Self-assembled targeting drug carrier nanoparticle and preparation method thereof
A nano-drug carrier and nano-particle technology, which is applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of large adverse reactions, poor selectivity of anti-cancer drugs, and treatment of toxic reactions, and achieve improved stability and water solubility , prolong circulation half-life, reduce toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
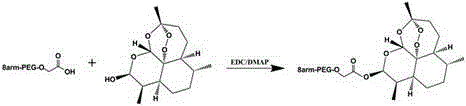
[0025] Synthesis of eight-armed polyethylene glycol (10 KDa)-dihydroartemisinin conjugate: Continuously inject N into a 50ml dry three-neck flask 2 ; at N 2 Under the protection of 0.28g dihydroartemisinin, 1.25g eight-arm carboxypolyethylene glycol (Mw 10,000) was dissolved in 15ml dichloromethane, then added 0.19g EDC, 0.12g DMAP, stirred and dissolved at 0 °C, Stir the reaction for 1 hour, then stir the reaction at room temperature for 24 hours; after the reaction is completed, remove the solvent by rotary evaporation, precipitate with 100ml glacial ether, and filter to obtain the crude product of eight-armed polyethylene glycol-dihydroartemisinin conjugate; use 20ml DMF / IPA (1:4 (v / v)) recrystallization, filter out insoluble matter, precipitate the product with 100ml ether, filter, and vacuum-dry the product to obtain an eight-arm polyethylene glycol-dihydroartemisinin conjugate with a purity of more than 95%. ;
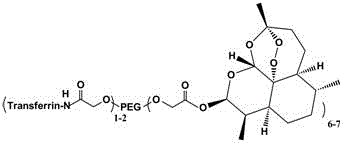
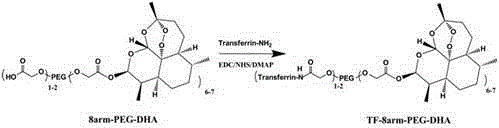
[0026] Synthesis of transferrin-eight-arm polyethylene gl...
Embodiment 2
[0029] Synthesis of eight-armed polyethylene glycol (20 KDa)-dihydroartemisinin conjugate: Continuously inject N into a 50ml dry three-neck flask 2 ; at N 2 Under the protection of 0.14g dihydroartemisinin, 1.25g eight-arm carboxypolyethylene glycol (Mw 20,000) was dissolved in 15ml tetrahydrofuran, then added 0.10g EDC, 0.06g DMAP, stirred to dissolve at 0 ° C, and stirred to react 1 hour, then stirred at room temperature for 24 hours; after the reaction was completed, the solvent was removed by rotary evaporation, 100ml of glacial ether was precipitated, and the crude product of the eight-armed polyethylene glycol-dihydroartemisinin conjugate was obtained after filtration; with 20ml DMF / IPA (1 : 4 (v / v)) recrystallization, filter out insoluble matter, the product is precipitated with 100ml ether, filters, and the product is vacuum-dried to obtain the eight-armed polyethylene glycol-dihydroartemisinin conjugate with a purity of more than 95%;
[0030] Synthesis of transferri...
Embodiment 3
[0033] Synthesis of eight-armed polyethylene glycol (40 KDa)-dihydroartemisinin conjugate: Continuously inject N into a 50ml dry three-neck flask 2 ; at N 2 Under the protection of 0.14g dihydroartemisinin, 2.50g eight-arm carboxypolyethylene glycol (Mw 40,000) was dissolved in 20ml 1,3-dioxane, then added 0.10g EDC, 0.06g DMAP, 0 ° Stir to dissolve at C, stir to react for 1 hour, then stir to react at room temperature for 24h; after the reaction is completed, the solvent is removed by rotary evaporation, 100ml of ice ether is precipitated, and the crude product of eight-armed polyethylene glycol-dihydroartemisinin conjugate is obtained after filtration; Recrystallize with 20ml DMF / IPA (1:4(v / v)), filter out the insoluble matter, precipitate the product with 100ml ether, filter, and dry the product in vacuum to obtain eight-armed polyethylene glycol-dihydro Artemisinin conjugates;
[0034]Synthesis of transferrin-8-arm polyethylene glycol (40 KDa)-dihydroartemisinin conjugat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com