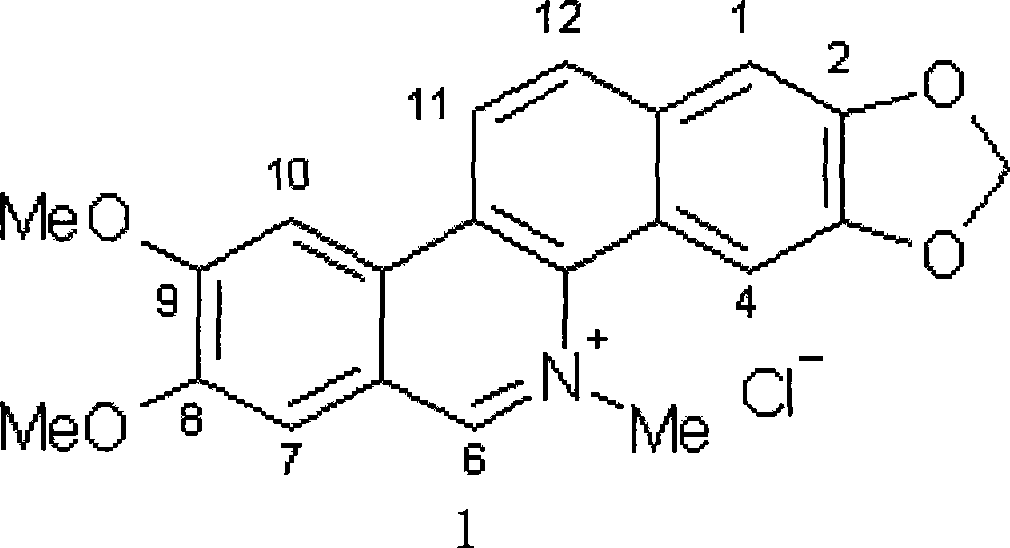
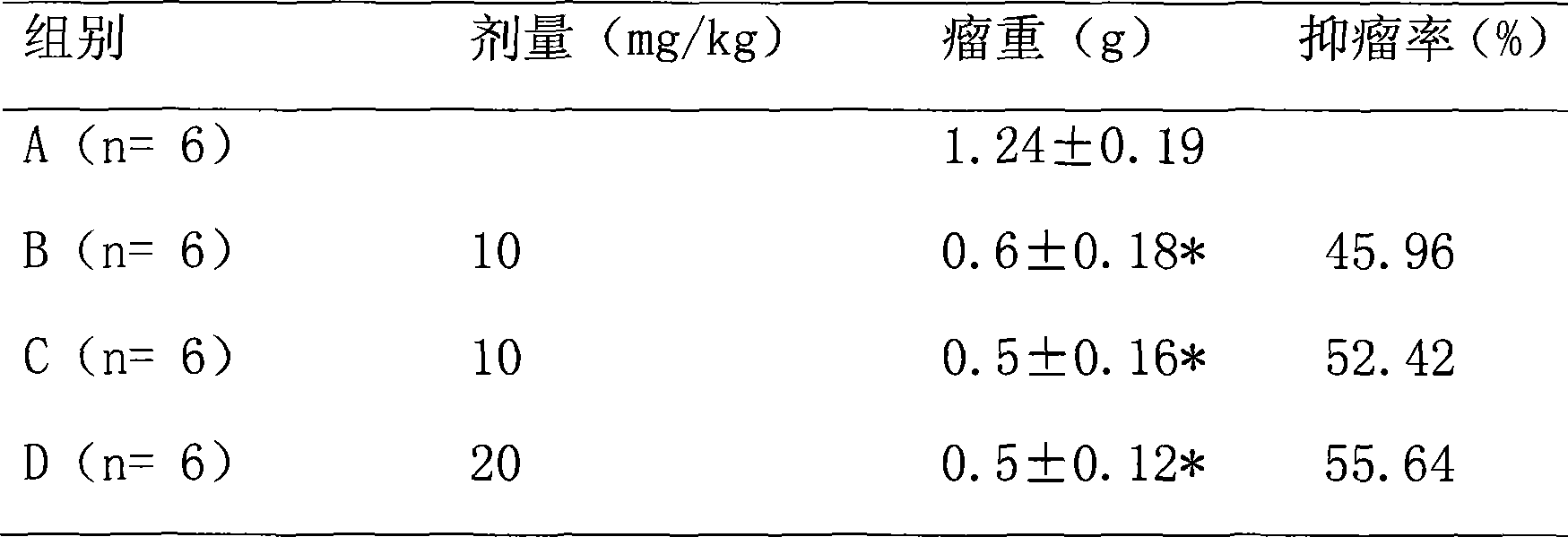
Preparation method of targeting antineoplastic medicine nitidine chloride complexes, product thereof and injection containing the product
A technique for chlorinated amploxacine and antitumor drug, which is applied to the preparation of water-soluble compounds and the preparation of targeted antitumor drug chloramphenicol compounds, and can solve the problems of poor water solubility, large toxic and side effects, and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1: Preparation of the targeted antineoplastic drug Echidine chloride complex.
[0059] (1) Pretreatment of chlorinated dimethicone:
[0060] Under the protection of nitrogen, accurately weigh the dihedral chloride and add it to an appropriate amount of concentrated hydroiodic acid, so that the concentration of the dihedral chloride is 0.001mol / L, heat up to 50°C while stirring; cool to 50°C after reacting for 6 hours At room temperature, adjust the pH of the mixed solution to about 10 with ammonia water, repeatedly extract the mixed solution three times with dichloromethane, evaporate the solvent in the water phase to dryness, dissolve the residue with methanol, add hydrochloric acid to adjust the pH to 2.5, and precipitate needle-shaped crystals after standing , which is the dihedral chloride crystal in which the methoxy group at the C-8 and / or C-9 position is replaced by a hydroxyl group.
[0061] Accurately weigh the needle-like crystals and malonic acid (1:1 molar ...
Embodiment 2
[0072] 1: Preparation of the targeted antineoplastic drug Echidine chloride complex.
[0073] Repeat the preparation method of Example 1 targeted antineoplastic chlorinated chlorinated compound, the difference is, with distearoylglycerol phosphatidylethanolamine-polyethylene glycol amine (DSPE-PEG-NH 2 , 10kDa) instead of biotin polyethylene glycol amine (Biotin-PEG-NH 2 , molecular weight is 5kDa), with 4-arm polyethylene glycol amino group (4-Arm-PEG-NH 2 , 5kDa) to replace the six-arm polyethylene glycol amino group (6-Arm-PEG-NH 2 , 10kDa).
[0074] 2: Preparation of the targeted antineoplastic drug Ephedrine Chloride Complex Injection.
[0075] Take 16.3g of polyether F-68 and dissolve it in 800mL of water for injection to obtain a 2% aqueous solution of polyether F-68 for subsequent use; accurately weigh 2.0g of the targeted antineoplastic drug chlorine Put the water-soluble compound of limpet base and single-walled carbon nanotubes in a 500mL volumetric flask, prepa...
Embodiment 3
[0079] 1: Preparation of the targeted antineoplastic drug Echidine chloride complex.
[0080] Repeat the preparation method of the targeted antineoplastic drug Echinacea chloride complex in Example 1, the difference is that adipic acid is used instead of malonic acid.
[0081] Two: Preparation of the targeted antineoplastic drug Echidine chloride complex powder injection.
[0082] Take the water-soluble complex (0.1%) of the targeted antineoplastic drug dihedral chloride and single-walled carbon nanotubes (0.1%), egg yolk lecithin (50%), cholesterol (20%) and polyether F-68 (5%) was dissolved in an appropriate amount of ethyl acetate, and the solvent was recovered under reduced pressure at 40°C with a rotary evaporator, and a lipid film was formed on the bottle wall; then an appropriate amount of aqueous solution with a pH of 6.5 was added to dissolve the lipid Membrane elution is dispersed in the aqueous solution, and the liposome solution of gained is supersonicated 30min u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com