Immunocompatible reversible universal pluripotent stem cells and application thereof
A kind of pluripotent stem cell and general-purpose technology, which is applied in immunocompatibility and reversible general-purpose pluripotent stem cell and its application field, can solve the problems of high risk of disease, no better solution to the problem, shrinking of antigen integrity and other problems, and achieve functional The effect of both sex and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1 A preparation method of immune compatible and reversible universal pluripotent stem cells and derivatives thereof
[0118] A method for preparing immune-compatible and reversible general-purpose pluripotent stem cells or derivatives thereof, wherein an inducible gene expression system and at least one immune-compatible molecule expression sequence are introduced into the genome of the pluripotent stem cells or their derivatives; The compatible molecule is used to regulate the expression of genes related to immune response in the pluripotent stem cell or its derivative; and the expression of the immune compatible molecule is regulated by an inducible gene expression system.
[0119] When carrying out immune-compatibility transformation, the transformation can be carried out on hPSCs first, and then differentiated into derivatives of pluripotent stem cells for use after the transformation is completed; it is also possible to carry out immune-compatibility transfo...
Embodiment 2
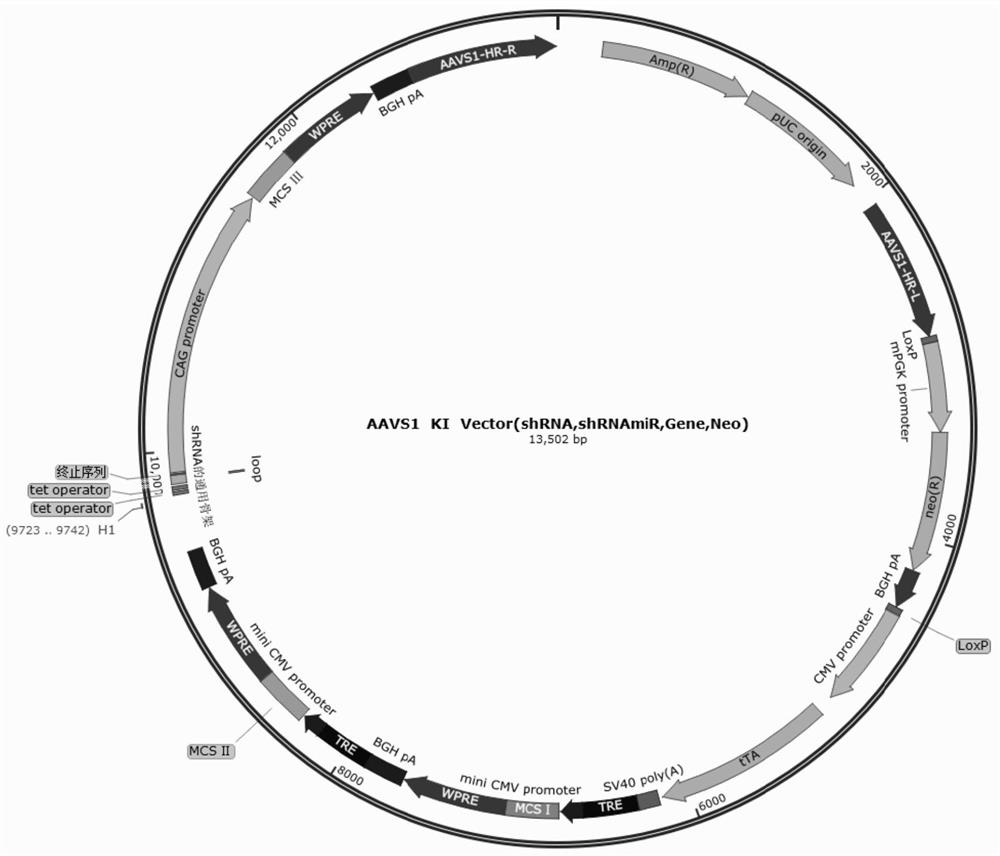
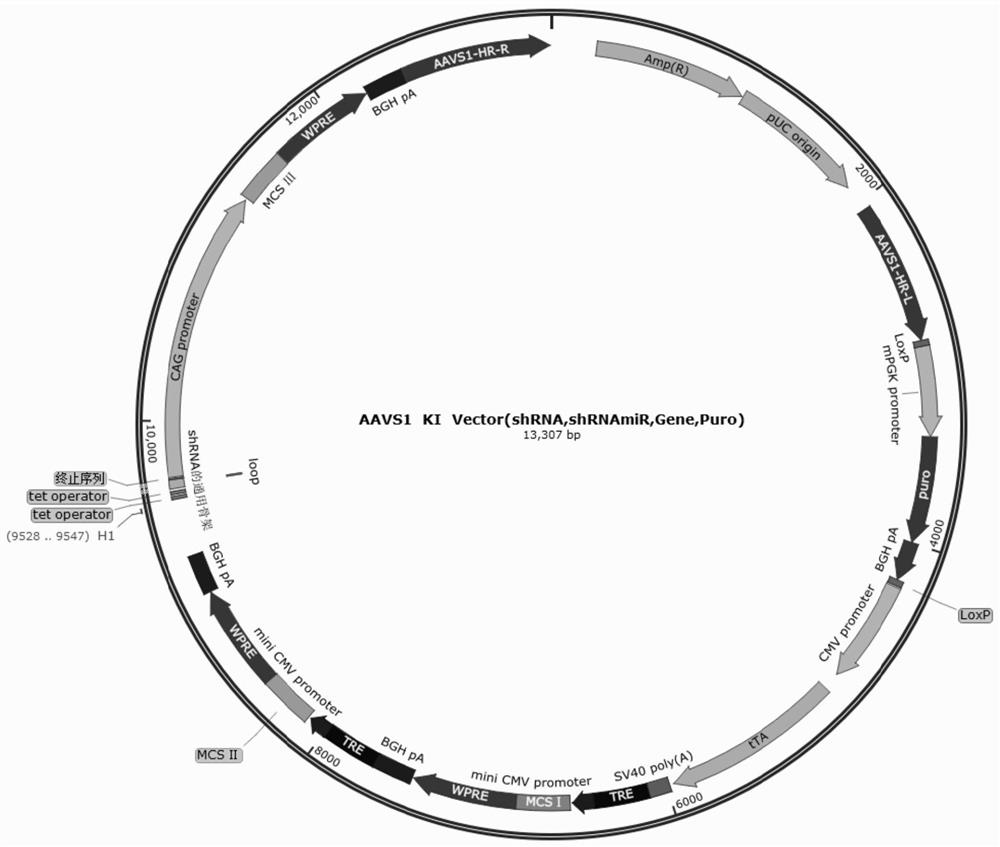
[0350] Using the method of Example 1, the Tet-Off system and immune compatibility molecule combination (Table 3-Table 6) were knocked into the genomic safety site AAVS1 of hPSCs-derived EBs to obtain EB immune-compatible cells.
[0351] use 51 Cr release test to detect the immune compatibility effect of EB ball immunocompatibility cells and T cells:
[0352] 1. Digest EB immune-compatible cells into individual cells as target cells;
[0353] 2. Will pass 51 Cr-labeled target cells and T cells were added to a 96-well culture plate at a ratio of 1:5 for post-reaction detection;
[0354] 3. According to 51 Detection of Cell Specificity of Immunocompatible Cells in EB Balloons by Cr Release Assay 51 Cr release rate, the results are shown in Table 9 and Figure 8 shown.
[0355] Table 9 EB ball immune compatible cells 51 Cr release rate
[0356]
[0357]
[0358] From Table 9 and Figure 8 It can be seen that the immunocompatibility effect of the hPSCs-derived EB sph...
Embodiment 3
[0360] This embodiment will test the effect of using an inducer (Dox) to prevent expression of the knocked-in immune-compatibility molecule in the case of graft lesions, and the steps are as follows:
[0361] (1) Add 6uM Dox to the culture medium of the EB sphere immunocompatibility cells prepared in Example 2 and treat for 48h;
[0362] (2) Digest the Dox-treated EB immune-compatible cells into individual cells as target cells;
[0363] (3) will pass 51 Cr-labeled target cells and T cells were added to a 96-well culture plate at a ratio of 1:5 for post-reaction detection;
[0364] (4) According to 51 Detection of Cell Specificity of Immunocompatible Cells in EB Balloons by Cr Release Assay 51 Cr release rate, the results are shown in Table 10 and Figure 9 shown.
[0365] Table 10 EB ball immune compatible cells after Dox treatment 51 Cr release rate
[0366]
[0367]
[0368] from Table 10 and Figure 9 It can be seen that after the hPSCs-derived EB sphere immuno...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com