bispecific aptamers can be used to treat cancer or virally infected cells. Generally, the bispecific aptamers bind to two surface proteins, preferably different proteins, on the same cell. In a preferred embodiment the bispecific aptamers bind to two different tumor markers, tumor antigens, tumor specific proteins and combinations thereof.
Owner:AUGUSTA UNIV RES INST INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com