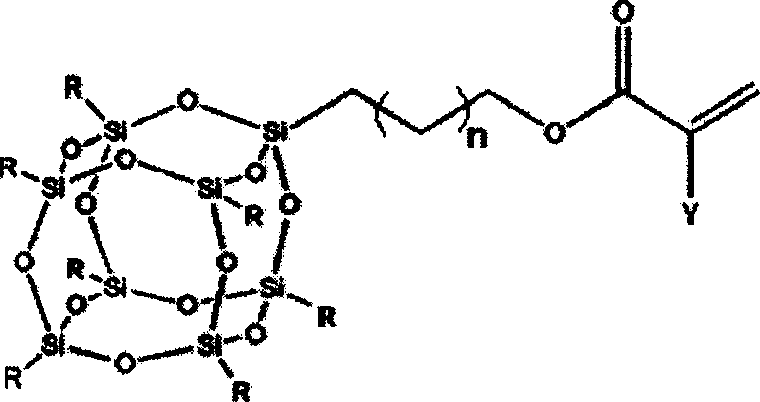
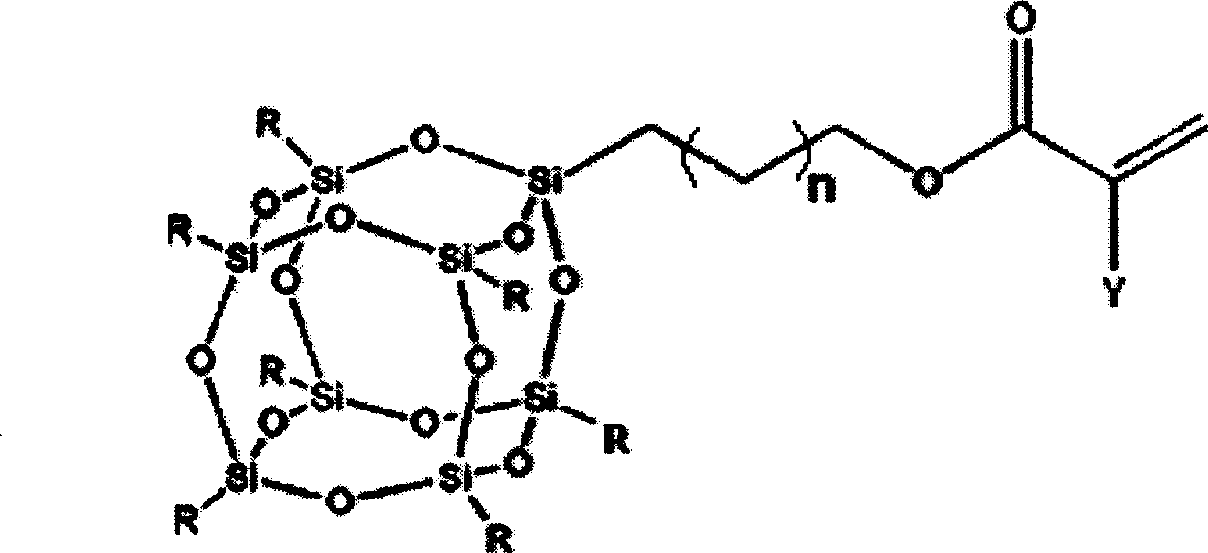
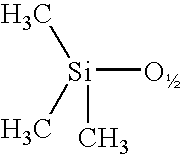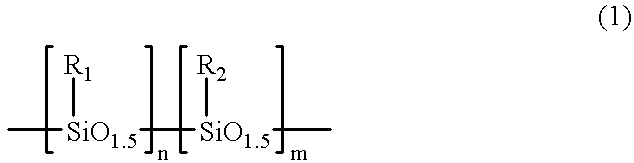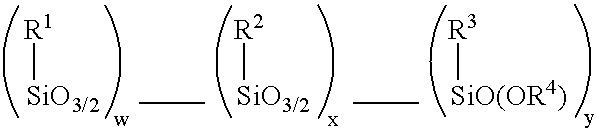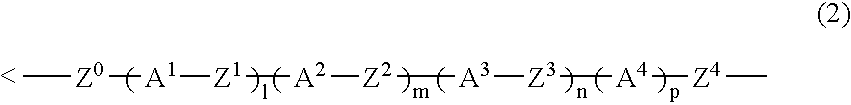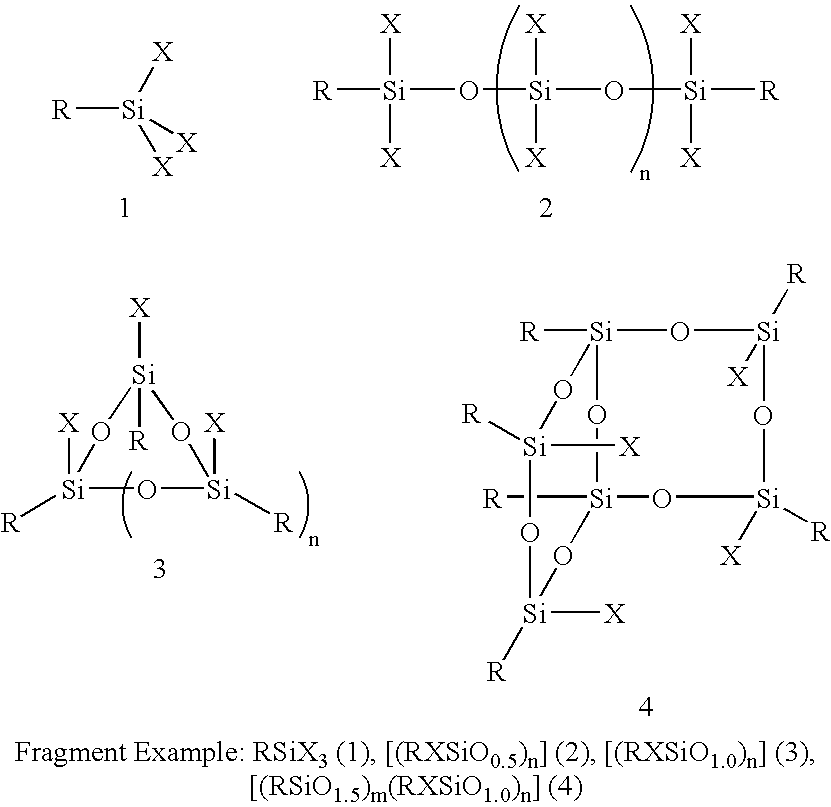Patents
Literature
3489 results about "Silsesquioxane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
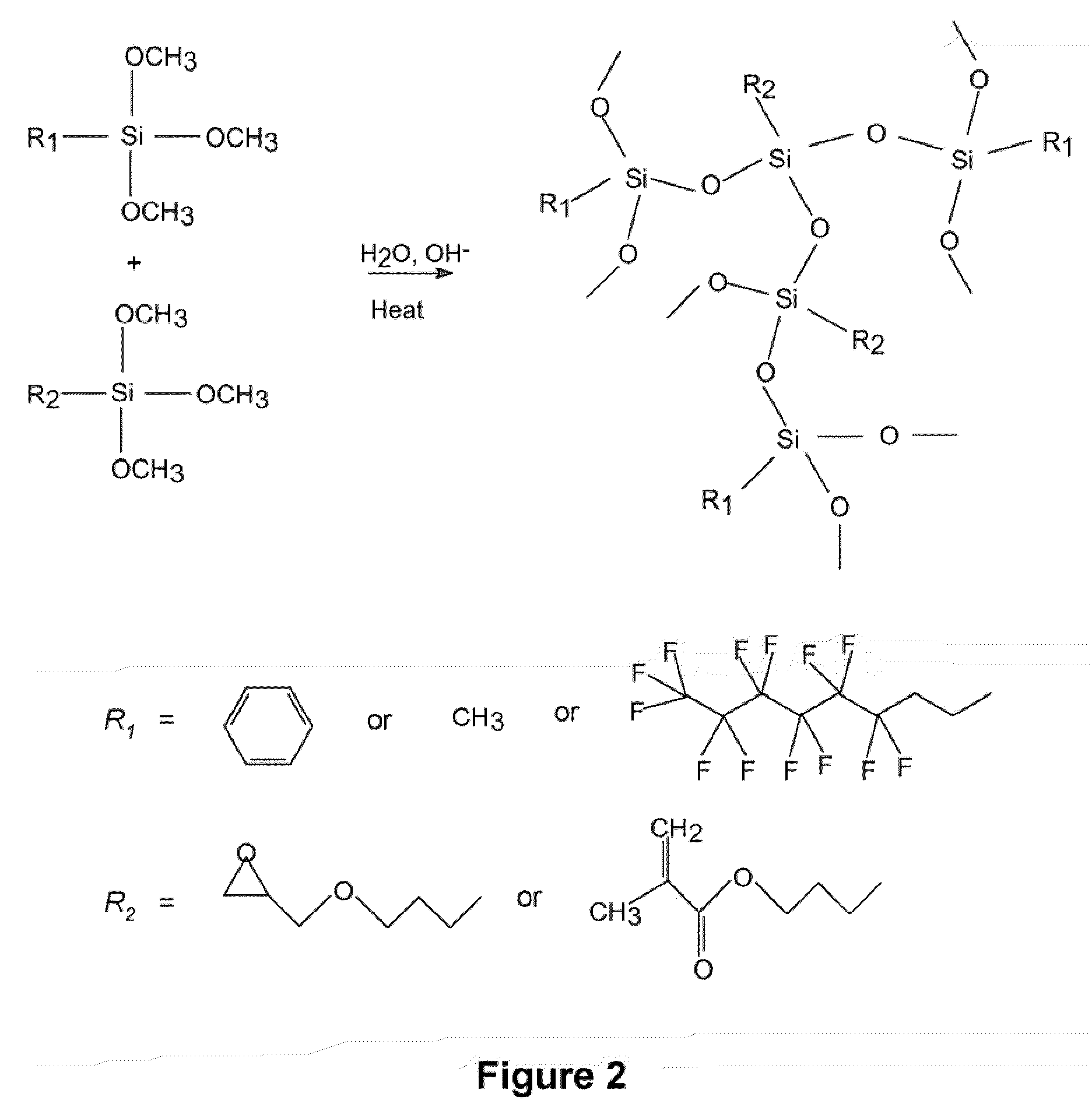
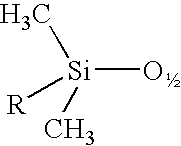
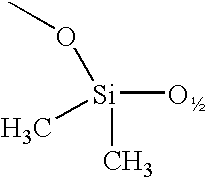
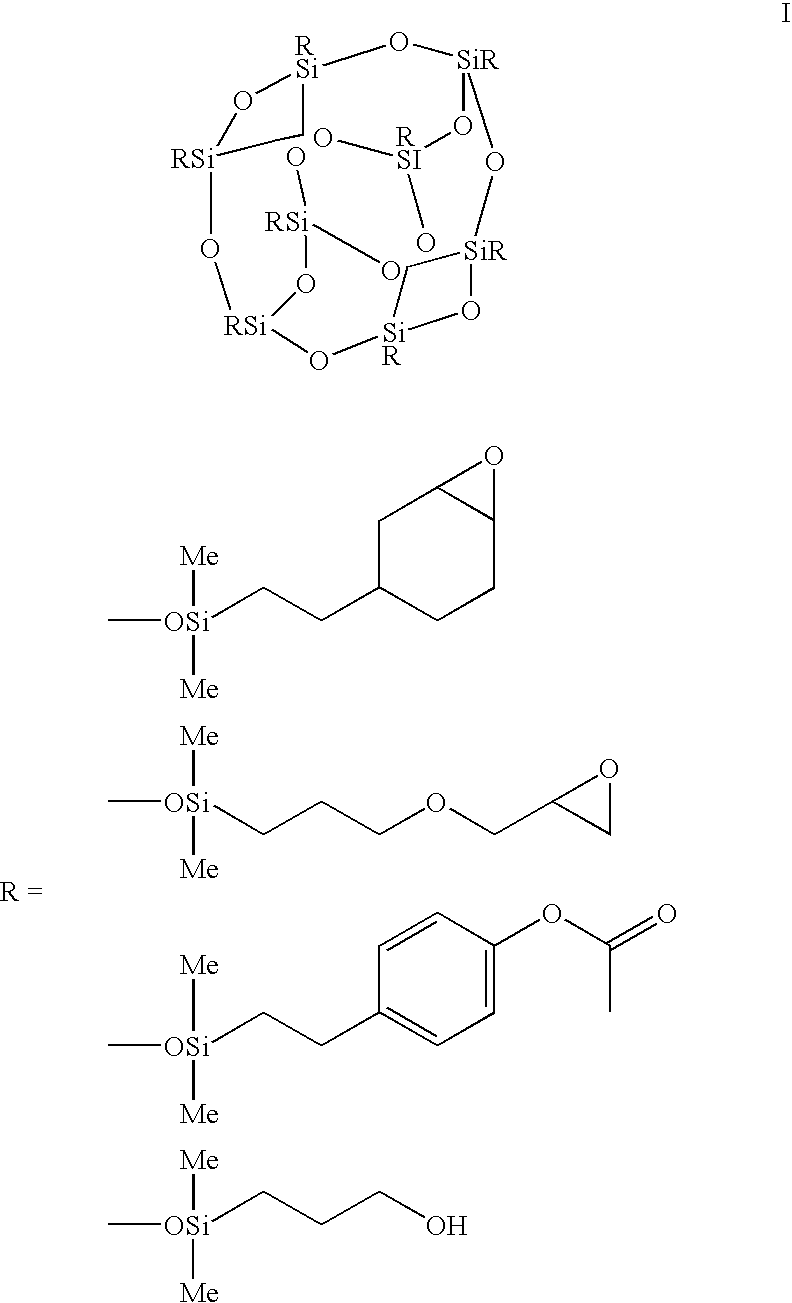
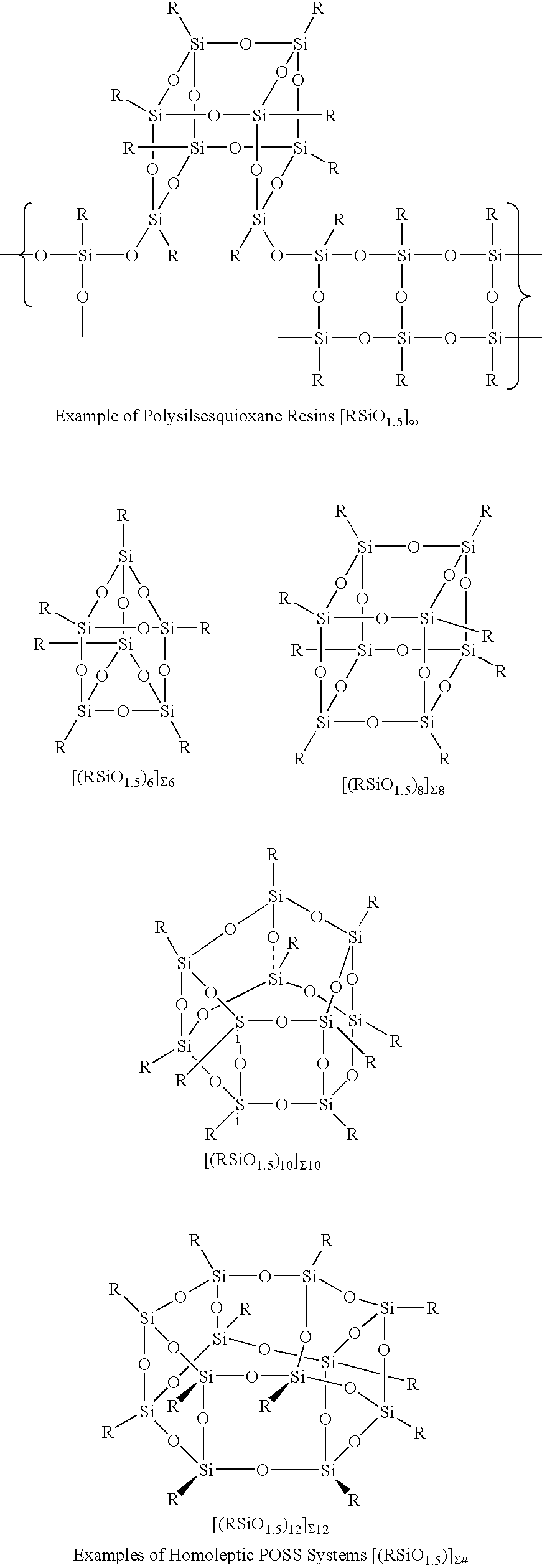
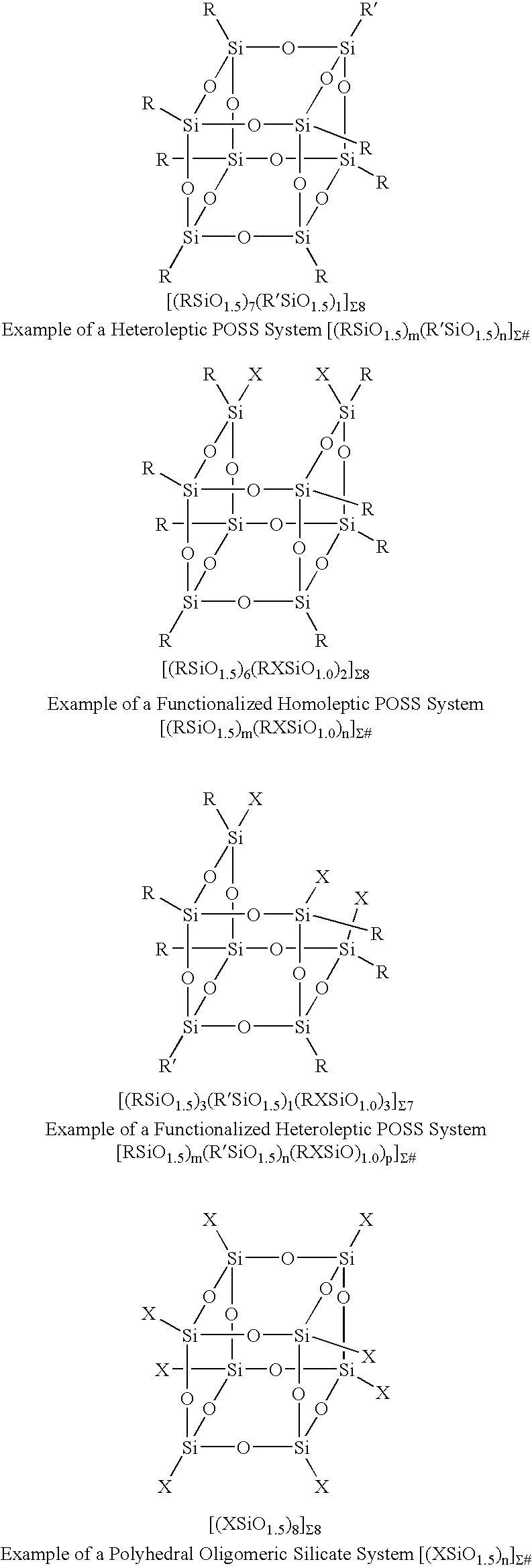
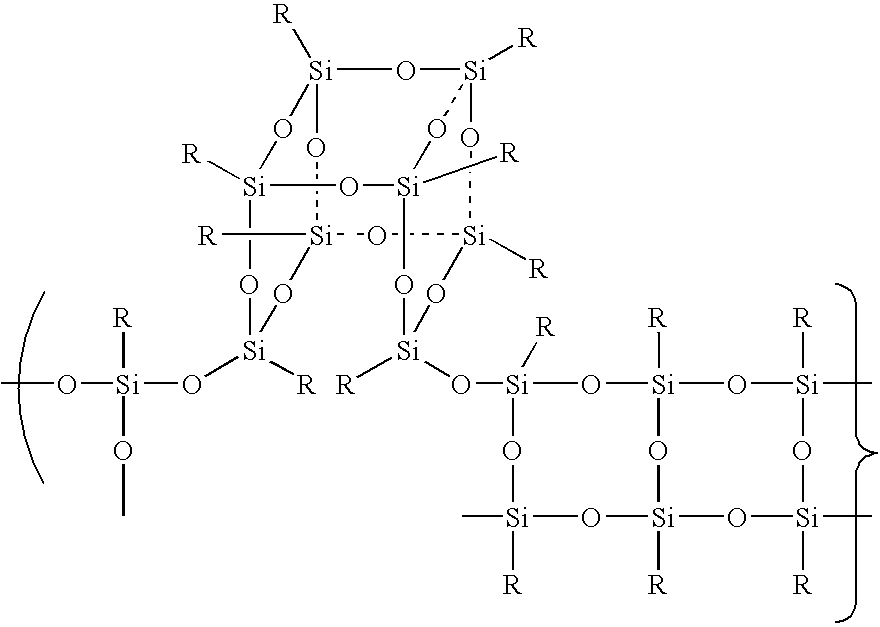
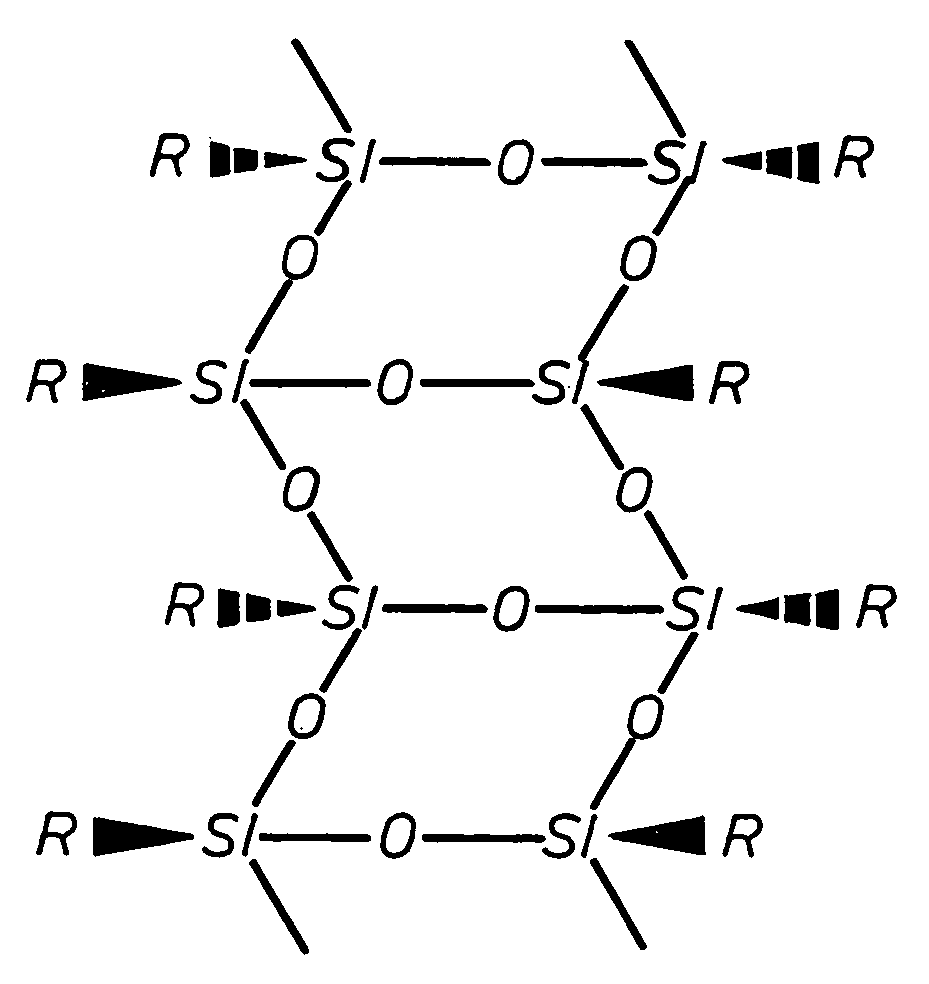
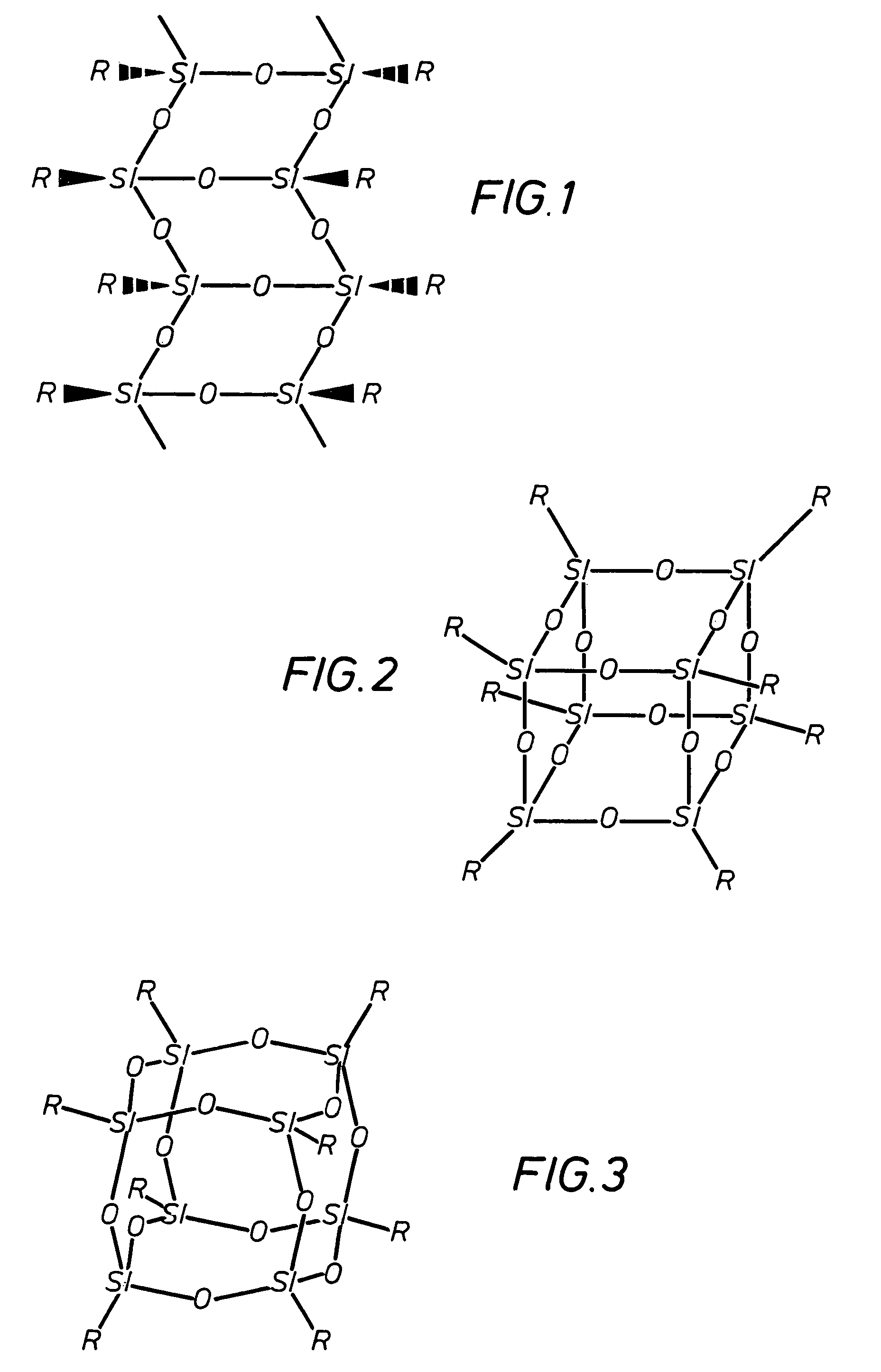
A silsesquioxane is an organosilicon compound with the chemical formula [RSiO₃⸝₂]ₙ (R = H, alkyl, aryl or alkoxyl). Silsesquioxanes are colorless solids that adopt cage-like or polymeric structures with Si-O-Si linkages and tetrahedral Si vertices. Silsesquioxanes are members of polyoctahedral silsesquioxanes ("POSS"), which have attracted attention as precursors to ceramic materials and nanocomposites. Diverse substituents (R) can be attached to the Si centers. The molecules are unusual because they feature an inorganic silicate core and an organic exterior. The silica core confers rigidity and thermal stability.
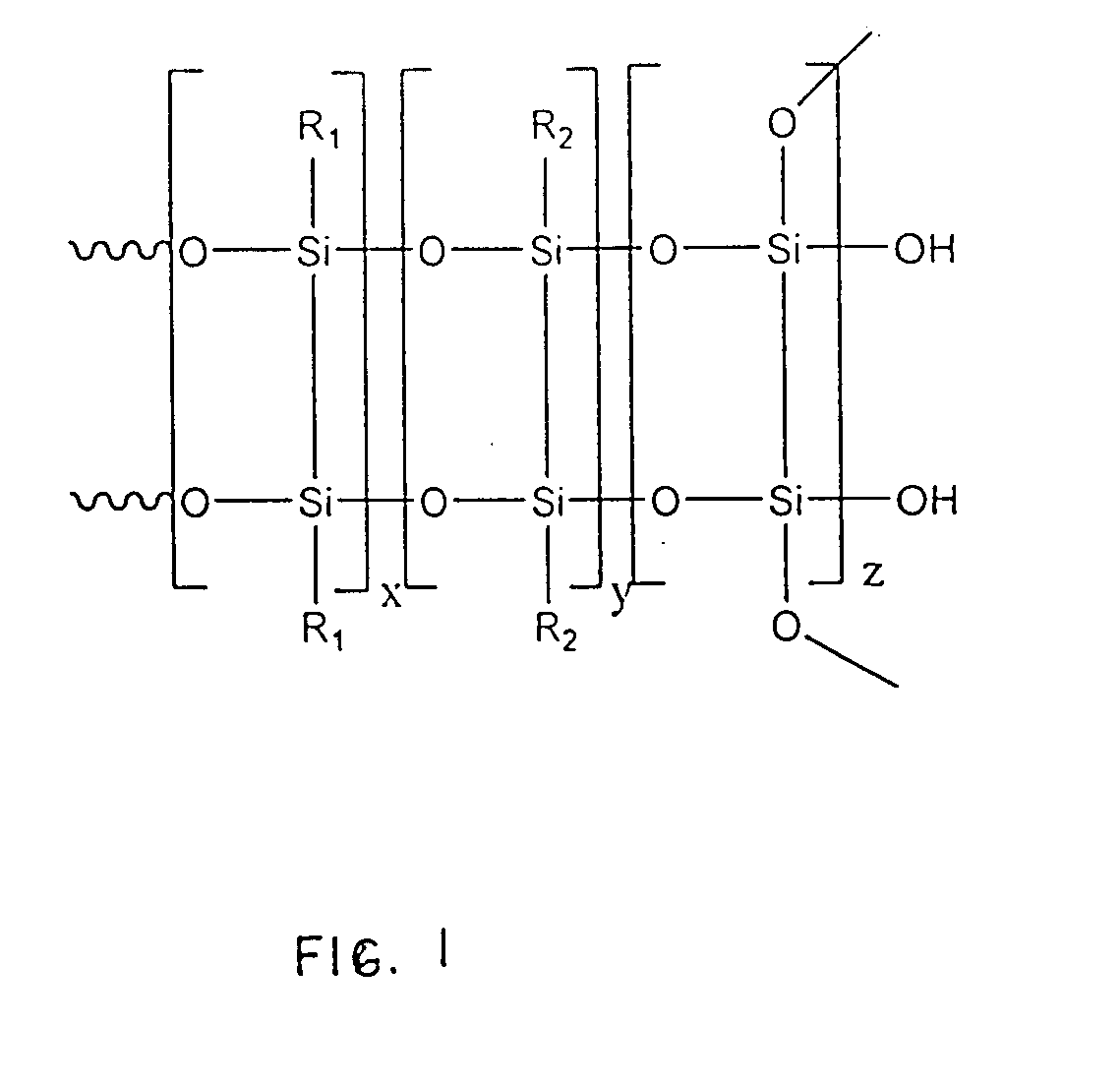
Silsesquioxane polymers, method of synthesis, photoresist composition, and multilayer lithographic method
InactiveUS6087064AReduce usageAvoid pollutionPhotosensitive materialsRadiation applicationsResistPolymer science
Novel silsesquioxane polymers are formed by methods which avoid the use of BBr3. The novel silsesquioxane polymers are especially useful in negative photoresist compositions and photolithographic processes. Alternatively, improved silsesquioxane polymer-containing negative photoresist compositions are obtained by using a polymer component containing a blend of silsesquioxane polymer and non-silsesquioxane polymer. The photoresist compositions provide improved dissolution characteristics enabling the use of 0.26 N TMAH developer. The photoresist compositions also provide improved thermal characteristics enabling use of higher processing temperatures. The photoresist compositions are especially useful in a multilayer photolithographic processes and are capable of producing high resolution.
Owner:GLOBALFOUNDRIES INC
UV Curable Silsesquioxane Resins For Nanoprint Lithography
ActiveUS20090256287A1Simple compositionImprove methodPhotosensitive materialsNanoinformaticsNanolithographyResist
Radiation-curable silsesquioxane resin materials are employed for micro- and nanolithography. The resin materials can include a radiation-curable silsesquioxane resin and a photo-initiator having low viscosity. The low viscosity of the liquid system allows imprinting with low pressure and low temperature; e.g. room temperature. The resist's dry etching resistance is increased and the cured film is more easily separated from the mask. Due to its high modulus after cure, the material allows the fabrication of micro- and nano-features having high aspect ratios while providing a high throughput. Various pattern sizes, for example, ranging from tens of microns to as small as a few nanometers, may be achieved with the UV-curable material system.
Owner:RGT UNIV OF MICHIGAN
Cosmetic compositions having enhanced wear properties
A cosmetic composition and process which provides enhanced wear and transfer resistance containing: (a) at least one polypropylsilsesquioxane film forming resin; (b) at least one solvent; and (c) optionally, at least one colorant.
Owner:LOREAL SA
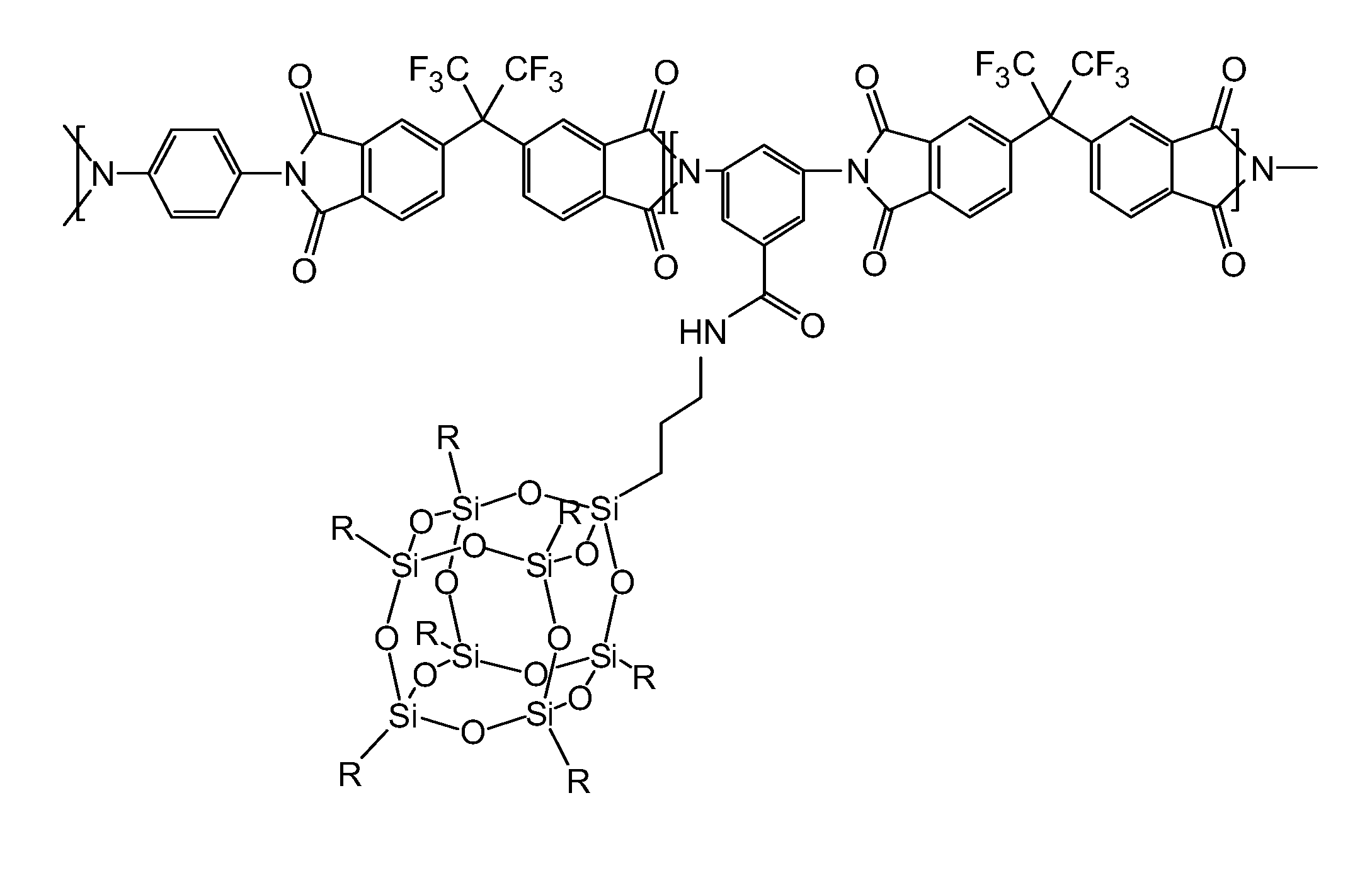
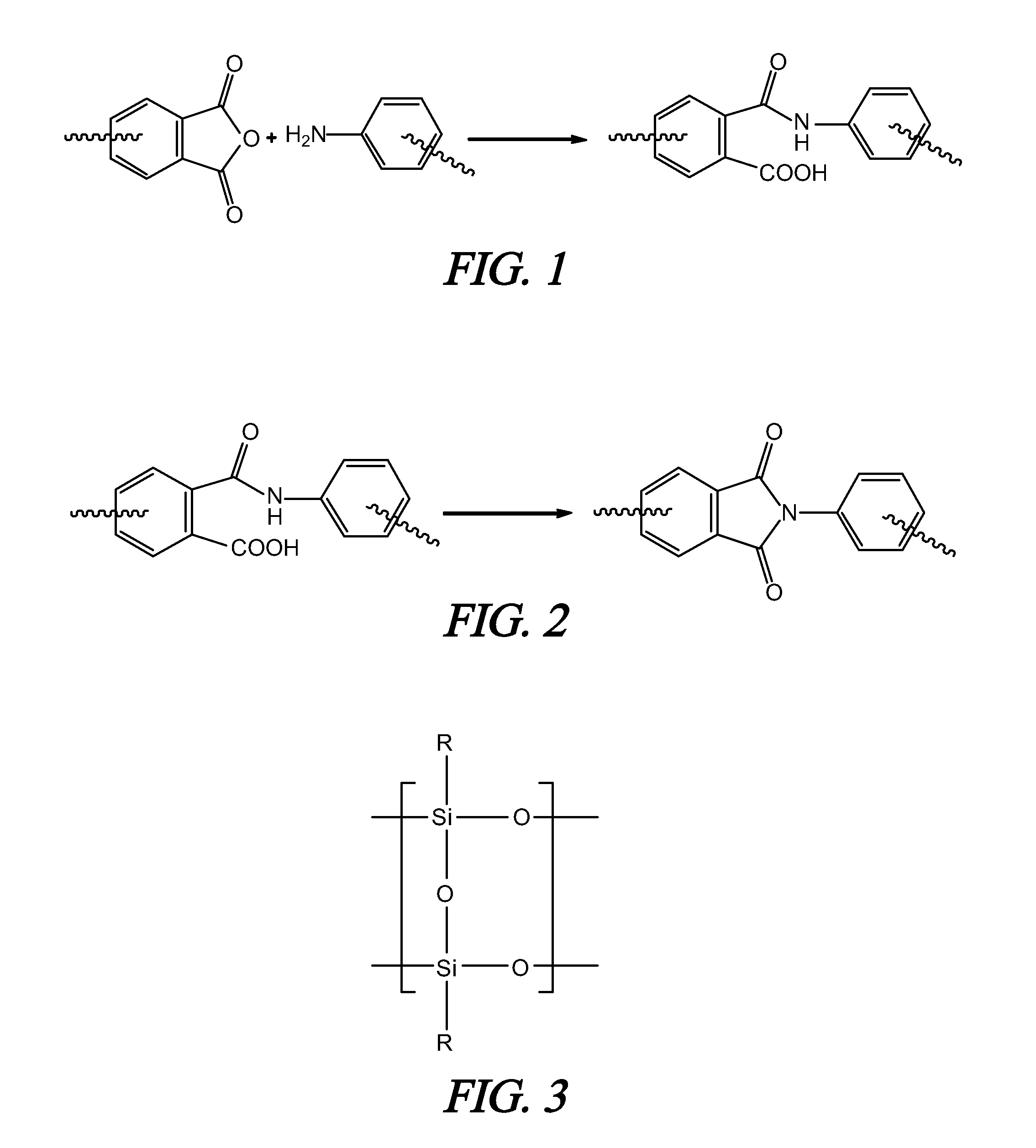
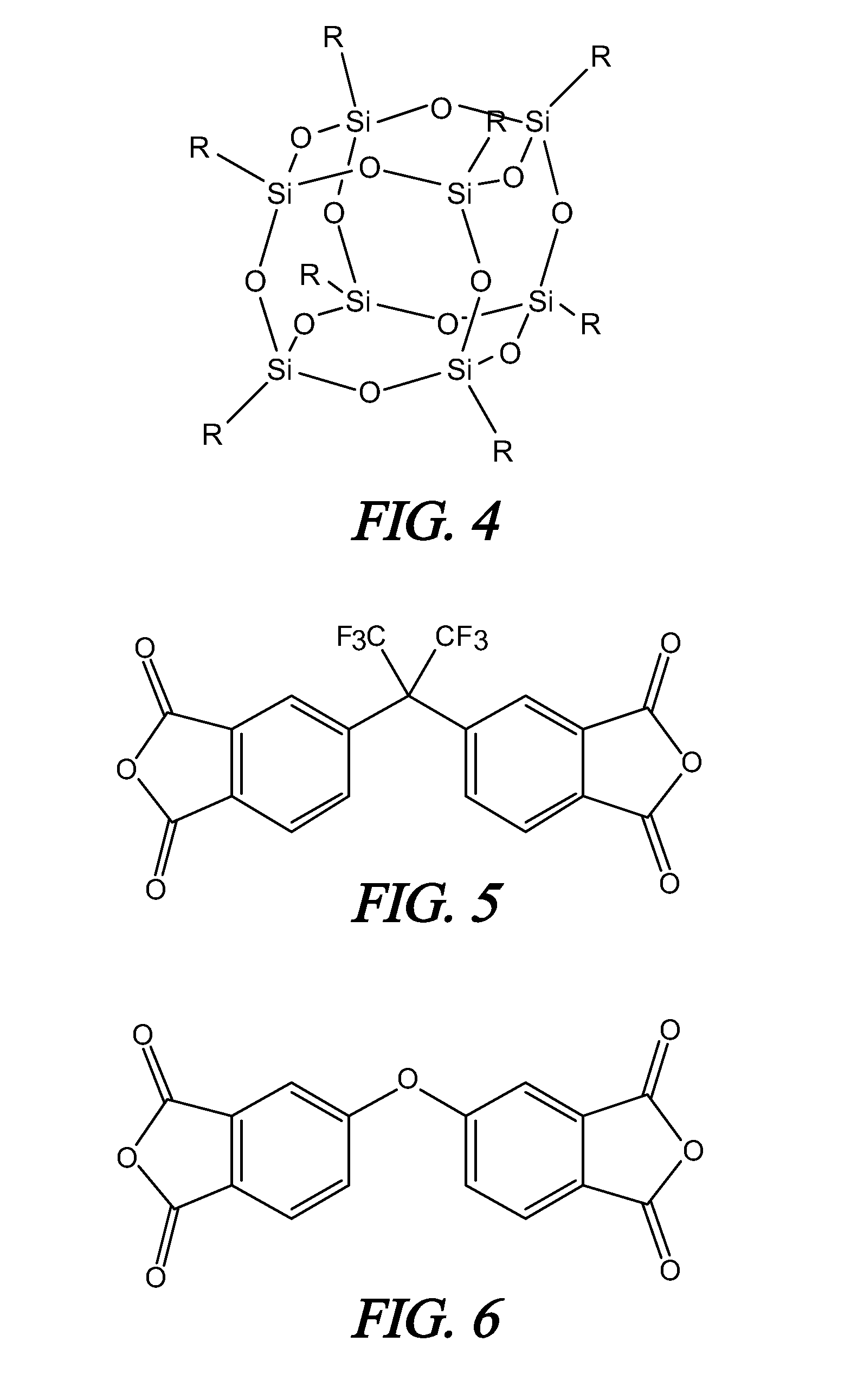
Polyimide polymer with oligomeric silsesquioxane
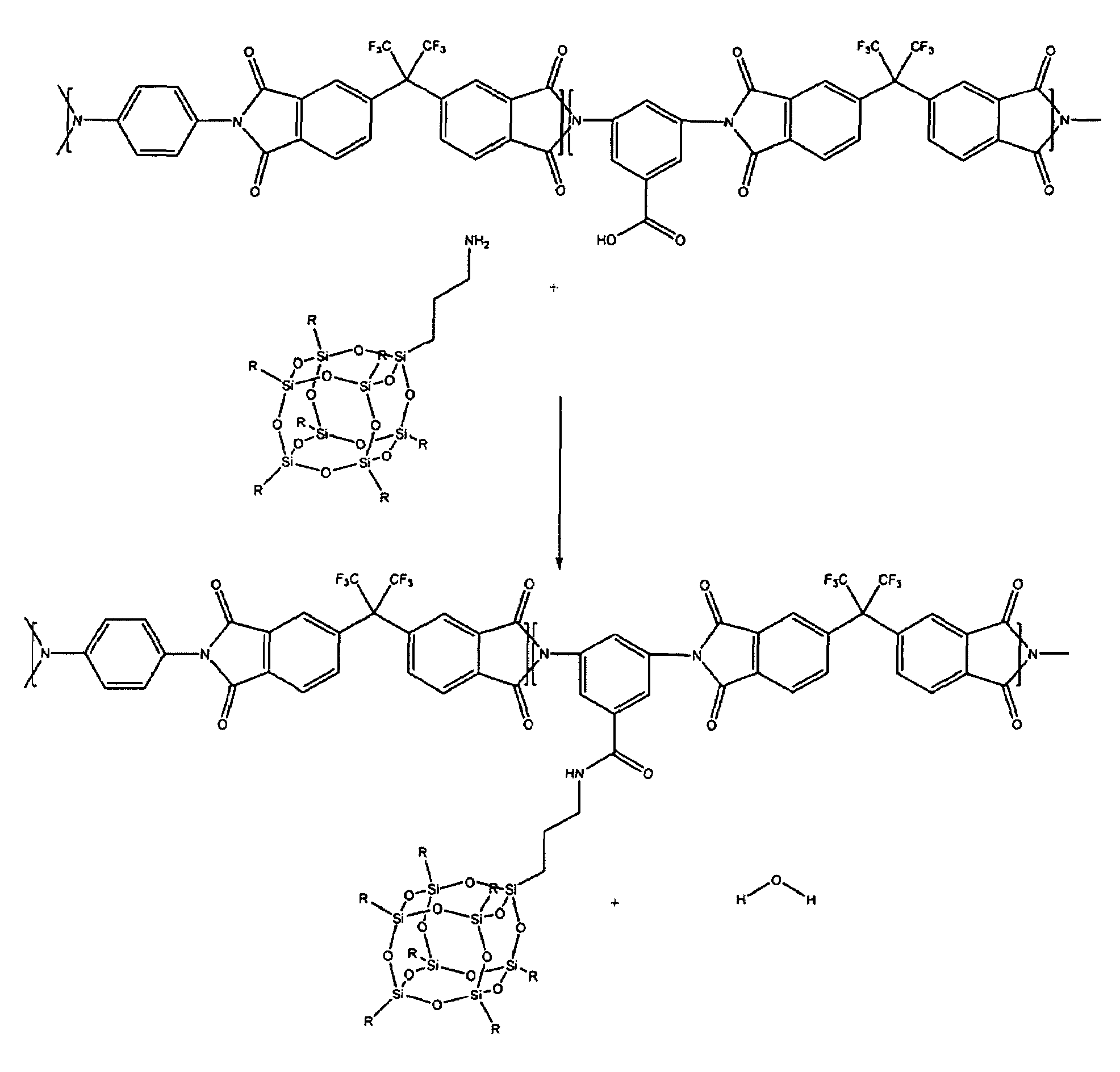
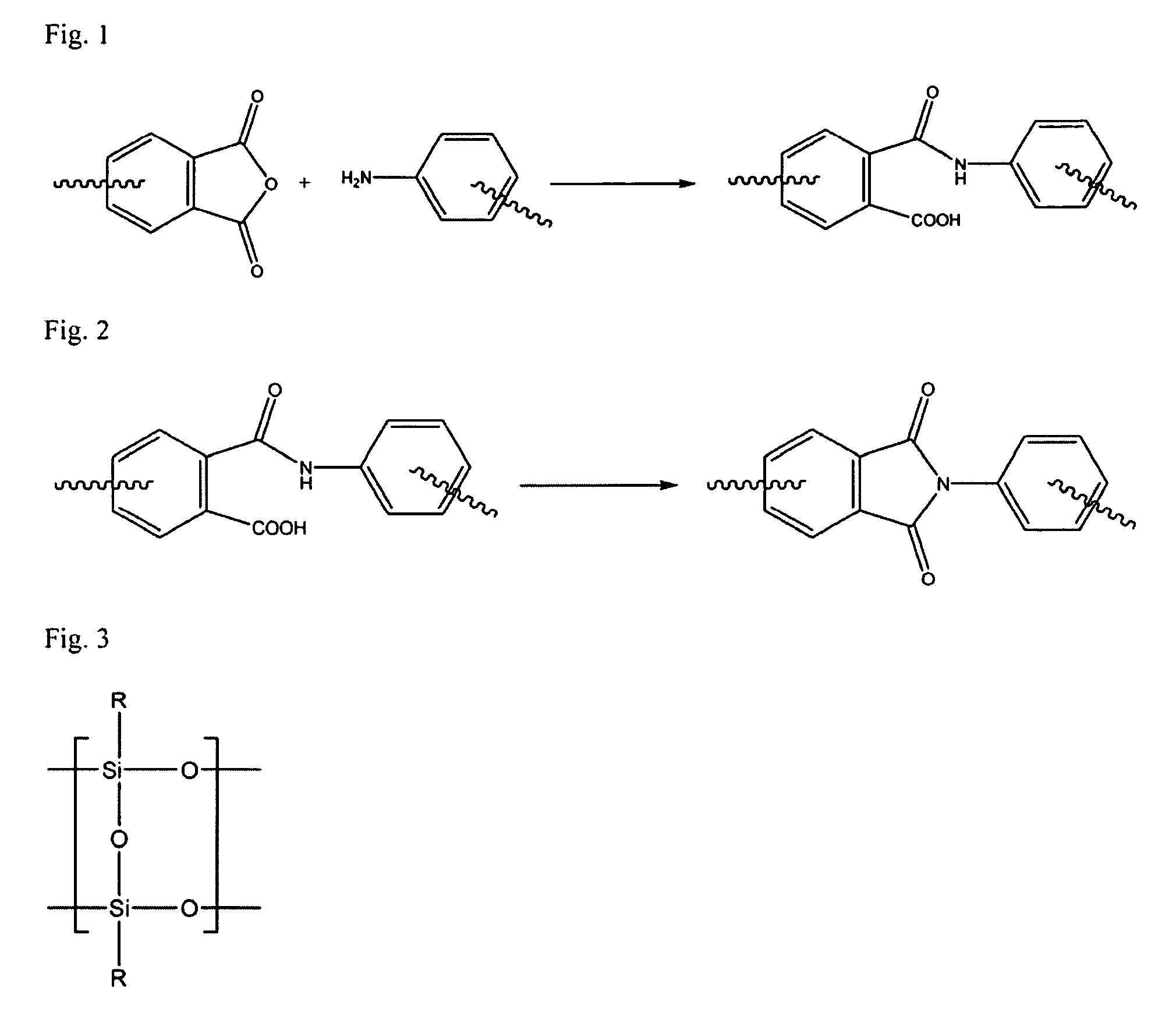
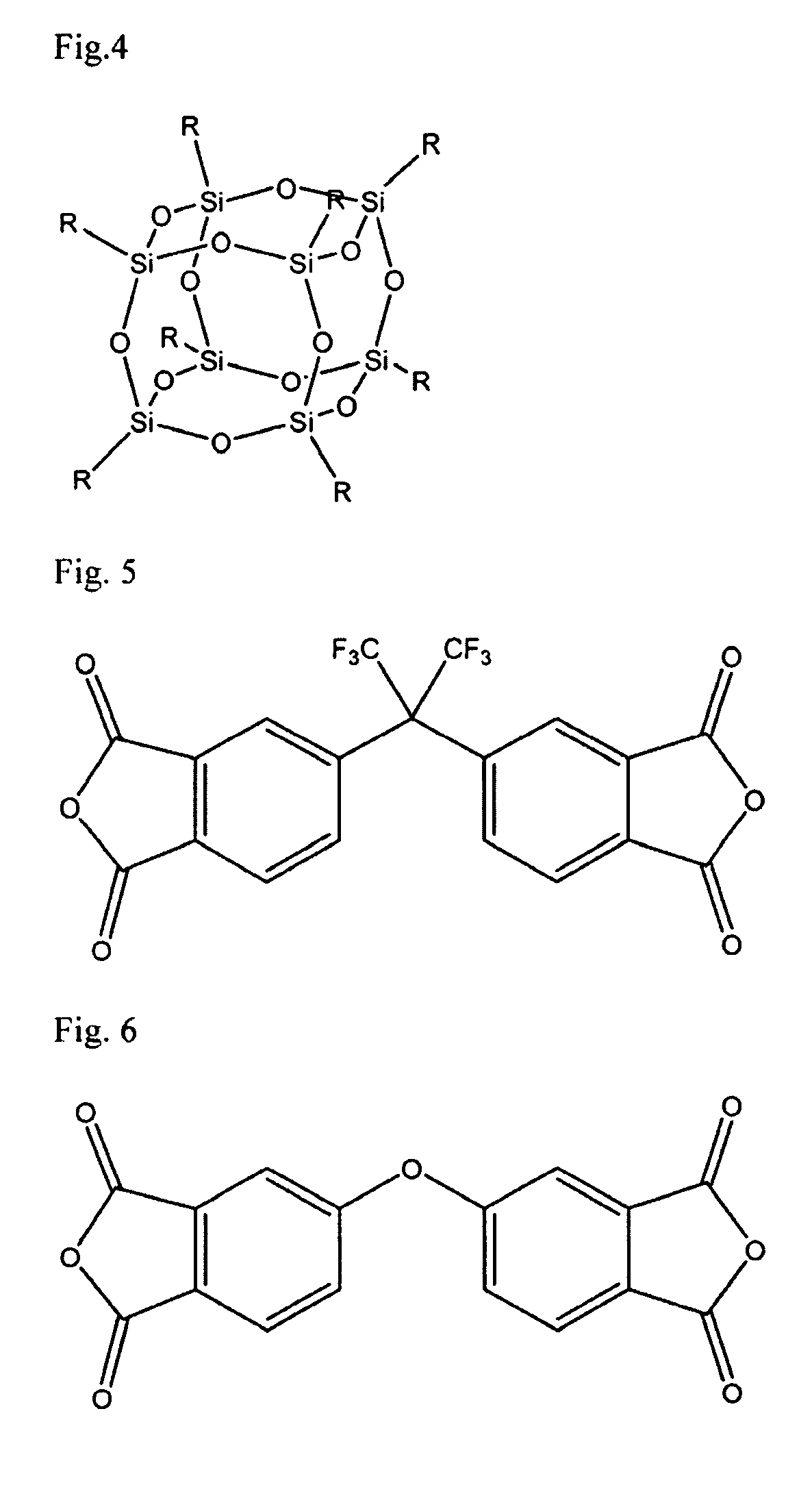
A soluble polyimide polymer with tethered oligomeric silsesquioxane compounds is produced using efficient, gentle reactions. A carboxylic acid attachment point on the polymer backbone is used to connect the oligomeric silsesquioxane. The oligomeric silsesquioxane compound includes an amine or an alcohol on an organic tether, which reacts with the carboxylic acid attachment point to produce either an amide or an ester bond. The amide or ester bond includes a carbonyl carbon directly connected to a phenyl group in the polymer backbone. The resultant polyimide polymer has many beneficial properties.
Owner:NEXOLVE HLDG CO LLC
Silsesquioxane polymers, method of synthesis, photoresist composition, and multilayer lithographic method
InactiveUS6340734B1Reduce usageAvoid pollutionSilicon organic compoundsRadiation applicationsResistPolymer science
Novel silsesquioxane polymers are formed by methods which avoid the use of BBr3. The novel silsesquioxane polymers are especially useful in negative photoresist compositions and photolithographic processes. Alternatively, improved silsesquioxane polymer-containing negative photoresist compositions are obtained by using a polymer component containing a blend of silsesquioxane polymer and non-silsesquioxane polymer. The photoresist compositions provide improved dissolution characteristics enabling the use of 0.26N TMAH developer. The photoresist compositions also provide improved thermal characteristics enabling use of higher processing temperatures. The photoresist compositions are especially useful in a multilayer photolithographic processes and are capable of producing high resolution.
Owner:GLOBALFOUNDRIES INC
Compounding silica-reinforced rubber with low volatile organic compound (VOC) emission
ActiveUS20060217473A1Enhanced rubber reinforcementEnhanced interactionSilicon organic compoundsSynthetic resin layered productsAlcoholReinforced rubber
Alkoxy-modified silsesquioxane compounds are described. The alkoxy-modified silsesquioxane compounds contain an alkoxysilane group that participates in an alkoxysilane-silica reaction as a silica dispersing agent in rubber, with the release of zero to about 0.1% by weight of the rubber of volatile organic compounds (VOC), especially alcohol, during compounding and further processing. Further described are methods for making alkoxy-modified silsesquioxanes, methods for making vulcanizable rubber compounds containing alkoxy-modified silsesquioxanes, vulcanizable rubber compounds containing alkoxy-modified silsesquioxanes, and pneumatic tires comprising a component that contains alkoxy-modified silsesquioxanes.
Owner:BRIDGESTONE CORP
Compound having silsesquioxane skeleton and its polymer
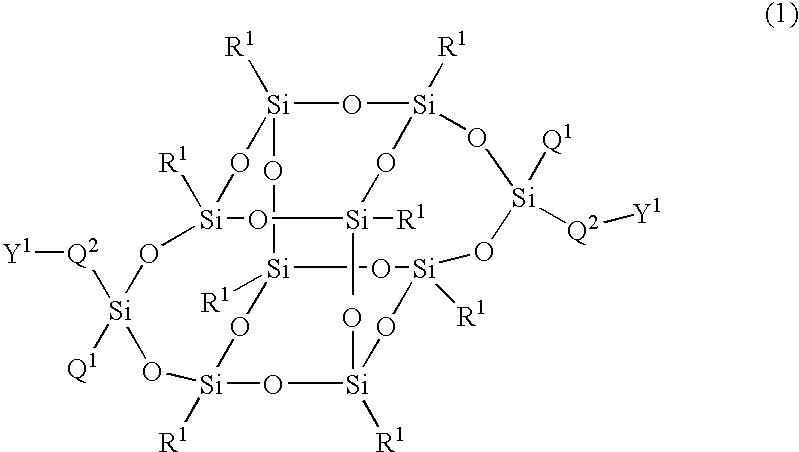
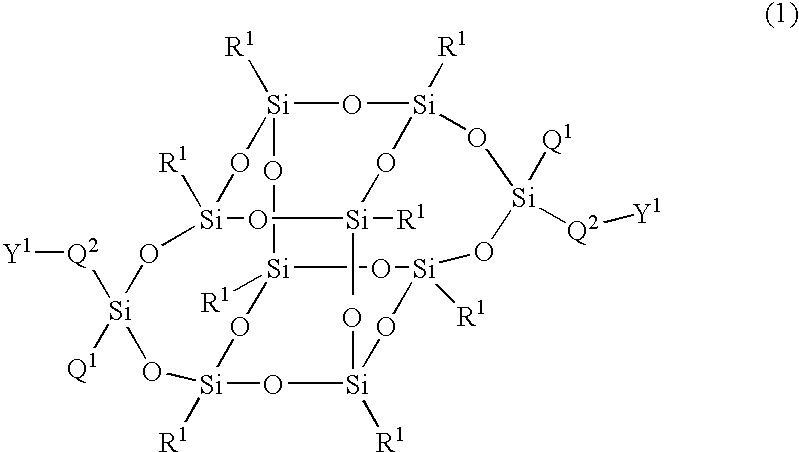
The present invention relates to a compound represented by Formula (1) and a polymer obtained using the compound: wherein R1 is phenyl which may have substituents, Q1 is hydrogen, halogen, alkyl having 1 to 10 carbon atoms, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexenyl or phenyl in which optional hydrogen may be replaced by halogen or alkyl having 1 to 5 carbon atoms, and Q2 is a group represented by Formula (2) wherein the code < represents a bonding point with silicon, l, m, n and p are independently 0, 1, 2 or 3, A1 to A4 are independently a single bond, 1,4-cyclohexylene, 1,4-cyclohexenylene, a condensed ring group having 6 to 10 carbon atoms which is a divalent group, or 1,4-phenylene, Z0 to Z3 are independently a single bond, —CH═CR—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms, and Z4 is a single bond, —CH═CH—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms. And Y1 in Formula (1) is the group defined in Claim 1.
Owner:JNC PETROCHEM CORP +1
Silsesquioxane derived hard, hydrophobic and thermally stable thin films and coatings for tailorable protective and multi-structured surfaces and interfaces
ActiveUS20110062619A1Attractive balanceImprove scratch resistanceNanoinformaticsPhotomechanical apparatusDiluentRare earth
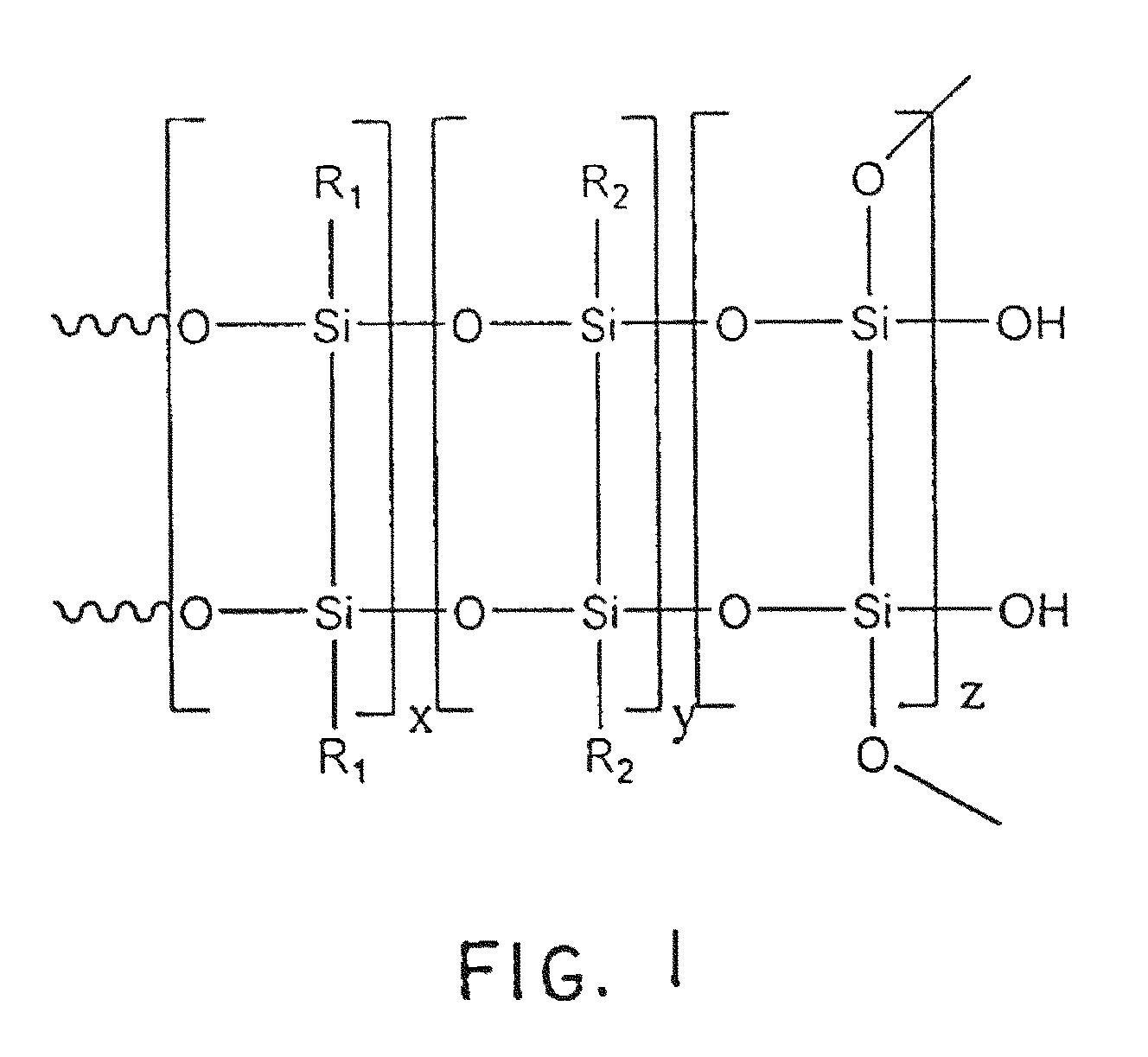
A method of forming a coating comprising the steps of dissolving an silsesquioxane (e.g., one that is primarily a cage compound with 8, 10, 12, 14 or related complete cages or with partially condensed cages containing primarily Si(O)4 units in the cage) in a solvent to form an silsesquioxane solution; introducing (e.g., dissolving) an additive in the solution (e.g., the additive being selected from a rare earth compound, an acid, an organic moiety, a precious metal or compound thereof, a transition metal compound, or any combination thereof, or any of their ionic constituents); and optionally mixing a diluent with the solution to form a coating that is applied to a substrate, wherein the resulting coating forms crosslinks between resulting pendant Si(OH)x groups and a substrate surface. The present invention also contemplates coatings and coated articles consistent with the present teachings.
Owner:LAINE RICHARD M
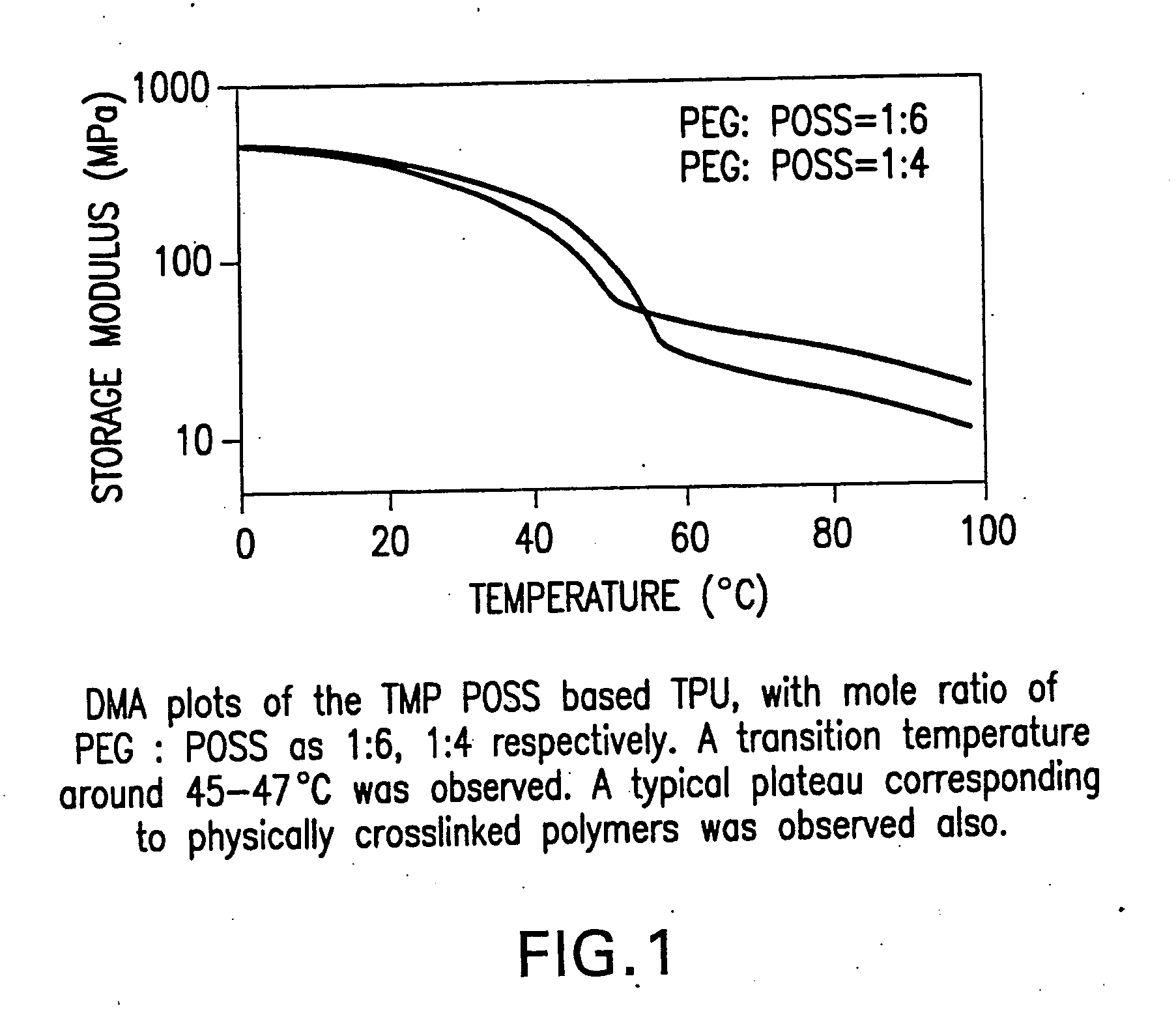
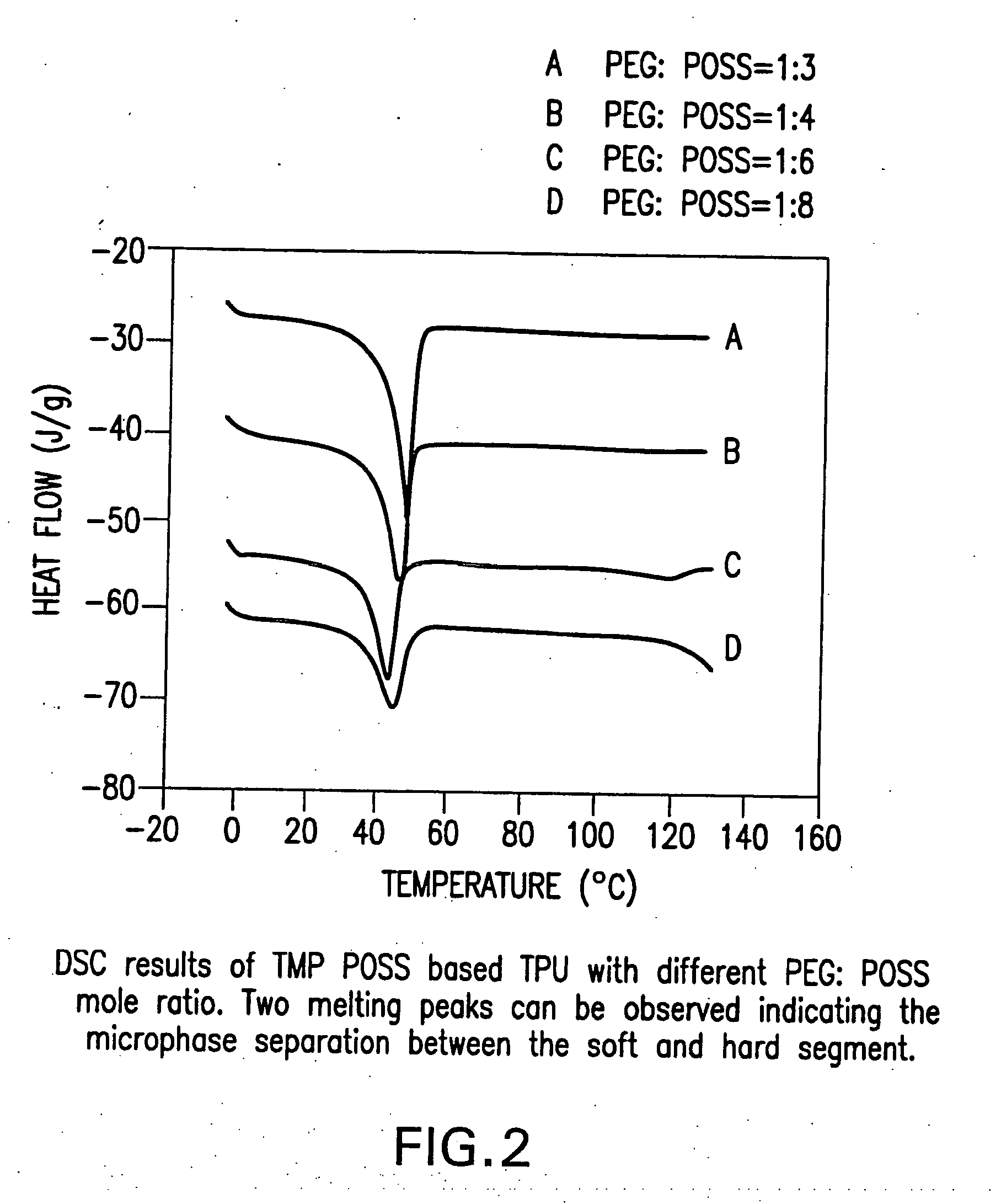
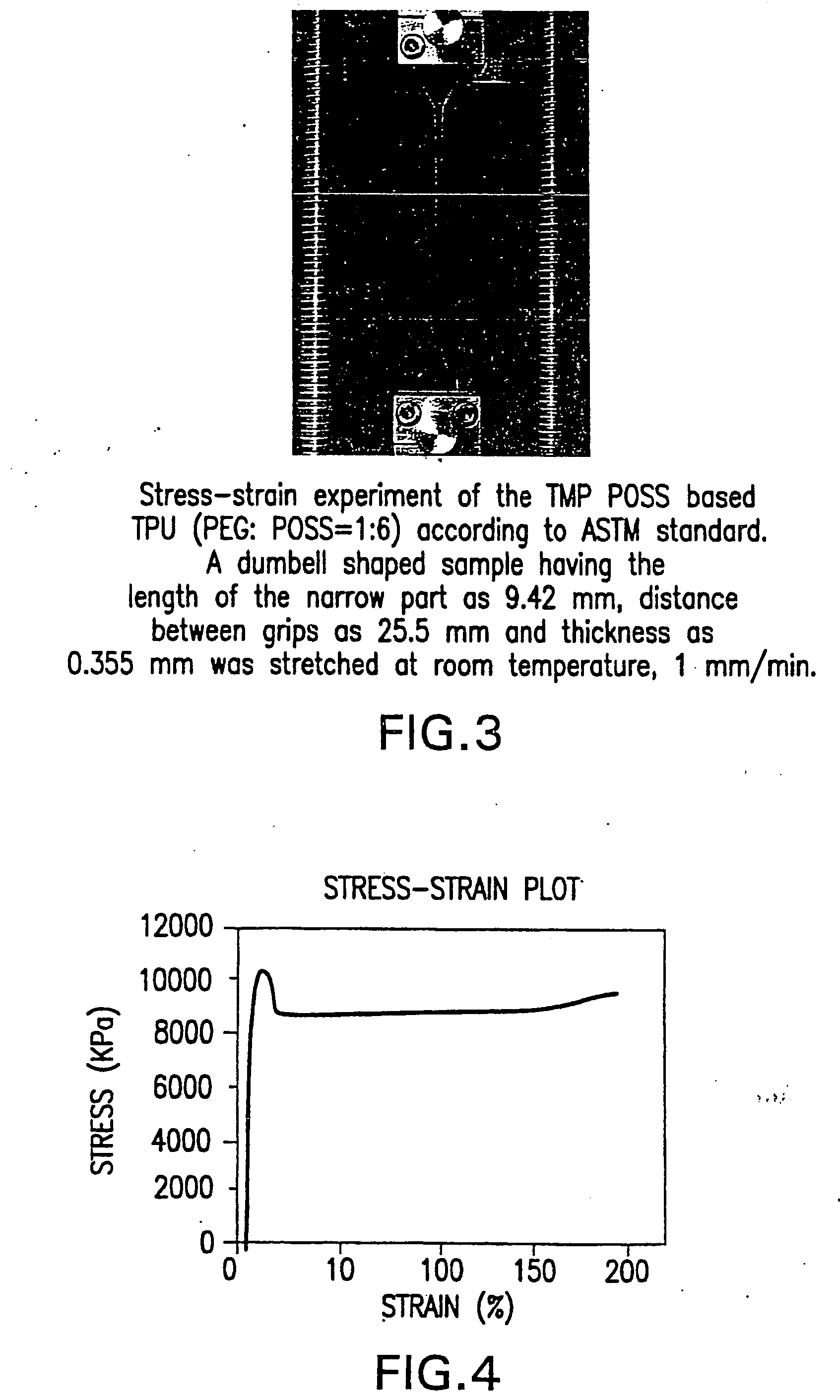
Shape memory polymers based on semicrystalline thermoplastic polyurethanes bearing nanostructured hard segments
InactiveUS20050245719A1Sharp and tunable transition temperatureAbove melting pointPolymer scienceAdhesive
Thermoplastic polyurethanes having an alternating sequence of hard and soft segments in which a nanostructured polyhedral oligosilsesquioxane diol is used as a chain extender to form a crystalline hard segment constituting SMPs. The polyurethanes are formed by reacting a polyol, a chain extender dihydroxyl-terminated polyhedral oligosilsesquioxane and a diisocyanate. The polyurethanes have multiple applications including for example, implants for human health care, drug delivery matrices, superabsorbant hydrogels, coatings, adhesives, temperature and moisture sensors, etc.
Owner:UNIV OF CONNECTICUT
Polymeric coating for protecting objects
ActiveUS8053492B2Provide protectionCosmonautic power supply systemsCosmonautic thermal protectionSilsesquioxanePhenyl group
A protective polymeric coating is applied to the surface of various objects which are to be exposed to a harsh environment. The protective polymeric coating covers the exposed surface, where the polymeric coating includes a polyimide polymer. The polyimide polymer in the polymeric coating has a backbone with at least one non-terminal phenyl group. A linkage is connected to the non-terminal phenyl group, where the linkage can be an amide linkage or an ester linkage. An oligomeric silsesquioxane compound is connected to the linkage through an organic substituent, where the oligomeric silsesquioxane is not incorporated into the polymer backbone. The polymeric coating provides protection to the underlying object.
Owner:NEXOLVE HLDG CO LLC
Radiation curable ink compositions suitable for ink-jet printing
InactiveUS20040163570A1Improve stabilityImprove compatibilityPhotosensitive materialsRadiation applicationsHydrogenSilsesquioxane
A radiation curable ink composition comprising at least one initiator and at least one polyhedral oligomeric silsesquioxane (POSS) represented by the following empirical formula [R(SiO1.5)]n wherein n=4,6,8,10,12,14,16 and larger and each R is independently hydrogen, an inorganic group, an alkyl group, an alkylene group, an aryl group, an arylene group, or non-heterocyclic group-containing organo-functional derivatives of alkyl, alkylene, aryl or arylene groups; and a process for obtaining a colourless, monochrome or multicolour ink jet image comprising the steps of jetting one or more streams of ink droplets having the above-mentioned composition onto an ink-jet ink receiver material, and subjecting the obtained image to radiation curing.
Owner:AGFA NV
Organic semiconductor device having an active dielectric layer comprising silsesquioxanes
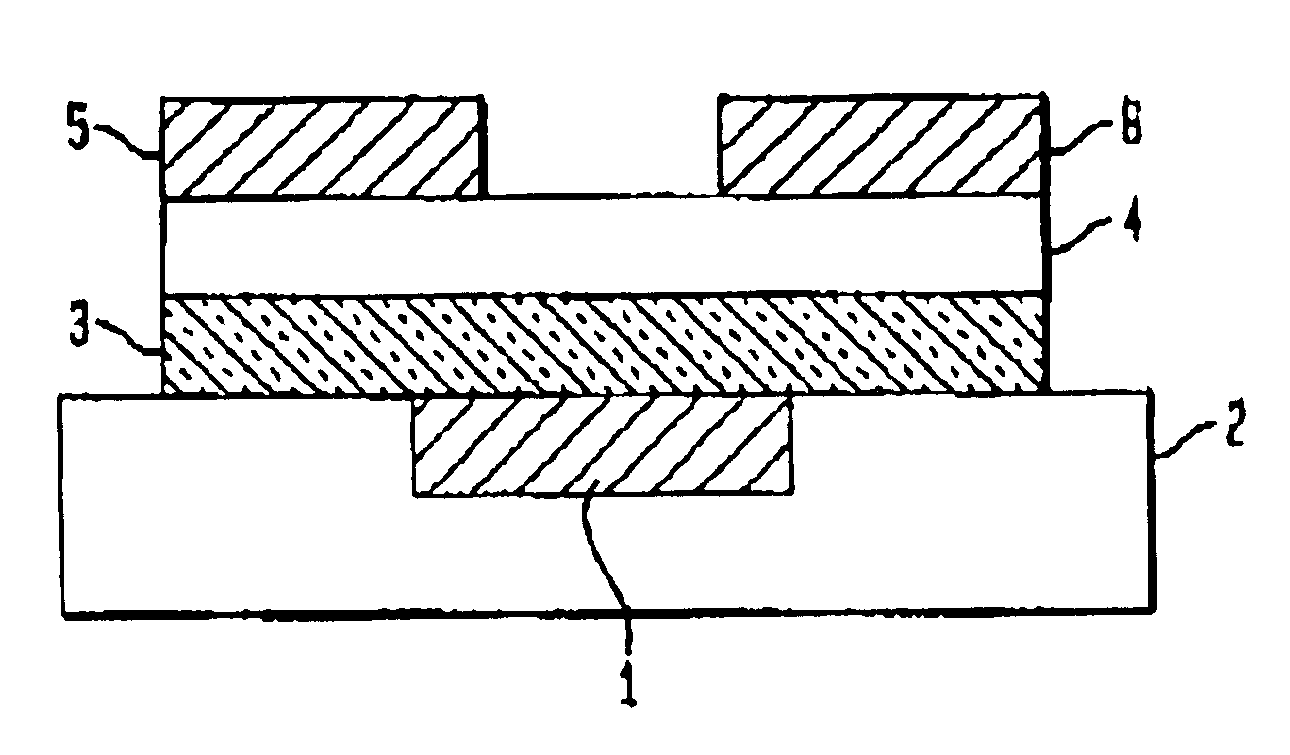
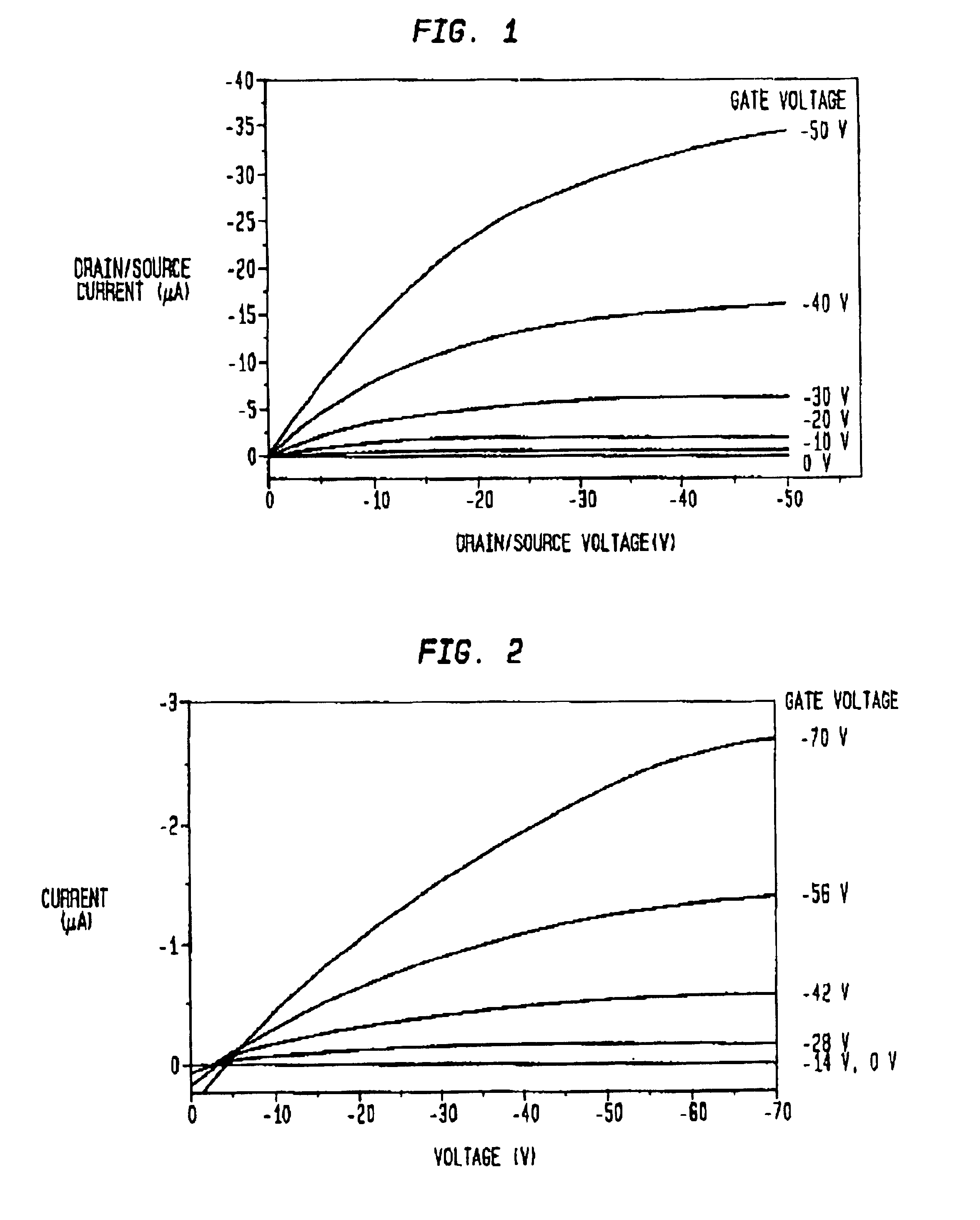
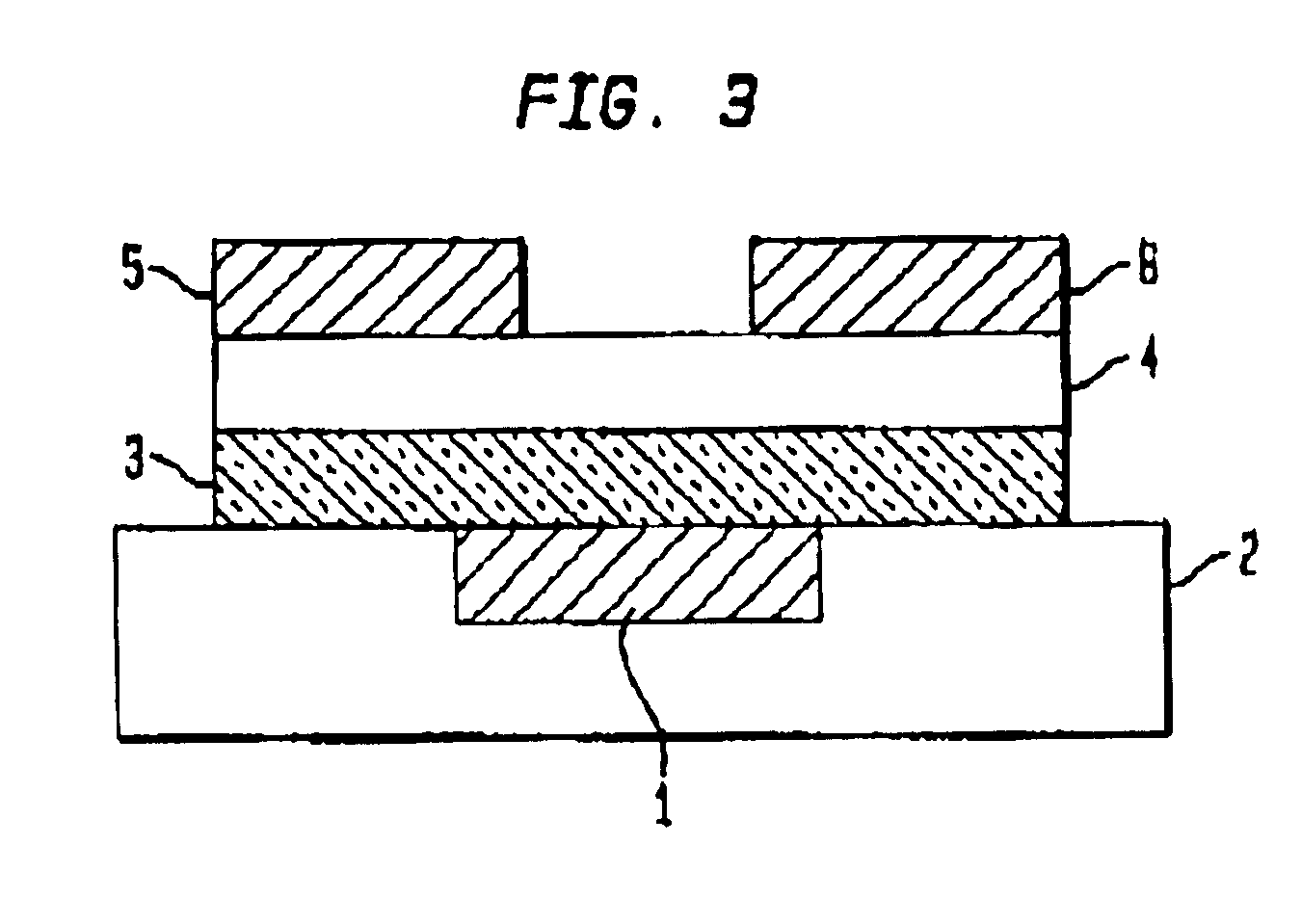
InactiveUS6891237B1Solid-state devicesPretreated surfacesOrganic field-effect transistorLow temperature curing
An organic field effect transistor (FET) is described with an active dielectric layer comprising a low-temperature cured dielectric film of a liquid-deposited silsesquioxane precursor. The dielectric film comprises a silsesquioxane having a dielectric constant of greater than 2. The silsesquioxane dielectric film is advantageously prepared by curing oligomers having alkyl(methyl) and / or alkyl(methyl) pendant groups. The invention also embraces a process for making an organic FET comprising providing a substrate suitable for an organic FET; applying a liquid-phase solution of silsesquioxane precursors over the surface of the substrate; and curing the solution to form a silsesquioxane active dielectric layer. The organic FET thus produced has a high-dielectric, silsesquioxane film with a dielectric constant of greater than about 2, and advantageously, the substrate comprises an indium-tin oxide coated plastic substrate.
Owner:ALCATEL-LUCENT USA INC +1
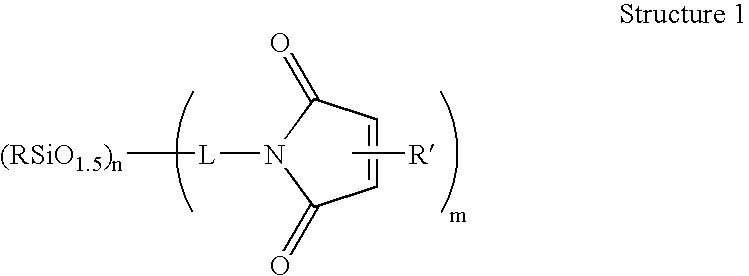
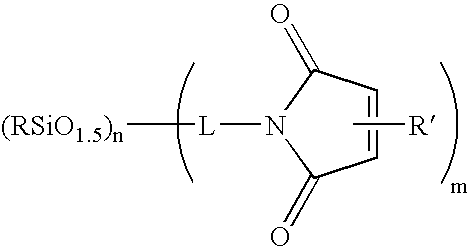
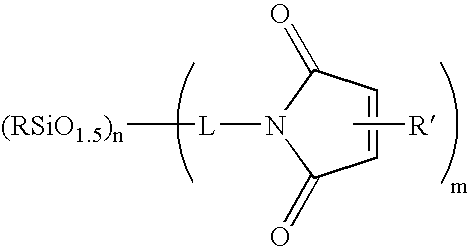
Compositions containing maleimide-substituted silsesquioxanes and methods for use thereof
InactiveUS20060009578A1Easy to prepareEasily incorporated into resin compositionSynthetic resin layered productsPhotomechanical apparatusCompound (substance)Silsesquioxane
The present invention is based on the discovery that maleimide-substituted polyhedralsilsesquioxane compounds are useful as components in photosensistive resin compositions. These compounds can be readily prepared from available precursors, and are easily incorporated into resin compositions by appropriate formulating conditions. The incorporation of a variety of polyhedralsilsesquioxane frameworks into a photosensitive resin composition is readily accomplished via the crosslinkable maleimide moiety, which crosslinks under a variety of conditions (e.g., chemically and radiatively). Indeed, the compounds described herein contain at least one maleimide moiety attached to a polyhedralsilsesquioxane framework.
Owner:DESIGNER MOLECULES
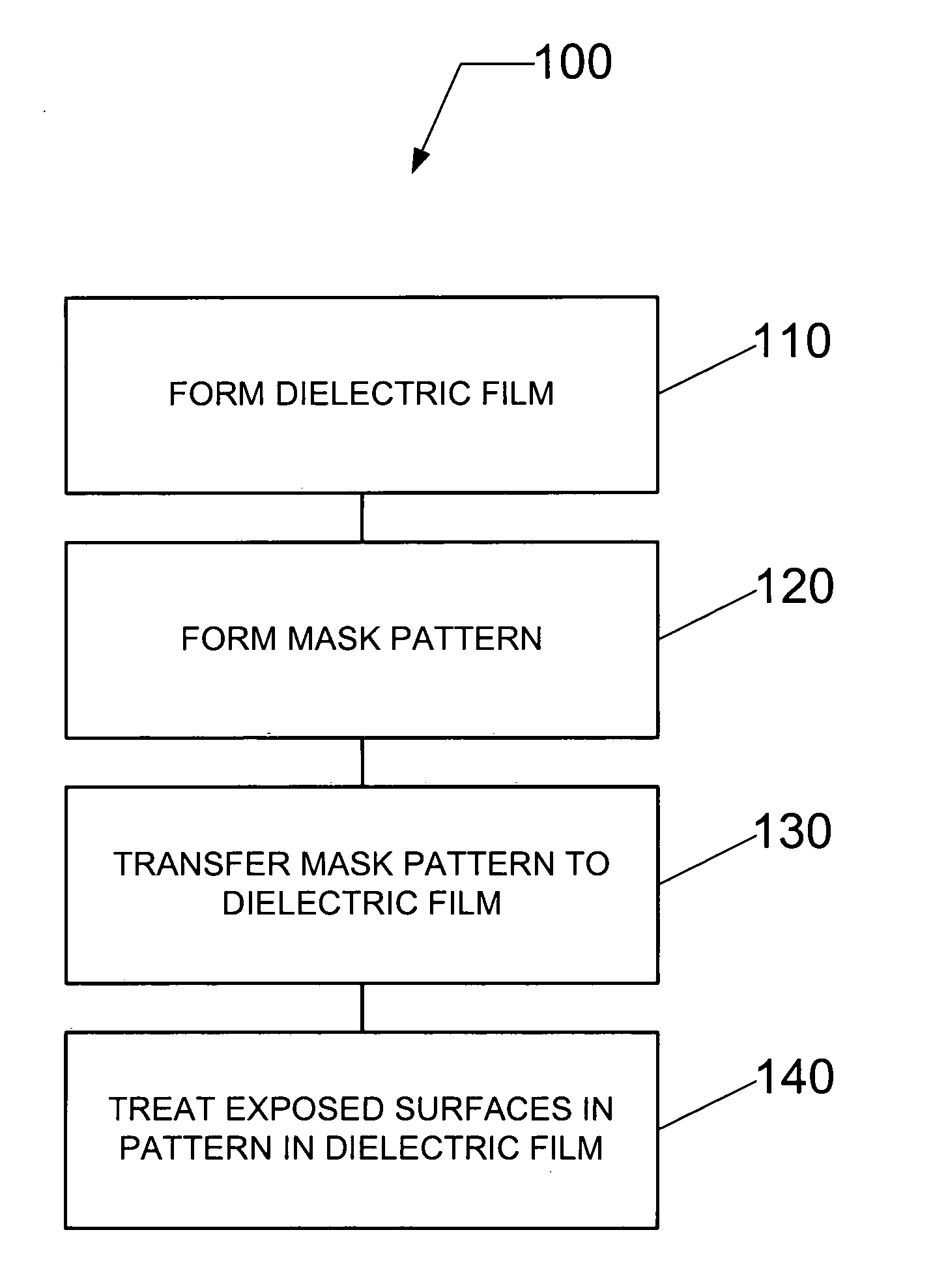
Method and system for treating a dielectric film
InactiveUS20050215072A1Reduce diffuseImprove adhesionSemiconductor/solid-state device detailsSolid-state devicesArylSilanes
A method and system for treating a dielectric film includes exposing at least one surface of the dielectric film to an alkyl silane, an alkoxysilane, an alkyl siloxane, an alkoxysiloxane, an aryl silane, an acyl silane, a cyclo siloxane, a polysilsesquioxane (PSS), an aryl siloxane, an acyl siloxane, or a halo siloxane, or any combination thereof. The dielectric film can include a low dielectric constant film with or without pores having an etch feature formed therein following dry etch processing. As a result of the etch processing or ashing, exposed surfaces in the feature formed in the dielectric film can become damaged, or activated, leading to retention of contaminants, absorption of moisture, increase in dielectric constant, etc. Damaged surfaces, such as these, are treated by performing at least one of healing these surfaces to, for example, restore the dielectric constant (i.e., decrease the dielectric constant) and cleaning these surfaces to remove contaminants, moisture, or residue. Moreover, preparation for barrier layer and metallization of features in the film may include treating by performing sealing of sidewall surfaces of the feature to close exposed pores and provide a surface for barrier film deposition.
Owner:TOKYO ELECTRON LTD
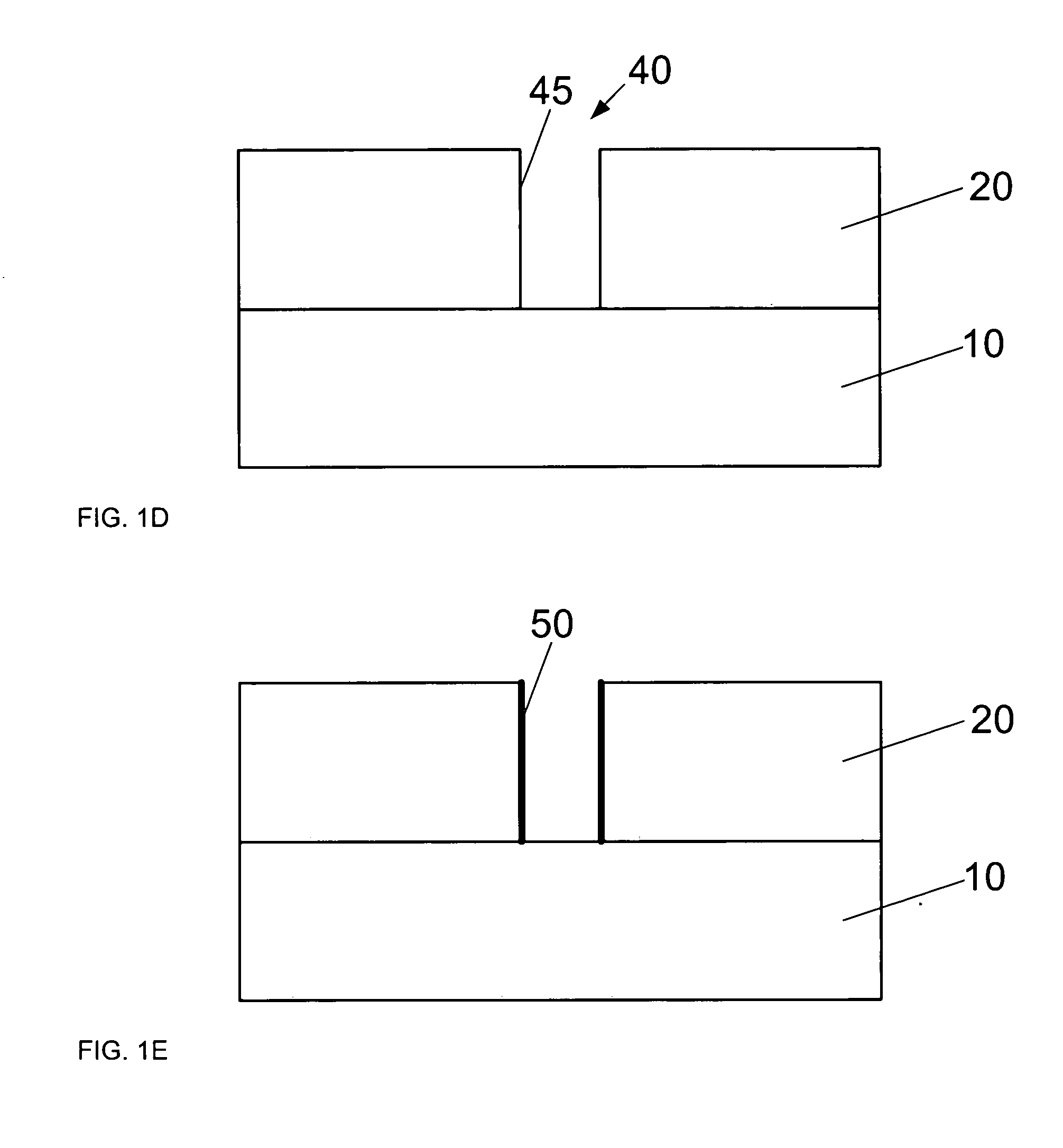
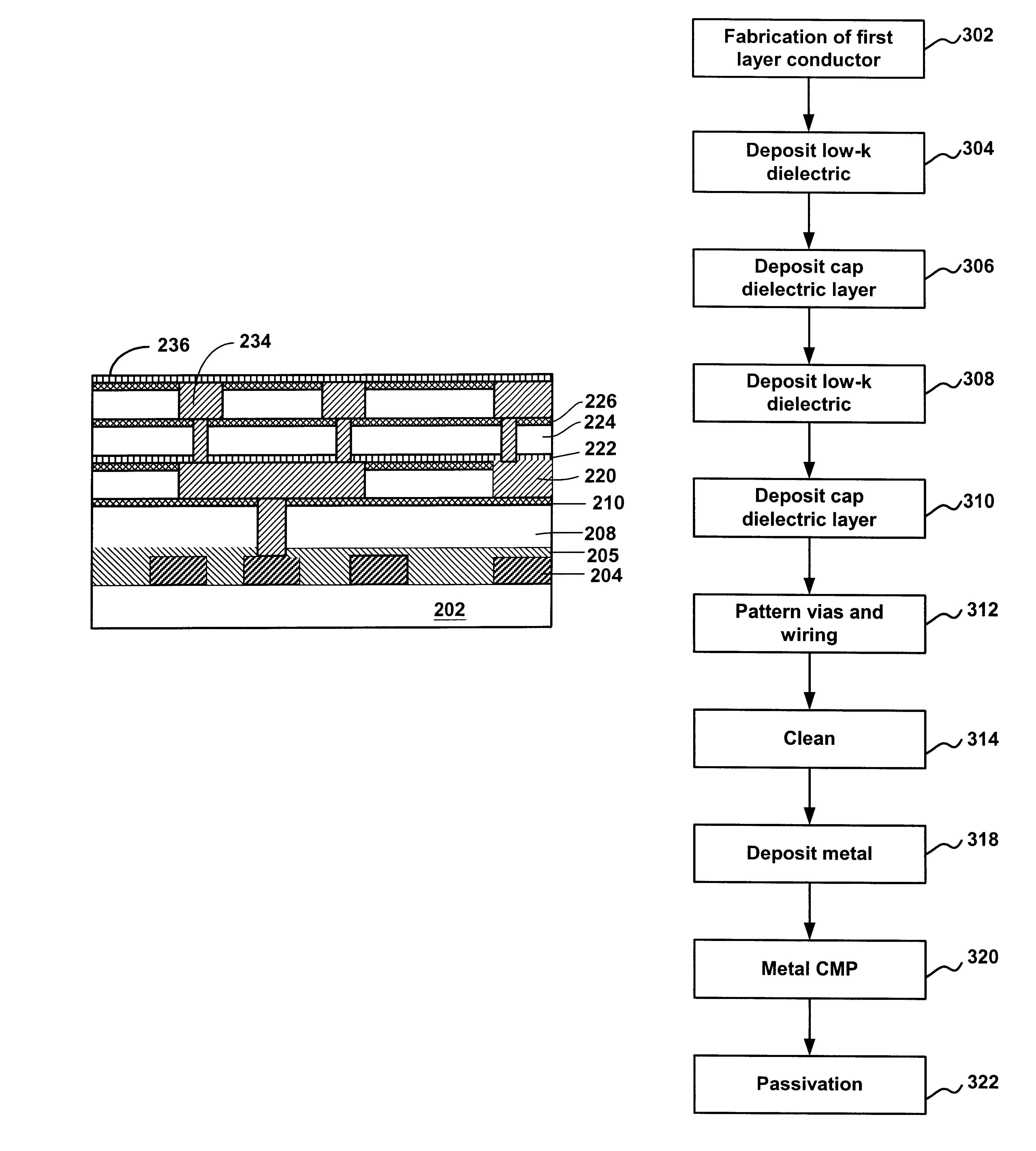
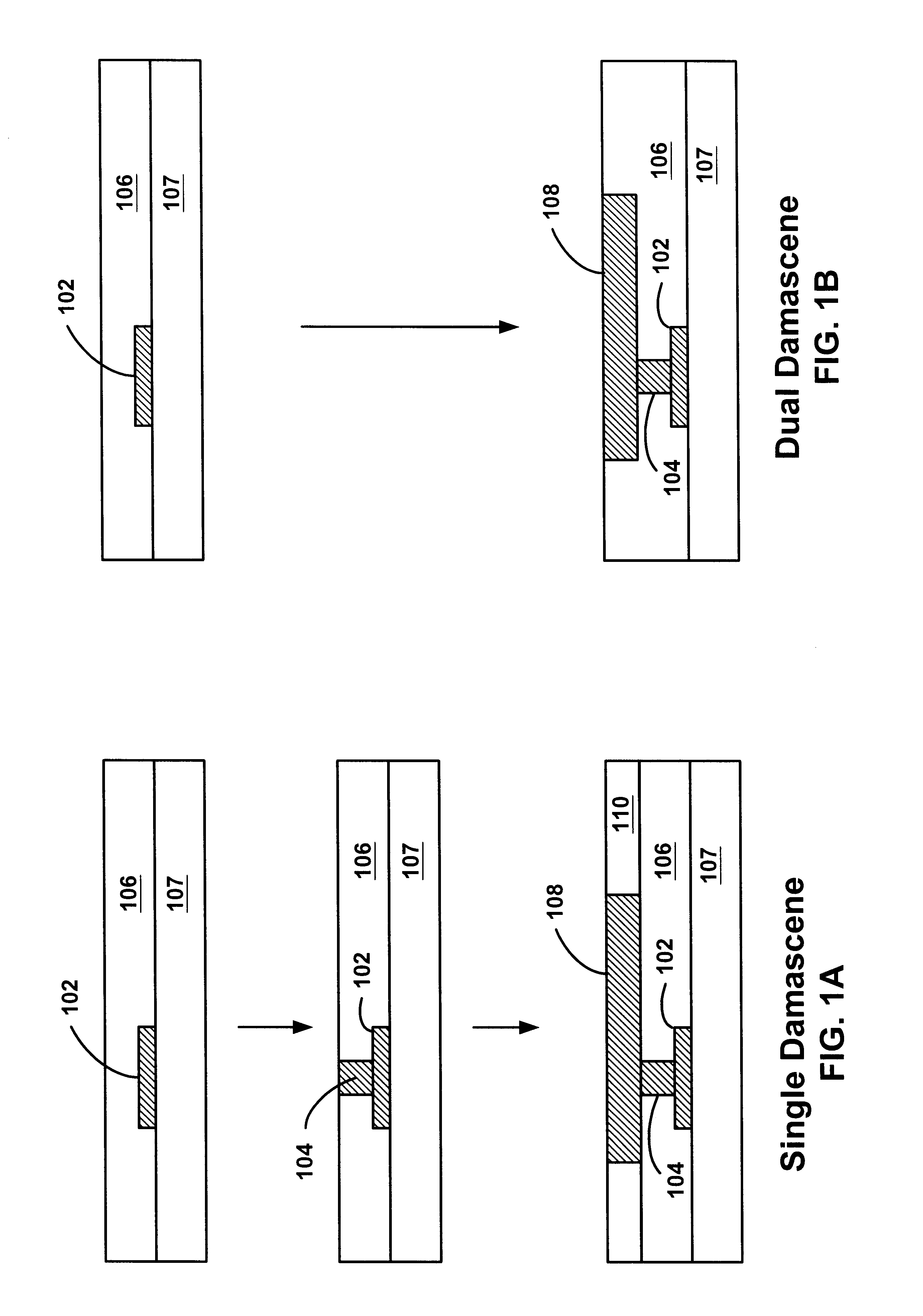
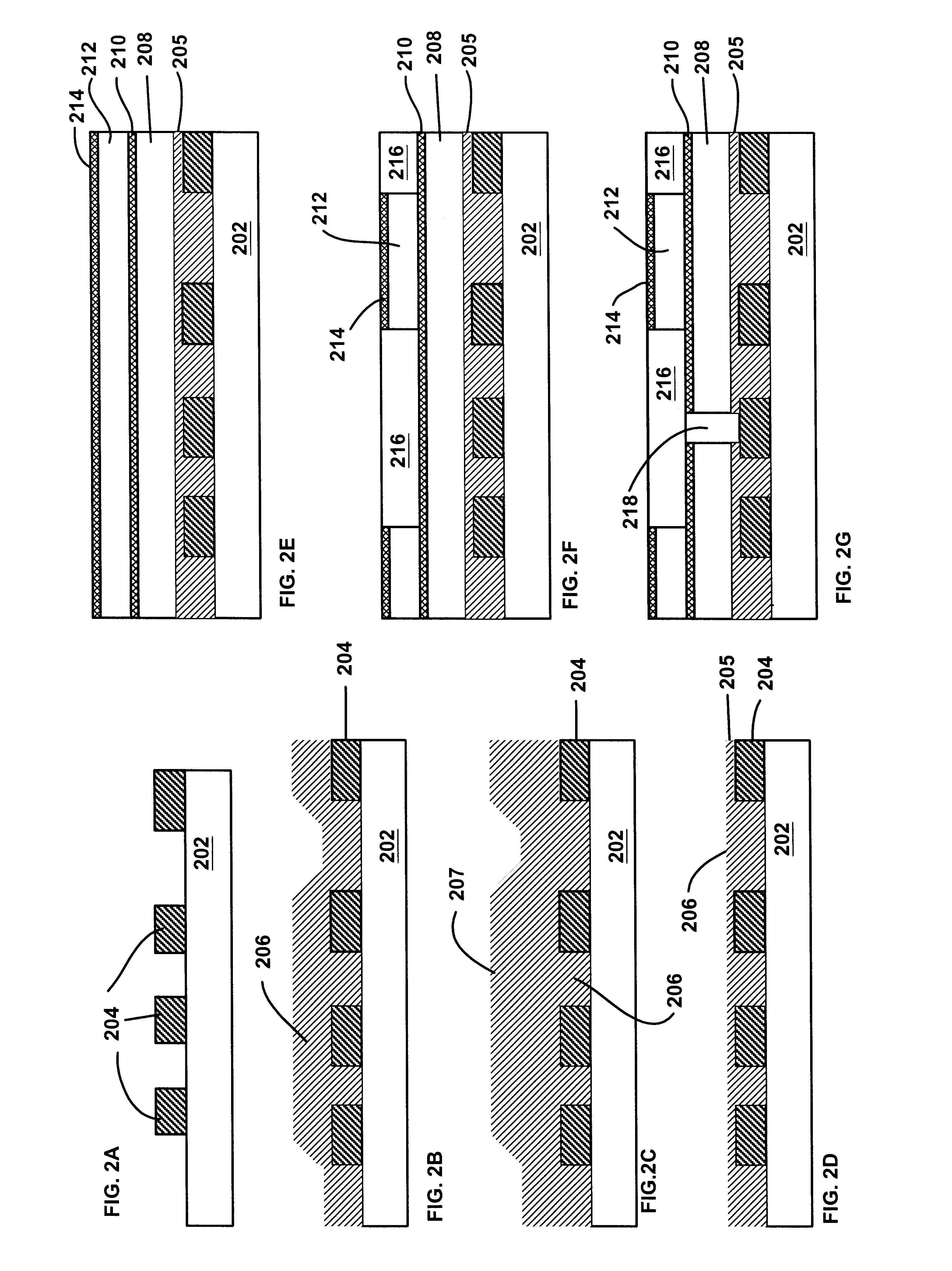
Method of forming dual-damascene interconnect structures employing low-k dielectric materials
InactiveUS6627539B1Semiconductor/solid-state device detailsSolid-state devicesCapacitanceEngineering
Owner:NEWPORT FAB
Silsesquioxane resin wax
ActiveUS20070149703A1Enhances durability and substantivityCosmetic preparationsMake-upWaxSilsesquioxane
A silsesquioxane resin wax composition, method for its preparation, and use in personal, household, automotive and medical care compositions are disclosed. The silsesquioxane resin wax can also find utility in a variety of oil and gas field applications, such as for crude oil wax control.
Owner:DOW SILICONES CORP
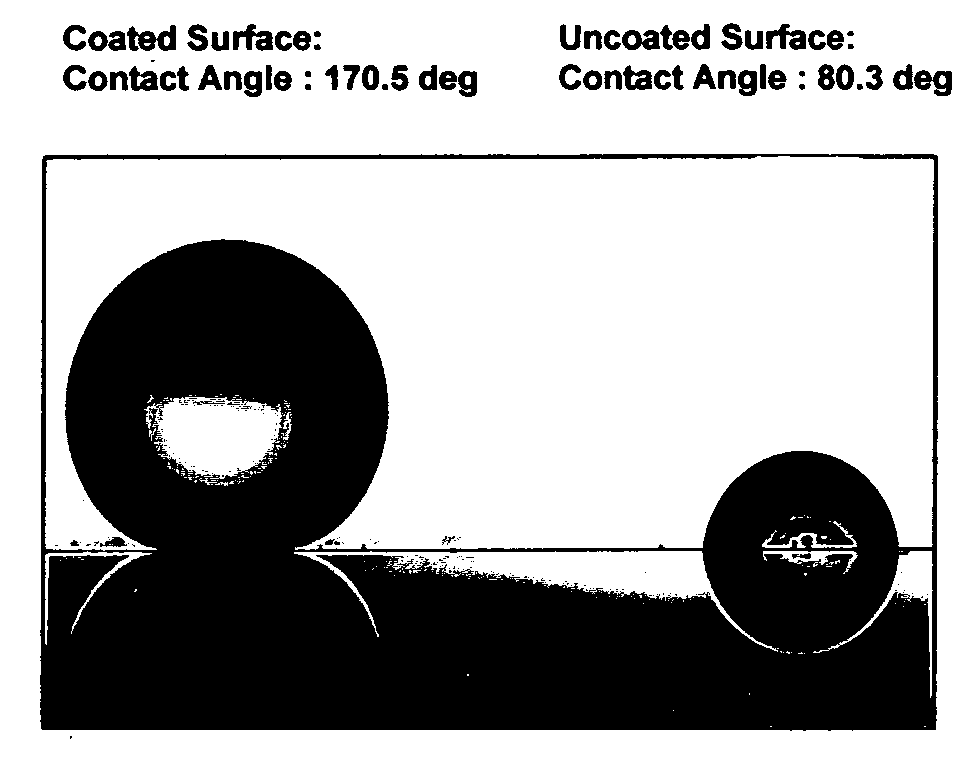
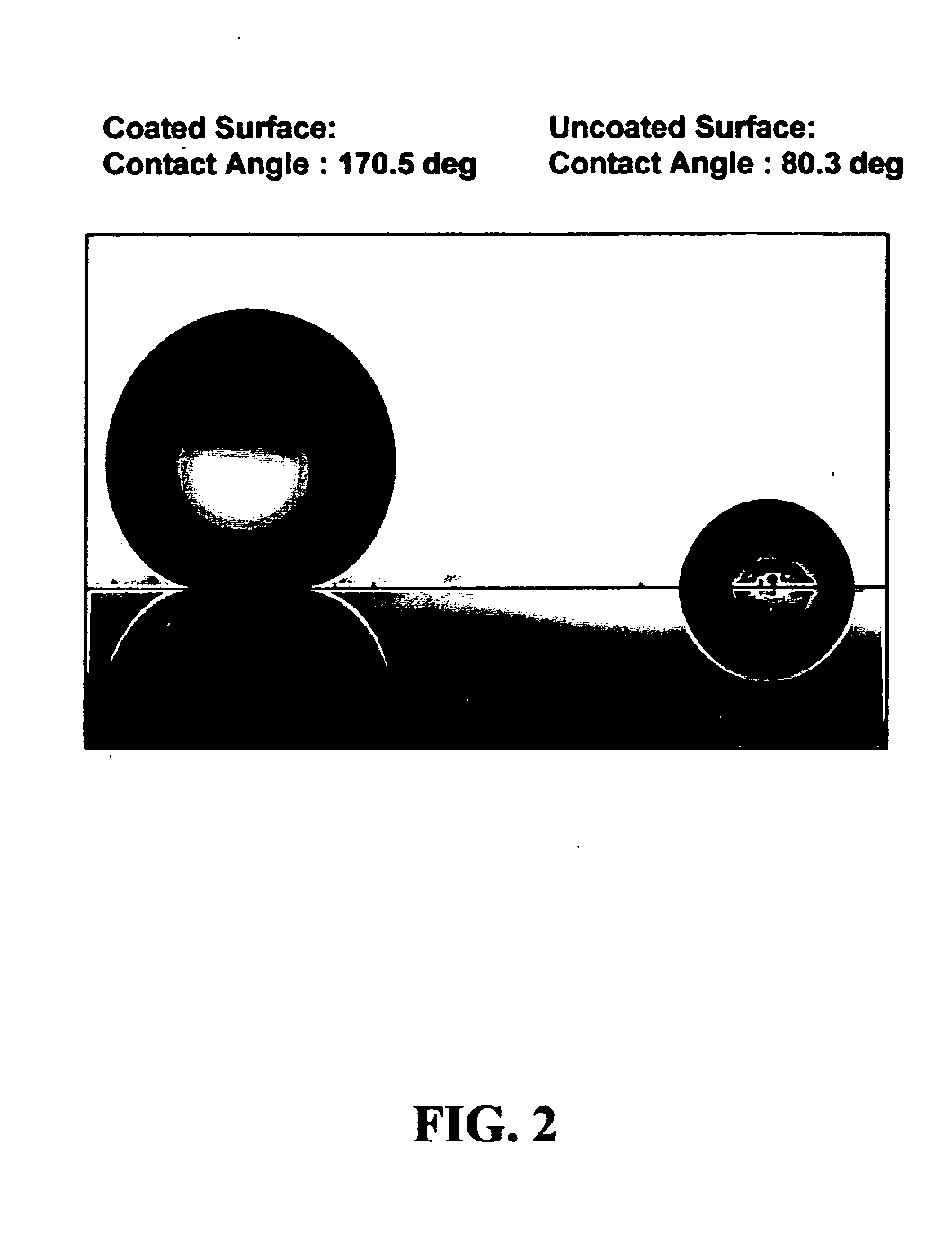
Coating compositions for producing transparent super-hydrophobic surfaces
InactiveUS20080221263A1Poor to abrasionImproves UV resistanceCoatingsThin material handlingNanoparticleSilsesquioxane
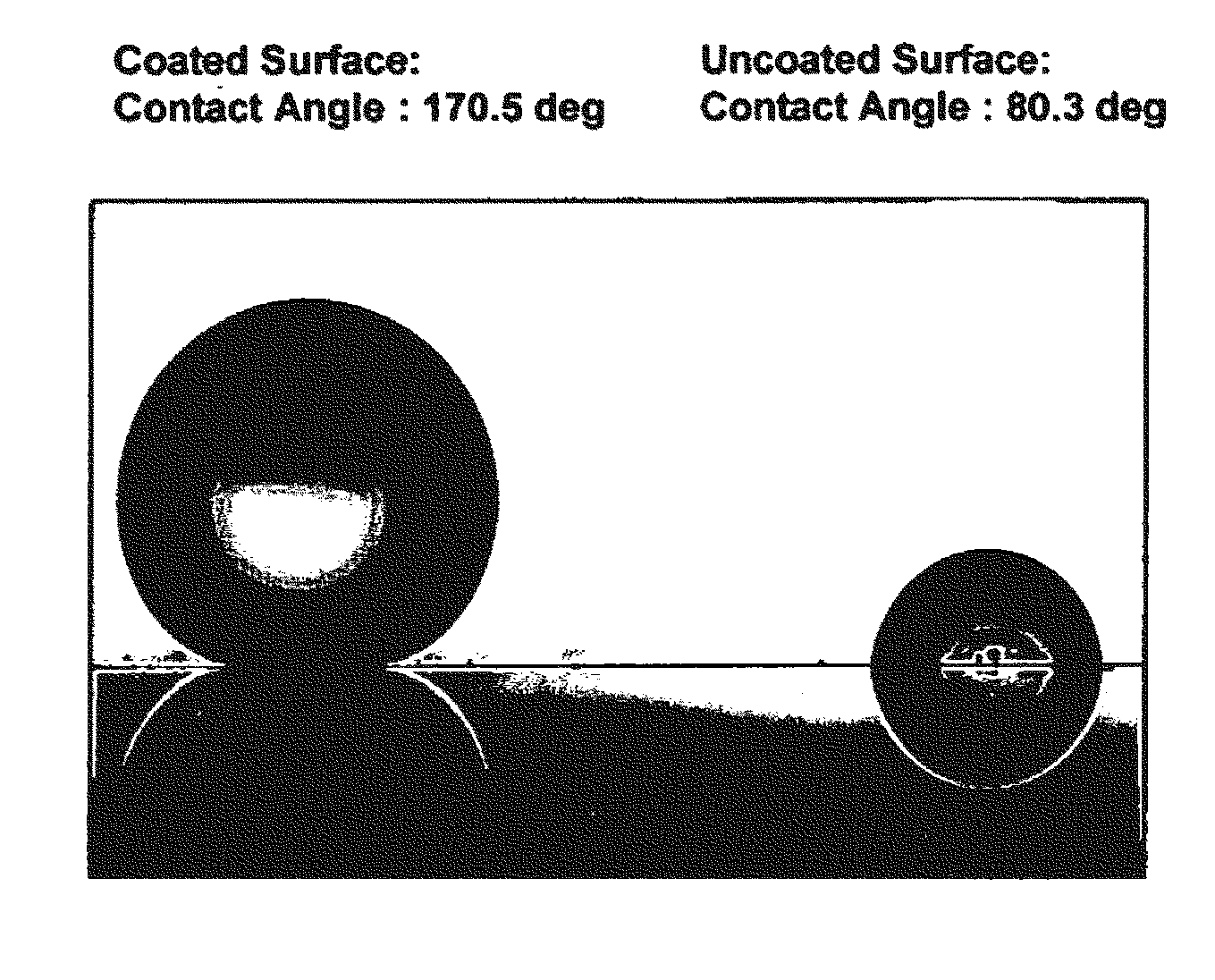
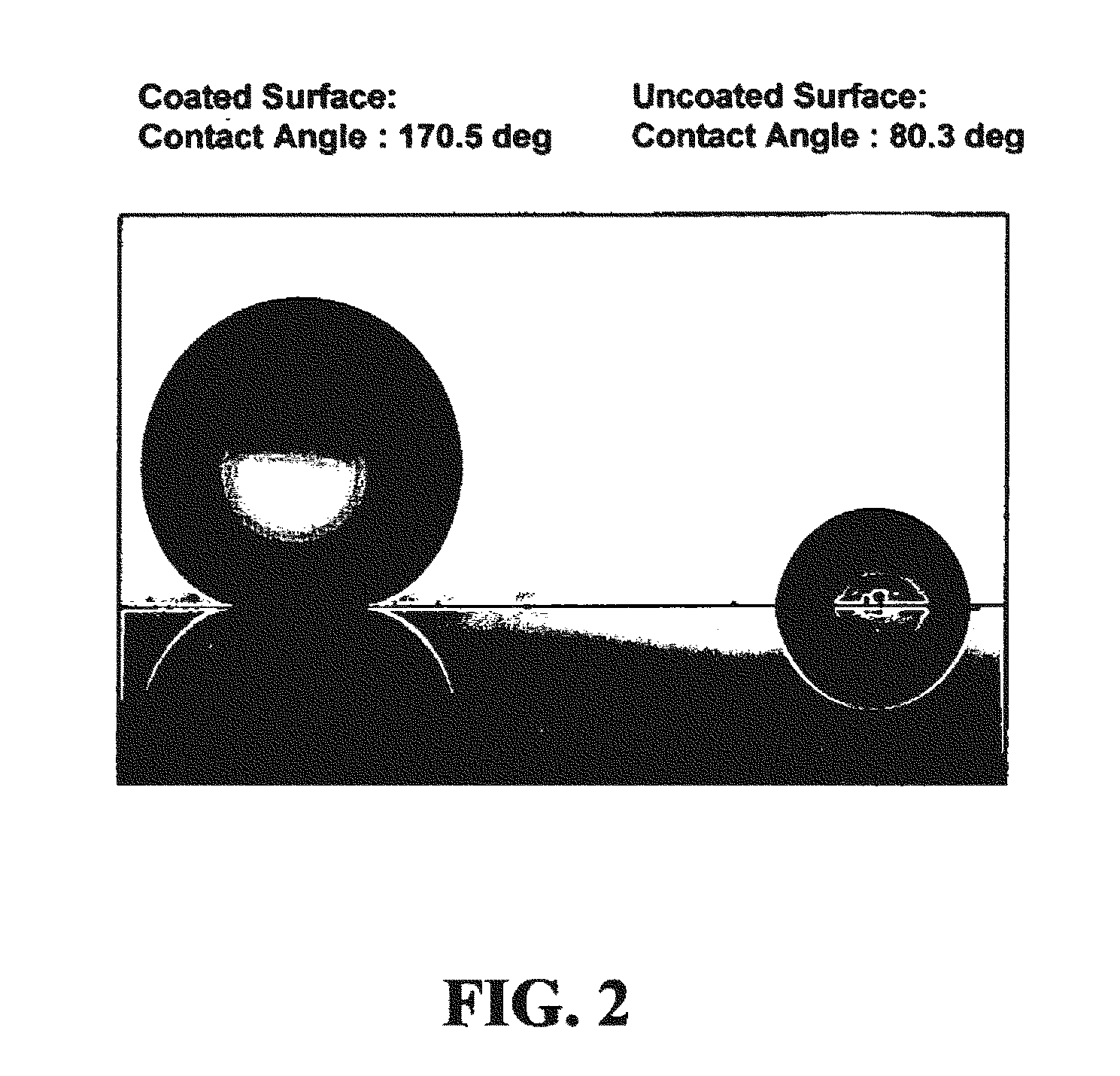
A coating composition and process for generating transparent, near-transparent, and semi-transparent super-hydrophobic coatings on surfaces having a contact angle of greater than 165 degrees. The composition comprises hydrophobic nanoparticles of silsesquioxanes containing adhesion promoter groups and low surface energy groups.
Owner:ASHLAND LICENSING & INTPROP LLC
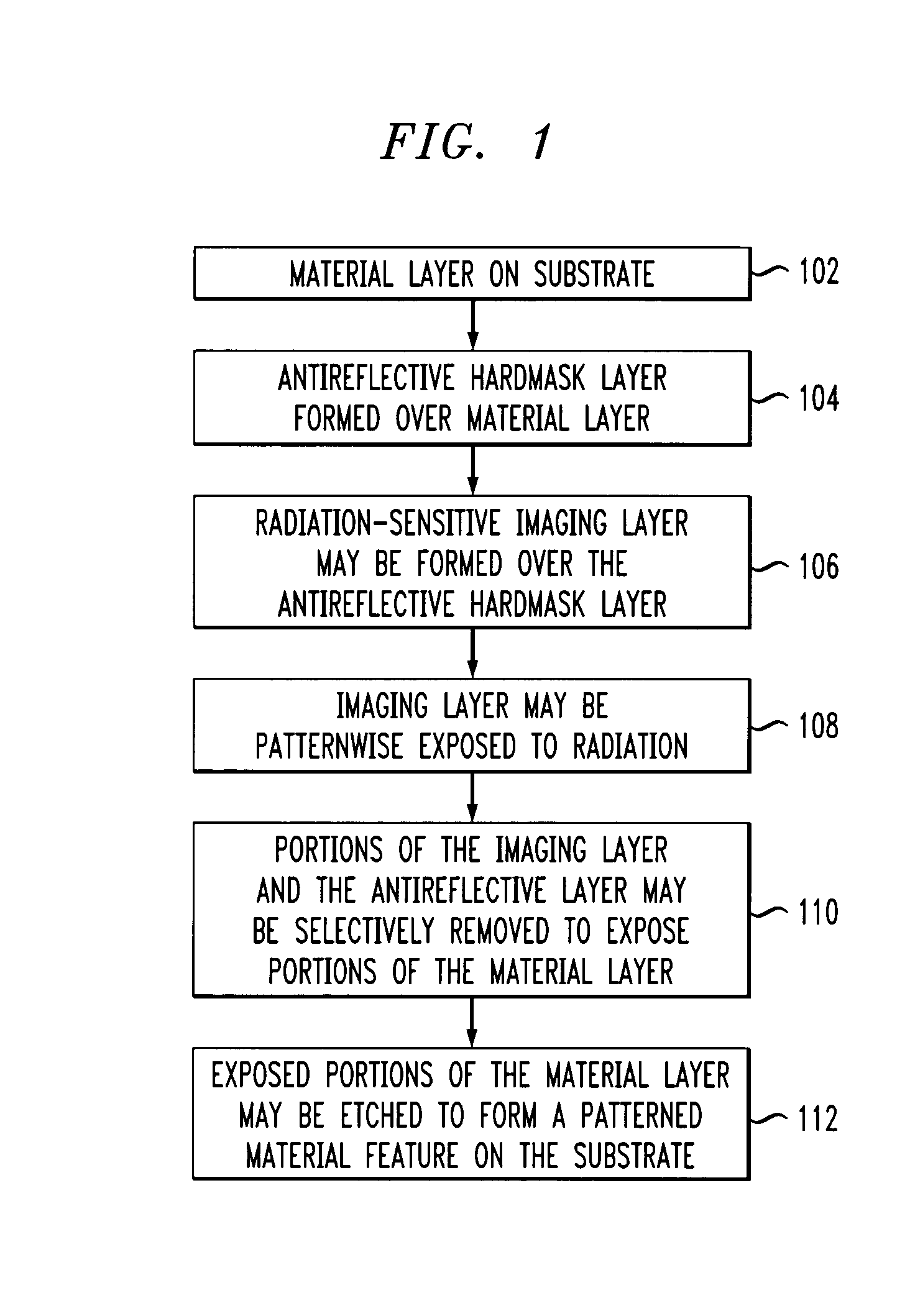
Lithographic antireflective hardmask compositions and uses thereof
InactiveUS20050031964A1Photosensitive materialsSemiconductor/solid-state device manufacturingDevice materialProviding material
Compositions and techniques for the processing of semiconductor devices are provided. In one aspect of the invention, an antireflective hardmask composition is provided. The composition comprises a fully condensed polyhedral oligosilsesquioxane, {RSiO1.5}n, wherein n equals 8; and at least one chromophore moiety and transparent moiety. In another aspect of the invention, a method for processing a semiconductor device is provided. The method comprises the steps of: providing a material layer on a substrate; forming an antireflective hardmask layer over the material layer. The antireflective hardmask layer comprises a fully condensed polyhedral oligosilsesquioxane, {RSiO1.5}n, wherein n equals 8; and at least one chromophore moiety and transparent moiety.
Owner:GLOBALFOUNDRIES INC
Shape memory polymers based on semicrystalline thermoplastic polyurethanes bearing nanostructured hard segments
InactiveUS7091297B2High modulusSufficient cross-linkingStentsOther chemical processesPolymer scienceAdhesive
Thermoplastic polyurethanes having an alternating sequence of hard and soft segments in which a nanostructured polyhedral oligomeric silsesquioxane diol is used as a chain extender to form a crystalline hard segment constituting SMPs. The polyurethanes are formed by reacting a polyol, a chain extender dihydroxyl-terminated POSS and a diisocyanate. The polyurethanes have multiple applications including for example, implants for human health care, drug delivery matrices, superabsorbant hydrogels, coatings, adhesives, temperature and moisture sensors, etc.
Owner:UNIV OF CONNECTICUT
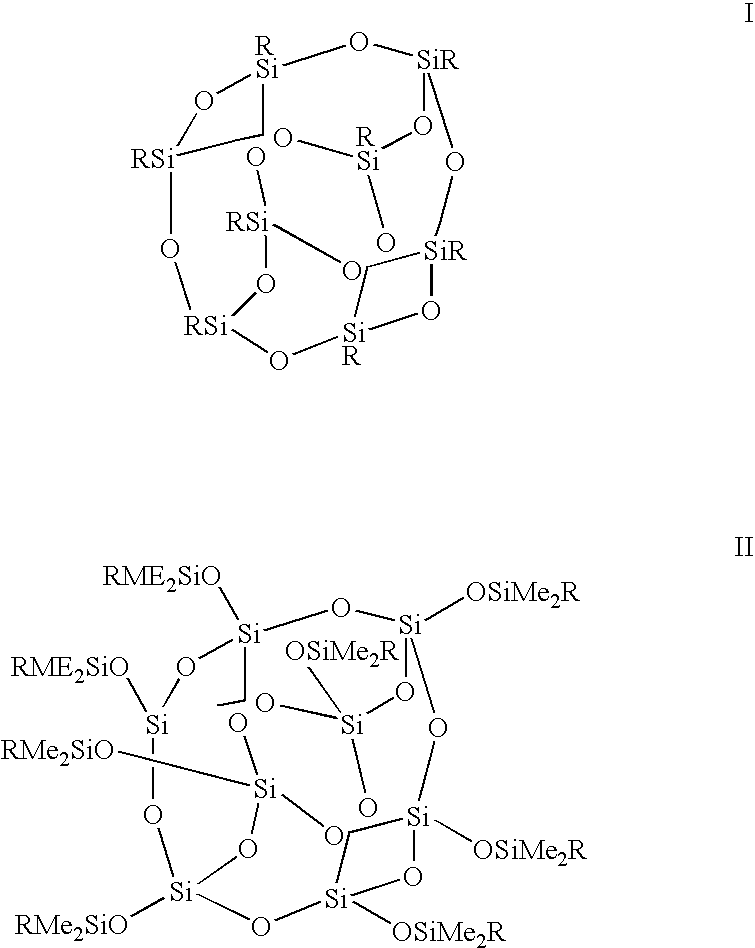
Process for the formation of polyhedral oligomeric silsesquioxanes
Three processes for the manufacture of polyhedral oligomeric silsesquioxanes (POSS) which utilize the action of bases that are capable of either attacking silicon or any compound that can react with a protic solvent (e.g. ROH, H2O etc.) and generate hydroxide [OH]−, alkoxide [RO]−′, etc. The first process utilizes such bases to effectively redistribute the silicon-oxygen frameworks in polymeric silsesquioxanes [RSiO1.5]28 where ∞=1-1,000,000 or higher into POSS nanostructures of formulas [(RSiO1.5)nΣ#, homoleptic, [(RXSiO1.5)n]Σ#, functionalized homoleptic, [(RSiO1.5)m(R′SiO1.5)n]Σ#, heteroleptic, and {(RSiO1.5)m(RXSiO1.0)n}Σ#, functionalized heteroleptic nanostructures. The second process utilizes base to aid in the formation of POSS nanostructures of formulas [(RSiO1.5)n]Σ# homoleptic and [(RSiO1.5)m(R′SiO1.5)n]Σ# heteroleptic and [(RSiO1.5)m(RXSiO1.0)n]Σ# functionalized heteroleptic nanostructures from silanes RSiX3 and linear or cyclic silsesquioxanes of the formula RX2Si—(OSiRX)m—OSiRX2 where m=0-10, X=OH, Cl, Br, I, alkoxide OR, acetate OOCR, peroxide OOR, amine NR2, isocyanate NCO, and R. The third process utilizes base to selectively ring-open the silicon-oxygen-silicon (Si—O—Si) bonds in POSS structures to form POSS species with incompletely condensed nanostructures. These processes also afford stereochemical control over X. The three processes result in new POSS species that can undergo additional chemical manipulations to ultimately be converted into POSS-species suitable for polymerization, grafting, or other desirable chemical reactions.
Owner:HYBRID PLASTICS INC
Photopatternable dielectric materials for beol applications and methods for use
InactiveUS20090233226A1Electric discharge tubesPhotosensitive materialsThermal energyCooking & baking
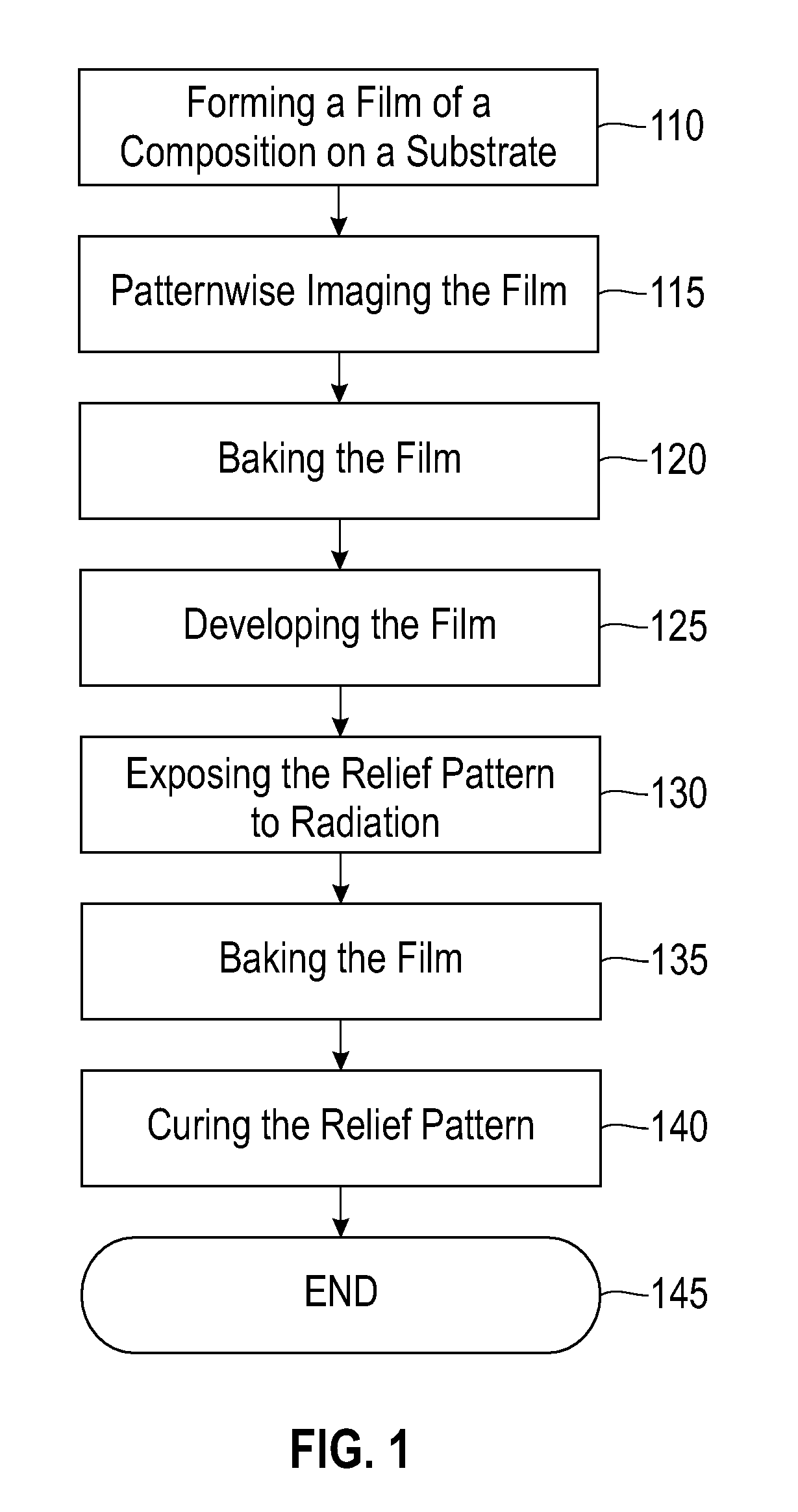
Compositions, a method, and a photopatternable blend. The compositions include a blend of a first and a second polymer. The first polymer is a substituted silsesquioxane copolymer. The second polymer is a substituted silsesquioxane polymer. The second polymer is configured to undergo chemical crosslinking with the first polymer, the second polymer, or a combination thereof, upon exposure to light, thermal energy, or a combination thereof. The compositions include a photosensitive acid generator. The method includes forming a film. The film is patternwise imaged, and at least one region is exposed to radiation. After the imaging, the film is baked, wherein at least one exposed region is rendered substantially soluble. After the baking, the film is developed, wherein a relief pattern remains. The relief pattern is exposed to radiation. The relief pattern is baked. The relief pattern is cured. A chemically amplified positive-tone photopatternable blend is also described.
Owner:GLOBALFOUNDRIES INC
Resist resin, resist resin composition and method of forming pattern using resist resin and resist resin composition
InactiveUS6303268B1Excellent in oxygen plasma etching resistanceHigh resolutionElectric discharge tubesPhotosensitive materialsResistMeth-
A resist resin comprising a copolymer in which a (meth)acrylic structure having a side chain group decomposable with an acid and a polyorganosilsesquioxane structure represented by formula (1) are present in one molecule or a mixture of polymers in which these structures are each present in different molecules as well as a method of forming a pattern using the resist resin:wherein a reference character or numeral represents the same meaning as recited in the specification.The resist resin of the present invention has high sensitivity to a radiation having a short wavelength of 220 nm or less and is capable of forming a fine pattern of 0.15 mum or less.
Owner:SHOWA DENKO KK
Reactive grafting and compatibilization of polyhedral oligomeric silsesquioxanes
InactiveUS6933345B1Rapid low cost modificationEasy to controlMaterial nanotechnologyFiberSilsesquioxane
The nanoscopic dimensions of polyhedral oligomeric silsesquioxanes (POSS) and polyhedral oligomeric silicates (POS) materials ranges from 0.7 nm to 5.0 nm and enables the thermomechanical and physical properties of polymeric materials to be improved by providing nanoscopic reinforcement of polymer chains at a length scale that is not possible by physically smaller aromatic chemical systems or larger fillers and fibers. A simple and cost effective method for incorporating POSS / POS nanoreinforcements onto polymers via the reactive grafting of suitably functionalized POSS / POS entities with polymeric systems amenable to such processes is described. The method teaches that the resulting POSS-grafted-polymers are particularly well suited for alloying agents by nongrafted POSS entitles such as molecular silicas. The successful alloying of POSS-polymers is aided because their interfacial tensions are reduced relative to non-POSS containing systems.
Owner:HYBRID PLASTICS INC
Fire resistant flexible ceramic resin blend and composite products formed therefrom
InactiveUS20100304152A1Improve fire performanceConducive to lightweightSynthetic resin layered productsCellulosic plastic layered productsAdhesiveComposite laminates
High heat resistant elastic composite laminates, sealants, adhesives, and coatings developed from a resin blend. The resin blend is made up of methyl and optionally phenyl silsequioxane resins selected to produce silanol-silanol condensation silicone polymers formed in a slowly evolving reaction mass containing submicron boron nitride, silica and boron oxide fillers. The required ratio of submicron boron nitride to silica has been discovered for assuring the formation of a high temperature resistant elastic composite blend that will form intermediate flexible ceramic products up to 600 deg C., then continue to form preceramic then dense ceramic products from 600 to 1000 deg C. The thermal yield of the composite is generally greater than 90 wt. % at 1000 deg C. Composite products with different levels of heat transformation can be fabricated within the same product depending upon the thickness of the layers of reinforcement.
Owner:FLEXIBLE CERAMICS
Durable hydrophobic surface coatings using silicone resins
Owner:PENNZOIL QUAKER STATE CO
Inertial separator
An inertial separator comprising a tubular body, the tubular body comprises a composition comprising a polymer selected from the group consisting of a cyclic olefin polymer and a cyclic olefin copolymer, and, a polyhedral oligomeric silsesquioxane and / or a flame retardant and / or an anti-static additive, is disclosed.
Owner:PALL CORP
Coating material containing POSS acrylate copolymer and preparing method
The invention relates to a manufacturing method for coat material containing POSS acrylate copolymer. It takes free radical copolymerized to acr monomer and other acr monomer, and uses as modifier adding into the base compounding of UV coat, after taking UV solidification the coat material of high rigidity would be gained. The constituent includes 40-55% light-cured resin, 5-20% active modifier, 20-45% spike, 1-10% photoinitiator, 2-10% anti-foam additive. The rigidity of coat could reach to 6H, and the shrinkage ratio could reach 2%.
Owner:XIAMEN UNIV
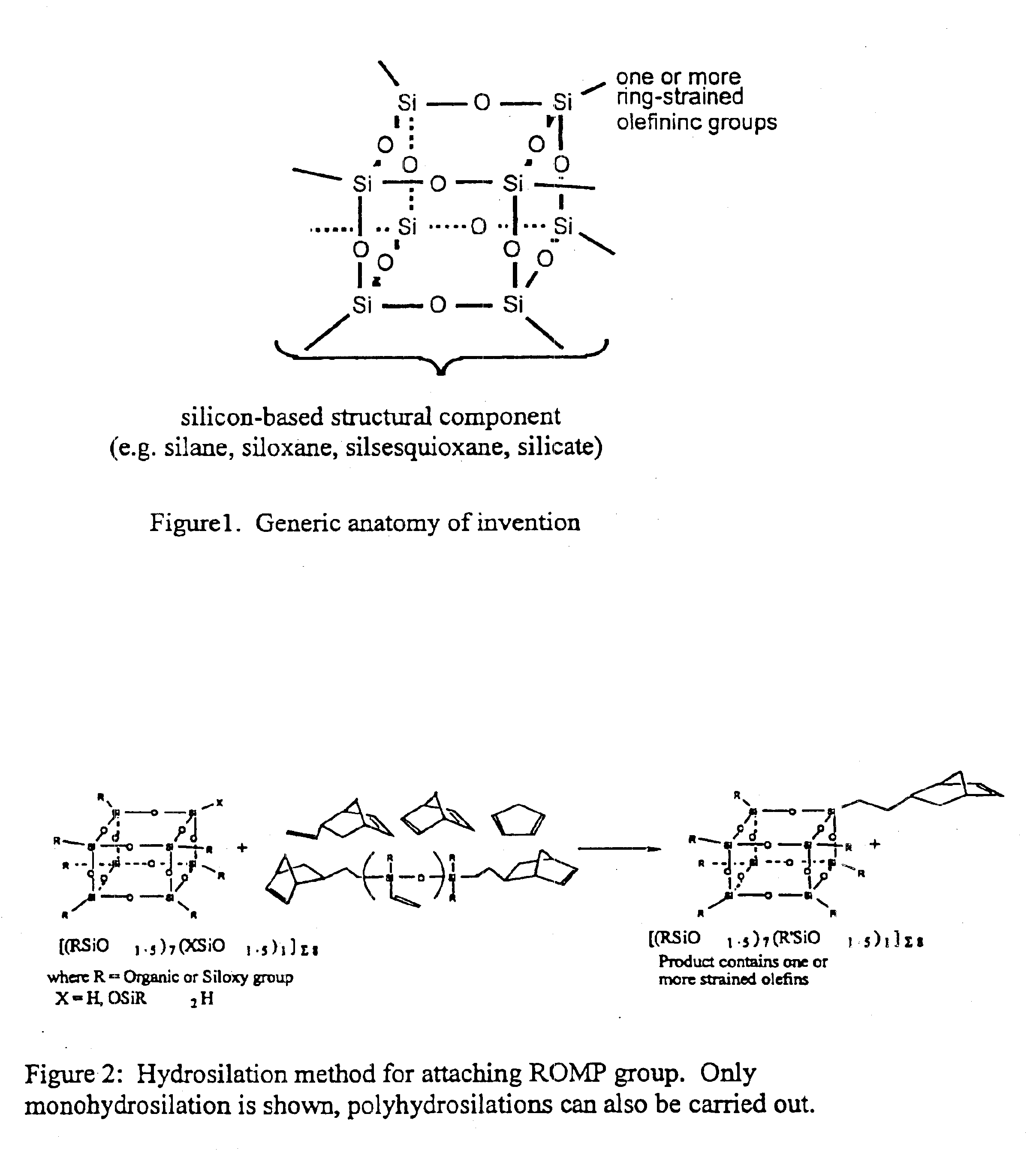
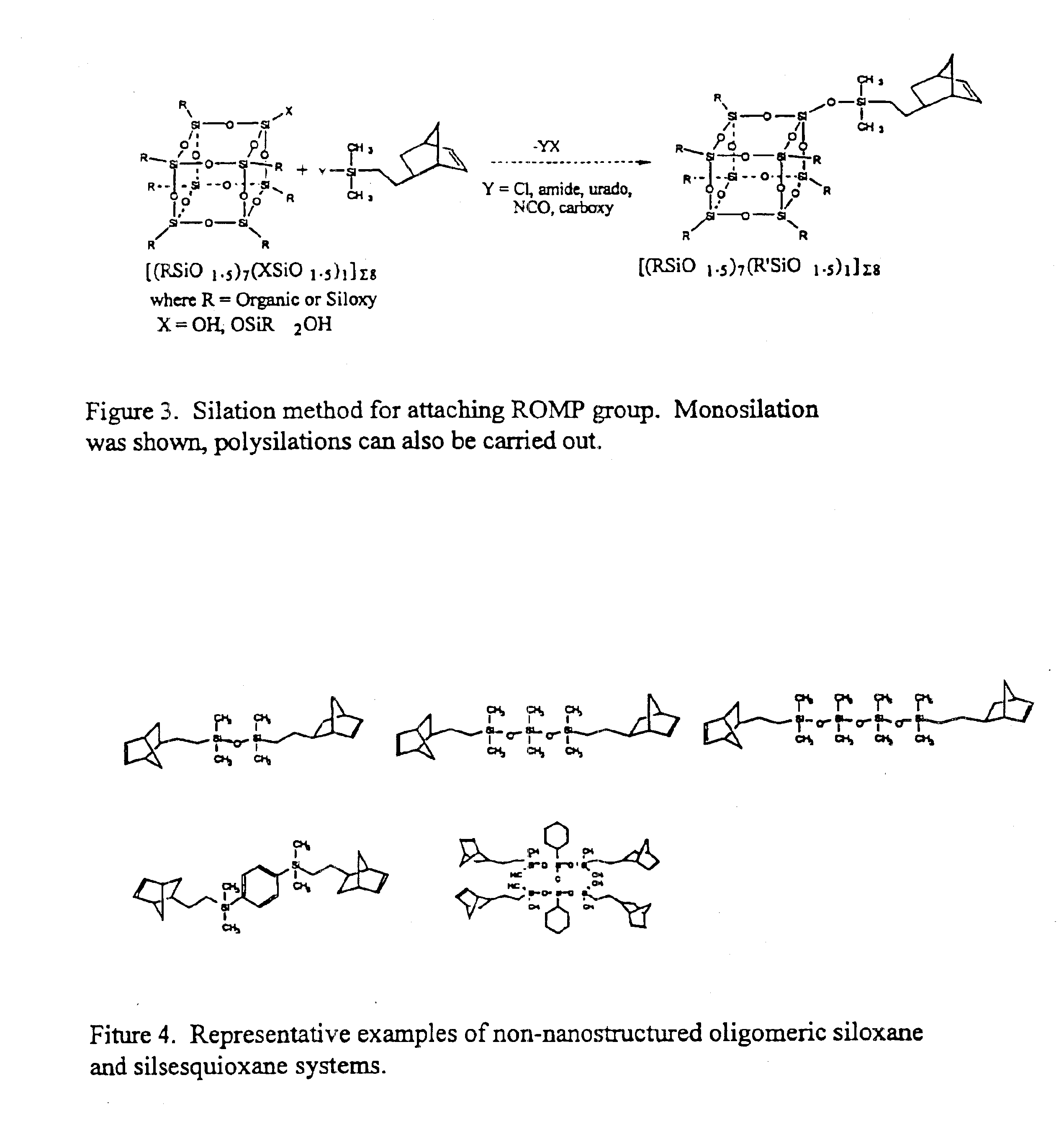
Polyhedral oligomeric -silsesquioxanes, -silicates and -siloxanes bearing ring-strained olefinic functionalities
InactiveUS6911518B2Improving biocompatabilityImprove fire resistanceSilicon organic compoundsGroup 6/16 organic compounds without C-metal linkagesAlkaline earth metalChemical reaction
Processes have been developed for the manufacture of polyhedral oligomeric silsesquioxanes (POSS), polysilsesquioxanes, polyhedral oligomeric silicates (POS), and siloxane molecules bearing reactive ring-strained cyclic olefins (e.g. norbornenyl, cyclopentenyl, etc. functionalities). The preferred manufacturing processes employ the silation of siloxides (Si—OA, where A=H, alkaline or alkaline earth metals) with silane reagents that contain at least one reactive ring-strained cyclic olefin functionality [e.g., X3-ySi(CH3)y(CH2)2 where y=1-2 and X=OH, Cl, Br, I, alkoxide OR, acetate OOCR, peroxide OOR, amine NR2, isocyanate NCO, and R]. Alternatively, similar products can be prepared through hydrosilation reactions between silanes containing at least one silicon-hydrogen bond (Si—H) with ring-strained cyclic olefin reagents [e.g., 5-vinyl, 2 norbornene CH2═CH, cyclopentadiene]. The two processes can be effectively practiced using polymeric silsesquioxanes [RSiO1.5]∞ where ∞=1-1,000,000 or higher and which contain unreacted silanol or silane groups at chain terminus or branch points, on POSS nanostructures of formulas [(RSiO1.5)n]Σ#, homoleptic, [(RSiO1.5)m(R′SiO1.5)n]Σ#, heteroleptic, and {(RSiO1.5)m(RXSiO1.0)n}Σ#, functionalized heteroleptic nanostructures, on silanes RSiX3, linear, cyclic, oligomeric and polymeric siloxanes (polymeric formula RX2Si—(OSiRX)m—OSiRX2 where m=0-1000, X=OH, Cl, Br, I, alkoxide OR, acetate OOCR, peroxide OOR, amine NR2, isocyanate NCO, and R). Each of the processes result in new chemical species bearing one or more ring strained olefins that can undergo polymerization, grafting, or other desirable chemical reactions to form polymeric products. These polymeric systems are most desirably utilized in polymerizations for the modification of properties of thermoplastic or thermoset resin systems or for the preparation of polymers with utility in electronics, medical devices, sporting goods, and aerospace as coatings and structural components.
Owner:HYBRID PLASTICS INC
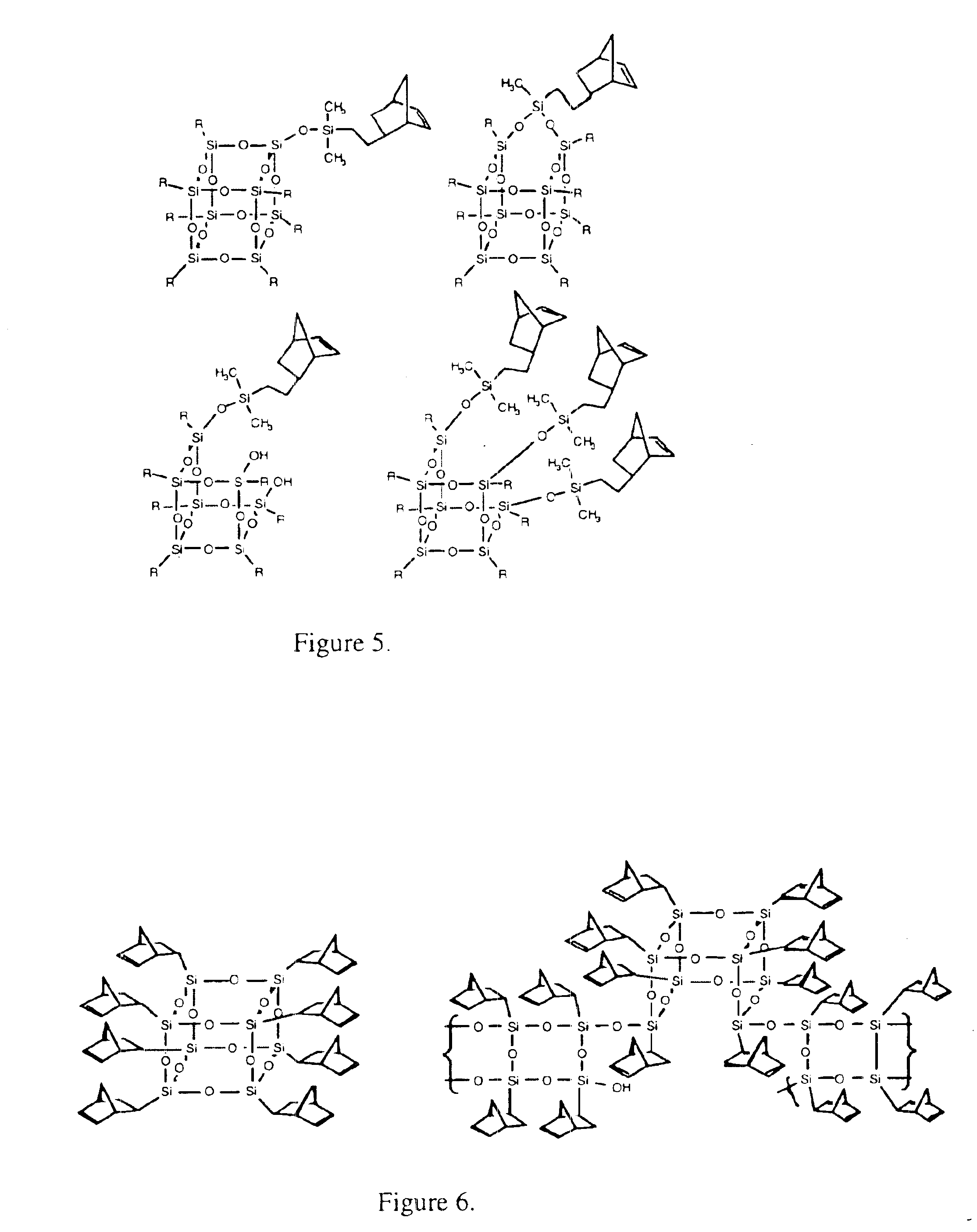
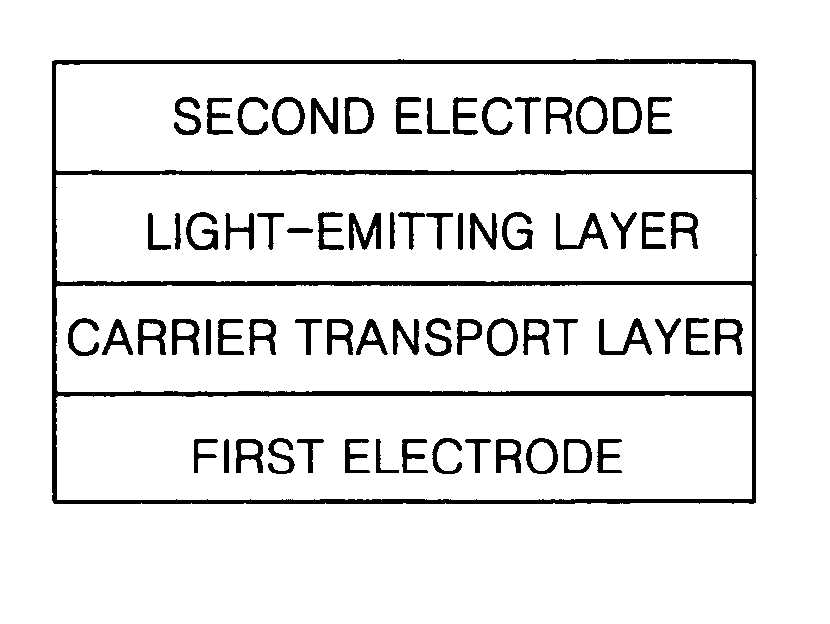
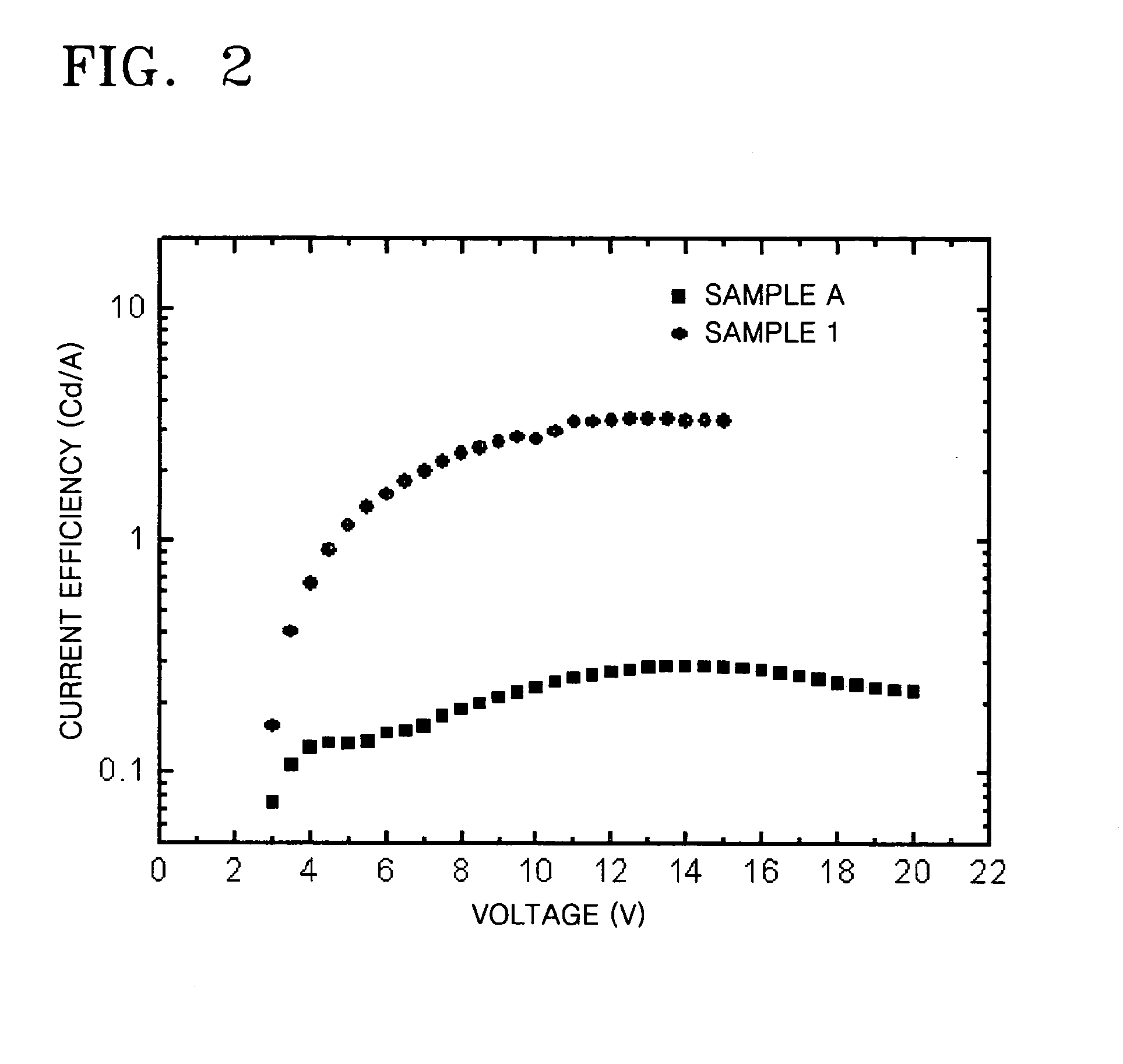
Silsesquioxane-based compound and organic light-emitting device including the same
InactiveUS20070045619A1Effective controlGood film smoothnessSilicon organic compoundsMaterial nanotechnologyOrganic light emitting deviceSilsesquioxane
A silsesquioxane-based compound represented by Formula 1 and an organic light-emitting device including the same: wherein R1, R2, R3, R4, R5, R6, R7, and R8 are as defined in the specification. The use of the silsesquioxane-based compound enables to produce an organic light-emitting device with improvement in electrical characteristics such as brightness and efficiency. The silsesquioxane-based compound can exhibit good film smoothness and adhesion, and at the same time, good electrical characteristics such as current efficiency and brightness, and thus, is suitable for use in an organic light-emitting device.
Owner:SAMSUNG DISPLAY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com