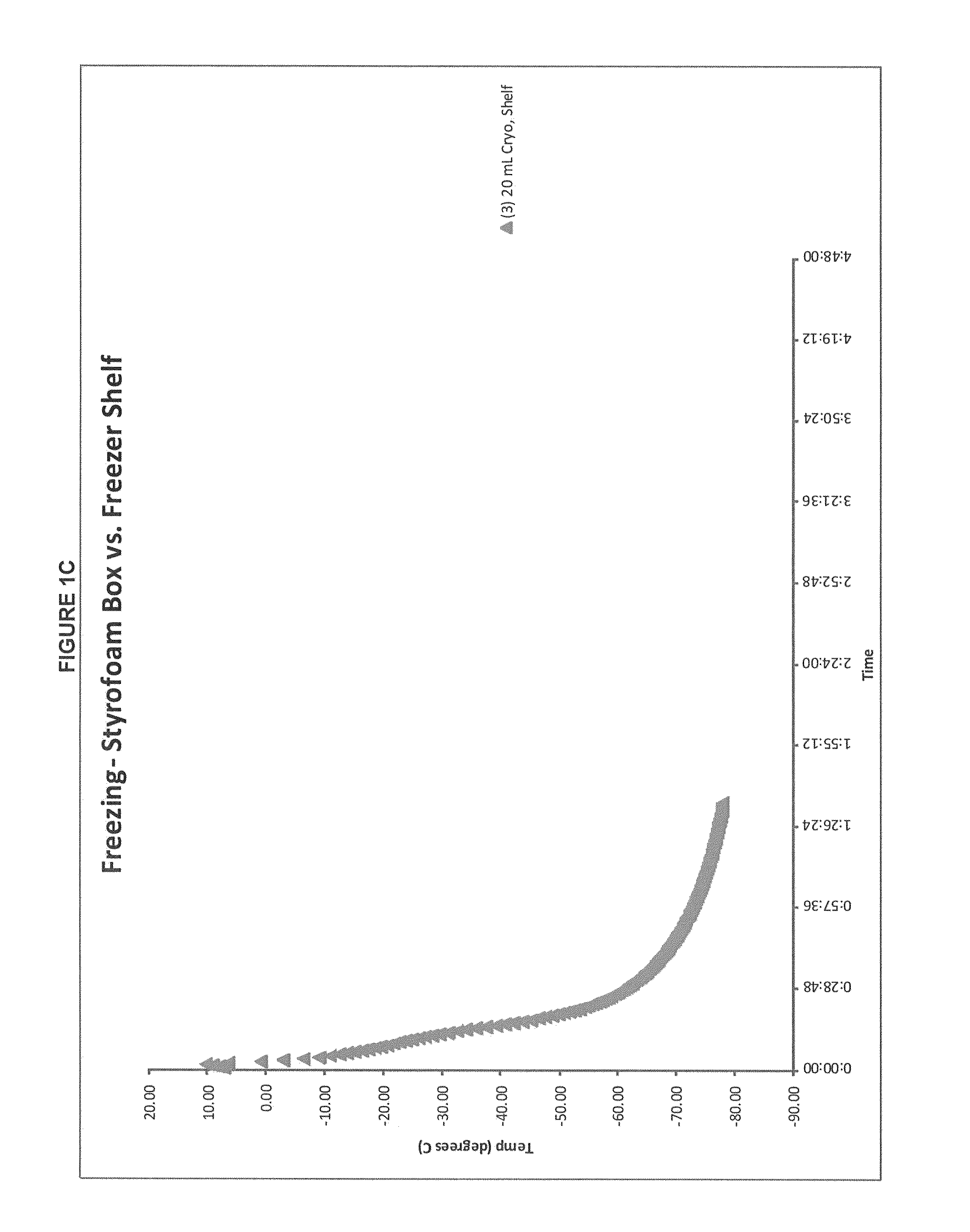
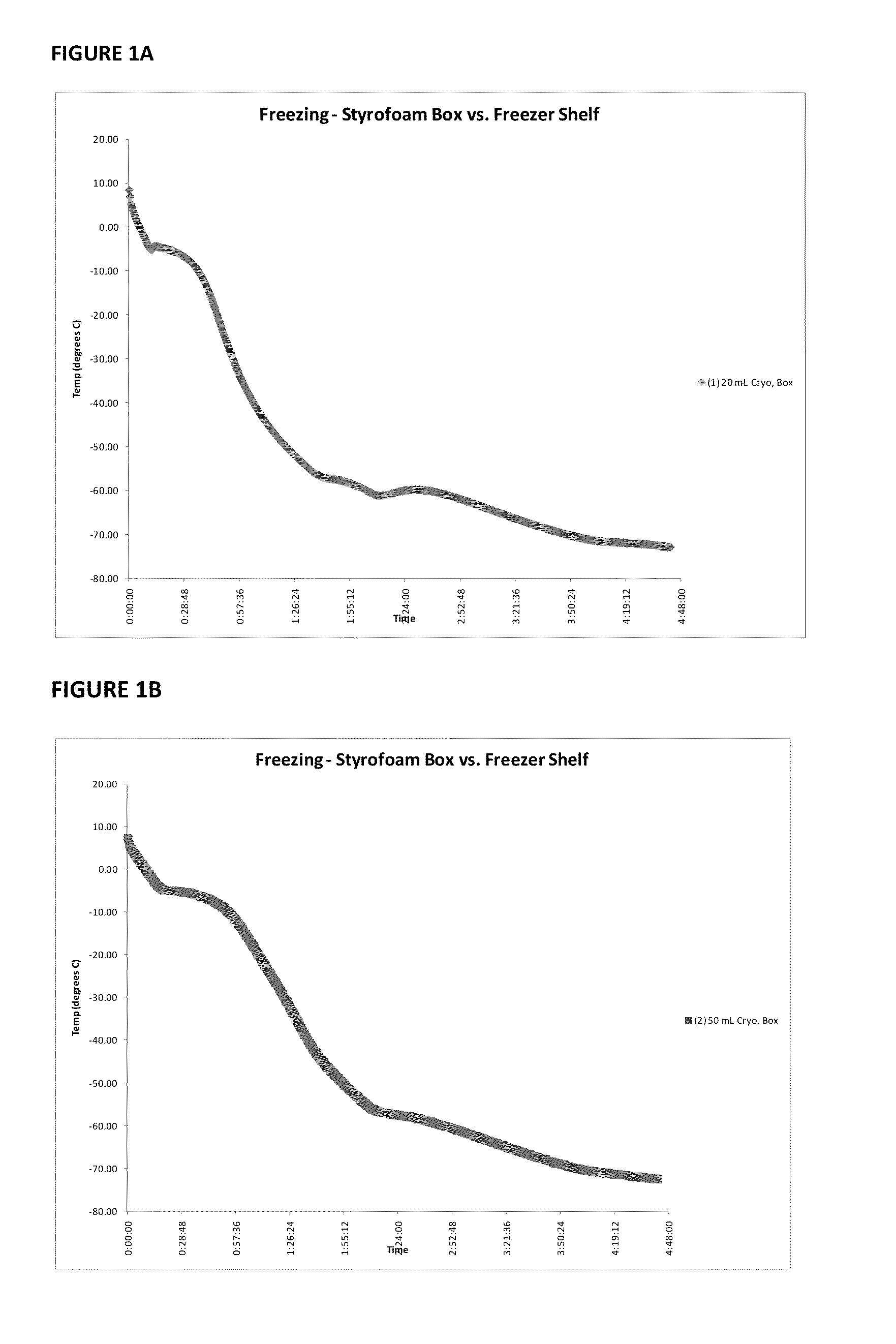
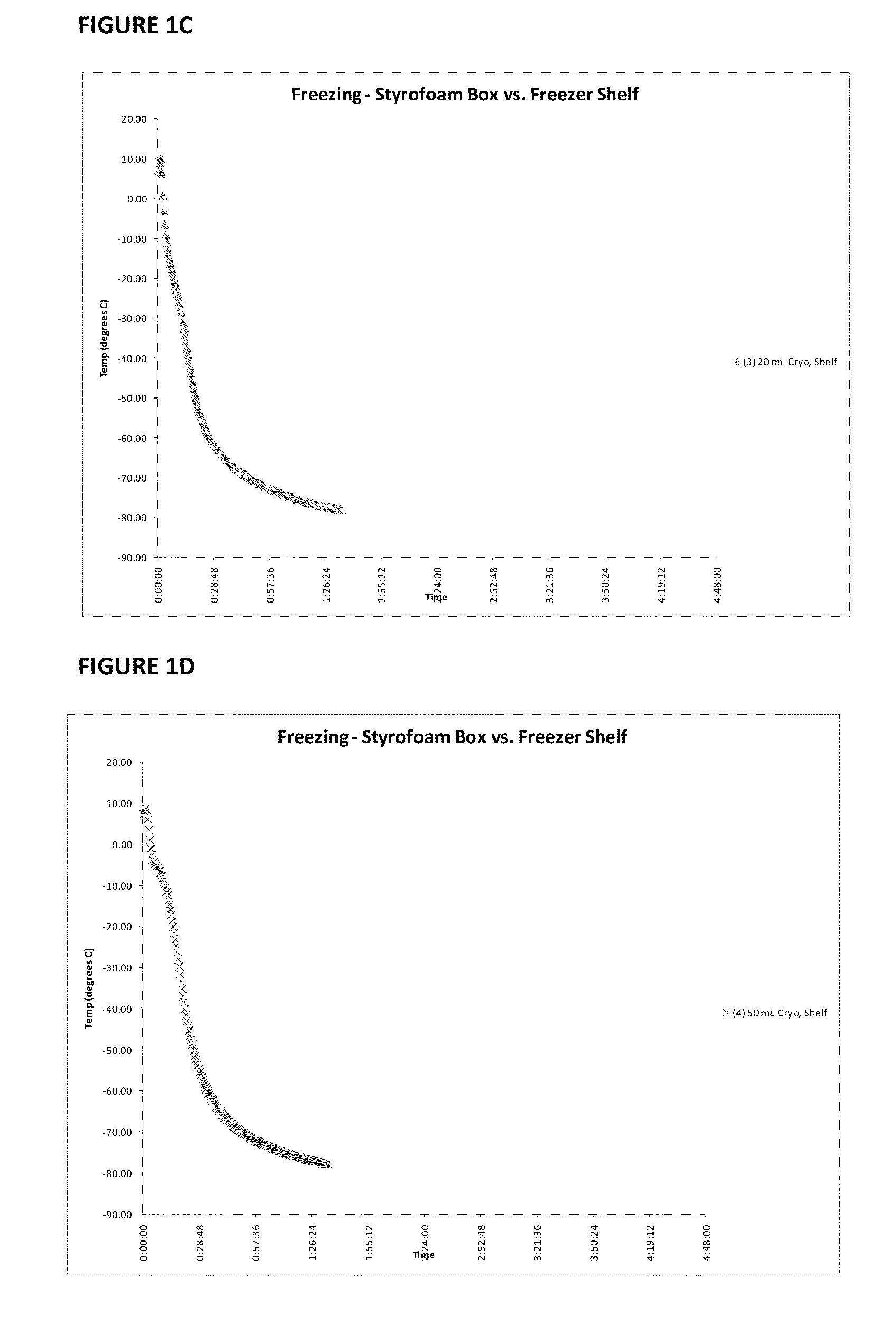
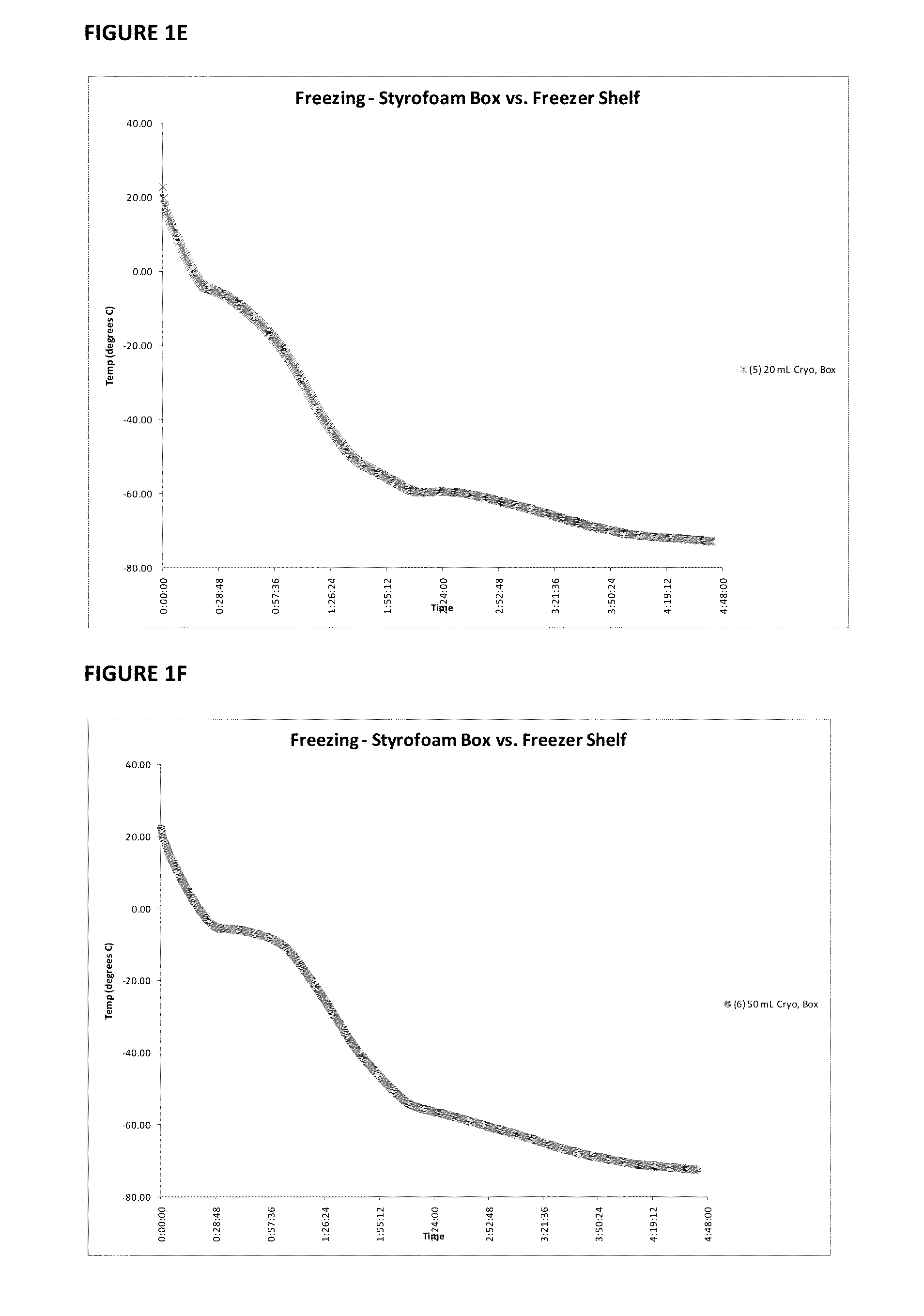
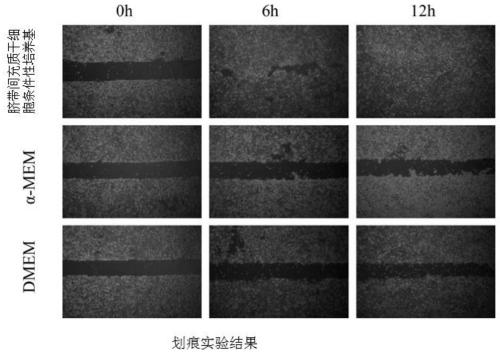
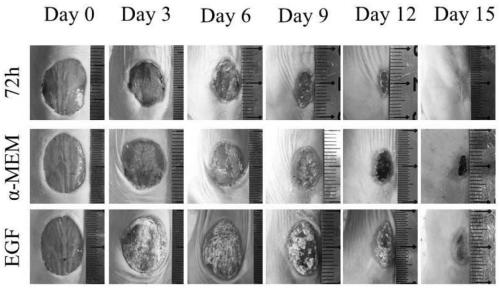
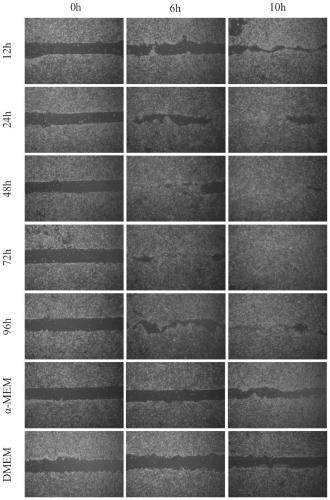
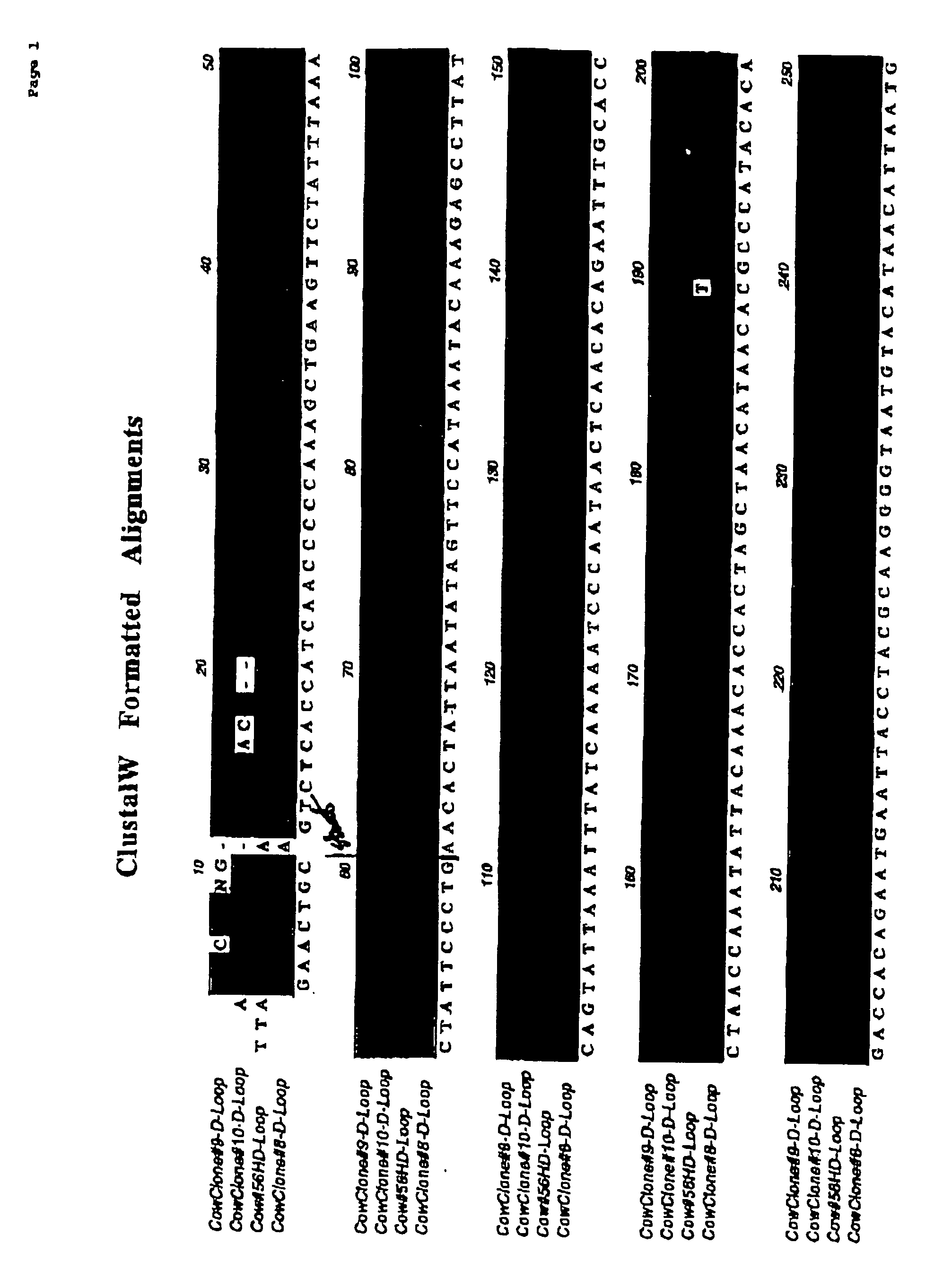
Patents
Literature
40 results about "Immunocompatibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunocompatibility (n.) 1. The degree of antigenic similarity between the tissues of different individuals, which determines the acceptance or rejection of allografts.
Methods of manufacture of immunocompatible amniotic membrane products
InactiveUS20110206776A1Superior wound healing propertyMaintain good propertiesPeptide/protein ingredientsCulture processObstetricsLigament
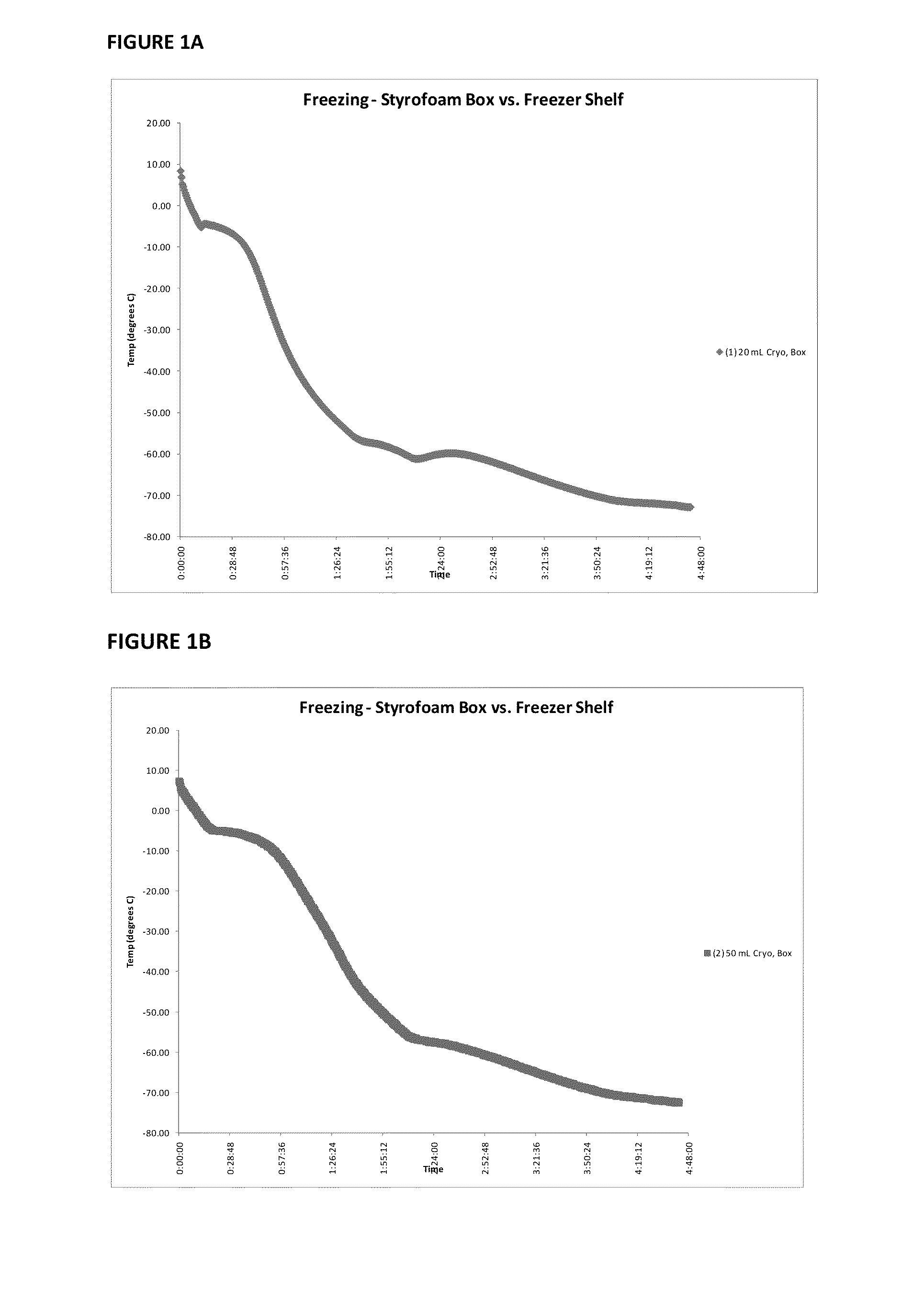
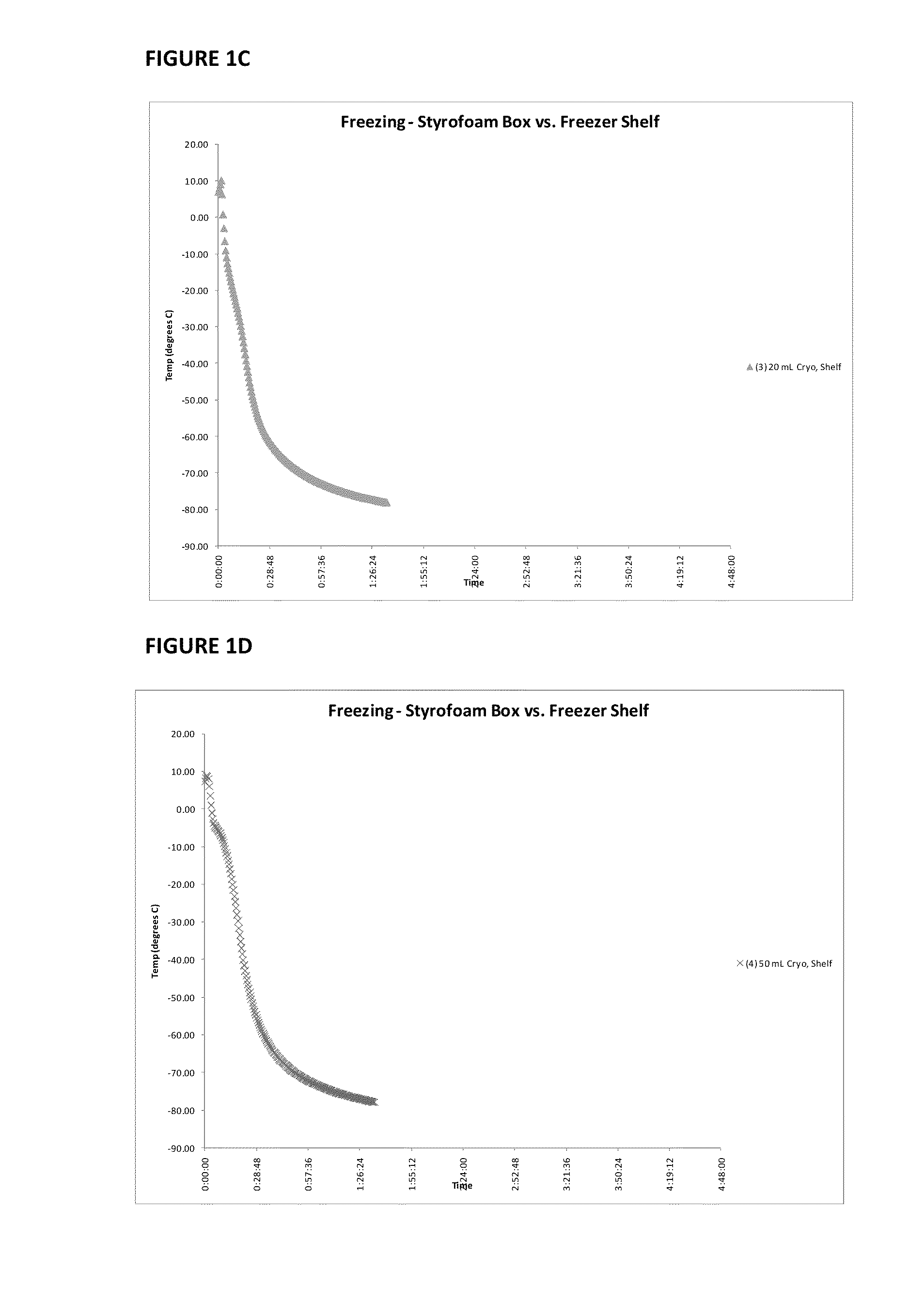
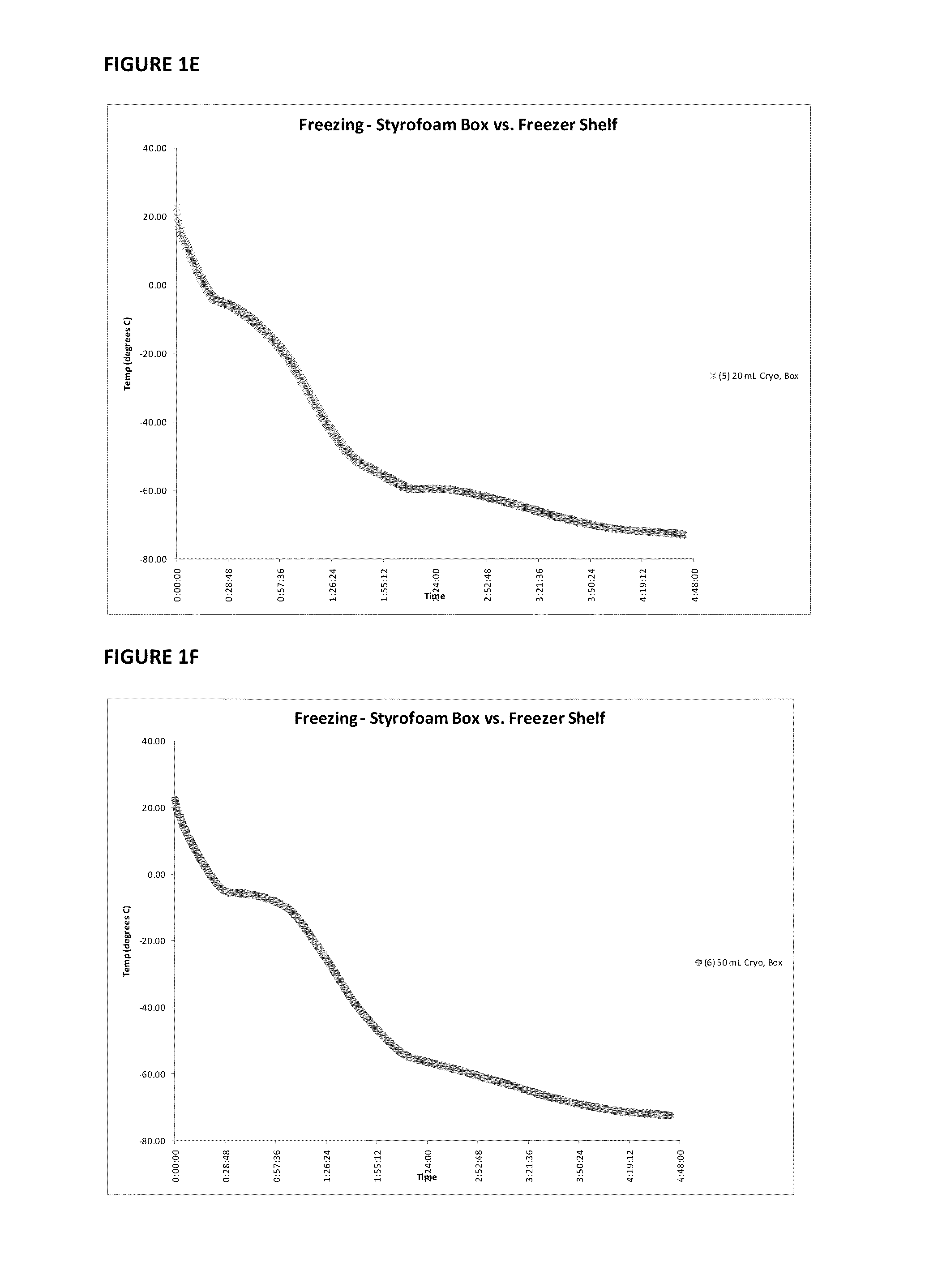
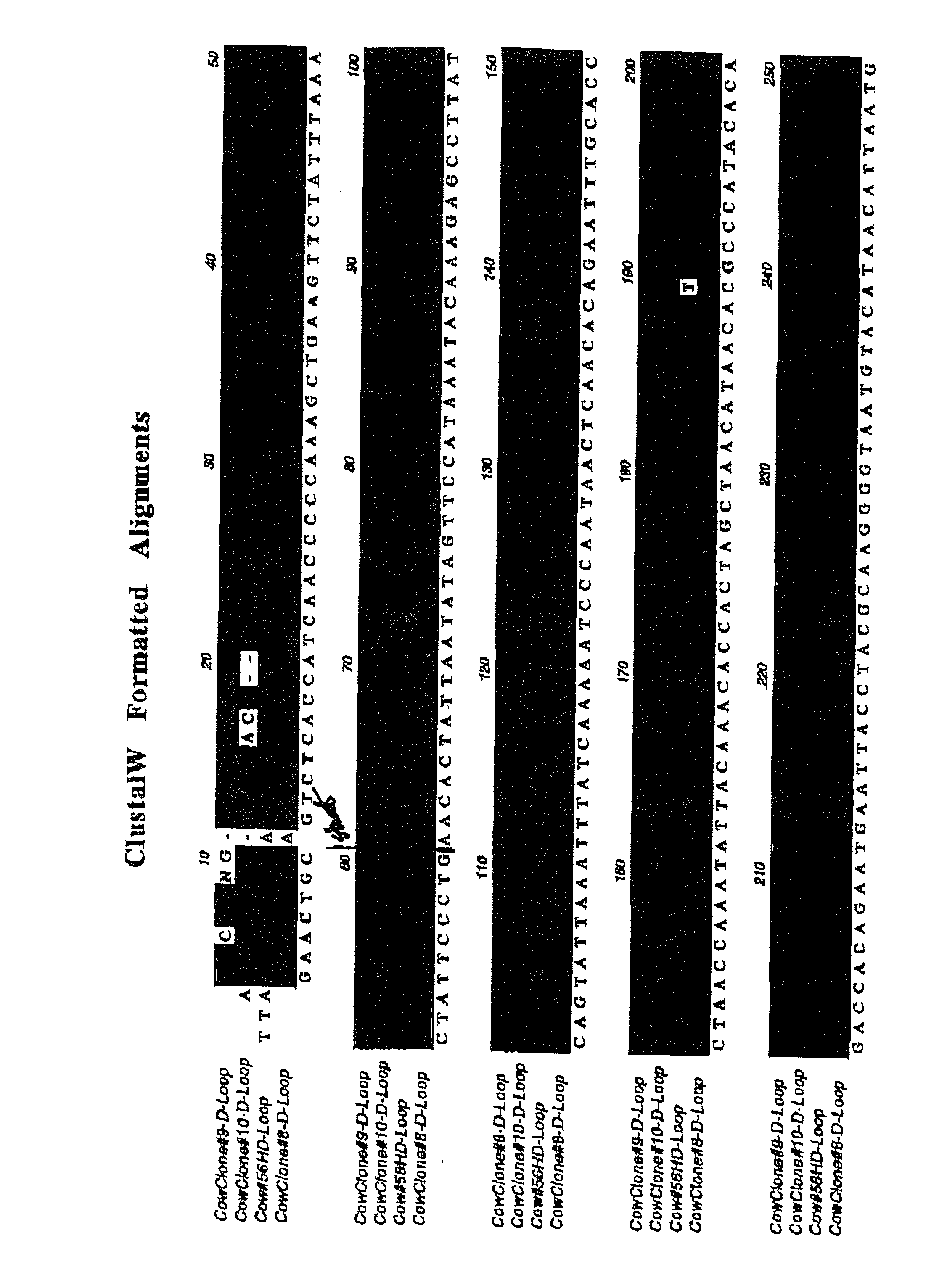
Provided herein is a placental product comprising an immunocompatible amniotic membrane. Such placental products can be cryopreserved and contain viable therapeutic cells after thawing. The placental product of the present invention is useful in treating a patient with a tissue injury (e.g. wound or burn) by applying the placental product to the injury. Similar application is useful with ligament and tendon repair and for engraftment procedures such as bone engraftment.
Owner:OSIRIS THERAPEUTICS
Prediction and assessment of immunogenicity
InactiveUS20060148009A1Low immunogenicityBiological material analysisVaccine efficacyImmunocompatibility
A system and method to predict and assess immunogenicity, especially prior to on-set of immunogenic conditions. Also disclosed are methods to identify relevant peptides associated with the formation of antibodies in patients treated with a given protein therapeutic. In various aspects, the present application is directed to methods of determining the immunological compatibility of a subject with a therapeutic agent such as a proteinaceous therapeutic agent, methods of determining vaccine efficacy by determining the immunological compatibility of a subject with a therapeutic agent, and selecting a therapeutic agent for a subject in need of treatment. Methods of designing a therapeutic agent with reduced immunogenicity for a subject and methods for designing vaccines with enhanced immunogenicity for a subject are also contemplated.
Owner:XENCOR
Immunocompatible chorionic membrane products
InactiveUS20110212158A1Maintain good propertiesBiocidePeptide/protein ingredientsObstetricsLigament structure
Provided herein is a placental product comprising an immunocompatible chorionic membrane. Such placental products can be cryopreserved and contain viable therapeutic cells after thawing. The placental product of the present invention is useful in treating a patient with a tissue injury (e.g. wound or burn) by applying the placental product to the injury. Similar application is useful with ligament and tendon repair and for engraftment procedures such as bone engraftment.
Owner:OSIRIS THERAPEUTICS
Immunocompatible amniotic membrane products
InactiveUS20150010610A1Reduce the amount requiredReduced activityBiocidePeptide/protein ingredientsObstetricsTissue repair
Provided herein is a placental membrane product comprising an immunocompatible amniotic membrane. Such placental membrane products can be cryopreserved and contain therapeutic factors and viable cells after thawing. The placental membrane products are useful in wound healing and tissue repair / regeneration as they are capable of promoting angiogenesis, reducing inflammation, inhibiting proteases and free radical oxidation, reducing scar formation, and other methods that promote healing. The present technology relates to products to protect injured or damaged tissue, or as a covering to prevent adhesions, to exclude bacteria, to inhibit bacterial activity, and / or to promote healing or growth of tissue. The field also relates to methods of manufacturing and methods of use of such membrane-derived products.
Owner:OSIRIS THERAPEUTICS
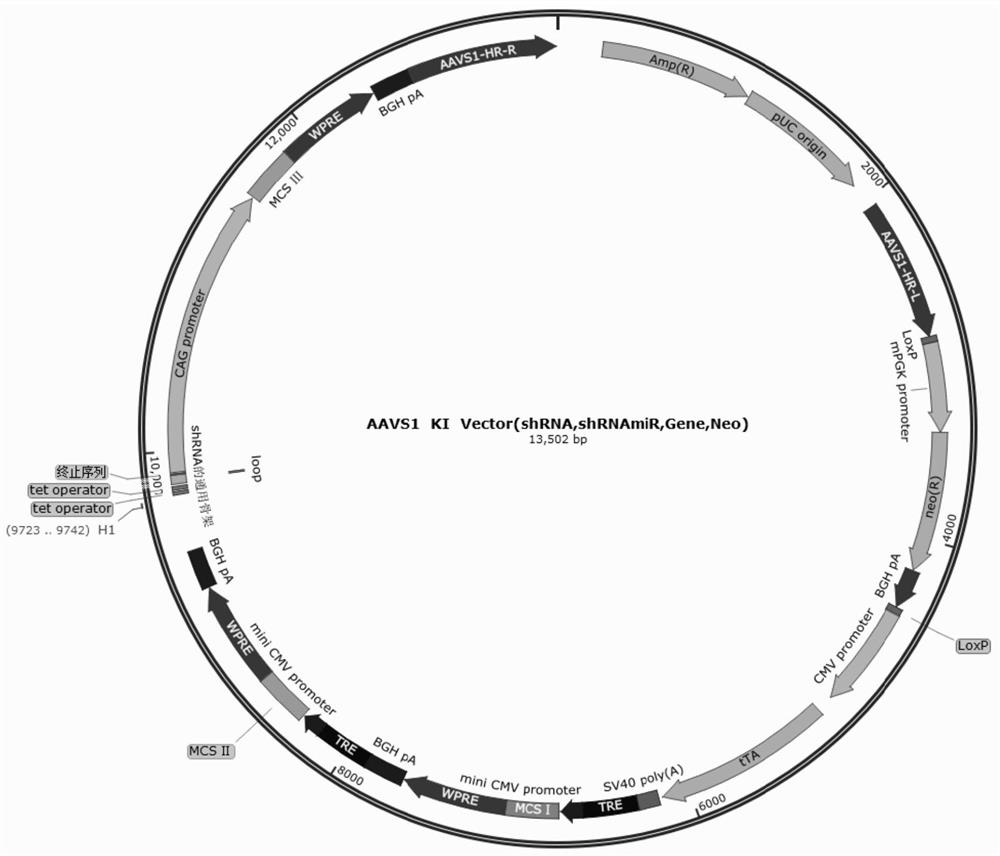
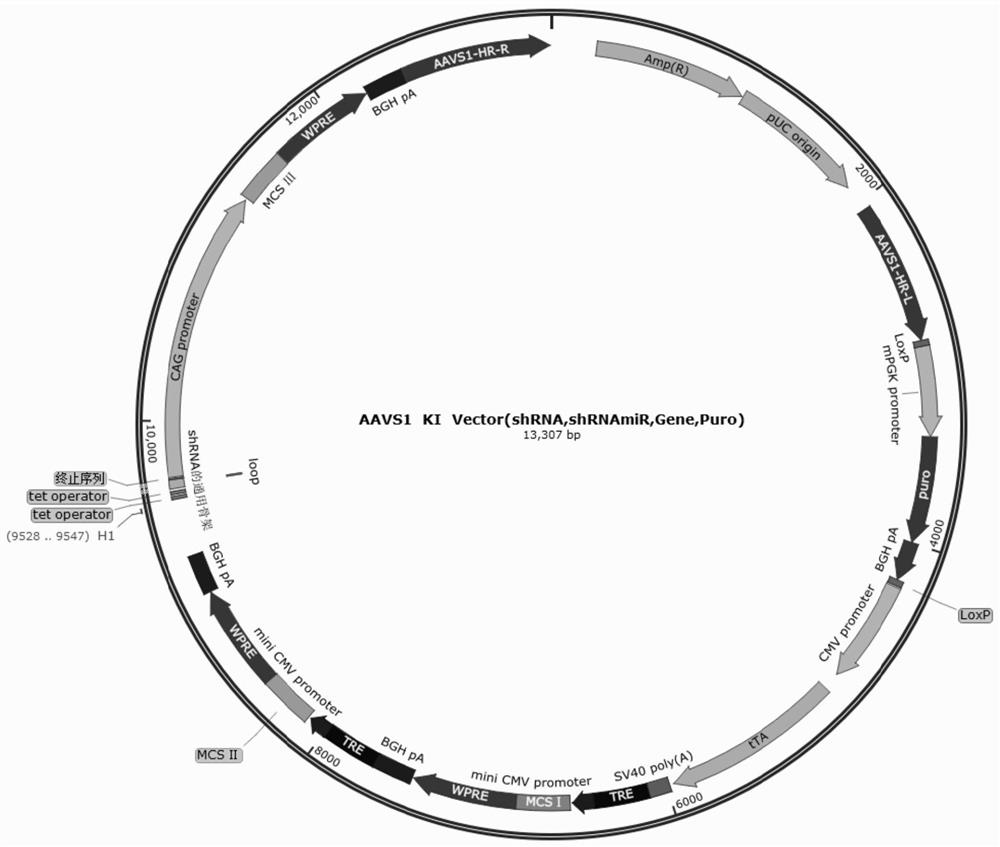
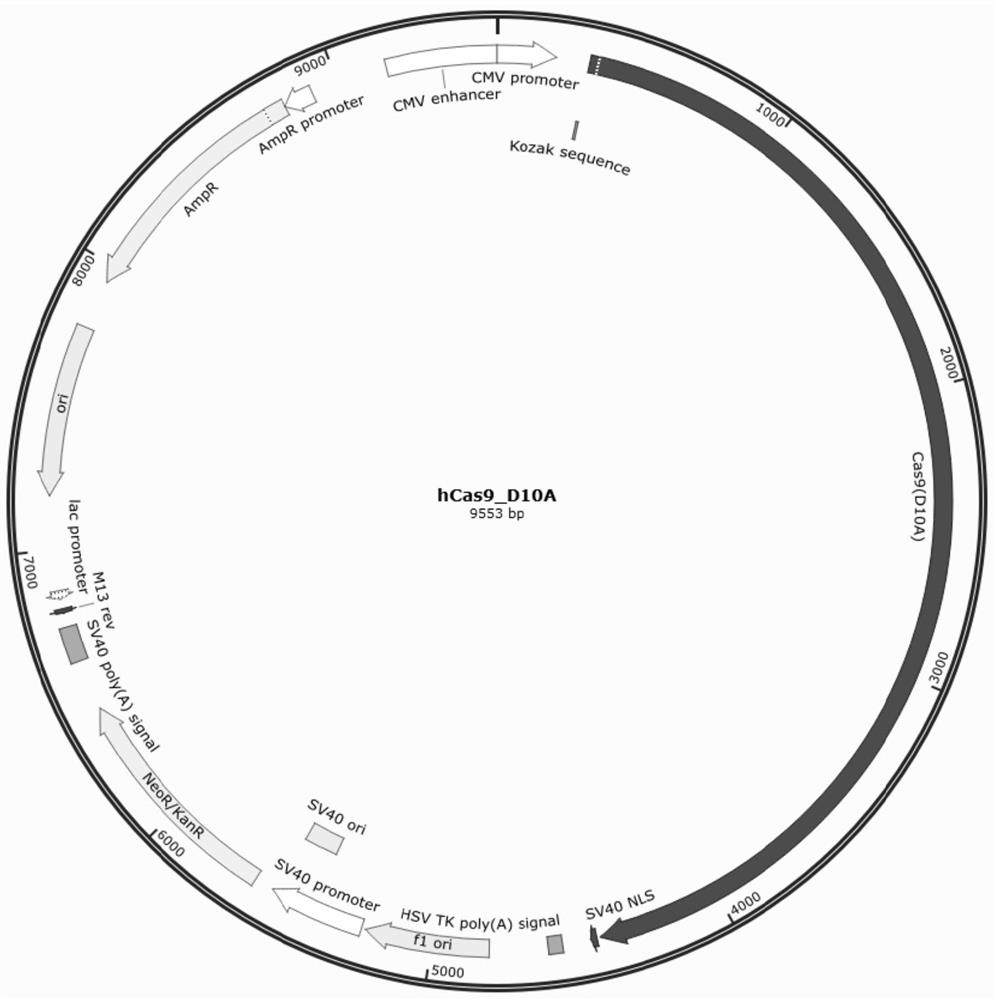
Method for generating immune-compatible cells and tissues using nuclear transfer techniques
InactiveUS6808704B1High incidenceImprove the level ofCompounds screening/testingBiocideNuclear transferTransgene
This invention relates to methods for making immune compatible tissues and cells for the purpose of transplantation and tissue engineering, using the techniques of nuclear transfer and cloning. Also encompassed are methods for determining the effect on immune compatibility of expressed transgenes and other genetic manipulations of the engineered cells and tissues.
Owner:ADVANCED CELL TECH INC
Immunocompatible chorionic membrane products
InactiveUS20150010609A1Prevent and reduce scarPrevent and reduce and contracture formationBiocidePeptide/protein ingredientsObstetricsDamages tissue
Provided herein is a placental membrane product comprising an immunocompatible chorionic membrane. Such placental membrane products can be cryopreserved and contain therapeutic factors and viable cells after thawing. The placental membrane products are useful in wound healing or tissue repair / regeneration as they are capable of promoting angiogenesis, reducing inflammation, reducing scar formation, and other methods that promote healing. The present technology relates to products to protect injured or damaged tissue, or as a covering to exclude bacteria, to inhibit bacterial activity, or to promote healing or growth of tissue. The field also relates to methods of manufacturing and methods of use of such membrane-derived products.
Owner:OSIRIS THERAPEUTICS
Method for preparing biomimetic artificial bone materials for biodegradable tissue engineering
ActiveCN101584884AShorten the preparation technology cycleReduce workloadProsthesisFreeze-dryingGenipin
The invention discloses a method for preparing biomimetic artificial bone materials for biodegradable tissue engineering. The method comprises the following steps: adding a hydroxyapatite suspension to a human-like collagen solution; adding Genipin for crosslinking; performing vacuum freeze-drying to obtain human-like collagen / hydroxyapatite powder; adding the human-like collagen / hydroxyapatite powder to a chitosan solution; using an NaOH solution to adjust the pH of the solution to 7.0; repeatedly adding the human-like collagen solution and the chitosan solution; adding a Genipin solution for crosslinking; freeze-drying a mixed solution; inoculating the obtained material with BMSCs of which the cell density is 5*104 cells / cm2; performing culture for 1 to 10 days; using an induction medium to culture BMSCs cells so as to induce the BMSCs cells to differentiate towards an osteogenesis direction; and forming bone repair composite materials with bone inducing activity. The artificial bone materials prepared by the method are excellent in bone inducing activity and immune compatibility, thereby thoroughly putting an end to inevitable viral hidden trouble of animal collagen scaffold materials and greatly improving use safety.
Owner:NORTHWEST UNIV(CN)
Method for generating immune-compatible cells and tissues using nuclear transfer techniques
InactiveUS20020046410A1Easily propagatableEasily transfectedCompounds screening/testingNew breed animal cellsNuclear transferTransgene
This invention relates to methods for making immune compatible tissues and cells for the purpose of transplantation and tissue engineering, using the techniques of nuclear transfer and cloning. Also encompassed are methods for determining the effect on immune compatibility of expressed transgenes and other genetic manipulations of the engineered cells and tissues.
Owner:LANZA ROBERT +1
Methods of manufacture of immunocompatible chorionic membrane products
InactiveUS20110212063A1Maintain good propertiesBiocidePeptide/protein ingredientsObstetricsImmunocompatibility
Provided herein is a placental product comprising an immunocompatible chorionic membrane. Such placental products can be cryopreserved and contain viable therapeutic cells after thawing. The placental product of the present invention is useful in treating a patient with a tissue injury (e.g. wound or burn) by applying the placental product to the injury. Similar application is useful with ligament and tendon repair and for engraftment procedures such as bone engraftment.
Owner:OSIRIS THERAPEUTICS
Method for preparing biodegradable blood vessel external scaffold material used in tissue engineering
InactiveCN101559238AStrong mechanical propertiesGood biocompatibilityProsthesisImmunocompatibilityRefrigerated temperature
The invention discloses a method for preparing a biodegradable blood vessel external scaffold material used in tissue engineering, comprising the steps as follows: using distilled water to dissolve human-like collagen to be a 1%-3% solution; using diluted acid to dissolve chitosan to be a 0.5%-1.5% solution; mixing the two solutions evenly, adding aqueous glutaric dialdehyde solution with the concentration of 20-50% according to mass percentage, mixing and stirring, and filtering followed by vacuum de-foaming; pouring the mixed solution into a tube-shaped die, cross-bonding at 4 DEG C for 1-3 days, placing the same into a refrigerator with the temperature being negative 80 DEG C for refrigerating, and then drying to obtain the blood vessel external scaffold material. Compared with the prior art, the prepared scaffold material has excellent mechanicalness, bio-compatibility, blood compatibility and immunity compatibility, is much lower in immunological rejection response, and can eradicate the potential virus hazard which can not be avoided by animal collagen, causing the security to be improved vastly.
Owner:NORTHWEST UNIV(CN)
Method for preparing blood vessel middle layer scaffold material used for biodegradable tissue engineering
ActiveCN101554490AHigh mechanical strengthControllable degradation rateProsthesisProtein solutionBiocompatibility Testing
The invention discloses a method for preparing blood vessel middle layer scaffold material used for biodegradable tissue engineering, comprising the steps: human-like collagen is dissolved to be solution with the mass percent concentration of 1-3% by distilled water; degumming silk protein is dissolved in 30-50% of CaCl2 solution to be dialyzed to obtain the silk protein solution with the concentration of 2-4%; the two obtained solutions are evenly mixed together, mixed with glutaric dialdehyde water solution with the mass percent concentration of 20-50% and stirred; after being filtered, the obtained solution is processed by vacuum defoamation; the mixed solution is injected into a tubular shape mould to be processed by cross linkage at the temperature of 4 DEG C for 2 days, and then put into a refrigerator with the temperature of -80 DEG C to be frozen and dried, so that the blood vessel middle layer scaffold is prepared. Compared with the prior art, the prepared scaffold material has excellent mechanical capacity, biocompatibility, blood compatibility and immunity compatibility as well as lower immunity rejection reaction, thoroughly avoids the virus hidden trouble which is inevitable for animal collagen, and greatly improves the safety.
Owner:NORTHWEST UNIV(CN)
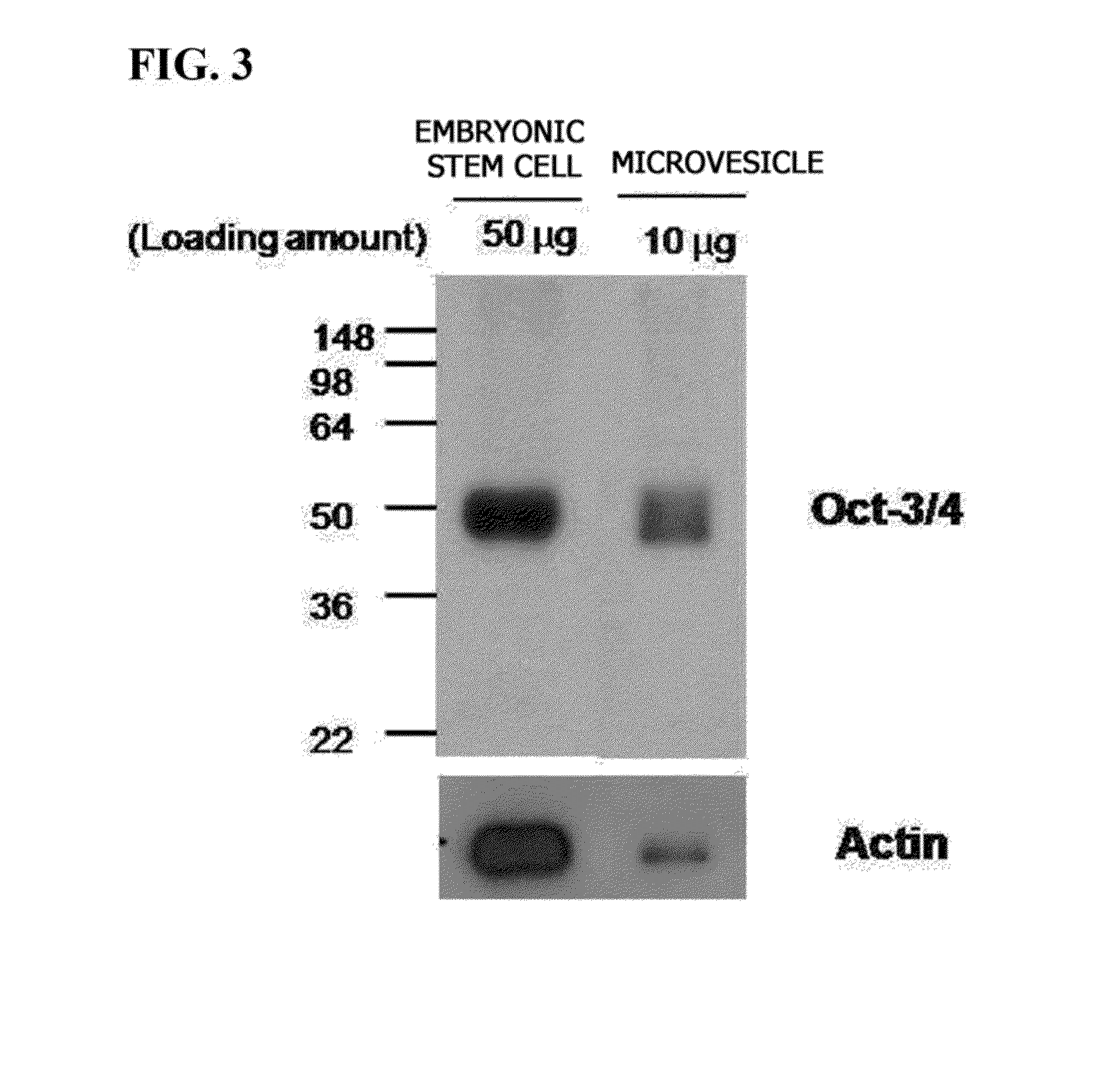
Method for preparing induced pluripotent stem cells using microvesicles derived from embryonic stem cells
InactiveCN103492554AFreely control the concentrationAvoid damageMammal material medical ingredientsSkeletal/connective tissue cellsSide effectSomatic cell
The present invention relates to a method for dedifferentiating somatic cells using microvesicles derived from embryonic stem cells. More particularly, the present invention relates to a method for preparing pluripotent stem cells by treating somatic cells with a composition having microvesicles derived from embryonic stem cells. The method for preparing pluripotent stem cells according to the present invention effectively facilitates the dedifferentiation of somatic cells without side-effects using the microvesicles, and further, is expected to be significantly useful in the development of cell therapy products having immune-compatibility suitable for specific individuals.
Owner:POSTECH ACAD IND FOUND
Skin repairing liquid and preparation method thereof
InactiveCN111166770AAvoid spreadingReduce usagePharmaceutical delivery mechanismMammal material medical ingredientsDiseaseConventional medicine
The invention discloses a skin repairing liquid and a preparation method thereof and belongs to the technical field of skin repairing. The skin repairing liquid is a conditional culture liquid derivedfrom umbilical cord mesenchymal stem cells. Compared with a method that mesenchymal stem cells are directly applied to treatment on skin wounds, the skin repairing liquid disclosed by the invention avoids security problems related to living cell transplanting and cell proliferation, such as immune compatibility, tumorigenicity and propagation of infectious diseases; in addition, security, dosagesand effects of components in a conditional culture medium can be evaluated by using a method similar to a method for evaluating conventional medicines; the skin repairing liquid disclosed by the invention is easy to produce and prepare, can be subjected to freeze drying preservation, is easy to package and transport, does not take the time for anabiosis, stem cell proliferation and the like before treatment, and is convenient to use.
Owner:YANAN HOSPITAL OF KUNMING CITY
Method for generating immune-compatible cells and tissues using nuclear transfer techniques
Owner:ADVANCED CELL TECH INC
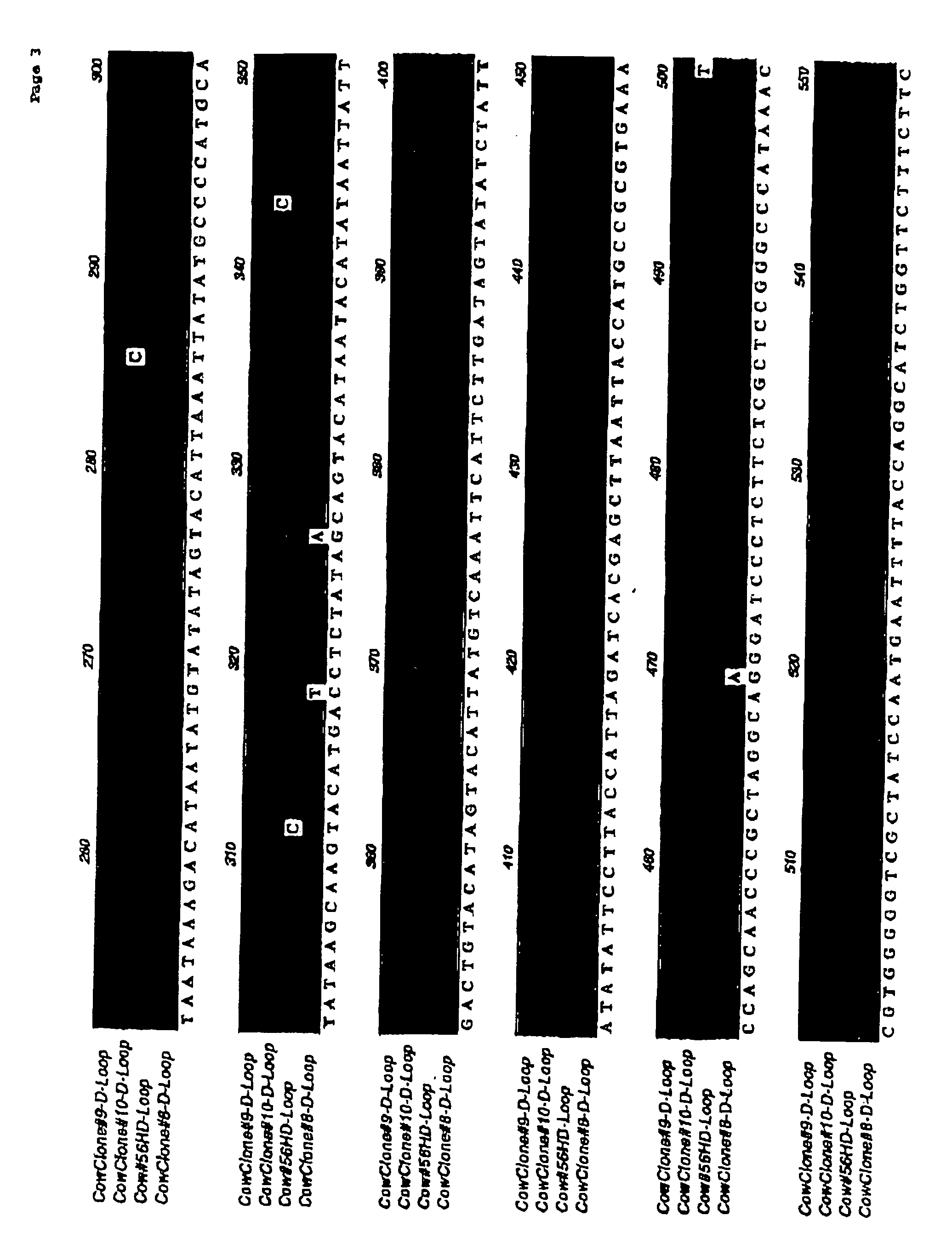
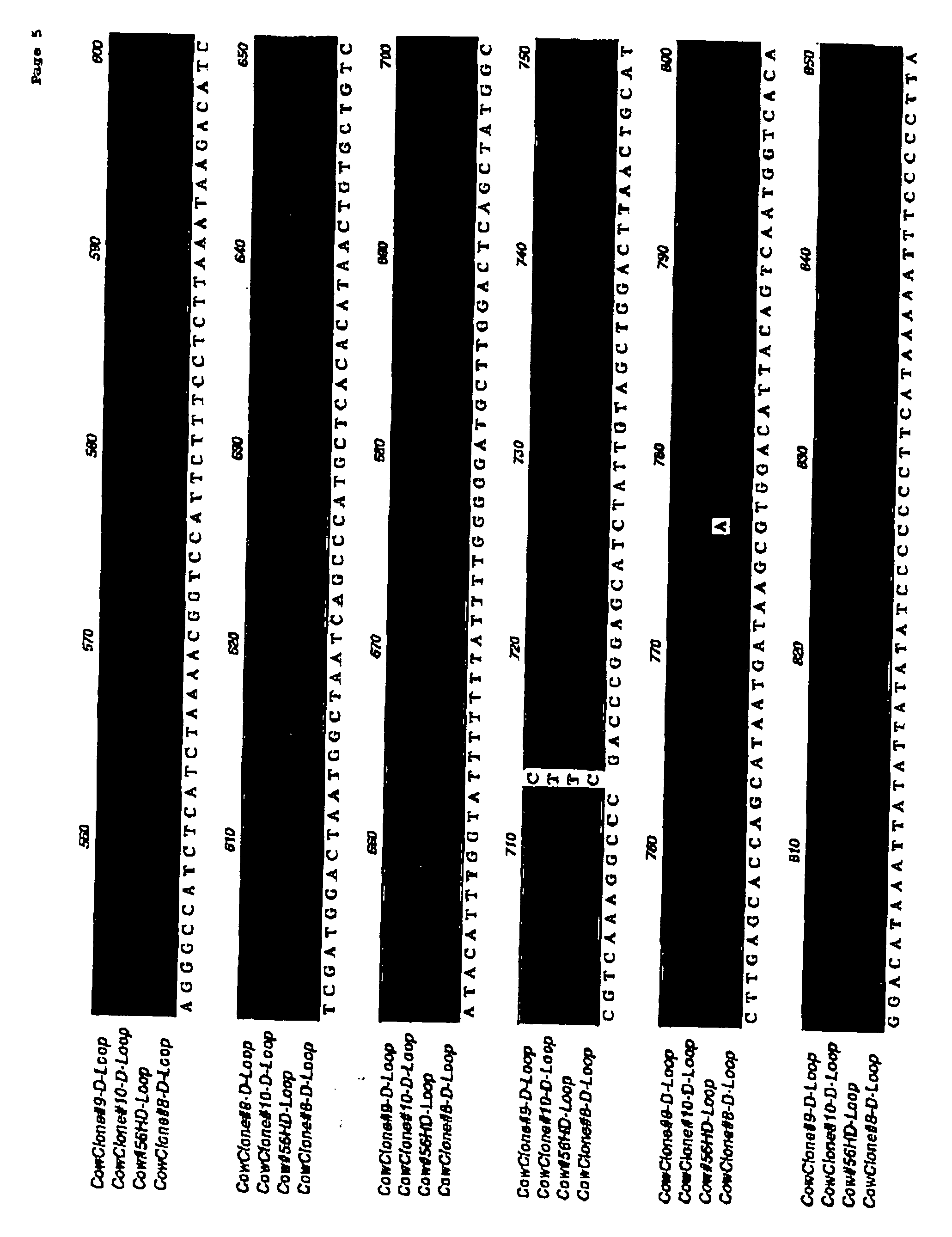
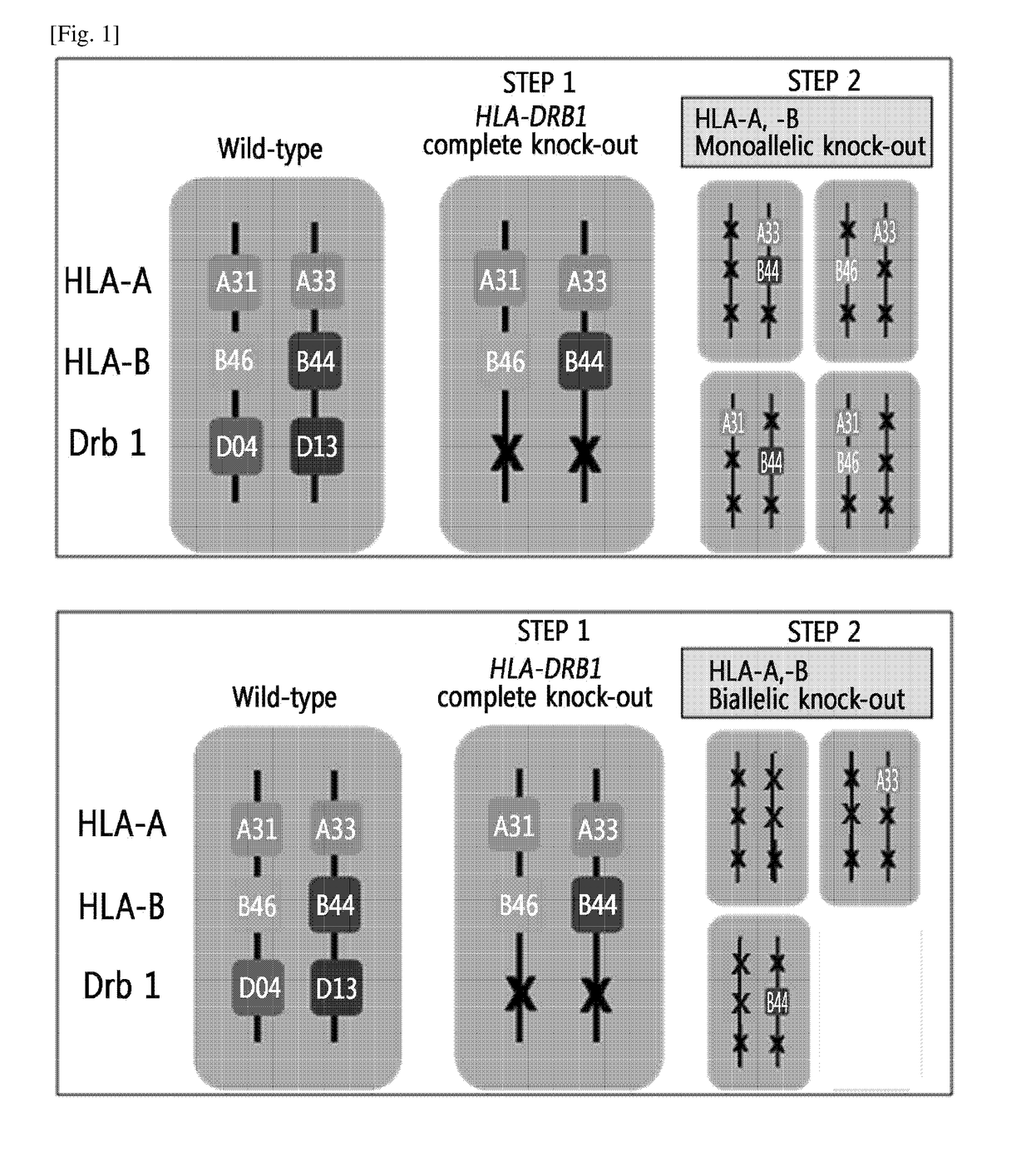
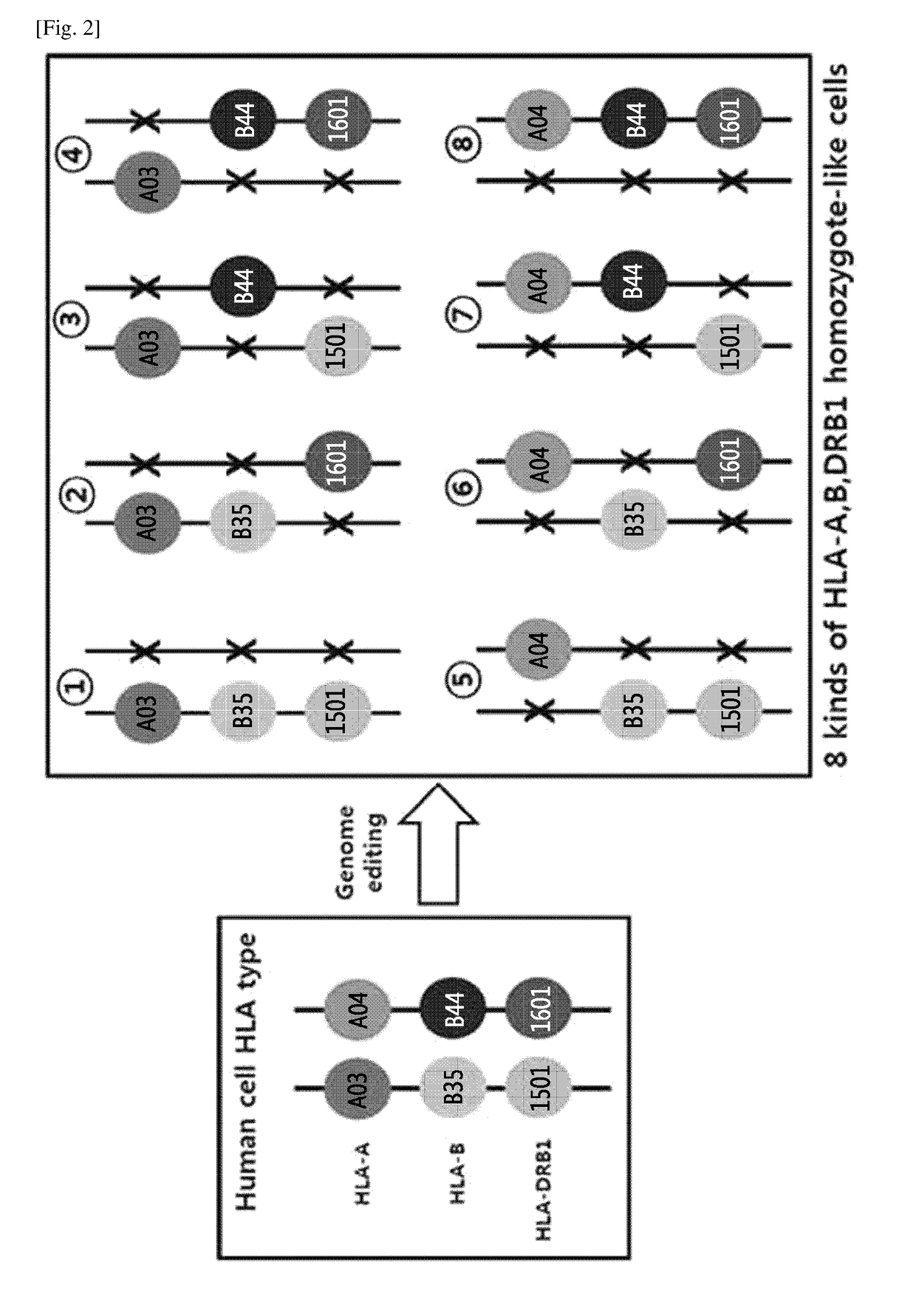
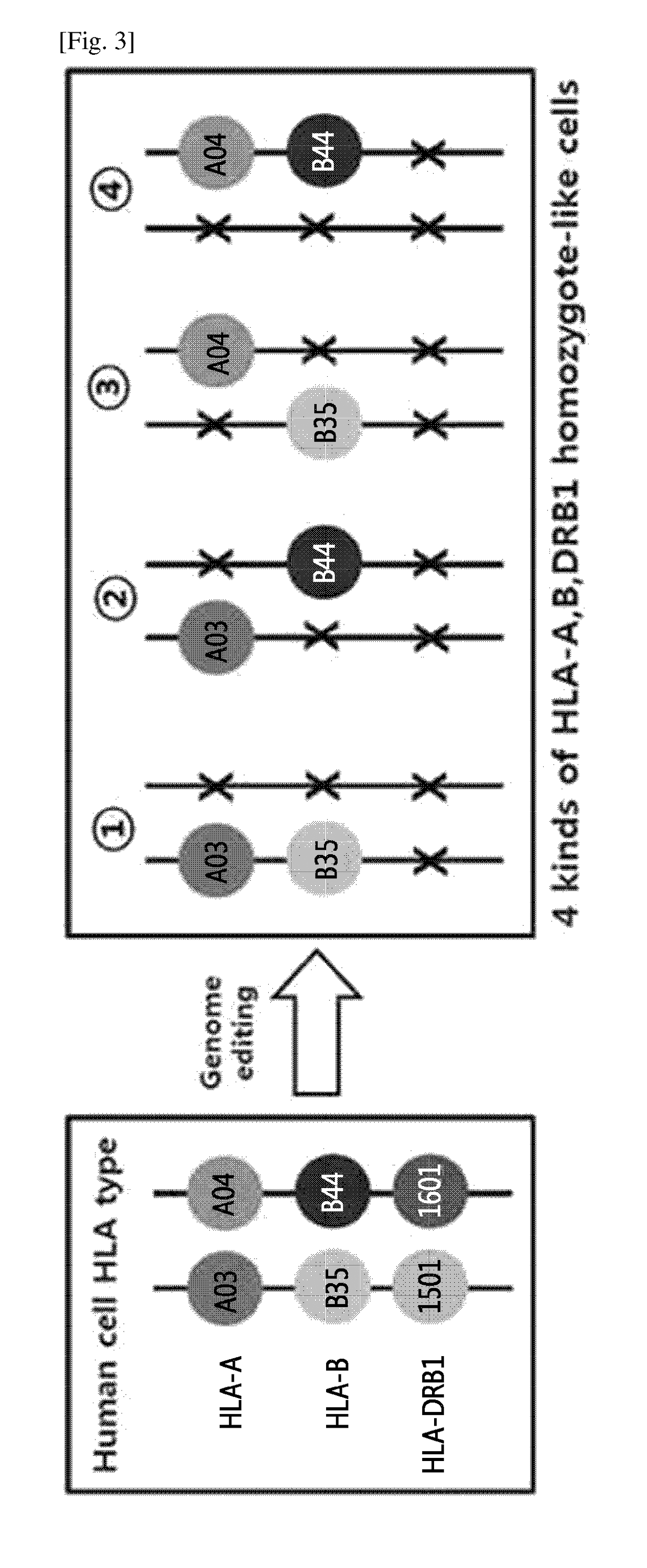
Immune-compatible cells created by nuclease-mediated editing of genes encoding HLA
The present invention relates to a method for producing immune-compatible cells or a cell population which comprises a step of editing one or two alleles of one or more immune-compatible antigen genes by gene deletion or modification in an isolated cell comprising at least one of the immune-compatible antigen genes selected from HLA (human leukocyte antigen)-A, HLA-B and HLA-DR, to immune-compatible cells produced by the method, and to a cell population comprising the immune-compatible cells produced by the method
Owner:COLLEGE OF MEDICINE POCHON CHA UNIV IND +1
GBS Toxin Receptor Compositions and Methods of Use
InactiveUS20090221804A1Ameliorate any conditionReduce severityImmunoglobulins against cell receptors/antigens/surface-determinantsCancer antigen ingredientsAutologous T-cellsImmunocompatibility
Methods are provided for preventing or attenuating pathoangiogenic conditions by administering at least one GBS toxin receptor polypeptide or at least one immunogenic fragment thereof. Also provided are a composition that includes a GBS toxin receptor polypeptide and a method for making such a composition. In another embodiment of the invention, immunized animals also receive GBS toxin, immunocompatible antibodies to the GBS toxin receptor, and / or expanded autologous T cells to the GBS toxin receptor. Also included in this invention are methods of identifying additional GBS toxin receptors.
Owner:VANDERBILT UNIV
Immunocompatible reversible universal pluripotent stem cells and application thereof
The invention discloses immunocompatible reversible universal pluripotent stem cells or derivatives thereof. An inducible gene expression system and an expression sequence of at least one immunocompatible molecule are introduced into the genome of the pluripotent stem cells or the derivatives thereof. The immunocompatible molecules are used for regulating and controlling the expression of genes related to immune response in pluripotent stem cells or derivatives of the pluripotent stem cells. The expression of the immunocompatible molecule is regulated and controlled by an inducible gene expression system. According to the technical scheme, the immunocompatibility between the graft and the receptor can be improved, and the antigen presentation capability of graft cells can be reversibly recovered, so that the clinical safety of such universal pluripotent stem cells and derivatives is improved, and the clinical application value of the universal pluripotent stem cells and derivatives isgreatly expanded.
Owner:FUTURE HOMO SAPIENS INST OF REGENERATIVE MEDICINE CO LTD (FHSR) +1
Method for inducing pluripotent stem cells and pluripotent stem cells prepared by said method
ActiveUS20170128336A1Cosmetic preparationsOrganic active ingredientsDiseaseInduced pluripotent stem cell
Owner:AMOREPACIFIC CORP +1
Method for preparing induced pluripotent stem cells using microvesicles derived from embryonic stem cells
InactiveUS20140170746A1Improve delivery efficiencyEffective protectionArtificial cell constructsUnknown materialsSide effectImmunocompatibility
Provided is a method of dedifferentiating somatic cells using embryonic stem cell-derived microvesicles. Particularly, a method of preparing induced pluripotent stem cells by treating a composition including embryonic stem cell-derived microvesicles to the somatic cells. According to the method of preparing induced pluripotent stem cells, the dedifferentiation of the somatic cells may be efficiently performed without side effects using the embryonic stem cell-derived microvesicles, and moreover, the method is expected to be very useful in developing a cell therapy product having immunocompatibilities by individuals.
Owner:POSTECH ACAD IND FOUND
Production of parthenogenetic stem cells and patient-specific human embryonic stem cells using somatic cell nuclear transfer
ActiveUS10017733B2Reduced feature requirementsNew breed animal cellsCell culture active agentsNuclear transferParthenogenesis
Immunocompatible pluripotent stem cells (pSCs), which include cells compatible with different patient populations or patient-specific cells, find wide application in regenerative medicine therapies. Described herein are immunocompatible pSCs generated using techniques such as parthenogenesis resulting in cells possessing desired haplotypes of reduced zygosity, antigenically compatible with multiple patient populations, or nuclear transfer allowing generation of patient-specific cells. Methods described herein related to parthenogenesis, nuclear transfer, or pSC cell line generation. Also described herein are compositions of immunocompatible pSCs and cell lines generated by the aforementioned techniques.
Owner:SUNG KWANG MEDICAL FOUND
Immunocompatible polymers
ActiveUS20140309313A1Ointment deliveryPharmaceutical non-active ingredientsPolymer scienceImmunocompatibility
Owner:MCMASTER UNIV
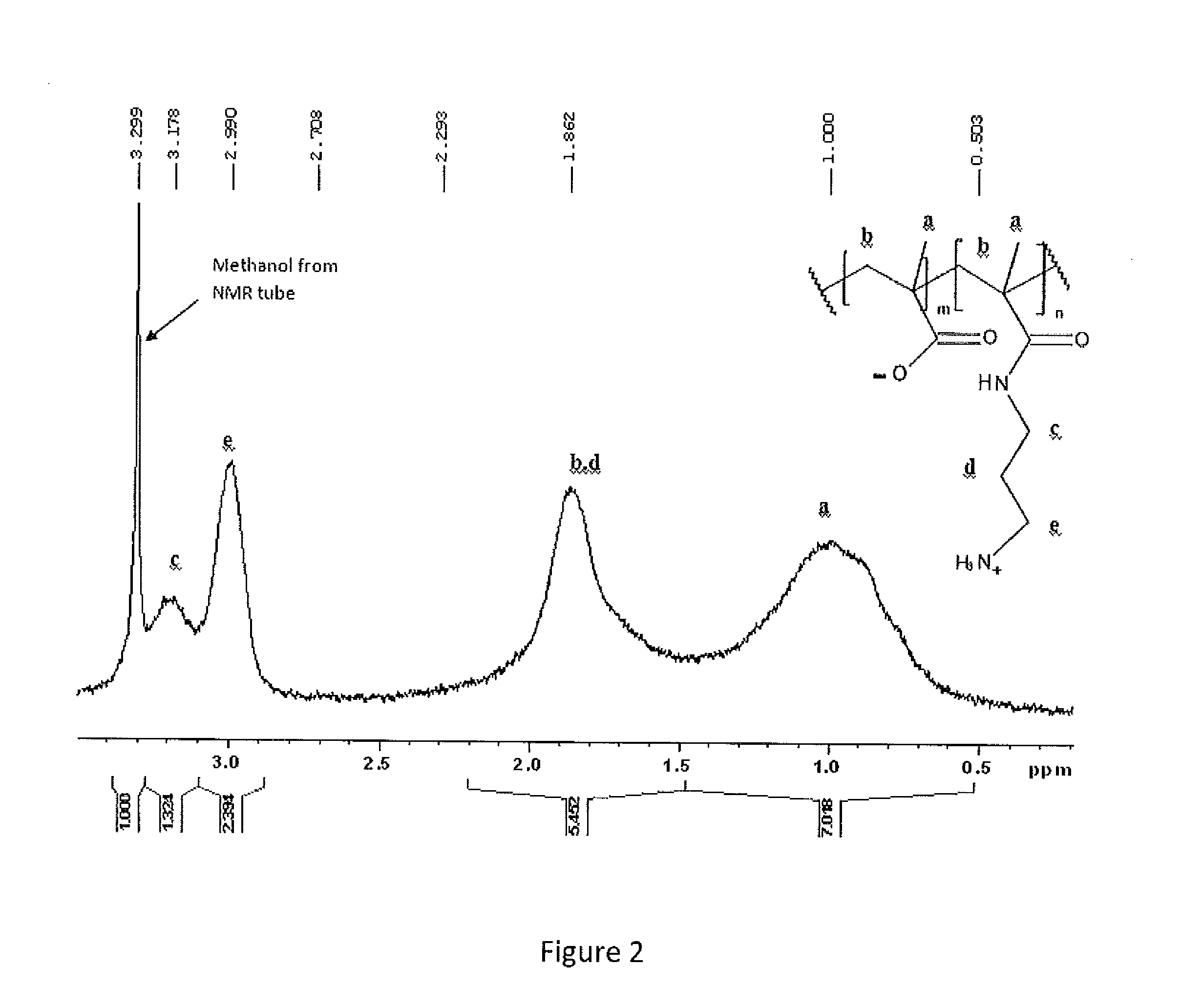
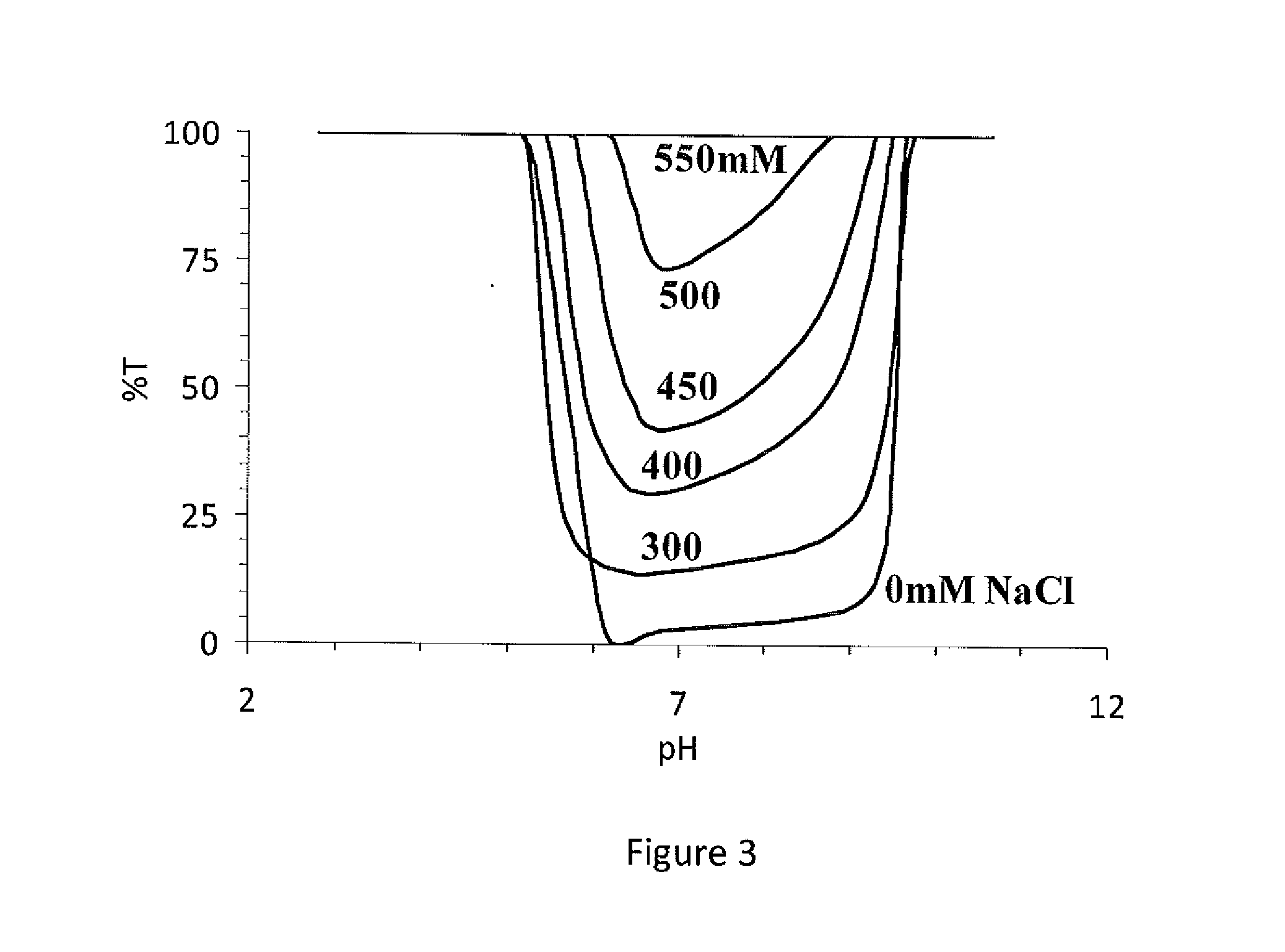
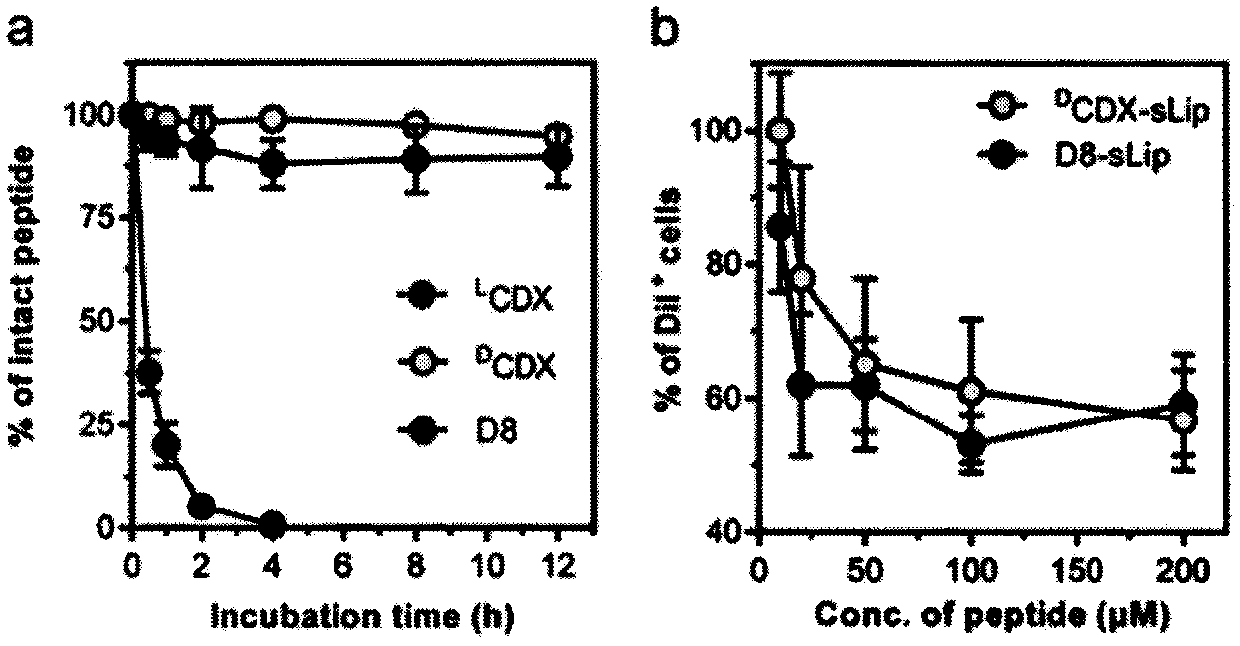
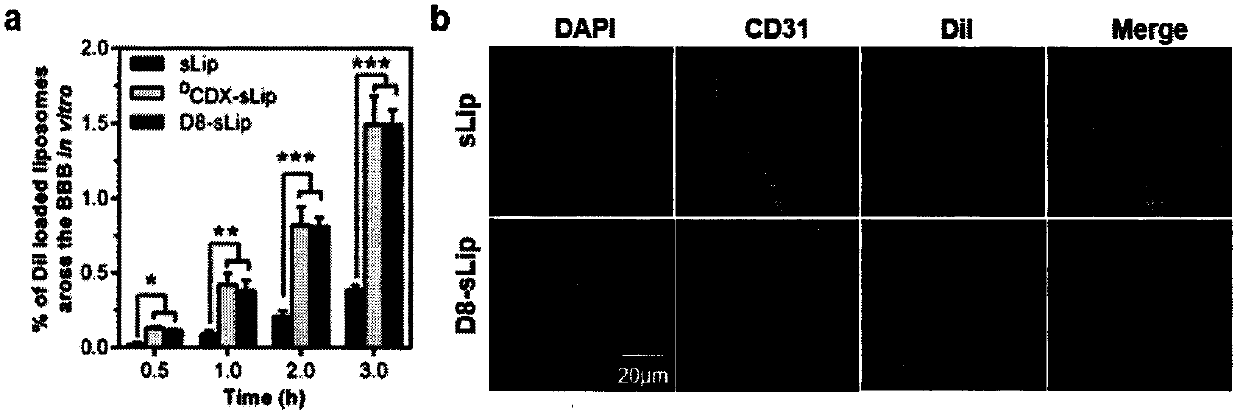
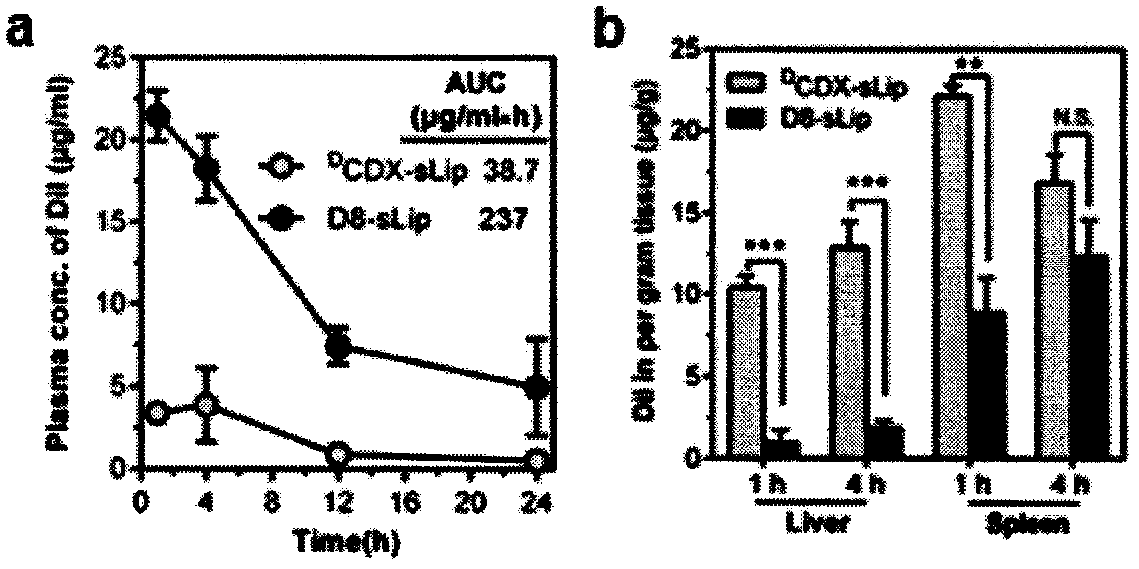
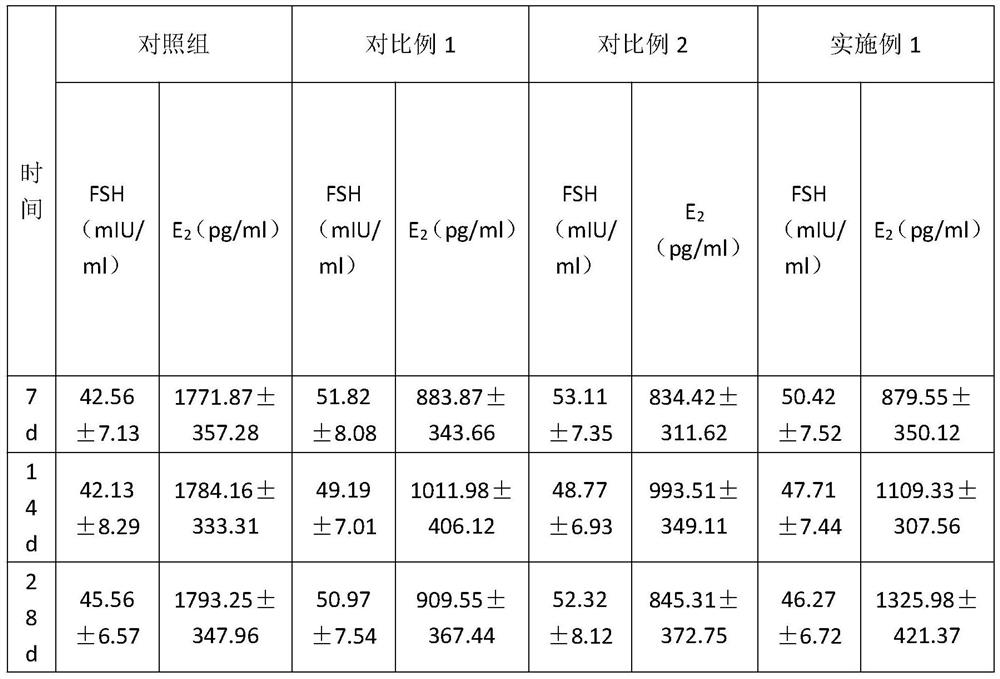
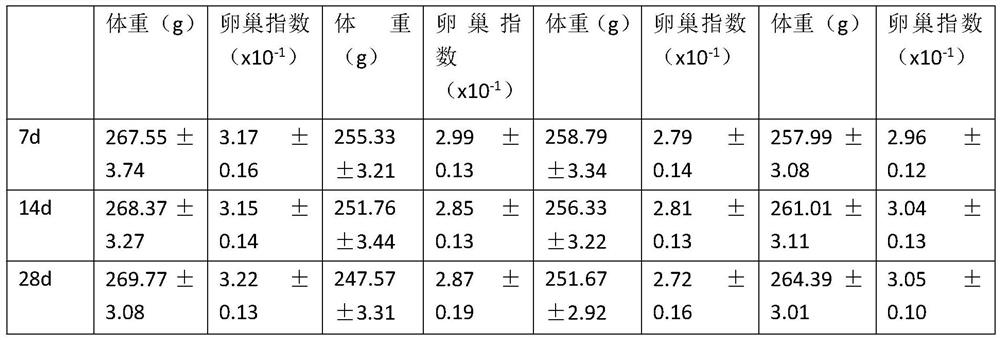
Specially-acetylcholine-receptor-targeted D8 polypeptide with biomembrane-crossing effect and brain-targeted drug delivery system thereof
ActiveCN110669101ALow immunogenicityExcellent immunocompatibilityOrganic active ingredientsPeptidesDiseaseSide effect
The invention, which belongs to the field of pharmacy, relates to a D8 polypeptide and a brain-targeted drug delivery system thereof, particularly to, a specially-acetylcholine-receptor-targeted D8 polypeptide with a biomembrane-crossing effect and a brain-targeted drug delivery system thereof. According to the invention, a nano-carrier modified by the polypeptide molecule D8 is specifically combined or taken by a positive cell expressing an acetylcholine receptor to realize strong brain targeting and imaging functions and the capability of affinity with a biomembrane barrier to form a cell. With a constructed nano drug delivery system, such as lipidosome, polymer micelle, a polymer disc, and a nanoparticle, an entrapped model drug can be delivered to a brain tissue and a cell expressing an acetylcholine receptor effectively, so that the treatment effect to diseases in brain can be improved obviously; the side effect is low; and the immunocompatibility is high. Therefore, the D8 polypeptide and the brain-targeted drug delivery system thereof have the broad application prospects in fields of diagnosis and targeted therapy of intracerebral diseases such as intracerebral tumors.
Owner:FUDAN UNIV
Stem cell film forming culture medium and application thereof
PendingCN114107188AHigh immunocompatibilityReduce adverse reactionsCell dissociation methodsCulture processPost transplantCollagenan
The invention relates to a stem cell film-forming culture medium and application thereof, the stem cell film-forming culture medium comprises a cell basal culture medium, fetal calf serum, growth factors, an attachment skeleton material and vitamins, and the method relates to preparation of the stem cell film-forming culture medium, separation of stem cells, culture of the stem cells and stem cell film formation. The method has the beneficial effects that the collagen is subjected to low-temperature centrifugal membrane forming by utilizing the dissolving property of the collagen, so that the operation cost and the technical requirements are low, and large-scale production is facilitated; the finally obtained stem cell biological patch is high in immunocompatibility, few in adverse reaction and thin in membrane thickness, the thickness can be controlled according to the temperature and the centrifugal time, the stem cells are embedded into the biological patch membrane, the stem cells are slowly released along with absorption of a matrix by a body after transplantation, and therefore the effect taking time is longer, and the survival rate of the stem cells is increased. Compared with a stem cell biological patch prepared by common culture and adsorption, the stem cell biological patch has a better ovary repairing effect.
Owner:BINZHOU MEDICAL COLLEGE +1
Xenograft Bone Matrix for Orthopedic Applications
InactiveUS20100196499A1Excellent immunocompatibilityDiminishing human to pig immunological responseHeart valvesBone implantPrimateGraft transplant
The invention provides for the use of an improved xenograft bone particulate with respect to osteo-integration and bone remodeling, while diminishing the primate-to-pig immunological response using established bone-processing technique. Work was carried out using undecalcified bone to determine immunocompatibilty and bone remodeling potential of processed porcine bone struts following onlay graft implantation. New bone formation was evident, including the infiltration of cellular materials responsible for fusion and bone reconstruction.
Owner:APERION BIOLOGICS
Method for preparing biomimetic artificial bone materials for biodegradable tissue engineering
ActiveCN101584884BShorten the preparation technology cycleReduce workloadProsthesisFreeze-dryingGenipin
Owner:NORTHWEST UNIV
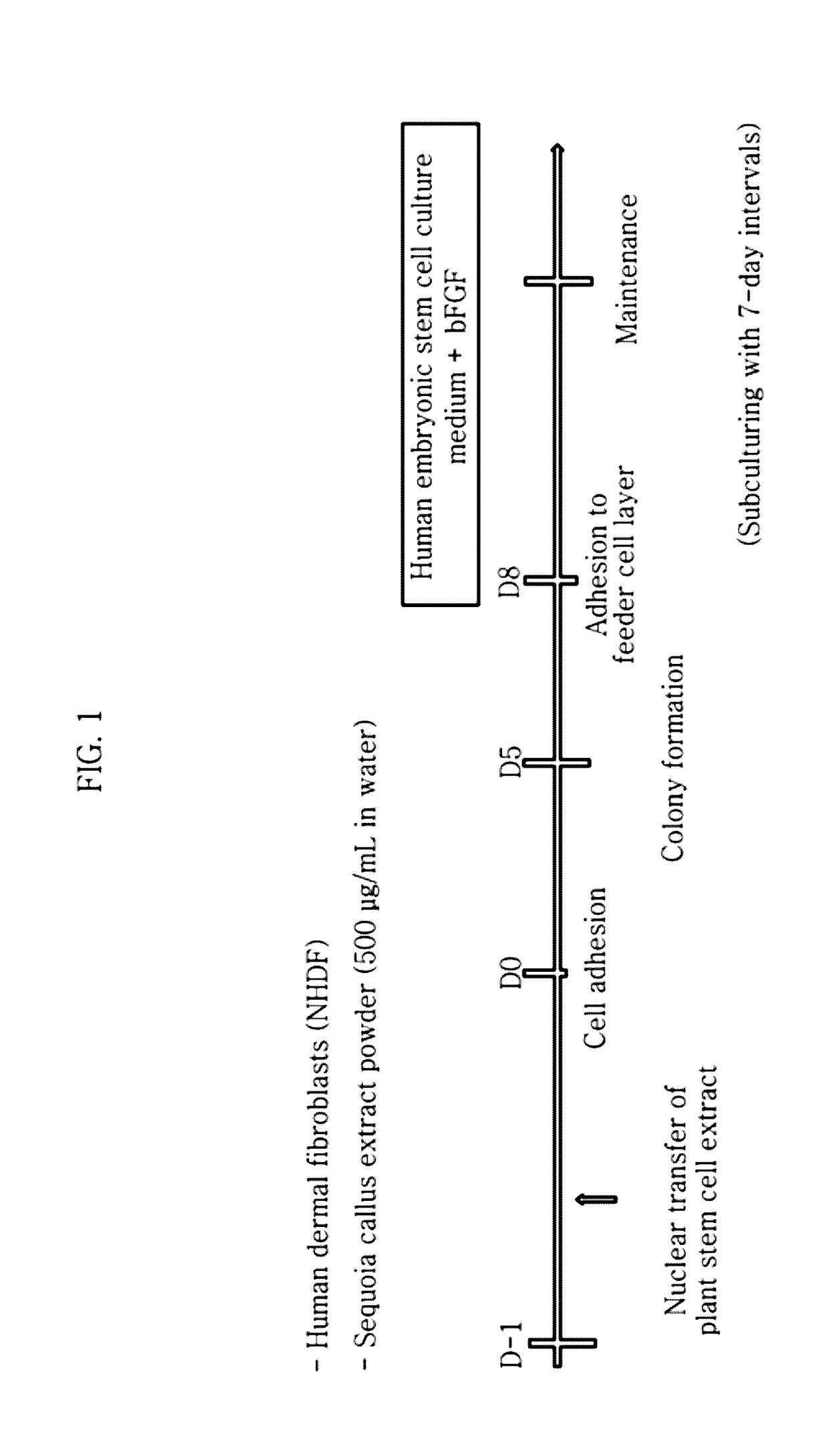
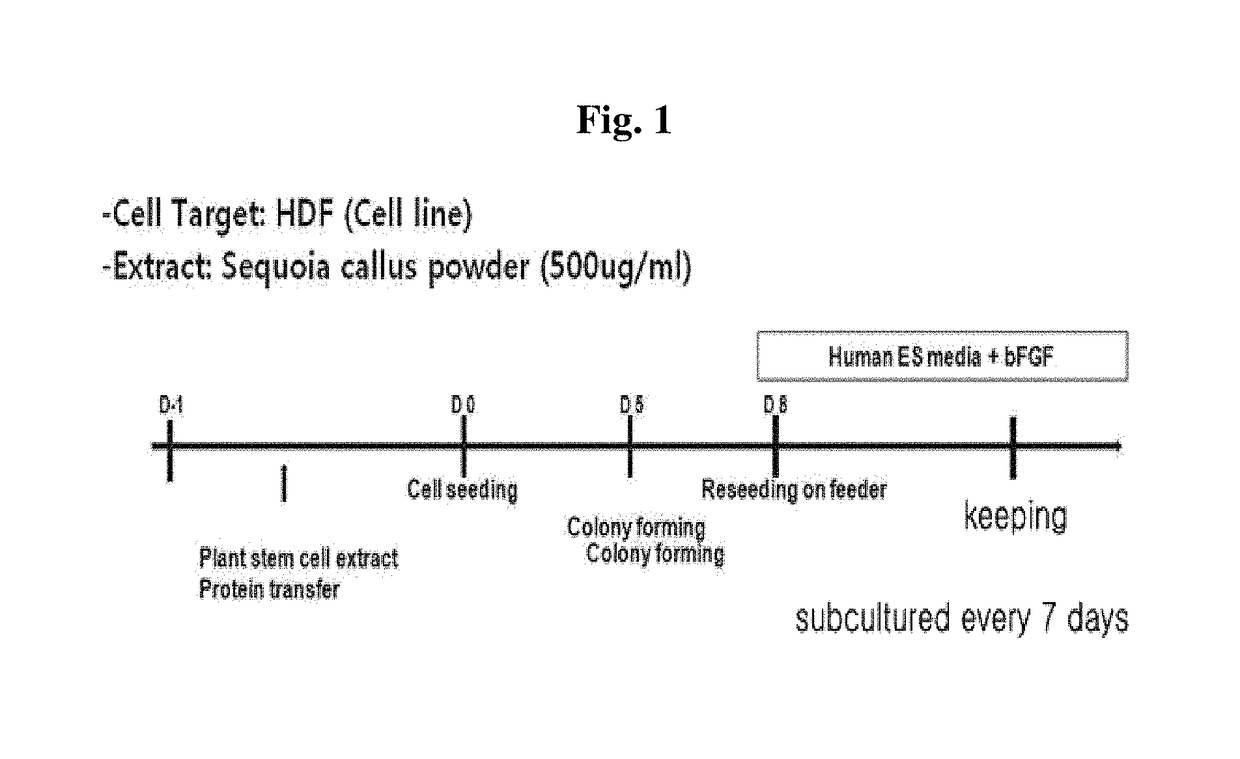
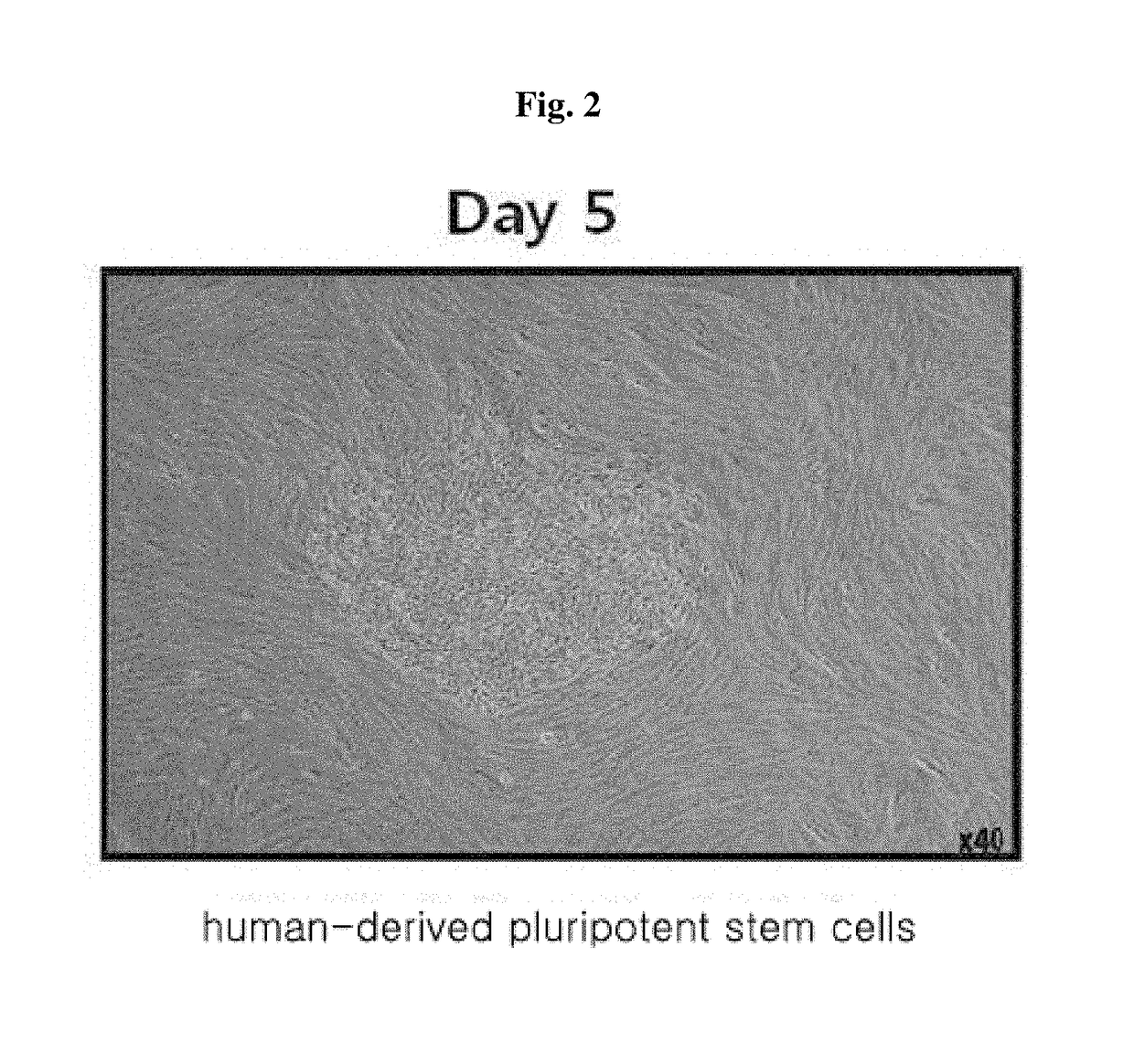
Method for inducing tailored pluripotent stem cells using extract of plant stem cells or plant dedifferentiated stem cells, and pluripotent stem cells produced by means of the method
ActiveUS9976118B2No ethical issueEfficient productionCosmetic preparationsToilet preparationsInduced pluripotent stem cellPlant stem
A method for inducing tailored pluripotent stem cells by reprogramming differentiated cells of an adult by using an extract of plant stem cells and plant dedifferentiated stem cells (callus); pluripotent stem cells produced by means of the method; and a cell therapy agent comprising the pluripotent stem cells are disclosed. It is possible to overcome ethical problems since eggs are not used in the making of pluripotent stem cells having abilities like embryonic stem cells. It is possible to produce pluripotent stem cells of ensured safety because a plant stem cell extract which above all has been verified to be harmless to the body is used. It is possible to develop an immunocompatible cell therapy agent tailored to different individuals.
Owner:SEOUL NAT UNIV R&DB FOUND
Method for generating immune-compatible cells and tissues using nuclear transfer techniques
InactiveCN1376196AAvoid transplant rejectionOrganic active ingredientsGenetic material ingredientsBiotechnologyImmunocompatibility
Owner:ADVANCED CELL TECH INC
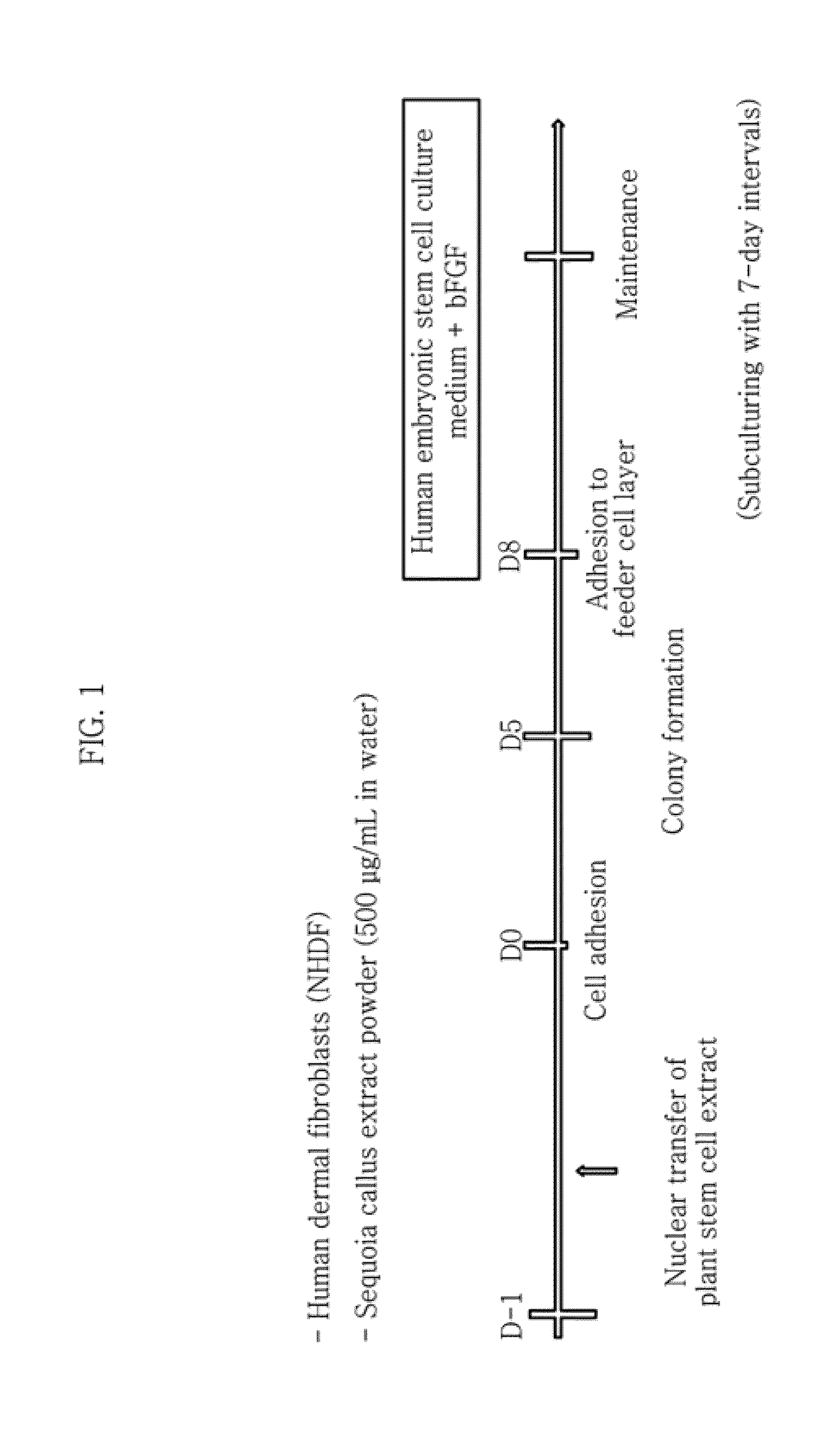
Method for inducing pluripotent stem cells and pluripotent stem cells prepared by said method
InactiveUS20160215269A1Cosmetic preparationsOrganic active ingredientsDiseaseInduced pluripotent stem cell
The present disclosure relates to a method for inducing pluripotent stem cells by inducing reprogramming and / or dedifferentiation of differentiated adult cells using shikimic acid, a plant extract or plant stem cells containing shikimic acid and an extract of dedifferentiated stem cells (callus), pluripotent stem cells prepared by the method and a composition containing the pluripotent stem cells. In accordance with the present disclosure, ethical concerns implicated with the use of eggs to prepare pluripotent stem cells such as embryonic stem cell can be resolved. And, because the plant stem cell extract unharmful to human is used, pluripotent stem cells with proven safety can be prepared and they may be used to develop immunocompatible cell therapy agents suited for individuals. In addition, by pluripotent stem cells from individuals having diseases, the present disclosure will be very useful in studying the cause of diseases and devolving therapeutic strategy.
Owner:AMOREPACIFIC CORP +1
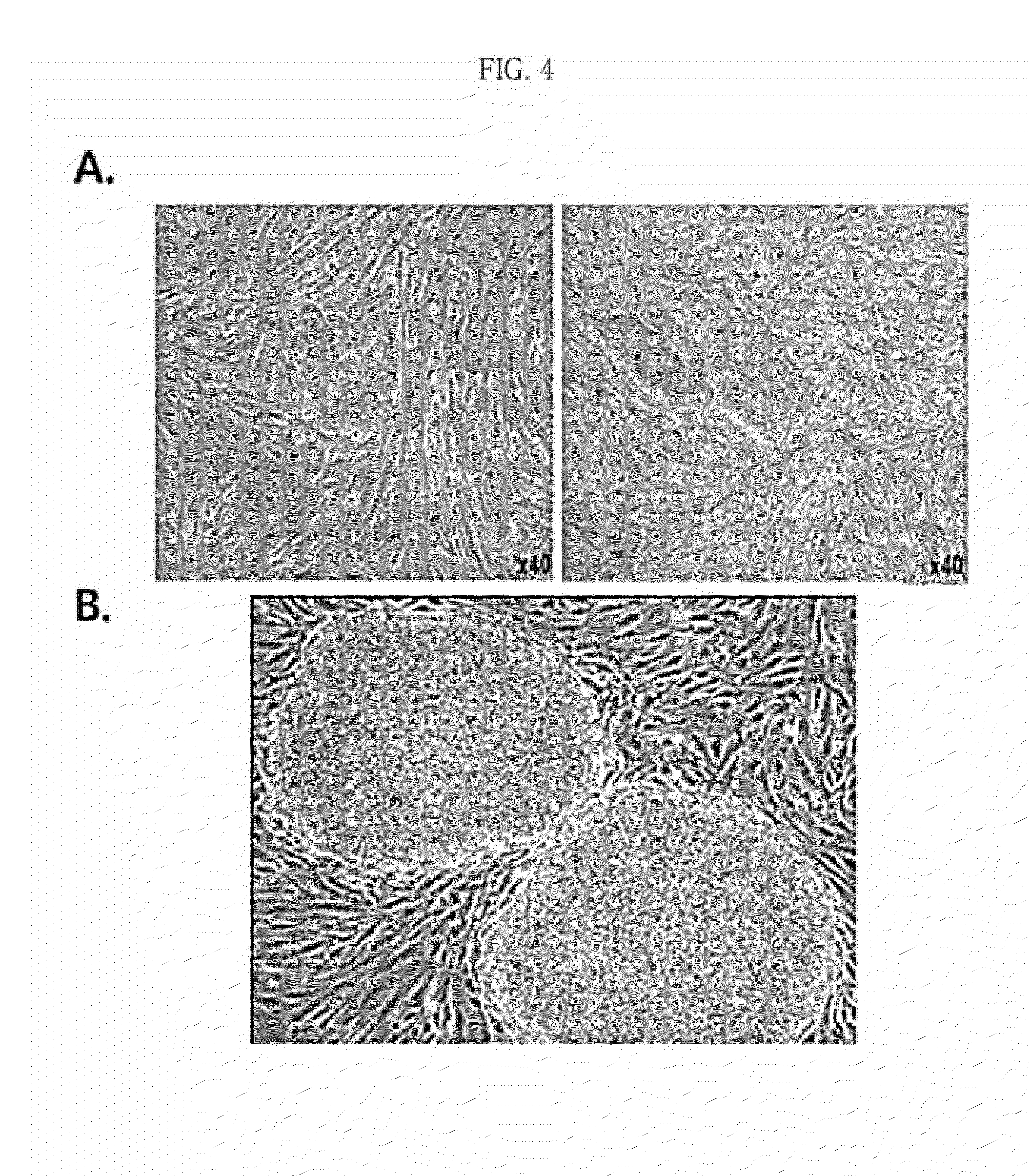
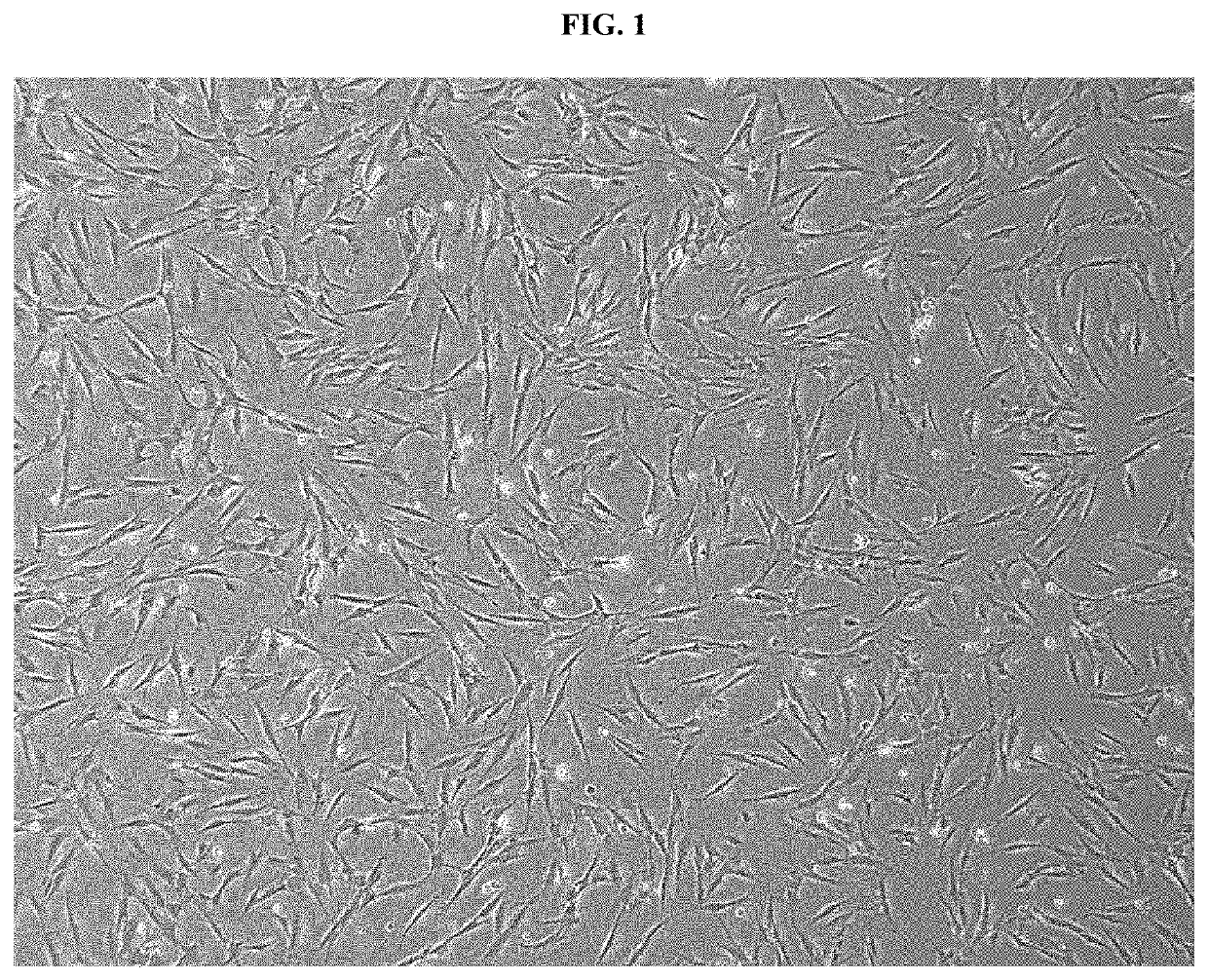
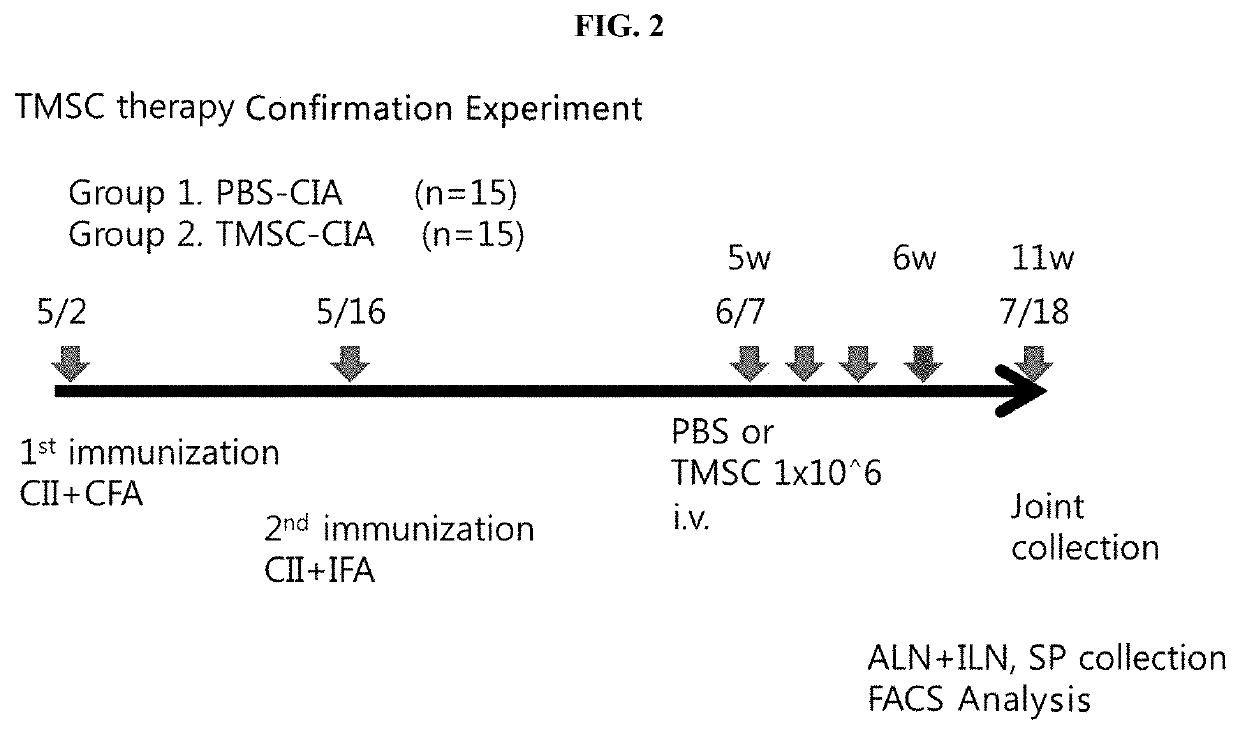
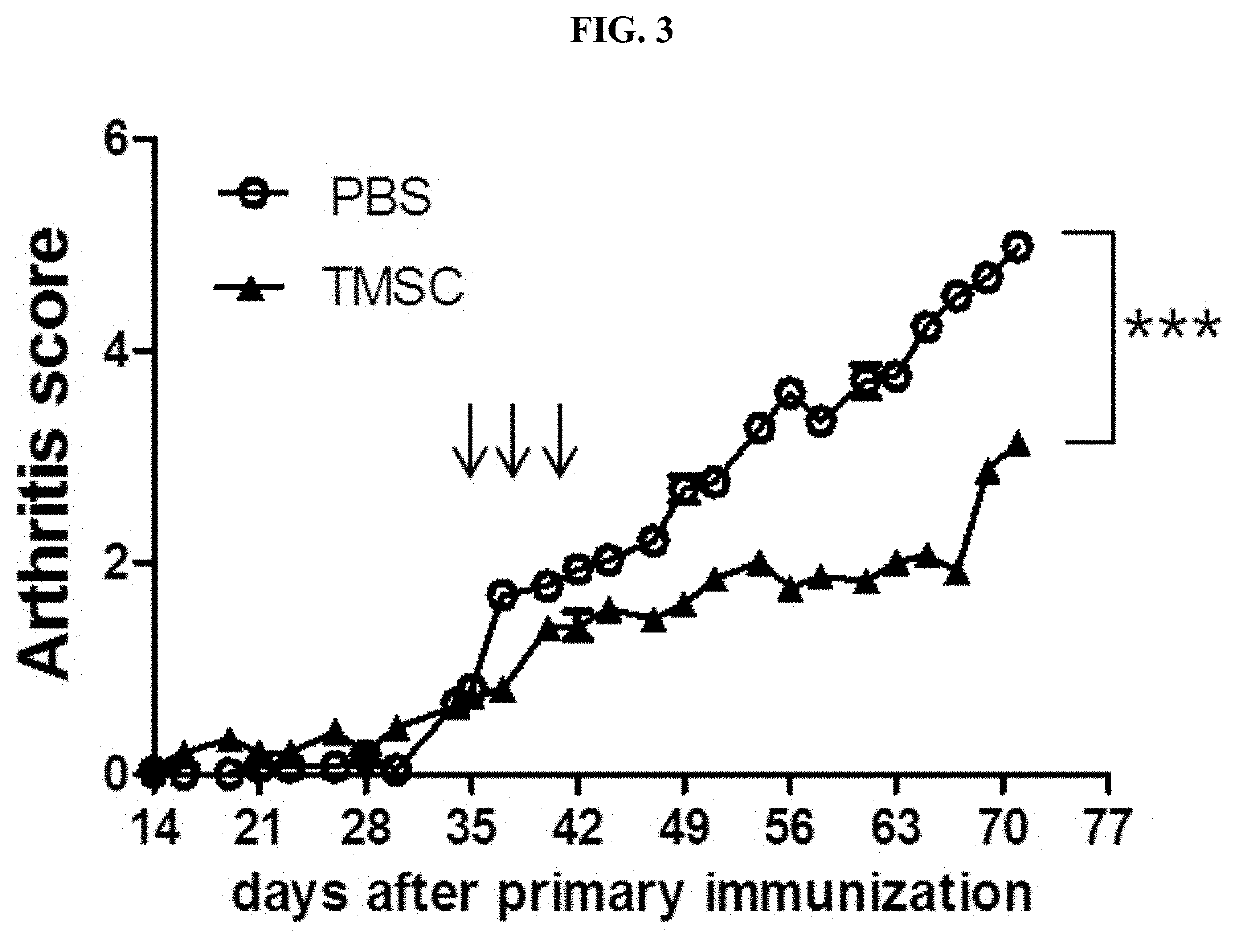
Pharmaceutical composition for preventing or treating rheumatoid arthritis comprising nasal inferior turbinate-derived mesenchymal stem cells as an active ingredient
ActiveUS20200254024A1Reduce productionProliferative abilitySkeletal disorderUnknown materialsWhite blood cellIntravenous gammaglobulin
The present invention relates to a pharmaceutical composition for preventing or treating rheumatoid arthritis, and a stem cell therapeutic agent for treating rheumatoid arthritis, comprising nasal inferior turbinate-derived mesenchymal stem cells as an active ingredient. Nasal inferior turbinate-derived mesenchymal stem cells of the present invention have the effects of reducing the production of interleukin-17A (IL-17A), tumor necrosis factor-α (TNF-α), immunoglobulin G2 (IgG2a) which is an inflammation-inducing factor, and / or the proliferative ability of lymph node T cells, increasing interleukin-10 (IL-10) and / or regulatory T-cells (Treg, CD4+CD25+foxp3+ cell) that contribute to immune tolerance in spleen cells, and inhibiting the proliferation of human T-cells. The nasal inferior turbinate-derived mesenchymal stem cells have a safer acquisition process than existing mesenchymal stem cells and can be acquired in sufficient amounts within a desired period of time, thus allowing mesenchymal stem cells to be acquired at low cost and high efficiency. Moreover, since the nasal inferior turbinate-derived mesenchymal stem cells have the same genetic origin as individuals administered therewith there are few side effects, the nasal inferior turbinate-derived mesenchymal stem cells can be usefully used for preventing or treating in individually tailored immunocompatible rheumatoid arthritis.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
Immunocompatible polymers
Owner:MCMASTER UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com