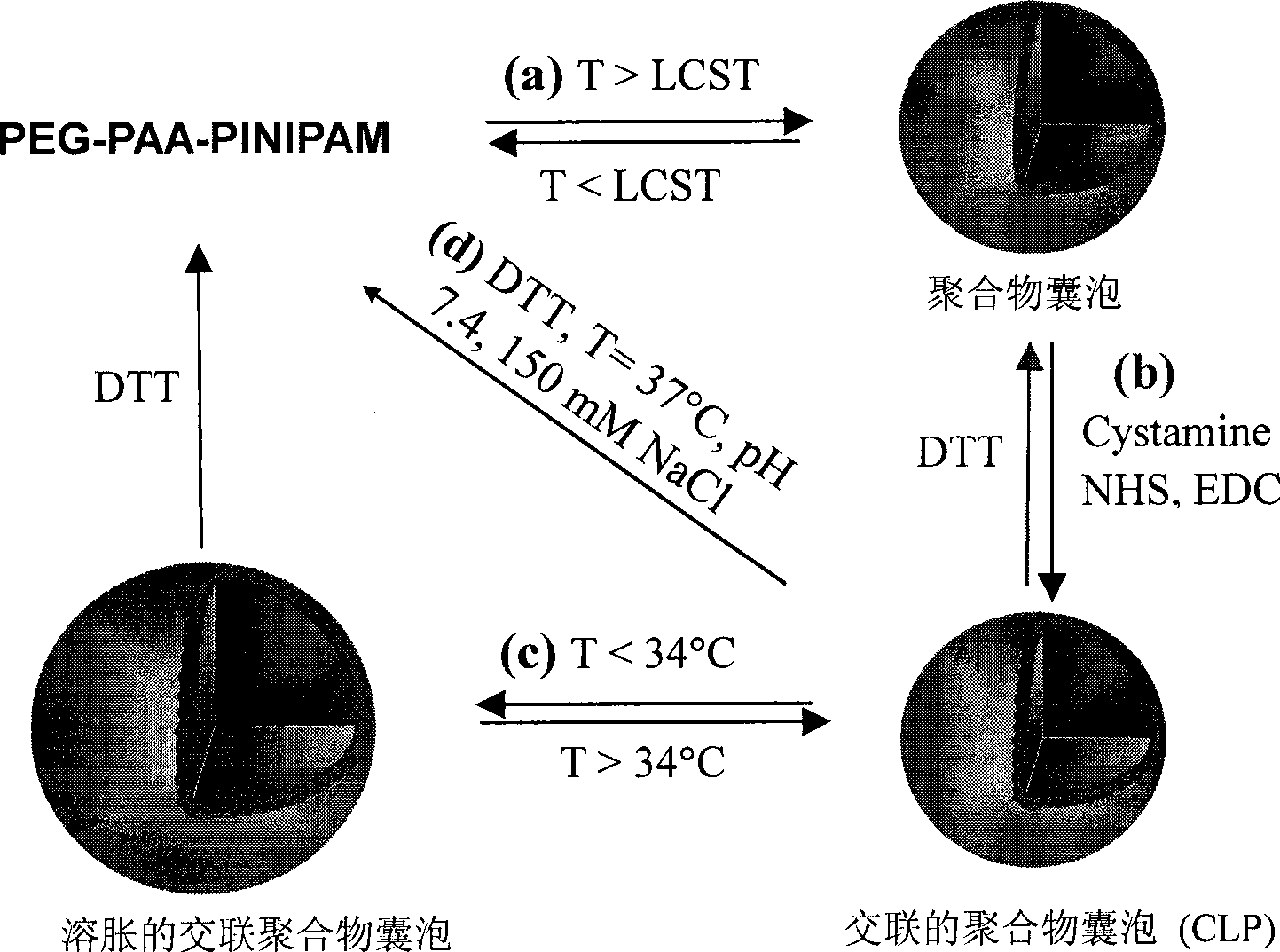
Interface-cross-linked temperature-sensitive polymer vesicle and use thereof
A technology of interfacial cross-linking and polymers, which is applied in the direction of medical preparations and pharmaceutical formulations of non-active ingredients, can solve the problems of premature drug release, low drug bioavailability, and low efficiency, and achieve high-efficiency entrapment and overcoming The effect of low drug entrapment efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
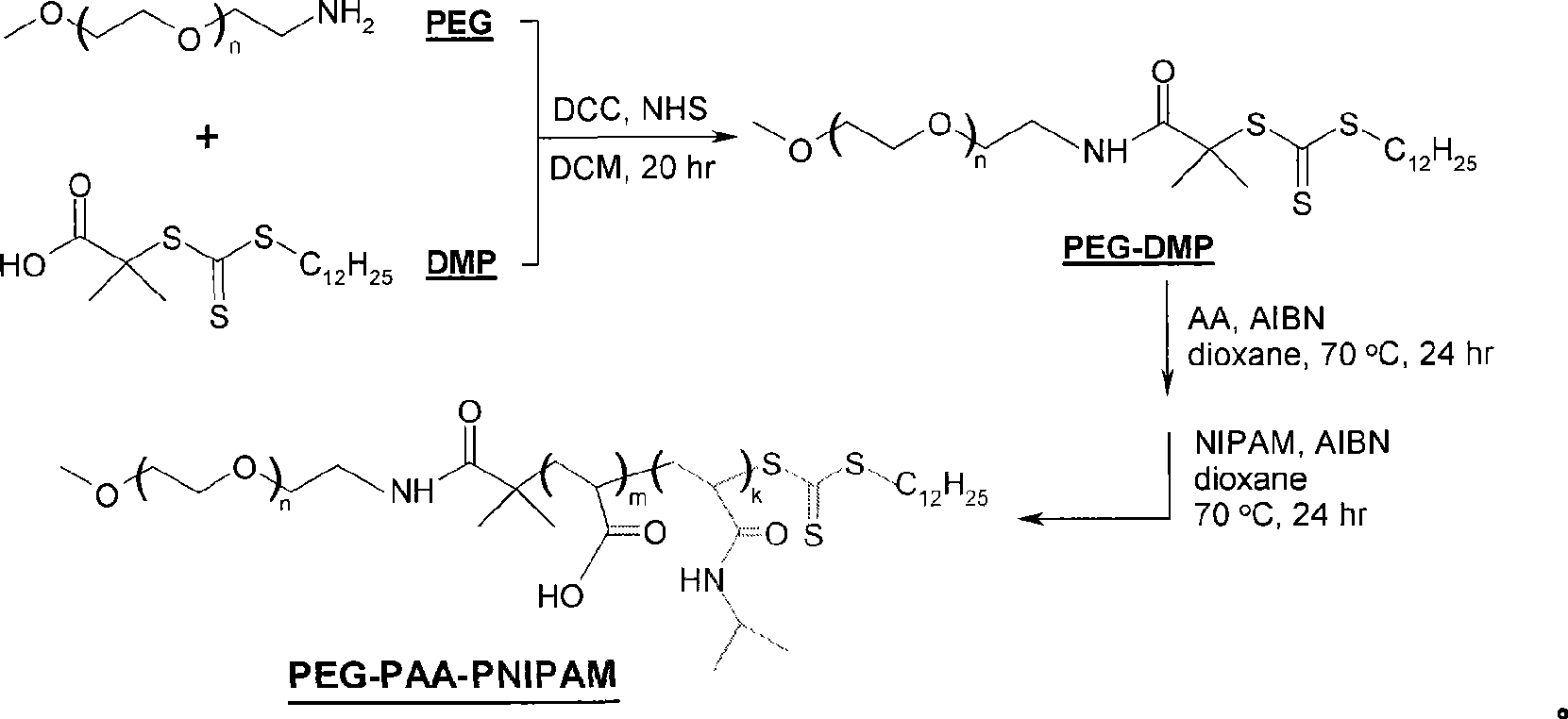
[0039] Example 1, RAFT polymerization to obtain triblock copolymer PEG-PAA-PNIPAM
[0040] Under argon protection, the initiator AIBN (0.62mg, 3.77μmol), the macromolecular RAFT reagent PEG-DMP (0.10g, 19μmol), acrylic monomer AA (0.0328g, 0.456mmol) and 5.0mL of dioxane Add it to a 10mL Schlenk vacuum-sealed bottle, put the bottle in an oil bath at 70℃ after 30 minutes of argon gas, stir and react for 24 hours, take a sample to determine the conversion rate of AA monomer, and add the second order to the rest NiPAAM (0.4260g, 3.8mmol) and some AIBN (0.2mg dissolved in 0.2mL dioxane) were allowed to react at 70°C for another 24 hours. After the reaction, it was precipitated in cold ether and dried under vacuum for 48 hours. , The triblock copolymer is obtained, and the yield is 70 to 86%. NMR results show that its structure is PEG 113 -PAA 24 -PNIPAM 211 PNIPAM accounts for about 78% of the molecular weight of the entire molecule, and its LCST in PBS is 38.5°C.
Embodiment 2
[0041] Example 2: RAFT polymerization to obtain block copolymer DEX-PMA-NIPAM
[0042] Under argon protection, the initiator AIBN (0.62mg, 3.77μmol), the macromolecular RAFT reagent DEX-CPDPA (0.127g, 20μmol), the methacrylic acid monomer MA (9.46mg, 110μmol) and 5.0mL of dioxane The ring was added to a 10mL Schlenk vacuum-sealed bottle. After argon for 30 minutes, the bottle was placed in an oil bath at 75°C. After stirring and reacting for 24 hours, a sample was taken to determine the conversion rate of the MA monomer, and the second was added to the rest Monomer NIPAAM (0.7230g, 6.4mmol) and some AIBN (0.2mg dissolved in 0.2mL dioxane) were allowed to react at 75°C for another 24 hours. After the reaction, it was precipitated in cold ether and then dried in vacuum 48 Within hours, a triblock copolymer is obtained with a yield of 70-75%. NMR results show that its structure is DEX37-PMA 5 -PNIPAM 356 , PNIPAM accounts for about 86% of the molecular weight of the entire molecule, ...
Embodiment 3
[0043] Example 3: RAFT polymerization to obtain block copolymer PEG 113 -PHA 10 -NIPAM 90
[0044] Under argon protection, the initiator AIBN (0.62mg, 3.77μmol), the macromolecular RAFT reagent PEG-CPDPA (0.10g, 19μmol), 5-hexenoic acid monomer HA (22.8mg, 200μmol) and 4.0mL of two Add oxane into a 10mL Schlenk vacuum-sealed bottle. After 30 minutes of argon, place the bottle in an oil bath at 75°C. After stirring and reacting for 24 hours, take a sample to determine the conversion rate of HA monomer, and add to the rest The second monomer NIPAM (0.1930g, 1.70mmol) and some AIBN (0.2mg dissolved in 0.2mL dioxane) were allowed to react at 70°C for another 24 hours. After the reaction, it was precipitated in cold ether and vacuum After drying for 48 hours, a triblock copolymer is obtained with a yield of 70-75%. NMR results show that its structure is PEG 113 -PHA 10 -NIPAM 90 PNIPAM accounts for about 61% of the molecular weight of the entire molecule, and its LCST in PBS is 38°C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com