Filters for infusion sets
A filter and infusion bag technology, which is applied in the field of protein therapy drug administration, can solve the problems of reducing the effective administration dose and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0010] The present invention provides a method of administering a positively charged protein therapeutic with a peripheral intravenous line comprising a 0.2 micron in-line intravenous filter, wherein the filter is a Baxter 0.2 micron high pressure extended life filter (e.g., 2C8671 and 2H5660), B. Braun Perifix (eg, 451550), Codan IVSTAR Plus 5 (eg, 76.3402), Pall Nanodyne ELD (eg, ELD96LLCE), Pall Posidyne ELD (eg, ELD96LL, ELD96LYL, and ELD96LLC), Rowe RoweFil 120 Nylon (eg, A-2356) and Terumo extension set TF-SW231H. The invention includes all code products of an infusion set having a filter identical to the disclosed filter, but other components of the infusion set (eg, tubing, valves, or needles) may differ.
[0011] The present invention provides a method of administering a positively charged protein therapeutic through a peripheral intravenous line comprising a 0.2 micron in-line intravenous filter, wherein the filter is a Baxter 0.2 micron high pressure extended life f...
example 1
[0051] Example 1: Analysis method
[0052] For the screens described in "Figure Legends", protein concentrations were determined by protein fluorescence analysis on a plate reader. In the following post-screening experiments, protein concentration was determined by Quantikine Human Relaxin-2 Immunoassay (R&D System Test Kit DRL200) (paragraphs 041, 043, and 044). Protein concentrations in the examples shown below were also determined by RP-HPLC measurements optimized by selection of suitable HPLC vials to achieve minimal adsorption loss of protein and by calibration experiments performed sequentially on samples with reference standards.
[0053] Biological activity is determined by a bioassay based on cell-based cAMP production.
[0054] The adsorption of H2 relaxin to infusion bags and infusion tubes filled with 5% dextrose or 0.9% saline was tested. Substantial absence of loss of H2 relaxin due to adsorption to infusion bags or threads was observed after 0, 1 or 30 hours o...
example 2
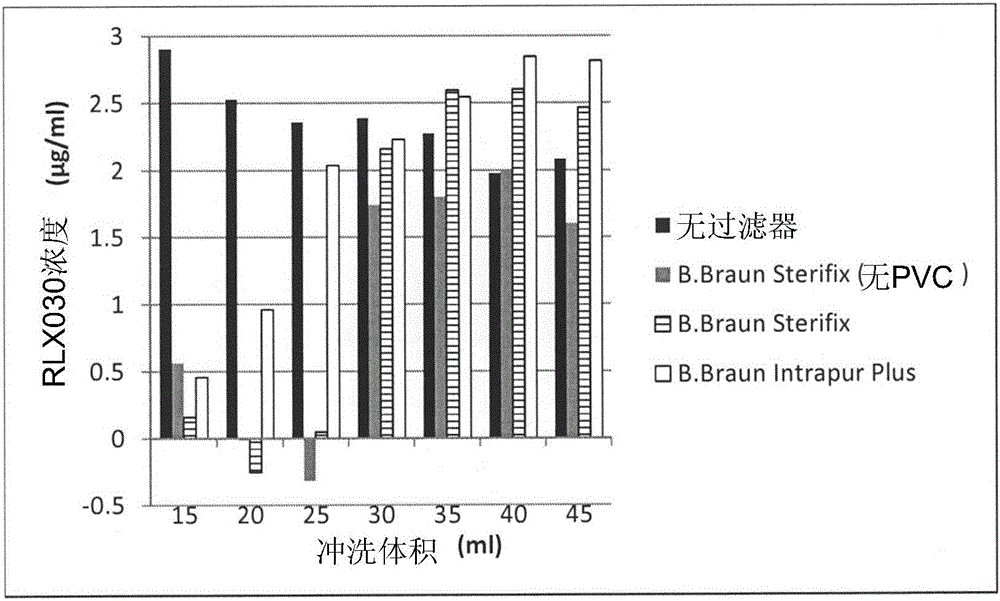
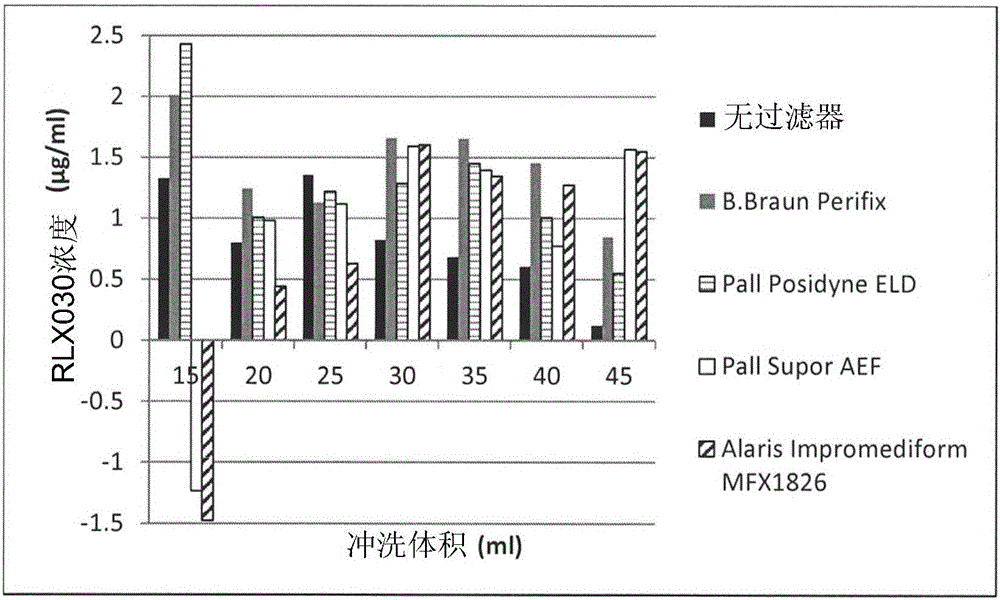
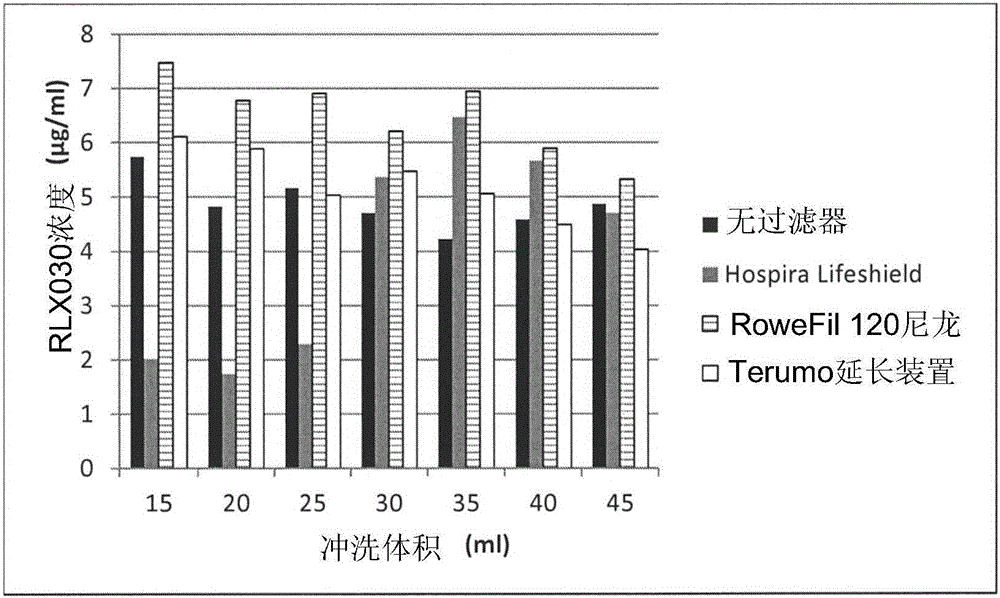
[0055] Example 2: Adsorption of Human Recombinant Relaxin 2 (Serelaxin) to Filters in 0.9% NaCl
[0056]
[0057]
[0058] 1 At a concentration of 5 μg / mL
[0059] 2 At a concentration of 30 μg / mL
[0060] Surprisingly, a positive charge on a filter did not predict whether it would adsorb positively charged proteins. Significant differences in adsorption were observed when different positively charged filters were tested. For example, little adsorption to neutral PES Baxter extension set 2C8671 and 2H5660 filters was observed and a flush volume of 20 mL was sufficient to achieve equilibrium. Partial adsorption to Pall Posidyne ELD ELD96LL was observed. The results are shown in Example 2 above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com