Preparation method of liposome entrapping water-soluble medicines
A technology for water-soluble drugs and liposomes, which is used in liposome delivery, pharmaceutical formulations, and devices that make drugs into special physical or taking forms, etc. The problem of small dosage and other problems can achieve the effect of saving dosage, simple preparation process and solving poor stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: insulin liposome
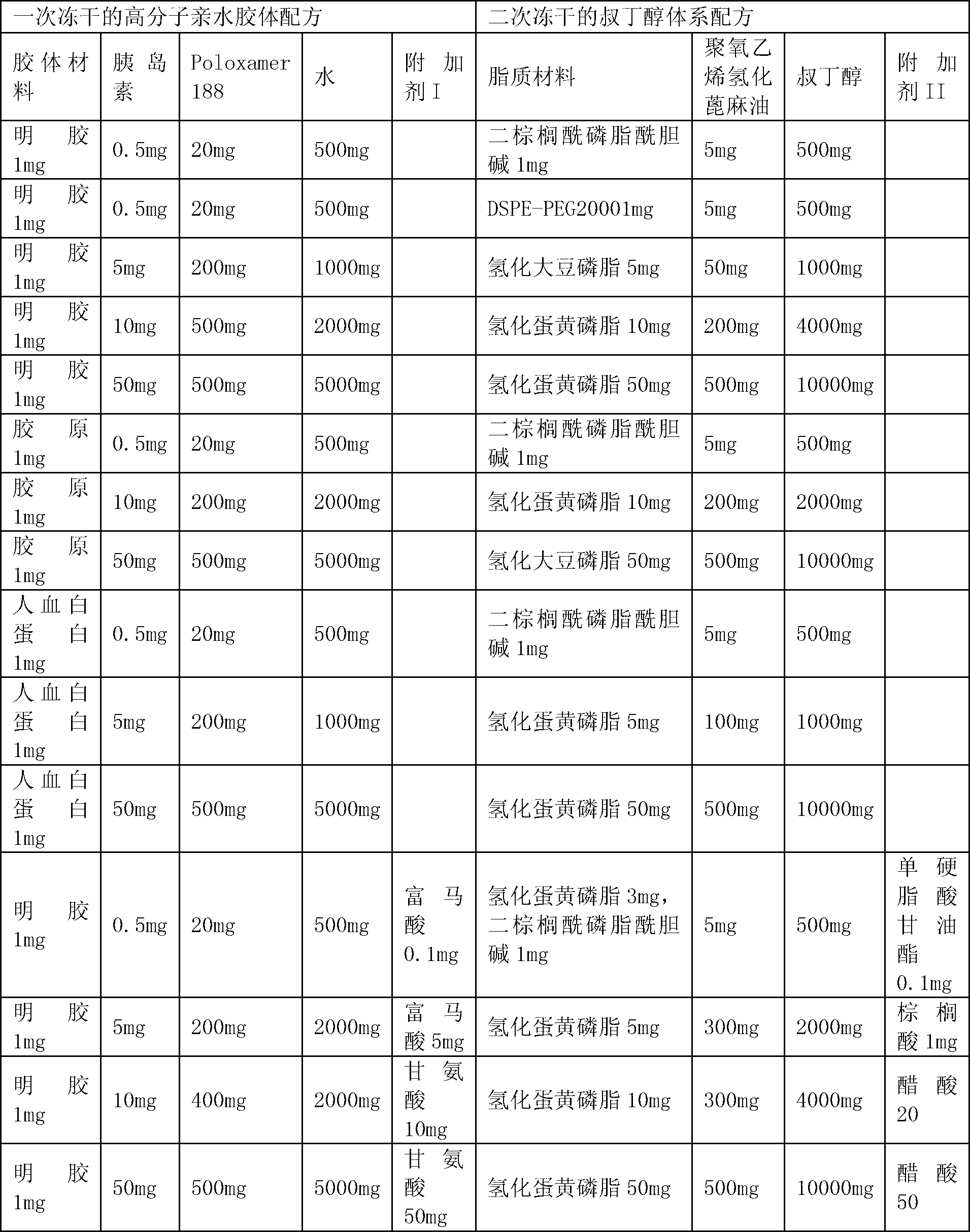
[0050] Insulin liposomes were prepared in this example, and the composition of insulin liposomes is shown in Table 1.
[0051] Table 1 Insulin liposome composition ratio
[0052]
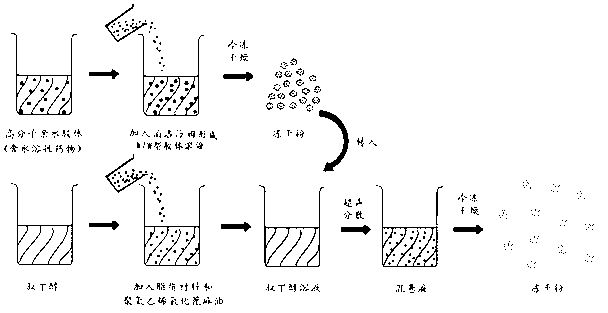
[0053] Insulin liposome lyophilized powder preparation method: as figure 2 As shown in the process flow, according to the mass ratio of the above components, weigh the components respectively, add the polymer hydrophilic colloid material into water to form a solution, add insulin, Poloxamer (Poloxamer), and additive I to dissolve, A hydrocolloid solution containing the drug is formed and freeze-dried to form freeze-dried powder I. Dissolve the lipid material, polyoxyethylene hydrogenated castor oil, and additive II in tert-butanol at 60°C, and mix well to form tert-butanol solution I. Add the lyophilized powder I to the tert-butanol solution I, ultrasonicate at 20KHz for 2 minutes to form the tert-butanol solution II, quickly freeze in liquid nitrogen t...
Embodiment 2
[0056] Embodiment 2: growth hormone liposome
[0057] This embodiment prepares growth hormone liposomes, and the composition of growth hormone liposomes is shown in Table 2.
[0058] Table 2 Somatotropin liposome composition ratio
[0059]
[0060] Preparation method of growth hormone liposome freeze-dried powder: according to the mass ratio of the above components, weigh the components respectively, add the polymer hydrophilic colloid material into water to form a solution, add growth hormone, poloxamer, and additives I is dissolved to form a hydrocolloid solution containing growth hormone, and freeze-dried to form a freeze-dried powder I. Dissolve lipid material, polyoxyethylene hydrogenated castor oil, and additive II in tert-butanol, and heat in a water bath at 55°C to form tert-butanol solution I. Add lyophilized powder I to tert-butanol solution I, ultrasonic (60KHz) for 1 min to form tert-butanol solution II, quick-freeze in liquid nitrogen to form a solid, and lyo...
Embodiment 3
[0061] Embodiment 3: the quality evaluation of growth hormone liposome
[0062] In this example, the growth hormone liposome prepared in Example 2 was used for quality evaluation.
[0063] Microscopic morphology and particle size analysis: Take 100mg of liposome freeze-dried powder and add 2ml of water to form a liposome solution, absorb a certain amount of liposome suspension and stain with 1% phosphotungstic acid, and observe the liposome under a scanning electron microscope. For the morphological characteristics of the plastids, the particle size of the liposomes was measured using a Coulter particle size analyzer.
[0064] Determination of encapsulation efficiency: take 1ml liposome solution, load it on a Sephadex G-50 gel column, use distilled water as the eluent, take different volumes of the eluted part, separate and receive the free growth hormone eluted part, use The content was analyzed by HPLC, and the encapsulation efficiency of the somatotropin liposome was calcu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com