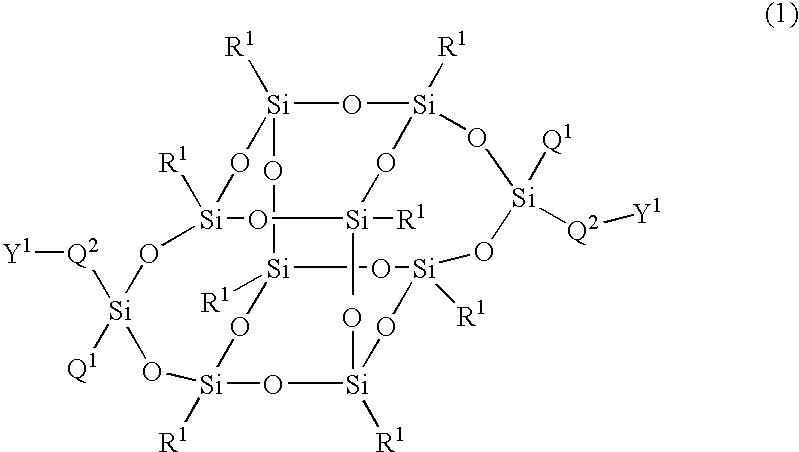
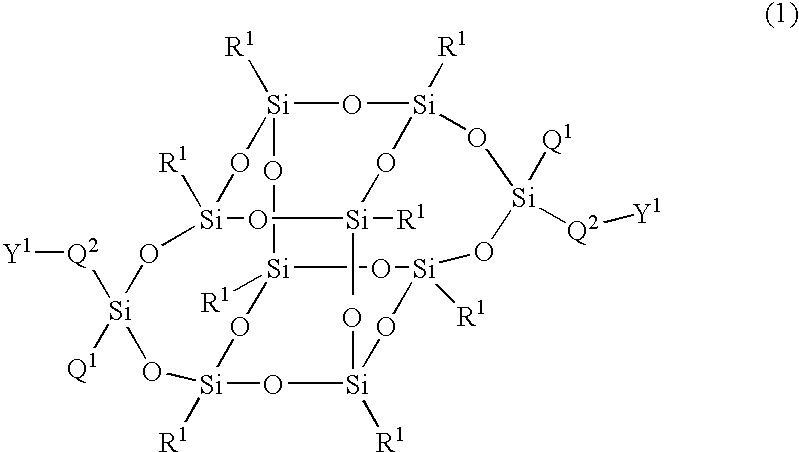
Compound having silsesquioxane skeleton and its polymer
a technology of silsesquioxane and polymer, which is applied in the direction of silicon organic compounds, group 4/14 element organic compounds, synthetic resin layered products, etc., can solve the problems of poor compatibility of polyorganosiloxane, prone to cloudiness, and prone to whitening of coating film obtained from the coating agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
<Production of Compound (1-3-7)>
Compound (1-3-7) was produced via the following route:
First Stage: Production of Allyl p-nitrophenyl Ether
Potassium carbonate (49.7 g, 0.36 mol) was added to an N,N-dimethylformamide (250 ml) solution of p-nitrophenol (25.0 g, 0.18 mol) under nitrogen atmosphere and suspended, and 3-bromopropene (21.7 g, 0.18 mol) was dropwise added thereto. After finishing dropwise adding, the solution was stirred at a room temperature for 5 hours, and then extracting practice with diethyl ether was carried out after adding water to the solution. The organic layer was washed with water and then dried on anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure, and the residue thus obtained was refined by means of silica gel chromatography (eluent solvent: toluene). Toluene was distilled off under reduced pressure, and then the resulting residue was recrystallized from ethanol to obtain allyl p-nitrophenyl ether (25.7 g).
Second St...
example 2
<Production of Compound (1-1-4)>
Compound (1-1-4) was produced via the following route:
THF (150 ml) was added to the compound (a) (50.0 g, 43.3 mmol) under nitrogen atmosphere and suspended, and a platinum-divinylsiloxane complex (3 wt % toluene solution, 320 μl) was added thereto and heated to 90° C. Allylsuccinic anhydride (14.5 g, 103.5 mmol) was dropwise added thereto in 5 minutes, and the solution was heated for 7 hours while refluxing. After standing to cool, the solvent was distilled off under reduced pressure, and then methanol (150 ml) was added to the resulting residue and stirred at a room temperature for 2 hours. The resulting solid matter was filtered and dissolved in THF (150 ml), and activated carbon (6 g) was added thereto, followed by stirring the mixture at a room temperature for 2 hours. After filtering off the activated carbon, THF was distilled off under reduced pressure to obtain Compound (1-1-4) 55.9 g.
1H-NMR (solvent: CDCl3): δ (ppm); 0.32 (s, 6H), 0...
example 3
<Production of Compound (1-1-1)>
Compound (1-1-1) was produced via the following route:
First Stage: Production of Compound (d)
3-Acetoxypropylmethyldichlorosilane (5.4 g, 25 mmol) was added to a mixture of a compound (c) (11.6 g, 10 mmol), triethylamine (2.5 g, 25 mmol) and THF (200 ml) under nitrogen atmosphere, and the solution was stirred at a room temperature for 3 hours. Toluene (200 ml) and water (100 ml) were added thereto and stirred, and the organic layer was washed with water and then dried on anhydrous magnesium sulfate. Toluene was distilled off under reduced pressure, and the residue thus obtained was washed with methanol and recrystallized from ethanol / ethyl acetate (100 ml) to obtain a compound (d) 6.51 g.
1H-NMR (solvent: CDCl3): δ (ppm); 0.31 (s, 6H), 0.72-0.75 (t, 4H), 1.70-1.74 (m, 4H), 1.88 (s, 6H), 3.91-3.94 (t, 4H), 7.18-7.52 (m, 40H). 29Si-NMR (solvent: CDCl3): δ (ppm); −17.8 (d, 2Si), −78.4 (s, 4Si), −79.3 (t, 4Si).
Second Stage: Production of Compo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Optical properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com