Peptide dentritic macromolecular drug and preparation method and application thereof
A technology of dendritic macromolecules and peptides, applied in the field of biomedicine, can solve the problems of difficult synthesis and purification, low cell uptake, and high production costs, and achieve the effects of rich surface functional groups, high anti-tumor activity, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation of peptide dendrimer
[0055] a) Protect the functional group of the amino acid: protect the amino acid according to the difference of the surface functional group of the core molecule of the peptide dendrimer to be prepared. If the surface functional group of the core molecule is an amino group or a hydroxyl group, protect the amino group of the amino acid, such as the core molecule If the surface functional group is a carboxyl group, the carboxyl group of the amino acid is protected;
[0056] b) Preparation of a first-generation dendrimer: Weigh branched cores (functionality n, n>1), amino acids (1.5n equivalents) containing protective groups, condensing agents (1.5n equivalents), catalysts (1.5 n equivalents) and an organic base (4n equivalents), at 0°C, under nitrogen protection conditions, add a solvent for dehydration condensation reaction; then react at room temperature, after the reaction, the resulting solution is washed and dried,...
Embodiment 2
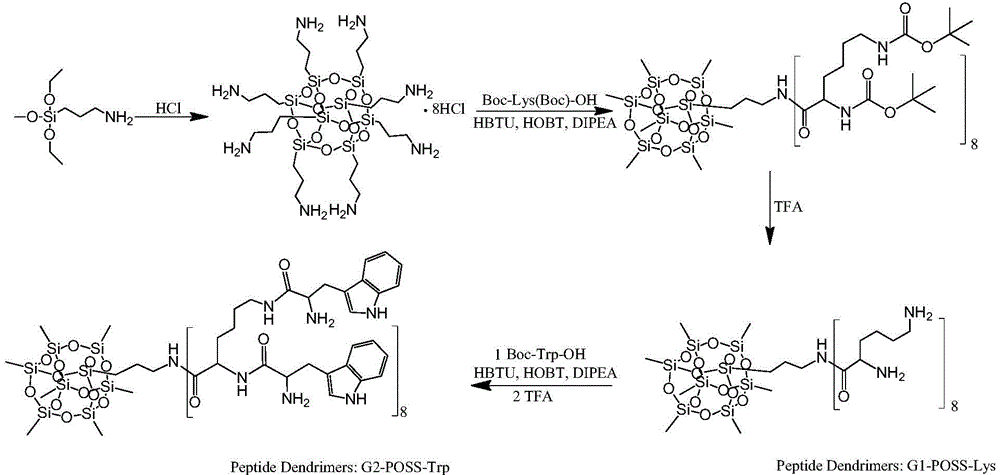
[0062] Embodiment 2: the specific synthesis example of a kind of peptide dendrimer drug (synthetic route such as figure 2 )
[0063] Synthesis of a new generation of peptide dendrimers (G1-Poss-Lys)
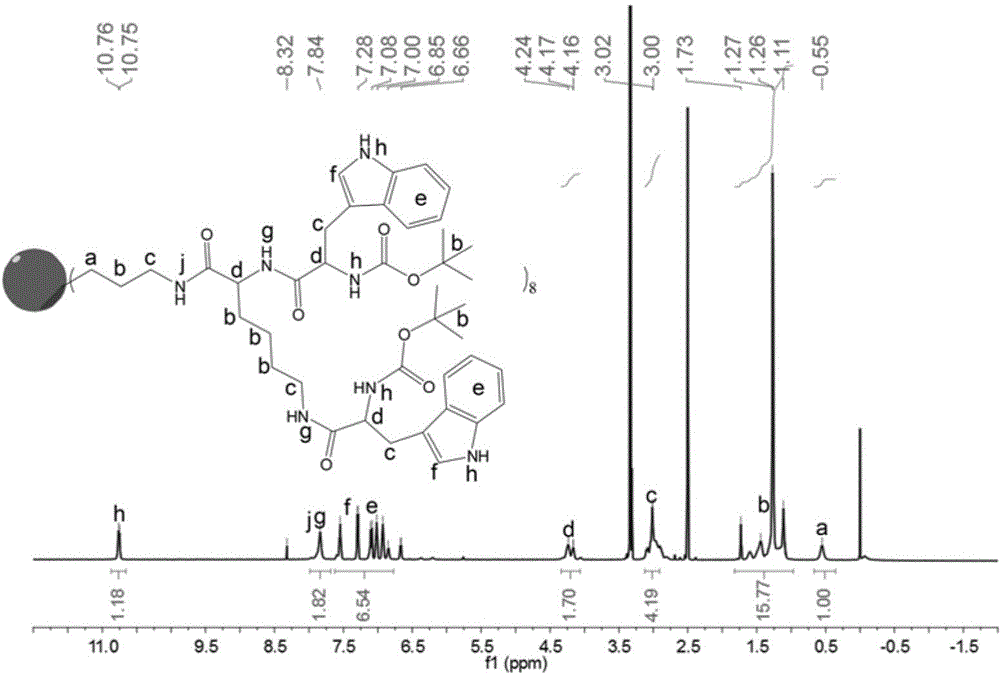
[0064] Add 30 mL of concentrated hydrochloric acid dropwise to 350 mL of methanol at 50°C, continue to raise the temperature to 90°C, slowly add 15 mL of 3-aminopropyltriethoxysilane dropwise, and react in a closed state for 24 hours, tetrahydrofuran precipitates to obtain octamer (3 - Aminopropyl)silsesquioxane hydrochloride (Poss.HCl). 1 H NMR (600 MHz, DMSO-d 6 )δ8.14 (s, 2H), 3.61-3.59 (t, 2H), 1.69 (m, 2H), 0.75-0.72 (t, 2H).
[0065] Weigh 2.0g cage-type octamer (3-aminopropyl) silsesquioxane hydrochloride (Poss HCl), 8.0g O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU) and 2.5 Add 1-g 1-hydroxybenzotriazole (HOBT) into a 100mL single-neck flask, add 5.0g Boc-lys(Boc)-OH into a 50mL constant pressure dropping funnel, vacuumize and fill with nitrogen. Add...
Embodiment 3
[0073] Embodiment 3: to the peptide dendrimer drug prepared in embodiment 2, it is carried out the detection of following aspects:
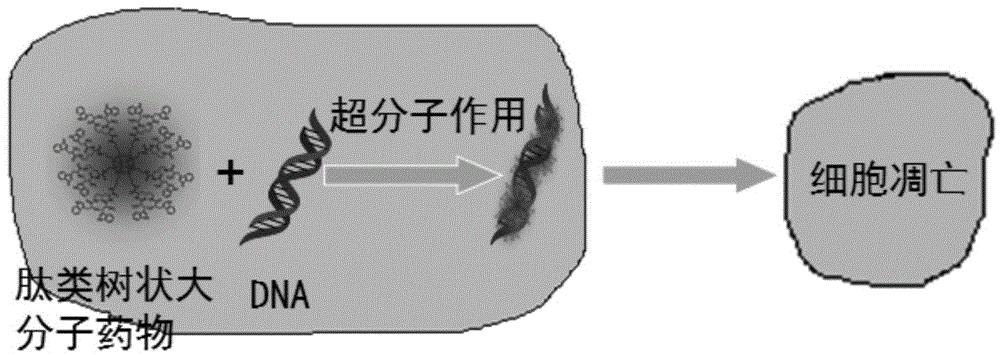
[0074] (1) Use gel electrophoresis retardation experiments to observe the ability of different amounts of peptide dendrimer drugs to interact with DNA. - EGFPC1 plasmid DNA (including 200ng DNA) was compounded at room temperature for 30 minutes, and the W / P ratio refers to the molar ratio of tryptophan (W) in the peptide drug to the phosphate group (P) in the DNA. The complex was subjected to 1% agarose gel electrophoresis at 100mV for 45min, stained with ethidium bromide, and observed under a 254nm ultraviolet light. Figure 6 The results showed that when the molar ratio of the peptide dendrimer drug to the p-EGFPC1 plasmid was greater than 10:1, it could effectively interact with the p-EGFPC1 plasmid and block its movement.
[0075] (2) At the same time, the fluorescence spectrum further indicated that there was a supramolecular interaction be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com